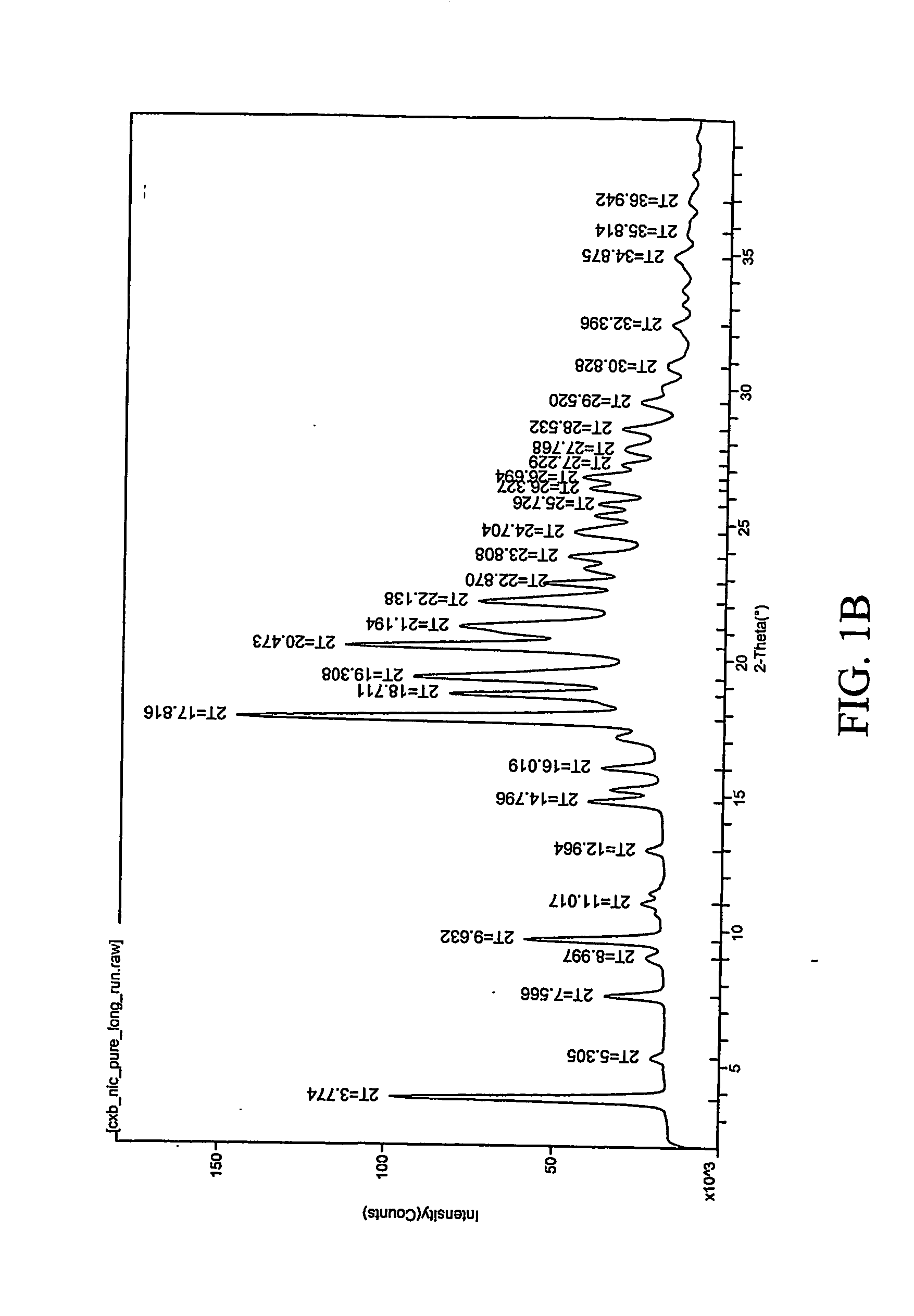
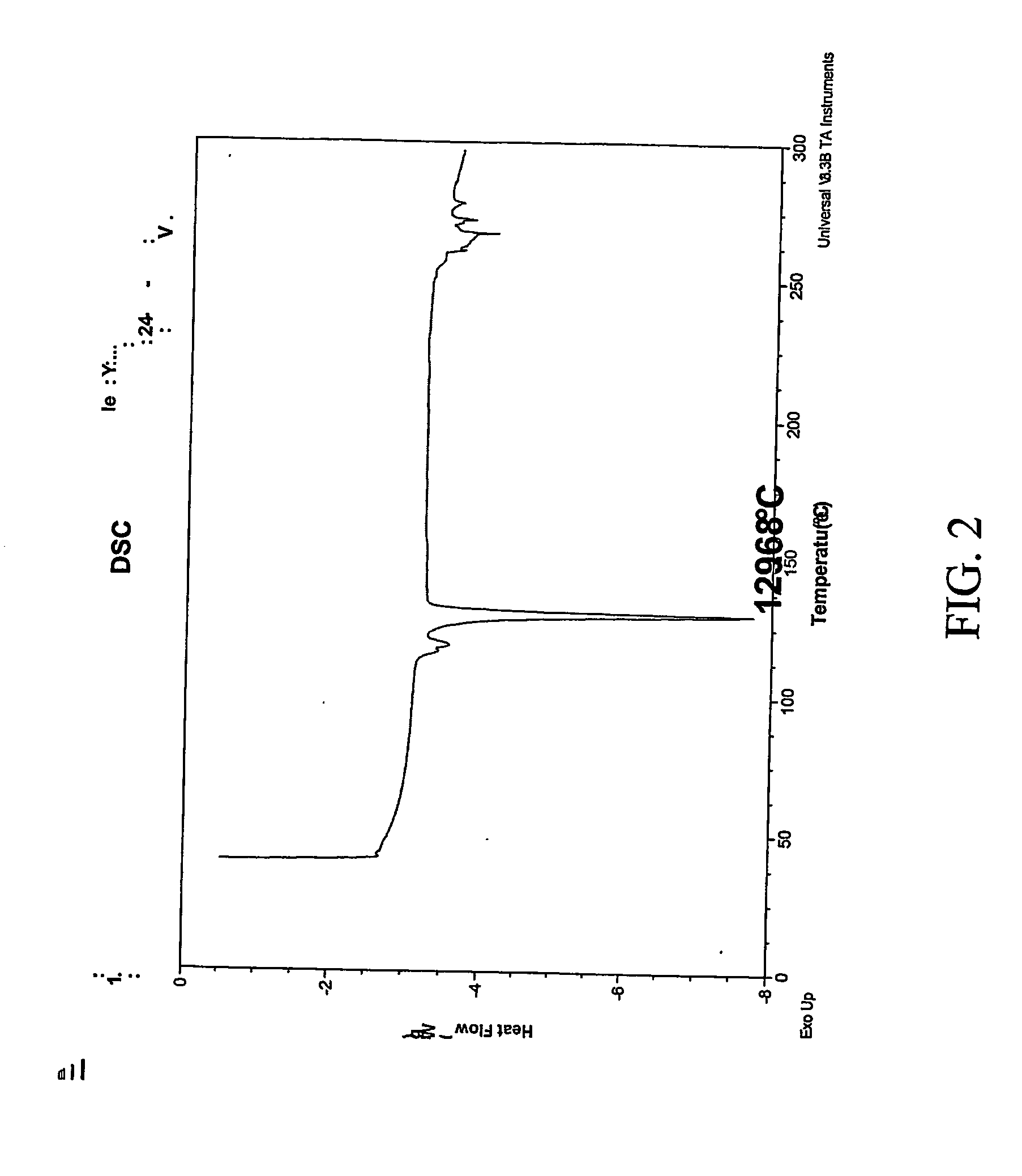
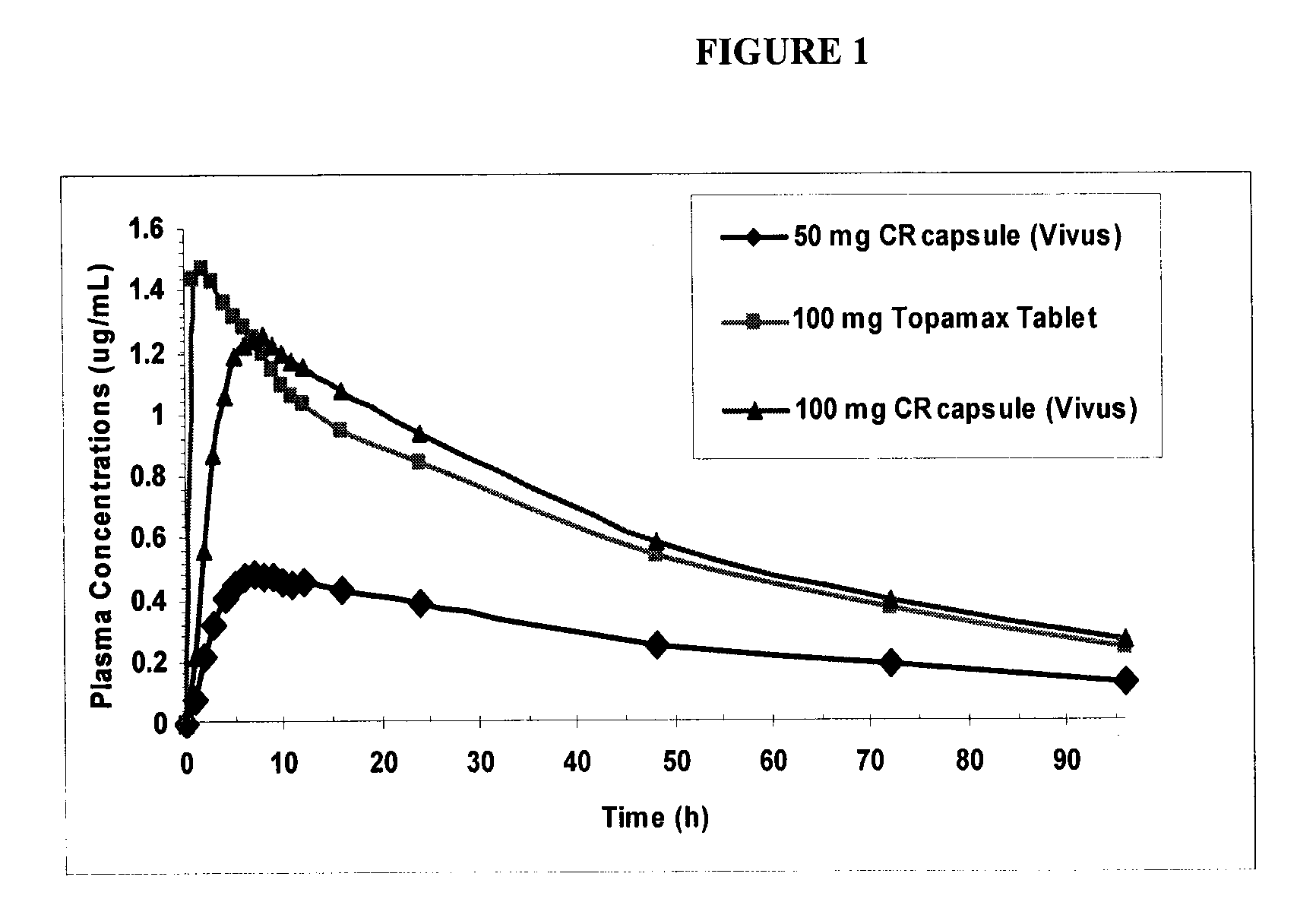
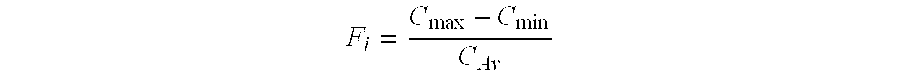Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
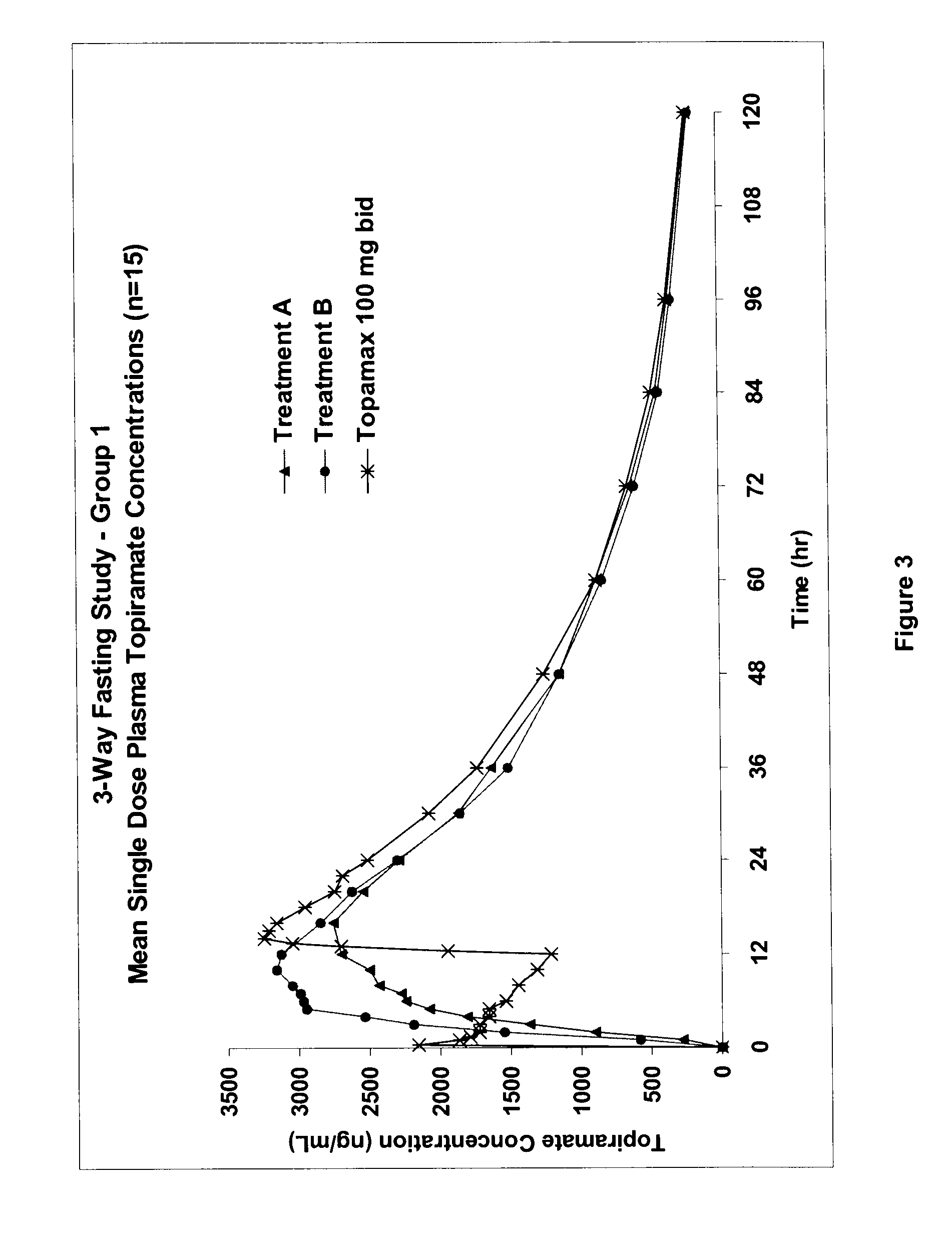
112 results about "Topiramate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Topiramate is used alone and with other medications to prevent and control seizures (epilepsy). It is also used to prevent migraine headaches.
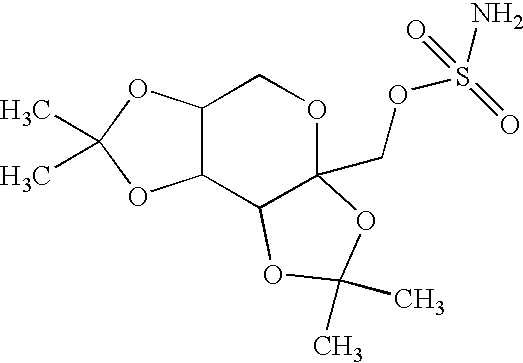
Topiramate sodium trihydrate
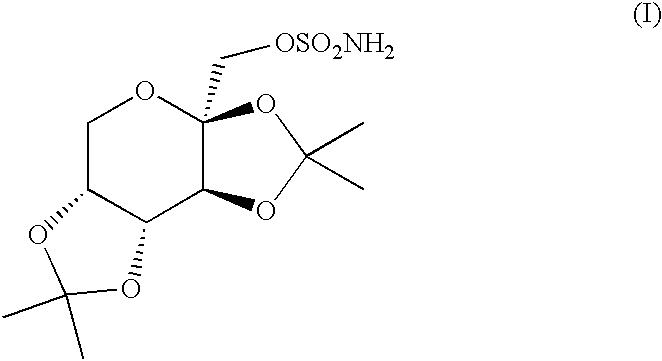
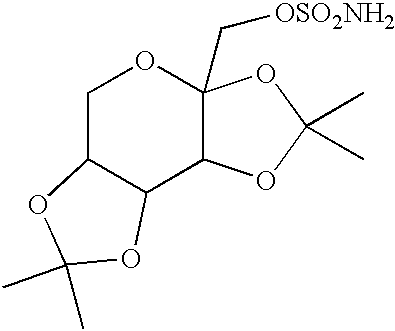
The invention encompasses novel salts of topiramate, and pharmaceutically acceptable polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, or amorphous forms thereof, as well as pharmaceutical compositions and pharmaceutical unit dosage forms containing the same. In particular, the invention encompasses pharmaceutically acceptable salts of topiramate, including without limitation topiramate sodium, topiramate lithium, topiramate potassium, or polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, and amorphous forms thereof. The invention further encompasses novel co-crystals or complexes of topiramate, as well as pharmaceutical compositions comprising them. The invention also encompasses methods of treating or preventing a variety of diseases and conditions including, but not limited to, seizures, epileptic conditions, tremors, cerebral function disorders, obesity, neuropathic pain, affective disorders, tobacco cessation, migraines, and cluster headache.
