Protein formulations and methods of making same
a technology of protein formulation and protein, applied in the field of pharmaceutical protein formulation, can solve the problems of protein solutions without such compounds that may encounter challenges with degradation, problems such as difficulty in maintaining the stability and solubility of proteins in solution, and difficulty in ensuring stability and solubility of proteins, so as to achieve low conductivity and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
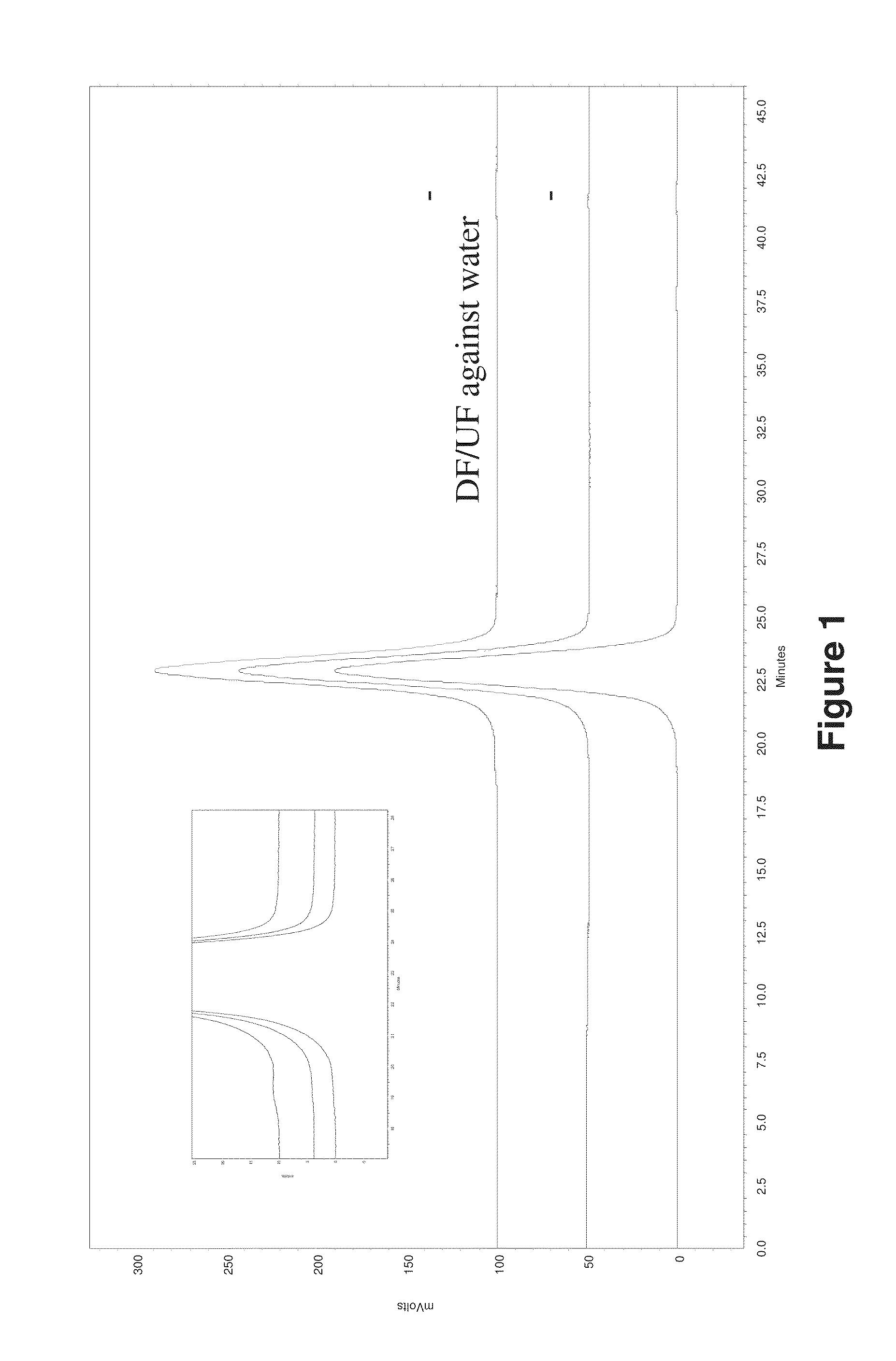
Diafiltration / Ultrafiltration with Adalimumab and J695
Materials and Methods
[0236]Adalimumab and J695 were diafiltered using pure water. After an at least 5-fold volume exchange with pure water, the protein solutions were ultrafiltered to a final target concentration of at least 150 mg / mL. Osmolality, visual inspection and protein concentration measurements (OD280) were performed to monitor the status of the proteins during DF / UF processing.
