Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Highly Pathogenic Avian Influenza Virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Avian influenza—known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. The type with the greatest risk is highly pathogenic avian influenza (HPAI).
Method and medicament for inhibiting the infection of influenza virus
InactiveUS8470771B2Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaPolynucleotide
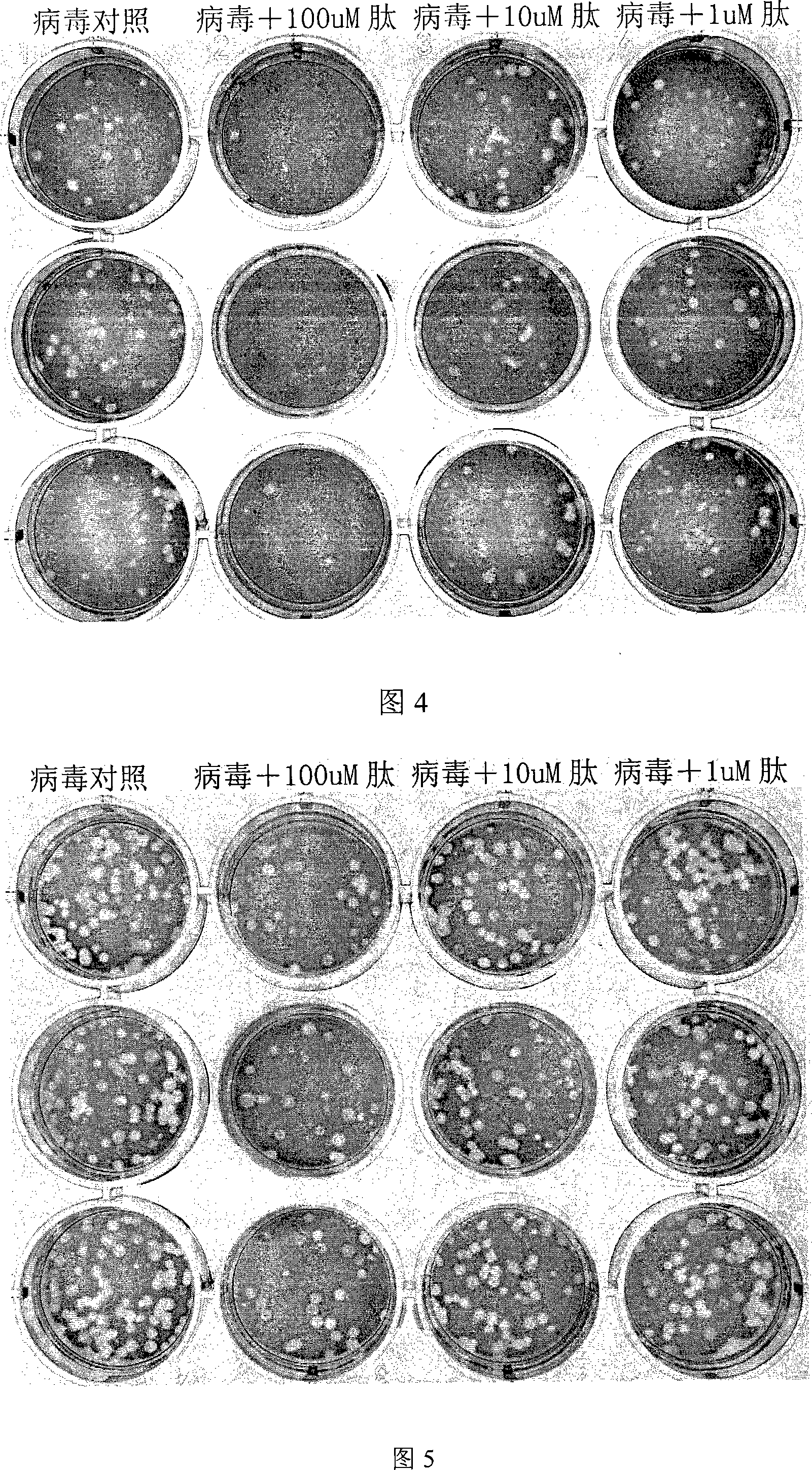
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Influenza Virus Vaccines
InactiveUS20080254065A1SsRNA viruses negative-senseViral antigen ingredientsFowlInfluenza virus vaccine
The invention provides a vaccine for protecting a human patient against infection by a human influenza virus strain, wherein the vaccine comprises an antigen from an avian influenza virus strain that can cause highly pathogenic avian influenza. The antigen can invoke an antibody response in the patient that is capable of neutralising said human influenza virus strain. Whereas the prior art used known non-pathogenic avian strains to generate antibodies in humans against known pathogenic avian strains, the invention uses known pathogenic avian strains to protect against emerging pathogenic human strains. Furthermore, whereas the prior art focused on achieving a close antigenic match between the vaccine strain and the target strain, the invention selects vaccine strains based on their pathogenicity, regardless of any perceived close antigenic relationship to the target strain. As the invention does not require detailed knowledge of an emerging strain, a vaccine can be provided further in advance to reduce the risk and potential effects of a human pandemic outbreak.
