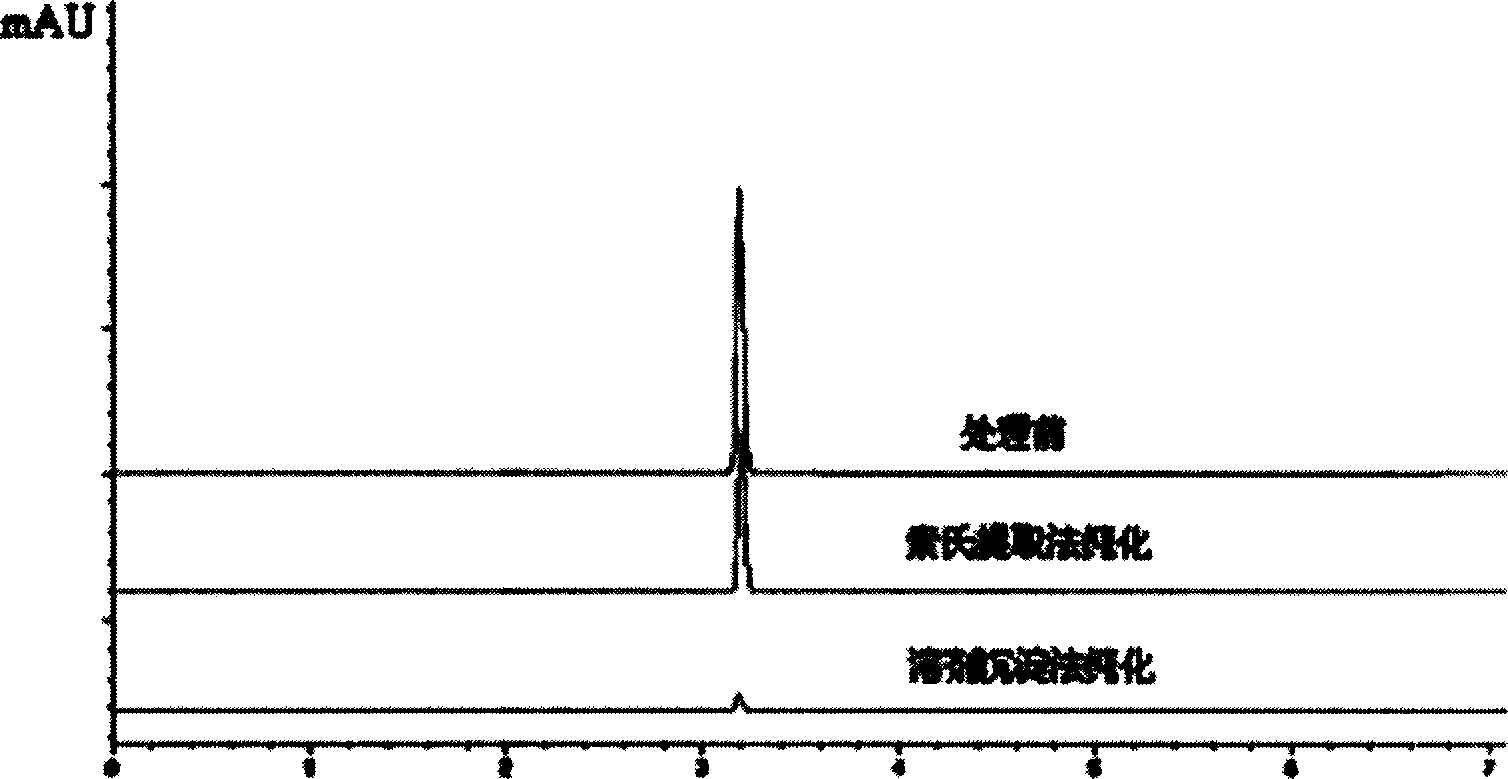
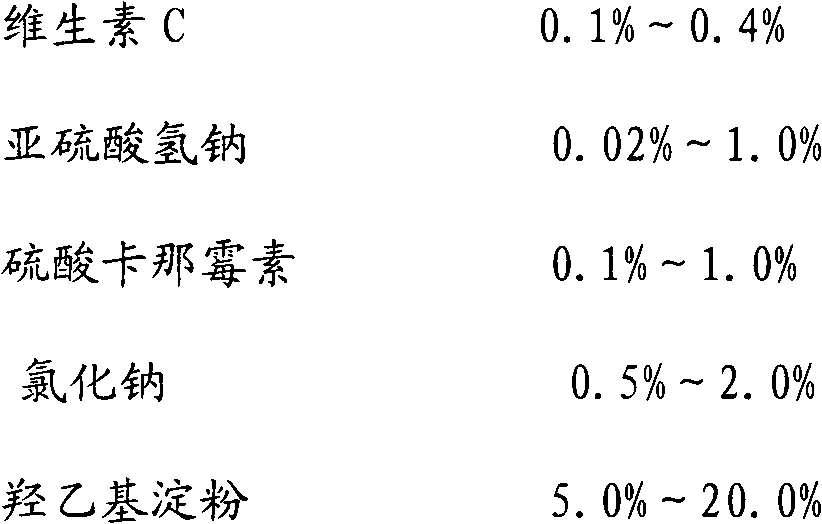
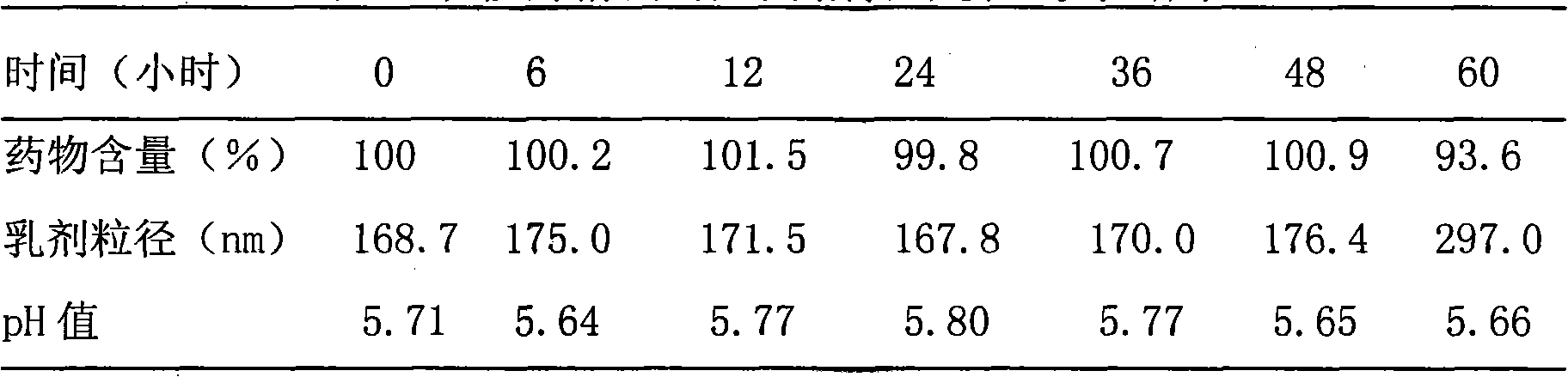
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2247 results about "Physiological saline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Physiological saline. n. A sterile solution of sodium chloride that is isotonic to body fluids, used to maintain living tissue temporarily and as a solvent for parenterally administered drugs.
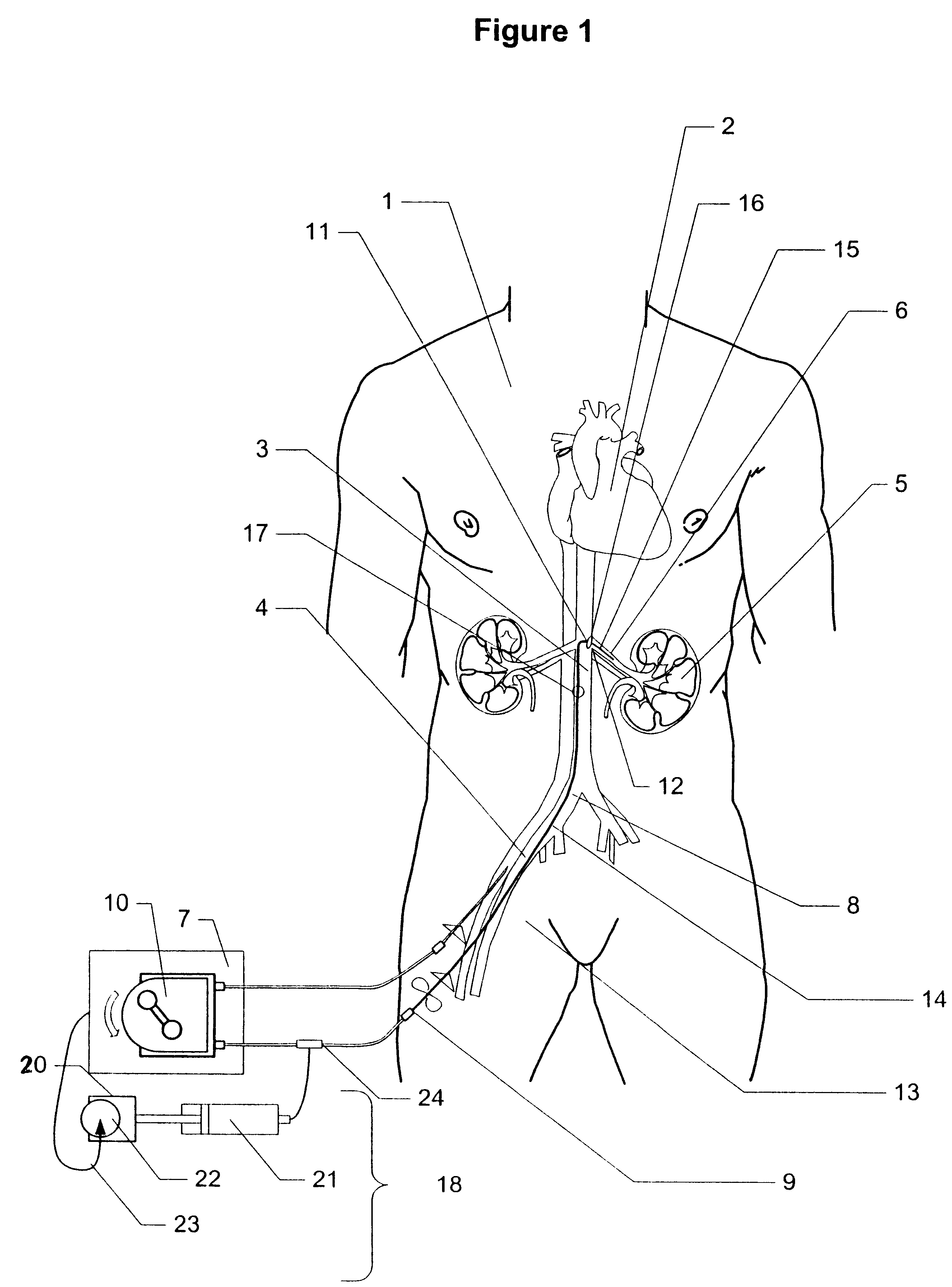
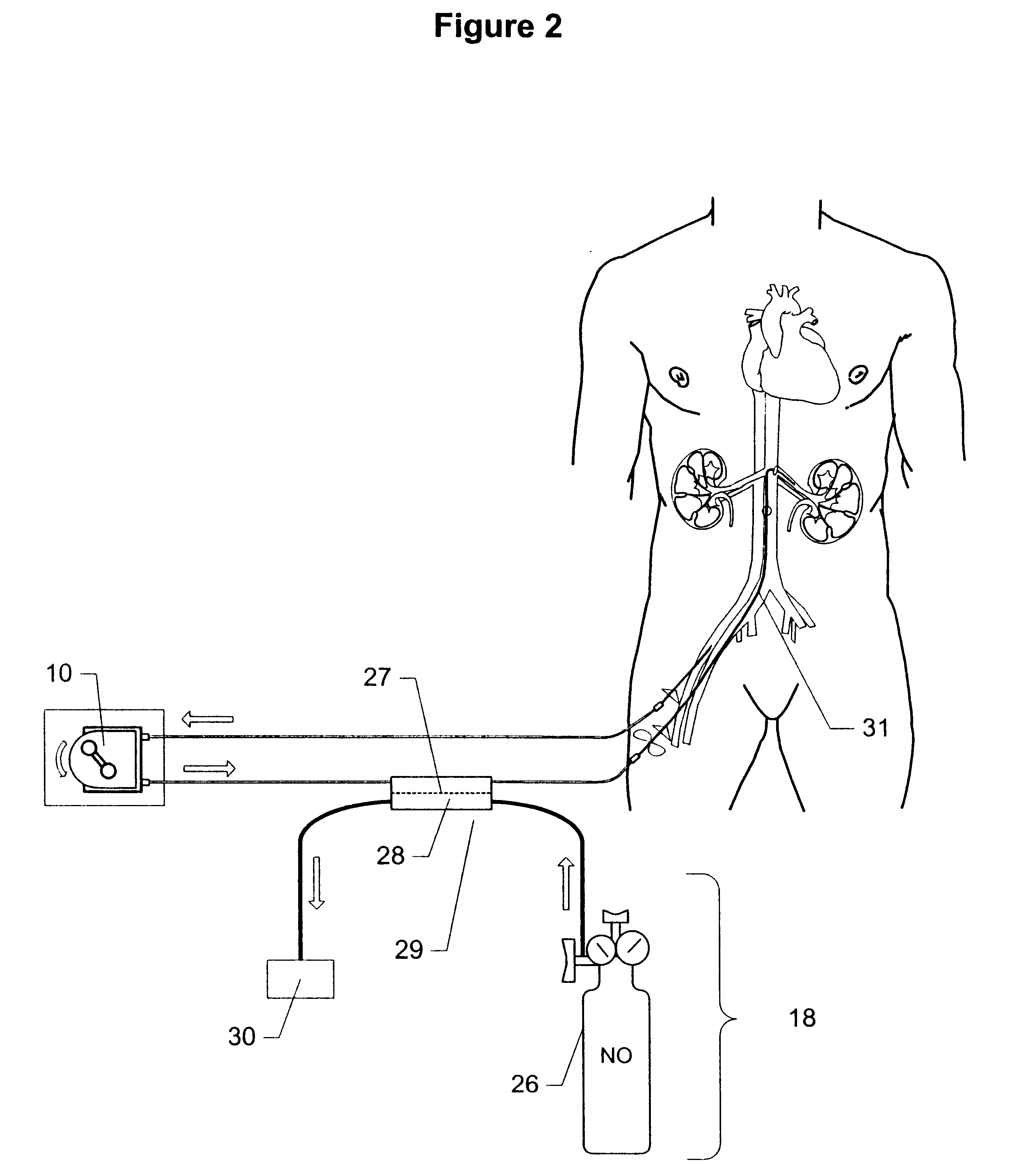
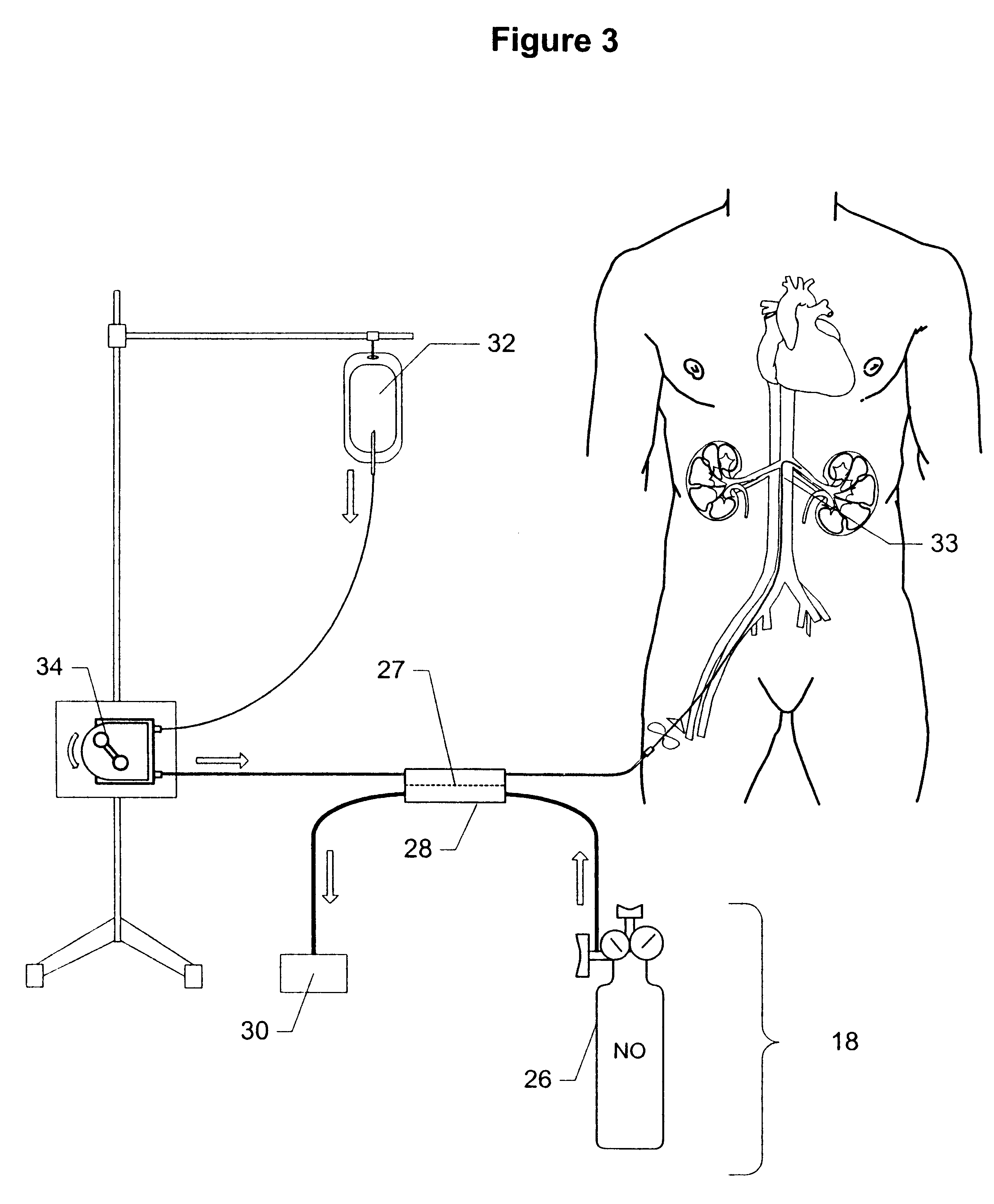
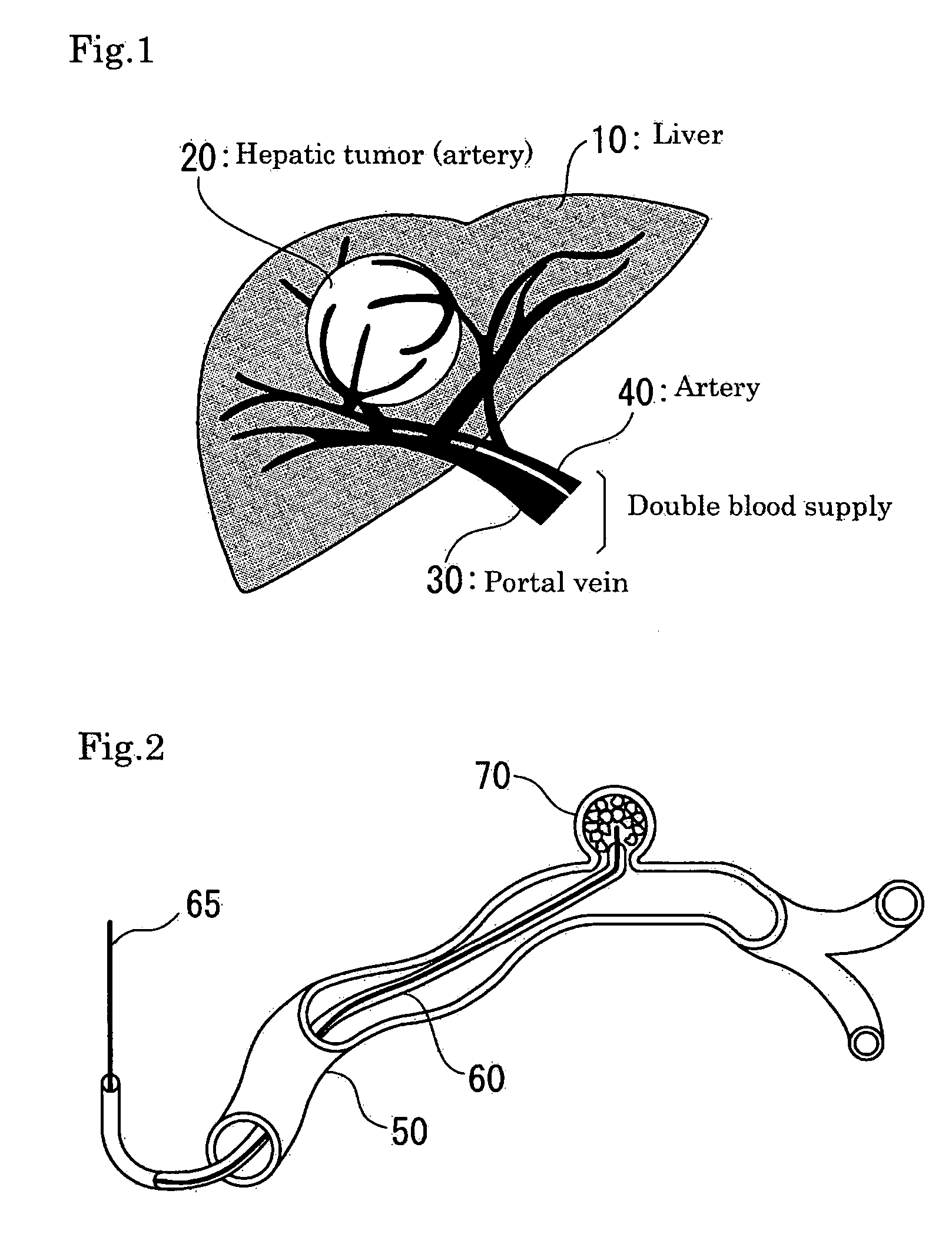
Method and apparatus for treatment of congestive heart failure by improving perfusion of the kidney by infusion of a vasodilator
InactiveUS6287608B1Improve the quality of lifeImprove survival rateBiocideInorganic active ingredientsRenin–angiotensin systemVascular dilatation
A method for treating congestive heart failure (CHF) has been developed that restores kidney renal functions by artificial vasodilation of at least one kidney. A vasodilator drug is locally delivered to the kidney via a kidney perfusion catheter. The drug can be mixed with the patient's blood, saline or other suitable solvent and the mixture directly applied to the kidney through the catheter. The restoration of kidney function assists the heart by removing excess fluid, urine and toxin from the patient, and by normalizing the patient's renin-angiotensin system and other neurohormonal substances. The method is applicable to treat chronic and acute CHF.
