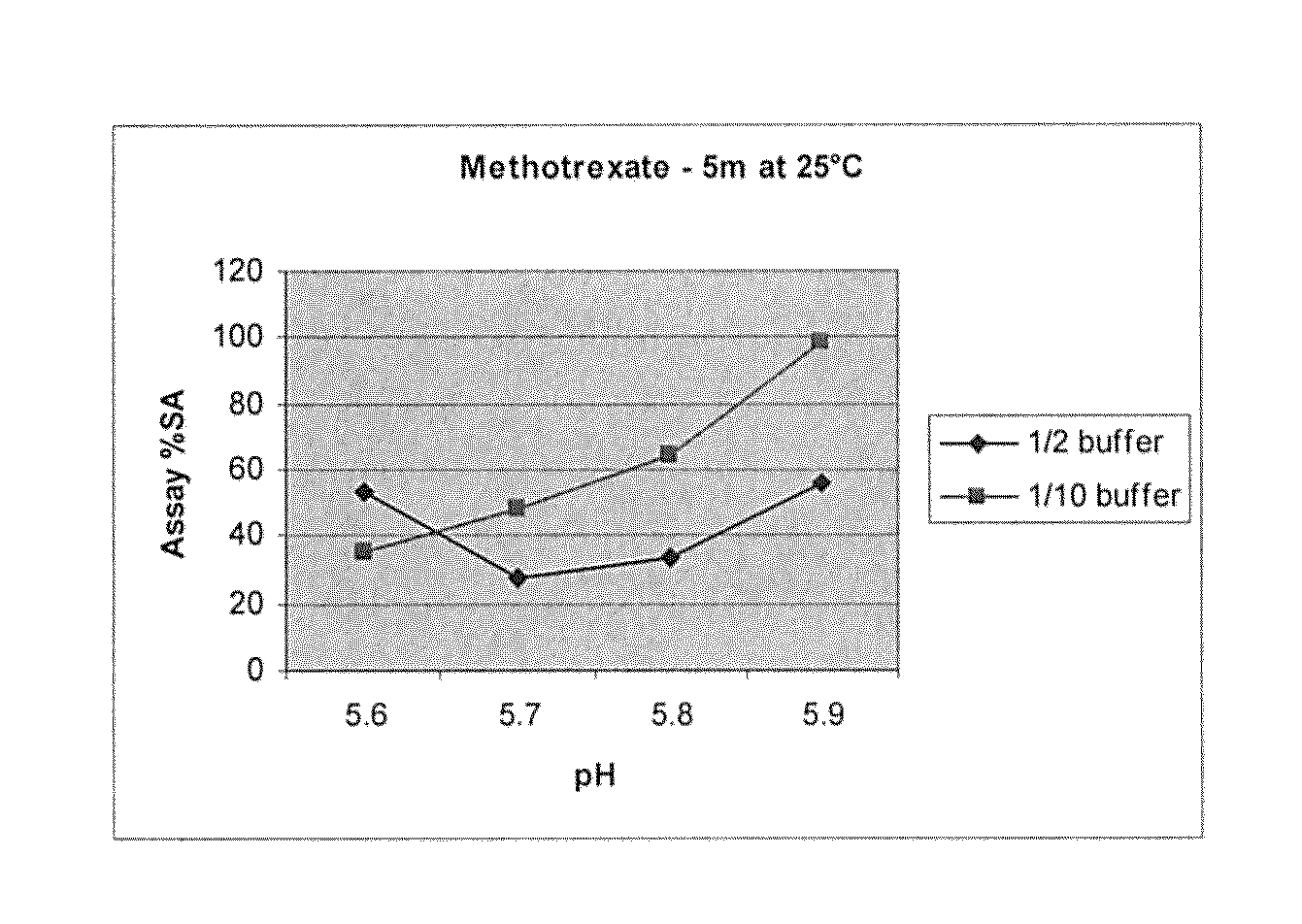
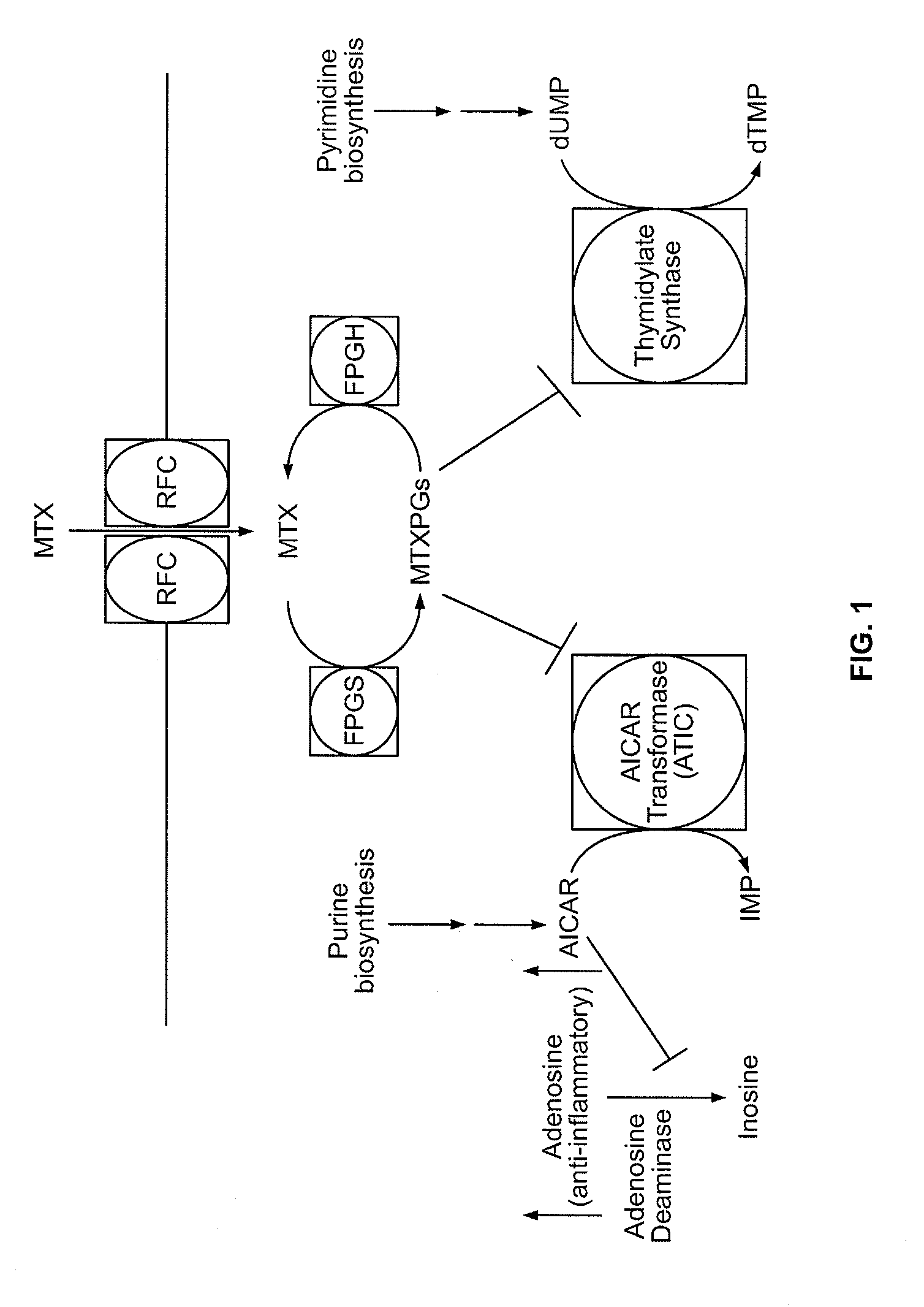
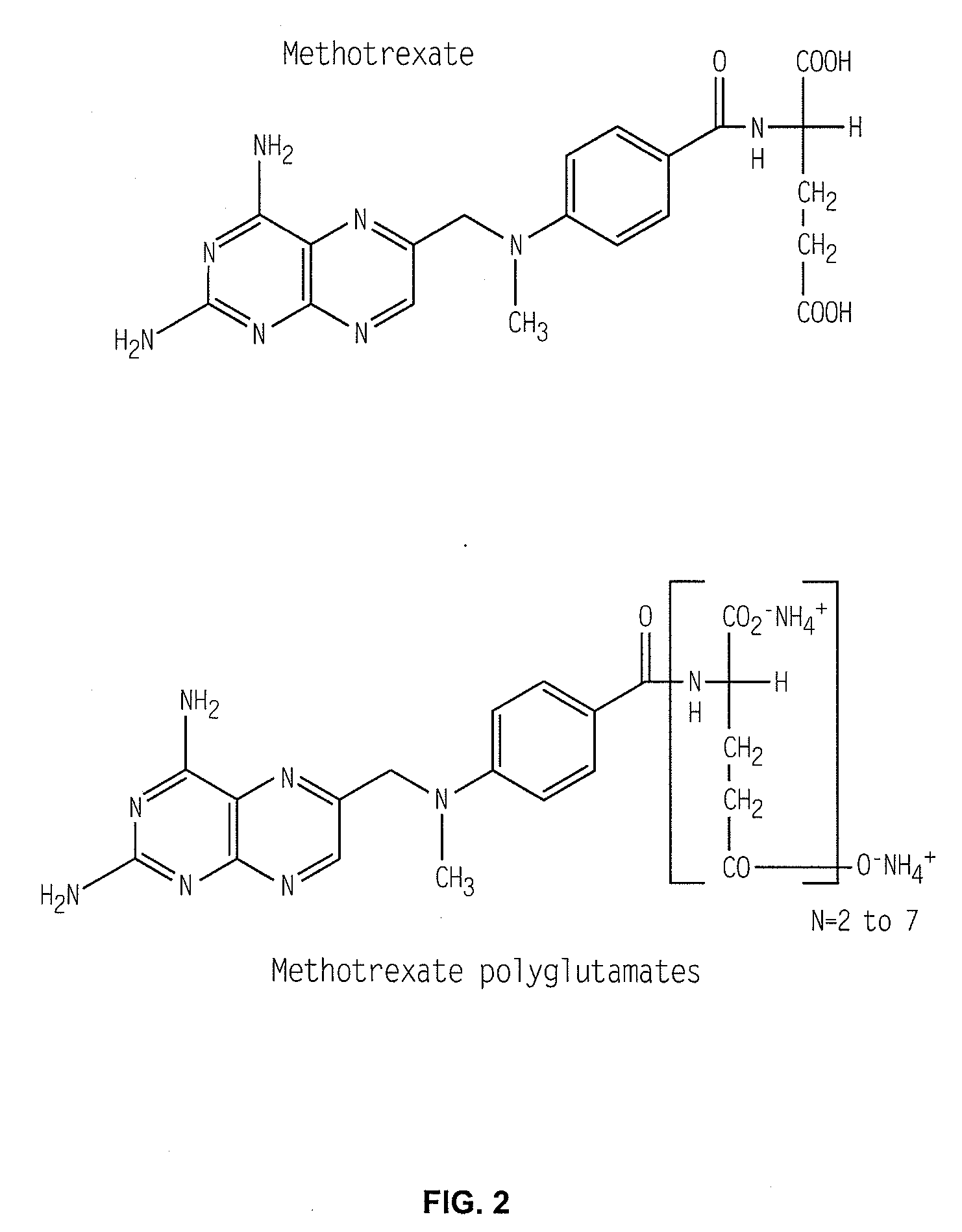
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
155 results about "Methotrexate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methotrexate is used to treat certain types of cancer or to control severe psoriasis or rheumatoid arthritis that has not responded to other treatments. It may also be used to control juvenile rheumatoid arthritis.
Corynebacterium glutamicum and production method of alpha-ketoglutarate through fermentation thereof
ActiveCN102391977AReduce manufacturing costThe fermentation process is simpleBacteriaMicroorganism based processesMethotrexateNitrogen source
Belonging to the technical field of bioengineering, the invention relates to corynebacterium glutamicum and a production method of alpha-ketoglutarate through fermentation of corynebacterium glutamicum. The bacterial strain of the invention is corynebacterium glutamicum GKG-047, with a preservation number of CGMCC No. 5481. With a strain of glutamic acid producing strain GDK-9 as a parent, the bacterial strain of the invention is a mutant strain that is screened out through mutagenesis and sensitive to methotrexate. Under the circumstance of a limited nitrogen source supply, the bacterial strain can accumulate alpha-ketoglutarate. During fermentation, the bacterial strain is characterized by aerobic fermentation, fast bacterium growth, and rapid acid production rate. After fermentation of the bacterial strain for 32h in a fermentation tank of 10L, the maximum output of alpha-ketoglutarate can be 47.2g / L. The method provided in the invention has the advantages of simple and easily controllable fermentation process, and low production cost of alpha-ketoglutarate, thus being in favor of the popularization and application of industrial production.
Owner:江苏澳创生物科技有限公司
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
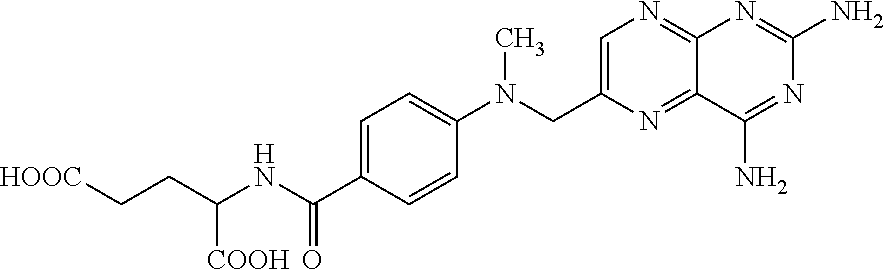
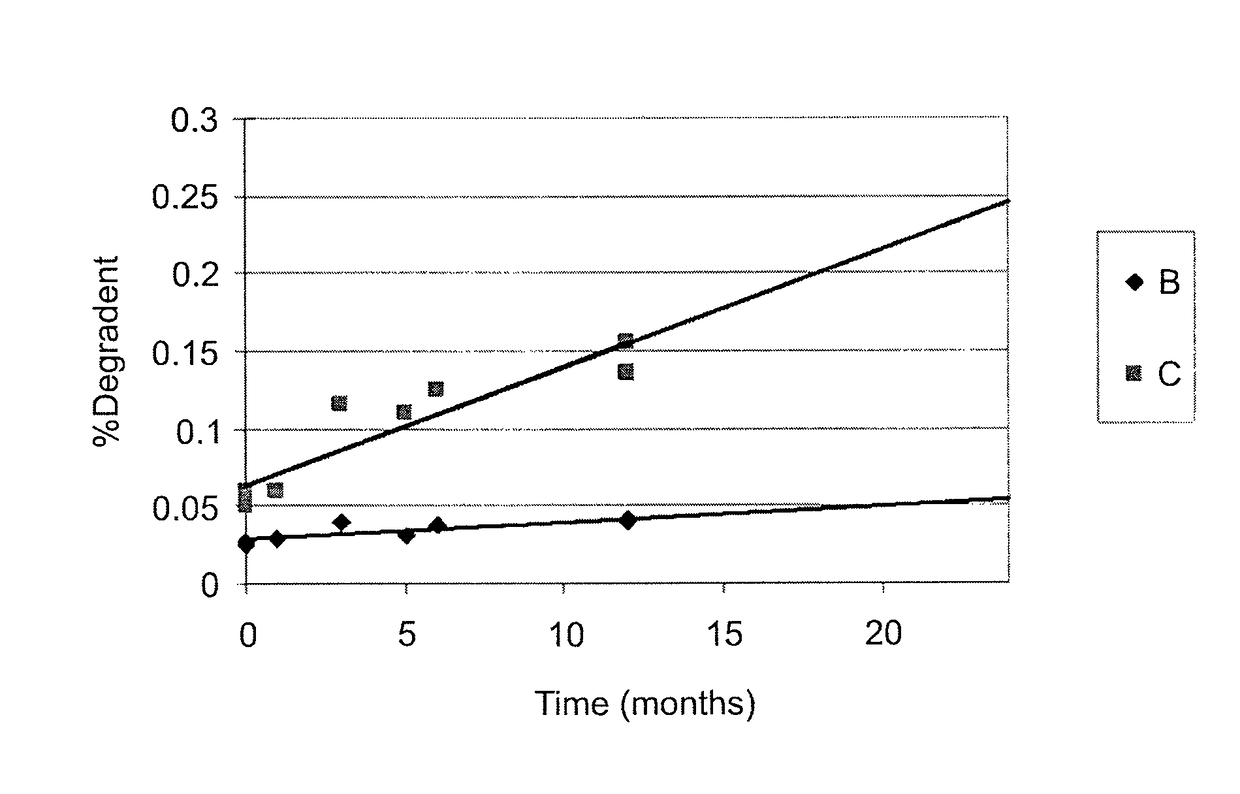
Methotrexate composition
ActiveUS9259427B2Prevent precipitationImprove stabilityOrganic active ingredientsDispersion deliveryMedicineMethotrexate
Owner:ROSEMONT PHARMA LTD
pH sensitive-type Fe3O4 coated LDH methotrexate-loaded nano drug particles, preparation method and application thereof
ActiveCN106552269AThe amount of coating is controllableOvercome the disadvantage of complicated operationOrganic active ingredientsPowder deliveryMedicineBiocompatibility Testing
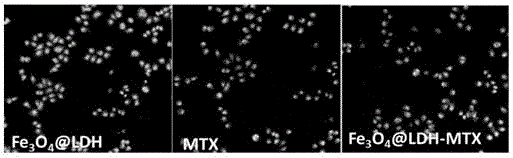
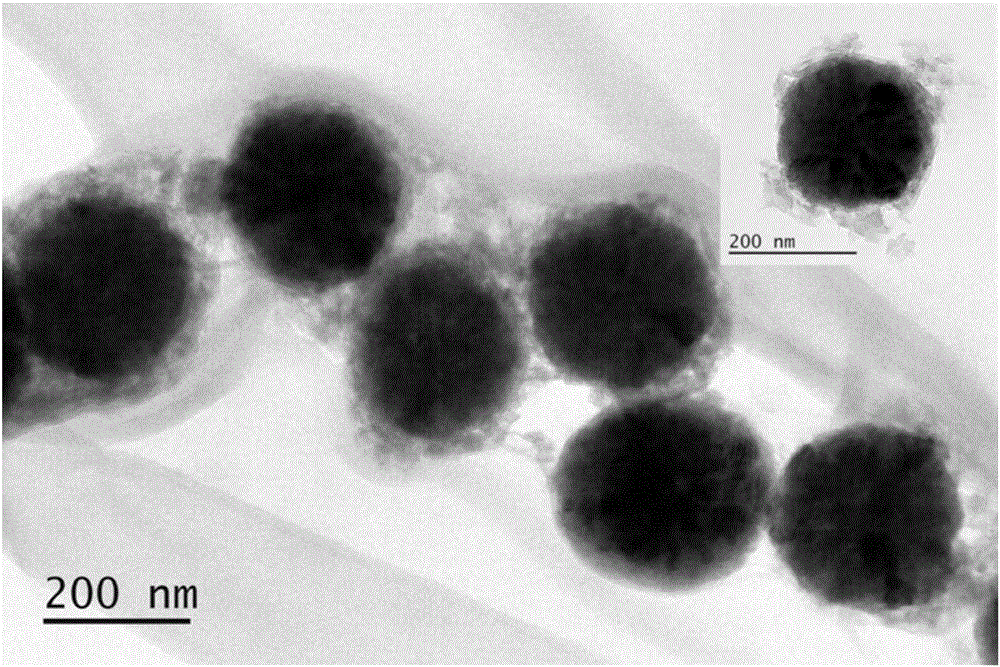
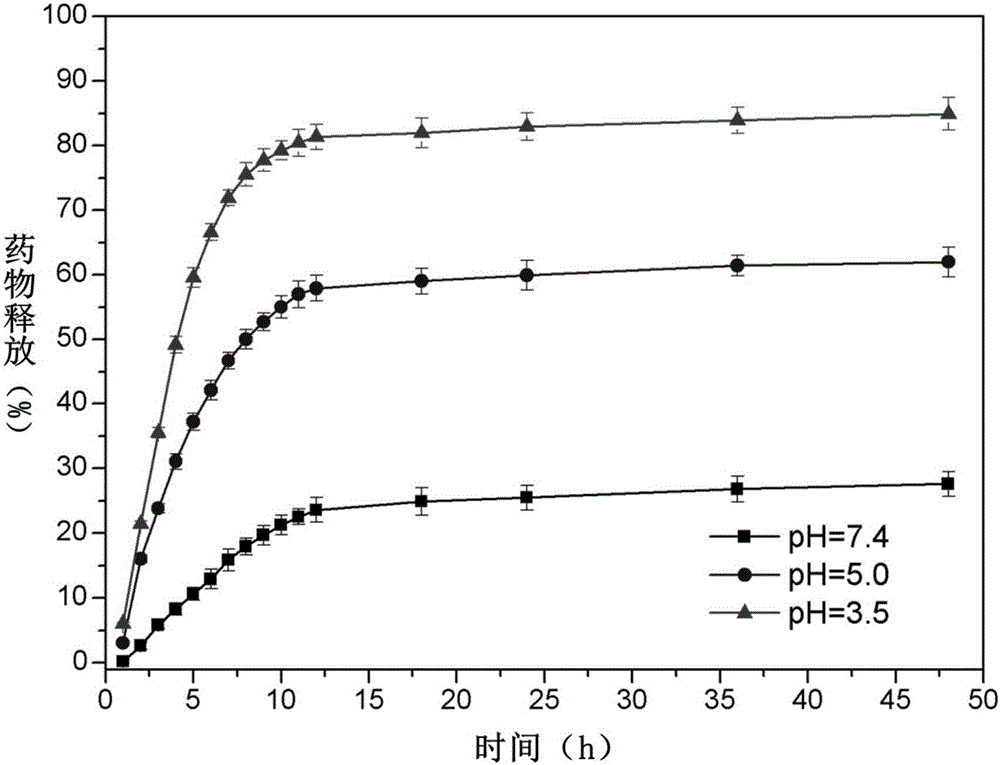
