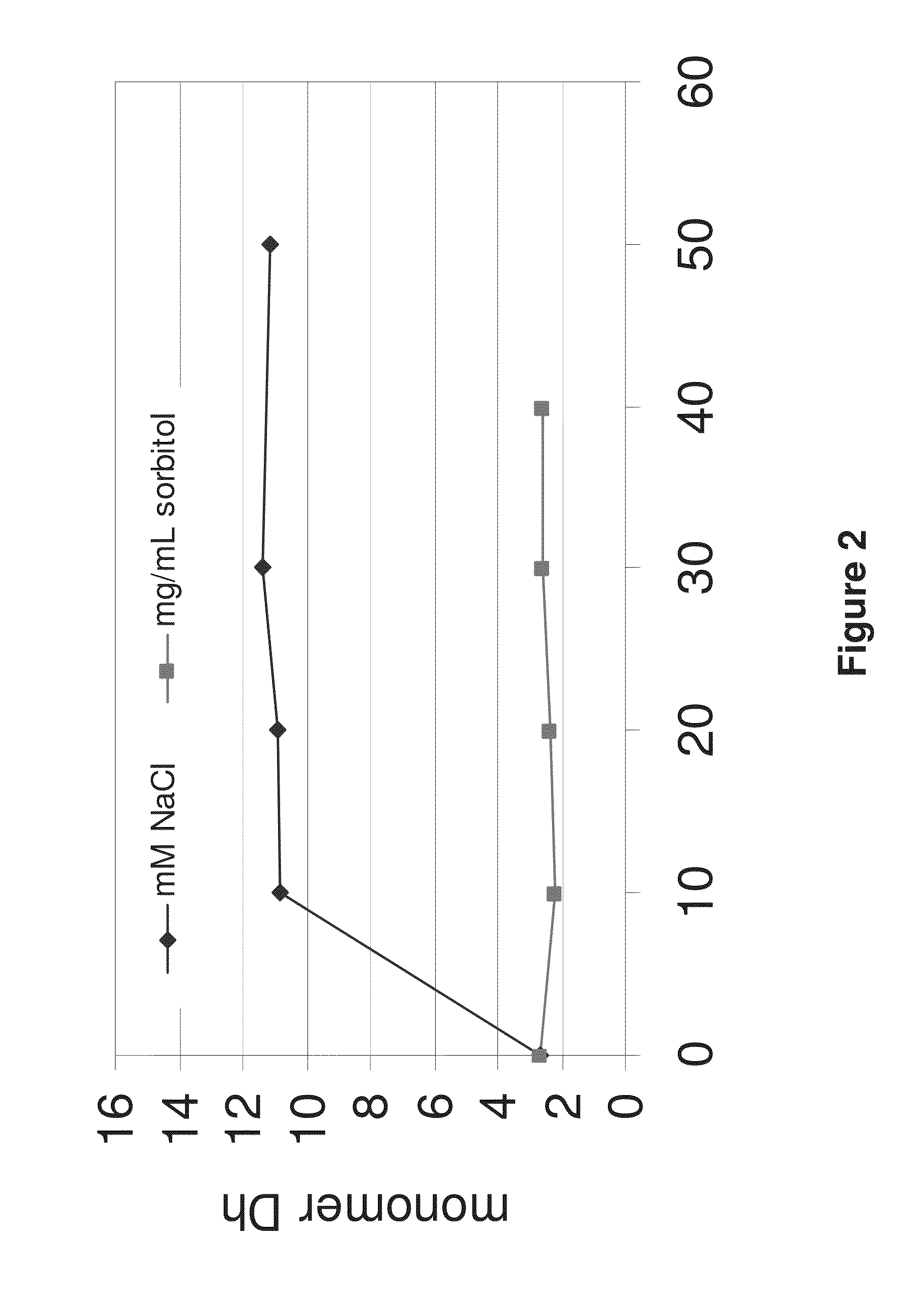
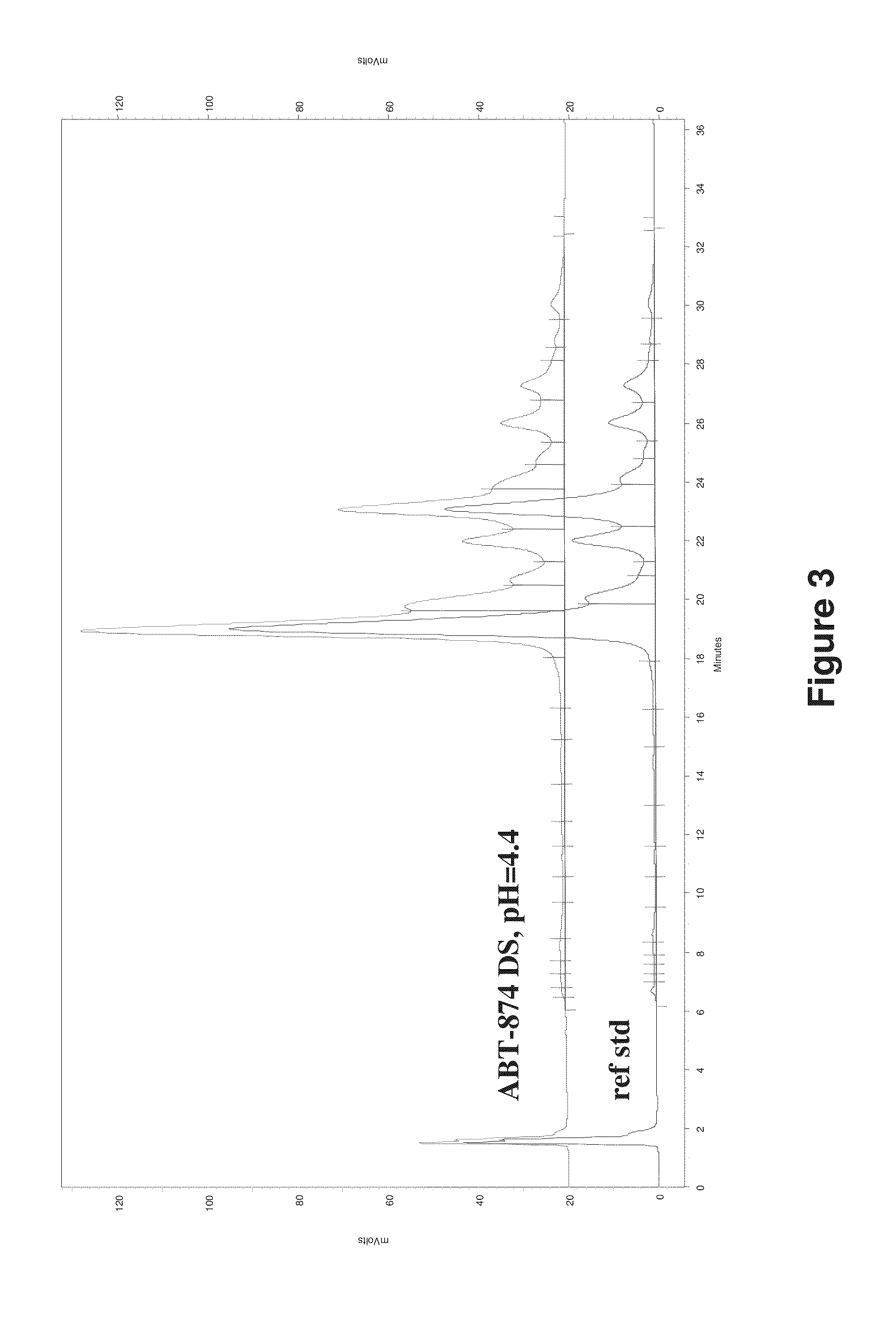
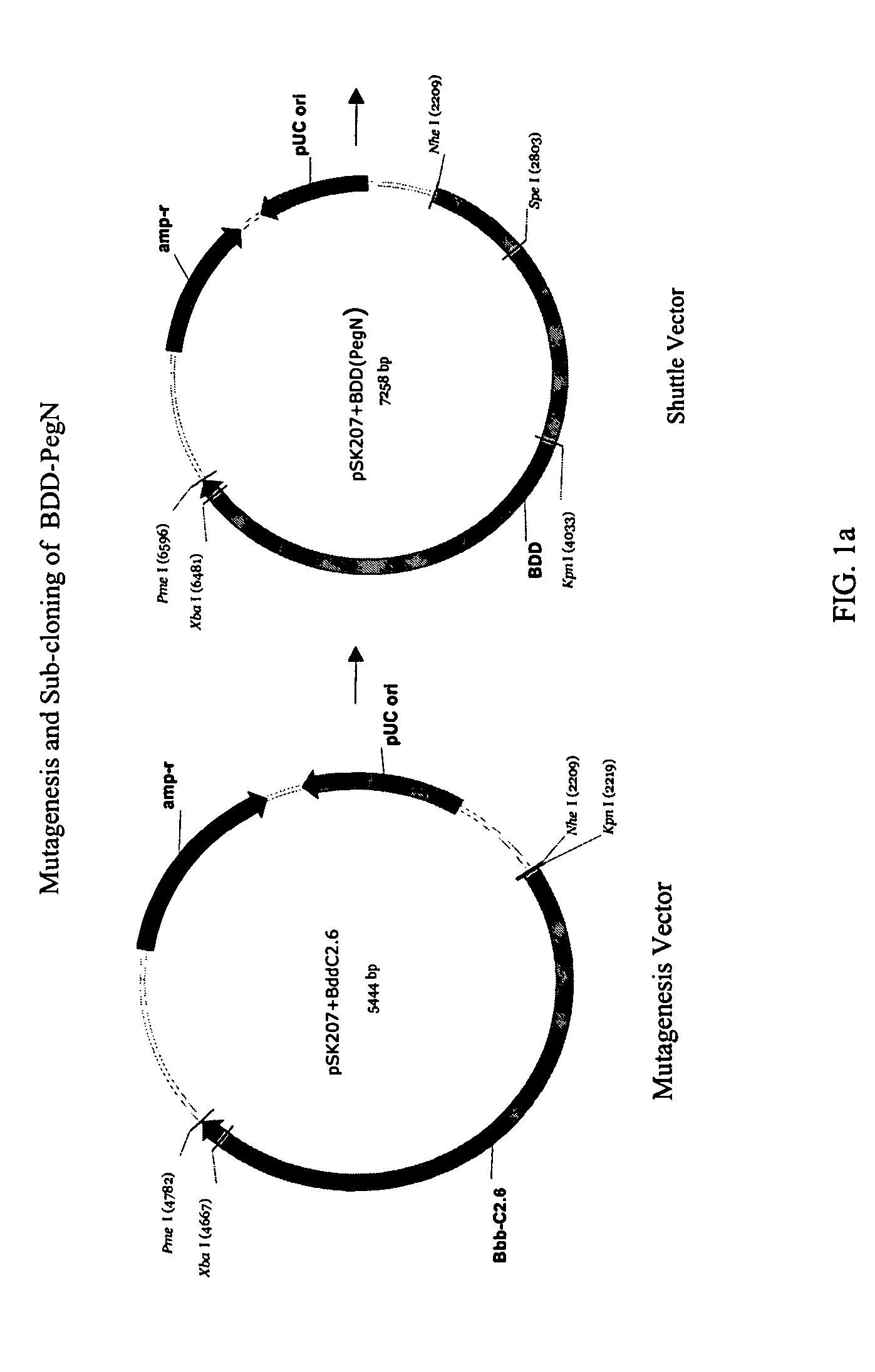
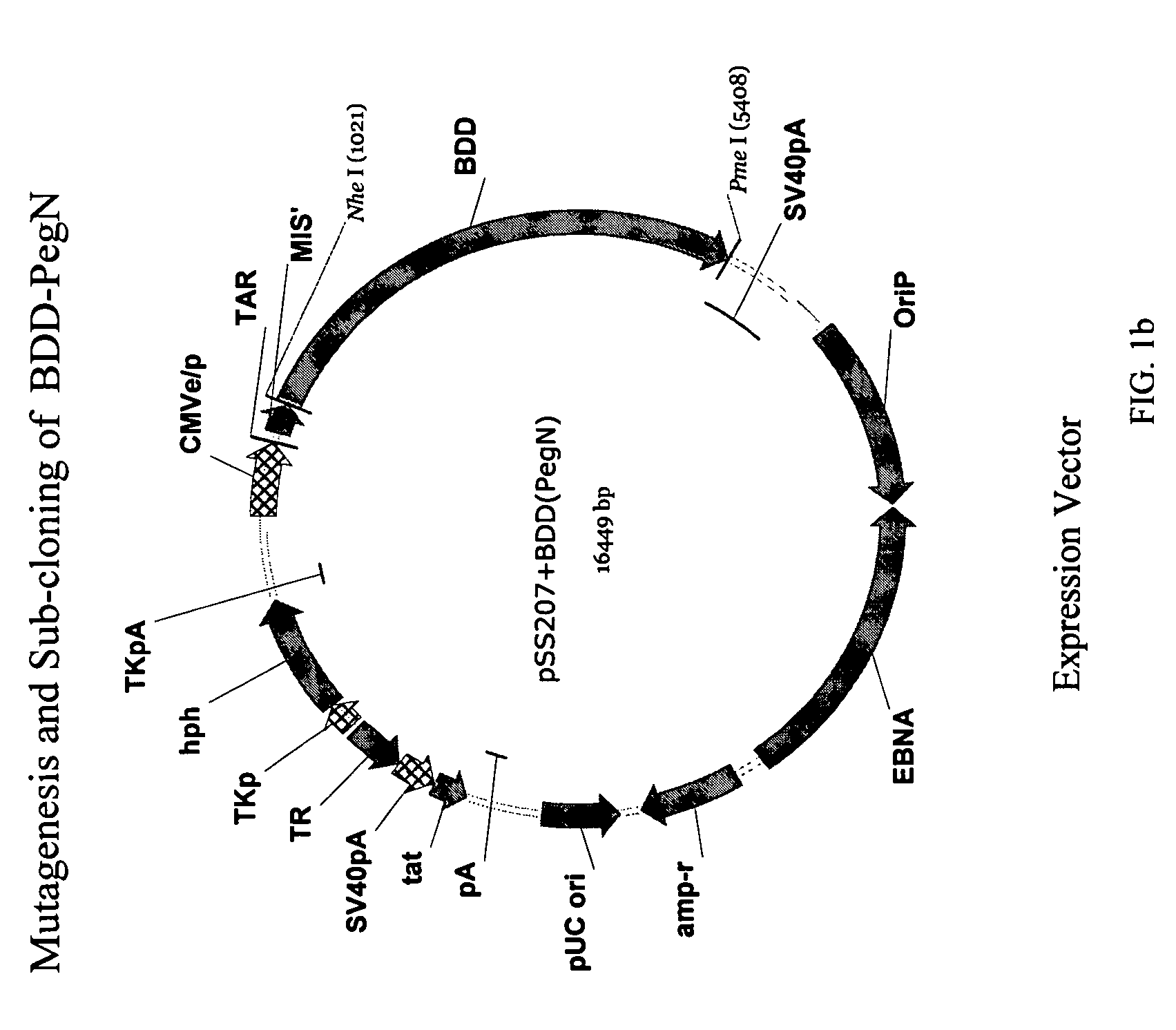
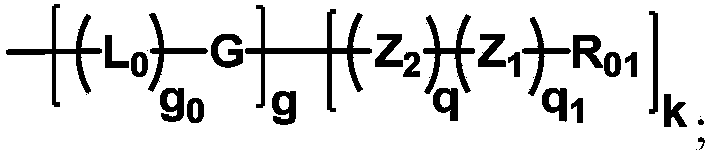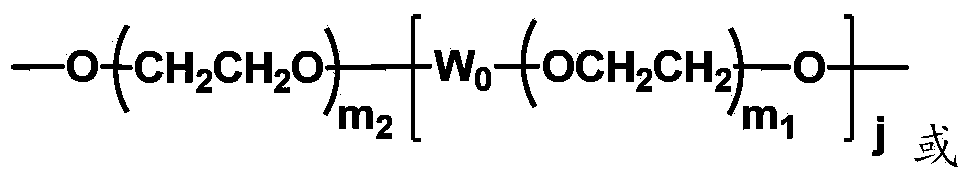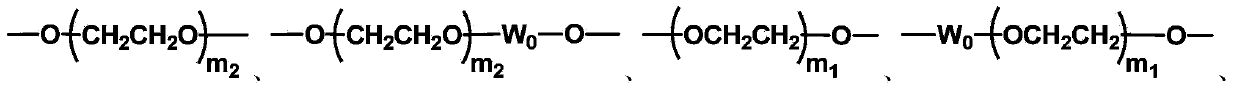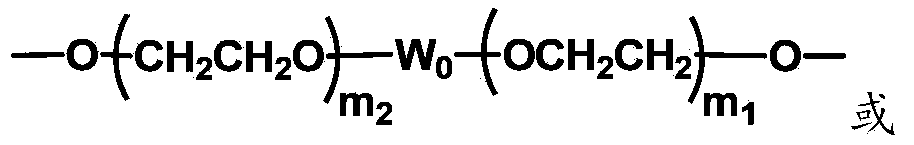Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2161results about How to "Low immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Protein formulations and methods of making same
ActiveUS20090291062A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntipyreticOsmolar ConcentrationExcipient
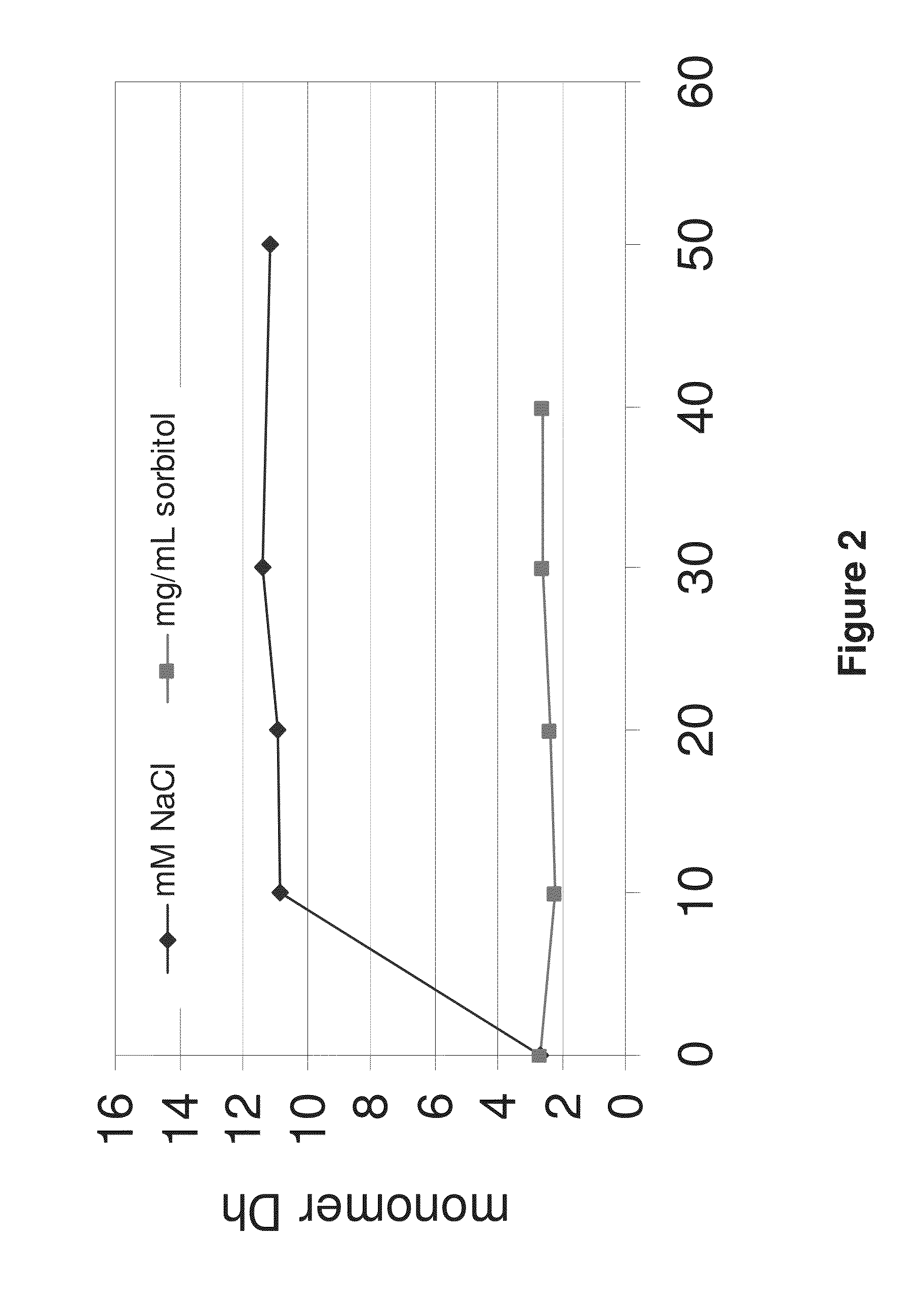
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductivity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
Methods of generating variant proteins with increased host string content and compositions thereof
ActiveUS20060008883A1Low immunogenicityMaximizes contentImmunoglobulins against cell receptors/antigens/surface-determinantsProteomicsMolecular biology
Owner:XENCOR INC
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
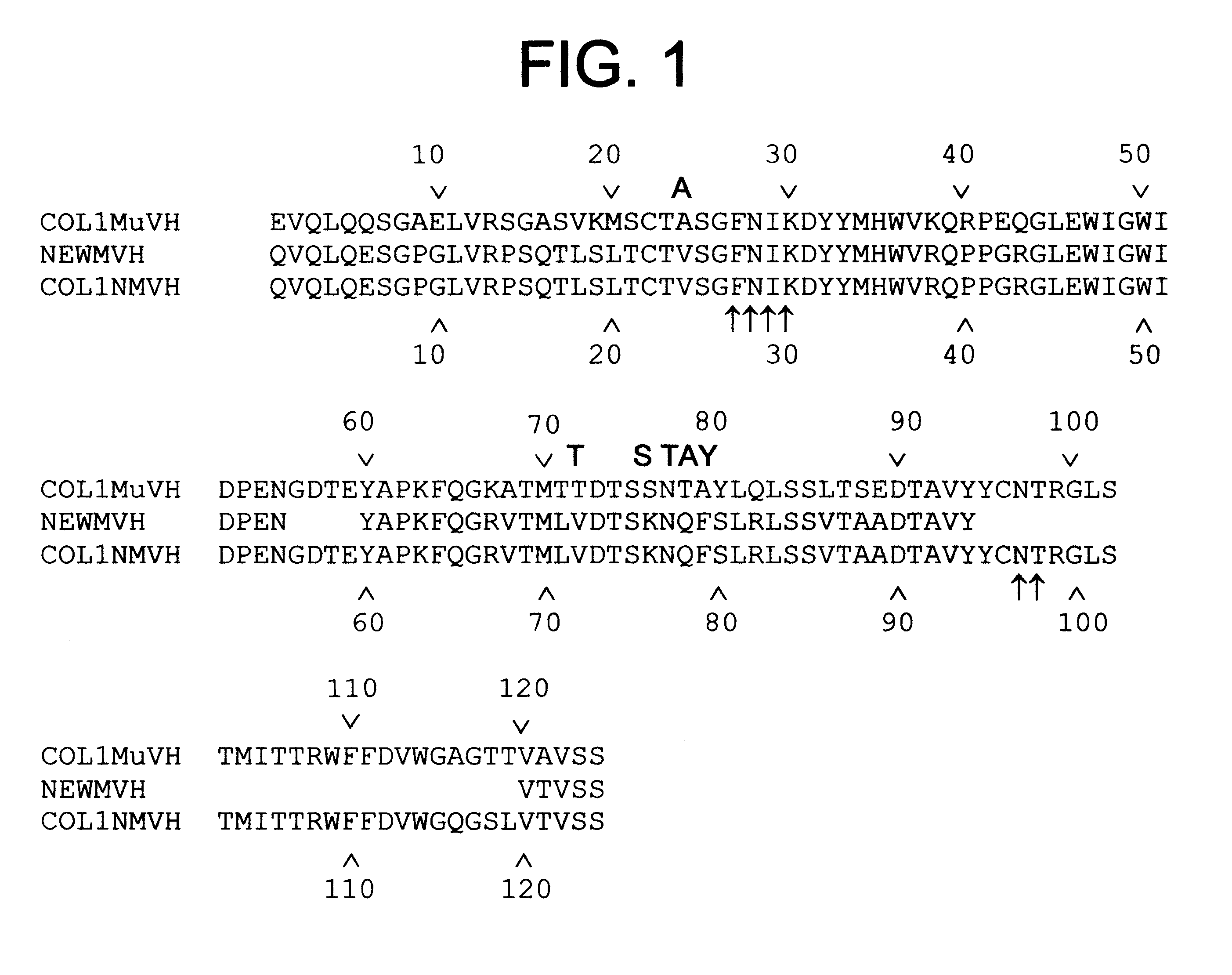
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Antibody producing non-human mammals
ActiveUS20100146647A1Low variabilityLow immunogenicityAnimal cellsAntibody mimetics/scaffoldsHuman animalDNA rearrangement
Owner:MERUS NV
Antibody formulations and methods of making same
ActiveUS8420081B2Minimal aggregationLow levelPeptide/protein ingredientsAntipyreticExcipientNuclear chemistry