Owner:GAMBRO LUNDIA AB
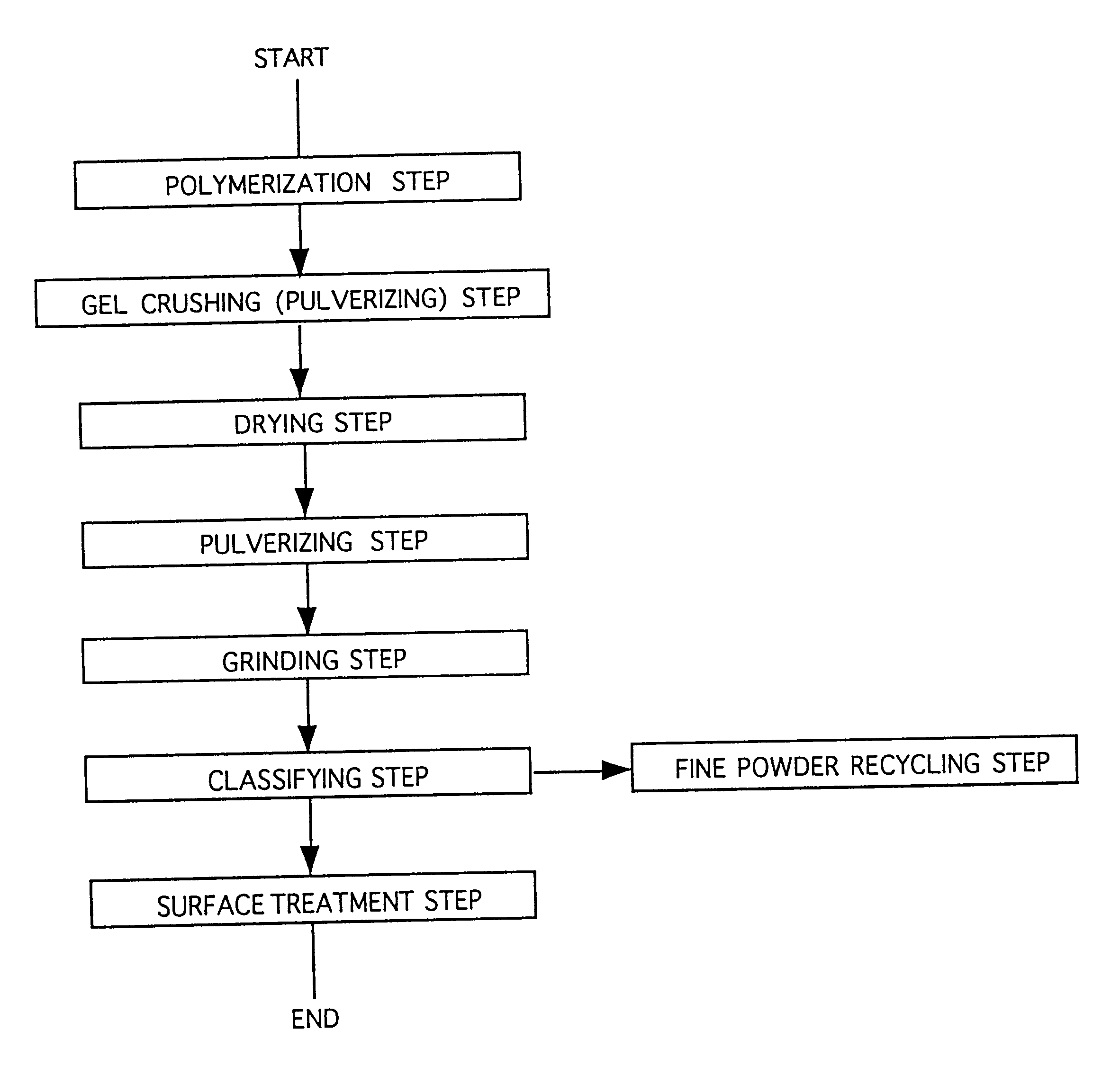
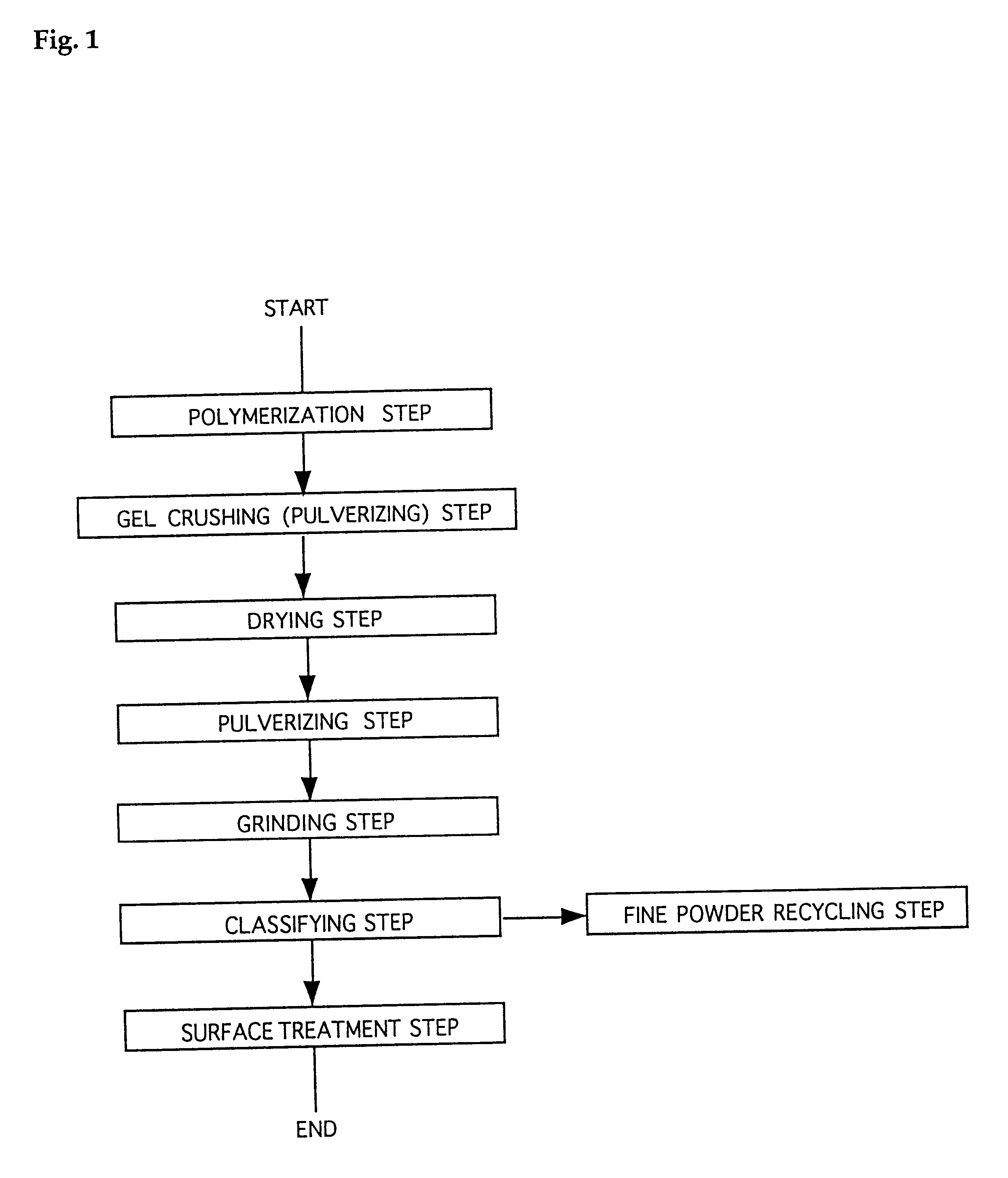
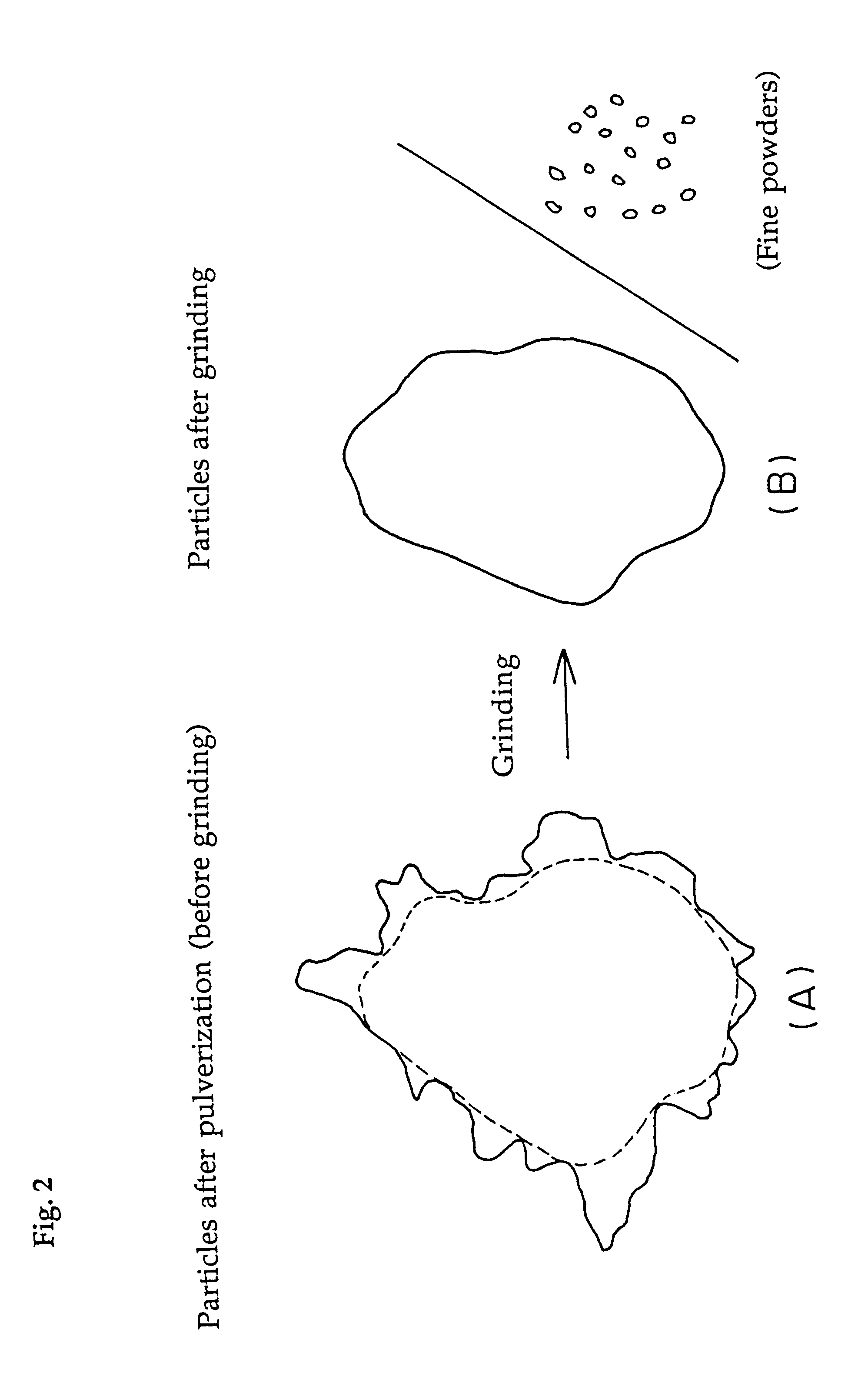
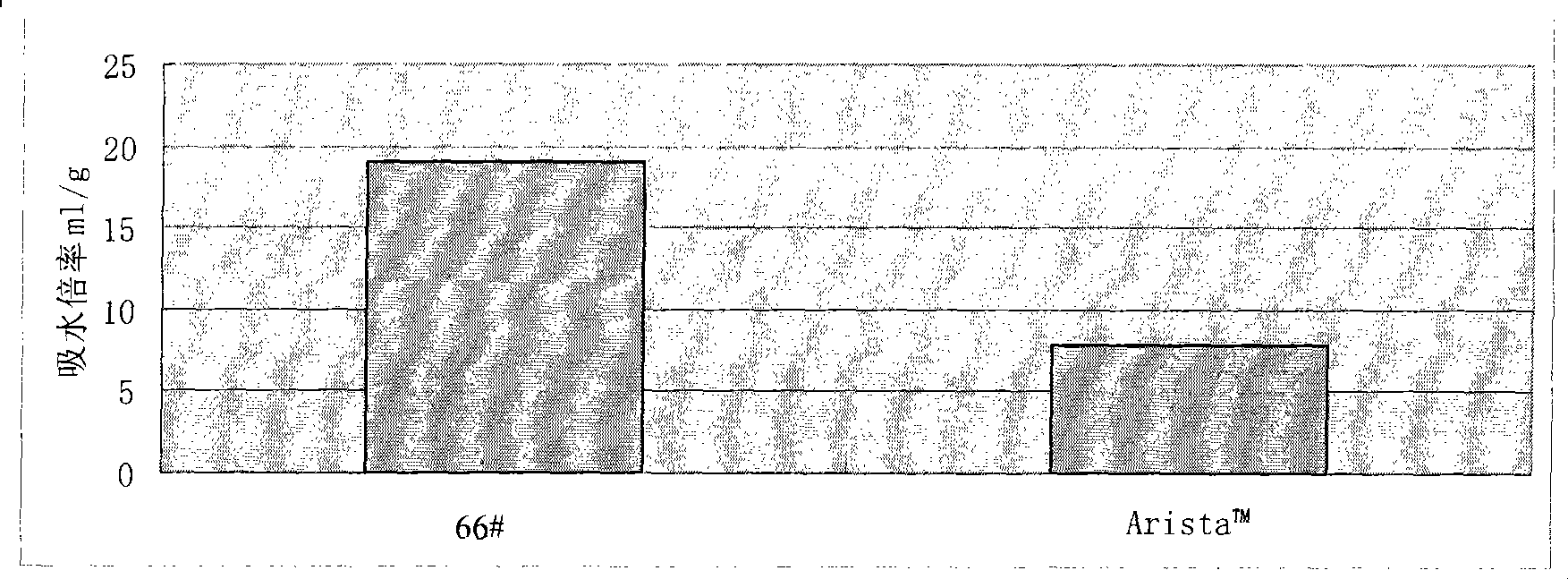
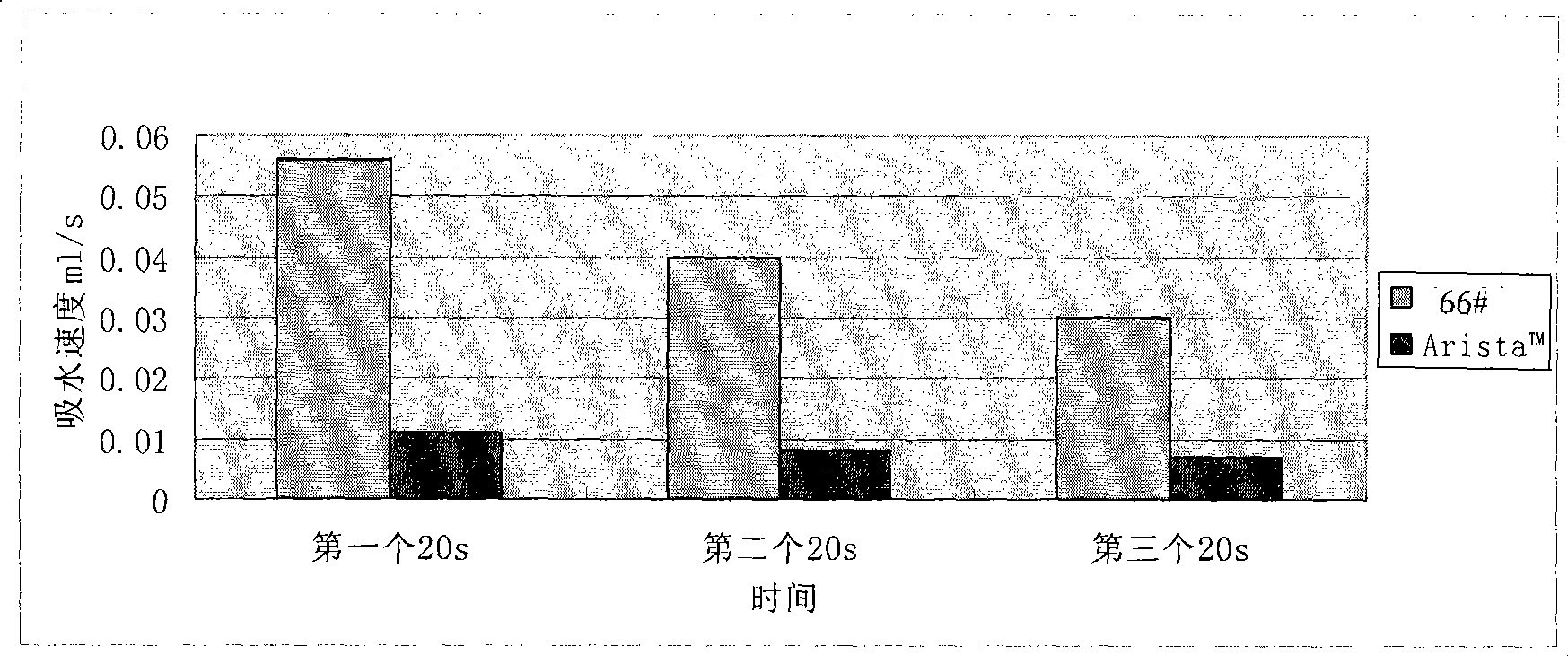
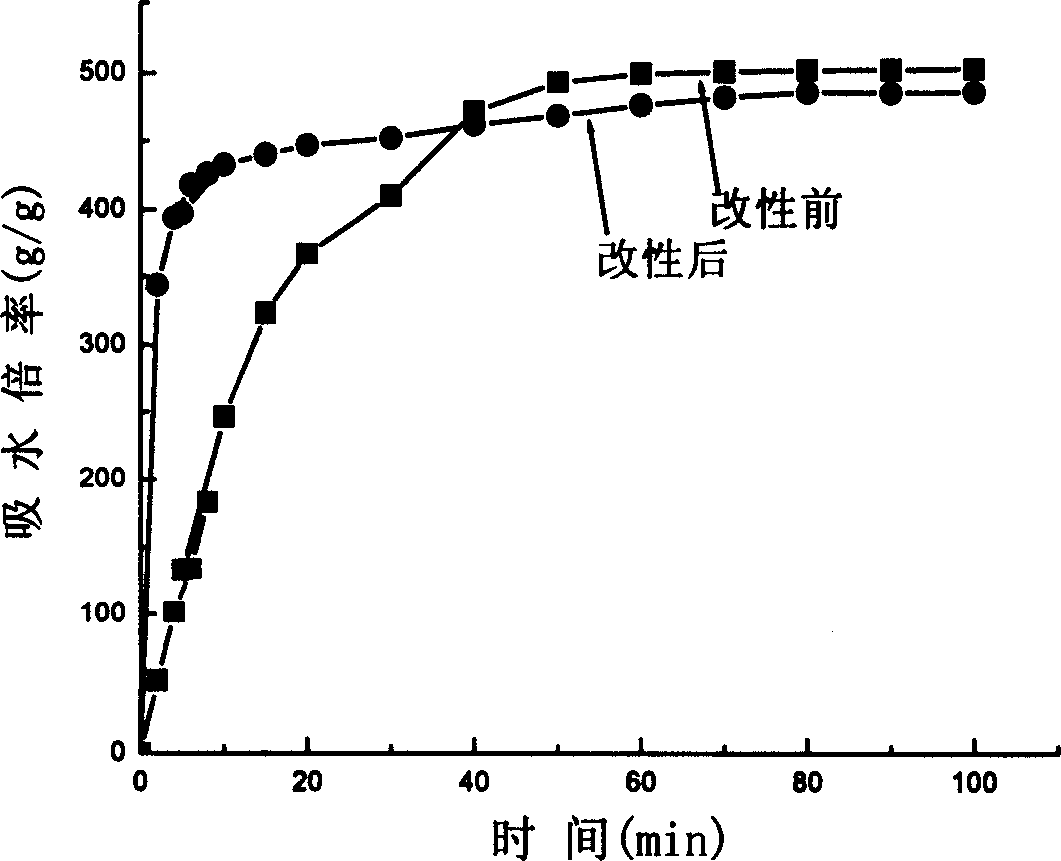
Water-absorbent resin powder and its production process and use
The present invention provides a water-absorbent resin powder and its production process and use, wherein the water-absorbent resin powder has high liquid permeability and high water absorbency. The production process for a water-absorbent resin powder, according to the present invention, comprises the step of obtaining water-absorbent crosslinked polymer particles by an aqueous solution polymerization step, and grinding the resultant crosslinked polymer particles until the bulk density thereof increases to not lower than 0.72 (g / ml). The water-absorbent resin powder is characterized by being arbitrarily pulverized and having a bulk density of not lower than 0.74 (g / ml) and a water absorption capacity of not lower than 20 (g / g) for 0.9 weight % physiological saline under a load of 0.7 psi (4.83 kPa). In addition, the absorbent structure comprises the above water-absorbent resin powder and a fibrous material. The absorbent article comprises an absorbent layer including the above absorbent structure.
