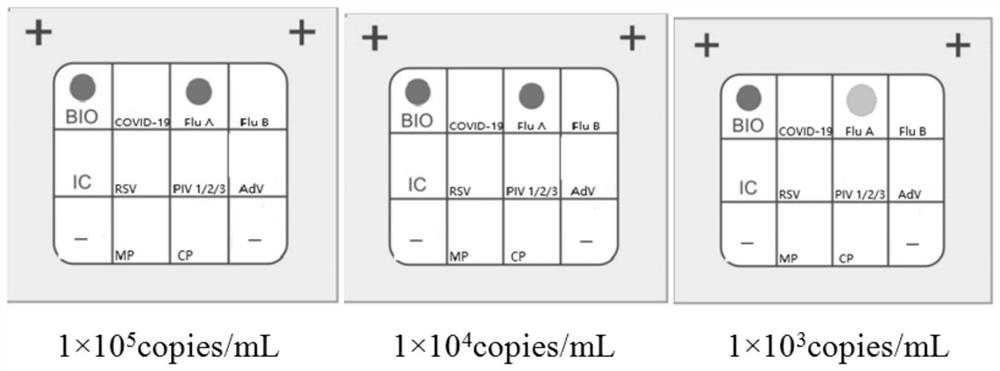
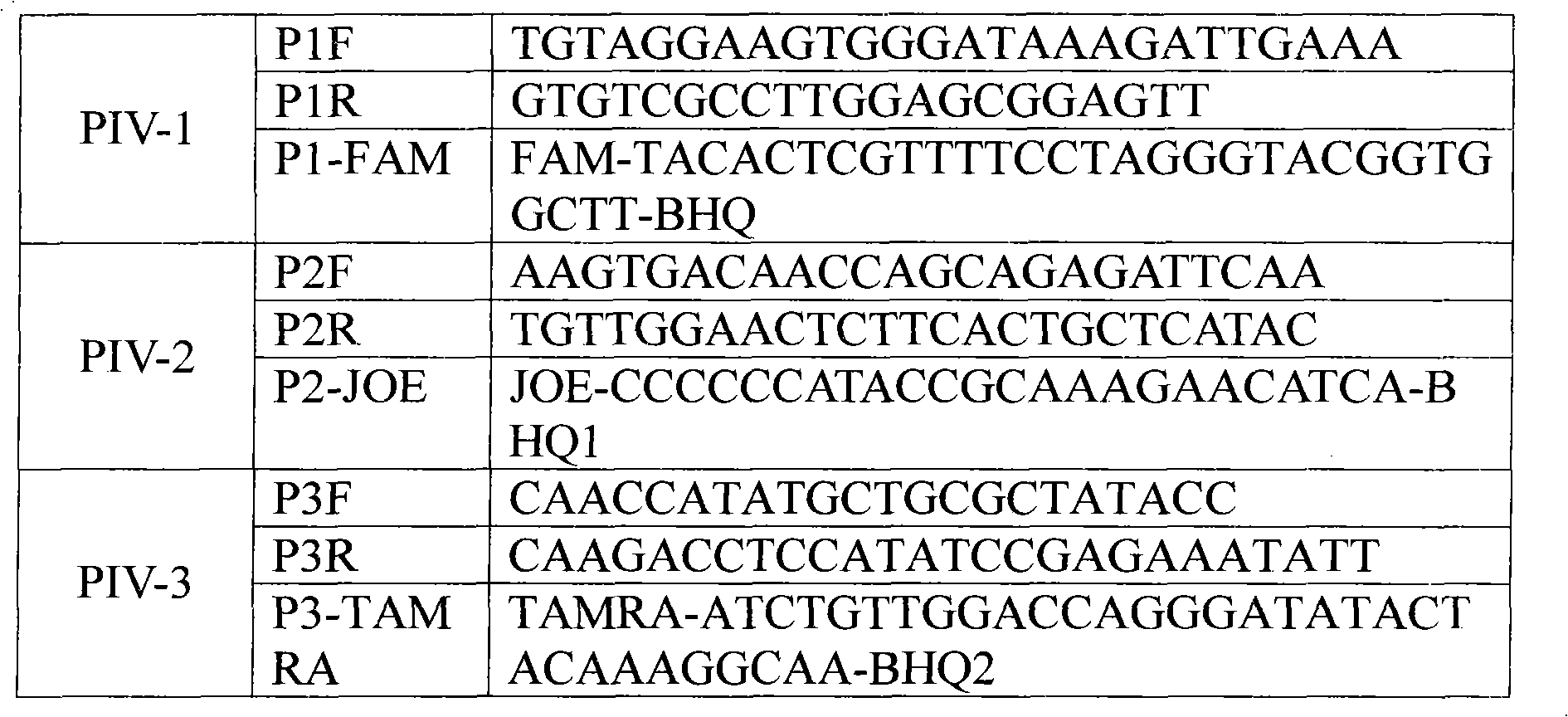
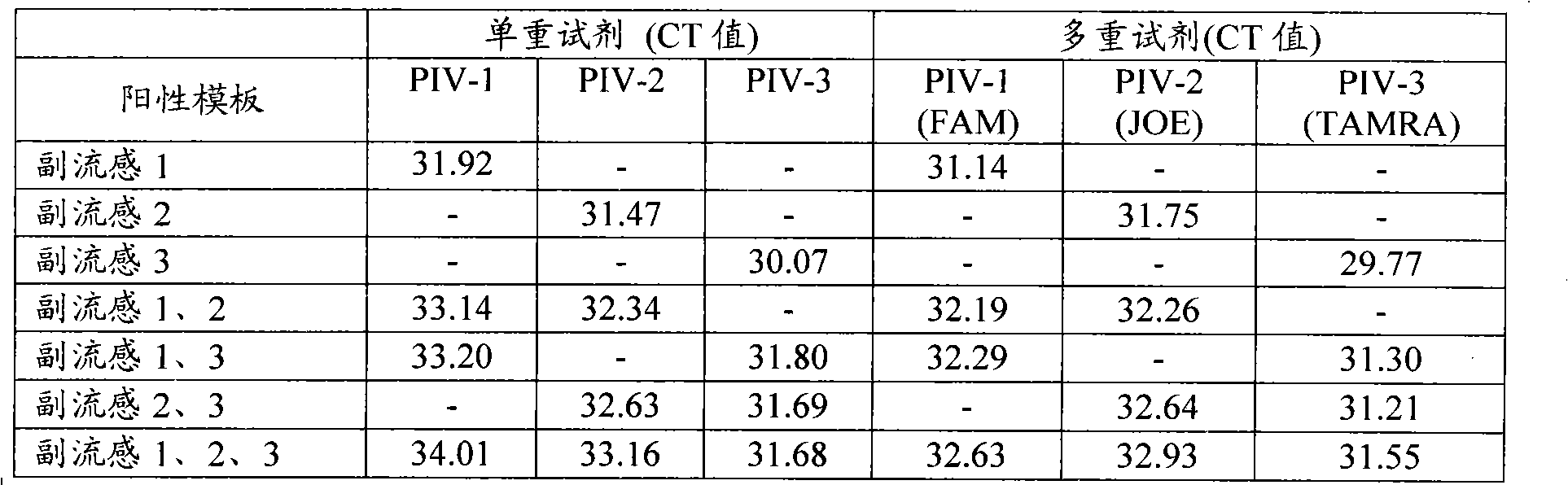
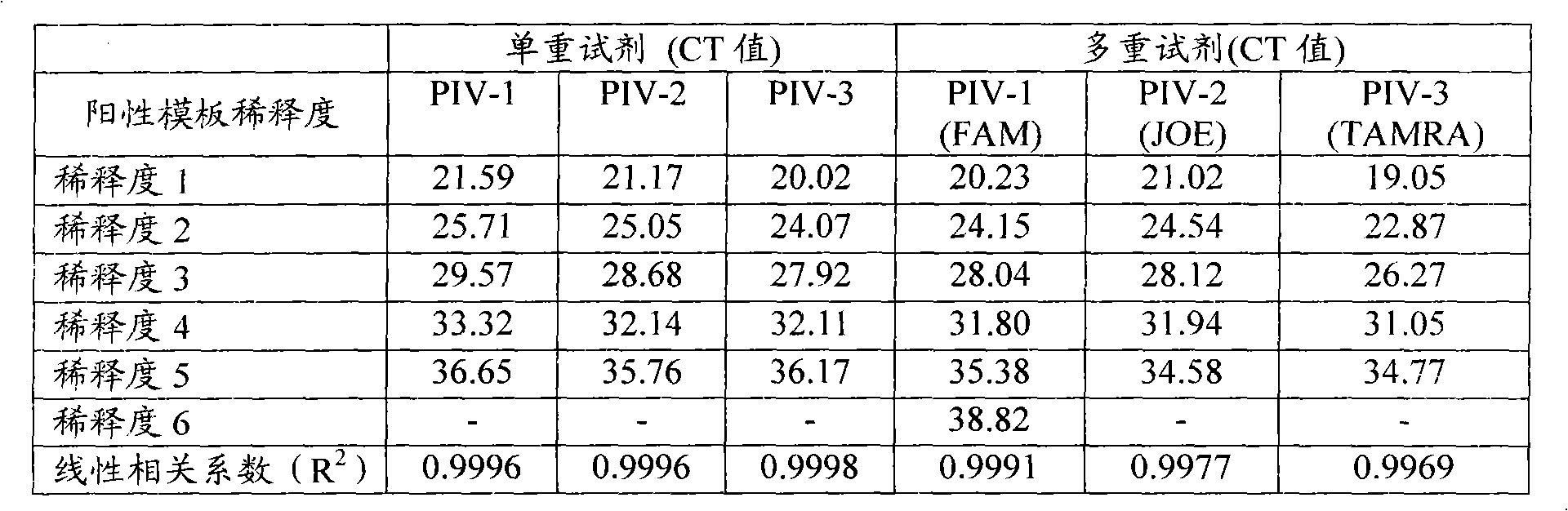
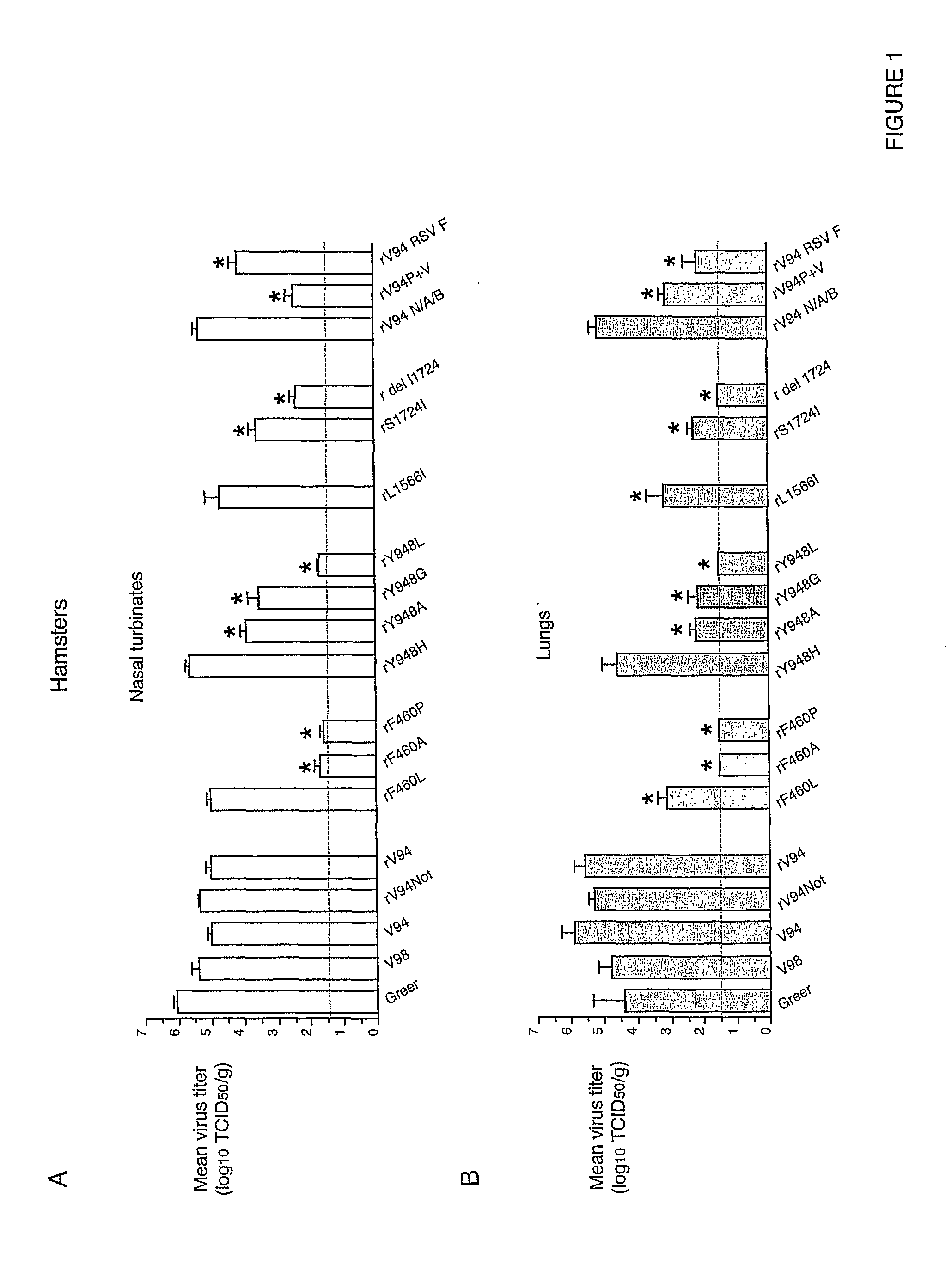
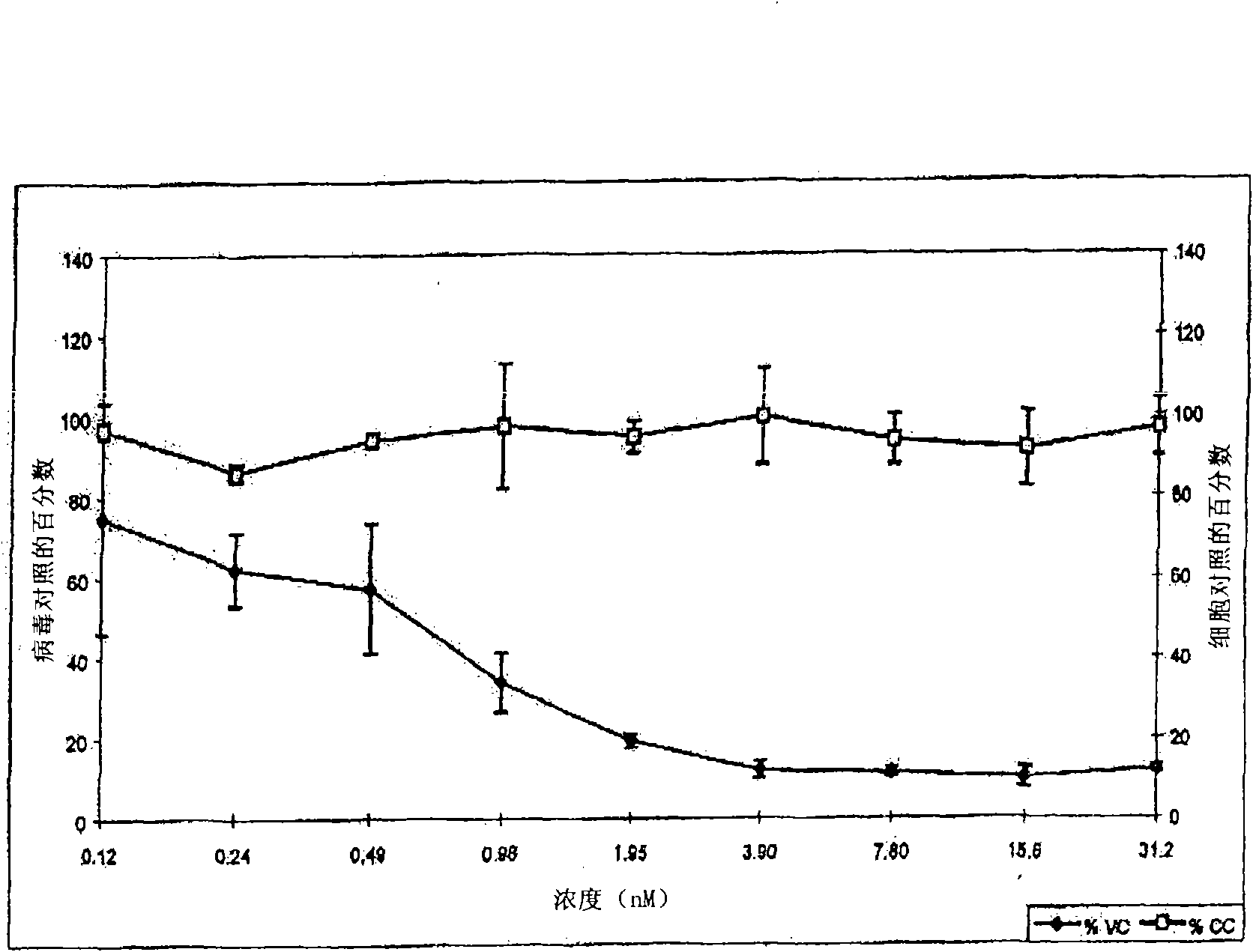
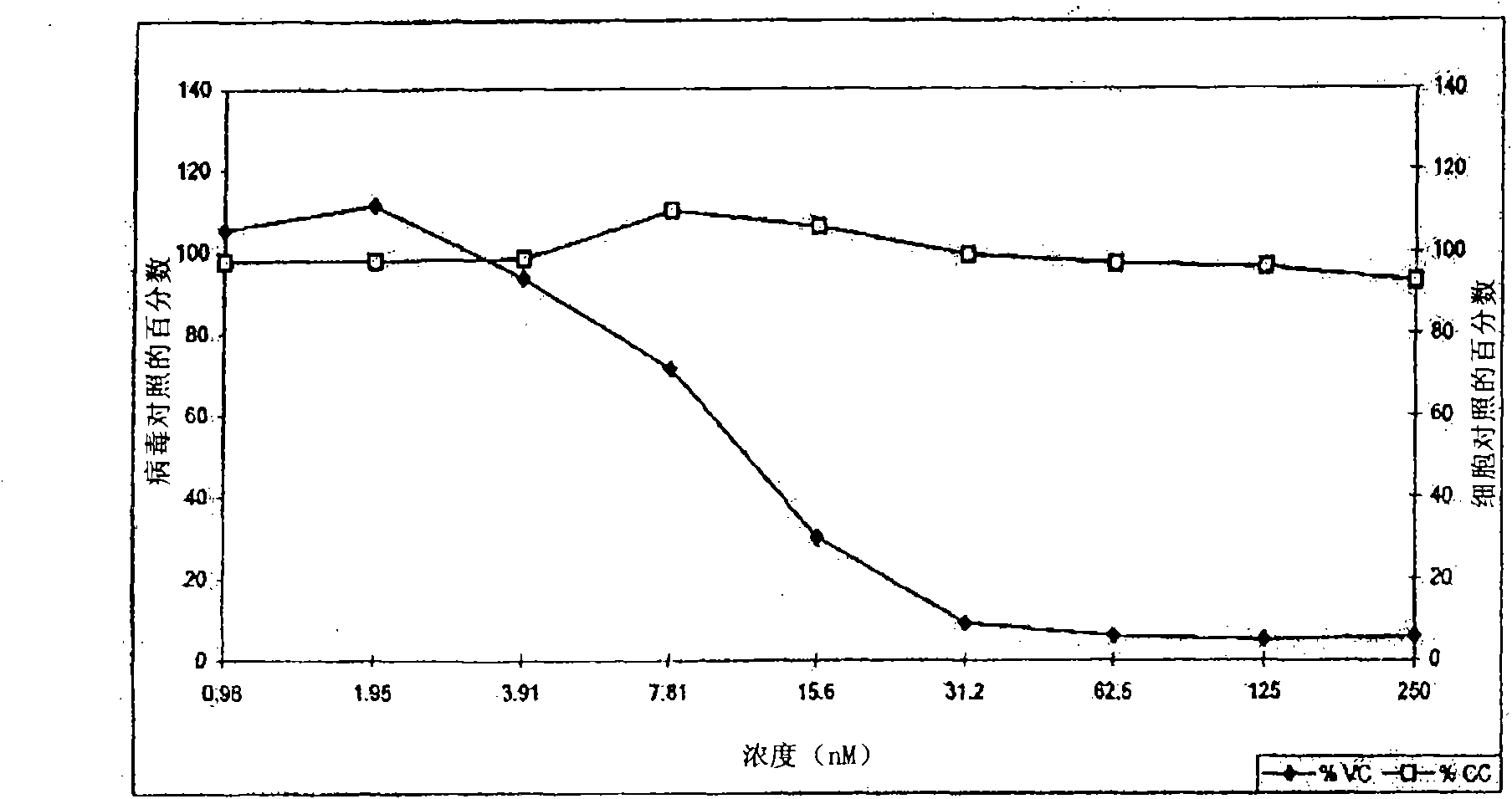
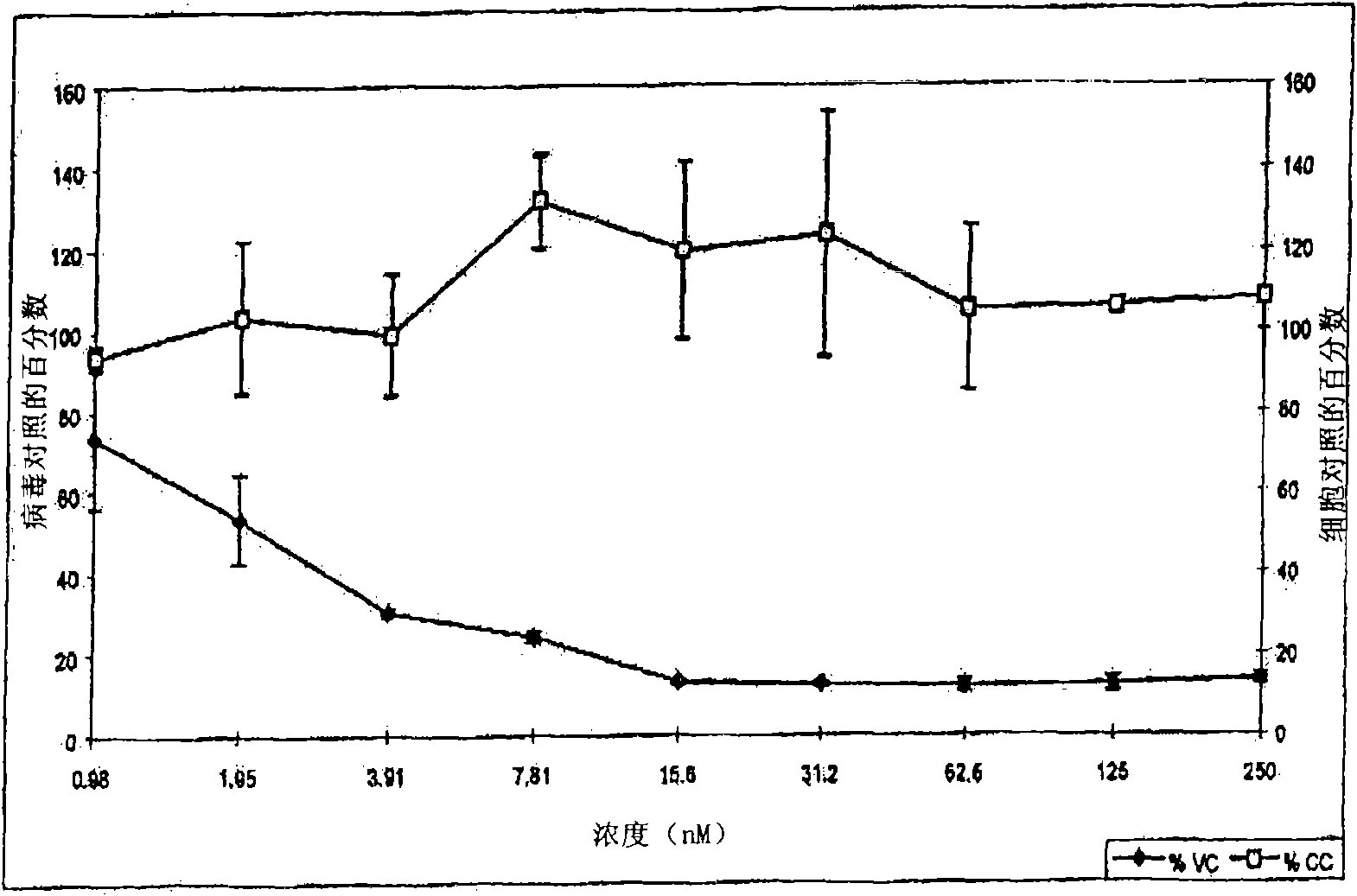
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Human Parainfluenza Virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
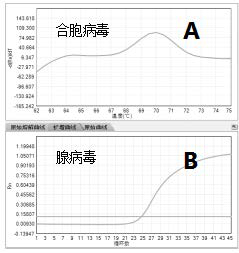
A non-taxonomic group of paramyxoviruses that are cause of respiratory tract infections in humans. Infection is most common in infants and young children and symptoms may include fever, runny nose, and cough. Infection is usually self-limiting but can also cause more severe illness, such as croup or pneumonia.
