Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2335 results about "Lysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
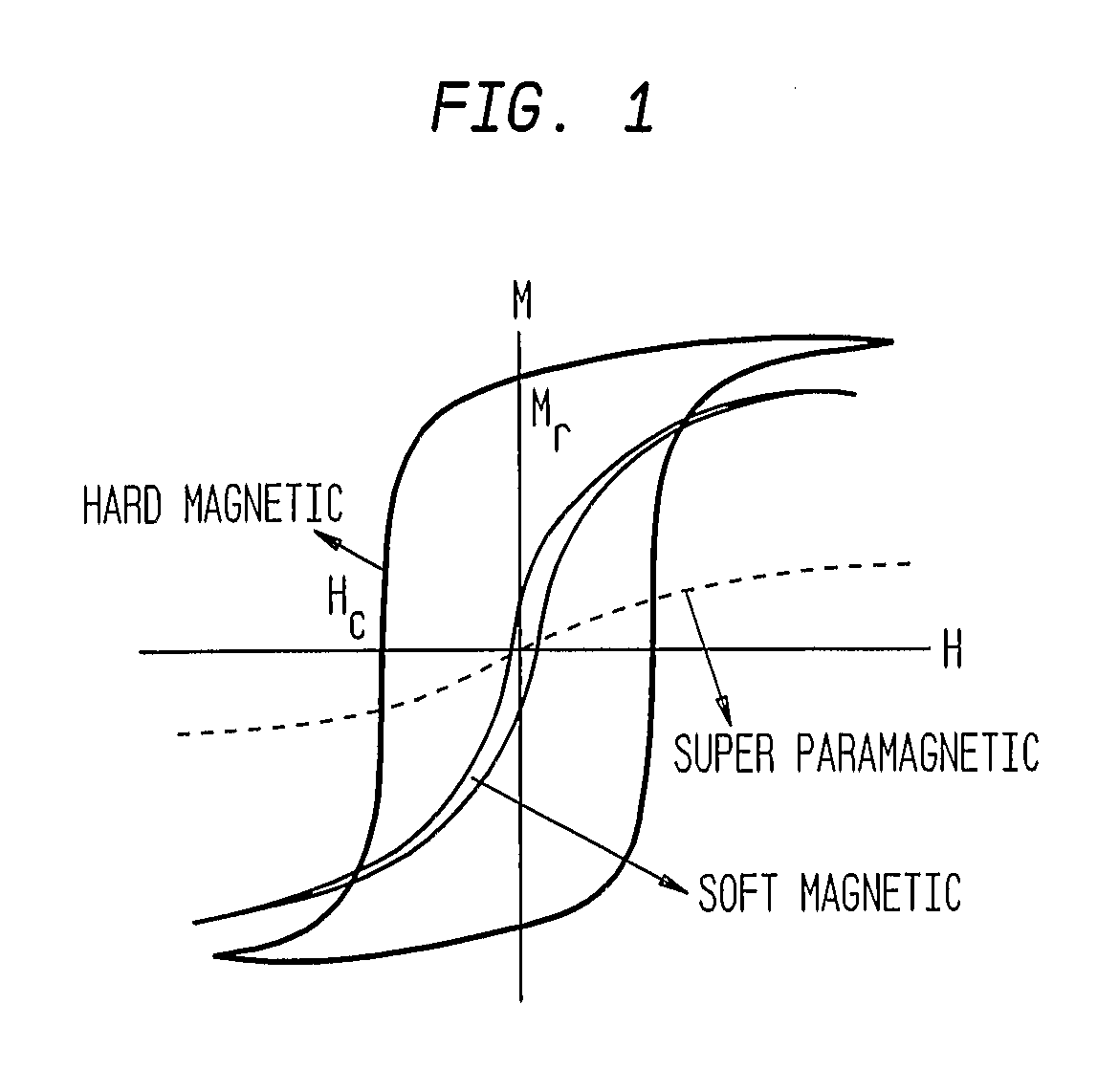
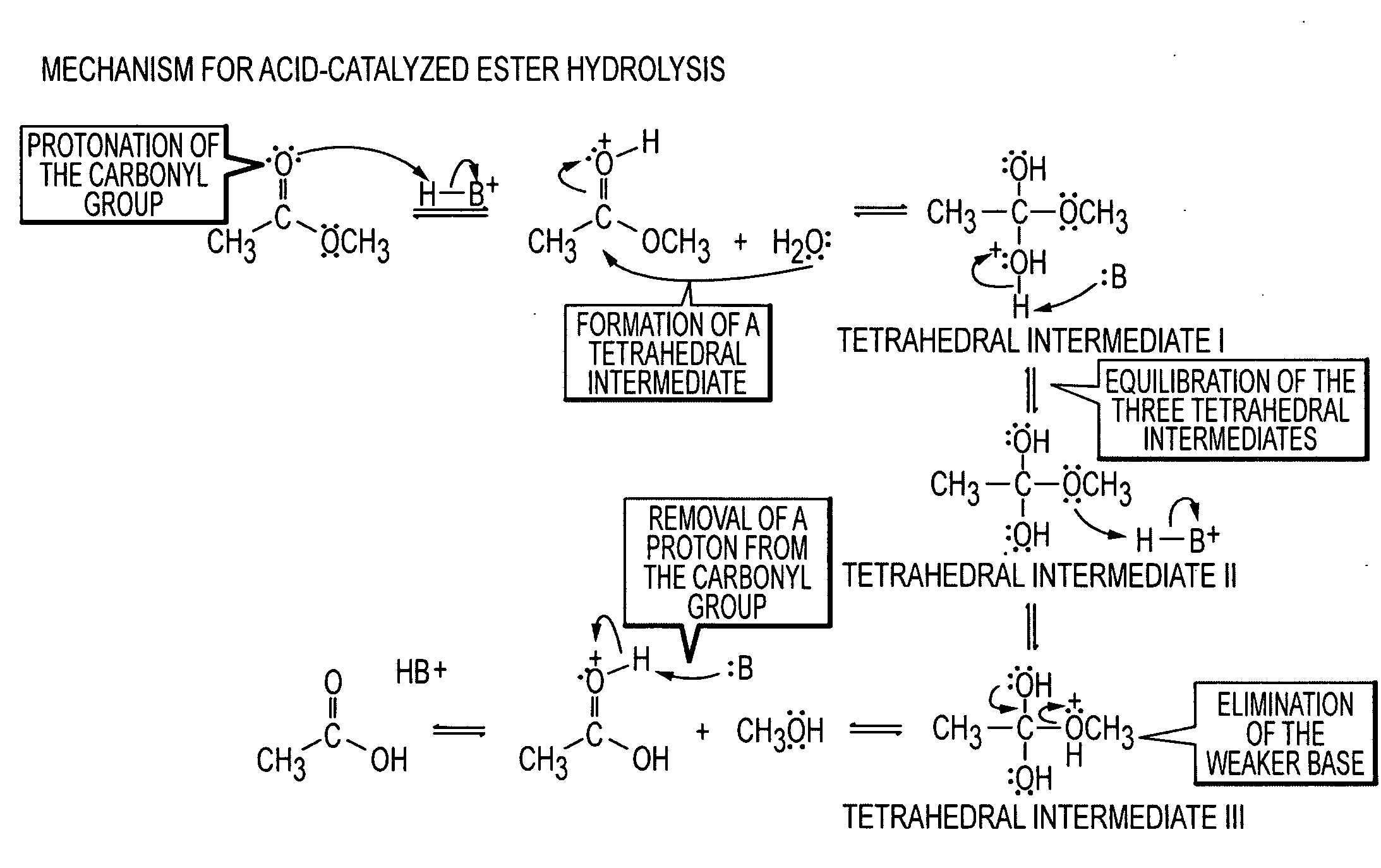
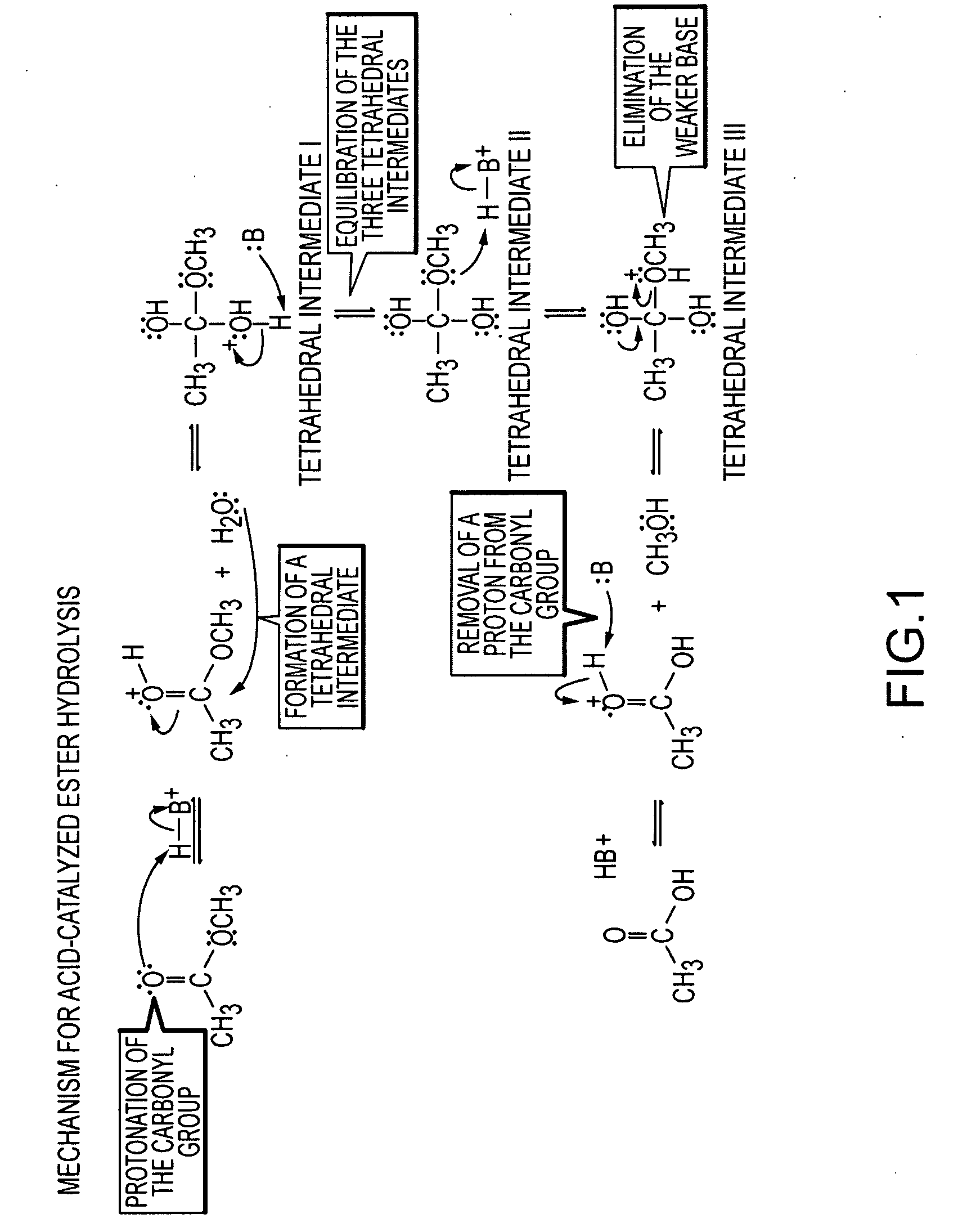
Lysis (/ˈlaɪsɪs/ LY-sis; Greek λύσις lýsis, "a loosing" from λύειν lýein, "to unbind") refers to the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic (that is, "lytic" /ˈlɪtɪk/ LIT-ək) mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a lysate. In molecular biology, biochemistry, and cell biology laboratories, cell cultures may be subjected to lysis in the process of purifying their components, as in protein purification, DNA extraction, RNA extraction, or in purifying organelles.
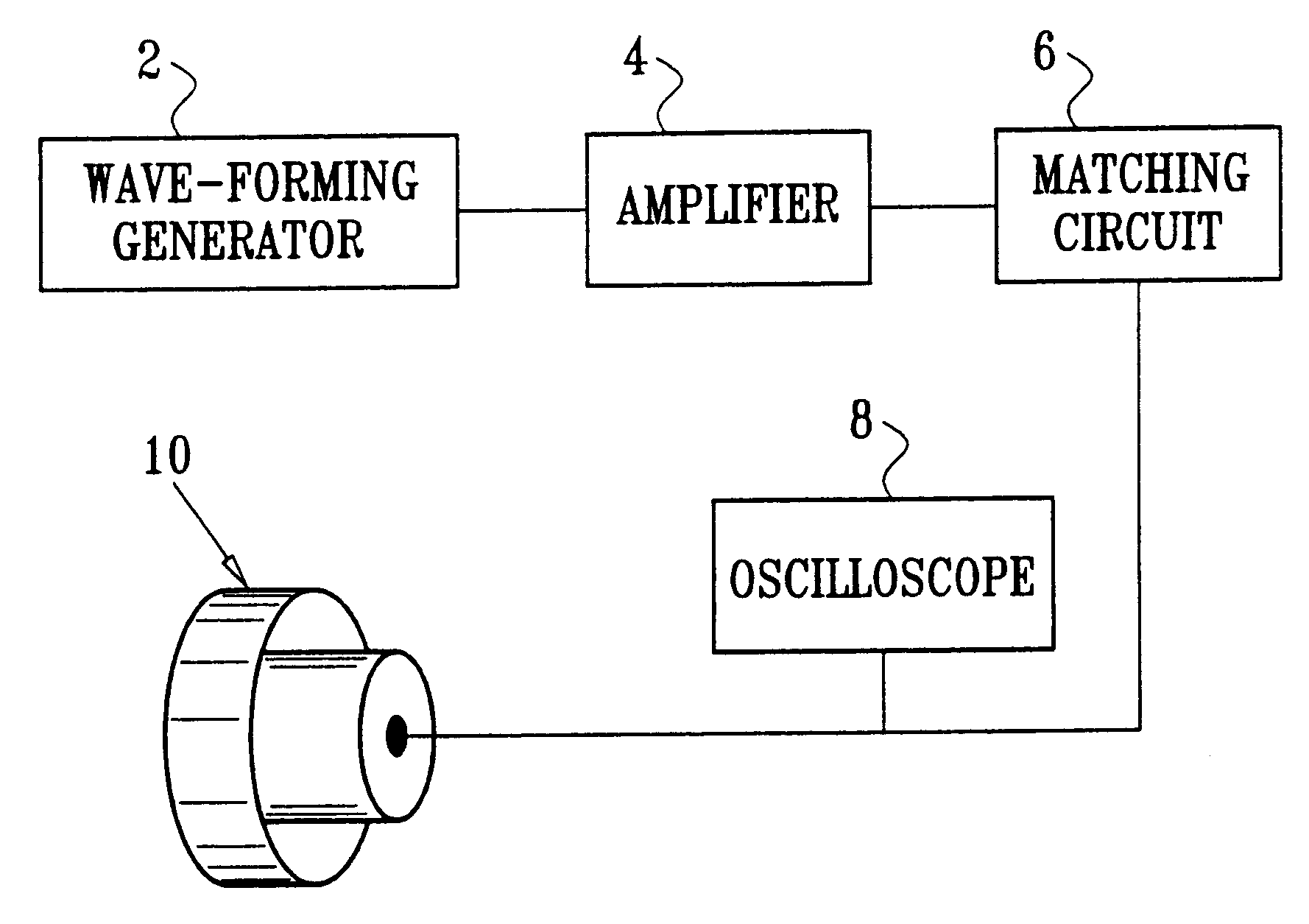
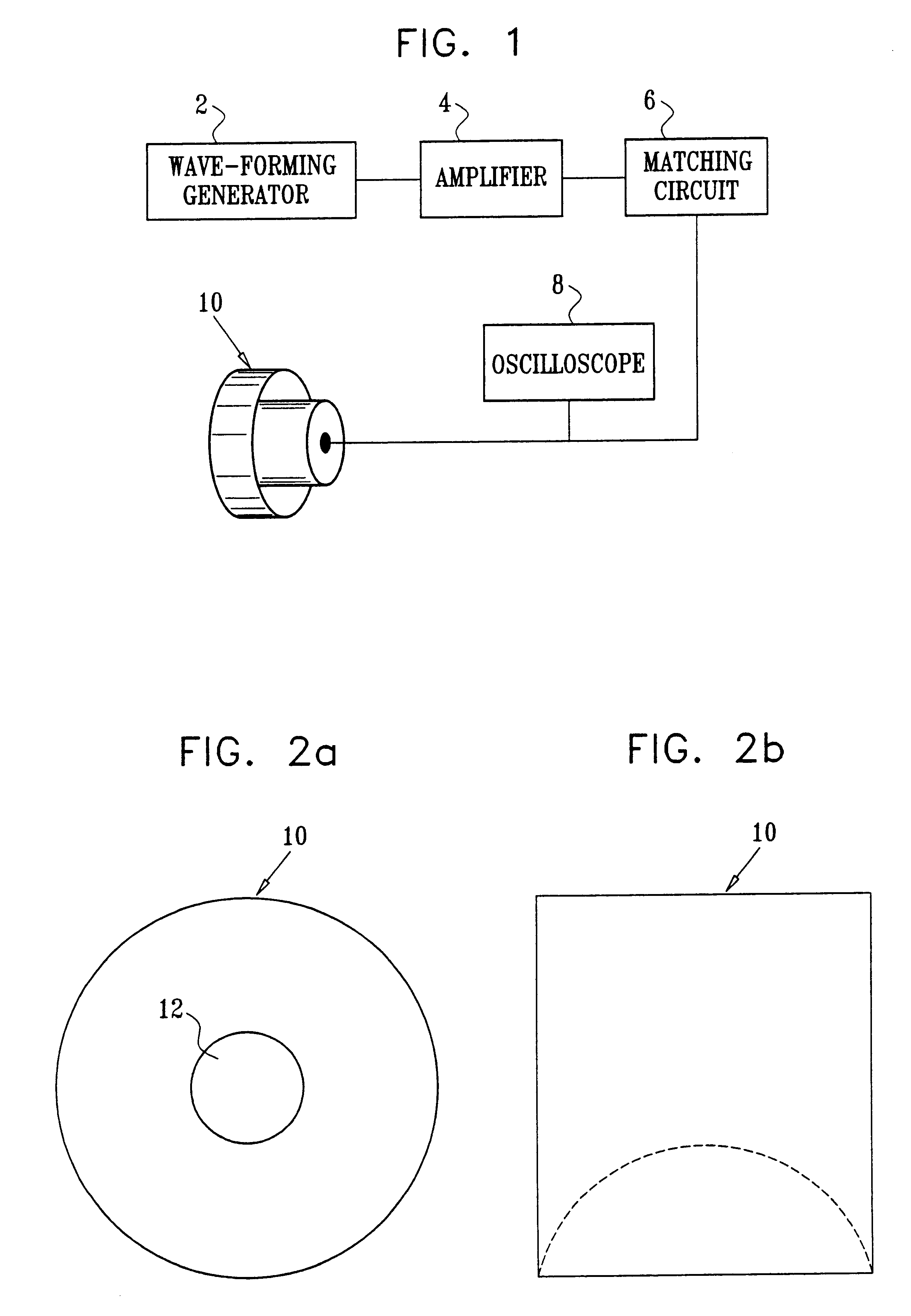
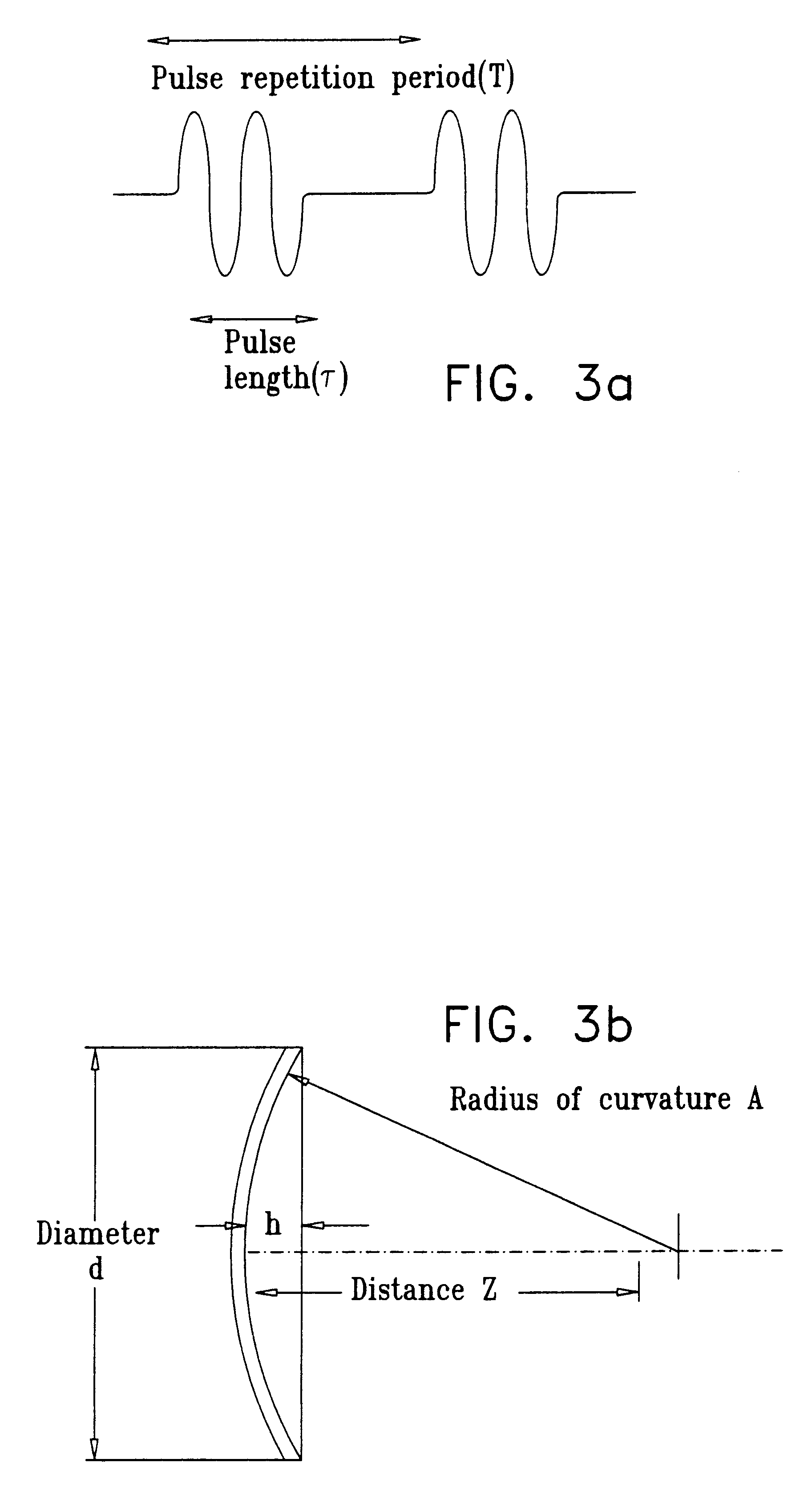
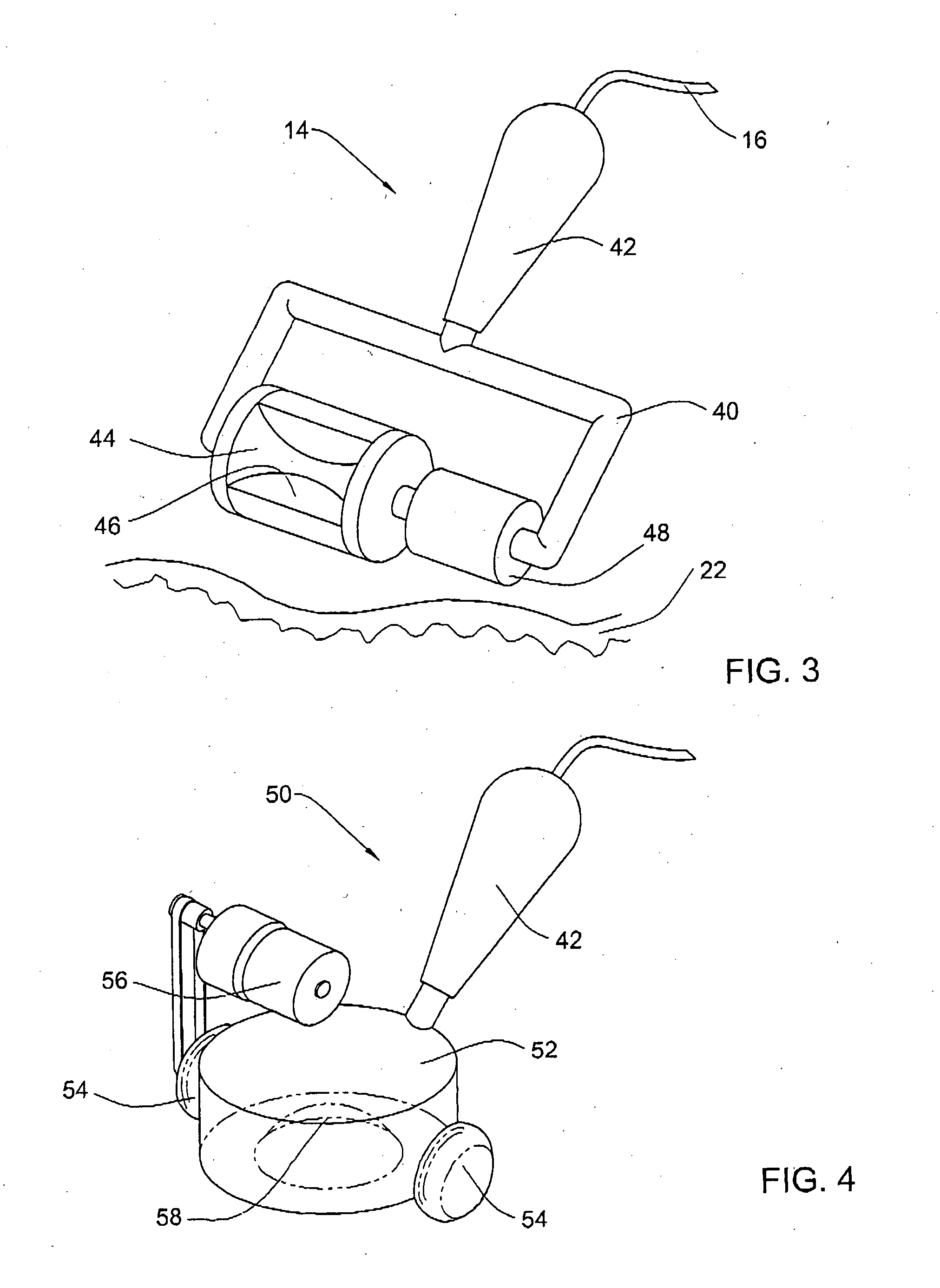
Method and apparatus for non-invasive body contouring by lysing adipose tissue
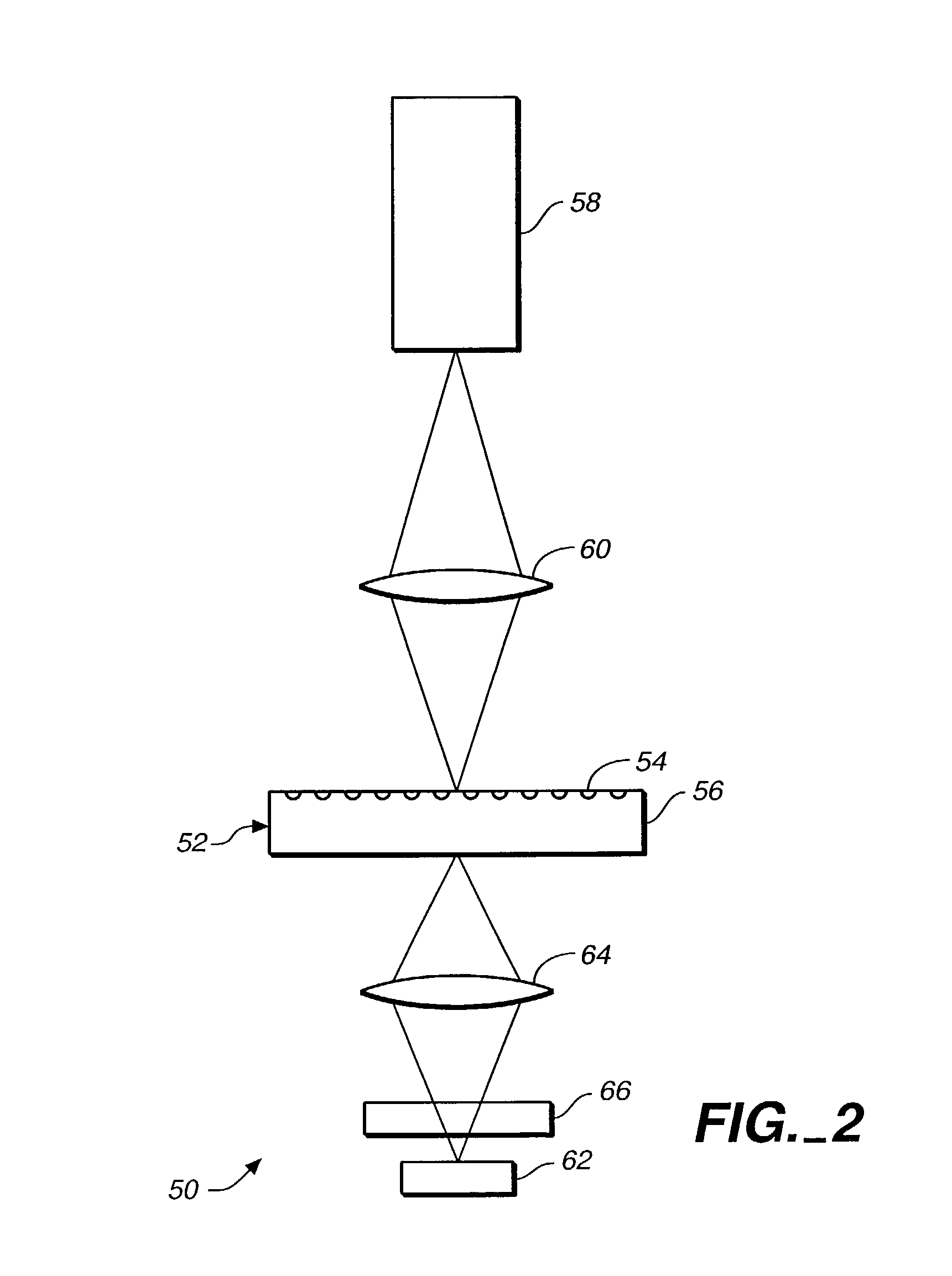
A method and apparatus for producing lysis of adipose tissue underlying the skin of a subject, by: applying an ultrasonic transducer to the subject's skin to transmit therethrough ultrasonic waves focussed on the adipose tissue; and electrically actuating the ultrasonic transducer to transmit ultrasonic waves to produce cavitational lysis of the adipose tissue without damaging non-adipose tissue.