Owner:ORTHO MCNEIL PHARM INC
Medication Combinations for the Treatment of Alcoholism and Drug Addiction
InactiveUS20110065628A1Decrease and cessationReverses effectBiocideNervous disorderAlcoholismsOndansetron
The present invention provides for the use of combinations of drugs to treat addictive disorders. More specifically, the present invention provides compositions and methods for treating disorders using combinations of drugs such as topiramate, ondansetron, and naltrexone.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Migraine treatment method using topiramate and related compounds
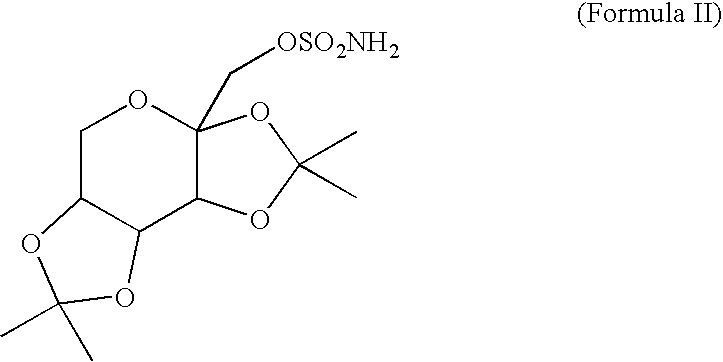
A method for treating migraine in non-epileptic subjects which involves administering to subjects an effective amount of a pharmaceutical composition comprising a sulfamate of the following formula:
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS
Topiramate compositions and methods of enhancing its bioavailability
ActiveUS20080085306A1Reduced adverse eventConvenient treatmentBiocideNervous disorderRegimenImmediate release
Owner:SUPERNUS PHARM INC
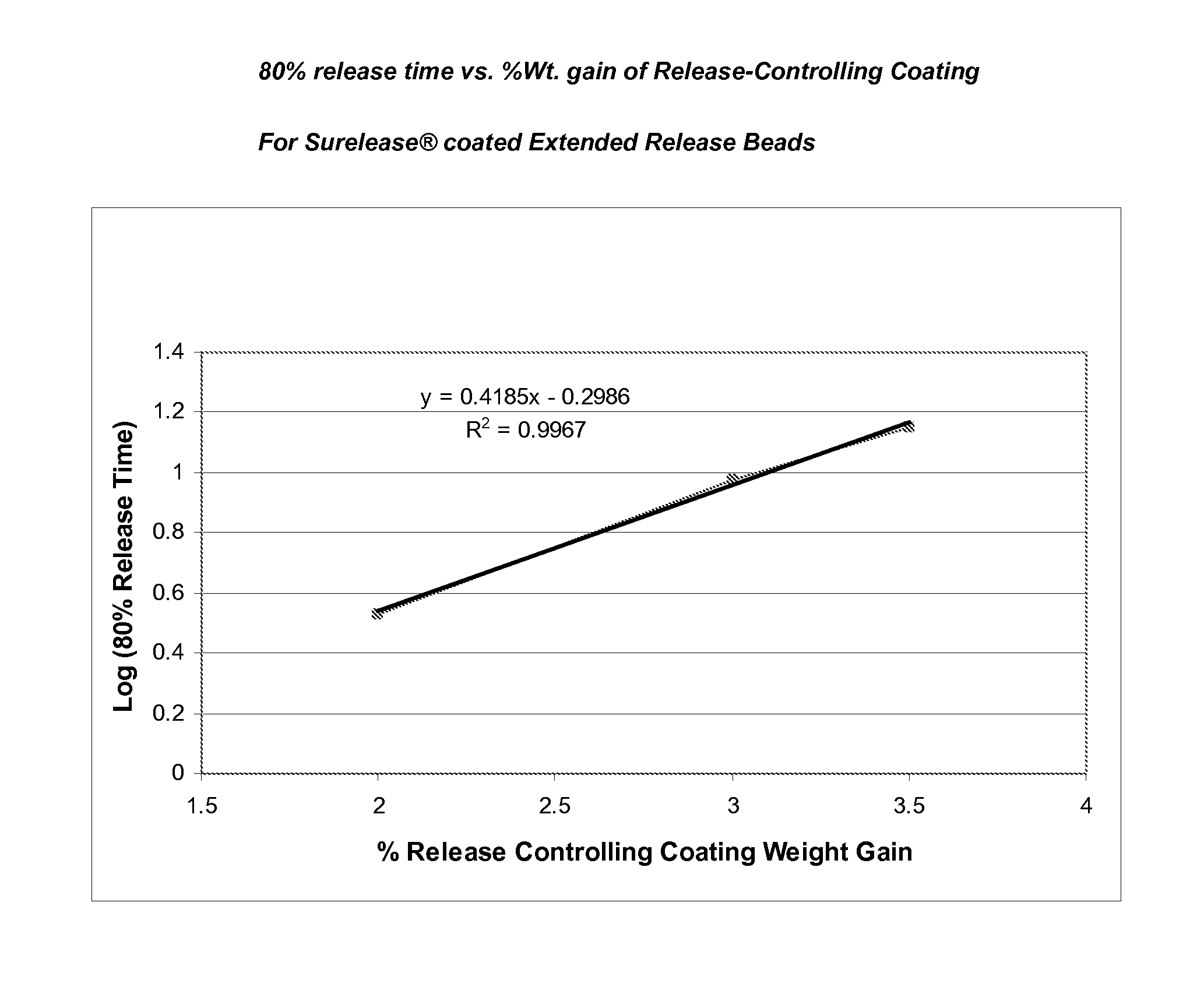
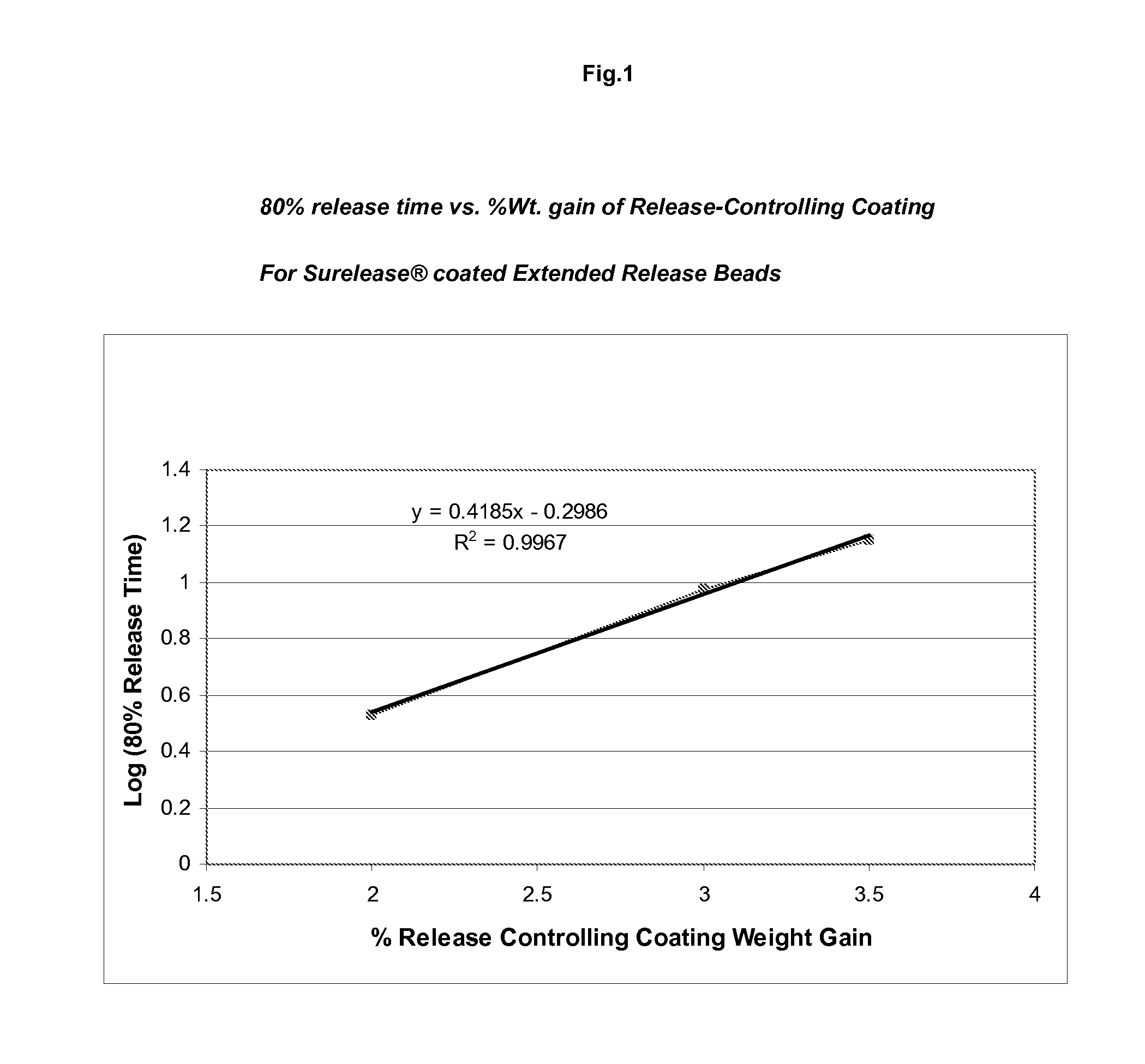
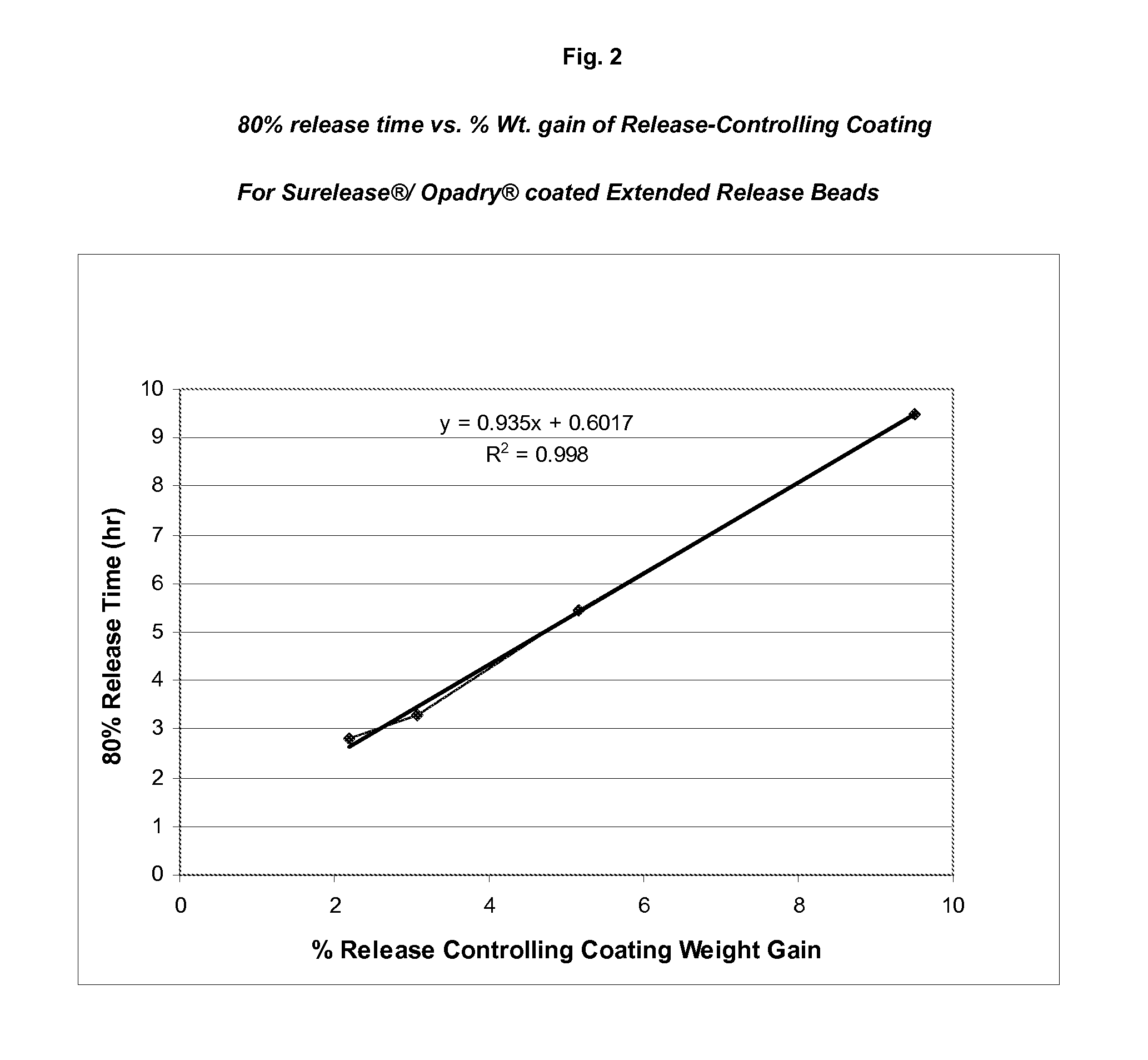
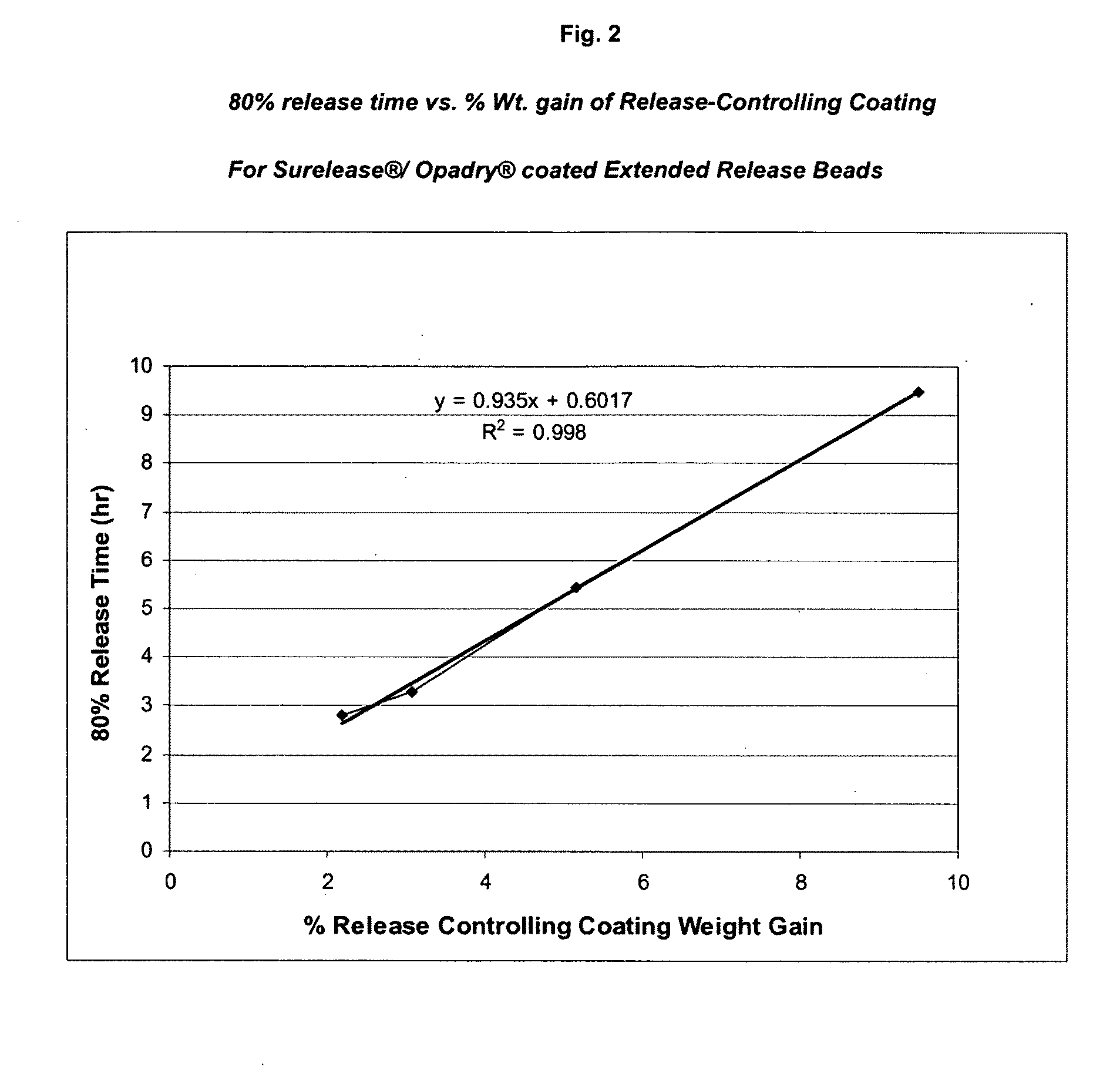
Sustained release topiramate
InactiveUS20030072802A1Avoid degradationInhibit growthBiocideCarbohydrate active ingredientsDepressantTopiramate
The present invention is an improvement in the treatment of mania and depression by administering topiramate in a sustained-release formulation. The sustained-release formulation of the present invention may also be co-administered with anti-psychotics and anti-depressants.
Owner:R T ALAMO VENTURES
Topiramate pharmaceutical composition
InactiveUS20060121112A1Improve bioavailabilityDifferential bioavailabilityBiocideCarbohydrate active ingredientsDiseaseGeneralized seizure
A once daily controlled-release pharmaceutical formulation which contains therapeutic amounts of topiramate and which is capable of being administered to specific regions along the gastrointestinal tract used to treat various types of conditions, for example, partial seizures with or without secondarily generalized seizures, primary generalized tonic-clonic seizures, seizures associated with Lennox Gastaut Syndrome, migraines, and obesity.
Owner:ALKERMES PHARMA IRELAND LTD
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Methods for treating macular degeneration with topiramate
InactiveUS6949518B1Prevent macular degenerationBiocideCarbohydrate active ingredientsNeuro degenerationTopiramate
A method for treating macular degeneration and / or treating optic nerve degeneration of a patient comprises administering topiramate with a dosage pharmaceutically effective to suppress degeneration or induce growth of new optic nerve fibers over a sustained period.
Owner:CHU PAO HSIEN +1
Novel topiramate compositions and an escalating dosing strategy for treating obesity and related disorders
InactiveUS20090304789A1Reduce exposureEliminate side effectsAntibacterial agentsBiocideSYMPATHOMIMETIC AGENTSTopiramate
The present invention is drawn to novel topiramate compositions as well as methods for treating obesity and related conditions, including conditions associated with and / or caused by obesity per se. The present invention also features a pharmaceutical composition that includes, e.g., topiramate alone or in combination with a sympathomimetic agent and a novel escalating dosing strategy for administering such compositions.
Owner:VIVUS
Method of treating and diagnosing sleep disordered breathing and means for carrying out the method
InactiveUS20040082519A1Eliminates and substantially reduces manifestation of OSA-relatedBiocidePowder deliveryOral medicationSleep disordered breathing
A method for treating snoring, sleep apnoea and other forms of sleep disordered breathing comprises administration to a patient of a therapeutically effective amount of topiramate over an appropriate period of time, such as a period substantially coniciding with the sleep period of a patient. A useful route of administration is per os. Also disclosed is the use of topiramate for the diagnosis of snoring, sleep apnoea and other forms of sleep disordered breathing.
Owner:HEDNER JAN +3
Topiramate salts and compositions comprising them
The invention encompasses novel salts of topiramate, and pharmaceutically acceptable polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, or amorphous forms thereof, as well as pharmaceutical compositions and pharmaceutical unit dosage forms containing the same. In particular, the invention encompasses pharmaceutically acceptable salts of topiramate, including without limitation topiramate sodium, topiramate lithium, topiramate potassium, or polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, and amorphous forms thereof. The invention further encompasses novel co-crystals or complexes of topiramate, as well as pharmaceutical compositions comprising them. The invention also encompasses methods of treating or preventing a variety of diseases and conditions including, but not limited to, seizures, epileptic conditions, tremors, cerebral function disorders, obesity, neuropathic pain, affective disorders, tobacco cessation, migraines, and cluster headache.
Owner:ORTHO MCNEIL PHARM INC
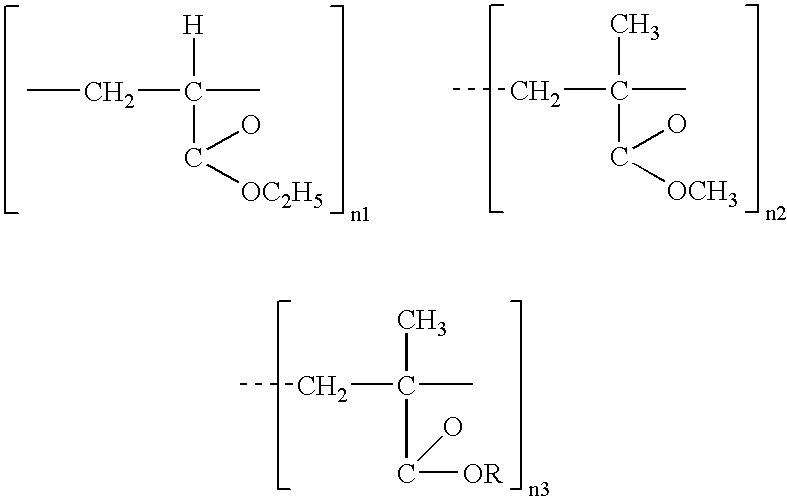
Controlled release formulations using intelligent polymers
InactiveUS20080292700A1Prevent burstImparts gastrointestinal stealth characteristicNervous disorderMetabolism disorderSmart polymerTopiramate
A controlled release pharmaceutical composition comprises (a) topiramate or a pharmaceutically acceptable salt thereof, (b) a first intelligent polymer component; and (c) a second intelligent polymer component having opposite wettability characteristics to the first intelligent polymer component. The polymer components are effective for controlled release of the pharmaceutically active substance from the composition.
Owner:VALEANT INT BARBADOS
Topiramate Compositions and Methods of Making and Using the Same
The present invention is directed to compositions comprising topiramate and a sulfoalkyl ether cyclodextrin, and methods of making and using the same.
