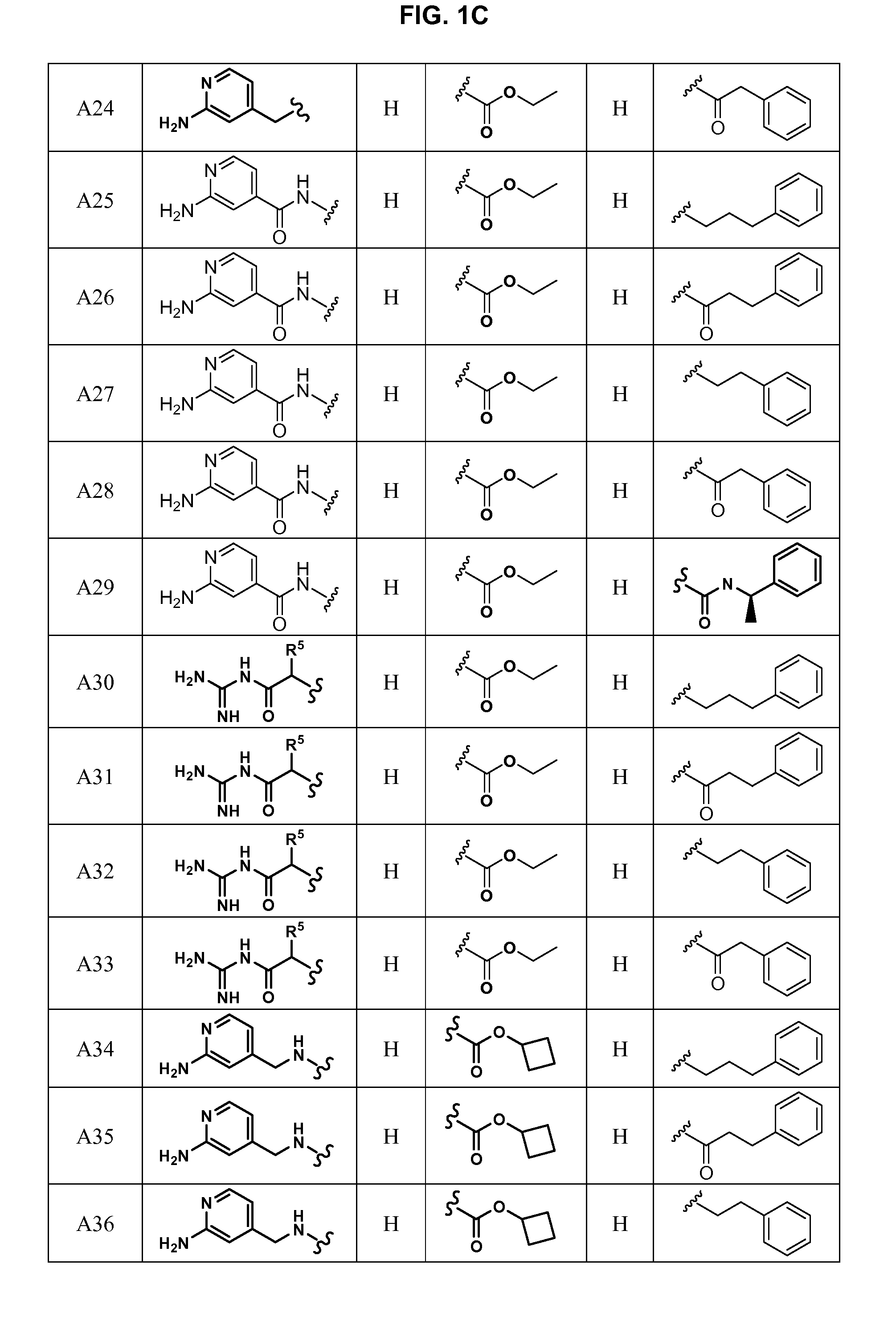
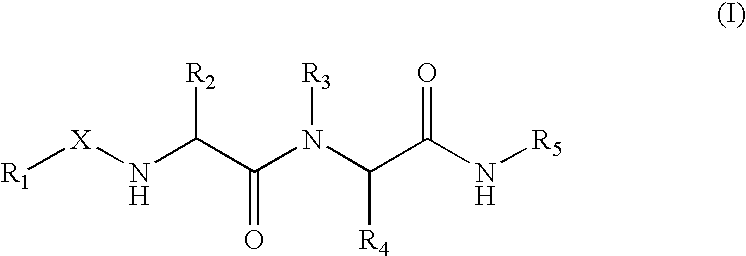
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
175 results about "Plasminogen activator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
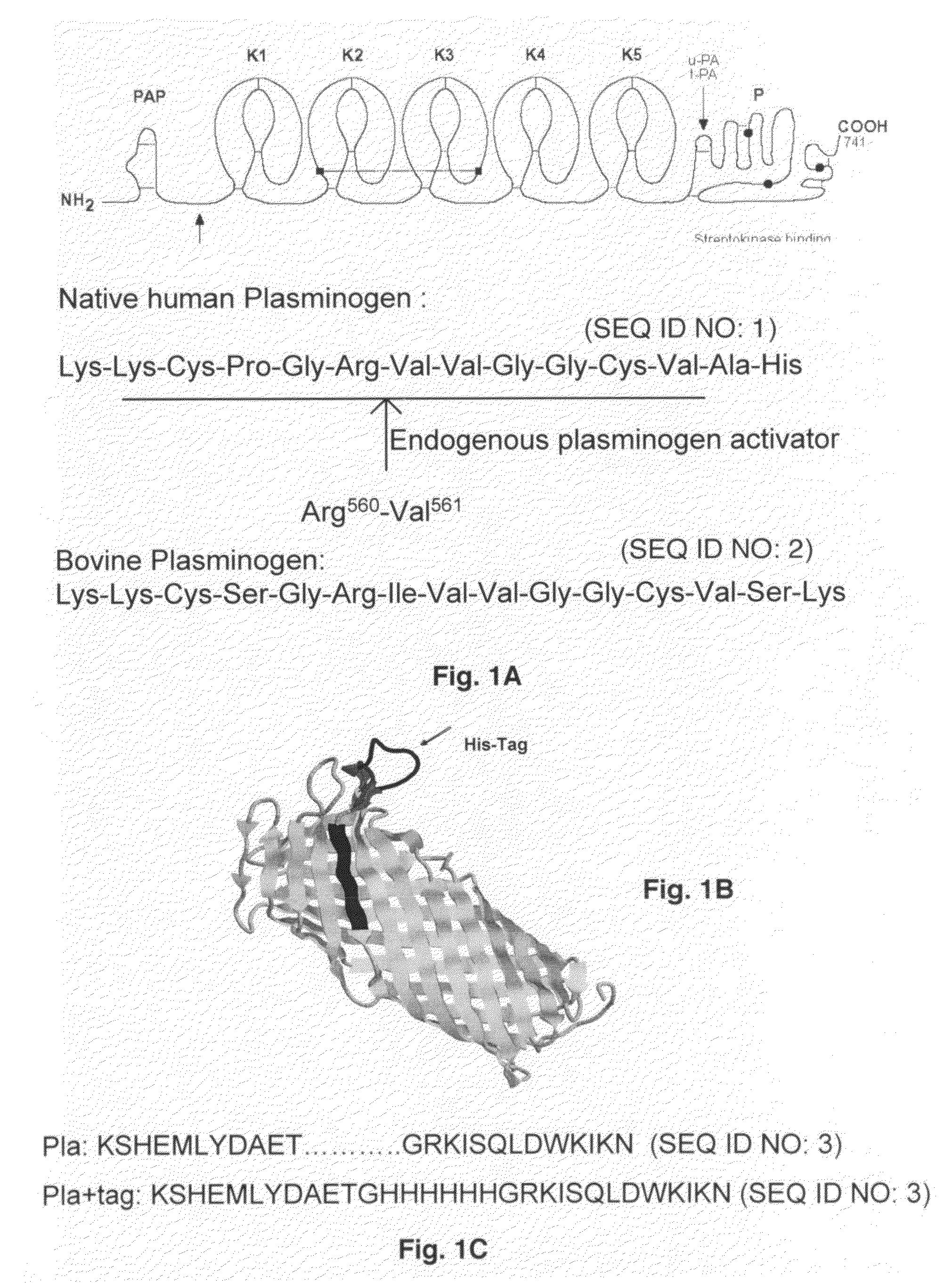
Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.
Compositions and techniques for localized therapy
InactiveUS7135189B2Little effectSurgical adhesivesPeptide/protein ingredientsThrombin activityNitric oxide
A therapeutic medical article is provided which comprises a medical article, a precursor compound and an activator compound. The medical article is adapted, upon administration to a patient, to release the precursor compound and the activator compound such that the activator compound interacts with the precursor compound and converts the precursor compound into activated form for local delivery. Specific examples of precursor and activator compound pairs include: (a) a nitrosothiol precursor and a nitric oxide donor, (b) plasminogen and plasminogen activator, and (c) fibrinogen and thrombin.
Owner:BOSTON SCI SCIMED INC
Method for producing recombinant proteins in micro-organisms
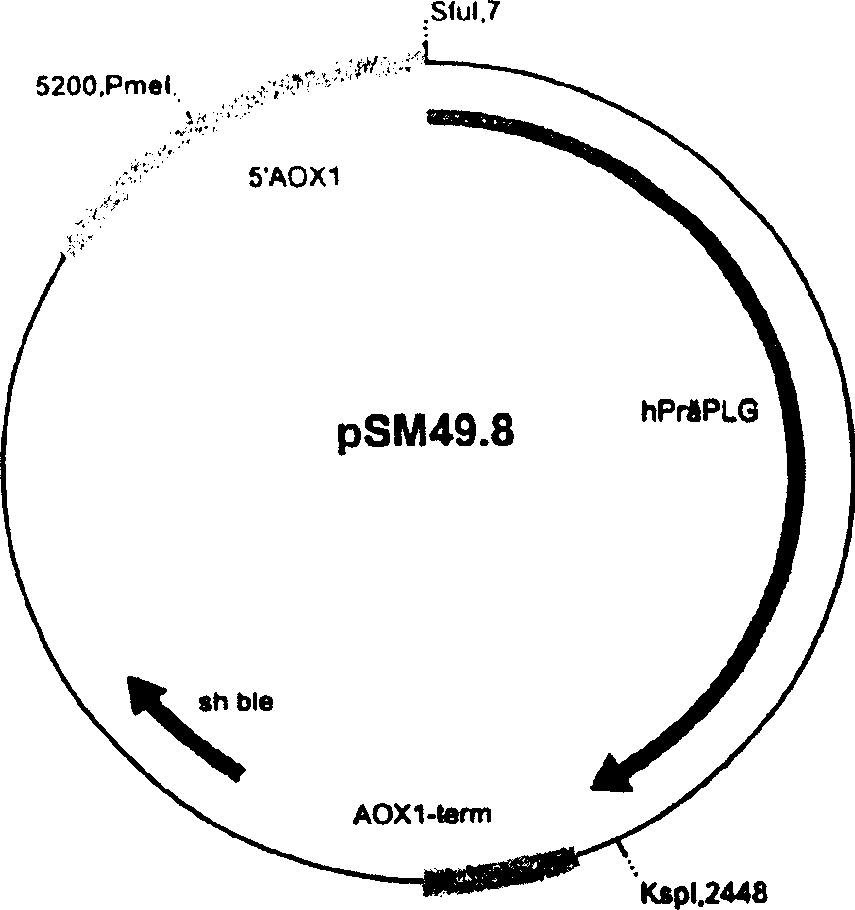
The invention relates to a method for producing a recombinant functional plasminogen in micro-organisms, and to a method for identifying plasminogen activators. The nucleic acid sequence coding for the functional part of the plasminogen is fused with a nucleic acid molecule coding for at least one signal peptide. The nucleic acid molecule coding for the plasminogen and the nucleic acid molecule coding for the signal peptide are combined with codons for interfaces of proteases which ensure the separation of the signal peptide. The recombinant plasminogen or the corresponding plasmine is suitable for treating wounds which are slow to heal or not healing, by application of the enzyme in an appropriate formulation.
