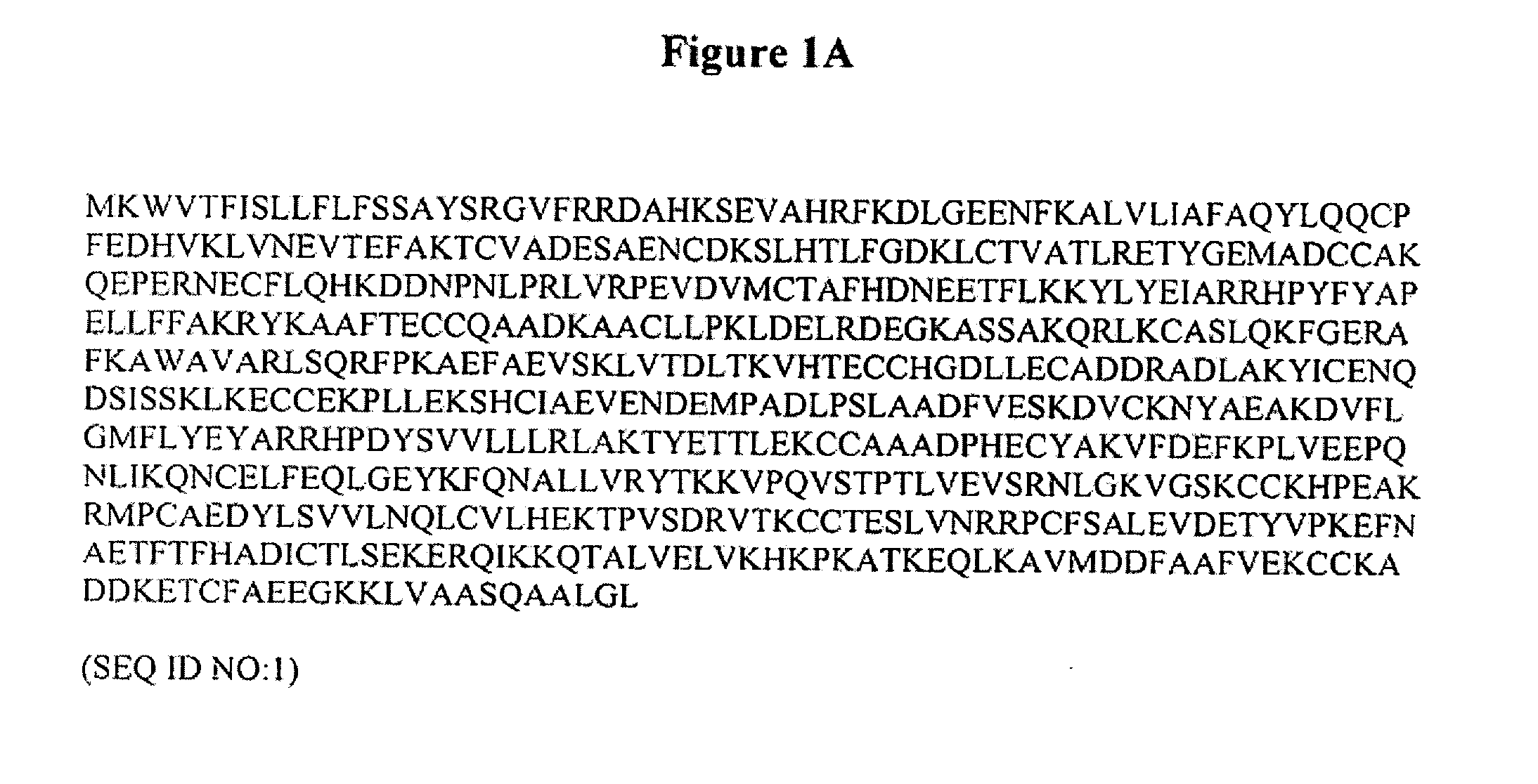
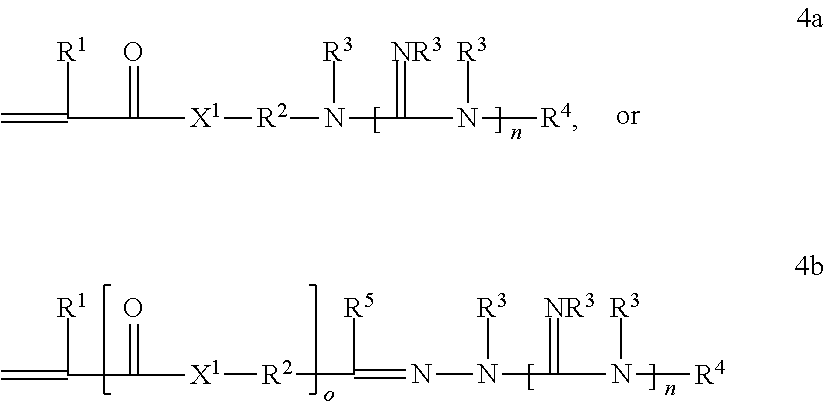
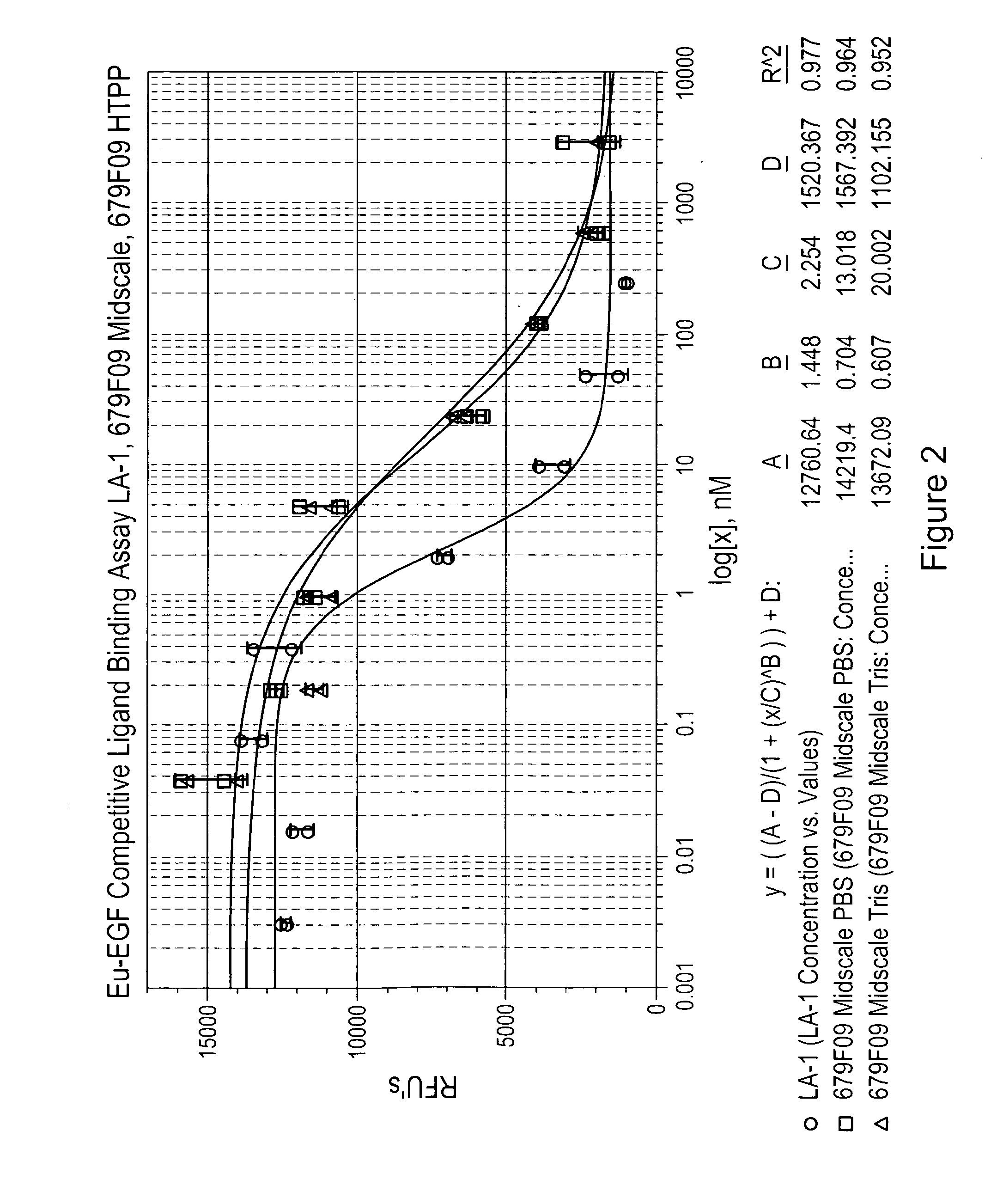
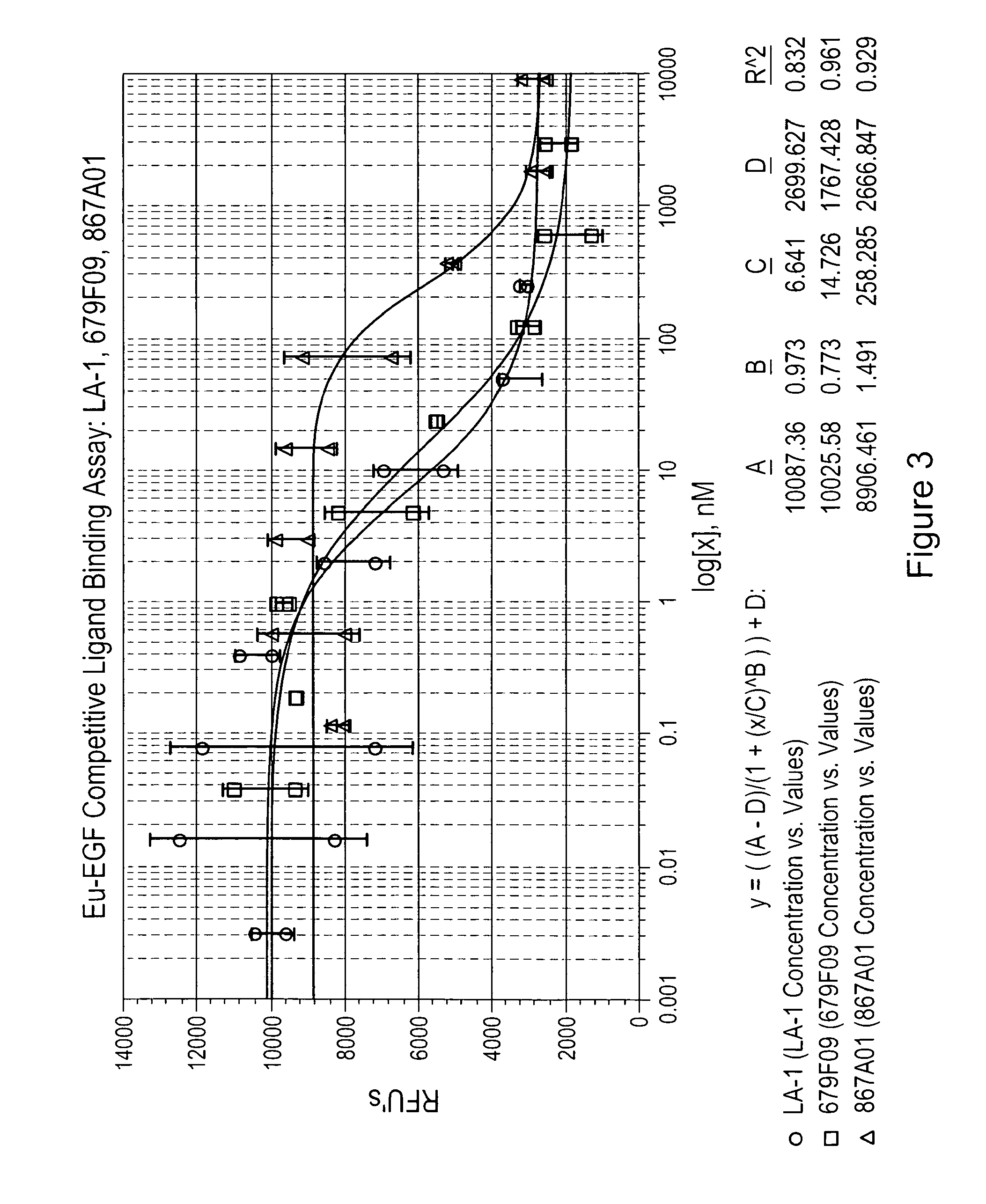
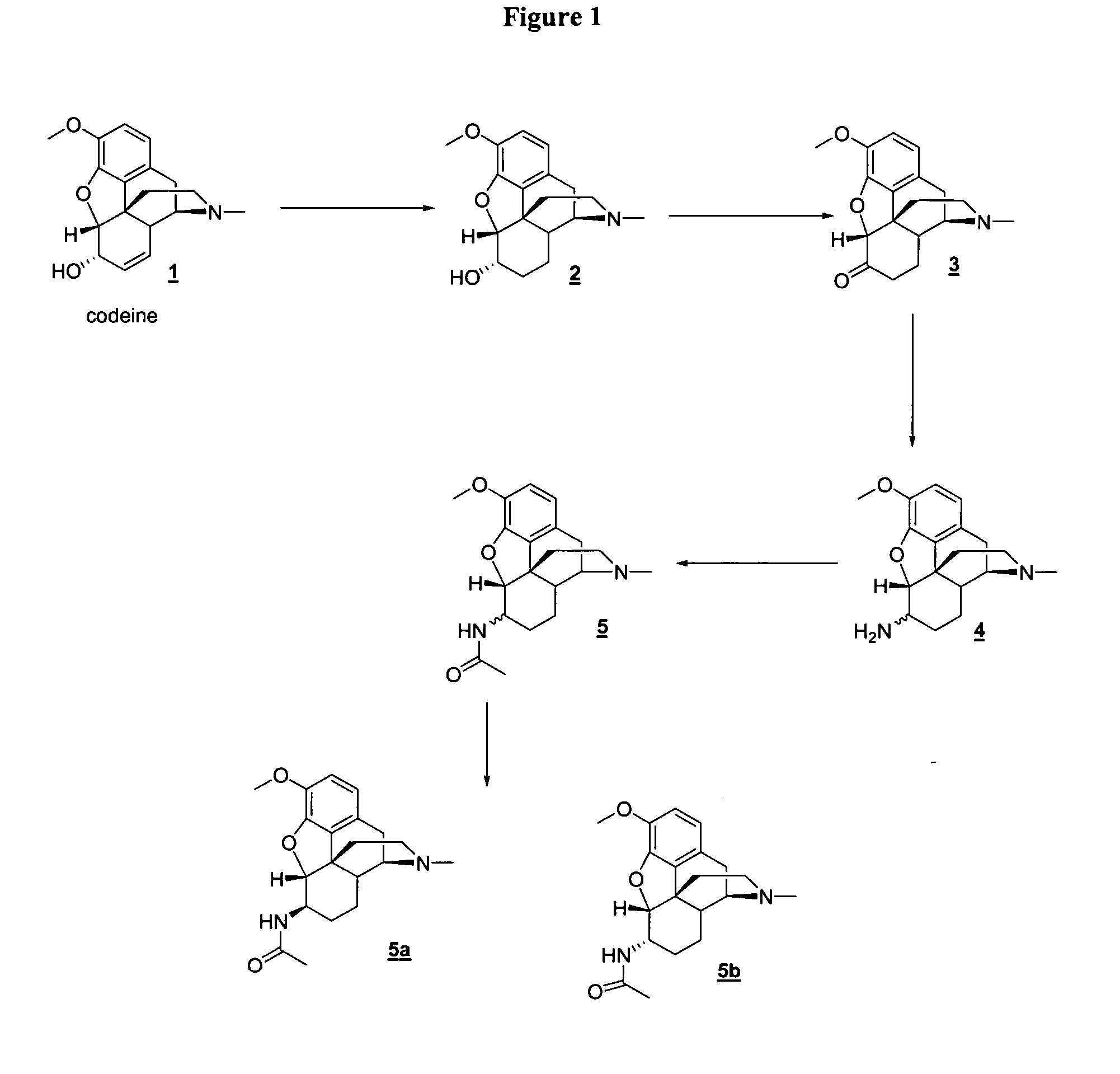
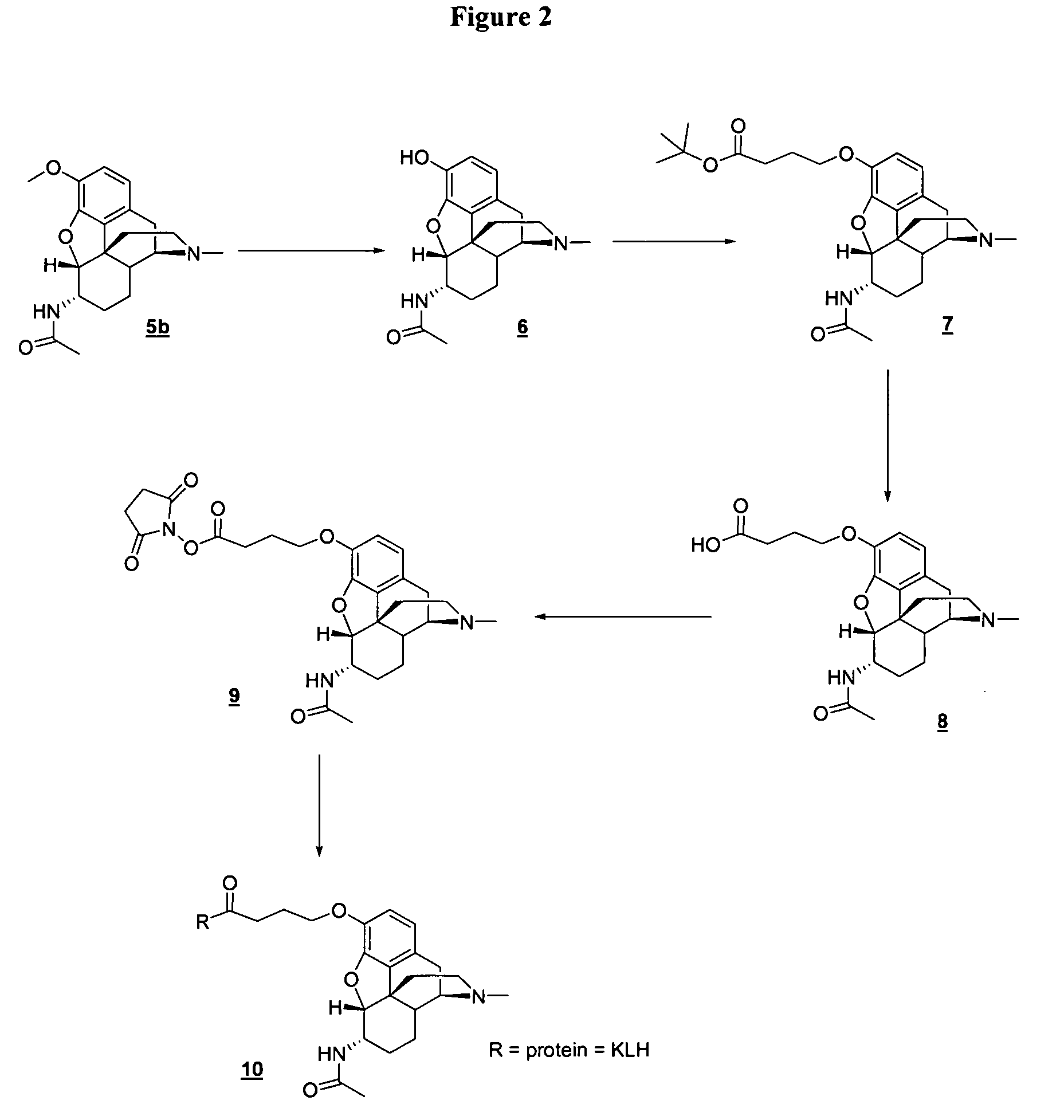
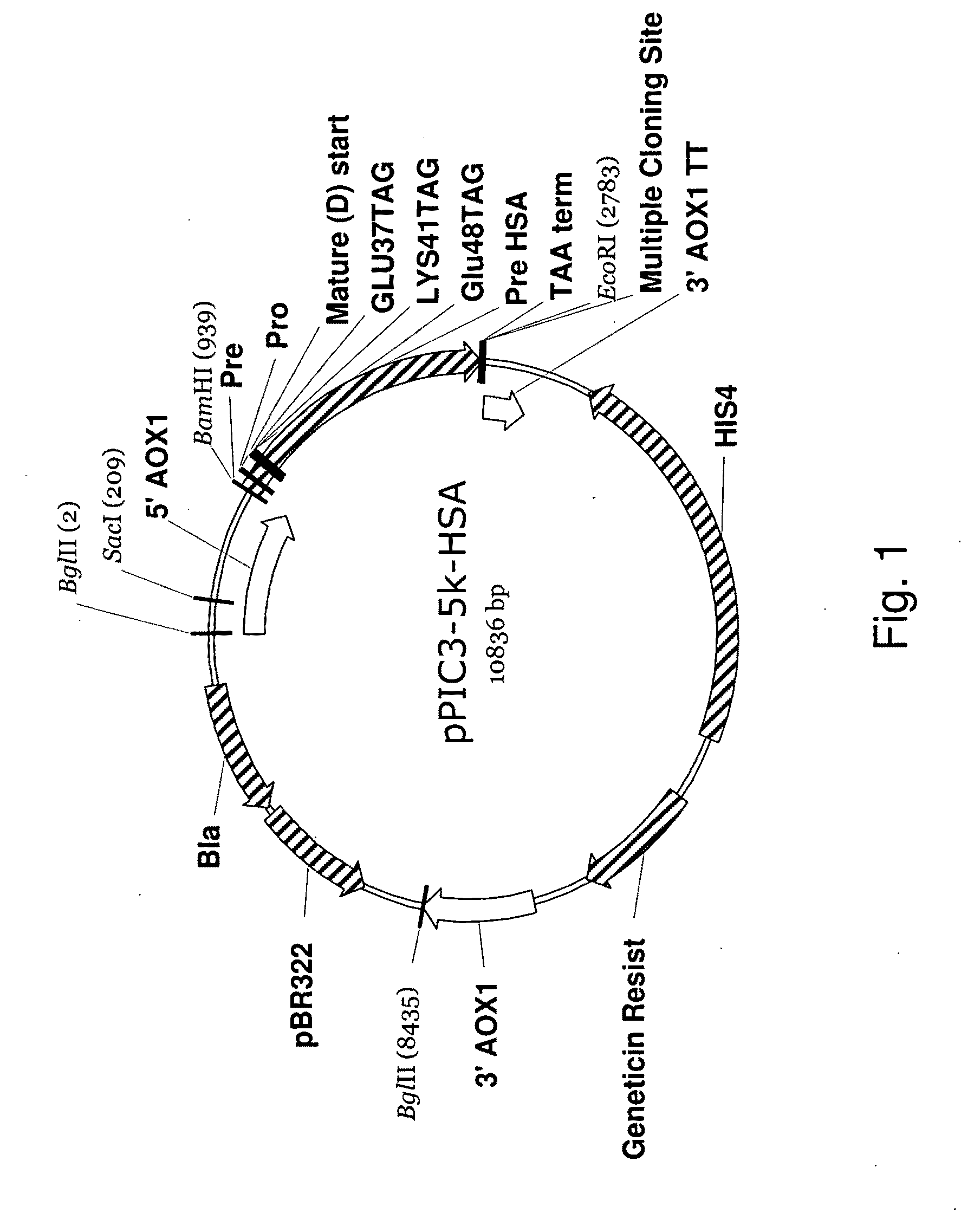
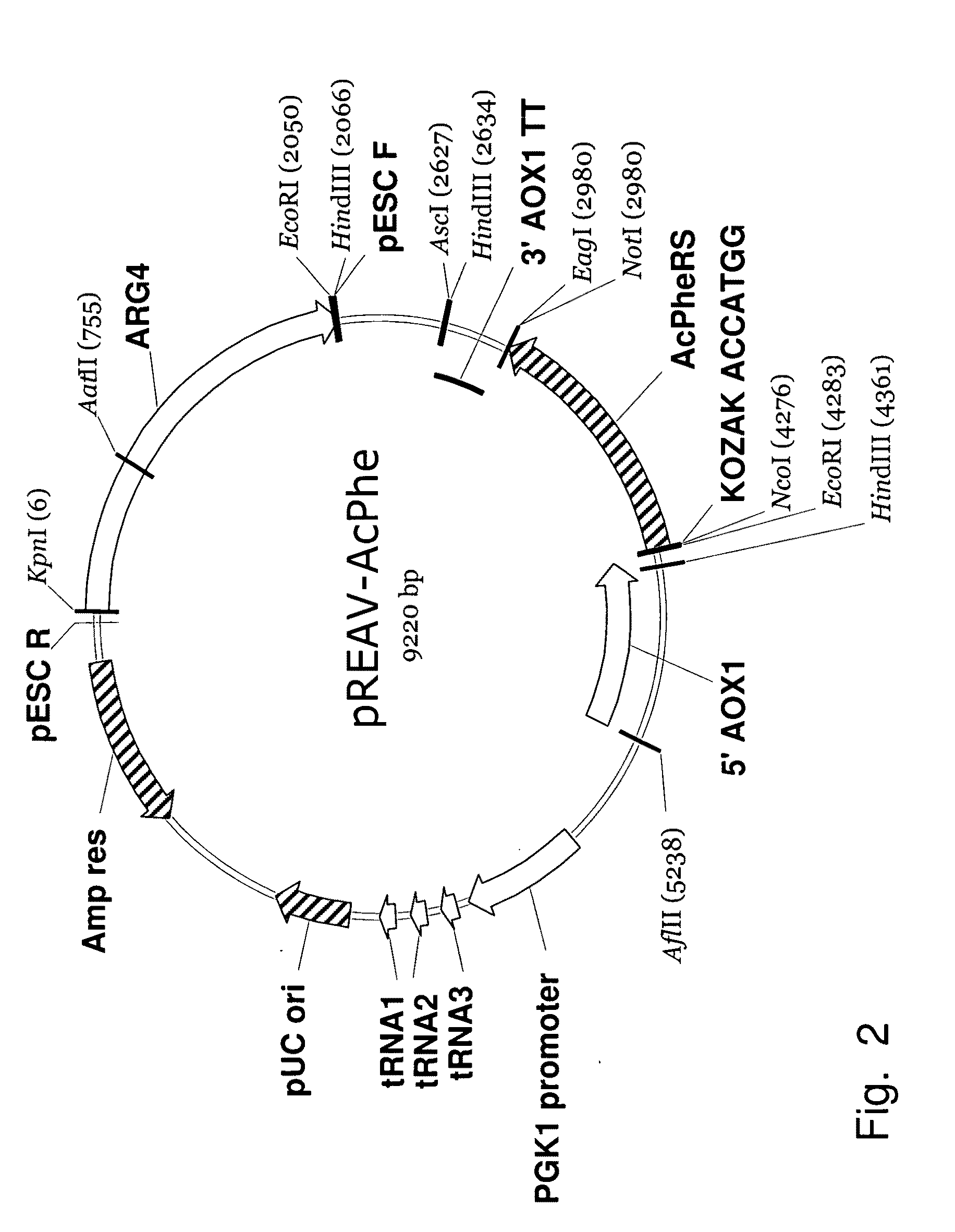
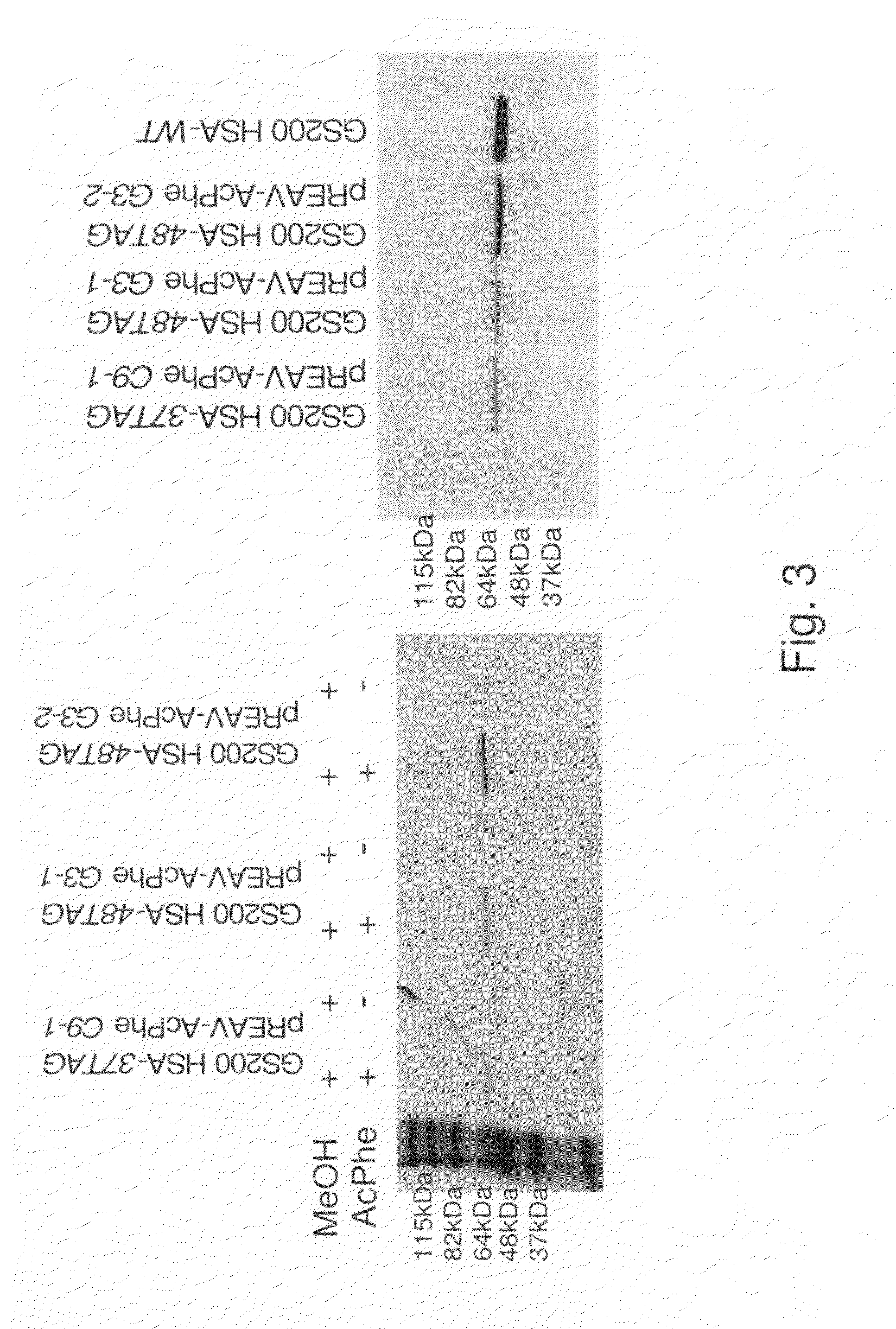
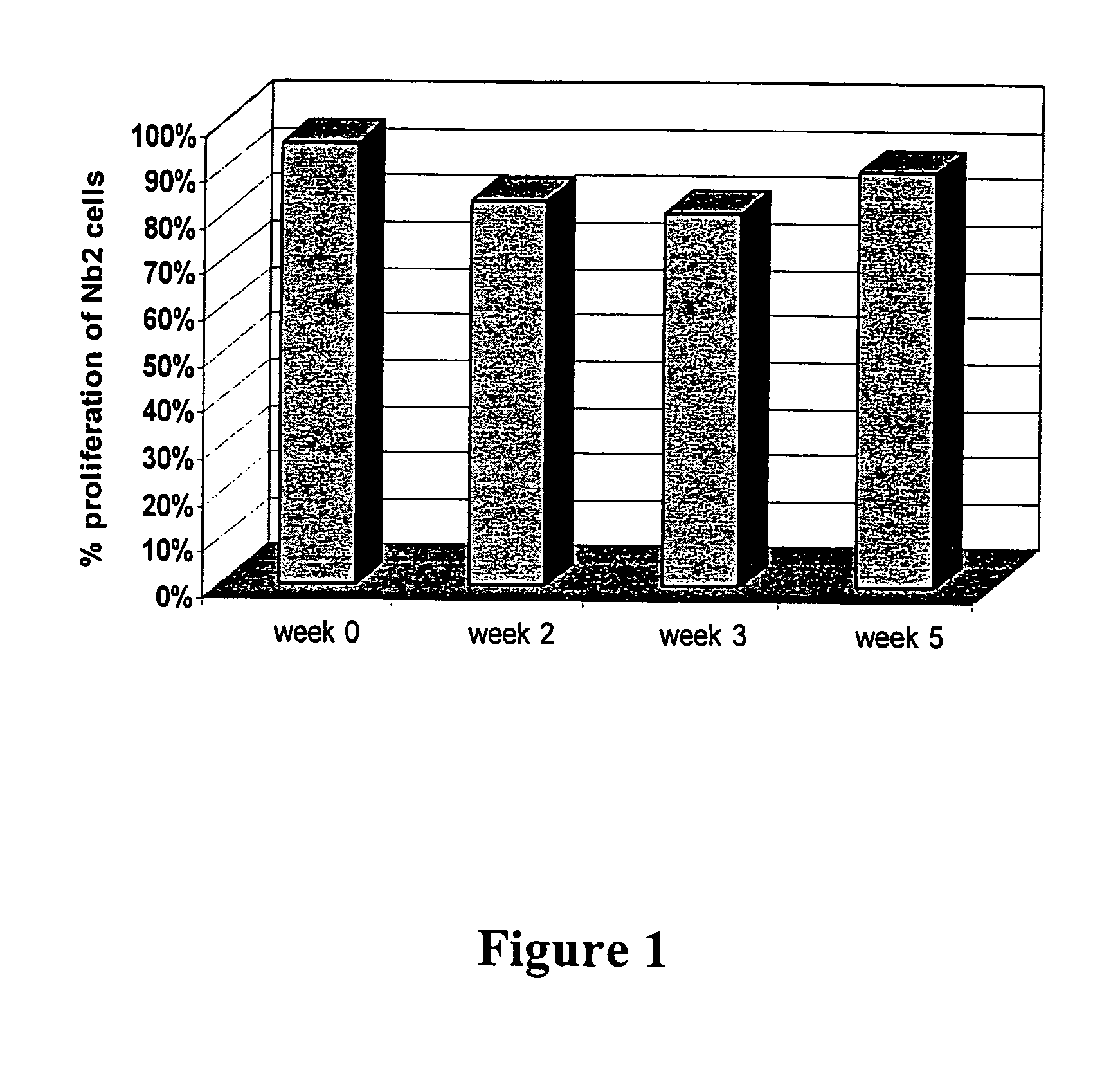
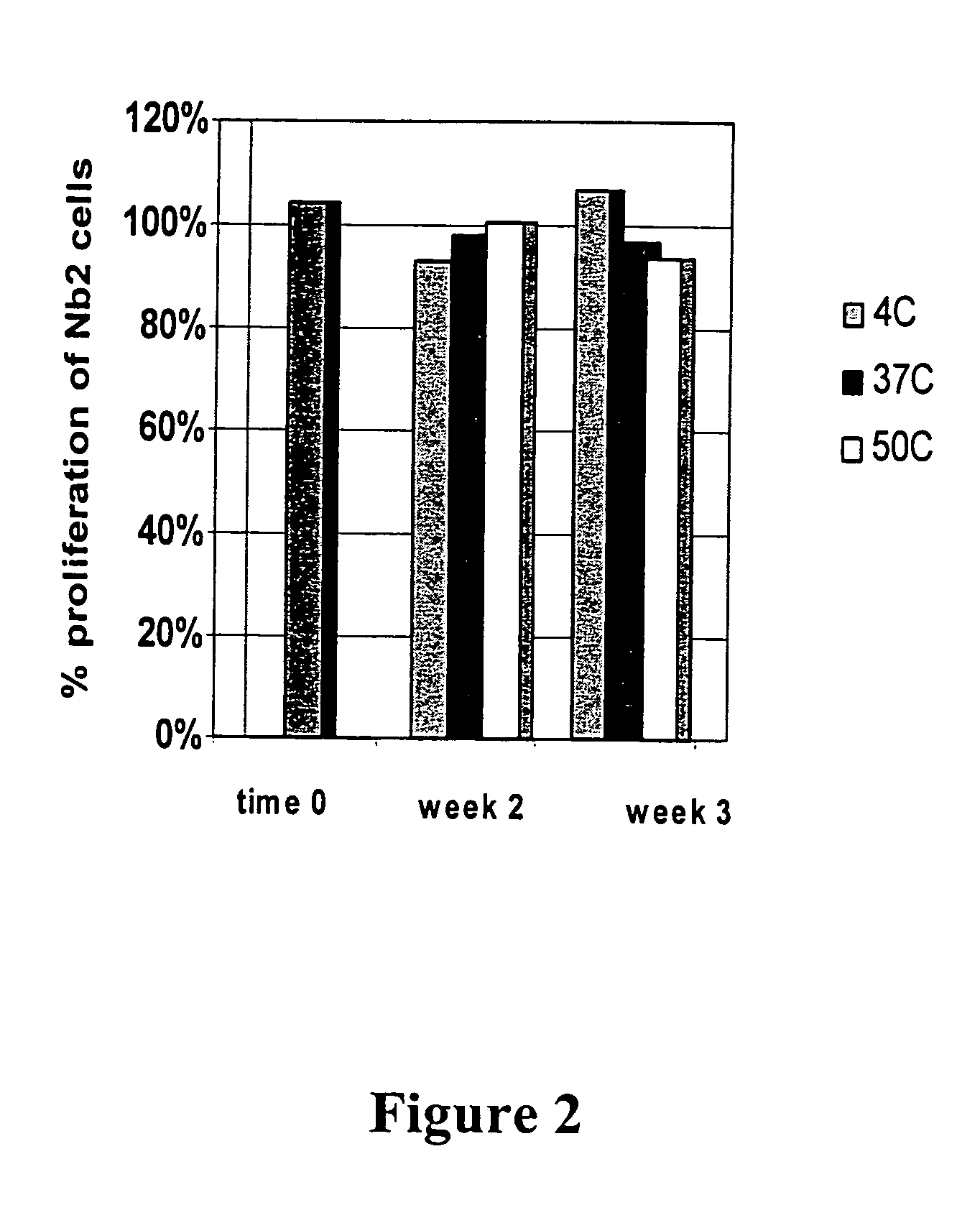
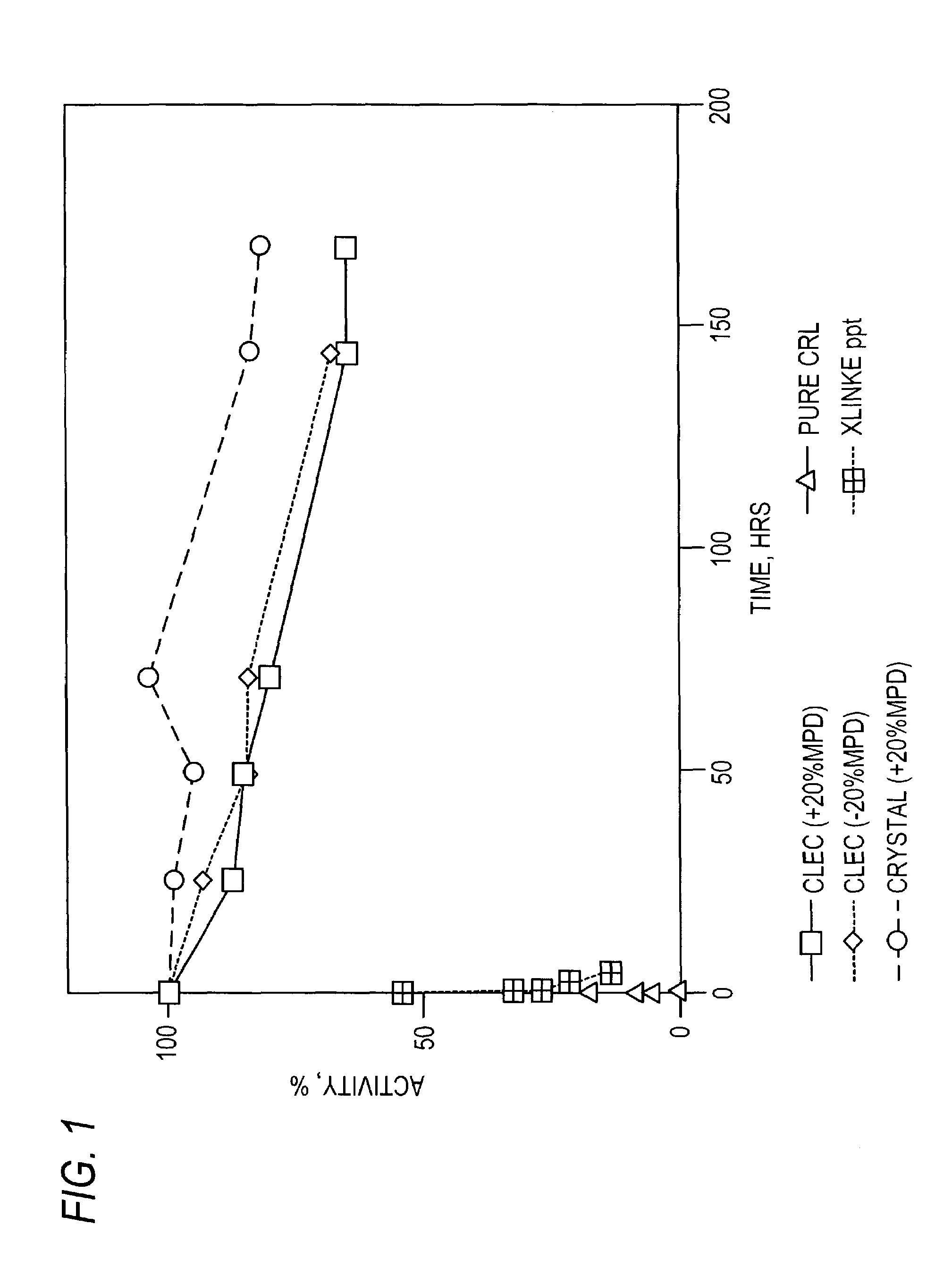
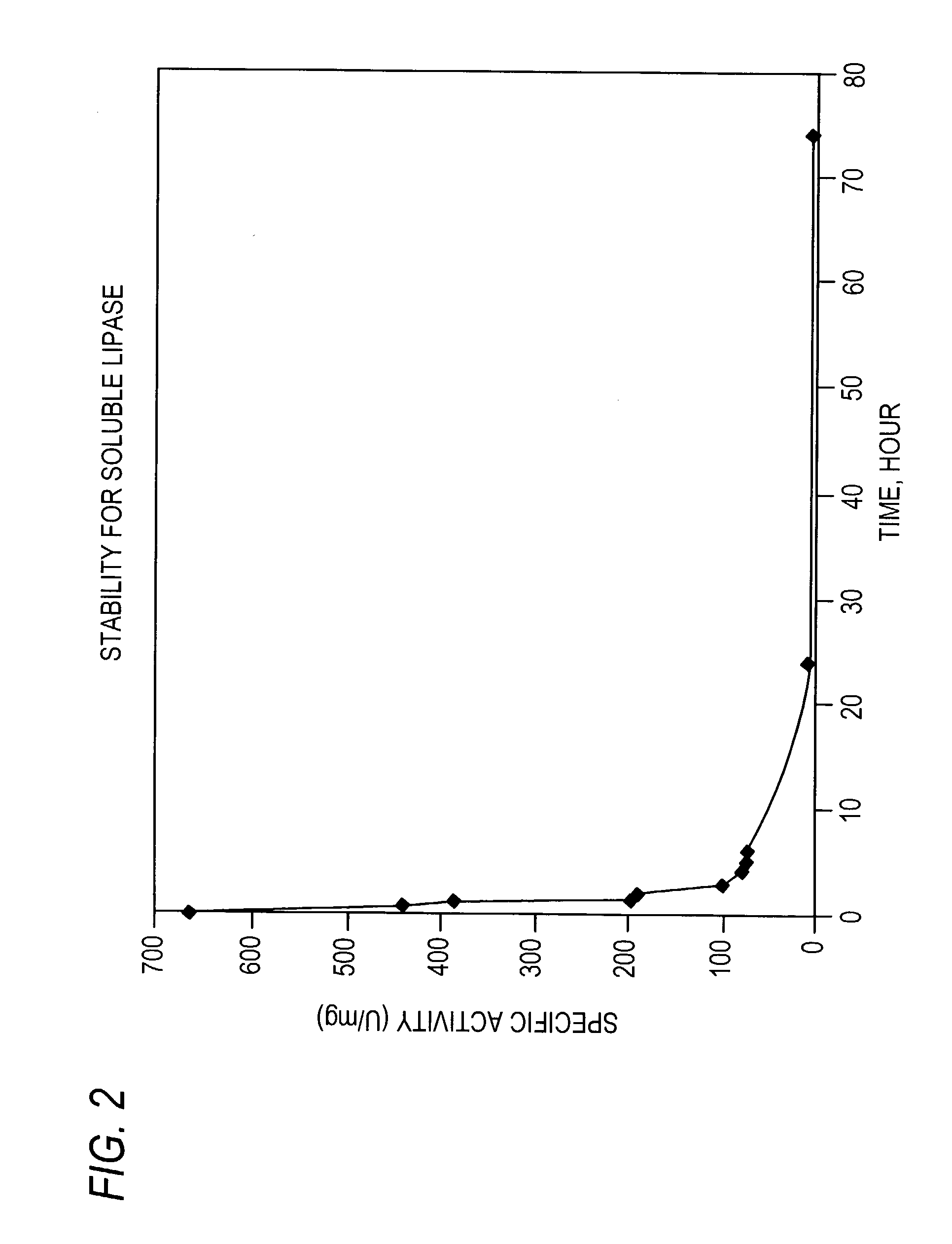
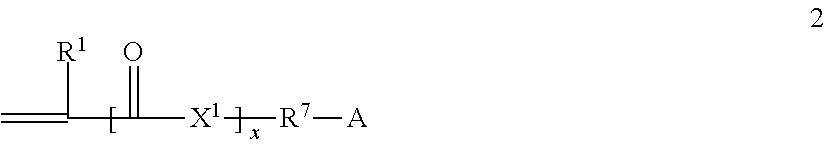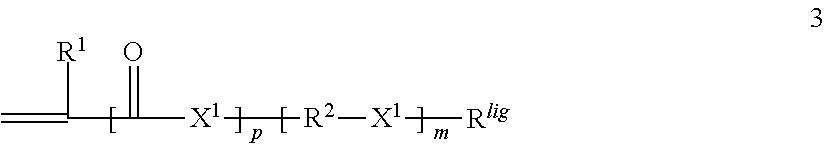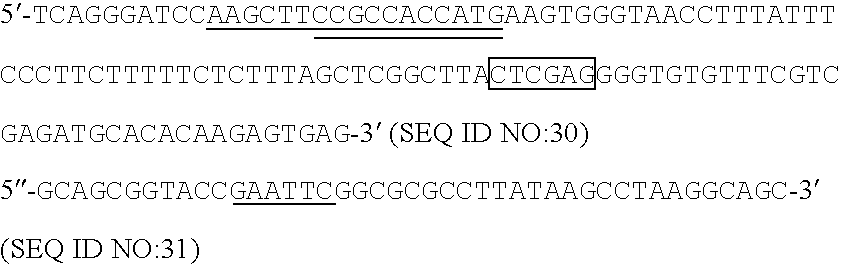