Owner:RGT UNIV OF MINNESOTA
Composition and method for compounded therapy
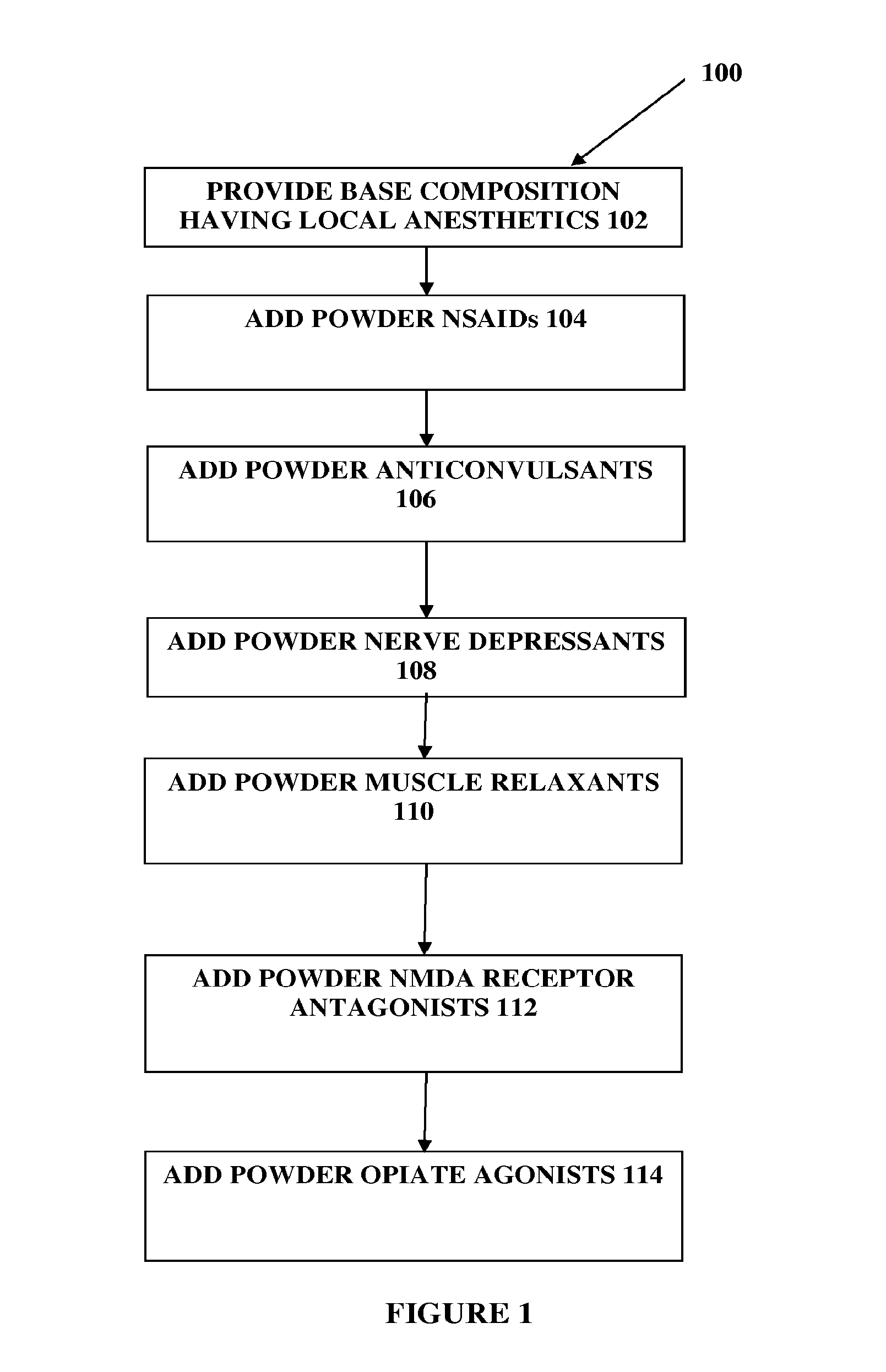
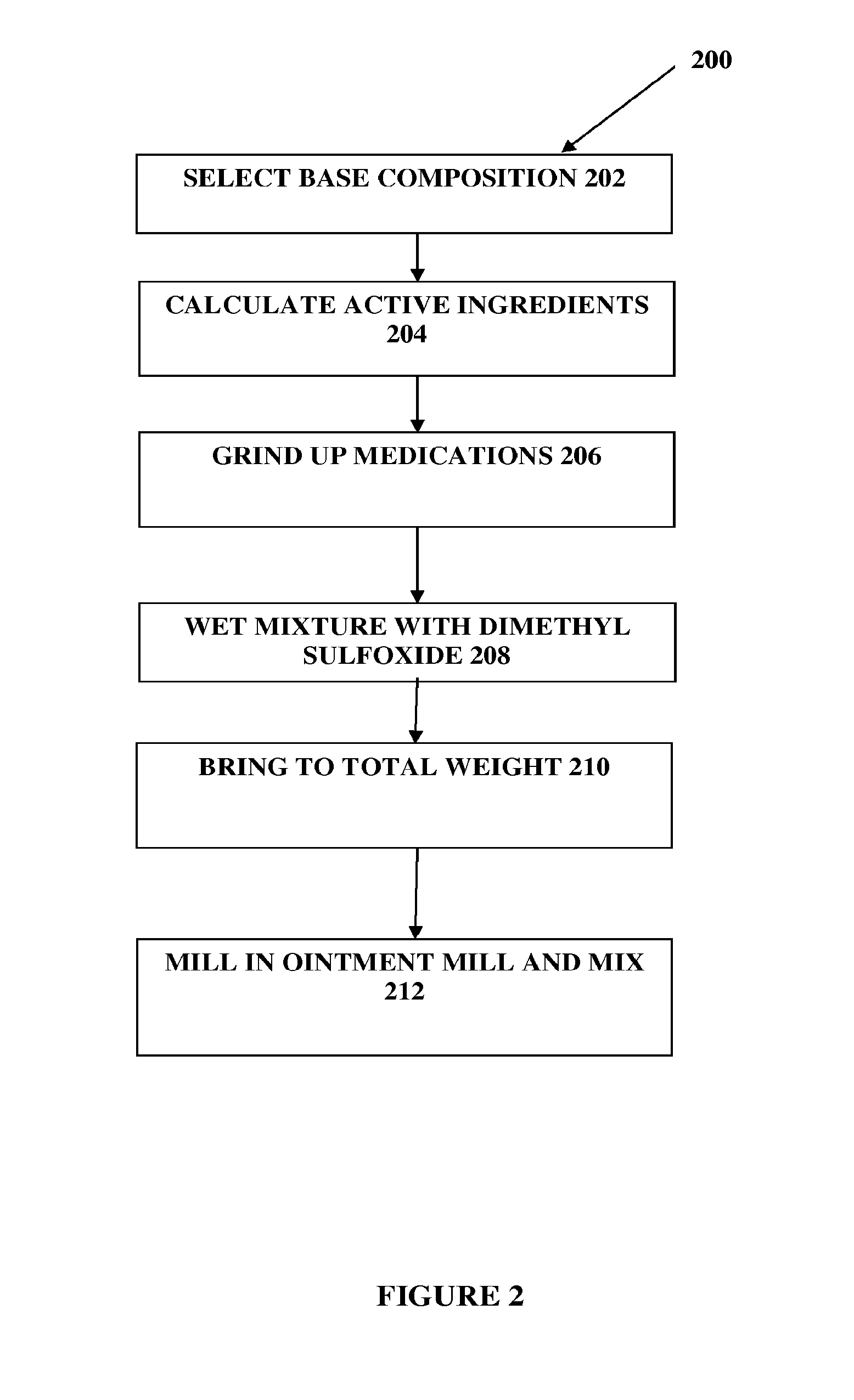
The present embodiments relate to topically delivered compounded medications. A transdermal cream may provide the effective topical administration of multiple medications simultaneously; may include low concentrations of local anesthetics, a NSAID, an anticonvulsant, and / or other active ingredients; and may include lidocaine, prilocaine, meloxicam, and lamotrigine and / or topiramate. Alternatively, the transdermal cream may include a lidocaine / prilocaine base cream to which is added a fine powder of one or more ground up medications to form a compounded medication. The compounded medication in powder form may be generated from grinding up tablets of NSAIDs, anticonvulsants, nerve depressants, antidepressants, muscle relaxants, NMDA receptor antagonists, opiate or opioid agonists, and / or other agents. The compounded medication in powder form may include meloxicam, lamotrigine, topiramate, other active ingredients, and DMSO or Sterile Water for Irrigation. In another aspect, the present embodiments relate to methods of compounding medications and transdermal creams or gels.
Owner:CMPD LICENSING
Dosage forms for low solubility and or low dissolution rate free acid pharmaceutical agents
InactiveUS20050287213A1Reduce solubilityLow dissolution ratePill deliveryAnhydride/acid/halide active ingredientsSolubilityTopiramate
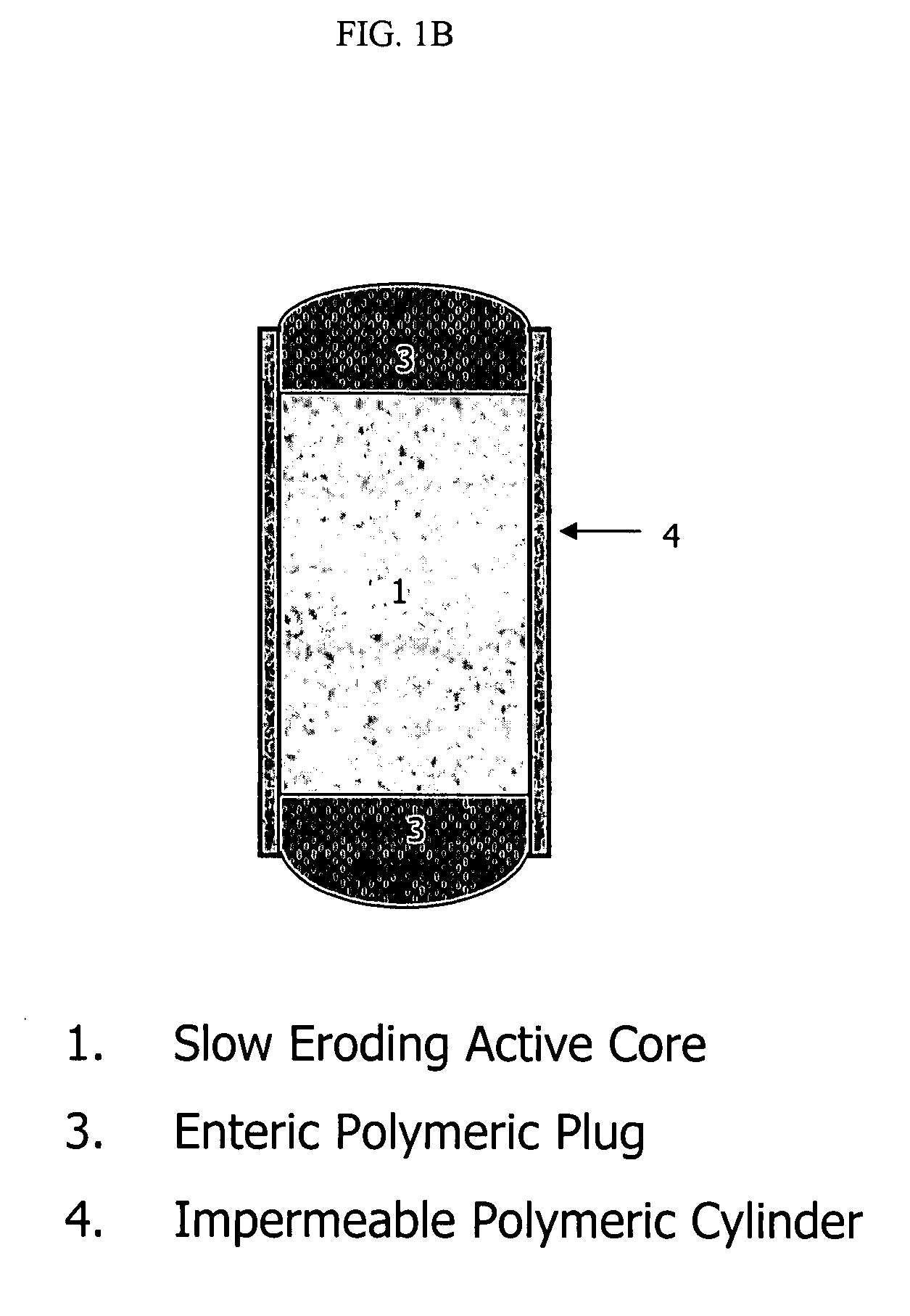
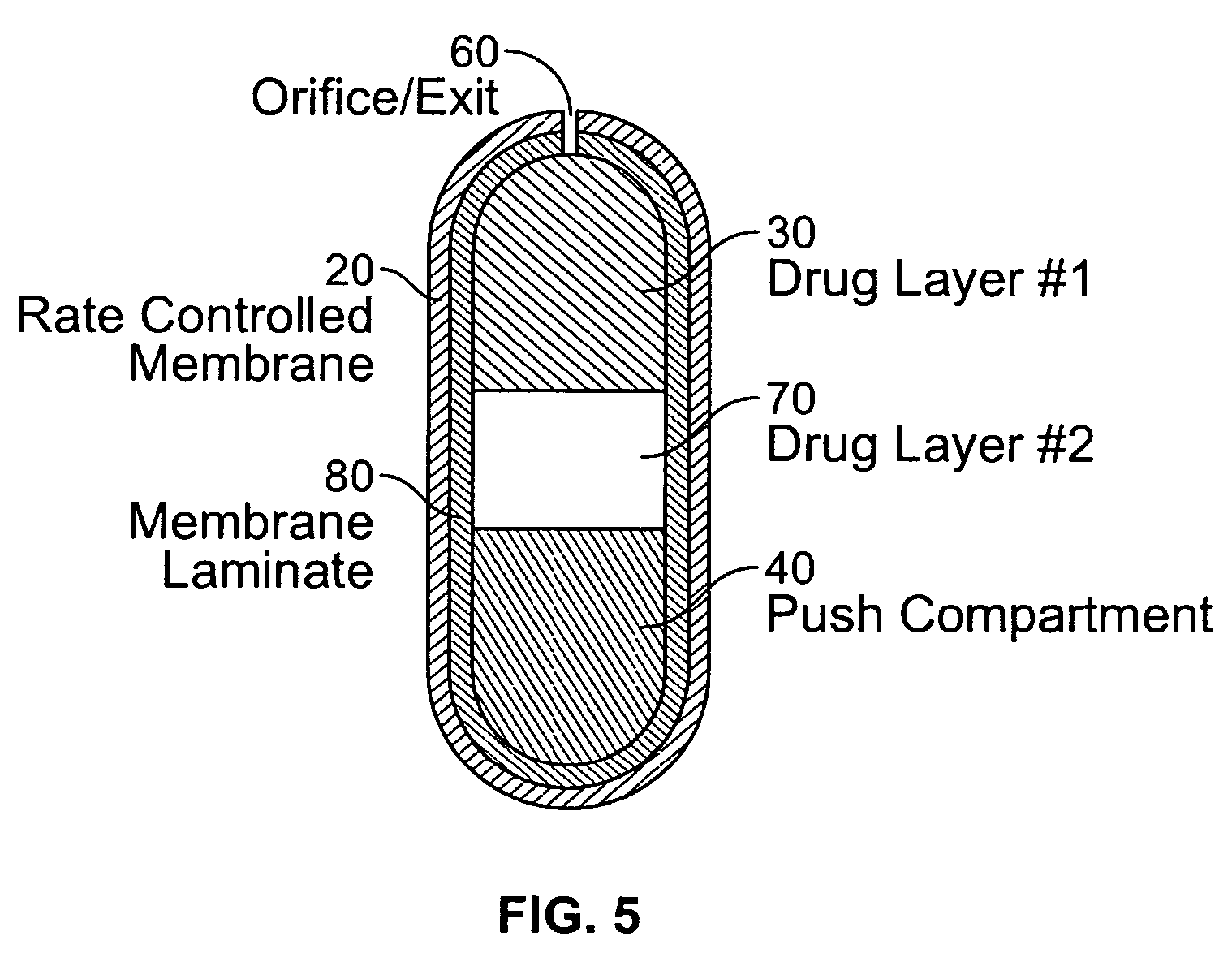
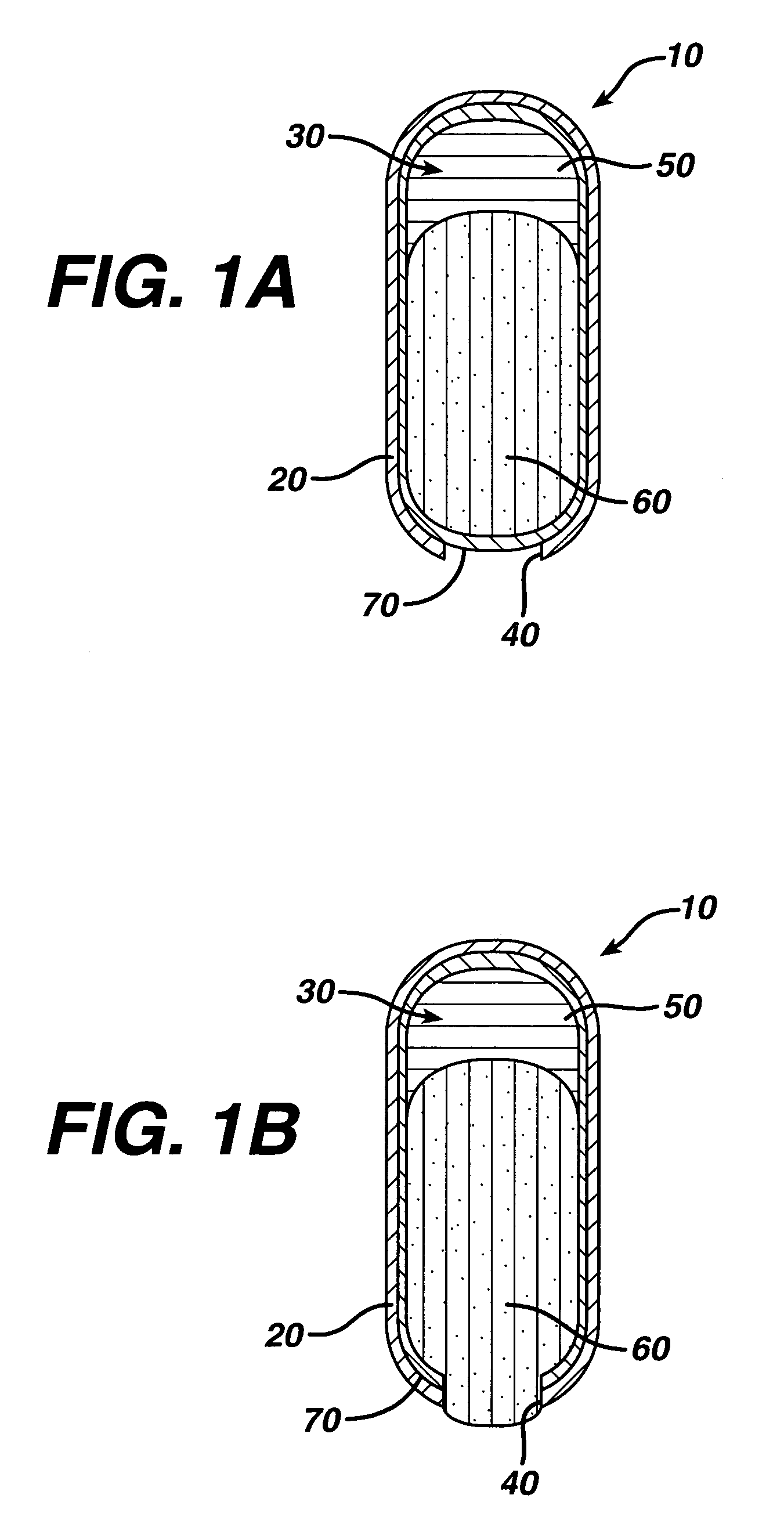
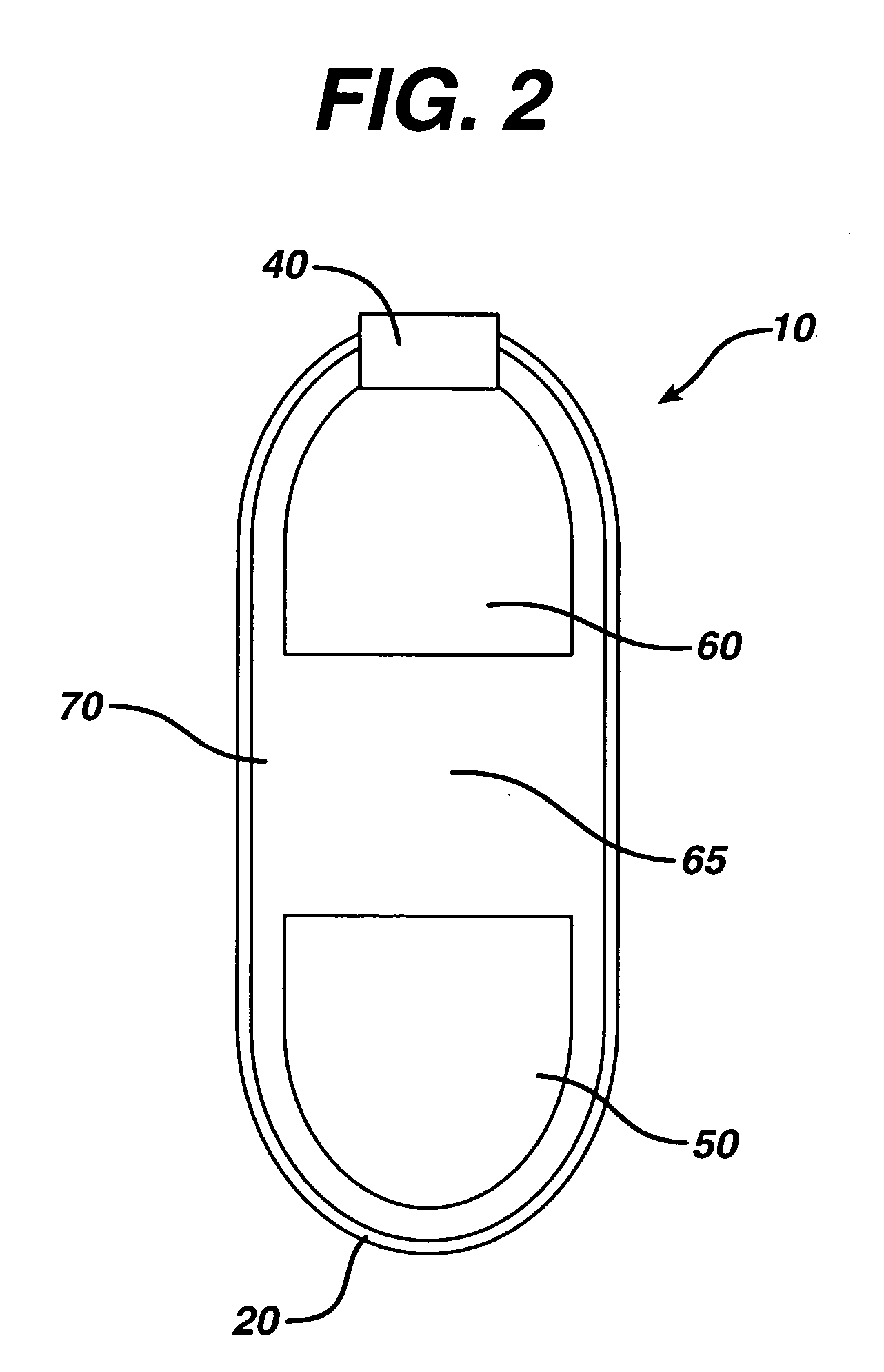
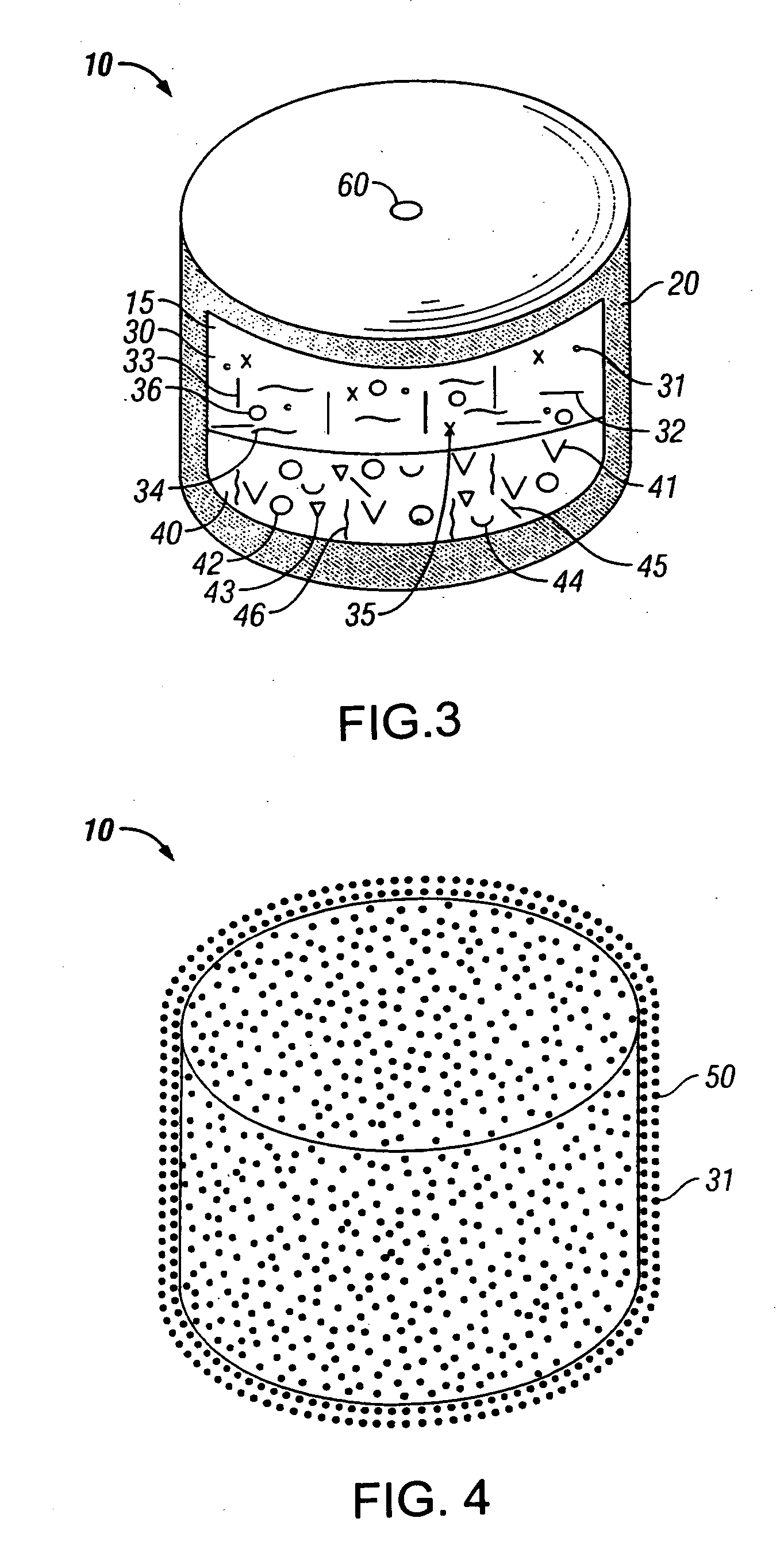
An osmotic controlled release dosage form is described comprising a core comprising a first drug composition, wherein the first drug composition comprises topiramate and / or its pharmaceutically acceptable salt; a semi-permeable wall surrounding the core; and an exit orifice through the semi-permeable wall for releasing the first drug composition from the dosage form over a prolonged period of time.
