Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Clot lysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clot lysis. the time required for a clot to lyse at 98.6°F (37°C) is a reflection of the plasmin content of the blood. Clot retraction and fibrinogen content of the blood sample are also influential.
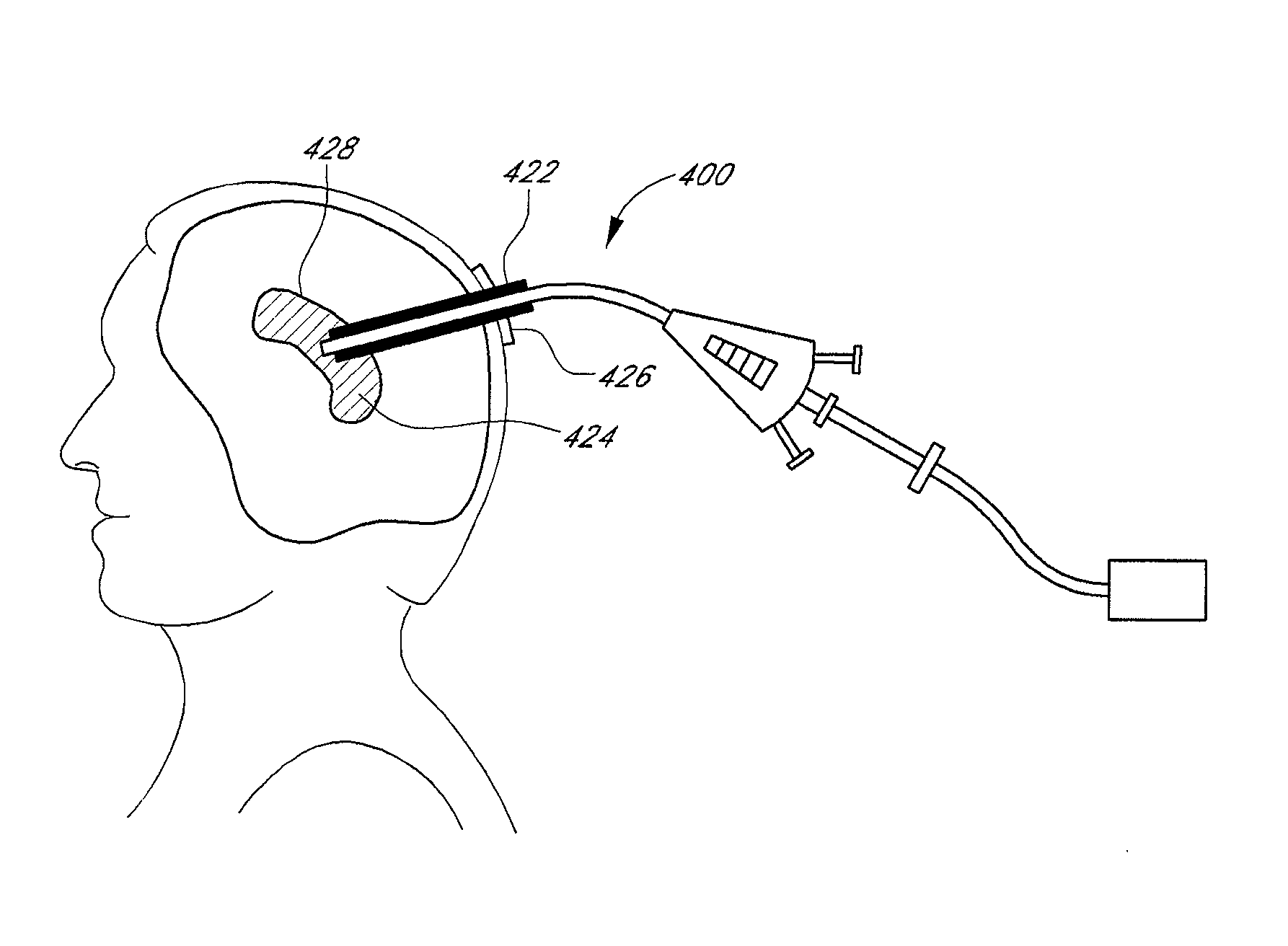
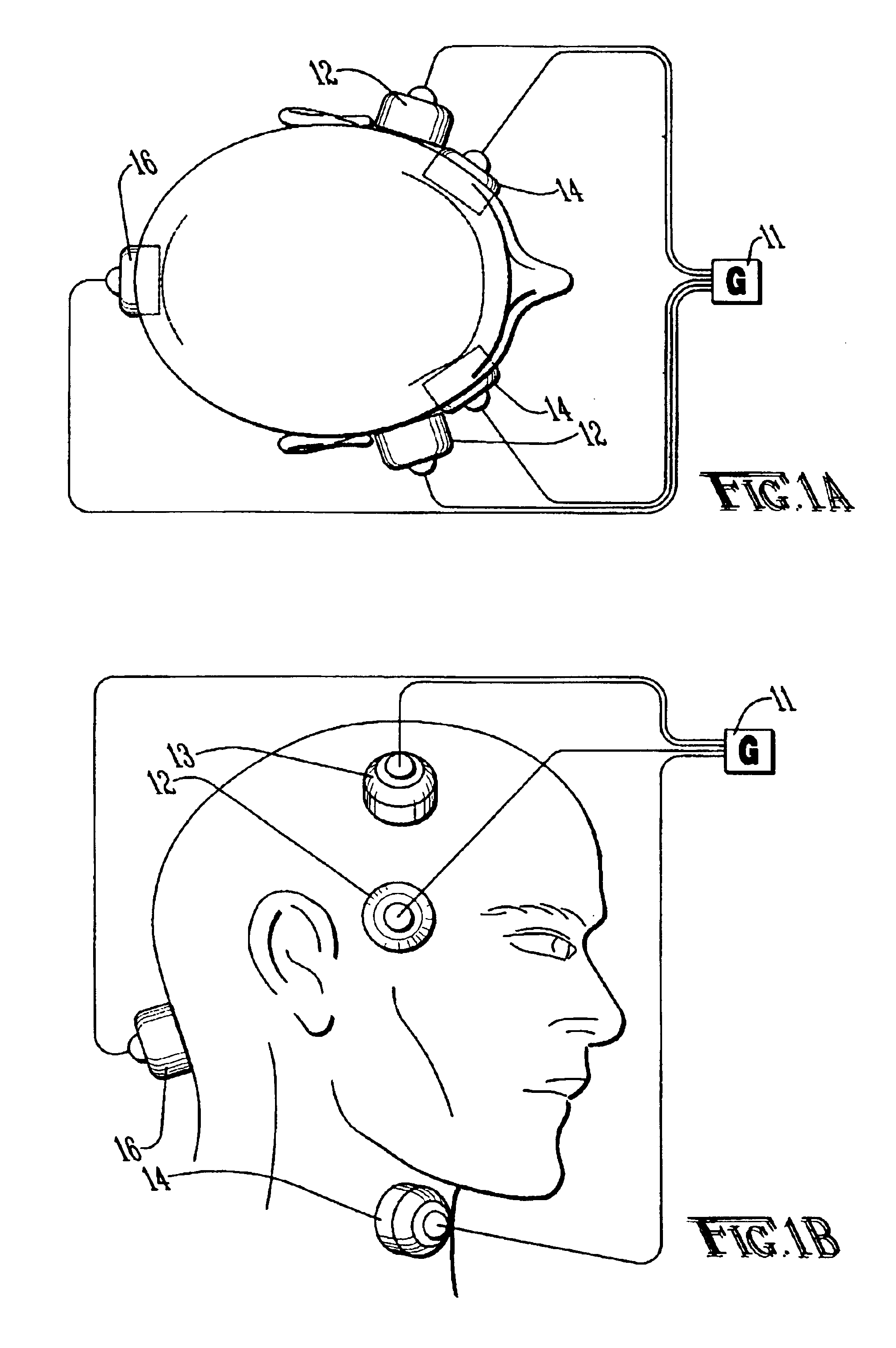
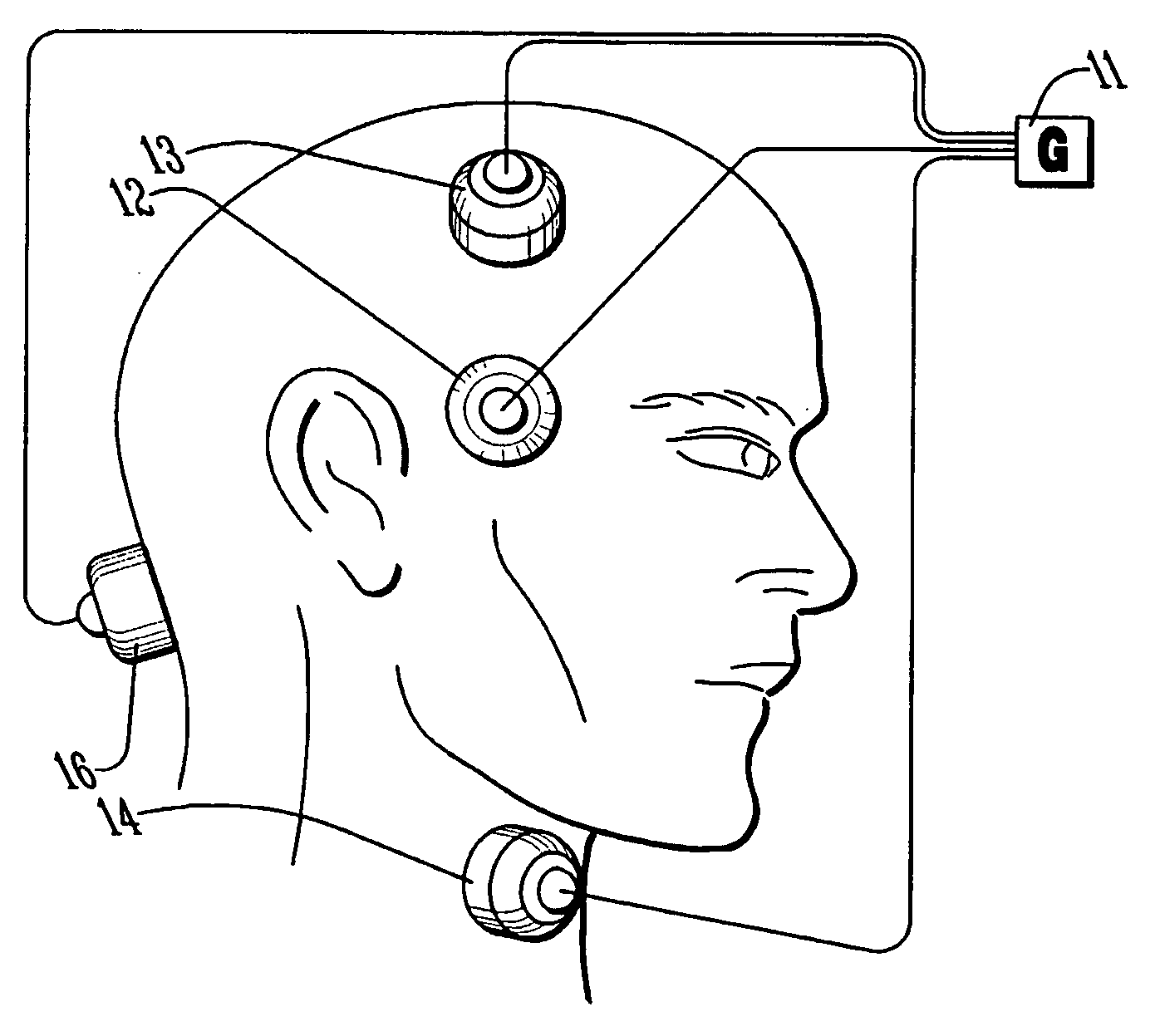
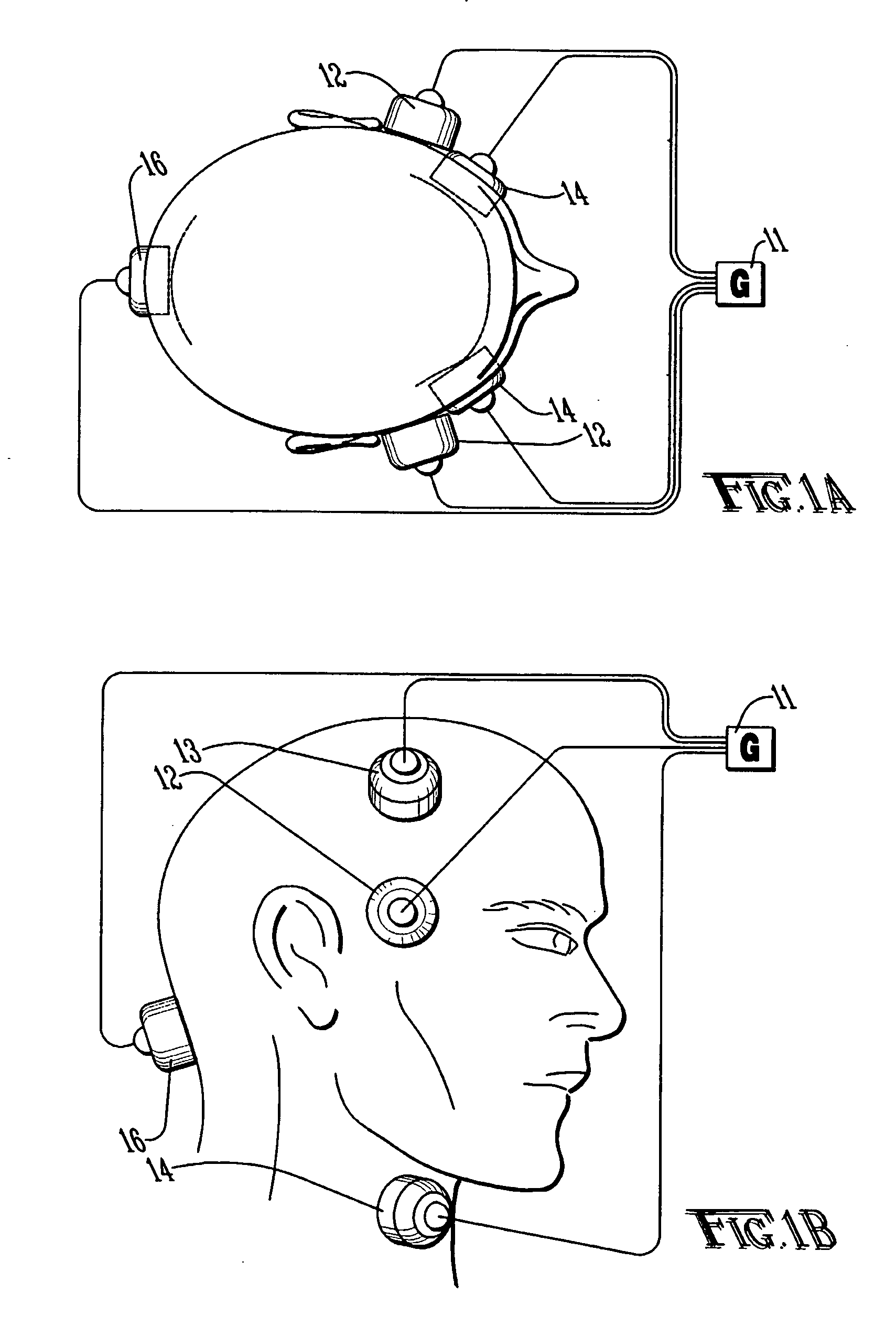
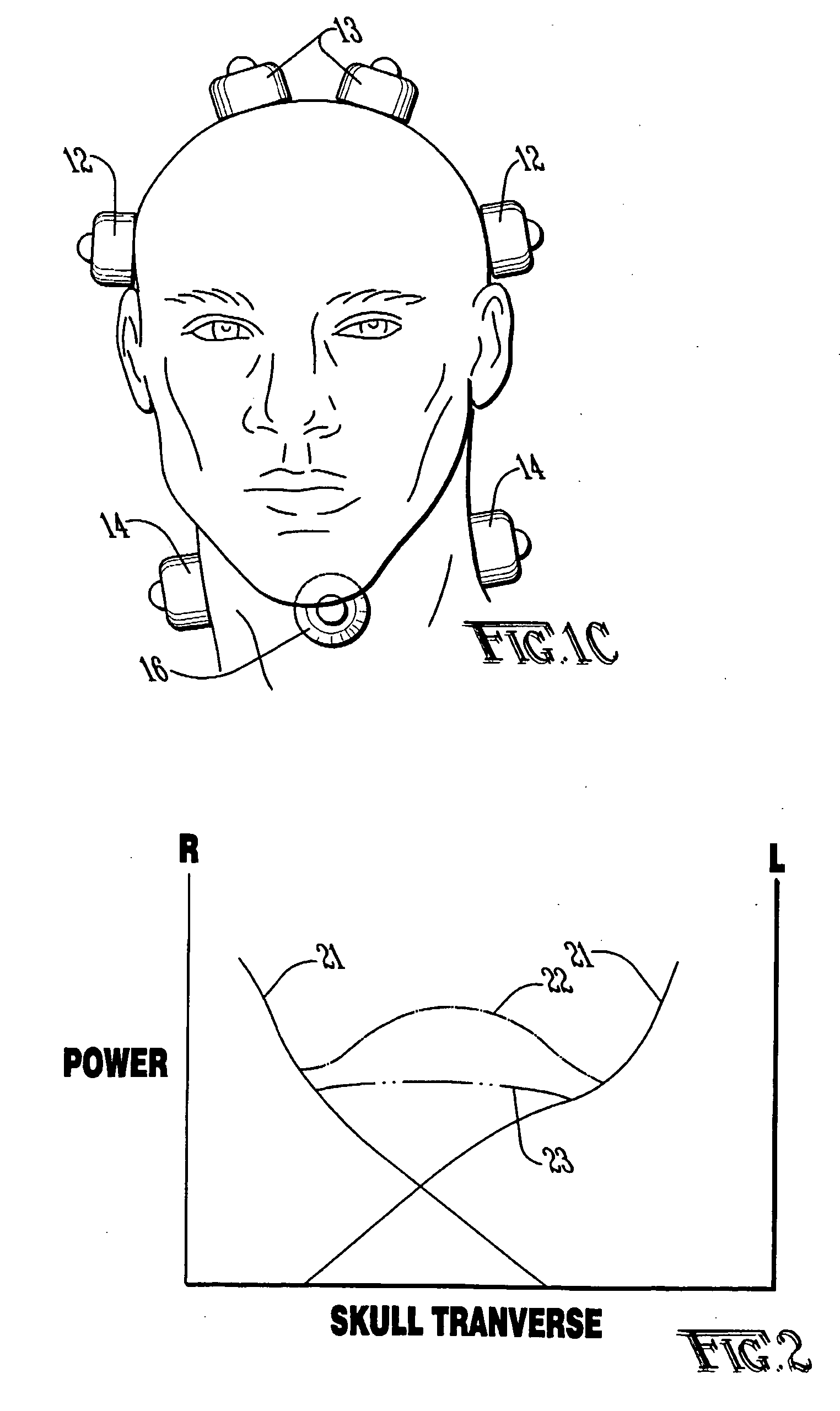
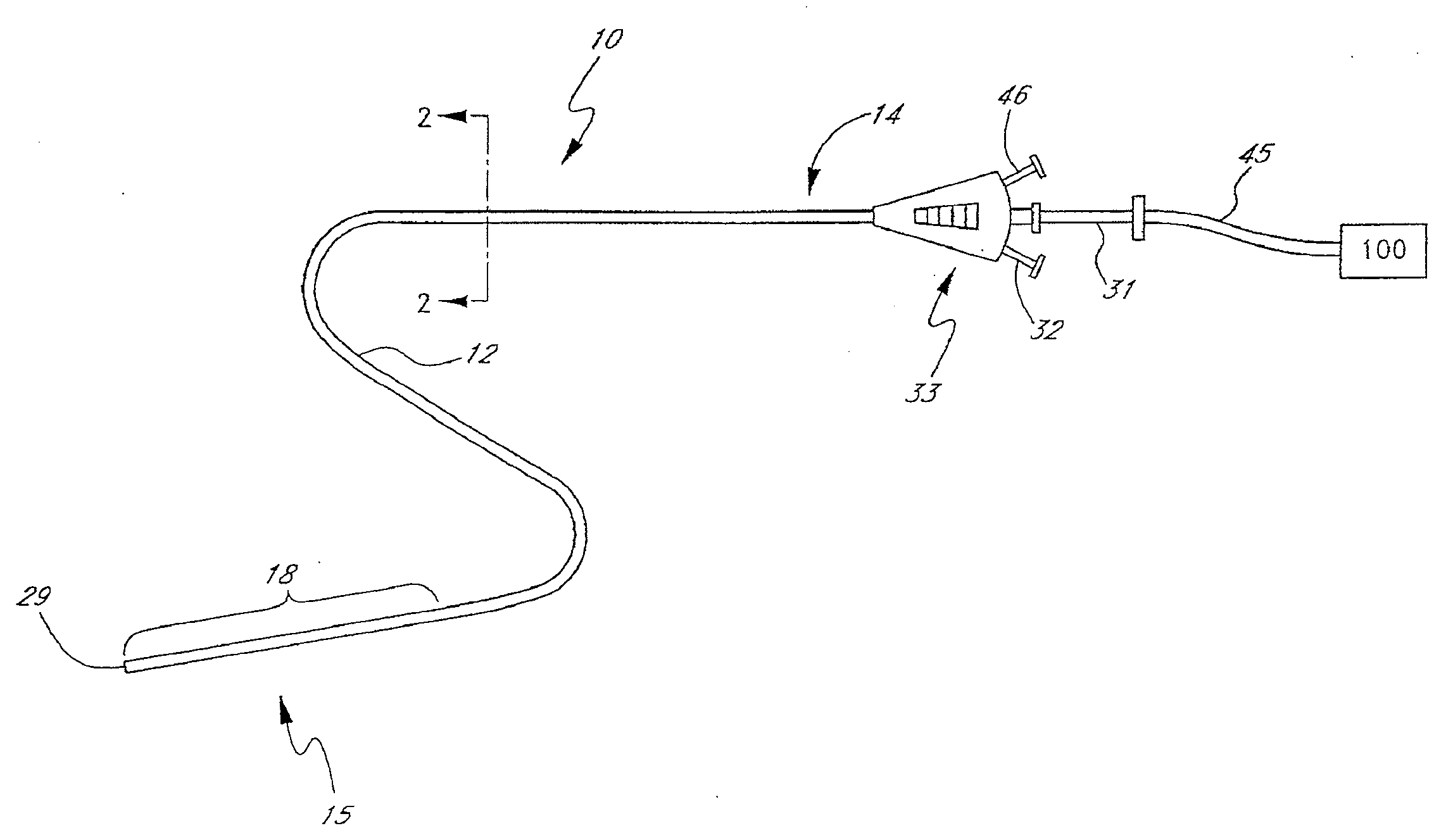
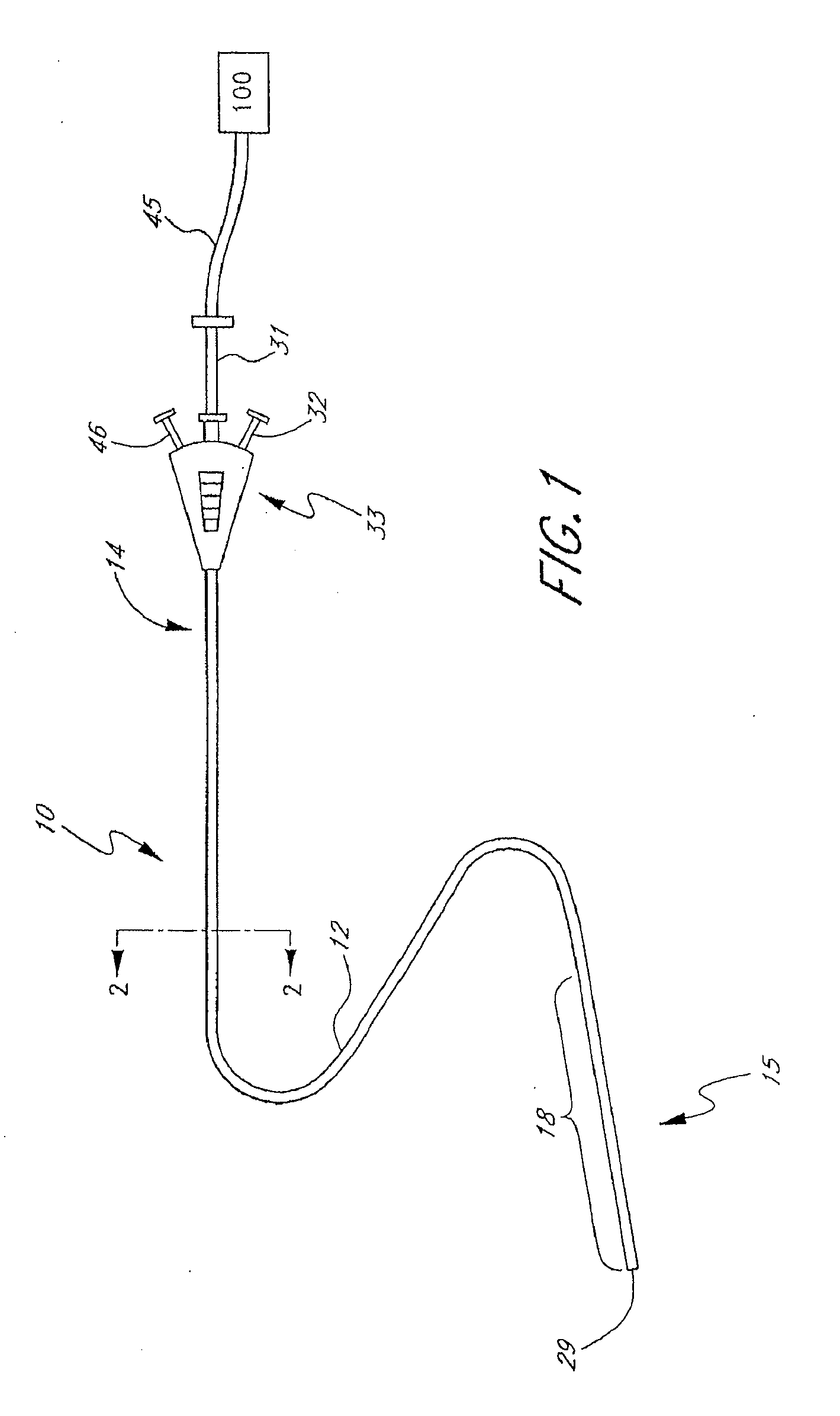
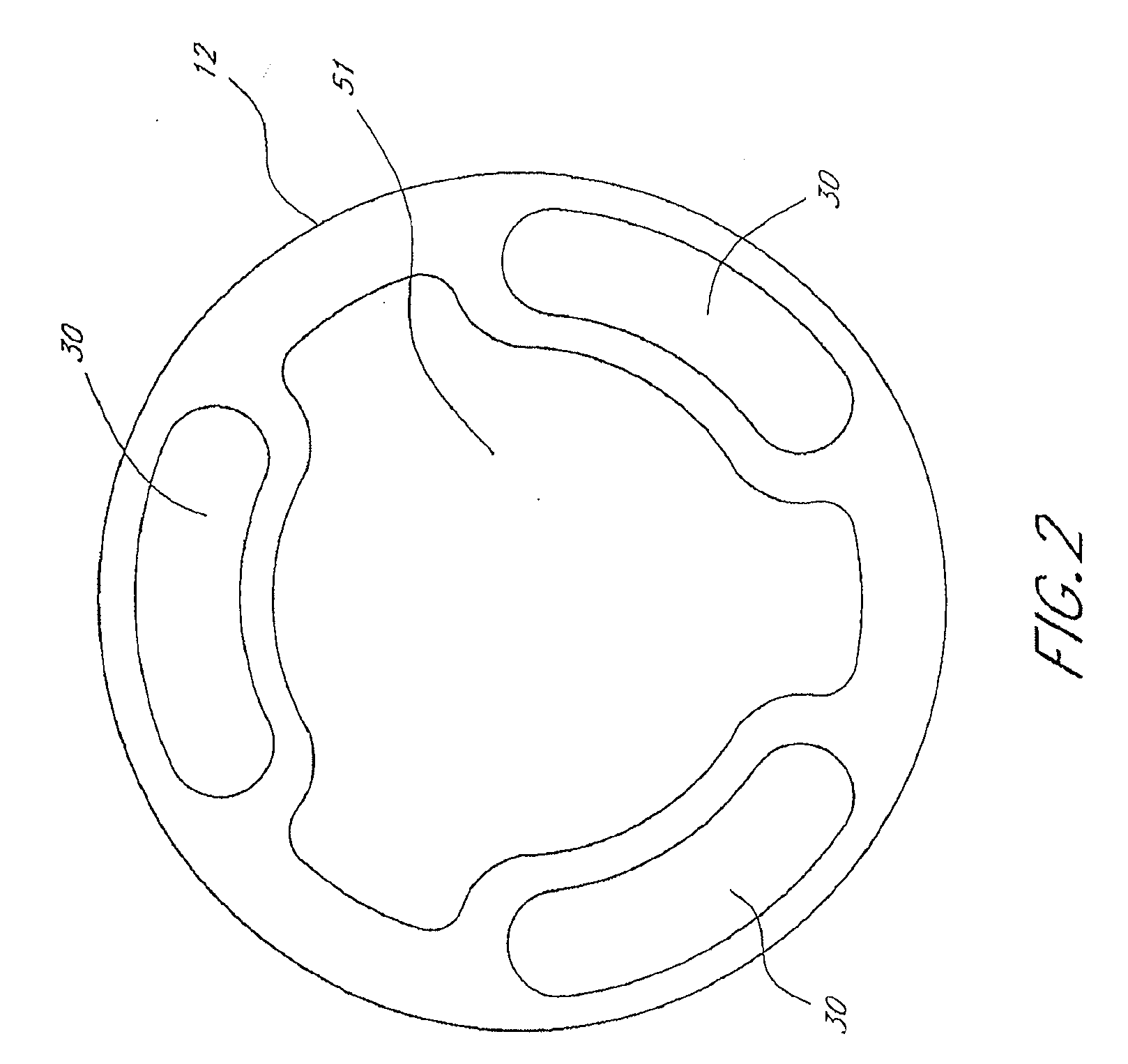