Owner:NIPPON SHOKUBAI CO LTD
Polymerization process and materials for biomedical applications
InactiveUS20050090612A1Avoid problemsPromote sportsOptical articlesOptical partsPolymer scienceAqueous solubility
A molded component or article for biomedical use is prepared from a crosslinkable non-water-soluble polymer which when crosslinked and saturated with water forms a hydrogel. The polymer is formulated as a composition containing a non-aqueous diluent in addition to the polymer, the diluent being present in a volumetric proportion that is substantially equal to the volumetric proportion of water in the hydrogel that would be formed when the polymer is crosslinked and saturated with water. The composition is cast in a mold where the composition is exposed to conditions that cause crosslinking to occur by a reaction to which the non-aqueous diluent is inert. The crosslinking reaction produces a molded non-aqueous gel which is then converted to a hydrogel by substituting an aqueous liquid such as water or physiological saline for the non-aqueous diluent. The use of a molding composition whose curing consists essentially entirely of crosslinking results in a molding process that entails little or no shrinkage, and dimensional integrity is maintained up through the formation of the hydrogel by using the non-aqueous diluent in essentially the same volumetric proportion as water in the hydrogel.
Owner:ZMS LLC
Dressing material containing medicine chitoholosida and its preparation method
InactiveCN1579559AHas therapeutic effectSustained releaseAbsorbent padsBandagesSolid componentPhosphate
The invention produces polyethylene alcohol hydrogel dressing containing medicine and chitosan with 60Co gamma-radial or high energy electron beam radial cross linking. Additional, adds in some humectant, plasticizer, medicine, the solvent is the secondary distilled water, physiological saline or phosphate neutral buffer liquid. The product can release medicine slowly and has natural amylose chitosan with biology sterilization activity, it has active sterilization function, at the same time, it has high water quantity, and good water reserving performance, the mechanical intensity is moderate, and the light penetration and air penetration are excellent. It can accord the demands for curing each kind of wound. It can used as the permanent dressing for light skin injuries, and it also can be applied to the temporally close of severe skin organization wound or burn wound.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Particulate water absorbent agent and production method thereof, and water absorbent article
ActiveUS20050209352A1Improve liquidityImprove water absorptionOther chemical processesAbsorbent padsParticulatesSaline water
A particulate water absorbing agent of the present invention includes a water absorbent resin, having a cross-linking structure, whose surface has been cross-linked by adding a surface treatment agent, wherein: (i) a mass average particle diameter (D50) ranges from 200 to 600 μm and 95 to 100 wt % of a particulate water absorbing agent whose particle diameter ranges from less than 850 μm to not less than 150 μm is contained with respect to 100 wt % of whole the particulate water absorbing agent, and (ii) a logarithmic standard deviation (σζ) of particle size distribution ranges from 0.25 to 0.45, and (iii) a compressibility rate defined by a following equation ranges from 0 to 18%, and (iv) a surface tension of a supernatant liquid obtained in 4 minutes after dispersing 0.5 g of the particulate water absorbing agent in 50 ml of physiological saline whose temperature is 20° C. is 55 mN / m or more, the compressibility rate (%)=(P−A) / P×100 where P represents a tapped bulk density of the particulate water absorbing agent and A represents a loose bulk density of the particulate water absorbing agent.
