Modified starch absorbable hemostasia material and preparation method thereof
A technology of modified starch and hemostatic materials, applied in the direction of absorbent pads, pharmaceutical formulations, applications, etc., can solve the problems of affecting the hemostatic effect, low viscosity, difficult to achieve hemostasis, etc., to promote wound tissue healing, good biocompatibility, The effect of improving the hemostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] A modified starch absorbable hemostatic material, including carboxymethyl starch, which is made from native starch (potato starch) through etherification and denaturation to make carboxymethyl starch. The carboxymethyl starch raw material is placed in a boiling machine at 40-50°C , Adding distilled water, coagulating, pelletizing, and sieving to prepare hemostatic material 66# (manufacturer Starch Medical Inc. batch number 070717, degree of substitution is 2 to 4). The molecular weight of the carboxymethyl starch 66# product is 15,000-2,000,000, and the particle size is 10-1000μm. Among them, starch particles with a particle size of 30-500μm account for not less than 95% of the total starch particles. More preferably, the particle size is less than 95%. The viscosity of the 6.67% starch solution at 50~250μm at 37℃ is 557.9mPa·s, and the viscosity work of the modified starch when water is saturated at room temperature is 68.1g·mm.
Embodiment 2
[0105] A modified starch absorbable hemostatic material, including hydroxyethyl starch, which is prepared from native starch (potato starch) through etherification and denaturation to produce hydroxyethyl starch. The raw material of hydroxyethyl starch is placed in a boiling machine at 40-50°C. Distilled water was added, polymerized, pelletized, and sieved to prepare hemostatic material 88# (manufacturer Starch Medical Inc. batch number 071122). The molecular weight of the hydroxyethyl starch 88# product is 15,000-2,000,000, and the particle size is 10-1000μm. Among them, starch particles with a particle size of 50-500μm account for not less than 95% of the total starch particles. More preferably, the particle size is less than 95%. The viscosity of the 6.67% starch solution at 50~250μm at 37℃ is 30.6mPa·s, and the viscosity work of the modified starch when water is saturated at room temperature is 75.2g·mm.
[0106] The water absorption performance of the invention is measured by...