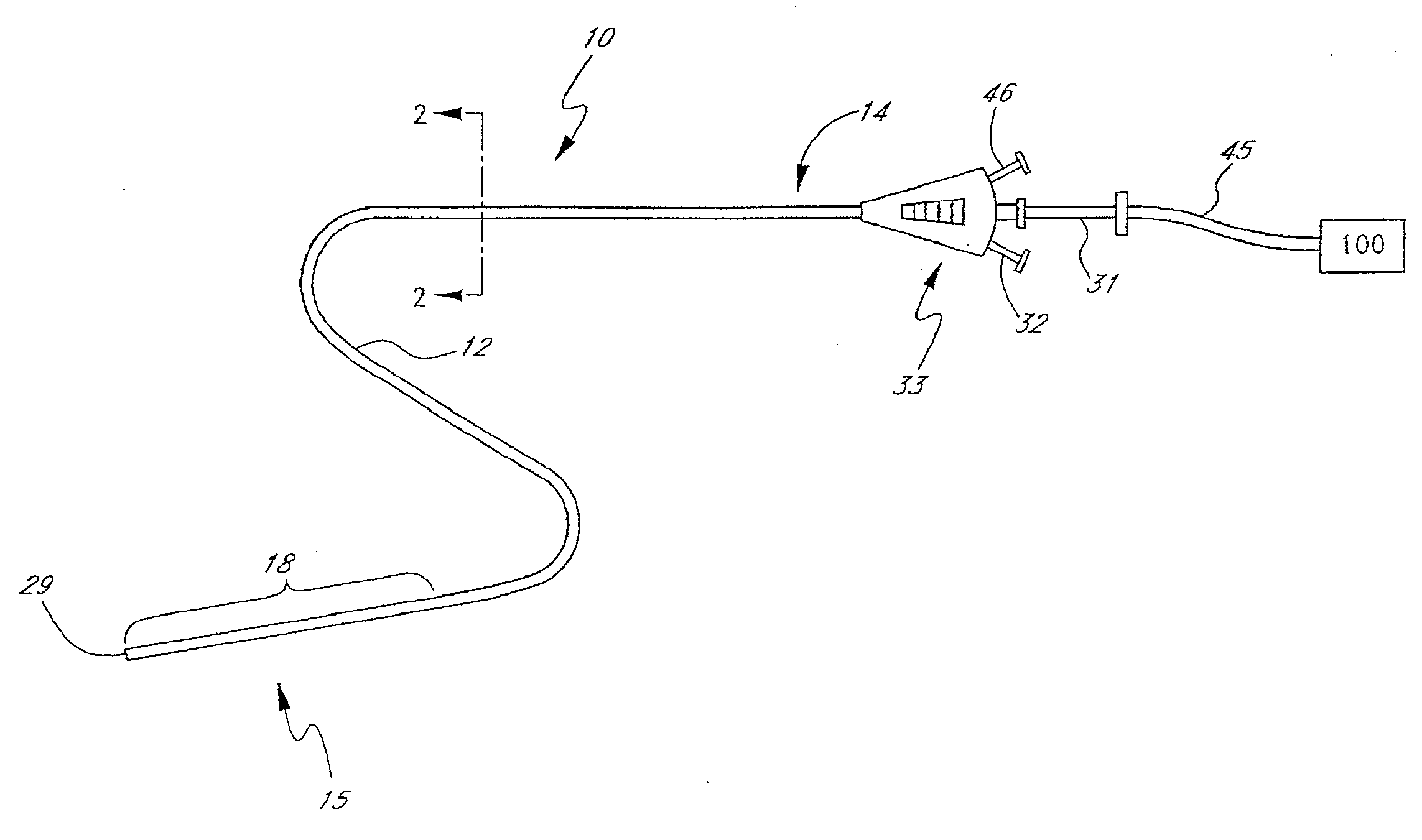
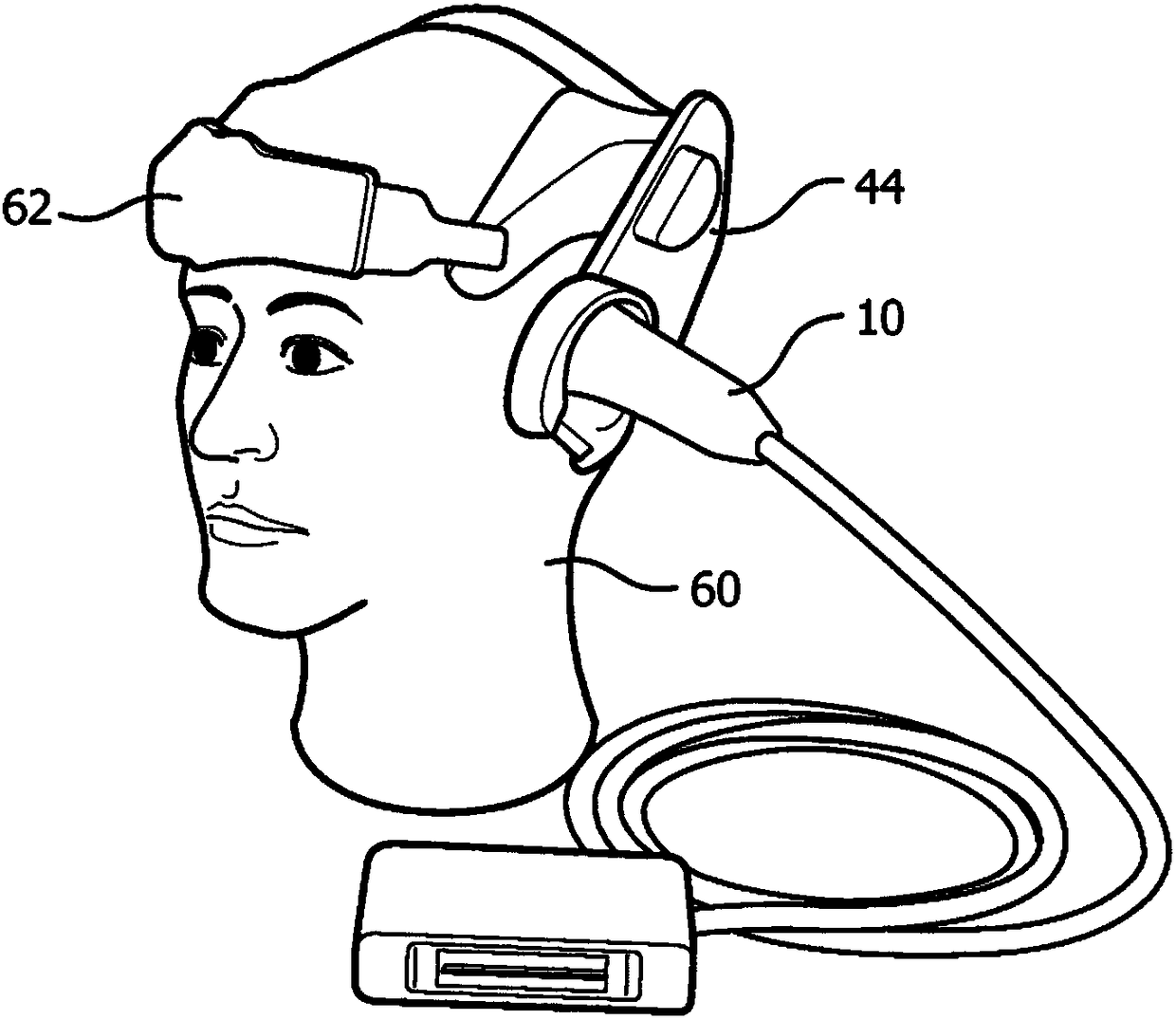
Method and apparatus for treatment of intracranial hemorrhages
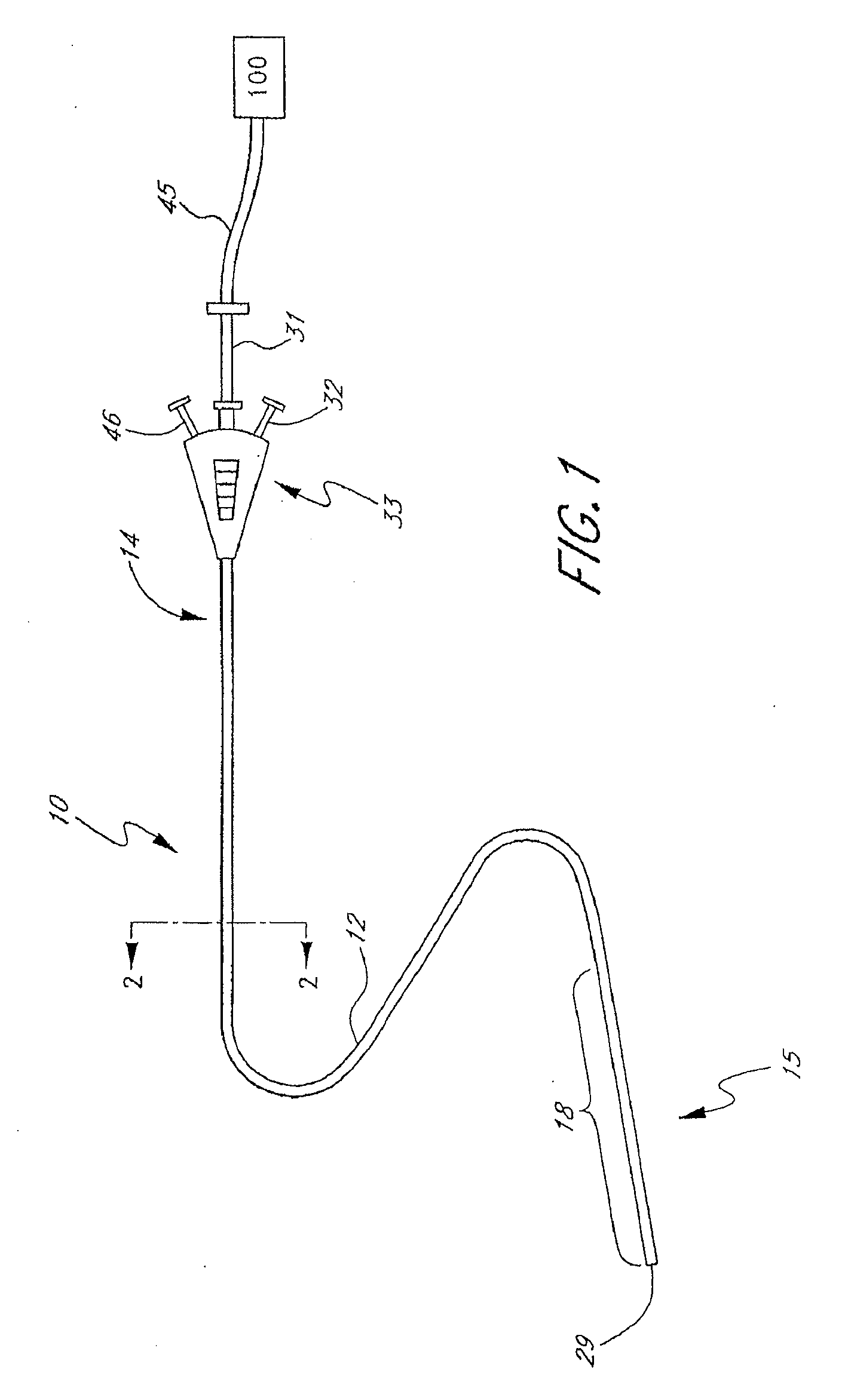
An ultrasound catheter with fluid delivery lumens, fluid evacuation lumens and a light source is used for the treatment of intracerebral hemorrhages. After the catheter is inserted into a blood clot in the brain, a lytic drug can be delivered to the blood clot via the fluid delivery lumens while applying ultrasonic energy to the treatment site. As the blood clot is dissolved, the liquefied blood clot can be removed by evacuation through the fluid evacuation lumens.
