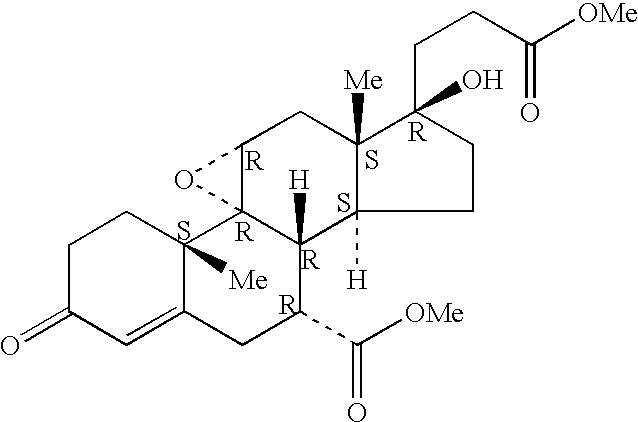
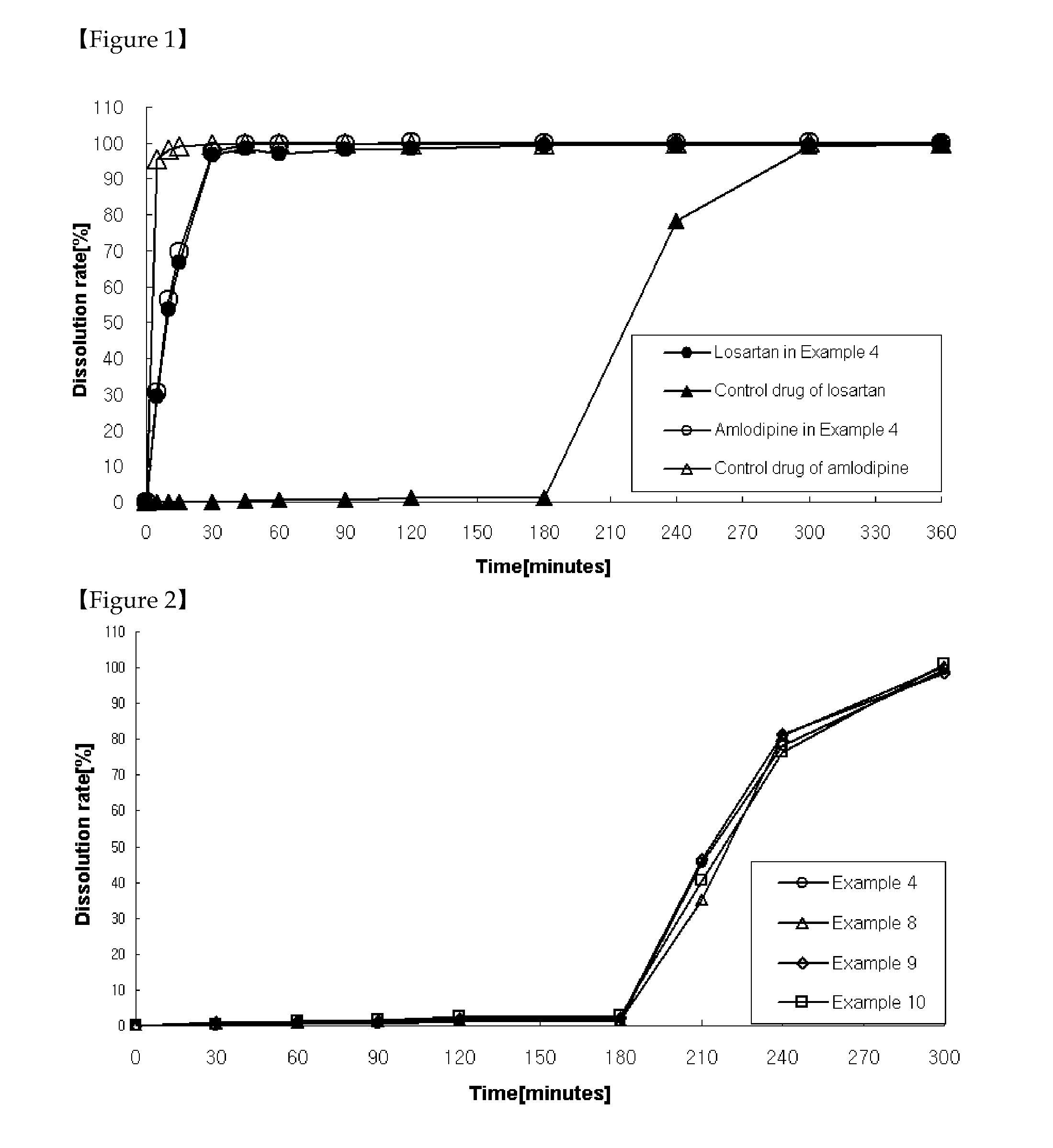
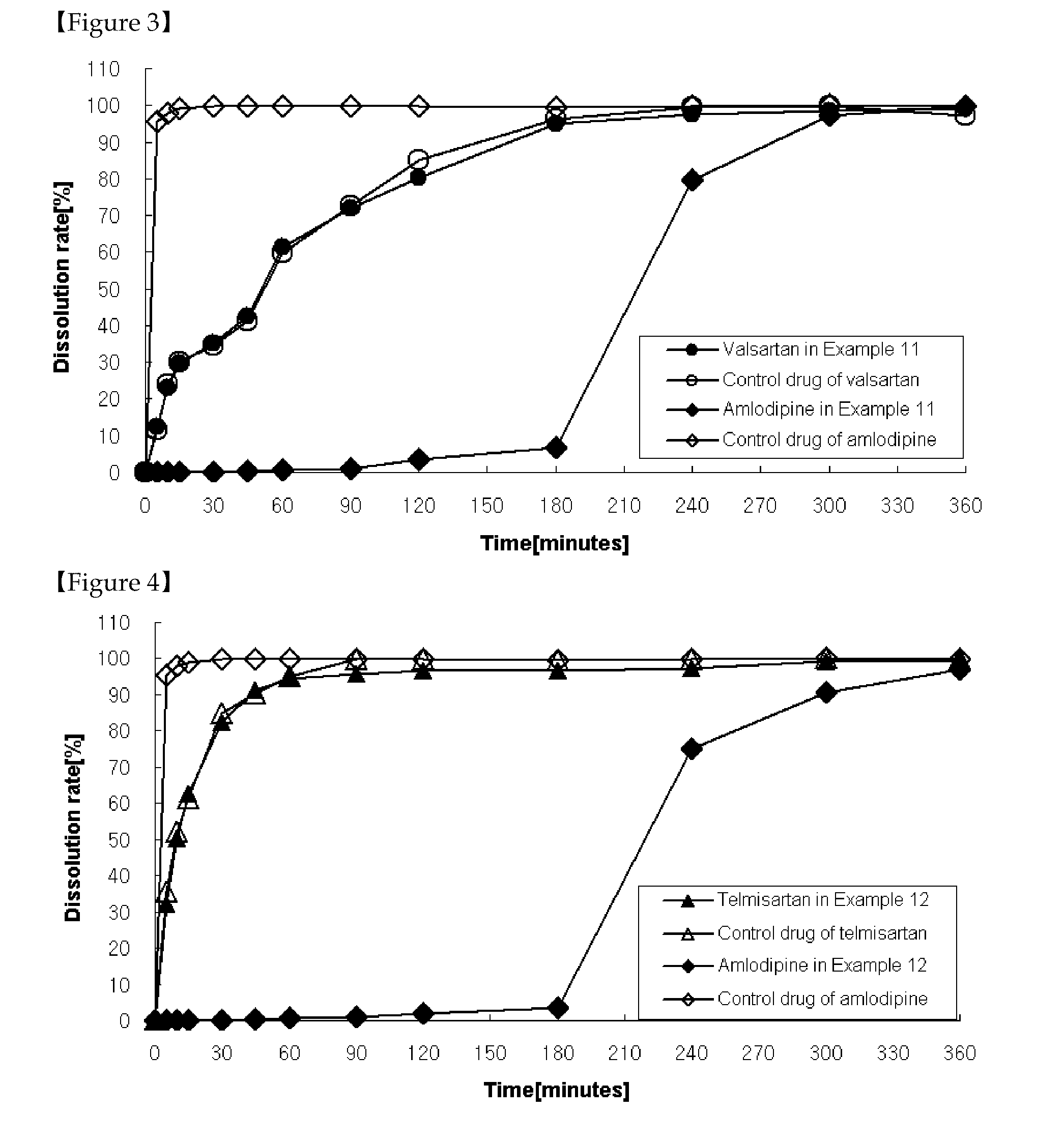
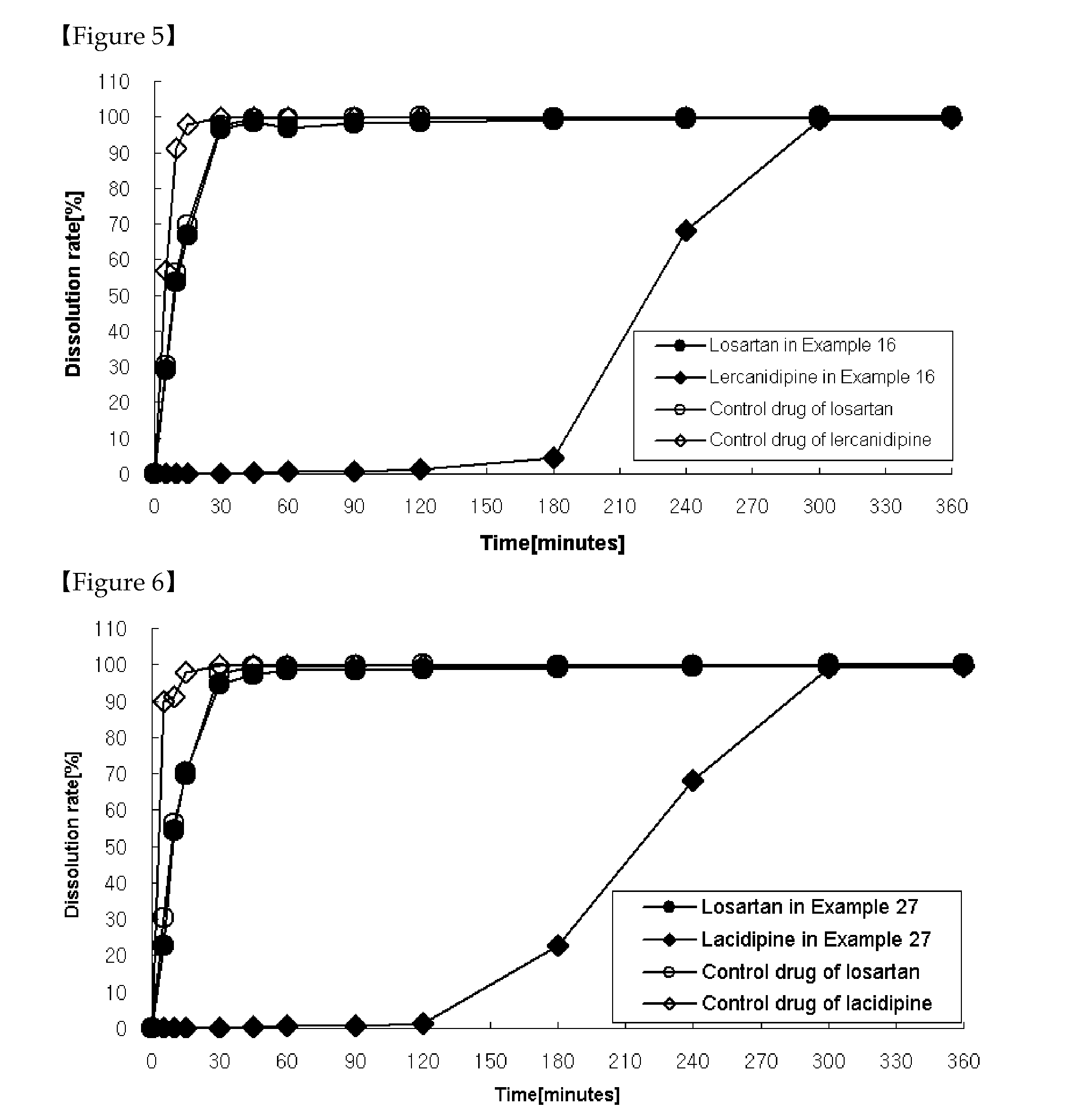
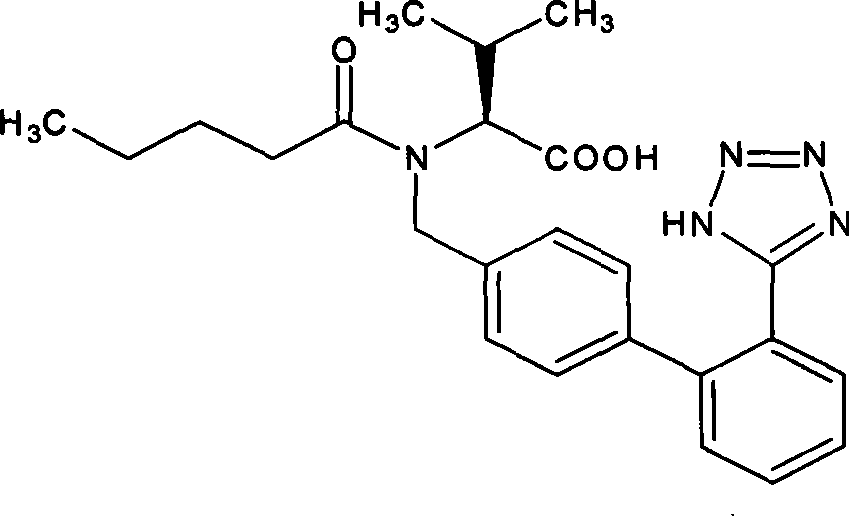
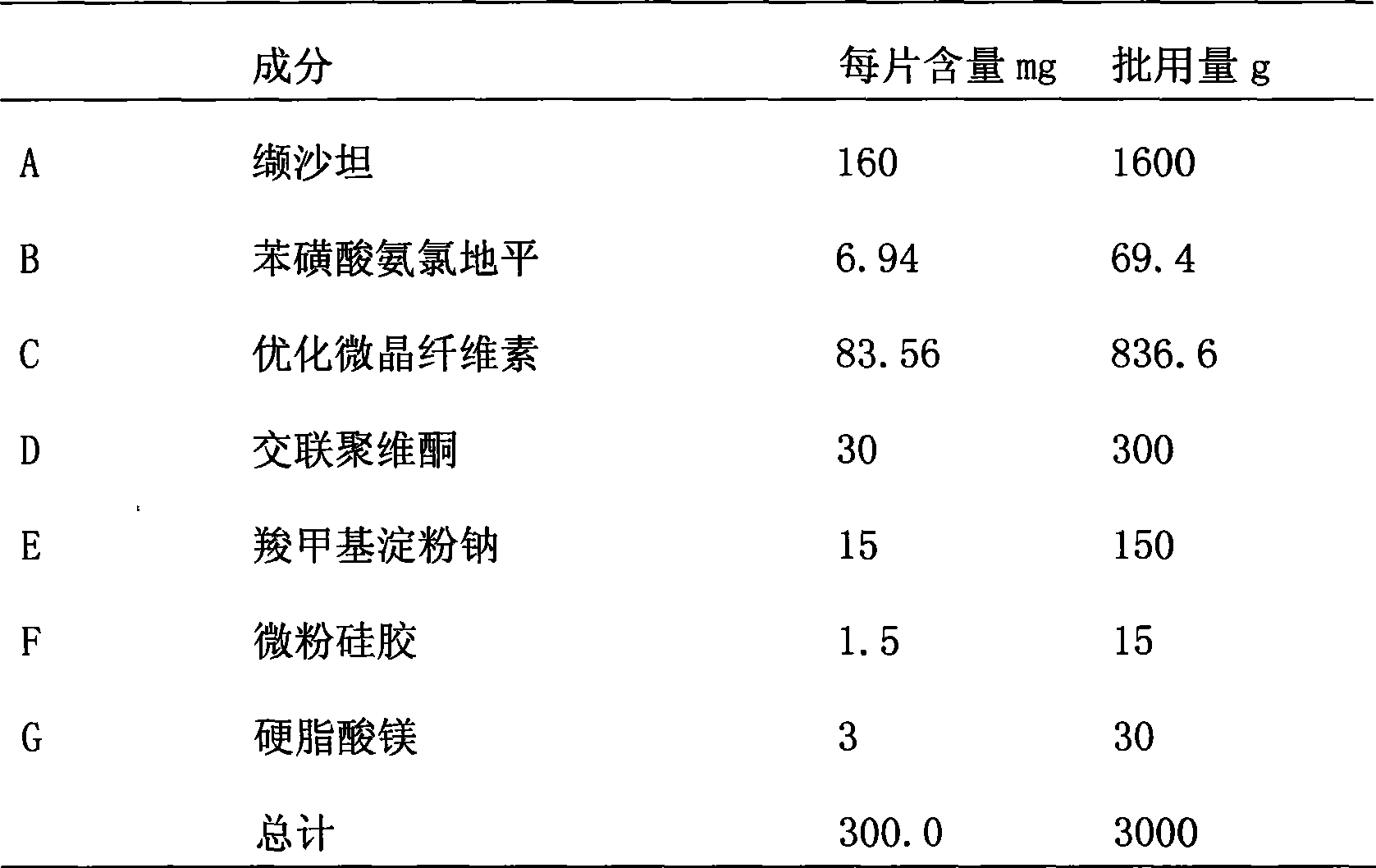
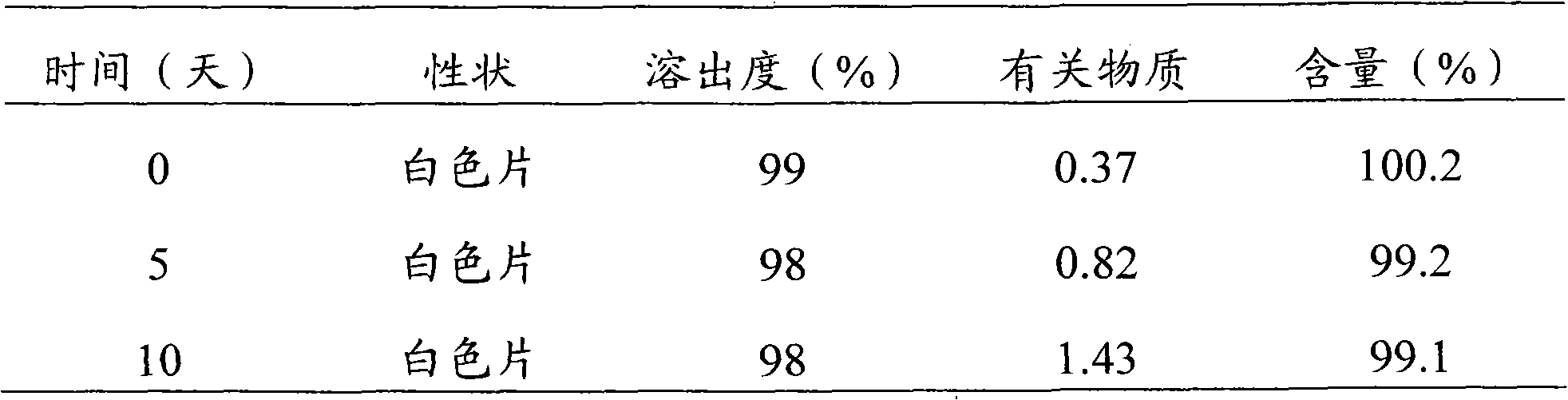
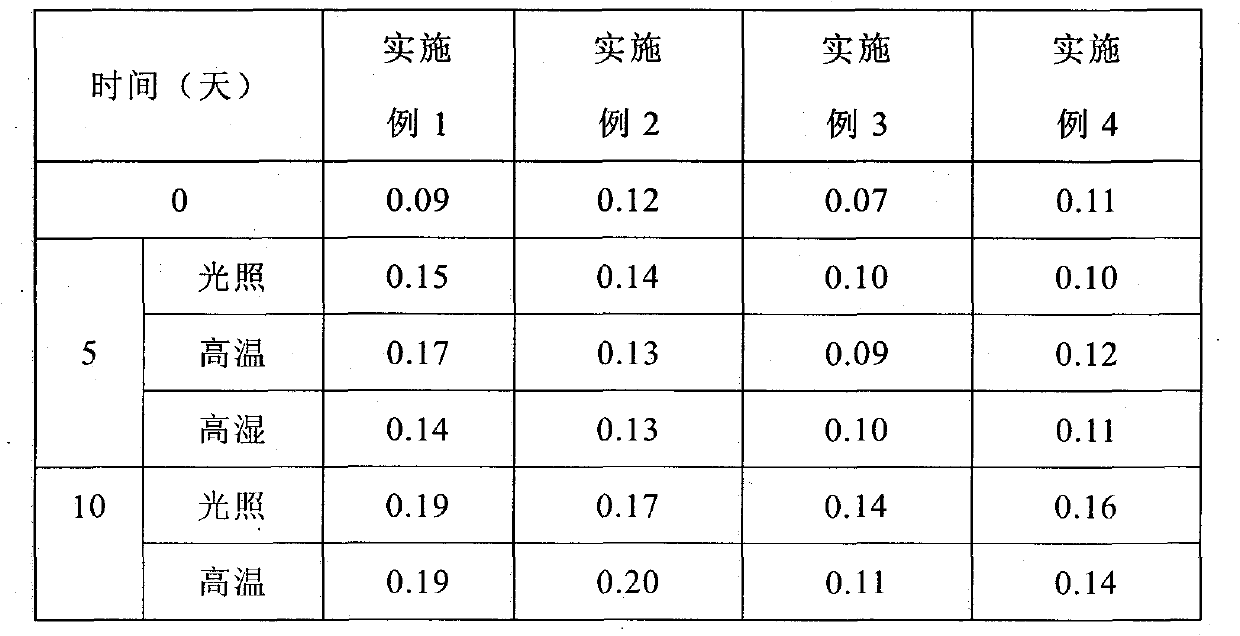
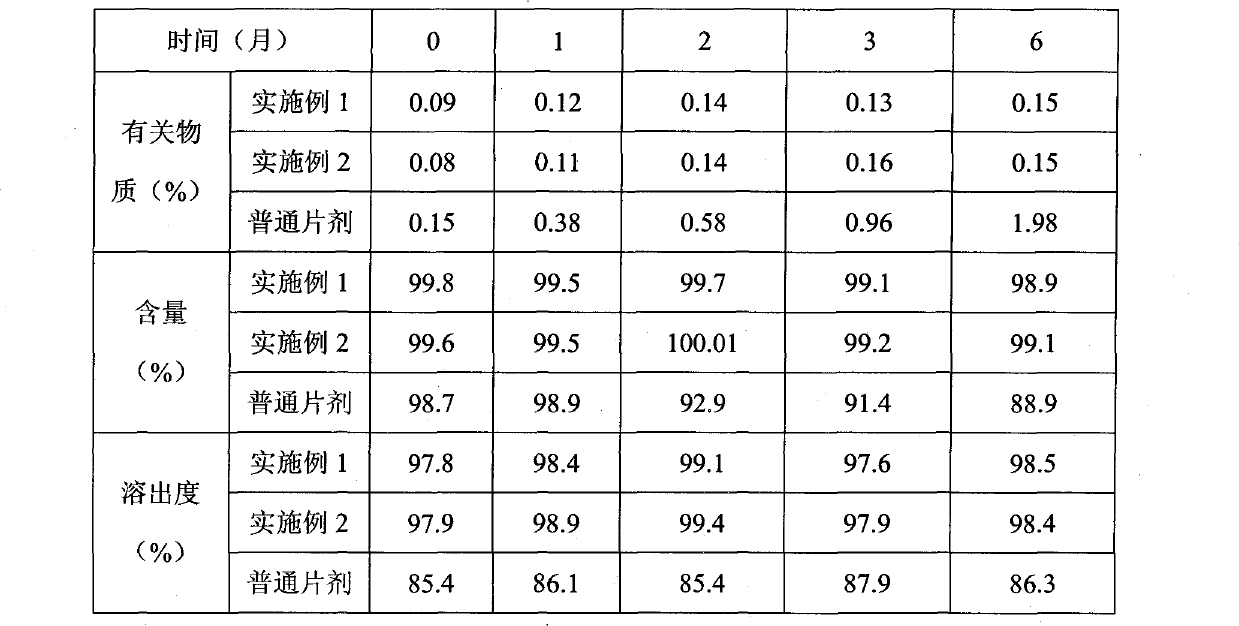
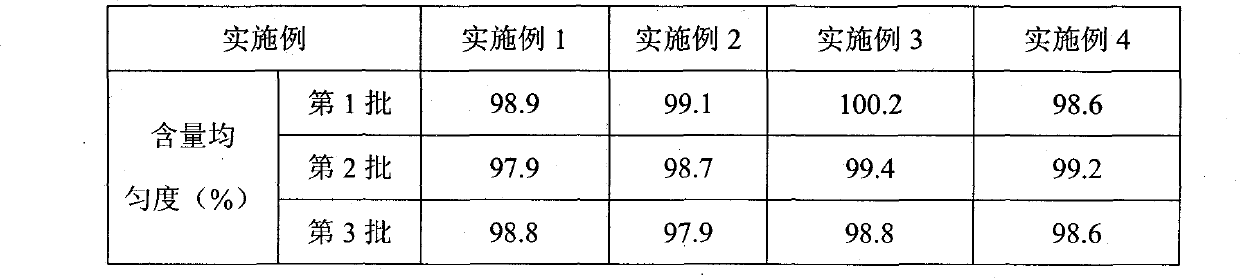
