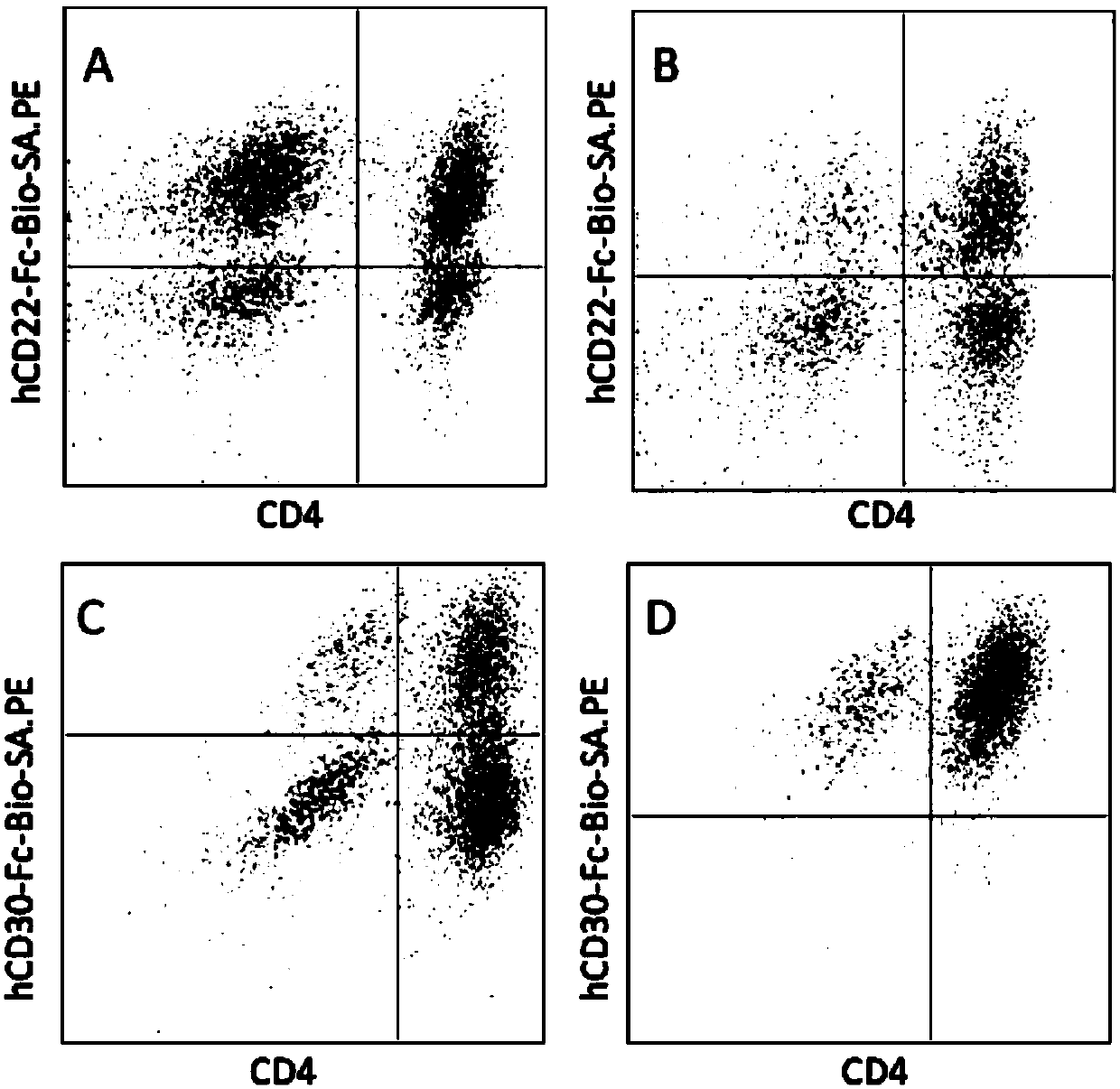
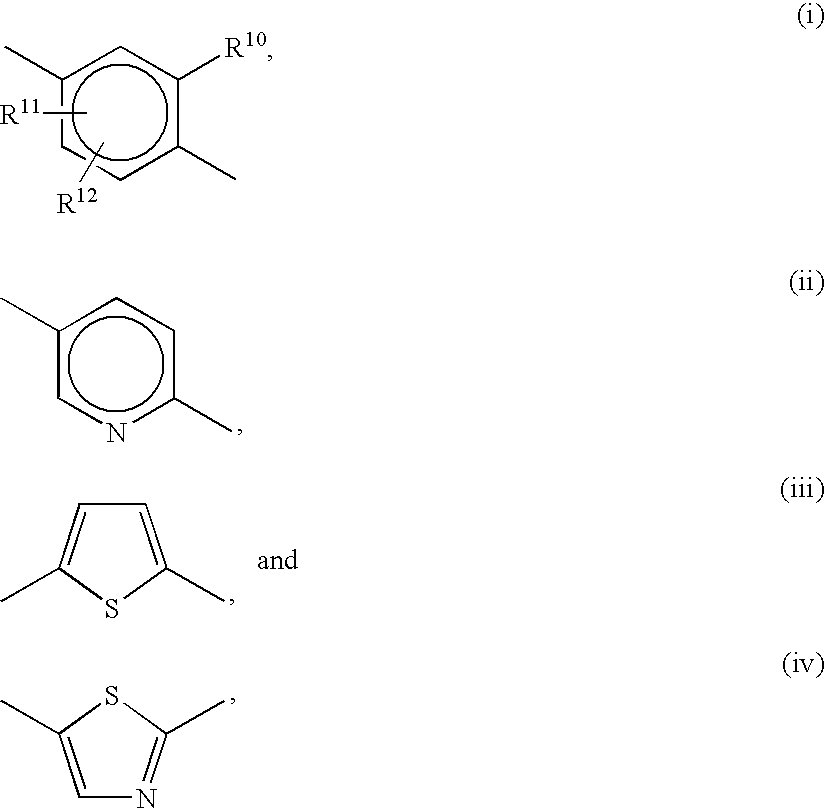
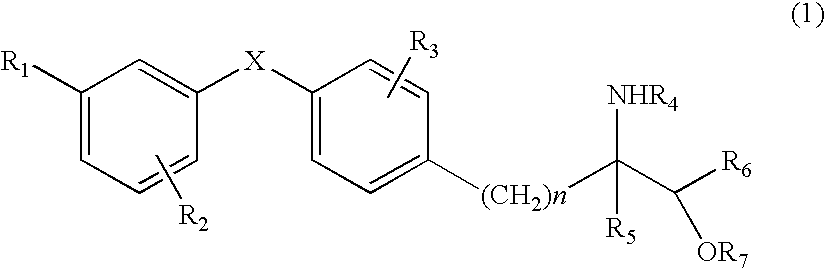
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
282 results about "Immunosuppressive effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Side effects of immunosuppressants may include: Diarrhea. Higher susceptibility to infection. Nausea. Vomiting.
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Diaryl sulfide derivative, addition salt thereof, and immunosuppressant