Owner:EKOS CORP
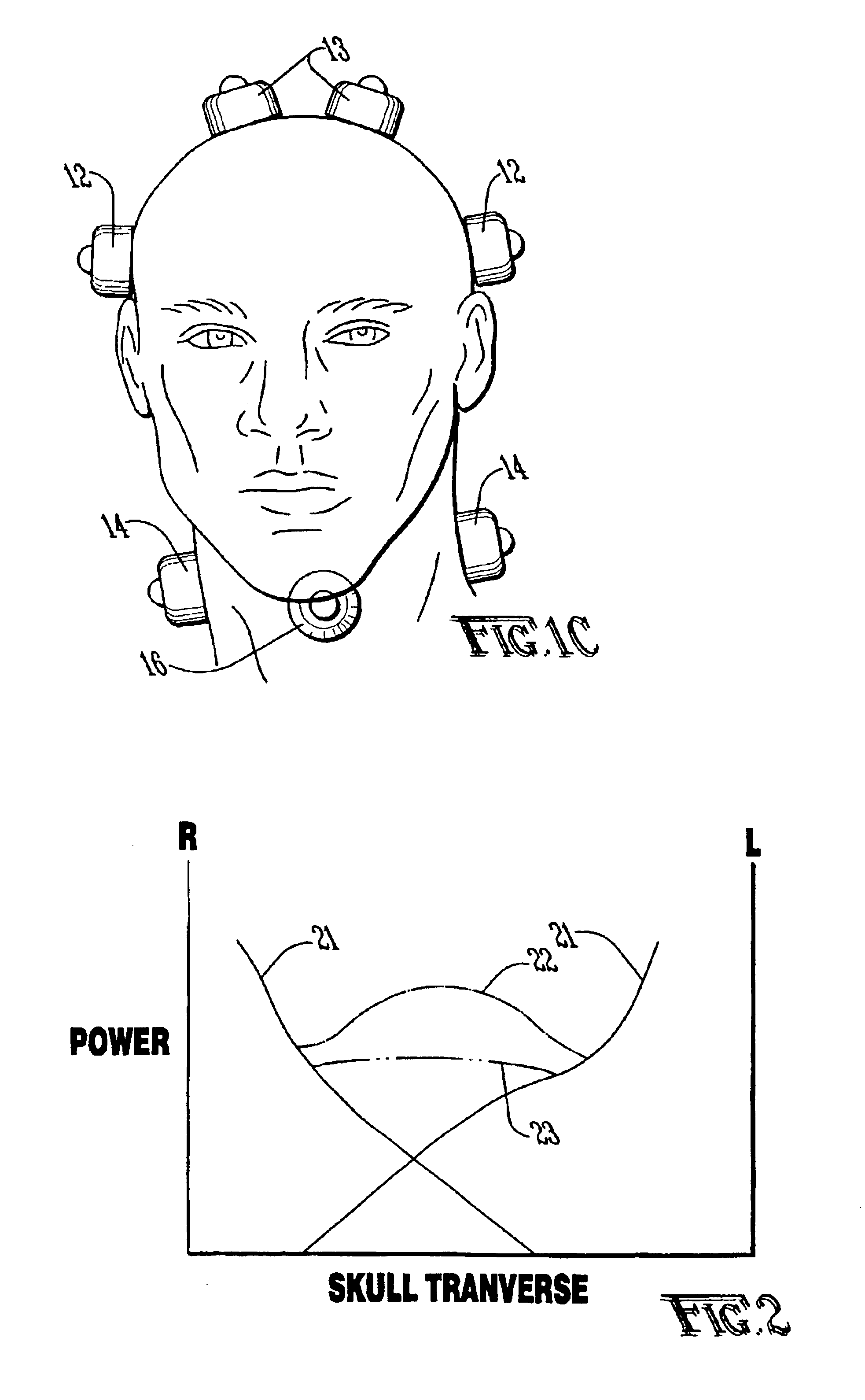
Ultrasound apparatus and method for augmented clot lysis
ActiveUS6945937B2Overcome problemsReduce thermal effectsUltrasonic/sonic/infrasonic diagnosticsUltrasound therapySonificationUltrasonic sensor
An apparatus and method for using ultrasound augmented with microbubbles, thrombolytic drugs or other agents for clot lysis wherein at least one ultrasound transducer generates a plurality of acoustic signals and time, amplitude, phase and frequency modulation of the signals provide more uniform power delivery with fewer gaps in the ultrasound field. Interference patterns from one or multiple transducers are constantly shifted in position. A phased array of transducers may generate a beam that is swept over the area to be treated. In another embodiment, an array of transducers may generate ultrasound at a number of slightly varying frequencies to produce an interference pattern that sweeps in and out through the targeted tissue. A single array may be used to produce both effects simultaneously or separately.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
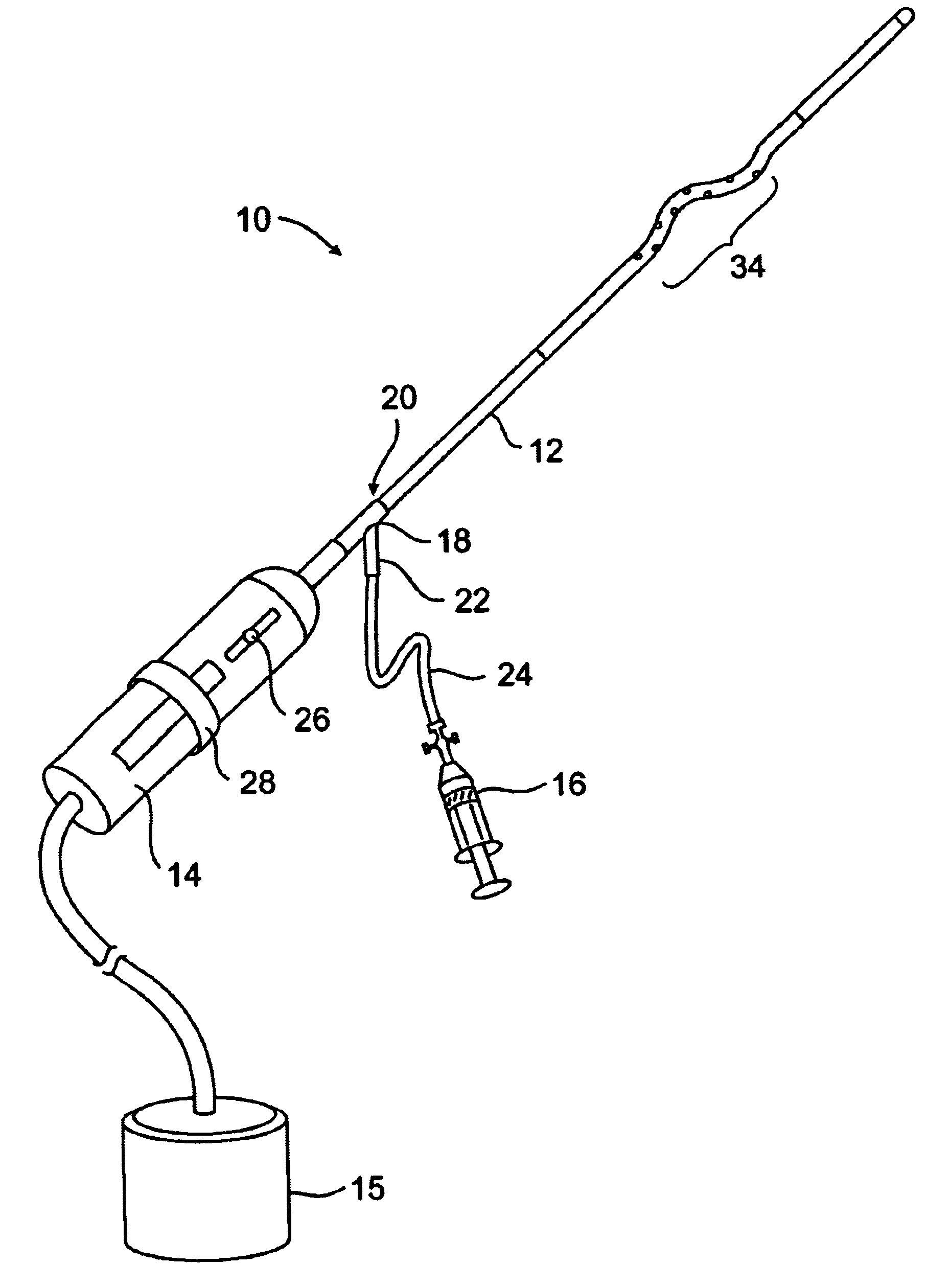
System and methods for clot dissolution
Clot disruption and dissolution are achieved using a catheter having both an agitator and the ability to deliver a thrombolytic agent. The catheter is introduced to a target region with a blood vessel and the agitator manipulated to engage and disrupt a region of clot therein. The thrombolytic agent, such as tPA, streptokinase, or urokinase, is directly released into the clot at the point where the agitator is engaging the clot. In this way, the thrombolytic activity of the agent is enhanced and the dissolution of the clot is improved.
Owner:TYCO HEALTHCARE GRP LP
Ultrasound apparatus and method for augmented clot lysis
ActiveUS20050085748A1Limited rangeOvercome problemsUltrasonic/sonic/infrasonic diagnosticsUltrasound therapySonificationUltrasonic sensor
An apparatus and method for using ultrasound augmented with microbubbles, thrombolytic drugs or other agents for clot lysis wherein at least one ultrasound transducer generates a plurality of acoustic signals and time, amplitude, phase and frequency modulation of the signals provide more uniform power delivery with fewer gaps in the ultrasound field. Interference patterns from one or multiple transducers are constantly shifted in position. A phased array of transducers may generate a beam that is swept over the area to be treated. In another embodiment, an array of transducers may generate ultrasound at a number of slightly varying frequencies to produce an interference pattern that sweeps in and out through the targeted tissue. A single array may be used to produce both effects simultaneously or separately.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
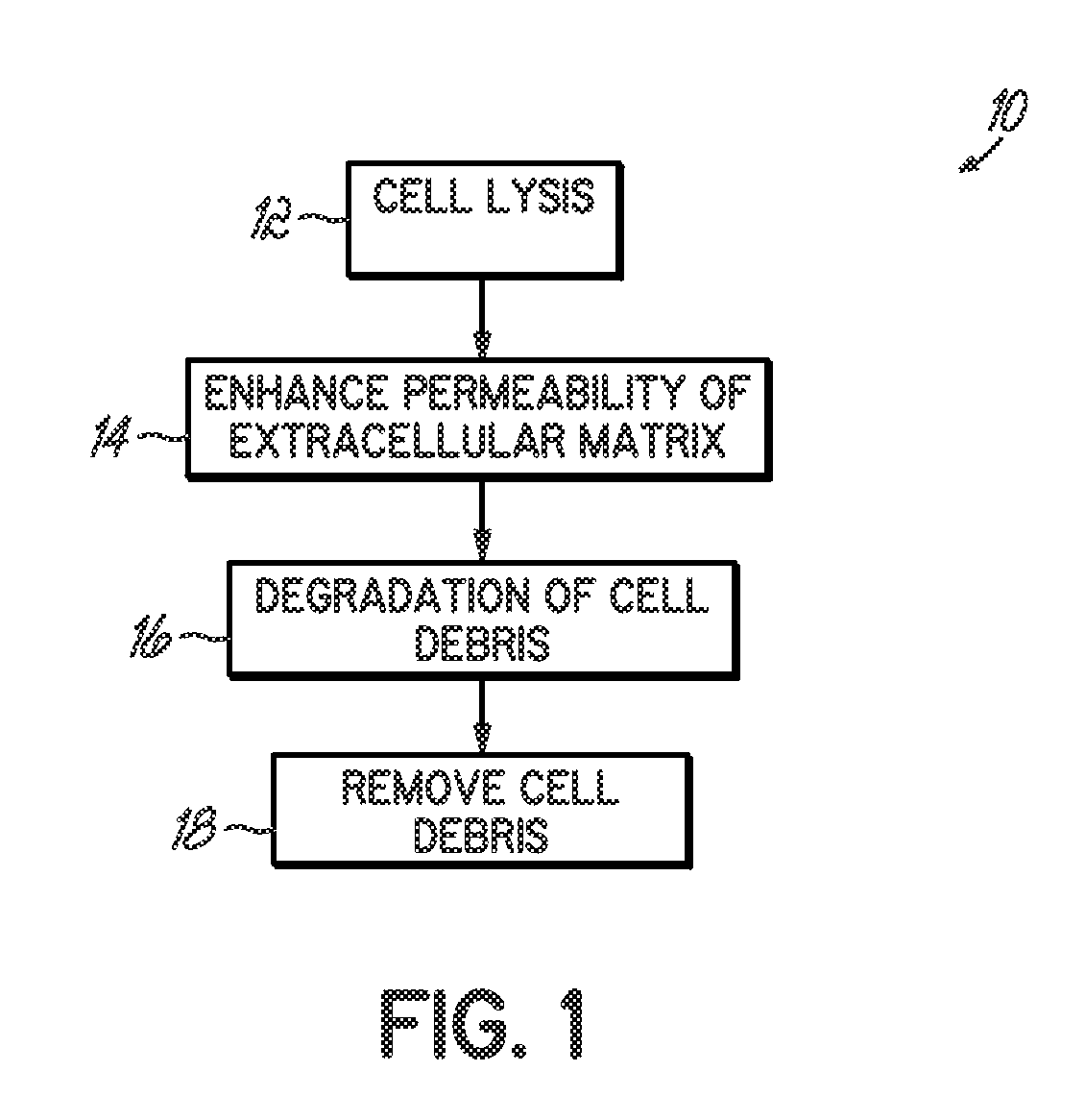
Tissue processing for nonimmunogenic implants
InactiveUS20080306610A1Improve breathabilityUnknown materialsTissue cultureLysisCell-Extracellular Matrix
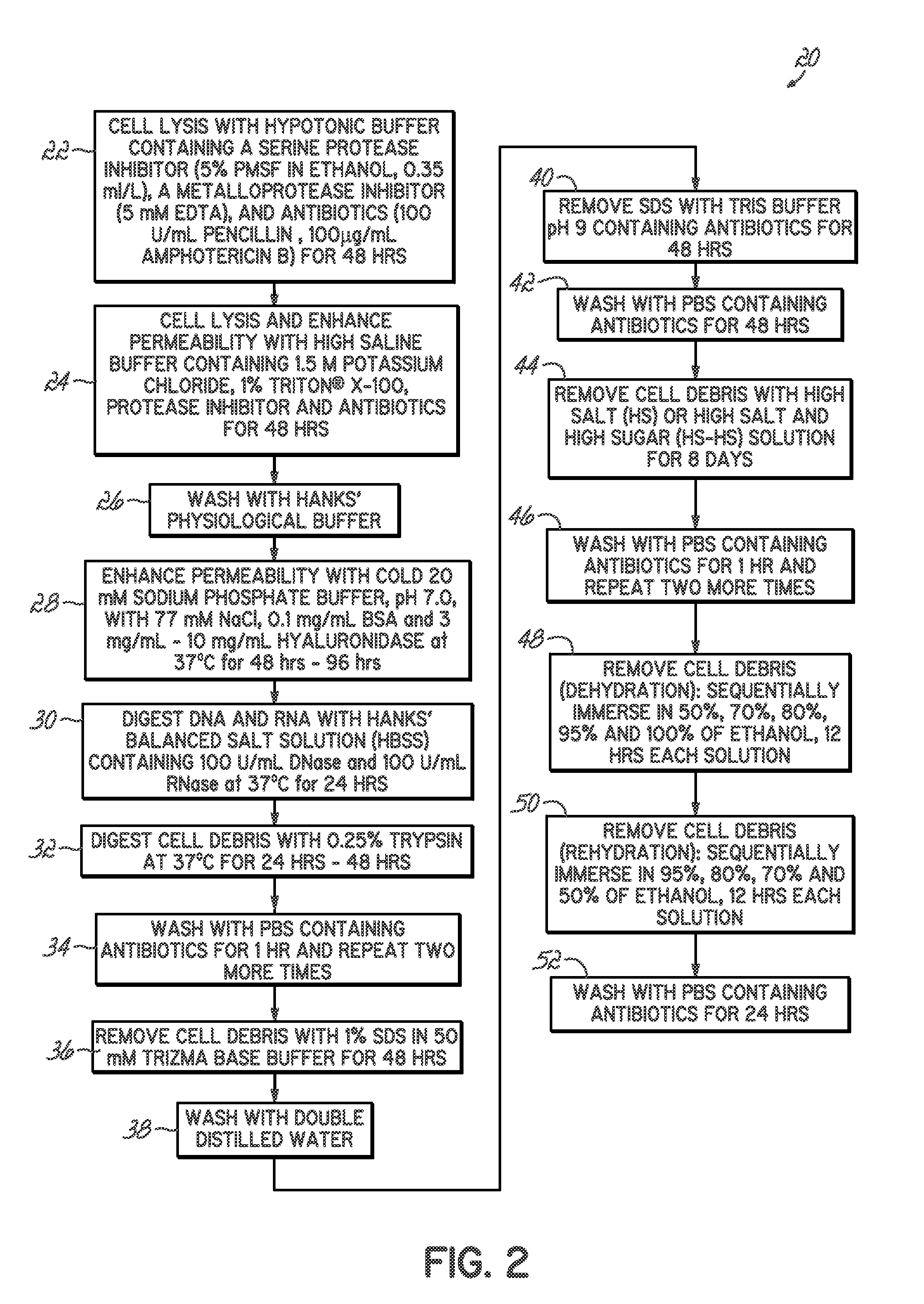
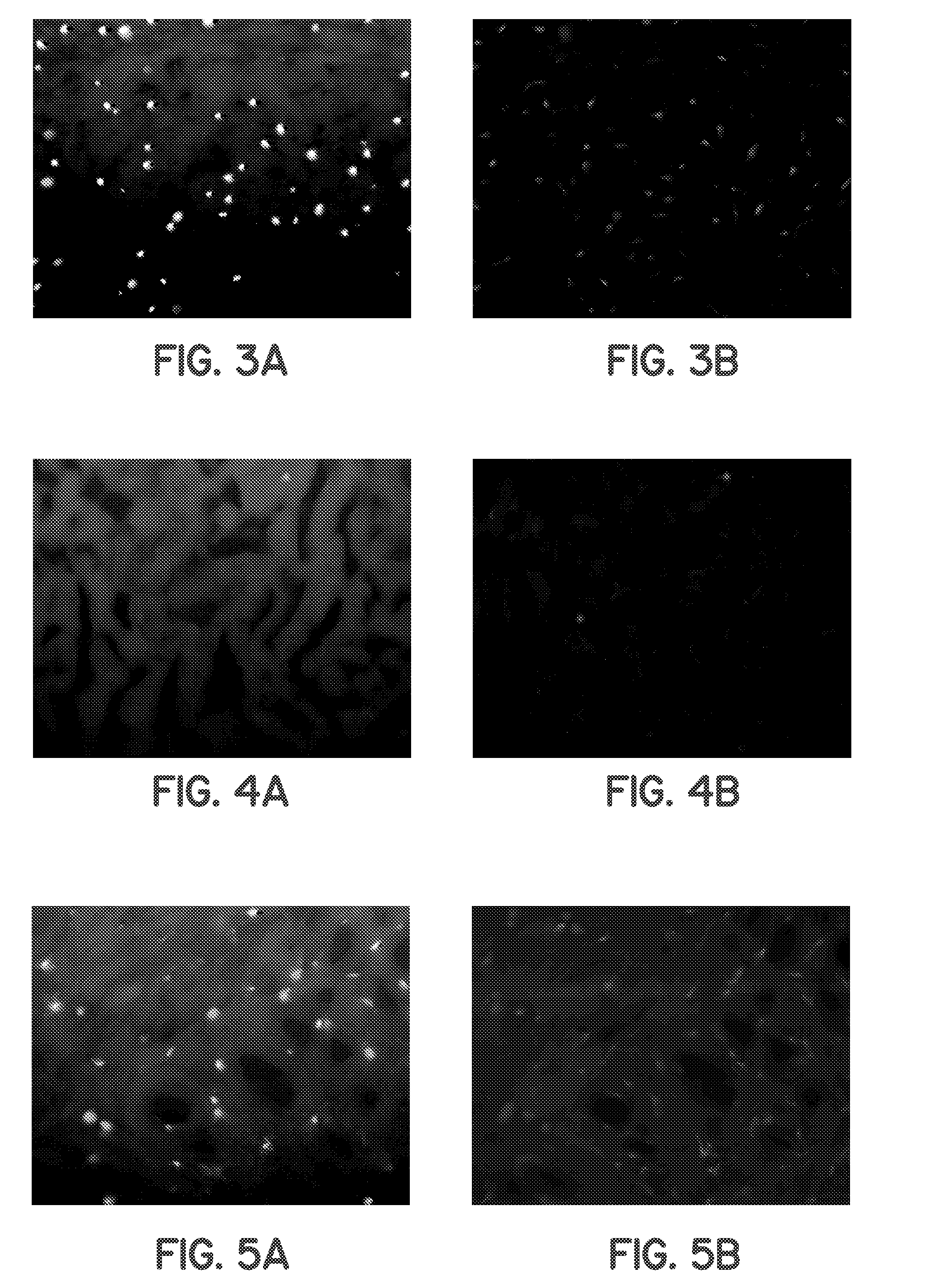
Methods for processing tissues to render them suitable for implantation, e.g. in an orthopedic site. Tissues are rendered substantially acellular and substantially nonimmunogenic by exposure to processes that result in cell lysis, increasing permeability of the extracellular matrix, degrading the debris from lysis, and removing the debris. Methods of forming tissue implants, kits for processing tissue implants, and methods of using tissue implants are also disclosed.