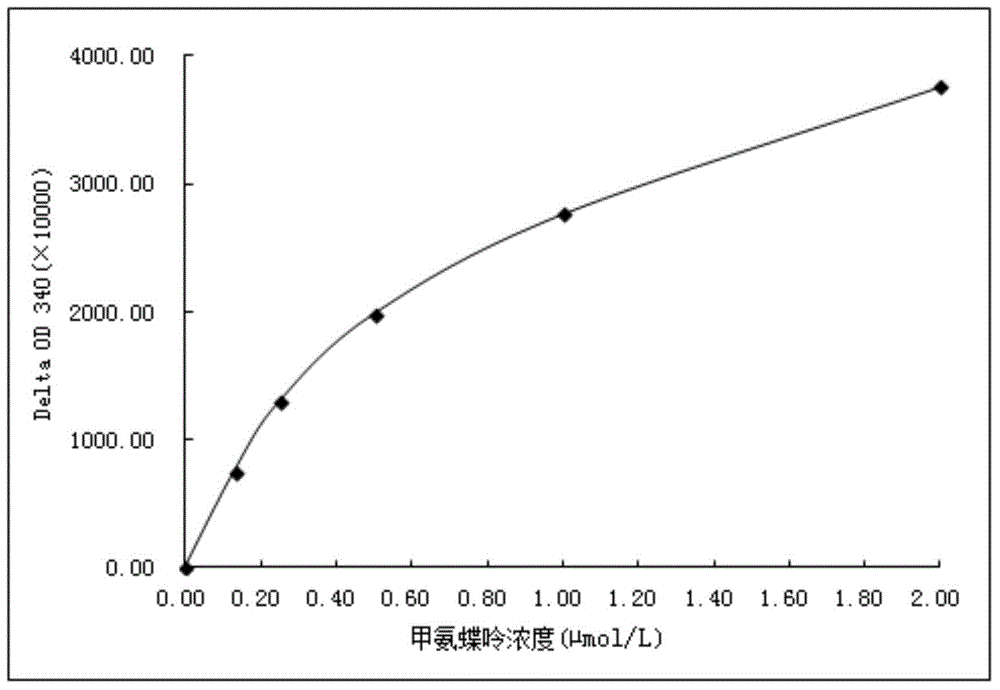
The invention discloses pH sensitive-type Fe3O4 coated LDH methotrexate-loaded nano drug particles. According to the invention, LDH is directly added in a preparation system of Fe3O4 nanoparticles, while the Fe3O4 nanoparticles are formed, at the same time, the surface of the Fe3O4 nanoparticles is coated with LDH through a self-assembling mode, a Fe3O4 coated LDH carrier is formed, and MTX can effectively loaded on the Fe3O4 coated LDH carrier through a subject and object exchange technology. The employed LDH can increase the biocompatibility of the Fe3O4 nanoparticles, and a special structure of LDH can greatly increase the load capacity of an anticancer medicine. The prepared Fe3O4 coated LDH-MTX has excellent medicine slow release performance, has obvious inhibition effect on tumor cells, has good antineoplastic effect, and has large application potential in the field of targeting tumour treatment.
Owner:NANJING UNIV OF SCI & TECH
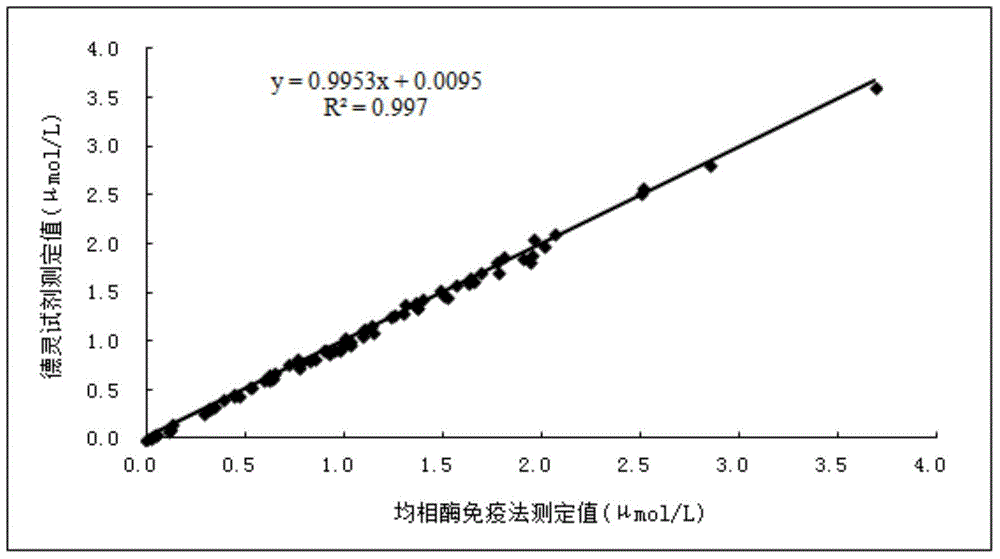
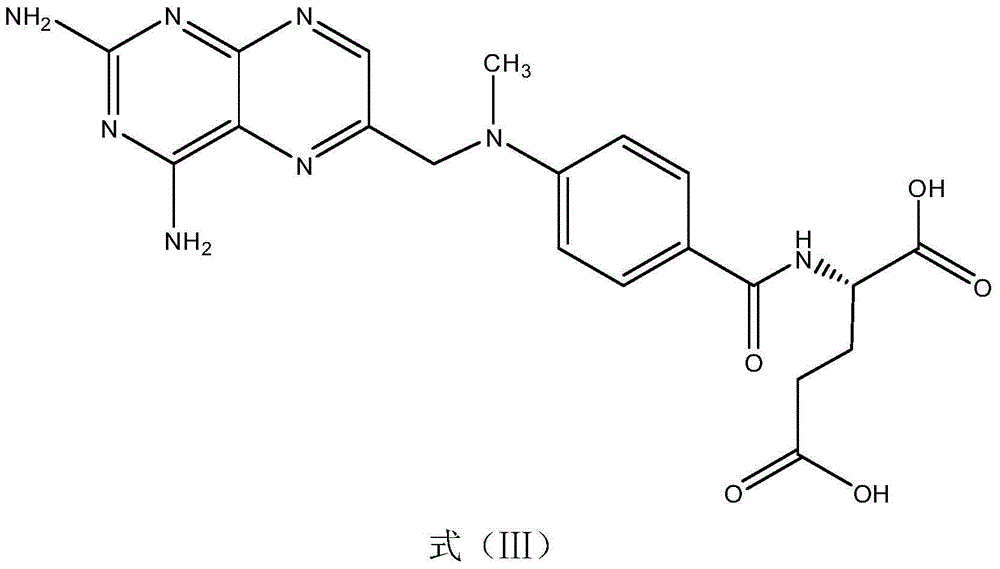
Methotrexate homogenous enzyme immunoassay reagent as well as preparation method and detection method thereof
ActiveCN104569373AStrong immunogen specificityStrong specificityMaterial analysisHigh fluxMethotrexate
The invention relates to a methotrexate detection reagent as well as a preparation method and a detection method thereof, and specifically relates to a methotrexate homogenous enzyme immunoassay reagent as well as a preparation method and a detection method thereof. The methotrexate homogenous enzyme immunoassay reagent comprises an anti-methotrexate specific antibody, and an indication reagent for detecting an anti-methotrexate specific antibody-methotrexate compound, wherein the anti-methotrexate specific antibody is obtained from immune animals with methotrexate immunogen. The methotrexate homogenous enzyme immunoassay reagent disclosed by the invention has the following beneficial effects: the methotrexate immunogen is high in specificity and immunogenicity, and the prepared anti-methotrexate specific antibody is high in specificity and valence, and free from any cross reaction with 62 common medicines; the homogenous enzyme immunoassay reagent containing the anti-methotrexate specific antibody is capable of conveniently, rapidly and accurately determining the content of methotrexate in a sample and measuring a plurality of samples on a fully-automatic biochemical analyser to realize high-flux rapid measurement for methotrexate, is high in accuracy and high in specificity, and is capable of greatly improving the accuracy and the detection efficiency.
