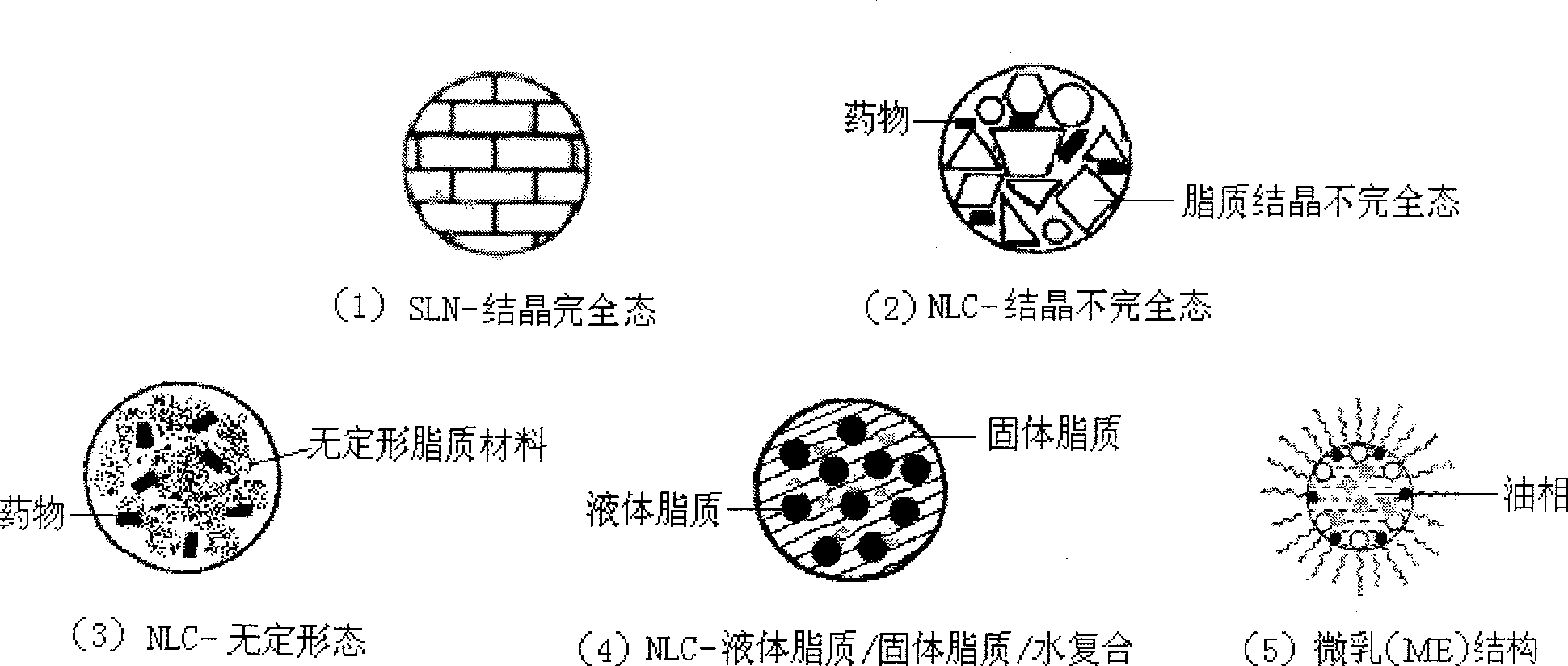
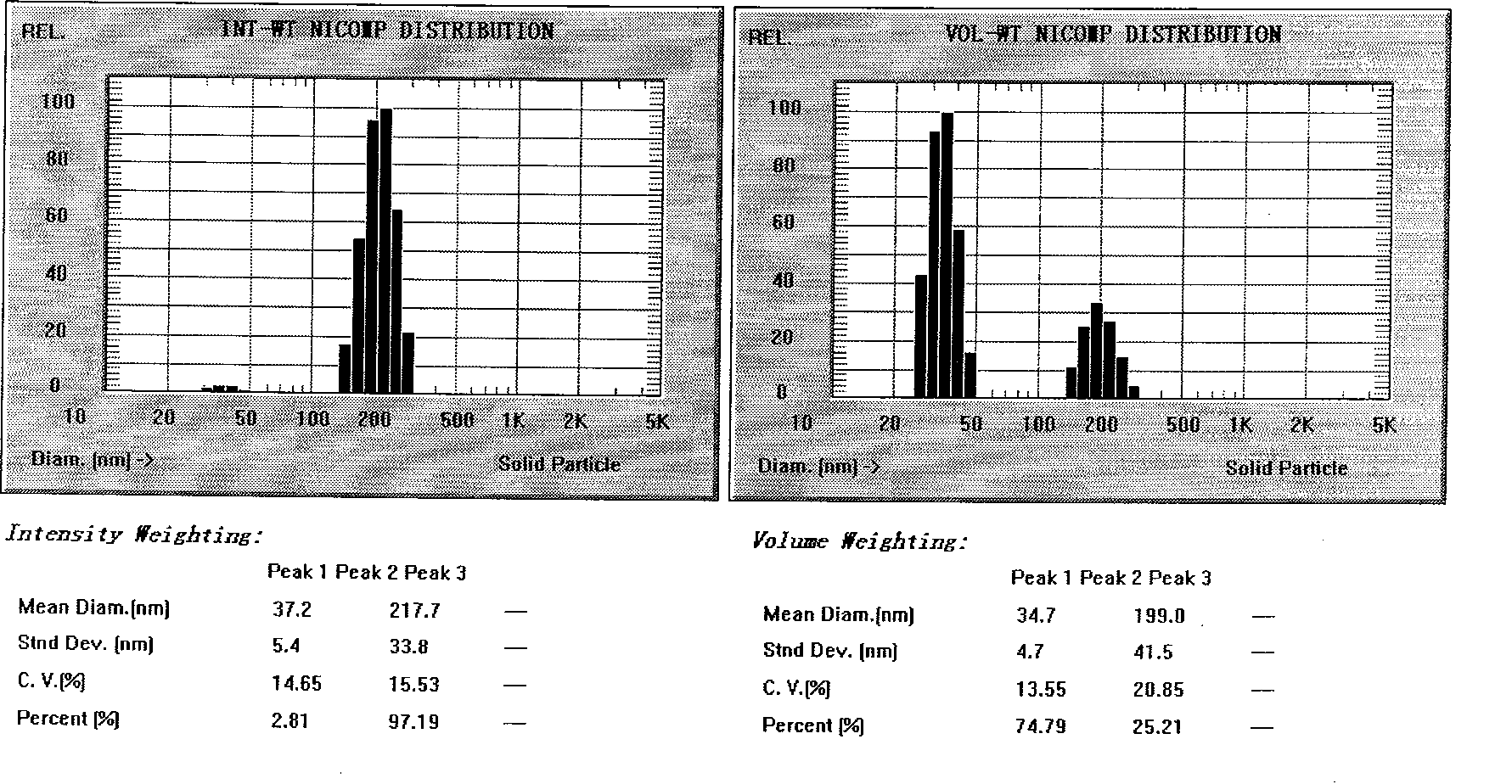
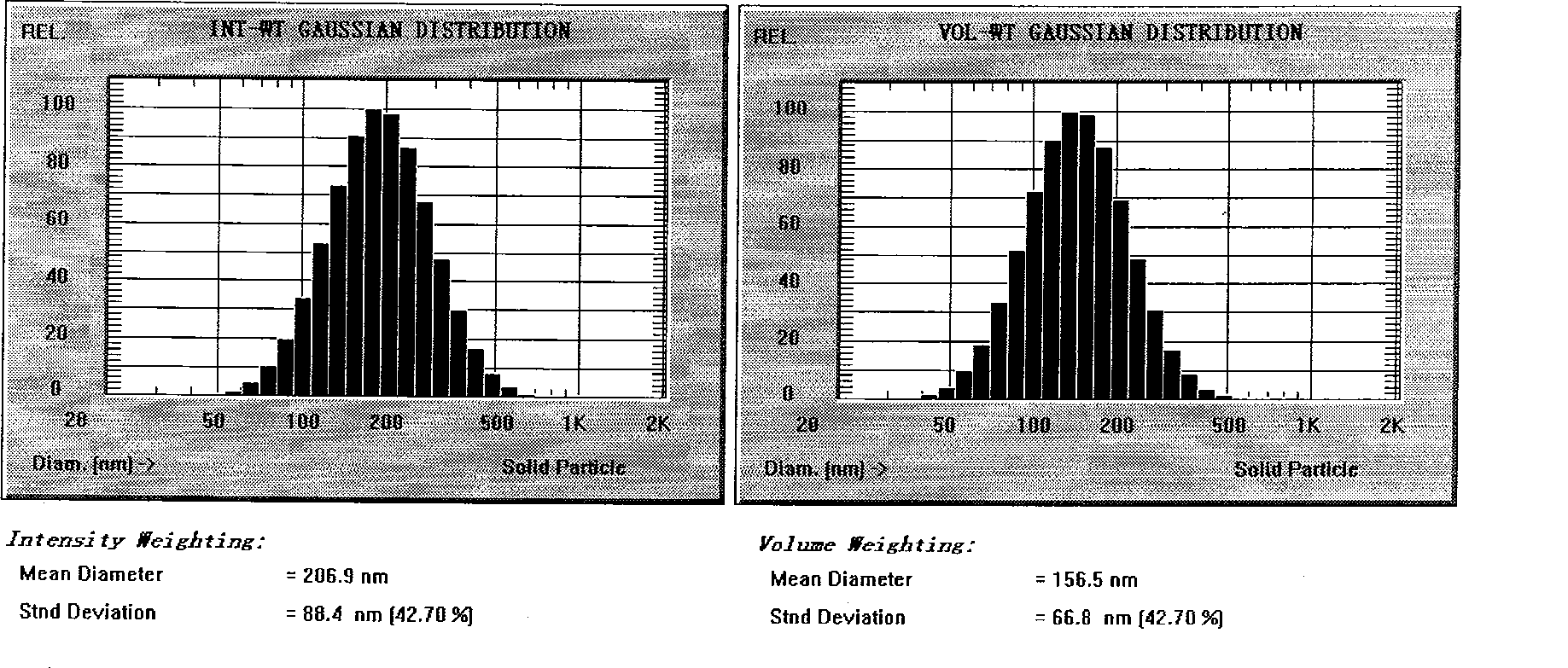
Novel nano-lipid carrier for injection embodying paclitaxel series substances and preparation method thereof
A nano-lipid carrier, paclitaxel technology, applied in the field of medicine, can solve problems such as limited patents, and achieve the effects of improving release properties, increasing drug loading, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Weigh 10 g of triolein, 90 g of safflower oil, 20 g of soybean lecithin, and 5 g of docetaxel in a water bath, heat and stir to melt, and keep the temperature at 70°C as the oil phase; (2) add 4 g of Poloxamer188 to Add an appropriate amount of water for injection, heat and stir to dissolve it, and keep the temperature at 70°C as the water phase. Under stirring, the water phase is dropped into the oil phase, and the stirring is continued to form colostrum; (3) the colostrum is homogenized 6 times through 750bar high-pressure emulsification, and the docetaxel nano-lipid carrier suspension can be obtained by rapid cooling and solidification. 