Owner:NIPPON SHOKUBAI CO LTD
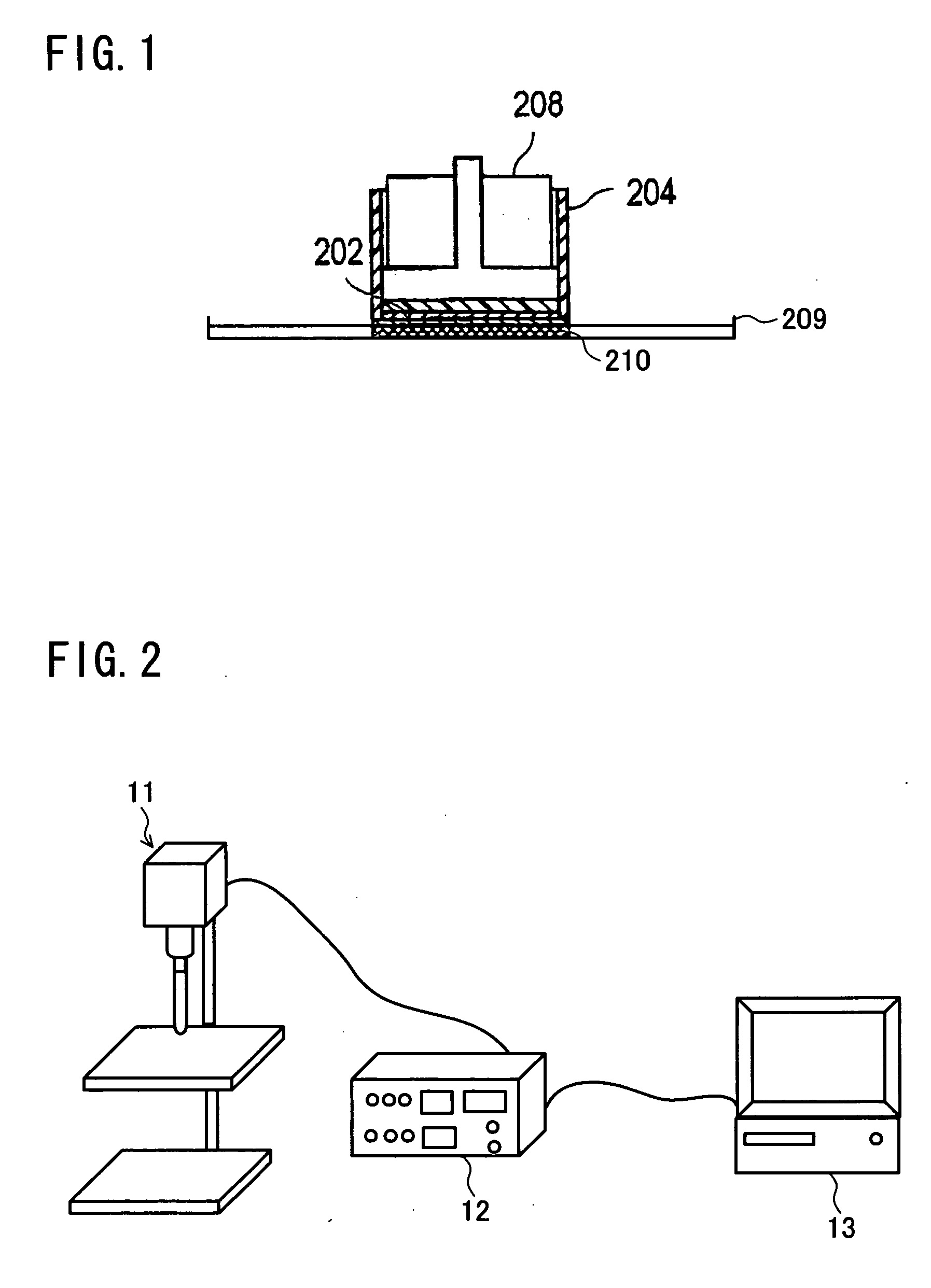
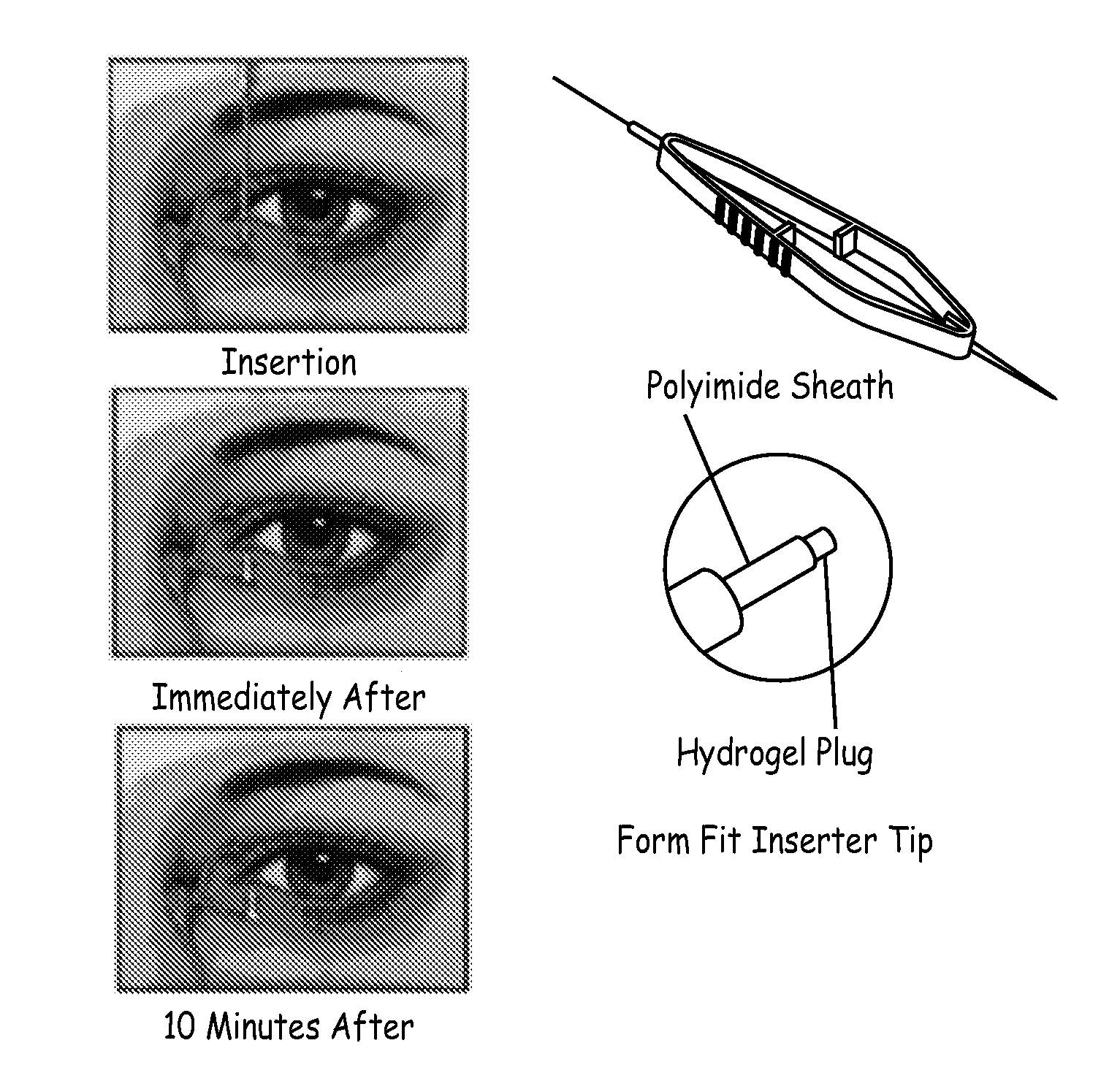
Drug delivery through hydrogel plugs
ActiveUS20100209478A1Improve securityIncrease chanceAntibacterial agentsBiocideProsthesisWater content
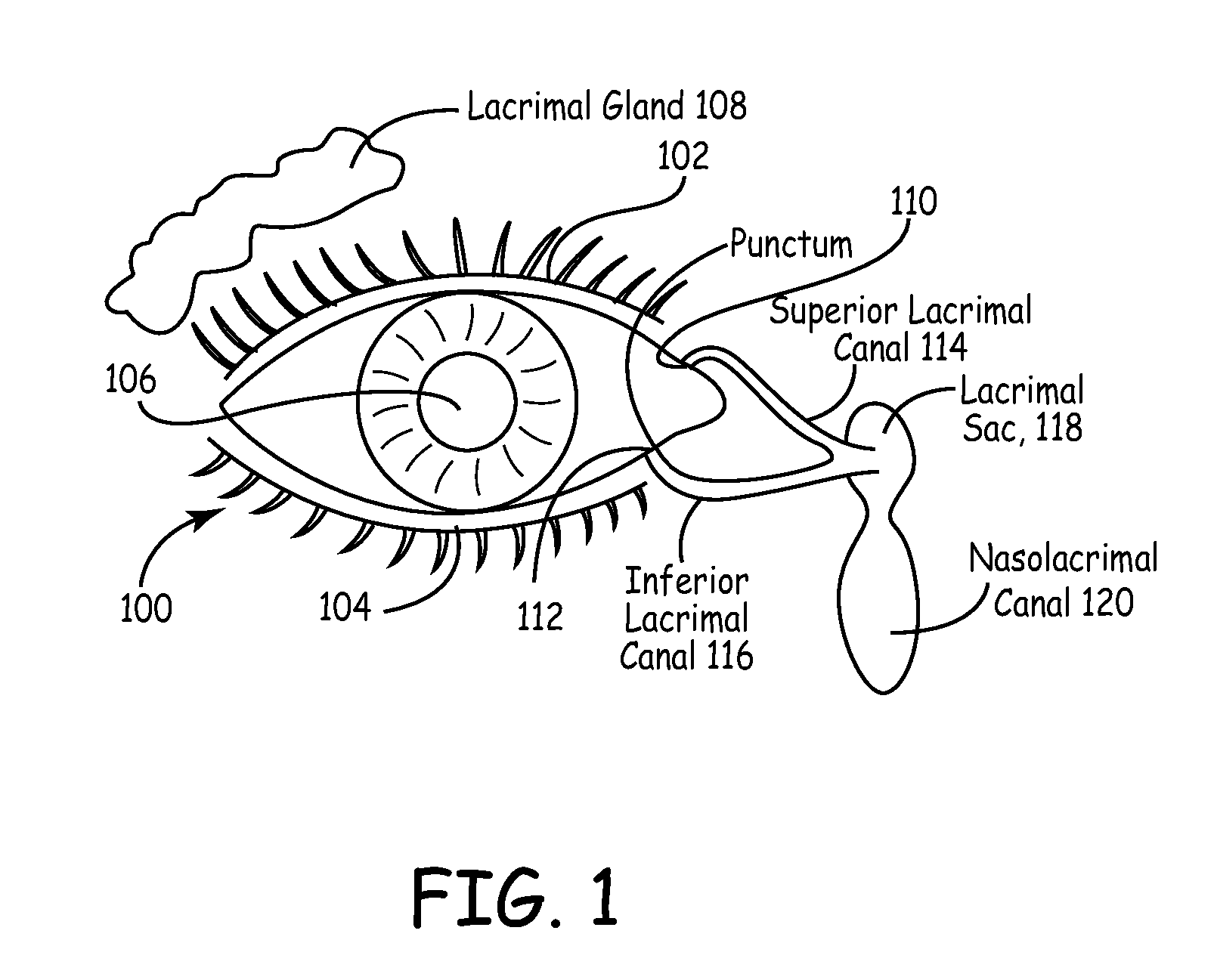
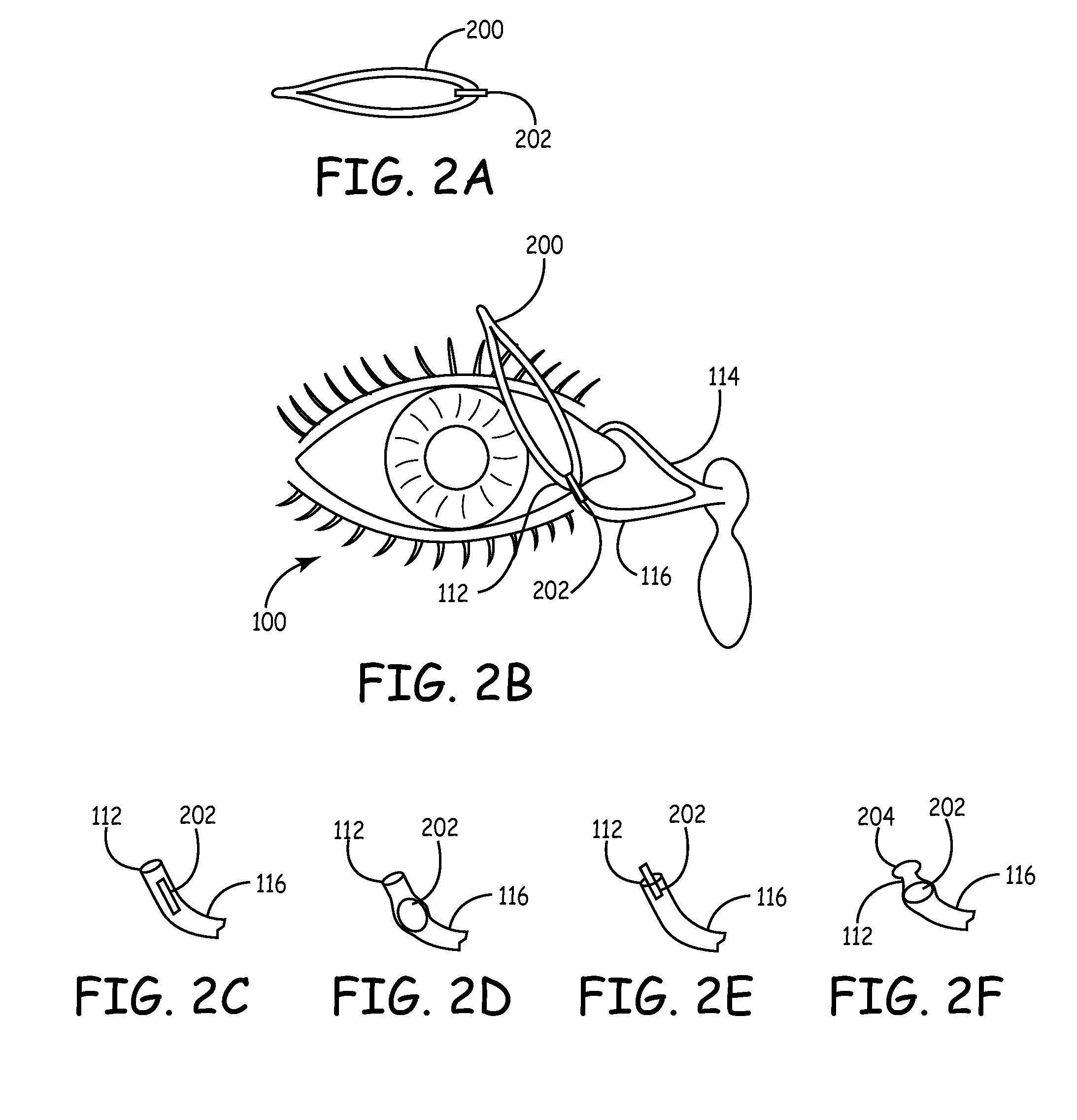
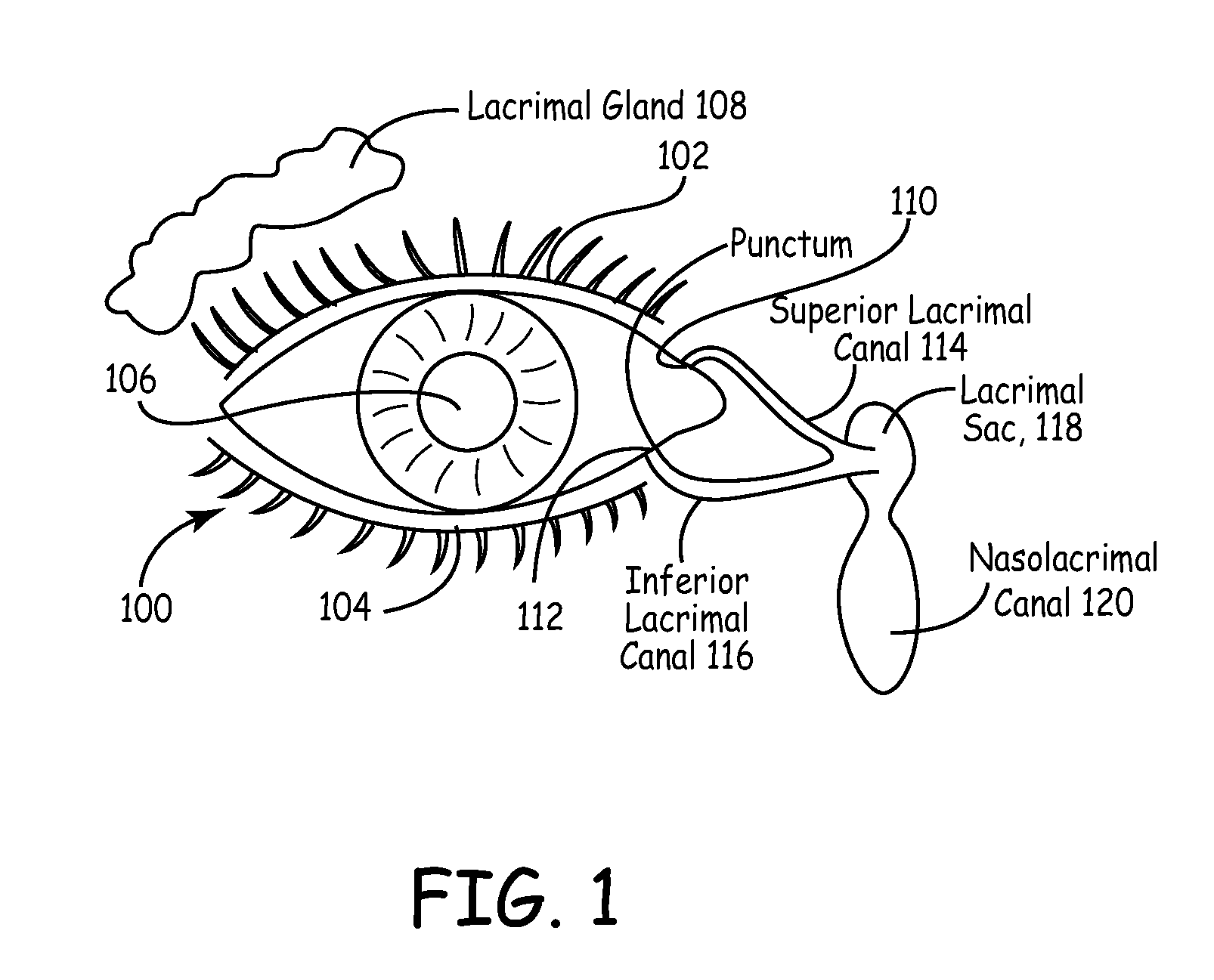
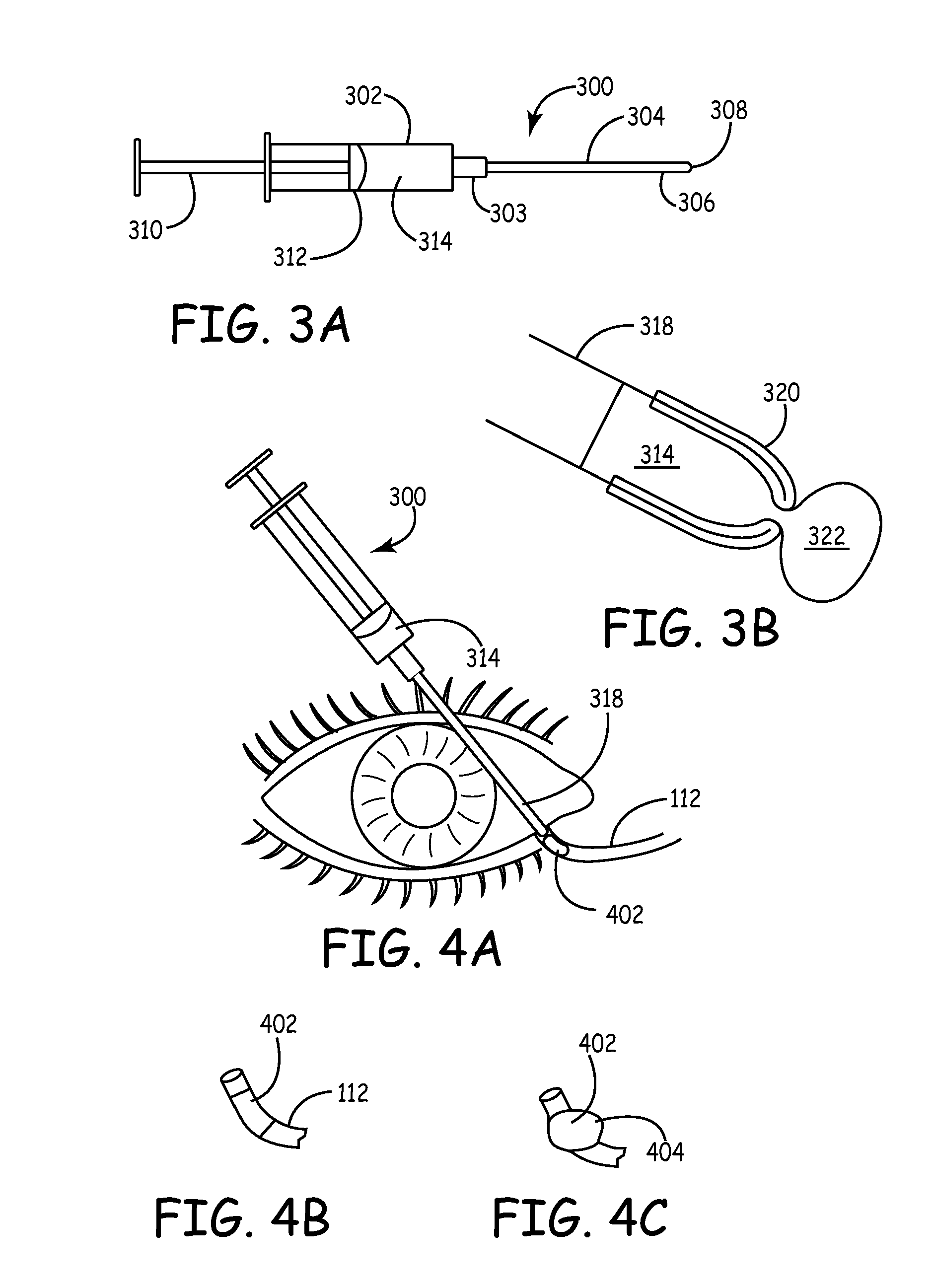
An embodiment is a medical prosthesis for blocking or reducing tear flow through a punctum or canaliculus of a human eye and delivering a drug to the eye that comprises a dehydrated covalently crosslinked synthetic hydrophilic polymer hydrogel with dimensions to pass through a puncta lacrimali, with the dehydrated hydrogel absorbing physiological water to swell to at least 1 mm in cross-sectional width and conformably fit a canaliculus, with the hydrogel comprising a therapeutic agent dispersed through the hydrogel for release to an eye, with the hydrogel having a water content of at least about 50% by weight or volume when allowed to fully hydrate in vitro in physiological saline.
Owner:INCEPT LLC
Particulate water absorbing agent with water-absorbing resin as main component
InactiveUS20070066167A1Satisfactory performancePractical to useSynthetic resin layered productsAbsorbent padsParticulatesSaline water
The present invention provides a water absorbing agent which maintains excellent water absorbing properties for a long time, even when urine composition of human urine varies depending. A particulate water absorbing agent comprising a water-absorbing resin obtained by crosslinking polymerization of an unsaturated monomer, which exhibits Centrifuge retention capacity in a physiological saline solution of not lower than 32 g / g, mass median particle size (D50) of 200 to 400 μm, ratio of particles with diameter of smaller than 150 μm of 0 to 2% by weight, and increased extractables by deterioration of 0 to 15% by weight and extractables for one hour in deterioration test liquid of 0.1 to 30% by weight.
Owner:NIPPON SHOKUBAI CO LTD
Modified starch absorbable hemostasia material and preparation method thereof
ActiveCN101361986AHigh viscosityImprove water absorption speedSurgical adhesivesPharmaceutical delivery mechanismWound healingBiomedical engineering
Owner:BEIJING UNIVERSAL LIKANG TECH CO LTD
Apparatus for detecting the position of a percutaneously-inserted intravenous catheter
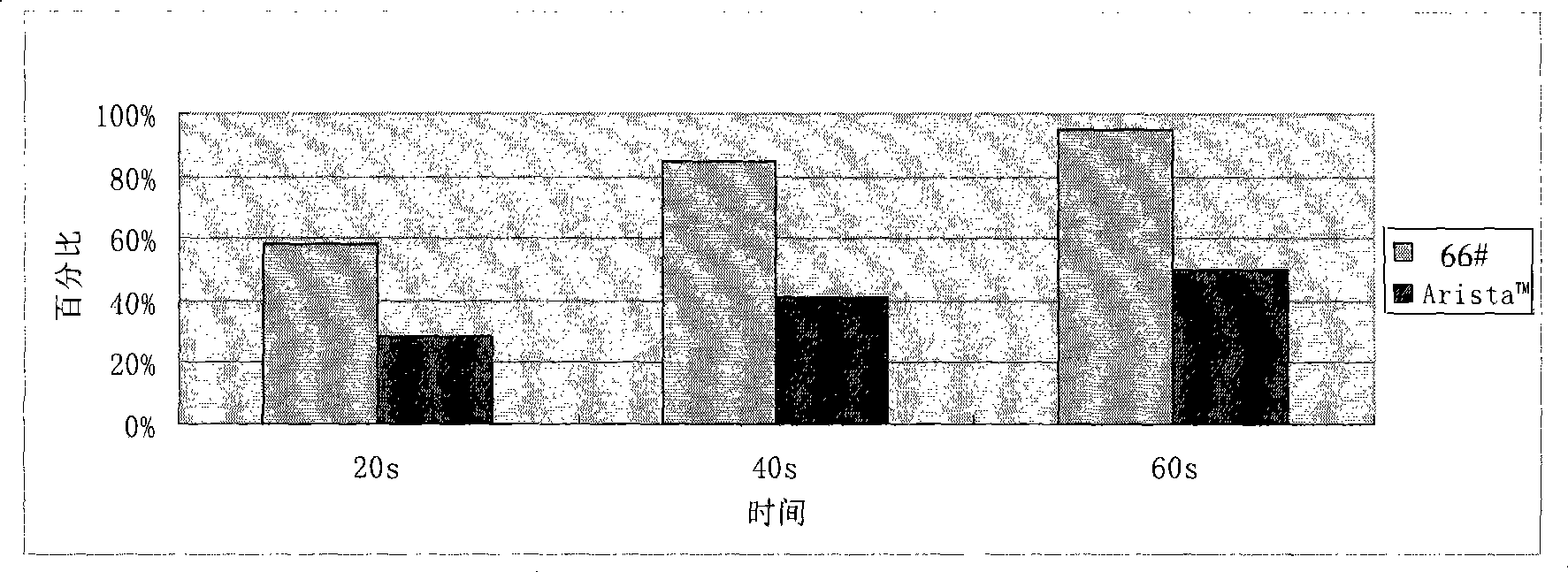
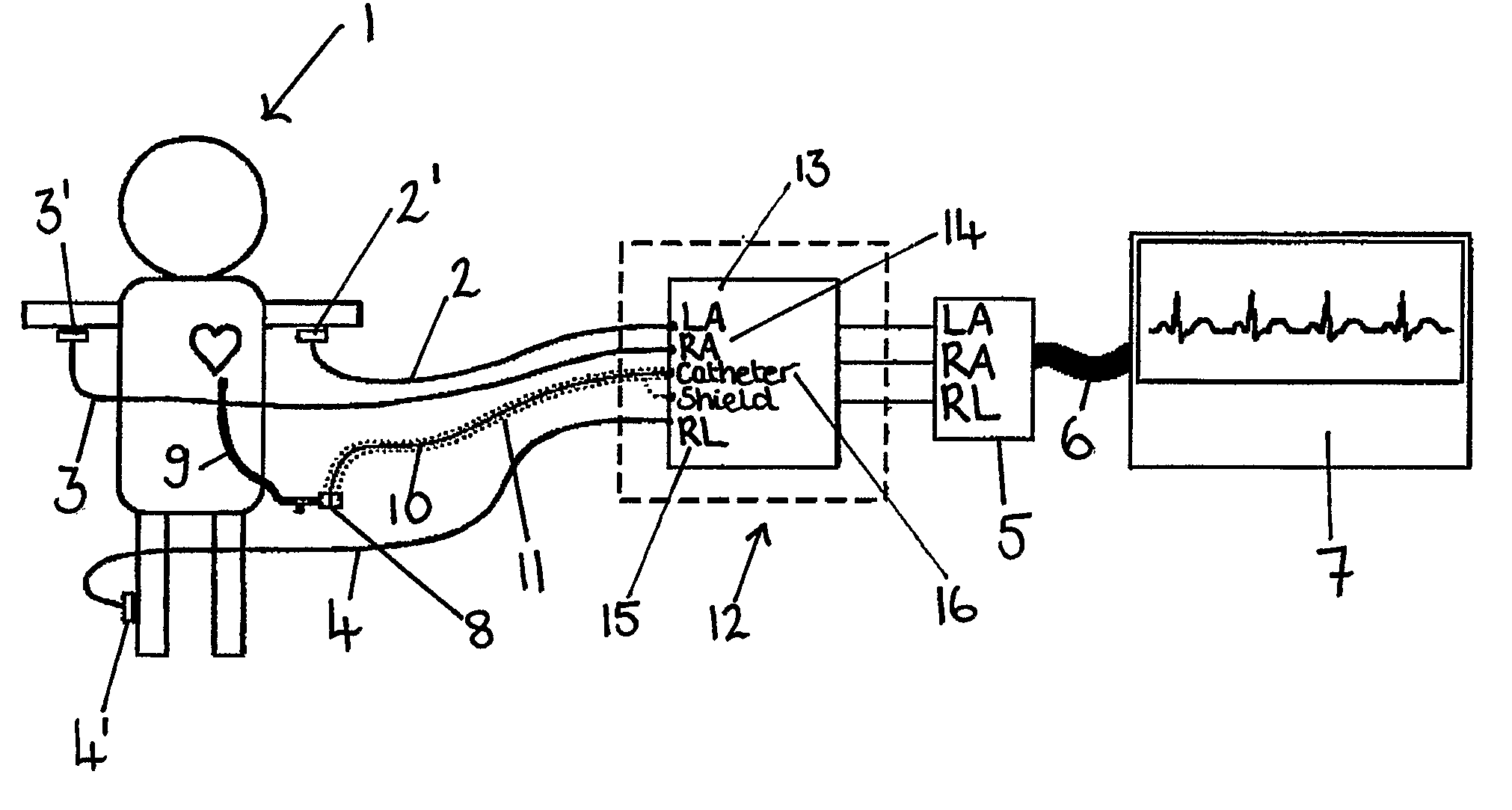
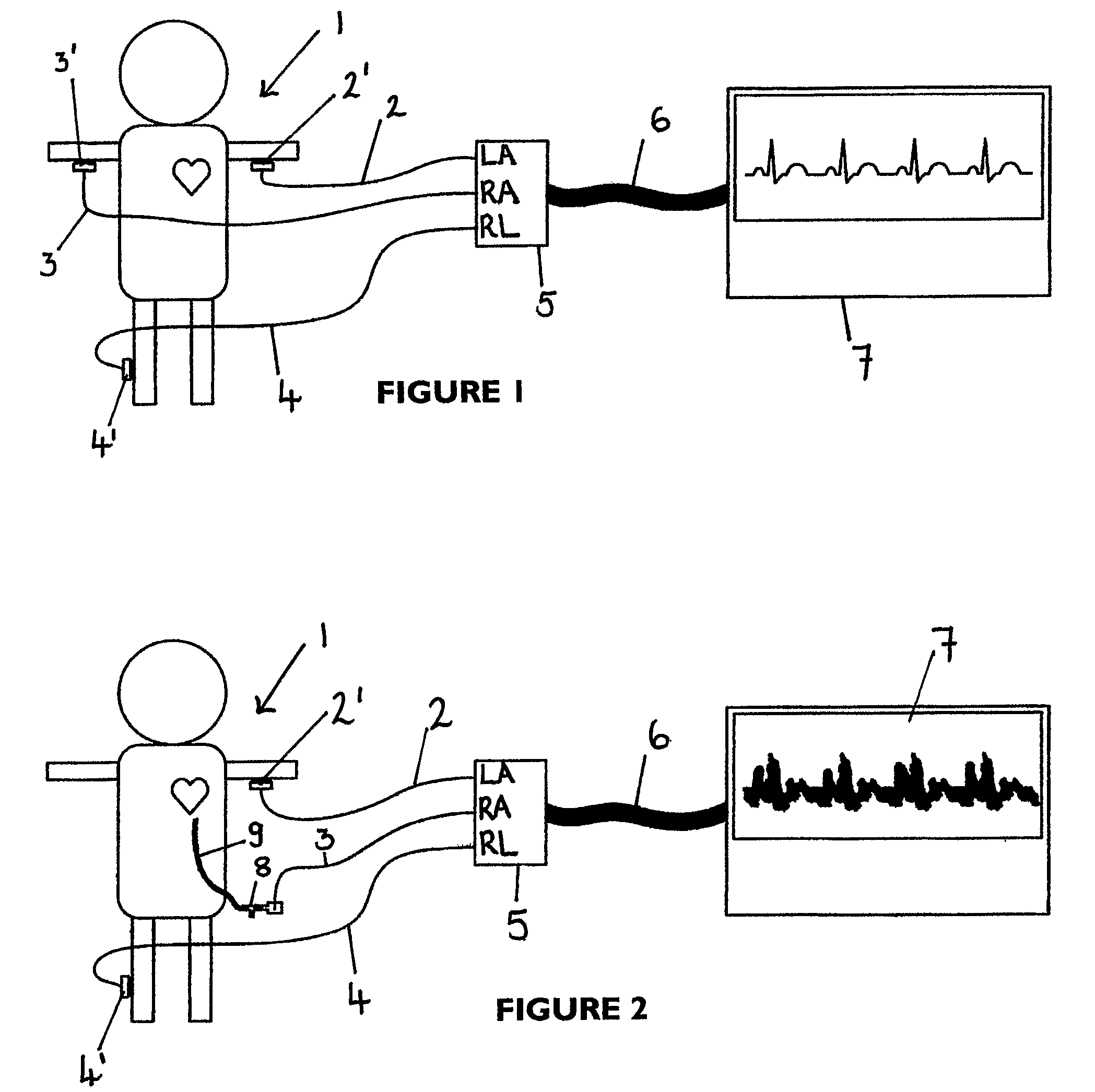
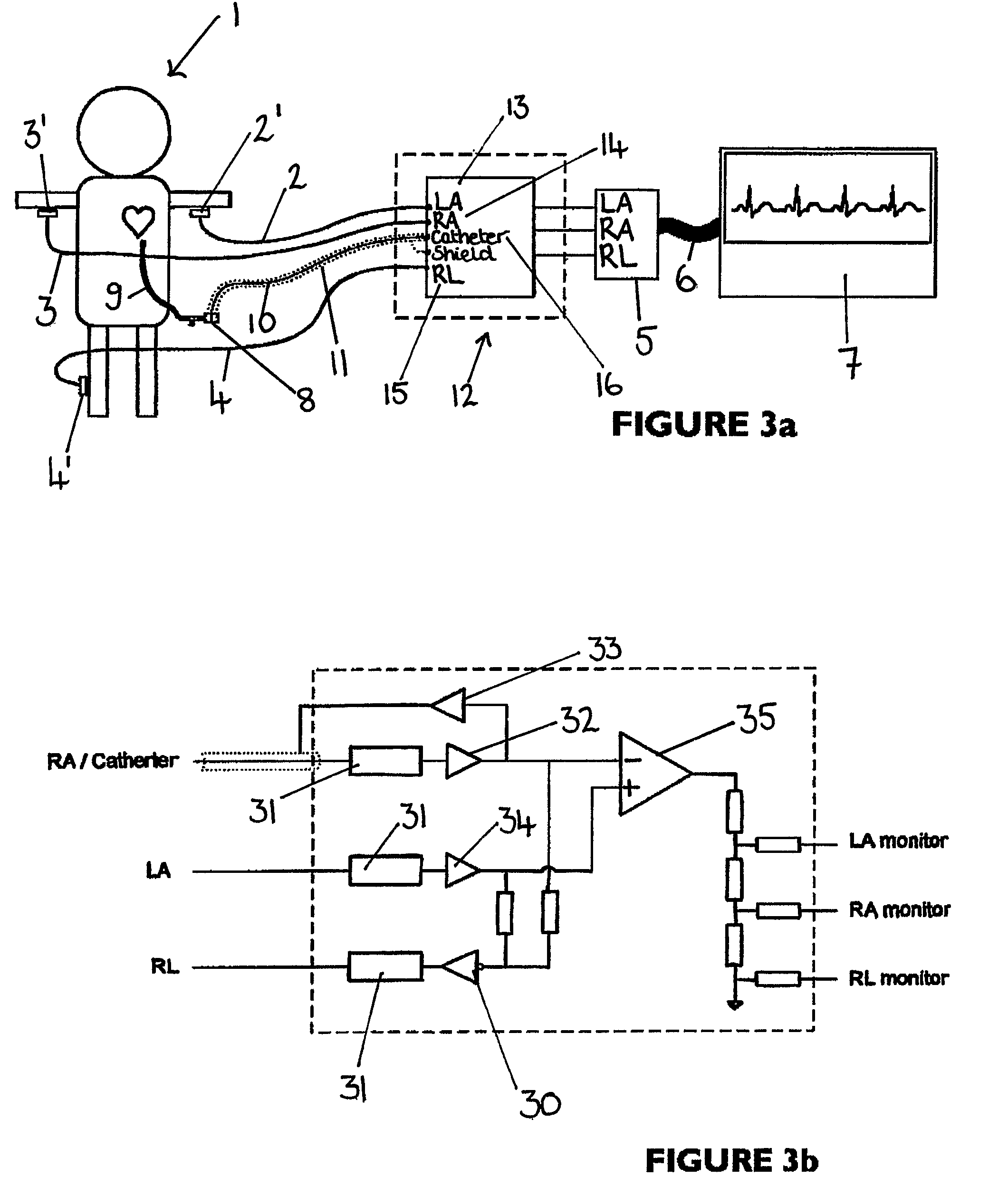
An apparatus that is able to detect the position of a catheter. The apparatus utilizes a catheter filled with electrically-conductive physiological saline and a connector for establishing an electrical connection between the saline of the catheter and an input of a controller. The controller includes at least one output connectable to a standard ECG lead connector, wherein the controller includes circuitry for generating a low impedance output signal. The controller provides an output which replicates the input a standard ECG patient lead connector is configured to receive. The apparatus provides a more convenient and cost effective solution for providing specialized ECG functions without having to replace a hospital's existing ECG beside monitoring equipment.