Owner:苏州博源医疗科技有限公司
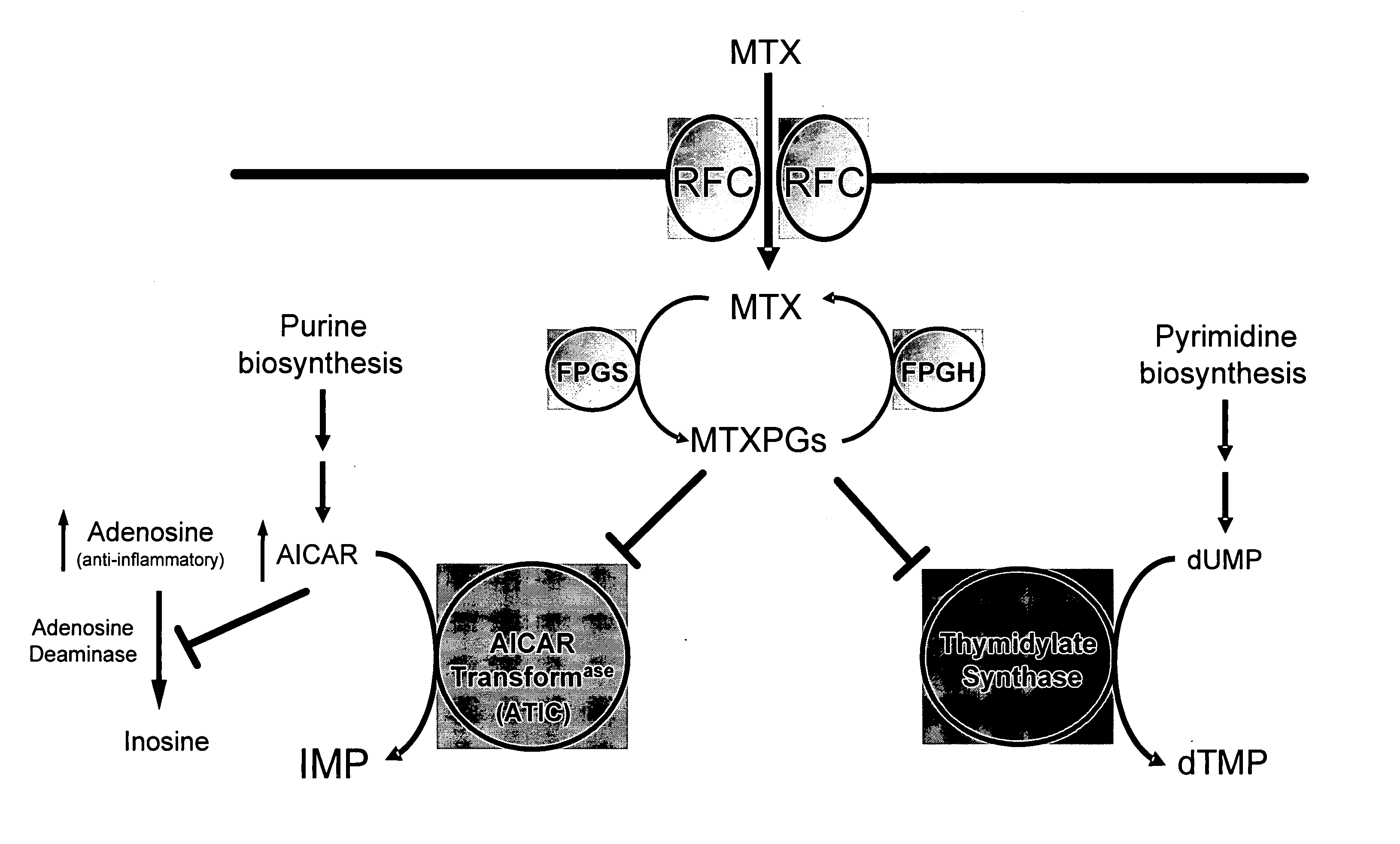
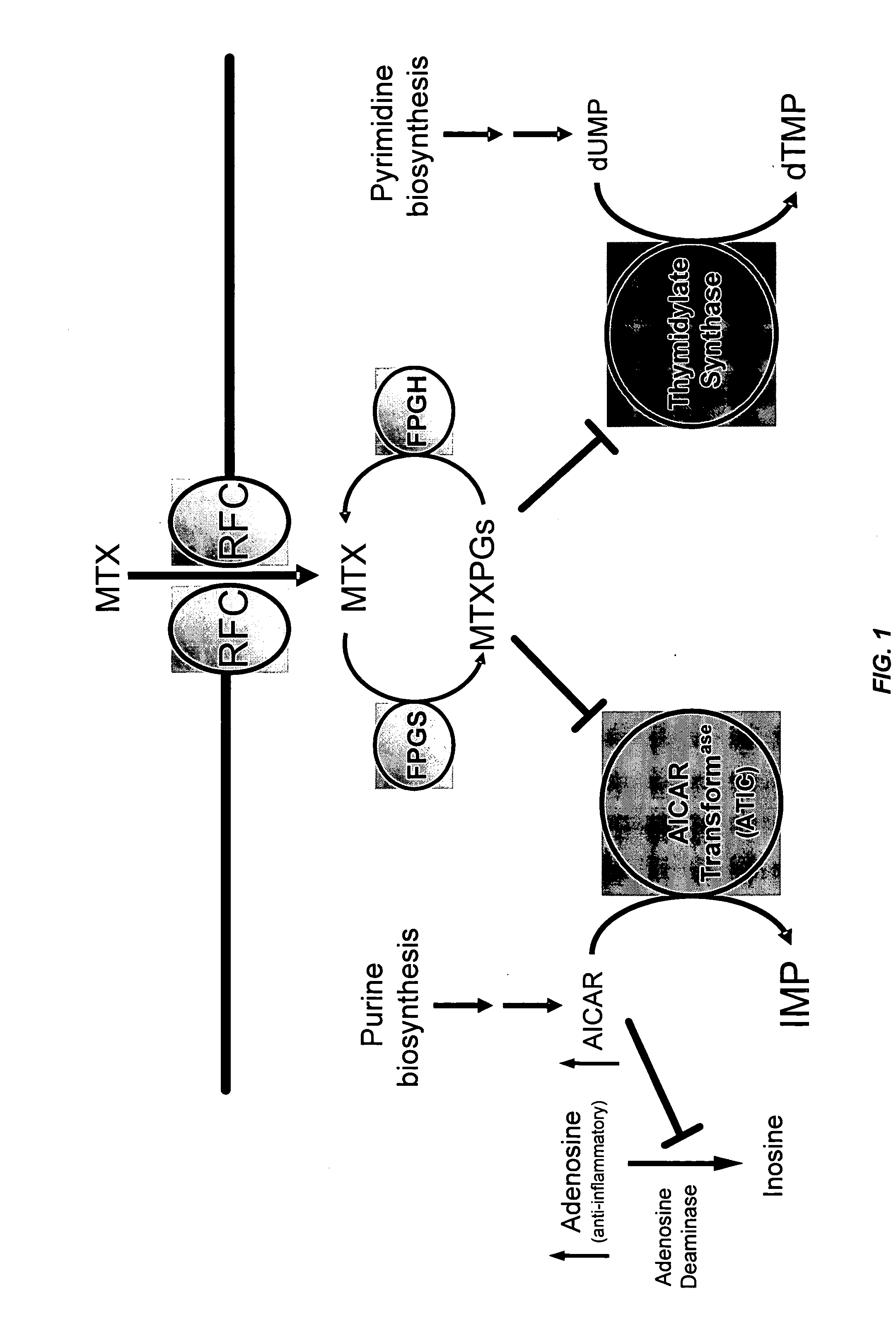
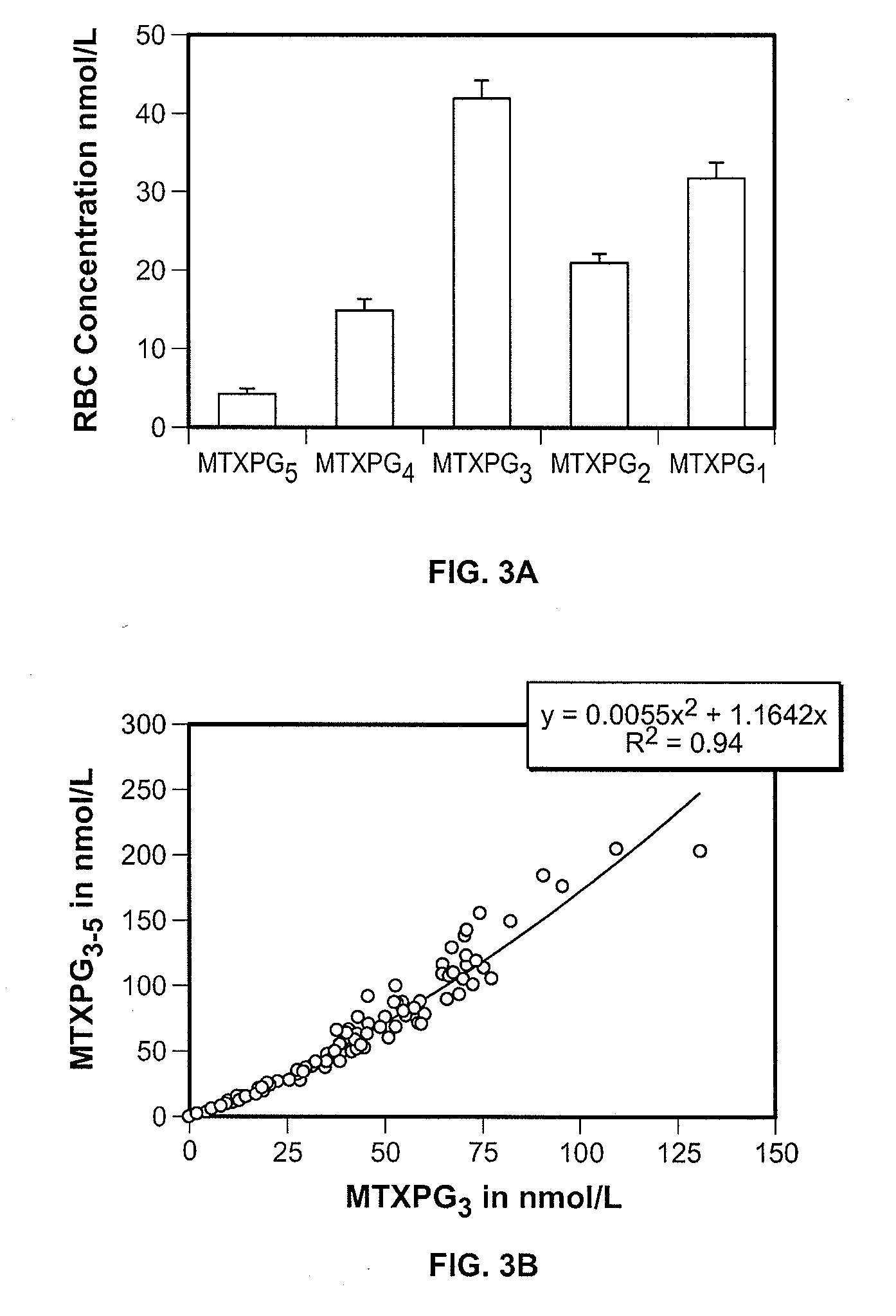
Methods for optimizing clinical responsiveness to methotrexate therapy using metabolite profiling and pharmacogenetics
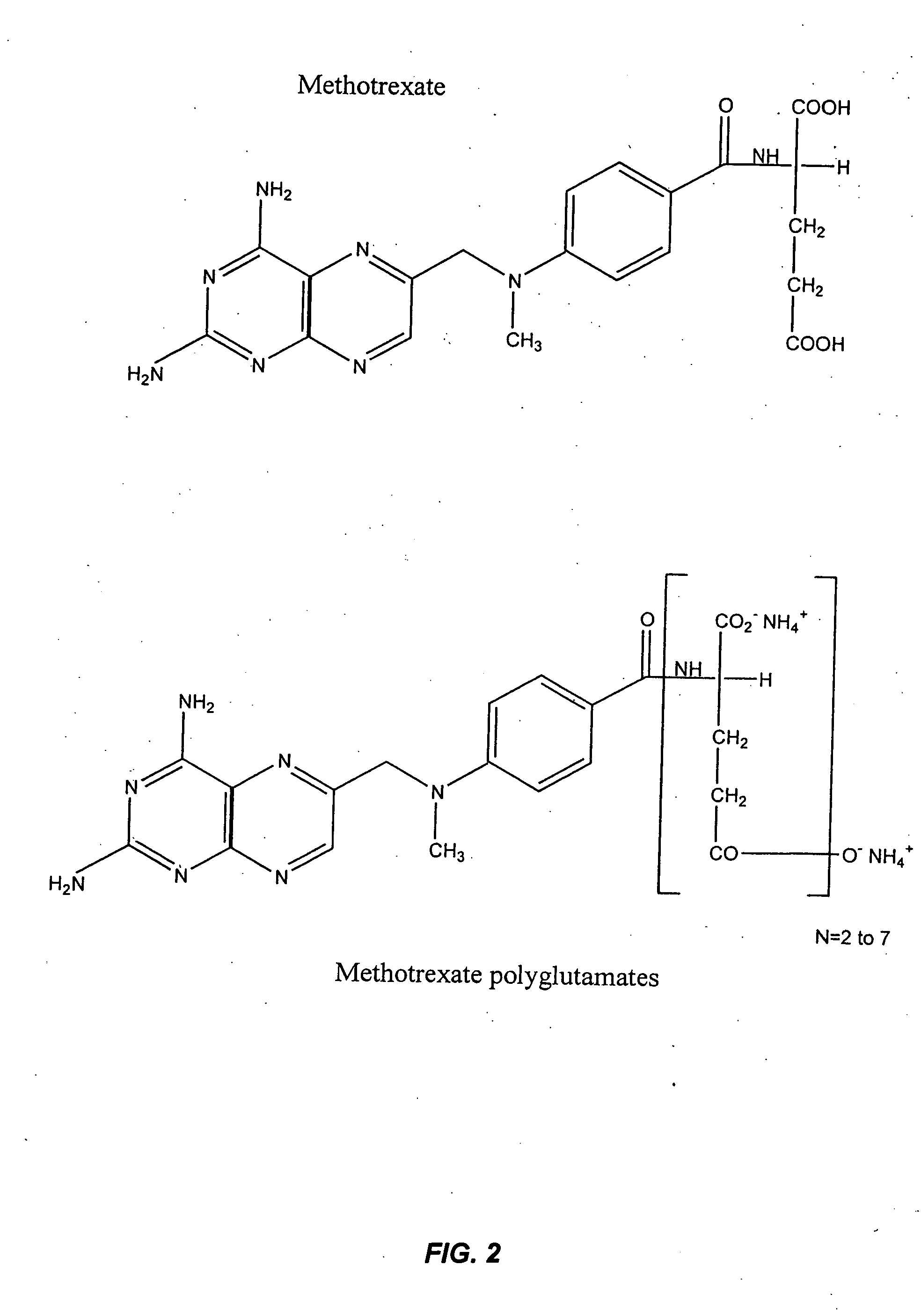
The present invention provides methods for optimizing clinical responsiveness to chemotherapy in an individual through genotypic analysis of polymorphisms in at least one gene. The methods of the present invention may further comprise determining the level of at least one long-chain methotrexate polyglutamate (MTXPG) in a sample obtained from the individual. The present invention also provides methods for generating a pharmacogenetic index for predicting clinical responsiveness to chemotherapy in an individual through genotypic analysis of polymorphisms in at least one gene. In addition, the present invention provides methods for optimizing therapeutic efficacy of chemotherapy in an individual by calculating the level of at least one long-chain MTXPG in a sample obtained from the individual.
Owner:EXAGEN INC
Low Fucose Cell Lines And Uses Thereof
A method of selecting cells having zero fucose level useful as host cells for expressing recombinant proteins is disclosed. The method comprises: (d) introducing genetic mutations into a population of CHO cells by contacting the cells with a methotrexate (MTX), (e) contacting the population of CHO cells comprising mutated cells with a non-toxic fucose binding agent for an amount of time that allows binding of the fucose binding agent to a fucose moiety on a cell membrane of the population of cells, wherein the amount of time does not allow killing of the cells; and (f) depleting from the population of cells comprising mutated cells, a subpopulation of cells which bind the fucose binding agent, thereby selecting cells useful as host cells for expressing recombinant proteins, the selected cells having zero fucose content. There are also disclosed cells and cell lines useful as host cells for expressing recombinant proteins.
Owner:MERCK SERONO SA
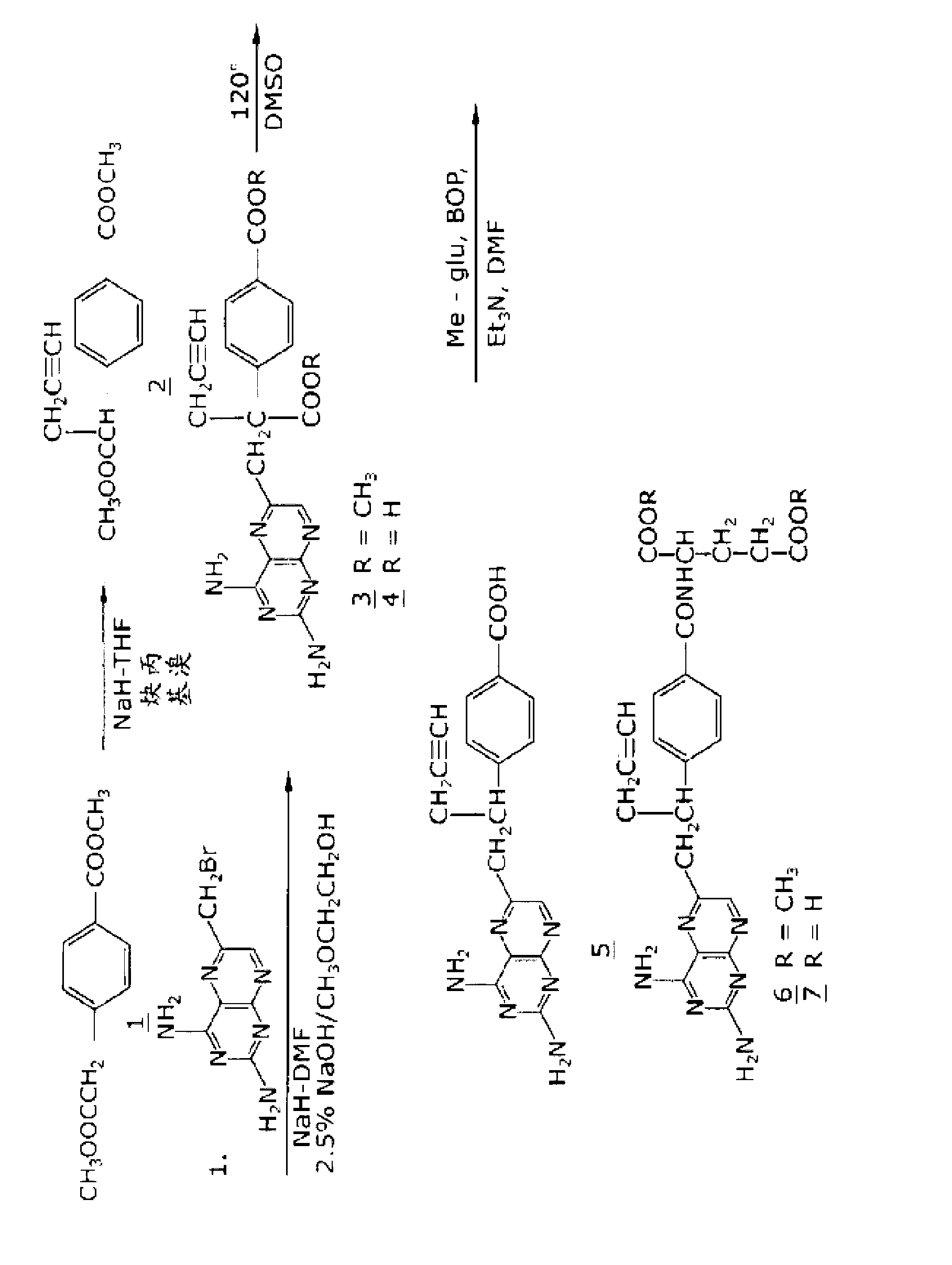
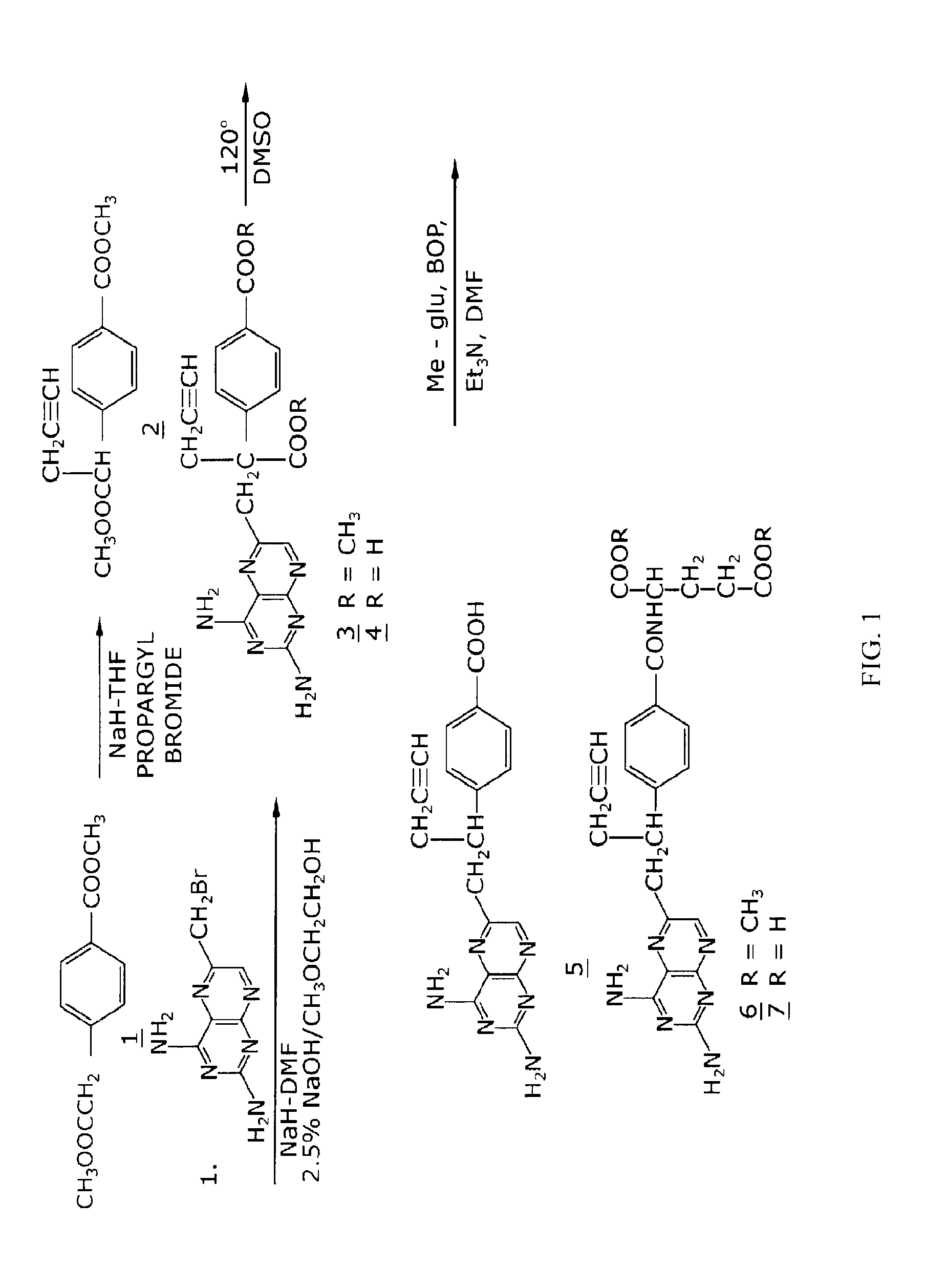
Methods for treating methotrexate-resistant disorders with 10-propargyl-10-deazaaminopterin
The present invention relates to a method for treating a methotrexate-resistant disorder in an individual, wherein the method comprises administering to the individual an effective amount of 10-propargyl-10-deazaaminopterin or its pharmaceutically acceptable salts.