InactiveUS6960692B2Good effectLittle side effectsGroup 4/14 element organic compoundsBiocideArylSide effect
Owner:PRIOTHERA LTD
IL28 and IL29 TRUNCATED CYSTEINE MUTANTS AND ANTIVIRAL METHODS OF USING SAME
InactiveUS20070053933A1Organic active ingredientsPeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
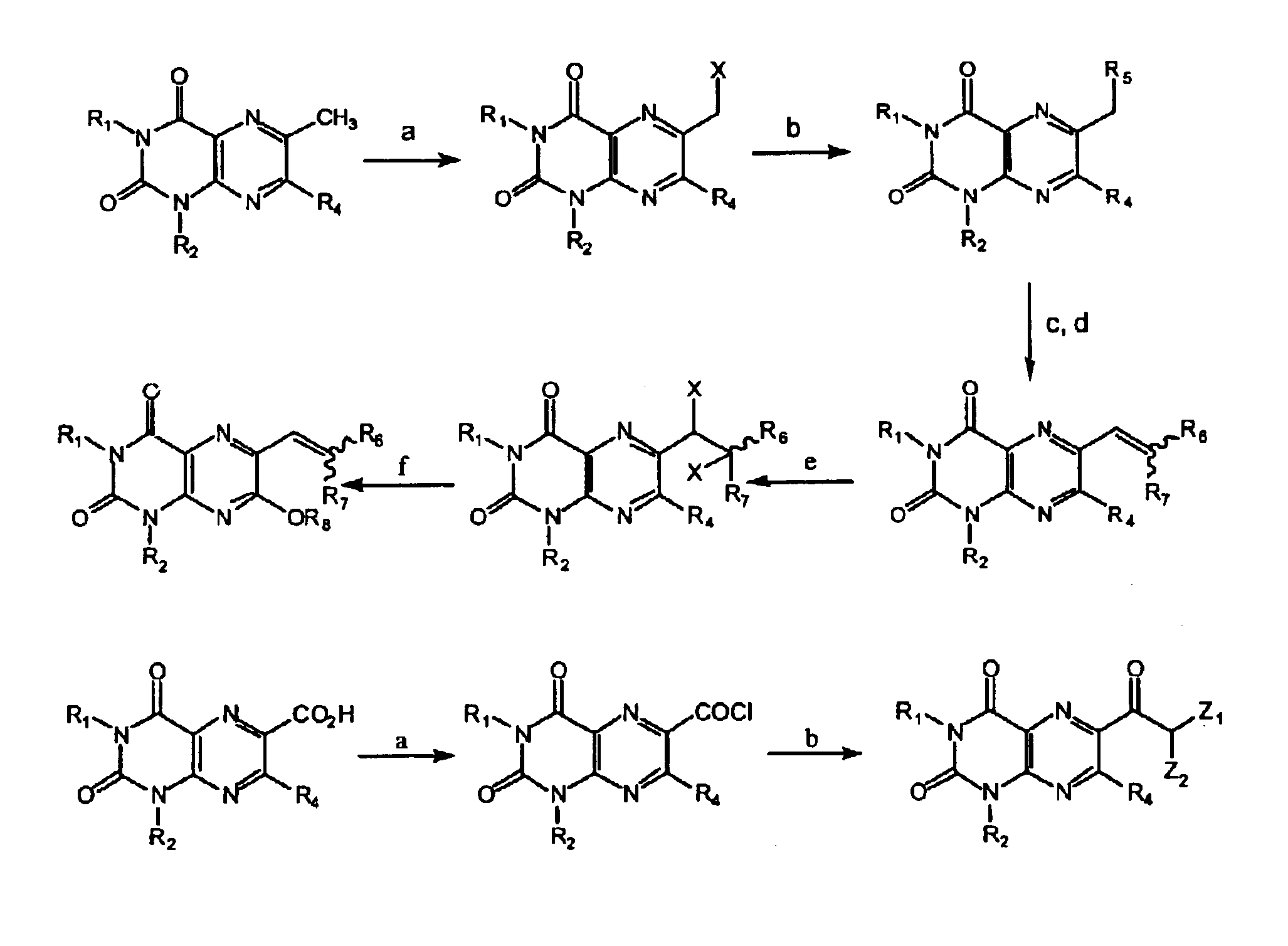
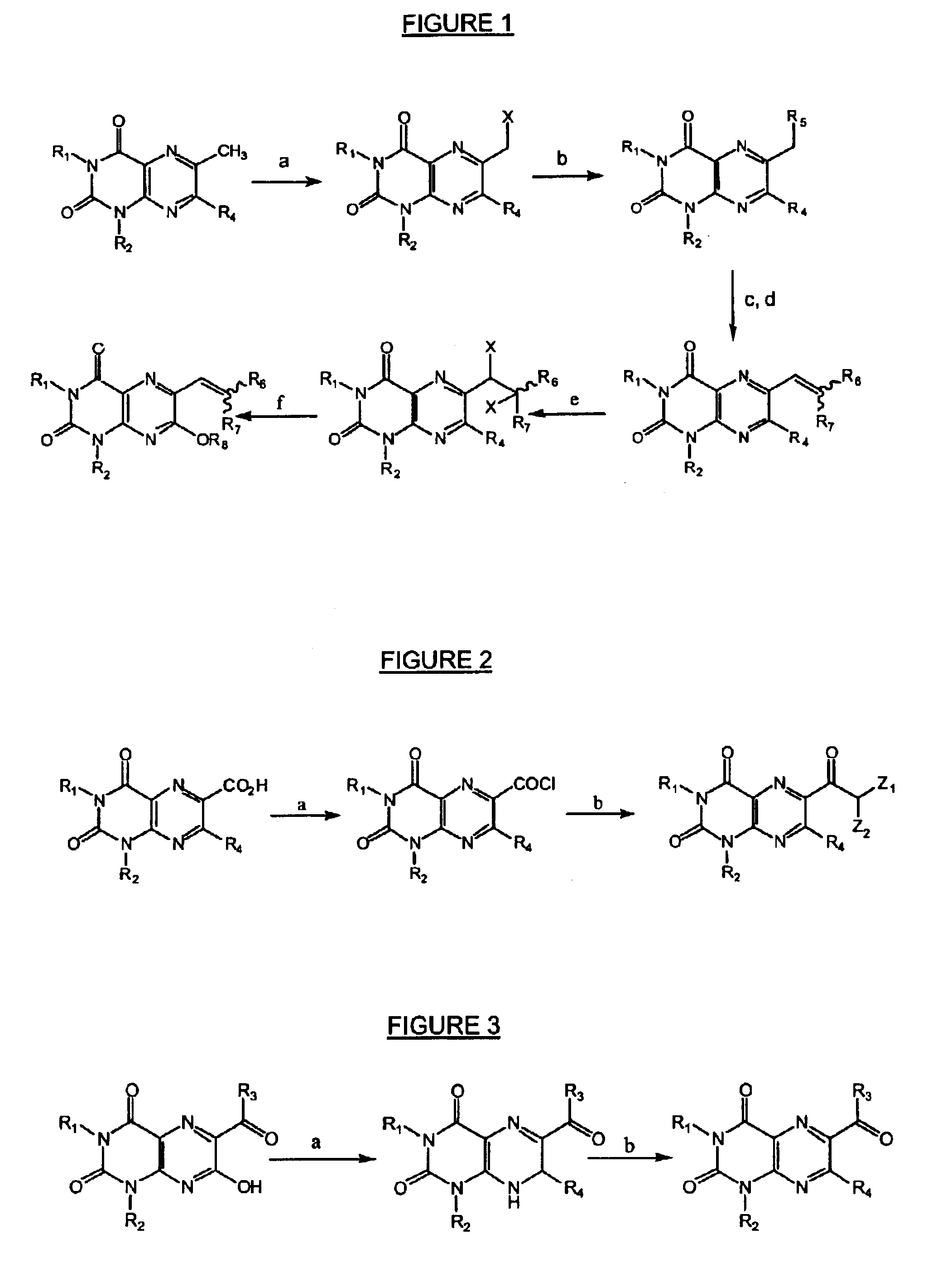
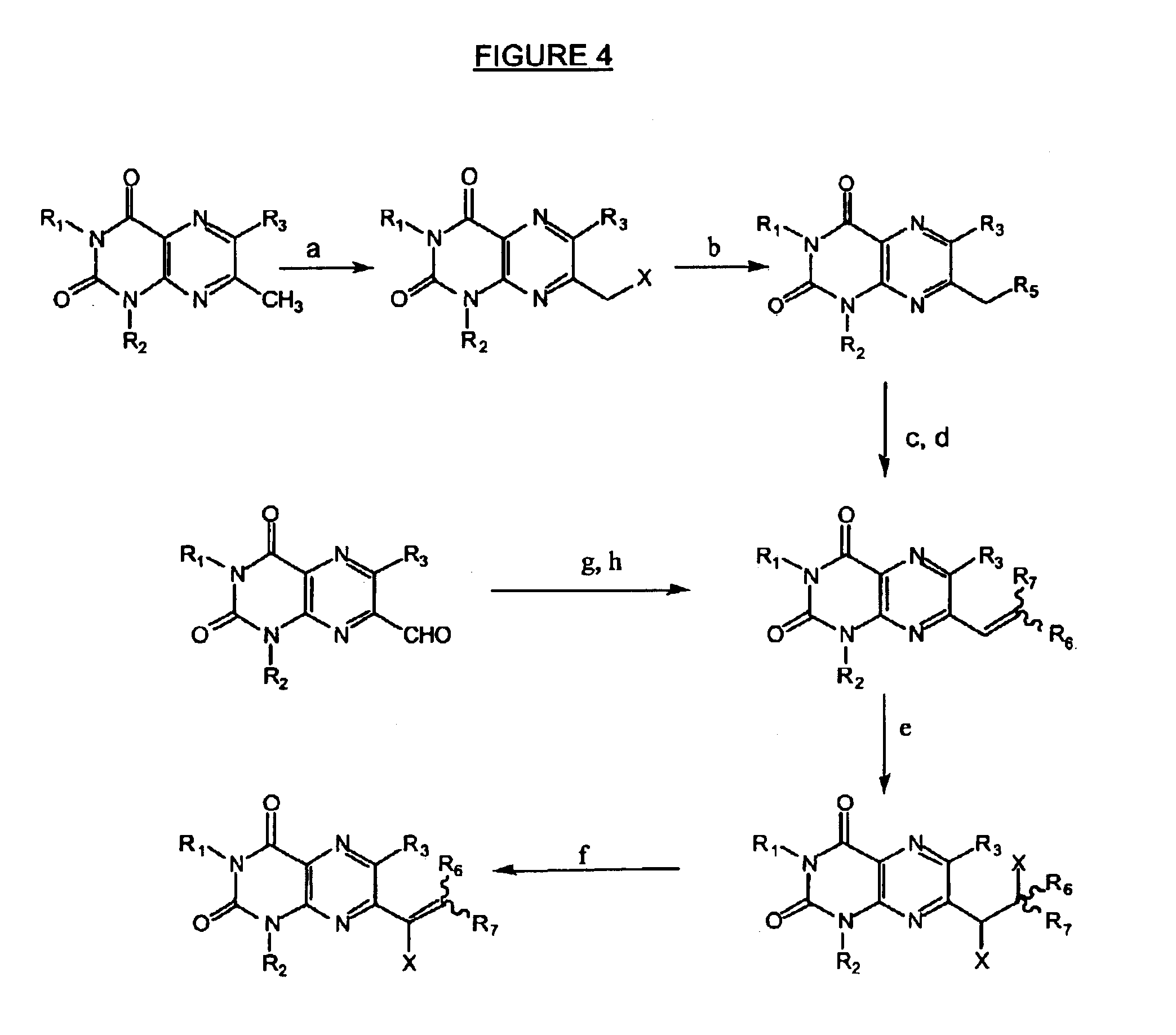
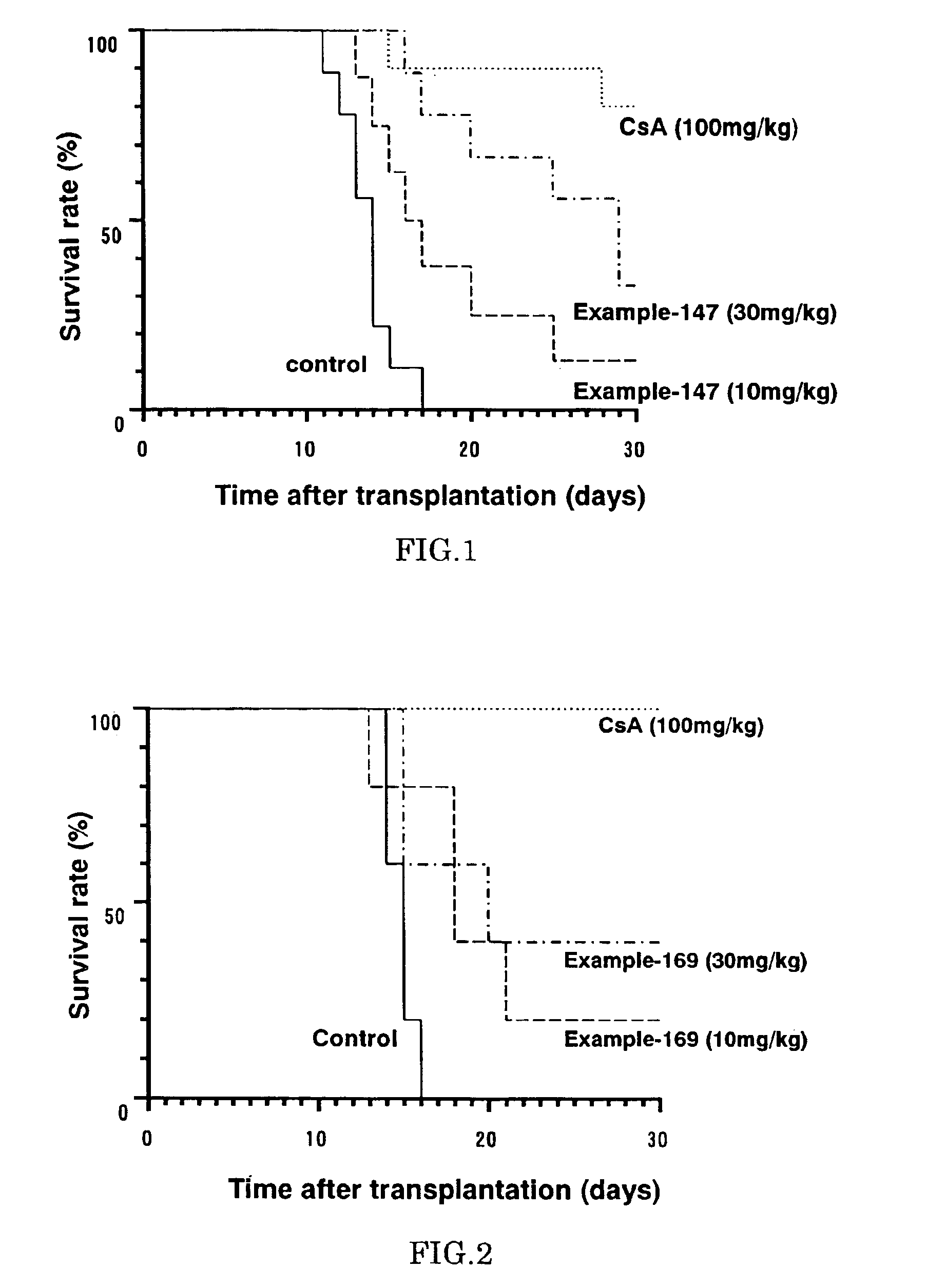
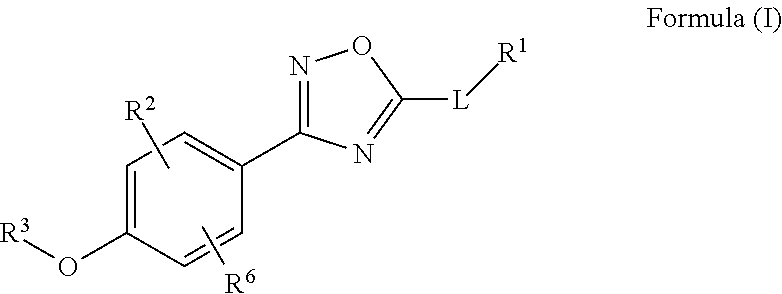
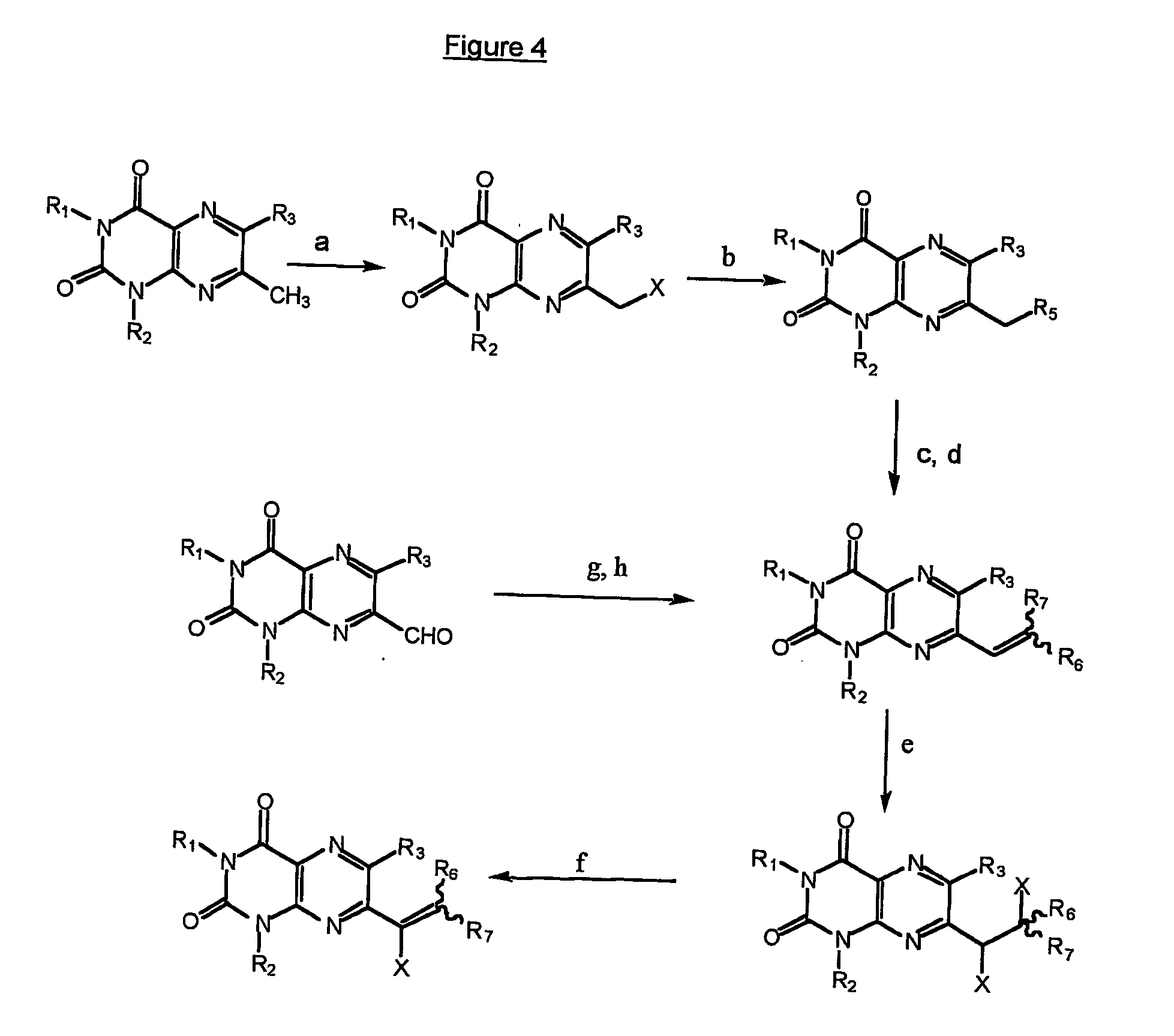
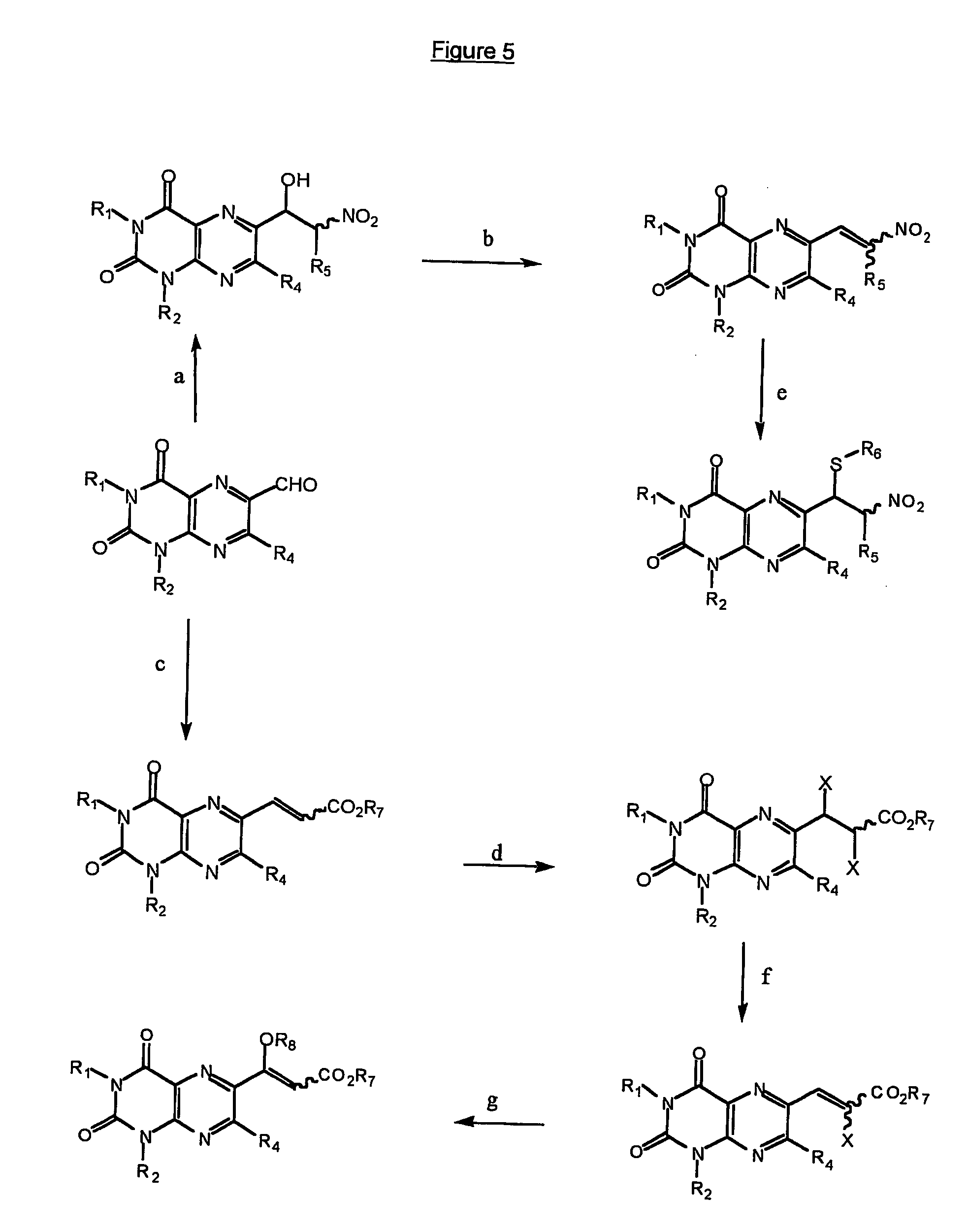
