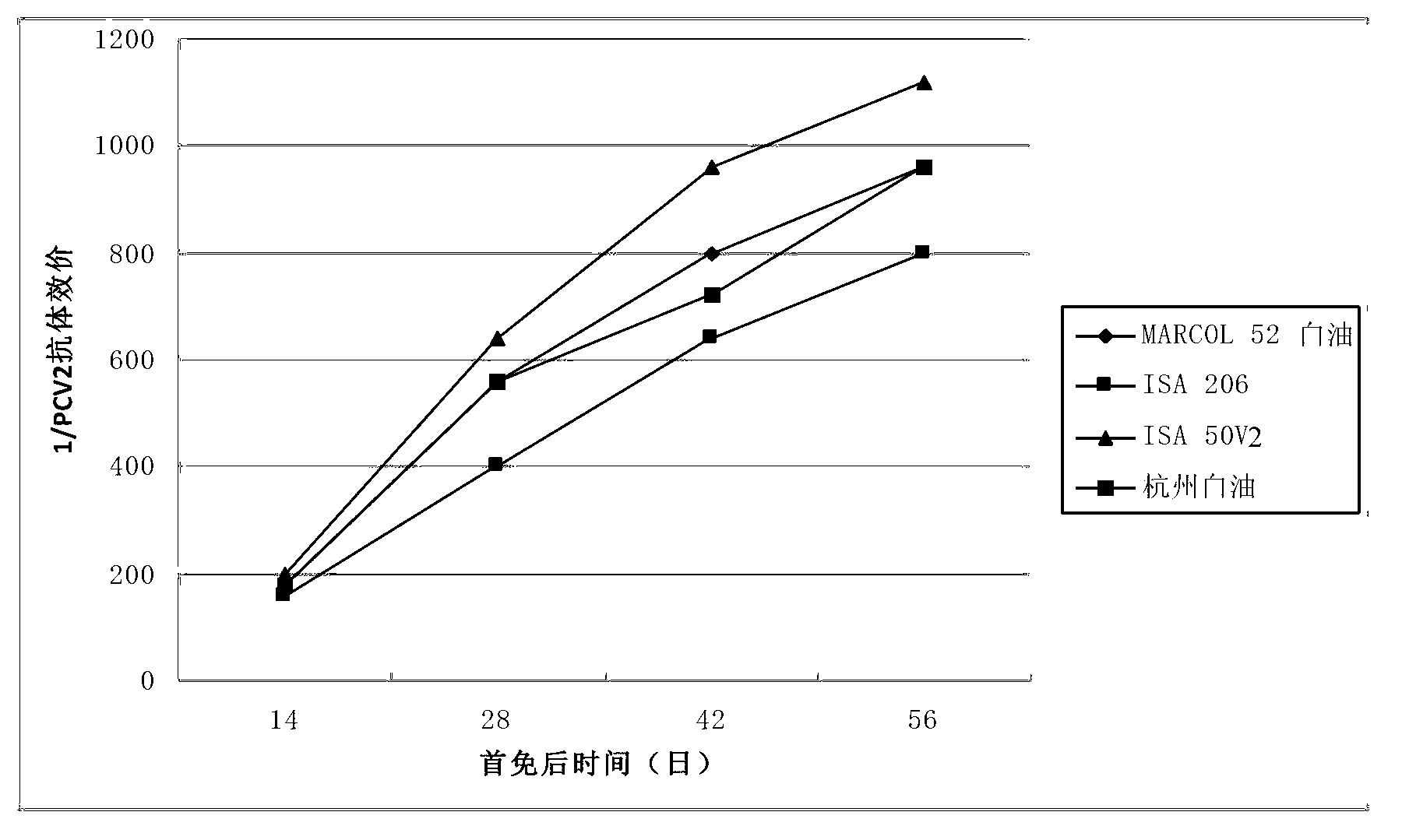
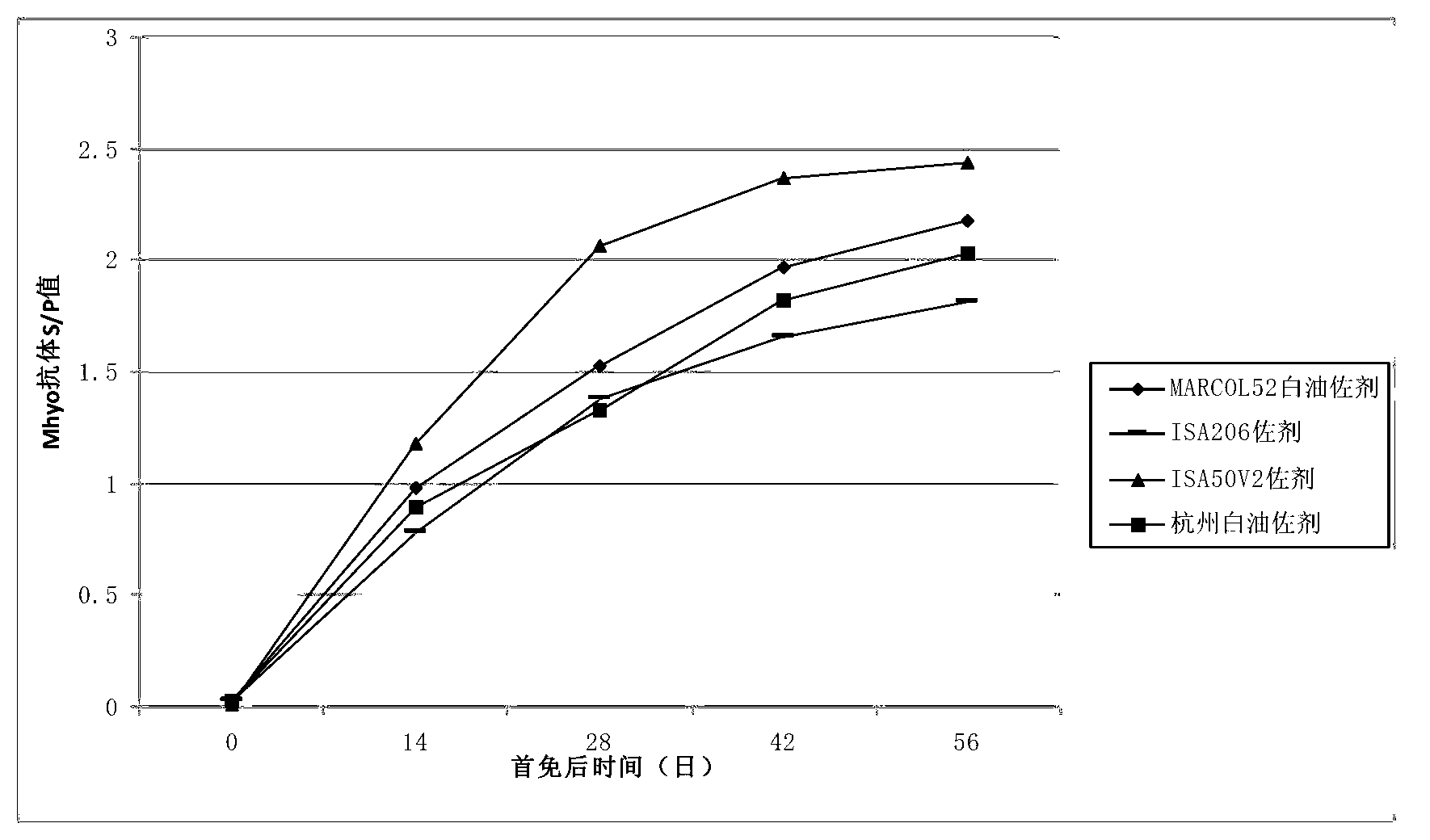
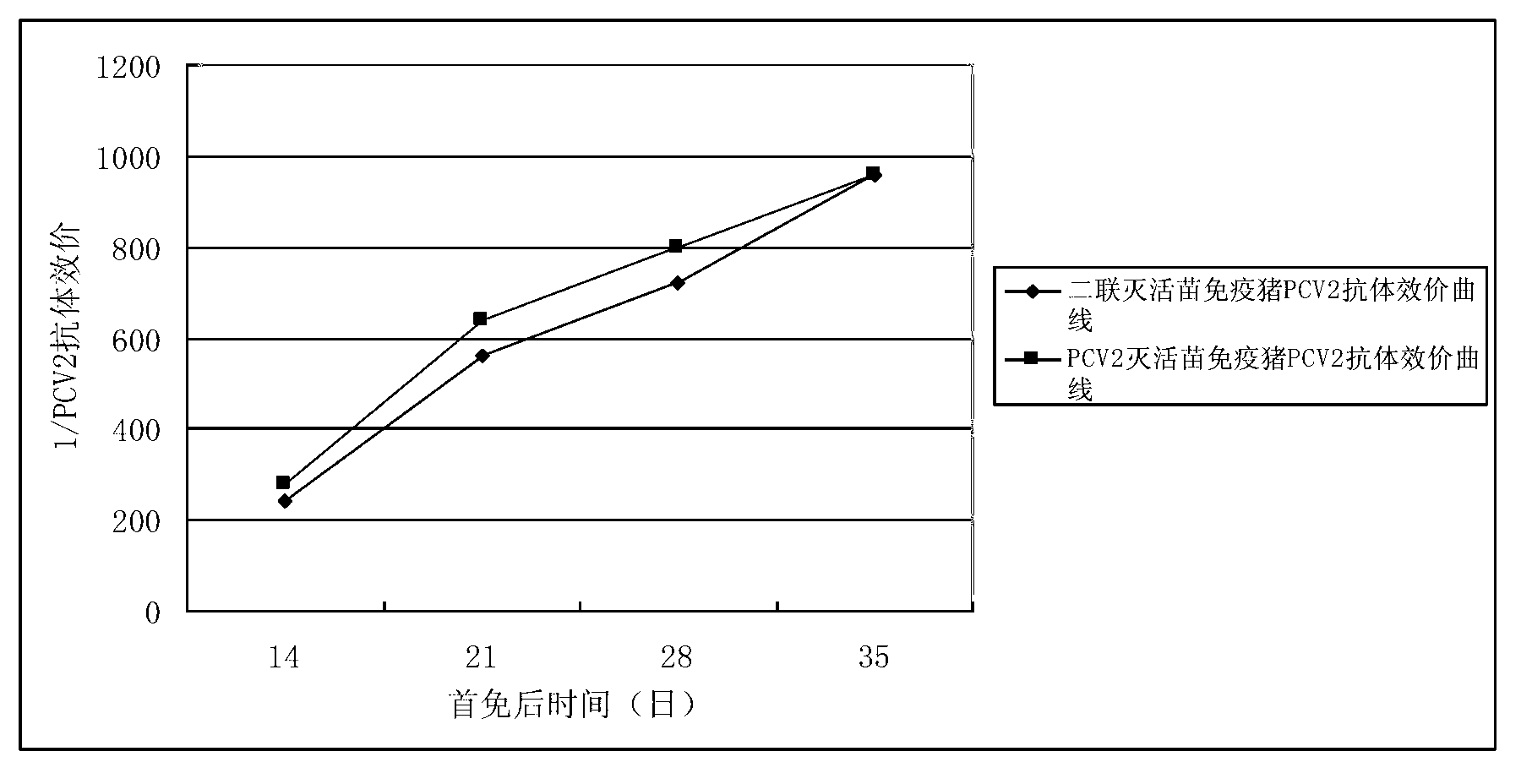
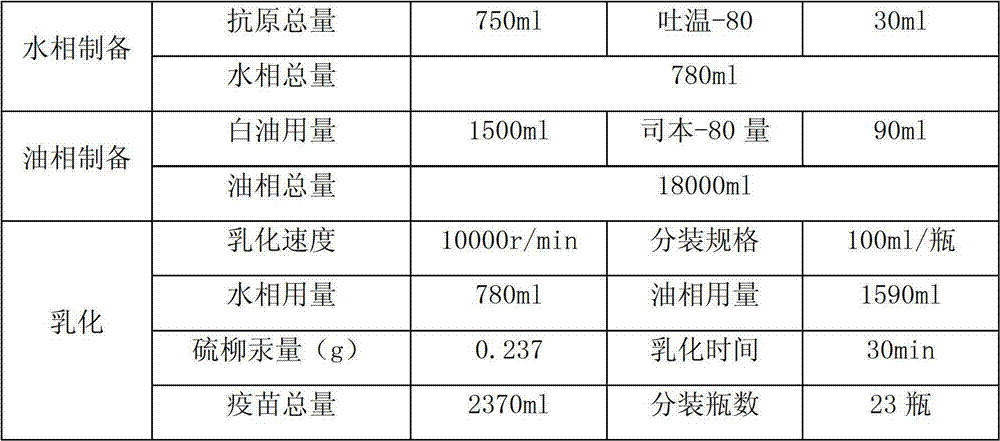
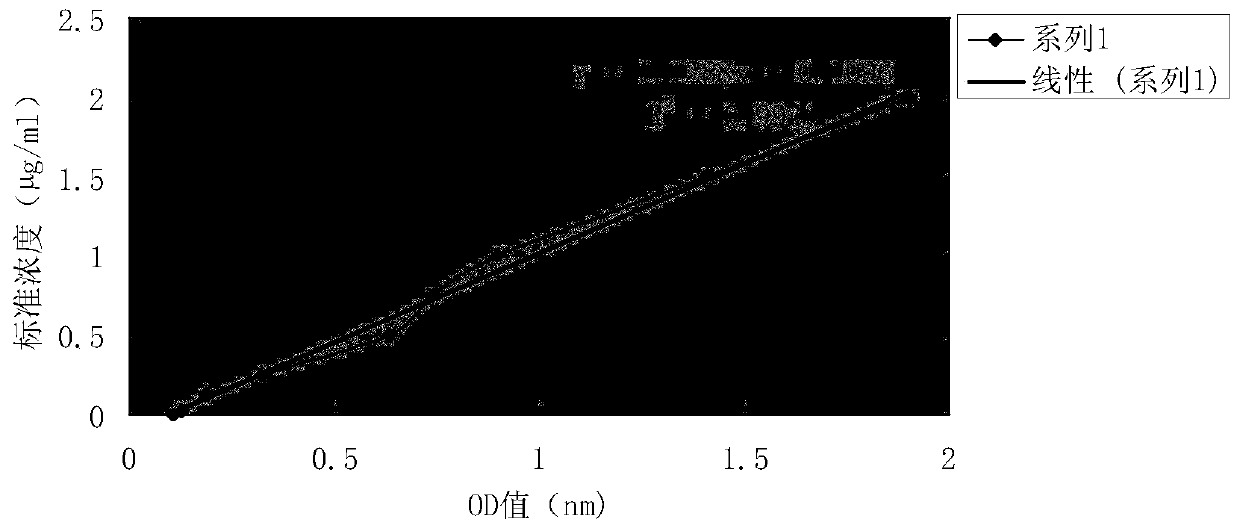
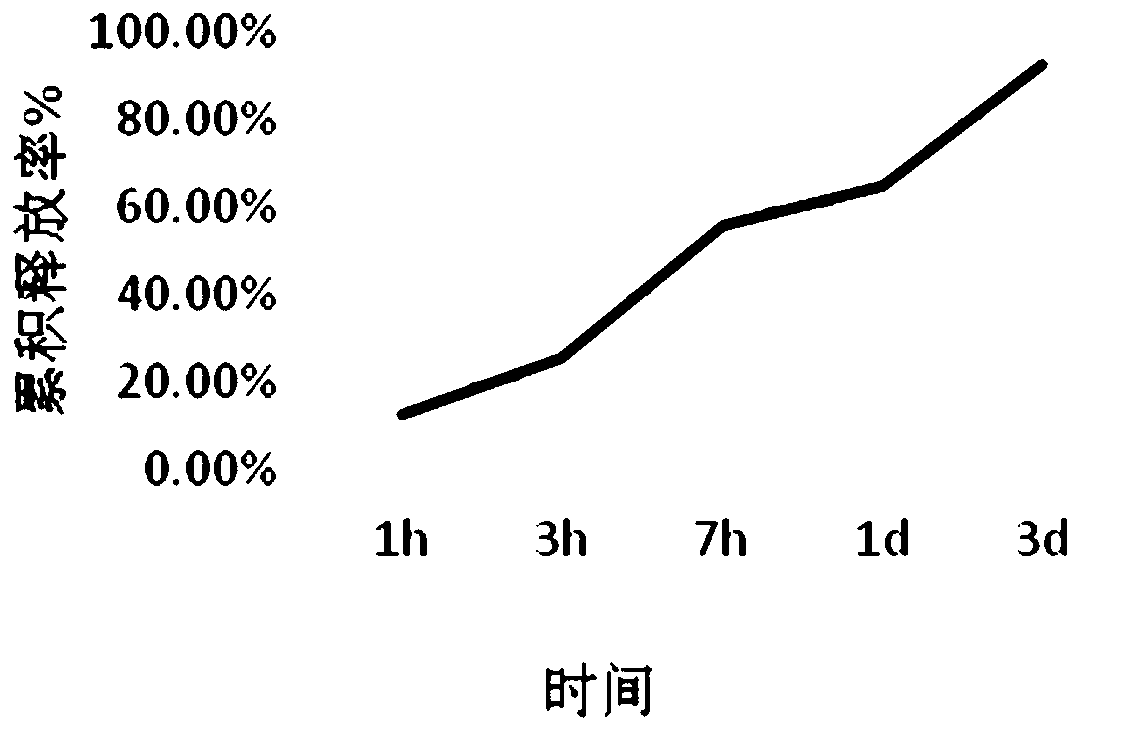
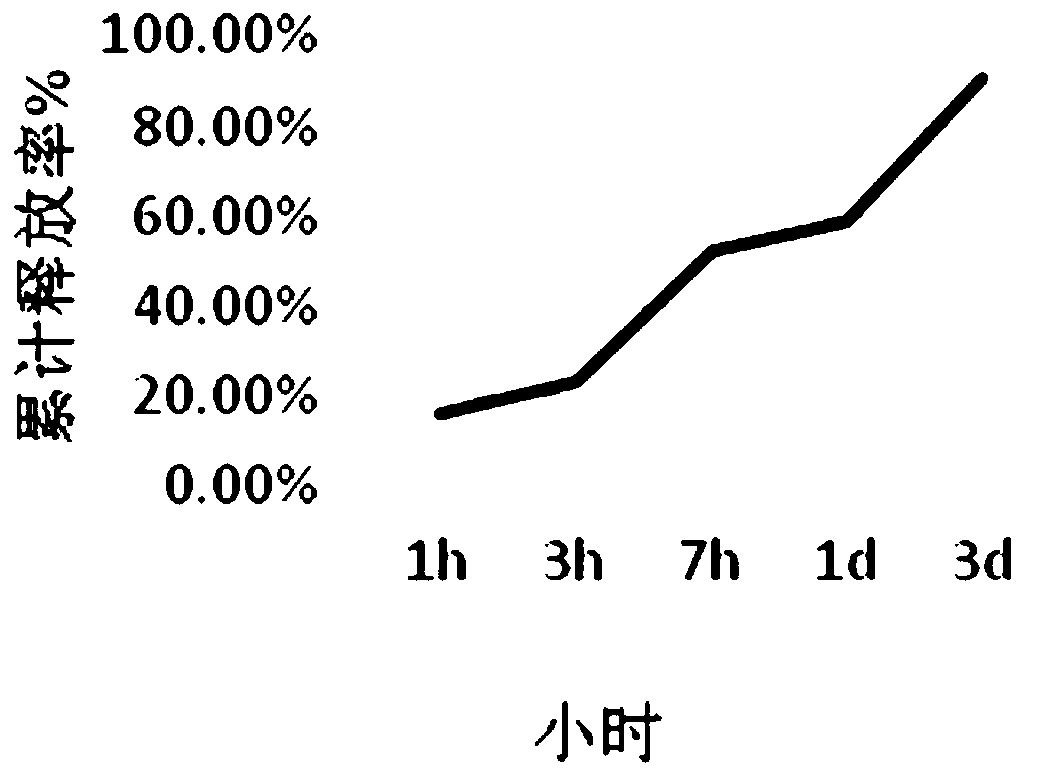
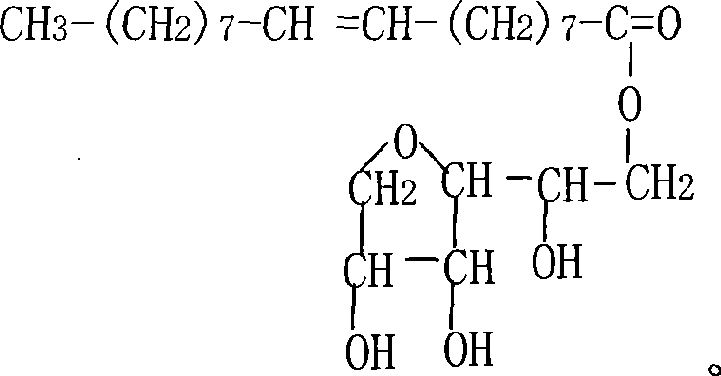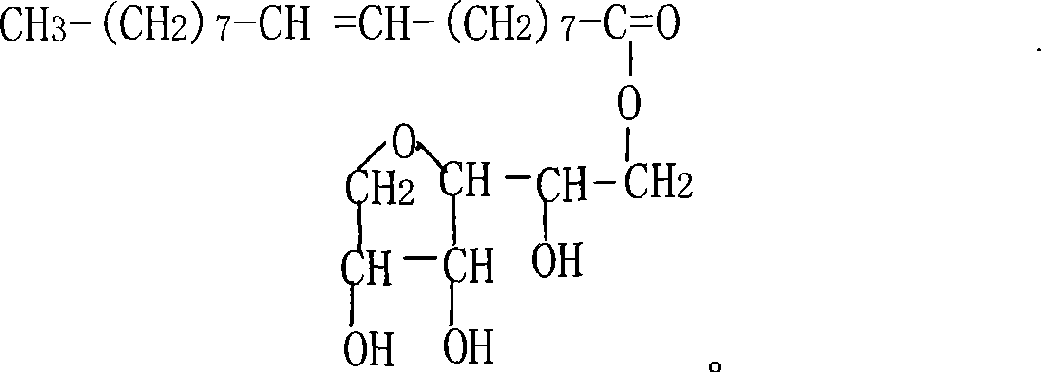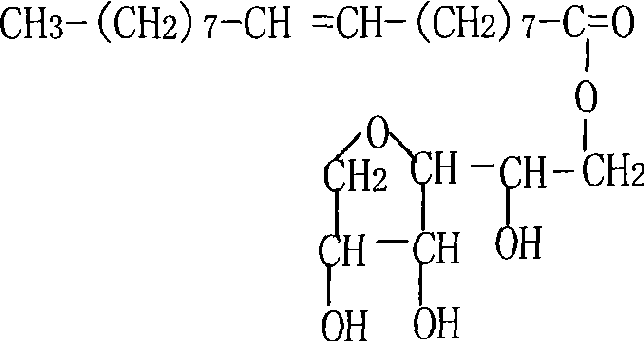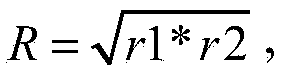Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
156 results about "Oil adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oil adjuvants are petroleum (PO) or methylated vegetable or seed oils (MSO) plus an emulsifier for dispersion in water. The emulsifier, the oil class (petroleum, vegetable, etc.), and the specific type of oil in a class all influence effectiveness of an oil adjuvant.