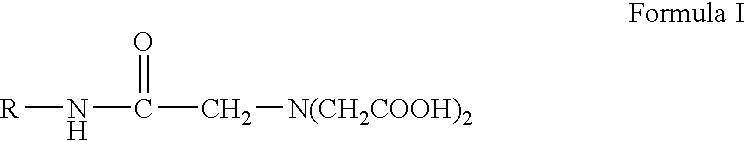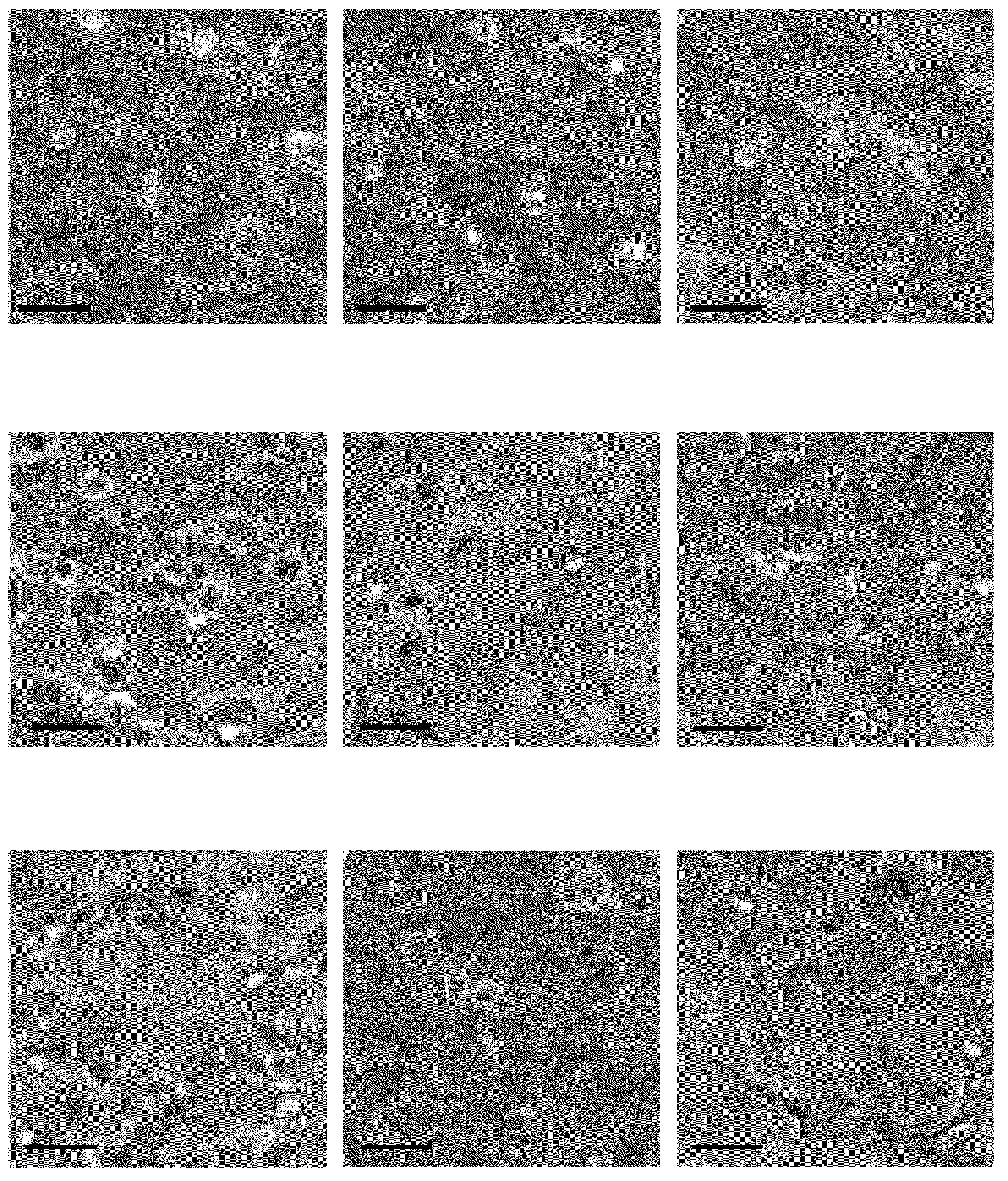Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2136results about "Serum albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
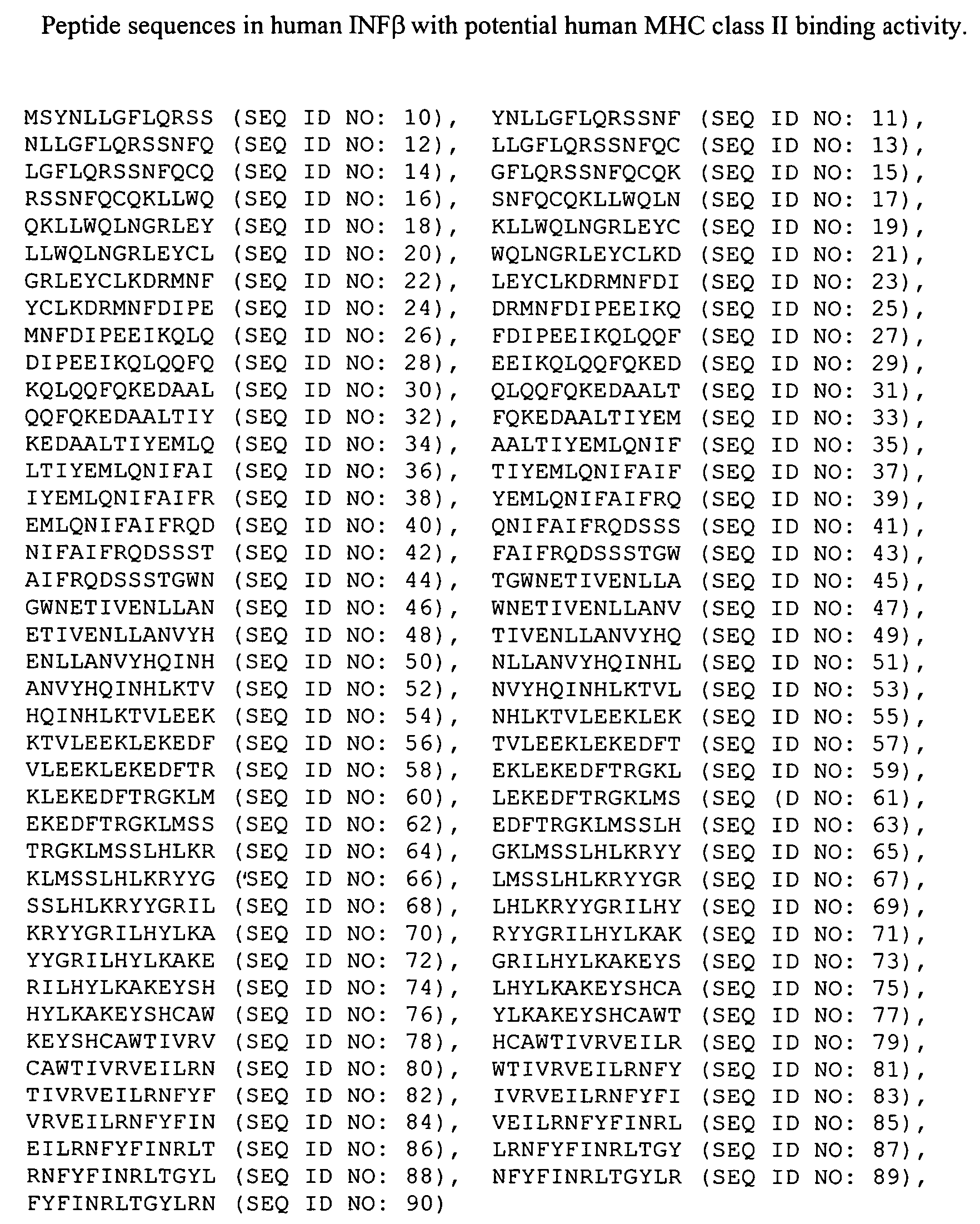
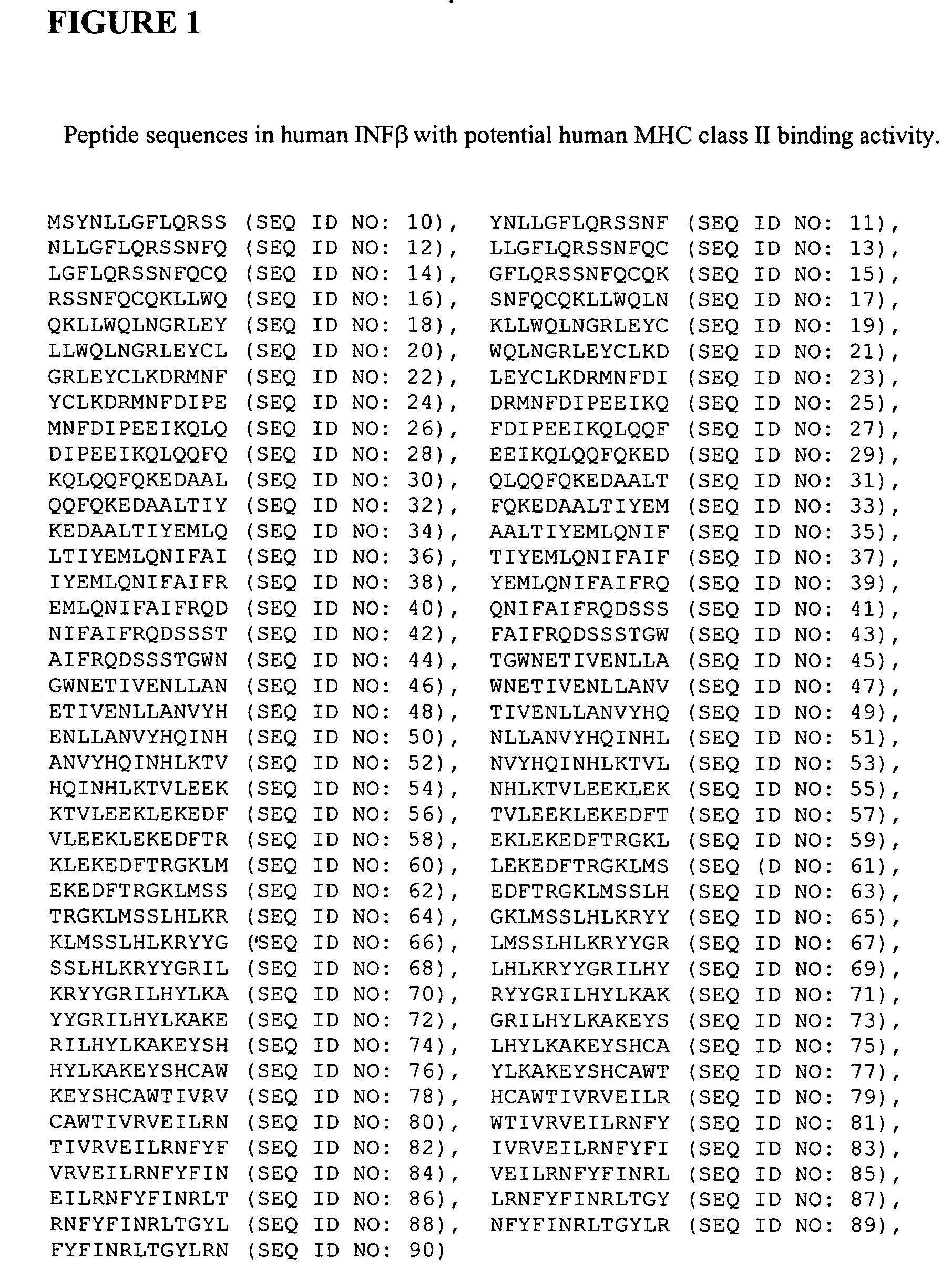
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Albumin fusion proteins
InactiveUS6946134B1Extended shelf lifeRetain activityPeptide/protein ingredientsSerum albuminDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
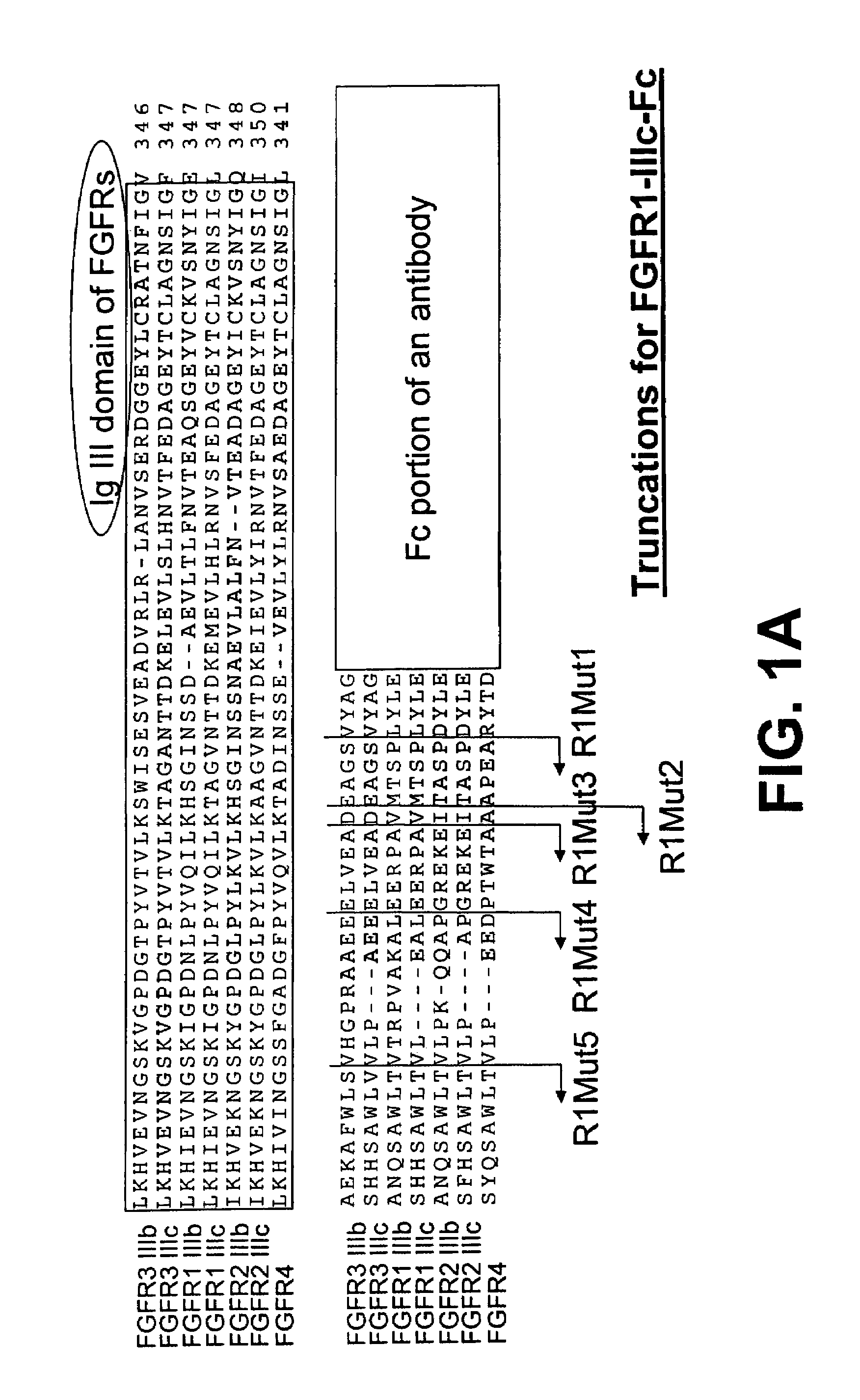
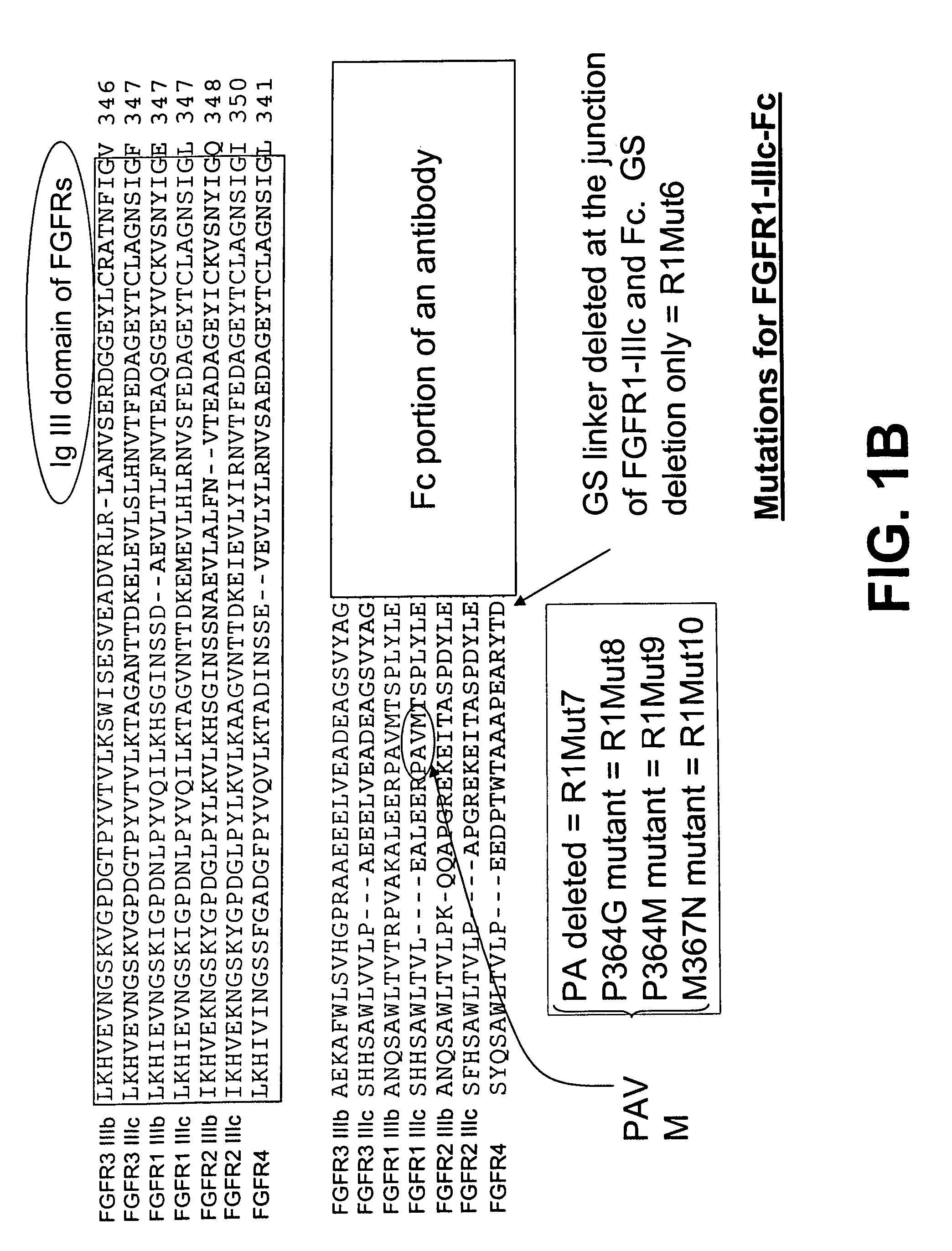
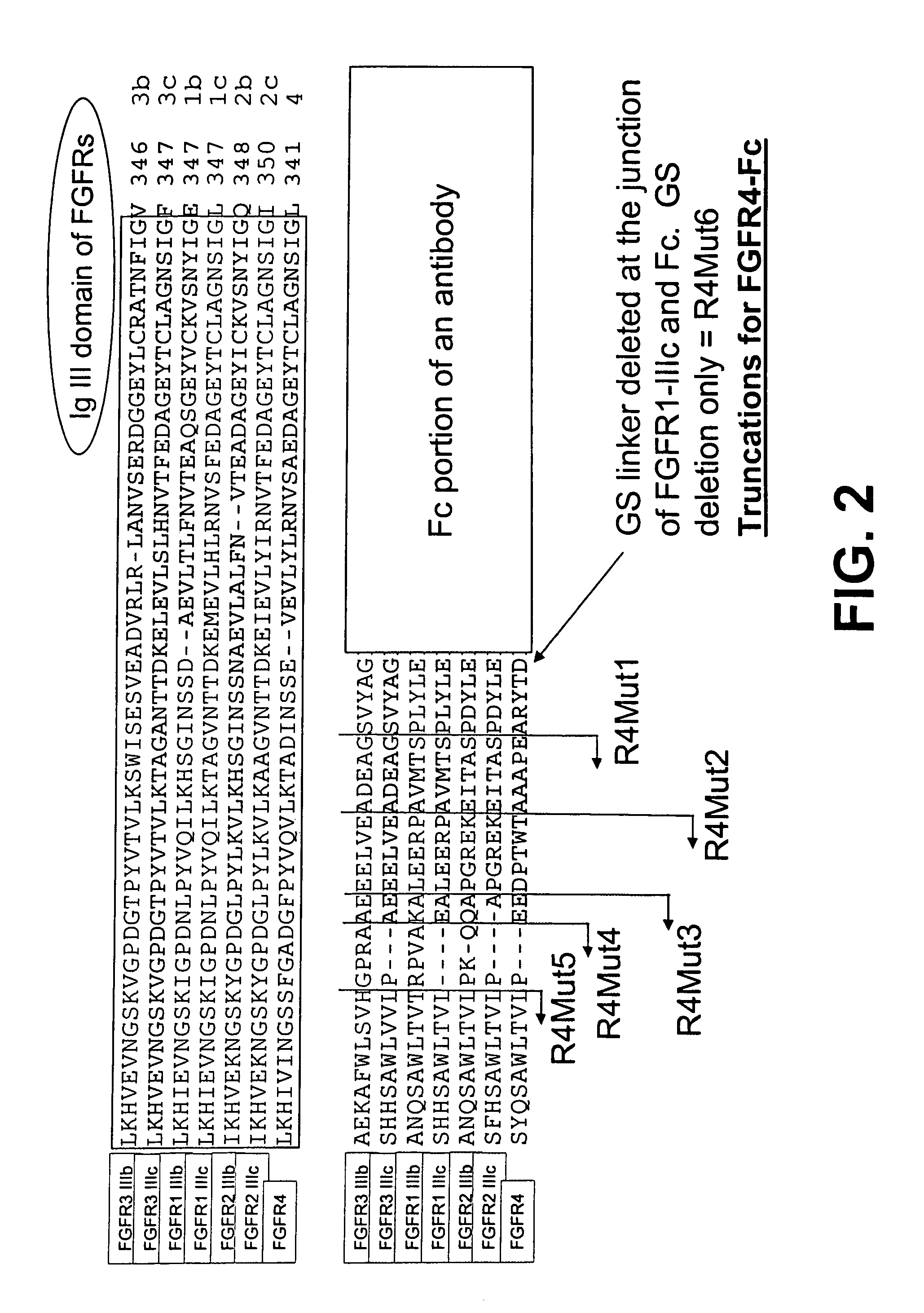
Compositions and methods of treating disease with FGFR fusion proteins
