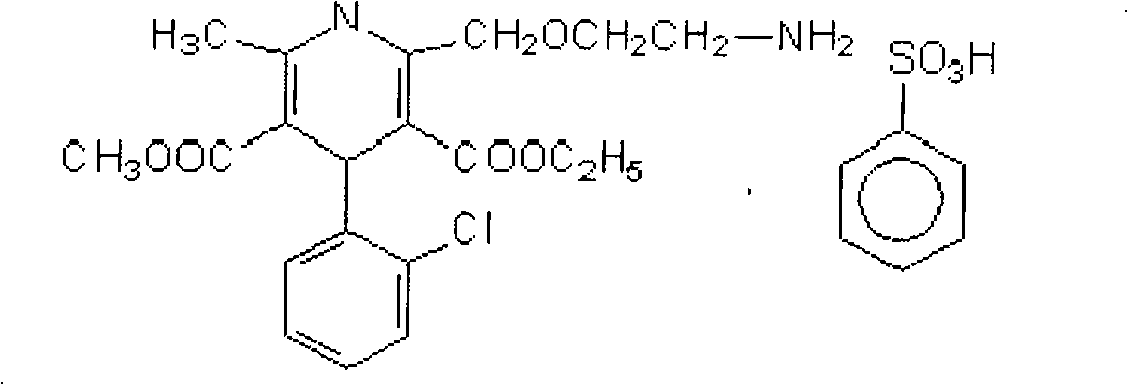Valsartan and amlodipine compound preparation and preparation method thereof
A valsartan-amlodipine compound formula and amlodipine technology are applied in the field of preparation of pharmaceutical preparations, can solve problems such as poor fluidity, great dissolution influence, unsuitability for industrialized production, and the like, and achieve the effects of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of valsartan amlodipine tablet
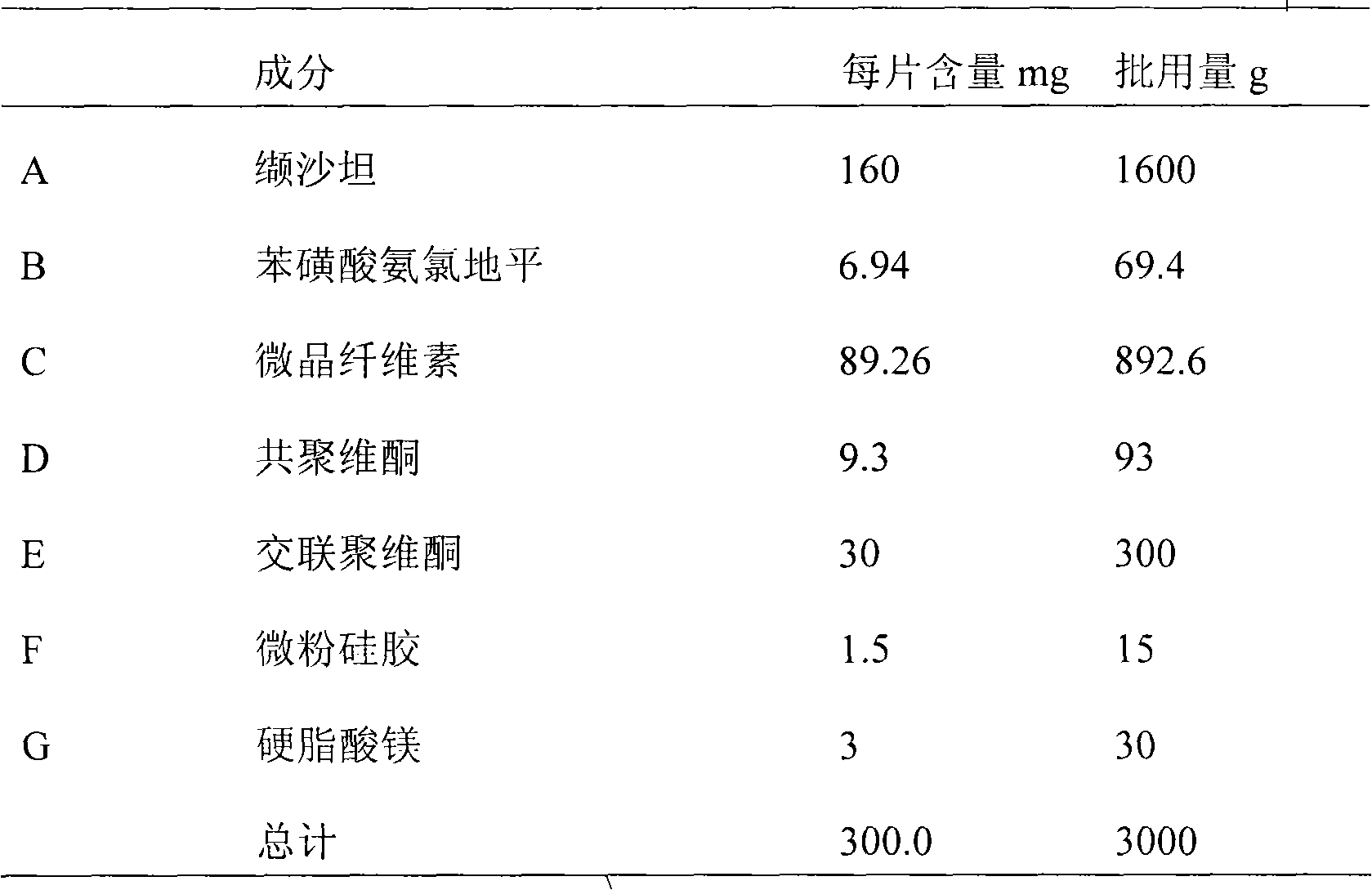
[0053] The material composition is shown in Table 2;
[0054] Table 2 Material Composition Ratio
[0055]
[0056]
[0057] Preparation of tablet cores: the valsartan and microcrystalline cellulose of prescription quantity, crospovidone, copovidone, micropowder silica gel and the magnesium stearate (1) of added part are mixed in total mixing barrel, adopt dry Granulate with French granulator (Alexander WP120V) to obtain dry granules. Pressure: 40bar, roller speed: 3rpm. The prescribed amount of amlodipine besylate is mixed with the above-mentioned dry granules, and an additional part of magnesium stearate (2) is added and mixed, and then compressed by a high-speed tablet machine.
[0058] Preparation of coated tablets: Weigh Ouba A coating solution is prepared to coat the plain tablets. Until the sheet has gained about 3% in weight. The indicators of the coated tablets meet the relevant regulati...
Embodiment 2
[0068] Embodiment 2: the preparation of valsartan amlodipine tablet
[0069] The material composition is shown in Table 6.
[0070] Table 6 Material Composition Ratio
[0071]
[0072]