Embodiment 3
[0136] A biocompatible modified starch used for hemostasis, including cross-linked carboxymethyl starch, which is made from original starch (potato starch) through etherification and cross-linking and denaturation to make cross-linked carboxymethyl starch, raw material of cross-linked carboxymethyl starch Place it in a boiling machine at 40-50°C, add distilled water, polymerize, pelletize, and sieve to make cross-linked carboxymethyl starch hemostatic material 66#+ (manufacturer StarchMedical Inc. batch number 071108). The cross-linked carboxymethyl starch 66#+ product has a molecular weight of 15,000-2,000,000 and a particle size of 10-1000μm. Among them, starch particles with a particle size of 50-500μm account for not less than 95% of the total starch particles.
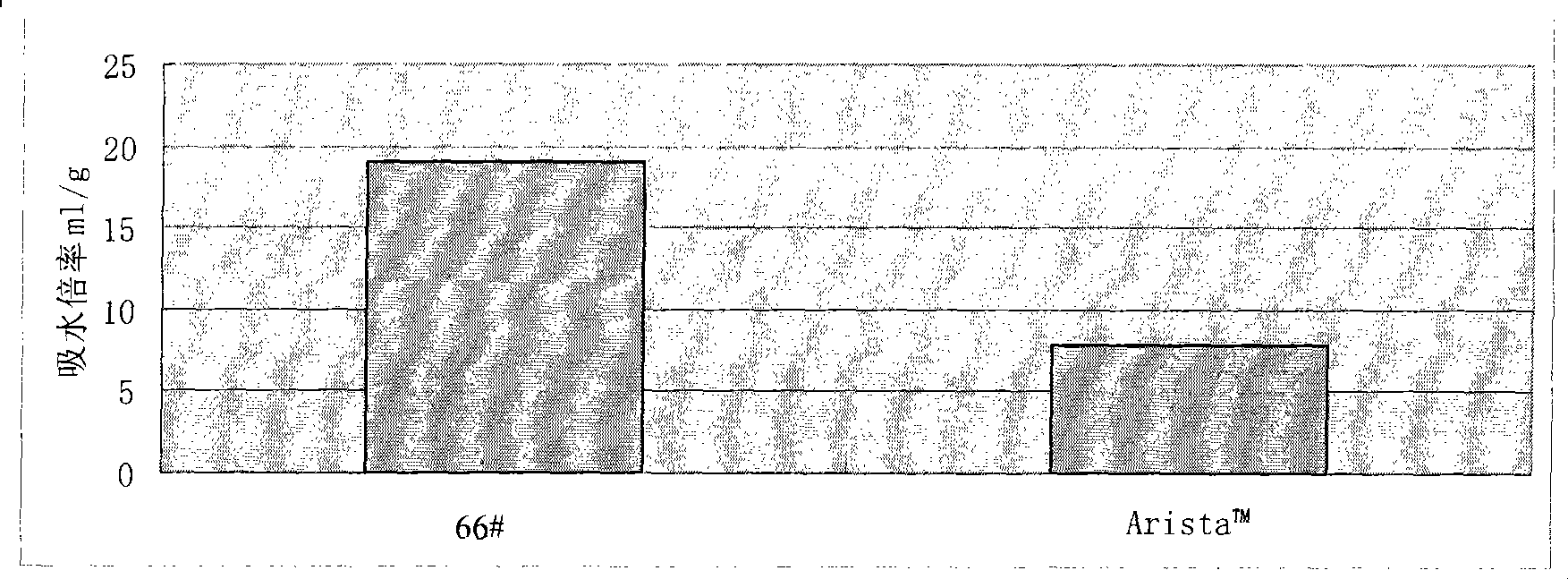
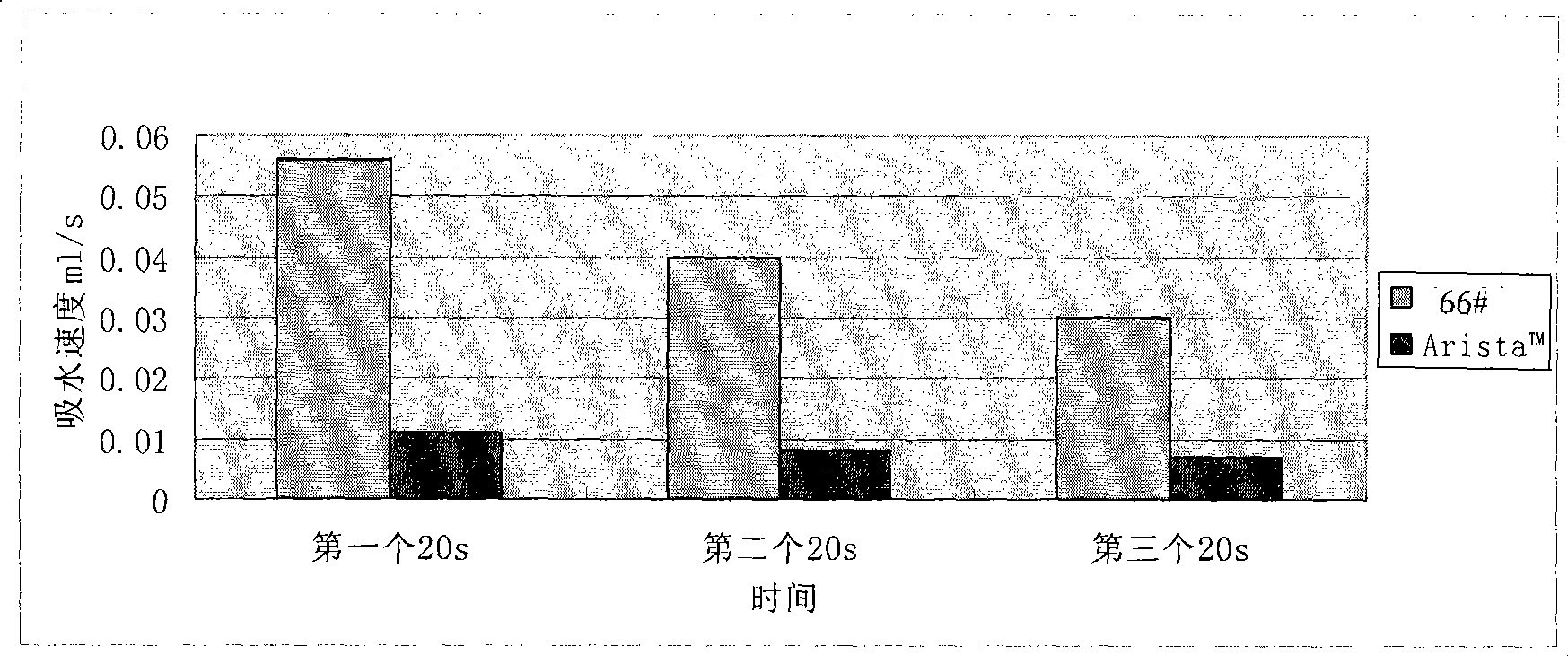
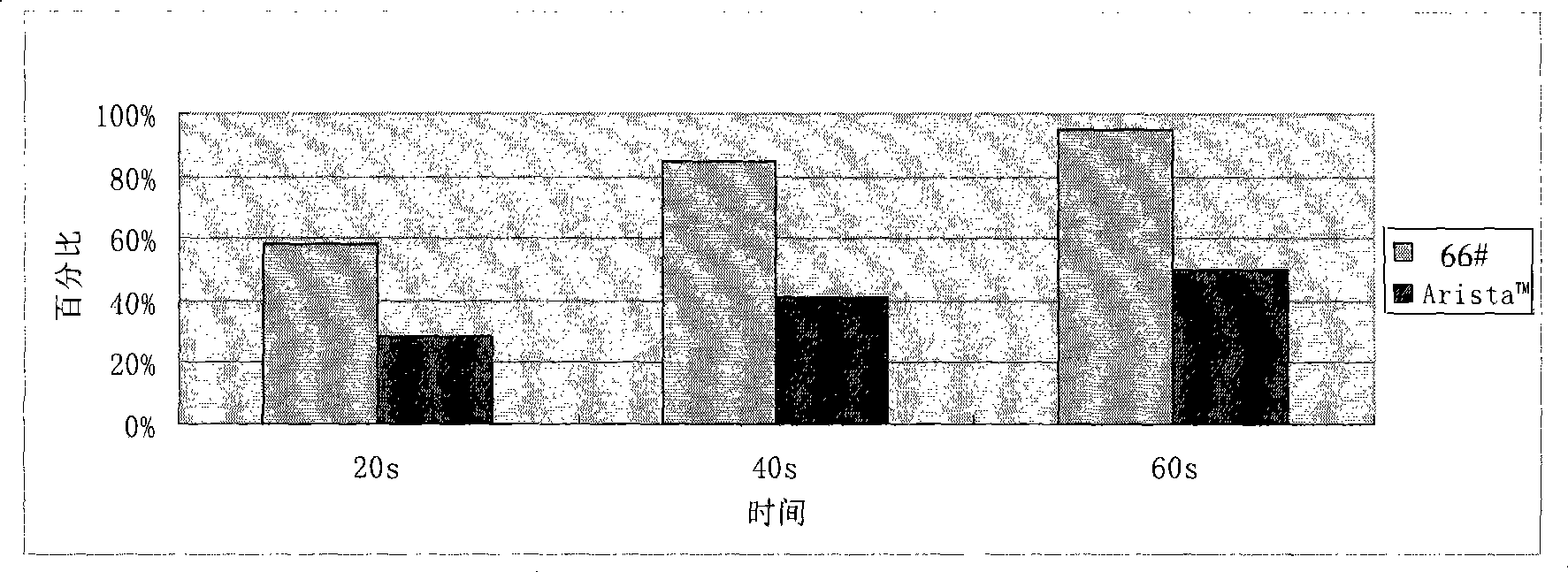
[0137] For each modified starch and Arista in Examples 1, 2, and 3 of the present invention TM Hemostatic powder (medafor, USA) was subjected to centrifugal method to determine the water absorption rate, and the resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com