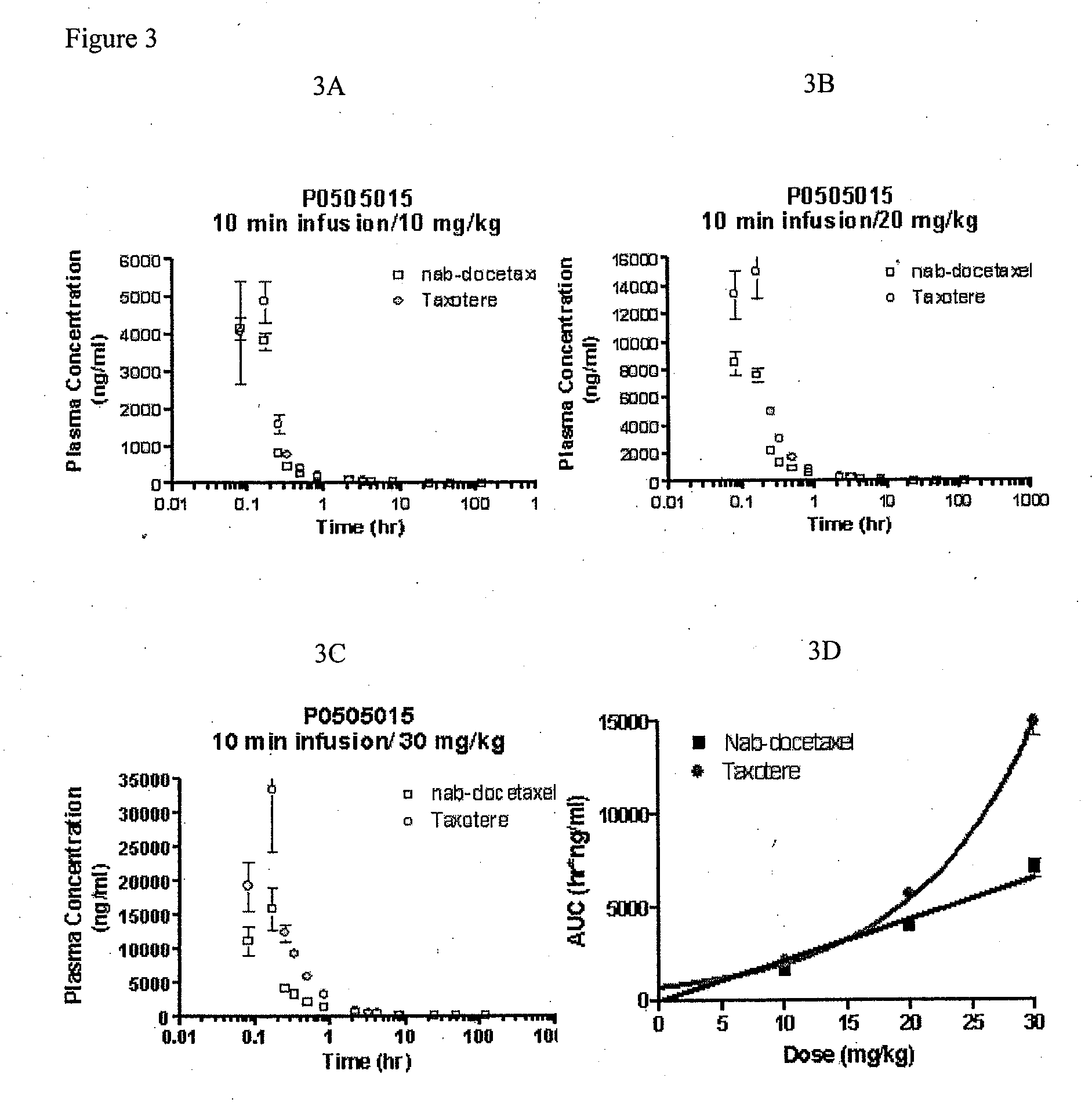
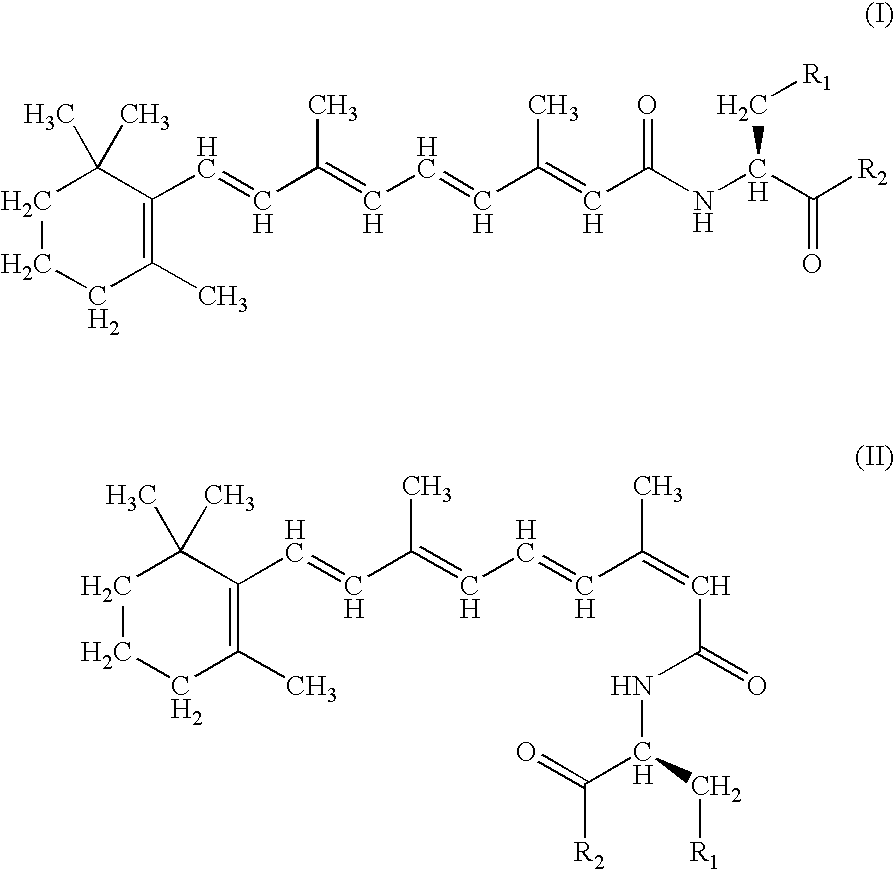
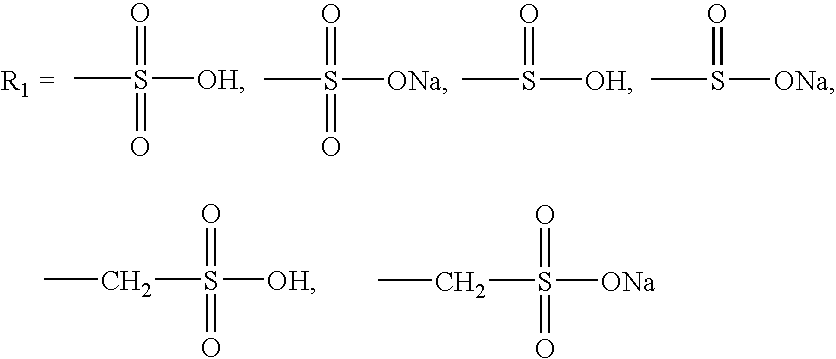
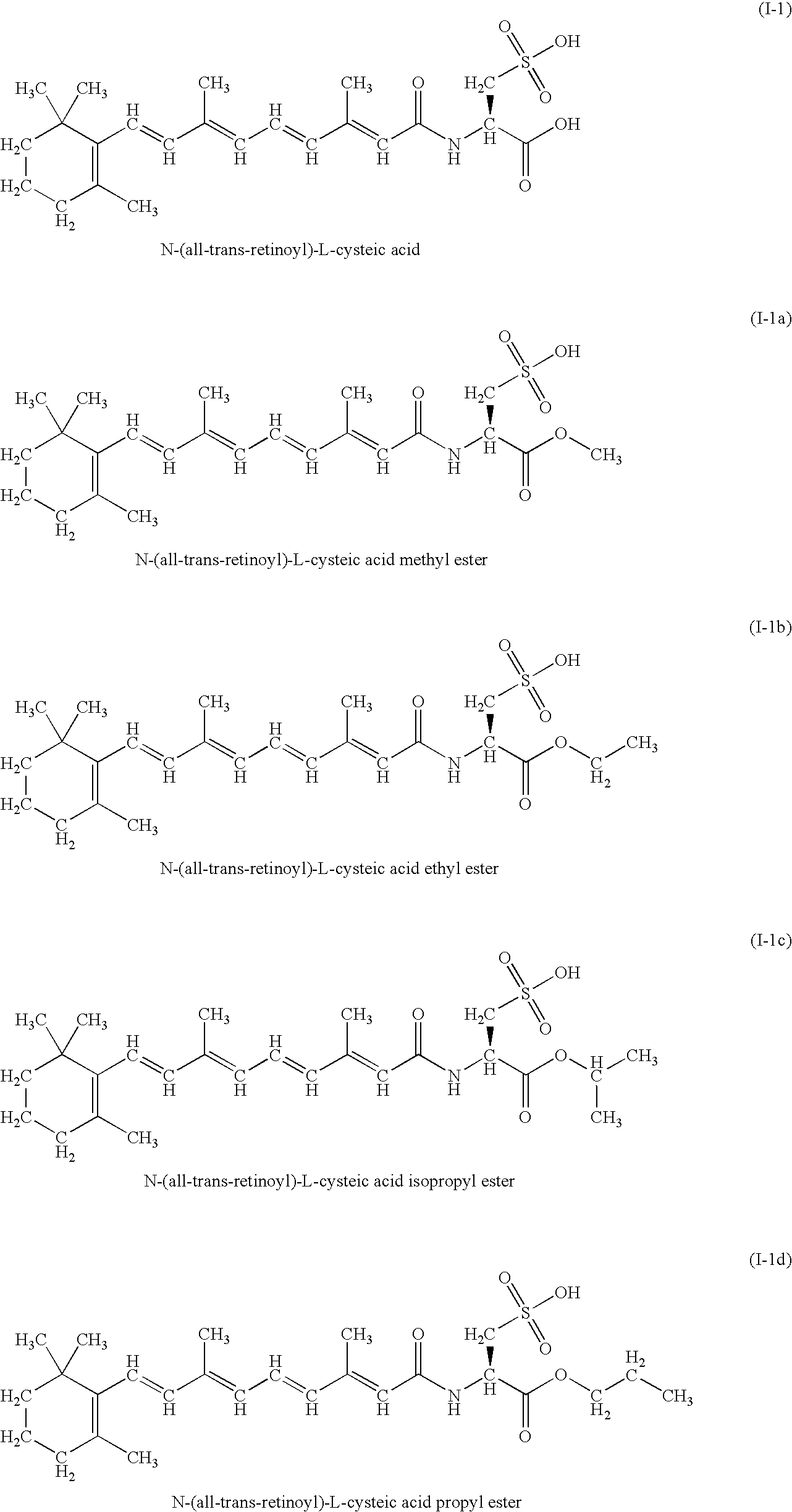
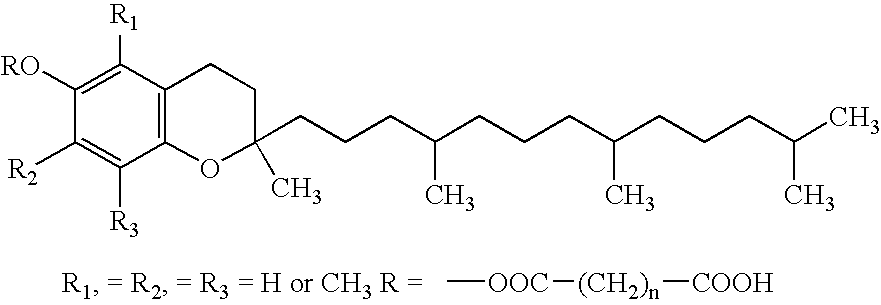
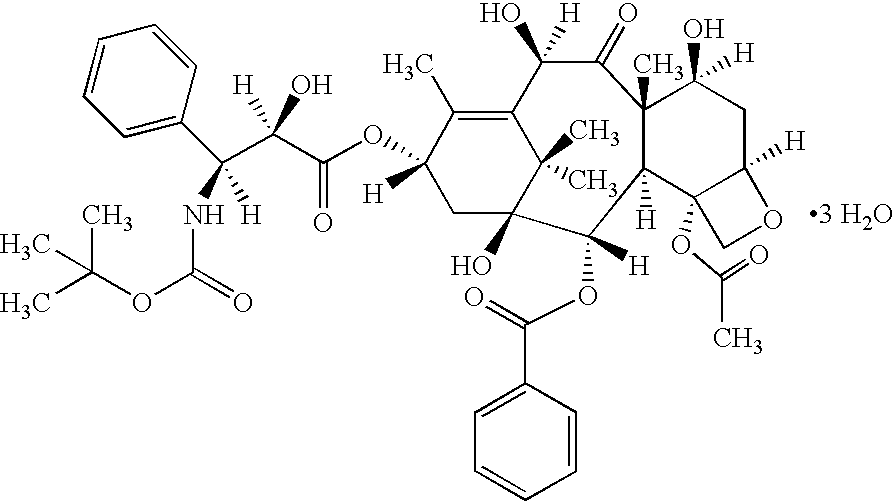
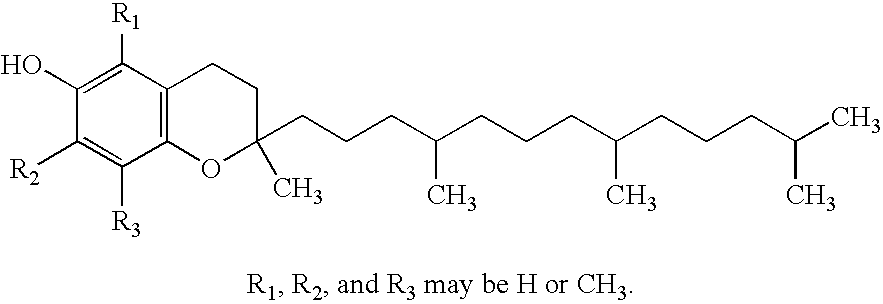
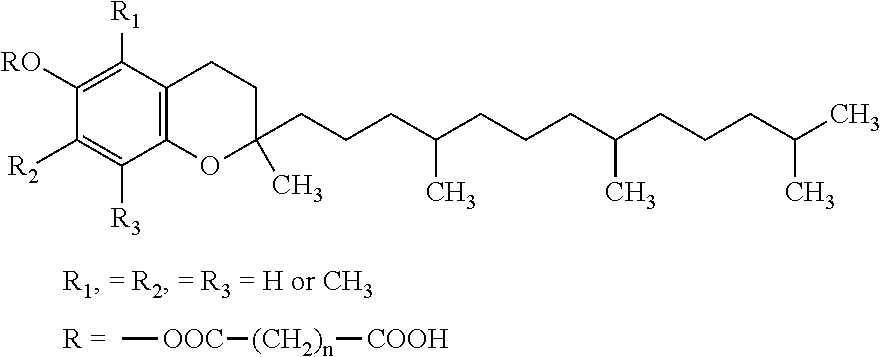
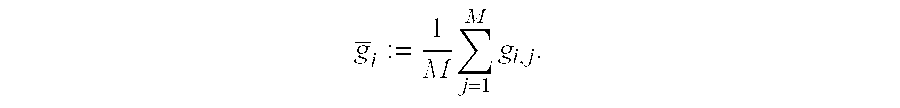Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
320 results about "Docetaxel-PNP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
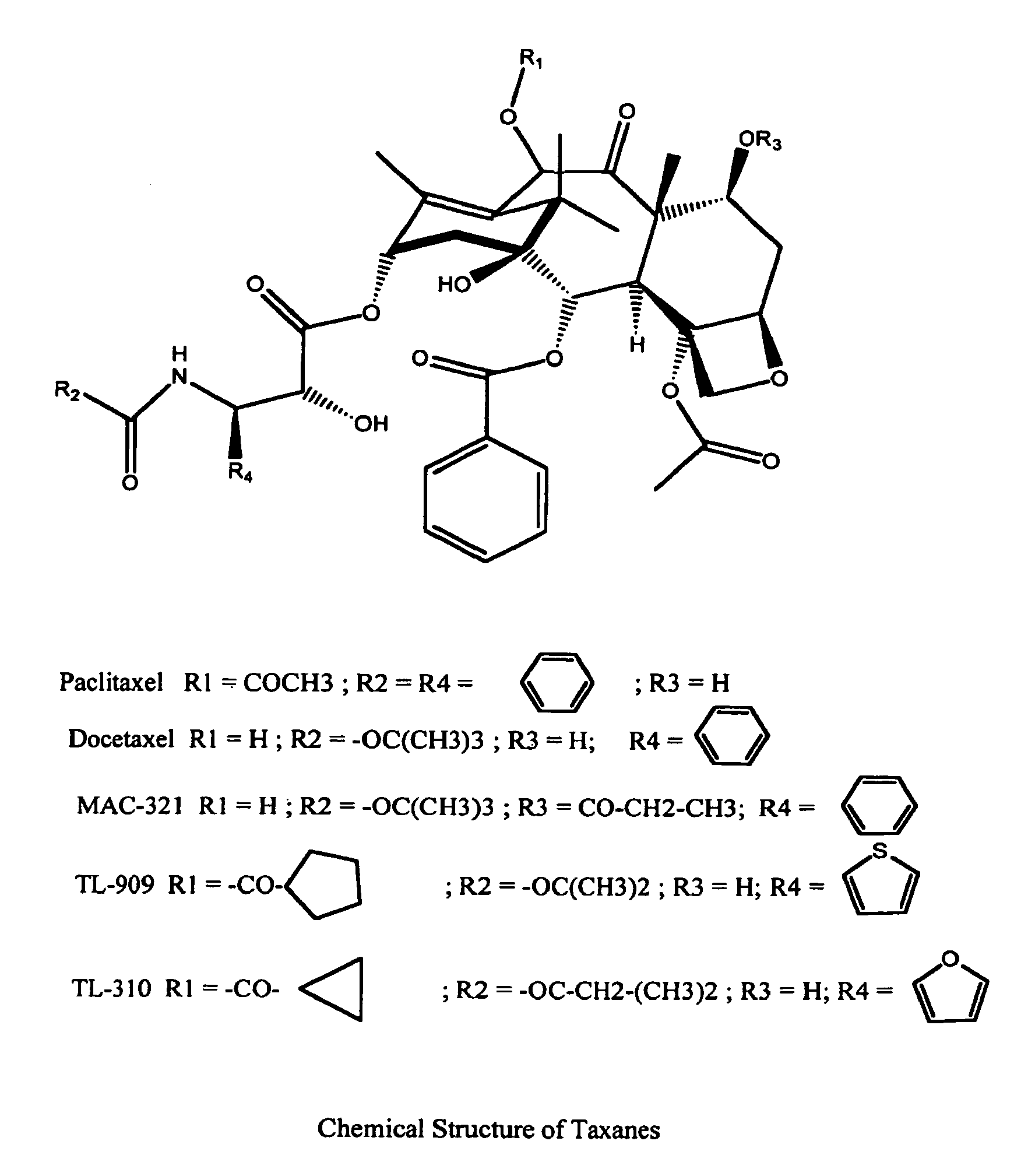
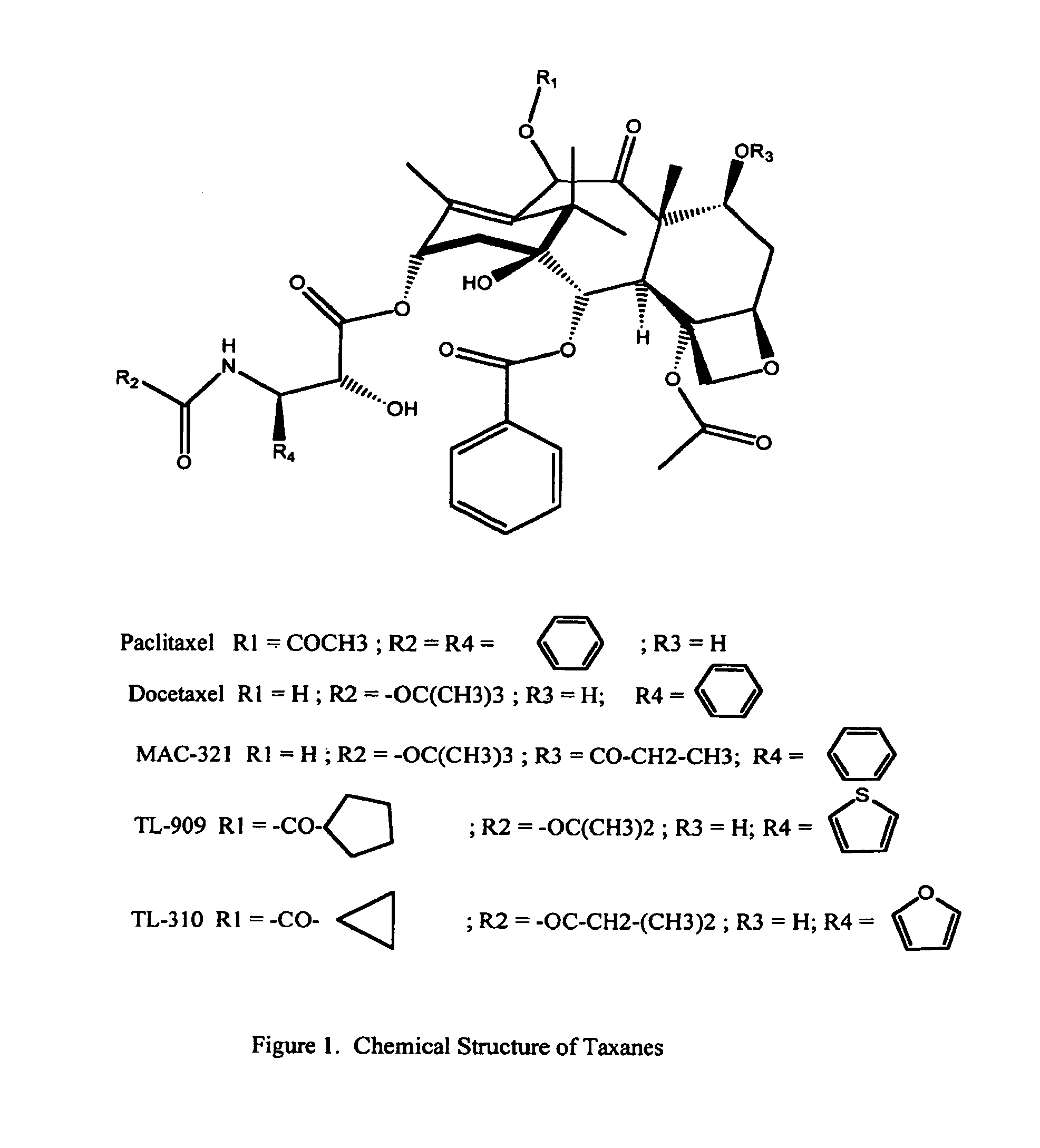
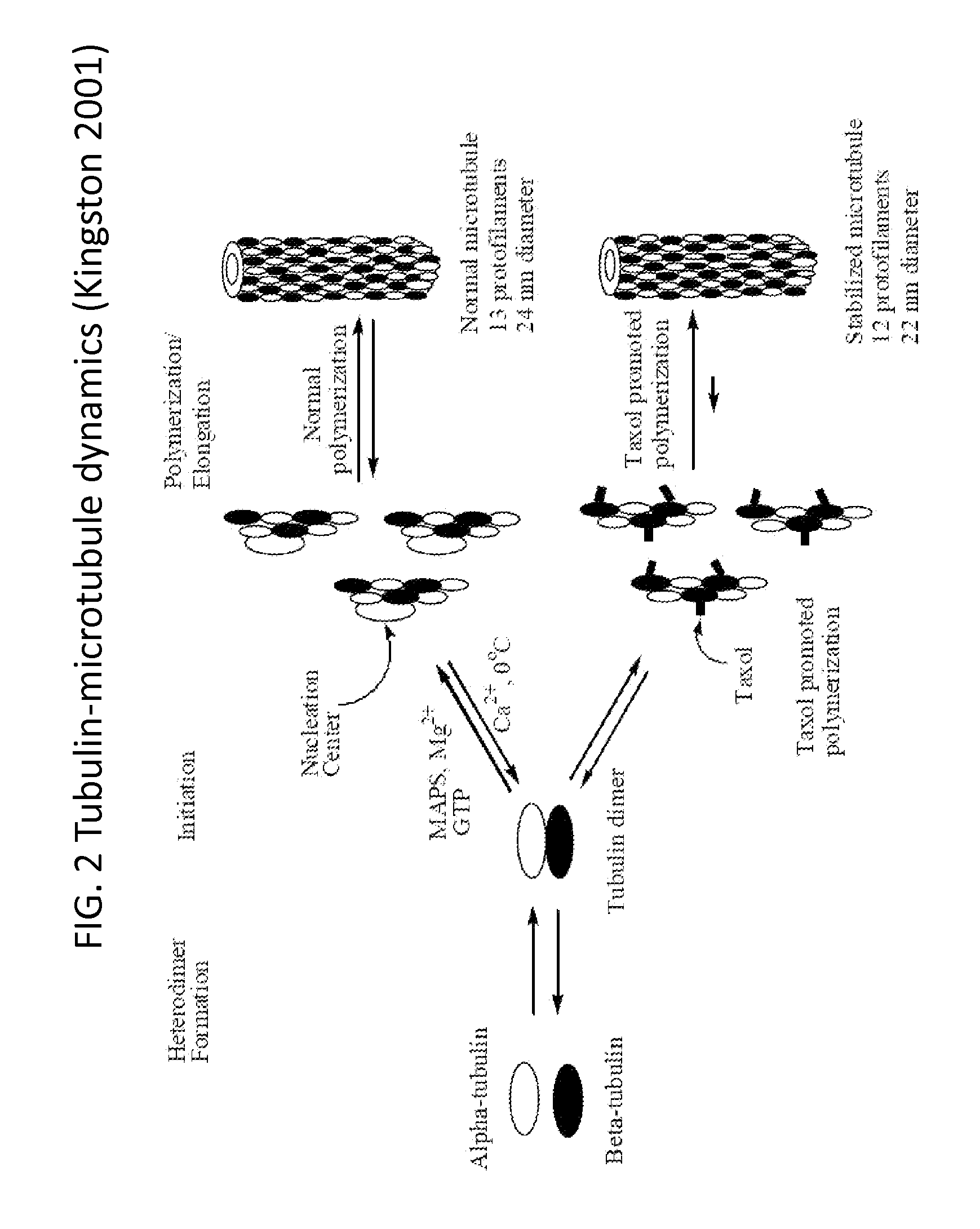
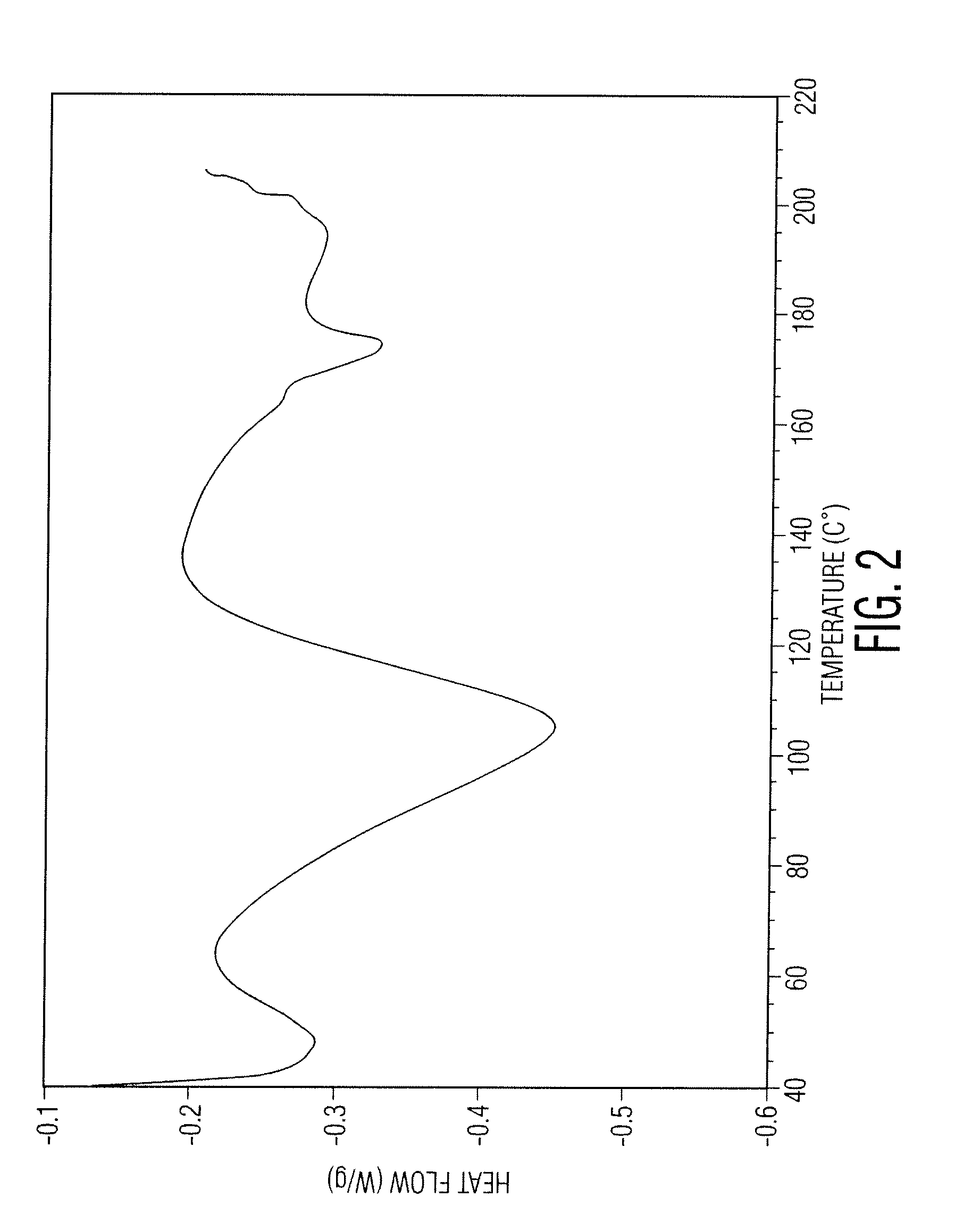
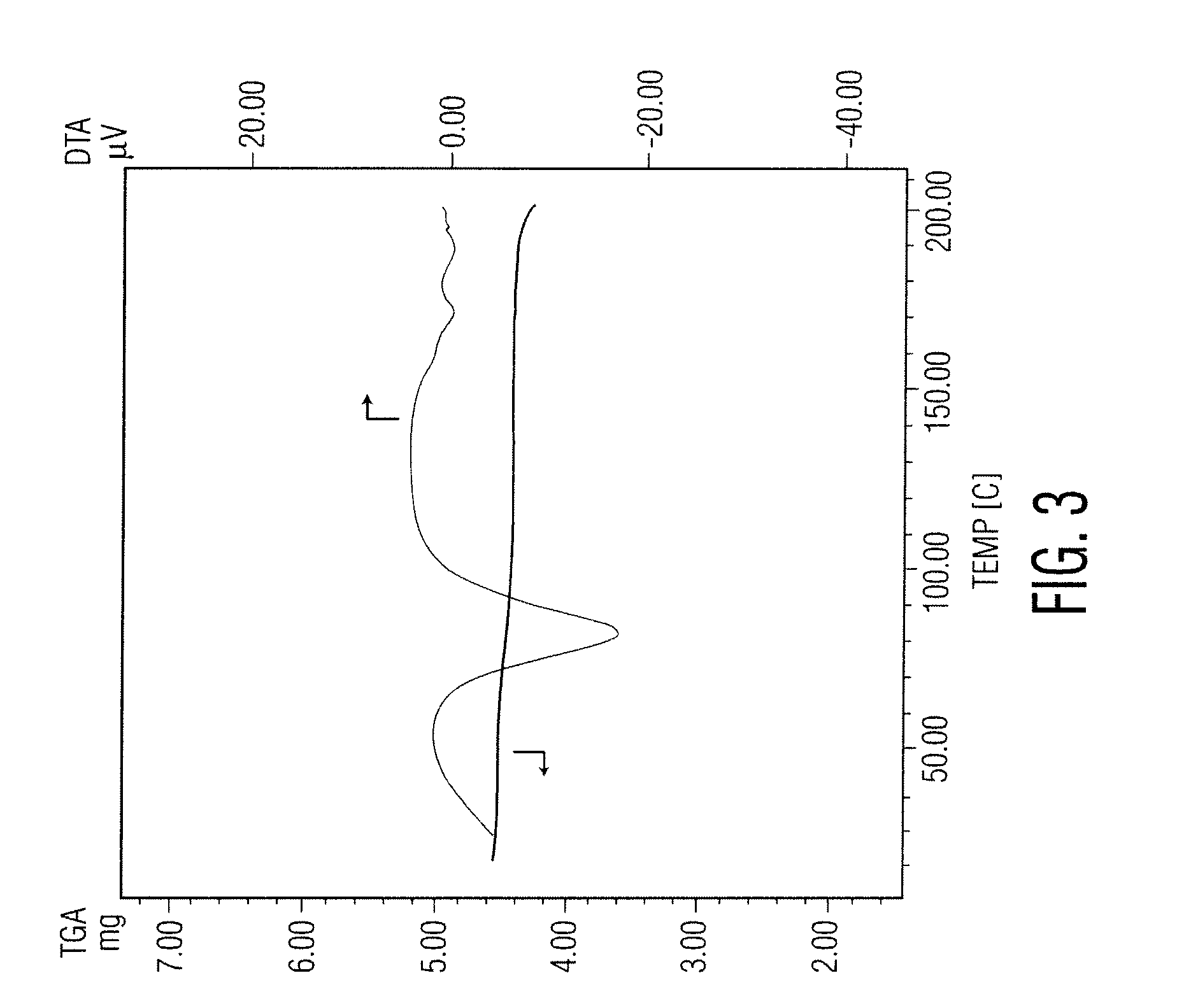
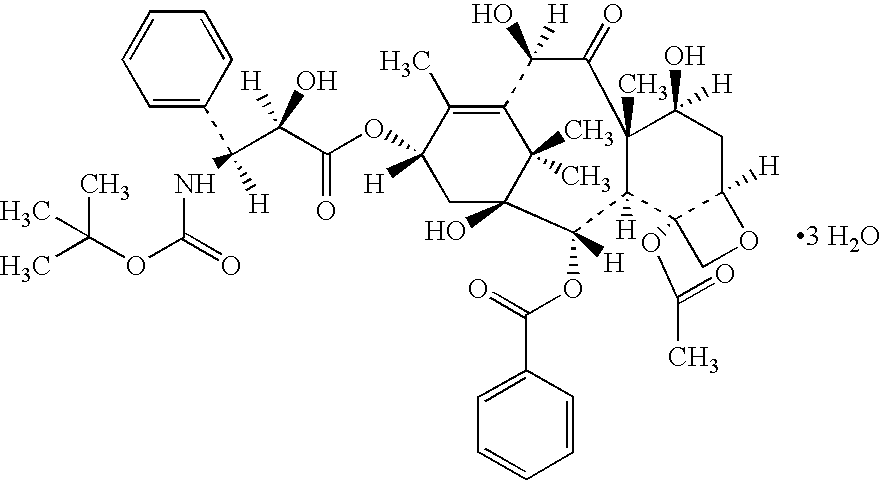