Owner:ALZA CORP
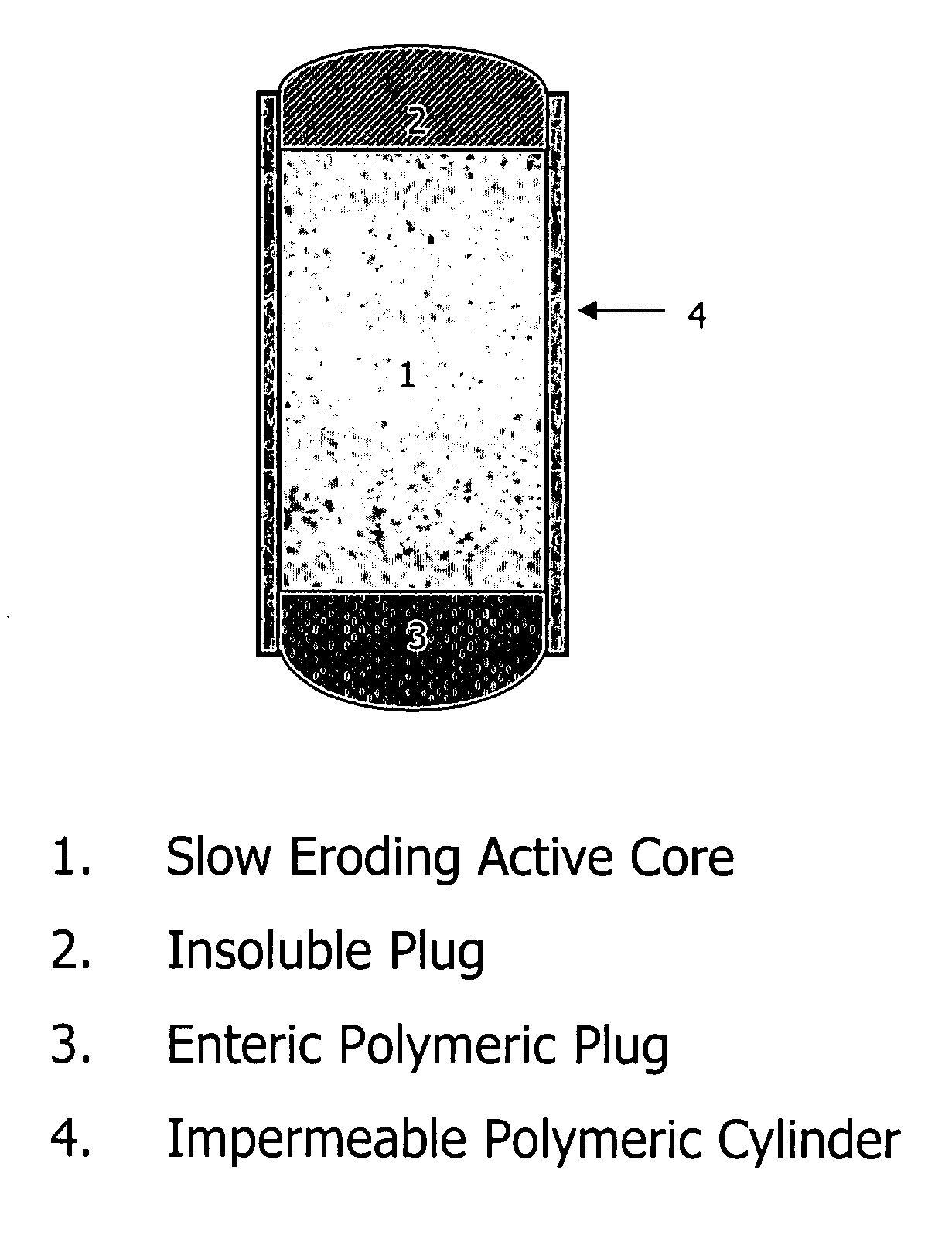
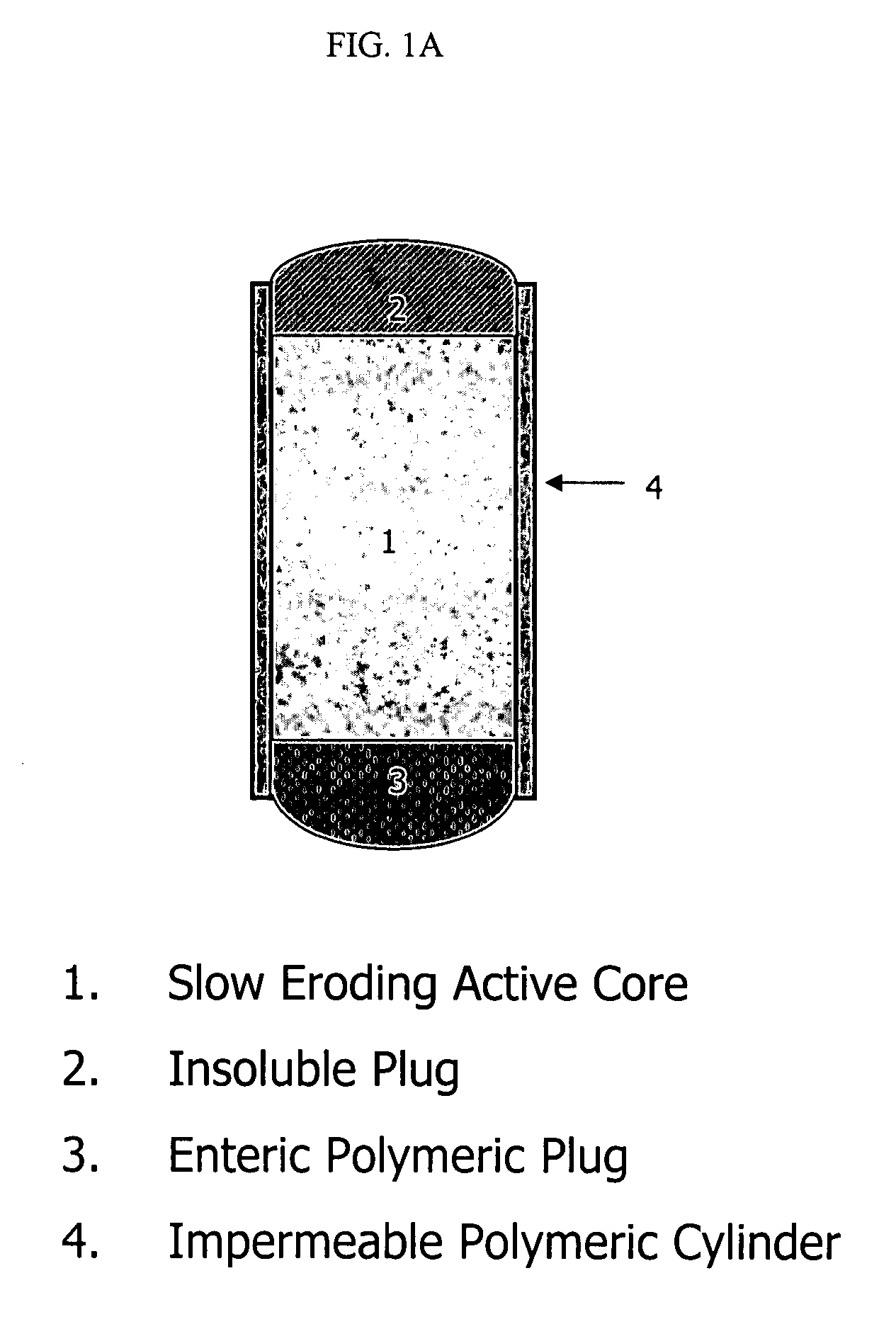
Stepwise delivery of topiramate over prolonged period of time
InactiveUS20050136108A1Improve bioavailabilityPromote absorptionNervous disorderOsmotic deliveryTopiramateControlled Release Dosage Form
Compositions and dosage forms for enhanced dispersion of topiramate in a controlled release dosage form released from the dosage form as a dry or substantially dry erodible solid over a prolonged period of time at a stepwise increasing rate of release are described.
Owner:YAM NOYMI V +4
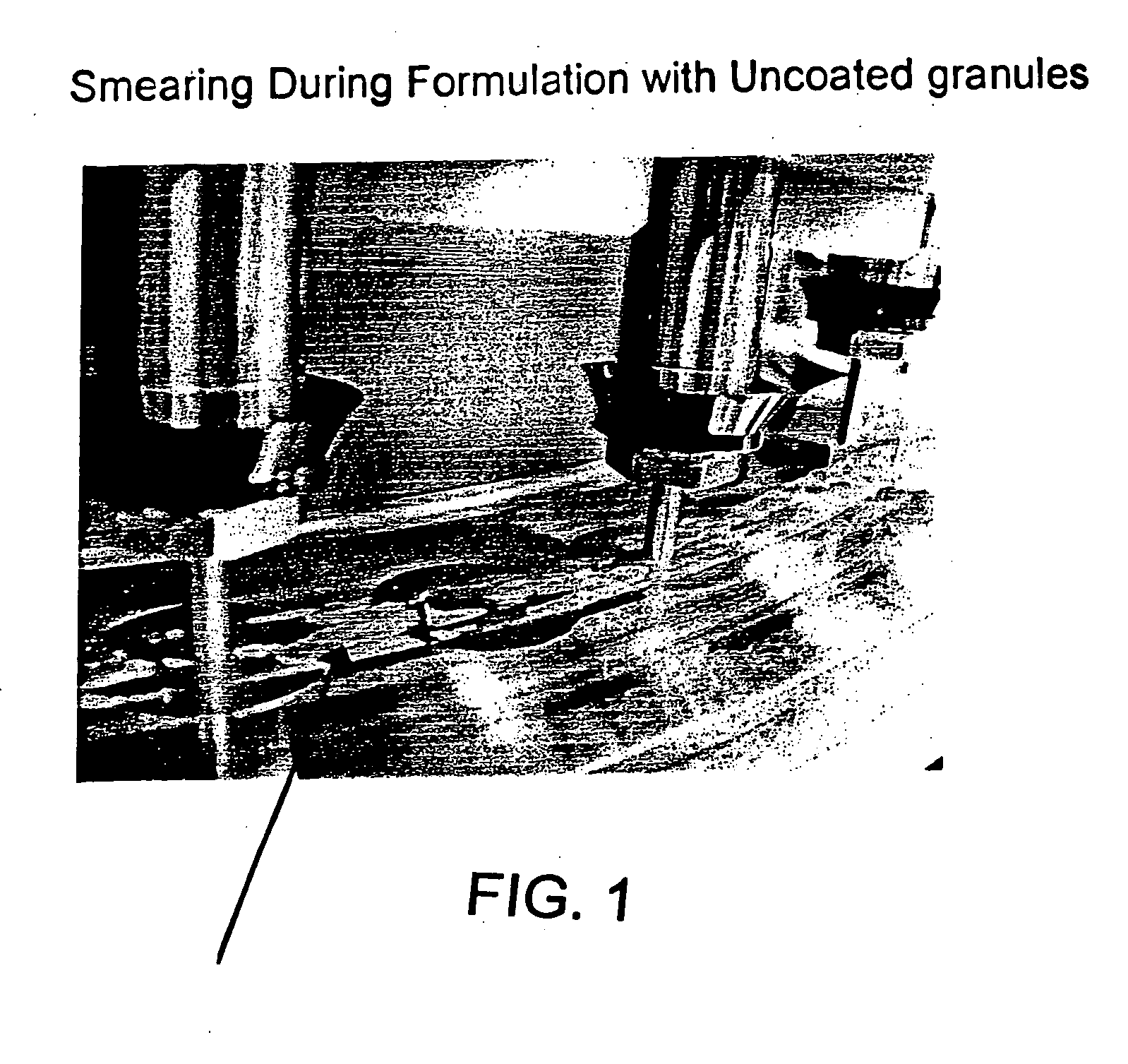
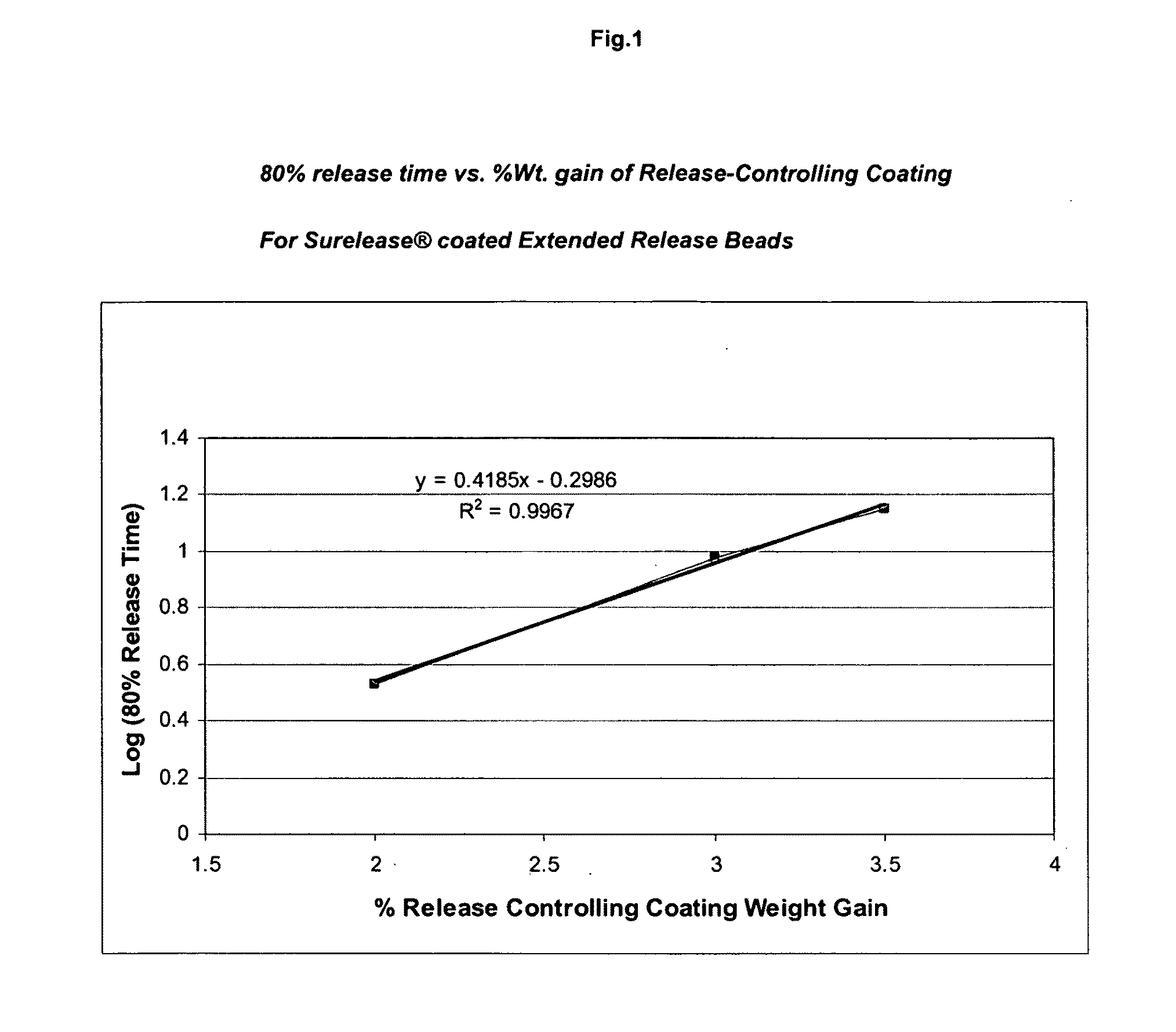
Drug granule coatings that impart smear resistance during mechanical compression
InactiveUS20050175696A1Low dissolution rateExtension of timeBiocideCarbohydrate active ingredientsSolubilityHydrophilic polymers
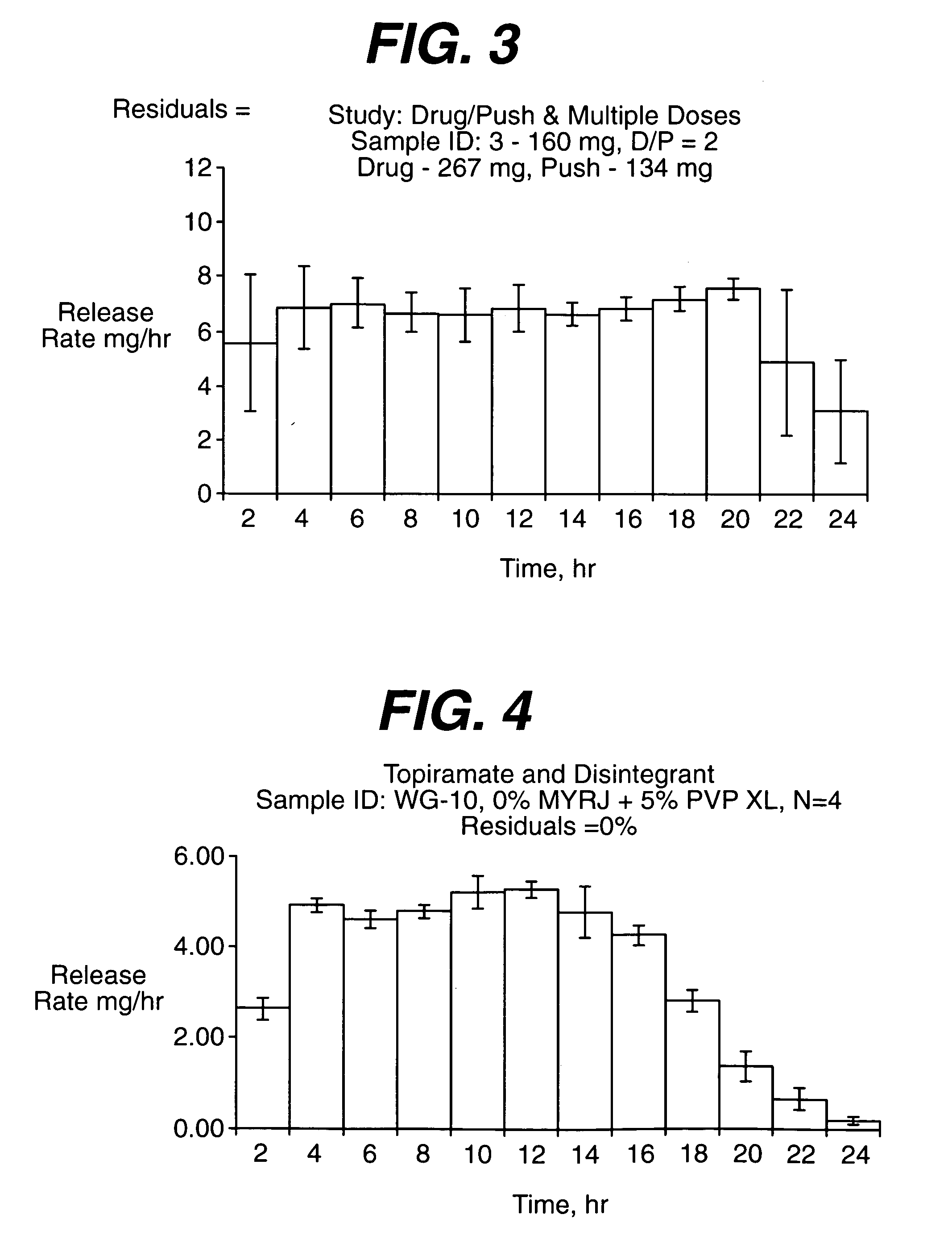
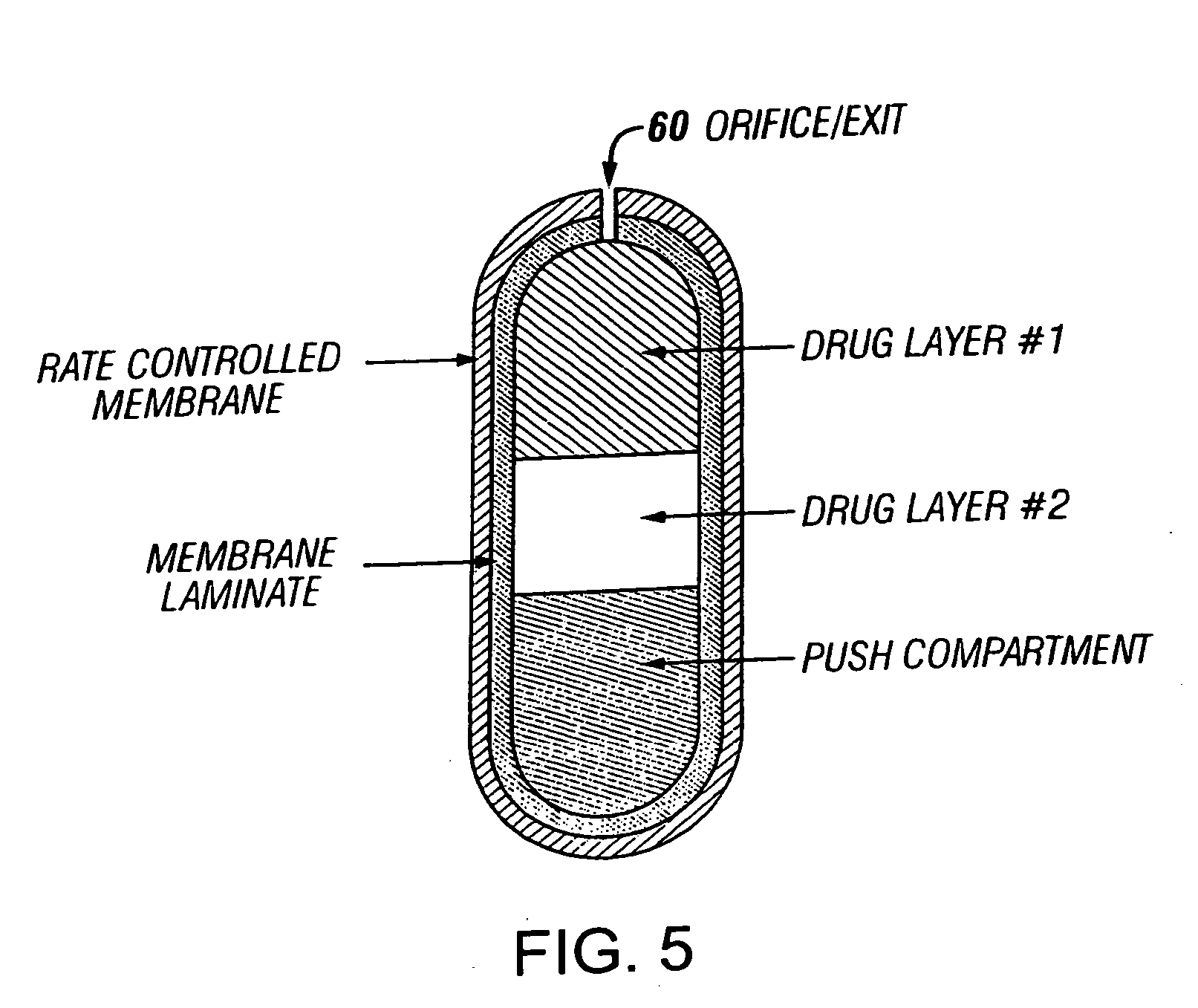
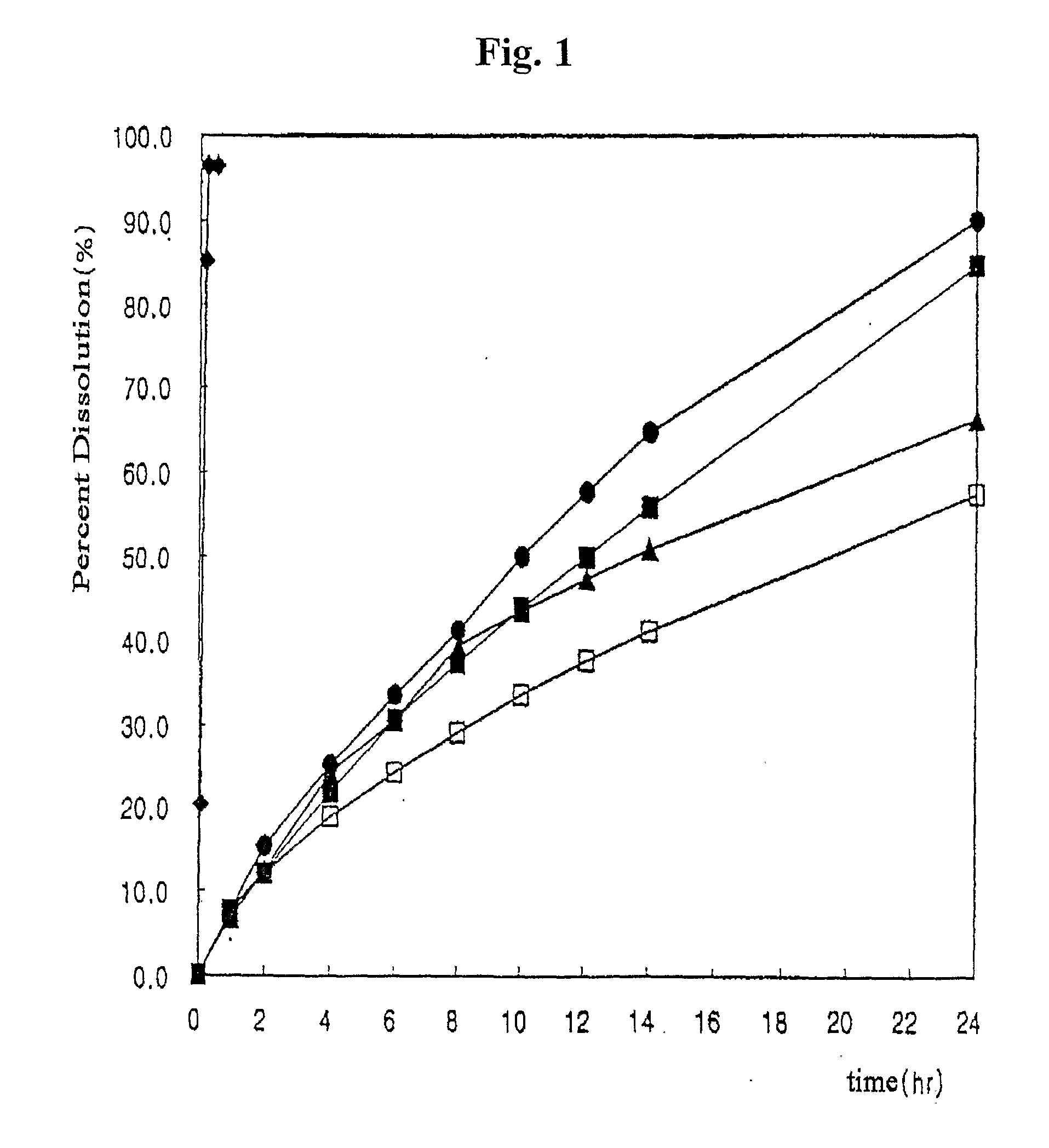
A drug formulation is disclosed comprising granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a drug formulation consisting of a tablet core made by mechanical compression, wherein said tablet core comprises granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a dosage form for oral administration of topiramate, comprising a tablet core and an osmotic delivery system. Methods for controlling topiramate release patterns by altering the composition of the topiramate dosage form are also disclosed.
Owner:ALZA CORP
Topiramate plus naltrexone for the treatment of addictive disorders
InactiveUS20120302592A1Treatment safetyLess susceptible to neuroadaptationBiocideNervous disorderDiseaseTopiramate
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
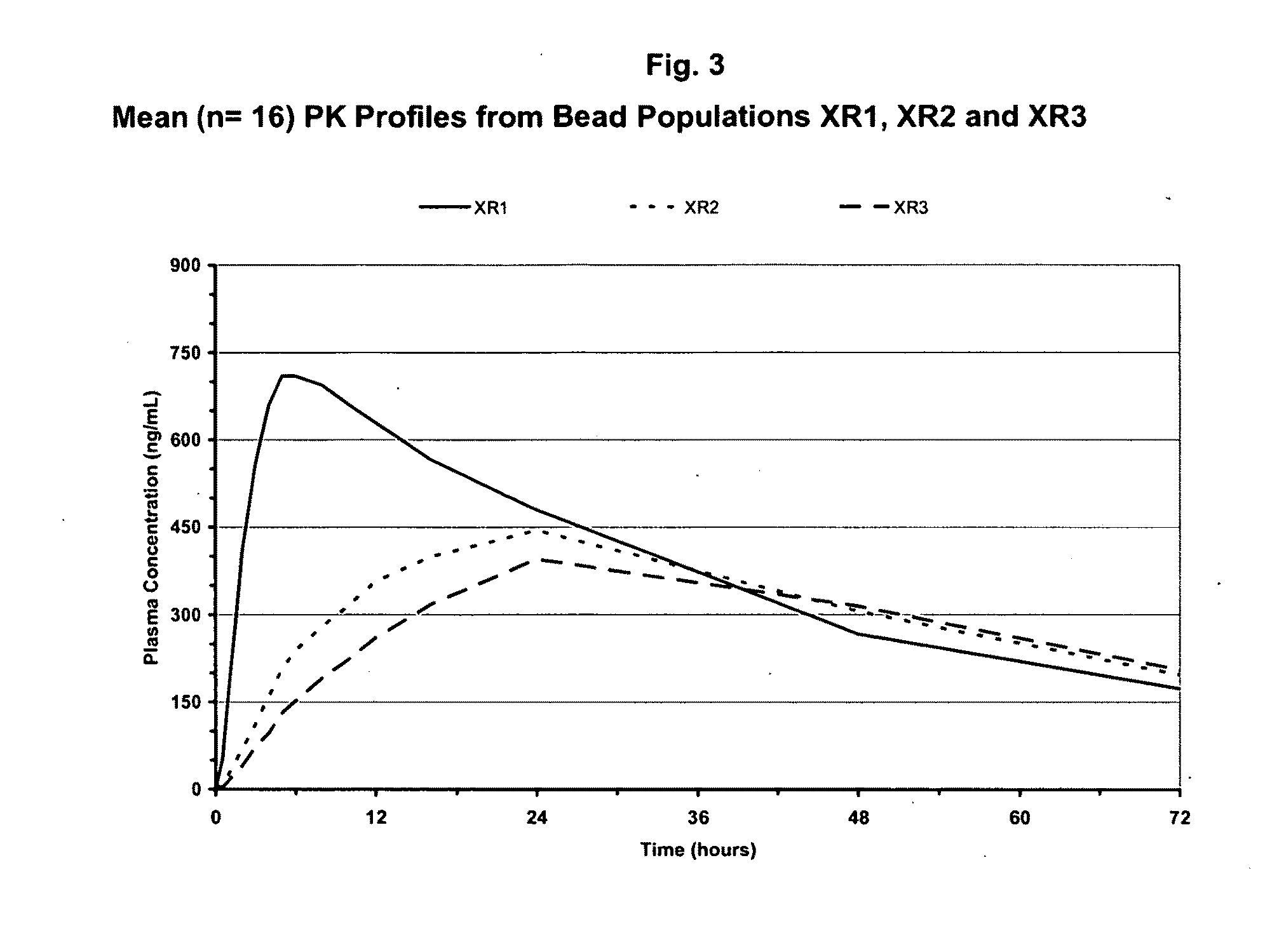
Sustained-release formulations of topiramate
ActiveUS20080118557A1Reduction in severity and frequencyBiocideSenses disorderOral medicationPharmaceutical drug
