Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3709results about How to "Inhibition release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
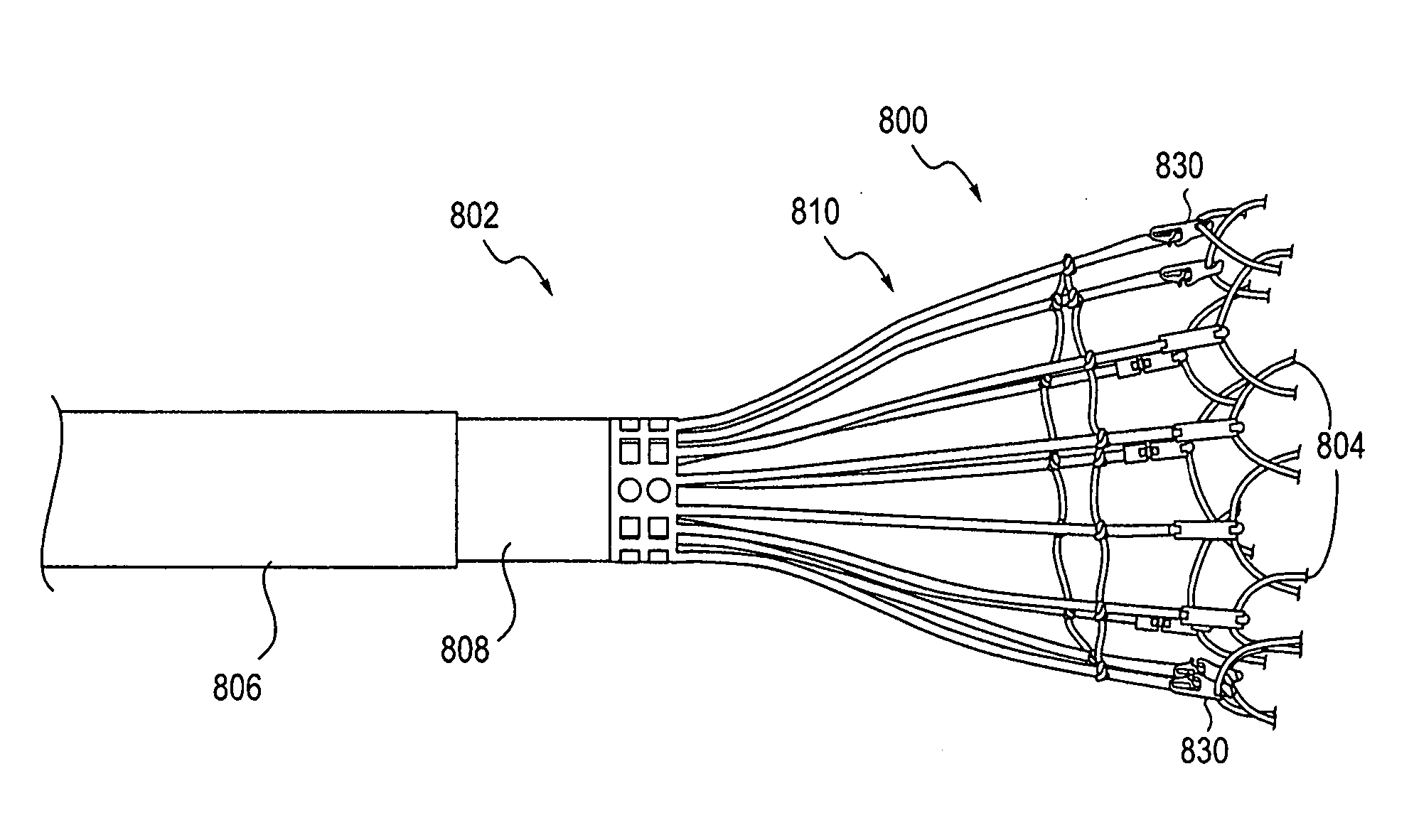
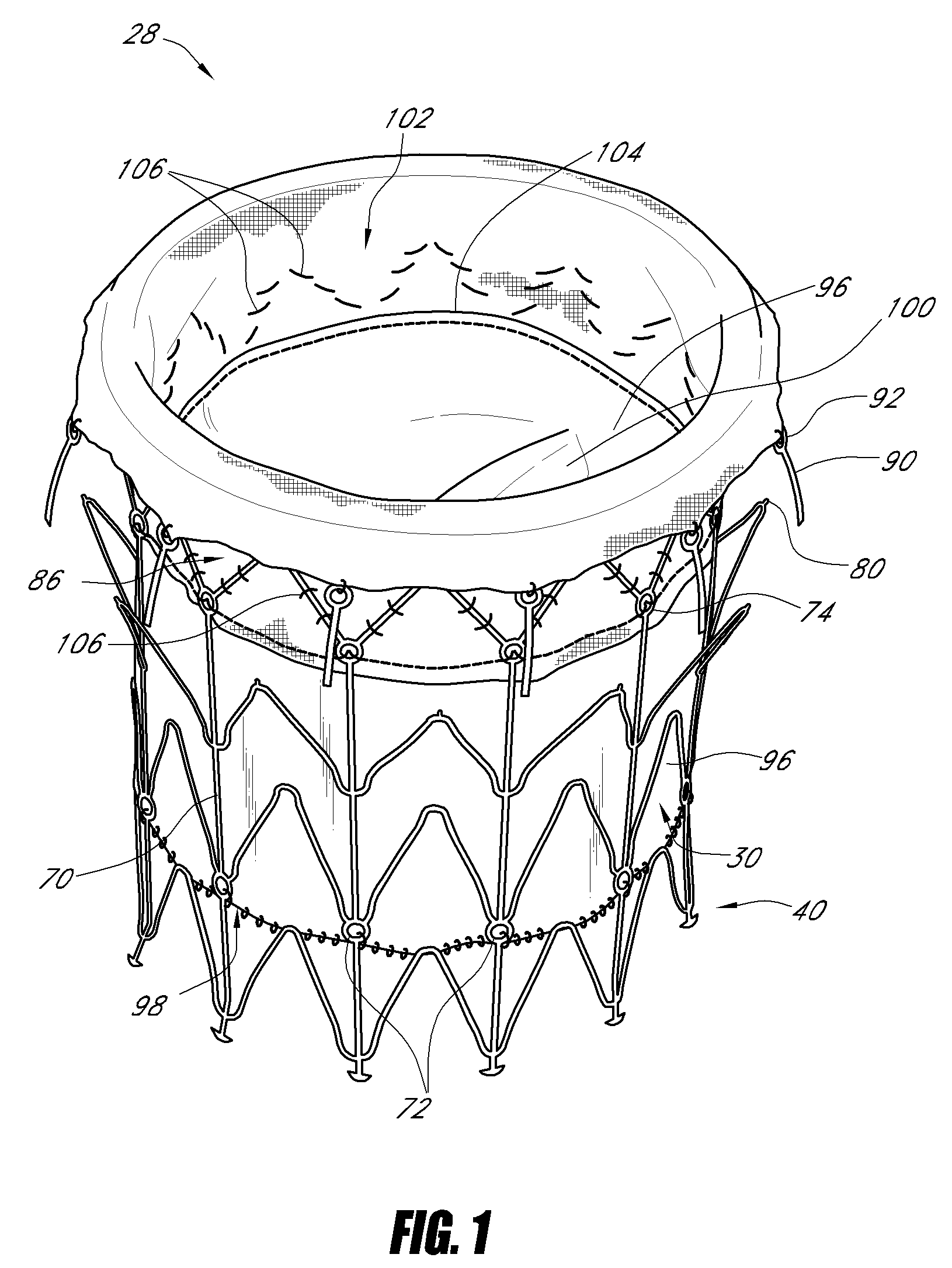
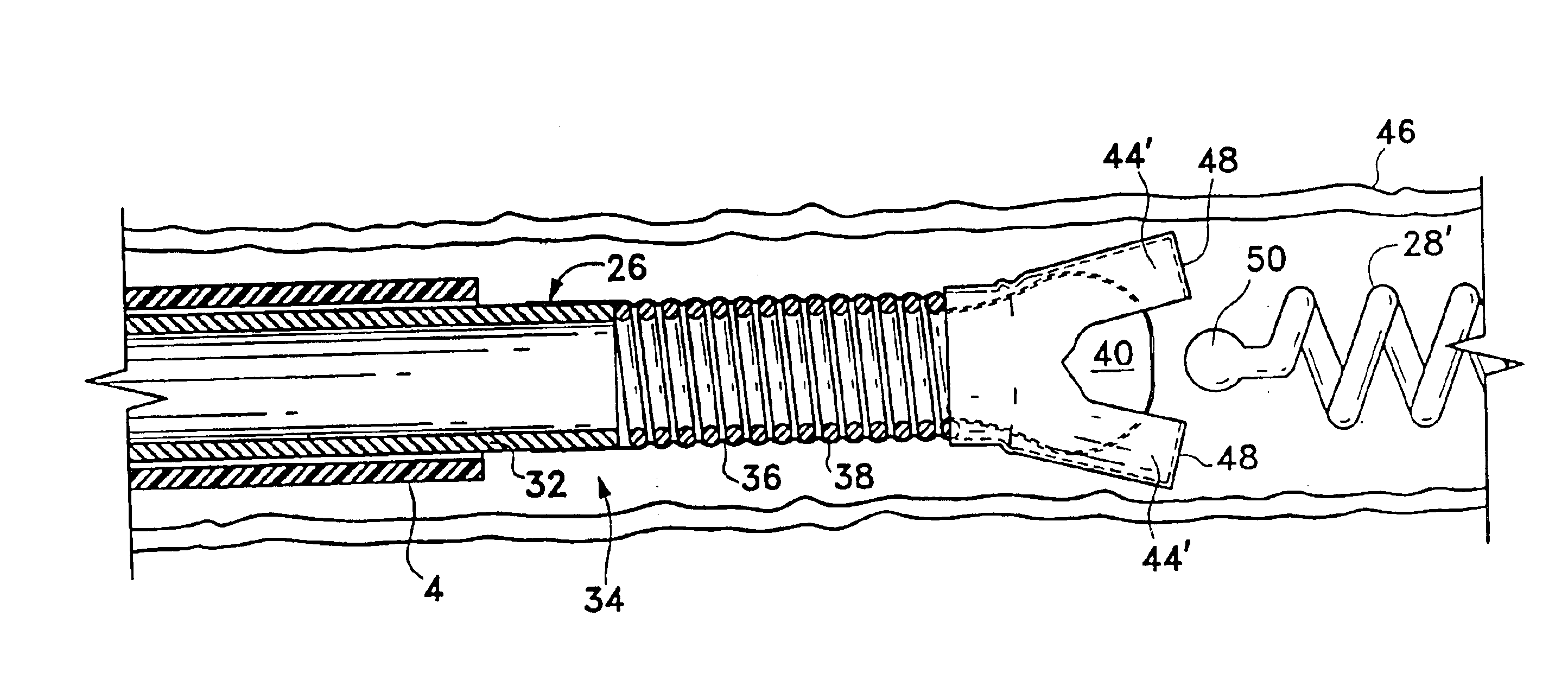
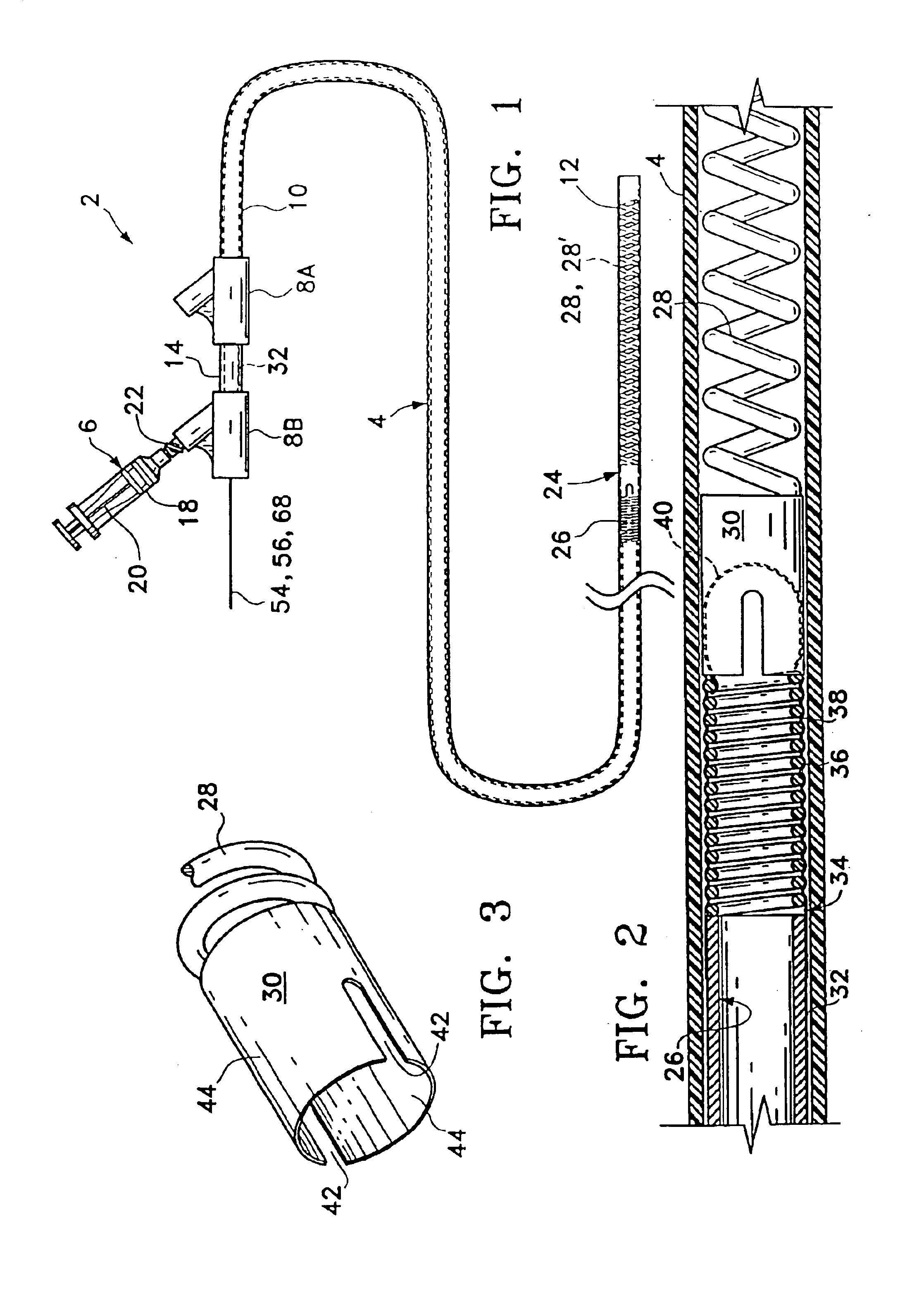
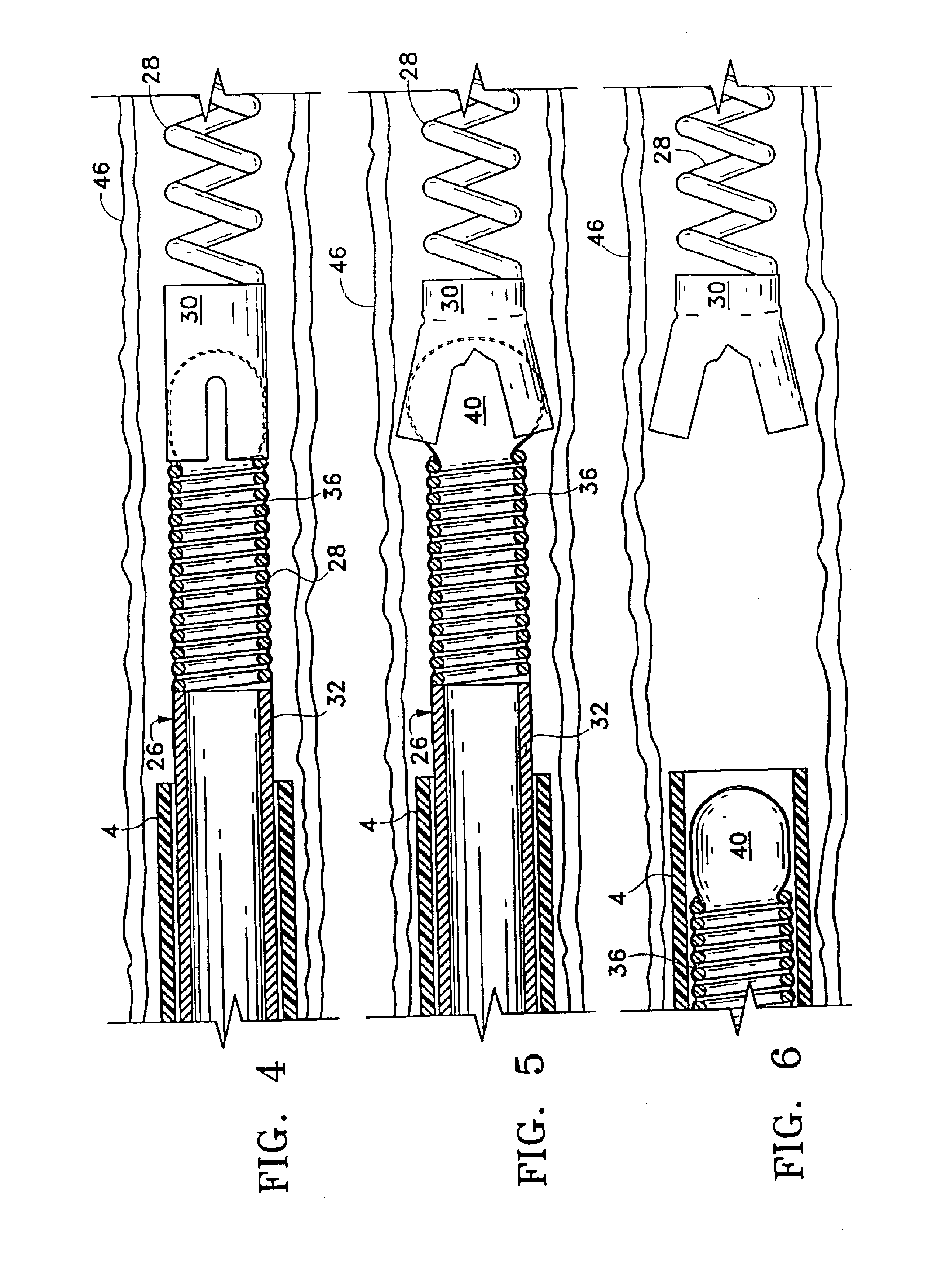
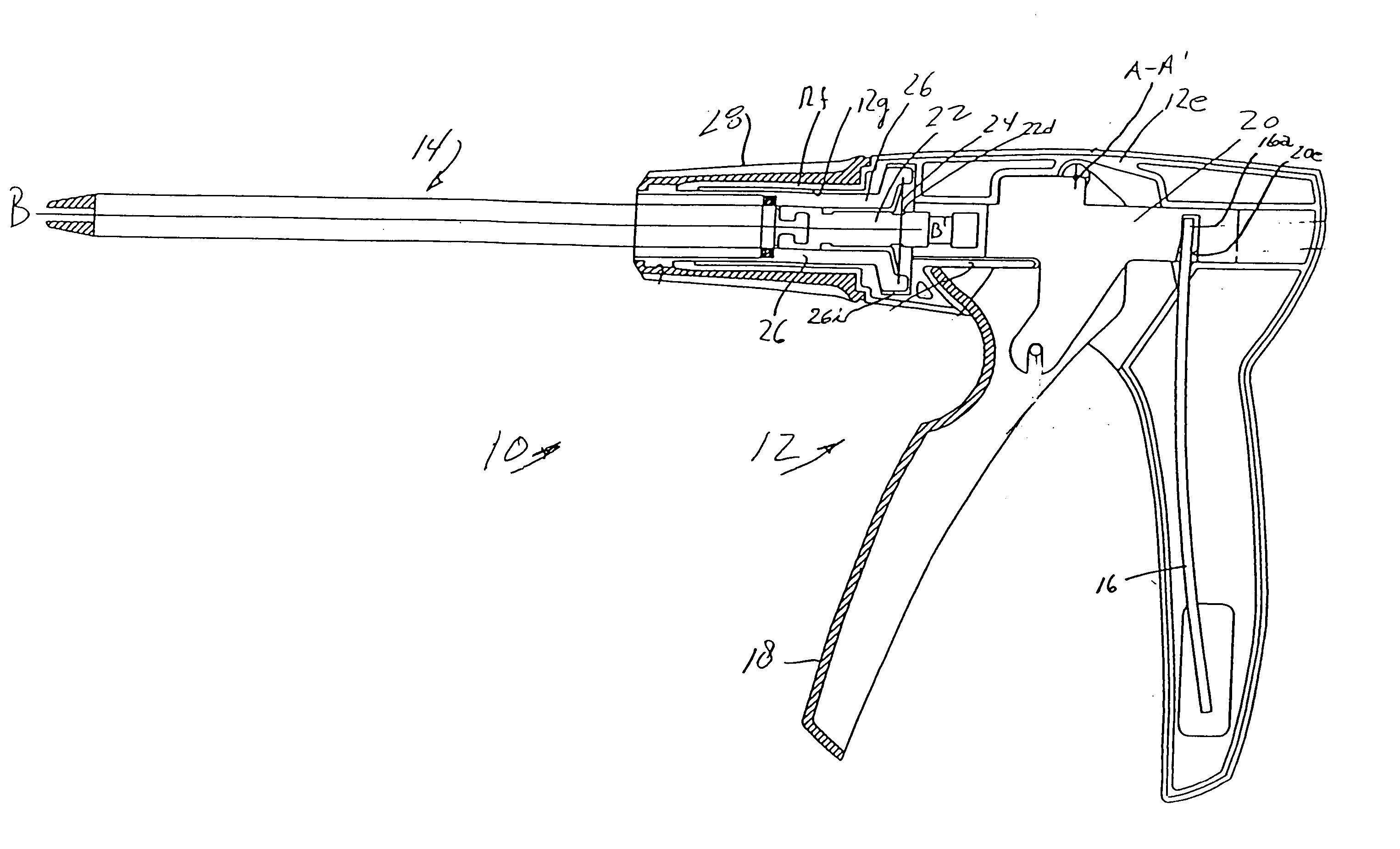
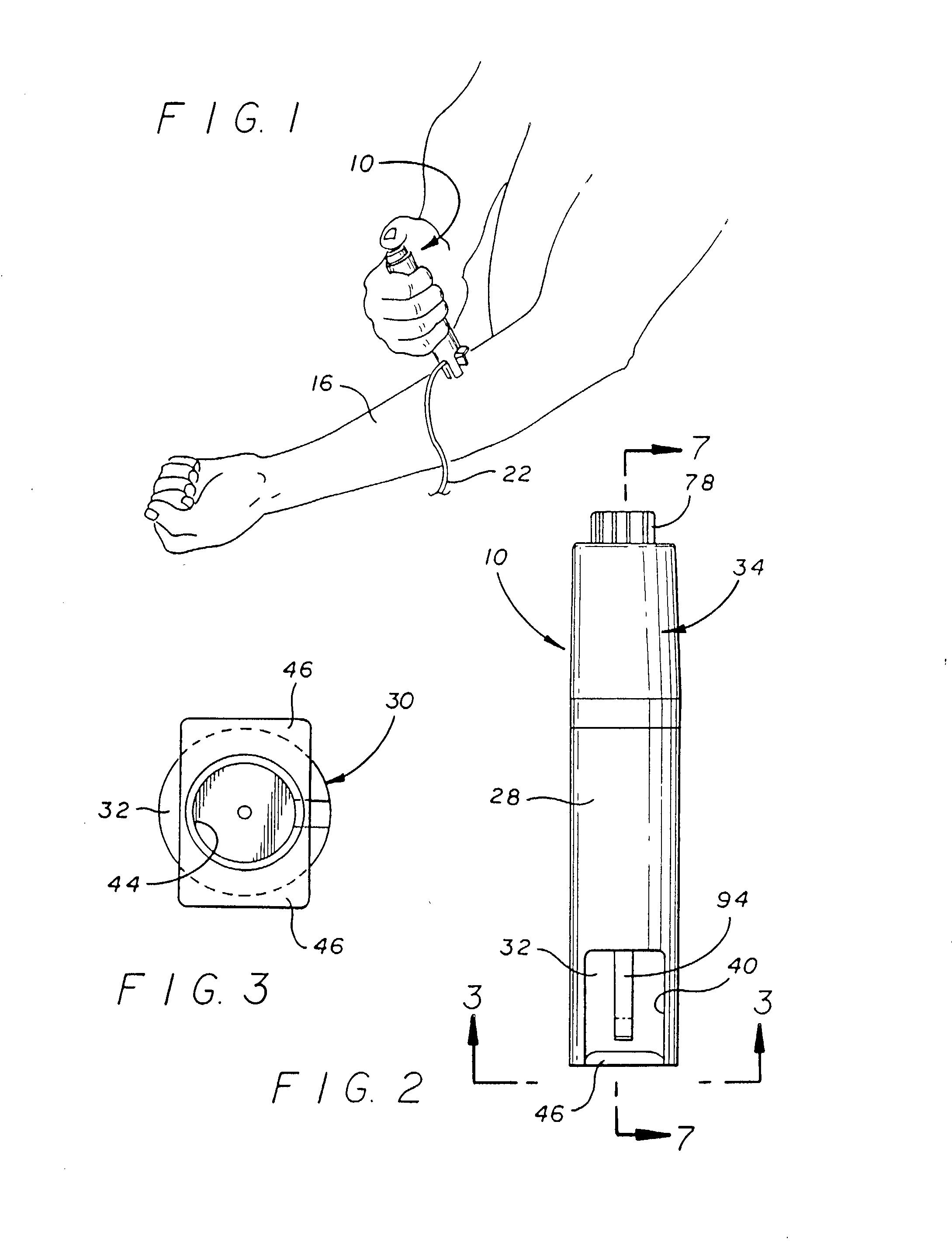
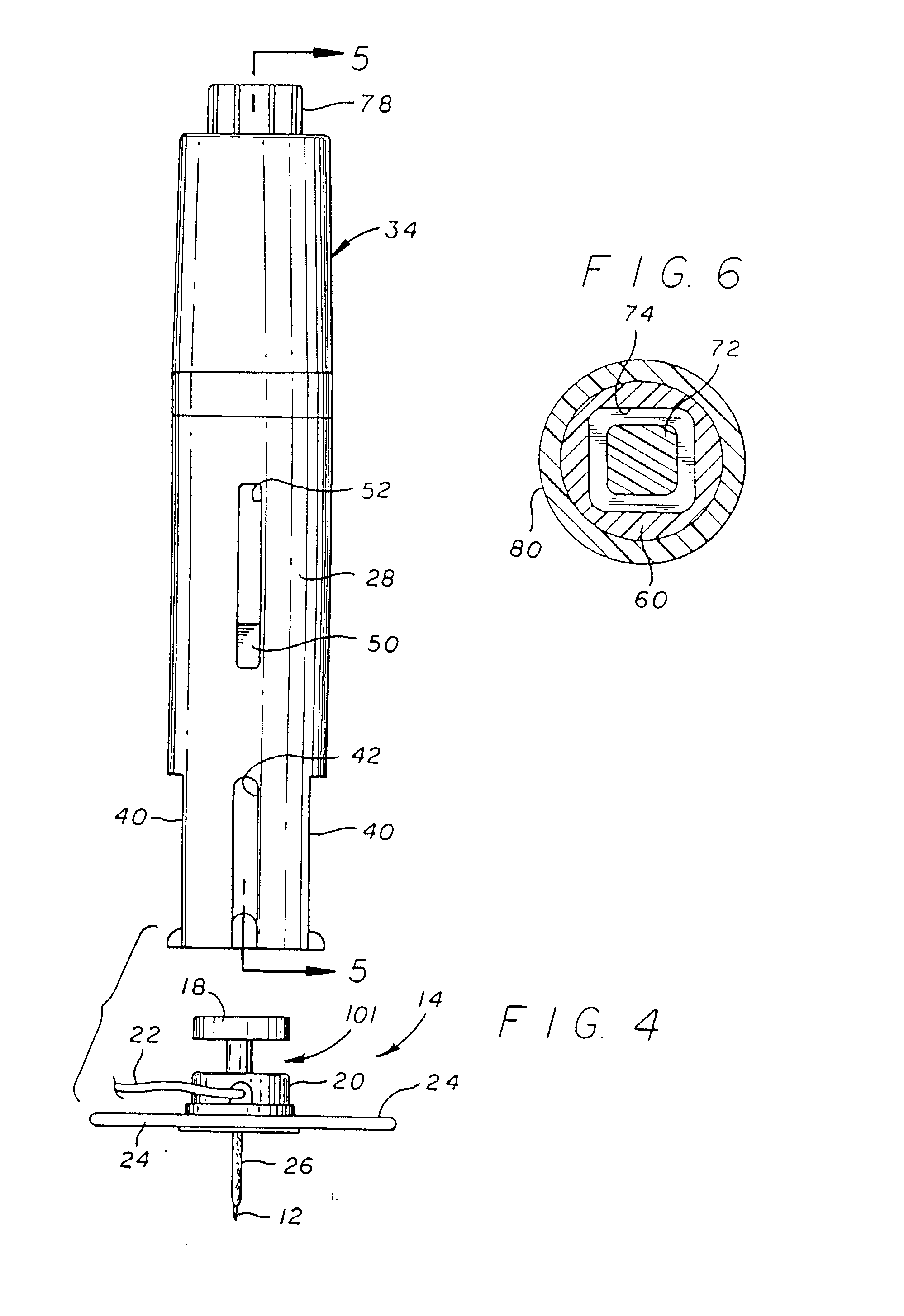
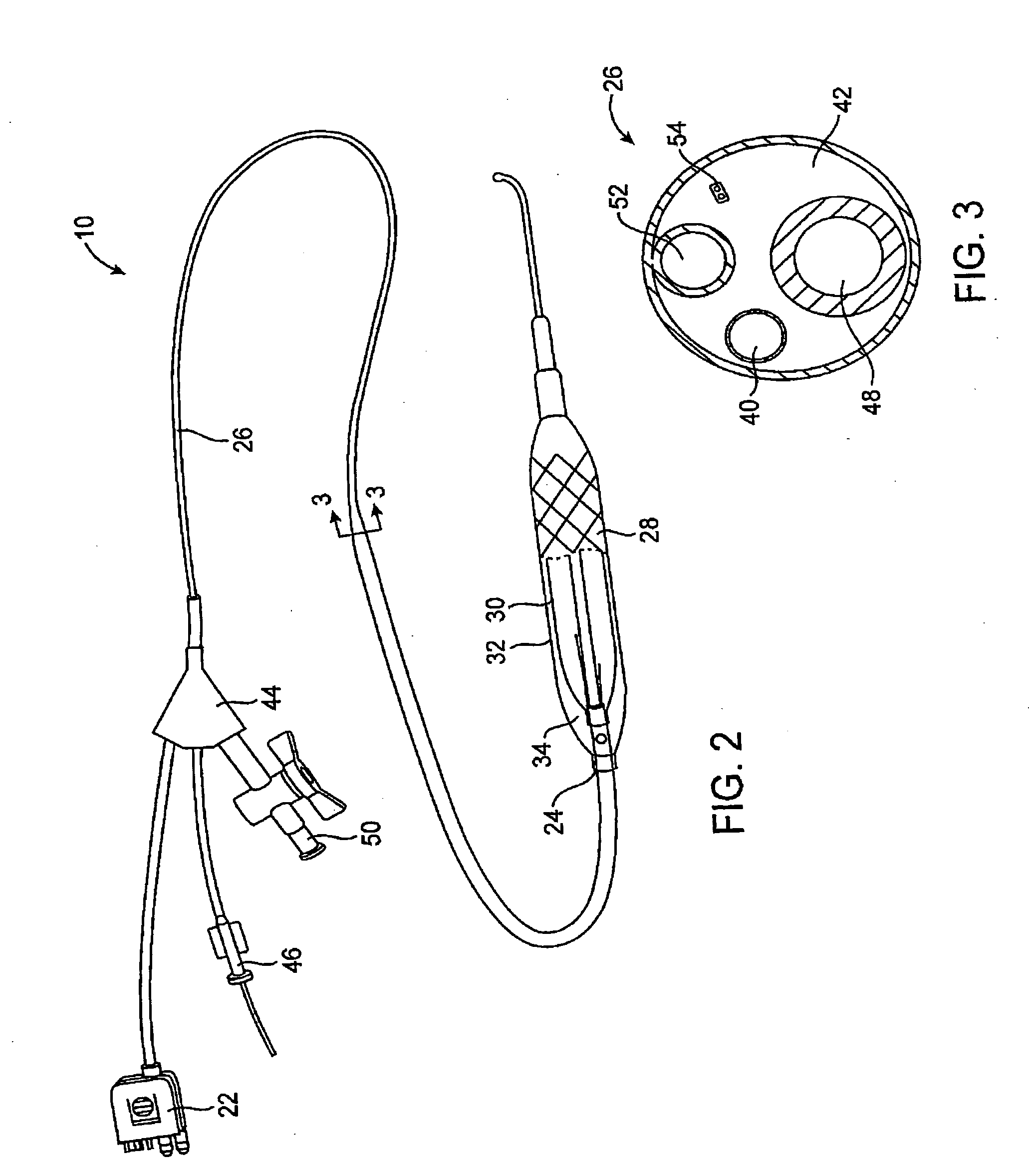
Systems and methods for delivering a medical implant
The present invention relates to apparatus and methods for endovascularly delivering and releasing a prosthesis, e.g., an aortic prosthesis, within and / or across a patient's native heart valve, referred to hereinafter as replacing the patient's heart valve. In some embodiments the delivery system comprises a plurality of first actuatable element adapted to engage a plurality of second elements in a first configuration to capture the implant within the delivery system, and wherein the plurality of first actuatable element are adapted to engage the plurality of second elements in a second configuration and to release the implant from the delivery system.