Owner:ZIMMER ORTHOBIOLOGICS
Microfluidic device comprising electrolysis device for cell lysis and method for electrochemically lysing cells using the same
Owner:SAMSUNG ELECTRONICS CO LTD
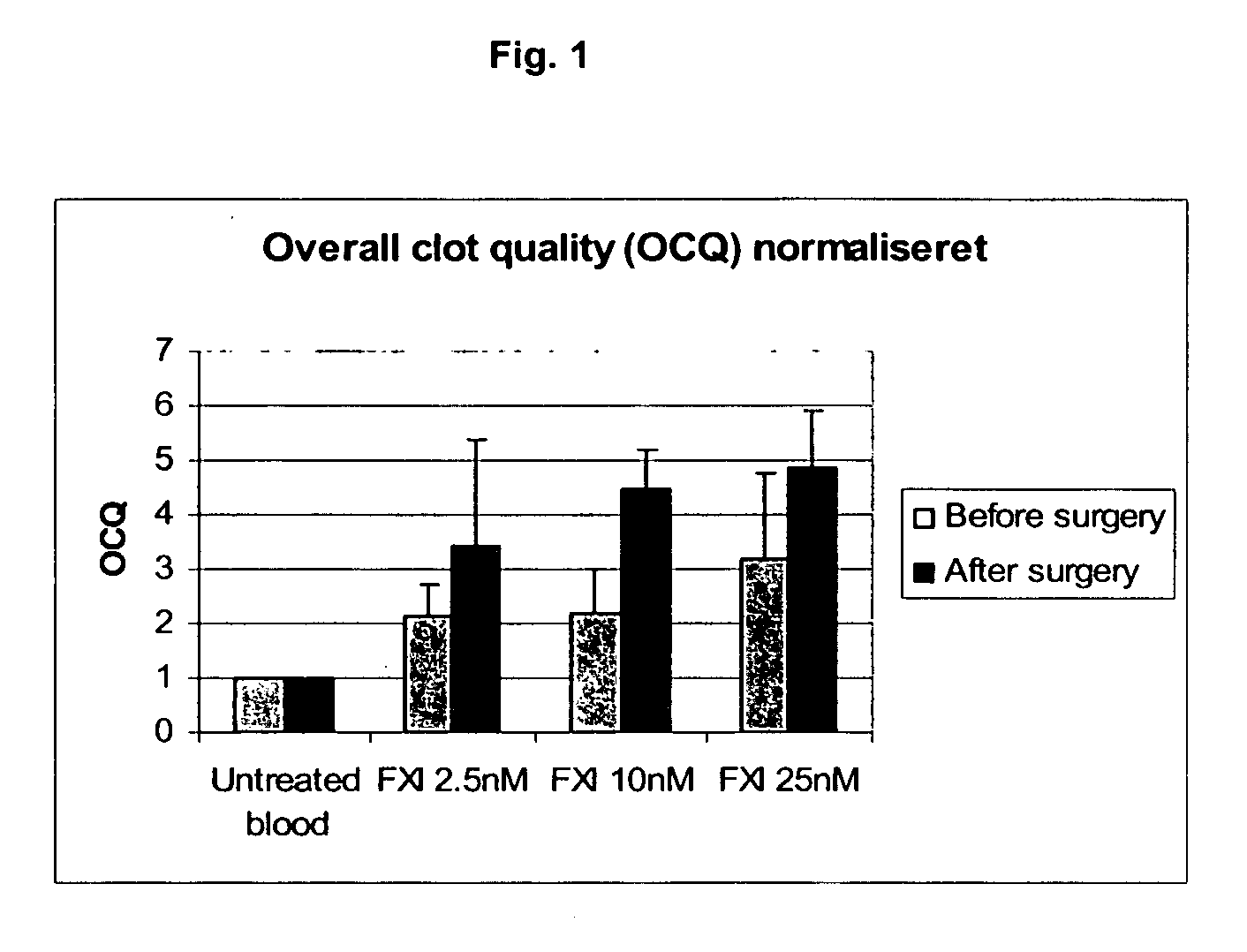
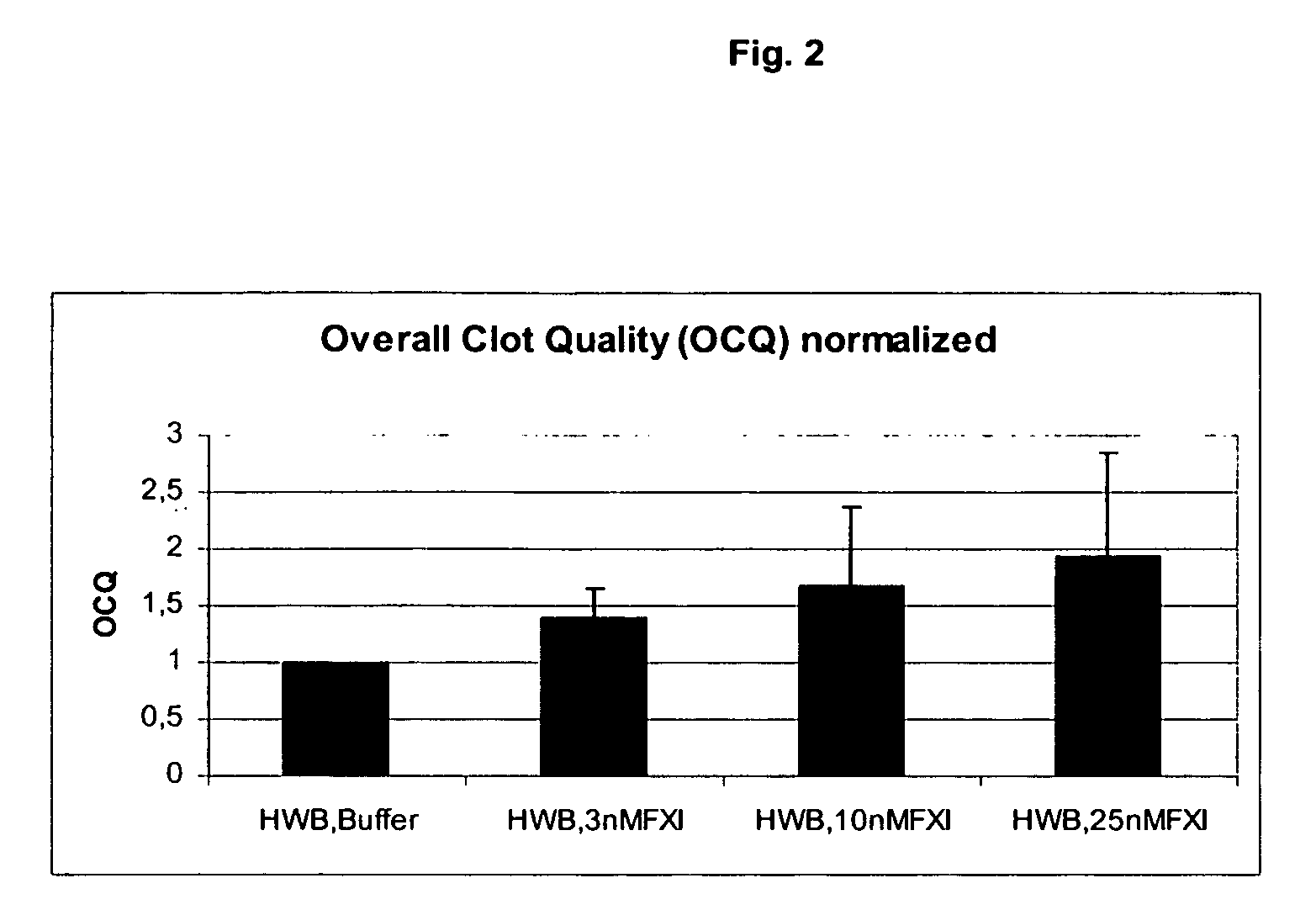
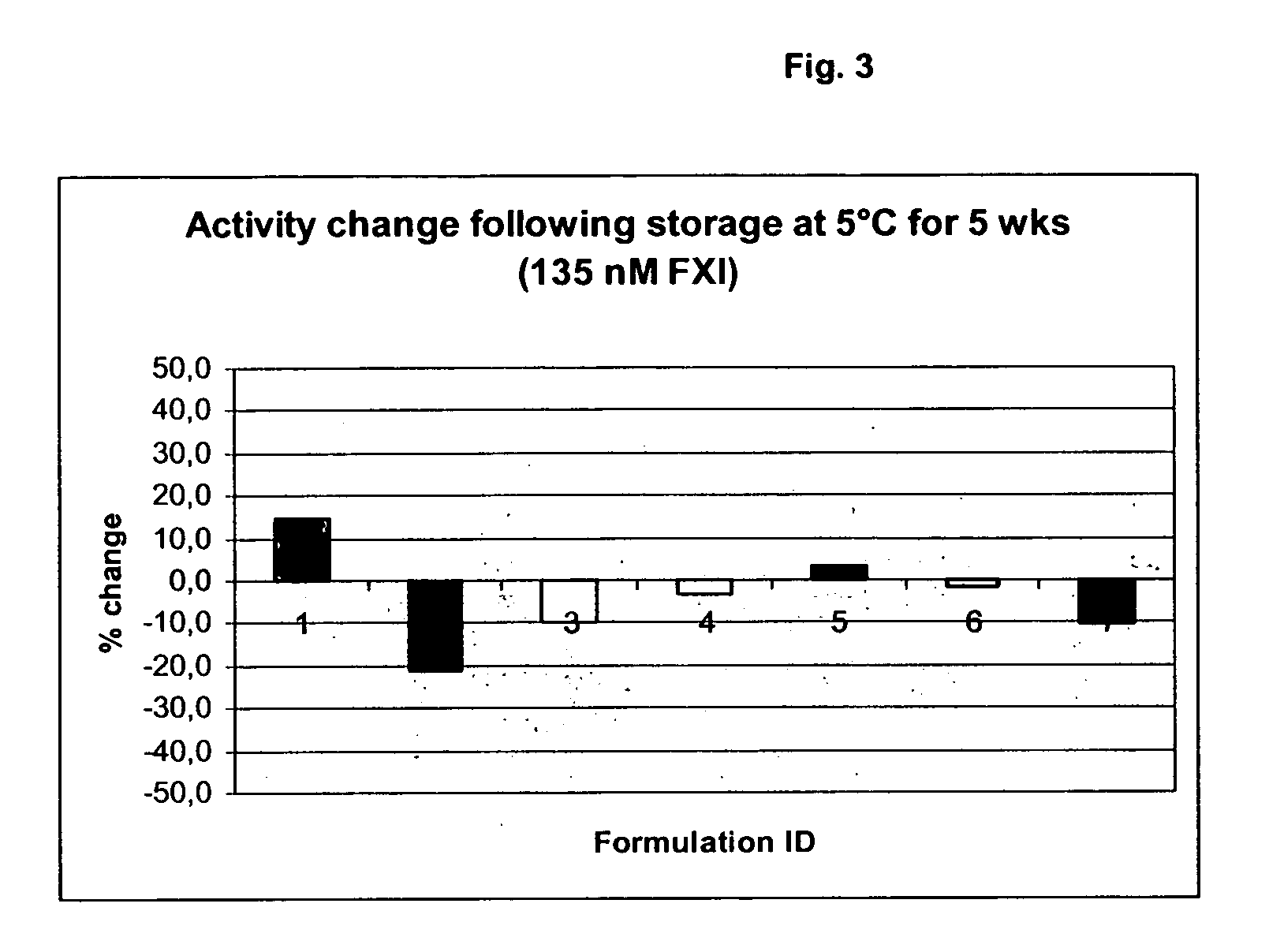
Therapeutic use of factor XI
InactiveUS20050181978A1Shorten clotting timeGood hemostasisPeptide/protein ingredientsBlood disorderMedicineFactor XI
The present invention provides methods and compositions for treating bleeding episodes. The methods are carried out by administering to a patient in need thereof a preparation comprising a factor XI polypeptide, in an amount effective for such treatment. The methods of the invention result in one or more of: reduced clotting time; enhancement of hemostasis; increase in clot lysis time; increase in clot strength; and / or increase in overall clot quality (OCQ) in said patient.
Owner:ROJKJAER RASMUS +4
Lysis Indication
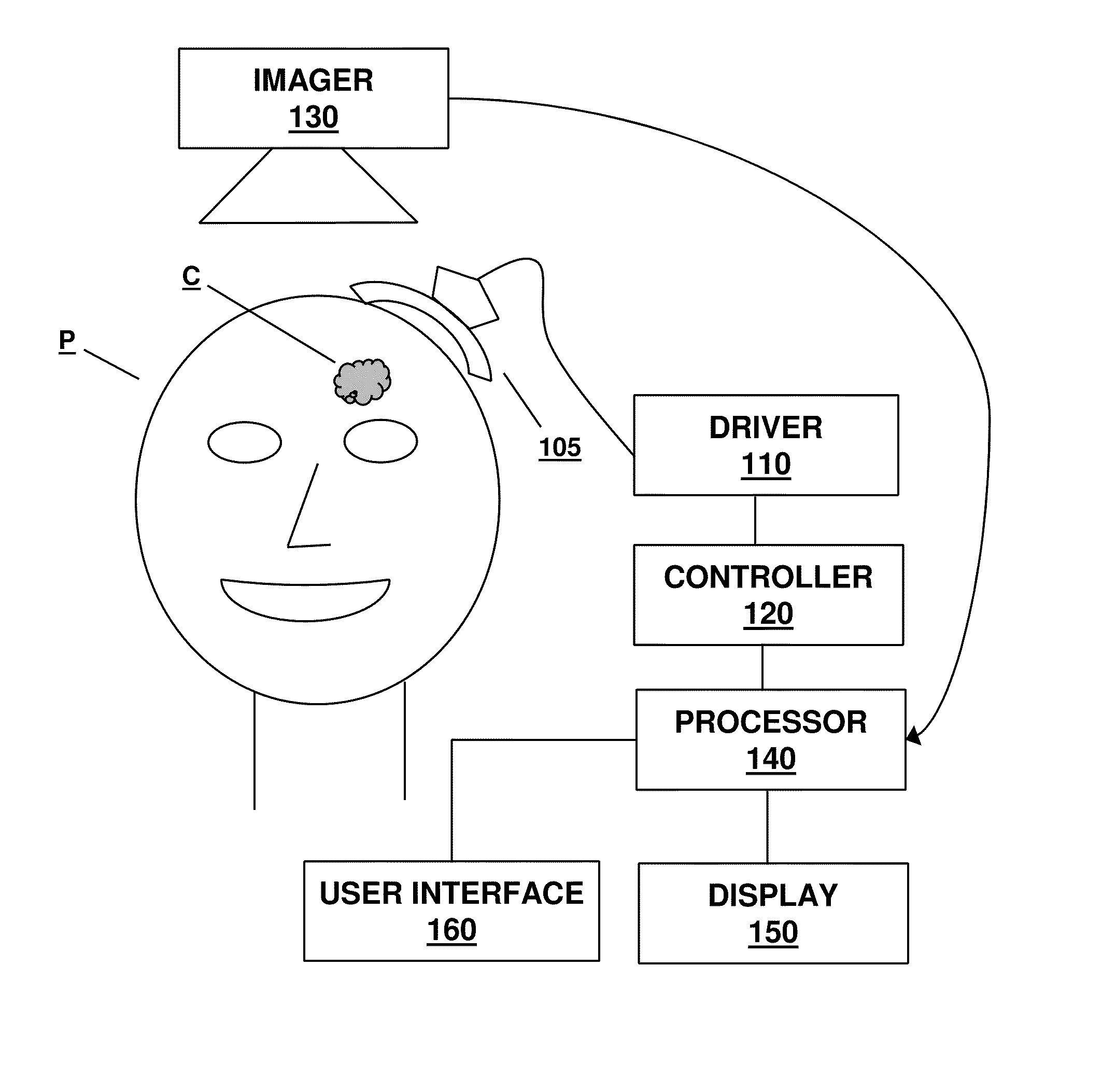
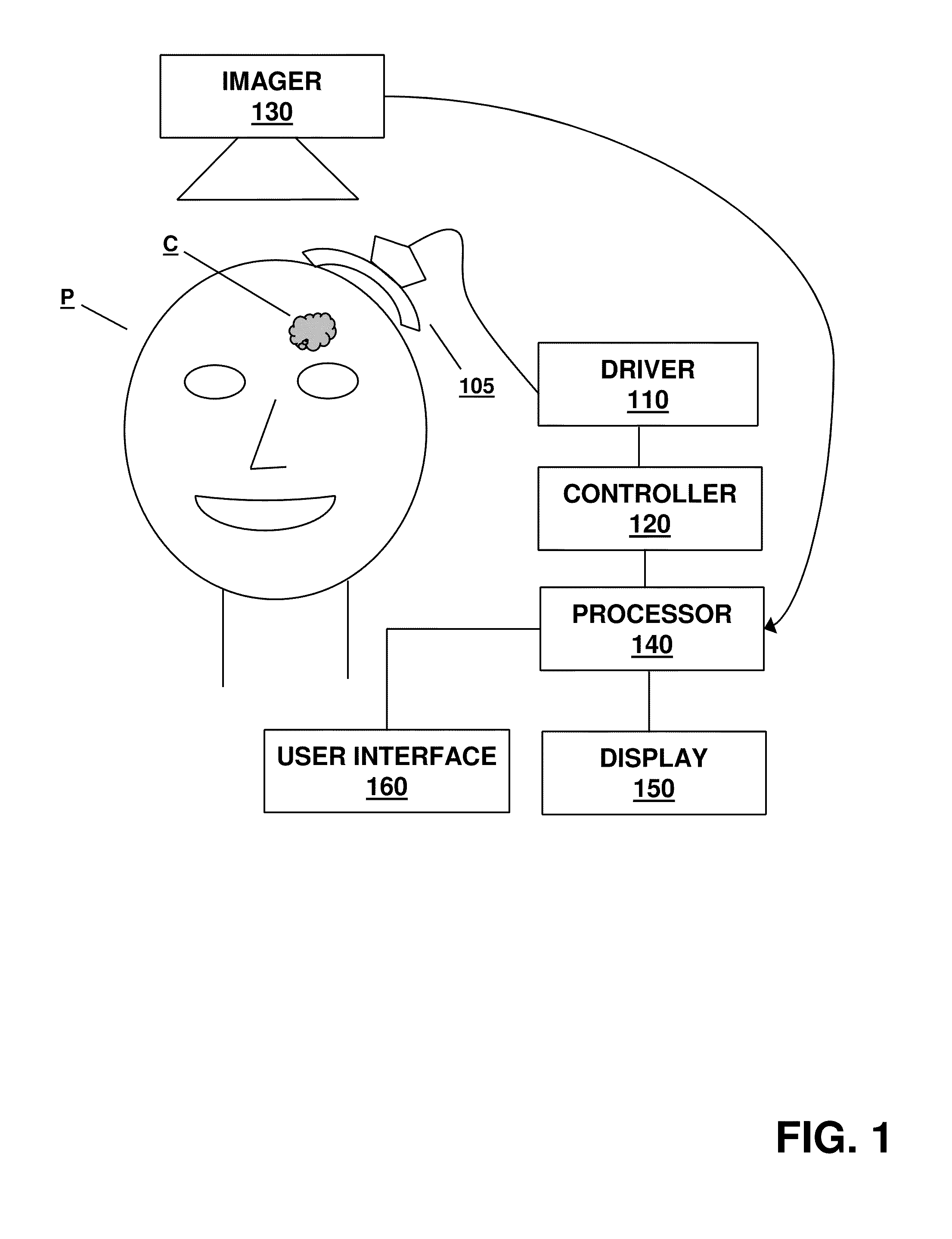
A method for monitoring clot dissolution in a patient's vasculature is disclosed. After a catheter is positioned at a treatment site in the patient's vasculature, a clot dissolution treatment procedure can be performed at the treatment site. The thermal parameter is measured at the treatment site. The measured thermal parameter and / or the changes in the measured thermal parameter is then displayed.
Owner:EKOS CORP
Lysis Indication
A method for monitoring clot dissolution in a patient's vasculature is disclosed. After a catheter is positioned at a treatment site in the patient's vasculature, a clot dissolution treatment procedure can be performed at the treatment site. The thermal parameter is measured at the treatment site. The clot dissolution treatment is then modified in response to the measured thermal parameter.
Owner:EKOS CORP
Closed-loop clot lysis
ActiveUS8425424B2Increasing and decreasing overall pressure and energyFocusUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyMedicineClosed loop
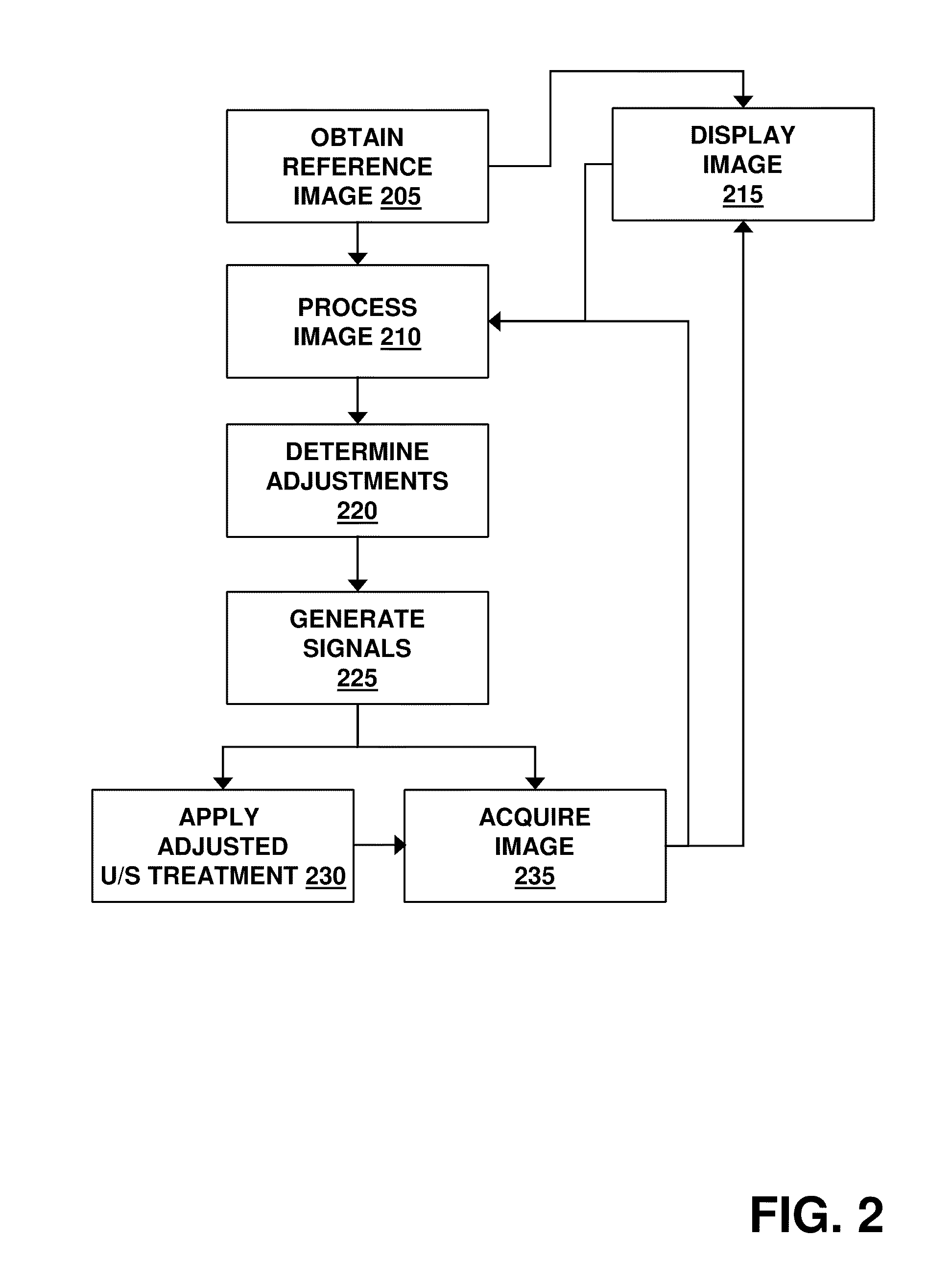
The present invention provides procedures and systems that use a closed-loop approach for directing ultrasound energy at a clot while monitoring blood flow and / or liquification of the clot tissue so as to allow automated and / or manual adjustments to various treatment parameters.