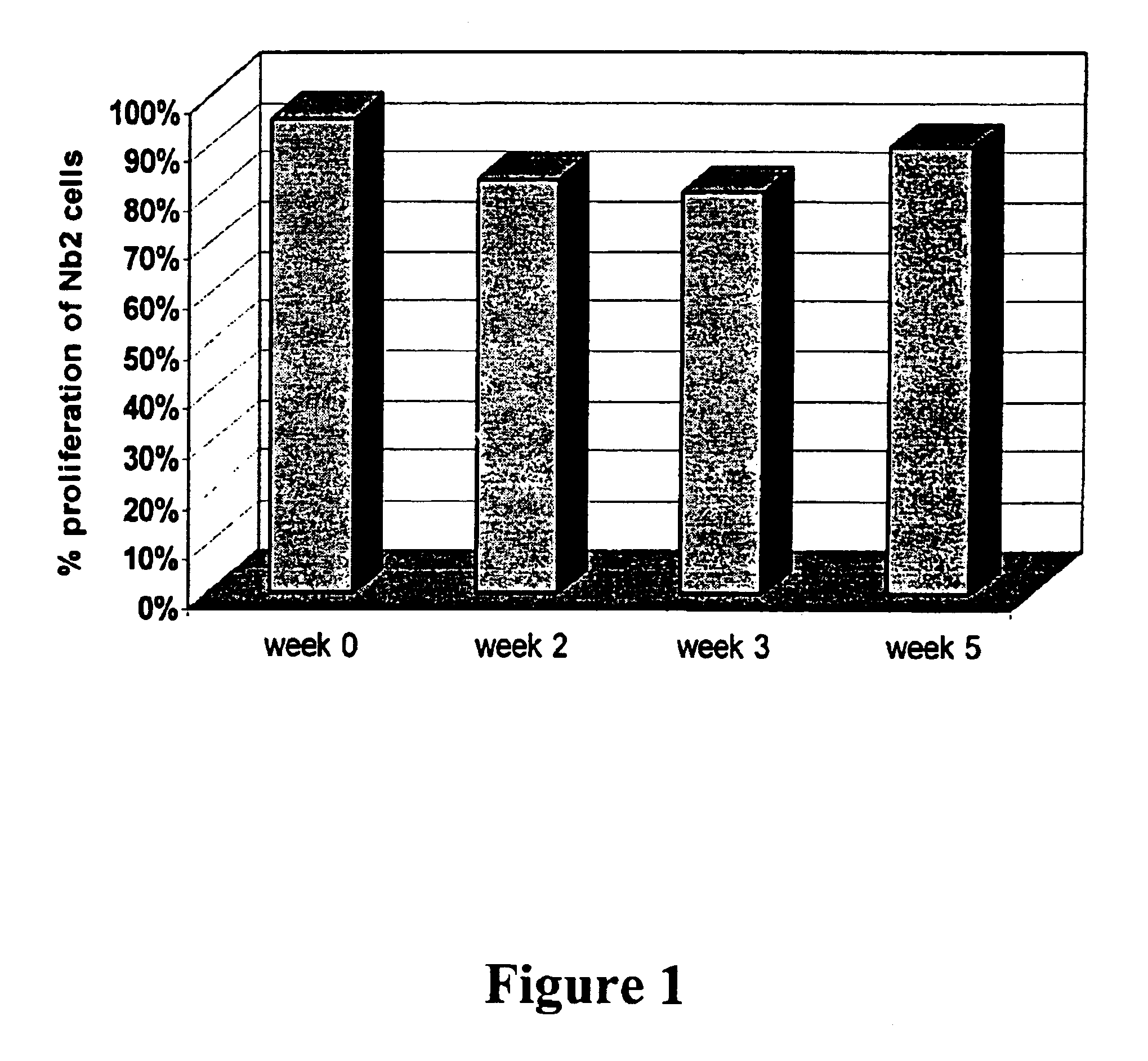
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
Modified Human Plasma Polypeptide or Fc Scaffolds and Their Uses
InactiveUS20080125574A1Improve stabilityGood water solubilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBlood plasmaChemistry
Owner:AMBRX
Ligand functional substrates
A substrate comprising a crosslinked polymer primer layer, and grafted thereto a ligand-functionalized polymer is provided. The grafted polymer has the requisite affinity for binding neutral or negatively charged biomaterials, such as cells, cell debris, bacteria, spores, viruses, nucleic acids, and proteins, at pH's near or below the pI's of the biomaterials.
Owner:3M INNOVATIVE PROPERTIES CO
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Acryloyloxyethylphosphorylcholine Containing Polymer Conjugates and Their Preparation
InactiveUS20100166700A1Prevents and reduces efficiencyExtended half-lifeFactor VIINervous disorderPolymer sciencePharmaceutical drug
The present invention relates to polymeric reagents and conjugates thereof, methods for synthesizing the polymeric reagents and conjugates, pharmaceutical compositions comprising the conjugates and methods of using the polymer conjugates including therapeutic methods where conjugates are administered to patients.