Owner:SEQIRUS UK LTD
Method for detecting flu and H5N1 avian influenza virus by using liquid chip
InactiveCN101392298AHigh sensitivityShort reaction timeMicrobiological testing/measurementHighly Pathogenic Avian Influenza VirusInfluenza a
The invention relates to a method for detecting influenza viruses, in particular to a method for detecting influenza viruses and H5N1 subtype avian influenza virus by a liquid-phase chip, belonging to the technical field of medical monitoring. The method is a fast and highly efficient one which can identify FluA and FluB influenza viruses including the H5N1 subtype avian influenza virus by types. With the utilization of a liquid-phase chip technology and the application of bioinformatics knowledge and related bioinformatics software, the liquid-phase chip which can specifically distinguish H5 and N1 gene fragments, NP gene fragment of the FluA influenza virus and HA gene fragment of the FluB influenza virus is manufactured. The method has the advantages that fast detecting and early-stage diagnosis of the FluA and the FluB influenza viruses including the H5N1 subtype highly pathogenic avian influenza virus can be carried out; and a basis for developing other fast and highly effective detecting methods for detecting various subtype influenza viruses is provided. The method is characterized by detecting multiple types of viruses, short response time and high sensitivity, thus determining that the method has wide application prospect.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Human anti-H7N9 avian influenza virus neutralizing antibody 1F7L and its use
InactiveCN107226861AEffective neutralizationAvoid infectionImmunoglobulins against virusesAntiviralsNatural Killer Cell Inhibitory ReceptorsNeutralizing antibody
The invention discloses a human anti-H7N9 avian influenza virus neutralizing antibody 1F7L screened by a single cell sorting technology. The amino acid sequences in the light and heavy chain variable regions are shown in the formulas of SEQ ID No. 2 and SEQ ID No. 5. The antibody has the ability to neutralize the H7N9 influenza viruses in vitro and mediates the killing (ADCC) of the H7N9 influenza virus-infected cells with effector cells mainly comprising NK cells. The antibody can be used as a treatment drug for highly pathogenic avian influenza infection and can also be used for the development of H7N9 influenza virus antigen detection reagents.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Method for inhibiting influenza virus infection and medicament thereof
InactiveCN101186637APeptide/protein ingredientsGenetic material ingredientsHighly pathogenicHuman influenza
The invention relates to a method for restraining enveloped virus infection, relative polypeptide and protein drug, belonging to biological medicine technical field. The invention comprises a method for restraining influenza virus, particularly for restraining highly pathogenic influenza virus and human influenza virus (as H1N1 hypotype and H3N2 hypotype), relative polypeptide and protein, relative nucleic acid encoding the polypeptide and protein, and relative carriers and cells for representing the polypeptide and protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Method and medicament for inhibiting the infection of influenza virus
InactiveUS20100249021A1Limit scopeClear descriptionBiocidePeptide/protein ingredientsHuman influenzaMedicine use
The invention relates to a process for inhibiting the infection of influenza viruses and a polypeptide or protein medicine used therein. More particularly, the invention involves a process for inhibiting the highly pathogenic avian influenza virus (such as H5N1 subtype) infection and human influenza virus (such as H1N1 subtype and H3N2 subtype) infection, as well as the polypeptide or protein involved therein, and a polynucleotide encoding the polypeptide or protein and a vector or host cell expressing said polypeptide or protein.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Device for preventing and treating highly pathogenic avian influenza and its usage
InactiveCN1926966AIncrease freshnessDisinfect thoroughlyAnimal housingDeodrantsDevice formEngineering
The invention relates to a device for resisting and controlling pathogenicity Avian Influenza Virus, and relative application. Wherein, it based on the character and transmission path of causal agent, uses high temperature to disinfect the causal agent of air, and baffle the transmission path, to resist and control the Avian Influenza Virus transmission. It comprises that heating the air into chicken coop to 70Deg. C, using the circulated spray device formed by water pump, tube, ejector, spray room and liquid storage device to disinfect with chicken coop; reducing the temperature to room temperature, and dedusting, using dehumidifying network to keep the humidity at 60%; entering the sterilize air into the chicken coop sealed by films. The invention has simple process, automatic process, and saved energy, without pollution. And it can replace temperature insulator and temperature reducer, in winter and summer.
Owner:王广信
Kit for detecting highly pathogenic avian influenza virus subtype h5n1
InactiveUS20120028246A1Reduce pathogenicityMicrobiological testing/measurementBiological material analysisH5N1 virusWater soluble
Disclosed by the invention are an immunoassay kit and an immunoassay method for detecting highly pathogenic avian influenza virus subtype H5N1 rapidly, conveniently and specifically. Also disclosed are an immunochromatographic detection kit and an immunochromatographic detection method for detecting the virus subtype H5N1 rapidly, conveniently and specifically. It is found that a monoclonal antibody 4G6 produced by using the virus subtype H5N1 as an immunogen does not react with the subtype H5N2 virus or a subtype H5N3 virus and reacts only with a subtype H5N1 virus specifically. It is also found that only an avian influenza virus subtype H5N1 can be detected specifically by an immunoassay utilizing the monoclonal antibody 4G6. It is further found that the sensitivity of the detection of immunochromatography can be increased by adding a nonionic surface and a water-soluble vinyl polymer having a polar group containing an oxygen atom and a nitrogen atom to a developing solution to be used in the immunochromatography.
Owner:OSAKA UNIV +1
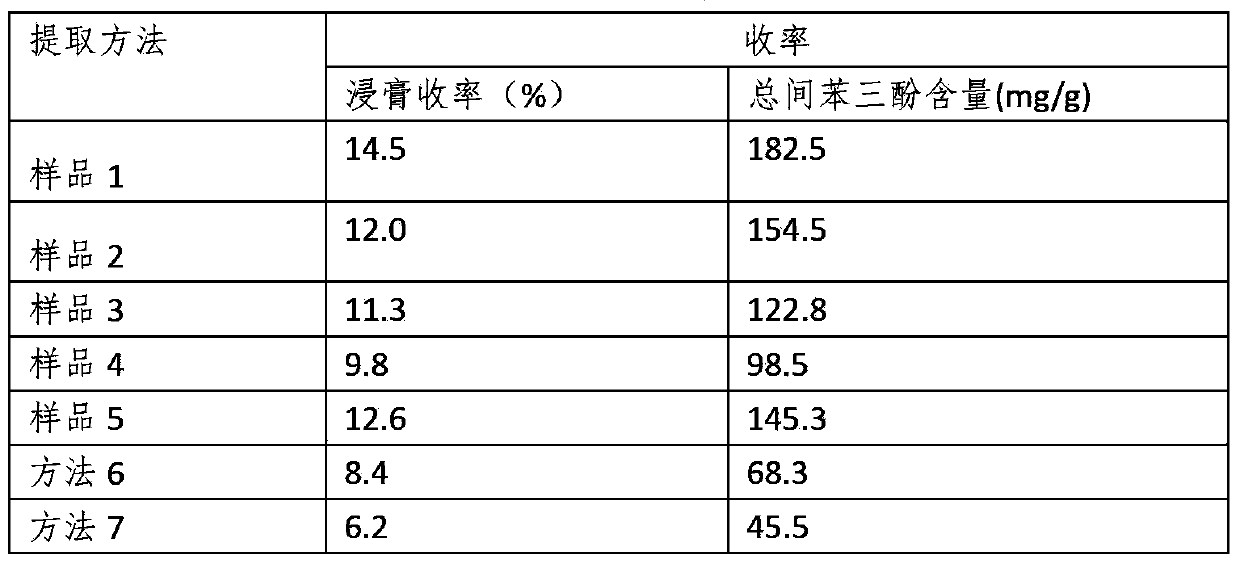
Composition of starwort sulphonic acid or vitriolic acid polyoses ester total phenolic glycoside and method of preparing the same and antiviral application
The invention relates to a kind of natural medicine of broad spectrum antibiotic. At present, the broad spectrum antibiotic medicine with high effect and safety is at shortage all round the world. The invention is intended to extract laminarinsulphate or sulphonic acid sugar ester or sulphosalts from plant chickweed or other chickweed plant with two resin adsorption methods or a water extraction and alcohol precipition method. The spectrum antibiotic in the invention is distributed under 50,000 in the formula weight formed by carbon glycosidic bond and / or oxide glycosidic bond with phenol, especially the total flavones comprising apigenin. However, the invention mainly acts as total phenolic glycoside with the formula weight under 4,000. Besides, the invention can form brownish compound with the total flavones comprising apigenin and the glycosidic ingredients without sulfur element, so as to be applied as broad spectrum antibiotic drug. Therefore, the compound in the invention can be applied to cure ADIS virus, hepatitis virus, influenza virus and parainfluenza virus comprising SARS, adenovirus, verruca acuminate virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella. No toxic effect has been found in the application. What is more, the invention can be made into 10 sorts of formulation, disinfector and health-improving products.