Owner:ALLOS THERAPEUTICS
Methods for reducing microsatellite instability induced by chemotherapy and methods for screening antioxidants that suppress drug-induced microsatellite instability while enhancing the cytotoxicity of chemotherapeutic agents
InactiveUS20160022605A1Reducing microsatellite instabilityStrong cytotoxicityBiocideCompound screeningAntioxidantCiclopirox
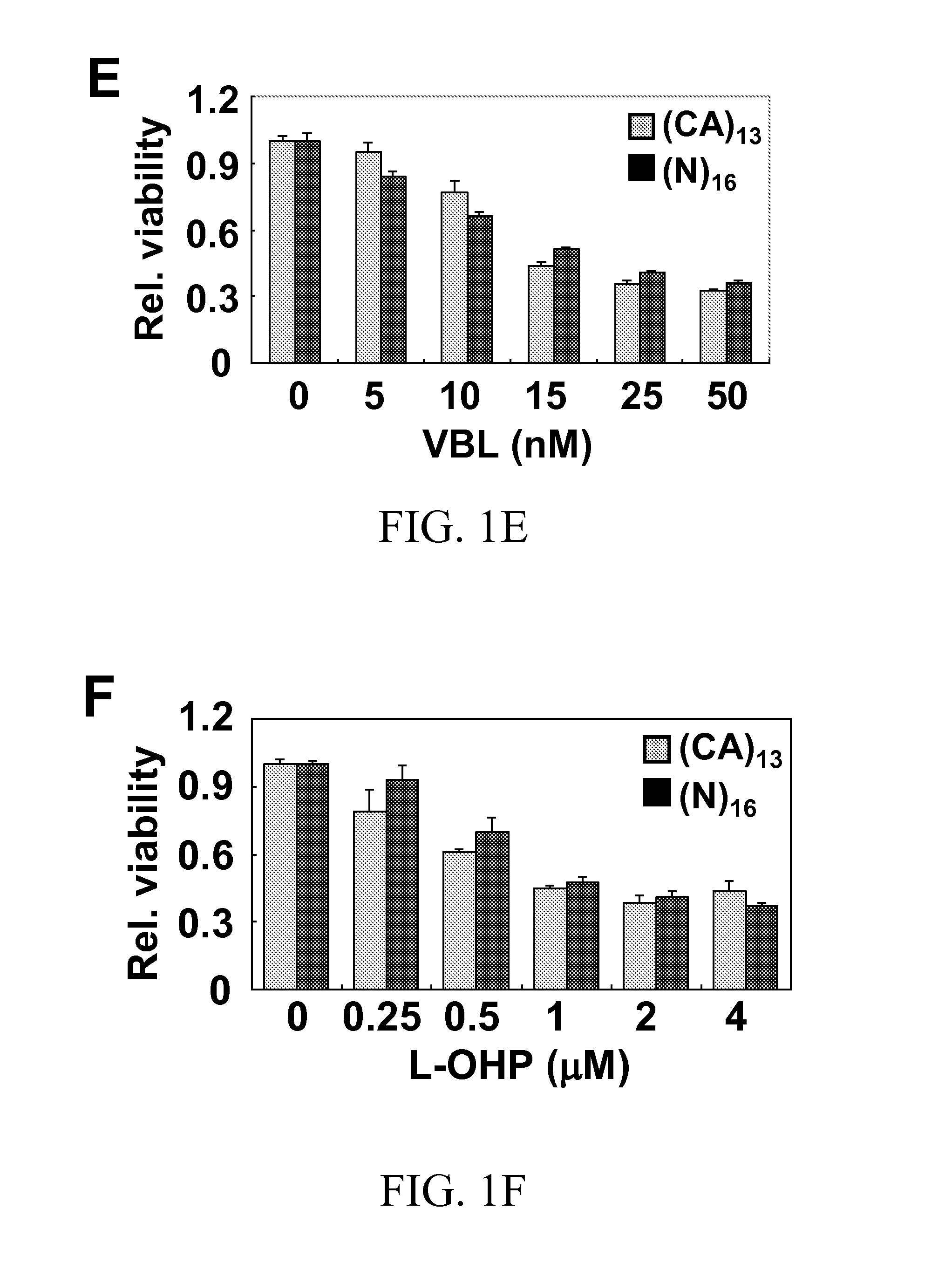
A therapeutic approach to prevent drug resistance and chemotherapy-related secondary cancer associated with DNA mismatch repair (MMR) deficiency is disclosed based on screening antioxidants for reducing microsatellite instability (MSI) while enhancing the cytotoxicity of chemotherapeutic agents. The work is based on experiments using antioxidants to target reactive oxygen species generated by oxaliplatin, a commonly used chemotherapeutic agent, and is applicable to other chemotherapeutic agent, and in particular 5-fluorouracil, methotrexate, CCNU, etoposide and vinblastine. In particular oxaliplatin is co-treated with an antioxidant, including CDC, CAPE, ciclopirox ethanolamine, hinokitiol, gossypol, n-Octyl caffeate, baicalein, or curcumin.
Owner:CHANG CHRISTINA LING
Method for preparing nanocapsule and nanocapsule composite microsphere
InactiveCN101401792AUniform particle sizeUniform sizePowder deliveryOrganic active ingredientsUltrasonic emulsificationMicrosphere
The invention discloses a method for preparing a nanometer capsule and a method for preparing a nanometer capsule composite microsphere. The method for preparing the nanometer capsule comprises the following: step 1, preparing a methotrexate alkaline solution and a PLGA-CH2Cl2 solution; step 2, injecting the methotrexate alkaline solution into the PLGA-CH2Cl2 solution to obtain a W / O colostrum after ultrasonic emulsification; step 3, adding the colostrum into sodium cholate to obtain a W / O / W multiple emulsion after the ultrasonic emulsification; and step 4, placing the obtained multiple emulsion in the sodium cholate to be evaporated through decompression to remove CH2Cl2 so as to obtain a nanometer capsule particle suspension. The method for preparing the nanometer capsule composite microsphere comprises the following: step a, preparing a nanometer capsule; and step b, preparing the nanometer capsule composite microsphere. The nanometer capsule particle prepared by the invention has the characteristics of even size, higher drug loading and encapsulation efficiency, peak value drug release, and smooth drug release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Combinations comprising methotrexate and dhodh inhibitors
InactiveUS20110280831A1Inhibit inflammationGood curative effectBiocideNervous disorderDitazolePharmaceutical medicine
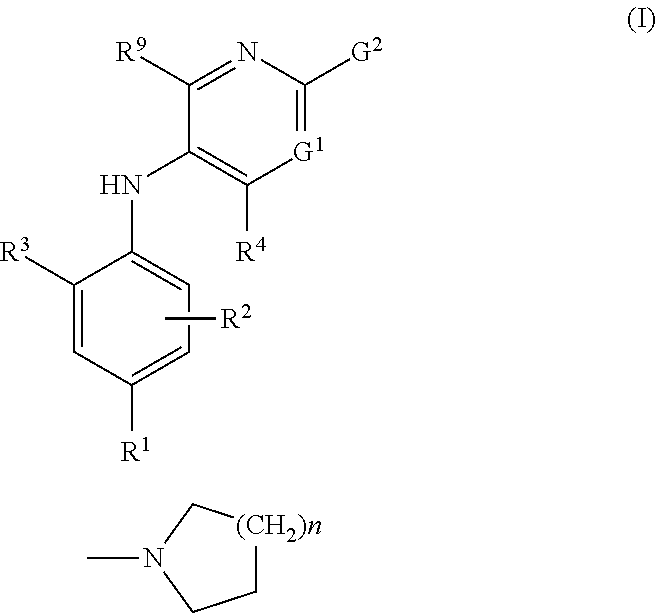
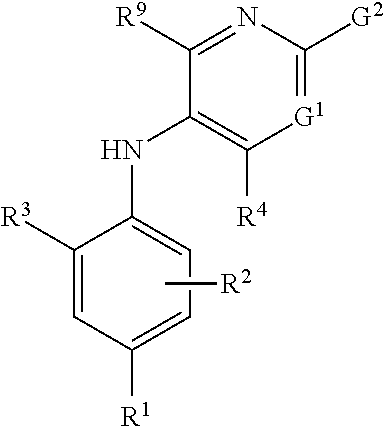
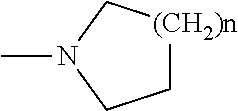
The present invention provides a combination which comprises (a) methotrexate and (b) a non-hepatotoxic DHODH inhibitor of formula (I): wherein: R1 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, —CF3 and —OCF3, R2 is selected from the group consisting of hydrogen atoms, halogen atoms and C1-4 alkyl groups, R3 is selected from the group consisting of —COOR5, —CONHR5, tetrazolyl, —SO2NHR5 and —CONHSO2R5 groups, wherein R5 is selected from the group consisting of a hydrogen atom and linear or branched C1-4 alkyl groups, R4 is selected from the group consisting of a hydrogen atom and a C1-4 alkyl group, R9 is selected from the group consisting of a hydrogen atom and a phenyl group, G1 represents a group selected from N and CR6 wherein R6 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, C1-4 alkoxy, —CF3, —OCF3, monocyclic N-containing C5-7 heteroaryl, monocyclic N— containing C3-7 heterocyclyl groups and C6-10 aryl groups which C6-10 aryl groups are optionally substituted with one or more substituents selected from halogen atoms and C1-4 alkyl groups, G1 represents a group selected from N and CR6 wherein R6 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, C1-4 alkoxy, —CF3, —OCF3, mono-cyclic N-containing C5-7 heteroaryl, monocyclic N— containing C3-7 heterocyclyl groups and C6-10 aryl groups which C6-10 aryl groups are optionally substituted with one or more substituents selected from halogen atoms and C1-4 alkyl groups, G2 represents a group selected from: a hydrogen atom, a hydroxy group, a halogen atom, a C3-4 cycloalkyl group, a C1-4 alkoxy group and —NRaRb, wherein Ra represents a C1-4 alkyl group and Rb is selected from a group consisting of C1-4 alkyl group and C1-4alkoxy-C1-4 alkyl group, or Ra and Rb together with the nitrogen atom to which they are attached form a saturated 6 to 8 membered heterocyclic ring optionally containing one oxygen atom as an additional heteroatom, a monocyclic or bicyclic 5 to 10 membered heteroaromatic ring containing one or more nitrogen atoms which is optionally substituted by one or more substituents selected from halogen atoms, C1-4 alkyl, C1-4 alkoxy, C3-4 cycloalkyl, C3-4 cycloalkoxy, —CF3, —OCF3, and —CONR7R8, wherein R7 and R8 are independently selected from hydrogen atom, linear or branched C1-4 alkyl groups, C3-7 cycloalkyl groups, or R7 and R8 together with the nitrogen atom to which they are attached form a group of formula wherein n is an integer from 0 to 3, and a phenyl group which is optionally substituted by one or more substituents selected from halogen atoms, C1-4 alkyl, hydroxyl, C1-4 alkoxy, C3-4 cycloalkyl, C3-4 cycloalkoxy, cyano, —CF3, —OCF3, —CONR7R8, oxadiazolyl, triazolyl, pyrazolyl and imidazolyl groups, which oxadiazolyl, triazolyl, pyrazolyl and imidazolyl groups are optionally substituted by C1-4 alkyl or C3-7 cycloalkyl groups and wherein R7 and R8 are independently selected from hydrogen atom, linear or branched C1-4 alkyl groups, C3-7 cycloalkyl groups, or R7 and R8 together with the nitrogen atom to which they are attached form a group of formula wherein n is an integer from 0 to 3 or, when G′ represents CR6, G2 together with R6 forms a non-aromatic C5-10 carbocyclic group or a C6-10 aryl group, and the pharmaceutically acceptable salts and N-oxides thereof.
Owner:ALMIRALL
Elemental nanoparticles of substantially water insoluble materials
InactiveUS20050129777A1Improve efficacyMinimize multiple dosingPowder deliveryOrganic active ingredientsWater insolubleProgesterones
This invention relates to a novel process of manufacture of nanoparticles of substantially water insoluble materials from emulsions. The emulsions have the ability to form a single liquid phase upon dilution of the external phase, instantly producing dispersible solid nanoparticles. The formed nanoparticles have average diameter of about 10 to 200 nm and are suitable for drug delivery and targeting of water insoluble therapeutic or diagnostic agents. Examples of such agents are methotrexate, progesterone, testosterone, prednisolone, and ibuprofen. Such agents can be used in a wide range of therapeutic and diagnostic treatments including treatment for cancer, hormonal therapy, and pain management.
Owner:HASSAN EMADELDIN M
Targeted fluorescent liposome based on low-frequency ultrasonic response, preparation method of liposome and application
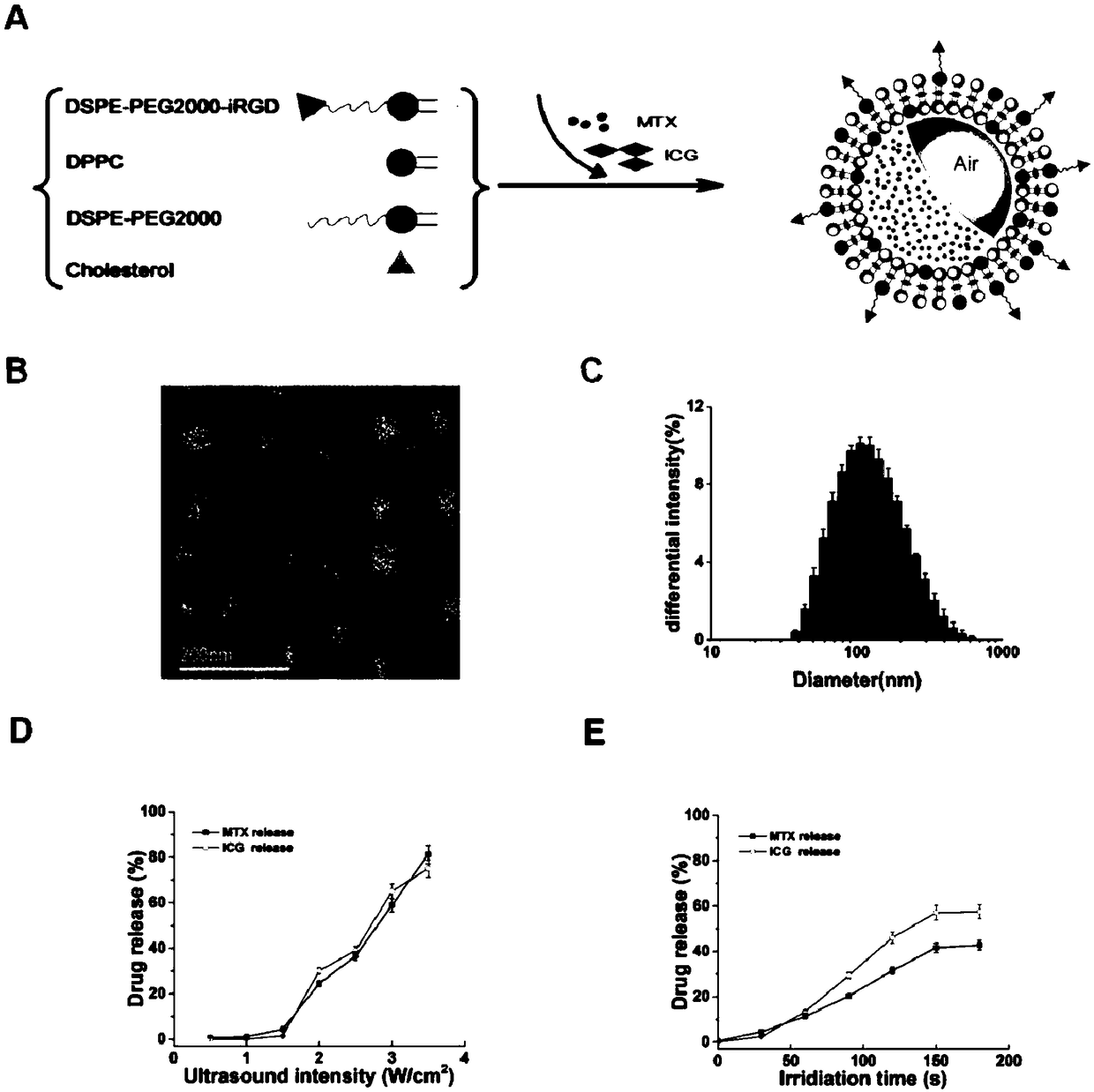
InactiveCN108888782AAvoid safety hazardsImprove responsivenessOrganic active ingredientsAntipyreticFluorescenceCholesterol
The invention provides a targeted fluorescent liposome based on low-frequency ultrasonic response, a preparation method of the liposome and an application, and particularly provides an active targeting medicine carrying fluorescent acoustic liposome which comprises a bimolecular lamellar lipid membrane, medicines and fluorescent agents, wherein the bimolecular lamellar lipid membrane comprises dipalmitoyl phosphatidyl choline (DPPC), cholesterol (CHO), polyethylene glycol modified distearoyl phosphoethanolamine (DSPE-PEG) and (DSPE-PEG-iRGD), and the acoustic liposome wraps the medicines and the fluorescent agents. The medicines are selected from methotrexate (MTX), the fluorescent agents are selected from indocyanine green (ICG), and polyethylene glycol (PEG) in the polyethylene glycol modified distearoyl phosphoethanolamine (DSPE-PEG) is selected from PEG1000-PEG3000.
Owner:GUANGDONG NO 2 PROVINCIAL PEOPLES HOSPITAL
Folic acid targeted antitumor medicine sustained-releasing carrier and preparation method of same
ActiveCN107744593AWide variety of sourcesLow priceOrganic active ingredientsPharmaceutical non-active ingredientsChemistryBone tissue engineering
The invention discloses a folic acid targeted antitumor medicine sustained-releasing carrier and a preparation method of same, and belongs to the field of biomedical materials. The sustained-releasingcarrier is prepared through the steps of: 1) preparing mono-dispersed micro-nano bioactive glass through a sol-gel method with combination of organic template self-assembly technology; 2) modifying the surface of the micro-nano bioactive glass by grafting amino groups via a silane coupling agent; 3) conjugating the amino groups with a gamma-carboxyl group on folic acid; and 4) supporting an antitumor medicine, methotrexate, onto the material through covalent combination. According to the invention, the folic acid is combined with a folate receptor on surface of a tumor cell membrane, and thenthe medicine carrier is transferred into a cell through the endocytosis of the cell membrane; with degradation of the bio-glass, the antitumor medicine is slowly released. The folic acid targeted antitumor medicine sustained-releasing carrier has good biocompatibility and improves efficiency that the carrier enters a tumor cell. The sustained-releasing carrier can be used in the fields of targeted therapy of tumor cells, medicine sustained releasing, bone tissue engineering, etc.
