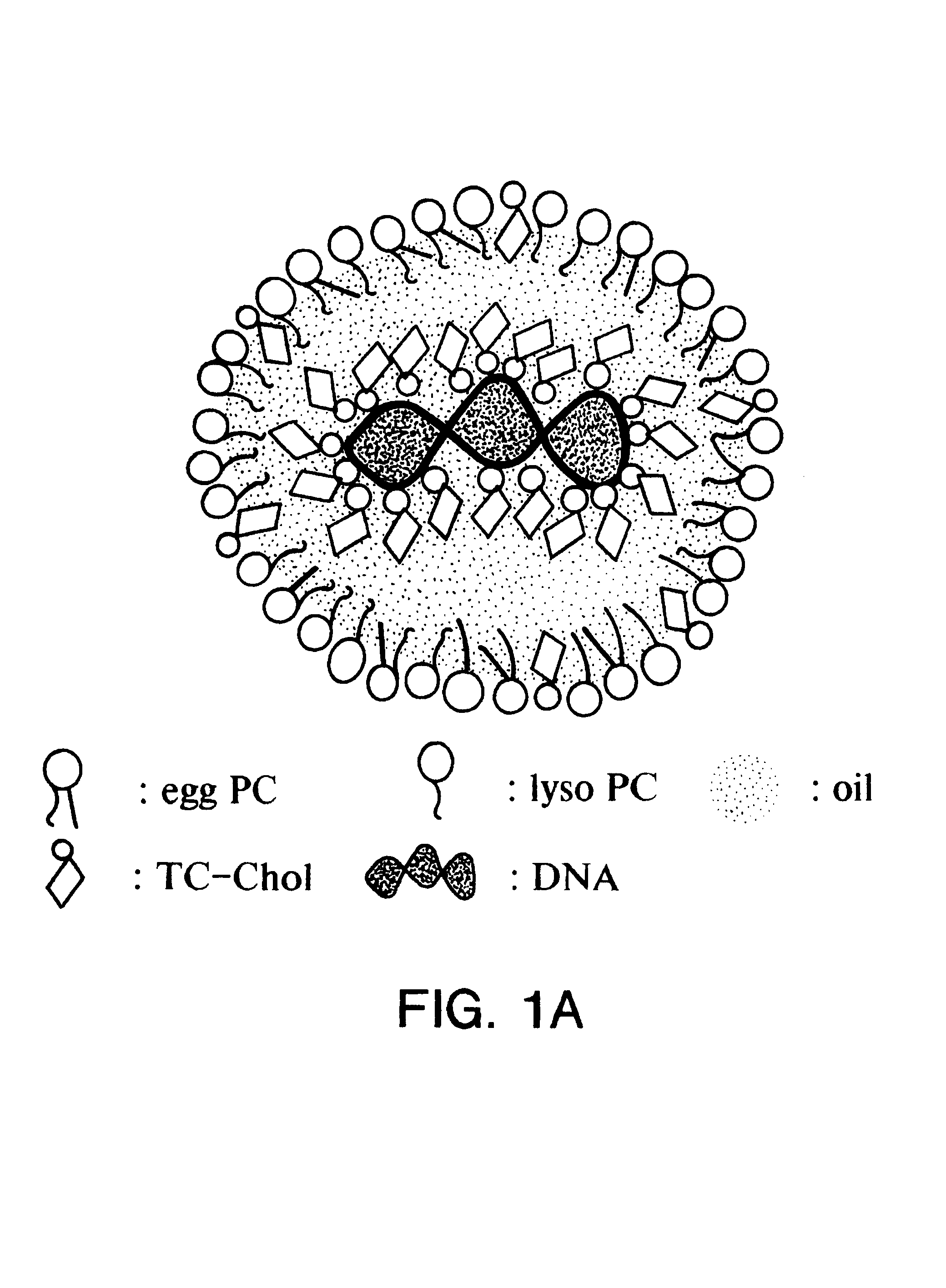
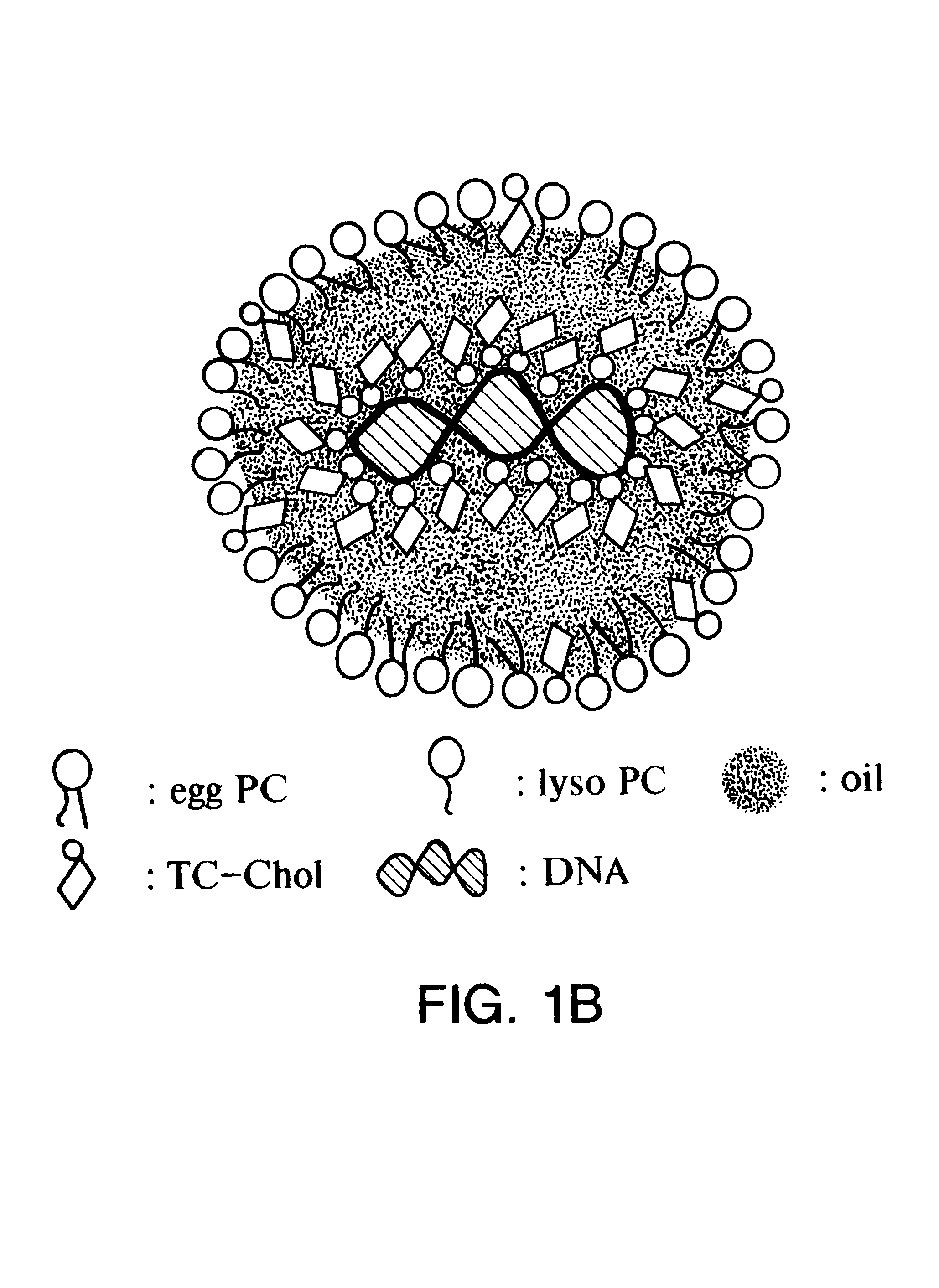
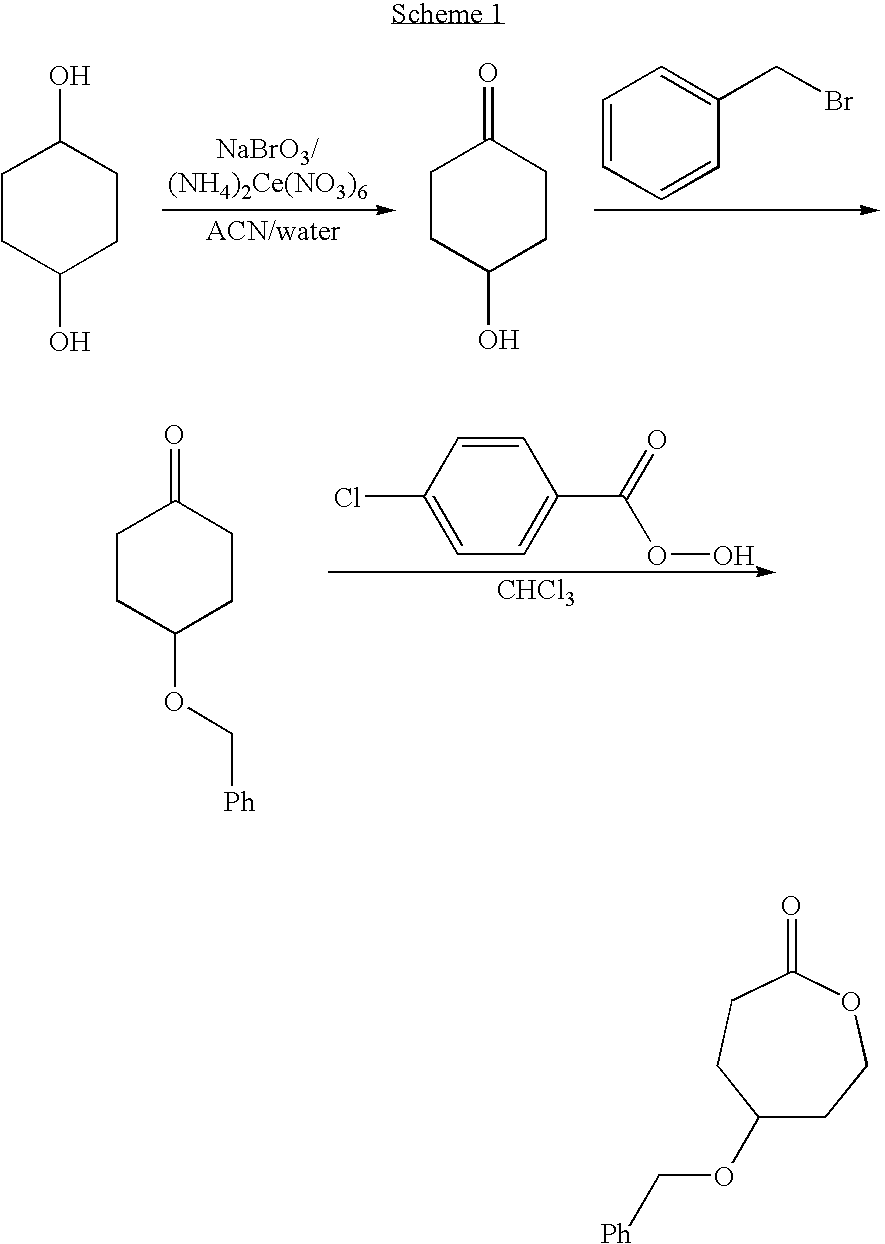
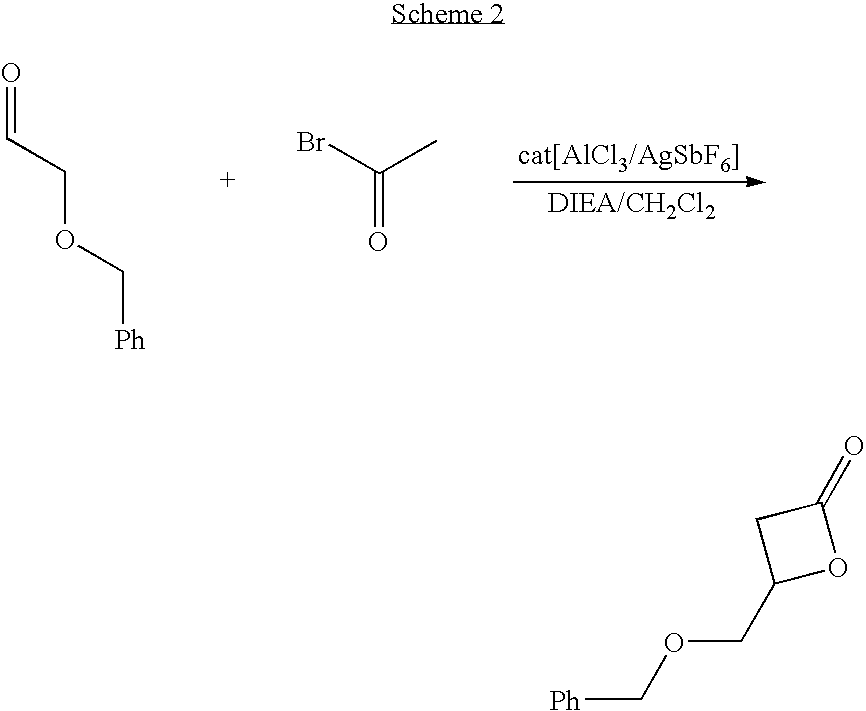
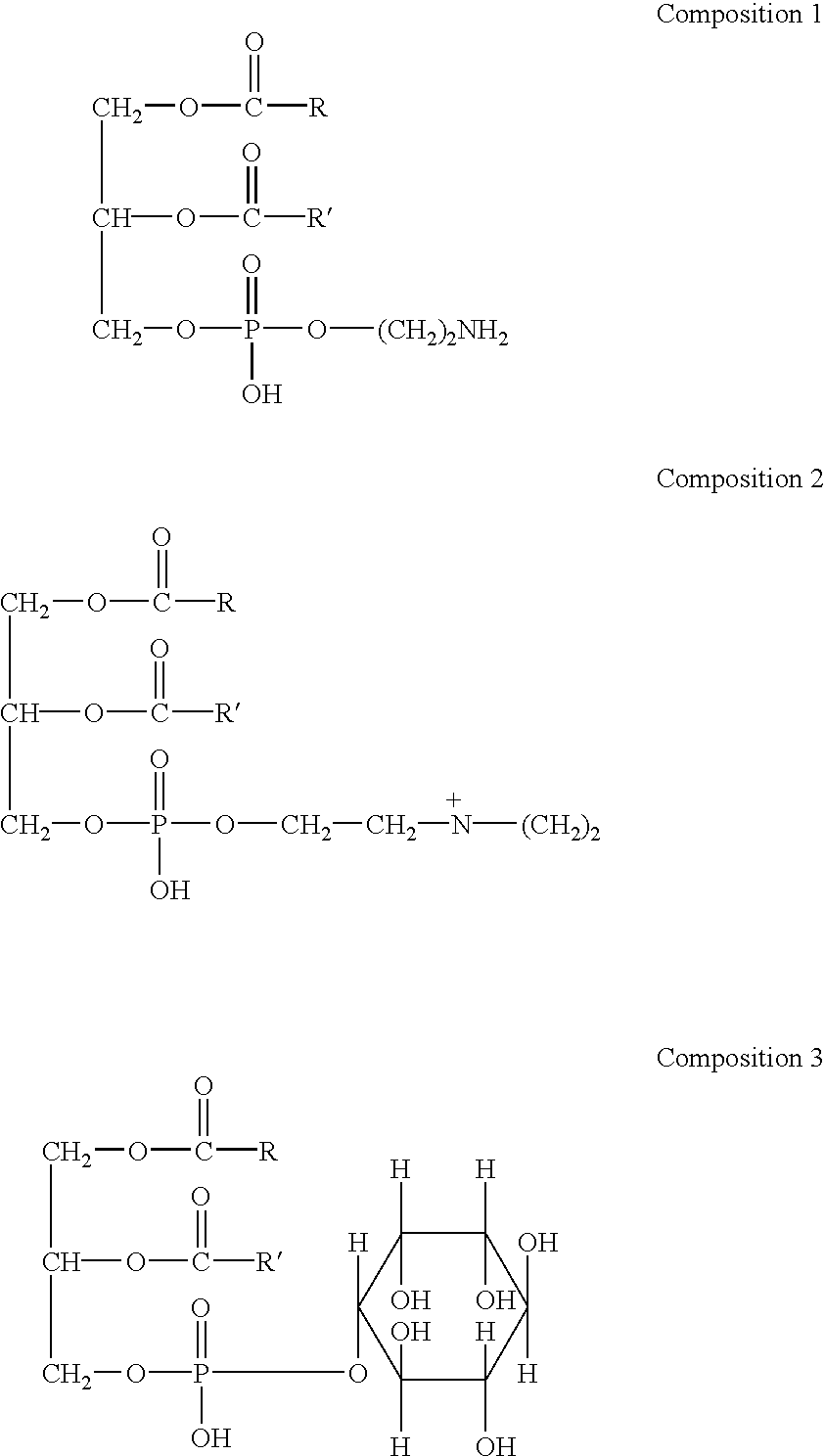Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5966 results