Pharmaceutical compositions of topiramate for once-a-day oral administration are provided. The formulations comprise a sustained-release component and an optional immediate-release component, the compositions of which can be selectively adjusted, respectively, to release the active ingredient along a pre-determined release profile. Method of treating or preventing pathological disorders in mammalian subjects comprising the administration of the novel formulations disclosed herein is also provided.
Owner:SUPERNUS PHARM INC
Sustained-release preparations containing topiramate and the producing method thereof
InactiveCN1988889AOrganic active ingredientsNervous disorderTopiramateSustained-Release Preparations
Disclosed herein are a sustained-release topiramate preparation and a method for producing the topiramate preparation. The sustained-release topiramate preparation is produced using double granules obtained by granulating topiramate or a pharmaceutically acceptable salt thereof using a solid dispersant by a solid dispersion method (first granulation), and further by granulating the granules using a release sustaining material by dry or wet granulation (second granulation).
Owner:AMOREPACIFIC CORP
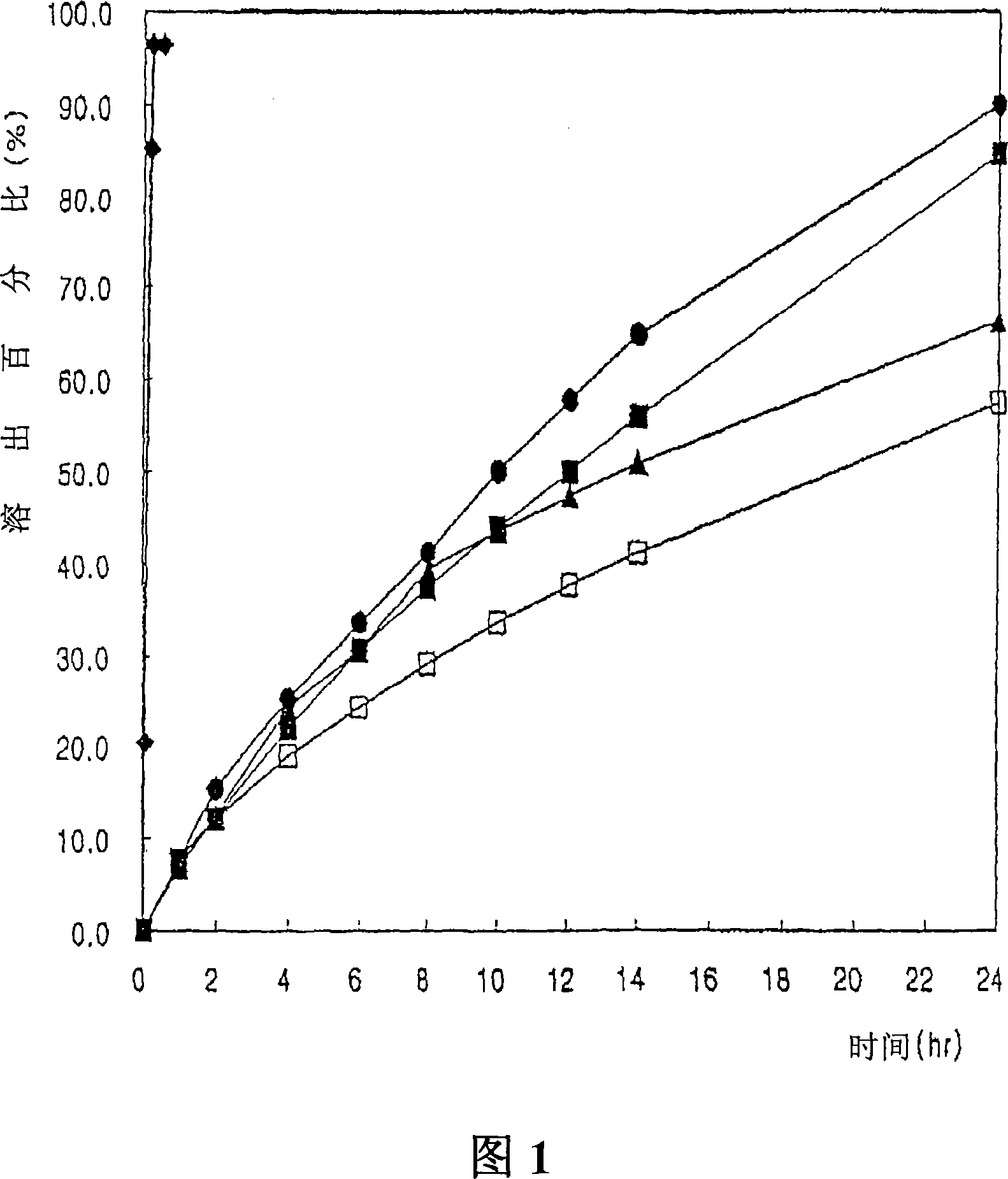
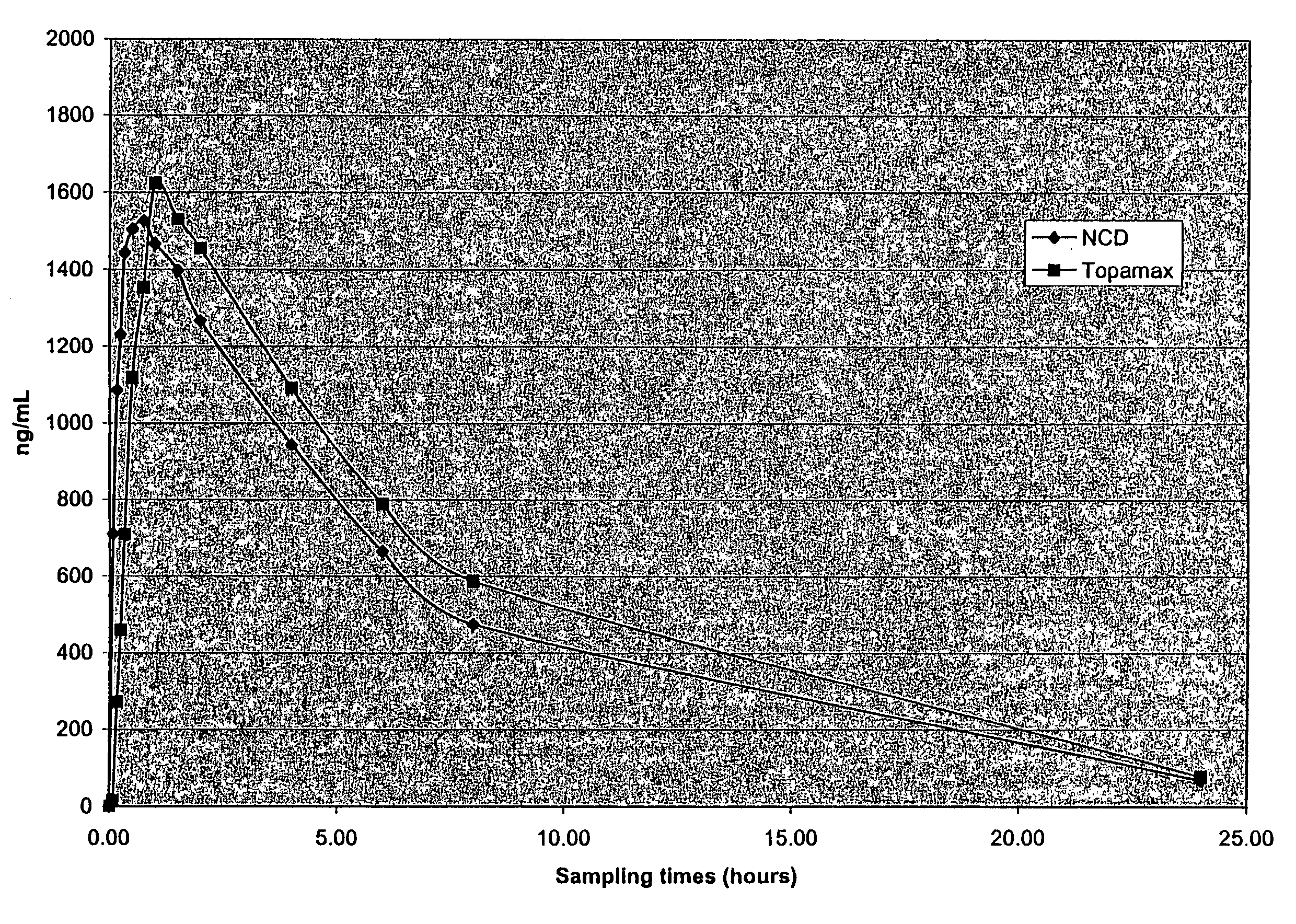
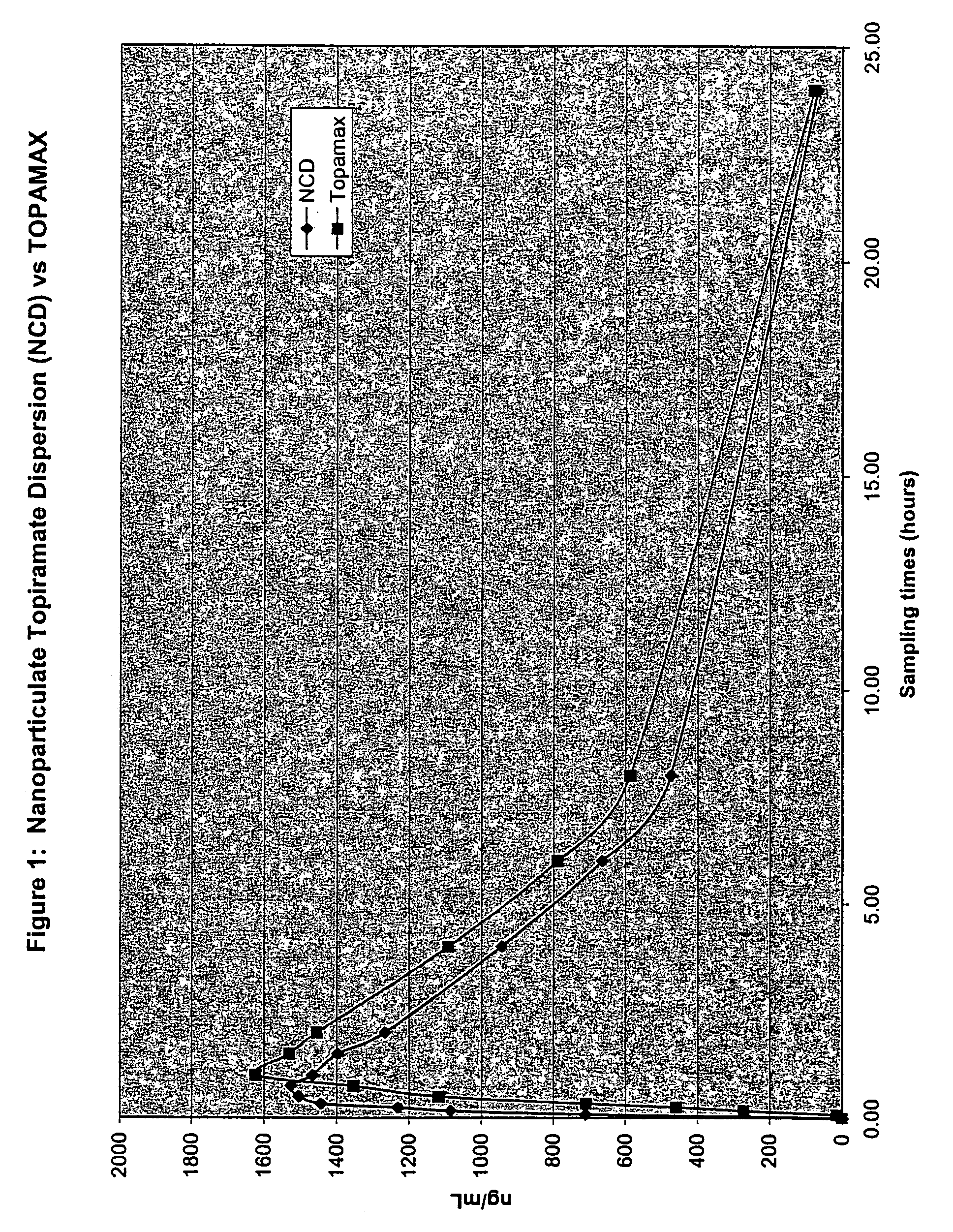
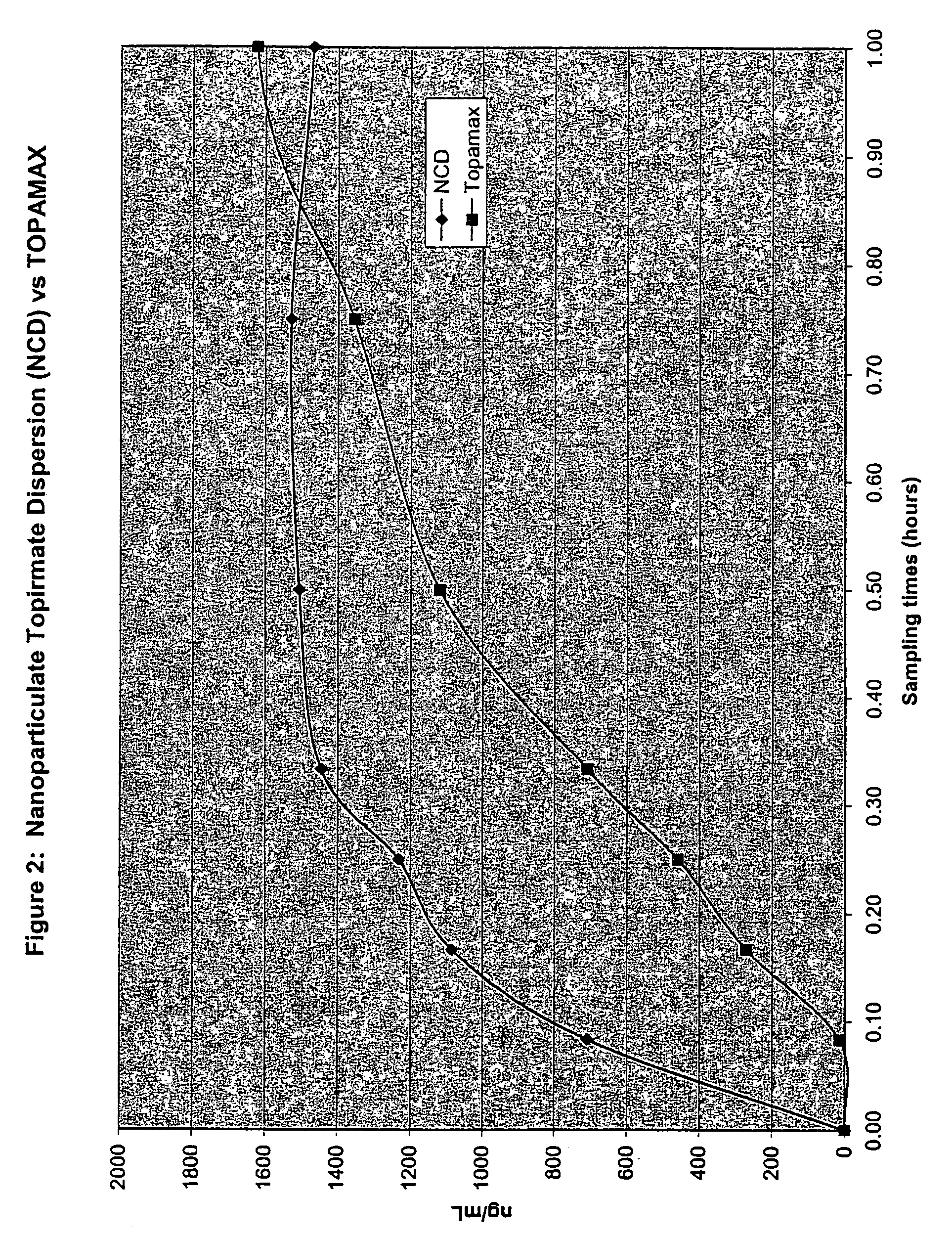
Nanoparticulate topiramate formulations
InactiveUS7390505B2Improved pharmacokinetic profileDecrease and eliminate adverse effect of drugPowder deliveryOrganic active ingredientsMedicineNanoparticle
The present invention is directed to nanoparticulate compositions comprising topiramate. The topiramate particles of the composition have an effective average particle size of less than about 2 microns.
Owner:ALKERMES PHARMA IRELAND LTD
Novel drug compositions and dosage forms of topiramate
InactiveUS20070243254A1Prolonged controlled deliveryFunction increaseNervous disorderPill deliverySolubilitySolvent