Owner:SOUTH CHINA UNIV OF TECH
Preparation methods of methotrexate and lecithin compound, nano-particles of methotrexate and lecithin compound as well as targeted sustained release preparation of methotrexate and lecithin compound
InactiveCN105055322AImprove stabilityGood tumor targetingOrganic active ingredientsPowder deliveryOrganic solventTreatment effect
The invention relates to a preparation method of a methotrexate and lecithin compound, which comprises the following steps: dissolving methotrexate and lecithin in an organic solvent A, carrying out mixed reaction at 20-40 DEG C for 12-24h, removing the organic solvent A in the mixed liquor after mixed reaction to obtain a dried product, and then purifying the dried product in which the organic solvent A is removed by adopting an organic solvent B, so as to obtain the methotrexate and lecithin compound. The invention also relates to a preparation method of nano-particles of the methotrexate and lecithin compound as well as a preparation method of a targeted sustained release preparation of the methotrexate and lecithin compound. The nano-particles of the methotrexate and lecithin compound, prepared by the method provided by the invention, have particle sizes of 100-500nm, good stability and better tumor targeting and sustained release characteristics. The targeted sustained release preparation of the methotrexate and lecithin compound, prepared by the method provided by the invention, has the advantages of targeting property, long circulation, low toxicity and good curative effect.
Owner:XIAMEN CHITOSAN BIO TECH
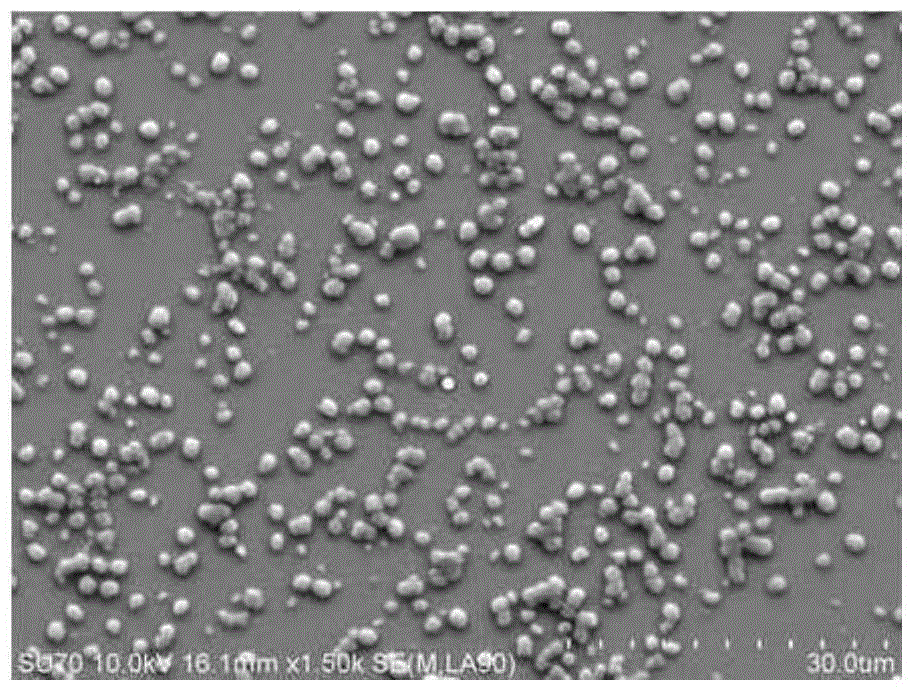
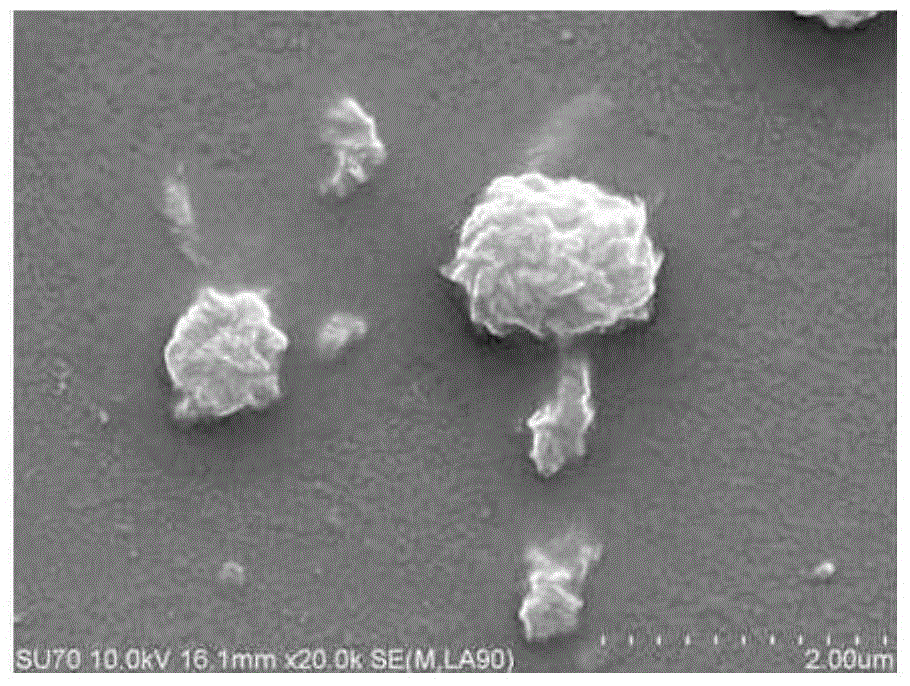
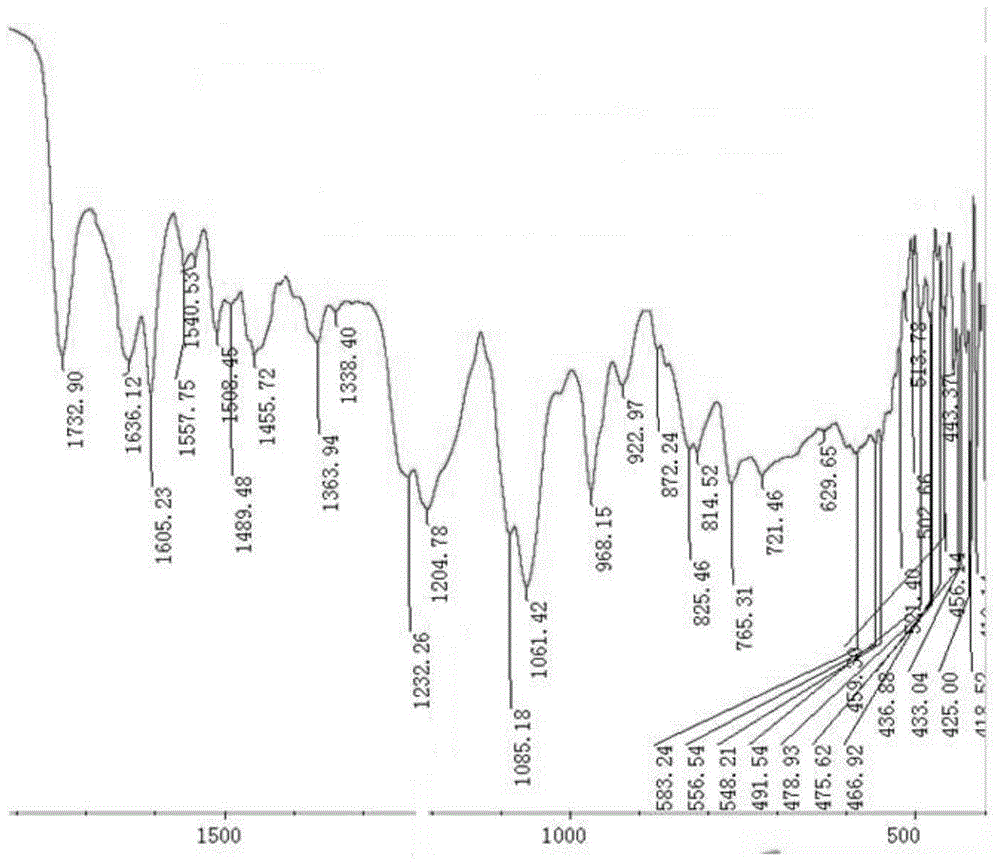
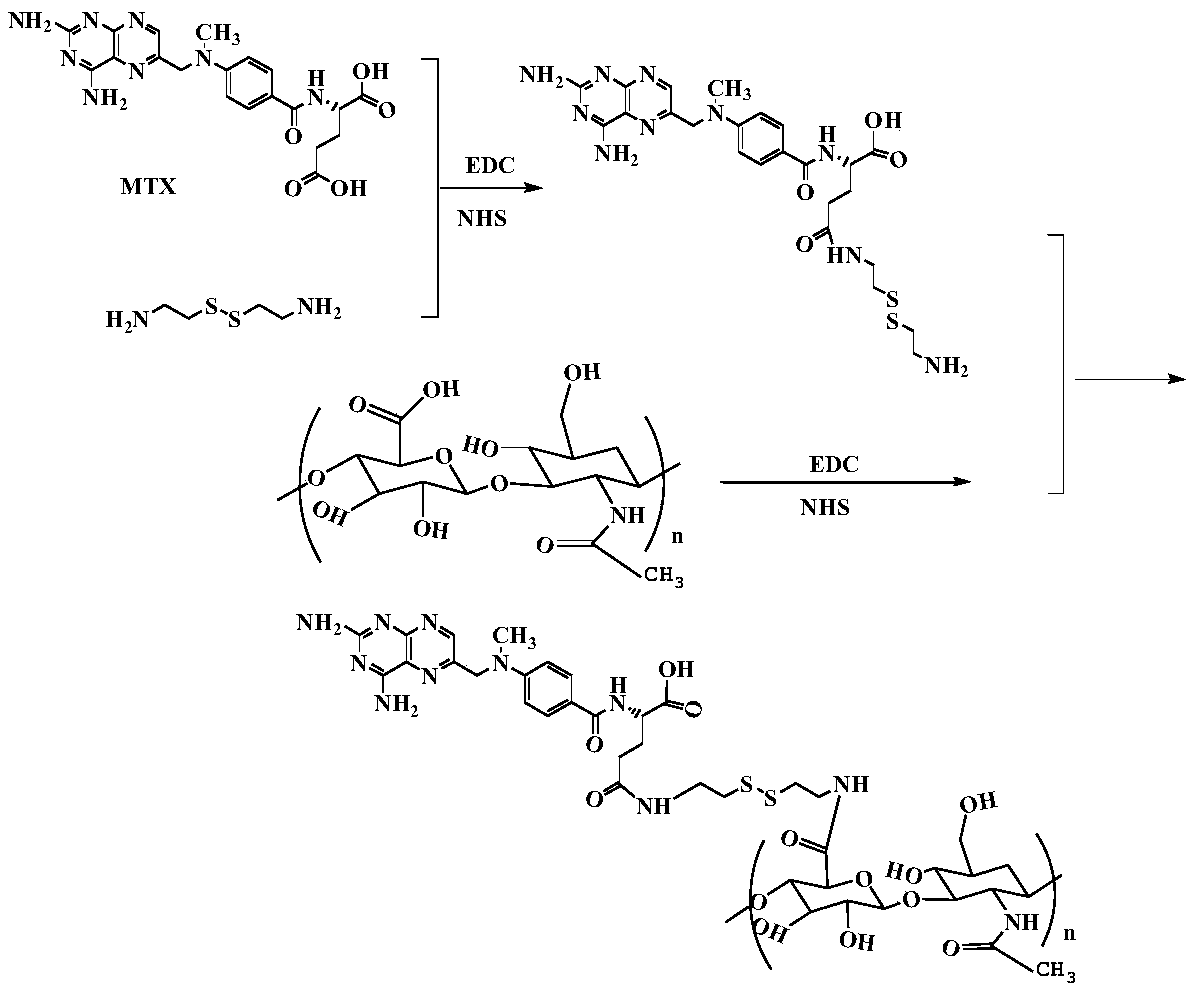
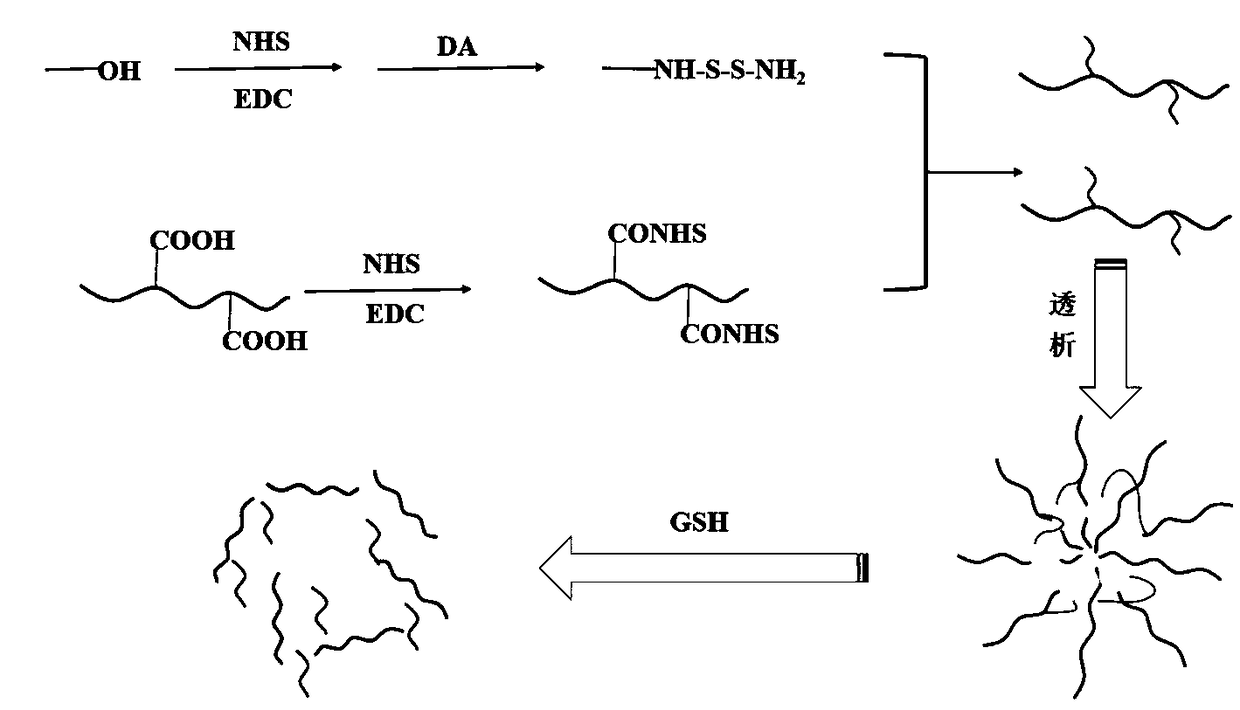
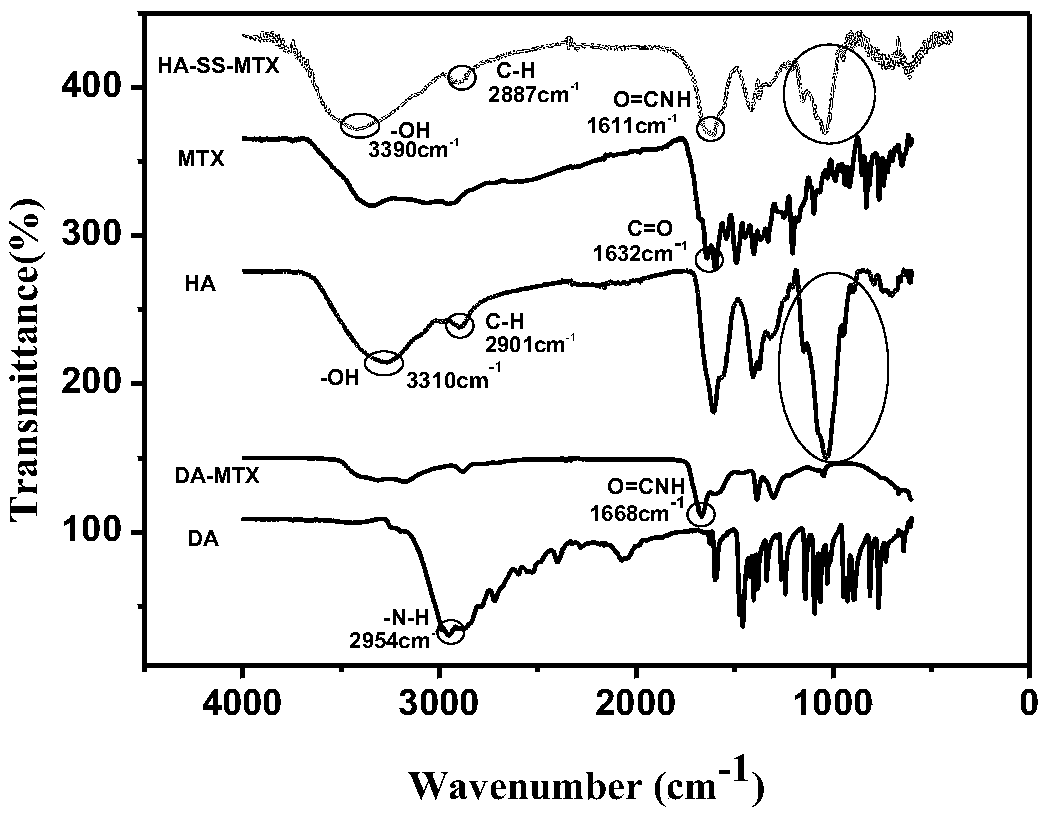
Hyaluronic acid-methotrexate self-assembled nanomicelle and preparation method thereof
InactiveCN108888775ATargetedEasy to controlOrganic active ingredientsPharmaceutical non-active ingredientsDisulfide bondLesion
The invention belongs to the technical field of nano pharmaceutical preparations and discloses a hyaluronic acid-methotrexate self-assembled nanomicelle and a preparation method thereof. Hyaluronic acid and methotrexate are connected through disulfide-bond cystamine to prepare the hyaluronic acid-methotrexate self-assembled nanomicelle through dialysis. By adoption of the disulfide-bond cystamineas a linkage factor, MTX and hyaluronic acid are connected to form the self-assembled nanomicelle, on the one hand, hyaluronic acid which can be specifically combined with tumor cells enables a targeting effect of the polymeric micelle, and medicines can be targetedly loaded to a lesion; on the other hand, by glutathione responsiveness of disulfide bond, controlled release of the medicines can berealized.