Owner:INSIGHTEC
Stent with auxiliary treatment structure
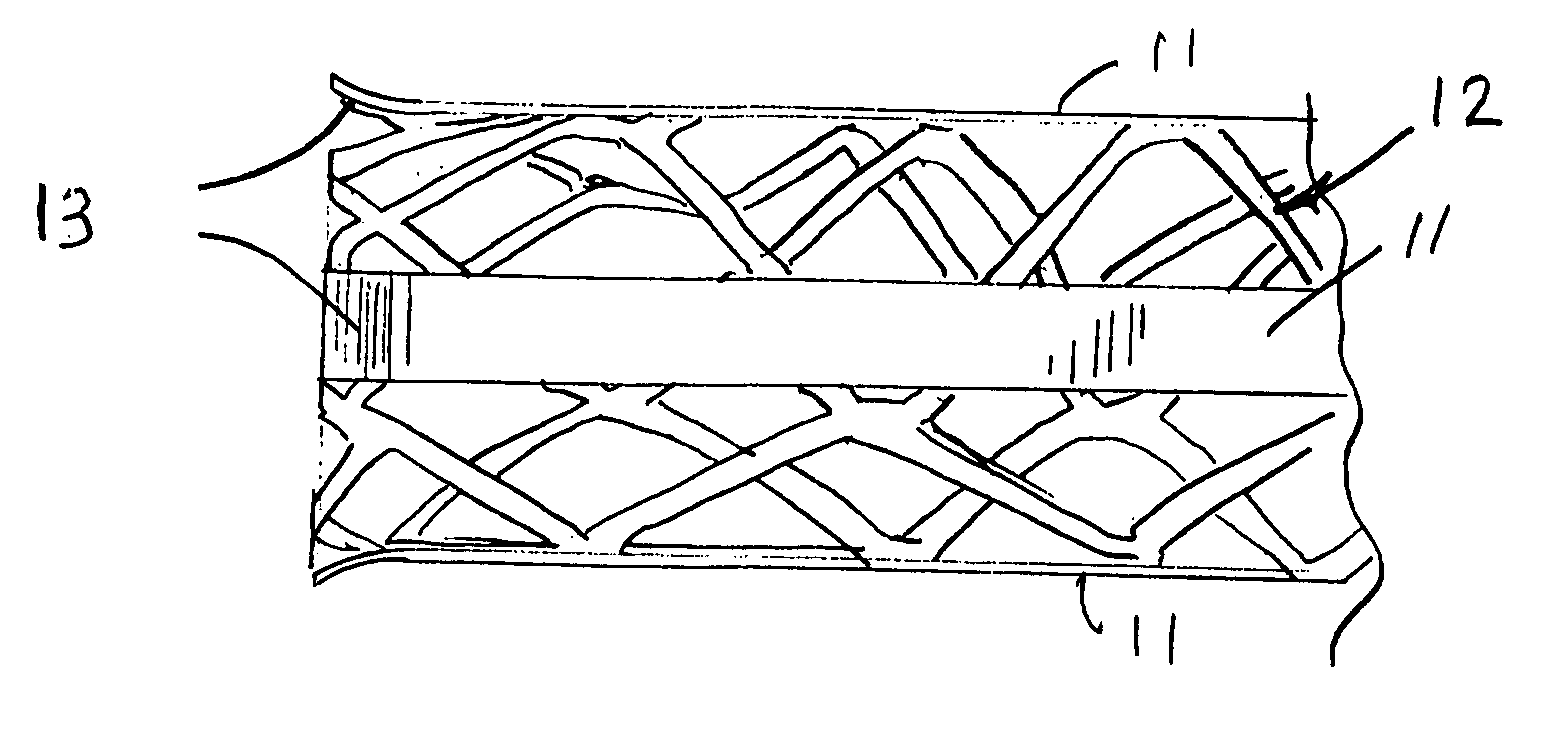
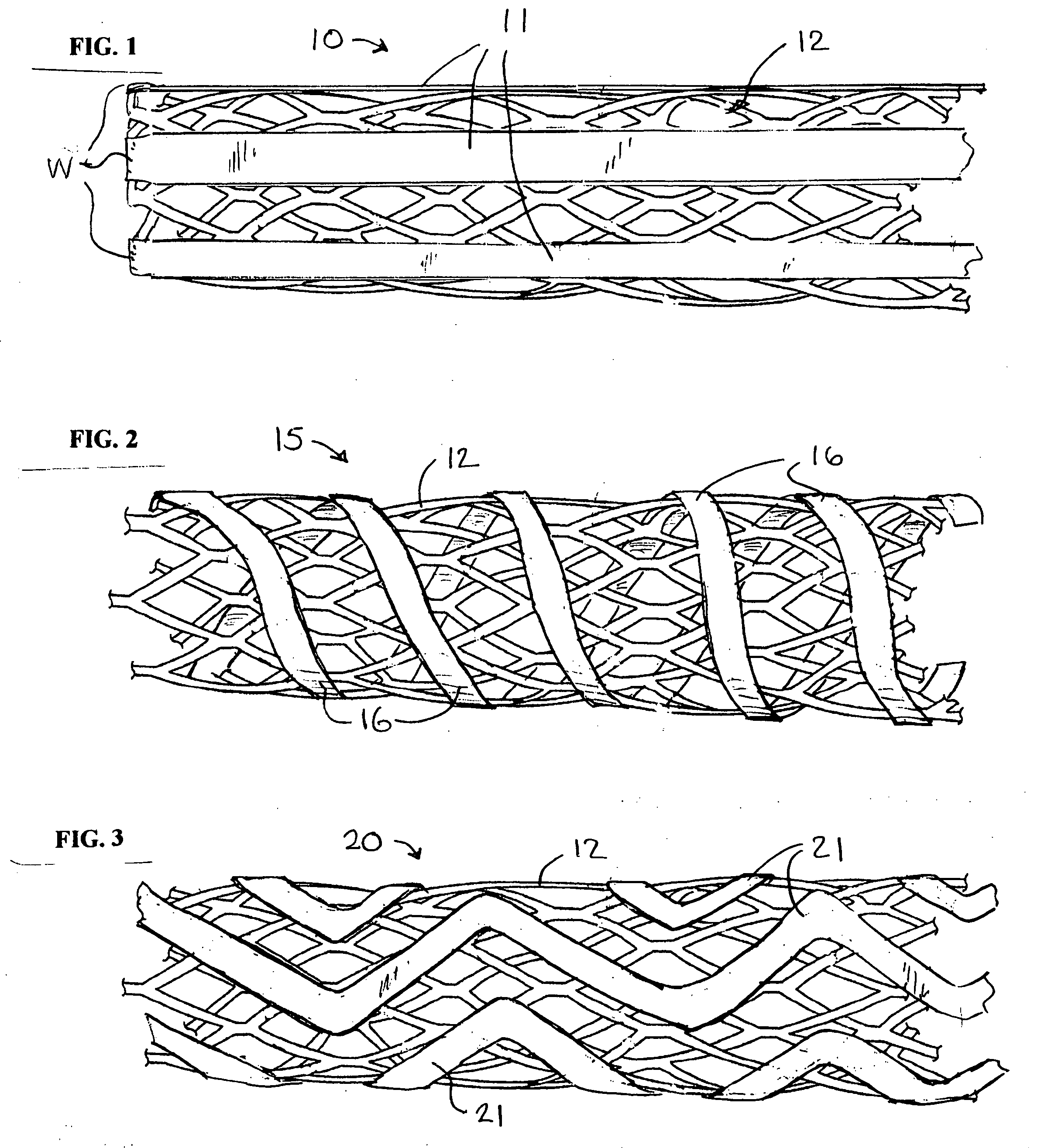
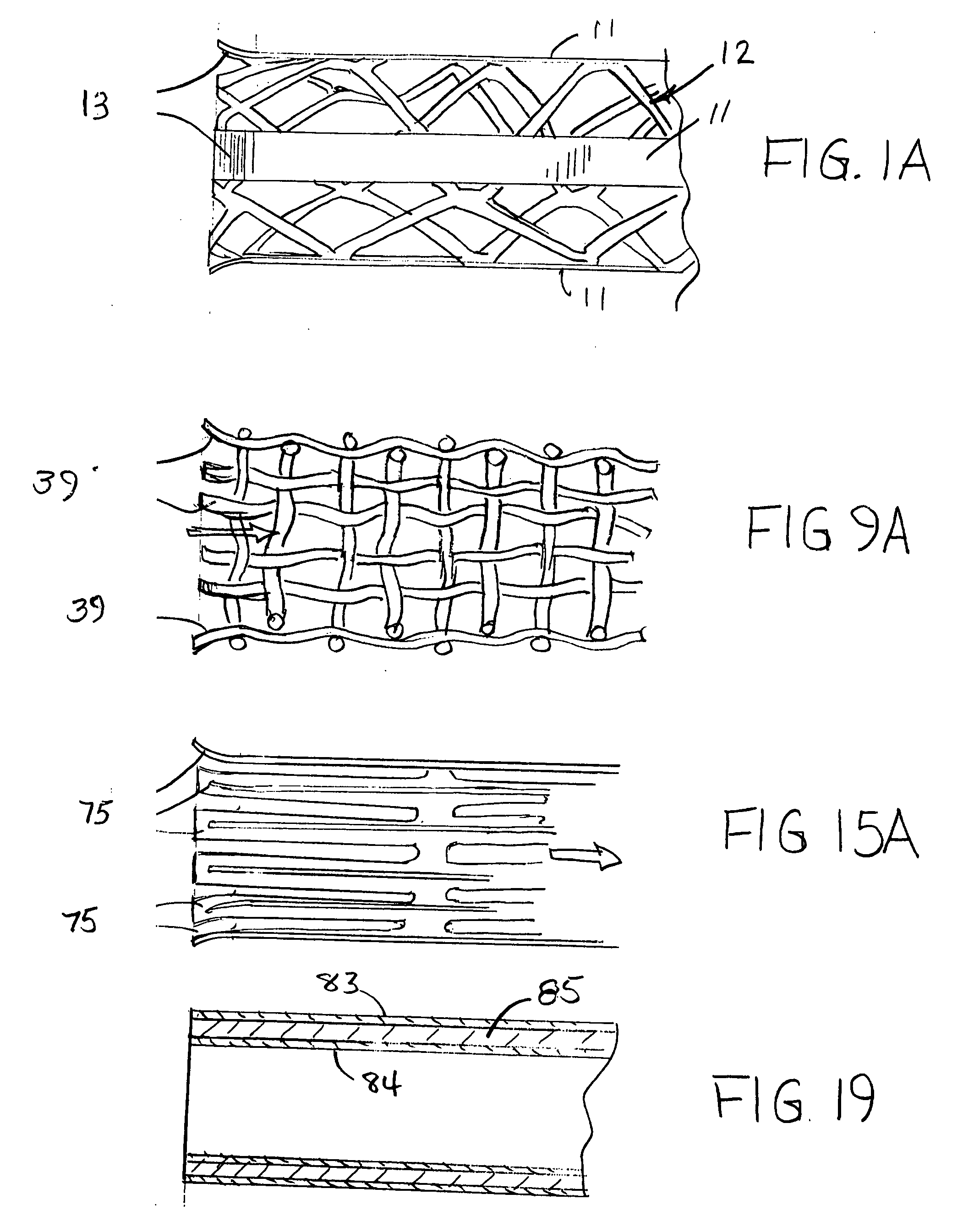
InactiveUS20090264982A1Reduce fluid turbulenceAvoid problemsStentsBlood vesselsCholesterolReticular formation
A medical device for treatment of a stenosed body lumen, includes an open-ended cylindrical body carried on a distal end of a catheter for insertion of the device into the body lumen and placement at the stenosed site. The cylindrical body is movable between a collapsed position for insertion into the body lumen, and a radially expanded position pressed against the wall of the body lumen. In one embodiment the body sidewall is formed by a plurality of interconnected struts or elements defining openings therebetween, and at least one elongate ribbon is attached to an outer surface thereof for carrying a therapeutic agent. In another embodiment, the body is formed of interwoven elements defining a mesh-like structure, and the elements may comprise dissimilar materials, such as, e.g., copper and silver. In a further embodiment the body is formed of layers of different materials such as, e.g., copper, silver, and / or steel, laminated together. In a still further embodiment the device is designed for temporary placement of the catheter and body in a body lumen for treatment of a stenosed site, after which the catheter and body are withdrawn. In all forms the body may have an outwardly flared inlet end to reduce turbulence of fluid flowing through it, and / or a gel-like coating of a cholesterol-dissolving or blood clot dissolving agent may be placed on the device.
Owner:LIM WALTER K
Device for carrying out cell lysis and nucleic acid extraction
Owner:NORCHIP AS
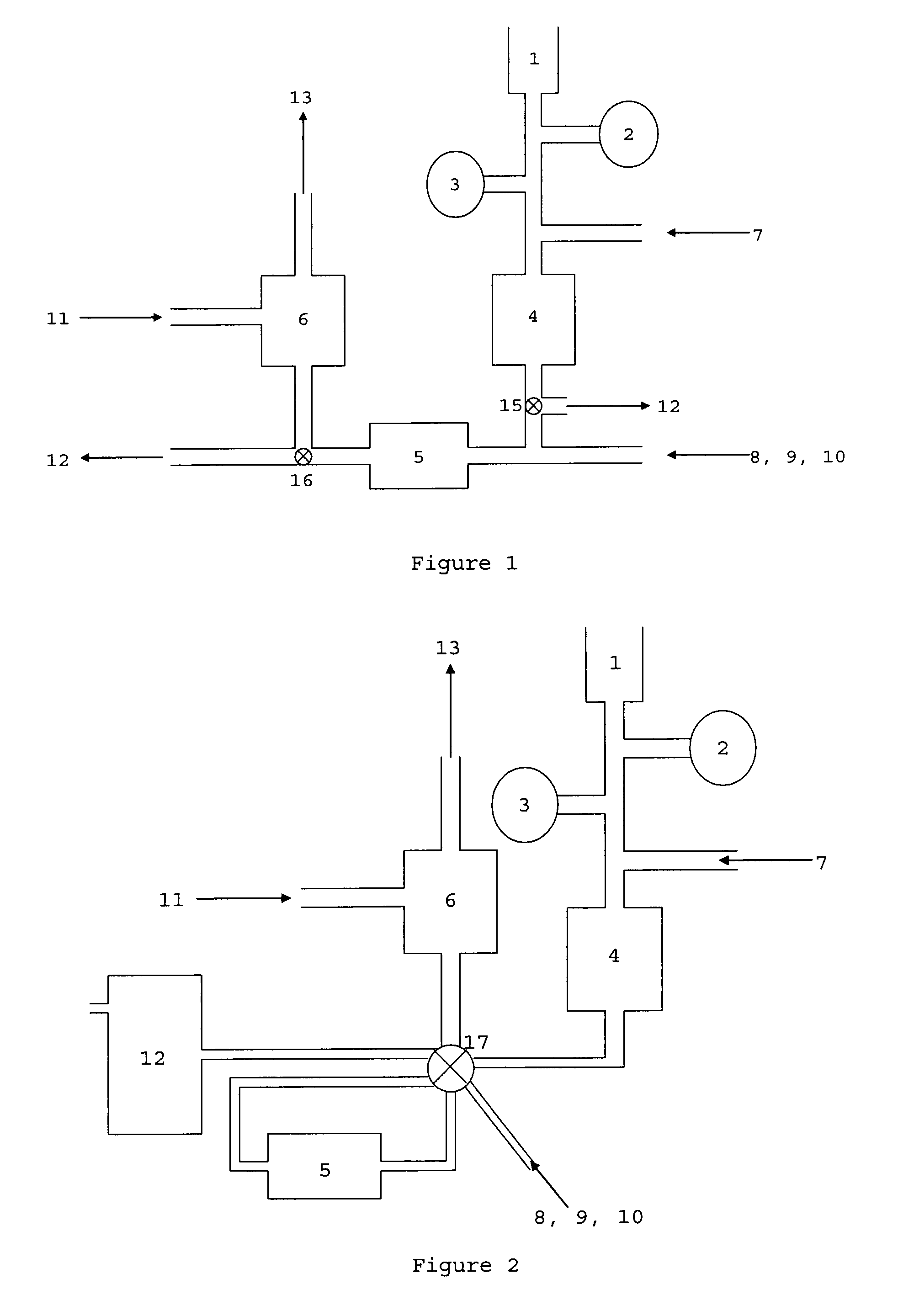
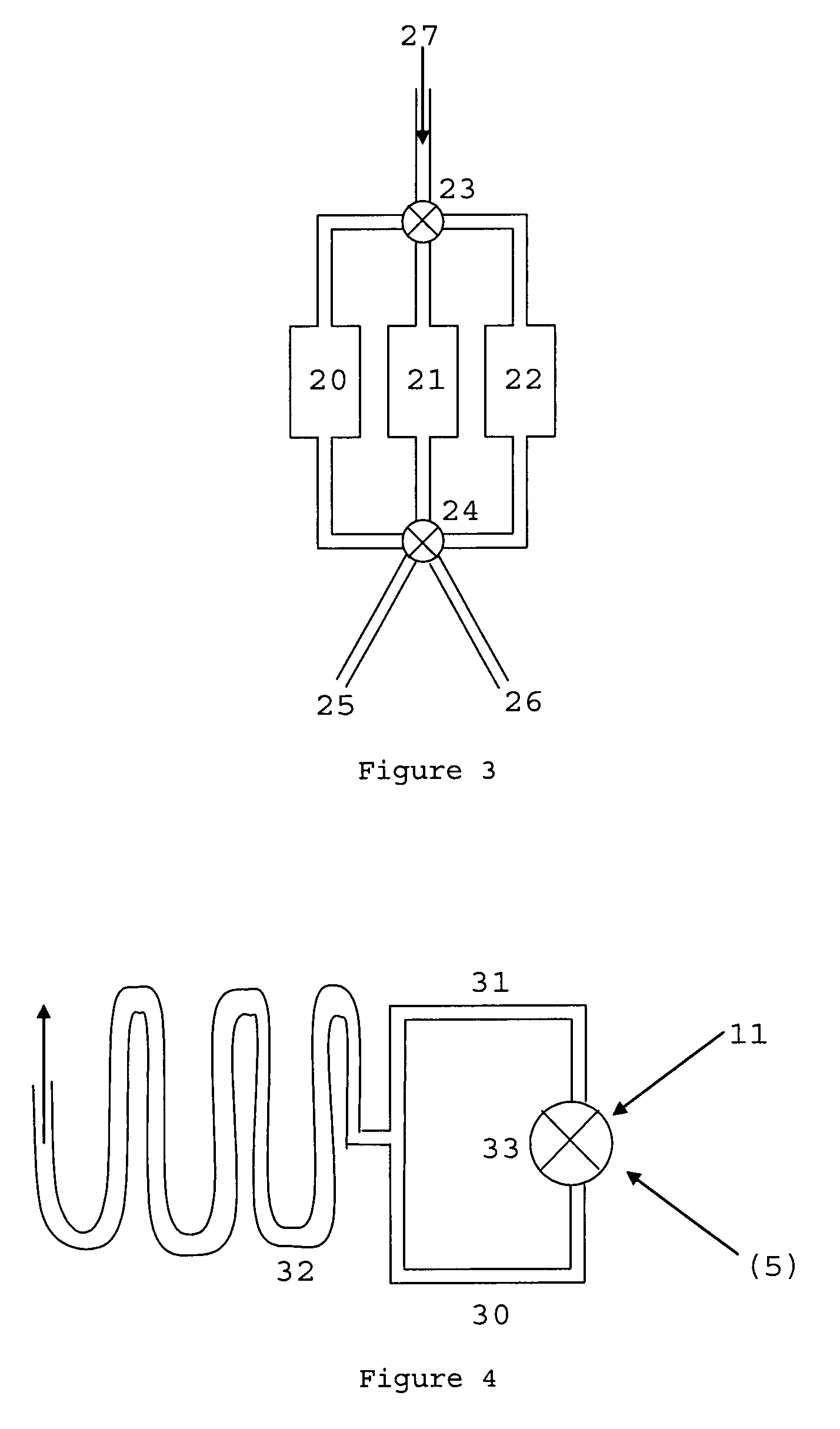
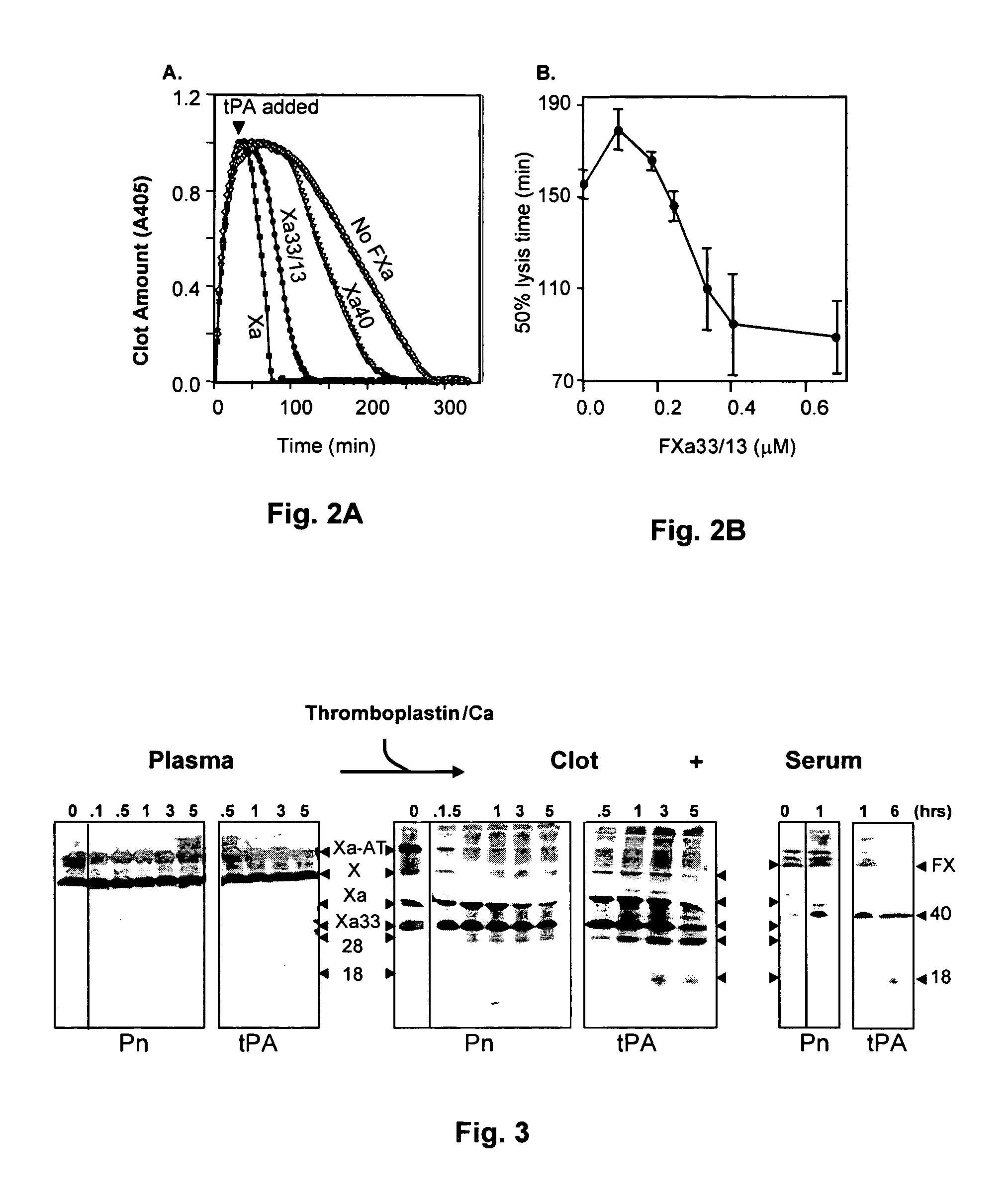
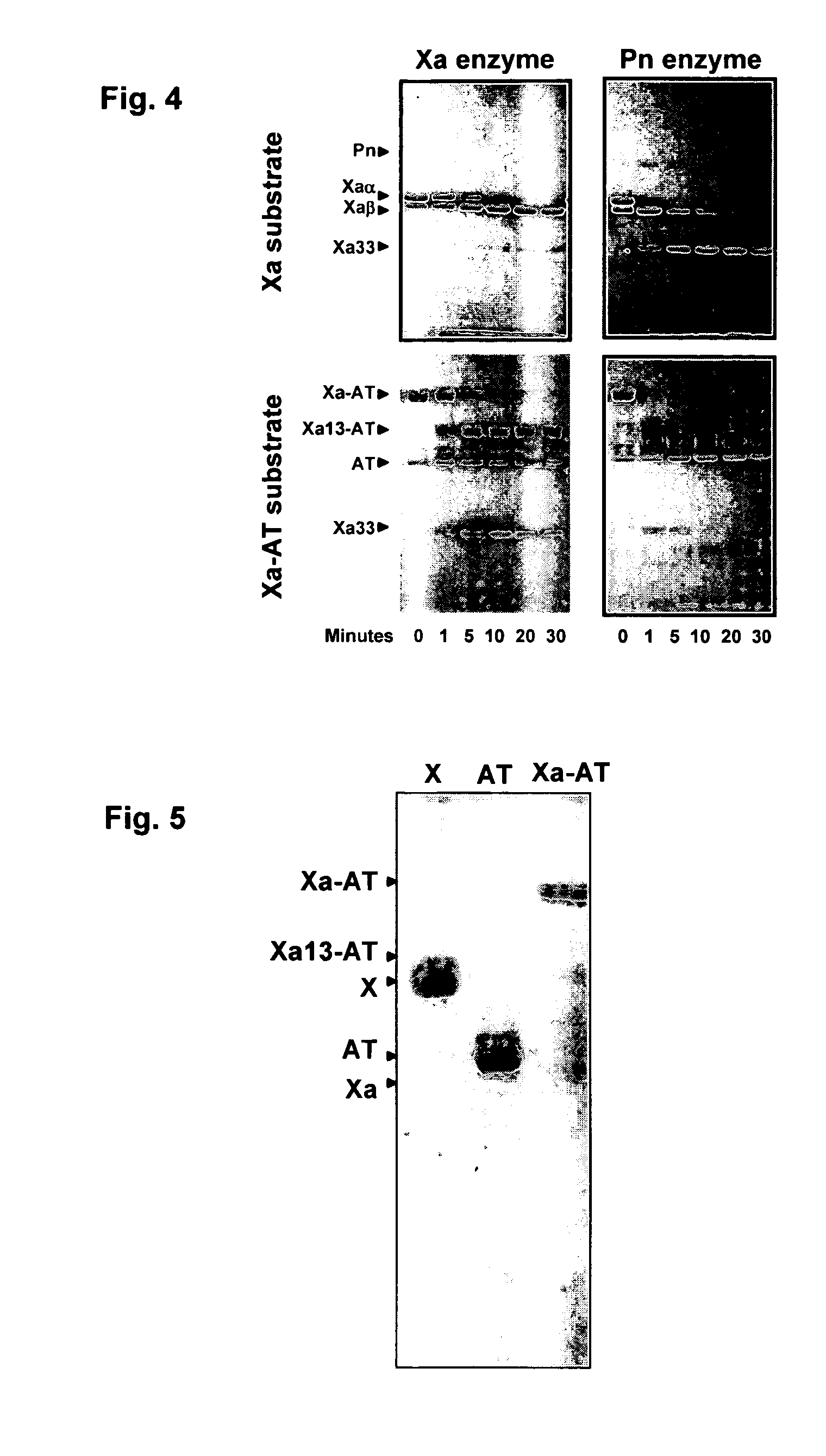
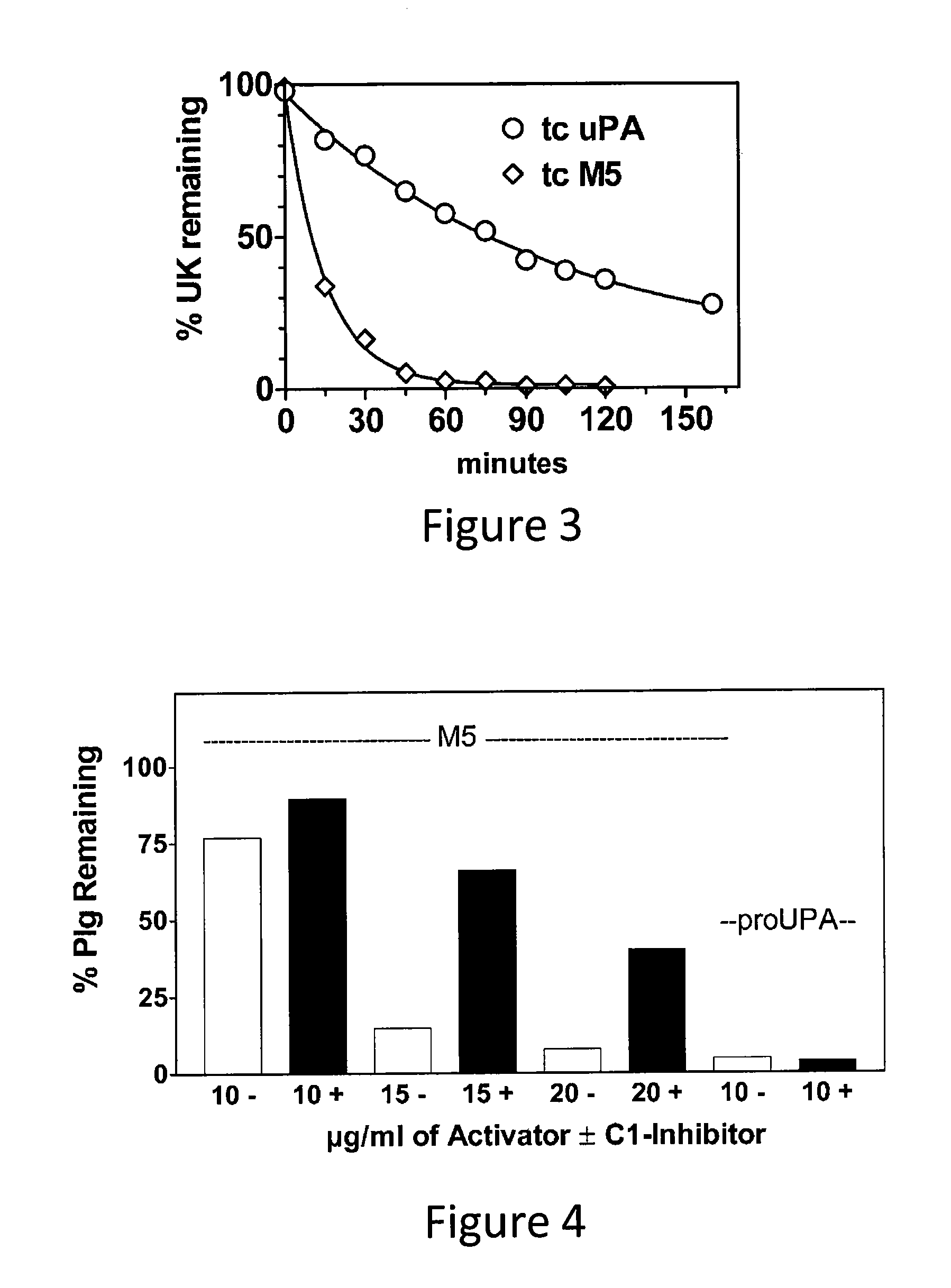
C-1 inhibitor prevents non-specific plasminogen activation by a prourokinase mutant without impeding fibrin-specific fibrinolysis
ActiveUS7837992B2Attenuation of rateHigh dose tolerancePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
Owner:THROMBOLYTIC SCI +1
Multicomponent conjugates which bind to target molecules and stimulate cell lysis
InactiveUS20040068100A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsLysisT cell
The invention relates to immunoconjugates of formula: A-B-(C)n where B may be present or absent, A is a specific binding protein such as an antibody or an antibody binding fragment, or a ligand binding to a receptor present on target cells, B comprises at least one molecule to which "A" and "C" bind, such as an avidin / strepavidin complex, "C" is an MHC molecule, and "n" is a whole number ranging from 1 to 10. The conjugates provide the exquisite binding specificity of antibodies, combined with an ability to stimulate cytotoxic T cells to identify and to destroy cells on which the conjugate is bound and oligomerized. The conjugates are useful both therapeutically and diagnostically.
Owner:LUDWIG INST FOR CANCER RES +1
Methods for the treatment of thrombosis
A fibrinolytically active metalloproteinase polypeptide (called “novel acting thrombolytic”) which is useful for blood clot lysis in vivo and methods and materials for its production by recombinant expression are described.
Owner:AMGEN INC
Coagulation proteins, coagulation-anticoagulation protein complexes, derivatives thereof and their uses
InactiveUS20070025979A1Enhance dissolving said blood clotHigh dissolution rateOrganic active ingredientsFibrinogenSide effectFactor X
