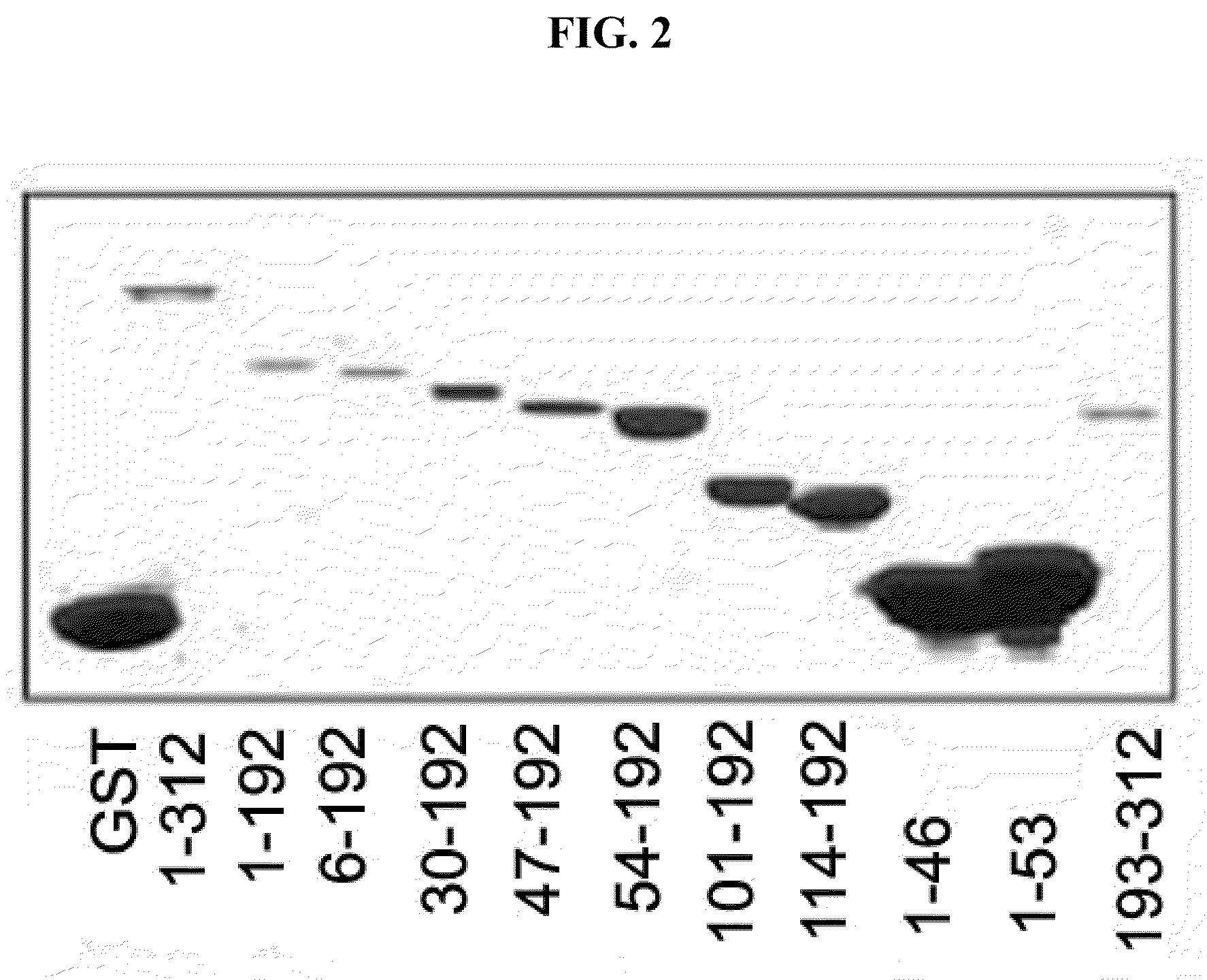
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
583results about How to "High titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
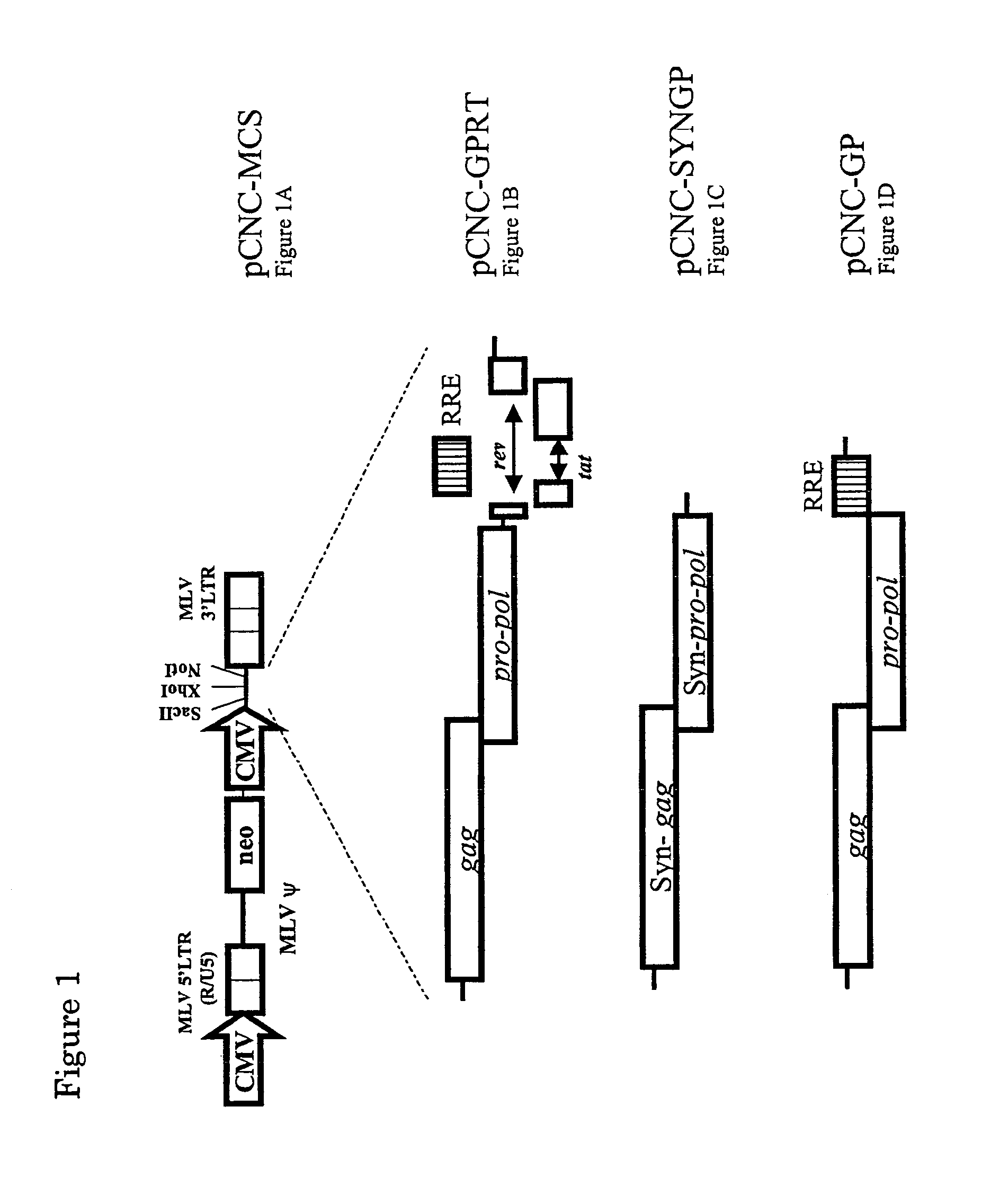
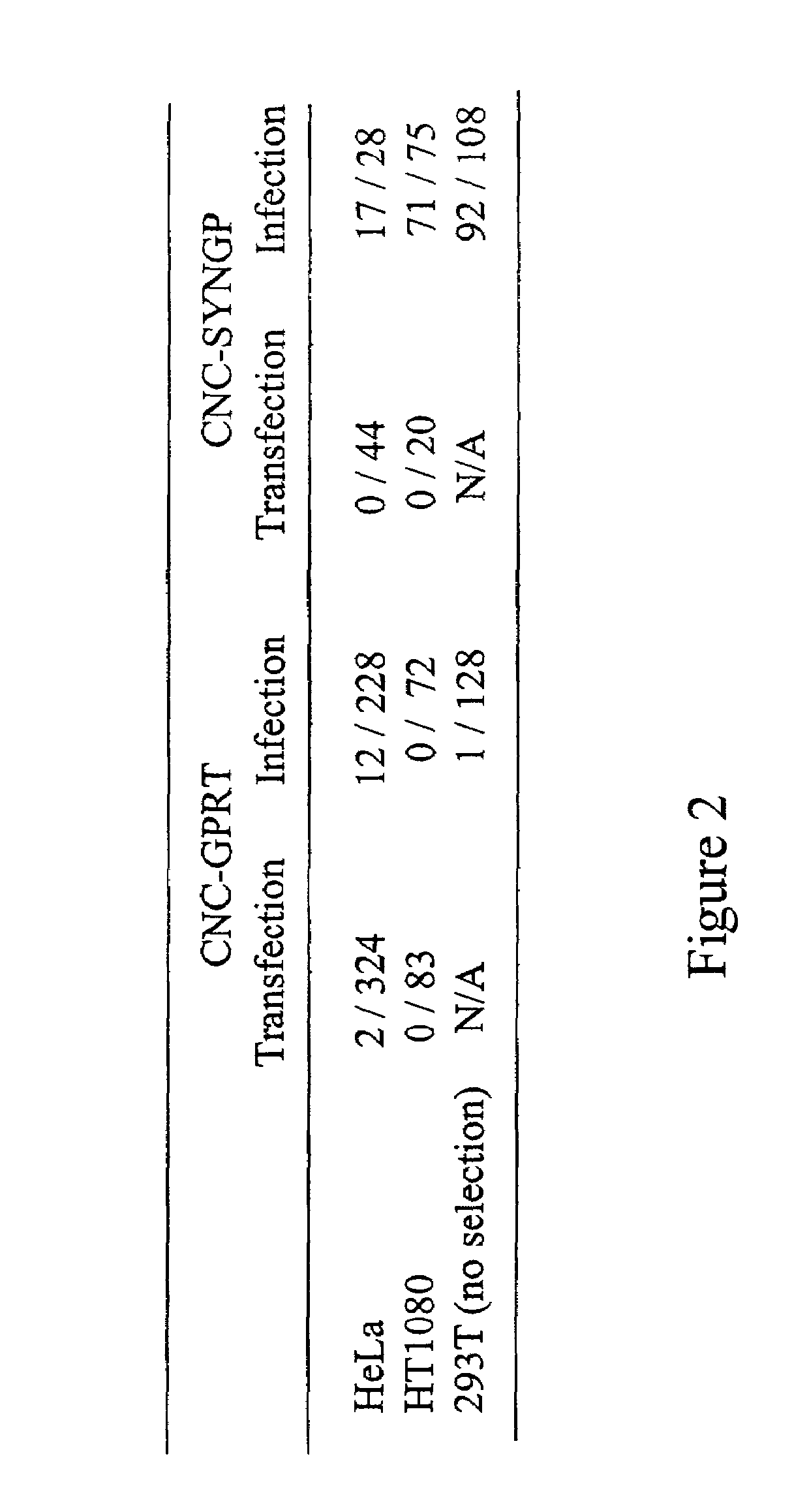
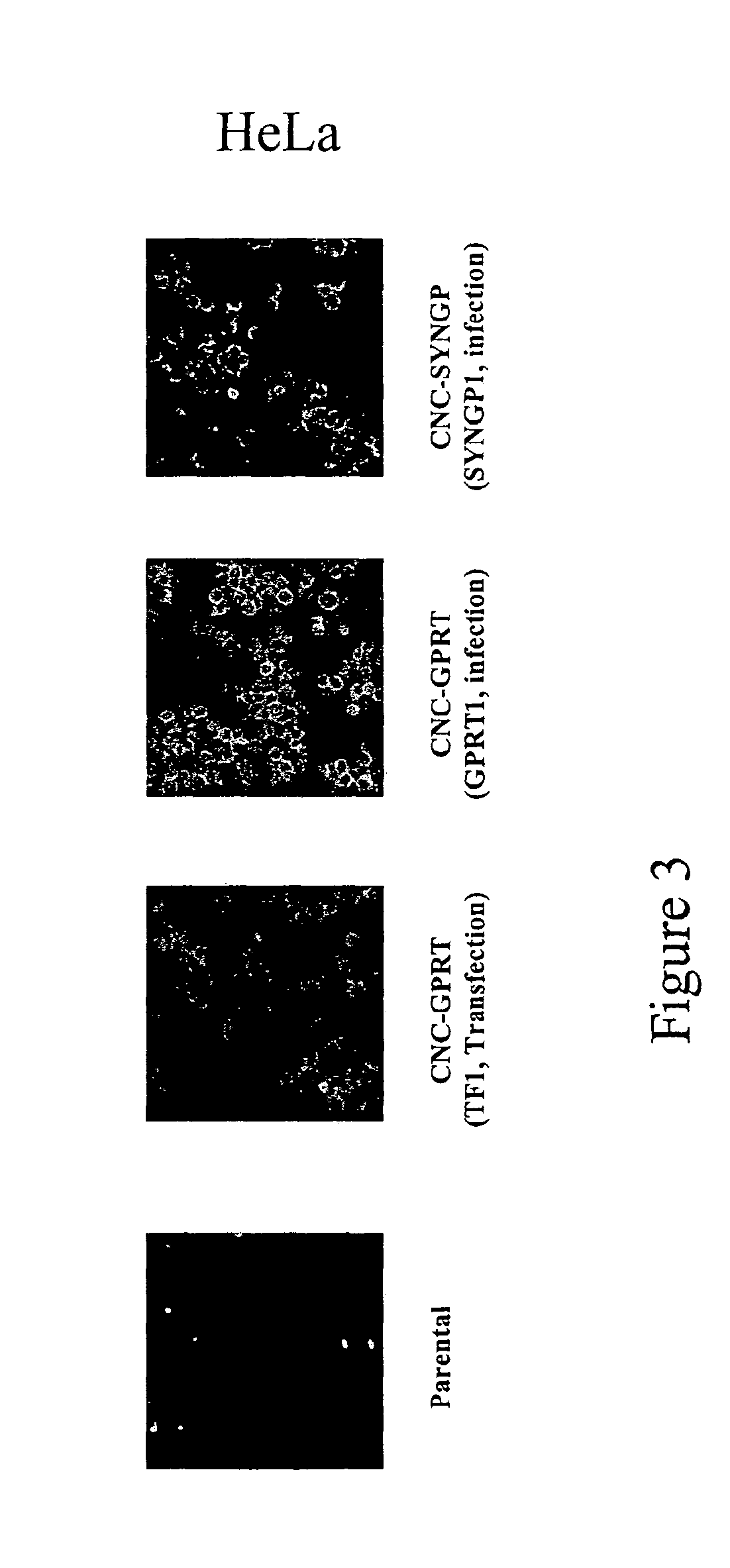
Retroviral vector
InactiveUS7351585B2High titerKeep for a long timeGenetic material ingredientsArtificial cell constructsNucleotideRetrovirus
Provided herein is a retroviral vector comprising, and capable of expressing, a nucleotide of interest (NOI), wherein the NOI encodes an RNA or protein which is harmful to a cell.
