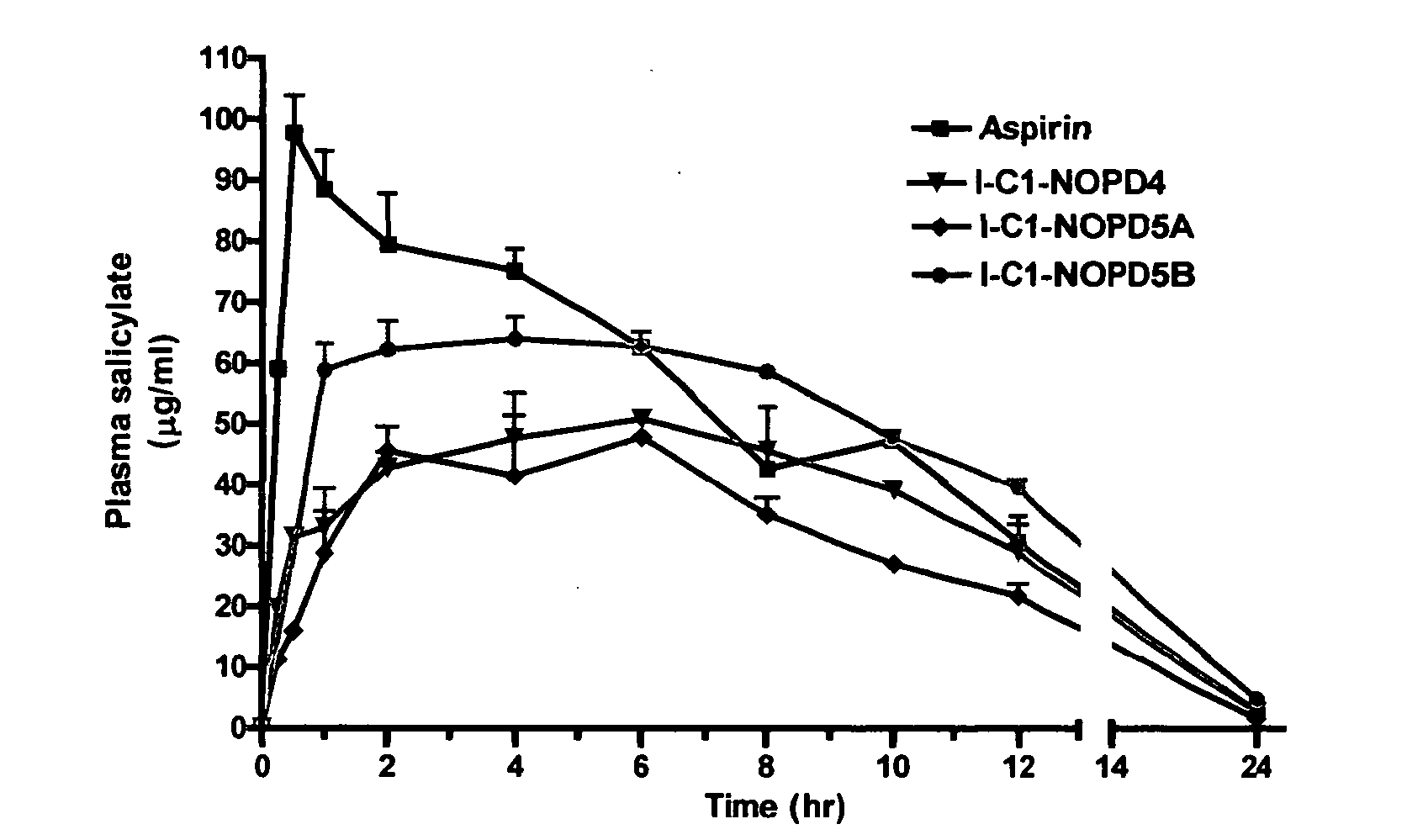
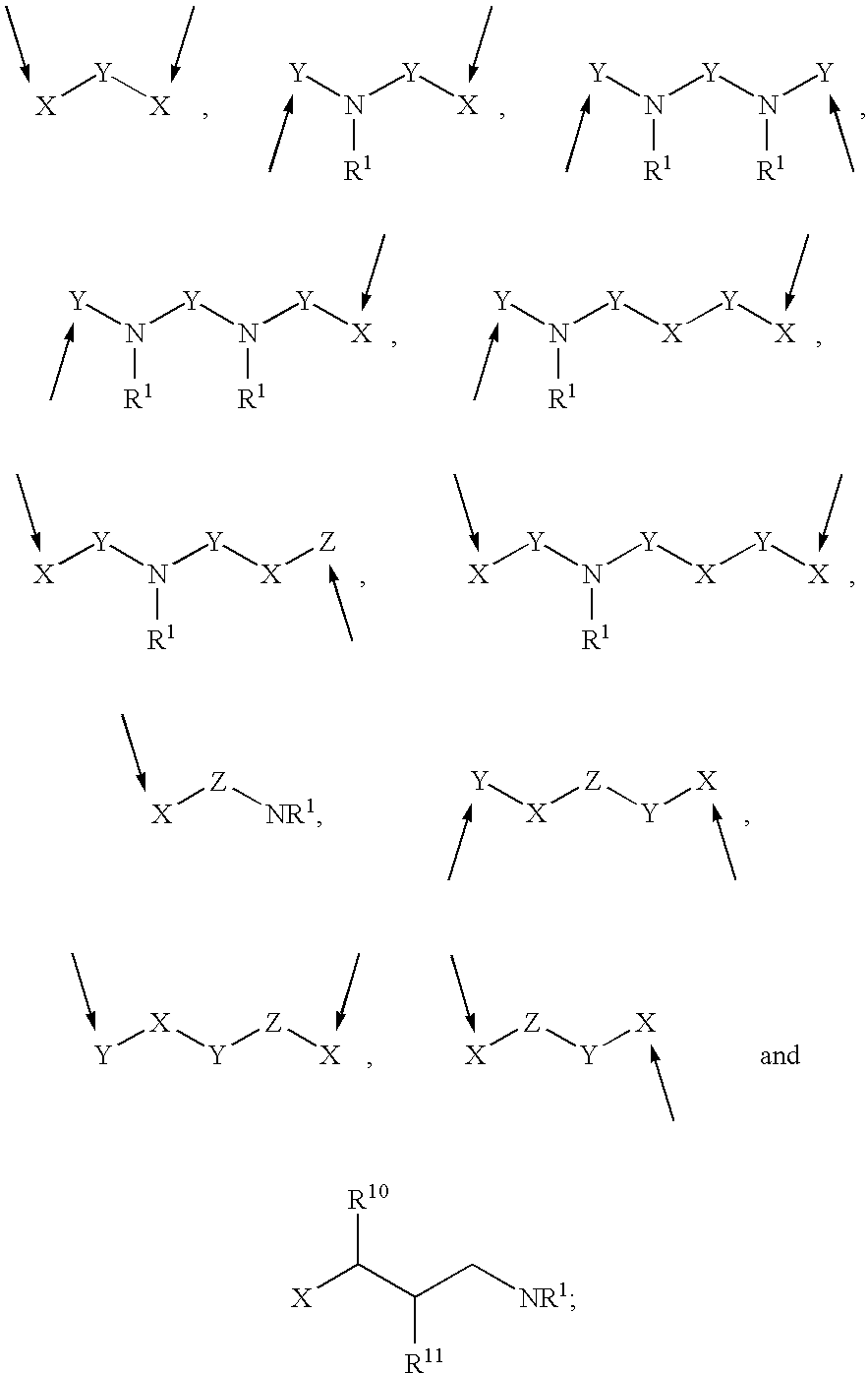
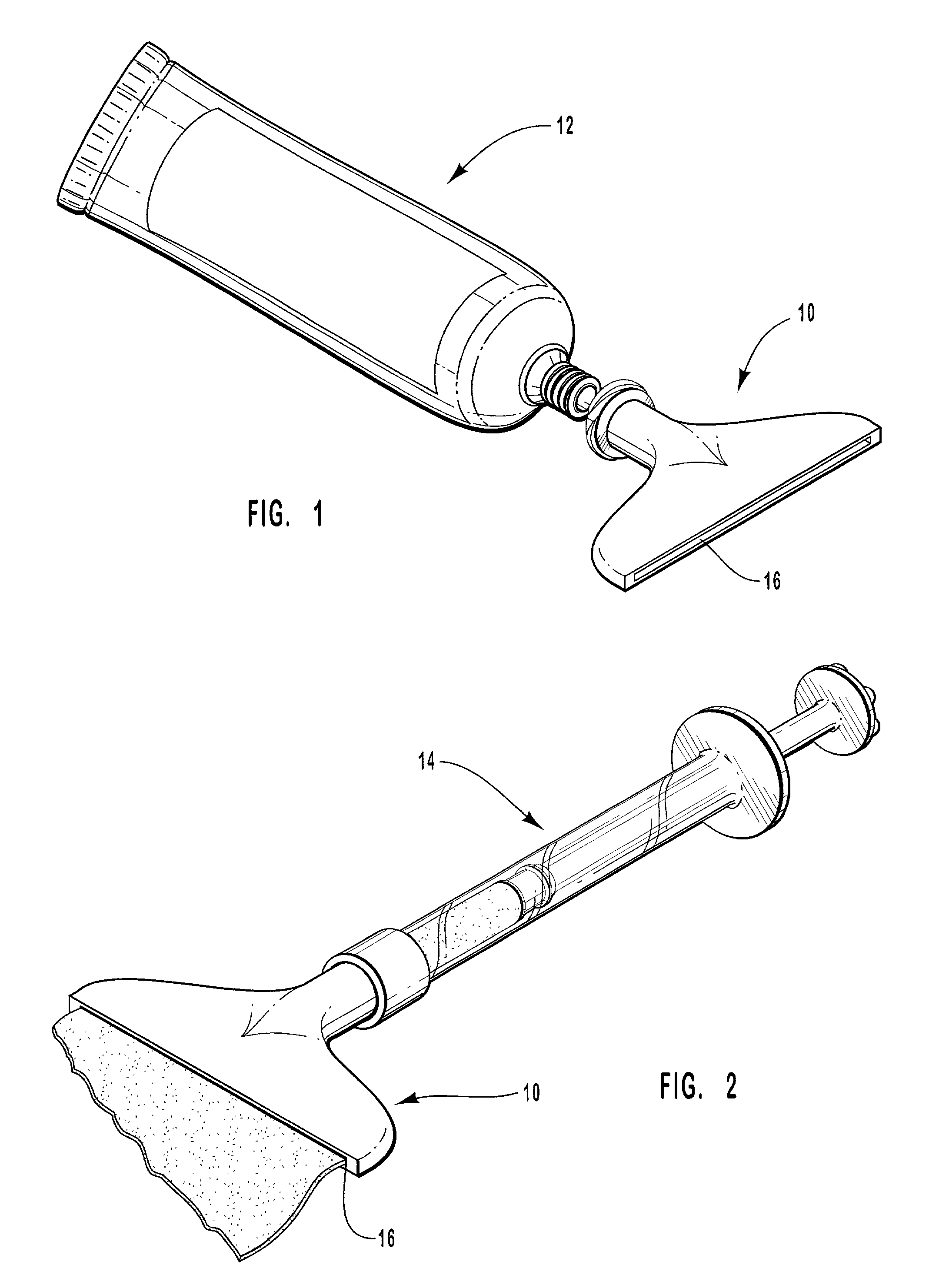
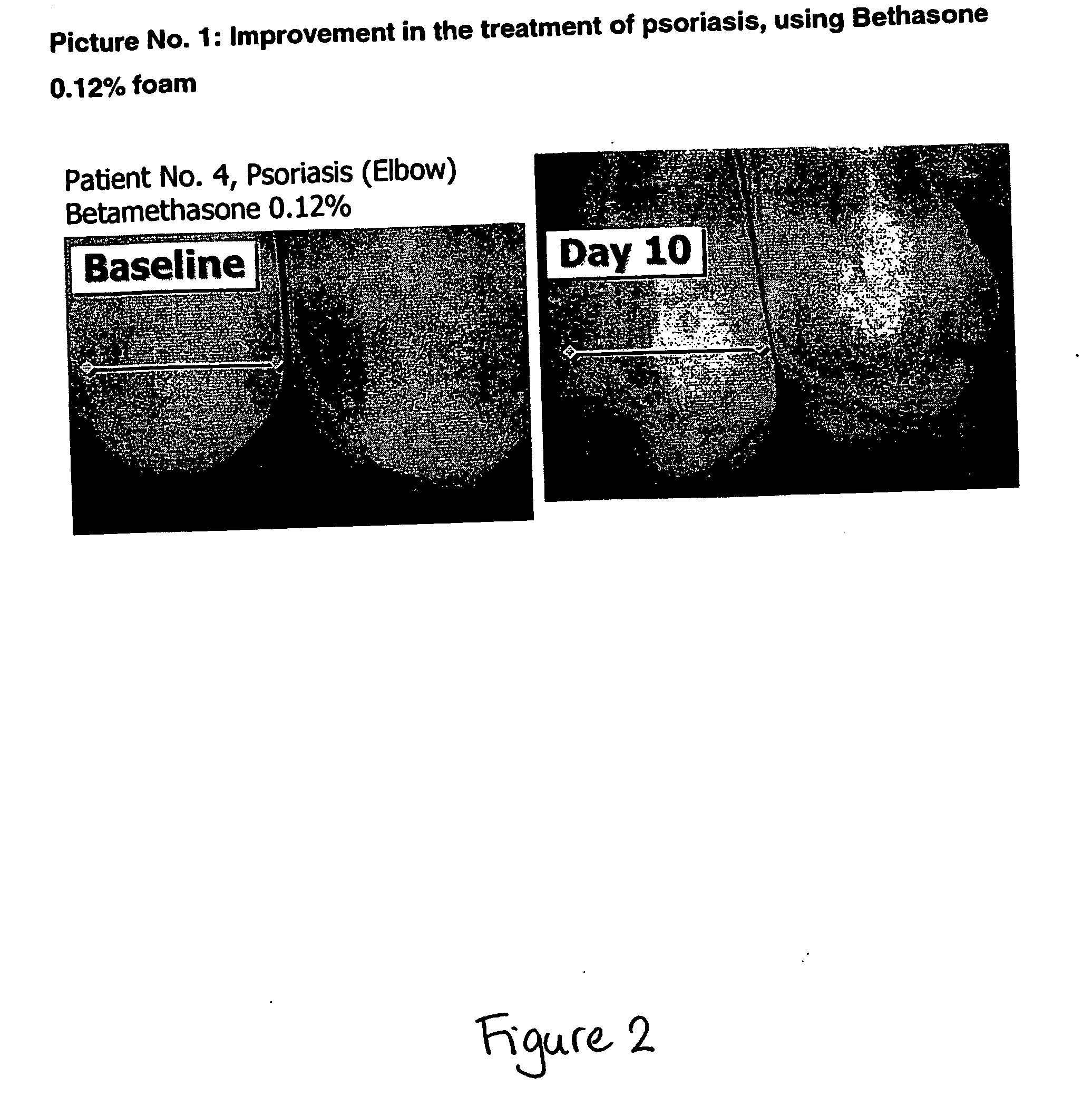
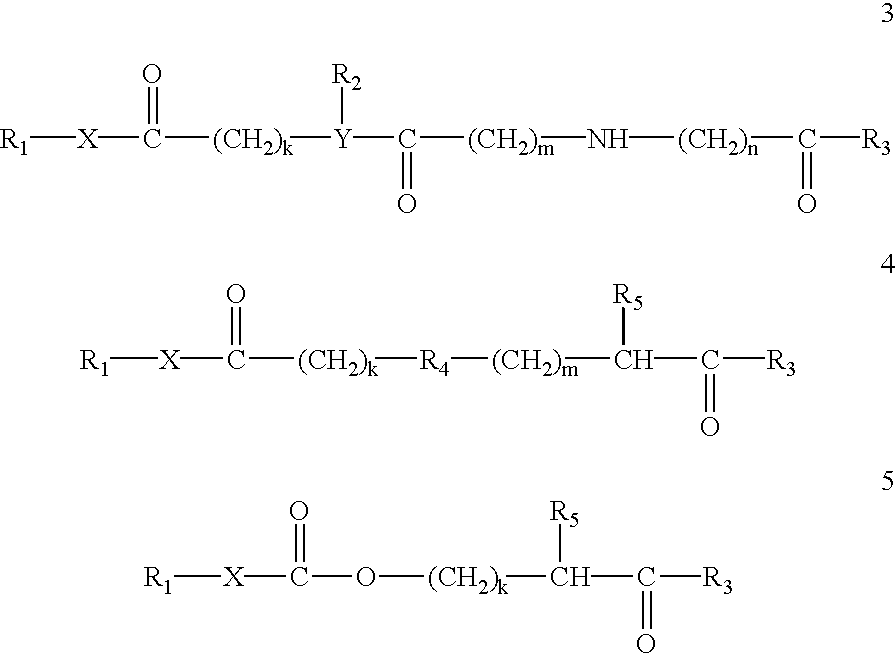
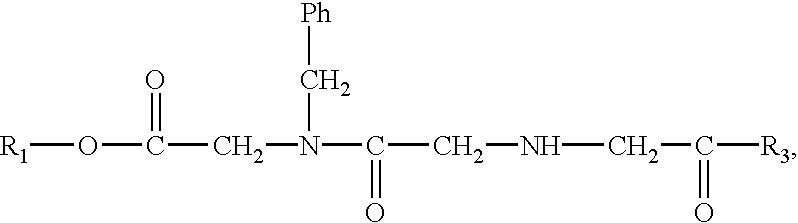
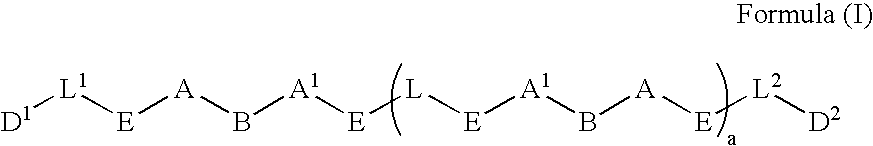