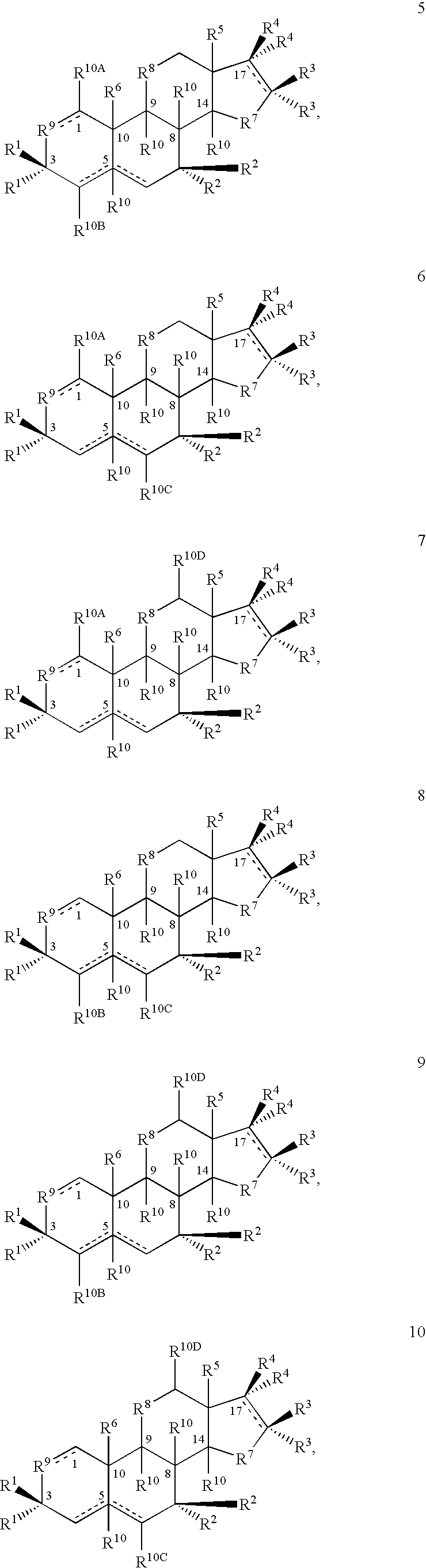
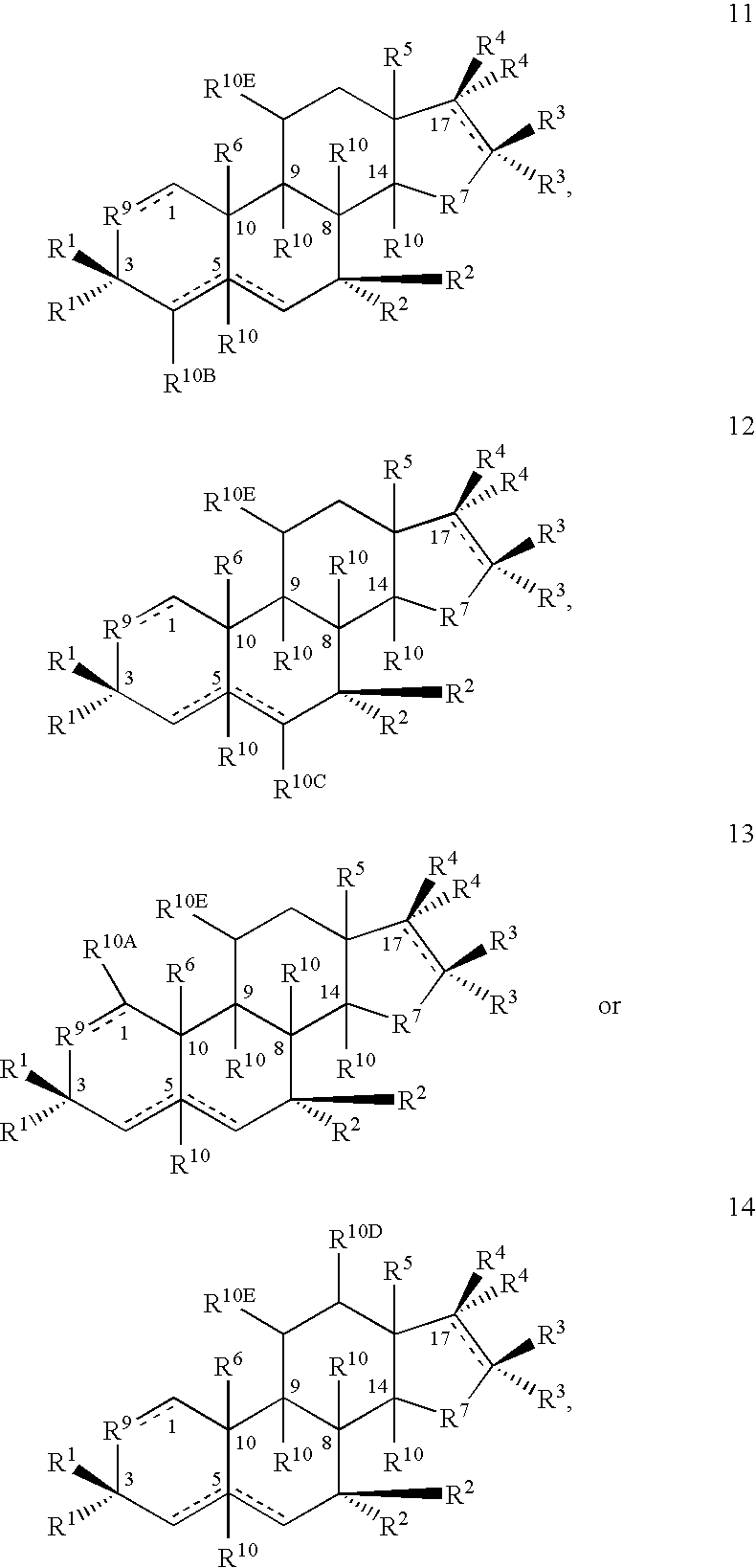
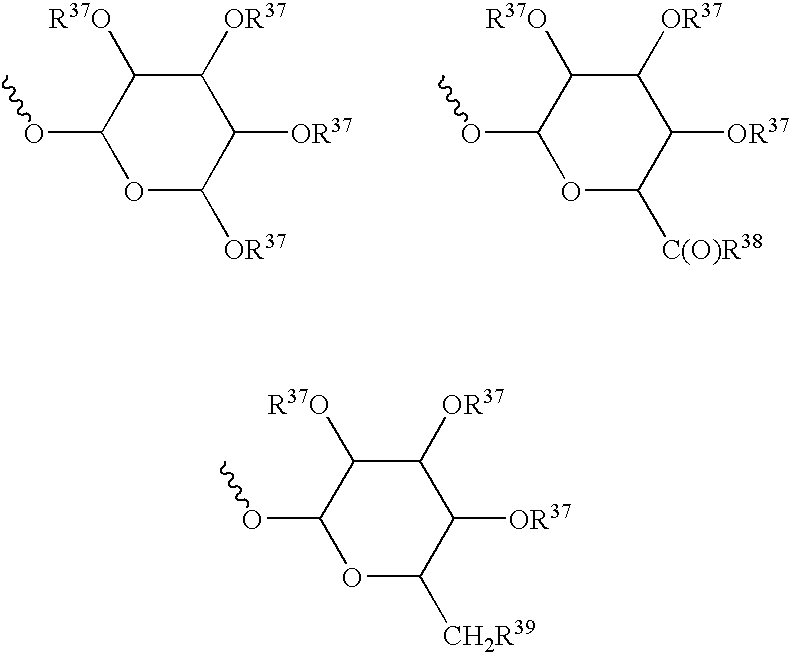
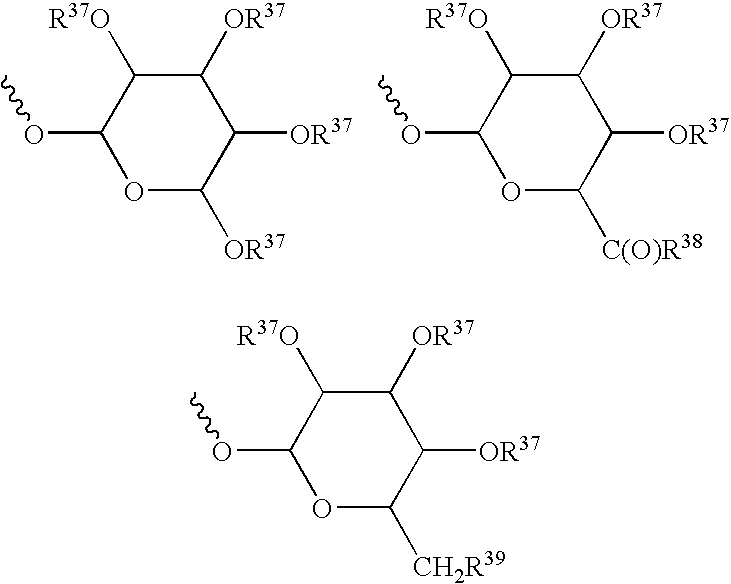
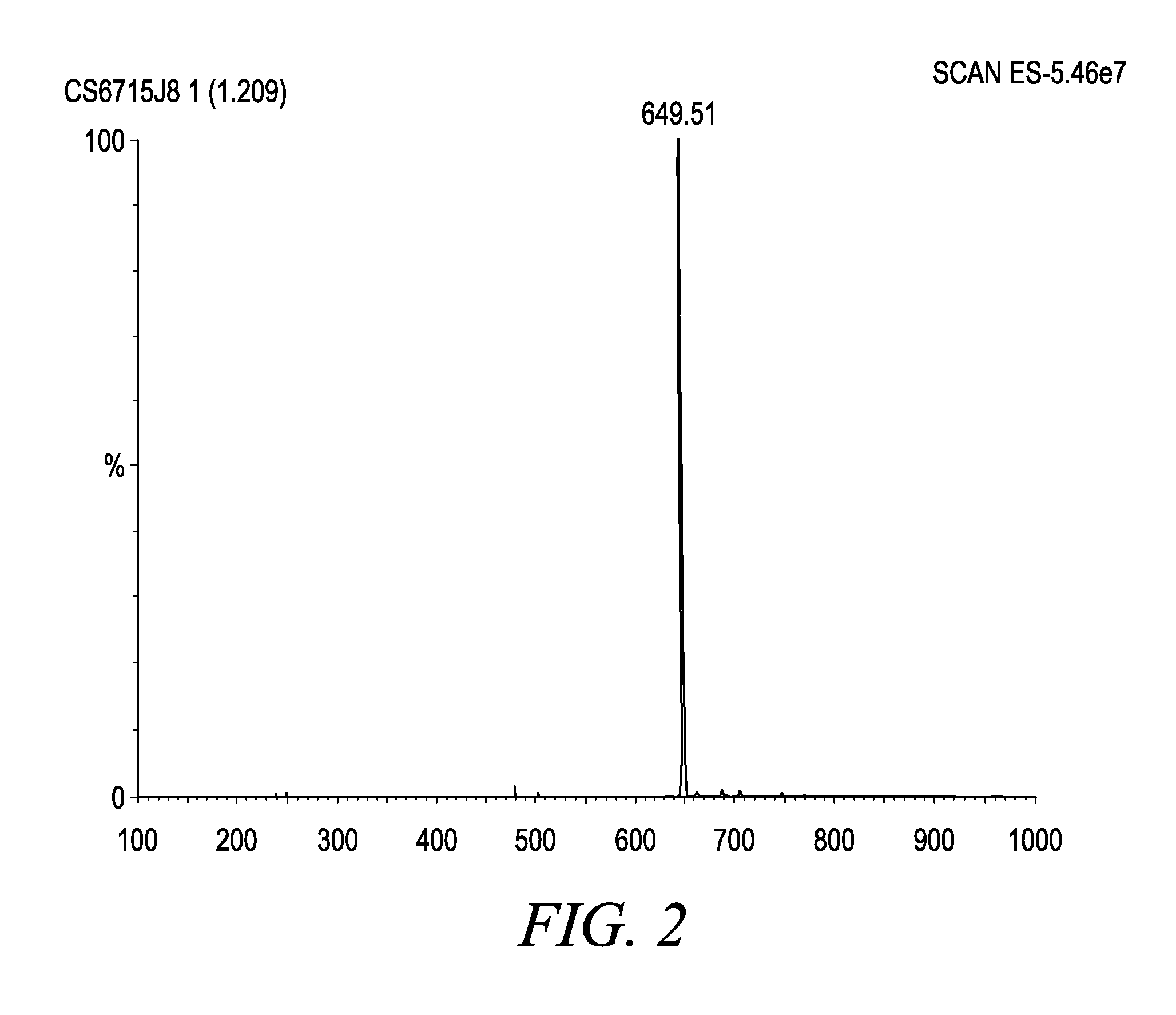
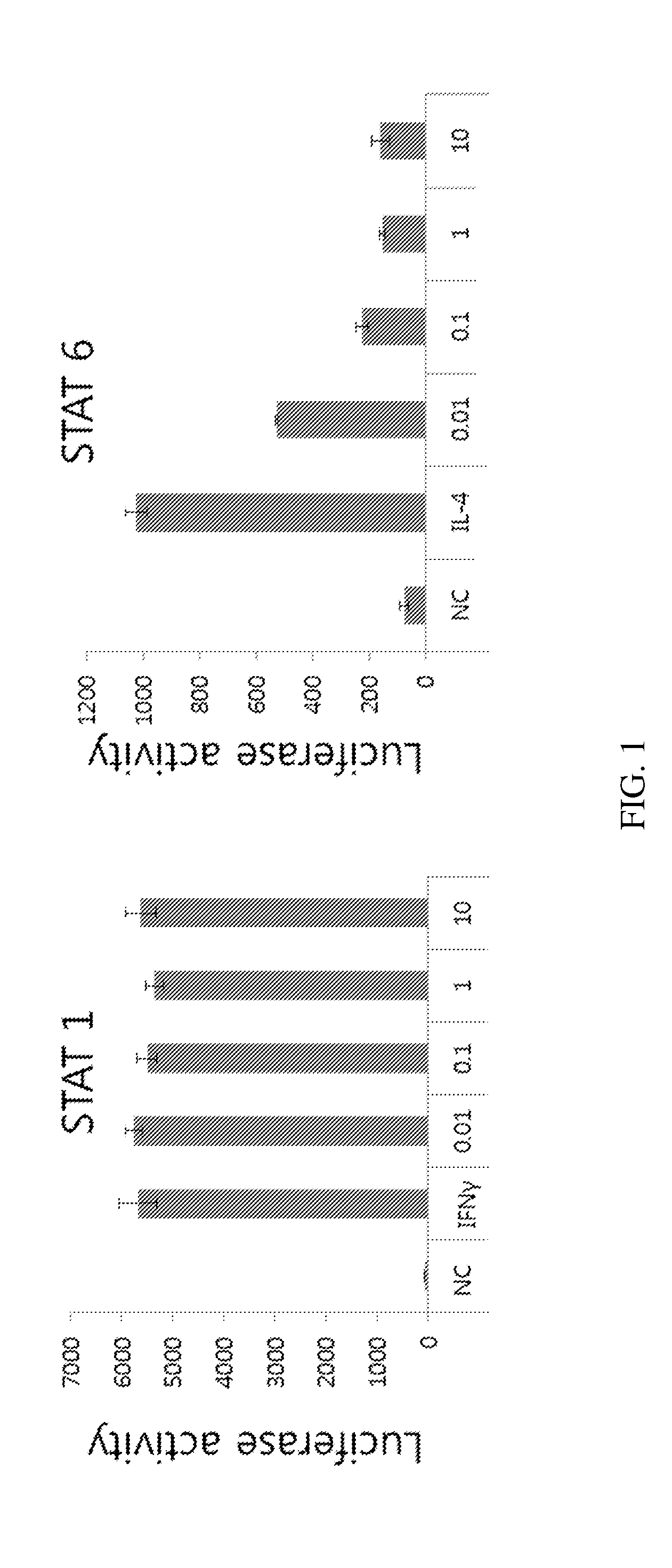
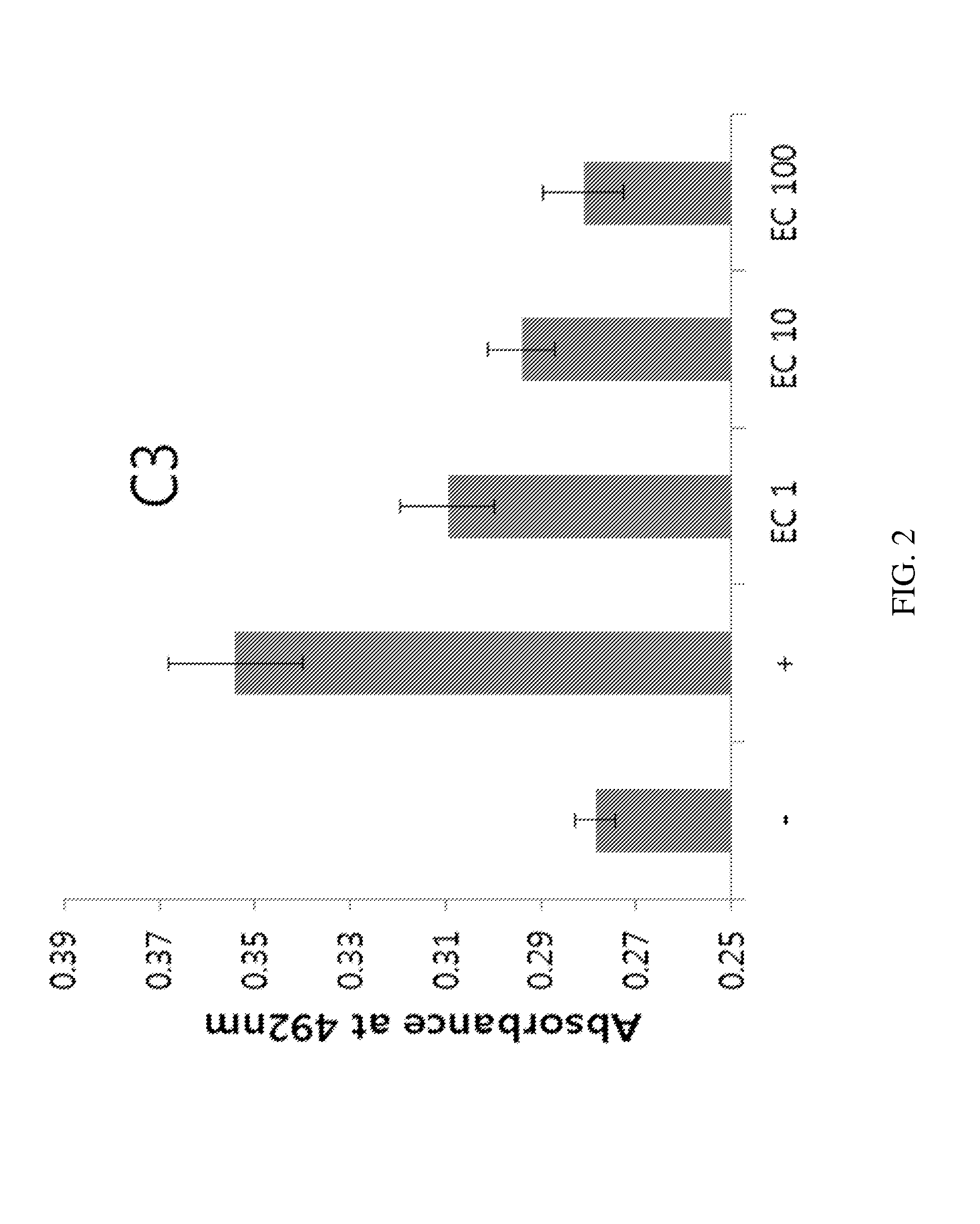Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Neutropenia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition characterized by abnormally low levels of white blood cells called neutrophils.
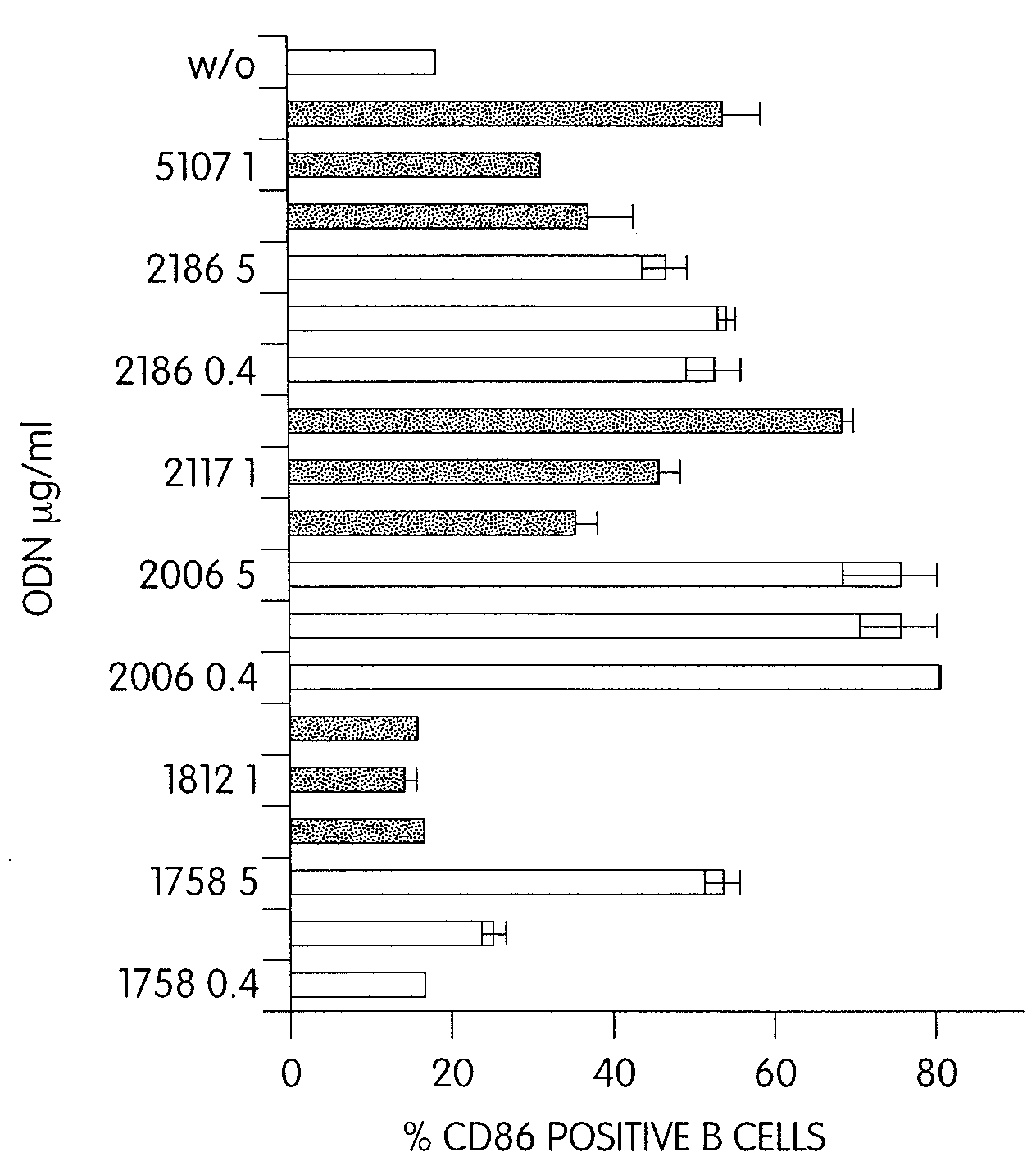
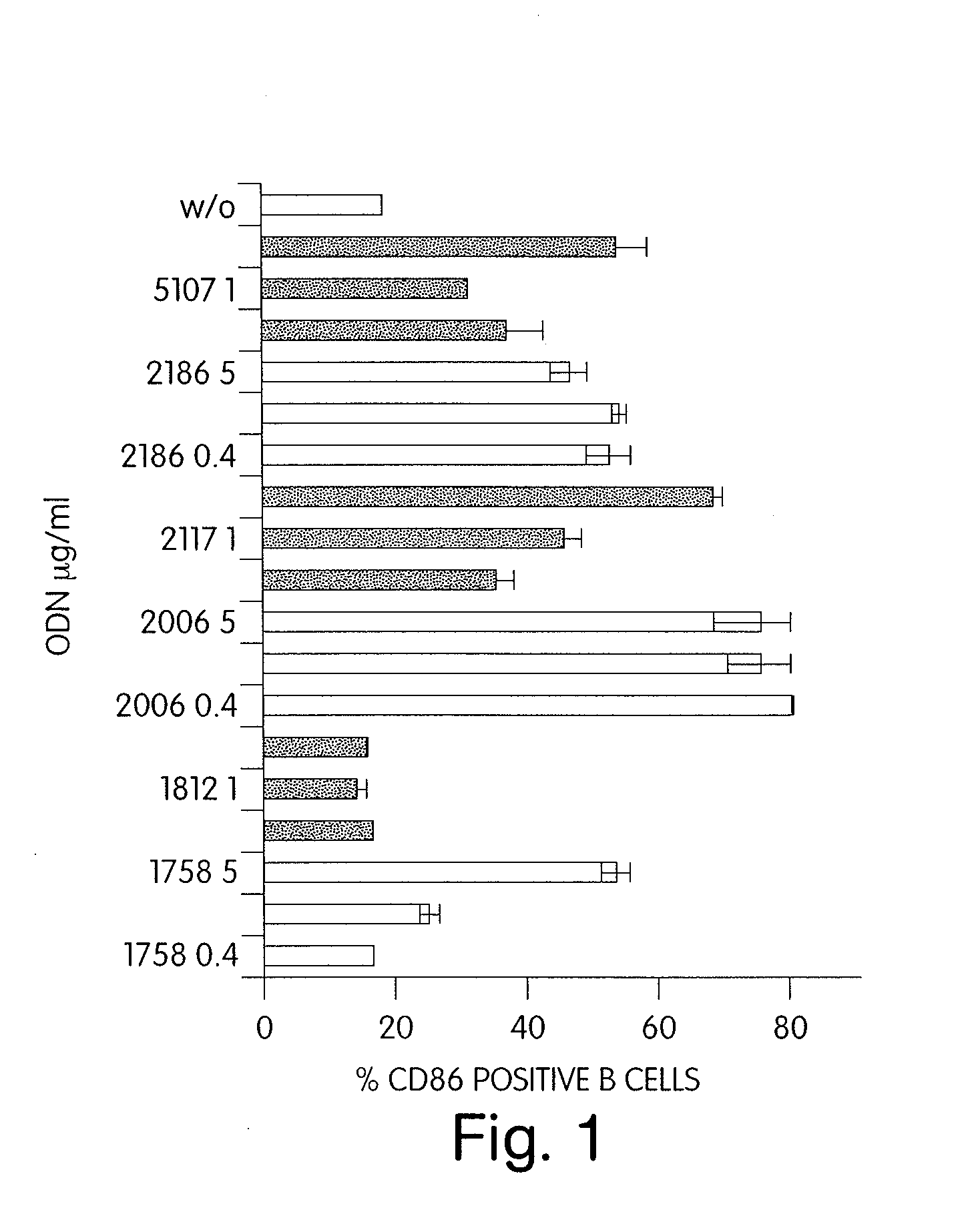
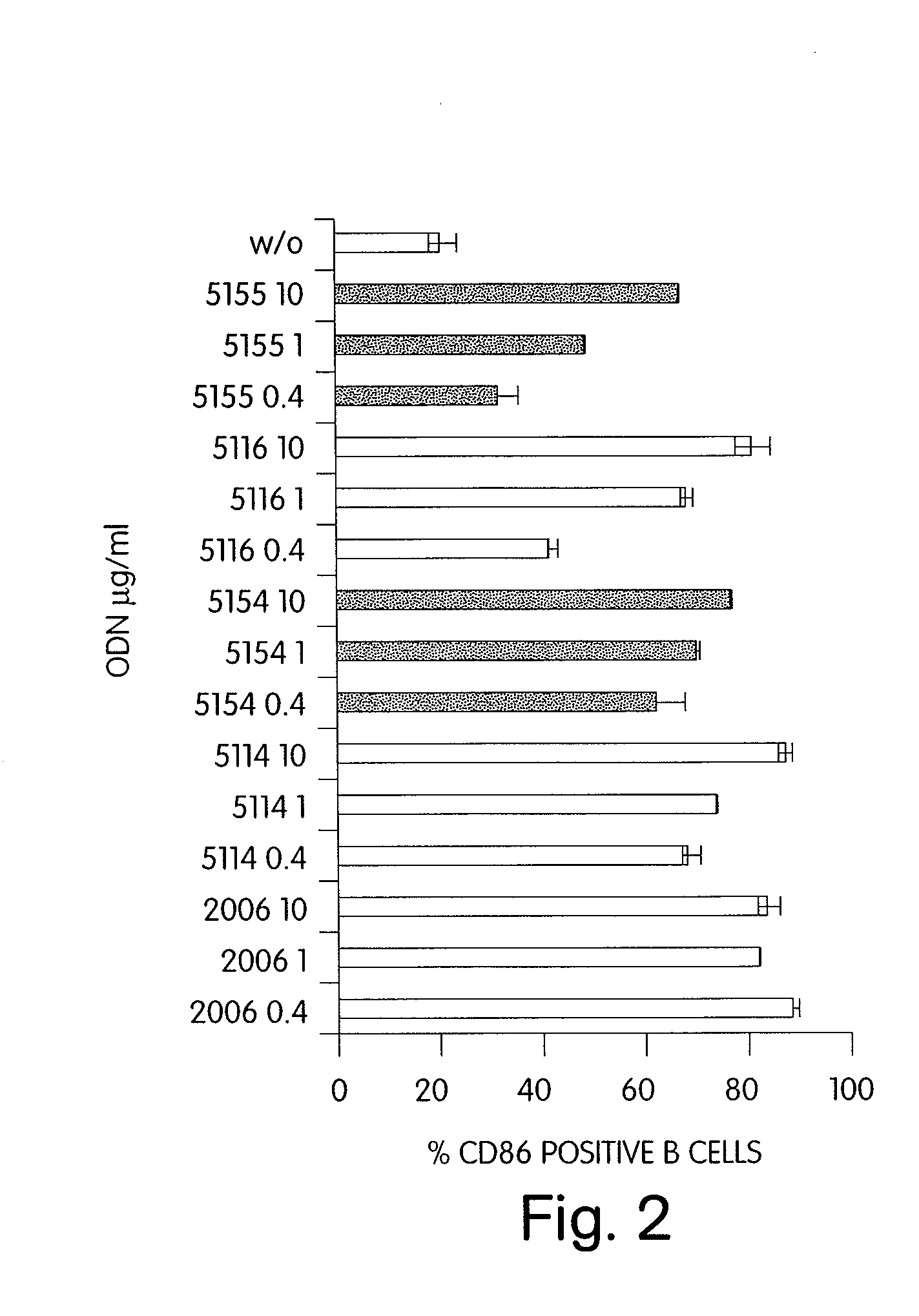
CPG-like nucleic acids and methods of use thereof
InactiveUS20080226649A1Enhancing neutrophil proliferationOrganic active ingredientsSugar derivativesAllergyPurine
Immunostimulatory compositions described as CpG-like nucleic acids are provided, including nucleic acids having immunostimulatory characteristics of CpG nucleic acid, despite certain substitutions of C, G, or C and G of the CpG dinucleotide. The substitutions can include, among others, exchange of methylated C for C, inosine for G, and ZpY for CpG, where Z is cytosine or dSpacer and Y is inosine, 2-aminopurine, nebularine, or dSpacer. Also provided are methods for inducing an immune response in a subject using the CpG-like nucleic acids. The methods are useful in the treatment of a subject that has or is at risk of developing an infectious disease, allergy, asthma, cancer, anemia, thrombocytopenia, or neutropenia.