Owner:OXFORD BIOMEDICA (UK) LTD
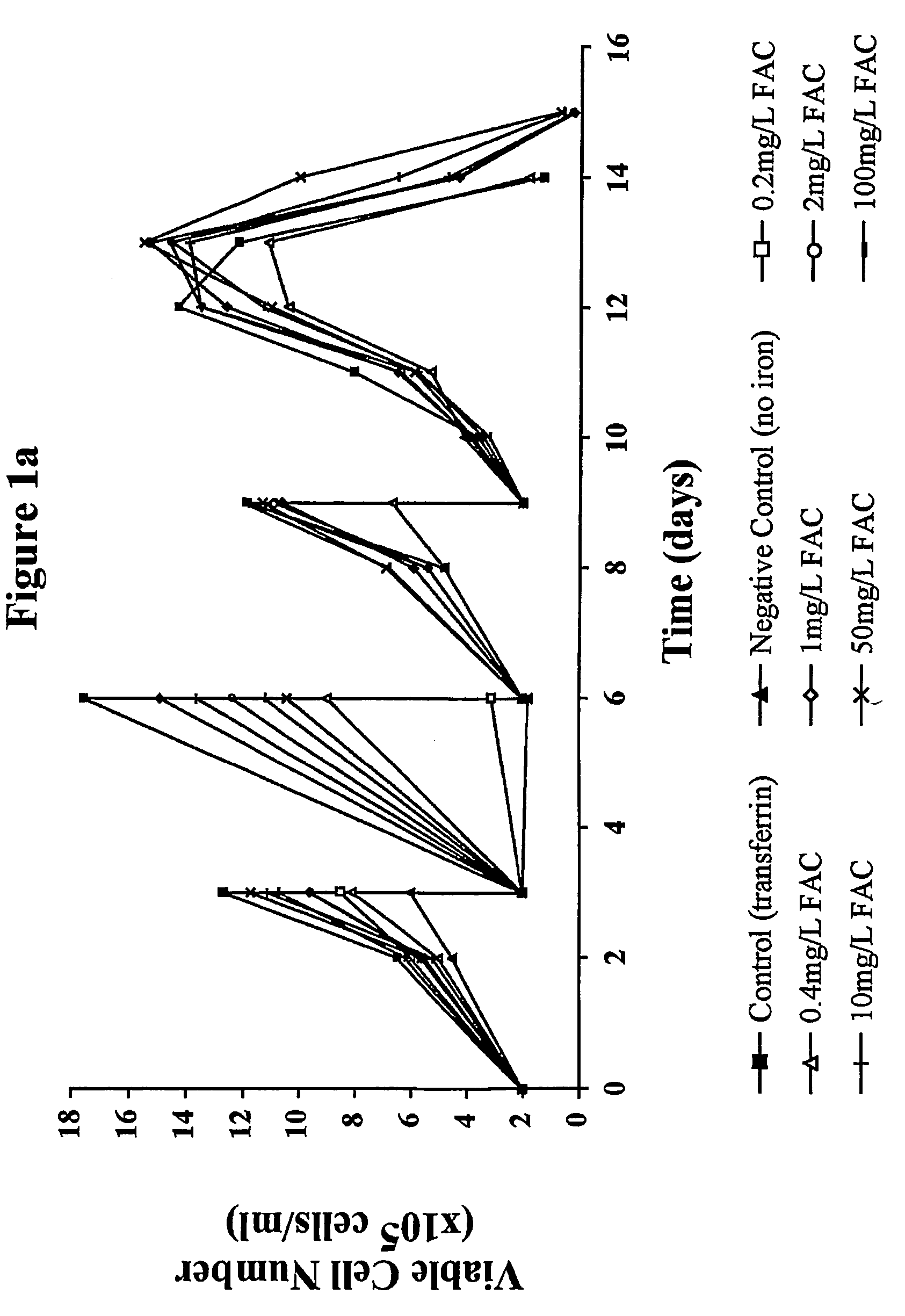
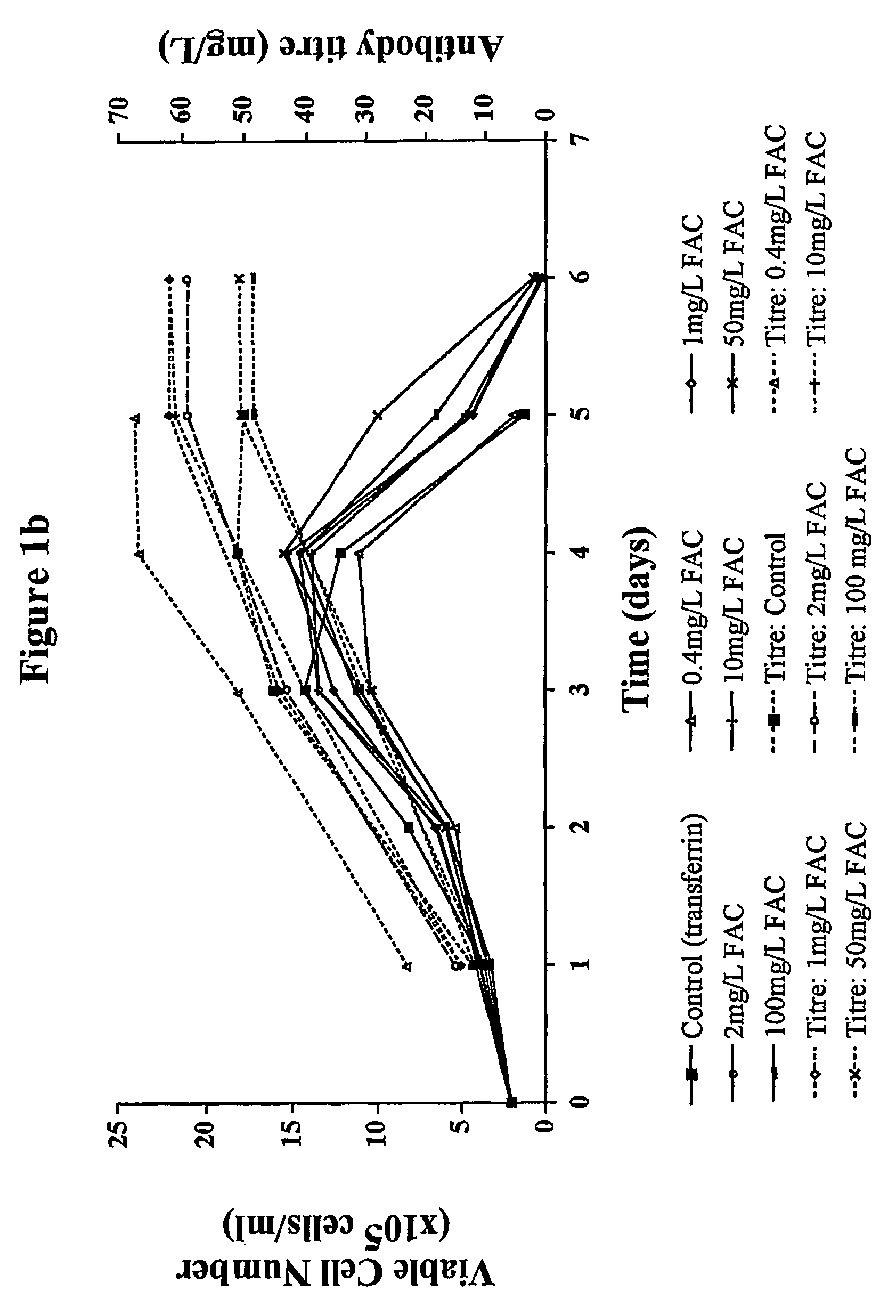
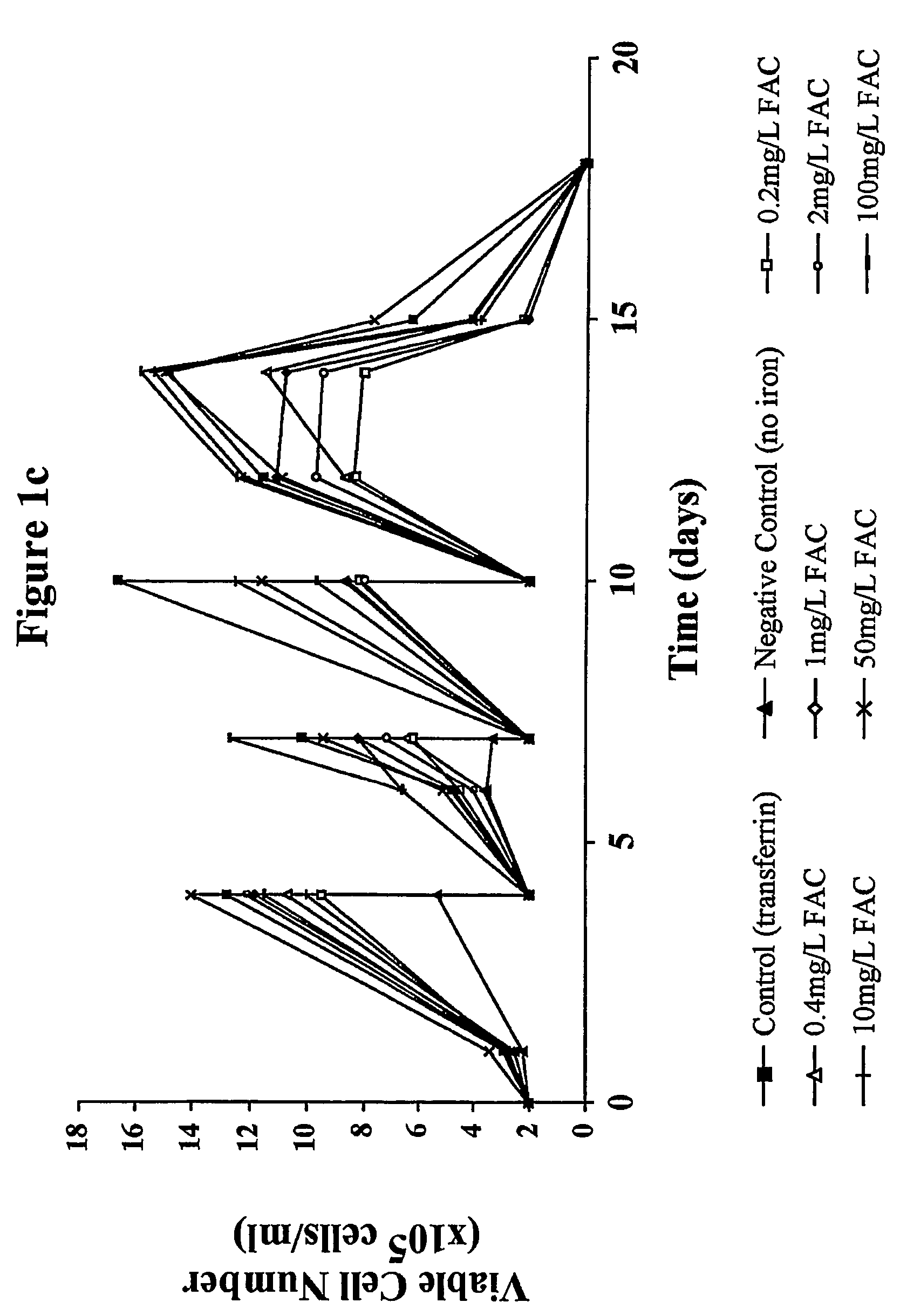
Myeloma cell culture in transferrin-free low iron medium
ActiveUS8361797B2High titerSupport growthGenetically modified cellsCulture processCulture cellMicrobiology
The present invention relates to a method for culturing mammalian cells in a culture medium which is transferrin free and which contains no lipophilic or synthetic nitrogen-containing chelators. Also provided is the use of the medium and a process for providing a mammalian product by culturing cells capable of producing the product in the medium.