Owner:N-酶生物技术有限公司
Non-covalent inhibitors of urokinase and blood vessel formation
InactiveUS6586405B2Promote cell adhesionImprove cell adhesionTripeptide ingredientsDepsipeptidesBiochemistryIn vivo
Owner:KEVIN S HELMBACHER GENERAL COUNSEL +1
Leader sequences for use in production of proteins
InactiveUS20070141666A1Efficient secretionEasy to handlePolypeptide with localisation/targeting motifSugar derivativesDNA constructTissues types
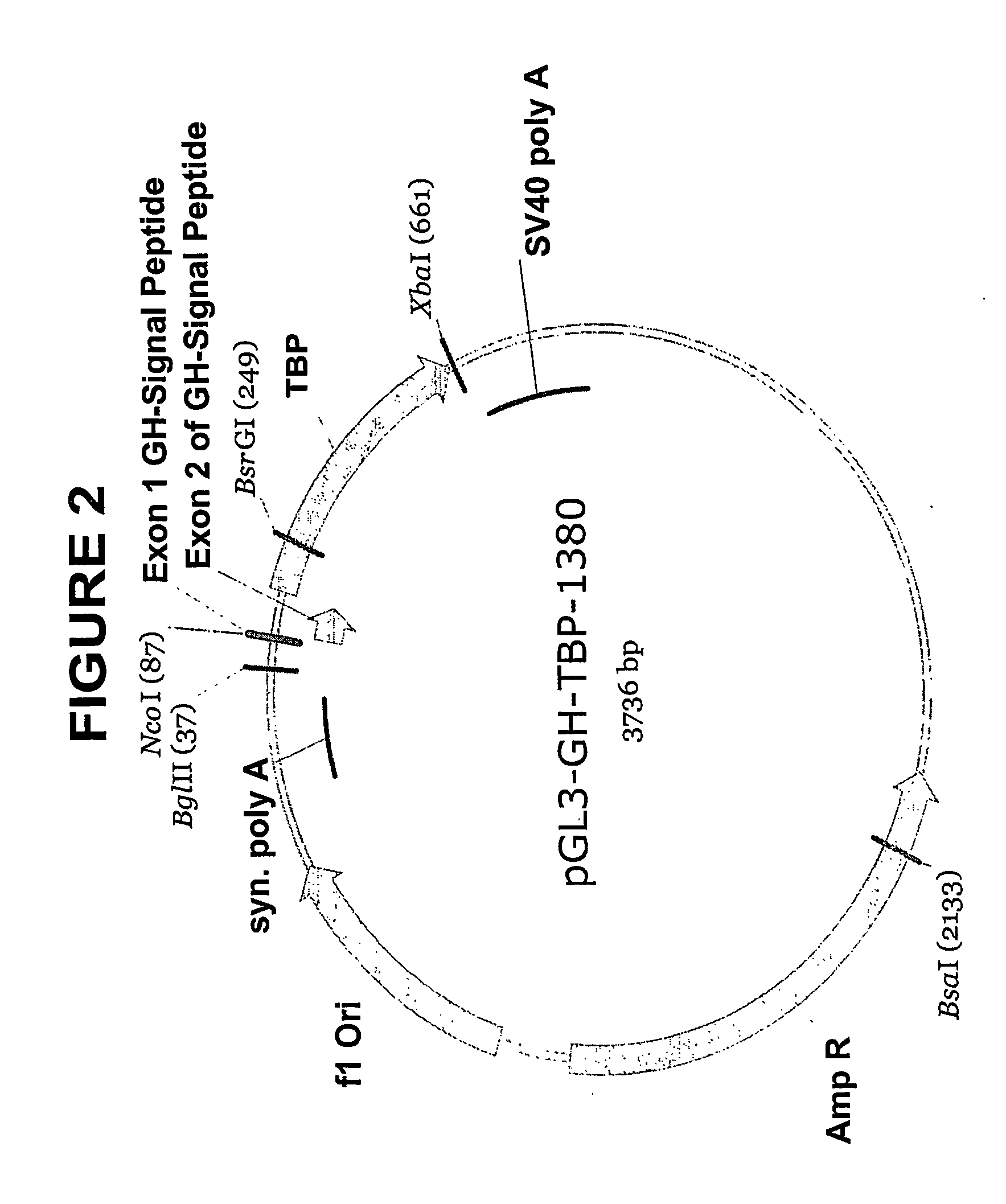
This invention encompasses novel leader sequences for production of proteins. More specifically, the invention relates to DNA constructs encoding leader sequences comprising an immunoglobulin signal peptide fused to a tissue-type plasminogen activator propeptide, and to DNA constructs encoding leader sequences comprising a truncated human tissue-type plasminogen activator propeptide. The invention further relates to the use of these DNA constructs for producing proteins in mammalian cells.
Owner:MERCK SERONO SA
Non-covalent inhibitors of urokinase and blood vessel formation
InactiveUS20020037857A1Promote cell adhesionImprove cell adhesionDipeptide ingredientsDepsipeptidesUrokinase Plasminogen ActivatorIn vivo
Novel compounds having activity as non-covalent inhibitors of urokinase and having activity in reducing or inhibiting blood vessel formation are provided. These compounds have Pi a group having an amidino or guanidino moiety or derivative thereof. These compounds are useful in vitro for monitoring plasminogen activator levels and in vivo in treatment of conditions which are ameliorated by inhibition of or decreased activity of urokinase and in treating pathologic conditions wherein blood vessel formation is related to a pathologic condition.
Owner:KEVIN S HELMBACHER GENERAL COUNSEL +1
Process for generating plasmin in the vitreous of the eye and inducing separation of the posterior hyaloid from the retina
InactiveUS6899877B2Inhibiting retinal tearReducing and preventing effectSenses disorderEye implantsUrokinase Plasminogen ActivatorVascular proliferation
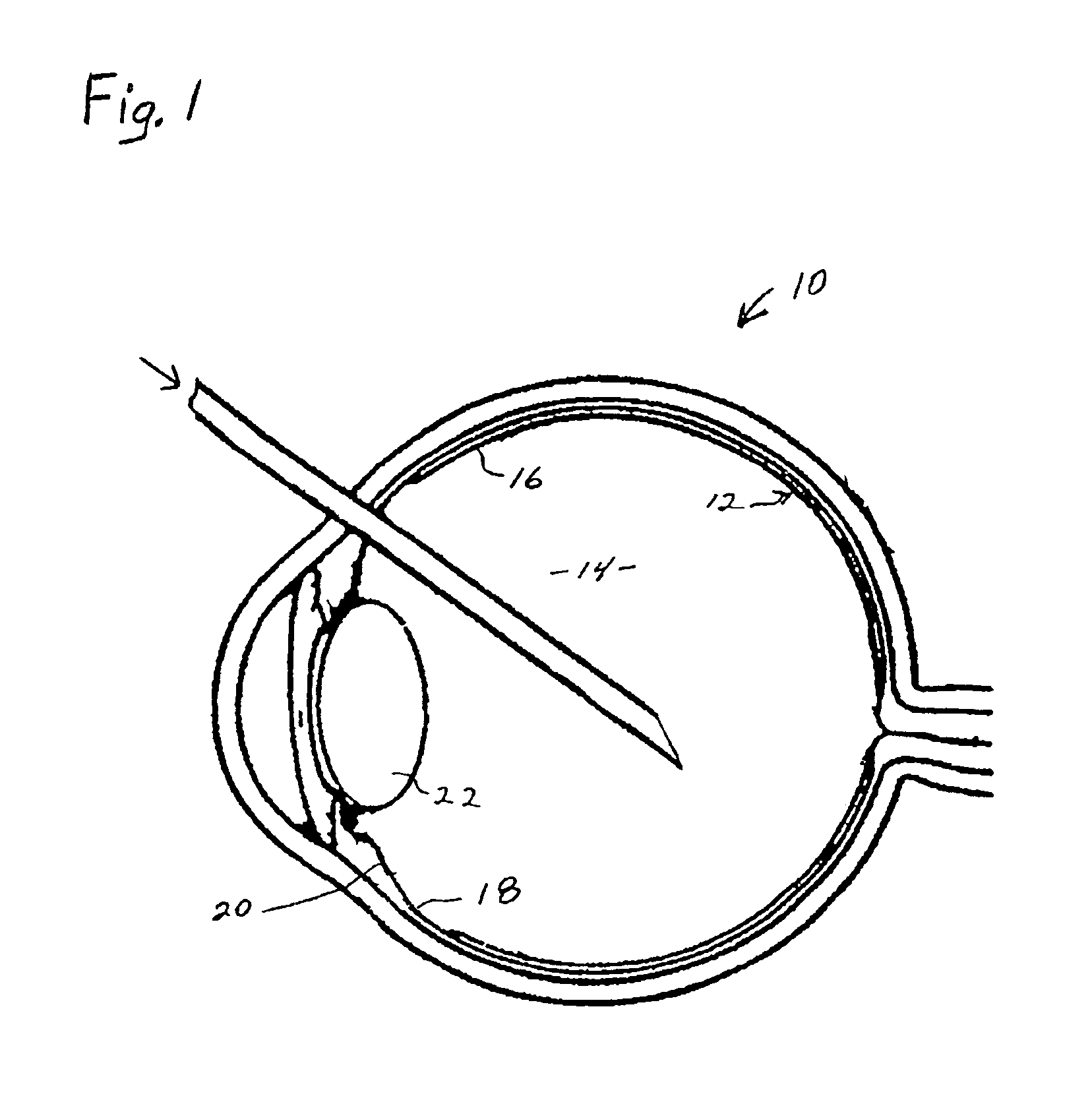
A process for inhibiting vascular proliferation separately introduces components into the eye to generate plasmin in the eye in amounts to induce complete posterior vitreous detachment where the vitreoretinal interface is devoid of cortical vitreous remnants. The process administers a combination of lysine-plasminogen, at least one recombinant plasminogen activator selected from the group consisting of urokinase, streptokinase, tissue plasminogen activator, chondroitinase, pro-urokinase, retavase, metaloproteinase, and thermolysin and a gaseous adjuvant to form a cavity in the vitreous. The composition is introduced into the vitreous in an amount effective to induce crosslinking of the vitreous and to induce substantially complete posterior vitreous detachment from the retina without causing inflammation of the retina. The gaseous adjuvant material is introduced into the vitreous simultaneously with or after the lysine-plasminogen and recombinant plasminogen activator such as recombinant urokinase to compress the vitreous against the retina while the composition induces the complete posterior vitreous detachment.