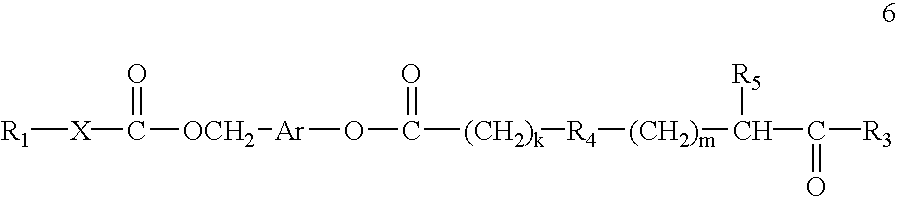Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2976results about "Anaesthetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
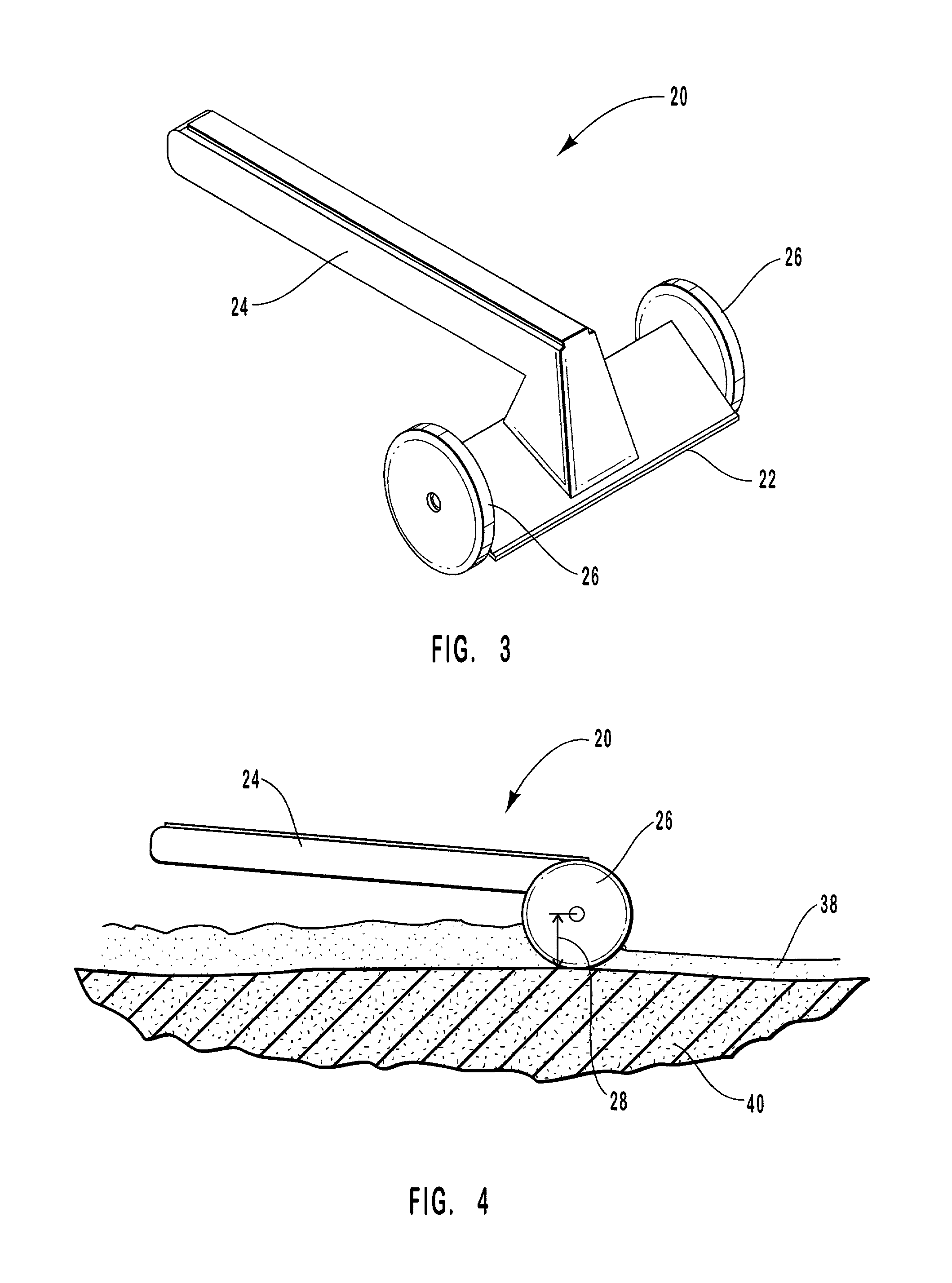
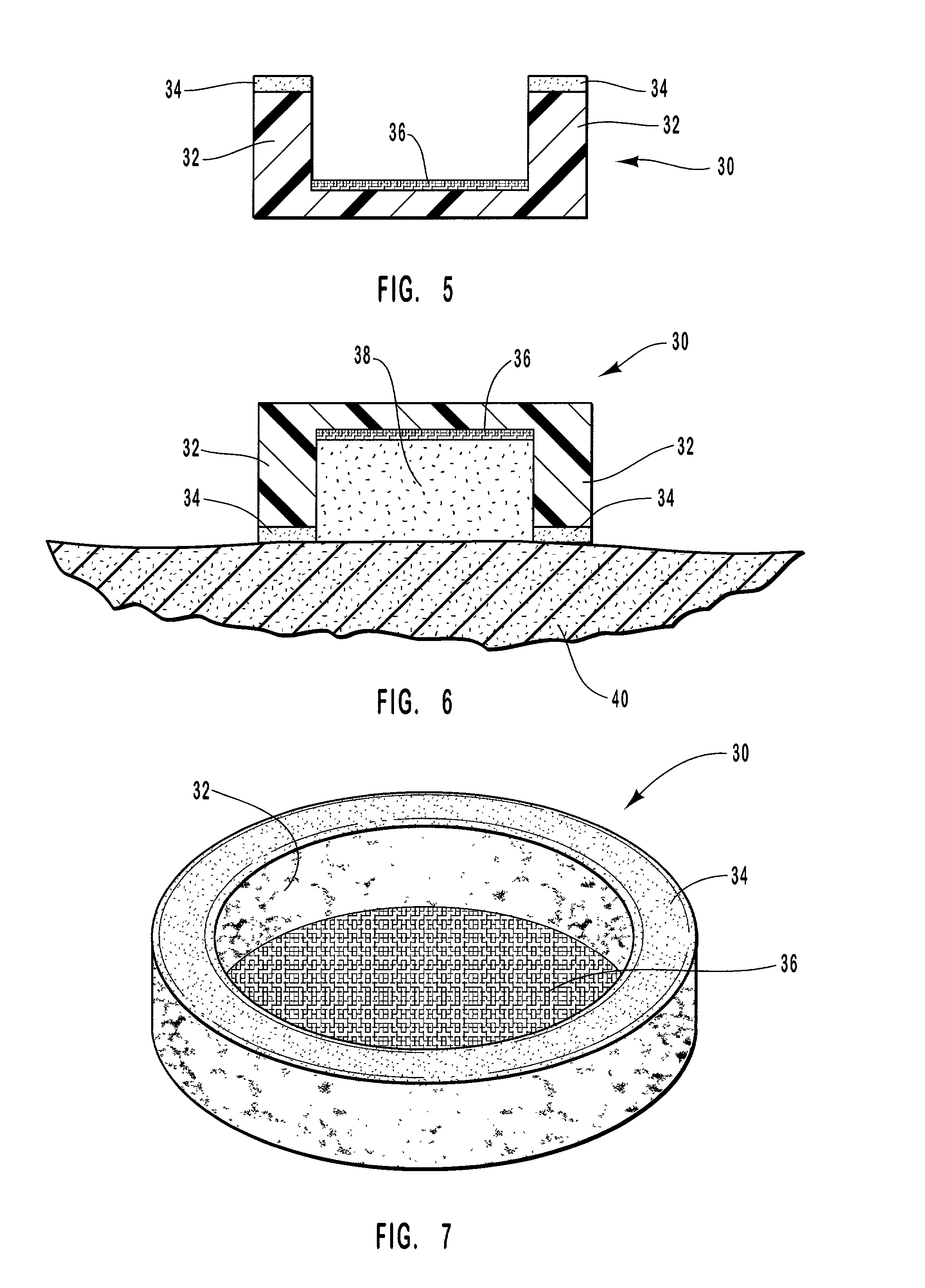
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
