Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
831 results about "Influenza A virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza A virus causes influenza in birds and some mammals, and is the only species of the genus Alphainfluenzavirus of the virus family Orthomyxoviridae. Strains of all subtypes of influenza A virus have been isolated from wild birds, although disease is uncommon. Some isolates of influenza A virus cause severe disease both in domestic poultry and, rarely, in humans. Occasionally, viruses are transmitted from wild aquatic birds to domestic poultry, and this may cause an outbreak or give rise to human influenza pandemics.
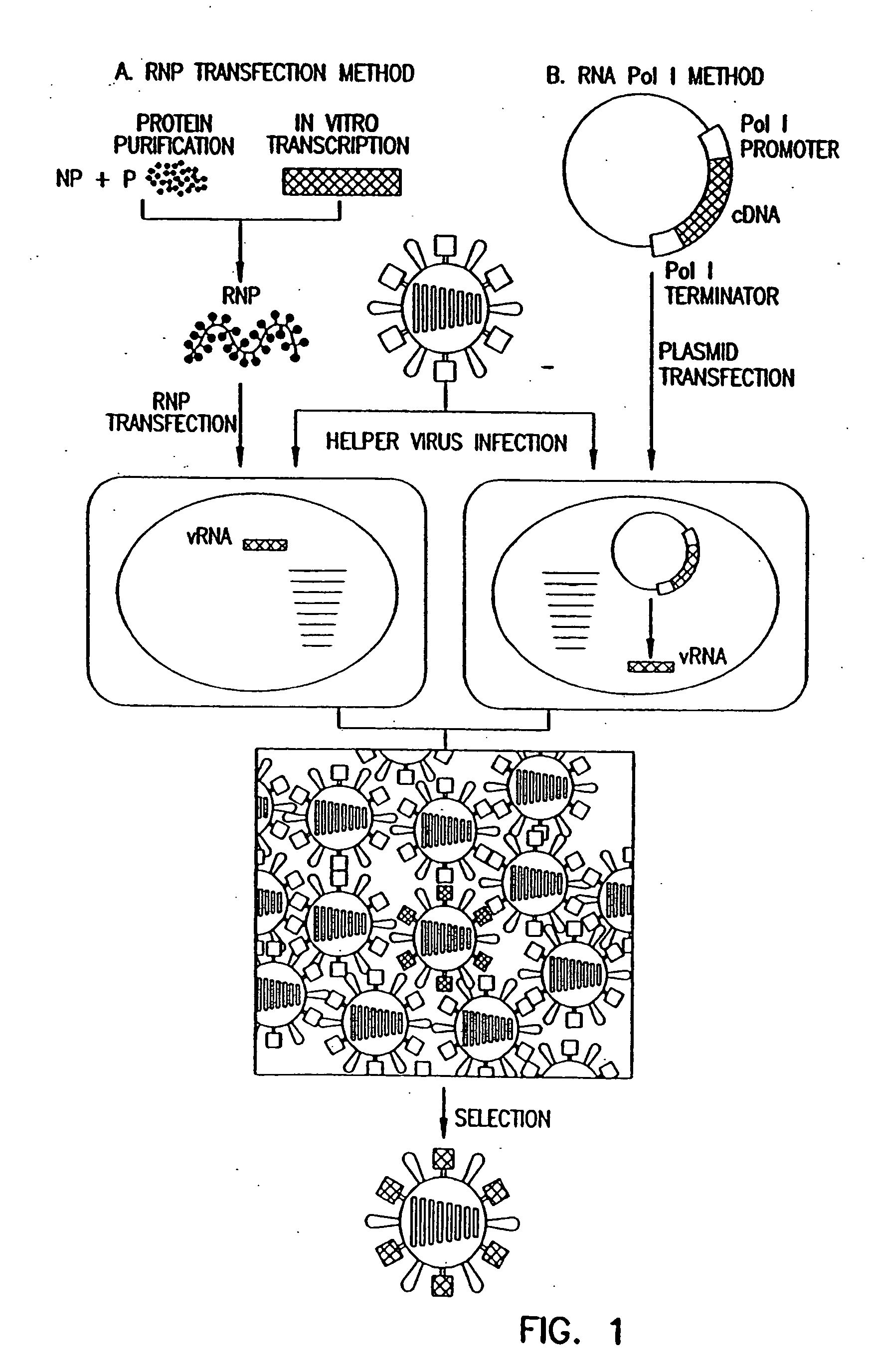
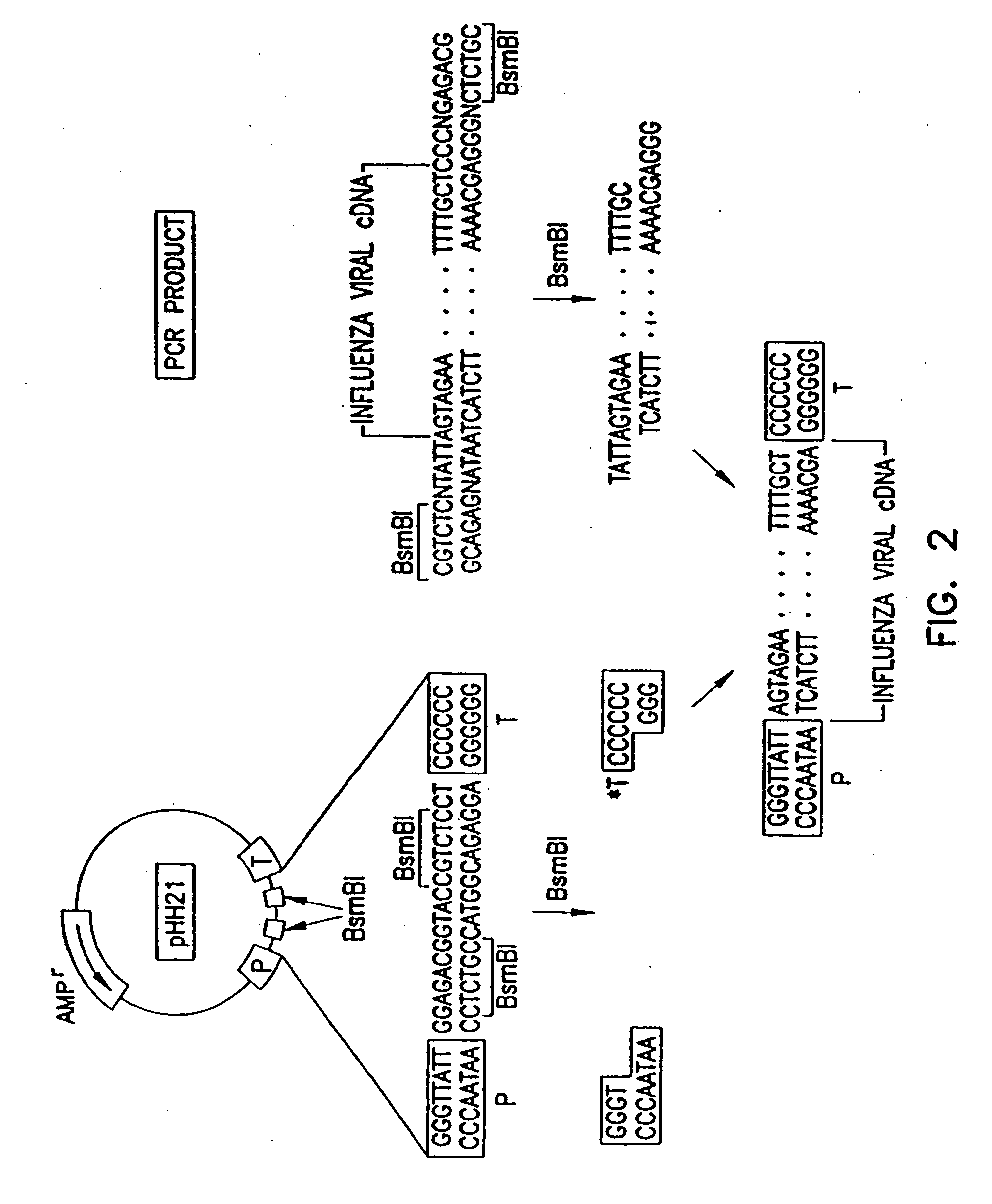
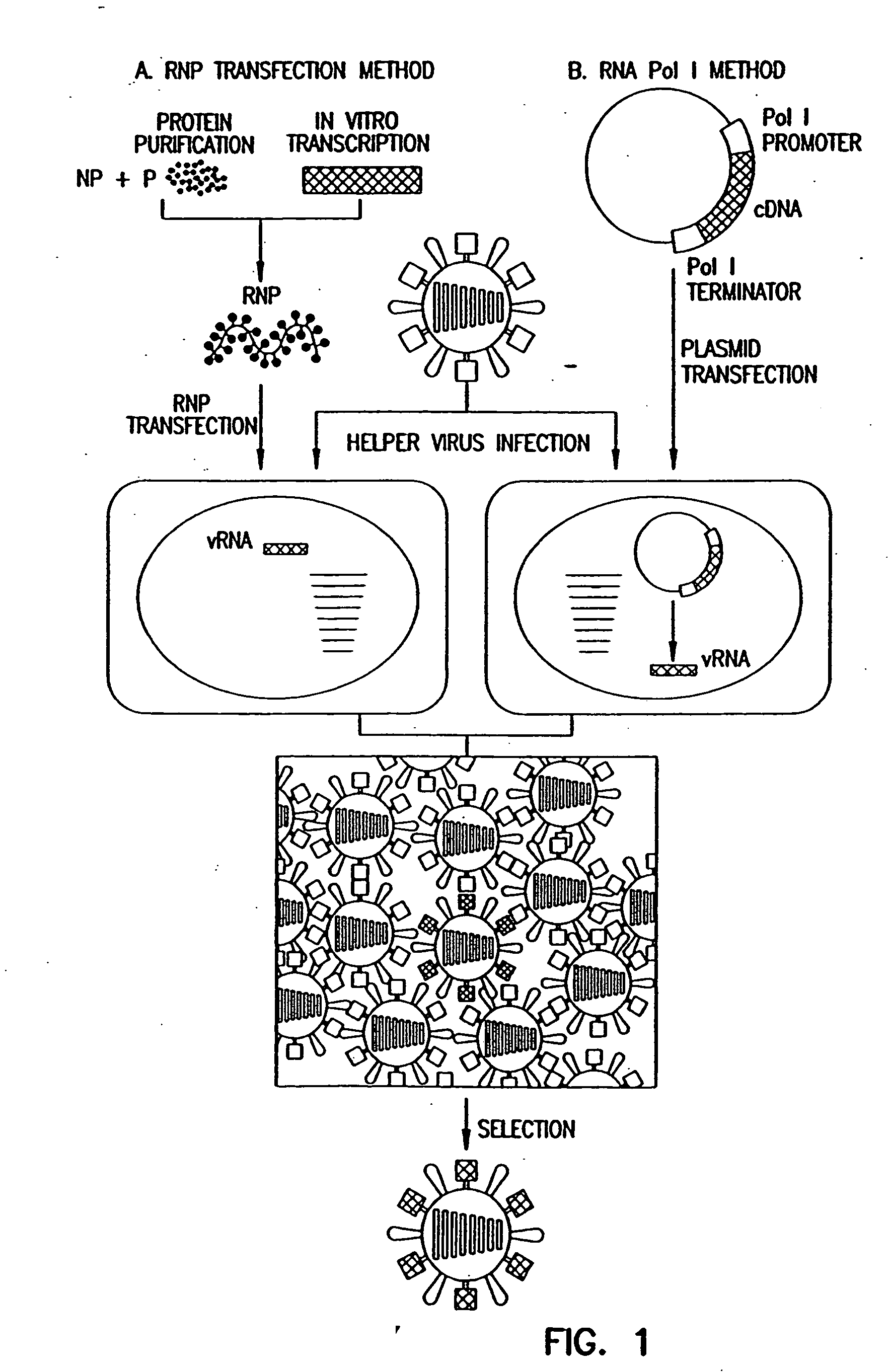
Method for generating influenza viruses and vaccines
InactiveUS7037707B2Grows more efficientlyEfficient growth processSsRNA viruses negative-senseBiocideMammalian cellGene
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Method and medicament for inhibiting the infection of influenza virus
InactiveUS8470771B2Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaPolynucleotide
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Influenza virus-like particles (VLPS) comprising hemagglutinin produced within a plant
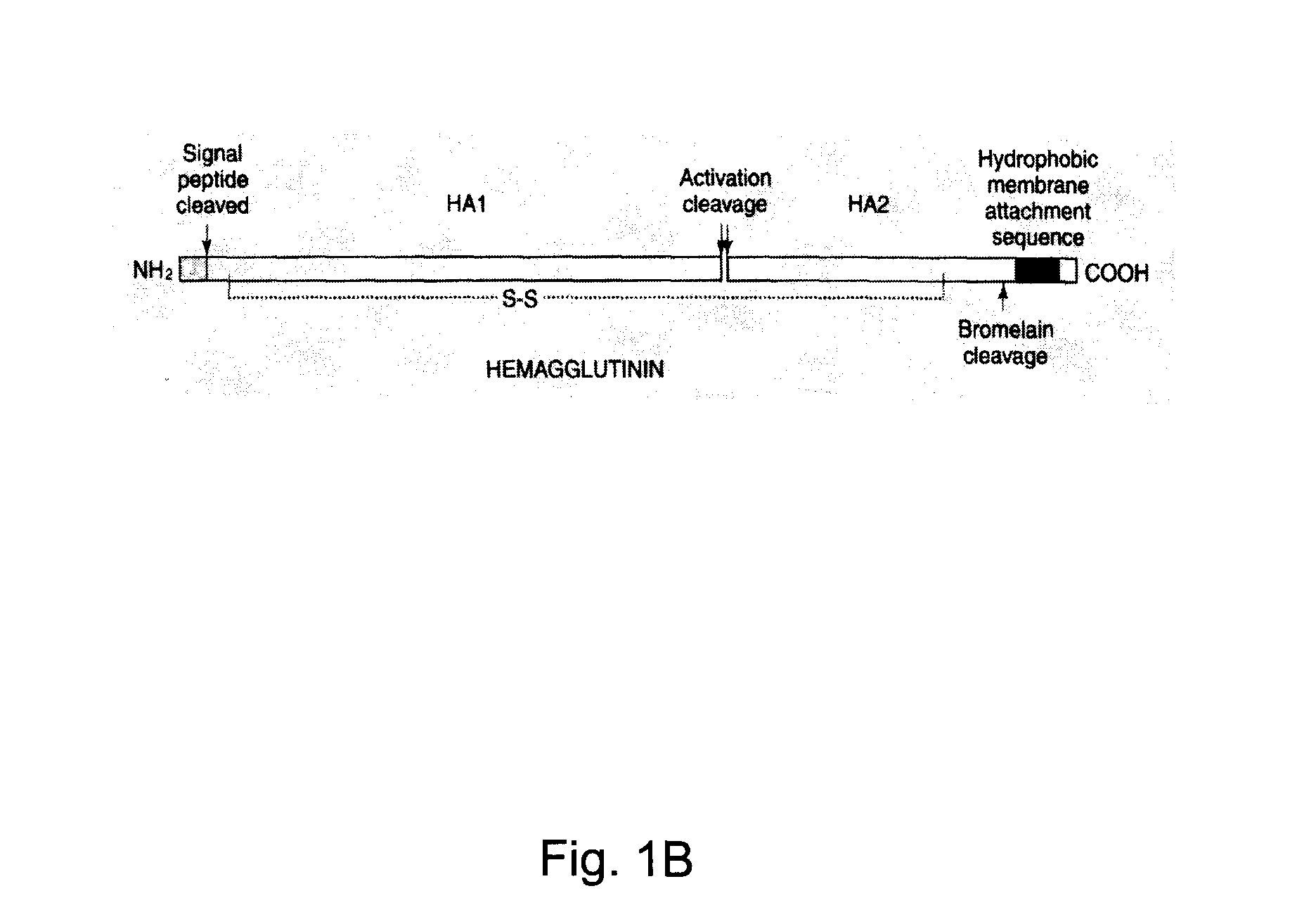
InactiveUS20100239610A1Enhance immune responseEasy to captureSsRNA viruses negative-senseVirus peptidesHemagglutininLipid formation
