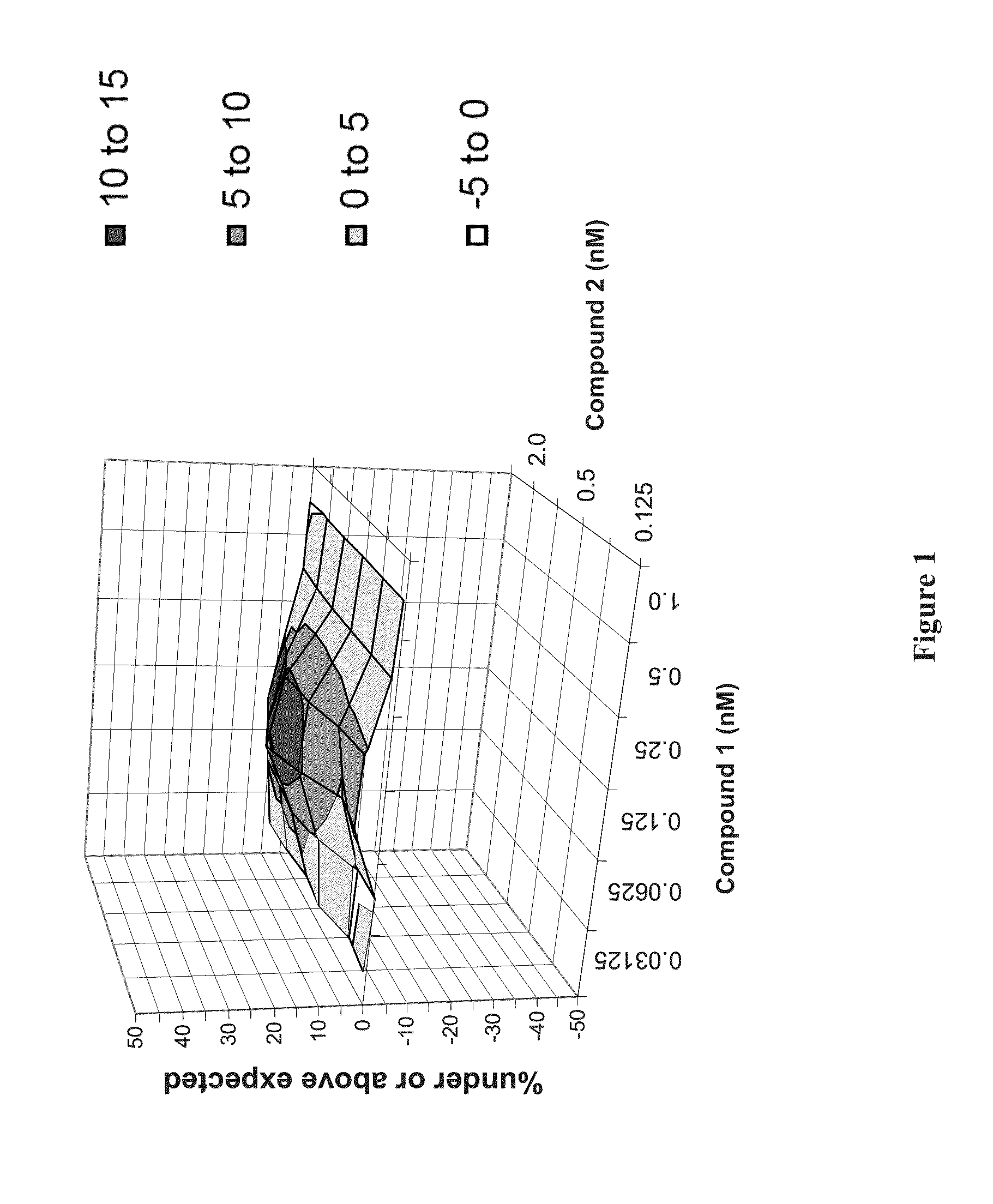
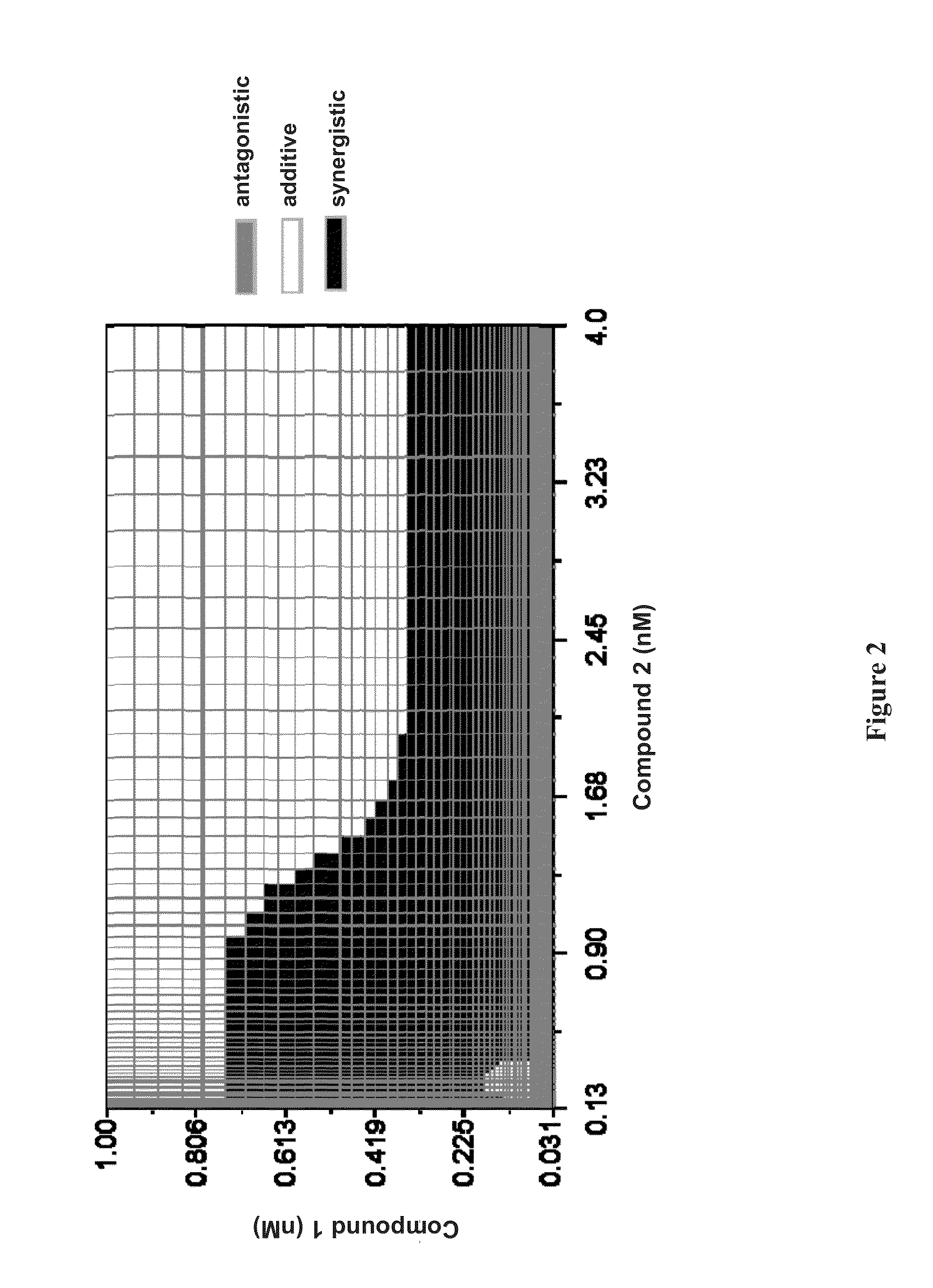
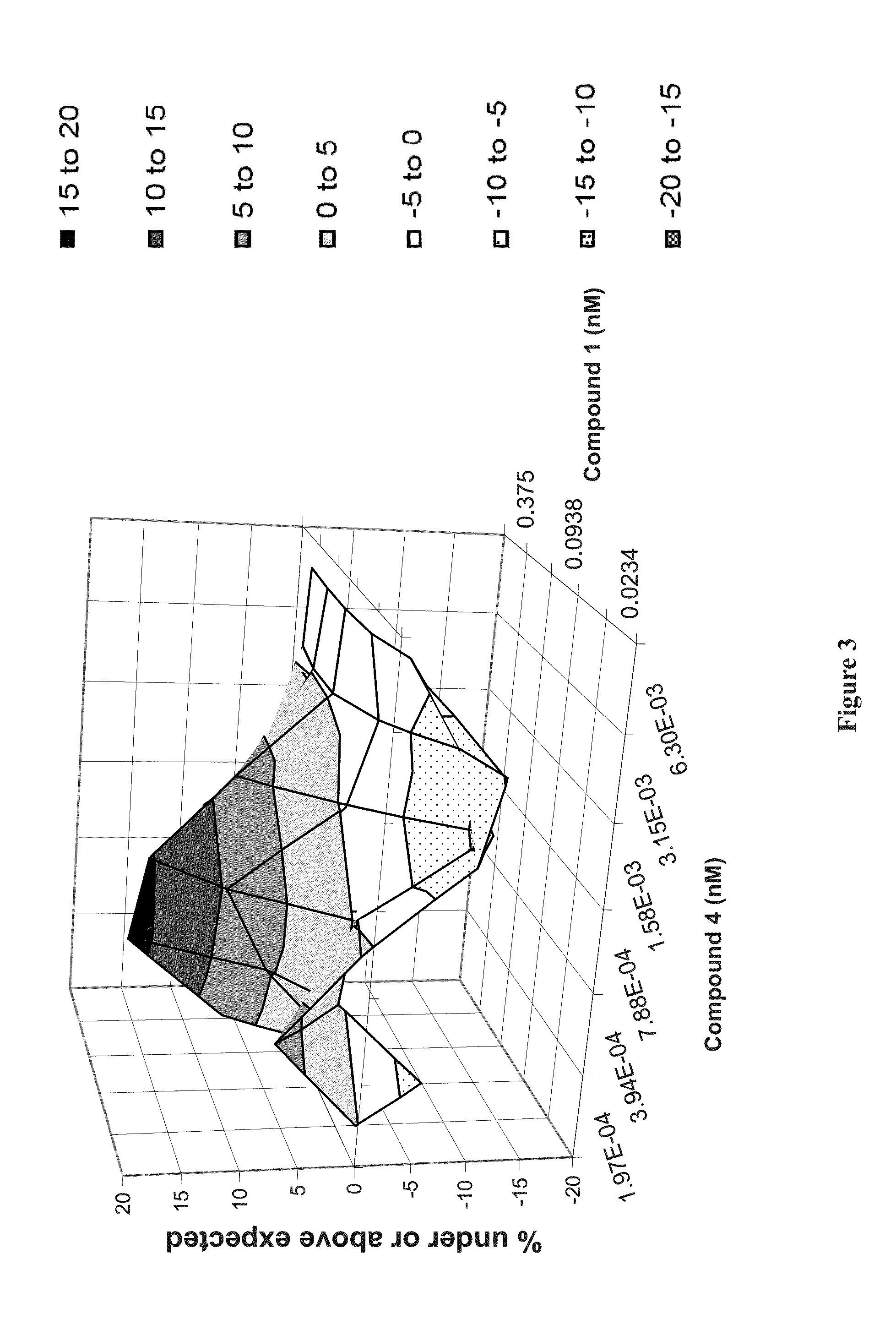
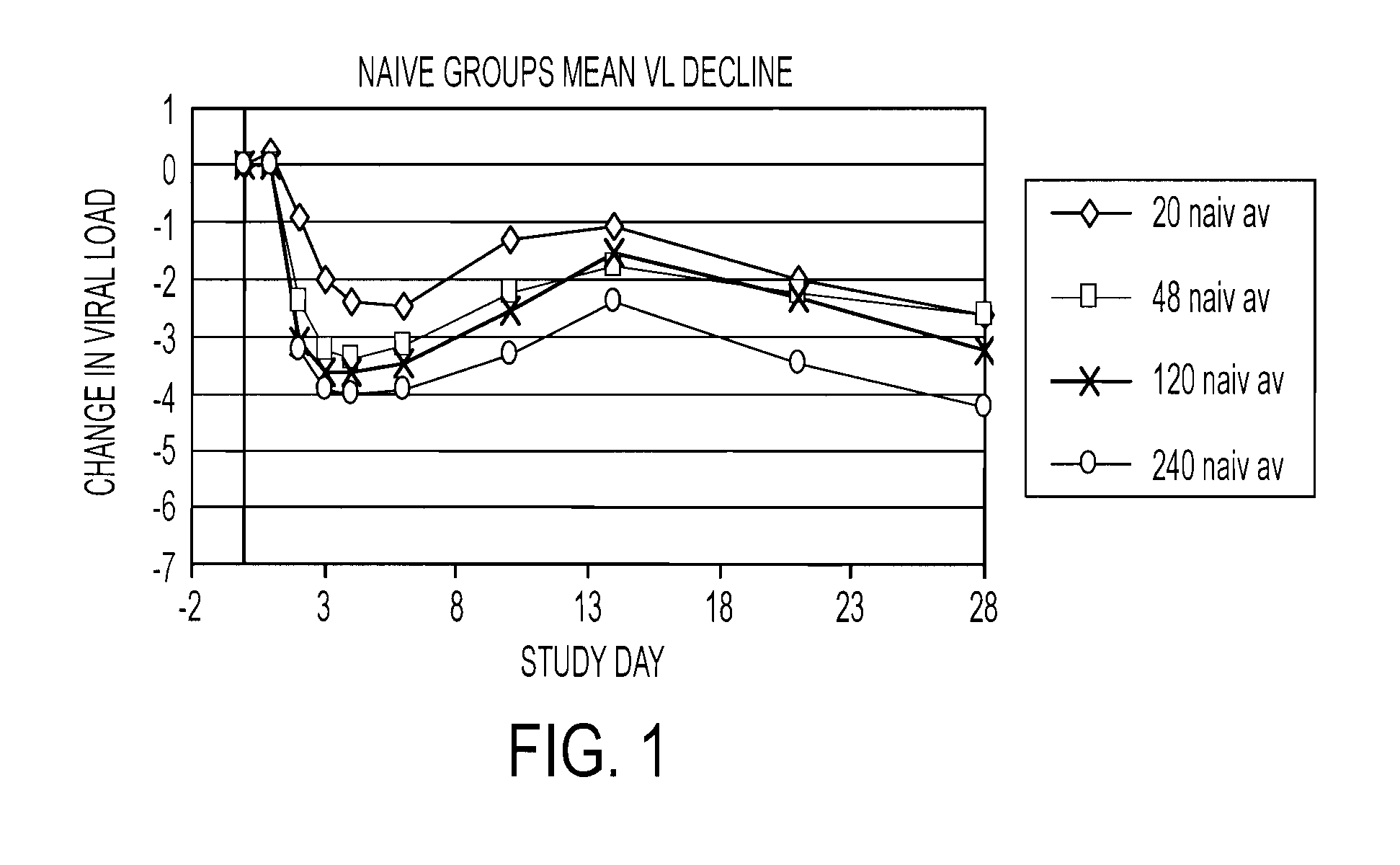
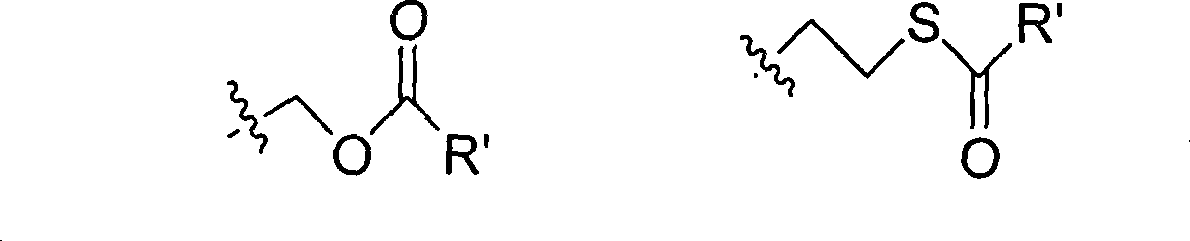Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
403 results about "Ribavirin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ribavirin is used in combination with other antiviral medications (such as interferon, sofosbuvir) to treat chronic (long-lasting) hepatitis C, a viral infection of the liver.
Drug for reducing side effects in ribavirin interferon combination therapy
InactiveUS20060088502A1Eliminate side effectsReduces side effects found in ribavirin/IFNBiocideElcosanoid active ingredientsChronic viral hepatitis CSide effect
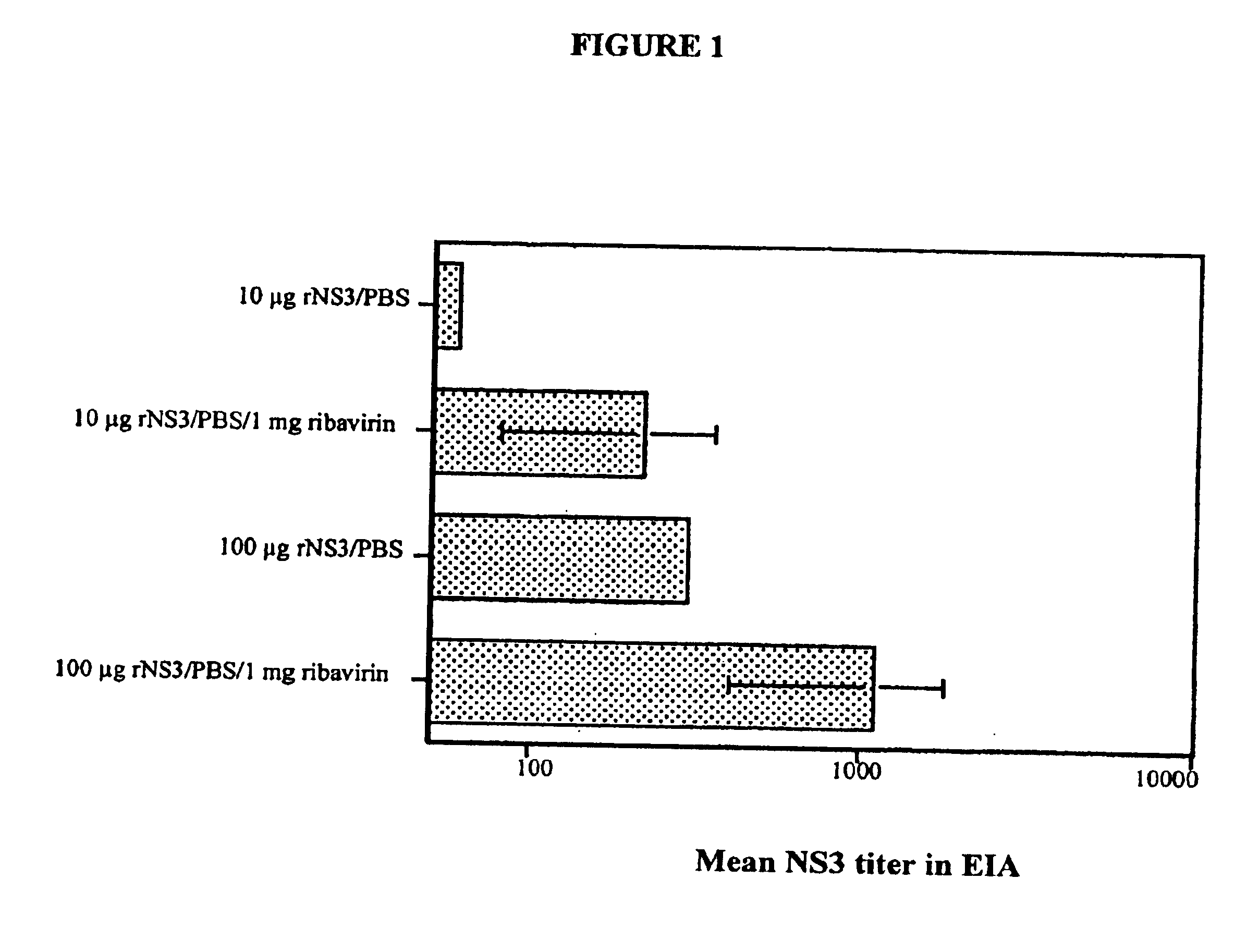
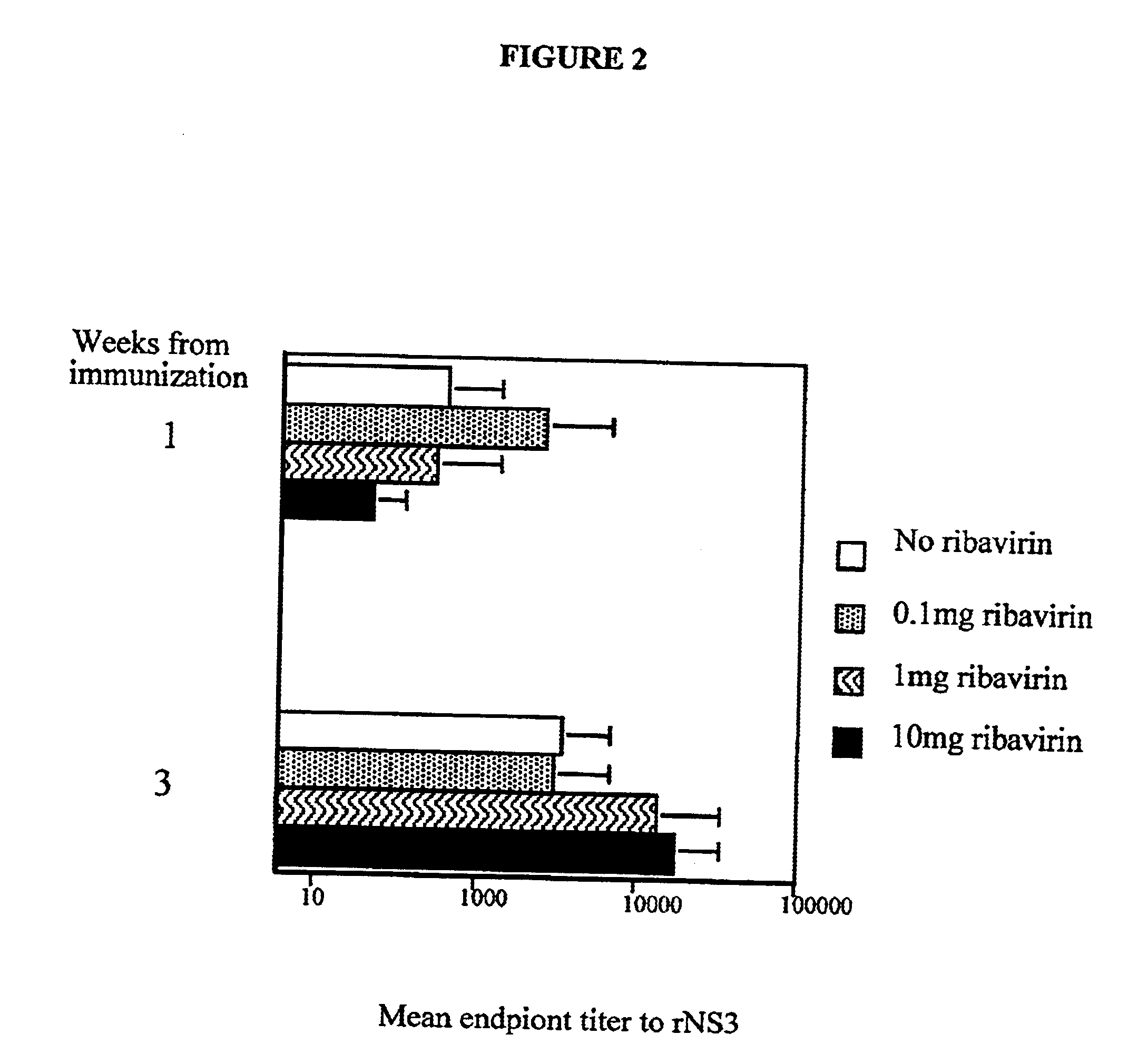
There is provided a drug for reducing side effects, anemia in particular, in combination therapy of chronic hepatitis C with ribavirin and interferon, which contains as the active ingredient at least one member selected from the group consisting of eicosapentaenoic acid (EPA) and pharmaceutically acceptable salts and esters thereof.