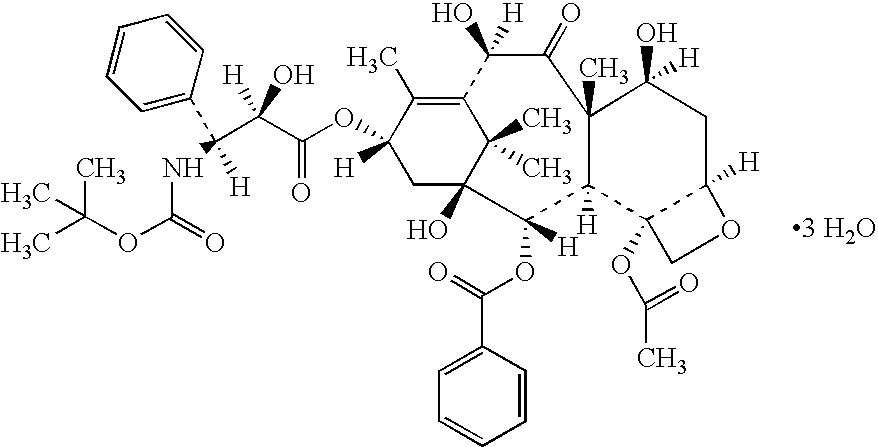
A polymeric nanoparticle (PNP) formulation containing the taxane docetaxel, a semi-synthetic analogue of paclitaxel, with antineoplastic activity. Docetaxel binds specifically to the beta-tubulin subunit of the microtubule, stabilizing tubulin and inhibiting microtubule disassembly, which results in cell-cycle arrest at the G2/M phase, preventing cell proliferation. This agent also inhibits pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and induces various mediators of the inflammatory response. Compared to docetaxel alone, the PNP formulation may enhance stability and improve delivery.
Treatment of metastatic breast cancer
InactiveUS20090317387A1Improve survivalOrganic active ingredientsAntibody ingredientsBiologic therapiesDocetaxel-PNP