A method for synthesizing influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of influenza HA in plants and the purification by size exclusion chromatography. The invention is also directed towards a VLP comprising influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Infectious disease epidemic situation predicative analysis method based on nonlinear and coefficient variation predictive model
InactiveCN101794342ASpecial data processing applicationsSusceptible populationSevere acute respiratory syndrome
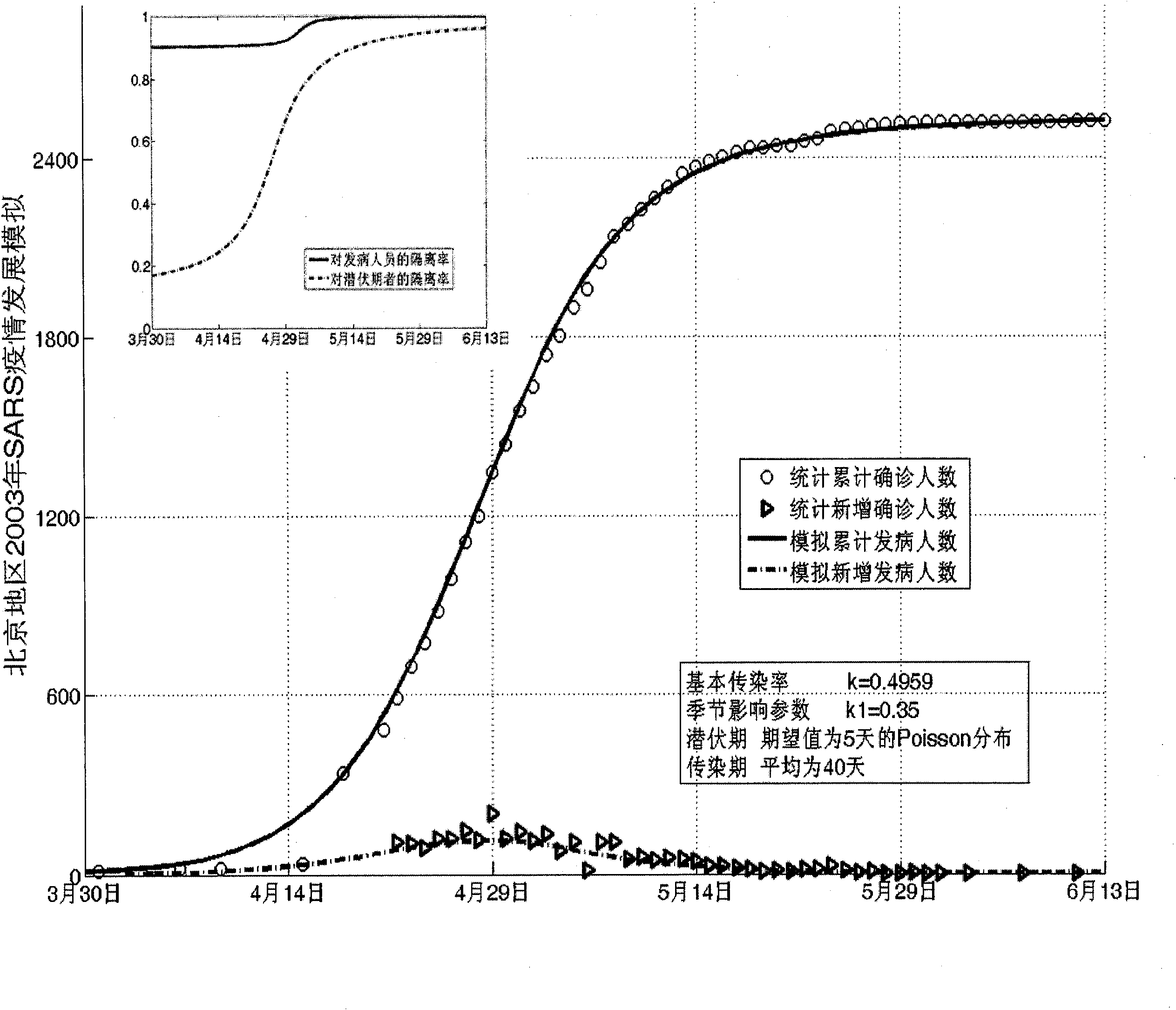
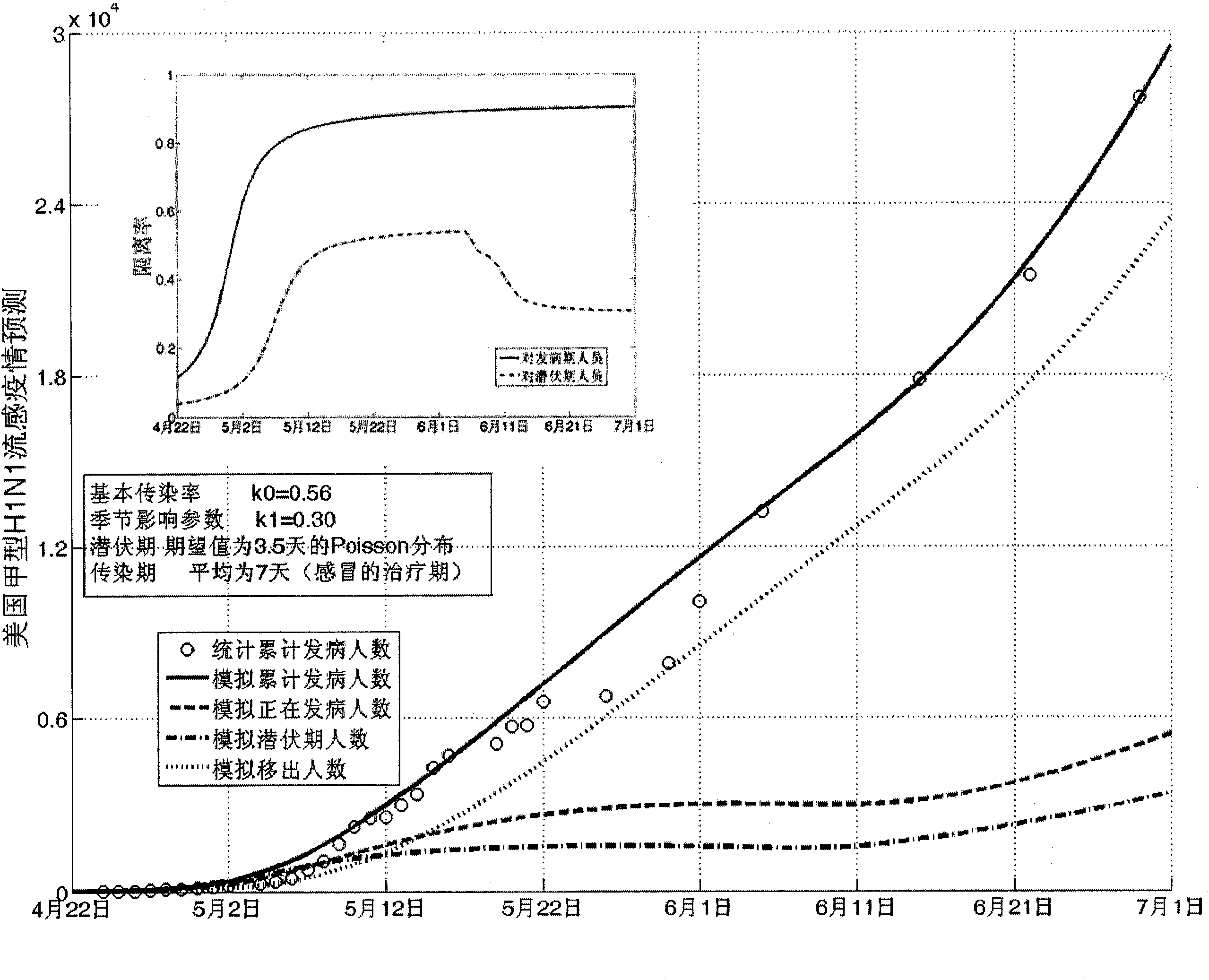
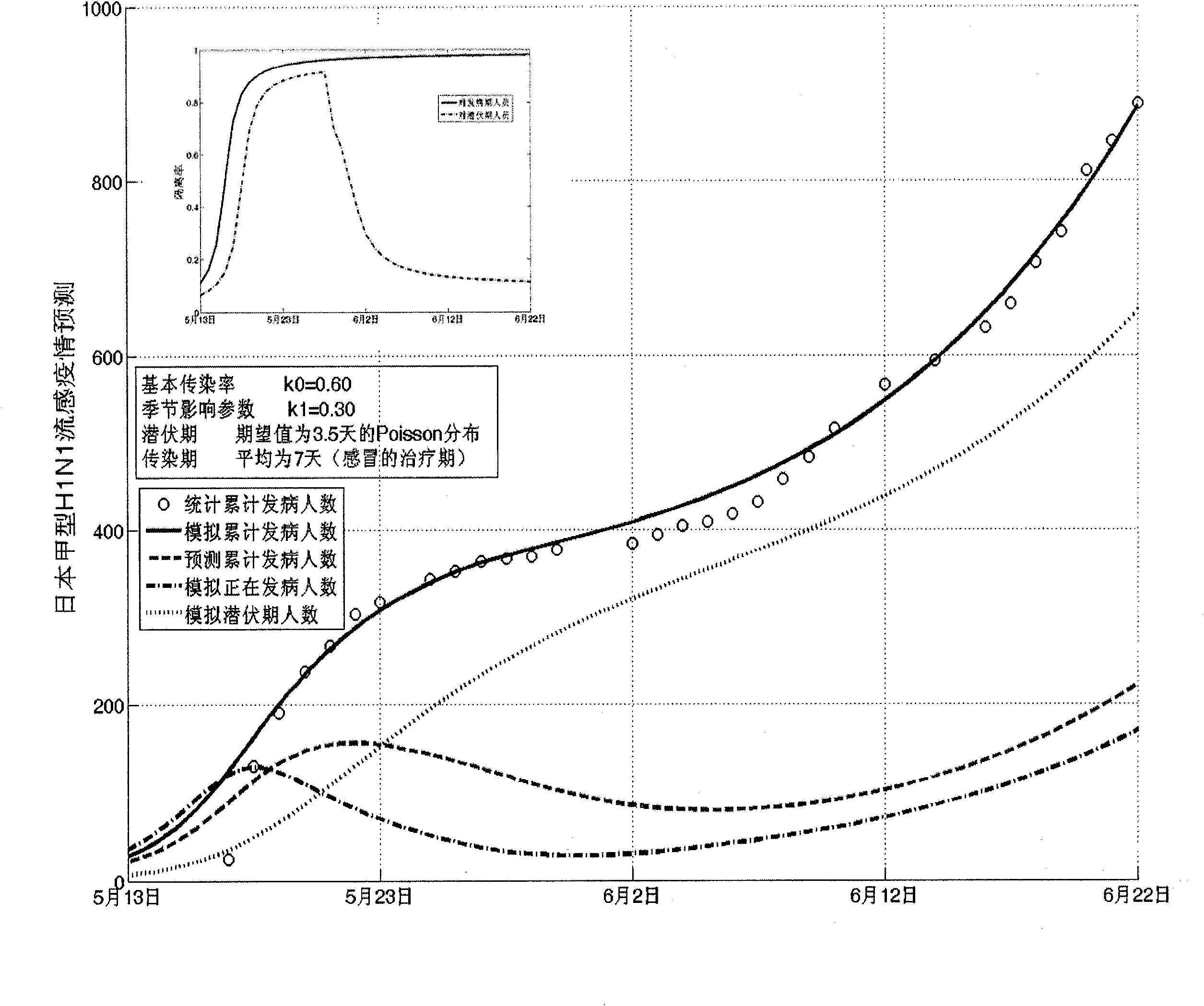
The invention establishes a nonlinear and coefficient variation infectious disease predictive model aiming at epidemic diseases with viruses which have infectivity at a latent period and a period of onset, provides an epidemic situation control function directly related to the model, simulating and predicting effects of different control measures and different control degrees on the basis of prediction in consideration of the control measures, considers the epidemic situation control as a continuous change process, integrally simulating and predicting development and control of the epidemic situation and provides crucial quantitative information for decision-making departments to optimally decide and control the epidemic situation with the smallest cost. By adopting the invention, a relative error for simulating SARS (Severe Acute Respiratory Syndromes) in Beijing areas in 2003 is 0.98% and predictive results for influenza A virus subtype H1N1 in US and Japan are well matched with the actual epidemic situation development, a quantified control factor for preventing the influenza A virus subtype H1N1 at an initial development stage and controlling the spread of the epidemic situation is obtained and epidemic situation development conditions of different control intensities and different susceptible people are predicted.
