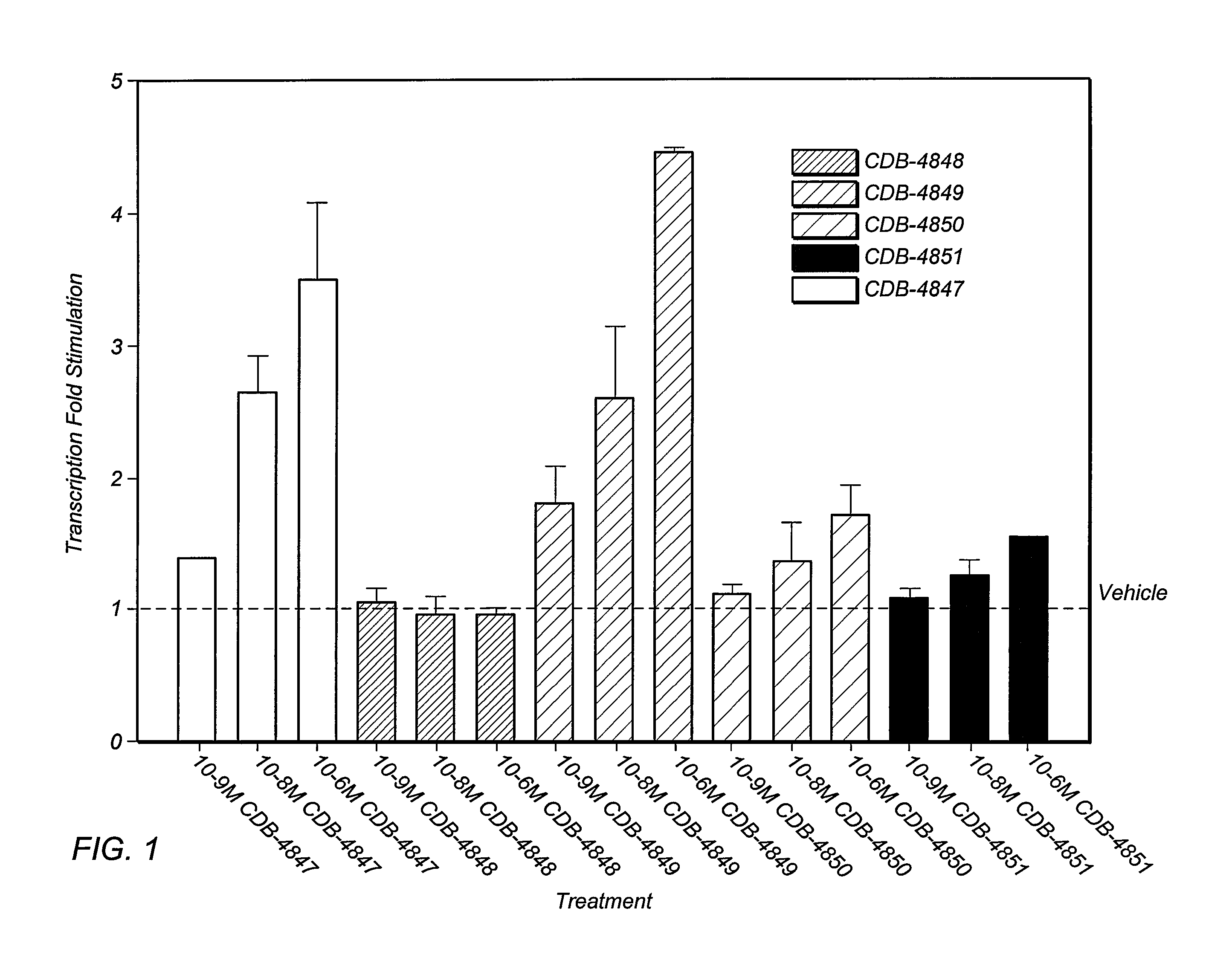
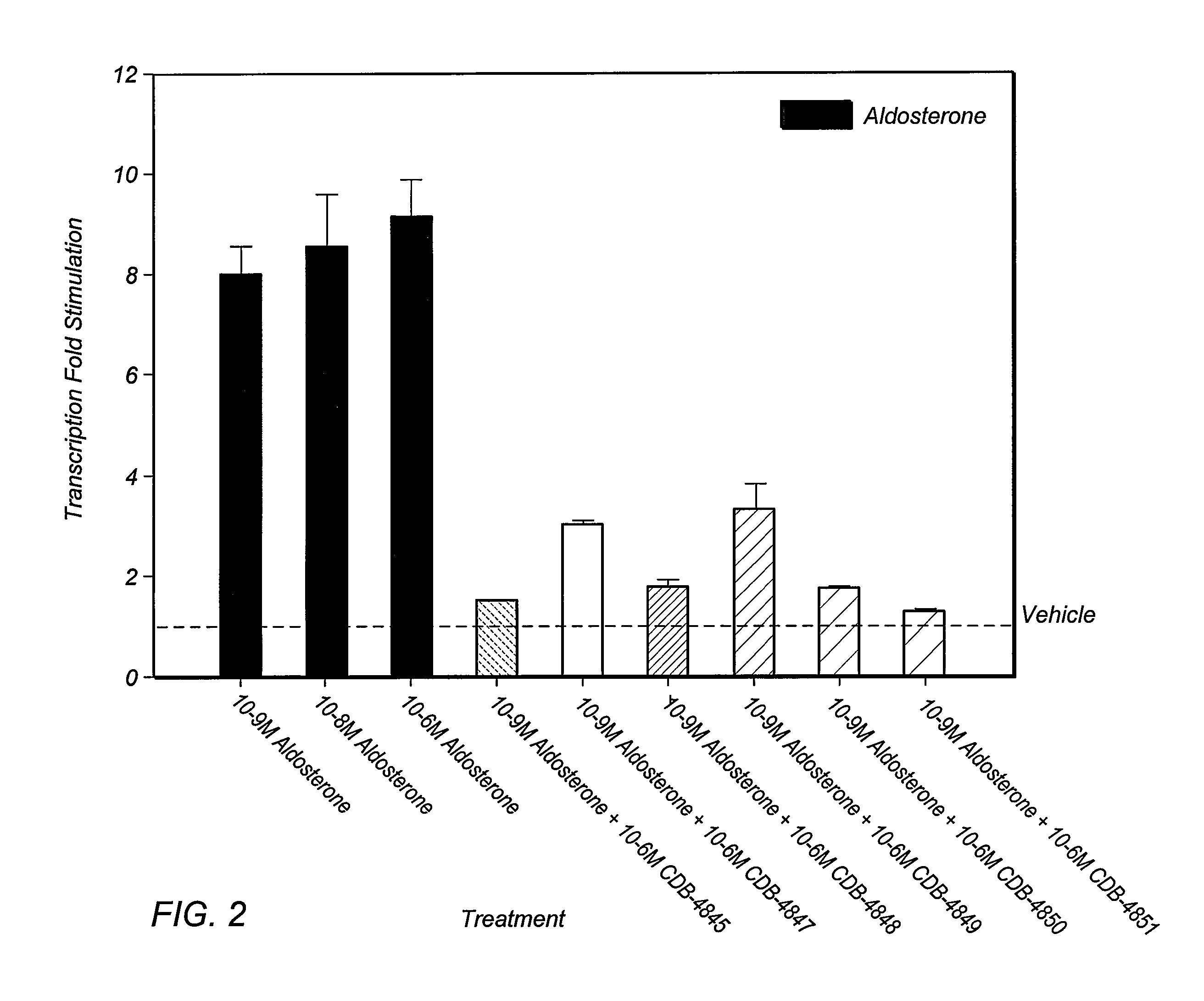
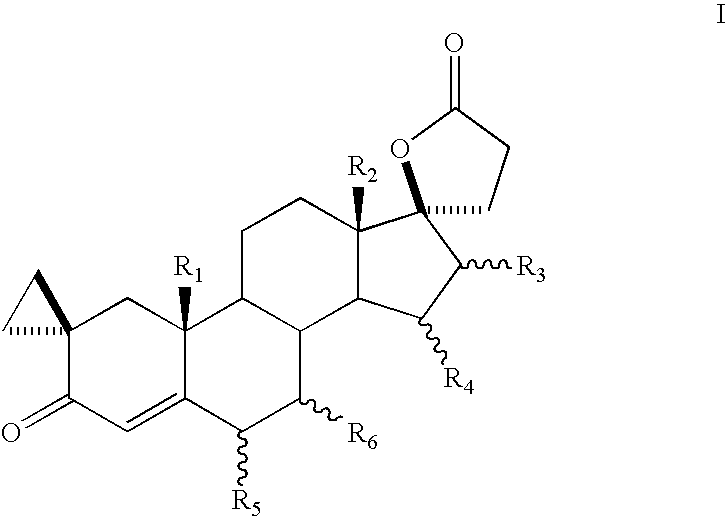
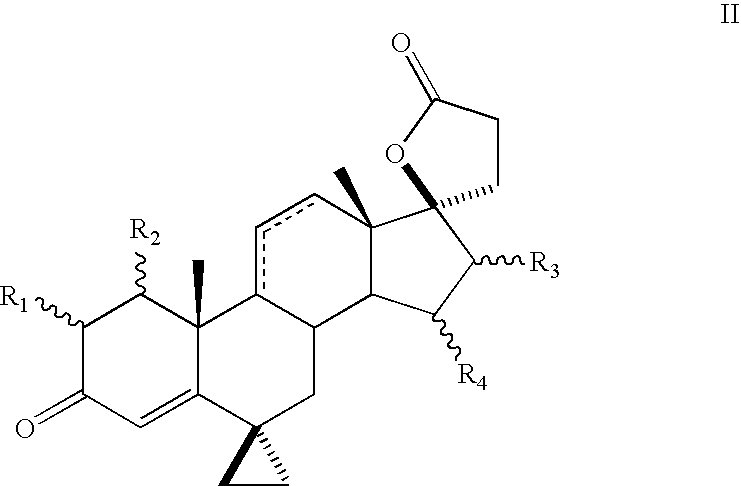
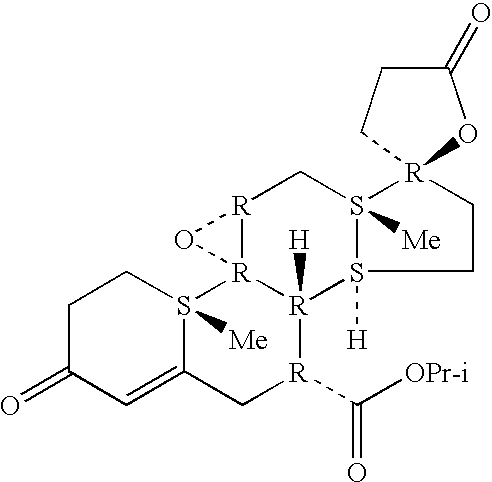
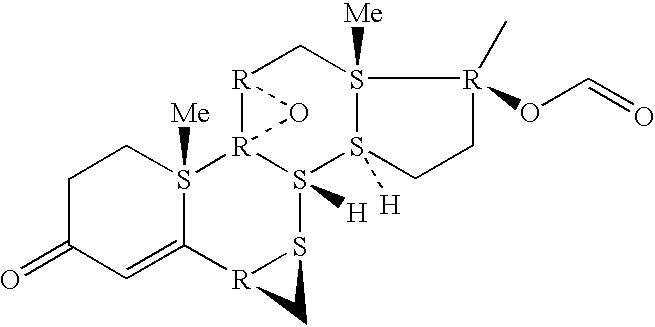
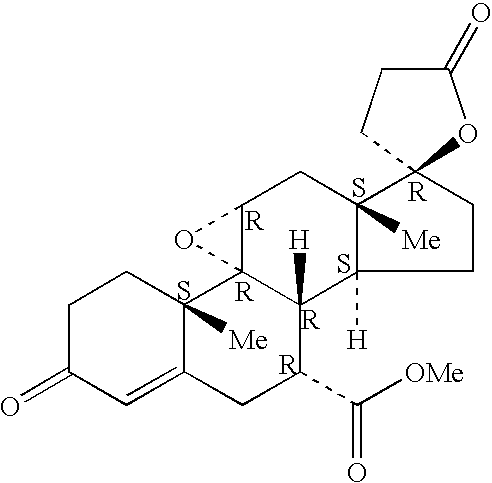
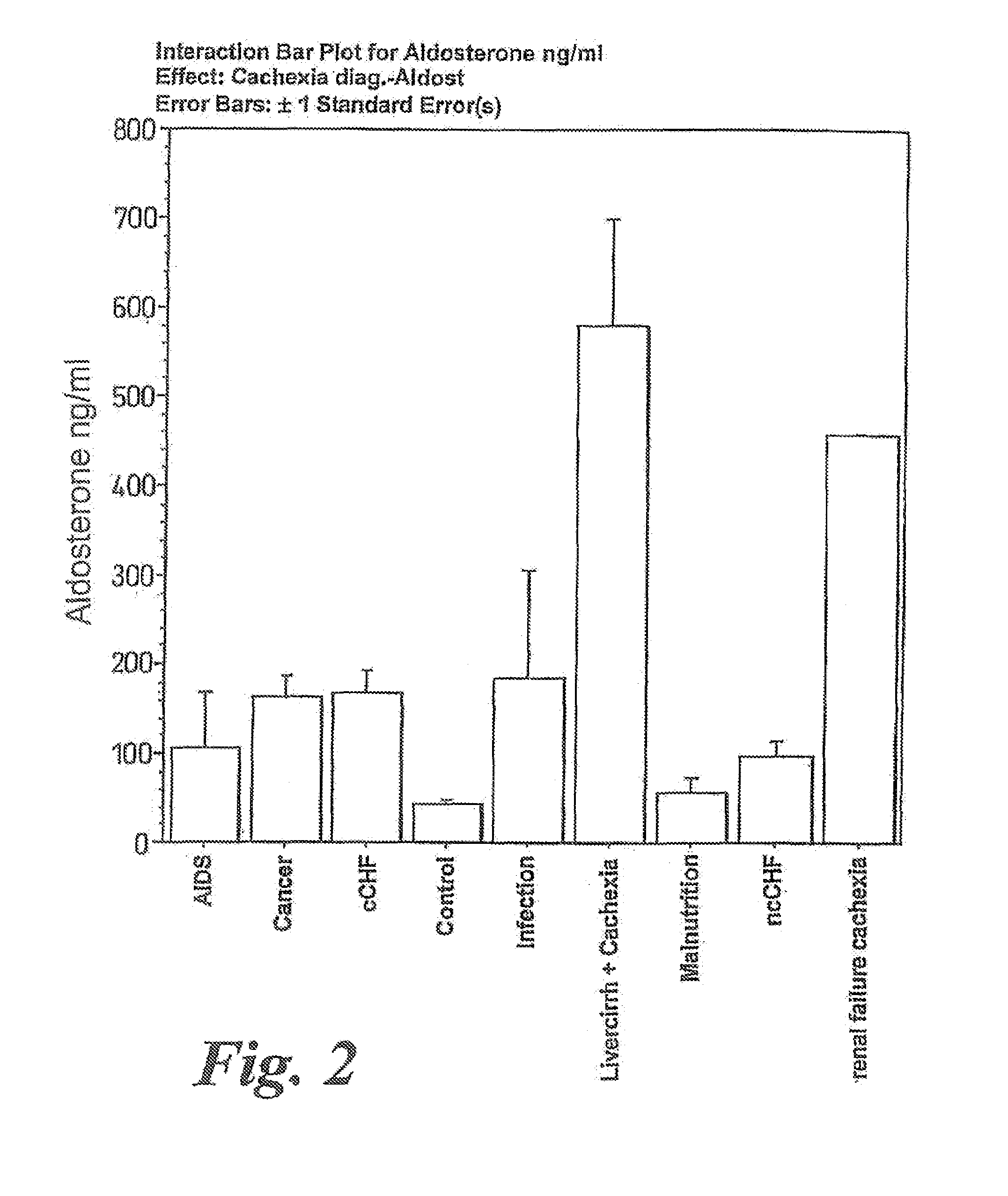
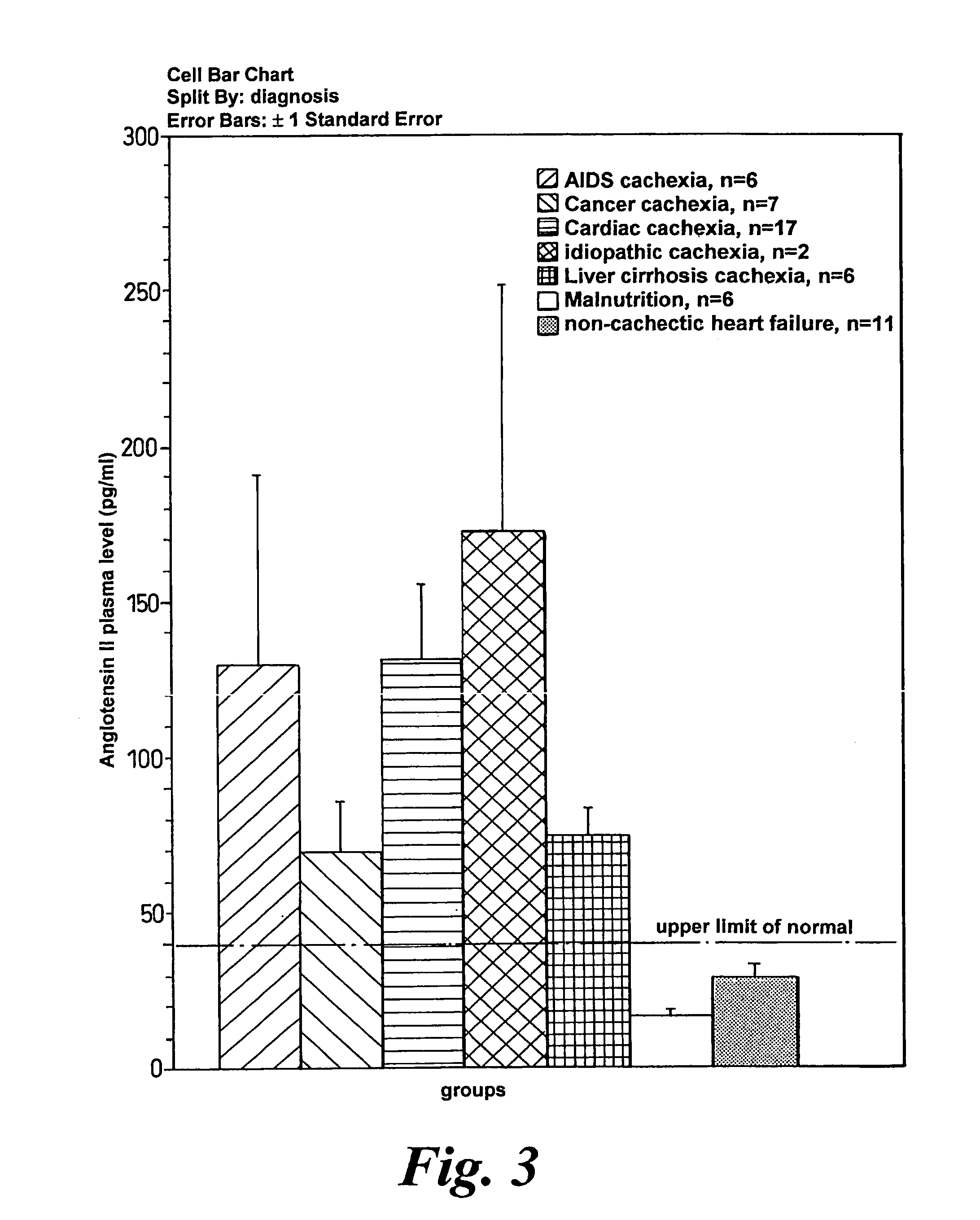
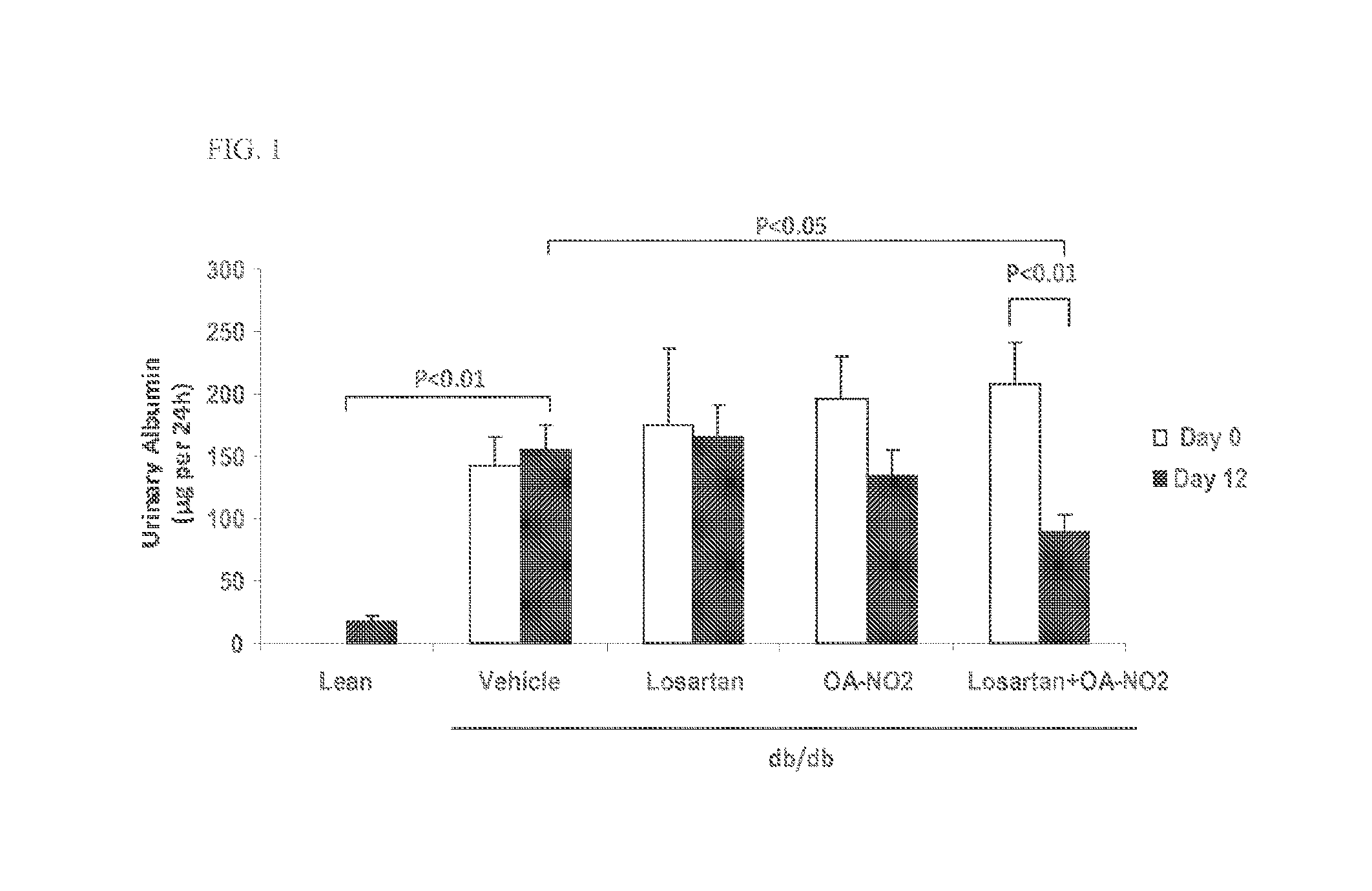
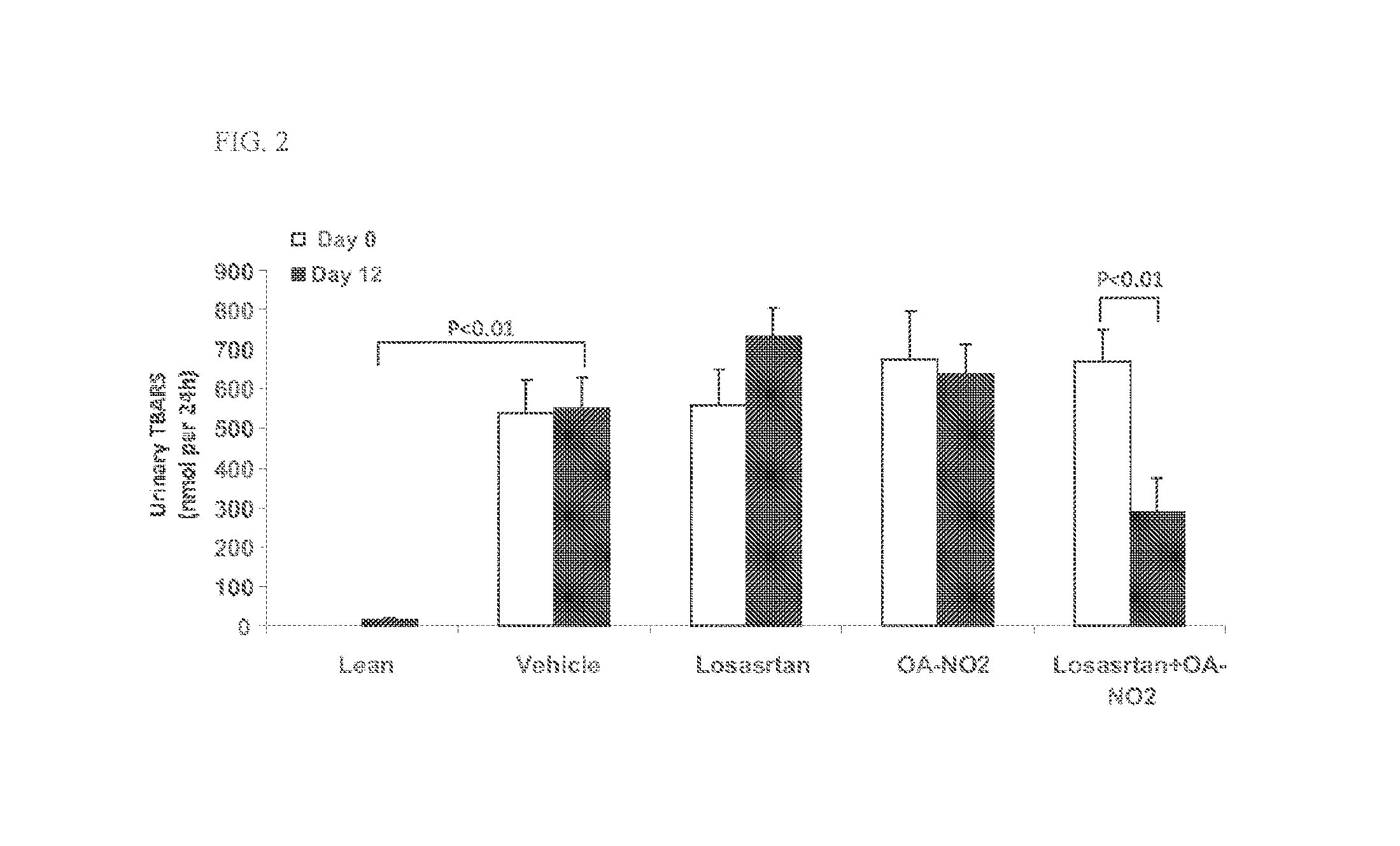
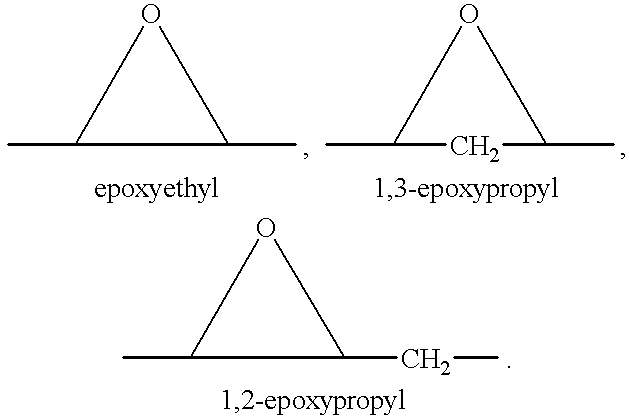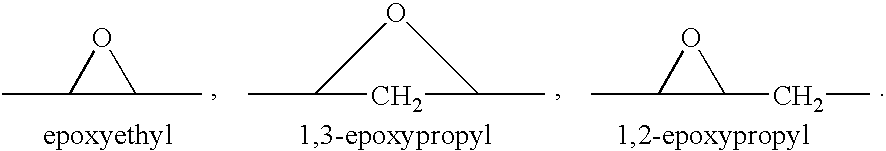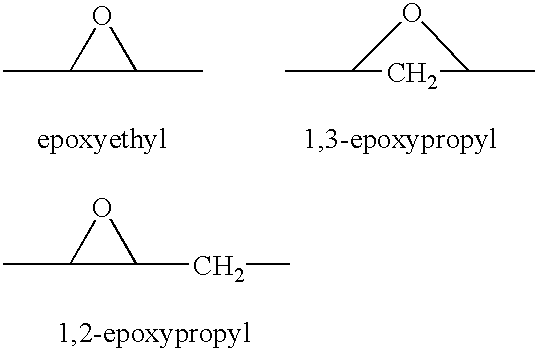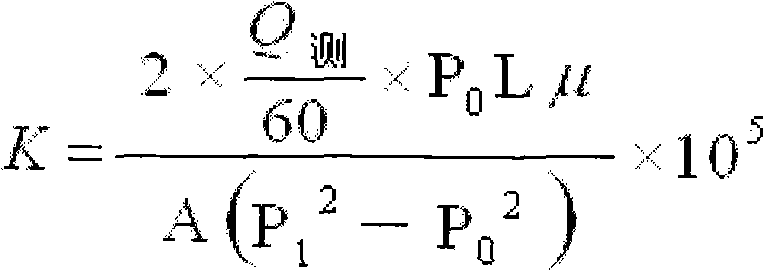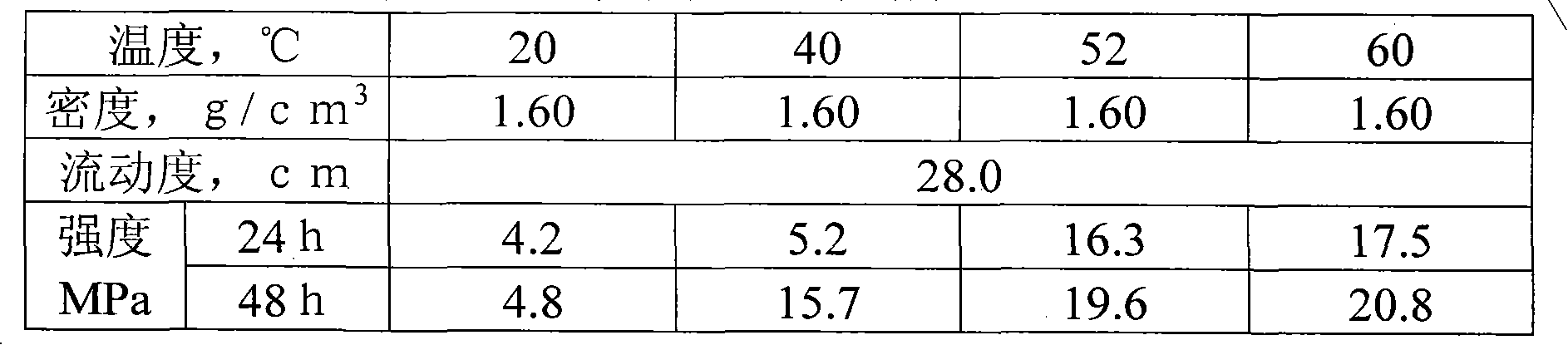Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
176 results about "Aldosterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aldosterone, the main mineralocorticoid hormone, is a steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na⁺), and potassium (K⁺) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.
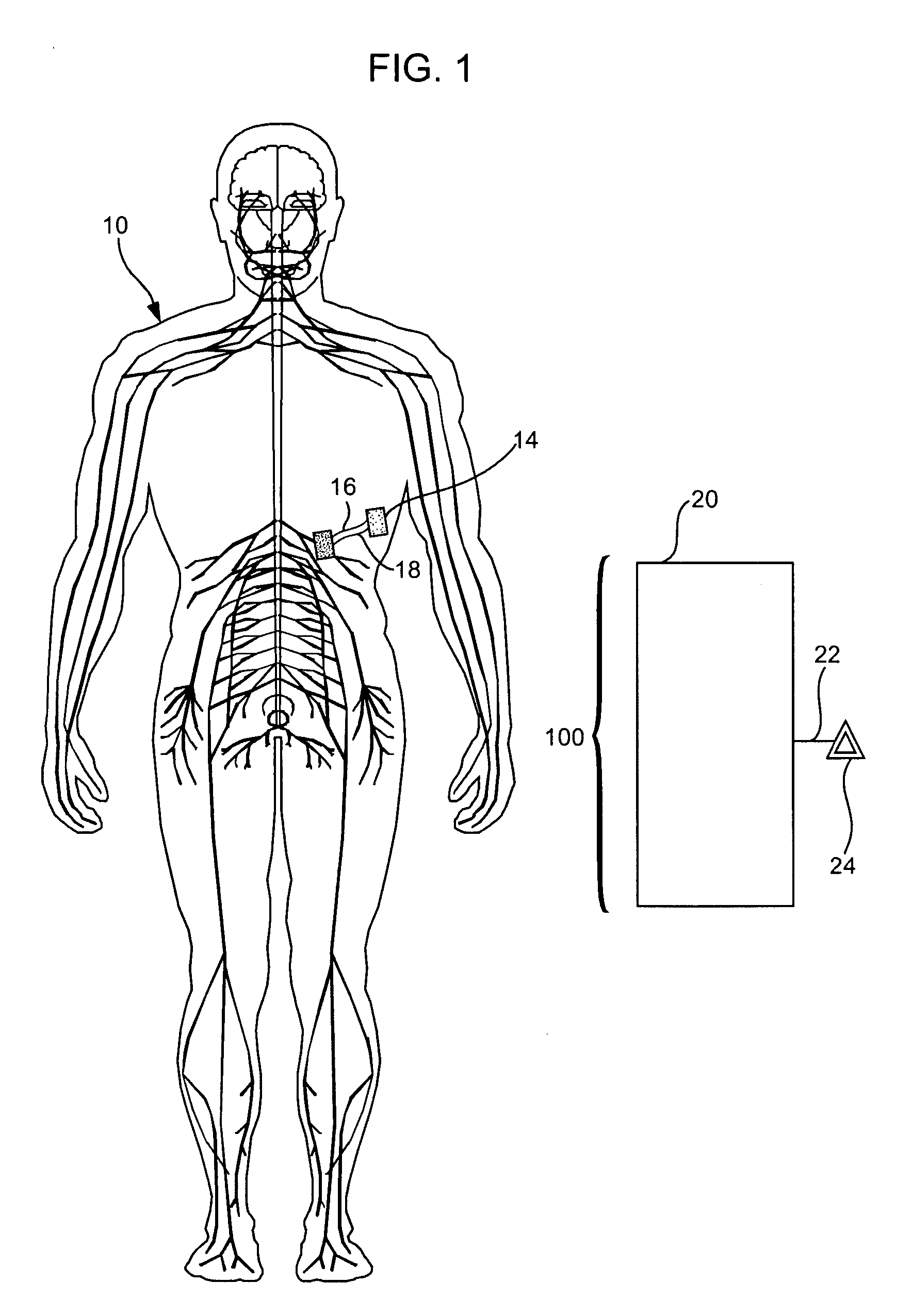
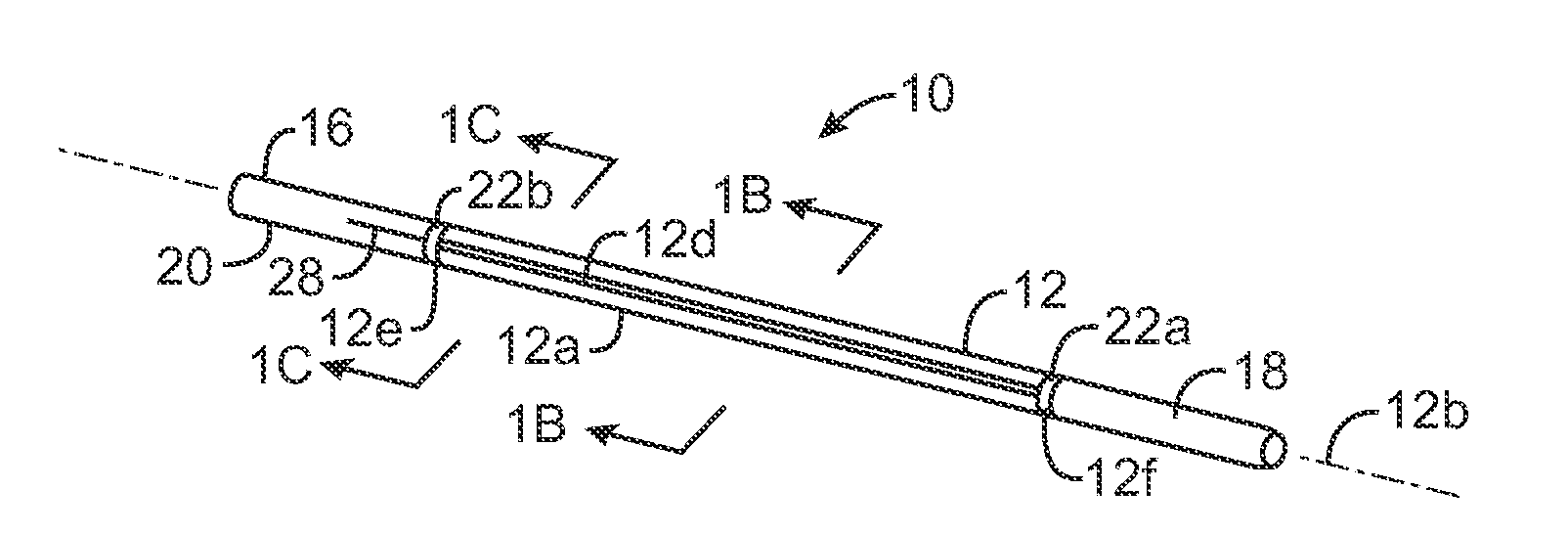
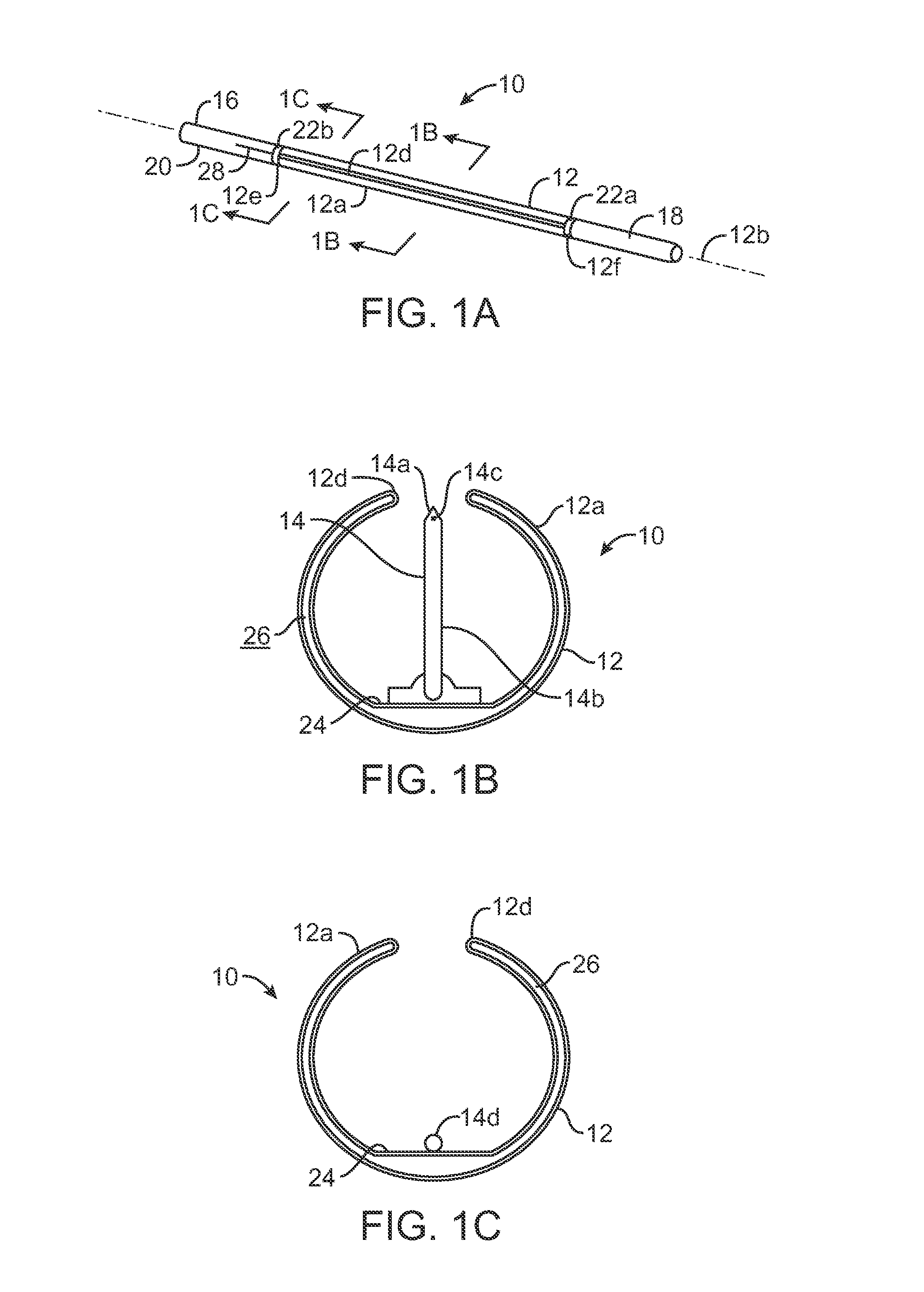
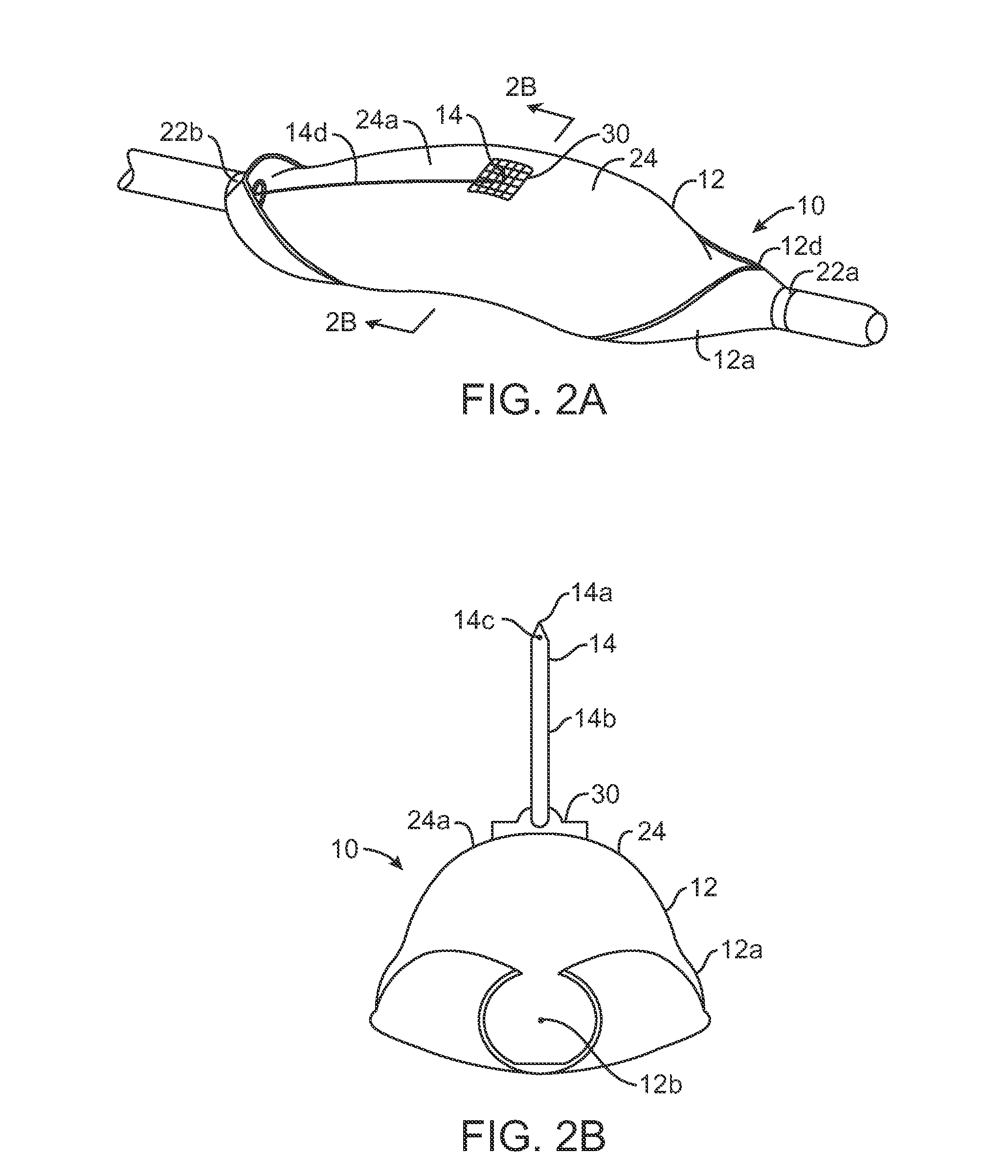
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Treatment of renal hypertension or carotid sinus syndrome with adventitial pharmaceutical sympathetic denervation or neuromodulation
ActiveUS20110104061A1Improve concentrationOrganic active ingredientsBacterial antigen ingredientsRenal HypertensionsCvd risk