Owner:BOSTON SCI SCIMED INC
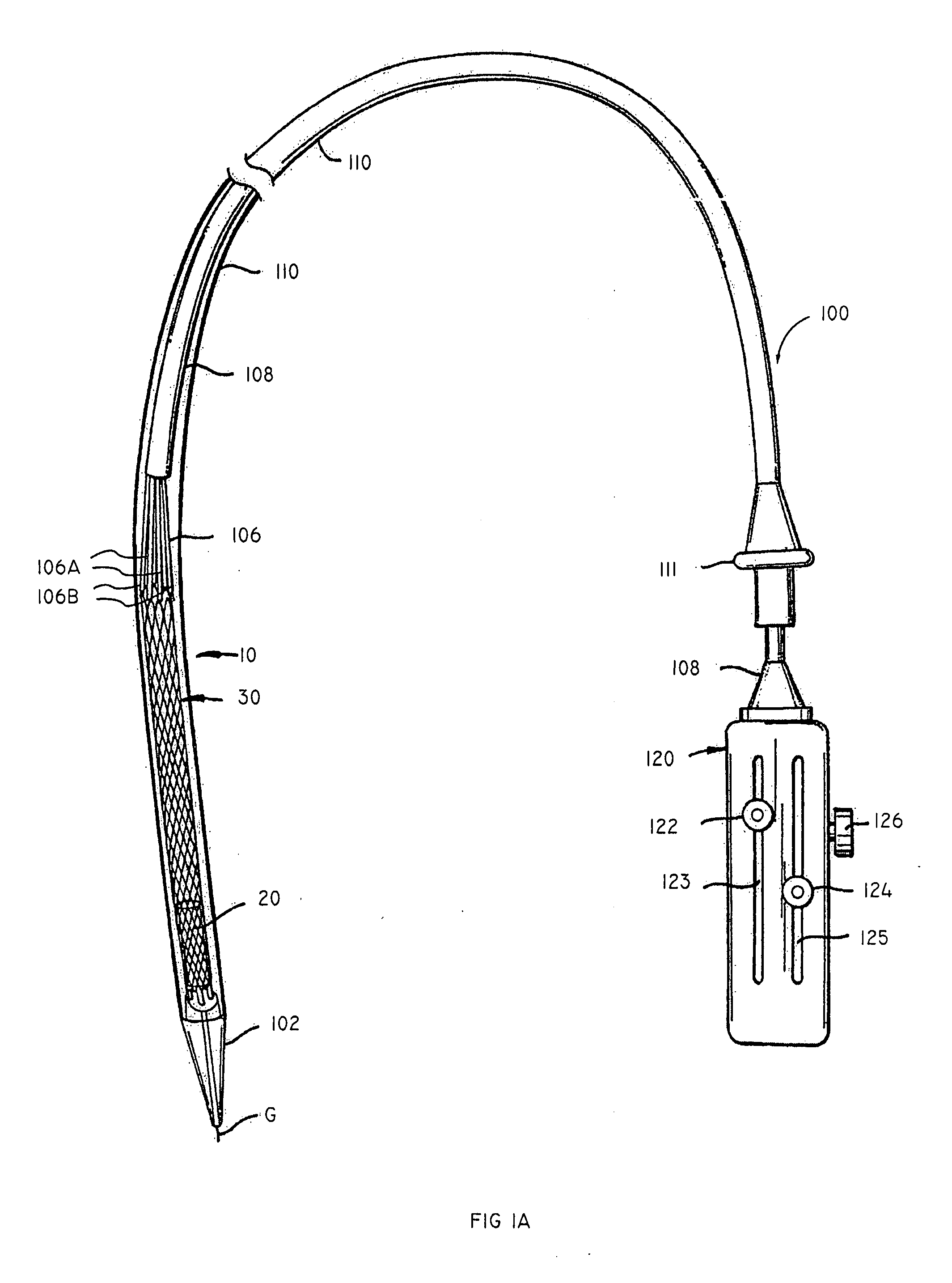
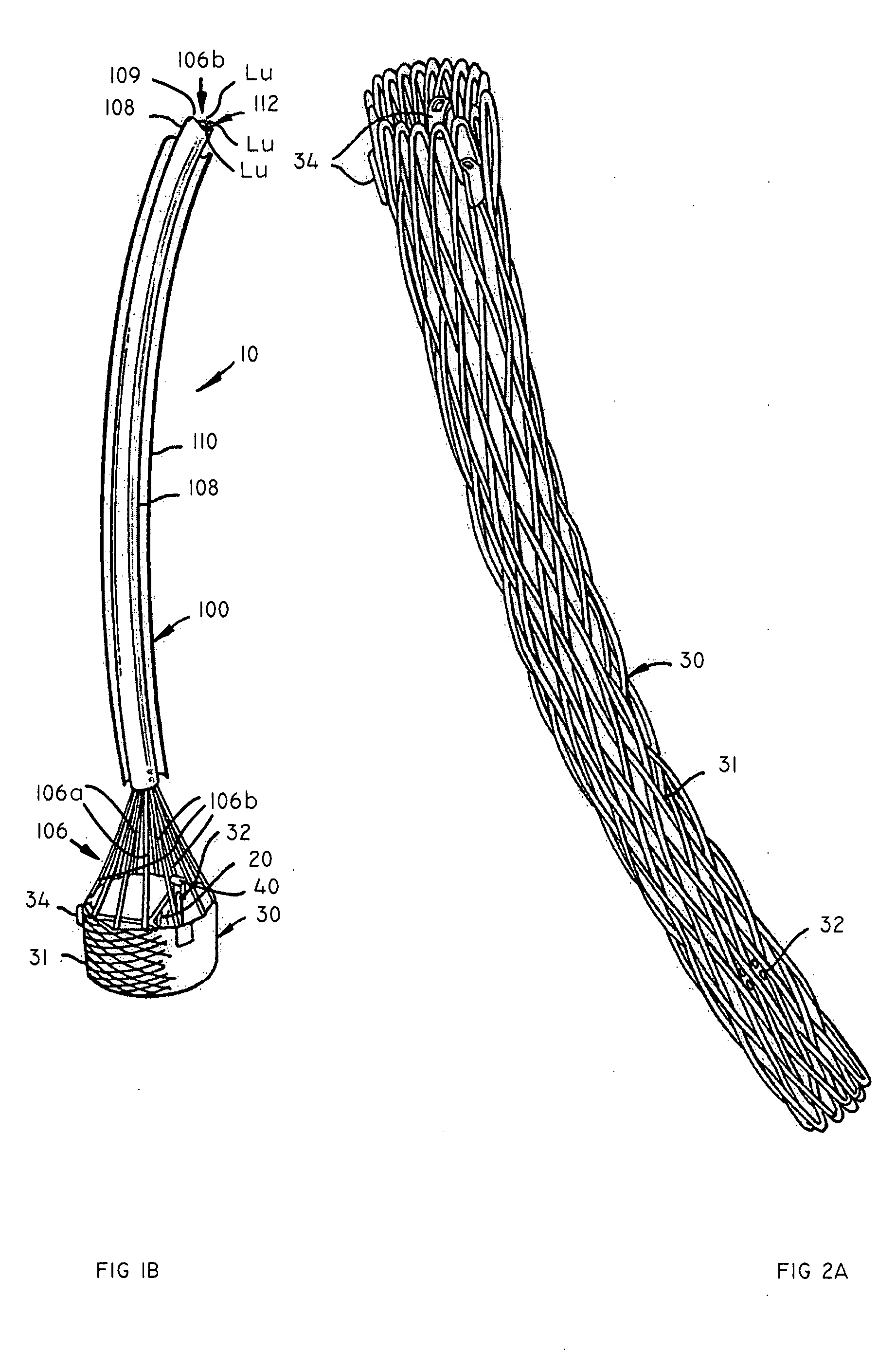
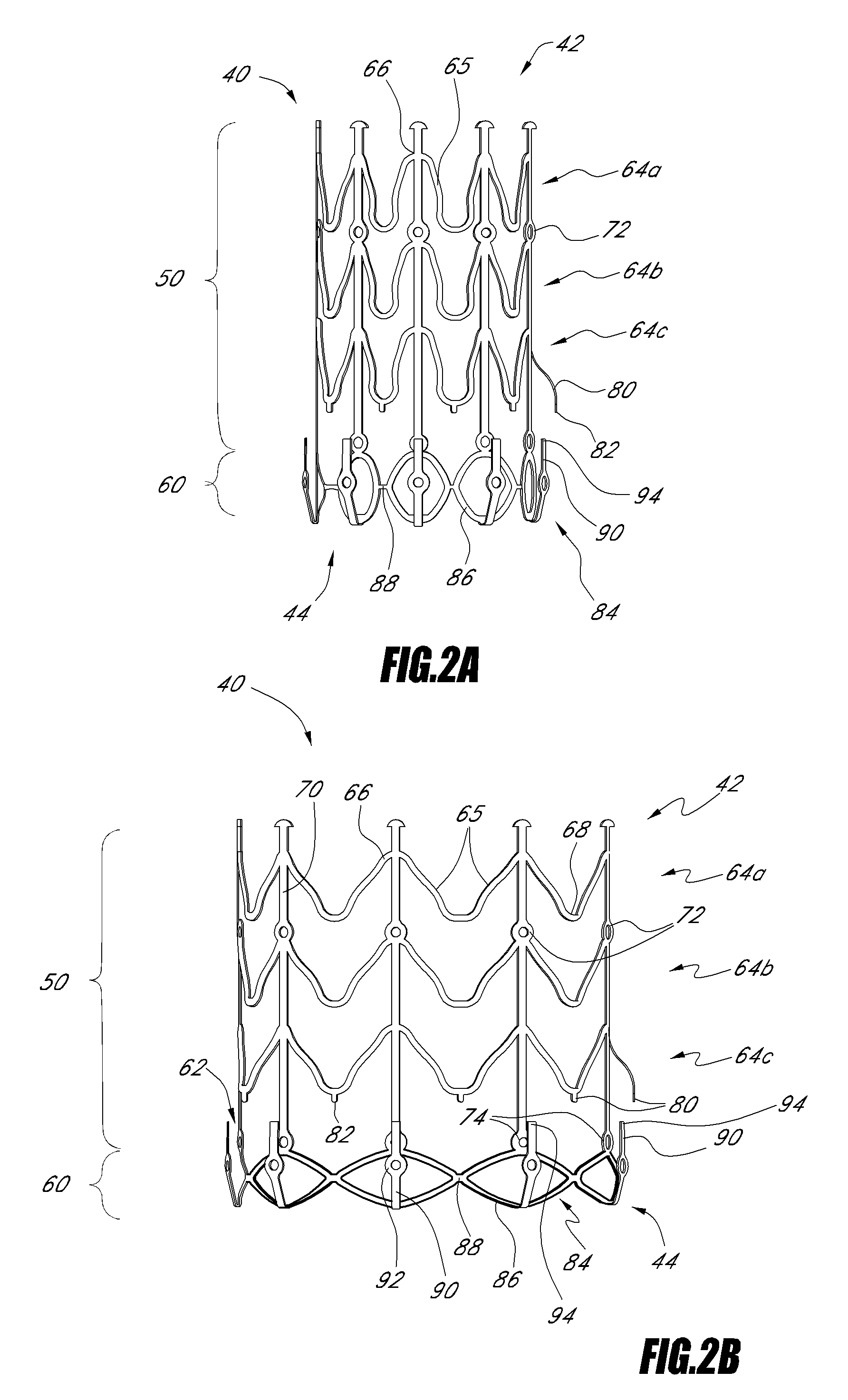
Vascular implant and delivery system
ActiveUS8414644B2Minimize axial movementPrevent radial movementStentsHeart valvesVascular implantLocking mechanism
A compacted vascular implant can be delivered to a target location within a delivery device. During delivery, the implant can be partially released from the delivery device before full deployment. The delivery device can include an elongated support tube, a locking mechanism, and a sheath. The locking mechanism can be provided on the support tube. The sheath can be configured to slide over the elongated support tube and can be configured to cover the locking mechanism and to restrain at least a portion of the implant.
Owner:EDWARDS LIFESCI CARDIAQ
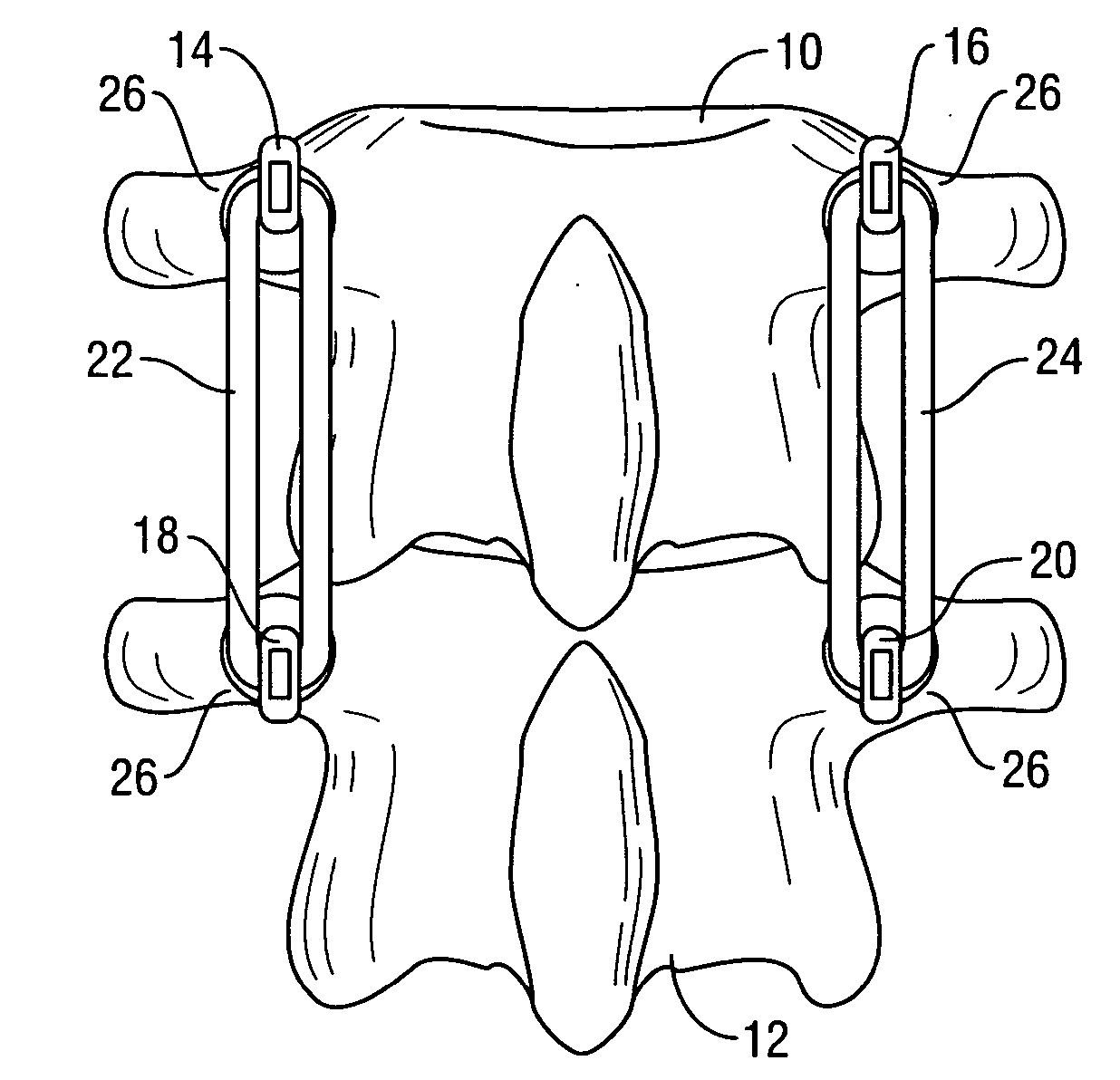
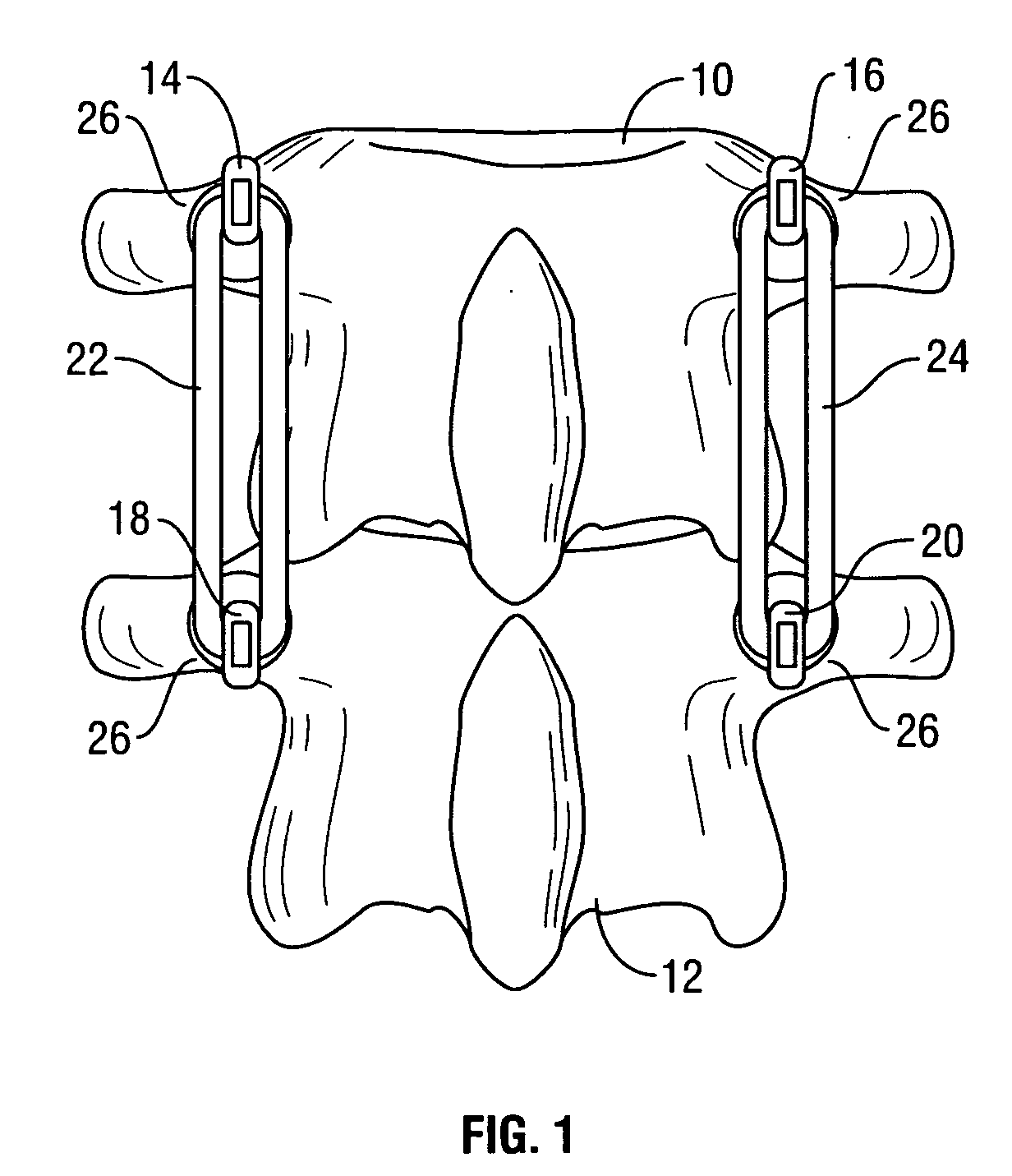
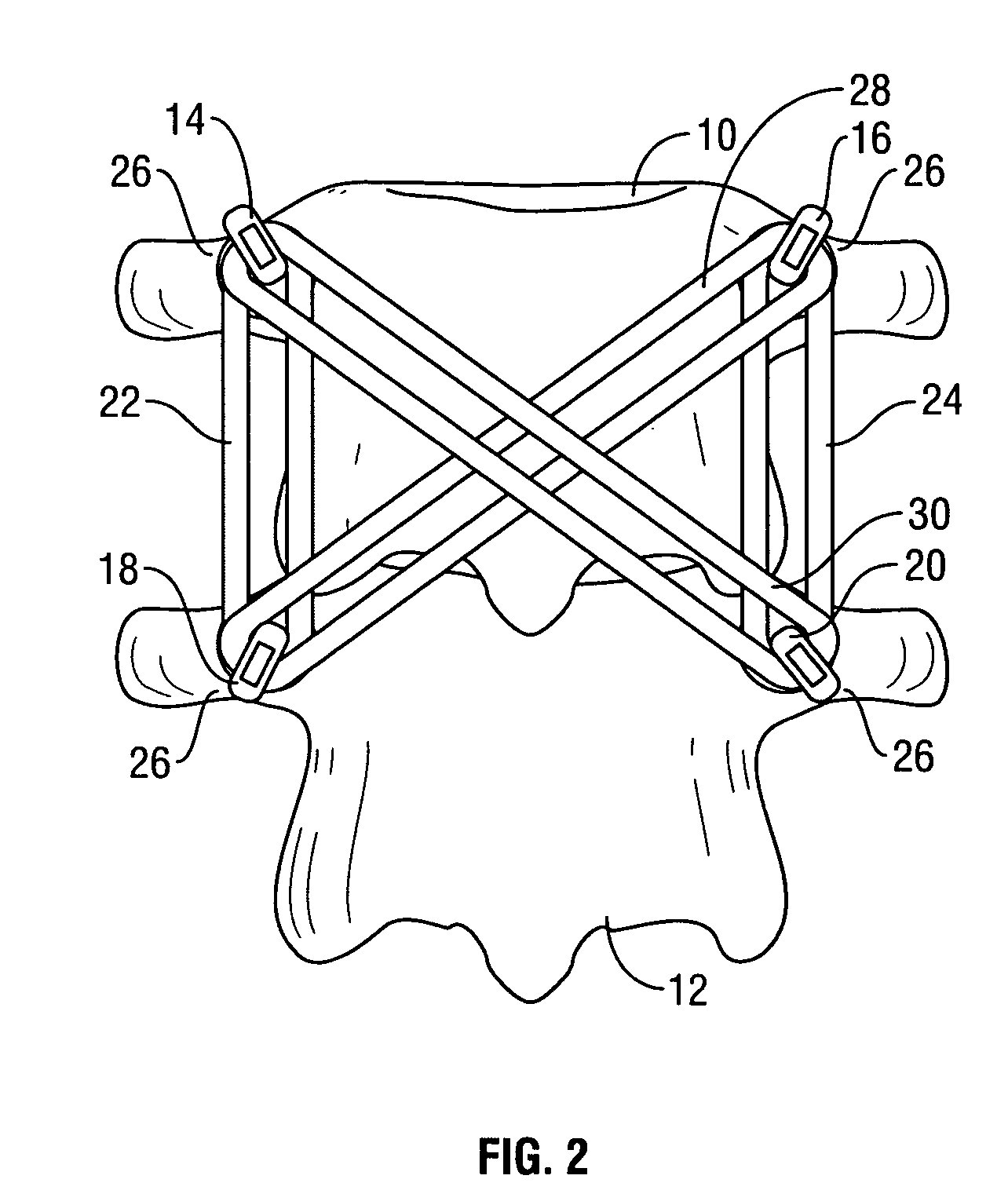
Spinal stabilization system to flexibly connect vertebrae
InactiveUS20050267470A1Preventing excessive motionPrecise alignmentInternal osteosythesisJoint implantsSpinal columnDeformity
A surgically implanted spinal stabilization system uses posterior anchor hooks attached to vertebrae to retain elastic bands to retain flexibility and mobility while maintaining alignment and preventing excessive motion and deformity. The elastic bands may parallel the longitudinal axis of the spine, or, for enhanced promotion of alignment, they may also arranged in a diagonally crossing configuration. Multi-level fixation can be achieved using the spinal stabilization system with longer elastic bands. A method of applying the spinal stabilization system using an elastic band application tool facilitates simple, rapid application of the system to a patient.