Owner:KODIAK SCI
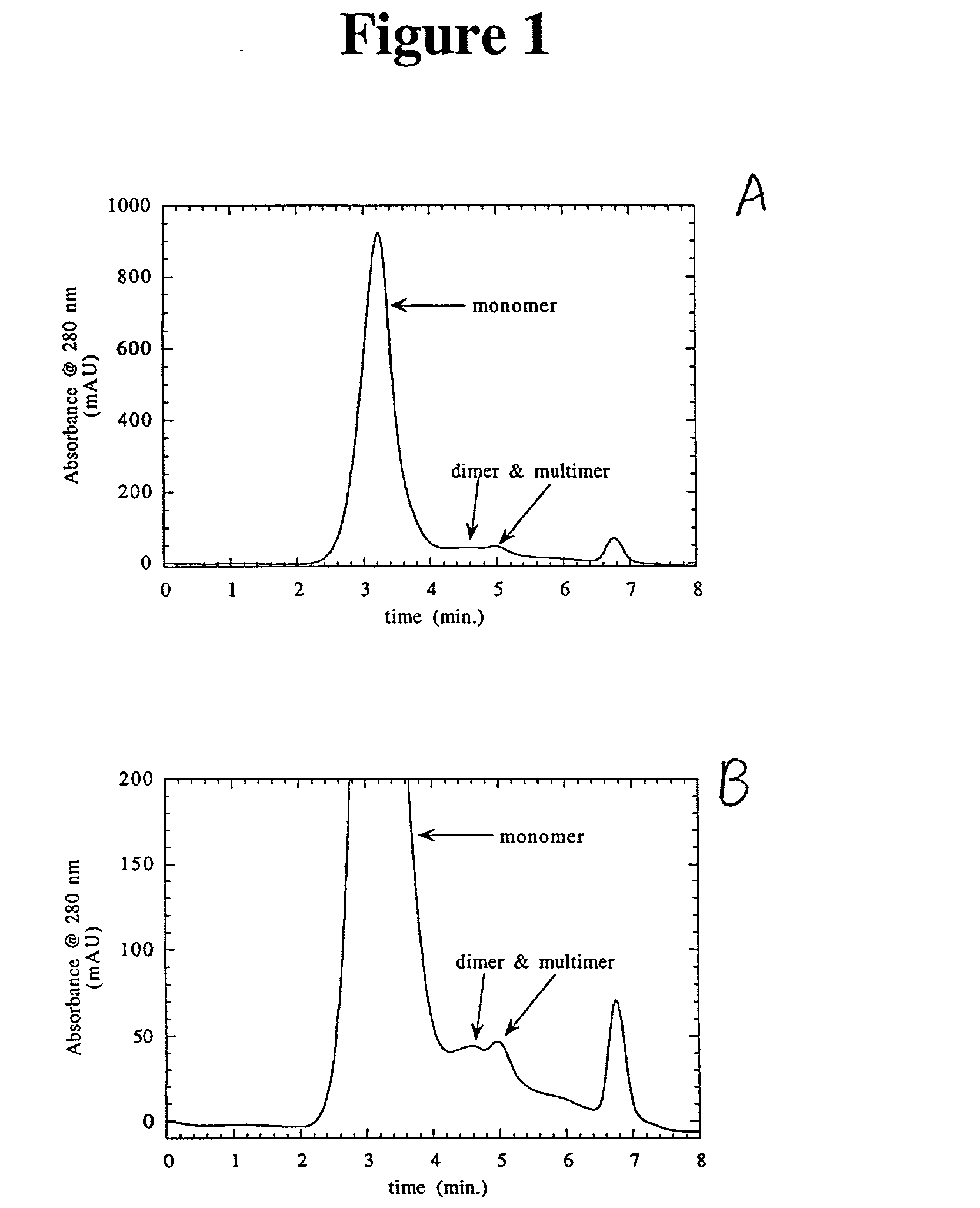
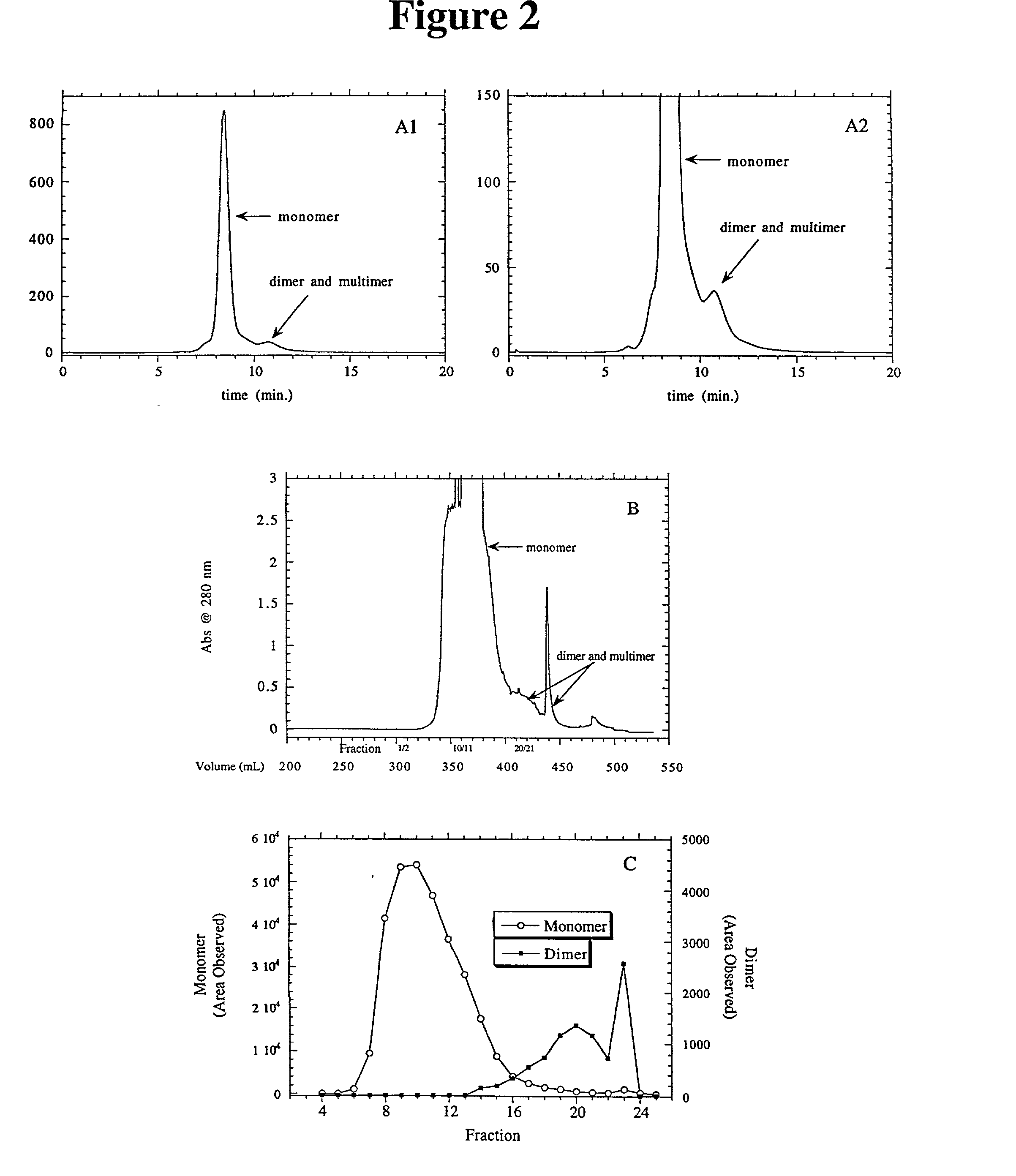
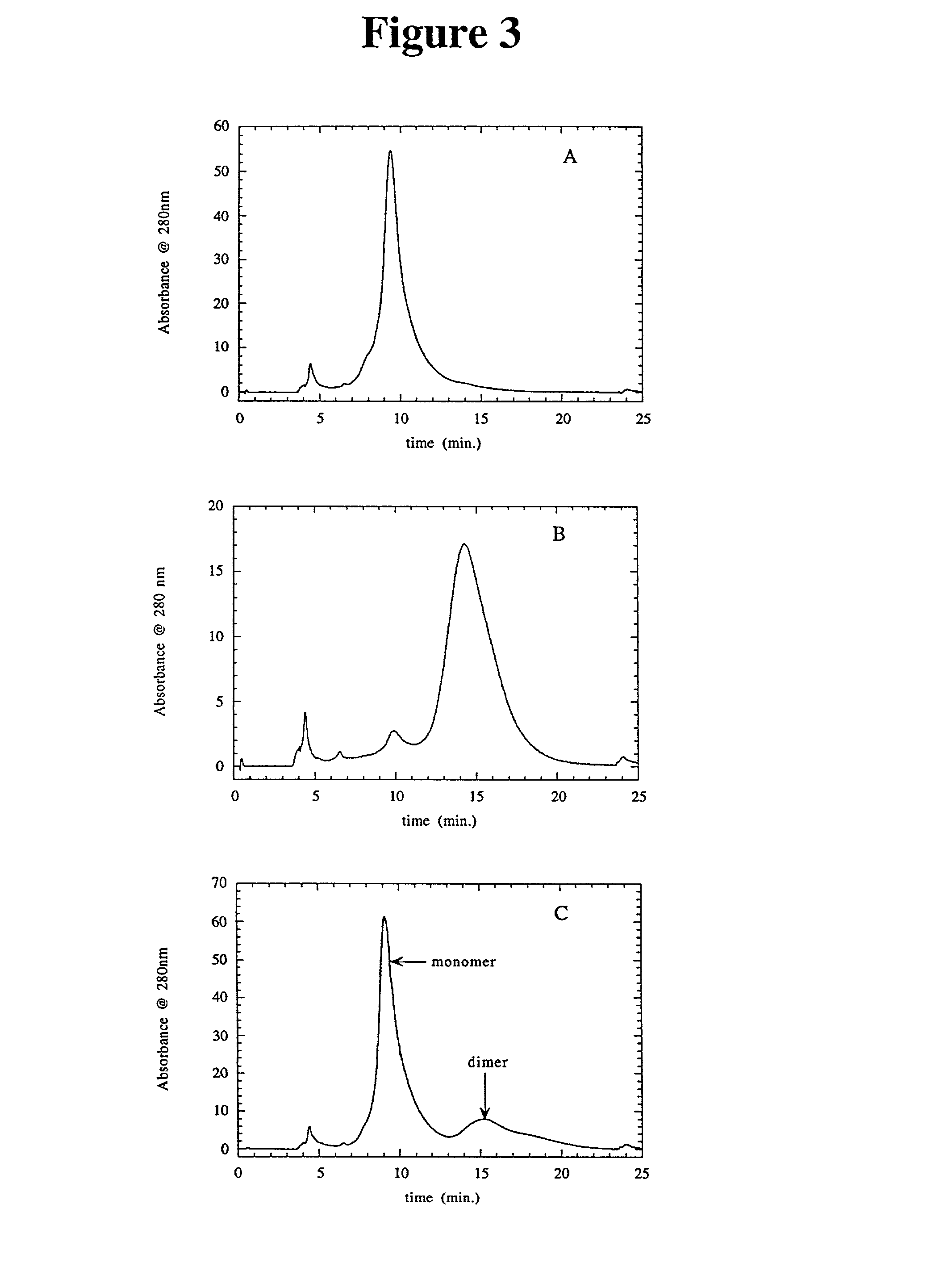
Separation of polypeptide monomers
InactiveUS20020010319A1Easy to separateInexpensiveComponent separationOther chemical processesAnion-exchange chromatographyAnion Exchange Proteins
A method is disclosed for separating a polypeptide monomer from a mixture comprising dimers and / or multimers. The method comprises applying the mixture to either a cation-exchange chromatography resin or an anion-exchange chromatography resin and eluting the mixture at a gradient of about 0-1 M of an elution salt, wherein the monomer is separated from the dimers and / or multimers present in the mixture.
Owner:GENENTECH INC
Process for promoting proper folding of human serum albumin using a human serum albumin ligand
InactiveUS20050209441A1Reduce concentrationSerum albuminPeptide preparation methodsHuman serum albuminProtein refolding
The present invention is a process for refolding and renaturing human serum albumin protein to substantially native conformation by the addition of a human serum albumin refolding ligand to a solution containing the protein under conditions conducive to refolding of the protein.
Owner:BLUE MOUNTAIN TEHNOLOGY DEV LAB
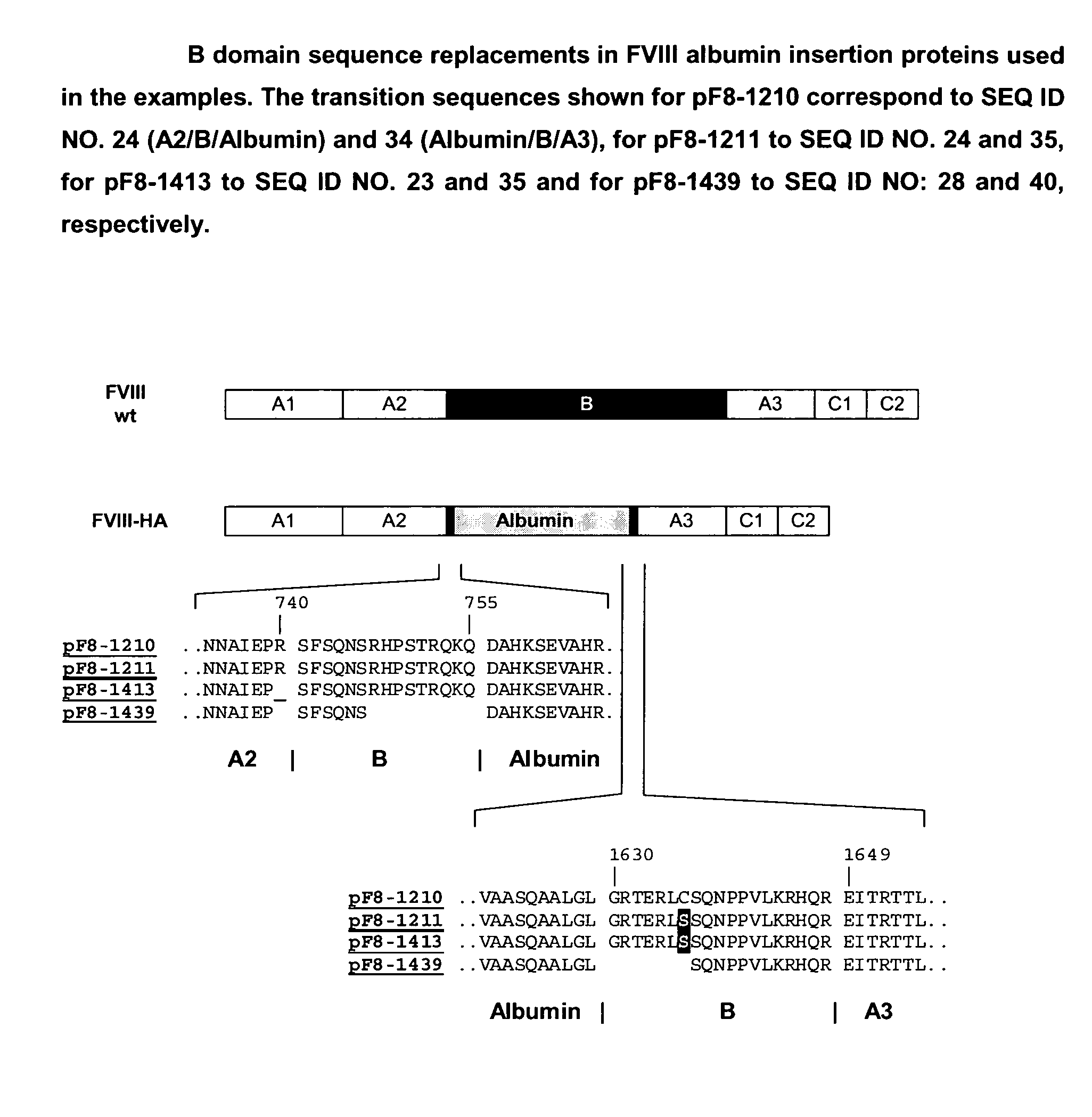
Modified coagulation factors with prolonged in vivo half-life
InactiveUS20100120664A1Increase productionHigh expressionFactor VIIPeptide/protein ingredientsHalf-lifeNucleic acid sequencing
The present invention relates to nucleic acid sequences coding for modified coagulation factors, preferably coagulation factor VIII, and their derivatives; recombinant expression vectors containing such nucleic acid sequences; host cells transformed with such recombinant expression vectors; and recombinant polypeptides and derivatives coded for by said nucleic acid sequences, whereby said recombinant polypeptides and derivatives have biological activities and prolonged in vivo half-lives compared to the unmodified wild-type proteins. The invention also relates to corresponding sequences that result in improved in vitro stability. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such nucleic acid sequences.