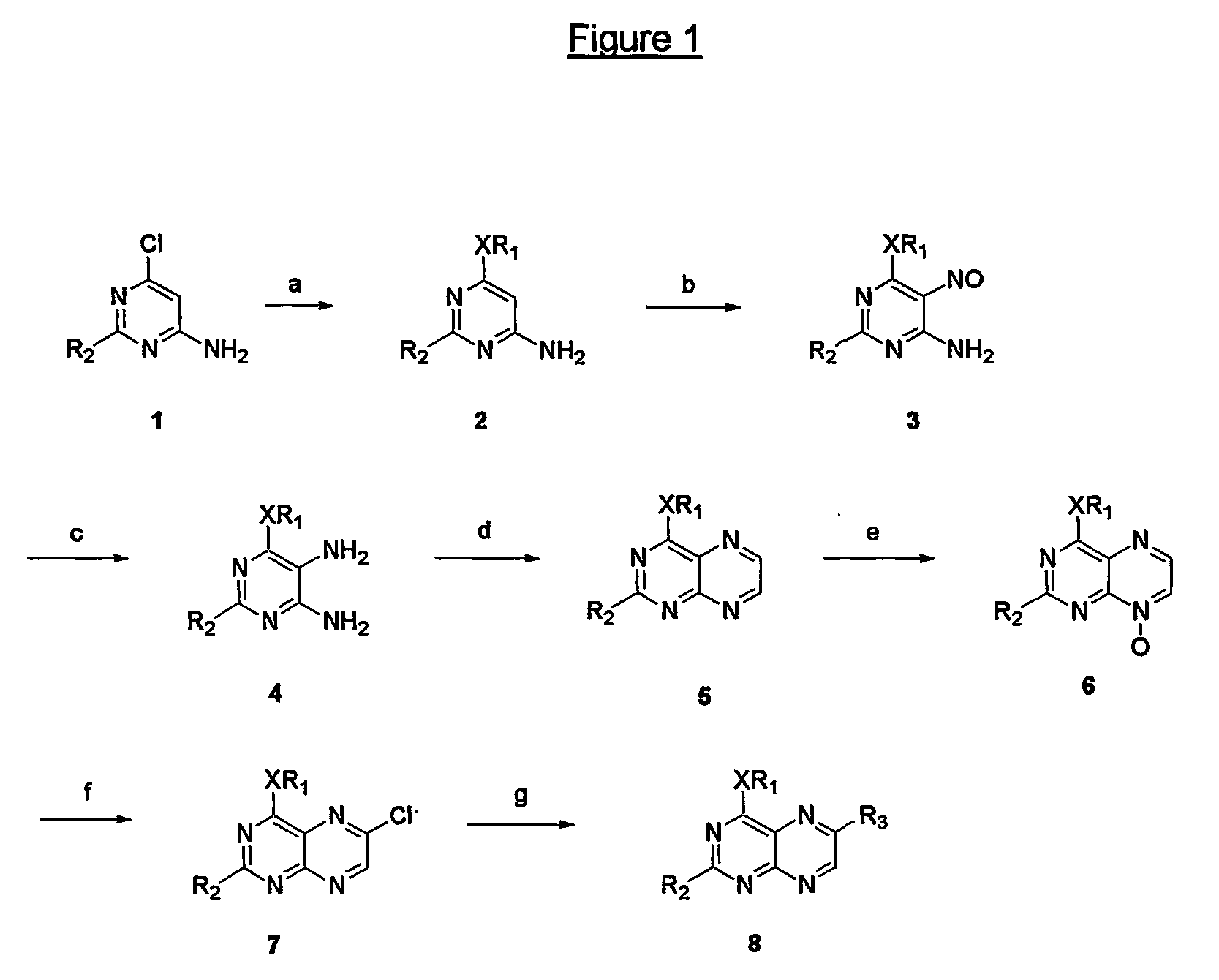
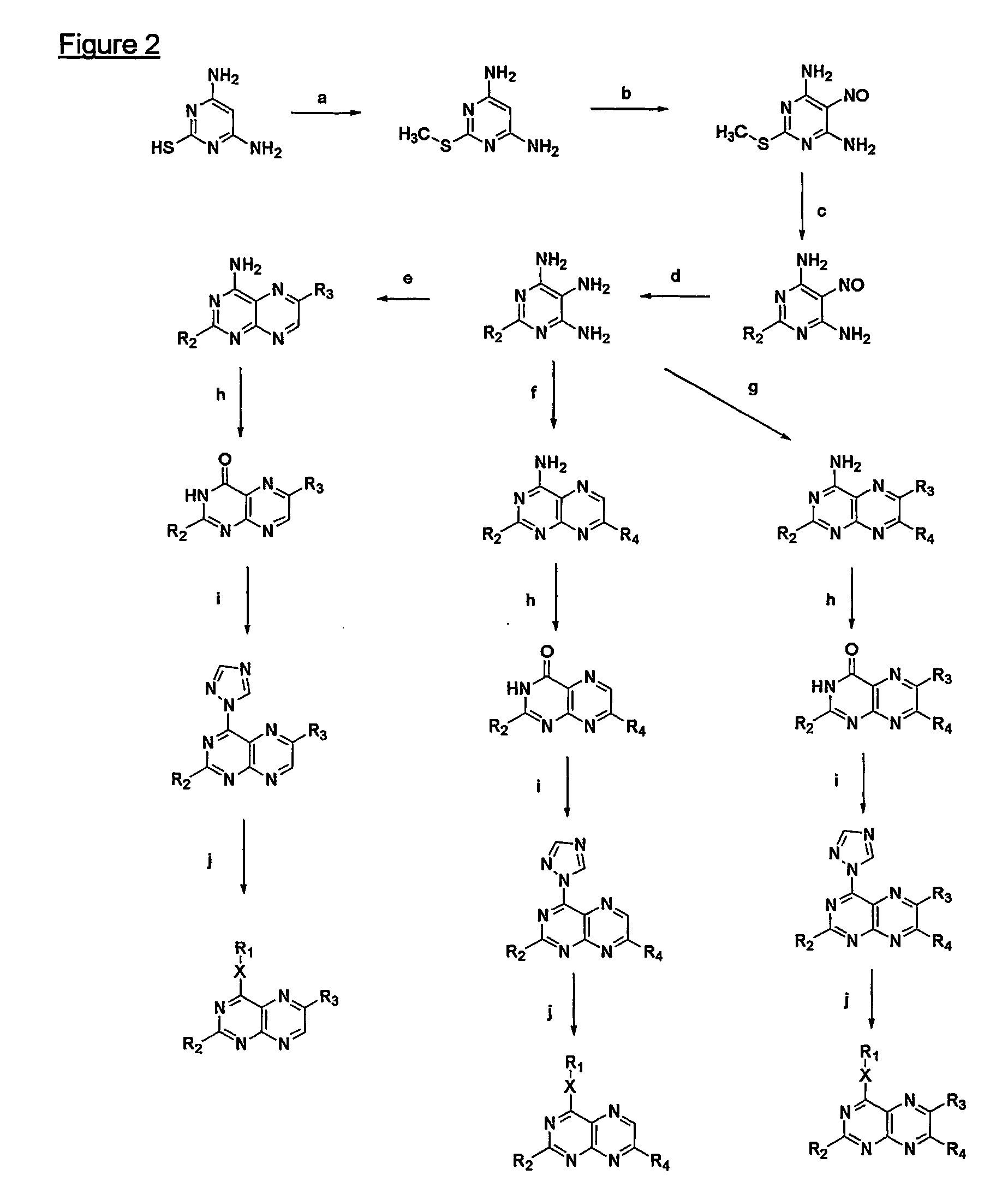
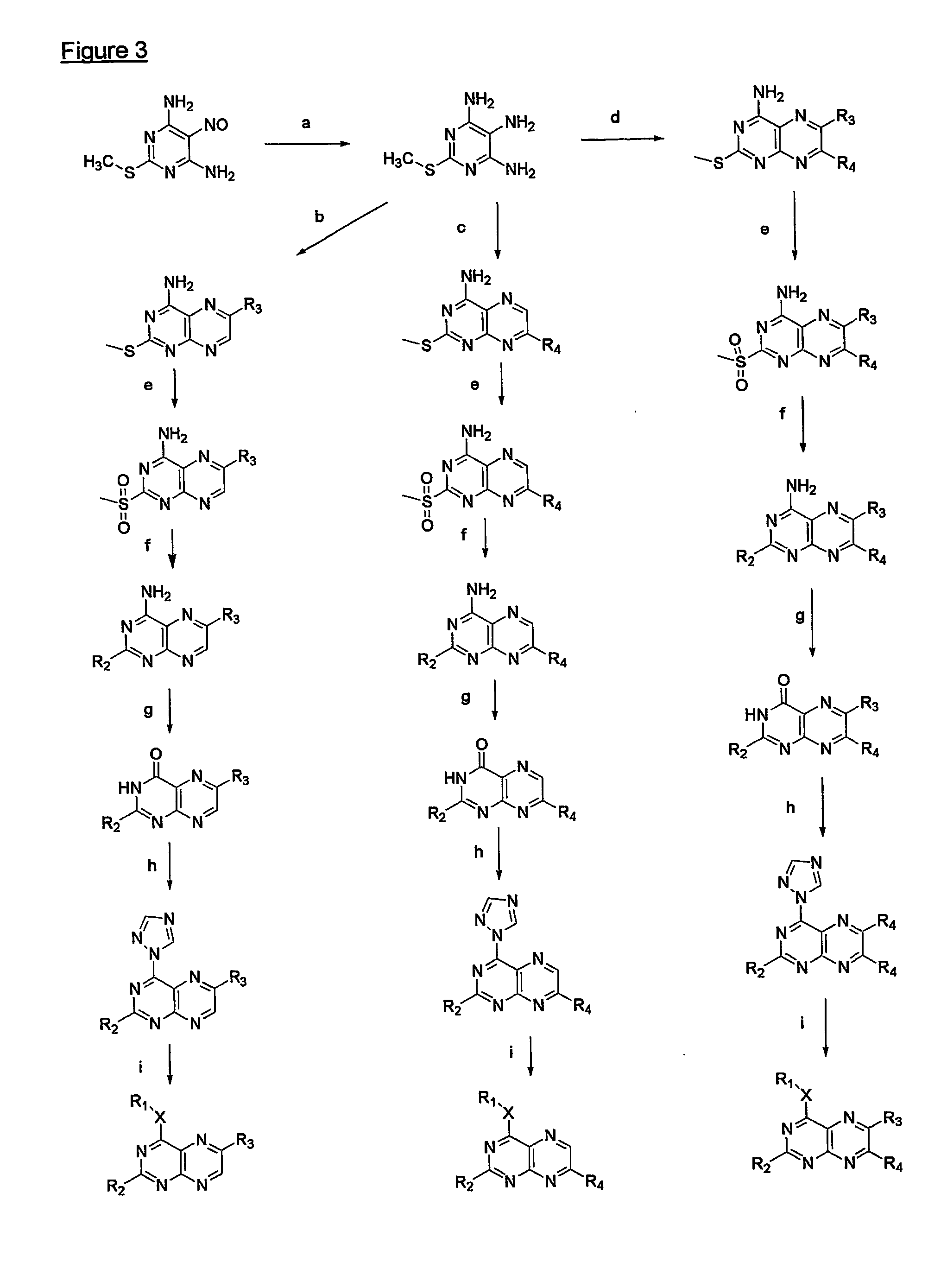
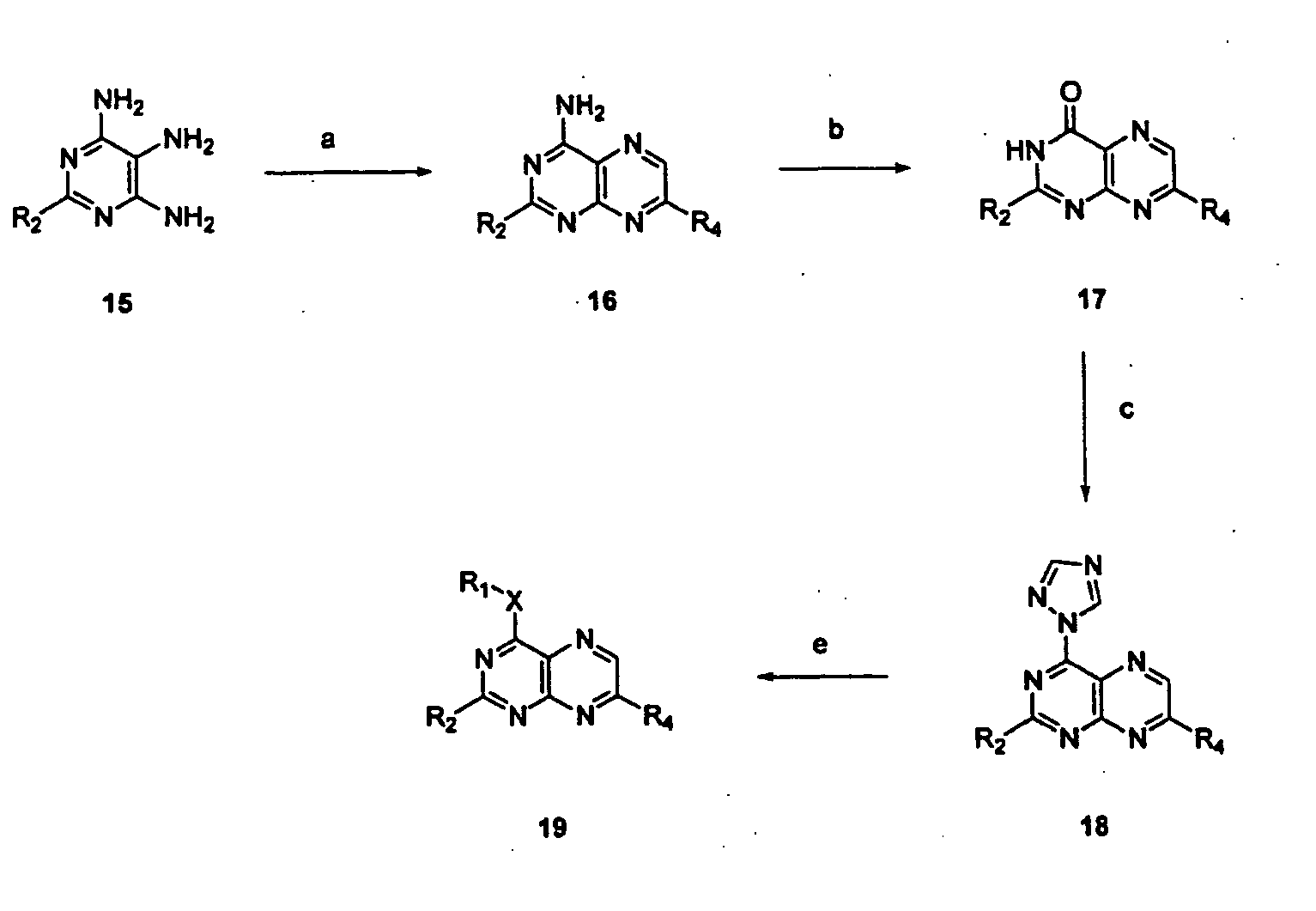
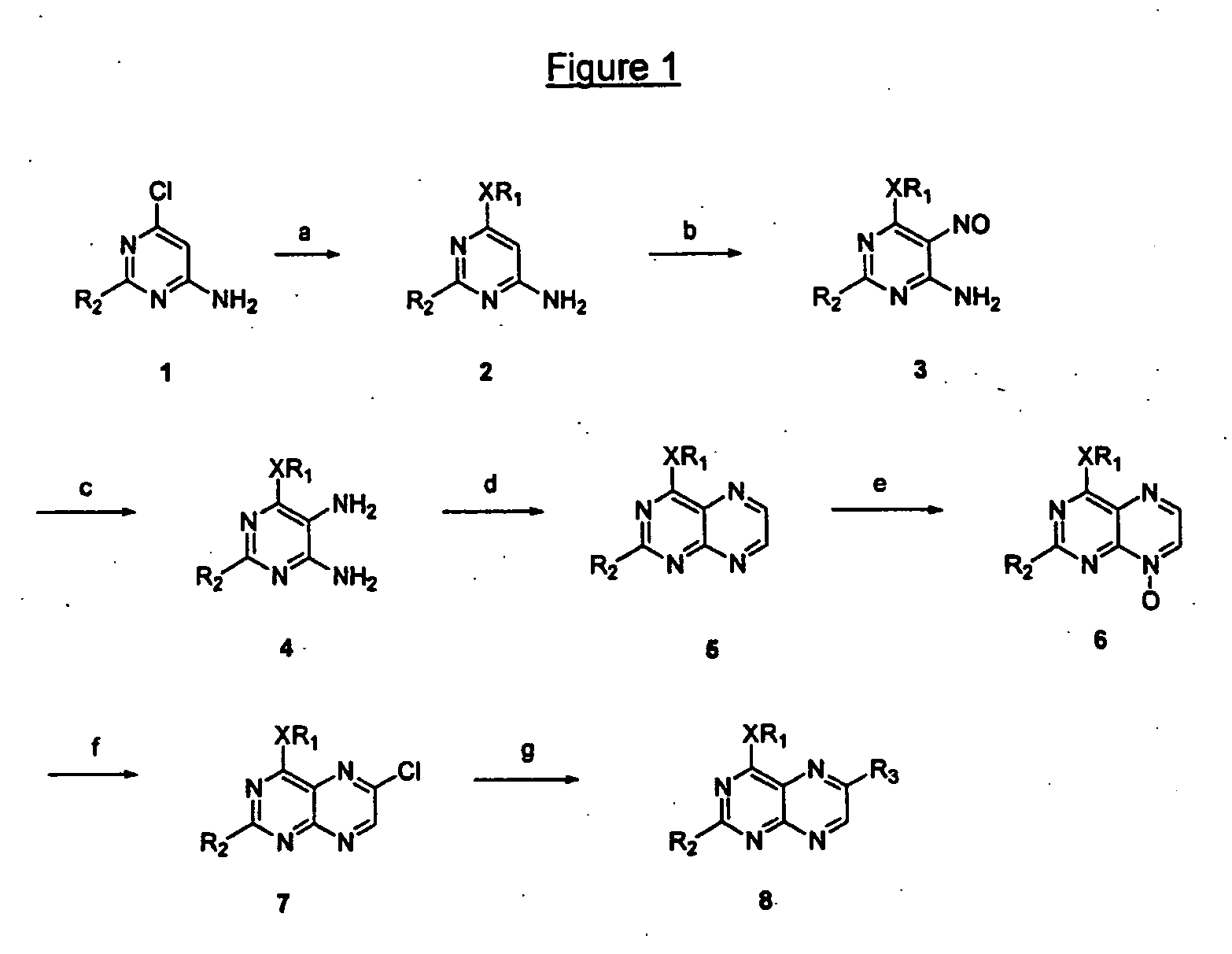
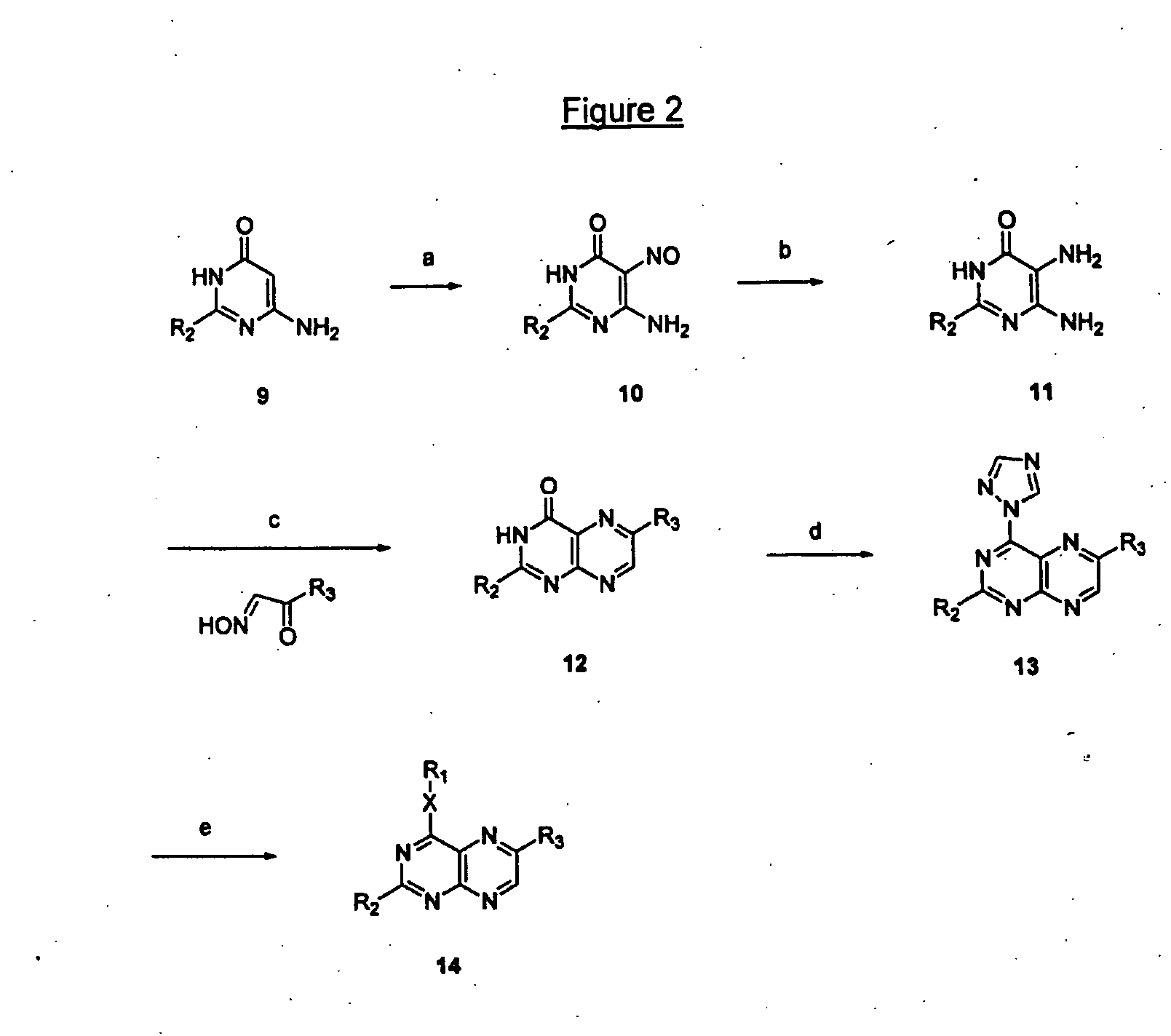
Immunosuppressive effects of pteridine derivatives
Novel poly-substituted pteridinediones (lumazines), and mono- or polysubstituted 2-thiolumazines, 4-thiolumazines or 2,4-dithiolumazines, having disclosed substituents in positions 1, 3, 6 and 7 of the pteridine ring, and pharmaceutically acceptable salts thereof, being represented by the general formula (I). are useful as biologically active ingredients in preparing pharmaceutical compositions especially for the treatment or prevention of a CNS disorder, a cell proliferative disorder, a viral infection, an immune or auto-immune disorder or a transplant rejection. Combinations of the pteridine derivatives of the invention with an immunosuppressant or immunomodulator drug, an antineoplastic drug or an antiviral agent, providing potential synergistic effects, are also disclosed.
