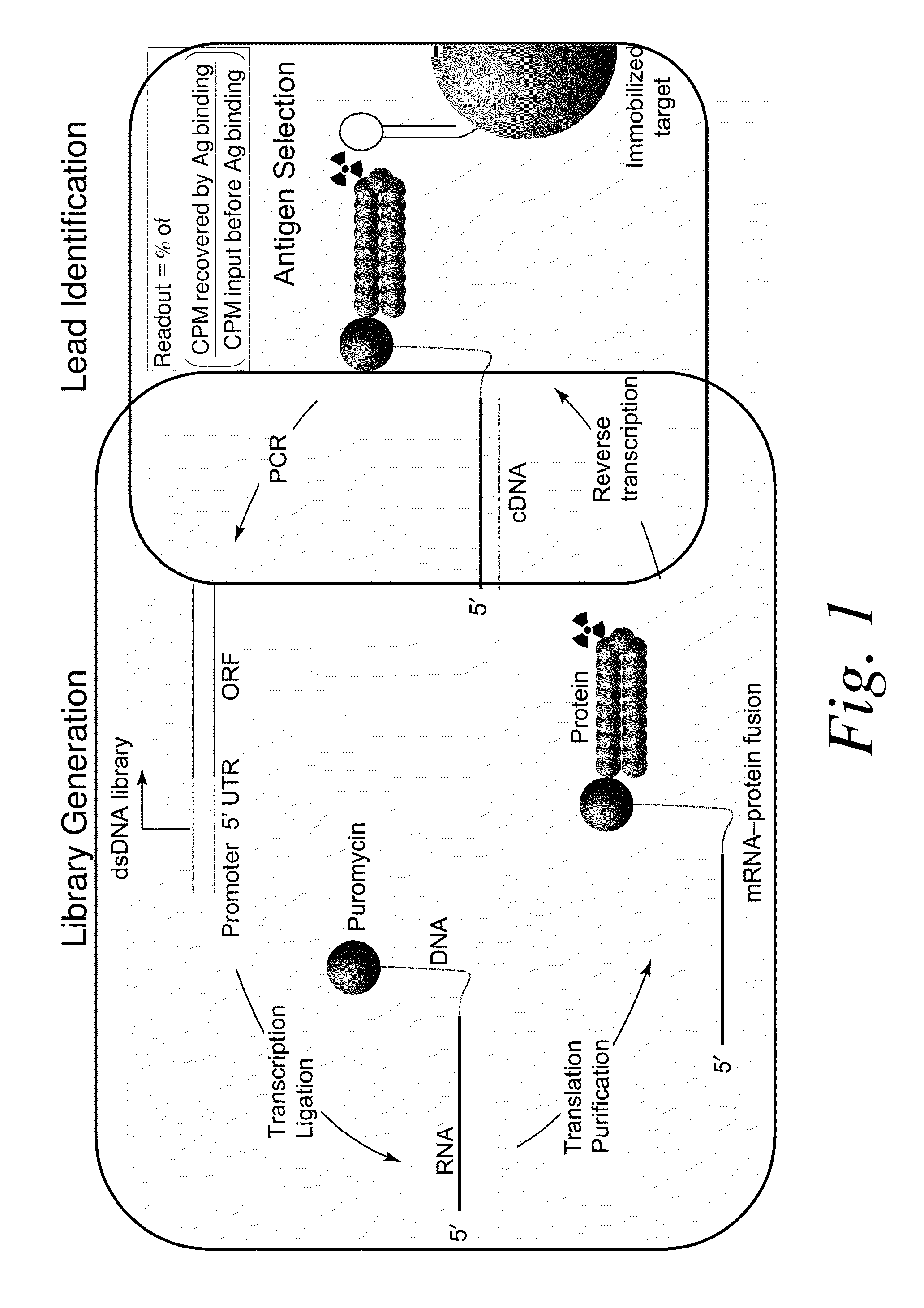
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "ScFv Antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody Libraries
InactiveUS20100099103A1Sugar derivativesMicrobiological testing/measurementExpression LibraryScFv Antibodies
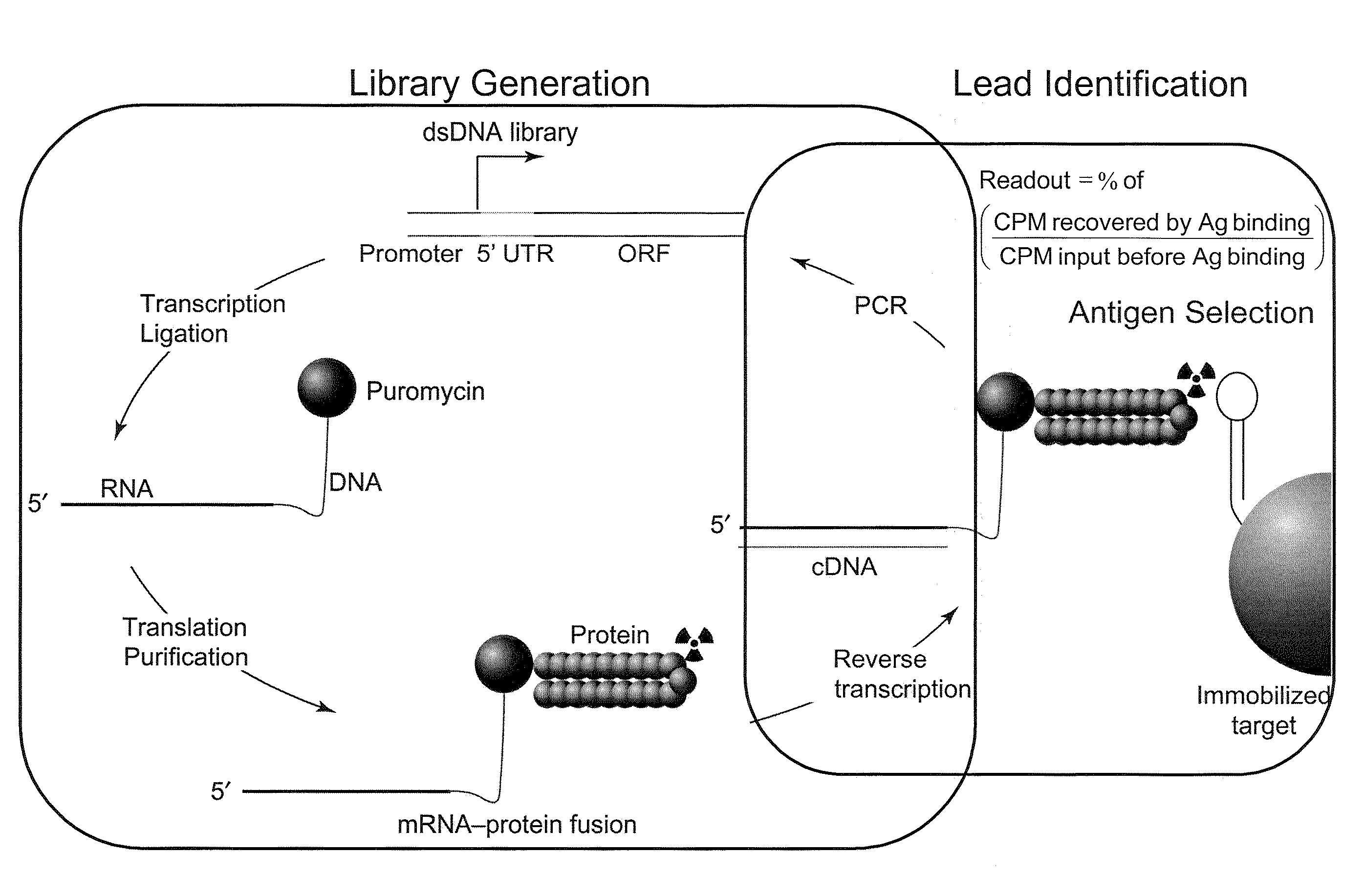
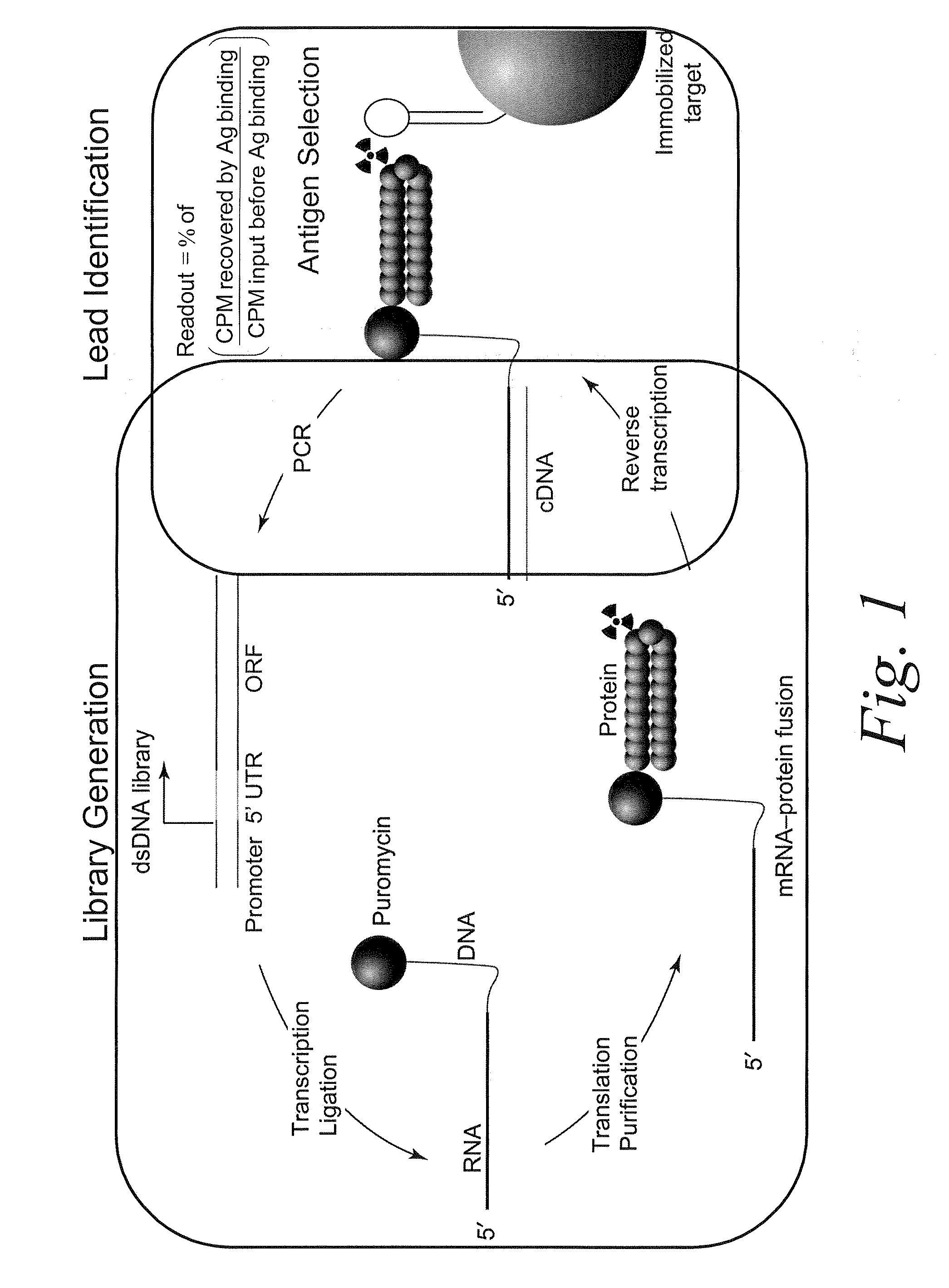
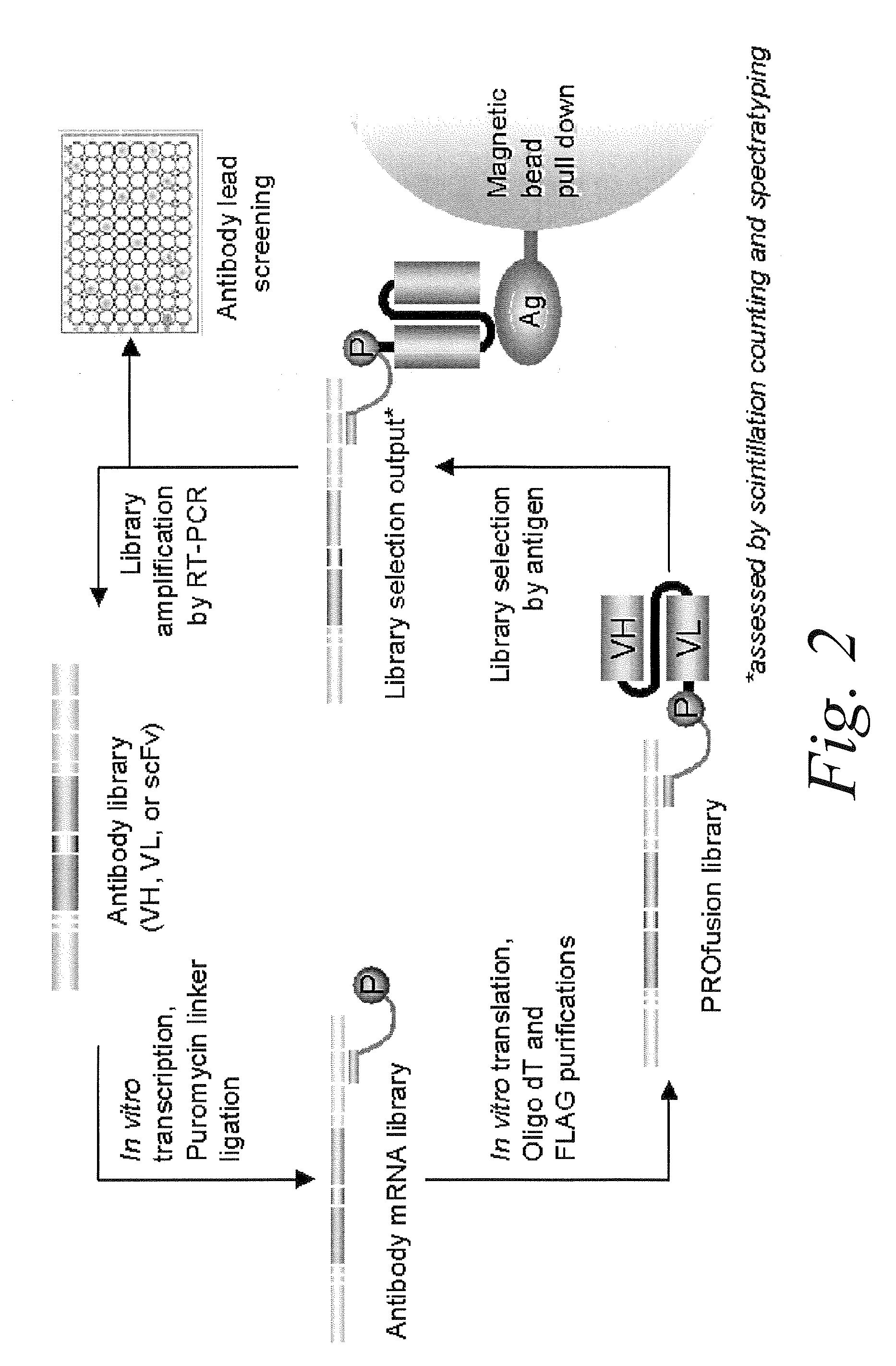
The present invention features improved in vitro RNA display libraries to allow reliable expression and selection of scFv antibody molecules from expression libraries. The scFv antibody libraries of the invention contain an optimized, shortened inter-domain linker that improves expression scFv antibody expression. The scFv antibody libraries also include short nucleic acid barcodes that allow for identification of individual library clones, libraries or subsets thereof. Primers for generating, amplifying and spectratyping the scFv antibody libraries of the invention are also provided.
