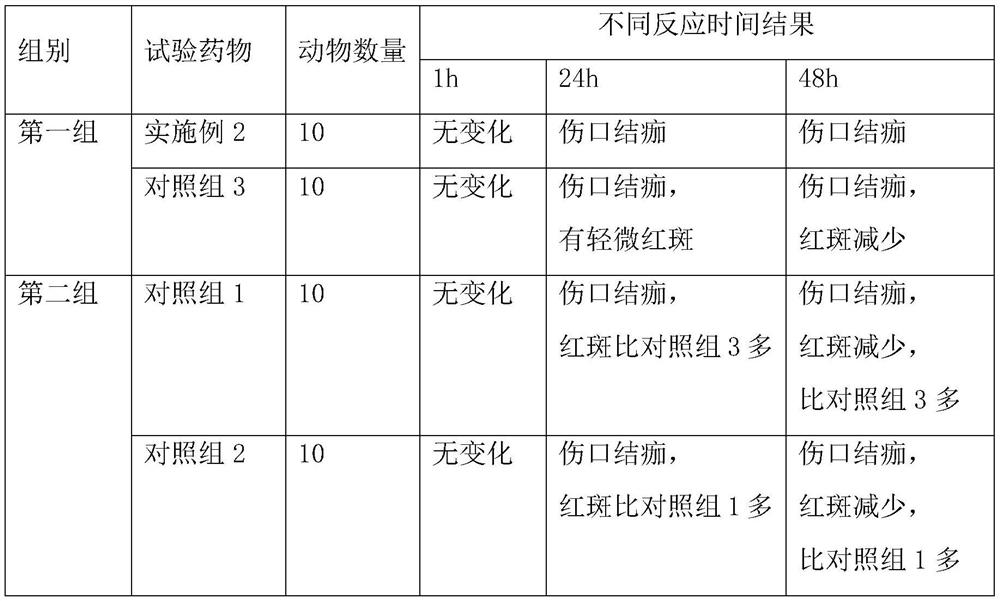
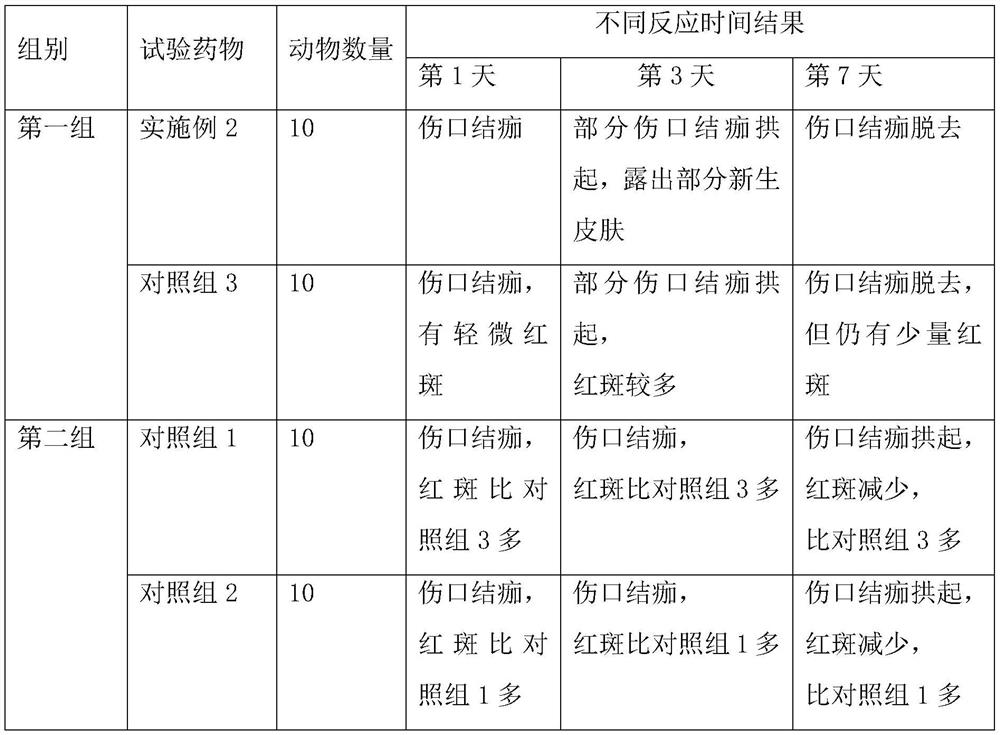
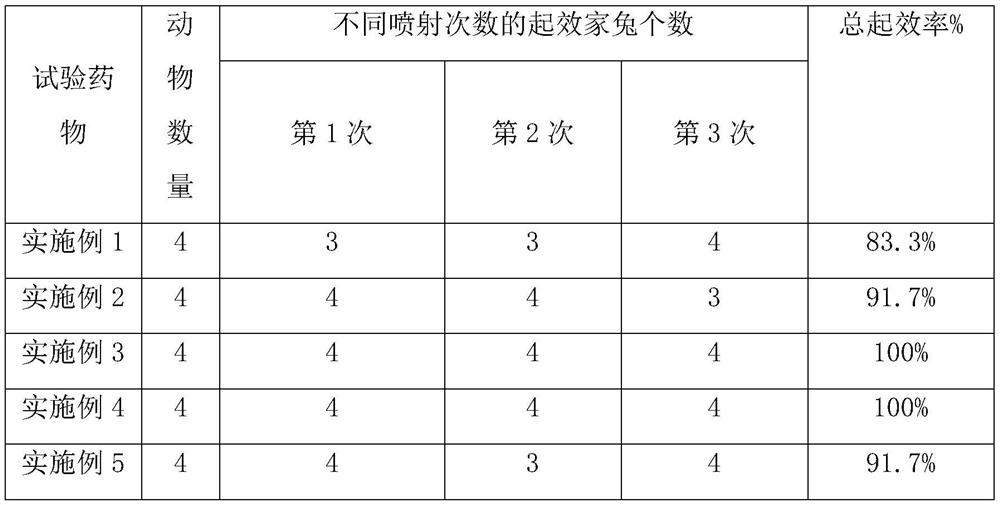
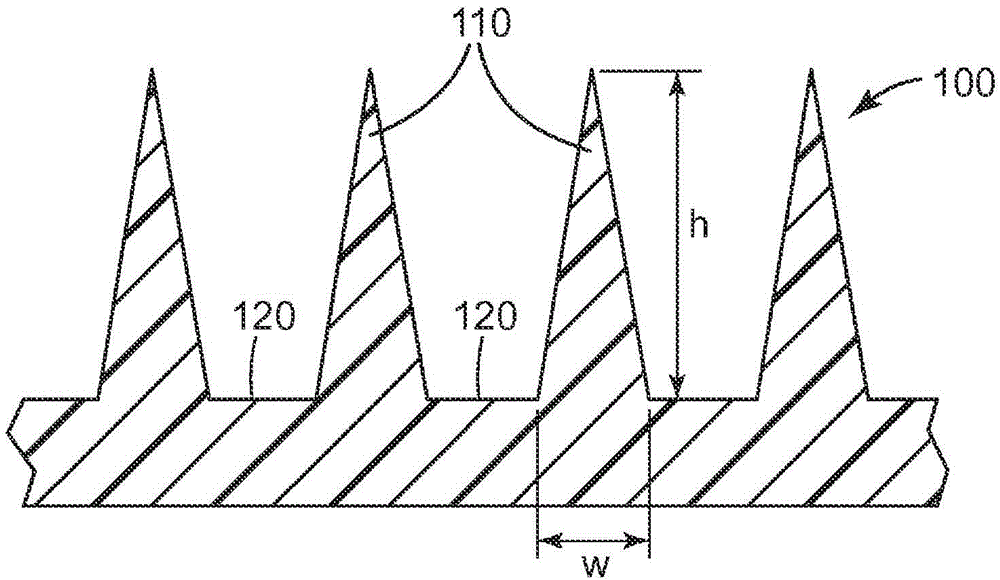
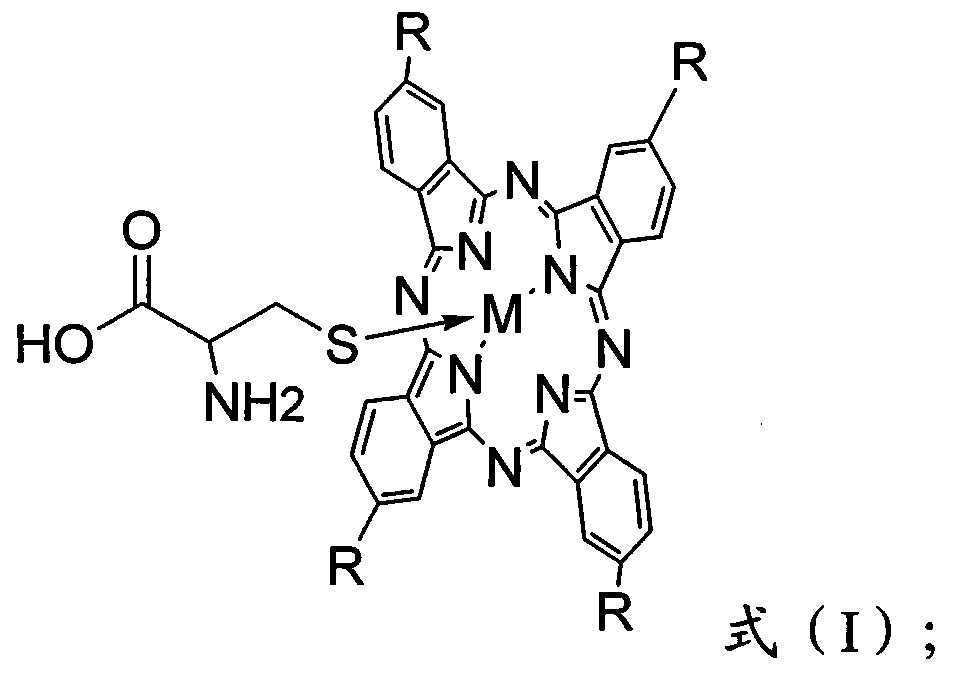
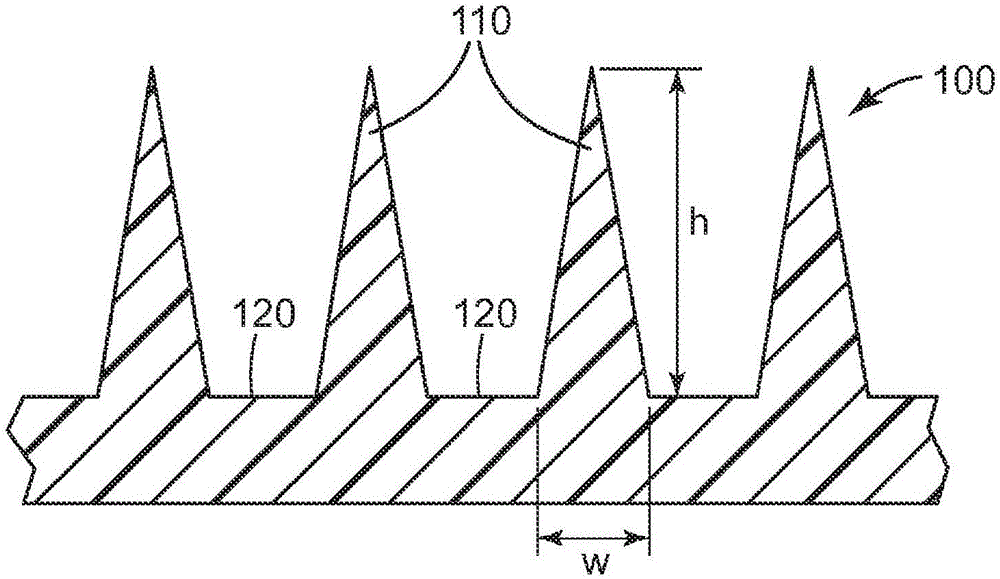
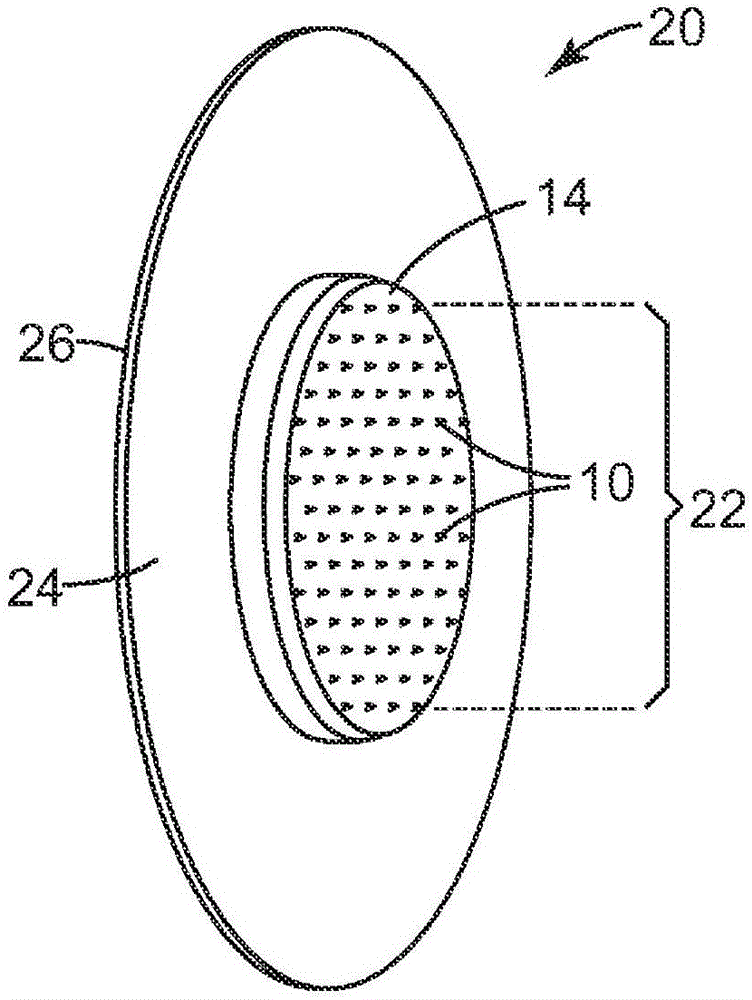
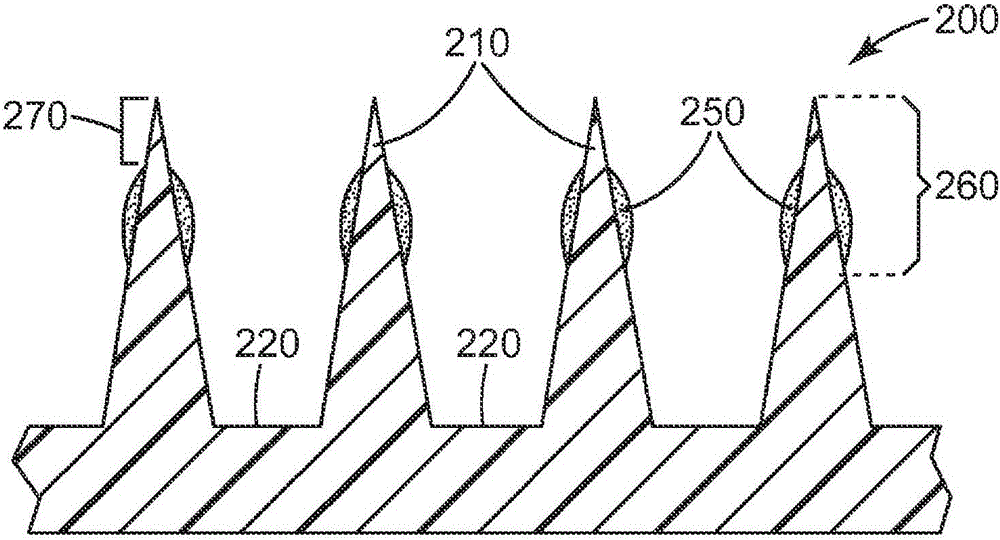
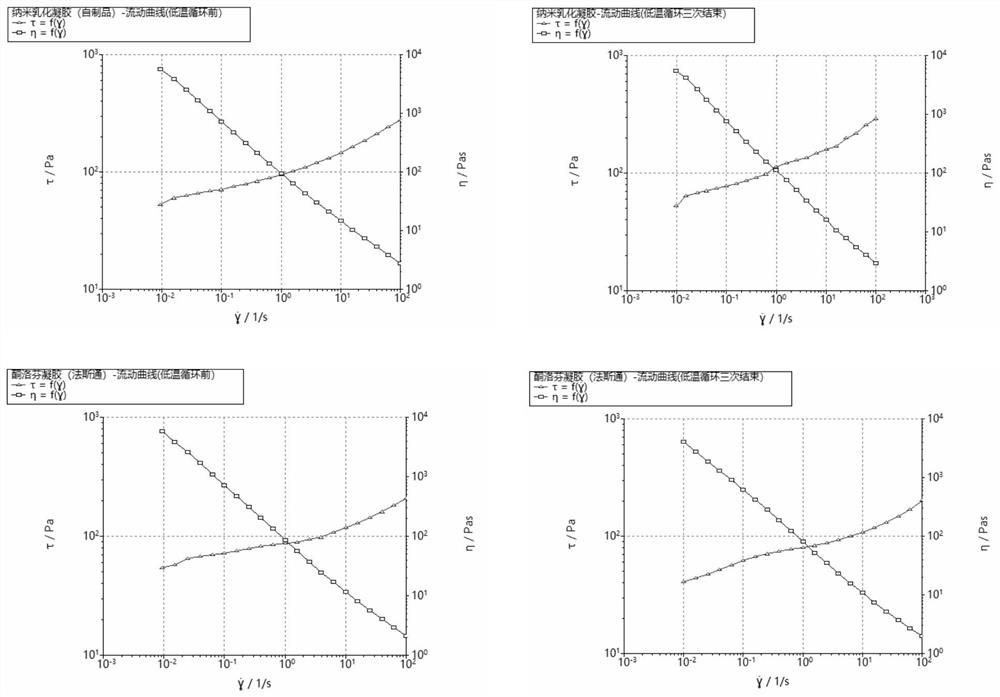
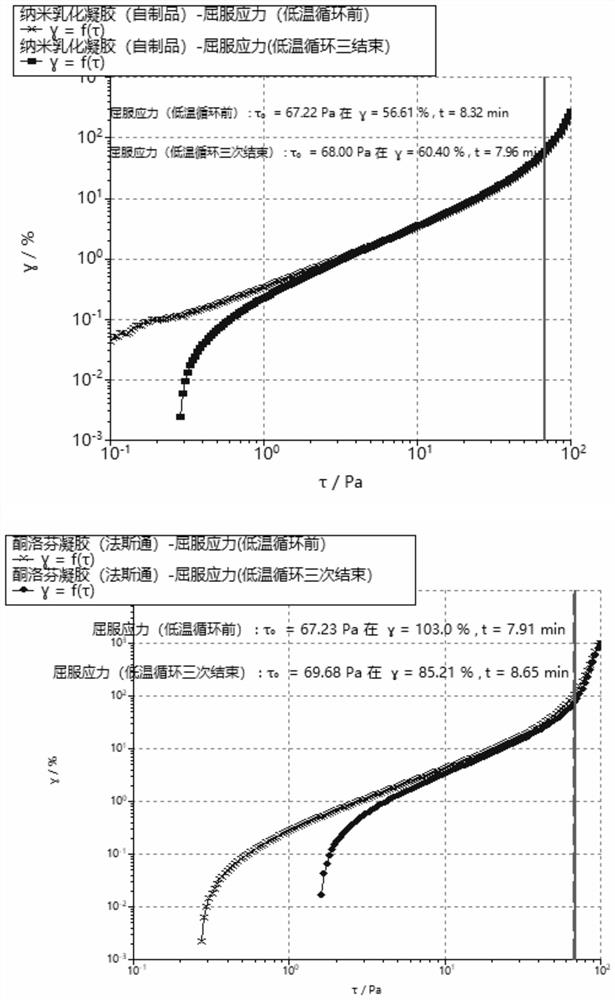
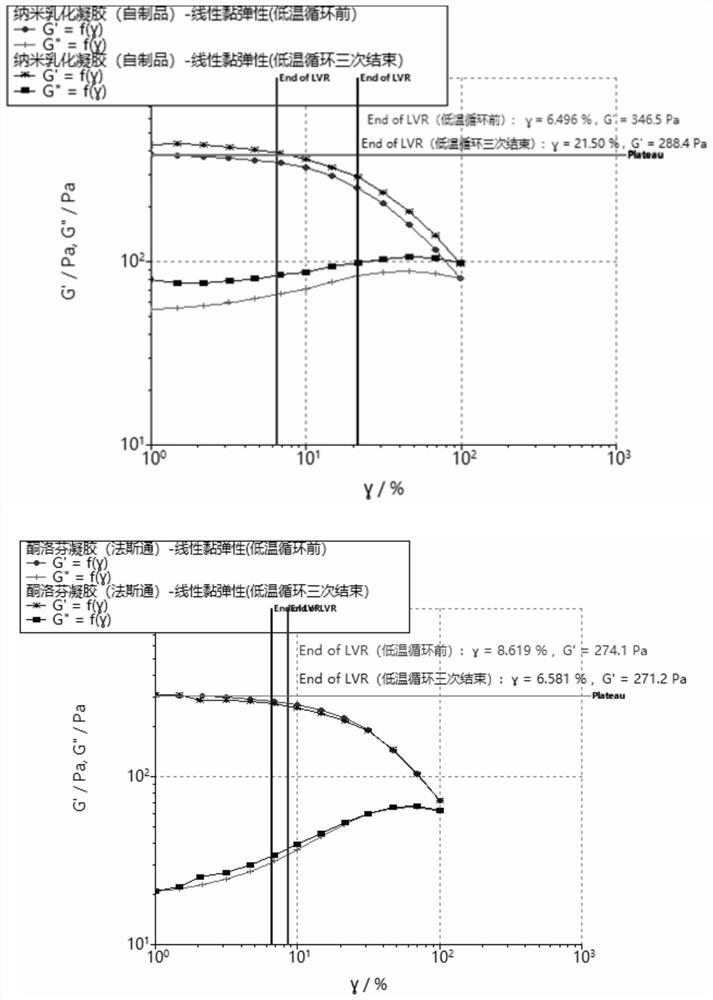
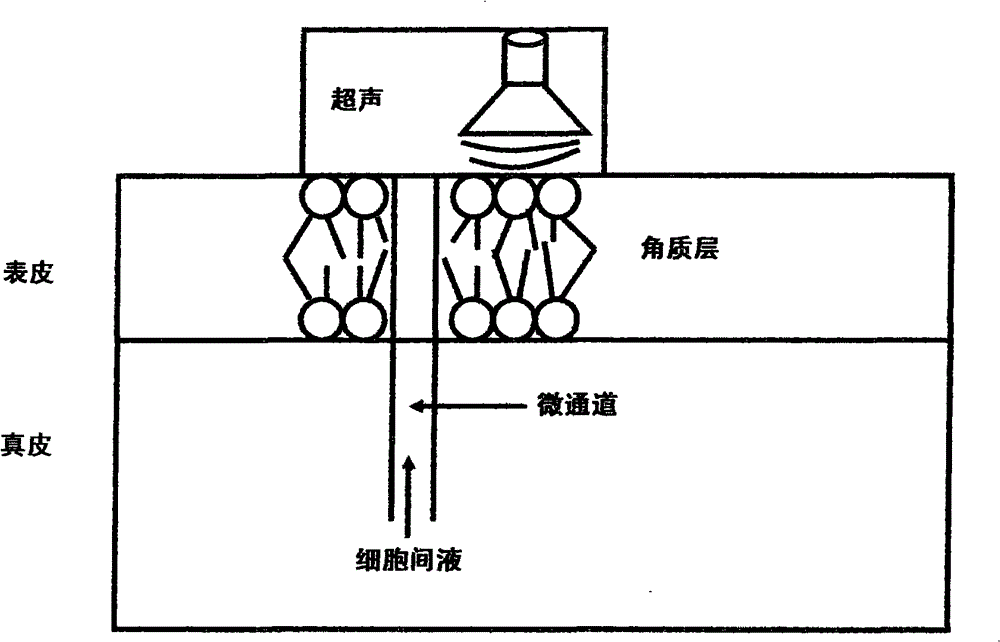
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Prilocaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
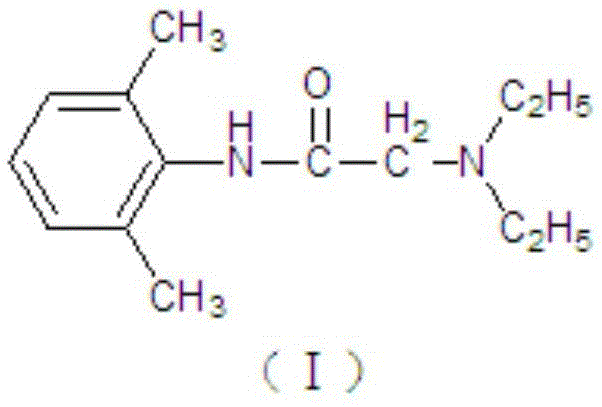
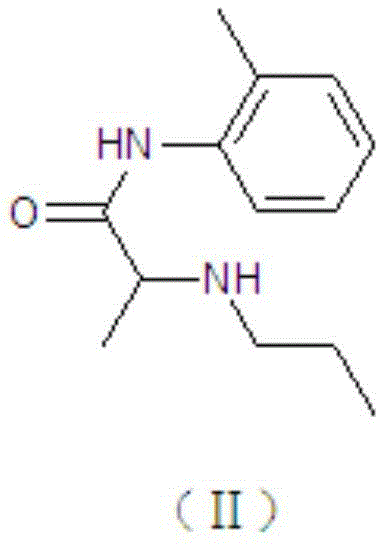
Prilocaine (/ˈpraɪləˌkeɪn/) is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils Löfgren. In its injectable form (trade name Citanest), it is often used in dentistry. It is also often combined with lidocaine as a topical preparation for dermal anesthesia (lidocaine/prilocaine or EMLA), for treatment of conditions like paresthesia. As it has low cardiac toxicity, it is commonly used for intravenous regional anaesthesia (IVRA).
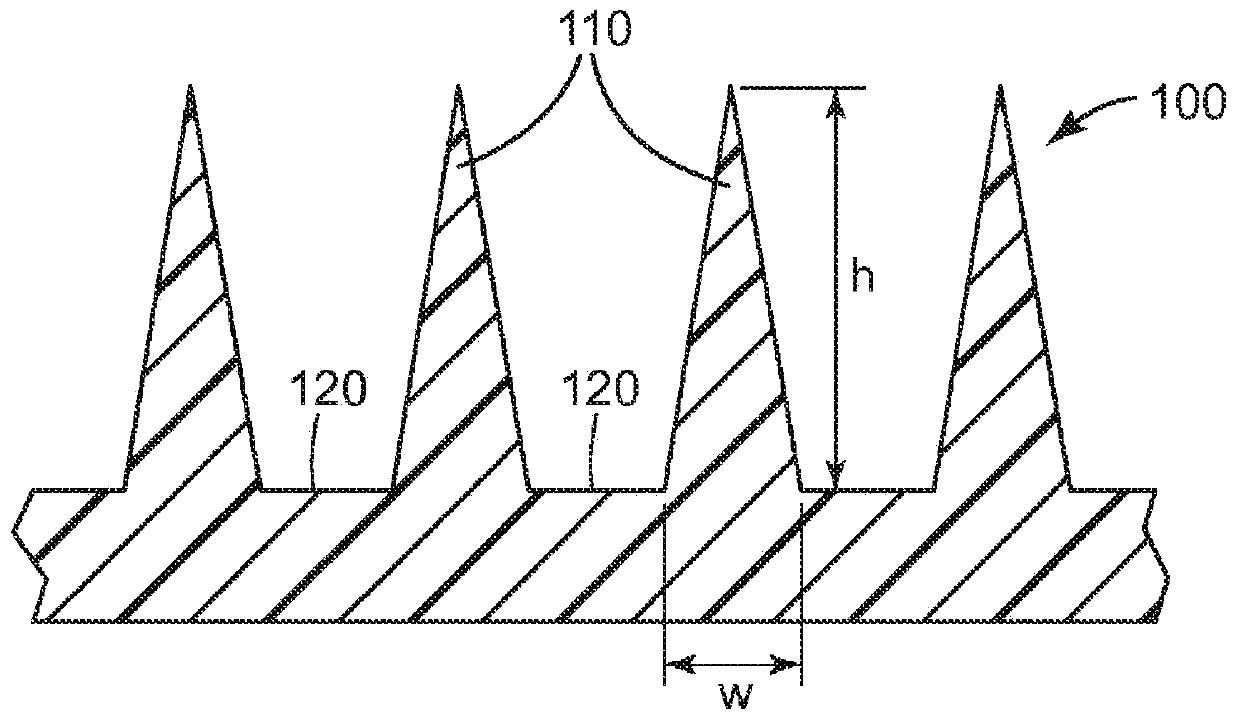