Owner:MINU
High-performance environment-friendly pig feed and preparation method thereof
InactiveCN101589774AImprove qualityLow costAnimal feeding stuffAccessory food factorsAnimal scienceGARLIC POWDER
The invention discloses a high-performance environment-friendly pig feed. The feed for pork pigs and mountain pigs is prepared by substituting raw materials such as earthworm powder, snail powder, fly maggot powder and yeast for protein raw materials such as fish meal, meat and bone meal and the like, substituting peanut powder for dregs of beans, substituting minor cereals-cassava powder for staple food grain such as corn, wheat and the like, and substituting polypeptide substances in the fly maggot powder, enzyme of the earthworm powder, complex enzyme of a multi-functional plasminogen activator, a flora of microbial probiotics, mechanism actions such as pathogenic bacteria inhibition for chemical growth hormones and chemical medicament antibiotics. The pig feed is characterized in that the pig feed contains a plurality of raw materials in portion by weight: 30 to 60 portions of detoxicated cassava powder, 10 to 25 portions of earthworm powder, 5 to 20 portions of snail powder, 2 to 10 portions of fly maggot powder, 6 to 30 portions of yeast powder, 5 to 20 portions of peanut powder, 1 to 4 portions of garlic powder, 1 to 4 portions of mineral trace elements, 2 to 5 portions of various amino acids, 0.5 to 2 portions of lecithin, 0.5 to 2 portions of various vitamins, 0.5 to 3 portions of multi-functional plasminogen activator, and 0.5 to 3 portions of microbial probiotics. The invention has the advantages of improving the quality of feeds, reducing production cost, promoting the safety probability of meat product and the like.
Owner:郭伟 +2
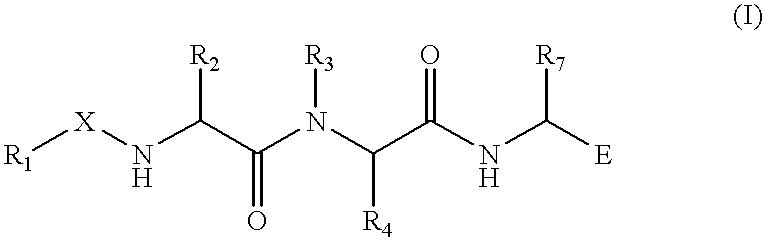
Substituted azetidinones
Owner:EXITHERA PHARMA
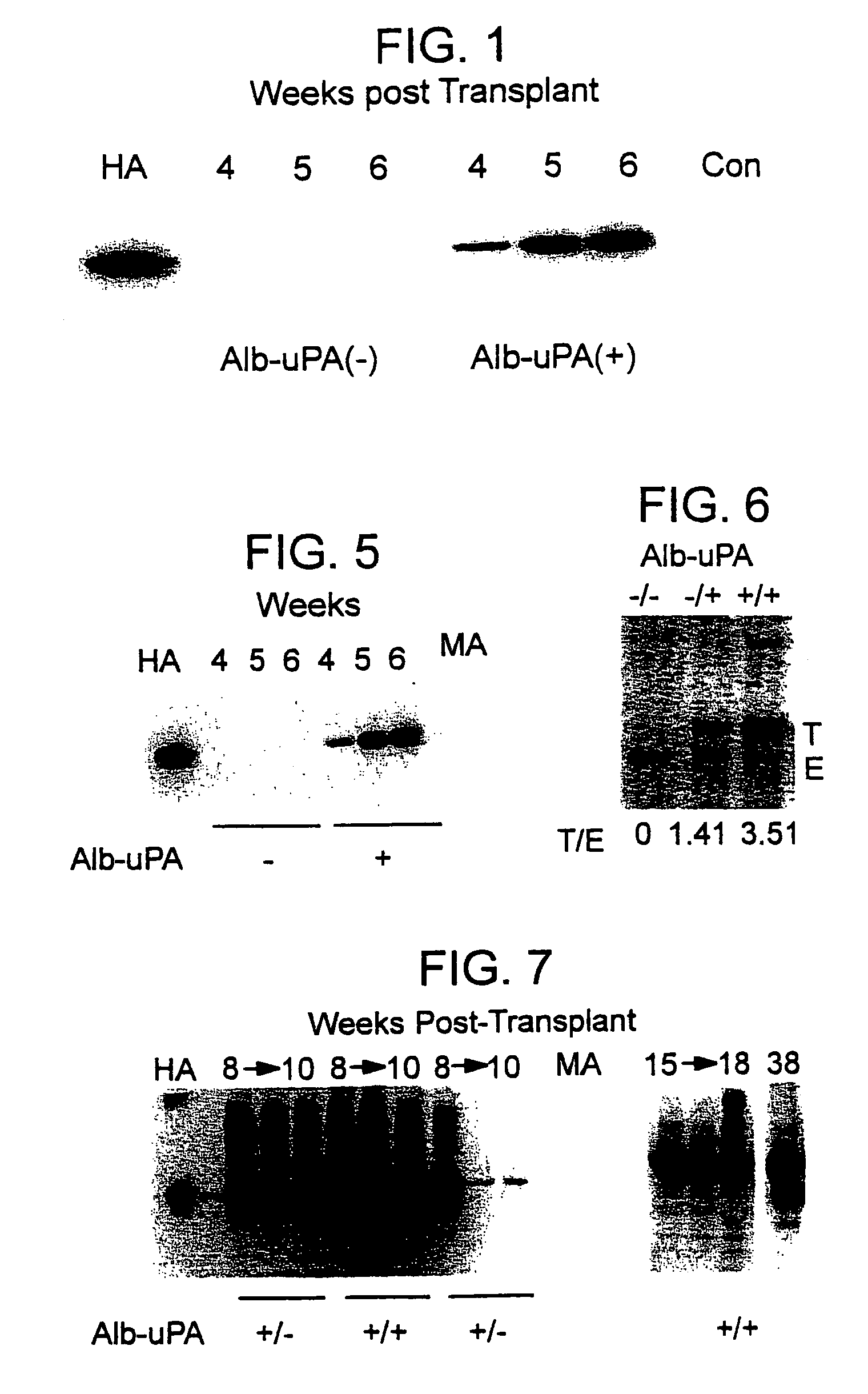
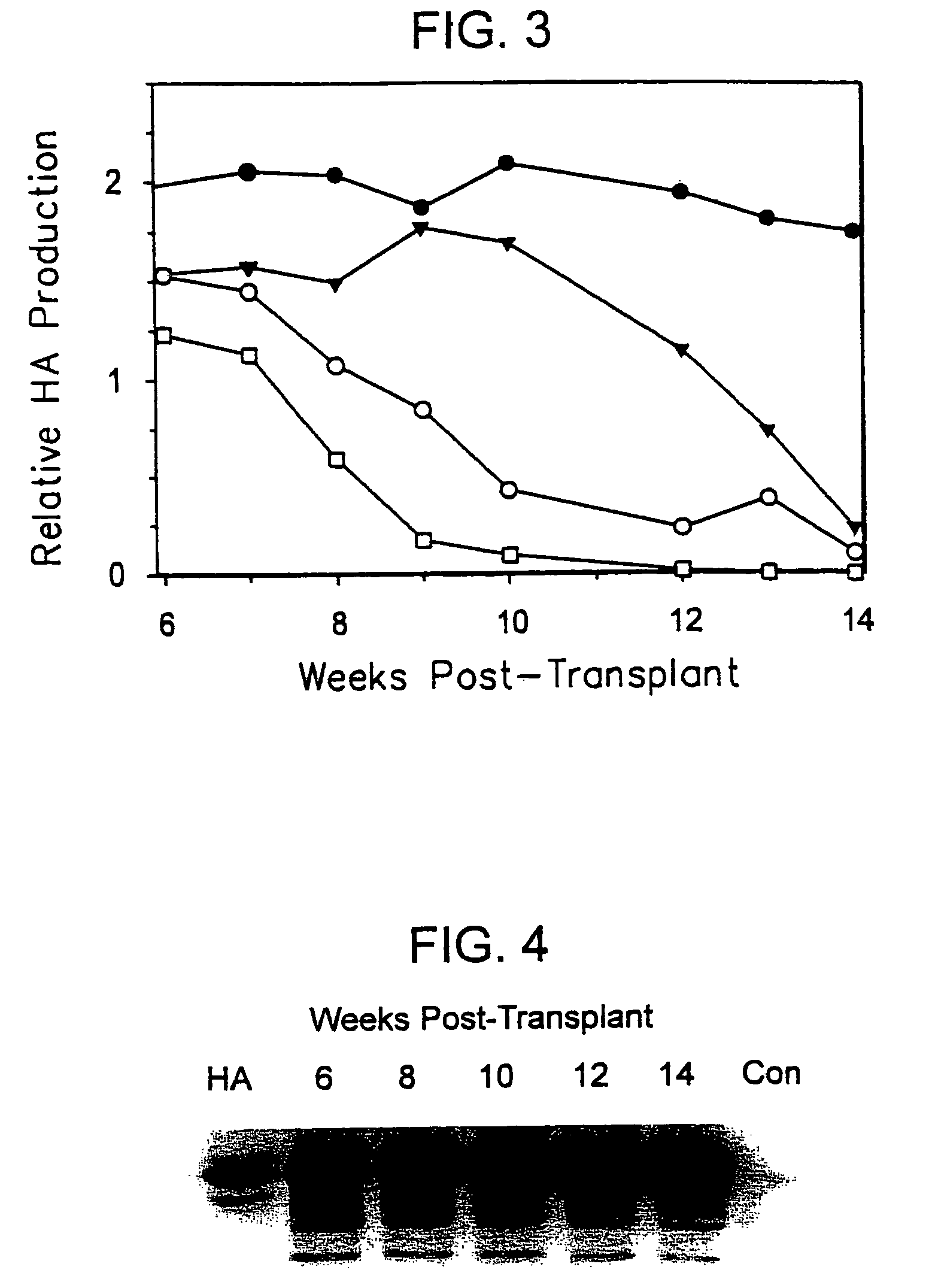
Animal model having a chimeric human liver
The present invention features a non-human animal model that is susceptible to infection by human hepatotrophic pathogens, particularly human hepatitis C virus (HCV). The model is based on a non-human, immunocompromised transgenic animal having a human-mouse chimeric liver, where the transgene provides for expression of a urokinase-type plasminogen activator in the liver. The invention also features methods for identifying candidate therapeutic agents, e.g., agents having antiviral activity against HCV infection. The animals of the invention are also useful in assessing toxicity of various agents, as well as the activity of agents in decreasing blood lipids.
Owner:KMT HEPATECH
Plasminogen activator variant formulations
InactiveUS20070014779A1Good curative effectIncrease concentrationPeptide/protein ingredientsRespiratory disorderThrombusSterile water
A solution is provided comprising about 0.01-0.05 mg / mL of tenecteplase in sterile water for injection or bacteriostatic water for injection and normal saline. Such solution is useful for delivery from a catheter and for treating a thrombotic disorder by exposing fibrin-rich fluid from the disorder to an effective amount thereof, as well as in kits. In a preferred embodiment, peripheral thrombosis is treated in a mammal comprising delivering to the mammal via a catheter an effective amount of this solution.