Owner:ABBVIE INC
Receptor Tyrosine Kinase-Like Orphan Receptor 1 (ROR1) Single Chain FV Antibody Fragment Conjugates and Methods of Use Thereof
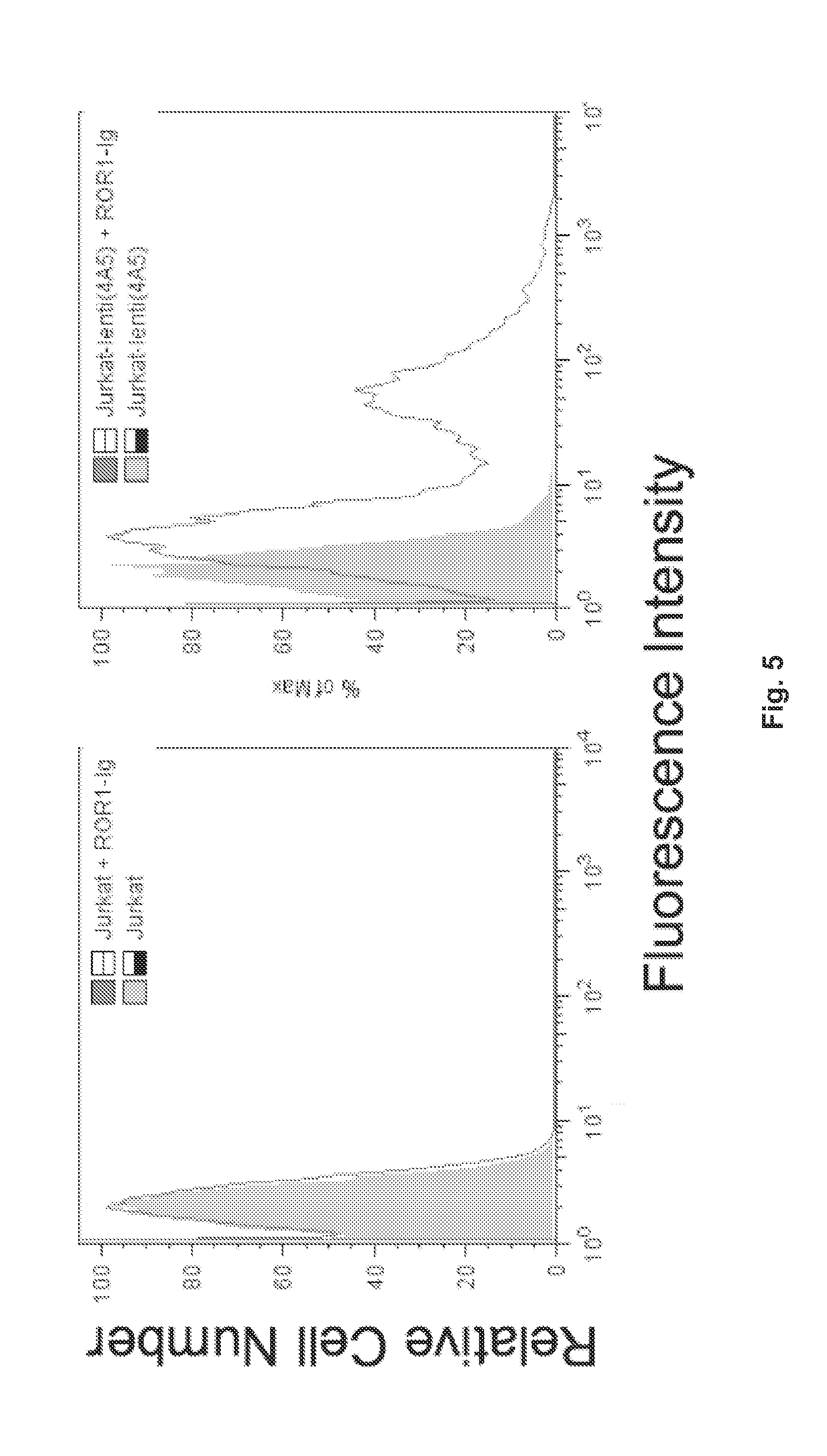
ActiveUS20130101607A1Peptide/protein ingredientsAntibody mimetics/scaffoldsCancer cellScFv Antibodies
Compositions including an antibody single-chain variable fragment (scFv) conjugate that specifically binds to ROR1 tumor-associated antigen are provided. The anti-ROR1 scFv antibody and conjugates may include a biologically-active molecule. Such conjugates may comprise a chimeric receptor to direct T cells to respond to ROR1 cancer cells, Methods to use the scFV conjugates to target cells expressing ROR1 for therapeutic and diagnostic purposes are also provided.