Owner:COLEY PHARMA GMBH
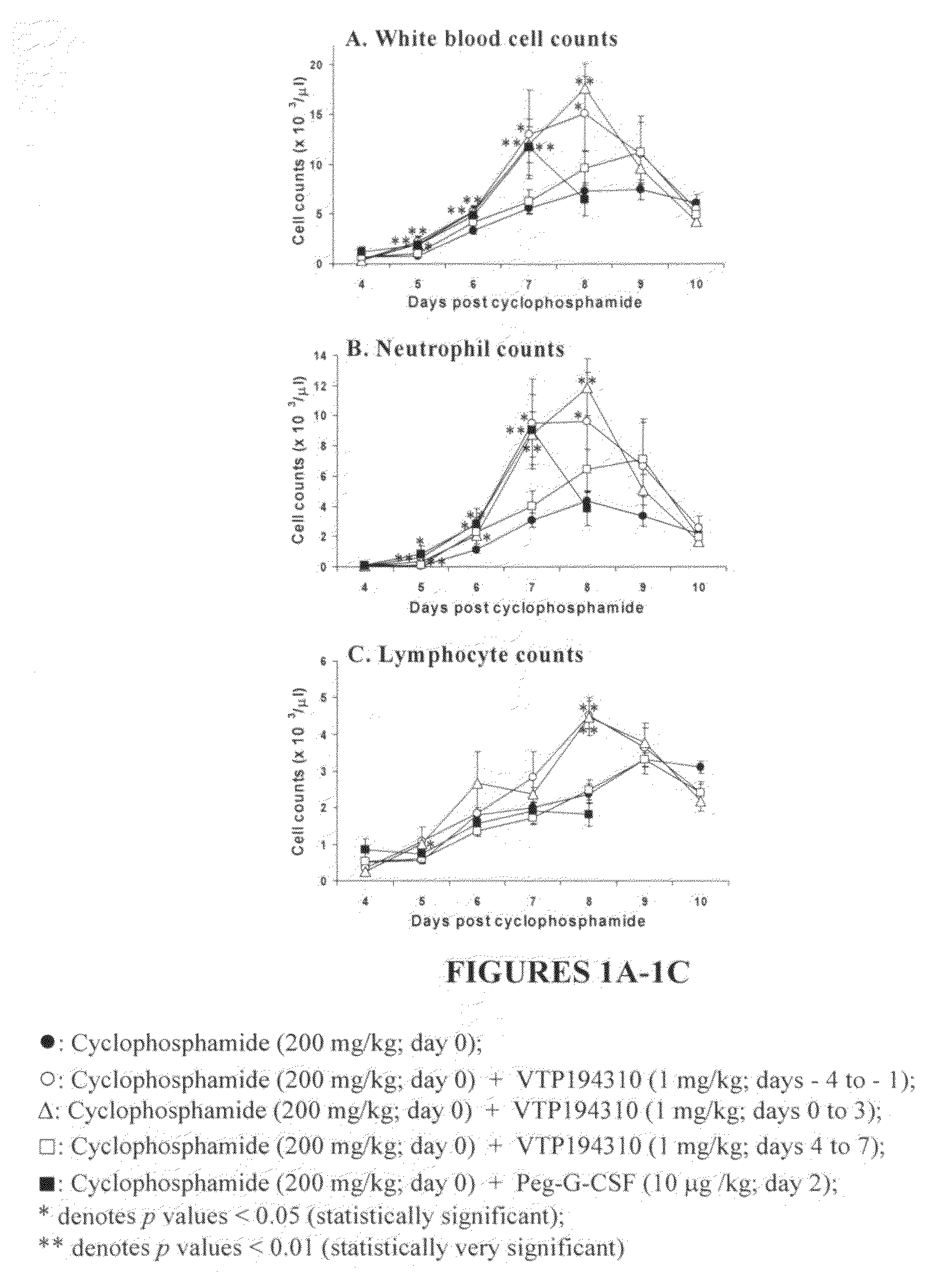
Methods for treating chemotherapy and radiation therapy side effects
ActiveUS20090176862A1Reduced hematopoietic functionDegraded immune functionAntibacterial agentsBiocideSide effectRadical radiotherapy
A method for treating chemo therapy or radiation therapy side effects in a mammal undergoing chemotherapy and / or radiation therapy, the method comprising a step of administering to the mammal a therapeutically effective amount of a RAR antagonist or RAR inverse agonist which binds to receptors of the RARα, RARβ and RARγ subtypes is disclosed. Such side effects include chemoradiotherapy-induced alopecia, chemoradiotherapy-induced thrombocytopenia, chemoradiotherapy-induced leucopenia and chemoradiotherapy-induced neutropenia.
Owner:IO THERAPEUTICS
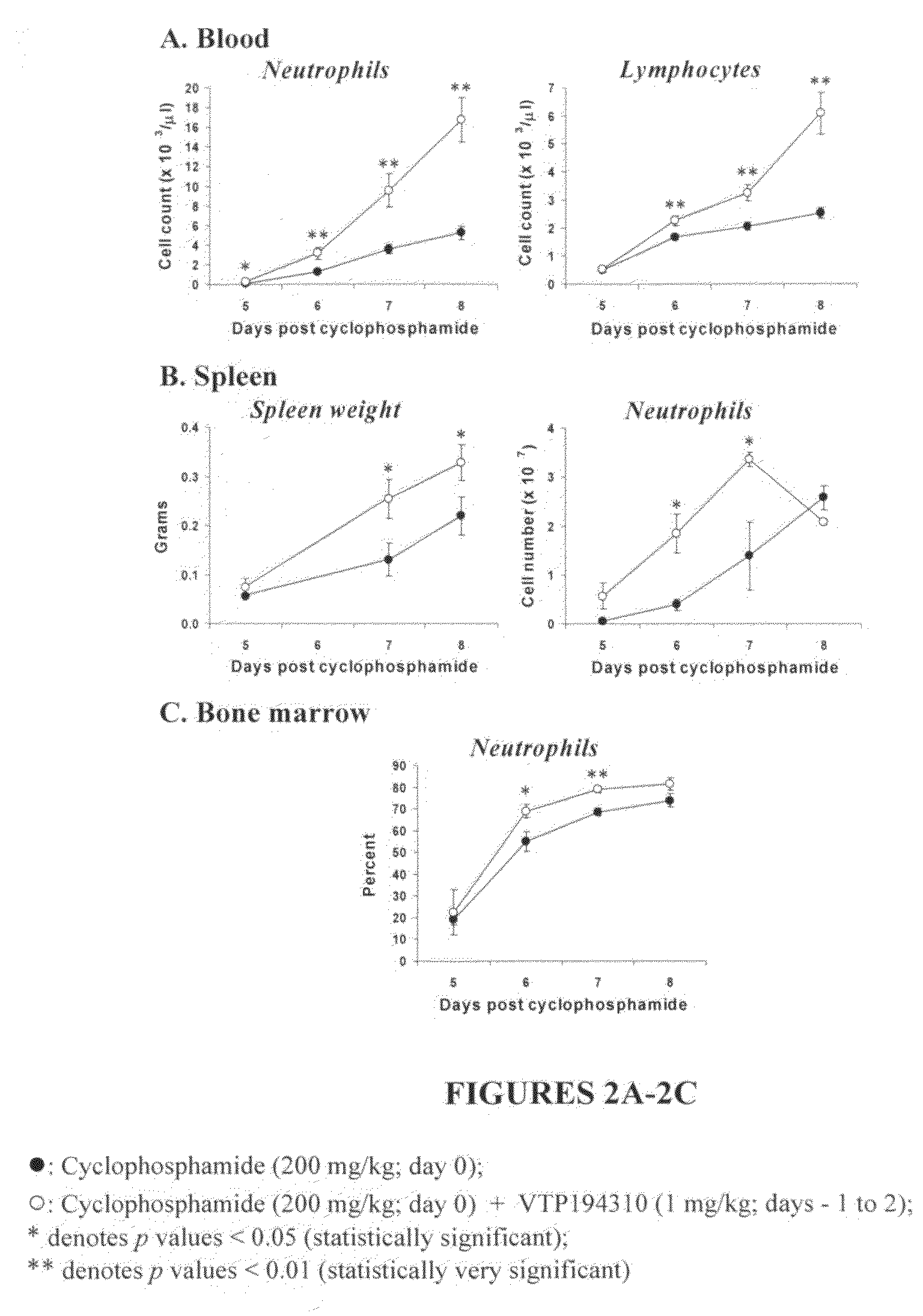
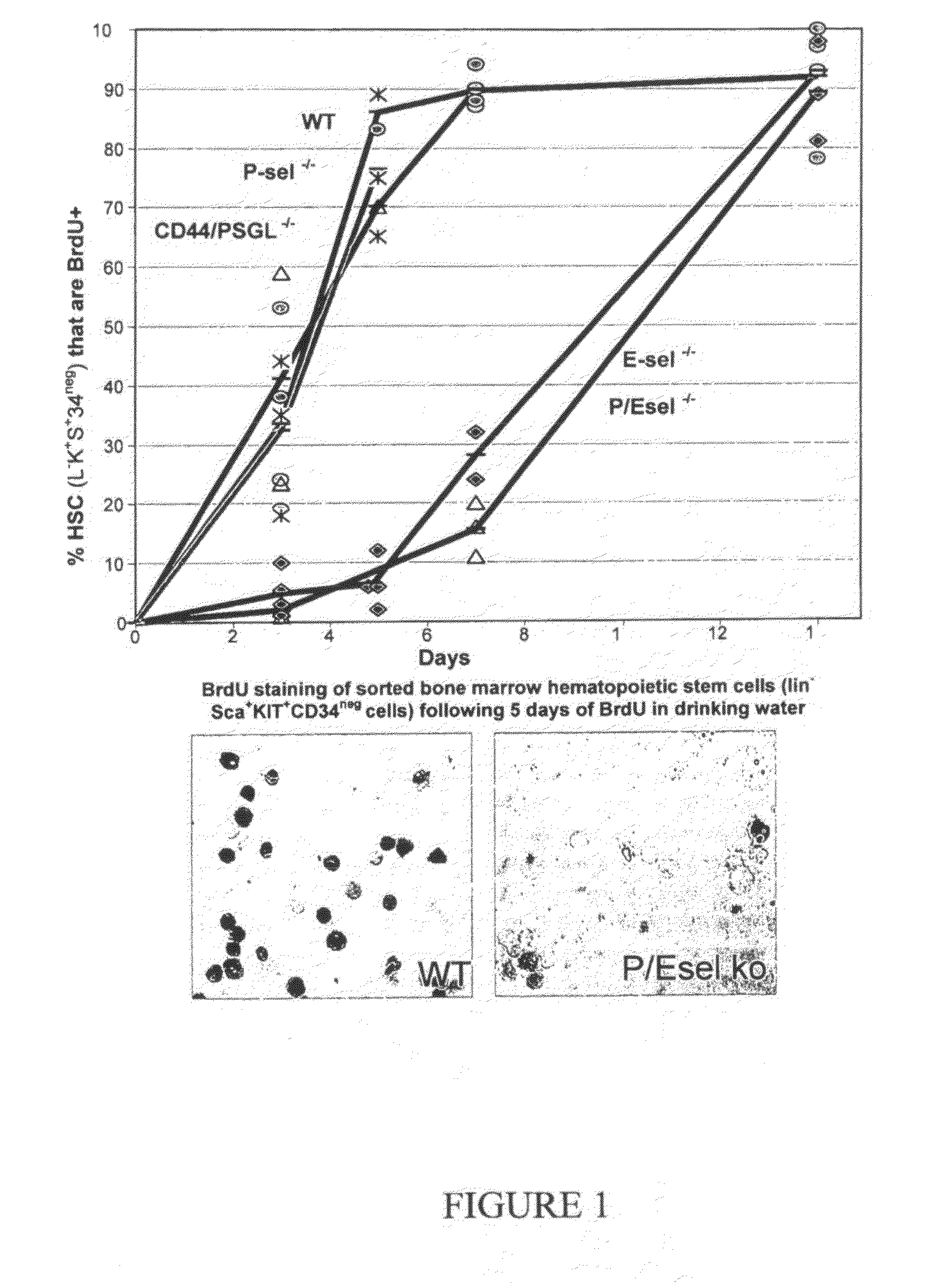
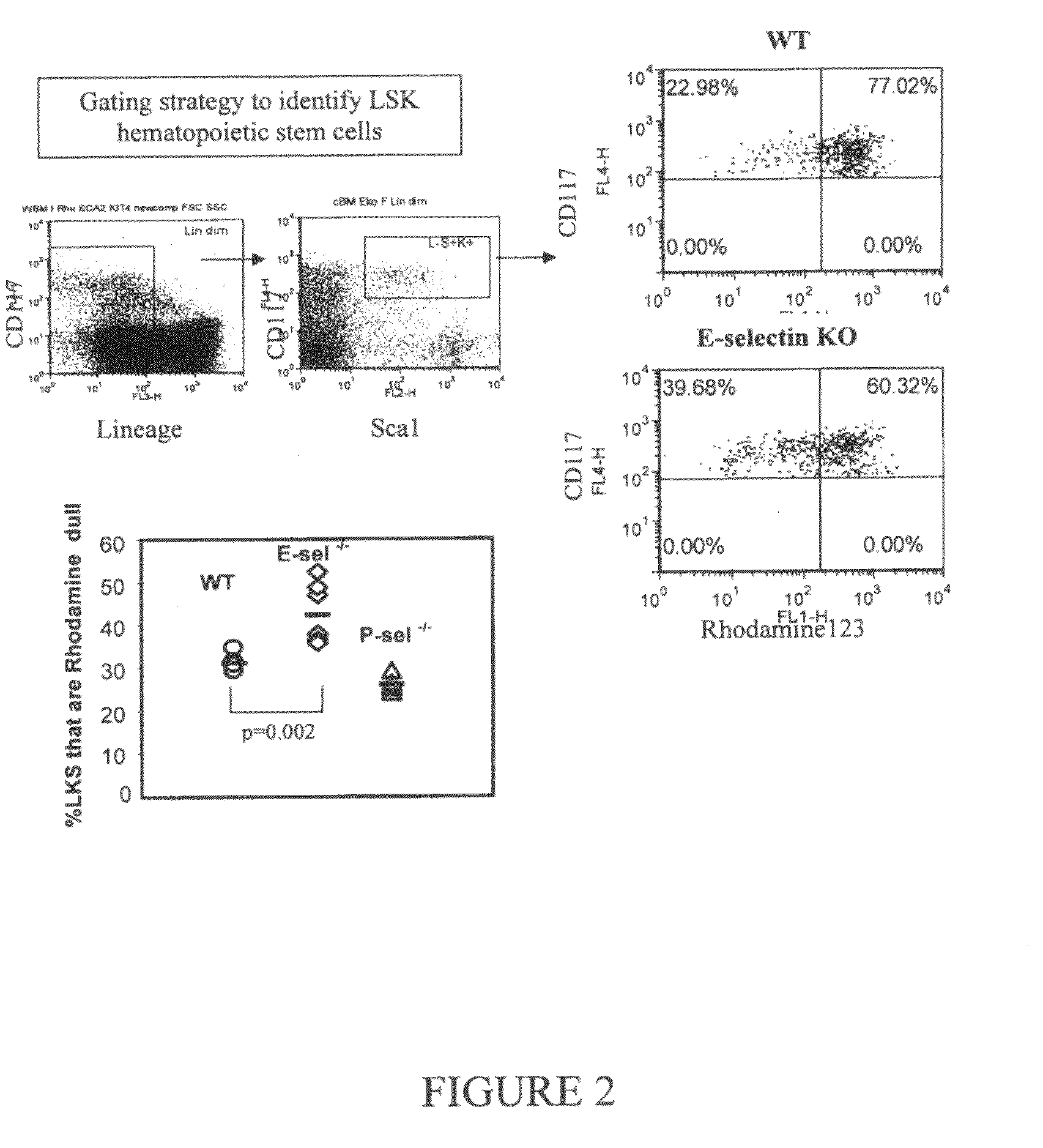
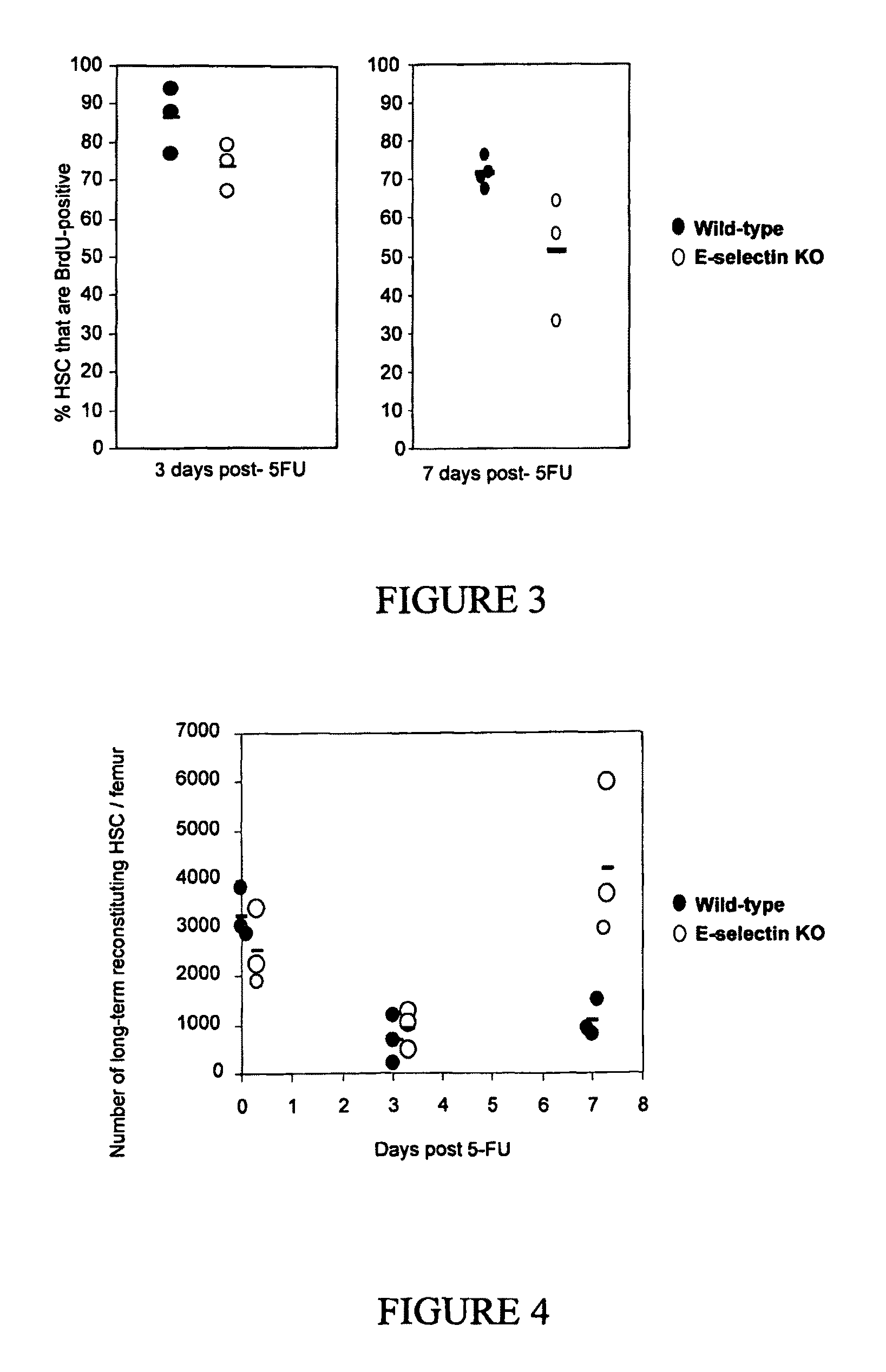
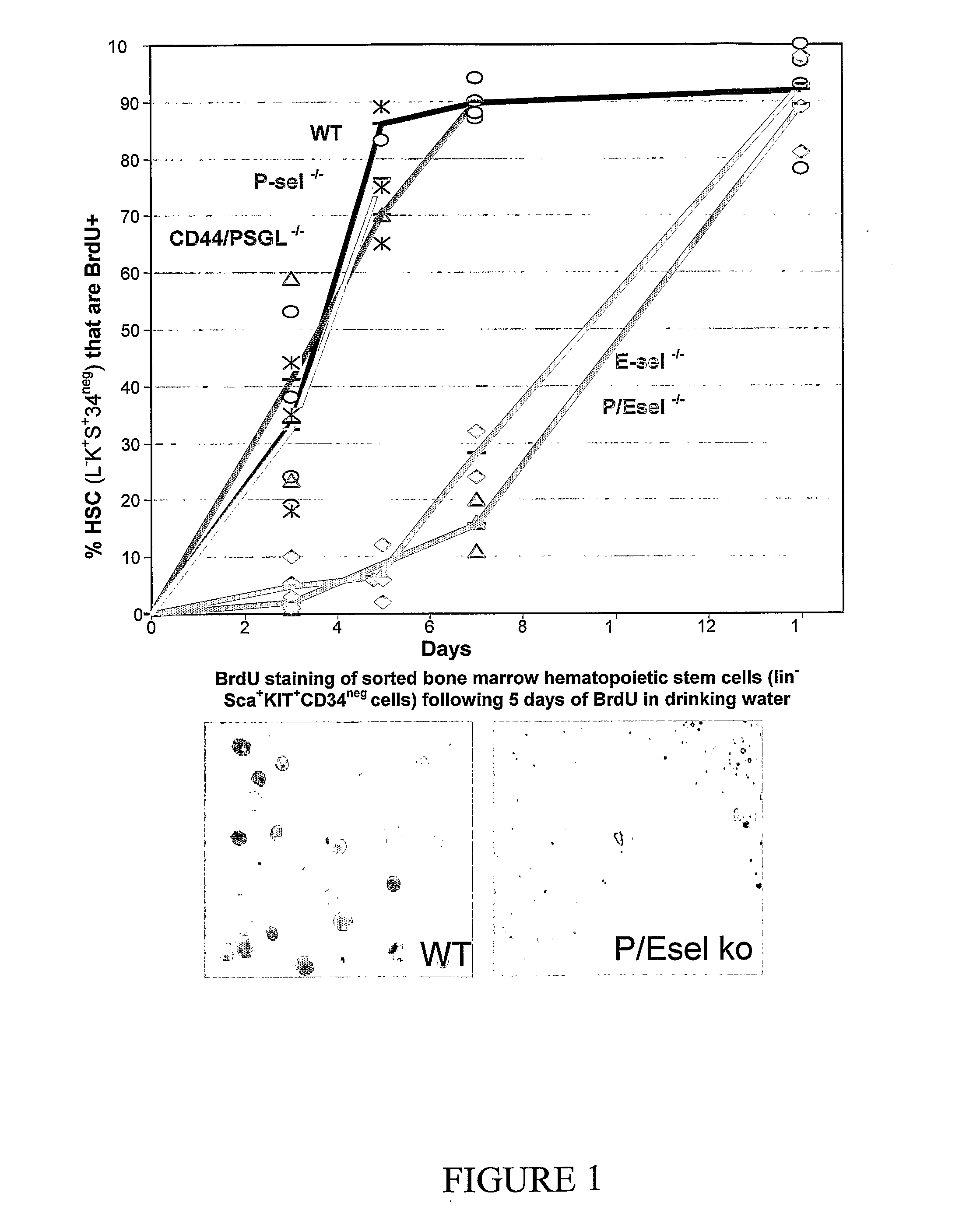
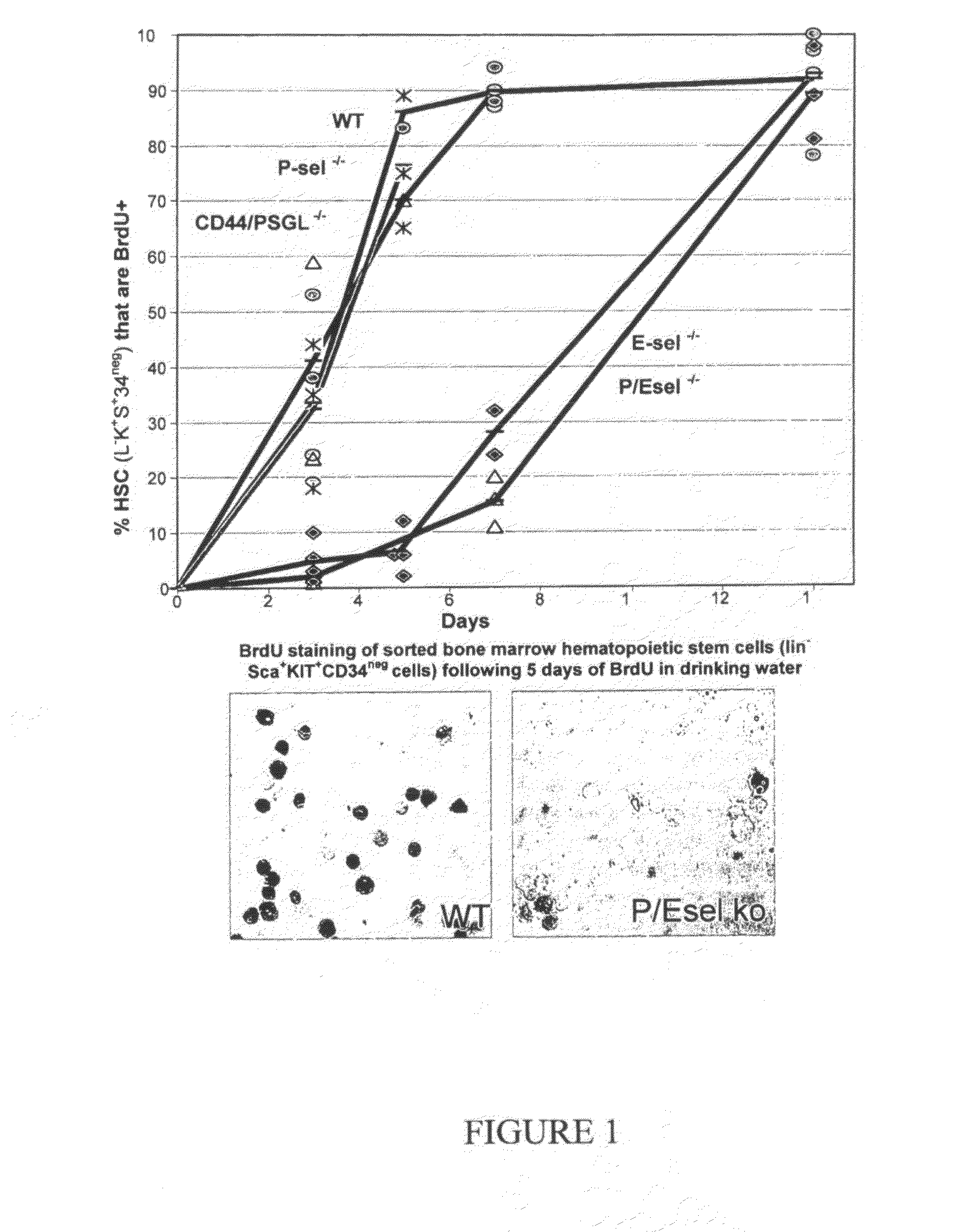
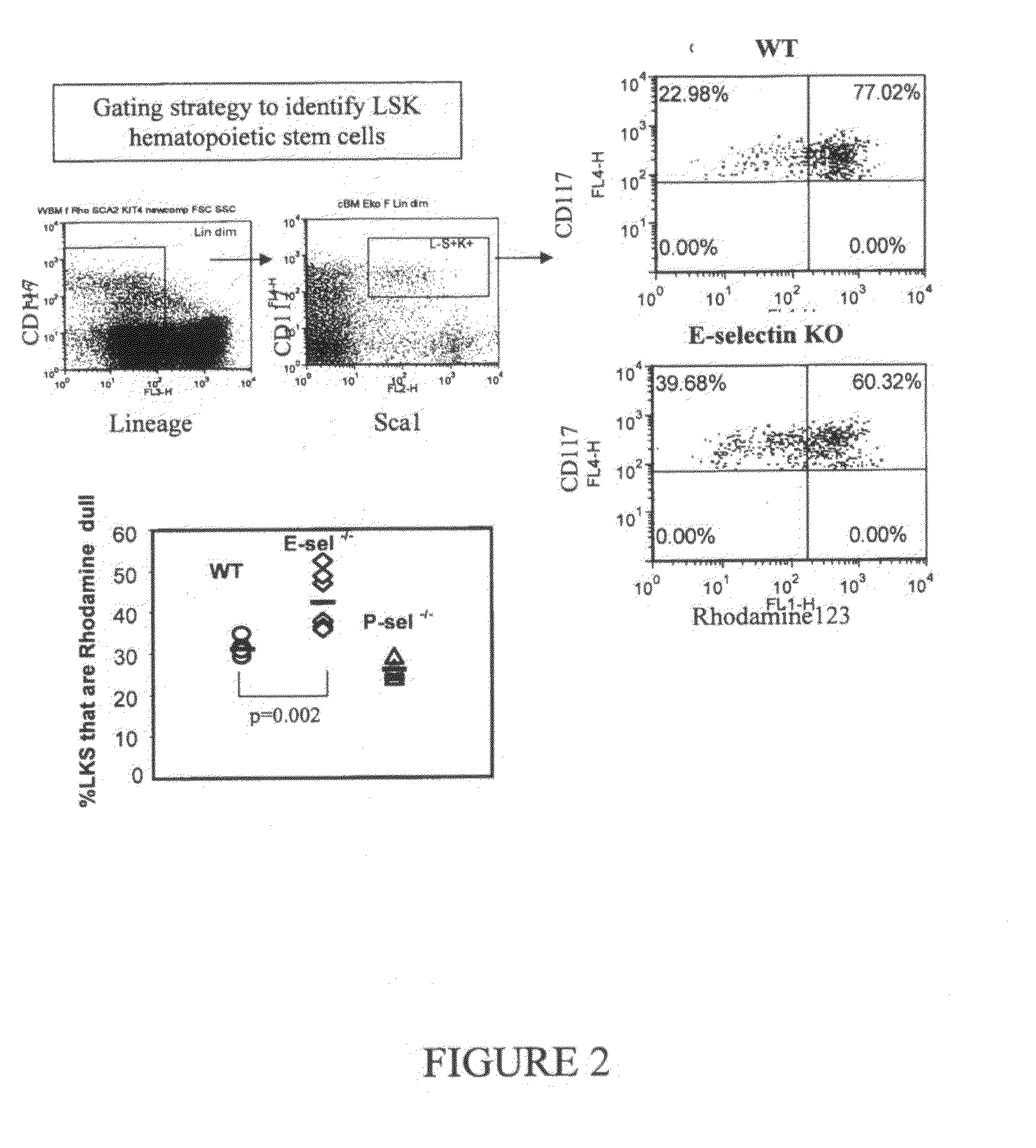
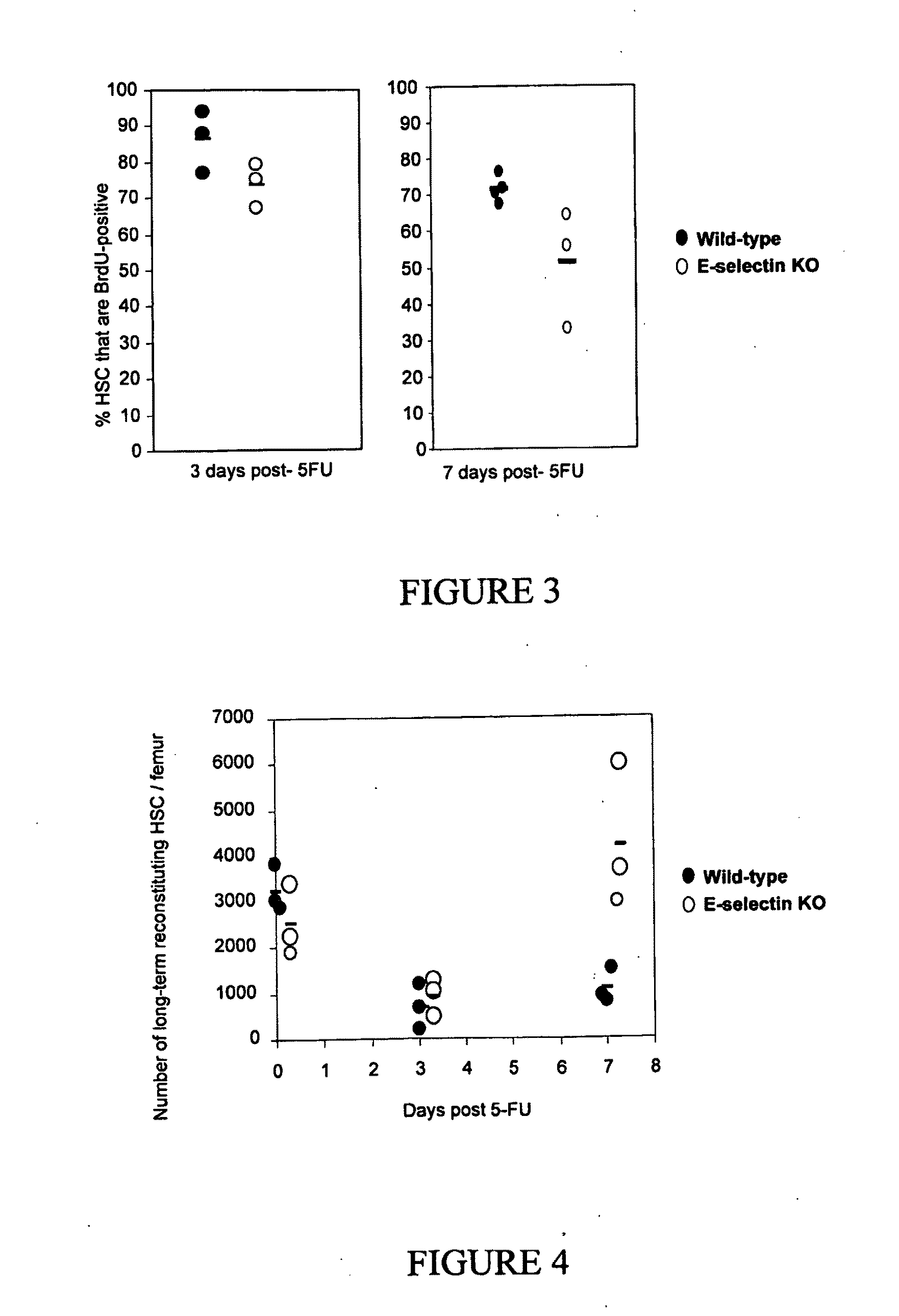
Compositions comprising E-selectin antagonists and uses therefor
ActiveUS9254322B2Stimulating and enhancing hematopoiesisAvoid adjustmentPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsProgenitorSurgery