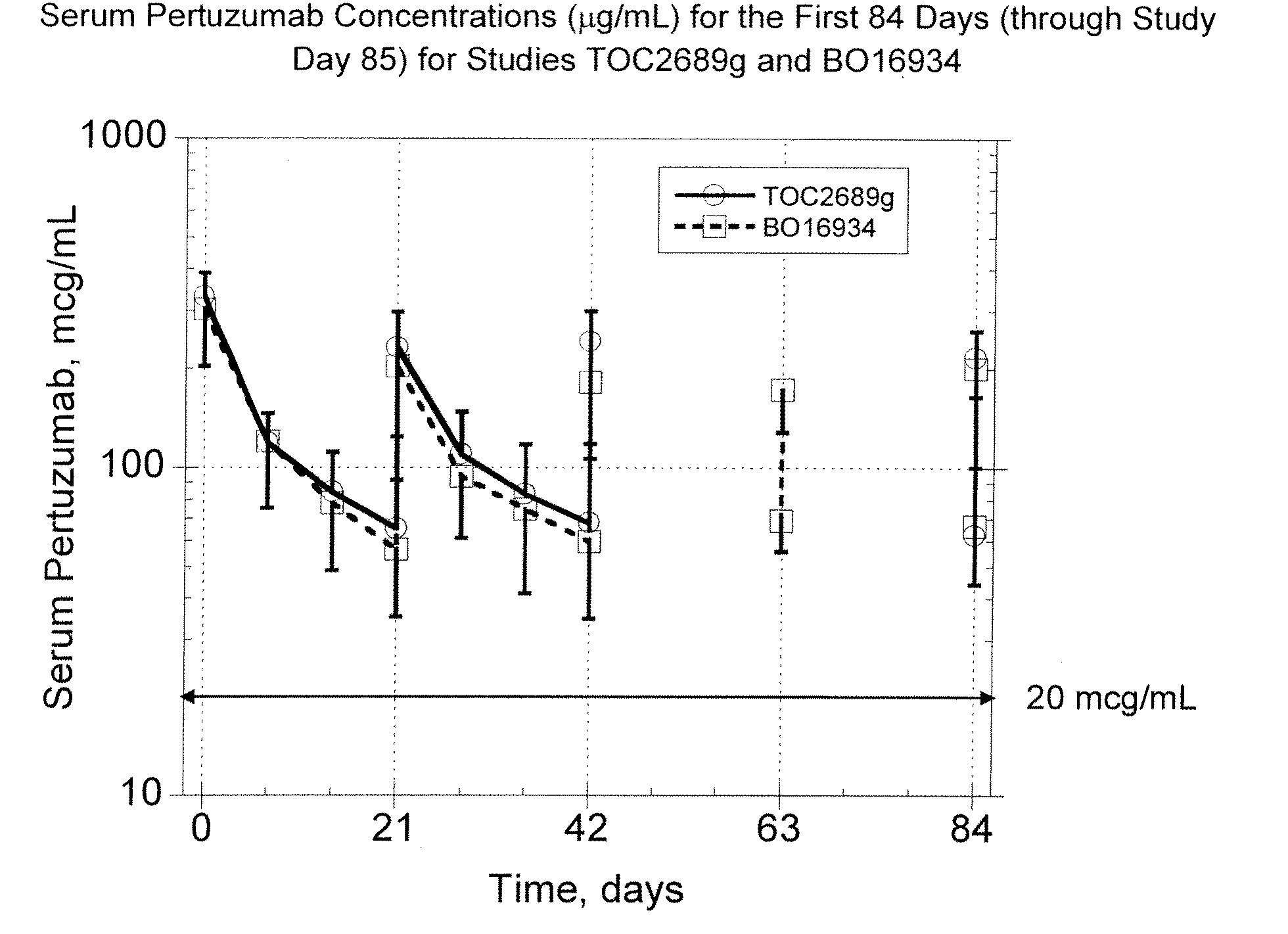
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
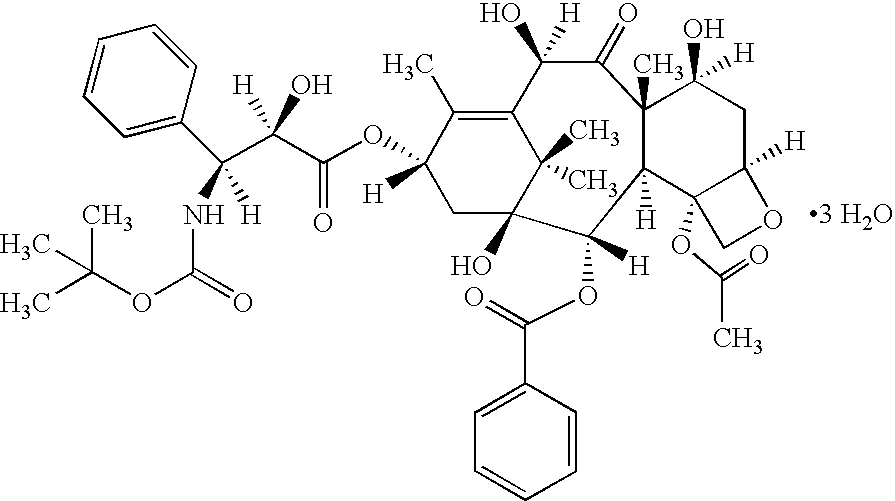
Process for preparation of paclitaxel trihydrate and docetaxel trihydrate
A process for converting paclitaxel or docetaxel to the respective trihydrate characterized by very high purity, comprises dissolving either paclitaxel or docetaxel in a mixture of alkane and chlorinated alkane to provide a crude product of 65-75% assay and dissolving the crude product in an alkyl ketone, followed by addition of an alkane to provide a product of increased chromatographic purity; dissolving the product of increased chromatographic purity in an aliphatic nitrile, with addition of water to precipitate taxane trihydrate.