about "Phospholipid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
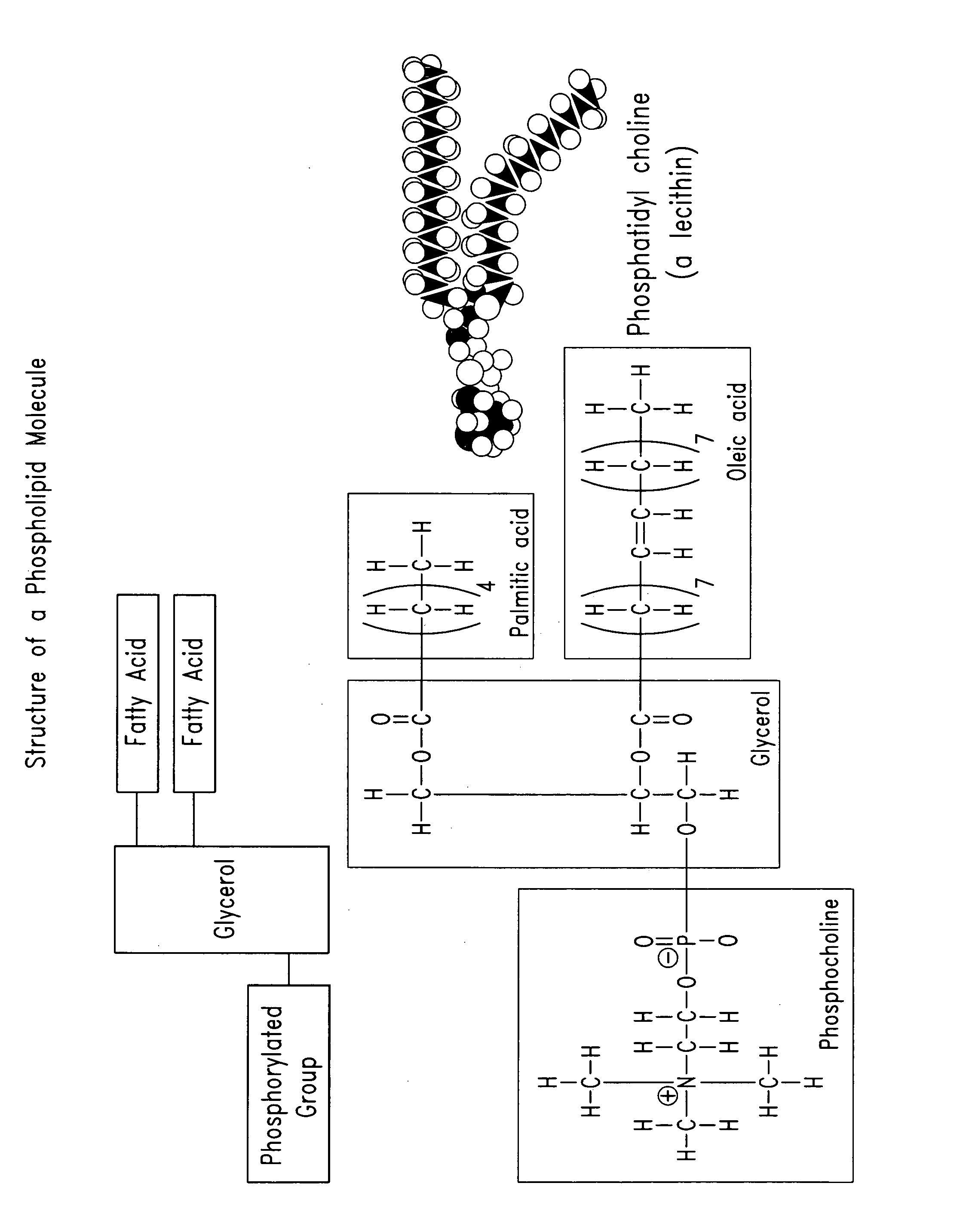
Phospholipids are a class of lipids that are a major component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. The structure of the phospholipid molecule generally consists of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group. The two components are usually joined together by a glycerol molecule. The phosphate groups can be modified with simple organic molecules such as choline, ethanolamine or serine.