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Water-in-oil-in-water adjuvant vaccine and preparation method thereof
ActiveCN103223164APromote absorptionGenerate fastAntiinfectivesAntibody medical ingredientsEngineeringImmunity response
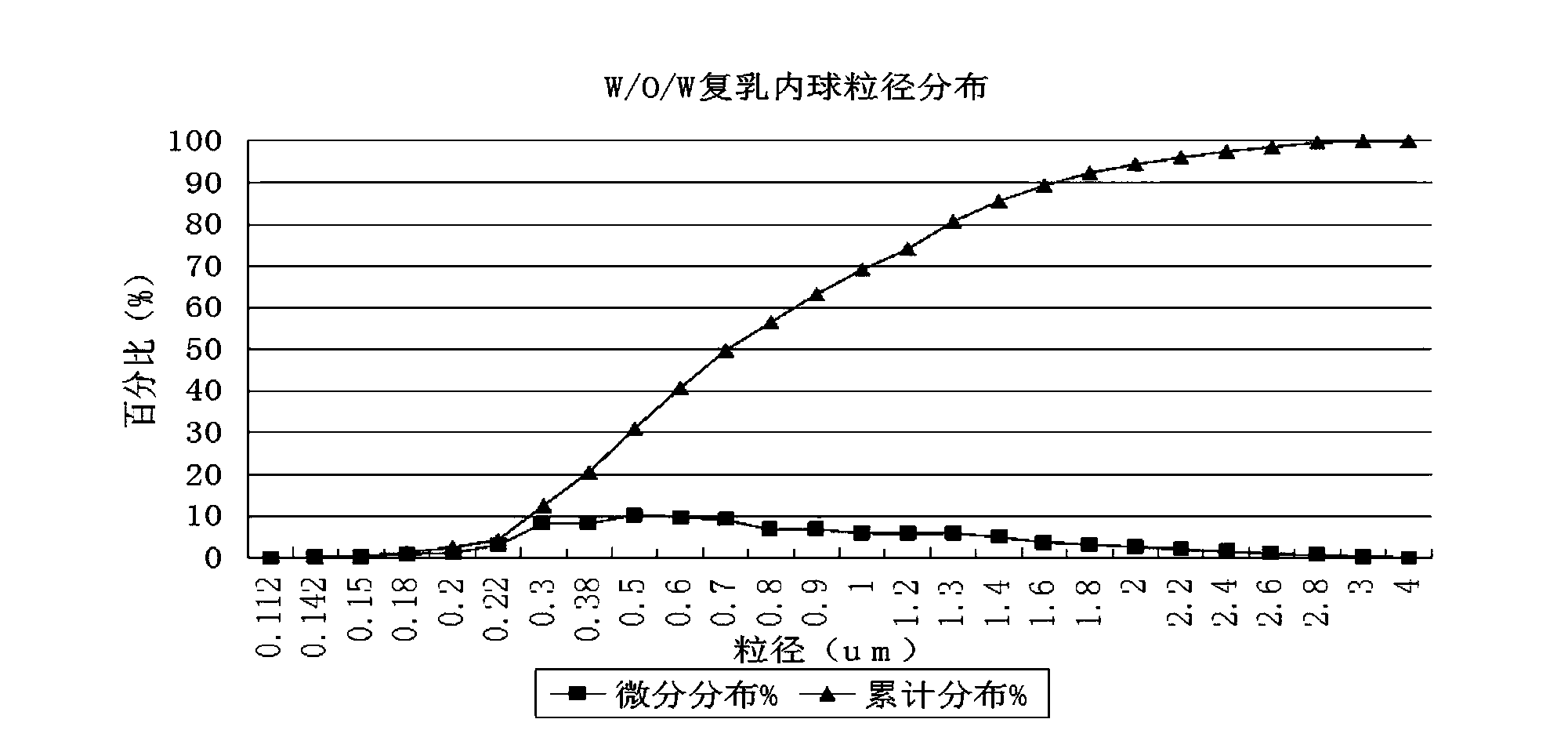
The invention discloses a water-in-oil-in-water adjuvant vaccine and a preparation method thereof. The adjuvant vaccine is composed of an internal water phase, a middle oil phase used for cladding the internal water phase, and an external water phase for cladding the middle oil phase. The external water phase comprises: a composite surfactant, a hydrophilic surfactant and mormal saline. The middle oil phase consists of: a lipophilic surfactant, a stabilizer and mineral oil. The internal water phase comprises: an immunopotentiator solution, the hydrophilic surfactant, and an inactivated antigen solution. The W / O / W (water-in-oil-in-water) type adjuvant vaccine provided in the invention can induce an immunoreaction earlier than traditional W / O (water-in-oil) type adjuvant vaccines, and can make experimental animals obtain earlier protective antibodies. The vaccine has good absorption, and causes a small inflammatory response at an inoculation site. The adjuvant vaccine disclosed in the invention has better stability than existing W / O / W type vaccines, and the antibody generation speed is fast.
Owner:SOUTH CHINA AGRI UNIV +1
Rhodococcus ruber and application of same as immunologic adjuvant in preparing vaccine
ActiveCN109576180AWill not cause accidental infectionReduce pollutionBacteriaMicroorganism based processesSide effectShort terms
The invention discloses rhodococcus ruber and application of same as an immunologic adjuvant in preparing vaccine. The rhodococcus ruber is also called rhodococcus ruber RDC-01, and the preservation number is CGMCC (China General Microbiological Culture Collection Center) NO. 16640. The rhodococcus ruber disclosed by the invention has the function of increasing and regulating the body immunity andis capable of nonspecifically enhancing the activity of TB (Tuberculosis) lymphocyte, macrophagocyte and NK cells and inducing multiple cell factors such as interferon, and the rhodococcus ruber canbe used as the immunologic adjuvant after being inactivated so as to be added in an oil-adjuvant inactive vaccine, so that generation of an animal antibody induced by the vaccine can be obviously promoted; compared with single use of the oil-adjuvant inactive vaccine, a high-titre antibody can be generated, the use is safe, long-term and short-term toxic and side effects are not generated, and anapplication prospect in the field of preparation of vaccines for animals is good.
Owner:北京利昂盛生物技术有限公司
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
Method for preparing duck virus hepatitis divalent refined egg yolk antibody
ActiveCN102827275AImprove securityHigh protection rateEgg immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusFreeze thawing
The invention relates to a method for preparing a duck virus hepatitis divalent refined egg yolk antibody. The method is that vaccines of a duck virus hepatitis virus 1 type YBH1 strain and novel YBHX strain with good immunogenicity are selected from duck virus hepatitis superior prevalent strains to prepare strains, the strains are respectively inoculated to a chicken embryo and a duck embryo, a dead embryo body and the embryo juice are respectively obtained, the virus liquid is collected after grinding and freeze thawing, ultrafiltration concentration and inactivation with formaldehyde solution are carried out, and oil adjuvant is added to mix and emulsify to produce the vaccine. The vaccine is inoculated to a laying hen, the egg with high immunity is obtained, the yolk is separated, inactivated, extracted and refined to produce the duck virus hepatitis divalent refined egg yolk antibody which can prevent the duck virus hepatitis which is caused by the 1 type and novel duck hepatitis viruses, so the egg yolk has the advantages of high efficiency, good safety, high protection ratio and the like.
Owner:YEBIO BIOENG OF QINGDAO
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
New castle disease and H9 subtype bird flu bivalent vaccine
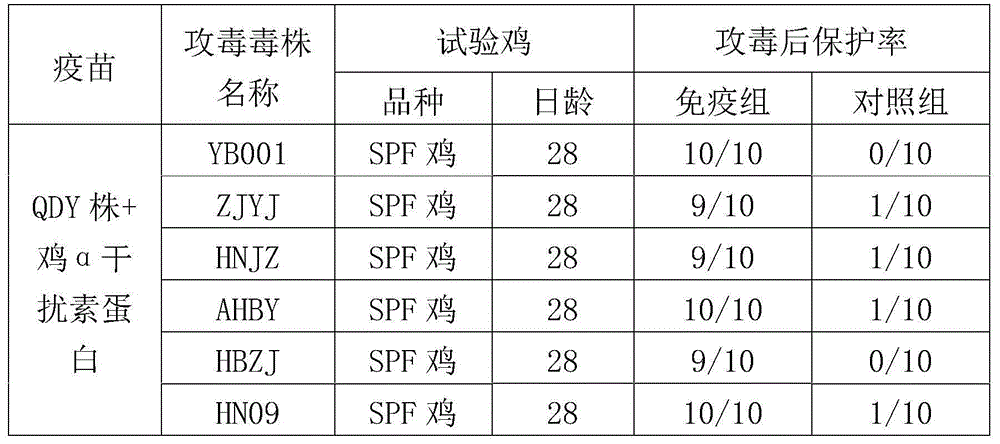
ActiveCN104922663AImproving immunogenicitySmall dose of immunizationViral antigen ingredientsAntiviralsDiseaseOil adjuvant
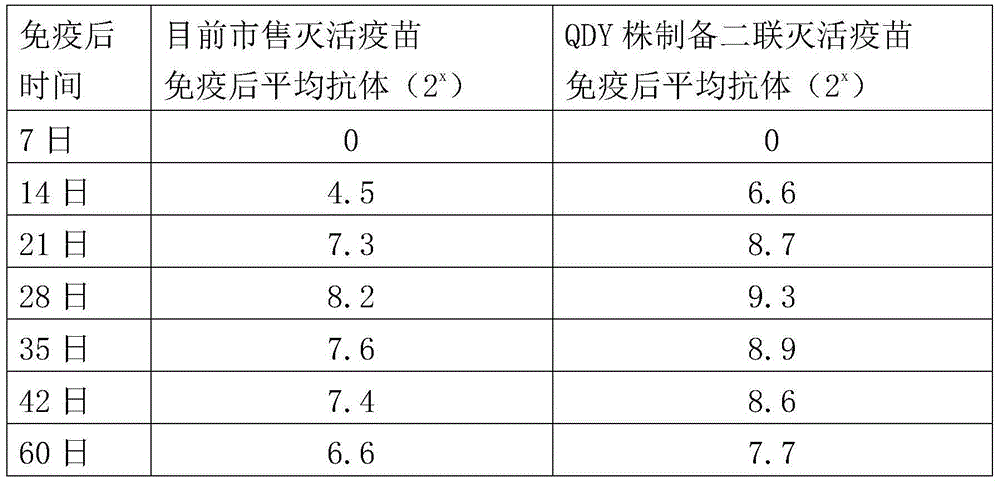
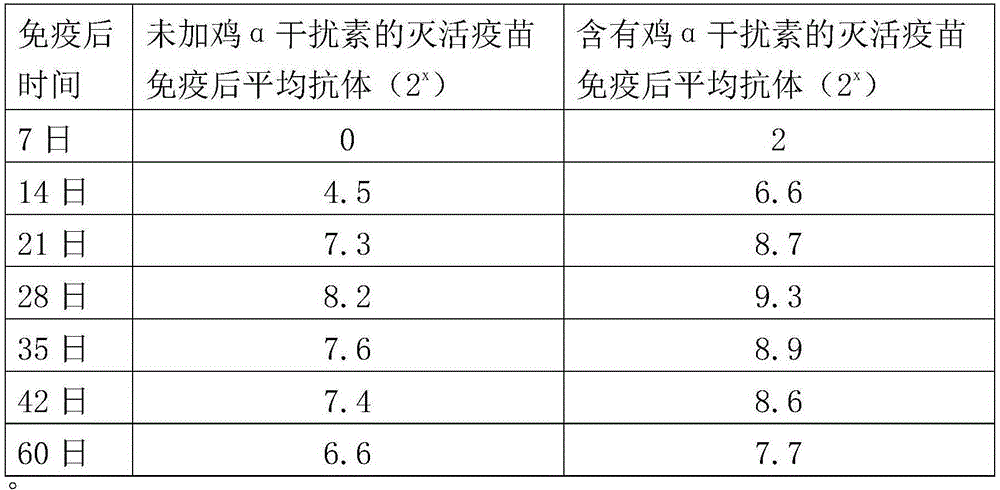
The invention aims at providing a new castle disease and H9 subtype bird flu bivalent vaccine. The new castle disease and H9 subtype bird flu bivalent vaccine contains antigens and adjuvant. The antigens are inactivated H9 subtype bird flu viruses and new castle disease viruses. The H9 subtype bird flu viruses are QDY strains, and the preservation number of the H9 subtype bird flu viruses is CCTCC v201517. The QDY strains of the H9 subtype bird flu viruses and a Lasota strain of the new castle disease viruses are inoculated to chick embryos respectively, and then virus liquid is collected; the virus liquid and the oil adjuvant are mixed and emulsified into the vaccine after the virus liquid is inactivated through a formaldehyde solution. The new castle disease and bird flu bivalent inactivated vaccine is good in immunogenicity, antibody production is fast after immunity, the produced antibody titer is high, the produced antibody holding time is long, the retention period is long, the immunizing dose is small, the selected adjuvant is easy to inject, and two kinds of diseases can be prevented through one-time injection. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Emulsifiable Concentrates Containing Adjuvants
InactiveUS20080248955A1Improve compatibilityLow viscosityBiocideDead animal preservationOil adjuvantPropionic acid derivatives
The present invention relates to stable emulsifiable concentrates comprising an oil adjuvant and at least one member selected from the group consisting of herbicidally active 2-[4[(5-chloro-3-fluoropyridin-2-yloxy)-phenoxy]-propionic acid derivatives and quinoline derivative safeners and to the use thereof as a pesticide.