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict medication regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
Treatment of hypertension by renal vascular delivery of guanethidine
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or sympathetic nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
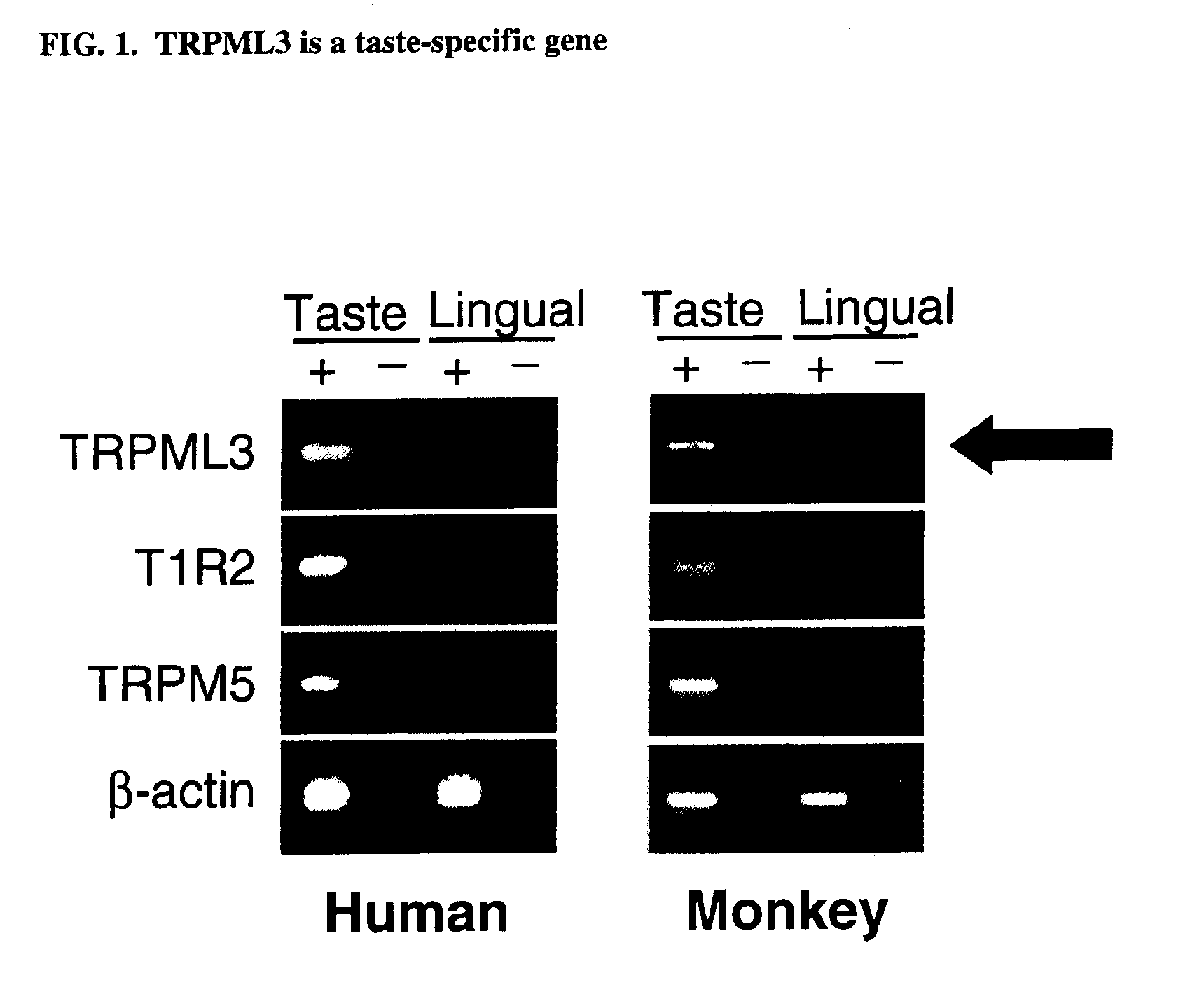
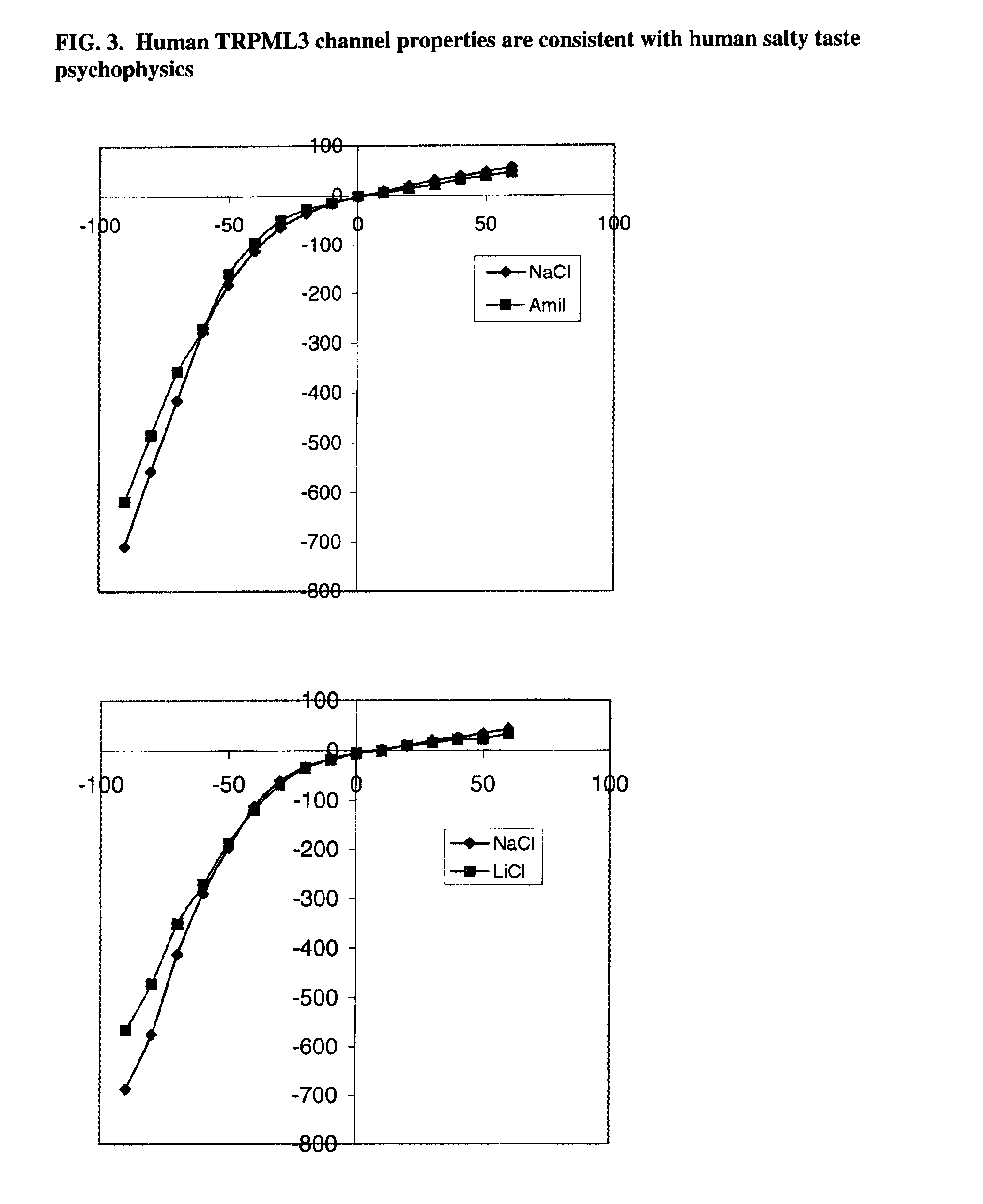
Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone, and/or vasopressin production or release
InactiveUS20090210953A1Reduce volumeIncrease pressureCompound screeningApoptosis detectionSalty taste perceptionReceptor
The present invention relates to the elucidation that TRPML3 is involved in salty taste perception in primates including humans and likely other mammals and based thereon high-throughput mammalian and medium-throughput oocyte-based electrophysiological assays for identifying human TRPML3 modulators, preferably TRPML3 enhancers. Compounds that modulate TRPML3 function in the assay are expected to affect salty taste in humans. The inventive electrophysiological assays, such as the two-electrode voltage-clamp technique, facilitate the identification of compounds which specifically modulate human TRPML3. The assays of the invention provide a robust screen useful to detect compounds that facilitate (enhance) or inhibit TRPML3 function. Compounds that enhance or block TRPML3 channel activity should thereby modulate salty taste. In addition, these compounds may be used to regulate sodium excretion, urinary output and other biological functions relating to sodium levels and TRPML3 related functions.