This invention discloses the use of an E-selectin antagonist and a mobilizer of hematopoietic stem cells or progenitor cells in methods and compositions for treating or preventing immunocompromised conditions resulting from medical treatment. The present invention is particular relevant for prophylaxis and / or treatment of hematopoeitic disorders including neutropenia, agranulocytosis, anemia and thrombocytopenia in individuals receiving or proposed to receive treatments that target rapidly dividing cells or that disrupt the cell cycle or cell division.
Owner:THE UNIV OF QUEENSLAND
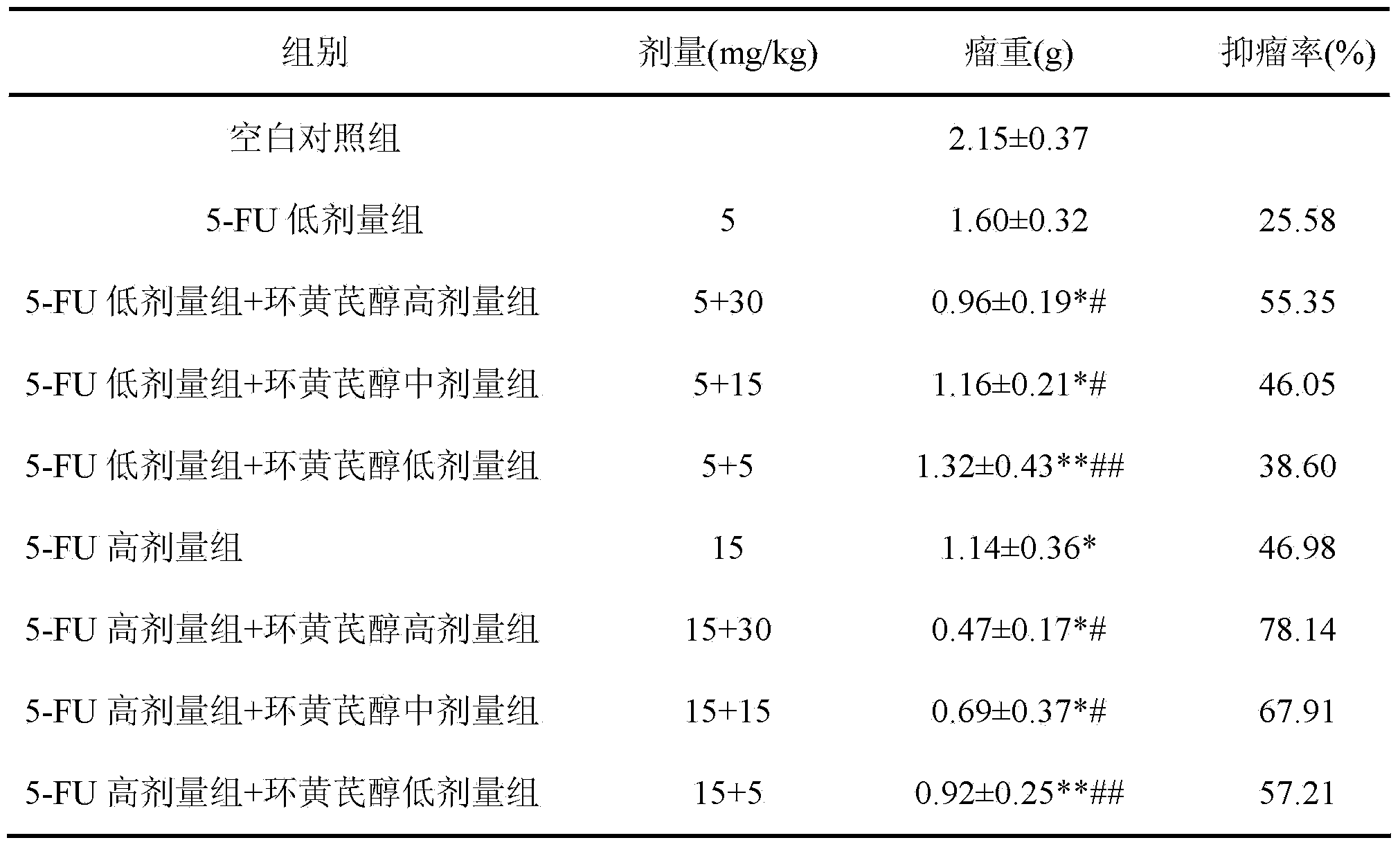
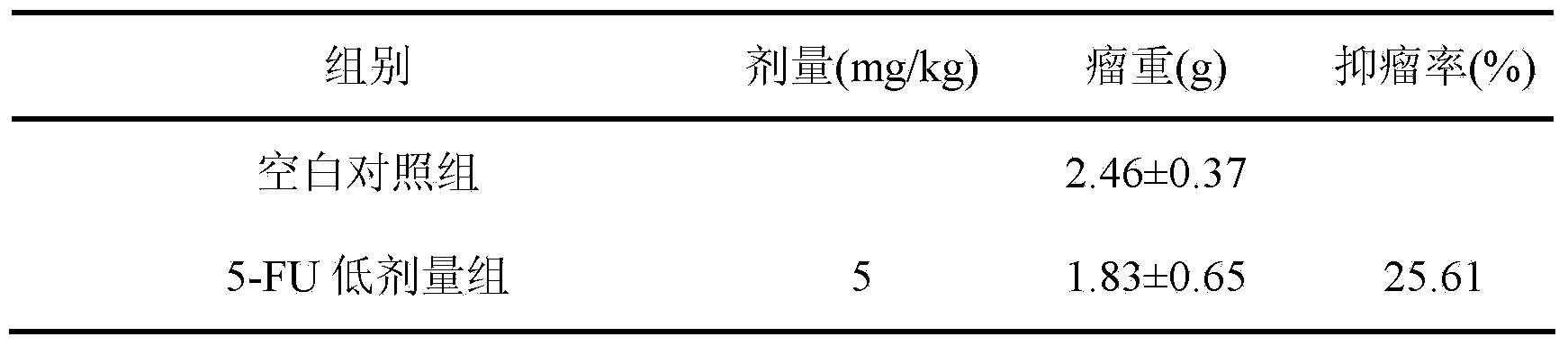
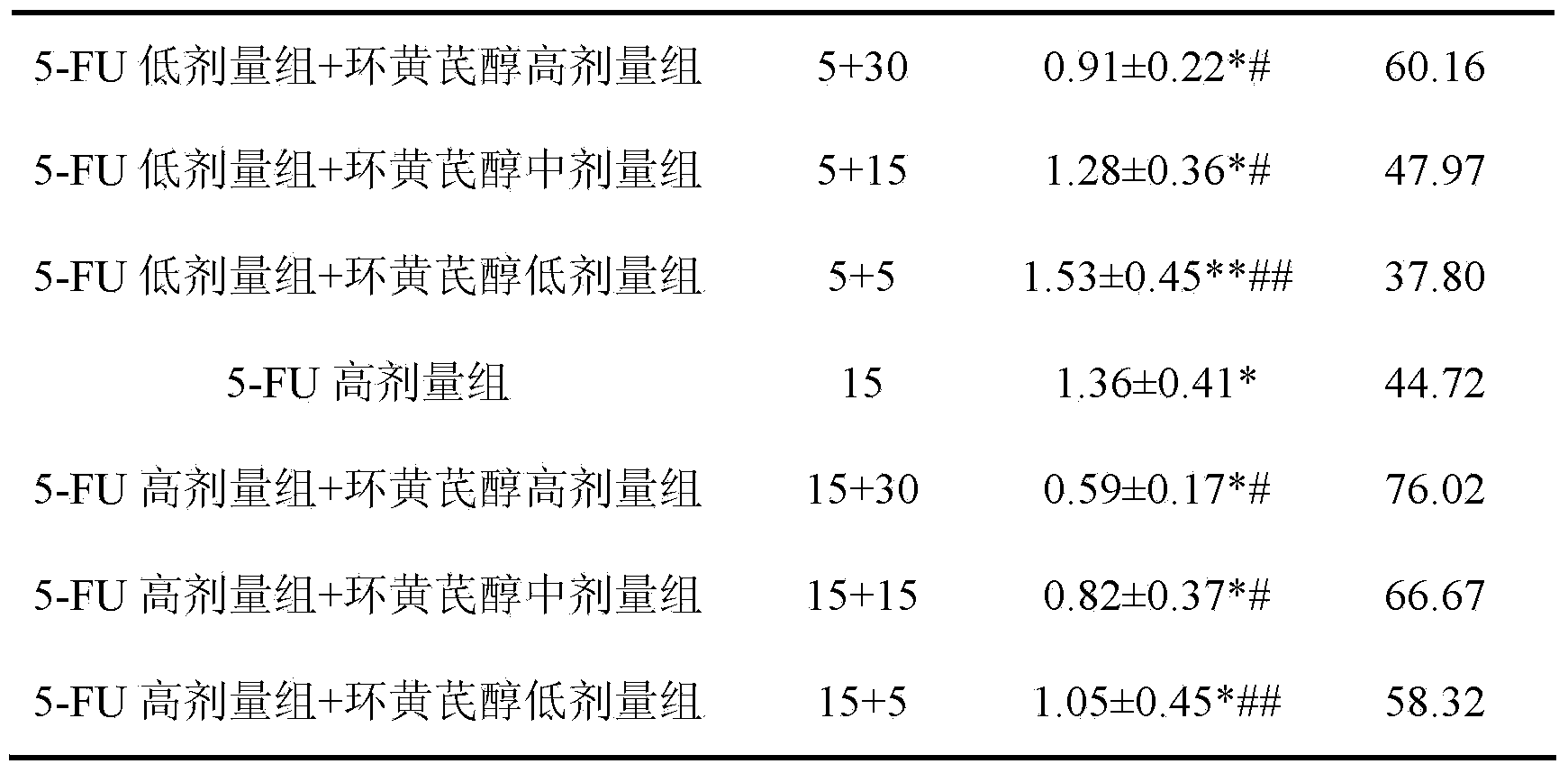
Preparation method and application of cycloastragenol
ActiveCN103880910AImprove qualityEfficient removalSteroidsAntineoplastic agentsAstragalosideAdjuvant
The invention discloses a preparation method and application of cycloastragenol. The preparation method of the cycloastragenol comprises the following steps: by using astragaloside or astrasieversianin as a raw material, oxidizing, reducing, hydrolyzing, extracting and purifying to obtain high-purity cycloastragenol. The method is simple to operate, gentle in condition, good in product quality and high in yield. The cycloastragenol can be applied to preparation of anticancer adjuvant therapeutic medicine. An anticancer adjuvane therapeutic medicine prepared by using the cycloastragenol as a pharmaceutical active ingredient has the anticancer adjuvant therapeutic effect of enhancing the anticancer therapeutic effect, reducing the toxicity of the anticancer medicine, preventing and treating the neutropenia caused by the anticancer medicine therapy.