Owner:MEDIMMUNE LTD
Method for screening drug target genes based on CRISPR/Cas9 high-throughput technology
InactiveCN106399377ADetermining infection efficiencyDetermine the MOI valueMicrobiological testing/measurementFermentationInfected cellCancer cell
The invention relates to a method for screening drug target genes based on a CRISPR / Cas9 high-throughput technology. The method comprises the steps of firstly establishing a sgRNA library; secondly packaging the sgRNA library by using slow viruses, and collecting the viruses; thirdly screening the sgRNA library in a cancer cell line; fourthly extracting cells obtained by screening and genome DNA of the cells before screening; and finally enriching sgRNA in genome DNA. Compared with the prior art, the method has the advantages that a CRISPR / Cas cell screening process is improved, the virus infection efficiency is determined with a simple and convenient method by utilizing puromycin resistance of infected cells, and MOI values of the viruses are determined; more importantly, a virus packaging method is greatly optimized, so that the virus packaging efficiency is improved to be more than 5 times that of a conventional method, and large-scale drug target screening cost can be greatly reduced; and the method is used for promoting industrialization of cancer drug target screening.
Owner:TONGJI UNIV
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
Novel polypeptide having Anti-tumor activity
InactiveUS20110124582A1Prevent proliferationEasy to useBacteriaPeptide/protein ingredientsApoptosisPolynucleotide
The present invention relates to a novel polypeptide having anti-tumor activity through inducing apoptosis of endothelial cell and use thereof. More particularly, the present invention relates to a method for inducing apoptosis of endothelial cell, and for preventing or treating cancer, comprising administering to a subject in need thereof an effective amount of (a) an isolated polypeptide having the amino acid sequence of SEQ ID NO: 9 or the amino acid sequence having at least 90% sequence homology to the amino acid sequence of SEQ ID NO: 9; or (b) an isolated polynucleotide encoding the polypeptide of (a).
Owner:ATYR PHARM INC
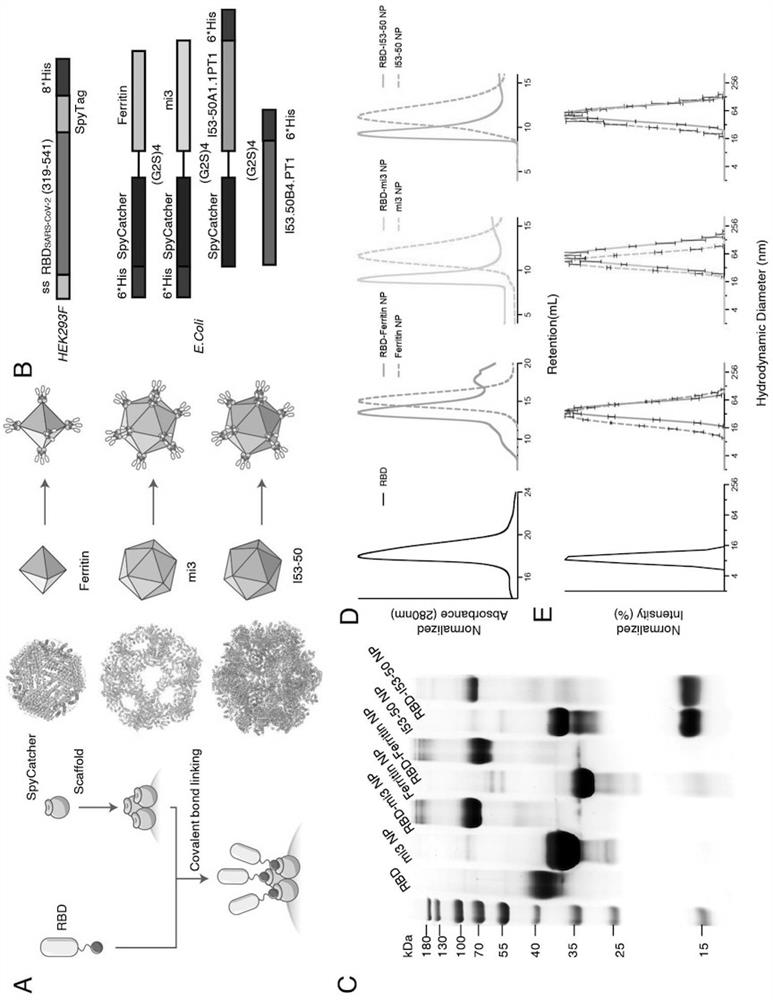
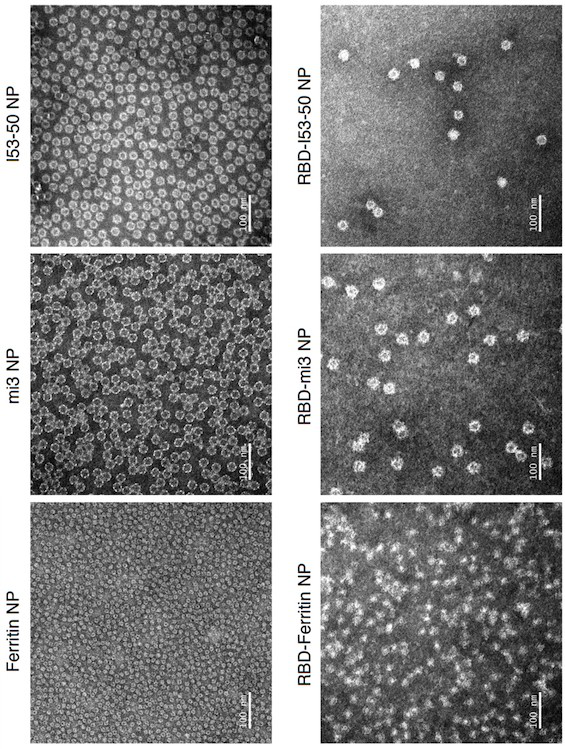
SARS-CoV-2 RBD conjugated nanoparticle vaccine
ActiveCN111991556AStrong titerImprove bindingSsRNA viruses positive-senseAntibody mimetics/scaffoldsNanoparticleCarrier protein
The invention relates to the field of immunomedicine, in particular to an SARS-CoV-2 RBD conjugated nanoparticle vaccine. The vaccine comprises an immunogenic compound, and the immunogenic compound comprises: a) a nanoparticle carrier obtained by self-assembly with a vector protein subjected to fusion expression with SpyCatcher; and b) an RBD antigen of the SARS-CoV-2 virus, which is subjected tofusion expression with SpyTag, the vector protein is selected from Ferritin, mi3 and I53-50, and the vector protein and the antigen are in covalent linkage through SpyCatcher-SpyTag.