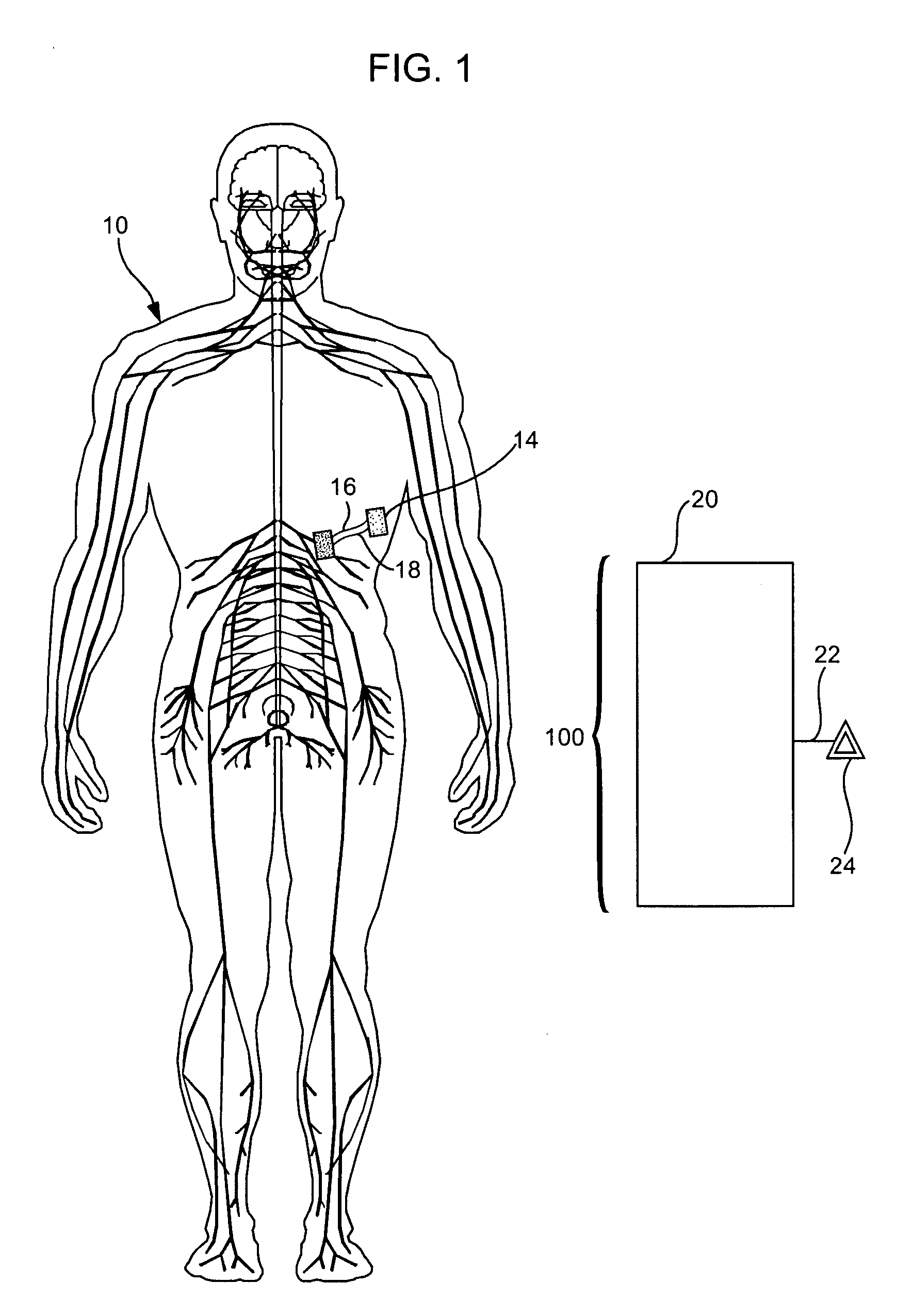
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
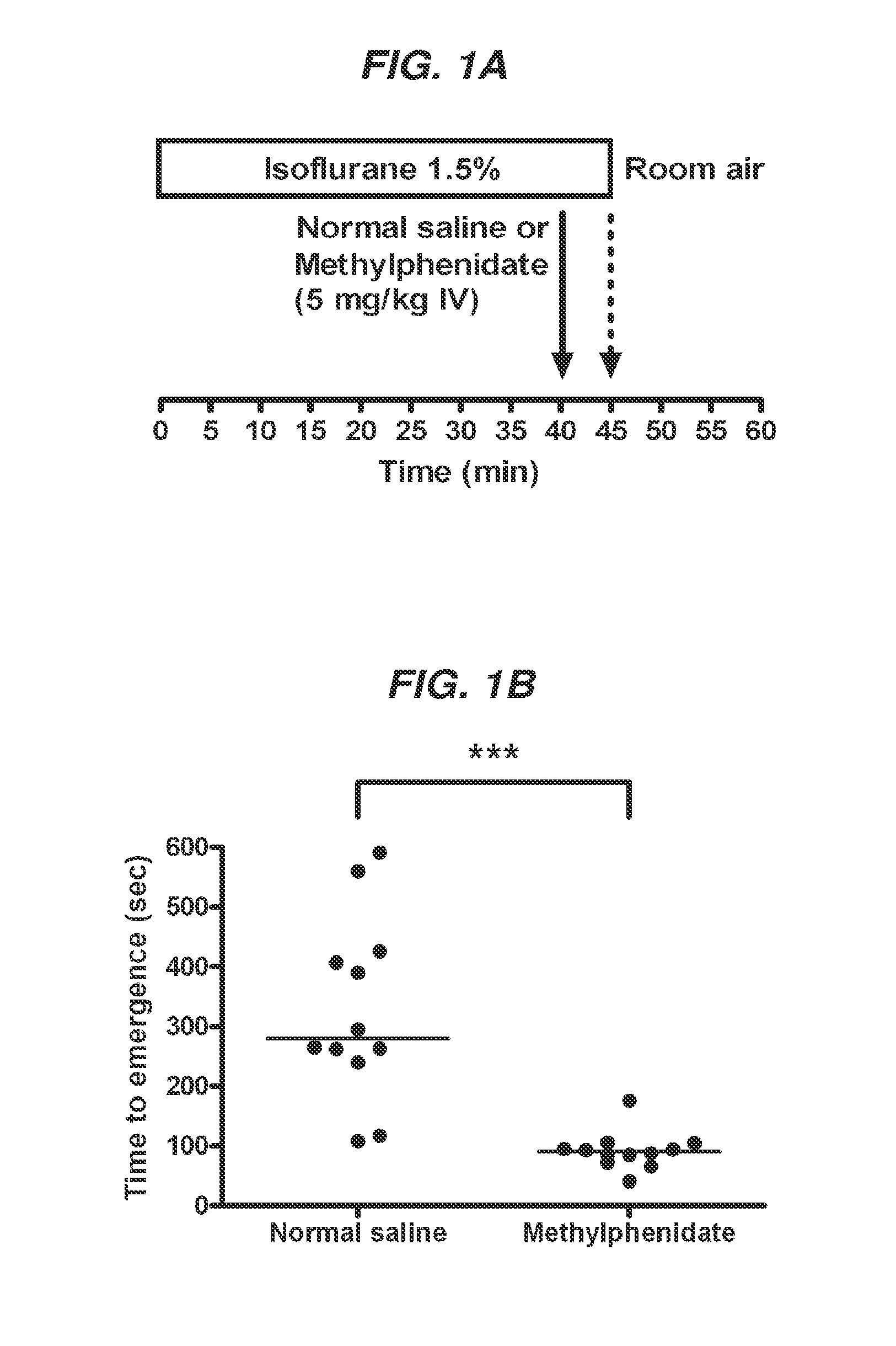
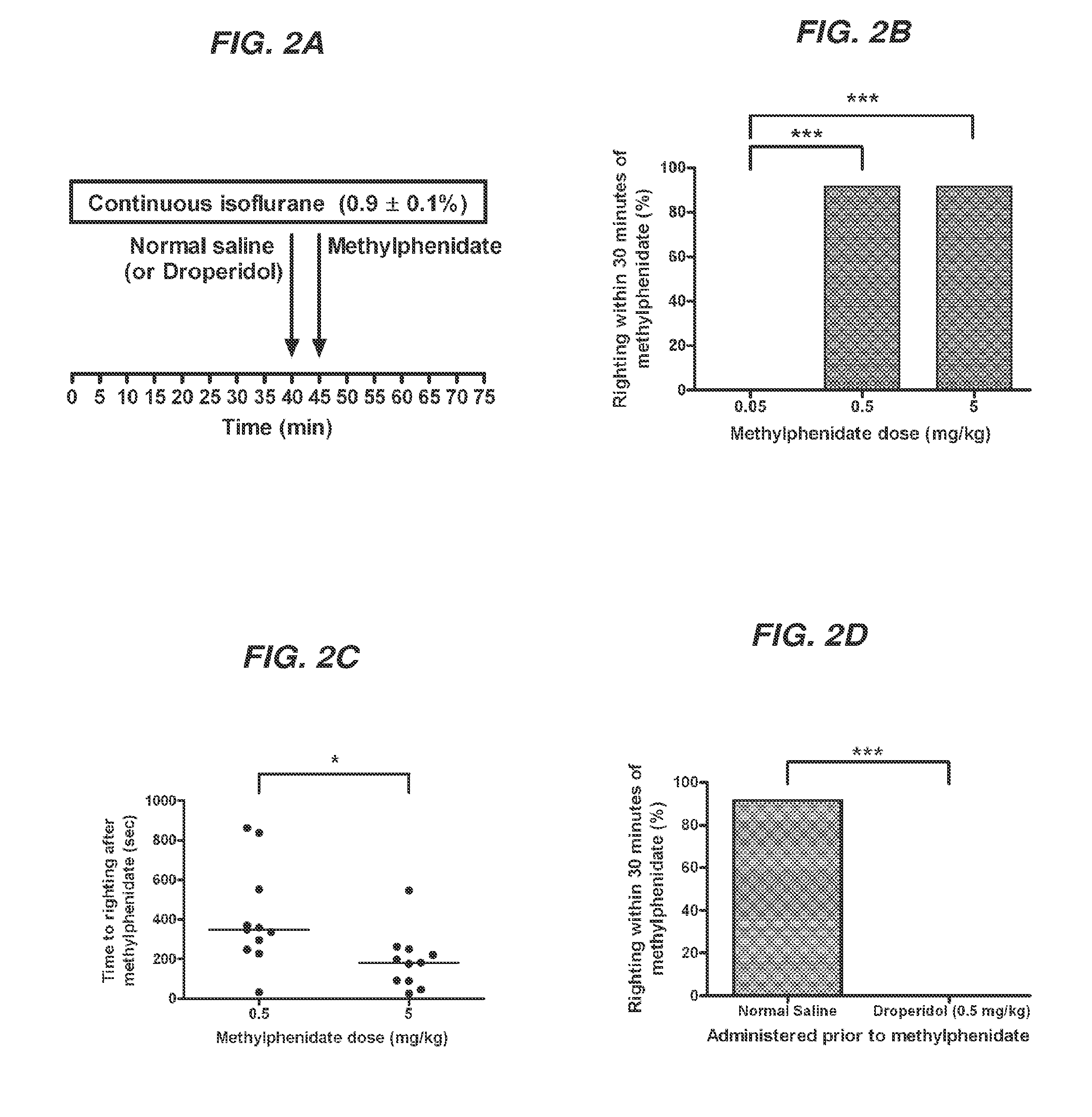
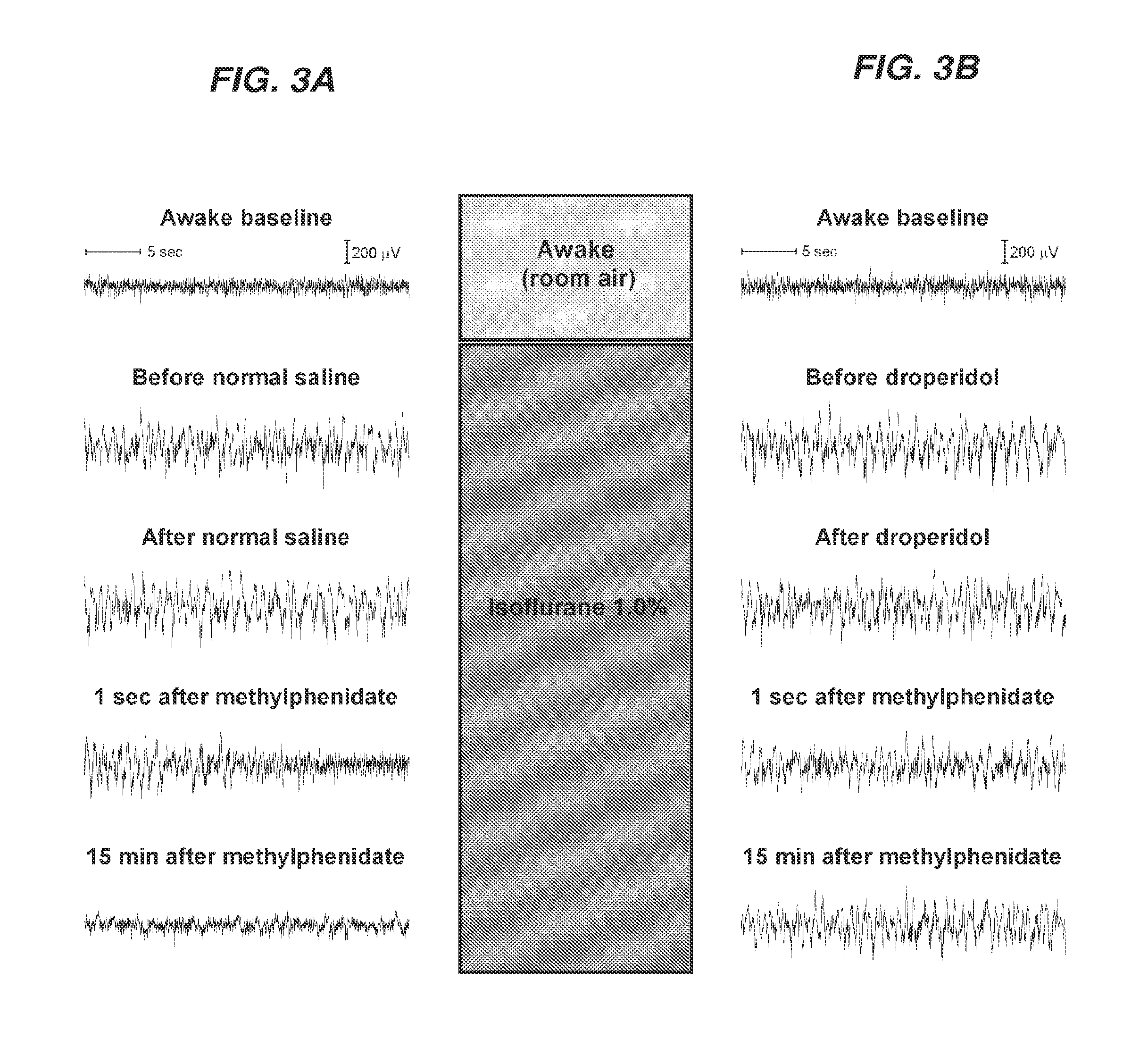
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