Owner:ABBVIE BIOTECHNOLOGY LTD
G-CSF conjugates
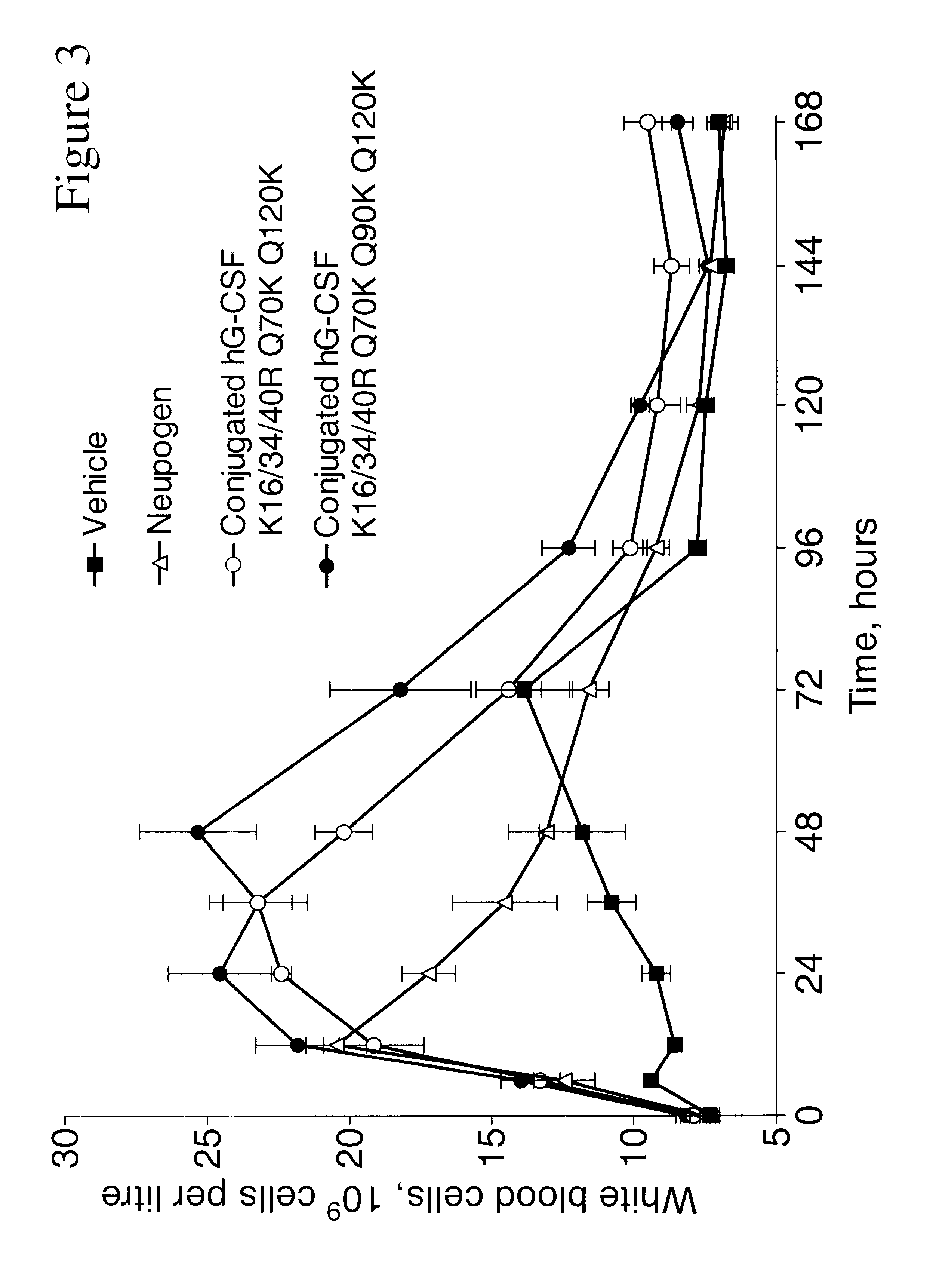
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Antibody producing non-human mammals
InactiveUS20100069614A1Easy to openEasy maintenanceAntibody mimetics/scaffoldsImmunoglobulins against virusesHuman animalDNA rearrangement
Described are transgenic, non-human animals comprising a nucleic acid encoding an immunoglobulin light chain, whereby the immunoglobulin light chain is human, human-like, or humanized. The nucleic acid is provided with a means that renders it resistant to DNA rearrangements and / or somatic hypermutations. In one embodiment, the nucleic acid comprises an expression cassette for the expression of a desired molecule in cells during a certain stage of development in cells developing into mature B cells. Further provided is methods for producing an immunoglobulin from the transgenic, non-human animal.
Owner:MERUS NV
Optimized proteins that target the epidermal growth factor receptor
InactiveUS20050142133A1Efficient effector functionEffective functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBiochemistryProtein
The present invention relates to optimized proteins that target the Epidermal Growth Factor Receptor (EGFR), and their application, particularly for therapeutic purposes.
Owner:XENCOR INC
Soluble, degradable poly(ethylene glycol) derivatives for conrollable release of bound molecules into solution