Owner:NEWCASTLE UPON TYNE HOSPITALS NAT HEALTH SERVICE TRUST
Drug delivery through hydrogel plugs
An embodiment is a medical prosthesis for blocking or reducing tear flow through a punctum or canaliculus of a human eye and delivering a drug to the eye that comprises a dehydrated covalently crosslinked synthetic hydrophilic polymer hydrogel with dimensions to pass through a puncta lacrimali, with the dehydrated hydrogel absorbing physiological water to swell to at least 1 mm in cross-sectional width and conformably fit a canaliculus, with the hydrogel comprising a therapeutic agent dispersed through the hydrogel for release to an eye, with the hydrogel having a water content of at least about 50% by weight or volume when allowed to fully hydrate in vitro in physiological saline.
Owner:INCEPT LLC
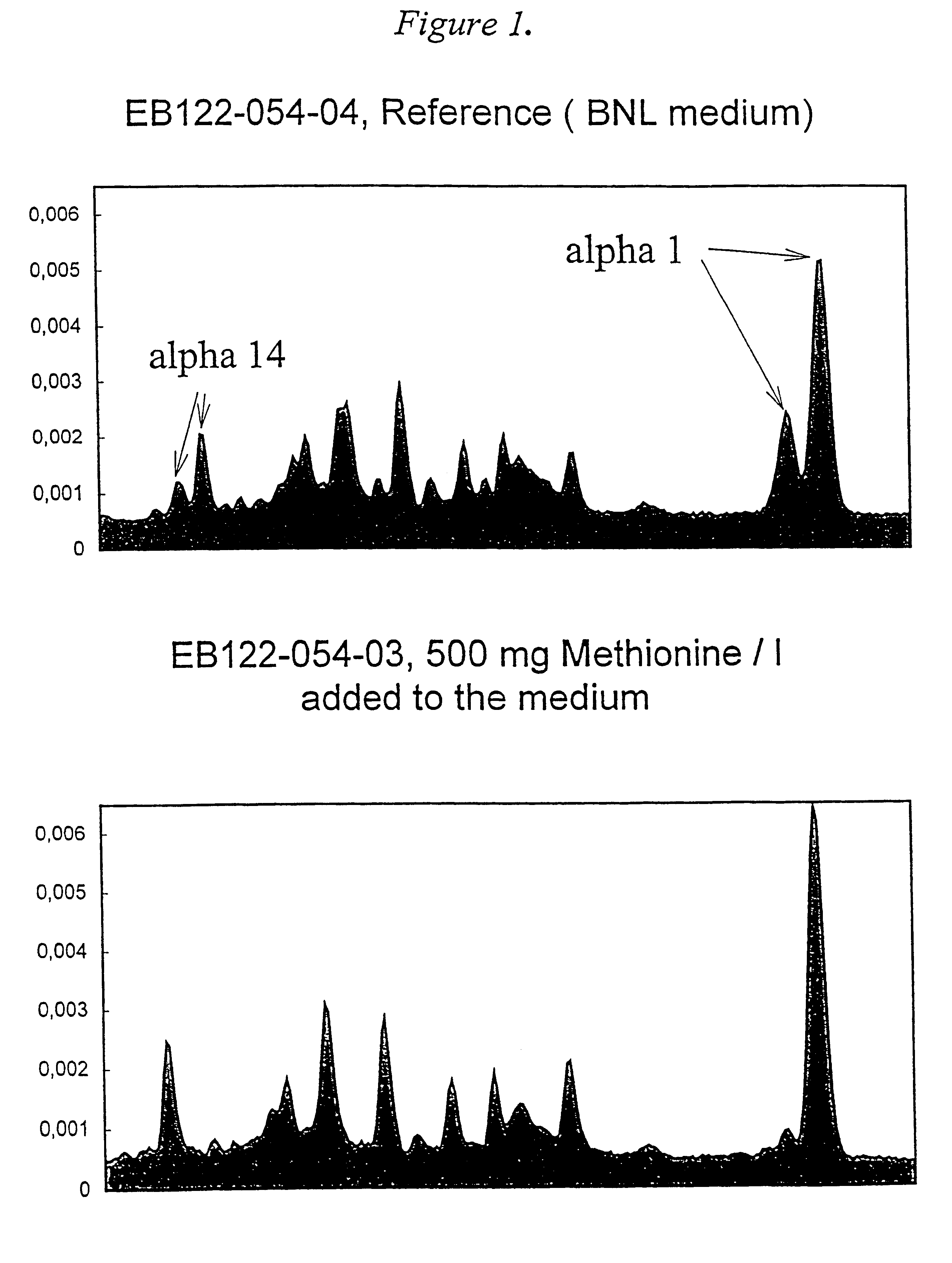
Methionine containing animal cell culture medium and its use
InactiveUS6309862B1Facilitates purification of desired productEasy to prepareCulture processCell culture mediaBiotechnologyWhite blood cell
A nutrient medium for protein producing cells, characterized in that said medium consists essentially of the following components: a physiological saline containing Ca2+, K+, Mg2+ and Na+, an energy source, a pH buffer and methionine in an amount of 0.015-2.0 g / liter, and optionally antibiotics. The invention further relates to use of the nutrient medium for cells and human leukocytes.
Owner:SWEDISH ORPHAN INT
Preparation of deuterium-depleted water, and application thereof
The invention relates to an apparatus system, wherein water electrolysis, hydrogen-oxygen recombination and a rectification technology are combined into the integration by the apparatus system, and deuterium-containing water in purified water and protium-containing water in purified water are separated by using the apparatus system. With adopting the apparatus system, the main protium-containing water can be effectively separated from the purified water, and the deuterium-containing water content in the purified water can be reduced to less than 100 ppm from 150 ppm after the separation. According to the present invention, the prepared deuterium-depleted water can be adopted for preparation of deuterium-depleted water wine, deuterium-depleted water beverages, deuterium-depleted water cosmetics and deuterium-depleted water physiological saline.
Owner:廖文加 +1
Preparation method and application of immobilized bacteria for improving denitrification efficiency of constructed wetland
InactiveCN102392011AHigh mechanical strengthImprove stabilityTreatment using aerobic processesSustainable biological treatmentConstructed wetlandIndustrial waste water
The invention discloses a preparation method and an application of immobilized bacteria for improving the denitrification efficiency of a constructed wetland. 1. The preparation steps are as follows: 1) screening and culturing aerobic denitrification strains which are screened out of the constructed wetland in a thermotank; 2) selecting aerobic denitrification bacteria in slope conservation, inoculating into a sterile enrichment culture medium and aerating to get bacterial suspension; 3) using sodium alginate-polyvinyl alcohol to embed so as to get the immobilized aerobic denitrification bacteria; and 4) taking pellets of the aerobic denitrification bacteria out of a refrigerator, washing with double distilled water or physiological saline, soaking in the physiological saline, and aerating to activate the immobilized pellets. 2. An application of the immobilized bacteria in the constructed wetland is as follows: filling the immobilized pellets into a simulation column of the constructed wetland for performing waste water denitrification treatment. The preparation method of the immobilized bacteria is simple, the production cost is low, the application in the industrial waste water treatment is easy, the denitrification efficiency is high, the accumulation amount of nitrite nitrogen is low, and the service life is long.
Owner:INST OF AQUATIC LIFE ACAD SINICA
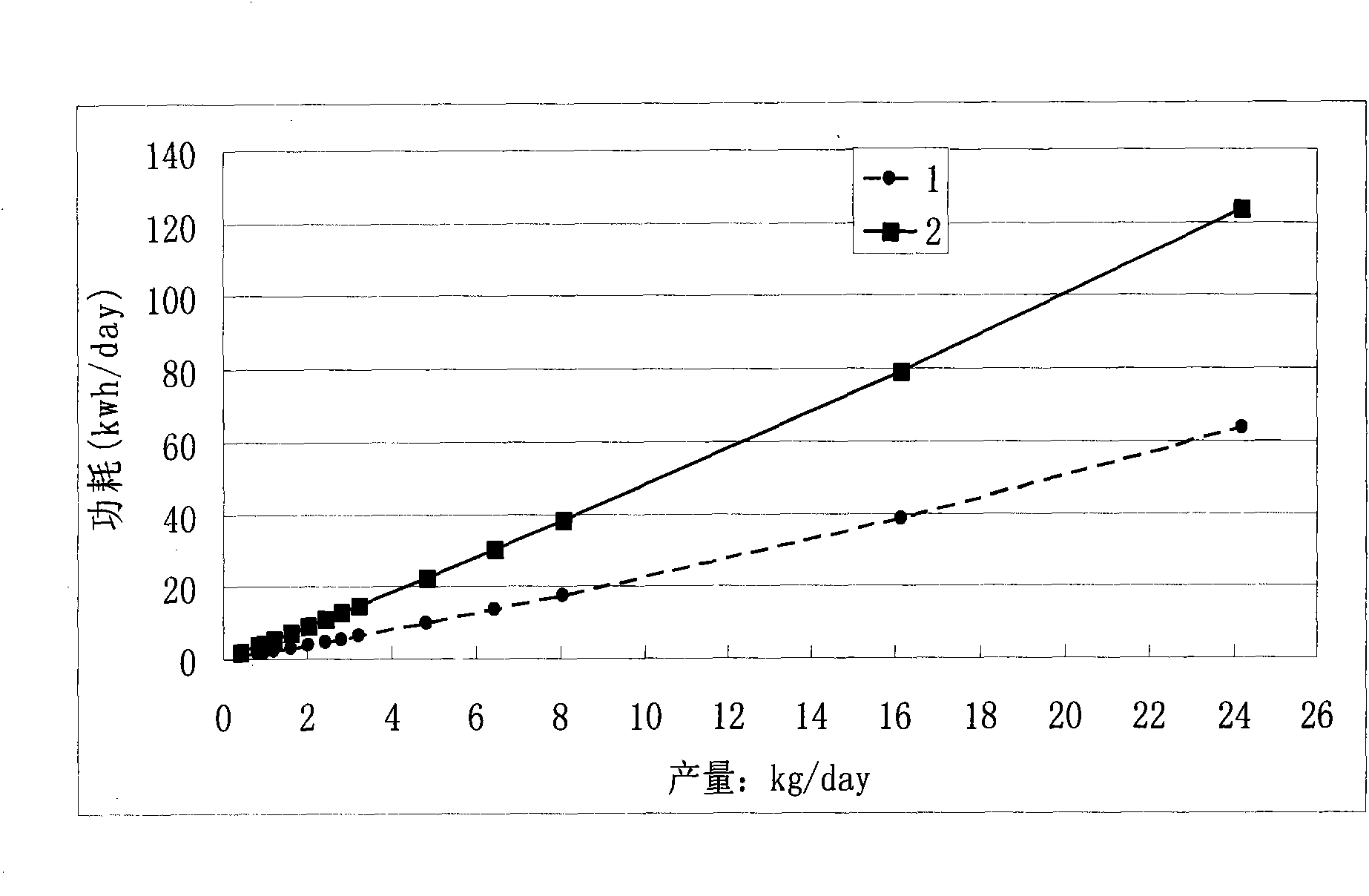
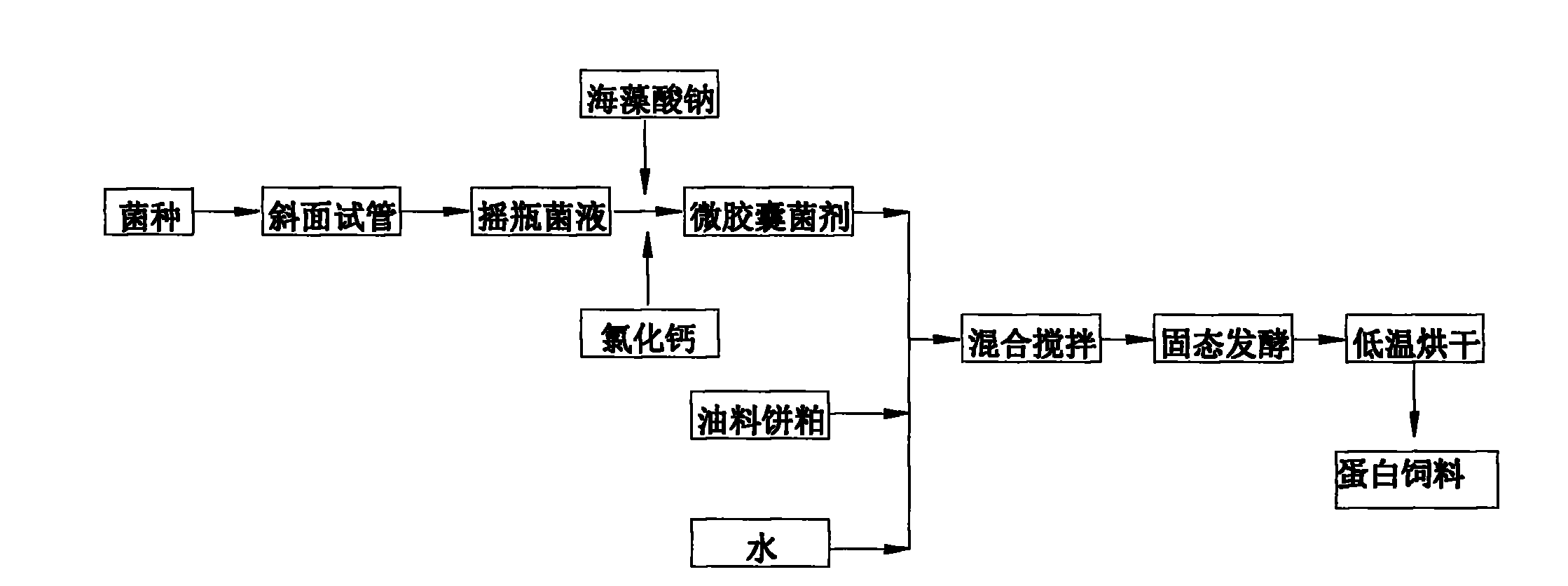
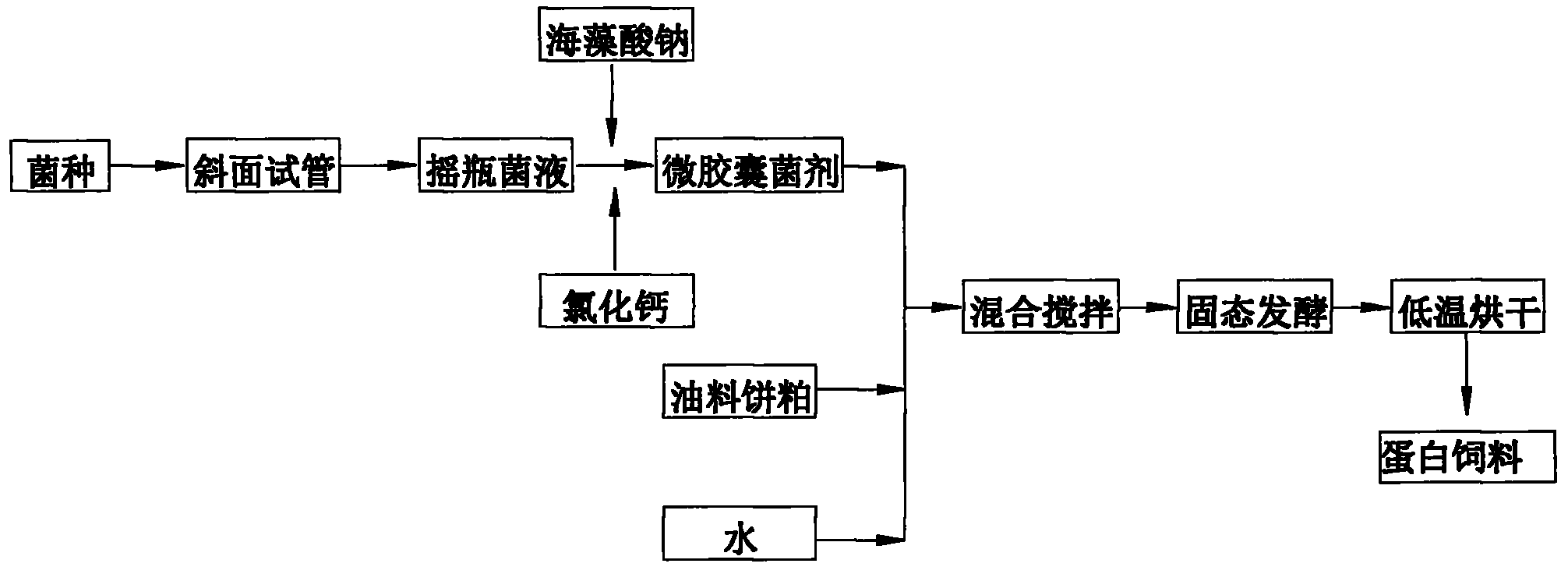
Method for preparing oil cake protein feedstuff through low moisture solid state fermentation
InactiveCN102318732AEasy to prepareLow priceFood processingAnimal feeding stuffMicroorganismRoom temperature
The invention belongs to the technical field of microbial fermentation, specifically relates to a method for preparing an oil cake protein feedstuff through low moisture solid state fermentation. The method for preparing the oil cake protein feedstuff through the low moisture solid state fermentation is characterized in that: the method comprises the following steps: 1) preparing a bacterial inoculum: injecting a mixed solution (comprising a bacterial liquid and a sodium alginate solution) in a CaCl2 solution through an injector, washing three times through sterilized physiological saline after curing to obtain a microcapsule bacterial inoculum, wherein a volume ratio of the sodium alginate solution to the CaCl2 solution is 1:2-1:10; 2) mixing and fermenting cake base materials: uniformly mixing and stirring the microcapsule bacterial inoculum, oil cake and water, carrying out standing fermentation for 24-96 hours at a room temperature to obtain a fermented product; 3) adopting one of the following two methods: (1) carrying out drying, wherein the fermented product is dried after completing the fermentation to obtain the oil cake protein feedstuff; (2) directly carrying out vacuum packaging for the fermented product after completing the fermentation to obtain the oil cake protein feedstuff. The method has characteristics of high survival rate and low production cost.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
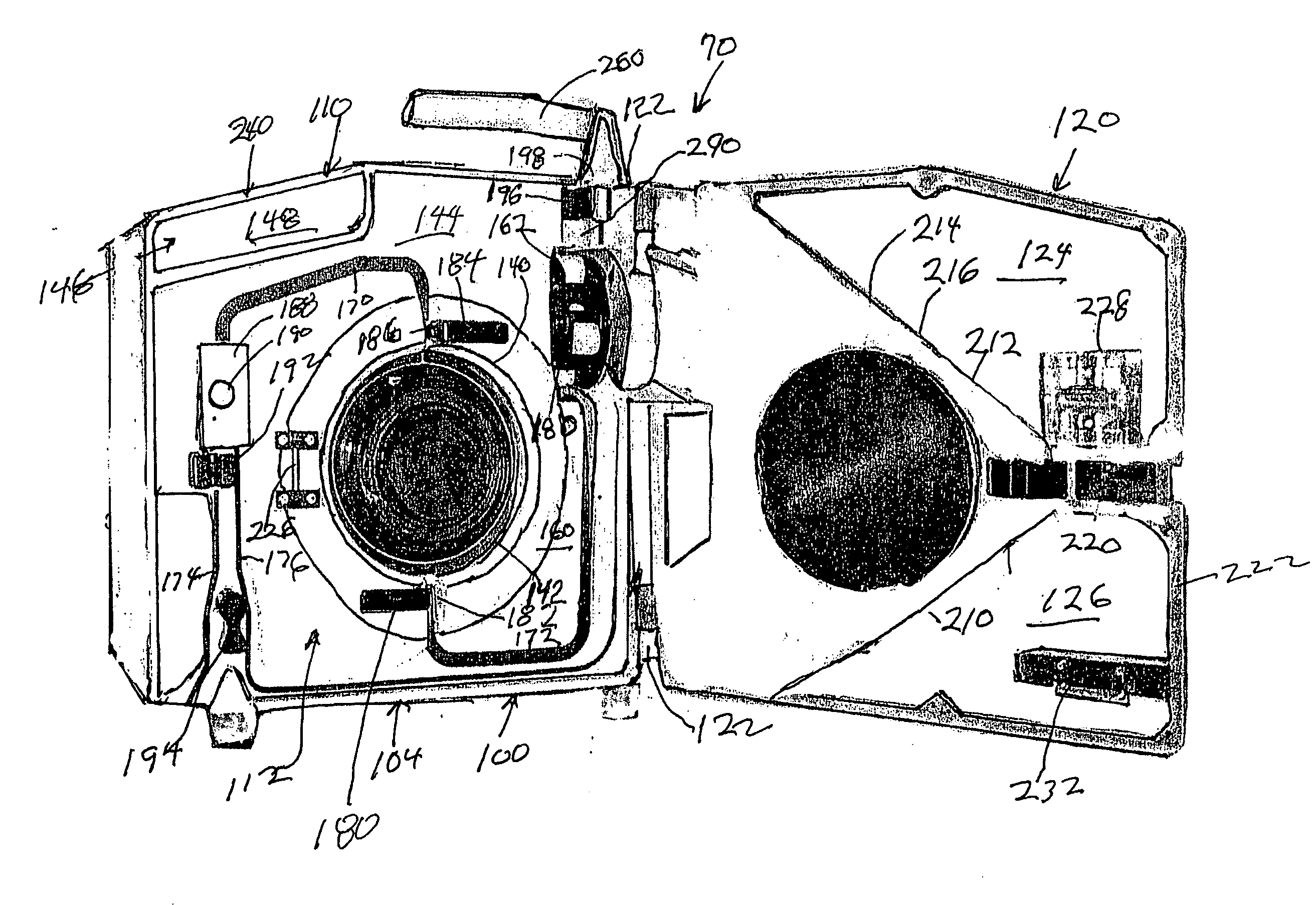
Hyperthermia, system, method and components
ActiveUS7819835B2Effective and safe for patientElectrotherapyInfusion devicesIntensive care medicineHigh body temperature
An IV pole mountable, therapeutic infusate processing device is incorporated into a hypothermia system to receive therapeutic fluid(s), such as normal saline, peritioneal dialysis solution, or other crystalloid solution, to heat such therapeutic fluid(s) a few degrees centigrade above normal body temperature and to direct the resulting heated infusate to and through a selected anatomical portion of a patients body to raise the temperature of that body portion so as to affect any cancerous or other tumors that may be located therein. The processing device is provided with touch screen controls and visual indicators to facilitate its proper use; while the system further includes temperature and pressure sensors to monitor the hyperthermia processing to insure patient safety.