Owner:SYNGENTA CROP PROTECTION INC
Preparation process and application of genetic engineering subunit vaccine of infectious bursal disease
ActiveCN1754959AAntigenic activityReduce manufacturing costBacteriaViral antigen ingredientsEscherichia coliVaccine Production
The invention relates to a bacterium for the production of genetically-engineered subunit oil-adjuvant vaccines against infectious bursal disease, i.e. Escherichia coli BL21 / pET-28a-VP2, CCTCC No: M204038. The process for constructing the bacterium consists of inserting VP2 protein gene of the infectious bursal disease virus onto expression plasmid pET-28a to construct prokaryotic expression plasmid, then using the prokaryotic expression plasmid to transform the escherichia coli BL21, using lactose as inducer to induce Escherichia coli BL21 / pET-28a-VP2 expression VP2 protein, purifying and charging oil-adjuvant to obtain the vaccines.
Owner:YEBIO BIOENG OF QINGDAO
Oil emulsion vaccine for broilers and preparation method thereof
InactiveCN102579339APromote absorptionQuick resultsViral antigen ingredientsAntiviralsOil emulsionOil phase
The invention discloses an oil emulsion vaccine for broilers and a preparation method thereof. The oil emulsion vaccine comprises a water phase and an oil phase, wherein the water phase contains concentrated and inactivated ND (newcastle disease) and H9 AI (avian influenza) venom and Tween-80; the oil phase contains an oil adjuvant formed by white oil, Span-80 and aluminium stearate; the volume ratio of the water phase to the oil phase is (1:1.6) to (1:2); and the kinematic viscosity (40 DEG C) of the selected white oil is 4-8mm<2> / s. The oil emulsion vaccine has the characteristics of easiness in absorption, quick effect and high level of the generated antibodies, is suitable for growth of the broilers, can effectively increase the immune levels of the broilers, can effectively protect the broilers from being invaded by ND and H9 AI viruses and avoids ND and H9 AI in the immune phase.
Owner:TIANJIN RINGPU BIO TECH
H9 subtype avian influenza virus strain
ActiveCN104946600AGuaranteed immune effectImprove securityMicroorganism based processesAntiviralsImmune effectsOil adjuvant
The invention aims at providing an H9 subtype avian influenza virus strain. Vaccine is prepared by the virus strain to solve the problem that existing H9 subtype avian influenza virus vaccine is poor in immune effect caused by a novel virus. The H9 subtype avian influenza virus QDY strain (Avian Influenza Virus) is preserved in the typical culture preservation center in China in Wuhan University on April 29, 2015, and the preservation number is CCTCC NO: V201517. After the H9 subtype avian influenza virus (QDY strain) is inoculated to a chick embryo, a virus solution is collected, after ultrafiltration concentration and formaldehyde solution inactivation are performed, oil adjuvant is added, and then mixing and emulsifying are performed to prepare the vaccine. According to the prepared vaccine, the antibody level after immunization can be improved, the antibody uniformity after immunization can be improved, and the immune effect of the vaccine can be guaranteed; the vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Ginsenoside-containing vegetable oil adjuvant and preparation method and application thereof
InactiveCN103751775ARaise antibody levelsHigh activityImmunological disordersAntibody medical ingredientsOil adjuvantVegetable oil
Owner:ZHEJIANG UNIV
Method for preparing nanoparticle oil adjuvant vaccine
InactiveCN102580083ASmall particle sizeLarge particle sizeViral antigen ingredientsAntiviralsOil adjuvantNewcastle disease virus NDV
The invention relates to a method for preparing a nanoparticle oil adjuvant vaccine. The method comprises the following steps of: adding newcastle disease virus liquid into a chitosan solution or an N-2-hydroxypropyl trimethyl ammonium chloride chitosan solution to obtain a solution A; adding sodium tripolyphosphate and a phosphate buffer solution (PBS) into the solution A, stirring, adding span-80 to obtain a solution B; centrifuging the solution B, extracting supernate to obtain a solution B1, suspending precipitation by using deionized water, and performing suspension to obtain a solution B2; sterilizing white oil for injection at high temperature, adding the span-80 and cooling to obtain a solution C1; adding tween-80 into the solution B1 to obtain a solution C2; adding the solution C2 into the solution C1, and stirring to obtain a solution C; and adding the solution B2 into the solution C, and stirring to obtain the nanoparticle oil adjuvant vaccine. The nanoparticle oil adjuvant vaccine is small in particle sizes of virus-loaded nanoparticles, high in envelop rate and large in medicine-carrying quantity, and the method has a mild preparation condition and a simple preparation process, and is low in production cost.
Owner:HEILONGJIANG UNIV
Porcine parvovirus L strain and use thereof in preparation of porcine parvovirus inactivated vaccines
The invention discloses a porcine parvovirus L strain and use thereof in preparation of porcine parvovirus inactivated vaccines. In the invention, microbial preservation number of the separated strain is CGMCC No. 3352. The strain is highly homologous with an NADL-2 strain and a China strain and keeps high reproduction, and the viruses are stable. Oil adjuvant is added into antigen liquid which inactivates the viruses by using binary ethylenimine to prepare the bidirectional oil-emulsion inactivated vaccines; and the prepared inactivated vaccines are applied in a vaccine inoculation experiment of 10,276 pigs (most of which are first farrowing sows), and in the experiment, an immune dosage of 2 millimeters is given to each pig through muscle injection, and each time of inactivated vaccine inoculation can obtain an immune period of over 6 months. Under the conditions that a 10 millimeter overdose vaccine is injected into a pig and that single dose inoculation is repeatedly carried out for three times, no abnormal reaction happens, which proves that the inactivated vaccines have high immunogenicity and safety.
Owner:哈药集团生物疫苗有限公司
Newcastle disease-H9 subtype avian influenza bivalent dual adjuvant inactivated vaccine and preparation method thereof
InactiveCN102416177APrevent immune failureAvoid immune failureViral antigen ingredientsAntiviralsImmune effectsOil adjuvant
The invention discloses a Newcastle disease-H9 subtype avian influenza bivalent dual-adjuvant inactivated vaccine and a preparation method thereof. The bivalent vaccine consists of a concentrated and inactivated ND and AI (H9) venom, levamisole hydrochloride and an oil adjuvant, wherein every milliliter of inactivated vaccine contains 0.5-16 mg of levamisole hydrochloride immunopotentiator. When the synergic ND-AI (H9) bivalent dual-adjuvant inactivated vaccine prepared with the method is applied to immunized chicken, the humoral immunity and cell immune levels of table poultry and laying hens can be raised effectively, the growth is promoted, and rate of live weight growth is increased. The bivalent dual-adjuvant inactivated vaccine has an immune effect which is remarkably superior to that of the conventional chicken ND-AI bivalent oil emulsion inactivated vaccine, and can be used for effectively protecting chicken flocks from being intruded by the ND virus and AI (H9) virus and preventing the Newcastle disease and H9 subtype avian influenza at the immune period.
Owner:TIANJIN RINGPU BIO TECH
Oil adjuvant vaccine
InactiveUS20050158330A1Good storage stabilityImprove immunitySnake antigen ingredientsSynthetic polymeric active ingredientsOil adjuvantSide effect
The present invention provides a W / O / W type oil adjuvant vaccine containing an outer aqueous phase containing 0.5 wt %-20 wt % of a polyethylene glycol derivative having a molecular weight of 400-20,000, and an inner aqueous phase containing a biologically acceptable and effective amount of an antigen. The constitution of the present invention that a polyethylene glycol derivative having a specific molecular weight is contained in the outer aqueous phase enables preparation of a W / O / W type oil adjuvant vaccine showing a high adjuvant effect, reduced side effects such as topical response, superior preparation stability and superior workability to allow a person to give an injection easily due to the lowered viscosity.