InactiveUS20050171328A1Reduce rateEnhance solubility and circulation lifetimePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsSolubilityIncreased sizes
PEG and related polymer derivatives having weak, hydrolytically unstable linkages near the reactive end of the polymer are provided for conjugation to drugs, including proteins, enzymes, small molecules, and others. These derivatives provide a sufficient circulation period for a drug-PEG conjugate, followed by hydrolytic breakdown of the conjugate and release of the bound molecule. In some cases, drugs that demonstrate reduced activity when permanently coupled to PEG maintain a therapeutically suitable activity when coupled to a degradable PEG in accordance with the invention. The PEG derivatives of the invention can be used to impart improved water solubility, increased size, a slower rate of kidney clearance, and reduced immunogenicity to a conjugate formed by attachment thereto. Controlled hydrolytic release of the bound molecule into an aqueous environment can then enhance the drug's delivery profile by providing a delivery system which employs such polymers and utilizes the teachings provided herein.
Owner:NEKTAR THERAPEUTICS INC
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
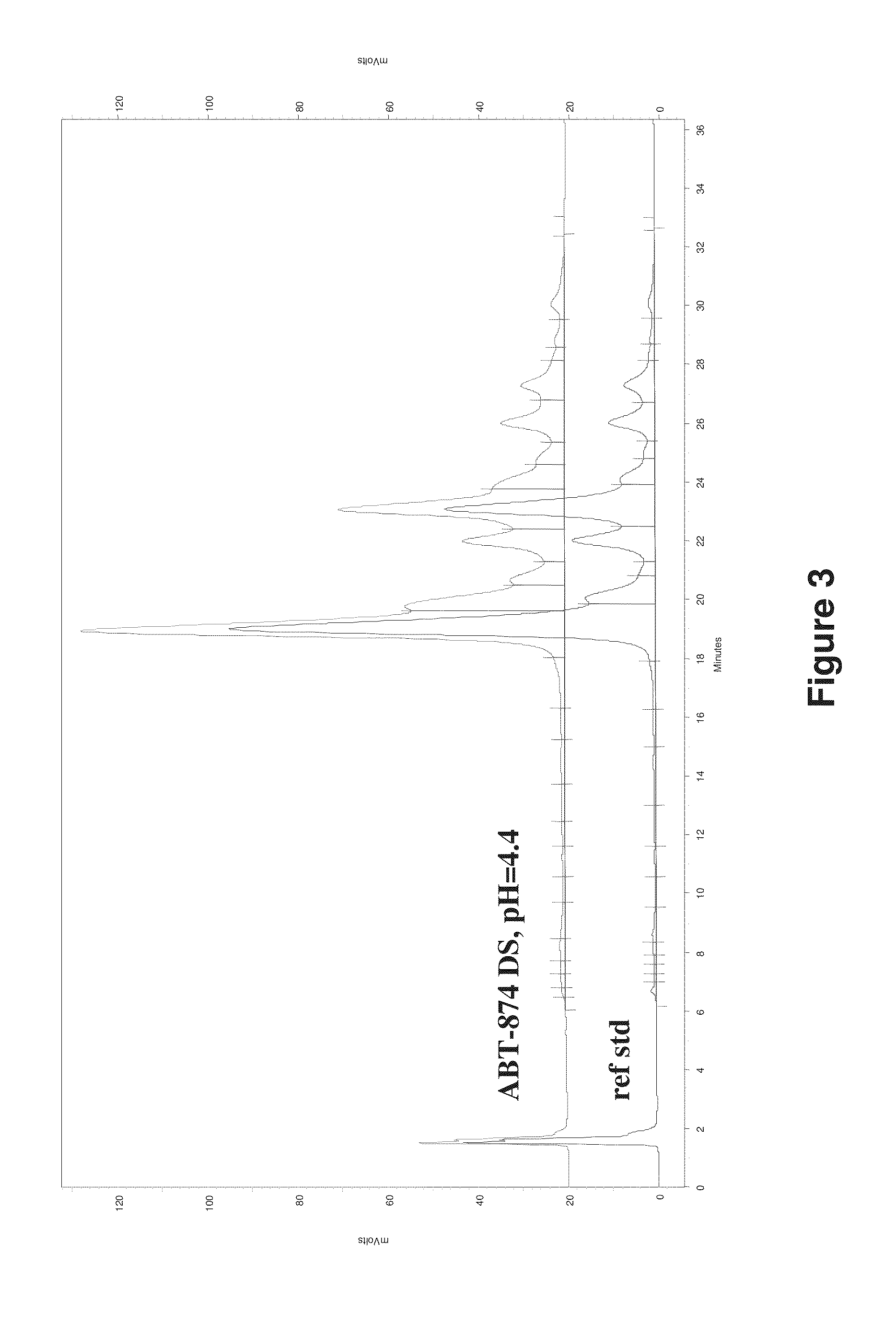
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Monitoring microrna expression and function
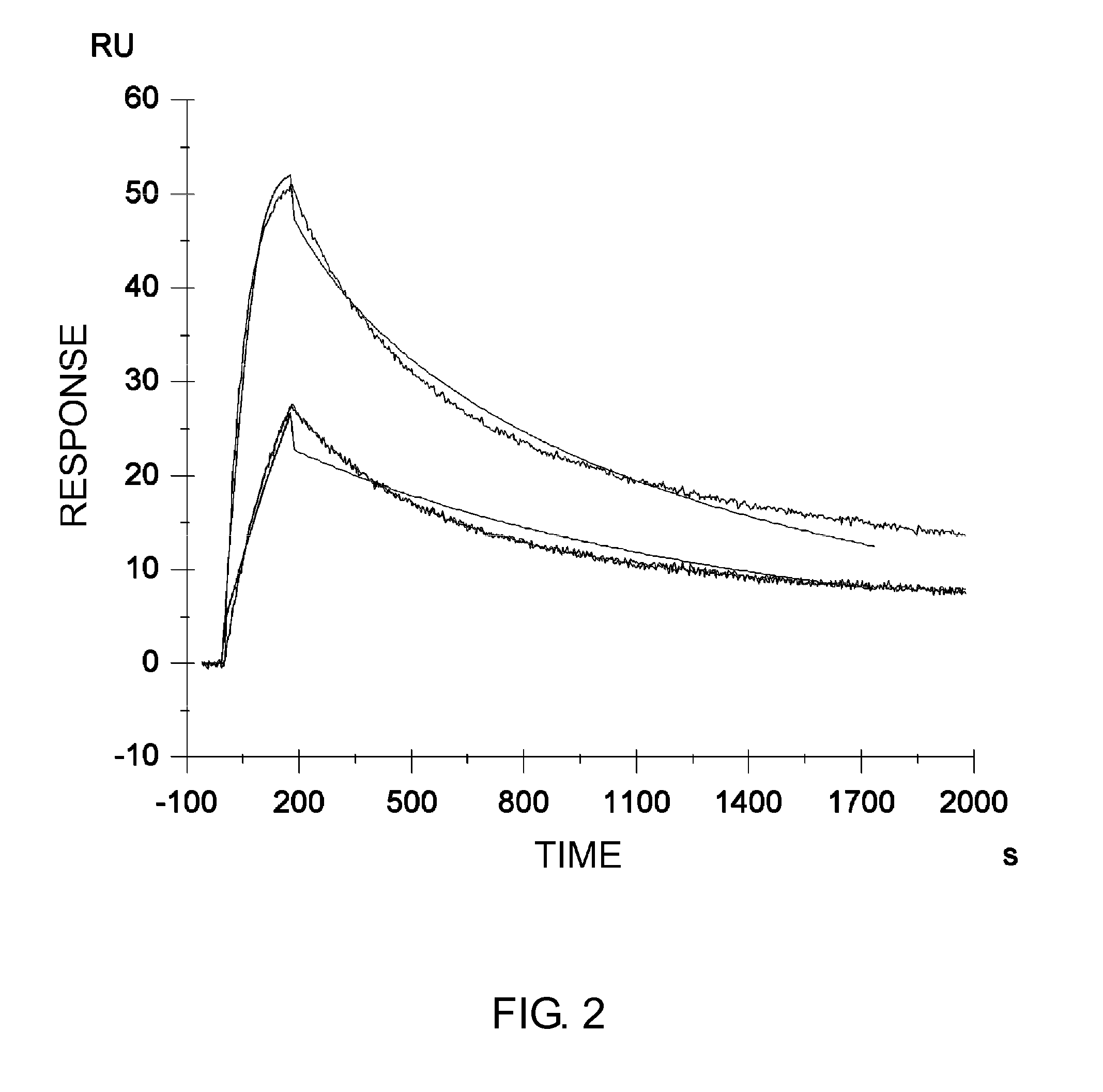
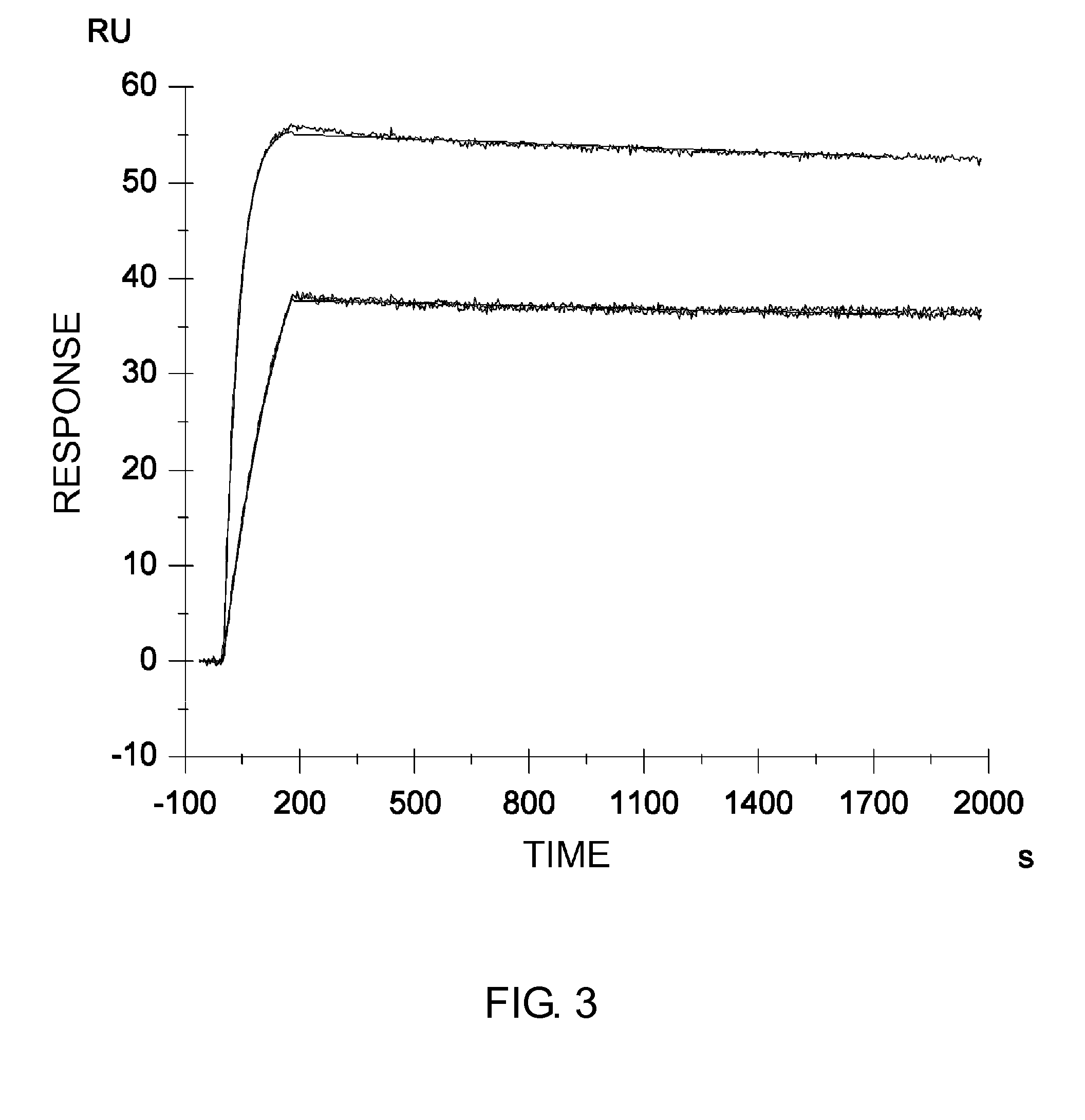
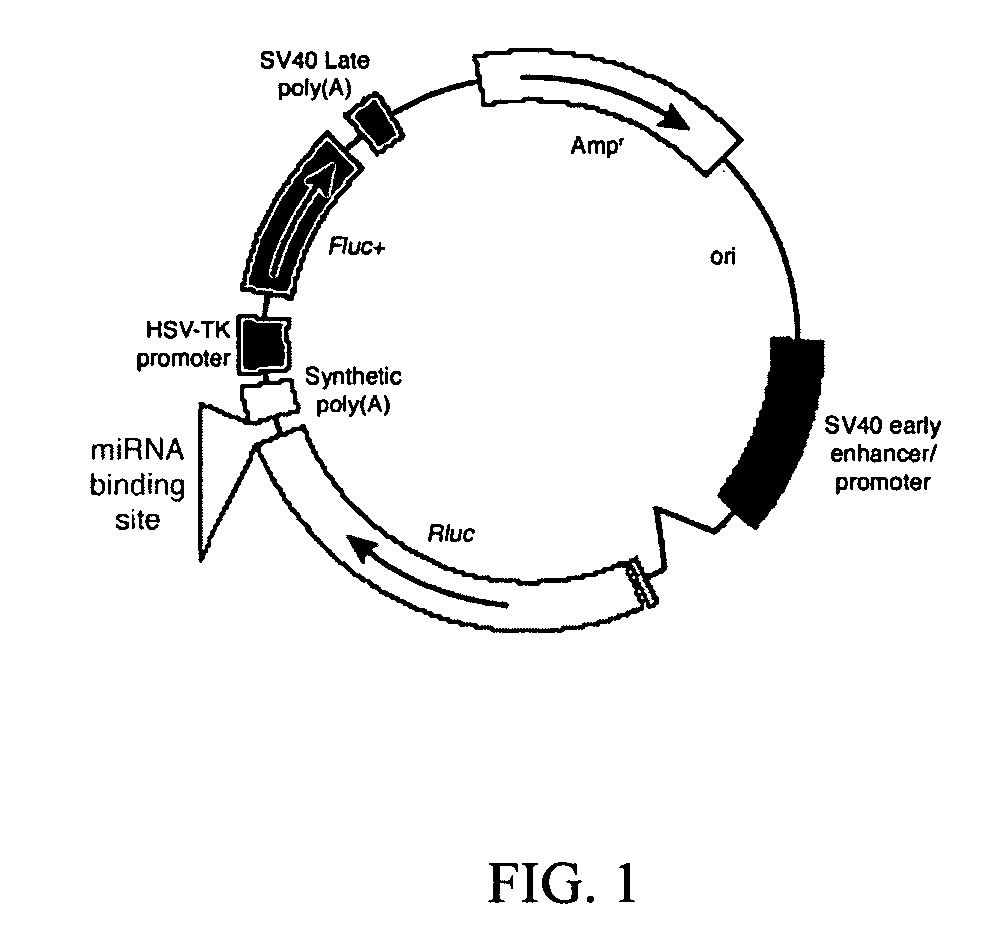