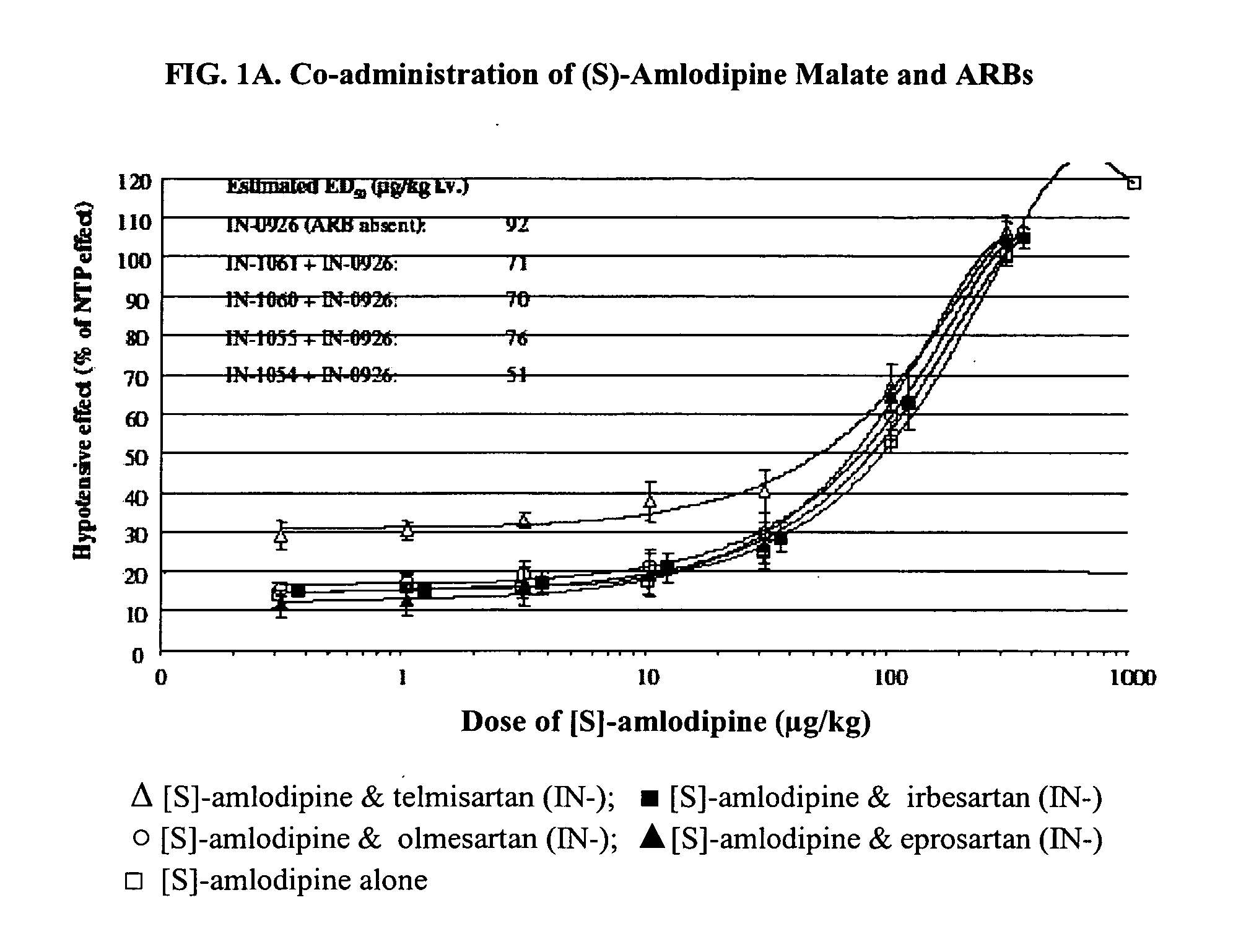
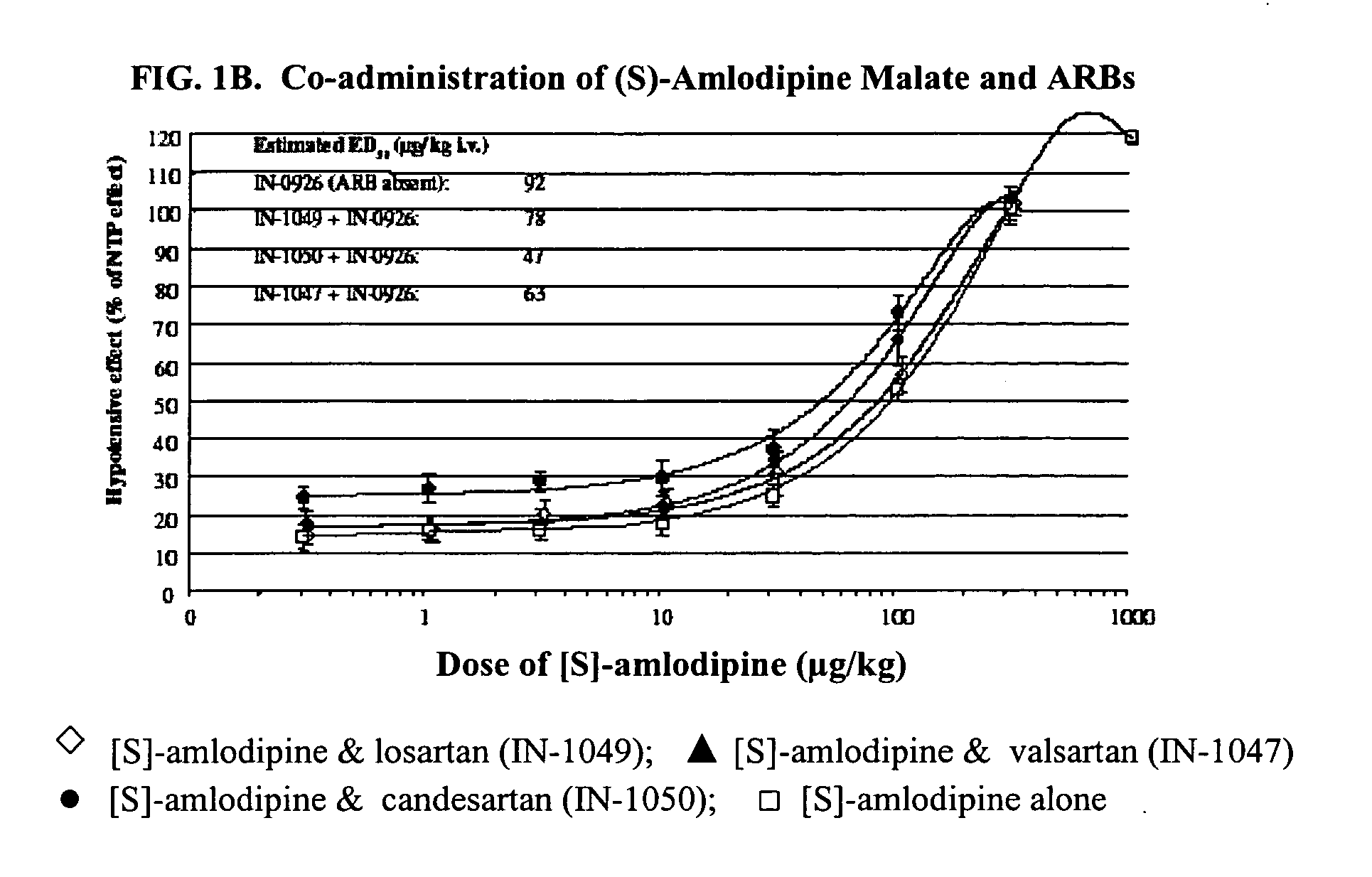
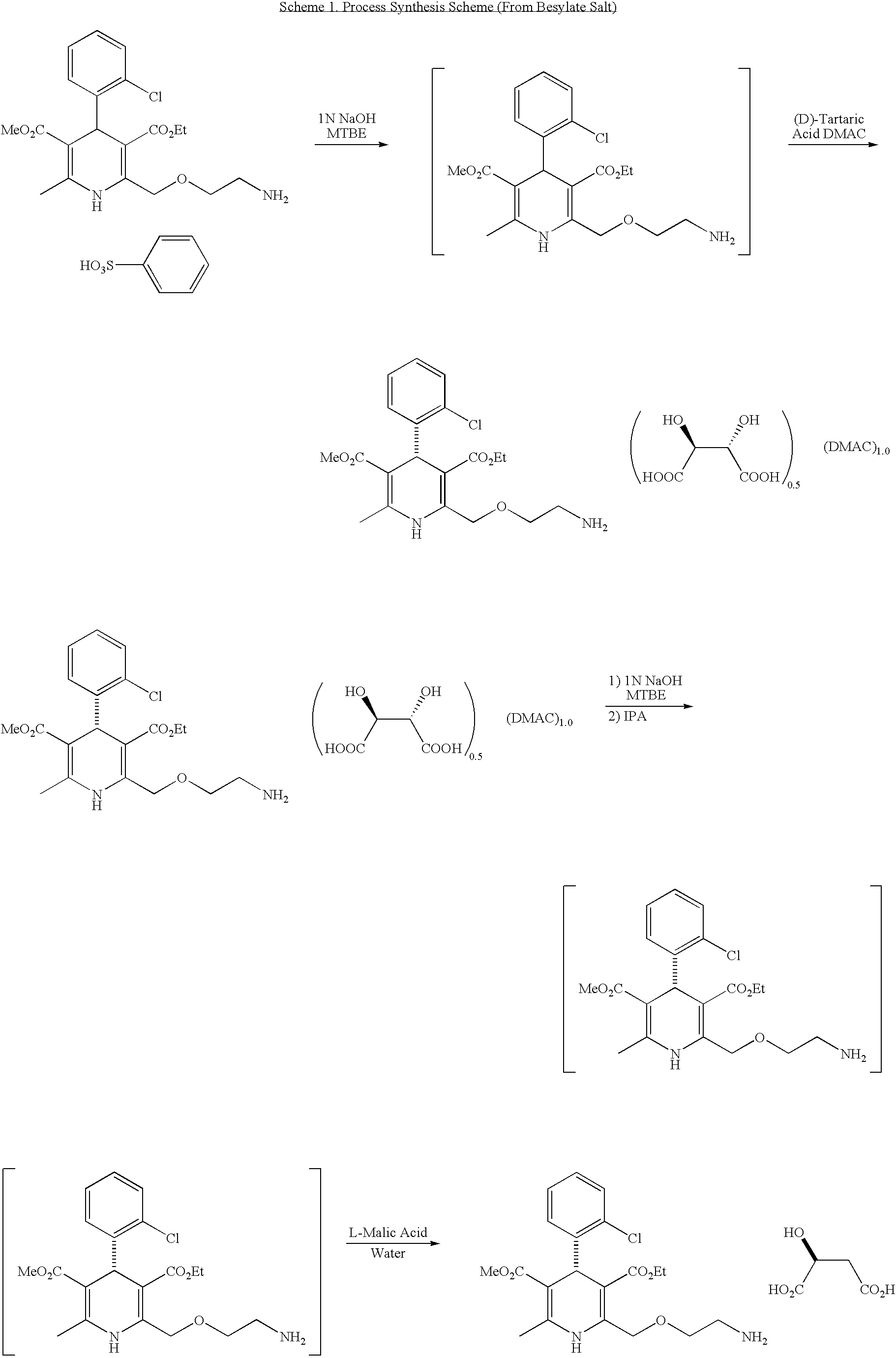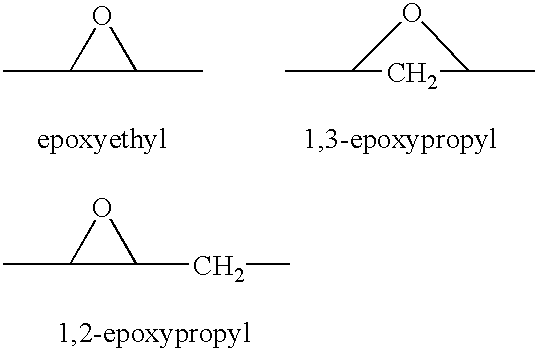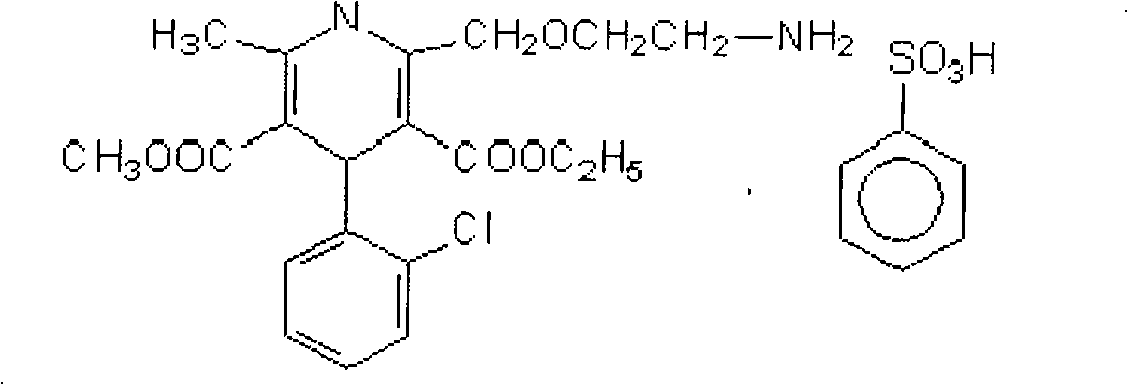Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
474 results about "Amlodipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amlodipine is used with or without other medications to treat high blood pressure.