Pharmaceutical aerosol composition
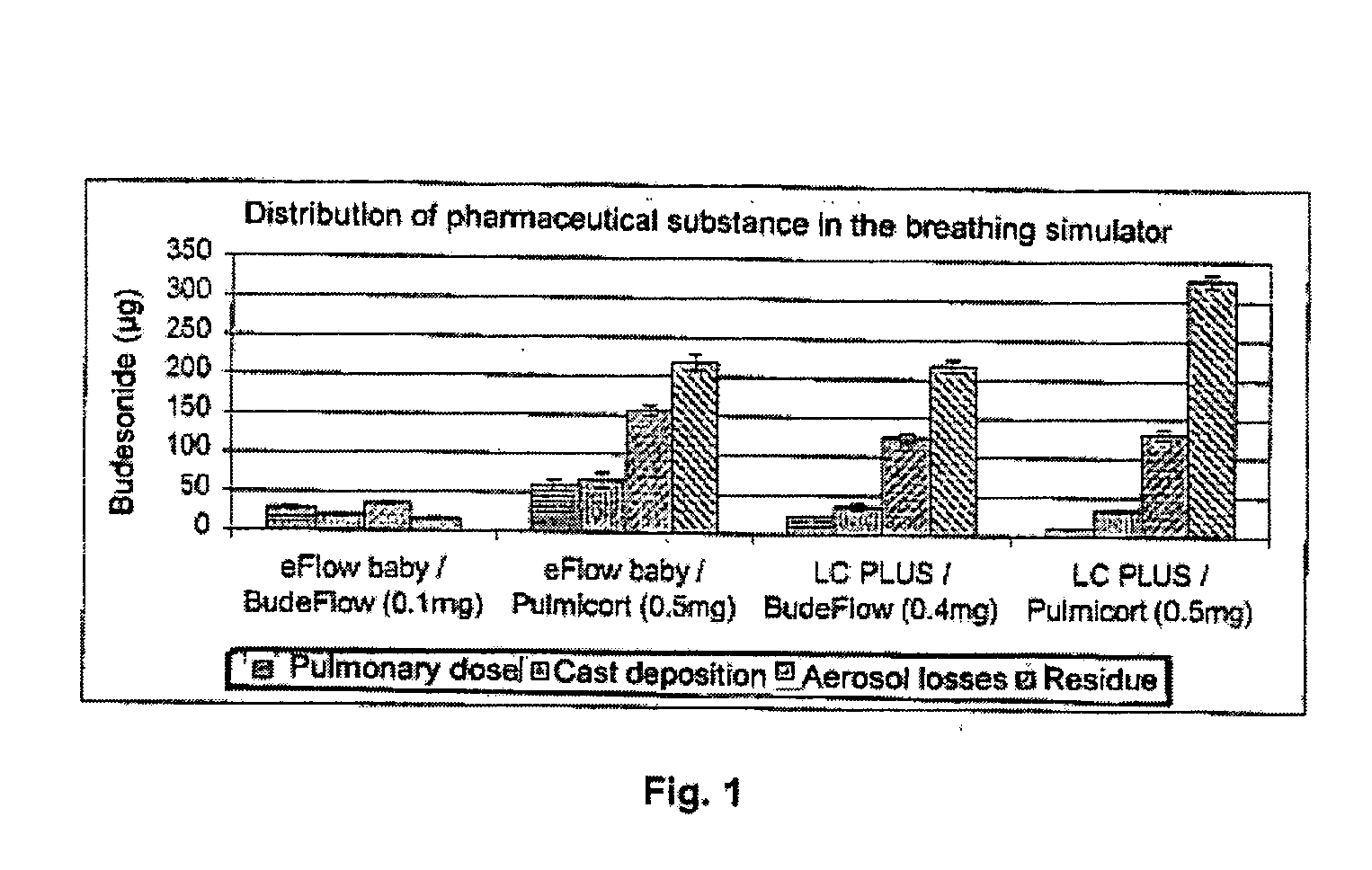
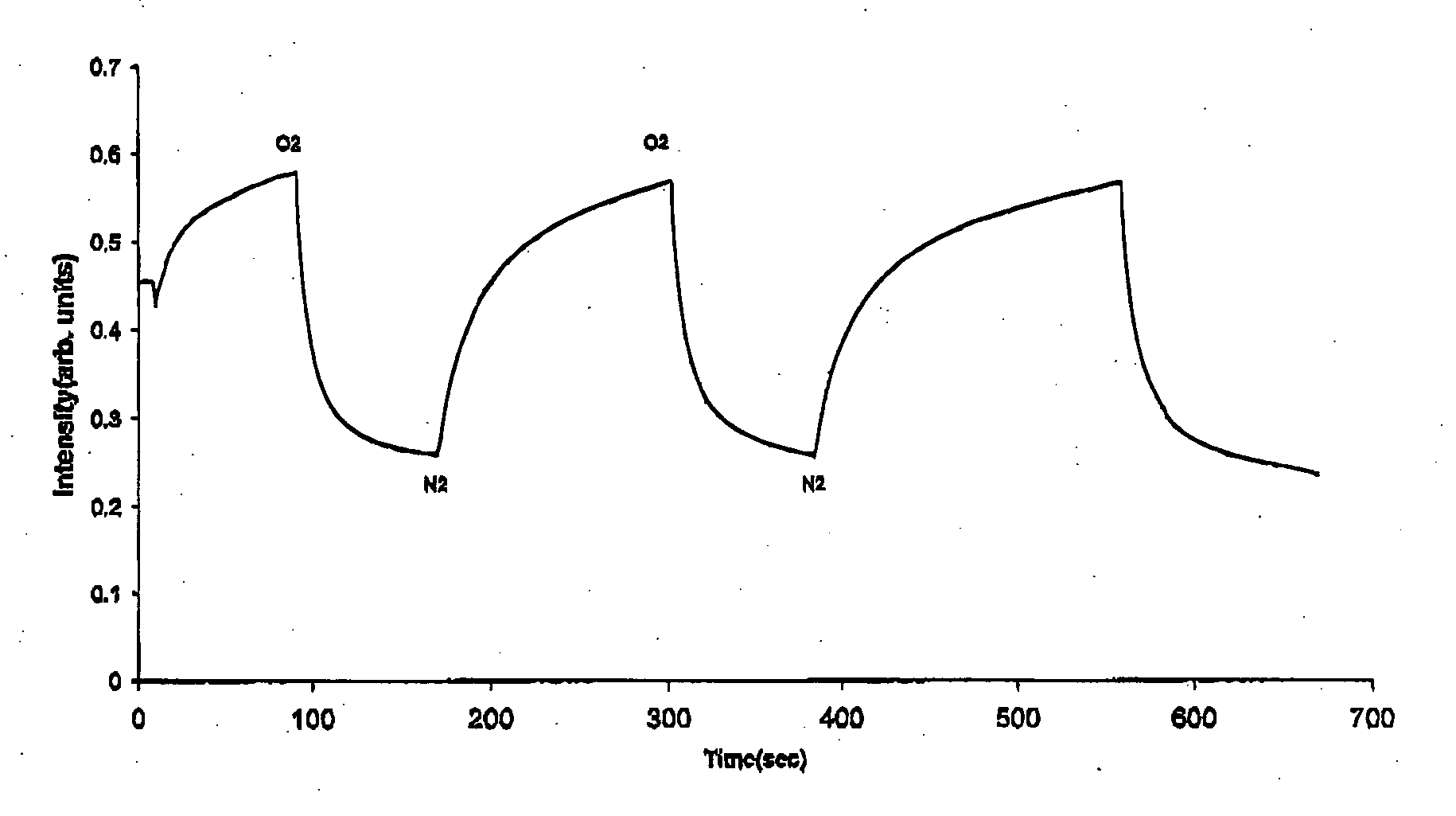
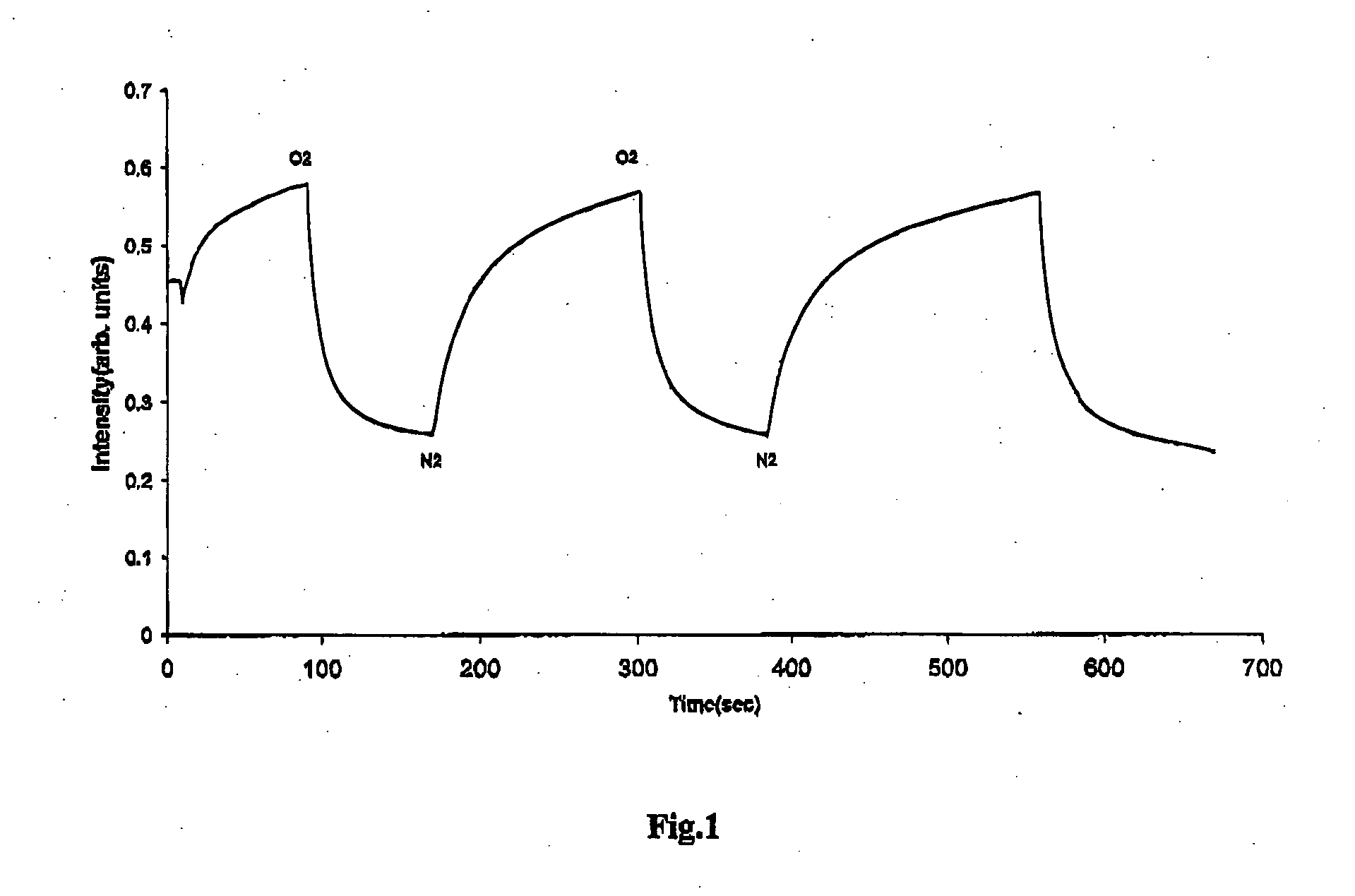
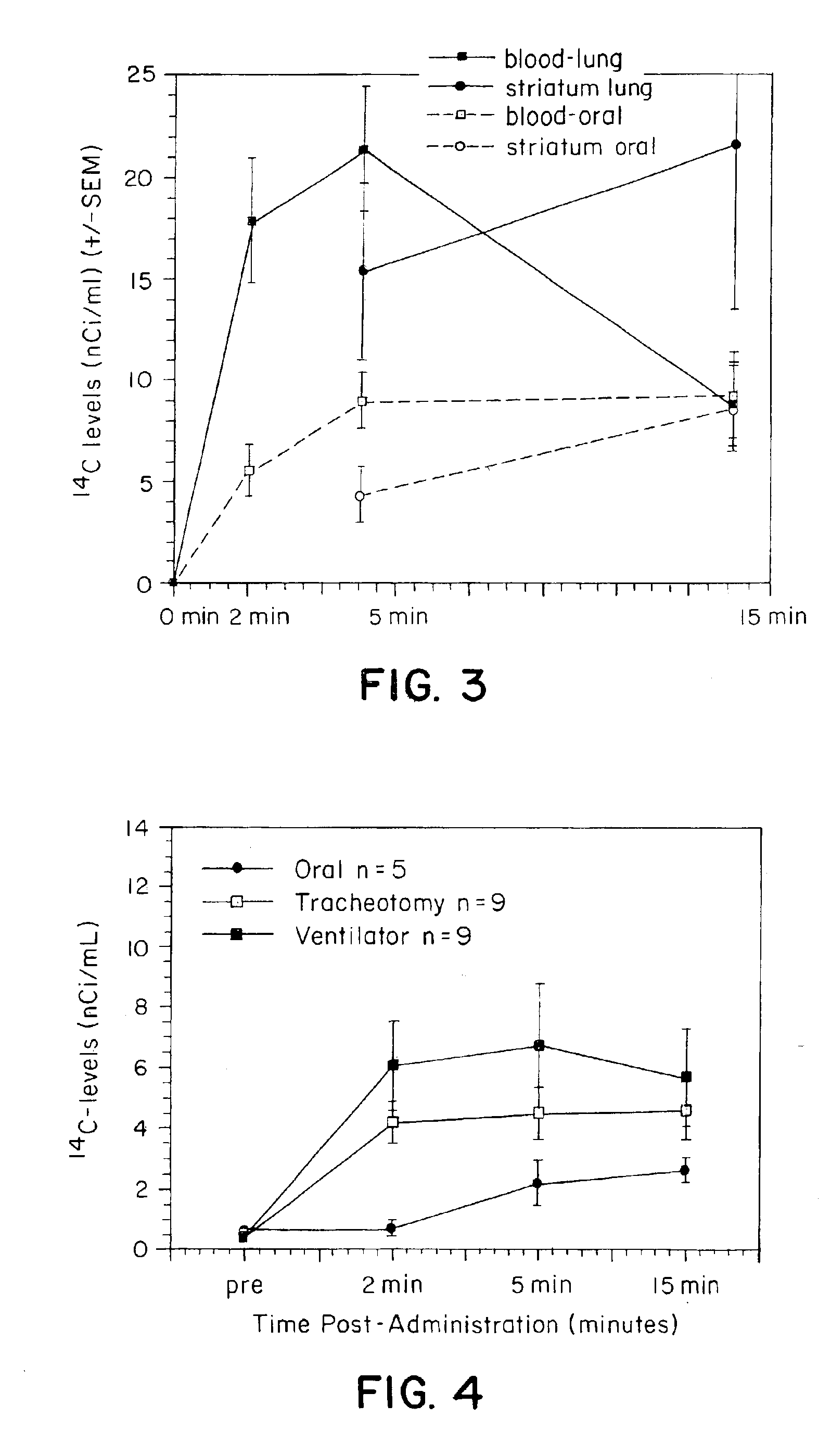
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
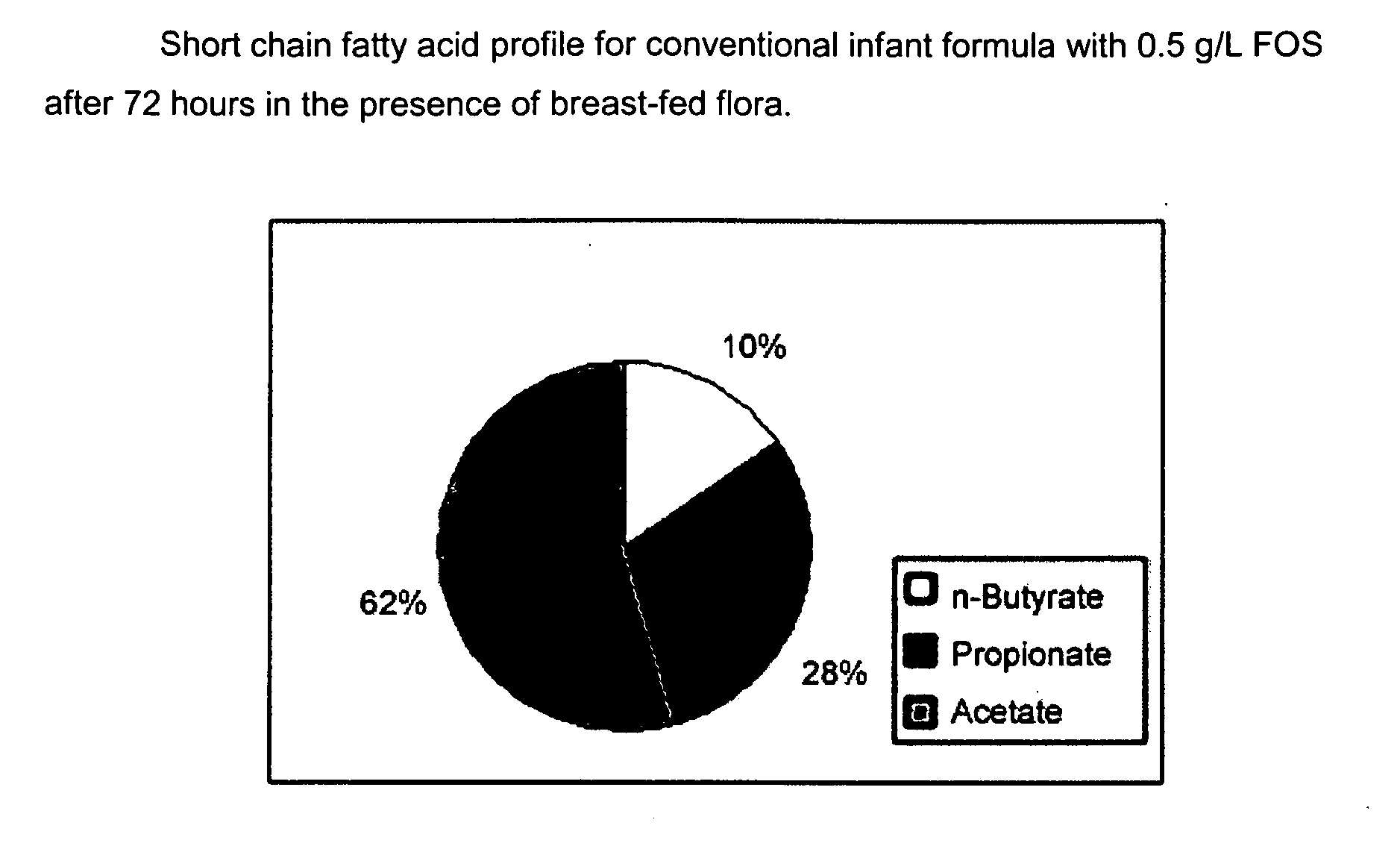
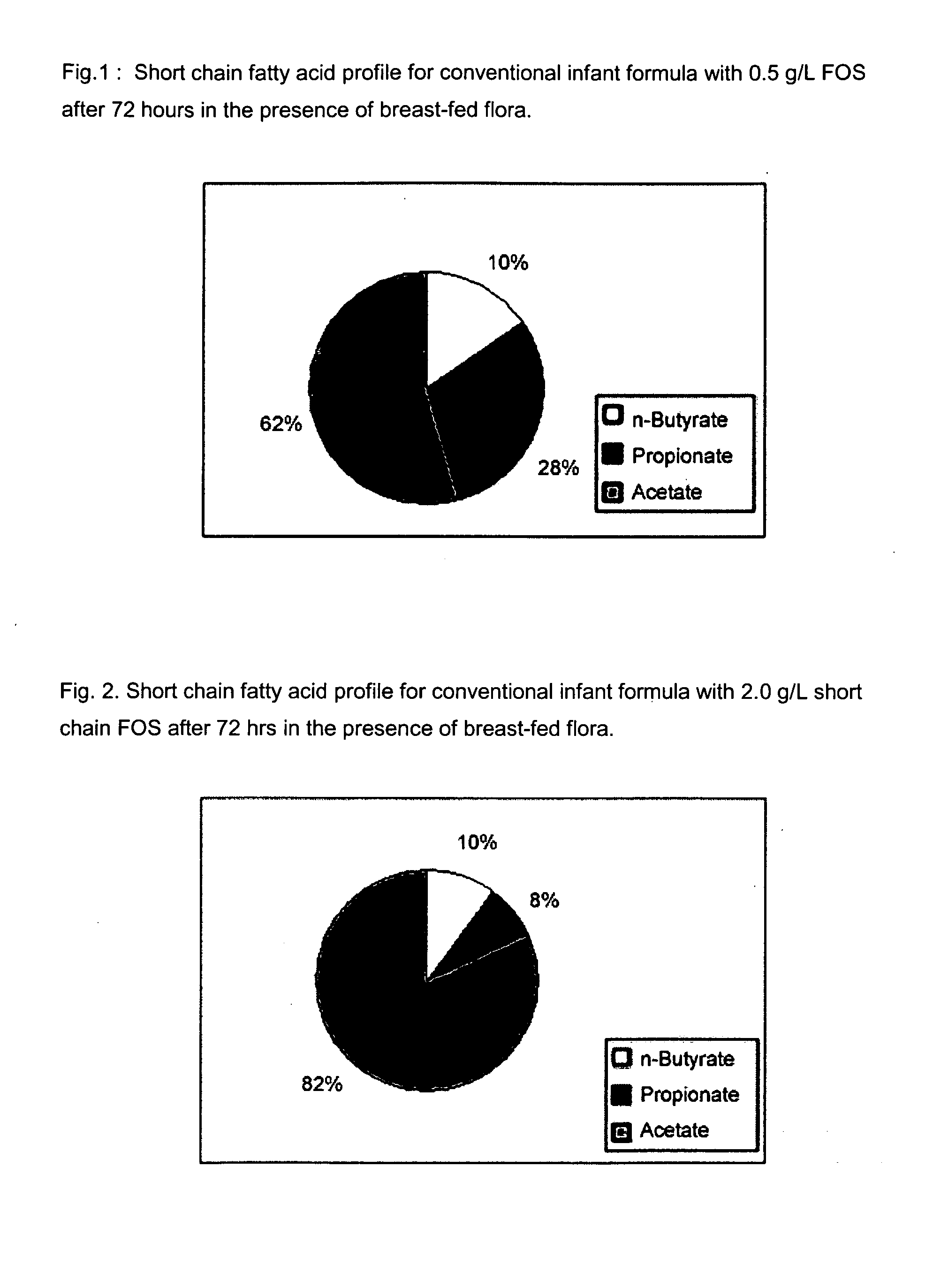
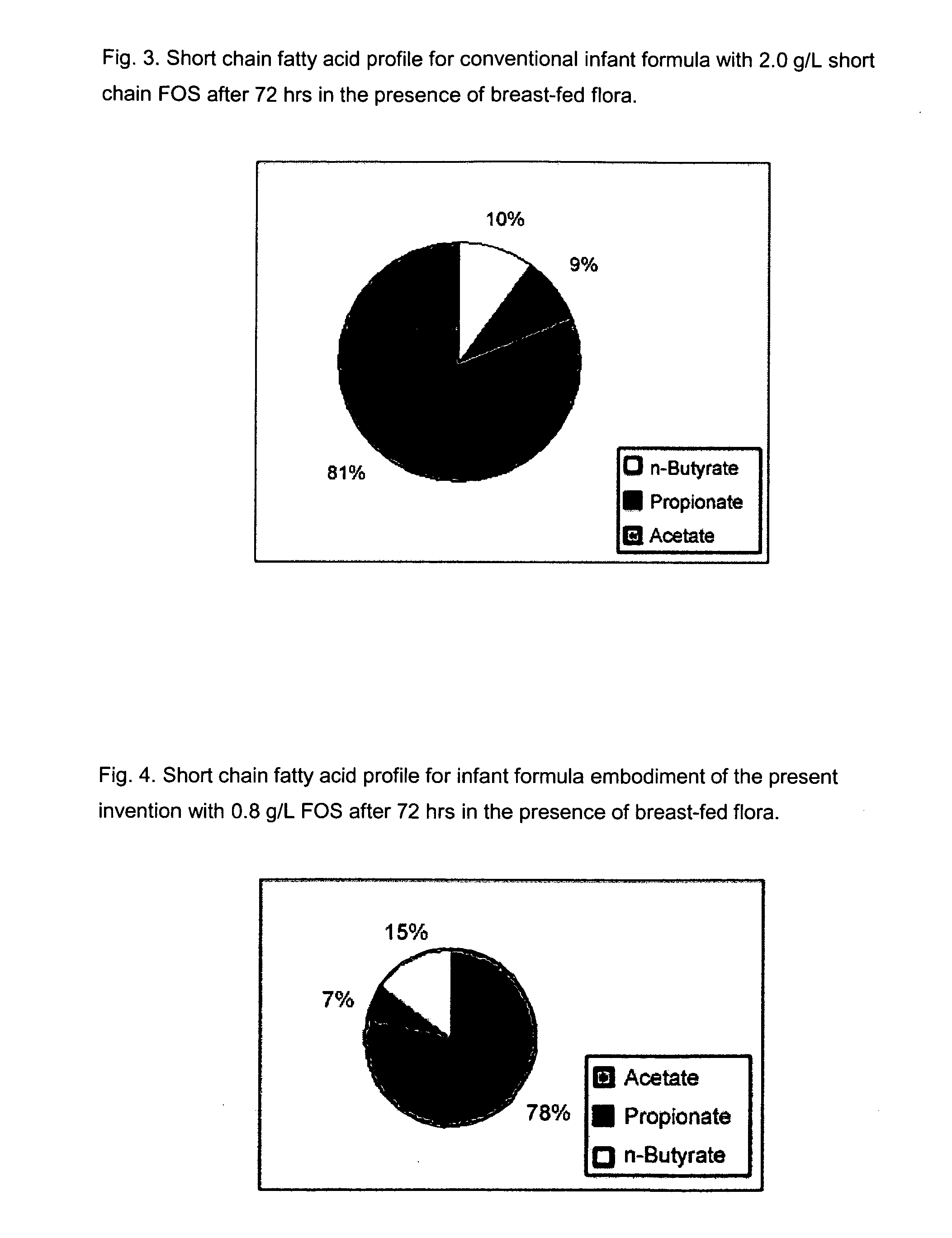
Infant formulas for early brain development
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis, at least about 5 mg / L of gangliosides, at least about 150 mg / L of phospholipids, at least about 70 mg / L of total sialic acid with at least about 2.5% as lipid-bound sialic acid, at least about 0.13% docosahexaenoic acid by weight of total fatty acids, and at least about 0.25% arachidonic acid by weight of total fatty acids. Also disclosed are methods of accelerating brain development, neural migration, and cognitive development in an infant by administering the infant formulas during the first 2-4 months of life, preferably as a sole source of nutrition.
Owner:ABBOTT LAB INC
Phospholipid compositions and methods for their preparation and use
The present invention provides compositions that comprise a phospholipid component (that contains one or more phospholipids) and a pharmaceutically acceptable fluid carrier, where the phospholipid component is in the range from about 10% to about 90% of the total weight. Optionally, the compositions may further comprise non-phospholipid filler materials, where the amount of the non-phospholipid filler materials is in the range from about 5% to about 50% of the total weight. In certain embodiments, the compositions may be injectable, non-liposomal, and / or in form of a gel or a paste. The compositions of the present invention are useful for repairing and augmenting soft and / or hard tissues or for sustained local drug delivery.