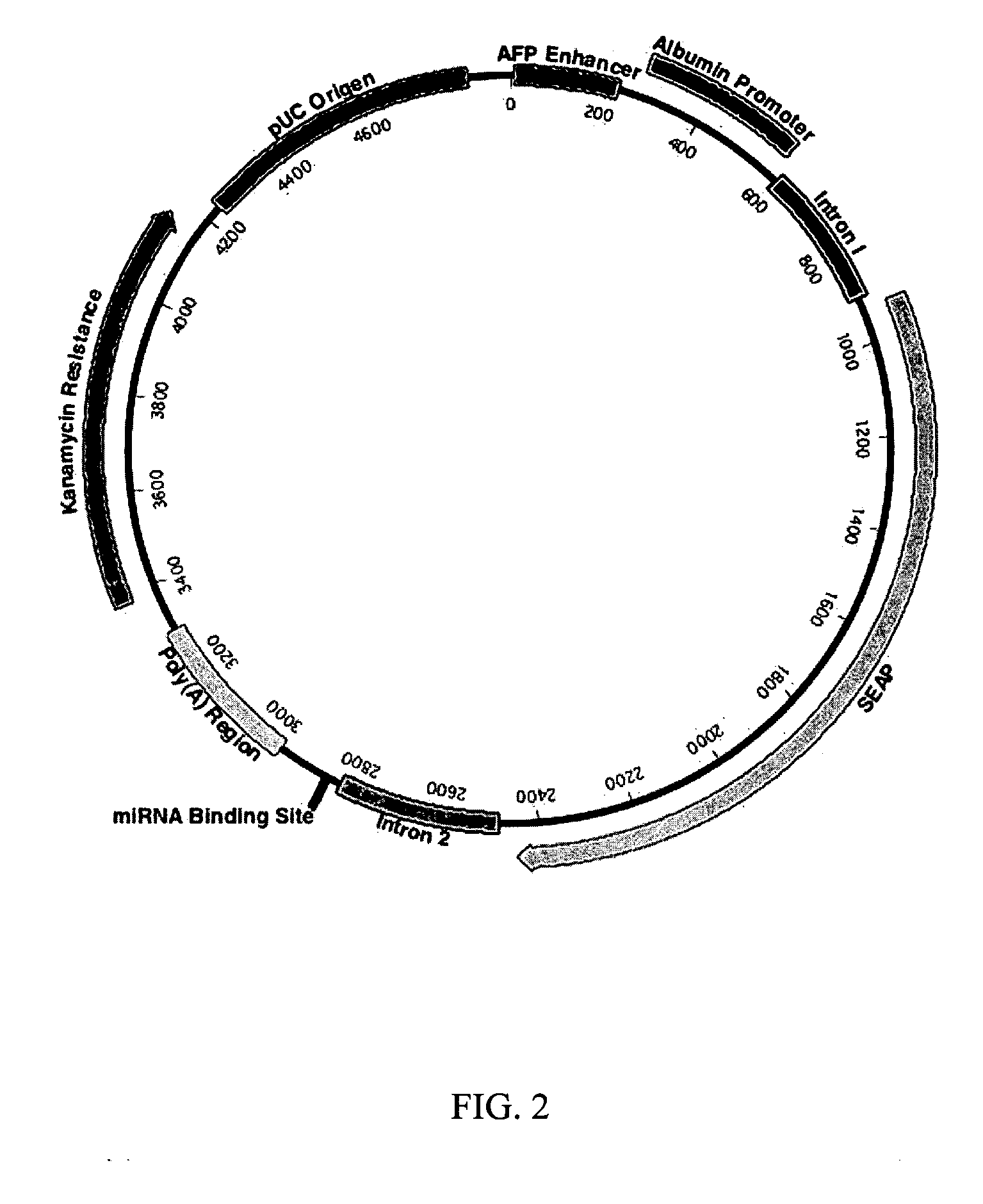
InactiveUS20060265771A1Reduce immunogenicityLong term expressionMicrobiological testing/measurementLibrary screeningReporter geneDevelopmental stage
In vivo endogenous microRNA (miRNA) activity can be observed over time using miRNA sensor plasmids capable of long term expression. Using reporter genes whose expression can be monitored without sacrificing the animal enables the investigator to follow changes in miRNA expression though developmental stages or in response to environmental factors or treatment regimens.
Owner:LEWIS DAVID +2
Compositions and methods for protein design
InactiveUS20060160138A1Improve stabilityLower immunogenicityPeptide librariesLibrary screeningBiologyProtein design
In certain aspects the present invention provides methods and compositions related to rational protein design.
Owner:CODON DEVICES
Modified plant virus particles and uses therefor
InactiveUS20120015899A1Broadening arrayEasy to assembleFusion with RNA-binding domainBiocideVirus-like particleCoat Proteins
Aspects of the invention provide modified virus-like particles that are designed for therapeutic applications. In particular, aspects of the invention provide CCMV coat proteins that are modified to generate virus-like particles, including mosaic virus-like particles, that can package and / or deliver one or more diagnostic and / or therapeutic agents. The invention also provides methods for treating subjects with one or more modified virus-like particles.