Owner:GENENTECH INC
Method and composition for treating prostate cancer
A method of treating prostate cancer in a living mammal includes local administration of a composition that includes a therapeutically effective concentration of collagenase. In one embodiment, a method of treating prostate cancer in a living mammal includes local administration of a composition that includes a therapeutically effective concentration of collagenase and at least one of a glycosidase, a protease, a nuclease, a lipase, an esterase, a plasminogen activator, a streptokinase, and combinations thereof. Preferably a glycosidase, such as, for example, hyaluronidase, is administered. Compositions used in methods for treating prostate cancer can also include or be administered with calcium ions, a nonionic surfactant, such as, for example, Triton® X-100, and an antibiotic, such as, for example, gentamicin. Another method of treating prostate cancer in a living mammal includes activating PSA in vivo by, for example, locally administering calcium ions.
Owner:IMMUNOLYTICS
Inhibitors of urokinase and blood vessel formation
InactiveUS6432922B1Positive maintenanceInhibition effectSenses disorderAntipyreticPLG - PlasminogenArginine
Novel compounds having activity inhibitors of urokinase and in reducing or inhibiting blood vessel formation are provided. These compounds have an arginine or arginine mimic aldehyde or an arginine ketoamide group at P1. These compounds are useful in vitro for monitoring plasminogen activator levels and in vivo in treatment of conditions which are ameliorated by inhibition of or decreased activity of urokinase and in treating pathologic conditions wherein blood vessel formation is related to a pathologic condition.
Owner:DENDREON PHARMA INC
Fusion protein of urokinase type plasminogen activator a chain and melittin and preparation thereof
InactiveCN101337992AAvoid stickingInhibit migrationFungiPeptide/protein ingredientsMelittinProtein C