Owner:RGT UNIV OF CALIFORNIA
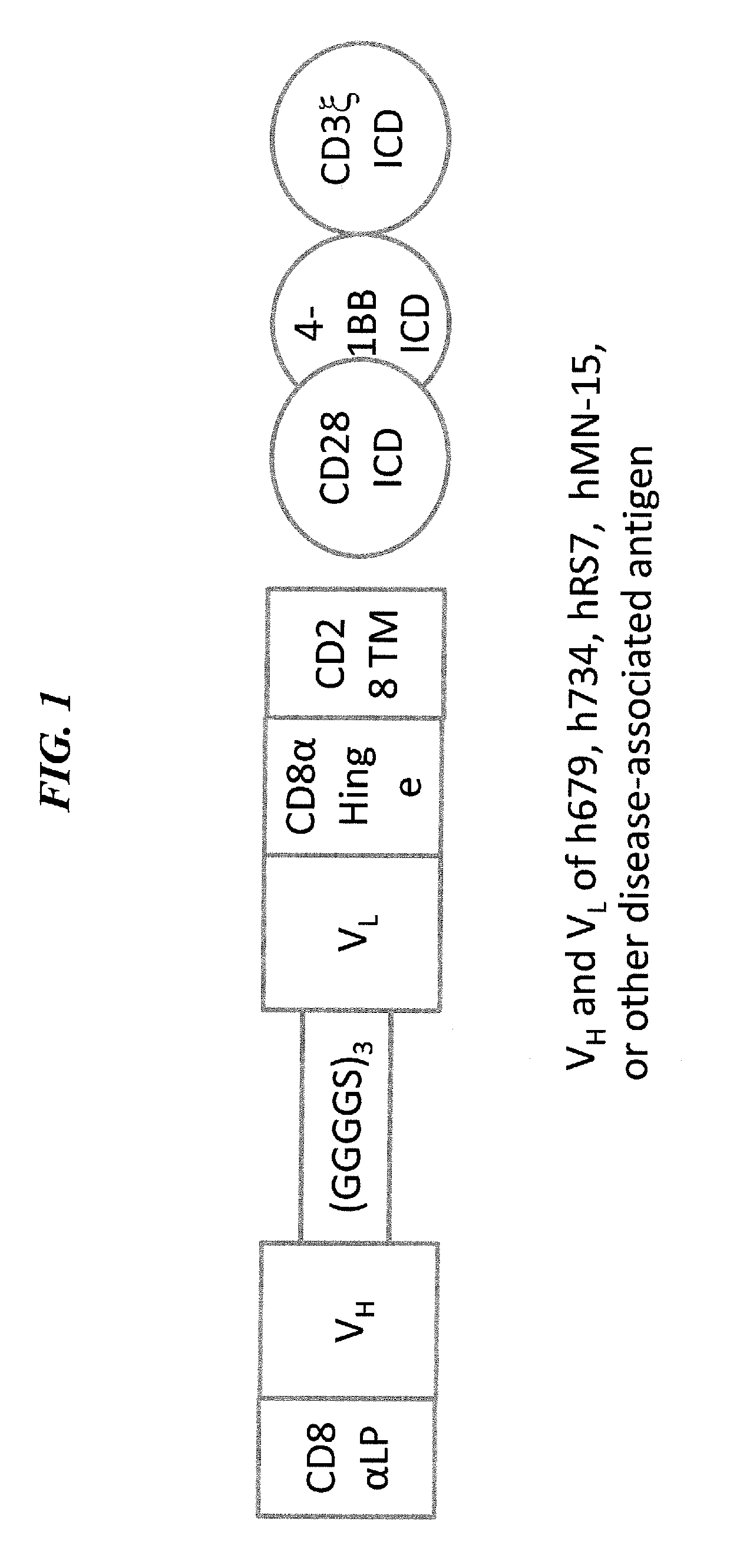
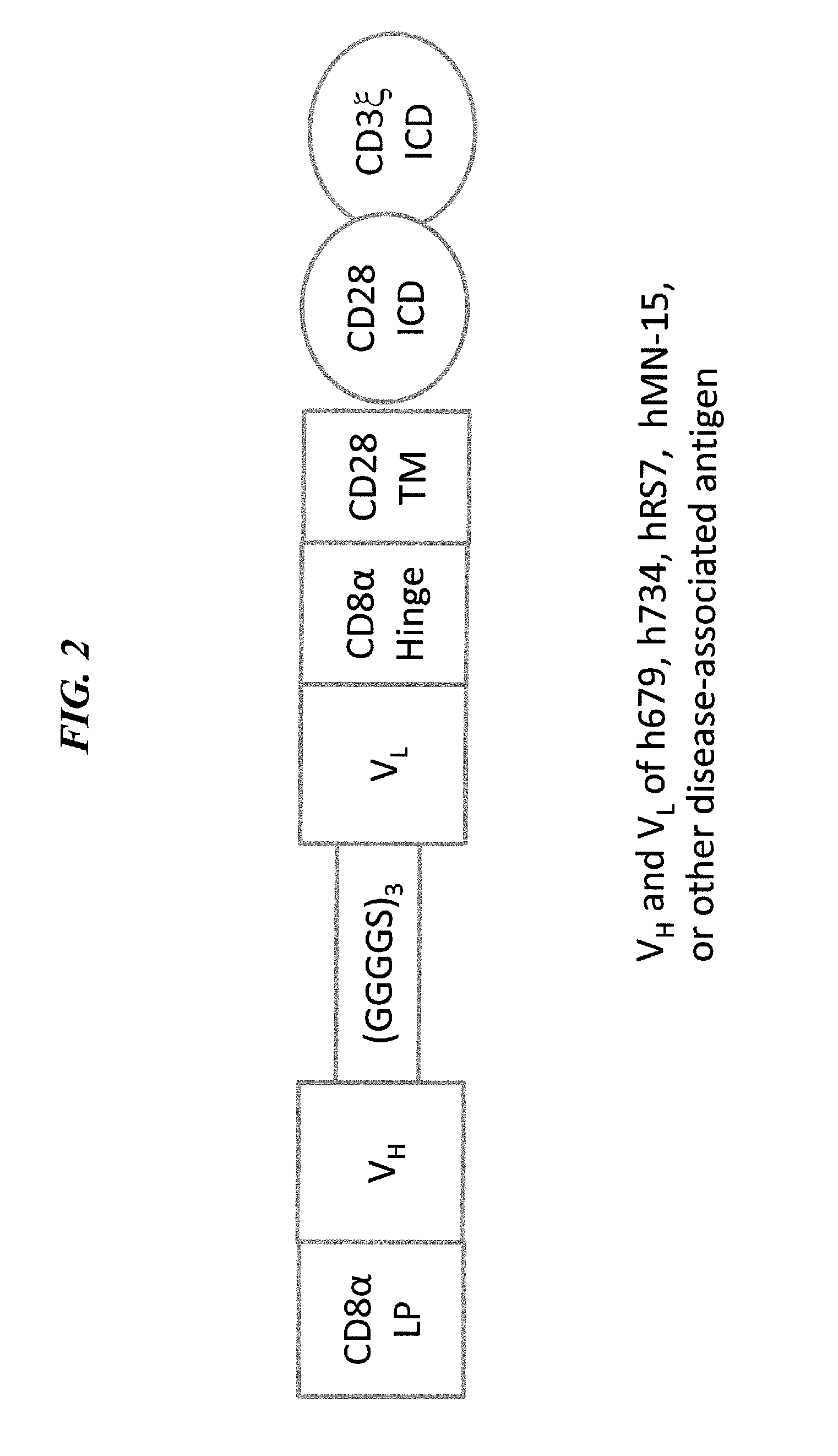
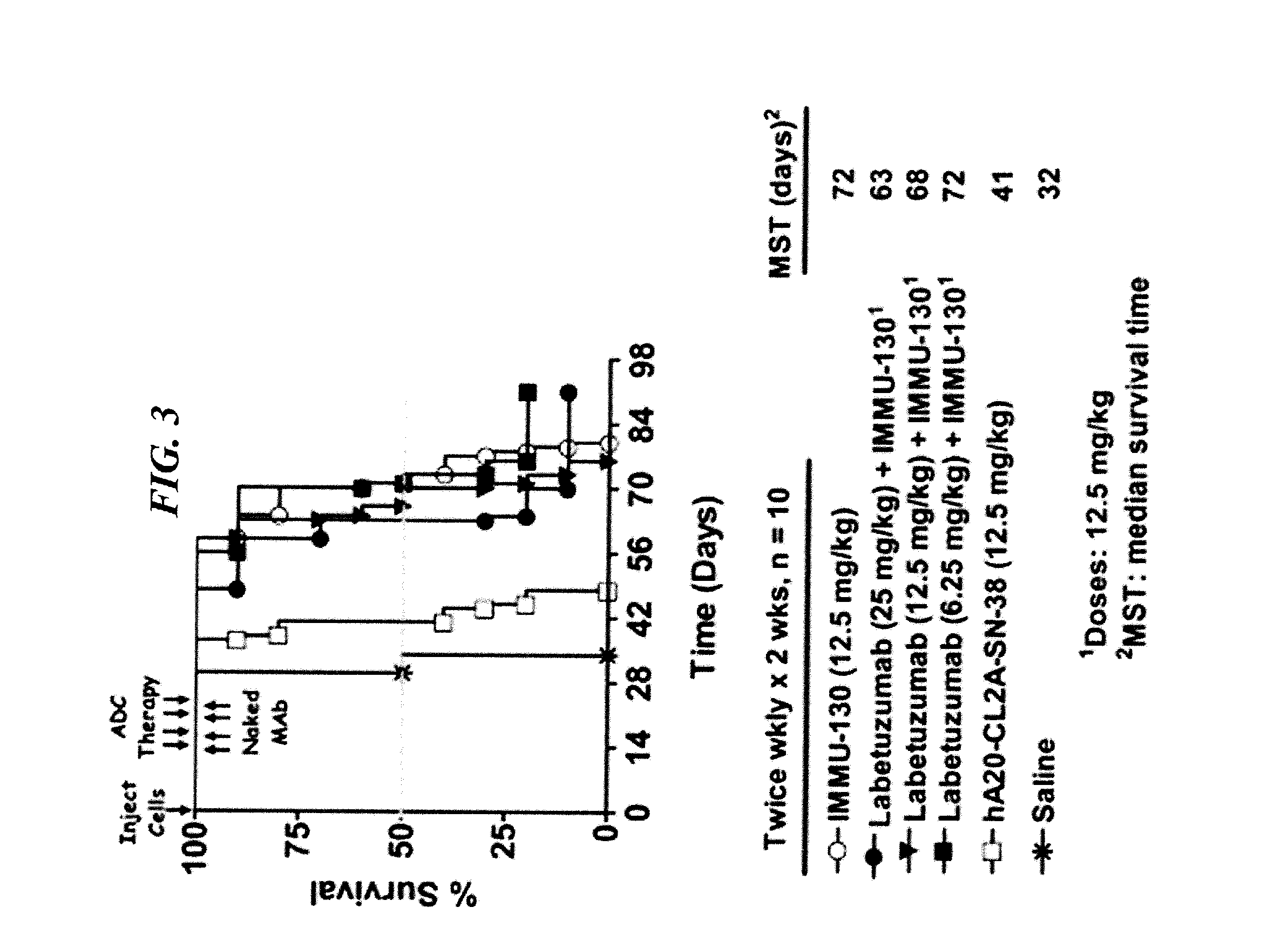
Disease therapy with chimeric antigen receptor (CAR) constructs and t cells (car-t) or nk cells (car-nk) expressing car constructs
InactiveUS20160361360A1Maintain self-toleranceModulate durationAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionDebulking Procedure
The present invention concerns CAR, CAR-T and CAR-NK constructs, preferably comprising a scFv antibody fragment against a disease-associated antigen or a hapten. More preferably, the antigen is a TAA, such as Trop-2. The constructs may be administered to a subject with a disease, such as cancer, autoimmune disease, or immune dysfunction disease, to induce an immune response against disease-associated cells. Where the constructs bind to a hapten, the subject is first treated with a hapten-conjugated antibody that binds to a disease associated antigen. Therapy may be supplemented by other treatments, such as debulking procedures (e.g., surgery, chemotherapy, radiation therapy) or coadministration of other agents. More preferably, administration of the construct is preceded by predosing with an unconjugated antibody that binds to the same disease-associated antigen. Most preferably, an antibody against CD74 or HLA-DR is administered to reduce systemic immunotoxicity induced by the constructs.
Owner:IMMUNOMEDICS INC
Methods of RNA Display
ActiveUS20100105569A1Solve problemsFunction increaseLibrary screeningDNA preparationExpression LibraryScFv Antibodies
The present invention features improved methods of in vitro RNA display to allow reliable expression and selection of scFv antibody molecules from expression libraries. The improved methods, in part, involve the use of mildly reducing conditions, which favor of scFv intra-chain disulphide bond and thus correct folding of the scFv antibody molecules. Although particularly suited to expression and selection of scFv antibody molecules, the methods of the invention are also expedient for in vitro RNA display of all classes of protein.
Owner:BRISTOL MYERS SQUIBB CO +1
Antibodies against tumor surface antigens
ActiveUS7232888B2Simple methodExtended retention timePeptide/protein ingredientsMammal material medical ingredientsCarcinoembryonic antigenScFv Antibodies
The present invention relates to improved antibodies against tumor surface antigens and their use in the treatment of tumors. Of particular interest are highly stable, humanized, high affinity antibodies against carcinoembryonic antigen (CEA), especially the antibody we have termed sm3E, which is derived from the scFv antibody MFE-23. Such antibodies have the potential for improved therapeutic efficacy.