The present invention is directed to novel drug compositions and dosage forms comprising said drug compositions. The drug compositions of the present invention comprise a pharmaceutical agent and a solubilizing agent. The drug compositions of the present invention are particularly advantageous for use with low solubility and / or low dissolution rate pharmaceutical agents. The present invention is further directed to methods for manufacturing of said drug compositions and dosage forms. The present invention is further directed to methods of treatment comprising administration of said drug compositions and dosage forms.
Owner:EDGREN DAVID +13
Novel drug compositions and dosage forms of topiramate
The present invention is directed to novel drug compositions and dosage forms comprising said drug compositions. The drug compositions of the present invention comprise a pharmaceutical agent and a solubilizing agent. The drug compositions of the present invention are particularly advantageous for use with low solubility and / or low dissolution rate pharmaceutical agents. The present invention is further directed to methods for manufacturing of said drug compositions and dosage forms. The present invention is further directed to methods of treatment comprising administration of said drug compositions and dosage forms.
Owner:ALZA CORP
Topiramate Plus Naltrexone for the Treatment of Addictive Disorders
InactiveUS20100076006A1Treatment safetyLess susceptible to neuroadaptationBiocideNervous disorderDiseaseTopiramate
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
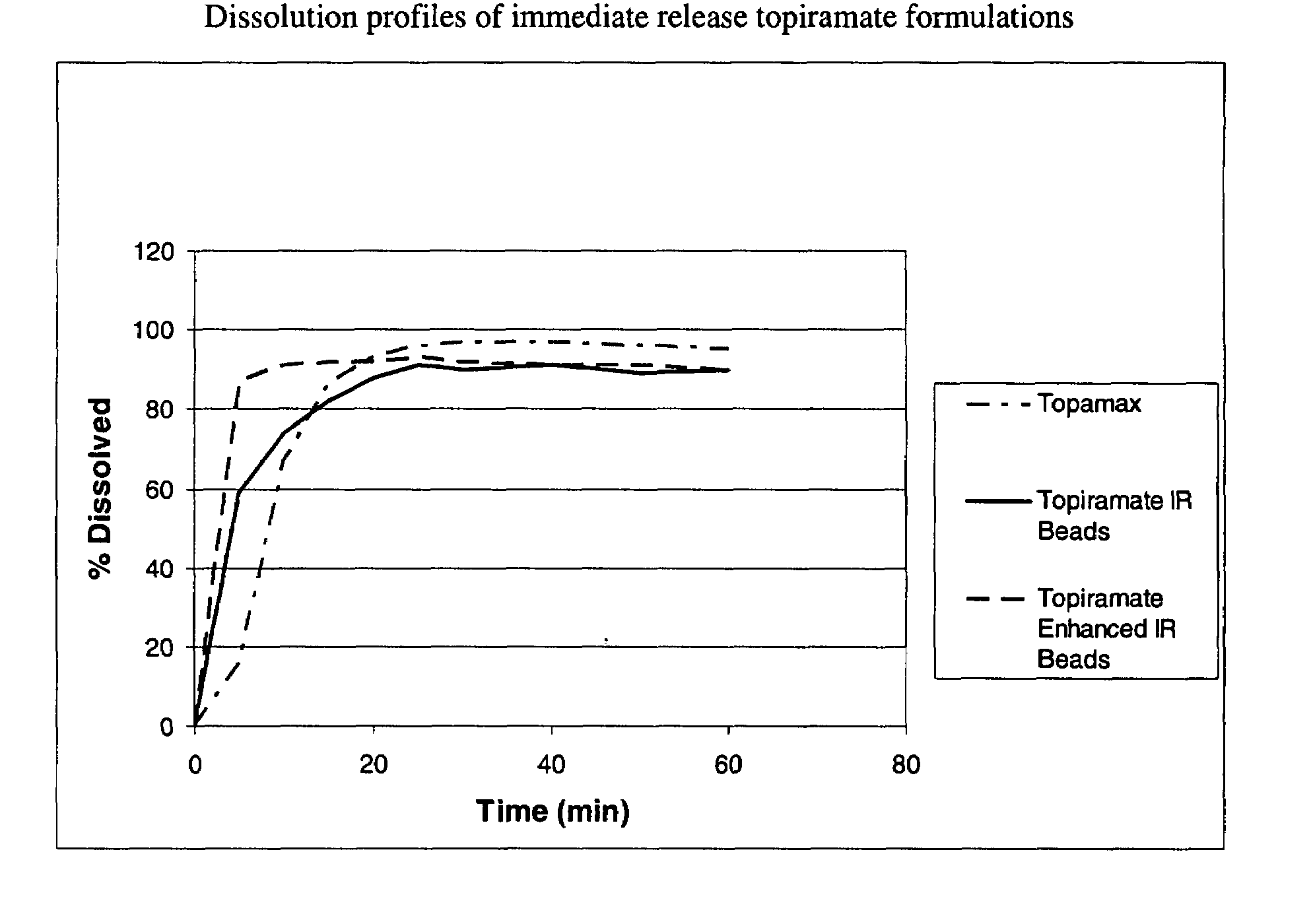
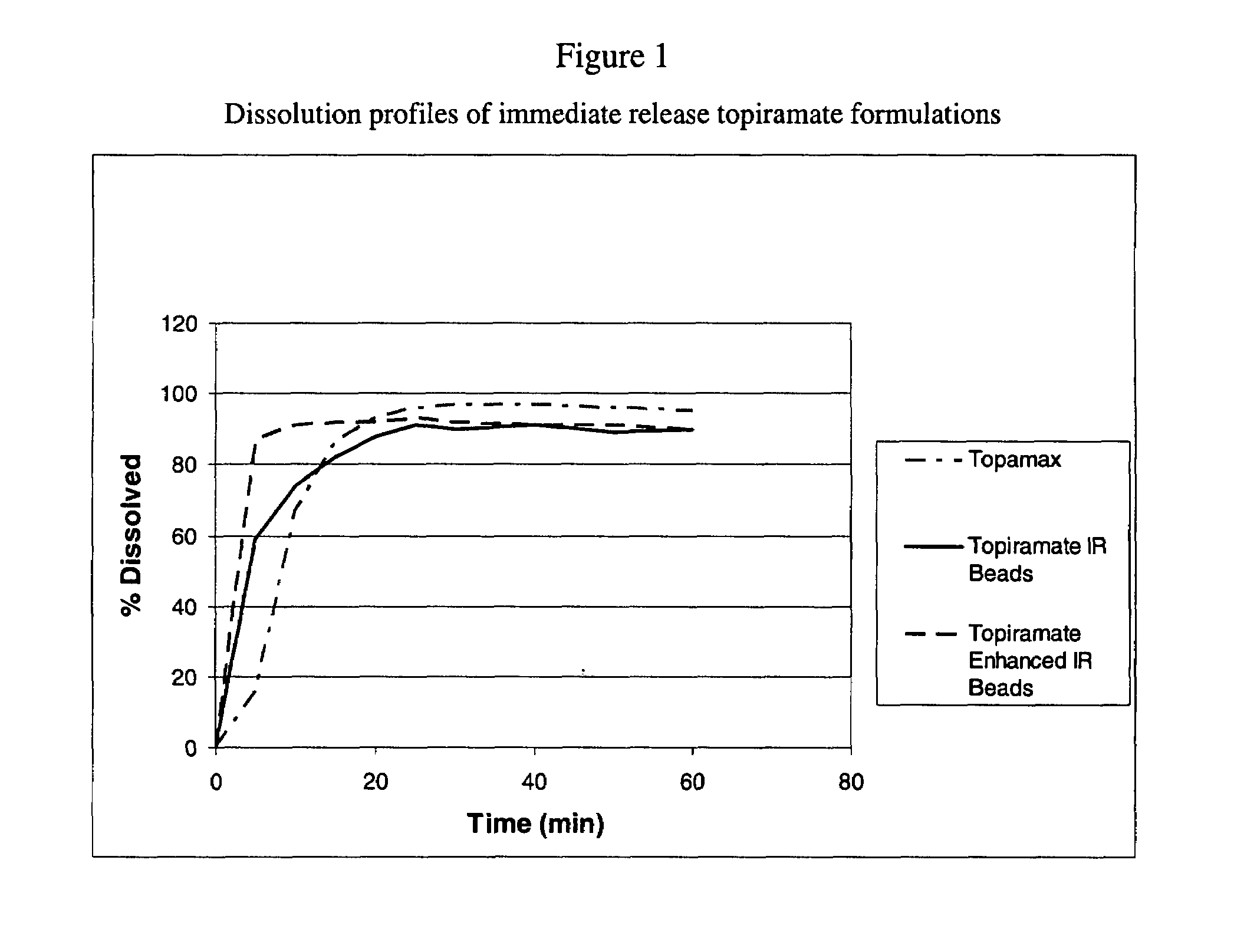
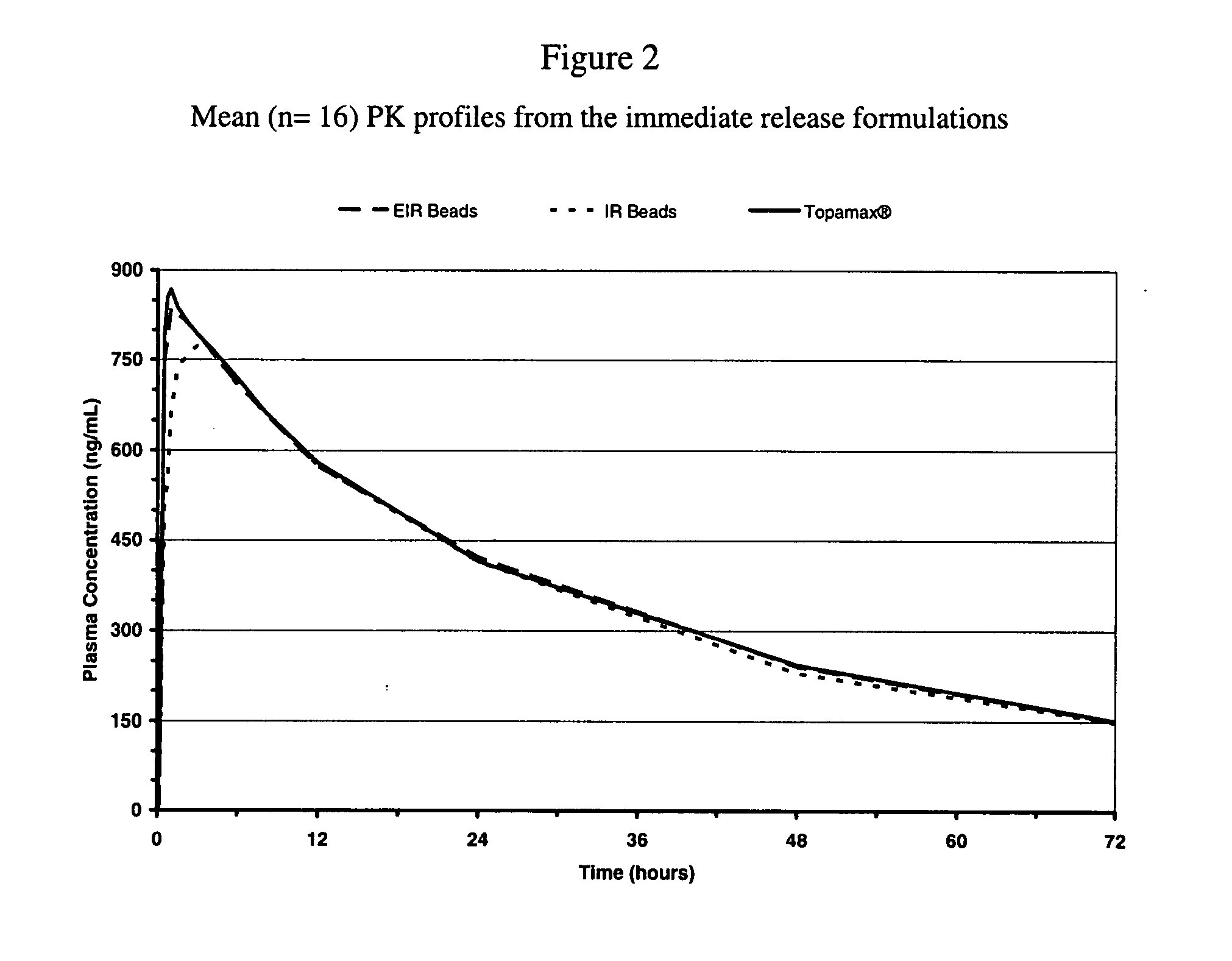
Enhanced immediate release formulations of topiramate
The present invention provides enhanced immediate release formulations of topiramate, in which 80% of the active ingredient is released in the period of time of not more than 30 min. These formulations may be advantageously used for the treatment of acute neurological conditions, such as migraine.