Owner:SUN YAT SEN UNIV +1
Serum free culture medium for growing various cells derived from kidney tissue
InactiveCN101974481AHigh biosecurityHigh titerArtificial cell constructsVertebrate cellsNutrientGlycerol
The invention relates to a serum free culture medium for growing various cells derived from the kidney tissue. In the serum free culture medium, dulbecco's modified eagle medium (DMEM) / nutrient mixture F-12(F12)(1:1) is used as a basic culture medium and comprises the components including yeast autolysate, soy protein hydrolysate, amino acids, a fatty acid combining agent, an antioxidant reagent, reduced glutathione, a polyamine mixture, ethanolamine, beta-mercaptoethanol, glycerol, minerals, Pluronic F-68 and the like, and the pH value is 7.2. The culture medium of the invention can be used for culturing various cell lines derived from the kidney tissue, such as HEK293 cells, Marc145 cells, Vero cells and BHK21 cells and for propagating viruses.
Owner:国家兽用生物制品工程技术研究中心
Method for inhibiting HIV-1 infectious agent from infecting primary lymphocyte by utilizing CRISPR/Cas9
ActiveCN104694573AEasy to operateStrong specificityFermentationPlant genotype modificationAnti virusGenetic engineering
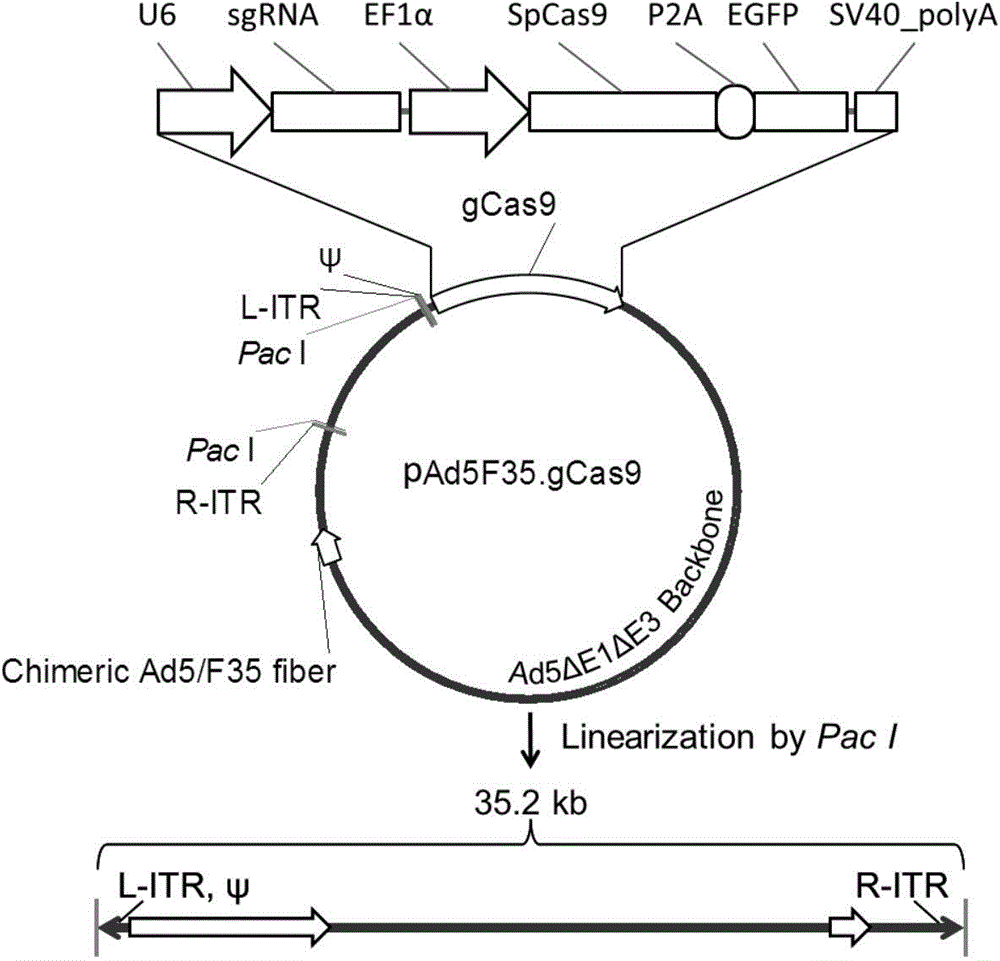
The invention discloses a method for inhibiting HIV-1 infectious agent from infecting primary lymphocyte by utilizing CRISPR / Cas9 and relates to genetic engineering and an anti-virus infection technology. According to the method, by utilizing the latest CRISPR / Cas9 nuclease system, through the combination of Ad5F35 mosaic type adenovirus vectors of efficient targeted primary T lymphocyte, by editing CCR5 expression of human CD4+T lymphocyte, HIV-1 virus infection is effectively inhibited. According to the method, the CRISPR / Cas9 system is packaged by utilizing the Ad5F35 type adenovirus vectors, the advantages of the CRISPR / Cas9 system and the Ad5F35 type adenovirus vectors are organically combined, the novel method for resisting HIV-1 virus infection is provided, and the method has the potential for being applied for HIV-1 gene treatment research; meanwhile, the Ad5F35 type adenovirus vectors carrying with the CRISPR / Cas9 system can be used for conducting targeted editing to other genes in the primary lymphocyte or gene editing research of other hematopoietic system cells.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Peptides which elicit a high neutralizing antibody titer, cytotoxic T lymphocyte response and T helper cell response in a broad range of MHC type recipients
InactiveUS7094405B1High titerHigh titer of neutralizing antibodyPeptide/protein ingredientsAntibody mimetics/scaffoldsV3 loopT helper cell
Peptide constructs comprised of multideterminant T helper peptides from the envelope glycoprotein of HIV previously identified to induce proliferative responses in four different haplotypes of mice and IL-2 responses in 52-73% of HIV positive, flu positive patients (cluster peptides), were co-linearly synthesized with the peptide 18 of the V3 loop of HIV-1 gp 160, corresponding to the principal neutralizing determinant of HIV-IIIB and also shown to contain a dominant CTL epitope. Cognate help for peptide 18 antibody was elicited following a single immunization in all strains of mice which had previously responded to a T cell epitope encompassed by the peptides. In two strains of mice, the level of neutralizing antibody achieved was comparable to levels adequate for protection from homologous viral challenge in chimpanzees. After a single boost, much higher antibody titers for 90% neutralization in the range of 1:1000 to 1:16,000 were achieved. Spleen cells from mice of three distinct MHC haplotypes sharing the Dd class I MHC molecule but with different class II molecules, immunized with the compound peptides, exhibited enhanced gp160-specific CTL activity.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Novel coronavirus antigen epitope and application thereof
ActiveCN111848753AImproving immunogenicityHigh titerSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigen epitopeCoronavirus vaccination
The invention provides a novel coronavirus antigen epitope and application thereof. A super computer is used for simulating S, E, M and N protein structures of the novel coronavirus, and novel coronavirus epitopes SEQ ID NO 1-38 are obtained through calculation. The epitopes have very good immunogenicity; an antibody generated by inducing a part of epitope polypeptide has the effect of neutralizing the novel coronavirus; the antigen epitope can be combined with antibodies in serum of novel coronavirus patients at home and abroad, has the potential of resisting various novel coronavirus strains, and also has the potential of identifying and applying different strains. The epitope provided by the invention can be used for (1) research and development of novel coronavirus vaccines and universal coronavirus vaccines such as MERS viruses and SARS viruses; and (2) preparing a novel coronavirus antibody, and further detecting and typing viruses and treating diseases caused by the viruses. Thenovel coronavirus antigen epitope has a wide application prospect in the aspects of prevention of coronavirus and propagation and outbreak thereof, and detection and diagnosis of virus strains.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Porcine pseudorabies virus strain as well as inactivated vaccine and applications thereof
ActiveCN103305474APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsLaboratory cultureVirus strain
The invention discloses a porcine pseudorabies virus strain as well as an inactivated vaccine and applications thereof, belonging to the field of separation and application of the porcine pseudorabies virus strain. The invention firstly provides a porcine pseudorabies virus BJ strain separated from diseased pig tissues, and the microbial preservation number of the porcine pseudorabies virus BJ strain is CGMCC (China General Microbiological Culture Collection Center) No.7351. The invention discloses a method for preparing the inactivated vaccine by applying the porcine pseudorabies virus BJ strain. The method comprises the steps of culturing a virus strain to obtain a virus solution; adding an inactivator, and inactivating and concentrating the virus solution; and evenly mixing an adjuvant and the virus solution, and emulsifying to obtain the inactivated vaccine. The technological parameters of the inactivated vaccine preparation method are further optimized, and the immune protection efficacy and safety of the inactivated vaccine can be improved. Shown by the immune protection efficacy and safety tests, the porcine pseudorabies inactivated vaccine prepared has good immune protection efficacy and safety, and can be clinically used for preventing or treating porcine pseudorabies.