Owner:ULTRASHAPE INC +1
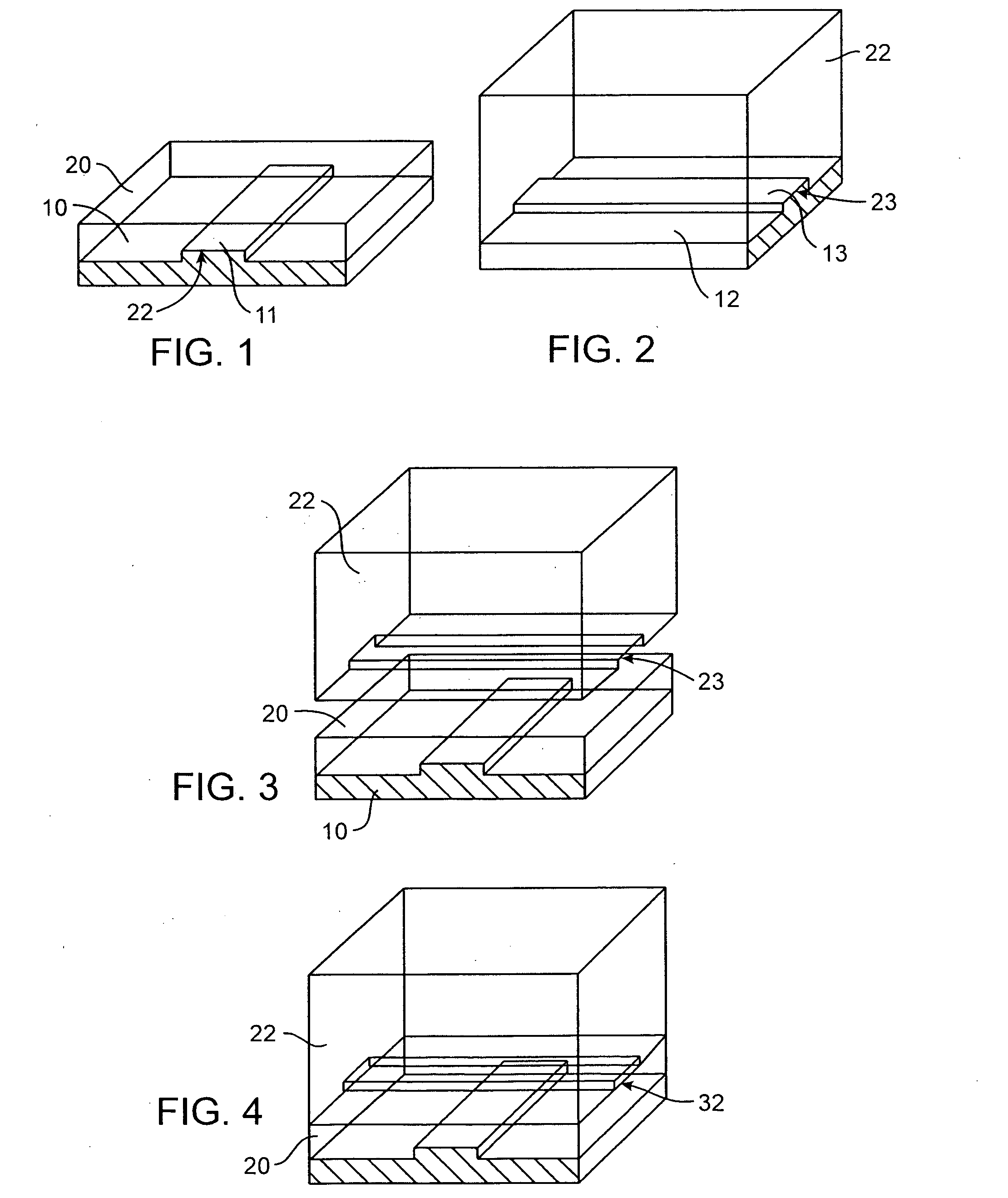
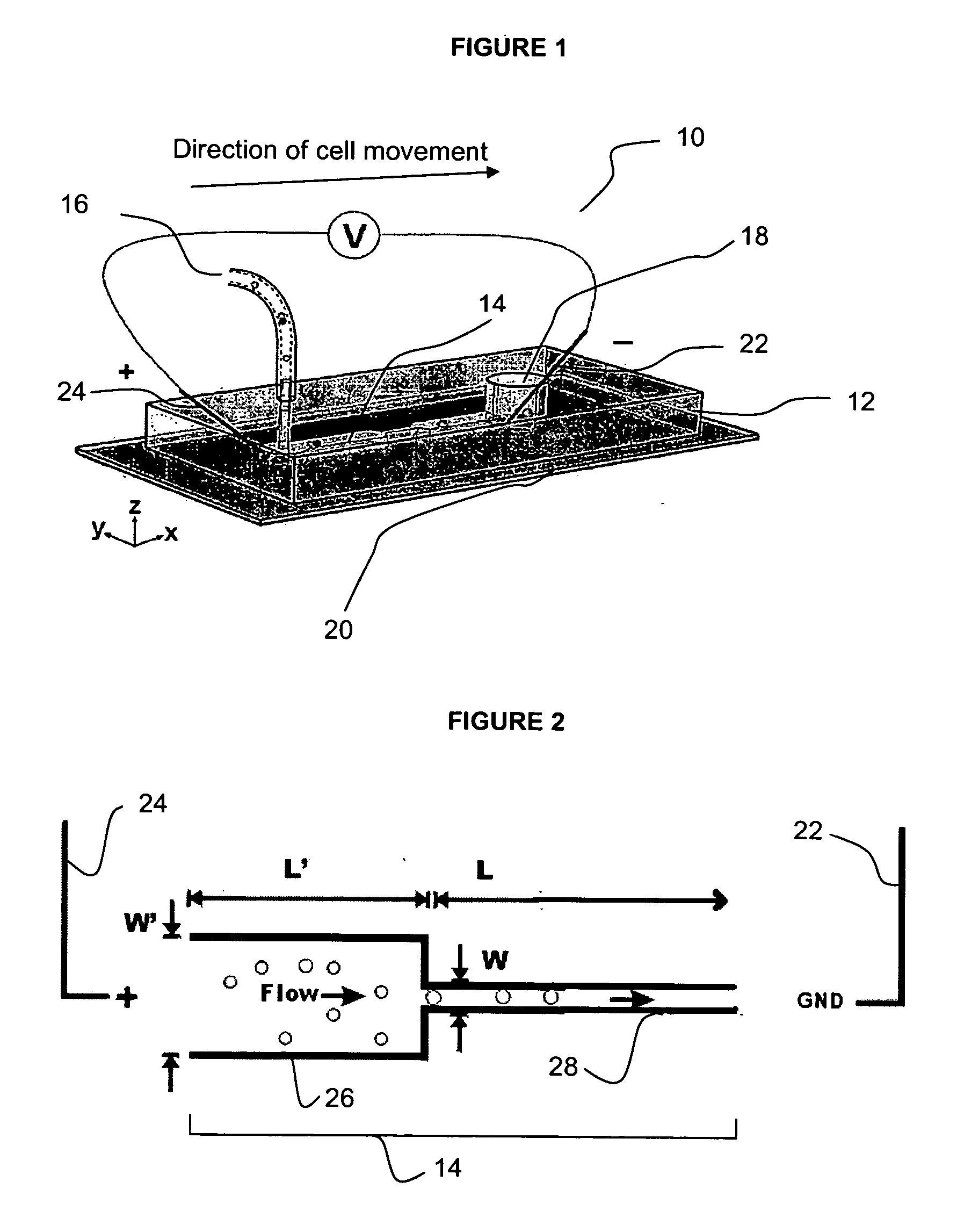
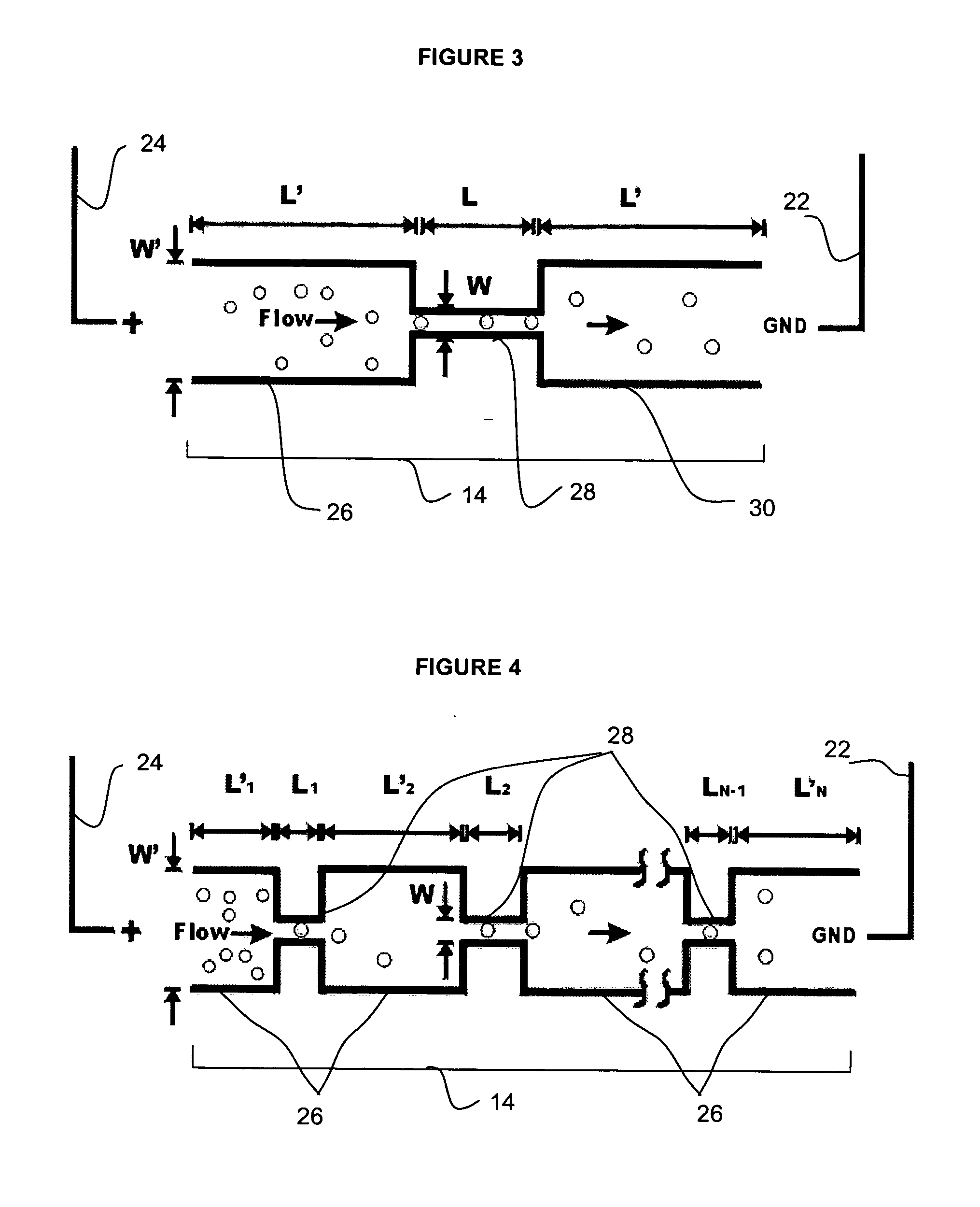
Microfluidic nucleic acid analysis
ActiveUS20050053952A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCDNA libraryCells isolation
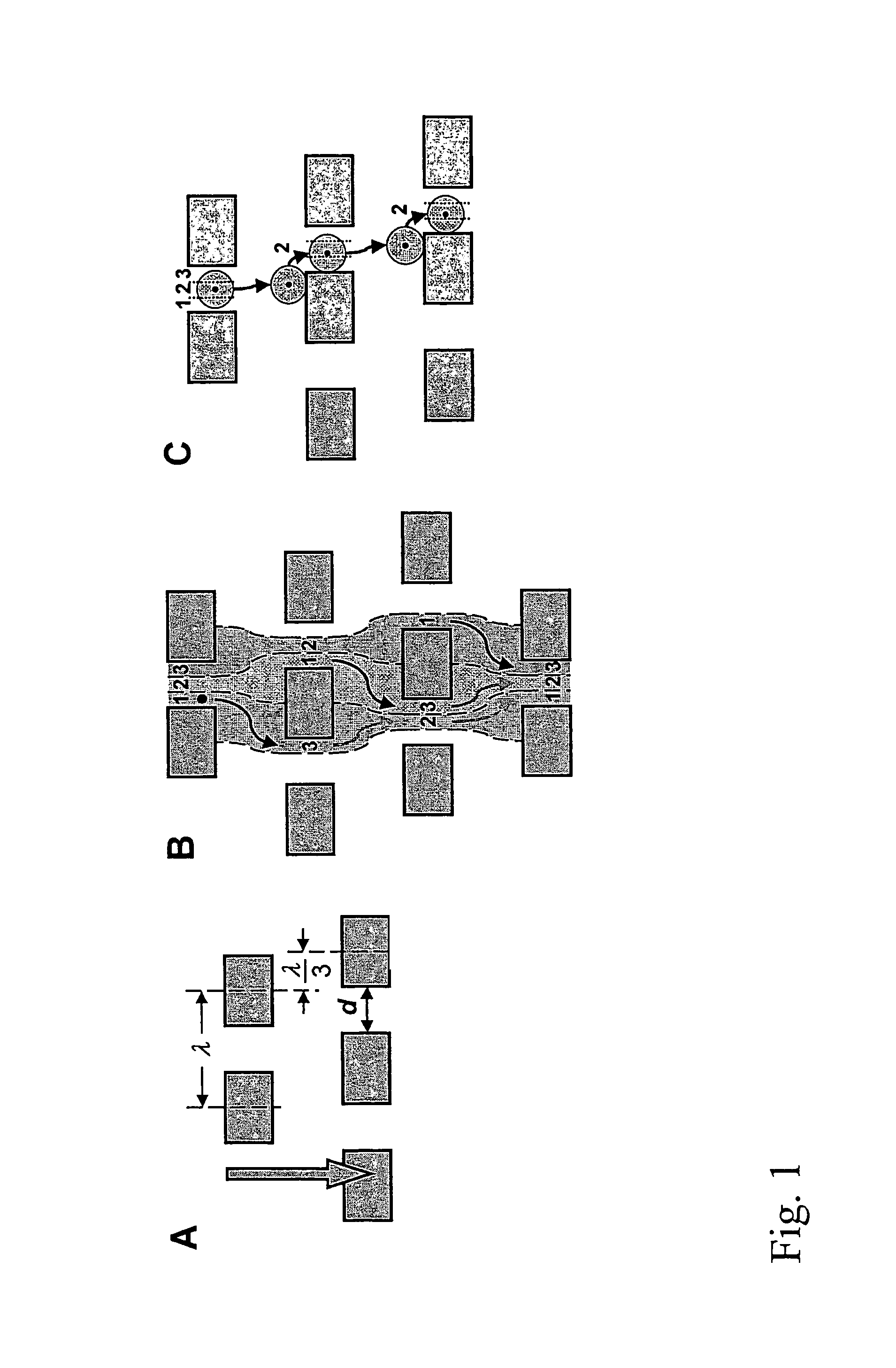
Nucleic acid from cells and viruses sampled from a variety of environments may purified and expressed utilizing microfluidic techniques. In accordance with one embodiment of the present invention, individual or small groups of cells or viruses may be isolated in microfluidic chambers by dilution, sorting, and / or segmentation. The isolated cells or viruses may be lysed directly in the microfluidic chamber, and the resulting nucleic acid purified by exposure to affinity beads. Subsequent elution of the purified nucleic acid may be followed by ligation and cell transformation, all within the same microfluidic chip. In one specific application, cell isolation, lysis, and nucleic acid purification may be performed utilizing a highly parallelized microfluidic architecture to construct gDNA and cDNA libraries.
Owner:CALIFORNIA INST OF TECH
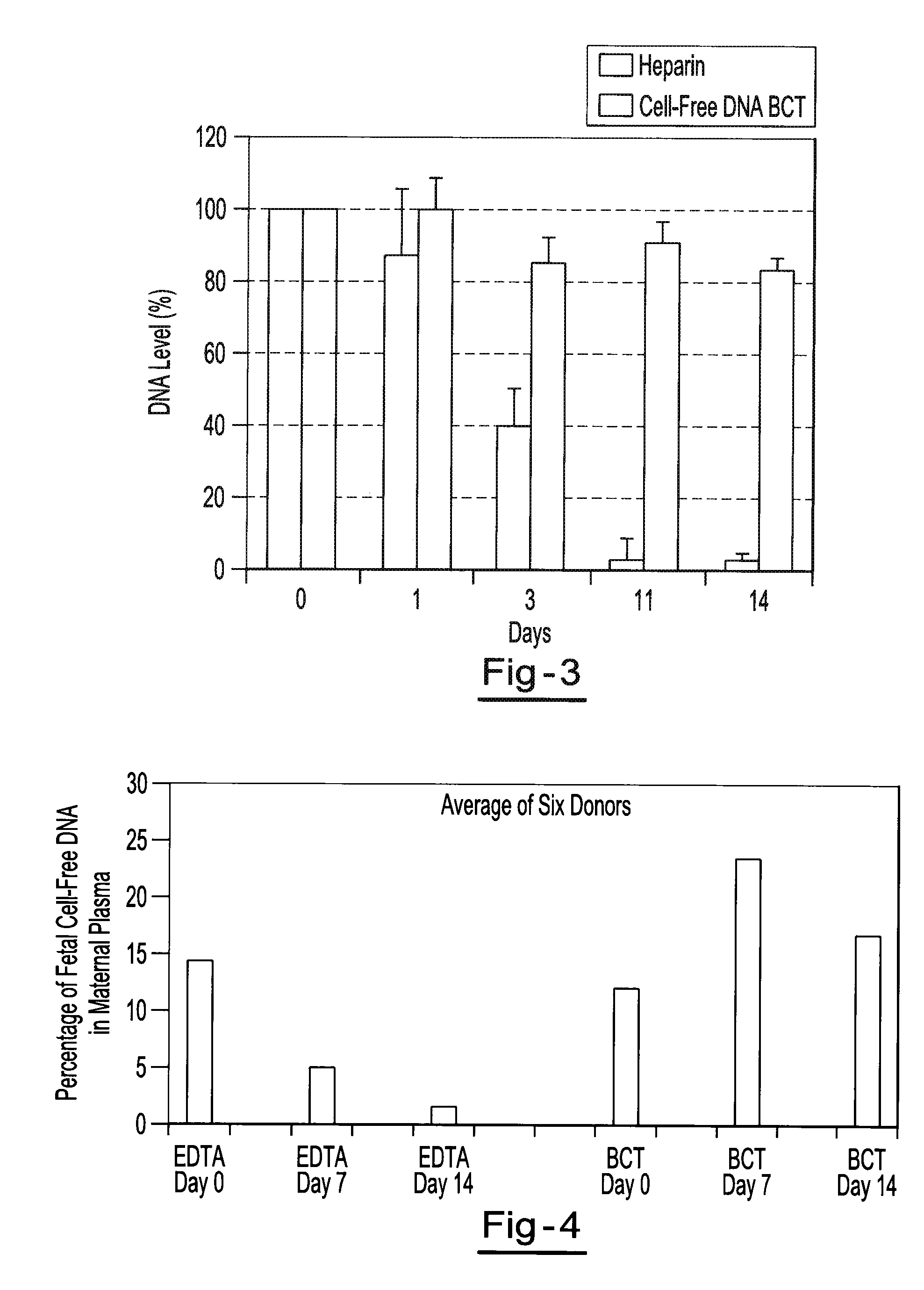
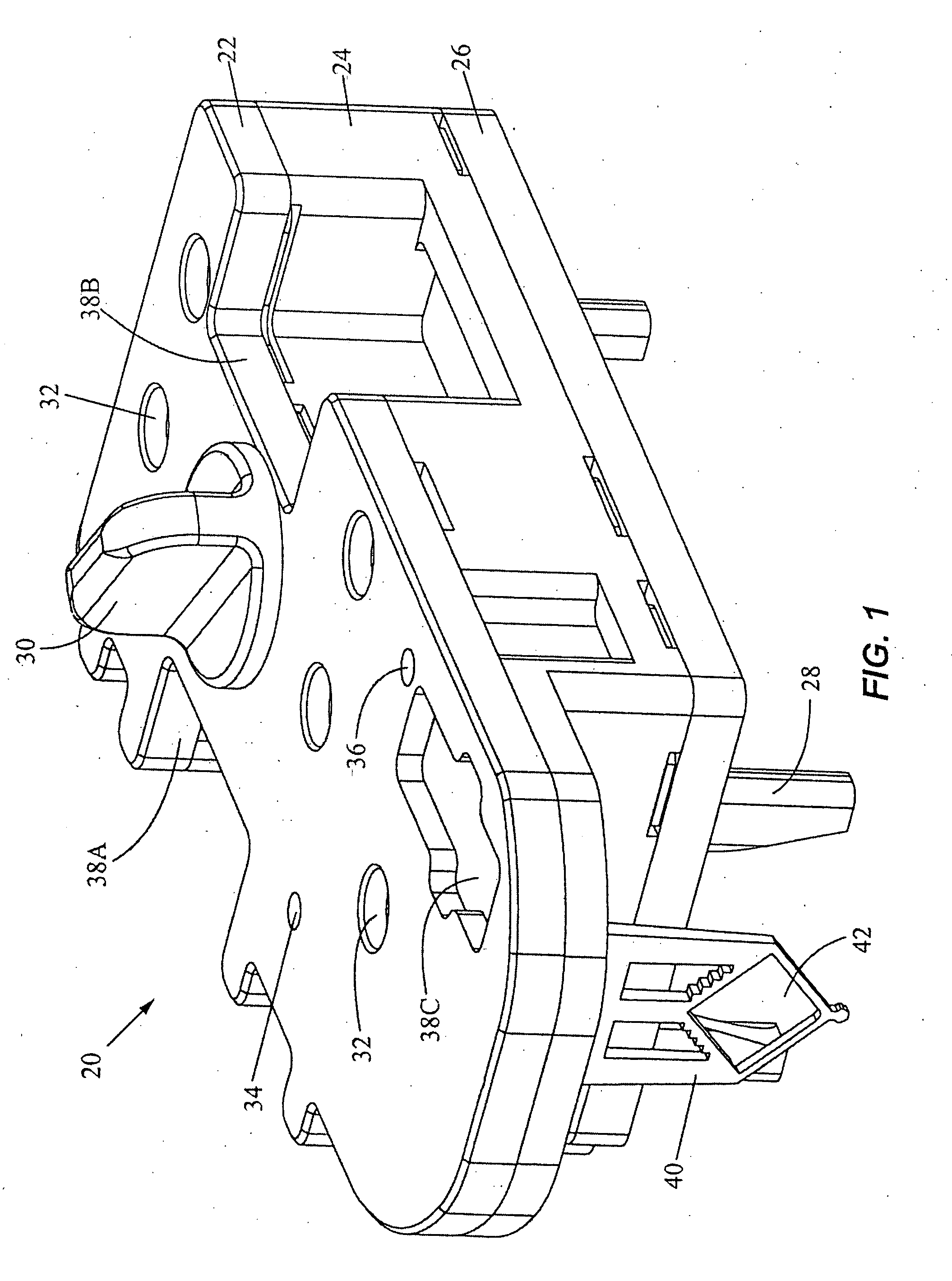
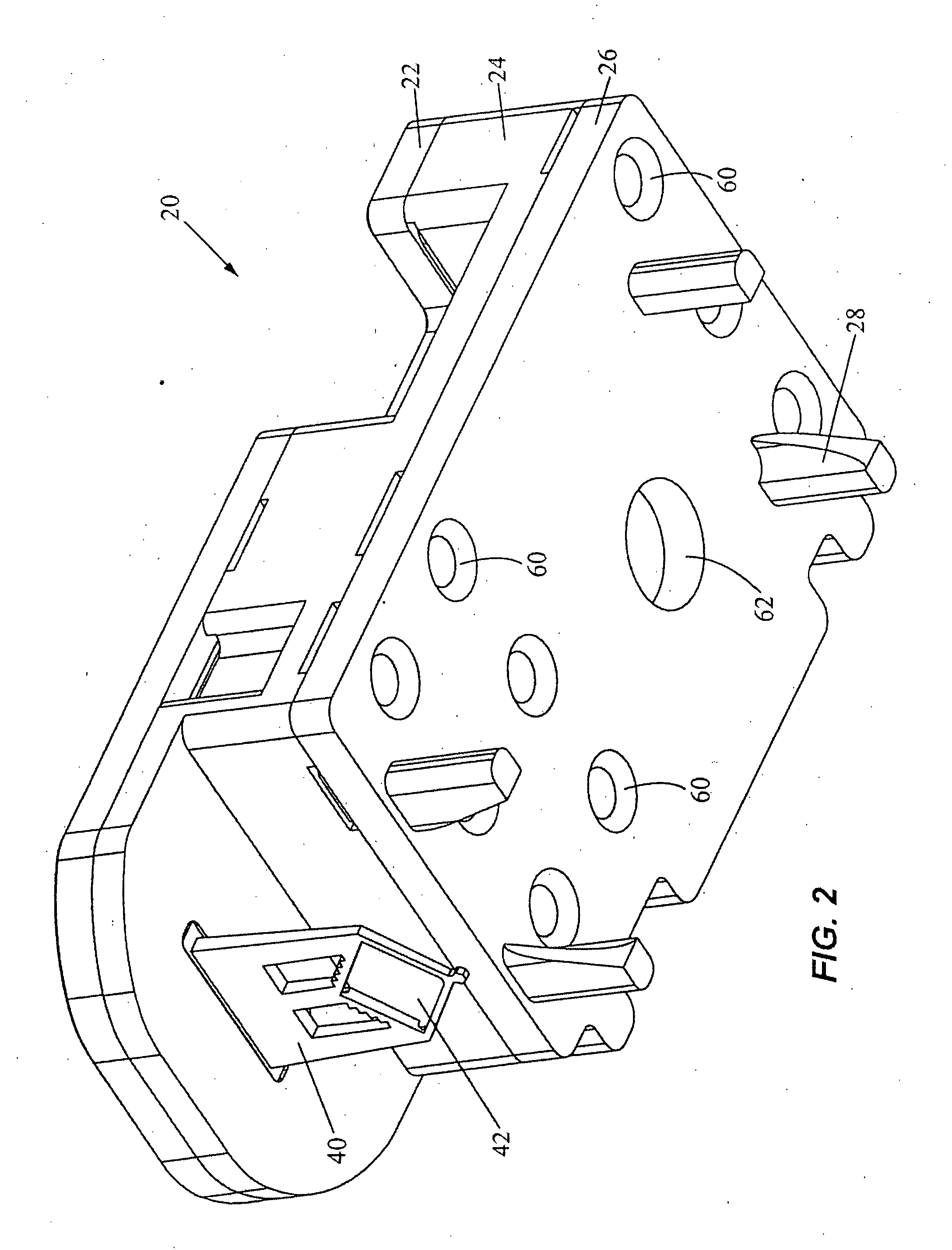
Preservation of fetal nucleic acids in maternal plasma
ActiveUS20100184069A1Maintain structural integrityInhibition releaseMicrobiological testing/measurementRibonucleaseLysis
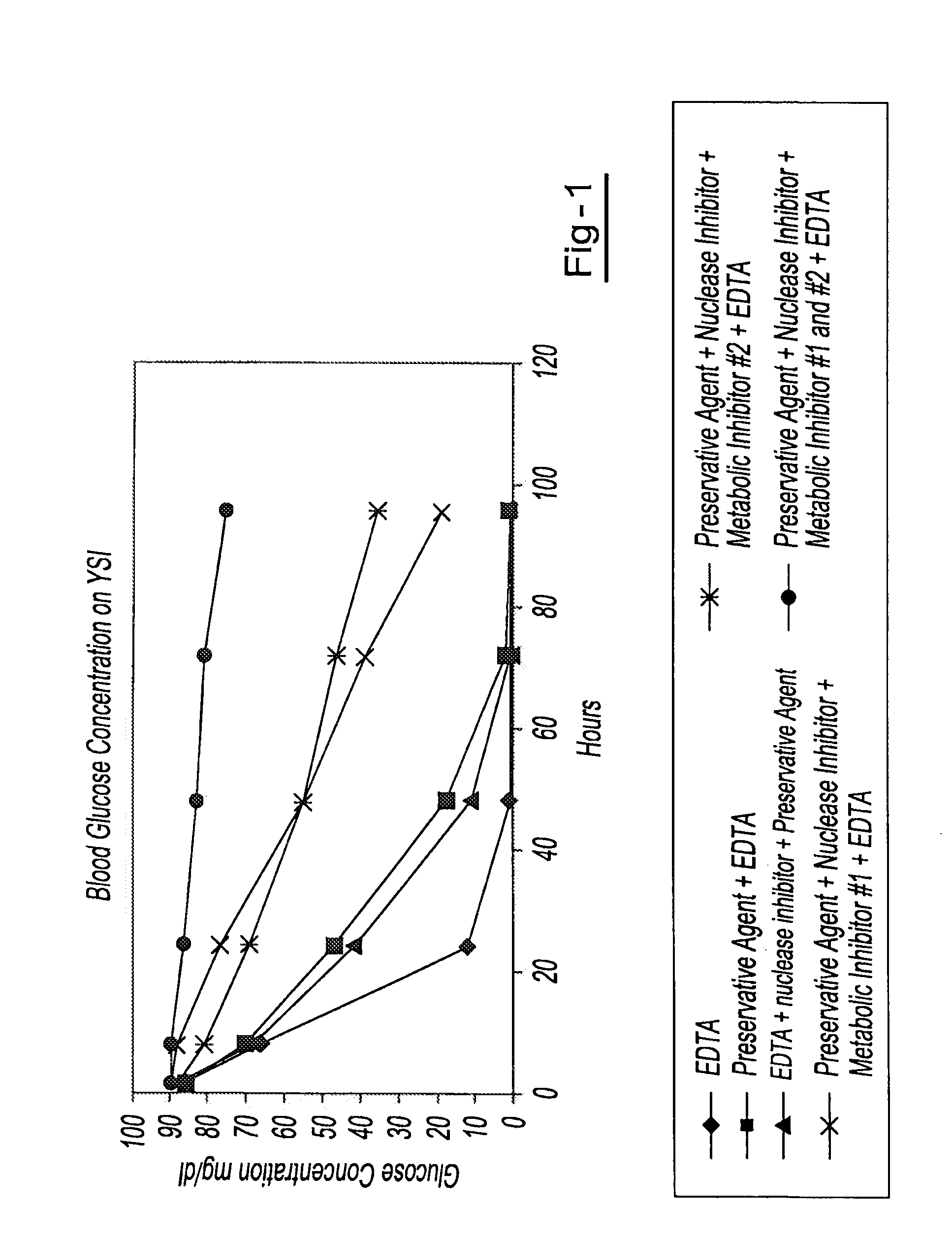
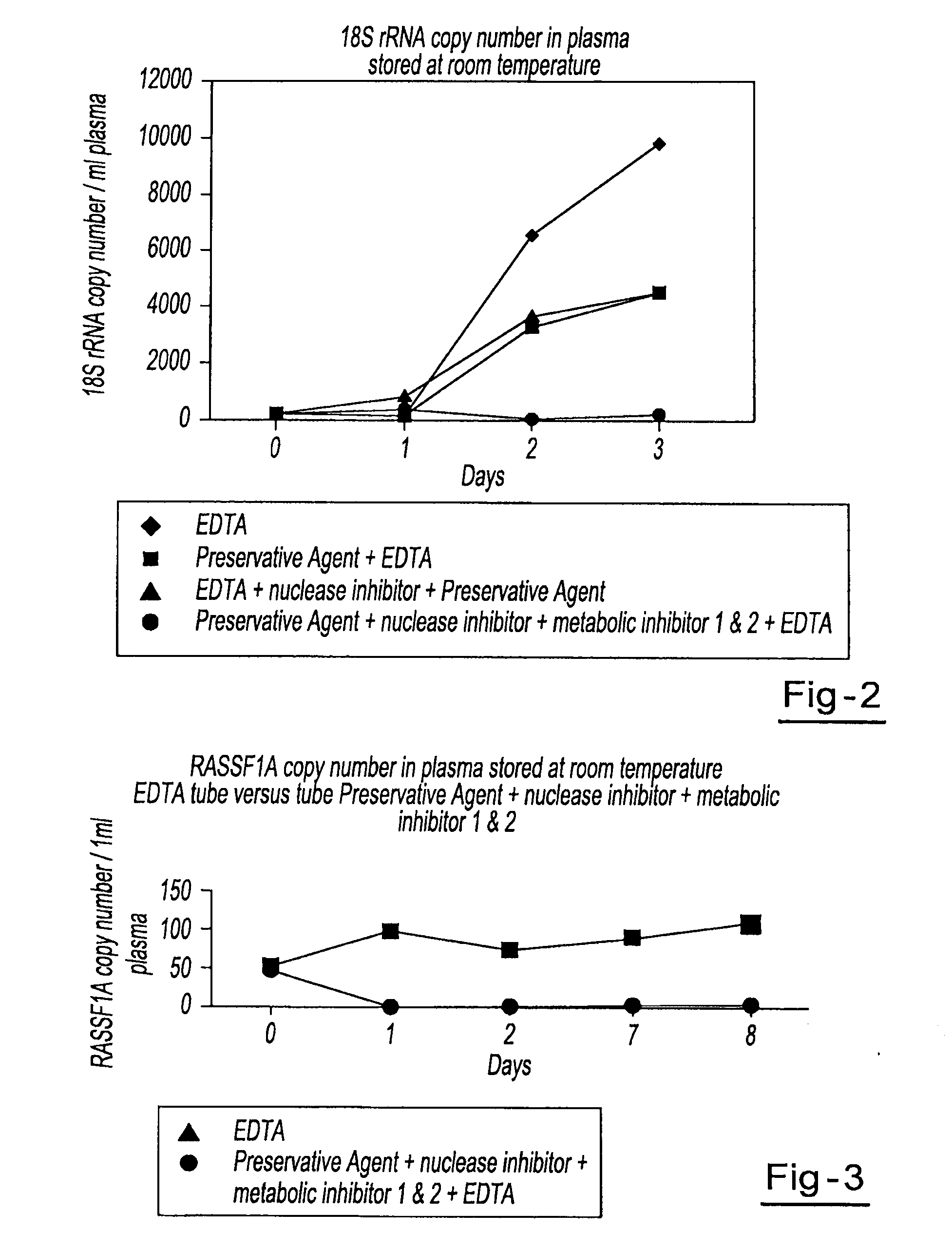
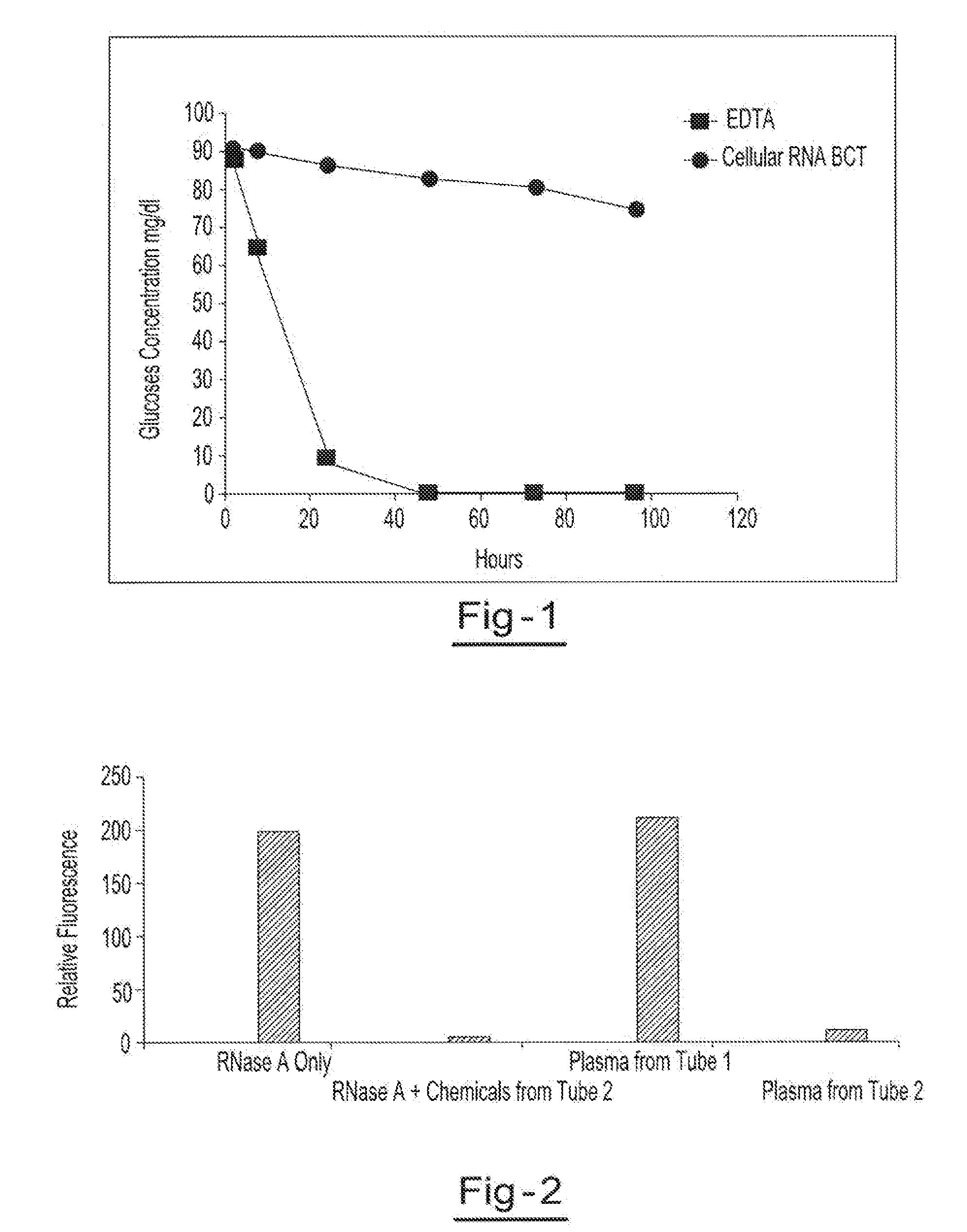
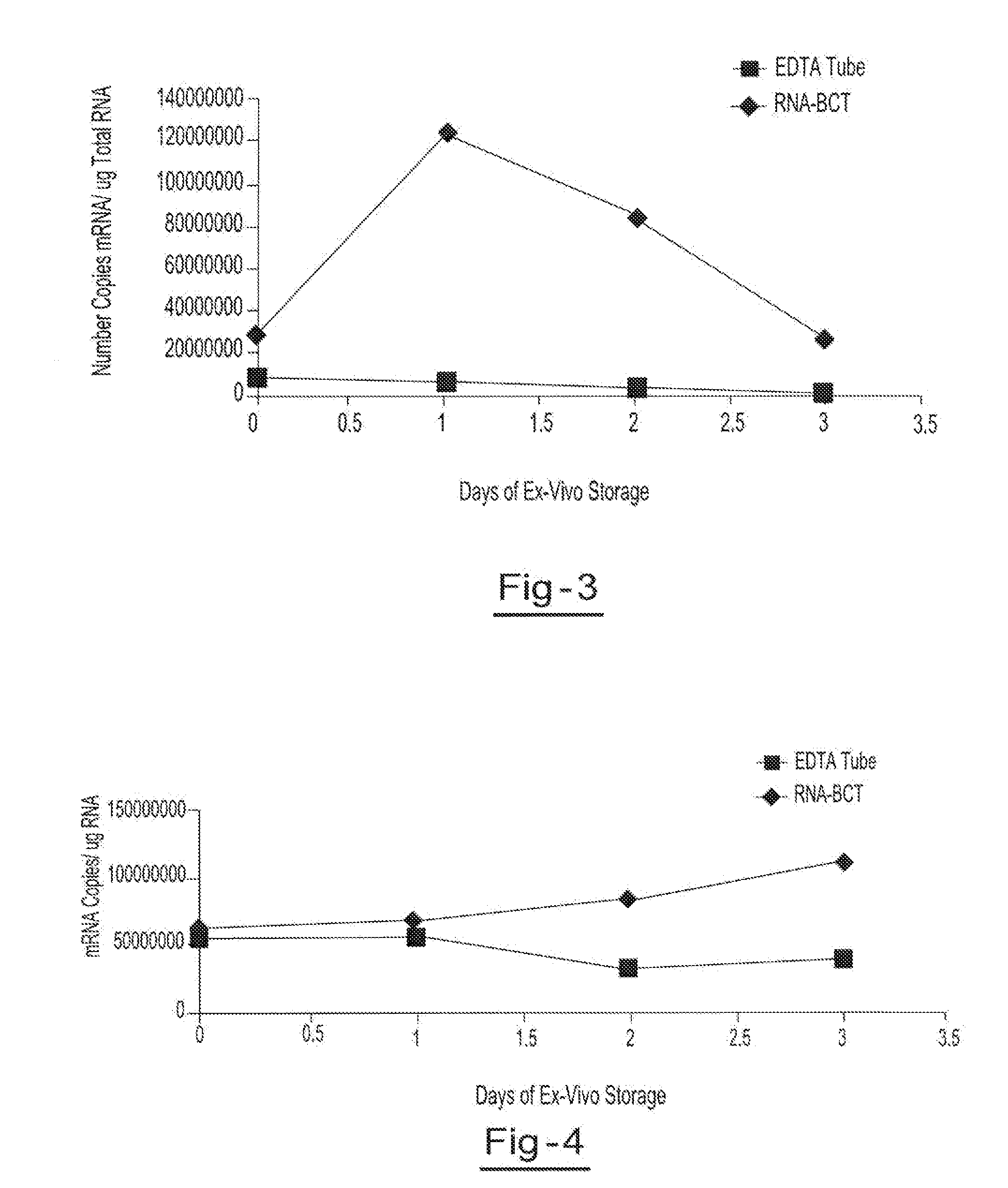
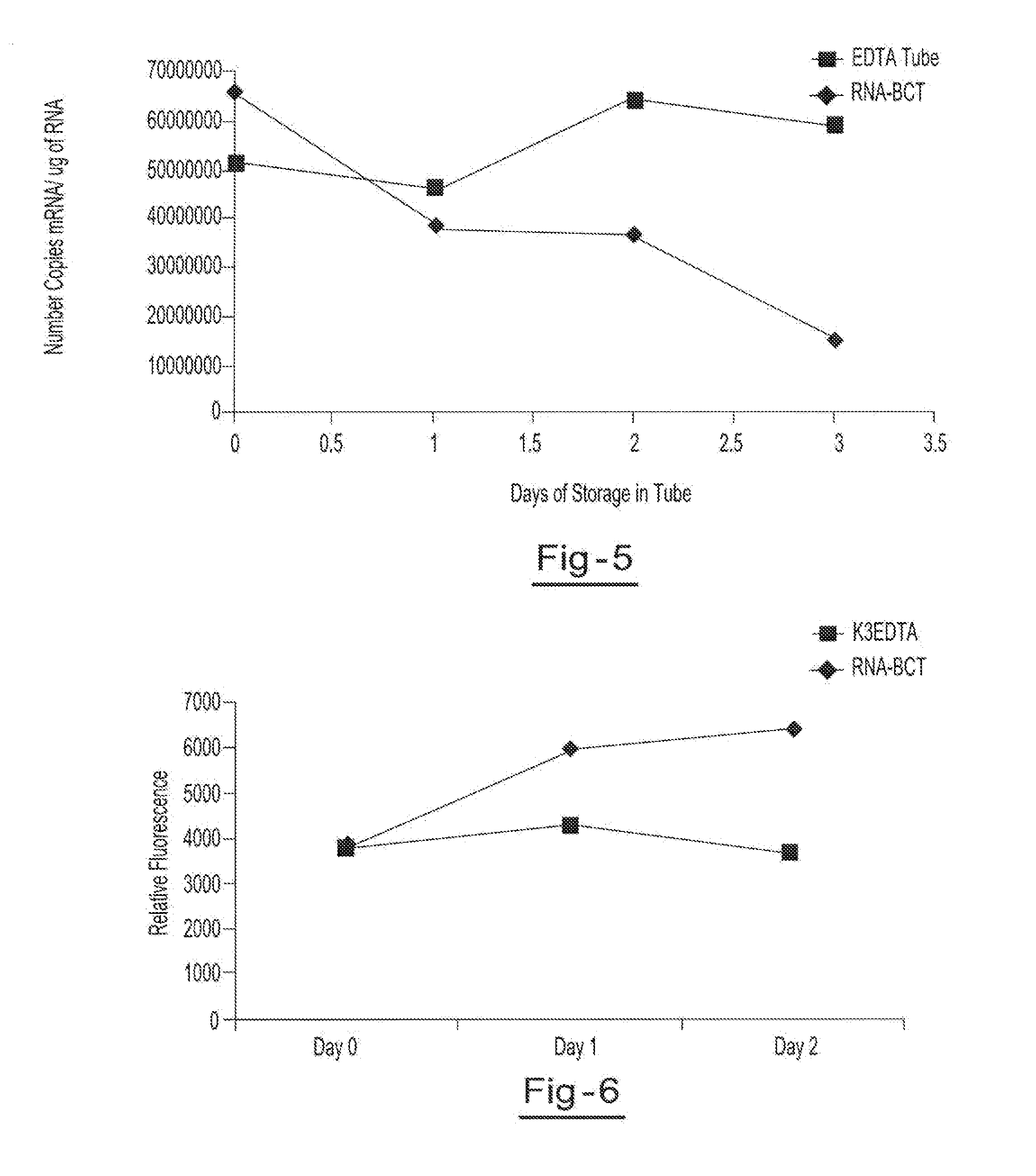