Owner:NORTHWEST UNIV(CN)
Topical Pharmaceutical Composition, Method for Producing the Topical Pharmaceutical Composition, Use of the Topical Pharmaceutical Composition and Method for the Topical Treatment of Psoriasis, Atopic Dermatitis or Chronic Eczema
This invention relates to a topical pharmaceutical composition comprising a combination of methotrexate, alpha bisabolol and allantoin; a process for producing the same and the use of the composition in the treatment of plaque psoriasis (psoriasis vulgaris), atopic dermatitis and chronic eczema. The composition of this invention can be used alone or in combination with other topical or systemic therapies. The present invention further discloses a process for producing the pharmaceutical composition.
Owner:BIOLAB SANUS FARMACEUTICA LTD
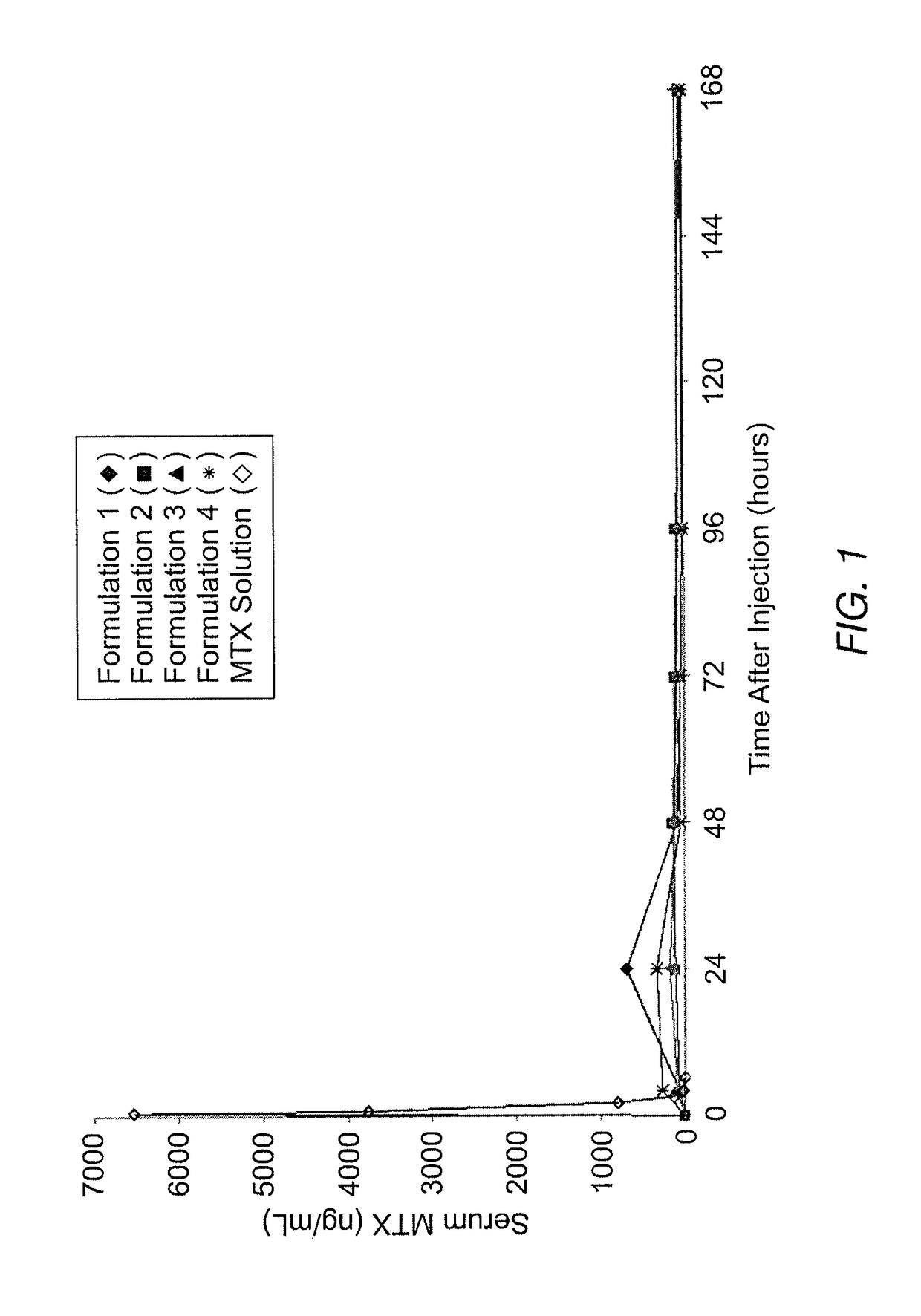
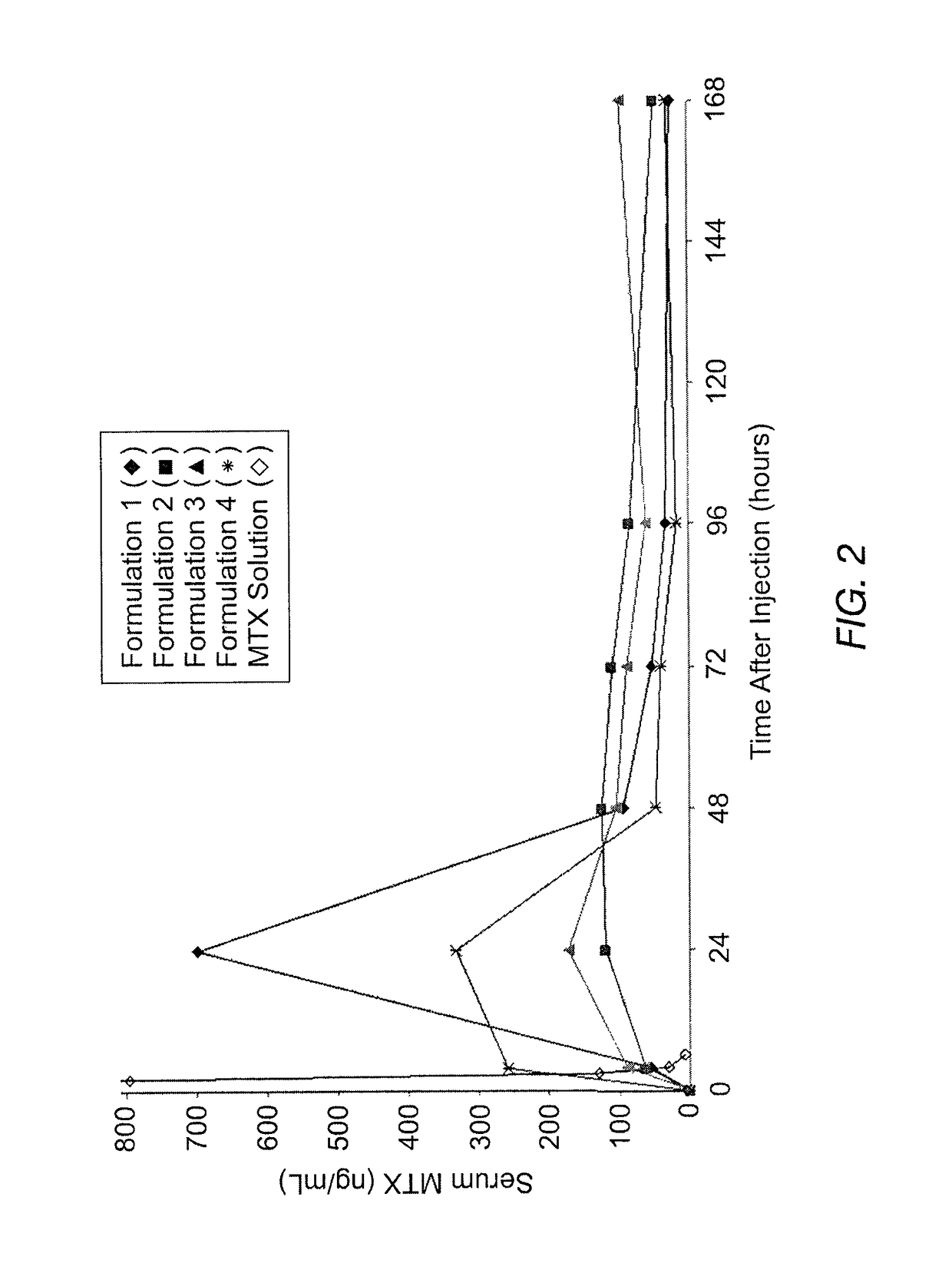
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
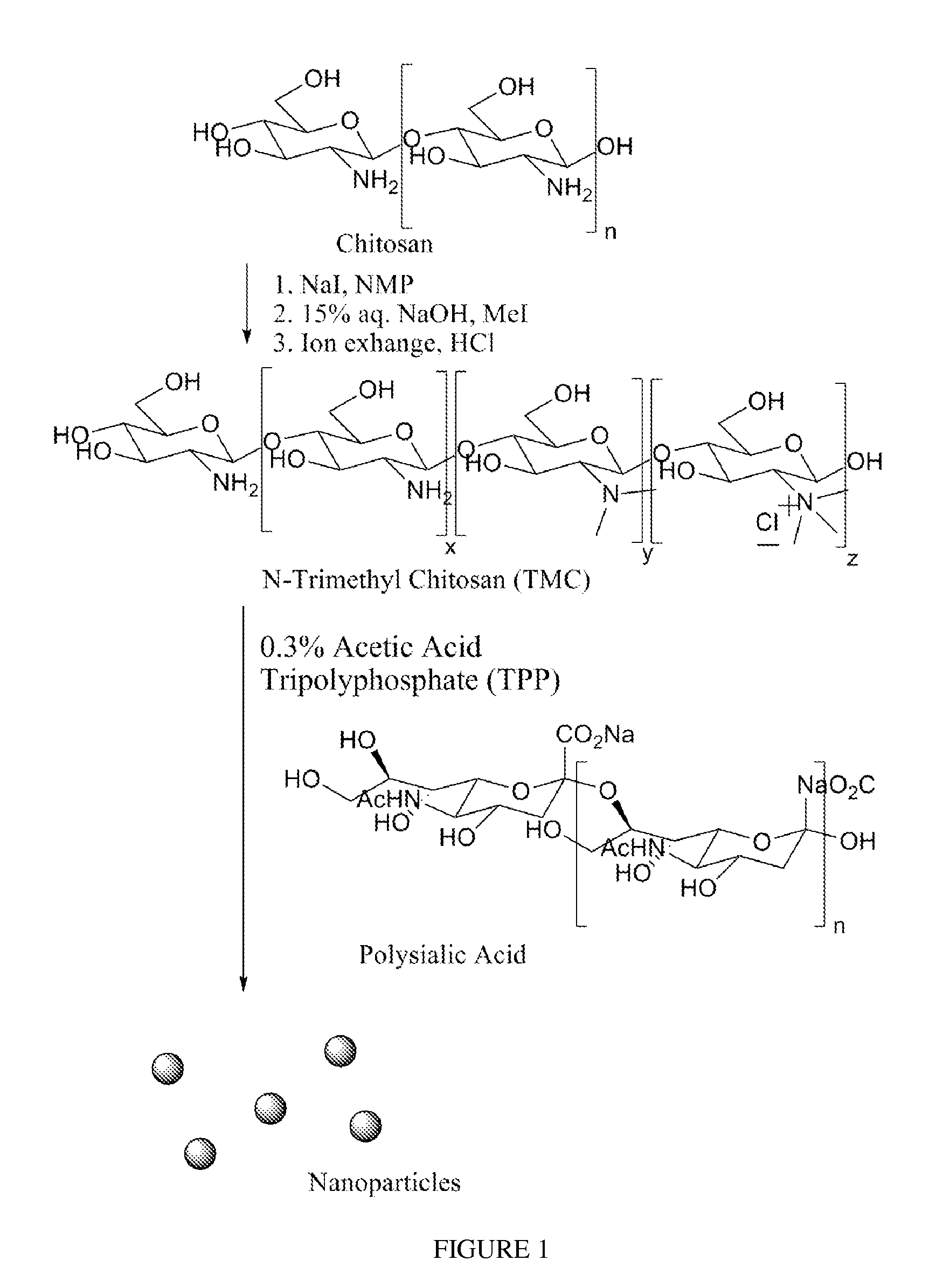
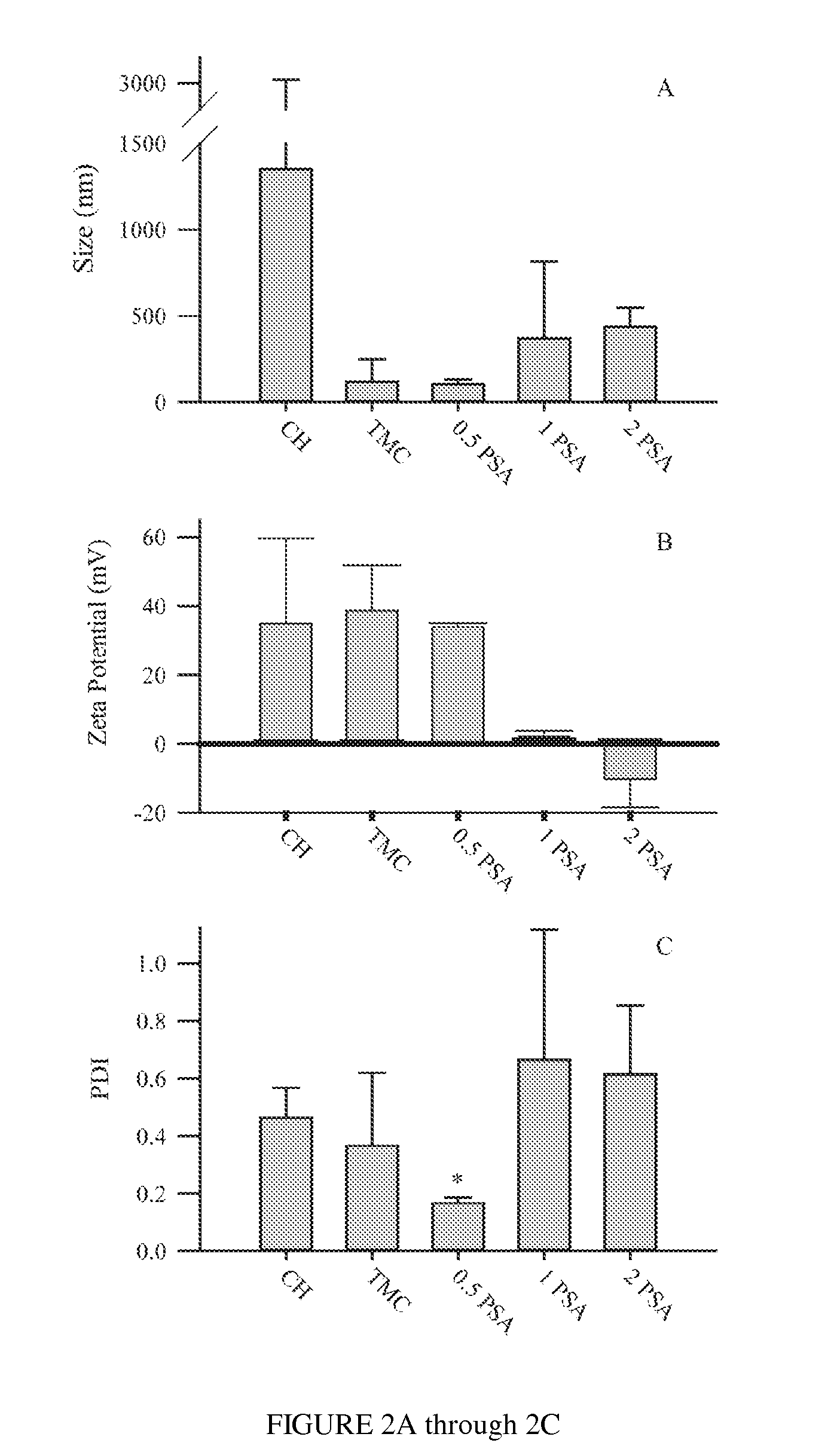
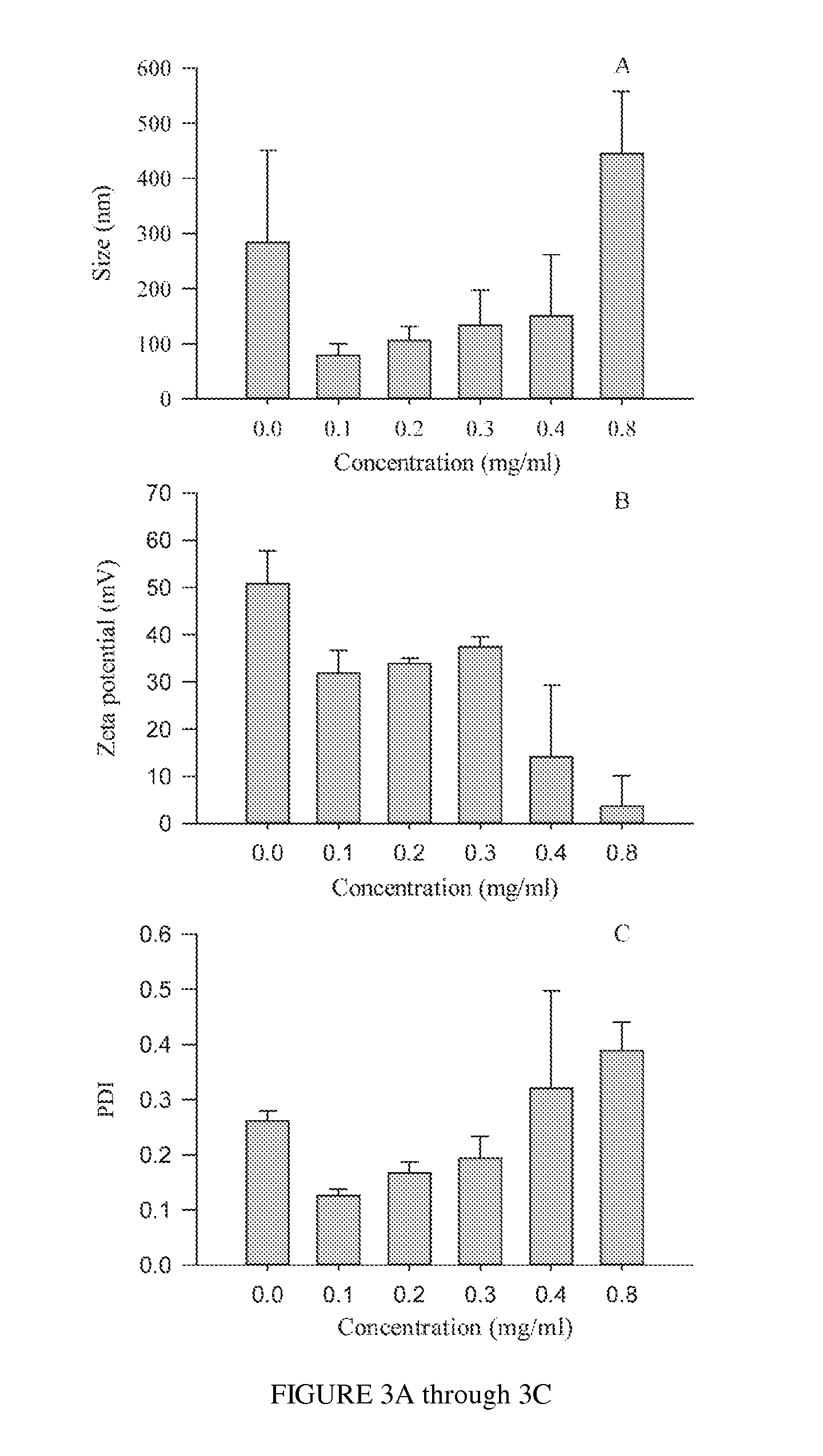
Polysialic acid-based n-trimethyl chitosan gel nanoparticles for systemic drug delivery
ActiveUS20120294904A1Safely and effectively deliverReduce sizeMaterial nanotechnologyBiocideDrug compoundMethotrexate
Gel nanoparticles for encapsulating and delivering a pharmaceutical compound to a patient. The nanoparticles are formed from N-trimethyl chitosan and polysialic acid, preferably in the presence of sodium tripolyphosphate. A ratio of polysialic acid to N-trimethyl chitosan of about 0.5 to 1 produces nanoparticles having diameter of about 100 nm (plus or minus 25 nm) and a zero potential above 30 milivolts that can stability contain a pharmaceutical compound, such as methotrexate, for delivery to a patient.