Owner:朱耕新
Transdermal immune influenza multivalent vaccine and preparation method thereof
InactiveCN101450209AEnhanced antigen presentation functionFacilitated DiffusionPowder deliveryAntiviralsBALB/cAdjuvant
The invention provides a transdermal immunity flu polyvalent vaccine and a preparing method. The transdermal immunity flu polyvalent vaccine comprises transdermal immunity adjuvant, flu polyvalent vaccine antigen, permeation agent and medical dressing. The flu polyvalent inactivation or attenuated live vaccine is differ from prior vaccine immunity approach and adjuvant. A result through permeation purpose immunity to Balb / c mouse, ferret, monkey and human body improves that the transdermal immunity flu polyvalent vaccine can generate IgA and IgG antibody with high valency, namely, can induce immune system and mucosal immune simultaneously, also can be used for immunoprophylaxis to popular flu and highly pathogenic avian influenza.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Polypeptide or derivative thereof and application of polypeptide or derivative in influenza virus infection
ActiveCN104151403AGood anti-influenza activityShort peptide chain lengthSsRNA viruses negative-senseGenetic material ingredientsHemagglutininInfluenza virus A hemagglutinin
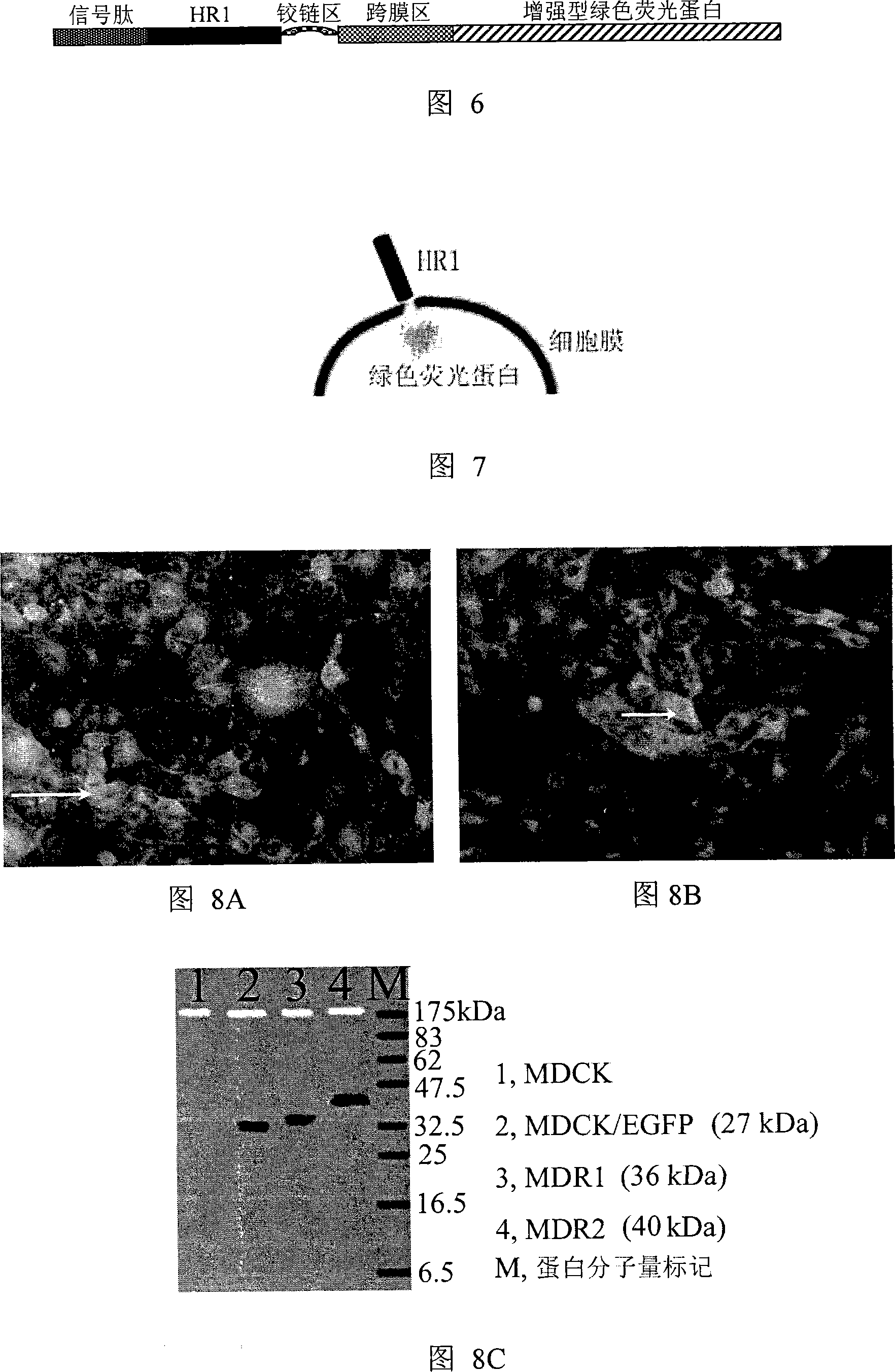
The invention relates to polypeptide, protein or peptide-like medicine from influenza virus hemagglutinin, and a method of the polypeptide, belongs to the technical field of biological medicines, and in particular relates to eight influenza virus hemagglutinin fragment peptides which can block influenza virus infection and have the serial numbers SEQ ID NO.1 to SEQ ID NO.8. The fragment peptides can inhibit and block infection of different species and influenza viruses of different subtypes to a host, including multiple influenza virus strains such as highly pathogenic avian influenza virus, seasonal human influenza virus and the like. The invention provides the peptide sequence (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), derivative peptides (including amino acid sequences of peptides and polynucleotide sequences of encoded peptides), peptide compositions and independent or united applications of peptides in preventing or treating influenza virus, such as medicine combination of peptides provided by the invention and other anti-influenza medicines.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