A method for preserving and processing fetal nucleic acids located within maternal plasma is disclosed, wherein a sample of maternal blood containing fetal nucleic acids is treated to reduce both cell lysis of the maternal blood cells and deoxyribonuclease (DNase) and ribonuclease (RNase) activity within the fetal nucleic acids. The treatment of the sample aids in increasing the amount of fetal nucleic acids that can be identified and tested while maintaining the structure and integrity of the fetal nucleic acids.
Owner:STRECK LLC
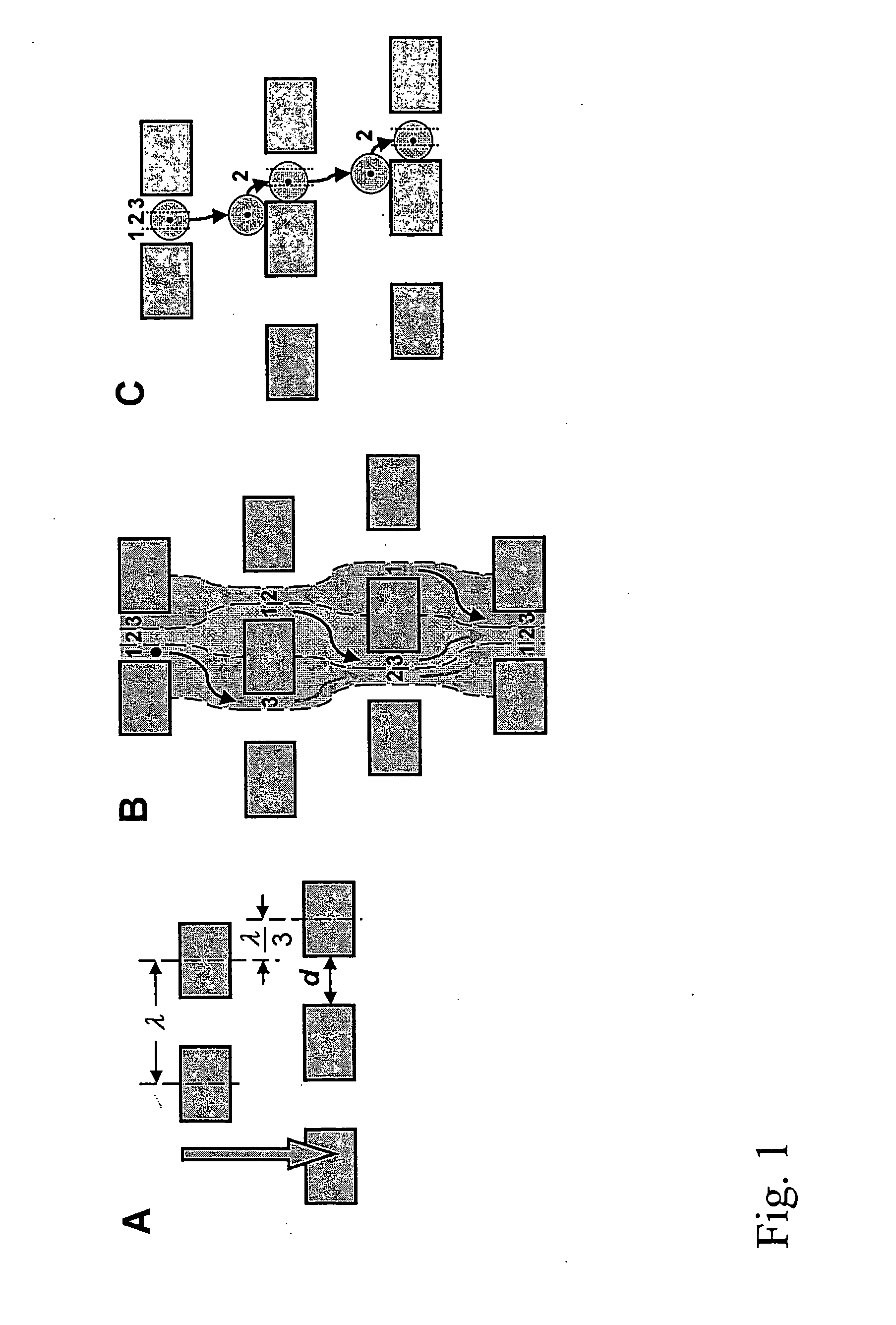
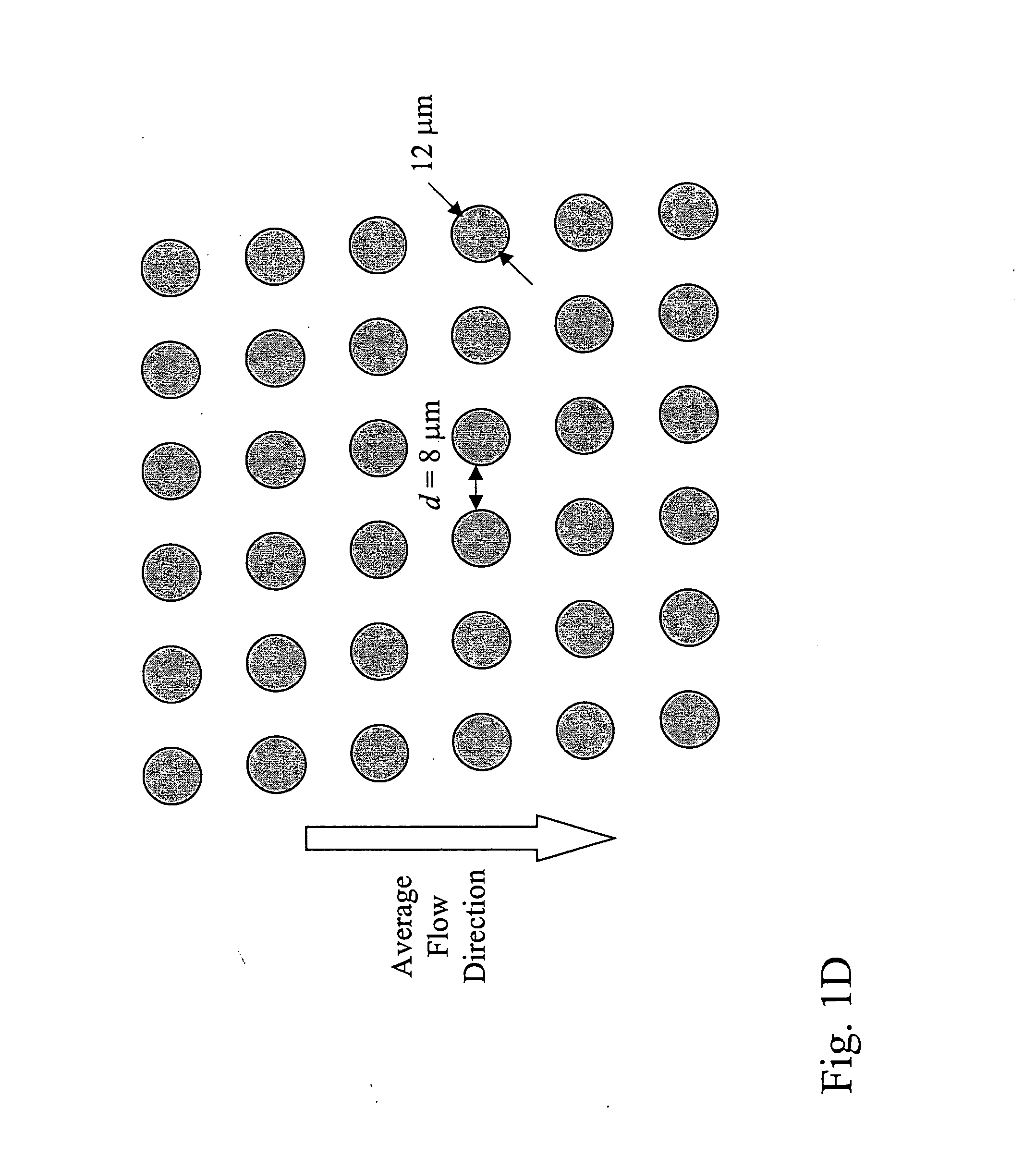
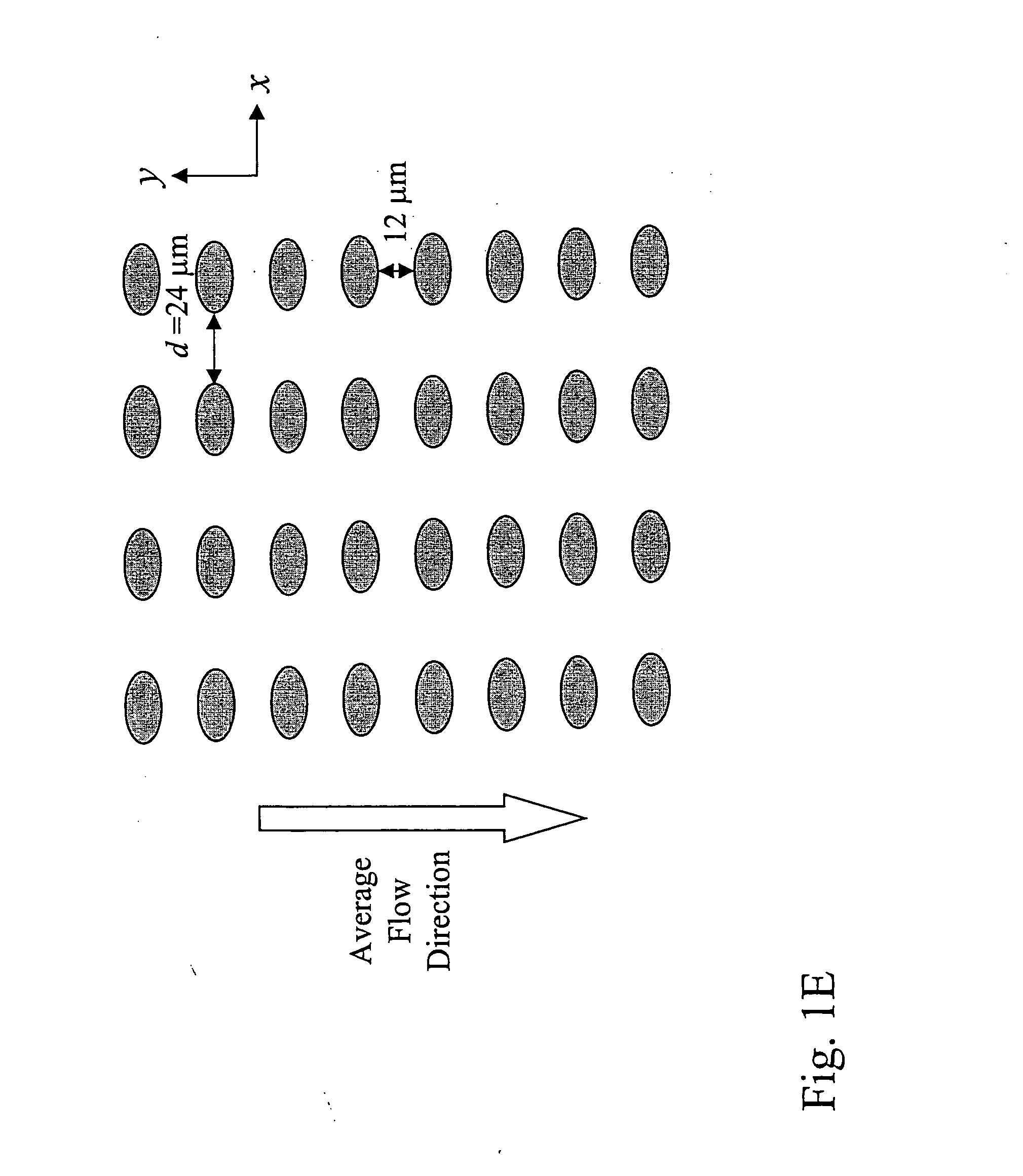
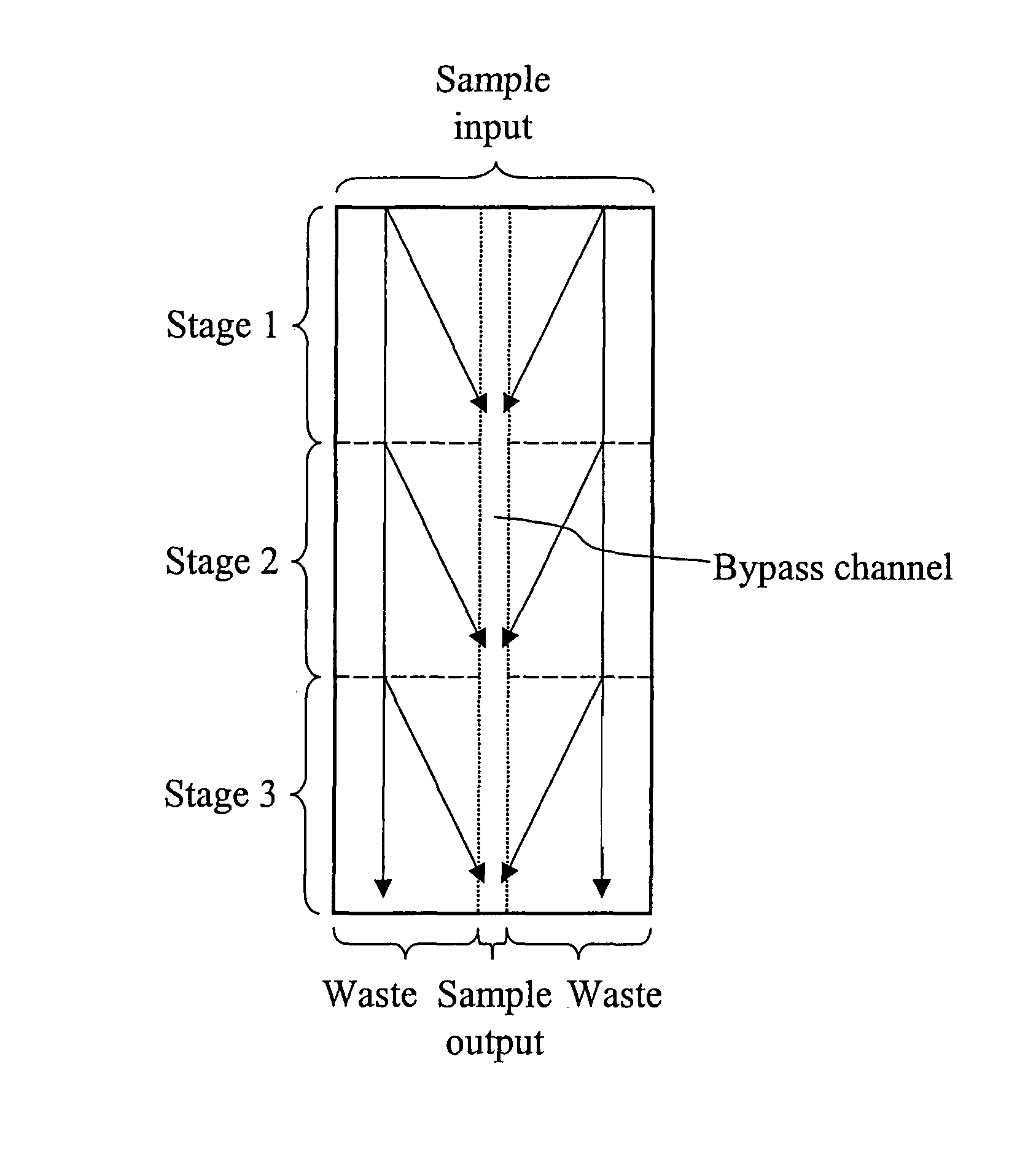
Devices and methods for enrichment and alteration of cells and other particles
ActiveUS20070026381A1Increase volumeReduced deformabilityBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentLysis
The invention features devices and methods for the deterministic separation of particles. Exemplary methods include the enrichment of a sample in a desired particle or the alteration of a desired particle in the device. The devices and methods are advantageously employed to enrich for rare cells, e.g., fetal cells, present in a sample, e.g., maternal blood and rare cell components, e.g., fetal cell nuclei. The invention further provides a method for preferentially lysing cells of interest in a sample, e.g., to extract clinical information from a cellular component, e.g., a nucleus, of the cells of interest. In general, the method employs differential lysis between the cells of interest and other cells (e.g., other nucleated cells) in the sample.