The invention provides a fusion protein formed by the combination of urokinase-type plasminogen activator a chain and melittin, the preparation method thereof, and the application of the fusion protein in tumor treatment.
Owner:吉林圣元科技有限责任公司
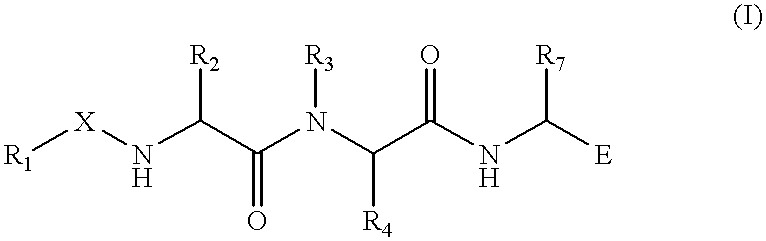
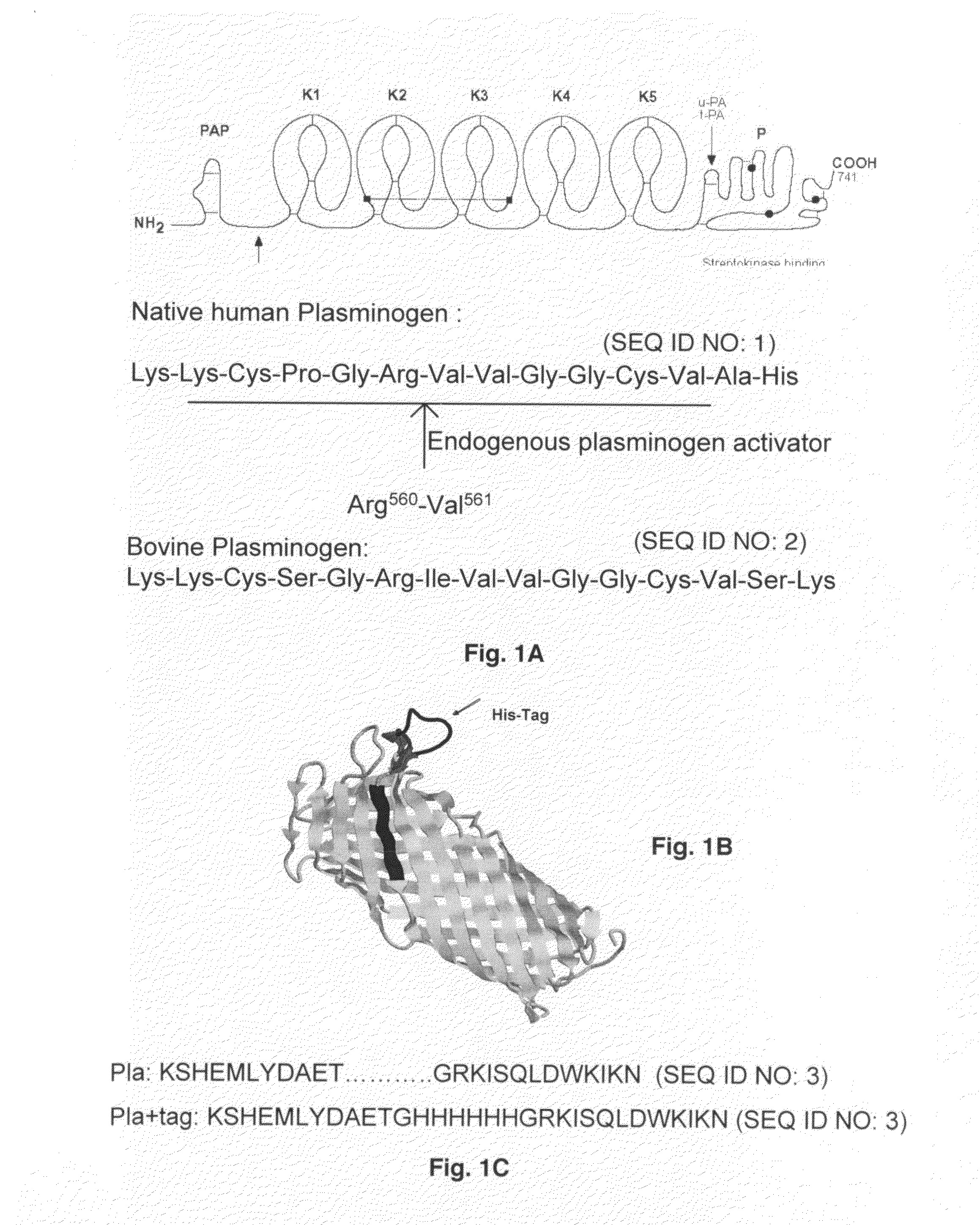
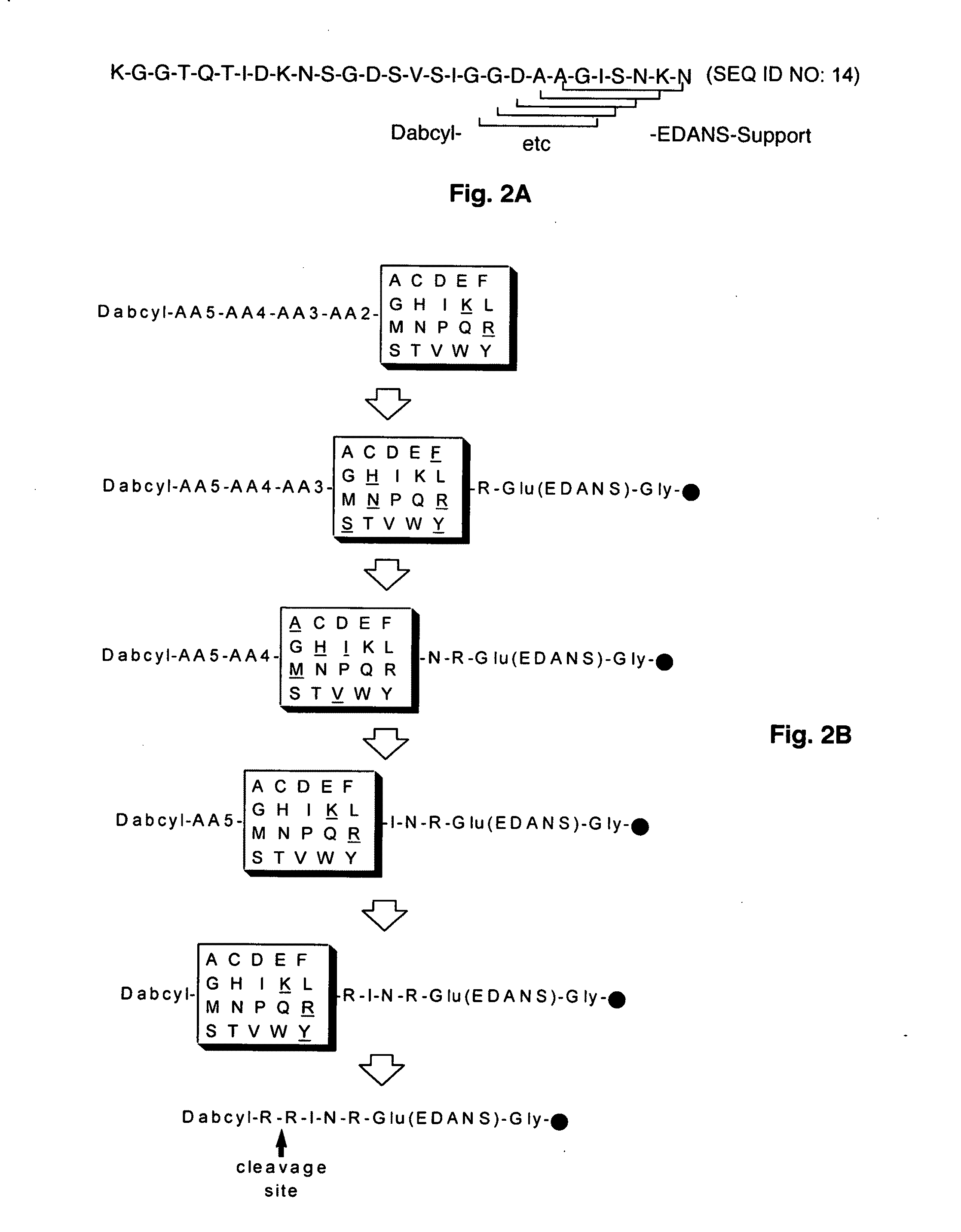
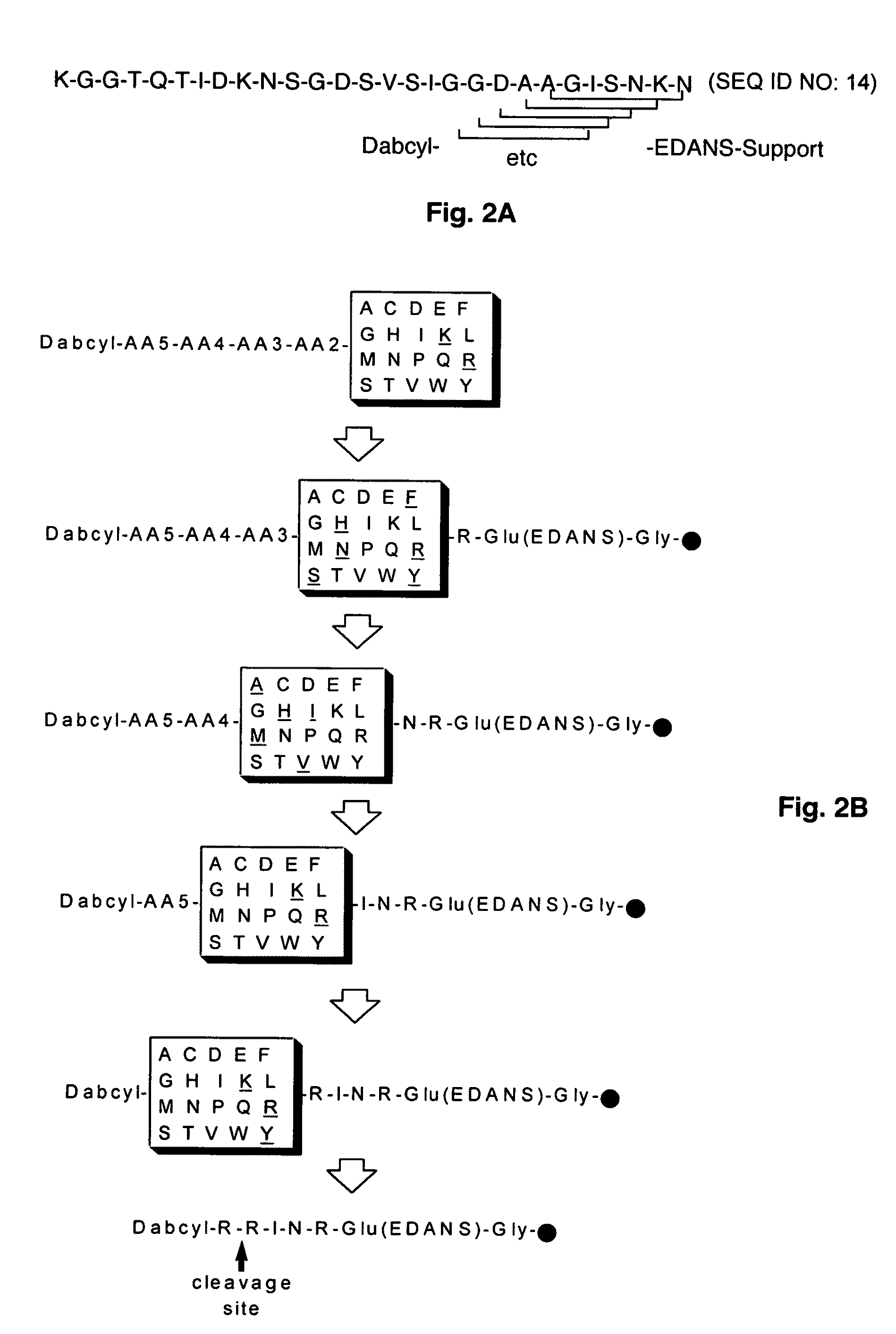
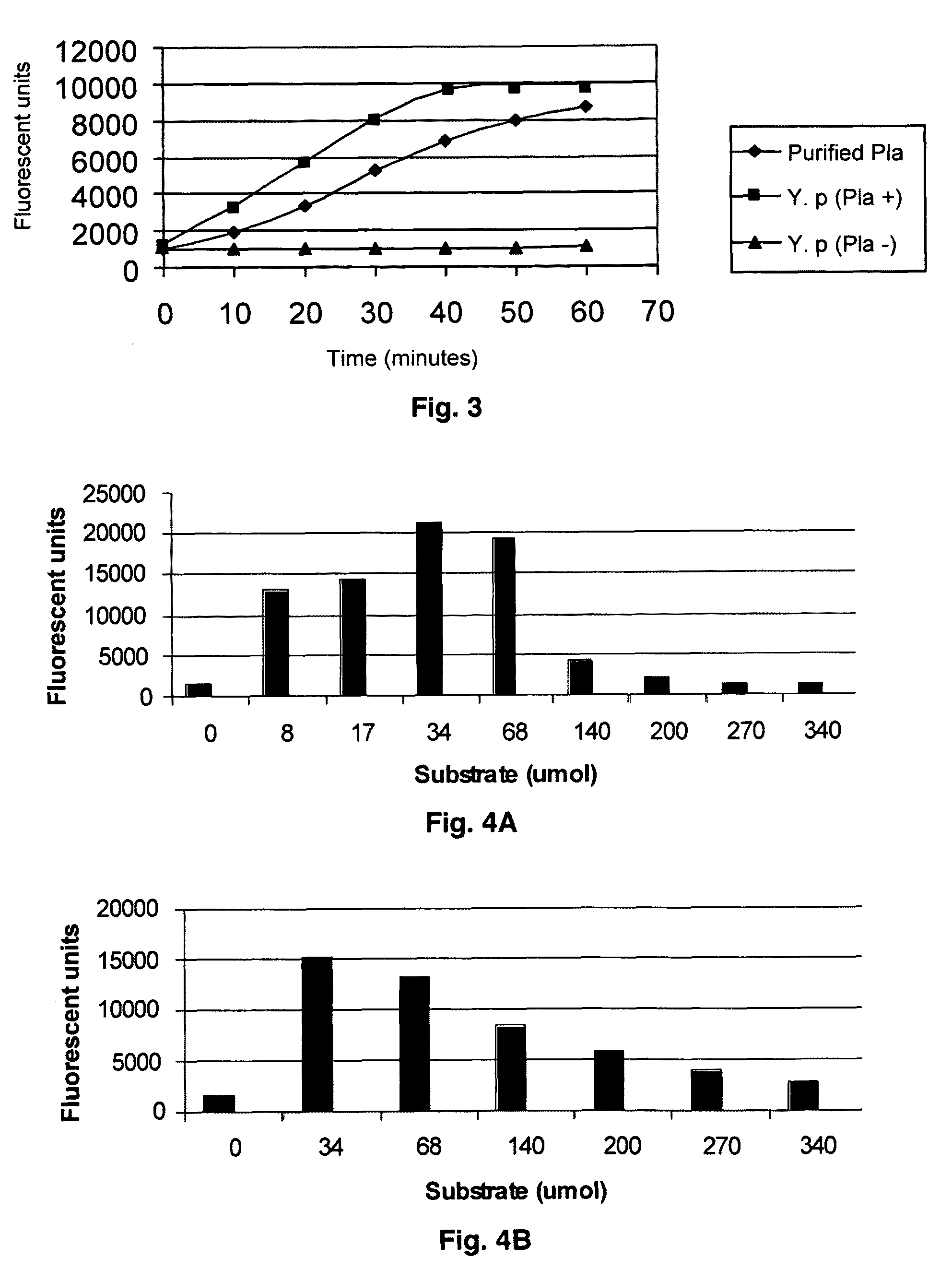
Substrate peptide sequences for plague plasminogen activator and uses thereof
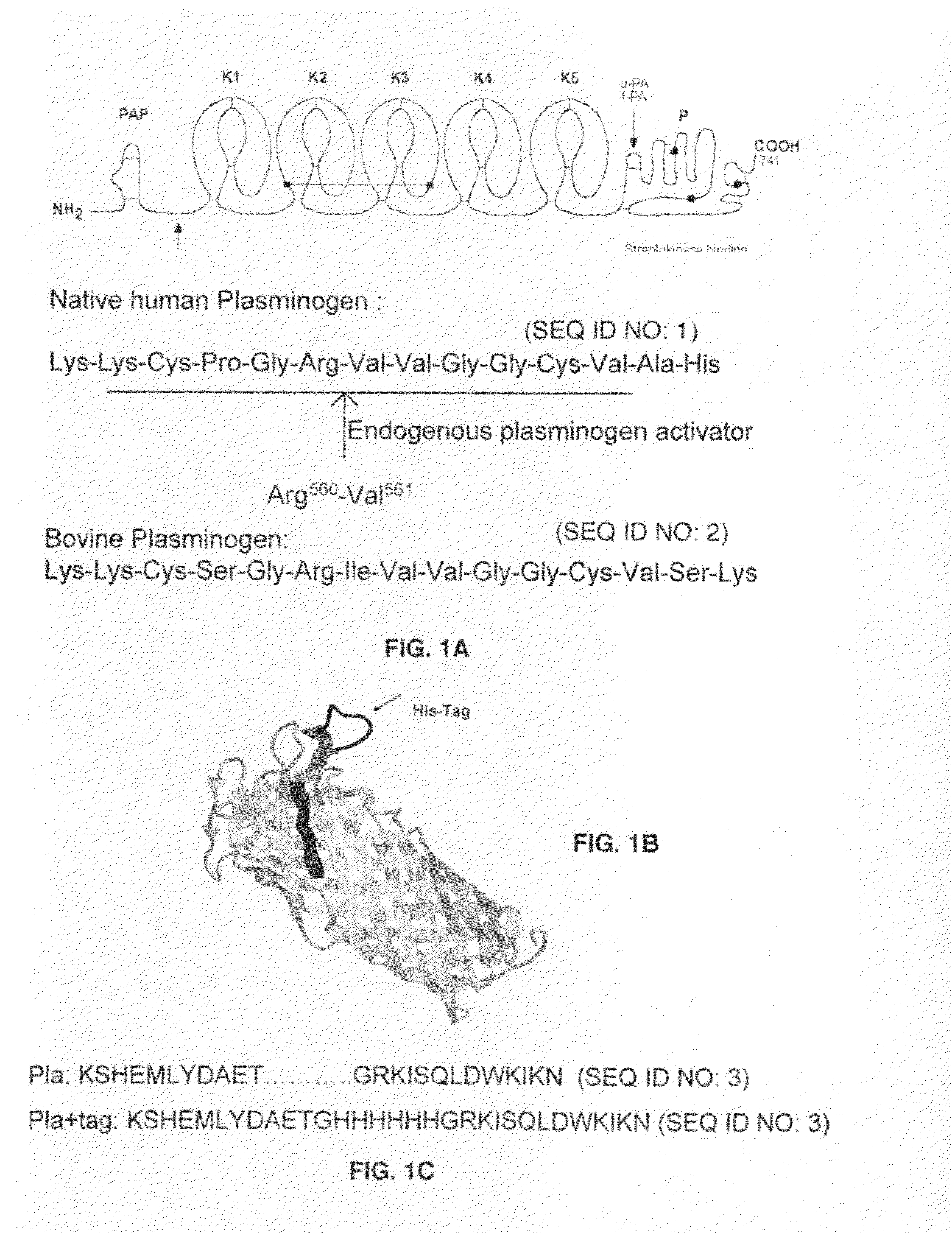
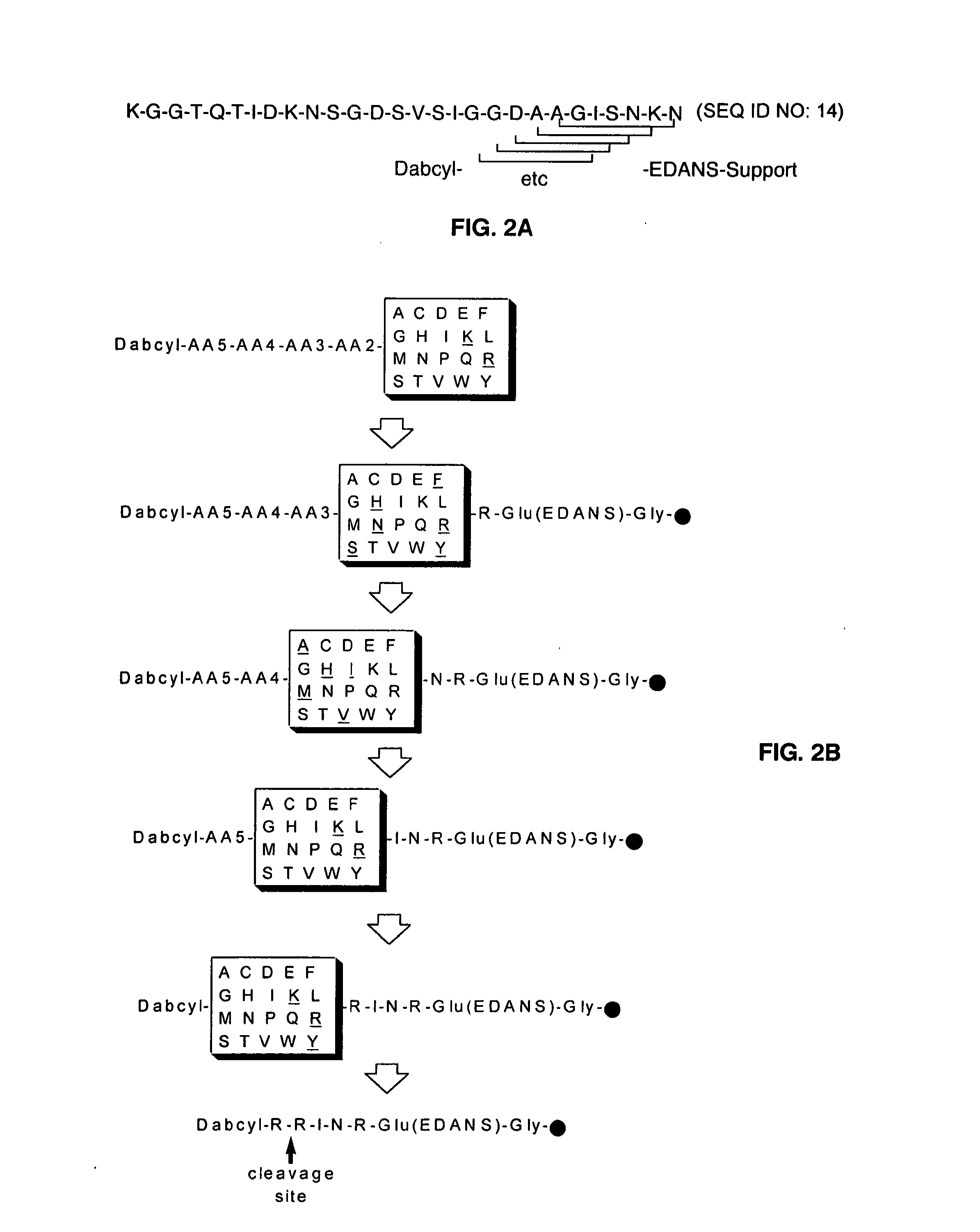
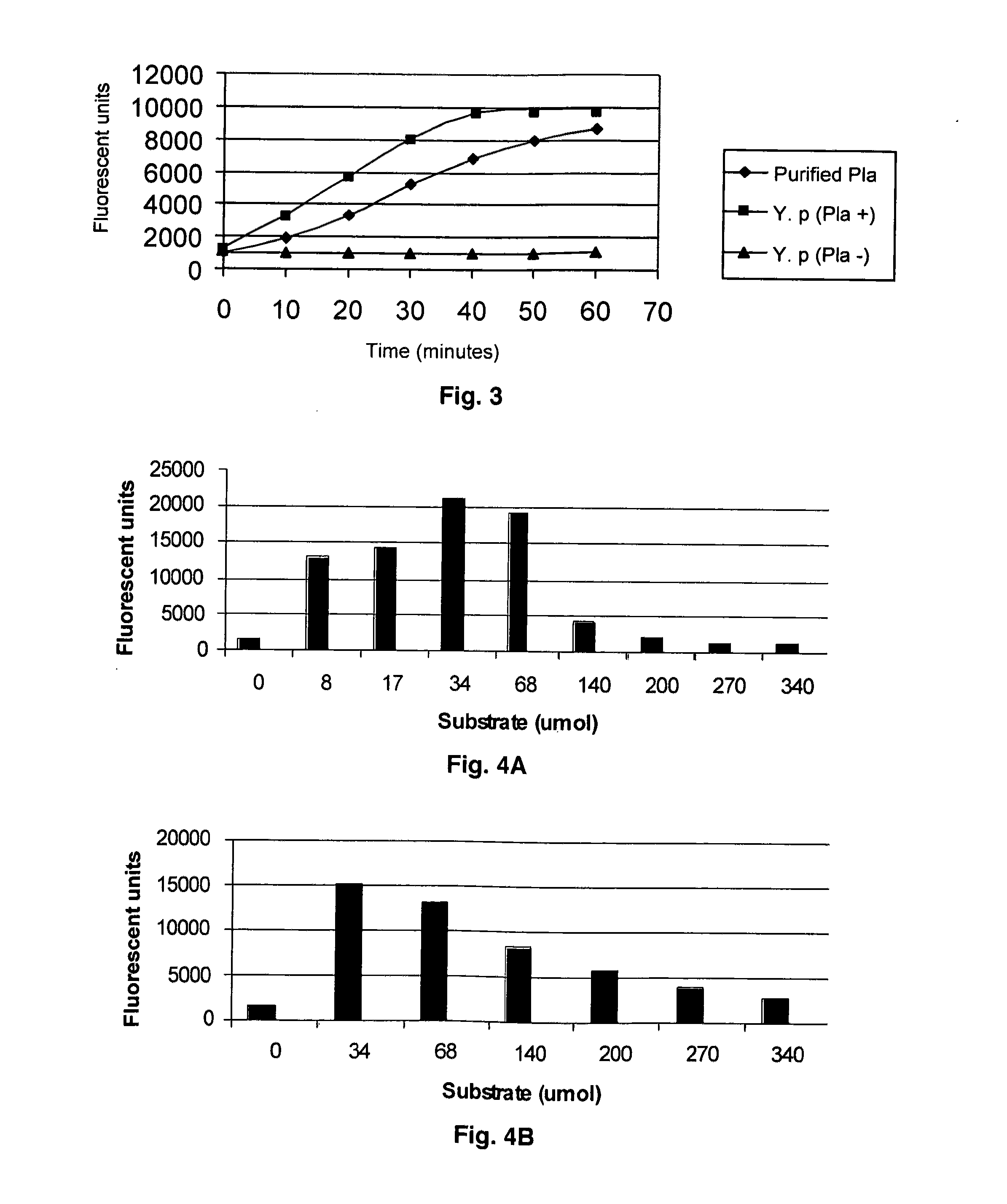
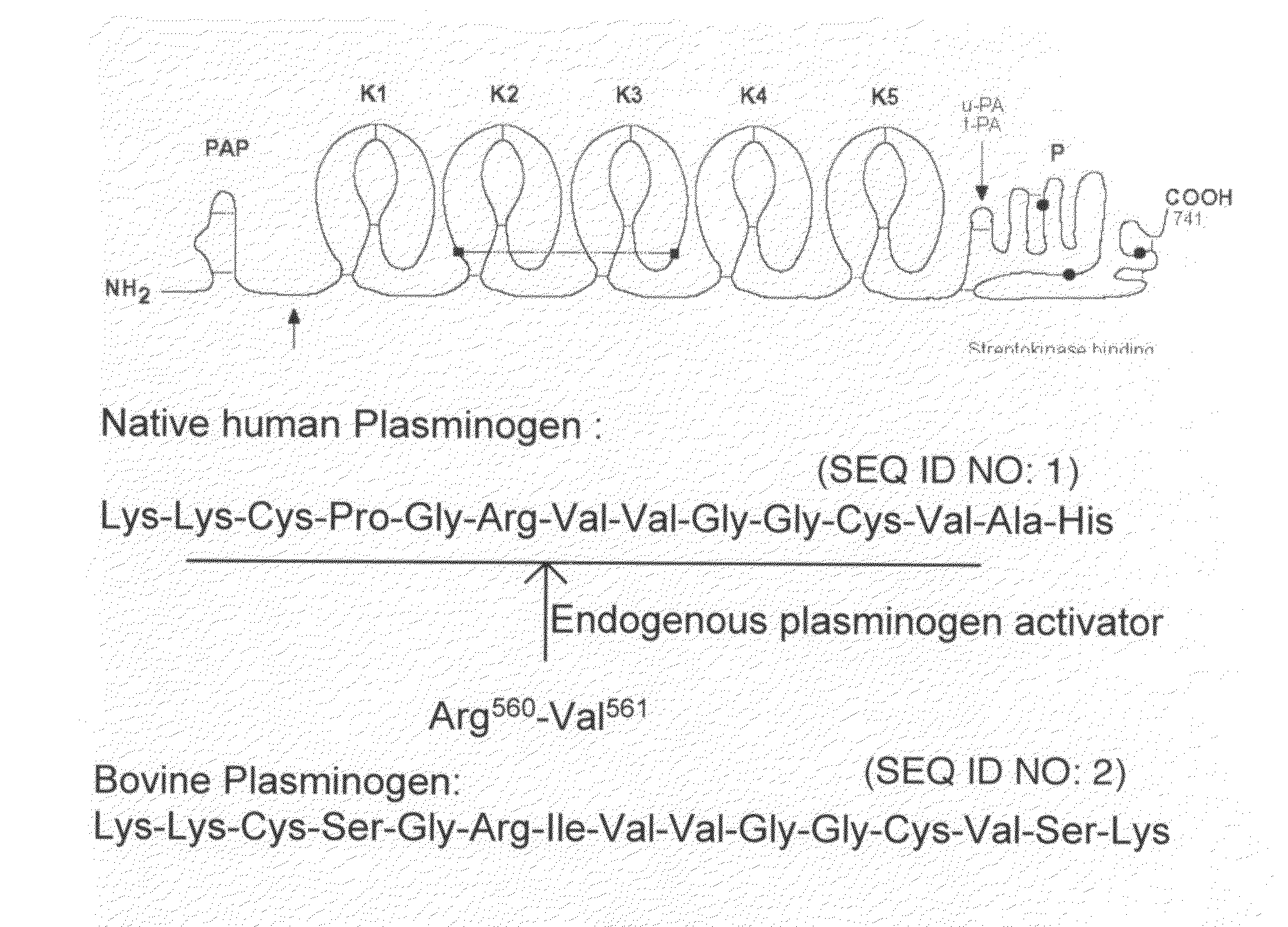
The present invention is directed to peptide sequences that were identified from combinatorial libraries and could serve as substrates of plague plasminogen activator (Pla). Another aspect of the present invention is drawn to peptides derived from the substrates for Pla as a result of chemical modifications leading to specific inactivation of the proteolytic activity of Pla. Additionally, the present invention is directed to the use of the substrates identified herein in the detection of bacteria expressing omptin family of proteases which includes Y. pestis. Furthermore, the present invention is also directed to the use of the inhibitors identified herein in the prevention and treatment of infection caused by these bacteria.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Substrate peptide sequences for plague plasminogen activator and uses thereof
ActiveUS20090069248A1Antibacterial agentsMicrobiological testing/measurementEgg proteinProteinase activity
The present invention is directed to peptide sequences that were identified from combinatorial libraries and could serve as substrates of plague plasminogen activator (Pla). Another aspect of the present invention is drawn to peptides derived from the substrates for Pla as a result of chemical modifications leading to specific inactivation of the proteolytic