Owner:SOUTHWEST JIAOTONG UNIV
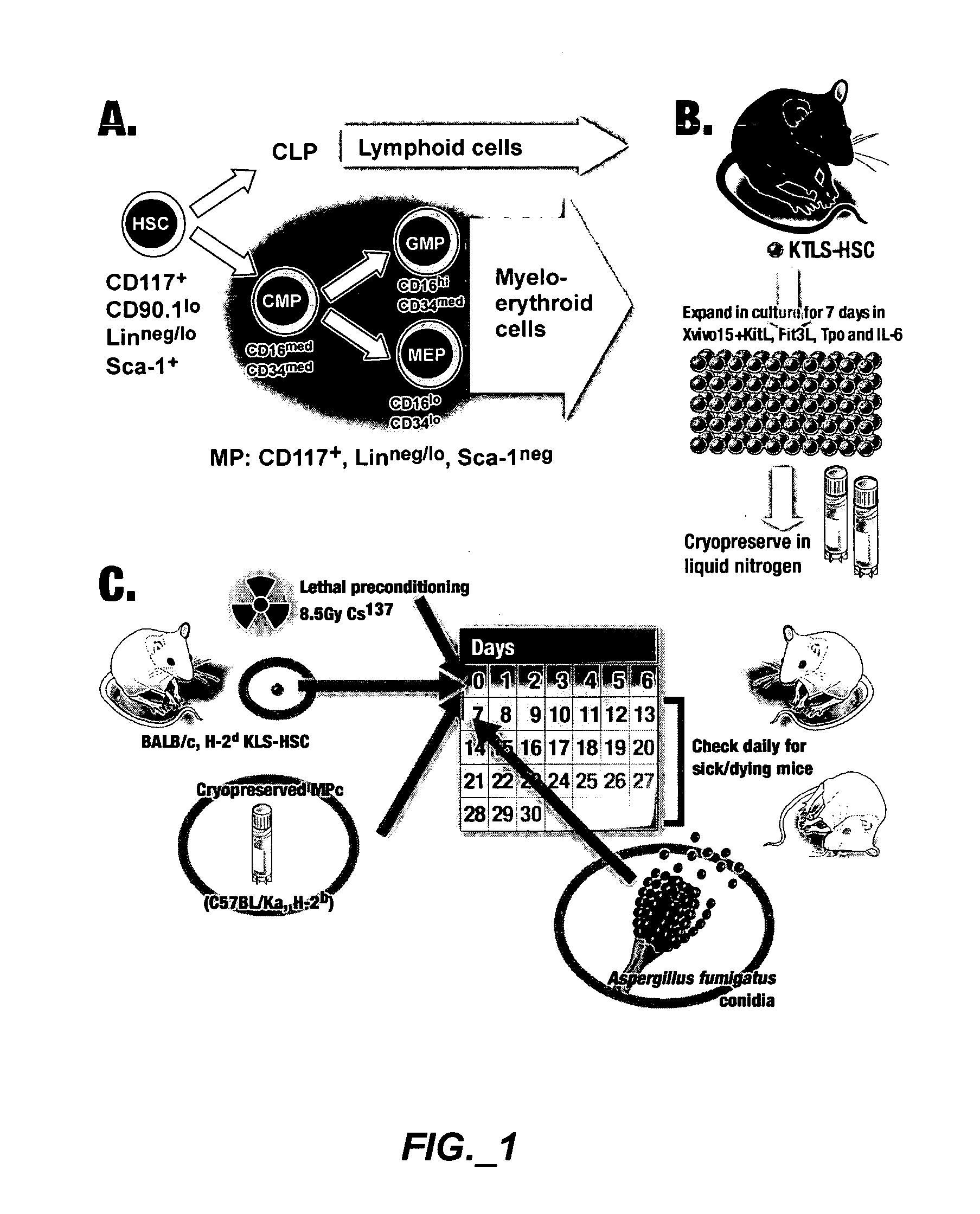
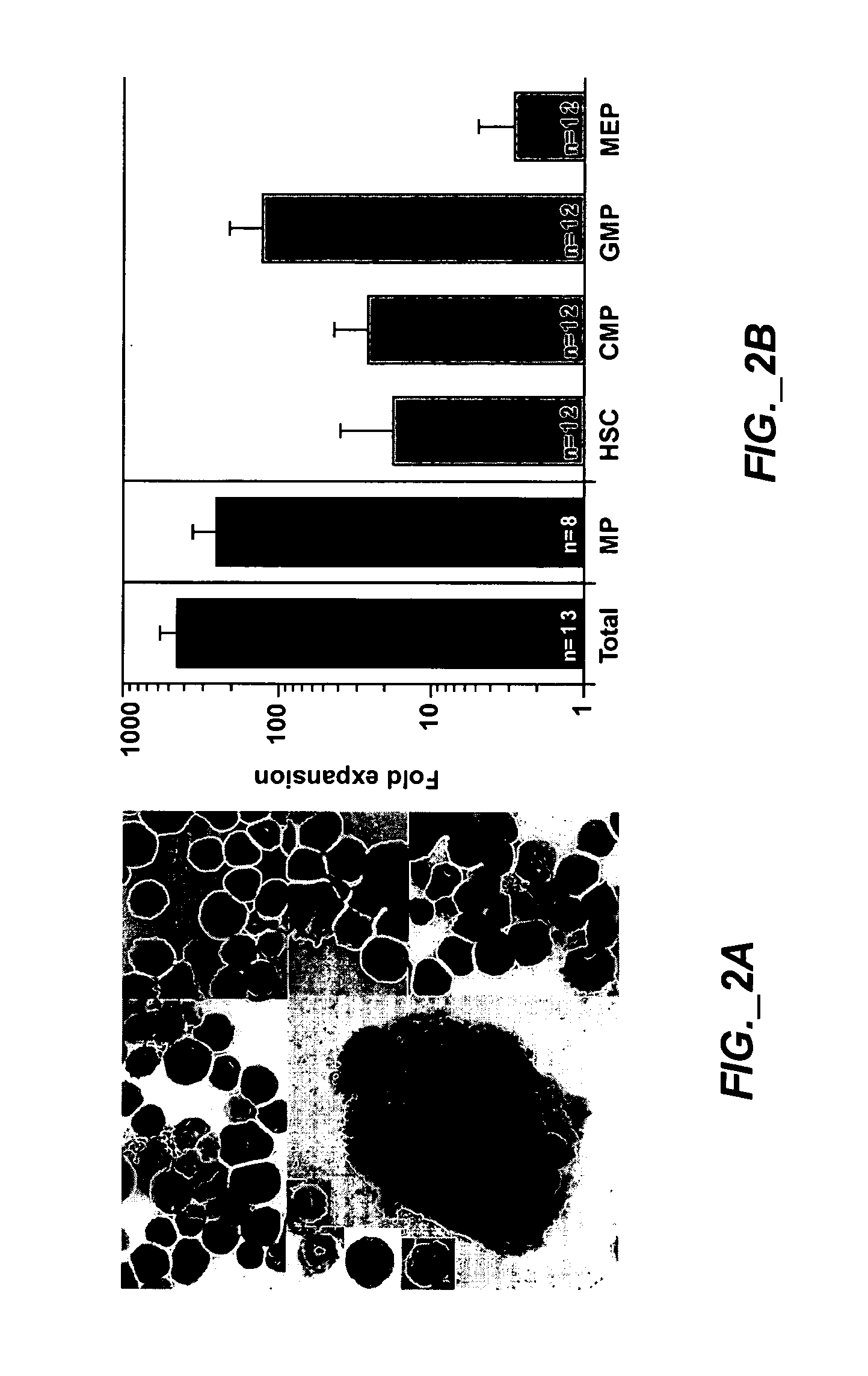
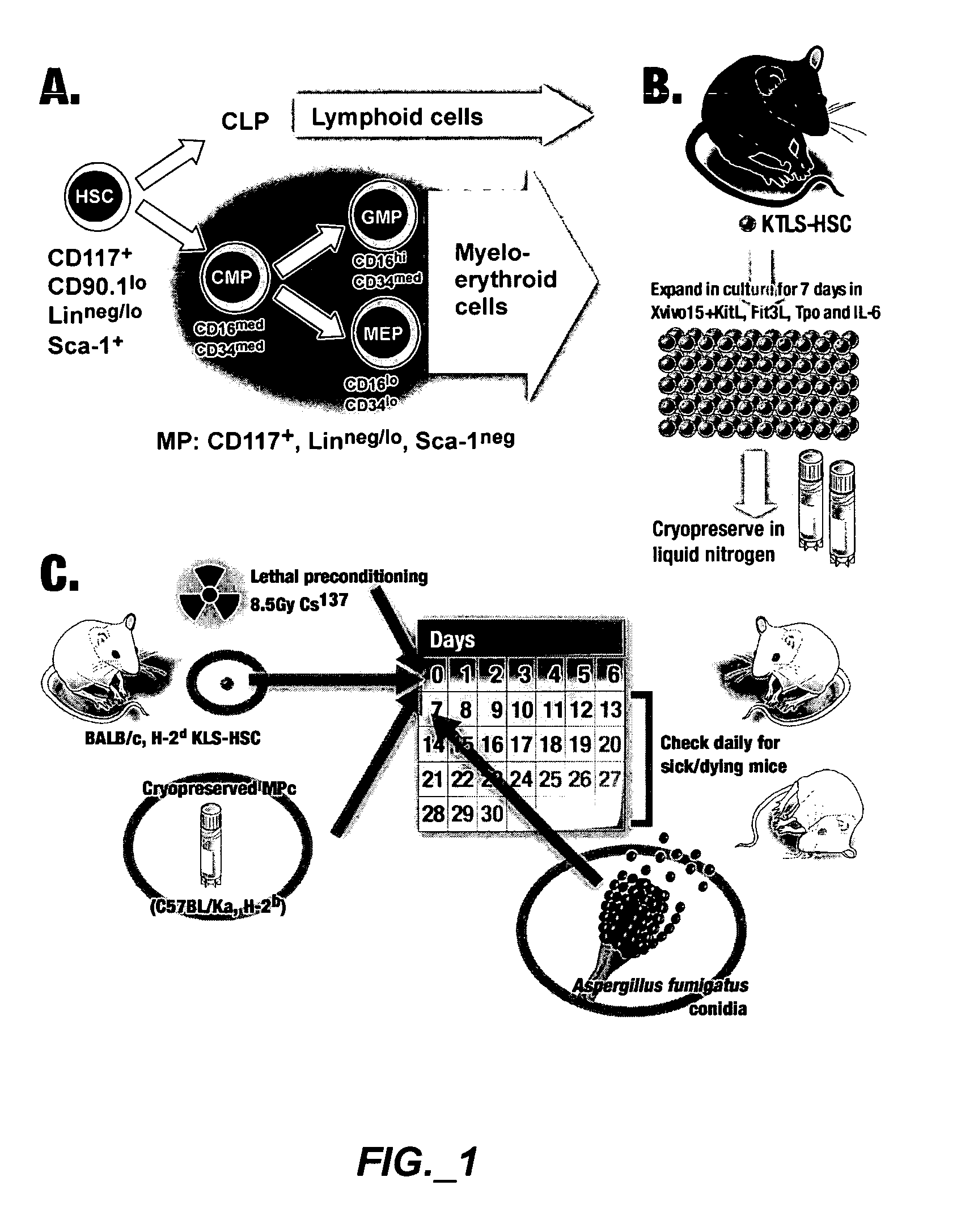
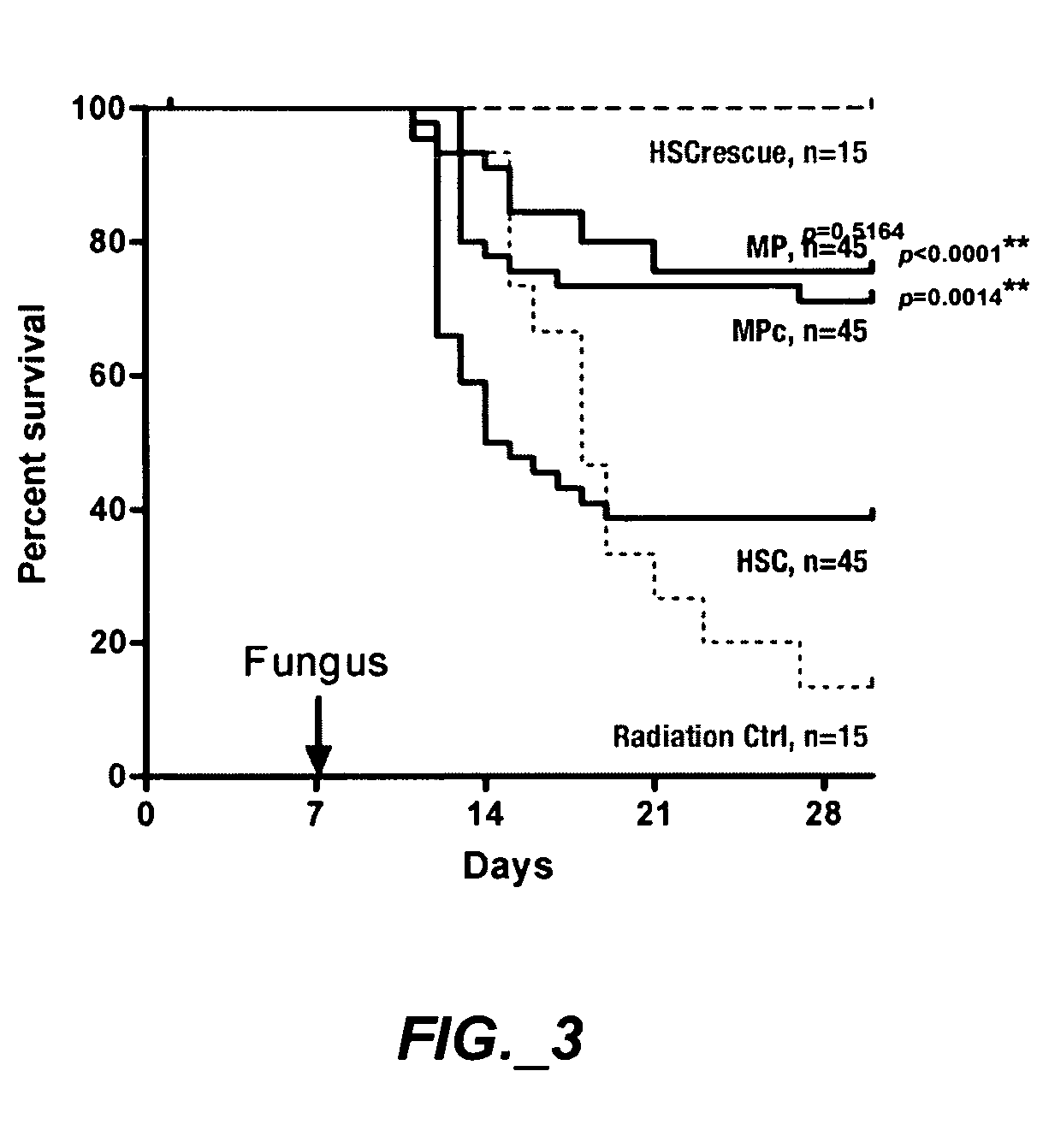
Methods of expanding myeloid cell populations and uses thereof
ActiveUS20060134783A1Increase differentiationAntibacterial agentsAntimycoticsMyeloid Progenitor CellsHematopoietic stem cell transplantation
The present disclosure relates to a method of expanding myeloid progenitor cells by culturing an initial population of cells in a medium comprising a mixture of cytokines and growth factors that promote growth and expansion of the myeloid progenitor cells. The expanded cell population provides a source of cells as therapeutic treatments for neutropenia and / or thrombocytopenia arising in patients subjected to myeloablative therapy and hematopoietic stem cell transplantation.
Owner:CELLERANT THERAPEUTICS INC
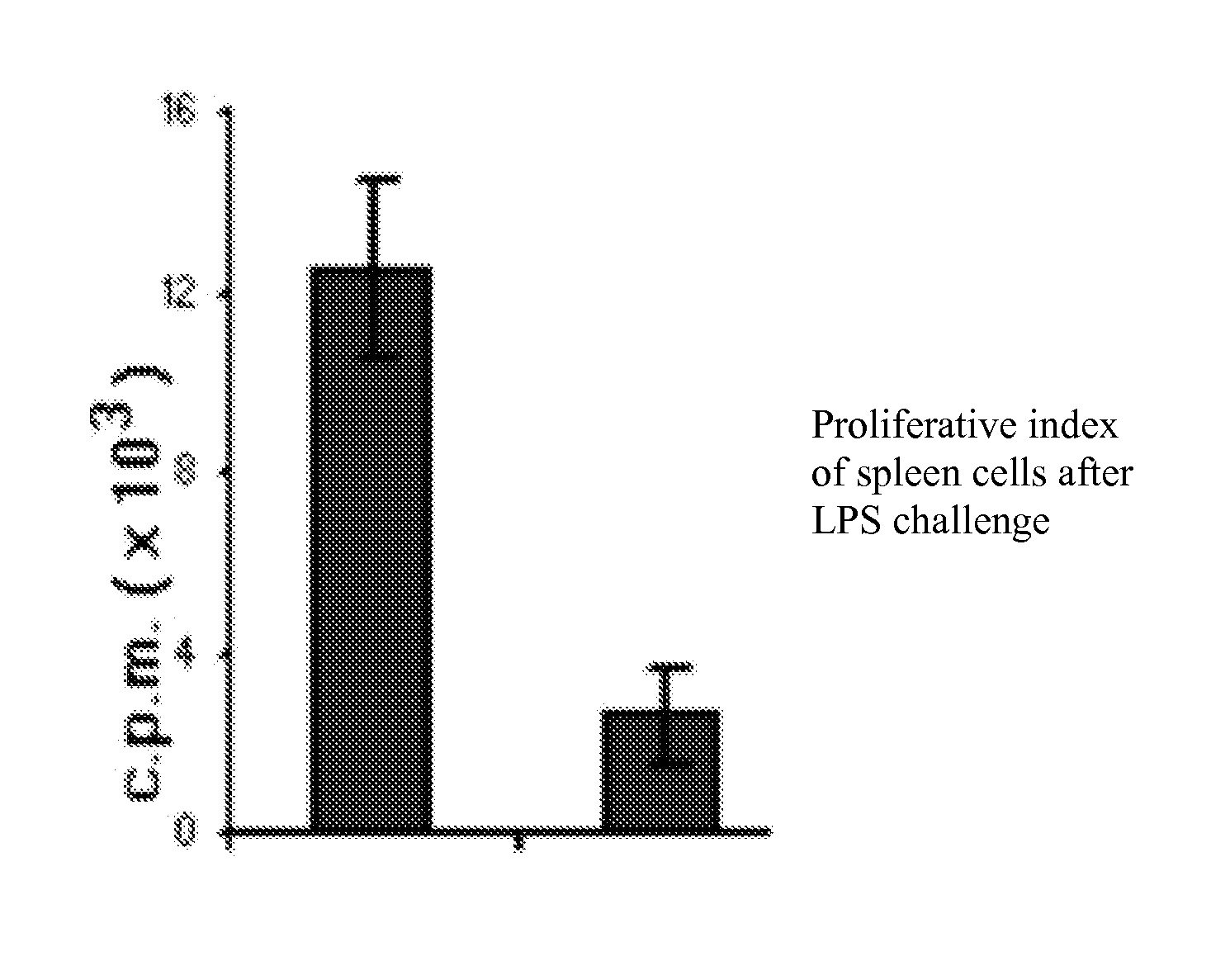
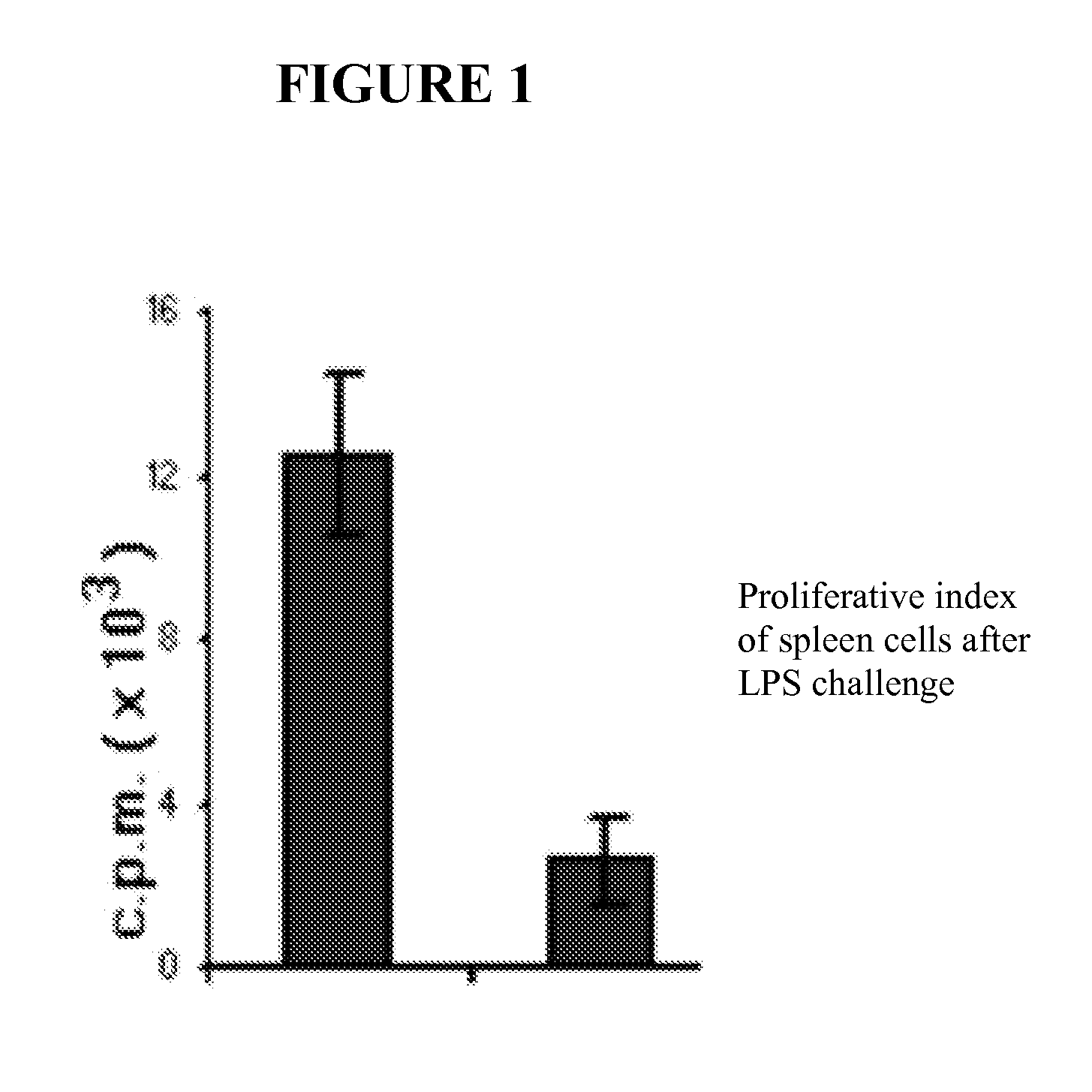
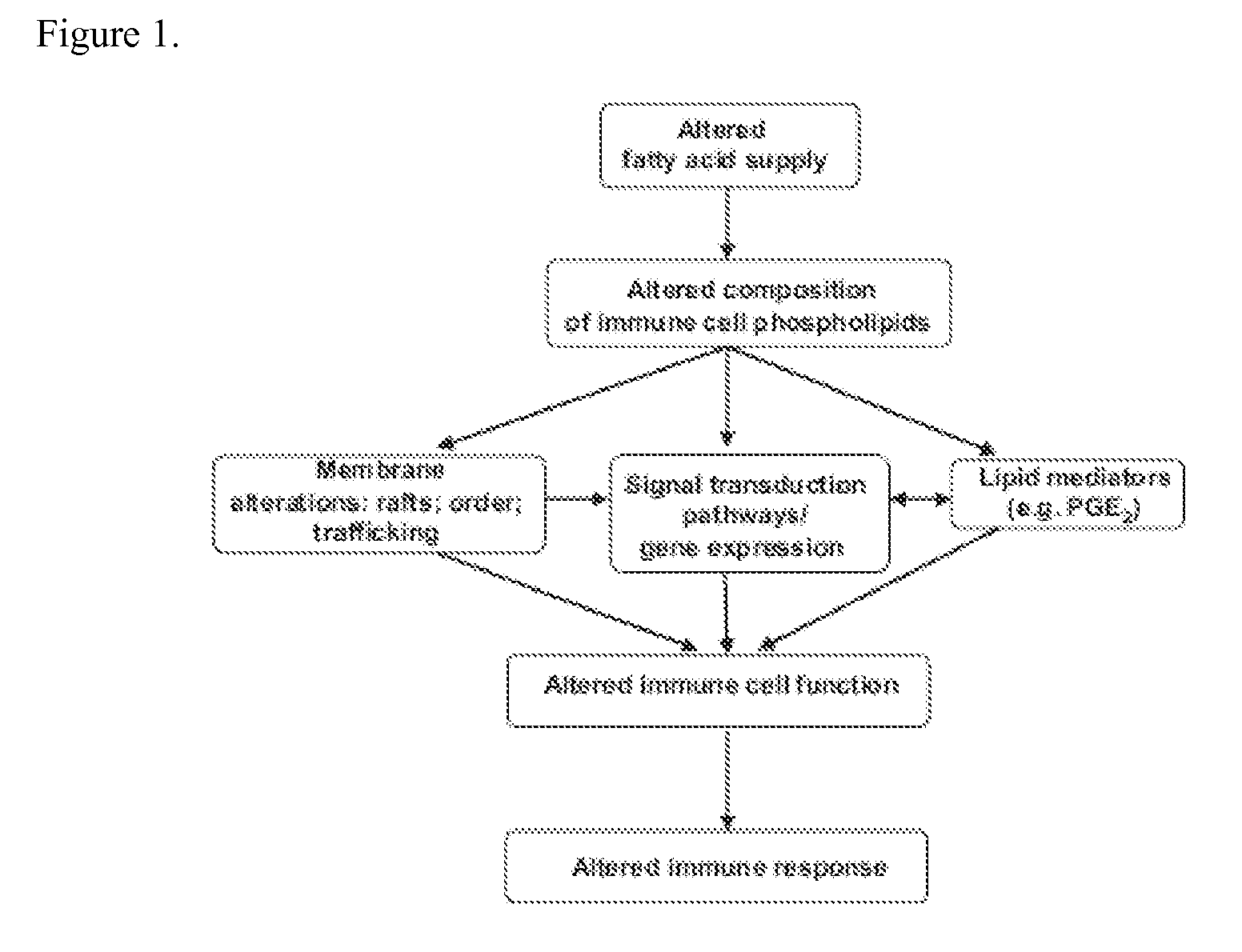
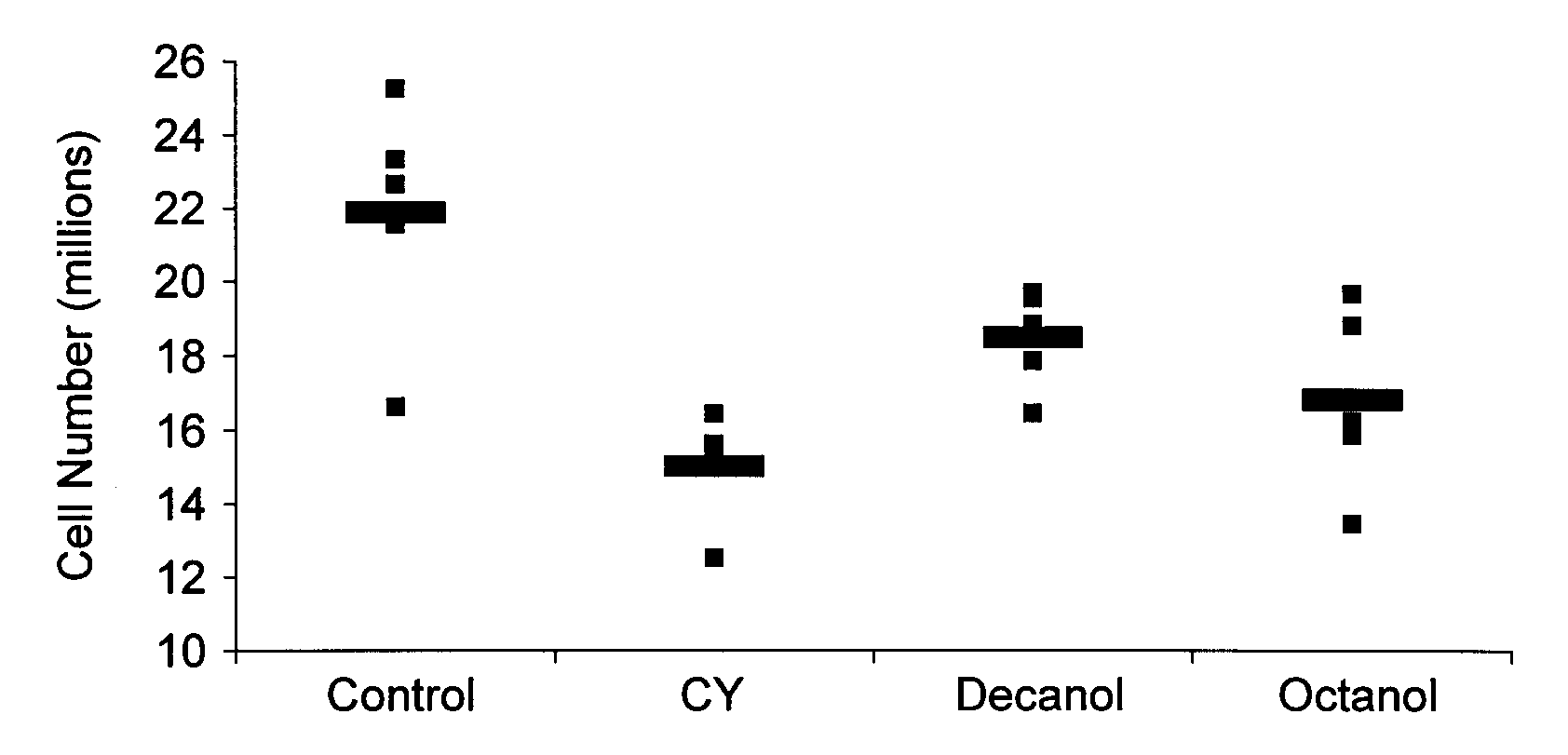
Nutritional support to prevent or moderate bone marrow paralysis or neutropenia during Anti-cancer treatment
InactiveUS20110229447A1Preserve activation capacityPreserve cell viabilityBiocideVitamin food ingredientsTolerabilityImmunocompetence
The present invention relates to methods and immunonutritional compositions for preventing the impairment of the immune function during anti-cancer therapy, thereby attaining a better efficacy of the treatment. More particularly, the present invention relates to methods and immunonutritional compositions that can transiently preventing or moderating, bone marrow paralysis or neutropenia of a subject undergoing anti-cancer therapy-induced apoptosis or necrosis or other cell damage such that the innate and adaptive immune functions and normal physiology of the bone marrow are preserved, at least in part, which, in turn, lead to (i) a better tolerance and increased efficacy to anti-cancer therapy; (ii) transient augmentation or enhancement of immunocompetence of the immune cell; and (iii) optimization of the effects of and increase of immunocompetence of the immune cell weakened by anti-cancer therapy.