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
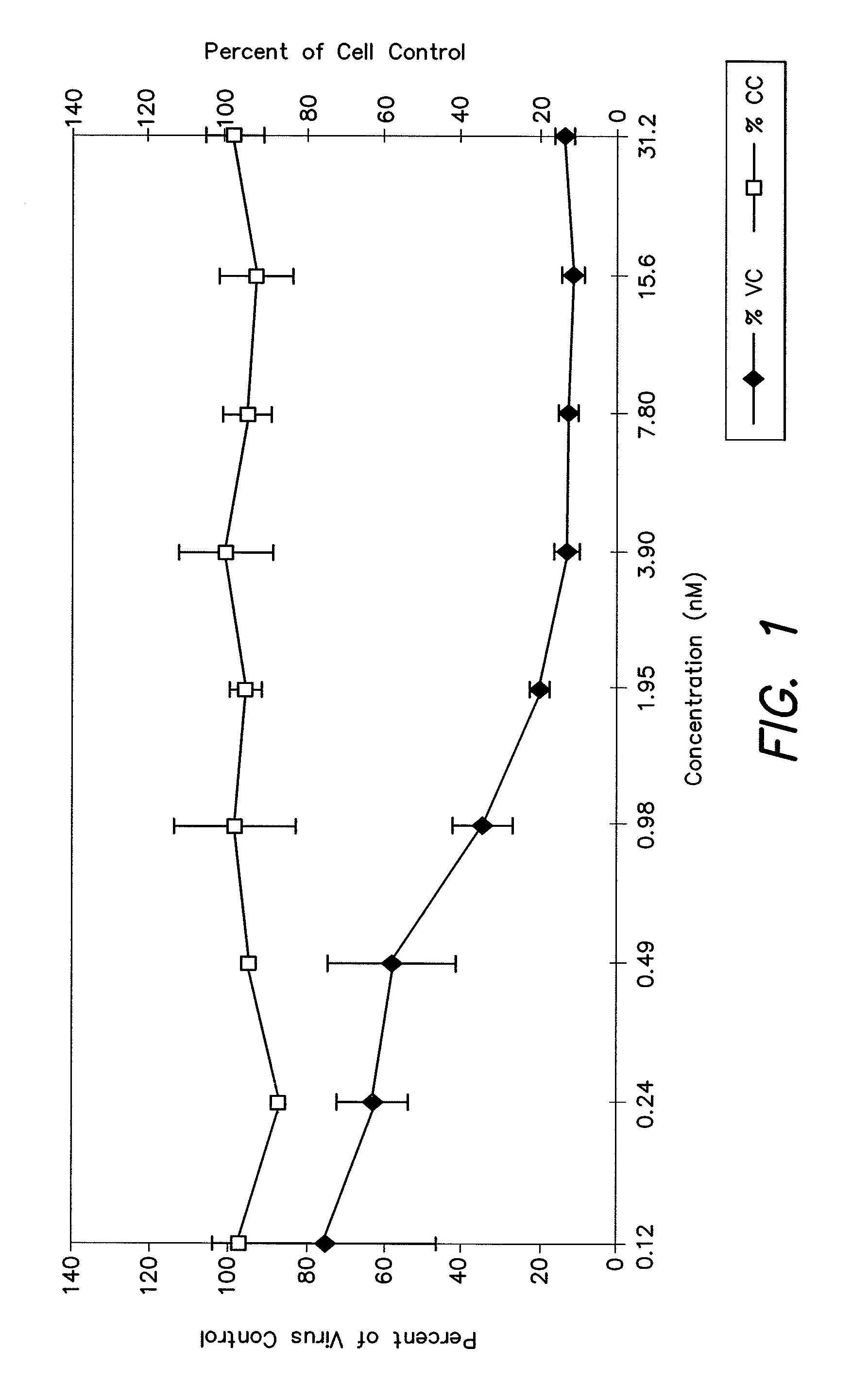
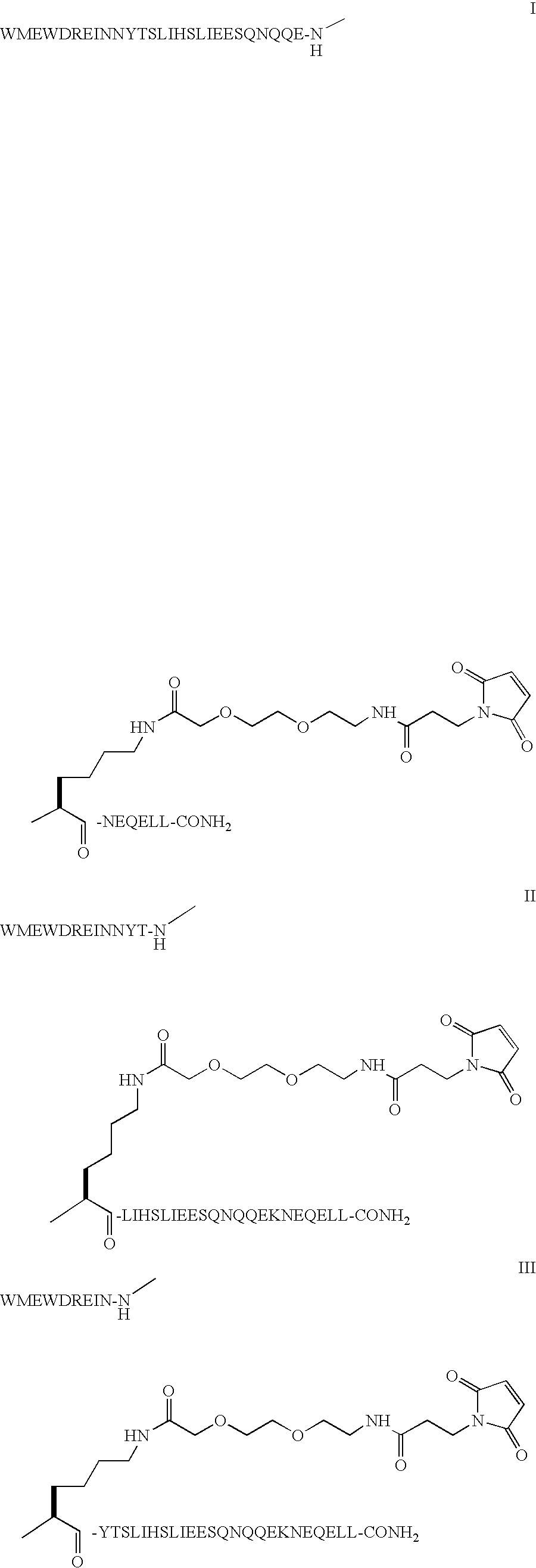
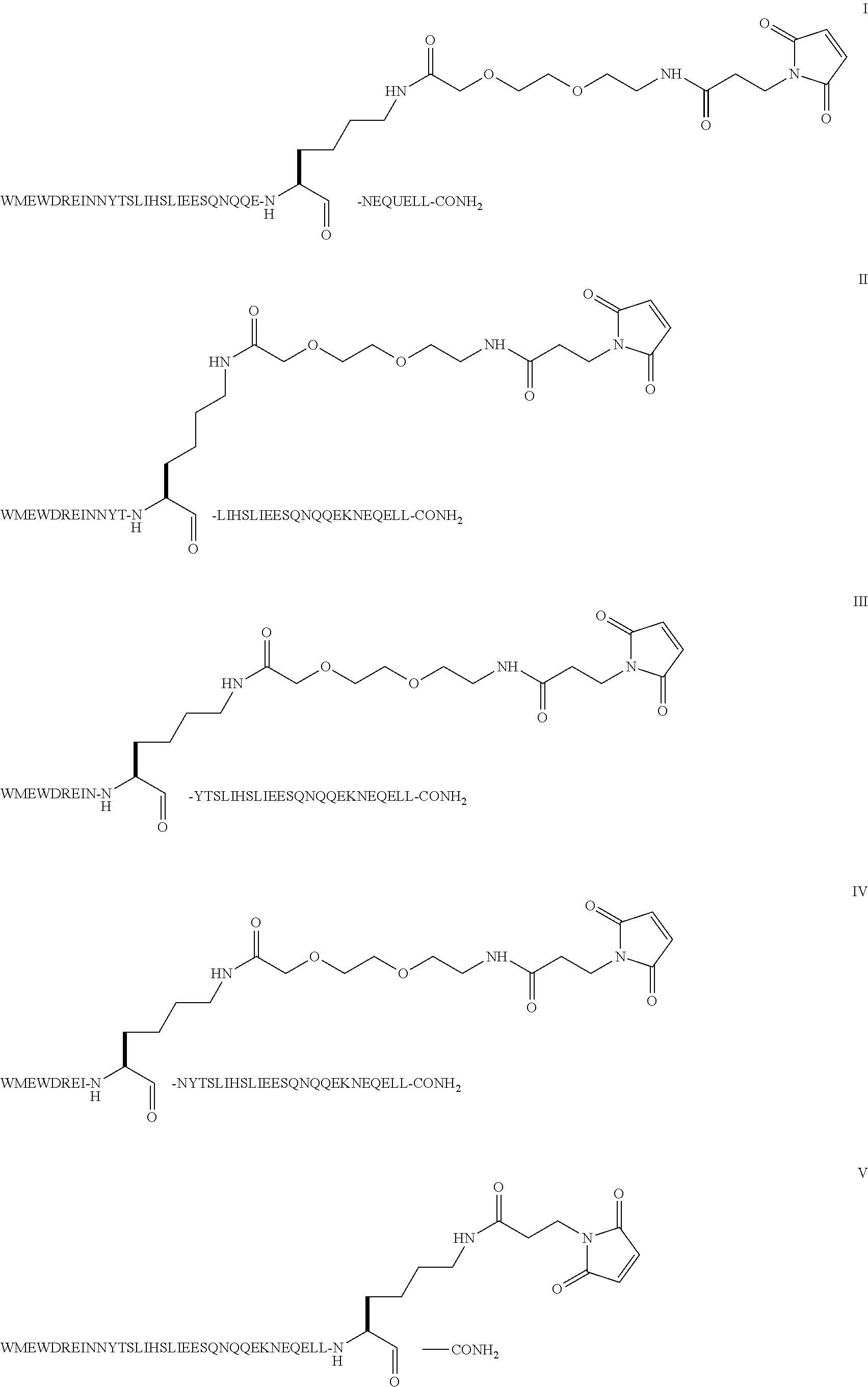
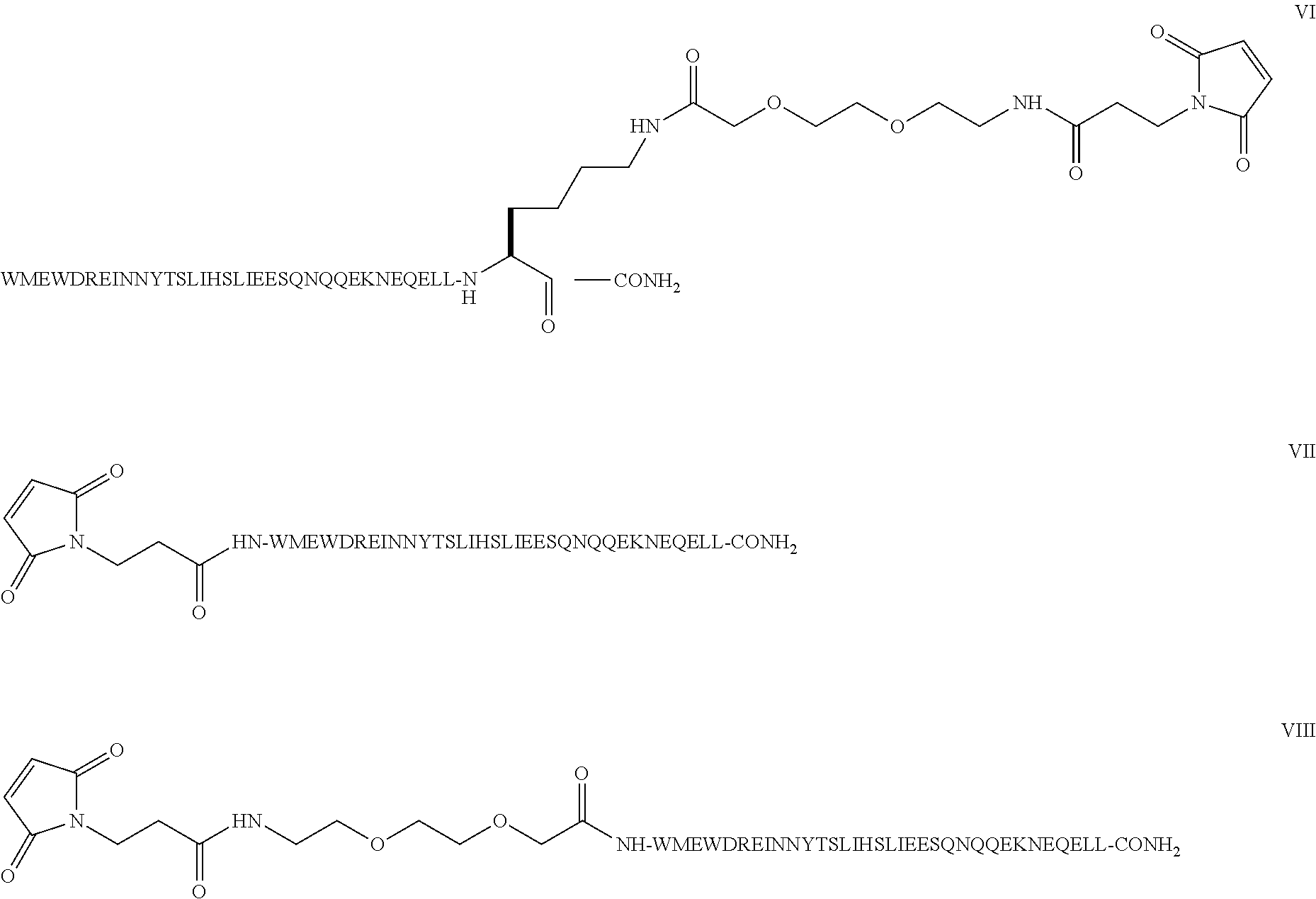
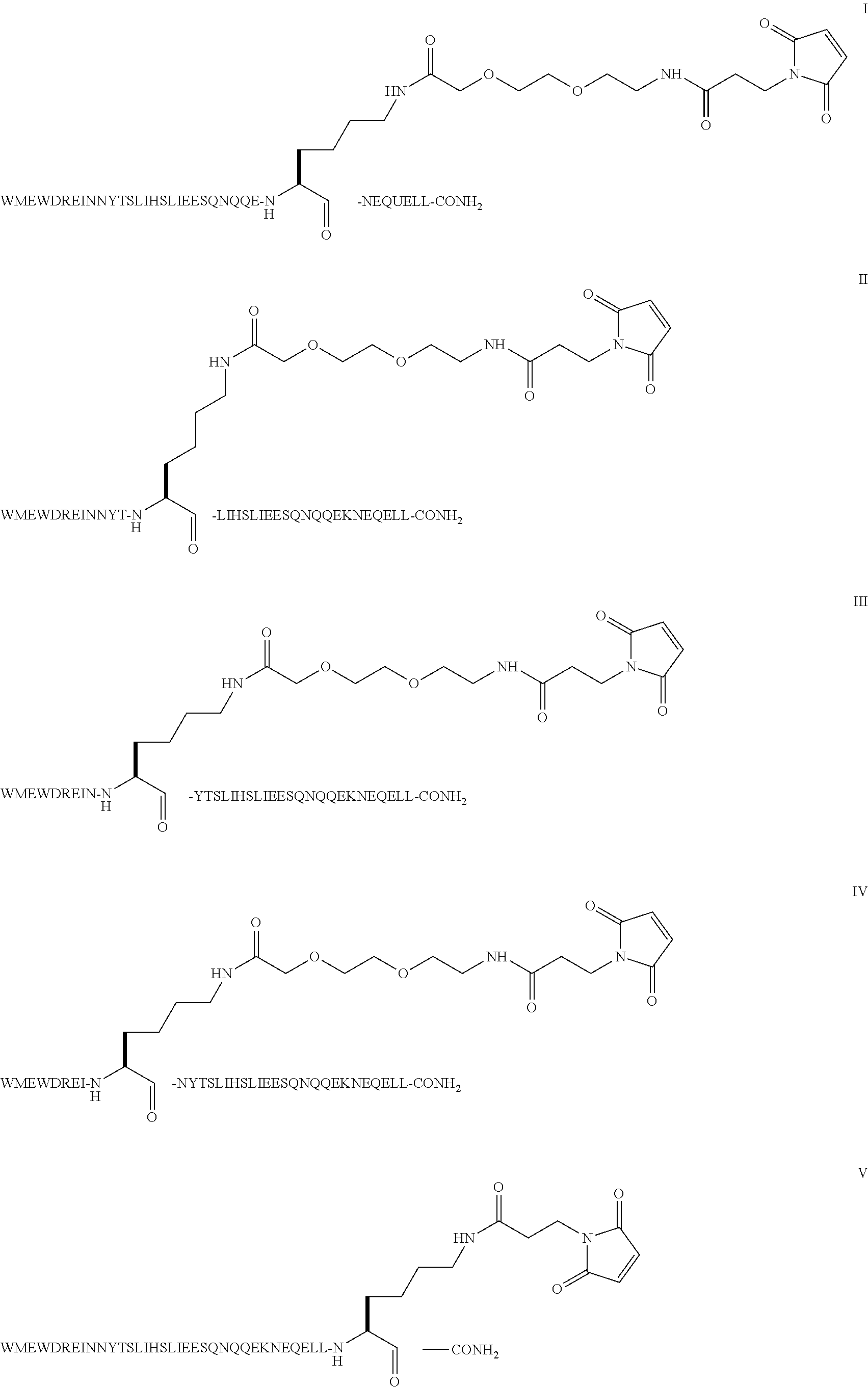
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Anti-viral treatment and assay to screenfor Anti-viral agent
InactiveUS20130085133A1Improve palatabilityImprove stabilityBiocideSugar derivativesCytopathic effectAntiviral drug
The present disclosure relates to novel compounds of formulas (1) through (19) and to a method for treating humans infected with a virus including various respiratory viruses such as members of the Paramyxoviridae family (respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV), measles virus, and mumps virus) with a compound of formulas (1) through (19). The present disclosure also relates to a cytopathic effect (CPE)-based assay that will assess virus-induced CPE for screening of compounds for treating viral diseases or inhibiting a virus.