Owner:SENOMYX INC
Treatment of hypertension by renal vascular delivery of guanethidine
ActiveUS8399443B2Improve concentrationBiocidePharmaceutical delivery mechanismRegimenRenal Hypertensions
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or sympathetic nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST INC
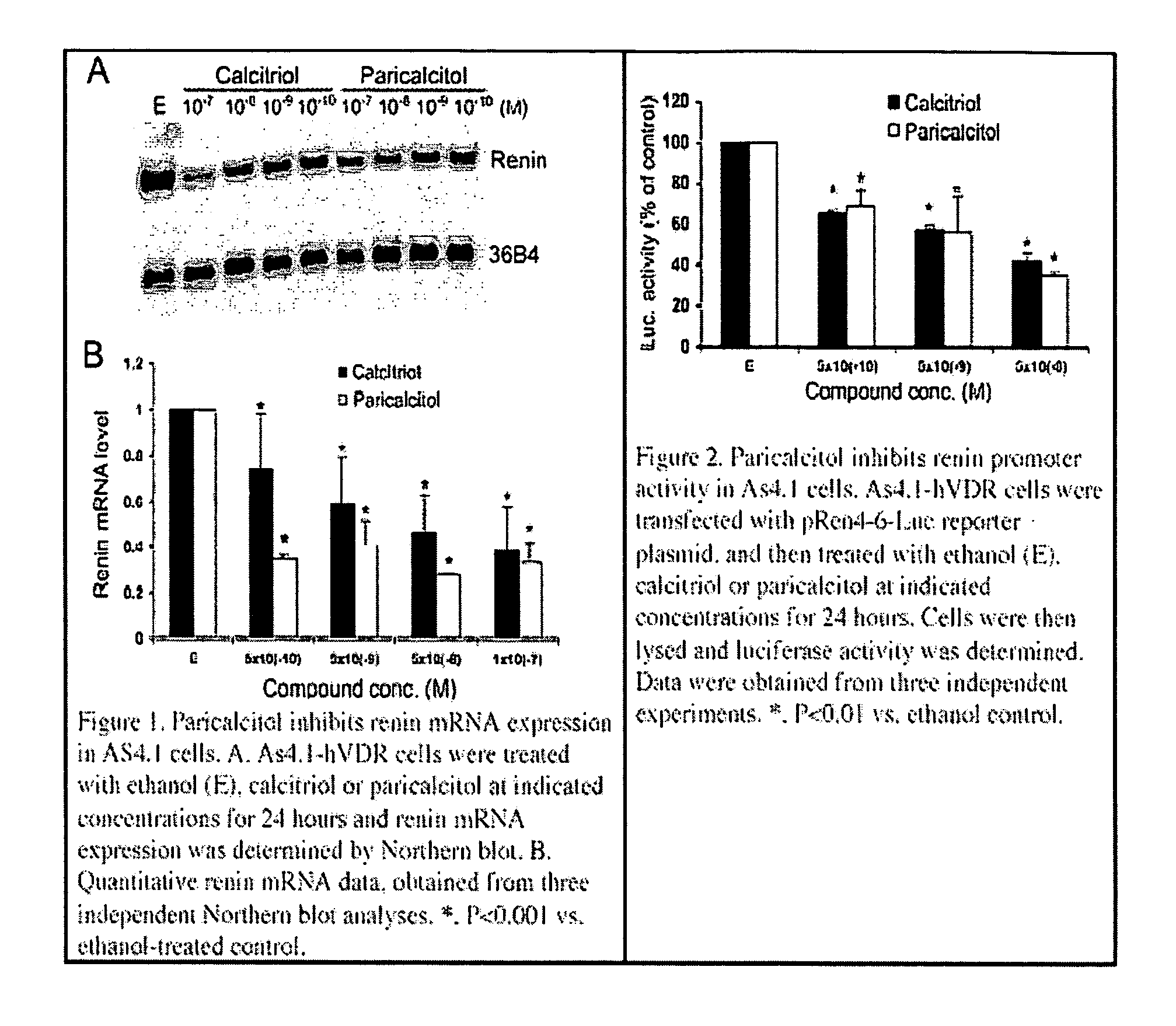
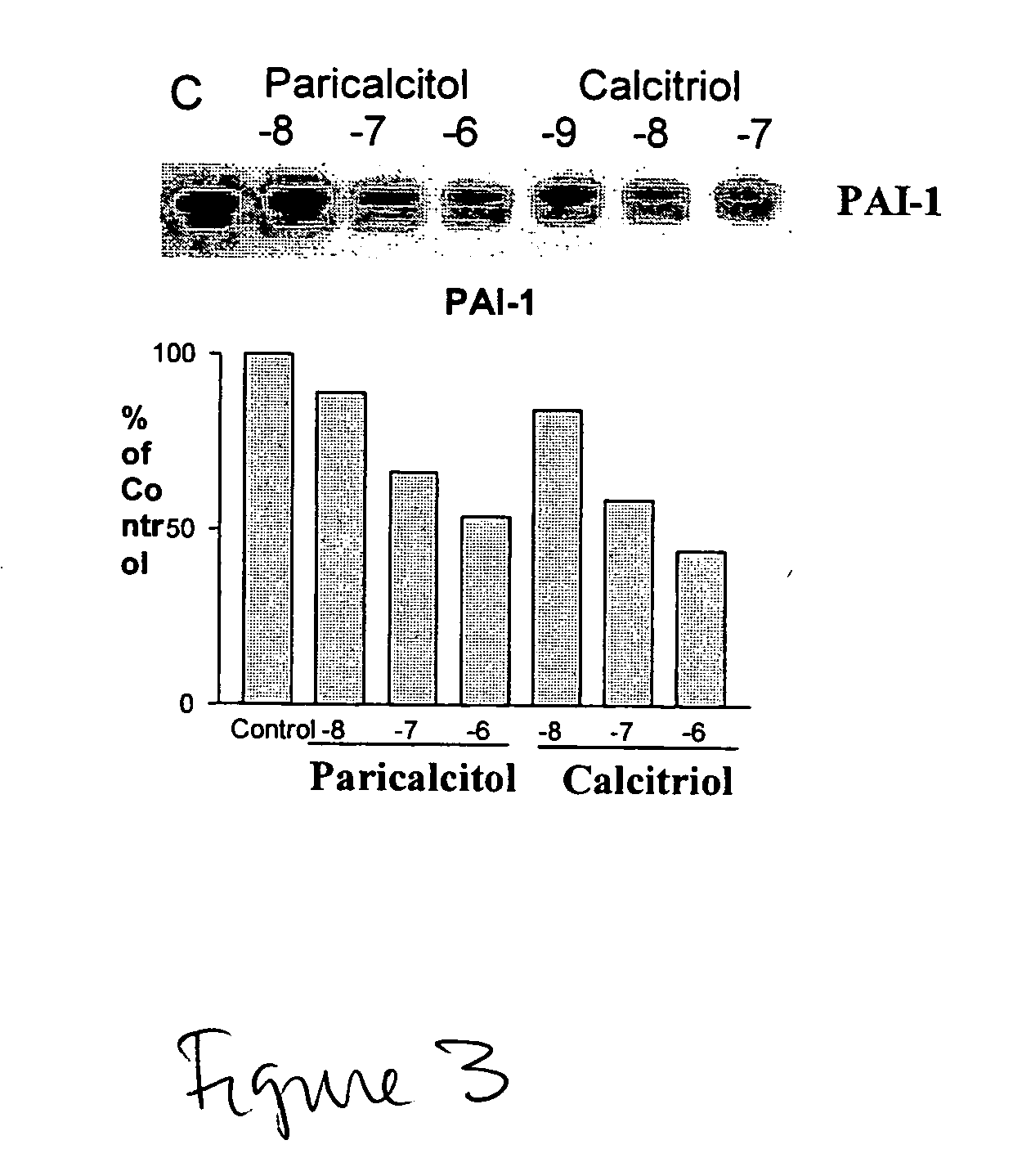
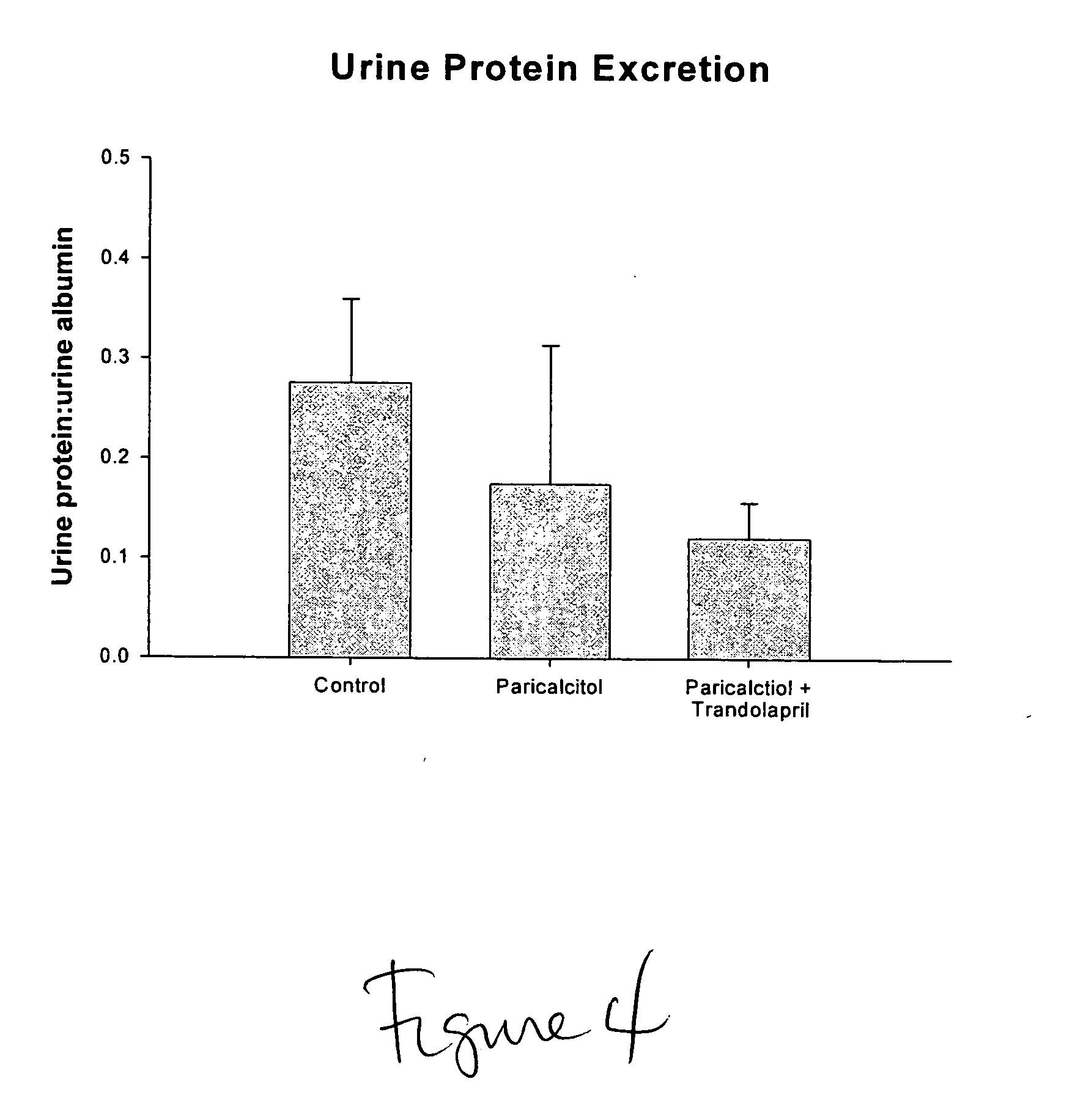
Use of vitamin Ds to treat kidney disease
InactiveUS20050124591A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAngiotensin Receptor Blockers
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:ABBOTT LAB INC
Combination of an aldosterone receptor antagonist and an anti-diabetic agent
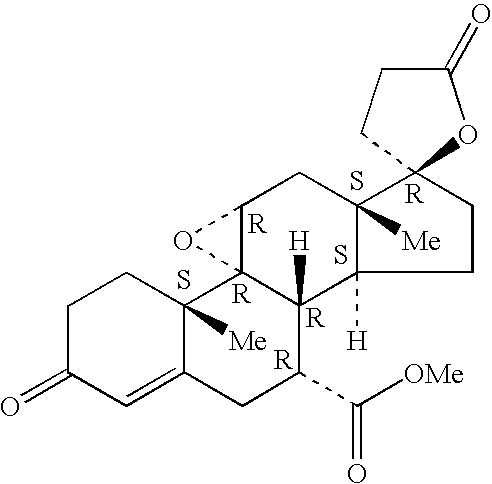
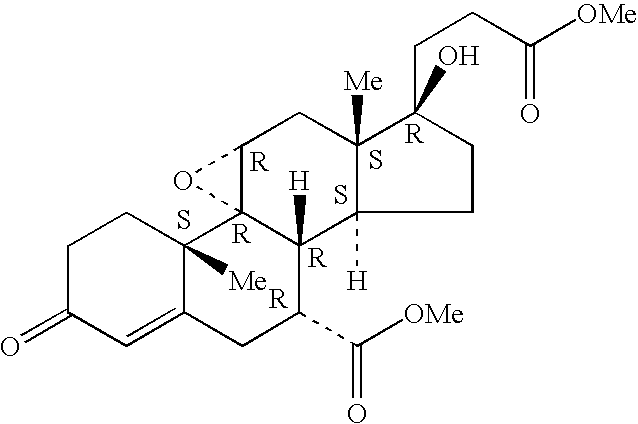
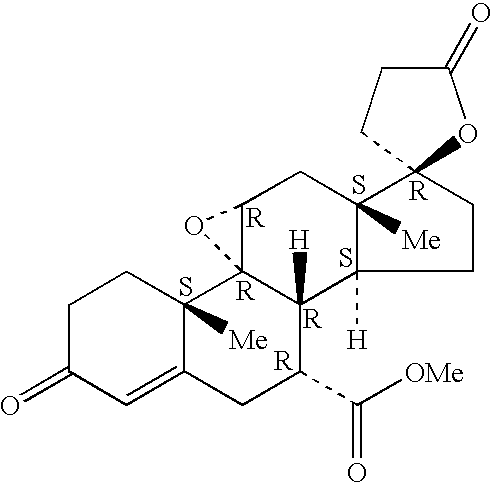
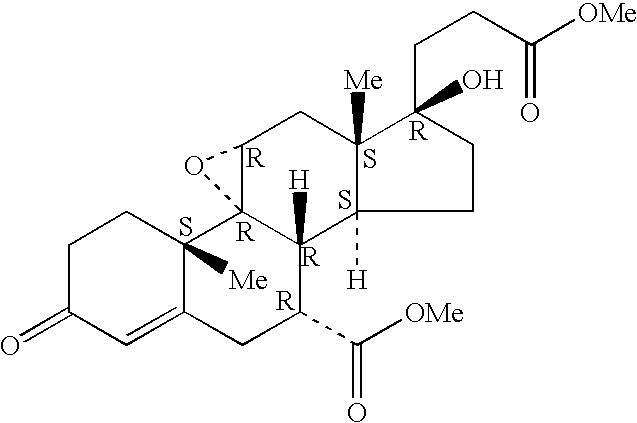
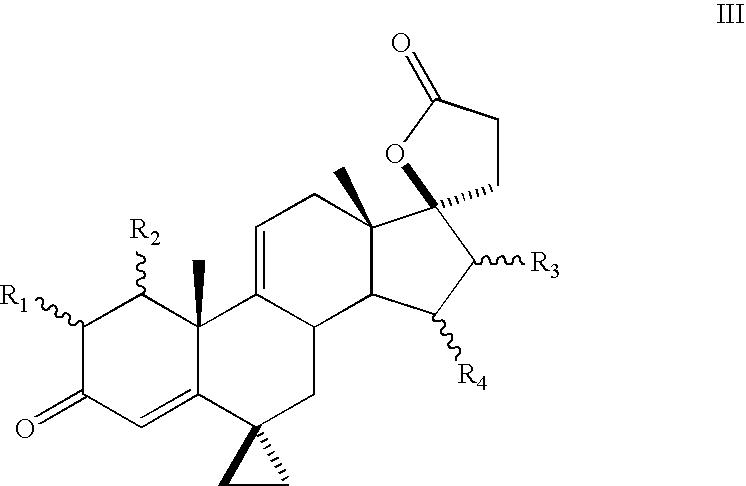
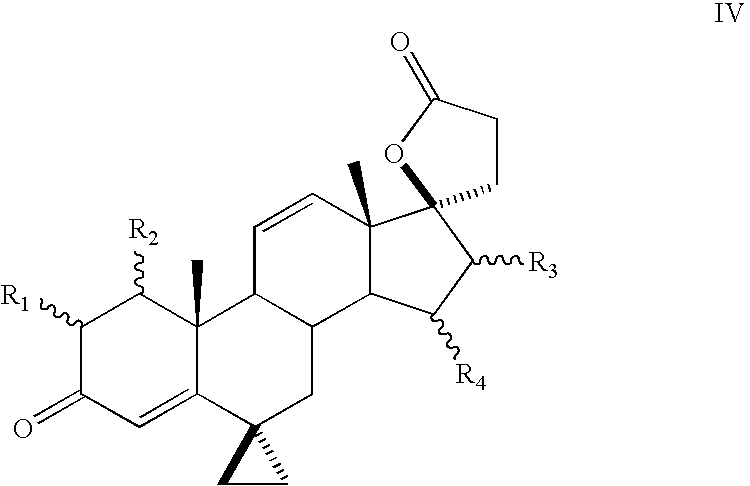
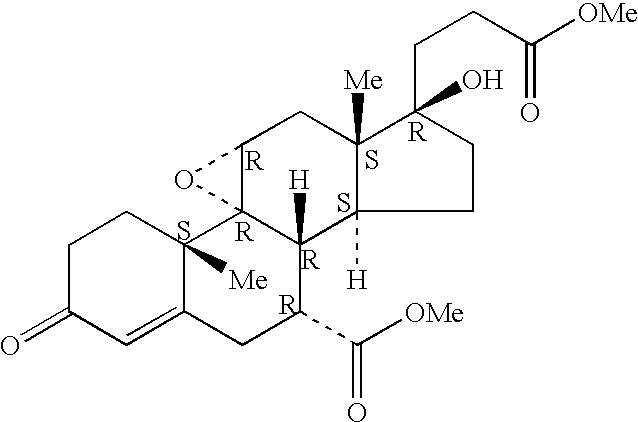
A combination therapy comprising a therapeutically-effective amount of an aldosterone receptor antagonist and a therapeutically-effective amount of an anti-diabetic agent is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred antidiabetic agents are those compounds having high potency and oral or parenteral bioavailability. Preferred aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9α,11α-substituted epoxy moiety.