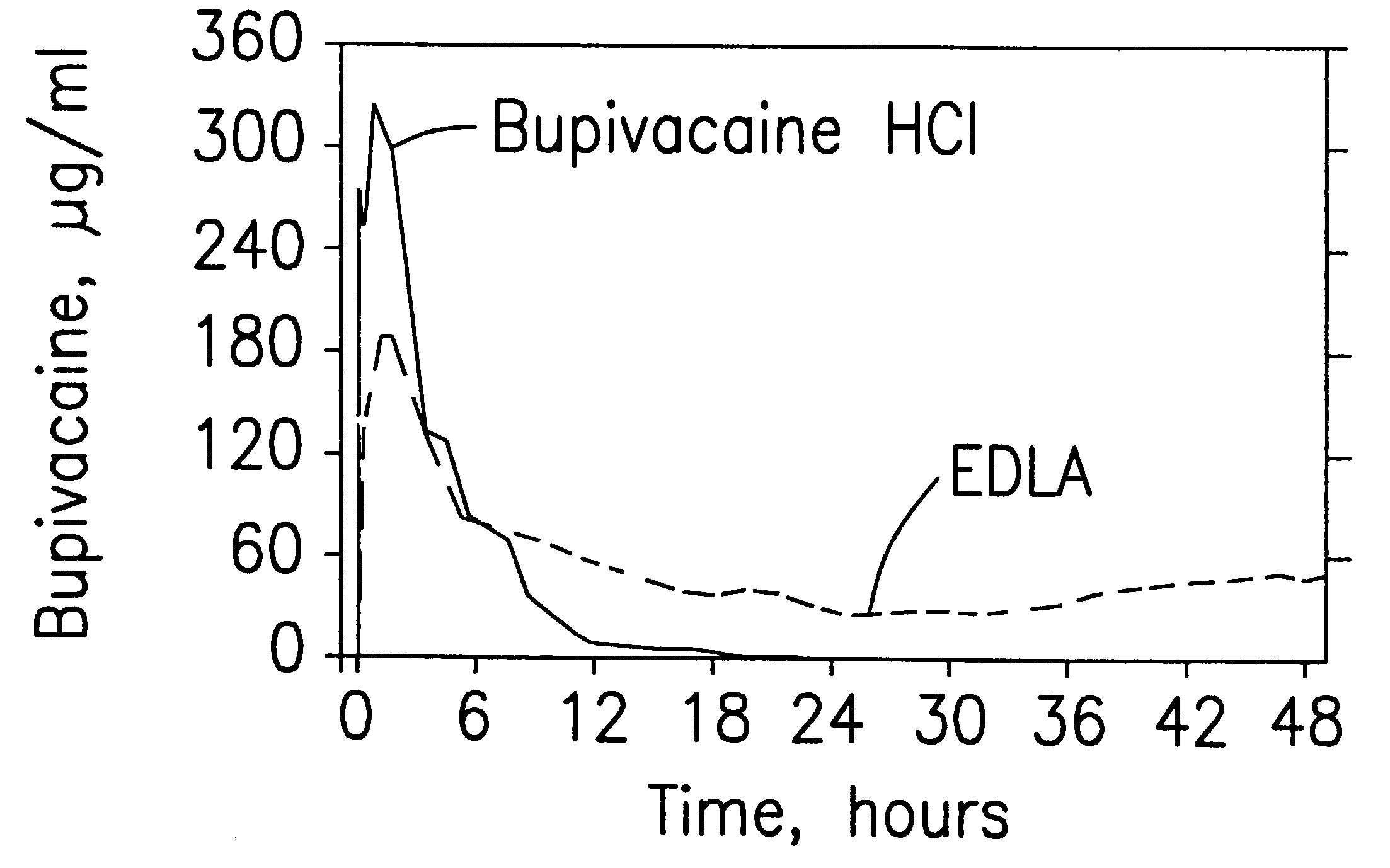
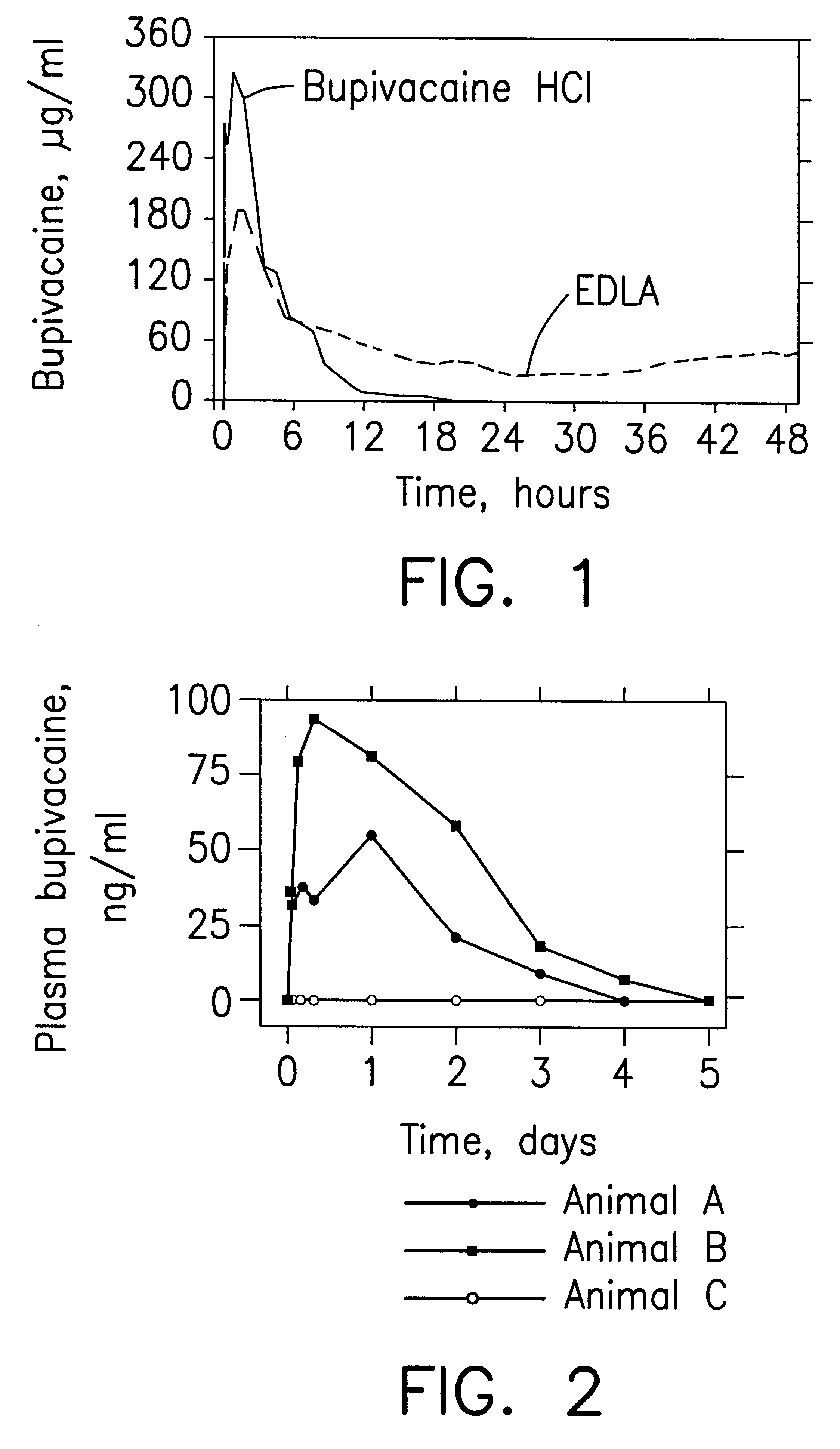
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
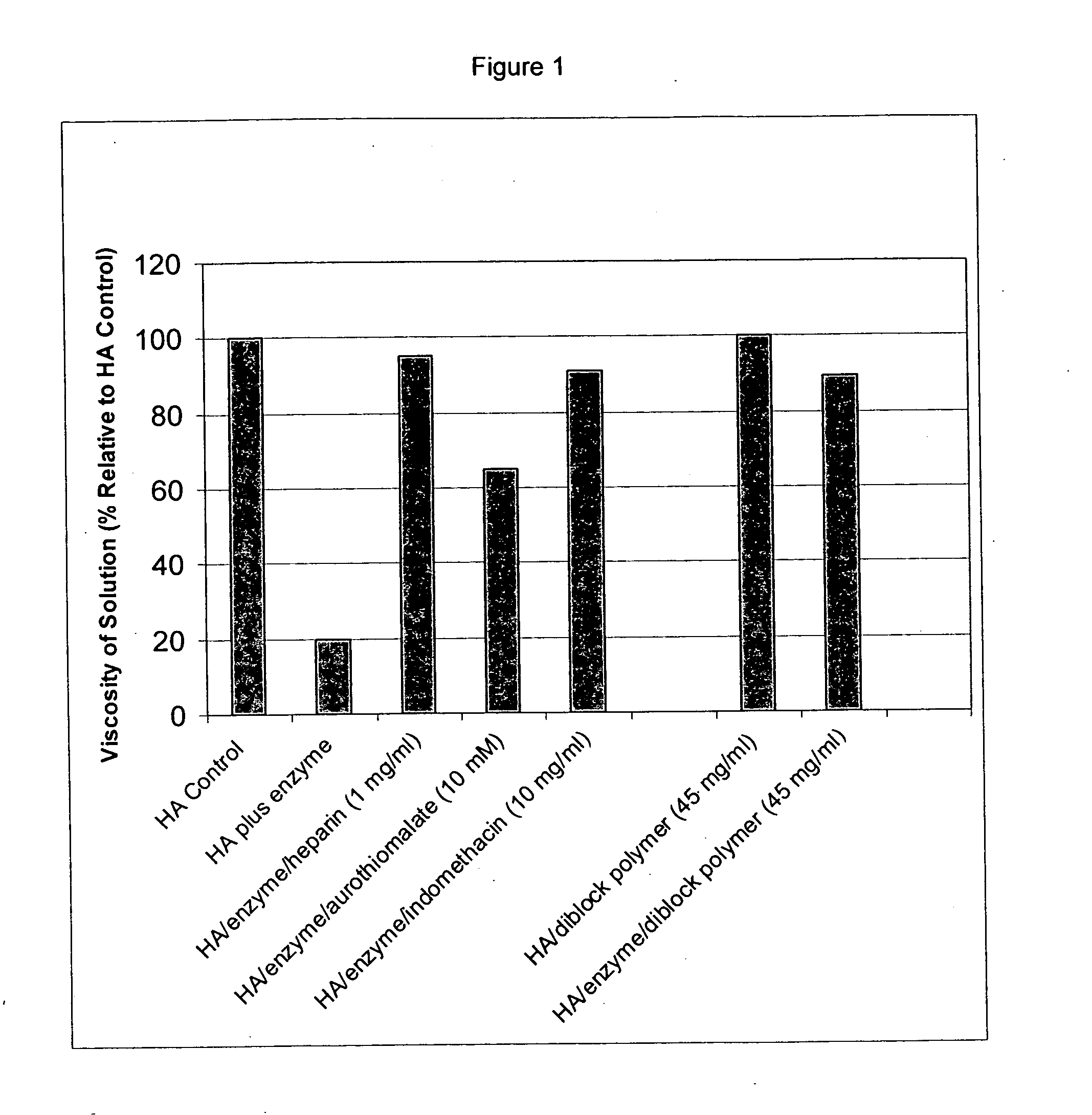
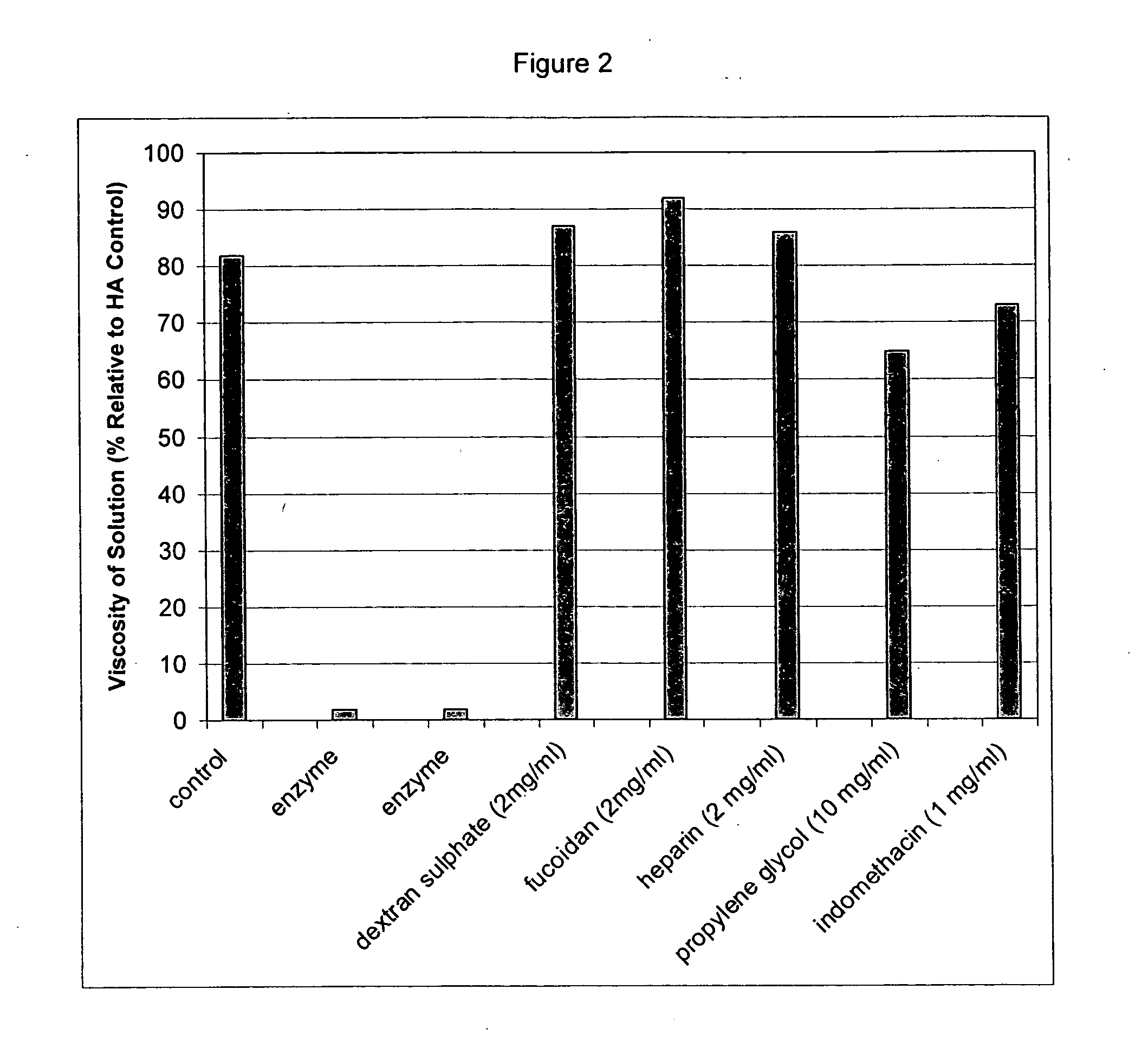
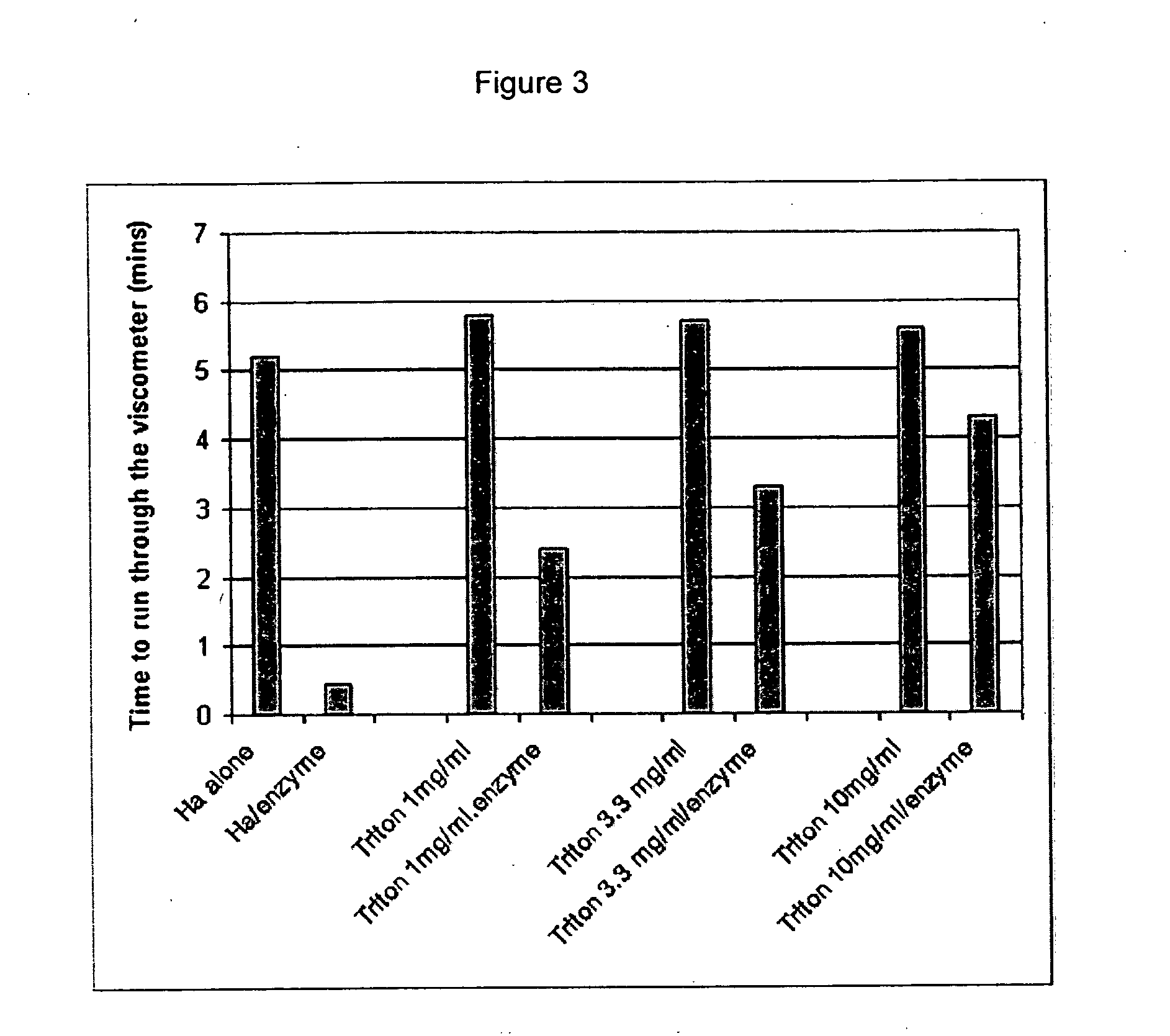
Compositions and methods using hyaluronic acid
InactiveUS20060040894A1Relieve painPreventing surgical adhesionBiocideHeavy metal active ingredientsCompound (substance)Hyaluronic acid
Compositions and devices including hyaluronic acid and a compound that inhibits degradation of hyaluronic acid, and methods of making and using same.