[0073] Preparation of tablet cores: Mix the prescribed amount of valsartan and microcrystalline cellulose PH101, crospovidone, micropowder silica gel and the magnesium stearate (1) added in the total mixing tank, and use a dry granulator (Alexander WP120V) to obtain dry granules. Pressure: 60bar, roller speed: 5rpm. Mix the prescribed amount of amlodipine besylate and microcrystalline cellulose PH102, add to the above dry granules for mixing, then add an additional part of magnesium stearate (2) for mixing, and then use a high-speed tablet machine for tableting.
[0074] Preparation of coated tablets: Weigh Ouba A coating solution is prepared to coat the plain tablets. Until the sheet weight gain is 3%. The indexes of the coated tablets meet the relevant regulations, and the resul...
Embodiment 3
[0080] Embodiment 3: the preparation of valsartan amlodipine tablet
[0081] The material composition is shown in Table 9.
[0082] Table 9 Material Composition Ratio
[0083]
[0084] Prepare tablet core: the valsartan of recipe quantity and microcrystalline cellulose PH101, crospovidone, copovidone, micropowder silica gel and polyethylene glycol are mixed in total mixing tank, adopt dry granulator (Alexander WP120V ) granulation to obtain dry granules. Pressure: 60bar, roller speed: 3rpm. After mixing the amlodipine besylate and microcrystalline cellulose PH102 in the prescribed amount, they are added to the above dry granules for mixing, then magnesium stearate is added for mixing, and then the tablets are compressed with a high-speed tablet machine.
[0085] Preparation of coated tablets: Weigh Ouba A coating solution is prepared to coat the plain tablets. Until the weight gain of the tablet is about 3%, each index of the coated tablet meets the relevant regulatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com