Owner:NESTEC SA
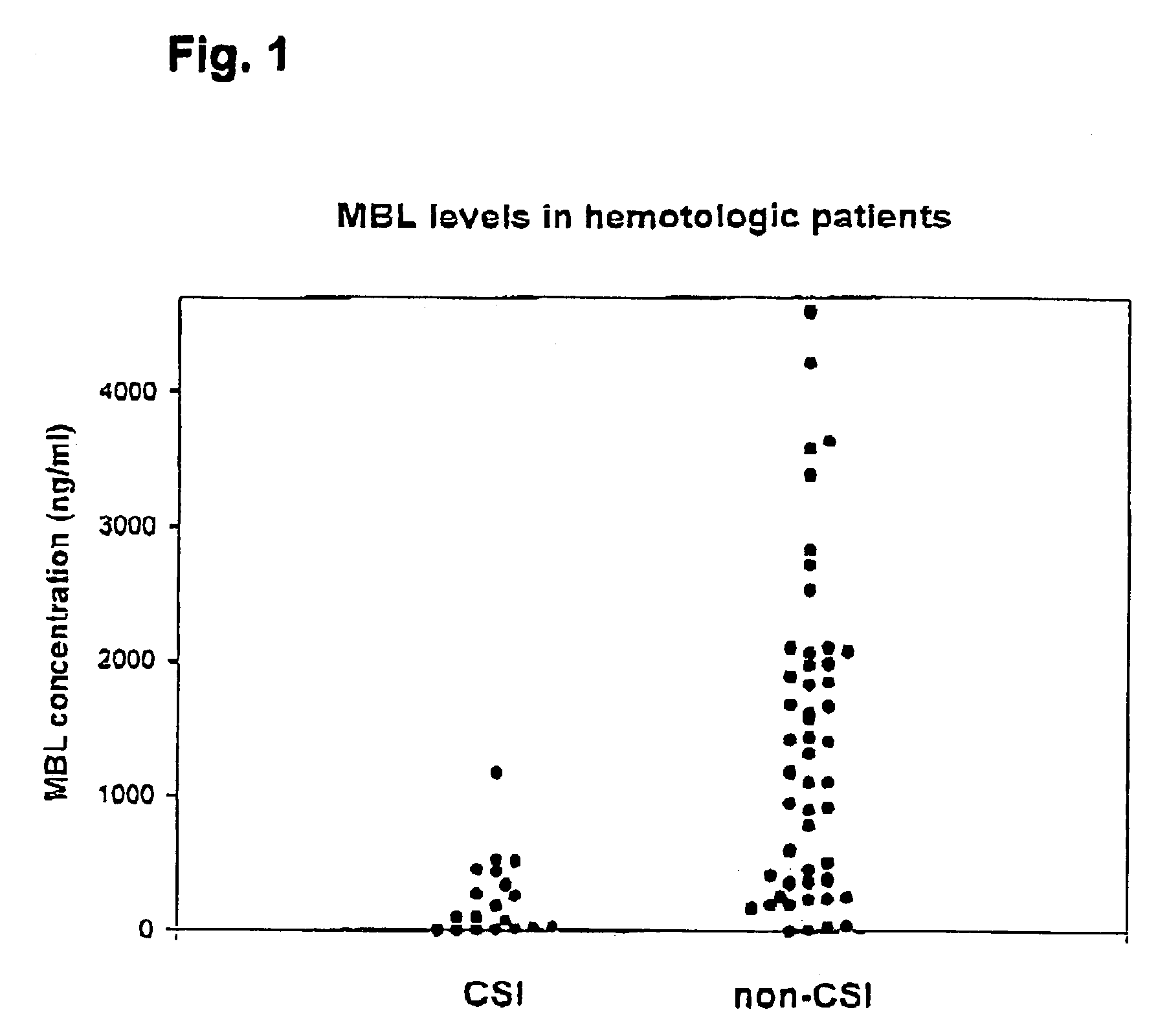
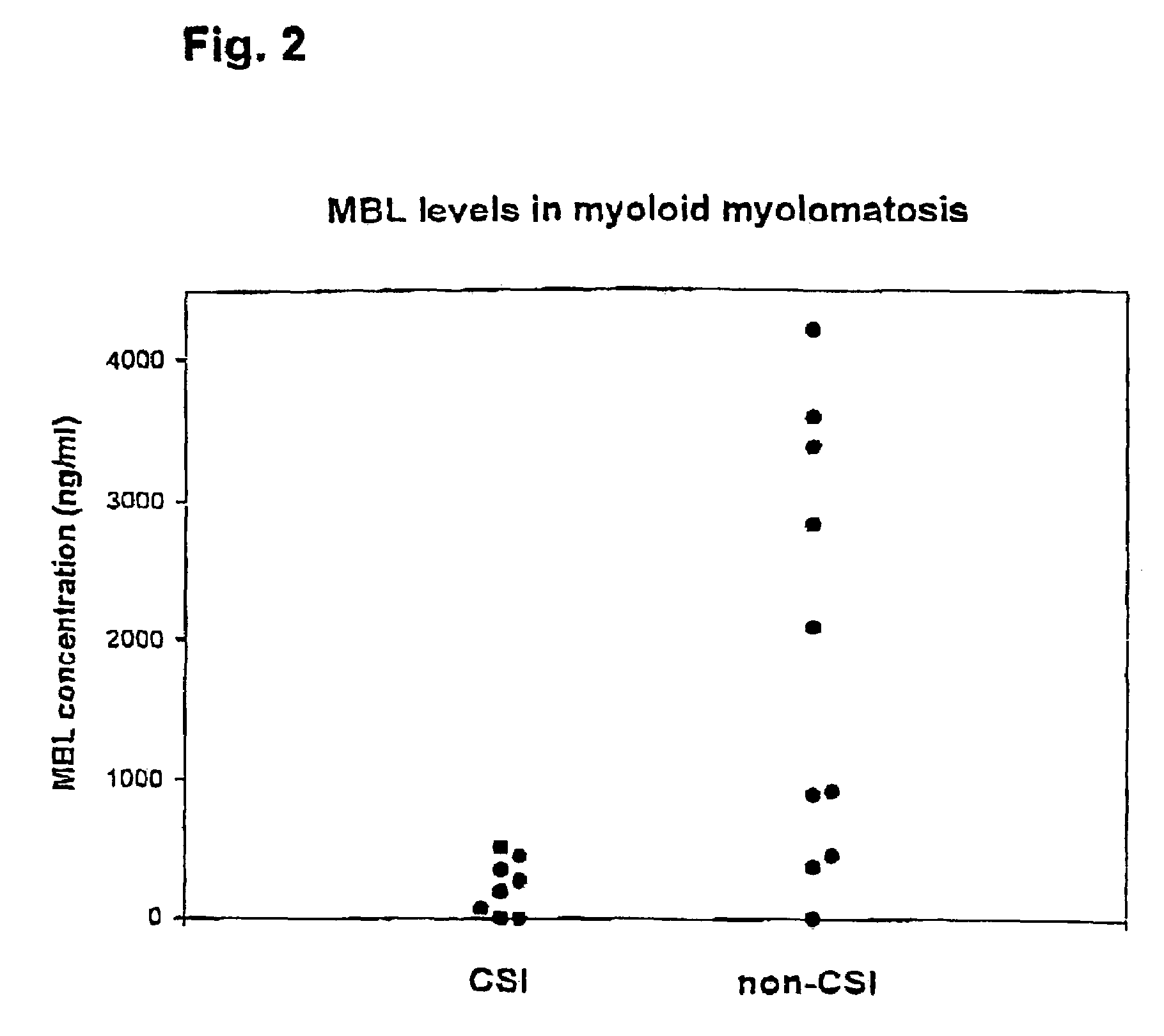
Indications of mannan-binding lectin (MBL) in the treatment of immunocompromised individuals
InactiveUS7202207B2Reduce riskIncreased risk of infectionAntibacterial agentsOrganic active ingredientsOligomerImmunologic function
The present invention relates to the use of a composition comprising at least one mannan-binding lectin (MBL) subunit, or at least one mannan-binding lectin (MBL) oligomer comprising the at least one mannan-binding lectin (MBL) subunit, in the manufacture of a medicament for prophylaxis and / or treatment of infection. In particular the invention relates to prophylaxis and / or treatment of infection in an individual having an immunocompromised condition; and / or an individual being at risk of acquiring an immunocompromised condition resulting from a medical treatment. The present invention is particular relevant for prophylaxis and / or treatment of infection in individuals suffering from neutropenia, in particular as prophylaxis and / or treatment of infection in individuals receiving or going to receive chemotherapy or similar treatment. The individuals may be treated independent on their serum MBL level, and it has been shown that in particular individuals having a serum MBL level in the range of from 50 ng / ml serum to 500 ng / ml serum may benefit from the prophylaxis and / or treatment.
Owner:ENZON PHARM INC
Treatment and prophylaxis
ActiveUS20110002881A1Reduce riskKill and inhibit growthBiocideHeavy metal active ingredientsCell divisionE-selectin
This invention discloses the use of an E-selectin antagonist in methods and compositions for treating or preventing immunocompromised conditions resulting from medical treatment. The present invention is particular useful for prophylaxis and / or treatment of hematopoietic disorders including neutropenia, agranulocytosis, anemia and thrombocytopenia in individuals receiving or proposed to receive treatments that target rapidly dividing cells or that disrupt the cell cycle or cell division.
Owner:THE UNIV OF QUEENSLAND
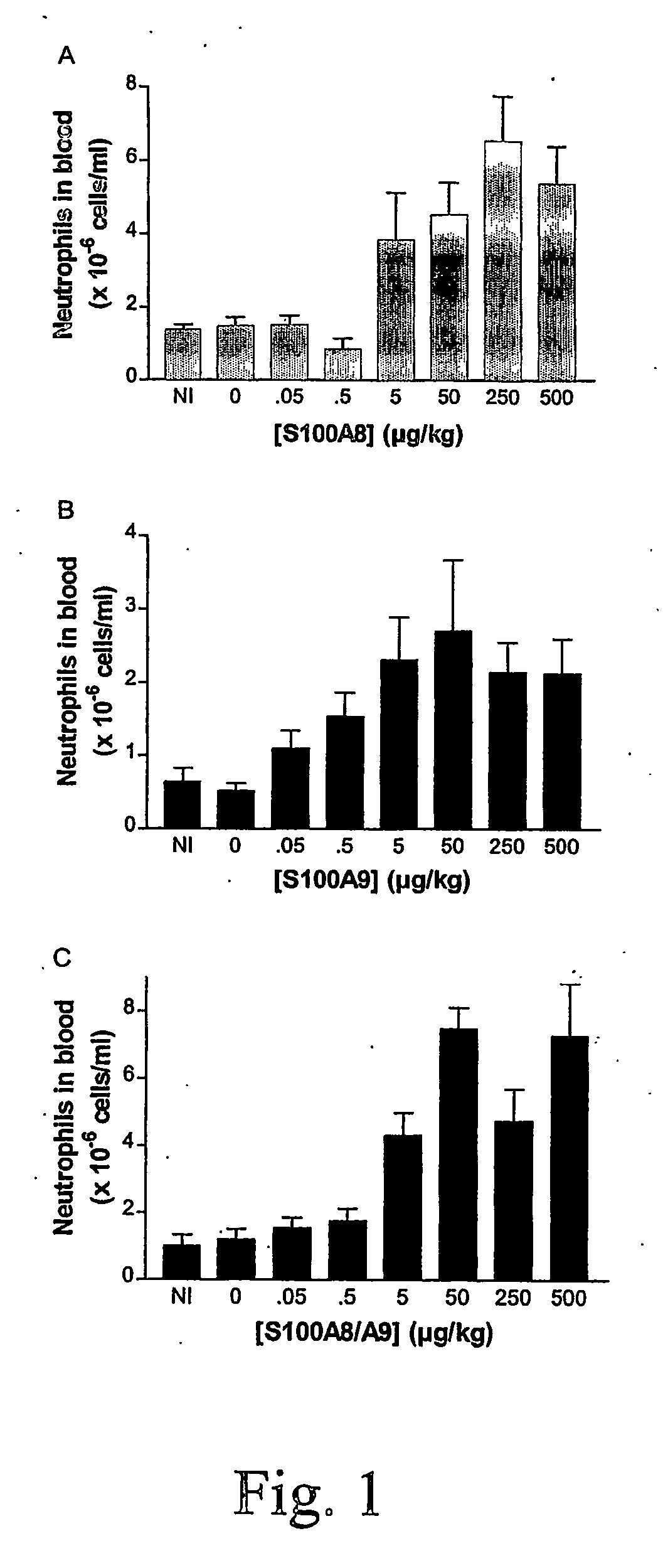
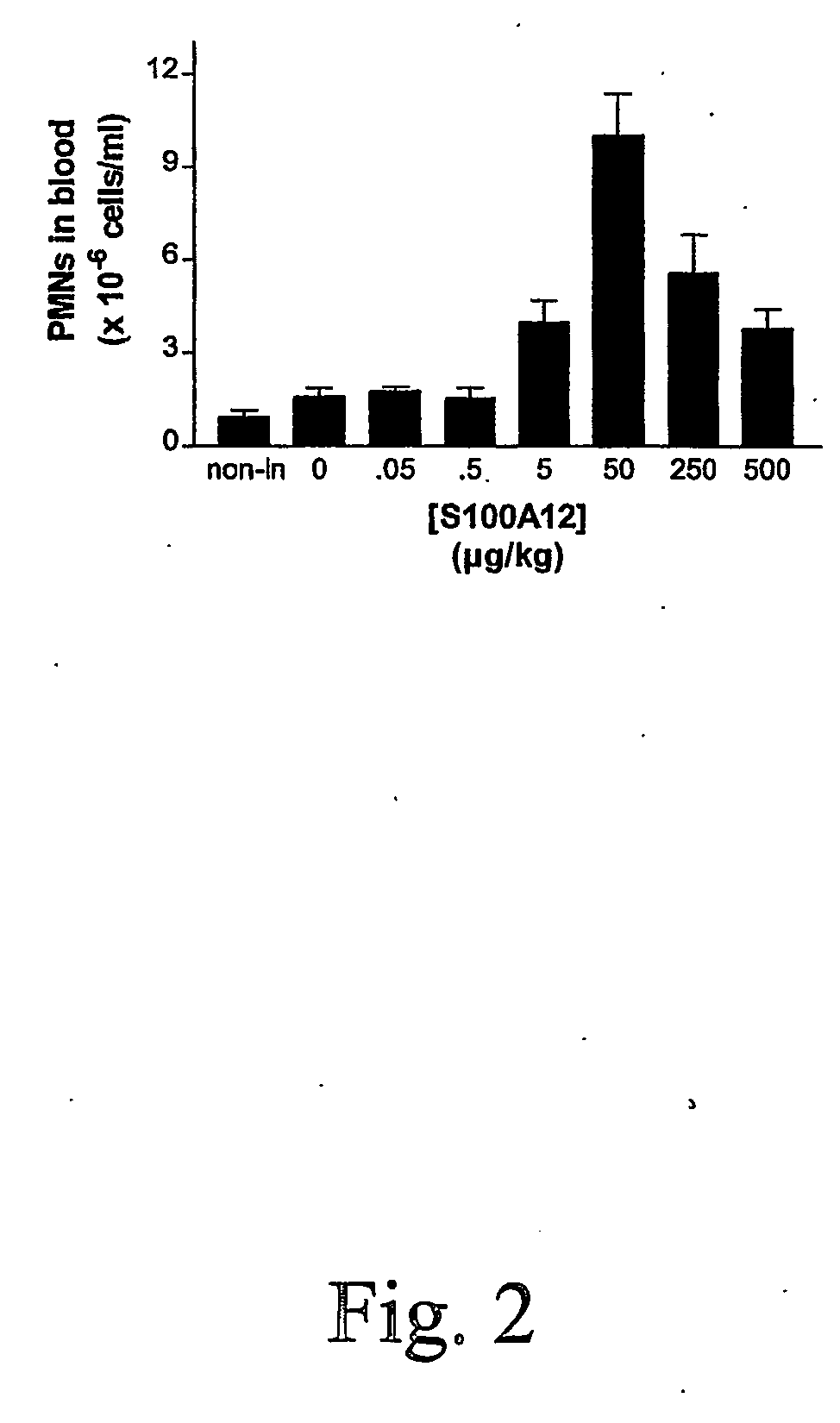
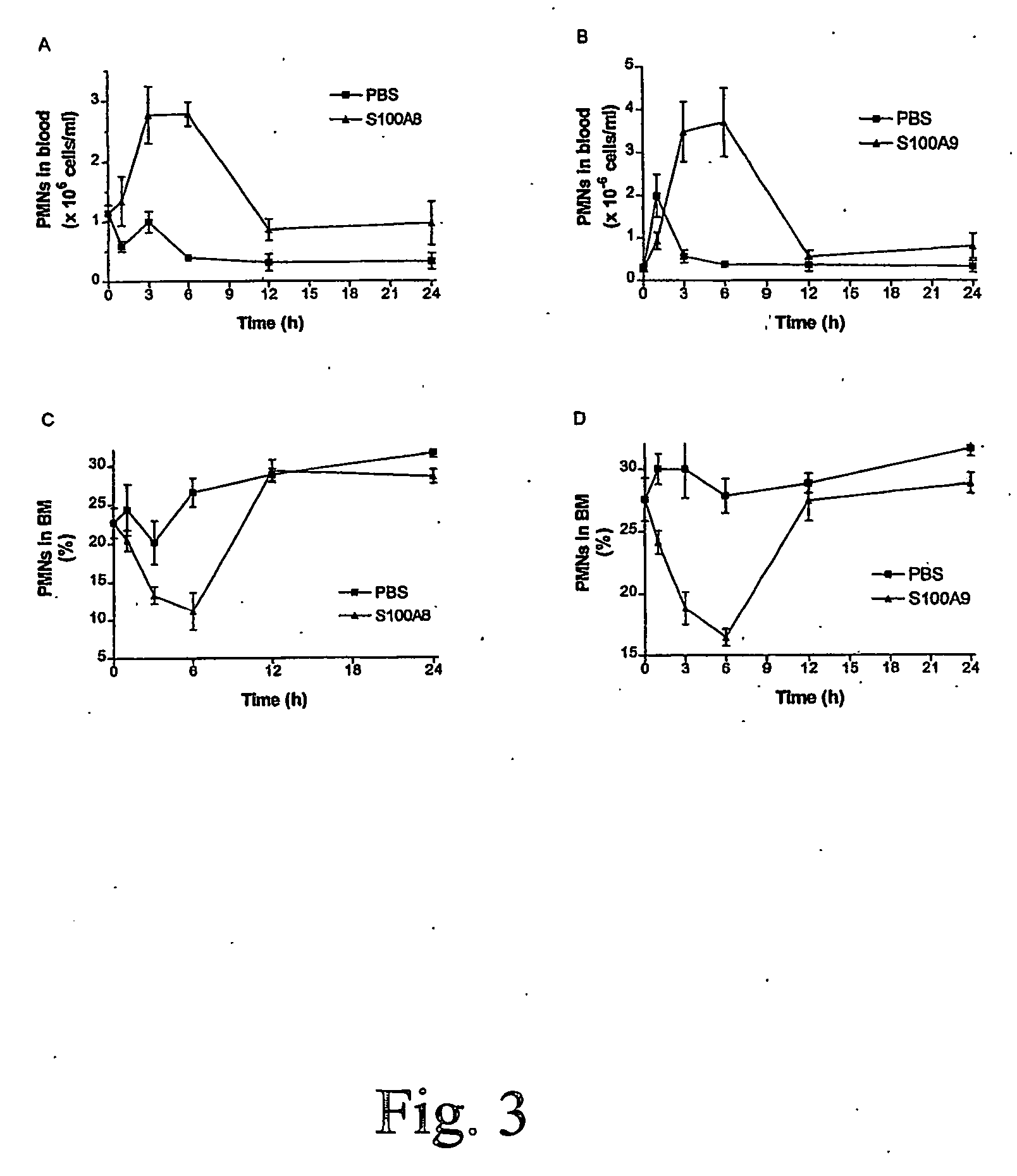
S100 protein as neutrophil activator for alleviating neutropenia in cancer treatment
InactiveUS20060281674A1Reduce riskBiocidePeptide/protein ingredientsNeutrophil granulocyteS100 protein
The present invention relates to a method and composition for inducing lymphocyte proliferation and migration, and for reducing the risks of microbial infections in patients immuno-supressed. The present invention particularly relates to the use of S100 protein, such as MRP, to induce the proliferation, differentiation and release of immune cells from bone marrow. More particularly, S100A8, S100A9, S100A12 and S100A8 / A9 are administered to patients with lowered neutrophil blood concentrations.