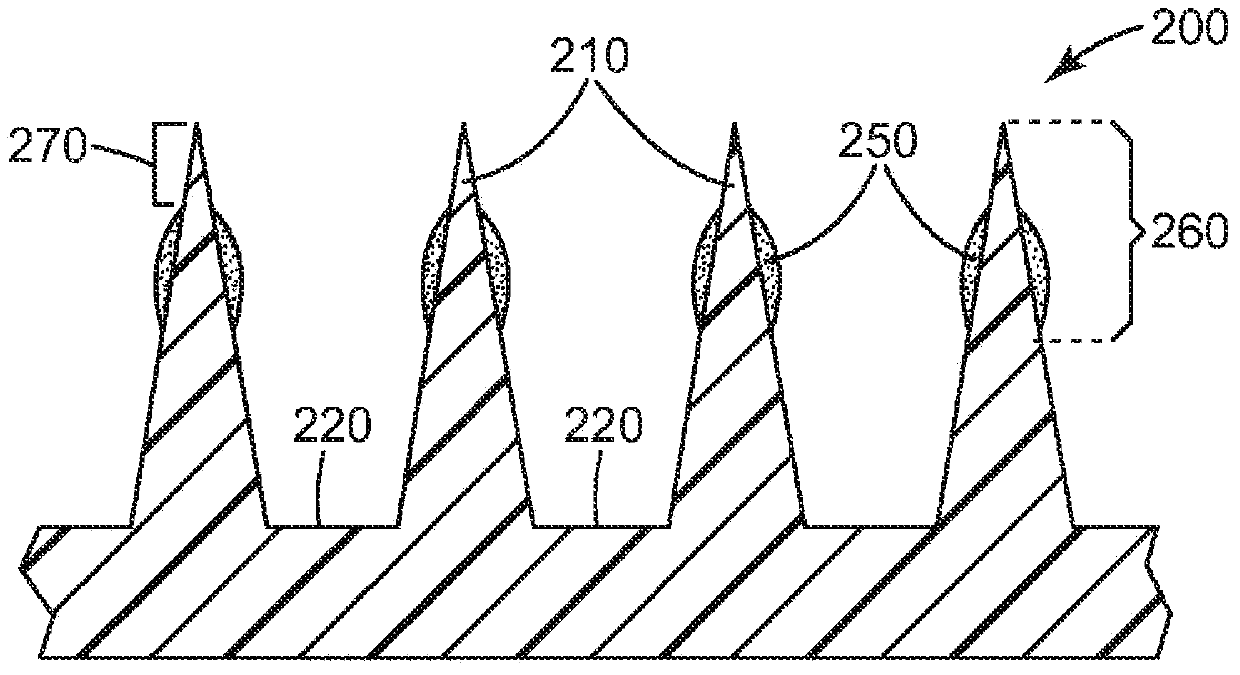
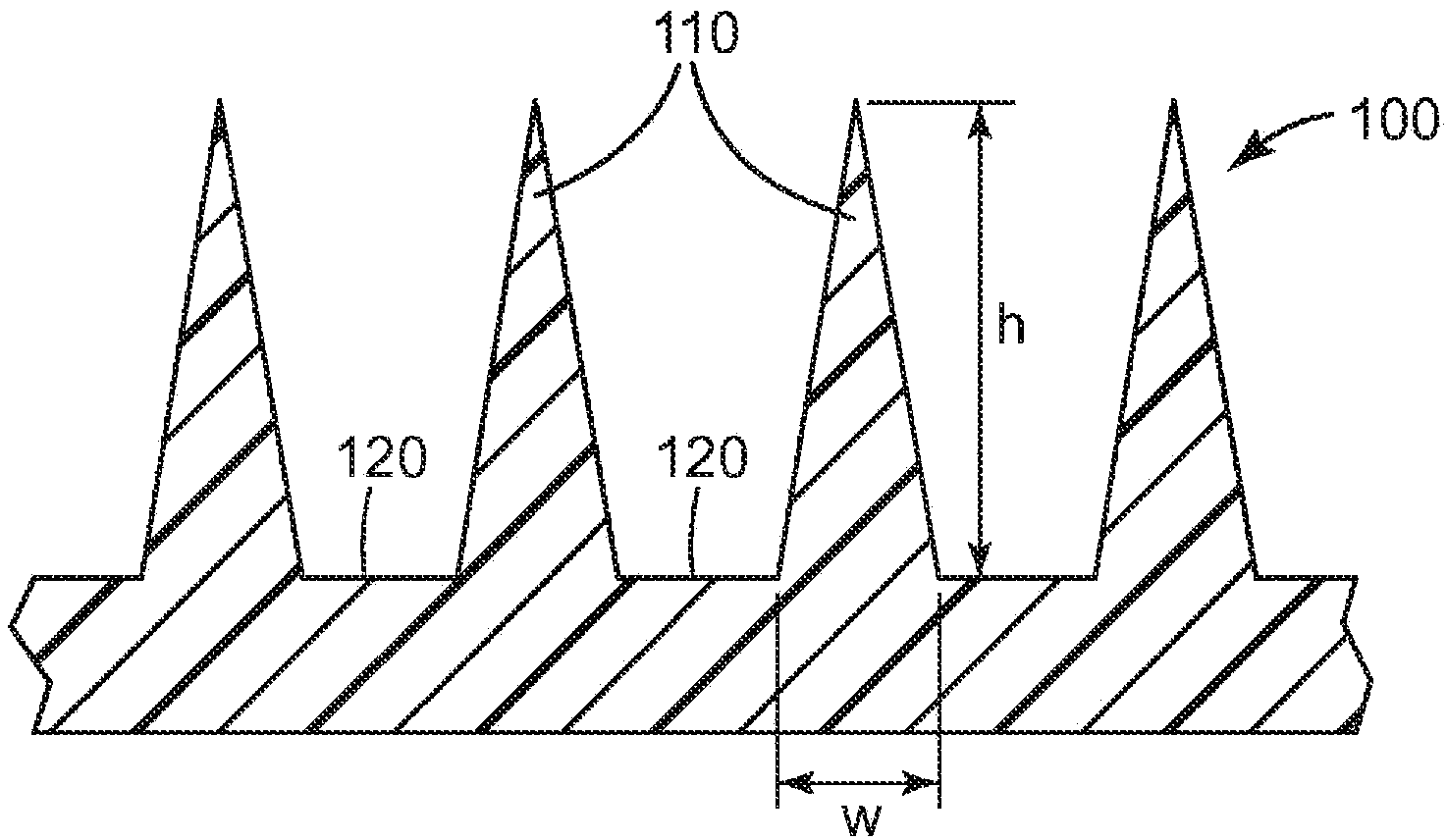
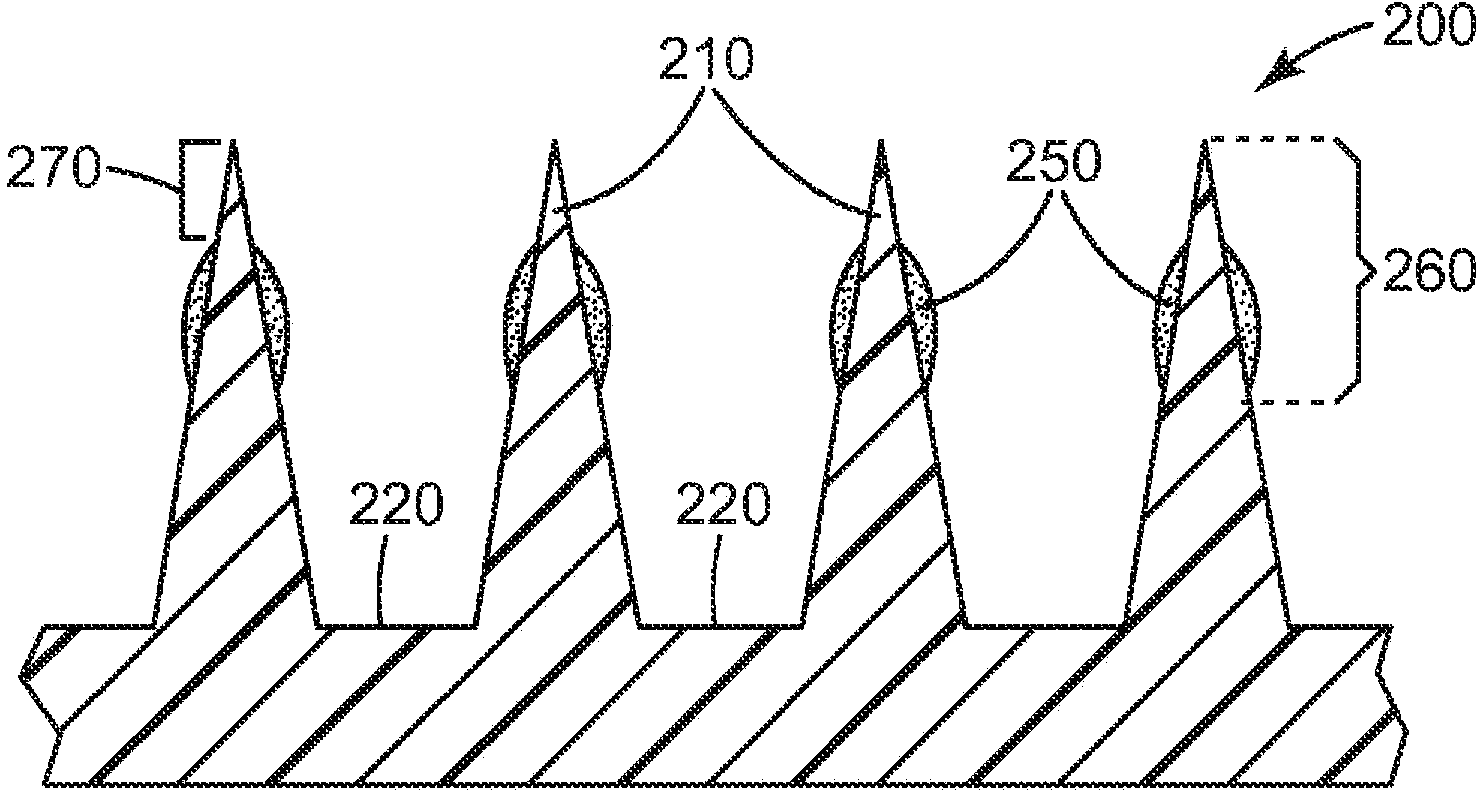
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
Anesthetic composition, formulation and method of use
InactiveUS20150010528A1Easy to managePromote rapid formationAmpoule syringesNervous disorderProcaineCarbocaine
An anesthetic composition for use e.g. in the administration of a local anesthetic by injection comprises a first component, which comprises hyaluronidase, and a second component which comprises an anesthetic preparation. The composition is both effective and highly shelf stable, and has as an advantage that it may be stored and administered at room temperature. In a particular embodiment, the hyaluronidase is prepared in dry powder form, as by lyophilization. The anesthetic component may be selected from a group of known anesthetics, such as lidocaine, polocaine, xylocaine, novocaine, procaine, prilocaine, bupivacaine, mepivacaine, carbocaine, etidocaine and chincocaine. The composition may be prepared in unit dosage forms, including a single dosage form, for a variety of purposes, and such unit dosage forms may be prepared in a plural chambered syringe or like dispenser, whereby the components are not mixed until administration.