Owner:SOUTHERN RES INST & IP
Long lasting fusion peptide inhibitors for hiv infection
InactiveUS20050065075A1Prolong half-life in vivoBiocidePeptide/protein ingredientsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
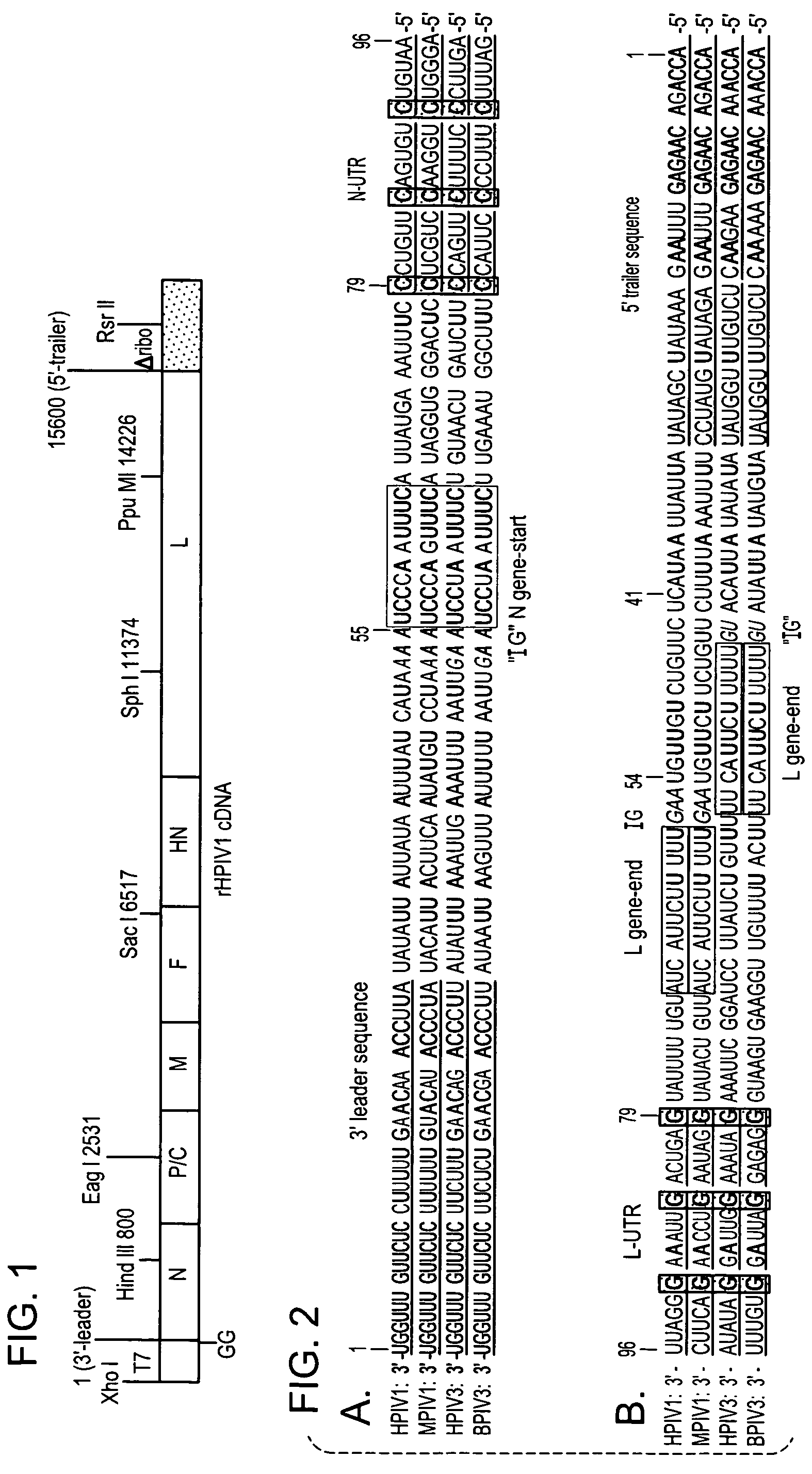
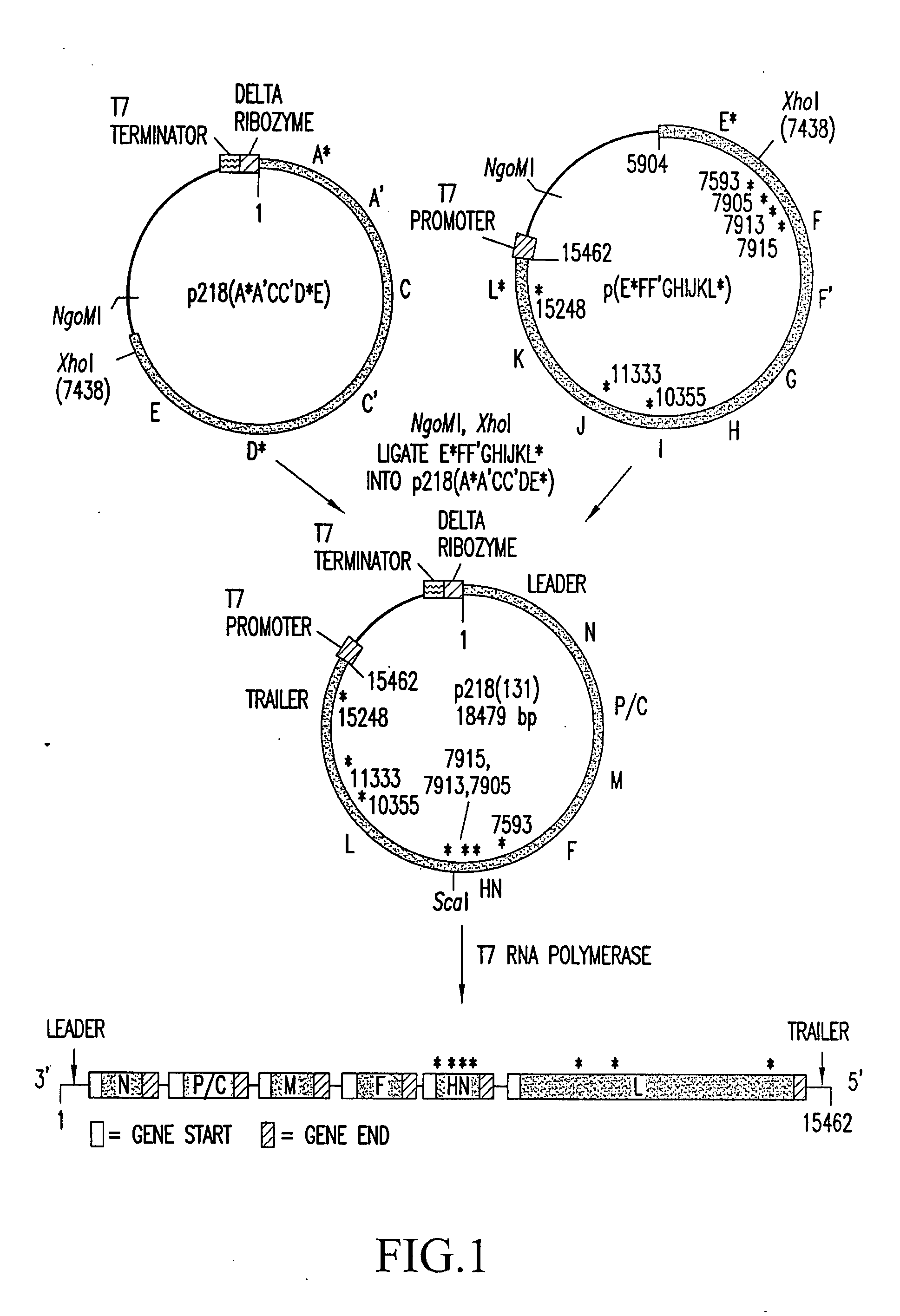
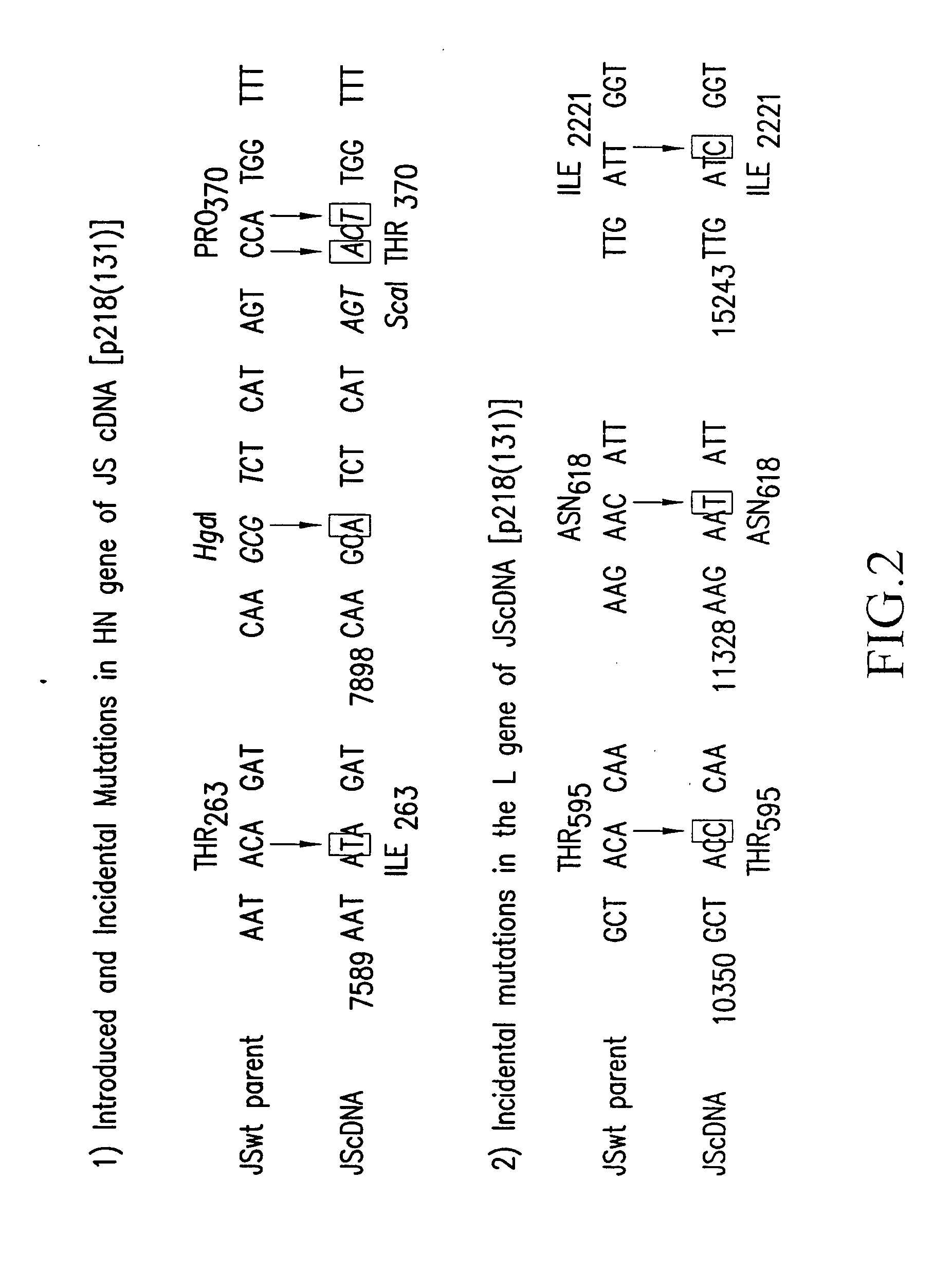
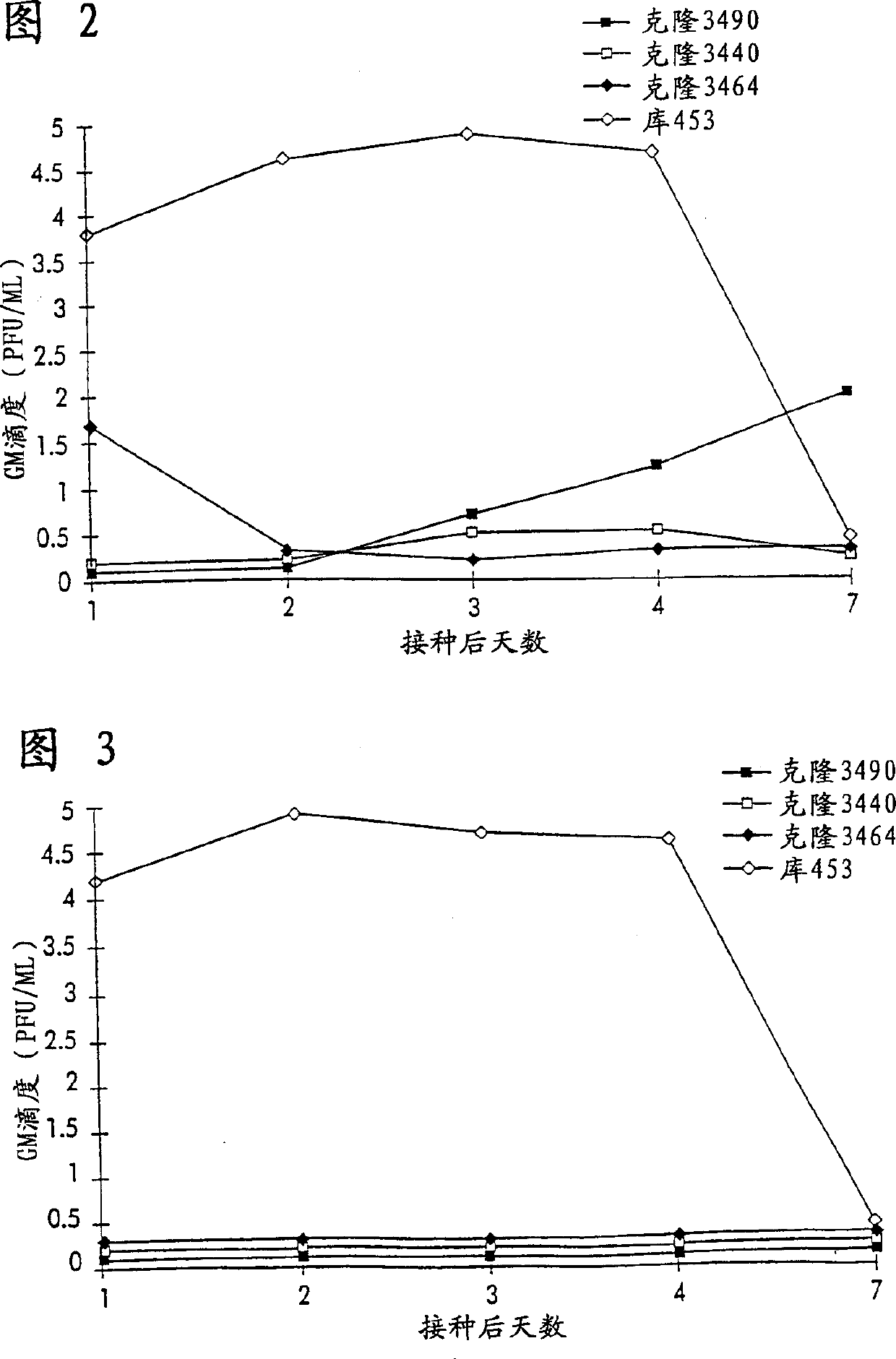
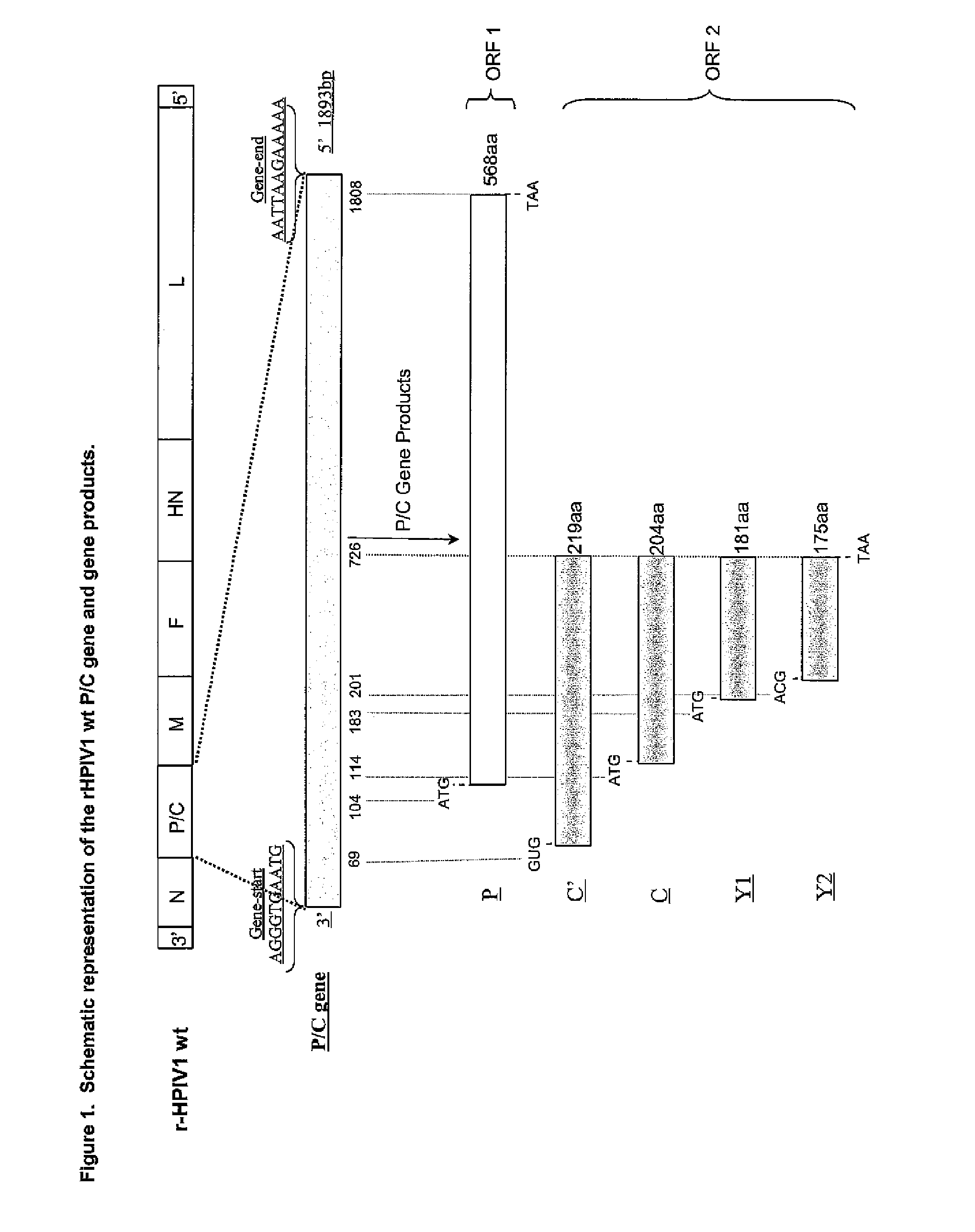
Recovery of recombinant human parainfluenza virus type 1 (HPIV1) from cDNA and use of recombinant HPIV1 in immunogenic compositions and as vectors to elicit immune responses against PIV and other human pathogens
InactiveUS7704509B2Mass productionBoost the response to both HPIVSsRNA viruses negative-senseViral antigen ingredientsHuman Parainfluenza VirusParainfluenza virus
Recombinant human parainfluenza virus type 1 (HPIV1) compostions, formulations and methods are provided. The recombinant HPIV1 viruses and HPIV1 chimeric and chimeric vector viruses provided according to the invention are infectious and attenuated in permissive mammalian subjects, including humans, and are useful in immunogenic composition s for eliciting an immune responses against one or more PIVs, against one or more non-PIV pathogens, or against a PIV and a non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a recombinant HPIV1 genome or antigenome.