Owner:MCBRIDE DUNCAN Q
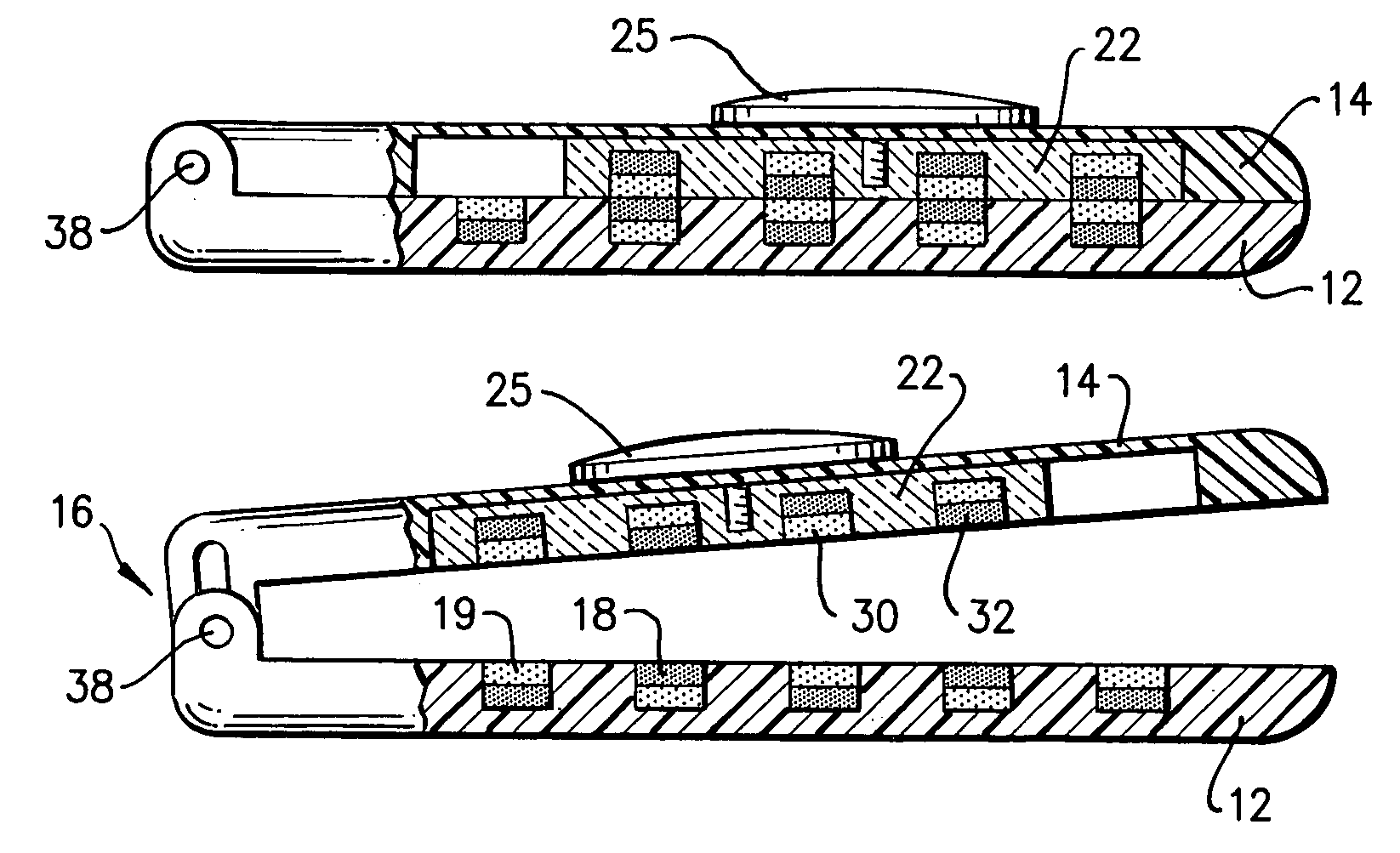
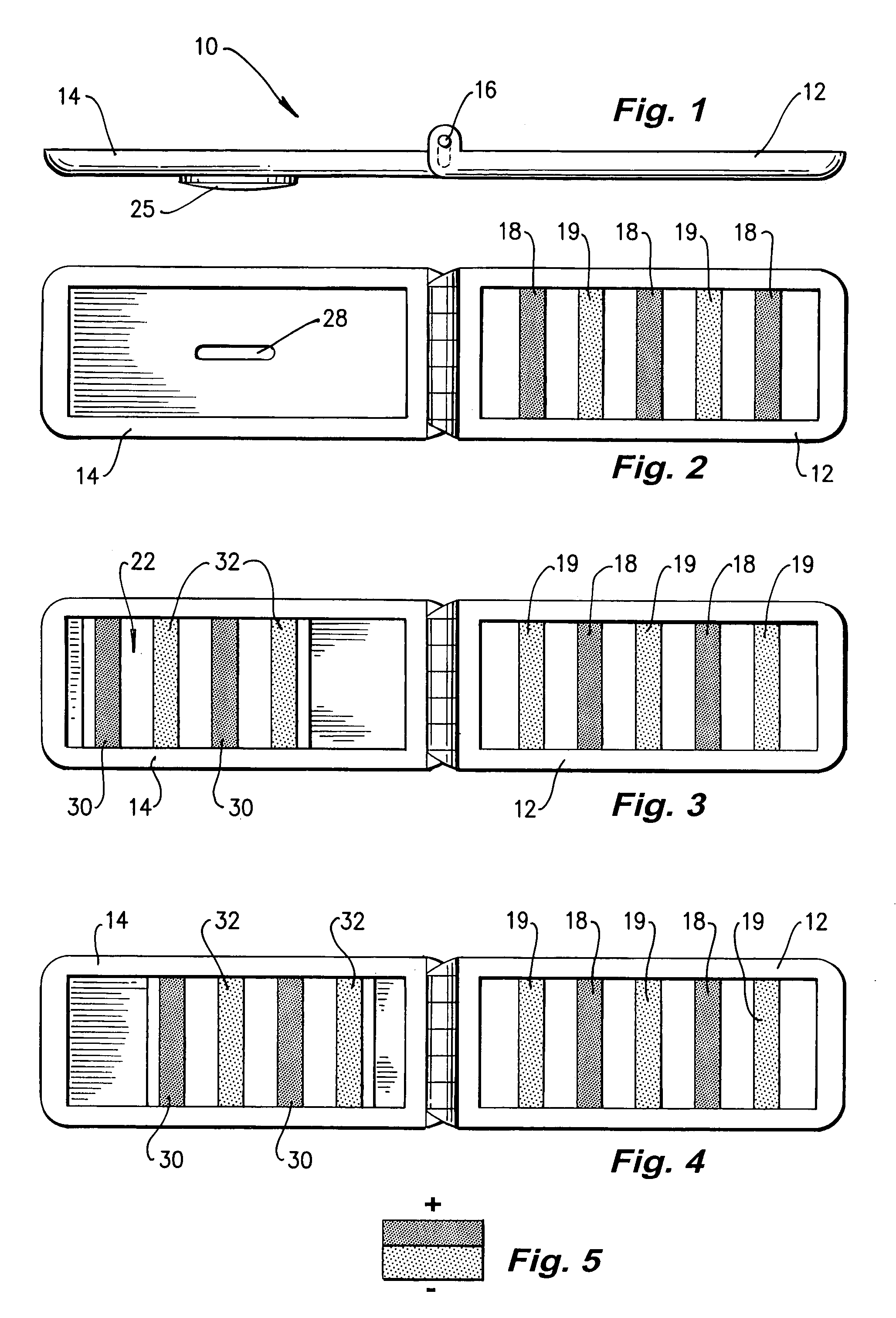
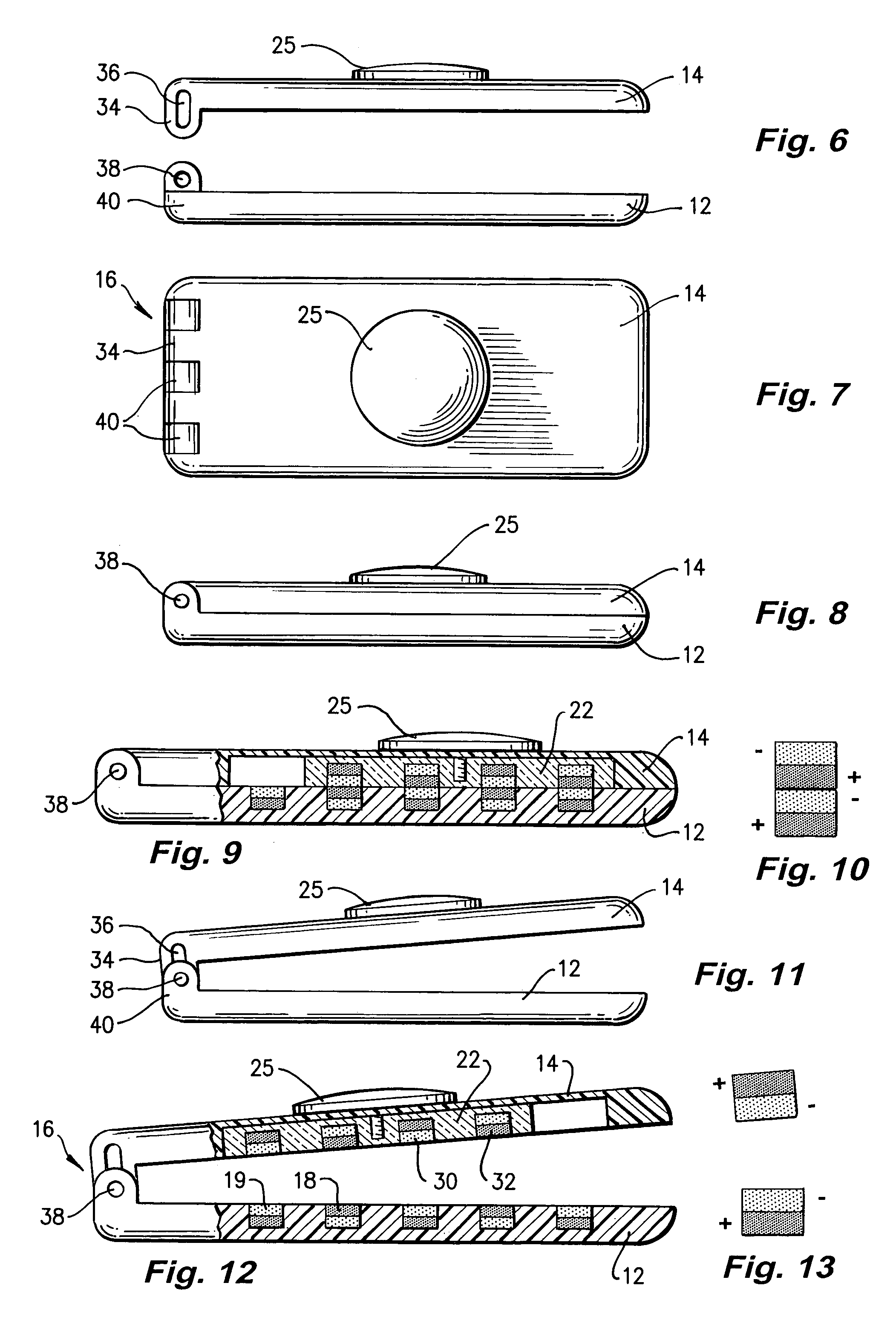
Clip
A clip (10) for holding banknotes has a first clasp portion (14) and a second clasp portion (12) for holding banknotes therebetween. The first clasp portion (14) has a first magnetic portion (22) including magnetic material (30,32) and the second clasp portion has a second magnetic portion including magnetic material (18,19). The first magnetic portion (22) is moveable relative to the second magnetic portion between: a closed position whereby the interaction of the magnetic material of the first magnetic portion (30,32) and second magnetic portion (18,19) is such that there is a net force of attraction to hold the first and second clasp portions (14,12) together; and an open position whereby the first and second clasp portions (14,12) are released apart.
Owner:HALSTEAD PAUL ANTHONY
Abuse-safeguarded dosage form
A pharmaceutical dosage form that is safeguarded against abuse containing at least one active substance that is susceptible to abuse and at least two of the following constituents (a) through (d): (a) at least one substance that irritates the nasal and / or pharyngeal region; (b) at least one viscosity increasing agent that together with a required minimum quantity of an aqueous liquid forms a gel in an extract obtained from the dosage form, which gel can still be discerned after being introduced into an additional quantity of aqueous liquid; (c) at least one antagonist for the at least one active substance that is susceptible to abuse; and (d) at least one emetic.
Owner:GRUNENTHAL GMBH
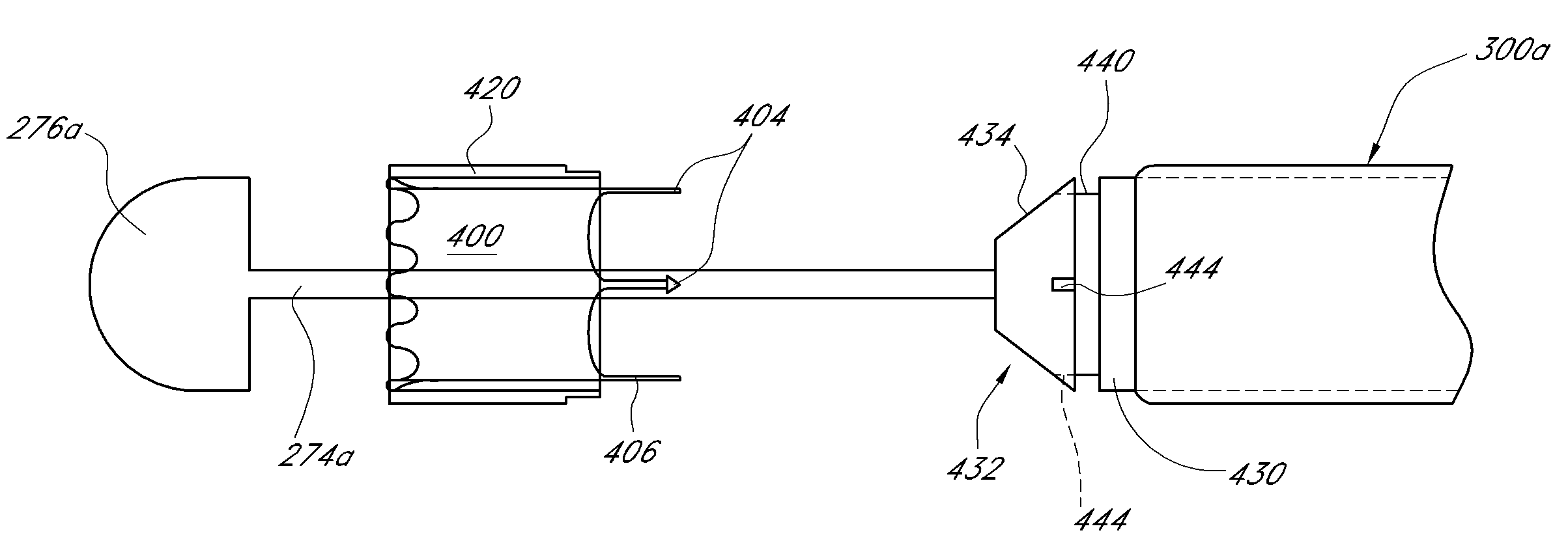
Implant delivery assembly with expandable coupling/decoupling mechanism
An occlusive implant delivery assembly includes a rapid response decoupling or detachment mechanism that does not effect significant migration of the implant during release. The assembly includes an occlusive implant device, such as an embolic coil, a pusher or device to carry the implant to the selected location, and an expandable coupling-decoupling mechanism for releasing the implant at the selected site. The mechanical construction provides rapid release times. In addition, the releasing mechanism generally operates without exerting any significant force on the implant, thereby avoiding any significant displacement of the implant during release.