Owner:THE GENERAL HOSPITAL CORP +1
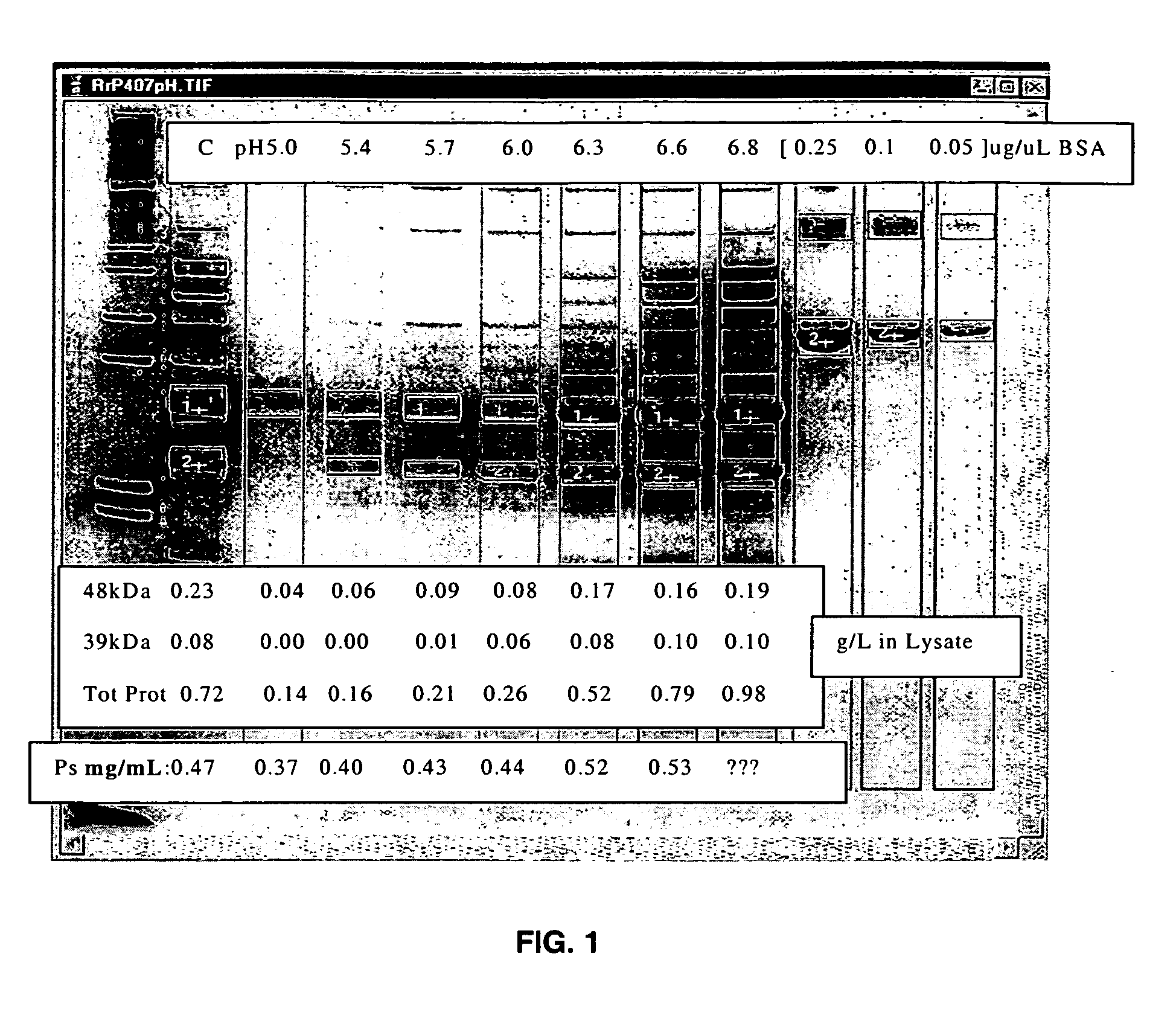
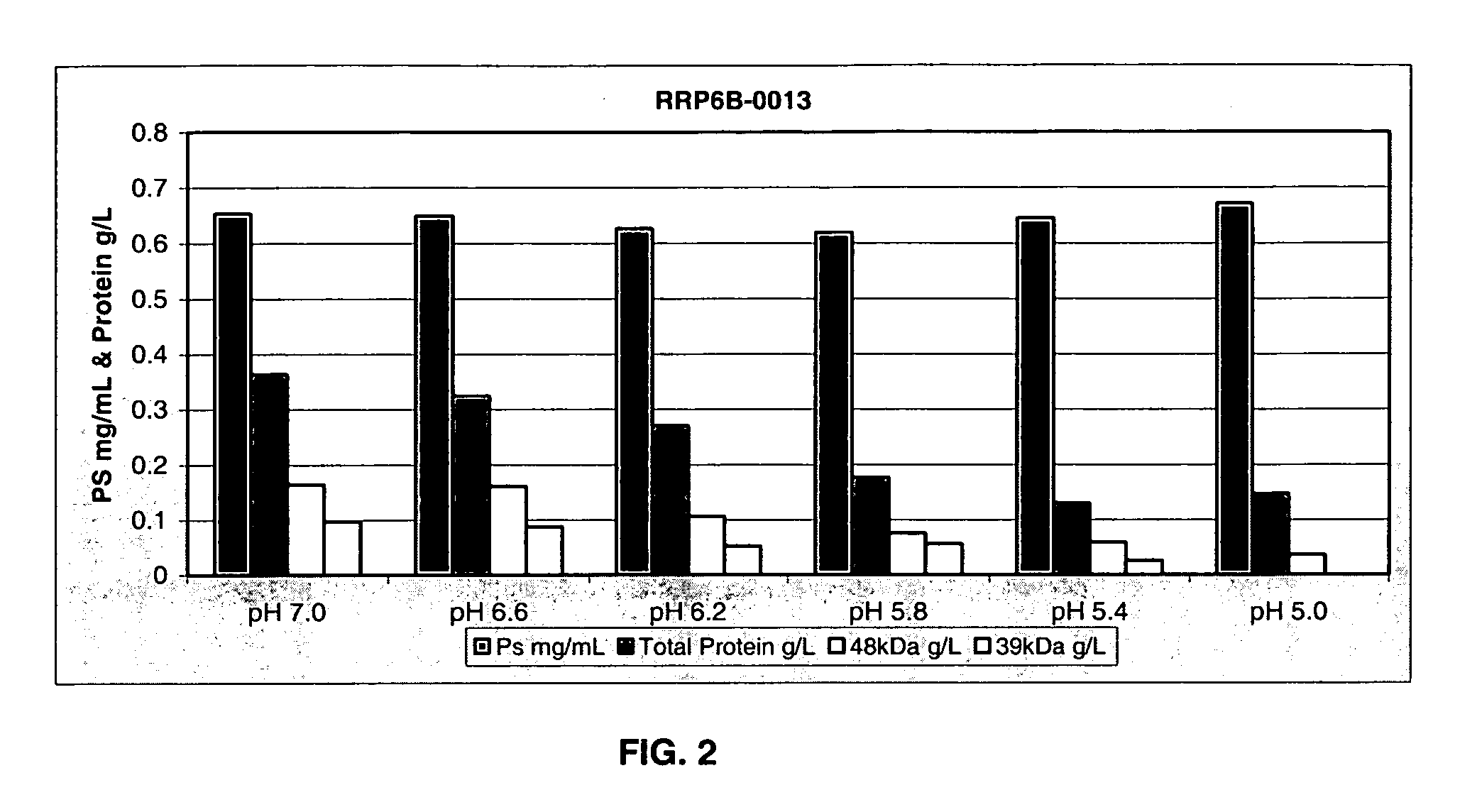
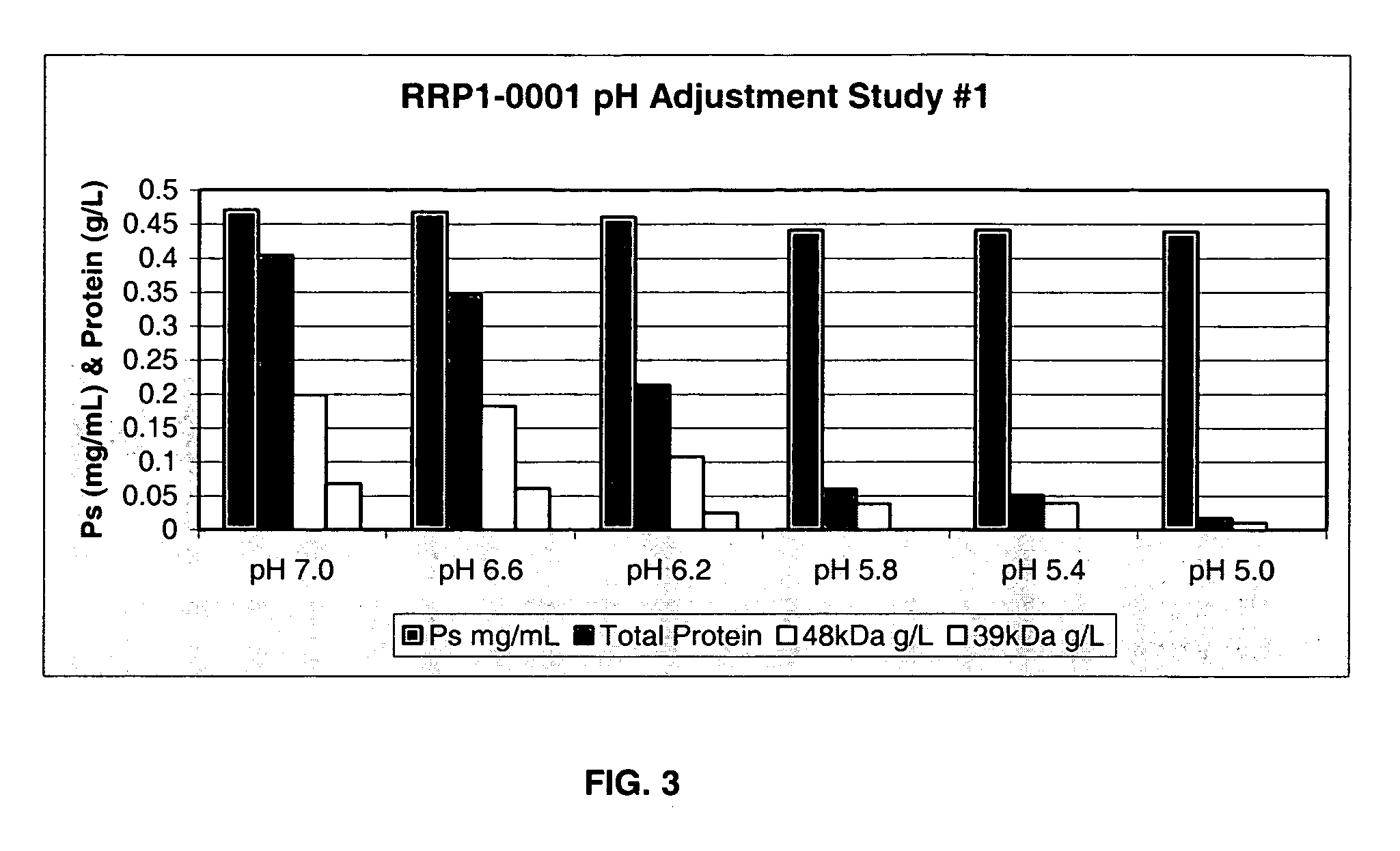
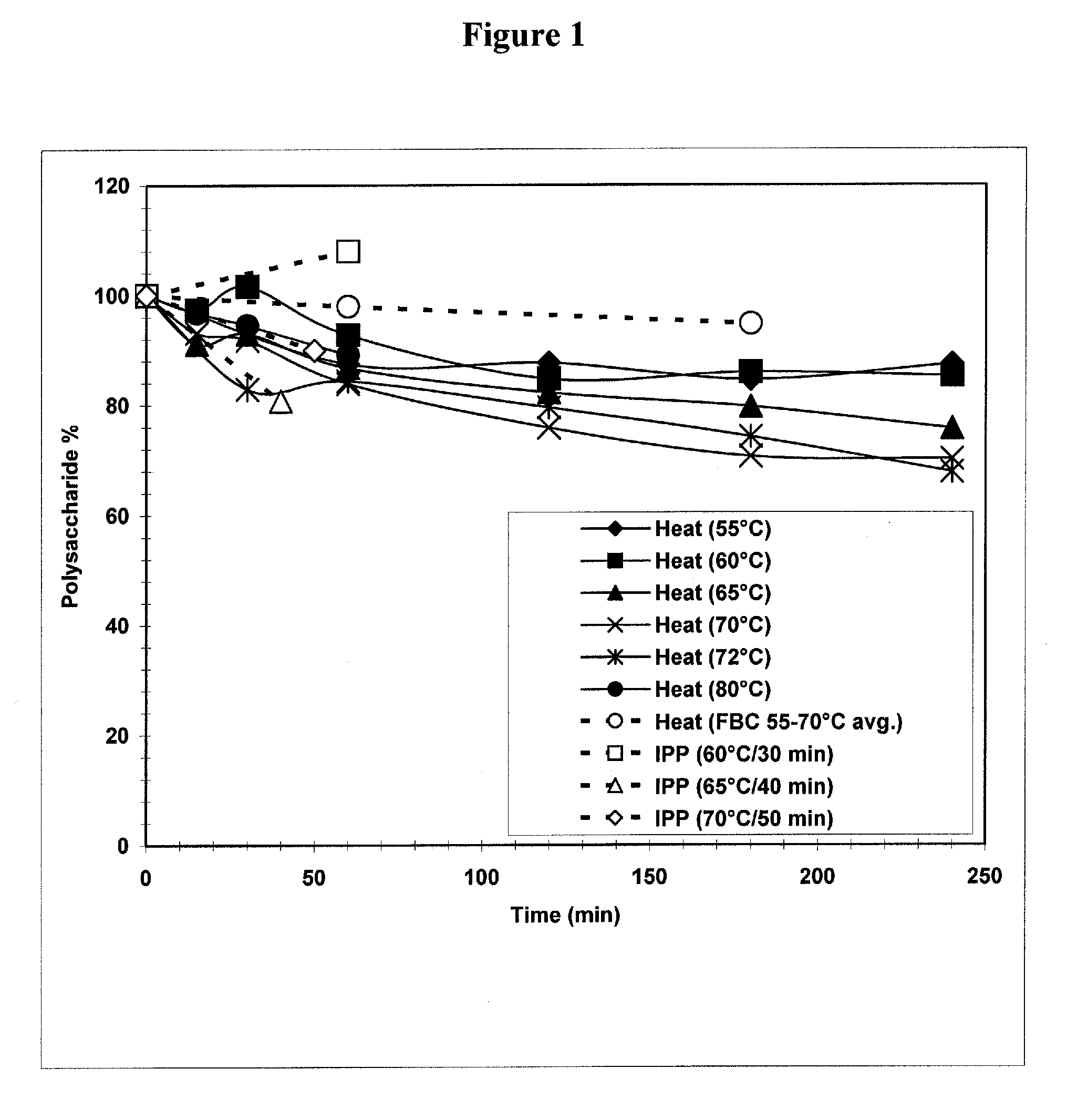
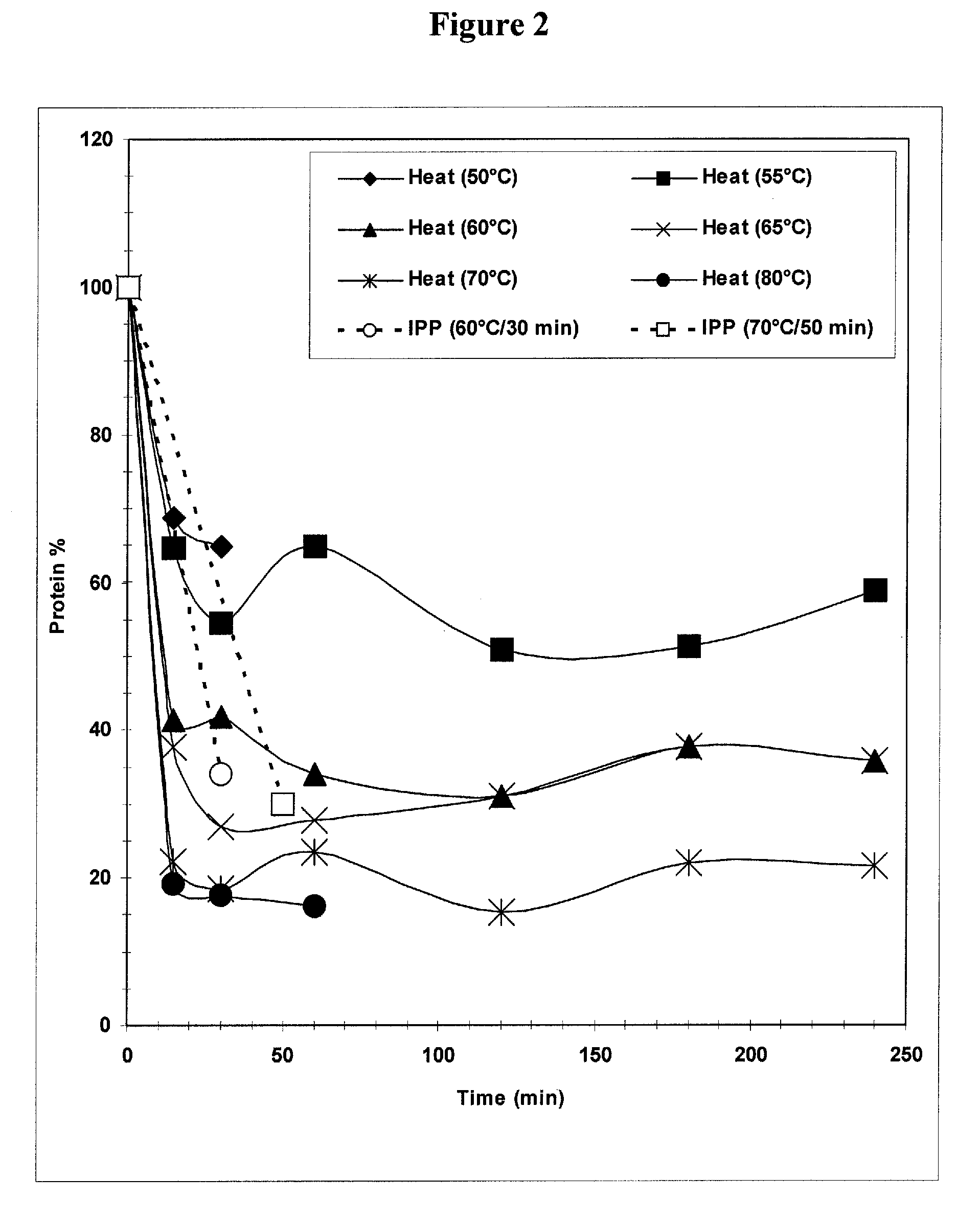
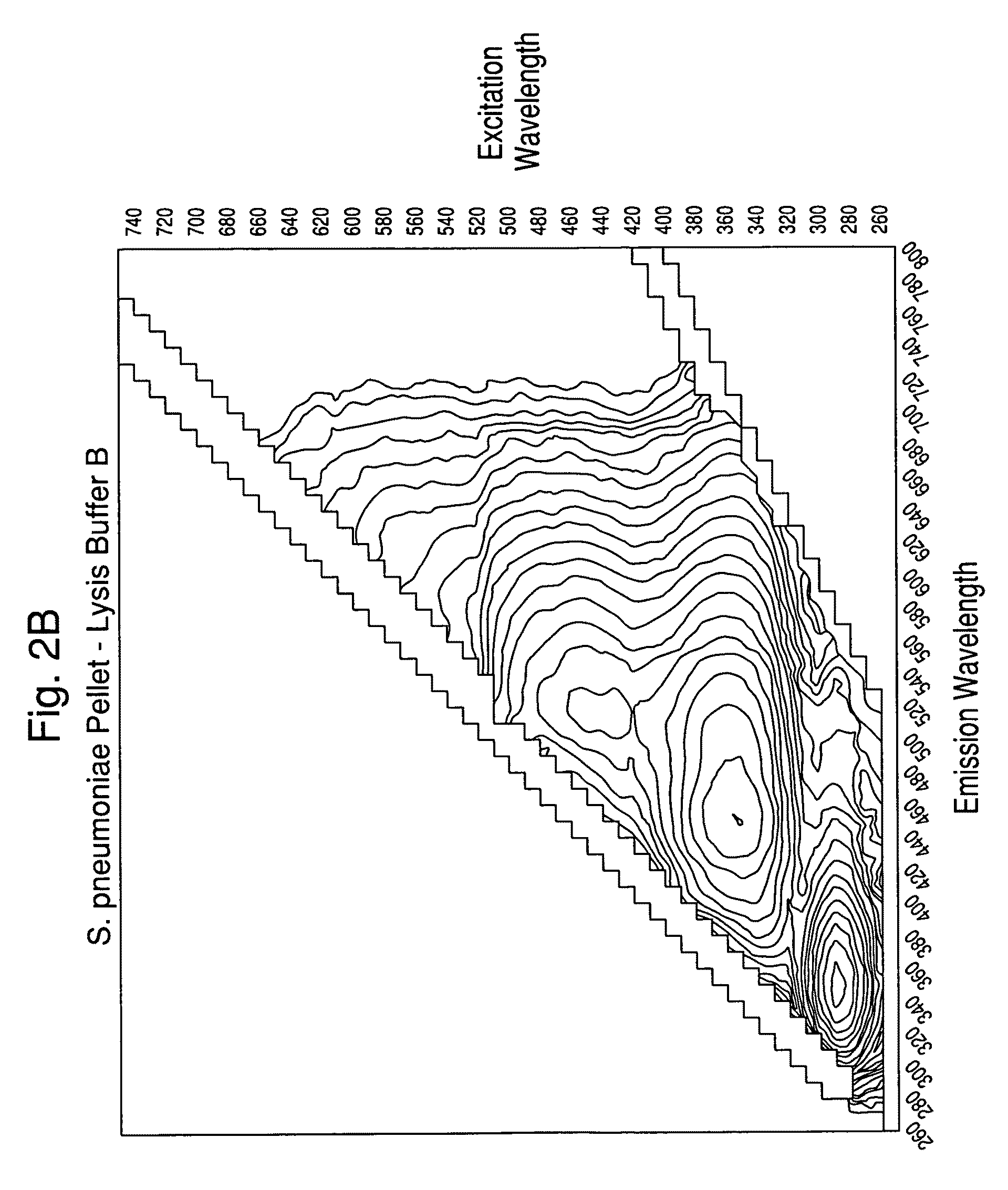
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
ActiveUS20060228381A1Soluble protein is effectively reducedBacterial antigen ingredientsSugar derivativesStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
Devices and methods for enrichment and alteration of cells and other particles
ActiveUS8021614B2Increases the hydrodynamic radius of a particleIncrease volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentClinical information
The invention features devices and methods for the deterministic separation of particles. Exemplary methods include the enrichment of a sample in a desired particle or the alteration of a desired particle in the device. The devices and methods are advantageously employed to enrich for rare cells, e.g., fetal cells, present in a sample, e.g., maternal blood and rare cell components, e.g., fetal cell nuclei. The invention further provides a method for preferentially lysing cells of interest in a sample, e.g., to extract clinical information from a cellular component, e.g., a nucleus, of the cells of interest. In general, the method employs differential lysis between the cells of interest and other cells (e.g., other nucleated cells) in the sample.
Owner:THE GENERAL HOSPITAL CORP +1
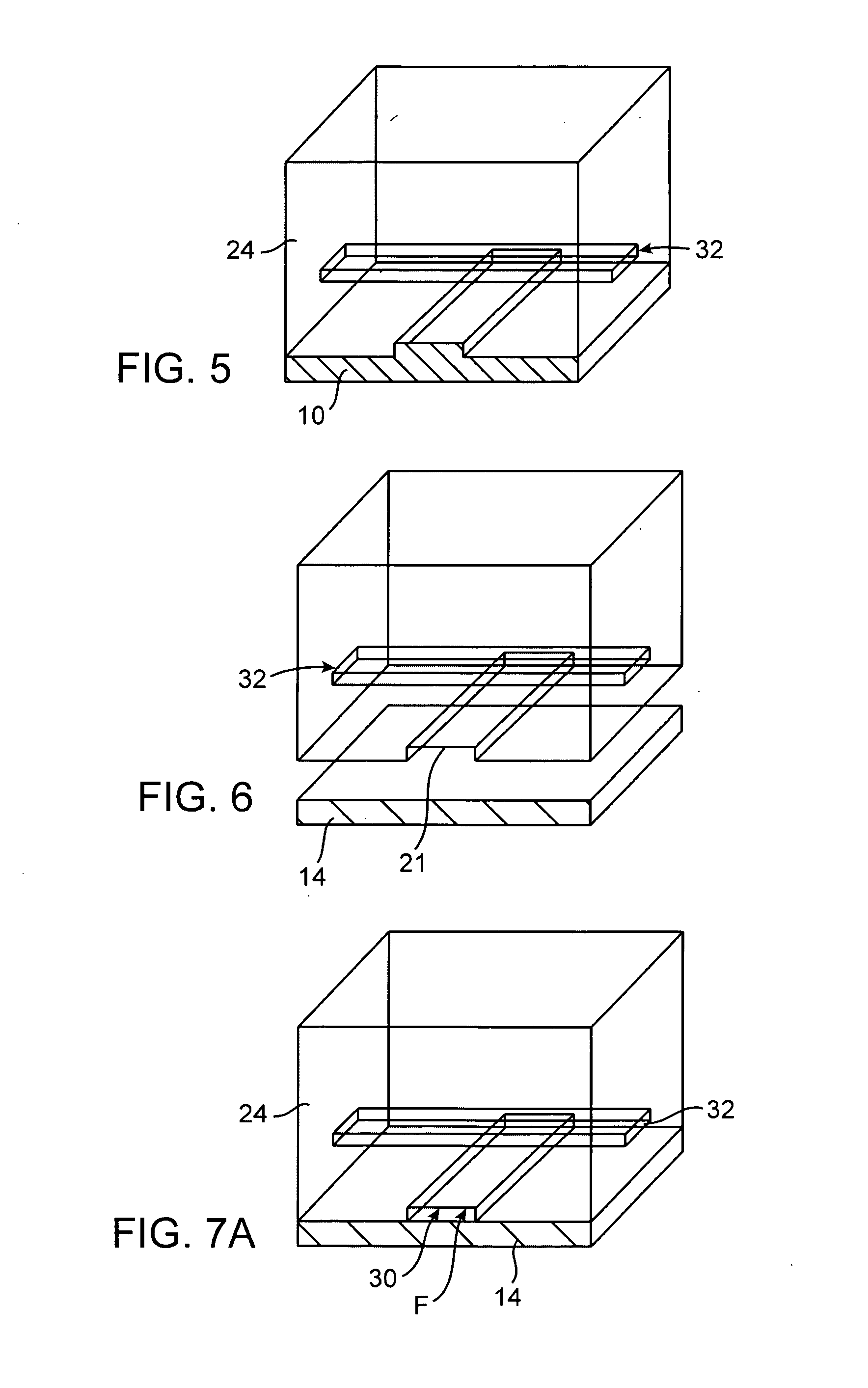
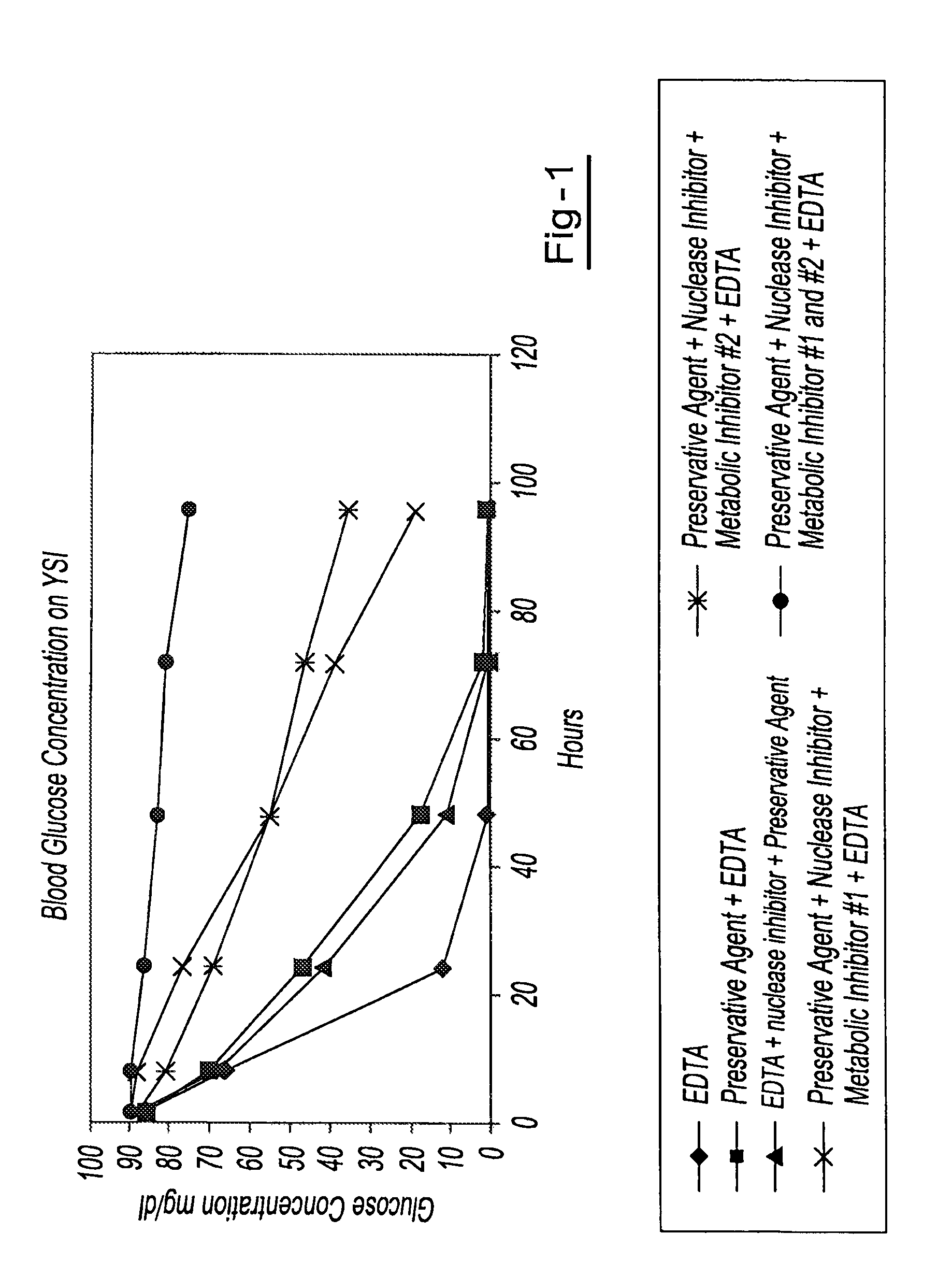
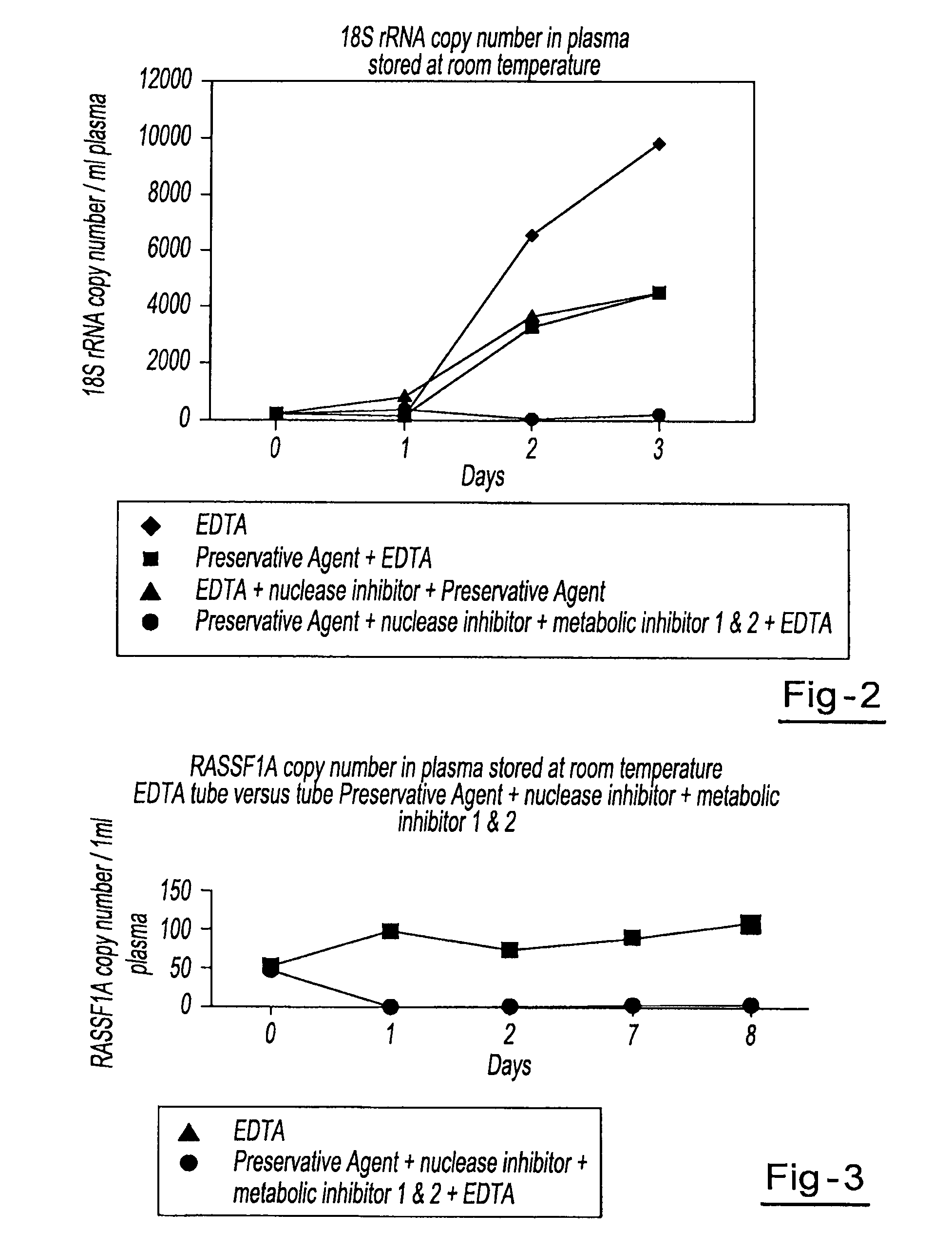
Preservation of cell-free nucleic acids
ActiveUS20100209930A1Prevent leakageInhibit synthesisMicrobiological testing/measurementChemical inhibitorsLysisNuclease
A method for preserving and processing cell-free nucleic acids located within a blood sample is disclosed, wherein a blood sample containing cell-free nucleic acids is treated to reduce both blood cell lysis and nuclease activity within the blood sample. The treatment of the sample aids in increasing the amount of cell-free nucleic acids that can be identified and tested while maintaining the structure and integrity of the nucleic acids.