Owner:4 AZA IP NV
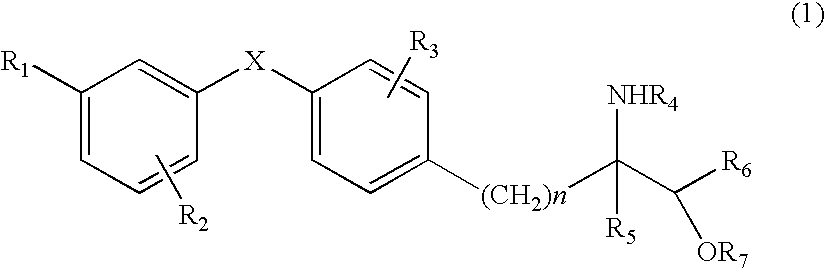
Diaryl ether derivative, addition salt thereof, and immunosuppressant
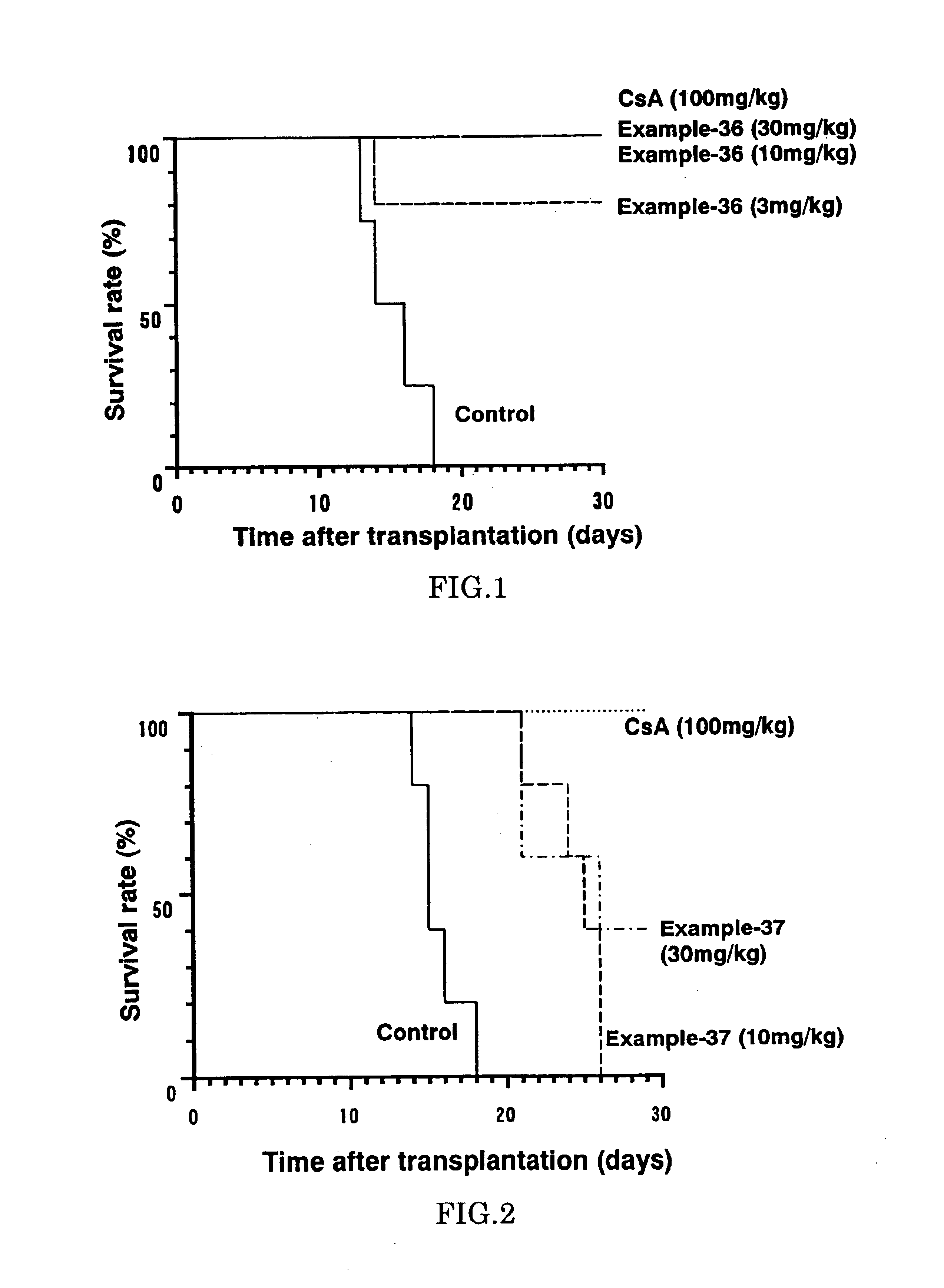
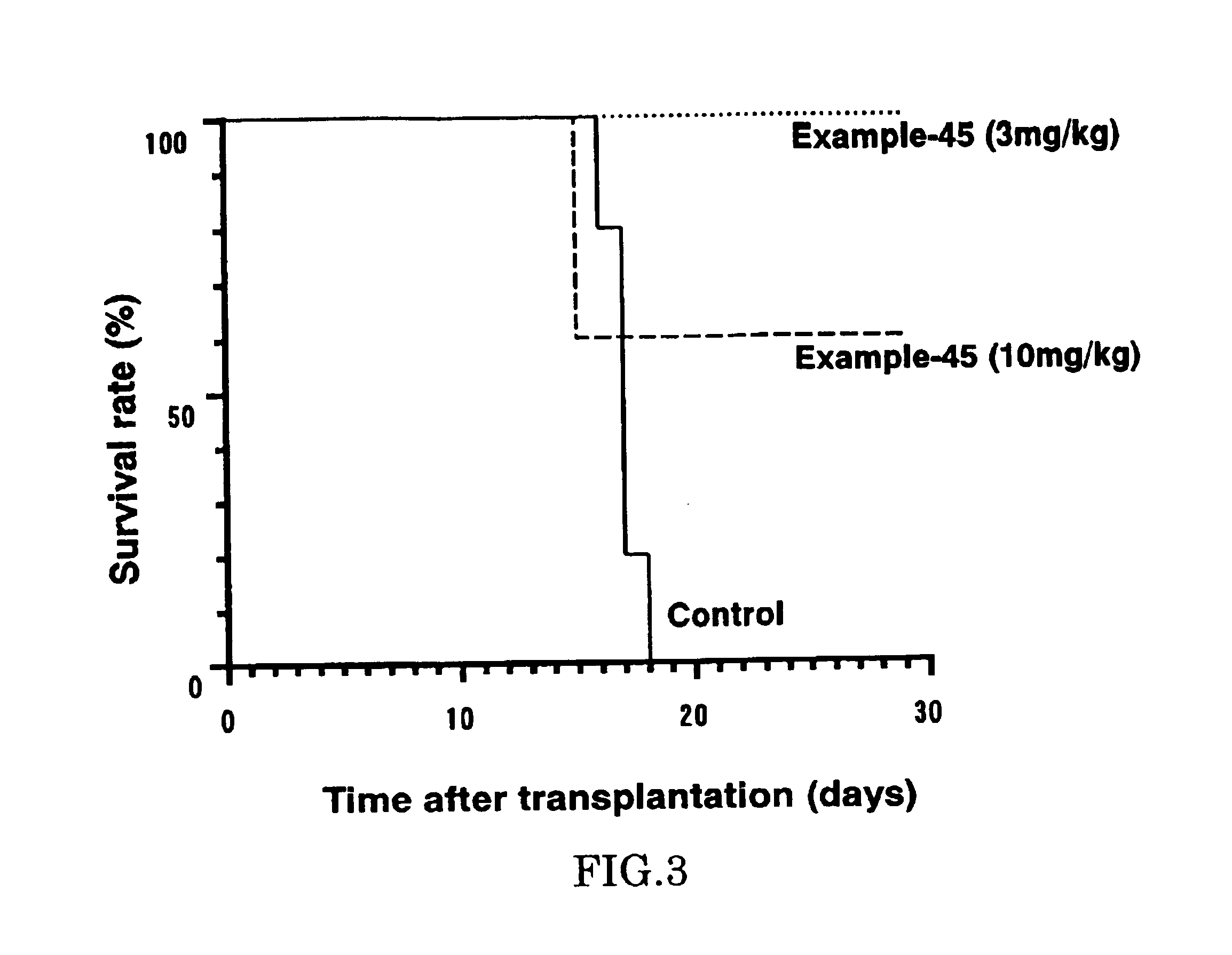
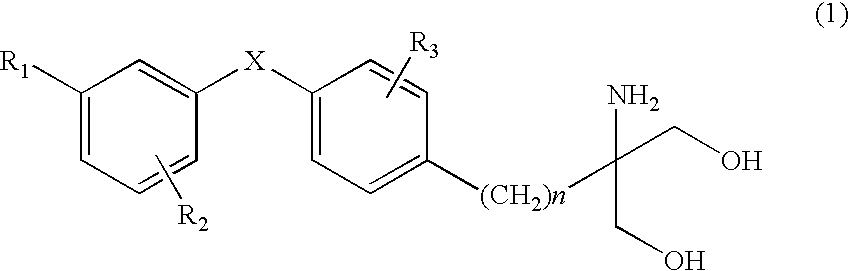
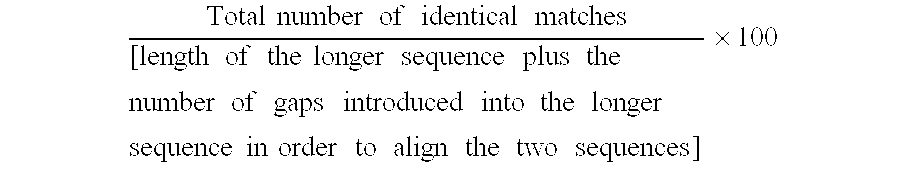
The present invention provides diaryl ether derivatives that exhibit significant immunosuppressive effects with less side effects.The diaryl derivatives of the present invention are represented by the following general formula (1): one example is 2-amino-2-[4-(3-benzyloxyphenoxy)-2-chlorophenyl]propyl-1,3-propanediol.
Owner:KYORIN PHARMA CO LTD
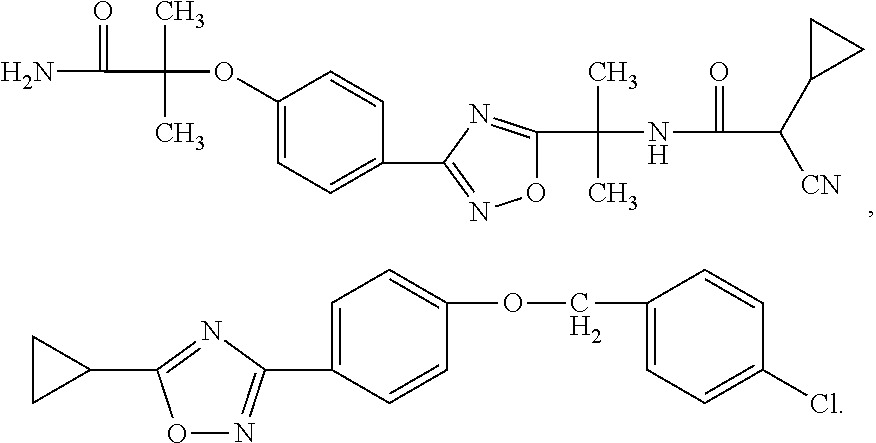
Oxadiazole compounds
ActiveUS7834039B2Reduce in quantityGood effectBiocideSenses disorderDiseaseG protein-coupled receptor
Novel oxadiazole compounds, pharmaceutical compositions containing such compounds and the use of those compounds or compositions as agonists or antagonists of the S1P family of G protein-coupled receptors for treating diseases associated with modulation of S1P family receptor activity, in particular by affording a beneficial immunosuppressive effect are disclosed.
Owner:ABBVIE INC
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20070087020A1Reduce and eliminate and ameliorate inappropriate immune responseStimulate immune responseProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish a transitory parasitic helminth infection and or to simulate in a parasitic helminth infection, thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TREG) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate IgE immune response including, but not limited to an aberrant and or enhanced IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual.
Owner:MILESTONE RES
Novel Oxadiazole Compounds
InactiveUS20110207704A1Reduce in quantityImprove immunosuppressionBiocideNervous disorderG protein-coupled receptorAgonist
Novel oxadiazole compounds, pharmaceutical compositions containing such compounds and the use of those compounds or compositions as agonists or antagonists of the S1P family of G protein-coupled receptors for treating diseases associated with modulation of S1P family receptor activity, in particular by affording a beneficial immunosuppressive effect are disclosed.
Owner:ABBOTT LAB INC
Immunosuppresive effects of pteridine derivatives
Novel poly-substituted pteridinediones (lumazines), and mono- or polysubstituted 2-thiolumazines, 4-thiolumazines or 2,4-dithiolumazines, having disclosed substituents in positions 1, 3, 6 and 7 of the pteridine ring, and pharmaceutically acceptable salts thereof, are useful as biologically active ingredients in preparing pharmaceutical compositions especially for the treatment or prevention of a CNS disorder, a cell proliferative disorder, a viral infection, an immune or auto-immune disorder or a transplant rejection. Combinations of the pteridine derivatives of the invention with an immunosuppressant or immunomodulator drug, an antineoplastic drug or an antiviral agent, providing potential synergistic effects, are also disclosed.
Owner:4 AZA IP NV
Polypeptide, nucleic acid for coding polypeptide, polypeptide-modified T lymphocyte and application of T lymphocyte
ActiveCN107827990AEffective treatmentIncrease the number ofMammal material medical ingredientsImmunoglobulinsFunctional activitySignalling pathways
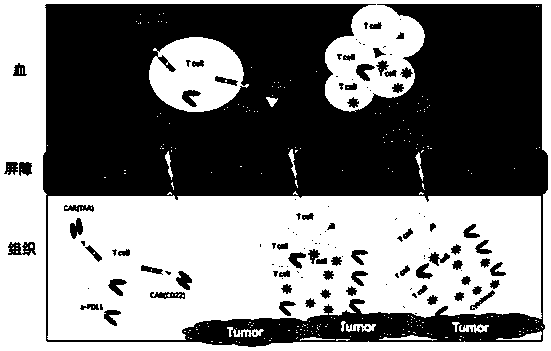
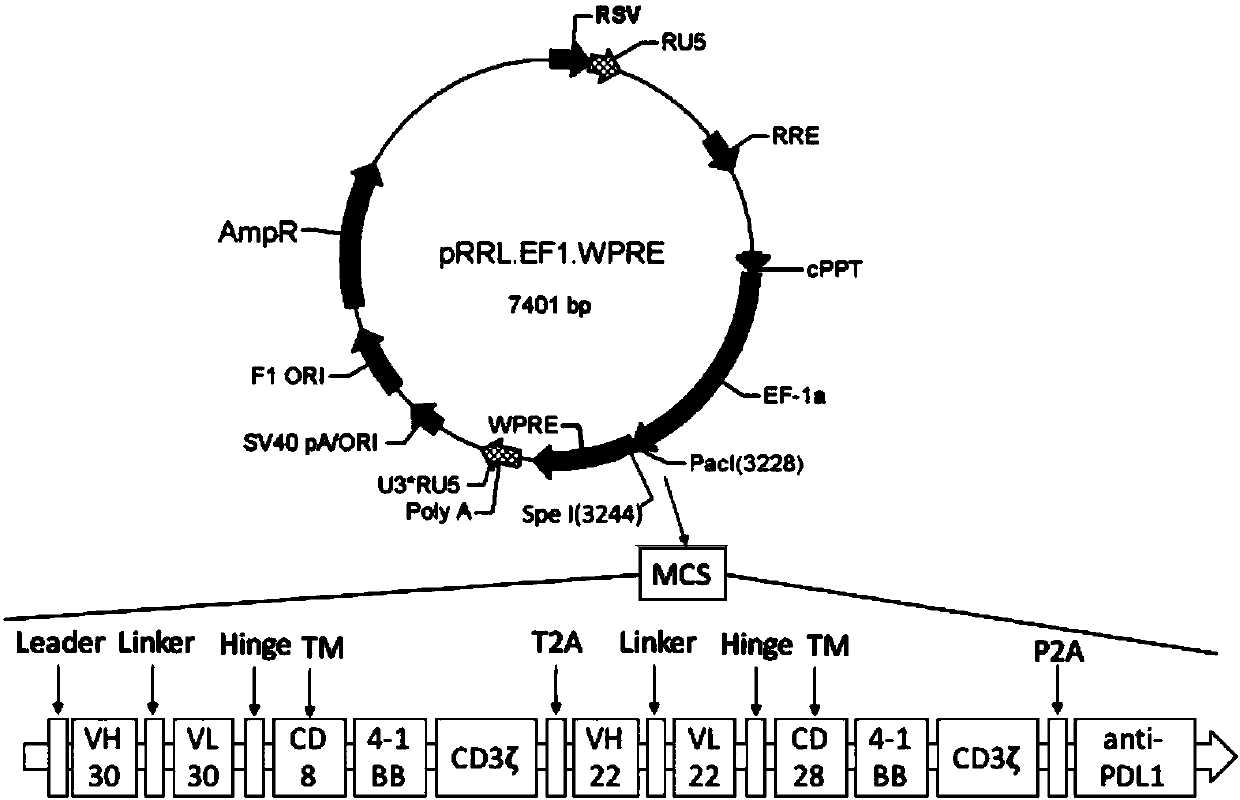
The invention belongs to the technical field of biology, and discloses a polypeptide containing two chimeric antigen receptor structures, nucleic acid for coding the polypeptide, a modified T lymphocyte, and a preparation method and application of the T lymphocyte. The polypeptide provided by the invention contains the two chimeric antigen receptor (CAR) structures, wherein one chimeric antigen receptor structure specifically targets a tumor cell, and the other one specifically targets a normal B lymphocyte, so that a CAR-T cell is effectively amplified through recognizing the B lymphocyte ofa blood circulation system, and more CAR-T cells arrive at a solid tumor part so as to specifically kill the tumor cell for improving a treatment effect on the solid tumor. The polypeptide further comprises an ScFv structure of an anti-PD-L1 monoclonal antibody, and the CAR-T cells can simultaneously secrete a PD-L1 monoclonal antibody which interdicts an immunosuppressive action medicated by a PD1 / PDL1 signal pathway and T cell failure, so that the treatment effect on the solid tumor is improved, the functional activity of immune cells in vivo is prolonged at the same time, and finally the tumor recurrence is retarded and even avoided.