Owner:SYRACUSE UNIVERSITY
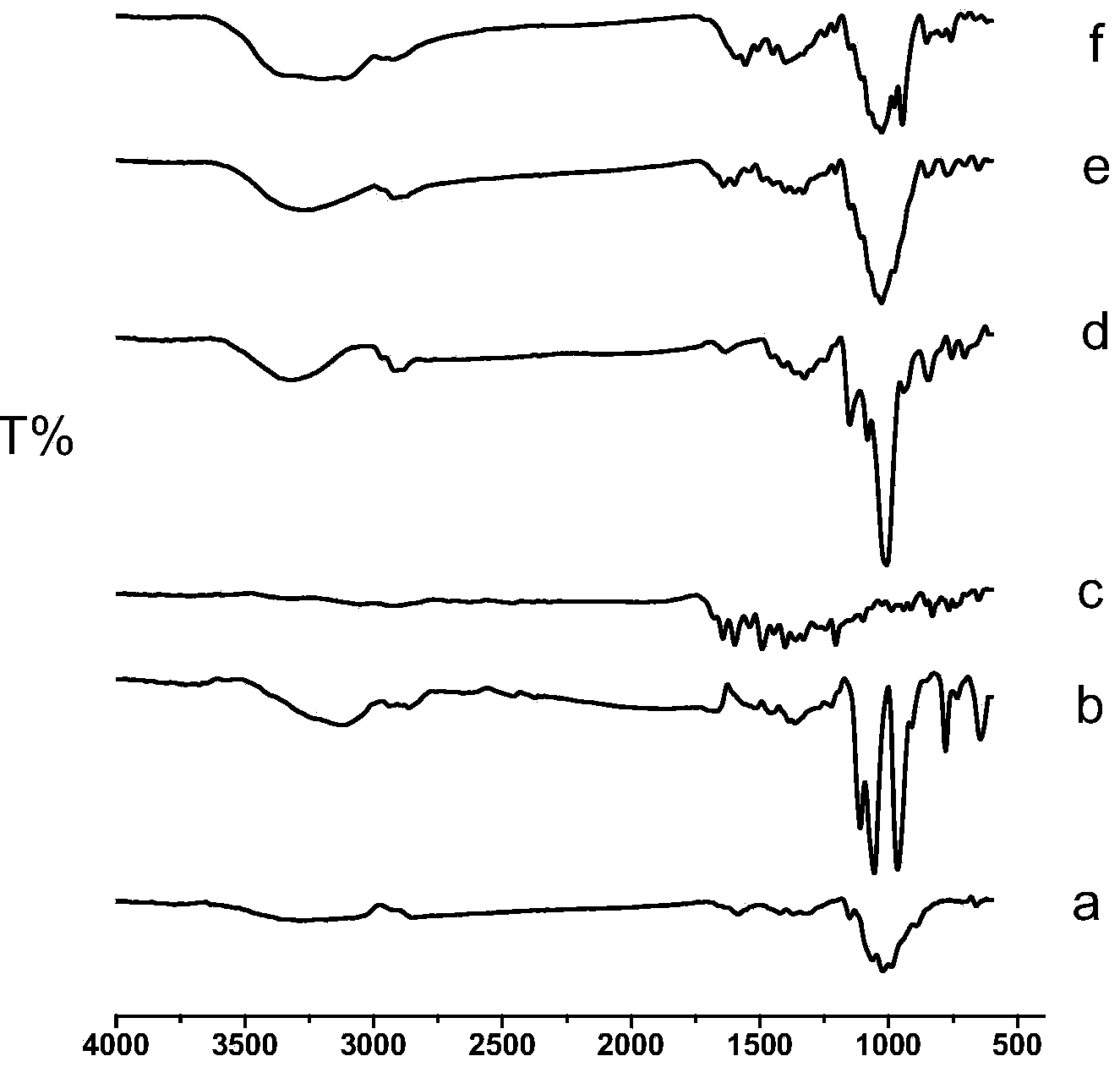
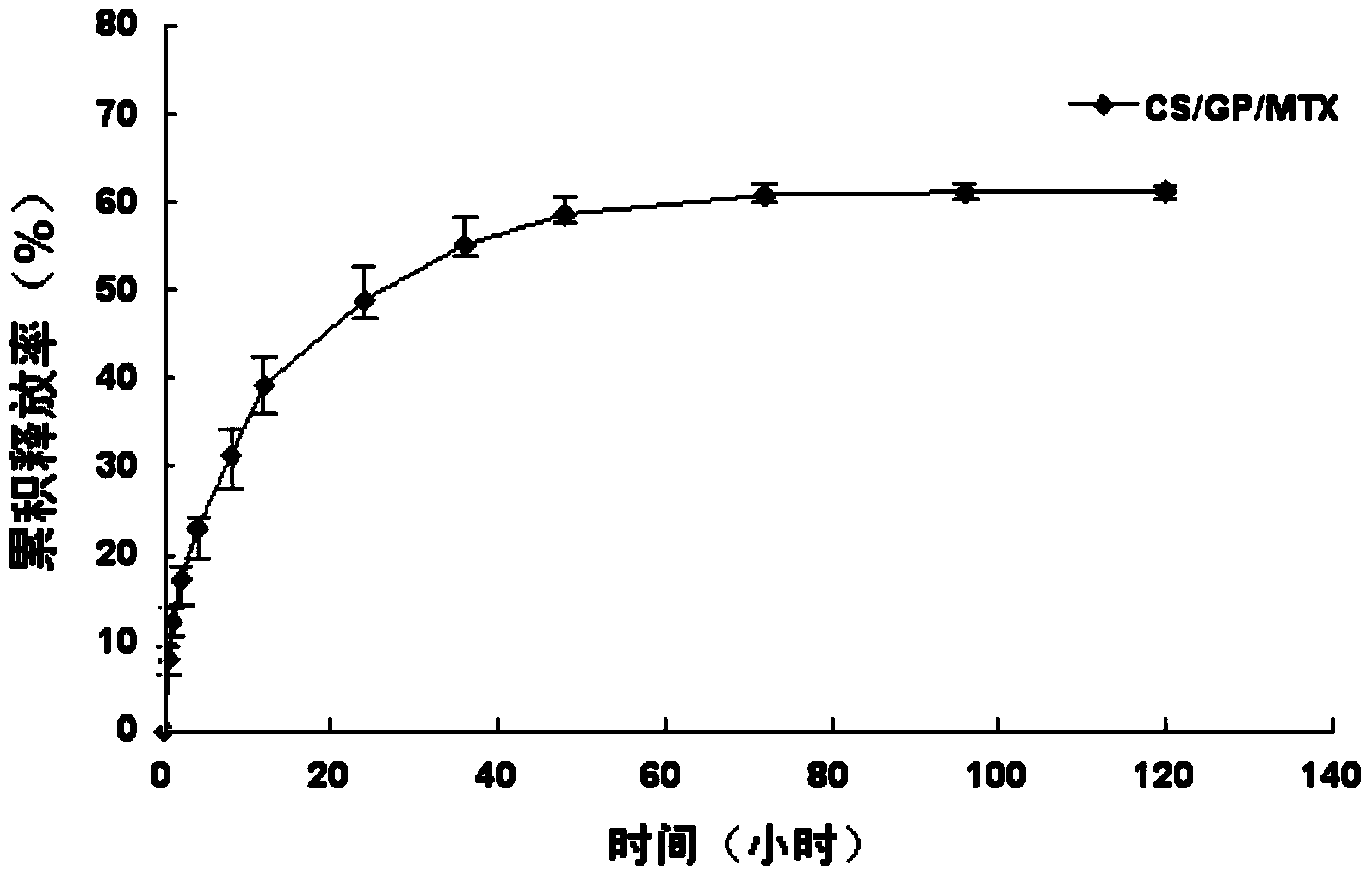
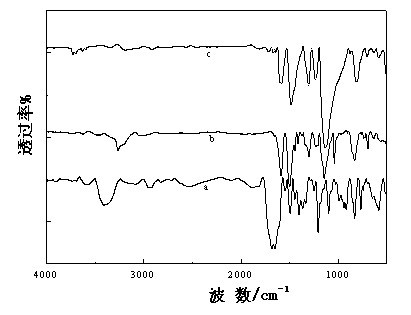
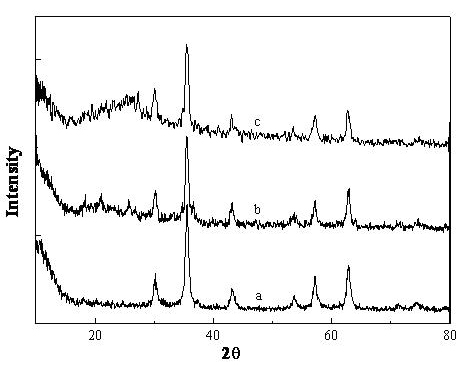
CS/GP/MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel) and application thereof
ActiveCN103735501AStable in natureQuality is easy to controlOrganic active ingredientsAntipyreticPhosphateBeta-glycerophosphate
The invention provides MTX subcutaneous sustained-release injection CS / GP / MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel). Every liter of aqueous solution of the CS / GP / MAX contains 16 to 22 grams of chitosan, 0.05 to 0.1 mole of acetic acid, 70 to 150 grams of beta-glycerophosphate, 0.1 to 0.12 mole of methotrexate, 0.1 to 0.12 mole of a solubilizer and 5 to 50 grams of an isoosmotic adjusting agent and pH modifier. The CS / GP / MAX has stable properties and controllable quality, and the external properties, tgel (the time of gelation), eta, pH value, content and preliminary stability of the CS / GP / MAX meet requirements on thermosensitive hydrogel; 70 percent of drugs of the CS / GP / MAX in a PBS (Phosphate Buffer Solution) are released within 5 days under the condition of 37 DEG C; compared with those of MTX injection, the Cmax (Maximum Plasma Drug Concentration) is reduced by 82 percent, the AUC0-t (area under the plasma concentration time curve from time 0 to t hours) is enlarged by 2.37 times, and the t0.02muM (the length of time the MTX concentration-time curve remained above 0.02 muM in one week) is prolonged by 12.93 times after the CS / GP / MAX is subcutaneously injected to the back of a rabbit; by the CS / GP / MAX, adverse effects can be reduced, and curative effects can be improved.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
Sustained-release injection containing methotrexate and synergist thereof
InactiveCN101396338AOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectWhole body
The invention relates to a sustained release injection containing methotrexate, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise effective anti-cancer component and sustained release auxiliary material; and the menstruum is ordinary menstruum or special menstruum containing suspending agent. The effective anti-cancer component is the combination of the methotrexate and the methotrexate synergist selected from platinum compound, topoisomerase inhibitor and / or tetrazine compound. The sustained release auxiliary material is selected from one or the combination of the copolymer of polylactic acid, polyglycolic acid and glycolic acid, ethylene vinyl acetate copolymer and the copolymer of polifeprosan, FAD and decanedioic acid. The suspending agent is selected from carboxymethyl cellulose, etc. The suspending agent is used for suspending the effective anti-cancer component or sustained release particle or microsphere containing the effective anti-cancer component, so as to be made into the sustained release injection. The sustained release injection is injected into tumor, which can not only reduce the general toxic reaction of the drug, but also improve the partial drug concentration at the tumour selectively and enhance the treatment effect of non-operative treatments, such as chemotherapeutic drug, radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Preparation method for conductive superparamagnetic nanometer gamma-ferric-oxide/polyaniline-methotrexate
InactiveCN102058598AThe method is simple and economicalImprove electrochemical activityOrganic active ingredientsSurgical adhesivesChemical synthesisMethotrexate
The invention provides a preparation method for conductive superparamagnetic nanometer gamma-ferric-oxide / polyaniline-methotrexate and relates to the technical field of chemical synthesis, in particular to a method for synthesizing the novel gamma-ferric-oxide / polyaniline-methotrexate. The preparation method prepares the nanometer gamma-ferric-oxide / polyaniline-methotrexate with conductive superparamagnetism by a doping method. In the preparation method, a doping mechanism of polyaniline is adopted, and methotrexate is doped into the conductive superparamagnetic nanometer gamma-ferric-oxide / polyaniline. The method for fixing medicaments has the unique advantage of avoiding influence on the treatment group of the fixed methotrexate. The gamma-ferric-oxide / polyaniline-methotrexate can suspend in water for three months or even longer. The method is simple and economic; the conductive superparamagnetic nanometer gamma-ferric-oxide / polyaniline-methotrexate has good electrochemical activity and good superparamagnetism under a nearly neutral environment, can be regulated and controlled to a specific position under the control of an external magnetic field and is expected to be an ideal targeted medicament, and to treat tumors in human bodies.