Owner:STRYKER CORP +1
Anti-backup mechanism for repeating multi-clip applier
InactiveUS20060009790A1Avoid dangerAvoid loadDisinfectionSurgical forcepsLinear motionSurgical operation
An instrument for applying clips in surgery having an operating handle with operating components and a clip cartridge with clip applying mechanism. The handle operating components generate reciprocal linear motion imparted to the clip applying mechanism and accommodate rotation of the cartridge about a cartridge axis. An anti-backup mechanism constrains operating components to complete first and second strokes of reciprocal linear motion.
Owner:BLAKE JOSEPH W III +1
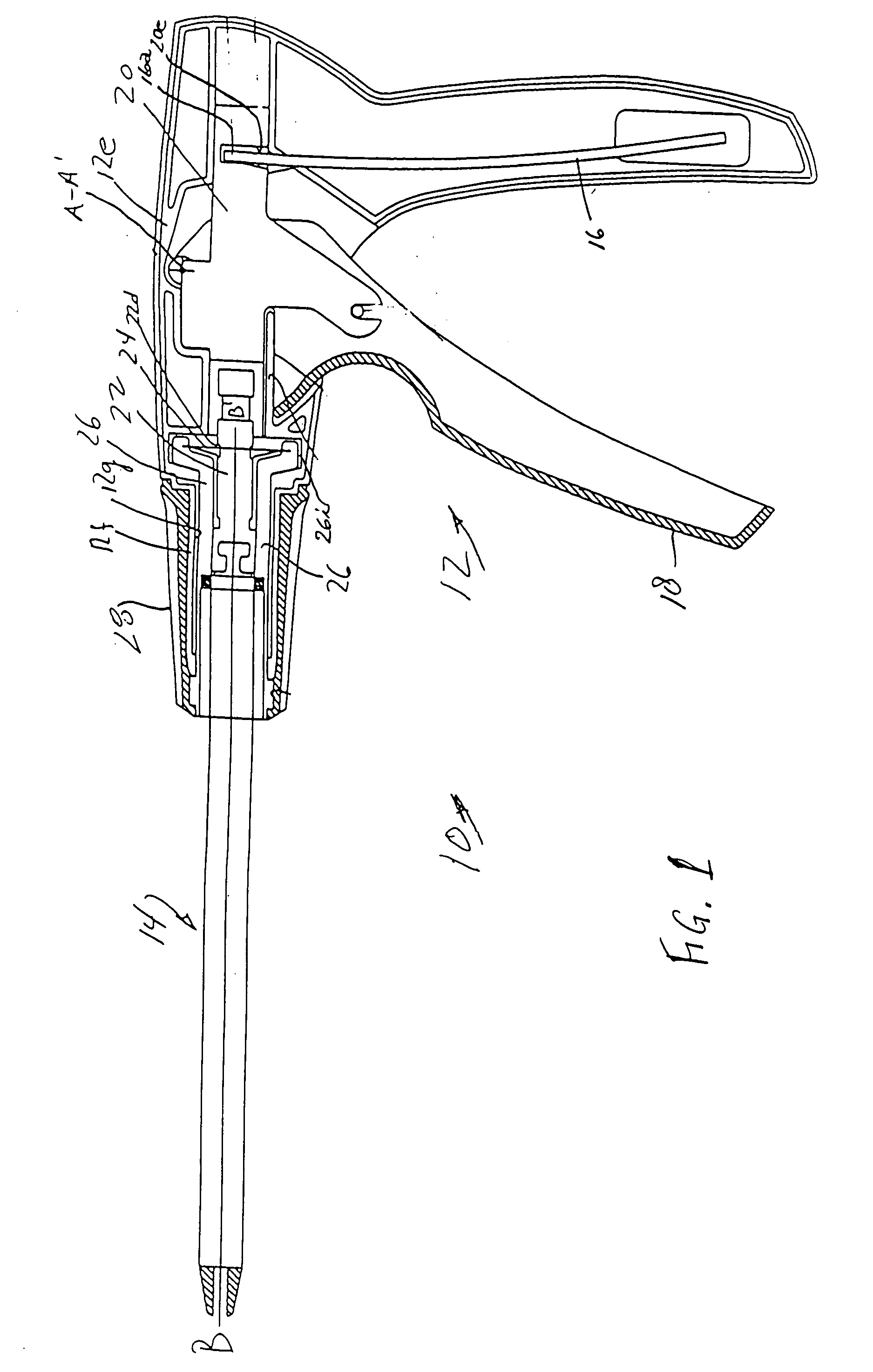
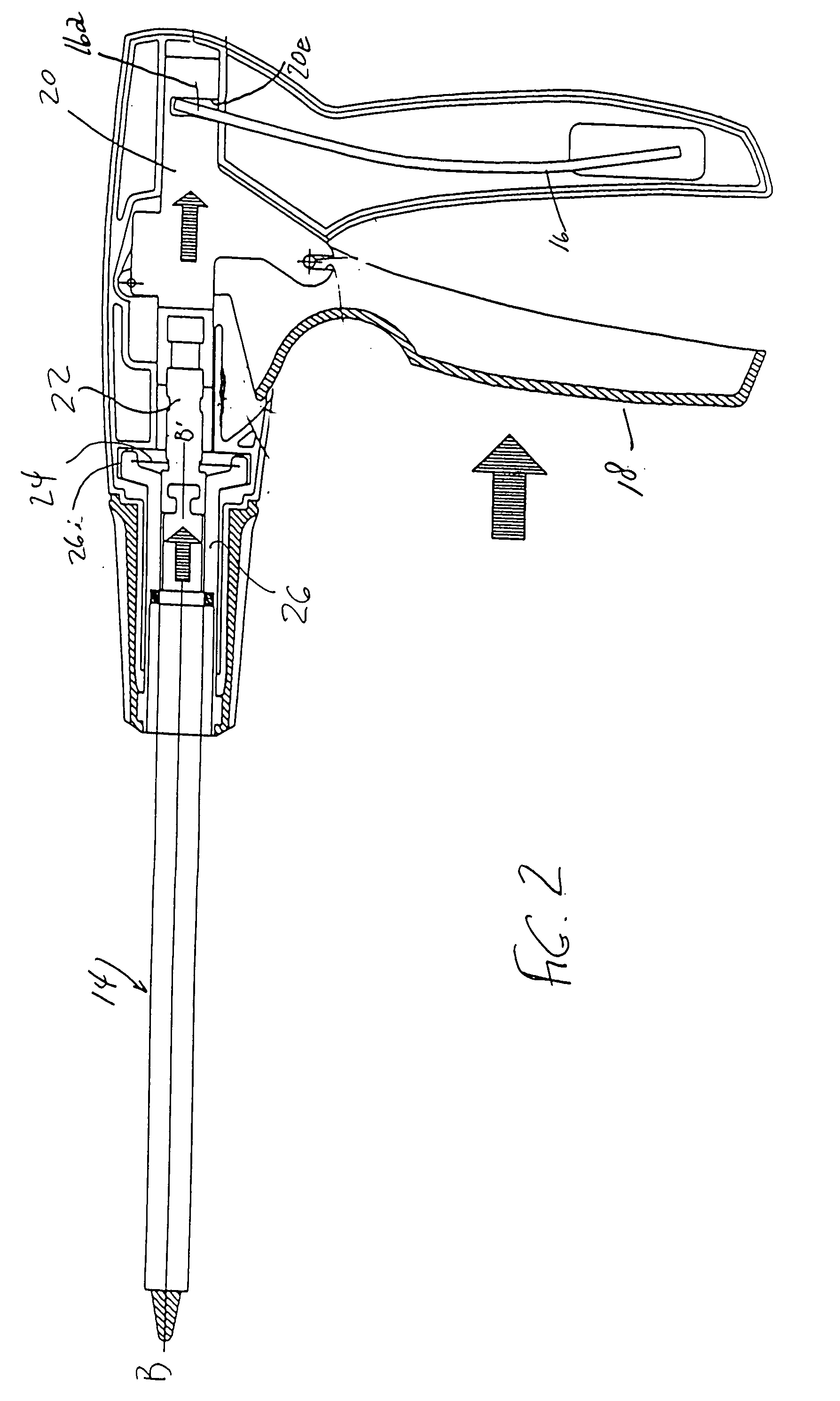
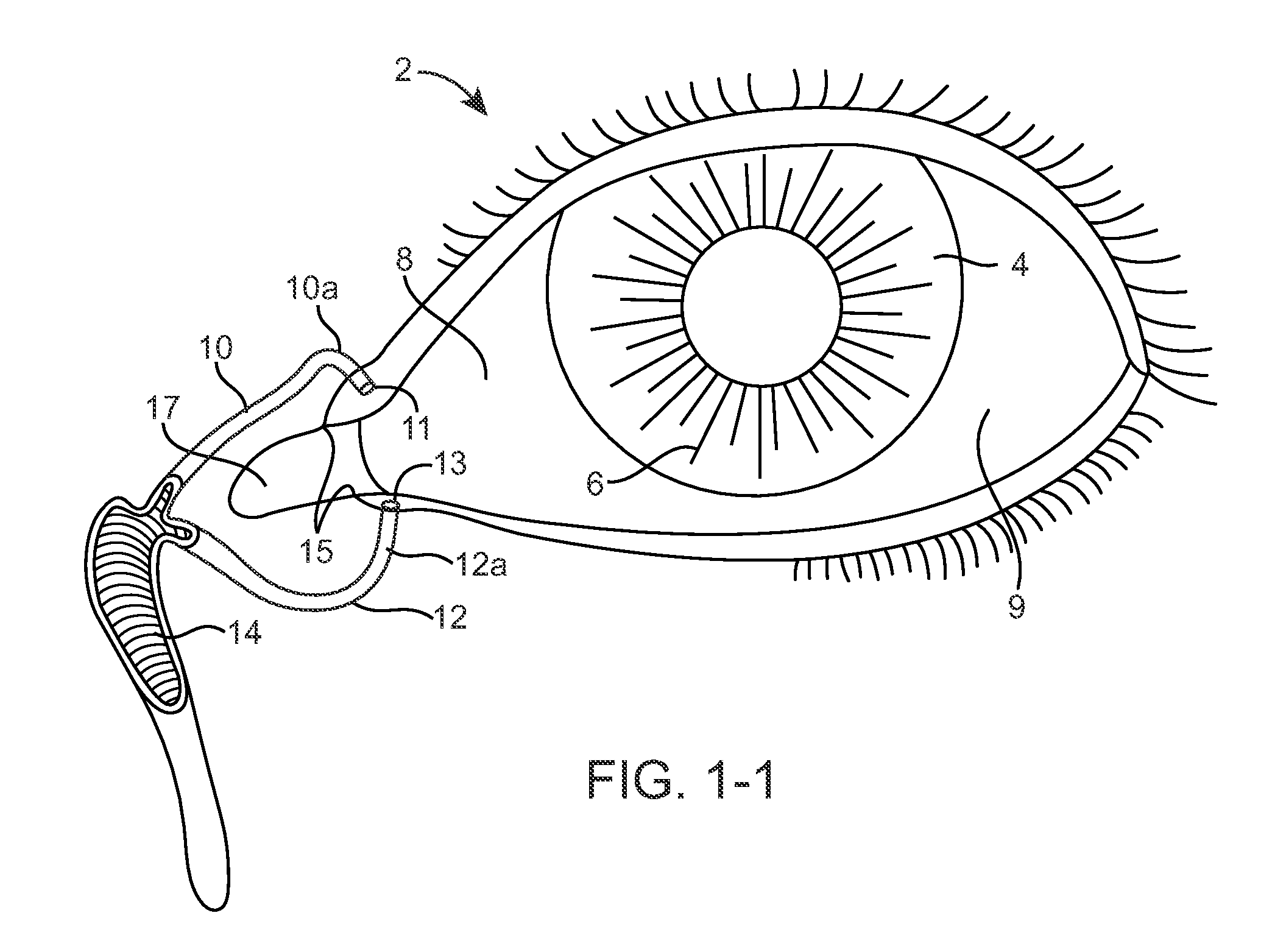
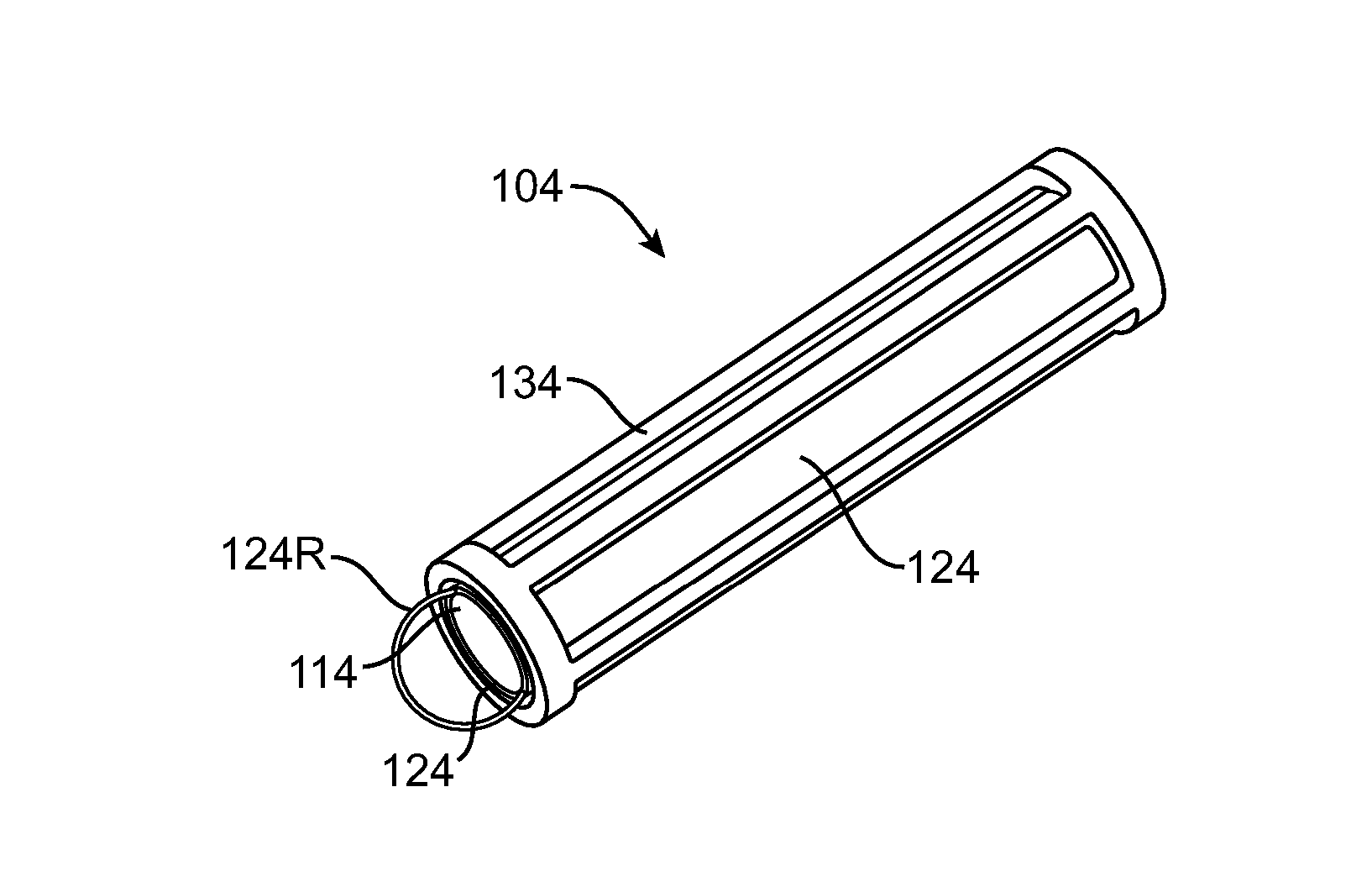
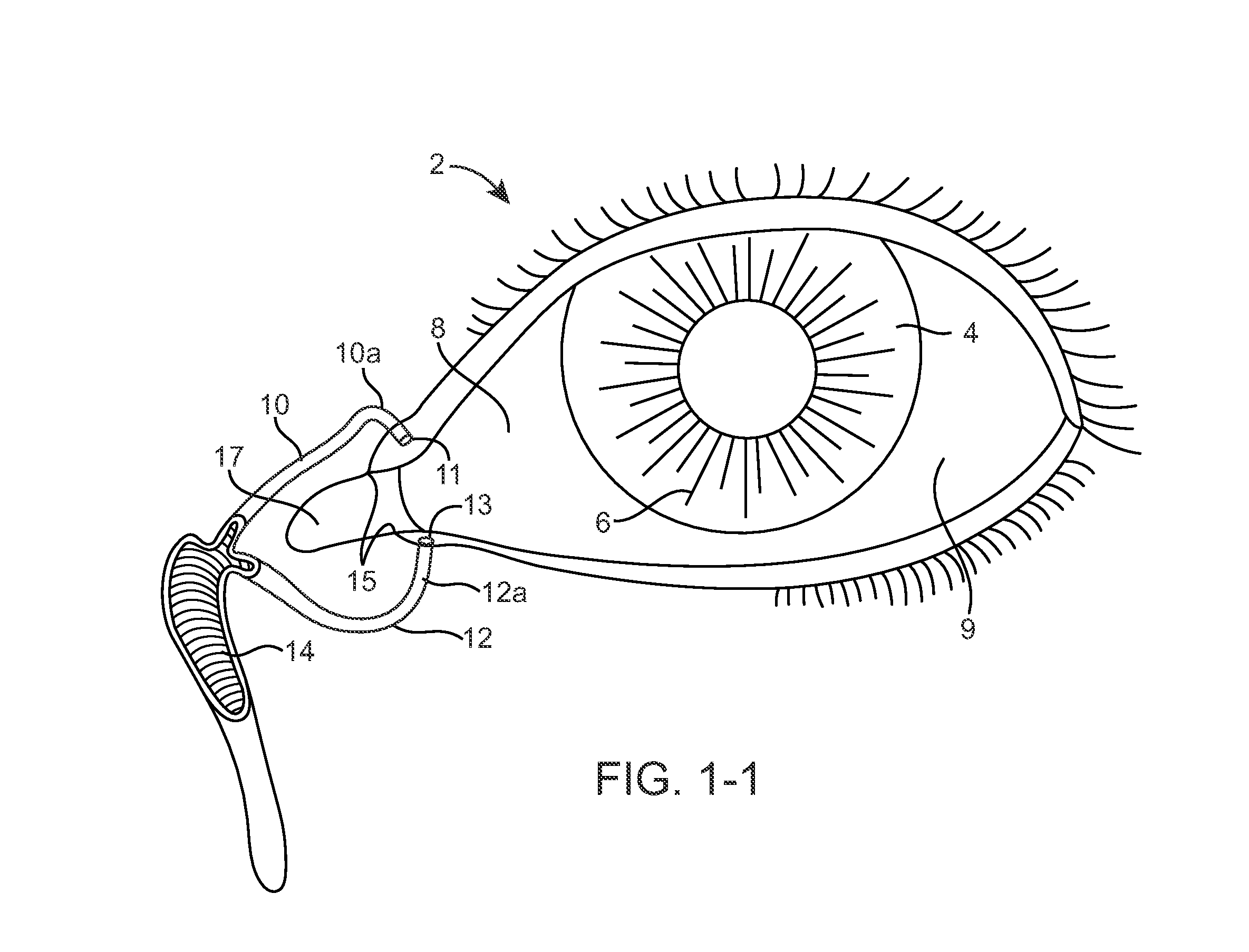
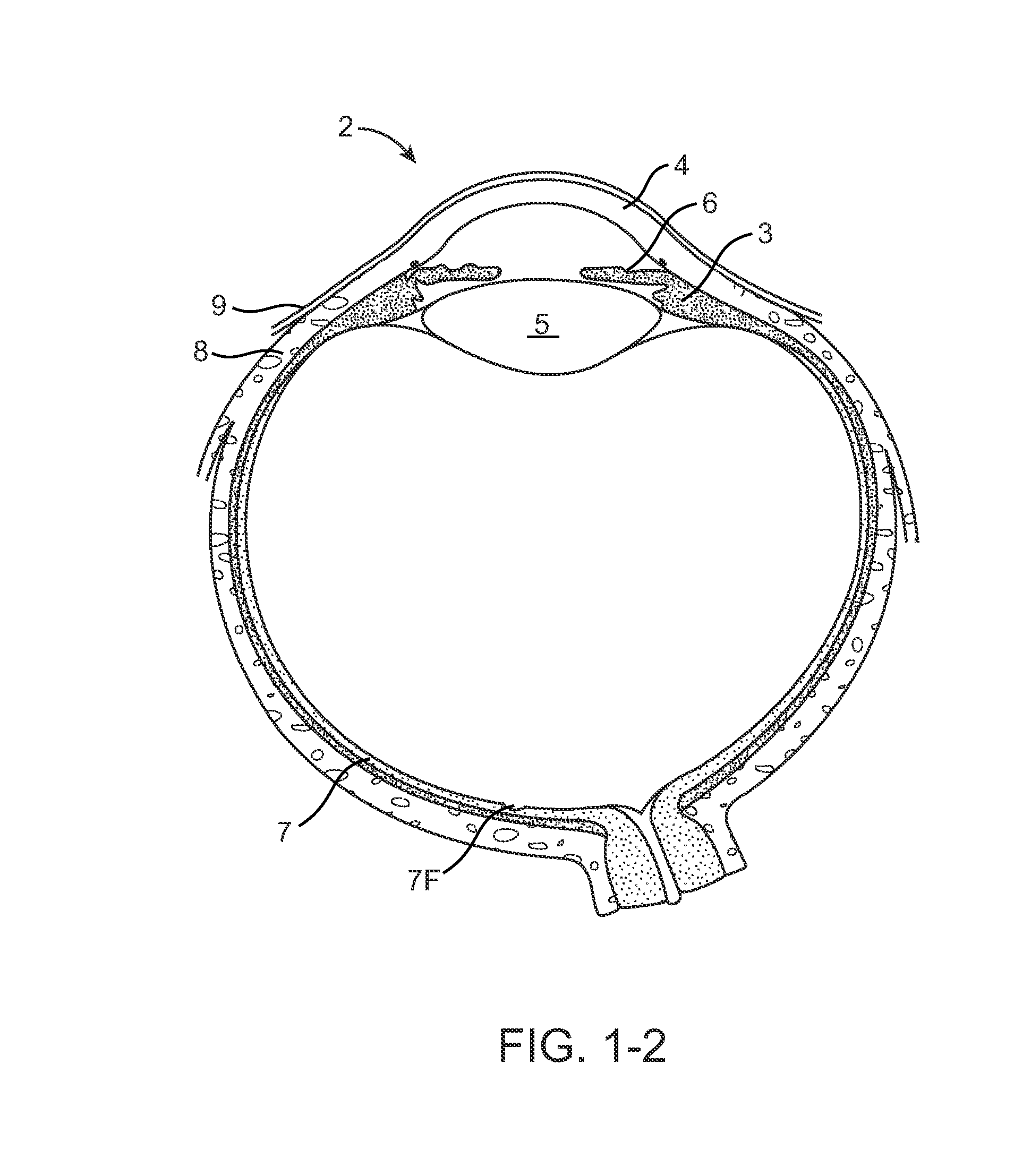
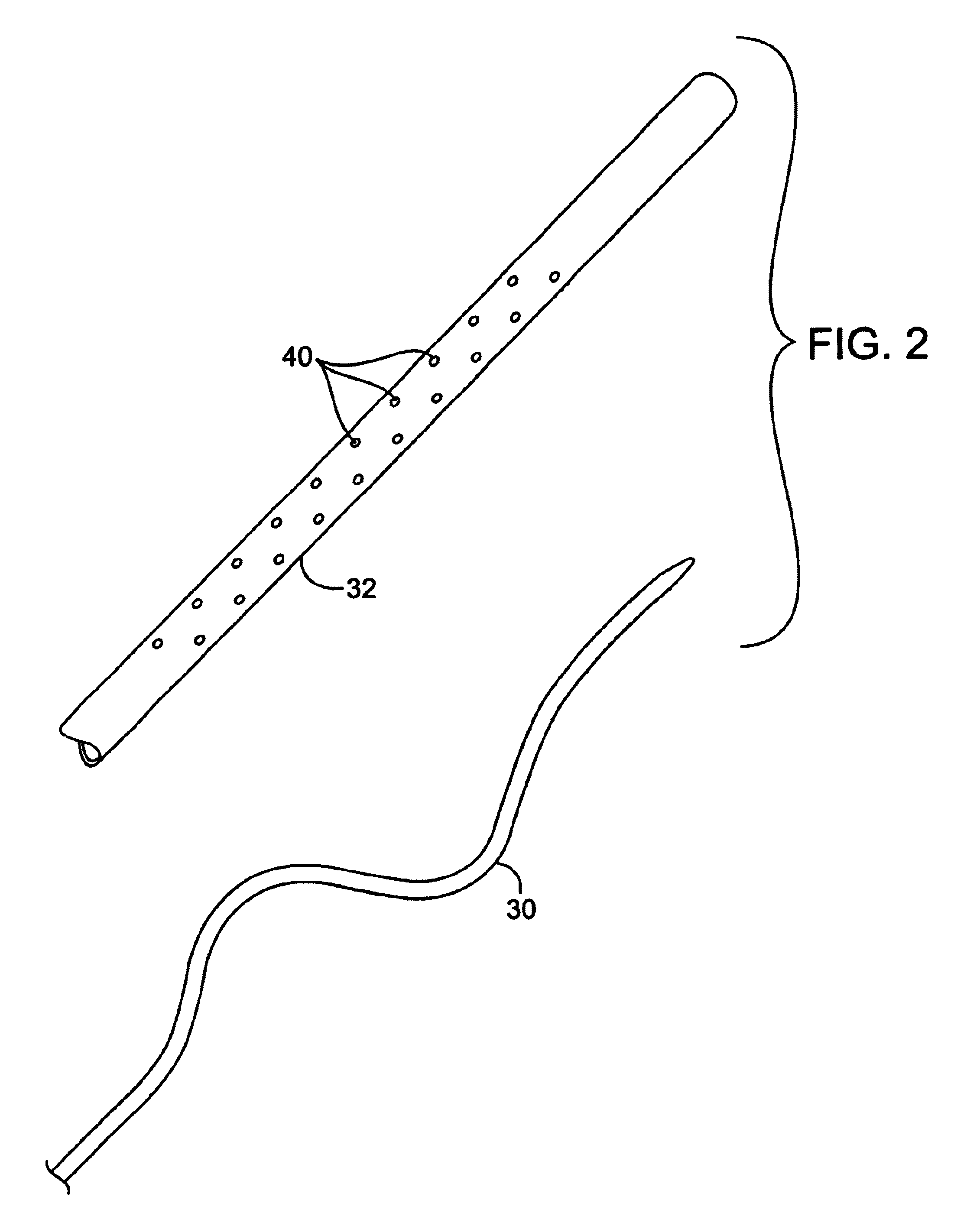
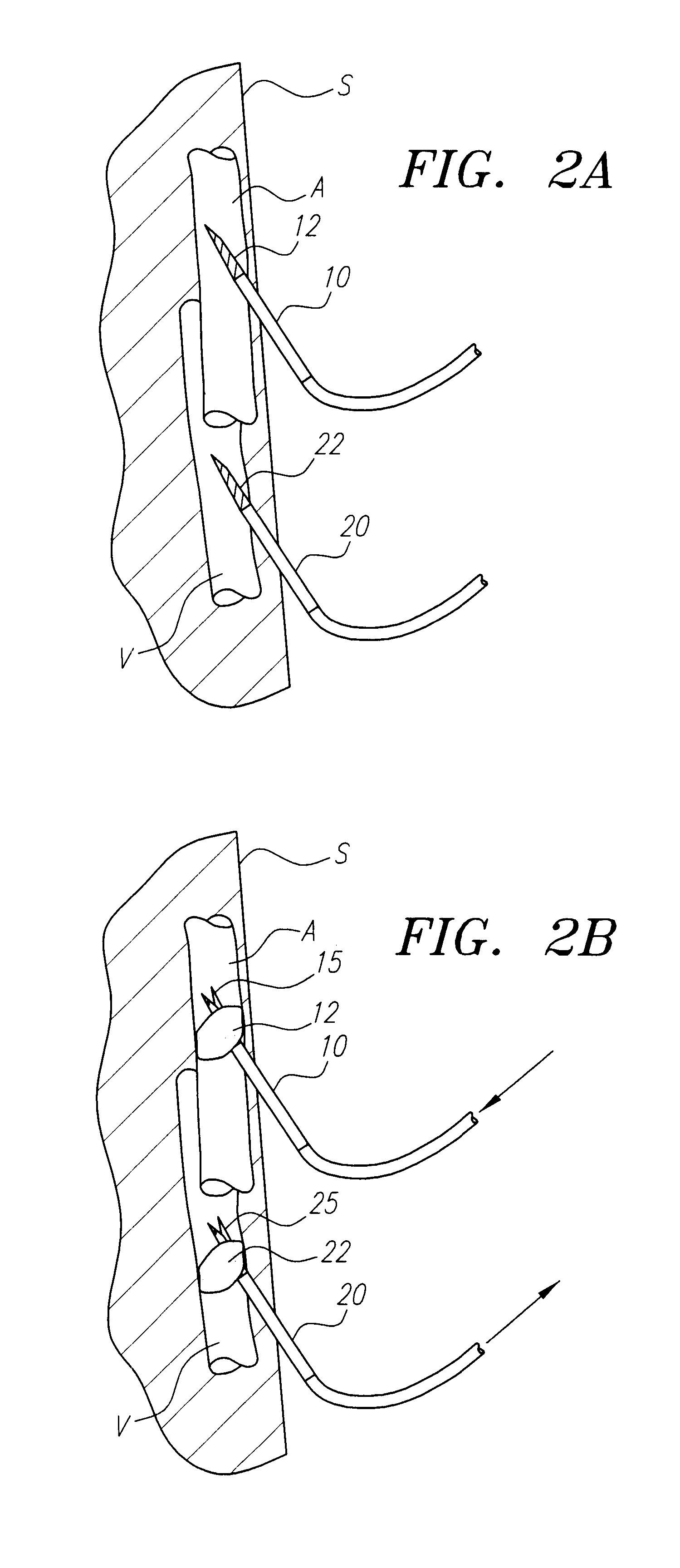
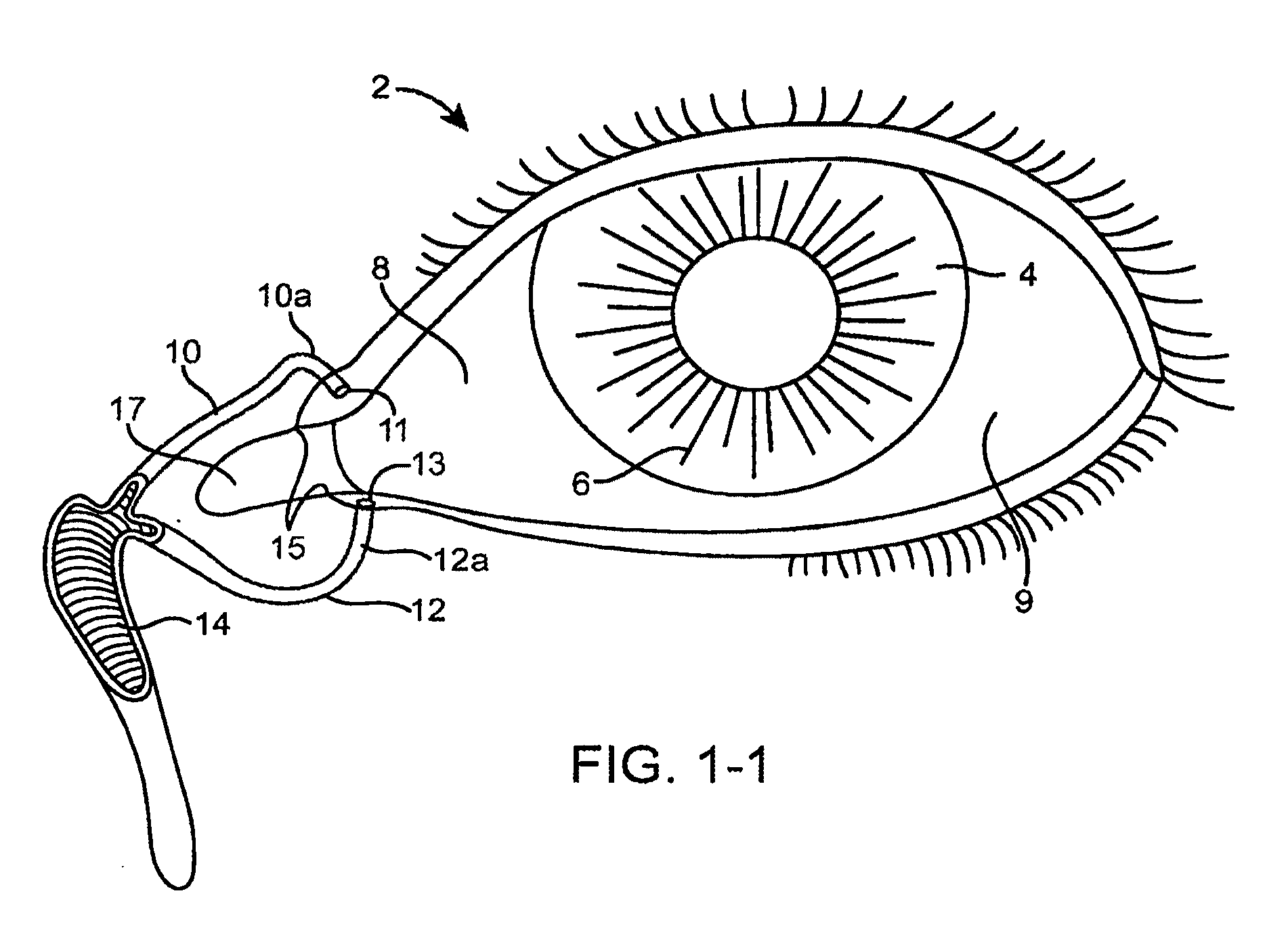
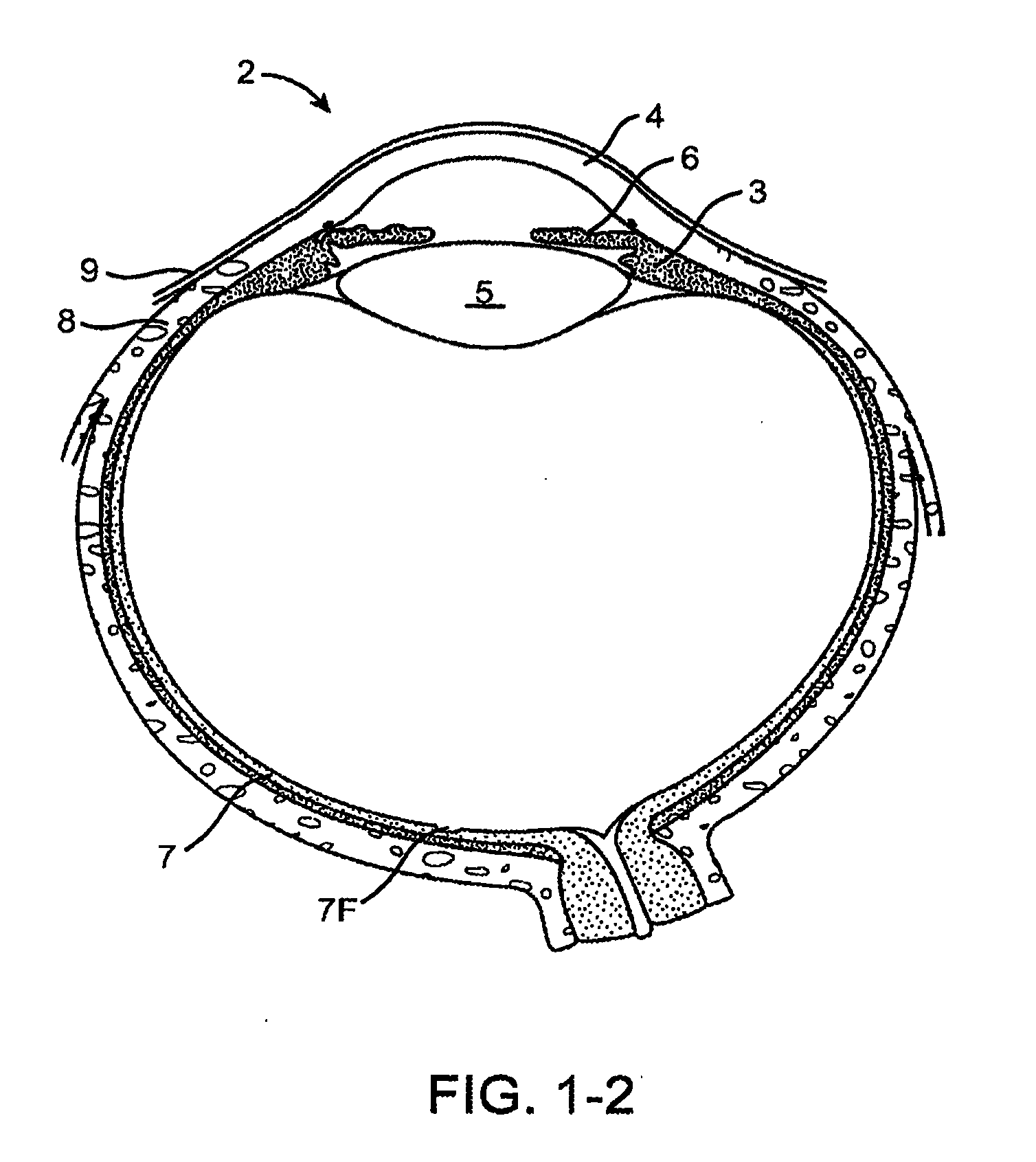
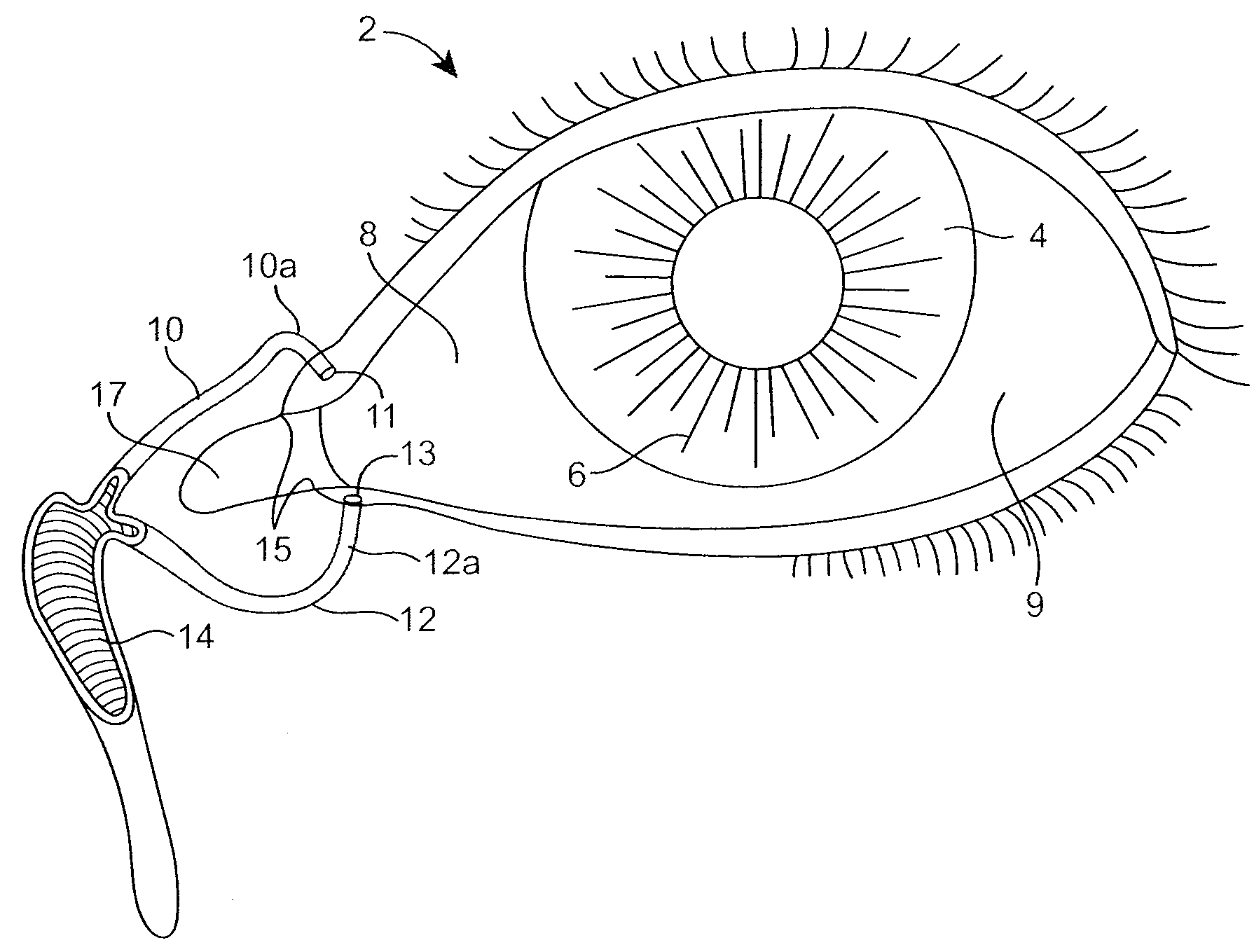
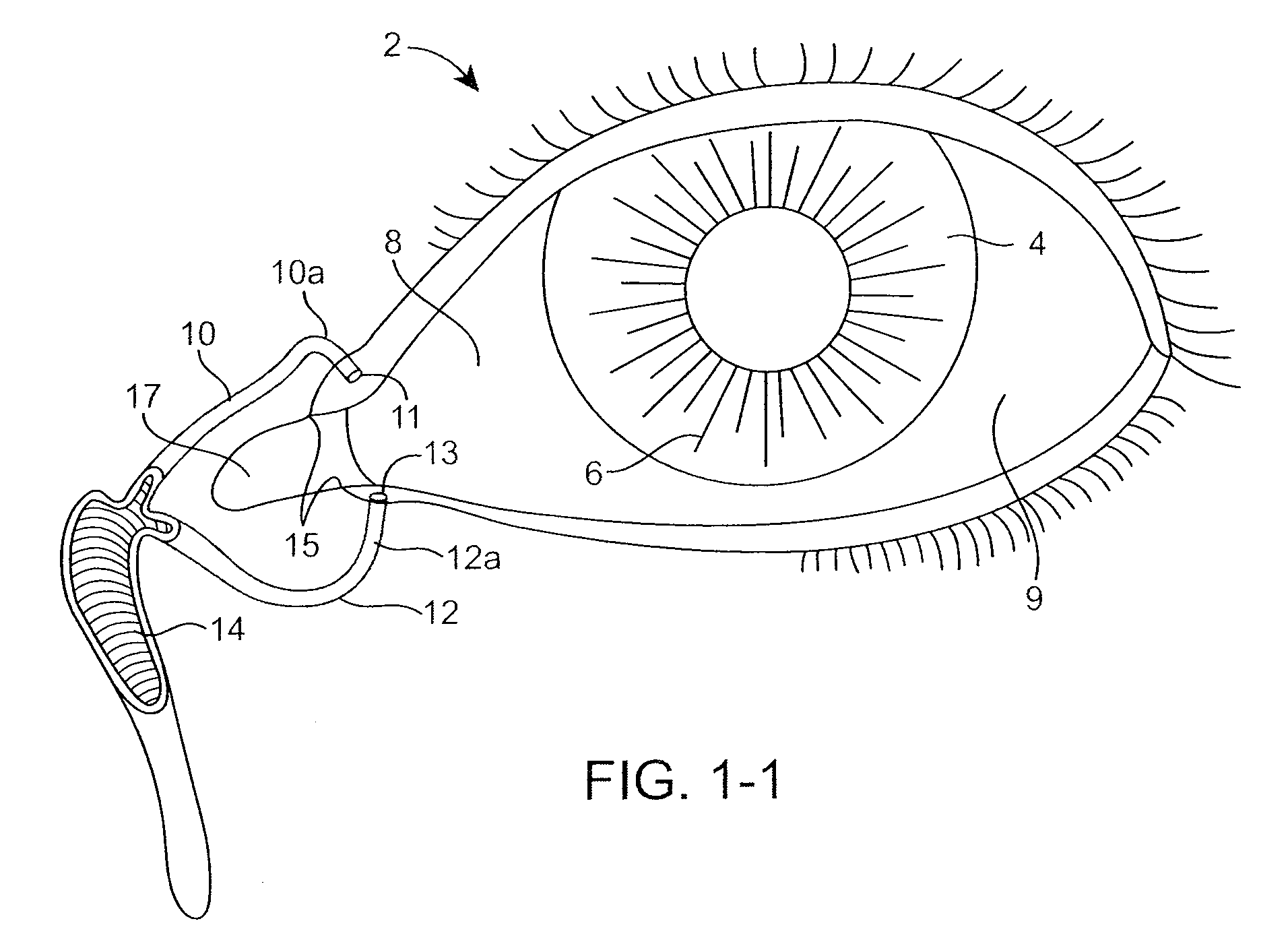
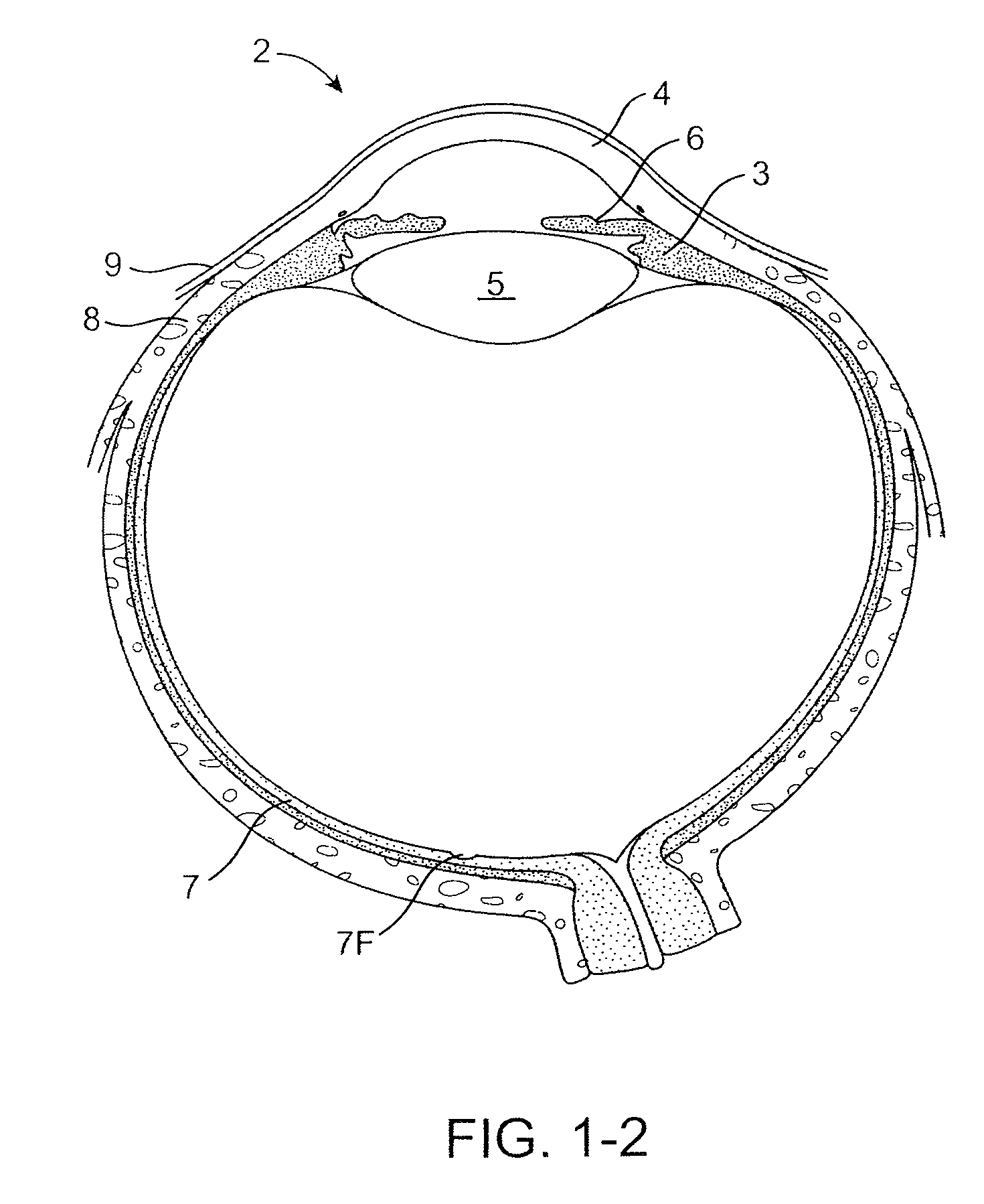
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
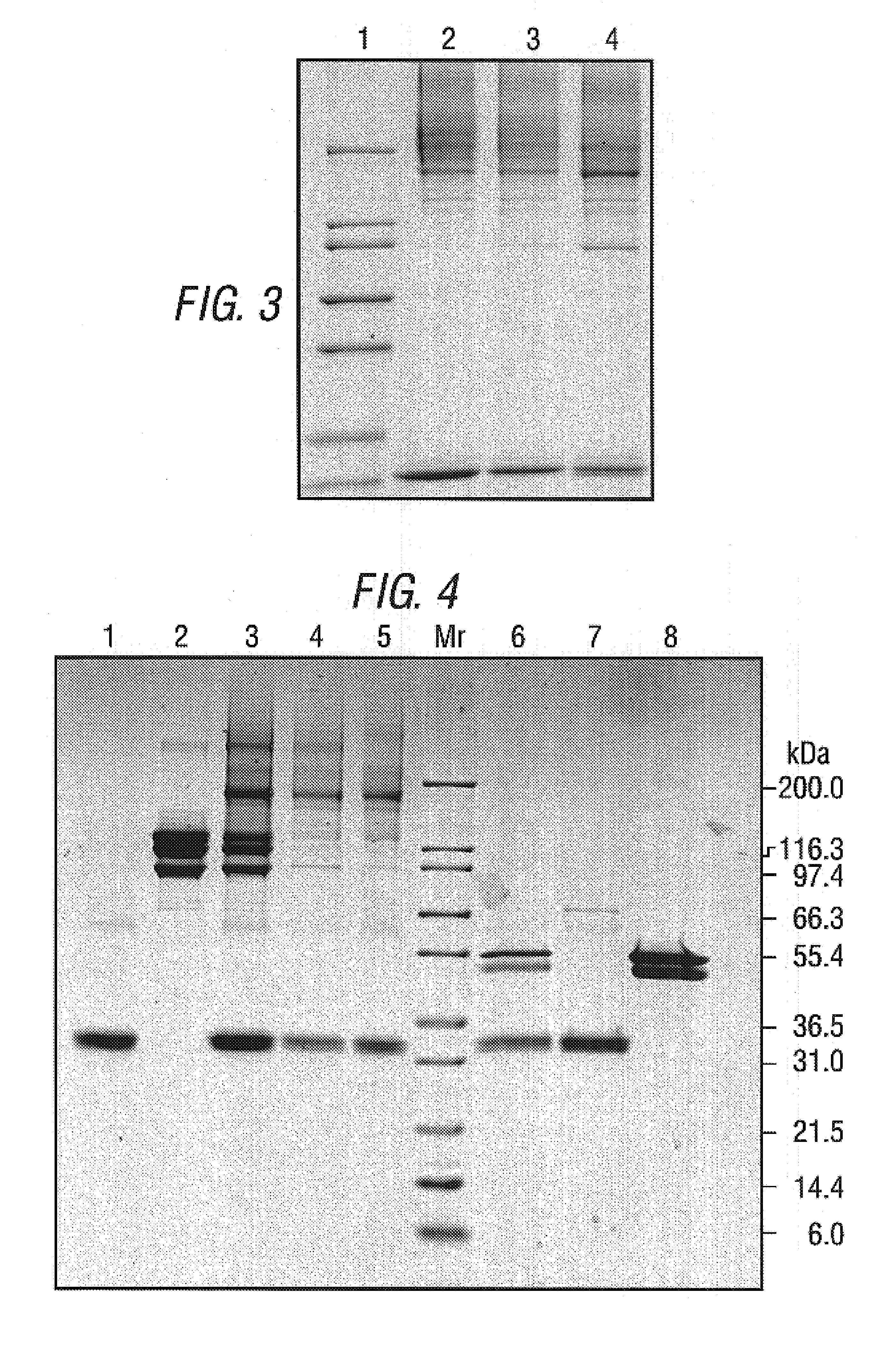
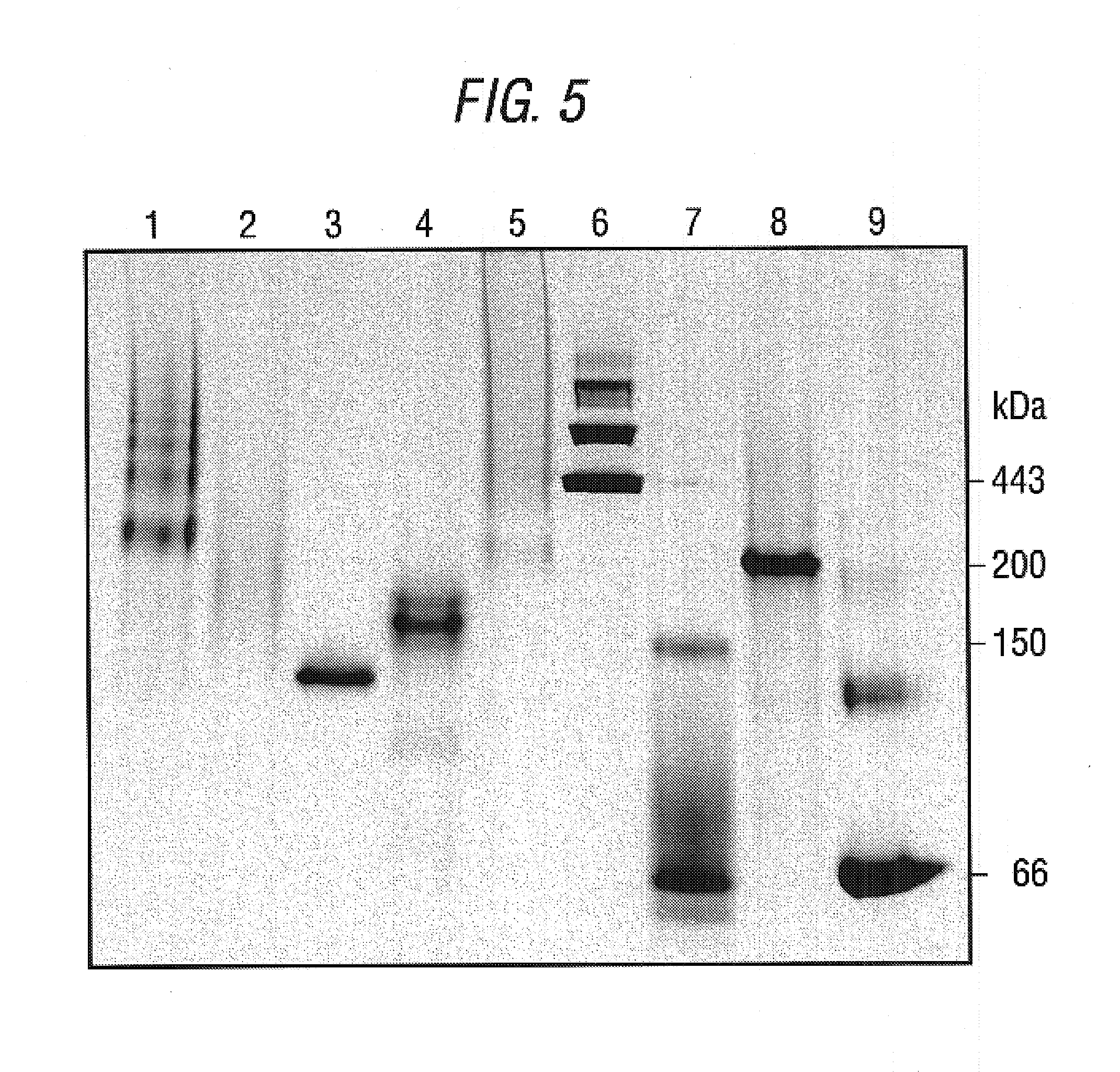
Antibodies that specifically bind to TIM3
ActiveUS8841418B2Promote growthReduce inflammationHeavy metal active ingredientsPeptide/protein ingredientsCancer cellAntibody
Provided herein are antibodies specific for TIM3 that can be used to detect cancer cells, in particular, cancer stem cells. The antibodies can also be used in therapeutic compositions for treating cancer and reducing inflammation.
Owner:ONK THERAPEUTICS LTD
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
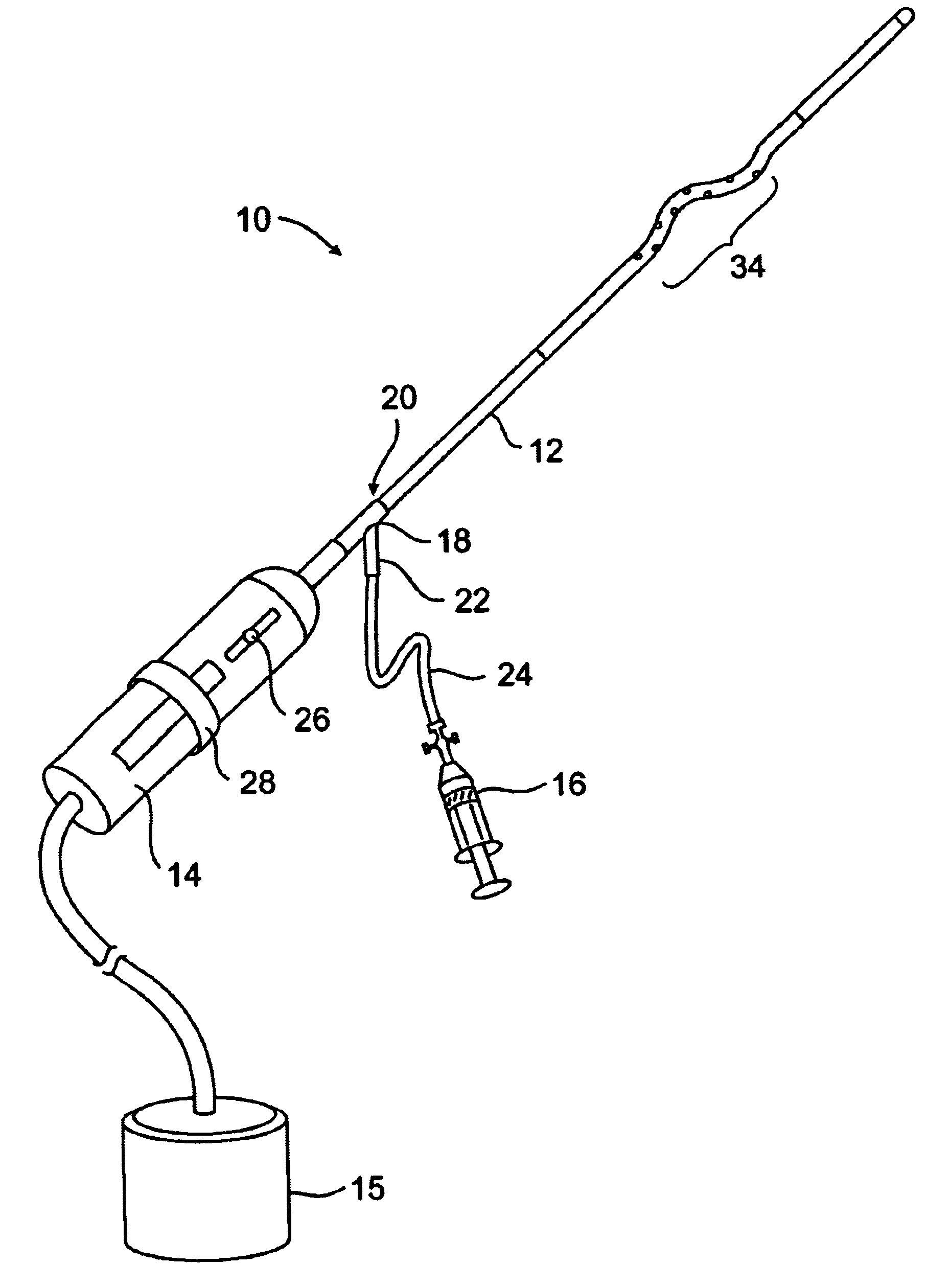