Owner:WEG STUART L
Anesthetic composition, formulation and method of use
InactiveUS20090143436A1Increased shelf stabilityExtended shelf lifeBiocideNervous disorderProcaineCarbocaine
An anesthetic composition for use e.g. in the administration of a local anesthetic by injection comprises a first component, which comprises hyaluronidase, and a second component which comprises an anesthetic preparation. The composition is both effective and highly shelf stable, and has as an advantage that it may be stored and administered at room temperature. In a particular embodiment, the hyaluronidase is prepared in dry powder form, as by lyophilization. The anesthetic component may be selected from a group of known anesthetics, such as lidocaine, polocaine, xylocaine, novocaine, procaine, prilocaine, bupivacaine, mepivacaine, carbocaine, etidocaine and chincocaine. The composition may be prepared in unit dosage forms, including a single dosage form, for a variety of purposes, and such unit dosage forms may be prepared in a plural chambered syringe or like dispenser, whereby the components are not mixed until administration.
Owner:WEG STUART L
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
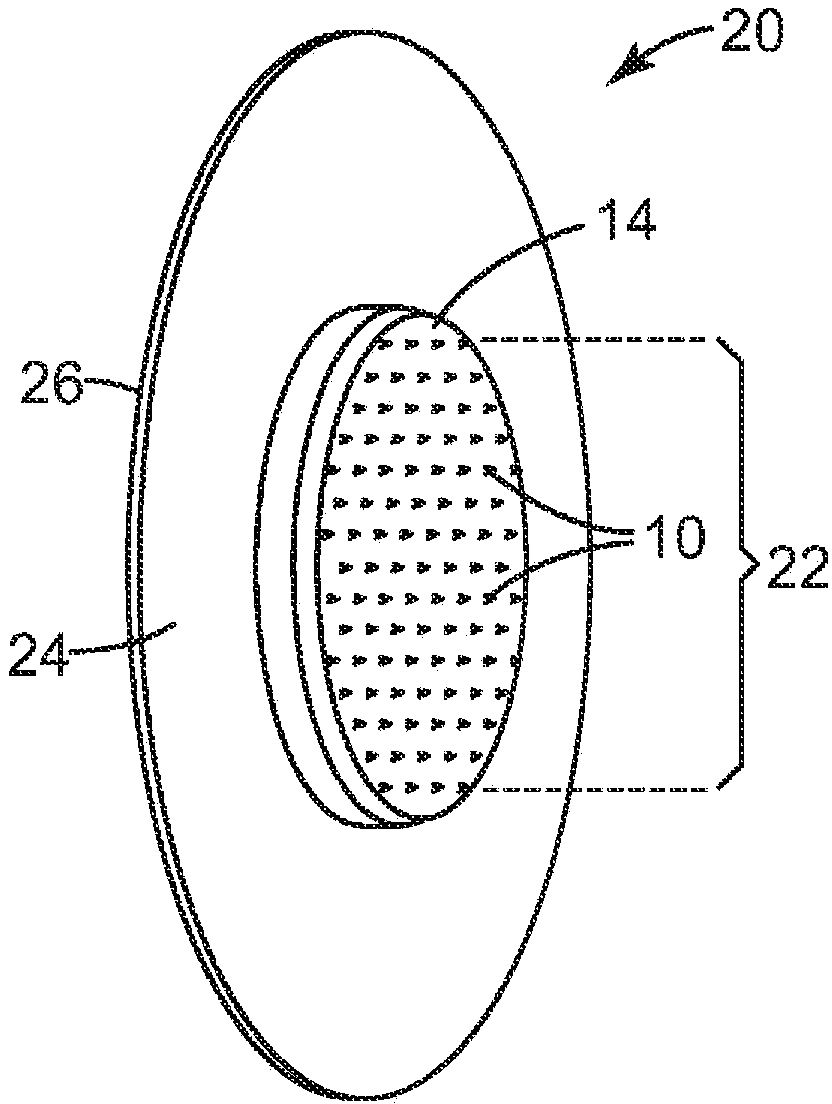
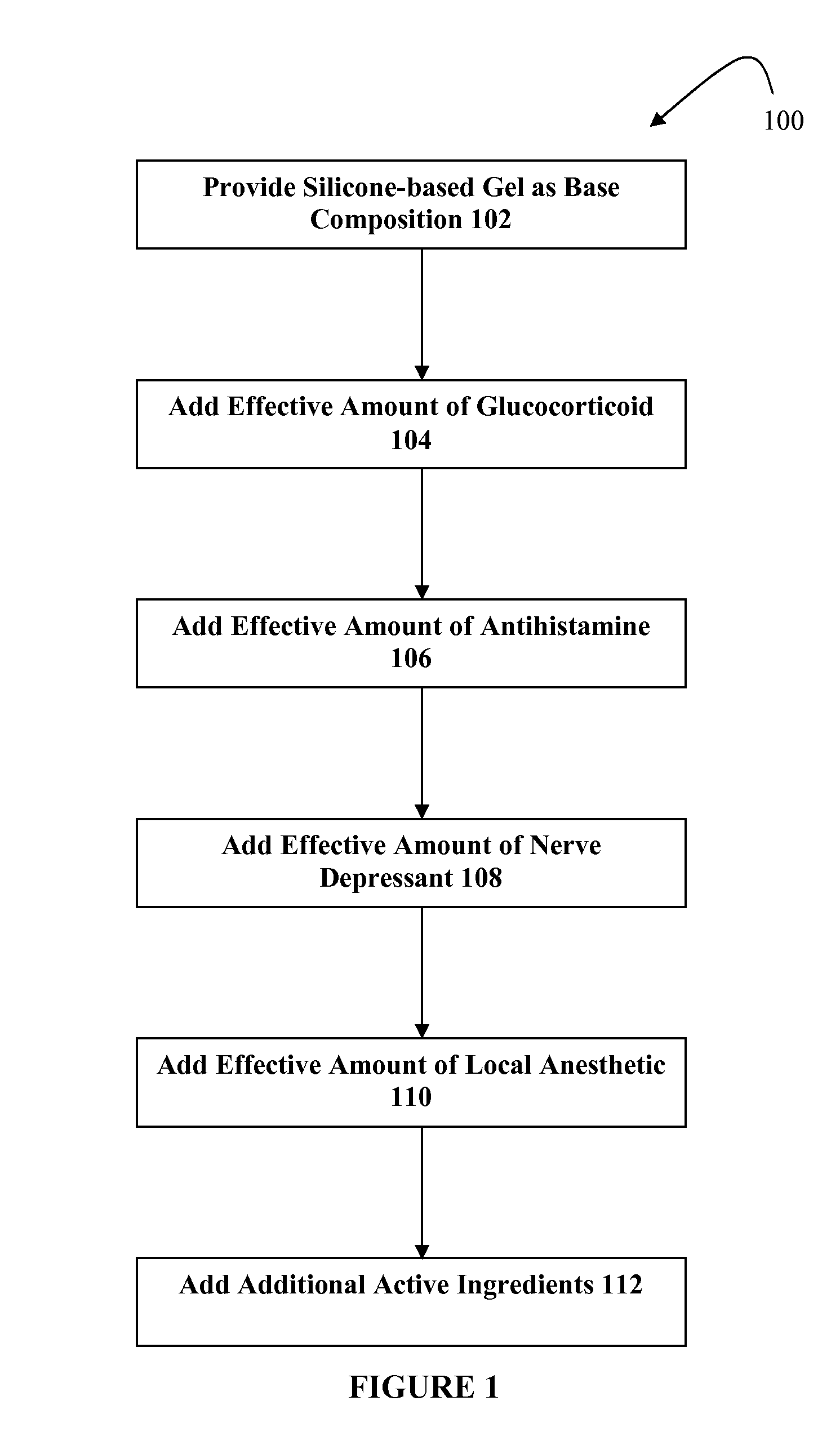
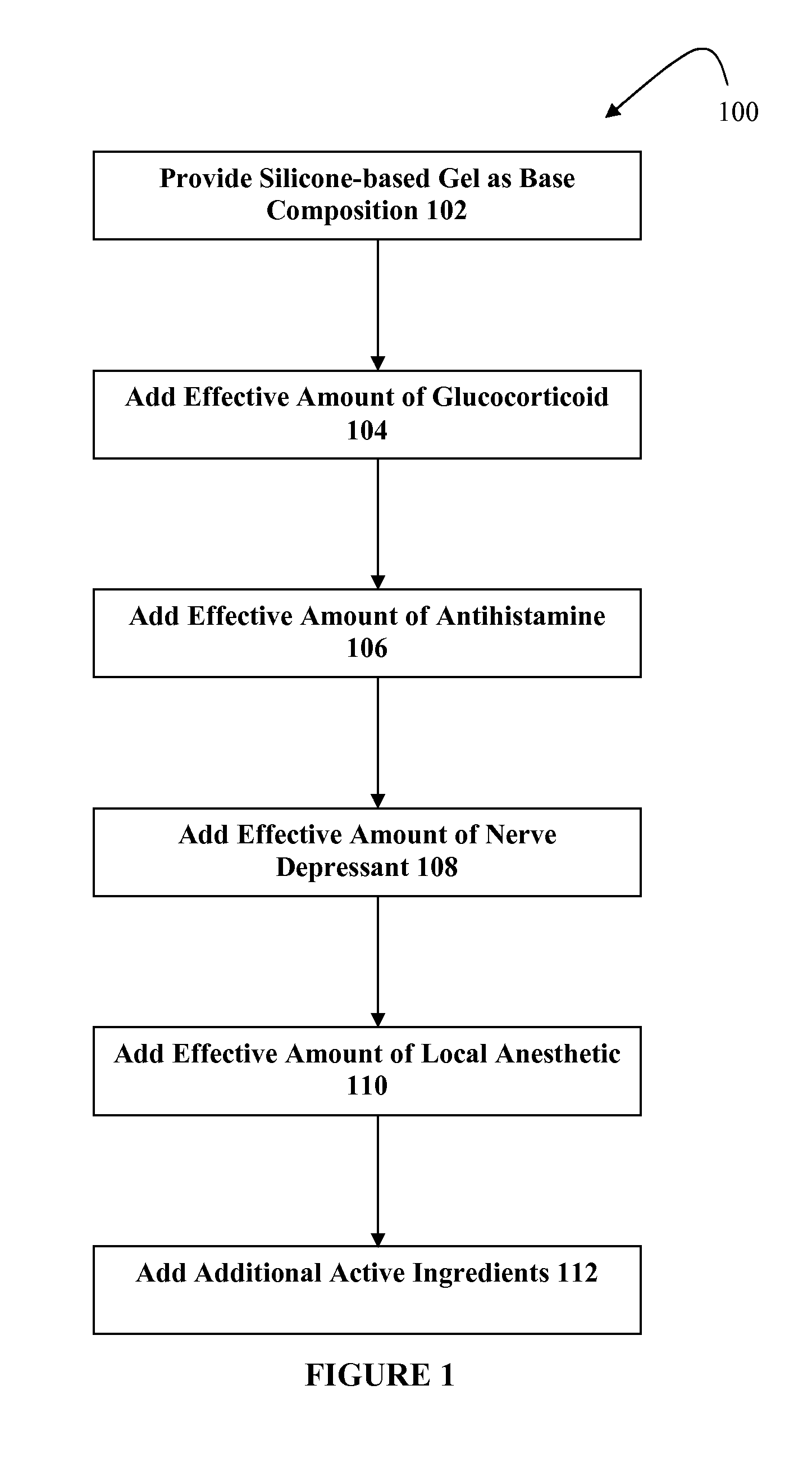
Silicone-based composition for skin treatment
The present embodiments may relate to topically delivered compounded medications for the treatment of scar tissue, skin disorders, and / or other ailments. In one aspect, a transdermal cream or gel may provide for the effective administration of multiple medications simultaneously. Preferably, a silicone-based gel may be provided as a base composition and may have a non-zero percentage of silicone or silicone variant. The silicone-based gel may comprise cyclopentasiloxane, polysilicone-11, dimethicone, and C30-45 alkyl cetearyl dimethicone crosspolymer, and include several active ingredients, such as glucocorticoids, antihistamines, and nerve depressants. The silicone-based gel may include a combination of fluticasone, loratadine, and gabapentin. The concentrations