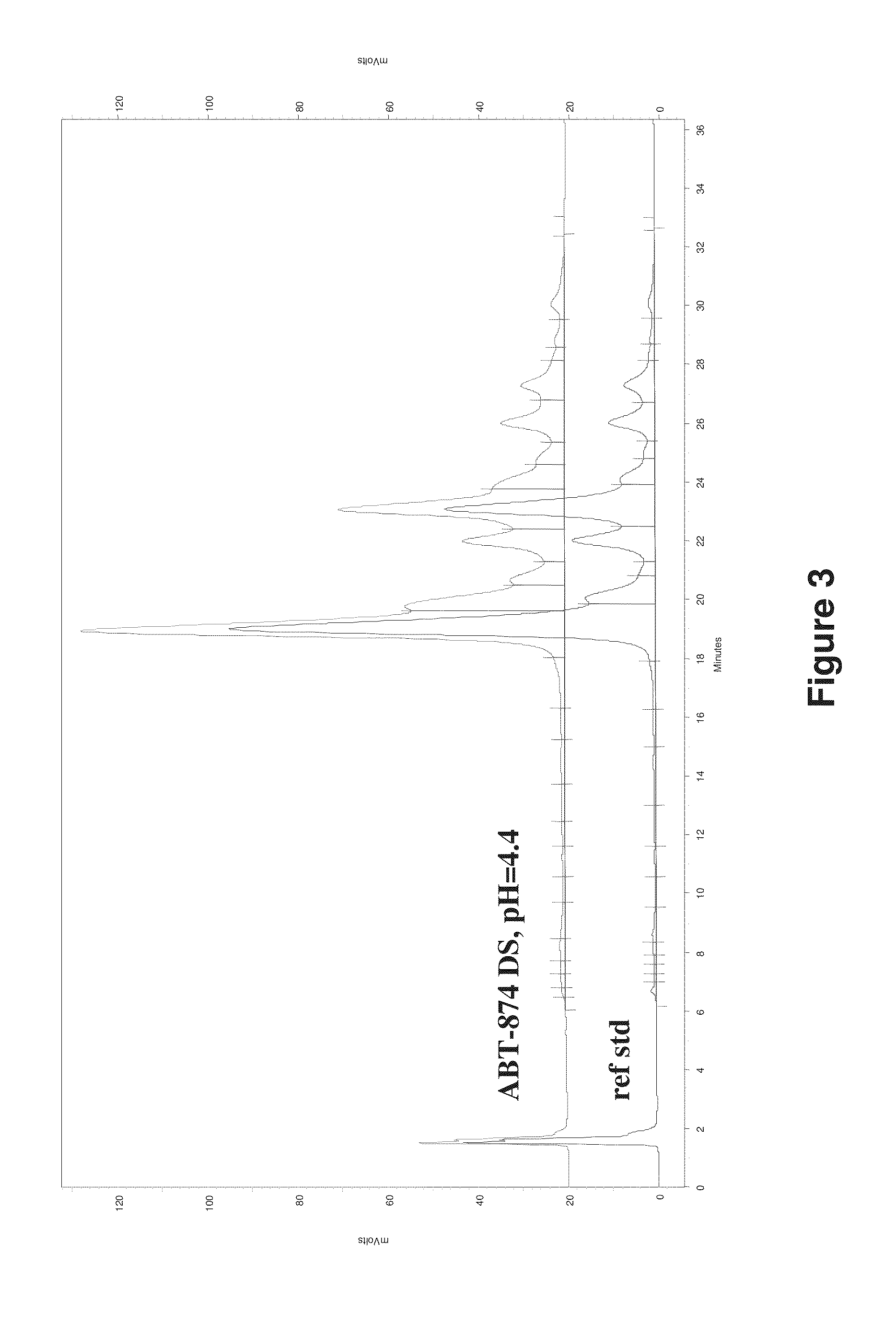
[0237]Size exclusion chromatography and ion exchange chromatography were used to characterize protein stability in each final DF / UF product as compared to the starting formulation, e.g., drug substance (DS) starting material and protein standard. Drug substance or “DS” represents the active pharmaceutical ingredient and generally refers to a therapeutic protein in a common bulk solution.[0238]Adalimumab Drug Substance, (Adalimumab extinction coefficient 280 nm: 1.39 mL / mg cm). Drug Substance did not contain polysorbate 80. DS composition: 5.57 mM ...