Owner:PLANT BIOSCI LTD +1
Biomaterials with enhanced properties and devices made therefrom
ActiveUS20120059487A1Reduce thicknessLow profile insertionHeart valvesSurgeryCell membraneLong term durability
Biomaterials with enhanced properties such as improved strength, flexibility, durability and reduced thickness are useful in the fabrication of biomedical devices, particularly those subjected to continuous or non-continuous loads where repeated flexibility and long-term durability are required. These enhanced properties can be attributed to elevated levels of elastin, altered collagen types, and other biochemical changes which contribute to these enhanced properties. Examples of devices which would be improved by use of such tissue include heart valves, including percutaneous heart valves, and vascular grafts, patches and the like. Such enhanced materials can be sourced from specific populations of animals, such as neonatal calves, or in range-fed adult cattle, or can be fabricated or created from cell populations exhibiting such properties. In one embodiment, glutaraldehyde-fixed neonatal pericardial tissue is used to create leaflets in a percutaneous heart valve, and may be used without chemical fixation, with or without processes to remove residual cellular membranes, and utilized as a scaffold material for tissue engineering.
Owner:SOUTHERN LIGHTS VENTURES 2002
Cross-linked polysaccharide gels
InactiveUS20100035838A1Improve the immunityGood degradation propertiesOrganic active ingredientsBiocideCross-linkPolysaccharide
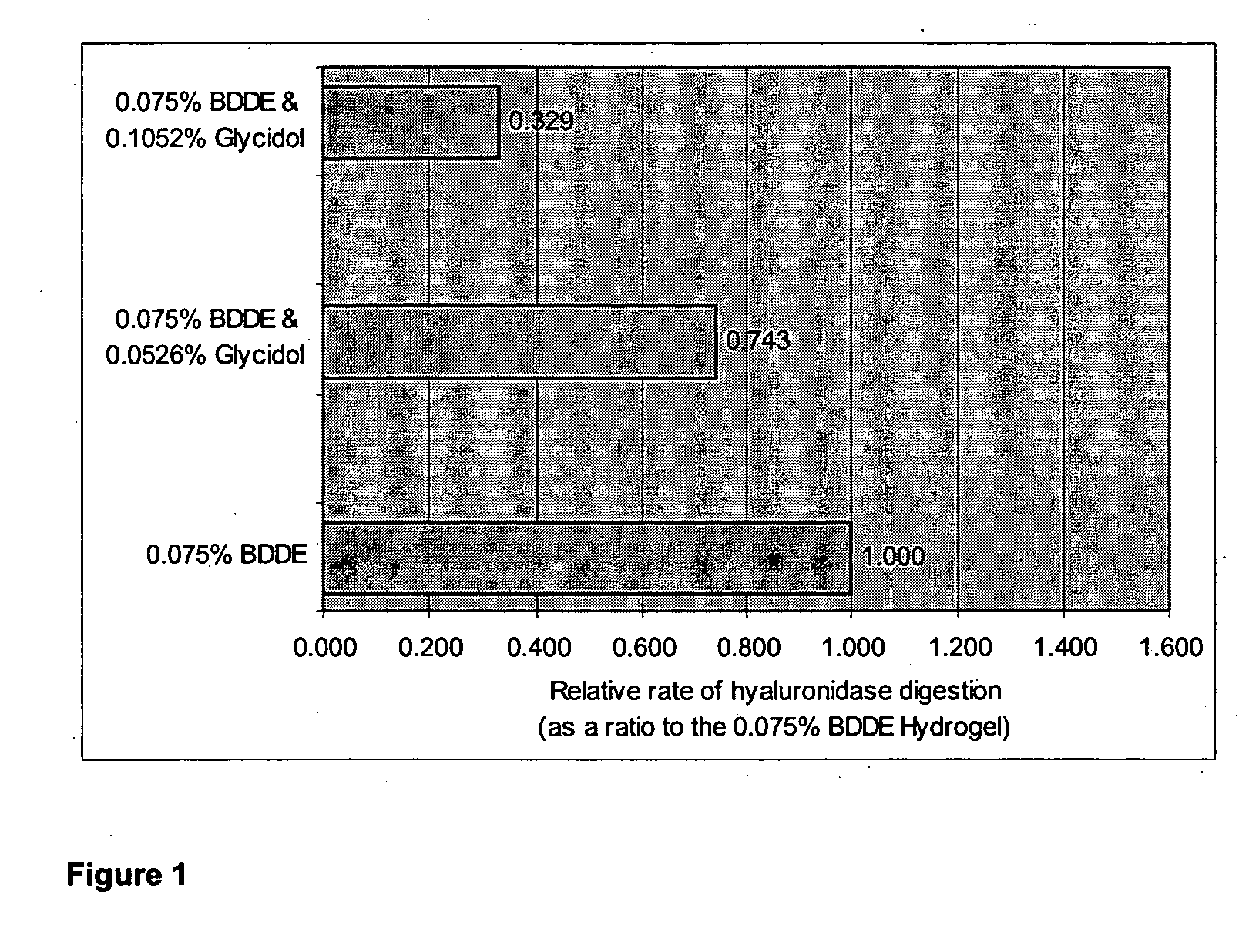
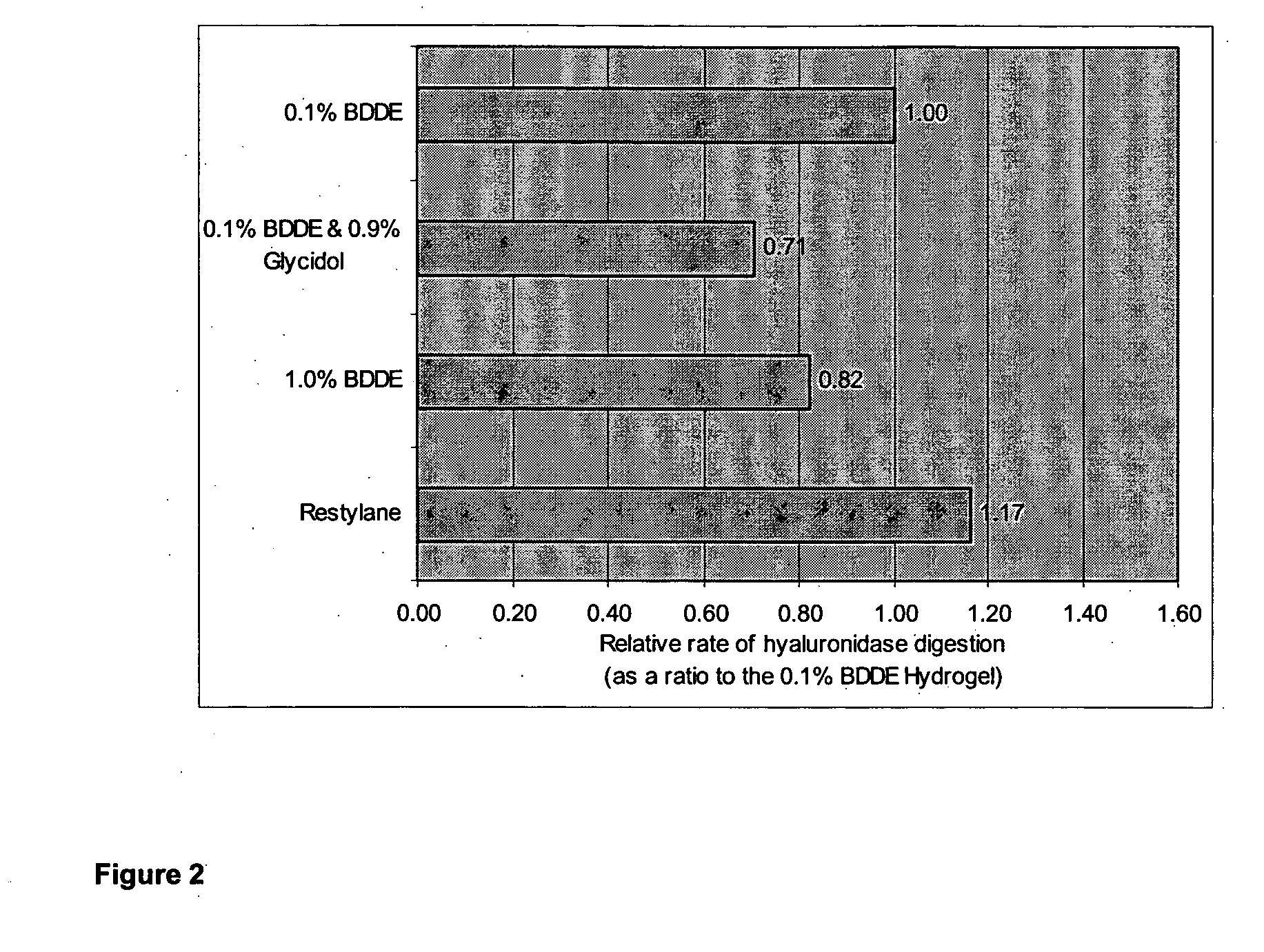
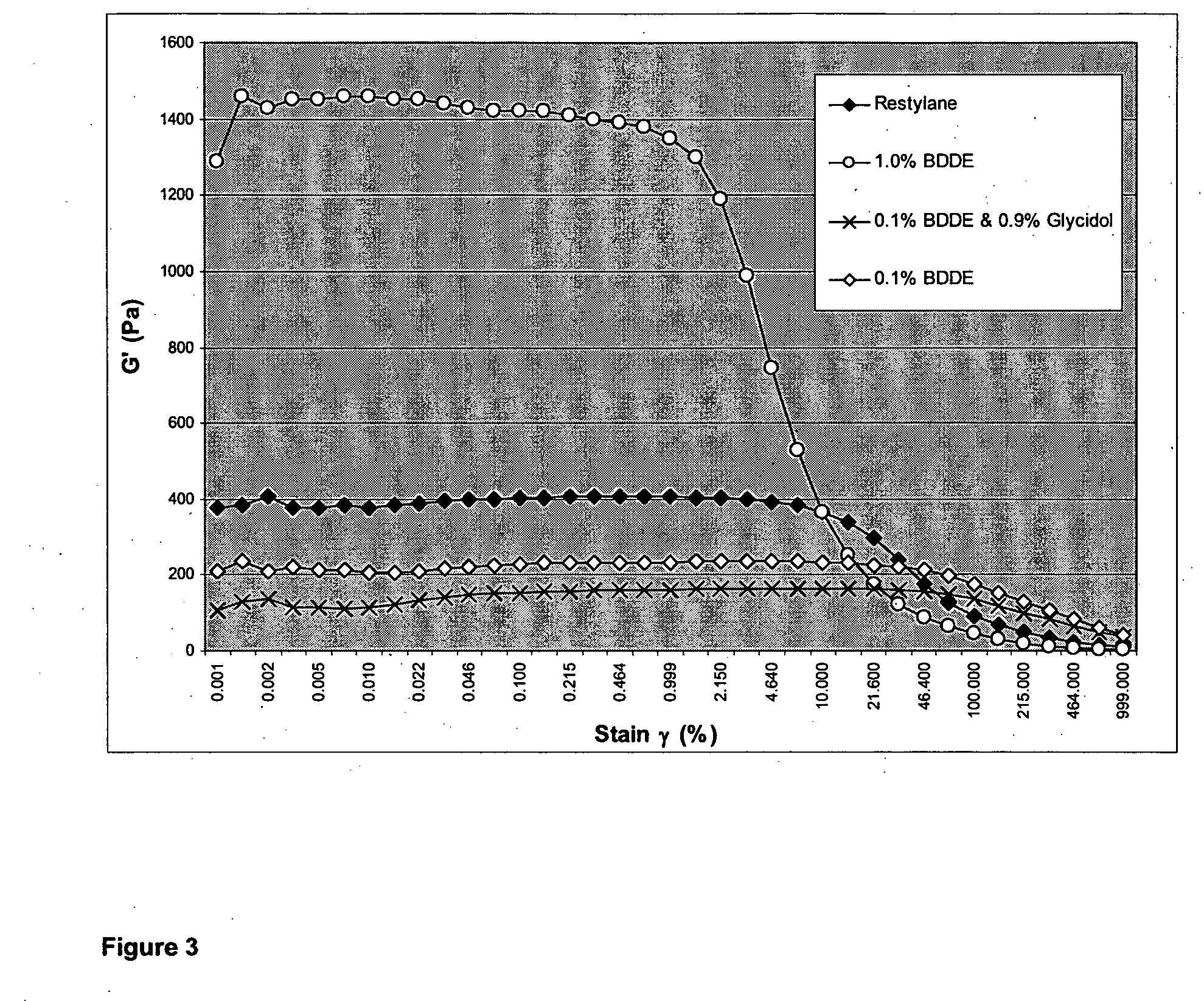
The present invention relates to a process for preparing a cross-linked polysaccharide gel comprising contacting a polysaccharide with a cross-linking agent and a masking agent to form a cross-linked polysaccharide gel having resistance to degradation under physiological conditions.