Owner:MOCHIDA PHARM CO LTD
Process for preparation of nucleoside phosphoric acid ester compound and application thereof
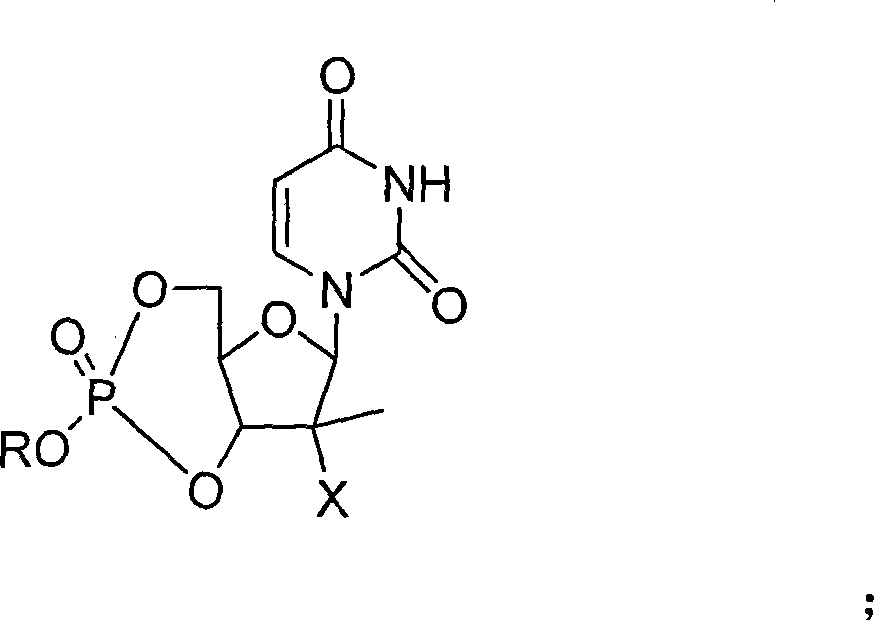
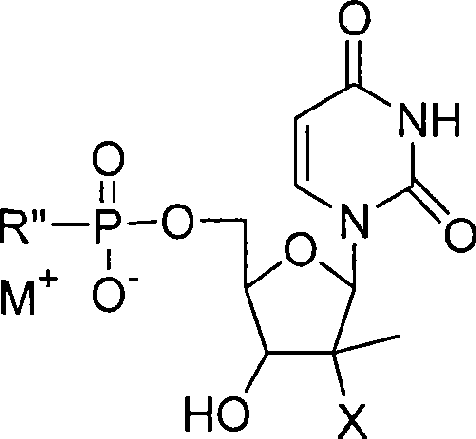
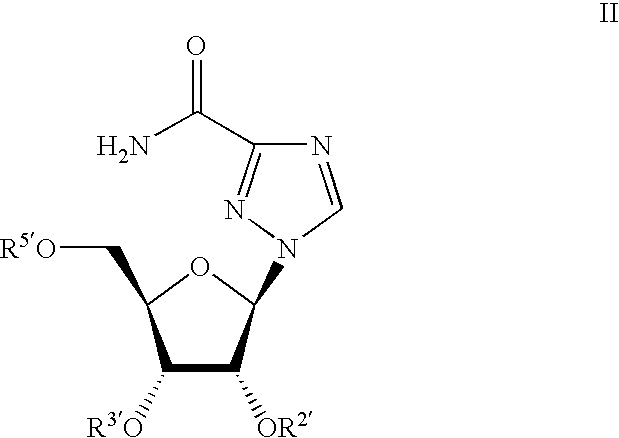
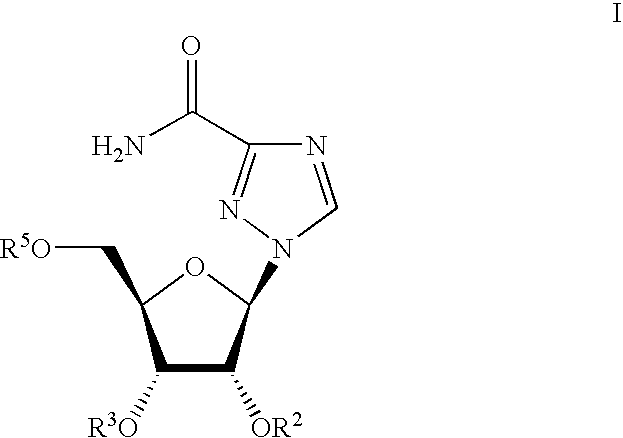
The invention provides the nucleoside phosphate compound and the preparation and application, which comprises the nucleoside 3', 5'-cyclic phosphate and the 5'-phosphodiester compound. Wherein, the 3', 5'-cyclic phosphate and the derivatives work as the novel HCV inhibitor and the structural formula is shown in the drawing (I), wherein, X equals to OH and F; R equals to H, NH4, R' 4N, R' 3NH and metals such as Na, K, Ca and Li or the structural formula in the drawing (II), R' equals to linear chain or substituted alkane or cyclane, aromatic hydrocarbon, or substituted aromatic hydrocarbon or heterocyclic aromatic hydrocarbon; the four R' and three R' in the amine are the same or different. The invention has the advantages that the nucleoside phosphate compound is used for treating the HCV or is combined with the Alpha-interferon, ribavirin or other anti-HCV drugs to treat the infection of the hepatic c virus or HCV.
Owner:冷一欣
Methods for Treating HCV
ActiveUS20130102526A1Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
HCV combination therapy
InactiveUS6849254B1Ameliorate ribavirin-related hemolysisLow viral-RNABiocidePeptide/protein ingredientsChronic viral hepatitis CHemolysis
Methods of treating patients having susceptible viral infections, especially chronic hepatitis C infection by administering to said patient a therapeutically effective amount of a combination therapy of interferon-alfa and ribavirin for a time sufficient to lower HCV-RNA in association with a therapeutically effective amount of an antioxidant for a time sufficient to ameliorate ribavirin-related hemolysis are disclosed.