The present invention relates to the use of coagulation proteins and complexes thereof with anticoagulation proteins for the lysis of blood clots or other applications affected by accelerated plasmin production. More specifically, the present invention provides a method for accelerating the dissolution of a blood clot through the administration of at least one coagulation protein, with or without being in complex with a serpin, comprising a basic C-terminal amino acid, wherein the coagulation protein may be a derivative of Factor X or Factor V or a combination thereof. Pharmaceutical compositions for the treatment and prophylaxis of blood clots are also provided, wherein, the methods and products of the present invention advantageously accelerate clot dissolution while potentially minimizing the adverse side-effects, such as hemorrhaging, seen with other clot dissolving agents. The present invention also provides a method for detecting a fibrinolytic potential in a subject.
Owner:CANADIAN BLOOD SERVICES
C1-Inhibitor Prevents Non-Specific Plasminogen Activation by a Prourokinase Mutant without Impeding Fibrin-Specific Fibrinolysis
ActiveUS20110081334A1Attenuation of rateHigh dose tolerancePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
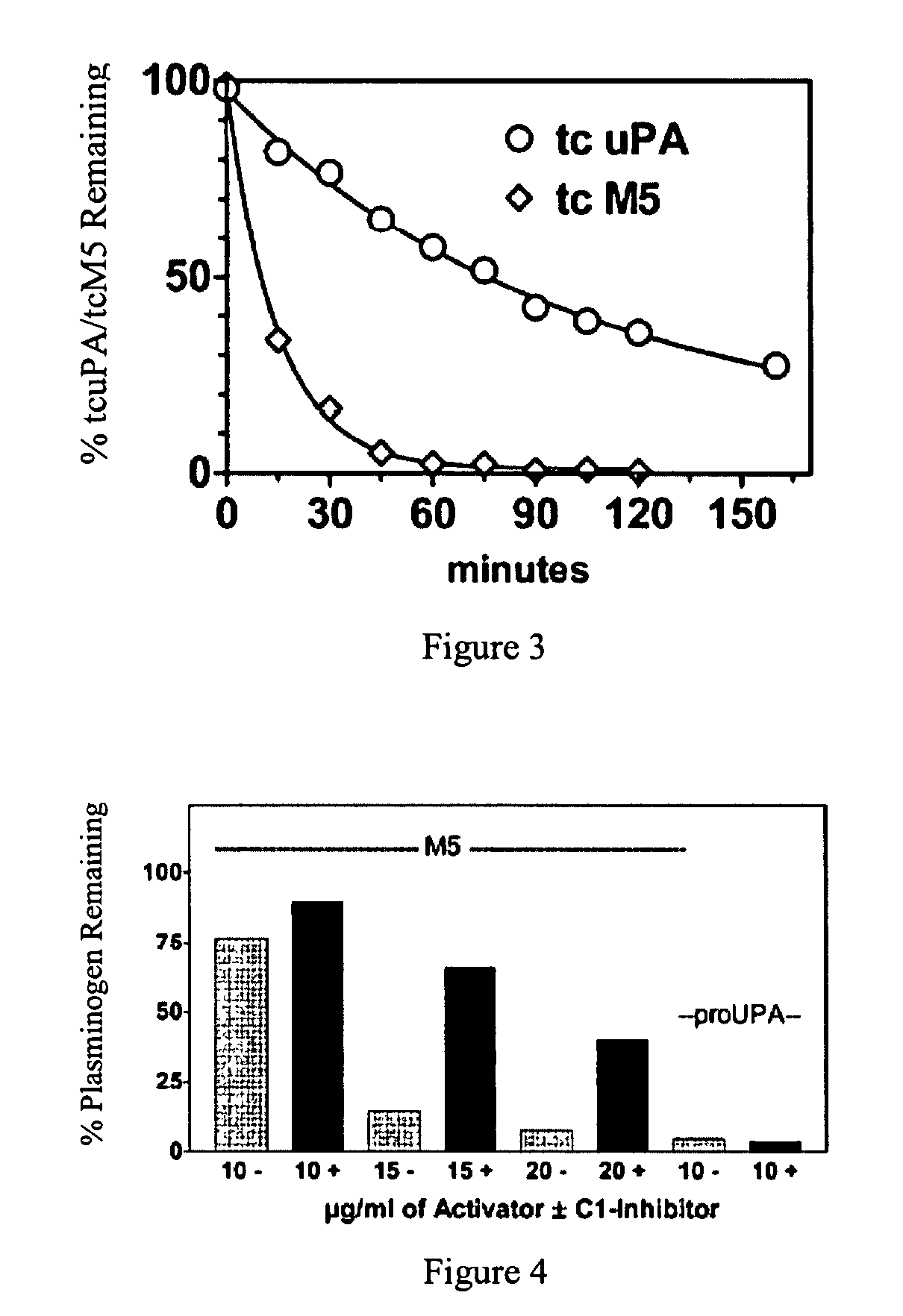
A mutant prourokinase plasminogen activator (M5) was developed to make prouPA less subject to spontaneous activation during fibrinolysis. C1-inhibitor complexes with tcM5. The effect of C1-inhibitor on fibrinolysis and fibrinogenolysis by M5 was determined. Supplemental C1-inhibitor restores the stability of M5 but not that of prouPA. Clot lysis by M5 with supplemental C1-inhibitor showed no attenuation of the rate of fibrinolysis, whereas fibrinogenolysis was prevented by C1-inhibitor. Due to higher dose tolerance of M5 with C1-inhibitor, the rate of fibrin-specific lysis reached that achievable by nonspecific fibrinolysis without inhibitor. Plasma C1-inhibitor stabilized M5 in plasma by inhibiting tcM5 and thereby non-specific plasminogen activation. At the same time, fibrin-specific plasminogen activation remained unimpaired. This unusual dissociation of effects has significant implications for improving the safety and efficacy of fibrinolysis. Methods of reducing bleeding and non-specific plasminogen activation during fibrinolysis by administering M5 along with exogenous C1-inhibitor are disclosed.
Owner:THROMBOLYTIC SCI
Method for continuous visualization of a blood clot or plaque in body lumen
InactiveUS20050063904A1Early detectionUltrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsCoelomContrast medium
A method for visualizing a blood clot or plaque disposed within a body lumen comprising: binding a contrasting complex comprising a contrast material and a thrombolytic material or a clot dissolving agent to the blood clot or plaque; and visualizing the blood clot or plaque over a period time by a visualizing system.
Owner:MED GENESIS LLC
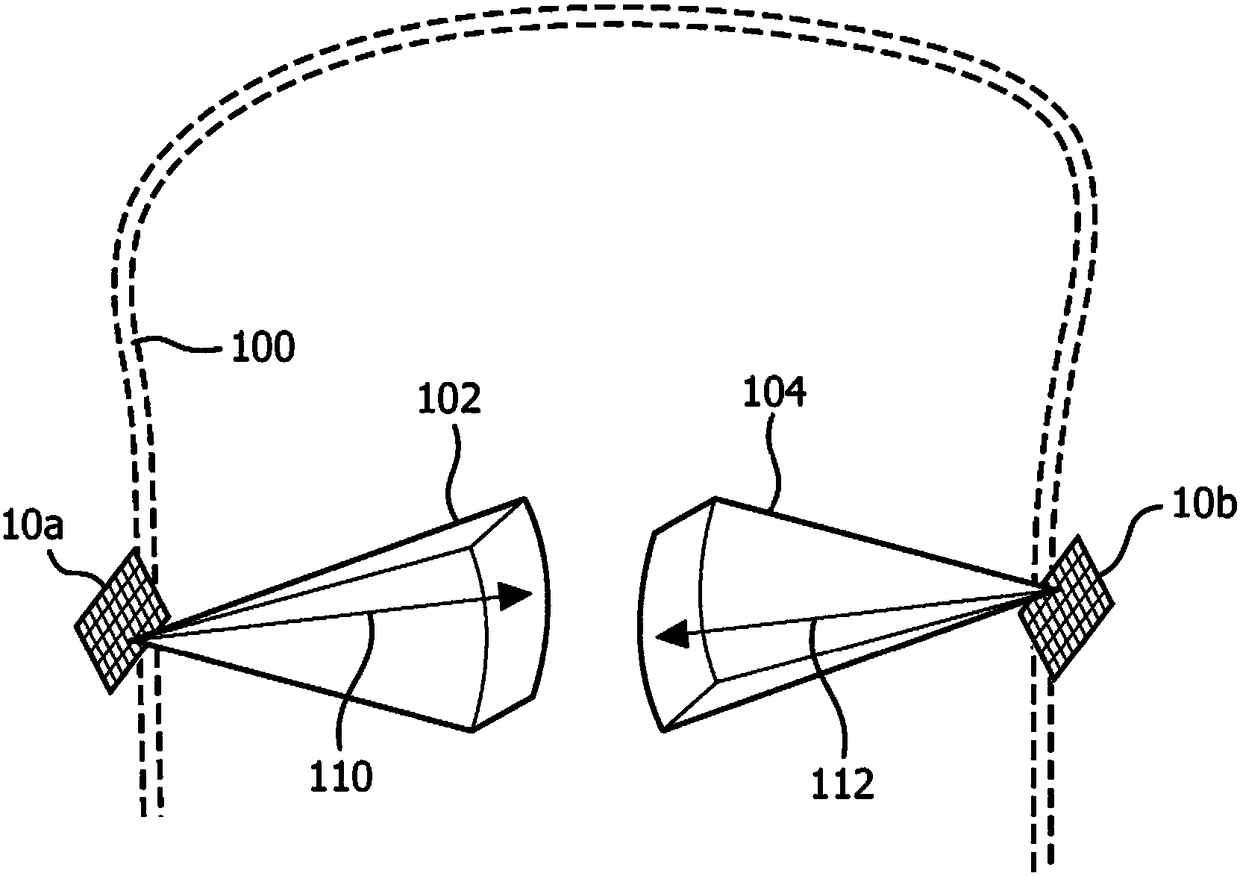
Interleaved beam pattern for sonothrombolysis and other vascular acoustic resonator mediated therapies
ActiveCN108601948AUltrasound therapyBlood flow measurement devicesTherapeutic ultrasoundBlood vessel
A therapeutic ultrasound system transmits a staggered or interleaved pattern of therapy beams for use in sonothrombolysis and other Vascular Acoustic Resonators (VAR) mediated therapy. The inventive technique minimizes VAR, e.g. microbubble, destruction due to adjacent beams, ensures uniform sonication of the targeted region by filling in the spaces between the beams in subsequent passes, and further provides a means for bubble replenishment to maximize the clot lysis from ultrasound. The technique is also applicable to diagnostic ultrasound, VAR mediated drug delivery and blood brain barrieropening.