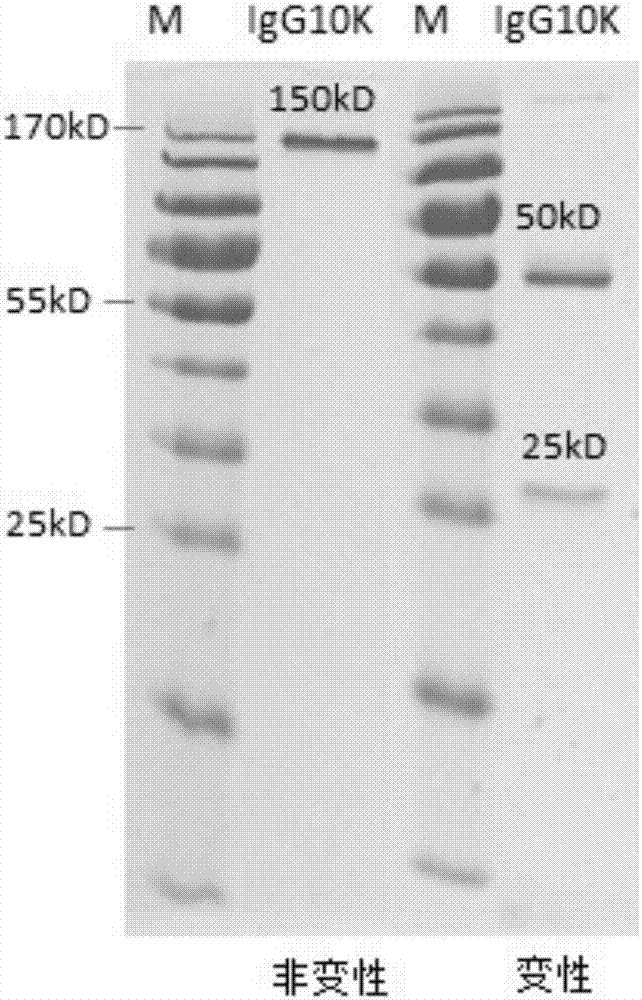
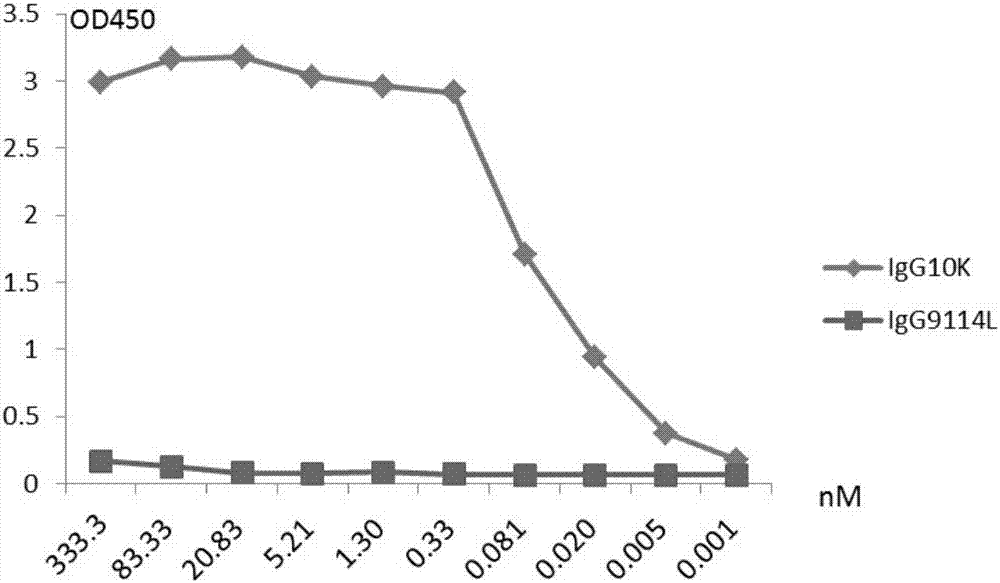
Humanized anti-H7N9 avian influenza virus high-affinity antibody 10K and application thereof
InactiveCN107056938AEfficient combinationImmunoglobulins against virusesAntiviralsHeavy chainEffector cell
The invention discloses a humanized anti-H7N9 avian influenza virus high-affinity antibody 10K filtered and obtained based on a single cell separation technology, the amino acid sequences of light chain and heavy chain variable regions of the antibody are shown as SEQ ID No. 2 and SEQ ID No. 5 respectively. The high-affinity specificity of the antibody is combined with H7N9 avian influenza virus 7 type hemagglutinin protein, and can mediate the kill and wound (ADCC) of effector cells using NK cells as main parts for H7N9 influenza virus infected cells. The antibody 10K can be used for therapeutic development of highly pathogenic avian influenza infection, and also can be used for development of H7N9 influenza virus antigen dectection reagents.
Owner:深圳普兰达科技有限公司
Method for quickly detecting nucleic acid of H5 subtype highly pathogenic avian influenza virus
The invention belongs to the technical field of biology and determines a method for quickly detecting nucleic acid of H5 subtype highly pathogenic avian influenza virus. The method comprises three technical points: determining a primer sequence required for nucleic acid detection; determining detection reaction types; determining a detection reaction system and reaction conditions. The method is useful in scientific research of H5 subtype avian influenza virus and clinical diagnostic detection for animals or humans.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Adenovirus vector avian influenza recombinant vaccine
ActiveCN101475641AImprove immunityGenetic material ingredientsAntiviralsProtective antigenHemagglutinin
The invention discloses an adenovirus carrier avian influenza recombined vaccine. The vaccine takes the hemagglutinin antigen gene of highly pathogenic avian influenza H5N1 as a main protective antigen gene which is fused with a braided mycobacterium tuberculosis heat shock protein coding gene. After being transferred into an adenovirus carrier to obtain a recombined adenovirus and immunize an animal, the fusion gene can produce a high titer antibody of the hemagglutinin antigen HA inside the animal and keep a high antibody titer inside the animal for a long period.