Owner:MASSACHUSETTS INST OF TECH +1
Stable and soluble antibodies inhibiting TNFalpha
Owner:NOVARTIS AG
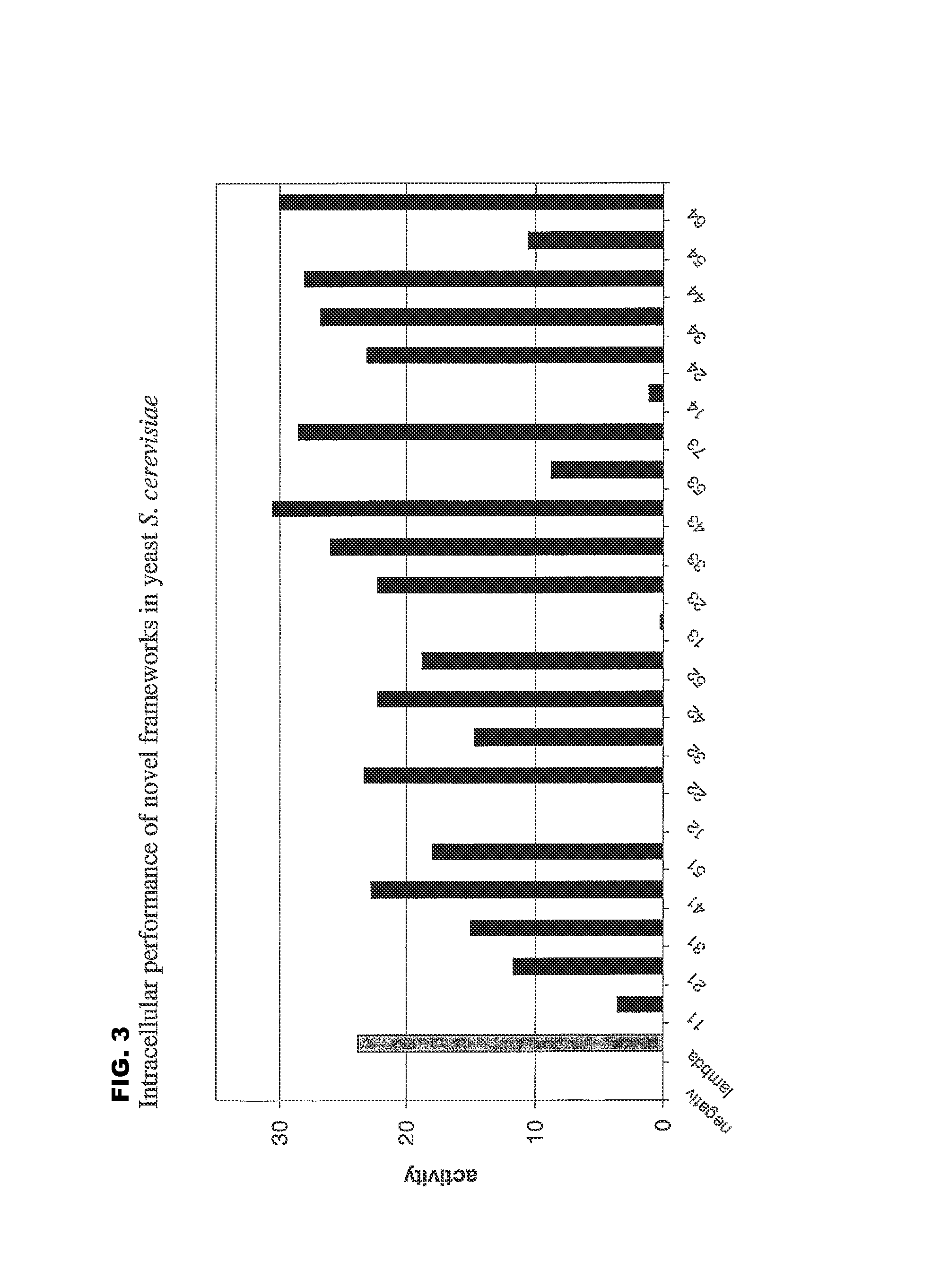
Immunoglobulin frameworks which demonstrate enhanced statbility in the intracellular envioronment and methods of identifying same
ActiveUS20060035320A1Improve solubilityImprove stabilityAnimal cellsSugar derivativesSolubilityAntibody fragments
Compositions are provided, which can be used as frameworks for the creation of very stable and soluble single-chain Fv antibody fragments. These frameworks have been selected for intracellular performance and are thus ideally suited for the creation of scFv antibody fragments or scFv antibody libraries for applications where stability and solubility are limiting factors for the performance of antibody fragments, such as in the reducing environment of a cell. Such frameworks can also be used to identify highly conserved residues and consensus sequences which demonstrate enhanced solubility and stability.
Owner:NOVARTIS AG
Antibodies against tumor surface antigens
ActiveUS20050147614A1Good effectExtended retention timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthCarcinoembryonic antigen
The present invention relates to improved antibodies against tumor surface antigens and their use in the treatment of tumors. Of particular interest are highly stable, humanized, high affinity antibodies against carcinoembryonic antigen (CEA), especially the antibody we have termed sm3E, which is derived from the scFv antibody MFE-23. Such antibodies have the potential for improved therapeutic efficacy.
Owner:MASSACHUSETTS INST OF TECH +1
Human antibody and expression thereof
The invention discloses a full-human anti-EGFR scFv antibody segment and entire antibody, which contains heavy chain of antibody and nucleic acid and amino acid of variable area of light chain as well as high-effective expressing method of entire antibody in the CHO cell.
Owner:SINO CELL TECH INC