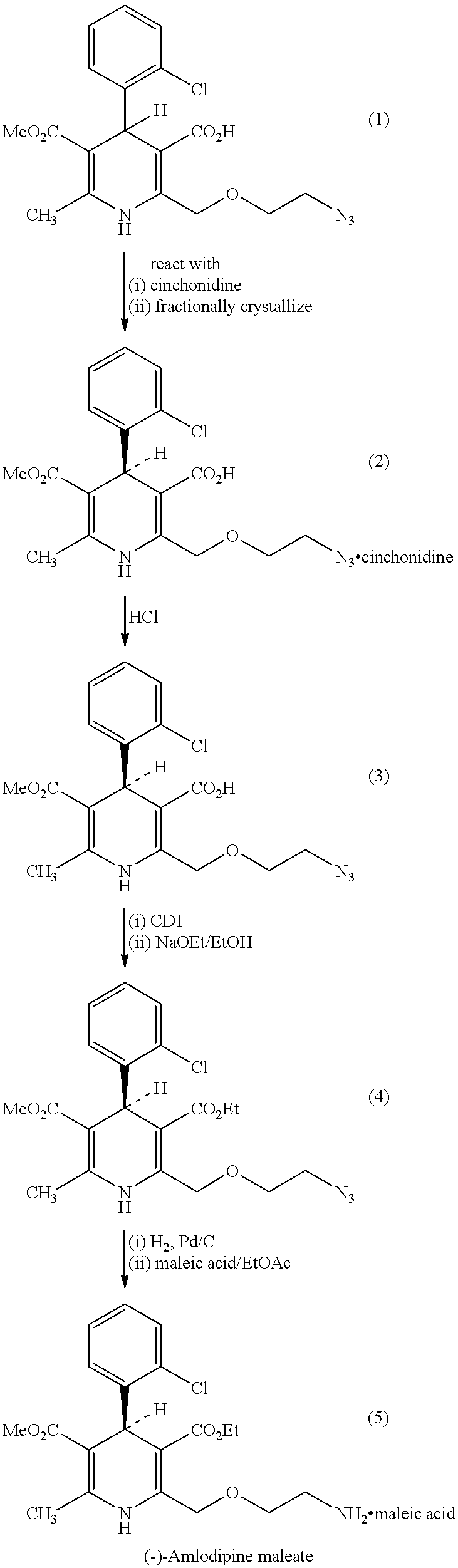
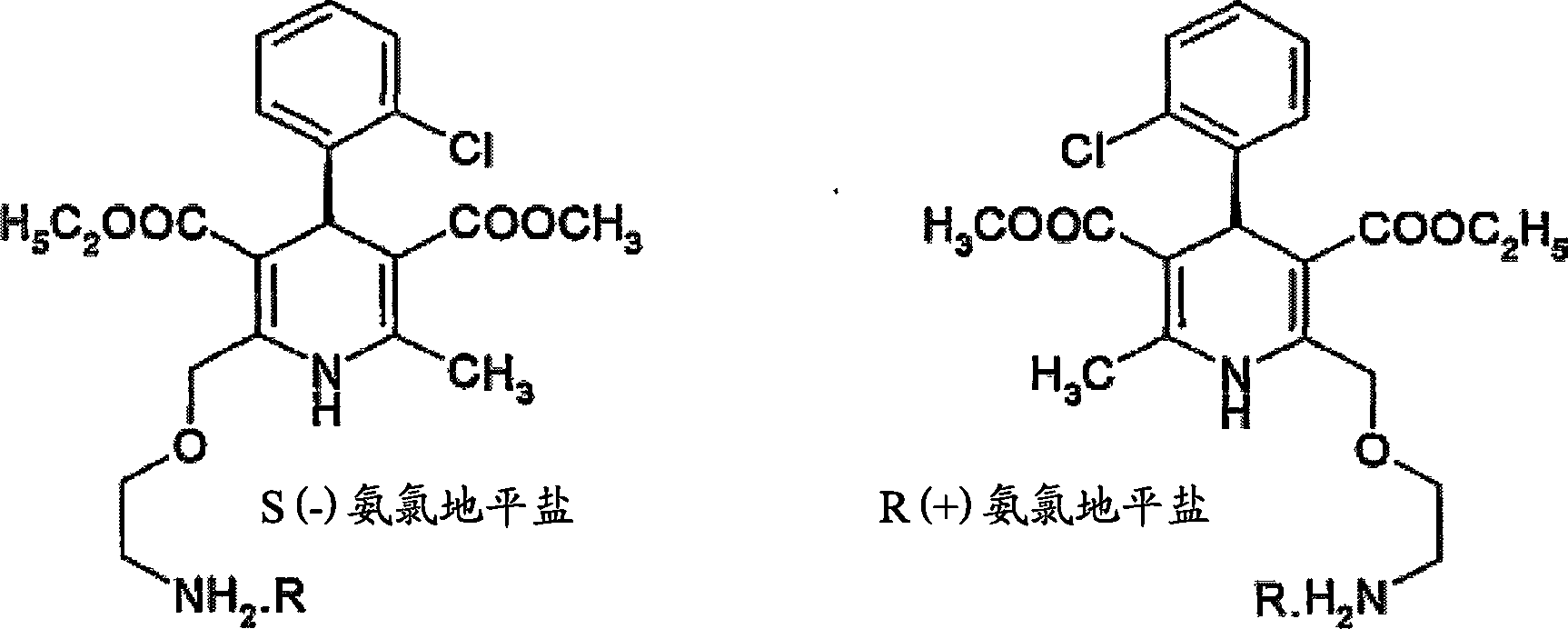
Methods and compositions for treating conditions caused by excessive calcium influx in cells using optically pure (-) amlodipine
Methods are disclosed utilizing the optically pure (-) isomer of amlodipine. This compound is a potent drug for the treatment of hypertension while avoiding the concomitant liability of adverse effects associated with the racemic mixture of amlodipine. The (-) isomer of amlodipine is also useful for the treatment of angina and such other conditions as may be related to the activity of (-) amlodipine as a calcium channel antagonist such as cerebral ischemia, cerebral disorders, arrhythmias, cardiac hypertrophy, coronary vasospasm, myocardial infarction, renal impairment and acute renal failure, without the concomitant liability of adverse effects associated with the racemic mixture of amlodipine.
Owner:SEPACOR INC
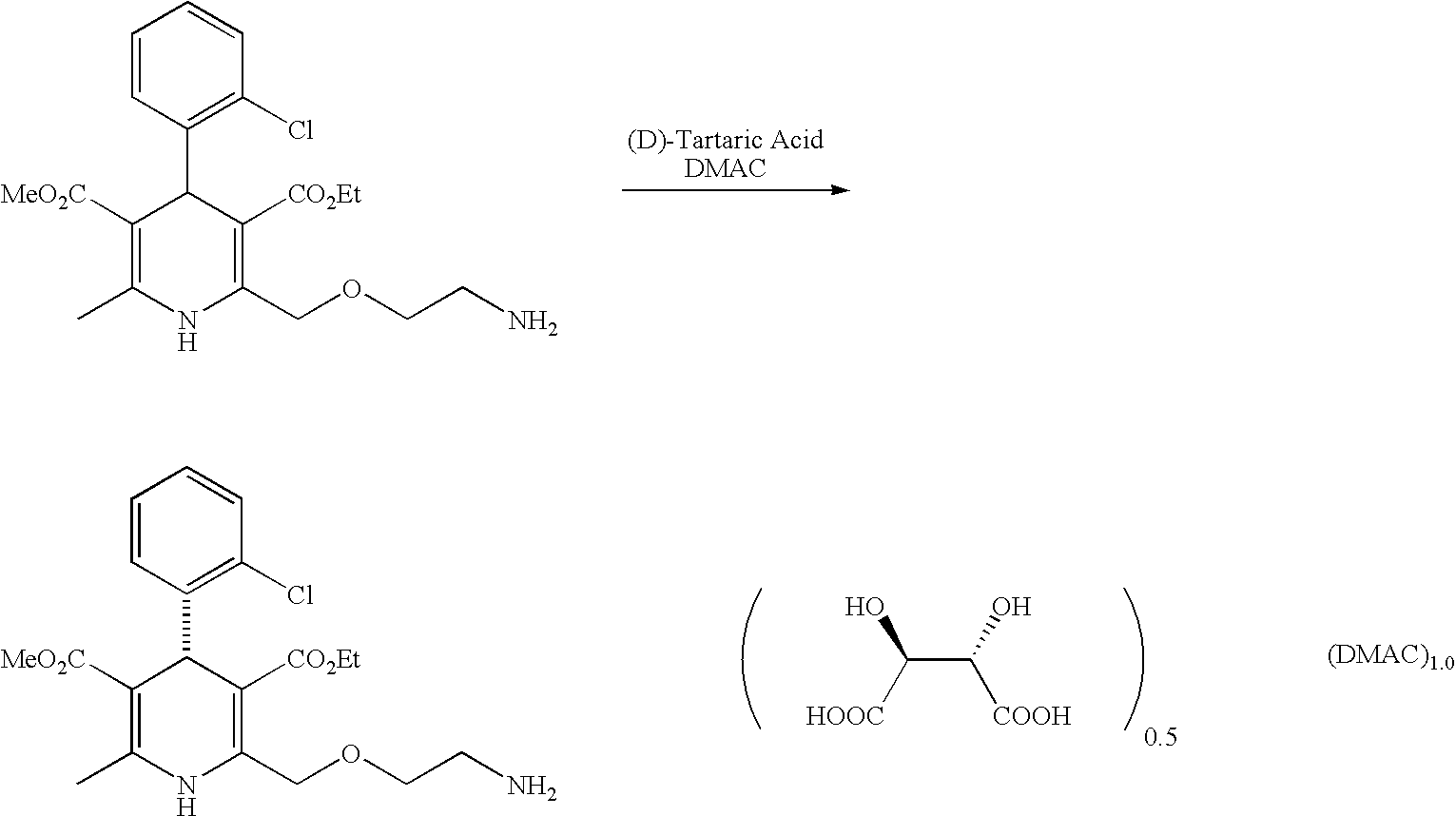
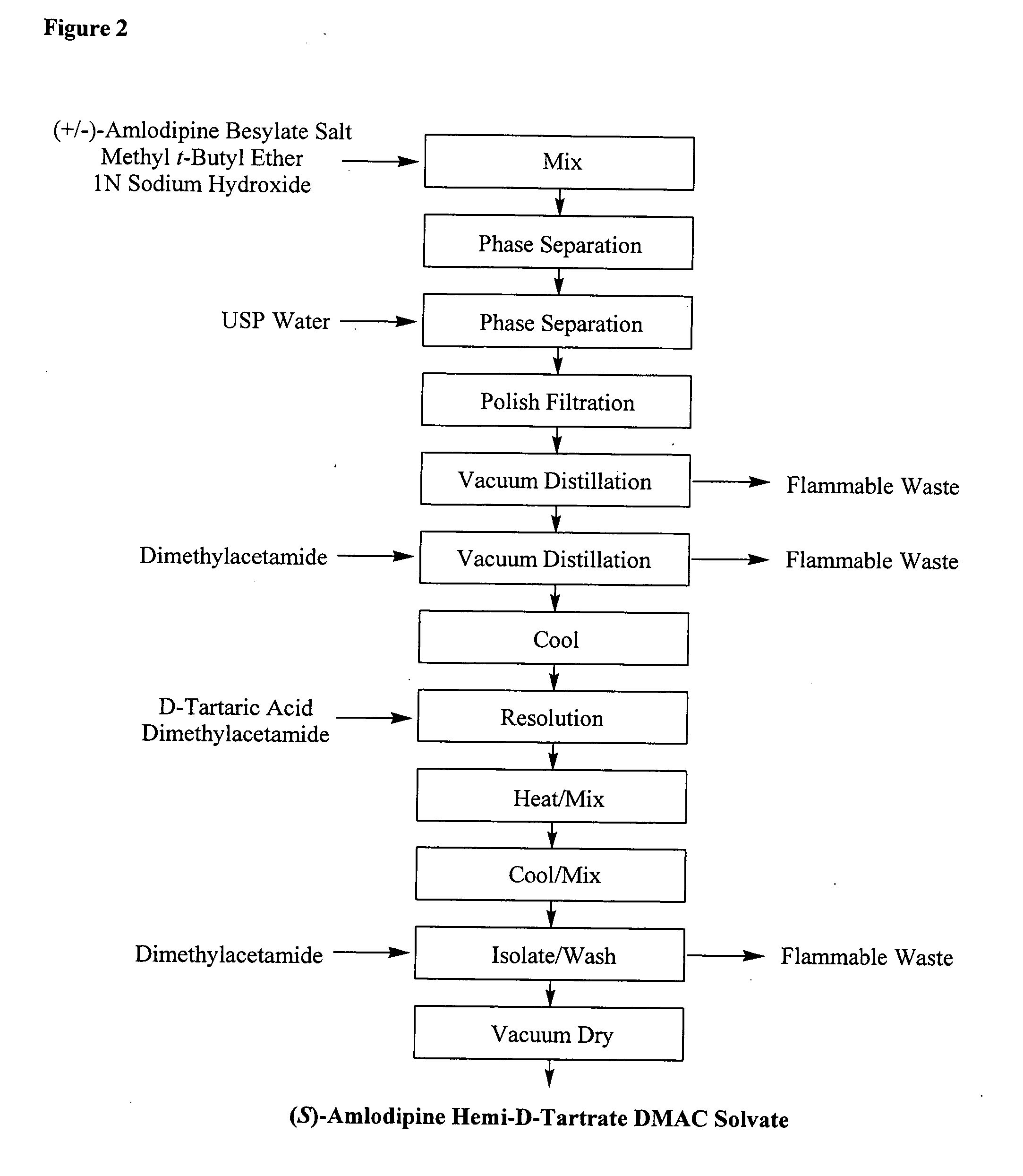
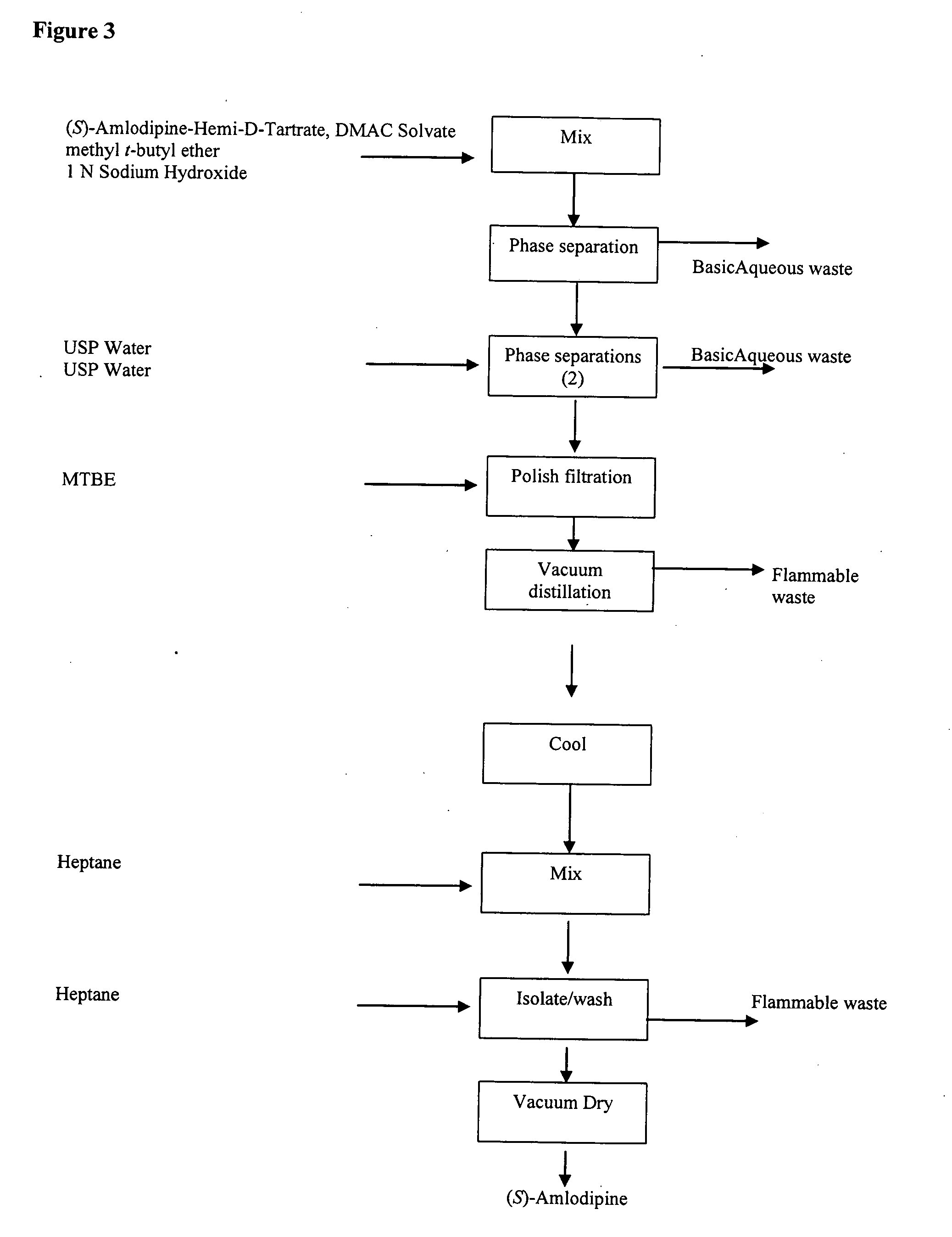