Owner:UNIV LAVAL
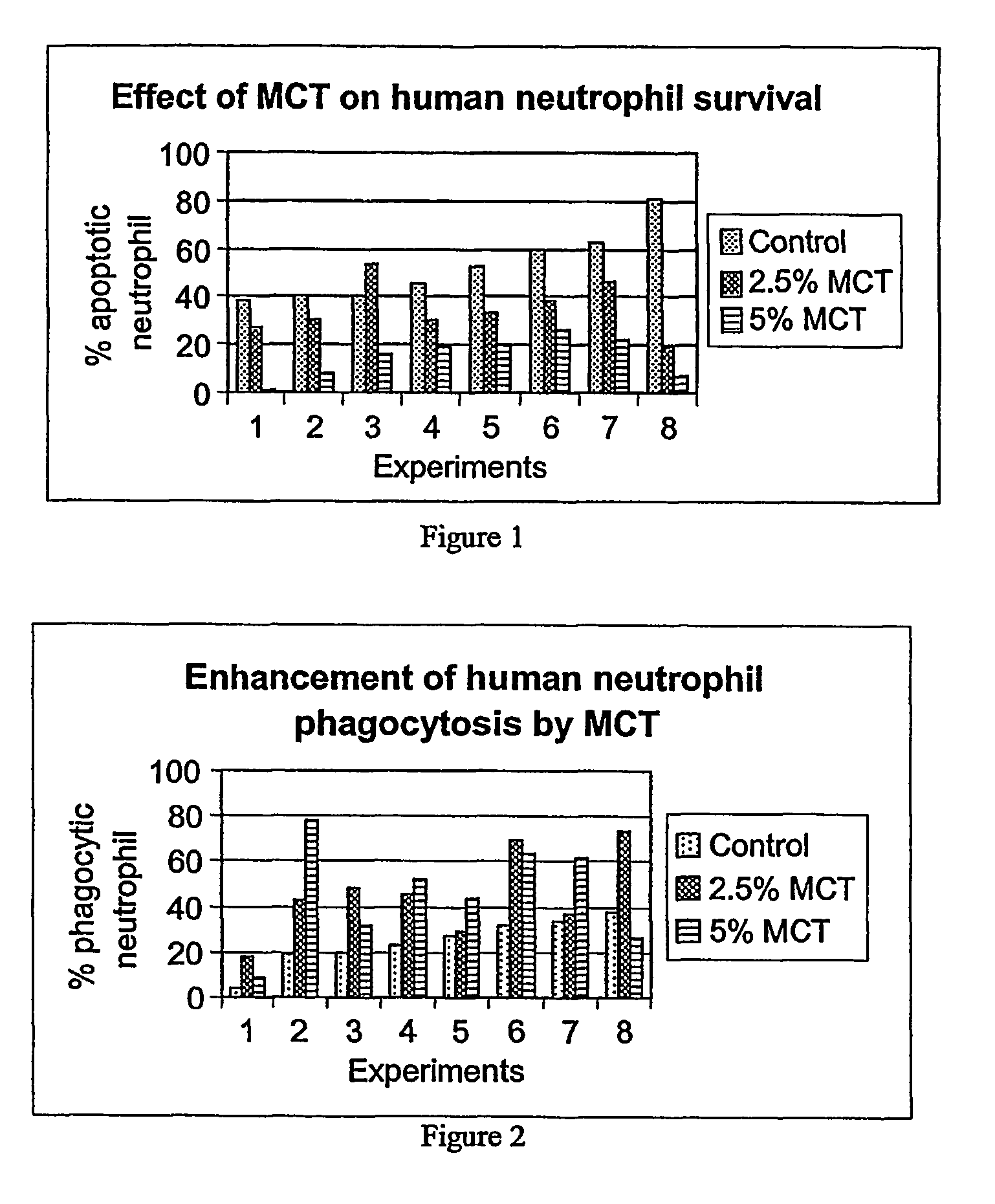
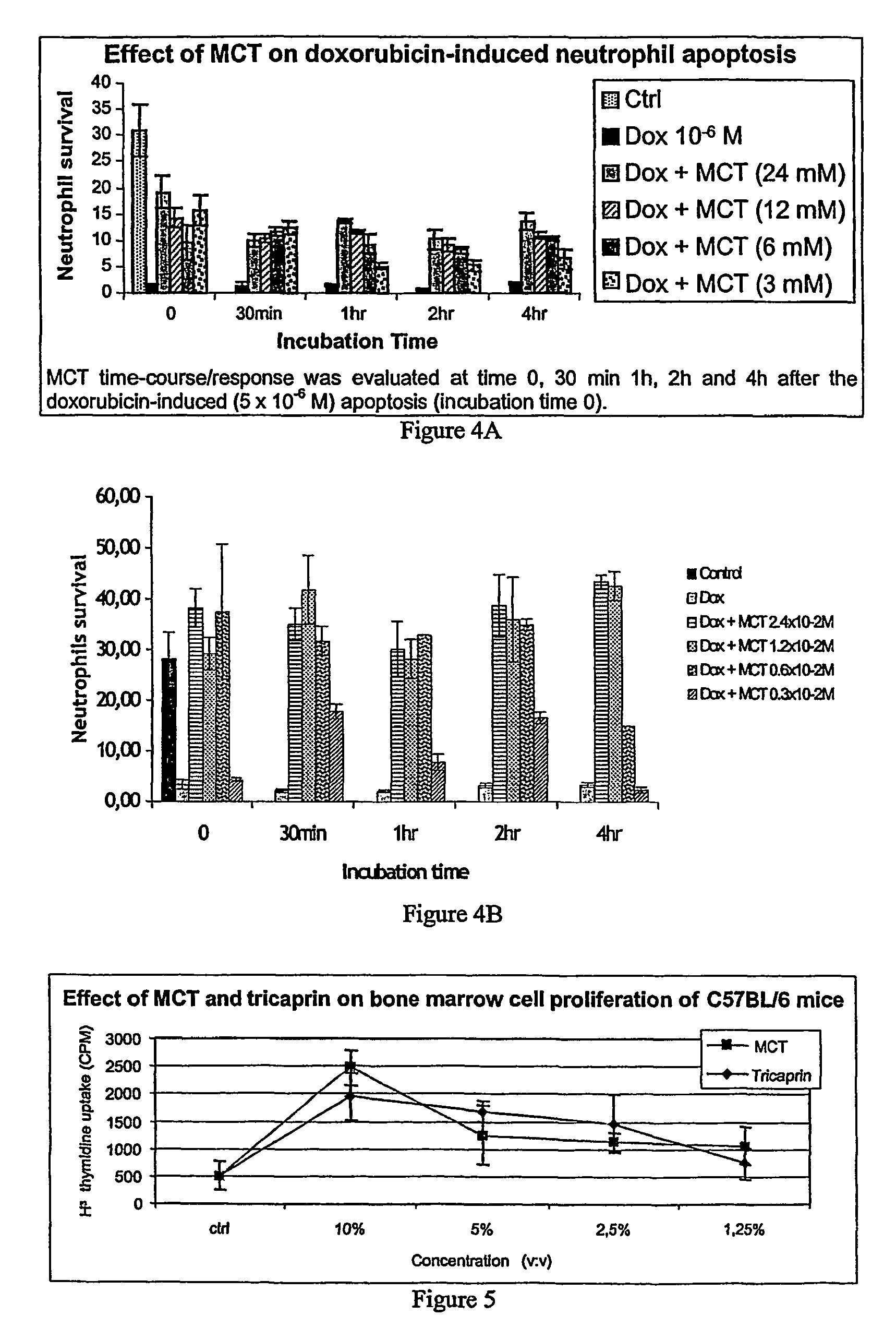
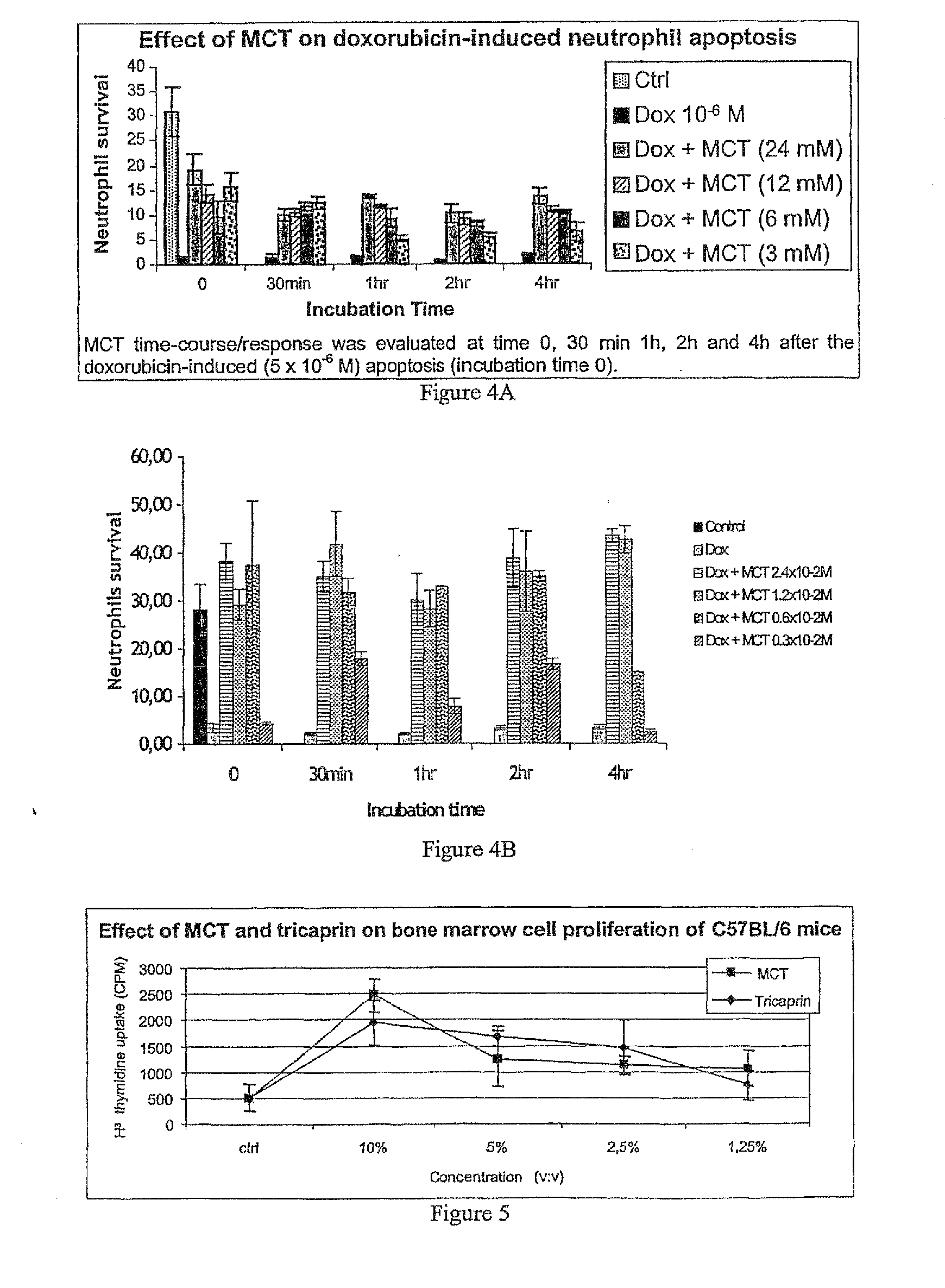
Medium-chain length fatty acids, glycerides and analogues as neutrophil survival and activation factors
ActiveUS7745488B2Meet actual needsIncreased activationBiocideFatty acid esterificationDiseaseSide effect
A composition and method for promoting neutrophil survival and activation such as the treatment of neutropenia arising as an undesirable side effect of chemotherapy and radiation therapy. A composition containing medium-chain fatty acids, such as Capri acid or caprylic acid, or salts or triglycerides thereof, or mono- or diglycerides or other analogues thereof or medium-chain triglycerides (MCT) is administered to a human or animal needing treatment in an amount sufficient to reduce or eliminate neutropenia. The composition is administered in an amount effective to treat the disorder. The methods are also useful in the management of bone narrow transplantation and in the treatment of various neutropenic diseases.
Owner:PROMETIC PHARMA SMT LTD
Treatment and prophylaxis
ActiveUS20110020270A1Stimulating and enhancing hematopoiesisAvoid adjustmentOrganic active ingredientsPeptide/protein ingredientsDiseaseWhite blood cell
This invention discloses the use of an E-selectin antagonist and a mobilizer of hematopoietic stem cells or progenitor cells in methods and compositions for treating or preventing immunocompromised conditions resulting from medical treatment. The present invention is particular relevant for prophylaxis and / or treatment of hematopoeitic disorders including neutropenia, agranulocytosis, anemia and thrombocytopenia in individuals receiving or proposed to receive treatments that target rapidly dividing cells or that disrupt the cell cycle or cell division.
Owner:THE UNIV OF QUEENSLAND
Methods of expanding myeloid cell populations and uses thereof
ActiveUS8252587B2Antibacterial agentsAntimycoticsMyeloid Progenitor CellsHematopoietic stem cell transplantation
The present disclosure relates to a method of expanding myeloid progenitor cells by culturing an initial population of cells in a medium comprising a mixture of cytokines and growth factors that promote growth and expansion of the myeloid progenitor cells. The expanded cell population provides a source of cells as therapeutic treatments for neutropenia and / or thrombocytopenia arising in patients subjected to myeloablative therapy and hematopoietic stem cell transplantation.
Owner:CELLERANT THERAPEUTICS INC
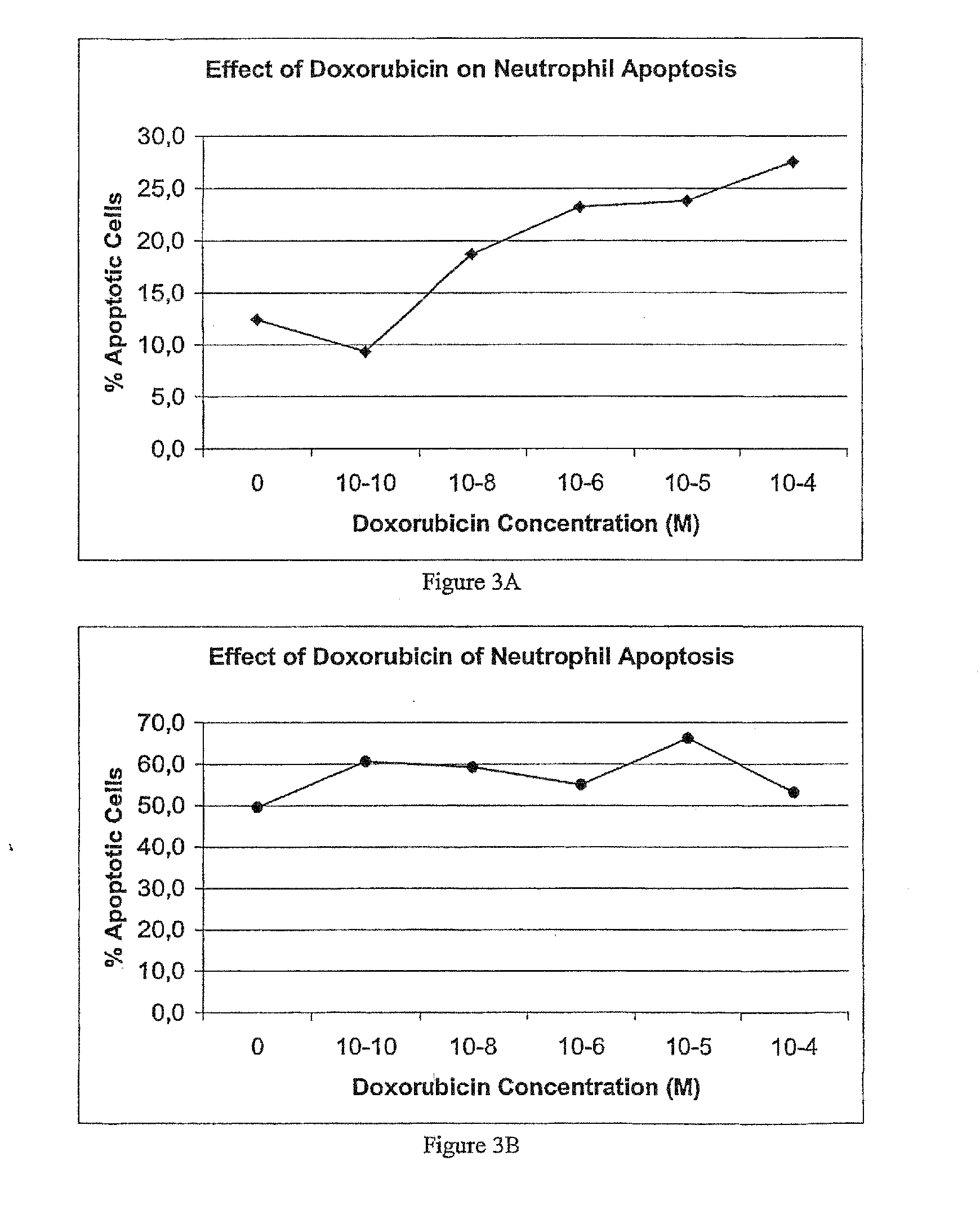
Catechin Adjuvants
InactiveUS20070082073A1Ameliorate immunosuppressive effectImprove morbidityBiocidePhosphorous compound active ingredientsAdjuvantMortality rate
A combination cancer therapy based upon catechins, the major biologically active polyphenol in plant products, including green tea extract, and one or more chemotherapeutic agents. A common complication of cancer chemotherapy is neutropenia, and in spite of advances in its prophylactic management is a major cause of risk for the development of serious microbial infections leading to increased morbidity and mortality in both humans and animals. The use of cathechins such as those found in green tea (Camellia sinensis), including but not limited to epigallocatechin gallate (EGCG) as nontoxic adjuvant to aid in the prevention of opportunistic microbial infections in patients undergoing immunosuppressive chemotherapy is a novel application. Also contemplated are methods using catechins to ameliorate the immunosuppressive effects of cancer chemotherapy by administering the compound to a patient in need thereof.
Owner:FLORIDA VETERINARY SPECIALIST & CANCER TREATMENT CENT +1
Medium-Chain Length Fatty Acids, Glycerides and Analogues as Neutrophil Survival and Activation Factors
InactiveUS20100279959A1Meet actual needsIncreased activationBiocideFatty acid esterificationDiseaseMonoglyceride
A composition and method for promoting neutrophil survival and activation such as the treatment of neutropenia arising as an undesirable side effect of chemotherapy and radiation therapy. A composition containing medium-chain fatty acids, such as Capri acid or caprylic acid, or salts or triglycerides thereof, or mono- or diglycerides or other analogues thereof or medium-chain triglycerides (MCT) is administered to a human or animal needing treatment in an amount sufficient to reduce or eliminate neutropenia. The composition is administered in an amount effective to treat the disorder. The methods are also useful in the management of bone narrow transplantation and in the treatment of various neutropenic diseases.
Owner:PROMETIC PHARMA SMT LTD
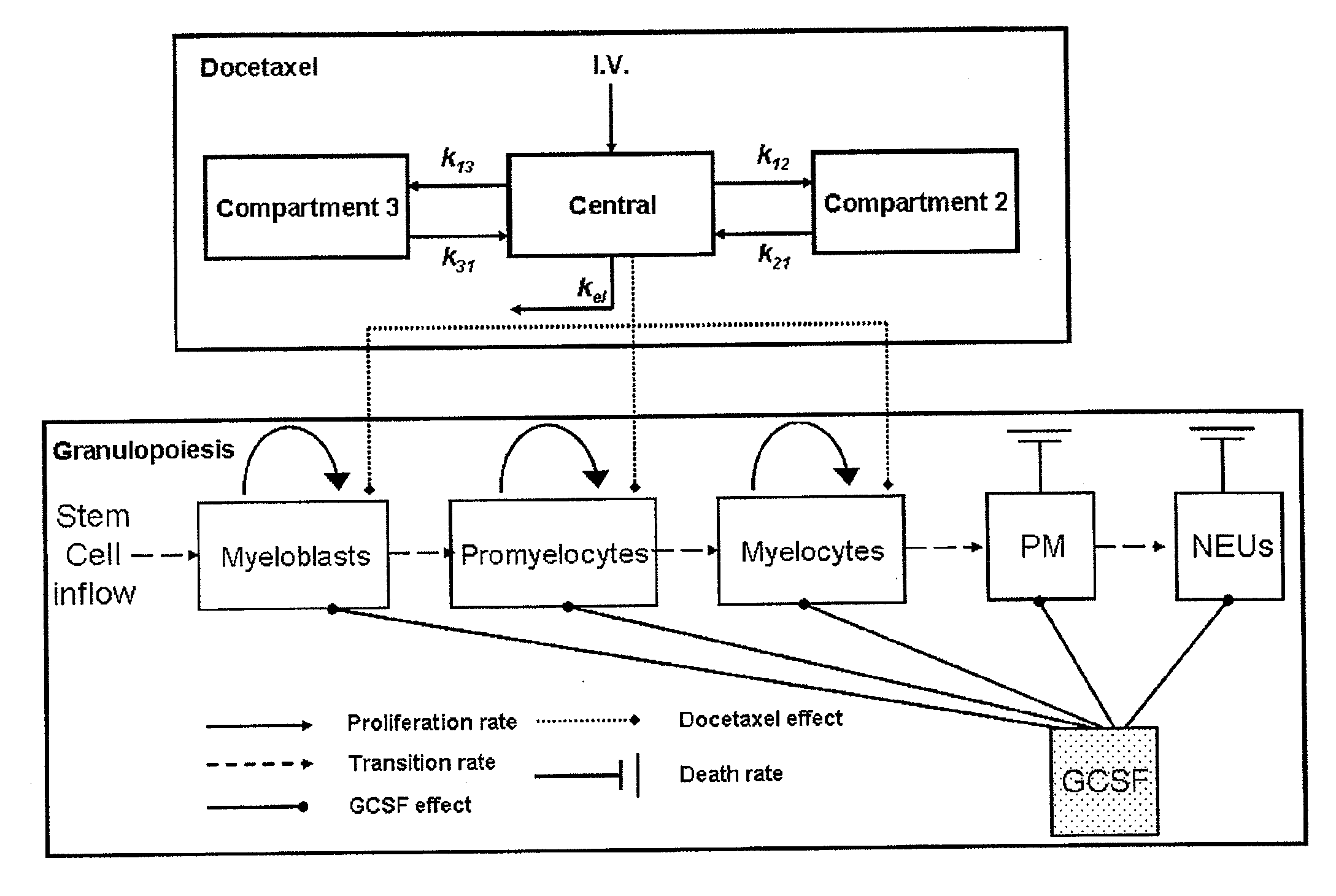
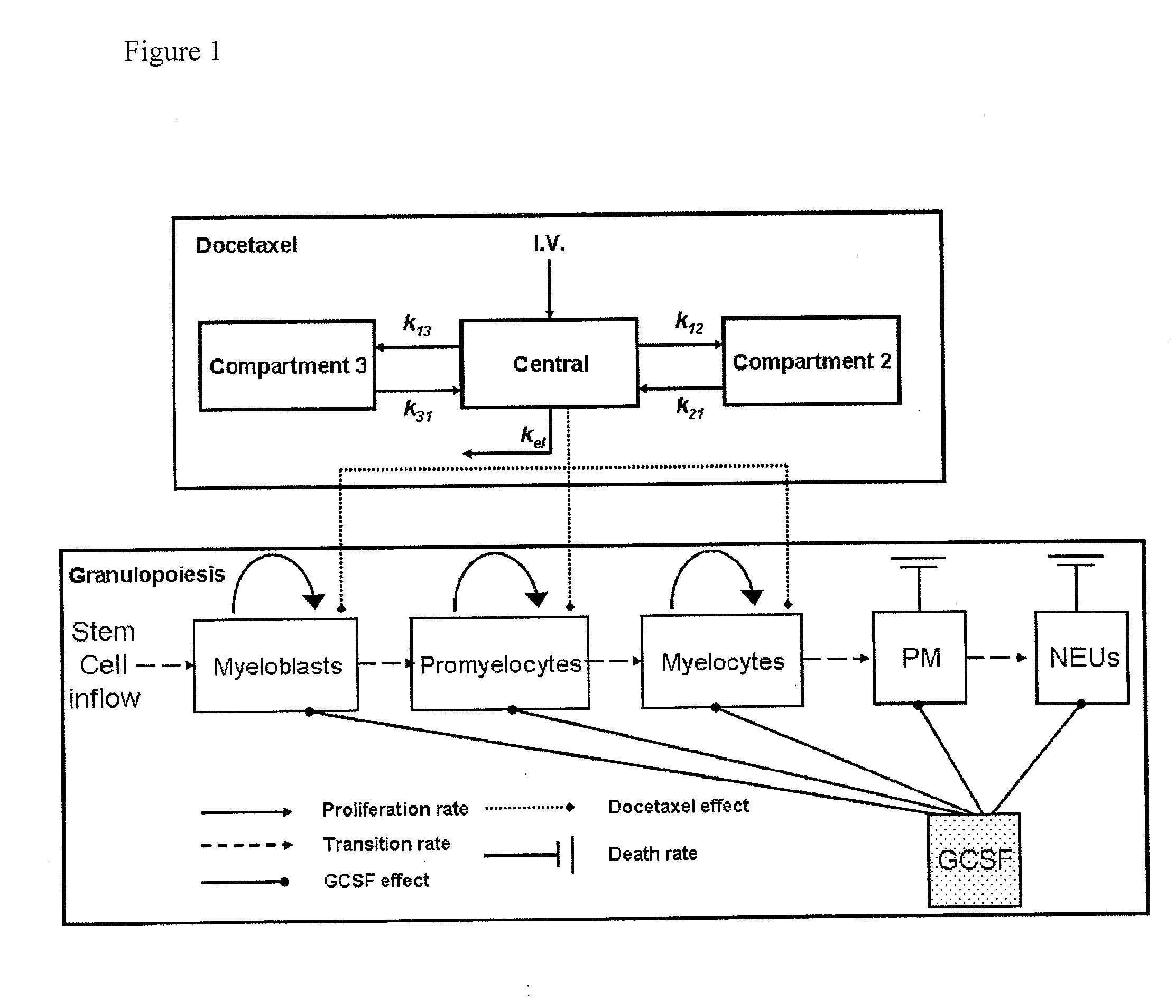
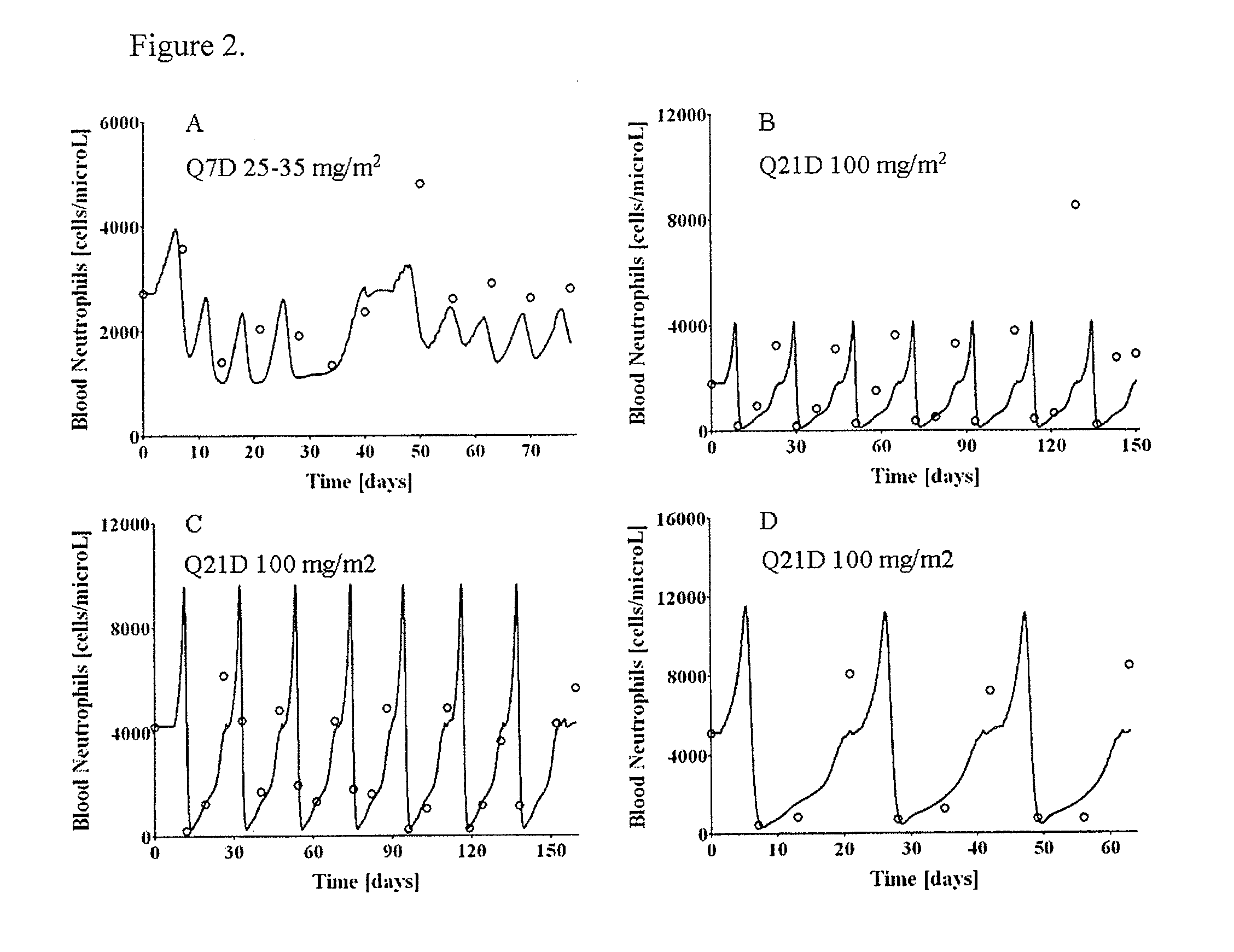
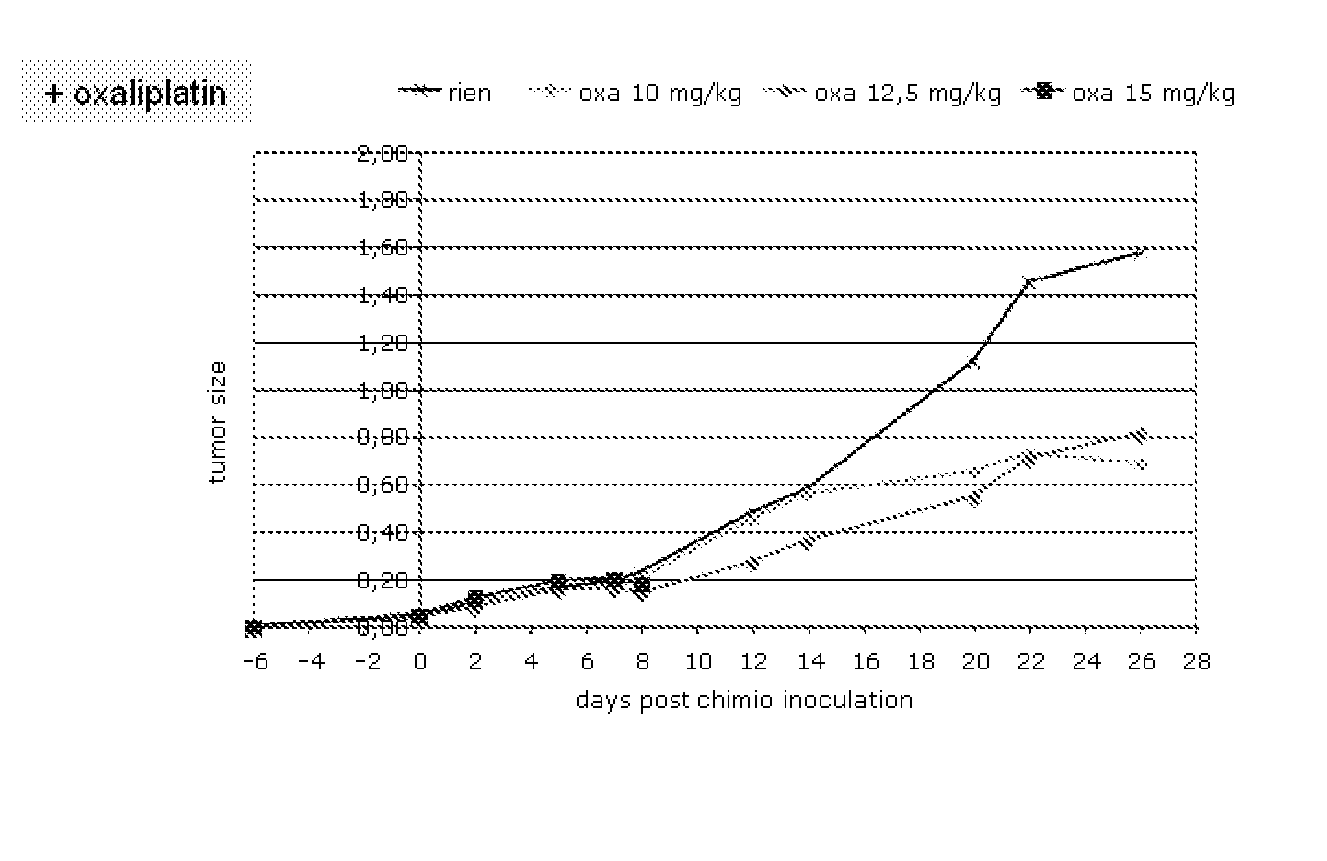
Cancer therapy by docetaxel and granulocyte colony-stimulating factor (g-csf)
Neutropenia is the dose-limiting toxicity of the tri-weekly docetaxel (Taxotere®) schedule. Here, we evaluate in Metastatic Breast Cancer (MBC) patients (N=38) a computerized method for predicting docetaxel-induced neutropenia, and use the model to identify improved docetaxel and Granulocyte Colony Stimulating Factor (G-CSF) regimens. Pharmacokinetics / pharmacodynamics (PK / PD) models were created and simulated concomitantly with a mathematical granulopoiesis model. Individual baseline neutrophil counts and docetaxel schedules served as inputs. Our trial validated the model accuracy in predicting nadir timings (r=0.99), grade 3 / 4 neutropenia (86% success) and neutrophil profiles (r=0.62). Model was robust to CYP3A-induced variability, except for slightly less accurate grade 3 / 4 neutropenia predictions. Simulations confirm smaller toxicity of the weekly docetaxel regimen than the tri-weekly one, and suggest an optimal G-CSF support for alleviating neutropenia, 60 μg / day QD×3, 6-7 days post-docetaxel, administered tri- and bi-weekly, and 4 days post weekly docetaxel>33 mg / m2.