example 2
Formulation Comprising High TNFα Antibody Concentration
2.1: Diafiltration
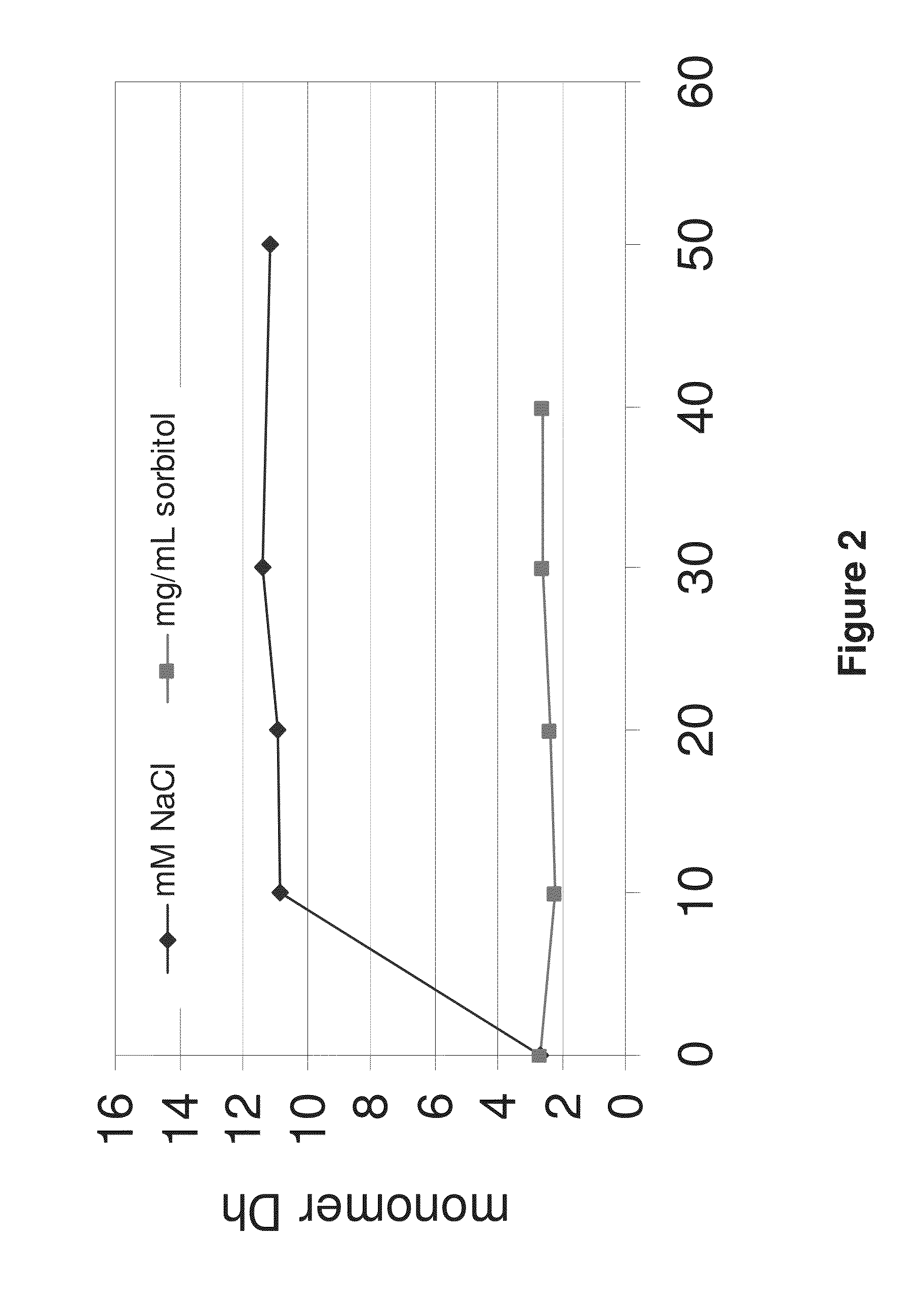
[0342]Prior to diafiltration, Adalimumab (49.68 mg / mL) was diluted with water for injection to a concentration of approximately 15 mg / mL. Therefore 140.8 mL Adalimumab solution (49.68 mg / mL) were filled in a 500 mL volumetric flask. The flask was filled up to the calibration mark with water for injection. The volumetric flask was closed and gently shaken for homogenization of the solution. The TFF labscale system was flushed with water. Then the membrane (PES) was adapted and was also flushed with 1 L distilled water. Next, the TFF labscale system and the membrane were flushed with approximately 300 mL of water for injection. The diluted Adalimumab solution was then filled in the reservoir of the TFF. A sample for an osmolality measurement (300 μL), UV spectrophotometry (500 μL) and a sample for SEC analysis (120 μL) were pulled. The system was closed and diafiltration was started. The DF / UF (diafiltration / ultr...
example 3
Formulation Comprising High Concentration 11-12 Antibody
3.1: Diafiltration
[0366]Prior to diafiltration, IL-12 antibody J695 (54 mg / mL) was diluted with water for injection to a concentration of approximately 15 mg / mL. This was done by placing 150 mL J695 solution (54 mg / mL) in a 500 mL volumetric flask and filling the flask to the calibration mark with water for injection. The volumetric flask was closed and gently shaken for homogenization of the solution. The TFF labscale system was flushed with water. Then the polyethersulfone membrane (PES) was adapted and was also flushed with 1 L of distilled water. Afterwards the TFF labscale system and the membrane were flushed with approximately 300 mL of water for injection. Next, the diluted J695 solution was placed in the reservoir of the TFF. A sample for osmolality measurement (300 μL), UV spectrophotometry (500 μL) and a sample for SEC analysis (120 μL) were pulled. The system was closed and diafiltration was started. After 200 mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mw | aaaaa | aaaaa |
| conductivity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com