Owner:PHARMACIA CORP
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of congestive heart failure
InactiveUS20020042405A1Reduce pathogenicityImprove the level ofMetabolism disorderBlood disorderCalcium channel blockerAldosterone
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker verapamil HCl (Benzenacetonitrile, (±)-alpha[3[[2-(3,4-dimethoxyphenyl) ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)hydrochloride) and the aldosterone receptor antagonist epoxymexrenone.
Owner:SCHUH JOSEPH R
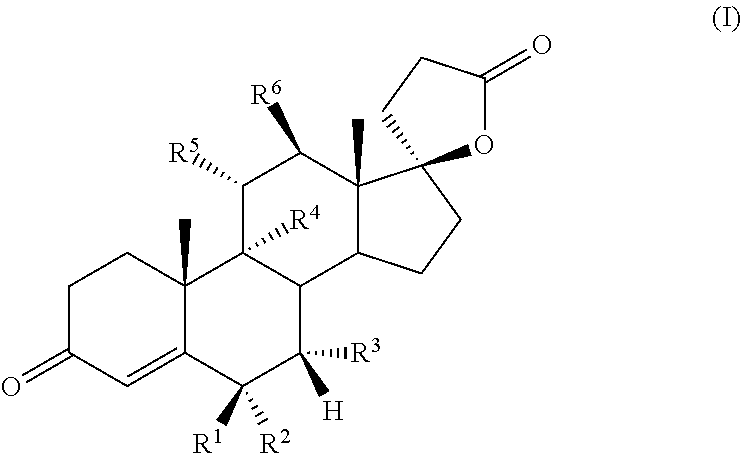
Bismethylene-17A carbolactones and related uses
Described herein are bismethylene-17α carbolactone derivatives having progestational and / or antimineralocorticoid and / or aldosterone antagonistic activity. Also described herein are methods of preparing and using these novel compounds.
Owner:EVESTRA
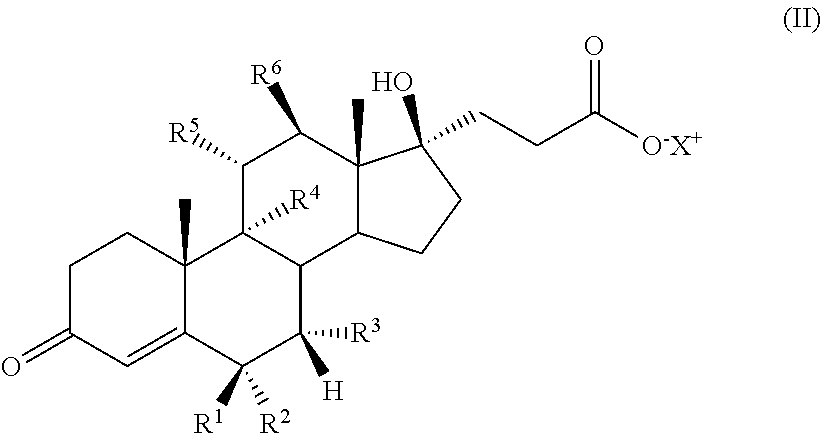
Progestational 3-(6,6-ethylene-17B-hydroxy-3-oxo-17A-pregna-4-ene-17A-yl)propionic acid G-lactones
Owner:EVESTRA
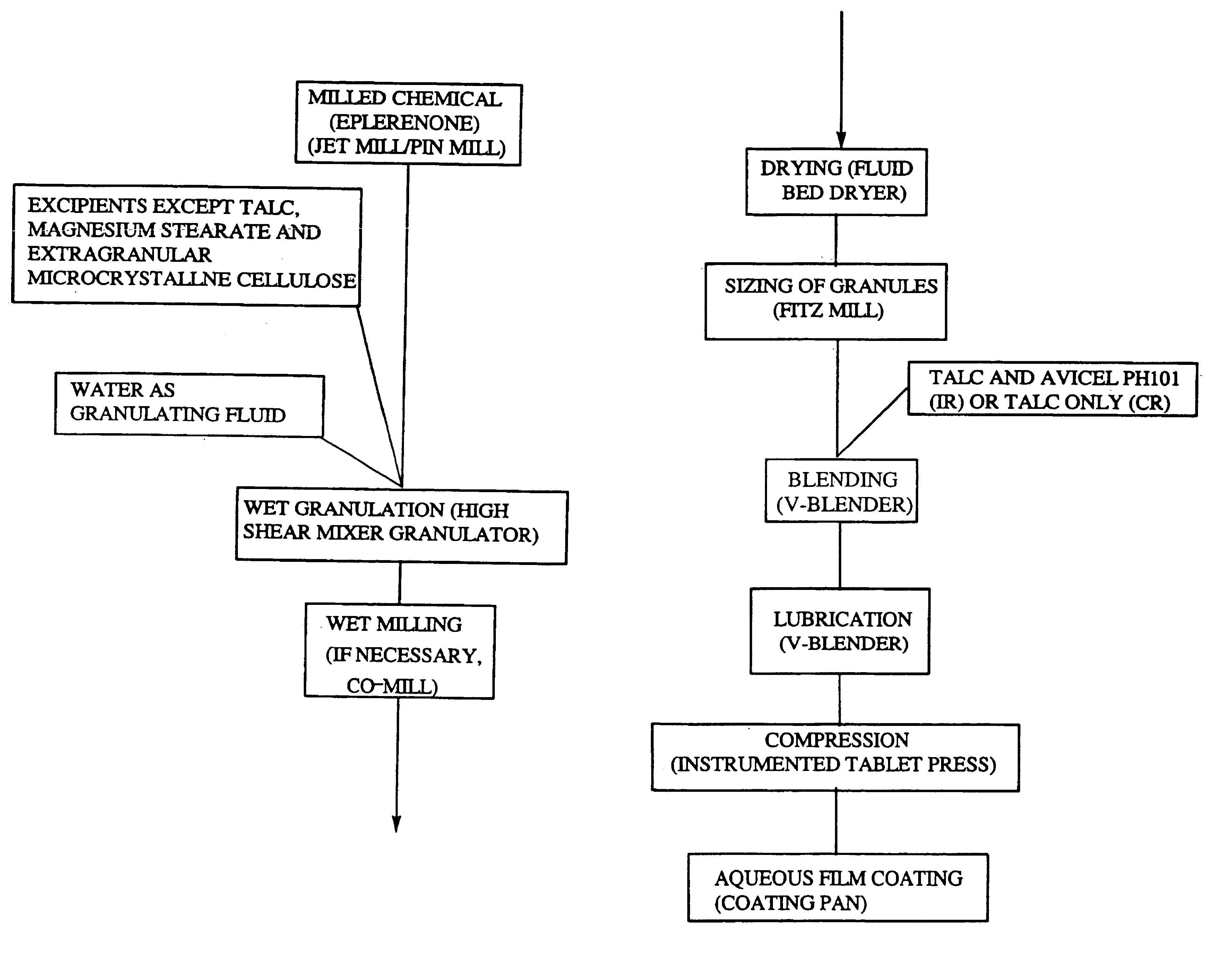
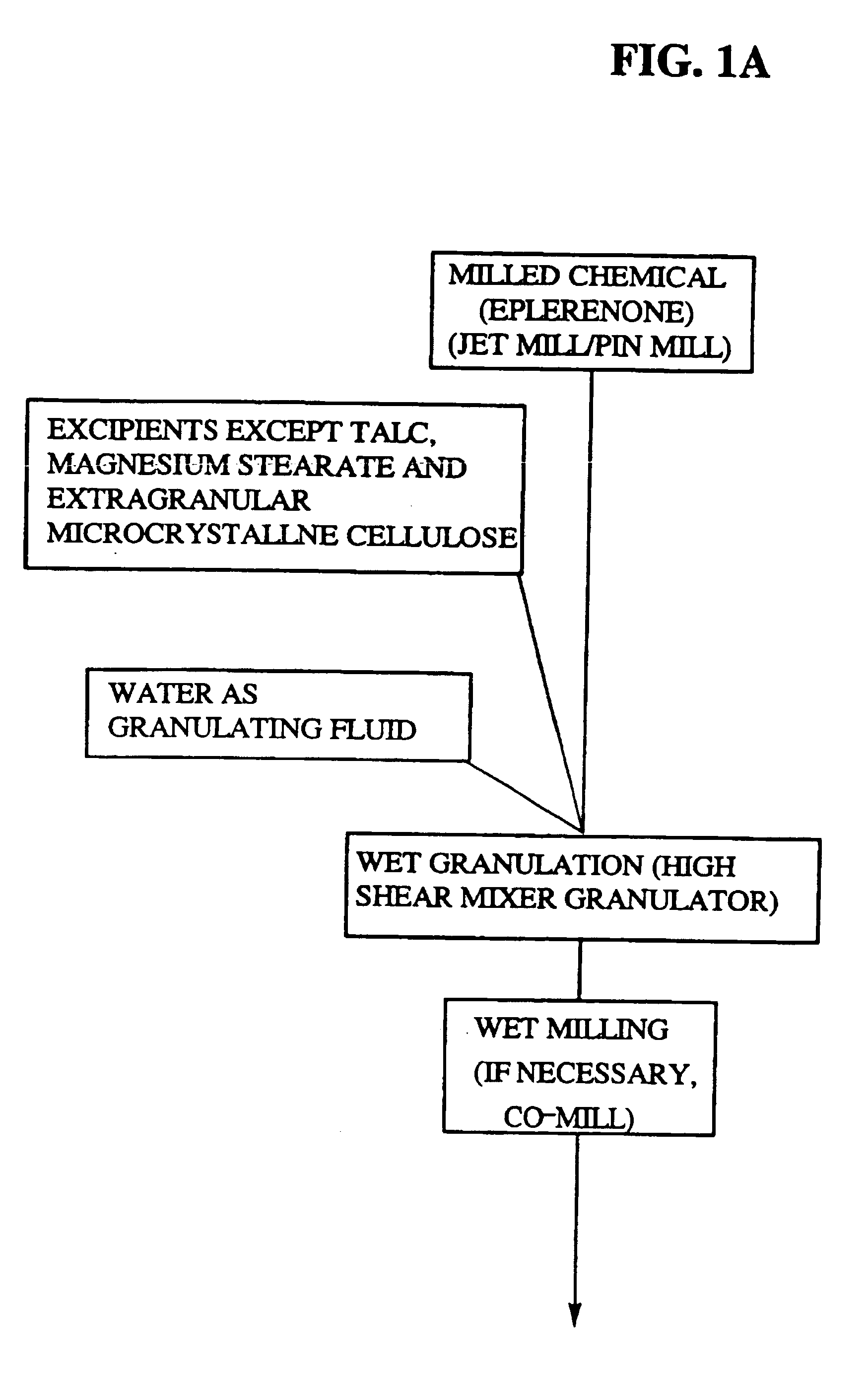
Immediate release eplerenone compositions
The invention relates to oral pharmaceutical compositions useful as aldosterone receptor blockers comprising the active agent micronized eplerenone in an amount of about 10 mg to about 1000 mg and one or more carrier materials.