Owner:BELMONT INSTR LLC
Method for preparing aerobiotic nitrifier immobilizing vector
InactiveCN103013973ALarge specific surface areaImprove adsorption capacityWater contaminantsOn/in organic carrierMicroorganismNitrogen removal
The invention discloses a method for preparing an aerobiotic nitrifier immobilizing vector, and belongs to the field of water treatment. The method comprises the steps of: firstly, carrying out acid washing on thermally modified attapulgite; adsorbing the nitrifier using the nanometer grade attapulgite; carrying out rapid inclusion by using the mixture gel of polyvinyl alcohol (PVA) and sodium alginate (SA); dropping the mixture into a crosslinking agent to soak for 24 hours using a granulator to obtain an immobilized nitrifier pellet; and finally, repeatedly washing the immobilized nitrifier pellet for three times using normal saline to obtain a vector particle. By taking advantage of pretty large specific surface area and pretty strong adsorption performance of the modified attapulgite, an adsorption vector which is conductive to the growth and reproduction of the aerobiotic nitrifier is prepared; the modified attapulgite is capable of ammonia and nitrogen adsorption, and can increase the ammonia and nitrogen removal rate in water; the carrier particle composited from the modified attapulgite and a macromolecular material can improve the activity and ammonia and nitrogen removal capacity of denitrification microorganisms; and the repeated use rate of the carrier is high.
Owner:CHANGZHOU UNIV
Biocompatibility pre-gelatinized modified starch and preparation thereof
InactiveCN101497670AAbsorbentSpeed up absorptionSurgical adhesivesFibre treatmentBiocompatibility TestingHigh pressure
The invention relates to a biocompatible pre-gelatinized modified starch. The water absorbency is not less than one time, and the biocompatible pre-gelatinized modified starch is taken as biocompatible hemostatic material, biocompatible anti-blocking material, biocompatible tissue-healing promoting material, biocompatible surgical sealant or biocompatible wound closure tissue glue. The invention has the advantages that the biocompatible pre-gelatinized modified starch is directly acted on the wounded area with blood for immediately stopping bleeding, has obviously increased water absorbency and speed of water absorption and greater viscosity and stickiness and further plays the role in preventing the tissue and the blood vessel from being damaged during the process of stopping bleeding; the modified starch is easy to swell or dissolve in water, and is washed by normal saline after the bleeding stopping so as to reduce the residual in the body, to be favorable for wound healing and to avoid the pain due to tearing the gauze and the bandage out; the pre-gelatinized modified starch has the actions of bacterial resistance and anti-inflammatory; and the pre-gelatinized modified starch is stable, not easy to decompose, long in guarantee period, convenient for storage, resistant at high pressure and low pressure, resistant at high temperature and low temperature and not easy to change the physicochemical characteristics.
Owner:纪欣
Method for preparing edible mushroom synbiotics microcapsule
InactiveCN101401638AEnhanced inhibitory effectLarge inhibition zoneFood shapingFood preparationSynbioticsFreeze-drying
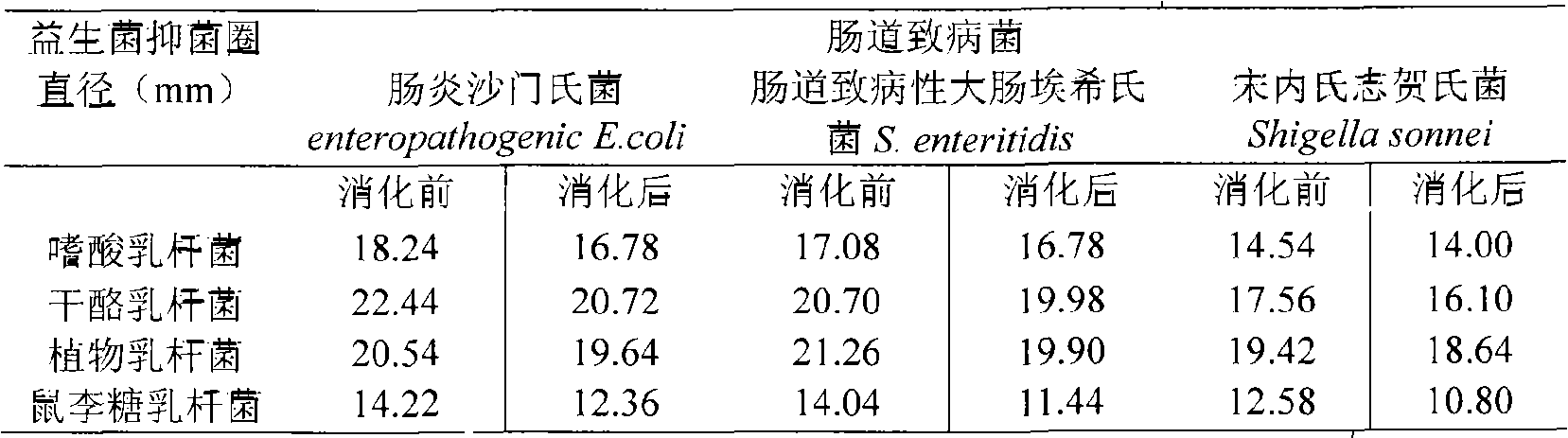
The invention provides a method for preparing edible bacteria synbiotic microcapsules. The method comprises the following steps: firstly, zymotic fluids of four strains of probiotic bacteria, namely Lactobacillus acidophilus, lactobacillus plantarum, lactobacillus casei and lactobacillus rhamnosus are prepared, then supernatants are removed after the speed of 5000r / min, and bacterial sludges are embedded for standby. 10g of each bacterial sludge of the probiotic bacteria to be embedded and 4g of edible bacteria Agaricus blazei powder with the fineness of 200 meshes are mixed with 40g of mixed colloid containing 2.5 percent of sodium alginate and 0.5 percent of flaxseed gum respectively; the first stirring is performed for 20min at a rotating speed of 200 r / min; the mixture is poured into a triangular flask which contains 1.5 times of volume of edible oil; the second stirring is performed for 20min at a rotating speed of 400r / min; a 1.5 percent CaCl2 solution is added into the mixture to be calcified for 3h; sterilized physiological saline is used to wash gel beads for three times; the gel beads are placed into a 0.6 percent chitosan solution with a pH value of 5.5 to perform a film-forming reaction for 15min, taken out, washed by the sterilized physiological saline and then placed into a citric acid solution with the concentration of 55mM to be liquefied for 30min; and wet microcapsules are obtained after the washing of the sterilized physiological saline, collected by centrifugating for 10min at a rotating speed of 150r / min, precooled, and finally freeze-dried to obtain the microcapsules.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
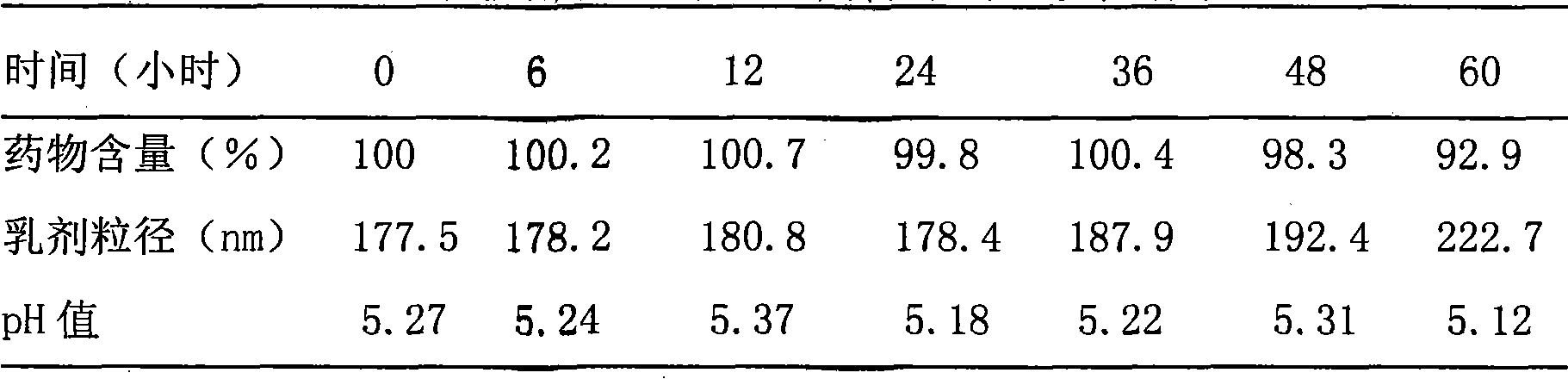
Paclitaxel submicron emulsion using lipid composite as middle carrier
ActiveCN101396343AOvercome the shortcomings that are not conducive to making liquid formulationsHigh drug loadingOrganic active ingredientsEmulsion deliveryLipid formationFreeze-drying
The invention discloses a paclitaxel submicron emulsion with a lipid compound as an intermediate carrier. With the paclitaxel lipid compound as the intermediate carrier, the paclitaxel is solved in oil phase, and water phase, emulsifier and auxiliary emulsifier are added. Emulsion droplet grain diameter is less than 600nm; the proportion of the oil phase and the water phase is 5 to 95 to 35 to 65; and the drug loading quantity is 0.25mg / ml to 5mg / ml if counted according to the paclitaxel. The prepared submicron emulsion has high drug loading quantity, is resistant for autoclaving and has stable quality after long time storage. The submicron emulsion can be made into intravenous drip transfusion directly, as well as into dry emulsion by a freeze drying technology, and when being used, the submicron emulsion is added with physiological saline or glucose to be diluted into intravenous drip.. The submicron emulsion uses nontoxic refined plant oil as the oil phase and phospholipid as the emulsifier, drug is coated in the oil phase, and thereby the submicron emulsion reduces the irritation and the adverse reaction of the paclitaxel preparation and has good safety.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
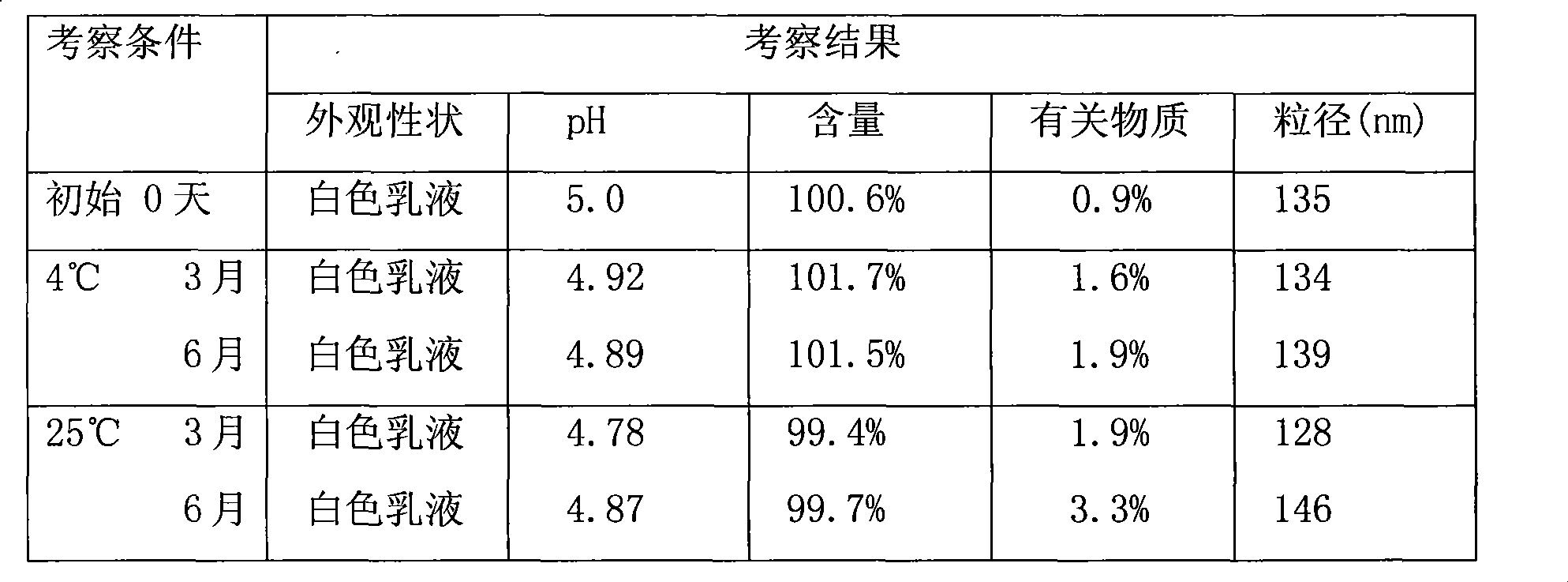
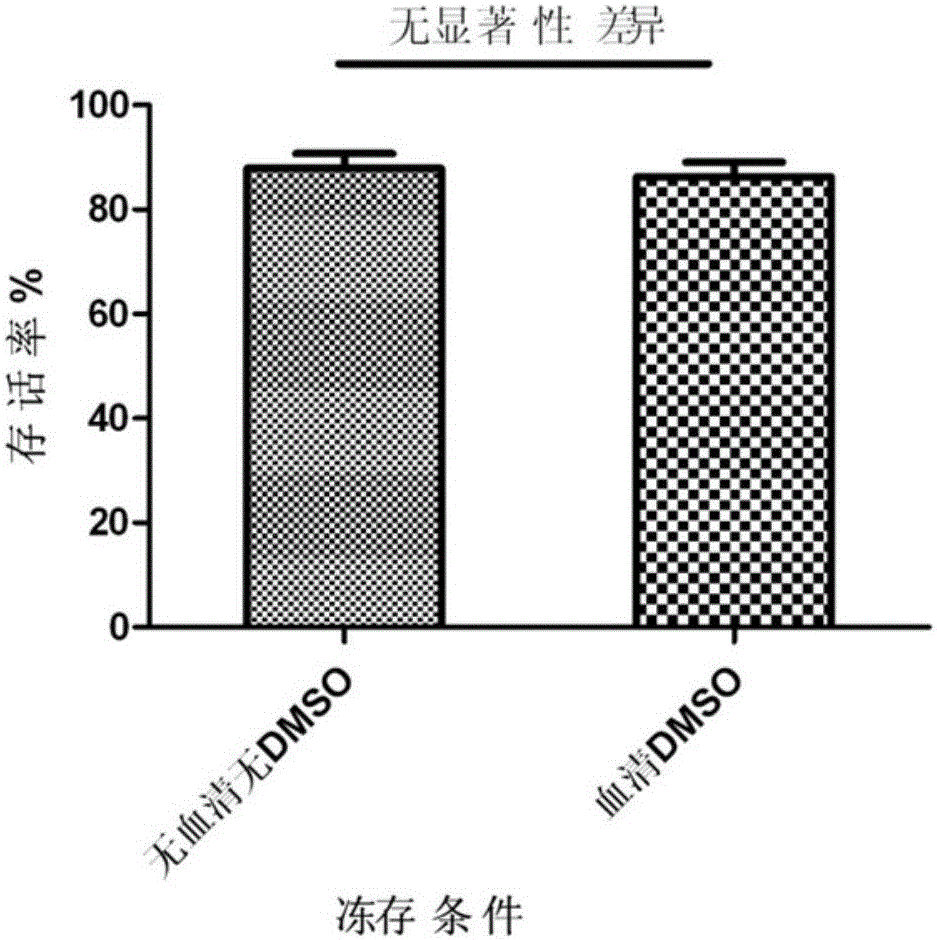
Cell cryopreservation fluid
InactiveCN105961374AImprove freezing effectClear ingredientsDead animal preservationAdditive ingredientPolyethylene glycol
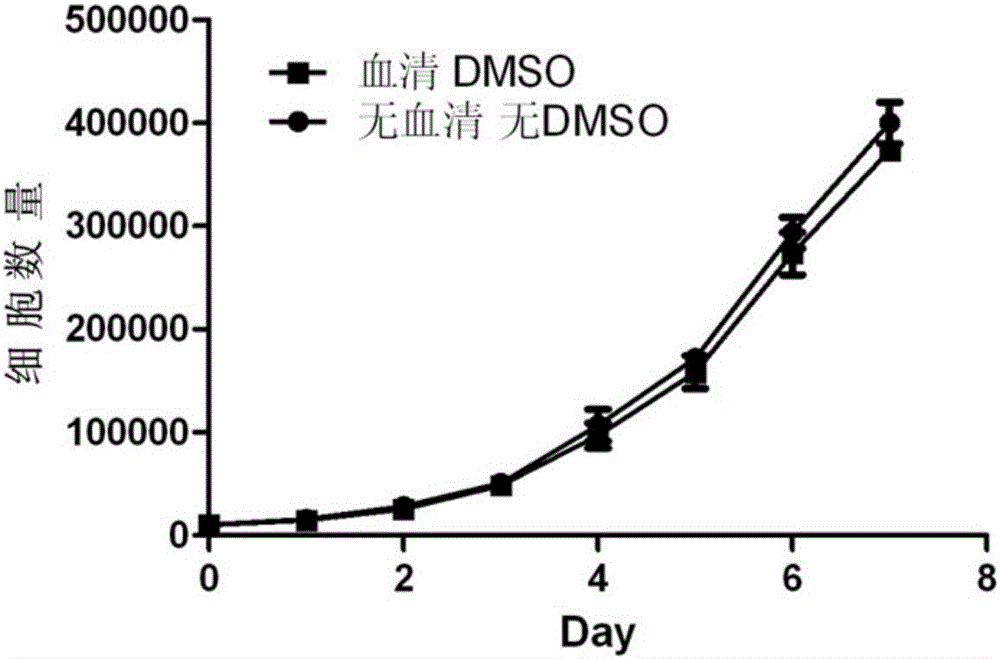
The invention discloses a cell cryopreservation fluid which can be used for performing cryopreservation on mesenchymal stem cells or other cells. The cell cryopreservation fluid is prepared from the following ingredients: PBS or normal saline or a basal culture medium serving as a main ingredient, as well as one or more of polyethylene glycol, propanediol, Ectoin, albumin, trehalose, proline and poloxamer 188 which serve as additive ingredients. The cell cryopreservation fluid disclosed by the invention does not contain serums and DMSO, have the definite additive ingredients, and is controllable in quality, high in batch stability and high in safety. By virtue of preserving cells with the cell cryopreservation fluid, revived cells are high in survival rate, and the cellular morphology, the multiplication capacity and the differentiation potential are not influenced; the cell cryopreservation fluid can be used for replacing cryopreservation fluids containing the serums and the DMSO.