of fluticasone and loratadine may be relatively low, while that of gabapentin moderately high. The silicone-based gel may also have one or more local anesthetics, such as prilocaine and / or lidocaine. The silicone-based gel may include additional active ingredients, such as NSAIDs, anticonvulsants, antidepressants, muscle relaxants, and / or other active ingredients.
Owner:CMPD LICENSING
Compound xylocaine cream pharmaceutical composition and preparation method thereof
ActiveCN106806338AQuality improvementControl contentOrganic active ingredientsAerosol deliveryIrritationMedicine
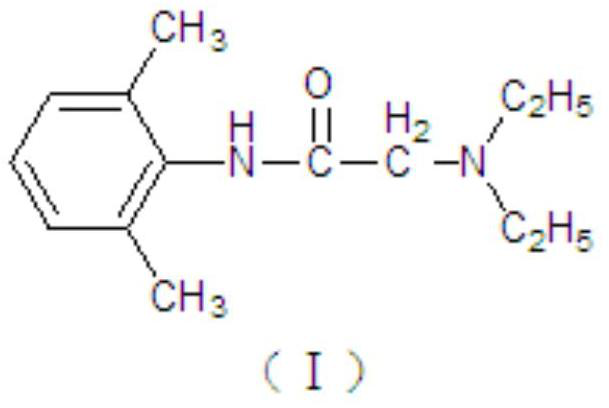
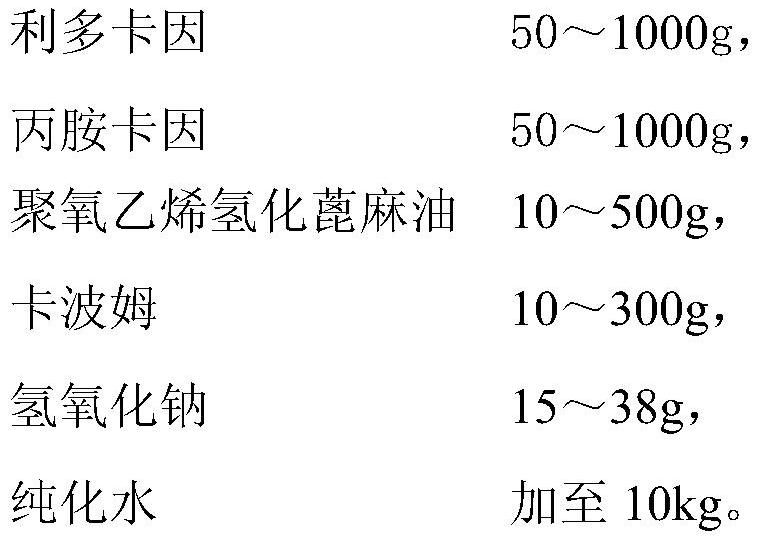
The invention provides a compound xylocaine cream pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is prepared by using effective quantities of xylocaine and prilocaine as main medicinal materials and using polyoxyethylene hydrogenated castor oil, carbomer, sodium hydroxide and purified water as medicinal auxiliary materials. The cream has the advantages of high moisture content, favorable spreadability, low impurity content, high quality and small skin irritation.