Owner:SUPERNUS PHARM INC
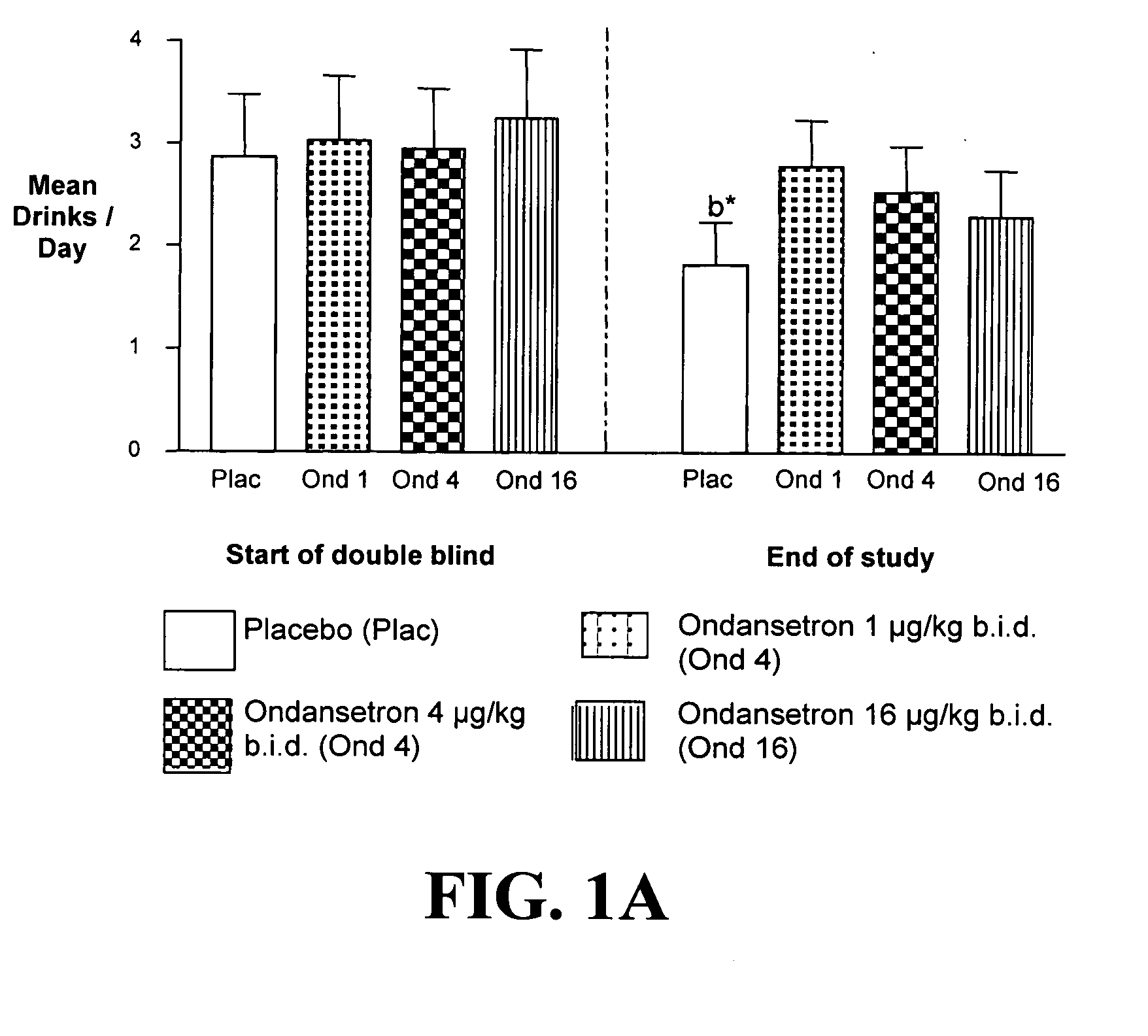
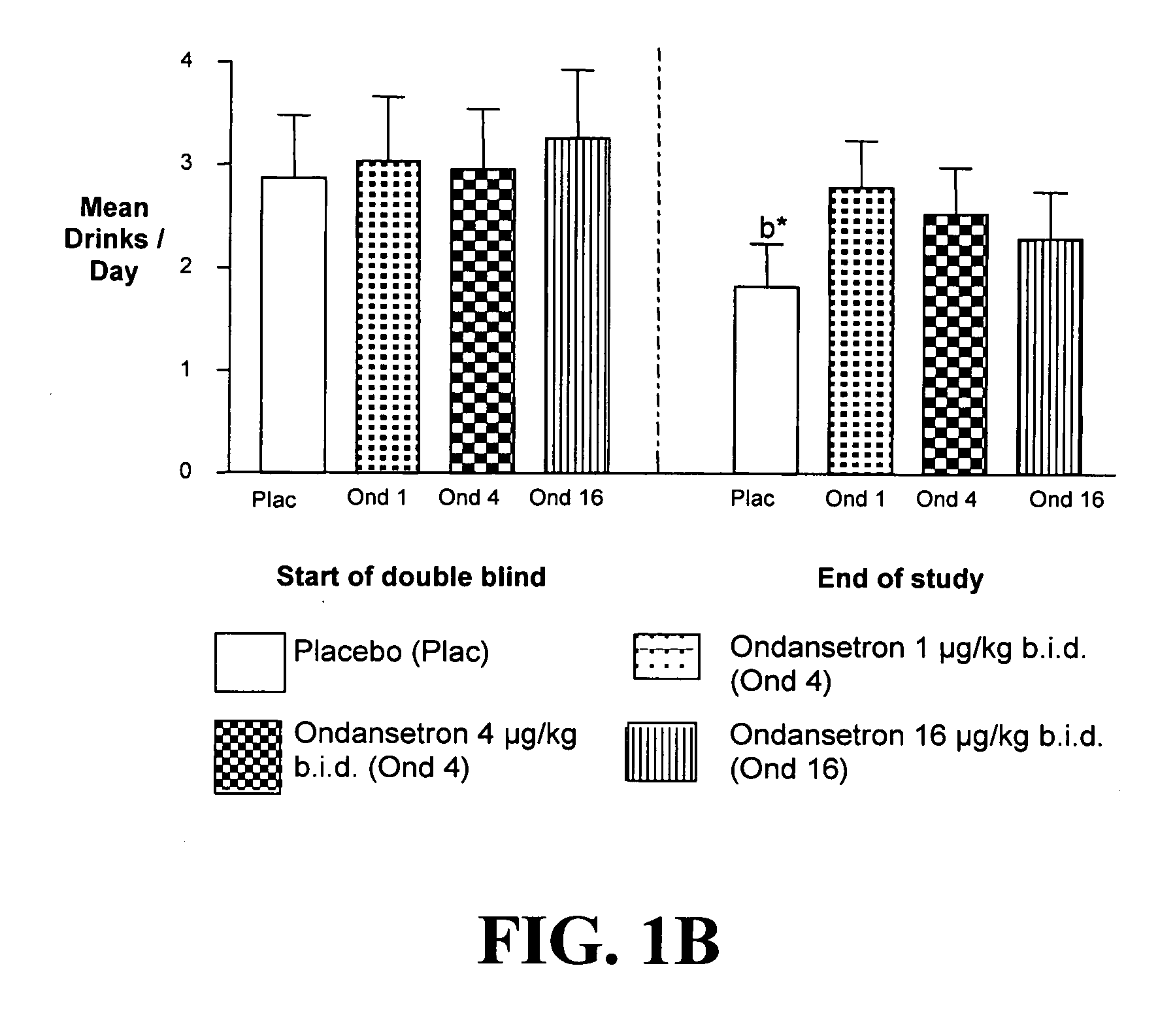
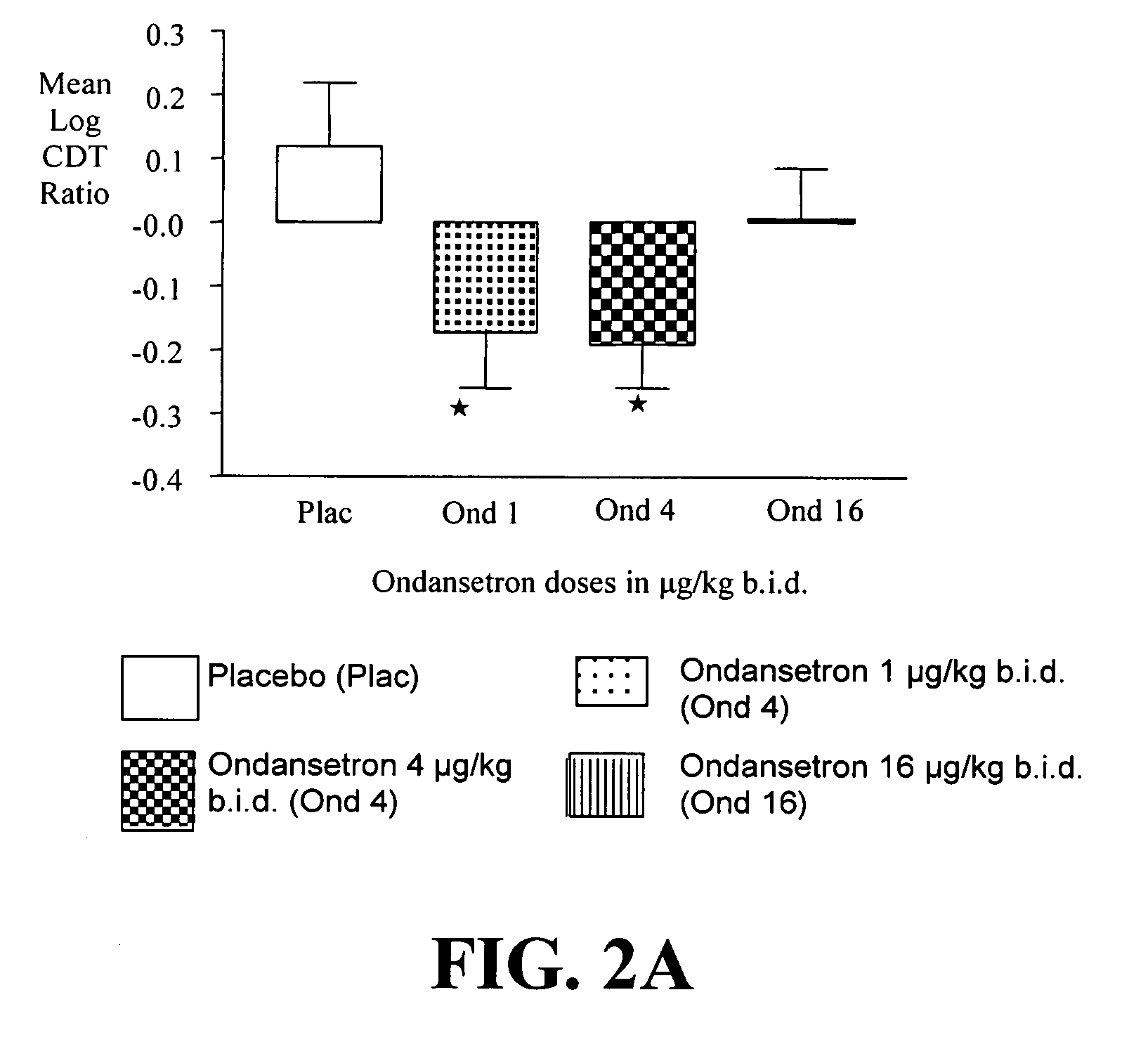
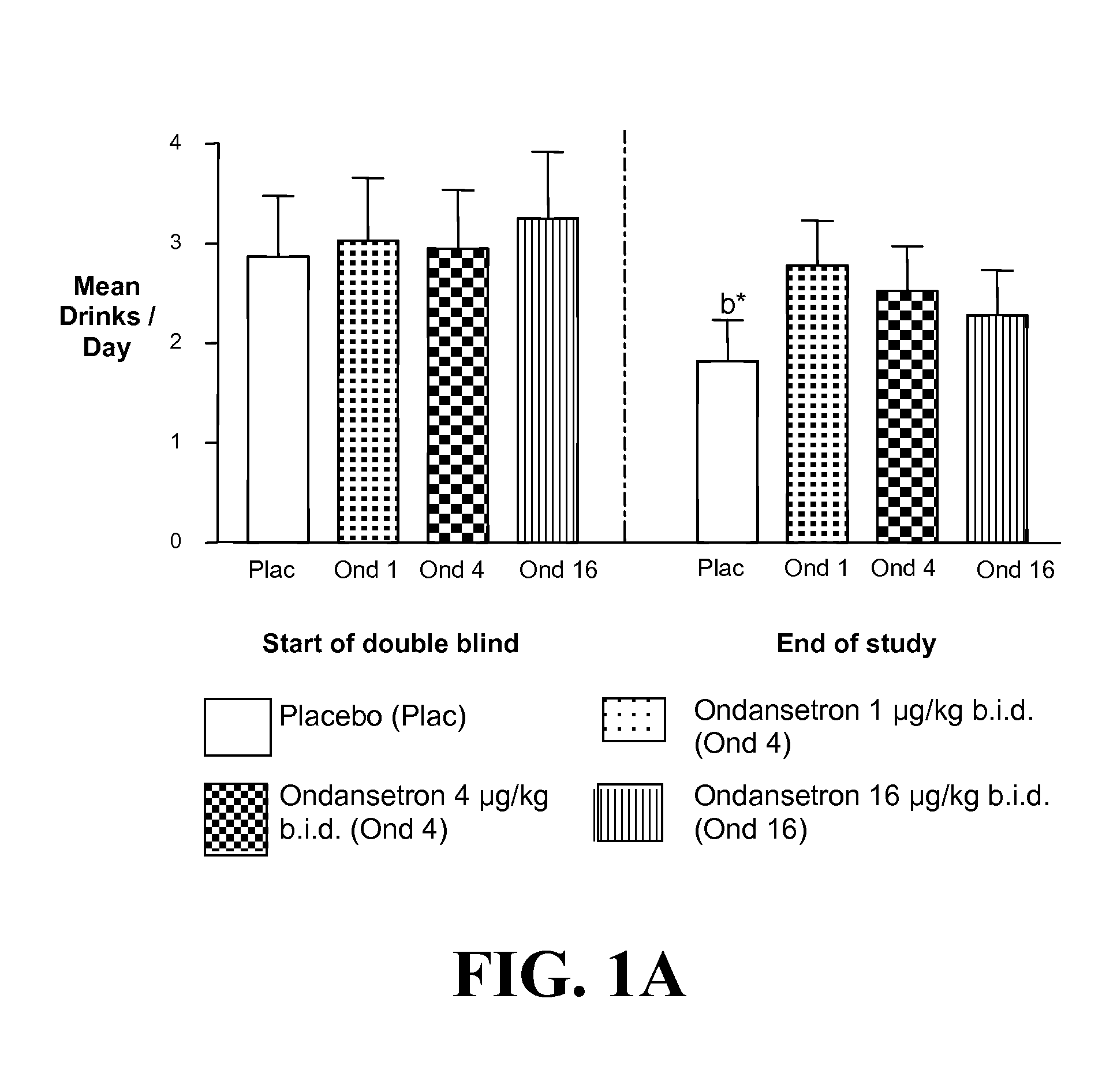
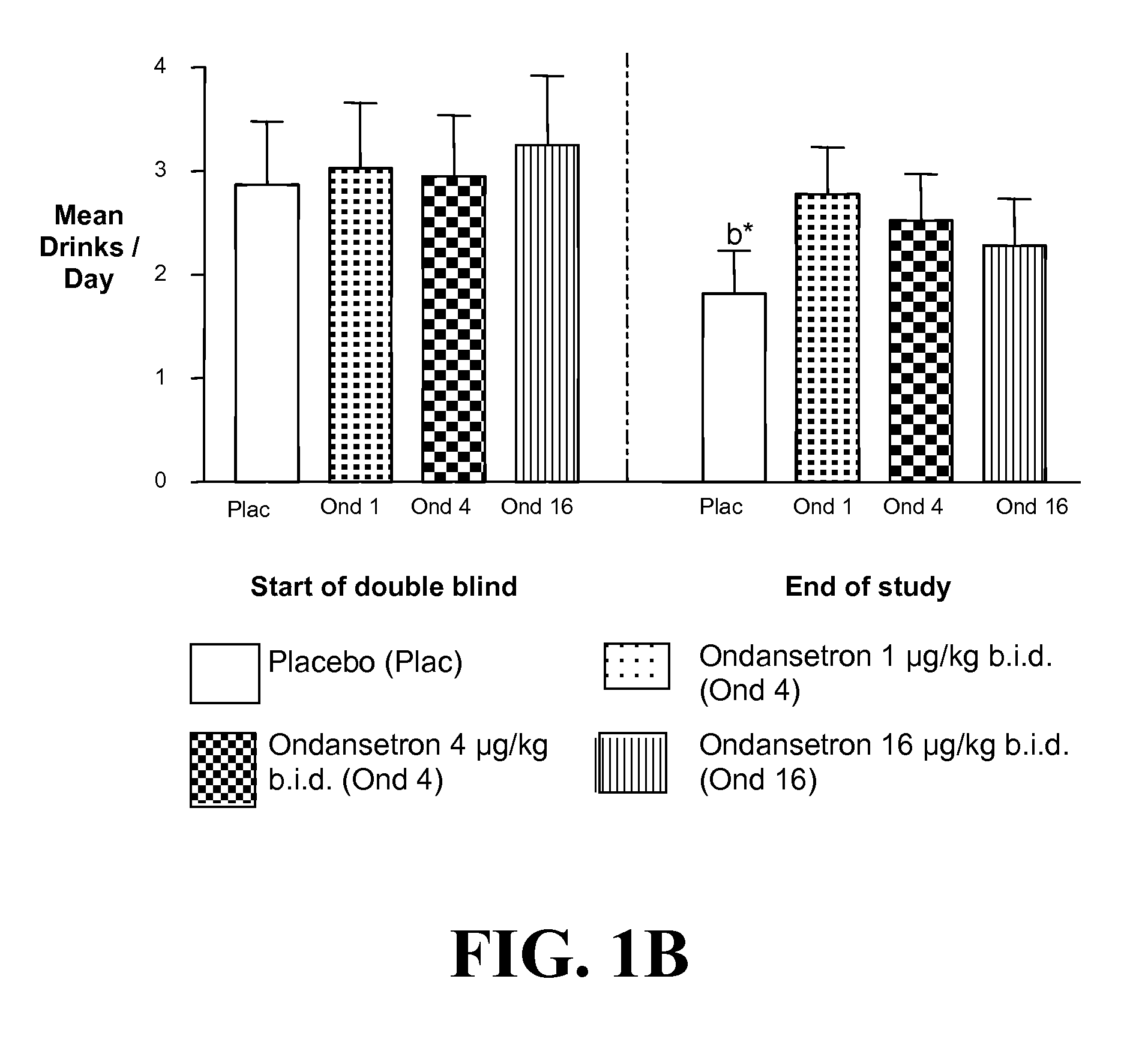
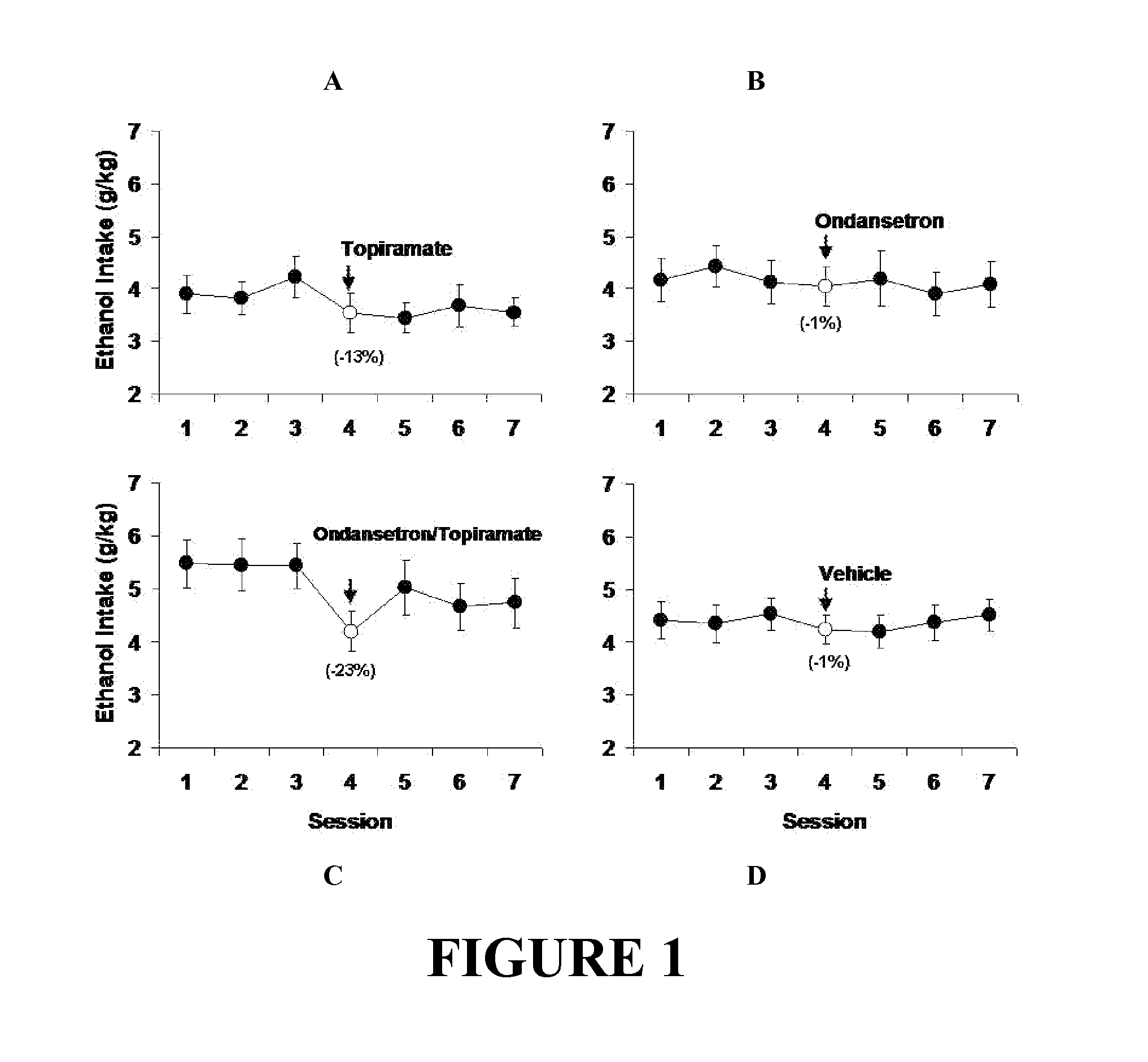
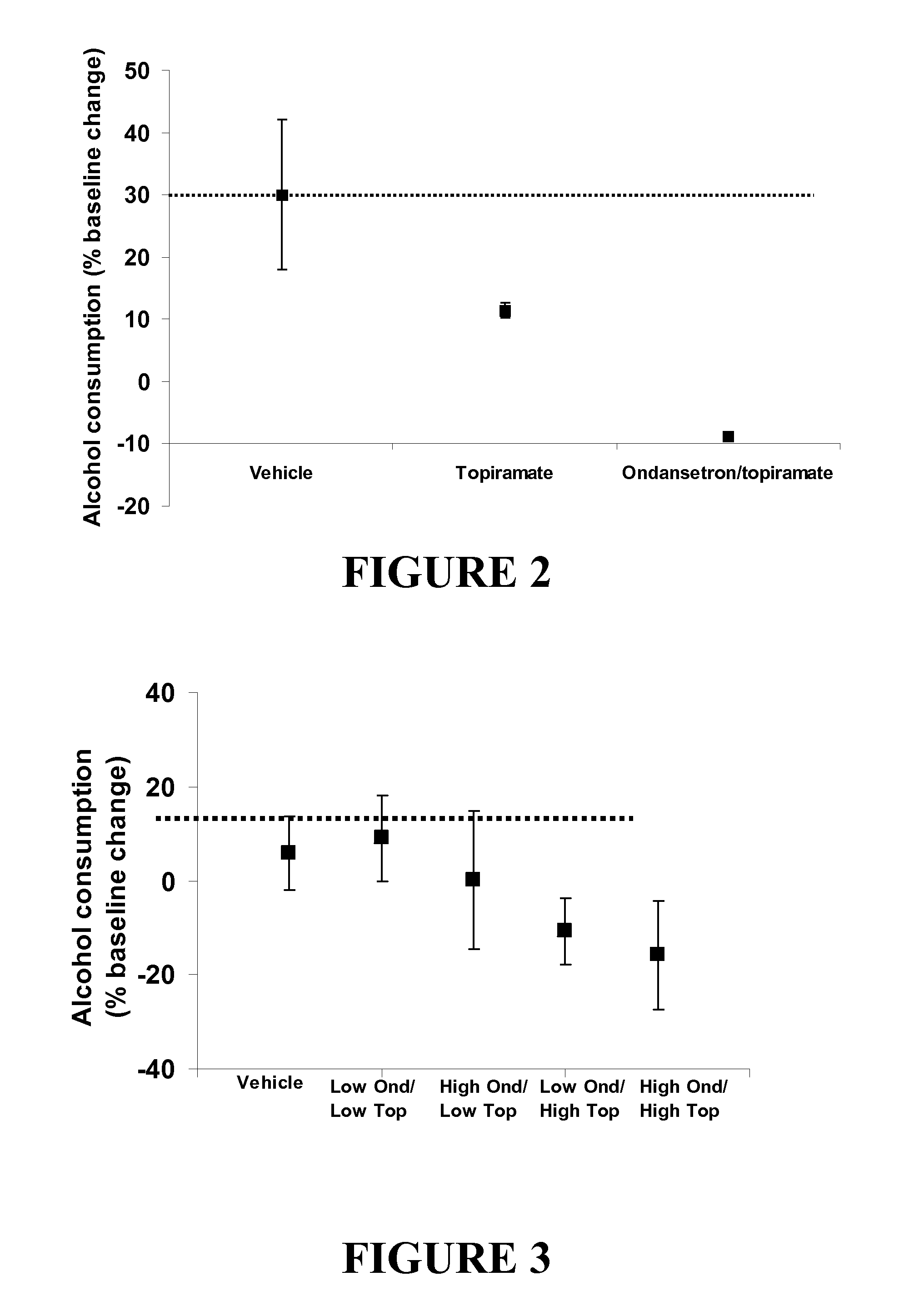
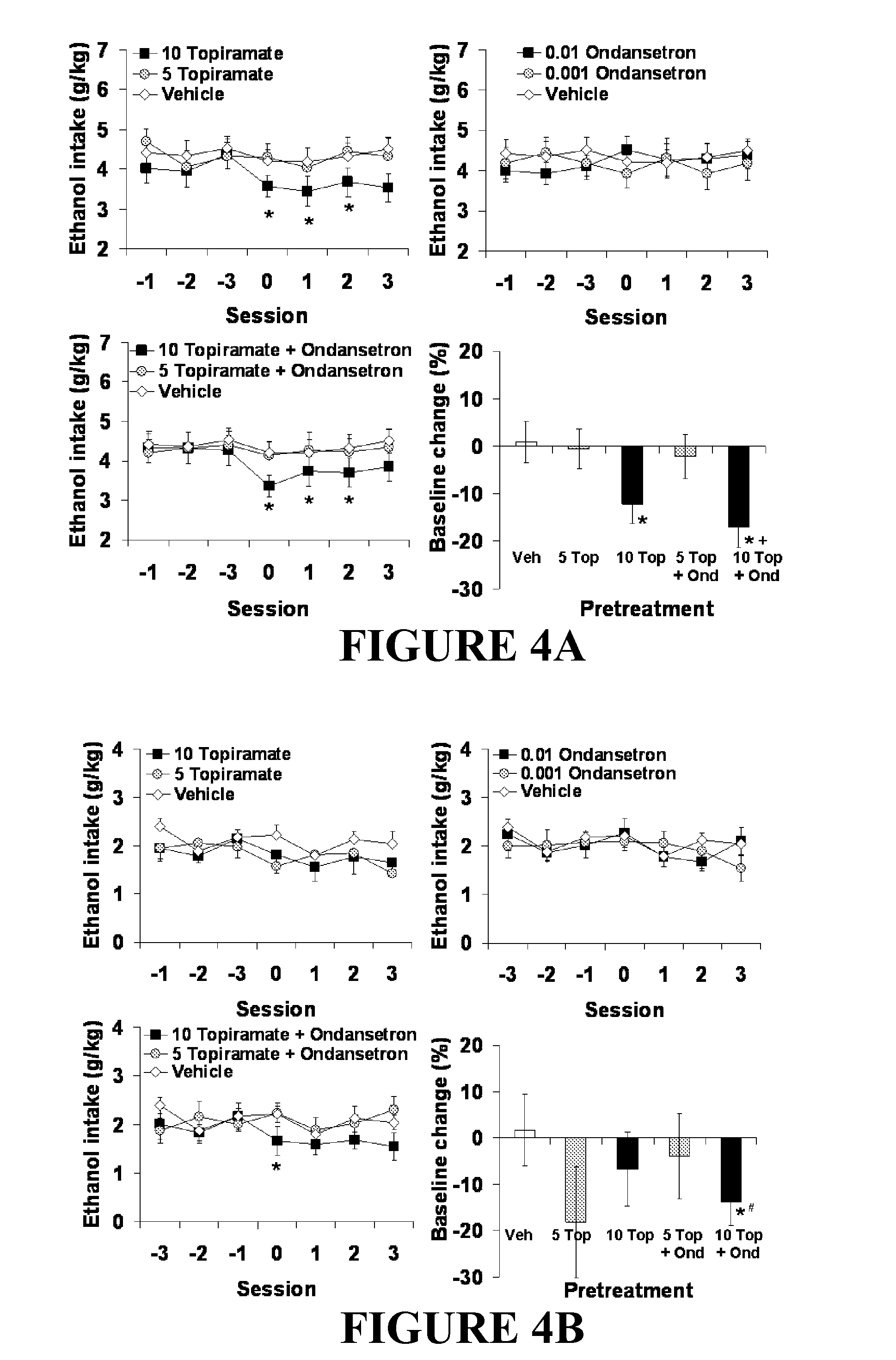
Combined Effects of Topiramate and Ondansetron on Alcohol Consumption
InactiveUS20100041689A1Avoid controlReduce impulseBiocideNervous disorderDiseaseBehavioral interventions
The present invention provides for the use of combinations of drugs to treat addictive disorders. More specifically, the present invention relates the use of drugs in conjunction with behavioral intervention to treat alcohol-related diseases and disorders as well as treatment of obesity and regulating weight.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Sustained-release formulations of topiramate
ActiveUS20110287099A1Reduction in severity and frequencyBiocideSenses disorderImmediate releaseTopiramate
Owner:SUPERNUS PHARM INC
Controlled release preparations comprising tramadol and topiramate
This invention relates to an oral pharmaceutical preparation, suitable for dosing every 24 hours, comprising a substrate, which substrate comprises a pharmaceutically effective amount of tramadol or a salt thereof and a pharmaceutically effective amount of topiramate and wherein said substrate may be coated with a controlled release coating; said preparation having a specific dissolution rate in vitro.
Owner:CILAG
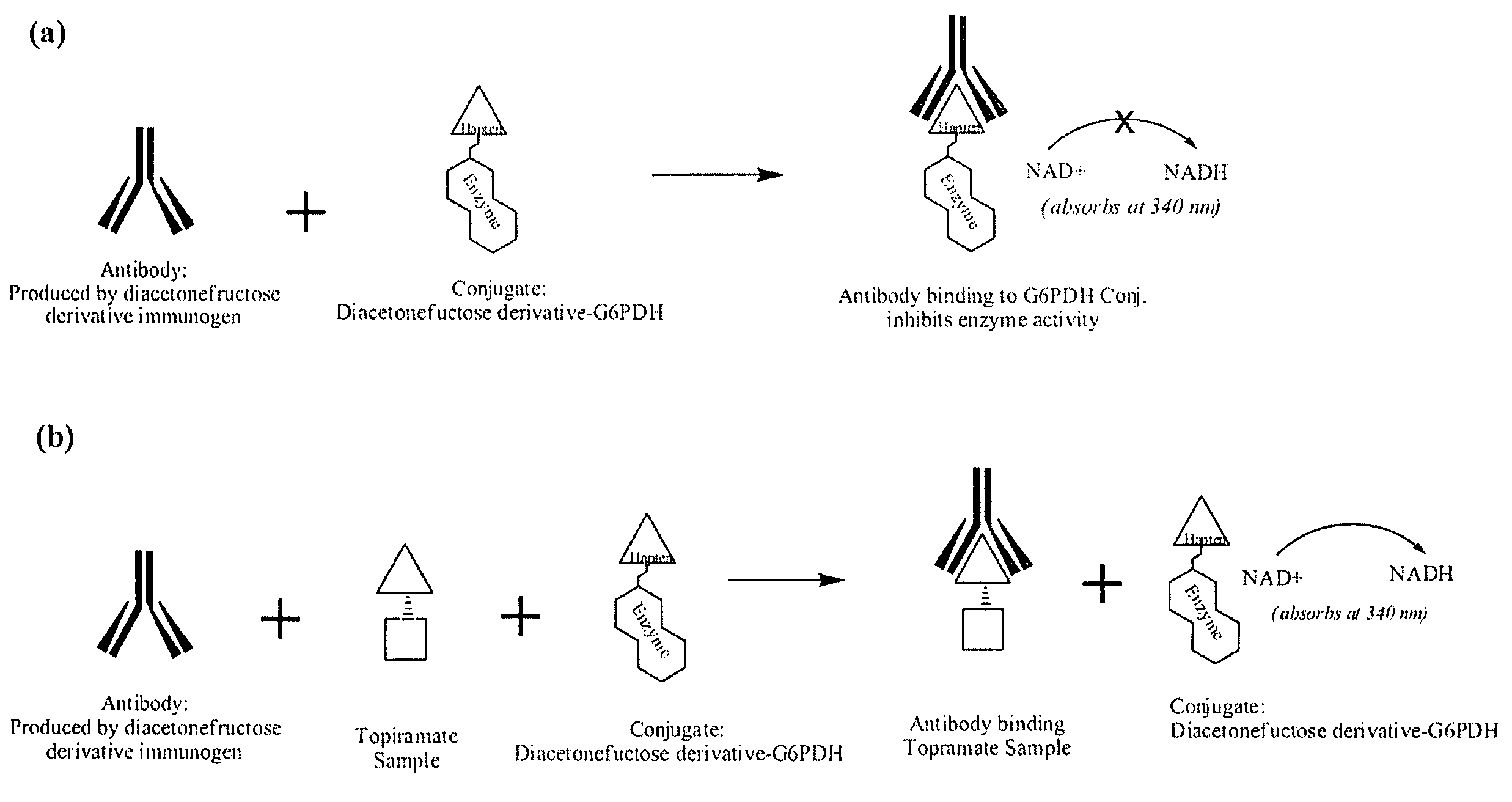
Topiramate Immunoassays