Owner:STRECK LLC
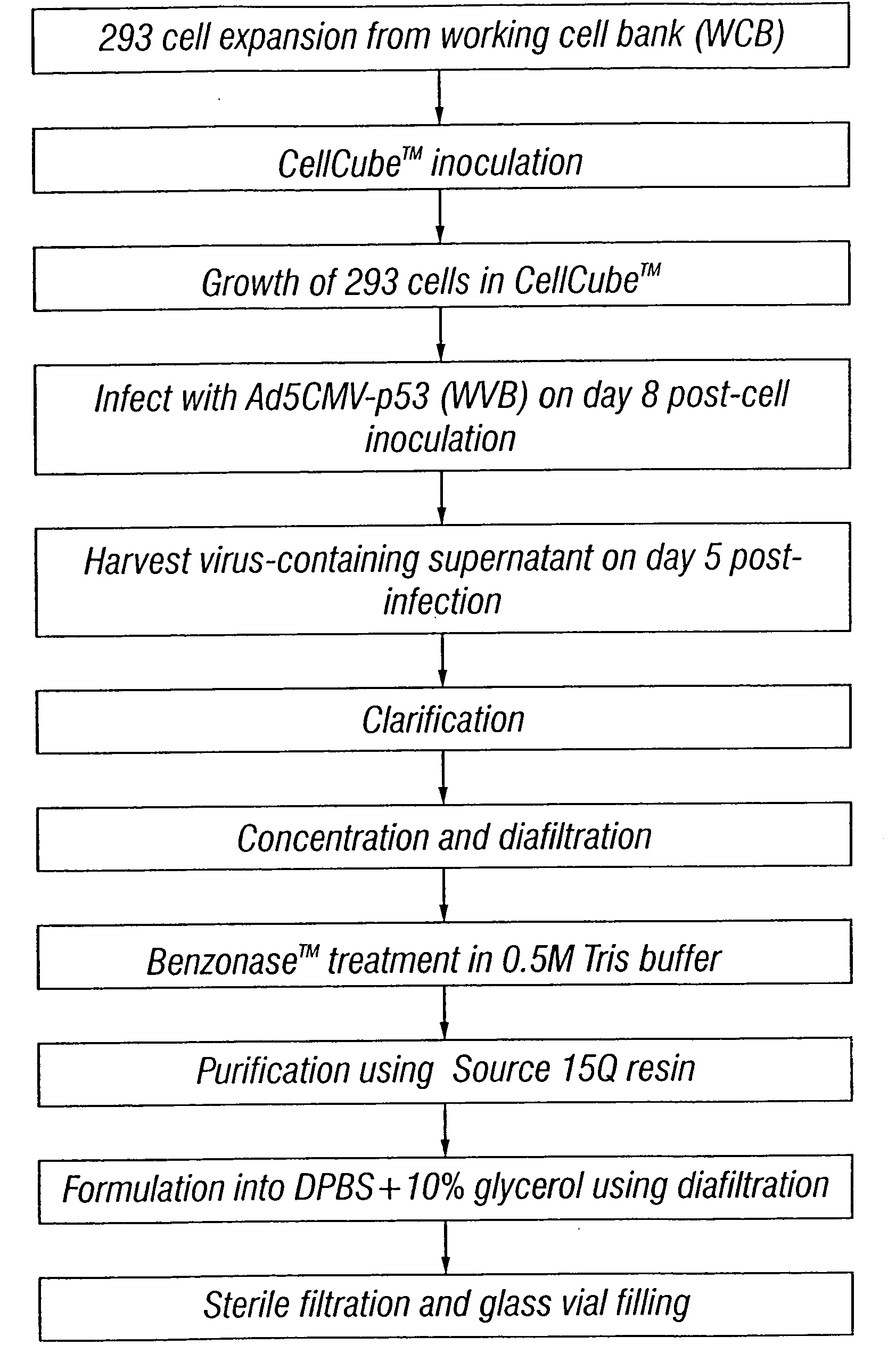
Method for the production and purification of adenoviral vectors
InactiveUS20080050770A1Peptide/protein ingredientsGenetic material ingredientsSerum igeUltracentrifuge
Owner:JANSSEN VACCINES & PREVENTION BV
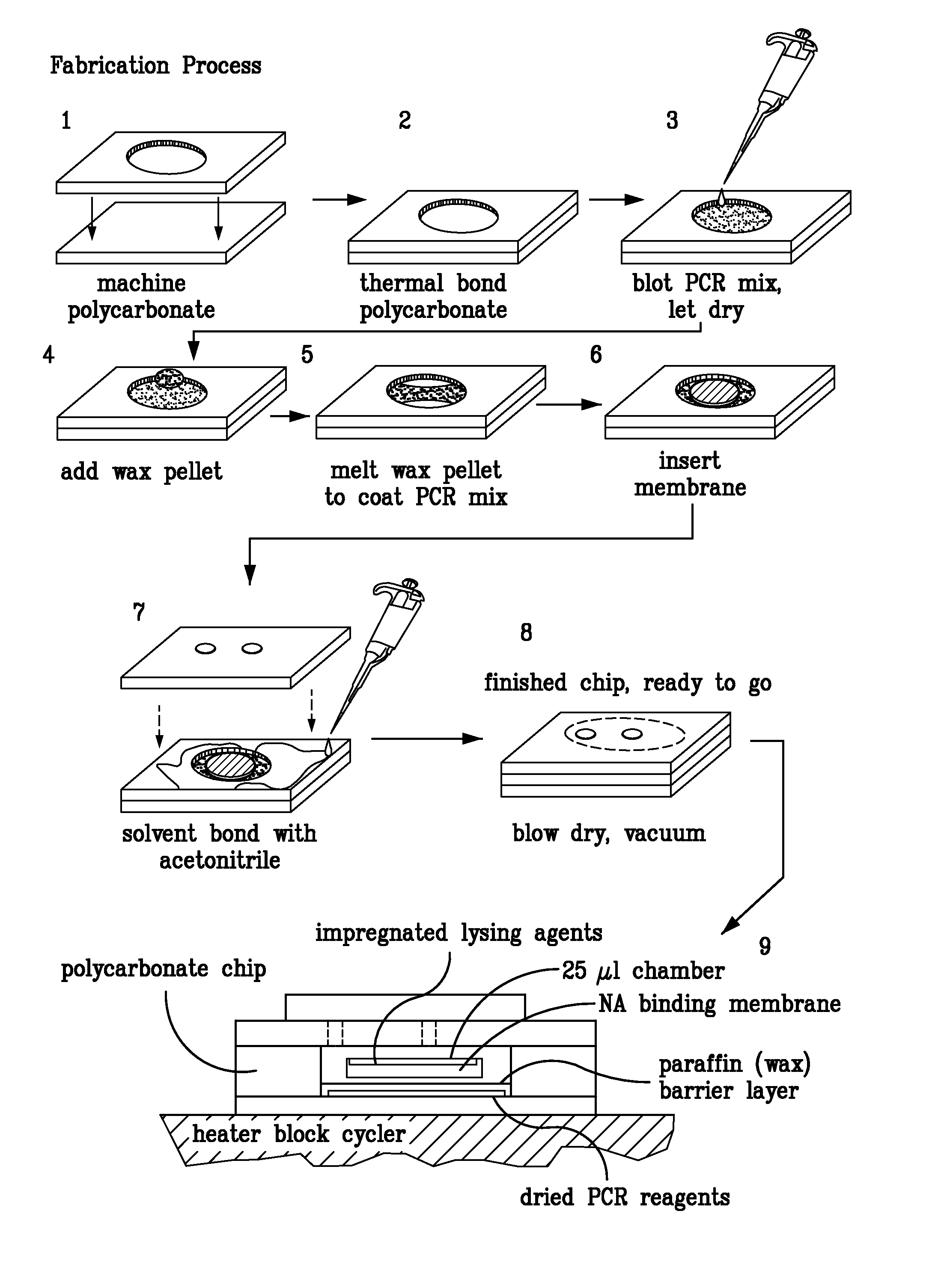
Multilayered microfluidic DNA analysis system and method
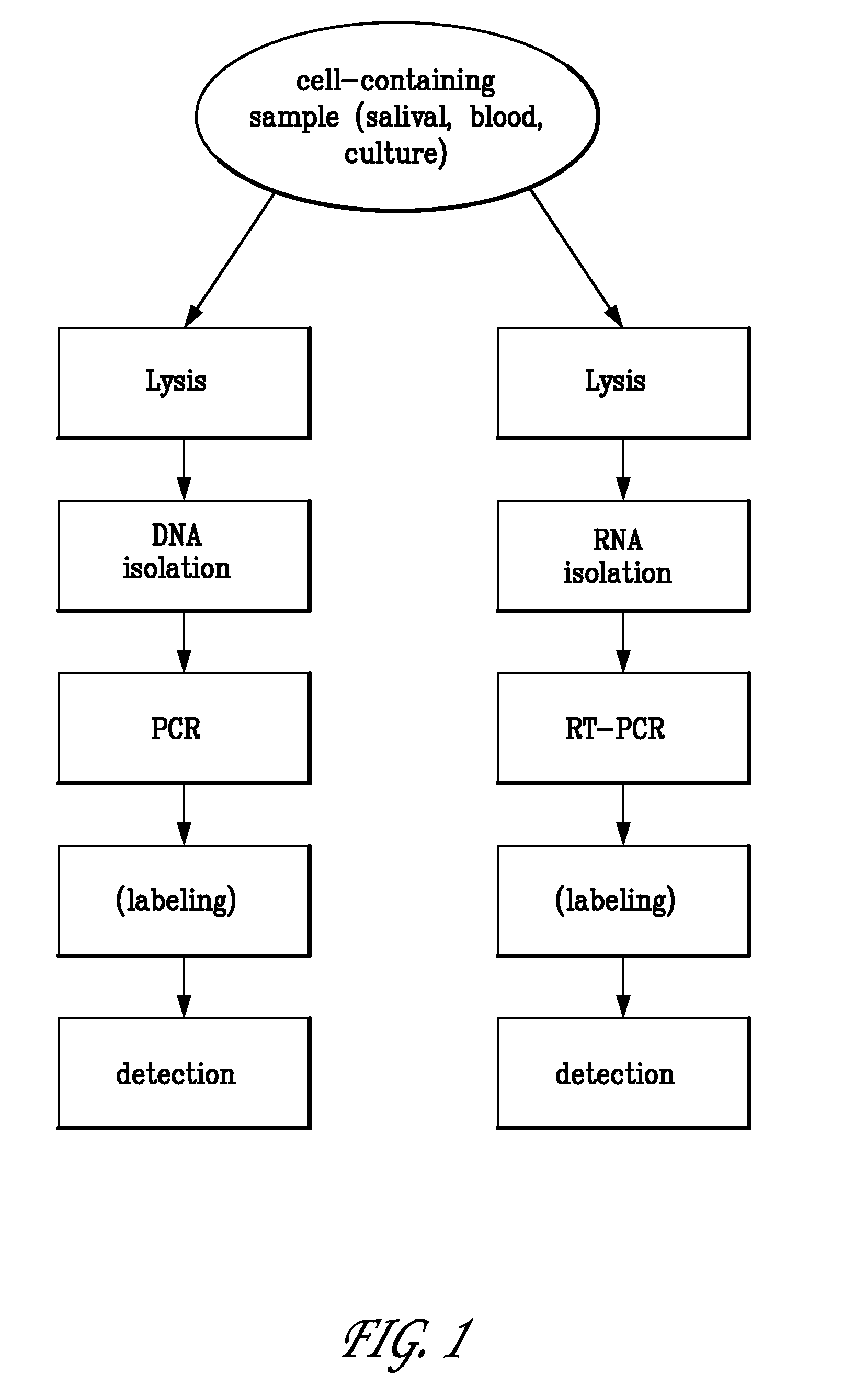
A multilayered microfluidic DNA analysis system includes a cell lysis chamber, a DNA separation chamber, a DNA amplification chamber, and a DNA detection system. The multilayered microfluidic DNA analysis system is provided as a substantially monolithic structure formed from a plurality of green-sheet layers sintered together. The substantially monolithic structure has defined therein a means for heating the DNA amplification chamber and a means for cooling the DNA amplification chamber. The means for heating and means for cooling operate to cycle the temperature of the DNA amplification chamber as required for performing a DNA amplification process, such as PCR.
Owner:GOOGLE TECH HLDG LLC
Universal anti-tag chimeric antigen receptor-expressing T cells and methods of treating cancer
ActiveUS9233125B2Efficient productionPromote efficient proliferationBiocideAntibody mimetics/scaffoldsHuman cancerCancer cell
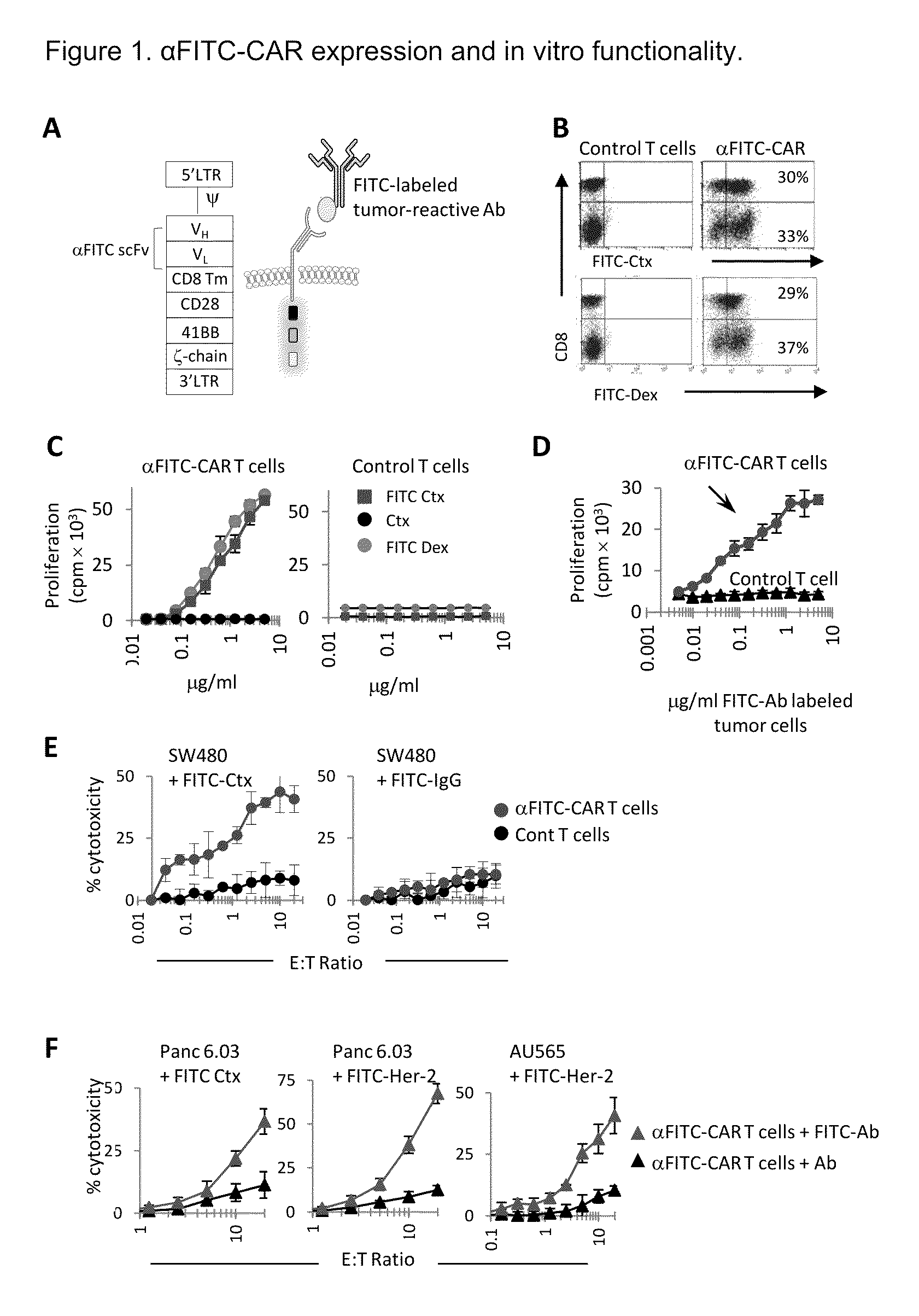
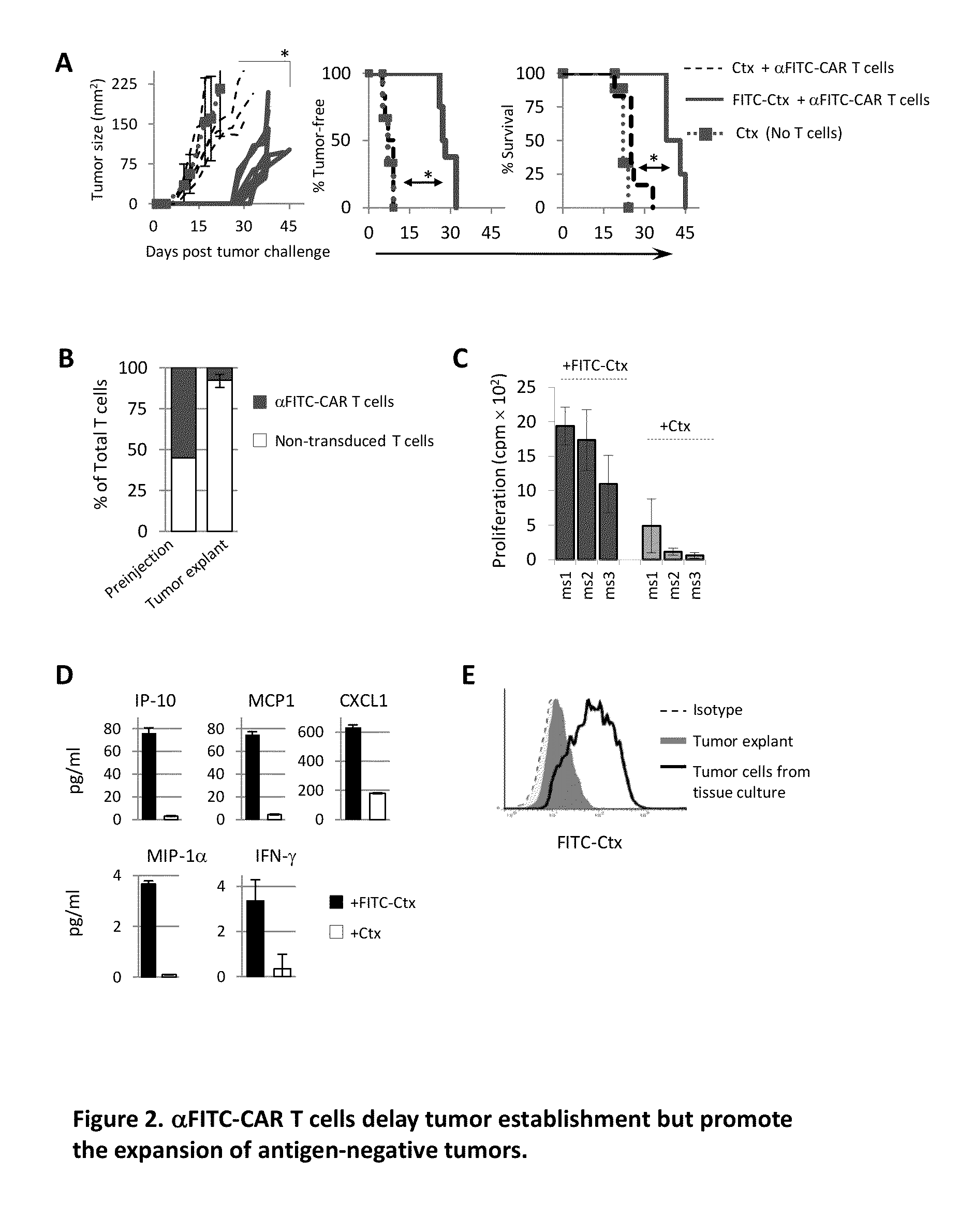
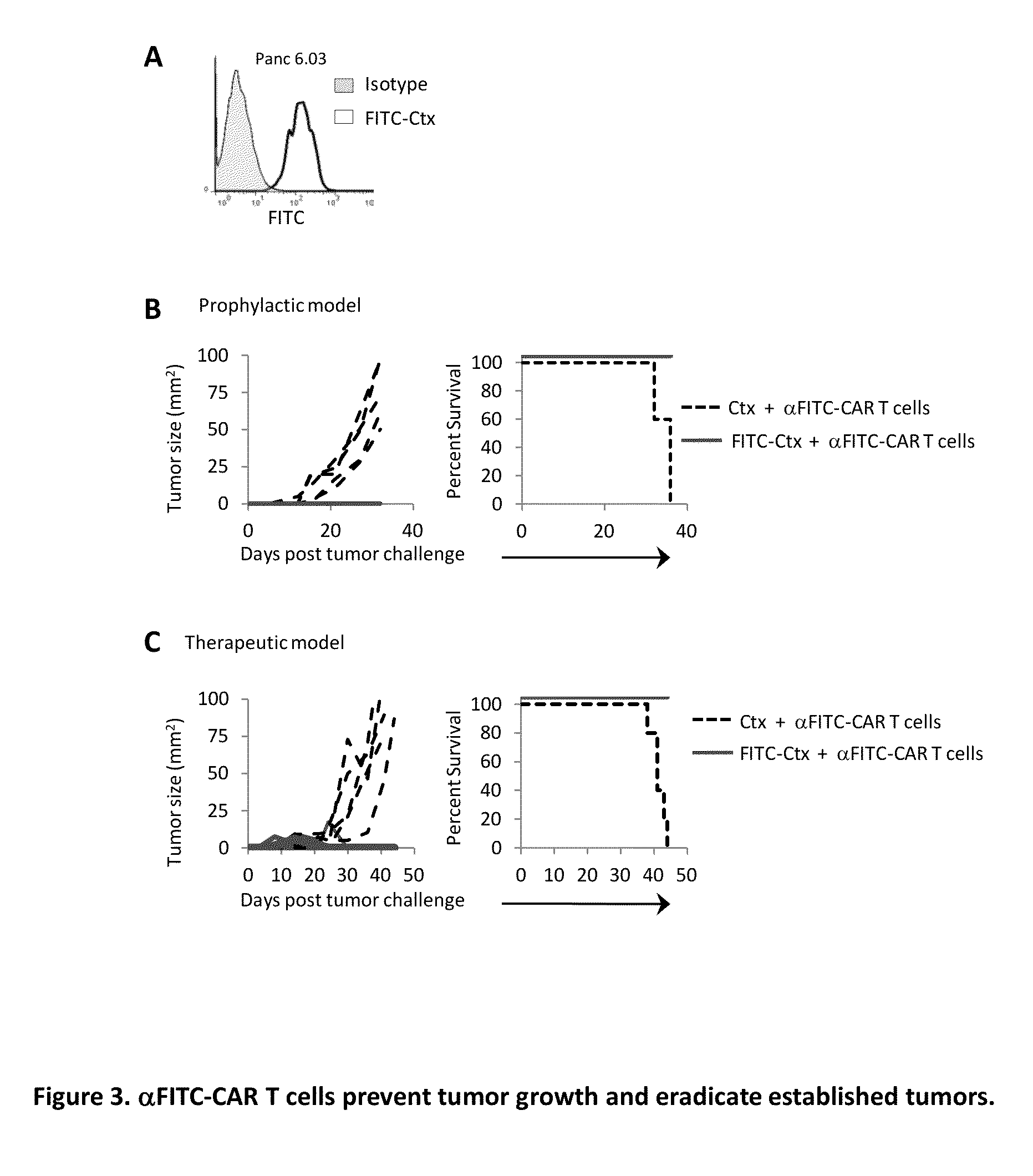
The present invention provides a universal, yet adaptable, anti-tag chimeric antigen receptor (AT-CAR) system which provides T cells with the ability and specificity to recognize and kill target cells, such as tumor cells, that have been marked by tagged antibodies. As an example, αFITC-CAR-expressing T cells have been developed that specifically recognize various human cancer cells when those cells are bound by cancer-reactive FITC-labeled antibodies. The activation of αFITC-CAR-expressing T cells is shown to induce efficient target lysis, T cell proliferation, and cytokine / chemokine production. The system can be used to treating subjects having cancer.
Owner:UNIV OF MARYLAND BALTIMORE
Automated sample-to-microarray system
InactiveUS20070092901A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLysisOligonucleotide
An apparatus having within or as part of a housing; a sample port; a microarray port; a lysis module; a purification module for containing a solid phase for binding of oligonucleotides; a thermocycling module for containing a polymerase chain reaction; a fragmentation module; and a microarray module for holding a microarray and a liquid in contact with the microarray. The apparatus is configured to be coupled to a device for: pumping a liquid through, in order, the lysis, purification, thermocycling, fragmentation, and microarray modules; sonicating any contents of the lysis module; thermocycling the thermocycling module to perform the polymerase chain reaction; heating the fragmentation module to fragment any oligonucleotides contained therein; circulating a fluid over the surface of the microarray; and performing one or more washing or staining steps on the microarray.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
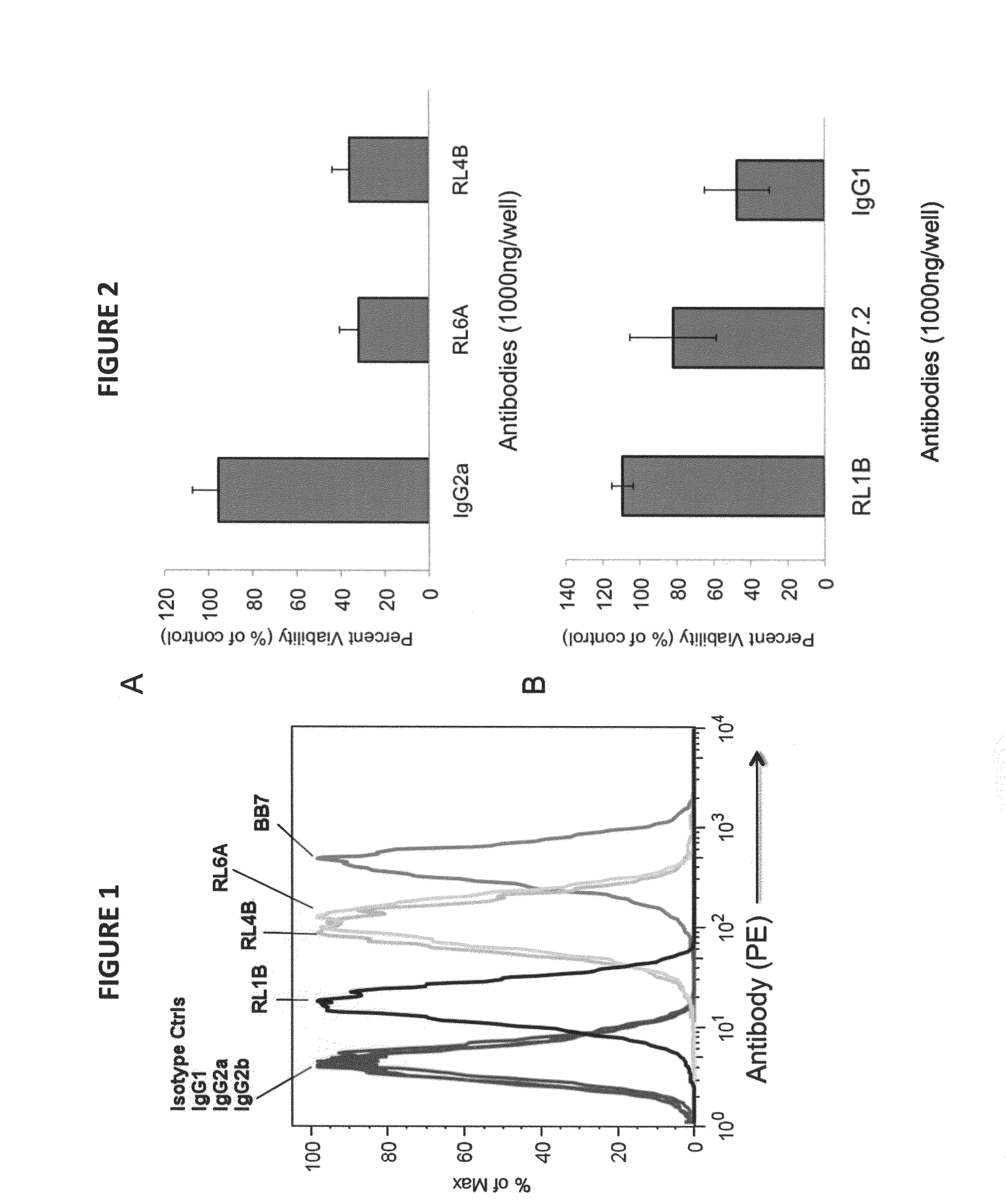
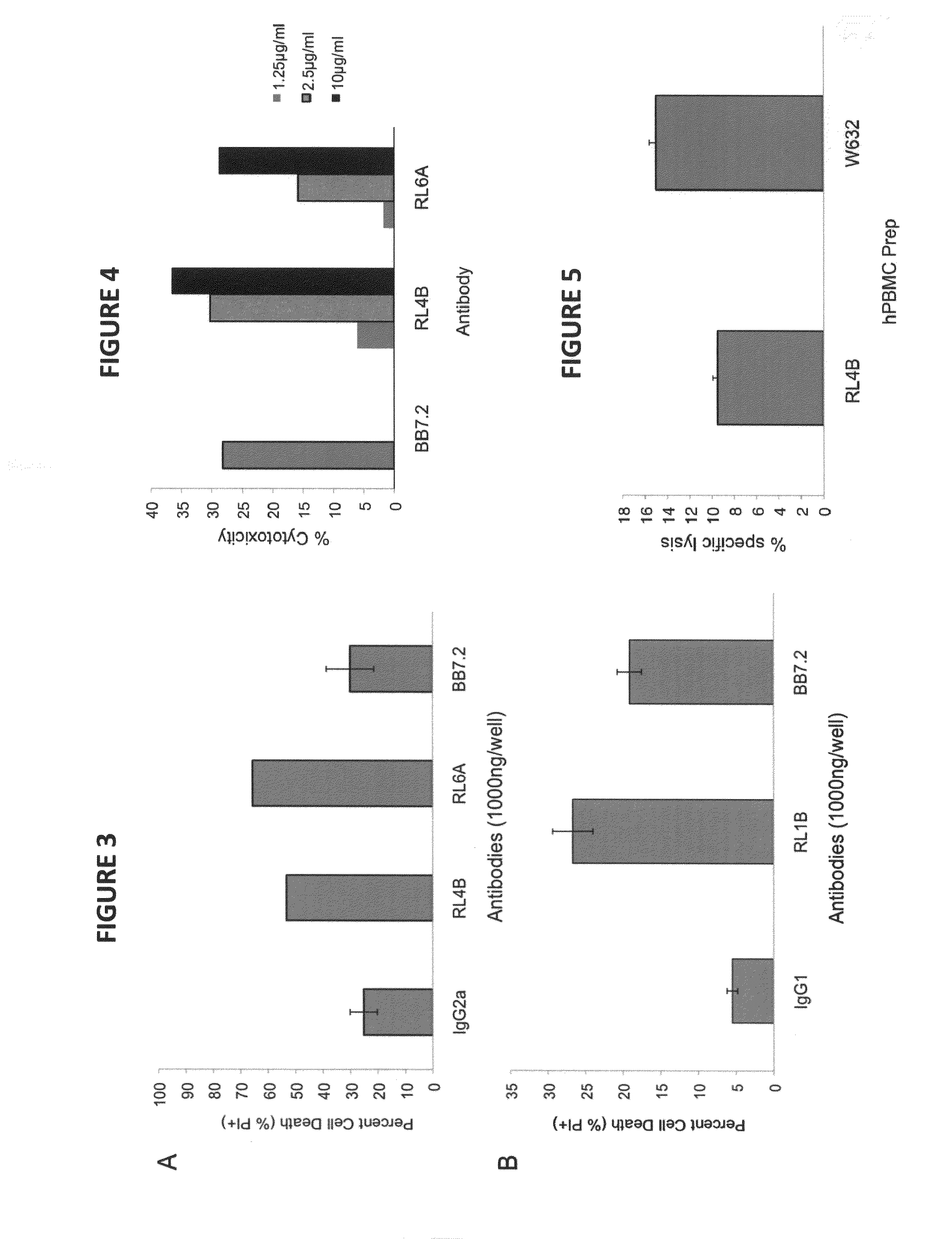
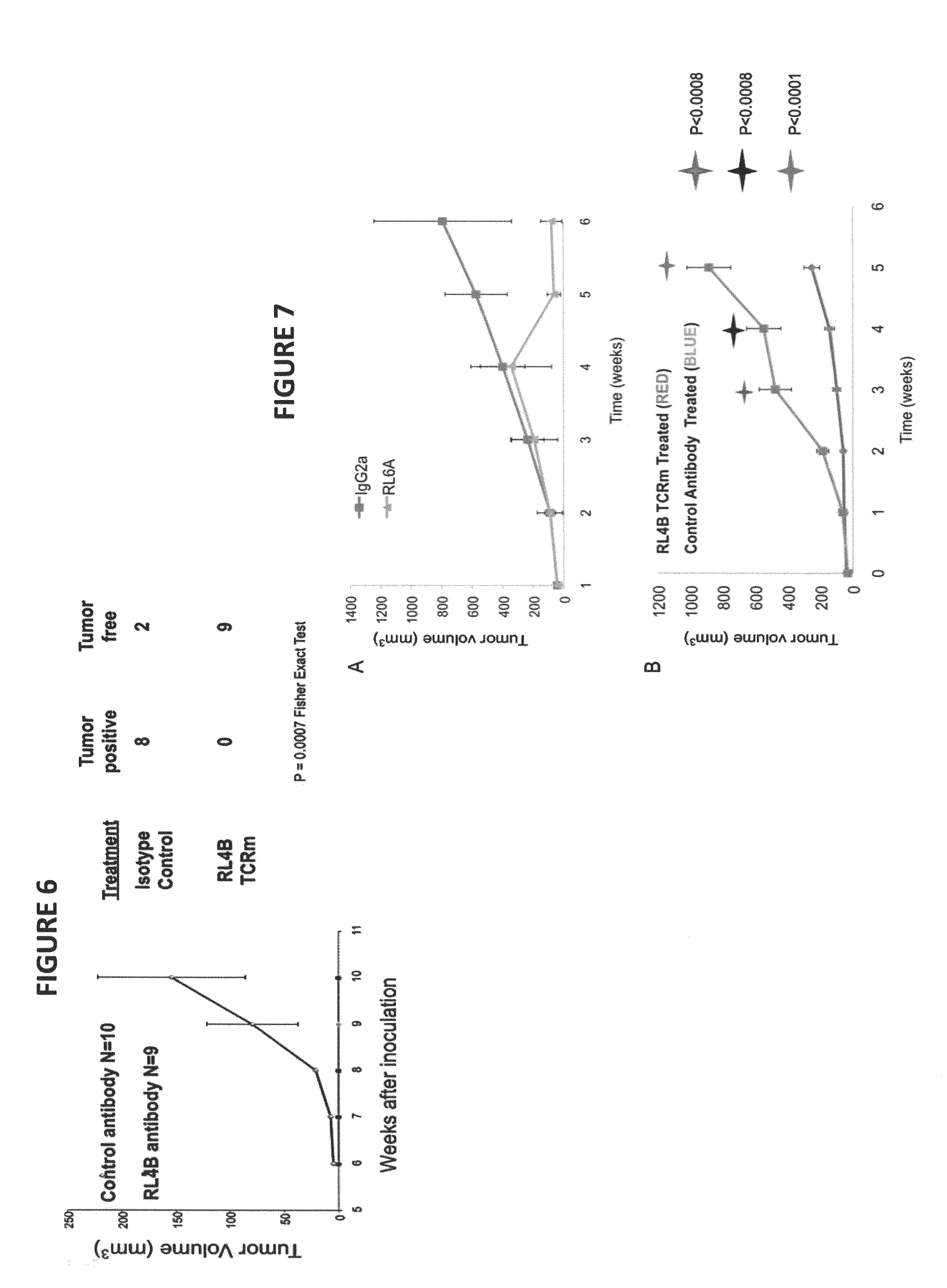
Antibodies as T cell receptor mimics, methods of production and uses thereof
The presently disclosed and claimed invention relates to a methodology of producing and utilizing antibodies that recognize peptides associated with a tumorigenic or disease state, wherein the peptides are displayed in the context of HLA molecules. These antibodies may be utilized in therapeutic methods of mediating cell lysis.