Owner:SICHUAN HAISCO PHARMA CO LTD
External preparation of lidocaine-prilocaine medicinal composition and application thereof
PendingCN112438963AImprove securityImprove drug utilizationOrganic active ingredientsAnaestheticsSide effectPharmaceutical medicine
The invention discloses an external preparation of a lidocaine-prilocaine medicinal composition. The content of the lidocaine-prilocaine medicinal composition is 1%-10%. The external preparation comprises a patch containing lidocaine-prilocaine oily eutectic, a non-hydrophilic matrix material and pharmaceutically acceptable external preparation auxiliary materials, and a gel patch containing lidocaine-prilocaine O / W microemulsion, a hydrophilic gel matrix material and pharmaceutically acceptable external preparation auxiliary materials. The patch and the gel patch can be used for local superficial anesthesia or relieving local pains such as post-injection pain, post-puncture pain, pain caused by intravenous injection, postherpetic neuralgia, touch pain caused by herpes zoster and protrusion of intervertebral disc. The external preparation and the application have the advantages that the lidocaine-prilocaine eutectic is taken as an active ingredient, so that the percutaneous penetrationrate and the absorption degree of lidocaine and prilocaine can be obviously improved, the beneficial effects of synergy are produced, meanwhile, the occurrence rate of toxic and side effects of the lidocaine and the prilocaine is reduced, and a new choice is provided for analgesia and anesthesia requirements of patients and medical staff.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
Compound isopropyl phenol injection contg. local anesthetic and prepn. method therefor
InactiveCN1903187ADefinite curative effectMature technologyHydroxy compound active ingredientsPharmaceutical delivery mechanismRopivacainePhenols
A compound isopropylphenol injection containing local anesthetic contains proportionally isopropylphenol, the local anesthetic chosen from procaine, lidocaine, etc, the refined plant oil for injection, emulsifier, isotonic regulator, antioxidant, pH regulator and the water for injection. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Hypoallergenic soothing epidermis anesthesia cream as well as preparation method and application thereof
ActiveCN112691076AReduce risk of sensitizationLess irritatingOrganic active ingredientsAerosol deliveryHypoallergenicBiology
The invention provides a hypoallergenic soothing epidermis anesthesia cream as well as a preparation method and application thereof. The cream comprises the following components of 5-20 g of an anesthetic, 0.5-2 g of a soothing agent, 8-15 g of an emulsifier, 8-15 g of a thickener and the balance of 900-950 ml of purified water. By adding specific soothing components, the sensitization risk caused by lidocaine and prilocaine can be greatly reduced, and particularly, the cream can also effectively relieve postoperative adverse reactions.
Owner:山东光普医疗科技有限公司
Silicone-based composition for skin treatment
The present embodiments may relate to topically delivered compounded medications for the treatment of scar tissue, skin disorders, and / or other ailments. In one aspect, a transdermal cream or gel may provide for the effective administration of multiple medications simultaneously. Preferably, a silicone-based gel may be provided as a base composition and may have a non-zero percentage of silicone or silicone variant. The silicone-based gel may comprise cyclopentasiloxane, polysilicone-11, dimethicone, and C30-45 alkyl cetearyl dimethicone crosspolymer, and include several active ingredients, such as glucocorticoids, antihistamines, and nerve depressants. The silicone-based gel may include a combination of fluticasone, loratadine, and gabapentin. The concentrations of fluticasone and loratadine may be relatively low, while that of gabapentin moderately high. The silicone-based gel may also have one or more local anesthetics, such as prilocaine and / or lidocaine. The silicone-based gel may include additional active ingredients, such as NSAIDs, anticonvulsants, antidepressants, muscle relaxants, and / or other active ingredients.
Owner:CMPD LICENSING
Pharmaceutical composition, patch, and preparation methods and application of pharmaceutical composition and patch
PendingCN110038130AGuaranteed thicknessGet Continuous Pain ReliefNervous disorderAntipyreticProcaineBULK ACTIVE INGREDIENT
Owner:张洁
Compound medicament with double effects of alleviating pain and inhibiting bacteria
InactiveCN103006699AReduce adverse reactionsLow cost of treatmentAntibacterial agentsOrganic active ingredientsUse medicationPharmaceutical drug
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Compound lidocaine aerosol and preparation method thereof
ActiveCN112274498ALess irritatingComposition is stableOrganic active ingredientsAntipyreticPolyvinyl alcoholActive agent
The invention discloses a compound lidocaine aerosol and a preparation method thereof. The compound lidocaine aerosol comprises the following components in parts by weight: 0.2-3.5 parts of lidocaine,0.2-3.5 parts of propylamine caine, 1-3.5 parts of 0.1 M sodium hydroxide, 2-35 parts of propellant, 1-17.6 parts of polyvinyl alcohol and 0.1-1.75 parts of rutin derivative. Under the condition of not containing penetrant and surfactant components, lidocaine and propylamine caine in a basic group form are released to the subcutaneous layer, and polyvinyl alcohol is used for forming a film on thesurface of the skin, so that the dosage of lidocaine and propylamine caine is reduced, and the safety of clinical use is enhanced; the treatment of male premature ejaculation is mainly used for inhibiting the expansion of the minor artery and capillary anterior sphincter of the cavernous body of the penis, so that the smooth muscle is relaxed, and the blood flow volume is increased; the rutin derivative is added, the elasticity and permeability of the drug are improved by improving the capillary function, and adverse reactions such as vascular bleeding caused by long-term use of traditional lidocaine drugs are avoided.
Owner:北京中泰邦医药科技有限公司
Microneedle devices and methods
The present invention provides a medical device, which comprises: a microneedle array, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from lidocaine, Prilocaine and combinations thereof; and local anesthetic dose sustained-release components selected from tetracaine, ropivacaine, bupivacaine, procaine and combinations thereof; wherein based on the solids in the coating The total weight of the local anesthetic is present in an amount of at least 1% by weight, and wherein the weight ratio of the local anesthetic and the dose-extending component is a non-eutectic weight ratio; a medical device comprising An array of dissolving microneedles comprising: a dissolvable matrix material; at least 1% by weight of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and combinations thereof; and a local anesthetic dose sustained release component , which is selected from tetracaine, ropivacaine, bupivacaine, procaine, and combinations thereof; wherein the weight ratio of the local anesthetic and the dose-sustaining component is a non-eutectic weight ratio, and wherein the weight % based on the total weight of solids in all portions of the dissolvable microneedles containing the local anesthetic; a method of using the device to provide sustained release of a locally delivered dose of local anesthetic in mammalian tissue; and a method of manufacturing method of the device.
Owner:3M INNOVATIVE PROPERTIES CO
Antibacterial itching relieving preparation and preparation method thereof
InactiveCN104906173AGood treatment effectOrganic active ingredientsAntisepticsPolyvinyl chlorideTherapeutic effect
The invention provides an antibacterial itching relieving preparation. The preparation comprises cysteine coordination bonded metal phthalocyanine, a ketoconazole ointment, mometasone furoate, prilocaine, lidocaine, vitamin B2, white vaseline, moroxydine, furazolidone and soybean oil. The invention also provides a preparation method of the antibacterial itching relieving preparation. The compound preparation provided by the invention has various functions of virus resistance, sterilization, bacteriostasis and itching relieving and has obvious treatment effects on skin diseases caused by various reasons, such as dermatitis, eczema, folliculitis, acnes and beriberi. Meanwhile, the preparation has obvious effects on pruritus and inflammations caused when emulsions, nitrile products, PVC (polyvinyl chloride) products, and the like are used in the medical treatment, physicochemical and industrial fields and other fields.
Owner:ZHEJIANG SCI-TECH UNIV
Microneedle devices and methods
The present invention provides a medical device, which comprises: a microneedle array, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from lidocaine, Prilocaine and combinations thereof; and a local anesthetic dose sustained release component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists and combinations thereof; wherein based on the coating The total weight of solids, the local anesthetic is present in an amount of at least 1% by weight, and wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001; a medical device comprising An array of dissolving microneedles comprising: a dissolvable matrix material; at least 1% by weight of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and combinations thereof; and a local anesthetic dose sustained release component , the local anesthetic dose-sustaining release component is selected from α1 adrenergic agonist, α2 adrenergic agonist and combinations thereof; wherein the weight ratio of the dose-sustaining component / local anesthetic is at least 0.0001, and wherein the weight % based on the total weight of solids in all portions of the dissolvable microneedles containing the local anesthetic; a method of using the device to provide sustained release of a locally delivered dose of local anesthetic in mammalian tissue; and a method of manufacturing method of the device.
Owner:3M INNOVATIVE PROPERTIES CO
Combined medicine emulsifiable paste for anesthesia, and preparation method thereof
ActiveCN113975261ALarge particle sizeSmall particle sizeHydroxy compound active ingredientsRotary stirring mixersPharmaceutical drugDrug efficiency
The invention discloses a combined medicine emulsifiable paste for anesthesia, and a preparation method thereof. The combined medicine emulsifiable paste comprises, by mass, 30-100 parts of lidocaine, 30-100 parts of prilocaine, 5-10 parts of a soothing agent, 20-35 parts of an emulsifier, 15-30 parts of a thickening agent and 9000-9500 parts of purified water. The above medicinal composition disclosed by the invention has the effects of being small in micro-emulsion particle size, narrow in distribution, good in transdermal effect, quick in anesthesia effect, long in anesthesia maintenance time and capable of preventing cold vibration after anesthesia, and through the synergistic effect of all the components, the drug effect of lidocaine can be more fully exerted, so a prepared anesthetic has a better anesthetic effect, a patient is prevented from being hurt due to insufficient anesthesia, and the sensitization risk caused by lidocaine and prilocaine is reduced.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
A kind of compound lidocaine cream medicinal composition and preparation method thereof
ActiveCN106806338BQuality improvementControl contentOrganic active ingredientsAerosol deliveryCream drugPharmaceutical Aids
The invention provides a compound lidocaine cream pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is prepared from effective doses of lidocaine and prilocaine as main drug raw materials, and polyoxyethylene hydrogenated castor oil, carbomer, sodium hydroxide and purified water as pharmaceutical auxiliary materials. The cream body of the present invention has good moisturization and applicability, low impurity content, good quality and little skin irritation.
Owner:SICHUAN HAISCO PHARMA CO LTD
Anesthetic chewing gum
InactiveUS20190054020A1Readily apparentOrganic active ingredientsPill deliveryAnesthetic AgentAdditive ingredient
The anesthetic chewing gum includes lidocaine HCL and prilocaine HCL as the anesthetic ingredients, as well as a chewing gum base. The anesthetic chewing gum may also include one or more sweeters, an anti-adherent, a lubricant, an opacifier, a glidant, a flavoring agent, and a flavor enhancer.
Owner:AL MELH MANAL MANSOUR ABU
A kind of compound lidocaine aerosol and preparation method thereof
ActiveCN112274498BLess irritatingComposition is stableOrganic active ingredientsAntipyreticPolyvinyl alcoholActive agent
The invention discloses a compound lidocaine aerosol and a preparation method thereof. 2 to 35 parts of propellant, 1 to 17.6 parts of polyvinyl alcohol and 0.1 to 1.75 parts of rutin derivatives; the present invention releases base forms of lidocaine and Prilocaine reaches the subcutaneous layer, and because polyvinyl alcohol is used to form a thin film on the skin surface, the dosage of lidocaine and prilocaine is reduced, and the safety of clinical use is enhanced; and because the treatment of male premature ejaculation is mainly to inhibit the penis The arterioles and capillary anterior sphincter dilate to relax the smooth muscle, thereby increasing the blood flow; the present invention adds rutin derivatives, improves capillary function, increases its elasticity and permeability, and avoids the traditional lidocaine Adverse reactions such as vascular bleeding caused by long-term use of drugs.