Owner:DABUR PHARM LTD
Compositions and methods for preparation of poorly water soluble drugs with increased stability
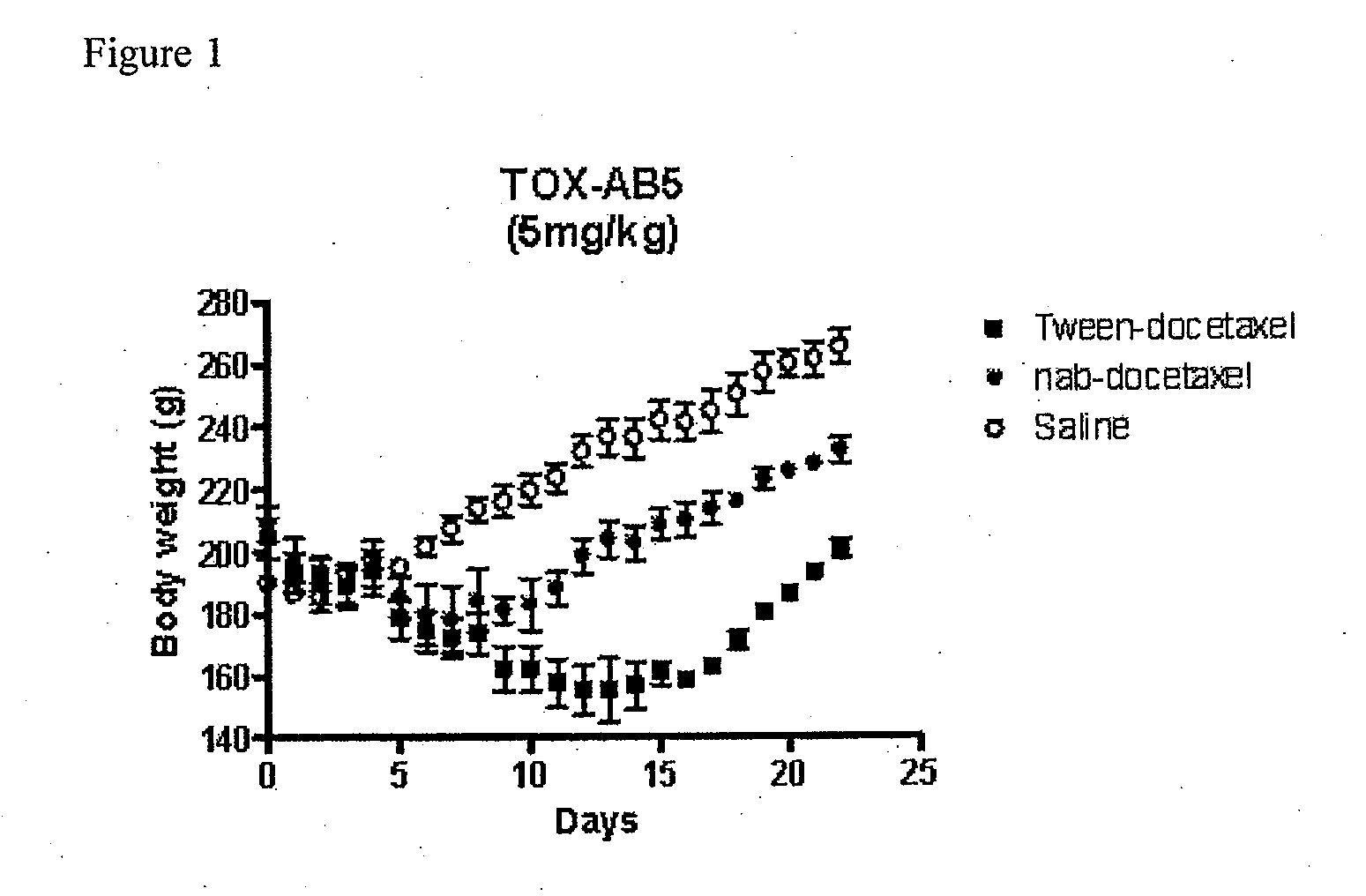
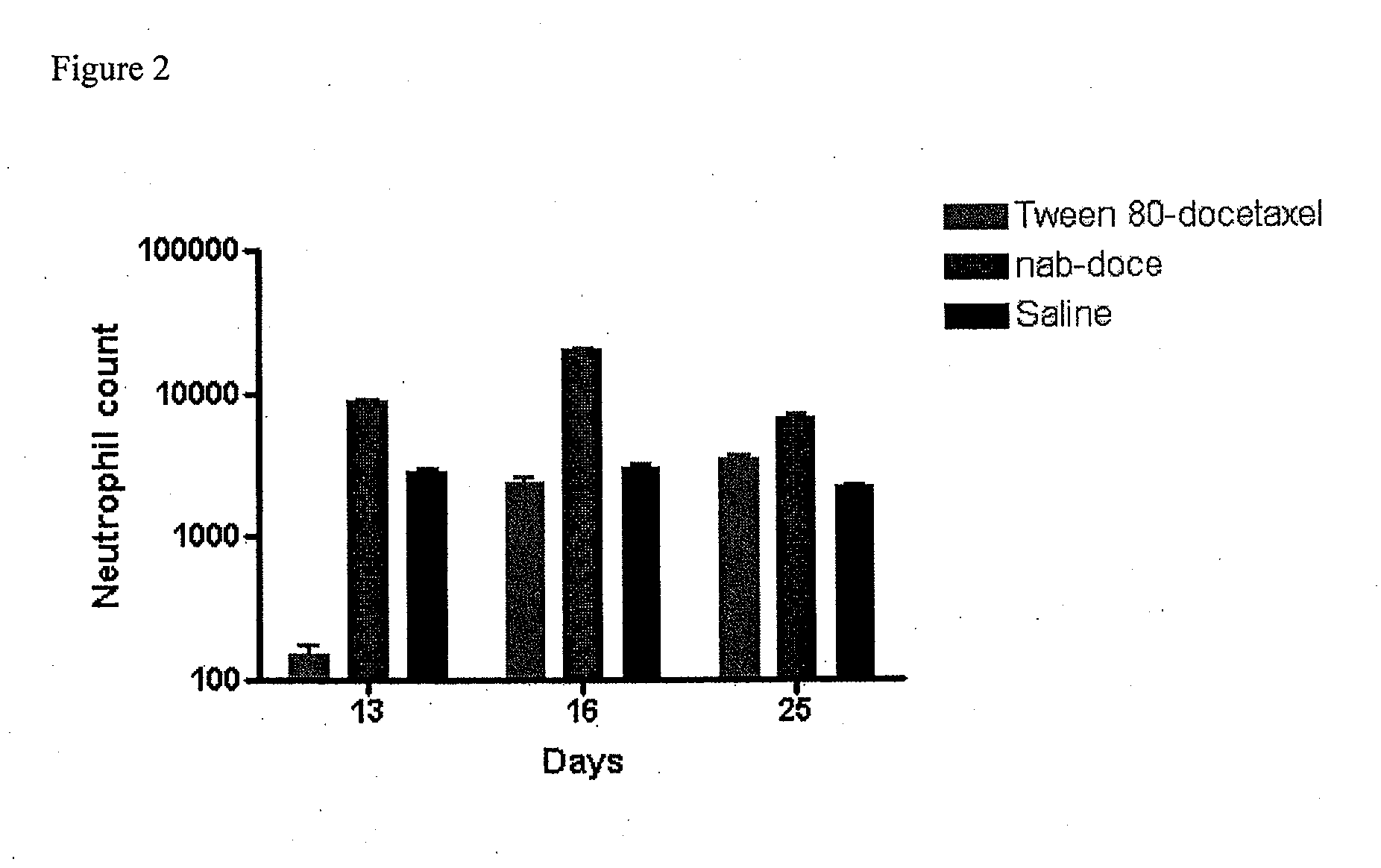
The present invention provides stable pharmaceutical compositions of poorly water soluble pharmaceutical agents and stabilizing agents which function to increase stability of the compositions. The use of stabilizing agents provide extended stability of nanoparticle suspensions and other formulations of poorly water soluble pharmaceutical agents such as docetaxel under certain conditions, for example upon dilution for administration.
Owner:ABRAXIS BIOSCI LLC
Lyophilized solid taxane composition, a process for preparing said solid composition, a pharmaceutical formulation and a kit for said formulation
InactiveUS20090215882A1High level of chemical degradationImprove solid solubilityOrganic active ingredientsBiocideDocetaxel-PNPDocetaxel
A lyophilized solid composition of taxane (preferably docetaxel and paclitaxel), is suitable to prepare a pharmaceutical formulation to be administered to mammals, particularly humans, comprising a taxane, a tensoactive, a lyophilizing excipient, and acid; also essentially free from organic solvents. The solid composition is free from polysorbate 80 and polyoxyethylated castor oil; it is sterile; it is soluble in aqueous solutions in the absence of organic solvent and it has an apparent density from 0.05 g / ml to 0.45 g / ml. A procedure of double lyophilization obtains a solid composition of taxane. A pharmaceutical formulation of a taxane comprises a solid composition of lyophilized taxane and a solubilizing composition. A kit comprises the compositions and a syringe.