Owner:MERCK SHARP & DOHME CORP
Combination therapy for treating hcv infection
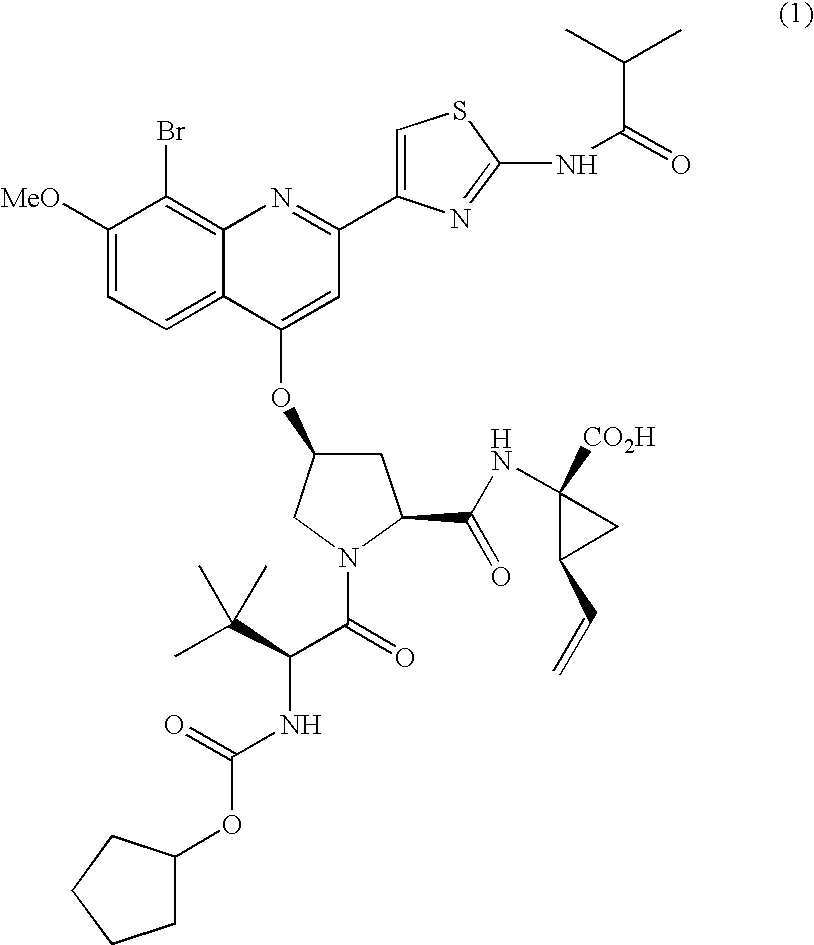
The present invention relates to therapeutic combinations comprising (a) Compound (1), or a pharmaceutically acceptable salt thereof, as herein described, (b) an interferon alfa and (c) ribavirin. Compound (1) is a selective and potent inhibitor of the HCV NS3 serine protease. The present invention also relates to methods of using such therapeutic combinations for treating HCV infection or alleviating one or more symptoms thereof in a patient.
Owner:BOEHRINGER INGELHEIM INT GMBH
Vaccines containing ribavirin and methods of use thereof
Compositions and methods for enhancing the effect of vaccines in animals, such as domestic, sport, or pet species, and humans are disclosed. More particularly, vaccine compositions comprising ribavirin and an antigen, preferably an antigen that has an epitope present in Hepatitis C virus (HCV), are disclosed for use in treating and preventing disease, preferably HCV infection.
Owner:TRIPEP
Combination therapy for HCV infection
The present invention relates to therapeutic combinations comprising VX-497, ribavirin, and interferon. The present invention also relates to methods using the therapeutic combinations of the present invention for treating HCV infection or alleviating one or more symptoms thereof in a patient. The present invention also provides kits comprising the combinations of the present invention.
Owner:VERTEX PHARMA INC
Methods for treating HCV
ActiveUS8492386B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Large dose ribavirin formulations
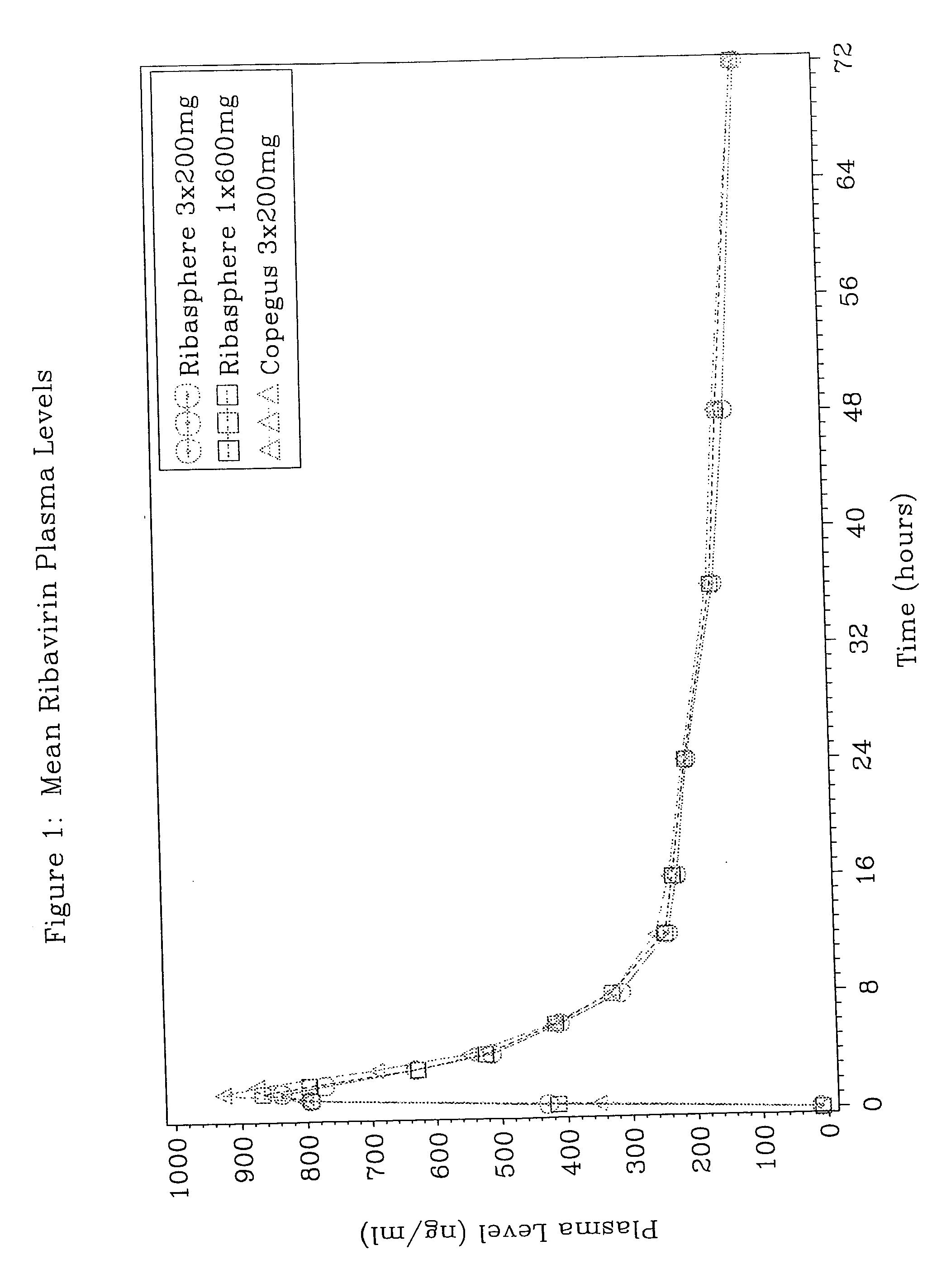
The present invention is related to pharmaceutical dosage forms of ribavirin which are designed to increase patient compliance to a ribavirin therapy. Examples of such dosage forms include 400 mg to 600 mg tablets. These dosage forms are bioequivalent to multiple doses of tablets containing small amounts of ribavirin.