Owner:中国人民解放军防化指挥工程学院
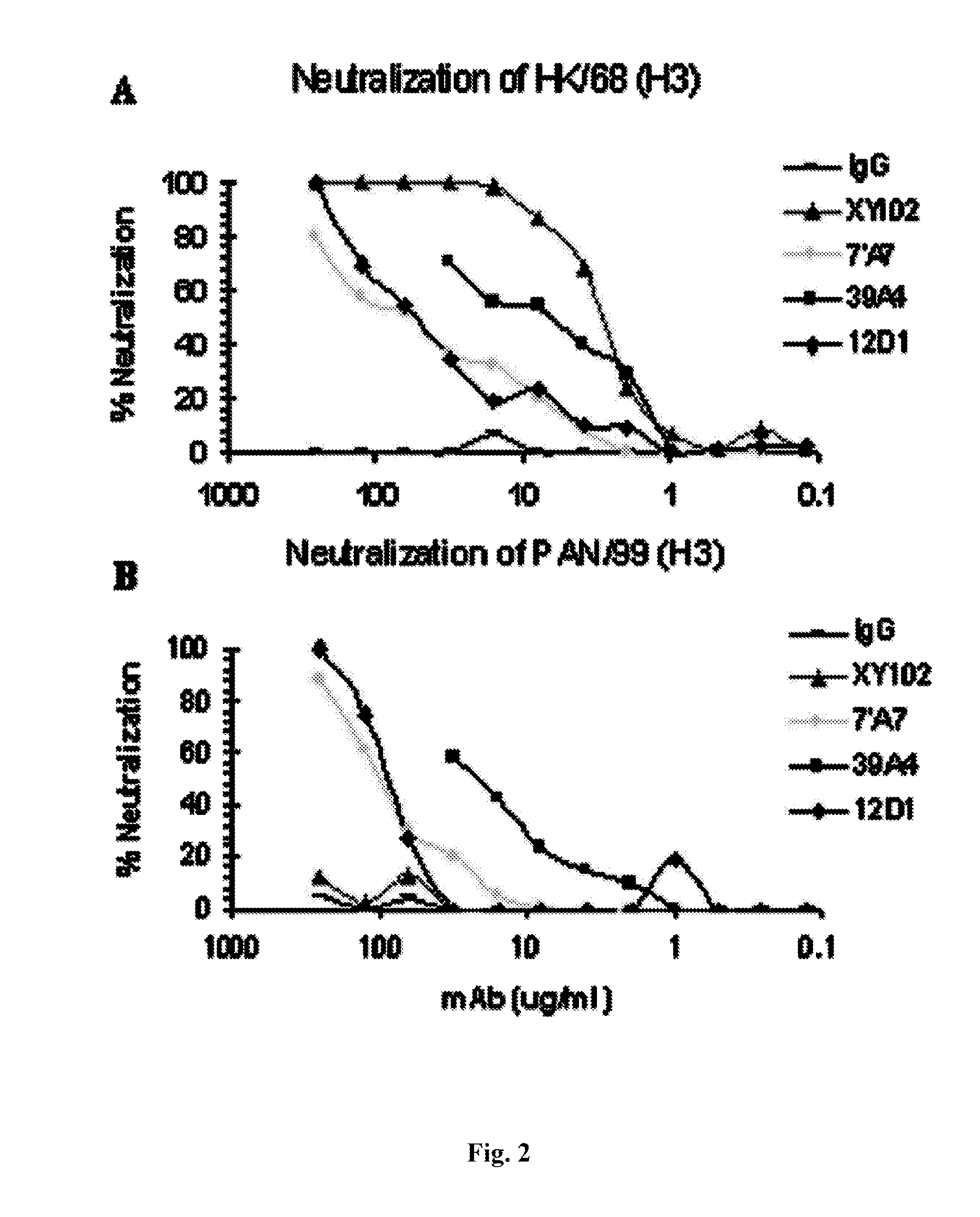
Neutralizing Anti-influenza a virus antibodies and uses thereof
ActiveUS20100080813A1Neutralization is narrowLimited successBiocidePeptide/protein ingredientsHemagglutininAntigen Binding Fragment
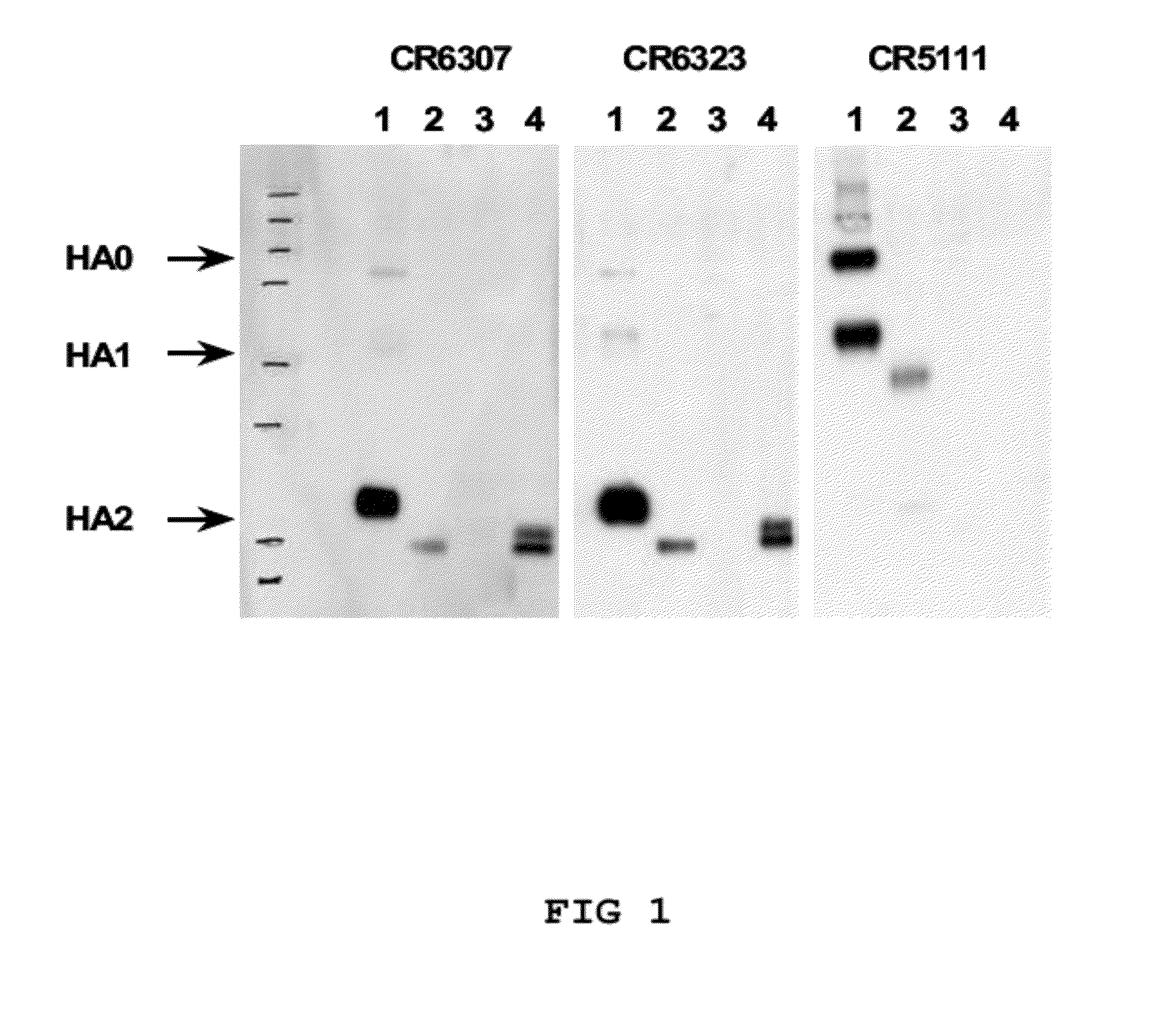
The invention relates to antibodies, and antigen binding fragments thereof, that bind to hemagglutinin and neutralize a group 1 subtype and a group 2 subtype of influenza A virus. The invention also relates to nucleic acids that encode, immortalized B cells and cultured single plasma cells that produce, and to epitopes that bind to such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis, treatment and prevention of influenza A virus infection.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Neutralizing Antibodies to Influenza Viruses
The present invention concerns methods and means for identifying, producing, and engineering neutralizing antibodies against influenza A viruses, and to the neutralizing antibodies produced. In particular, the invention concerns neutralizing antibodies against various influenza A virus subtypes, including neutralizing antibodies against two or more of H1, H2, H3, H5, H7 and H9, such as, for example all of H1, H2, H3, and H5 subtypes, and methods and means for making such antibodies. More specifically, the invention concerns antibodies capable of neutralizing more than one, preferably all, isolates of an influenza A virus subtype.