Owner:ANGIOTECH INT AG (CH) +1
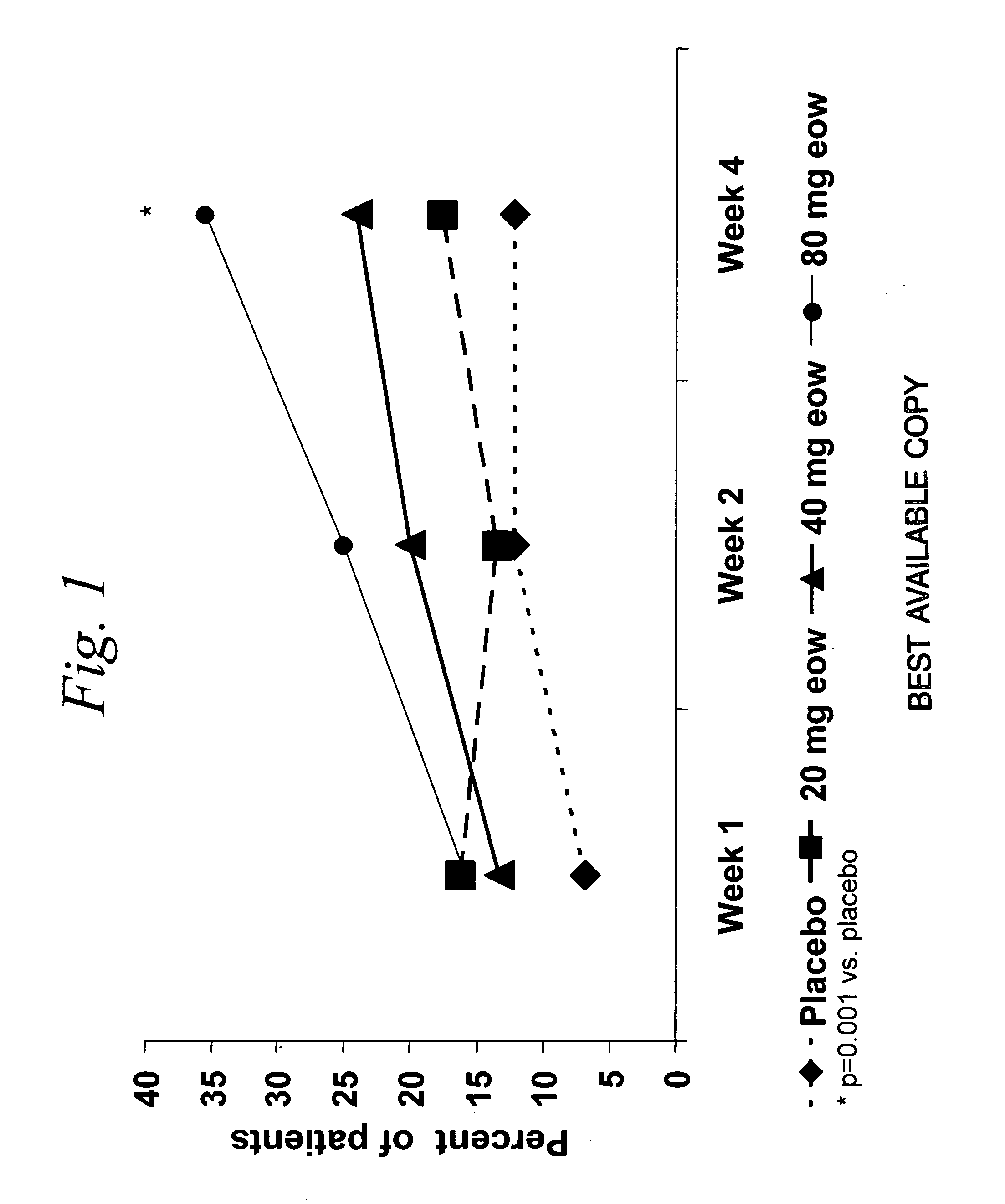
Multiple-variable dose regimen for treating TNFalpha-related disorders
ActiveUS20060009385A1Reduce decreaseIncrease the areaBiocideOrganic active ingredientsDosing regimenRegimen
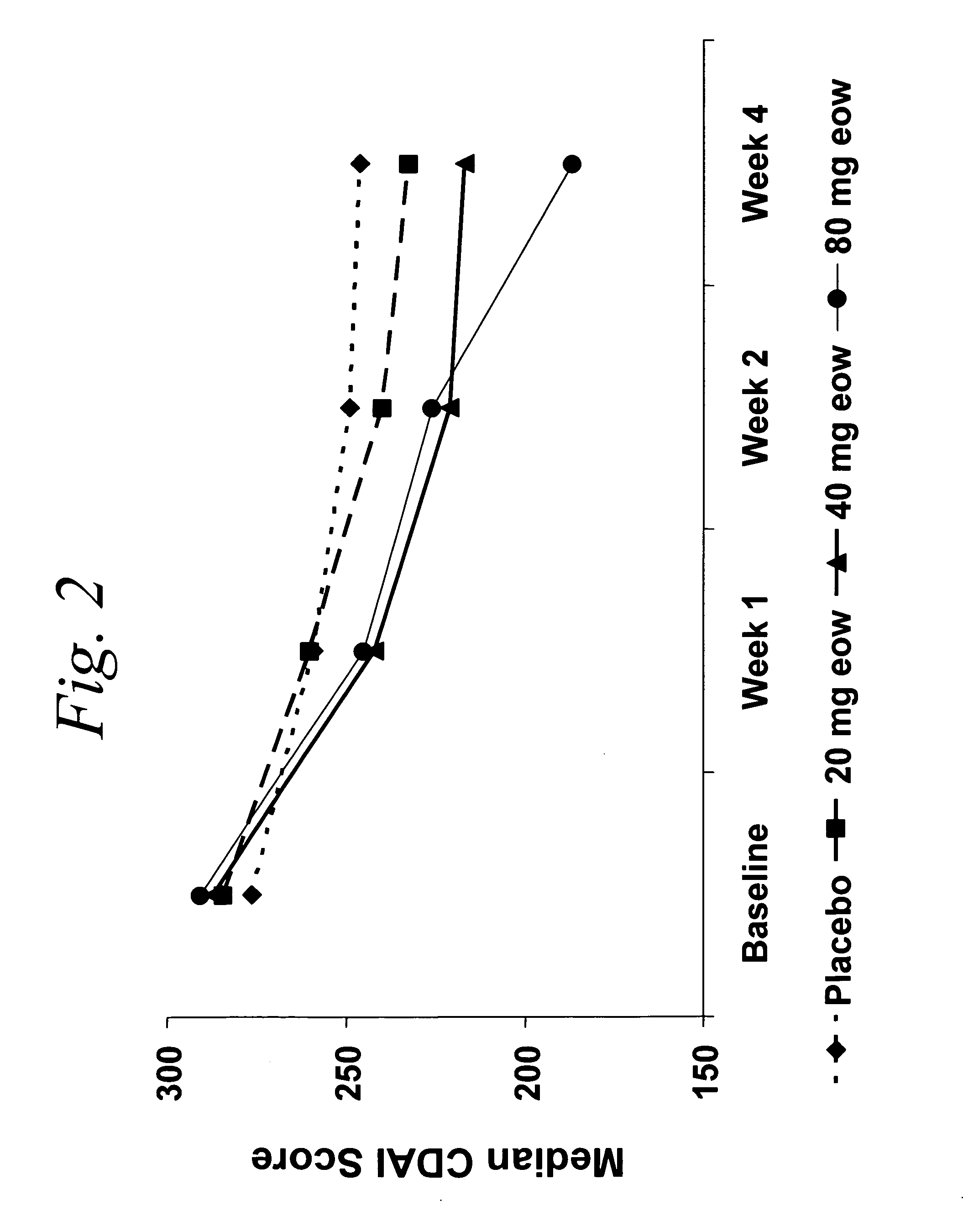
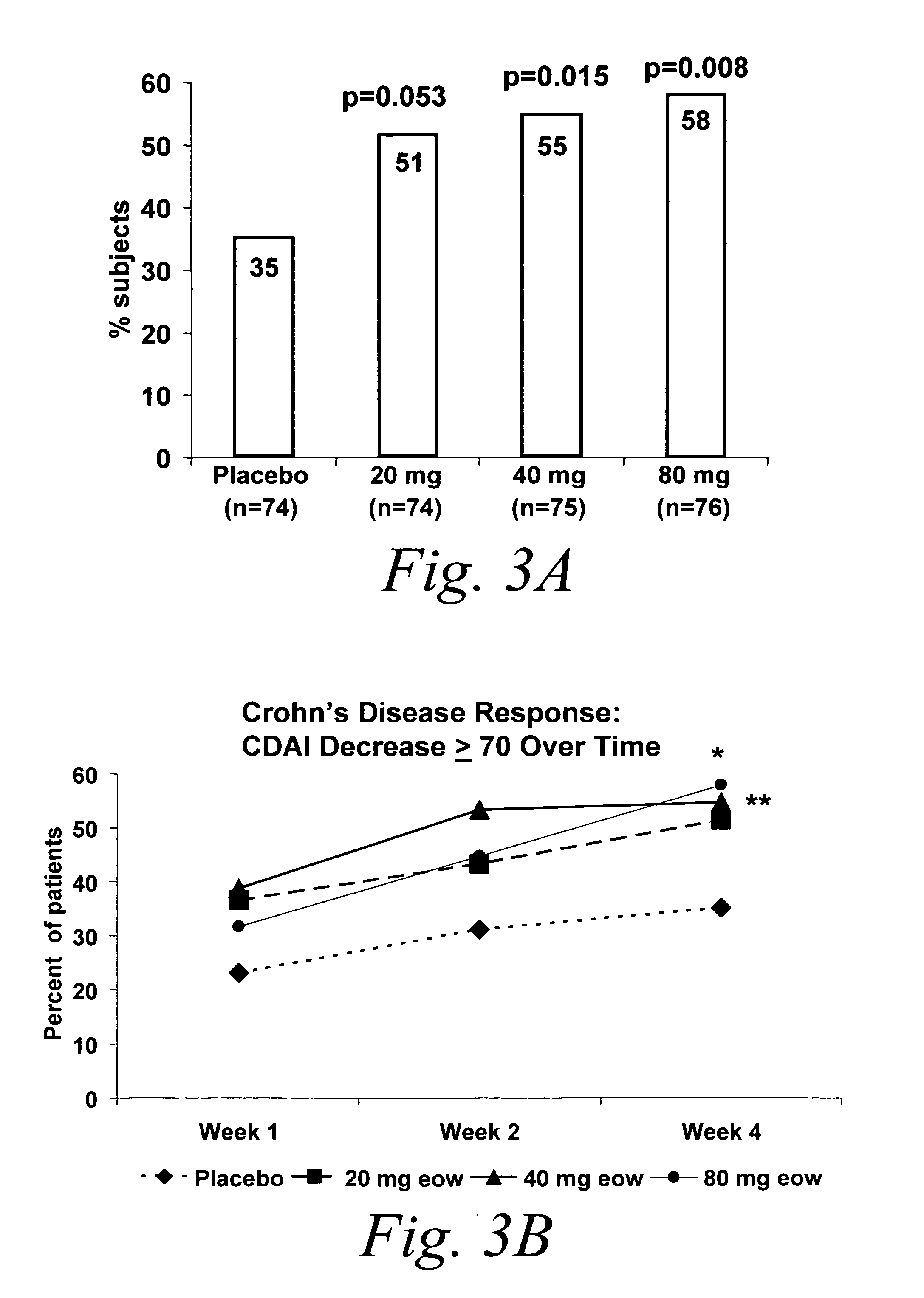
Multiple-variable dose methods for treating TNFα-related disorders, including Crohn's disease and psoriasis, comprising administering TNFα inhibitors, including TNFα antibodies, are described. Multiple-variable dose methods include administration of a TNF-inhibitor in an induction or loading phase followed by administration of the agent in a maintenance or treatment phase, wherein the TNF-inhibitor is administered in a higher dosage during the induction phase.