Owner:泰州博莱得利生物科技有限公司 +2
Use of recombinant parainfluenza viruses (PIVs) as vectors to protect against infection and disease caused by PIV and other human pathogens
InactiveUS7192593B2Prone to infectionHigh titerSsRNA viruses negative-senseSugar derivativesAntigenDisease
Chimeric parainfluenza viruses (PIVs) incorporate a PIV vector genome or antigenome and one or more antigenic determinant(s) of a heterologous PIV or non-PIV pathogen. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, or against a PIV and non-PIV pathogen. Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric Ply genome or antigenome which includes a partial or complete PIV vector genome or antigenome combined or integrated with one or more heterologous gene(s) or genome segment(s) encoding antigenic determinant(s) of a heterologous PIV or non-PIV pathogen.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE DEPT OF
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
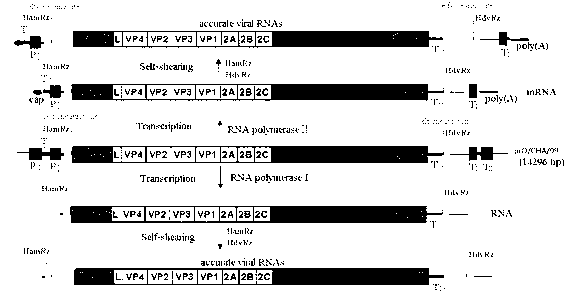
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Fish broad-spectrum vibrio subunit vaccine and preparation method
InactiveCN102512674AImprove resistanceImprove immunityAntibacterial agentsMicroorganism based processesFlagellinVibrio alginolyticus
The invention discloses a broad-spectrum vibrio subunit vaccine for preventing fish pathogenic vibrio infection and a preparation method, belonging to the technical field of biology. The fusion protein OmpK-FlaA of an outer membrane protein OmpK with a conserved structure and a flagellin protein FlaA on the vibrio parahaemolyticus surface serves as an antigen component of the vaccine. The method is characterized by comprising the following steps of: superposing and extending Ppolymerase chain reaction (PCR) to the ompK and flaA genes of the vibrio parahaemolyticus to obtain a fusion protein gene flaA-ompK; constructing flaA-ompK-pET-28a recombinant plasmids; and obtaining the fusion FlaA-OmpK with high purity through exogenous induction expression and purification. The vaccine prepared bythe invention is safe and nontoxic and has no side effects; injection immune can be adopted; and the immune can also be realized by taking enteric microsphere vaccine orally; cross immunity protection can be acted to fish pathogenic vibrio (vibrio parahaemolyticus, vibrio alginolyticus, vibro harveyi, vibrio anguillarum and vibrio vulnificus).
Owner:FUZHOU UNIVERSITY
Method for preparing porcine parvovirus inactivated vaccines
InactiveCN101947318AIncrease surface areaIncreased ST cell densityAntiviralsViruses/bacteriophagesTiterVirus inactivation
The invention discloses a method for preparing porcine parvovirus inactivated vaccines. The preparation method comprises the following steps of: recovery culture of ST cells or IBRS-2 cells, bioreactor micro-carrier culture of the ST cells or the IBRS-2 cells, continuous culture of bioreactor micro-carriers of the ST cells or the IBRS-2 cells, inoculated porcine parvovirus culture, collection of virus liquid, and virus inactivation for preparing the porcine parvovirus inactivated vaccines. The method for preparing the porcine parvovirus inactivated vaccines is a porcine parvovirus process for culturing the ST cells by adopting the bioreactor micro-carriers; the micro-carriers provide larger surface area, and can improve the density of the ST cells and the virus titer; the reactors can automatically monitor the growth of the cells or the optimal biochemical conditions during propagation of viruses; and the viruses produced on each batch of reactors have uniform titer, small batch and stable quality.
Owner:扬州优邦生物药品有限公司 +1
Recombination virus particles for expressing 2-typed porcine circovirus nucleocapsid protein Cap gene
InactiveCN101289658ANon-pathogenicImprove replication efficiencyViral antigen ingredientsAntiviralsAntigenShuttle vector
The invention relates to construction and application of recombinant nucleocapsids of Cap genes of porcine circovirus expression type 2 nucleocapsid protein, belonging to the genetic engineering bacterin field. C-terminal gene fragments of porcine parvovirus (PPV) VP2 genes are cloned into a type 5 adenovirus shuttle vector of the human beings, and recombinant adenovirus rAd-deltaVP2 is obtained; deltaVP2 proteins are expressed successfully and highly efficiently and can be self-assembled into the nucleocapsids [PPV:VLPs]; the PPV VP2 nucleocapsids are used as antigen transport vectors and 165 to 200 sites of amino acid (deltaCap) genes of the porcine circovirus type 2 (PCV2) nucleocapsid proteins (Cap) are embedded into an N-terminal (deltaVP2) of the PPV VP2, and then recombinant adenovirus rAd-deltaCap-deltaVP2 is obtained; embedded VP2 (deltaCap-deltaVP2) proteins are expressed successfully and highly efficiently and can be self-assembled into nucleocapsids [PPV:VLP(PCV2)]. The invention also relates to application of the recombinant virus and recombinant PPV VP2 nucleocapsids of the expression Cap genes of the recombinant virus in the aspects of bacterin immunity and so on.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Human papillomaviruse type hybrid virus-like particles and preparation method thereof
ActiveCN102747047AIncrease production costProne to cross protectionViral antigen ingredientsInactivation/attenuationDiseaseHuman papillomavirus
The present invention relates to human papillomavirus (HPV) type hybrid virus-like particles and a preparation method thereof. The virus-like particles can be used for preventions two or more HPV infections and diseases caused by HPV infections. The present invention further relates to uses of the protein and the virus-like particles in preparations of drug compositions or vaccines, wherein the drug compositions or the vaccines are provided for preventions of HPV infections and diseases caused by HPV infections, and the diseases comprise cervical cancer, condyloma acuminatum, and the like.
Owner:XIAMEN UNIV +1
New castle disease and H9 subtype bird flu bivalent vaccine
ActiveCN104922663AImproving immunogenicitySmall dose of immunizationViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Novel coronavirus vaccine and application thereof
ActiveCN112266411AHigh titerImprove expression levelSsRNA viruses positive-senseVirus peptidesCoronavirus vaccinationPharmaceutical drug
The invention relates to a novel coronavirus vaccine and application thereof, in particular to a truncated Spike protein, a fusion protein containing the truncated Spike protein, a nucleic acid molecule containing a nucleotide sequence encoding the truncated Spike protein or the fusion protein, and a vector and a host cell containing the nucleic acid molecule. The invention further relates to a pharmaceutical composition containing the truncated Spike protein, fusion protein, nucleic acid molecule or vector.