Owner:ERIOCHEM SA
Water soluble paclitaxel derivatives
InactiveUS6884817B2Surprising antitumor activityImprove efficacyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
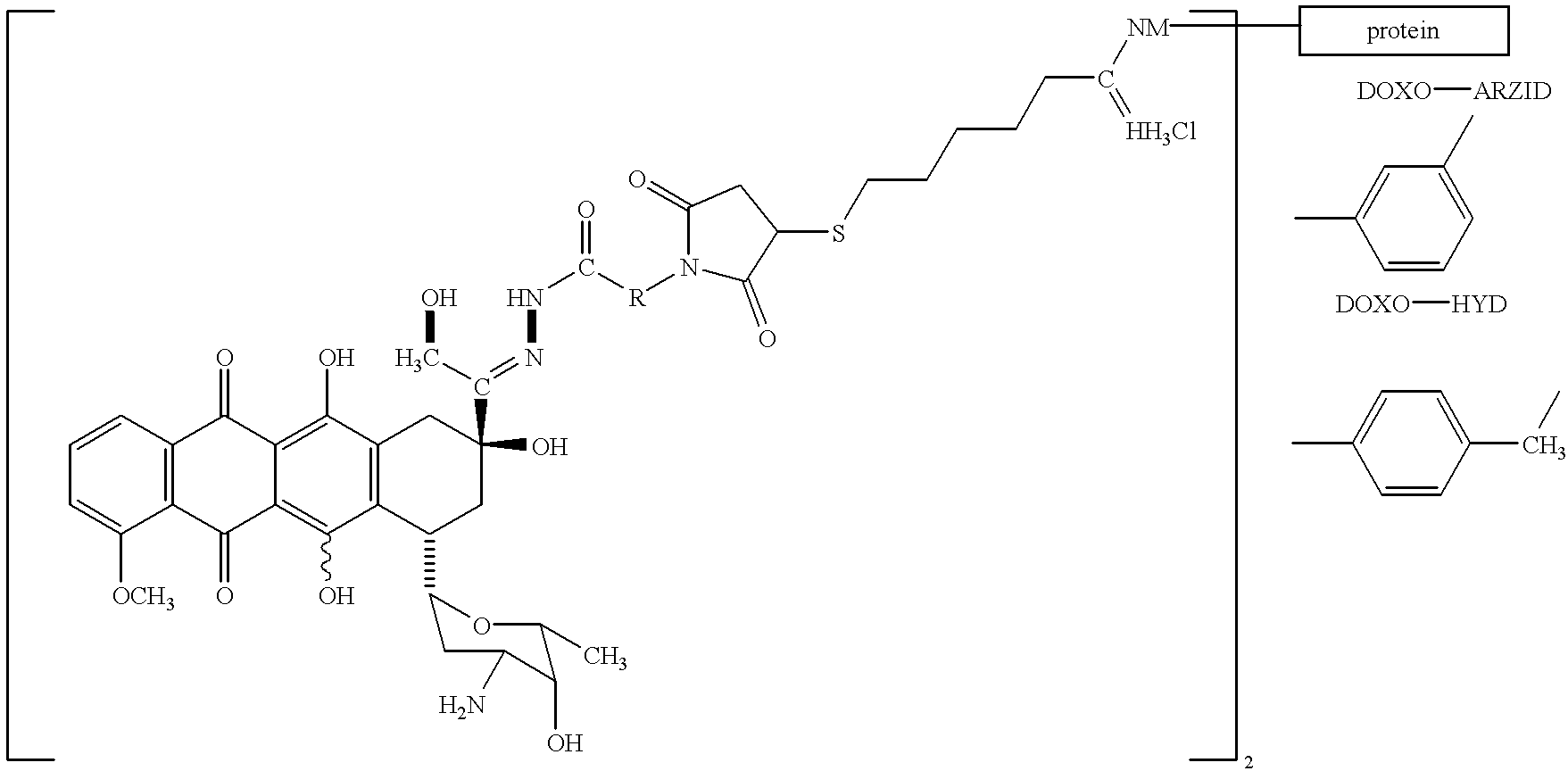
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
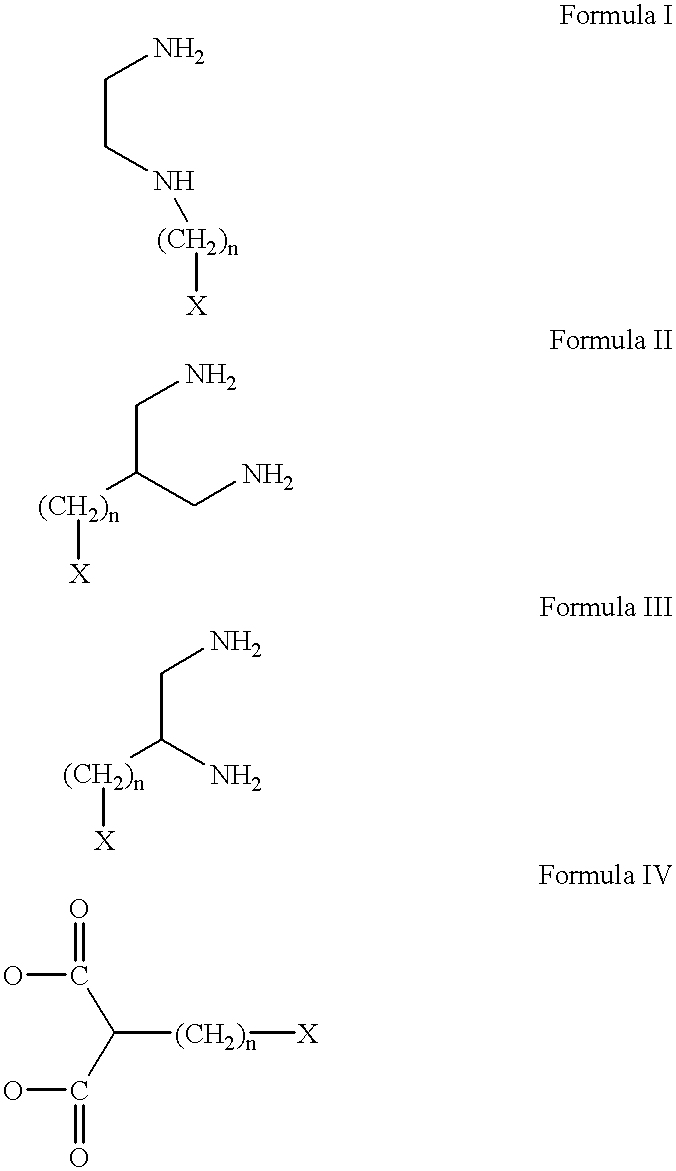
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Solid nanoparticle formulation of water insoluble pharmaceutical substances with reduced ostwald ripening
ActiveUS8728527B2Good dispersionReduced and substantially no particle growthBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
The present invention belongs to the fields of pharmacology, medicine and medicinal chemistry. The present invention provides novel pharmaceutical compositions composed of solid nanoparticles dispersed in aqueous medium of substantially water insoluble pharmaceutical substances such as docetaxel with reduced Ostwald ripening.
Owner:AUSTIN BIOSCIENCES CORP
Method of treating cancer, especially soft tissue sarcoma utilizing gemcitabine in combination with docetaxel and anti-VEGF therapy (bevacizumab)
InactiveUS20070065449A1Great likelihoodLong median survivalBiocideGenetic material ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to a pharmaceutical cocktail, in particular, effective amounts of gemcitabine, in combination with effective amounts of docetaxel and angiogenesis inhibitor, especially a vascular endothelial growth factor (VEGF) inhibitor, such as bevacizumab for the treatment of cancer, in particular sarcoma, especially soft tissue sarcoma. Pharmaceutical compositions and methods of treating cancer, including sarcoma, especially soft tissue sarcoma (prolonging the patient's life, eliminating the tumor, improving the patient's quality of life, shrinking the tumor, prolonging survival and / or preventing the tumor's metastases) are additional aspects of the present invention.
Owner:STC UNM
Compositions and methods for preparation of poorly water soluble drugs with increased stability
Owner:ABRAXIS BIOSCI LLC
Therapeutic compounds
InactiveUS20040048923A1Improve pharmacological activityGood curative effectBiocideOrganic compound preparationDocetaxel-PNPTreatment effect
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:VIVESTO AB
Methods of treating cancer
ActiveUS20120070502A1High elongationLow toxicityPowder deliveryOrganic active ingredientsCarboplatinPlatinum
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin). The present application also provides methods of treating prostate cancer by administering to the individual a) an effective amount of a composition comprising nanoparticles comprising docetaxel and an albumin; and b) an effective amount of a steroid.
Owner:ABRAXIS BIOSCI LLC
Methods of treating cancer
ActiveUS20140072643A1Many symptomShorten the progressOrganic active ingredientsHeavy metal active ingredientsCarboplatinDocetaxel-PNP
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin). The present application also provides methods of treating prostate cancer by administering to the individual a) an effective amount of a composition comprising nanoparticles comprising docetaxel and an albumin; and b) an effective amount of a steroid.
Owner:ABRAXIS BIOSCI LLC
Antibodies against csf-1r
ActiveUS20110243947A1Prevent dimerizationInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDocetaxel-PNPDocetaxel
The invention provides a human antibody that binds human CSF-1R with high affinity. Antibodies of the present invention have significant advantages over the antibodies known in the art by being multifunctional: inhibiting signaling of CSF-1R, internalizing and inducing CSF-1R degradation and stimulating ADCC in cell including tumors, macrophages and monocytes. They are also shown to be effective in treating leukemia, breast, endometrial and prostate cancer alone or in combination with docetaxel, paclitaxel, Herceptin® or doxorubicin.
Owner:IMCLONE SYSTEMS
Compositions and methods for preparation of poorly water soluble drugs with increased stability
The present invention provides stable pharmaceutical compositions of poorly water soluble pharmaceutical agents and stabilizing agents which function to increase stability of the compositions. The use of stabilizing agents provide extended stability of nanoparticle suspensions and other formulations of poorly water soluble pharmaceutical agents such as docetaxel under certain conditions, for example upon dilution for administration.
Owner:ABRAXIS BIOSCI LLC
Docetaxel process and polymorphs
InactiveUS20100197944A1Low toxicityImprove performanceOrganic chemistryDocetaxel-PNPMedicinal chemistry
Owner:DR REDDYS LAB LTD +1
Novel improved compositions for cancer therapy
InactiveUS20100166872A1Reduced chemotherapy-induced side-effectsOrganic active ingredientsBiocideDocetaxel-PNPSide effect
The present invention relates to novel and improved compositions of anticancer drugs, preferably taxanes, such as paclitaxel and docetaxel, their derivatives or their analogues, methods of manufacturing these compositions and methods of fractionating the particles in particular size range and methods of treating cancer patients with these compositions, which provide reduced chemotherapy-induced side-effects especially reduced chemotherapy-induced-alopecia. The composition is such that there is substantially no free drug in the said composition.
Owner:PANACEA BIOTEC
Process for preparation of paclitaxel trihydrate and docetaxel trihydrate
A process for the preparation of paclitaxel trihydrate and doctaxel trihydrate comprising (a) treating taxane selected from paclitaxel and docetaxel with a mixture of alkane and chlorinated alkane to obtain a crude product of 65-75% assay, (b) dissolving the crude product thus obtained in alkyl ketone followed by slow addition of an alkane to increase chromatographic purity, (c) dissolving the taxane of step (b) in an aliphatic nitrile at a temperature of 50-70° C., (d) adding purified water to the product of step (c) to precipitate taxane trihydrate; and (e) filtering and drying the product of step (d) to obtain taxane trihydrate of C.P. >99.5% and 98-102% assay.
Owner:DABUR PHARM LTD
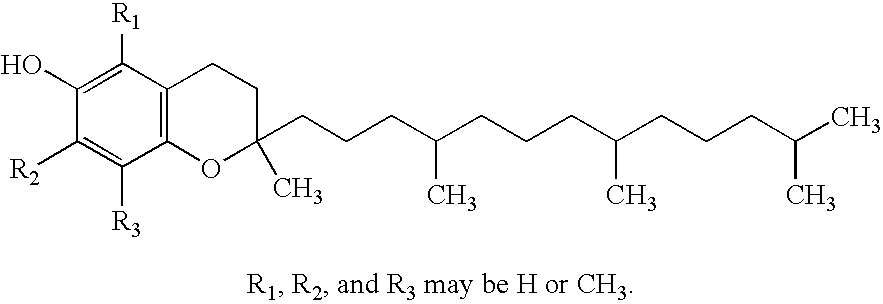
Vitamin E succinate stabilized pharmaceutical compositions, methods for the preparation and the use thereof
The present invention provides vitamin E succinate (VES)-stabilized compositions, methods for the preparation thereof and methods useful for the in vivo delivery of substantially water insoluble and optionally chemically unstable pharmacologically active agents (such as docetaxel).
Owner:CHEN ANDREW XIAN
Vitamin E succinate stabilized pharmaceutical compositions, methods for the preparation and the use thereof
The present invention provides vitamin E succinate (VES)-stabilized compositions, methods for the preparation thereof and methods useful for the in vivo delivery of substantially water insoluble and optionally chemically unstable pharmacologically active agents (such as docetaxel).
Owner:CHEN ANDREW XIAN
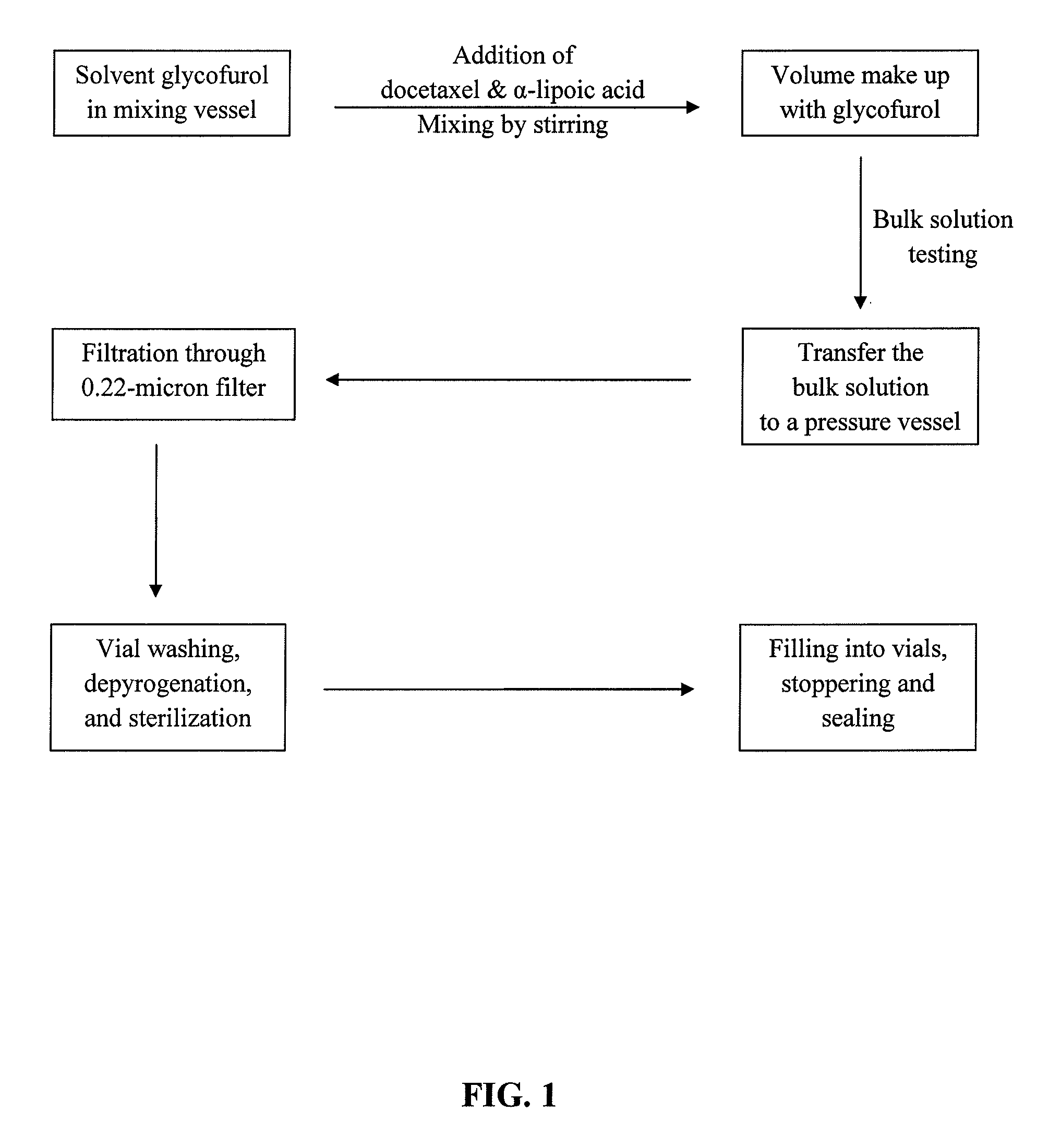
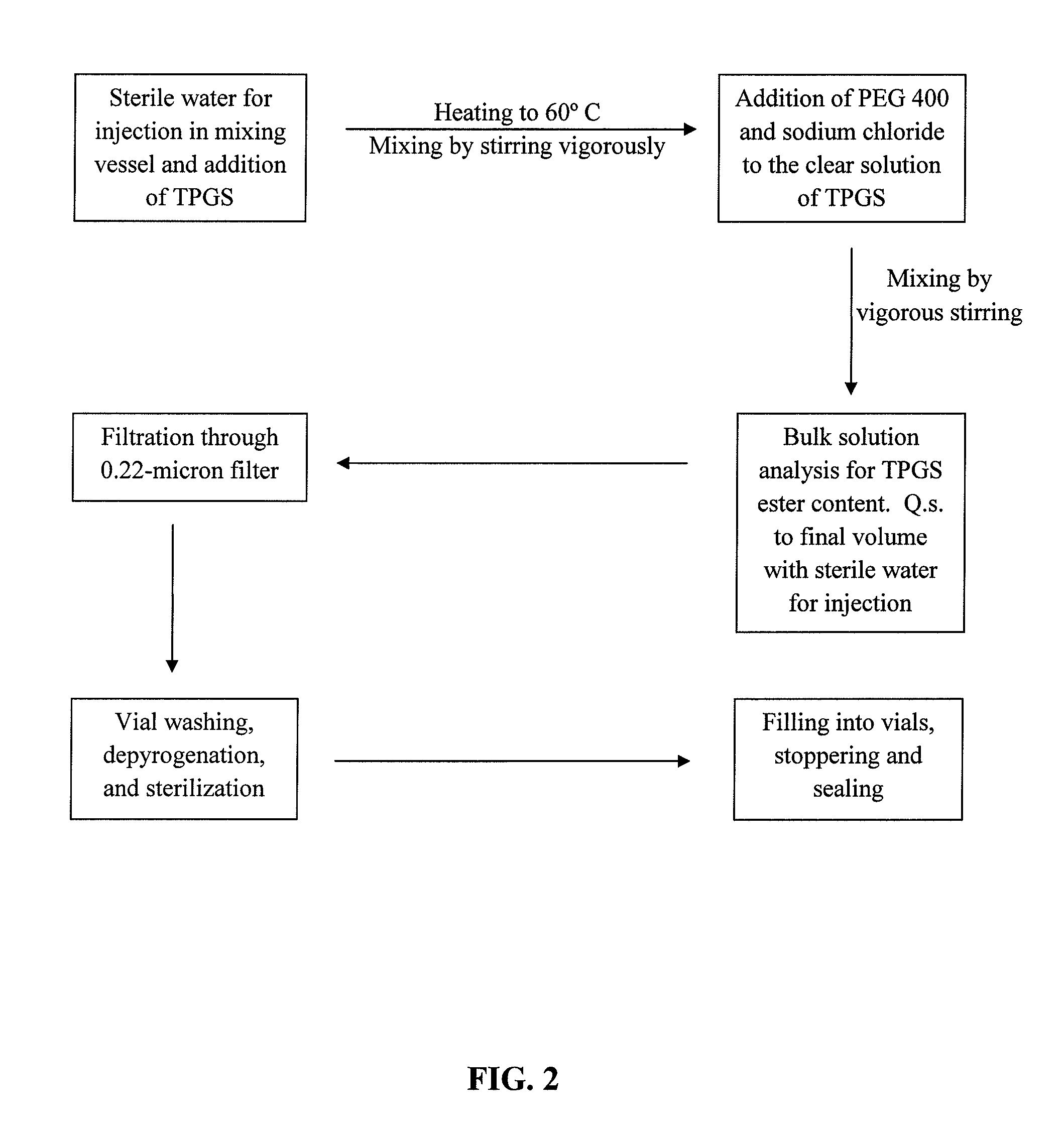
Docetaxel formulations with lipoic acid
Pharmaceutical formulations comprising docetaxel, solubilizer, and α-lipoic acid, wherein the formulation is substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol, acetic acid, benzyl alcohol, or ethanol. The α-lipoic acid, at certain concentrations, may impart stability and prevent degradation of docetaxel while the formulations are in storage. The formulations may be combined with a diluent, which comprises one or more hydrotropes such as tocopherol polyethylene glycol succinate and polyethylene glycol. The formulations combined with the diluent also exhibit stability after storage. Methods of administering docetaxel comprise preparing the formulation comprising docetaxel, solubilizer, and α-lipoic acid; mixing the formulation with a diluent; diluting the resulting formulation in saline, water for injection, or the like; and then injecting the formulations into patients in need thereof.