System and methods for clot dissolution
Clot disruption and dissolution are achieved using a catheter having both an agitator and the ability to deliver a thrombolytic agent. The catheter is introduced to a target region with a blood vessel and the agitator manipulated to engage and disrupt a region of clot therein. The thrombolytic agent, such as tPA, streptokinase, or urokinase, is directly released into the clot at the point where the agitator is engaging the clot. In this way, the thrombolytic activity of the agent is enhanced and the dissolution of the clot is improved.
Owner:TYCO HEALTHCARE GRP LP
Organic Compounds
InactiveUS20090186022A1Reducing required dosagingReduce potential side effectsSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsInflammatory Bowel DiseasesAtopic dermatitis
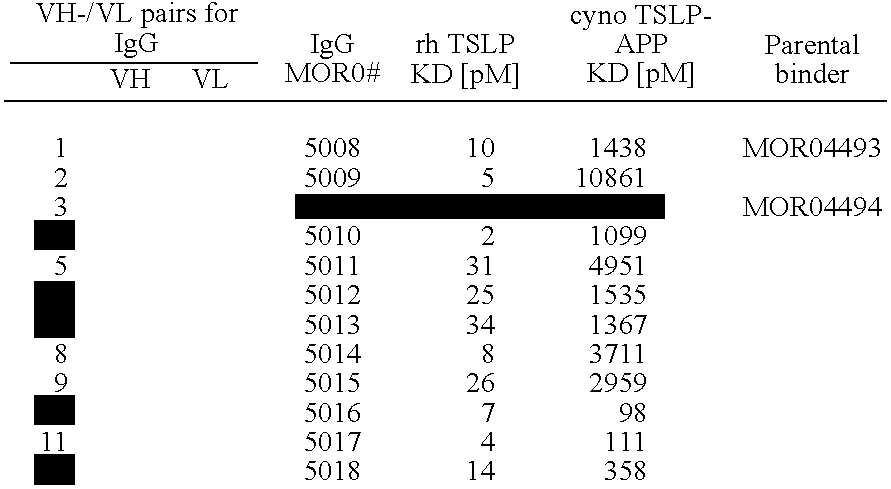
The present invention relates to human thymic stromal lymphopoietin (hTSLP) antibodies and especially those which neutralize hTSLP activity. It further relates to methods for using anti-hTSLP antibody molecules in diagnosis or treatment of hTSLP related disorders, such as asthma, atopic dermatitis, allergic rhinitis, fibrosis inflammatory bowel disease, and Hodgkin's lymphoma.
Owner:NOVARTIS AG
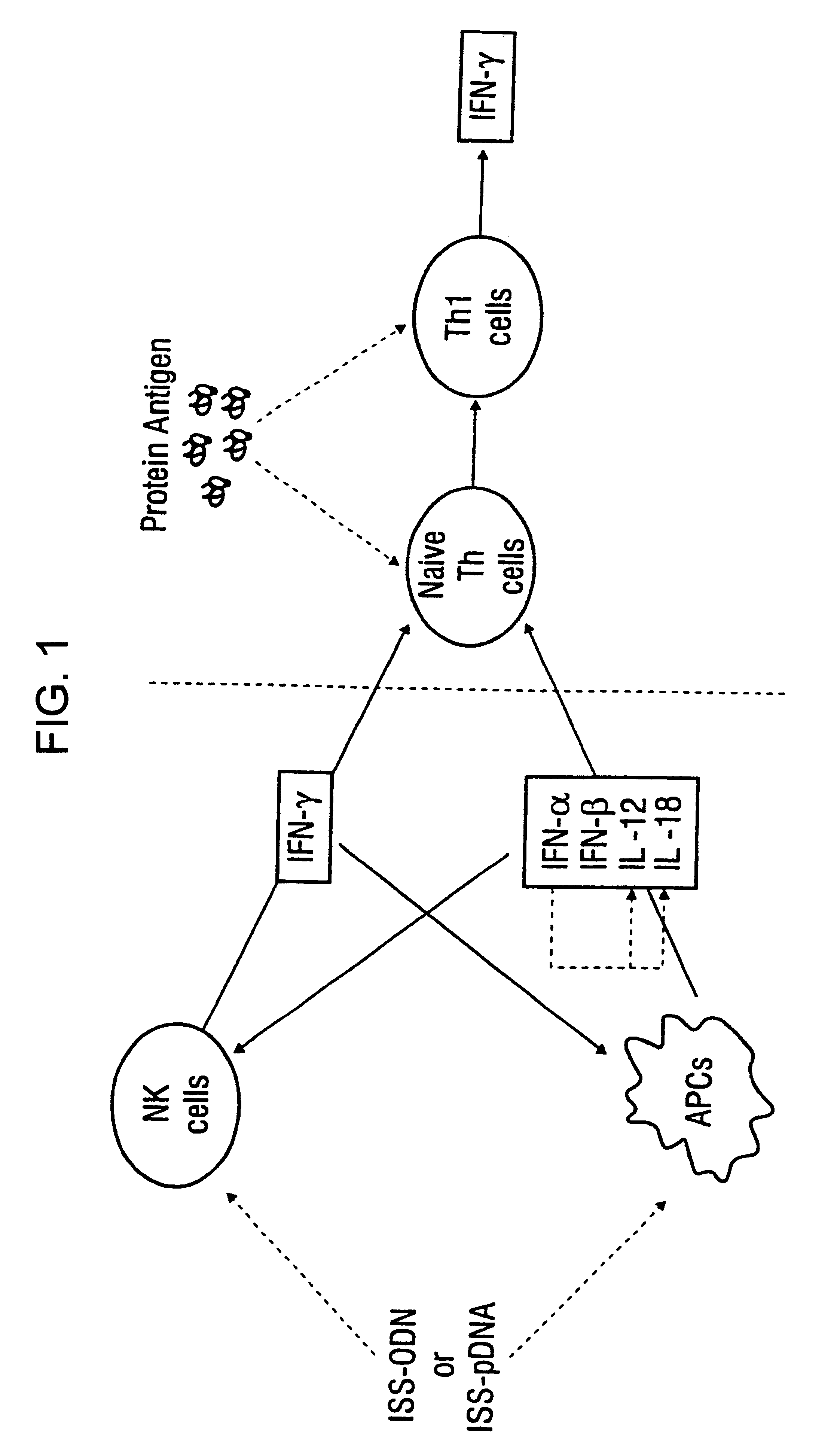
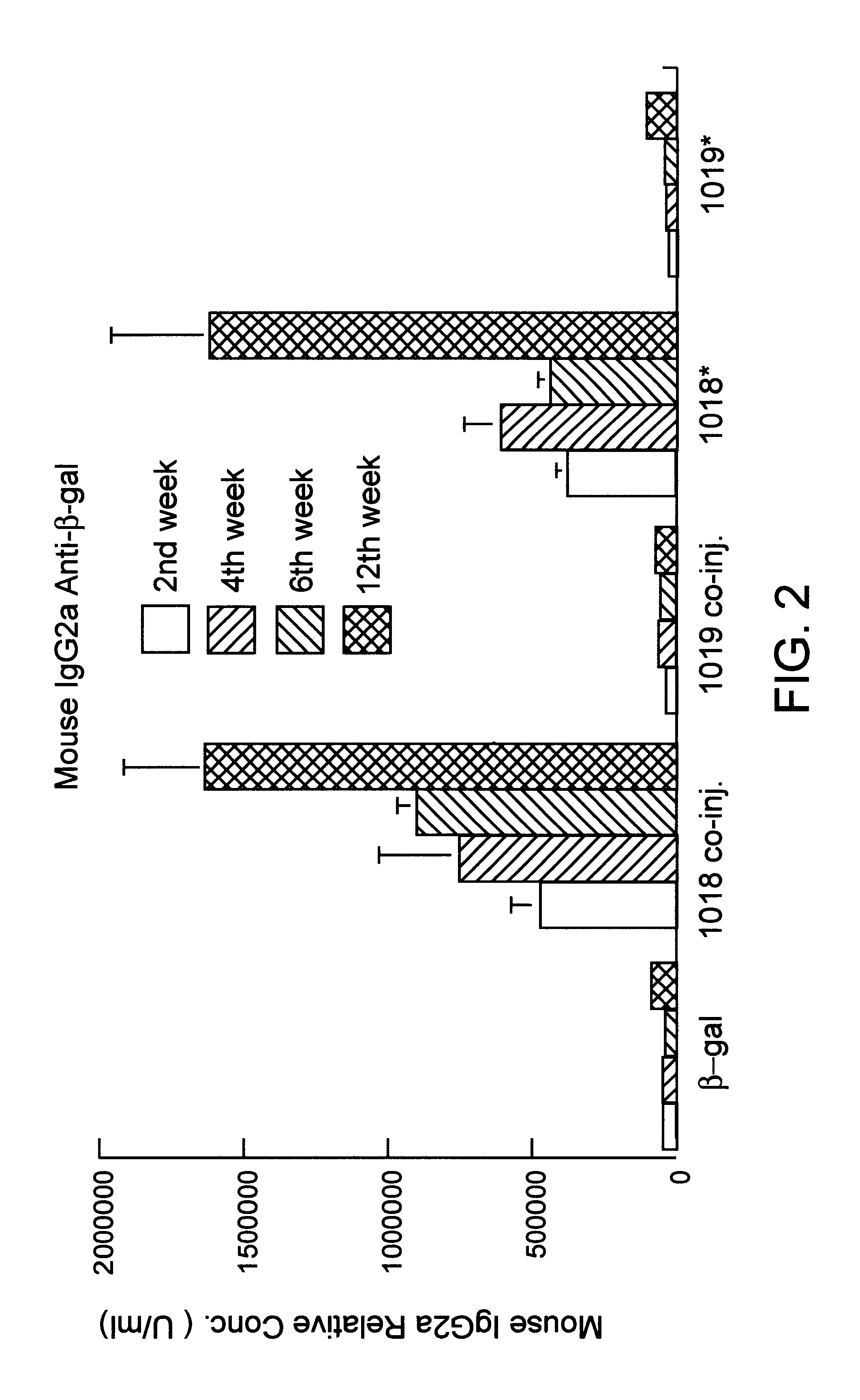
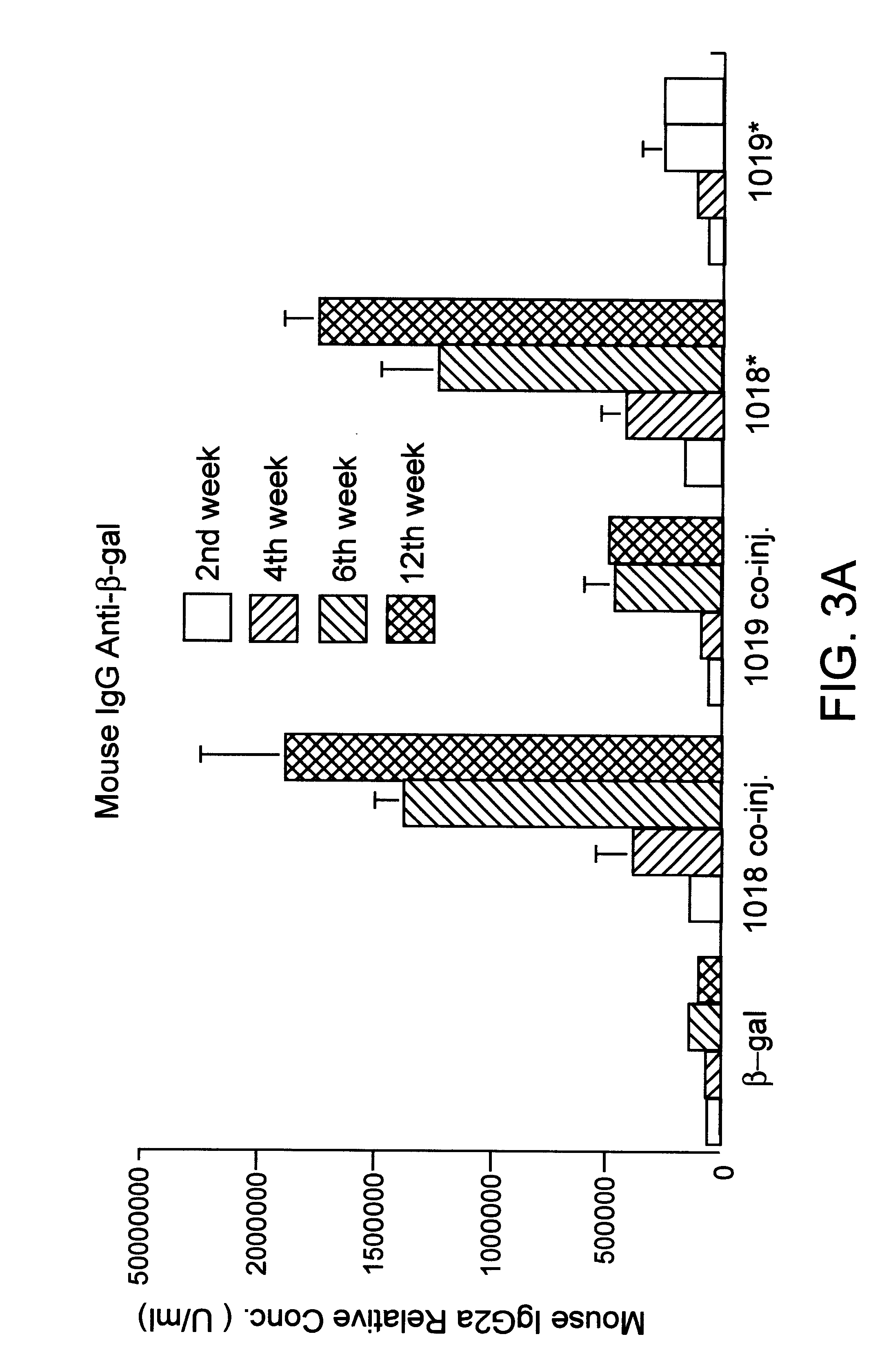
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a Th1 phenotype
InactiveUS6498148B1Treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsSenses disorderAntigen stimulationTherapeutic intent
The invention relates to methods for preventing or reducing antigen-stimulated, granulocyte-mediated inflammation in tissue of an antigen-sensitized mammal host by delivering an immunostimulatory oligonucleotide to the host. In addition, methods for using the immunostimulatory oligonucleotides to boost a mammal host's immune responsiveness to a sensitizing antigen (without immunization of the host by the antigen) and shifting the host's immune responsiveness to a Th1 phenotype to achieve various therapeutic ends are provided. Kits for practicing the methods of the invention are also provided.