Owner:UNITED STATES OF AMERICA
Kit for jointly detecting respiratory tract pathogen through multiple fluorescent PCR method
ActiveCN107058622ANo further action requiredShorten the course of the diseaseMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlFluorescence
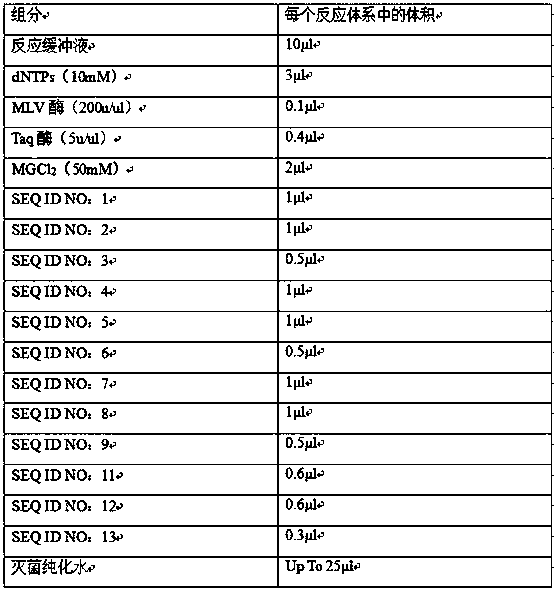
The invention provides a kit for jointly detecting respiratory tract pathogen through a multiple fluorescent PCR method. The kit comprises six components: reaction liquid A, reaction liquid B, reaction liquid C, enzyme mixed liquid, positive control and negative control, and comprises 11 common respiratory tract pathogen detections (general type of influenza virus A, influenza virus B, respiratory syncytial virus, 1 / 2 / 3 type of human parainfluenza virus, adenovirus, mycoplasma pneumoniae, chlamydia pneumonia, legionella pneumophila, streptococcus pneumonia, haemophilus influenza, A streptococcal); the amplification is performed through three reaction buffers, and each reaction buffer contains four fluorescent channels, 90% pathogen infection on the clinic can be checked.
Owner:DEBIQI BIOTECH XIAMEN
Multiplex PCR detection kit for nucleic acids of twelve respiratory viruses
ActiveCN105734168ASuitable for detectionReduce testing costsMicrobiological testing/measurementAgainst vector-borne diseasesInfluenza h1n1Multiplex pcrs
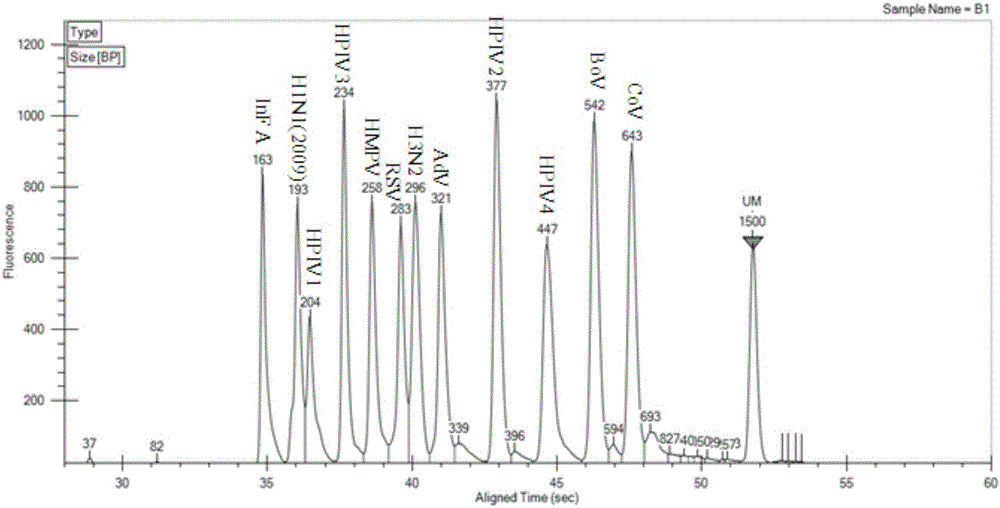
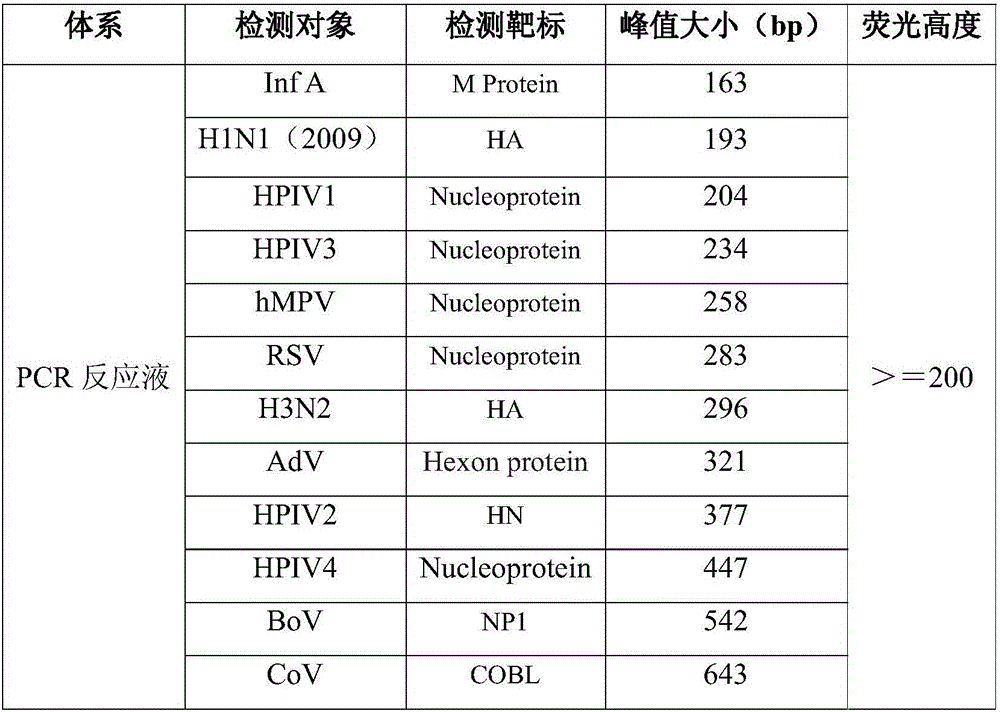
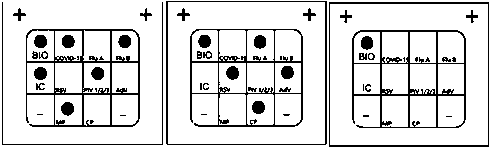
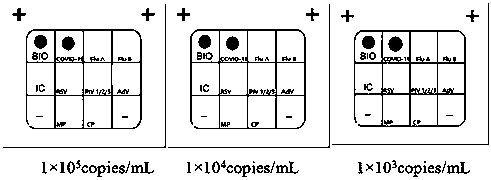
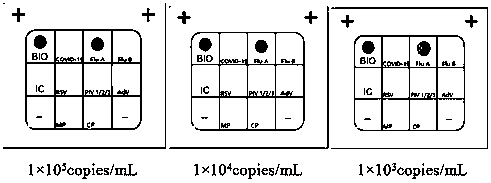
The invention discloses a Multiplex PCR detection kit for nucleic acids of twelve respiratory viruses. The kit includes primers for amplifying the specific gene loci of the following twelve respiratory viruses: influenza A virus (Inf A), influenza A H1N1 (2009), influenza A H3N2, human parainfluenza virus (HPIV1), human parainfluenza virus (HPIV2), human parainfluenza virus (HPIV3), human parainfluenza virus (HPIV4), human metapneumovirus (hMPV), respiratory adenovirus (AdV), respiratory syncytial virus (RSV), bocavirus (BoV) and coronavirus (CoV). The kit allows multiplex detection, has high sensitivity and is convenient and quick to use. Specific primer sequences ensure reliability of detection results. A detection method is simple in operation, save time and labor intensity, has high detection throughput, is low in cost of reagent and consumable, and can directly detect the nucleic acids extracted from a respiratory pathogen sample, is low in requirement on detection platform and operation technology, and can be widely promoted in common detection.
Owner:NANJING MOKOBIO BIOTECH
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Cysteic acid derivatives of Anti-viral peptides
InactiveUS20090088377A1Easy to processEasy to purifyPeptide/protein ingredientsVirus peptidesFeline immunodeficiency virusImmunodeficiency virus
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Long lasting fusion peptide inhibitors for HIV infection
InactiveUS7741453B2Prolong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Probes and methods for the simultaneous detection and identification of multiple viruses that cause respiratory infections in humans
InactiveCN101107366AShorten the lengthSynthetic economyMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusSevere acute respiratory syndrome
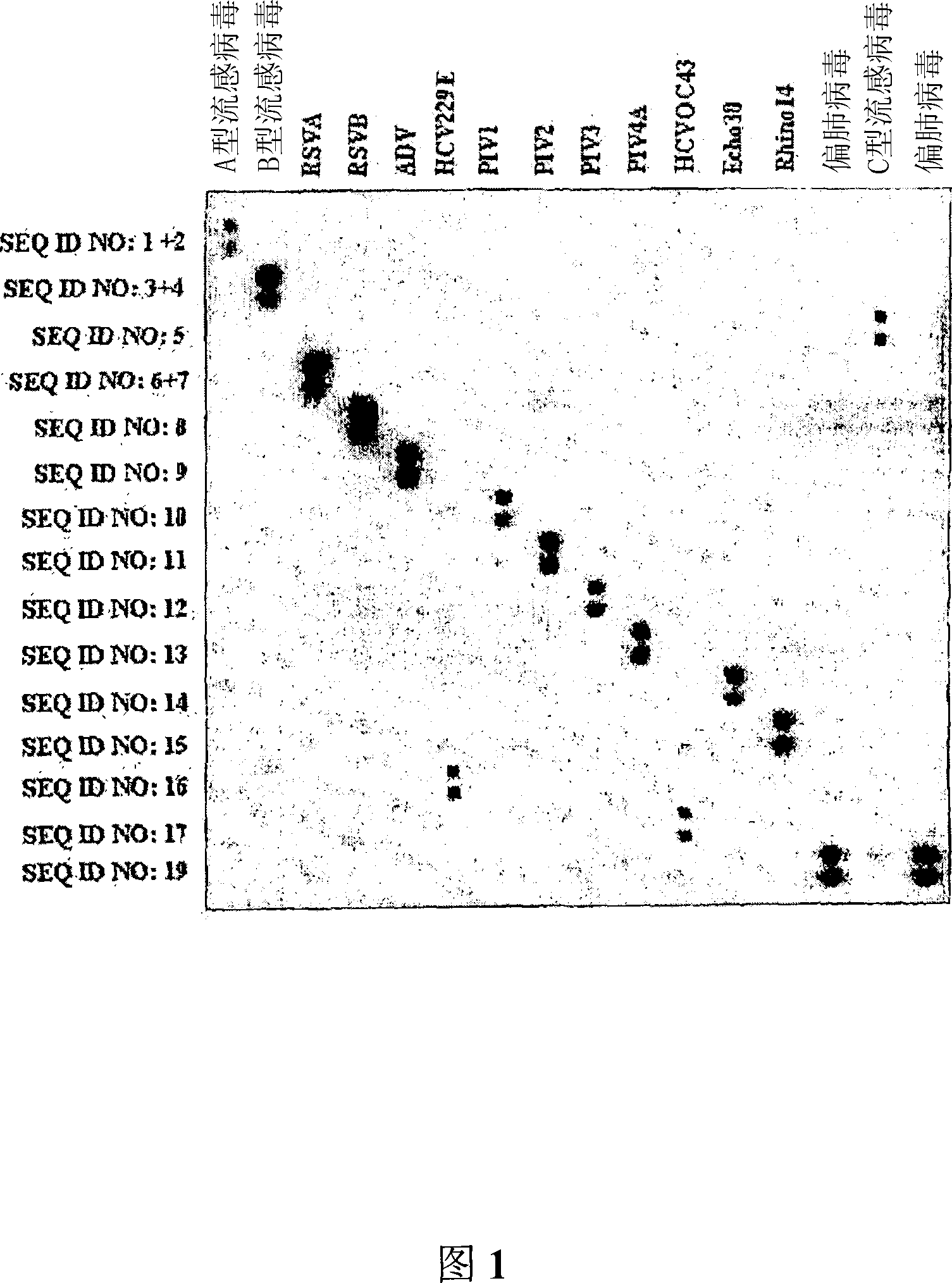
The invention relates to probes and assays which are used for the simultaneous detection, in a single assay sample, of a plurality of nucleic acid sequences of viruses that cause respiratory infections in humans, selected from among influenza virus type A, influenza virus type B, influenza virus type C, human respiratory syncytial virus type A, human respiratory syncytial virus type B, human adenovirus, human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3, human parainfluenza virus types 4A and 4B, enterovirus, rhinovirus, human coronavirus type 229E, human coronavirus type OC43, coronavirus that causes severe acute respiratory syndrome (SARS), human metapneumovirus and combinations thereof.