Owner:CHARTWELL PHARM LLC
Composition containing ribavirin and use thereof
InactiveUS20050019406A1Improved ribavirin compositionMinimal variationBiocideCarbohydrate active ingredientsMedicineRibavirin
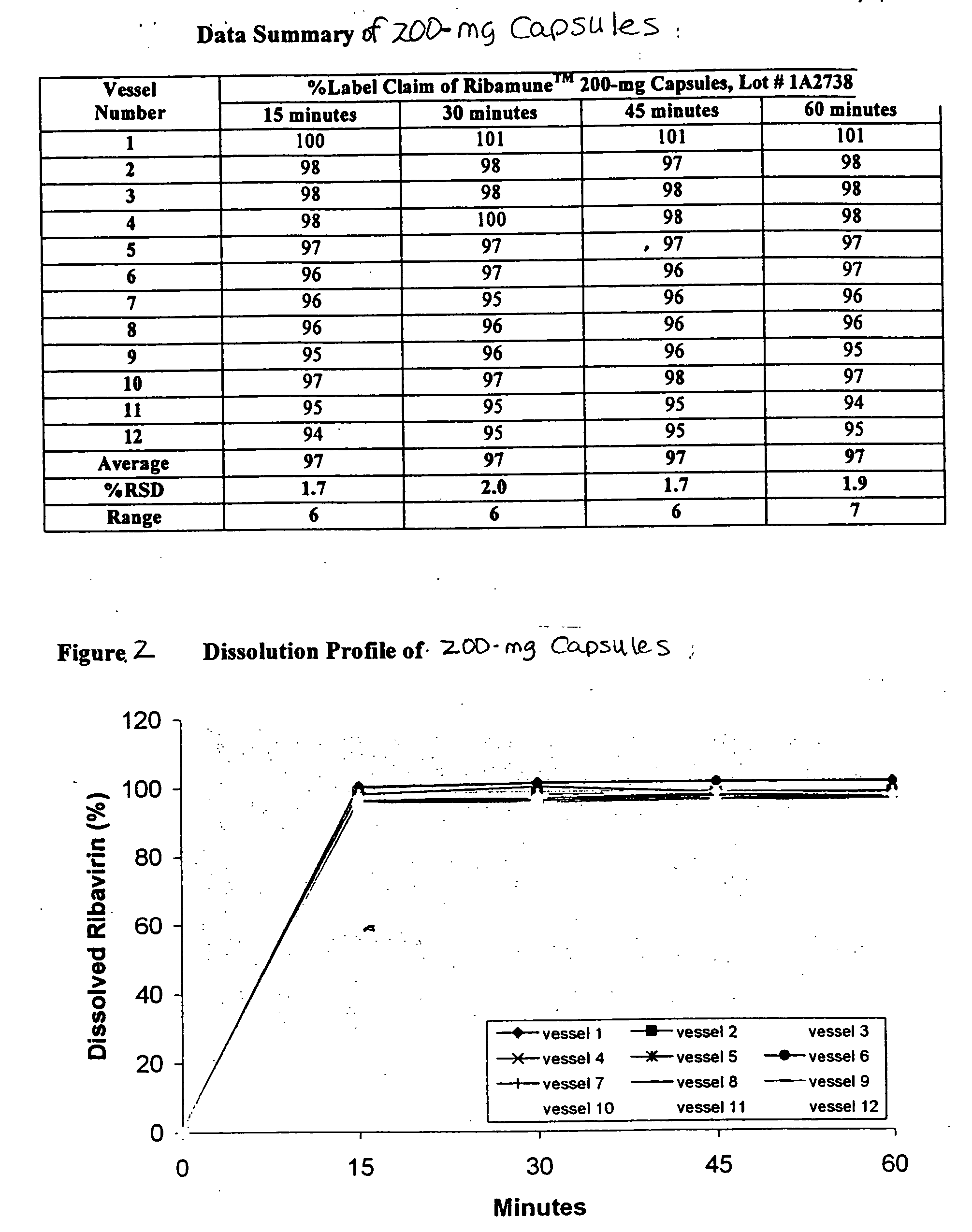
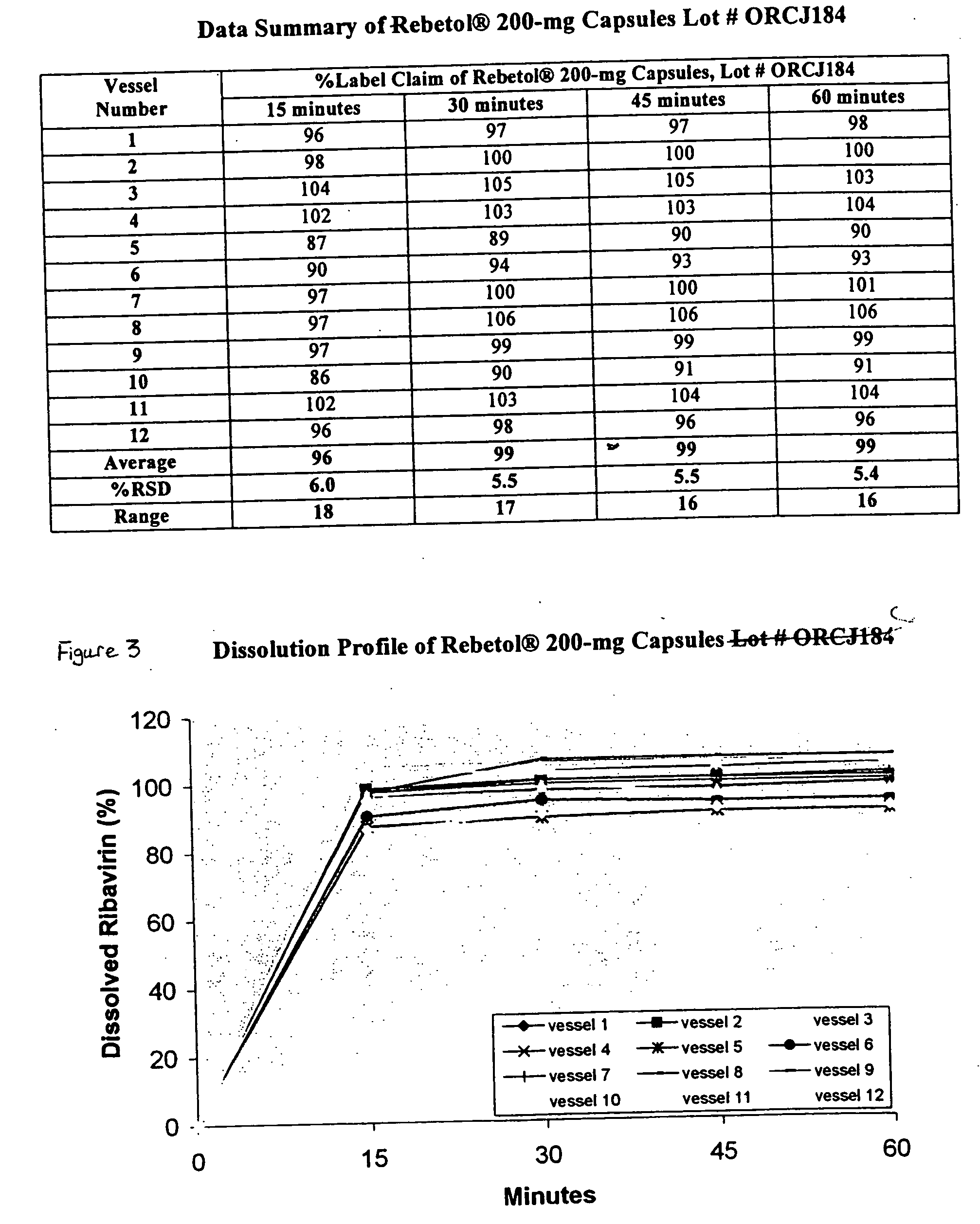
Ribavirin formulations are disclosed for use in capsules or tablets as well as processes for their preparation and methods for their administration.
Owner:KADMON PHARMA LLC
Treatment of viral infections using the L-isomer of ribavirin
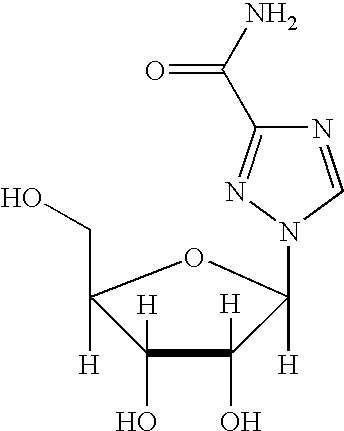
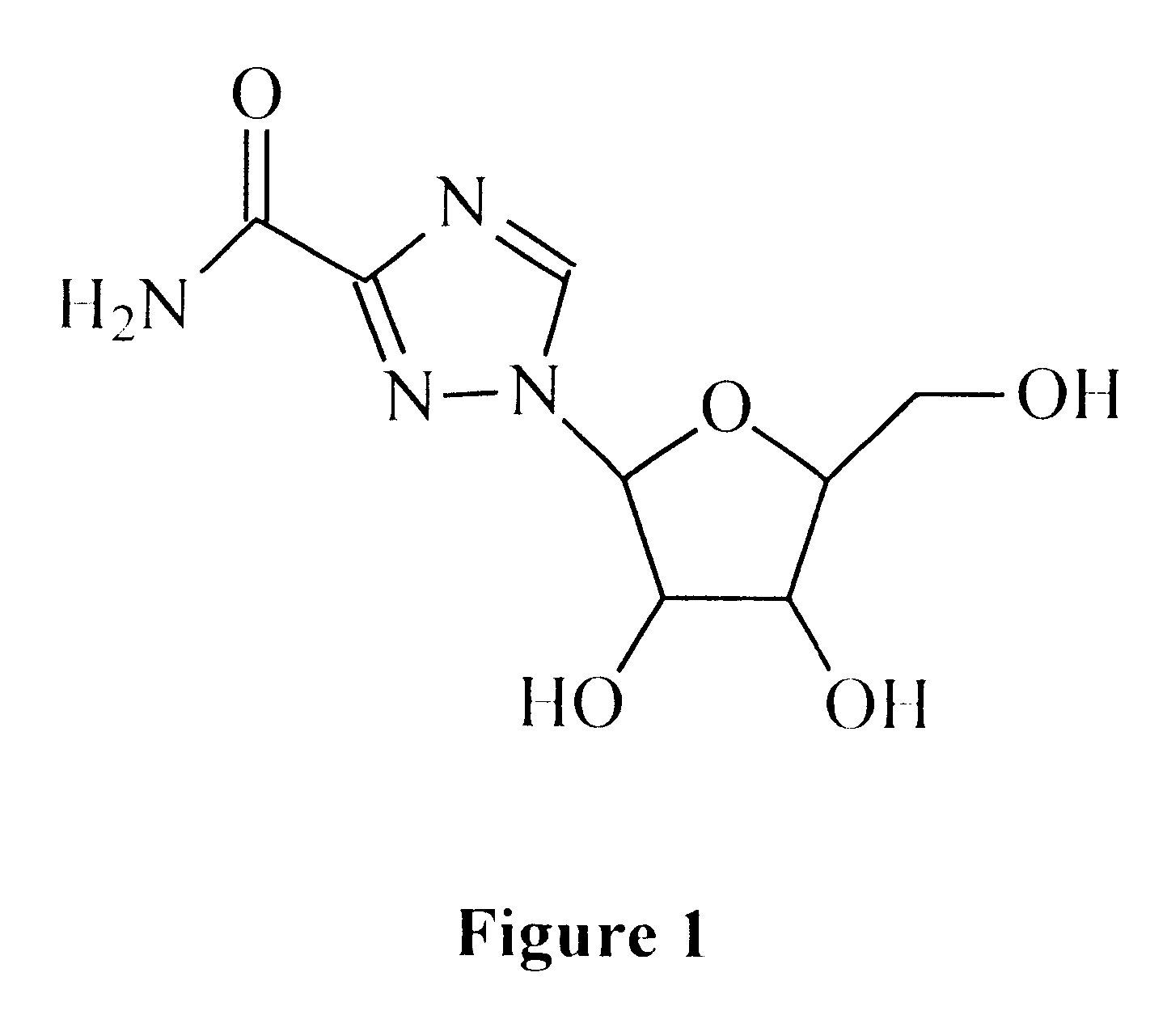
A 1-(beta-L-ribofuranosyl)-1,2,4-triazole-3-carboxamide is administered in a method of treatment of a viral infection in a patient, including HIV infection, HCV infection, or BHV infection.