Owner:SHENZHEN HORNETCORN BIOTECH
Active capillary
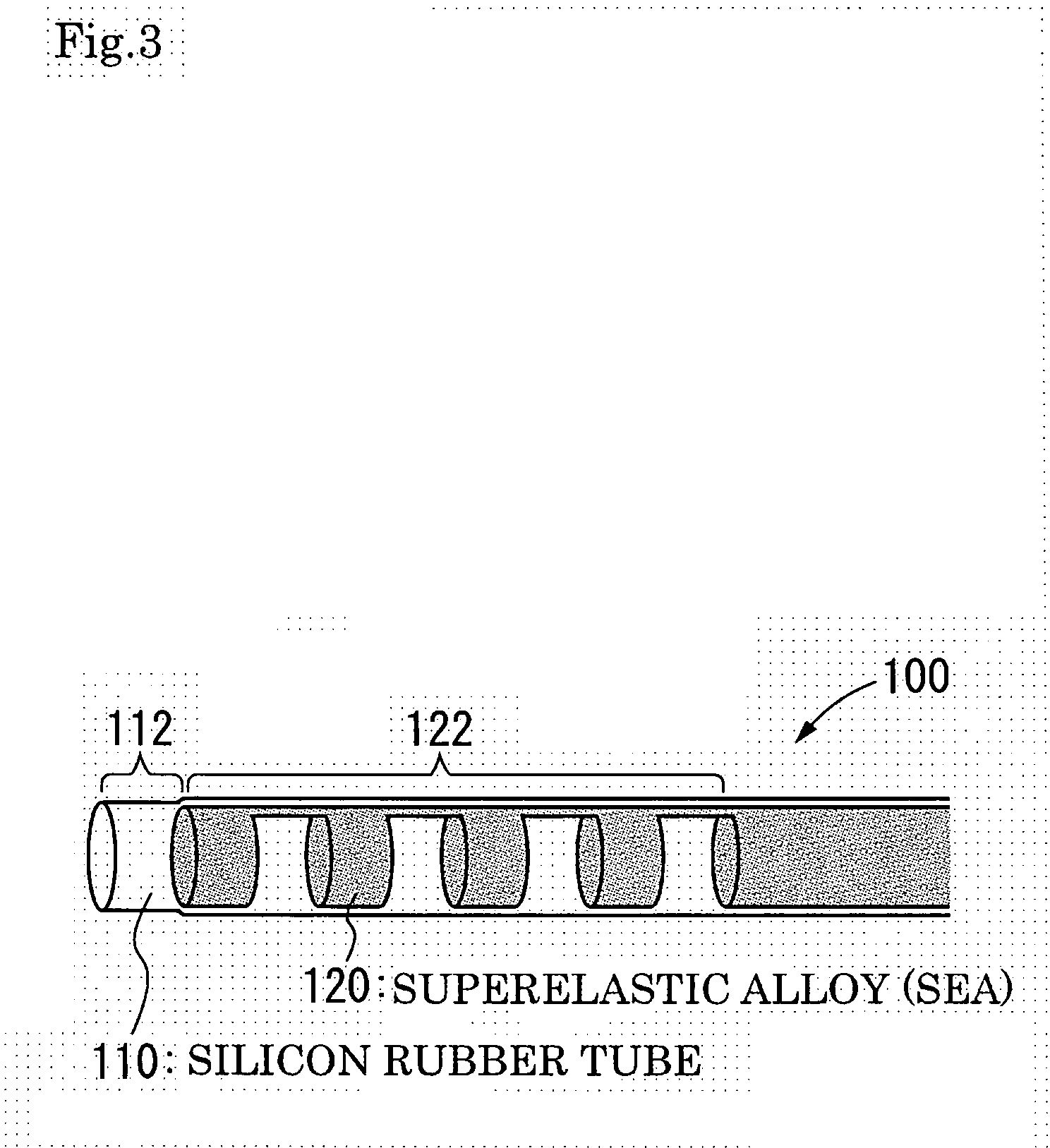
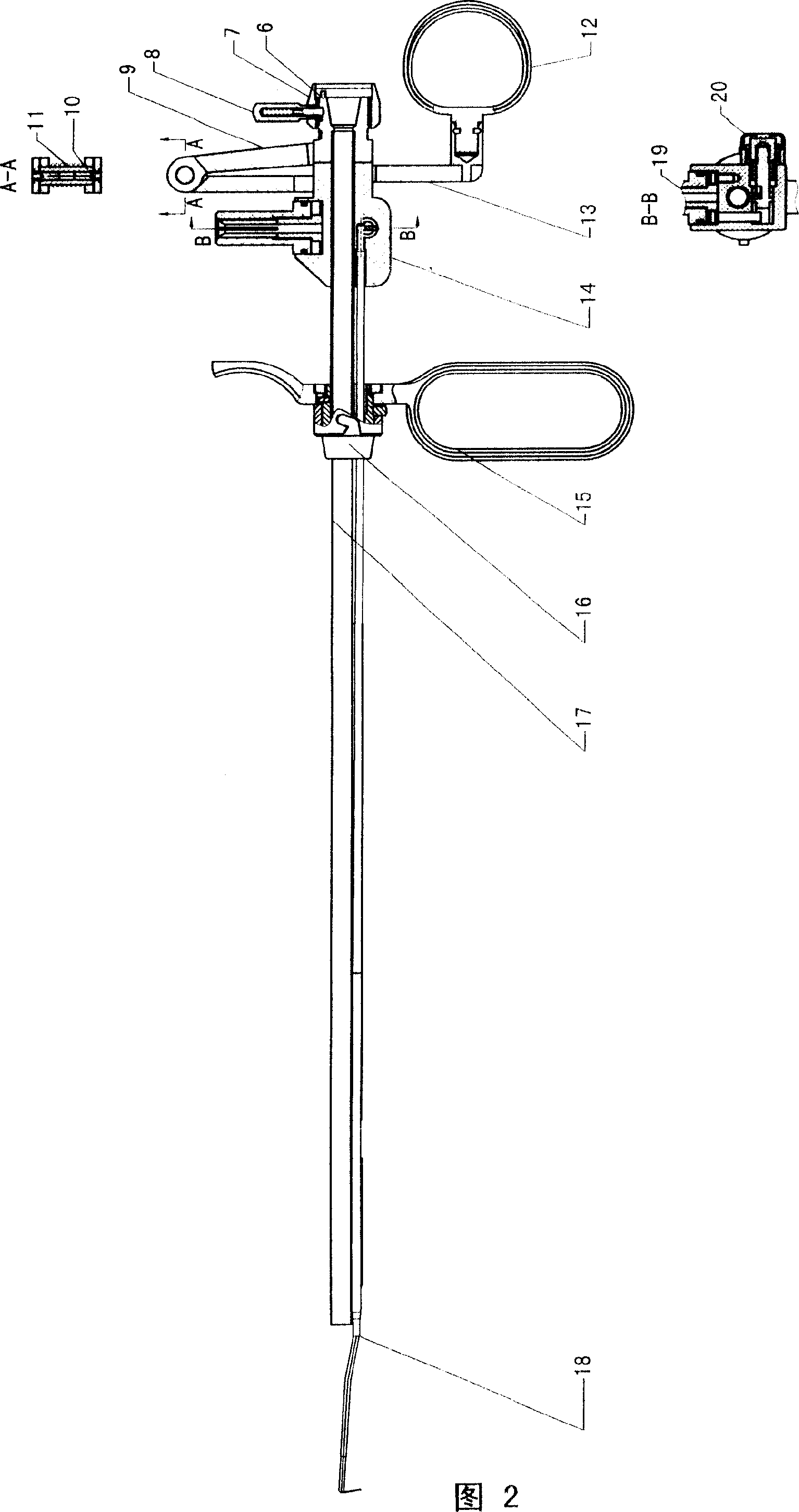
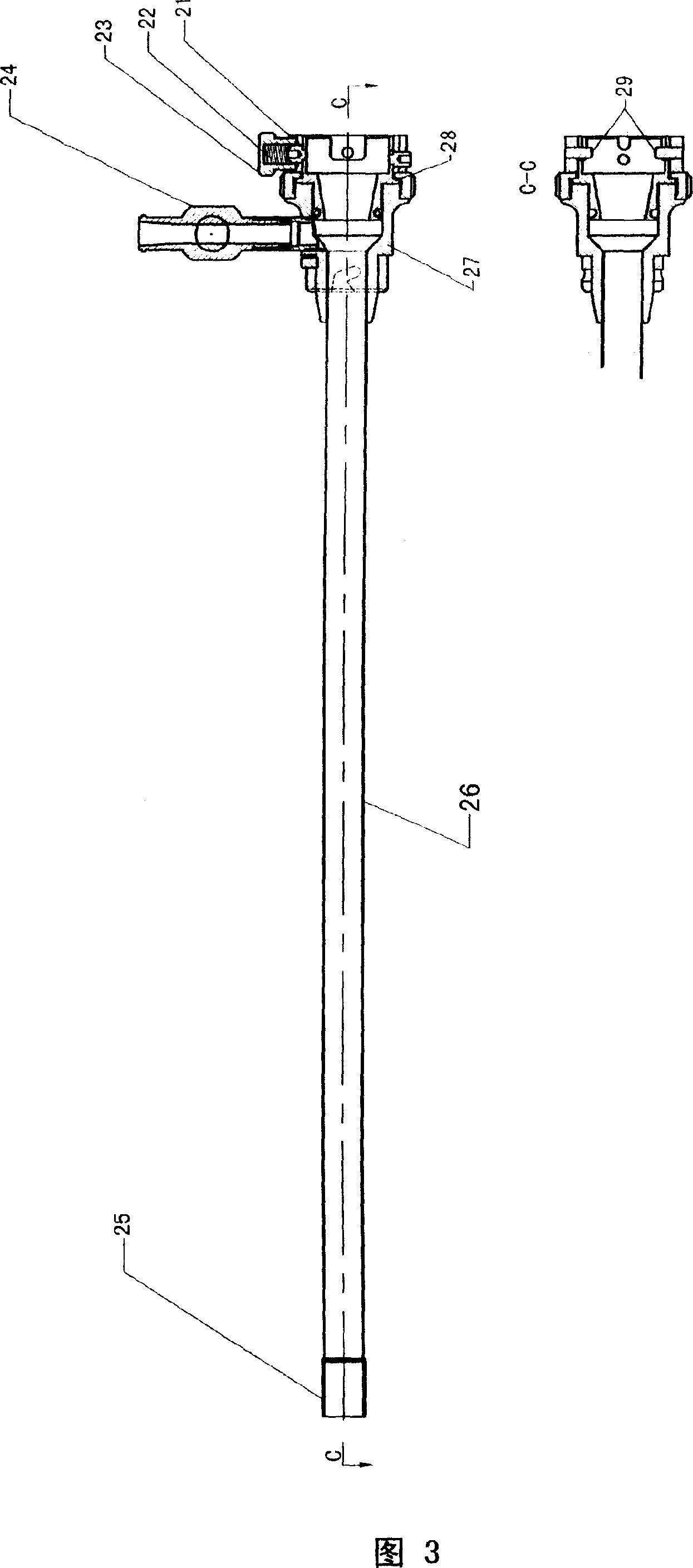
Catheter of such a structure that Ti—Ni superelastic allow (SEA) tube has its outside covered with thin-film silicone rubber tube. The SEA tube at part whose flexion is desired is wrought to cut off multiple grooves (crenas) with thin joints left. The covering with the silicone rubber tube is effected without the use of its front end portion in the covering. When a negative pressure is applied to physiological saline placed therein, the front end portion works as a valve, and the silicone rubber tube at the wrought part yields inward so that flexion of the catheter occurs at that part.
Owner:TOHOKU TECHNO ARCH CO LTD
Vaporization electric excision scope for medical purpose
InactiveCN101108138AAvoid cross infectionEasy to operateSuture equipmentsInternal osteosythesisRESECTOSCOPEHigh pressure
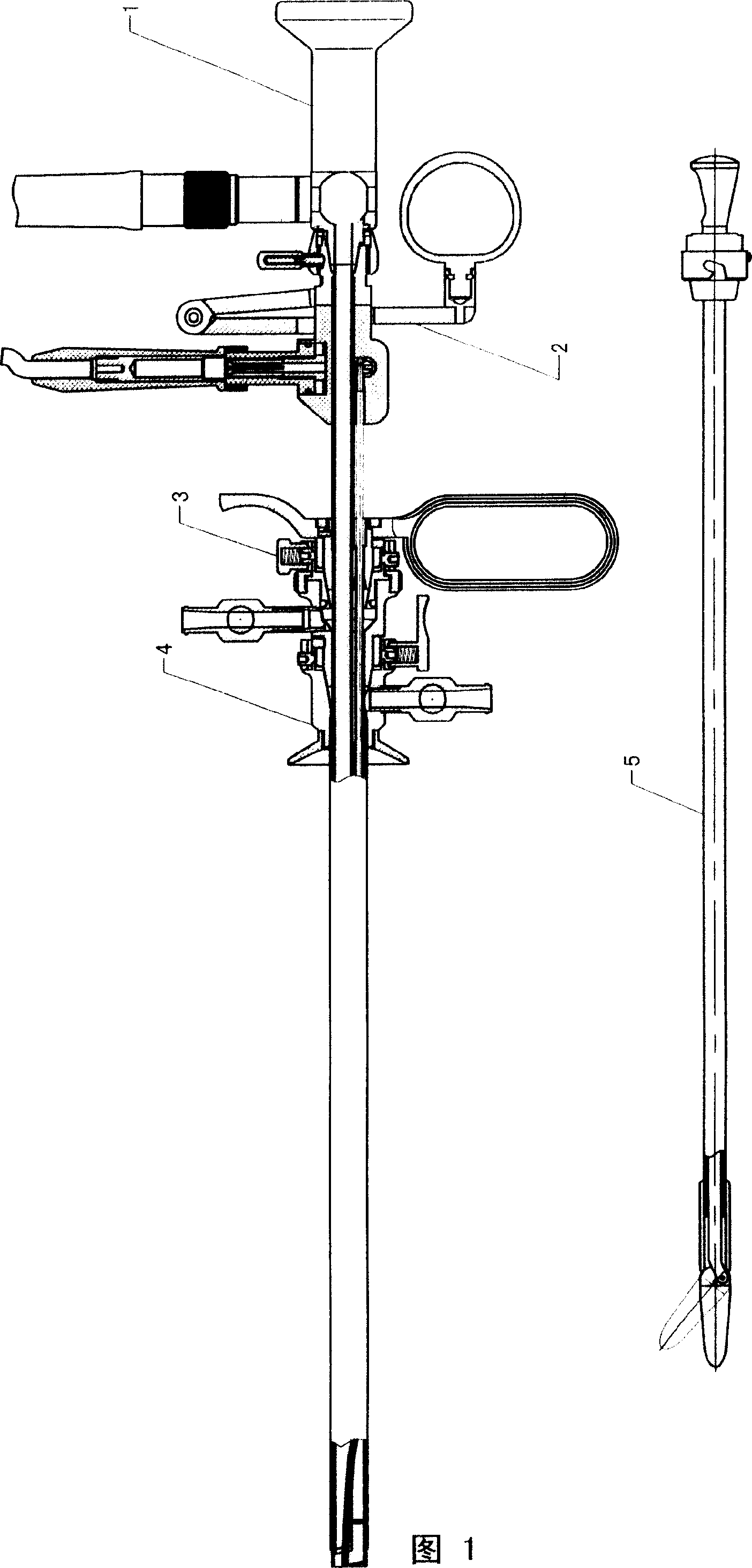
The invention relates to a medical gasification resectoscope for inspecting and electric-cutting in urinary system. The opened operation of the traditional urinary system has the defects of large cut and damnifies, a plurality of blooding during operation and stimulated symptoms, long pathogenesis and enormous pains after the operation. The invention provides the gasification resectoscope, which comprises a main body, a sheath, an endotheca capable for inserting into the sheath and an inserting piece capable of inserting into the endotheca. The invention has the advantages of unnecessary of operating, small pain, light blooding, quick for recovery and reliable effects, which enables the operation safer, more precious and thorough, thus largely deducing the operation time, releasing the pain of the patients and shortening the curing time; besides, the invention is capable of being sterilized with high temperature and pressure and features uneasy for rusting; in addition, the physiological saline and excision enter and exit through different cannuls, thus enabling the cutting scope easy for control and has simple operation and clear version.
Owner:王水良
Method for preparing probiotic microcapsules by using electrostatic spraying
InactiveCN101856604AUniform particle sizeGood encapsulation performanceFood shapingMicroballoon preparationChemical industrySaline water
The invention discloses a method for preparing probiotic microcapsules by using electrostatic spraying. Sodium alga acid and gelatin are taken as wall materials, probiotic suspension or microporous starch-adsorbed probiotics are taken as core materials, and calcium chloride is taken as solidifying solution. The method comprises the following steps of: a) mixing 1.5 percent solution of sodium alga acid and 1 percent gelatin in the same volume, and uniformly mixing with the probiotic suspension or microporous starch-adsorbed probiotics to form mixed solution; b) putting the mixed solution into a container, and injecting the mixed solution into 2 to 3 percent continuously stirred solution of calcium sodium chloride in the electrostatic spraying mode by using a microcapsule molding device to form microcapsules; c) standing and solidifying the encapsulated probiotic microcapsules for 20 minutes in the solution of calcium chloride, and filtering and cleaning the solution by using physiological saline; and d) saving the probiotic microcapsules in the physiological saline. The prepared microcapsules have the advantages of uniform grain diameter, good encapsulation performance, simple process, readily available wall materials, and low cost, and can be widely applied to the fields of food processing, biomedicines, chemical industry, and the like.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Process for preparing chitosan base macroporous high water absorptive resin
InactiveCN1616505AThe synthesis steps are simpleProcess conditions are easy to controlCross-linkPotassium persulfate
The present invention relates to the preparation process of chitosan base macroporous high water absorption resin. After chitosan is dissolved in dilute acetic acid solution, acrylic acid is made to graft on the molecular chain of chitosan under the initiation of ammonium or potassium persulfate and the product is cross-linked with cross-linking agent, so as to produce high water absorption chitosan grafted acrylic resin with distilled water absorption of 560-1210 g / g and physiological saline absorption of 76-125 g / g. The prepared resin is further modified via solvent deposition process to form macroporous structure to raise the water absorbing rate greatly. High efficiency liquid phase chromatographic test shows that the resin has greatly reduced acrylic acid monomer residue.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
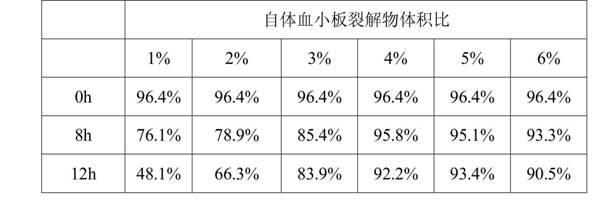
Mesenchymal stem cell self-preserving liquid
ActiveCN102349500AAdapt to the living environmentMaintain acid-base balanceDead animal preservationVitamin injectionPeroxidase
The invention discloses a mesenchymal stem cell self-preserving liquid which is prepared from the following raw materials according to volume ratios: 1-6% of self platelet lysate, 95-97% of solution medium and 0-1% of vitamin C injection, wherein the solution medium is a mixed sugar electrolyte injection or physiological saline injection. The mesenchymal stem cell self-preserving liquid has the advantages that: growth factors contained in the mesenchymal stem cell self-preserving liquid can maintain the activity state of cells, the vitamin C can maintain the activity of various peroxidases, at the same time, the mixed sugar electrolyte injection is especially suitable for cell preservation, and osmotic pressure is also favorable for nutrient absorption of the cells so as to carry out metabolism; the activity of the mesenchymal stem cells which are preserved in the mesenchymal stem cell self-preserving liquid within 8 hours is still larger than 90%, the cell activity change is not larger than 7%, and the activity of the mesenchymal stem cells which are preserved in the mesenchymal stem cell self-preserving liquid within 12 hours is still maintained at 85%; and by using the mesenchymal stem cell self-preserving liquid, the requirements for human intravenous back-transfusion safety can be met, and the cell preserving problem of long-time transport is solved, thus more patients have opportunity to undergo mesenchymal stem cell treatment.