Owner:GD SEARLE & CO
Use of Vitamin Ds to treat kidney disease
InactiveUS20050148557A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAldosterone escape
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:UNIVERSITY OF CHICAGO
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of congestive heart failure
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 2O-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker verapamil HC1 (Benzenacetonitrile, (±) -alpha[3[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)hydrochloride) and the aldosterone receptor antagonist epoxymexrenone.
Owner:GD SEARLE & CO
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of cardiovascular disorders
InactiveUS20030220312A1Reduce pathogenicityImprove the level ofOrganic active ingredientsCapsule deliveryAnginaCongestive heart failure chf
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, angina and congestive heart failure. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker amlodipine and the aldosterone receptor antagonist eplerenone.
Owner:GD SEARLE & CO
Compositions and treatment of heart deficiency in non-human animals
InactiveUS20100183718A1Reduce morbidityImprove heart functionBiocideDipeptide ingredientsHuman animalPhysiology
The invention relates to novel compositions including an aldosterone antagonist and administered according to a predetermined dosage for treating heart deficiency in non-human mammals.
Owner:CEVA SANTE ANIMALE
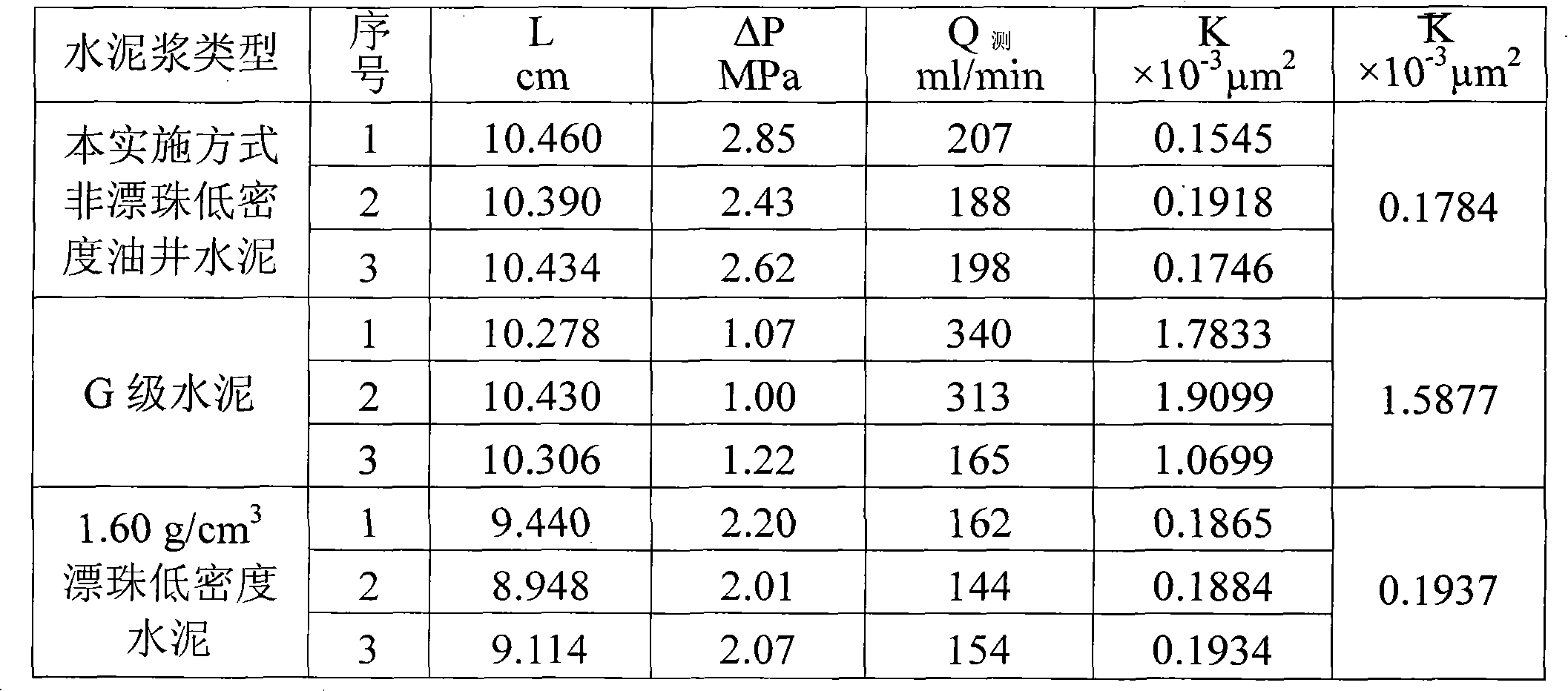
Non-floating bead low-density oil-well cement
The invention relates to low-density oil-well cement, in particular to non-floating bead low-density oil-well cement. The invention solves the problems of low early strength, poor slurry flowability and stability and short service life of the traditional non-floating bead pulverized fuel ash low-density cement. The non-floating bead low-density oil-well cement comprises oil-well cement, pulverized fuel ash, an aldosterone sulfonate condensate dispersing agent, nano silicon ash stone and an early strength agent, and the mass content of SiO2 in the nano silicon ash stone is larger than or equalto 96 percent. The cement has good stability, good compactness, high early strength, long service life, good rheological property and controllable densification time and meets the requirement of a low-pressure stratum oil field cementation well on the performance of the cement.
Owner:哈尔滨太行兴隆水泥有限公司
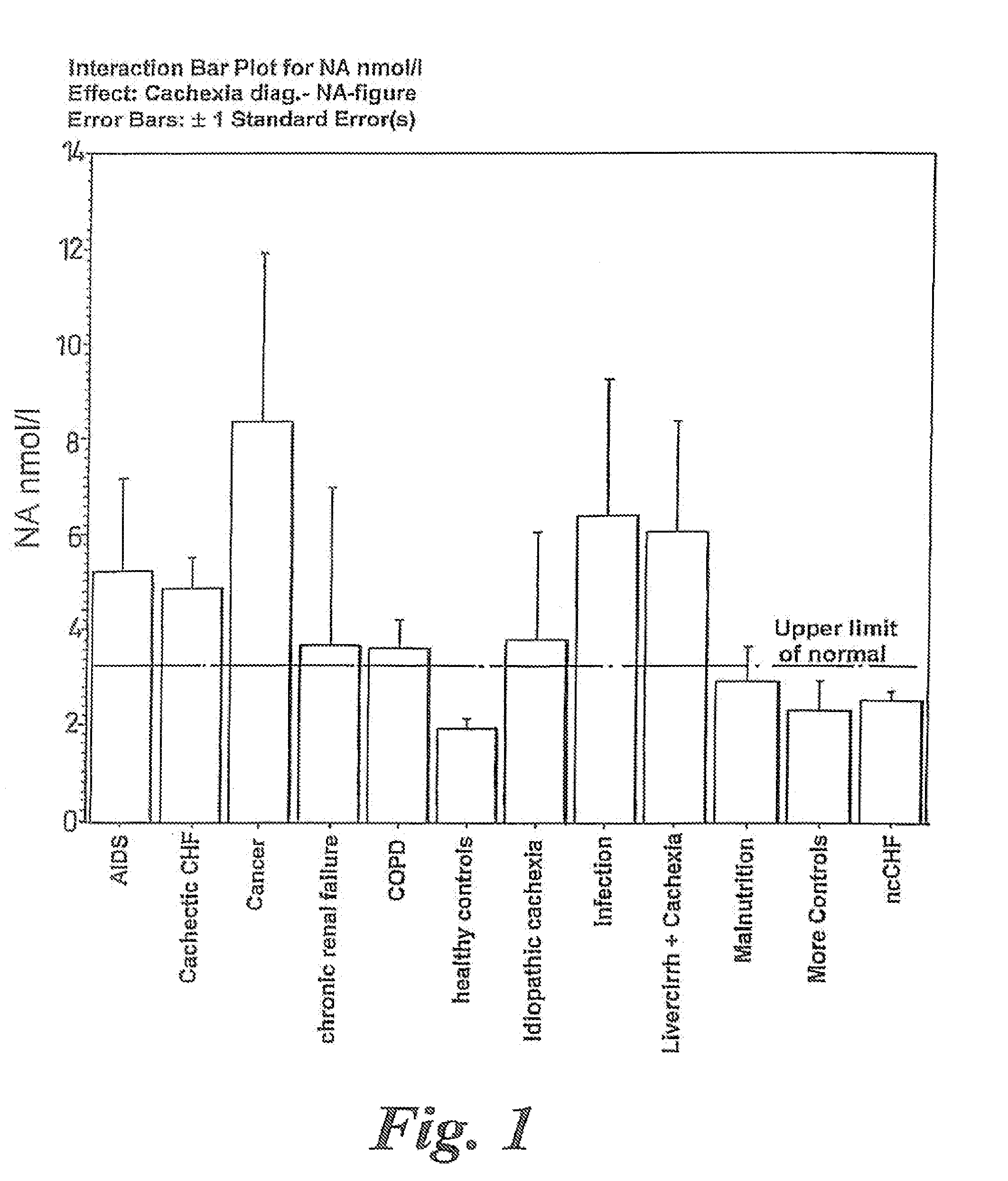
Methods of treating cachexia
InactiveUS7417038B1Reduced metabolic rateIncrease blood flowPeptide/protein ingredientsMetabolism disorderDiseaseImidazoline receptor
A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the 10 method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a 13 receptor blocker; an imidazoline receptor antagonist; a centrally acting tx receptor antagonist; a peripherally acting ct receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduce SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
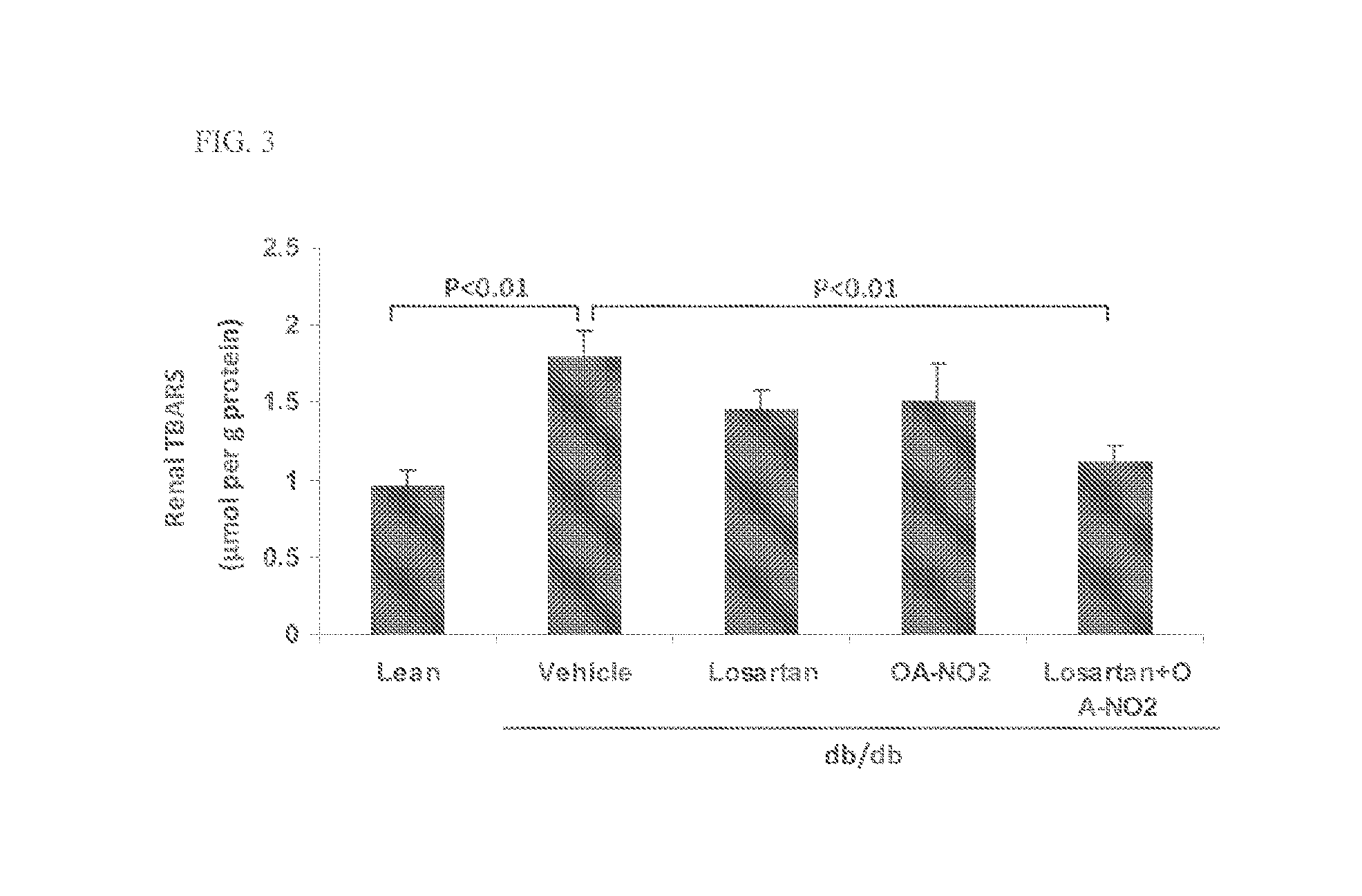
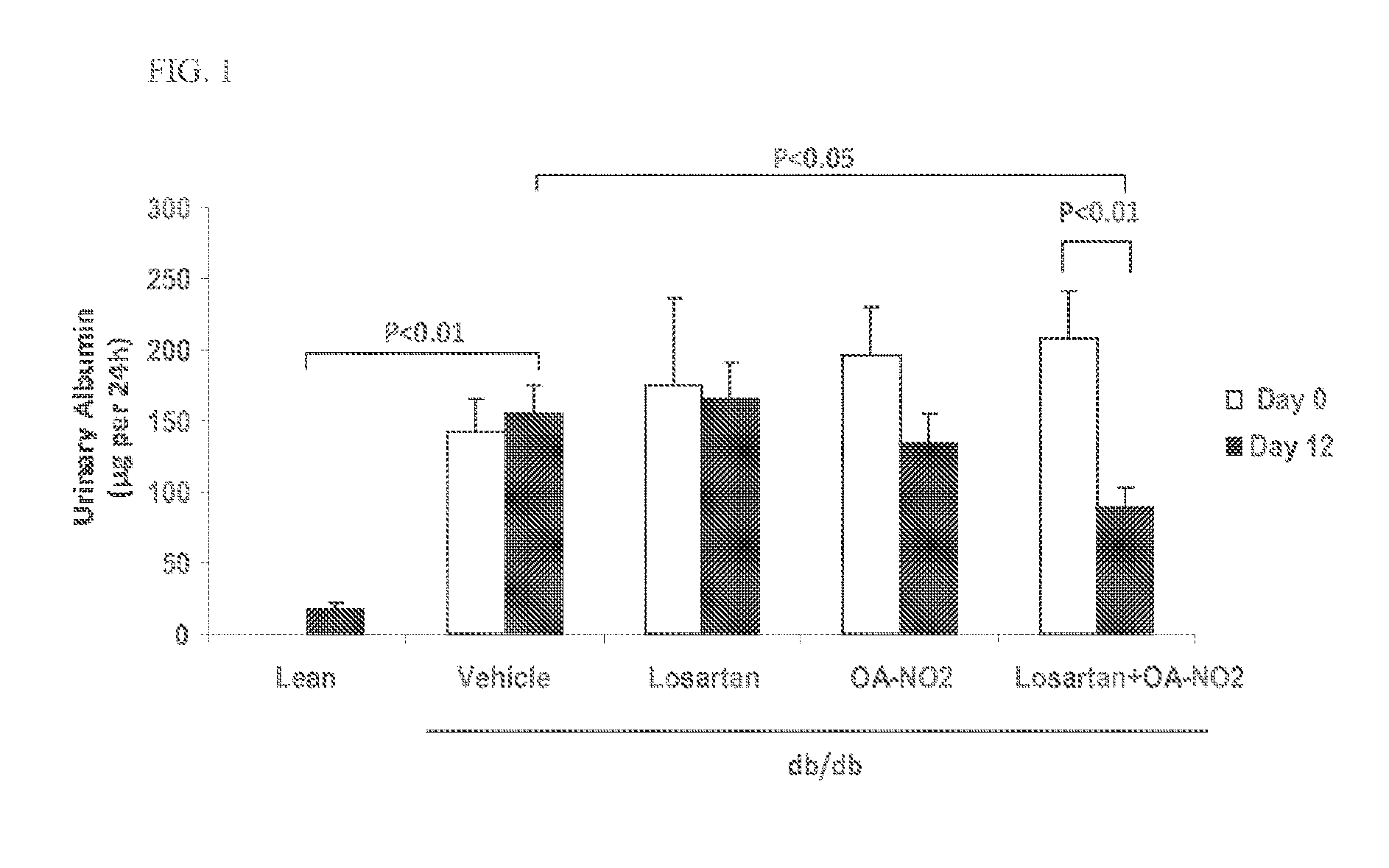
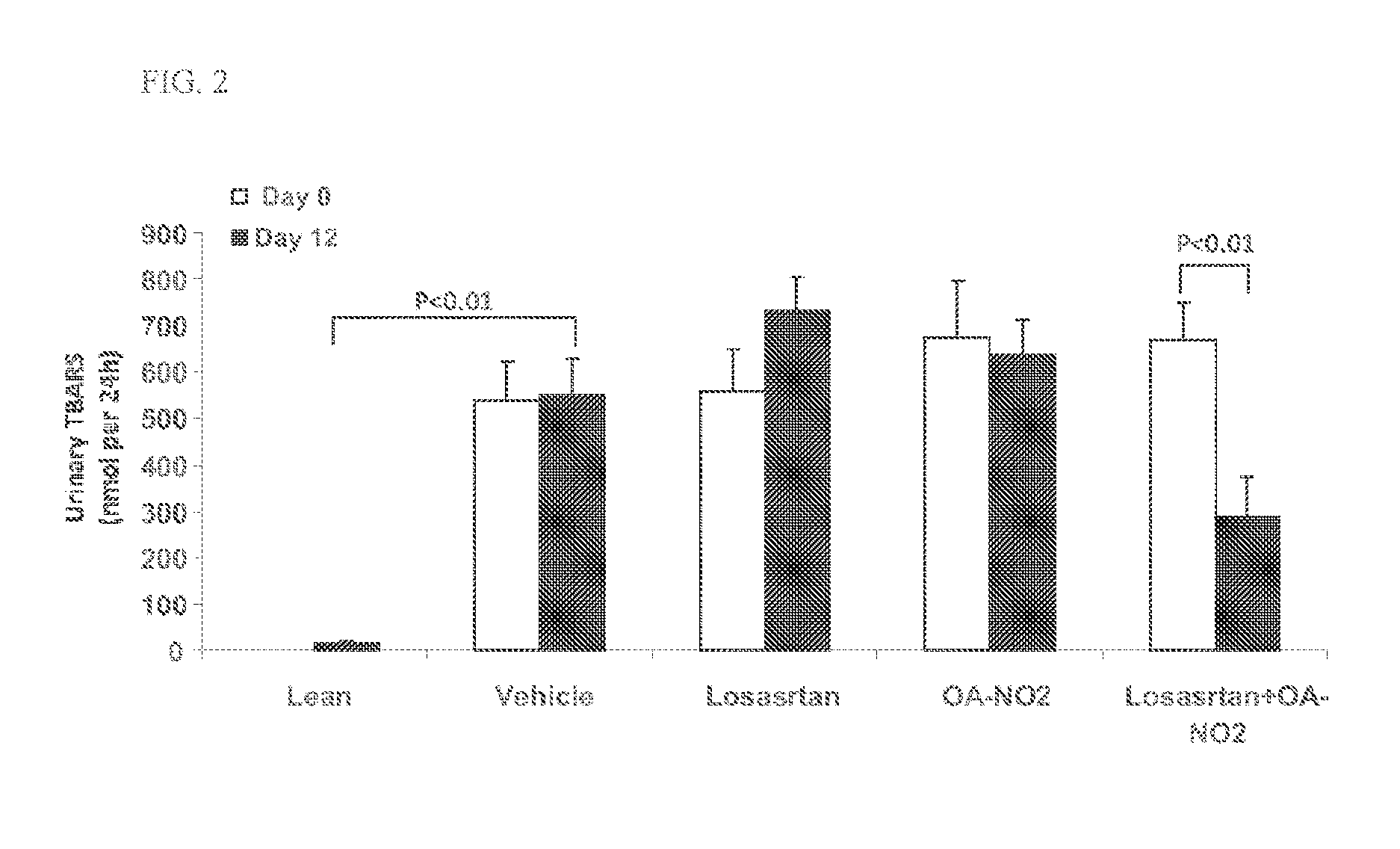
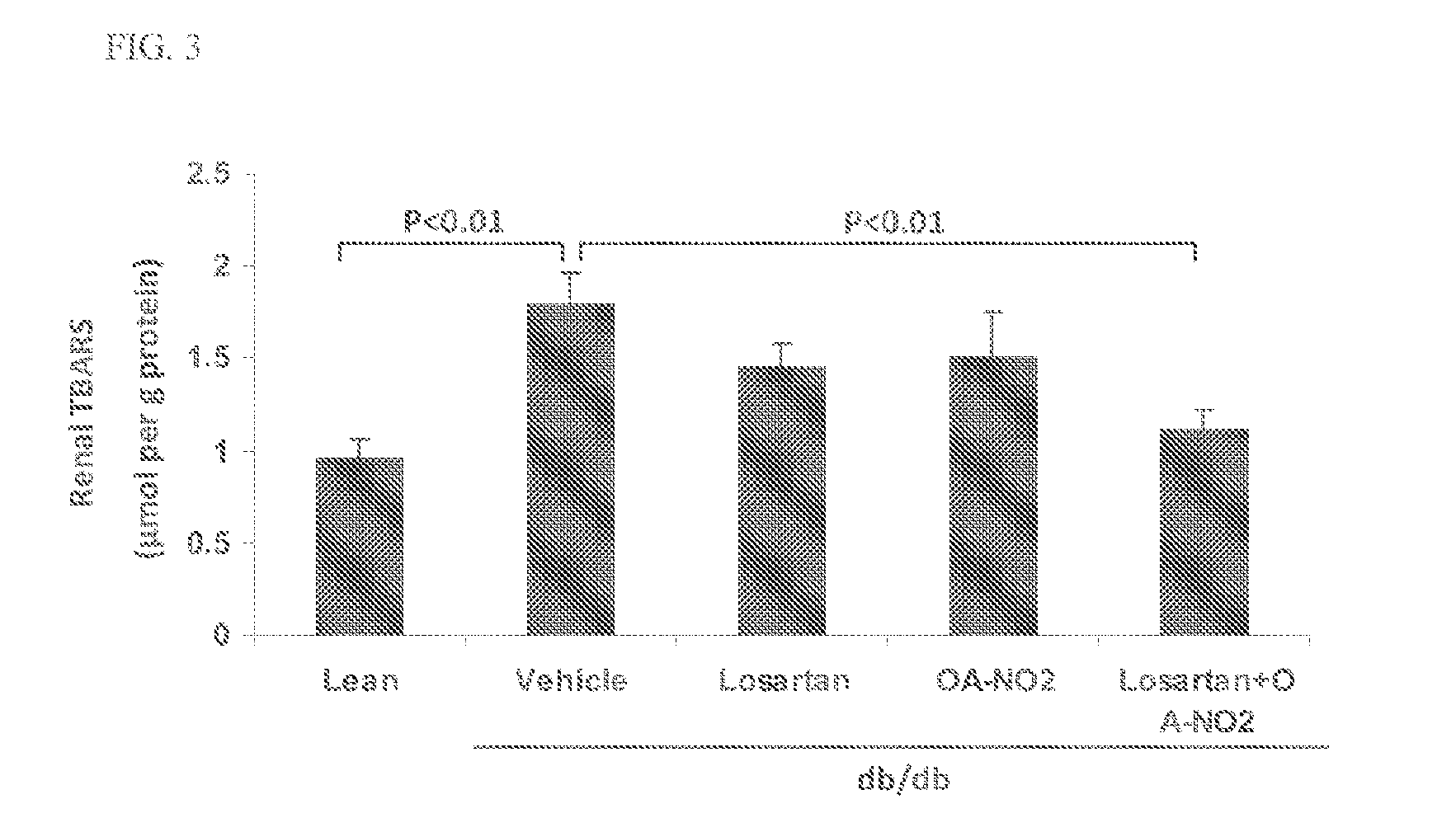
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS9192600B2Urinary disorderAnhydride/acid/halide active ingredientsNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Medicine composition application for preparing hypertension medicine
An application of an invented medicinal composition in preparing the medicines for treating primary hypertension and protecting heart and kidney is disclosed.