Owner:INST DE SALUD CARLOS III
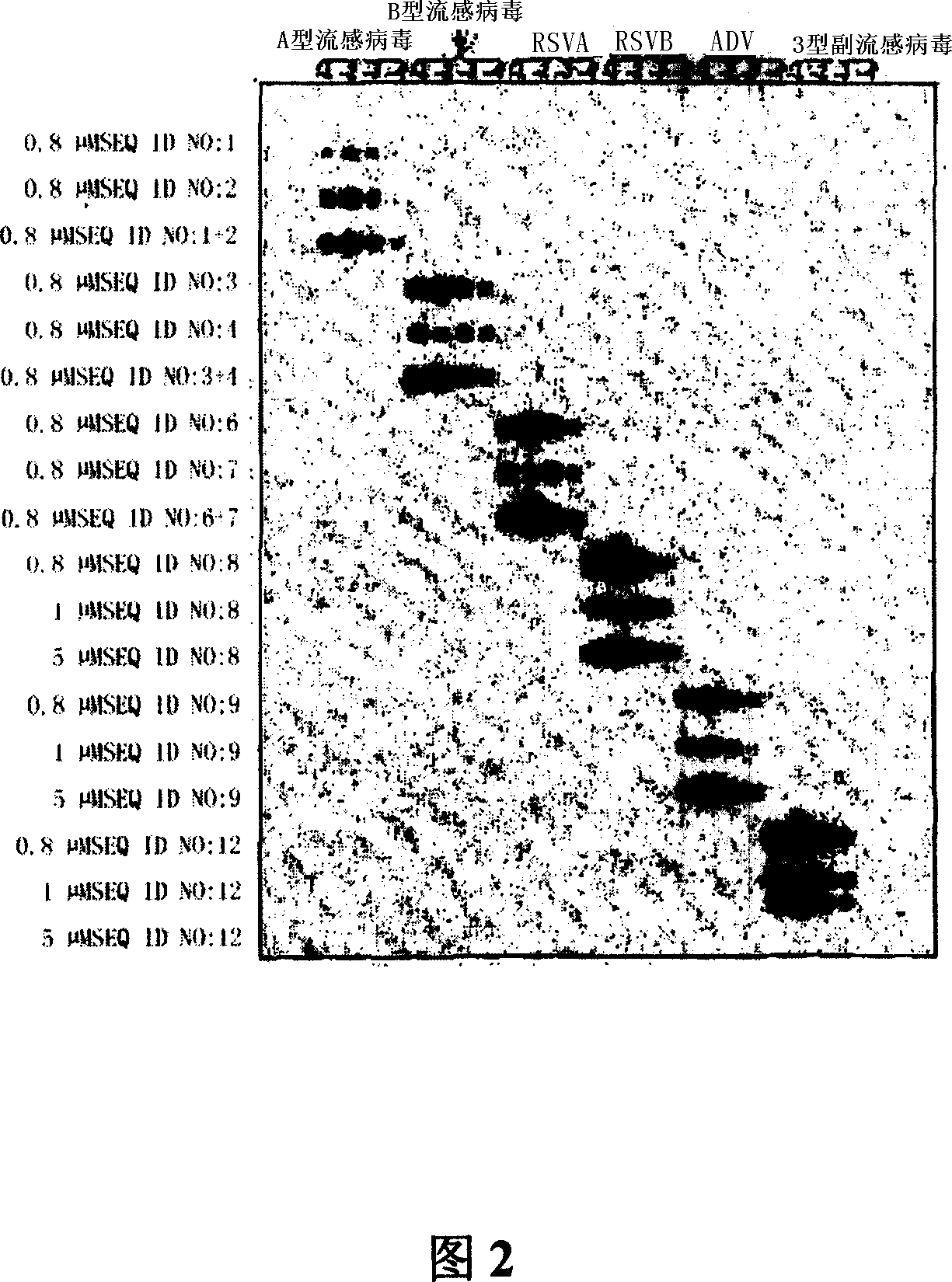
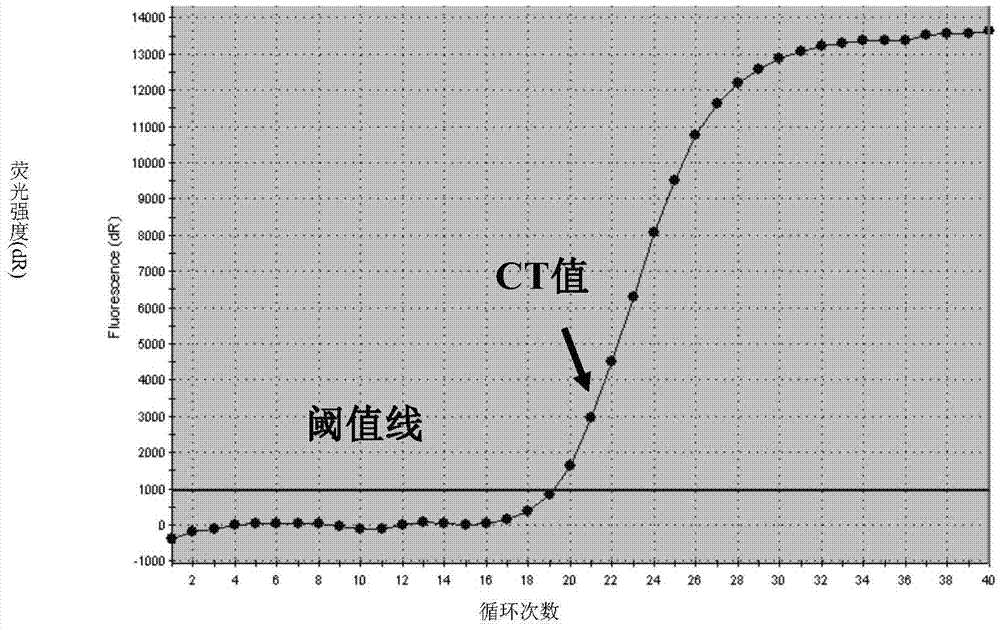
Human parainfluenza virus distinguishing and quantitative detection regent kit
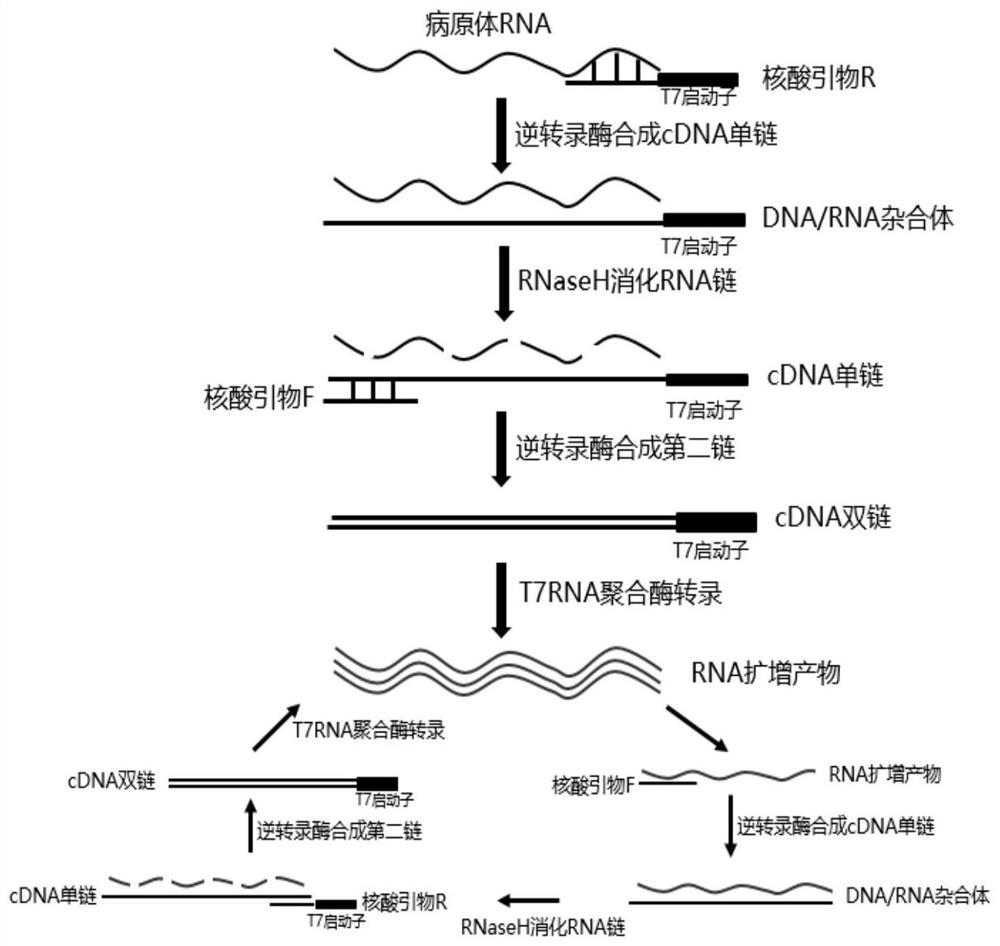
ActiveCN101550455AAvoid the "plateau effect"Increased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHuman Parainfluenza VirusFluorescence
The invention relates to three reagent kits for detecting human parainfluenza viruses 1, 2, 3 type real-time fluorescence polymerase chain reaction, which respectively adopts a one-step method RT-PCR, a two-step method RT-PCR and a multicolor fluorescence method RT-PCR to distinguish types of the human parainfluenza viruses of multi-type specimens and fix an amount of the human parainfluenza viruses of the multi-type specimens. The detection methods of the reagent kits have simple operation, short consuming time and high sensitivity and specificity and can be extensively used in a plurality of fields, such as the auxiliary diagnosis of the infection of the human parainfluenza viruses, clinical medicine direction, epidemiology retrospective study, and the like.
Owner:广州达安临床检验中心有限公司
Kit for simultaneously detecting nucleic acids of seven respiratory pathogens and application thereof
ActiveCN110964854AEasy to degradeLower requirementMicrobiological testing/measurementMicroorganism based processesRNA extractionMycoplasma pneumoniae pneumonia
The invention discloses a kit for simultaneously detecting nucleic acids of seven respiratory pathogens and application of the kit. The kit is based on a double amplification technology (RNA isothermal amplification and multi-biotin signal amplification), and can detect H1N1 / H3N2 type influenza A viruses, influenza B viruses, respiratory syncytial viruses, 1 / 2 / 3 type human parainfluenza viruses, B / E type adenoviruses, mycoplasma pneumoniae and chlamydia pneumoniae. The kit of the invention does not need RNA extraction, and is not prone to pollution, high in sensitivity and good in specificityin the process of detection, and can be widely applied to detection of the nucleic acids of the above seven respiratory pathogens.
Owner:武汉中帜生物科技股份有限公司
Respiratory tract infection pathogen nucleic acid combined detection kit
PendingCN112280897AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acidChlamydophila Pneumonia
The invention discloses a respiratory tract infection pathogen nucleic acid combined detection kit. The invention develops a primer and probe combination for detecting various respiratory tract infection pathogens such as novel corona virus, influenza A virus, influenza B virus, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumoniae and chlamydia pneumoniae by combining a multiple fluorescent quantitative PCR technology and a diversion hybridization gene chip technology. The nucleotide sequences of the primer and probe combination are shown in SEQ ID NO: 1-36in sequence. The respiratory tract infection pathogen nucleic acid combined detection kit is constructed. Synchronous joint detection of eight respiratory tract infection pathogens can be realized, the detection accuracy is good, the specificity is strong, the sensitivity is high, the repeatability is good, false negative and false positive are low, the detection time is short, the cost is low, apatient can be comprehensively detected, the pathogens can be accurately positioned, timely treatment is carried out or corresponding isolation measures are carried out, and the kit has important significance for effectively controlling respiratory tract infection to prevent related infectious infection outbreak.