Owner:BEIJING NORTHLAND BIOTECH
Exhibiting method of preparing antibody by antigen utilizing bacteriophage exhibiting technique
InactiveCN1485432AImproving immunogenicityHigh titerImmunoglobulins against animals/humansOther foreign material introduction processesPolyclonal antibodiesTroponin
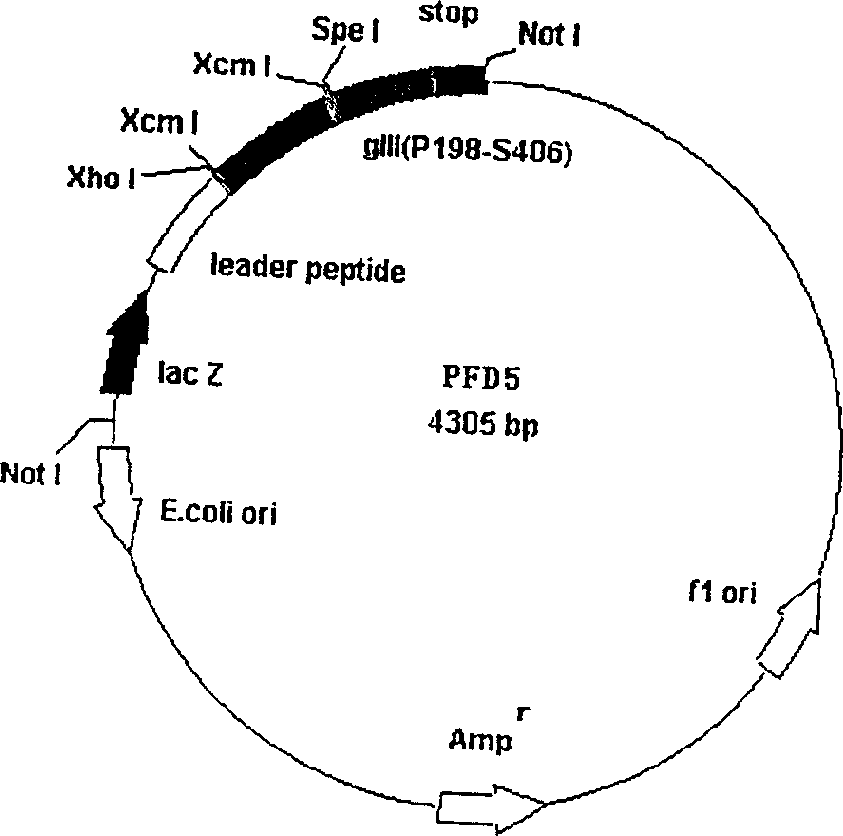
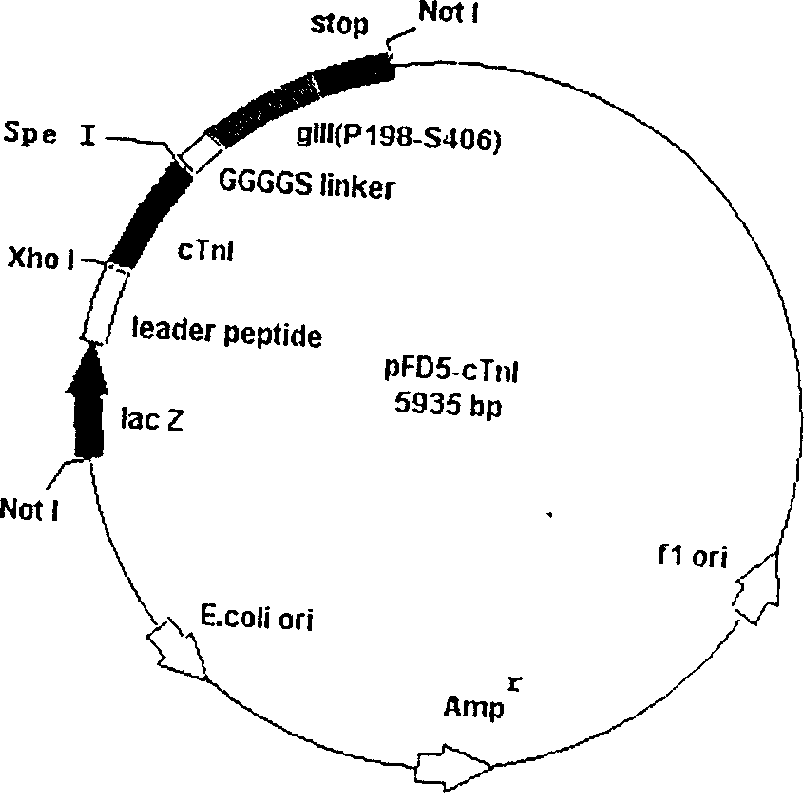
The invention comprises, fusing exogenous antigen genes and filobactivirus III gene proteins and expressed onto the tail of filobactivirus, fusing antigen epitopes and filobactivirus III gene proteins and expressed onto the surface of filobactivirus, preparing antibodies taking filobactivirus with exogenous antigen and antigen epitopes as the immunogen. The invention claims:1)a method of preparing monoantibodies and polyantibodies based on filobactivirus showing technology;2) a method of preparing cTnI monoantibody and polyantibody based on filobactivirus showing technology showing antigen and antigen epitopes of human troponin; 3) a PIII filobactivirus showing carrier pFD5; 4) a filobactivirus showing fusion expressing carrier pFD5-cTnI;5) a PIII filobactivirus showing fusion expressing carrier PC89-cTnI[95??08];6)taking filobactivirus particles showing cTnI and cTnI[95í½108] as immunogens, immunizing new zealand white rabbit to get cTnI antibody.
Owner:CHINA PHARM UNIV
Porcine epidemic diarrhea virus inactivated vaccine and preparation method thereof
ActiveCN107050447AImproving immunogenicityPassive immunity is goodSsRNA viruses positive-sensePeptide/protein ingredientsEpidemic diarrheaMicroorganism
The invention provides a porcine epidemic diarrhea virus inactivated vaccine. The porcine epidemic diarrhea virus inactivated vaccine contains the inactivated porcine epidemic diarrhea virus and an adjuvant, wherein the adjuvant is prepared from the following components in percentage by weight: 5% of squalane, 1% of oleic acid, 1% of Tween 80, 92% of 0.005M sodium citrate buffer solution, and 1% of beta-glucan, the porcine epidemic diarrhea virus is prepared by inactivating the porcine epidemic diarrhea virus strain PEDV-KB2013-4, is assigned with the microbial accession number of CGMCC No.12663, has the classification name of Porcine Epidemic Diarrhea Virus (PEDV), and is preserved in the China General Microbiological Culture Collection Center (CGMCC) on Aug 23, 2016, and the preservation address is the Institute of Microbiology of Chinese Academy of Sciences located in 3, Courtyard 1, West Beichen Road, Chaoyang District, Beijing.
Owner:陕西诺威利华生物科技有限公司 +1
Method for improving proliferation capability of mycoplasma gallisepticum in vaccine production
ActiveCN102168073AImprove proliferative abilityHigh titerAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureVaccine Production
The invention relates to a method for improving proliferation capability of mycoplasma gallisepticum in vaccine production, vaccines are prepared through the process steps of preparing seeds, preparing a culture medium of the mycoplasma gallisepticum, preparing vaccine-making bacterial liquid and preparing the inactivated vaccines, after improvement, the content of PPLO (pleuropneumonia-like organism) broth, porcine serum and glucose is increased, MEM (minimum essential medium) ingredients are removed, and the mixture ratio of the ingredients in the culture medium are more scientific and the nutritional value is higher after adjustment. The bacterial liquid concentration of a semi-finished product prepared by the method is as high as 1.0*1013CCU / ml-1.0*1014CCU / ml, and the bacterial liquiddoes not need to be concentrated and can be directly or indirectly used for preparing the inactivated vaccines after dilution, thereby not only simplifying the production process, but also reducing the production cost.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Attenuated vaccine strain of VII type new castle disease virus with mutated L gene and preparation method thereof
ActiveCN104962526AHigh titerGenetically stableMicroorganism based processesViruses/bacteriophagesAntigenDisease
The invention relates to an attenuated vaccine strain of a VII type new castle disease virus with a mutated L gene which is produced by reverse genetic operation technology and a preparation method thereof, and the invention belongs to the fields of biological products and biological technology. The virulence of the attenuated vaccine strain of the VII type new castle disease virus with the mutated L gene is obviously decreased, simultaneously the attenuated vaccine strain has the characteristics of high titer, stable heritability and high matching with the antigen of the epidemic virus according to the chicken embryo experiment; the preparation method is suitable for large scale production of vaccines, and is used for producing vaccines; compared with the conventional vaccines (gene I and gene II types), the attenuated vaccine strain of the VII type new castle disease virus with the mutated L gene has wide application prospects in the aspects of morbidity and epidemic control of new castle disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Adeno-associated virus vector and application thereof
PendingCN111349148AImprove efficiencyHigh infection efficiencySenses disorderVirus peptidesNucleic acid sequencingMutant
The present invention relates to an adeno-associated virus (AAV) capsid protein mutant, a polynucleotide containing a nucleic acid sequence encoding the AAV capsid protein mutant, a vector containingthe polynucleotide, and a host cell containing the vector. The invention also relates to an adeno-associated vector comprising the AAV capsid protein mutant, a recombinant adeno-associated virion which is constructed from the AAV vector and carries a gene expression cassette, a method for producing the adeno-associated virus vector or virion, and an application of the adeno-associated virus vectorand virion in treating diseases.