0.2% citric acid to adjust the pH to 5.5; or (4) adding 20% glucose to the suspension of docetaxel nano-lipid carrier, and freeze-drying to obtain solid powder of docetaxel nano-lipid carrier for injection.
Embodiment 2
[0058] (1) Weigh 40g of glyceryl trilaurate, 40g of medium-chain oil, 30g of egg yolk phospholipid, 10g of paclitaxel, heat and stir in a water bath to melt, and keep the temperature at 75°C as the oil phase; (2) Weigh polyoxyethylene-8 - Add 7.5 g of caprylic acid / capric glyceride (Labrasol) into an appropriate amount of water for injection, heat and stir to dissolve, and keep the temperature at 75° C. as the water phase. Under stirring, the water phase is dropped into the oil phase, and the stirring is continued to form colostrum; (3) the colostrum is homogenized 5 times through 1000bar high-pressure emulsification, and rapidly cooled and solidified to obtain the docetaxel nano-lipid carrier suspension. Adjust the pH to 4.0 with 0.1 mol / L hydrochloric acid; or (4) add 10% sucrose to the paclitaxel nano-lipid carrier suspension, and freeze-dry to obtain the docetaxel nano-lipid carrier solid powder for injection.
Embodiment 3
[0060] (1) Weigh 62.5g of glyceryl monostearate, 12.5g of soybean oil, 45g of TPGS, 75g of HS-15, 0.1g of oleic acid, and 5g of paclitaxel, heat and stir in a water bath to melt, and keep the temperature at 75°C as the oil phase (2) Weigh 5.0 g of sodium deoxycholate and add it to an appropriate amount of water for injection, heat and stir to dissolve it, and keep the temperature at 75° C. as the water phase. Under stirring, drop the water phase into the oil phase, and continue to stir to form colostrum; (3) the colostrum is homogenized 6 times through 1200bar high-pressure milk, and the paclitaxel nano-lipid carrier suspension can be obtained by rapid cooling and solidification. / L NaOH to adjust the pH to 7.0; or (4) adding 15% mannitol to the paclitaxel nano lipid carrier suspension and spray drying to obtain the paclitaxel nano lipid carrier solid powder for injection. Spray drying conditions: inlet temperature 160°C, outlet temperature 91°C, air volume 100%, flow rate 0.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com