Owner:TEXAS TECH UNIV SYST
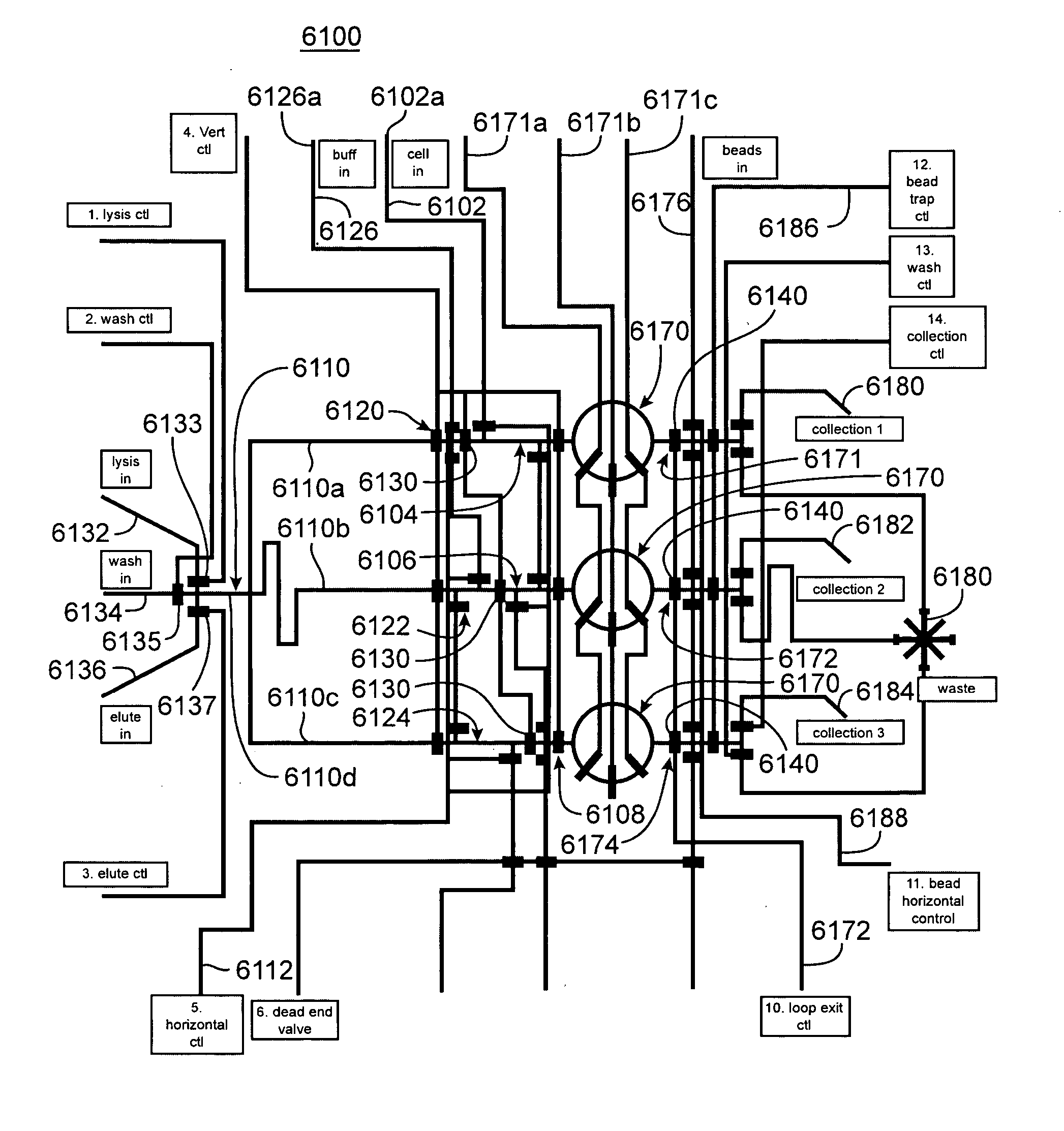
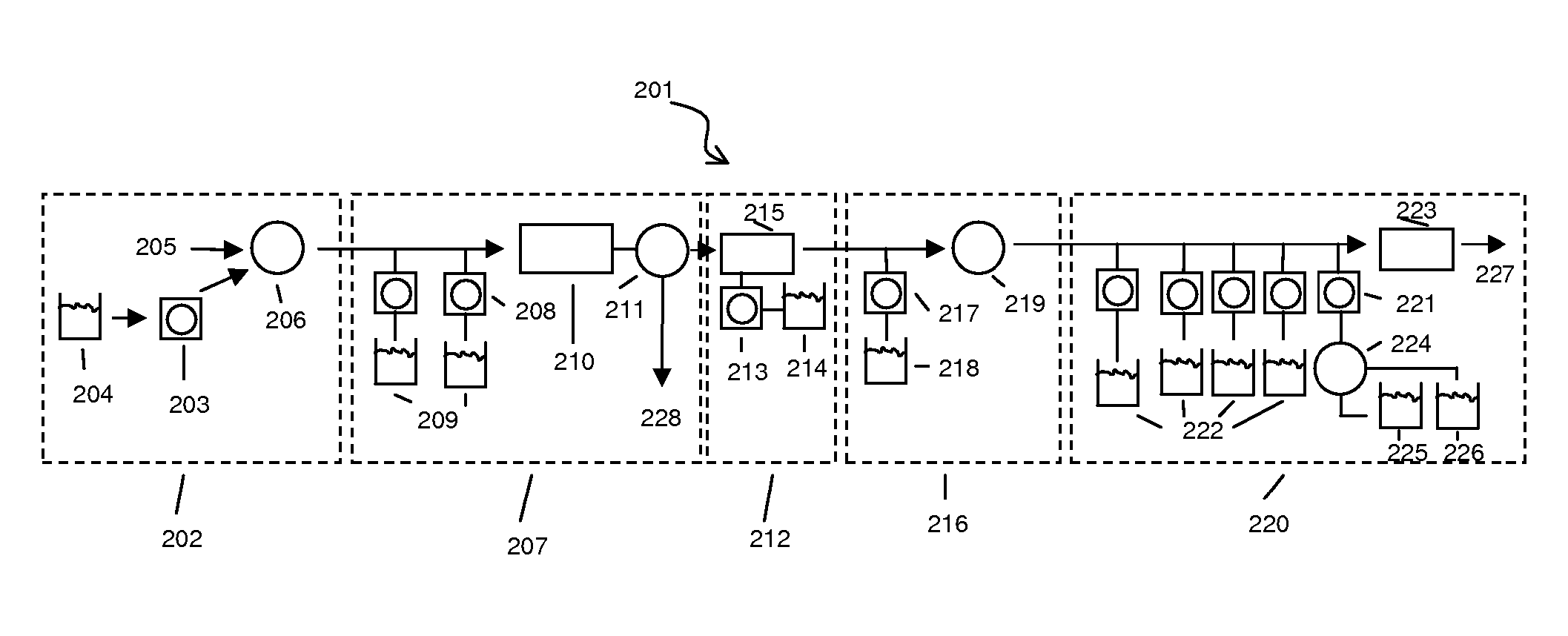
Device for extracting nucleic acid from a sample
InactiveUS20080057572A1Efficient processingAccurate detectionBioreactor/fermenter combinationsHeating or cooling apparatusChemical reactionAnalyte
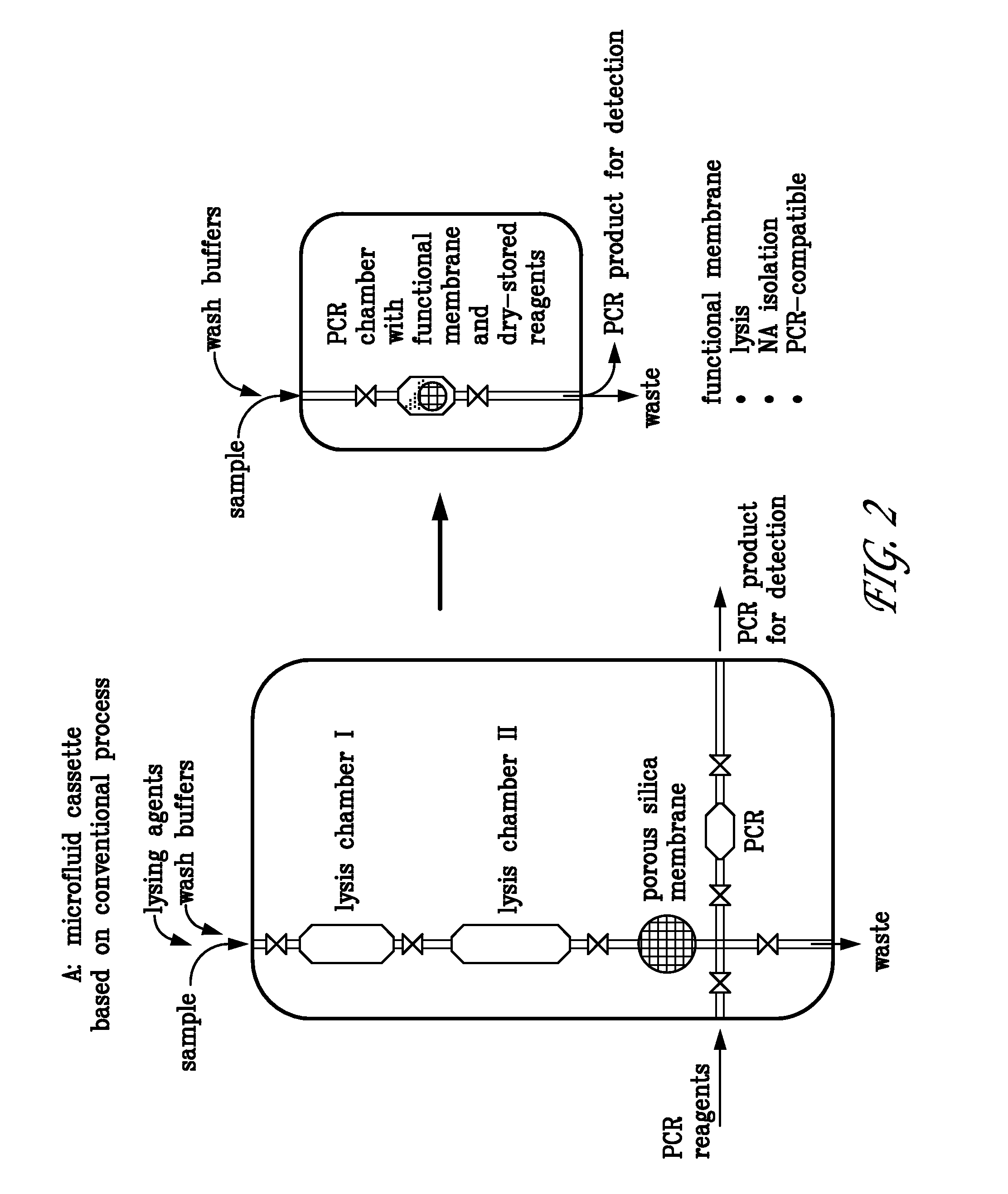
The present invention provides a cartridge for analyzing a fluid sample. The cartridge provides for the efficient separation of cells or viruses in the sample from the remaining sample fluid, lysis of the cells or viruses to release the analyte (e.g., nucleic acid) therefrom, and optionally chemical reaction and / or detection of the analyte. The cartridge is useful in a variety of diagnostic, life science research, environmental, or forensic applications for determining the presence or absence of one or more analytes in a sample.
Owner:CEPHEID INC
Preservation of cell-free RNA in blood samples
ActiveUS8304187B2Prevent leakageInhibit synthesisSugar derivativesMicrobiological testing/measurementLysisNuclease
Owner:STRECK LLC
DNA isolation method
InactiveUS6852851B1Not readyBioreactor/fermenter combinationsBiological substance pretreatmentsNuclear membraneLysis
Owner:GYROS
Methods for the separation of streptococcus pneumoniae type 3 polysaccharides
ActiveUS20080102498A1Reducing and removing impurityImprove filtering effectAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeLysis
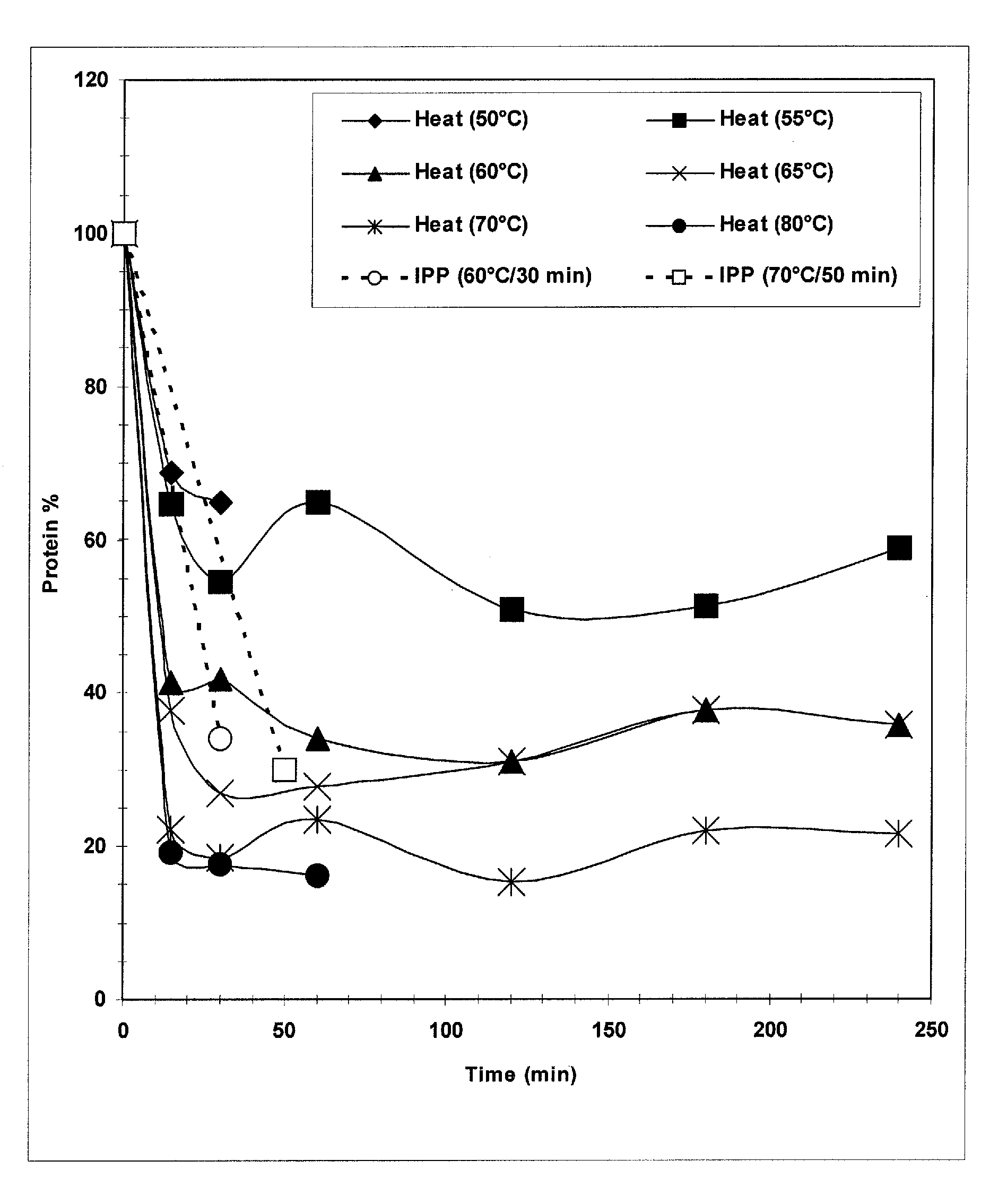
The present invention provides improved methods for the reduction or removal of protein impurities from a complex cellular Streptococcus pneumoniae lysate or centrate comprising serotype 3 polysaccharides involving steps relating to post-lysis heating or pH adjustment. In certain methods, the lysate is heated for a time and at a temperature sufficient to denature proteins present in the lysate and cause their aggregation and precipitation. In one embodiment, the lysate is heated to at least 60° C. for at least 30 minutes to cause protein aggregation and precipitation, more particularly about 60° C. to about 70° C. for about 30 to about 50 minutes, and even more particularly about 65° C. for about 40 minutes. In other methods, the pH of the lysate or centrate is increased to at least 8.0 to improve filterability, more particularly about 8.0 to 8.4, and even more particularly about 8.2. In further methods, heating and pH adjustment steps are combined to cause the aggregation and precipitation of proteins as well as to improve filterability of the lysates or centrates. In other methods, the pH of the lysate or centrate is lowered to about 3.0 to about 5.0 to cause protein aggregation and precipitation. Such methods allow for the production of substantially purified serotype 3 polysaccharide-containing lysates or centrates.
Owner:WYETH LLC
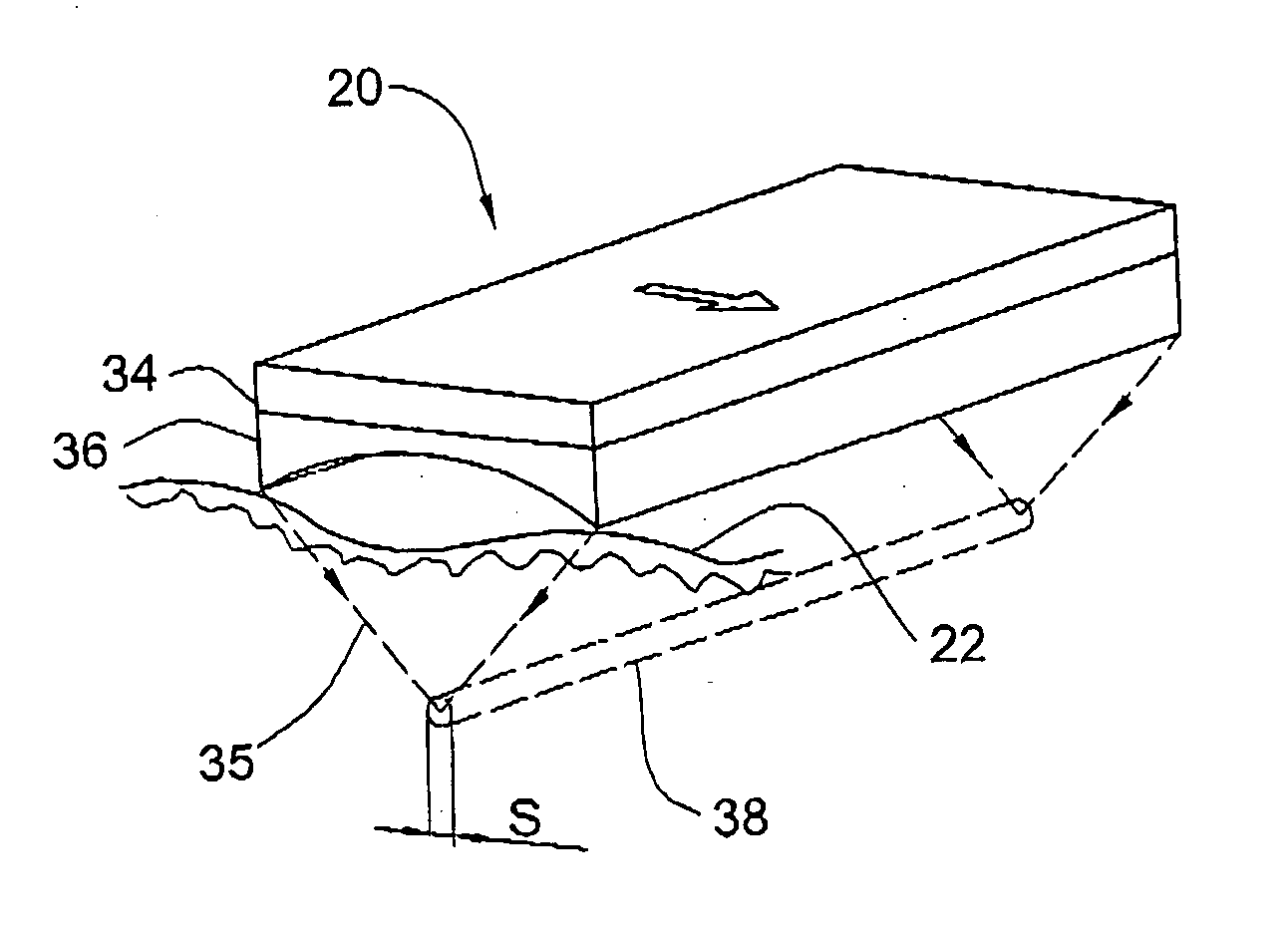
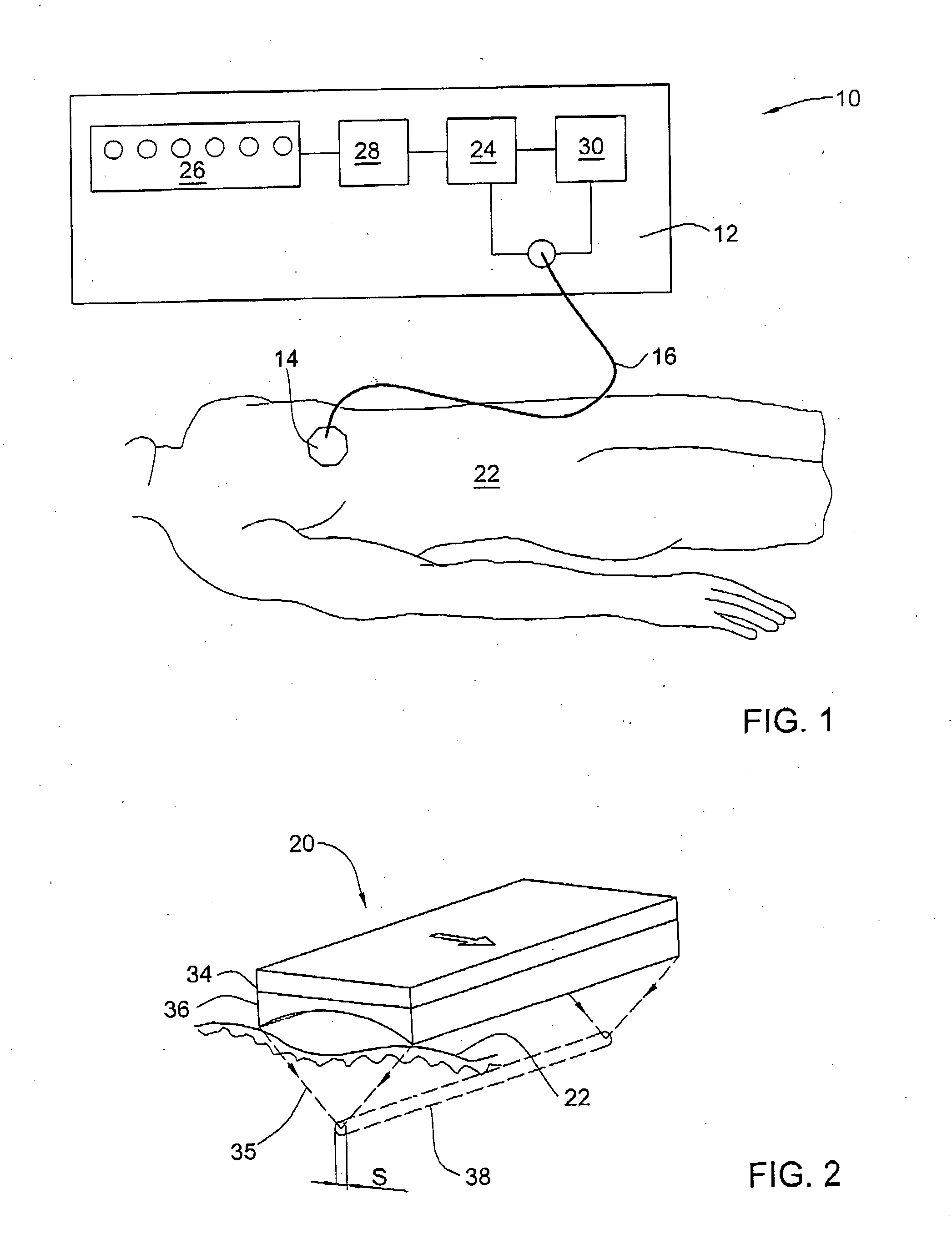
Method and device for sub-dermal tissue treatment
InactiveUS20050055018A1Avoid heat damageAvoid tissue damageUltrasound therapySurgical instruments for heatingCavitationHand held
System and method for non-invasive lysis of sub-dermal tissue by means of focused ultrasonic energy, the system comprising: a source of ultrasonic energy adapted to operate in continuous wave mode and to focus ultrasonic energy in a focal zone within the sub-dermal tissue, the ultrasonic energy being adapted to induce tissue cavitation in the focal zone; means for continuous displacement of the source over the skin surface; and means for determining a safe speed for the displacement, the safe speed allowing to avoid thermal tissue damage. The source of ultrasonic energy is accommodated in a hand-held applicator including a wheeled traction system powered by an electric drive.
Owner:SYNERON MEDICAL LTD
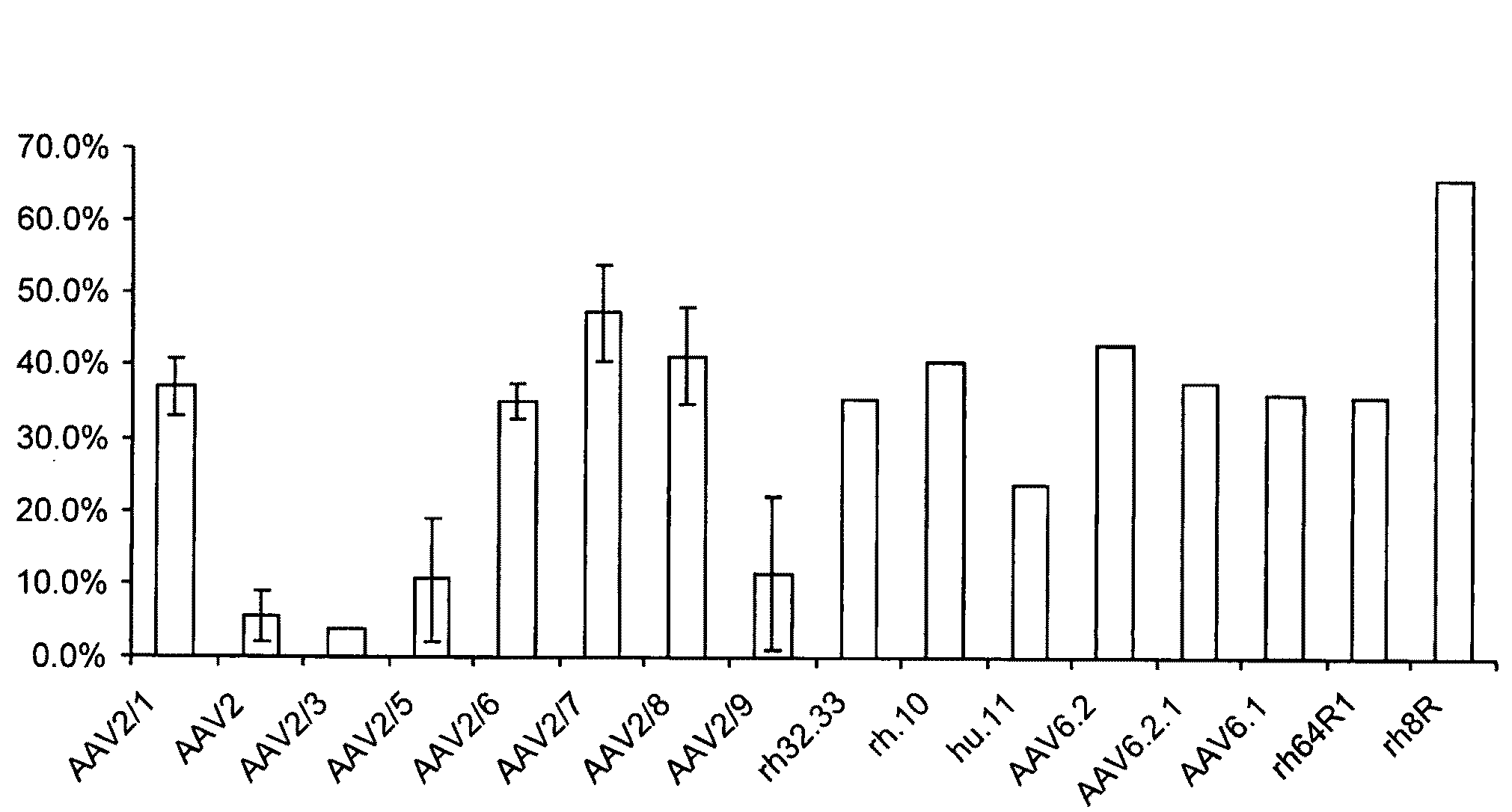
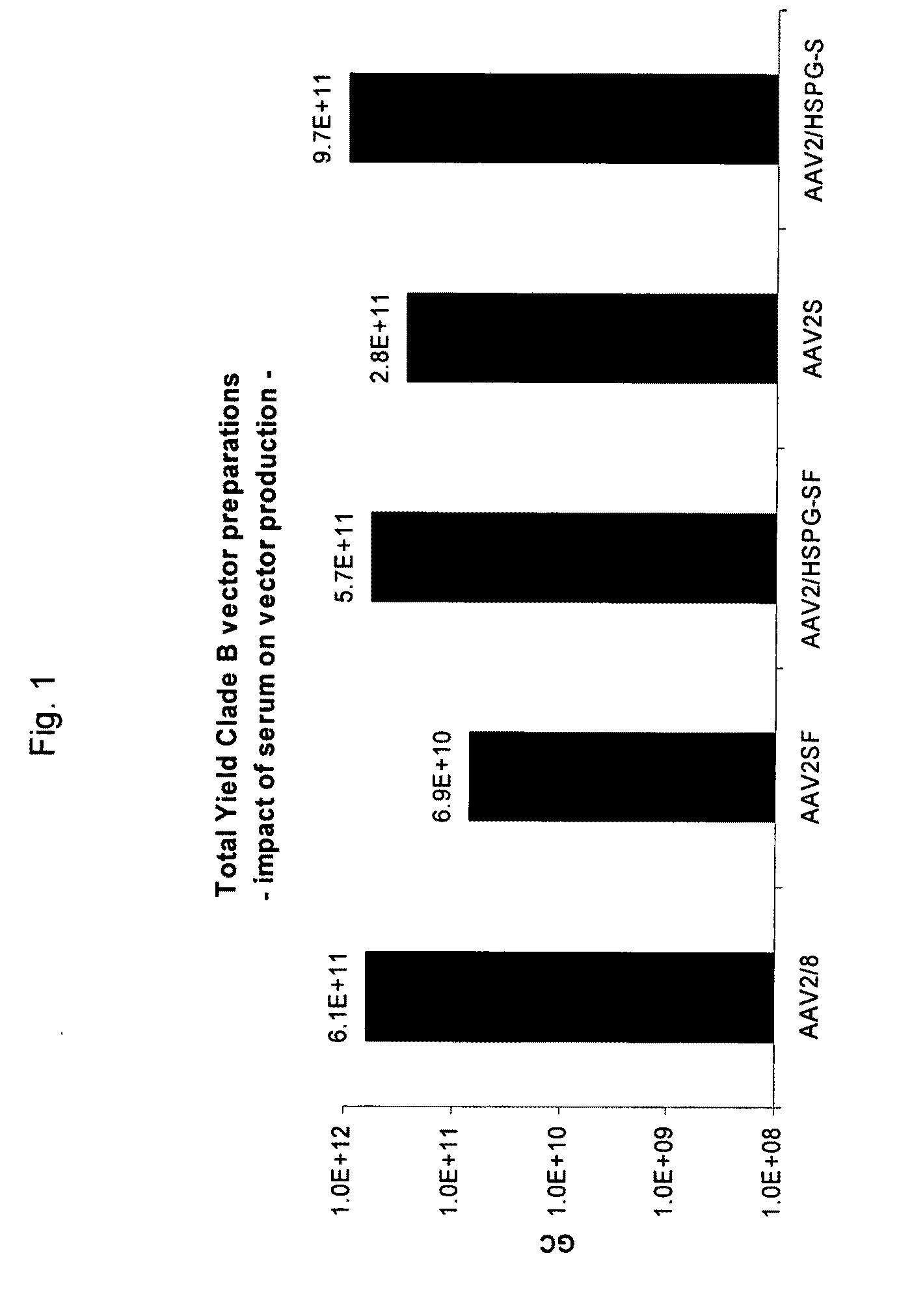
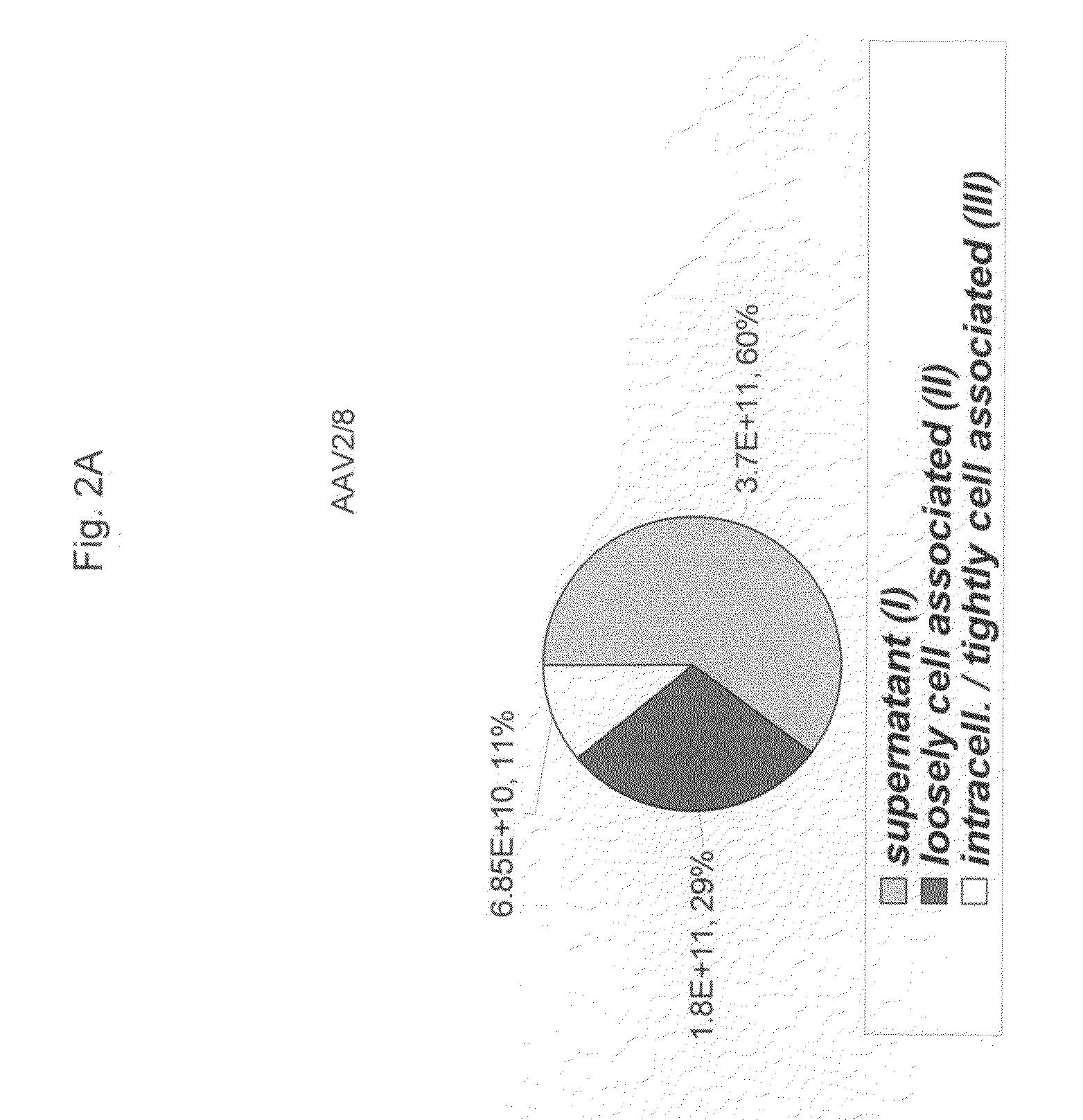
Scalable Production Method for AAV
ActiveUS20090275107A1Reduce and eliminate bindingHigh yieldGenetic therapy composition manufactureVirus peptidesLysisBinding site
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
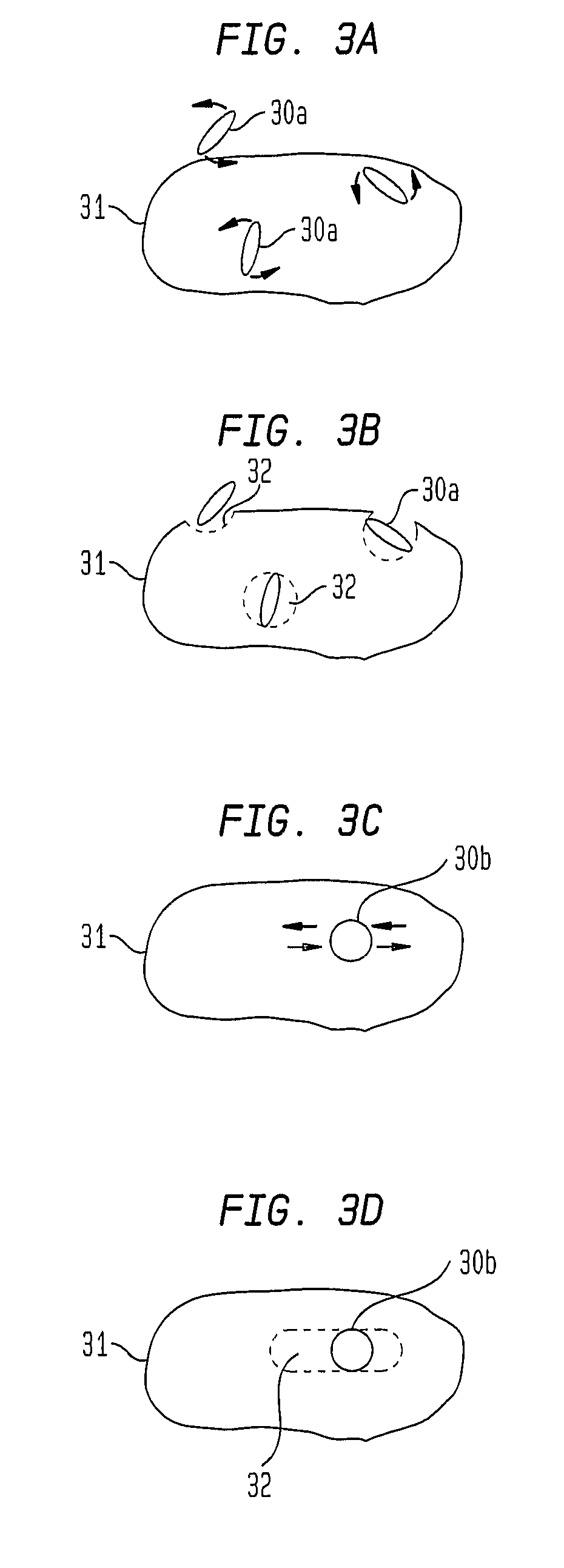
Method and articles for remote magnetically induced treatment of cancer and other diseases, and method for operating such article
InactiveUS20070196281A1Avoid damagePowder deliveryHeart defibrillatorsAbnormal tissue growthCancer cell
This invention describes unique treatment methods and innovative articles that can be placed in a human or animal body to enable controlled destruction of diseased tissue. The methods include destruction of diseased cells and tissues by magnetically controlled motion and an externally controllable drug delivery process with a capability to start and stop the drug delivery at any time, for any duration. This invention provides two approaches to diseased cell destruction, (1) magneto-mechanical disturbance of cell structure (e.g. cancer cells) for cell lysis and (2) magnetically activated drug release at local regions (e.g. tumors) from a magnetic-particle-containing drug reservoir. The invention also provides combinations of both the above treatments for dual therapy. It further combines one or both of the treatments with magnetic hyperthermia for multifunctional cell destruction therapy. The approaches can be combined with magnetic MRI for monitoring the accuracy of placement as well as for following up the cancer destruction progress and appropriate reprogramming of the magneto-mechanical therapy and remote-controlled drug release.