Separation of the enantiomers of amlodipine via their diastereomeric tartrates
InactiveUS6046338AGood yield andenantiomeric puritySimple and economic and efficientOrganic chemistryOrganic solventEnantiomer
A method for the separation of R-(+)- and S-(-)-isomers of amlodipine (I) from mixtures thereof, which comprises the reaction of the mixture of isomers with either L- or D-tartaric acid in an organic solvent containing sufficient dimethyl sulphoxide (DMSO) for the precipitation of, respectively, a DMSO, solvate of an L-tartate salt of R-(+)-amlodipine, or a DMSO solvate of a D-tartrate salt of S-(-)-amlodipine.
Owner:PFIZER INC
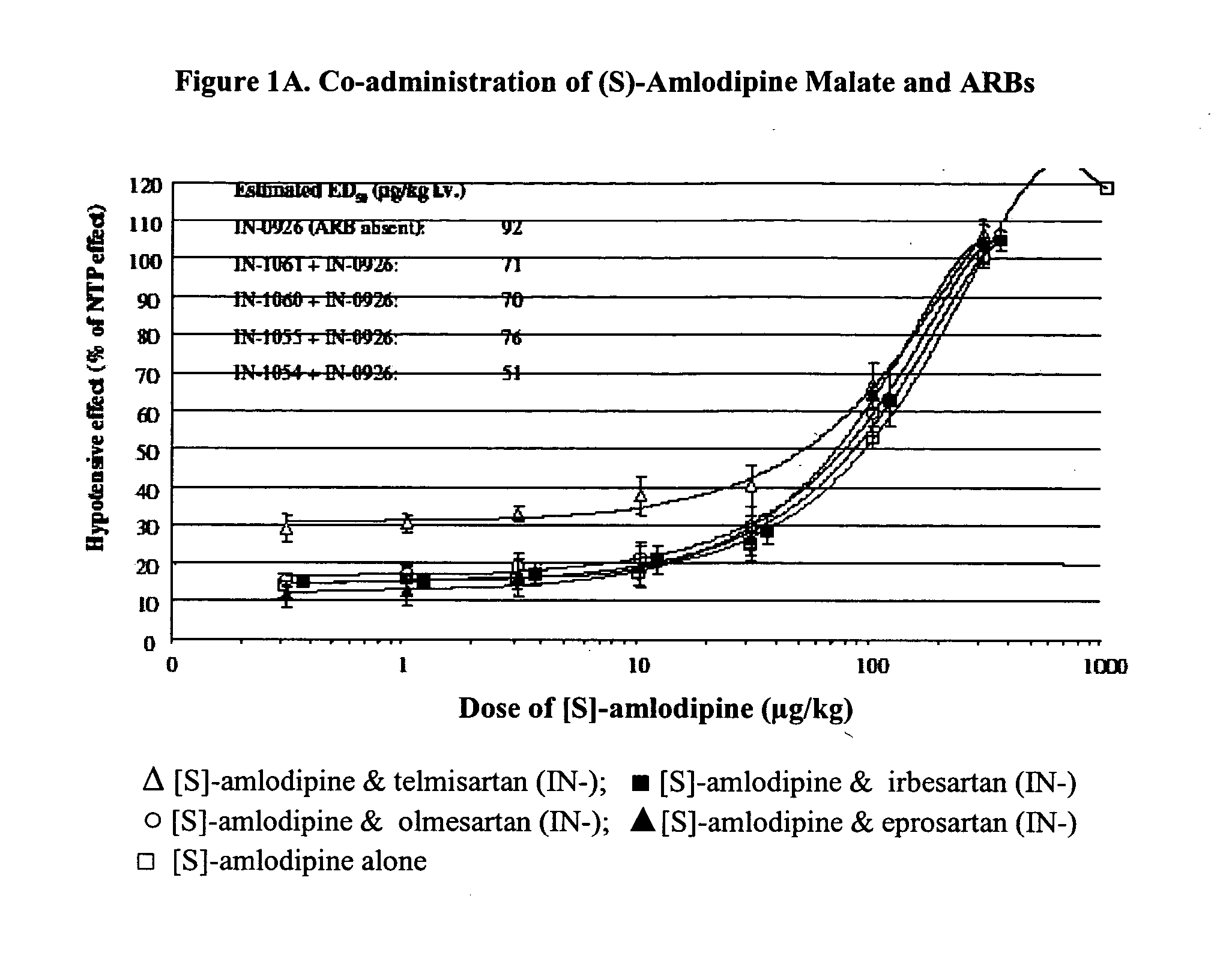
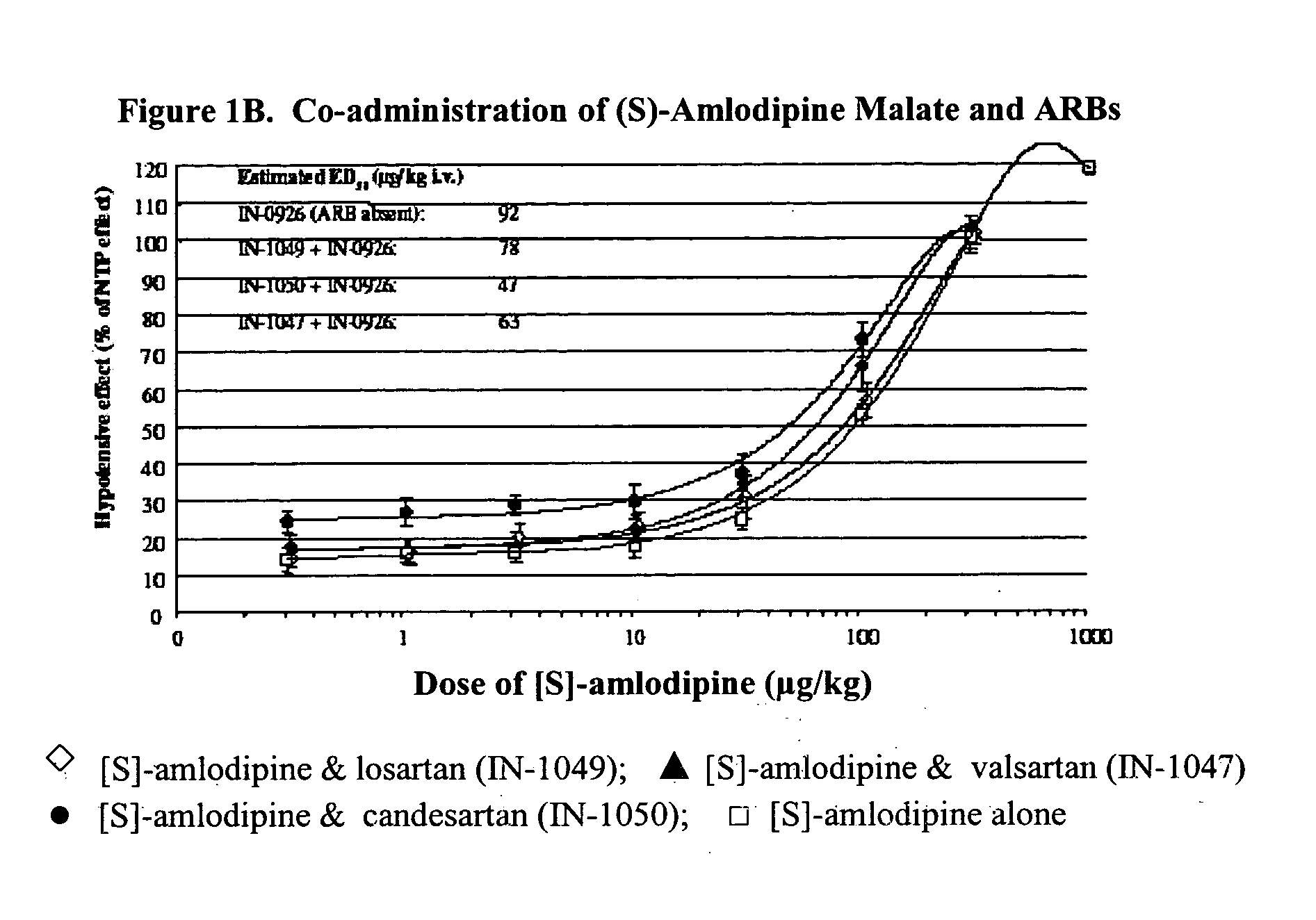
Compositions comprising (S)-amlodipine and an angiotensin receptor blocker and methods of their use
A pharmaceutical composition comprising enantiomerically pure (S)-amlodipine, an ARB and optional other active agents, and methods of treating, preventing and managing cardiovascular diseases and disorders, and symptoms thereof, using the composition, are disclosed.