Owner:VALEANT RES & DEV
Ribavirin-interferon alfa combination therapy for eradicating detectable HCV-RNA in patients having chronic hepatitis C infection
Ribavirin derivatives represented by the formula II, pharmaceutical compositions containing them as well as methods of using the ribavirin derivatives represented by the formula II for the treatment of susceptible viral infections, for example, chronic hepatitis C infections administrating, the ribavirin derivatives being represented by formula II are disclosed.
Owner:SCHERING CORP
Combination therapy for treating hcv infection
InactiveUS20120135949A1Relieve symptomsBiocideOrganic chemistryCompound (substance)Combination therapy
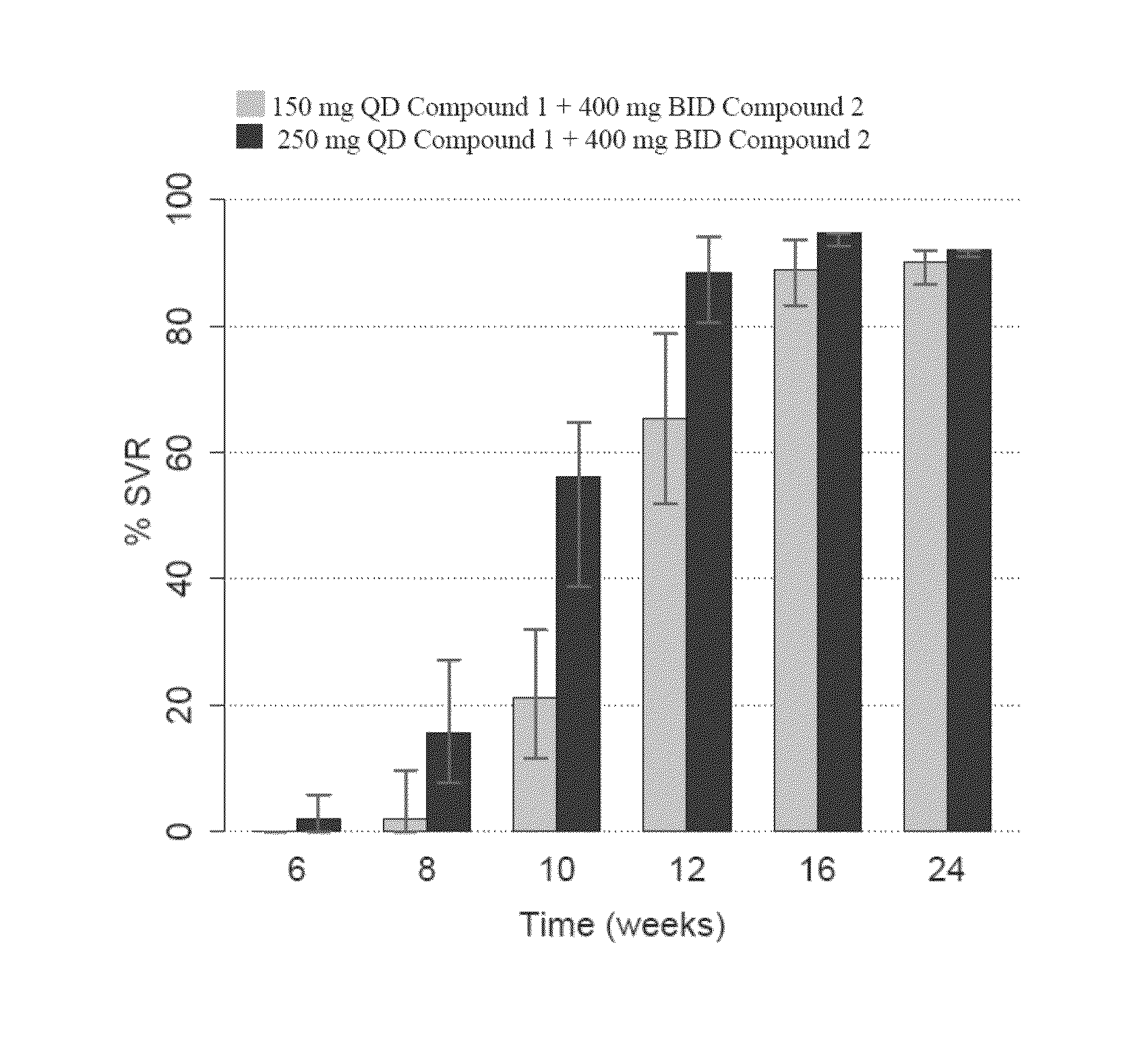
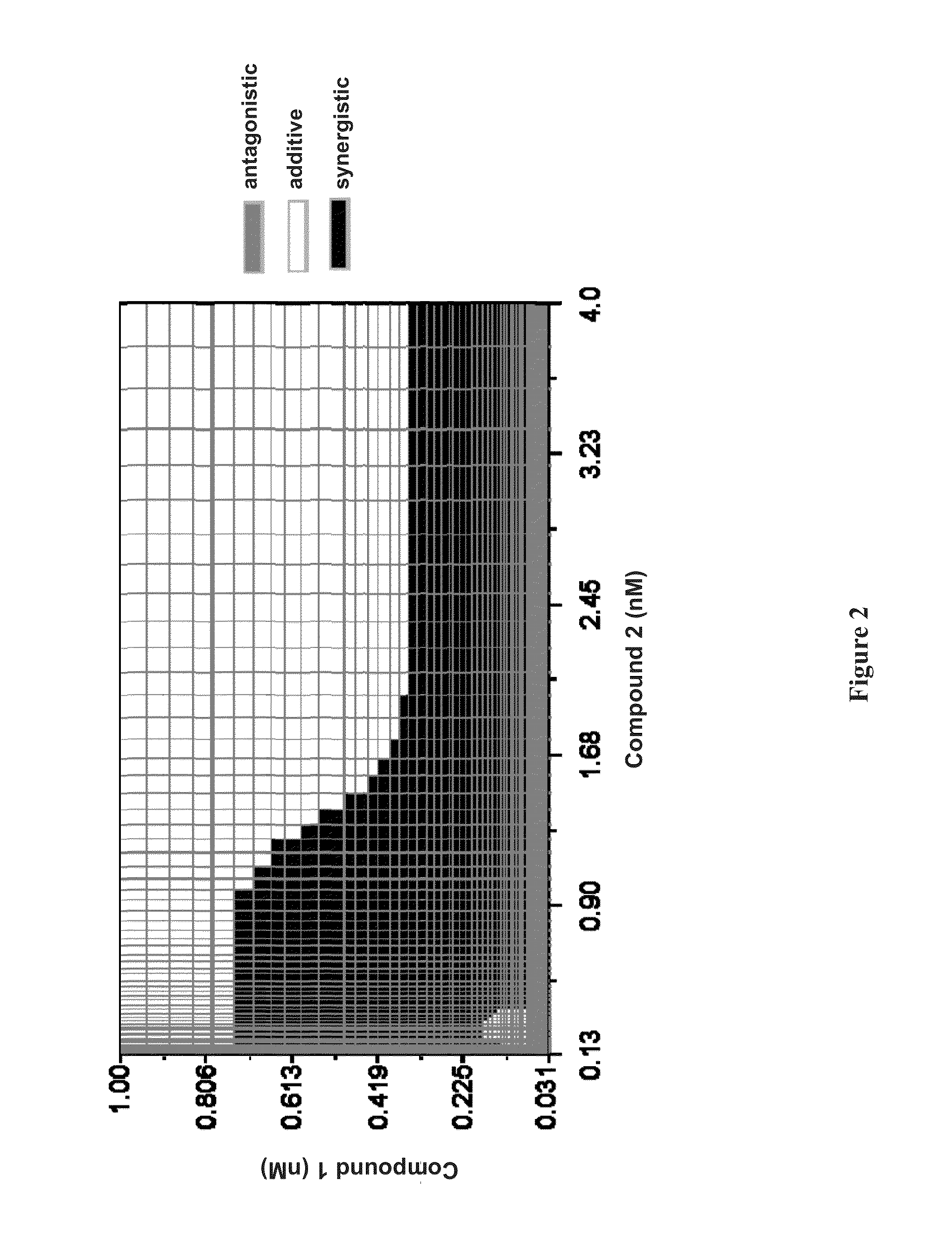
The present invention relates to therapeutic combinations comprising (a) Compound (1), or a pharmaceutically acceptable salt thereof, as herein described, (b) Compound (2), or a pharmaceutically acceptable salt thereof, as herein described, and optionally (c) ribavirin, and methods of using such therapeutic combinations for treating HCV infection or alleviating one or more symptoms thereof in a patient.