Owner:KONINKLJIJKE PHILIPS NV +1
A cervical cell lysis kit and a cell lysis method
ActiveCN109207473ACracking is effectiveInhibit DNase activityDNA preparationHigh concentrationCervical cells
The invention provides a cervical cell lysis kit and a cell lysis method, belonging to the technical field of cell lysis. The kit includes a lysis buffer and proteinase K. The lysis buffer comprises the following components in concentration: 10-500mM of Tris-HCl, 1-80 mM of EDTA, 0.05-2 wt% of SDS and 100-250 mM of NaCl. The cell lysis method comprises the following steps: after cleaning a cervical cell sample solution with PBS buffer, uniformly mixing the solution with lysis buffer, adding proteinase K, and digesting at 53-58 DEG C for 10-14 hours; After 30-60 s of vortex vibration, digestingthe cells at 53-58 DEG C for 1.5-2.5 h to complete cell lysis. The kit and the lysis method can fully lyse cervical cells in the sample, dissociate high-concentration and high-quality DNA, and facilitate subsequent DNA extraction and amplification.
Owner:DALIAN MEDICAL UNIVERSITY
C-1 inhibitor prevents non-specific plasminogen activation by a prourokinase mutant without impeding fibrin-specific fibrinolysis
ActiveUS20070298023A1High catalytic activityReduce ratePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
A mutant prourokinase plasminogen activator (M5) was developed to make prouPA less subject to spontaneous activation during fibrinolysis. C1-inhibitor complexes with tcM5. The effect of C1-inhibitor on fibrinolysis and fibrinogenolysis by M5 was determined. Supplemental C1-inhibitor restores the stability of M5 but not that of prouPA. Clot lysis by M5 with supplemental C1-inhibitor showed no attenuation of the rate of fibrinolysis, whereas fibrinogenolysis was prevented by C1-inhibitor. Due to higher dose tolerance of M5 with C1-inhibitor, the rate of fibrin-specific lysis reached that achievable by nonspecific fibrinolysis without inhibitor. Plasma C1-inhibitor stabilized M5 in plasma by inhibiting tcM5 and thereby non-specific plasminogen activation. At the same time, fibrin-specific plasminogen activation remained unimpaired. This unusual dissociation of effects has significant implications for improving the safety and efficacy of fibrinolysis. Methods of reducing bleeding and non-specific plasminogen activation during fibrinolysis by administering M5 along with exogenous C1-inhibitor are disclosed.
Owner:THROMBOLYTIC SCI +1
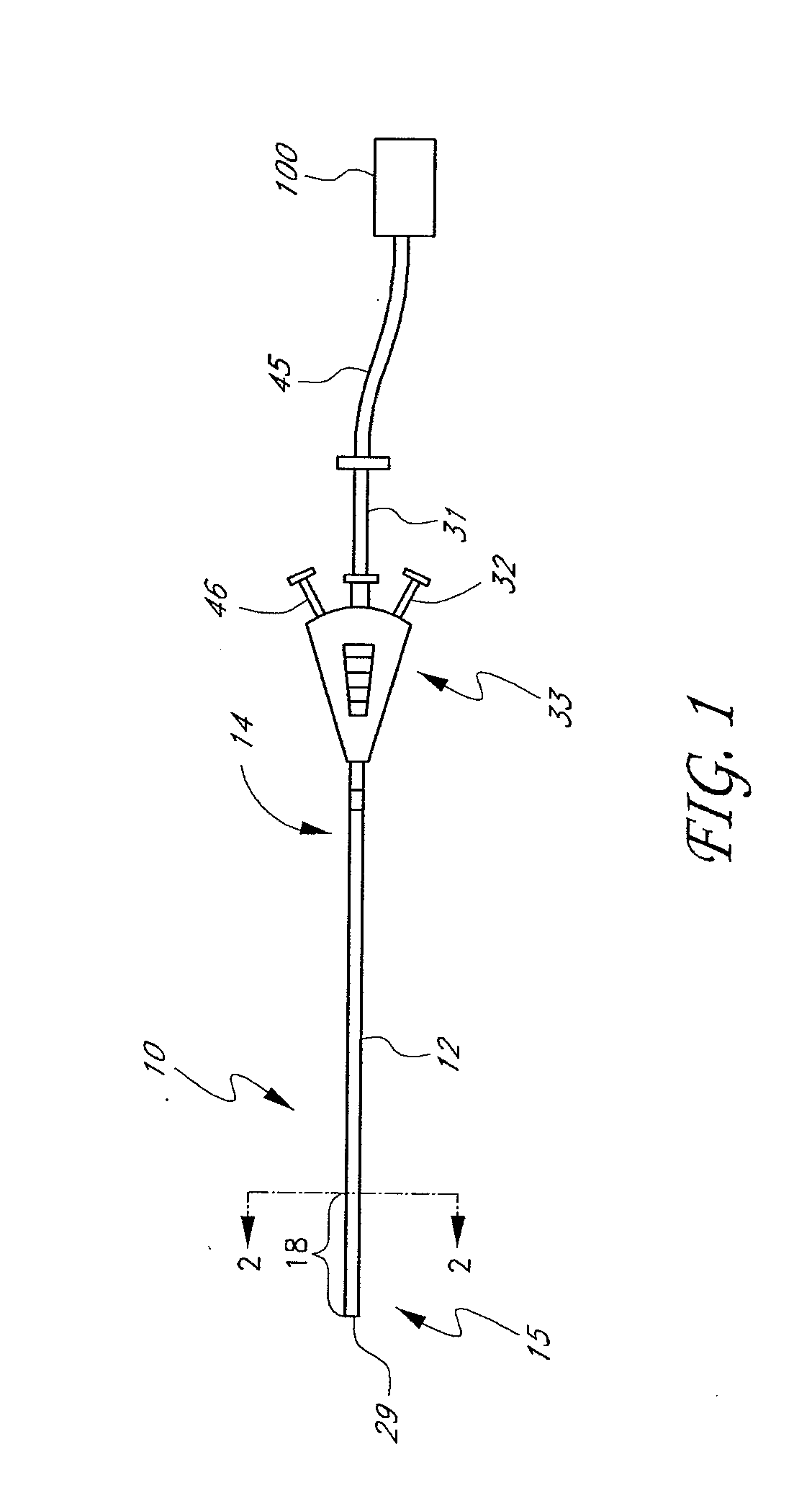
Apparatus and Cartridge for Hemostasis Testing
ActiveUS20150316534A1Material analysis using sonic/ultrasonic/infrasonic wavesFlow propertiesBlood componentClot formation
A sample testing cartridge is usable to perform a variety of tests on a visco-elastic sample, such hemostasis testing on a whole blood or blood component sample. The cartridge includes a sample processing portion that is in fluid communication with a sample retention structure. A suspension, such as a beam, arm, cantilever or similar structure supports or suspends the sample retention portion relative to the sample processing portion in a unitary structure. In this manner, the sample retention portion may be placed into dynamic excitation responsive to excitation of the cartridge and correspondingly dynamic, resonant excitation of the sample contained within the sample retention portion, while the sample processing portion remains fixed. Observation of the excited sample yields data indicative of hemostasis. The data may correspond to hemostasis parameters such as time to initial clot formation, rate of clot formation, maximum clot strength and degree of clot lysis.
Owner:HAEMONETICS
Methods for the treatment of thrombosis
A fibrinolytically active metalloproteinase polypeptide (called “novel acting thrombolytic”) which is useful for blood clot lysis in vivo and methods and materials for its production by recombinant expression are described.
Owner:AMGEN INC
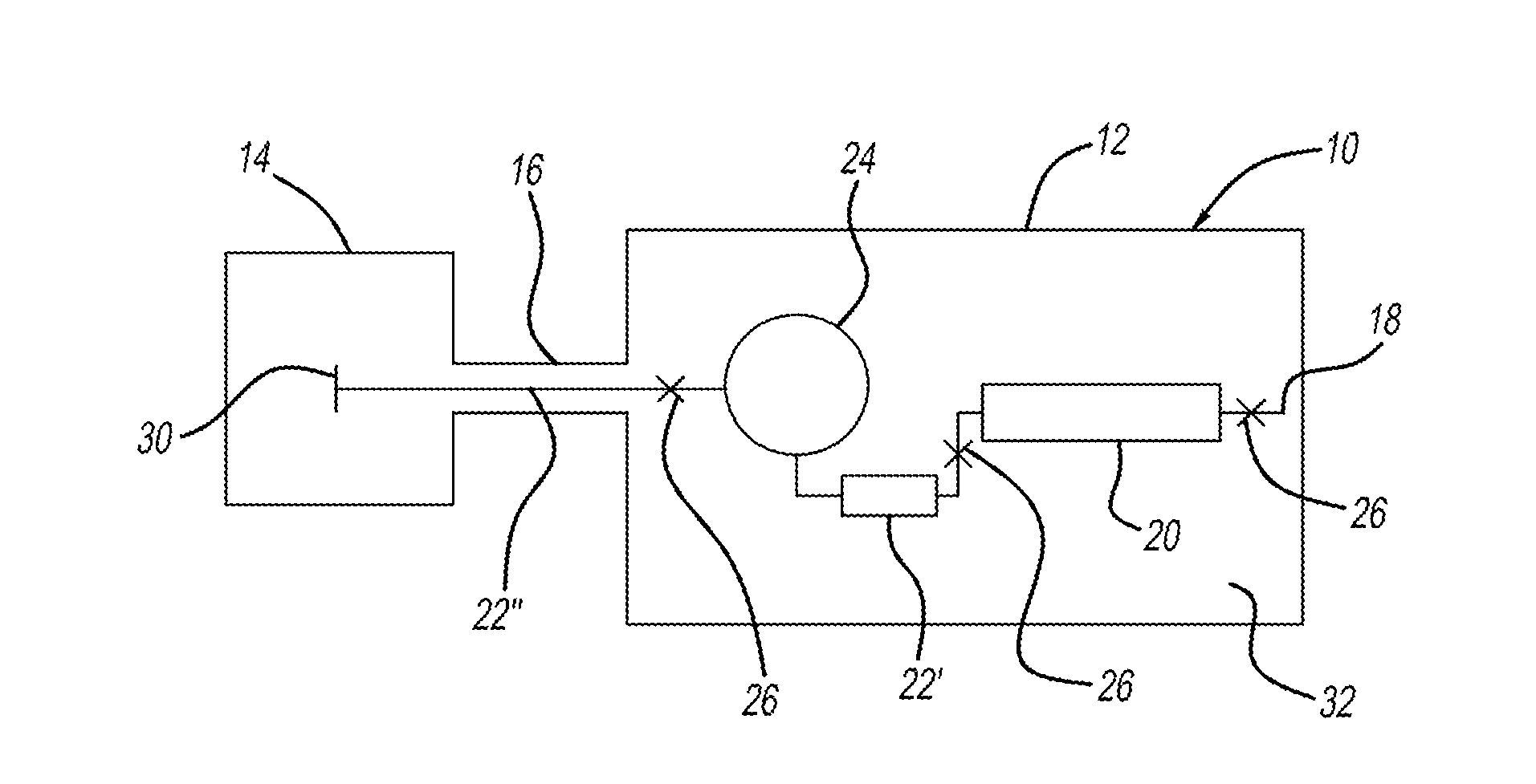
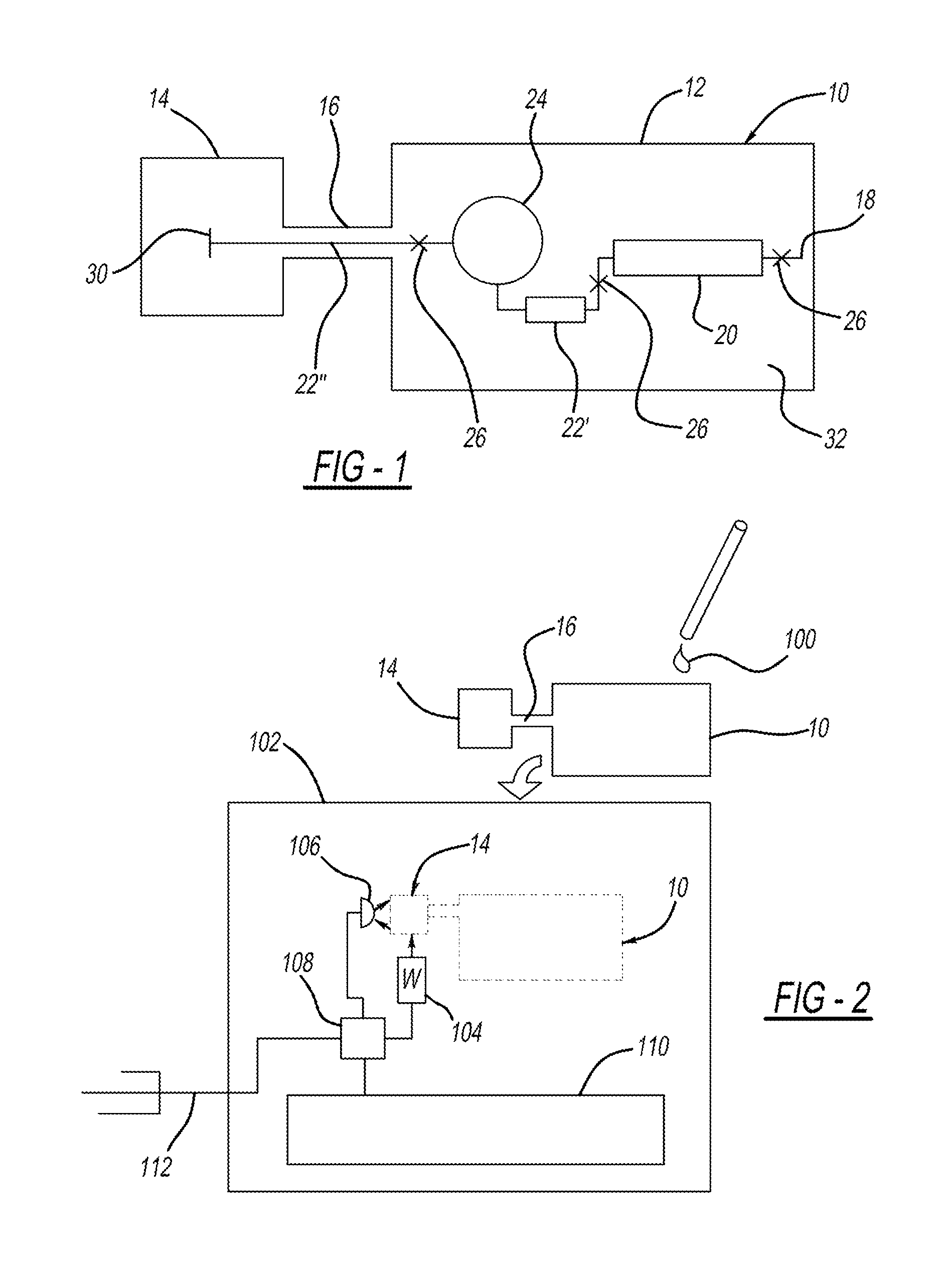
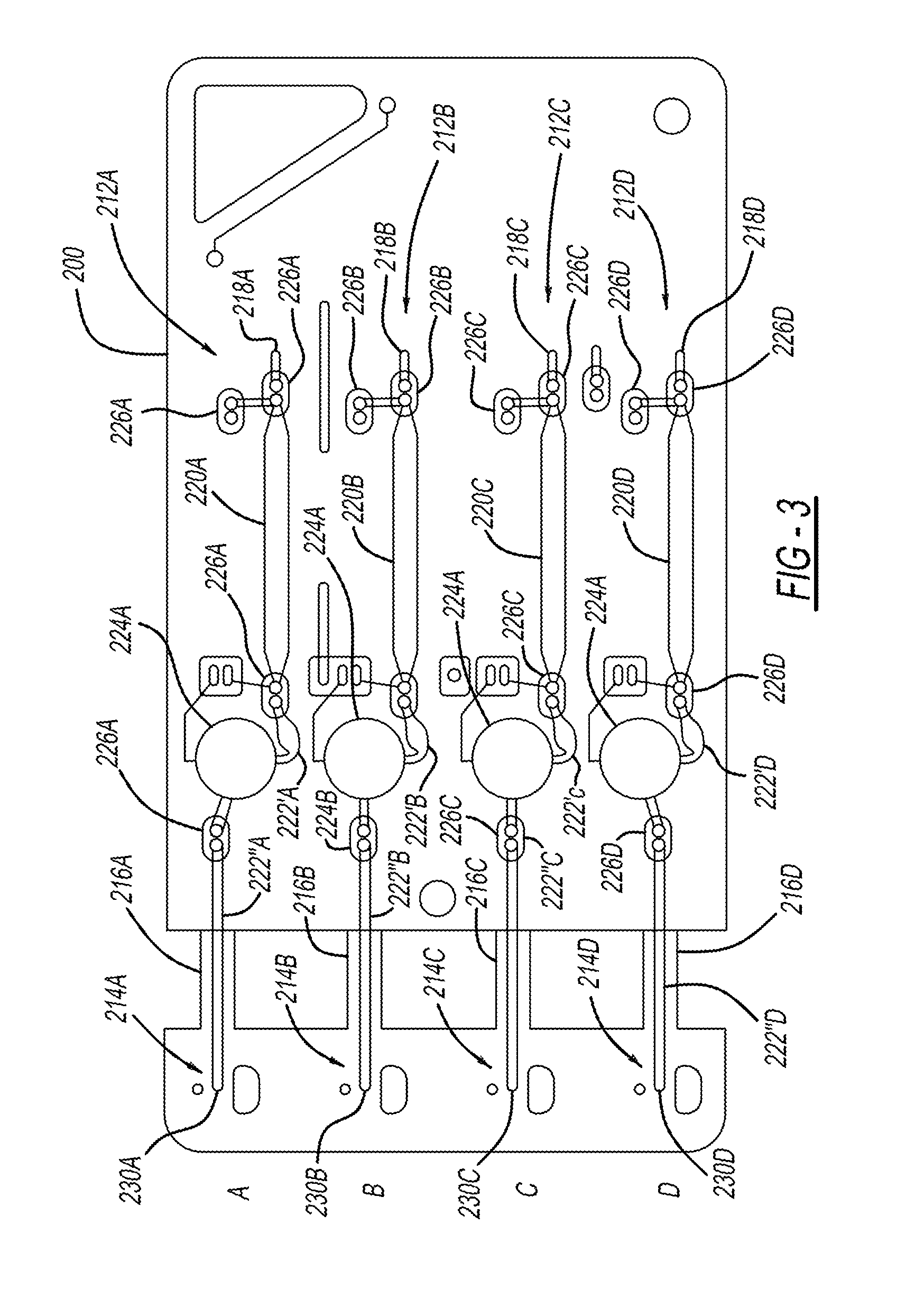
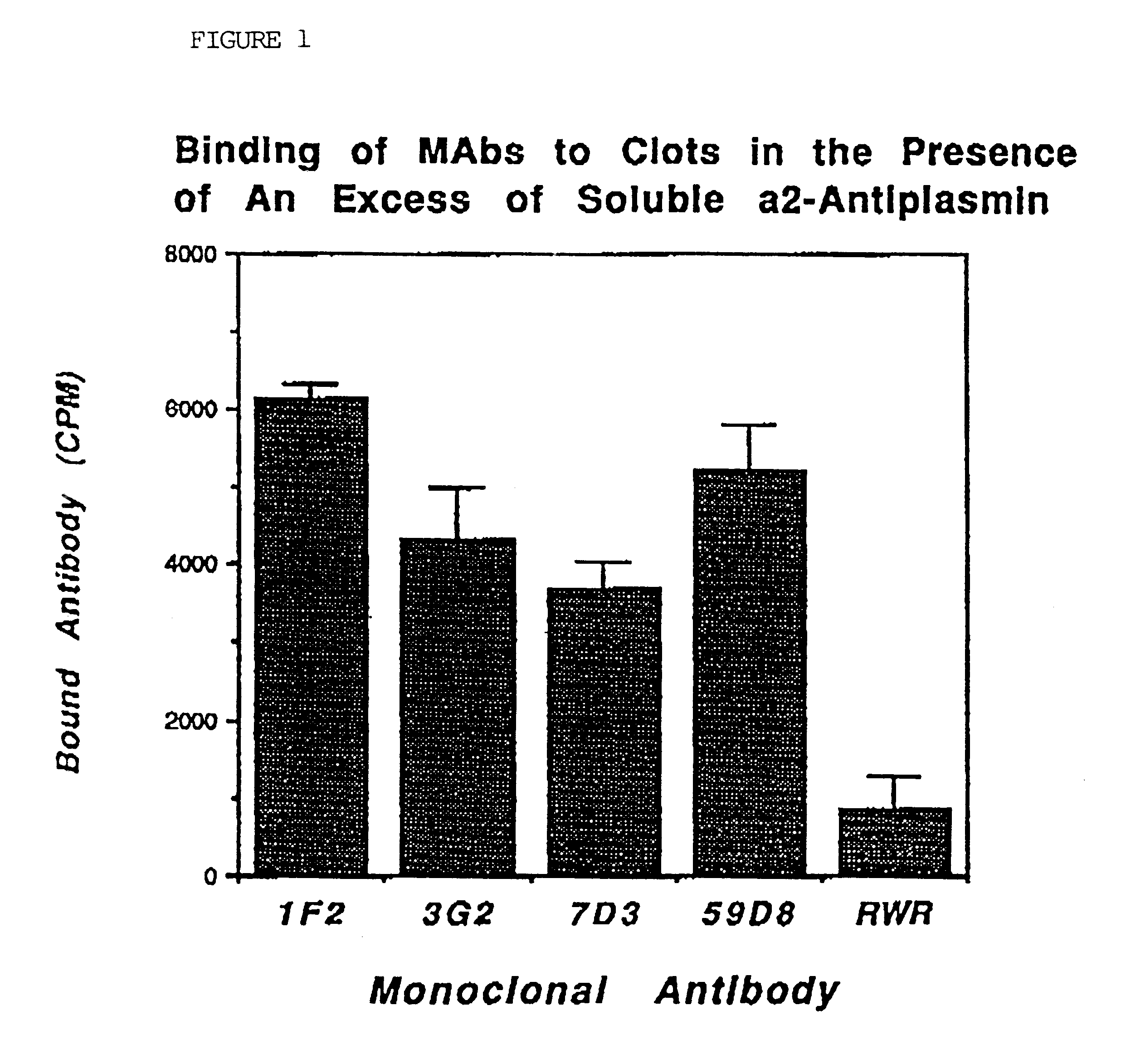
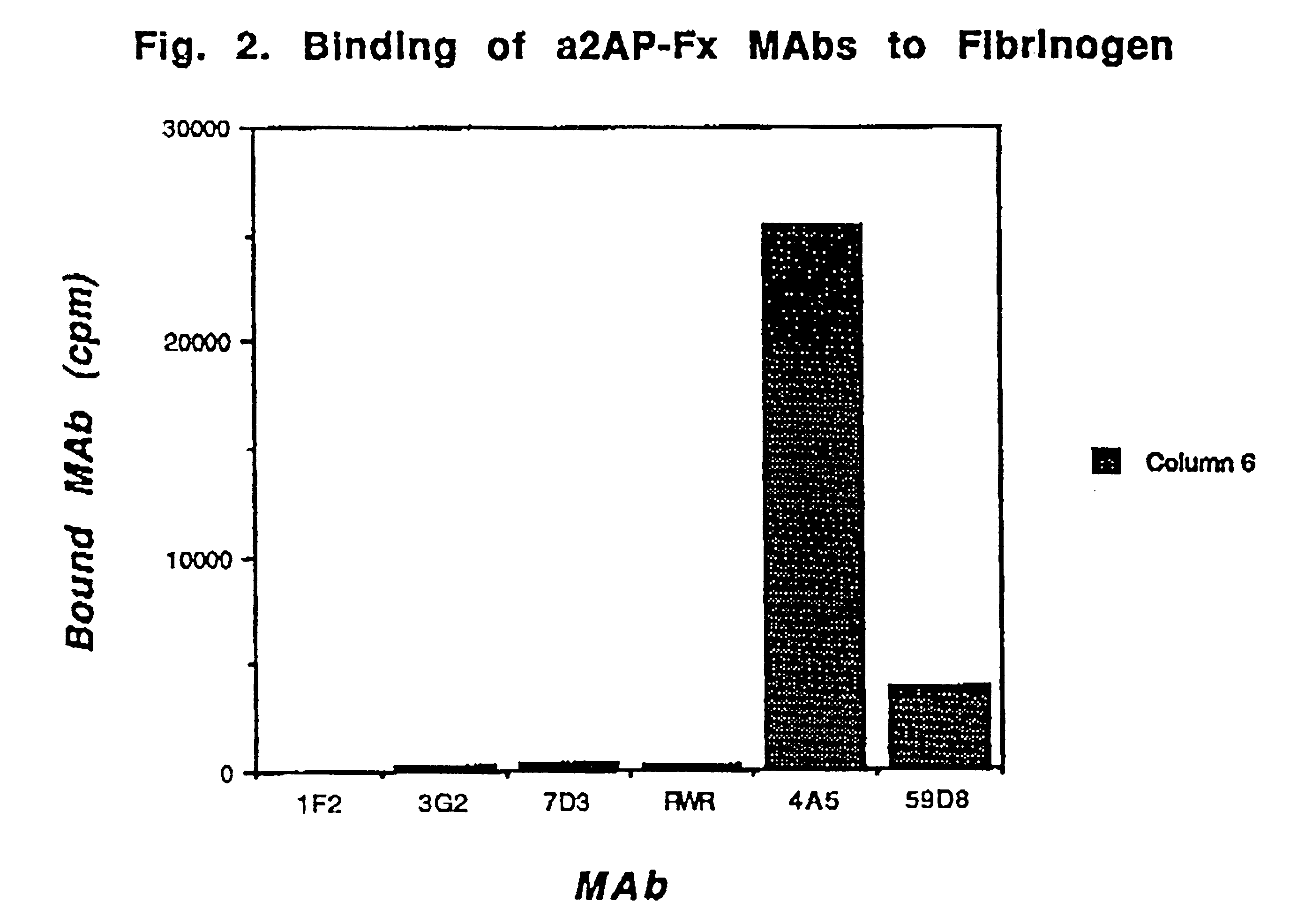
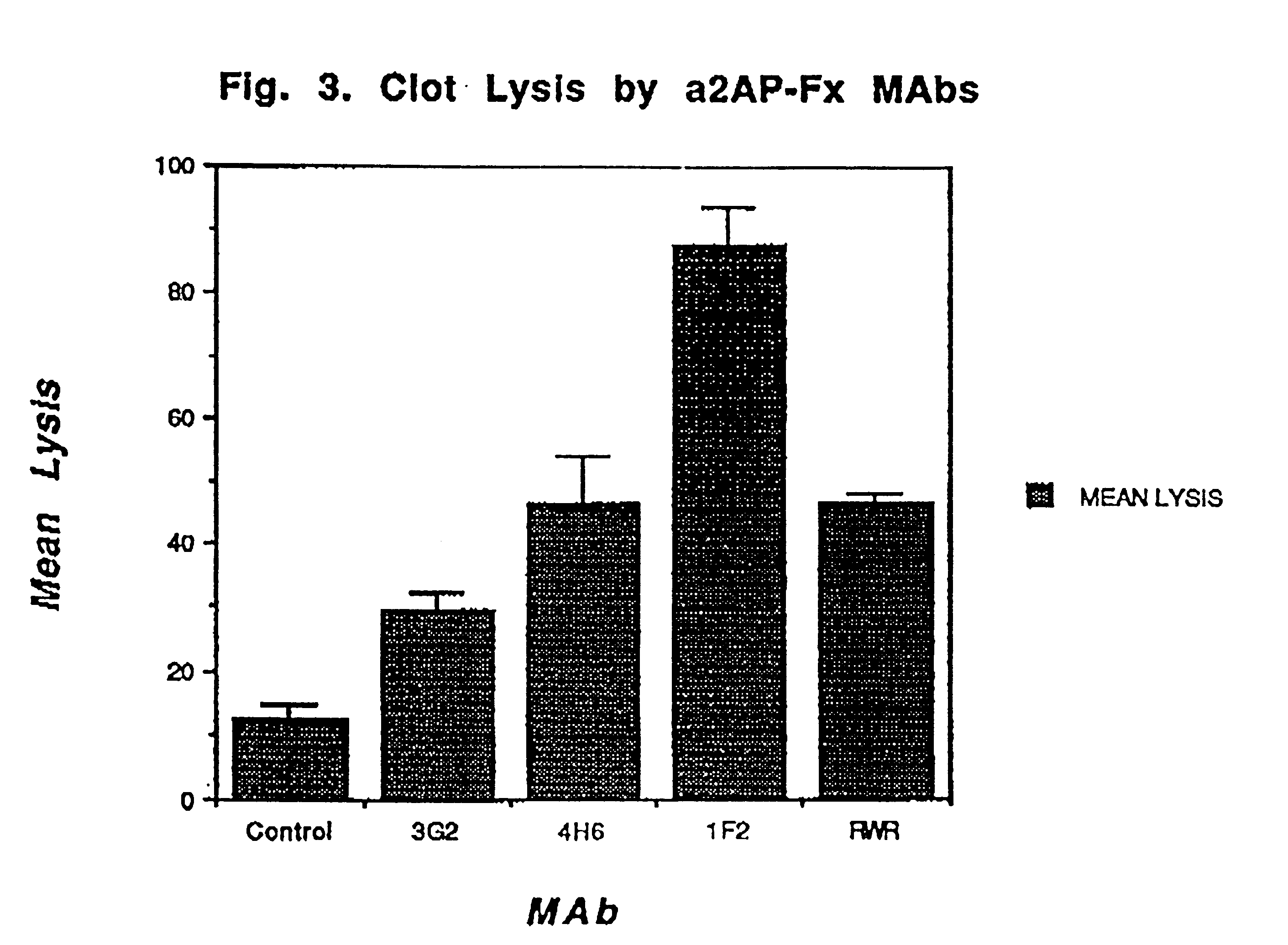
Antibodies that bind to alpha2-antiplasmin crosslinked to fibrin which do not inhibit plasma alpha2-antiplasmin
The present invention relates to a treatment for myocardial infarction and blood clots within a patient, and more specifically to a therapy which enhances clot lysis comprising administering to a patient an antibody directed to alpha2-antiplasmin crosslinked to fibrin (alpha2AP-FX) which does not inhibit plasma alpha2-antiplasmin (alpha2AP). The invention also relates to a treatment for enhancing clot lysis comprising administering an antibody directed toward alpha2-antiplasmin crosslinked to fibrin which does not inhibit plasma alpha2AP together with a thrombolytic agent.
Owner:THE GENERAL HOSPITAL CORP
Method for cell lysis and PCR within the same reaction chamber
ActiveUS20130203120A1Improve throughputHigh throughput methodMicrobiological testing/measurementTransferasesLysisLiving cell
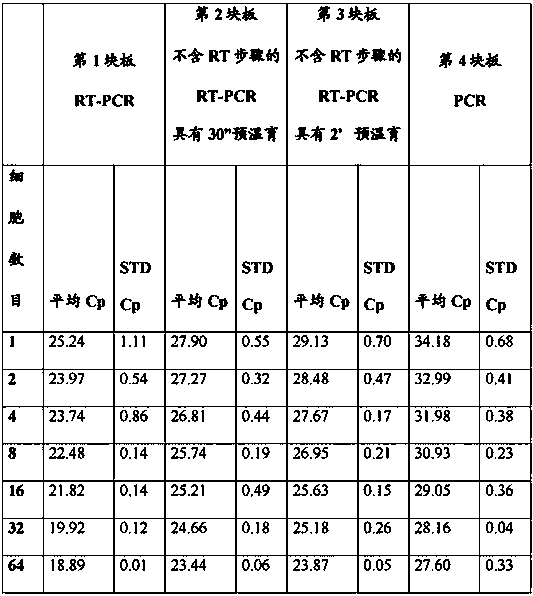