Antibodies Against Influenza Virus and Methods of Use Thereof
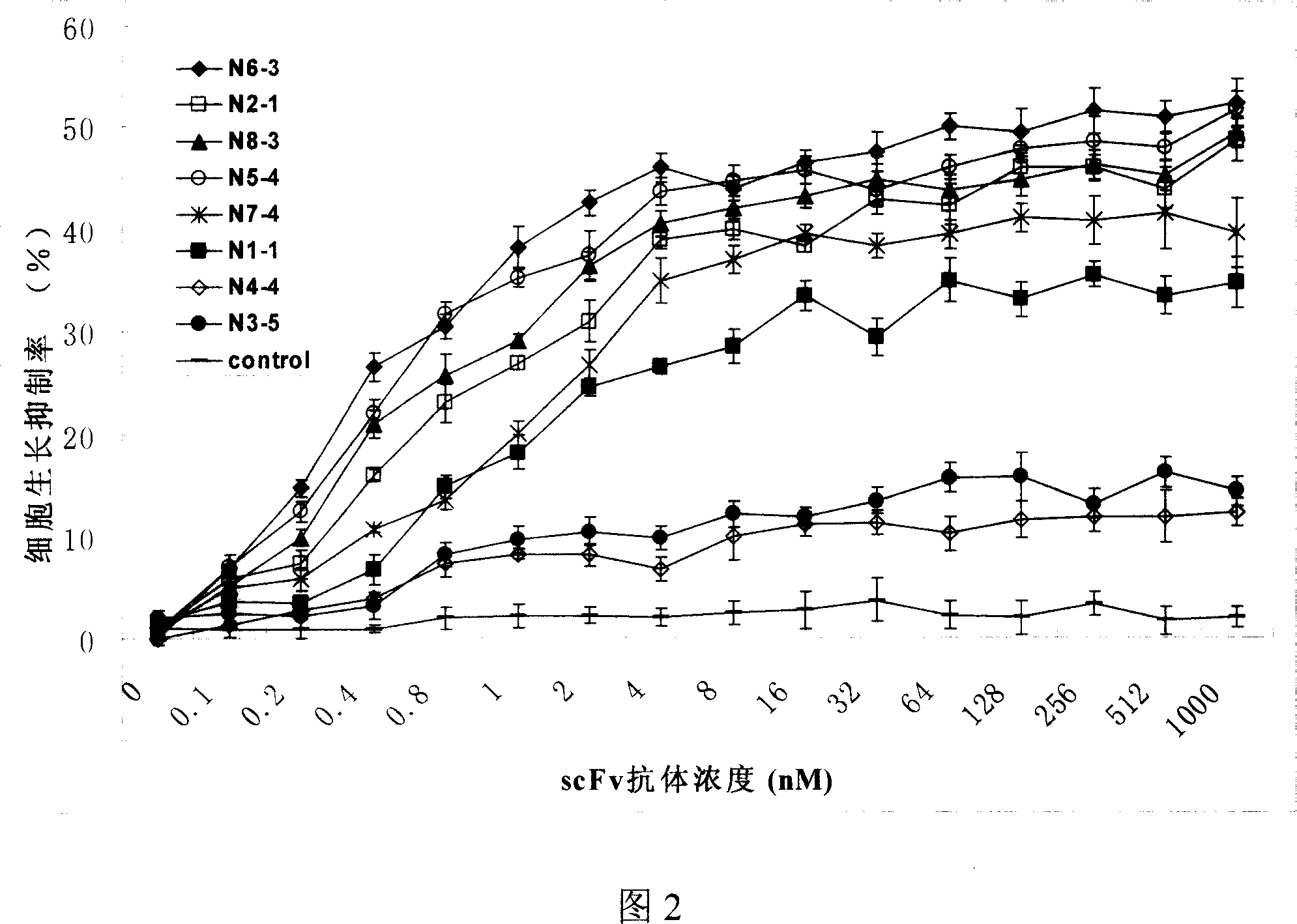
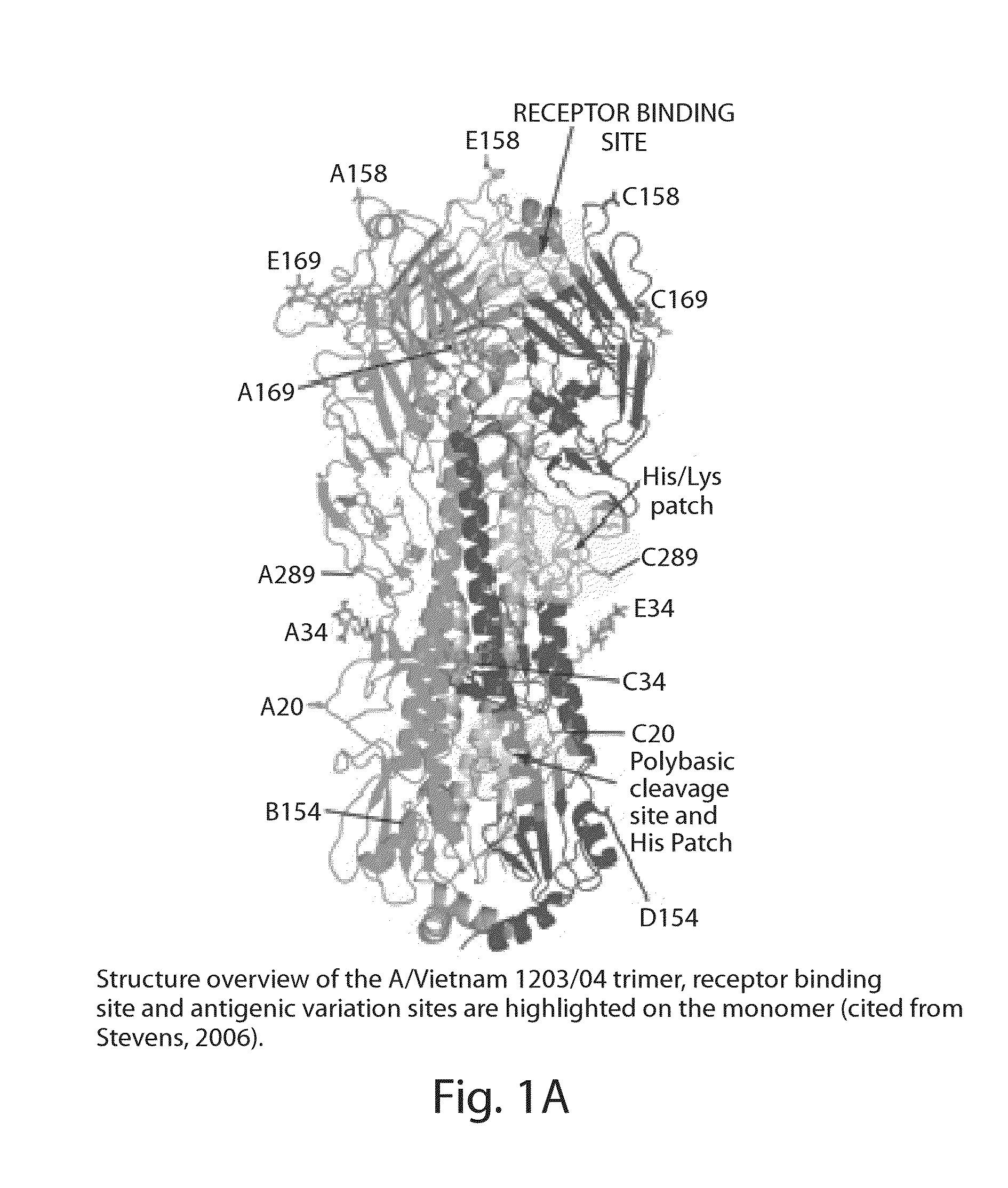
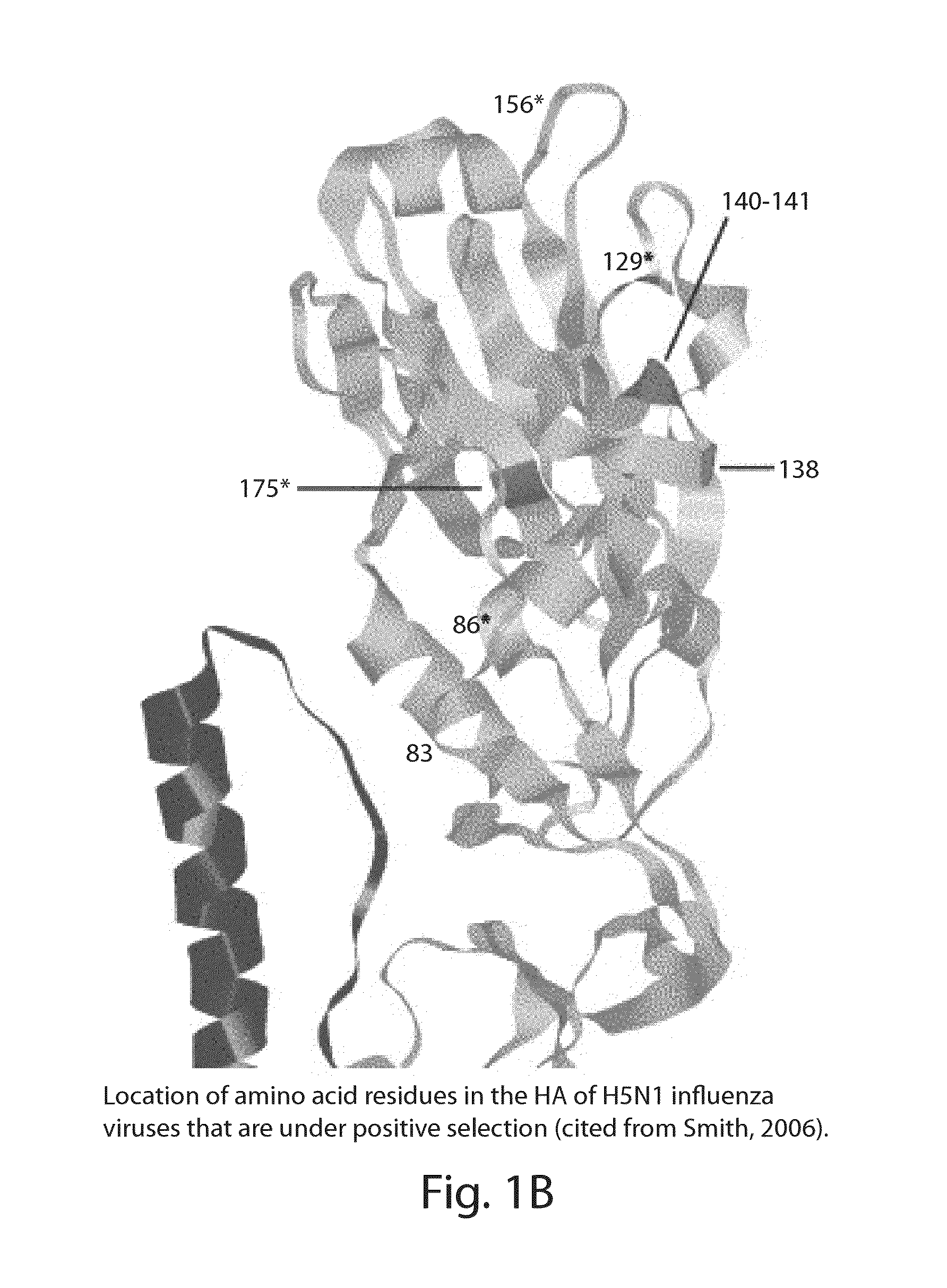
The invention provides human scFv antibodies and monoclonal antibodies that neutralize influenza virus. Also provided are methods of treating and / or preventing a influenza related disease or disorder such bird flu The invention also provides methods of vaccinating a patient against influenza. Also provided are methods of diagnosing influenza-related diseases or disorders and methods of detecting the presence of a influenza in a sample.
Owner:BURNHAM INST FOR MEDICAL RES +1
Antibodies against SARS-CoV and methods of use thereof
InactiveUS20050249739A1Enhance neutralization activityStrong neutralizing activitySsRNA viruses positive-senseAntibody mimetics/scaffoldsVaccinationMonoclonal antibody
The invention provides scFv antibodies and monoclonal antibodies that neutralize SARS-CoV. Also provided are methods of treating and / or preventing a coronavirus-related disease or disorder such as SARS. The invention also provides methods of vaccinating a patient against SARS-CoV. Also provided are methods of diagnosing coronavirus-related diseases or disorders and methods of detecting the presence of a coronavirus in a sample. The invention additionally provides methods of screening for compounds that modulate the binding of SARS-CoV and the SARS-CoV receptor ACE2 as well as for compounds useful to treat SARS-CoV-related diseases or disorders.
Owner:DANA FARBER CANCER INST INC
Methods of modifying antibodies, and modified antibodies with improved functional properties
InactiveUS20090074780A1Improve functional propertiesImprove solubilityAntibody ingredientsImmunoglobulinsSolubilitySingle-Chain Antibodies
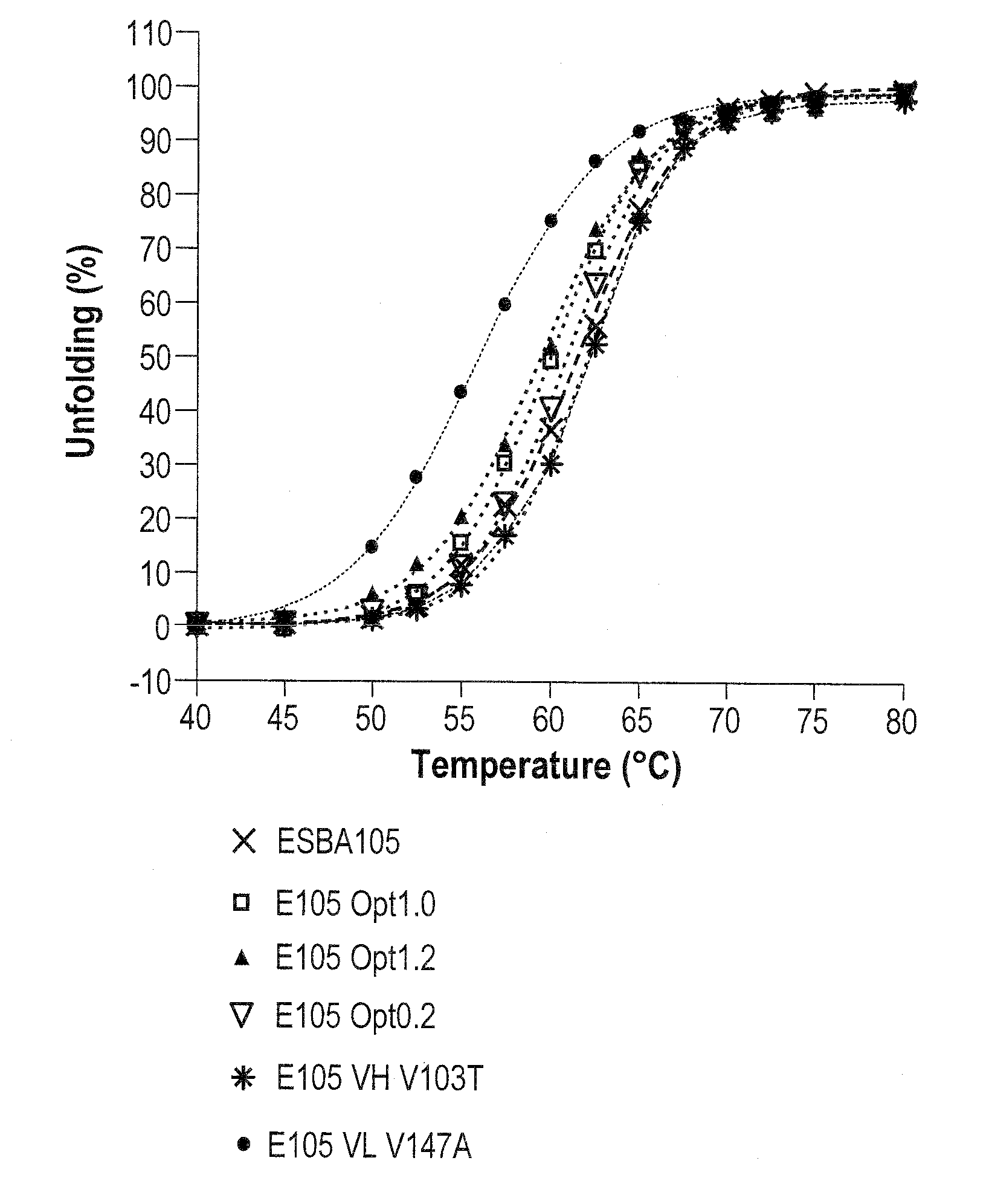
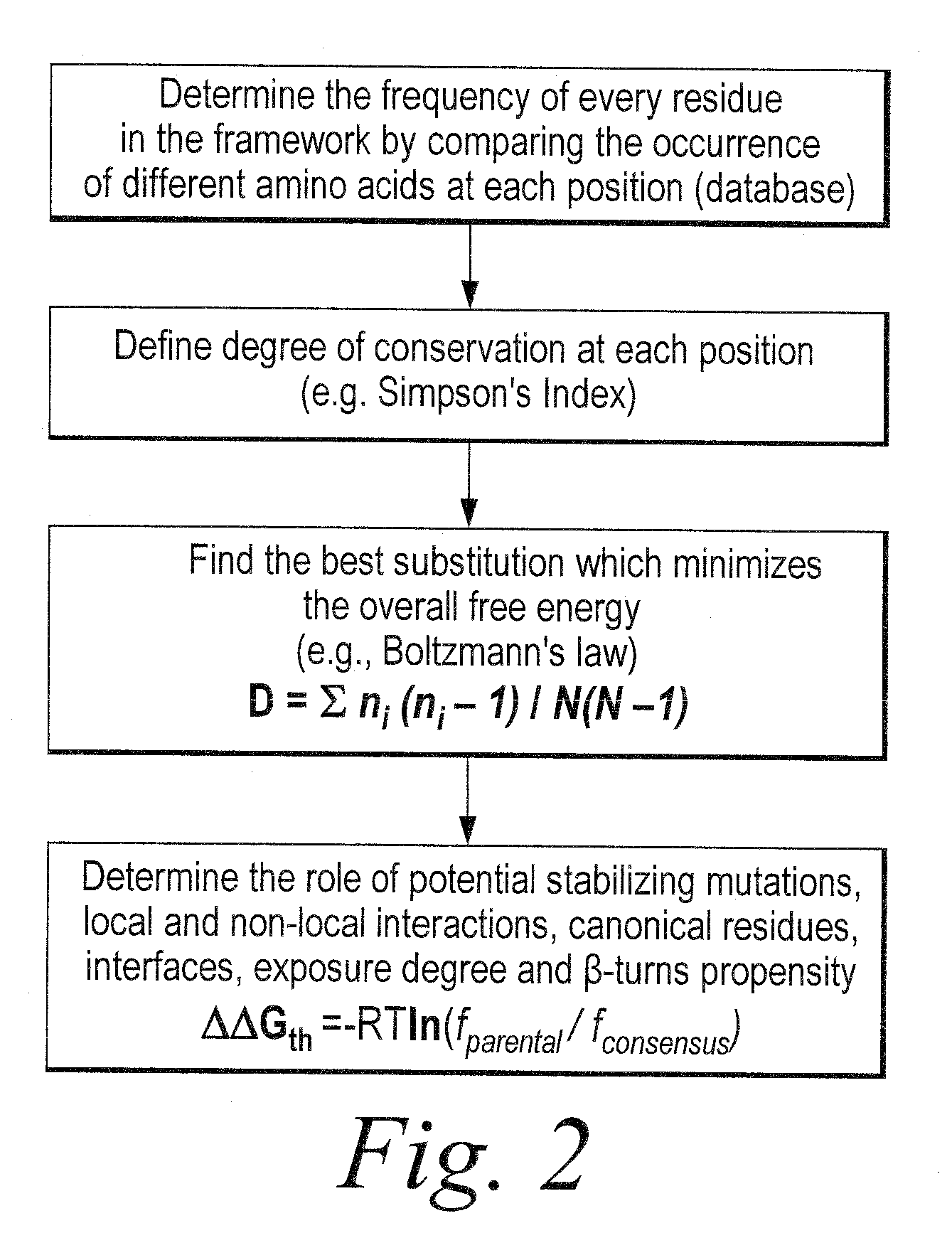
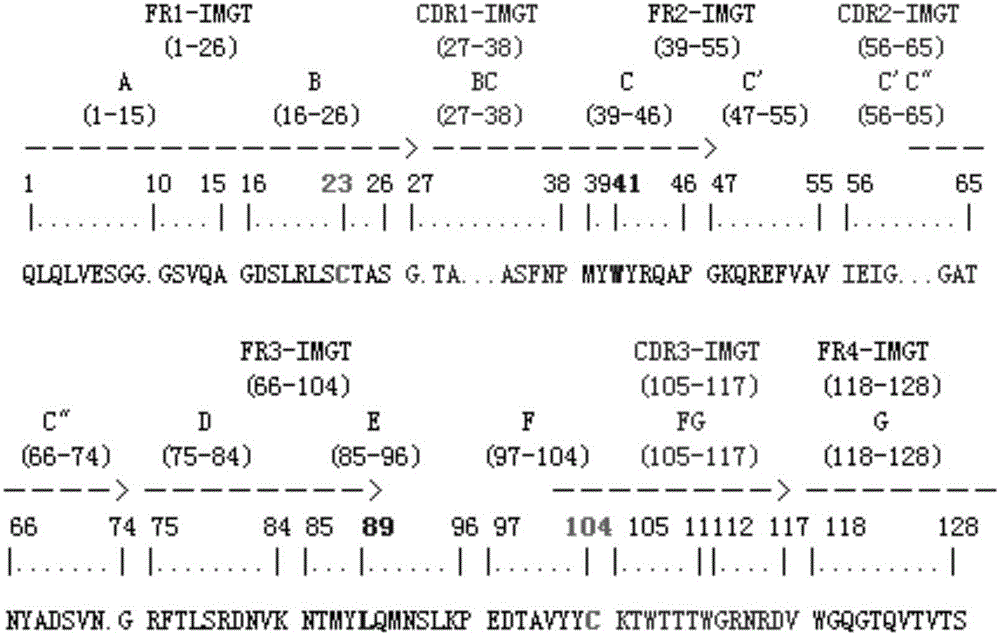
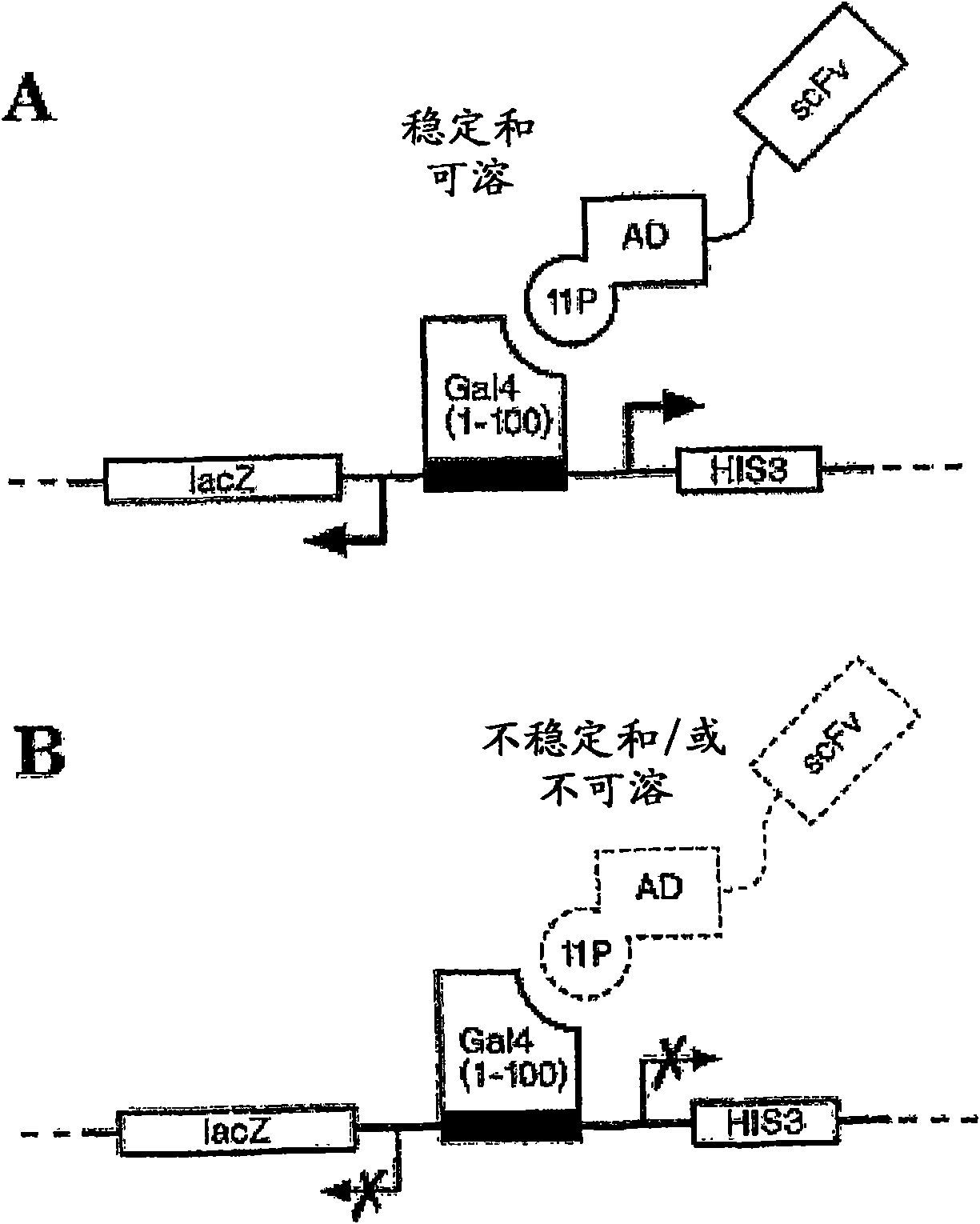