Owner:NOF CORP +1
Compound oil adjuvant as well as preparation method and application thereof
ActiveCN102813922ASolve the difficult problem of preparationEasy injectionViral antigen ingredientsAntiviralsAnimals vaccinesEngineering
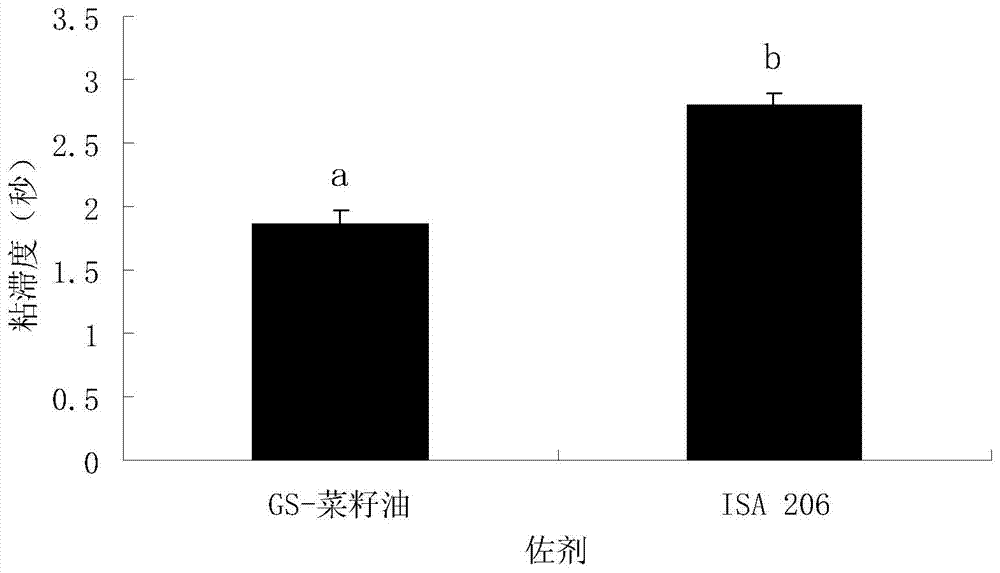
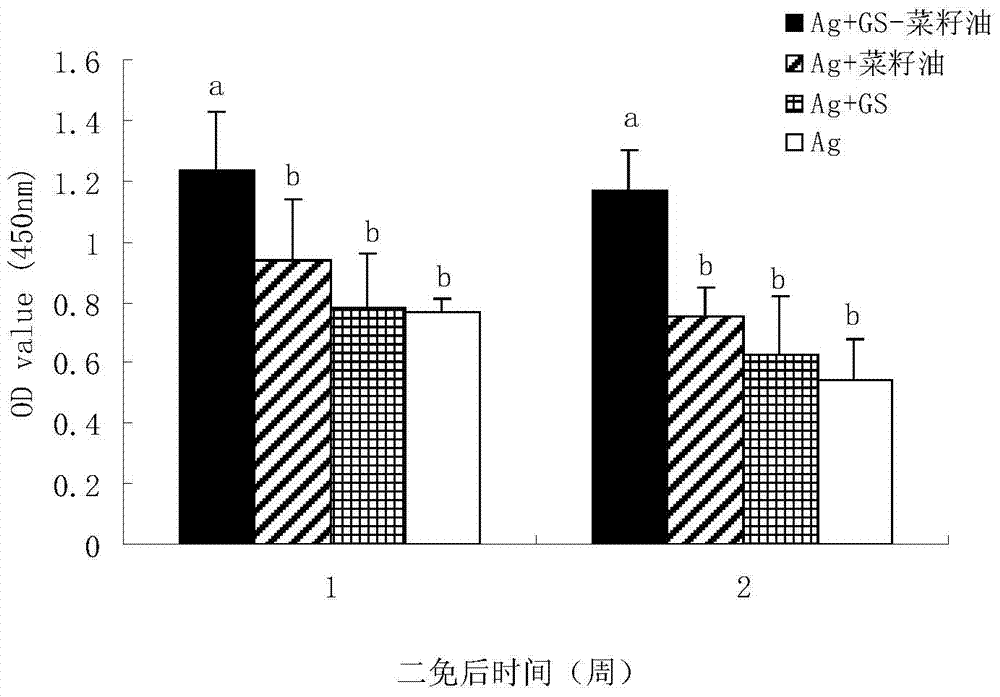
The invention relates to the technical field of vaccine adjuvant, in particular relates to a compound oil adjuvant. The compound oil adjuvant comprises three ingredients, namely white oil, oleyl alcohol polyoxyethylene ether and polyoxyethylene ether stearate. The white oil accounts for 80-95%, the oleyl alcohol polyoxyethylene ether accounts for 1-10%, and the polyoxyethylene ether stearate accounts for 1-10%. Molecular weight of the oleyl alcohol polyoxyethylene ether is 400-600, and the molecular weight the polyoxyethylene ether stearate is 400-600. The preparation method of the compound oil adjuvant disclosed by the invention comprises the steps of respectively weighing the white oil, the oleyl alcohol polyoxyethylene ether and the polyoxyethylene ether stearate in proportion of a prescription and mixing to be uniform. The invention also discloses an application of the compound oil adjuvant in preparation of an animal vaccine. The invention successfully solves the problem that a W / O / W type emulsion vaccine is difficult to prepare, the prepared emulsion vaccine is convenient for injection and also has good immunogenicity, and stability of the prepared emulsion vaccine is better than that of the W / O / W type emulsion vaccine prepared by ISA206.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Oil adjuvant of inactivated vaccine
ActiveCN101474403AEasy to useStable manufacturingOrganic chemistryAntibody medical ingredientsOil adjuvantMANNITOL/SORBITOL
The invention relates to an oil adjuvant of inactivated vaccine. The oil adjuvant consists of white oil for injection and mannitol olein which are mixed in volume proportion of 90:10 to 100:8. The oil adjuvant of the inactivated vaccine has the advantages that: 1) the oil adjuvant of the inactivated vaccine is convenient for use, the process for manufacturing the inactivated vaccine is simplified, the inactivated vaccine is manufactured by one step in stead of three steps at present, and the cost of the vaccine is reduced; 2) the oil adjuvant of the inactivated vaccine is almost nontoxic, the content of aromatic hydrocarbon of the white oil used in the invention is low and is less than 50ppm, after the vaccine is injected into an animal body, the vaccine causes very small erythema and does not influence the quality and the appearance of meat; and 3) the oil adjuvant of the inactivated vaccine has low viscosity coefficient, has low resistance for pushing a pin when being used for animal epidemic prevention injection and is convenient for injecting.
Owner:兆丰华生物科技(南京)有限公司 +1
Bovine capsular serotype A Pasteurella mutocida, validation identification and application thereof
The invention discloses bovine capsular serotype A Pasteurella mutocida and application thereof. The bovine capsular serotype A Pasteurella mutocida is separated from disease samples with haemorrhagic septicomia of cattle in six provinces (cities) with the microbial preservation number of CGMCC No.3619. The bovine capsular serotype A Pasteurella mutocida is cultured by BHI, oil adjuvant is adopted to prepare inactivated immunogen, and a mouse model proves that the bovine capsular serotype A Pasteurella mutocida has complete protecting function on serotype A Pm velogenic attck, and does not have crossing protection with serotype B Pm. Testing results prove that vaccine can be prepared by the bovine capsular serotype A Pasteurella mutocida for preventing the haemorrhagic septicomia of cattle caused by the capsular serotype A Pasteurella mutocida. The strain cannot cause diseases when inoculated to chicks, which proves that the strain is chicken isolated capsular serotype A Pasteurella mutocida.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Egg yolk antibody used for preventing and treating Muscovy duck source gosling plague
ActiveCN105949307AHigh potencyHigh protection rateEgg immunoglobulinsMicroorganism based processesYolkFreeze thawing
The invention provides an egg yolk antibody used for preventing and treating Muscovy duck source gosling plague. The egg yolk antibody is prepared with a Muscovy duck source gosling plague virus with the preservation serial number of CCTCC NO: V201620 as an antigen. Muscovy duck embryos are inoculated with the Muscovy duck source gosling plague virus strain YBGPV-M, idiosome and blastochyle of dead embryos are obtained respectively, virus liquid is obtained after grinding and freeze thawing, an oil adjuvant is added for mixing emulsification after ultrafiltration concentration and formaldehyde solution inactivation, and a vaccine is obtained; laying hens are inoculated with the vaccine, eggs are obtained, yolks are separated, inactivation, extraction and refining are carried out, and the antibody is obtained. The prepared antibody can prevent young Muscovy duck gosling plague virus infection caused by the gosling plague virus; the egg yolk antibody has the advantages of being efficient, good in safety, high in protection rate and the like.
Owner:YEBIO BIOENG OF QINGDAO
Muscovy duck parvovirus inactivation vaccine and application thereof
ActiveCN103830724ALow toxicityRaise antibody levelsAntiviralsAntibody medical ingredientsMaternal antibodyTGE VACCINE
The invention provides a Muscovy duck parvovirus inactivation vaccine which comprises an antigen and a vaccine adjuvant, wherein the antigen is inactivated Muscovy duck parvovirus, and the collection number of the Muscovy duck parvovirus is CGMCC No.8504. The Muscovy duck parvovirus inactivation vaccine provided by the invention is prepared by the steps of screening a Muscovy duck parvovirus YBMDP strain with low toxicity and high immunogenicity; inoculating and collecting the virus liquid; performing ultrafiltration concentration and formaldehyde solution inactivation; adding an oil adjuvant and mixing and emulsifying to obtain the vaccine. The vaccine provided by the invention can improve the antibody level of Muscovy duck, guarantee the maternal antibody level of the filial generation and prevent the duckling parvovirus infection caused by the Muscovy duck parvovirus; the vaccine has the advantages of high efficiency and good safety.