Owner:I2 PHARMA INC
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060057116A1Improve efficiencyIncrease productionSsRNA viruses negative-senseBiocideHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:KAWAOKA YOSHIHIRO +1
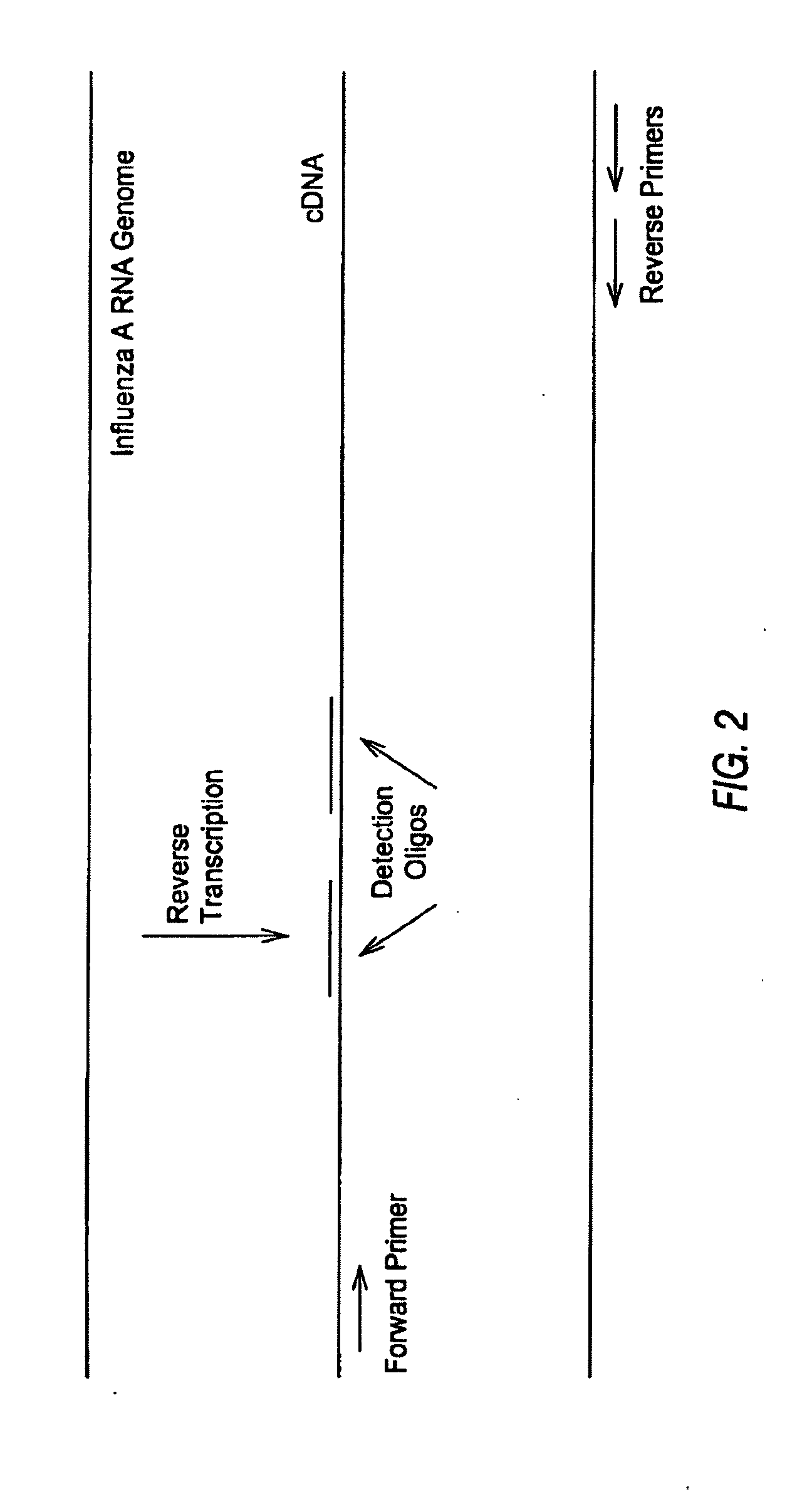
Method for detecting various respiratory viruses and primers and probes thereof
InactiveCN101985665AEasy to operateStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceMicrosphereNucleotide
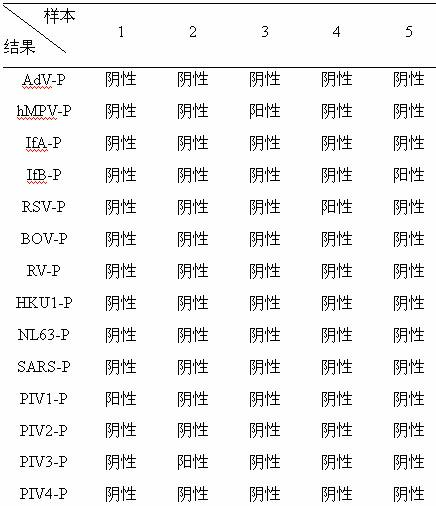
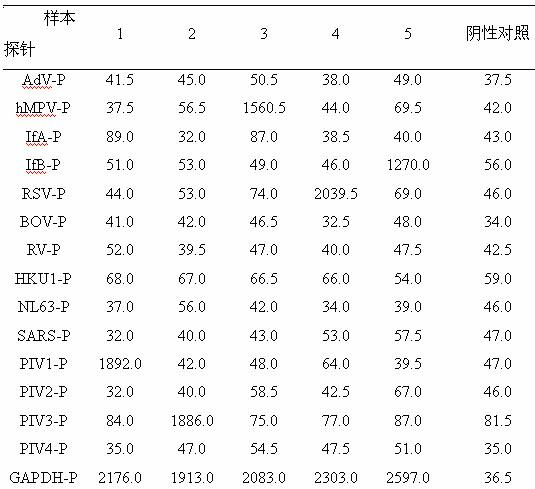
The invention belongs to the technical fields of biochips and diagnostic reagents, and discloses a method for detecting various respiratory viruses, and primers and probes thereof. In the invention, nucleotide sequences of 14 respiratory viruses, namely adenovirus, human metapneumovirus, influenza virus A, influenza virus B, respiratory syncytial virus, bocavirus, rhinovirus, coronavirus (HKU1, NL63 and SARS), and parainfluenza virus (type I, type II, type III and type IV) are analyzed, and corresponding reverse transcription primers, PCR primers and specific probes are designed. Specific gene segments are amplified by reverse transcription and multiple asymmetric PCR methods; a fluorescence-coded microsphere group coupled with the virus specific probes and the PCR amplification product are incubated and hybridized by liquid phase chip technology; and finally the Bio-PlexTM200 is used for detection. The detection method has the advantages of high flux, high specificity and sensitivity, stable results and good repeatability, the detection method is easy to operate, and the detection speed is high.