Owner:OPTIMATA
Sepsis Treatment Methods
Owner:HARBOR DIVERSIFIED +2
Pharmaceutical compositions
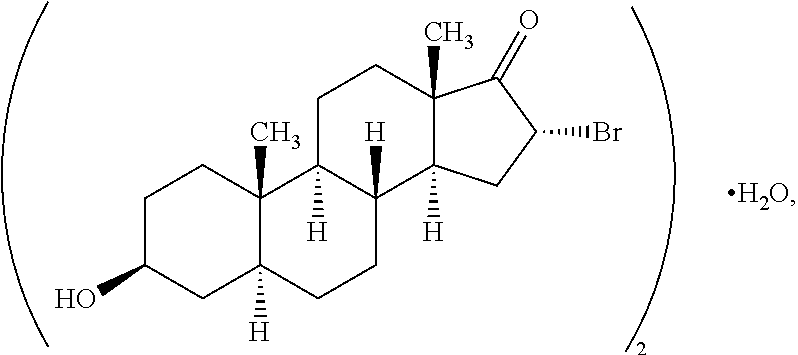
The invention relates to the use of compounds to ameliorate or treat an condition such as a cystic fibrosis, neutropenia or other exemplified conditions. Exemplary compounds that can be used include 3β-hydroxy-17β-aminoandrost-5-ene, 3β-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3α-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3β-hydroxy-16β-fluoro-17β-aminoandrost-5-ene, 1α,3β-dihydroxy-4α-fluoroandrost-5-ene-17-one, 1α,3β,17β-trihydroxy-4α-fluoroandrost-5-ene, 1β,3β-dihydroxy-6α-bromoandrost-5-ene, 1α-fluoro-3β,12α-dihydroxyandrost-5-ene-17-one, 1α-fluoro-3β,4α-dihydroxyandrost-5-ene and 4α-fluoro-3β,6α,17β-trihydroxyandrostane.
Owner:HOLLIS EDEN PHARMA +2
Methods for treating leukopenia and thrombocytopenia
ActiveUS20160128966A1Easy to produceEnhance platelet productionBiocideHeavy metal active ingredientsCompound (substance)Leukopenia
The disclosure provides methods for treating, controlling or mitigating leukopenia (e.g. neutropenia) and / or thrombocytopenia, for example in the context of cancer chemotherapy, comprising administration of a monoacetyl-diacyl-glycerol compound, as well as compositions useful therefor.
Owner:ENZYCHEM LIFESCI CORP
Method of treatment for feline leukemia virus infections
InactiveUS6350443B1Reduce feverReduce in quantityBiocidePeptide/protein ingredientsGranulocytopeniasInterferon alpha
A method of treatment for feline leukemia virus infections by continuously administering a feline interferon preparation containing a feline interferon as a main component daily to a cat is disclosed. As a feline interferon, a feline omega (omega)-interferon is preferably used, and more particularly, a recombinant interferon is preferably used. A method of treatment using a therapeutic agent containing a feline omega-interferon as a main component in accordance with the present invention is a novel and superior method suitable for treating feline leukemia virus infections, and in particular, for treating neutropenia.
Owner:TORAY IND INC
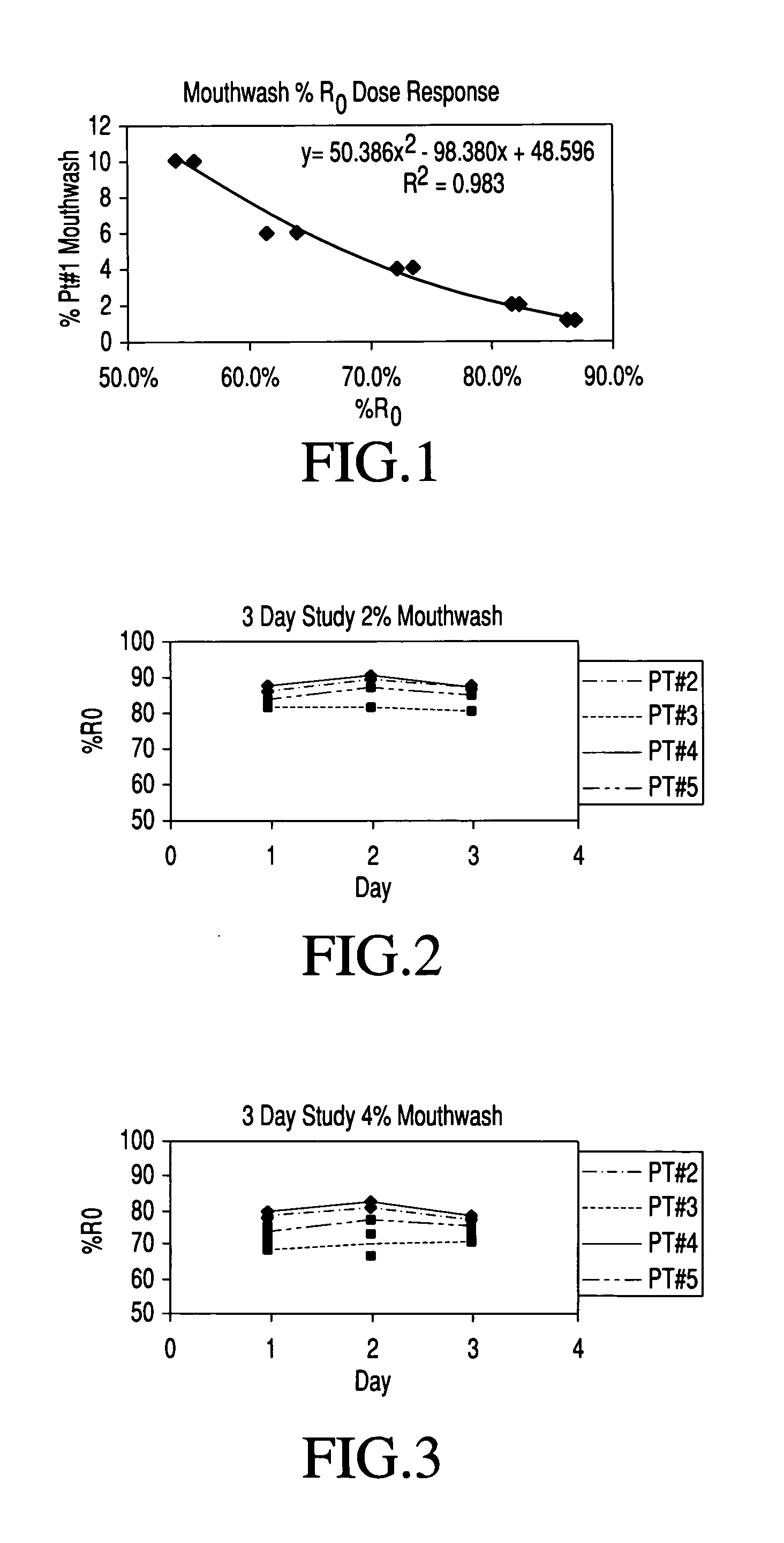
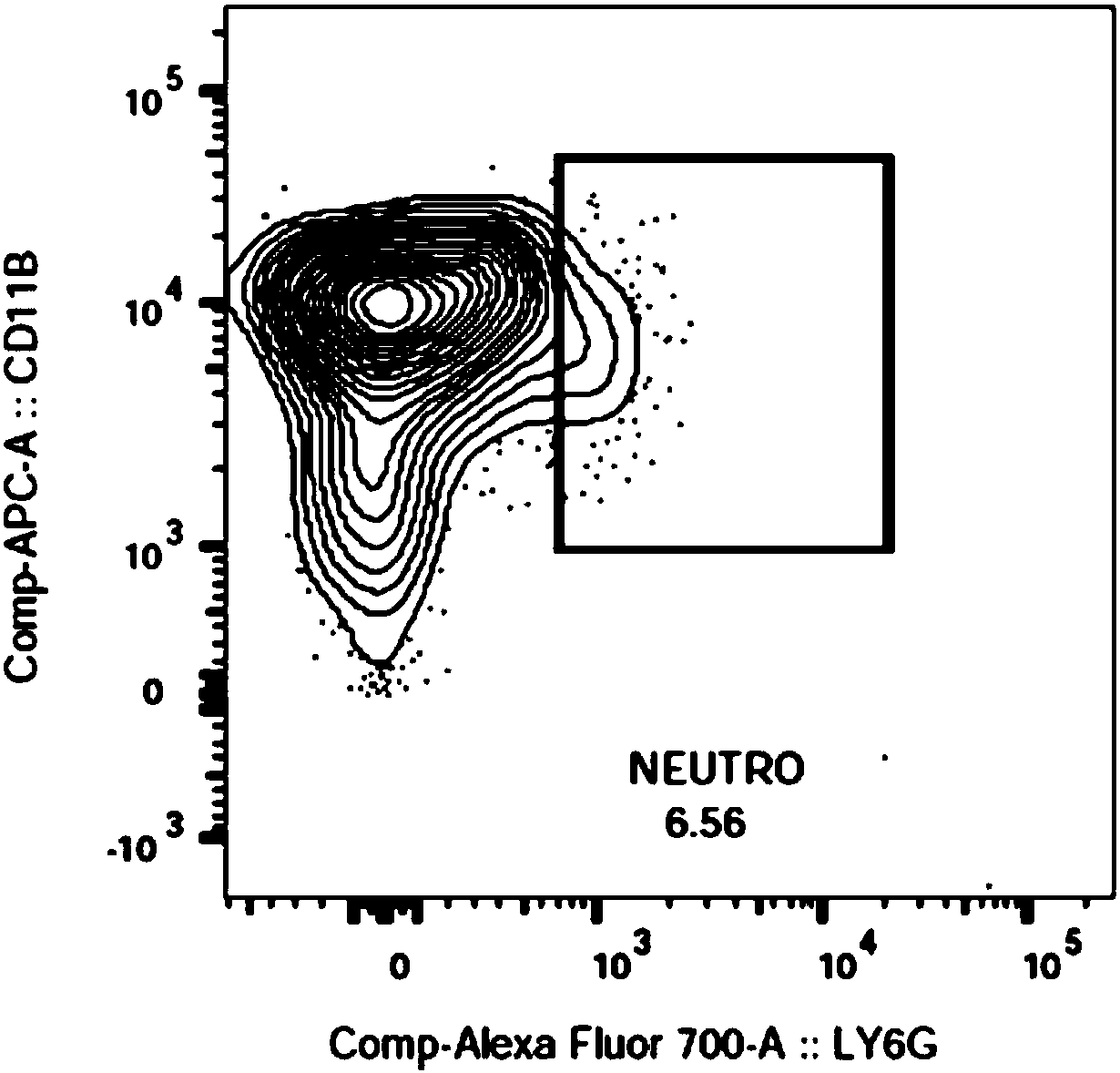
Method for determining mucosal neutrophil counts in neutropenia patents
InactiveUS20050220712A1Enhance self-administrationGood test resultUltrasonic/sonic/infrasonic diagnosticsMicrobiological testing/measurementFluorescenceNeutrophil granulocyte
Based on recent investigations showing iatrogenic, profound neutropenia (and the fever spikes that often accompany it, which must be medicated immediately to avoid the risk of life-threatening infection) can most accurately be monitored by obtaining daily mucosal neutrophil counts from the patient's oral mucosa rather than obtaining daily counts of the patient's blood neutrophils as in the past, a mouth wash method has been developed for collecting muscosal neutrophils. The mouth wash samples so collected are delivered directly, or in aqueous dilution to a sample pad supported on a strip which sample pad has deposited thereon reagents enabling a colorimetric, fluorescent or chemiluminescent assay of the quantity of an enzyme characteristic of human neutrophils that is present in the sample. This measured quantity can be correlated to mucosal neutrophil count. The method shows outstanding sensitivity, precision and accuracy relative to microscopic methods of counting mucosal neutrophils.
Owner:WRIGHT DANIEL G +2
Shp2 phosphatase inhibitors and methods of use thereof
ActiveUS20190307745A1Inhibiting SHP phosphatase activityOrganic chemistryAntineoplastic agentsDiseaseHER2 Positive Breast Cancer
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for inhibiting the activity of SHP2 phosphatase with the compounds and compositions of the invention. The present invention further relates to, but is not limited to, methods for suppressing tumor cell growth, ameliorating the pathogenesis of systemic lupus erythematosus, and the treatment of various other disorders, including Noonan syndrome, diabetes, neutropenia, neuroblastoma, melanoma, juvenile leukemia, juvenile myelomonocytic leukemia, chronic myelomonocytic leukemia, acute myeloid leukemia, and other cancers associated with SHP2 deregulation with the compounds and compositions of the invention, alone or in combination with other treatments. Other cancers associated with SHP2 deregulation include HER2-positive breast cancer, triple-negative breast cancer, ductal carcinoma of the breast, invasive ductal carcinoma of the breast, non-small cell lung cancer, esophageal cancer, gastric cancer, squamous-cell carcinoma of the head and neck (SCCHN), and colon cancer.
Owner:D E SHAW RES & DEV LLC +1
Compositions and treatment methods-4
The invention relates to the use of compounds to ameliorate or treat an condition such as a cystic fibrosis, neutropenia or other exemplified conditions. Exemplary compounds that can be used include 3β-hydroxy-17β-aminoandrost-5-ene, 3β-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3α-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3β-hydroxy-16β-fluoro-17β-aminoandrost-5-ene, 1α,3β-dihydroxy-4α-fluoroandrost-5-ene-17-one, 1α,3β,17β-trihydroxy-4α-fluoroandrost-5-ene, 1β,3β-dihydroxy-6α-bromoandrost-5-ene, 1α-fluoro-3β,12α-dihydroxyandrost-5-ene-17-one, 1α-fluoro-3β,4α-dihydroxyandrost-5-ene and 4α-fluoro-3β,6α,17β-trihydroxyandrostane.
Owner:BIOVIE INC
use of rhamnolipids as a drug of choice in the case of nuclear disasters in the treatment of the combination radiation injuries and illnesses in humans and animals
InactiveUS20120322751A1Reduce resistanceIncrease ratingsBiocideNervous disorderRadiation injuryRhamnolipid
This invention is related to the use of one or more rhamnolipids based on structure Formula 1 where the composition of the mixture is useful in the treatment of combination radiation illnesses and regeneration of cells and / or tissues by different application ways of any desirable mentioned rhamnolipids. These rhamnolipid composition mixture are very suitable in the treatment of combination radiation injuries and illnesses and for the regeneration of human and animal cells and tissues after nuclear catastrophes; comprising two or more injuries / illnesses and for cell and tissue regenerations which include radiation damages combined with burns, mechanical injuries, infections of digestive system, infections of lungs, neutropenia, sepsis, atherosclerosis, depression, schizophrenia, atopic eczema and other illnesses in connection with radiation injuries.
Owner:PILJAC GORAN
Steroids having 7-oxgen and 17-heteroaryl substitution
The invention relates to the use of compounds to ameliorate or treat an condition such as a cystic fibrosis, neutropenia or other exemplified conditions. Exemplary compounds that can be used include 3β-hydroxy-17β-aminoandrost-5-ene, 3β-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3α-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3β-hydroxy-16β-fluoro-17β-aminoandrost-5-ene, 1α,3β-dihydroxy-4α-fluoroandrost-5-ene-17-one, 1α,3β,17β-trihydroxy-4α-fluoroandrost-5-ene, 1β,3β-dihydroxy-6α-bromoandrost-5-ene, 1α-fluoro-3β,12α-dihydroxyandrost-5-ene-17-one, 1α-fluoro-3β,4α-dihydroxyandrost-5-ene and 4α-fluoro-3β,6α,17β-trihydroxyandrostane.
Owner:BIOVIE INC
Methods for treating leukopenia and thrombocytopenia
ActiveUS20160151323A1Reduce neutrophil migrationInhibit transferBiocideHeavy metal active ingredientsLeukopeniaThrombasthenia
The disclosure provides methods for treating, controlling or mitigating leukopenia (e.g. neutropenia) and / or thrombocytopenia, for example in the context of cancer chemotherapy, comprising administration of a monoacetyl-diacyl-glycerol compound, as well as compositions useful therefor.
Owner:ENZYCHEM LIFESCI CORP
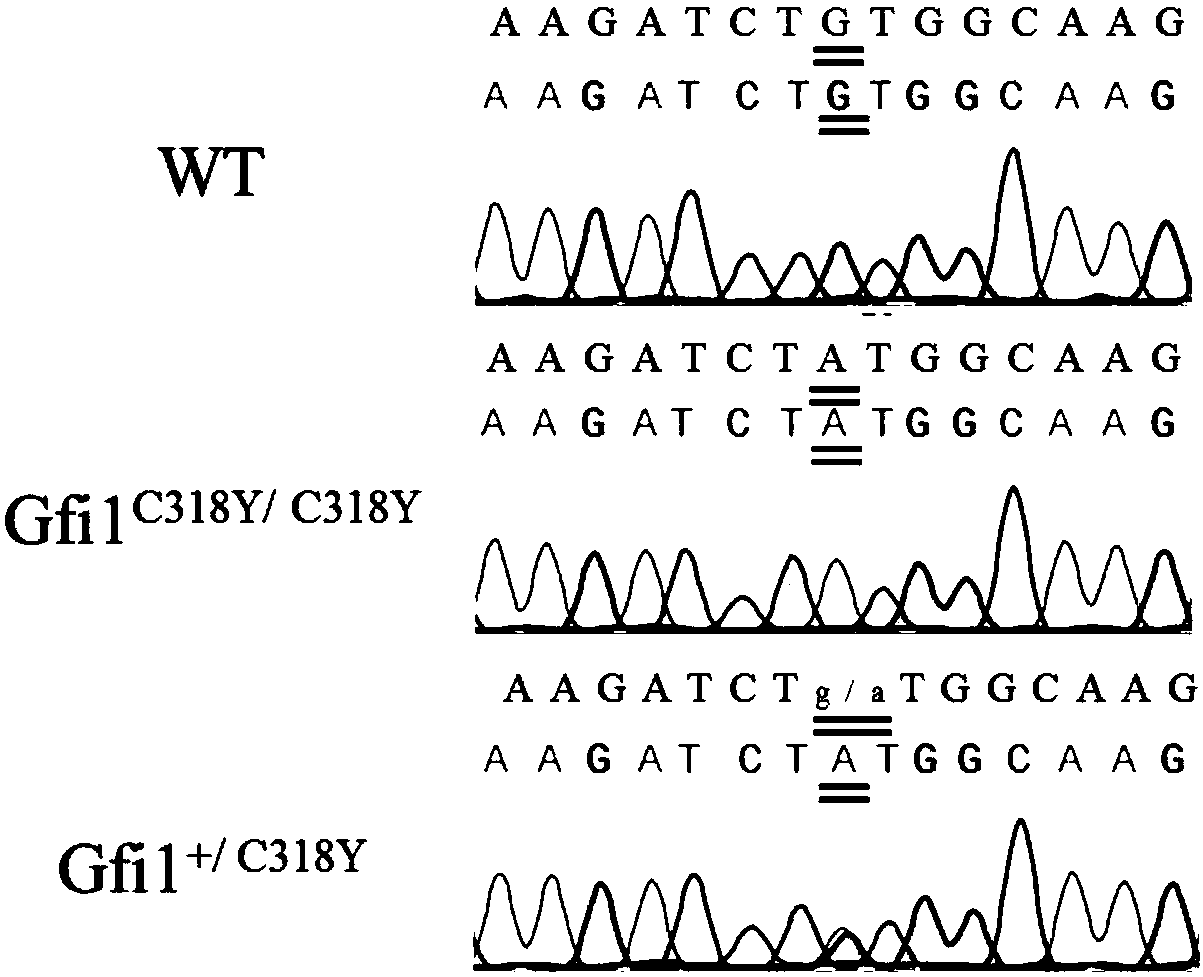
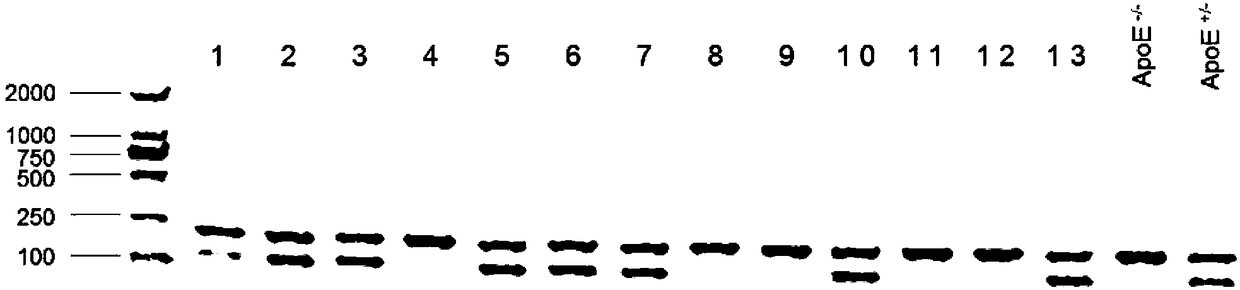
Establishing method of neutropenia atherosclerosis model rat
ActiveCN108271740ASuccessful breedingAlleviate disease symptomsMicrobiological testing/measurementAnimal husbandryNeutrophil granulocyteAtheroma
The invention relates to an establishing method of a neutropenia atherosclerosis model rat, and belongs to the technical field of genetic engineering and genetic modification. A first neutropenia atherosclerosis rat genetic model is established by introducing the Gfi1 point mutation into an ApoE-knocked-out rat gemone for the first time. It is proved through experiments that in the model rat, atherosclerosis plaque is remarkably reduced and atherosclerosis symptoms are remarkably relieved; it is shown that the neutropenia atherosclerosis model rat is successfully established and the atherosclerosis lesion degree can be relieved by neutropenia. The obtained neutropenia cocurrent atherosclerosis model rat has quite important potential application value in the disclosing of the effects of neutrophils on forming and repairing of atherosclerosis and in the diagnosis and treatment of atherosclerosis.
Owner:XINXIANG MEDICAL UNIV
Nutritional support to prevent and/or mitigate bone marrow toxicity from a cancerous tumor
ActiveUS20110223136A1Preserve activation capacityPreserve cell viabilityBiocideNervous disorderAbnormal tissue growthTolerability
The present invention relates to methods and immunonutritional compositions for preventing or mitigating paralysis of the bone marrow, caused by a tumor or neoplasm, between cycles of and after anti-cancer therapy, thereby attaining a better efficacy of the treatment. More particularly, the present invention relates to methods and immunonutritional compositions that can transiently preventing or moderating, bone marrow paralysis or neutropenia of a subject tumor-induced apoptosis or necrosis or other cell damage such that the innate and adaptive immune functions and normal physiology of the bone marrow are preserved, at least in part, which, in turn, lead to (i) a better tolerance and increased efficacy to treatment; (ii) transient augmentation or enhancement of immunocompetence of the immune cell; and (iii) optimization of the effects of and increase of immunocompetence of the immune cell weakened due to paralysis of the bone marrow, caused by a tumor or neoplasm.