Owner:TIANJIN TASLY PHARMA CO LTD
Tgf-beta antagonists combined with renin-angiotensin-aldosteron-system antagonists for treating renal insufficiency
InactiveUS20060286105A1Loss of renal functionPrevent and reduce riskBiocidePeptide/protein ingredientsRenal disorderFibrosis
The disclosure provides methods for treating, preventing, and reducing risk of occurrence of renal insufficiency in mammals. The disclosed methods include administering to a subject susceptible to, or afflicted with, renal disorder therapeutically effective amounts of a TGF-β antagonist and a renin-angiotensin-aldosterone system (RAAS) antagonist so as to maintain desirable levels of renal function. The populations treated by the methods of the invention include but are not limited to patients suffering or at risk for the development of renal insufficiency, such as those afflicted with type I or type II diabetes, hypertension, (auto)immune disease, systemic fibrosis, etc.
Owner:LEDBETTER STEVEN +2
Implantable medical device for drug delivery and method of use
An apparatus and method of preparing a cardiac harness for use in controllably delivering a medicament such as an mTOR inhibitor or aldosterone blockade from the surface of the cardiac harness to the epicardium of a patient's heart for the site-specific treatment of cardiac and non-cardiac maladies. The delivery of the medicament from the cardiac harness is achieved by: coating the medicament on the surface of the cardiac harness; coating the cardiac harness with a polymer material or dielectric material and impregnating the coating with the medicament; or by loading the medicament into an implant attached to the cardiac harness.
Owner:PARACOR MEDICAL
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for aldosterone, and preparation method of kit
InactiveCN102998442AAnalytical sensitivity is not highStrong specificityChemiluminescene/bioluminescenceAntiendomysial antibodiesHorse radish peroxidase
The invention discloses a quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for aldosterone. The kit comprises aldosterone calibrators, magnetic particle suspension coupled with streptavidin, an aldosterone antibody labeled with biotin, an aldosterone enzyme combination, an aldosterone quality controller, chemiluminescence liquor A, chemiluminescence liquor B, 20 times concentrated washing liquor, and a reaction tube, wherein enzyme adopted by the aldosterone enzyme combination is horse radish peroxidase with the purity RZ being more than or equal to 3.0 and the activity being more than or equal to 250U / ml. The invention also discloses a preparation method of the kit. Compared with the conventional kit, the quantitative detection kit is simple and convenient to operate, is safe, does not cause environment pollution, and also has the advantages of wide concentration range, low cost, good stability and the like of detection samples.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Biaryl amide derivative or pharmaceutically acceptable salt thereof