Owner:RGT UNIV OF CALIFORNIA
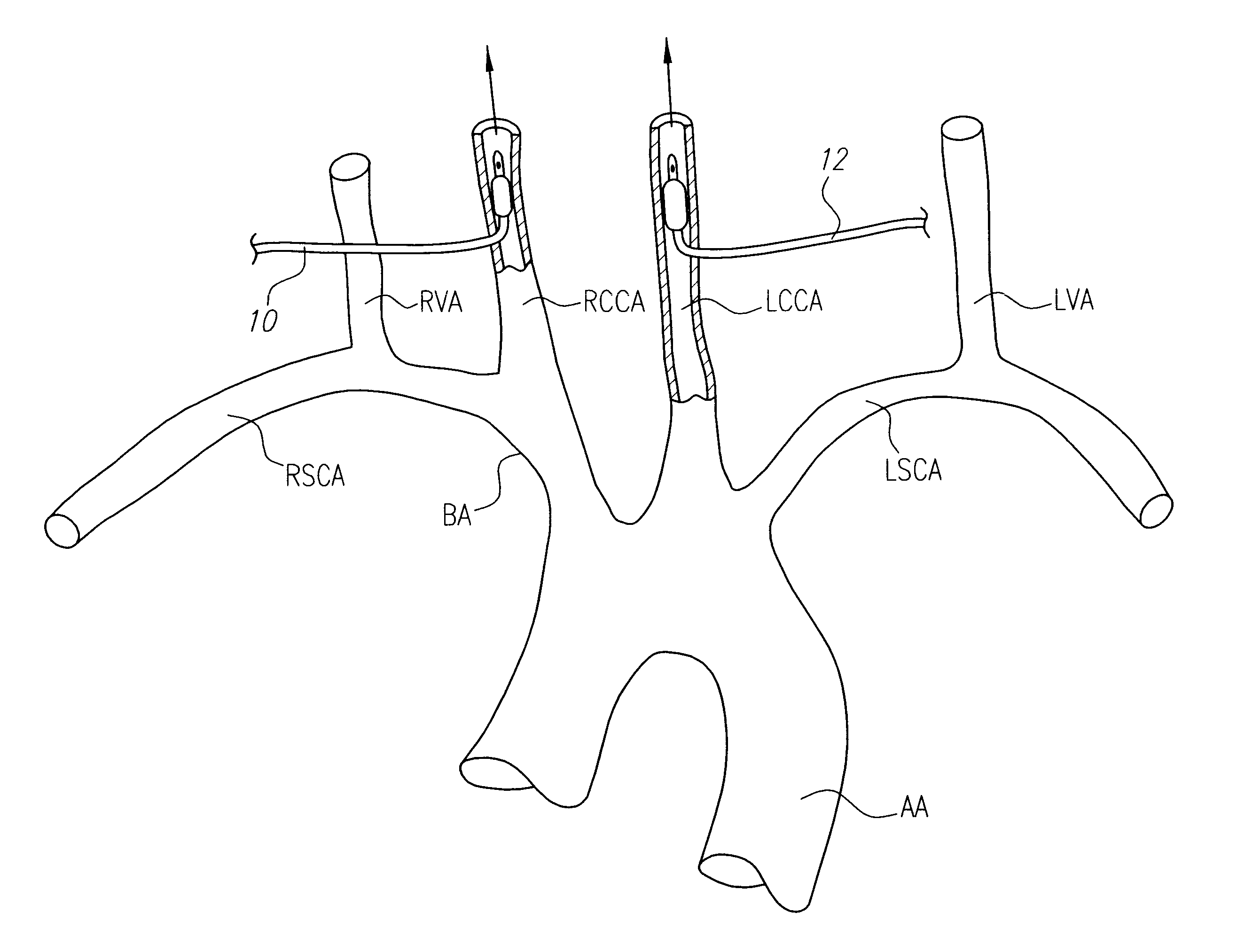
Intravascular methods and apparatus for isolation and selective cooling of the cerebral vasculature during surgical procedures
InactiveUS6555057B1Reduce riskIncrease perfusionOther blood circulation devicesMedical devicesRisk strokeSurgical department
Patients having diminished circulation in the cerebral vasculature as a result of stroke or from other causes such as cardiac arrest, shock or head trauma, or aneurysm surgery or aortic surgery, are treated by flowing an oxygenated medium through an arterial access site into the cerebral vasculature and collecting the medium through an access site in the venous site of the cerebral vasculature. Usually, the cold oxygenated medium will comprise autologous blood, and the blood will be recirculated for a time sufficient to permit treatment of the underlying cause of diminished circulation. In addition to oxygenation, the recirculating blood will also be cooled to hypothermically treat and preserve brain tissue. Isolation and cooling of cerebral vasculature in patients undergoing aortic and other procedures is achieved by internally occluding at least the right common carotid artery above the aortic arch. Blood or other oxygenated medium is perfused through the occluded common carotid artery(ies) and into the arterial cerebral vasculature. Usually, oxygen depleted blood or other medium leaving the cerebral vasculature is collected, oxygenated, and cooled in an extracorporeal circuit so that it may be returned to the patient. Occlusion of the carotid artery(ies) is preferably accomplished using expansible occluders, such as balloon-tipped cannula, catheters, or similar access devices. Access to the occlusion site(s) may be open surgical, percutaneous, or intravascular.
Owner:BARBUT DENISE +3
Strip for whitening tooth surfaces
InactiveUS6514483B2Inhibition releaseCosmetic preparationsImpression capsWhitening AgentsEthylene oxide
A thin, flexible film which when applied to stained teeth is hydrated by saliva and is effective in such form to whiten teeth, the film comprising an anhydrous water hydratable ethylene oxide polymer matrix containing a solid peroxide whitening agent whereby upon application to stained tooth surfaces, the peroxide whitening agent is solublilized by saliva present in the oral cavity into active whitening activity when the film is positioned and placed on the teeth.
Owner:COLGATE PALMOLIVE CO
Drug delivery implants for inhibition of optical defects
InactiveUS20100114309A1Avoid side effectsInhibition releaseSenses disorderEye surgeryFar-sightednessHomatropine
An implant for use with an eye comprises an implantable structure and a therapeutic agent. The therapeutic agent is deliverable from the structure into the eye so as to therapeutically effect and / or stabilize a refractive property of the eye. In many embodiments, the refractive property of the eye may comprise at least one of myopia, hyperopia or astigmatism. The therapeutic agent can comprise a composition that therapeutically effects or stabilizes the refractive property of the eye. The therapeutic agent may comprise at least one of a mydriatic or a cycloplegic drug. For example, the therapeutic agent may include a cycloplegic that comprises at least one of atropine, cyclopentolate, succinylcholine, homatropine, scopolamine, or tropicamide. In many embodiments, a retention element can be attached to the structure to retain the structure along a natural tissue surface.
Owner:MATI THERAPEUTICS
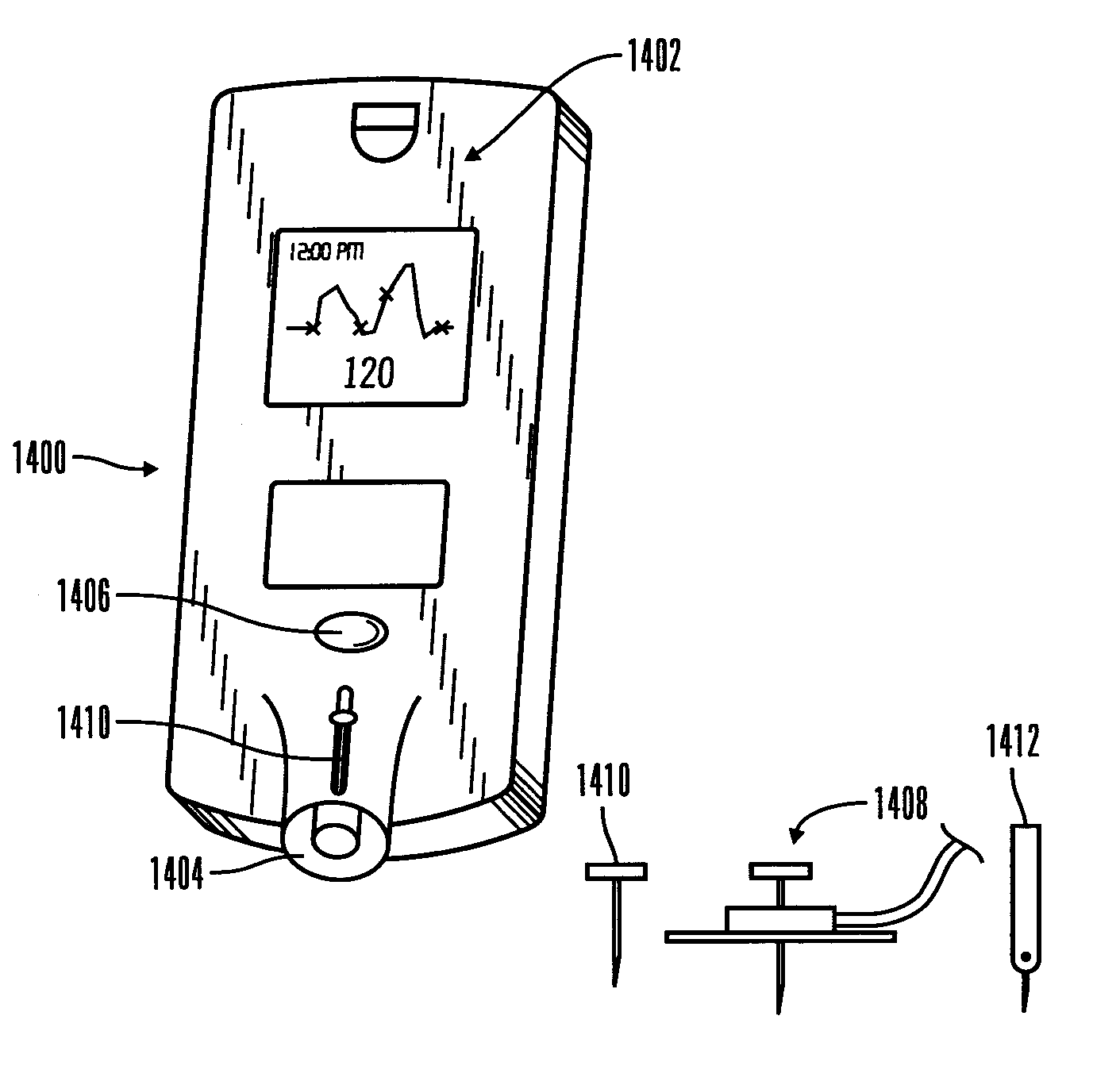
Insertion device for an insertion set and method of using the same
InactiveUS20070142776A9Minimal patient discomfortAccurate placementAutomatic syringesDiagnostic recording/measuringInsertion deviceVariable angle
An insertion device and insertion set. The insertion device for inserting at least a portion of at least one piercing member of an insertion set through the skin of a patient includes a device housing, a carrier body and a driver. The carrier body is slidably received within the device housing for movement between an advanced position and a retracted position. The carrier body also includes a receiving structure to support the insertion set in a position with the at least one piercing member oriented for insertion through the skin of the patient at a predetermined or variable angle relative to the skin of the patient upon movement of the carrier body from the retracted position to the advanced position. The driver is operatively coupled between the device housing and the carrier body to urge the carrier body from the retracted position toward the advanced position to place at least a portion of the at least one piercing member of the insertion set thorough the skin of the patient to install the insertion set to the patient. The receiving structure of the carrier body is removable from the insertion set while maintaining the installation of the insertion set to the patient.
Owner:MEDTRONIC MIMIMED INC
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
InactiveUS20090092654A1Avoid expulsionInhibition releaseAntibacterial agentsBiocideEffective treatmentBioabsorbable polymer
An implant for insertion into a punctum of a patient comprises a body. The body has a distal end, a proximal end, and an axis therebetween. The distal end of the body is insertable distally through the punctum into the canalicular lumen. The body comprises a therapeutic agent included within an agent matrix drug core. Exposure of the agent matrix to the tear fluid effects an effective therapeutic agent release into the tear fluid over a sustained period. The body has a sheath disposed over the agent matrix to inhibit release of the agent away from the proximal end. The body also has an outer surface configured to engage luminal wall tissues so as to inhibit expulsion when disposed therein. In specific embodiments, the agent matrix comprises a non-bioabsorbable polymer, for example silicone in a non-homogenous mixture with the agent.
Owner:MATI THERAPEUTICS
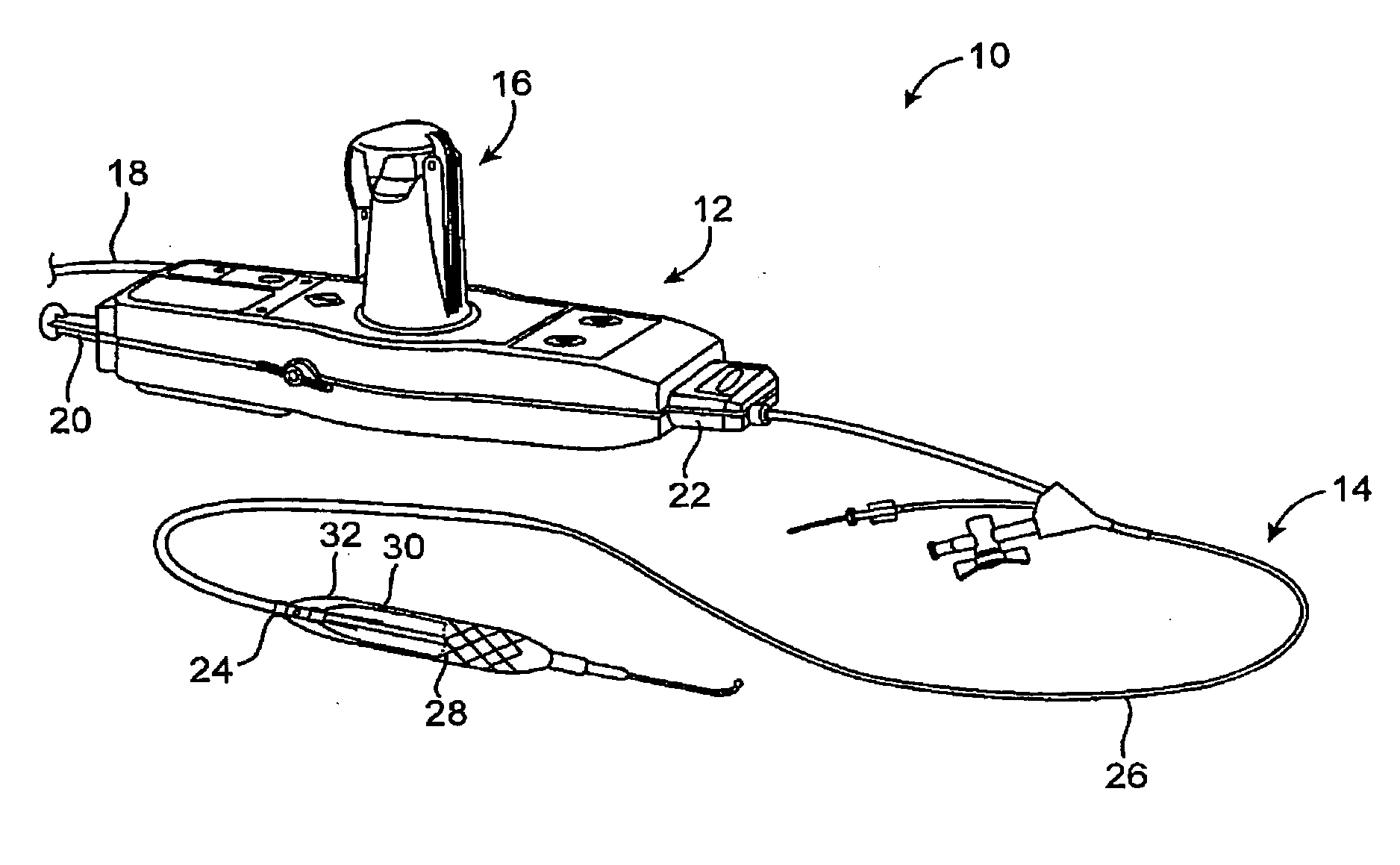
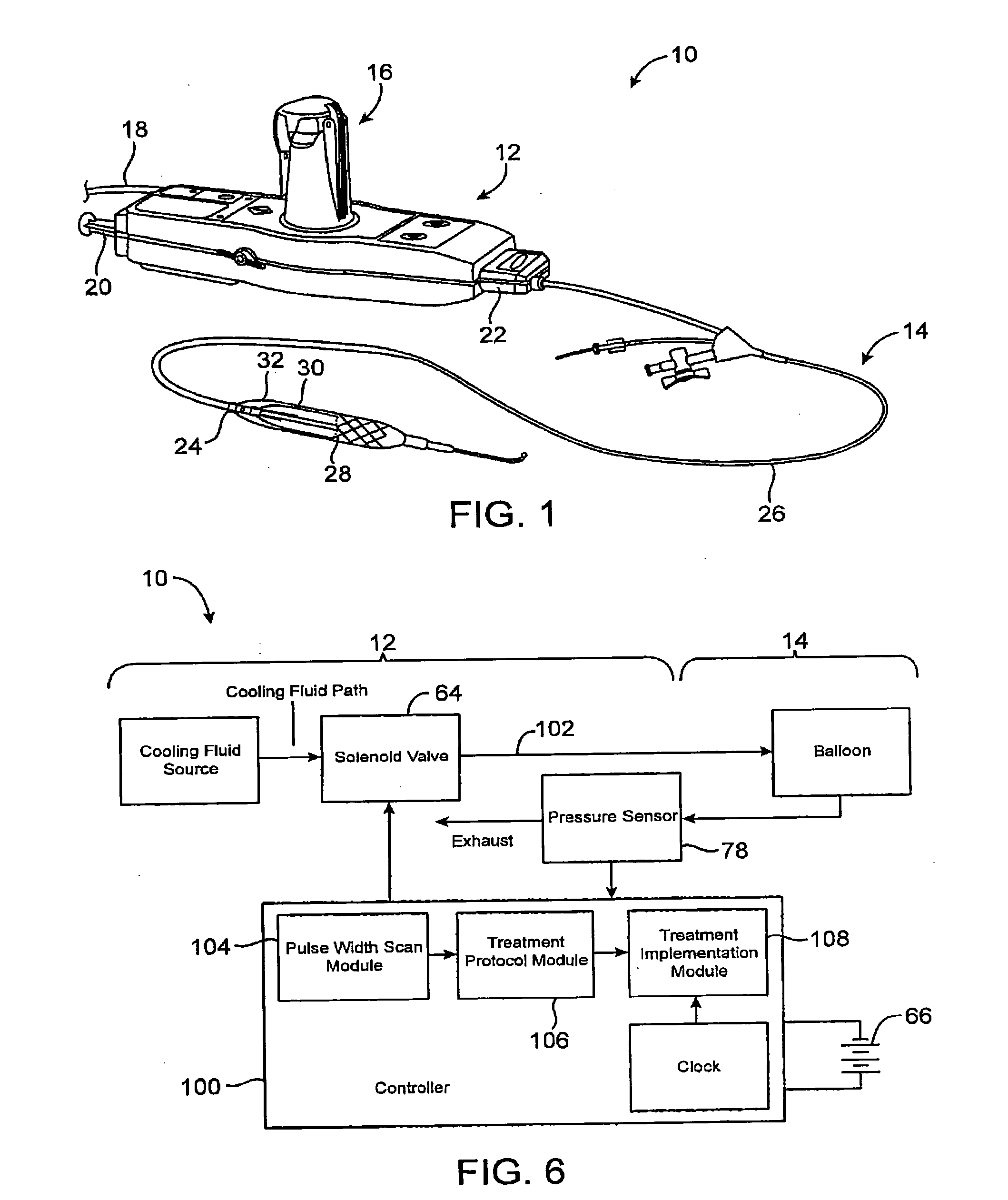
Efficient controlled cryogenic fluid delivery into a balloon catheter and other treatment devices
ActiveUS20060129142A1Avoiding unnecessary releaseEasy to copyOperating means/releasing devices for valvesCatheterSolenoid valveBalloon catheter
Devices, systems, and methods efficiently dilate and / or cool blood vessels and other body tissues. Controlled cooling with balloon catheters and other probes may be effected by a change in phase of a cryogenic fluid, often after measuring a minimum pulse width for actuating an individual solenoid valve along the cooling fluid path, with the measured pulse width allowing gradual inflation of a balloon without excessive venting of cooling fluid.