The invention provides methods of using sequence based analysis and rational strategies to modify and improve the structural and biophysical properties of immunobinders, and in particular of single chain antibodies (scFvs), including such properties as stability, solubility, and / or antigen binding affinity. The invention provides methods of engineering immunobinders, and in particular scFvs, by performing one or more substitutions at amino acid positions identified by analysis of a database of selected, stable scFv sequences, wherein preferred amino acid residues for substitution have been identified. The invention also provides immunobinders prepared according to the engineering methods of the invention. The invention also provides preferred scFv framework scaffolds, into which CDR sequences can be inserted, as well as scFv antibodies made using these preferred framework scaffolds.
Owner:NOVARTIS AG
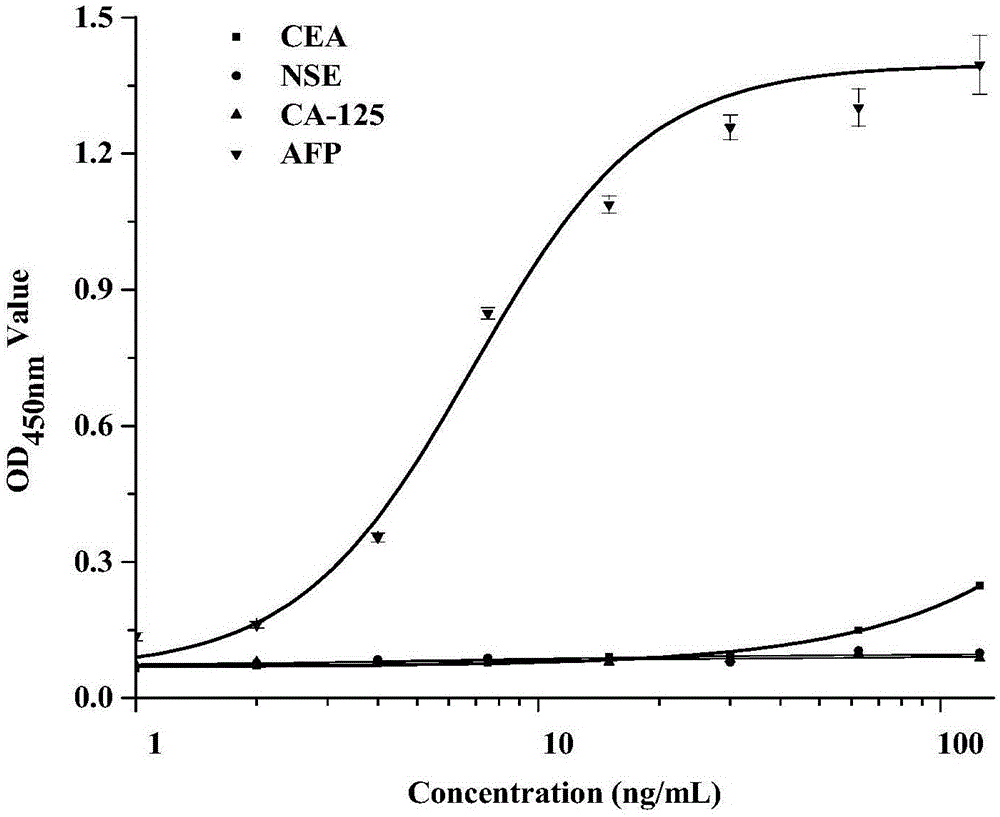
AFP nanometer antibody A83 based on AFP antigen
ActiveCN105037544AUnique molecular structureSmall molecular weightImmunoglobulins against animals/humansBiological testingAntigenDisease
The invention belongs to the technical field of biology, and relates to a camel source immunity nanometer antibody (single-domain heavy-chain antibody, VHH) specifically bound with AFP and application of the antibody. The amino acid sequence of the antibody is SEQ ID NO.:1. The invention further relates to nucleotides for encoding amino acids. The AFP nanometer antibody can be used as the antibody to be applied to immunological detection of AFP not for disease diagnosis and treatment. Compared with a Scfv antibody and a Fab antibody, the camel source immunity VHH has the higher specificity and affinity, more stable in structure, resistant to acid, base and high temperature, high in detection flexibility and the like, so that the immunity detection stability is greatly improved, and meanwhile the environment resistance is greatly improved.