Owner:HUIGENE THERAPEUTICS CO LTD
Canine parvovirus strain CPV-YH and applications thereof
ActiveCN106591242AProliferate fastHigh titerMicrobiological testing/measurementInactivation/attenuationCanine parvovirusRaw material
The invention provides a canine parvovirus strain CPV-YH and applications thereof, and belongs to the technical field of microbes. The preservation number of the provided canine parvovirus strain CPV-YH is CGMCC No.12990. The invention also provides applications of the canine parvovirus strain CPV-YH in the preparation of canine parvovirus vaccines and canine parvovirus diagnostic reagents. Based on the canine parvovirus strain CPV-YH, the invention also provides a vaccine composition for preventing and / or treating diseases caused by canine parvovirus and a reagent for detecting a canine parvovirus strain and / or diagnosing diseases caused by canine parvovirus. A novel content and direction are provided for the research on the variation of epidemic virus; a novel strain is provided for the canine parvovirus vaccine development; and a high quality raw material and choice are provided for the vaccine development.
Owner:北京世纪元亨动物防疫技术有限公司
Process for preparing veterinary rabies inactivated and freeze-dried vaccine through suspension culture cell
ActiveCN102228686AQuality improvementSmall difference between batchesInactivation/attenuationAntiviralsAntigenHamster
The invention relates to a process for preparing a veterinary rabies inactivated vaccine, in particular to a process for preparing a veterinary rabies inactivated and freeze-dried vaccine through a suspension culture cell. The method has the key point that: a bioreactor is used for large-scale suspension culture of baby hamster kidney BHK21-C13 cells and rabies virus is inoculated, and the rabiesvirus is subjected to mass propagation through a fed-batch and perfusion technology, so that a high-concentration and high-titer rabies antigen is obtained and concentrated, inactivated and purified to prepare the veterinary rabies inactivated vaccine; and thus, technical problems such as complexity, low antigen content, low effectiveness, large dosage, poor batch-to-batch variation and the like existing in the conventional process are solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
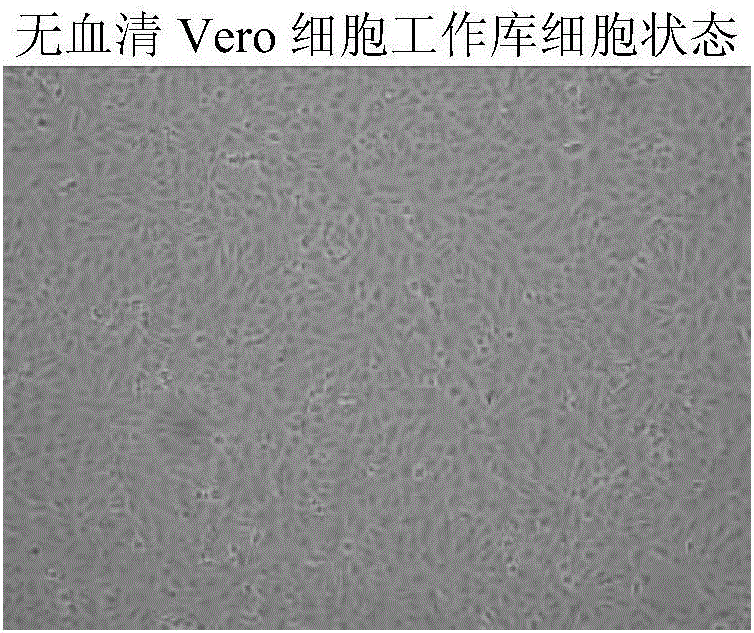
Method for preparing rabies vaccine stock solution from serum-free Vero cells and serum-free rabies vaccine product
InactiveCN106834239ARemove inhibitionHigh titerSsRNA viruses negative-senseViral antigen ingredientsUltrafiltrationVirus strain
The invention provides a method for preparing a rabies vaccine stock solution from serum-free Vero cells. The method comprises the following steps: culturing Vero cells with a serum-free culture medium to obtain a serum-free culture medium adaptive cell line; establishing a serum-free Vero cell seed bank by utilizing the serum-free culture medium adaptive cell strain; recovering, culturing, performing passage and amplifying cells of the Vero cell working seed bank by using the serum-free culture medium to serve as basal cells for bioreactor culture, and continuously perfusing to culture high-density Vero cells with the serum-free culture medium by applying a bioreactor and a microcarrier after cell expansion; inoculating virus seeds of a rabies vaccine virus strain working seed bank, performing bioreactor microcarrier serum-free culture, starting to continuously perfuse to obtain a virus fluid when the virus is amplified to peak to obtain titer of the virus fluid; and performing clarifying, ultrafiltration concentration, inactivation and purification treatment to obtain the serum-free human rabies vaccine stock solution.
Owner:AB&B BIO TECH CO LTD JS
Preparation method of inactivated newcastle disease vaccine
ActiveCN103110942AReduce in quantityEnhance immune functionViral antigen ingredientsAntiviralsOil phaseAntibody titer
The invention relates to a preparation method of an inactivated newcastle disease vaccine. The preparation method comprises the steps of virus liquid inactivation, preparation of a compound traditional Chinese medicine extract, preparation of an aqueous phase, preparation of an oil phase and emulsification. According to the preparation method, the compound traditional Chinese medicine extract serving as an immunopotentiator is added to the inactivated newcastle disease vaccine so as to well cause cellular immunologic response and enhance the immunologic function of an organism, so that an antibody is generated in advance, and the antibody titer is obviously improved.
Owner:山东滨州沃华生物工程有限公司
Plate for detecting immunity of cannabis and tetrahydrocannabinol monoclonal antibody through collaurum tag
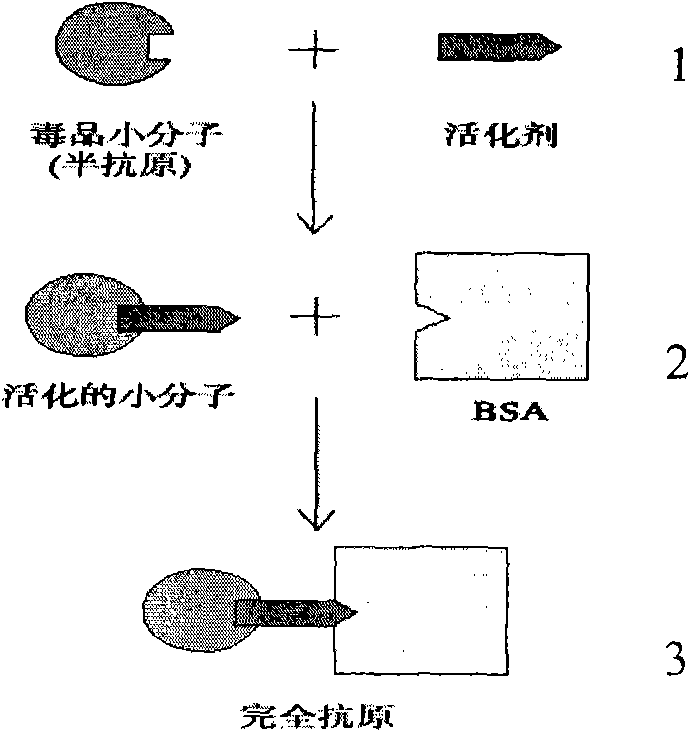
ActiveCN101580544AImproving immunogenicityPreserved immunoreactivityTissue cultureImmunoglobulinsMonoclonal antibodyImmuno detection
The invention discloses a complete antigen for detecting tetrahydrocannabinol and preparing an antibody. The invention also discloses an anti-tetrahydrocannabinol monoclonal antibody prepared by the complete antigen and a tetrahydrocannabinol monoclonal antibody immunity detection plate through a collaurum tag for detecting the tetrahydrocannabinol in medicine, a urine specimen or other human specimens. Compared with HPLC and other methods, the detection plate is simple, portable and easy to carry, can carry out field detection and does not need expensive equipment. When the detection plate is used to detect the tetrahydrocannabinol, the whole test can be completed within 1.5 minutes; the detecting sensitivity can reach 0.5ng; and the detection plate has no crossed reaction with more than 60 kinds of common medicaments, drugs and internal metabolins of the tetrahydrocannabinol.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com