Owner:FUDAN UNIV +1
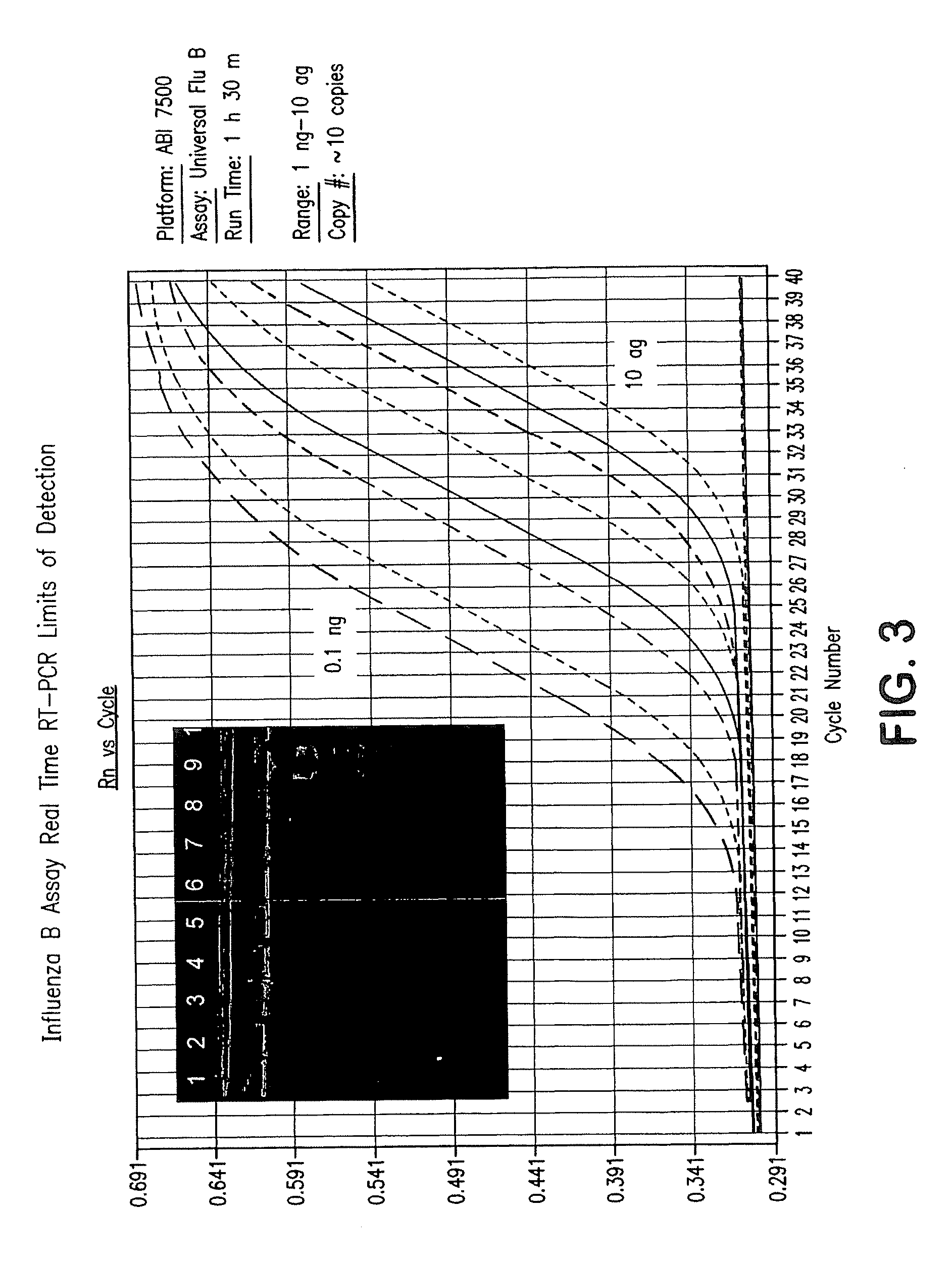
Compositions and method for rapid, real-time detection of influenza a virus (H1N1) swine 2009
ActiveUS20100009343A1Quick checkQuick identificationBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic Acid ProbesField analysis
Disclosed are oligonucleotide amplification primers and detection probes specific for the amplification and detection of pathogenic organisms, including for example, specific Influenza A H1N1 viral isolates. Also disclosed is a biological organism identification kit including the disclosed nucleic acid probes and primers, as well as thermal cycling reagents that is both portable and durable, and may also be self-contained for remote, or in-field analysis and identification of particular influenza isolates from a variety of biological specimen types.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
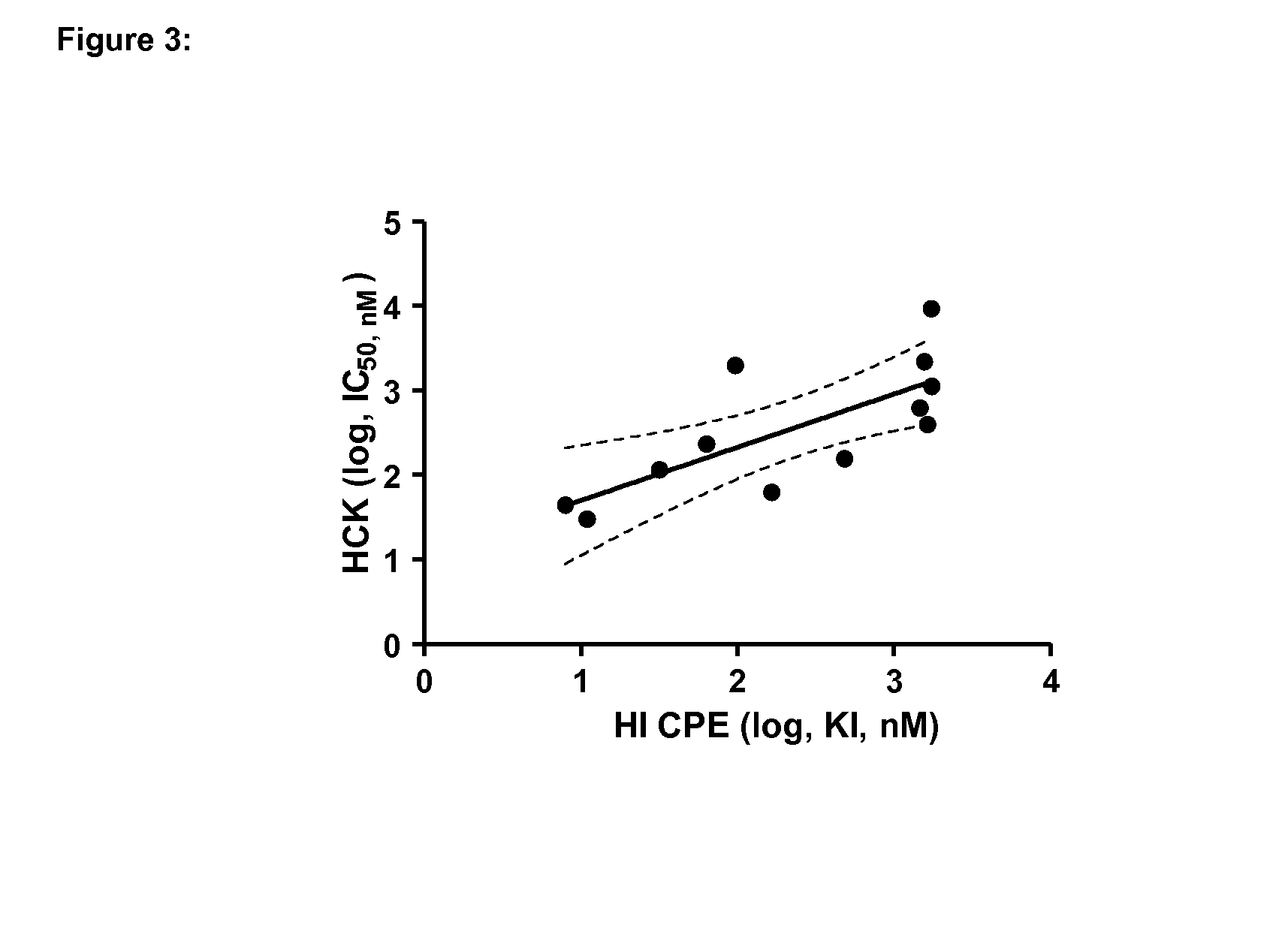
Inhibitors of hemopoietic cell kinase (p59-hck) and their use in the treatment of influenza infection
InactiveUS20120244120A1Efficient methodRisk minimizationBiocideCompound screeningHEMOPOIETIC CELL KINASEViral infection
The present invention relates inter alia to the treatment or prevention of influenza virus infection (including subtypes influenza A virus, influenza B virus, avian strain H5N1, A / H1N1, H3N2 and / or pandemic influenza) using compounds which inhibit the activity of p59-HCK and to a method of screening for a candidate drug substance intended to prevent or treat influenza virus infection in a subject, said method comprising identifying a test substance capable of inhibiting p59-HCK activity.
Owner:RESPIVERT
Neutralizing antibodies to influenza viruses
The present invention concerns methods and means for identifying, producing, and engineering neutralizing molecules against influenza A viruses, and to the neutralizing molecules produced. In particular, the invention concerns neutralizing molecules against various influenza A virus subtypes, including neutralizing antibodies against H5 and / or H3 and / or H1, such as, for example all of H1, H3, and H5 subtypes, and methods and means for making such molecules.