Owner:ZHEJIANG YEBIO BIOTECH +1
Composition for treating and/or preventing influenza, method and application thereof
ActiveCN109675042AHigh biosecurityHigh internal and external protection effectPeptide/protein ingredientsAntiviralsTherapeutic effectInfluenza a
The present invention provides a composition for treating and / or preventing influenza, a method and an application thereof. The composition contains protein treated with acid anhydride and another drug for treating and / or preventing influenza. The acid anhydride-treated protein prepared by the invention is acid anhydride-treated lactoglobulin. An in vitro experiment shows that the protein can broadly inhibit influenza virus infected cells of various subtypes. In animal in vivo experiments, the protein has a significant prevention and treatment effect on influenza virus infection. Influenza viruses have many subtypes and high mutation rates, so that the development of broad-spectrum anti-influenza preparations is very important. The acid anhydride-treated protein can not only inhibits the infection of many seasonal influenza viruses, but also significantly inhibits the highly pathogenic avian influenza virus H7N9, and the influenza virus H1N1 (2009) which caused a pandemic, suggesting that the protein can be developed as a highly effective and broad-spectrum biological preparation for the prevention and treatment of influenza viruses.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Method and device for anaerobic pyrolysis treatment of dead-of-disease livestock and municipal organic refuse
InactiveUS20160339488A1Bio-organic fraction processingCoke oven safety devicesChemical reactionDehydrogenation
Disclosed is a method for anaerobic pyrolysis treatment of dead-of-disease livestock and municipal organic refuse, which uses an anaerobic pyrolysis device to perform a harmlessness treatment on dead-of-disease pigs, the treatment being a chemical reaction process performed in a sealed, oxygen-free, non-combustible, high-temperature state, comprising heating the bodies of pigs to a high temperature under an anaerobic state, and by the action of thermal decomposition through reactions such as vaporization, pyrolysis, dehydrogenation, thermal condensation and carbonization, evaporating the moisture from the pig bodies, converting the organics therein to combustible gases and organic carbon, and killing various types of bacteria in the bodies of the dead pigs via the high temperature. Thus, a harmlessness and reutilization treatment of the dead-of-disease livestock is achieved, and environmental pollution and propagation of fatal animal diseases, such as foot-and-mouth disease, highly pathogenic avian influenza, highly pathogenic blue-ear pig disease and swine fever are avoided. Also disclosed is a device for realizing the above-mentioned method.
Owner:MISSISSIPPI INT WATER +1
Anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody and application thereof
The invention provides an anthropogenic H5N1-resisting hemagglutinin protein broad-spectrum neutralising antibody. The antibody is obtained by screening through a phage display technology. The sequence of the hypervariable region (CDRs) determining the specificities of the antibody is represented by figure 1. The antibody of the invention specifically identifies H5N1 highly pathogenic avian influenza virus particle antigen, has obvious fluorescence immune reaction and enzyme-coupled immune reaction with the avian influenza virus H5N1 aiming at avian influenza virus hemagglutinin protein HA, and has the neutralising activity function of resisting the avian influenza virus H5N1. The antibody of the invention can be made into specific antibody medicines clinically used for preventing and treating human avian influenza that is caused by the H5N1 avian influenza virus, so as to clinically prevent or treat human avian influenza that is caused by the H5N1 avian influenza virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
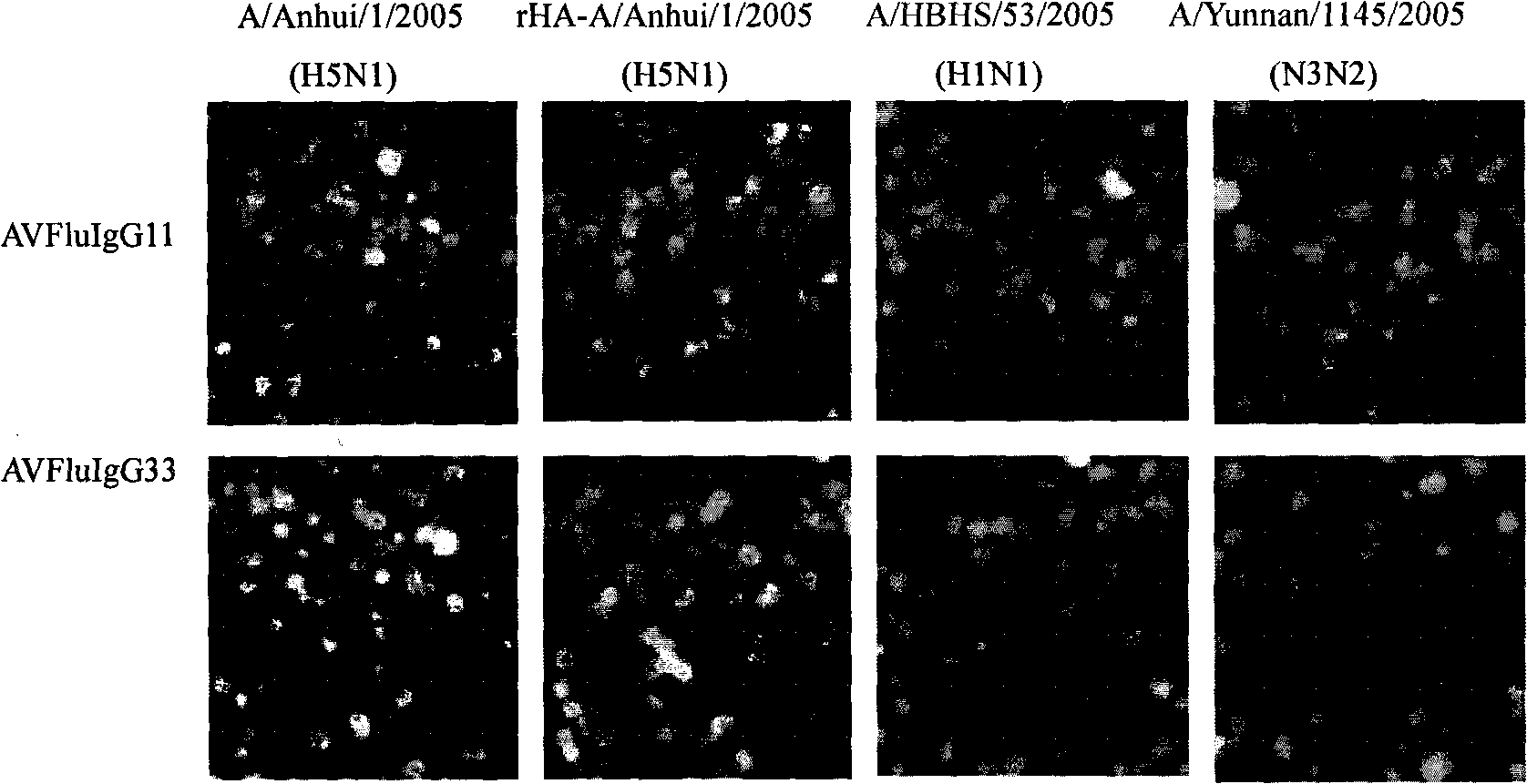
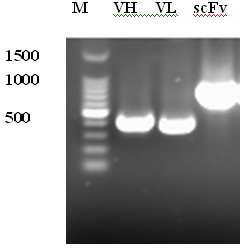
ScFv antibody for resisting H5N1 type highly-pathogenic avian influenza and application thereof
The invention relates to an scFv antibody for resisting H5N1 type highly-pathogenic avian influenza and an application thereof. A light chain amino acid sequence is shown as SEQIDNo: 1; a heavy chain amino acid sequence is shown as SEQIDNo: 2; a light chain nucleotide sequence is shown as SEQIDNo: 3; and a heavy chain nucleotide sequence is shown as SEQIDNo: 4. The scFv antibody for resisting the H5N1 type highly-pathogenic avian influenza is applied to the preparation of a medicine for resisting the H5N1 type avian influenza virus. Proven by protection tests of embryonated eggs, the 4F5 antibody has a 100% protective rate on prevention of the avian source and human source H5N1 type avian influenza virus infection, and the 4F5 antibody has a 100% protective rate on treatment of the avian source H5N1 type avian influenza virus infection. The highest protective rate on treatment of the human source H5N1 type highly-pathogenic avian influenza virus infection is 62.5%.