Owner:YANGZHOU UNIV
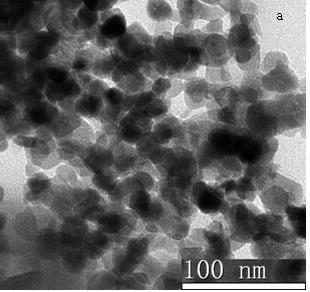
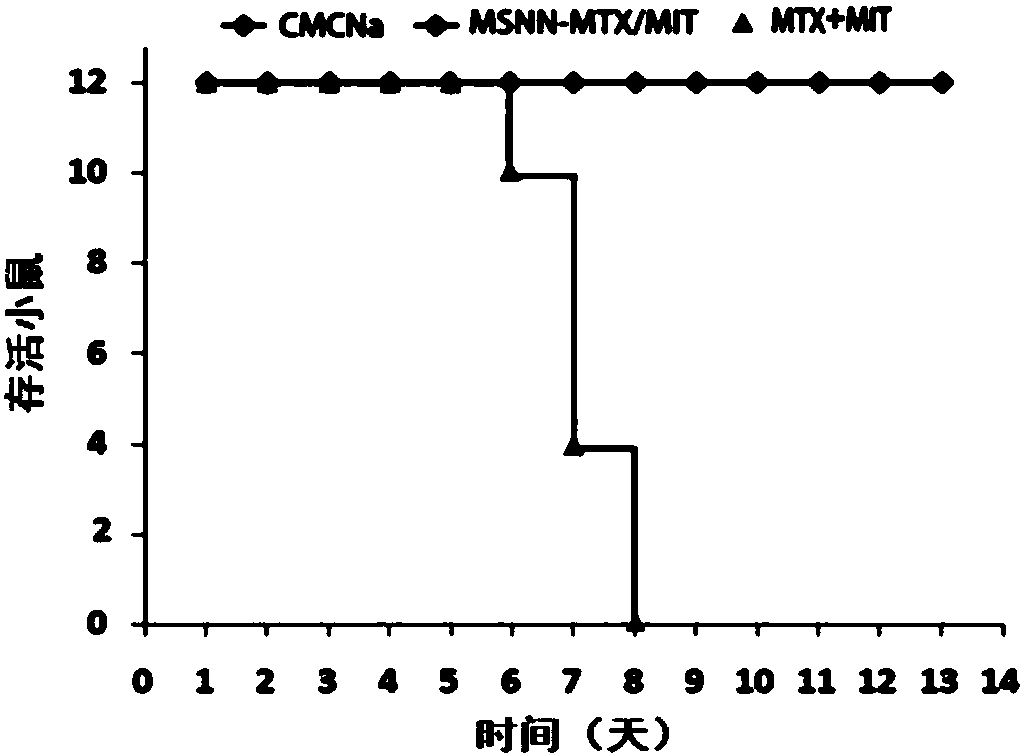
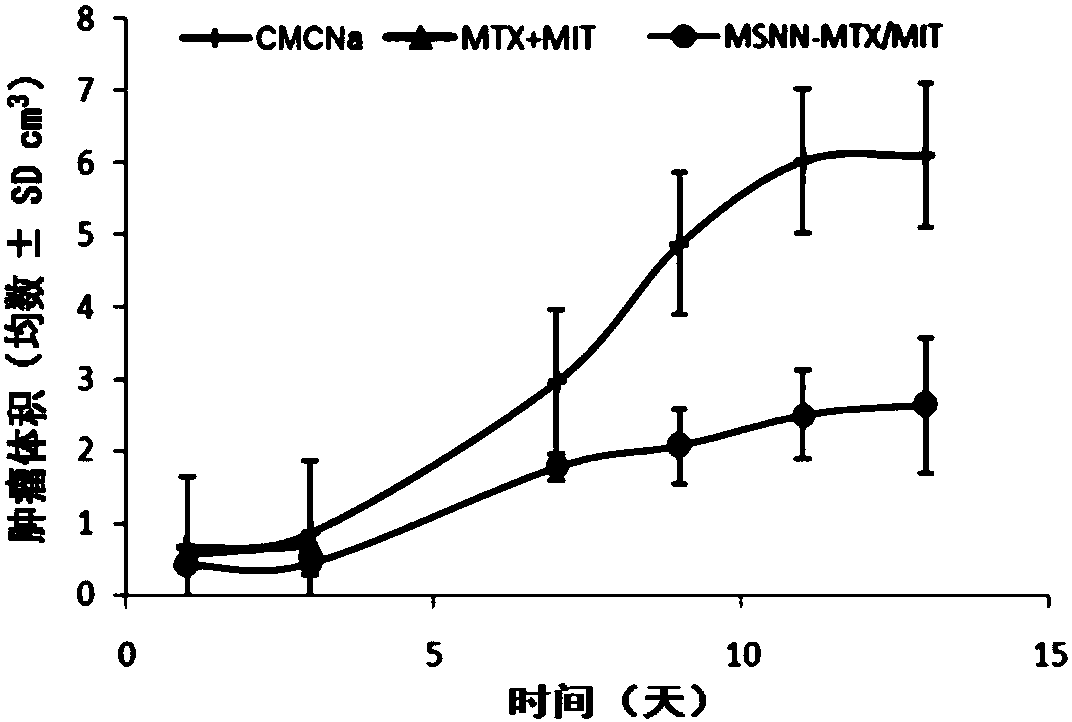
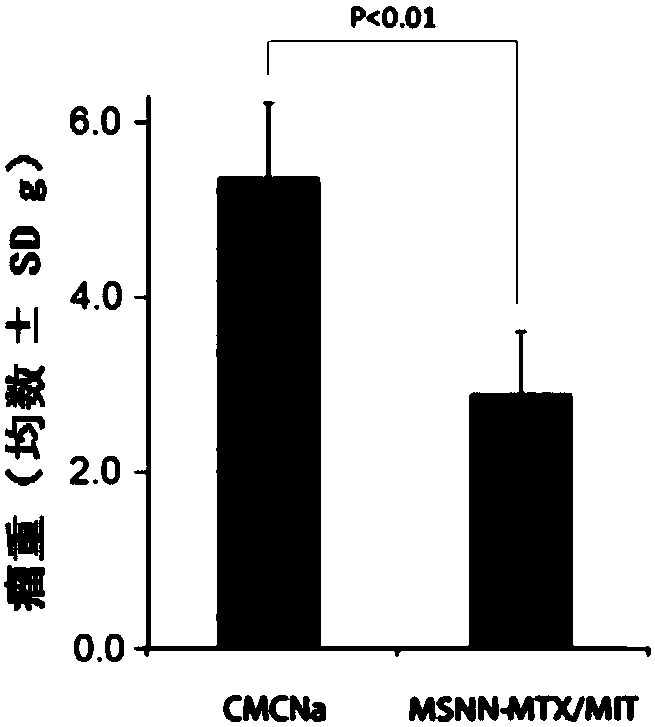
Mesoporous silicon dioxide-methotrexate-mitoxantrone nanoparticles as well as preparation, activity and application thereof
InactiveCN107684627AOrganic active ingredientsPowder deliveryMethotrexate/mitoxantroneSilica nanoparticles
The invention discloses nano-scale mesoporous silicon dioxide-methotrexate-mitoxantrone nanoparticles. Its preparation method is disclosed, that is, amino functionalized mesoporous silica nanoparticles (MSNN) are prepared by modifying amino groups on the surface of mesoporous silica nanoparticles, and then the amino groups of MSNN are covalently combined with MTX to form methotrexate ‑Amino-modified mesoporous silica nanoparticles (MSNN‑MTX), and finally load mitoxantrone into the nanopores of MSNN‑MTX to obtain nanoscale mesoporous silica‑methotrexate / mitoxantrone Quinone nanoparticles (MSNN‑MTX / MIT). Its antitumor effect is disclosed. Compared with the combined application of conventional MTX and MIT, MSNN‑MTX / MIT significantly prolongs the survival time of S180 mice, improves the curative effect and reduces systemic toxicity. Therefore, the present invention discloses the application of MSNN-MTX / MIT in the preparation of antitumor drugs combined with MTX and MIT. The MSNN-MTX / MIT of the present invention has a good clinical application prospect.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
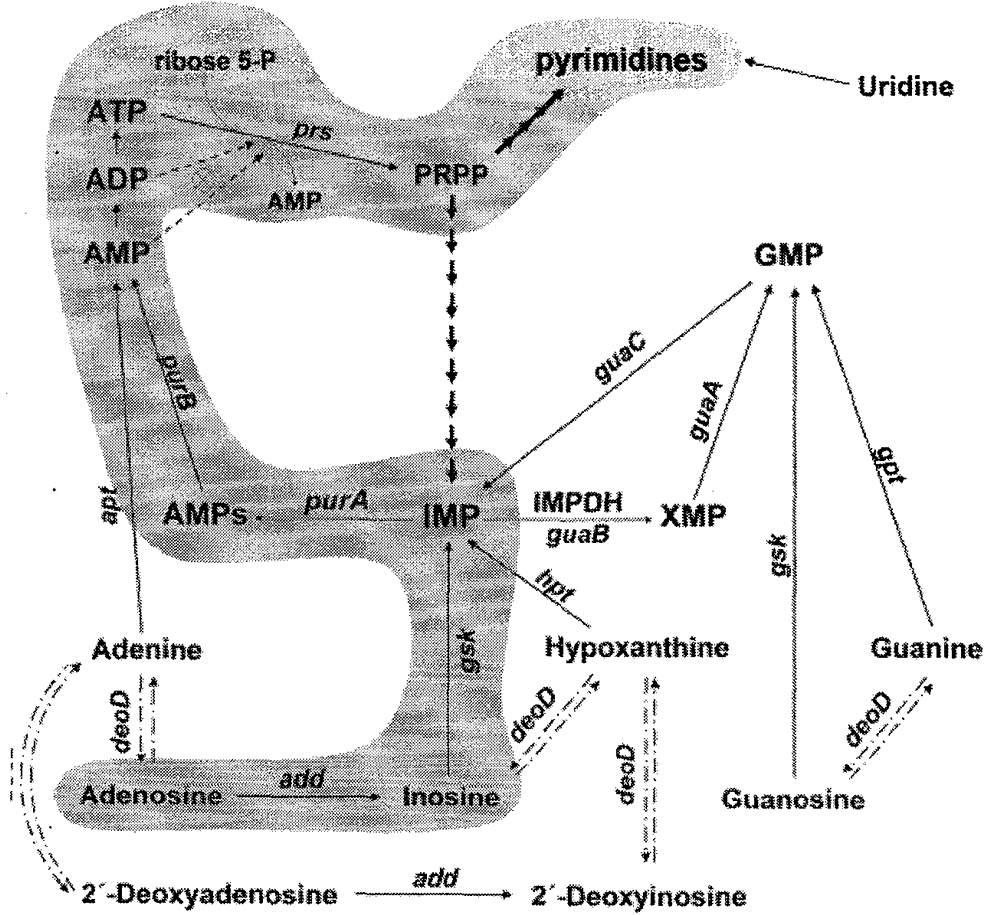
Bacterial strain mutagenesis cultivation in membrane bioreactor and adenosine fermentation method
The invention describes a method for producing a bacillus subtillis bacterial strain with adenosine having a concentration of 8g / L and uridine having a concentration of 6g / L through once or two-time mutagenesis. The bacillus subtillis bacterial strain is cultured in a high density in a membrane bioreactor by taking ribavirin, methotrexate, allopurine and bupirimate as enzyme inhibitors to perform cooperative mutagenesis of bacillus subtillis. The result shows that the fermentation rate is increased since the membrane bioreactor is utilized to ferment adenosine.
Owner:LUOYANG DESHENG BIO TECH CO LTD +1
Dual-drug controlled release system with pH and glutathione dual response and preparation method of dual-drug controlled release system
ActiveCN113018251APrevent leakageAvoid sudden releaseOrganic active ingredientsSilicaCytarabineCellulose
The invention belongs to the field of material synthesis and biological medicine, and relates to a dual-drug controlled release system with pH and glutathione dual response and a preparation method thereof.The dual-drug controlled release system is constructed by adopting mesoporous silica, and sodium hyaluronate serves as a gating material to block pore channels of the mesoporous silica; Mesoporous channels of the mesoporous silica are loaded with cytarabine. The mesoporous silica, the chitosan and the sodium carboxymethyl cellulose form a gel system, and methotrexate is loaded in the gel system. The preparation method has the beneficial effects that the prepared pH and glutathione dual-response dual-drug controlled release system can load two anti-cancer drugs at the same time, and realizes the successive release of the two drugs in a subacid tumor environment and a high-concentration glutathione environment. The double-drug controlled release system is simple to prepare and high in biocompatibility, and can be widely applied to the field of biological medicines.
Owner:CHANGZHOU UNIV
Recombinant CHO cell strain capable of stably expressing GPC3 and application thereof
ActiveCN111040999AStable integrationHigh expressionTumor rejection antigen precursorsMicroorganism based processesAntigenDihydrofolic acid
The invention discloses a recombinant CHO cell strain capable of stably expressing GPC3 and an application thereof. The preservation number of the CHO cell strain is CGMCC No.18881, the GPC3 is encoded by a cDNA sequence shown in the formula of SEQ ID No. 1, an antigen produced by the recombinant CHO cell strain is used for preparing a GPC3 monoclonal antibody, and the recombinant CHO cell strainis used for preparing a GPC3 antibody. The folate reductase defect type CHO cells are lack of endogenous dihydrofolate reductase, when an expression vector containing a dihydrofolate reductase gene and a target gene is co-transfected, the dihydrofolate reductase gene and the target gene are generally integrated at the same site of a host chromosome, and then in the presence of a competitive inhibitor-methotrexate of dihydrofolate reductase, the increasing of the copy number of the dhfr gene is driven to achieve the purpose of increasing the expression quantity of the target gene, so that a stable cell line for stably and efficiently expressing GPC3 is constructed, and the technical blank is filled.
Owner:南京拂晓生物科技有限公司
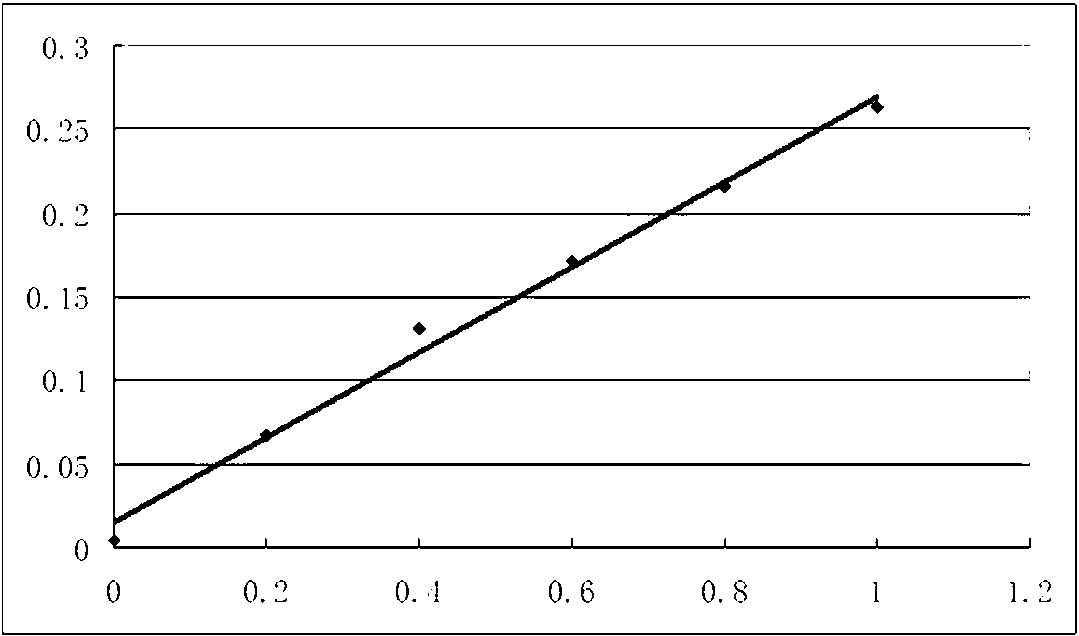
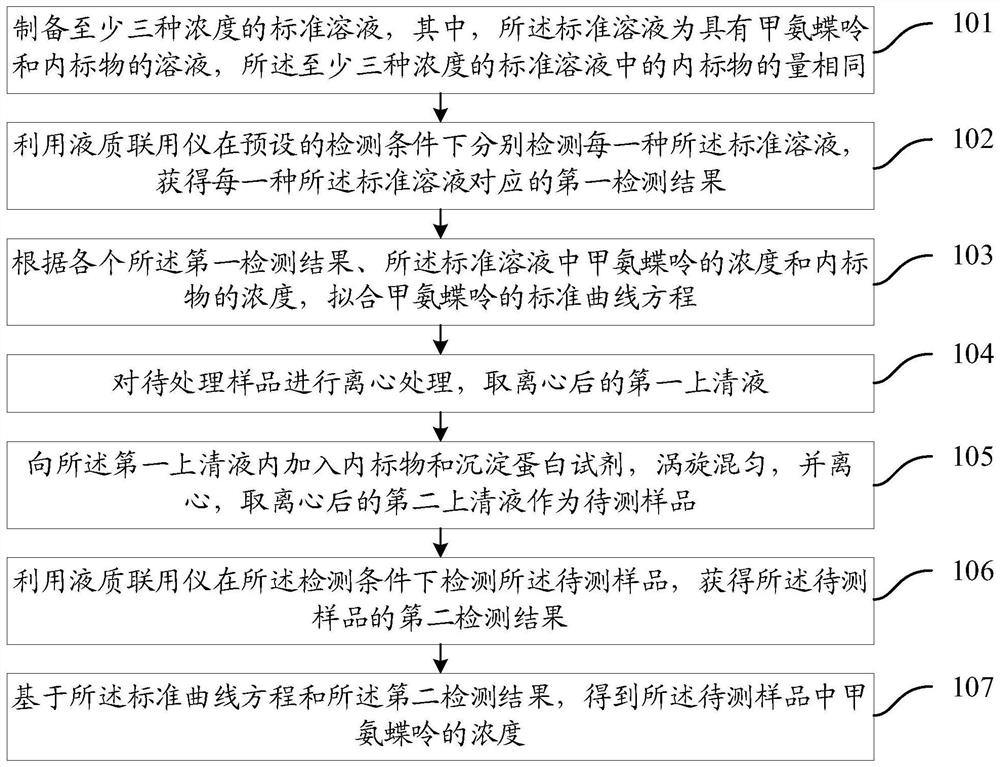
Methotrexate detection method
The invention provides a methotrexate detection method, and the method comprises the following steps: preparing standard solutions with at least three concentrations of methotrexate and internal standard substances, wherein the quantities of the internal standard substances in the standard solutions are the same; detecting each standard solution by using a liquid chromatograph-mass spectrometer under a detection condition to obtain a first detection result corresponding to the standard solution; fitting a standard curve equation of methotrexate according to each first detection result, the concentration of methotrexate in the standard solution and the concentration of the internal standard substance; taking a first supernatant of the sample to be treated after centrifugation; adding an internal standard substance and a precipitated protein reagent into the first supernatant, carrying out vortex mixing uniformly, centrifuging, and taking a centrifuged second supernatant as a sample to be detected; detecting the to-be-detected sample by utilizing the liquid chromatograph-mass spectrometer under the detection condition to obtain a second detection result of the to-be-detected sample;based on the standard curve equation and the second detection result, obtaining the concentration of methotrexate in the to-be-detected sample. The scheme can shorten the sample detection time.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Methods for optimizing clinical responsiveness to methotrexate therapy using metabolite profiling and pharmacogenetics
The present invention provides methods for optimizing clinical responsiveness to chemotherapy in an individual through genotypic analysis of polymorphisms in at least one gene. The methods of the present invention may further comprise determining the level of at least one long-chain methotrexate polyglutamate (MTXPG) in a sample obtained from the individual. The present invention also provides methods for generating a pharmacogenetic index for predicting clinical responsiveness to chemotherapy in an individual through genotypic analysis of polymorphisms in at least one gene. In addition, the present invention provides methods for optimizing therapeutic efficacy of chemotherapy in an individual by calculating the level of at least one long-chain MTXPG in a sample obtained from the individual.
Owner:CYPRESS BIOSCI +1
Preparation method of multi-component nanometer medicine with collaborative treatment effect and high drug loading
InactiveCN103239455ASuppress drug resistanceImprove efficacyOrganic active ingredientsAntineoplastic agentsTreatment effectMass ratio
The invention discloses a preparation method of a multi-component nanometer medicine with a collaborative treatment effect and high drug loading. The preparation method comprises the following steps of: 1) dissolving three hydrophobic cancer-fighting medicines, namely, methotrexate, 10-hydroxycamptothecine and taxol which are in a mass ratio of 1: 1: 1, into dimethylsulfoxide; ultrasonically treating for 5 minutes to produce mixed solution with concentration of 3mg / mL; 2) dropwise adding 400 micro-millimeters of the mixed solution into ultrapure water; and magnetically stirring to obtain suspension liquid of the multi-component nanometer medicine; and 3) adding 300 micro-millimetres of polymaleic anhydride 18 carbene-polyethylene glycol with concentration of 1mg / mL to the suspension liquid of the multi-component nanometer medicine; ultrasonically dispersing for 5 minutes; and standing for 3 minutes. The preparation method is simple, efficient, high in repeatability, excellent in controllability and high in universality, and provides experimental bases for preparing the nanometer medicine applied to multi-medicine collaborative treatment in the cancer field.
Owner:SUZHOU UNIV
Methods for Treating Methotrexate-Resistant Disorders with 10-Propargyl-10-Deazaaminopterin
The present invention relates to a method for treating a methotrexate-resistant disorder in an individual, wherein the method comprises administering to the individual an effective amount of 10-propargyl-10-deazaminopterin or its pharmaceutically acceptable salts.
Owner:ALLOS THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com