Owner:ABBVIE BIOTECHNOLOGY LTD
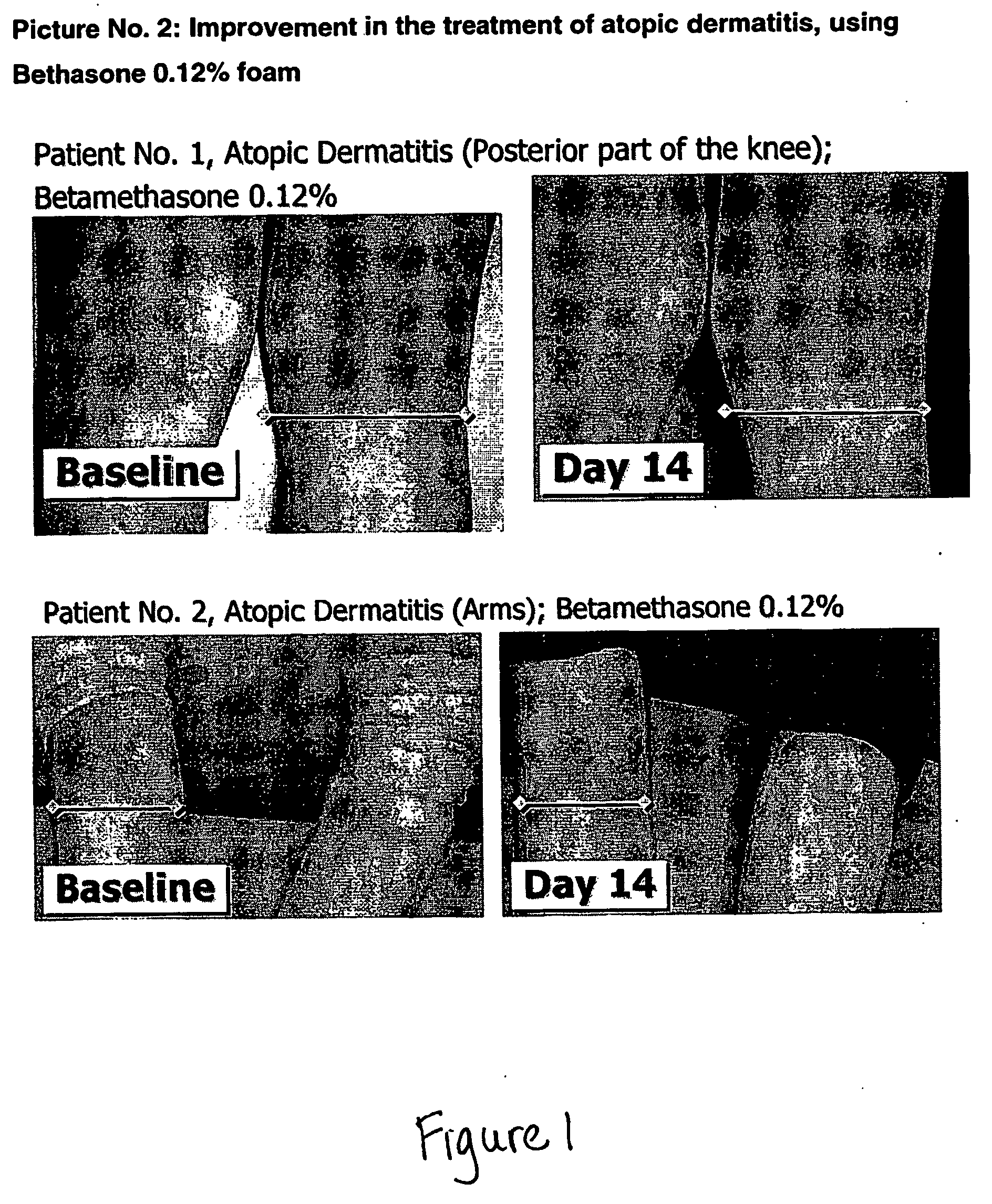
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20070292461A1Pleasant and easy to spreadPatient compliance is goodCosmetic preparationsMetabolism disorderActive agentPolyethylene glycol
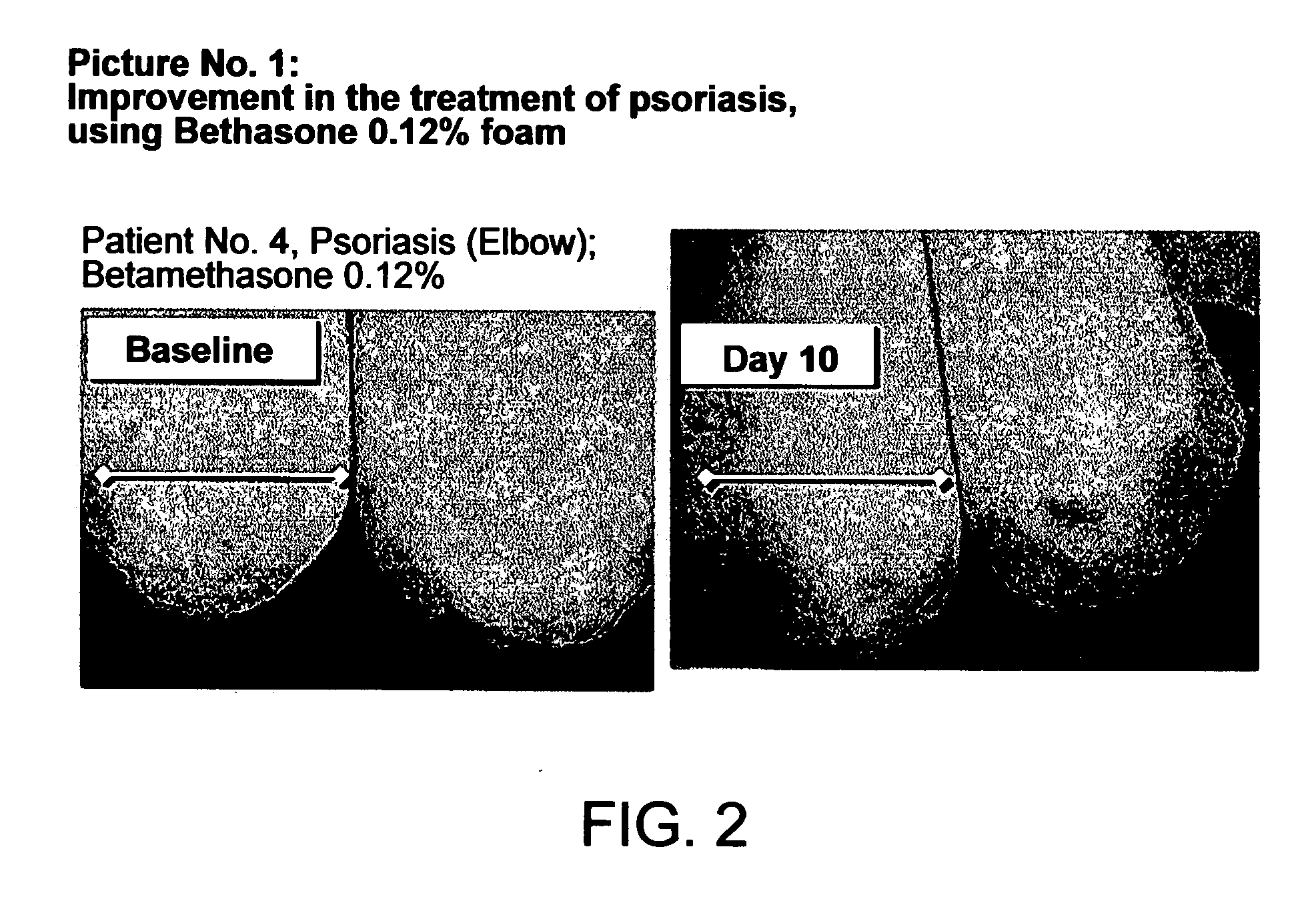
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
Implantable Drug Delivery Device and Methods for Treatment of the Bladder and Other Body Vesicles or Lumens
ActiveUS20090149833A1High plasma concentrationMinimize irritationBiocideMedical devicesDrug reservoirControlled drugs
An implantable medical device is provided for controlled drug delivery within the bladder, or other body vesicle. The device may include at least one drug reservoir component comprising a drug; and a vesicle retention frame which comprises an elastic wire having a first end, an opposing second end, and an intermediate region therebetween, wherein the drug reservoir component is attached to the intermediate region of the vesicle retention frame. The retention frame prevents accidental voiding of the device from the bladder, and it preferably has a spring constant selected for the device to effectively stay in the bladder during urination while minimizing the irritation of the bladder.
Owner:MASSACHUSETTS INST OF TECH
Aerosol pharmaceutical formulation for pulmonary and nasal delivery
InactiveUS6294153B1Antibacterial agentsPowder deliveryPharmaceutical formulationPharmaceutical preservatives
An aerosol pharmaceutical formulation for pulmonary and nasal delivery is provided. The formulation comprises a pharmaceutical agent, water, a phenol and a propellant. Optionally, an excipient such as glycerin or polyglycerin, lysine or polylysine, or other salts, flavoring or coloring agents, protease inhibitors or stabilizers can be added. A method of administering the formulation with a metered dose dispenser, and a dispenser containing the formulation are also provided.
Owner:GENEREX PHARMA
Compositions and methods for improved skin care
InactiveUS20070077292A1Safe and effective amount of collagenPrevent adverse side effectsOrganic active ingredientsCosmetic preparationsMedicineInstability
Compositions and methods for administering collagen to a human subject have been developed. The collagen-containing lipid vesicles of the invention provide a delivery system for human collagen which eliminates problems associated with chemical and physical instability of the collagen as well as immune responses to non-human collagen.
Owner:PINSKY MARK A
Prodrugs containing novel bio-cleavable linkers
Owner:PIRAMAL ENTERPRISES LTD
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
ActiveUS20080044444A1Convenient vehicle for topical deliveryGood treatment effectPowder deliveryBiocideDicarboxylic acidCarboxylic acid
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:VYNE THERAPEUTICS INC
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS20020004063A1Cleanly peeled from the skinMaintenance of such surfaceSalicyclic acid active ingredientsAnaestheticsPharmaceutical formulationSoft solids
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
Cosmetic and pharmaceutical foam
InactiveUS20080031907A1Efficient ConcentrationReduce sensitivityAntibacterial agentsBiocideAlcohol freeVegetable oil
The invention relates to uses of an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent as a flame retardant or flame resistant foam. The hydrophobic solvent is preferably mineral oil; medium chain triglycerides; isopropyl myristearate or octyl dodecanol, silicone oil or vegetable oil or mixtures thereof. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, also making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil-soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Methods for treating sinus headache
InactiveUS6838434B2Effective treatmentReduce and inhibit and eliminateAntibacterial agentsBiocideToxin typesSurgery
A sinus headache can be treated by administration of a botulinum toxin to a patient. The botulinum toxin can be botulinum toxin type A and the botulinum toxin can be administered to or to the vicinity of a sinus membrane of a patient with a sinus headache.
