Preparation method of tacrolimus solid dispersion, quick-release pharmaceutical composition and application
A technology of solid dispersion and tacrolimus, which is applied in the field of medicine, can solve the problems of many steps and inconvenient operation, and achieve the effect of stable quality and good uniformity of release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
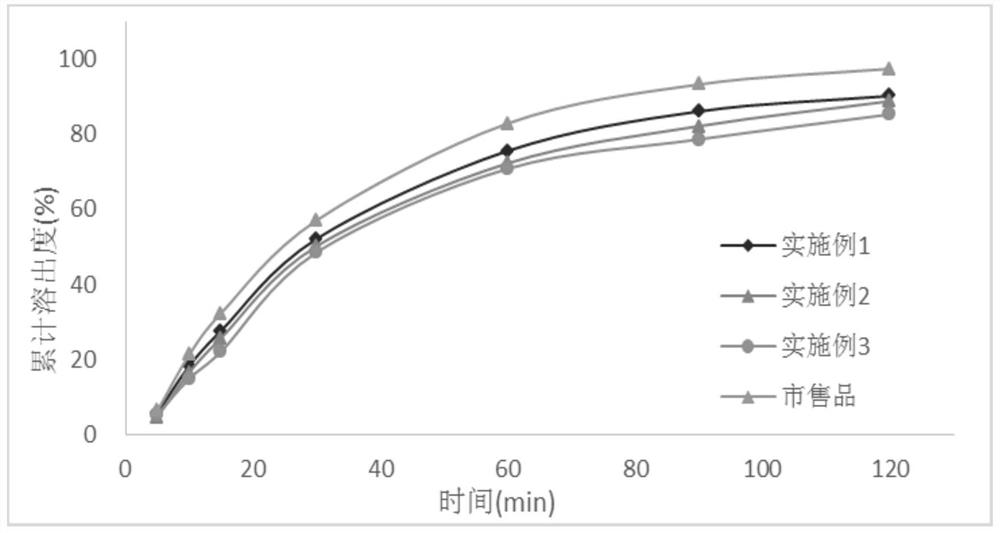
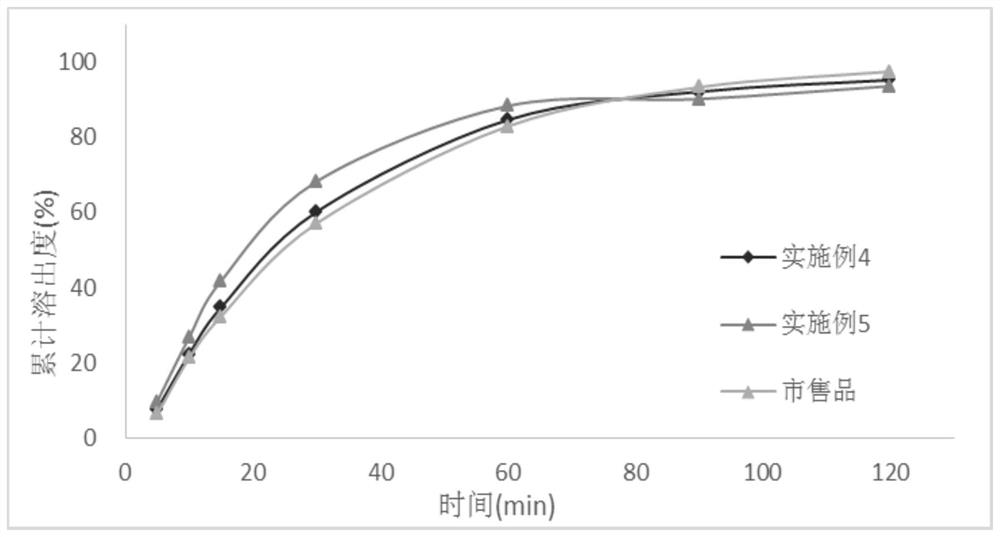
[0028] In the first aspect, an embodiment of the present invention provides a method for preparing a tacrolimus solid dispersion, comprising the following steps: using a fluidized bed one-step forming process, using a carrier material as a substrate, and spraying a tacrolimus solution After the granulation is completed, continue to dry in the fluidized bed to obtain a dried solid dispersion, wherein the tacrolimus solid dispersion contains 1-3 parts by mass of tacrolimus and 5- 10 parts by mass.
[0029] In an optional embodiment, the spray granulation parameters are: the air inlet temperature of the fluidized bed is 50°C-55°C, the material temperature is 20°C-35°C, and the air inlet temperature is a key parameter. If the temperature is too high, then Related substances will increase significantly; the air intake volume and atomization pressure will affect the size and uniformity of the prepared particles, which need to be adjusted in real time during the preparation process t...
Embodiment 1
[0042] The batch size is 15,000 capsules, and the capsule weight is 65mg / capsule.
[0043] prescription composition
[0044] Tacrolimus 15g Absolute ethanol 300g Hypromellose 615 45g Croscarmellose Sodium 6g lactose 900g Magnesium stearate 9.75g
[0045] The preparation method is as follows:
[0046] Dissolve tacrolimus in absolute ethanol, stir until clear and free of foreign matter; add hypromellose into the fluidized bed, set the air volume to 5m 3 / h, the air inlet temperature is 50°C, the material temperature is controlled at about 25°C, the atomization pressure is 0.05MPa, the backflush pressure is 0.06MPa, the peristaltic pump speed is 20r / min, the spray granulation is started, and the liquid medicine is dried at 50°C for 1 The weight loss on drying of the solid dispersion is less than 3% after 1 hour; add croscarmellose sodium, lactose, and magnesium stearate, mix well, and then fill the capsules, the capsule capacity is 6...
Embodiment 2
[0049] The batch size is 15,000 capsules, and the capsule weight is 65mg / capsule.
[0050] prescription composition
[0051] Tacrolimus 18g Absolute ethanol 360g Hypromellose 615 42g Croscarmellose Sodium 6g lactose 909.3g Magnesium stearate 9.75g
[0052] The preparation method and stripping method are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com