Owner:RGT UNIV OF CALIFORNIA
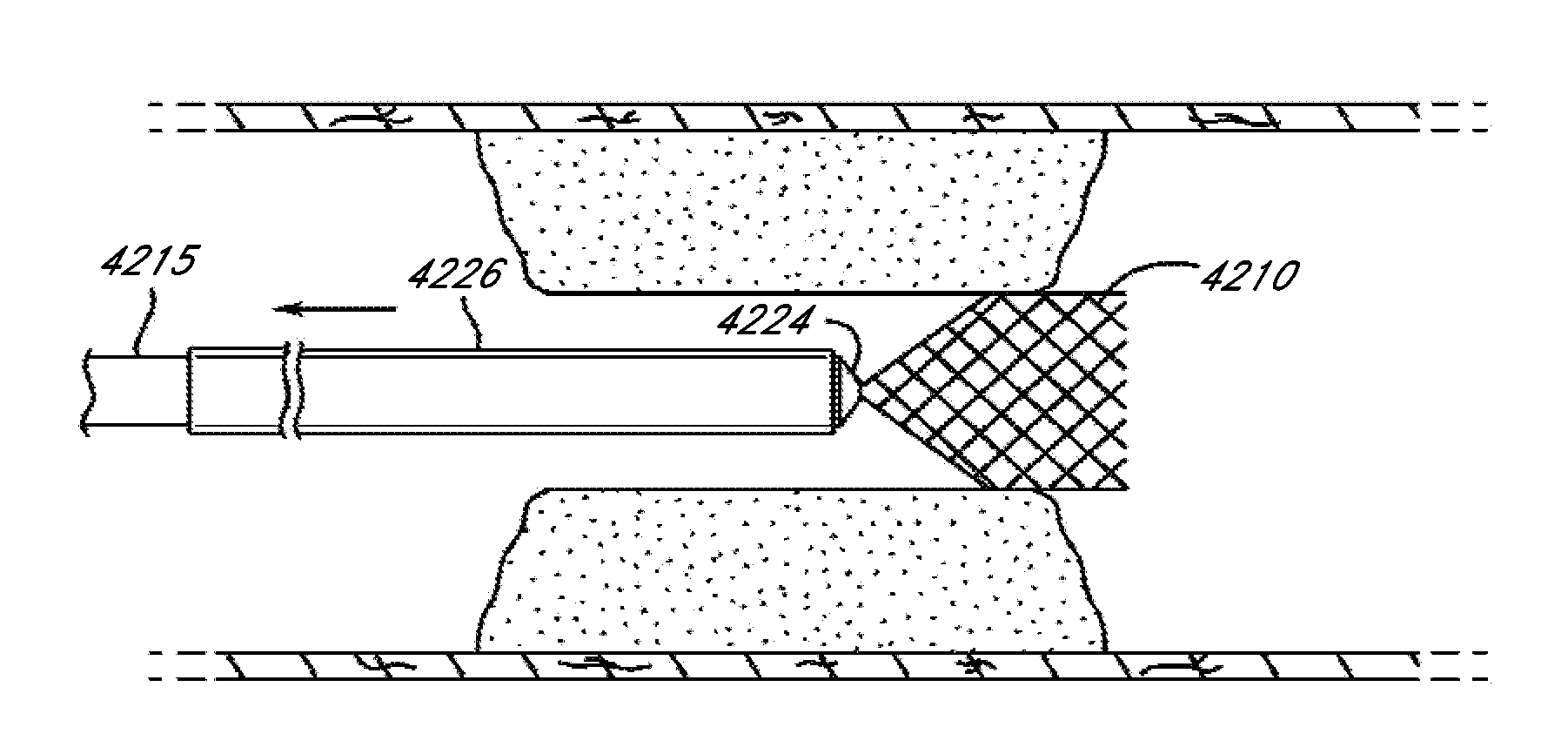
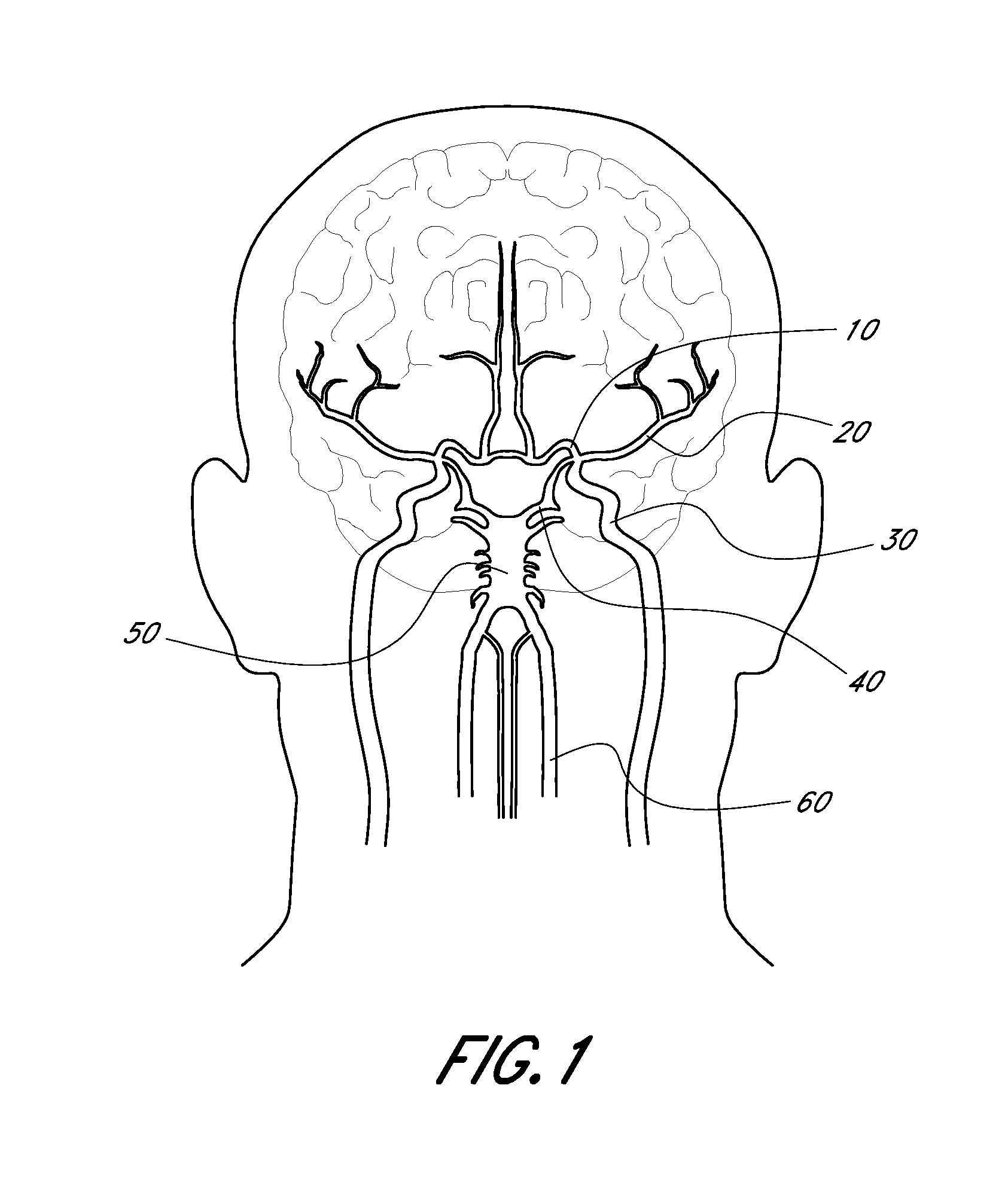
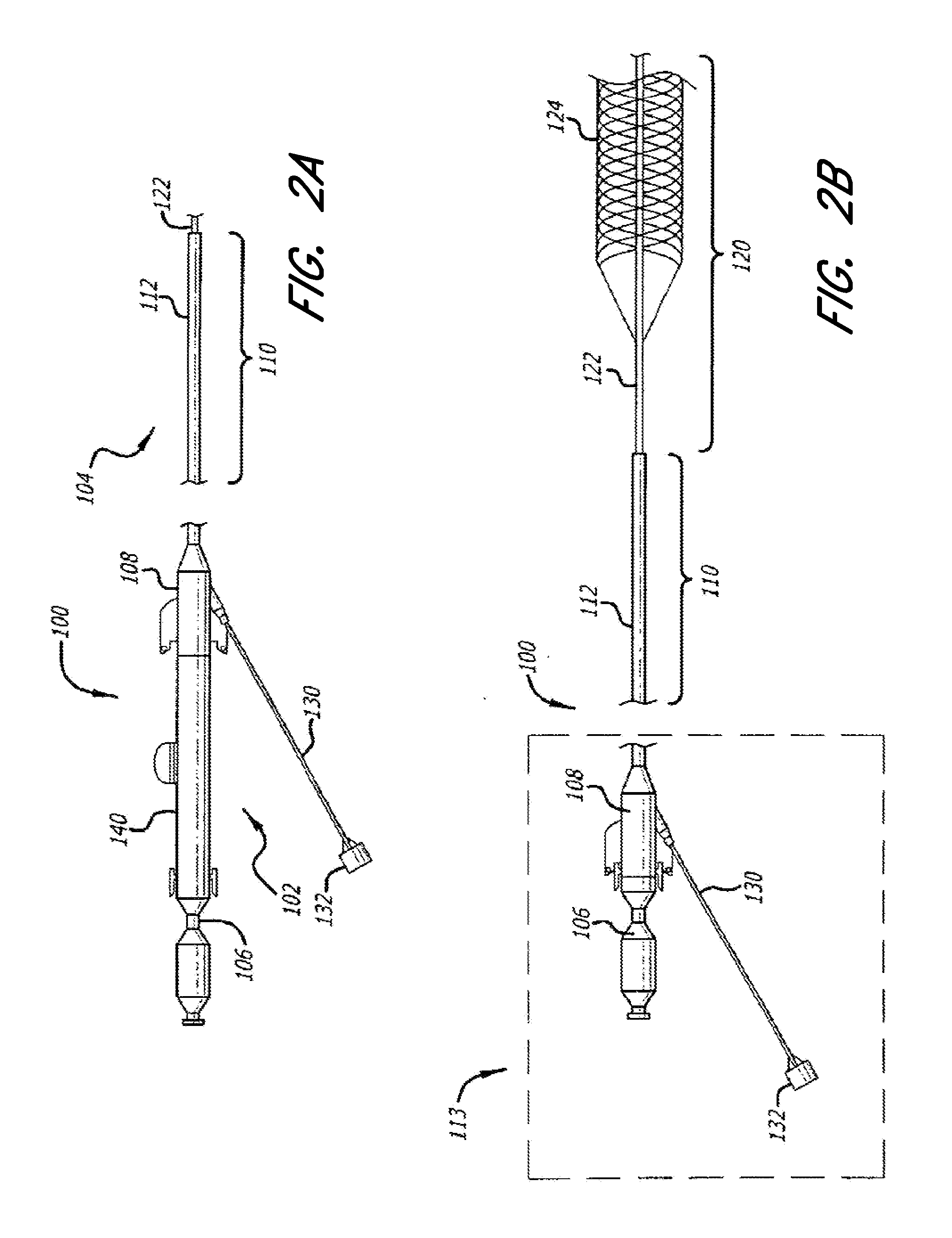
Method for providing progressive therapy for thrombus management
ActiveUS20110160742A1Avoid damageFacilitates natural lysisStentsIncision instrumentsLysisDistal embolization
Systems, methods, and devices for the treatment of acute ischemic stroke that provide immediate blood flow restoration to a vessel occluded by a clot and, after reestablishing blood flow, address the clot itself. Immediate blood flow restoration advantageously can facilitate natural lysis of the clot and also can reduce or obviate the concern for distal embolization due to fragmentation of the clot. Several embodiments of the invention provide for progressive, or modular, treatment based upon the nature of the clot. For example, the progressive treatment can comprise a three-step progressive treatment process that includes immediate restoration of blood flow, in-situ clot management, and / or clot removal depending on the particular circumstances of the treatment. The in-situ clot management can include, for example, lysis and maceration. The progressive, or modular, treatment can be provided by a system or kit of one or more treatment devices.
Owner:TYCO HEALTHCARE GRP LP
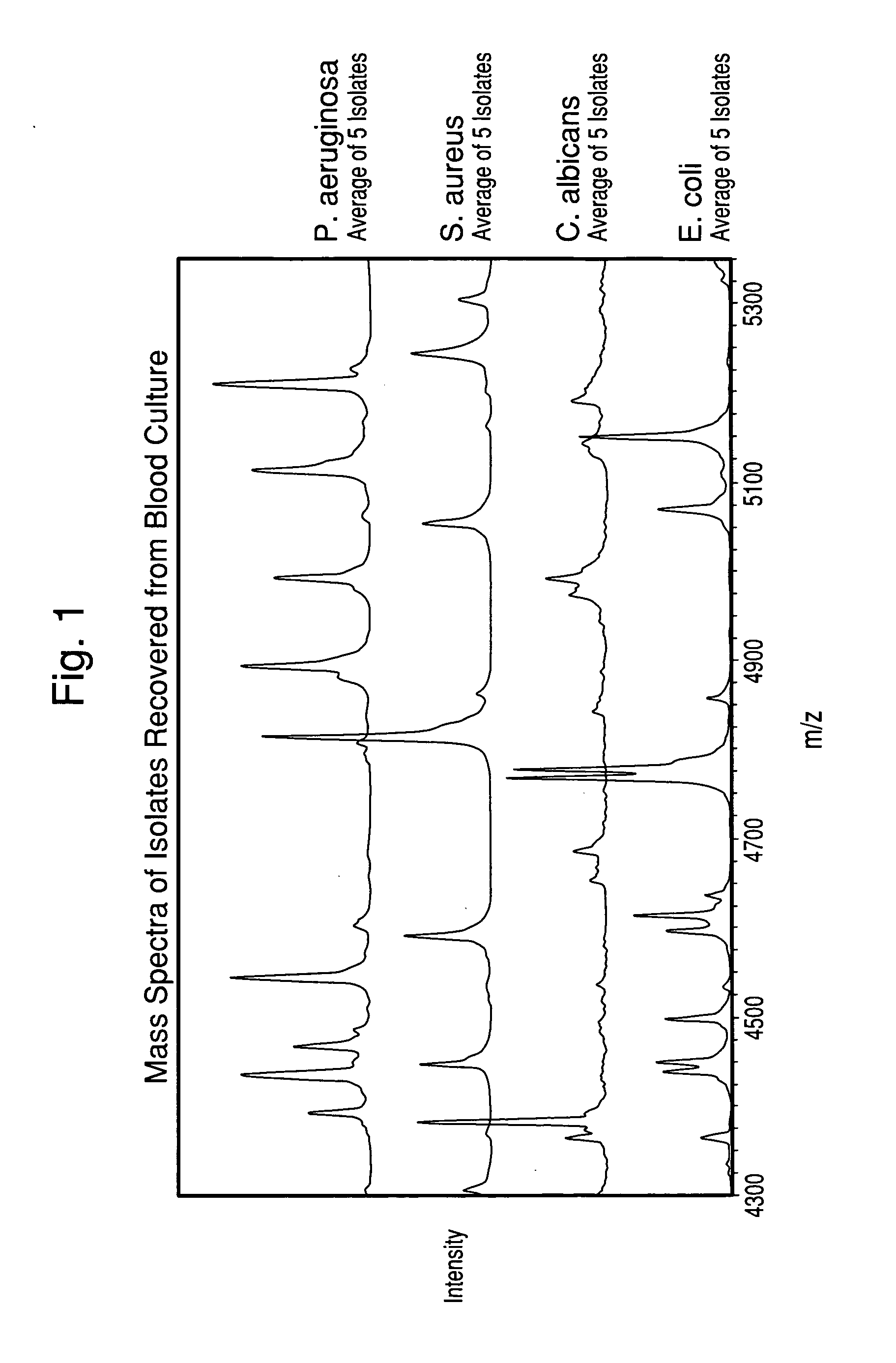
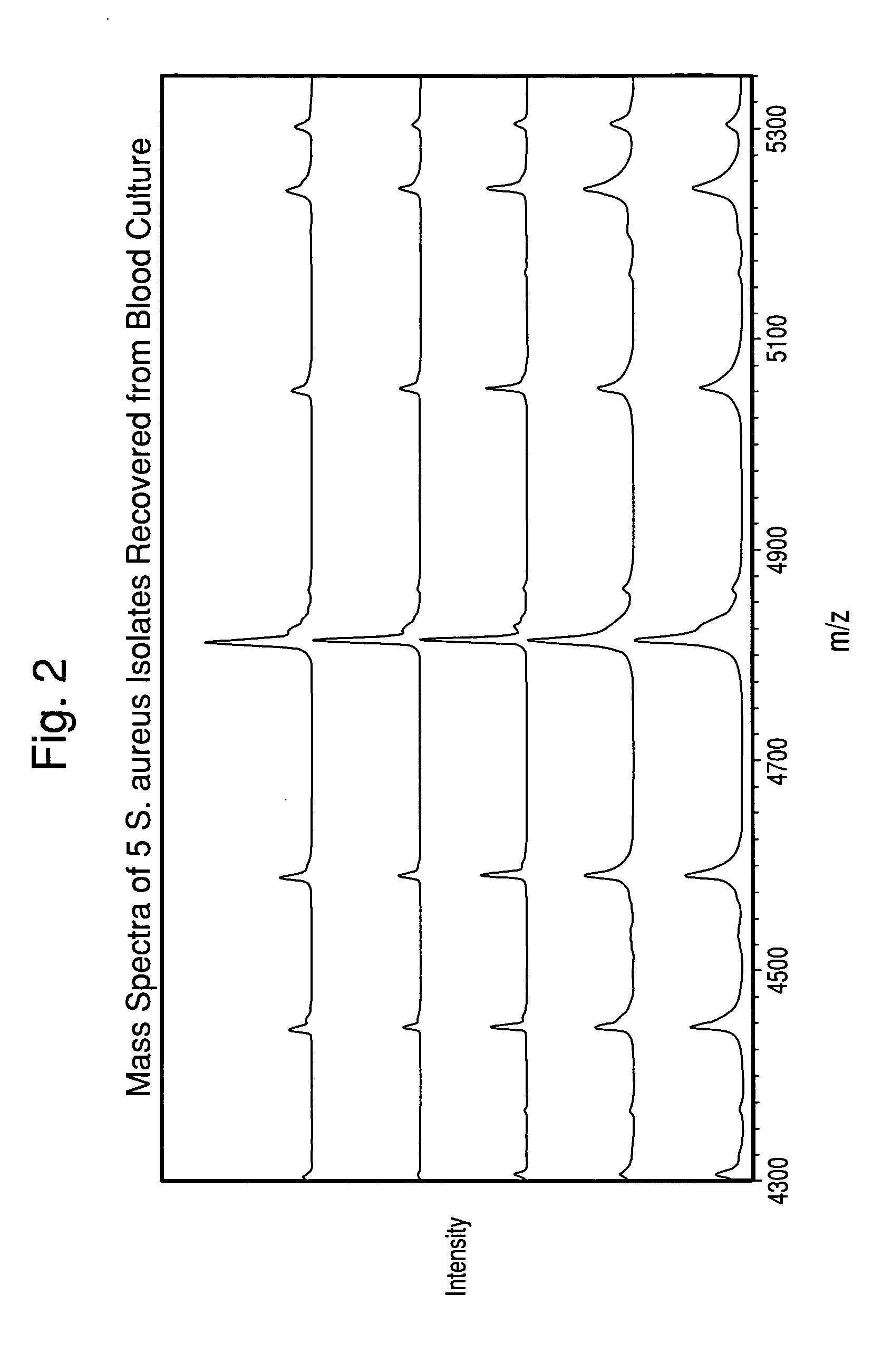
Methods for the isolation and identification of microorganisms
ActiveUS20100129857A1Rapid diagnosisReduce riskMicroorganismsMicrobiological testing/measurementMicroorganismLysis
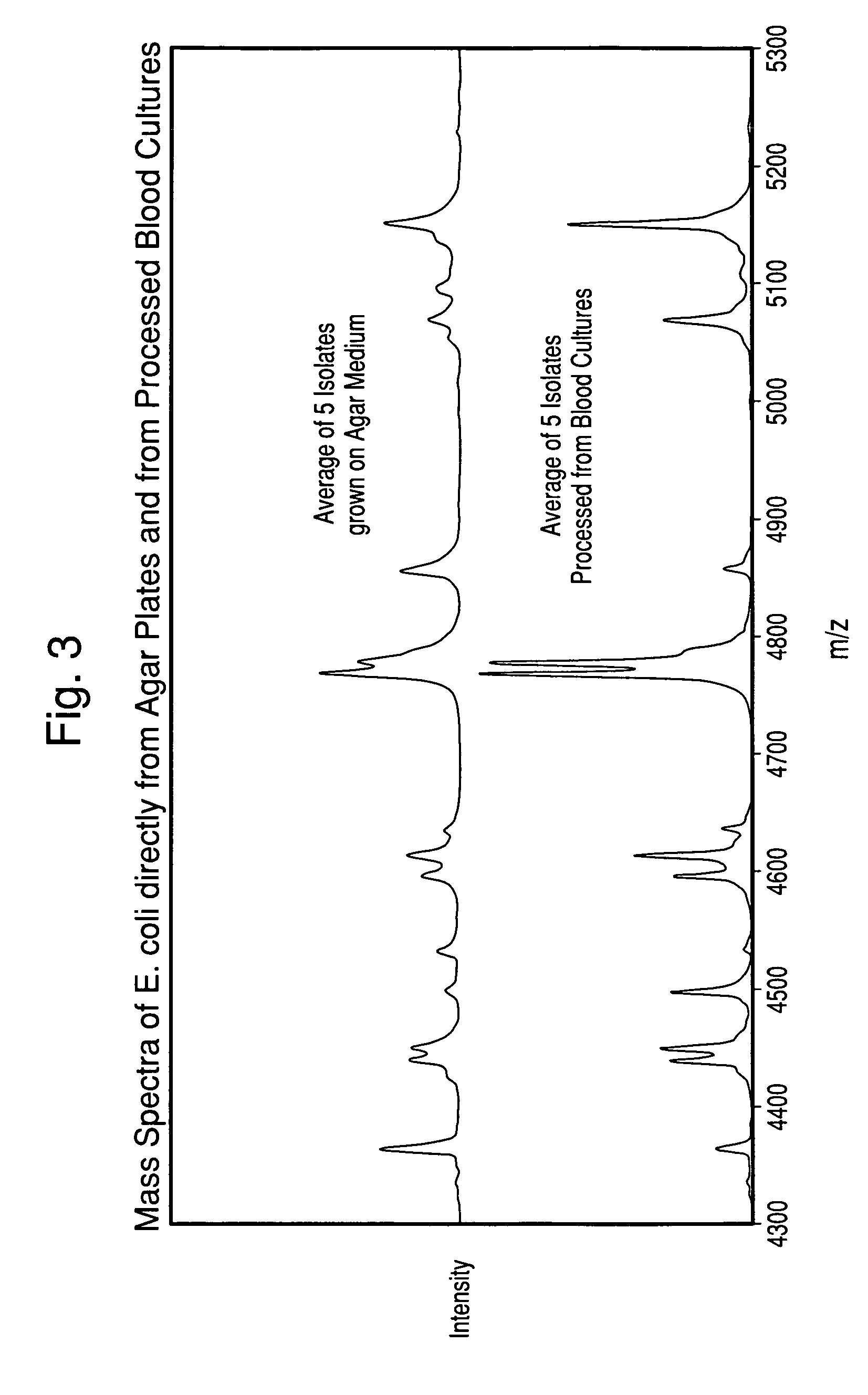
The present invention is directed to a method for separating, characterizing and / or identifying microorganisms in a test sample. The method of the invention comprises an optional lysis step for lysing non-microorganism cells that may be present in a test sample, followed by a subsequent separation step. The method may be useful for the separation, characterization and / or identification of microorganisms from complex samples such as blood-containing culture media. The invention further provides methods for separating, characterizing and / or identifying microorganisms in situ within a single system.
Owner:BIOMERIEUX INC
Stabilization of RNA in intact cells within a blood sample
InactiveUS20110111410A1Effective isolationEfficient testingMicrobiological testing/measurementTissue cultureLysisNuclease
A method for preserving and processing nucleic acids located within a blood sample is disclosed, wherein a blood sample containing nucleic acids is treated to reduce both blood cell lysis and nuclease activity within the blood sample. The treatment of the sample aids in increasing the integrity and amount of cellular nucleic acids that can be identified and tested while avoiding contamination of the isolated nucleic acids with cell-free nucleic acids.