Owner:CSL BEHRING GMBH
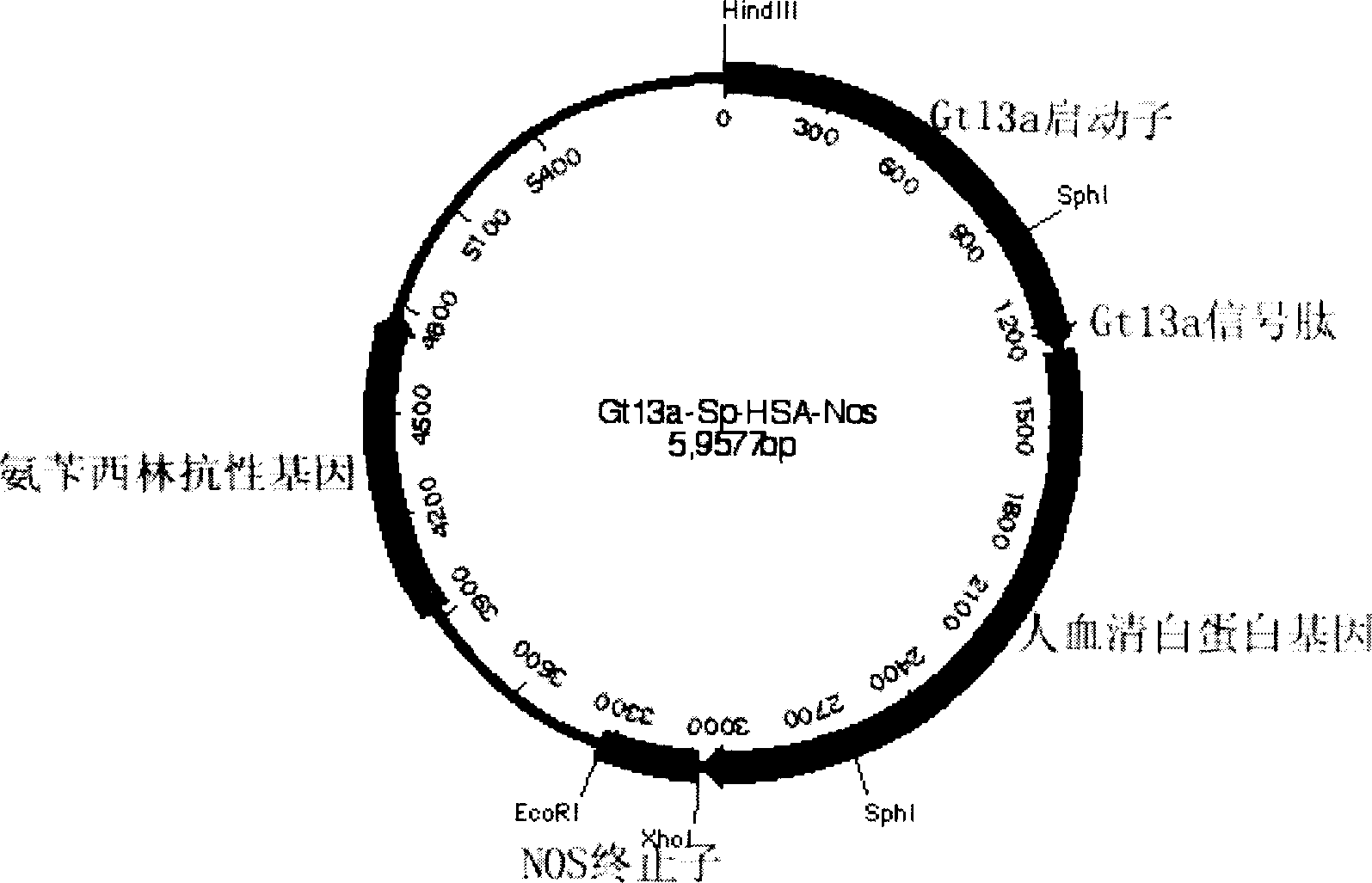
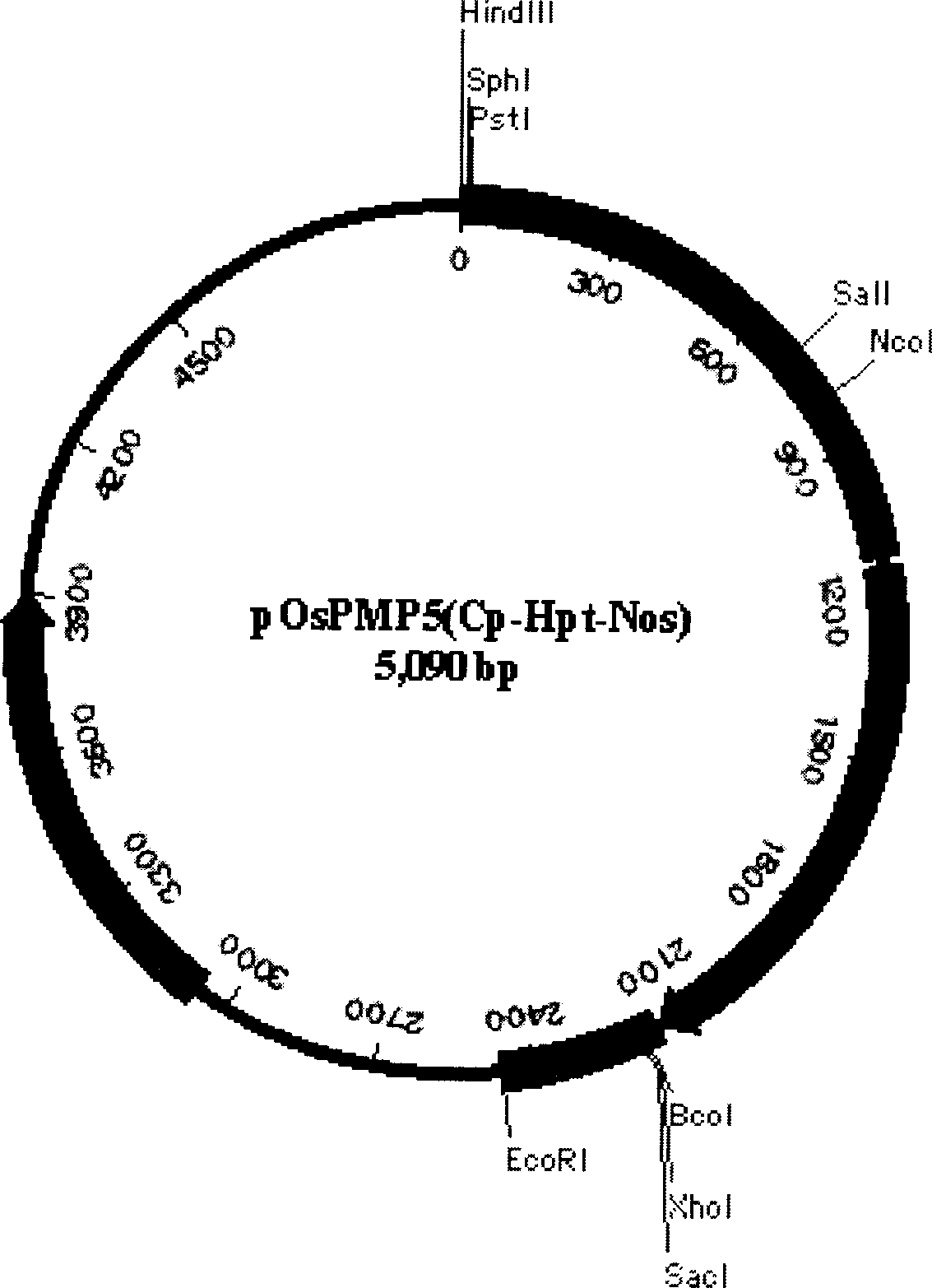
Production of recombinant human serum albumin with rice-embryo milk cell as biological reactor
ActiveCN1896239ANo pollution in the processEfficient expressionSerum albuminFermentationSerum protein albuminEmbryo
Production of recombinant human serum albumin with rice albuminous cell as biological reactor is carried out by taking rice albuminous cell protein as storage site for recombinant protein, taking rice albuminous specific expression starter and signal peptide, entering inductive recombinant human serum albumin into inner-film system of rice serum albumin cell and storing it in rice albuminous protein. It is cheap, has more yield and higher expression.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Affinity labeling libraries and applications thereof
InactiveUS6277583B1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsCombinatorial chemistryAffinity labeling
Novel methods and compositions are provided for preferentially bonding an oligomeric molecule to a macromolecular target where the target is a member of a complex mixture and / or there is preferential bonding at one of a plurality of available bonding sites of the macromolecular target. The methods represent a complete system for both producing and identifying affinity label molecules from a combinatorial library which preferentially bind to a marcomolecular target or target site and preferentially binding those affinity labels to a macromolecule of interest either ex vivo or in vivo. Macromolecular targets include a variety of cellular- and non-cellular-associated proteins.
Owner:CONJUCHEM
Albumin Fusion Proteins
ActiveUS20090029914A1Extended shelf lifeIncrease plasma stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
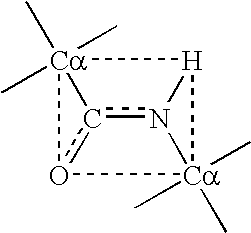
Photoactivated crosslinking of a protein or peptide
A method of crosslinking a protein or peptide for use as a biomaterial, the method comprising the step of irradiating a photoactivatable metal-ligand complex and an electron acceptor in the presence of the protein or peptide, thereby initiating a crosslinking reaction to form a 3-dimensional matrix of the biomaterial.
Owner:COOK MEDICAL TECH LLC
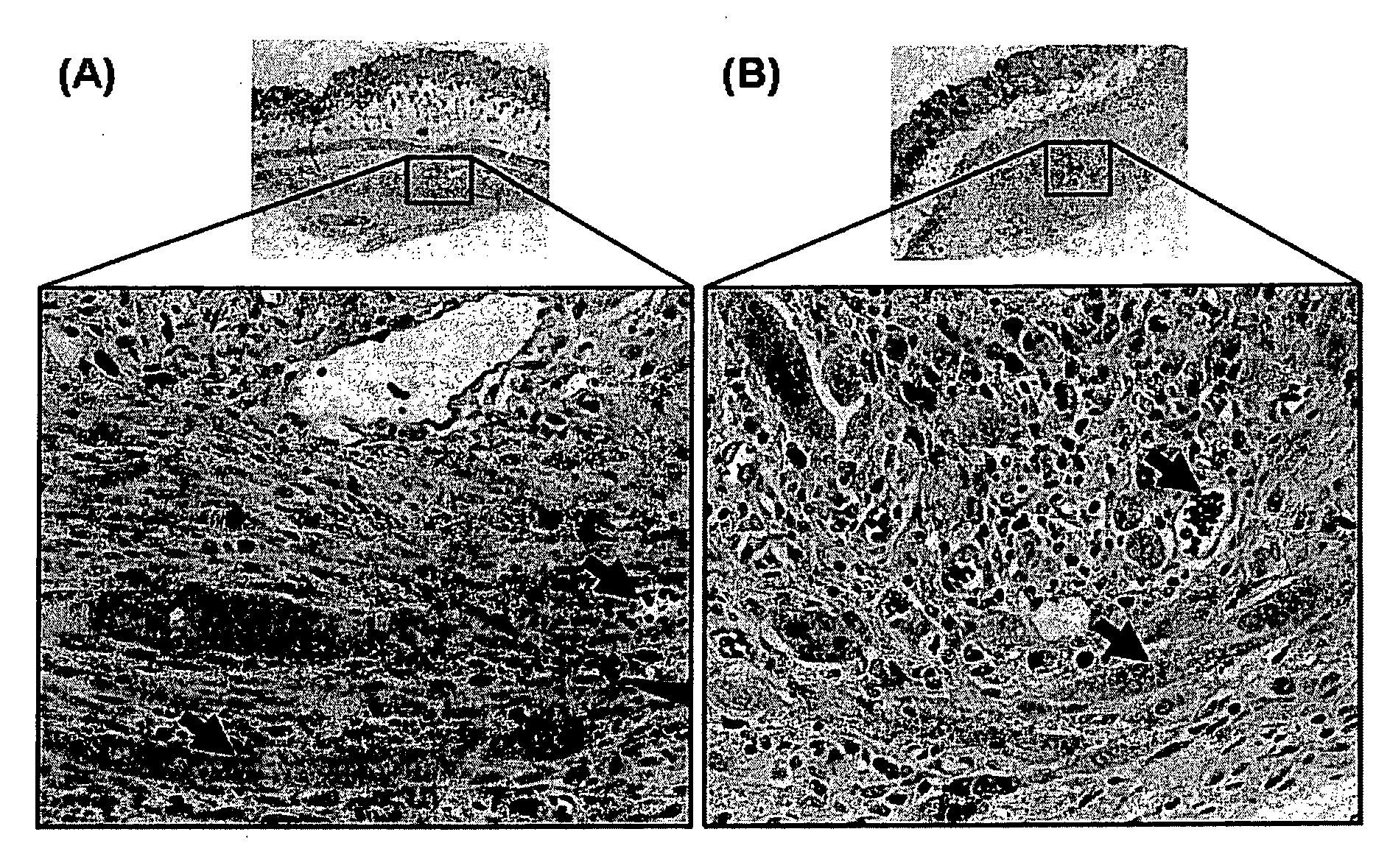