Owner:SCIDOSE
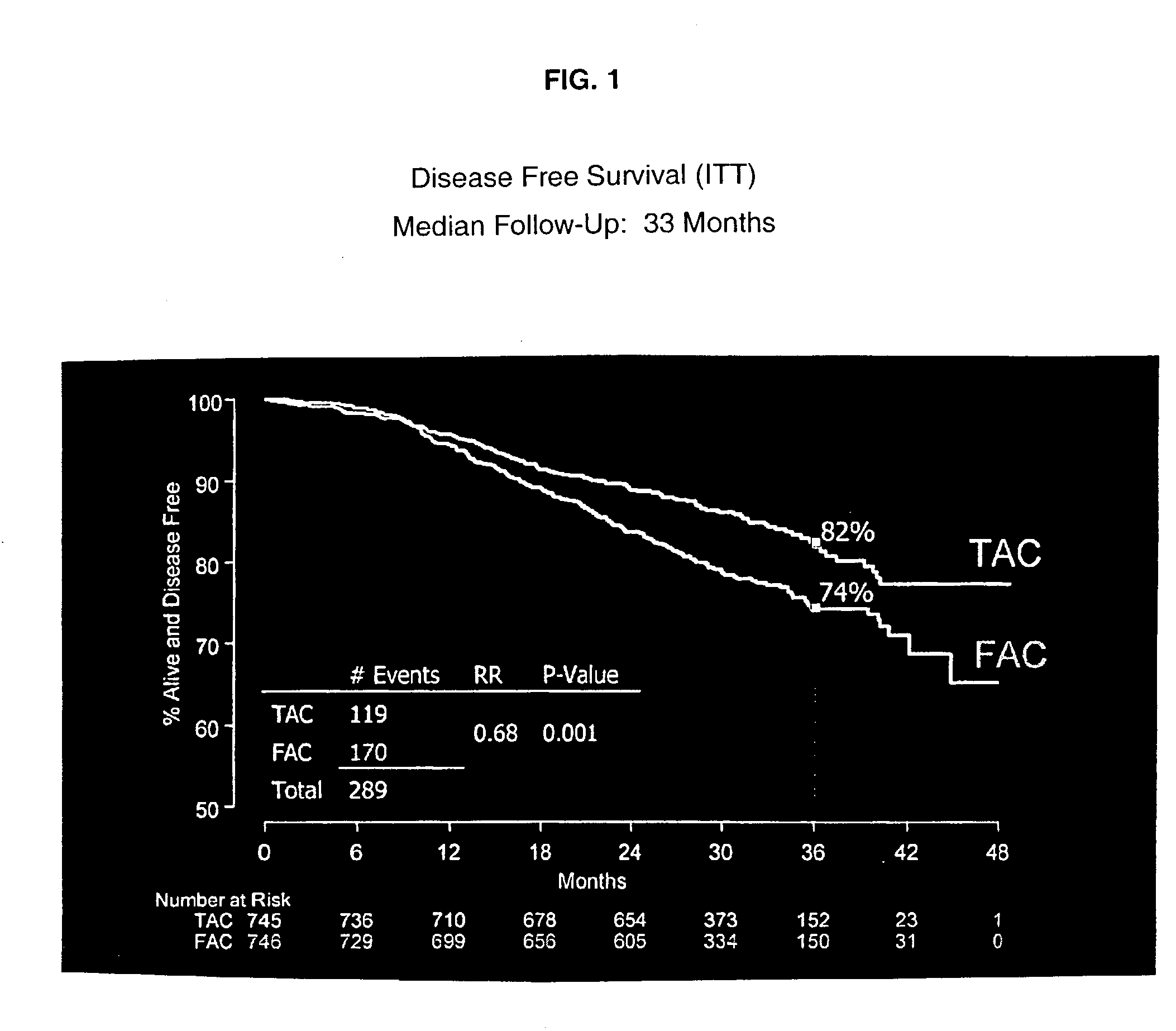
Use of docetaxel/doxorubicin/cyclophosphamide in adjuvant therapy
The present invention relates to a new method of adjuvant therapy in the treatment of early breast cancer, comprising administering six cycles of docetaxel, doxorubicin and cyclophosphamide to a patient in need thereof, wherein said dosages have a marked therapeutic effect when compared to other adjuvant therapies.
Owner:AVENTIS PHARMA SA (US)
Stable Pharmaceutical Composition Containing Docetaxel and a Method of Manufacturing the Same
InactiveUS20090163574A1Improve stabilityEasily prepared into injectionOrganic active ingredientsBiocideDocetaxel-PNPDocetaxel
The present invention relates to a stable pharmaceutical composition for injection containing docetaxel and a method of preparing the same. More particularly, the present invention relates to a pharmaceutical composition for injection containing docetaxel having better storage stability than conventional medications, which is prepared by dissolving docetaxel, a water-insoluble compound, in distilled water after mixing it with cyclodextrin (CD) and a water-soluble polymer such as hydroxypropyl methylcellulose (HPMC), polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP) and lyophilizing the mixture, and a method of preparing the same.
Owner:SK CHEM CO LTD
Process of purification of paclitaxel and docetaxel
InactiveUS20030225291A1Simple and cost-effectiveImprove scalabilityOrganic chemistryAlkaneDocetaxel-PNP
A process for the purification of paclitaxel comprising: (a) mixing crude paclitaxel with a mixture of solvents such as alkane and chlorinated alkane, filtering the solid followed by drying to obtain paclitaxel of increased purity; (b) repeating step (a) one or more times to obtain paclitaxel of increased purity; (c) dissolving the solid obtained from step (b) in alkyl ketone followed by adding alkane thereto, filtering and drying the solid thus formed to obtain paclitaxel of increased purity; (d) repeating step (c) one or more times to increase the purity of paclitaxel; (e) dissolving the paclitaxel obtained from step (d) in alkanol and then adding water, filtering and drying the solid thus formed, to obtain paclitaxel of increased purity; and (f) dissolving the solid obtained from step (e) in alkyl ketone, filtering, followed by adding alkane to the filtrate, filtering and drying the solid thus formed to obtain pure paclitaxel.