Owner:CHENGDU QINGKE BIOTECH
Preparation method of polyinosinic acid-polycytidylic acid lyophilized powder injection
InactiveCN102988303ASlow down the rate of oxidative degradationLong validity periodOrganic active ingredientsPowder deliverySide effectOrganic chemistry
The invention discloses a preparation method of a polyinosinic acid-polycytidylic acid lyophilized powder injection. The method comprises the preparation of a polyinosinic acid-polycytidylic acid solution and a preparation of a lyophilized powder injection thereof. According to the invention, proper amounts of polyinosinic acid and polycytidylic acid are respectively dissolved by using normal saline; the mixtures are mixed and stirred, such that the polyinosinic acid-polycytidylic acid solution is prepared; a lyophilization additive is added and well mixed; and filtering, sub-packaging, and lyophilization are carried out. The prepared injection is suitable for frost preservation under a temperature below 0 DEG C. Therefore, expiration date is postponed, oxidative degradation speed is reduced, toxic and side effect are reduced, and stability is improved.
Owner:天津泽世德生物医药有限公司
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
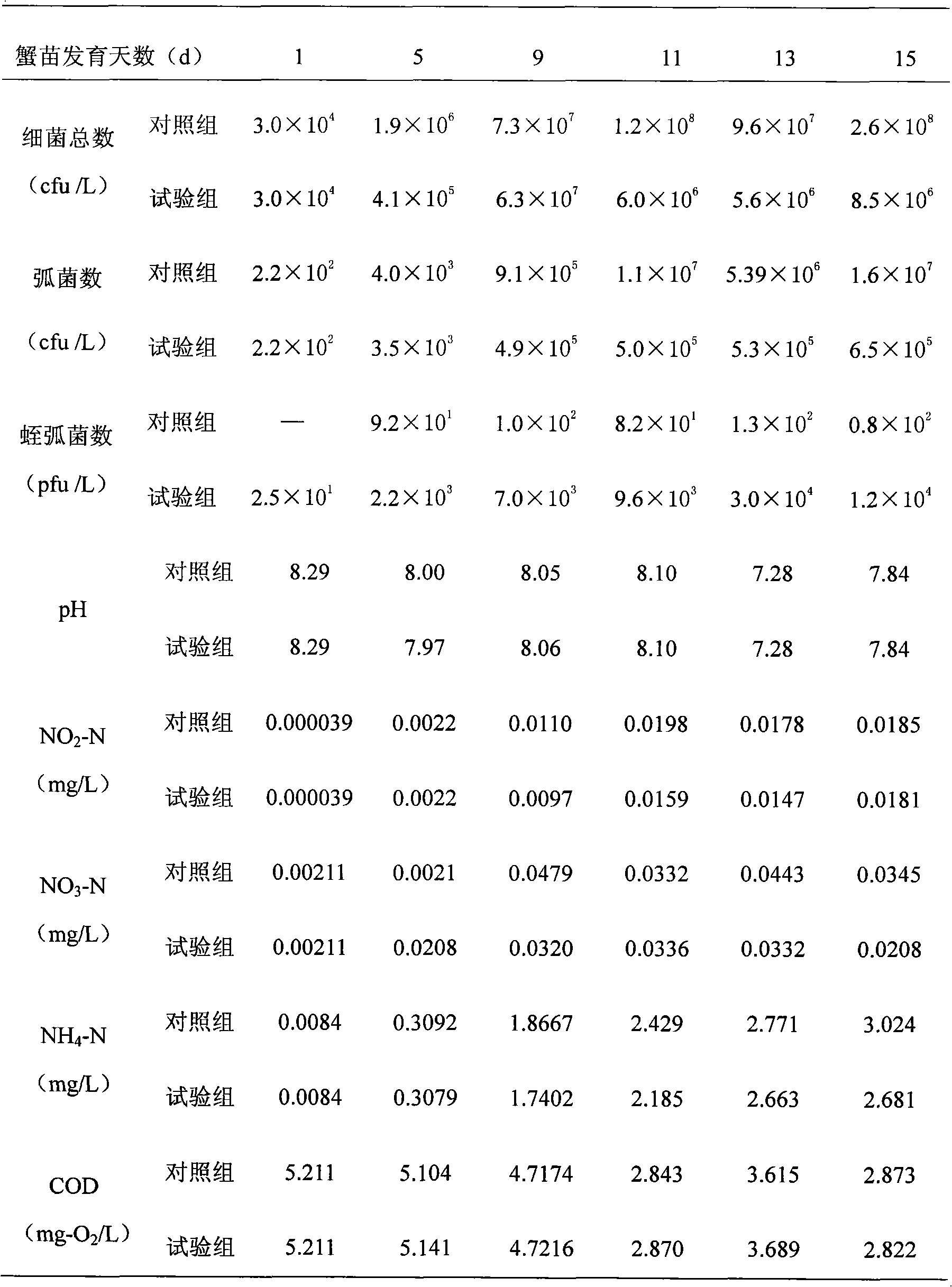
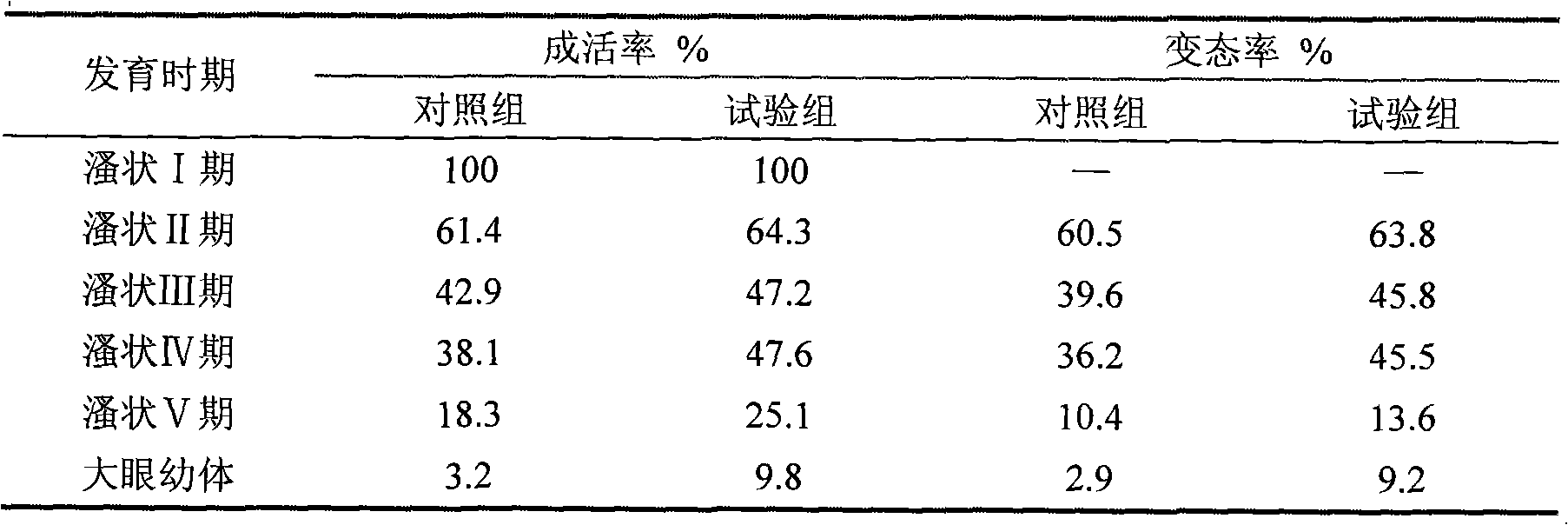
Preparation method and application of sea bdellovibrio bacteriovorus ecological preparation
InactiveCN101781627AGuaranteed lytic activityPromote lysisBiocideBacteriaNutrient brothCulture mediums
The invention provides a preparation method and application of a sea bdellovibrio bacteriovorus ecological preparation, and relates to a sea microbial ecological preparation and a preparation method of a bdellovibrio bacteriovorus preparation for mariculture. The preparation method comprises the following steps of: preparing a host bacteria suspension; separating and purifying bdellovibrio bacteriovorus; and propagating and culturing bdellovibrio bacteriovorus. The preparation method is characterized in that after pseudomonas stutzeri is connected with an incline and is activated, adding physiological saline in a test tube to prepare the bacterial suspension; then propagating and culturing with nutrient bouillon; adding a flocculating agent in the obtained bacterial suspension; standing and precipitating after uniformly shaking; adding the physiological saline to obtain 1012 cfu / mL of host bacteria suspension; separating and purifying the bdellovibrio bacteriovorus of collected sea and bottom sediment samples with a double agar plate method; pouring culture media containing the host bacteria suspension and the collected samples into sea agar in the lower layer for culture after uniformly mixing; and adding the host bacteria and the bdellovibrio bacteriovorus obtained by separation into sterilized natural sea, and culturing until the concentration of the bdellovibrio bacteriovorus is 108 to 109 pfu / mL for future use.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Hyperthermia, system, method and components
ActiveUS20090043256A1Effective and safe for patientInfusion devicesMedical devicesIntensive care medicineHigh body temperature
An IV pole mountable, therapeutic infusate processing device is incorporated into a hypothermia system to receive therapeutic fluid(s), such as normal saline, peritioneal dialysis solution, or other crystalloid solution, to heat such therapeutic fluid(s) a few degrees centigrade above normal body temperature and to direct the resulting heated infusate to and through a selected anatomical portion of a patients body to raise the temperature of that body portion so as to affect any cancerous or other tumors that may be located therein. The processing device is provided with touch screen controls and visual indicators to facilitate its proper use; while the system further includes temperature and pressure sensors to monitor the hyperthermia processing to insure patient safety.
Owner:BELMONT INSTR LLC
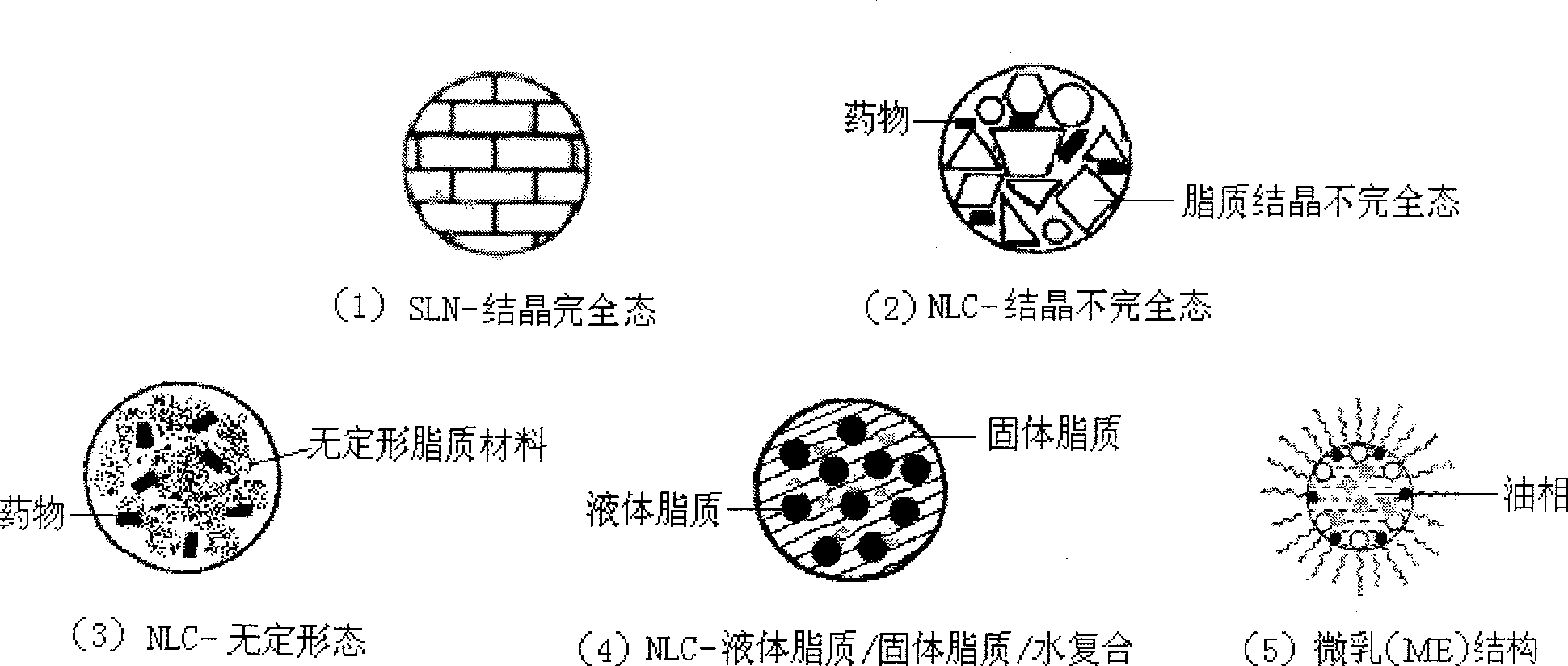
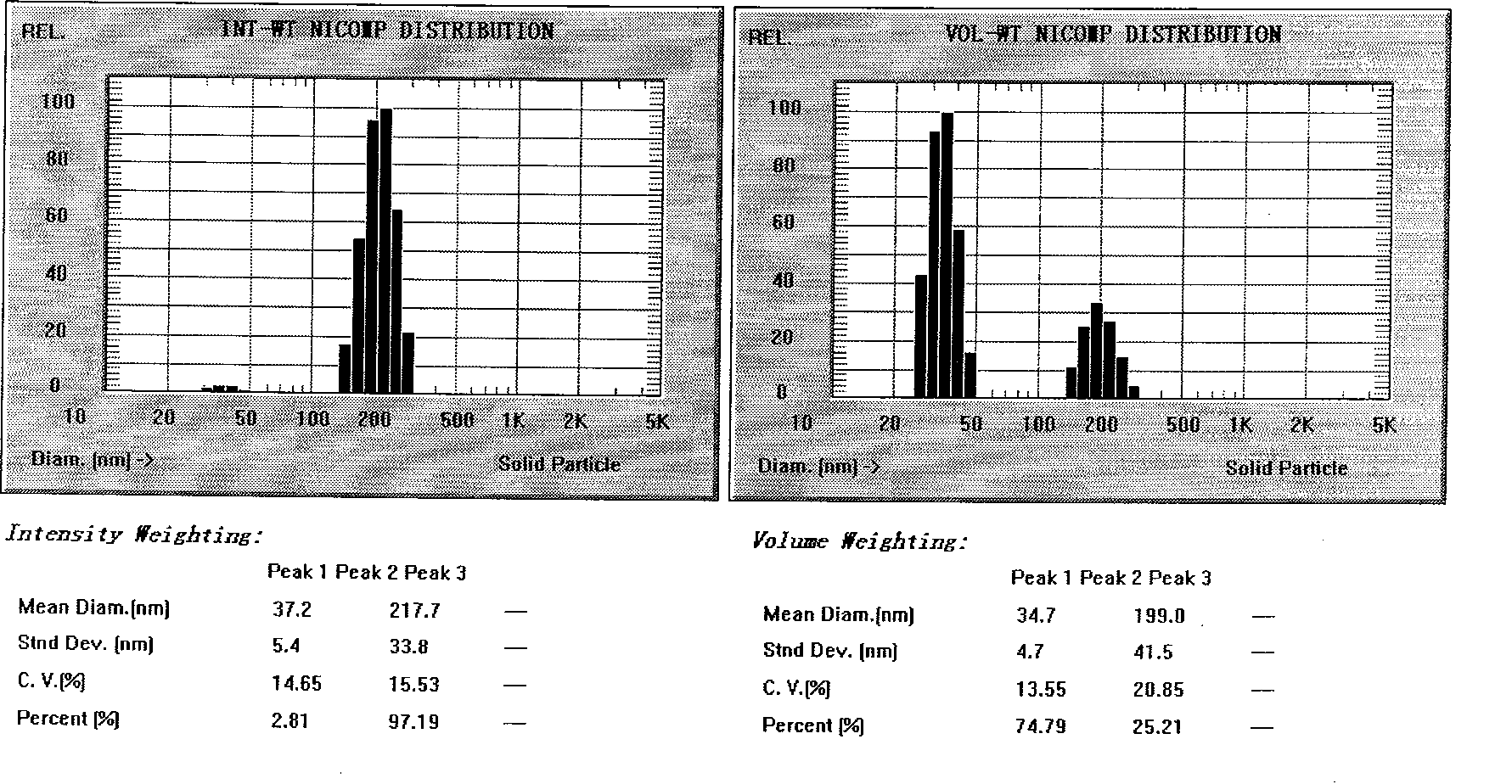
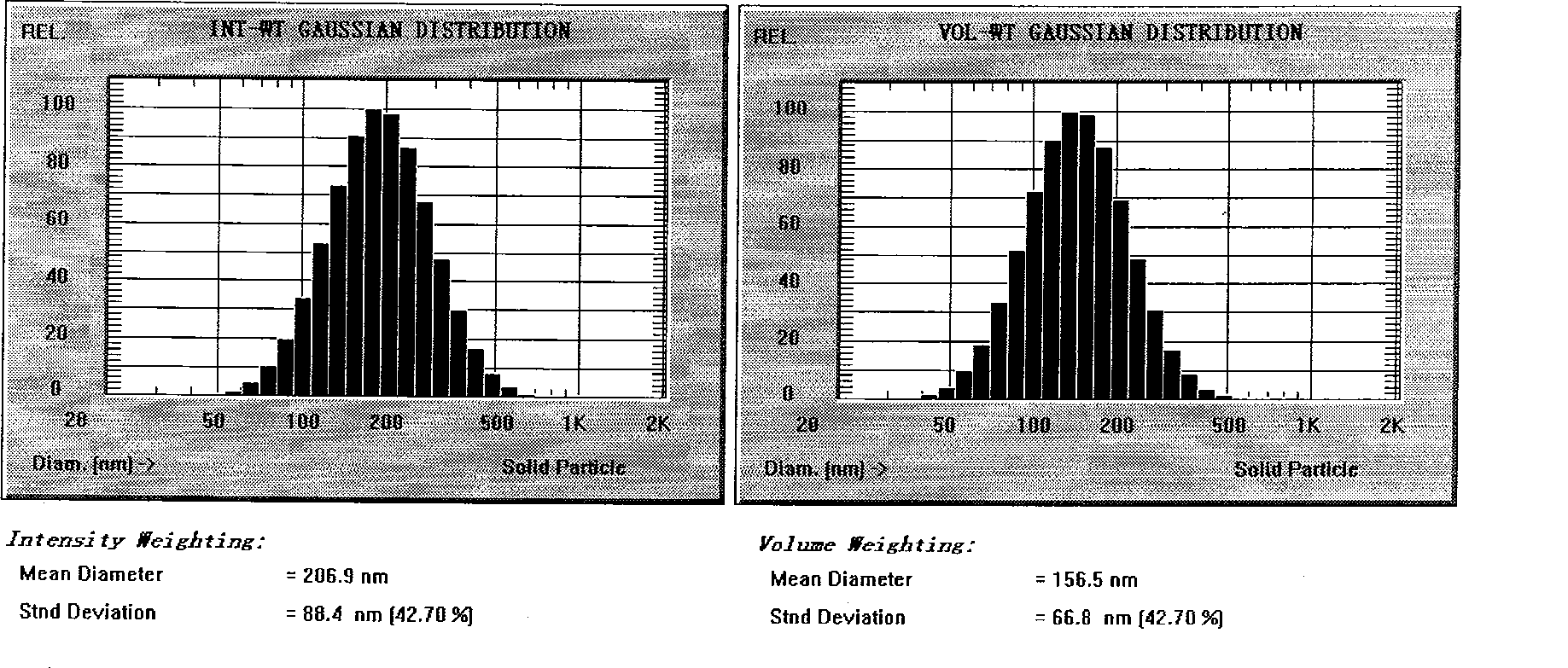
Novel nano-lipid carrier for injection embodying paclitaxel series substances and preparation method thereof
InactiveCN101366697AImprove complianceDoes not cause toxic reactionsOrganic active ingredientsAntineoplastic agentsLipid formationFreeze-drying
The invention provides a novel nanometer lipid carrier containing a taxol substance for injection and a method for preparing the same. Mixed lipids are used as materials; liquid lipid and solid lipid with different physical states are mixed according to the proportion of between 0 and 100 percent to obtain the nanometer lipid carrier (NLC) which is prepared by a mixed solid-liquid substrate and has different crystalline states; and microemulsion (ME) prepared by a full liquid substrate and solid lipid nanoparticles (SLC) prepared by a full solid substrate are also obtained. The new nanometer lipid carrier can increase or improve the encapsulation efficiency and the release property of drug and has the characteristics of high drug-loading amount, good stability and low toxicity. The taxol substance, a carrier material, a surfactant and injection water are respectively stirred to form two phases of oil and water; the oil phase and the water phase are mixed and emulsified to obtain primary emulsion; further taxol substance nanometer lipid carrier injection or suspension type injection which meets the requirement of intravenous injection is prepared by a high-pressure homogenization process; or a protective agent is added into the injection to prepare solid powder for injection by vacuum freeze drying or spray drying; before use, normal saline, a glucose solution or a Ringer solution is used for dilution; and the solid powder can be rapidly dissolved. Experiments show that the prepared nanometer lipid carrier containing the taxol substance for injection can improve targeting property to tumor cells, improve curative effect, greatly reduce toxic and side effects, has simple preparation process and low cost and is suitable for large-scaled industrialized production.
Owner:李淑斌
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com