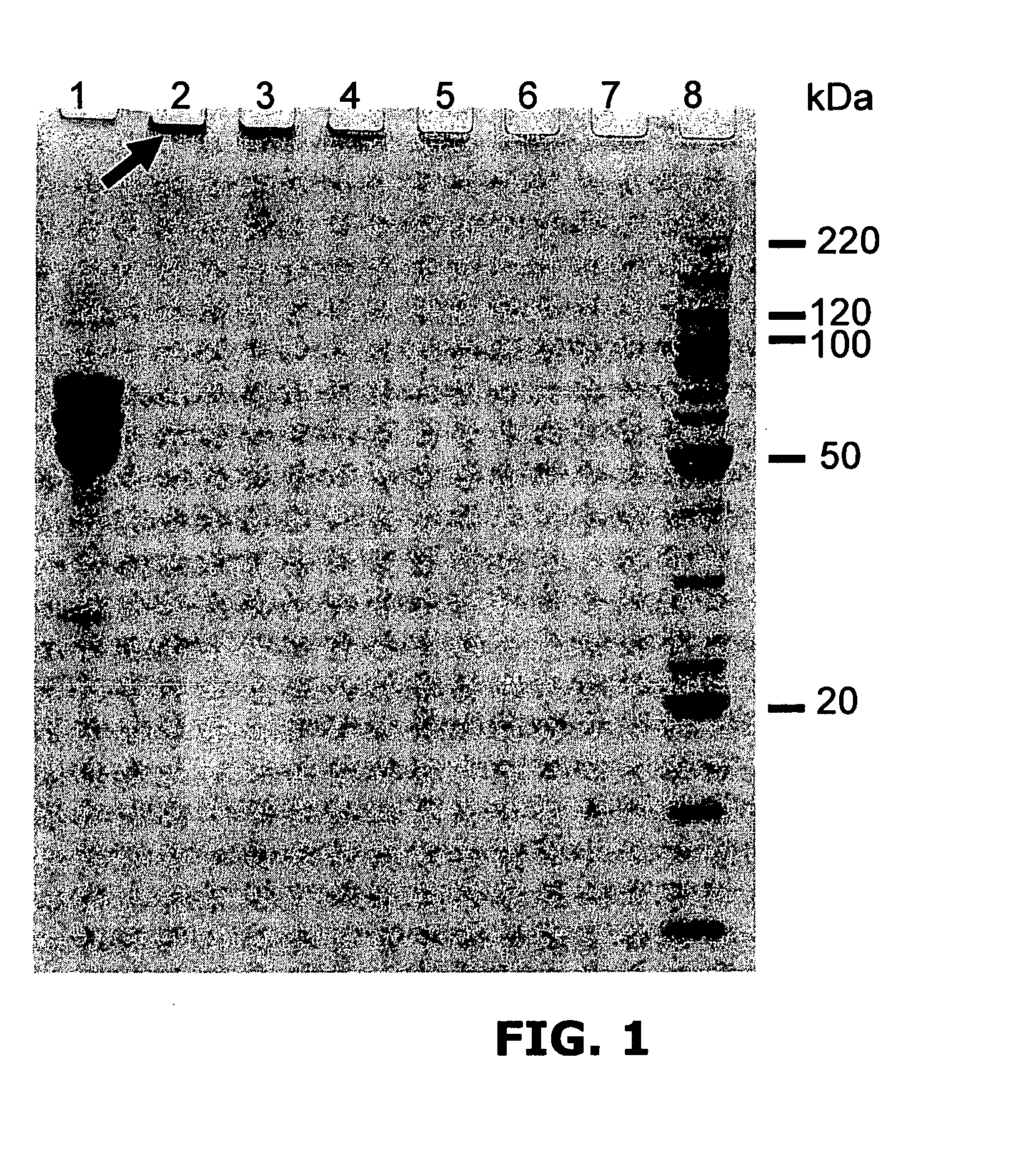
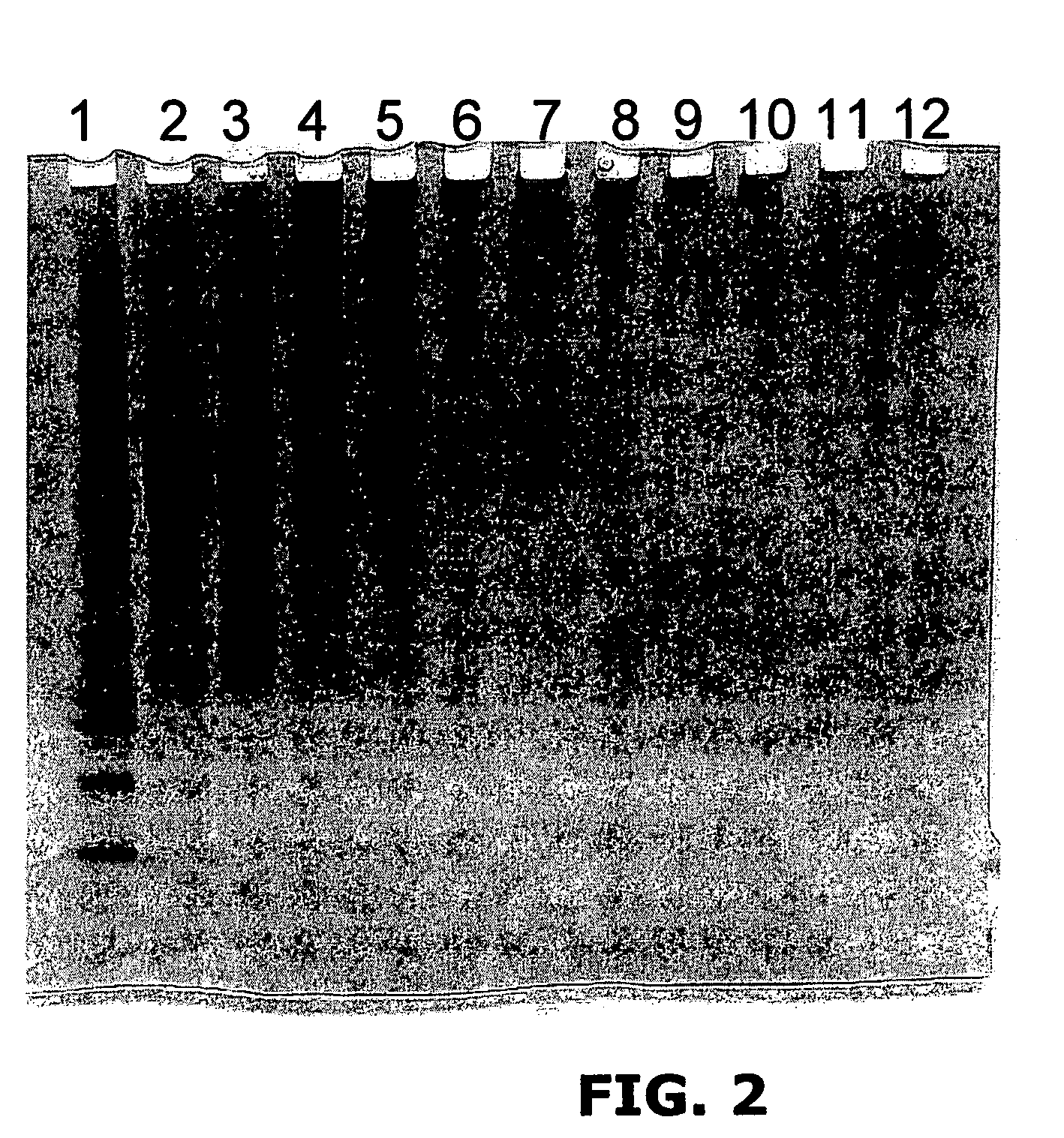
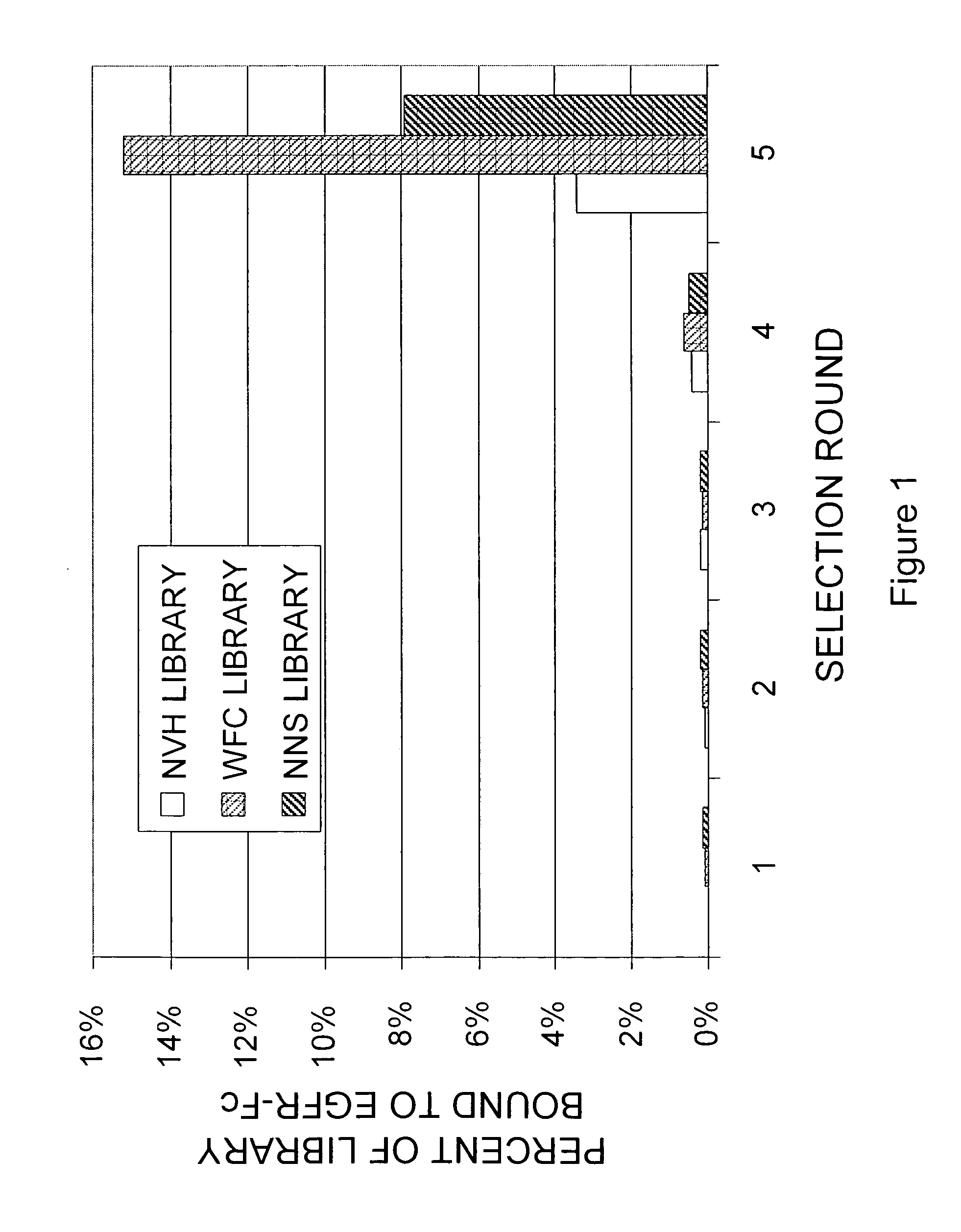
Targeted therapeutics based on engineered proteins that bind EGFR
ActiveUS8524244B2Improves one or more pharmacokinetic properties of the polypeptidesIncreased serum half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideADAMTS Proteins
Owner:BRISTOL MYERS SQUIBB CO
6-Monoacetylmorphine derivatives useful in immunoassay
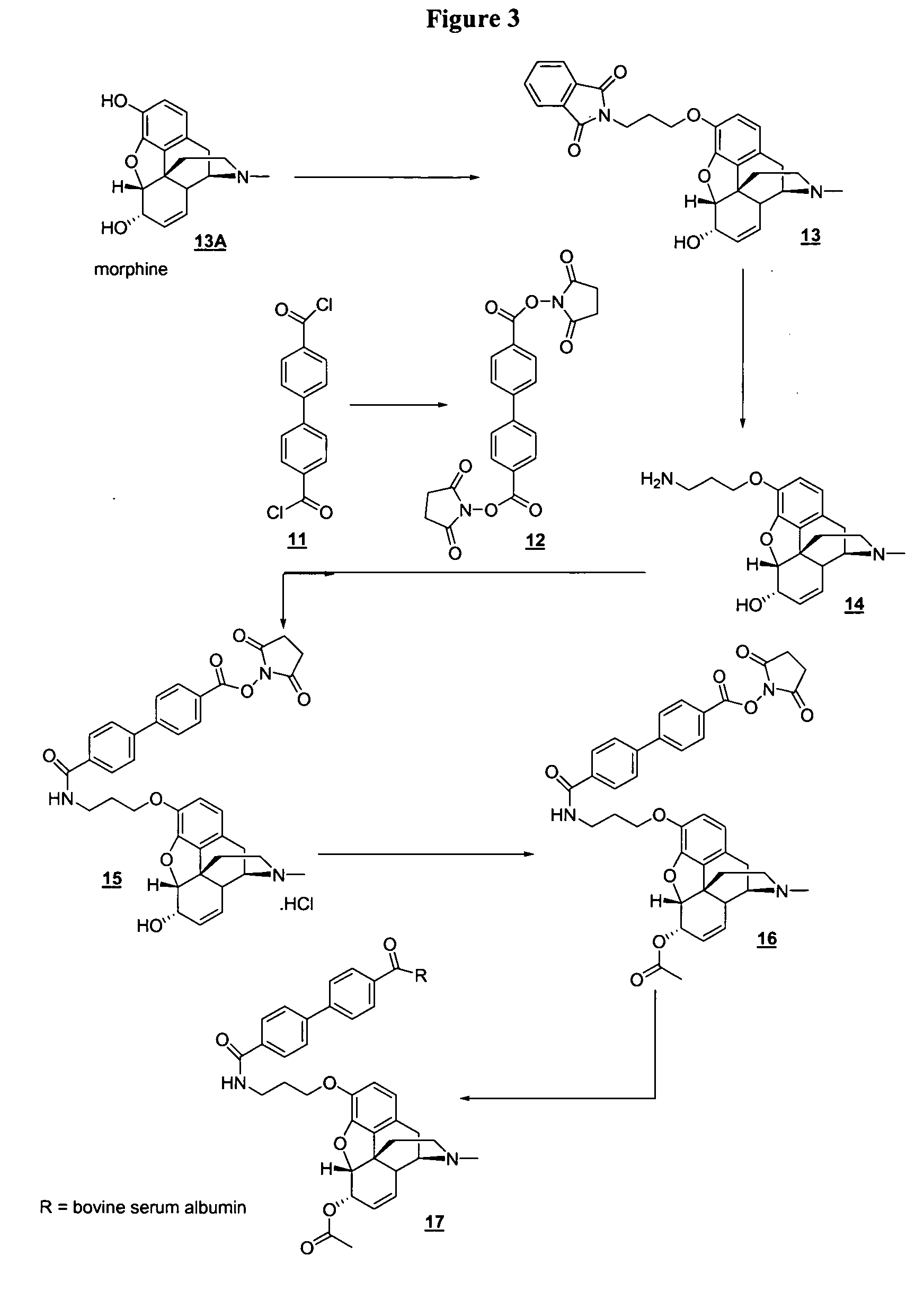
Analogs of 6-monoacetyl morphine (6-MAM) are described. These include analogs derivatized at either the C-3 position, the C-6 position, or the nor position of the molecule. These analogs allow for elaboration with linkers terminated by a functional group such as an activated ester, the functional groups being useful for attaching the molecule to other entities such as proteins, polysaccharides, and reporter groups.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
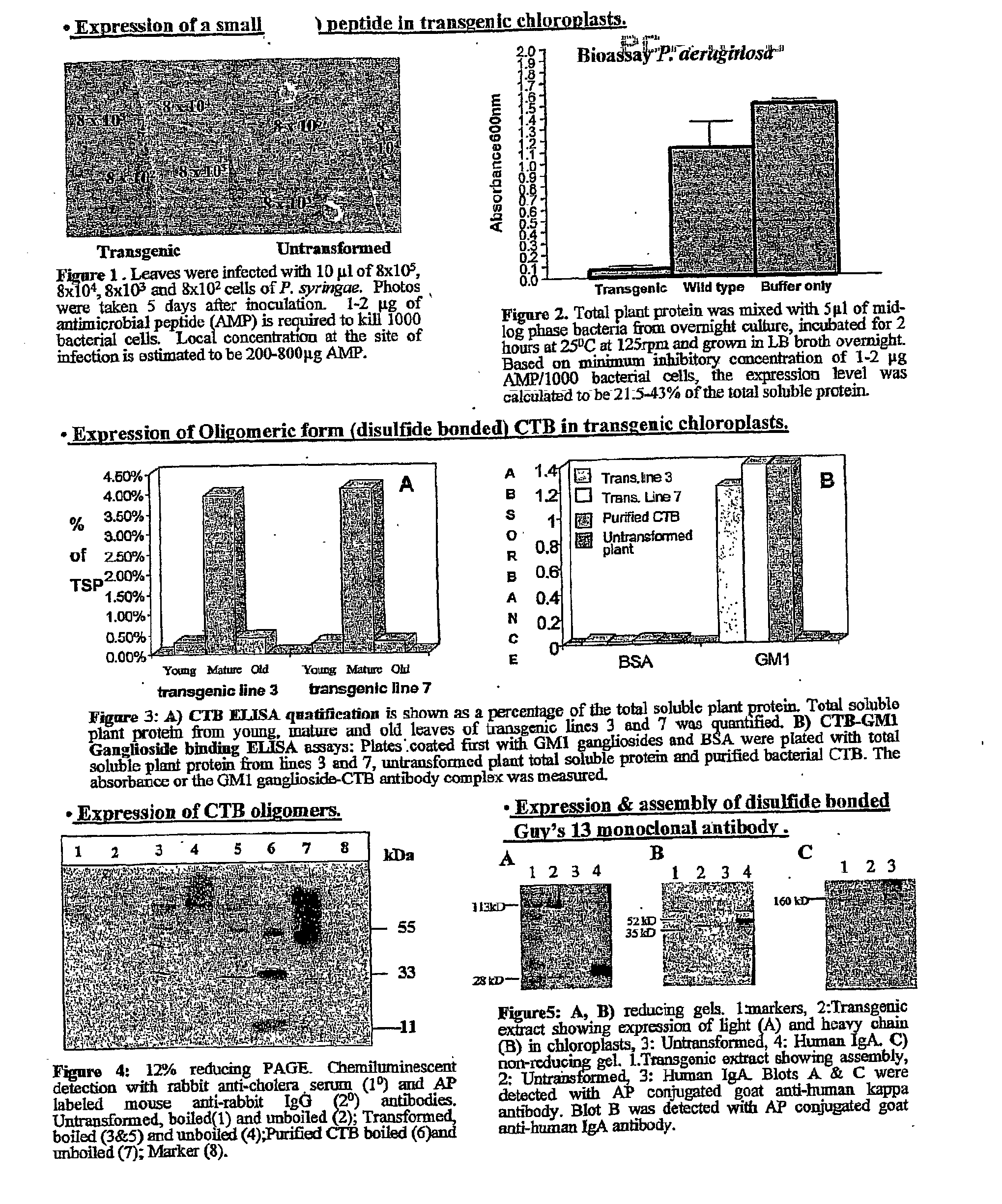
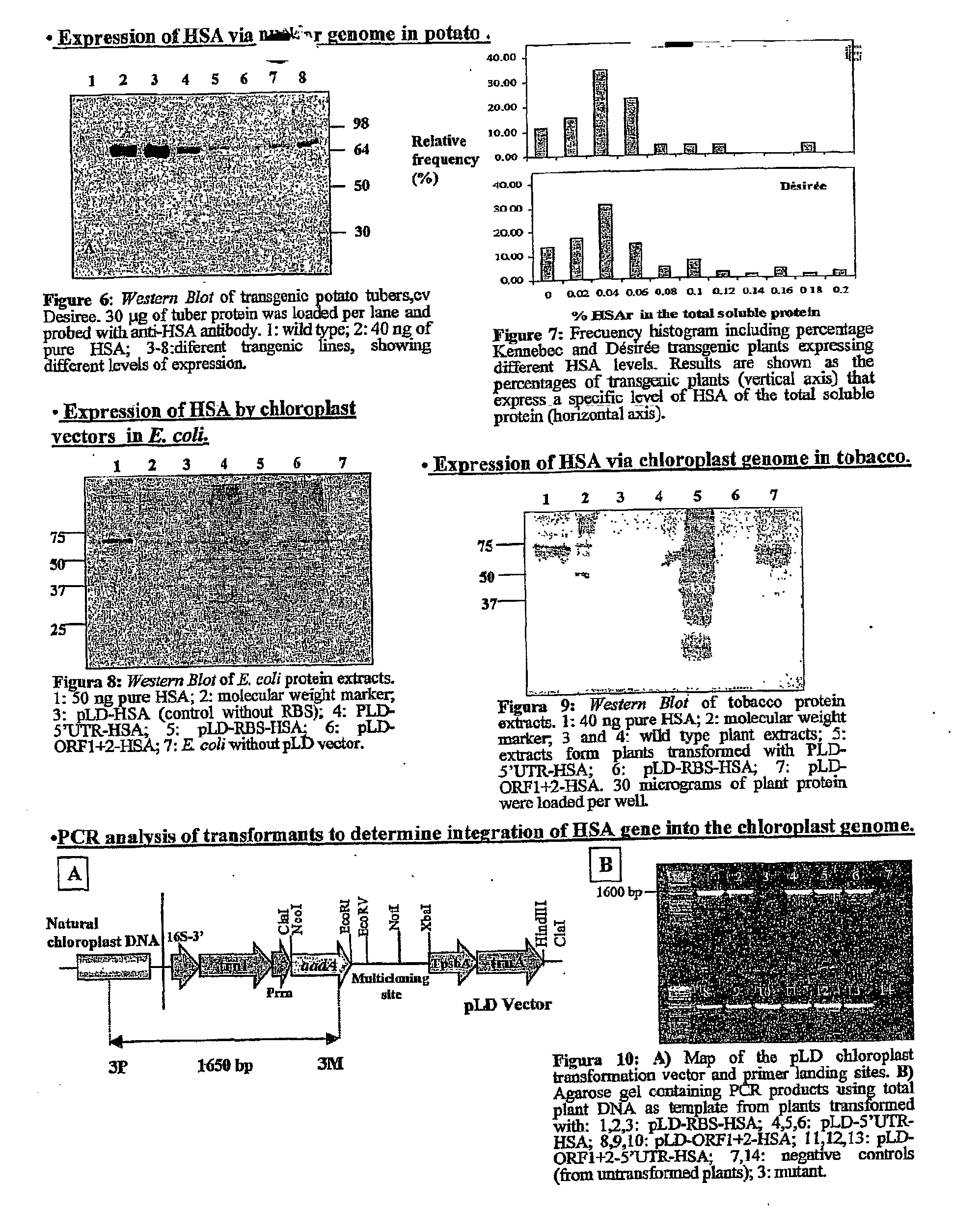
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Fragments or polymers of albumin with tunable vascular residence time for use in therapeutic delivery and vaccine development
InactiveUS20070041987A1Maximize the effect of treatmentMaximizing abilityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeResidence time
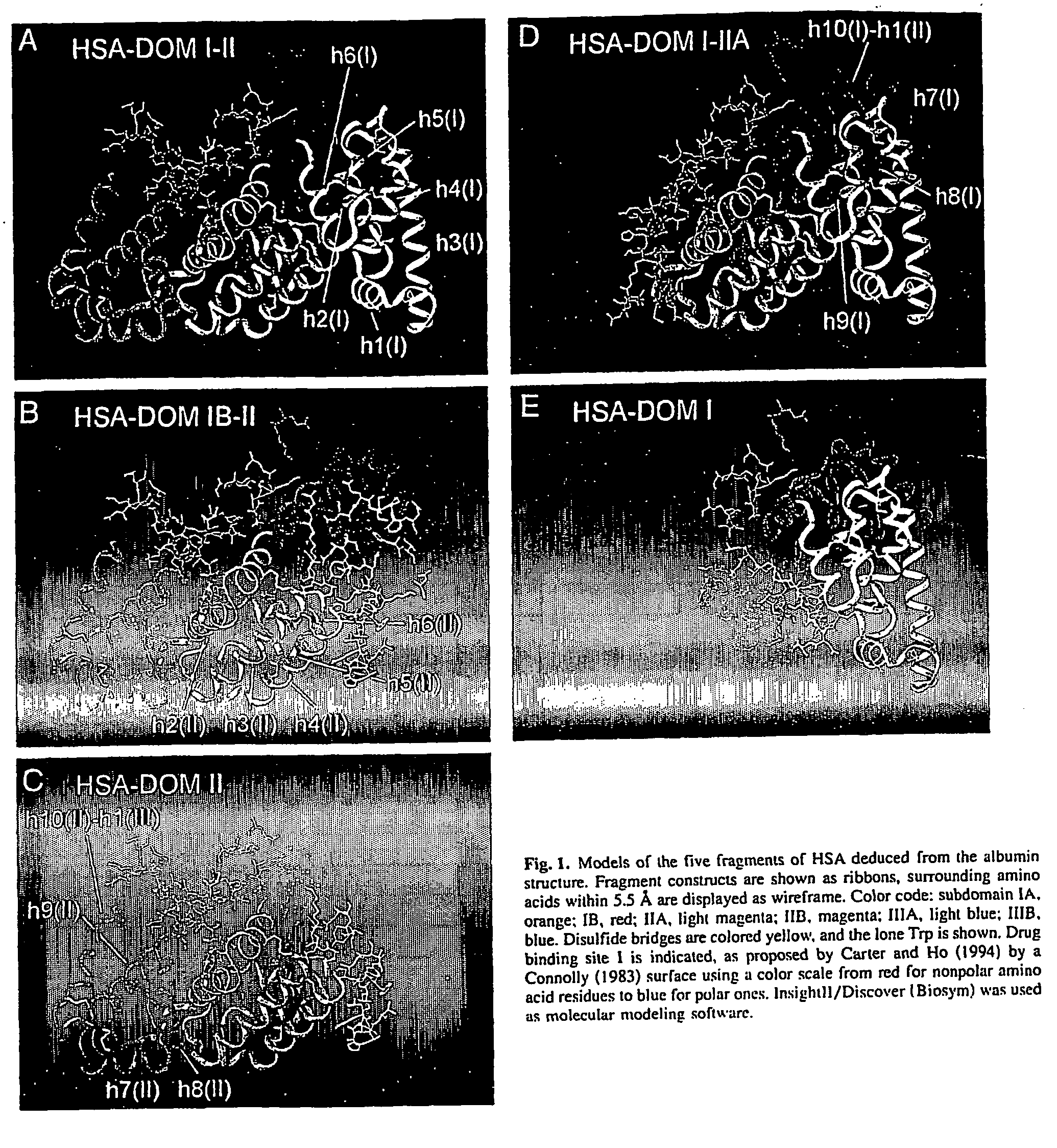
An isolated fusion protein is provided which is a conjugate of a therapeutic polypeptide and an albumin fragment, such as a fragment including an individual domain or subdomain of albumin, or a polymer of albumin (e.g., dimers, trimers, etc.) and which is used to optimize the half-life of that therapeutic agent in the bloodstream in a tunable fashion based on the molecular weight of the fragment or polymer. Albumin fragments useful in the invention include fragments containing any of the individual domains and subdomains of human serum albumin, as well as fragments including specific combinations of binding regions or subdomains. The present invention thus provides fragments or polymers which will allow for optimizing half-lives of therapeutic polypeptides depending on their molecular weight, and this will optimizes protein and vaccine therapeutics to have desired half lives for their greatest effectiveness.