Owner:BOEHRINGER INGELHEIM INT GMBH
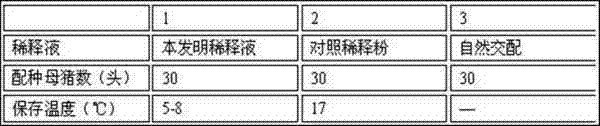
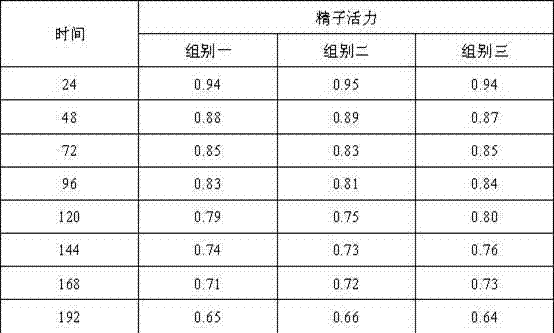
Boar antiviral long-acting semen diluent formula and preparation method
Belonging to the technical field of boar semen dilution, the invention relates to a boar antiviral long-acting semen diluent formula and a preparation method. The boar semen diluent comprises: alpha-D-glucopyranose powder, fructose, trisodium citrate dihydrate, ethylenediamine tetraacetic acid disodium, sodium bicarbonate, polyvinyl alcohol, trishydroxymethylaminomethane, inositol, vitamin C, arginine, a radix isatidis injection, a 5% ceftiofur sodium injection, and a 5% Ribavirin injection. The preparation method includes: dissolving all the components in distilled water, mixing the components evenly, and conducting sterilization to obtain the boar semen diluents. The formula involves rich nutrients, antibacterial Chinese and western medicine components and antibacterial components, thus ensuring normal sperm metabolism. After certain period of time at the lower limit of storage temperature, the effects of nutrition and sperm shock prevention can be realized, and the invasion of external bacterial viruses in an artificial insemination process can be avoided.
Owner:GUANGDONG ZHONGNONGLIAN BIOLOGICAL PHARMCO
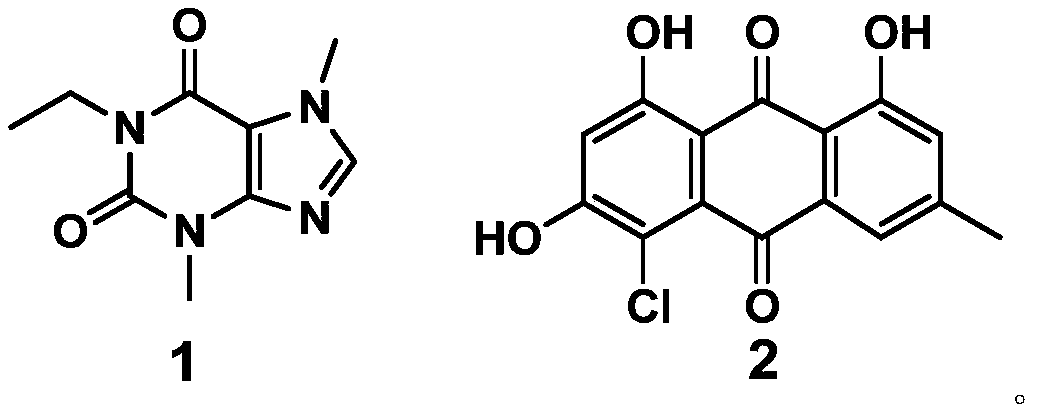
Application of compound to preparation of medicines for treating viral pneumonia
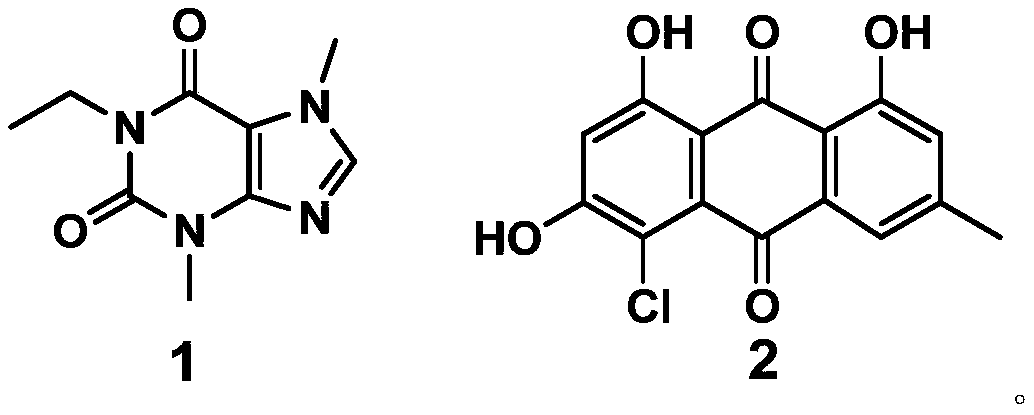
ActiveCN111228275AExcellent antiviral pneumonia effectStrong anti-virusAntiviralsRespiratory disorderPurinePharmacology
The invention discloses an application of a compound to preparation of medicines for treating viral pneumonia, and relates to the technical field of drugs. The technical problem that novel antiviral drugs are deficient in the prior art, can be solved. The compound comprises a purine compound adopting the structure as shown in the formula 1, and / or an anthraquinone derivative adopting the structureas shown in the formula 2. The purine compound adopting the structure as shown in the formula 1, and / or the anthraquinone derivative adopting the structure as shown in the formula 2 both can show predominant effect for resisting viral pneumonia when being separately used or used jointly. Drugs for treating viral pneumonia, are prepared by the compound provided by the invention are more notable inactivity compared with clinical frontline drugs, and are better than drugs of ribavirin, oseltamivir and the like.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
A kind of preparation method of ribavirin
ActiveCN102286046ARaise the level of industrial productionLow market priceSugar derivativesSugar derivatives preparationInosineAlcohol
The invention discloses a preparation method of ribavirin, comprising the following steps: A, by taking inosine as a raw material, adding acid and a catalyst I for acylation reaction to generate ribofuranose tetraacetate; B, respectively treating the ribofuranose tetraacetate obtained in the step A and 1,2,4-triazole-3-carboxylic acid methyl ester by using active carbon firstly, then uniformly mixing the ribofuranose tetraacetate and the 1,2,4-triazole-3-carboxylic acid methyl ester, and then adding a catalyst II for condensation reaction to obtain a condensation compound; C, subjecting the condensation compound obtained in the step B to ammonolysis in ammonia and methyl alcohol to generate crude ribavirin; and D, carrying out refinement on the crude ribavirin obtained in the step C to obtain pure ribavirin. The method is simple in operation, good in selectivity, clean and environment friendly and high in yield of ribavirin.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Methods of treating West Nile virus infection
The present invention provides methods of preventing or treating West Nile virus as well as infections caused by other viruses of the Flaviviridae family in animals comprising administering to the animal an effective amount of ribavirin and / or interferon alpha-2b.
Owner:THE NEW YORK HOSPITAL MEDICAL CENT OF QUEENS
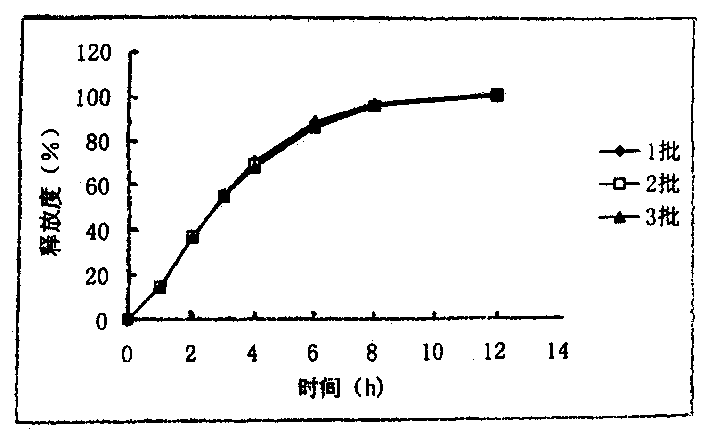
Libaweilin slow-released pill
InactiveCN1437936AImprove complianceSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismAntiviral drugMedicine
Owner:GUANGZHOU PUIS PHARMA FACTORY
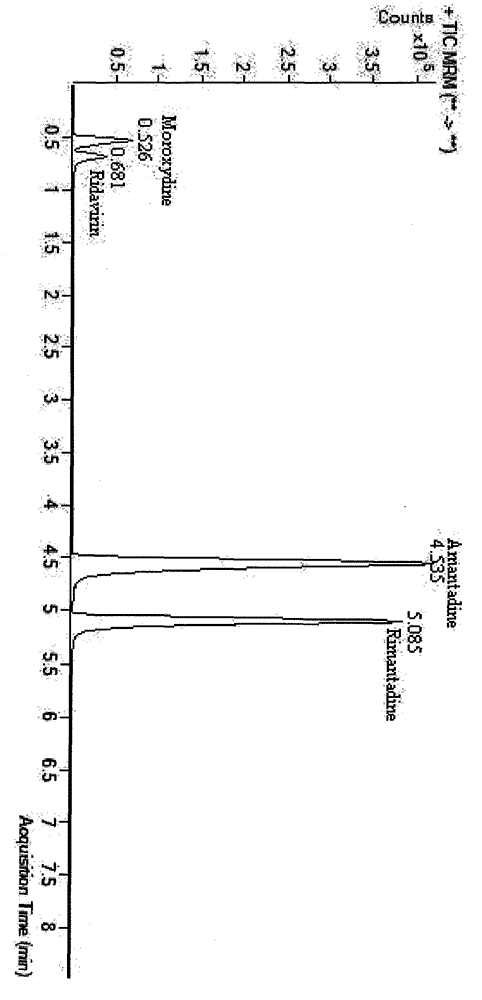
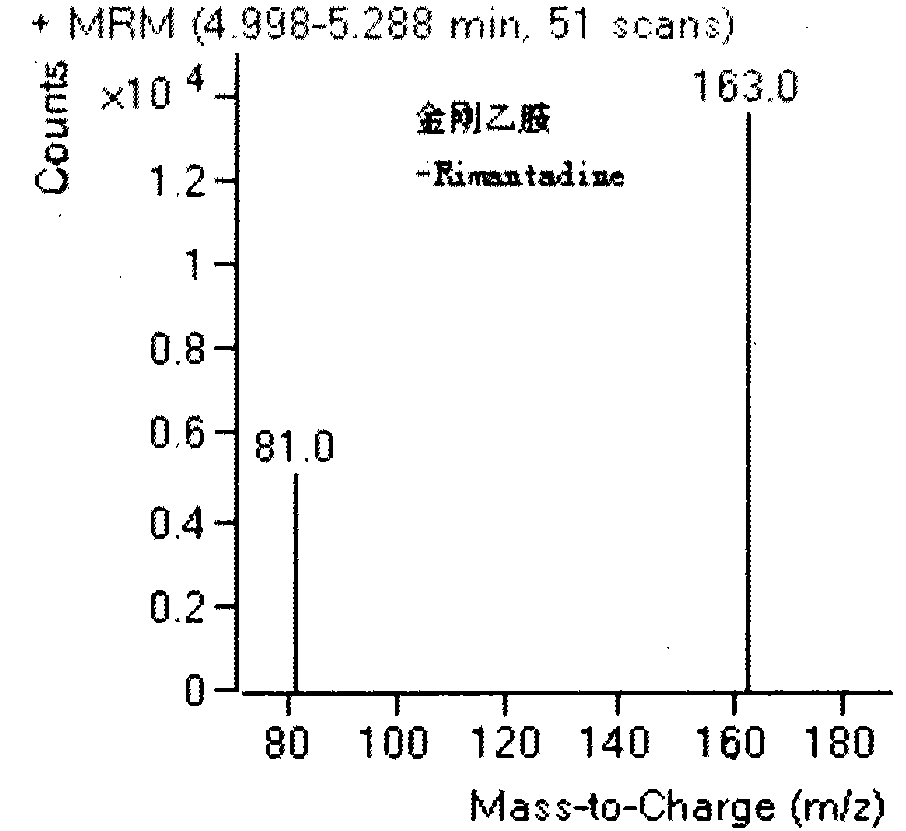
Combined detection method of amantadine, rimantadine, ribavirin and moroxydine residues in eggs
The invention belongs to the technical field of poultry product detection and relates to a combined detection method of amantadine, rimantadine, ribavirin and moroxydine residues in eggs. The method comprises the following steps: carrying out low temperature repetitive freeze-thawing on a sample, adding a formic acid-methanol solution, mixing, centrifuging, taking a supernatant for standby, adding water saturated n-hexane, mixing, carrying out ultrasonic treatment, centrifuging to remove n-hexane floccules on the upper layer with an extracting solution left at the lower layer, purifying with a cation exchange solid-phase extraction column, measuring by a liquid chromatography-tandem mass spectrometer provided with an ESI source, and carrying out accurate qualitative and quantitative analysis on residues of the four antiviral drugs in eggs. The method provided by the invention has high specificity, can accurately and simultaneously measure residual quantity of amantadine, rimantadine, ribavirin and moroxydine without pollution, has high sensitivity, and provides technical support for guaranteeing quality safety of eggs.