activity of Pla. Additionally, the present invention is directed to the use of the substrates identified herein in the detection of bacteria expressing omptin family of proteases which includes Y. pestis. Furthermore, the present invention is also directed to the use of the inhibitors identified herein in the prevention and treatment of infection caused by these bacteria.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
High-performance environment-friendly shrimp fodder and preparation method thereof
The invention discloses high-performance environment-friendly shrimp fodder and a preparation method thereof. The fodder for sea shrimps and freshwater shrimps is prepared by substituting raw materials, such as earthworm powder, snail powder, fly larvae powder, yeast, and the like for protein raw materials, such as fish meal, meat, bone meal, and the like, substituting peanut powder for soybean meal, substituting minor cereal tapioca for staple food grain, such as corn, wheat, and the like, and substituting the mechanism effects of peptide substances in maggot powder, enzymes of earthworm powder, enzymes of a multifunctional plasminogen activator, microbial probiotics flora, pathogenic bacteria inhibitor, and the like for chemical growth hormones and chemical medical antibiotics. The high-performance environment-friendly shrimp fodder is characterized by being prepared from the following raw materials by weight parts: 30-50 parts of virus-free cassava, 10-20 parts of earthworm powder, 5-20 parts of snail powder, 5-15 parts of fly larvae powder, 6-20 parts of yeast, 5-10 parts of peanut powder, 1-4 parts of garlic powder, 1-4 parts of mineral trace elements, 2-5 parts of amino acids, 0.5-2 parts of lecithin, 0.5-2 parts of vitamins, 0.5-3 parts of multifunctional plasminogen activator and 0.5-3 parts of microbial probiotics. The invention has the advantages of improved fodder quality, decreased production cost, improved safety rate of culture food, and the like.