Owner:STRECK INC
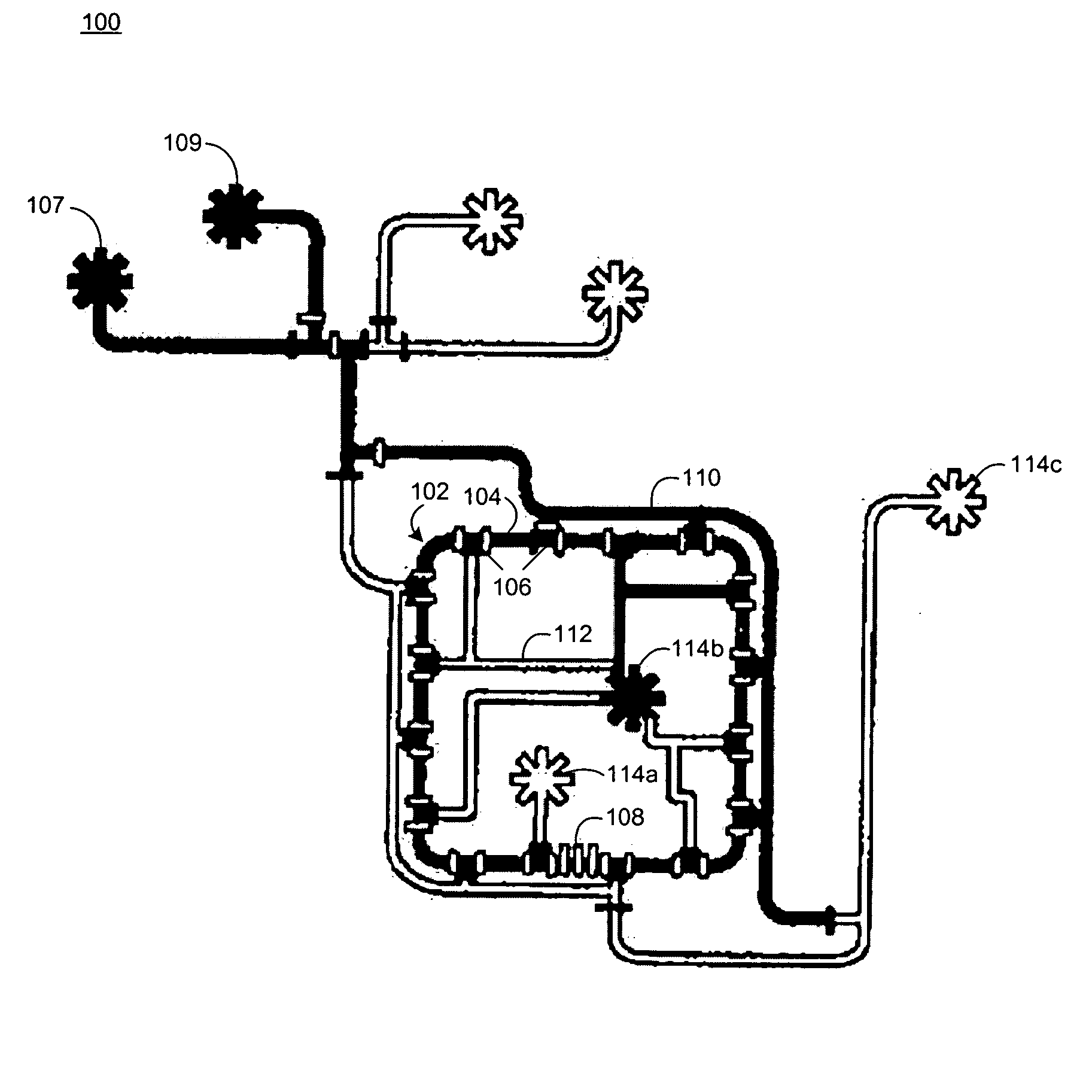
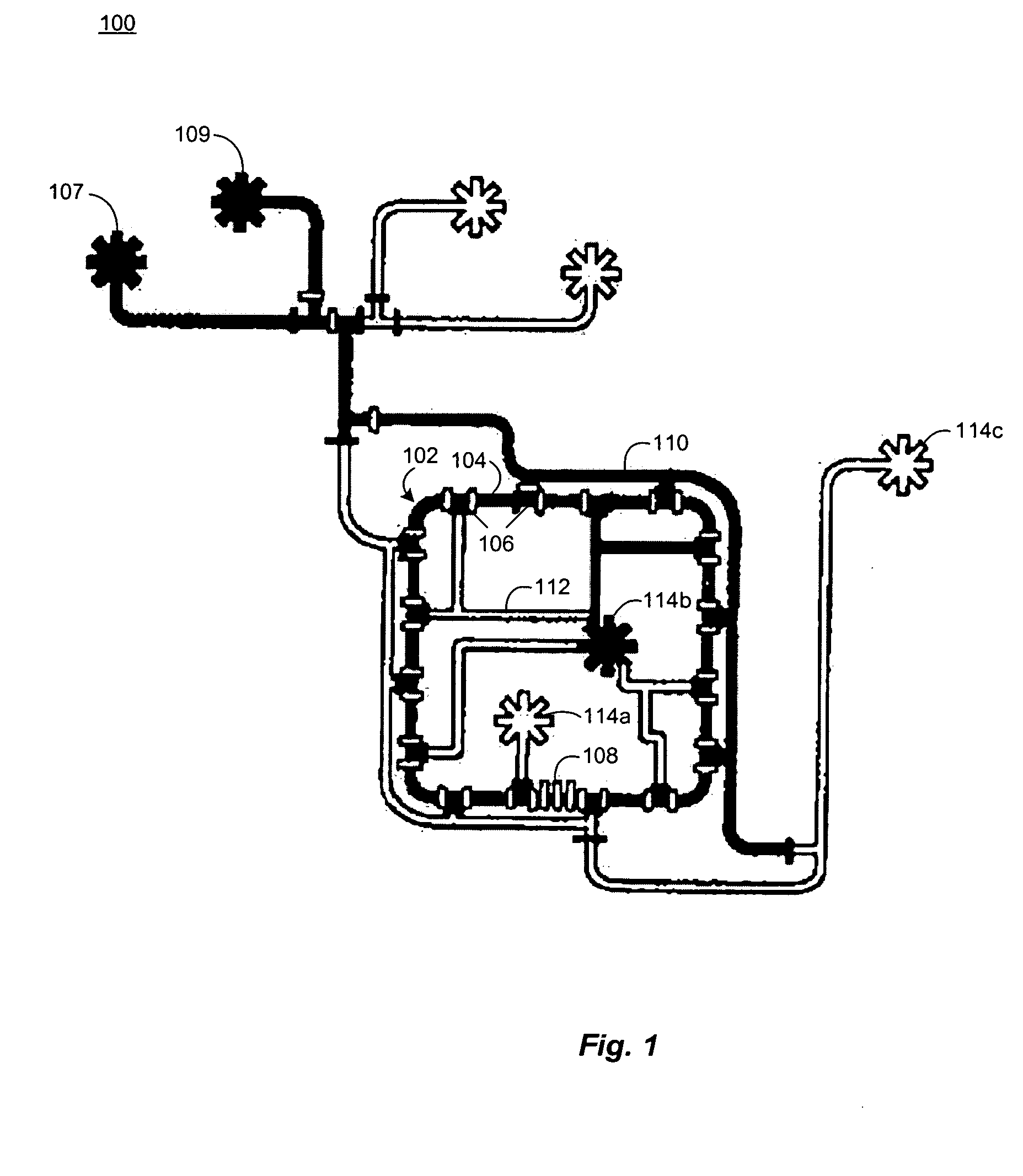
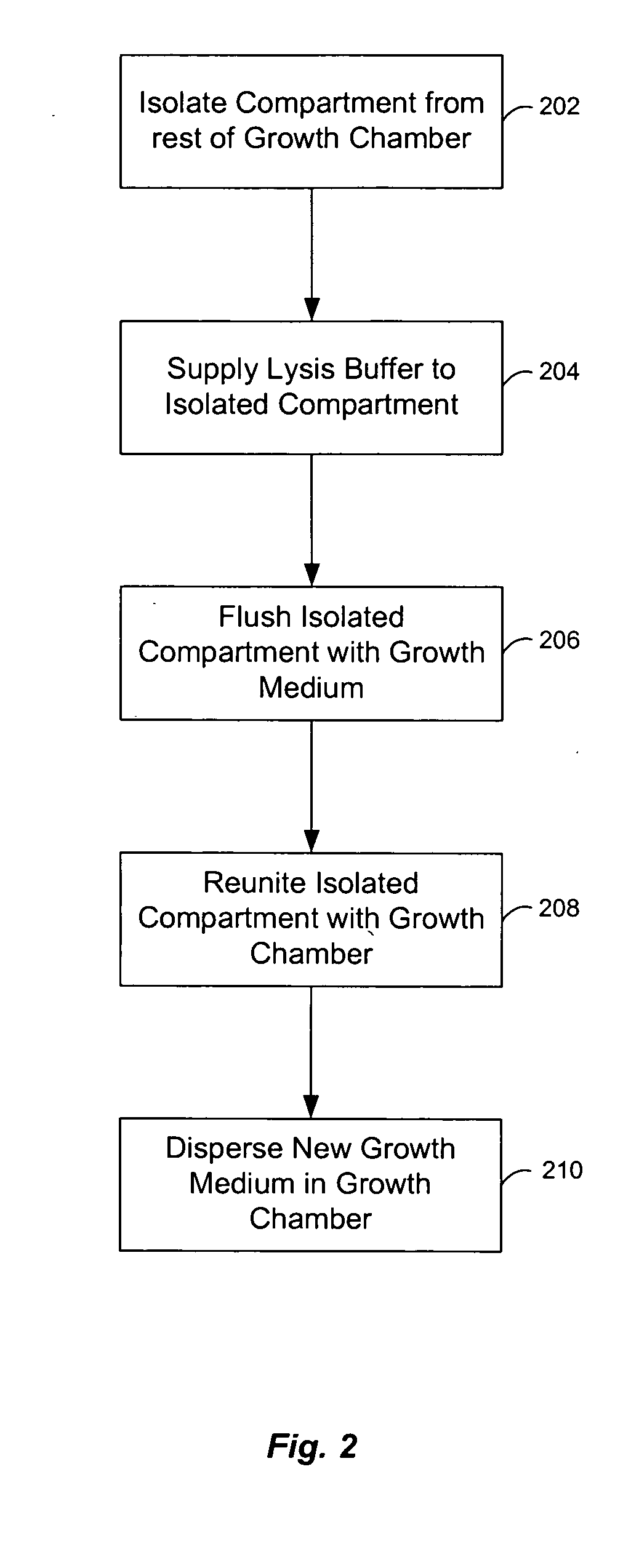
Microfluidic chemostat
InactiveUS20050164376A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiofilmLysis
A chemostat that includes a growth chamber having a plurality of compartments, where each of the compartments may be fluidly isolated from the rest of the growth chamber by one or more actuatable valves. The chemostat may also include a nutrient supply-line to supply growth medium to the growth chamber, and an output port to remove fluids from the growth chamber. Also, a method of preventing biofilm formation in a growth chamber of a chemostat. The method may include the steps of adding a lysis agent to a isolated portion of the growth chamber, and reuniting the isolated portion with the rest of the growth chamber.
Owner:CALIFORNIA INST OF TECH
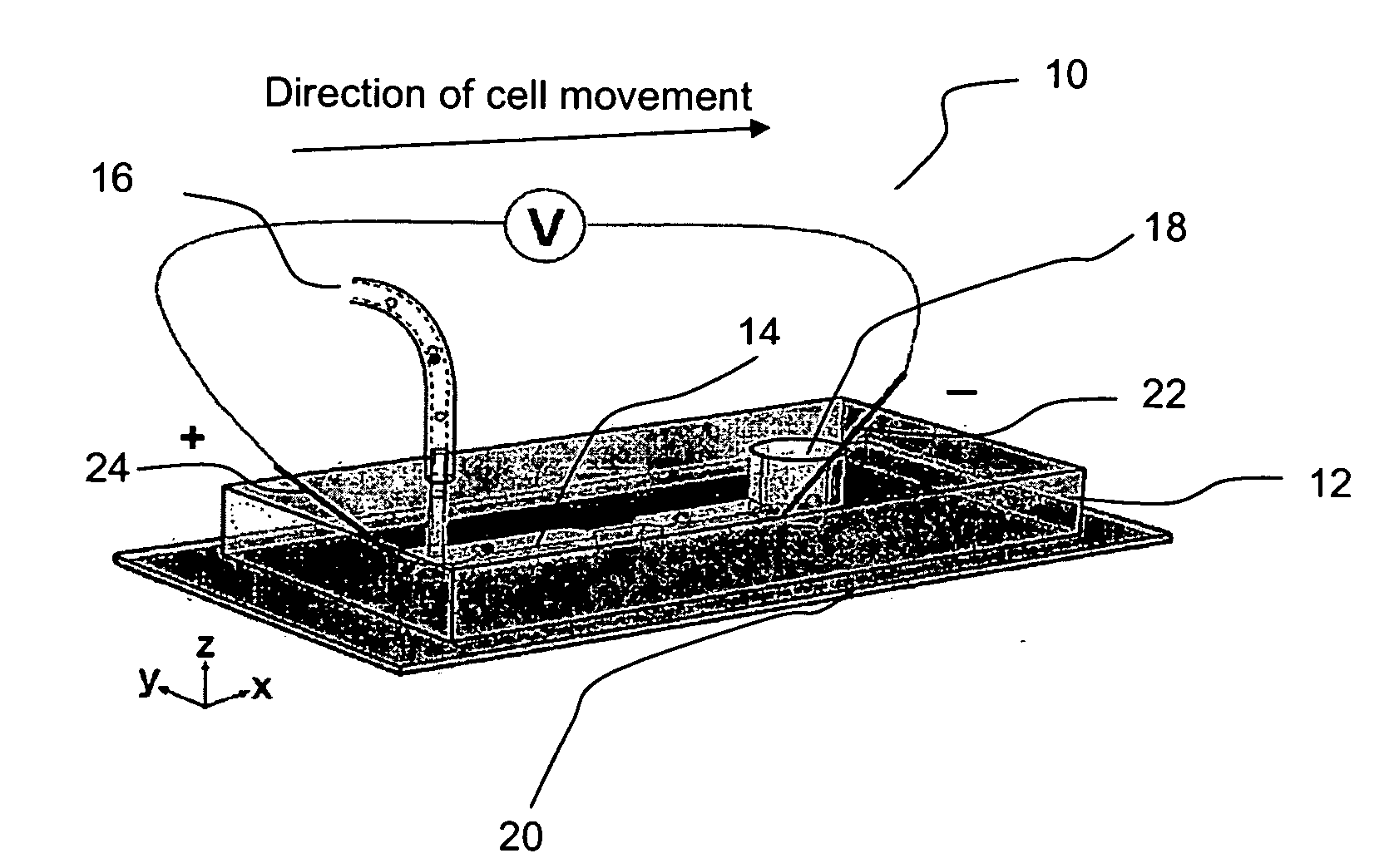
Fluidic device
InactiveUS20070105206A1Great electric field intensityBioreactor/fermenter combinationsBiological substance pretreatmentsLysisElectroporation
A fluidic device for cell electroporation, cell lysis, and cell electrofusion based on constant DC voltage and geometric variation is provided. The fluidic device can be used with prokaryotic or eukaryotic cells. In addition, the device can be used for electroporative delivery of compounds, drugs, and genes into prokaryotic and eukaryotic cells on a microfluidic platform.
Owner:PURDUE RES FOUND INC
Method for separation, characterization and/or identification of microorganisms using mass spectrometry
ActiveUS20100120085A1Rapid diagnosisRapid characterizationParticle separator tubesMicrobiological testing/measurementMicroorganismLysis
The present invention is directed to a method for separating, characterizing and / or identifying microorganisms in a test sample. The method of the invention comprises an optional lysis step for lysing non-microorganism cells that may be present in a test sample, followed by a subsequent separation step. The method may be useful for the separation, characterization and / or identification of microorganisms from complex samples such as blood-containing culture media. The invention further provides for interrogation of the separated microorganism sample by mass spectrometry to produce a mass spectrum of the microorganism and characterizing and / or identifying the microorganism in the sample using the mass spectrum obtained.
Owner:BIOMERIEUX INC
Method for the production and purification of adenoviral vectors
The present invention addresses the need to improve the yields of viral vectors when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of low-medium perfusion rates in an attached cell culture system provides for improved yields. In other embodiments, the inventors have shown that there is improved Ad-p53 production cells grown in serum-free conditions, and in particular in serum-free suspension culture. Also important to the increase of yields is the use of detergent lysis. Combination of these aspects of the invention permits purification of virus by a single chromatography step that results in purified virus of the same quality as preparations from double CsCl banding using an ultracentrifuge.
Owner:JANSSEN VACCINES & PREVENTION BV
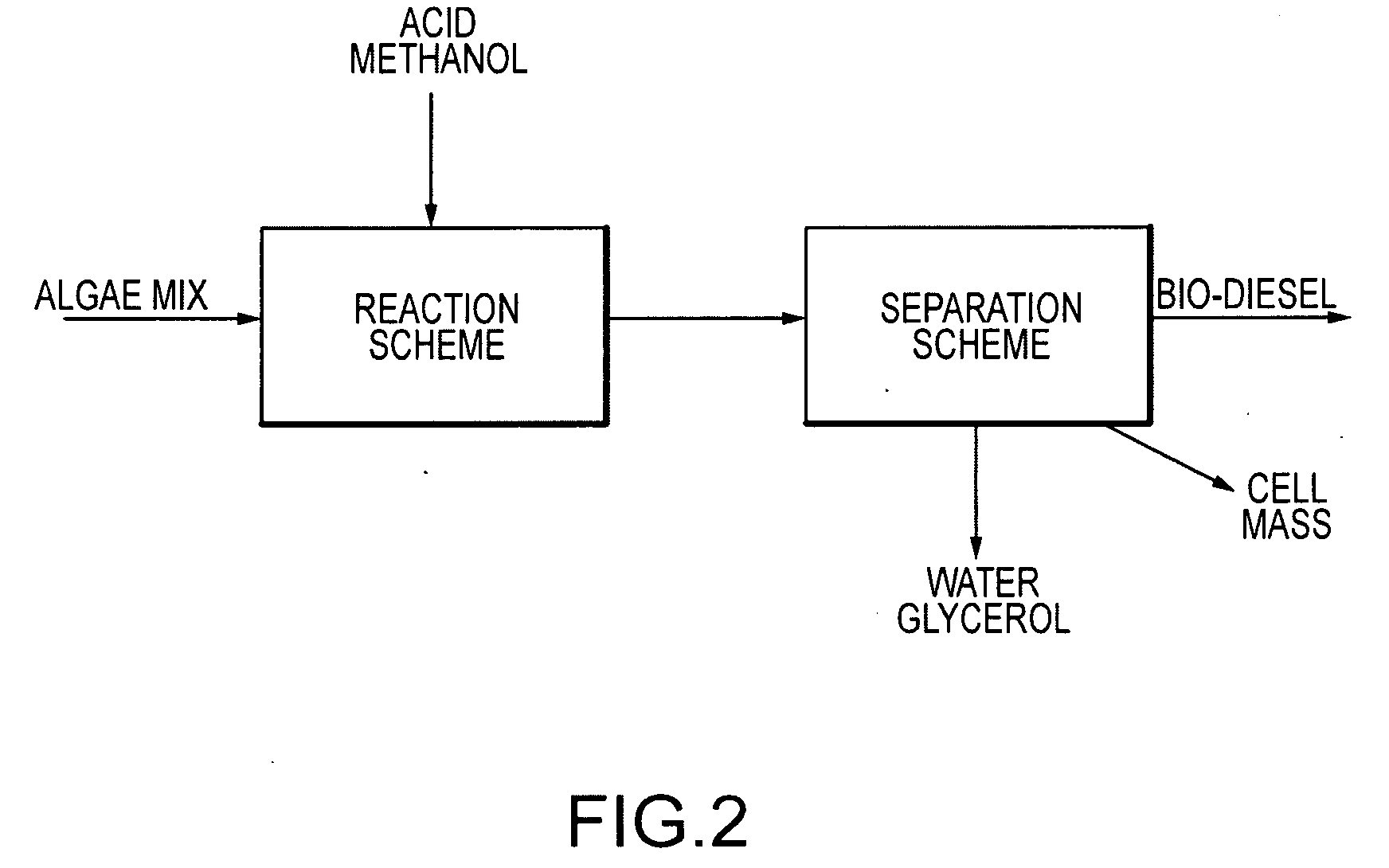
Continuous algal biodiesel production facility
Embodiments of the present invention concern methods, compositions, and apparatus for the continuous conversion of algal lipids into biodiesel. In some embodiments, the biodiesel is formed in a multi-step sequence, the first steps occurring in the presence of water and a strong acid wherein the lipids are released from the algae by means of mechanical and chemical action and are then hydrolyzed to free fatty acids. In a subsequent step, this free fatty acid mixture is reacted with methanol to generate fatty acid methyl esters (also known as biodiesel). Such methods produce biodiesel from algal lipids without the requirement for separate algal cell lysis or lipid extraction or purification prior to the acid catalysis sequence. In other embodiments, the multi-step acid catalysis sequence occurs at 100° C. at two atmospheres of pressure.
Owner:COLORADO STATE UNIVERSITY +1
Integrated PCR Reactor for Cell Lysis, Nucleic Acid Isolation and Purification, and Nucleic Acid Amplication Related Applications
ActiveUS20090186357A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle vesselLysis
Disclosed are integrated devices capable of performing a polymerase chain reaction within a single vessel. Also disclosed are related methods of sample analysis.
Owner:NEW YORK UNIV
Device and method for contacting picoliter volumes of fluid
InactiveUS20070099289A1Avoid adsorptionBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteLysis
The invention features devices for mixing fluids, e.g., for lysing cells, and methods of use thereof. One device is based on the ability to control the flow of fluids, e.g., by contact angle and channel size. Fluids in this device can be divided to form segments of controlled volume, which are then brought together to initiate midxing. An exemplary use of the device is for the lysis of single cells. Another device is based on the ability to two mix two fluids in a channel and affinity capture of analytes. The devices can be integrated on the same chip with other devices, for example, for cell handling or analysis of DNA, rnRNA, and proteins released from the lysis of a cell.
Owner:THE GENERAL HOSPITAL CORP
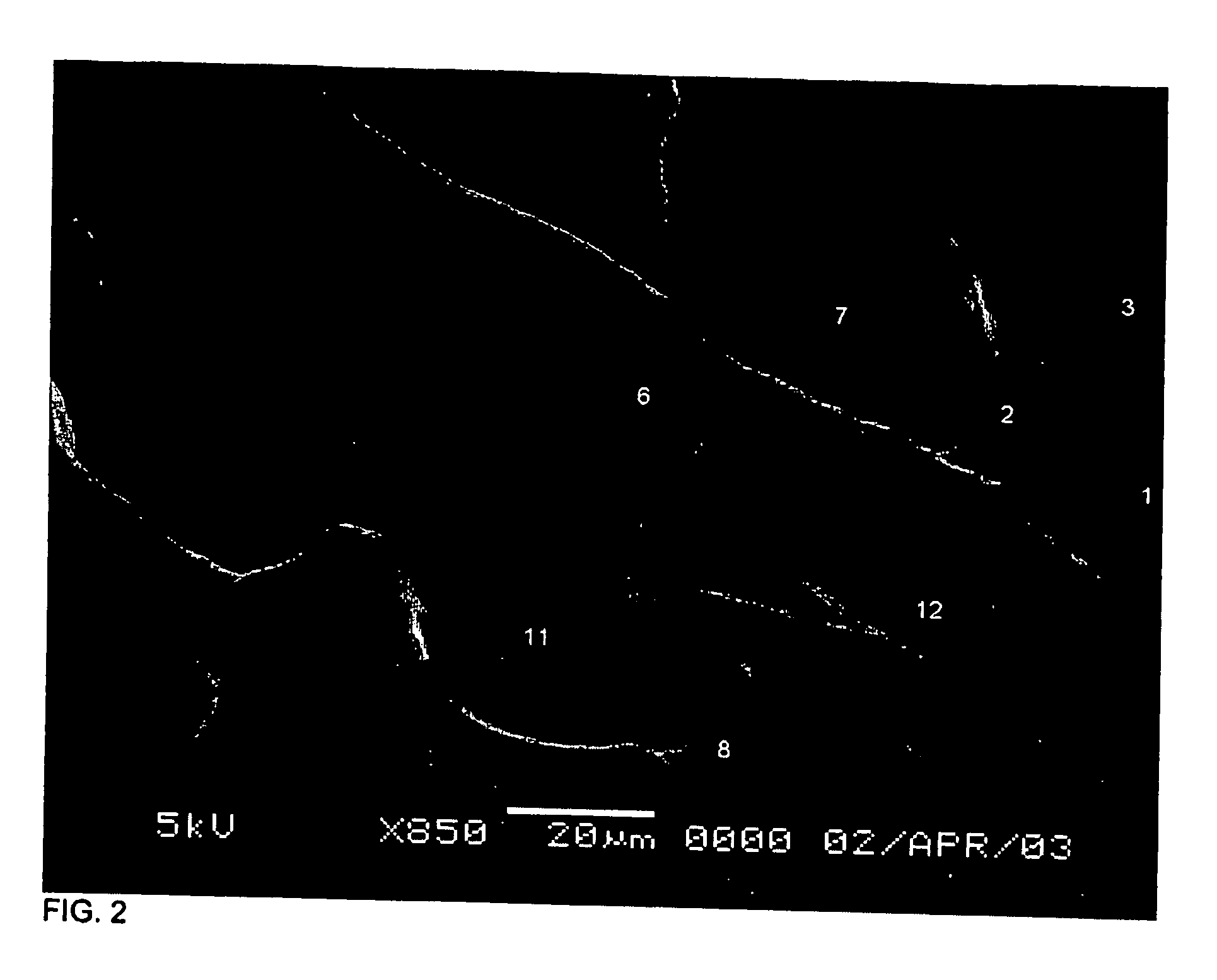
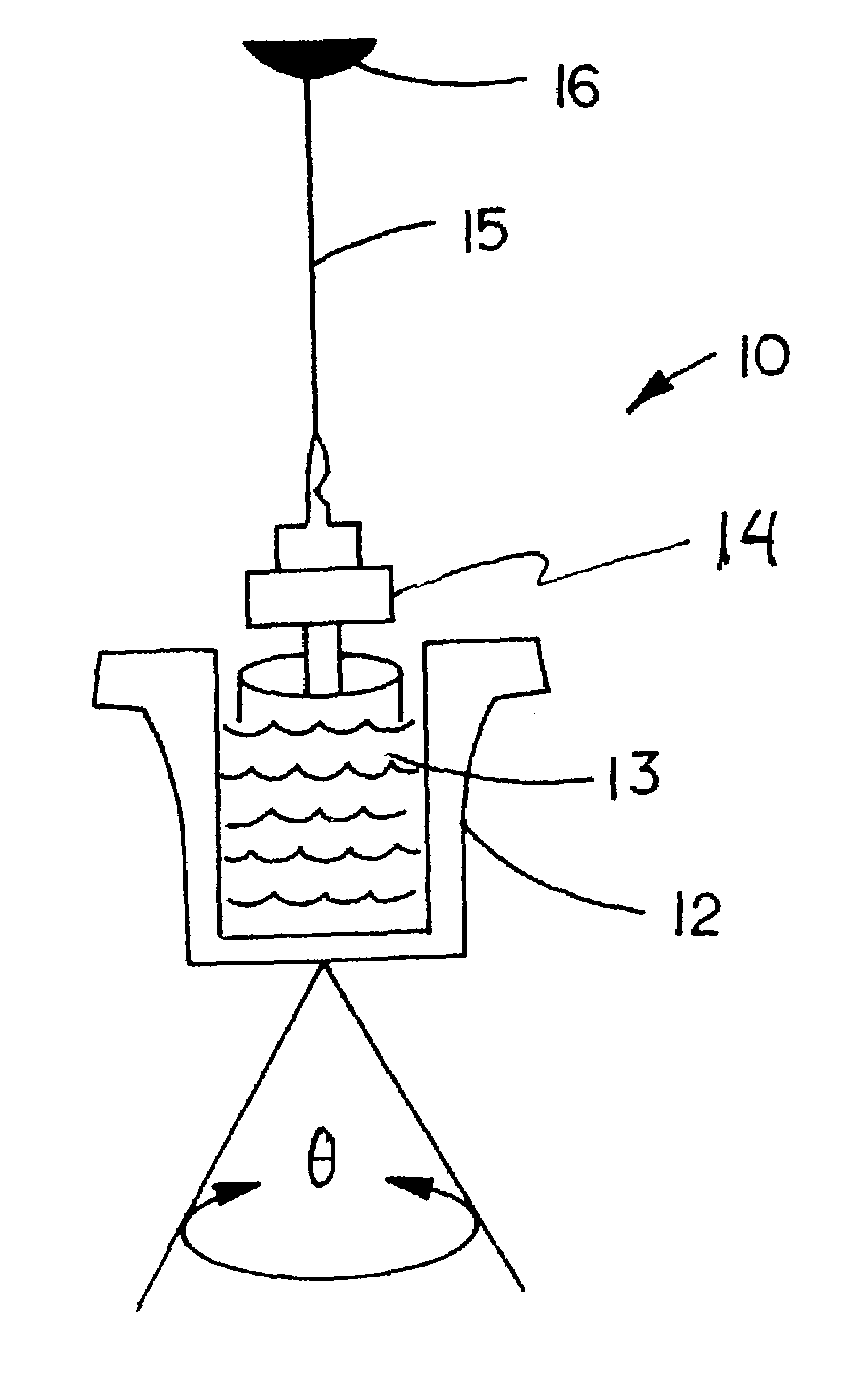
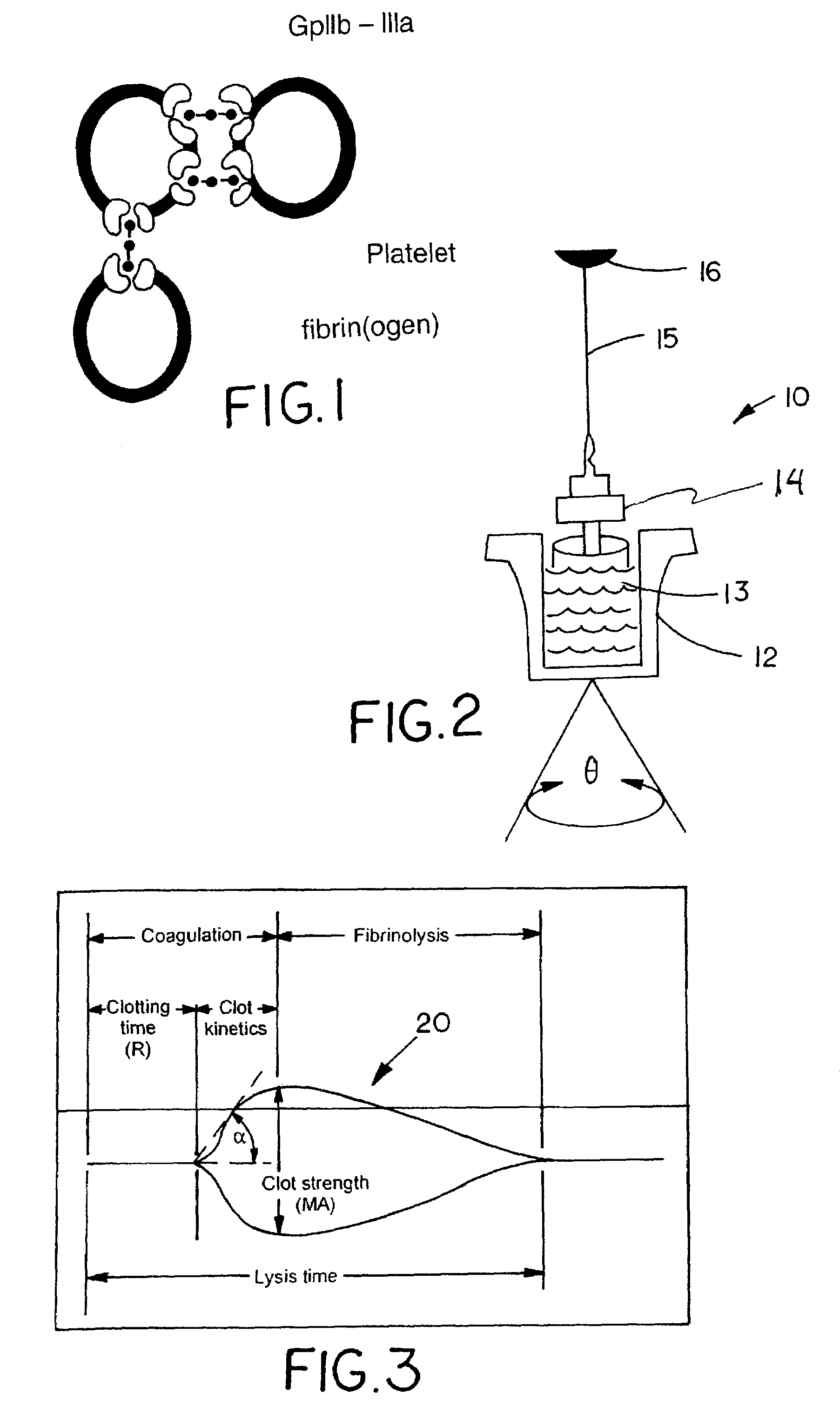
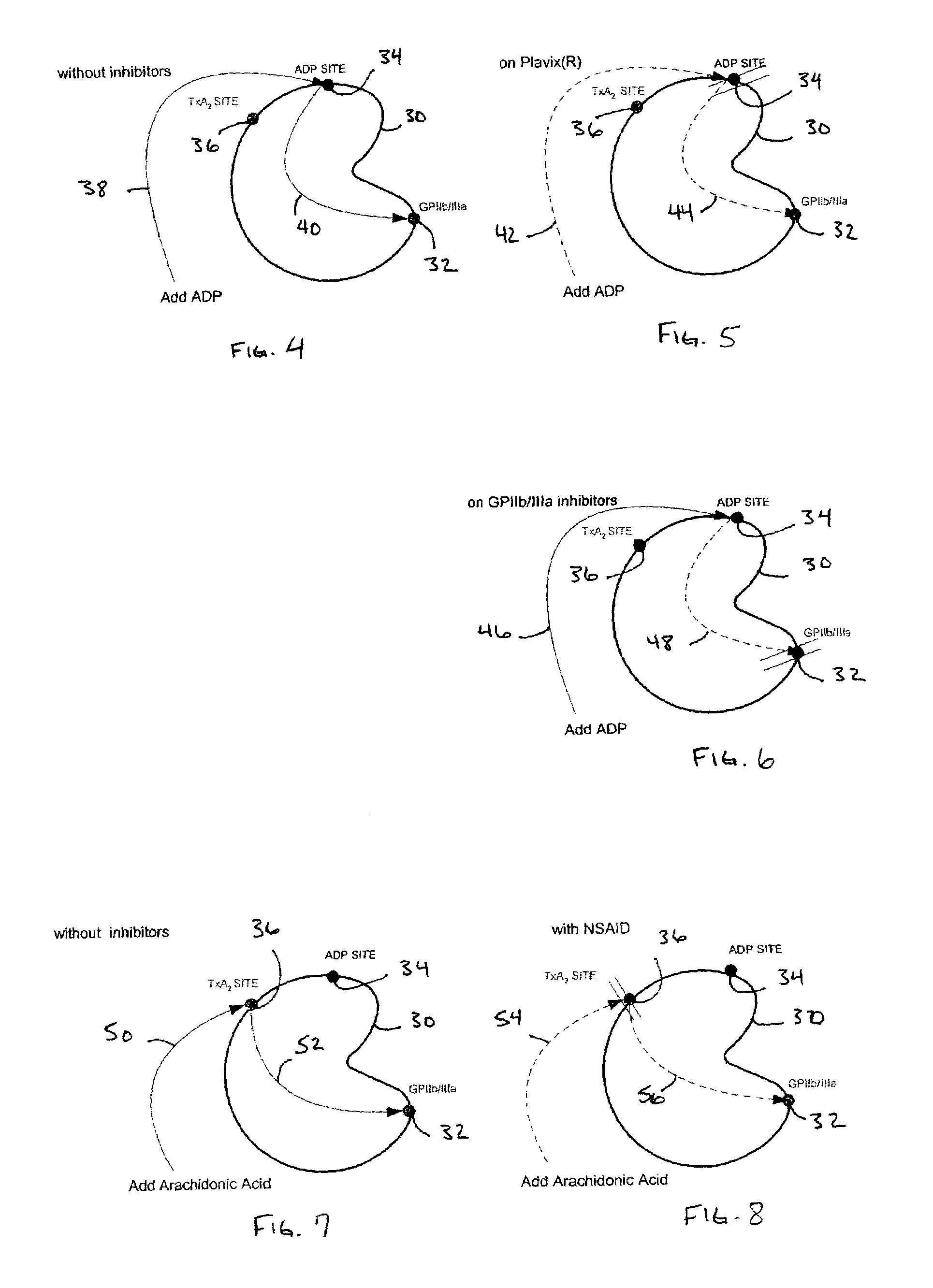
Protocol for monitoring platelet inhibition
A hemostasis analyzer, such as the Thrombelastograph® (TEG®) hemostasis analyzer is utilized to measure continuously in real time, the hemostasis process from the initial fibrin formation, through platelet-fibrin interaction and lysis to generate blood hemostasis parameters. The measured blood hemostasis parameters permit evaluation of platelet inhibition therapy.
Owner:HAEMONETICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com