Owner:HEBEI SENLANG BIOTECH CO LTD
Immunosuppressive effects of administration of a cyclooxygenase-2 inhibitor and a leukotriene A4 hydrolase inhibitor
Treatment with a cyclooxygenase-2 inhibitor and a leukotriene A4 hydrolase inhibitor is described as being useful in reducing recipient rejection of transplanted organs and for treatment of autoimmune diseases.
Owner:PHARMACIA CORP
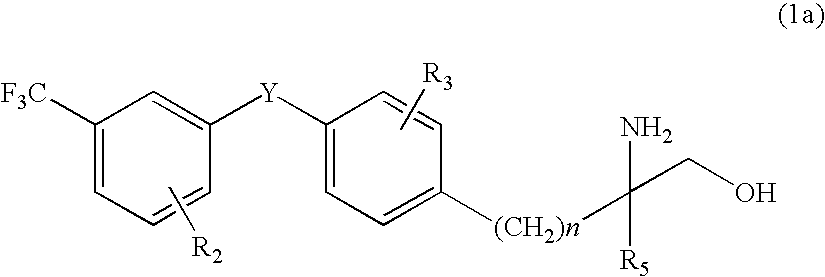
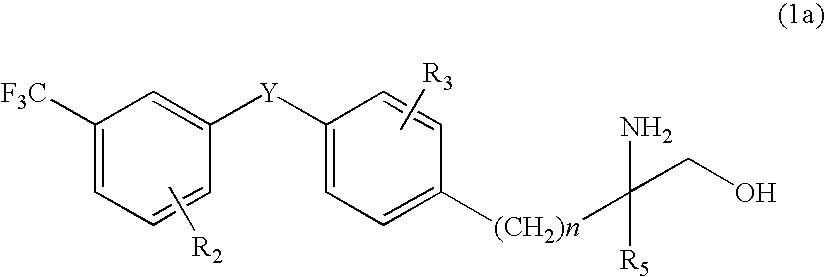
Amino alcohol derivative, addition salt thereof, and immunosuppressant
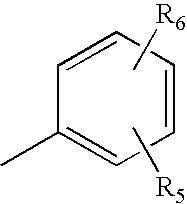
An amino alcohol derivative represented by the following general formula (1) (for example, (±)-2-amino-5-[4-(3-benzyloxyphenylthio)-2-chlorophenyl]-2-methylpentane-1-ol) exhibits strong immunosuppressive effect while causing less side effects:
Owner:KYORIN PHARMA CO LTD
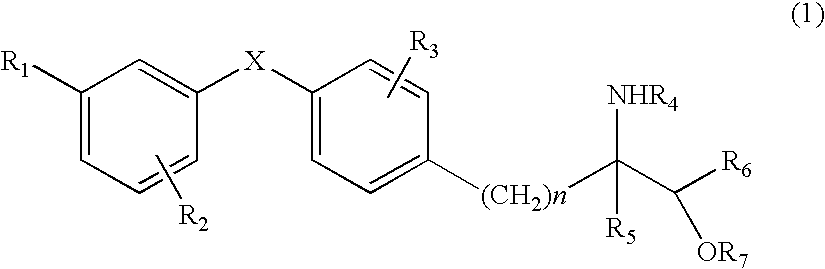
Amino alcohol derivative, addition salt thereof, and immunosuppressant
InactiveUS20060135622A1Improve immunosuppressionLess side effectsBiocideOrganic active ingredientsSide effectImmunosuppressive effect
An amino alcohol derivative represented by the following general formula (1) (for example, (±)-2-amino-5-[4-(3-benzyloxyphenylthio)-2-chlorophenyl]-2-methylpentane-1-ol) exhibits strong immunosuppressive effect while causing less side effects:
Owner:KYORIN PHARMA CO LTD
Immunosuppressive effects of pteridine derivatives
This invention relates to a group of trisubstituted and tetrasubstituted pteridine derivatives, their pharmaceutically acceptable salts, N-oxides, solvates, dihydro- and tetrahydro-derivatives and enantiomers, possessing unexpectedly desirable pharmaceutical properties, in particular which are highly active immunosuppressive agents, and as such are useful in the treatment in transplant rejection and / or in the treatment of certain inflammatory diseases. These compounds are also useful in preventing or treating cardiovascular disorders, allergic conditions, disorders of the central nervous system and cell proliferative disorders.
Owner:4 AZA IP NV
Immunosuppressive effects of pteridine derivatives
This invention relates to a group of trisubstituted and tetrasubstituted pteridine derivatives, their pharmaceutically acceptable salts, N-oxides, solvates, dihydro- and tetrahydroderivatives and enantiomers, possessing unexpectedly desirable pharmaceutical properties, in particular which are highly active immunosuppressive agents, and as such are useful in the treatment in transplant rejection and / or in the treatment of certain inflammatory diseases. These compounds are also useful in preventing or treating cardiovascular disorders, allergic conditions, disorders of the central nervous system and cell proliferative disorders.
Owner:4 AZA IP NV
Dexamethasone-RGD polypeptide conjugate, preparation method thereof and application
ActiveCN103044523AEnhanced inhibitory effectGood anti-inflammatory effectOrganic active ingredientsAntipyreticSide effectArg-Gly-Asp
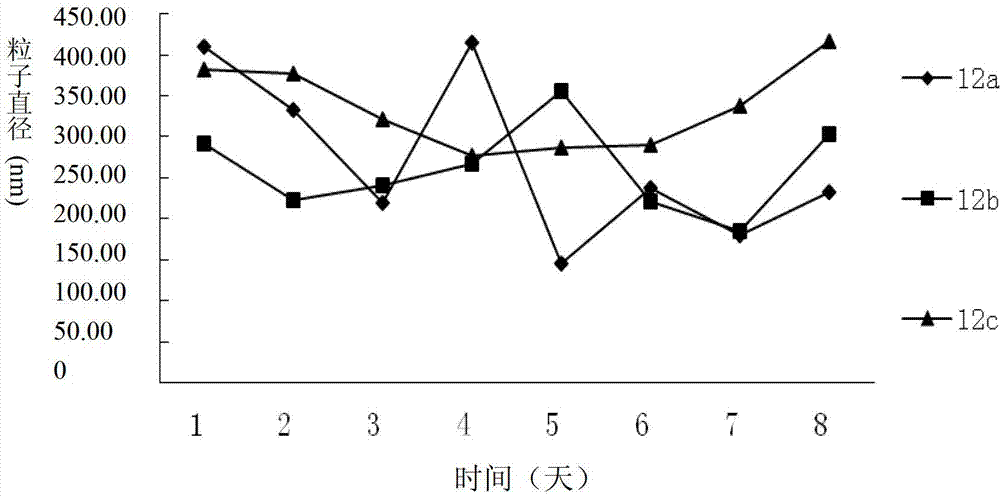
The invention relates to a dexamethasone-RGD polypeptide conjugate shown as general formula i, a preparation method thereof and application. In the formula i, RGDX is tetrapeptide Arg-Gly-Asp-X, wherein X is any amino acid of Val, Phe and Ser. Proved by experiments, the compound of the present invention has excellent immunosuppressive effect and anti-inflammatory effect, and the side effects of osteoporosis is reduced. The conjugate can be used as an immunosuppressant and anti-inflammatory agent for the side effect of low osteoporosis clinically. At the same time, the dexamethasone-RGD polypeptide conjugate of the present invention can self-assemble into stable nanoparticles with particle size of 200-500 nm, therefore the conjugate can be prepared into a target drug of immunosuppressive pharmacosome, microemulsion or liposome.
Owner:SHANDONG HUBBLE KISEN BIOLOGICAL TECH CO LTD
Prunella spike extract and its preparation method and use
An extract of prunella spike for treating rheumatoid arthritis and improving immune suppression contains phenolic acid (60-70%) and rosemaric acid (14-21%). Its preparing process is also disclosed.
Owner:JIANGSU KANION PHARMA CO LTD
Polypeptide Sequence Involved in the Modulation of the Immunosuppresive Effect of Viral Proteins
ActiveUS20080008683A1Stimulate immune responseReduce riskPeptide/protein ingredientsGenetic material ingredientsImmunosuppressive effectHomologous Sequences
The present invention relates to a polypeptide having a sequence of 7 to 20 amino acid residues, which is capable of modulating the immunosuppressive properties of a viral protein or a fragment thereof, against the host in which it is expressed (immunosuppression-modulatory sequence) when it substitutes the homologous sequence of the viral protein or fragment, the polypeptide including the minimum following consensus amino acid sequence: X1Y9Y10Y11CY12X2 wherein, X1 and X2 are selected to impact on the immunosuppressive properties, and Y9 to Y12 represent variable amino acid residues.
Owner:INSTITUT GUSTAVE ROUSSY +2
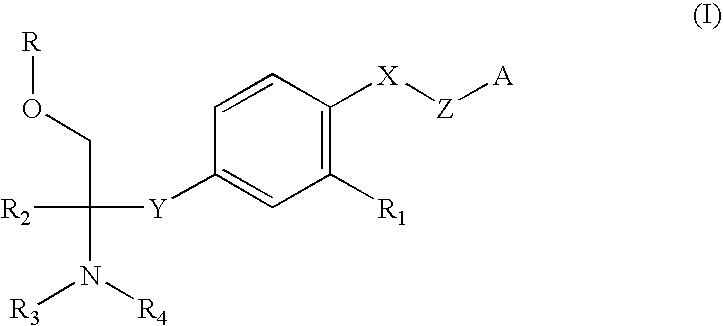
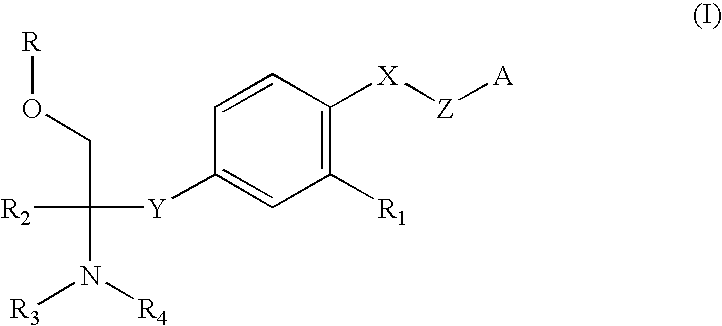
Amine Compound and Use Thereof for Medical Purposes
ActiveUS20090137530A1Superior peripheral blood lymphocyte decreasing actionEliminate side effectsBiocideSenses disorderCarbon numberSide effect
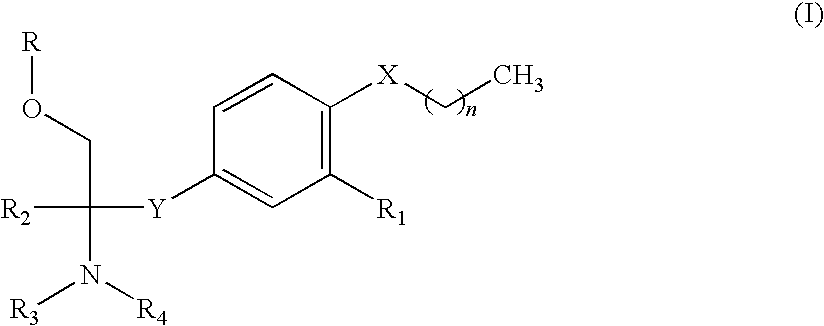
A novel amine compound represented by the following formula (I), which is superior in immunosuppressive action, rejection suppressive action and the like, and shows reduced side effects such as bradycardia and the like, or a pharmaceutically acceptable acid addition salt thereof, or hydrates thereof, or solvate, as well as a pharmaceutical composition containing this compound and a pharmaceutically acceptable carrier.wherein R is a hydrogen atom or P(═O)(OH)2, X is an oxygen atom or a sulfur atom, Y is CH2CH2 or CH═CH, R1 is cyano or alkyl having a carbon number of 1 to 4 and substituted by a halogen atom(s), R2 is alkyl having a carbon number of 1 to 4 and optionally substituted by a hydroxyl group(s) or a halogen atom(s), R3 and R4 may be the same or different and each is a hydrogen atom or alkyl having a carbon number of 1 to 4, and n is 5-8.
Owner:MITSUBISHI TANABE PHARMA CORP
Immunosuppressive effects of administration of a cyclooxygenase-2 inhibitor and a 5-lipoxygenase inhibitor
This invention is in the field of a combination comprising a therapeutically-effective amount of a cyclooxygenase-2 inhibitor, a 5-lipoxygenase inhibitor and an immunosuppressive drug selected from antiproliferative agents, antiinflammatory-acting compounds and inhibitors of leukocyte activation. This combination may be used, for example, to suppress the immune response associated with organ transplantation, graft versus host disease, and conditions with underlying autoimmune or inflammatory reactivities or responses.
Owner:GD SEARLE & CO
Catechin Adjuvants
InactiveUS20070082073A1Ameliorate immunosuppressive effectImprove morbidityBiocidePhosphorous compound active ingredientsAdjuvantMortality rate
A combination cancer therapy based upon catechins, the major biologically active polyphenol in plant products, including green tea extract, and one or more chemotherapeutic agents. A common complication of cancer chemotherapy is neutropenia, and in spite of advances in its prophylactic management is a major cause of risk for the development of serious microbial infections leading to increased morbidity and mortality in both humans and animals. The use of cathechins such as those found in green tea (Camellia sinensis), including but not limited to epigallocatechin gallate (EGCG) as nontoxic adjuvant to aid in the prevention of opportunistic microbial infections in patients undergoing immunosuppressive chemotherapy is a novel application. Also contemplated are methods using catechins to ameliorate the immunosuppressive effects of cancer chemotherapy by administering the compound to a patient in need thereof.