Owner:DABUR PHARM LTD
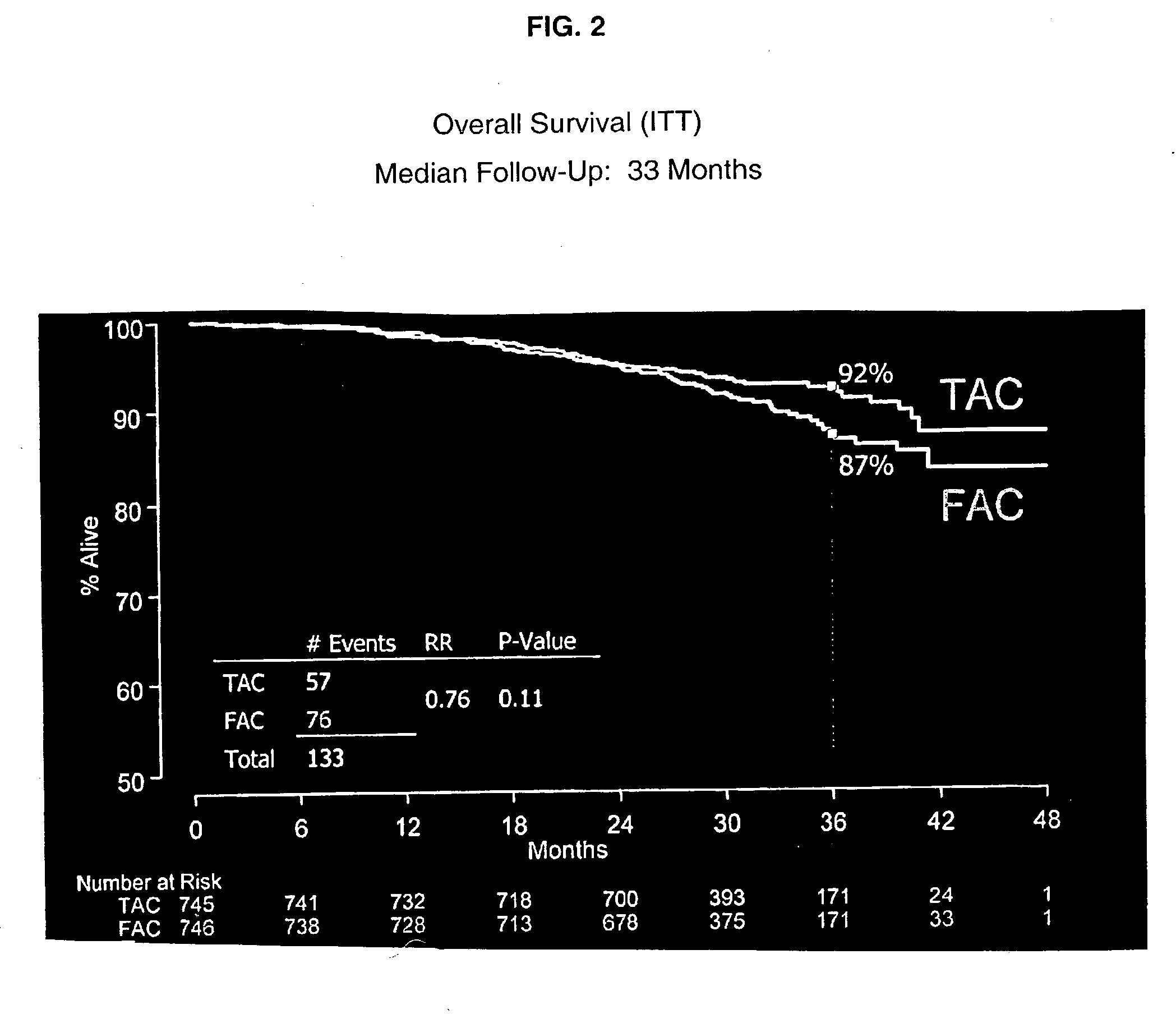
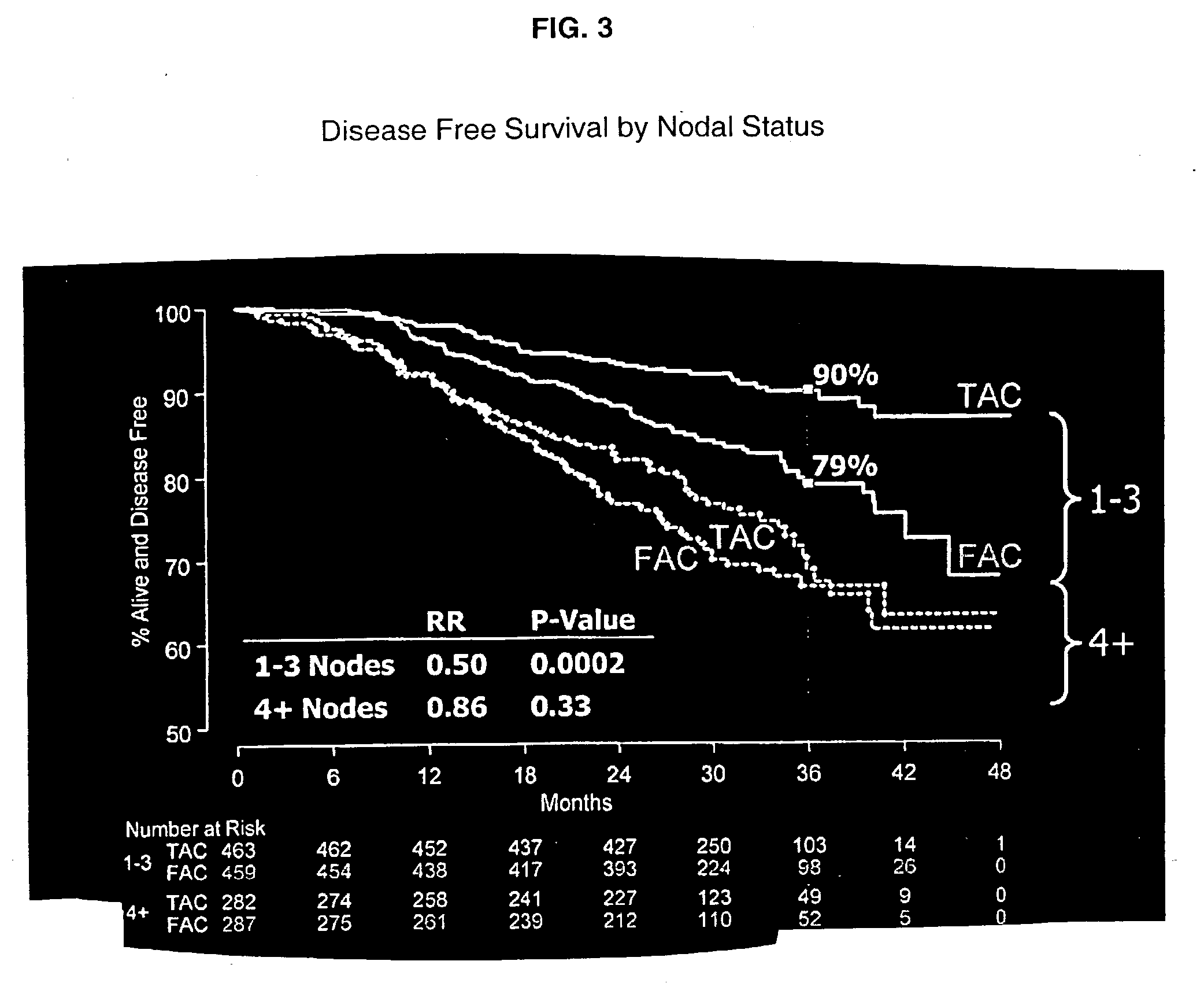
Prediction of Breast Cancer Response to Taxane-Based Chemotherapy
InactiveUS20090239223A1High degree of correlationReliable distinctionMicrobiological testing/measurementDocetaxel-PNPDocetaxel
The invention relates to methods and kits for the prediction of a likely outcome of chemotherapy in a cancer patient. More specifically, the invention relates to the prediction of tumour response to chemotherapy based on measurements of expression levels of a small set of marker genes. The set of marker genes is useful for the identification of breast cancer subtypes responsive to taxane based chemotherapy, such as e.g. a taxane-anthracycline-cyclophosphamide-based (e.g. Taxotere (docetaxel)-Adriamycin (doxorubicin)-cyclophosphamide, i.e. (TAC)-based) chemotherapy.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Mesoporous silica nano-preparation and its preparation method and use
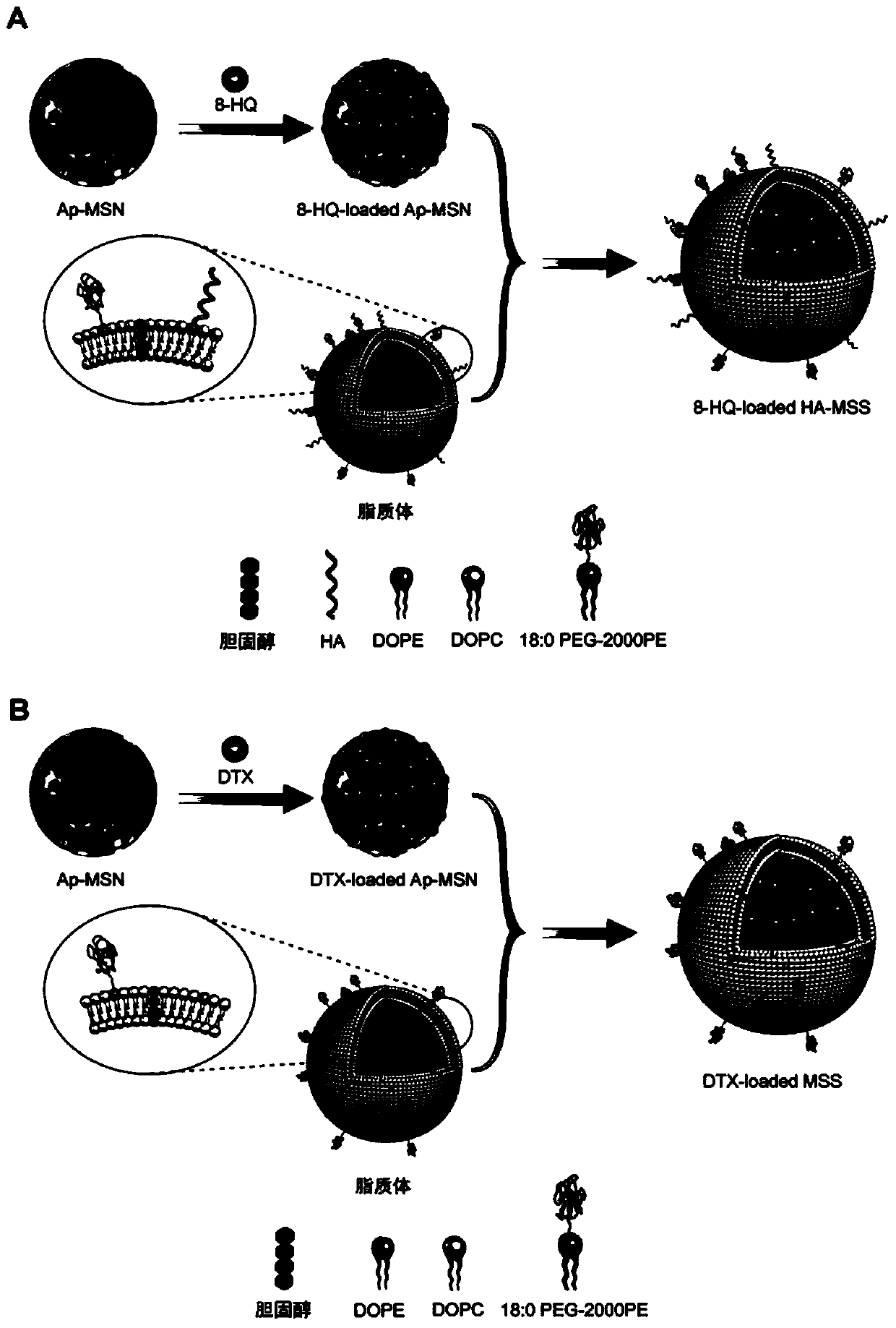
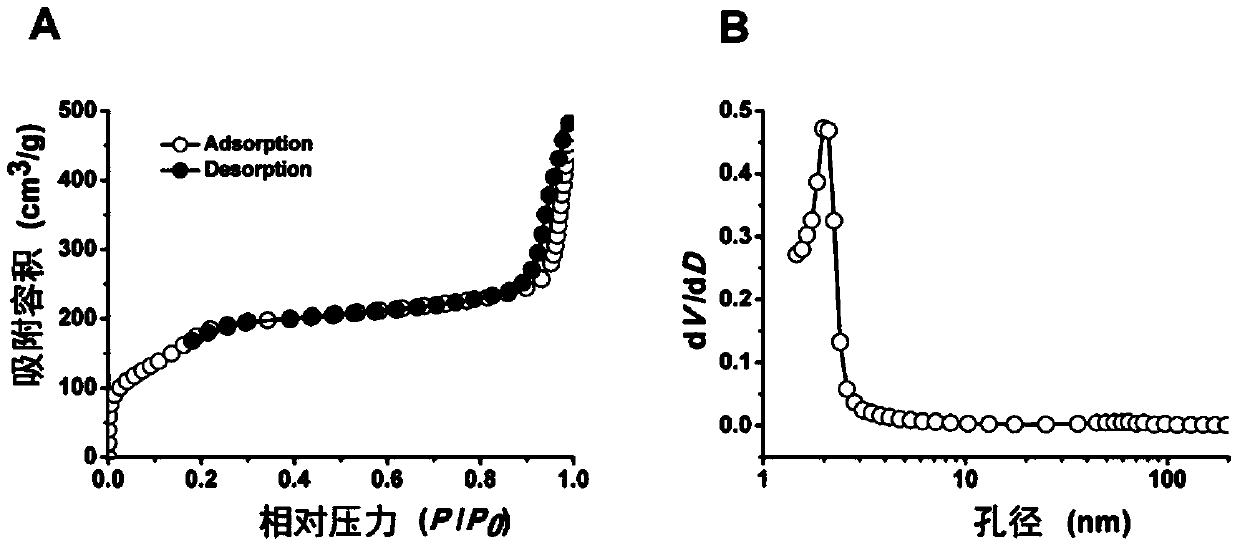
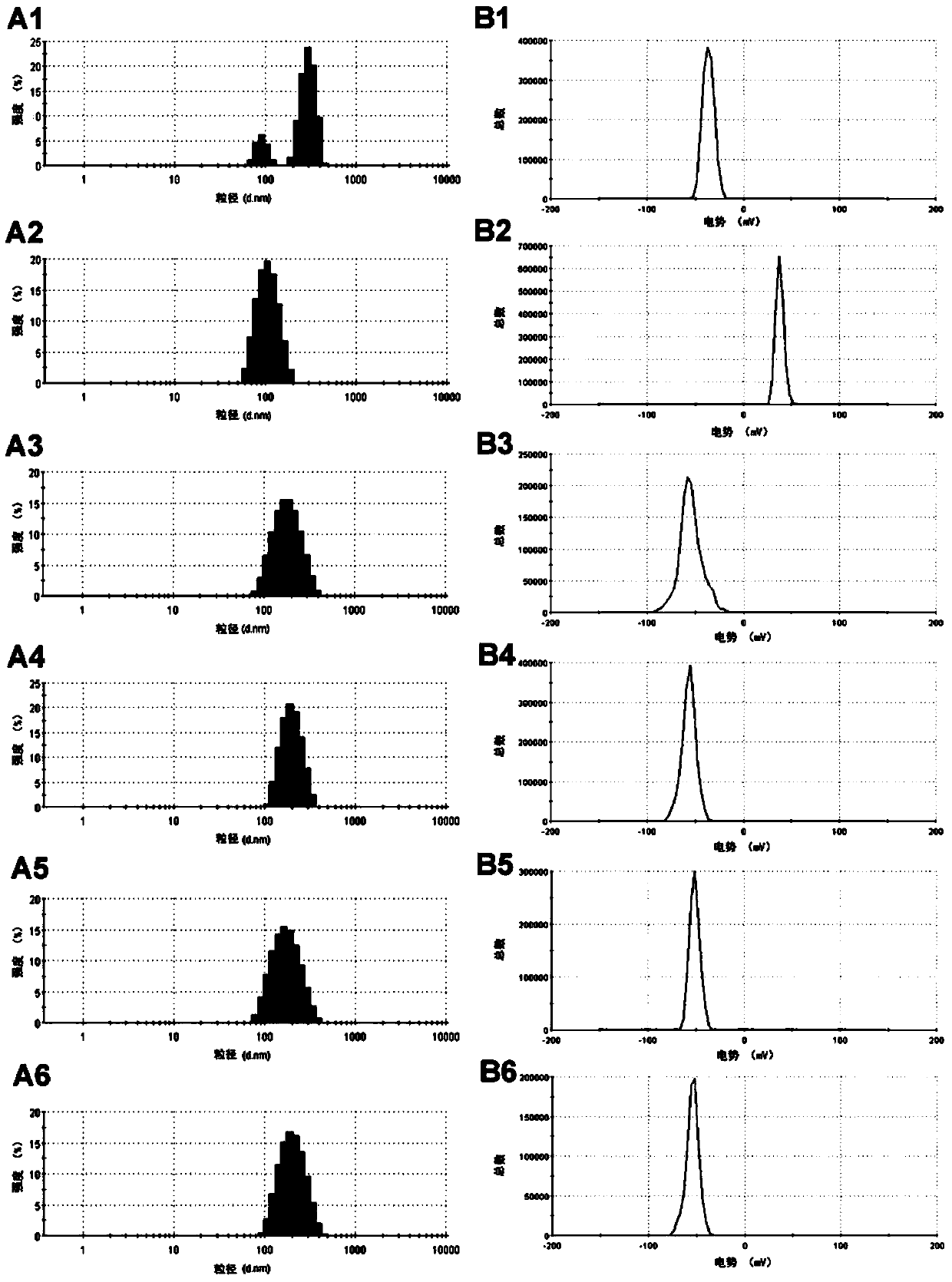
InactiveCN103990130ANo releaseQuick releaseNanomedicinePharmaceutical non-active ingredientsDocetaxel-PNPDocetaxel
The invention discloses a mesoporous silica nano-preparation. The mesoporous silica nano-preparation comprises docetaxel or 8-hydroxyquinoline, mesoporous silica nanoparticles, and a lipid double-molecule layer. The mesoporous silica nanoparticles carry docetaxel or 8-hydroxyquinoline. The surfaces of the mesoporous silica nanoparticles carrying docetaxel or 8-hydroxyquinoline are coated with the lipid double-molecule layer. The invention also discloses a preparation method and a use of the mesoporous silica nano-preparation. The mesoporous silica nano-preparation carrying docetaxel or 8-hydroxyquinoline can substantially improve antitumor activity of a drug, can effectively reduce tumor recurrence and nonspecific toxicity and has a very wide application prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Gamma radiation sterilized nanoparticulate docetaxel compositions and methods of making same
Owner:ELAN PHRMA INT LTD
Water soluble paclitaxel derivatives
InactiveUS7060724B2Surprising antitumor activityImprove efficacyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
Combination therapy for cancer
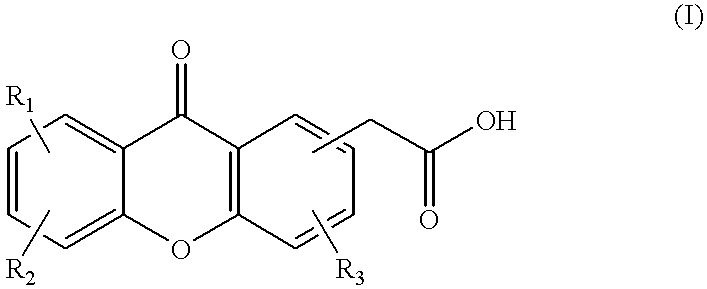
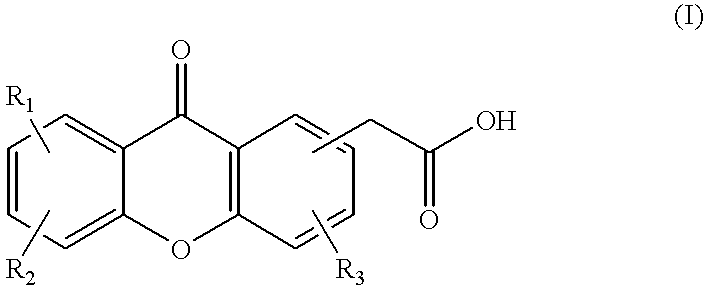
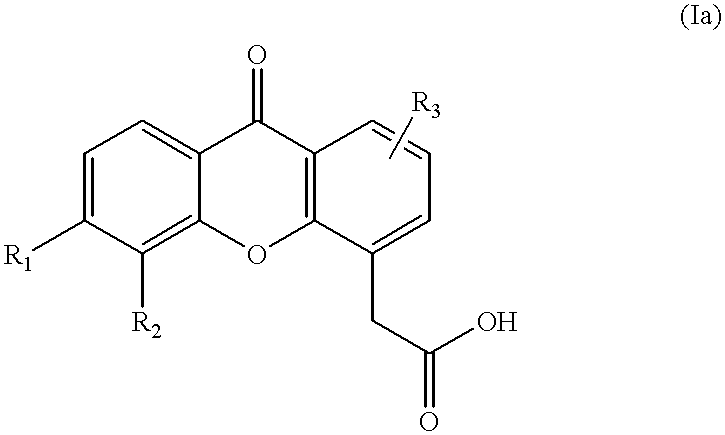