InactiveUS20130116227A1High binding affinityEliminate side effectsBiocideOrganic chemistryReceptorAcyl group
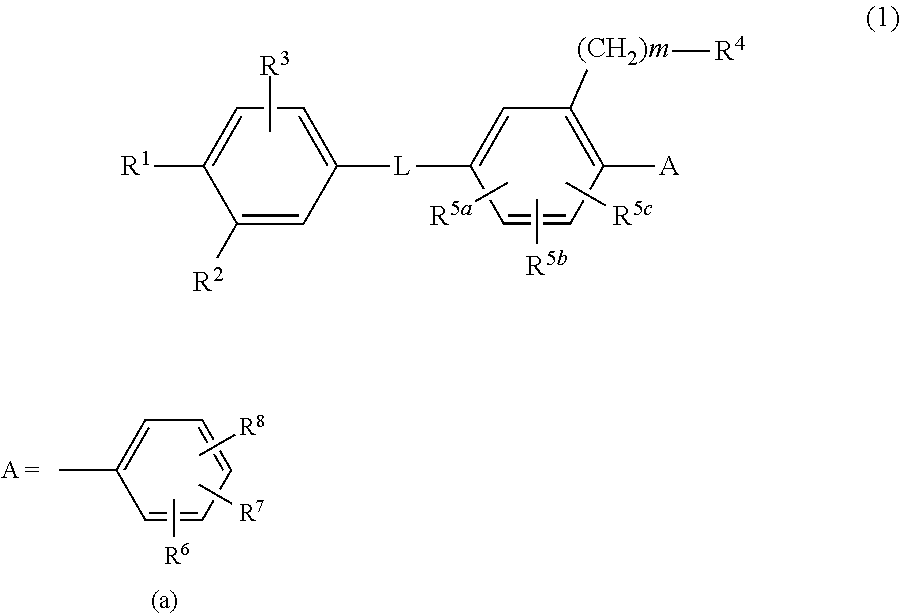
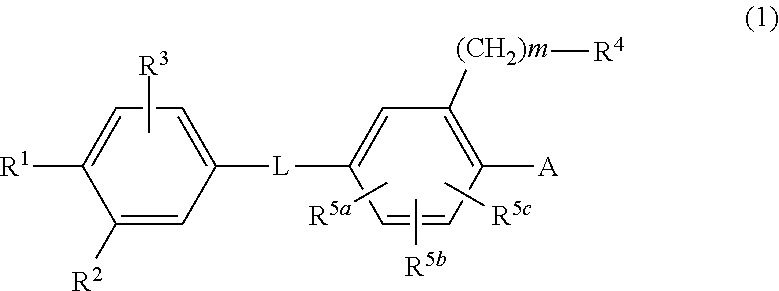
Disclosed is a novel biaryl amide derivative represented by formula (1) and having an affinity for the aldosterone receptor; also disclosed is a pharmaceutically acceptable salt thereof. (In the formula, A is any of the groups represented by formula (a); L is —CONH—, etc.; R1 is a substitutable aminosulfonyl group, etc.; R2 is a hydrogen atom, etc.; R3 is a hydrogen atom, etc.; R4 is a hydrogen atom, a halogen atom, hydroxy group, a substitutable amino group, a substitutable C1-6 alkoxy group, a substitutable 4- to 7-membered cyclic amino group, etc.; R5a, R5b and R5c are each independently hydrogen atoms, etc.; R6 is a halogen atom, a cyano group, etc.; R7 and R8 are each independently a hydrogen atom, etc.; and m is an integer such as 0.)
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
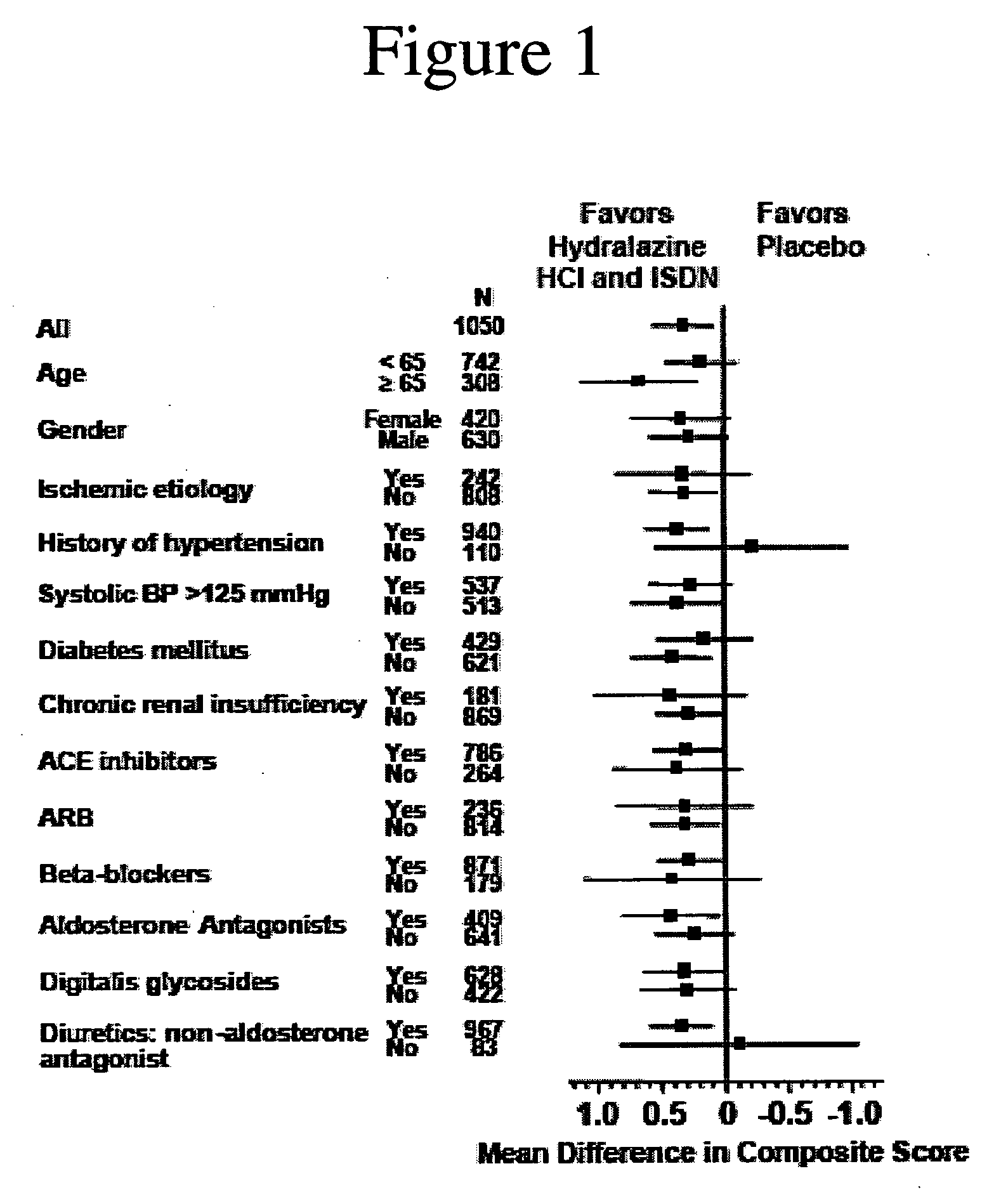
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
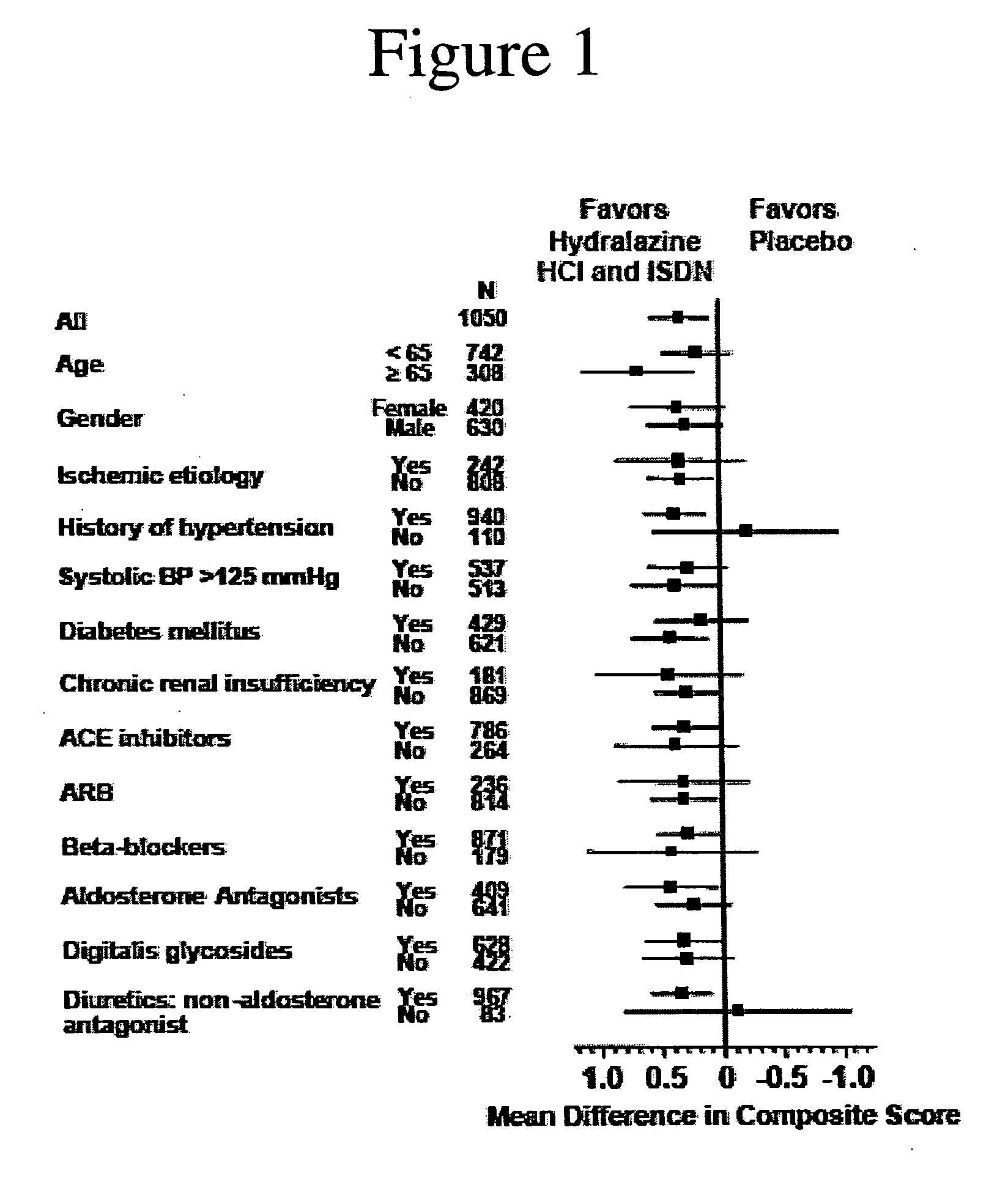
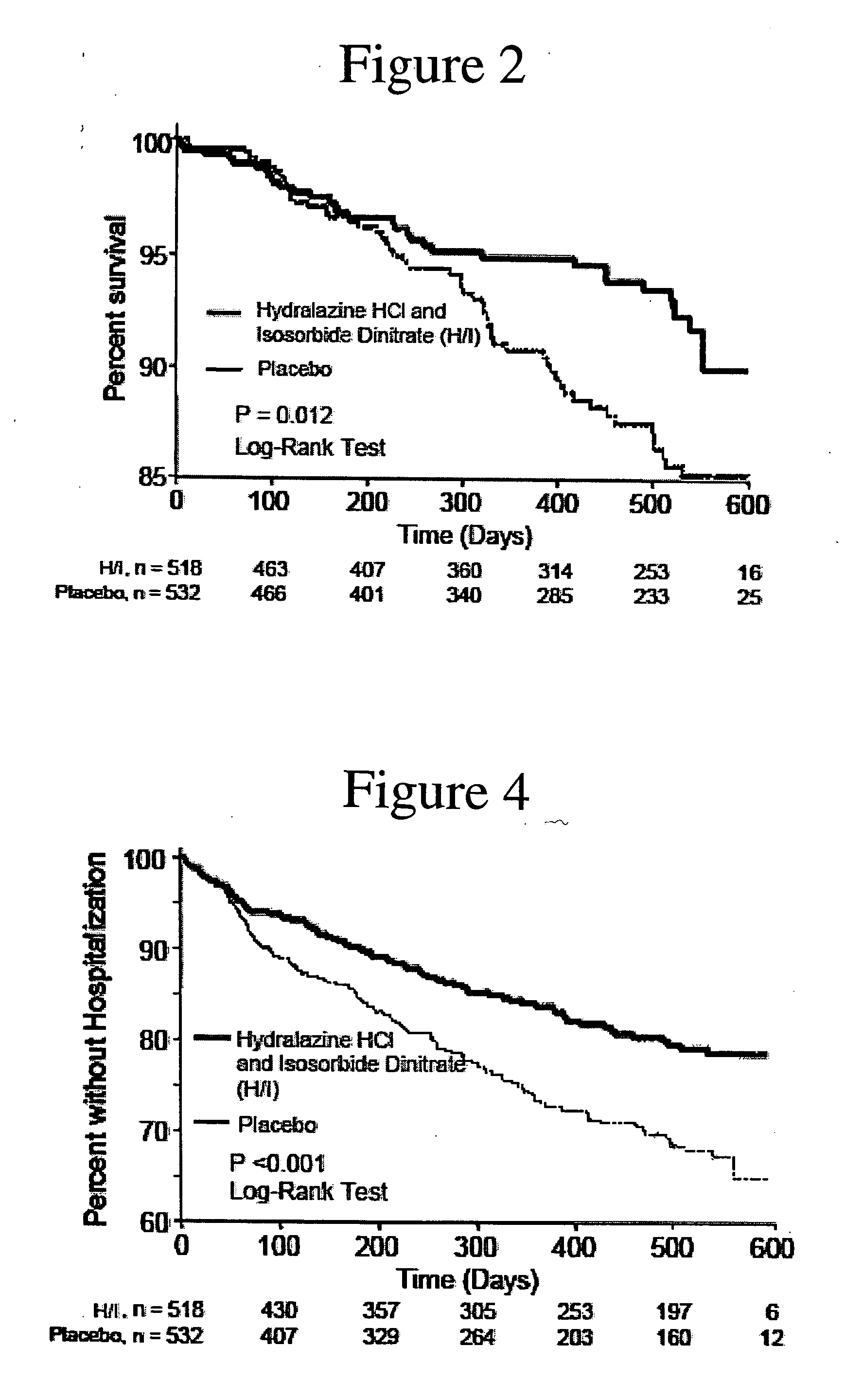
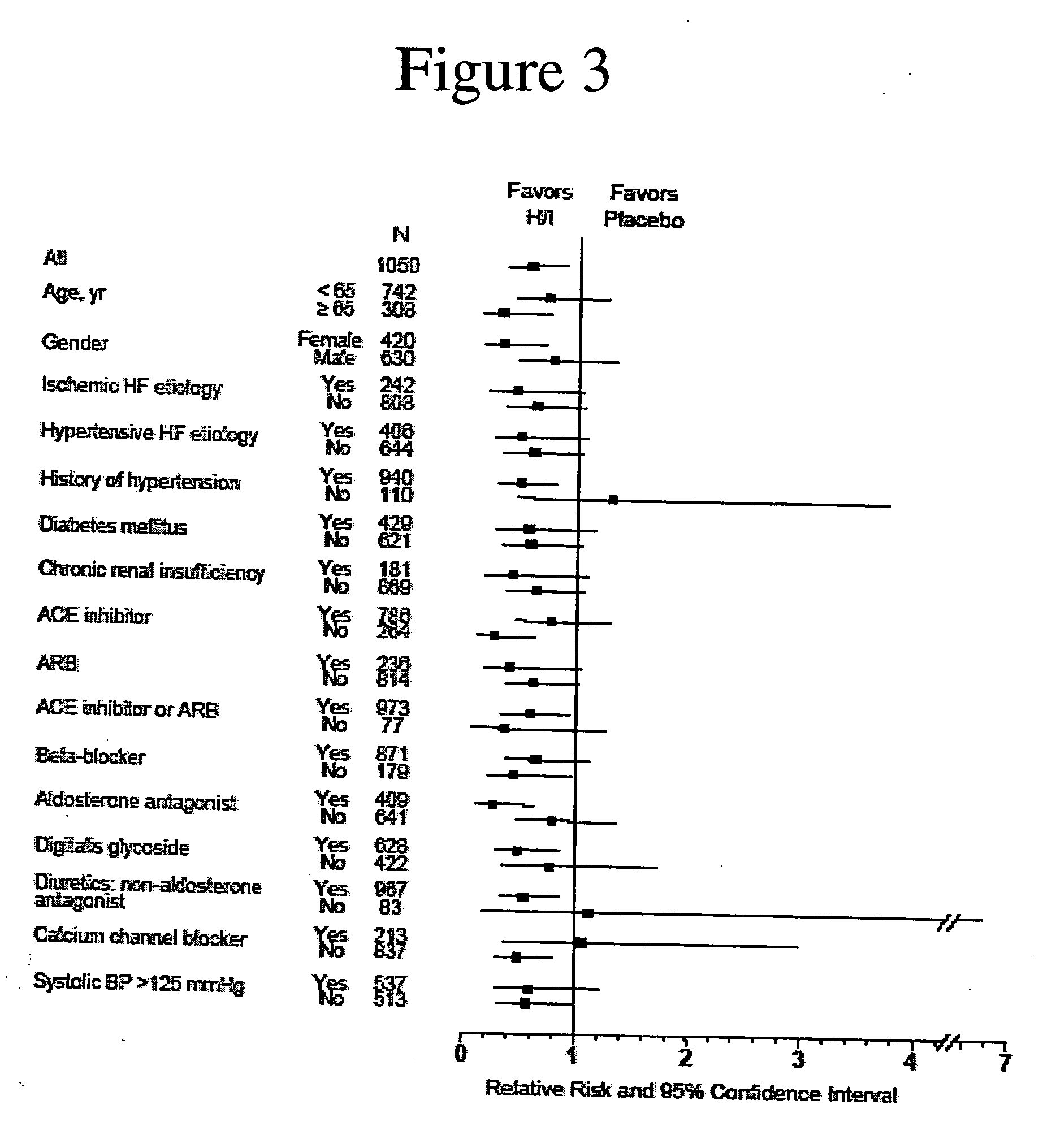
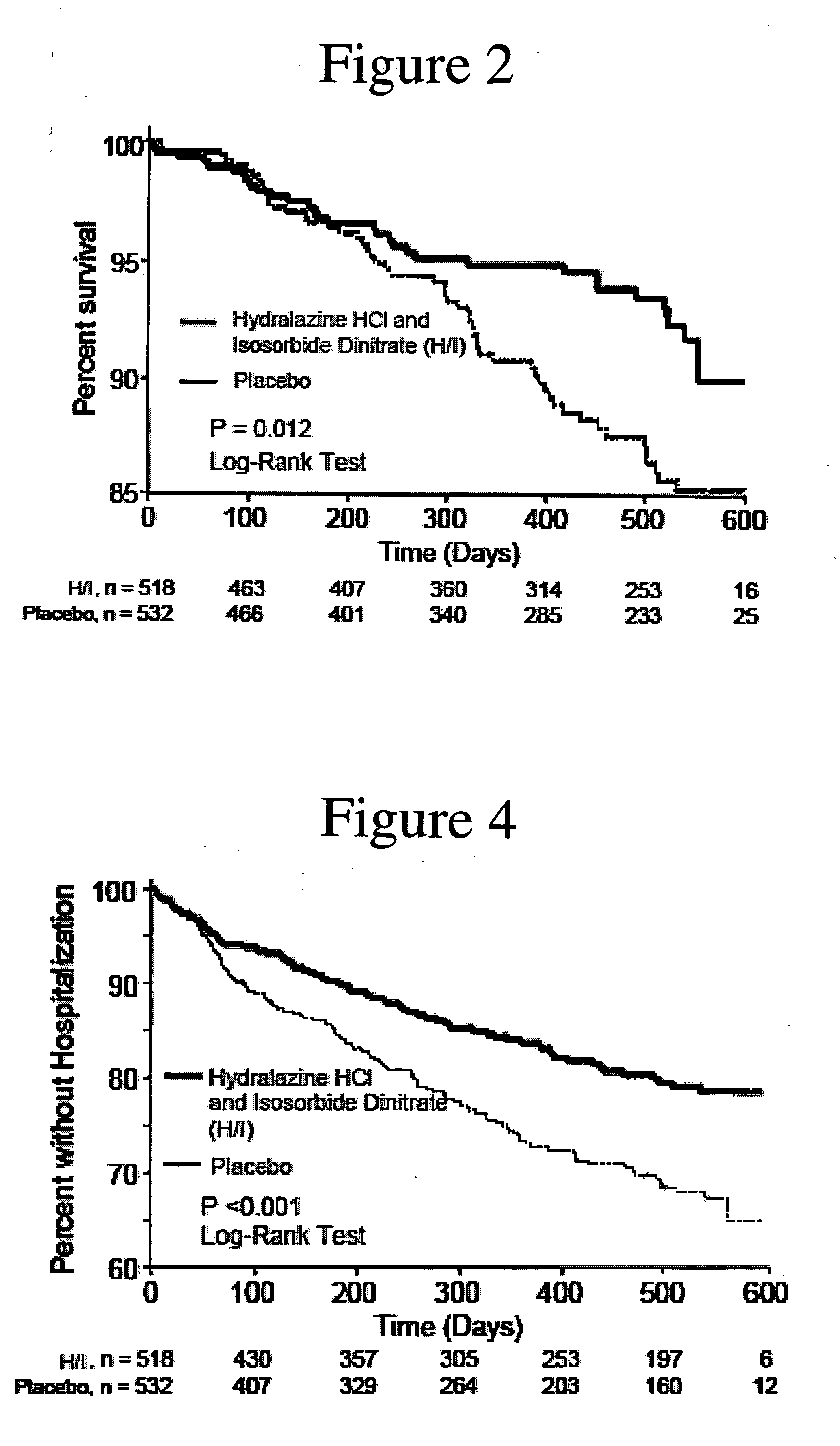
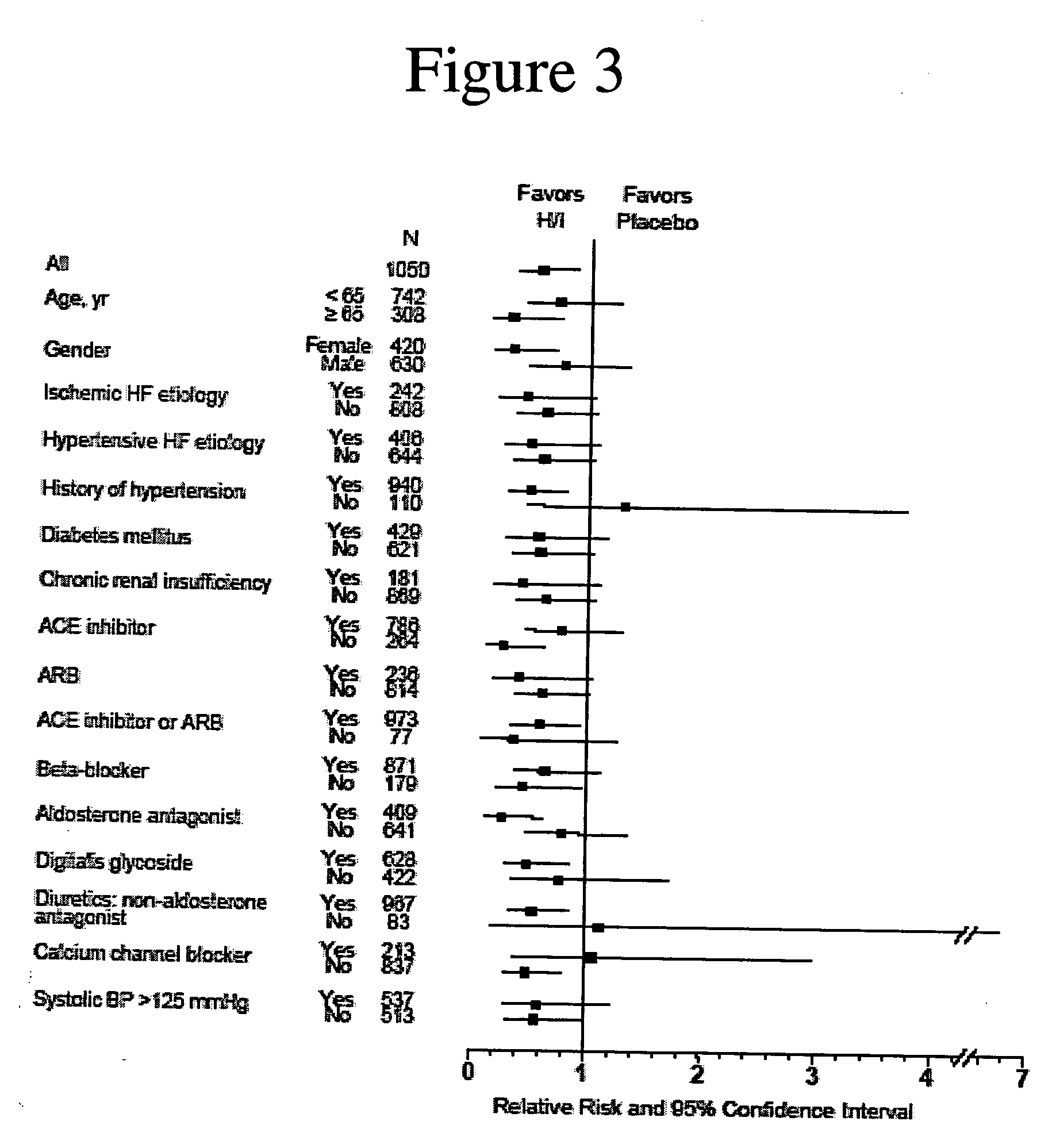
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
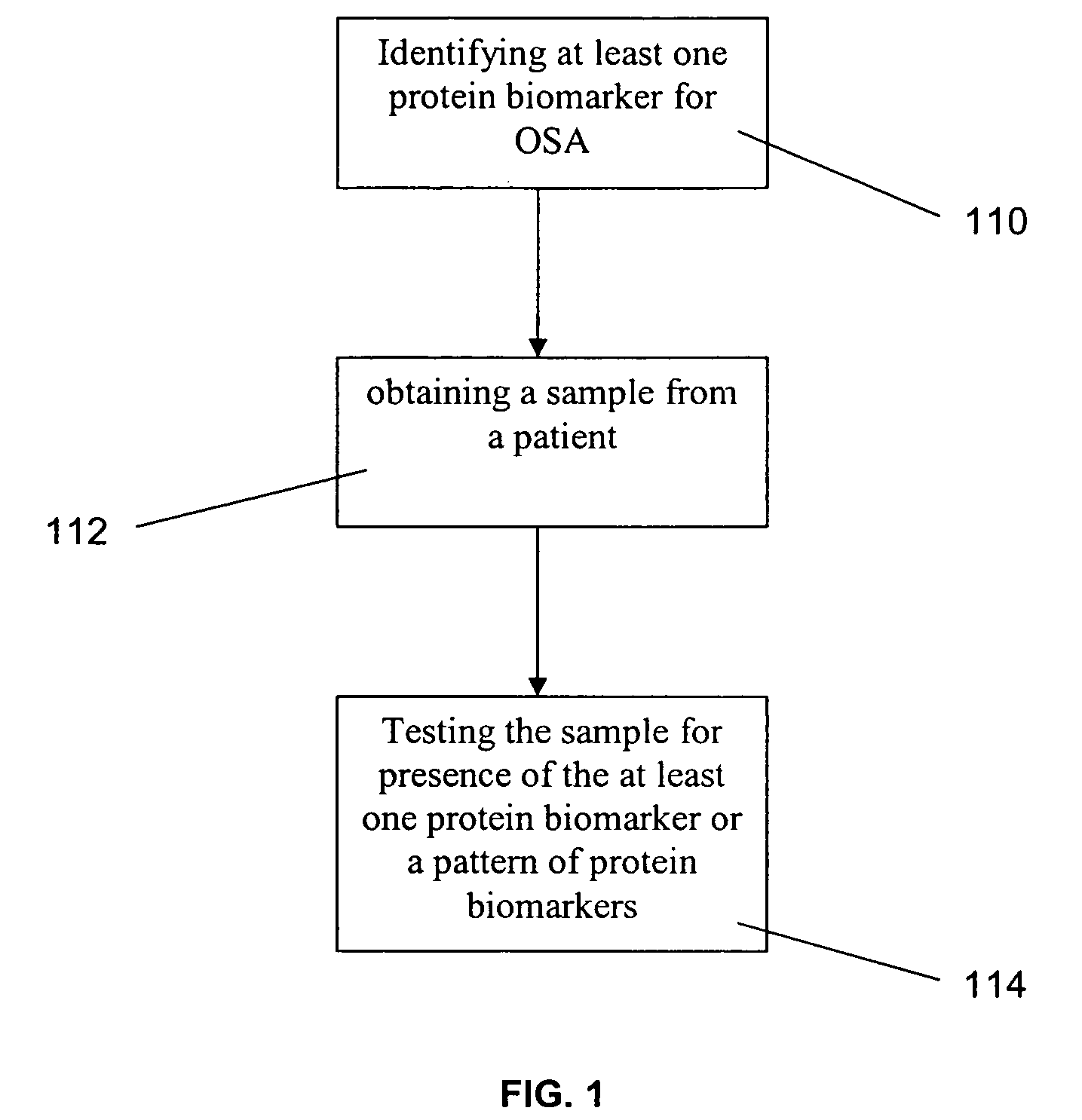
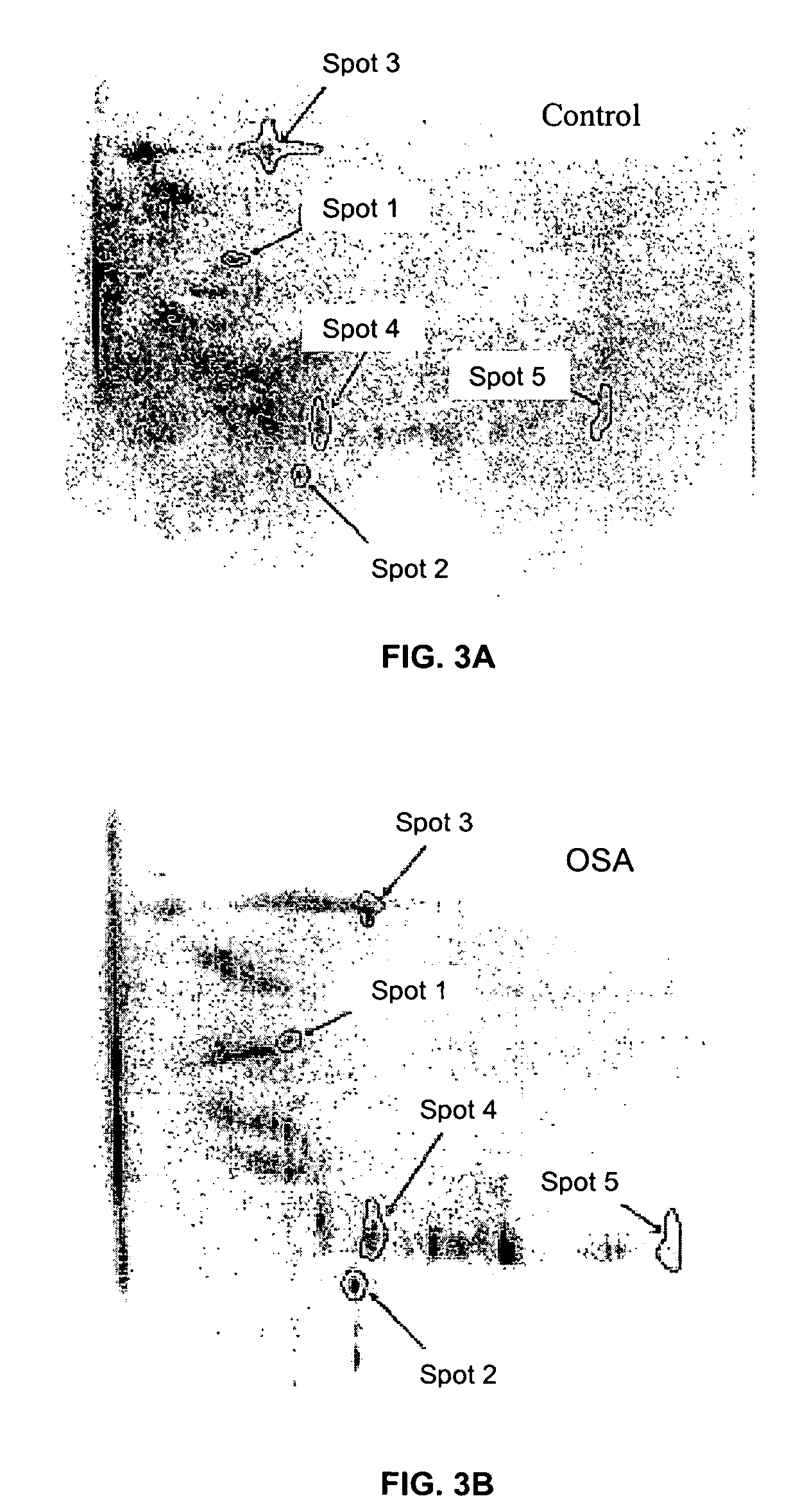
Method for diagnosing obstructive sleep apnea
A method for diagnosing obstructive sleep apnea in a patient comprises identifying at least one protein biomarker for obstructive sleep apnea; obtaining a sample from the patient; and testing the sample for presence of the at least one protein biomarker. The protein biomarkers may include alpha-1 B-glycoprotein; kallikrein, laminin, aldosterone-binding protein and / or urocortin-2 precursor. The presence of the protein biomarkers may be detected using antibodies. These antibodies may be provided in an array for detecting the presence of the at least one protein biomarker or a pattern of protein biomarkers.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS20140243380A1Reducing mRNA expressionHigh expressionBiocideUrinary disorderNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Treatment of sleep apnea with a combination of a carbonic anhydrase inhibitor and an aldosterone antagonist
InactiveUS20160045527A1Good for weight lossAlleviates sleep apneaBiocidePharmaceutical delivery mechanismCarbonic anhydrase IIPharmaceutical formulation
This invention relates generally to methods and pharmaceutical formulations useful in treating patients suffering from sleep apnea, including obstructive sleep apnea syndrome (OSAS). Treatment is effected by administering a carbonic anhydrase inhibitor to the patient in combination with an aldosterone antagonist. Formulations containing a therapeutically effective amount of a carbonic anhydrase inhibitor and a therapeutically effective amount of an aldosterone antagonist are provided as well.