Owner:YEBIO BIOENG OF QINGDAO
Inactivated vaccine of cow chlamydia, its preparation and inspection method
ActiveCN1698892AFight infectionInfection fromChlamydiaceae ingredientsAntiinfectivesMicrocosmic saltOil adjuvant
The invention relates to an inactivated vaccine of cow chlamydia, its preparation and the related inspection method during the vaccine preparation. The preparing process comprises diluting the Chlamydia psittaci SX 5 or NX with microcosmic salt buffering liquid or physiological saline, vaccinating to healthy chick embryo hatched at 37 deg. C for 6-7 days, harvesting vitelline membrane and allantois liquid of dead chick embryo after 72 hours as antigens, triturating the antigens, diluting and charging formaldehyde for deactivation, mixing the deactivated antigens with oil adjuvant by the proportion of 1:1, stirring homogeneously, carrying out homogeneous emulsion to obtain the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Avian influenza H9 subtype inactivated vaccine, preparation method and application thereof
ActiveCN103736088AGood protection against poisonImprove securityMicroorganism based processesAntiviralsOil adjuvantAvian influenza virus
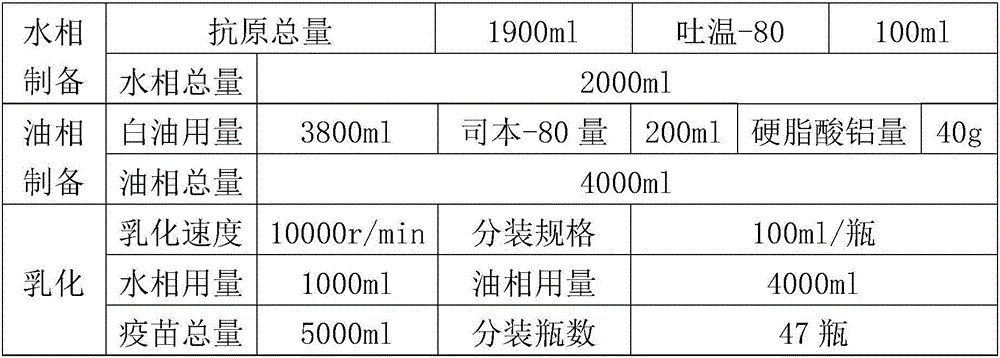
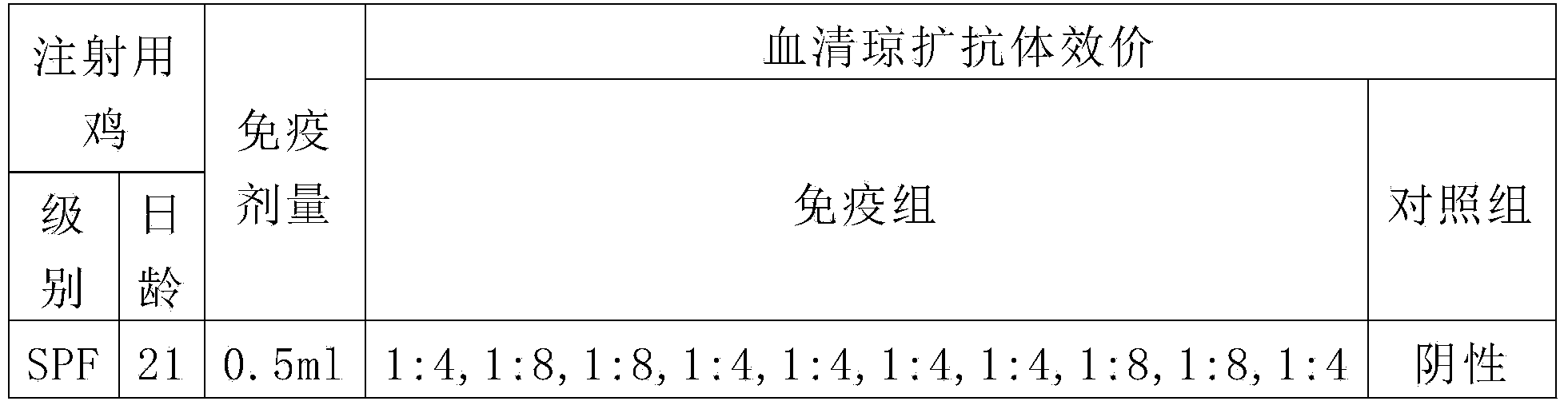
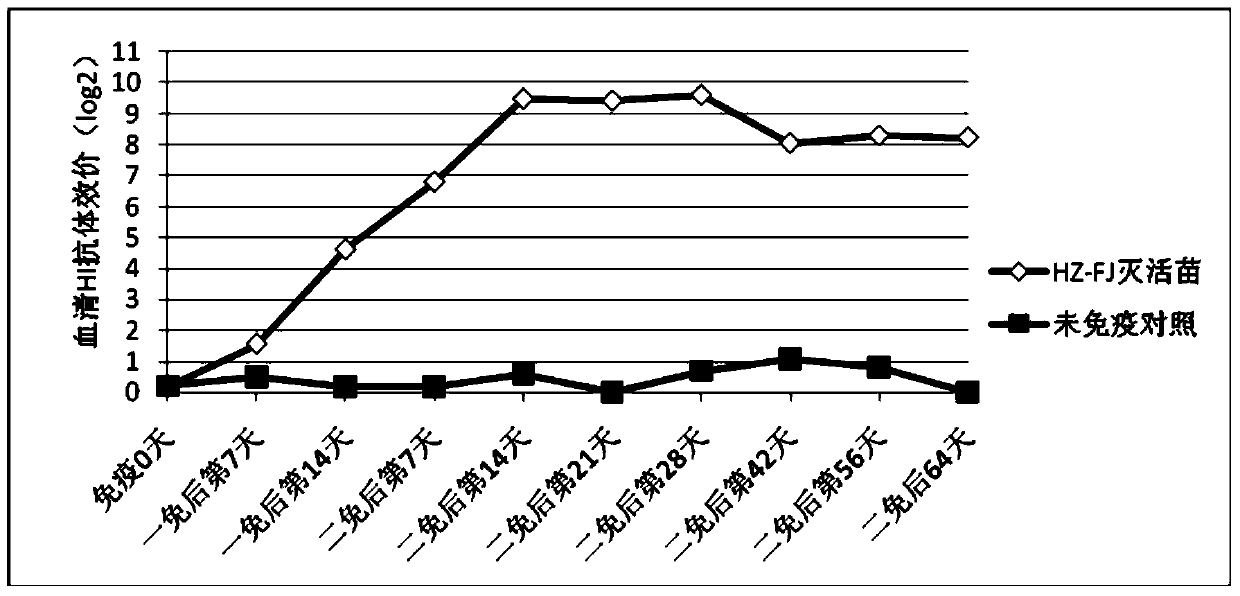
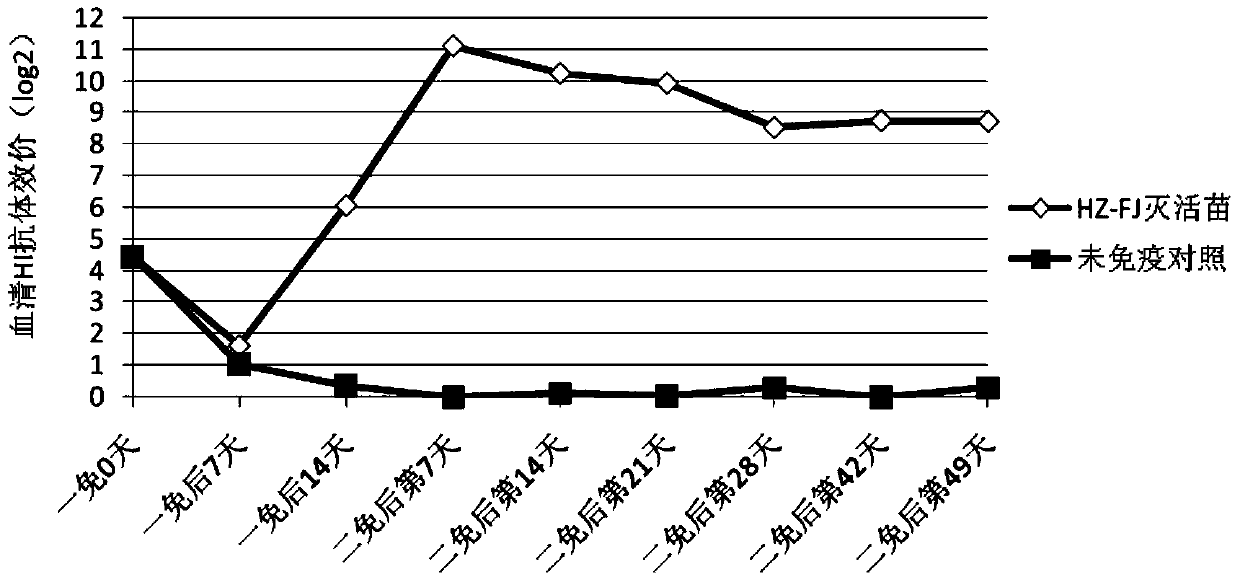
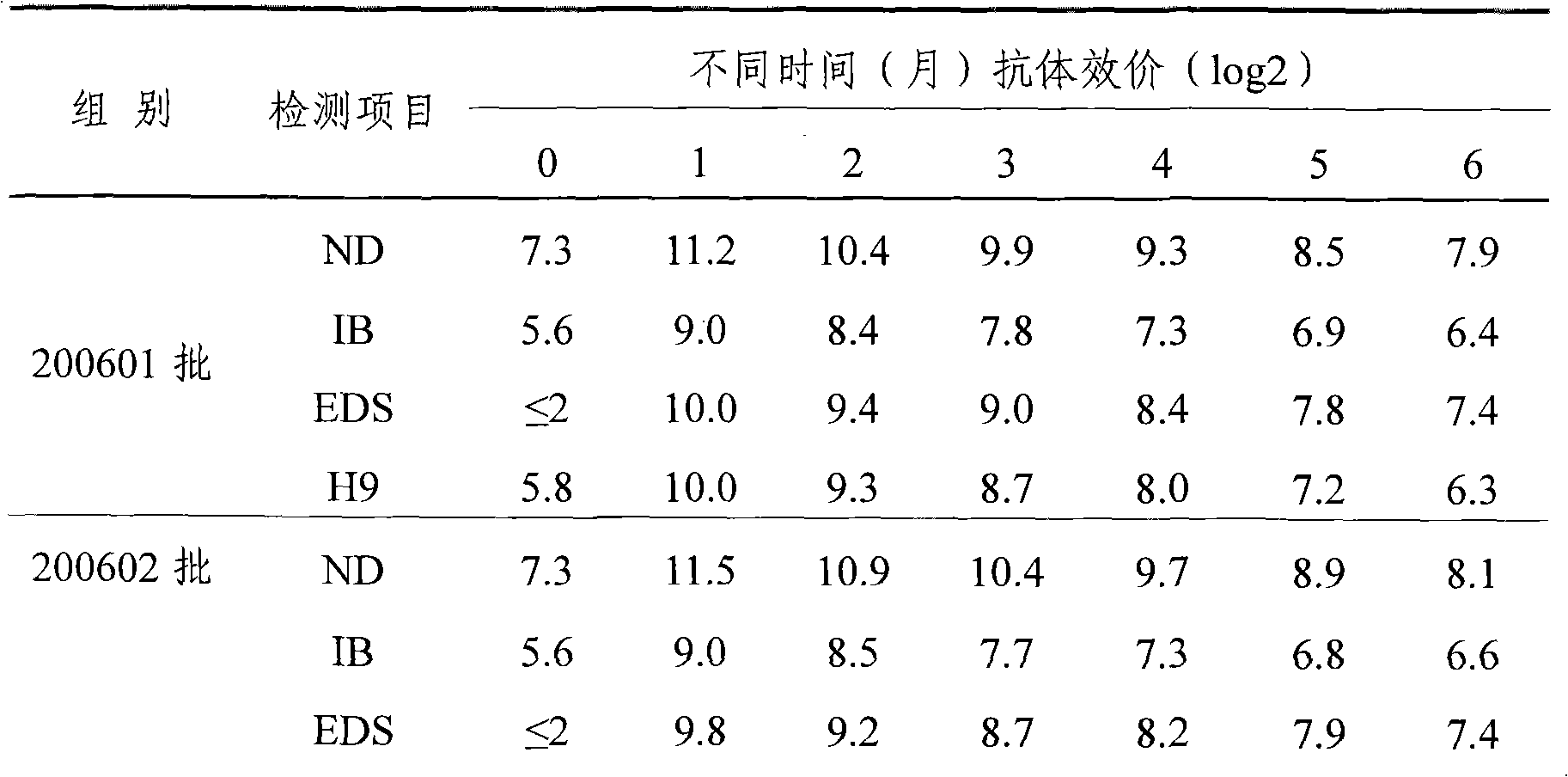
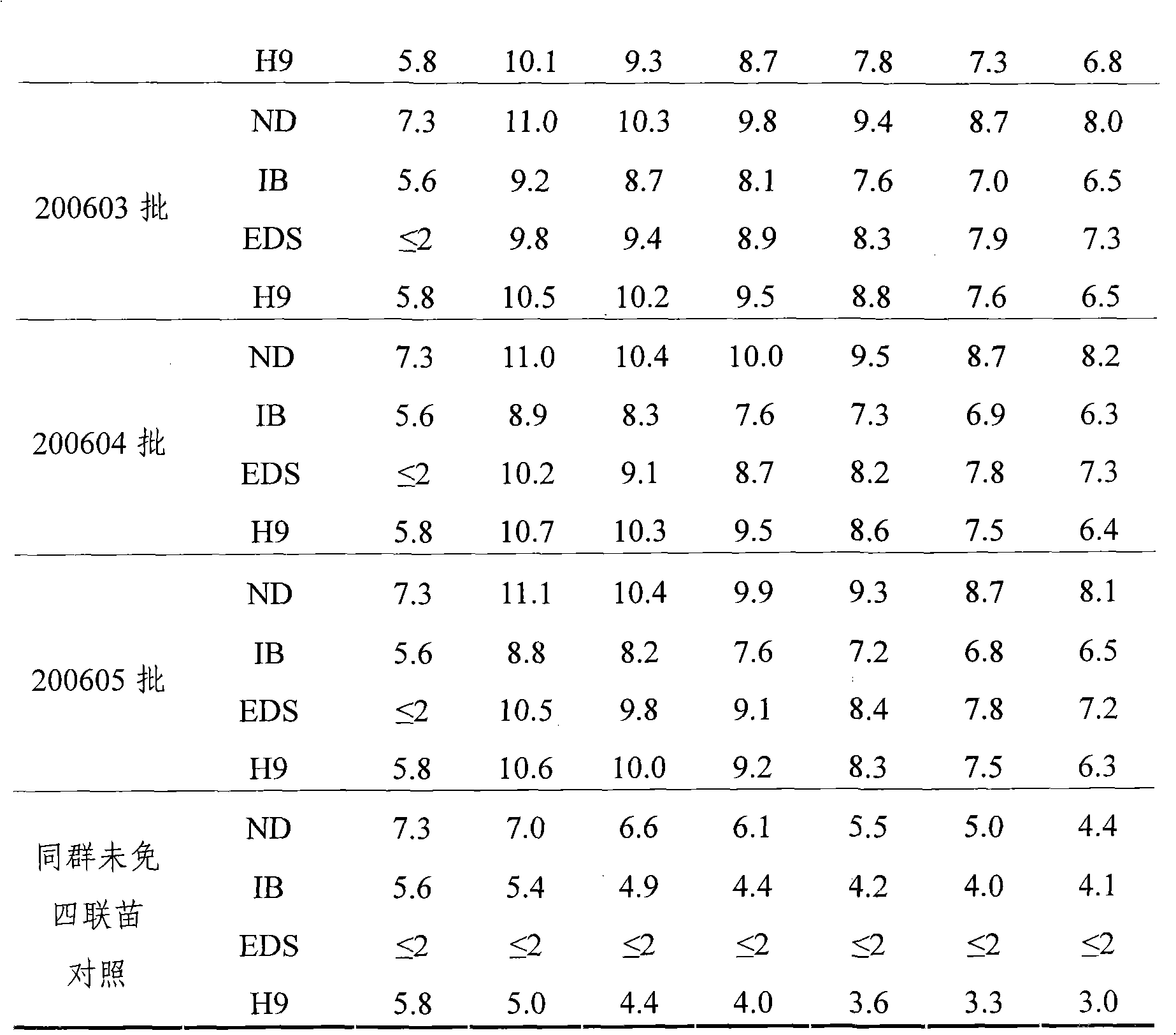
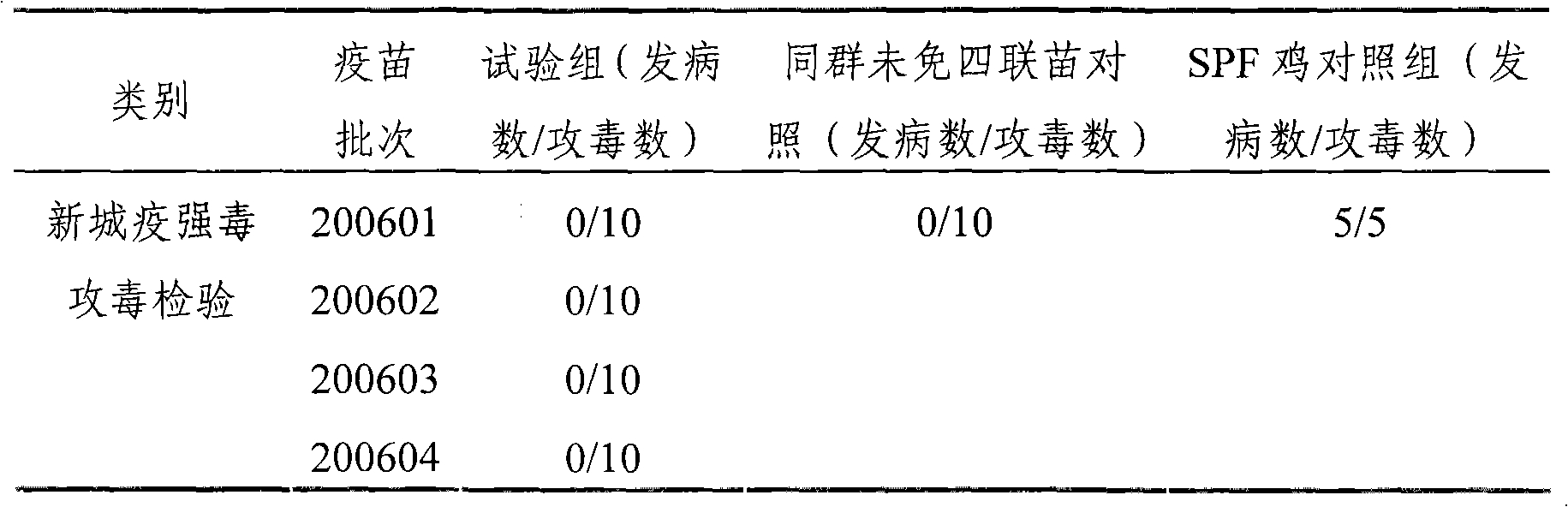
The present invention relates to an avian influenza H9 subtype inactivated vaccine, which is formed by mixing two strains of inactivated H9 subtype avian influenza viruses and an oil adjuvant, wherein the two strains of the inactivated H9 subtype avian influenza viruses are respectively the HZ strain and the FJ strain, the HA gene sequence of the HZ strain is represented by SEQ ID NO:1, and the HA gene sequence of the FJ strain is represented by SEQ ID NO:2. According to the preparation method, the virus solution of the HZ strain and the virus solution of the FJ strain of the H9 subtype avian influenza viruses are prepared and inactivated, the oil phase solution and the water phase solution are prepared, and emulsification is performed to obtain the finished product. According to the present invention, the two strains of the H9 subtype avian influenza viruses separated from different places are utilized to prepare the inactivated vaccine with characteristics of strong immunogenicity and good cross-protection property, wherein the inactivated vaccine can be used for prevention of chicken H9 subtype avian influenza diseases. In addition, after the inactivated vaccine is adopted to immunize chicken, the antibody titer is high so as to make the chicken have good virus challenge protection property on the H9 subtype strains epidemic in different places, the safety is high, and the efficacy is stable.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Quadruple inactivated vaccine for preventing chicken diseases