Owner:BOSTON SCI SCIMED INC
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
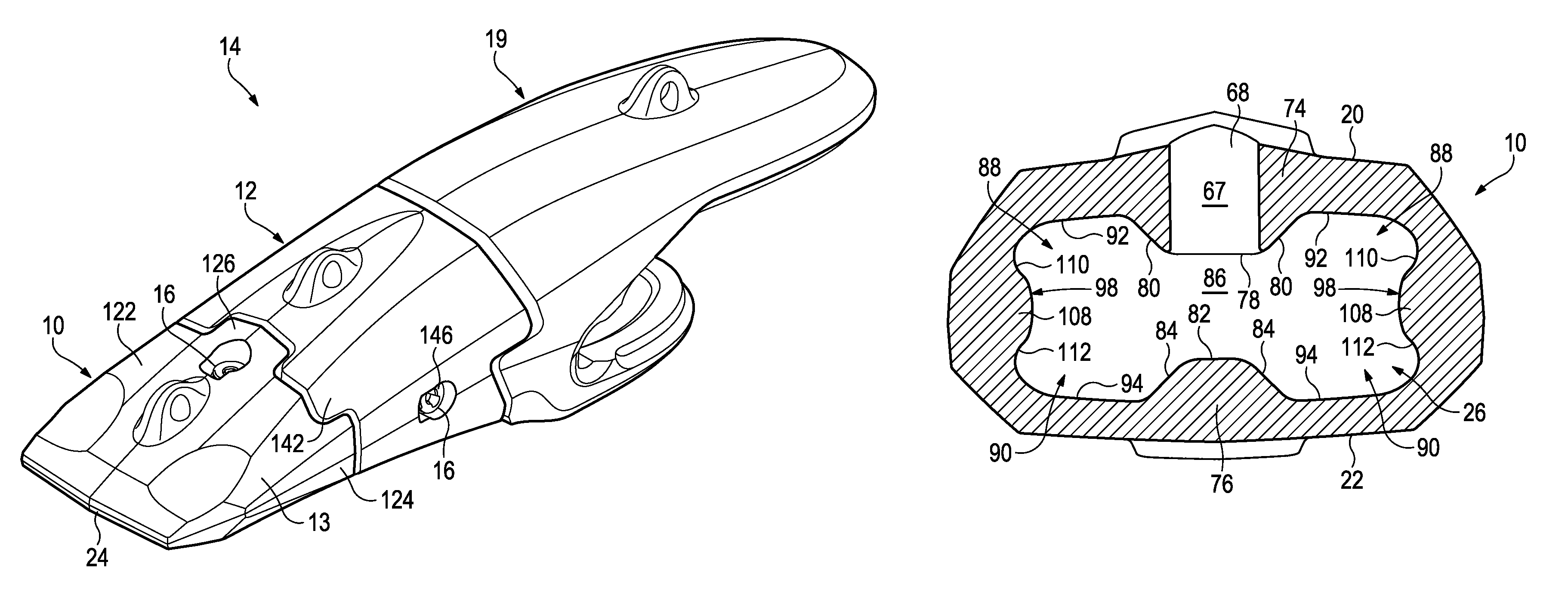
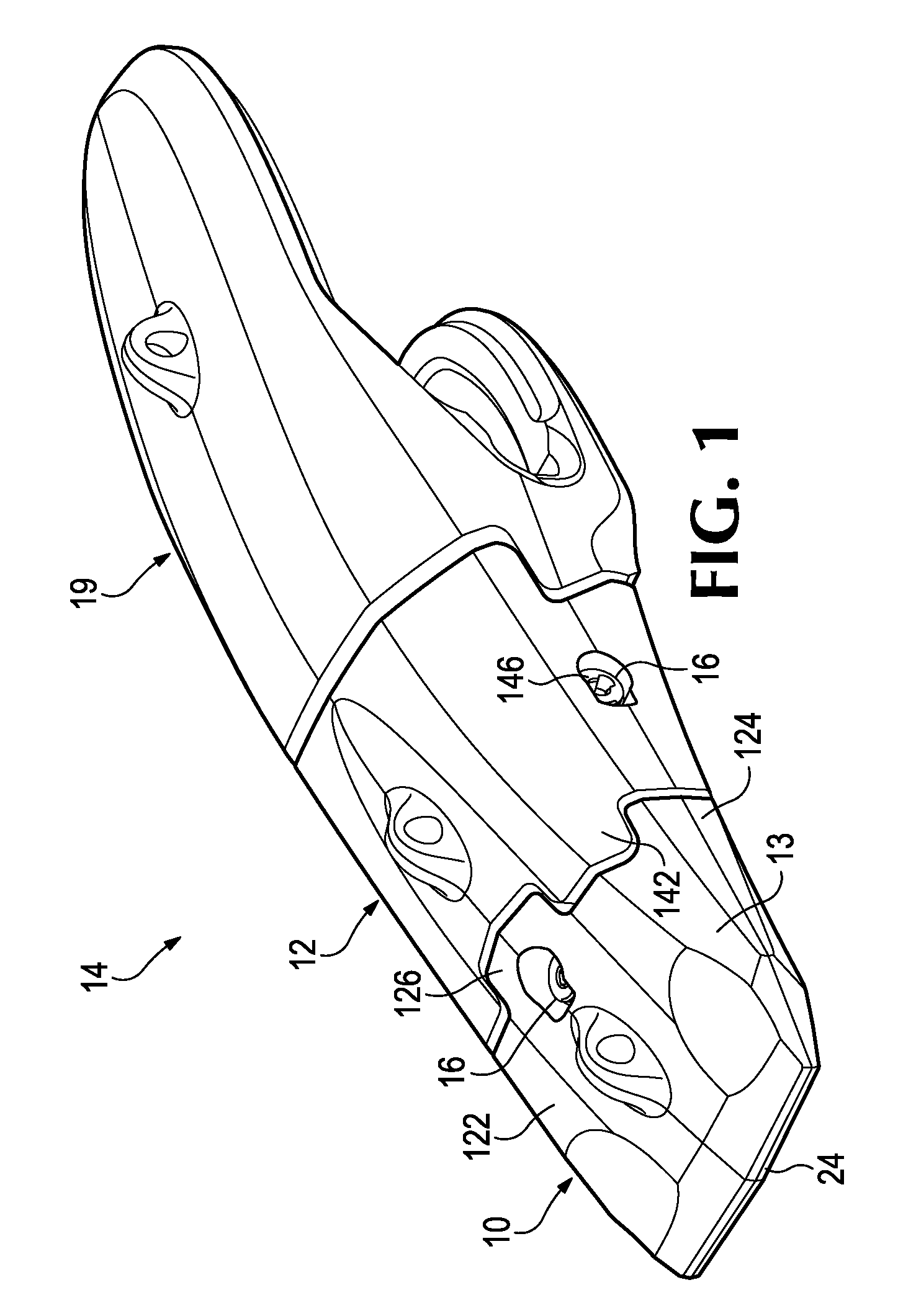
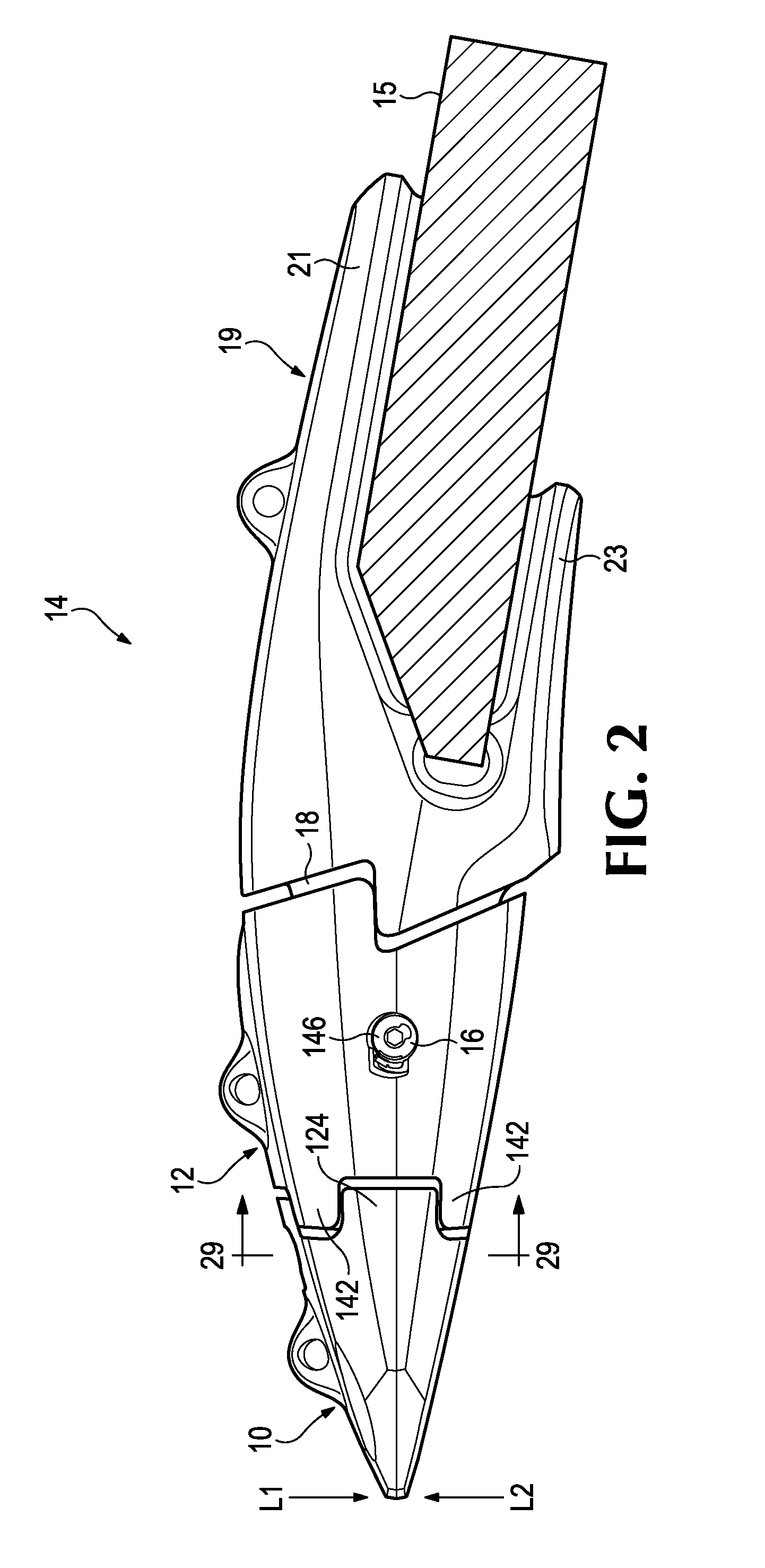
Wear assembly
ActiveUS9222243B2Lessens risk of ejection and lossImprove stable installationSoil-shifting machines/dredgersMechanical engineering
A wear assembly for use on various kinds of earth working equipment that includes a base with a supporting portion, a wear member with a cavity into which the supporting portion is received, and a lock to releasably secure the wear member to the base. The supporting portion is formed with top and bottom recesses that receive complementary projections of the wear member. These recesses and projections include aligned holes so as to receive and position the lock centrally within the wear assembly and remote from the wear surface. The lock includes a mounting component that defines a threaded opening for receiving a threaded pin that is used to releasably hold the wear member to the base. The separate mounting component can be easily manufactured and secured within the wear member for less expense and higher quality than forming the threads directly in the wear member.
Owner:ESCO GRP LLC
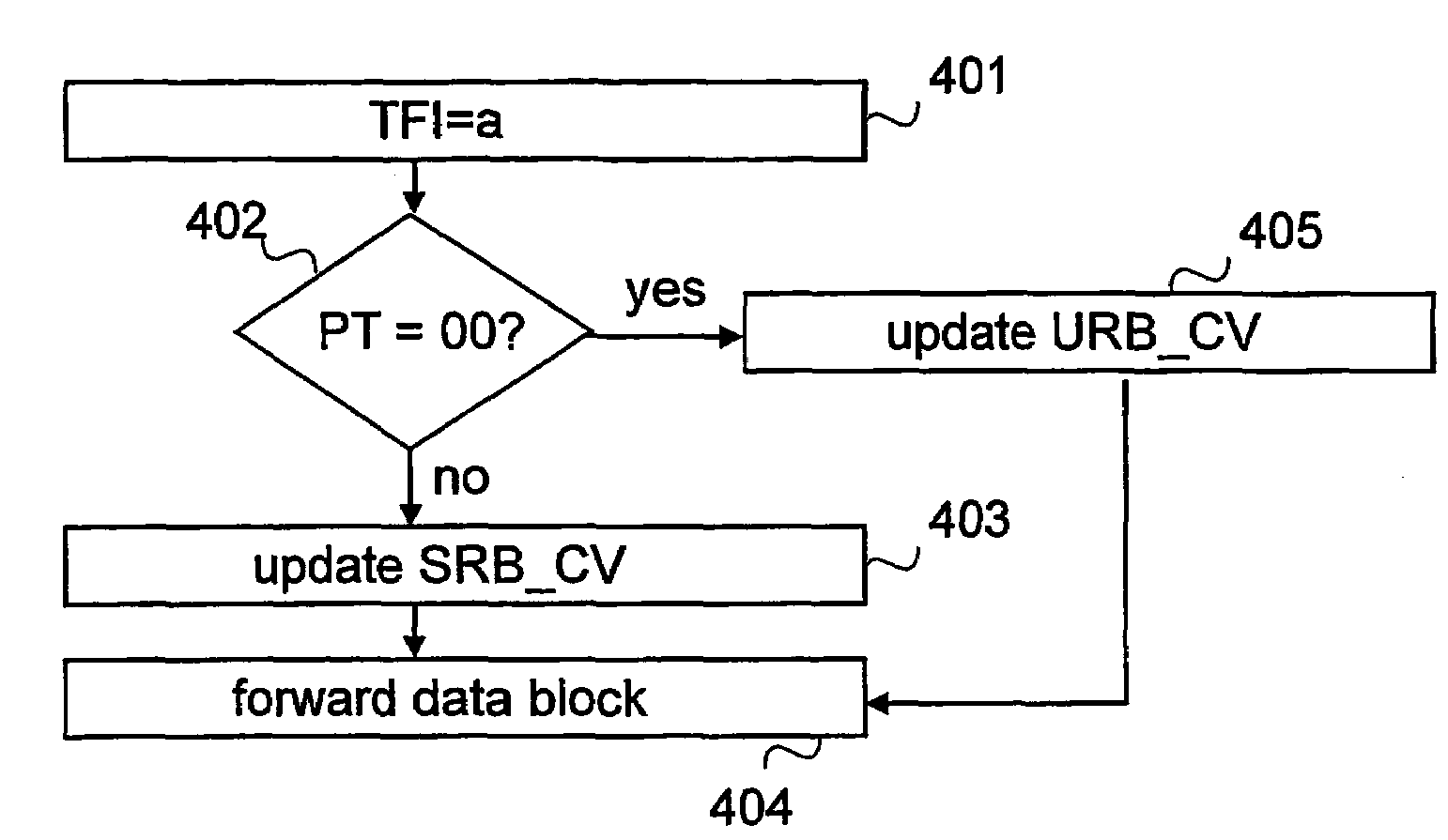
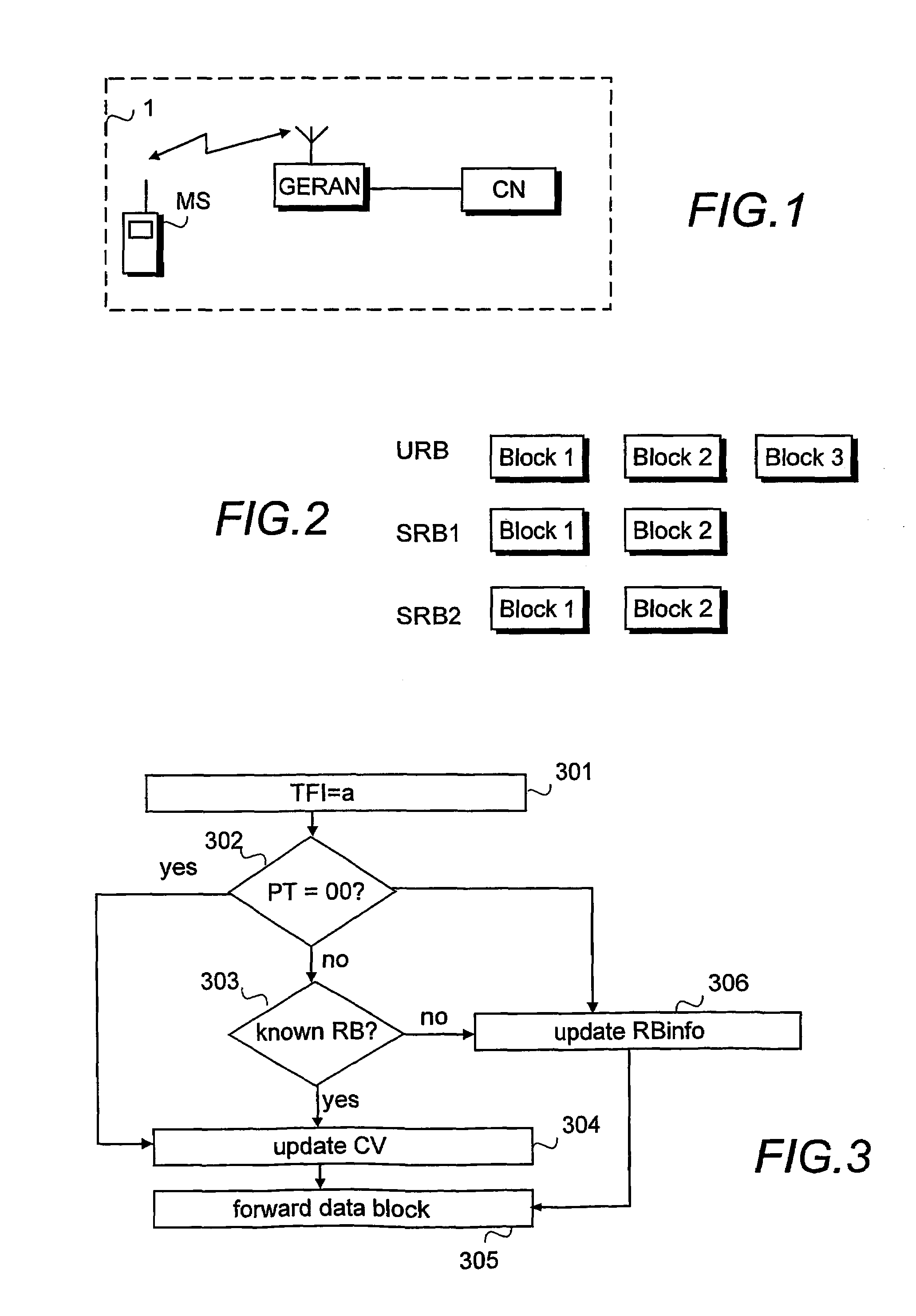
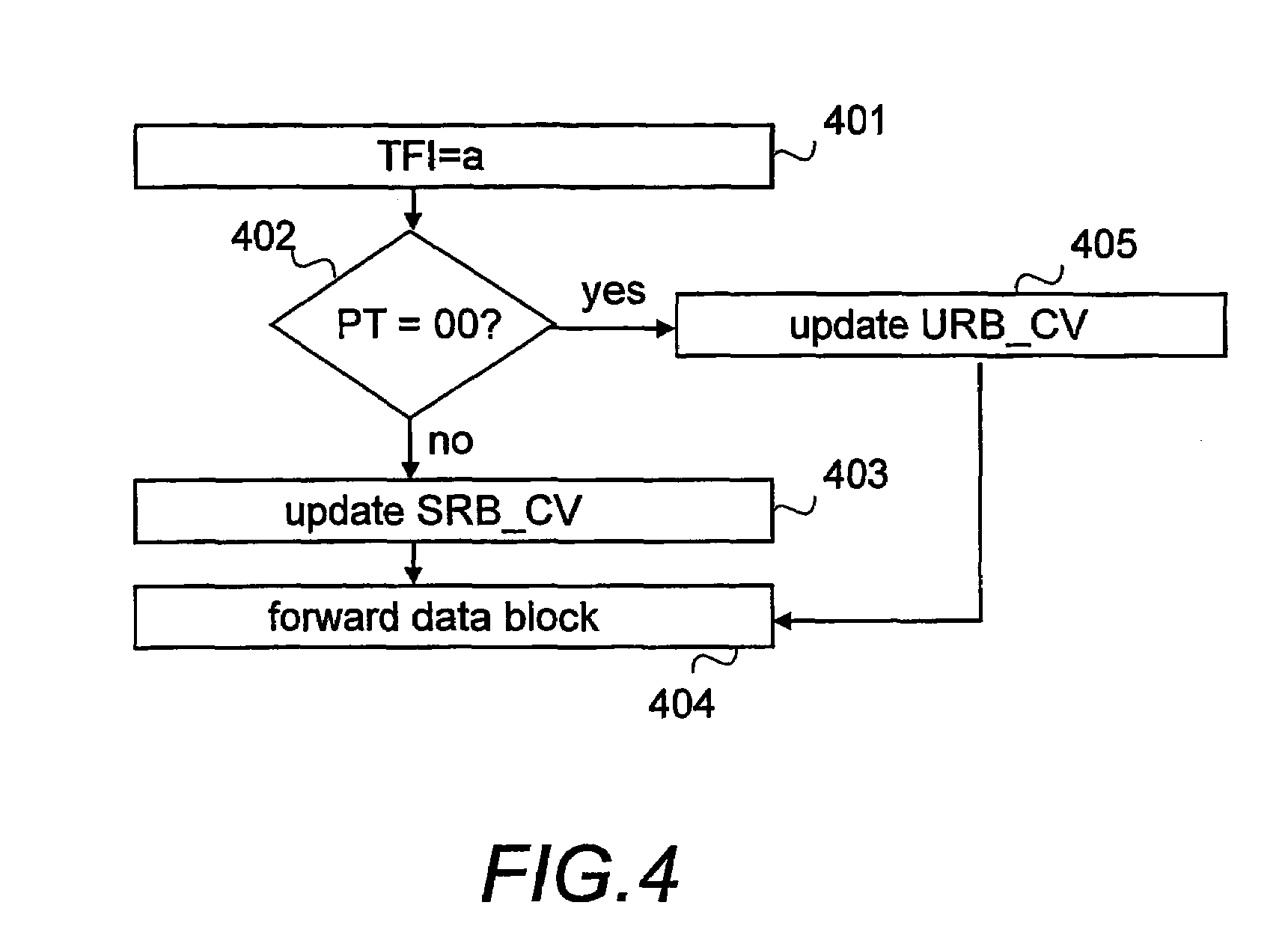
Informing network about amount of data to be transferred
ActiveUS7369508B2Inhibition releaseSufficient informationError preventionTransmission systemsOperating systemComputer network
When uplink signalling radio bearers steal capacity from a user bearer, at least the amount of data waiting for transmission on a TBF established for the user bearer should be informed to the network. This can be done by using separate countdown values for each radio bearer, using a first countdown value for the bearer the TBF was established for and a second countdown value which indicates the total amount of data on stealing bearers, or by calculating a common countdown value indicating the total amount of data on all bearers using the TBF.
Owner:INTELLECTUAL VENTURES I LLC
Conjugates of galactose-binding lectins and clostridial neurotoxins as analgesics
InactiveUS7052702B1Pain reliefReduce and preferably prevent transmissionNervous disorderBacteriaGalactose binding lectinProtein translocation
A class of novel agents that are able to modify nociceptive afferent function is provided. The agents may inhibit the release of neurotransmitters from discrete populations of neurones and thereby reduce or preferably prevent the transmission of afferent pain signals from peripheral to central pain fibers. They comprise a galactose-binding lectin linked to a derivative of a clostridial neurotoxin. The derivative of the clostridial neurotoxin comprises the L-chain, or a fragment thereof, which includes the active proteolytic enzyme domain of the light (L) chain, linked to a molecule or domain with membrane translocating activity. The agents may be used in or as pharmaceuticals for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Antibodies that specifically bind to tim3
ActiveUS20130022623A1Promote growthReduce inflammationHeavy metal active ingredientsPeptide/protein ingredientsCancer cellAntibody
Provided herein are antibodies specific for TIM3 that can be used to detect cancer cells, in particular, cancer stem cells. The antibodies can also be used in therapeutic compositions for treating cancer and reducing inflammation.
Owner:ONK THERAPEUTICS LTD
Waste storage device
InactiveUS20050044819A1Create efficientlySolve the lack of spaceRefuse receptaclesWrapper twisting/gatheringWaste disposalEngineering
The present invention discloses an automated sealing waste disposal apparatus using tubular material.
Owner:CHOMIK RICHARD S +5
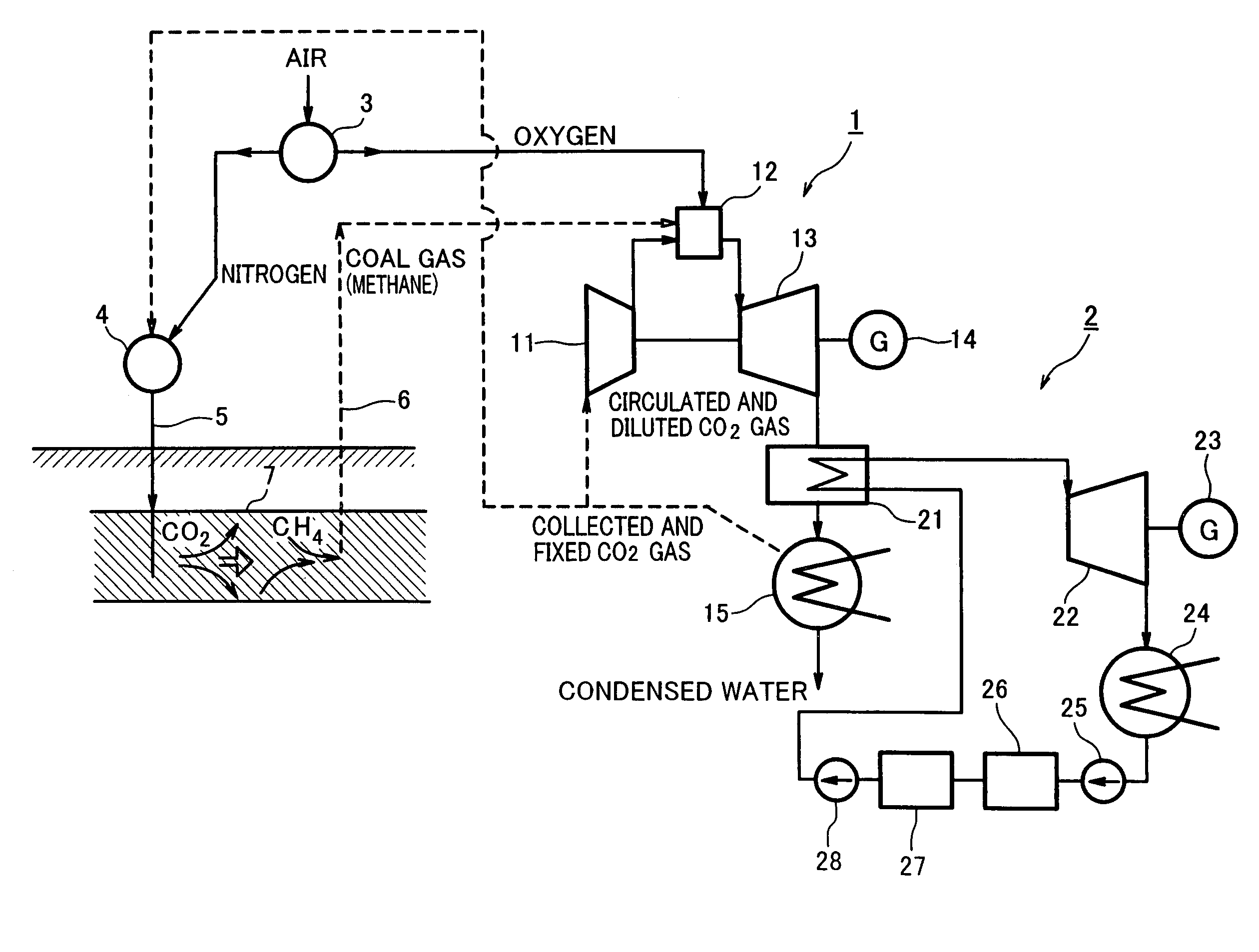
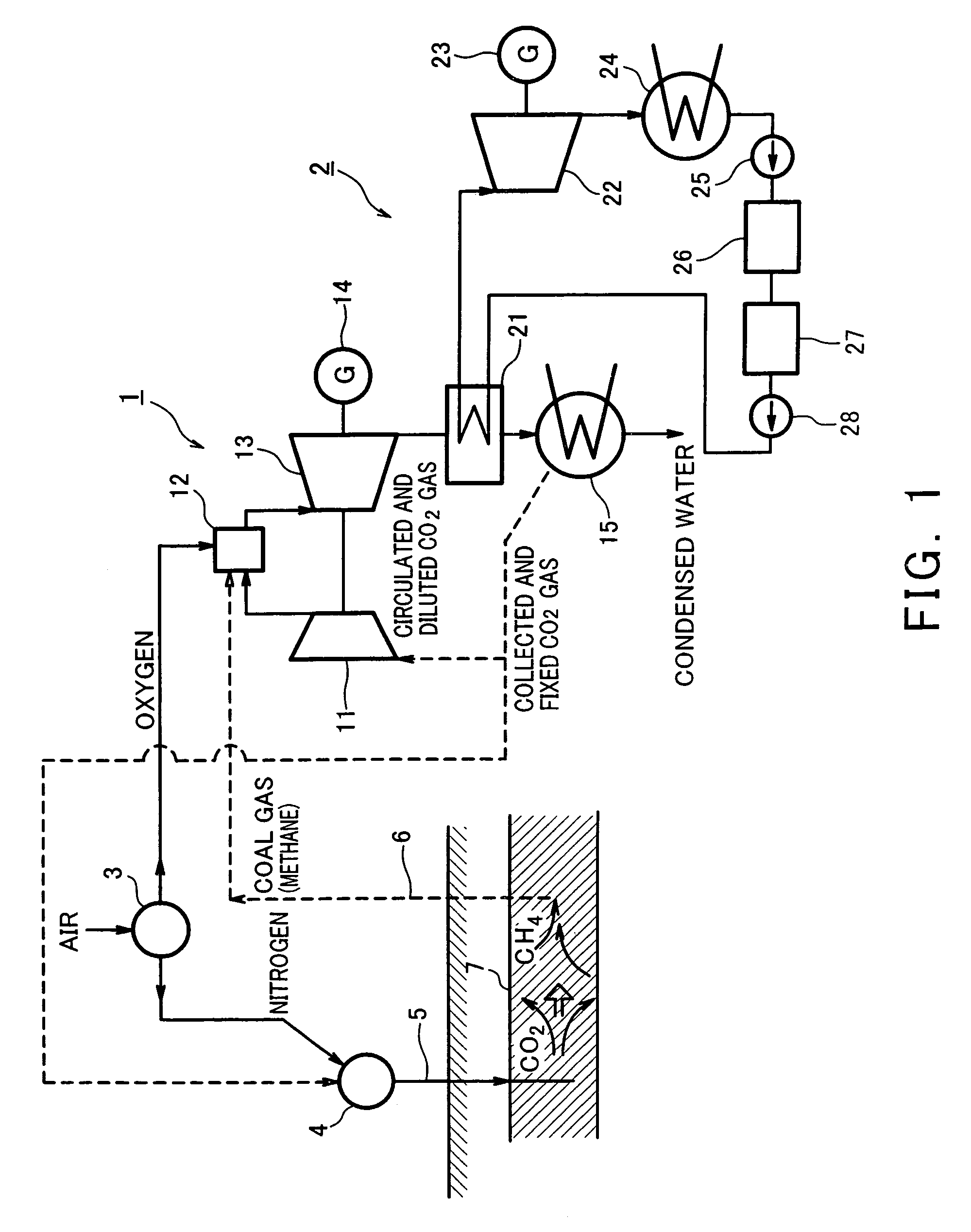
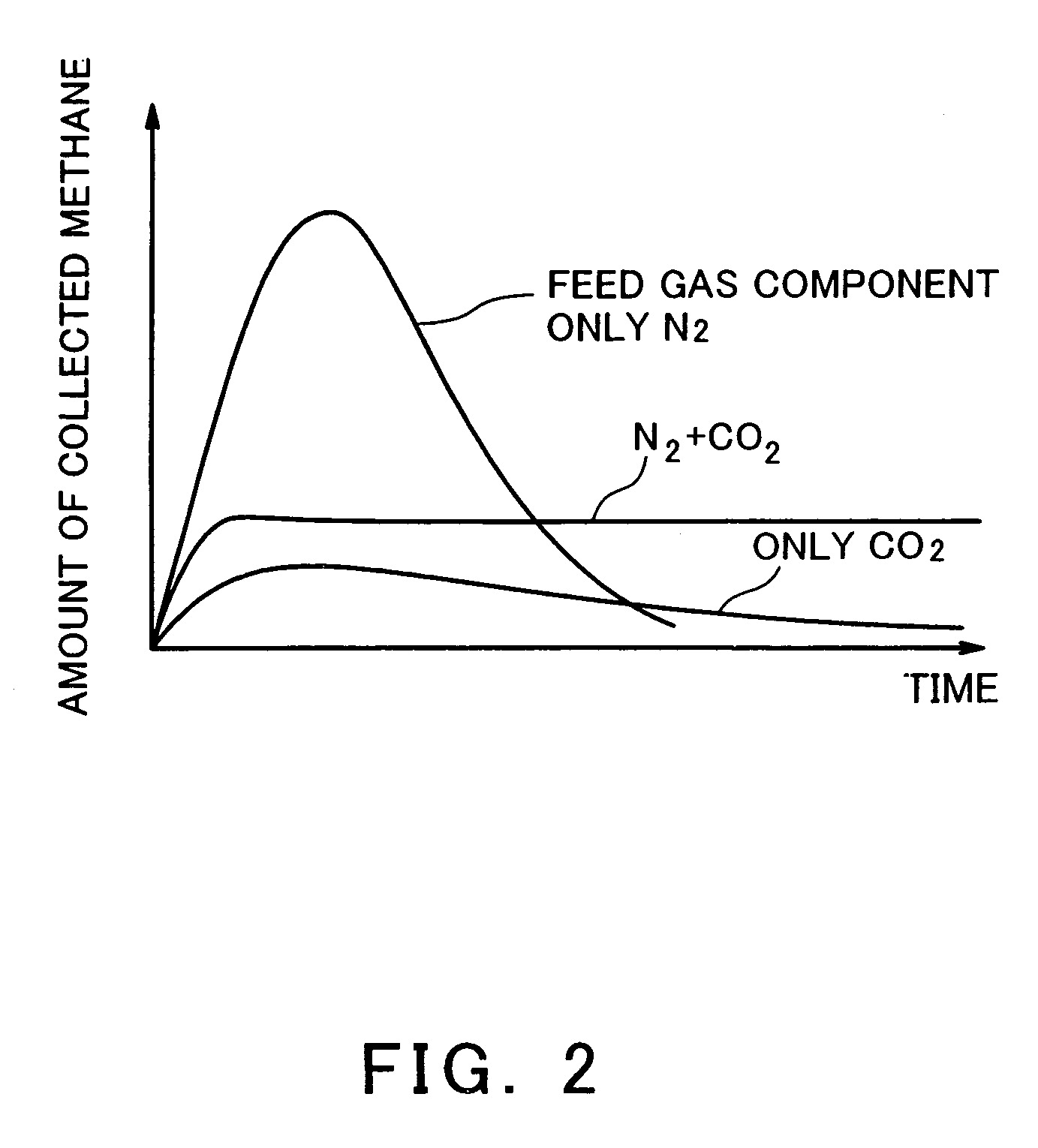
Gas turbine system comprising closed system of fuel and combustion gas using underground coal layer
InactiveUS7143572B2Inhibition releaseTurbine/propulsion fuel supply systemsWorking fluid for enginesCombustionProduct gas
A gas turbine system is configured to inhibit release of carbon dioxide to the atmosphere in such a manner that methane is combusted with oxygen that has been separated from air by an oxygen generator to allow an oxygen combustion type gas turbine to be driven. Carbon dioxide emitted from the turbine is fed under pressure into an underground coal bed along with an air component after separating the oxygen from the air and fixed into the coal bed, and the coal bed methane is collected above the ground by a gas drive action of the gas and supplied to the gas turbine as fuel.