Owner:SEPACOR INC
Pharmaceutical composition for treating hypertension and cardiovascular disease
InactiveCN1883478AImprove solubilityImprove bioavailabilityPill deliveryGranular deliveryVascular diseaseTreatment effect
Owner:CSPC OUYI PHARM CO LTD
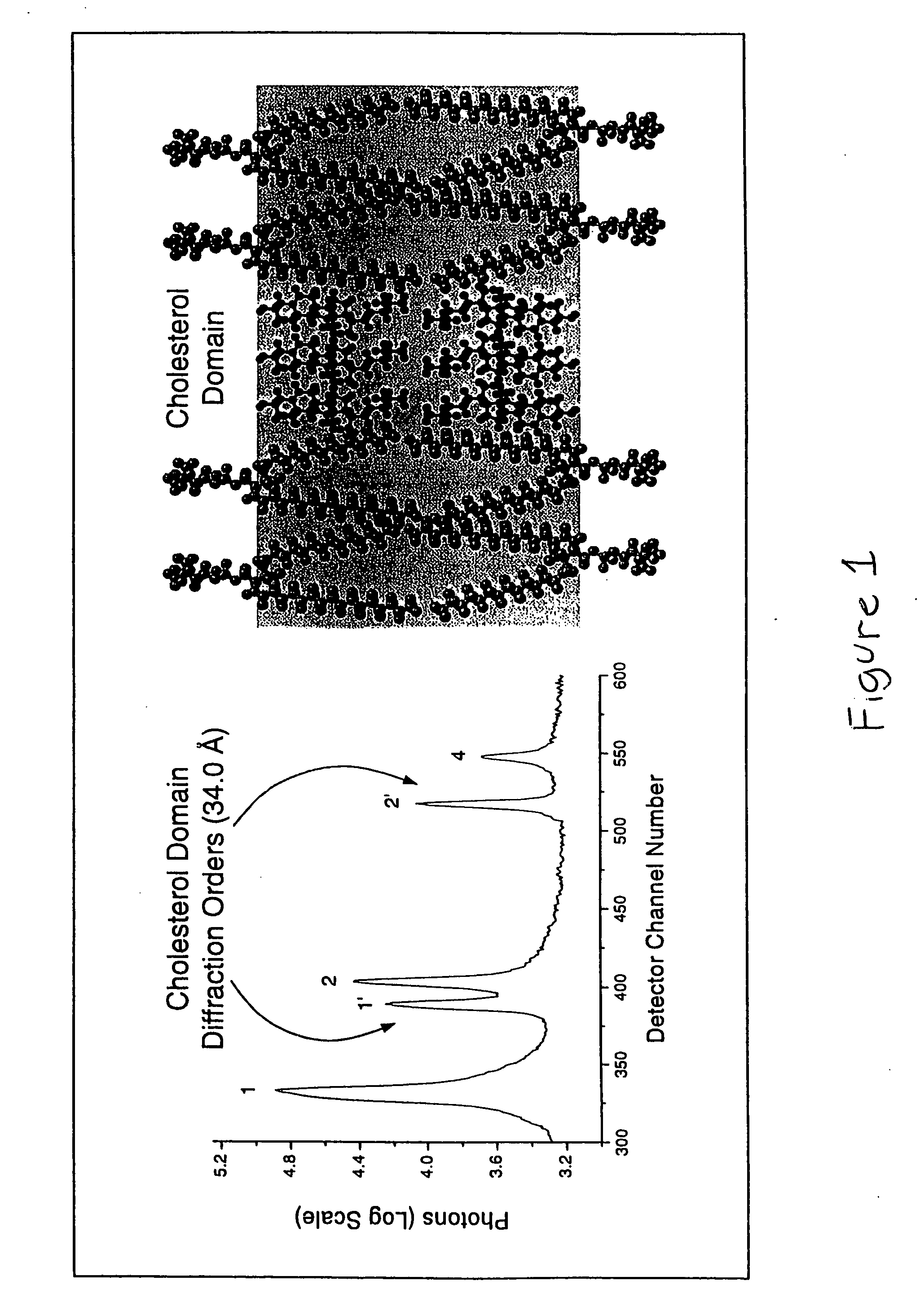
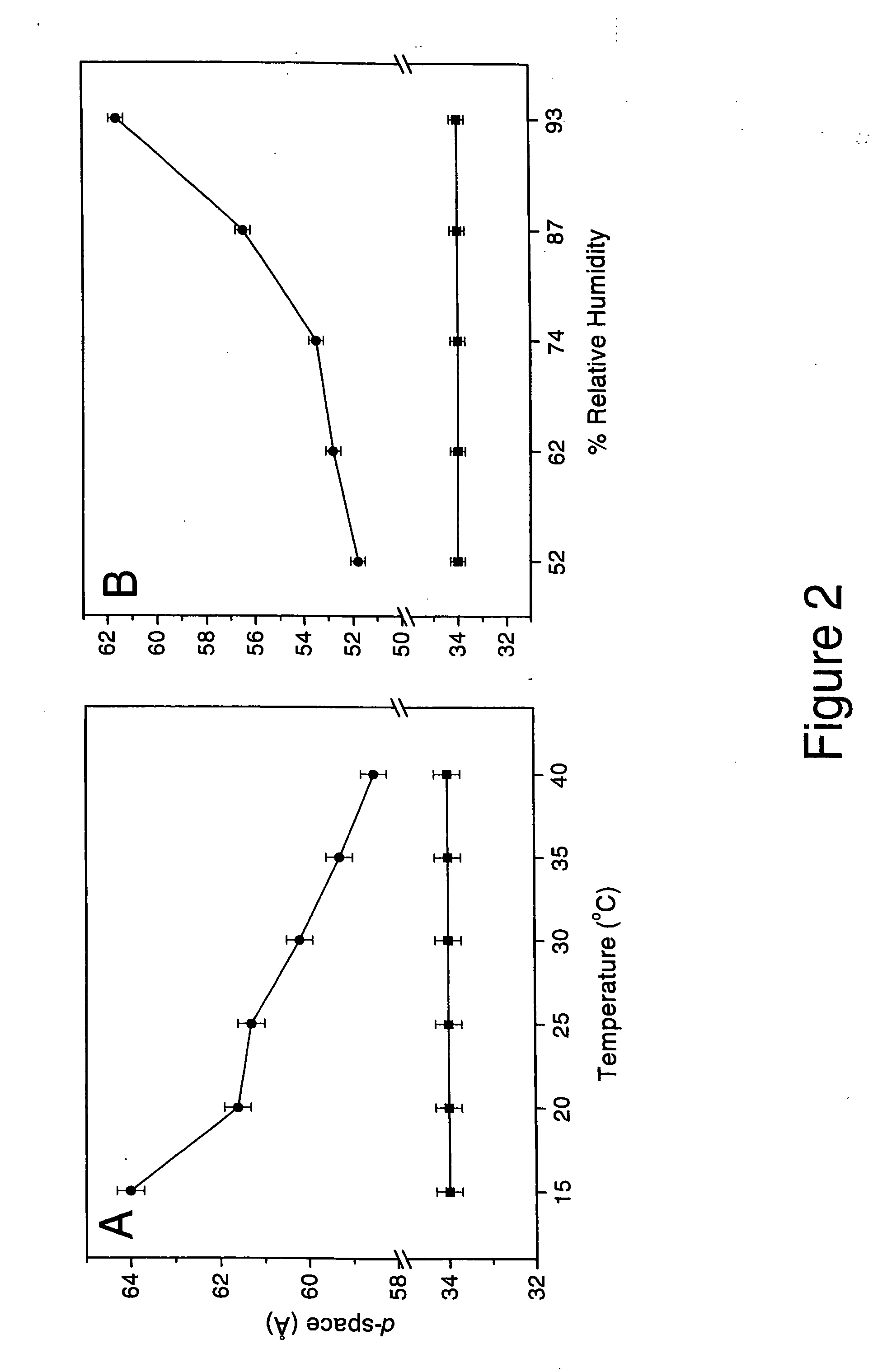
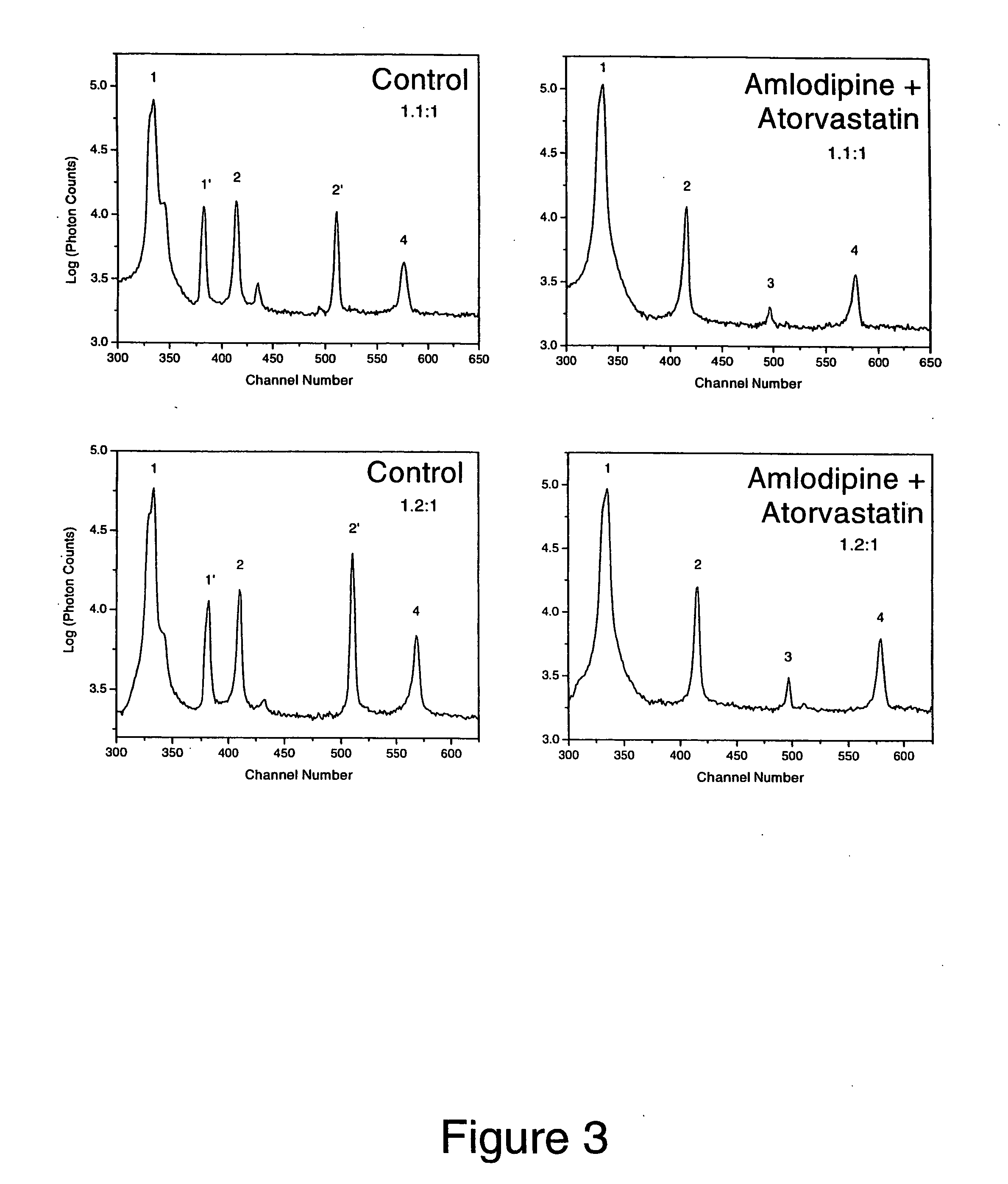
Synergistic effects of amlodipine and atorvastatin metabolite as a basis for combination therapy
The combination of amlodipine with atorvastatin metabolite shows a synergistic antioxidant effect on lipid peroxidation in human low-density lipoproteins and membrane vesicles enriched with polyunsaturated fatty acids. Inhibition of oxy-radical damage by this drug combination was observed at therapeutic levels in a manner that could not be reproduced by the combination of amlodipine with other statins or the natural antioxidant, vitamin E. The basis for this potent activity is attributed to the chemical structures of these compounds and their molecular interactions with phospholipid molecules, as determined by x-ray diffraction analyses. This combination therapy can be used to treat cardiovascular disorders, especially coronary artery disease, by increasing the resistance of low-density lipoproteins and vascular cell membranes against oxidative modification.
Owner:MASON R PRESTON
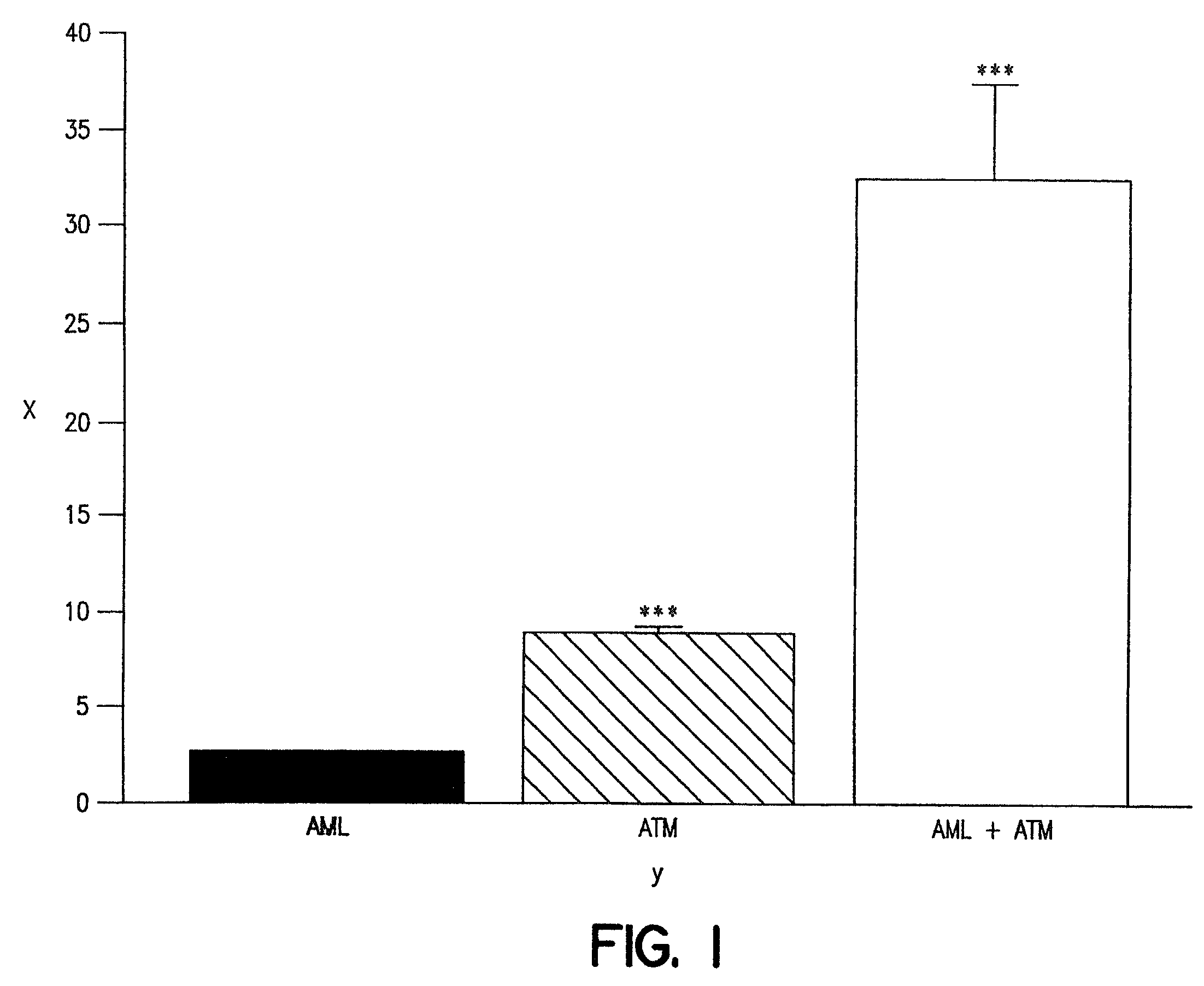
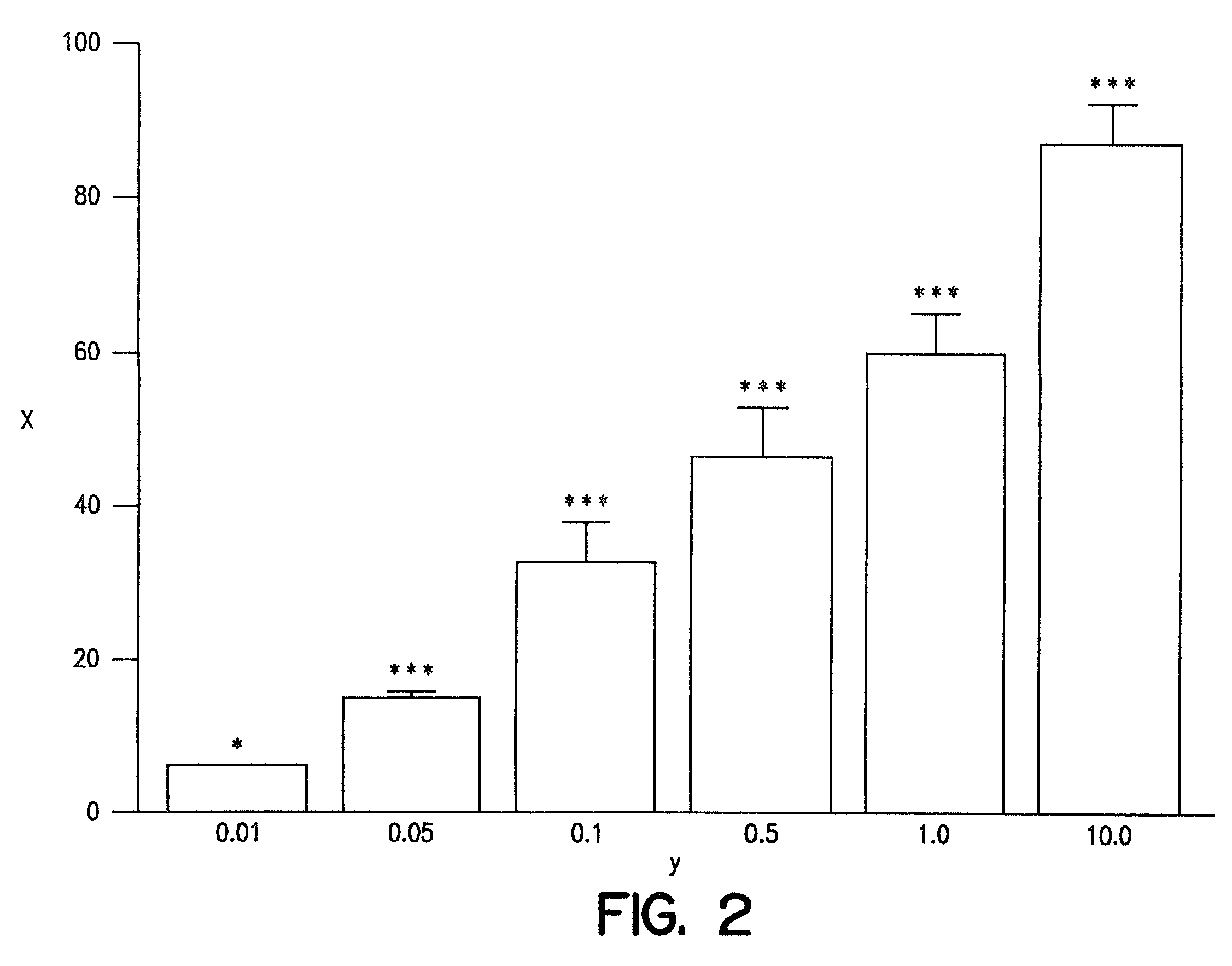
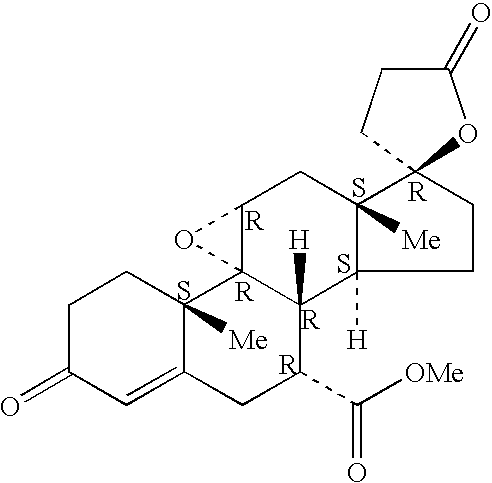
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of cardiovascular disorders
InactiveUS20030220312A1Reduce pathogenicityImprove the level ofOrganic active ingredientsCapsule deliveryAnginaCongestive heart failure chf
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, angina and congestive heart failure. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker amlodipine and the aldosterone receptor antagonist eplerenone.
Owner:GD SEARLE & CO
Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
InactiveUS20100047341A1Improve Medication AdherenceConstant controlBiocideAnimal repellantsCo administrationSide effect
The present invention relates to a functional combination preparation comprising a dihydropyridine-based calcium channel blocker such as amlodipine and an ARB (Angiotensin-2 receptor blocker) such as losartan. In particular, the present invention relates to a chronotherapeutical combination pharmaceutical formulations with controlled-release for the prevention or treatment of cardiovascular disease, which is formulated in accordance with xenobiotics and chronotherapy for enabling the two drugs to be chronotherapeutically released, thereby improving the therapeutic activity as compared to the co-administration of each drug in the form of a single pill, while reducing side effects and maintaining the therapeutic activity as high as possible at the time of day when the risk of a complication of cardiovascular disease is highest.