Owner:NANJING MEDICAL UNIV +1
Construction and application of recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein
ActiveCN110218706AThoroughly purifiedShorten the timeSsRNA viruses negative-senseViral antigen ingredientsTurkey HerpesvirusNucleotide sequencing
The invention discloses construction and application of a recombinant turkey herpesvirus expressing H7N9 subtype highly pathogenic avian influenza virus HA protein. The recombinant turkey herpesvirusis obtained by inserting an exogenous gene expression box between the HVT-065 and HVT-066 genes in a non-essential replication area of a coding sequence of a turkey herpesvirus (HVT), wherein the exogenous gene expression box is formed by connecting an MCMV promoter, an exogenous gene and SV40 poly A in sequence; the exogenous gene is the H7HA gene, and the nucleotide sequence of the exogenous gene is shown as SEQ ID NO:1. The construction method of the recombinant turkey herpesvirus is also provided. An ultrasonic cracking and breaking method is adopted for purification, the screening time isshortened, and the obtained recombinant turkey herpesvirus rHVT-H7HA provides good protection for the highly pathogenic H7N9 subtype avian influenza virus and can be used for subsequent development of new avian influenza virus vaccines.
Owner:SOUTH CHINA AGRI UNIV
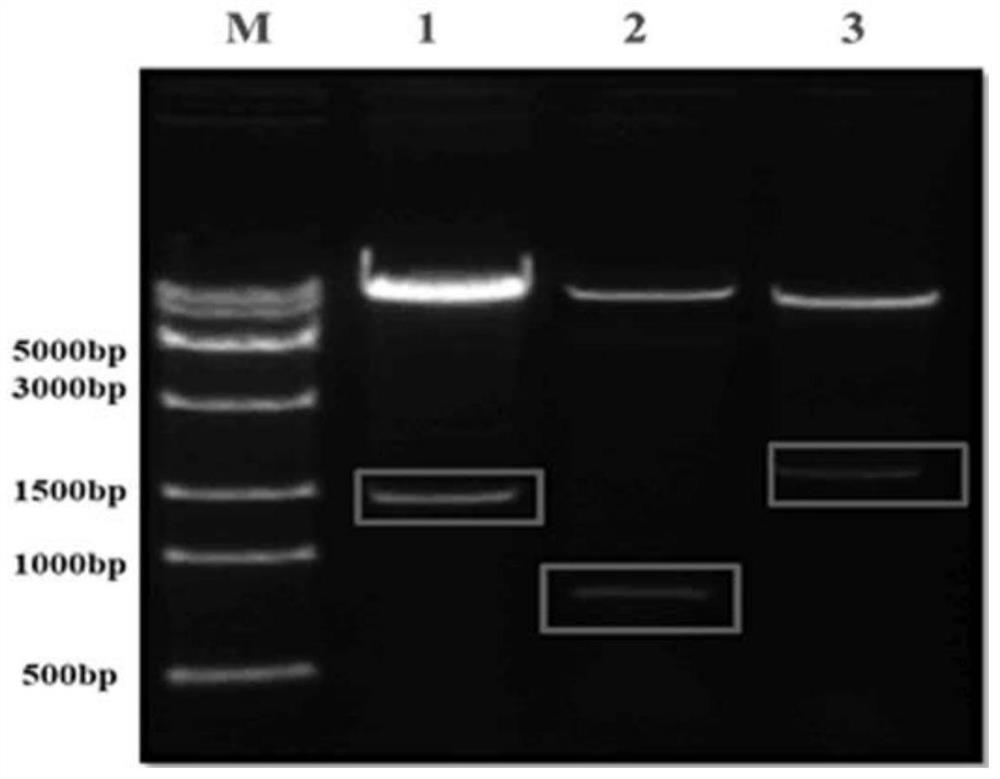
Recombinant H7N9 subtype avian influenza virus-like particle, and preparation method and application thereof
ActiveCN112079904AGood cross reactivityMild diseaseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunogenicity
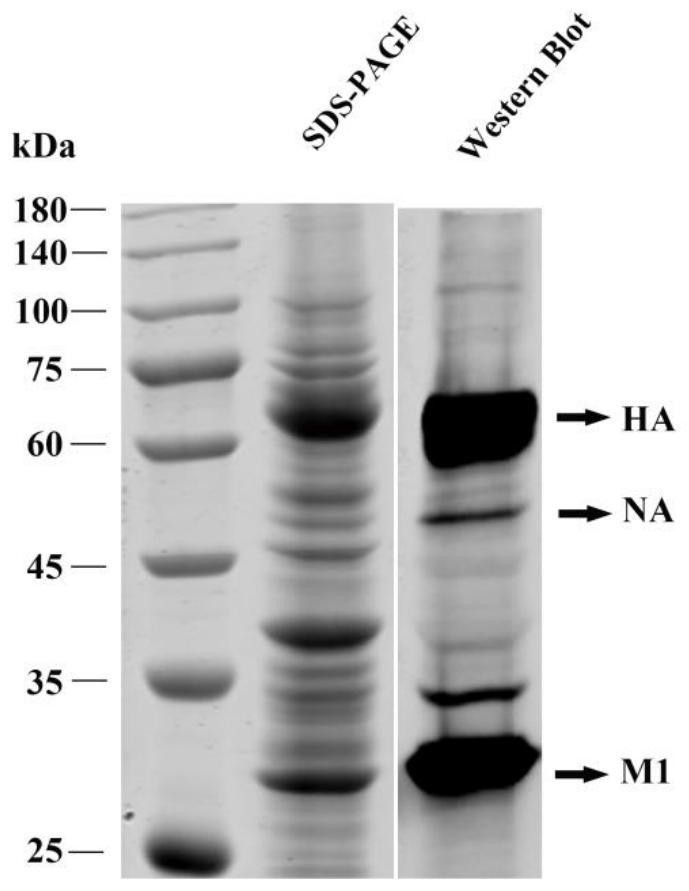
The invention discloses a recombinant H7N9 subtype avian influenza virus-like particle, and a preparation method and application thereof. Recombinant baculoviruses for expressing HA protein, NA protein and M1 protein of an H7N9 highly pathogenic avian influenza virus are respectively constructed on the basis of a recombinant baculovirus insect cell culture system. The three recombinant baculovirusstrains are used for co-infecting suspended insect cells, so that the H7N9 subtype avian influenza virus-like particle capable of being self-assembled in the cells can be obtained. After the concentration and purification with cane sugar with different gradient concentrations through an ultrafiltration tube, the H7N9 subtype avian influenza virus-like particle is mixed and emulsified with an