Owner:SHANGHAI CITY PUDONG NEW DISTRICT ZHOUPU HOSPITAL +2
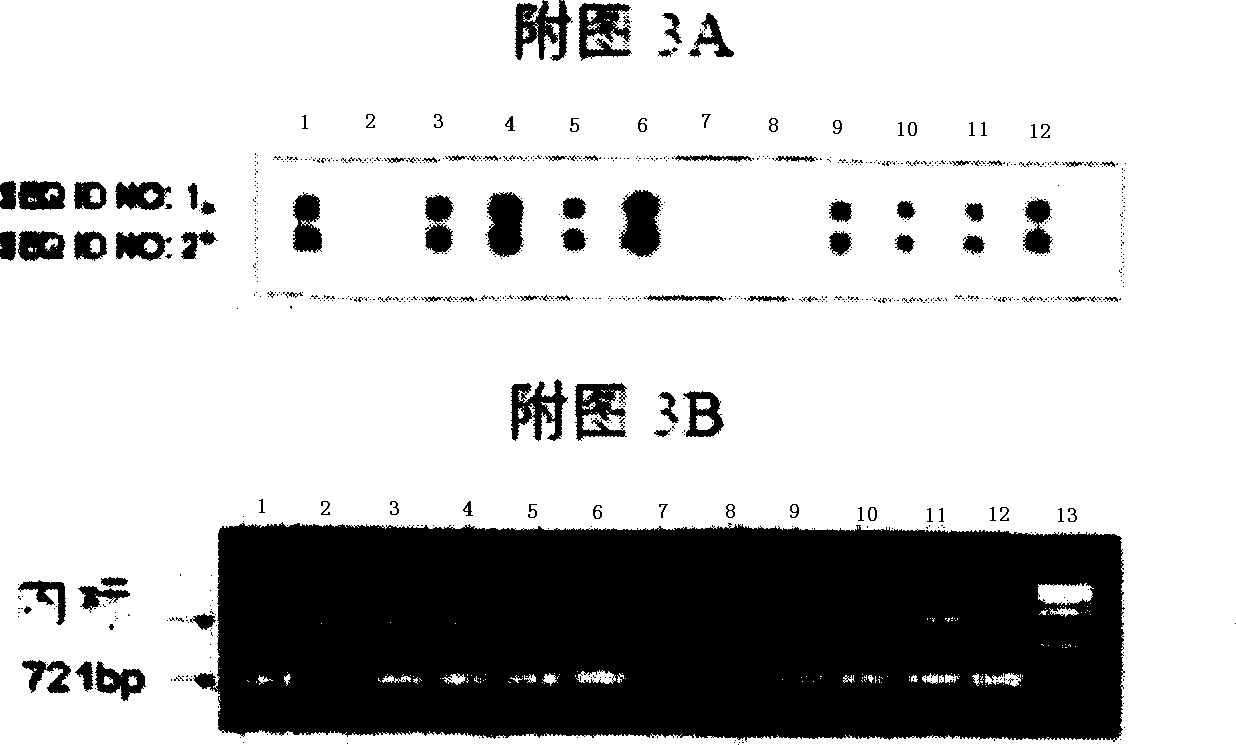
Single-tube multiplex fluorescence PCR (Polymerase Chain Reaction) detection method and kit for 1,2,3-type parainfluenza viruses
InactiveCN101948931ARealize multiple detection in one tubeStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceFluoresceinHuman Parainfluenza Virus
The invention provides a single-tube multiplex fluorescence PCR detection method for 1,2,3-type human parainfluenza viruses. In the method, a primer, the sequence of which is shown as SEQ ID NO:1-6 is adopted, and a probe the sequence of which is shown by SEQ ID NO:7-9 is also adopted. The invention also provides a single-tube multiplex fluorescence PCR detection kit for the 1,2,3-type parainfluenza viruses, comprising the primer and the probe. In the invention, the single-tube multiplex detection of the 1,2,3-type parainfluenza viruses is realized by adopting the self-designing PIV-1 / PIV-2 / PIV-3 specific primer and the Taqman probe and using an FAM / JOE / TAMRA multiplex fluorescein sign. The invention has the advantages of strong specificity, high sensitivity, rapidness, simple and convenient operation, low cost and the like, can be used as a reagent for detecting the 1,2,3-type human parainfluenza viruses and is used for scientific research and clinical application.
Owner:GUANGZHOU INST OF RESPIRATORY DISEASE
Attenuated parainfluenza virus (PIV) vaccines
InactiveUS20080096264A1Prone to infectionHigh titerSsRNA viruses negative-senseVectorsHuman Parainfluenza VirusParainfluenza virus
The invention provides isolated nucleic acids encoding recombinant genomes or antigenomes of Human Parainfluenza Viruses that are useful as vaccines. The recombinant genomes or antigenomes can be incorporated into expression vectors for production of recombinant viruses in vitro. The invention also provides recombinant Human Parainfluenza viruses having one or more mutations that attenuate replication of the virus in a host.
Owner:THE GOVERMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES (SEE PF37)
Human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit, and detection method thereof
ActiveCN105441589ASolve efficiency problemsResolve SensitivityMicrobiological testing/measurementDNA/RNA fragmentationHuman Parainfluenza VirusParainfluenza virus
The invention belongs to the technical field of virus detection kit, and provides a human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit, and a detection method thereof. The human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit comprises human parainfluenza virus specific primers and gene probes; and in the detection method, the human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit is used for detection. Compared with the prior art, the human parainfluenza virus I, II, III, and IV-type quadruple-PCR detection kit and the detection method possess following advantages: detection of human parainfluenza virus I, II, III, and IV types can be realized at the same time; sensitivity, specificity, and accuracy are high; and detection period is short.
Owner:深圳生科原生物有限公司
A detection kit for human parainfluenza virus nucleic acid extraction-free gene typing
InactiveCN108676913AGuaranteed reliabilityImprove accuracyMicrobiological testing/measurementMicroorganism based processesTypingHuman Parainfluenza Virus
The invention discloses a human parainfluenza virus nucleic acid extraction-free and rapid gene typing detection kit. The kit includes a PCR amplification reagent and a contrast reagent, the PCR amplification reagent is composed of a PCR reaction buffer solution A, an enzyme system B, and a HPIV1 / 2 / 3 / 4 primer probe mixed liquor, and the contrast reagent is mainly composed of a negative contrast and a positive contrast. The kit has advantages of direct sample amplification, fast multiple detection velocity, high sensitivity, good specificity and the like.
Owner:WUXI ENTRY EXIT INSPECTION & QUARANTINE BUREAU PEOPLES REPUBLIC OF CHINA
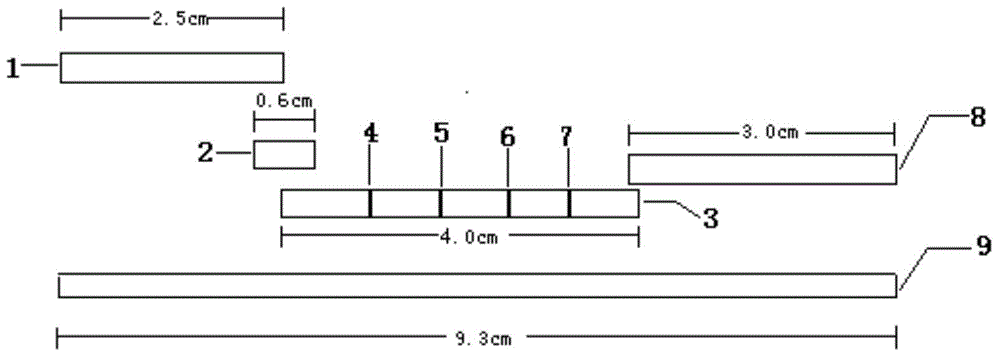
Human parainfluenza virus (HPIVs) IgM antibody detection test strip and preparation method thereof
The invention provides a human parainfluenza virus (HPIVs) IgM antibody detection test strip and a preparation method thereof. The human parainfluenza virus (HPIVs) IgM antibody detection test strip comprises a PVC bottom plate, a sample pad, a water absorption pad, a gold pad coated with an HPIVs specificity recombinant antigen, a detection line coated with a mouse anti-human IgM polyclonal antibody, and a nitrocellulose membrane (NC membrane) coated with a quality control line of a polyclonal antibody of an anti-HPIVs recombinant antigen. The human parainfluenza virus specific antibody IgM in serum of a human body is rapidly detected by applying an immunochromatographic method so that the acute infection of human parainfluenza viruses is conveniently subjected to differential diagnosis, and the certain significance is achieved for the clinical laboratory medicine.
Owner:秦志浩
Human parainfluenza virus quantum dot immunochromatography typing detection card, preparation method and applications
ActiveCN105277693AHigh sensitivityStrong specificityMaterial analysisNitrocelluloseHuman Parainfluenza Virus
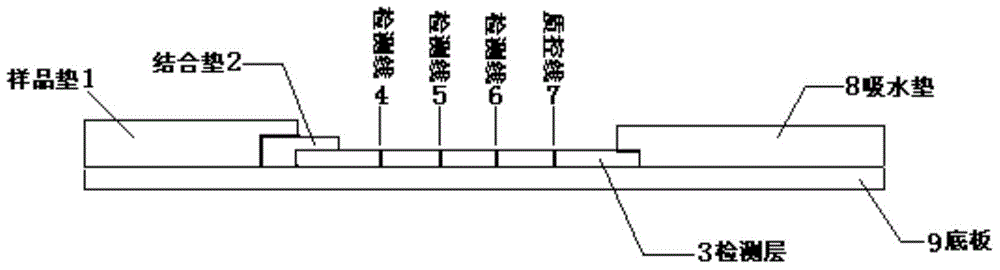
The invention provides a human parainfluenza virus quantum dot immunochromatography typing detection card, a preparation method and applications. The detection card comprises a base plate, a sample pad, a water absorption pad, a combination pad and a detection layer. The combination pad is coated with a mixture of rabit-anti I-type, II-type and III-type human parainfluenza virus HN protein polyclonal antibodies labeled with quantum dots respectively. The detection layer is composed of a solid phase nitrocellulose membrane with three detection lines and a quality control line. The three detection lines are coated with rabit-anti I-type, II-type and III-type human parainfluenza virus polyclonal antibodies respectively. The quality control line is coated with anti-rabit IgG. The detection layer is pasted on the base plate. The combination pad and the water absorption pad are arranged above two ends of the detection layer respectively, overlap with part of the detection layer and are pasted with the detection layer and the base plate respectively. The sample pad is arranged on the combination pad, overlaps with part of the combination pad and is pasted with the combination pad and the base plate. The provided detection card has advantages of simple operation, rapid detection, quantification, high sensitivity and the like.
Owner:湖北诺美华抗体药物技术有限公司
Attenuated human parainfluenza virus, methods and uses thereof
ActiveUS20130052718A1Elicit immune responseSsRNA viruses negative-senseVirus peptidesHuman Parainfluenza VirusPolynucleotide
The invention provides self replicating infectious recombinant paramyxoviruses. The recombinant paramyxovirus preferably have one or more attenuating mutations. In some embodiments, the recombinant paramyxovirus has a separate variant polynucleotide encoding a P protein and a separate monocistronic polynucleotide encoding a V protein. In some embodiments, recombinant paramyxovirus have at least one temperature sensitive mutation and one non-temperature sensitive mutation. Also provided are compositions and methods for using the recombinant paramyxoviruses as described herein.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Real-time fluorescent quantitative PCR probe primer group and kit for rapid qualitative typing detection of four types of human parainfluenza viruses
InactiveCN113322351AEasy to readSave time and costMicrobiological testing/measurementMicroorganism based processesThroat swabHuman Parainfluenza Virus
The invention discloses a real-time fluorescent quantitative PCR probe primer group and a kit for rapid and qualitative typing detection of four types of human parainfluenza viruses. The kit comprises the probe primer group specially designed for the four types of human parainfluenza viruses, i.e., HPIV-1, HPIV-2, HPIV-3 and HPIV-4; primer sequences in the probe primer group are as shown in SEQ ID NO.1-27; and probe sequences in the probe primer group are as shown in SEQ ID NO. 28 to 38. According to the invention, a reaction system of the real-time fluorescent quantitative PCR kit for rapid qualitative typing detection of the four types of human parainfluenza viruses aims at conserved regions of genomes of the four types of human parainfluenza viruses and can be used for rapid detection of parainfluenza viruses in nasopharynx swabs or sputum samples, and detection results can be used for auxiliary diagnosis of symptoms such as cough, fever, pneumonia and the like causedby parainfluenza and rapidly distinguishing parainfluenza patients from other fever and pneumonia patients.
Owner:北京华诺奥美基因生物科技有限公司
Fluorescence quantitative PCR (Polymerase Chain Reaction) detection method and kit for HPIV (Human Parainfluenza Virus)
InactiveCN102115795ASimple and fast operationEasy to operateMicrobiological testing/measurementMicroorganism based processesHuman Parainfluenza VirusFluorescence
The invention relates to a fluorescence quantitative PCR (Polymerase Chain Reaction) detection method and a kit for an HPIV (Human Parainfluenza Virus). A detection primer and a probe are designed in specific to an HPIV gene sequence conserved fragment, and RNA (Ribose Nucleic Acid) of the HPIV is detected qualitatively and quantificationally by using an improved one-step method and an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) real-time fluorescence amplification technology. The method has the advantages of easiness in operating, high repeatability and high specificity and sensitivity.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Cysteic acid derivatives of anti-viral peptides
InactiveCN101687911AImprove solubilitySimple purification processPeptide/protein ingredientsVirus peptidesHuman Parainfluenza VirusCysteic acid
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial vims (RSV), human parainfluenza virus (HPV), measles virus (MeV). and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM BIOTECH INC
Composition, test kit and method for detecting and typing 10 kinds of respiratory tract related viruses, and application of composition
PendingCN111808997ALow costImprove throughputMicrobiological testing/measurementMicroorganism based processesVirus typeHuman Parainfluenza Virus
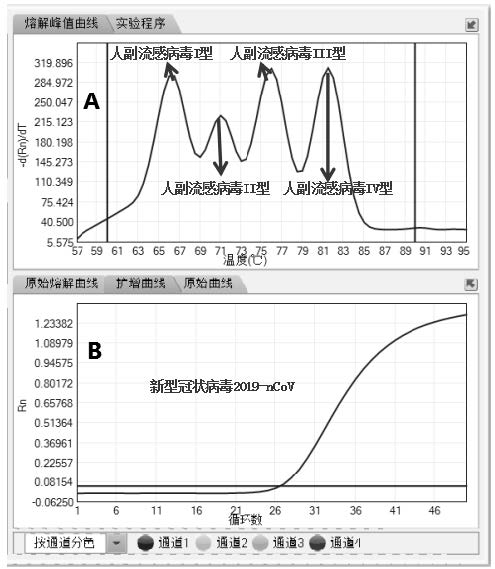
The invention relates to the field of molecular biological detection, specifically to detection of novel coronavirus 2019-nCoV, influenza virus type A, influenza virus type B, human parainfluenza virus type I, human parainfluenza virus type II, human parainfluenza virus type III, human parainfluenza virus type IV, respiratory adenovirus, respiratory syncytial virus and human metapneumovirus. Meanwhile, the invention also provides a test kit containing the composition, an application of the composition and a method for detecting and typing respiratory tract related viruses. The composition disclosed by the invention combines a fluorescent probe method and a melting curve method, can be used for simultaneously detecting and typing the respiratory tract related viruses in one tube, is low incost, high in flux and less in time consumption, greatly improves the detection efficiency, is simple and convenient to operate, and avoids false positive result and environmental pollution caused bycross of samples through single-tube operation.
Owner:SANSURE BIOTECH
Temperature-sensitive and cold-adapted human parainfluenza virus type 2(HPIV-2) and vaccines based on such virus
InactiveCN1314940ASsRNA viruses negative-senseViral antigen ingredientsHuman Parainfluenza VirusCold adapted
The present invention relates to isolated, attenuated viral strains of human parainfluenza virus 2 (HPIV-2), which are useful in live vaccine preparations. These strains exhibit a temperature sensitive and cold adapted phenotype useful for stimulating a protective immune response in an inoculated mammal without producing severe symptoms.