Owner:HANALL PHARMA CO LTD
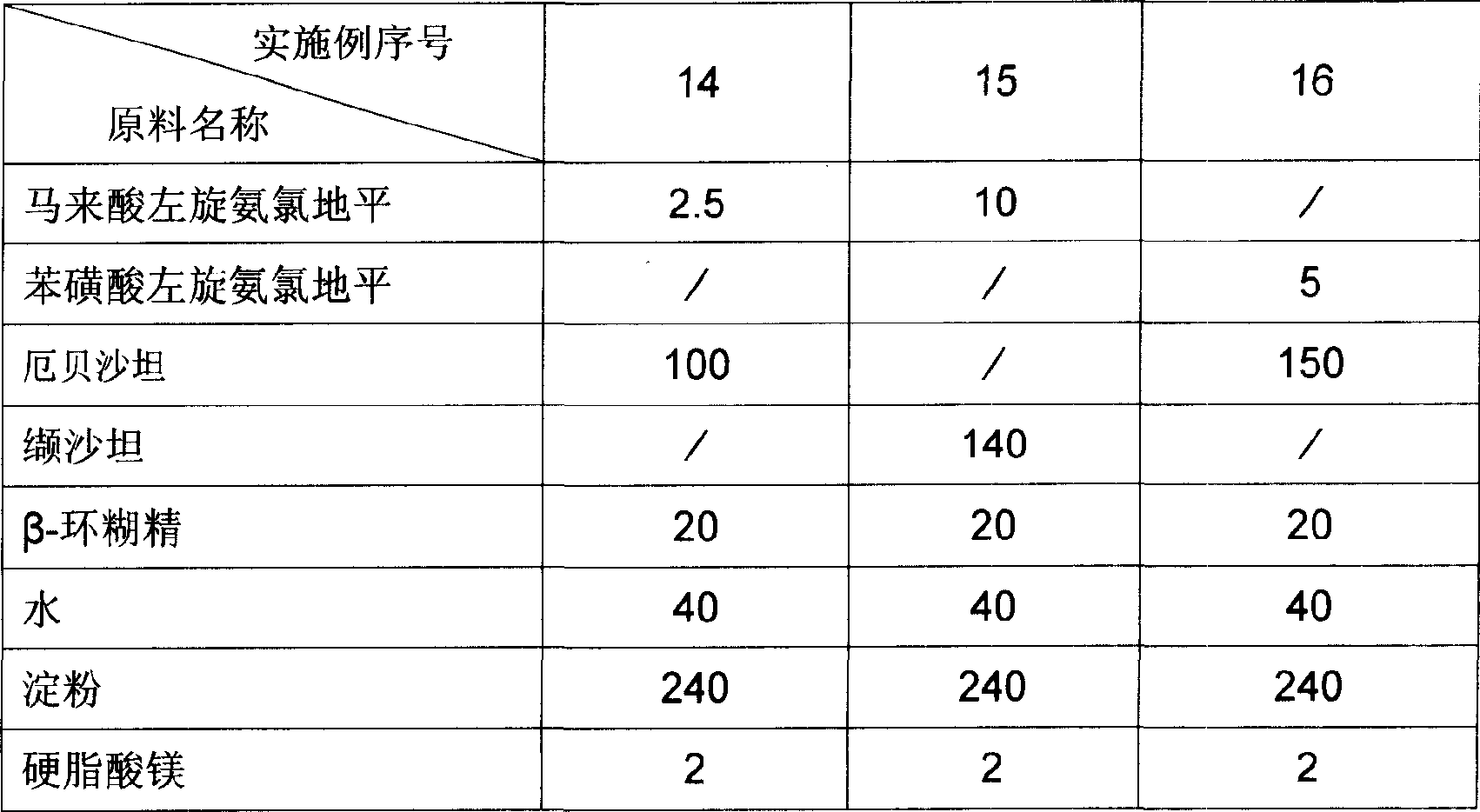
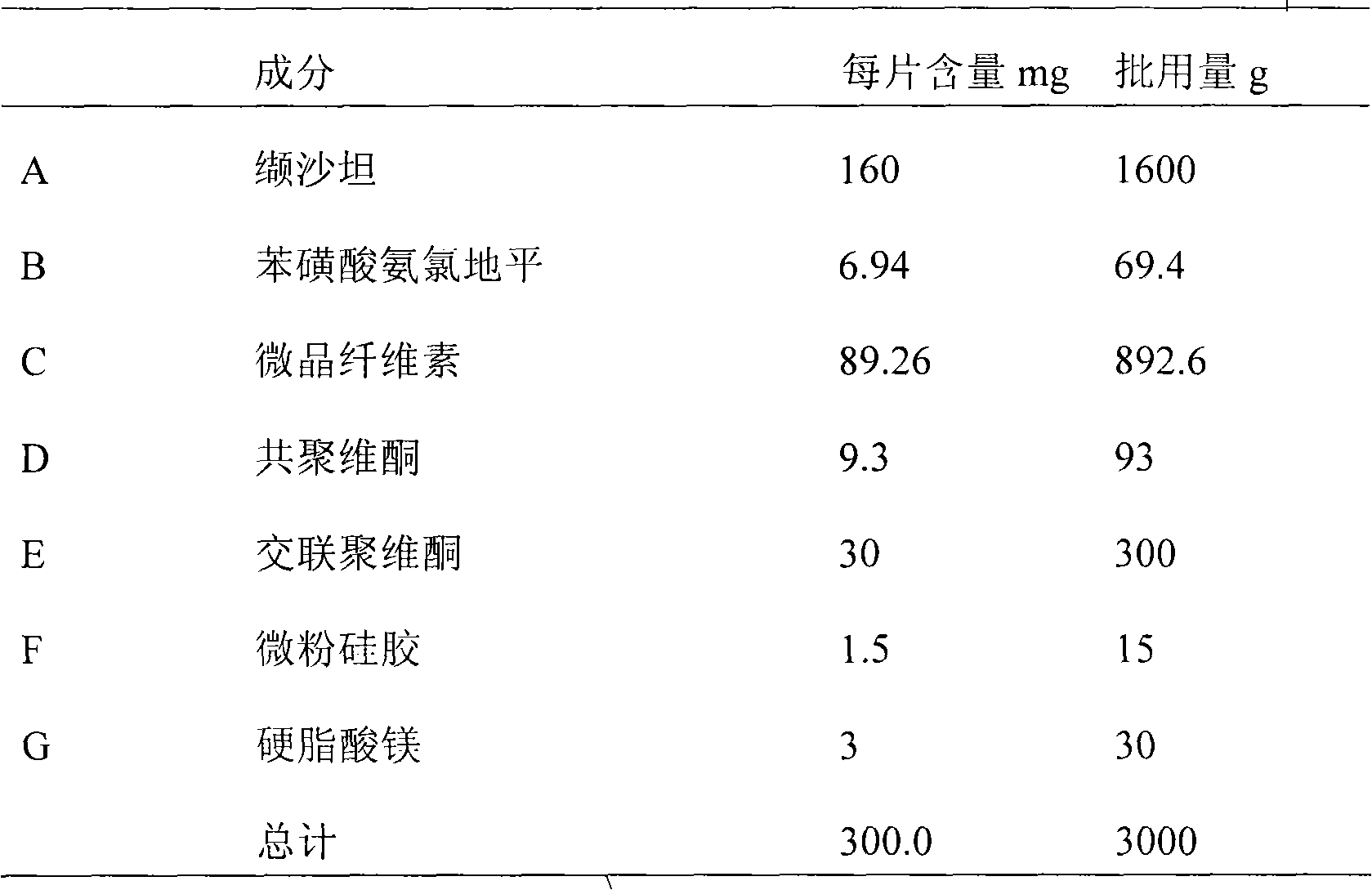
Diovan compound preparation and preparation method thereof
ActiveCN101485657AImprove liquidityNormal production processPharmaceutical product form changeCapsule deliveryValsartanBULK ACTIVE INGREDIENT
The invention discloses a valsartan compound preparation and a method for preparing the same. In the method, the valsartan or pharmacologically accepted salts of the valsartan and amlodipine or pharmacologically accepted salts of the amlodipine are used as active ingredients, and the active ingredients are pressed to prepare a compact by a rolling method; the compact is screened to prepare granules; and the granules are mixed with pharmaceutic adjuvants to prepare a tablet or a capsule. The method pretreats the active ingredients, so that materials have good fluidity; and the valsartan compound preparation and the method have the characteristics of simple process, low cost and suitability for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
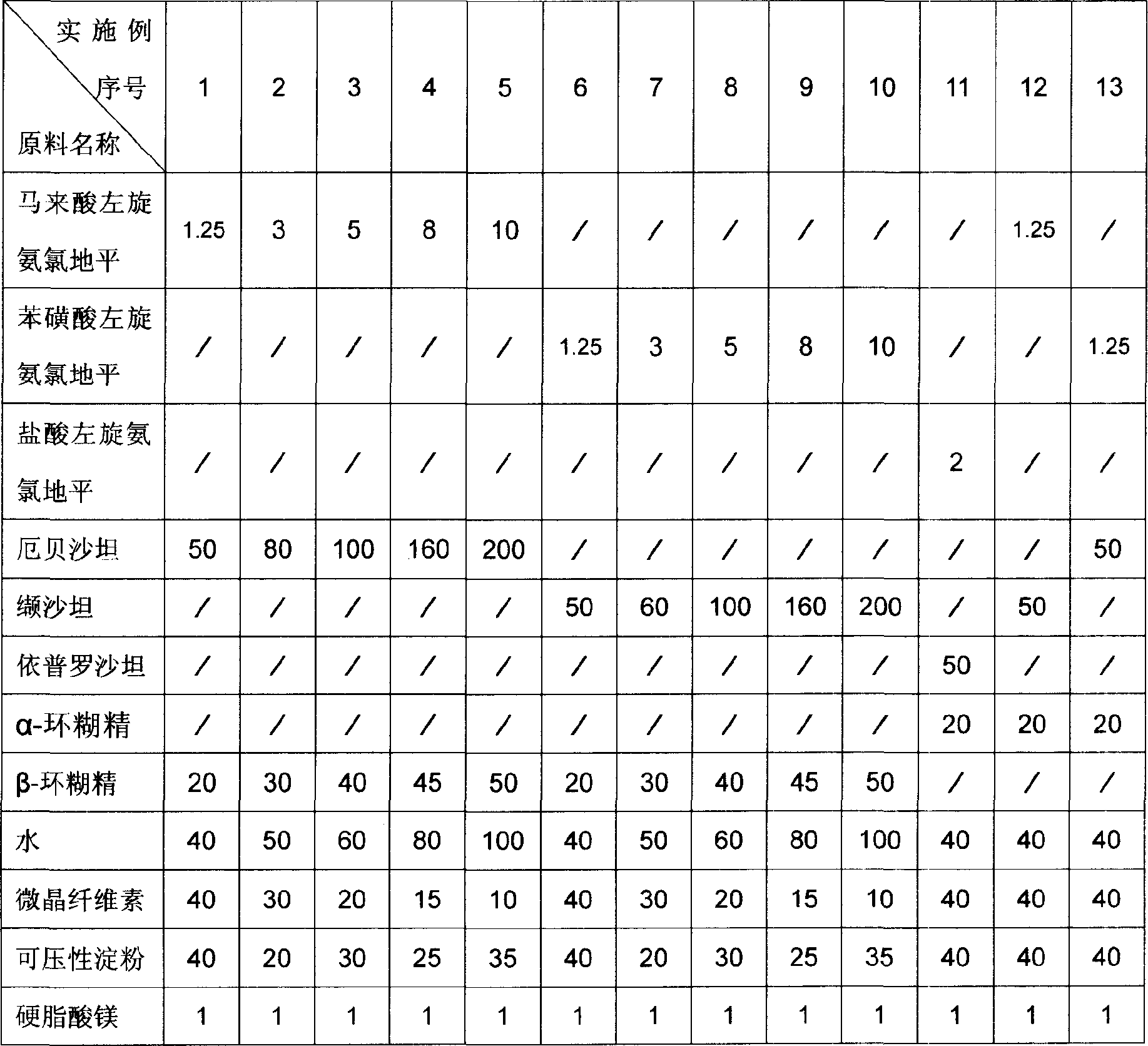
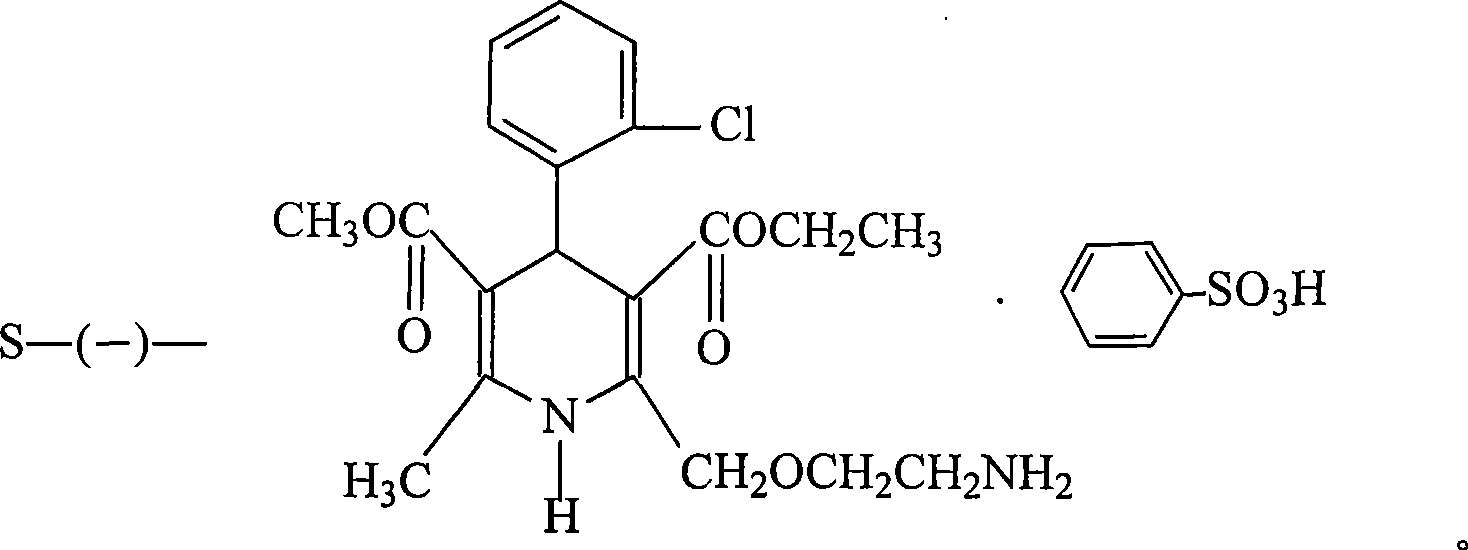
Benzene sulfonic acid levo-amlodipine pill and preparation method thereof
InactiveCN101559043AHigh dissolution rateReasonable designOrganic active ingredientsPill deliveryBenzeneMagnesium stearate
The invention discloses benzene sulfonic acid levo-amlodipine pills and a preparation method thereof. On the basis of 1000 pills, the benzene sulfonic acid levo-amlodipine pill comprises 1-10g of benzene sulfonic acid levo-amlodipine, preferably 2.5g; 50-100g of lactose, preferably 67-87g and most preferably 80g; 5-55g of low-substituted hydroxy propyl cellulose, preferably 20-40g and particularly preferred 30g; 2-20g of crosslinked polyethylene ketopyrrolidine, preferably 5g; and 0.5-2.5g of magnesium stearate, preferably 1.5g. The benzene sulfonic acid levo-amlodipine pills have more than 95 percent of dissolution rate and good production stability.
Owner:NANCHANG HELIOEAST PHARMA
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Levamlodipine beaylate tablets and preparation method thereof
ActiveCN101766582AImprove stabilityRapid dissolutionOrganic active ingredientsPharmaceutical delivery mechanismActive componentLevamlodipine
The invention belongs to the technical field of medicament, and provides levamlodipine beaylate tablets and a preparation method thereof. The tablets consist of tablet cores using the levamlodipine beaylate as an active component and film coatings coated on the outer layer, wherein diluent in the tablet cores contains diatomite or aerosil, or a mixture of diatomite and aerosil, and contains other pharmaceutically acceptable supplementary materials; and the outer film coating accounts for 8 to 12 percent of the weight of the tablets, and can play a role in resisting humidity and avoiding light to ensure that the medicinal stability can be greatly improved, and related substances are obviously reduced. Furthermore, the tablets have small specification, so the tablets ensure uniform content, and improve dissolution; and the method is simple and controllable, and ensures that the hygroscopicity of the medicament is obviously reduced.