Owner:NEW CENTURY PHARMA INC
In vivo unnatural amino acid expression in the methylotrophic yeast pichia pastoris
InactiveUS20090197339A1Enhanced and novel stericEnhanced and novel and chemicalFungiSerum albuminYeastIn vivo
The invention provides orthogonal translation systems for the production of polypeptides comprising unnatural amino acids in methylotrophic yeast such as Pichia pastoris. Methods for producing polypeptides comprising unnatural amino acids in methylotrophic yeast such as Pichia pastoris are also provided.
Owner:THE SCRIPPS RES INST
Albumin fusion proteins
InactiveUS20050244931A1Extended shelf lifeRetain activityFungiPeptide/protein ingredientsDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
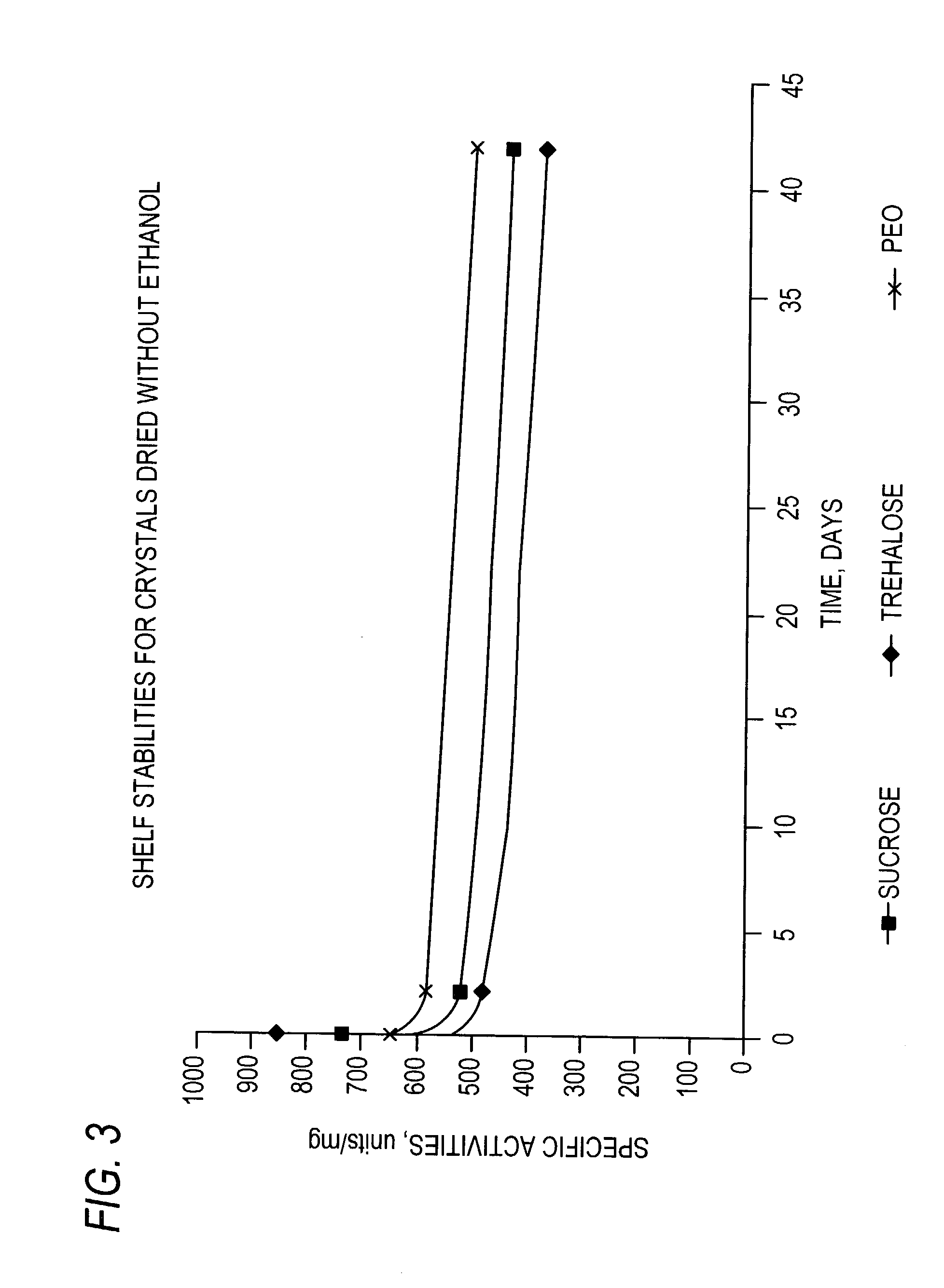
Stabilized protein crystals, formulations comprising them and methods of making them
InactiveUS7351798B2Further stabilizationComposition is stableImmobilised enzymesPowder deliveryPersonal careAdditive ingredient
This invention relates to methods for the stabilization, storage and delivery of biologically active macromolecules, such as proteins, peptides and nucleic acids. In particular, this invention relates to protein or nucleic acid crystals, formulations and compositions comprising them. Methods are provided for the crystallization of proteins and nucleic acids and for the preparation of stabilized protein or nucleic acid crystals for use in dry or slurry formulations. The present invention is further directed to encapsulating proteins, glycoproteins, enzymes, antibodies, hormones and peptide crystals or crystal formulations into compositions for biological delivery to humans and animals. According to this invention, protein crystals or crystal formulations are encapsulated within a matrix comprising a polymeric carrier to form a composition. The formulations and compositions enhance preservation of the native biologically active tertiary structure of the proteins and create a reservoir which can slowly release active protein where and when it is needed. Methods are provided preparing stabilized formulations using pharmaceutical ingredients or excipients and optionally encapsulating them in a polymeric carrier to produce compositions and using such protein crystal formulations and compositions for biomedical applications, including delivery of therapeutic proteins and vaccines. Additional uses for the protein crystal formulations and compositions of this invention involve protein delivery in human food, agricultural feeds, veterinary compositions, diagnostics, cosmetics and personal care compositions.
Owner:AJINOMOTO ALTHEA INC
Albumin-insulin fusion proteins
ActiveUS20080057004A1Efficient deliveryPowder deliveryPeptide/protein ingredientsInsulin activityIn vivo
Owner:TEVA BIOPHARM USA
Preparing monomeric metal ion chelator containing diacetyl glycine group linked to proteinaceous molecule
A precursor for the construction of chelated metal conjugates which demonstrate improved assay performance and utility in minimizing non-specific binding while maintaining specificity for target molecules is disclosed. The precursor has tridentate functionality towards multivalent ions such as iron and nickel and contains a diacetyl glycine group covalently linked via an amide to a molecule such as a proteinaceous molecule providing a primary amide group for amide bond formation. The precursor is preferably prepared in monomeric form by reacting nitrilotriacetic acid or a salt thereof in an aqueous medium at an alkaline pH of at least 8 with a proteinaceous molecule containing a primary amine group in the presence of a carbodiimide. The proteinaceous molecule may be bovine serum albumin or an enzyme such as alkaline phosphatase or horseradish peroxidase.
Owner:PIERCE BIOTECHNOLOGY
Protein containing serum albumin domain
InactiveUS7253259B2Improve functional activityImprove antioxidant capacityFungiBacteriaSerum igeADAMTS Proteins
A protein produced by gene recombinant technology, including at least one domain selected from domains I, II, and III of serum albumin but having a different structure from that of native albumin; and a method of producing the protein. The protein has an enhanced functional activity or activities selected from among various functional activities or serum albumin including antibacterial activity, antioxidative effect, inflammation inhibitory effect, in vivo substance transporting action, and enzymatic activity.
Owner:NIPRO CORP
Hiv inhibiting proteins
InactiveUS20060241027A1High activityProlong half-life in vivoBiocidePeptide/protein ingredientsHiv transmissionAlbumin
The invention relates to proteins comprising HIV fusion inhibiting peptides, such as T-20 and / or T-1249 peptides (including, but not limited to, fragments and variants thereof), which exhibit anti-retroviral activity, fused to albumin (including, but nbot limited to fragments or variants of albumin). These fusion proteins are herein collectively referred to as “albumin fusion proteins of the invention.” These fusion proteins exhibit extended shelf-life and / or extended or therapeutic activity. The invention encompasses therapeutic albumin fusion proteins, compositions, pharmaceutical compositions, formulations and kits. The invention also encompasses nucleic acid molecules encoding the albumin fusion proteins of the invention, as well as vectors containing these nucleic acids, host cells transformed with these nucleic acids and vectors, and methods of making the albumin fusion proteins of the invention using these nucleic acids, vectors, and / or host cells. The invention also relates to compositions and methods for inhibiting HIV-induced cell fusion. The invention further relates to compositions and methods for inhibiting HIV transmission to uninfected cells.
Owner:NOVOZYMES BIOPHARMA DK AS
Compositions and methods for scaffold formation
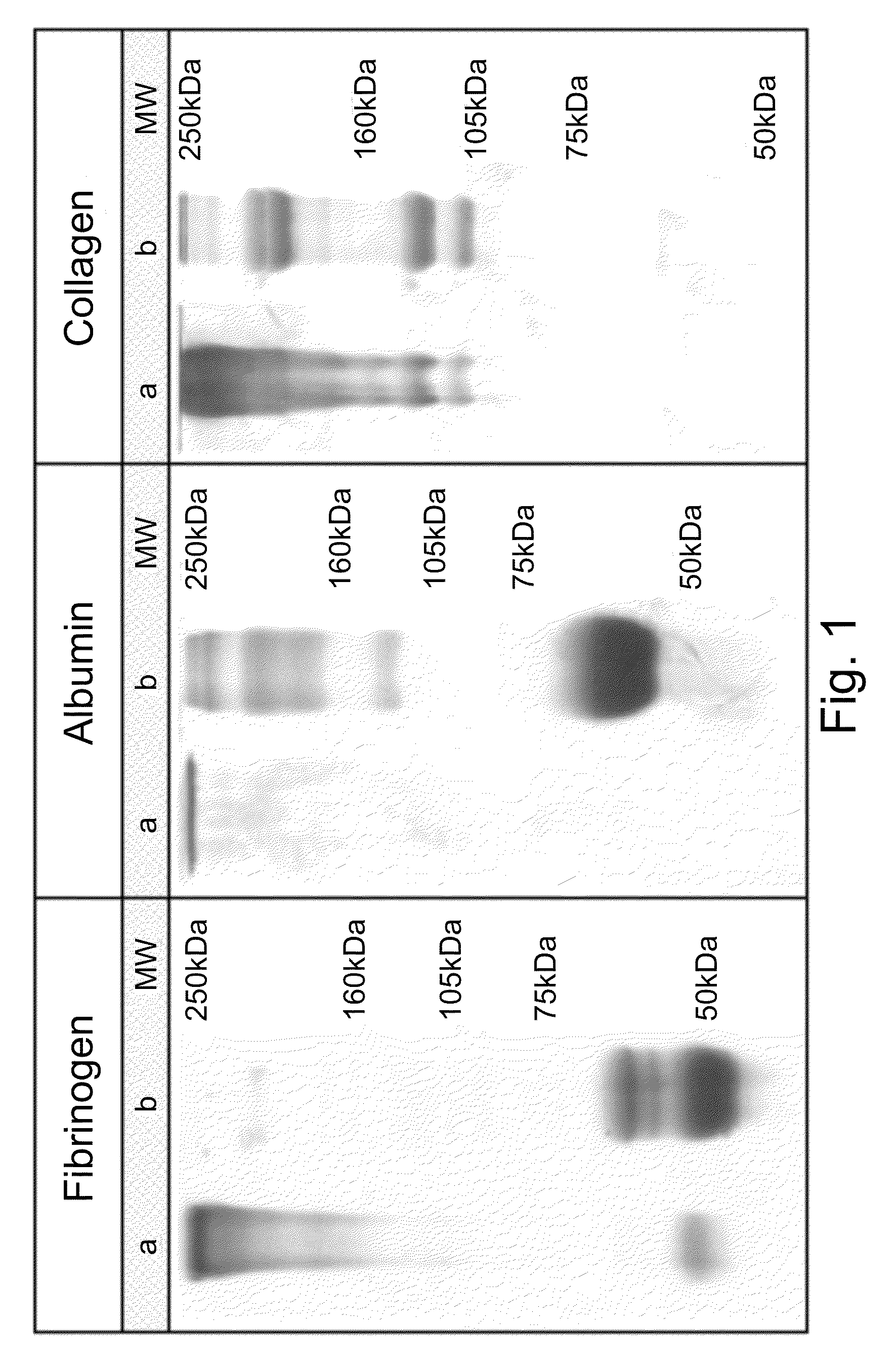
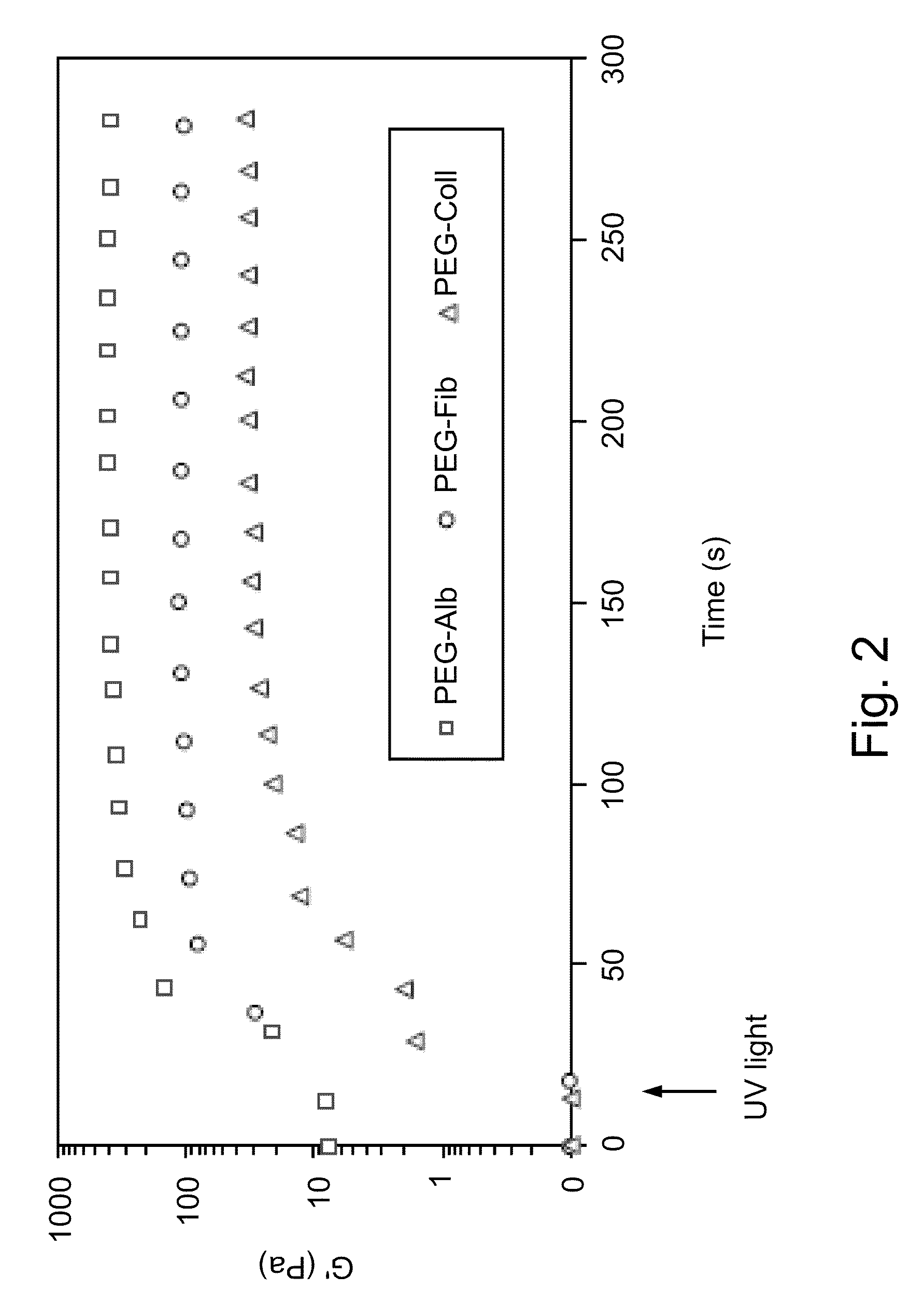
The present invention relates to scaffolds composed of a protein backbone cross-linked by a synthetic polymer. Specifically, the present invention provides PEGylated-thiolated collagen scaffolds and PEGylated albumin scaffolds and methods of generating and using same for treating disorders requiring tissue engineering.
Owner:REGENTIS BIOMATERIALS
Modified interferon beta with reduced immunogenicity
InactiveUS20050054052A1Extended circulation timeImproved propertyPeptide/protein ingredientsSerum albuminBiochemistryHuman Interferon Beta
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human interferon beta to result in proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Bovine serum albumin-platinum composite nanomaterial mimetic peroxidase
InactiveCN103433484ANo significant change in catalytic activityImprove stabilitySerum albuminPeroxidaseUltrafiltration
The invention discloses bovine serum albumin-platinum composite nanomaterial mimetic peroxidase as well as a preparation method thereof and application. Bovine serum albumin is used as a template, and the bovine serum albumin-platinum composite nanomaterial mimetic peroxidase is prepared through biomineralization. Bovine serum albumin-platinum composite nanomaterials are prepared through the following method that chloroplatinic acid aqueous solutions are added to bovine serum albumin aqueous solutions and are mixed, sodium hydroxide aqueous solutions are added to obtain mixed solutions, and water bath heating is carried out; ultrafiltration is carried out on the solutions, then the solutions are washed, and bovine serum albumin-platinum composite nanomaterial aqueous solutions are obtained. The bovine serum albumin-platinum composite nanomaterials have excellent peroxidase activity, and can catalyze hydrogen peroxide oxidation 3, 3', 5, 5'-tetramethyl benzidine hydrochloride to be in color development. Meanwhile, the mimetic peroxidase resists acid and base, high temperature and high salinity, and has excellent short-term indoor temperature stability and long-term indoor temperature stability.
Owner:FUJIAN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com