Owner:SAINT LOUIS UNIVERSITY
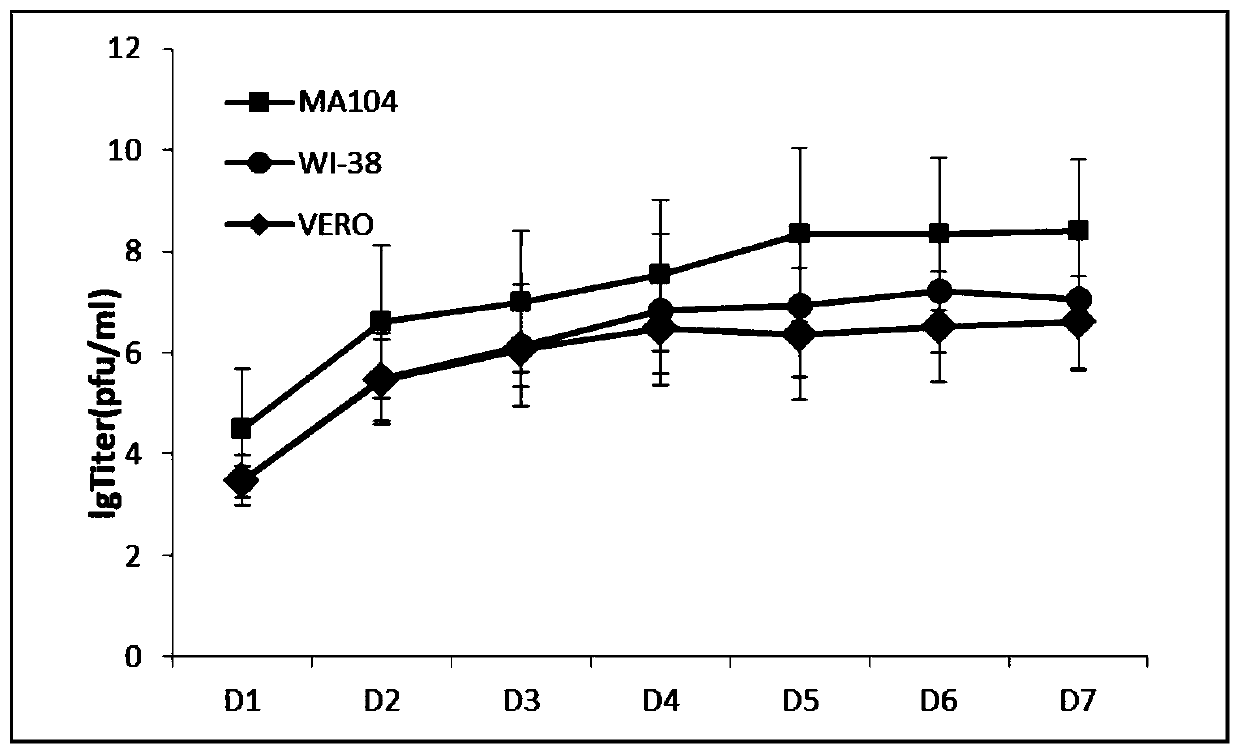
Human parainfluenza virus type 3 (HPIV-3) wild strain and application thereof
ActiveCN110628724AAvoid infectionHigh titerSsRNA viruses negative-senseViral antigen ingredientsHN ProteinF protein
The invention relates to a human parainfluenza virus type 3 (HPIV-3) wild strain and an application thereof. The preservation number of the wild strain is CCTCC NO:V201911, the wild strain has HN protein and F protein of an HPIV-3 virus, and the wild strain can be applied to an HPIV-3 virus infected mouse model and an HPIV-3 neutralizing antibody detection experiment, and can be applied to preparation of vaccines for preventing parainfluenza type 3. The human parainfluenza virus type 3 wild strain disclosed by the invention is a cloned purified high-titer HPIV3LZ1728C19 virus strain free fromspecific exogenous factor plaque formation, and a human parainfluenza virus type 3 HN protein subunit vaccine prepared from the virus can effectively prevent parainfluenza virus type 3 infection.
Owner:LANZHOU INST OF BIOLOGICAL PROD
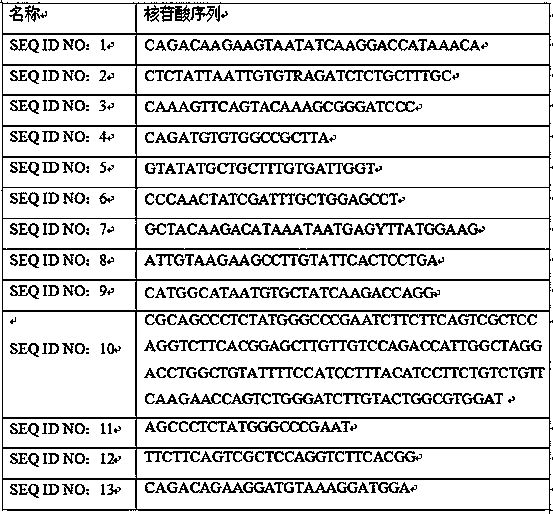
Detection kit for HPIV (human parainfluenza virus) triple nucleic acid
InactiveCN109097497ASolve efficiency problemsSolve the characteristicsMicrobiological testing/measurementMicroorganism based processesHuman Parainfluenza VirusPolynucleotide
The invention discloses a detection kit for HPIV (human parainfluenza virus) triple nucleic acid. The detection kit is characterized by comprising HPIV type 1, type 2 and type 3 nucleic acid PCR reaction liquid, wherein the PCR reaction liquid comprises reaction buffer liquid, deoxyribonucleoside triphosphate, upstream and downstream primers for amplification of HPIV type 1, type 2 and type 3 target polynucleotides and probes for detection of target polynucleotides, and the sequence table of the probes is shown as SEQ ID NO:1-9. The detection kit has the advantages of being rapid, convenient and simple to operate, good in detection specificity, high in sensitivity and wide in detection range. As the probes with higher specificity is applied to the kit, the kit can rapidly detect the HPIV type 1, type 2 and type 3 nucleic acids in unknown samples; reliable experimental basis is provided for diagnosing the HPIV type 1, type 2 and type 3 nucleic acids, and the technical problems of low efficiency, poor specificity and low sensitivity of existing kits are solved.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Kit for simultaneous detection of nucleic acids of seven respiratory pathogens and application thereof
ActiveCN110964854BEasy to degradeLower requirementMicrobiological testing/measurementMicroorganism based processesRNA extractionPneumonitis
Owner:武汉中帜生物科技股份有限公司
Long lasting fusion peptide inhibitors for HIV infection
InactiveUS20110166061A1Prolong half-life in vivoBiocidePeptide/protein ingredientsFeline immunodeficiency virusImmunodeficiency virus
The present invention is concerned with This invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Human parainfluenza viruses having separated p and c genes
InactiveUS20100330120A1SsRNA viruses negative-senseSugar derivativesHuman Parainfluenza VirusPolynucleotide
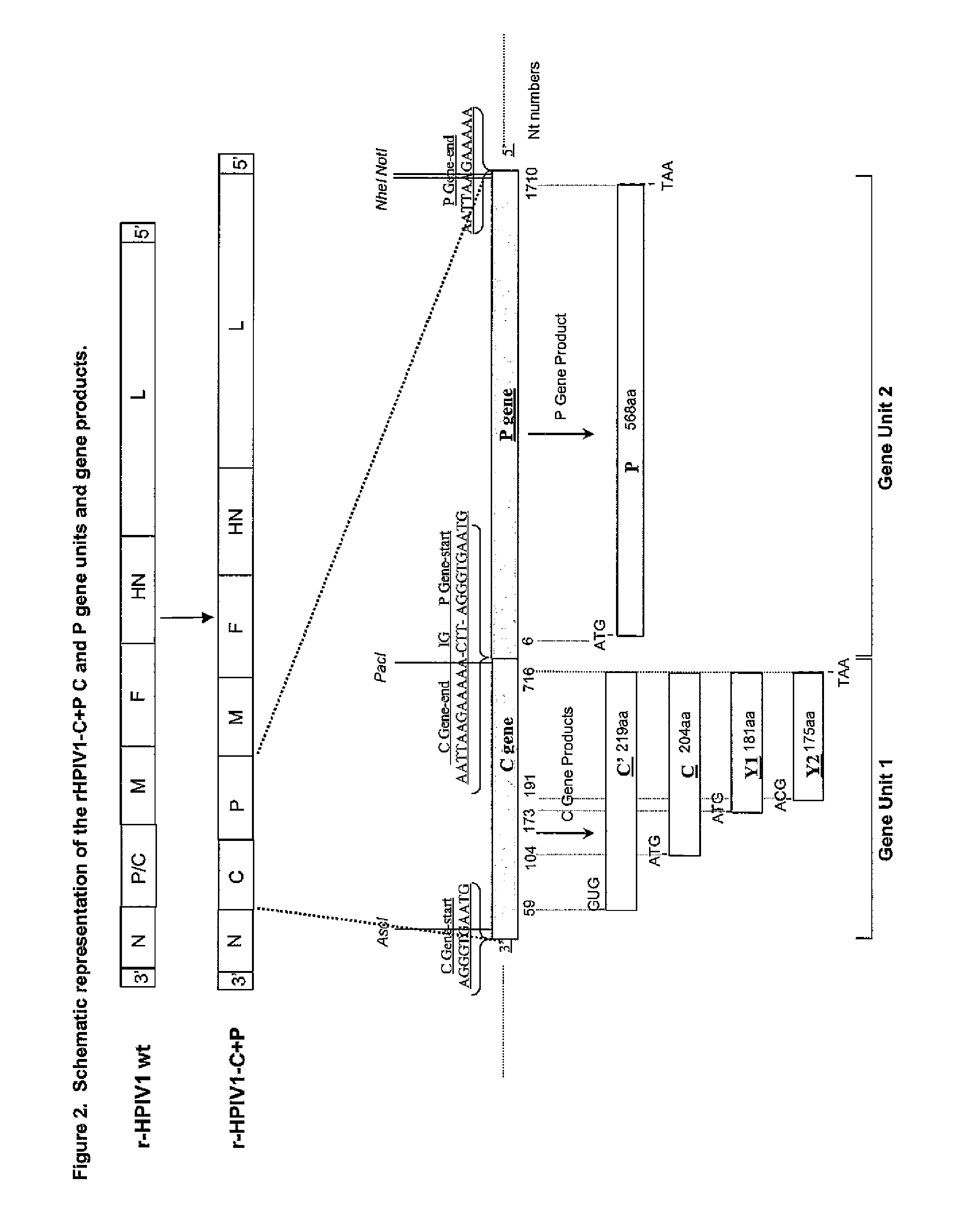
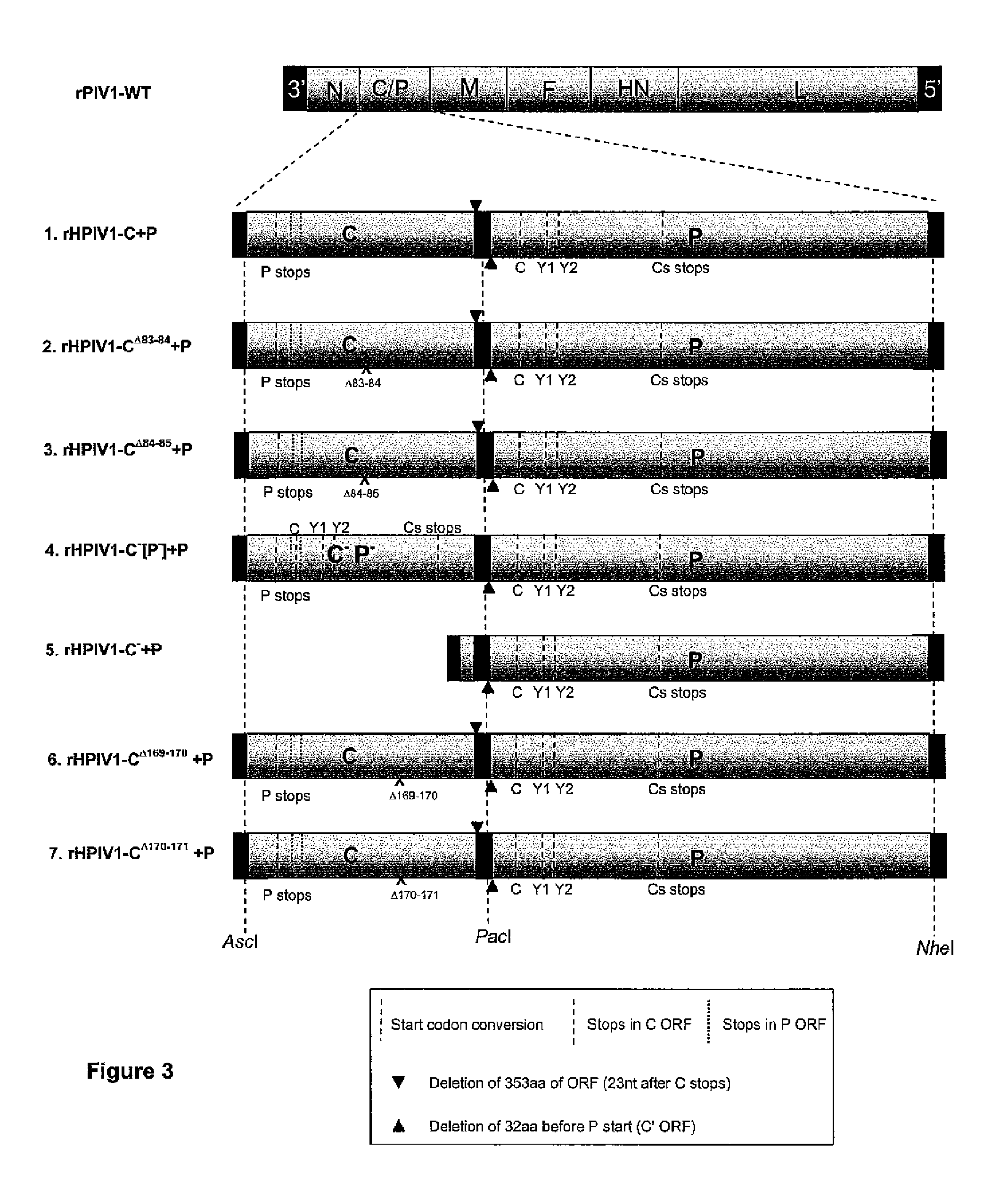
The invention provides self replicating infectious recombinant paramyxoviruses where the P and C genes are separated rated. The recombinant paramyxoviruses preferably have one or more attenuating mutations and / or at least one temperature sensitive mutation and one non-temperature sensitive mutation. In some embodiments, the recombinant paramyxovirus has a separate variant polynucleotide encoding a C protein and a separate monocistronic polynucleotide encoding a P protein. Also provided are compositions and methods for using the recombinant paramyxoviruses as described herein.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com