Owner:I2 PHARMA INC
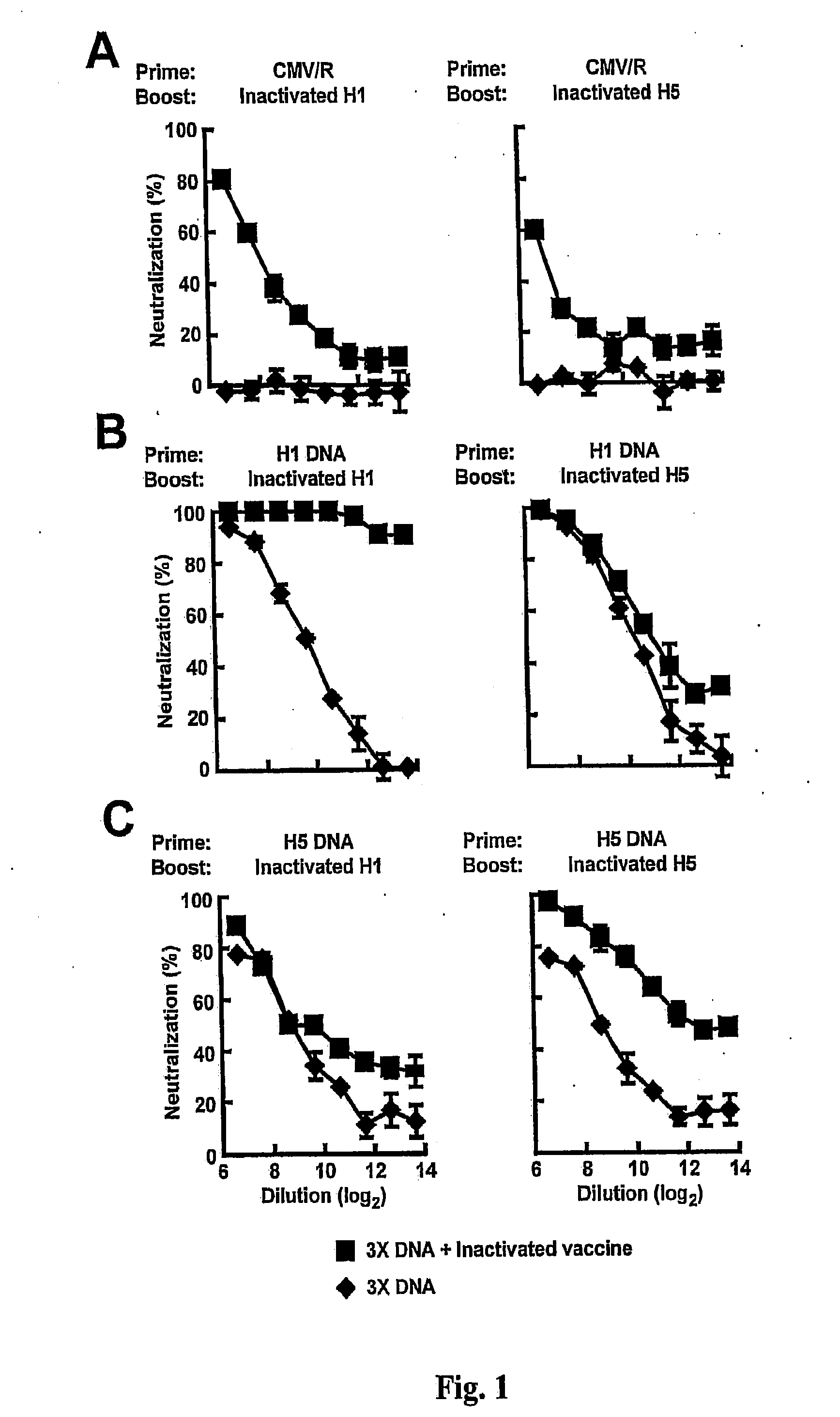
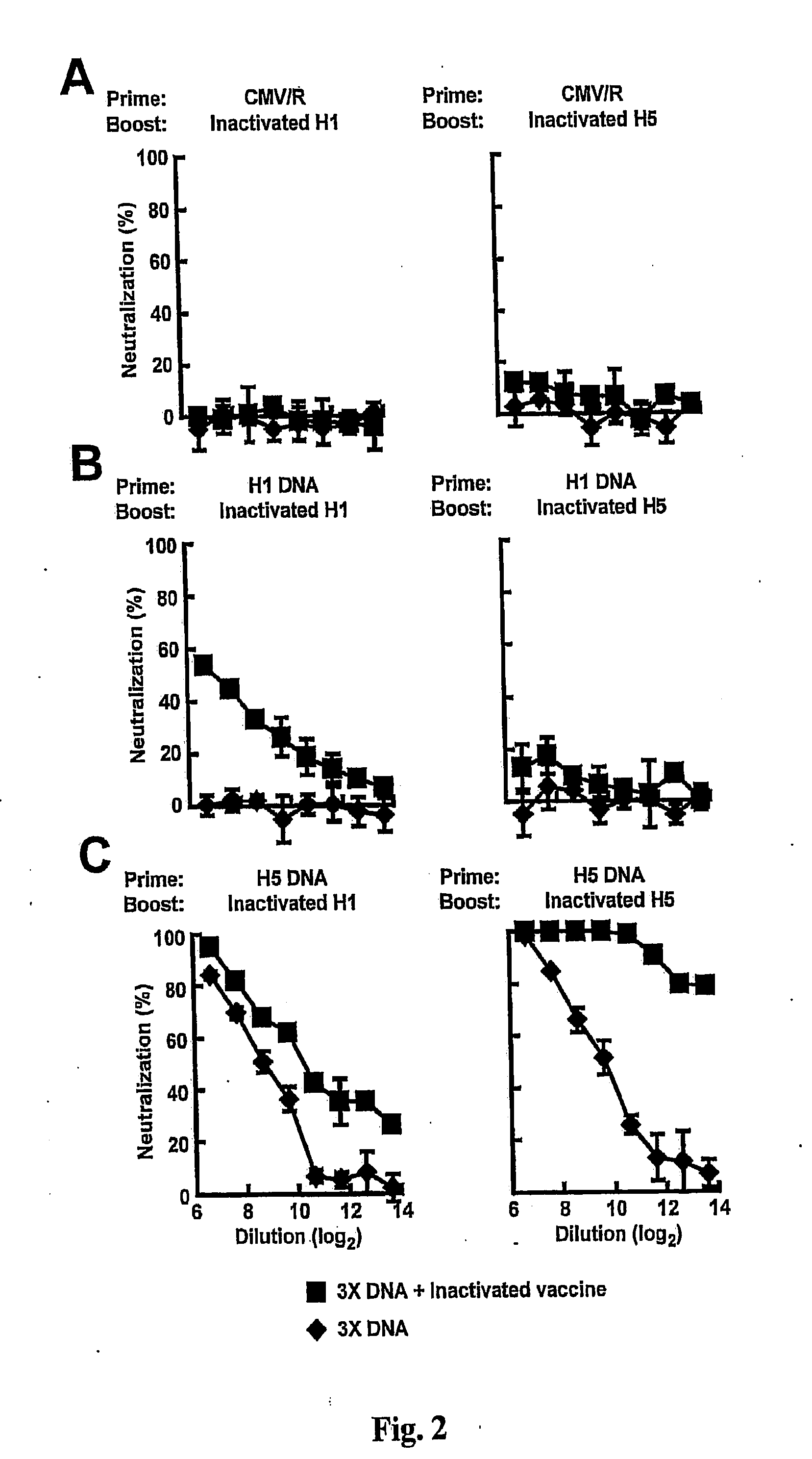
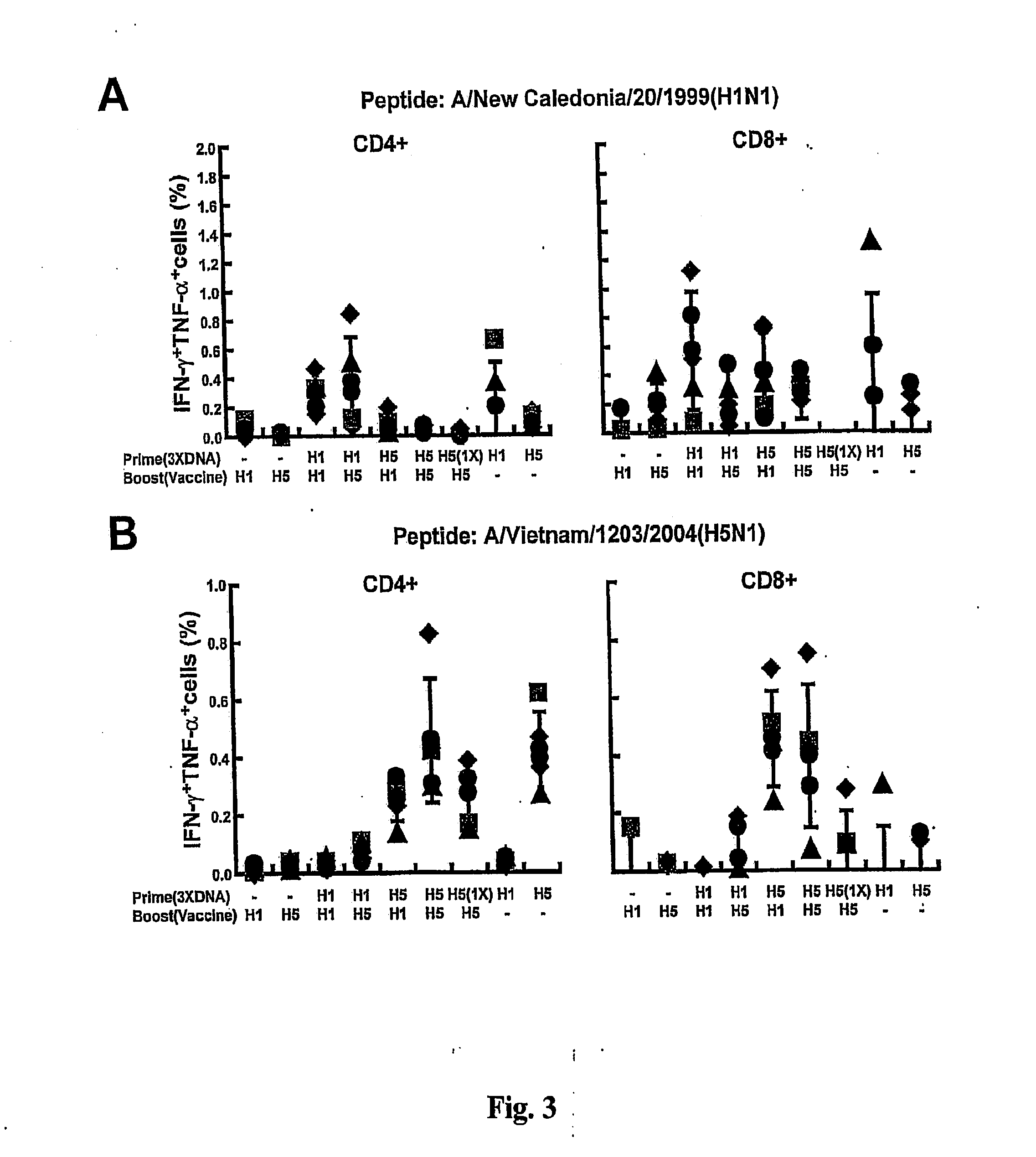
DNA prime/activated vaccine boost immunization to influenza virus
InactiveUS20110177122A1Enhance immune responseStimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininEpitope
The present invention relates to a combination of a priming composition and a boosting composition to prime and boost an immune response in a subject whereby the immune response resulting from administration of the priming composition to the subject is capable of being boosted. The priming composition comprises a DNA plasmid that comprises a nucleic acid molecule encoding an influenza virus hemagglutinin (HA) or an epitope-bearing domain thereof. The boosting composition comprises an influenza vaccine. The present invention also relates to a method to use such a combination to vaccinate a subject and to enhance an immune response to an influenza vaccine administered alone. Such a combination can elicit an immune response not only against at least one influenza virus strain from which the priming composition or boosting composition is derived but also to at least one heterologous influenza virus strain.