Owner:NANCHANG DAJIA TECH
Scfv antibodies which pass epithelial and/or endothelial layers
scFv antibodies which specifically bind selected antigens and are obtainable by a method comprising (i) selecting from a pool of soluble and stable antibody frameworks a soluble and stable framework matching best the framework of a non-human antibody against the antigen with a certain binding specificity, (ii) either providing said soluble and stable framework with CDRs that bind specifically to said antigen, or mutating the framework of said non-human antibody towards the sequence of said soluble and stable framework, to generate scFv antibodies, (iii) testing the generated antibody for solubility and stability, and testing the generated antibody for antigen binding, and (iv) selecting an scFV that is soluble, stable and binds to the antigen specifically. Also provided are pharmaceutical compositions comprising said scFv antibody, methods of treatment and diagnosis for diseases related to over expression of antigens that are specifically bound by said antibody.
Owner:NOVARTIS AG
Stable and soluble antibodies inhibiting TNFα
Owner:ESBATECH +1
Antibodies against SARS-CoV and methods of use thereof
InactiveUS7750123B2Inhibition formationInhibit syncytium formationSsRNA viruses positive-senseAntibody mimetics/scaffoldsScFv AntibodiesMonoclonal antibody
The invention provides scFv antibodies and monoclonal antibodies that neutralize SARS-CoV. Also provided are methods of treating and / or preventing a coronavirus-related disease or disorder such as SARS. The invention also provides methods of vaccinating a patient against SARS-CoV. Also provided are methods of diagnosing coronavirus-related diseases or disorders and methods of detecting the presence of a coronavirus in a sample. The invention additionally provides methods of screening for compounds that modulate the binding of SARS-CoV and the SARS-CoV receptor ACE2 as well as for compounds useful to treat SARS-CoV-related diseases or disorders.
Owner:DANA FARBER CANCER INST INC
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) single chain Fv antibody fragment conjugates and methods of use thereof
Compositions including an antibody single-chain variable fragment (scFv) conjugate that specifically binds to ROR1 tumor-associated antigen are provided. The anti-ROR1 scFv antibody and conjugates may include a biologically-active molecule. Such conjugates may comprise a chimeric receptor to direct T cells to respond to ROR1 cancer cells, Methods to use the scFV conjugates to target cells expressing ROR1 for therapeutic and diagnostic purposes are also provided.
Owner:RGT UNIV OF CALIFORNIA
Stable and soluble antibodies inhibiting TNF alpha
The present invention relates to particularly stable and soluble scFv antibodies and Fab fragments specific for TNF, which comprise specific light chain and heavy chain sequences that are optimized for stability, solubility, in vitro and in vivo binding of TNF, and low immunogenicity. Said antibodies are designed for the diagnosis and / or treatment of TNF-mediated disorders. The nucleic acids, vectors and host cells for expression of the recombinant antibodies of the invention, methods for isolating them and the use of said antibodies in medicine are also disclosed.
Owner:NOVARTIS AG
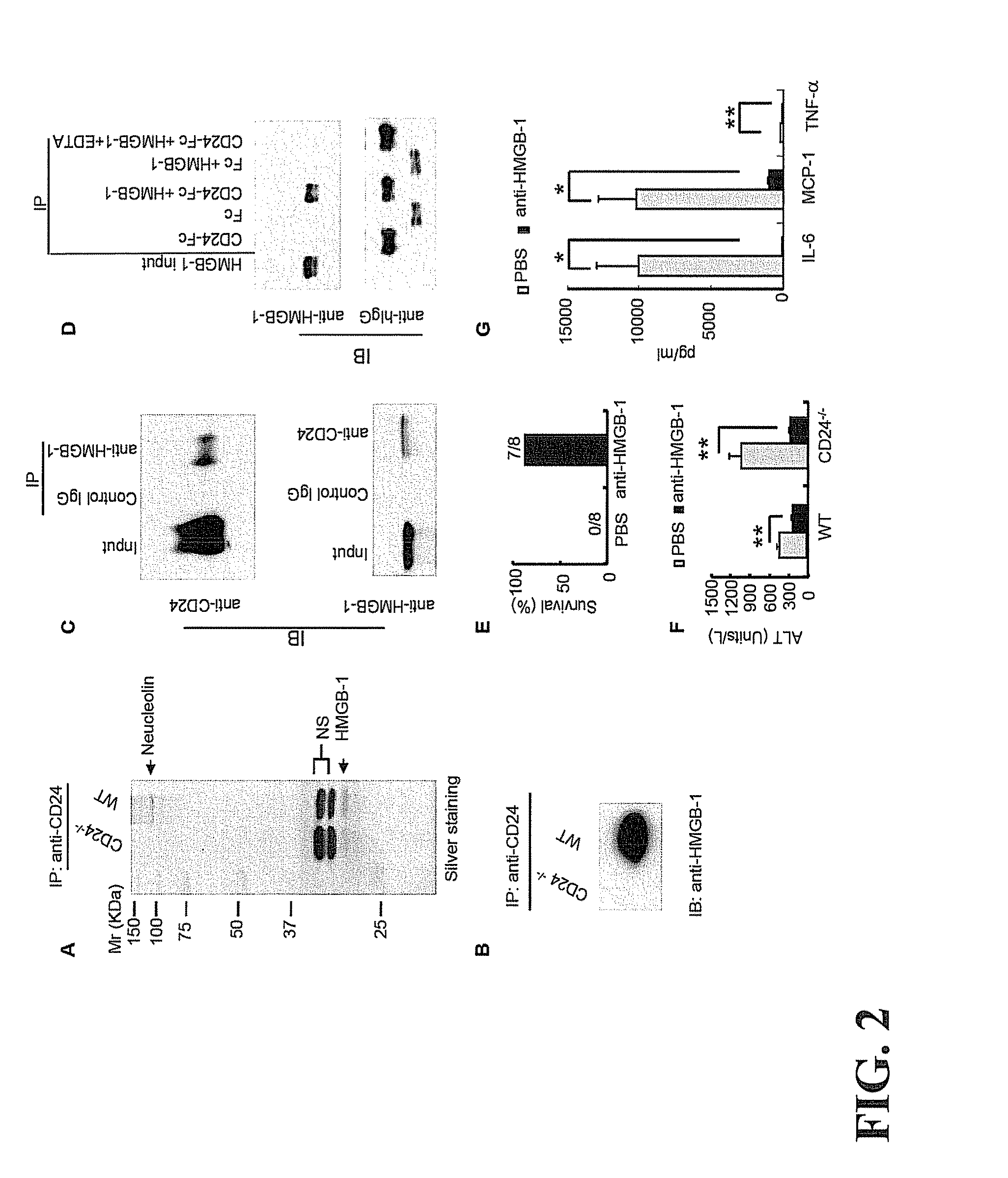
Treatment of drug-related side effect and tissue damage by targeting the cd24-hmgb1-siglec10 axis
ActiveUS20110064746A1Organic active ingredientsPeptide/protein ingredientsSide effectAntibody fragments
The present technology provides methods and compositions for the treatment of tissue-damage related immune dysregulation by administering a composition comprising one or more of CD24; CD24 fragments, variants and derivatives, CD24Fc fusion proteins; HMBG1-binding proteins, binding proteins to HMBG1 Box B; antagonists of HMGB1, polyclonal, monoclonal, recombinant, chimeric, humanized scFv antibodies and antibody fragments to HMGB1 or fragments of HMGB1 and antibodies that bind and suppress the activity of HMGB1 Box B; Siglec 10 agonists such as anti-Siglec 10 antibodies; and combinations thereof to a patient.
Owner:RGT UNIV OF MICHIGAN
Stable and soluble antibodies inhibiting TNF alpha
The present invention relates to particularly stable and soluble scFv antibodies and Fab fragments specific for TNFα, which comprise specific light chain and heavy chain sequences that are optimized for stability, solubility, in vitro and in vivo binding of TNFα, and low immunogenicity. Said antibodies are designed for the diagnosis and / or treatment of TNFα-related disorders. The nucleic acids, vectors and host cells for expression of the recombinant antibodies of the invention, methods for isolating them and the use of said antibodies in medicine are also disclosed.
Owner:ESBATECH +1
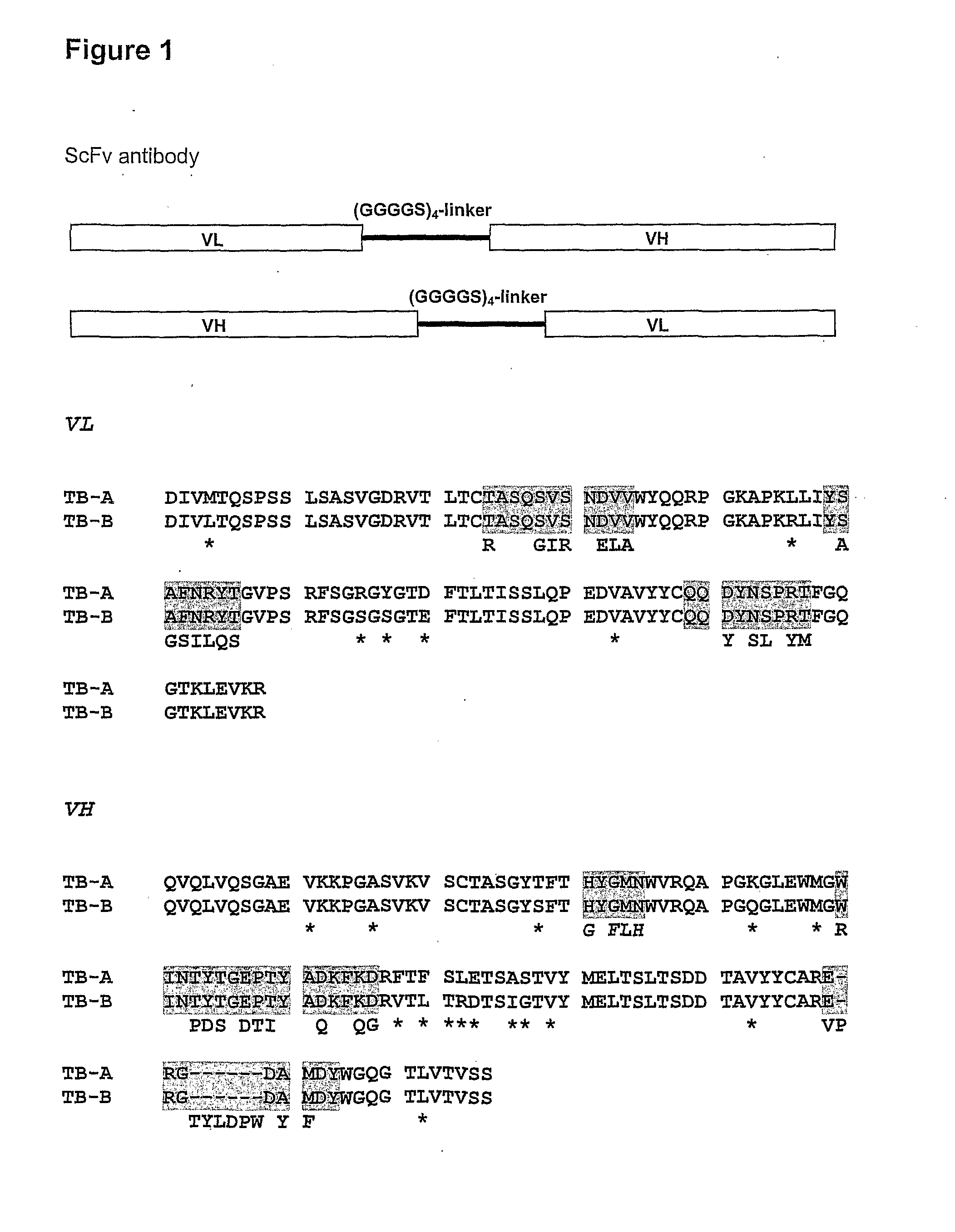
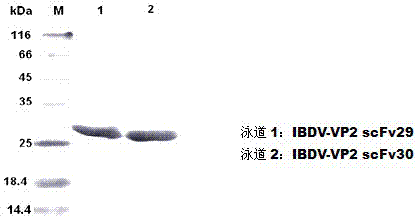
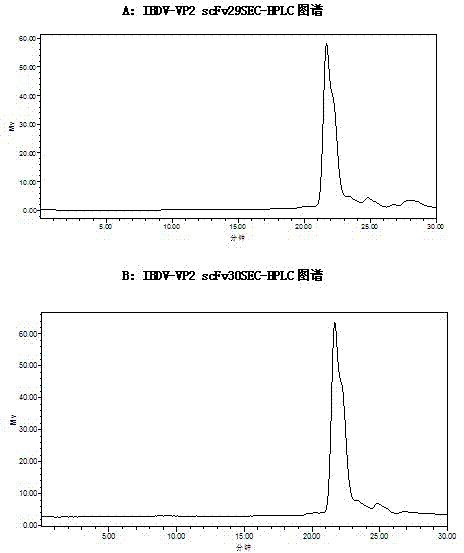
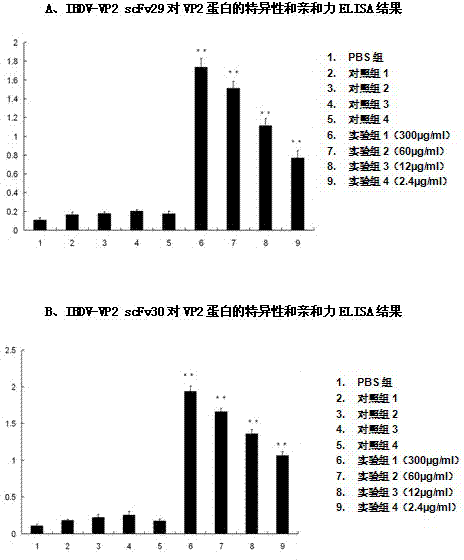
Two ScFv (Single Chain Variable Fragment ) antibodies, encoding genes and application thereof for preparing preparation for treating or preventing infectious bursal disease of chicken
InactiveCN103483449AStrong specificityGood treatment effectBacteriaImmunoglobulins against virusesAnimal virusDisease
The invention discloses two types of ScFv (Single Chain Variable Fragment) antibodies, encoding genes of the antibodies and applications of the antibodies for preparing the preparations for treating or preventing infectious bursal disease of chicken. Two single-chain antibodies (namely, the ScFv antibodies) are provided; and the ScFv antibodies can be specially bound with the protein 2 (VP2) (Virus Protein 2) in an IBDV (Infectious Bursal Disease Virus) structure and a plurality of IBDV strains to block the cytopathic effect (CPE) of the chicken embryo fibroblast by the IBDV so as to protect the young chicken infected with IBDV. The immune serum and egg yolk antibody are tedious in preparation, high in production cost, unstable in effect, difficult to control industrialized production quality, capable of causing horizontal disease spread and the like in a use process; the ScFv antibodies disclosed by the invention can overcome the above disadvantages and have the advantages of strong specificity, good treatment effect, controllable industrialized production quality, capability of preventing the horizontal disease spread caused by the egg yolk antibody and the like; and therefore, by virtue of the ScFv antibodies, a new situation is opened up in the IBDV prevention and treatment history, and even in the entire prevention and treatment history for animal virus diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Yeast cell surface display of proteins and uses thereof
InactiveUS20090280560A1Effectively mimickedHigh affinityFungiSaccharide peptide ingredientsSurface displayAgglutinin-B