A method for amplification of a target DNA, comprising the steps of (i) transferring a liquid with a first volume comprising at least one or more living cells into a vessel (ii) adding to said vessel a PCR reaction buffer with a second volume, whereas said second volume is at least 2× as large as said first volume (iii) lysing said at least one or more living cells within said vessel by means of incubation for at least 1 Minute at at least 90° C., and (iv) amplifying said target by means of a polymerase chain reaction without performance of an intermediate purification step.
Owner:ROCHE MOLECULAR SYST INC
Multifunctional intracerebral hematoma drainage tube with multiple secondary tubes
PendingCN108325051AImprove dissolution rateIncrease drainage speedWound drainsCatheterDiseaseGynecology
The invention discloses a multifunctional intracerebral hematoma drainage tube with multiple secondary tubes, and relates to the technical field of medical apparatus and instruments. The multifunctional intracerebral hematoma drainage tube has the advantages that the multiple secondary tubes are arranged in a header tube, secondary tube extension and retraction holes are formed in the front of theheader tube, and accordingly the secondary tubes can extend out of the header tube or can be retracted into the header tube; medicines can be administrated for multiple points when the fronts of thesecondary tubes extend out of the header tube, drainage can be implemented for the multiple points when the fronts of the secondary tubes extend out of the header tube, the header tube can rotate by 360-degree angles when the fronts of the secondary tubes are retracted into the header tube, the medicines further can be rotationally administrated at 360-degree angles by the multiple secondary tubesat different levels, the purposes of uniformly administrating the medicines for the multiple points on 360-degree circumferences and carrying out drainage for the multiple points on the 360-degree circumferences can be achieved, accordingly, the blood clot lysis and drainage speeds can be increased, compression of hematomas on main functional structures can be quickly relieved, and harm due to hypertensive cerebral hemorrhage diseases can be reduced.
Owner:崔建忠 +1
Method for continuous visualization of a blood clot or plaque in body lumen
InactiveUS7803352B2Ultrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsCoelomRadiology
A method for visualizing a blood clot or plaque disposed within a body lumen comprising: binding a contrasting complex comprising a contrast material and a thrombolytic material or a clot dissolving agent to the blood clot or plaque; and visualizing the blood clot or plaque over a period time by a visualizing system.
Owner:MED GENESIS LLC
Use of coagulation proteins to lyse clots
InactiveUS20060275277A1Enhance dissolving said blood clotHigh dissolution rateOrganic active ingredientsPeptide/protein ingredientsSide effectLysis
The present invention relates to the use of coagulation proteins for the lysis of blood clots. More specifically, the present invention provides a method for accelerating the dissolution of a blood clot through the administration of at least one coagulation protein comprising a basic C-terminal amino acid, wherein the coagulation protein may be a derivative of Factor X or Factor V or a combination thereof. Pharmaceutical compositions for the treatment and prophylaxis of blood clots are also provided, wherein, the methods and products of the present invention advantageously accelerate clot dissolution while potentially minimizing the adverse side-effects, such as hemorrhaging, seen with other clot dissolving agents. The present invention also provides a method for detecting a fibrinolytic potential in a subject.
Owner:CANADIAN BLOOD SERVICES
Method, device and system for targetted cell lysis
ActiveUS9629912B2Reduce cell deathField is lowElectrotherapySurgical instrument detailsSufficient timeLysis
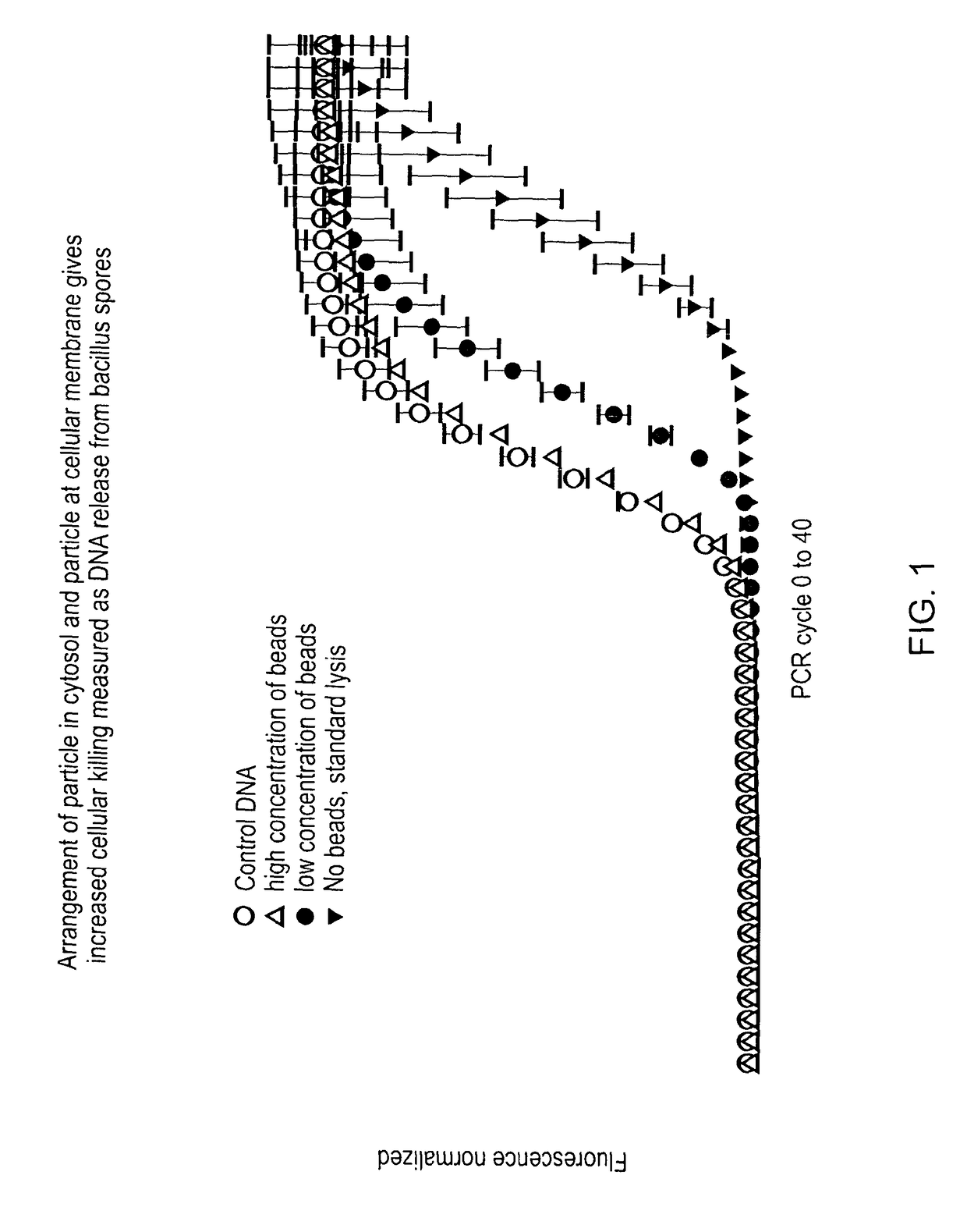
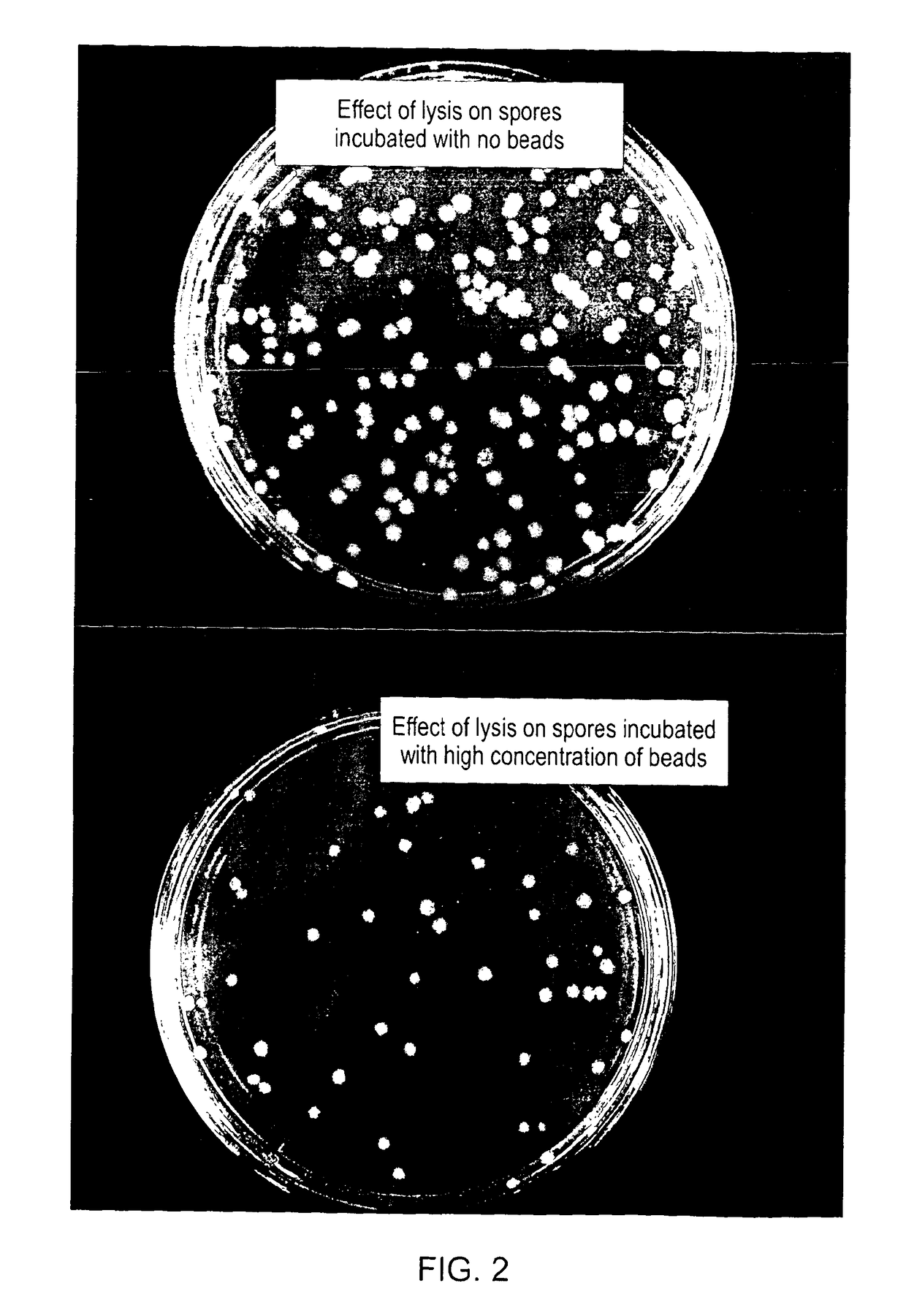
A method, device and system employs particles, such as nanoparticles, and an electric or electro-magnetic field, to cause cell death in target cells by non-thermal means. The method of causing targeted cell death comprises the steps of: introducing a particle to the interior of a target cell and exposing the target cell to a transient electromagnetic field for a sufficient time interval in order to cause cell death. The invention overcomes problems associated with similar methods as a result of the fact that a smaller electric field is applied because the particle enhances the effect of the electric field in its immediate vicinity, so reducing the field strength needed to achieve cell lysis and thereby reducing the risk of damage to healthy cells that may be in its vicinity. Apparatus for performing the method; as well as techniques of delivering particles and for producing particles are also described.
Owner:BIOVICES IPR HLDG AS +1
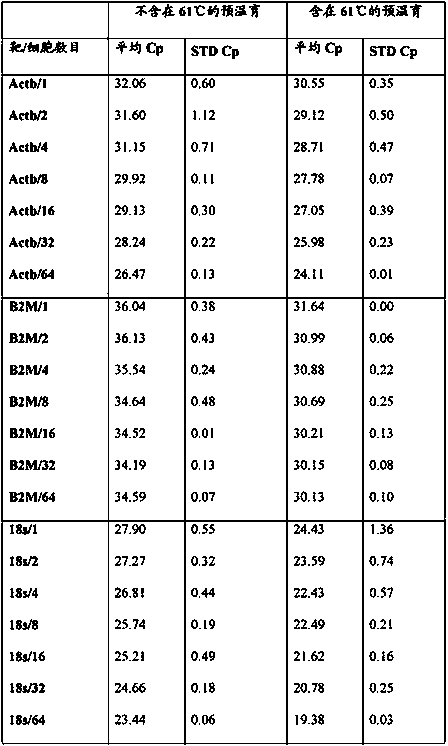
Method for cell lysis in rt‑PCR reaction buffer
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com