Owner:VIVUS
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
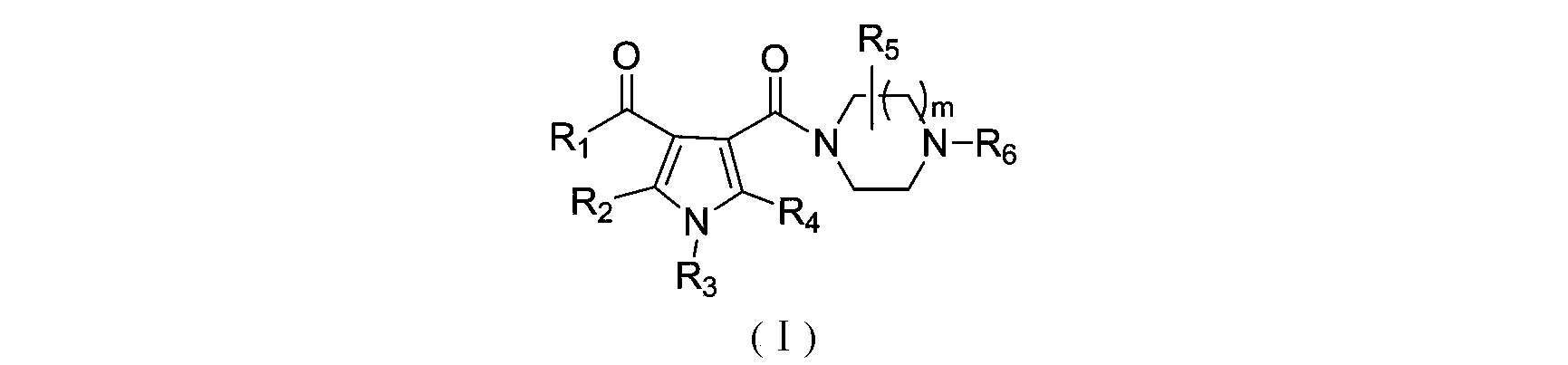
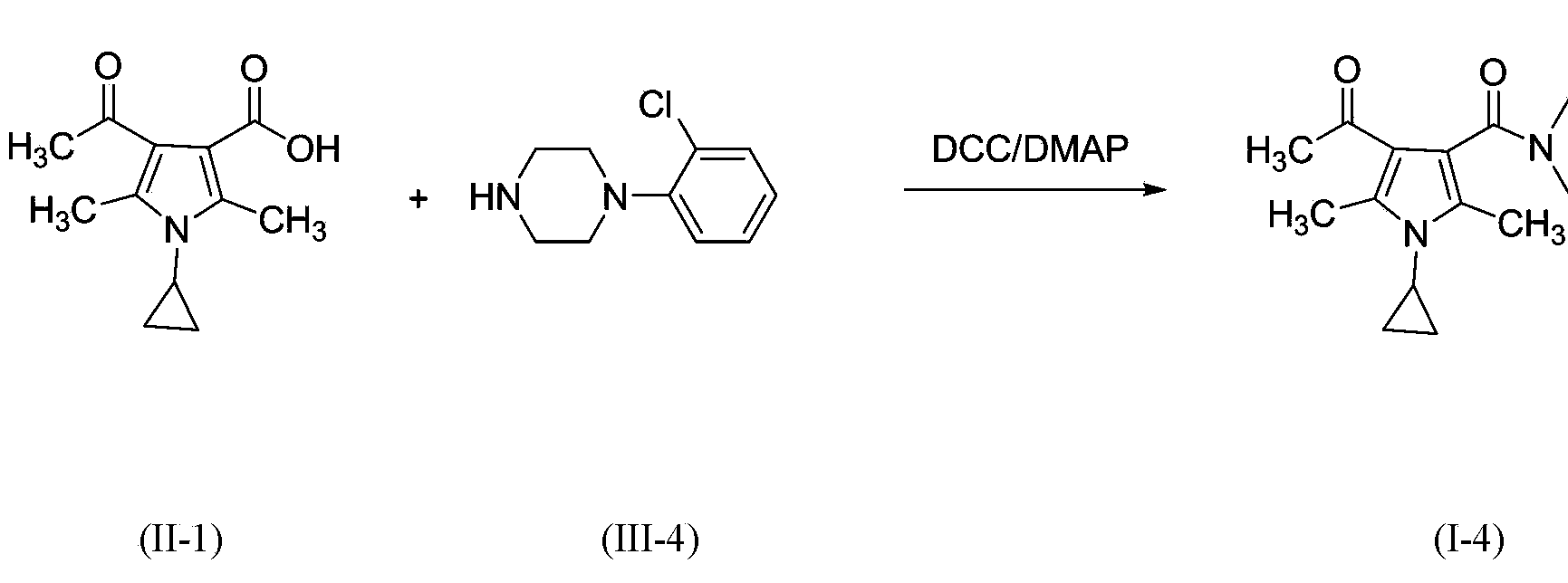
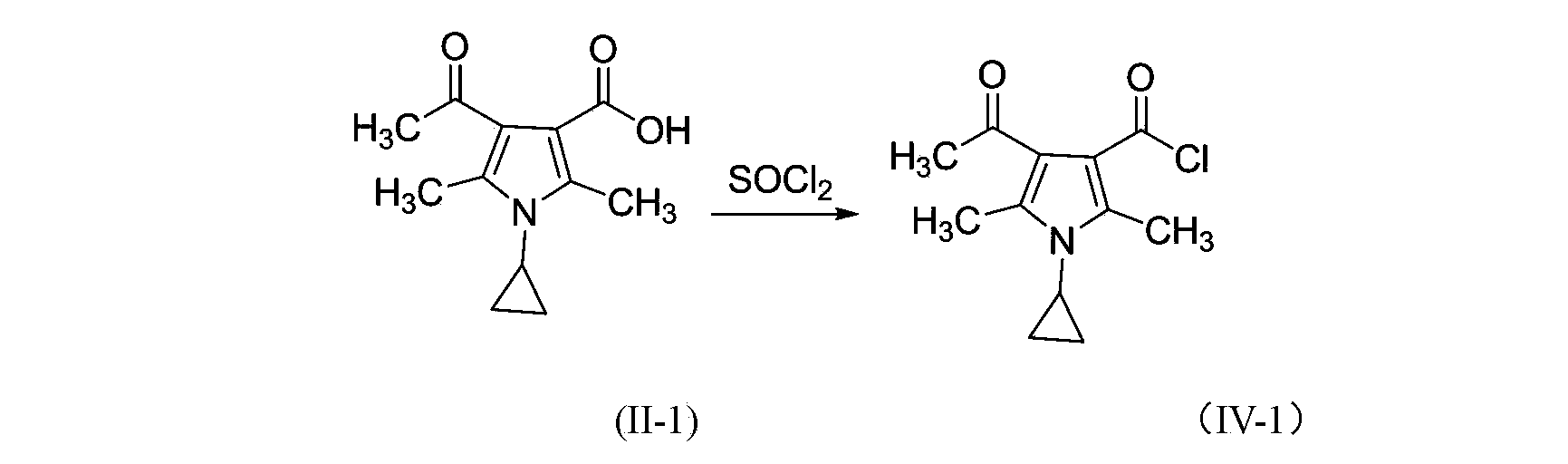
3-pyrrole carboxylic acid derivatives, and preparing method and application thereof
InactiveCN103420890AHas receptor antagonistic effectReceptor antagonismOrganic active ingredientsNervous disorderArginineAcid derivative
The invention relates to 3-pyrrole carboxylic acid derivatives, and a preparing method and an application thereof, and particularly relates to the 3-pyrrole carboxylic acid derivatives having a structure represented by formula I and pharmaceutically acceptable salts thereof and the preparing method of the derivatives, pharmaceutical compositions with the 3-pyrrole carboxylic acid derivatives having the structure represented by the formula I and the pharmaceutically acceptable salts of the 3-pyrrole carboxylic acid derivatives as active ingredients, and applications of the pharmaceutical compositions in prevention or treatment of diseases related with an arginine vasopressin V1a receptor, an arginine vasopressin V1b receptor, an arginine vasopressin V2 receptor, a sympathetic nervous system or a renin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
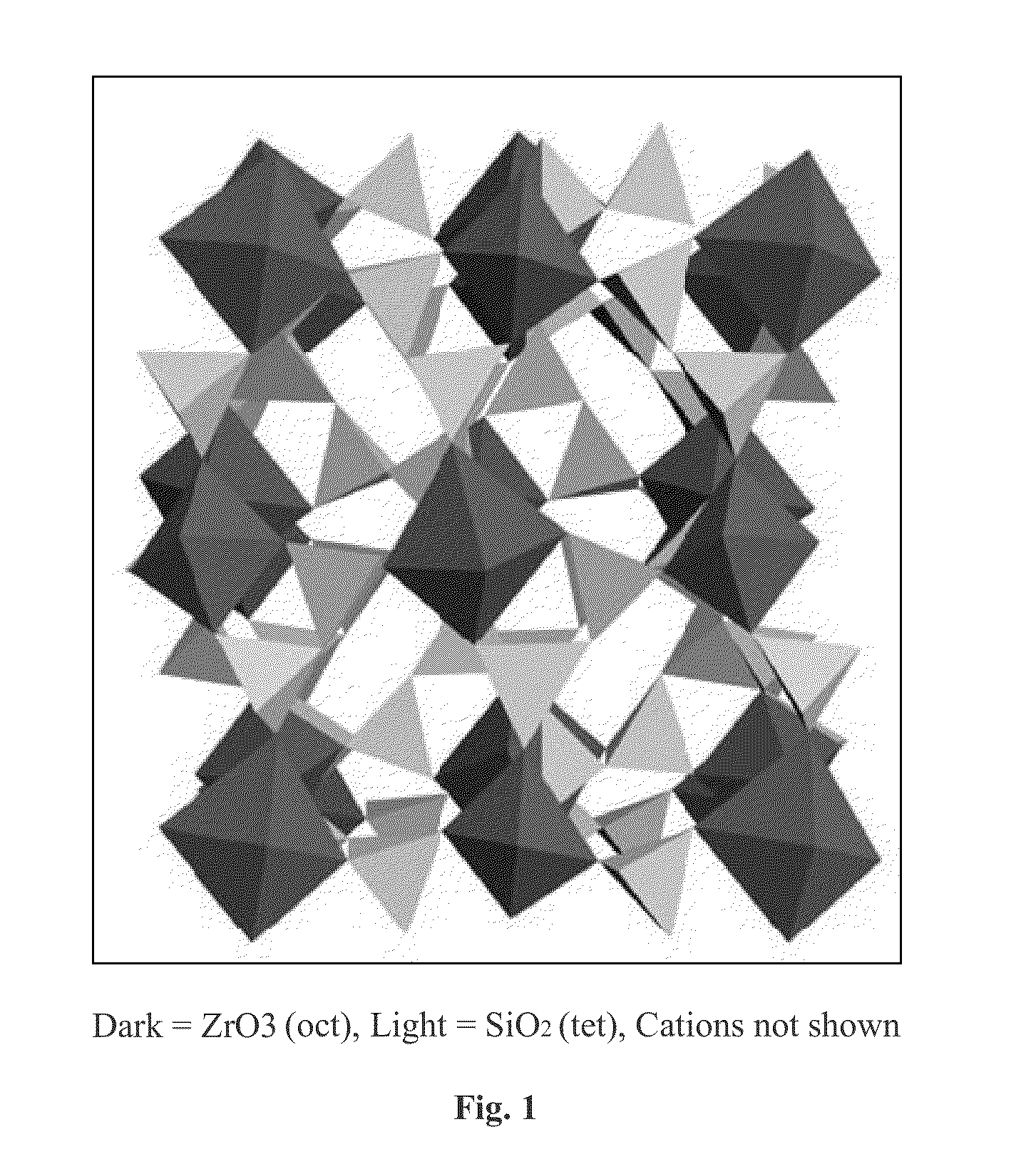
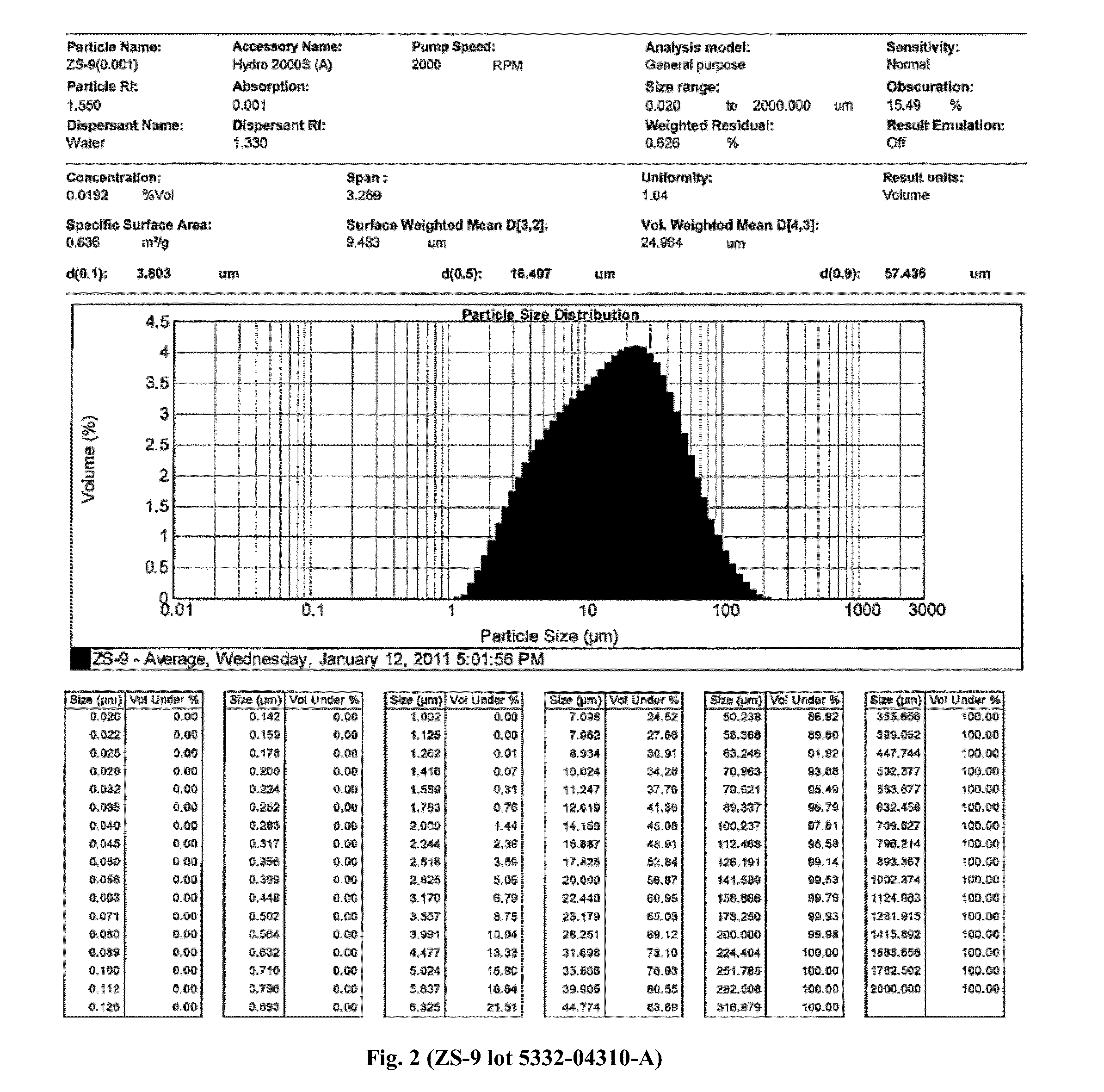
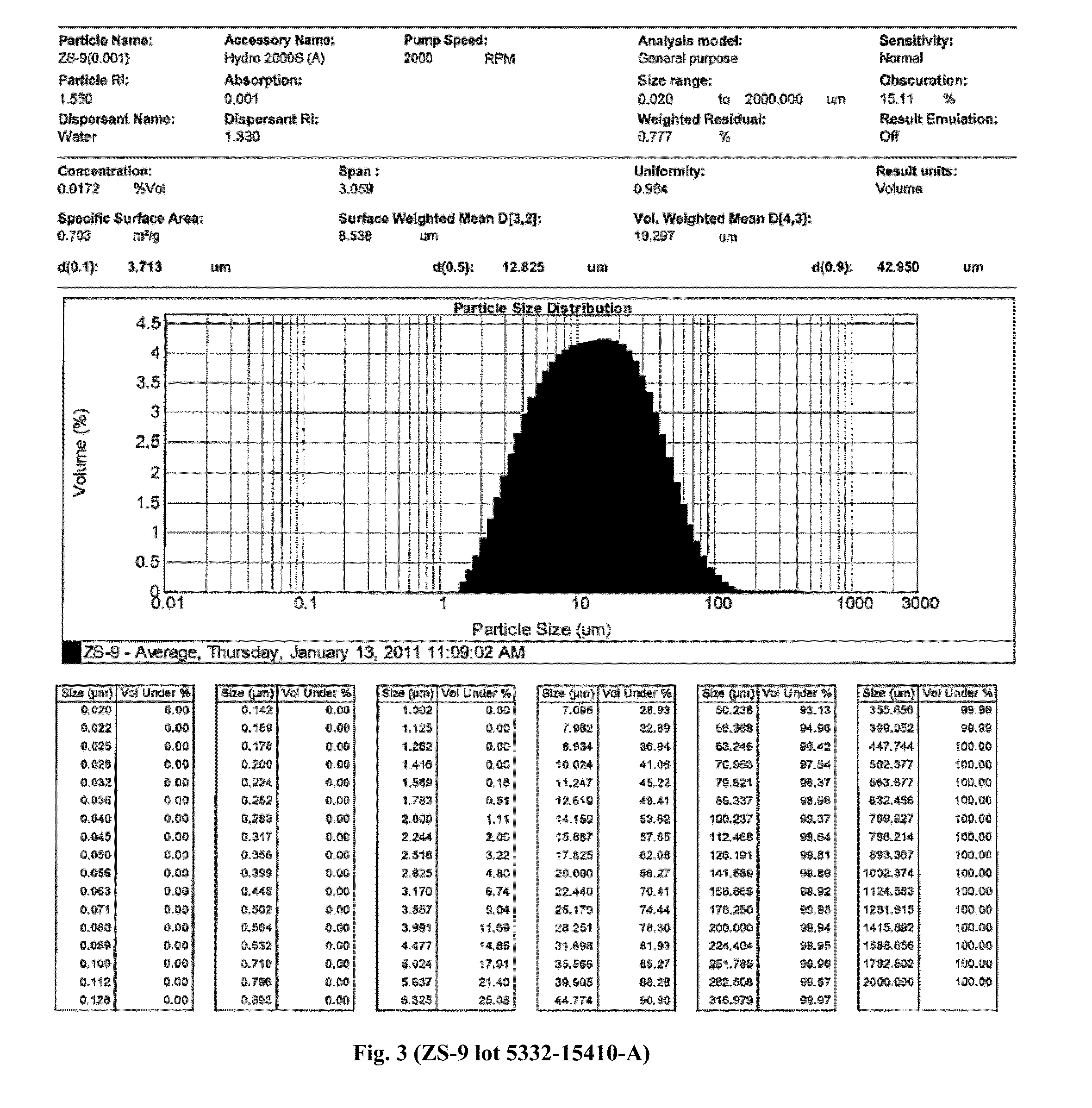
Microporous zirconium silicate and diuretics for the reduction of potassium and treatment of chronic kidney and/or chronic heart disease
The present invention relates to novel methods of using microporous zirconium silicate to reduce the risk of hyperkalemia and to lower aldosterone levels in the treatment of chronic kidney disease and / or chronic heart disease with therapies comprising diuretics. The invention provides a safe way to reduce the risk of hyperkalemia and to lower aldosterone. The invention also relates to treatment of other conditions that can occur either alone or in connection with hyperkalemia, chronic kidney disease, and / or chronic heart disease.
Owner:ZS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com