Owner:ALLERGAN INC
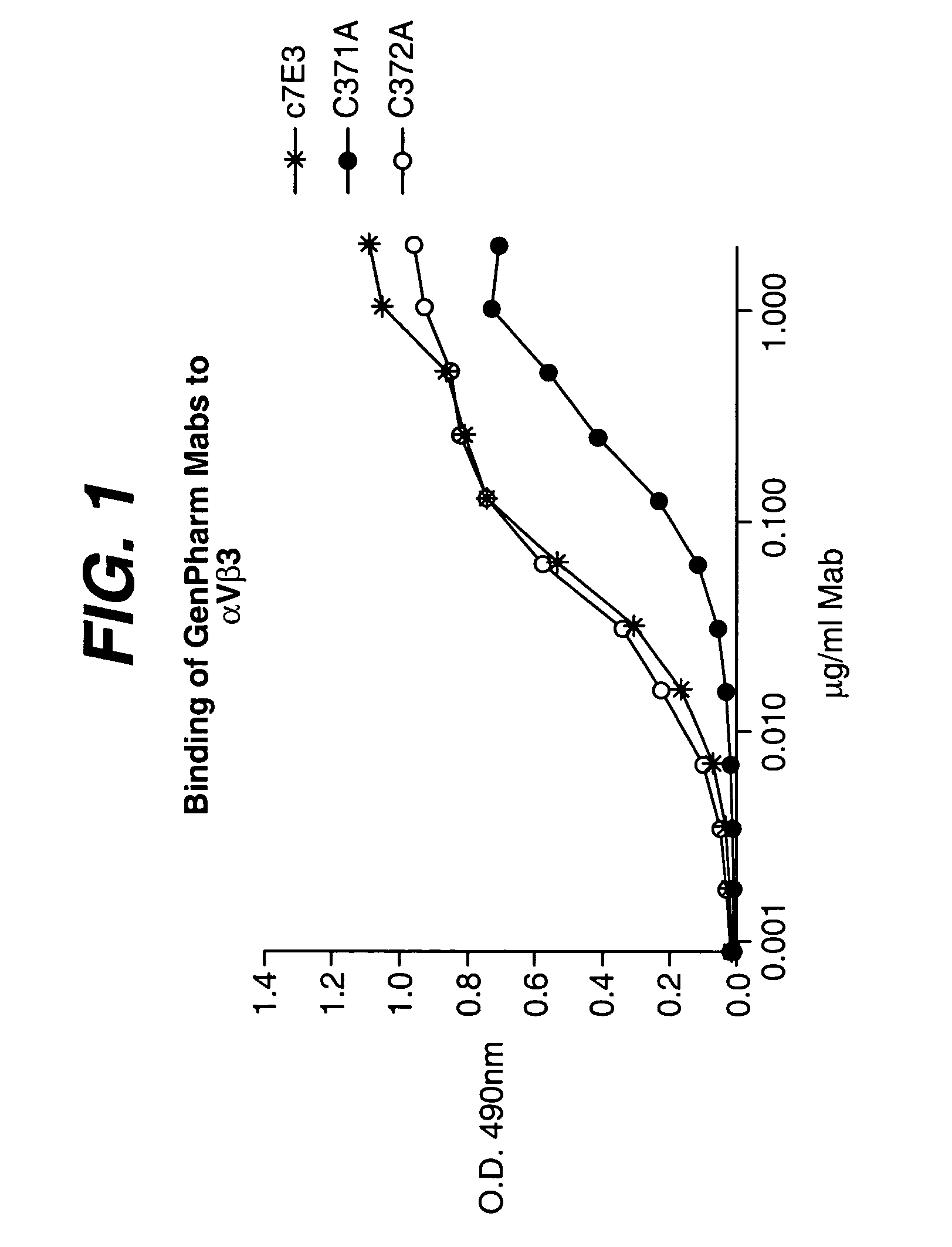
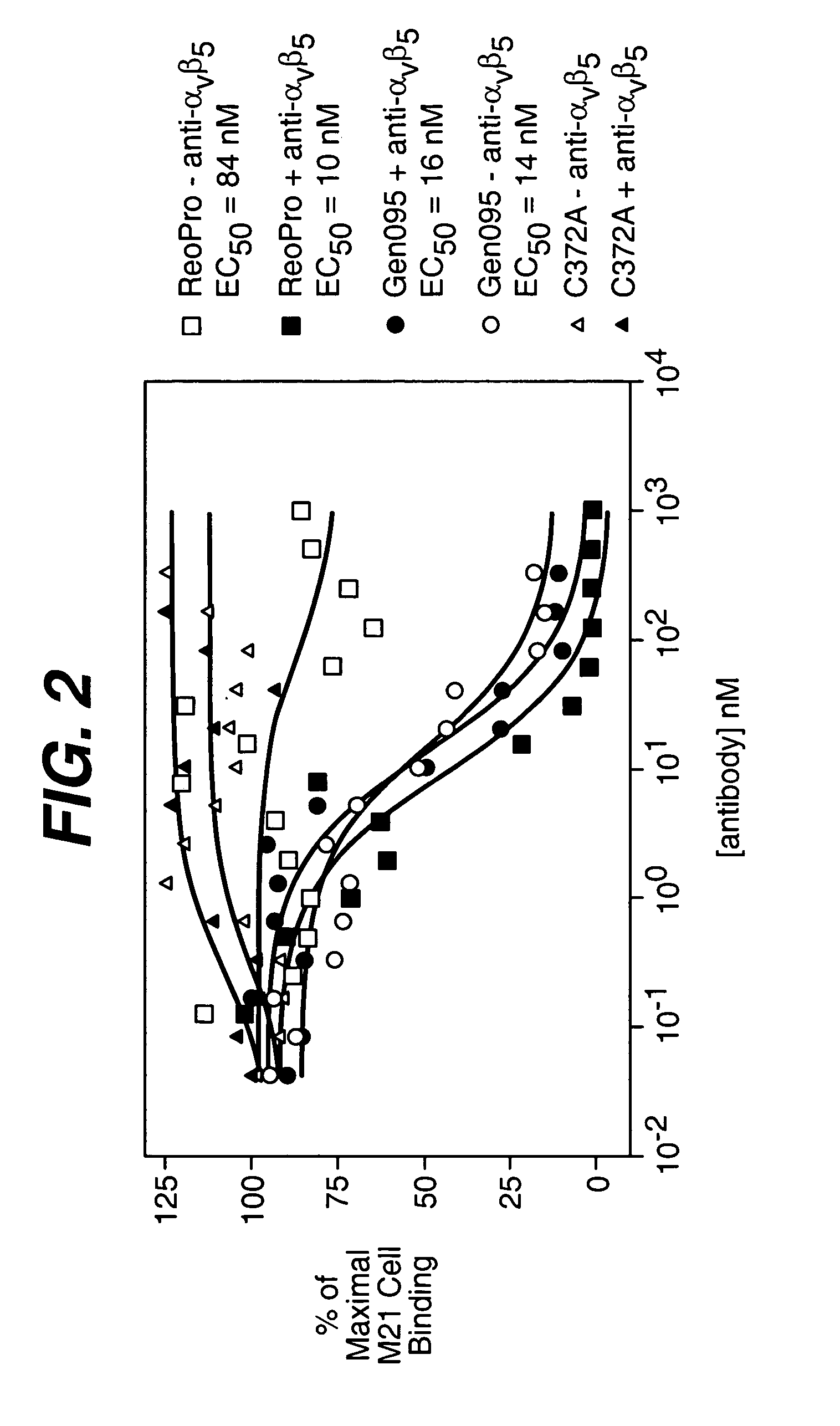
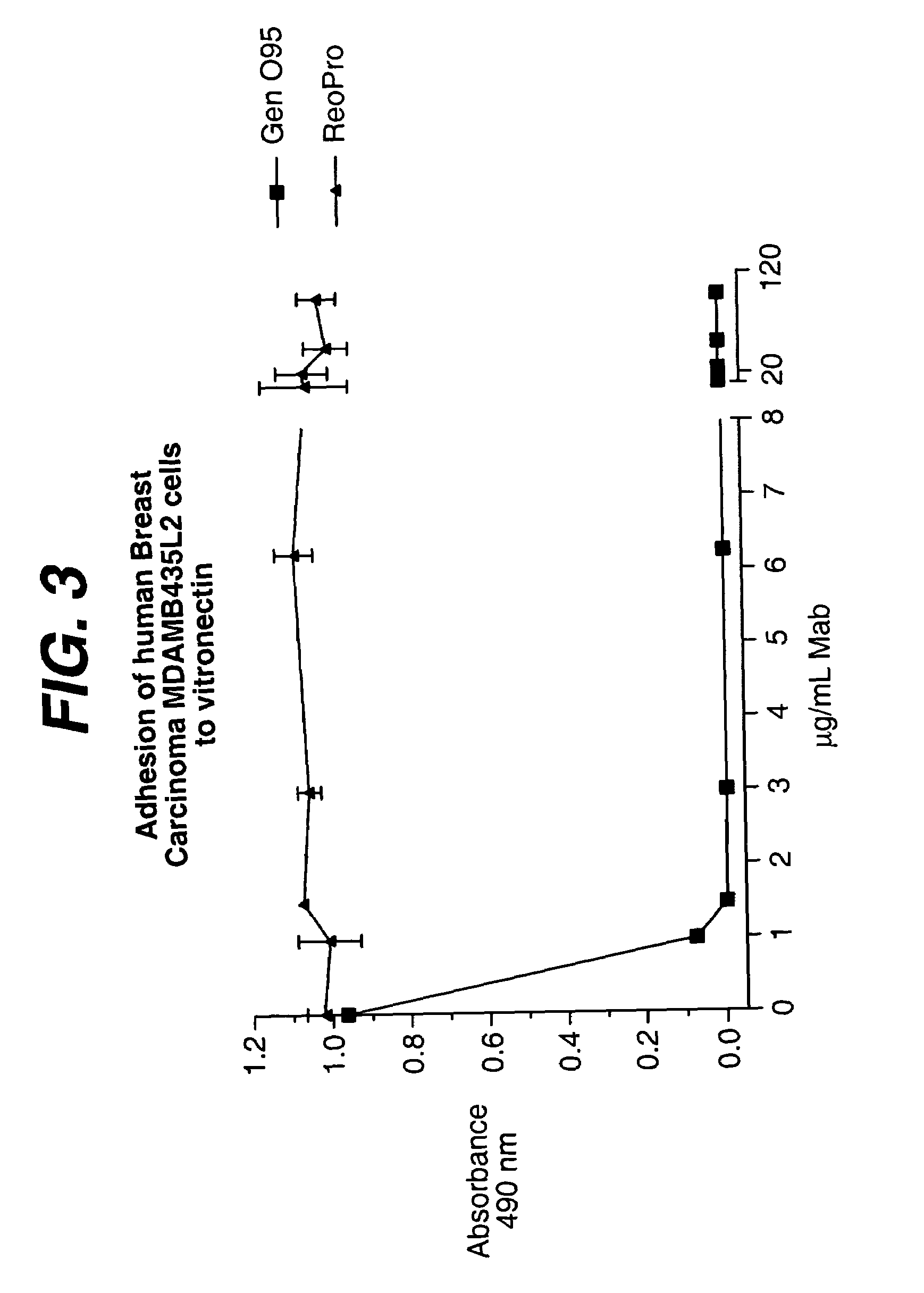
Anti-integrin antibodies, compositions, methods and uses
The present invention relates to at least one novel anti-alpha-V subunit antibodies, including isolated nucleic acids that encode at least one anti-alpha-V subunit antibody, alpha-V subunit, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:JANSSEN BIOTECH INC
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Cosmetic and pharmaceutical foam
InactiveUS20060140984A1Lower yield strengthRubbing easy and efficientCosmetic preparationsBiocideAlcohol freeAdjuvant
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Capsaicinoid gel formulation and uses thereof
InactiveUS20060148903A1Relieve painReduce the amount requiredBiocideNervous disorderSurgical sitePost-Procedural Pain
The present invention provides capsaicinoid gel formulations and methods for relieving pre- and post-surgical pain at a site in a human or animal by administering at a surgical site in a human or animal in need thereof a dose of capsaicinoid gel in an amount effective to attenuate post-surgical pain at the surgical site, the dose of capsaicin ranging from 100 μg to 10,000 μg.
Owner:ALGORX PHARMA INC
Compositions and methods for enhancing drug delivery across and into epithelial tissues
This invention provides compositions and methods for enhancing delivery of drugs and other agents across epithelial tissues, including the skin, gastrointestinal tract, pulmonary epithelium, and the like. The compositions and methods are also useful for delivery across endothelial tissues, including the blood brain barrier. The compositions and methods employ a delivery enhancing transporter that has sufficient guanidino or amidino sidechain moieties to enhance delivery of a compound conjugated to the reagent across one or more layers of the tissue, compared to the non-conjugated compound. The delivery-enhancing polymers include, for example, poly-arginine molecules that are preferably between about 6 and 25 residues in length.
Owner:KAI PHARMA
Tape formulation for percutaneous administration containing fentanyi
PCT No. PCT / JP97 / 01595 Sec. 371 Date Jan. 13, 1998 Sec. 102(e) Date Jan. 13, 1998 PCT Filed May 13, 1997 PCT Pub. No. WO97 / 42952 PCT Pub. Date Nov. 20, 1997A tape formulation for percutaneous administration containing fentanyl which comprises fentanyl or a salt thereof, a pressure sensitive adhesive and sodium acetate, is disclosed. The salt of fentanyl is preferably fentanyl citrate. The tape formulation of the present invention is little irritation to the skin and excellent in the percutaneous permeation of fentanyl and has a high stability even after the passage of time.
Owner:HISAMITSU PHARM CO INC
Method for treating neuromuscular disorders and conditions with botulinum toxin types A and B
A method of treating a patient suffering from a disease, disorder or condition includes the administration to the patient of a therapeutically effective amount of botulinum toxin of a selected serotype until the patient experiences loss of clinical response to the administered botulinum toxin and thereafter administering to the patient a therapeutically effective amount of another botulinum toxin of a different serotype.
Owner:SOLSTICE NEUROSCI
Temporarily Stiffened Mesh Prostheses
ActiveUS20070198040A1Low experience requirementReduce implantationPretreated surfacesAdhesive dressingsFiberSide effect
The present invention relates to medical prostheses and methods of manufacturing those devices. In particular, the prostheses are temporarily stiffened meshes with particular coatings to provide initial stiffness and thereby permit easier surgical handling for treatment or reconstruction of soft tissue defects. Preferred embodiments include surgical meshes coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The devices of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use. By including biologically active agents and / or drugs in the coating, the devices provide prophylaxis for and can alleviate side effects or complications associated with the surgery or use of prostheses in general.
Owner:MEDTRONIC INC
Phospholipid compositions and methods for their preparation and use
The present invention provides compositions that comprise a phospholipid component (that contains one or more phospholipids) and a pharmaceutically acceptable fluid carrier, where the phospholipid component is in the range from about 10% to about 90% of the total weight. Optionally, the compositions may further comprise non-phospholipid filler materials, where the amount of the non-phospholipid filler materials is in the range from about 5% to about 50% of the total weight. In certain embodiments, the compositions may be injectable, non-liposomal, and / or in form of a gel or a paste. The compositions of the present invention are useful for repairing and augmenting soft and / or hard tissues or for sustained local drug delivery.
Owner:ENCORE THERAPEUTICS
Body cavity foams
The invention relates to an alcohol-free cosmetic or therapeutic foam carrier comprising water, a hydrophobic organic carrier, a foam adjuvant agent, a surface-active agent and a gelling agent. The cosmetic or therapeutic foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble therapeutic and cosmetic agents.
Owner:VYNE THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com