Owner:HEBER GEOFFREY KENNETH +1
Protein Formulations and Methods of Making Same
ActiveUS20130156760A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntibody ingredientsExcipientNuclear chemistry
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
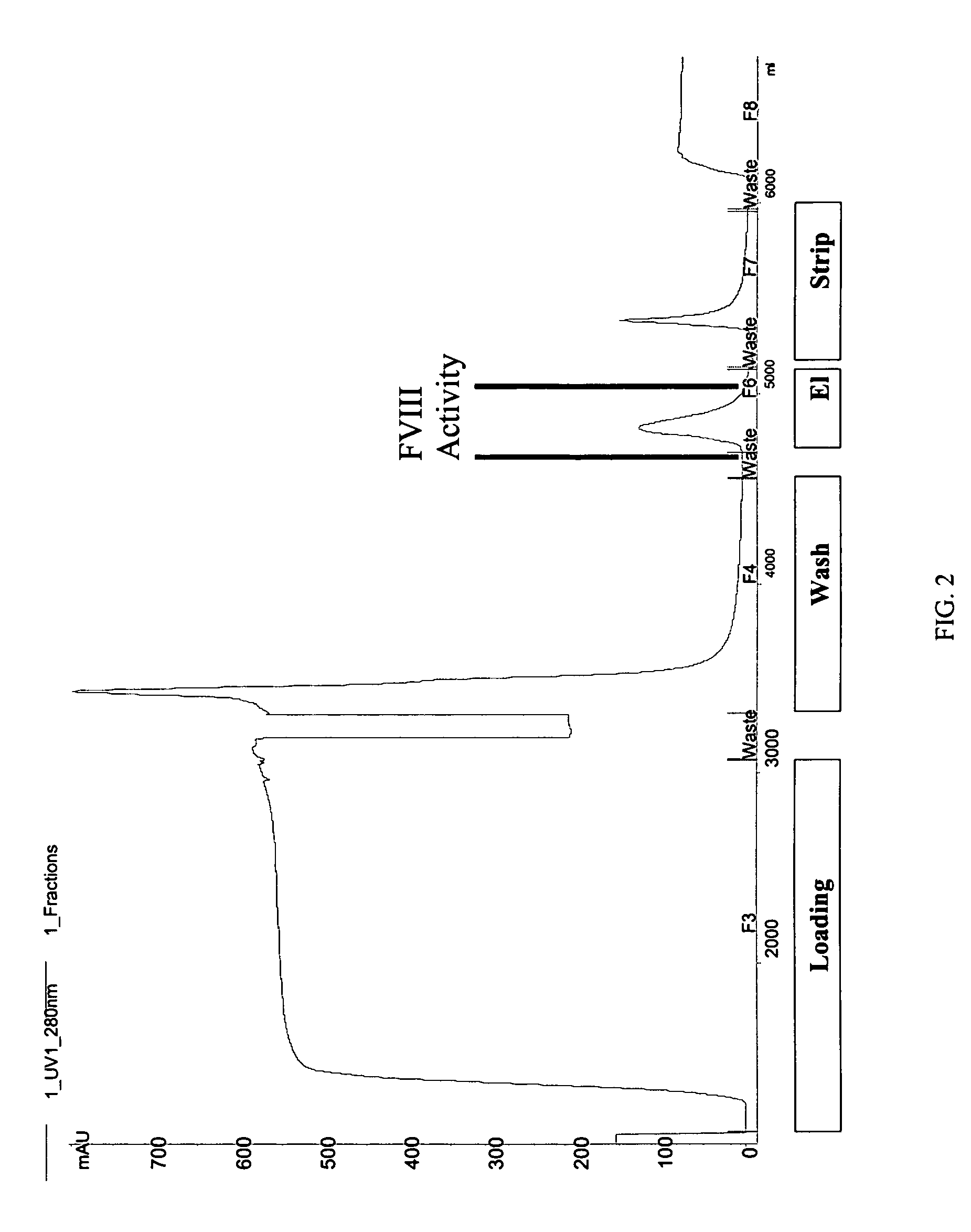
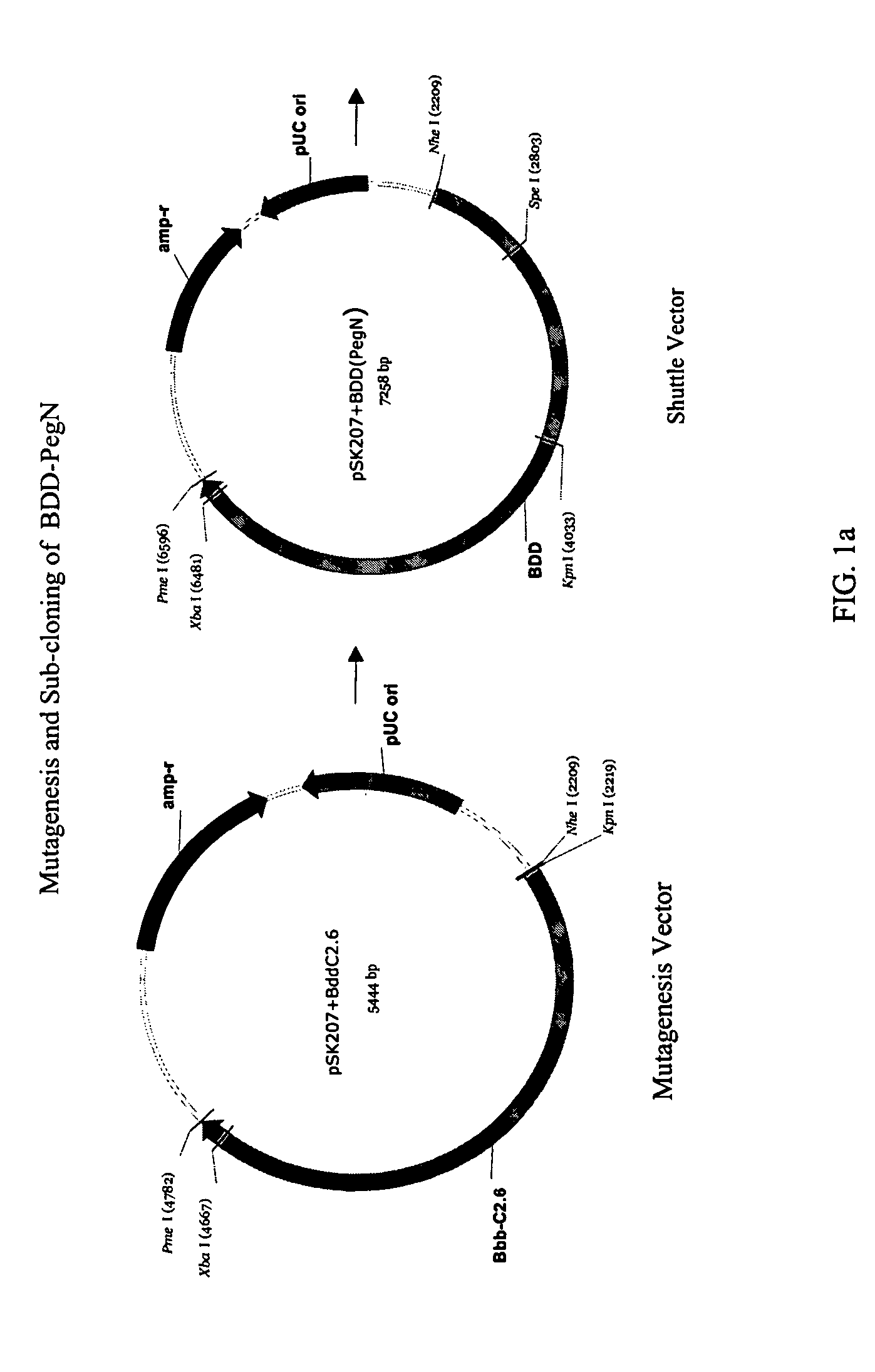
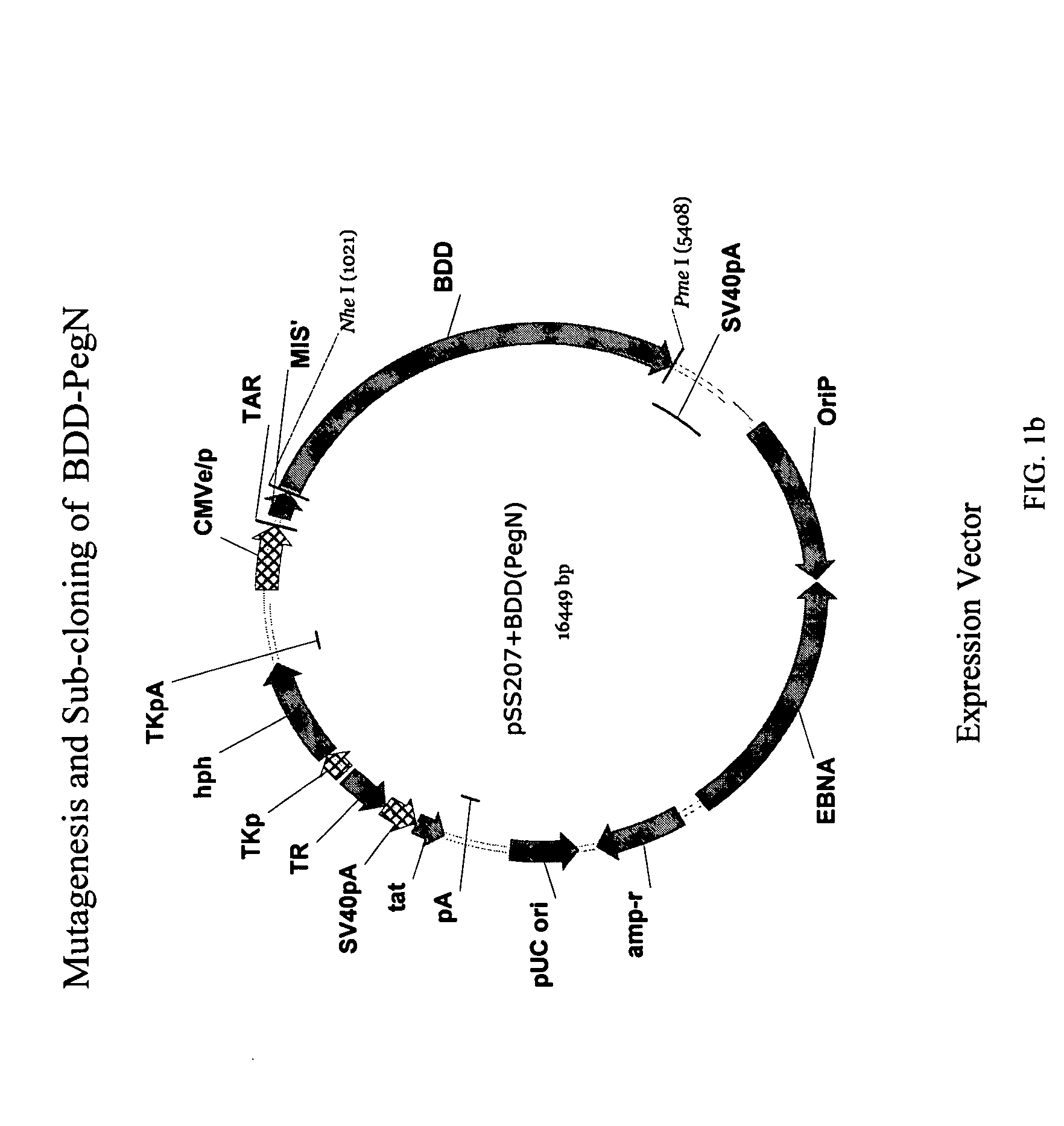
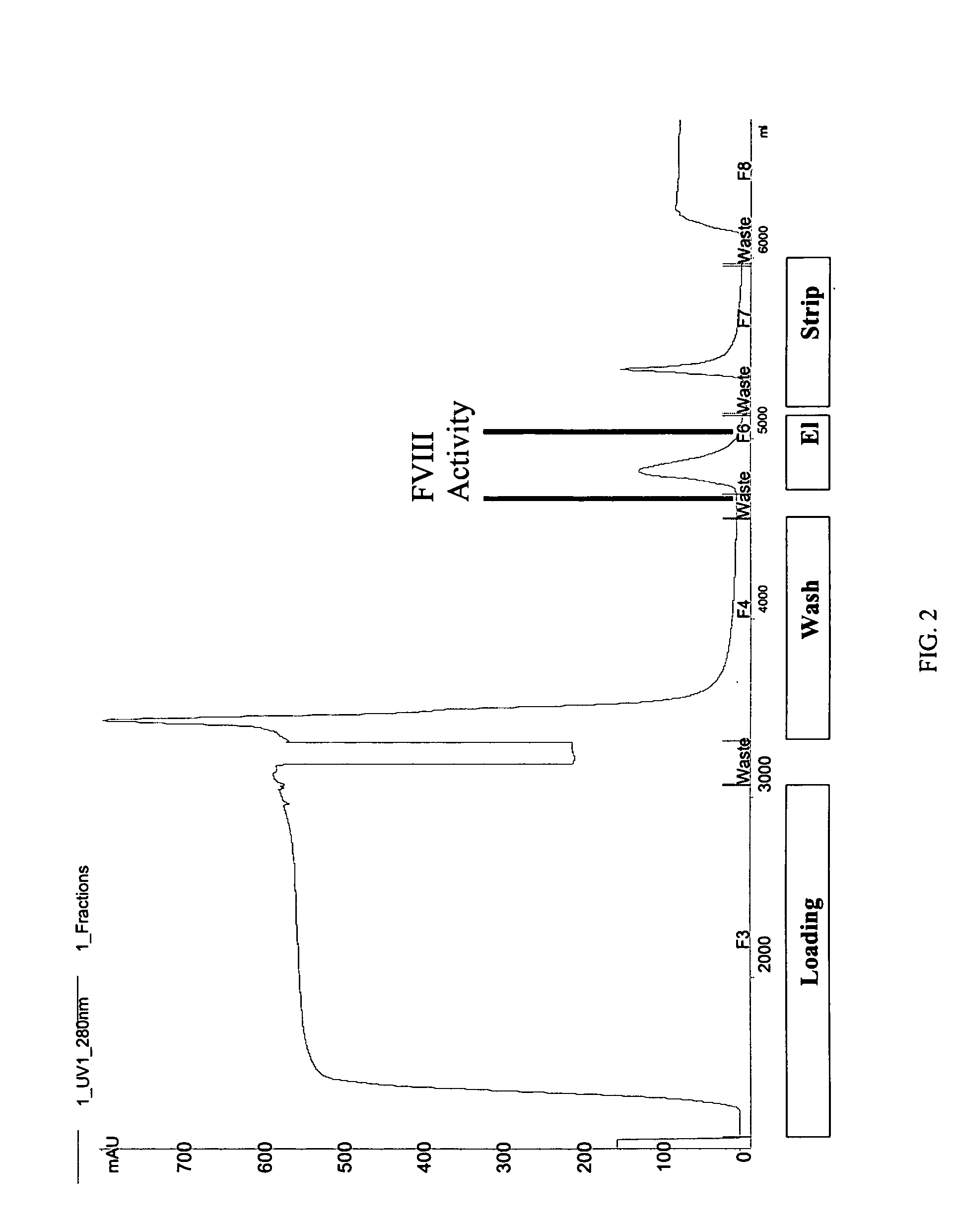
Site-directed modification of FVIII
ActiveUS7632921B2Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsFactor VIII deficiencyPolyethylene glycol
Owner:BAYER HEALTHCARE LLC
Site-directed modification of FVIII
ActiveUS20060115876A1Improve featuresImproved pharmacokinetic propertiesFactor VIIPeptide/protein ingredientsPolyethylene glycolMutant protein
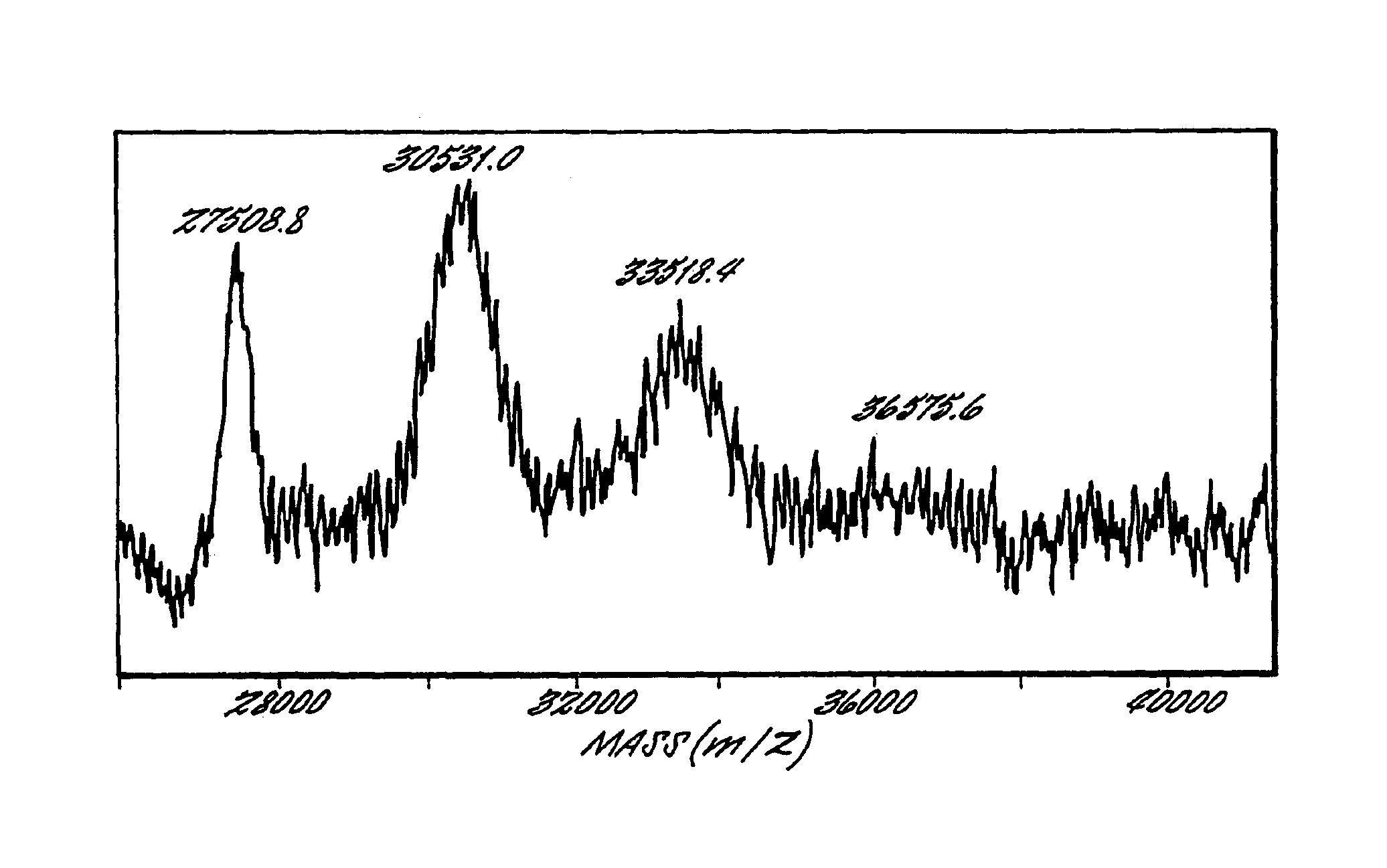
This invention relates to Factor VIII muteins that are covalently bound, at a predefined site that is not an N-terminal amine, to one or more biocompatible polymers such as polyethylene glycol. The mutein conjugates retain FVIII procoagulant activity and have improved pharmacokinetic properties.
Owner:BAYER HEALTHCARE LLC
Anti-CD19 antibodies with reduced immunogenicity
ActiveUS20070154473A1Reduce immunogenicityImproved biological propertyNervous disorderAntipyreticFramework regionHeavy chain
Anti-CD19 B4 antibodies with modified variable regions are disclosed. The modified anti-CD19 variable region polypeptides have alterations to one or more framework regions or complementarity determining regions of the heavy chain variable region or light chain variable region, thereby to reduce a T-cell response.
Owner:CANCER RES TECH LTD
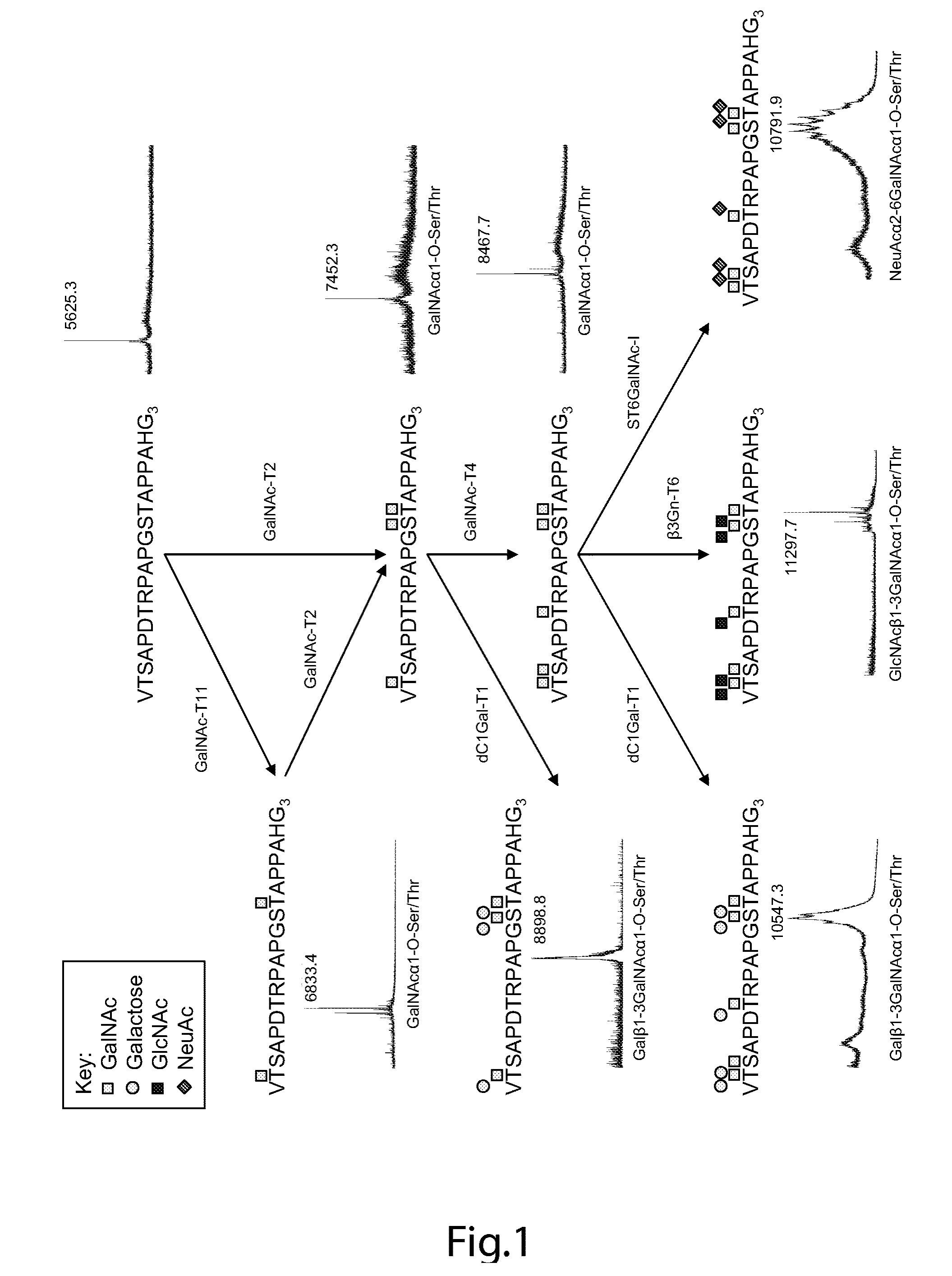
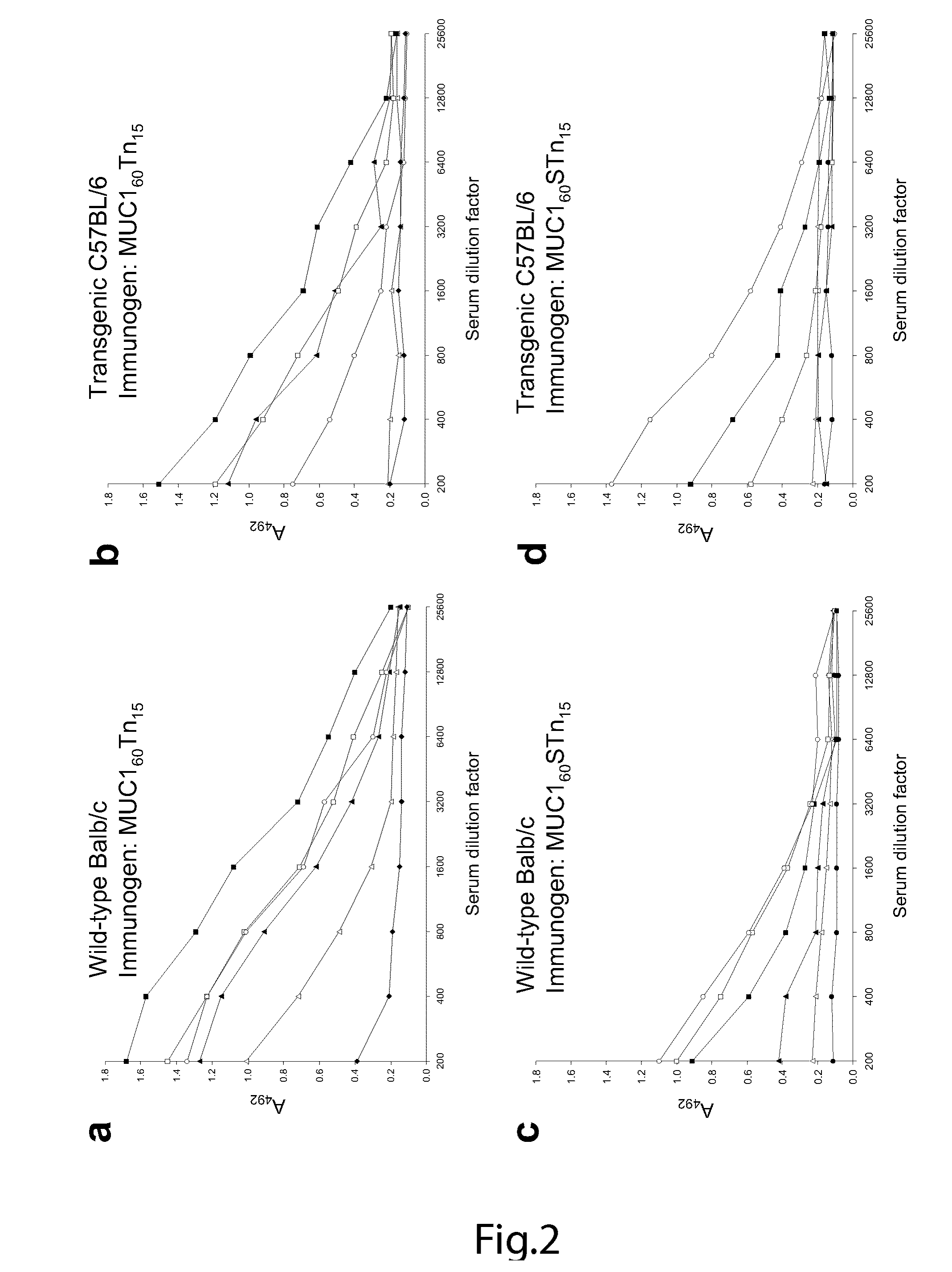
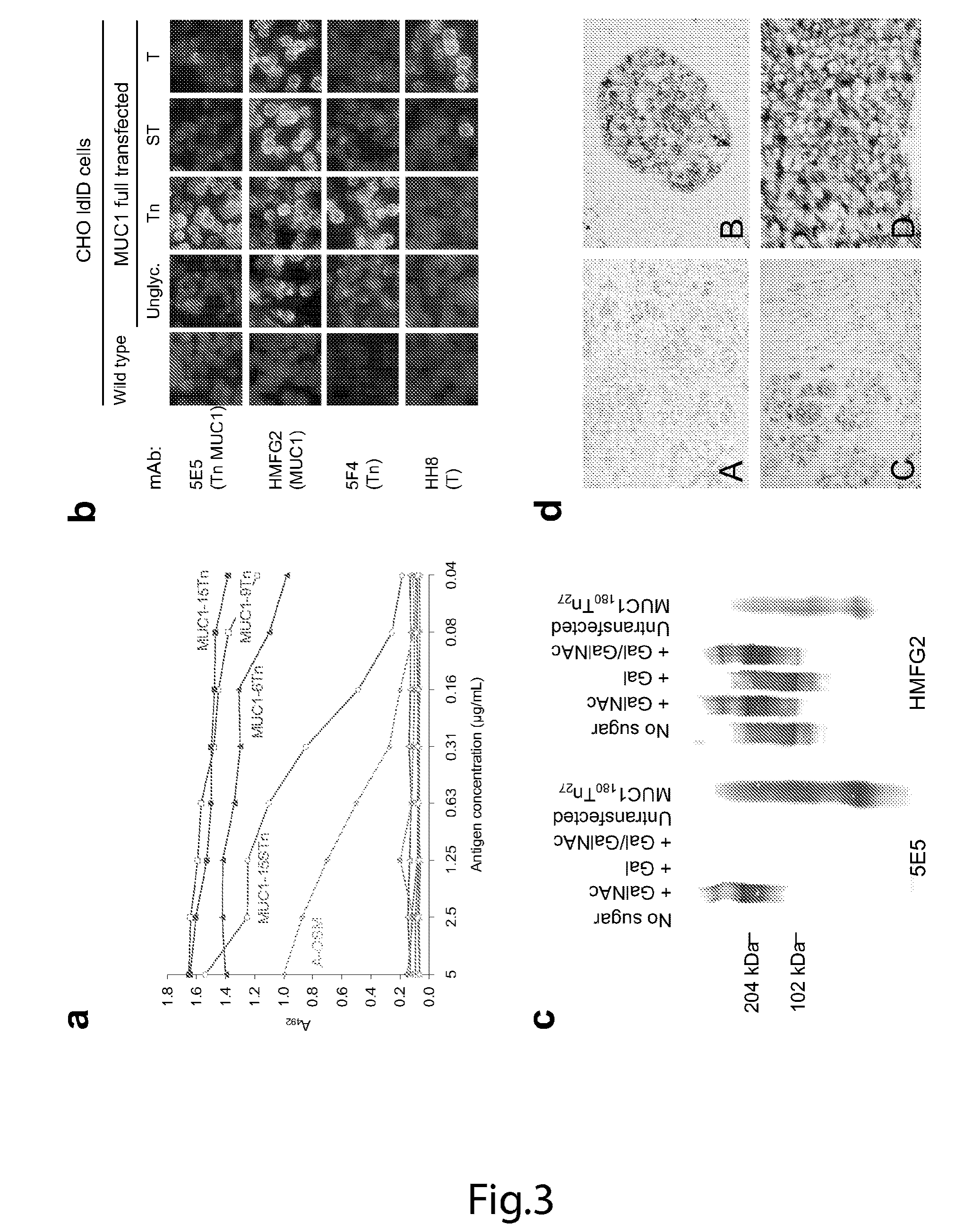
Generation of a cancer-specific immune response toward MUC1 and cancer specific MUC1 antibodies
The present invention provides a method for inducing a cancer specific immune response against MUC1 using an immunogenic glycopeptide. Other aspects of the invention are a pharmaceutical composition comprising the immunogenic glycopeptide and a cancer vaccine comprising the immunogenic glycopeptide. Another aspect is an antibody generated using the immunogenic glycopeptide and the use of said antibody in therapy and diagnosis.
Owner:UNIVERSITY OF COPENHAGEN +1
Protein formulations and methods of making same
ActiveUS8883146B2Minimal aggregationLow levelPowder deliveryPeptide/protein ingredientsExcipientNuclear chemistry
Owner:ABBVIE BIOTECHNOLOGY LTD
High affinity humanized anti-CEA monoclonal antibodies
InactiveUS6333405B1Reduce complicationsReduce and potentially eliminate elicitingSugar derivativesAntibody mimetics/scaffoldsCancer cellCarcinoembryonic antigen