Owner:郭伟 +2
Compositions and techniques for localized therapy
InactiveUS7947299B2Little effectOrganic active ingredientsSurgical adhesivesFibrinogenLocalized Therapy
Owner:BOSTON SCI SCIMED INC
Title inhibitors of urokinase
InactiveUS6576613B1Promote cell adhesionImprove cell adhesionSenses disorderDipeptide ingredientsZymogenPLG - Plasminogen
Novel inhibitors of urokinase are provided which have an arginine or arginine mimic aldehyde or an arginine ketoamide group at P1. These compounds are useful in vitro for monitoring plasminogen activator levels and in vivo in treatment of conditions which are ameliorated by inhibition of or decreased activity of urokinase.
Owner:DENDREON PHARMA INC
Substrate peptide sequences for plague plasminogen activator and uses thereof
ActiveUS8030447B2Antibacterial agentsMicrobiological testing/measurementProteinase activityPeptide sequence
The present invention is directed to peptide sequences that were identified from combinatorial libraries and could serve as substrates of plague plasminogen activator (Pla). Another aspect of the present invention is drawn to peptides derived from the substrates for Pla as a result of chemical modifications leading to specific inactivation of the proteolytic activity of Pla. Additionally, the present invention is directed to the use of the substrates identified herein in the detection of bacteria expressing omptin family of proteases which includes Y. pestis. Furthermore, the present invention is also directed to the use of the inhibitors identified herein in the prevention and treatment of infection caused by these bacteria.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
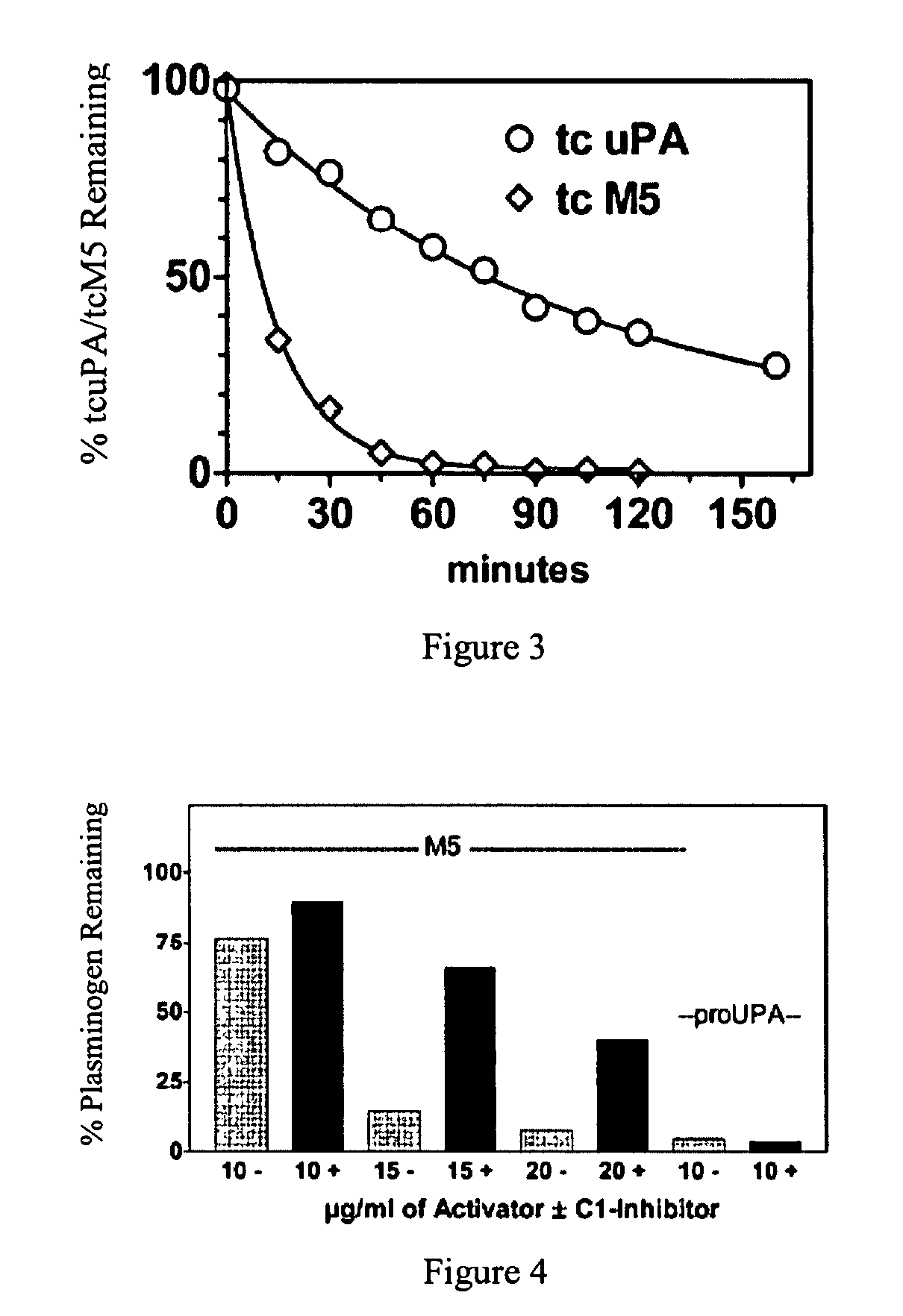
C-1 inhibitor prevents non-specific plasminogen activation by a prourokinase mutant without impeding fibrin-specific fibrinolysis
ActiveUS7837992B2Attenuation of rateHigh dose tolerancePeptide/protein ingredientsBlood disorderC1-inhibitorHigh doses
Owner:THROMBOLYTIC SCI +1
Animal model having a chimeric human liver
The present invention features a non-human animal model that is susceptible to infection by human hepatotrophic pathogens, particularly human hepatitis C virus (HCV). The model is based on a non-human, immunocompromised transgenic animal having a human-mouse chimeric liver, where the transgene provides for expression of a urokinase-type plasminogen activator in the liver. The invention also features methods for identifying candidate therapeutic agents, e.g., agents having antiviral activity against HCV infection. The animals of the invention are also useful in assessing toxicity of various agents, as well as the activity of agents in decreasing blood lipids.
Owner:KMT HEPATECH
Catheter Composition and Uses Thereof
InactiveUS20060257390A1Growth inhibitionPreserve stability and functionPeptide/protein ingredientsMedical devicesAlcoholMedicine
A composition useful for removal of fibrin-bound blood clots from a catheter comprises water, a fibrinolytically effective amount of a plasminogen activator, and a preservatively effective amount of a bacteriostatic organic alcohol. The composition does not comprise a chelating agent.
Owner:GENENTECH INC
Compositions and techniques for localized therapy
Owner:BOSTON SCI SCIMED INC
High-performance environment-friendly cattle feed and preparation method thereof
The invention discloses a high-performance environment-friendly cattle feed and a preparation method thereof. Raw materials such as earthworm powder, snail powder, maggot powder, yeast and the like are used for replacing protein raw materials such as fish meal, meat and bone meal and the like, peanut meal is used for replacing soya bean meal, coarse grain cassava meal is used for replacing staple food grains such as corn, wheat and the like, and polypeptide substances in the maggot powder, the enzymes of the earthworm powder, the complex enzymes of multifunctional plasminogen activators and the flora of microbial probiotics are used for inhibiting mechanism effects of pathogenic bacteria and the like to replace chemical growth hormones and chemical medical antibiotics, thereby preparing the feed of dairy cattle and beef cattle. The feed is characterized by being prepared from the following multiple raw materials in parts by weight: 30-60 parts of non-toxic cassava meal, 5-15 parts of earthworm powder, 5-20 parts of snail powder, 2-10 parts of maggot powder, 6-20 parts of yeast powder, 5-10 parts of peanut meal, 1-4 parts of garlic powder, 1-4 parts of mineral trace elements, 2-5 parts of multiple amino acids, 0.5-2 parts of lecithin, 0.5-2 parts of multiple vitamins, 0.5-3 parts of multifunctional plasminogen activators and 0.5-3 parts of microbial probiotics. The invention has the advantages of improving the quality of the feed, reducing the production cost, enhancing the safety probability of cultured foods, and the like.
Owner:郭伟 +2
Mice transplanted with human hepatocytes
Disclosed is a mouse having human hepatocytes transplanted therein. Specifically disclosed is a mouse having human hepatocytes transplanted therein. In the mouse, a foreign thymidine kinase gene or an urokinase-type plasminogen activator gene is retained so that the gene can be expressed specifically in the liver of the mouse, and hepatocytes of the mouse are substituted by human hepatocytes.