The present invention provides a genetic method for tethering polypeptides to the yeast cell wall in a form accessible for binding to macromolecules. Combining this method with fluorescence-activated cell sorting provides a means of selecting proteins with increased or decreased affinity for another molecule, altered specificity, or conditional binding. Also provided is a method for genetic fusion of the N terminus of a polypeptide of interest to the C-terminus of the yeast Aga2p cell wall protein. The outer wall of each yeast cell can display approximately 104 protein agglutinins. The native agglutinins serve as specific adhesion contacts to fuse yeast cells of opposite mating type during mating. In effect, yeast has evolved a platform for protein-protein binding without steric hindrance from cell wall components. As one embodiment, attaching an scFv antibody fragment to the Aga2p agglutinin effectively mimics the cell surface display of antibodies by B cells in the immune system for affinity maturation in vivo. As another embodiment, T cell receptor mutants can be isolated by this method that are efficiently displayed on the yeast cell surface, providing a means of altering T cell receptor binding affinity and specificity by library screening.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Anti to human alkaline fibroblast growth factor human s c F v antibody and application thereof
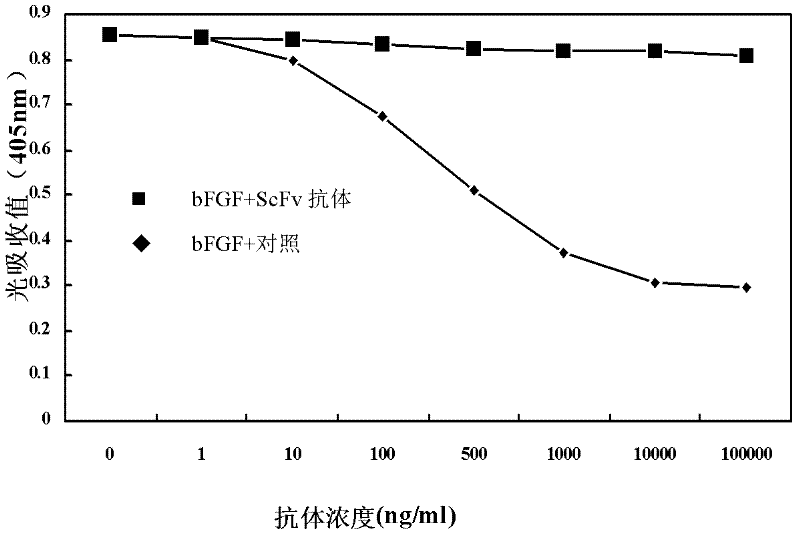
ActiveCN102558351AHigh affinityImprove featuresDigestive systemImmunoglobulins against growth factorsSingle-Chain AntibodiesFibrosis
The invention discloses a human single-chain antibody fragment (scFv) antibody of anti-recombination basic fibroblast growth factor and an application thereof. An amino acid sequence of heavy chain variable area of the human scFv antibody is shown in SEQ ID NO. 1, and an amino acid sequence of light chain variable area of the human scFv antibody is shown in SEQ ID NO. 2. A gene sequence encoding the heavy chain variable area is shown in SEQ ID NO. 3. A gene sequence encoding the light chain variable area is shown in SEQ ID NO. 4. The human scFv antibody has the characteristics of high affinity and specificity, and can be directly developed to be used as an antibody drug for human because the human scFv antibody is fully human antibody. The gene encoding the human scFv antibody can be constructed and expressed to obtain various forms of micromolecular genetic engineering antibodies, such as, fragment antigen-binding (Fab) antibody, F(ab)2, single chain antibody, Nanobody, antibody fusion protein, immunoglobulin G (IgG) complete antibody, etc., which can be used for preparing antibody drugs for diagnosing and treating tumor and / or inhibiting viscera fibrosis.
Owner:JINAN UNIVERSITY
Antibodies against influenza virus and methods of use thereof
The invention provides human scFv antibodies and monoclonal antibodies that neutralize influenza virus. Also provided are methods of treating and / or preventing a influenza related disease or disorder such bird flu The invention also provides methods of vaccinating a patient against influenza. Also provided are methods of diagnosing influenza-related diseases or disorders and methods of detecting the presence of a influenza in a sample.
Owner:BURNHAM INST FOR MEDICAL RES +1
Immunoglobulin frameworks which demonstrate enhanced stability in the intracellular environment and methods of identifying same
ActiveUS8853362B2Improve solubilityImprove stabilityAnimal cellsSugar derivativesSolubilityScFv Antibodies
Compositions are provided, which can be used as frameworks for the creation of very stable and soluble single-chain Fv antibody fragments. These frameworks have been selected for intracellular performance and are thus ideally suited for the creation of scFv antibody fragments or scFv antibody libraries for applications where stability and solubility are limiting factors for the performance of antibody fragments, such as in the reducing environment of a cell. Such frameworks can also be used to identify highly conserved residues and consensus sequences which demonstrate enhanced solubility and stability.
Owner:NOVARTIS AG
Methods of modifying antibodies, and modified antibodies with improved functional properties
Owner:NOVARTIS AG
AFP nanometer antibody A65 based on AFP antigen
ActiveCN105037546AUnique molecular structureSmall molecular weightImmunoglobulins against animals/humansPeptide preparation methodsDiseaseAntigen
The invention belongs to the technical field of biology, and relates to a camel source immunity nanometer antibody (single-domain heavy-chain antibody, VHH) specifically bound with AFP and application of the antibody. The amino acid sequence of the antibody is SEQ ID NO.:1. The invention further relates to nucleotides for encoding amino acids. The AFP nanometer antibody can be used as the antibody to be applied to immunological detection of AFP not for disease diagnosis and treatment. Compared with a Scfv antibody and a Fab antibody, the camel source immunity VHH has the higher specificity and affinity, more stable in structure, resistant to acid, base and high temperature, high in detection flexibility and the like, so that the immunity detection stability is greatly improved, and meanwhile the environment resistance is greatly improved.
Owner:NANCHANG DAJIA TECH
Anti-DON single-chain antibody ScFv and preparing method and application thereof
InactiveCN101983970AMicroorganism based processesImmunoglobulins against fungi/algae/lichensEscherichia coliSingle-Chain Antibodies
The invention relates to an anti-DON single-chain antibody ScFv and a preparing method and application thereof, belonging to biological product field. In the invention, variable region gene Fab section of heavy chain and light chain of antibody is cloned through a PCR method in a hybridoma cell strain McGill078 which secretes anti-DON monoclonal antibody; two genes are recombined through a gene engineering method; a prokaryotic expression plasmid vector is constructed and is transformed to Escherichia coli to express an ScFv antibody active fragment which can specifically recognize DON; and then the recombined single chain antibody ScFv is purified and renatured. The anti-DON recombined single-chain antibody ScFv has an activity for binding with DON and has the advantage of residual detection for DON owing to recognition and binding activity to DON and having antibody medicament prospect for treating DON poisoning after humanized modification; and the constructed transformation vector plasmid is easy to preserve and produce in a great quantity.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Immunoglobulin having particular framework scaffold and methods of making and using
InactiveUS20050037420A1Immunoglobulins against virusesBiological testingHigh level expressionScFv Antibodies
This invention relates to immunoglobulin molecules comprising light chain (VL) chimeric variable domains, heavy chain (VH) chimeric variable domains, e.g., scFv antibodies that are expressed at high levels within a host cell, preferably within particular cellular compartments such as, e.g., cytosol or apoplast. The VL, VH and scFv antibody molecules comprise framework scaffolds of particularly preferred framework regions. This invention also relates to nucleic acid molecules encoding the immunoglobulin molecules of this invention, vectors expressing the immunoglobulin molecules, hosts transformed with the nucleic acid molecules and vectors, and methods of using the immunoglobulin molecules. Also described are immunoglobulin libraries as well as host cells, including transgenic plants, expressing the VL, VH or scFv antibody molecules of this invention.
Owner:ZHANG MEI YUN +5
Methods for identifying immunobinders of cell-surface antigens
ActiveUS8227199B2Rapid and efficient identificationSugar derivativesPreparing sample for investigationCell Surface AntigensScFv Antibodies
The invention provides methods for identifying immunobinders, such as scFv antibodies, capable of specifically binding to cell surface antigens, and compositions identified according to said methods.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com