Owner:鲁南新时代生物技术有限公司
Levamlodipine besylate tablet, preparation process thereof and control method for relevant materials
ActiveCN102028662AGood treatment effectStable buckOrganic active ingredientsPill deliveryCross-linkSolubility
The invention relates to a levamlodipine besylate tablet, a preparation process thereof and a control method for relevant materials. Each 1000 levamlodipine besylate tablets provided by the invention comprise the following compositions: 2.5g of levamlodipine besylate (measured in besylate), 30 to 50g of lactose (as a filler), 20 to 40g of beta-cyclodextrin (as an inclusion agent), 25 to 45g of microcrystalline cellulose (as a disintegrating agent), 5 to 15g of cross-linked polyvinylpyrrolidone (as a disintegrating agent), 1 to 2g of magnesium stearate (as lubricant), and 50 to 80g of 2.5% HPMC (hydroxypropyl methylcellulose) and 50% ethanol (as an adhesive). The levamlodipine besylate tablet provided by the invention has the advantages of making multi-item improvements on the properties of the levamlodipine besylate, increasing the solubility and apparent dissolution rate of the tablet, improving the stability of the tablet, reducing the excitability of the tablet, significantly reducing the limit of the relevant material, and having better clinical treatment effect, so that the blood pressure lowering of the patient with hypertension is more stable.
Owner:JIANGXI SHIMEI PHARM CO LTD
Compositions comprising (S)-amlodipine malate and an angiotensin receptor blocker and methods of their use
A pharmaceutical composition comprising enantiomerically pure (S)-amlodipine malate, an ARB and optional other active agents, and methods of treating, preventing and managing cardiovascular diseases and disorders, and symptoms thereof, using the composition, are disclosed.
Owner:SEPACOR INC
Method for preparing an amlodipine microsphere
InactiveCN101530396ARound shapeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseMicrosphere
The method discloses a method for preparing an amlodipine microsphere. Medicament wrapped by the prepared amlodipine microsphere is amlodipine and organic acid salts of the amlodipine; a carrier material of the microsphere is polylactic acid (PLA), a polylactic acid-glycolic acid copolymer (PLGA), a polylactic acid-glycol block copolymer (PLA-mPEG) or other biodegradable materials; a surfactant solution, a monosaccharide or amylose solution, a polyalcohol solution, a cellulose solution and a colloid solution are used as a dispersion medium; and through an emulsion solvent evaporation method, the amlodipine microsphere is prepared under the mechanical stirring or high-speed shearing action. The microsphere has a round shape and even distribution of grain diameter; the grain diameter is within the range between 1 and 125 mu m; the medicine loading capacity can reach more than 1.5 percent; and the encapsulating rate is more than 70 percent.
Owner:XIAN LIBANG PHARMA TECH
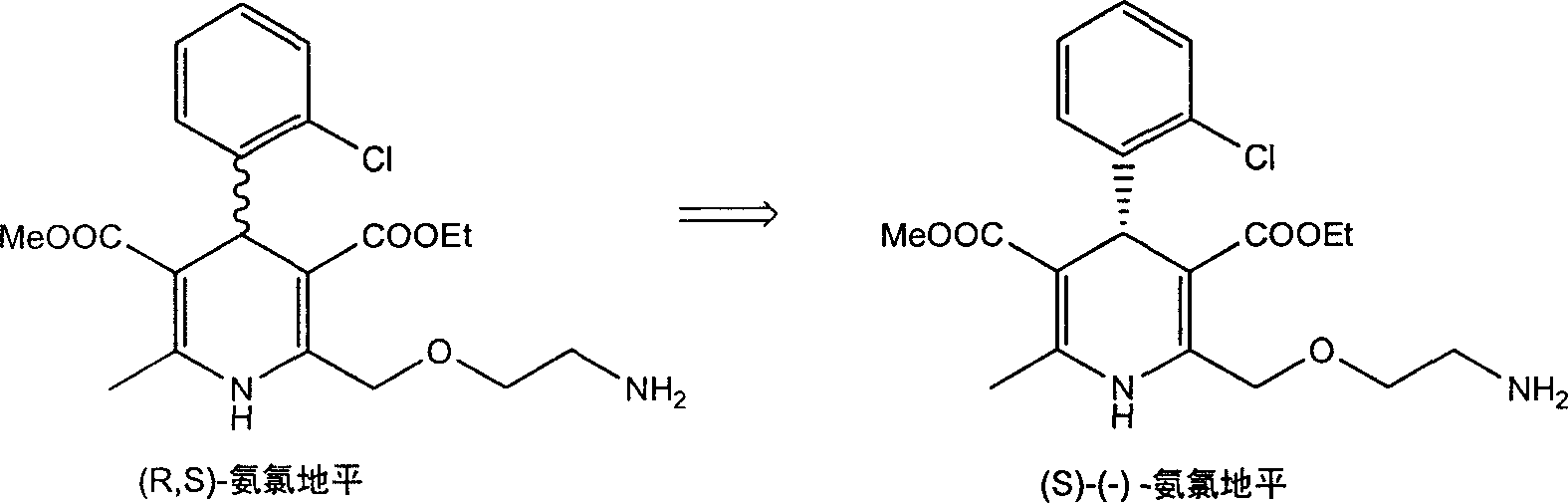
Method for splitting Amlodipine
This invention discloses a method for resolving amlodipine. The method uses tartaric acid as the chiral reagent, and N-methylpyrrolidone as the chiral aid. The method has such advantages as high resolving rate, high product optic purity and simple process, and is suitable for mass production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA +1
Levamlodipine beaylate tablets and preparation method thereof
InactiveCN101721384AGood compressibilityPromote dissolutionOrganic active ingredientsPharmaceutical delivery mechanismMedicineFiller Excipient
The invention discloses levamlodipine beaylate tablets which is prepared by comprising the following raw materials in parts by weight: 1-20 parts of levamlodipine beaylate, 20-150 parts of filling agent, 10-100 parts of disintegrating agent and 1-10 parts of lubricant. The preparation method of the levamlodipine beaylate tablets comprises the following steps of: evenly mixing the raw materials, crushing, screening with a 60-100 mesh sieve, evenly mixing, and preparing the levamlodipine beaylate tablets in a novel powder feeder for a tablet machine by using a direct dry powder tablet compressing method. Compared with the traditional tablet production technology, the levamlodipine beaylate tablets of the invention have the advantages of favorable compressibility, friability and tablet weight variation, uniform content and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
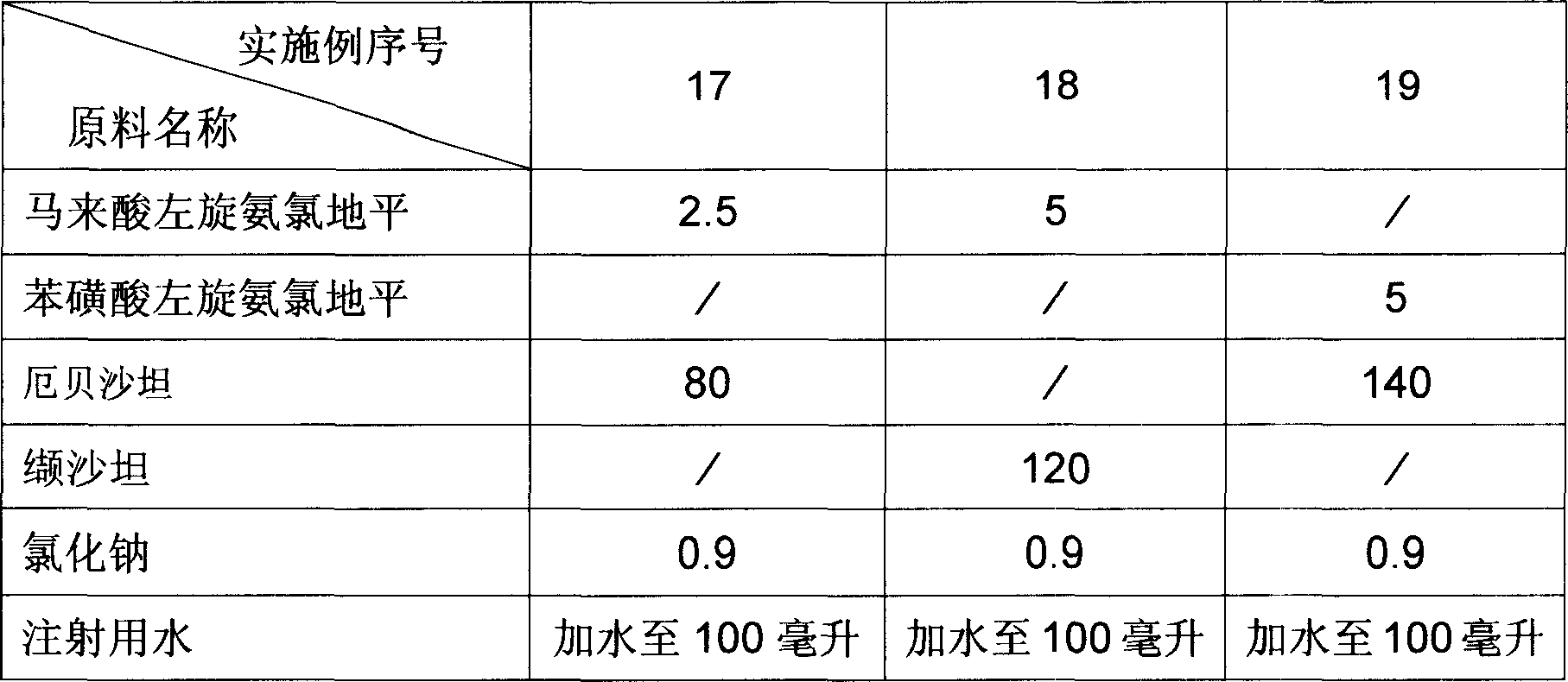
Aqueous oral preparation of stable amlodipine
InactiveUS20110294860A1Masking of bitter tasteEasy to carryBiocideOintment deliveryAmlodipineSURFACTANT BLEND
The invention provides a stable and rapidly disintegrable aqueous oral preparation (liquid or jelly preparation) of amlodipine. The liquid preparation comprises an anionic surfactant having a sulfuric acid group or a sulfonic acid group as a stabilizer in an aqueous solution of amlodipine, at pH 5-7, while the jelly preparation further comprises a gelling agent, a fine powder solid, and a gelling regulator.
Owner:MEDRX CO LTD
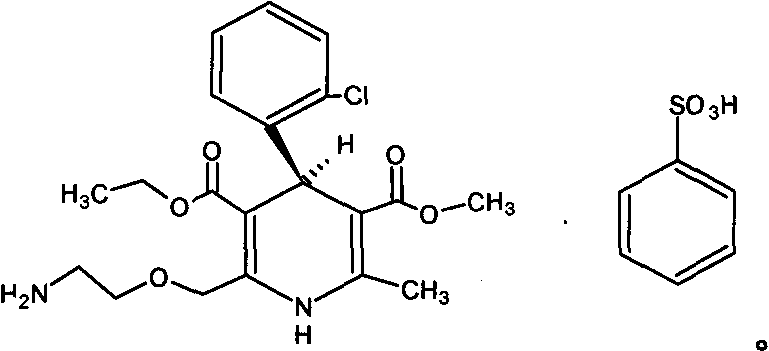
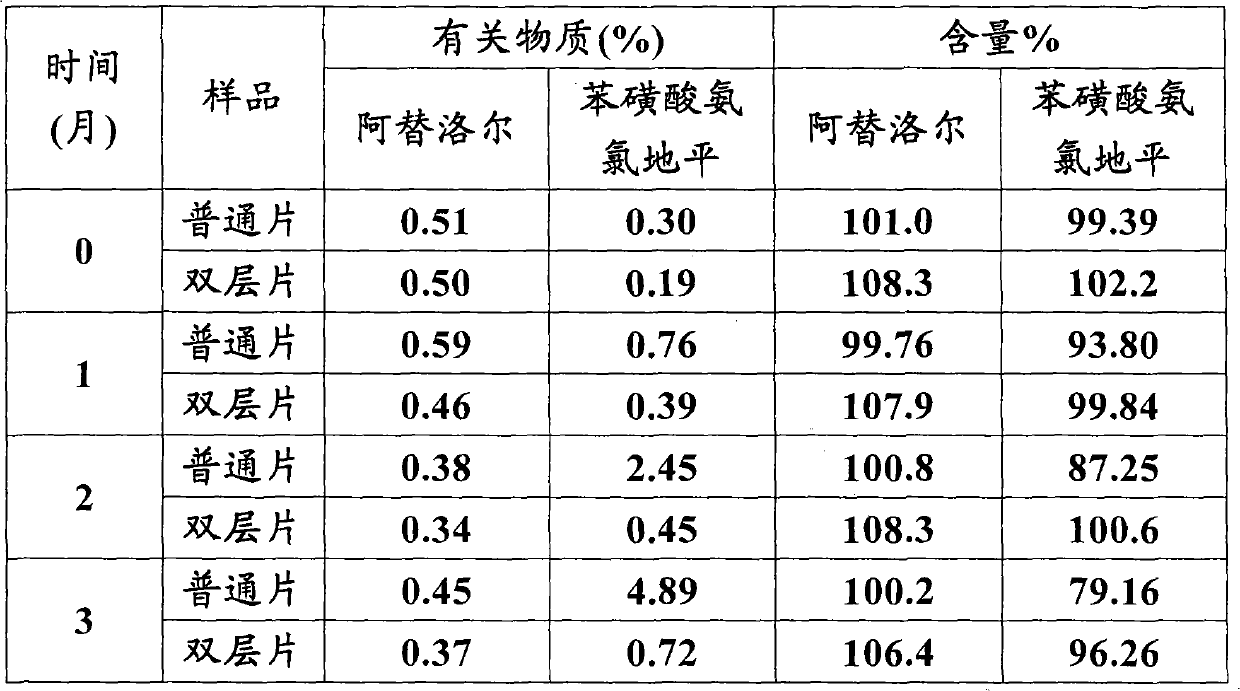
Atenolol and amlodipine bilayer tablet
ActiveCN102085201AOvercome unsatisfactory dissolutionOrganic active ingredientsPill deliveryDissolutionAmlodipine
The invention relates to an atenolol and amlodipine bilayer tablet. Particularly, the invention relates to a bilayer tablet comprising a first tablet layer and a second tablet layer, wherein the first tablet layer contains atenolol as an active constituent and a pharmaceutically acceptable excipient or accessory, and the second tablet layer contains amlodipine as an active constituent or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable excipient or accessory. The invention also provides a preparation method of the bilayer tablet. The bilayer tablet provided by the invention not only has favorable stability, but also has favorable dissolution rate.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Production method of levamlodipine besylate
The invention takes N, N-dimethylformamide as a chiral auxiliary and separates amlodipine with tartaric acid resolution reagent to prepare l-amlodipine. In addition, benzene sulfonic acid and l-amlodipine alkali are directly salified and refined, filtered and dried via a special filter to produce levoamlodipine besylate. The upper part and the lower part of the special filter adopted by the invention are respectively provided with a hemispherical top cap and a hemispherical bottom cap, the middle part is provided with a lauter tank and ring groove filter plates are respectively arranged between the top cap and the lauter tank or the bottom cap and the lauter tank. A feed pipe, inlet and outlet of inert gases, an outlet of cooling fluid and a temperature meter are installed on the top cap of the filter; a discharge pipe and the outlet of cooling fluid are installed on the bottom cap. The filter is provided with an insulating layer and an interlayer, thus can control the temperature, avoid light, be filled with inert gases and protect the feed liquid and filtrate from oxidation, illumination and high temperature damage. The filtration efficiency is high, the effect is good and the structure is simple, the filter operation, disassembly, assembly and cleaning are convenient and the levoamlodipine besylate enjoys high synthesis and production yield and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Resolution method of optically active amlodipine
ActiveCN1927836AMoisture content no special requirementsShort reaction timeOrganic chemistrySolventAmlodipine
The present invention relates to chemical method of resolving despinner Amlodipine into (S)-(-)-Amlodipine and (R)-(+)-Amlodipine. The resolving agent is L-tartaric acid or D-tartaric acid; and the solvent is methyl ethyl sulfoxide or mixed solvent containing methyl ethyl sulfoxide. The present invention has greatly shortened reaction period and no special requirement on the water content in the solvent, and is especially suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Solid preparation of angiotensin receptor inhibitor and amlodipine and new preparation method thereof
InactiveCN101507715AQuality improvementImprove stabilityPill deliveryPharmaceutical non-active ingredientsValsartanAngiotensin receptor
The invention relates to an angiotensin receptor inhibitor and a solid preparation of amlodipine and a novel method for preparing the same. The method comprises the following steps that: 1, valsartan and amlodipine are screened respectively until the grain size of the valsartan and amlodipine is qualified; 2, the valsartan and amlodipine are added with proper amount of excipient respectively and the mixture is evenly mixed; and 3, the evenly mixed powder are added with proper amount of bonding agent or wetting agent respectively to obtain grain in proper grain size, and the grain is dried.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for preparing S-(-)-amlodipine and R-(+)-amlodipine by chirally resolving racemic amlodipine
ActiveCN101798280AWide variety of sourcesCheap sourceOptically-active compound separationOrganic racemisationSolventTartrate
The invention discloses a method for preparing S-(-)-amlodipine and R-(+)-amlodipine by chirally resolving racemic amlodipine. The resolving solvent is ethanol or a mixed solvent containing ethanol, chiral dibenzoyl-D-tartaric acid or dibenzoyl-L-tartaric acid is used as the resolving agent, the chiral resolving agent and the racemic amlodipine selectively form chiral amlodipine dibenzoyl tartrate, and the chiral amlodipine dibenzoyl tartrate is alkalified to obtain the chiral amlodipine. The invention adopts the ethanol as the resolving solvent; and when using industrial ethanol as the resolving solvent, the invention can also acquire good resolving effect and obtain the qualified medicinal amlodipine raw material, thereby obviously reducing the cost. The invention has the characteristics of simple reaction operation, easy control, low toxicity, environmental protection, high efficiency and the like, and is suitable for green large-scale production.