Owner:UNITED STATES OF AMERICA
Monoclonal antibodies against influenza virus generated by cyclical administration and uses thereof
ActiveUS20110027270A1Reduce in quantityLower titerSsRNA viruses negative-senseVirus peptidesMonoclonal antibodyVirus diseases
Provided herein are methods of producing neutralizing monoclonal antibodies, by cyclical immunization, that cross-react with strains of Influenza virus of the same subtype or different subtypes. Also provided herein are compositions comprising such antibodies and methods of using such antibodies to diagnose, prevent or treat Influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060134138A1Improve efficiencyEnhances these viruses as vaccine vectorsSsRNA viruses negative-senseAnimal cellsHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:WISCONSIN ALUMNI RES FOUND
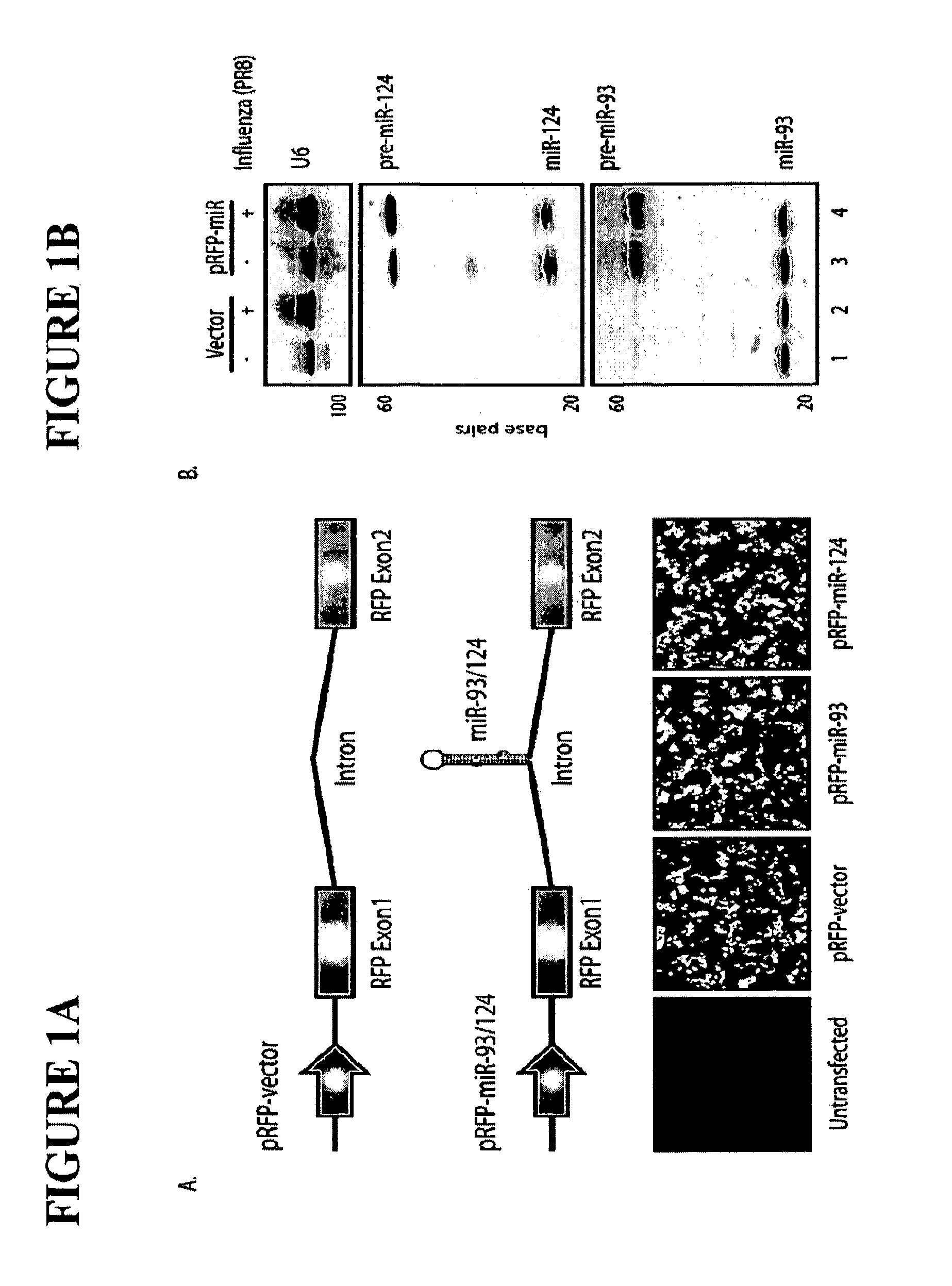
Live Attenuated Influenza Virus Vaccines Comprising Microrna Response Elements
ActiveUS20120148622A1Increase vaccine safetyQuick buildSsRNA viruses negative-senseSugar derivativesUltrasound attenuationInfluenza virus vaccine
The invention is directed to novel live attenuated influenza virus (LAIV) vaccines comprising one or more microRNA (miRNA) Response Element(s) (MRE) within an influenza virus genome. The MREs useful for the present invention can be derived from any miRNA which is highly expressed in influenza-targeted cells of an animal in need of vaccination but are not expressed or are expressed at very low levels in species (e.g., embryonated chicken eggs) or cell lines used for a large-scale vaccine production. This allows efficient vaccine production but renders the vaccine virus susceptible to attenuation in the influenza-targeted cells of vaccinated animals expressing a cognate miRNA.
Owner:MT SINAI SCHOOL OF MEDICINE
Cold-adapted influenza virus
InactiveUS7344722B1Promote growthSsRNA viruses negative-senseSugar derivativesNucleotideCold adapted
The cold-adapted master strain A / Ann Arbor / 6 / 60 7PI (H2N2) and progenitor wild type E2(3) viral strains have been deposited and their genomic sequences identified. Seven nucleotide differences were found between the sequences identified herein and the previously published sequences for cold-adapted A / Ann Arbor / 6 / 60 genes. The cold-adapted live influenza virus of the present invention can be reassorted with a variety of epidemic wild type influenza viruses and used to produce vaccines to prophylactically and therapeutically treat influenza.
Owner:RGT UNIV OF MICHIGAN
Compositions and methods of enhancing immune responses
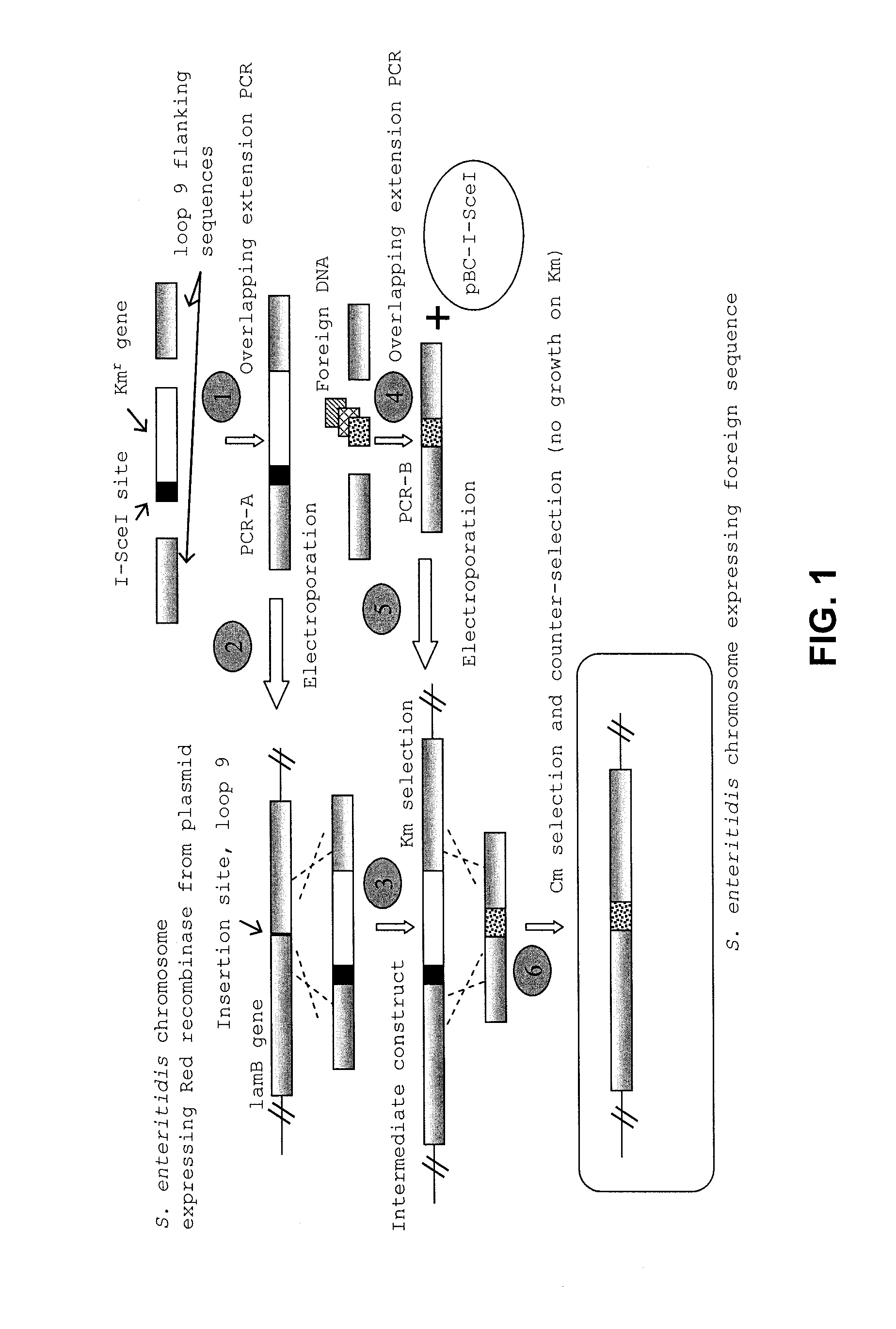
ActiveUS8604178B2Enhance immune responseReduce morbiditySsRNA viruses negative-senseBacteriaSalmonella enteritidisBacilli
Provided herein are Salmonella enteritidis 13A strains and compositions comprising these strains. Also provided are methods of enhancing an immune response against Influenza A and methods of reducing morbidity associated with an Influenza A infection. Methods of enhancing an immune response to a vaccine vector by expressing a polypeptide of CD 154 capable of binding CD40 are also disclosed. Methods of developing a bacterial vaccine vector are disclosed. Methods of generating scarless site-specific mutations in a bacterium are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Human binding molecules capable of neutralizing influenza virus h5n1 and uses thereof
Described are binding molecules such as human monoclonal antibodies that bind to influenza virus H5N1 and have neutralizing activity against influenza virus H5N1. Also described are nucleic acid molecules encoding the antibodies, and compositions comprising the antibodies and methods of identifying or producing the antibodies. The antibodies can be used in the diagnosis, prophylaxis, and / or treatment of an influenza virus H5N1 infection. In certain embodiments, the antibodies provide cross-subtype protection in vivo, such that infections with H5, H2, H6, H9, and H1-based influenza subtypes can be prevented and / or treated.
Owner:JANSSEN VACCINES & PREVENTION BV
Site-directed mutated influenza virus, and live vaccine, preparation method and application thereof
InactiveCN106929482ASsRNA viruses negative-senseViral antigen ingredientsGenomeAttenuated Live Vaccine
The invention relates to an influenza virus subjected to site-directed mutation or an influenza virus with simultaneous mutation of multiple sites. The influenza virus can be derived from human and other animals. The invention further relates to a method of the site-directed mutated influenza virus. The method includes introducing UAG into an influenza virus genome according to a reverse inheritance technology and introducing unnatural amino acid into an influenza virus gene in a site-directed way according to a gene codon extension technology. The invention further relates to application of the site-directed mutated influenza virus or the influenza virus with multiple mutation site combinations, such as serving as attenuated live vaccines and copying controllable safe influenza virus models.
Owner:PEKING UNIV
Compositions and method for rapid, real-time detection of influenza A virus (H1N1) swine 2009
ActiveUS8097419B2Rapid detection and identificationMinimize and eliminate contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsH1n1 virusNucleic Acid Probes
Disclosed are oligonucleotide amplification primers and detection probes specific for the amplification and detection of pathogenic organisms, including for example, specific Influenza A H1N1 viral isolates. Also disclosed is a biological organism identification kit including the disclosed nucleic acid probes and primers, as well as thermal cycling reagents that is both portable and durable, and may also be self-contained for remote, or in-field analysis and identification of particular influenza isolates from a variety of biological specimen types.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Respiratory pathogen multi-detection reagent kit
InactiveCN109355437AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoronavirus 229EFluorescence
The invention discloses a respiratory pathogen multi-detection reagent kit. The respiratory pathogen multi-detection reagent kit has the advantages that the respiratory pathogen multi-detection reagent kit is based on multi-PCR (polymerase chain reaction) technologies, detection results can be determined by the aid of fluorescence resonance energy transfer via the melting temperature ranges, the respiratory pathogen multi-detection reagent kit can be used for qualitatively simultaneously detecting 16 types of respiratory pathogens, the 16 types of respiratory pathogens include 12 types of RNA(ribonucleic acid) viruses (influenza A viruses, influenza B viruses, H1N1 influenza A viruses, type A and type B respiratory syncytial viruses, type -1 / -2 / -3 parainfluenza viruses, type OC43 coronaviruses, type 229E coronaviruses, rhinoviruses and human metapneumovirus), 2 types of DNA (deoxyribonucleic acid) viruses (adenoviruses and bocavirus) and 2 types of bacteria (mycoplasma pneumoniae andbordetella pertussis), the respiratory pathogen multi-detection reagent kit is high in detection sensitivity, and the sensitivity even can reach 1 copy / reaction; the multi-detection reagent kit is good in specificity, and negative results of pathogens which have identical sampling sites and similar pathogenic mechanisms and are not in the detection range of the respiratory pathogen multi-detectionreagent kit can be obtained; the respiratory pathogen multi-detection reagent kit is short in operation time and easy to operate and can be used for quickly detecting the 16 types of respiratory pathogens in a single tube of a reaction system, the results are clear and are easy to interpret, and the like.