Owner:KAWASAKI HEAVY IND LTD +1
Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same
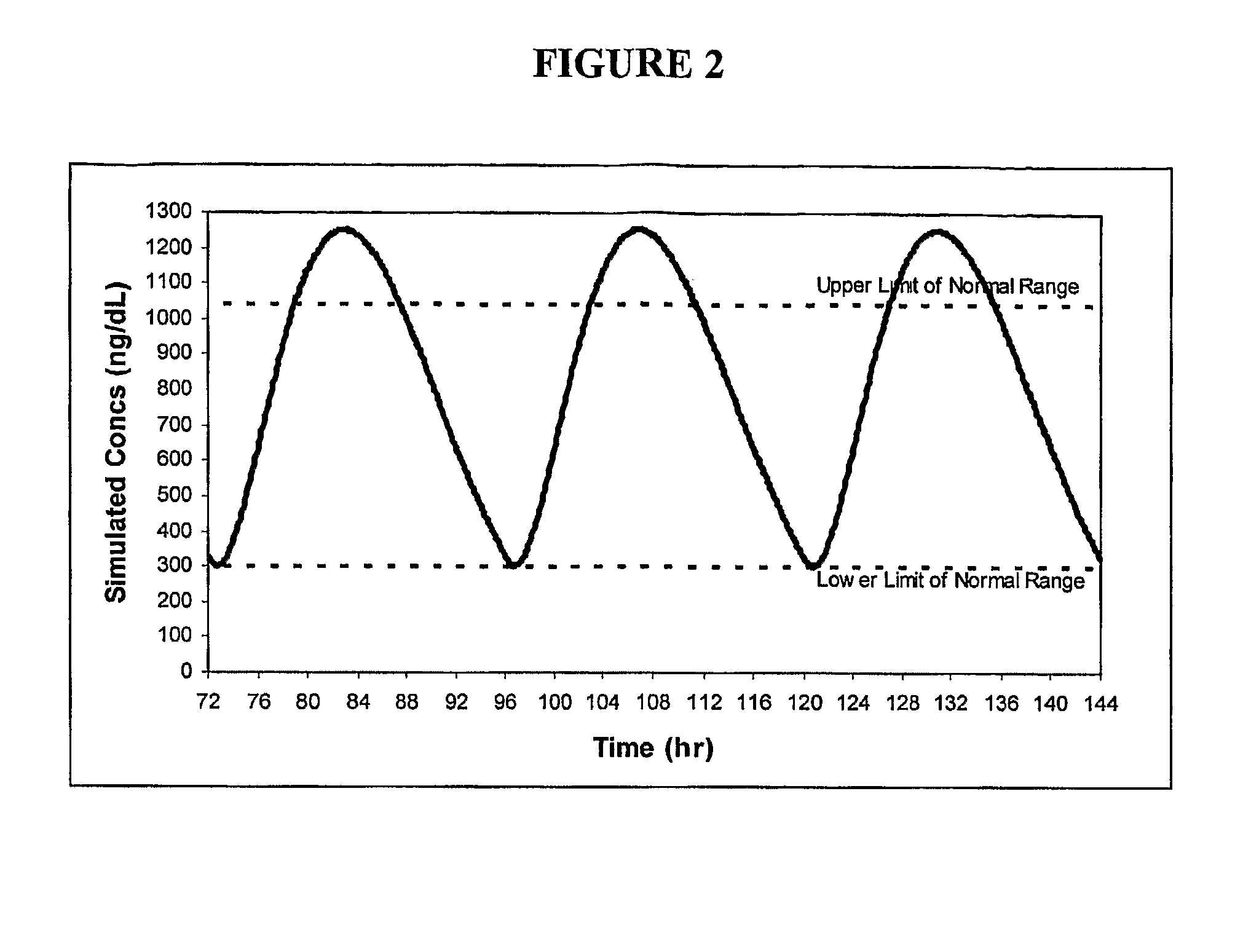
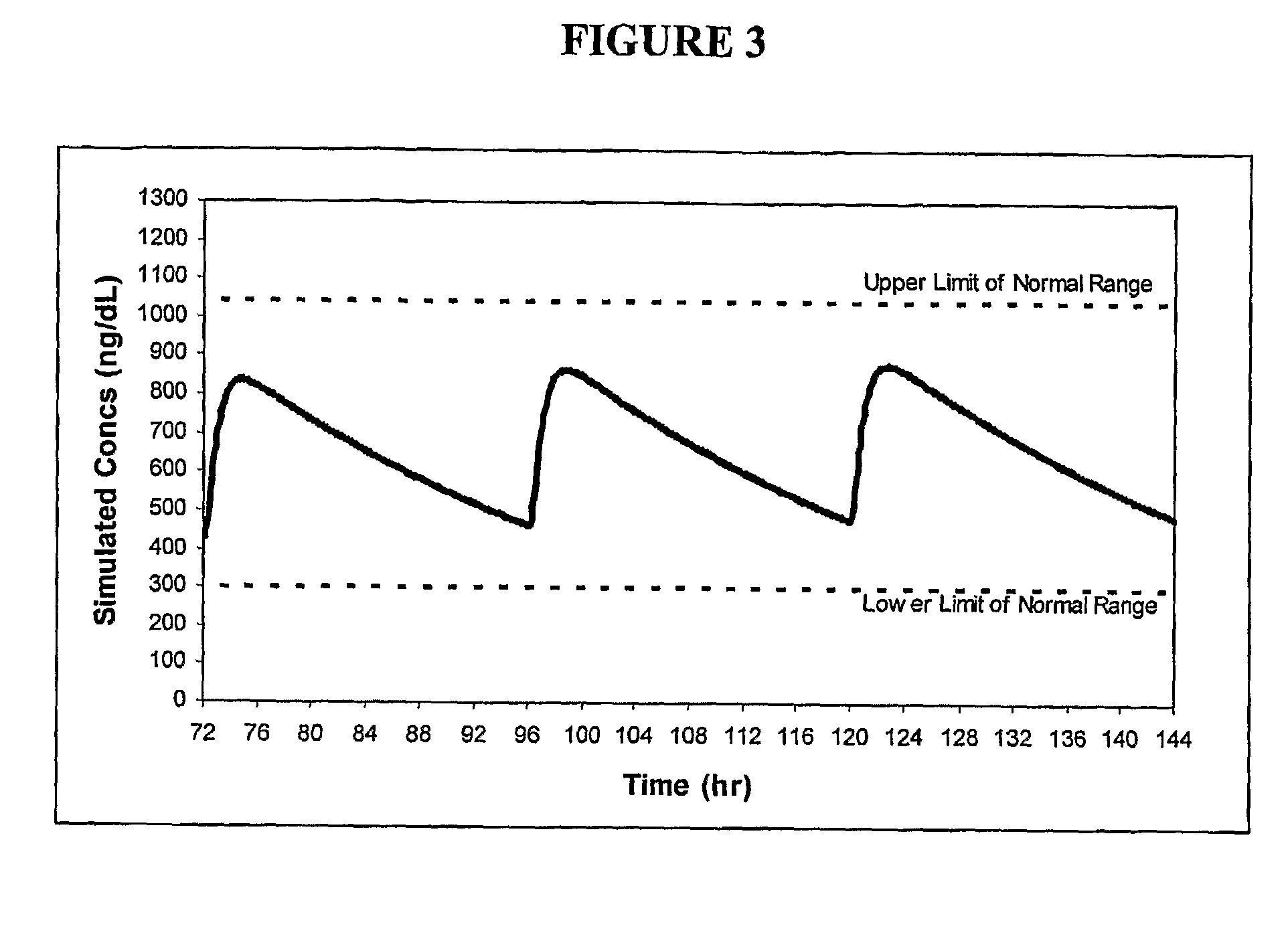
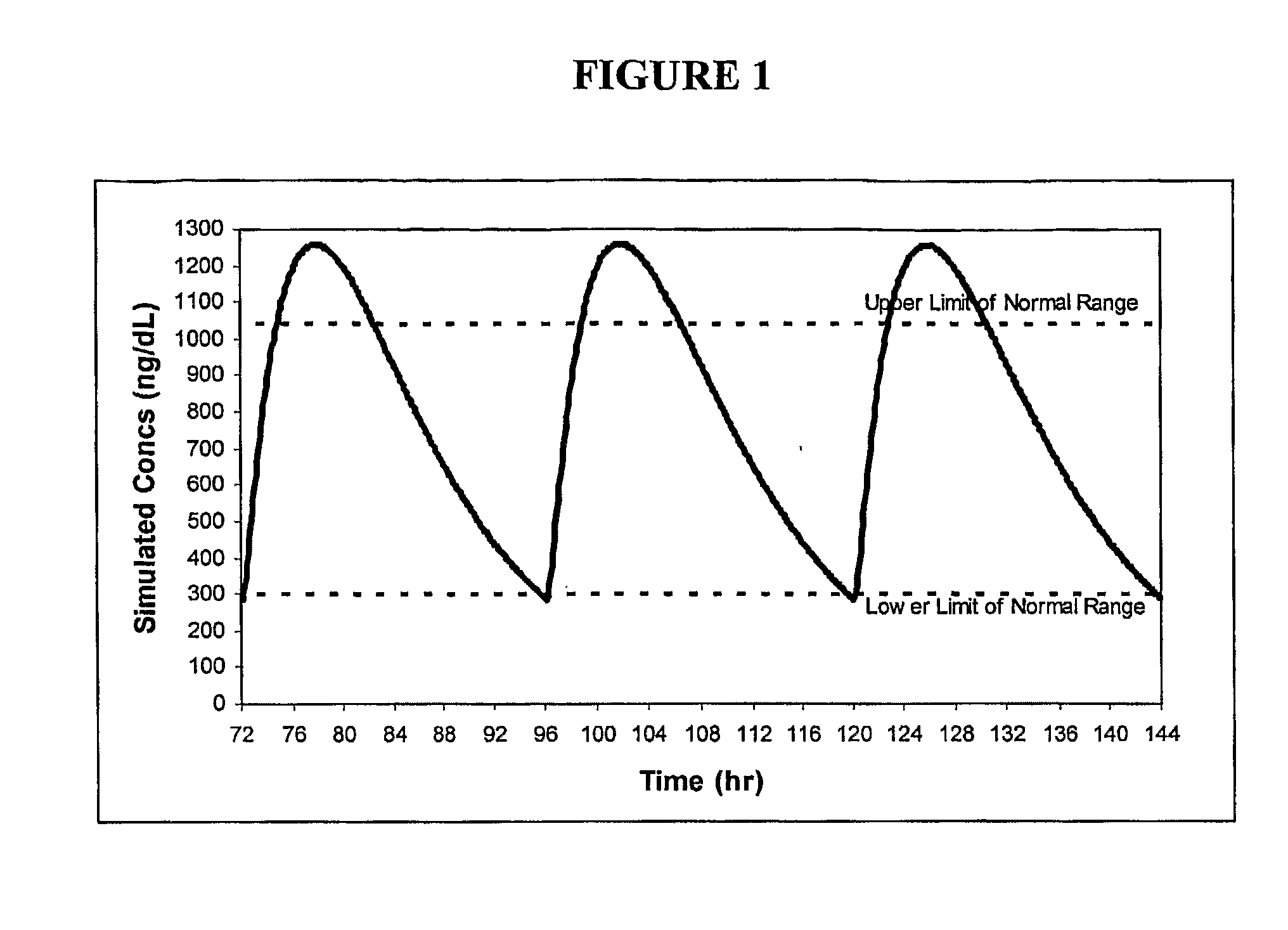
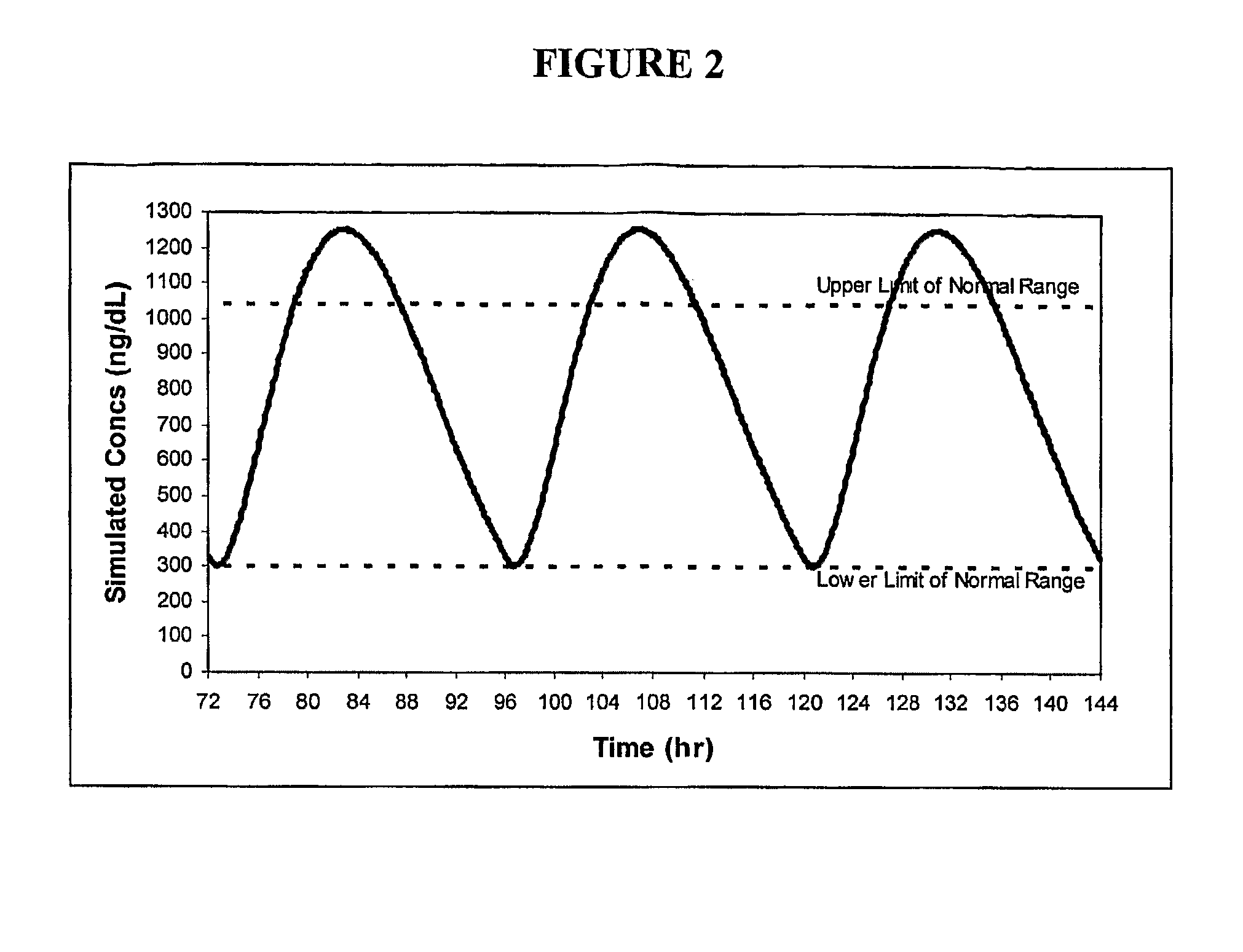
ActiveUS8241664B2Preventing and alleviating symptomInhibition releaseBiocideOintment deliveryOral medicationMedicine
A drug delivery system for oral administration of hydrophobic drugs with enhanced and extended absorption and improved pharmacokinetics is provided. In one embodiment, formulations comprising testosterone and testosterone esters, e.g., testosterone palmitate, are disclosed. Methods of treating a hormone deficiency or effecting male contraception with the inventive formulations are also provided.
Owner:TOLMAR INC
Pharmaceutical Delivery Systems for Hydrophobic Drugs and Compositions Compositions Comprising Same
ActiveUS20080317844A1Preventing and alleviating symptomInhibition releaseBiocideOintment deliveryOral medicationDelivery system
A drug delivery system for oral administration of hydrophobic drugs with enhanced and extended absorption and improved pharmacokinetics is provided. In one embodiment, formulations comprising testosterone and testosterone esters, e.g., testosterone palmitate, are disclosed. Methods of treating a hormone deficiency or effecting male contraception with the inventive formulations are also provided.
Owner:TOLMAR INC
Use of diazoxide for the treatment of metabolic syndrome and diabetes complications
InactiveUS6197765B1Reduce releaseReduce weightHeterocyclic compound active ingredientsHyperinsulinemiaSecondary hyperlipidemia
The present invention discloses a treatment for syndrome-X, and resulting complications, that include hyperlipidemia, hypertension, central obesity, hyperinsulinemia and impaired glucose intolerance. Diabetic complications include excess proinsulin levels.
Owner:VARDI PNINA +1
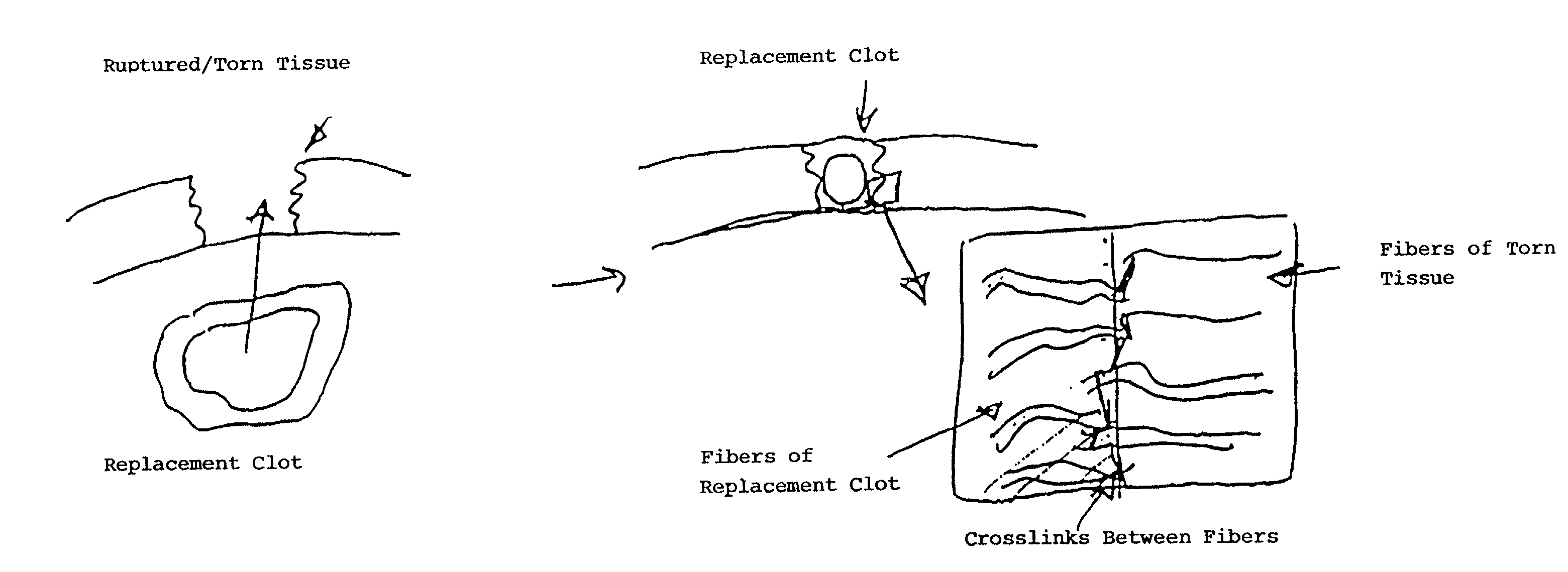
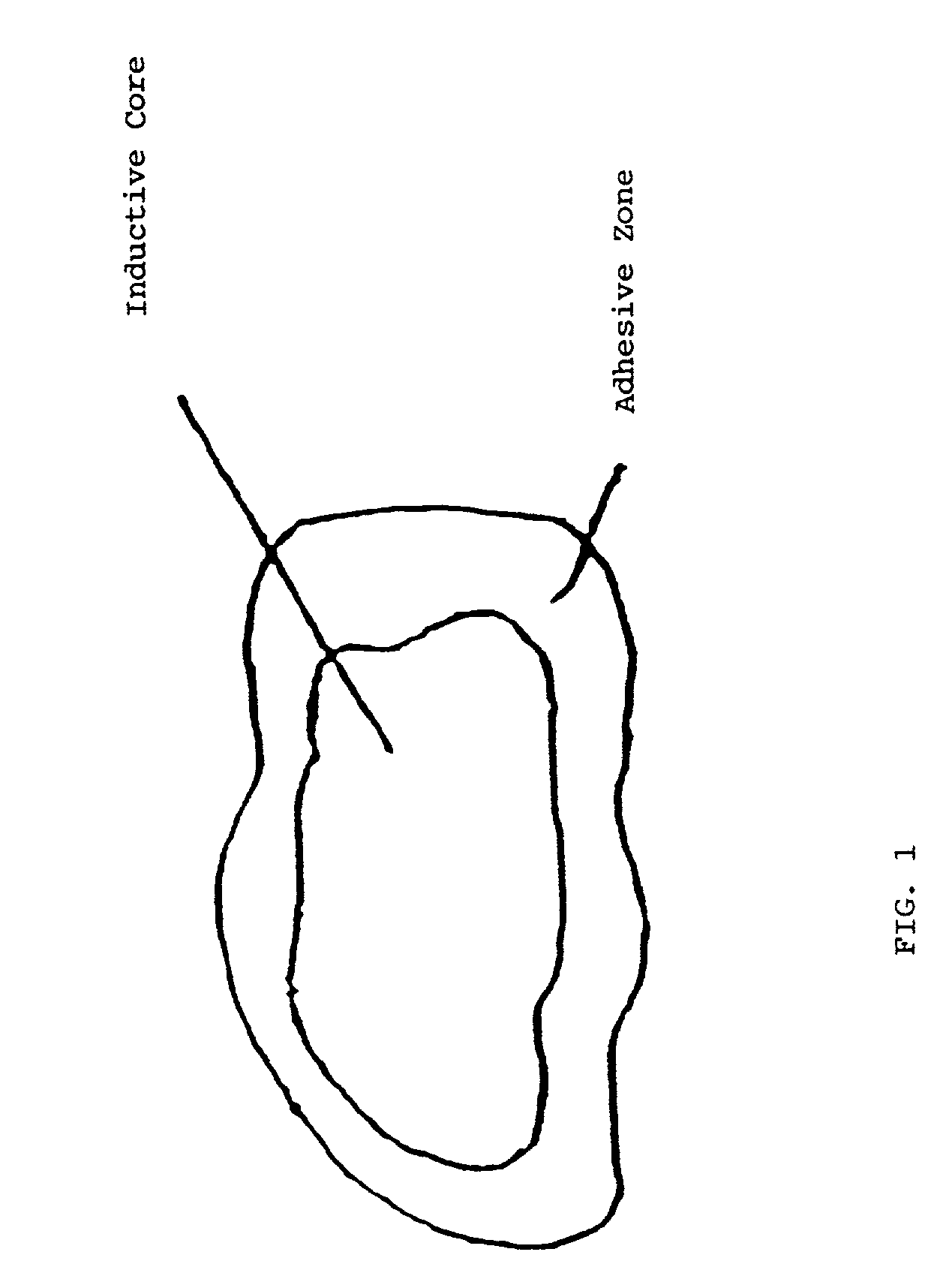
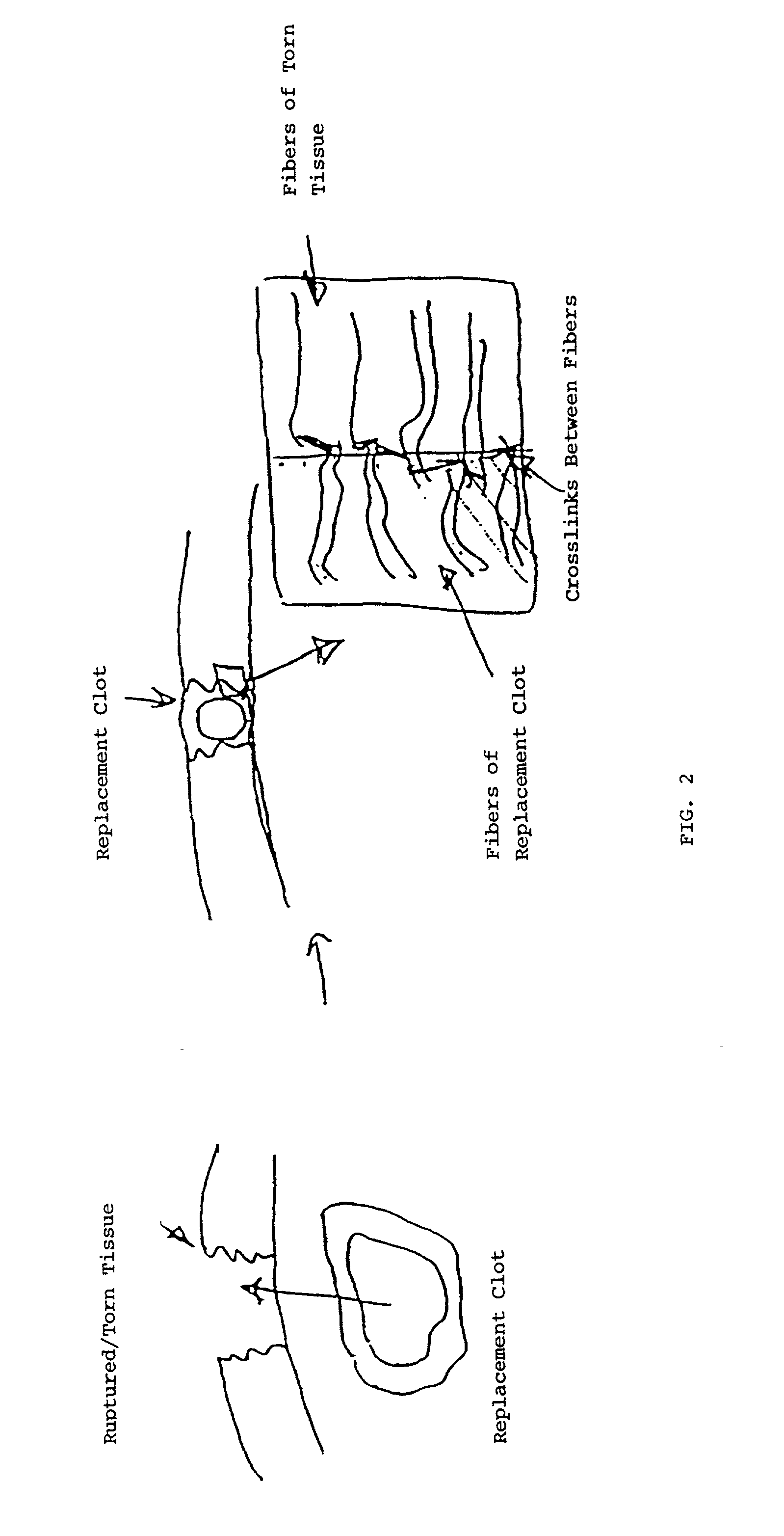
Biologic replacement for fibrin clot
InactiveUS20020123805A1Promote regenerationLess invasive treatmentSurgical adhesivesPeptide/protein ingredientsMedicineLigament
Owner:CHILDRENS MEDICAL CENT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com