Novel humanized monoclonal antibodies, fragments or derivatives thereof which specifically bind carcinoembryonic antigen (CEA) are provided as well as methods for their manufacture. These humanized antibodies are useful in the treatment of cancers which express CEA as well as for diagnostic purposes, e.g., for in vivo imaging of tumors or cancer cells which express CEA.
Owner:THE DOW CHEM CO
Soluble, degradable poly (ethylene glycol) derivatives for controllable release of bound molecules into solution
InactiveUS6864350B2Limit deliveryImparting sizePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsSolubilityKidney
PEG and related polymer derivatives having weak, hydrolytically unstable linkages near the reactive end of the polymer are provided for conjugation to drugs, including proteins, enzymes, small molecules, and others. These derivatives provide a sufficient circulation period for a drug-PEG conjugate and then for hydrolytic breakdown of the conjugate and release of the bound molecule. In some cases, drugs that previously had reduced activity when permanently coupled to PEG can have therapeutically suitable activity when coupled to a degradable PEG in accordance with the invention. The PEG of the invention can be used to impart water solubility, size, slow rate of kidney clearance, and reduced immunogenicity to the conjugate. Controlled hydrolytic release of the bound molecule in the aqueous environment can then enhance the drug delivery system.
Owner:NEKTAR THERAPEUTICS INC
Compositions of prokaryotic phenylalanine ammonia-lyase and methods of using compositions thereof
ActiveUS7534595B2High catalytic activityExtended half-lifeNervous disorderPeptide/protein ingredientsWild typeDrug biological activity
Owner:BIOMARIN PHARMA INC
Pericardial tissue sheet
InactiveUS20080195230A1Low antigenicityLow immunogenicityFluid jet surgical cuttersSurgical instruments for heatingThermal energyTissue material
A method of cutting tissue material of biology origin employs a plotted water-jet or RF cutting system. The cutting system is computer controlled and includes a water-jet or RF cutting means combined with a motion system. The cutting energy is selected so that communication of thermal energy into the segment beyond the edge is minimized to avoid damaging the segment adjacent the edge.
Owner:QUIJANO RODOLFO C +1
Biologically related substances modified by multifunctional H-type polyethylene glycol derivative
ActiveCN104530413ALow immunogenicityImprove distributionPharmaceutical non-active ingredientsFluorescencePolyethylene glycol
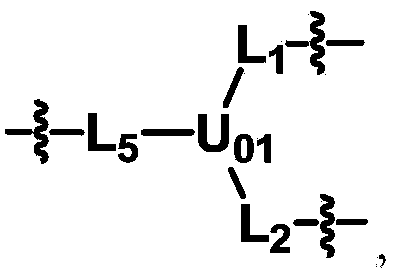
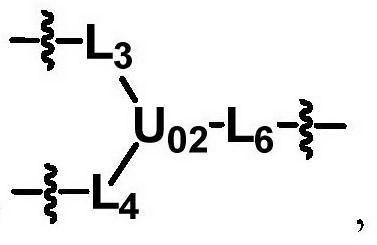