Owner:山东世通检测评价技术服务有限公司
Treatments for viral infections using IFN cytokines and ribavirin, alone or in combination
InactiveUS20060024271A1Reduce dosageReduced pro-inflammatory responseBiocidePeptide/protein ingredientsCytokineViral infection
A treatment or prophylaxis for viral infection using interferon (IFN) cytokines alone or in combination with ribavirin is provided. The treatments and prophylaxis allow for lowered dosages of IFNs, reduced pro-inflammatory responses, and delays the initiation time and reduced frequency of the IFN treatment required.
Owner:AFG BIOSOLUTIONS
Preparation for inhibiting coronavirus infections
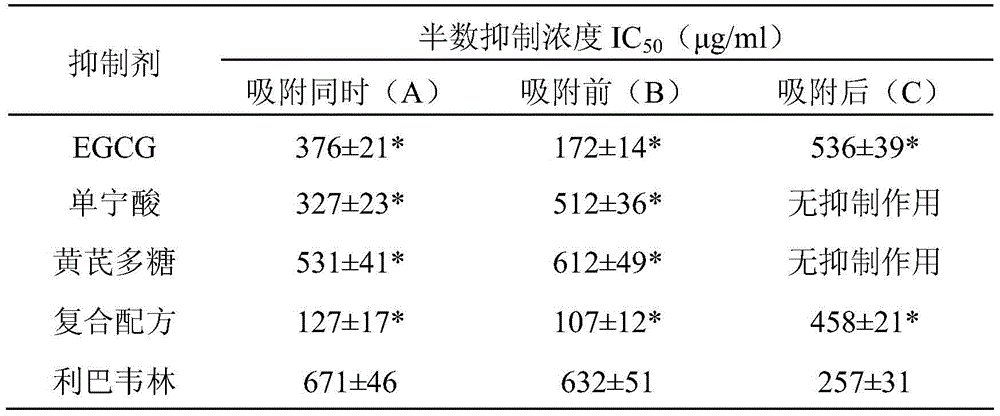
InactiveCN105687226AAvoid infectionLow cytotoxicityOrganic active ingredientsAntiviralsAstragalus polysaccharideCytotoxicity
The invention discloses a preparation for inhibiting coronavirus infections. The preparation provided by the invention is applied to (1) or (2) as follows: (1) preparing of products for inhibiting the coronavirus infections; (2) inhibiting of coronavirus infections. The preparation is prepared mainly through mixing epigallocatechin gallate, tannin and astragalus polysaccharides, wherein the mass proportioning ratio of the epigallocatechin gallate to the tannin to the astragalus polysaccharides is (0.5 to 1.0): (0.5 to 1.0): (0.5 to 1.5). The cytotoxicity of the compounded preparation provided by the invention is not higher than that of a control sample, i.e., ribavirin which has already obtained security permission, so that the preparation is safer. The coronavirus infections can be effectively inhibited through using the preparation provided by the invention in a preventive manner in case of safe working concentration, so that the preparation has a commercial value in being further researched and developed into a coronavirus infection inhibitor.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Synergistically effective combinations of dihaloacetamide compounds and interferon or ribavirin against HCV infections
ActiveUS20050129659A1Reach therapeutic levelReduce the amount requiredBiocideOrganic chemistryInterferon alphaVirus
The present invention relates to anti-HCV dihaloacetamide compounds in synergistic combination with an interferon and / or ribavirin and pharmaceutical compositions thereof for inhibition of the replication of HCV virus. The present invention also relates to the use of the compositions to inhibit HCV replication and / or proliferation and to treat or prevent HCV infections.
Owner:RIGEL PHARMA
Enhanced compliance antiviral medicaments and methods of manufacture and use
InactiveUS20050281872A1Reduce gastrointestinal side effectsReducing gastrointestinal bleeding and the resultant patient discomfortBiocideCarbohydrate active ingredientsSide effectPatient compliance
Reduced gastrointestinal side effect medicaments for treating viral infection in a patient suffering therefrom are provided comprising at least 500 mg of antiviral compound in an oral dosage form that can be administered in effective total daily dosages of antiviral compound ranging from 1000 mg to 2000 mg. The reduced gastrointestinal side effect medicaments enhance patient compliance with long term, multi-dose treatment regimens by reducing physiological and psychological side effects that can cause reductions or discontinuations of antiviral therapy. Exemplary antiviral compounds include nucleoside analogues such as ribavirin, levovirin, and viramindine which are effective when combined with interferon to treat acute or chronic viral infections including hepatitis, and particularly hepatitis C. Associated methods for the production and use of the medicaments also are provided.
Owner:AXIUM HEALTHCARE PHARMACY
Pyrimidopyrimidine compound, nucleoside analog derivative thereof, preparation method thereof and use thereof
ActiveCN102516339AInhibitory activityPotential to inhibit replicationOrganic active ingredientsSugar derivativesChemical compoundDiaminopyrimidine
The invention relates to a pyrimidopyrimidine compound and a nucleoside analog derivative thereof. The pyrimidopyrimidine compound and the nucleoside analog derivative thereof prepared in the invention have certain hepatitis virus inhibition activities, and 4,7-diamino-1-(beta-2-deoxy-D-ribofuranose)pyrimido[4,5-d]pyrimidine-2,5(1H,6H)-dione and 4,7-diaminopyrimido[4,5-d]pyrimidine-2,5(1H,6H)-dione perform obvious virus copying inhibition potentials and are better than ribavirin, so a new selection is provided for clinical medicines.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409AAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
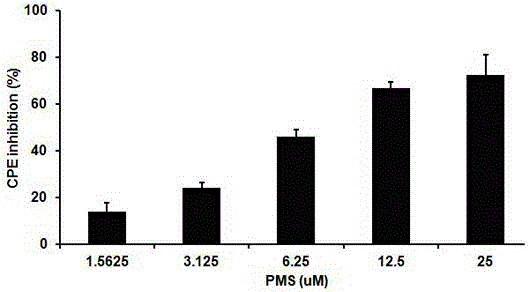
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Oral ribavirin pharmaceutical compositions
InactiveUS20070173464A1Maintain bioavailabilityExtension of timeBiocideCarbohydrate active ingredientsReservoir typeImmediate release
Owner:FLAMEL TECHNOLOGIES
Ribavirin inhalation powder atomizing agent and its preparing process and anti-virus infection use
The Ribavirin inhalation is a kind of respiratory tract mucous membrane absorbed preparation comprising superfine Ribavirin powder and fine medicinal carrier powder and is administrated with special administrating device. It has high and fast target effect, safe antiviral function, no stimulation to mucous membrane and other advantages. The present invention further expands the clinical application range of Ribavirin.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Methods for treating hcv
InactiveUS20140275099A1Avoid side effectsEfficient managementBiocideDigestive systemShort durationInterferon alpha
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20130102558A1Avoid side effectsImprove pharmacokineticsBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Application of ganoderma acid y in preparation of medicine for treating or preventing enterovirus 71 infection
InactiveCN102274234AAnti-infectionAnti-inflammatoryOrganic active ingredientsAntipyreticPositive controlTreatment effect
The invention discloses application of ganoderic acid Y to the preparation of a medicament for treating or preventing enterovirus 71 infection. The invention proves that the ganoderic acid Y has a good in-vitro antivirus effect in an enterovirus 71 infection cell test. Meanwhile, the ganoderic acid Y has a certain killing effect on enterovirus 71, can be used for preventing the infection of the enterovirus 71, and has a good treatment effect on cells infected by the enterovirus 71 and a good inhibiting effect on virus replication; and the acting effect of the ganoderic acid Y is more remarkable than ribavirin serving as a positive control medicament. Moreover, the ganoderic acid Y has a good inhibiting effect on inflammatory reactions caused by the enterovirus 71, and the prospect of the development of the medicament into an anti-enterovirus 71 medicament is disclosed.
Owner:WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com