Owner:石家庄润柏医药科技有限公司
Levamlodipine besylate crystal and preparation method and application thereof
ActiveCN105111137AClarify the main parametersClear crystal formOrganic chemistry methodsSulfonic acids salts preparationSolubilitySpace group
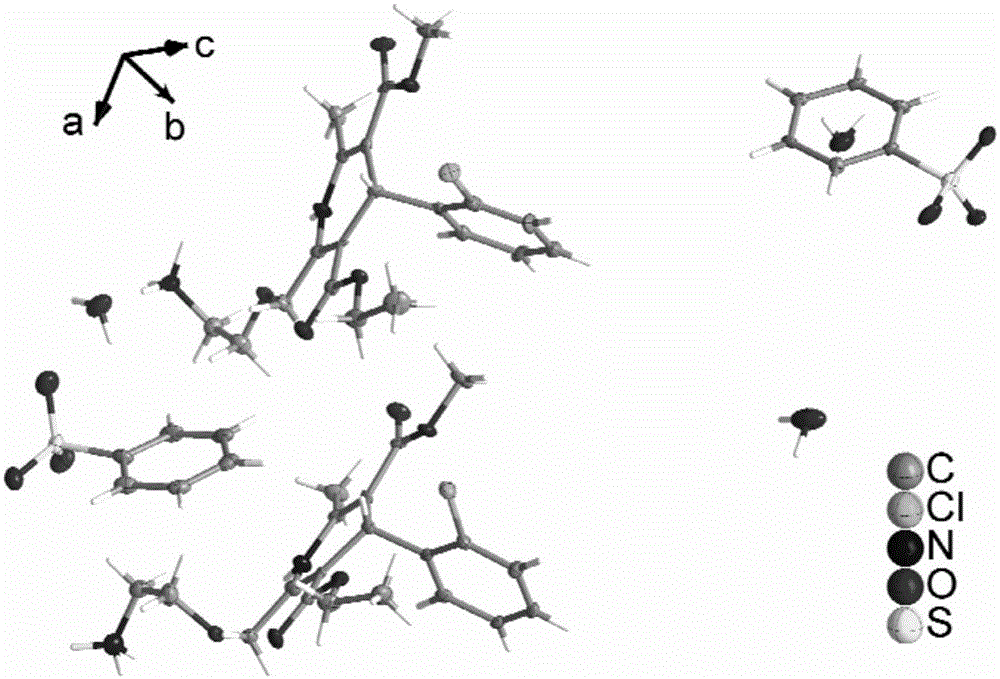
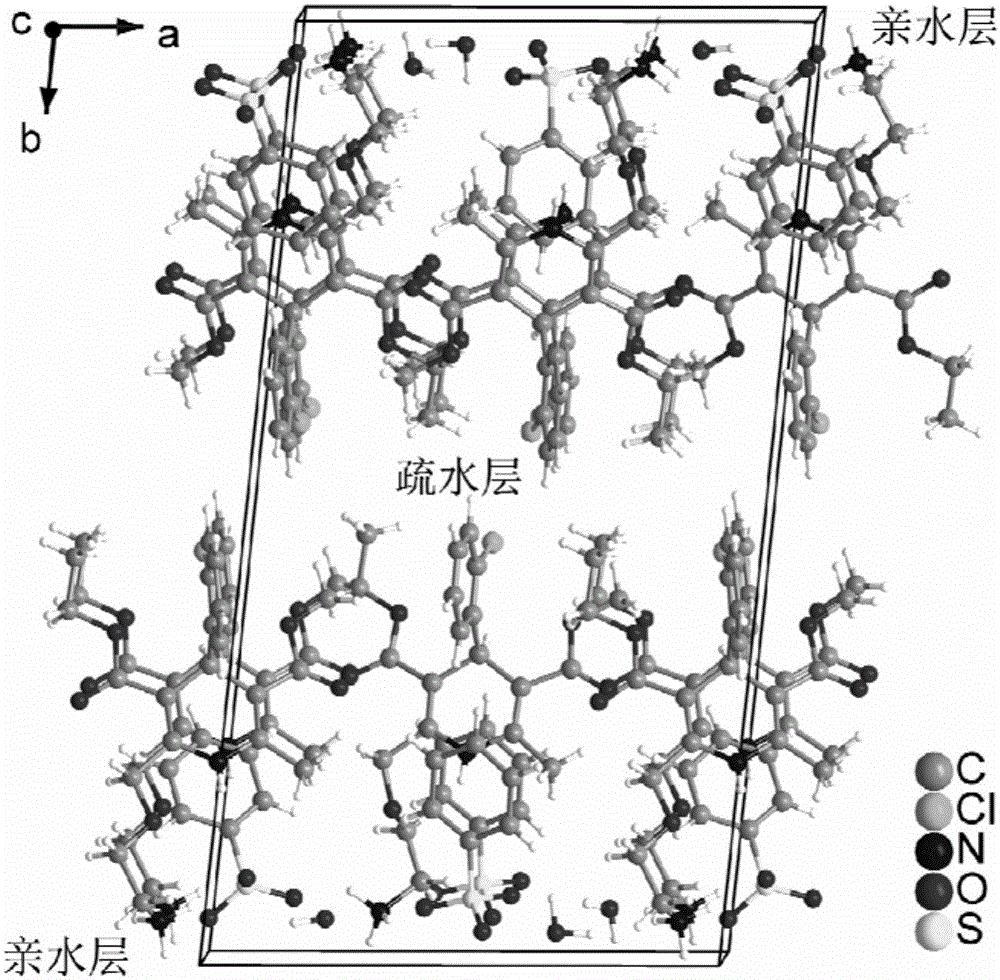
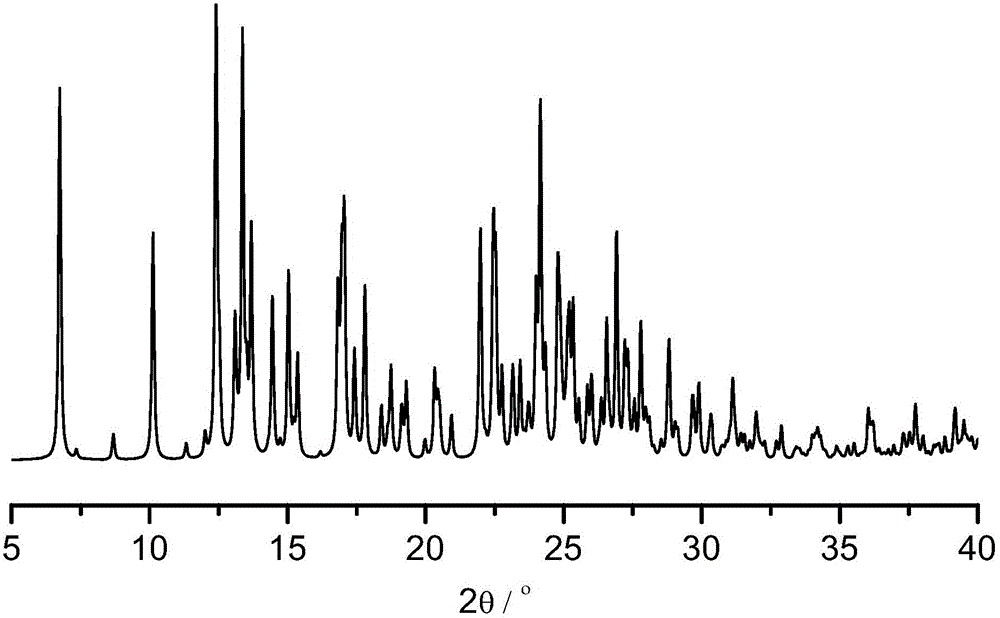
The invention provides a levamlodipine besylate crystal with the molecular formula being (C20H25ClN2O5) (C6H6O3S) (H2O) 1.5 and the molecular weight being 594.07. Crystallography measurement parameters are a monoclinic system and P21 chirality space groups, and the chirality absolute configuration is determined by crystallography Flack parameter being 0.08(6); the unit cell size is shown in the specification, wherein beta is 95.817(4) degrees, and V is 2880.1(11). The characteristic peak in an X-ray powder diffraction pattern (Cu-Kalpha) is displayed at 2thea being 6.70 degrees, 10.12 degrees, 12.40 degrees, 13.36 degrees, 13.68 degrees, 17.04 degrees, 22.46 degrees and 24.16 degrees. The invention further provides a preparation method and application of the levamlodipine besylate crystal. The levamlodipine besylate crystal has the specific crystal form and the amount of crystal water and specific crystallography main parameters and the exact atom spatial position, the solubleness and stability of existing levamlodipine besylate are improved, the stability and bioavailability of the levamlodipine besylate tablet can be improved, preparation is easy, the cost is low, and the obtained crystal is regular in form, uniform in particle size and suitable for large-scale application and popularization.
Owner:菲洋生物科技(吉林)有限公司
Process for preparation of chiral amlodipine salts
InactiveCN1882543AReduce usageIncrease workloadOrganic active ingredientsOptically-active compound separationOrganic acidAmlodipine
A process for the preparation of pharmaceutically acceptable salts of chiral Amlodipine namely S(-) Amlodipine and R(+) Amlodipine without isolation of the chiral free base wherein the product has optical purity ranging between 96-99% is described in the present invention. The process comprises resolving RS amlodipine base using L(+) or D(-) tartaric acid followed by reaction of the separated tartrate salt with an organic acid to obtain the salt corresponding to the acid used in ee ranging from 96-99%.
Owner:COUNCIL OF SCI & IND RES
Method for preparing S-(-)-amlodipine
InactiveCN101209991AOptically-active compound separationOrganic racemisationAmlodipineTetrahydrofuran
The invention relates to an economical and effective method used for preparing S-(-)-amlodipine by taking tetrahydrofuran as solvent and D-tartaric acid as resolution agent to resolve racemic amlodiphine.
Owner:钟南平
Preparation method of Levamlodipine besylate tablet
InactiveCN102846565AHigh dissolution rateConducive to the amount of controlOrganic active ingredientsPill deliveryLevamlodipineDissolution
A preparation method of a Levamlodipine besylate tablet includes pulverizing Levamlodipine besylate, a filler and a disintegrant, sieving, mixing, adding a lubricant, granulating, and tableting. The invention reduces the amount of related substance of the Levamlodipine besylate tablet, and improves stability. The method uses a fluidized bed granulation step to prepare the Levamlodipine besylate tablet, so as to simplify preparation step, shorten time, optimize process parameter, significantly increase the dissolution of the product and improve quality. The inventive method is suitable for industrial production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
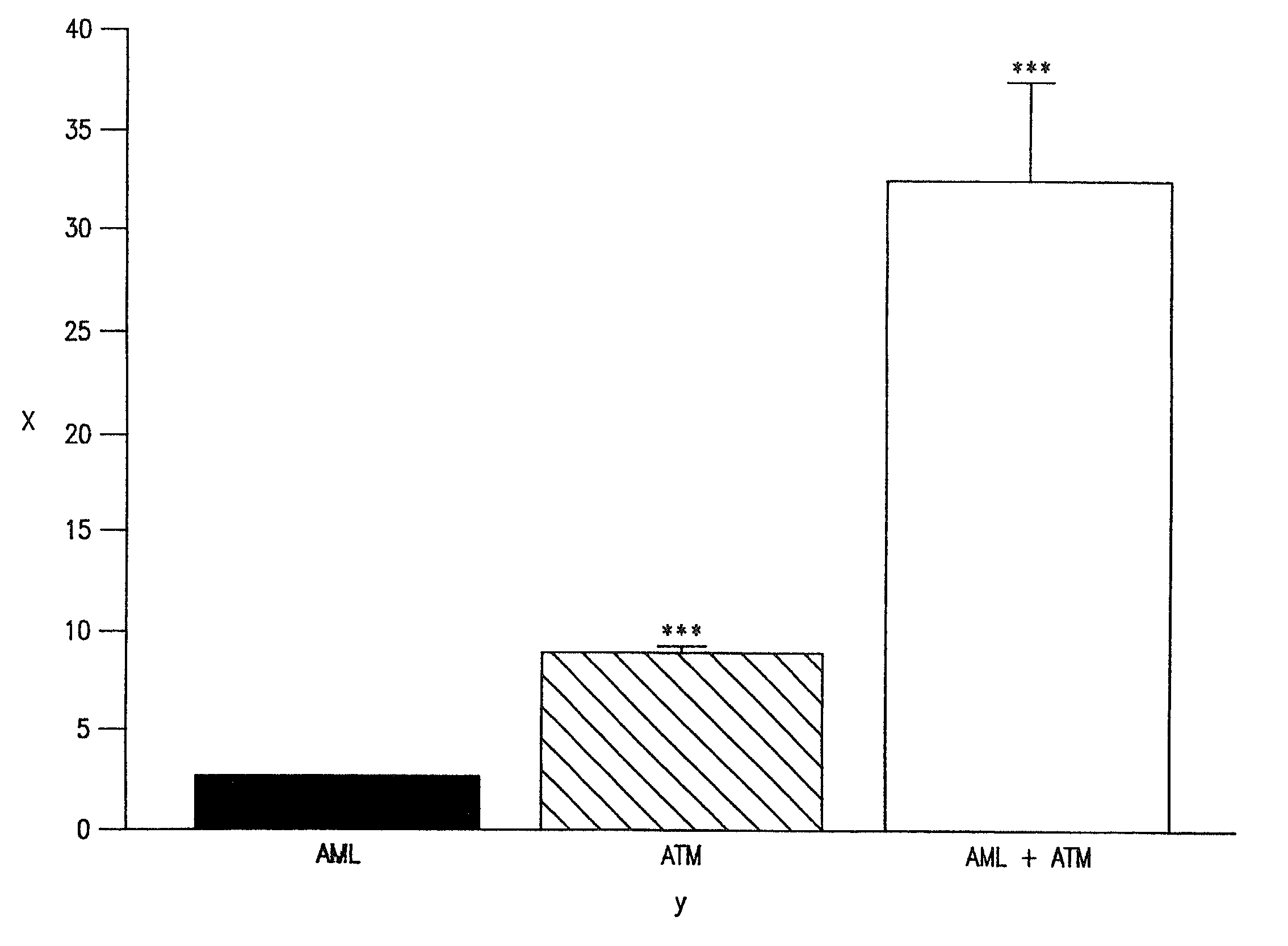
Synergistic effect of amlodipine and atorvastatin on aortic endothelial cell nitric oxide release
The combination of amlodipine and atorvastatin act to synergistically synthesize NO production. Moreover, the addition of a tertiary compound complements this combination of amlodipine and atorvastatin in NO production.
Owner:MASON R PRESTON
Valsartan and amlodipine compound preparation and preparation method thereof
InactiveCN102091069AEffective growth controlAvoid direct contactPill deliveryCapsule deliveryValsartanBULK ACTIVE INGREDIENT
The invention discloses valsartan and amlodipine compound preparation and a preparation method thereof. In the invention, valsartan or pharmaceutically acceptable salts thereof and amlodipine or pharmaceutically acceptable salts thereof are used as active ingredients, the valsartan and a pharmaceutical auxiliary material are mixed and rolled by a rolling process to form a pressed material; the pressed material is sieved to obtain a granular material; and the granular material is mixed with amlodipine to form tablets or capsules. In the invention, the valsartan and the auxiliary material are granulated first and then mixed with the amlodipine equally, so the liquidity of the material is improved; and the prepared product is very stable, and the process is simple and suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
(S)-amlodipine malate
One aspect of the present invention relates to optically pure (S)-amlodipine malate. Another aspect of the present invention relates to (rac)-amlodipine malate. In a preferred embodiment, the compound is optically pure (S)-amlodipine L-malate. Another aspect of the present invention relates to a pharmaceutical composition comprising optically pure (S)-amlodipine malate. Another aspect of the present invention relates to a method of preparing optically pure (S)-amlodipine malate, comprising admixing optically pure (S)-amlodipine with malic acid. Another aspect of the present invention relates to the various polymorphic and solvated forms of optically pure (S)-amlodipine malate. In another prefered embodiment the invention relates to polymorphic and solvated forms A-G. The present invention also relates to a method of preparing optically pure (S)-amlodipine malate, comprising combining a salt of optically pure (S)-amlodipine with a malate salt to give optically pure (S)-amlodipine malate. In a preferred embodiment, the malate salt is an optically pure L-malate salt.
Owner:SEPACOR INC
Process for preparing amlodipine benzenesulphonate
InactiveCN1927837ALow costShort reaction pathOrganic chemistryBulk chemical productionBenzaldehydeAmlodipine
The present invention discloses preparation process of Amlodipine benzene sulfonate. The preparation process includes the following steps: 1. the reaction between 4-[2-(tritylamindo)ethoxy] ethyl acetoacetate and amine compound to produce 3-amino-4-[2-( tritylamindo)ethoxy] ethylcrotonate; 2. the reaction between o-chluoro benzaldehyde and methyl acetoacetate under the catalysis of alkali to produce 2-(2-o-chluorobenzal)-ethyl acetoacetate; 3. the reaction of the products in the foregoing steps to obtain 4-(2-chlorophenyl)-6-methyl-2-((2-(tritylamido) ethoxy) methyl)-1, 4-dihydropyridyl-3-ethyl formate-5-methyl formate; and 4. further direct reaction with benzene sulfonic acid to eliminate protecting group and form Amlodipine benzene sulfonate.
Owner:上海开特生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com