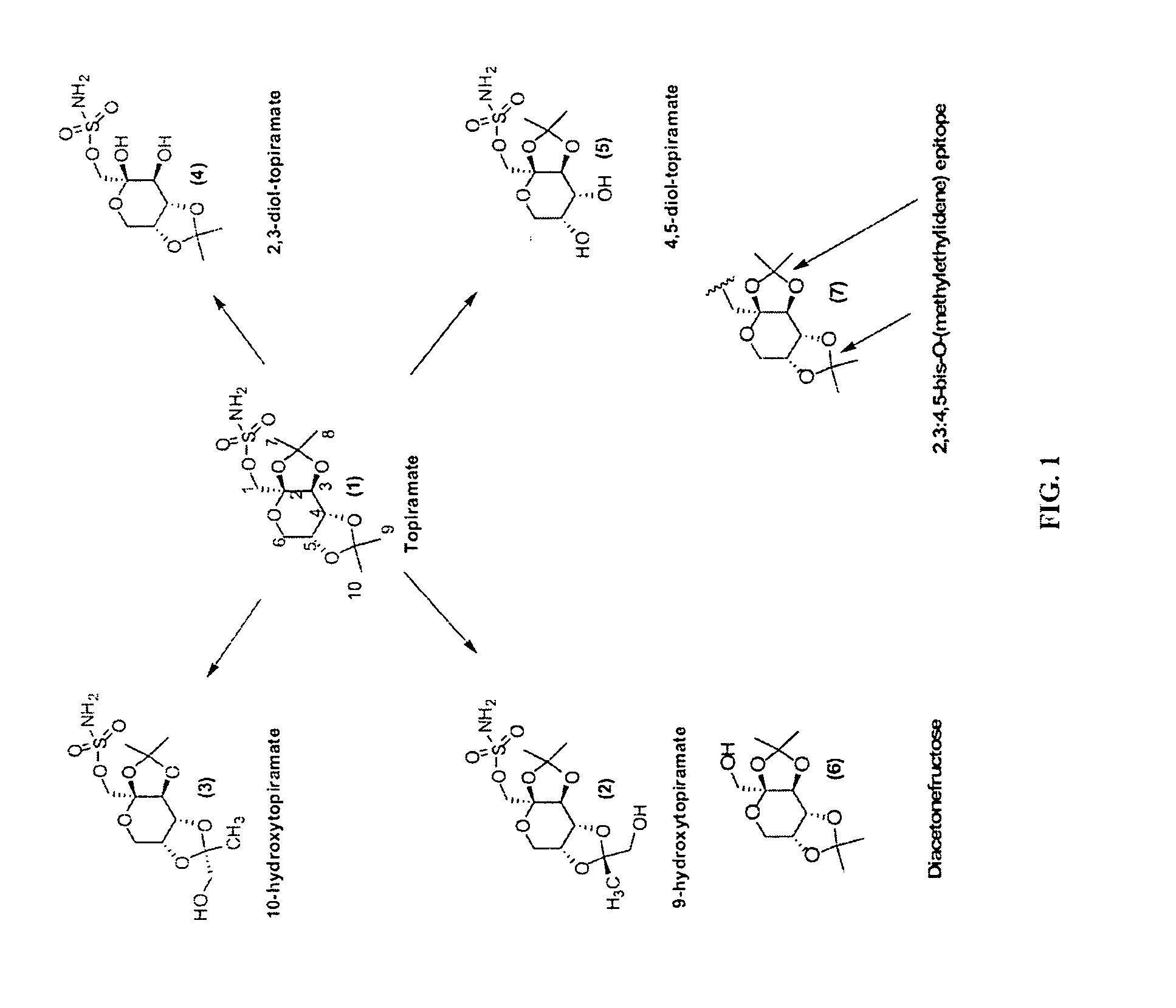
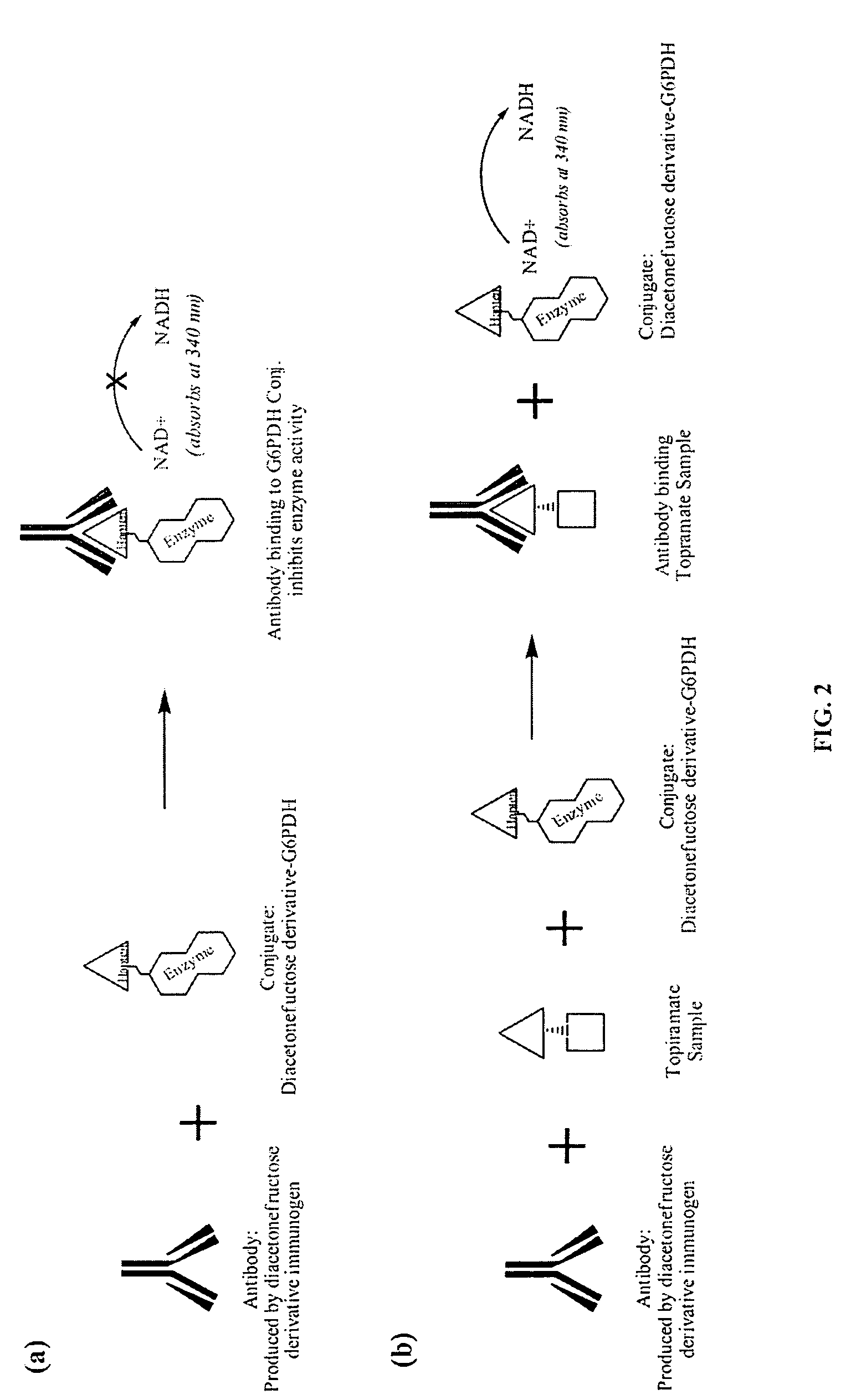
Diacetonefructose derivatives have substituents at the hydroxyl-position. Diacetonefructose derivatives may include immunogenic moieties to prepare anti-diacetonefructose derivative antibodies, or antigenic moieties for immunodiagnostic assays. Also, the diacetonefructose derivatives can include signal generating moieties for detecting the presence or amount of the diacetonefructose derivative in a sample. Additionally, the diacetonefructose derivatives can be used in immunodiagnostic assays to compete with topiramate for binding with anti-diacetonefructose derivative antibodies. Also, methods, compositions and kits are disclosed directed at diacetonefructose derivatives, immunogens, signal generating moieties and immunoassays for topiramate.
Owner:ARK DIAGNOSTICS
Sustained-Release Preparations Containing Topiramate and the Producing Method Thereof
InactiveUS20070224281A1Facilitates control of releaseImprove wettabilityOrganic active ingredientsNervous disorderMedicineTopiramate
Disclosed herein is a sustained-release topiramate preparation and a method for producing the topiramate preparation. The sustained-release topiramate preparation is produced using double granules obtained by granulating topiramate or a pharmaceutically acceptable salt thereof using a solid dispersant by a solid dispersion method (first granulation), and further by granulating the granules using a release sustaining material by dry or wet granulation (second granulation).
Owner:AMOREPACIFIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com