Owner:CENT INST FOR EXPERIMENTAL ANIMALS +1
Mutants of streptokinase and their covalently modified forms
ActiveUS20100034804A1Proteolytic stability is enhancedExtended plasma elimination half-lifeBacteriaPeptide/protein ingredientsFluorescenceHalf-life
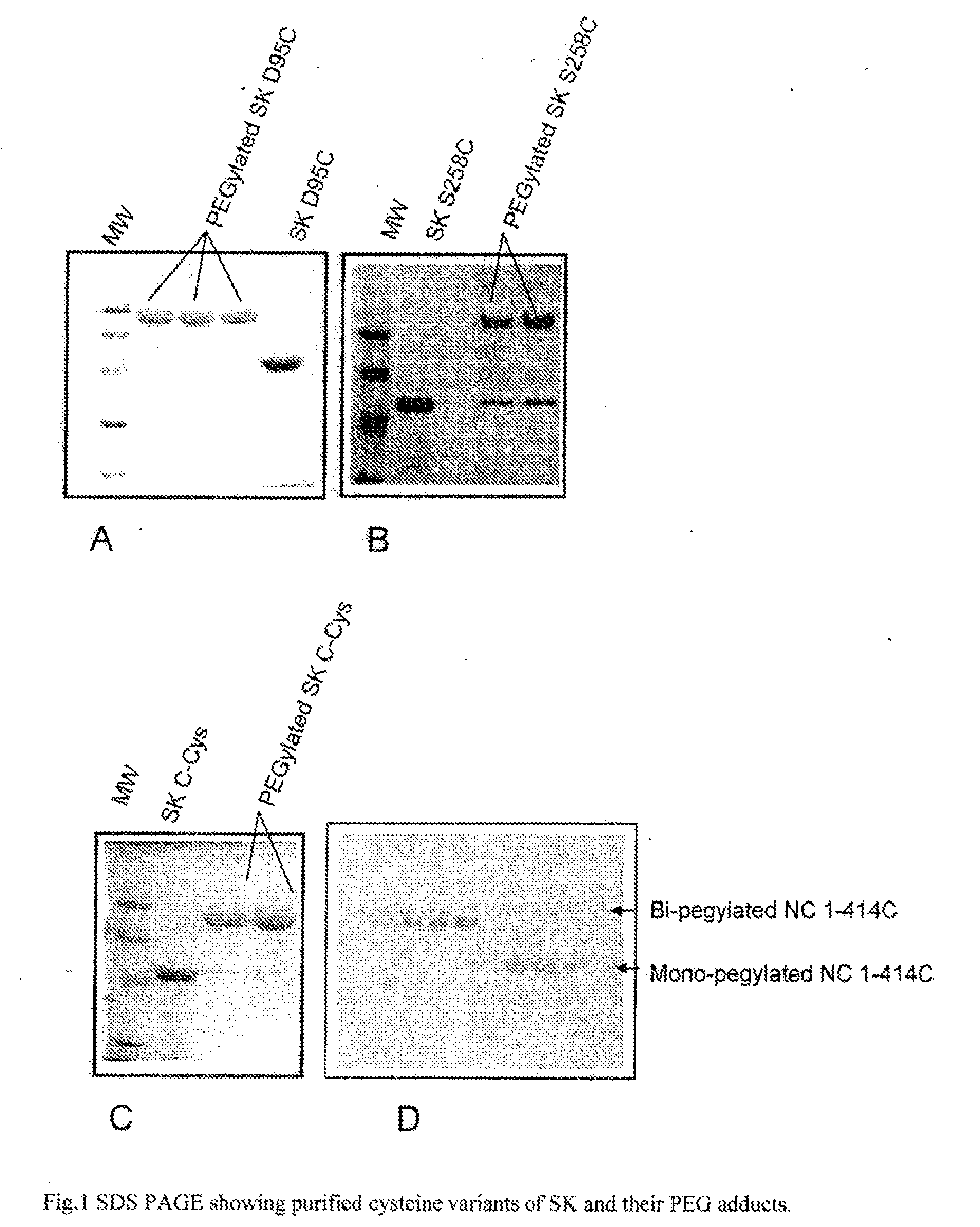
The present invention relates to novel mutants of Streptokinase, its functional fragments and covalently modified forms. Methods are provided for the preparation of the bacterial plasminogen activator protein, Streptokinase its muteins, species variants and their covalently modified variants that are characterized by improved therapeutic properties, such as increased proteolytic stability, extended plasma half-lives, reduced immuno-reactivity and enhanced fibrin clot specificity. The method involves either incorporating additional cysteine residues, or substituting cysteine residues for naturally occurring amino acids into non-essential regions of the protein such that the catalytic activity of the resultant protein remains largely unaltered. These cysteine variants were further modified by covalently attaching a cysteine reactive polymer such as polyethylene glycol (PEG) or sulfhydryl-reactive moieties from a group that includes fluorophore, spin labels or other small conjugates. Disclosed herein are site-specific biologically active conjugates of Streptokinases and its covalently modified variants.
Owner:COUNCIL OF SCI & IND RES
Recombinant tissue-type plasminogen activator, and preparation method and use thereof
ActiveCN103159860AHigh expressionImprove stabilityPeptide/protein ingredientsSurgeryHalf-lifeTissues types
The invention discloses a recombinant TNK-tPA-Fc fusion protein, and a preparation method and application thereof. The protein orderly contains human TNK-tPA, a flexible peptide joint and human IgG natural or variant Fc. The fusion protein has invitro and invivo biological activity similar to human TNK-tPA, and greatly prolonged plasma half-life.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
High-performance environment protective ostrich feed and preparation method thereof
InactiveCN101606638AImprove qualityLow costAnimal feeding stuffAccessory food factorsAnimal scienceHousefly
The invention discloses high-performance environment protective ostrich feed and a preparation method thereof. Earthworm meal, snail meal, housefly larva meal and yeast and other materials replace fish meal, meat and bone meal and other protein materials, peanut meal replaces soybean meal, side crops, such as cassava meal, replaces grain staple food, such as corn, wheat and the like, the polypeptide substance in the housefly larva meal, enzyme in the snail meal, enzyme complex in the multifunctional plasminogen activator, the flora of microbial probiotics, which restrain pathogenic bacteria and have other mechanism, replace chemical growth hormone and chemical drug antibiotic, and the raw materials are prepared into feed for adult ostrich and young ostrich. The feed is characterized in that the feed comprises the following raw materials according to part by weight: 30 to 60 parts of detoxicated cassava meal, 5 to 15 parts of earthworm meal, 5 to 20 parts of snail meal, 2 to 10 parts of housefly larva meal, 6 to 20 parts of yeast powder, 5 to 10 parts of peanut meal, 1 to 4 parts of garlic meal, 1 to 4 parts of mineral trace element, 2 to 5 parts of various amino acids, 0.5 to 2 parts of lecithin, 0.5 to 2 parts of various vitamins, 0.5 to 3 parts of multifunctional plasminogen activator, and 0.5 to 3 parts of microbial probiotics. The invention has the advantages that the quality of the feed is improved, the production cost is reduced, and the safety probability of the cultivated food is enhanced.
Owner:郭伟 +2
Ligands binding the complex of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) that inhibit downstream uPAR interactions: identification and use in diagnosis or therapy
Antibodies or other ligands specific for the binary uPA-uPAR complexes, for ternary complexes comprising uPA-uPAR and for complexes of uPAR and proteins other than uPA such as integrins inhibit the interaction of uPA and uPAR with additional molecules with which the complexed interact. Such antibodies or other ligands are used in diagnostic and therapeutic methods, particularly against cancer.
Owner:ATTENUON LLC
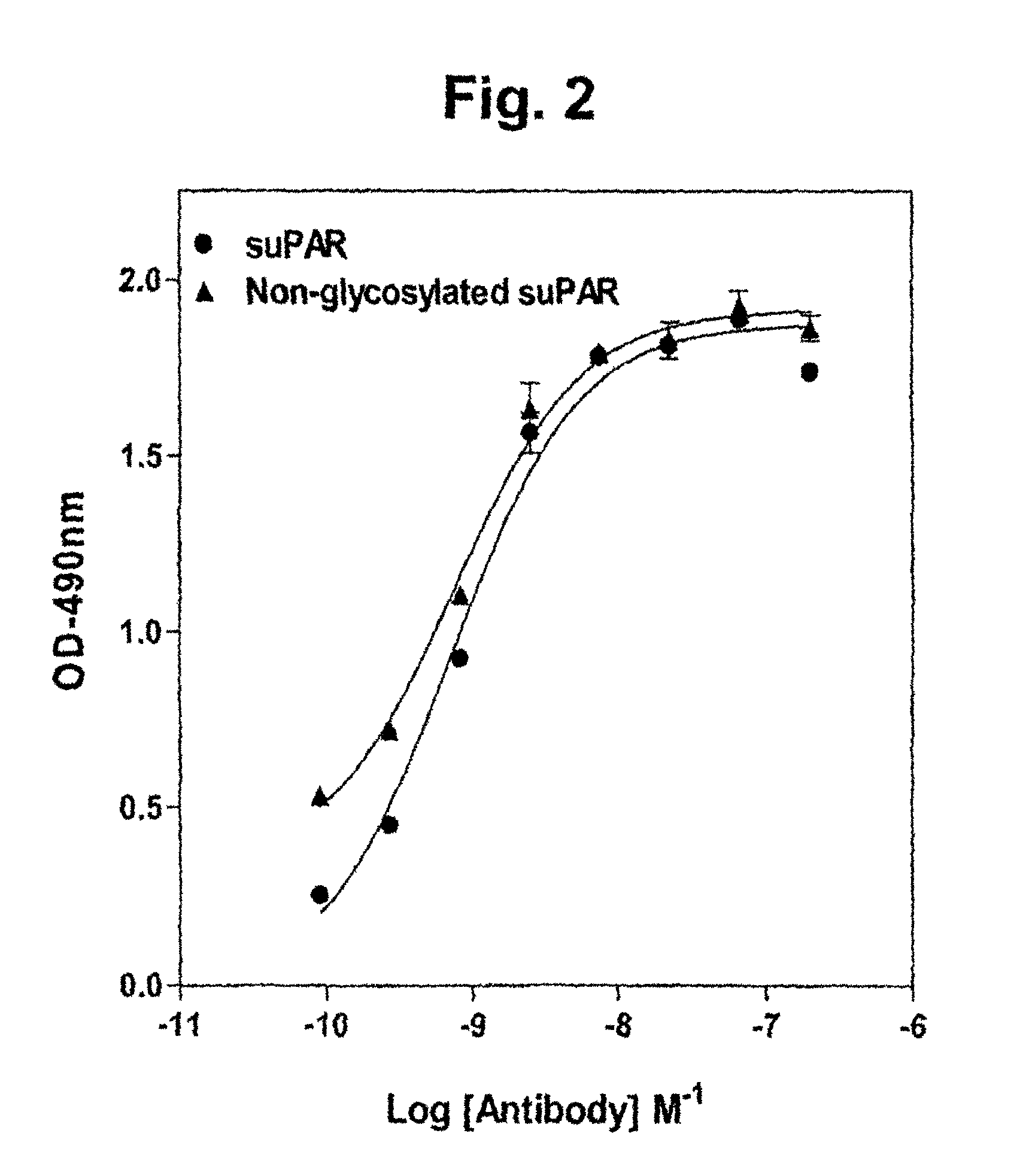
Antibodies that bind urokinase plasminogen activator
InactiveUS9908944B2Inhibitory activityCompound screeningApoptosis detectionUrokinase Plasminogen ActivatorAntibody
The present invention relates to a method of identifying urokinase plasminogen activator (uPA) antibodies. The invention also relates to antibodies that are capable of binding uPA and which are capable of reducing or inhibiting uPA activity. Furthermore, the invention relates to uses for such antibodies, such as therapeutic and pharmaceutical uses.
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com