adjuvant to prepare a vaccine. When a chicken is immunized by the prepared vaccine, the body can be induced to generate a specific antibody; and the advantages of high immunogenicity, good safety, high hereditary stability and the like are realized. After the attack by a lethal dose of H7N9 subtype highly pathogenic avian influenza viruses, the complete clinical protection can be provided, and virus expelling of the chicken is obviously inhibited. The invention provides a novel method for preventing H7N9 subtype avian influenza virus infection, and also lays a foundation for the development of a novel influenza virus vaccine.
Owner:YANGZHOU UNIV
Use of atractylone, atractylone-containing plant and atractylone extract in preparing influenza virus resisting medicine
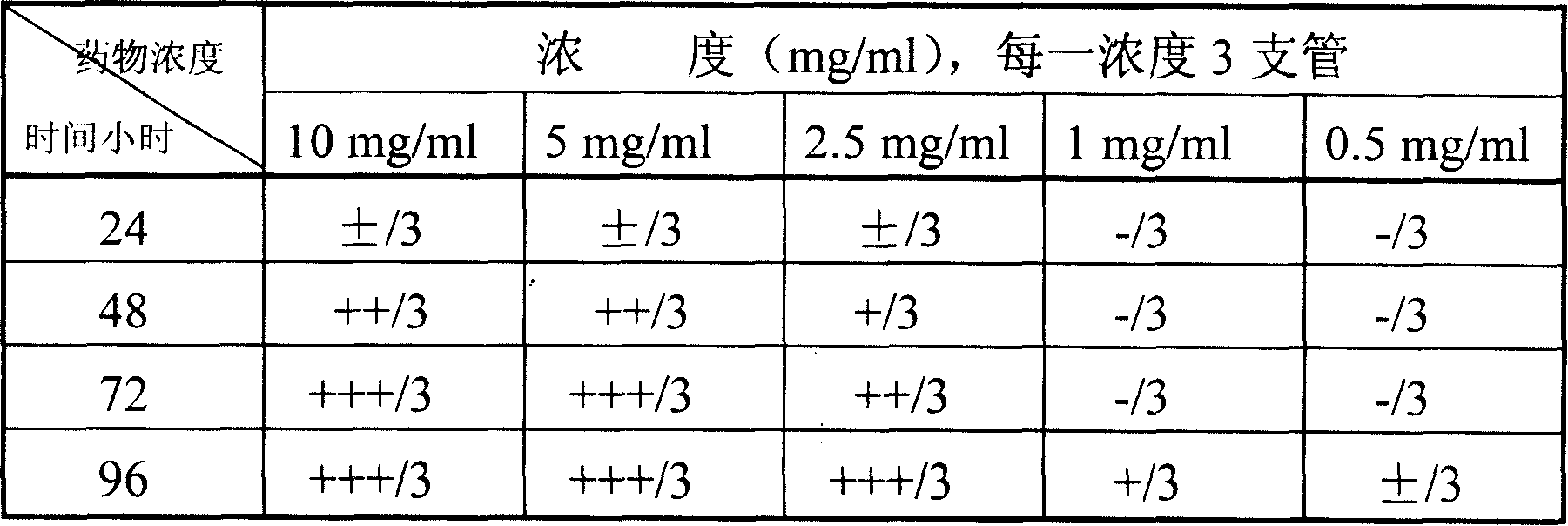
InactiveCN1857358AOrganic active ingredientsAerosol deliveryBird fluHighly Pathogenic Avian Influenza Virus
The present invention discloses the effective cold virus resisting effective component, atractylone, and features that atractylone and Chinese medicine or extract containing atractylone have effect of resisting influenza A and B virus. Extracorporeal experiments show that atractylone in concentration of 1 mg / ml has the effect of killing influenza A virus H3N2 subtype, high pathogenic bird flu virus H5N1 subtype and influenza B virus strain. All the said atractylone, Chinese medicine or extract containing atractylone may be used as the medicine for human body and animal to prevent and treat influenza and bird flu, and may be prepared into tablet, capsule, granule, oral liquid, injection, inhalant and spray.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Compound of sulfoacid flavonecosid component in chickweed and the antivirus application and the preparing method
The invention relates to a wide-spectrum antiviral natural drug, while present world is lack in high-effect safe wide-spectrum antiviral natural drug. The invention, via two the adsorptions of two different resins, extracts a compound from plant FanLu, while the compound contains oligosaccharide or / and polyose and flavonoid as brown flavonoid glycoside with sulfonic acid group formed with apione, whose molecule weight is lower than 4, 000. The inventive compound can be used to treat AIDS, hepatitis viruse, influenza virus, or the like, without toxicity, while it can be made into 10 kinds of agent and health-care products.