Owner:北京中泰邦医药科技有限公司
Transdermal delivery kits
Provided are kits containing a base composition and an active pharmaceutical ingredient for transdermal delivery of active pharmaceutical ingredients. Examples of active pharmaceutical ingredients include amitriptyline, baclofen, cyclobenzaprine HCl, ibuprofen, lidocaine HCl, naproxen, and tramadol, ketoprofen, diclofenac, phenylbutazone, mefenamic acid, flubiprofen, piroxicam, guaifenasin, prilocaine, bupivicaine, tetracaine, nifedipine, verapamil, orphenadrine, imipramine, ketamine, gabapentin, carbamazepine, menthol, capsaicin, clonidine, dexamethasone, dextromethorphan, testosterone, progesterone, and estrogens.
Owner:ASCLEMED USA INC DBA ENOVACHEM MFG
Skin anesthesia cream for western surgery and preparation method thereof
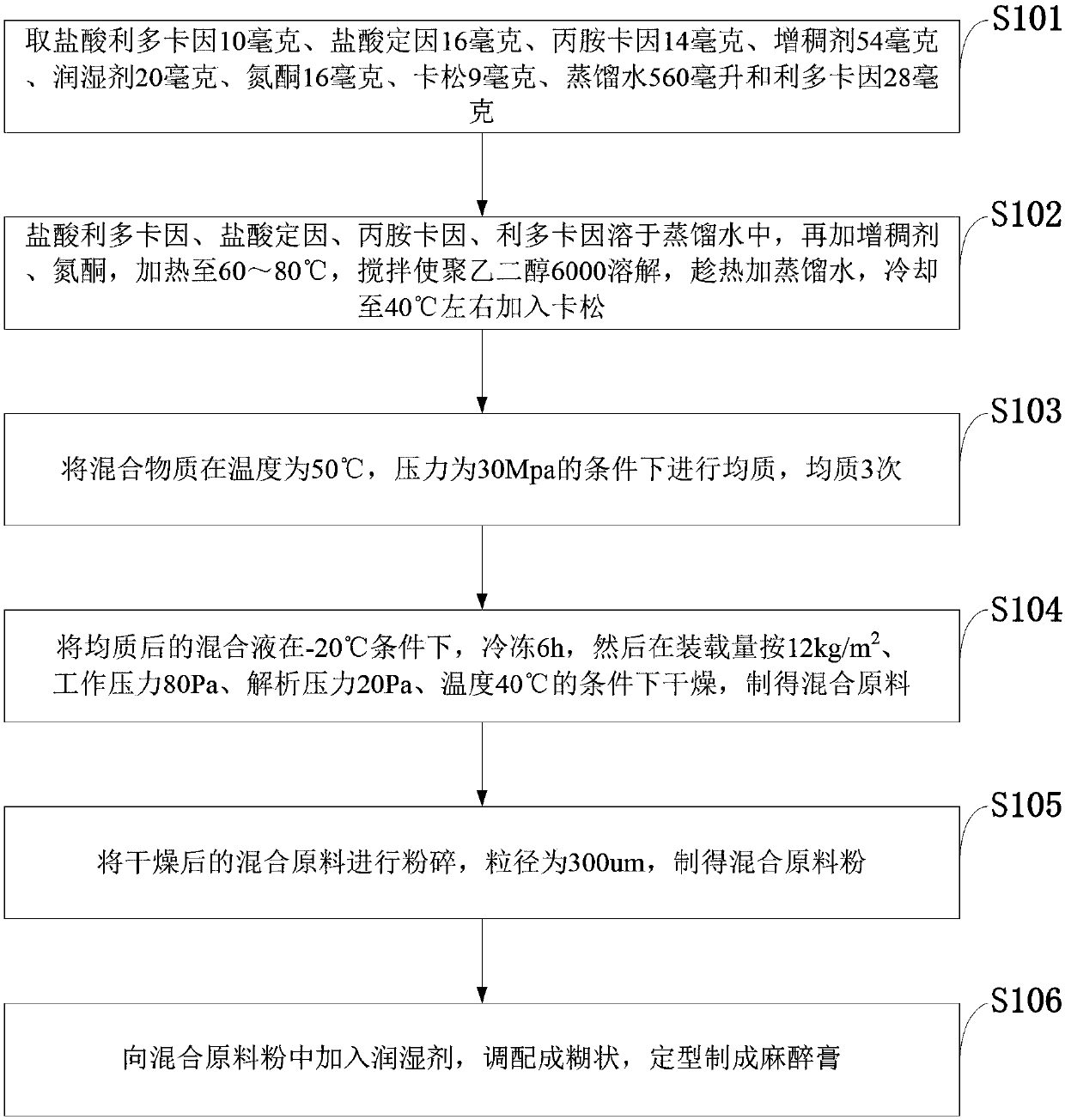
InactiveCN108272746AQuick effectImprove permeabilityOrganic active ingredientsAerosol deliveryIrritationPolyethylene glycol
The invention belongs to the technical field of biological pharmacy, and discloses a skin anesthesia cream for western surgery and a preparation method thereof. The skin anesthesia cream is prepared from the following components of 10mg of lidocaine hydrochloride, 16mg of tetracaine hydrochloride, 14mg of prilocaine, 54mg of thickener, 20mg of wetting agent, 16mg of azone, 9mg of cason, 560ml of distilled water, and 28mg of lidocaine, wherein the thickener is a carboxymethylcellulose and polyethylene glycol 6000 mixture; the wetting agent is a propanediol and glycerin mixture. The skin anesthesia cream for western surgery has the advantages that the permeation function on mucosa and skin is strong, the effect is quickly taken, the irritation or allergy reaction is avoided, the certain aging is maintained, and the cost is low; a preparation method of a novel surface anesthesia cream is provided.
Owner:佳木斯大学附属第二医院
A kind of compound lidocaine pharmaceutical composition and preparation method thereof
ActiveCN105769839BImproves transdermal penetrationImprove stabilityOrganic active ingredientsAerosol deliverySide effectOil phase
Owner:SHANXI YUANYANG PHARMA TECH
Compound lidocaine mask liquid for anesthesia as well as preparation method and application thereof
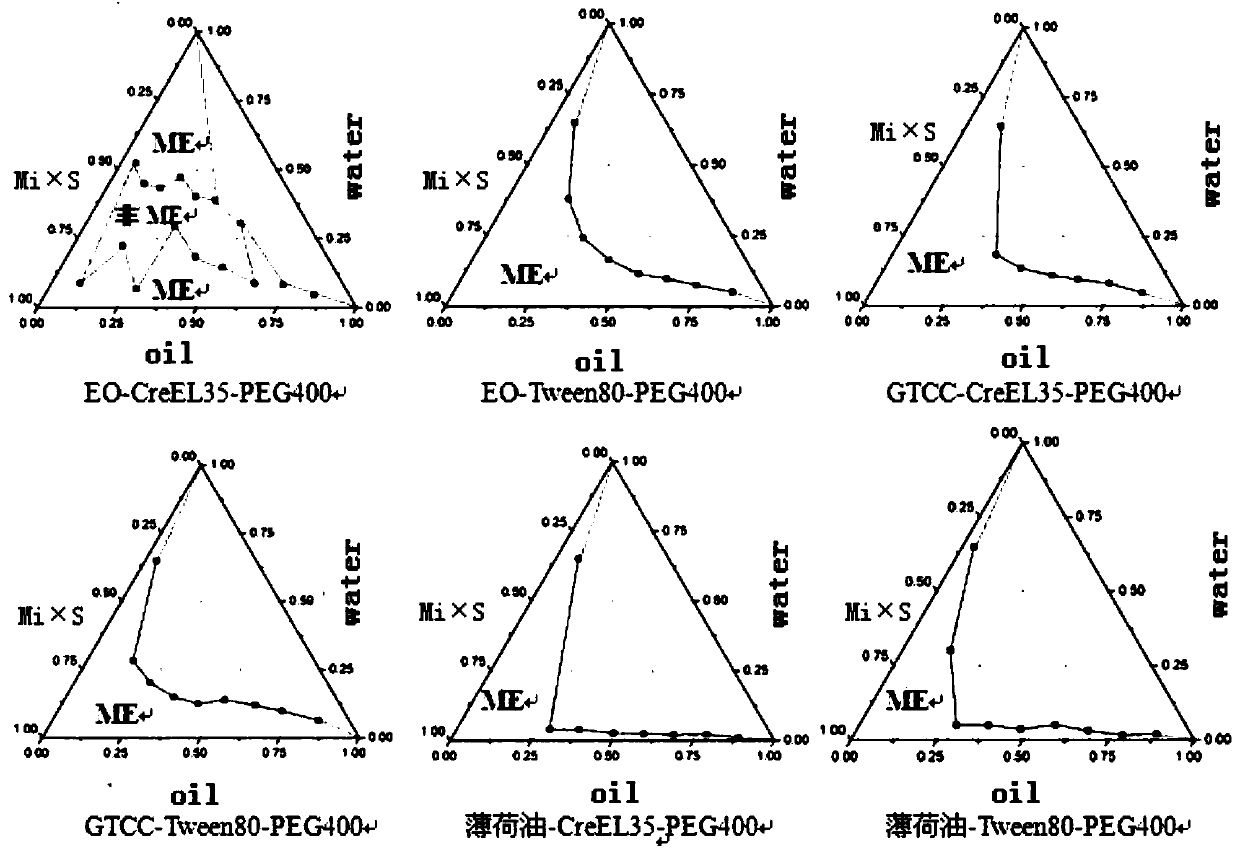
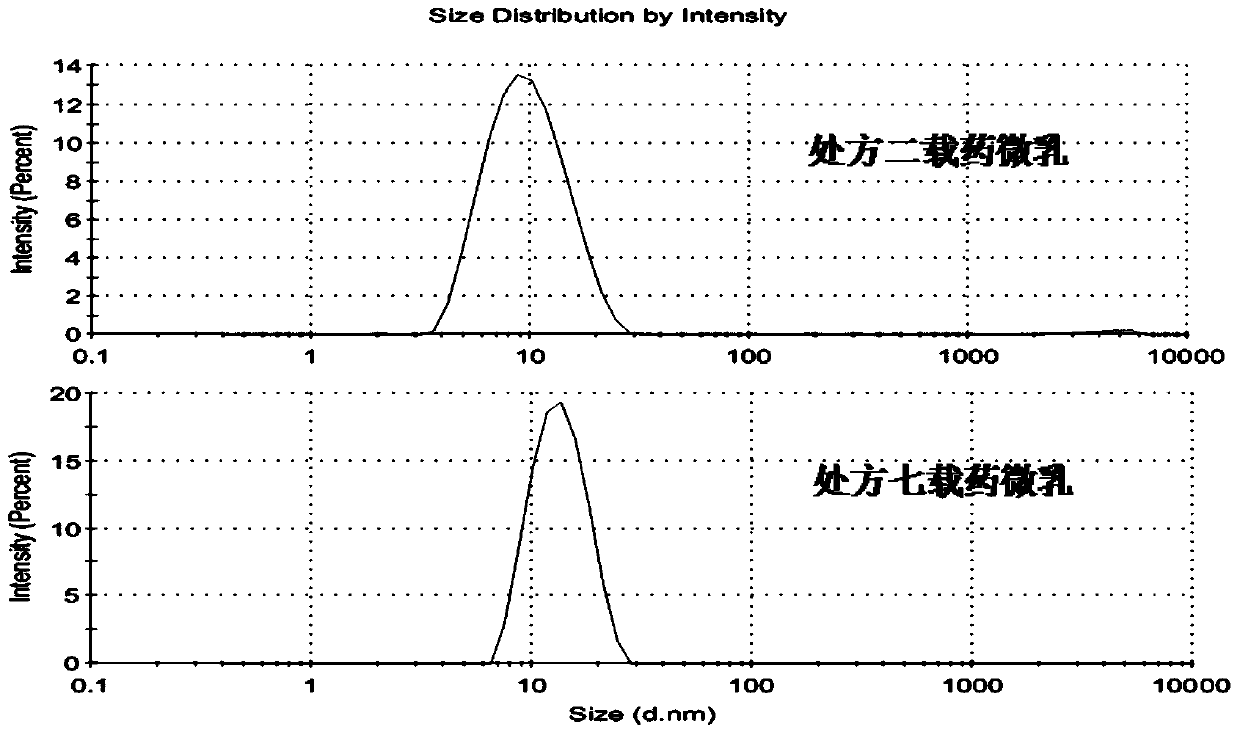
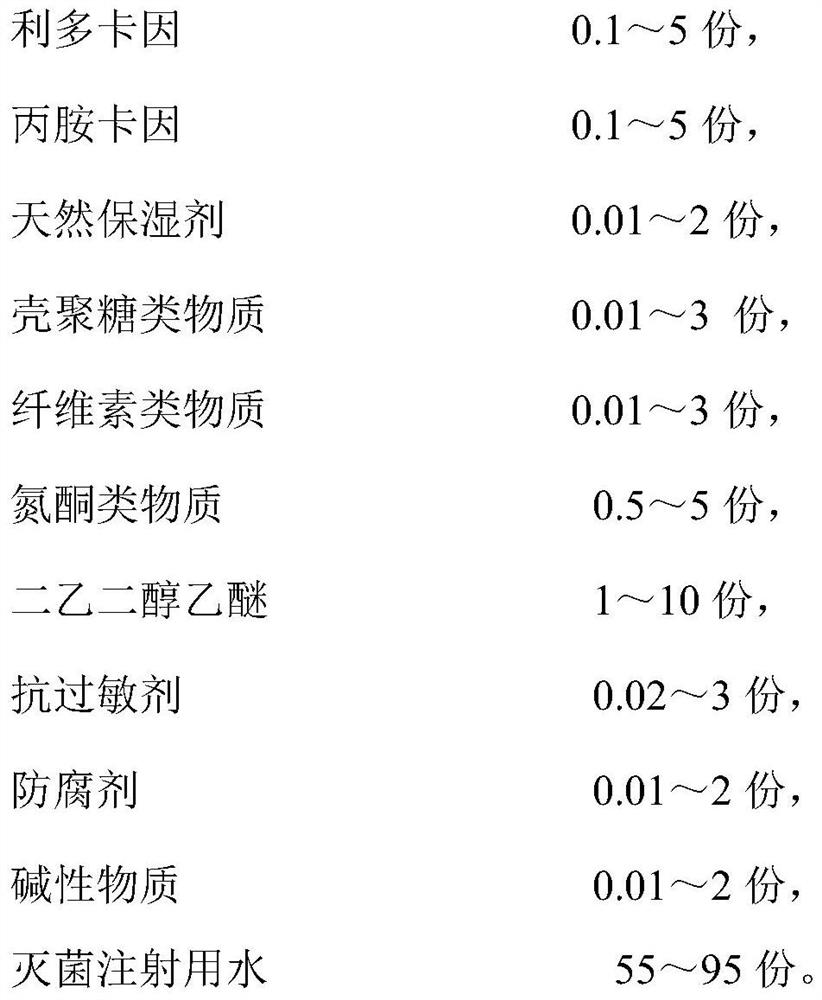
InactiveCN113797344AAvoid stratificationShorten the onset time of anesthesiaOrganic active ingredientsAnaestheticsCelluloseIrritation
The invention discloses a compound lidocaine mask liquid for anesthesia as well as a preparation method and application thereof. The mask liquid is prepared from the following components in parts by weight of 0.1-5 parts of lidocaine, 0.1-5 parts of prilocaine, 0.01-2 parts of a natural humectant, 0.01-3 parts of a chitosan substance, 0.01-3 parts of a cellulose substance, 0.5-5 parts of an azone substance, 1-10 parts of diethylene glycol diethylether, 0.02-3 parts of an antiallergic agent, 0.01-2 parts of a preservative, 0.01-2 parts of an alkaline substance and 55-95 parts of sterilized water for injection. The mask liquid has the characteristics of quick response, high safety, convenience in use, mildness, no irritation and the like; and in addition, the compound lidocaine mask liquid is simple in preparation method and low in cost, and has good economic benefits. The compound lidocaine mask liquid can also be used for making a mask, the compound lidocaine mask liquid is made into the mask, the mask is directly taken out and applied to a specific position during use, and the operation is simple.
Owner:安徽润朗集录生物医药科技有限公司
Pharmaceutical Composition for the Treatment of Solar Urticaria
InactiveUS20110294894A1Induces down-regulation or inhibition of the releaseBiocidePharmaceutical delivery mechanismSolar urticariaMedicine
The present invention refers to a local anesthetic, preferably selected from the group of aminoamide local anesthetics, in particular lidocaine or prilocaine, for use in the treatment or prevention of physical urticaria, in particular solar urticaria. The present invention further refers to a pharmaceutical composition, e.g. a cream, comprising such a local anesthetic, in particular a combination of lidocaine and prilocaine.
Owner:CHARITE UNIVS MEDIZIN BERLIN
Methods and compositions for treating tumors
PendingCN113893264AGrowth inhibitionPharmaceutical delivery mechanismAnaestheticsProcaineCopper chlorophyllin
The present invention relates to methods and compositions for treating tumors. On one hand, the invention relates to an injection which comprises 0.001%-0.2% of aluminum sulfate in terms of anhydrous substances and water for injection. The injection can also contain a local anesthetic, such as the local anesthetic selected from the group consisting of lidocaine, procaine, chloroprocaine, tetracaine, cocaine, dibucaine, prilocaine, mepivacaine, bupivacaine, etidocaine, mesocainum, ropivacaine, pirocaine or dyclonine, preferably lidocaine such as lidocaine hydrochloride. The injection can also comprise the following coloring agents of methylene blue, indigo blue, brilliant blue, patent blue V, gentian violet, copper sulfate, chlorophyll, sodium iron chlorophyllin, copper chlorophyllin, copper chlorophyllin complex salt, sodium copper chlorophyllin and fast green, preferably methylene blue. The invention also relates to application of the injection in preparation of medicines for treating tumors. The injection provided by the invention shows an excellent effect of inhibiting tumor growth.
Owner:GENERAL HOSPITAL OF PLA