ActiveCN102166355AEasy to prepareGood effectAntiviralsAntibody medical ingredientsDiseaseInfectious bronchitis
The invention discloses a quadruple inactivated vaccine for preventing chicken diseases, which is prepared by respectively vaccinating Newcastle disease virus ZM10 strain, infectious bronchitis virus M41 strain and H9 subtype avian influenza virus S2 strain in a susceptible chicken embryo, and collecting an infected chicken embryo solution; vaccinating egg drop syndrome virus AV-127 strain in a susceptible duck embryo, and collecting an infected duck embryo solution; and concentrating the collected virus solutions, inactivating, blending with an oil adjuvant, and emulsifying. The quadruple vaccine provided by the invention can be used for preventing diseases caused by Newcastle disease viruses, infectious bronchitis viruses, egg drop syndrome viruses and avian influenza (H9 subtype) viruses to achieve the effect of preventing four diseases simultaneously and reduce immune cost and immune stress reaction.
Owner:乾元浩生物股份有限公司
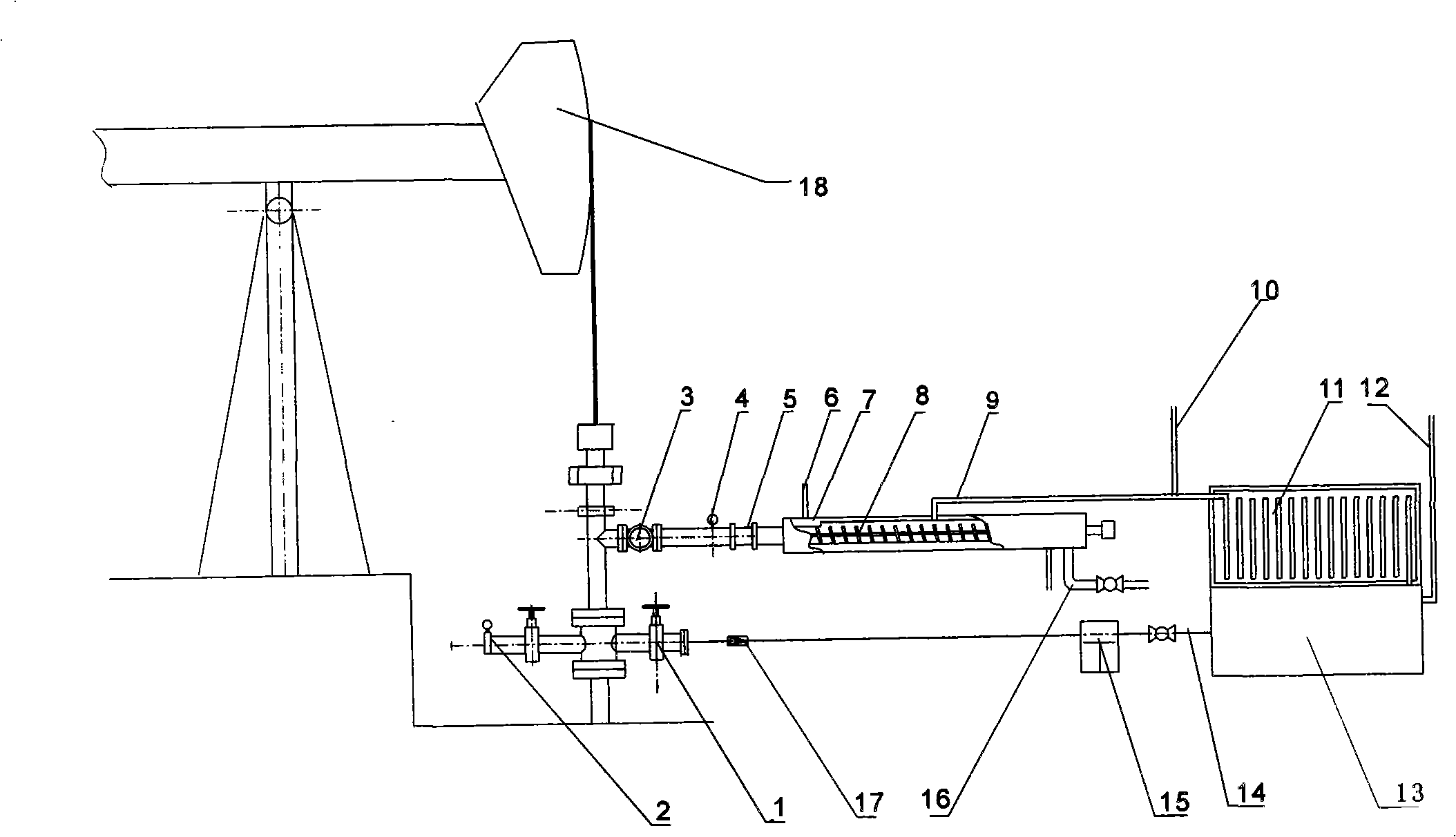
Process for assisting thick oil exploitation and device thereof
InactiveCN101525990AEnhanced overall recoveryQuality is not affectedInsulationFluid removalOil adjuvantHydrocarbon mixtures
The invention provides a process for assisting thick oil exploitation and a device thereof. The process comprises the following steps: selecting petroleum hydrocarbon mixture as a thick oil adjuvant; injecting the petroleum hydrocarbon mixture by a pump; separating on site to collect the petroleum hydrocarbon mixture; and using the petroleum hydrocarbon mixture in closed cycle. The device comprises a spiral separator, a light refrigeration coacervation device, a hydrocarbon mixture storage tank, a hydrocarbon injection pump, various brake values, pipelines and pipe fittings. The process and the device do not damage the oil layer, influence the quality of crude oil, or pollute the environment, have the advantages of simple process, convenient installation and the like, not only can be used for an individual well and a production platform, but also can be used for an oil production block, have strong process feasibility and applicability, can significantly improve the efficiency of an oil extractor, improve recovery ratio of the thick oil, and have excellent economical benefit.