This invention relates to a method of treating cancer, and particularly a method including the steps of administering to a mammal in need of such treatment, either simultaneously or sequentially, (i) a compound selected from a paclitaxel and docetaxel, and (ii) a compound of the formula or a pharmaceutically acceptable salt or ester thereof; wherein R1, R2 and R3 are each independently selected from the group consisting of H, C1-C6 alkyl, halogen, CF3, CN, NO2, NH2, OH, OR, NHCOR, NHSO2R, SR, SO2R or NHR, wherein each R is independently C1-C6 alkyl optionally substituted with one or more substituents selected from hydroxy, amino and methoxy, and wherein each of R1, R2 and R3 may be present at any of the available positions 1 to 8; and wherein in each of the carbocyclic aromatic rings in formula (I), up to two of the methine (-CH=) groups may be replaced by an aza (-N=) group; and wherein any two of R1, R2 and R3 may additionally together represent the group -CH =CH.CH =CH-, such that this group, together with the carbon or nitrogen atoms to which it is attached, forms a fused 6 membered aromatic ring.
Owner:CANCER RES TECH LTD
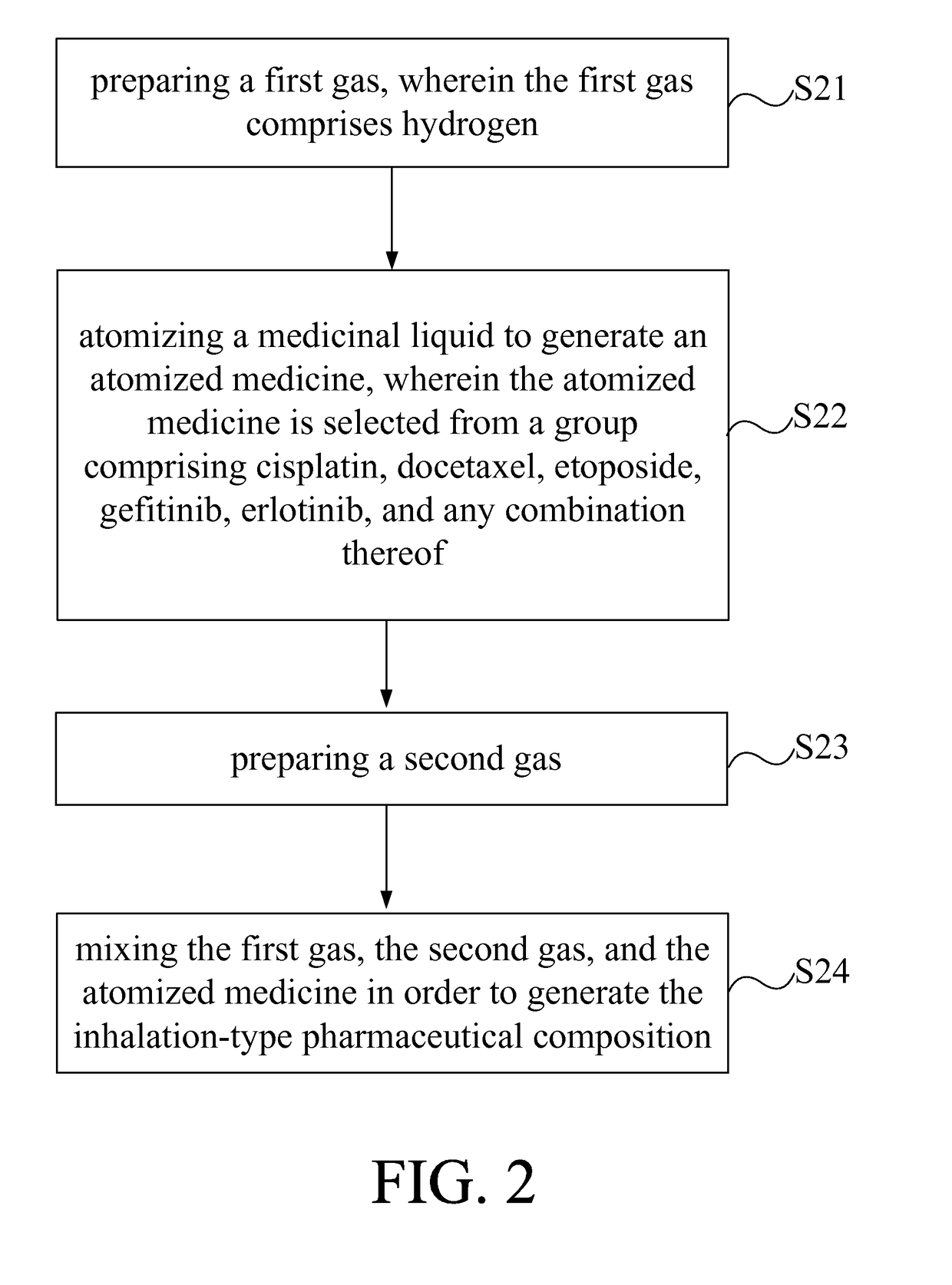
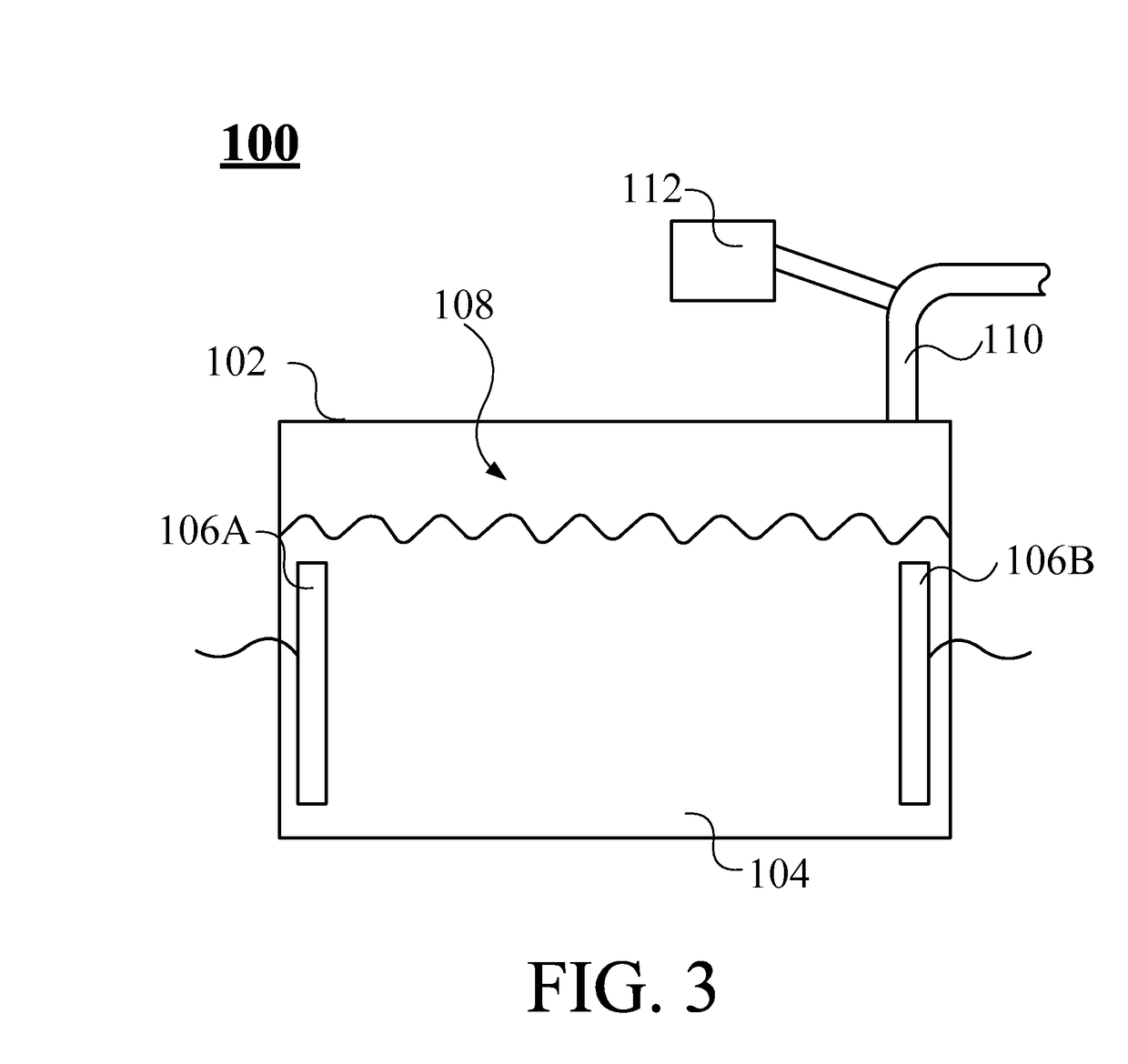
Inhalation-type pharmaceutical composition for the treatment of lung cancer and preparation method thereof
ActiveUS9763946B2Promote absorptionGood curative effectPowder deliveryOrganic active ingredientsSide effectErlotinib
The present invention provides an inhalation-type pharmaceutical composition for lung cancer and preparation method thereof, comprising a first gas and an atomized medicine. The first gas comprises hydrogen. The gas volume concentration of hydrogen in the inhalation-type pharmaceutical composition is between 2 to 96%. The atomized medicine is selected from a group comprising cisplatin, docetaxel, etoposide, gefitinib, erlotinib, and any combination thereof. The inhalation-type pharmaceutical composition of the present invention can remove harmful radicals in the body of the patient through the use of hydrogen while also increases the absorption effect of the medicine for the patient by using an atomized medicine. At the same time, because the use of the small amount of the vaporized pharmaceutical liquid can indirectly reduce the side effects on the user.
Owner:LIN HSIN YUNG
Solubilized formulation of docetaxel without tween 80
InactiveUS20080319048A1Avoid side effectsAvoid hypersensitivityBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Lyophilizates containing docetaxel and the use thereof in preparing concentrated liquid formulations, and ready to use formulations for injection, as well as such concentrates and ready to use formulations themselves are disclosed in which Tween surfactants are avoided so that hypersensitivity reactions to Tween surfactants can be avoided and docetaxel can be administered at higher doses and / or for longer periods of time and / or for additional treatment cycles.
Owner:SCIDOSE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com