Owner:SOC DES PROD NESTLE SA +1
Medium-Chain Length Fatty Alcohols as Stimulators of Hematopoiesis
InactiveUS20080051324A1Good curative effectReducing and eliminating chemotherapyBiocideHydroxy compound active ingredientsProgenitorNutritional deficiency
Owner:PROMETIC PHARMA SMT LTD
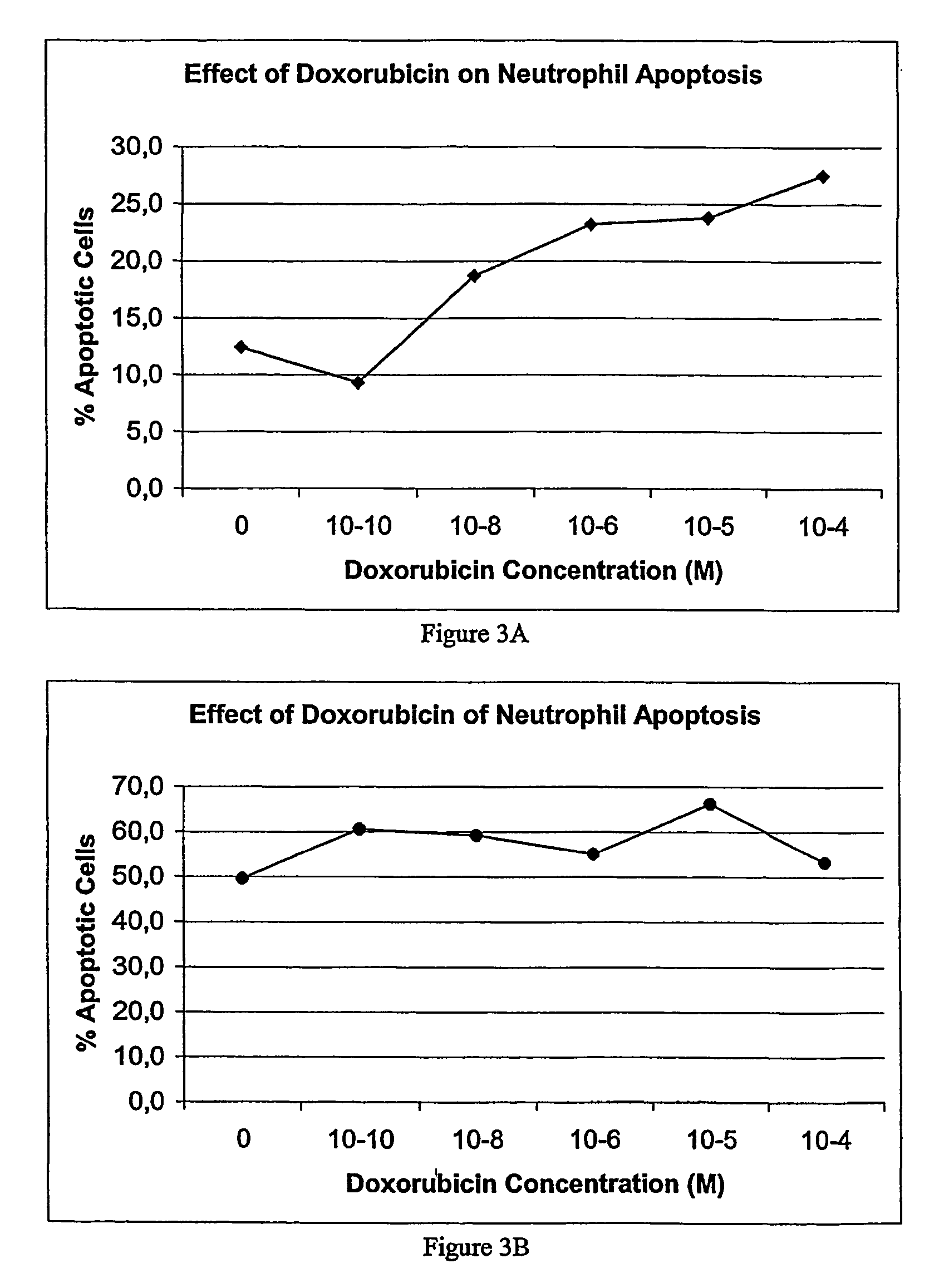
Compounds for the treatment of inflammation and neutropenia
InactiveUS20130059773A1Affect survivalEliminate the effects ofAntibacterial agentsCompound screeningDiseaseMedicine
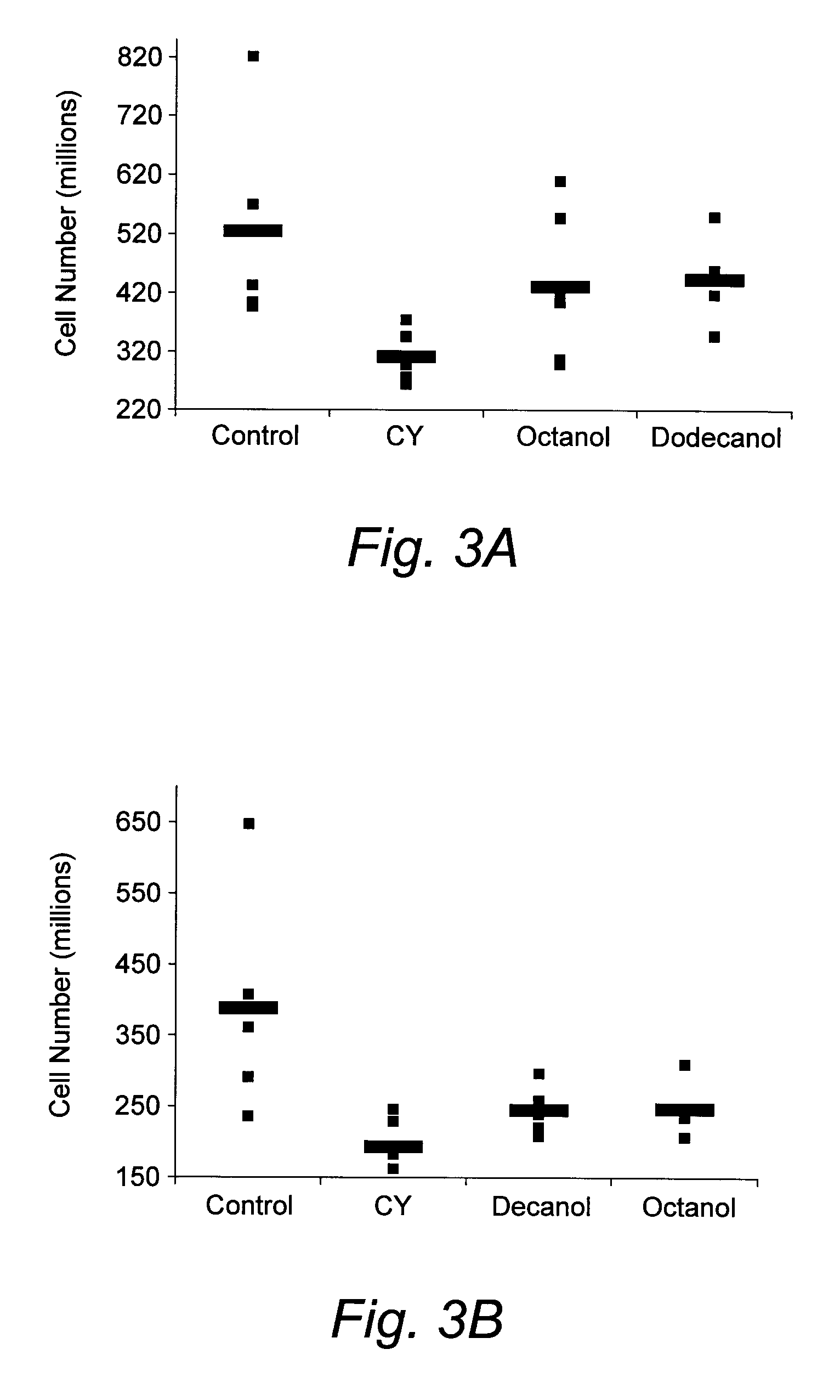
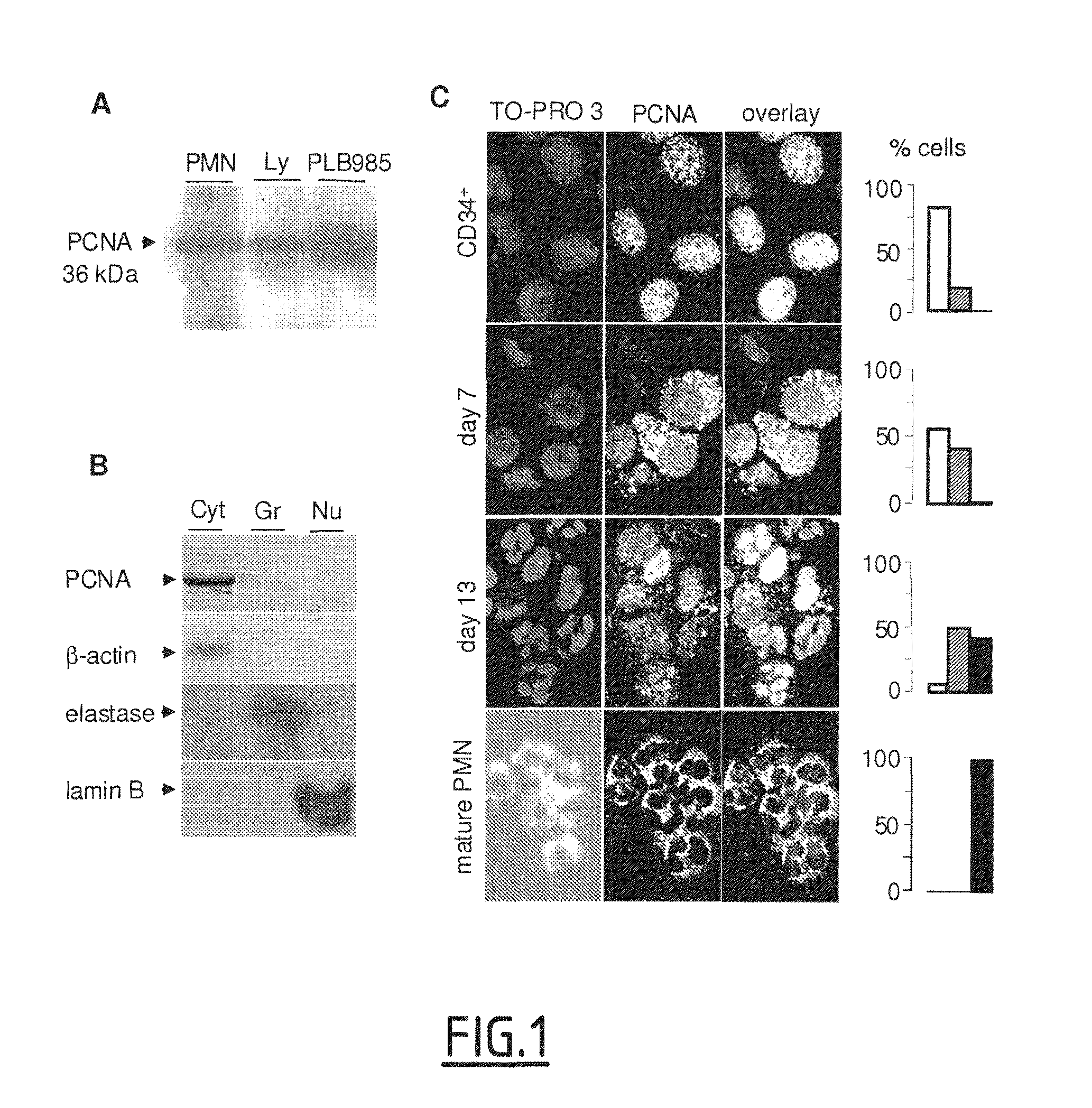
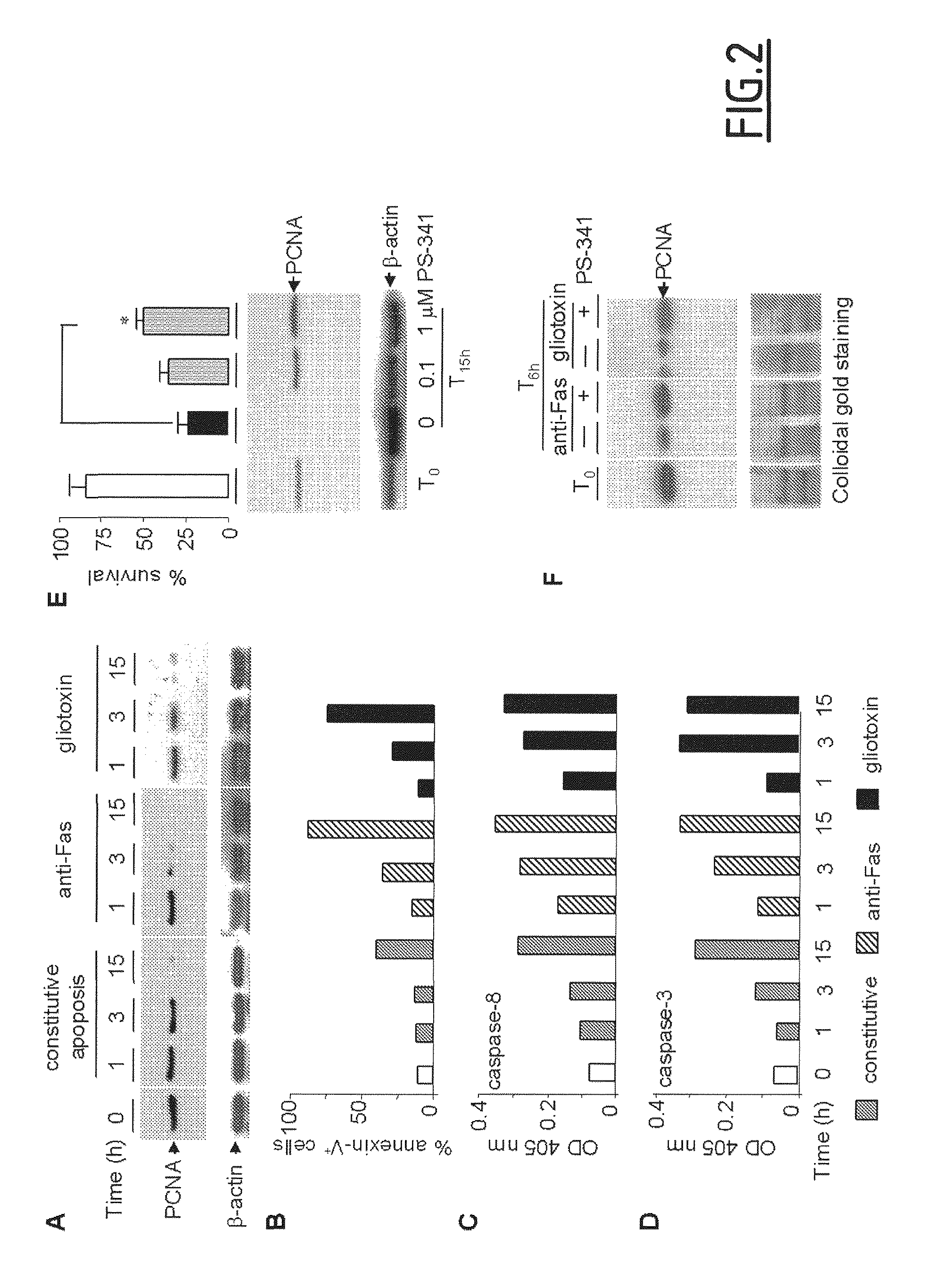
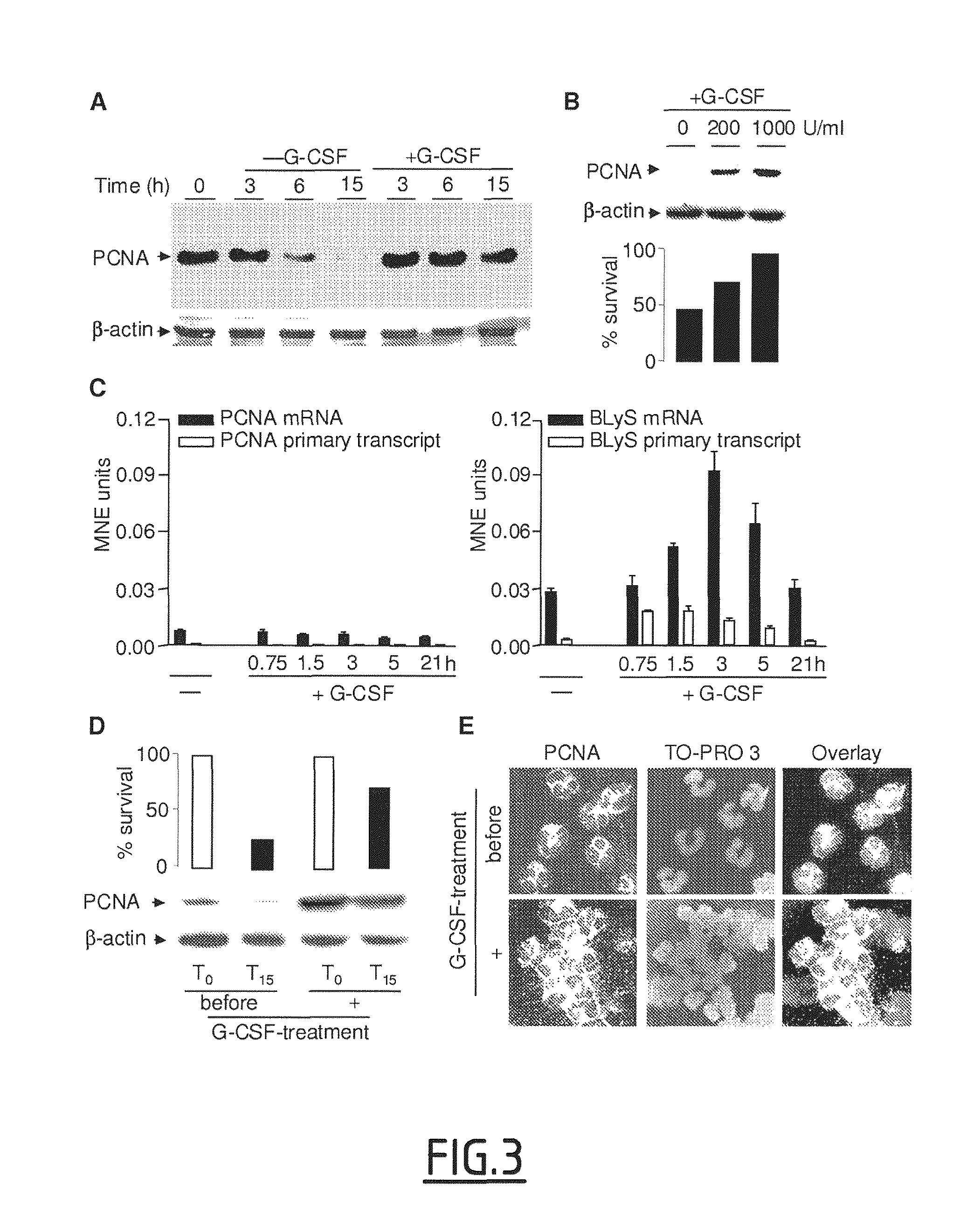
The present invention concerns compounds modulating apoptosis of neutrophil cells. In particular, the invention concerns compounds inhibiting an interaction between Proliferating Cell Nuclear Antigen (PCNA) and proteins binding to cytoplasmic PCNA in neutrophil cells, for use in the treatment of a disease involving a neutrophil-dependent inflammatory process. The invention also relates to a method for the identification of a compound for use in the treatment of a neutrophil-dependent inflammatory process. The invention further relates to peptides for use in the treatment of neutropenia.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
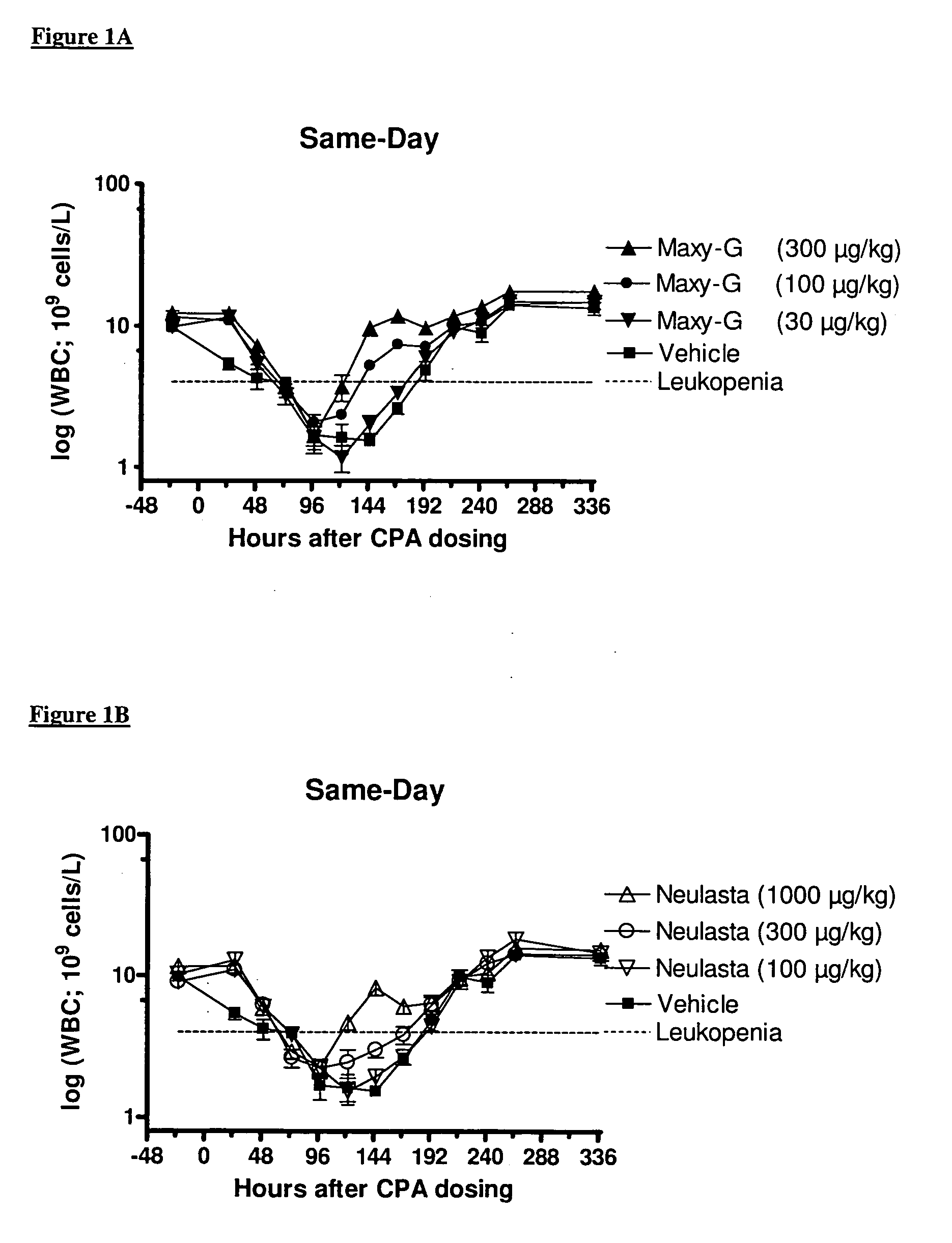
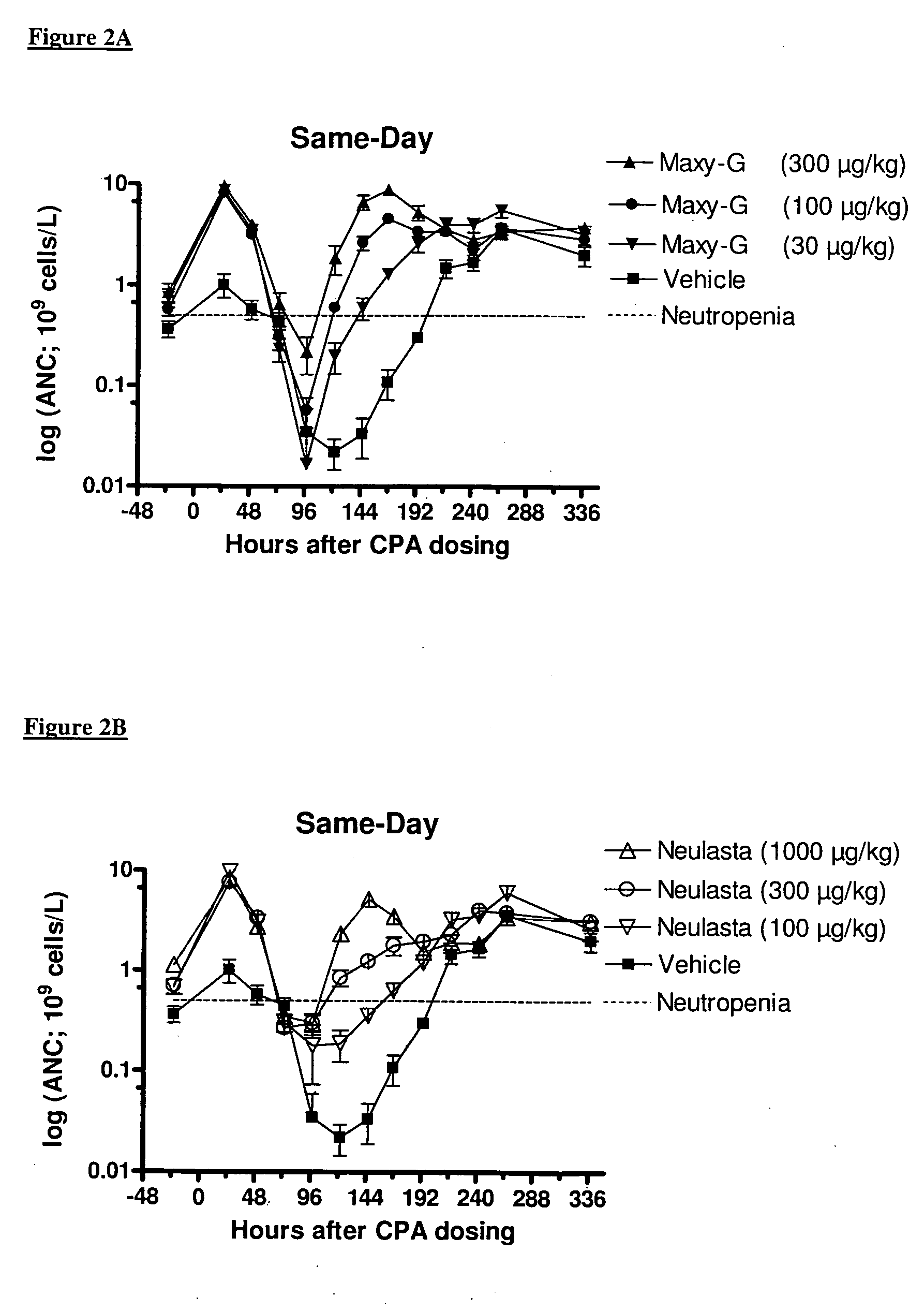
Method for the treatment of neutropenia by administration of a multi-pegylated granulocyte colony stimulating factor (G-CSF) variant
InactiveUS20090203601A1Reduce chemotherapy-induced neutropeniaTreating and preventing neutropeniaPeptide/protein ingredientsPharmaceutical non-active ingredientsPegylated granulocyte colony-stimulating factorGranulocytopenias
The invention relates to a method for treating or preventing neutropenia in a patient receiving chemotherapy by administering a multi-PEGylated granulocyte colony stimulating factor (G-CSF) variant on the same day that chemotherapy is administered.
Owner:MAXYGEN HLDG
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com