Owner:杨勇华 +1
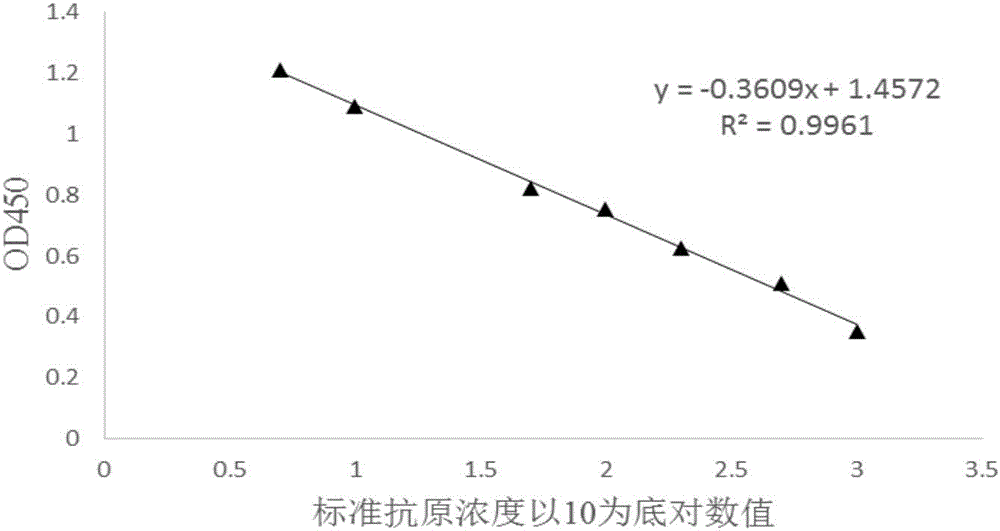
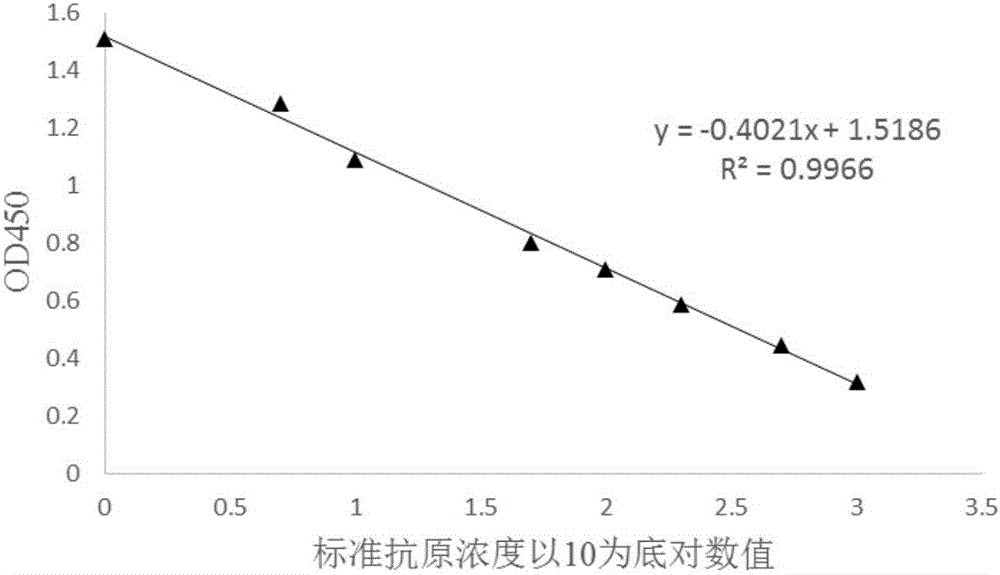
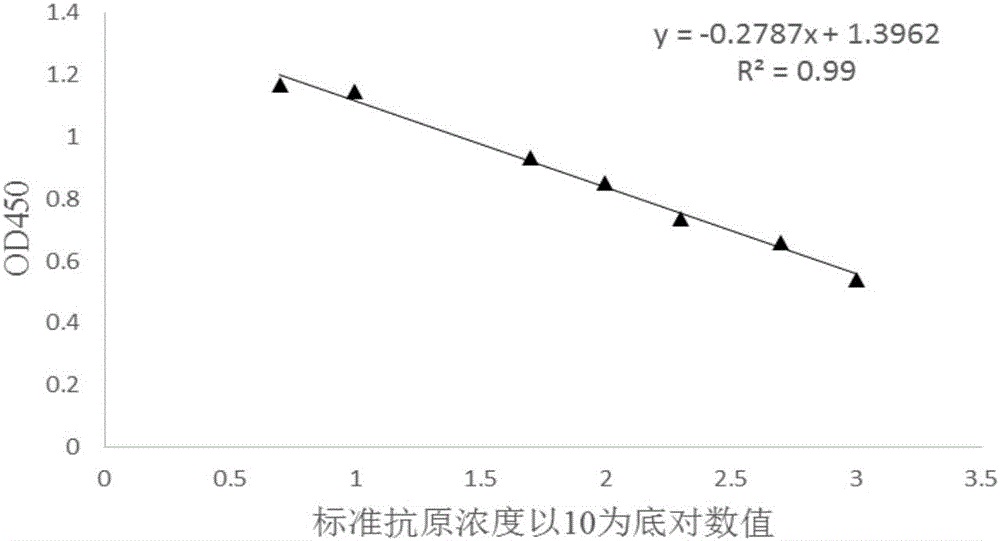
Competitive ELISA qualitative and quantitative detection method of oil adjuvant vaccine
InactiveCN106706924AAchieve complete suppressionHigh detection sensitivityBiological testingOil adjuvantPeptide antigen
The invention provides a competitive ELISA qualitative and quantitative detection method of an oil adjuvant vaccine. The method comprises the following steps of coating an antigen, diluting an antibody, diluting the antigen, drawing an antigen standard curve and quantitatively detecting the to-be-detected antigen. The method comprises the specific steps: firstly enabling the to-be-detected antigen to react with the antibody with known concentration to completely neutralizing the antibody and the antigen; and then enabling the neutralized solution to react with the antigen adsorbed in an ELISA plate in a solid-phase way to determine the concentration of the to-be-detected antigen. The standard curve slope obtained by the invention is able to be greater than that obtained through an indirect competition method, thereby greatly improving the detection sensitivity; the detection method disclosed by the invention is extensive in detection, and capable of detecting the synthetic peptide antigen finished product, semi-finished product and vaccine antigen; and an aqueous-phase sample obtained after vaccine demulsification can be determined without performing the purification treatment; the spent time is short in comparison with other detection method; and the lowest detection limit of the antigen sample according to the invention can achieve 1ng / mL-1.5ng / mL.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Protective agent for foot-and-mouth disease inactivated virus and preparation method of microcapsule vaccine
The invention provides a protective agent for a foot-and-mouth disease inactivated virus antigen. The protective agent consists of the following components: trehalose, raffinose, dextran, sorbitol, mannitol, inositol, xylitol, polyethylene glycol 3350, TPGS and propolis. The protective agent can prolong the preservation time of the antigen, protect the 146s antigen of the foot-and-mouth disease virus to be stable during production and cold chain transportation and is conducive to establishment of a foot-and-mouth disease virus antigen library and improvement of the low temperature storage stability of the 146s antigen in a foot-and-mouth disease virus antigen solution. The invention also provides a foot-and-mouth disease inactivated virus microcapsule vaccine containing the protective agent and the foot-and-mouth disease inactivated virus antigen as a core material and a preparation method thereof. The vaccine is orally taken by or injected to an animal for immunization, can produce ahigh titer FMDV antibody in 8 weeks and lasts longer than conventional oil adjuvant inactivated vaccines to achieve immune protection faster. The vaccine is directly injected for immunization withoutan adjuvant and can be inoculated to the animal by water drinking or feeding for prevention, and the clinical operation is convenient.
Owner:内蒙古必威安泰生物科技有限公司
Riemerella anatipestifer and escherichia coli bigeminal inactivated vaccine, and preparation method thereof
InactiveCN101690807AEasy to prepareImprove product performanceAntibacterial agentsBacterial antigen ingredientsEscherichia coliOil adjuvant
The invention belongs to the technical field of biological products for veterinary uses, in particular to a method for preparing a riemerella anatipestifer and escherichia coli bigeminal inactivated vaccine and a preparation method thereof. The vaccine is prepared by inoculating riemerella anatipestifer I type, riemerella anatipestifer II type and escherichia coli O78 type which have excellent immunogenicity into a culture medium for culture, using formaldehyde solution to inactivate the bacteria and emulsifying the resulting product by an oil adjuvant. The bigeminal inactivated vaccine is used for preventing and controlling infectious serositis and colibacillosis of a duck. The preparation method of the vaccine has a simple operation; and the prepared vaccine has stable performance and can effectively prevent and control the infectious serositis and the colibacillosis of the duck.
Owner:广东永顺生物制药股份有限公司
Pig breeding and respiratory syndrome NVDC-JXA1 strain-porcine parvovirus disease duplex inactivated vaccine and preparation method and application thereof
InactiveCN102716479AReduce immune densityAvoid mutual interferenceViral antigen ingredientsAntiviralsDiseaseOil adjuvant
The invention particularly relates to a pig breeding and respiratory syndrome NVDC-JXA1 strain-porcine parvovirus disease duplex inactivated vaccine and a preparation method and application thereof. The pig breeding and respiratory syndrome NVDC-JXA1 strain-porcine parvovirus disease duplex inactivated vaccine is prepared by enabling pig breeding and respiratory syndrome virus NVDC-JXA1 strain to inoculate with a Marc-145 cell, enabling a porcine parvovirus virulent strain to inoculate with an ST cell, respectively obtaining infection cell culture fluid to be inactivated by using formaldehyde solution, and conducting ultrafiltration, concentration and mixing to obtain mixed virus liquid to be mixed and emulsified with oil adjuvant. The inactivated vaccine comprises, by weight, 1-2 parts of the oil adjuvant and 1 part of mixed virus liquid aqueous phase, the inactivated vaccine is prepared by preparing pig breeding and respiratory syndrome virus liquid, porcine parvovirus liquid, an oil phase, the aqueous phase and emulsifying, and NVDC-JXA1 strain pig breeding and respiratory syndrome-porcine parvovirus disease duplex inactivated vaccine quality standards are built.
Owner:GUANGDONG DAHUANONG ANIMAL HEALTH PRODS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com