Owner:朱耕新
Traditional Chinese medicine effective part for treating influenza virus and its preparation process
The invention discloses an effective portion of traditional Chinese medicament for resisting influenza virus, which is prepared from selfheal 5-200 parts, mulberry leaf 5-1-00 parts, wild chrysanthemum flower 5-100 parts as raw material through disintegrating, and supercritical CO2 extraction. The effective portion can be prepared into tablets, capsules, granules, oral liquids, injections, inhalants, sprays or drop pills.
Owner:GUANGZHOU XINGQUN PHARMA
Polypeptide vaccine for treating diseased induced by high pathogenic avian influenza virus H5N1
InactiveCN101380466AEasy to store and transportFacilitate automated mass productionAntiviralsAntibody medical ingredientsDiseaseSide effect
The invention relates to a polypeptide vaccine for treating diseases caused by highly pathogenic avian influenza virus H5N1, which is characterize in that: the amino acid sequence is as follows: amino terminal-QIGNISIWV-carboxyl terminal; and lysine is adopted as a linker to connect a plurality of amino acid sequences to form a multi-copy series structure. The invention has the advantages of convenient transportation and preservation and automated mass production, and can activate specific cytotoxic T lymphocyte (CTL) which can effectively kill and lyses target cells with the N1 protein of the highly pathogenic avian influenza virus H5N1 without toxicity and side effects.
Owner:CHONGQING UNIV
Rhizoma Dryopteris Crassirhizomatis extract and its use in preparation of viral disease controlling medicines
ActiveCN103356717AImprove immunityFunction increaseAntiviralsPteridophyta/filicophyta medical ingredientsDiseaseSide effect
The invention provides a Rhizoma Dryopteris Crassirhizomatis extract and its use in the preparation of viral disease controlling medicines. The Rhizoma Dryopteris Crassirhizomatis extract is an extract obtained through ethyl acetate extraction-ethanol refluxing extraction, can be added to drinking water or feeds to improve the humoral immunity and cellular immunity functions of animals, can control highly pathogenic avian influenza, newcastle disease virus, infectious bursal disease viruses and weaned pig diarrhea, and can also control the occurrence and epidemic of immunosuppressive diseases. The Rhizoma Dryopteris Crassirhizomatis extract has the advantages of easily available raw material, low cost, and small side effects, and is suitable for controlling the epidemic diseases of animals having impaired immunities after failed immunities.
Owner:承德普润生物制药有限公司
Preparation method of CpG ODN with pig immune enhancement activity
InactiveCN106490361AImprove featuresImprove responseAccessory food factorsImmunological disordersBird fluImmunologic function
The invention relates to the technical field of veterinarian biological products, in particular to a preparation method of CpG ODN with the pig immune enhancement activity. The method comprises the following steps of 1 volume production of CpG ODN, 2 envelope additive preparation and 3 ingredient preparation. The body is induced to generate various immunology effects and promoted to secrete various cell factors, the specific and non-specific immunity reaction capability of the body are improved, the pig immunity can be effectively improved when the CpG ODN is added into feed, and the good immunity effect on great animal epidemic diseases such as the high pathogenicity pig ear disease, the high pathogenicity bird flu, foot-and-mouth diseases and pig fevers is achieved, the pig survival rate is effectively increased, diseases are reduced, and the breeding cost is lowered.
Owner:NANNING UNIV
Composition of starwort total glycopeptides and total flavone and preparation method and uses thereof
InactiveCN101390921ASafe broad-spectrum antiviral drug actionHigh-efficiency broad-spectrum antiviral drugsBiocideOrganic active ingredientsAlkaneDisease
Disclosed is a dark brown combination with the molecular of 1,000-100,000, which is composed of total glycopeptides ingredient and total flavonoid ingredient, and extracted from stellaria through three methods and used as safer and more efficient natural broad-spectrum anti-viral medicine. The total peptide part accounts for 15%-25% and is composed of 17 types of amino acids respectively according to the proportions, such as by proportion, such as aspartic acid, methionine and isoleucine; the total sugar accounts for 15%-30% and is composed of glucose, galactose and arabinose; the total flavonoid which is mainly apigenin glycoside accounts for 10%-40%; Oxygenated alkane which contains alcohols accounts for 25%-40%. The combination can be used as the medicines for the treatment of the diseases, including HIV, hepatitis viruses, influenza virus such as Highly-pathogenic avian influenza virus, condyloma virus, herpes virus and mumps virus, and have no toxicity after application; the combination can be made into more than 10 types of medicinal preparations and health care products, and avoid environmental pollution during the production process.
Owner:朱耕新
Adenovirus vector avian influenza recombinant vaccine
ActiveCN101993498AImprove immunityGenetic material ingredientsAntiviralsHemagglutininProtective antigen
The invention discloses an adenovirus vector avian influenza recombinant vaccine. A hemagglutinin antigen gene of highly pathogenic avian influenza H5N1 is used as a main protective antigen gene, and the gene is fused with a mycobacterium tuberculosis heat shock protein coding gene. The fusion gene can generate a high-valence antibody of the anticoagulin antigen hemagglutinin (HA) in animal bodies after being inoculated into an adenovirus vector, obtaining recombinant adenovirus and immunizing animals, and can keep high antibody valence in the animal bodies for a long time.
Owner:ZHEJIANG YEBIO BIOTECH +1
Gene for coding recombinant avian influenza virus HA protein, virus-like particle, vaccine, preparation and application
ActiveCN113862284AHigh expressionImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsNucleotideGenetic engineering
The invention belongs to the technical field of genetic engineering vaccines, and particularly relates to a gene for coding recombinant avian influenza virus HA protein, a virus-like particle, a vaccine, preparation and application. The gene has a nucleotide sequence as shown in SEQ ID NO: 1. The invention also provides an avian influenza virus-like particle, the avian influenza virus-like particle is assembled by HA protein, NA protein and M1 protein, and has a hemagglutination titer up to 13log2. The invention also provides an avian influenza virus-like particle vaccine containing the virus-like particle, the vaccine can provide complete clinical protection and significantly inhibit detoxification against lethal attack of homologous and wild H7N9 subtype highly pathogenic avian influenza viruses, and a new vaccine choice is provided for prevention and control of H7N9 subtype avian influenza.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com