The invention discloses biologically related substances modified by a multifunctional H-type polyethylene glycol derivative. The derivative comprises a linear main axis LPEG and four PEG branched chains, and n1, n2, n3 and n4 are polymerization degrees of the branched chains, respectively; U1 and U2 are trivalent branching groups connecting the main axis LPEG with two PEG branched chains; F1 and F2 contain functional groups or their protected forms R01, and the number of R01 is one or more than one. Any linking group in the molecule or linking groups formed with adjacent heteroatom groups are stable or degradable; any PEG chain segment in the molecule independently shows polydispersity or monodispersity. One H-type molecule can modify multiple types of or multiple biologically related substances; the modified product has a flexible branching structure, a high drug loading capacity, optimized pharmacokinetics and tissue distribution, and can also carry two biologically related substances with different functions, so as to generate fluorescence property or targeting function.
Owner:XIAMEN SINOPEG BIOTECH
Multifunctional H-type polyethylene glycol derivative and preparation method thereof
ActiveCN104530417AIncrease the number ofImprove modification efficiencyPolymer sciencePolyethylene glycol
The invention discloses a multifunctional H-type polyethylene glycol derivative and a preparation method thereof. The structure is as shown in formula (1) in the description, wherein a linear main axis LPEG and four PEG branched chains are included, and n1, n2, n3 and n4 are polymerization degrees of the branched chains, respectively; U1 and U2 are trivalent branching groups connecting the main axis LPEG with two PEG branched chains; F1 and F2 contain functional groups or their protected forms R01, and contain or do not contain branching groups G, and correspondingly, the number of R01 is one or more than one; F1 is the same as or different from F2; any linking group in the molecule or linking groups formed with adjacent heteroatom groups are stable or degradable; any PEG chain segment in the molecule independently shows polydispersity or monodispersity. The functionalized polyethylene glycol is diverse in branching structure and branching arm length, is adjustable and easily-controllable in various parameters and performance indexes, and is wide in application.
Owner:XIAMEN SINOPEG BIOTECH
Bispecific binding molecules for Anti-angiogenesis therapy
InactiveUS20110172398A1Reduce manufacturing costImproving desired propertySenses disorderSugar derivativesAngiogenesis EffectAntiangiogenic therapy
Bispecific binding molecules, in particular immunoglobulin single variable domains such as VHHs and domain antibodies, comprising a VEGF-binding component and a Dll4-binding component in one molecule. Pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with VEGF- and Dll4-mediated effects on angiogenesis. Nucleic acids encoding the bispecific binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com