Owner:FLORIDA VETERINARY SPECIALIST & CANCER TREATMENT CENT +1
Medical usage of periplocin A and E
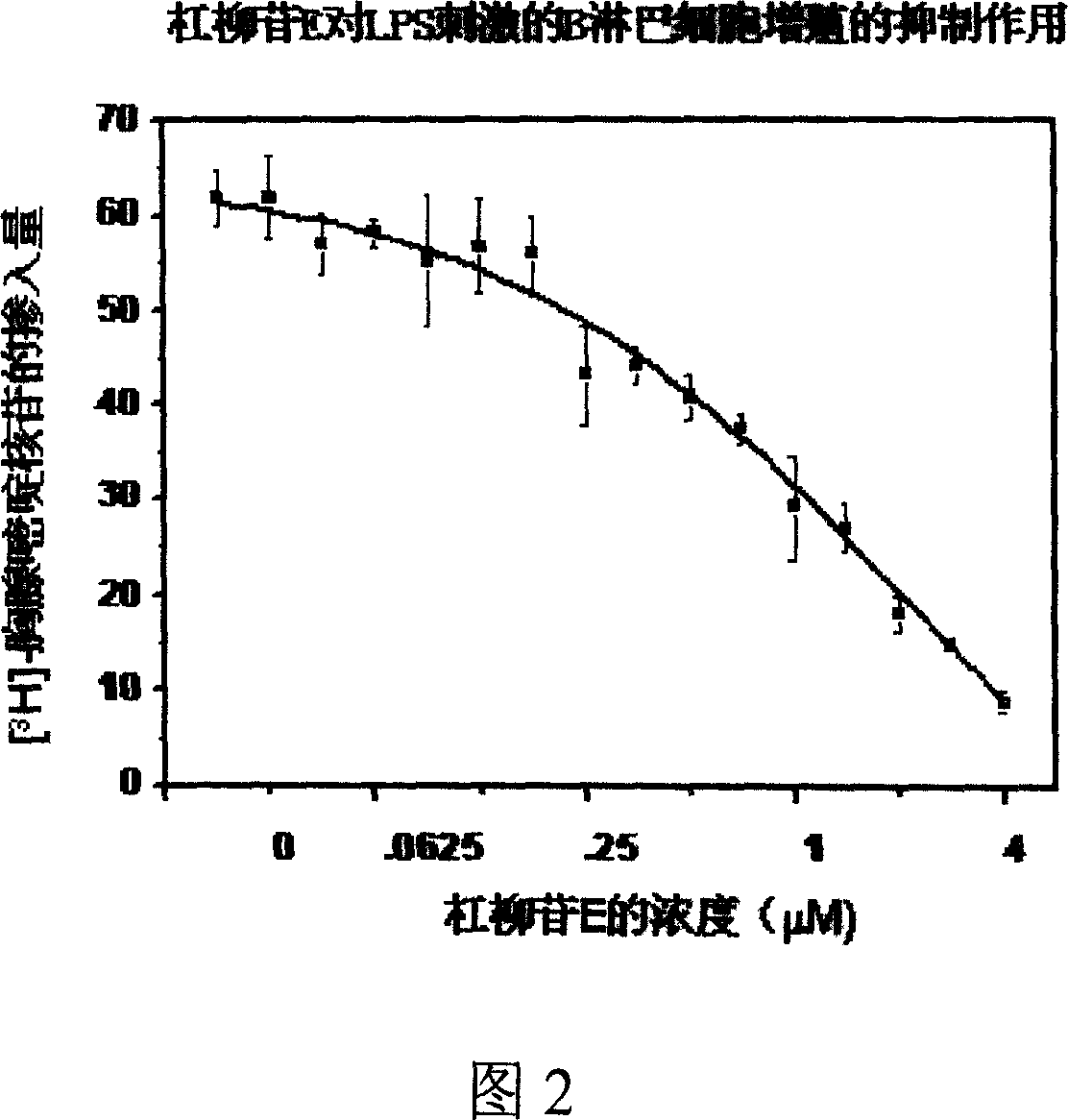
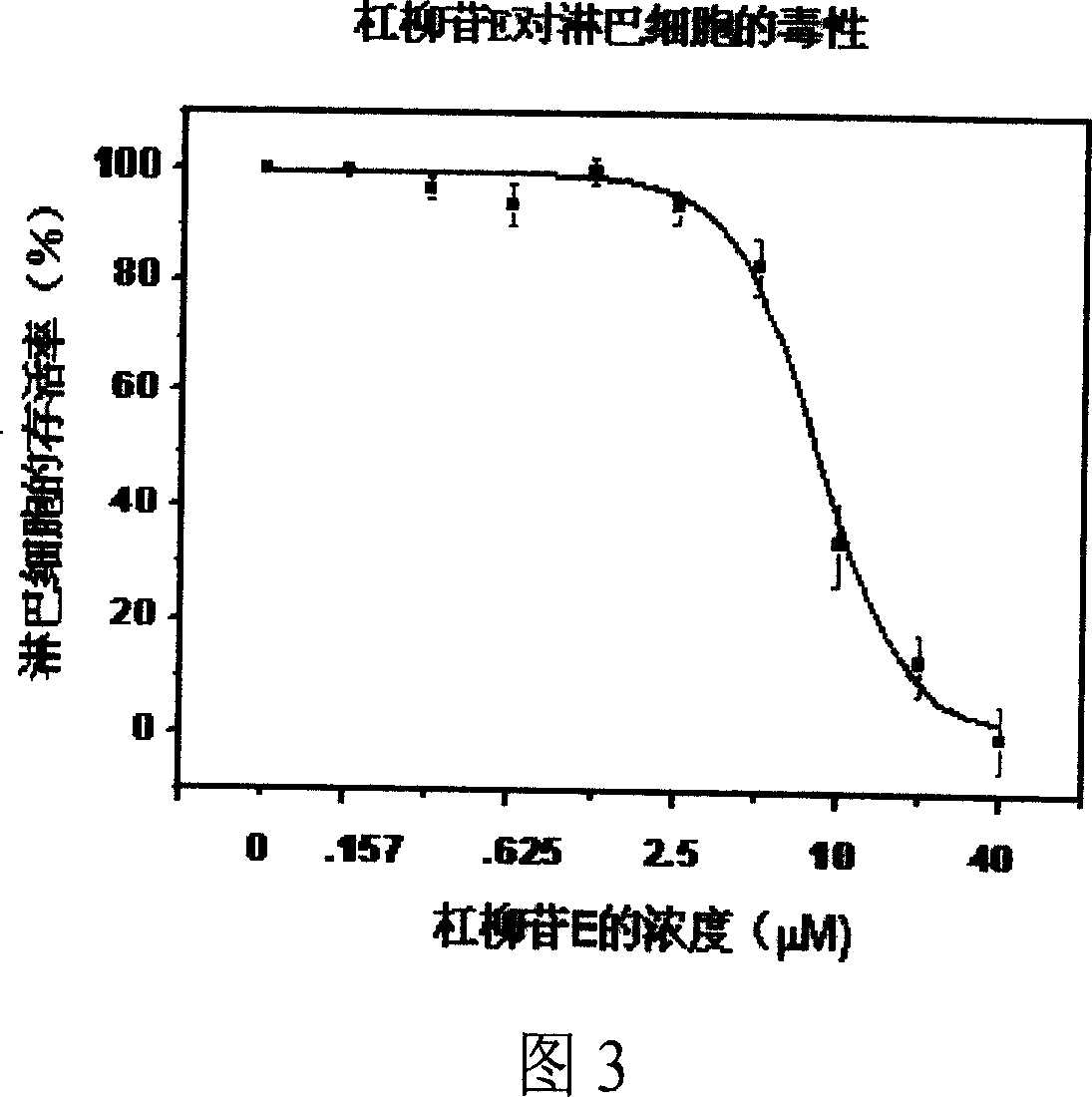
InactiveCN1939324AHas an immunosuppressive effectOrganic active ingredientsMetabolism disorderImmunosuppressive effectBiomedical engineering
A medical application of periplocoside A and E in preparing the immunodepress ant with high effect and low poison is disclosed.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Amine compound and pharmaceutical use thereof
ActiveUS20100179216A1Eliminate side effectsReduce actionBiocideCarbamic acid derivatives preparationSide effectChemical compound
Provided is a novel amine compound represented by the following formula (I) having a superior peripheral blood lymphocyte decreasing action and superior in the immunosuppressive action, rejection suppressive action and the like, which shows decreased side effects of, for example, bradycardia and the like, or a pharmaceutically acceptable acid addition salt thereof, or a hydrate thereof, or a solvate thereof.wherein each symbol is as defined in the specification.
Owner:MITSUBISHI TANABE PHARMA CORP
Process for the production of substances that have been bactericidally treated and/or exhibit immune-modulatory activity, and the use thereof
InactiveUS6303152B1Static indicating devicesMammal material medical ingredientsSerum igeImmunosuppressive effect
A process is disclosed for the production of immune-genetically active suspensions of which the initial substances are fluids or tissues from the human or animal body. Autohemotherapy, immune-stimulatory and / or immune-suppressive effects are employed. The suspensions thus produced can be used as pooled sera as desired.
Owner:KIEF HORST
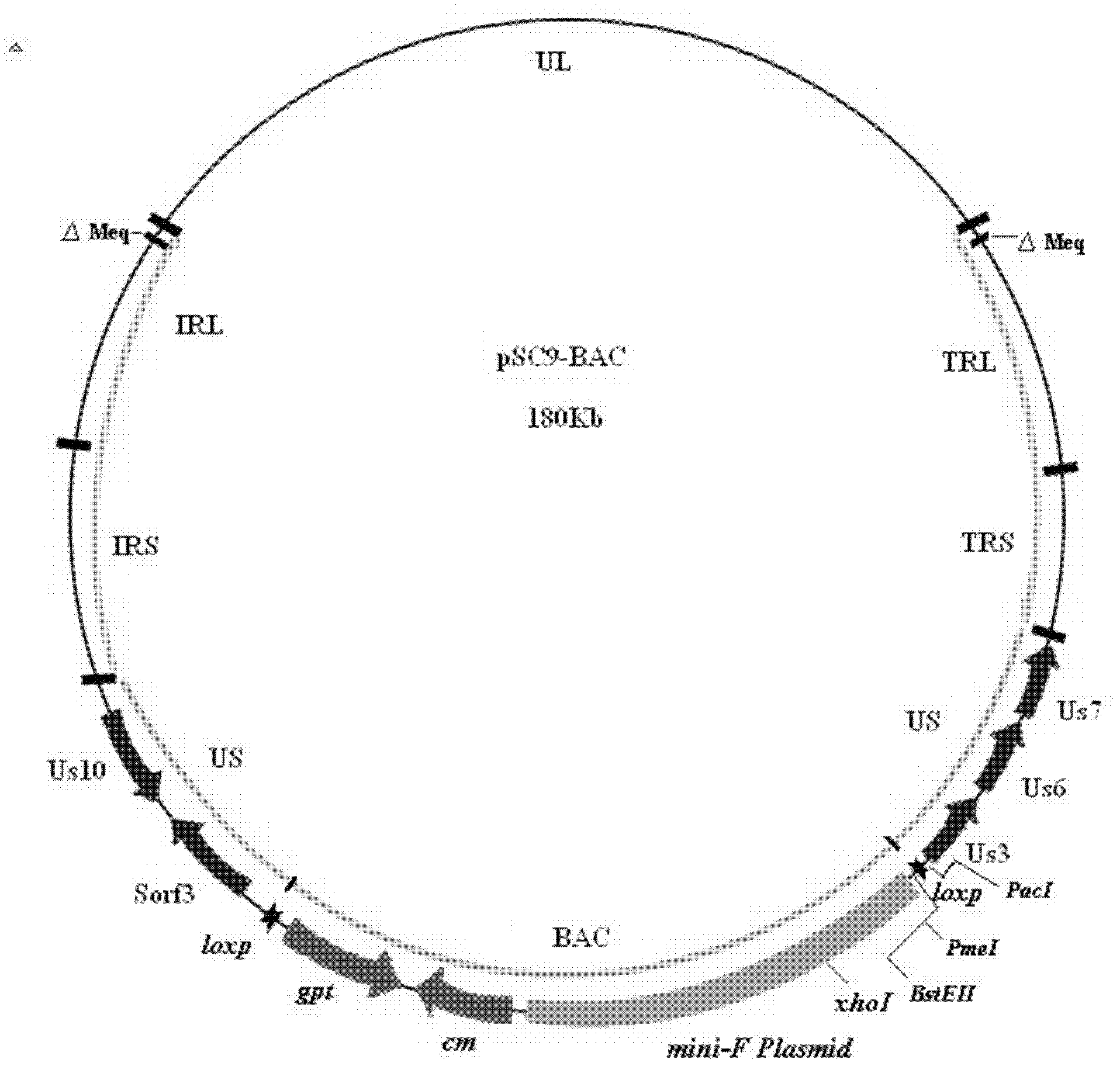
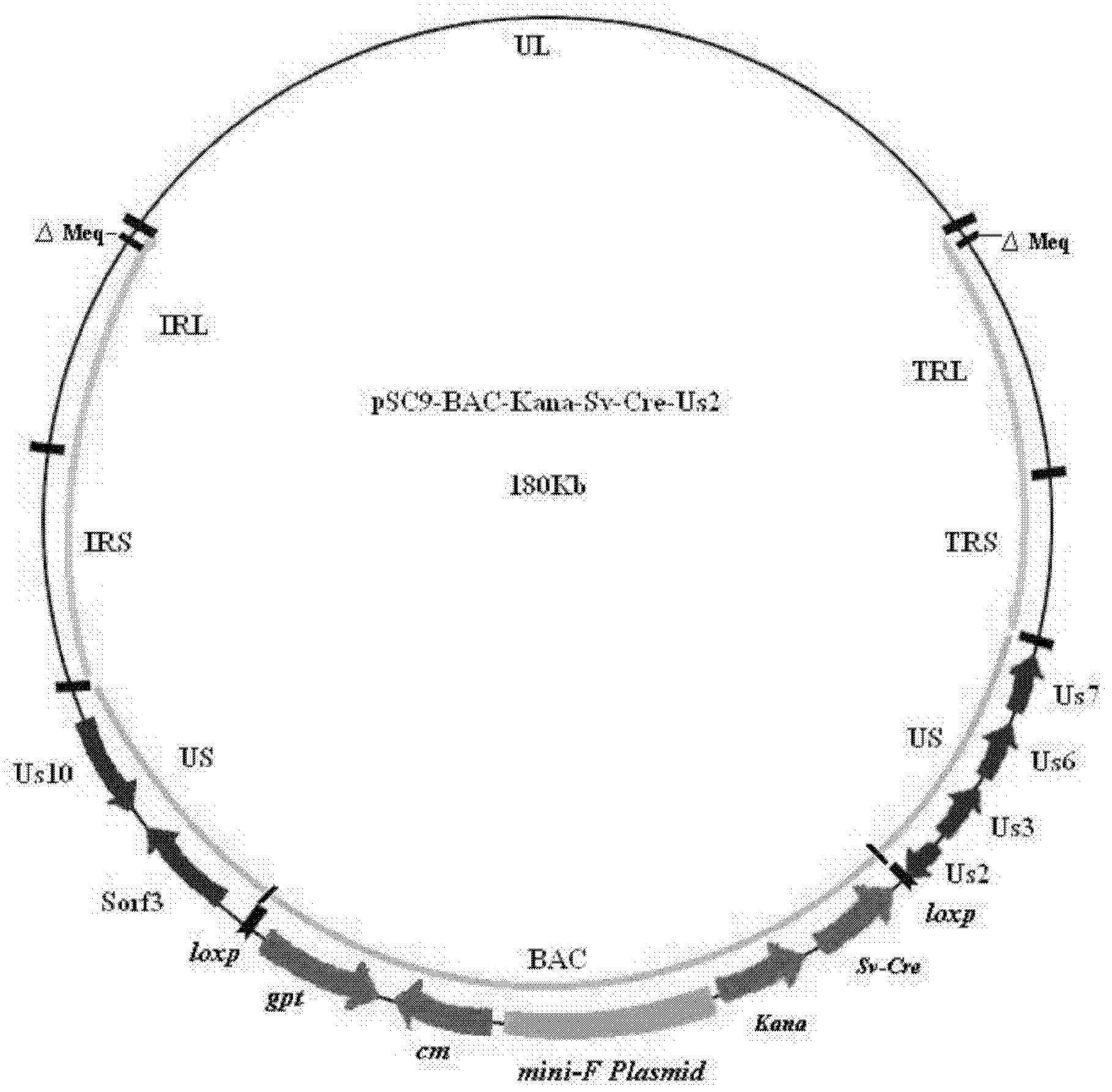
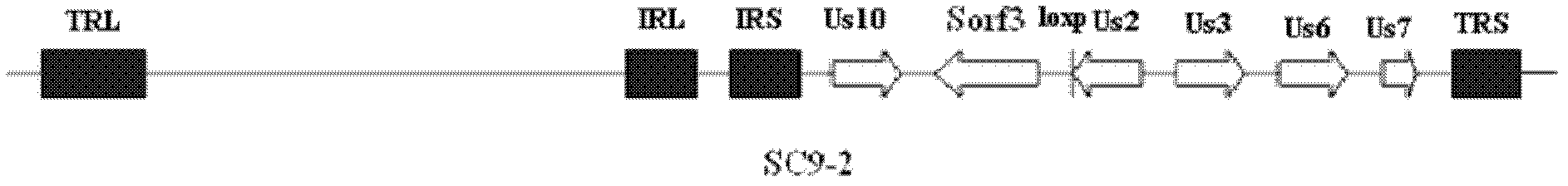
Construction and application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain
The invention relates to a construction and an application of recombinant Chicken Marek's Disease Virus SC9-1 strain and SC9-2 strain. The recombinant virus construction method solves the technical problem that kanr gene cannot be knocked out again by a present method after kanr gene containing flp recognition sites at two ends is continuously used twice to knock out two specific functional genes on the same virus genome. The obtained recombinant virus MDV SC 9-1 strain and MDV SC 9-2 strain are used as production strains of Marek's Disease live vaccine. The prepared vaccine prevents the very virulent or emerging very virulent plus MDV induced chicken Marek's disease. The protective immunity effect of the vaccine is superior to CVI988 / Rispens strain vaccine which is mostly widely used in foreign and domestic markets at present. The antigenicity of the recombinant virus is more similar to that of Chinese epidemic strain than the antigenicity of the similar virus rMd5deltameq which has been published in the United State. The strains provided by the invention will not induce tumor and has no immune suppression effect. Therefore, the strains are more applicable to China.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Immunosuppressant-anti-CD25 mosaic monoclonal antibody
InactiveCN101210049AReduce acute rejectionExcellent immunosuppressive effectImmunoglobulins against animals/humansAntibody ingredientsEpitopeHeavy chain
The invention discloses a monoclonal antibody resisting CD25 with special variable region of the light chain and variable region of heavy chain structure aiming at special CD25 epitope (IL-2 acceptor). The invention also discloses the coded sequence of the monoclonal antibody, the expression vector and host cell containing the coded sequence and the preparation method thereof. The monoclonal antibody of the invention has excellent immunosuppressive action and low immunogenicity and high specificity.