Owner:上海捷诺生物科技股份有限公司
Compounds and methods for the treatment of viral infections
High throughput and virtual screening methods are disclosed that can identify potential anti-viral agents. The virtual screening methods identify agents that interact with a viral nucleoprotein binding site. The high throughput methods identify compounds that inhibit viral infection by binding to viral nucleoprotein. Also disclosed are pharmaceutical formulations useful for treating or preventing viral infections, especially influenza A.
Owner:VERSITECH LTD
Influenza vaccine
InactiveUS20090304730A1Overcomes drawbackSsRNA viruses negative-senseViral antigen ingredientsEpitopeInfluenza vaccine
The present invention relates to influenza vaccines for human and veterinary use. In particular, the present invention provides a vaccine able to effect long term and cross-strain protection by including at least two influenza virus epitopes expressed as a chimeric polypeptide wherein at least one epitope is influenza A virus matrix protein epitope and the second epitope is a haemagglutinin peptide epitope.
Owner:YEDA RES & DEV CO LTD
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
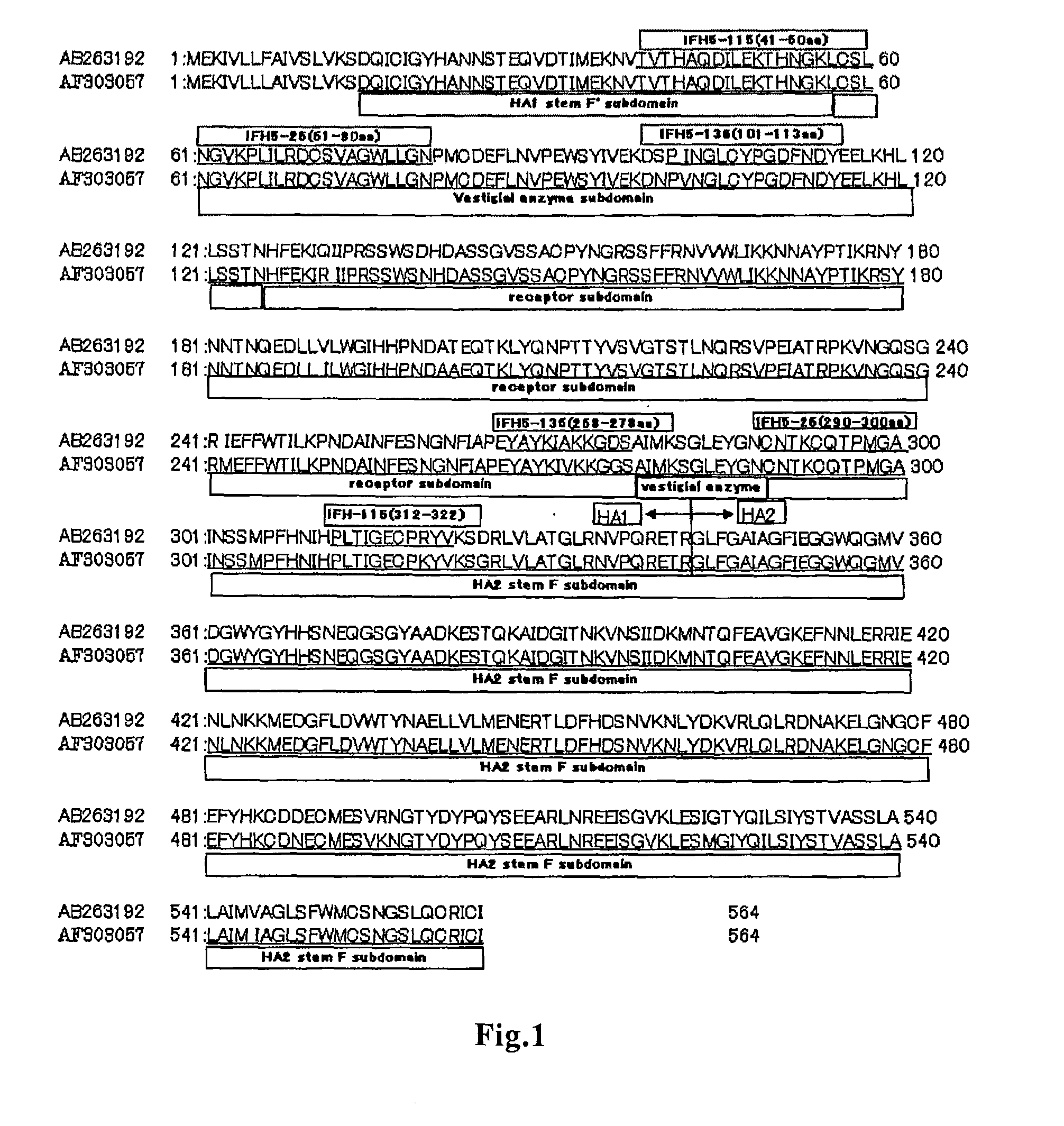
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
Influenza viruses and uses thereof
ActiveUS20120244183A1Reduced ability to reassortImprove securitySsRNA viruses negative-senseSugar derivativesDiseaseOpen reading frame
Described herein are chimeric influenza virus gene segments and nucleic acid sequences encoding such chimeric influenza virus gene segments. A chimeric influenza virus gene segment described herein comprises packaging signals found in the non-coding and coding regions of one type of influenza virus gene segment and an open reading frame of a different type of influenza virus gene segment or fragment thereof. Also described herein are recombinant influenza viruses comprising two or more chimeric influenza virus gene segments and the use of such viruses in the prevention and / or treatment of influenza virus disease.
Owner:MT SINAI SCHOOL OF MEDICINE
100% sequence identity detection methods for variable genomes
The present disclosure provides methods, reagents and kits for the detection of all known human variants of influenza A virus and at least 90% of avian and swine variants of influenza A virus in a biological sample, based on amplification primers and detection probes that are specific to a highly conserved region of the influenza A matrix gene.
Owner:GENEOHM SCI INC
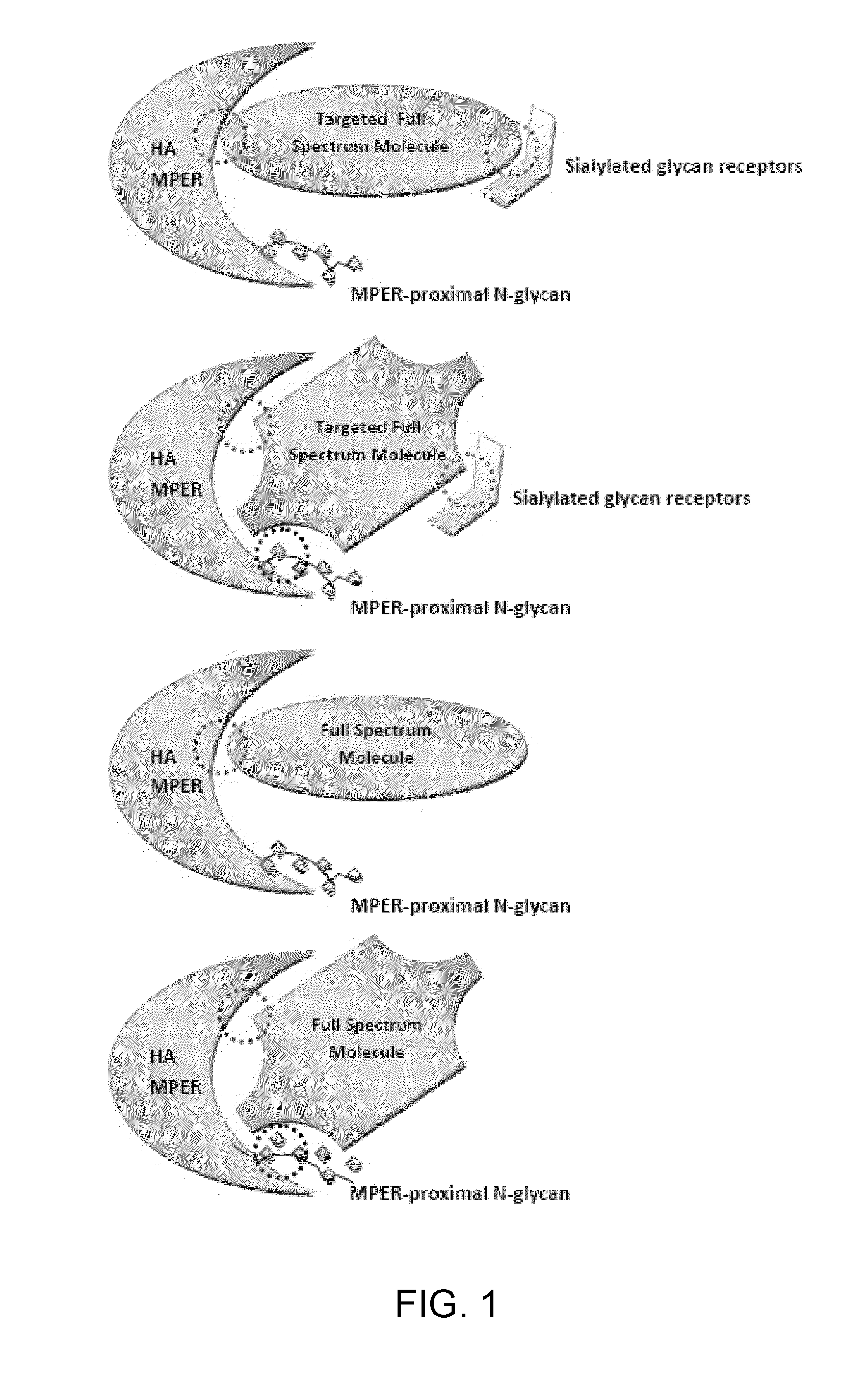
Engineered polypeptide agents for targeted broad spectrum influenza neutralization
ActiveUS20110201547A1Avoid infectionSsRNA viruses negative-sensePowder deliveryHemagglutininNovel agents
The present invention provides novel agents for broad spectrum influenza neutralization. The present invention provides agents for inhibiting influenza infection by bind to the influenza virus and / or hemagglutinin (HA) polypeptides and / or HA receptors, and reagents and methods relating thereto. The present invention provides a system for analyzing interactions between infolds and the interaction partners that bind to them.
Owner:MASSACHUSETTS INST OF TECH
Neutralizing molecules to influenza viruses
InactiveUS20120128671A1Reduced oxidative potentialSsRNA viruses negative-senseImmunoglobulins against virusesNeutralizing antibodyInfluenza a
The present invention concerns methods and means for identifying, producing, and engineering neutralizing antibodies against influenza A viruses, and to the neutralizing antibodies produced. In particular, the invention concerns neutralizing antibodies against various influenza A virus subtypes, and methods and means for making such antibodies.
Owner:I2 PHARMA INC
Neutralizing Anti-influenza a virus antibodies and uses thereof
The invention relates to antibodies and antigen binding fragments thereof, that bind to hemagglutinin and neutralize infection of at least two different group 1 subtypes or at least two different group 2 subtypes of influenza A virus. The invention also relates to nucleic acids that encode, immortalized B cells and cultured single plasma cells that produce, and to epitopes that bind to, such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis, treatment and prevention of influenza A virus infection.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com