Anorectal wet tissue and preparation method thereof
InactiveCN112675107AWith hemostasisAnti-inflammatoryAntibacterial agentsCosmetic preparationsAbsorption (skin)Biology
The invention relates to the technical field of wet tissues, in particular to an anorectal wet tissue and a preparation method thereof. At present, a wet tissue solution of anorectal wet tissues on the market does not contain a tranquilizer with an anesthetic effect, so that the wet tissues cannot effectively relieve pains of hemorrhoid patients. In order to solve the problem, the wet tissue solution of the provided anorectal wet tissue contains benzocaine, lidocaine hydrochloride, ropivacaine, prilocaine, articaine and other effective components with an analgesic effect and also contains a certain amount of a penetration enhancer. Due to the existence of the penetration enhancer, absorption of perianal skin to the tranquilizer and a wetting agent in the wet tissue solution can be effectively promoted, and an anti-microorganism effect of the wet tissue can be greatly improved.
Owner:ROOSIN MEDICAL CO LTD
Ketoprofen and prilocaine nano-emulsified gel preparation and preparation method thereof
ActiveCN114533713AGood heat and cold resistanceConvenient circulation and storageOrganic active ingredientsAntipyreticGel preparationActive agent
The invention discloses a nano emulsified gel preparation of ketoprofen and prilocaine and a preparation method of the nano emulsified gel preparation. The pharmaceutical composition comprises a medicine phase, a water phase matrix and a surfactant, wherein the medicine phase in the system is a compound of ketoprofen and prilocaine in a molten state; and the water-phase matrix is a water-soluble high polymer material. According to the nano-emulsified gel, the heat resistance and cold resistance of a gel system are improved, and the product can be conveniently circulated and stored around the world and used after being uncovered; the light stability of the product is superior to that of Fastone by utilizing the protection effect of the nano delivery system on the medicine, so that the product is convenient to store and use; compared with commercially available fasaston, the nano-emulsified gel has the advantages that the in-vitro release rate is stable, burst release of the medicine is avoided, the total release amount can be increased, and the stable medicine effect can be maintained for a long time; the prescription does not need ethanol, so that skin irritation caused by ethanol is avoided, and the effectiveness of the product is improved. In addition, the safety and operability of the production process can be improved.
Owner:NEOFORM BIOPHARMACEUTICAL LTD
Rapid transdermal delivery system of local anesthetics
ActiveCN101810908BExcellent transdermal penetrationMedical devicesUltrasonic transmissionRopivacaine
The invention belongs to the technical field of transdermal delivery, in particular to a rapid transdermal delivery system of local anesthetics, which directly uses a preparation that is loaded with the local anesthetic, characterized by nano-materials and can control the release for transdermal delivery, or adds the preparation into thermo-sensitive gel poloxamer F127 solution with the concentration of 5%-40%, prepares a gel or a cream by regulating the temperature and directly uses the gel or the cream for transdermal delivery, or realizes the rapid transdermal delivery through pretreatment or an ultrasonic transmission device and / or an iontophoresis device treating the skin simultaneously under the common action of ultrasonic introduction, iontophoresis introduction or the combination of the two methods. The rapid transdermal delivery system can lead the transdermal permeation amount, the anesthetic onset time and the anesthetic potency of the local anesthetics, such as lidocaine, tetracaine, prilocaine, bupivacaine, ropivacaine, dicaine and the like to be better than those of the ordinary surface anesthetic preparation, solve the defects of long onset time of the existing surface anesthetic and limited action effects, eliminate the obvious skin irritation and be used for symptomatic treatment of skin anesthesia and neuropathic pain with skin invasive operation.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Compounded lidocaine periodontal gel and preparation method therefor
InactiveCN112245445ALess irritatingIncrease irritationOrganic active ingredientsAerosol deliveryTherapeutic effectDentistry
The invention discloses compounded lidocaine periodontal gel and a preparation method therefor. The compounded lidocaine periodontal gel comprises the following ingredients in parts by weight: 0.1-4.5parts of lidocaine, 0.1-4.5 parts of prilocaine, 3-10 parts of poloxamer 188, 4-25 parts of poloxamer 407, 0.05-2 parts of vitamin p, 30-120 parts of purified water and 2-7 parts of diluted hydrochloric acid. According to the compounded lidocaine periodontal gel and the preparation method therefor, provided by the invention, the carry-over is convenient, the use is convenient, the treatment effect is good, and the effect taking speed is high. Compared with the traditional injection anesthetization, the gel has the advantages that the effect is remarkable during periodontal pocket anesthetization, the depth of a periodontal pocket and an attached level of gums cannot be affected in periodontal scaling and root face leveling treatment, and the aching feeling is lowered. The gel has a very good popularization value.
Owner:北京中泰邦医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com