Owner:SHANGHAI NEWSUMMIT BIOPHARMA +1
Saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester, synthetic method and application thereof
InactiveCN101899085AExcellent immunosuppressive effectTripeptide ingredientsPeptide preparation methodsAlcoholImmunosuppressive effect
The invention relates to 6 saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester conjugates with immunosuppressive activity in a general formula Glu-Asp-Gly-O-CH2-(CH2)nCH3 I. In saturated fatty chain alcohol, n is equal to 6, 8, 10, 12, 14 or 16. The invention also relates to a preparation method and application of the conjugates as an immunosuppressant. An experimental result of the saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester has the inhibition effects of splenic lymphocyte mitogen breeder reaction and macrophage phagocytosis activity indicates that the conjugates of the invention have excellent immunosuppressive action and can be clinically used as an immunosuppressant.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Conjugates of saturated aliphatic chain alcohol, dexamethasone, and Glu-Asp-Gly, preparation, nano structure, and applications thereof
InactiveCN104211760AExcellent immunosuppressive effectAntipyreticAnalgesicsNano structuringSide effect
The invention relates to conjugates of saturated aliphatic chain alcohol, dexamethasone, and Glu-Asp-Gly, preparation, a nano structure, and applications thereof. The invention discloses 6 saturated aliphatic chain alcohol modified dexamethasone-Glu-Asp-Gly conjugates represented by the formula 10 a-f, wherein in the formula the n represents 7, 9, 11, 13, 15, or 17. The invention also discloses a preparation method, a nano structure, immunity inhibition activity, inflammation inhibition activity, and pain relieving activity of the conjugates. The invention also finds that the conjugates do not have any side effect leading to osteoporosis. The researches on the inhibition effect of the conjugates on splenic lymphocyte mitogen proliferation reactions and the survival time after mouse retroauricular cardiac muscle transplant show that the conjugates have an excellent immunity inhibition effect. The researches on the effect of the conjugates on the swelling degree of mouse ears which are inflamed due to xylene show that the conjugates have an excellent anti-inflammation effect. The researches on the effect of the conjugates on mouse heat radiation tail flick time show that the conjugates have an excellent pain-relieving effect. The researches on the effect of the conjugates on the mouse thigh bones show that the conjugates cannot cause osteoporosis. So the conjugates have a wide application prospect in the preparation of immunity inhibition drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Cells with increased immuno-regulatory properties and methods for their use and manufacture
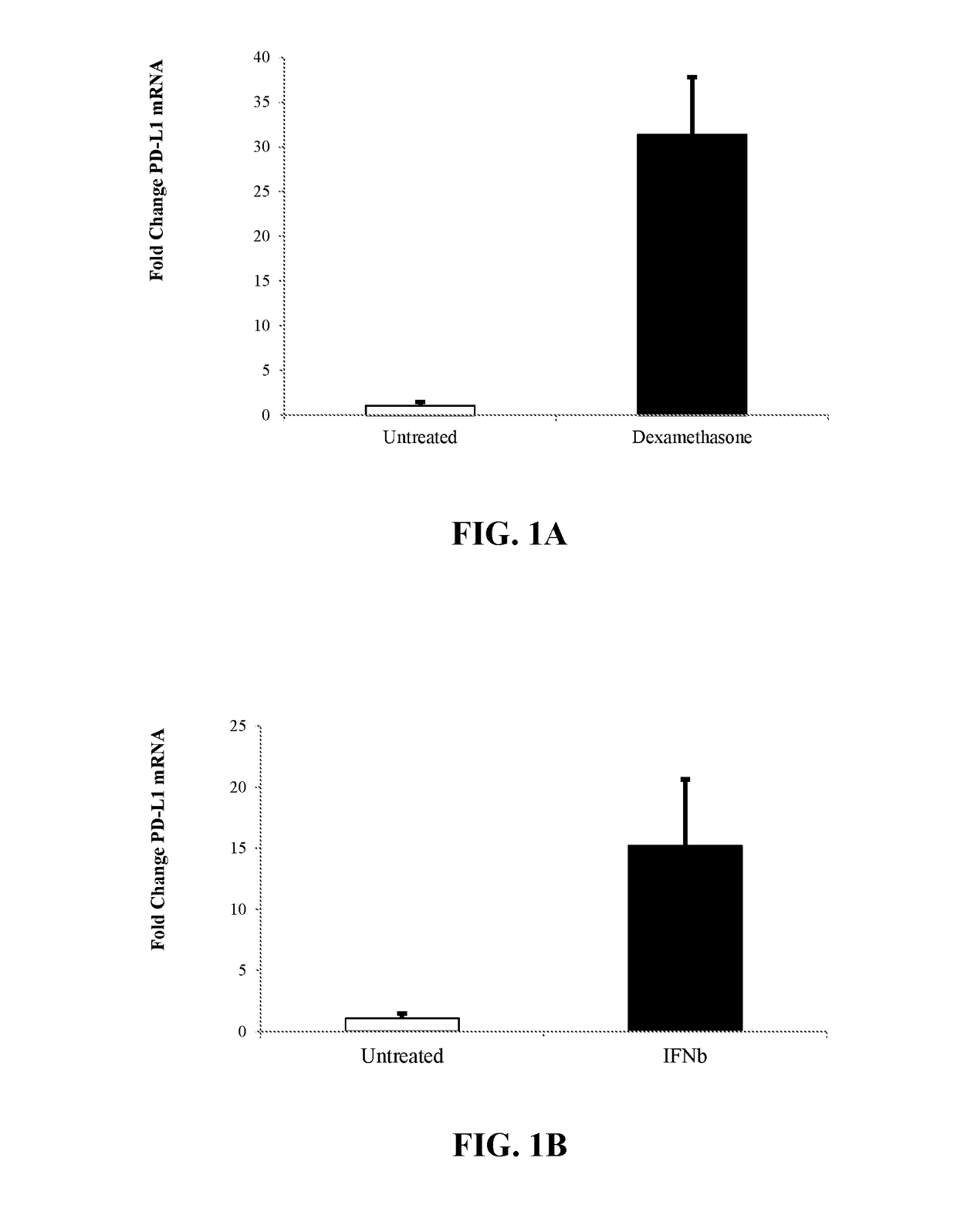
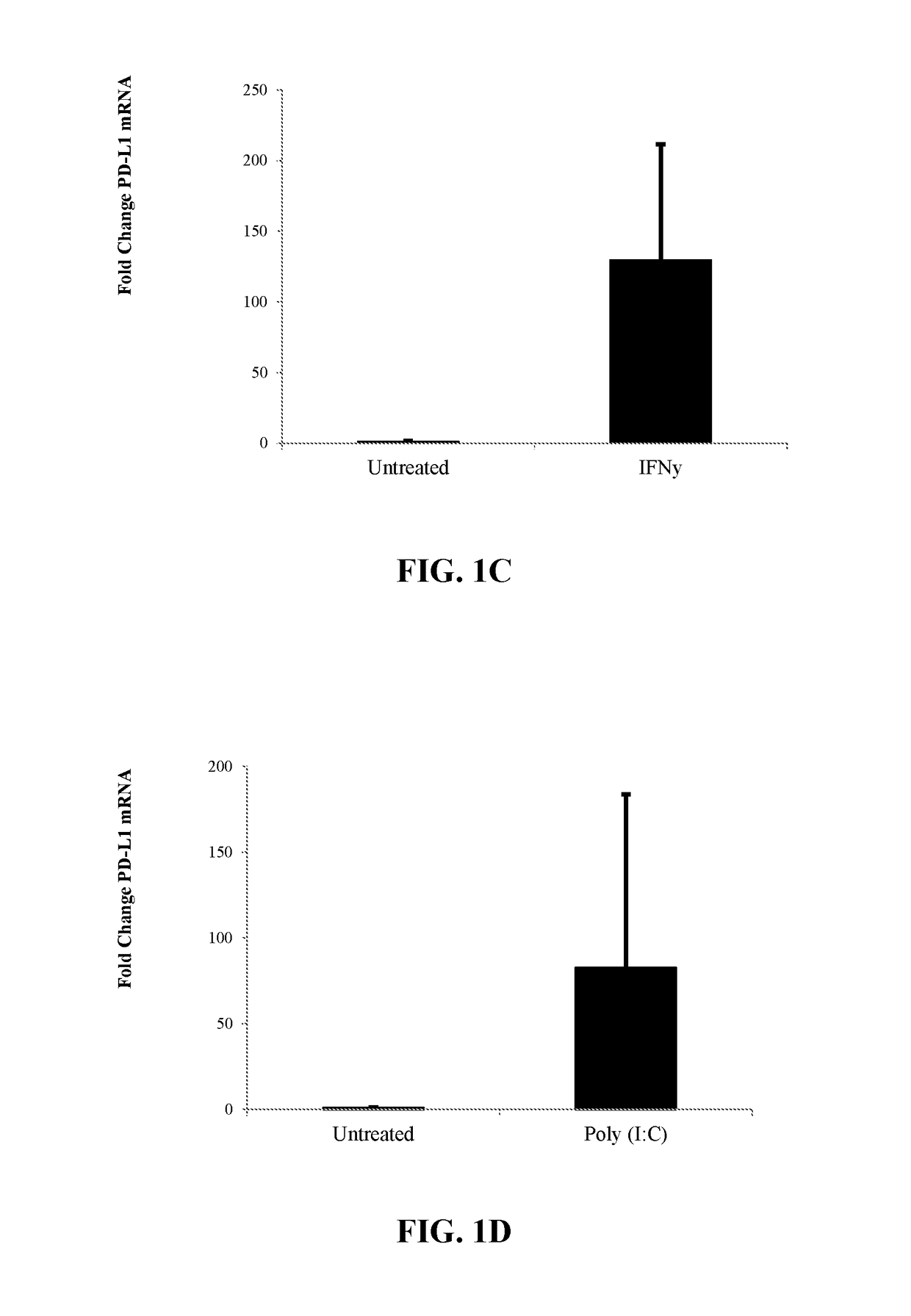
InactiveUS20180112180A1High expressionImprove the level ofPeptide/protein ingredientsAntipyreticPD-L1Immunosuppressive effect
The present invention is directed to compositions and methods to increase the expression of PD-L1 and / or IDO-1 in a population of cells, the modulated cells expressing increased PD-L1 and / or IDO-1, and methods related to the immunosuppressive effects obtained by cells expressing increased PD-L1 and / or IDO-1.
Owner:FATE THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com