Owner:ENCORE THERAPEUTICS
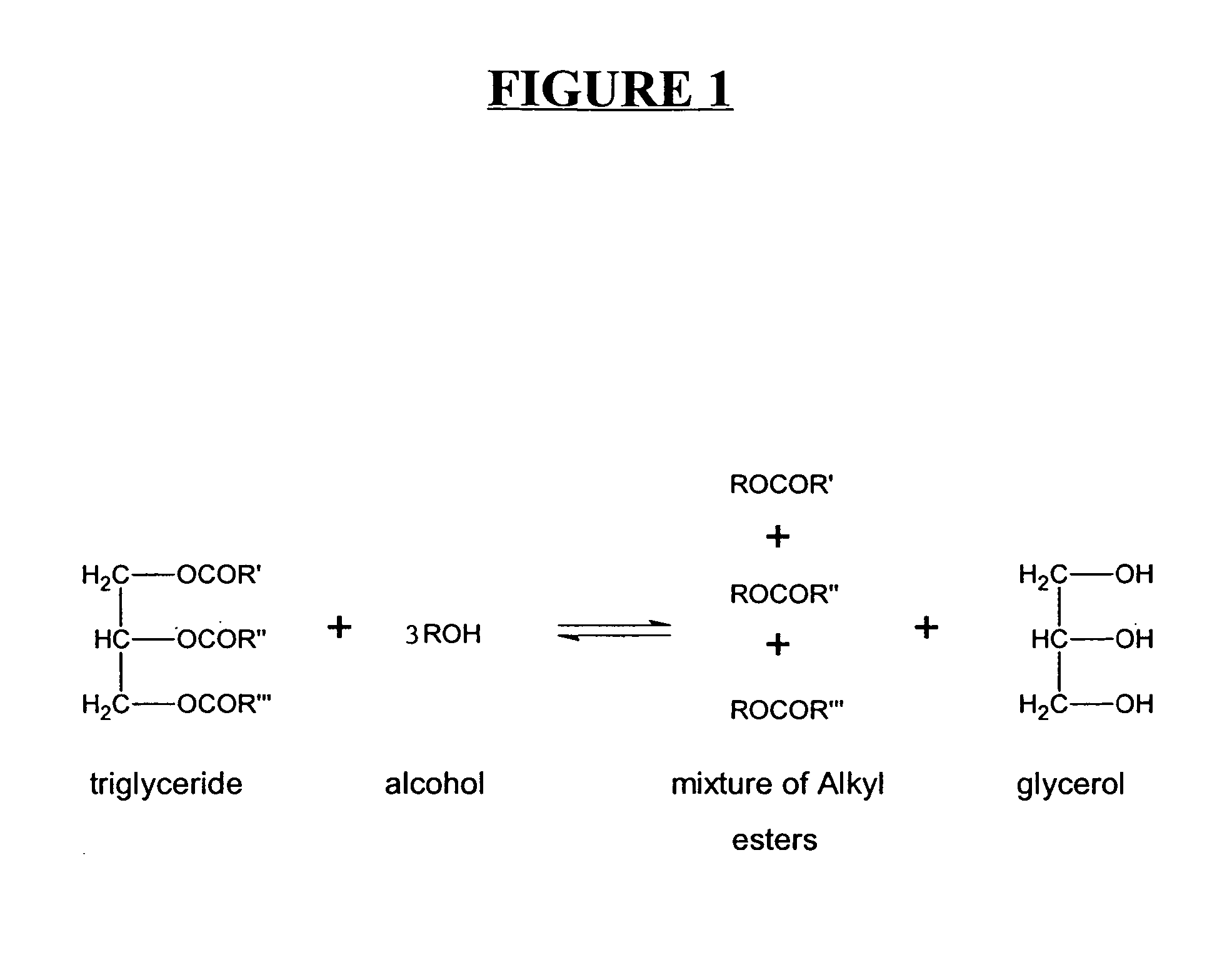
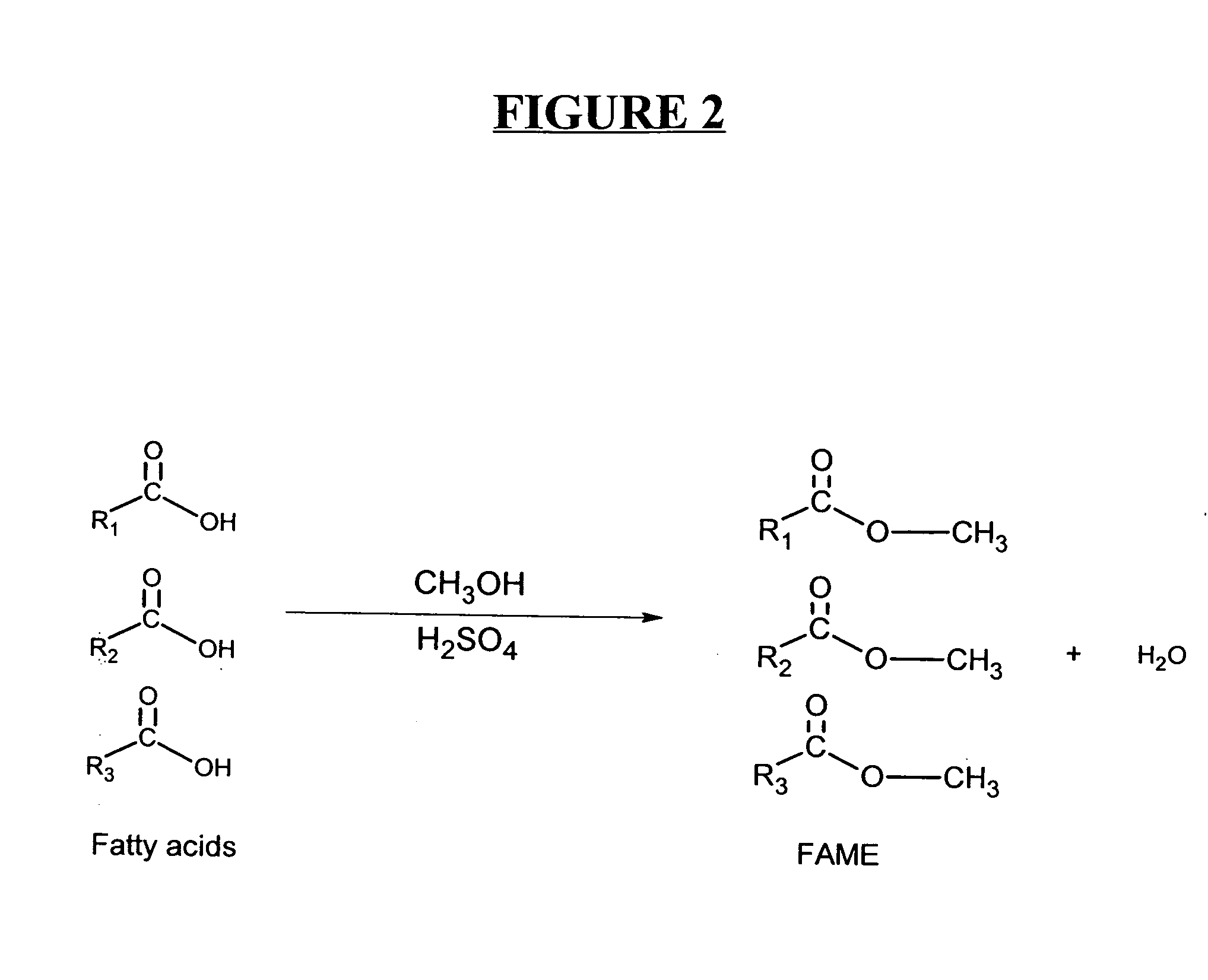
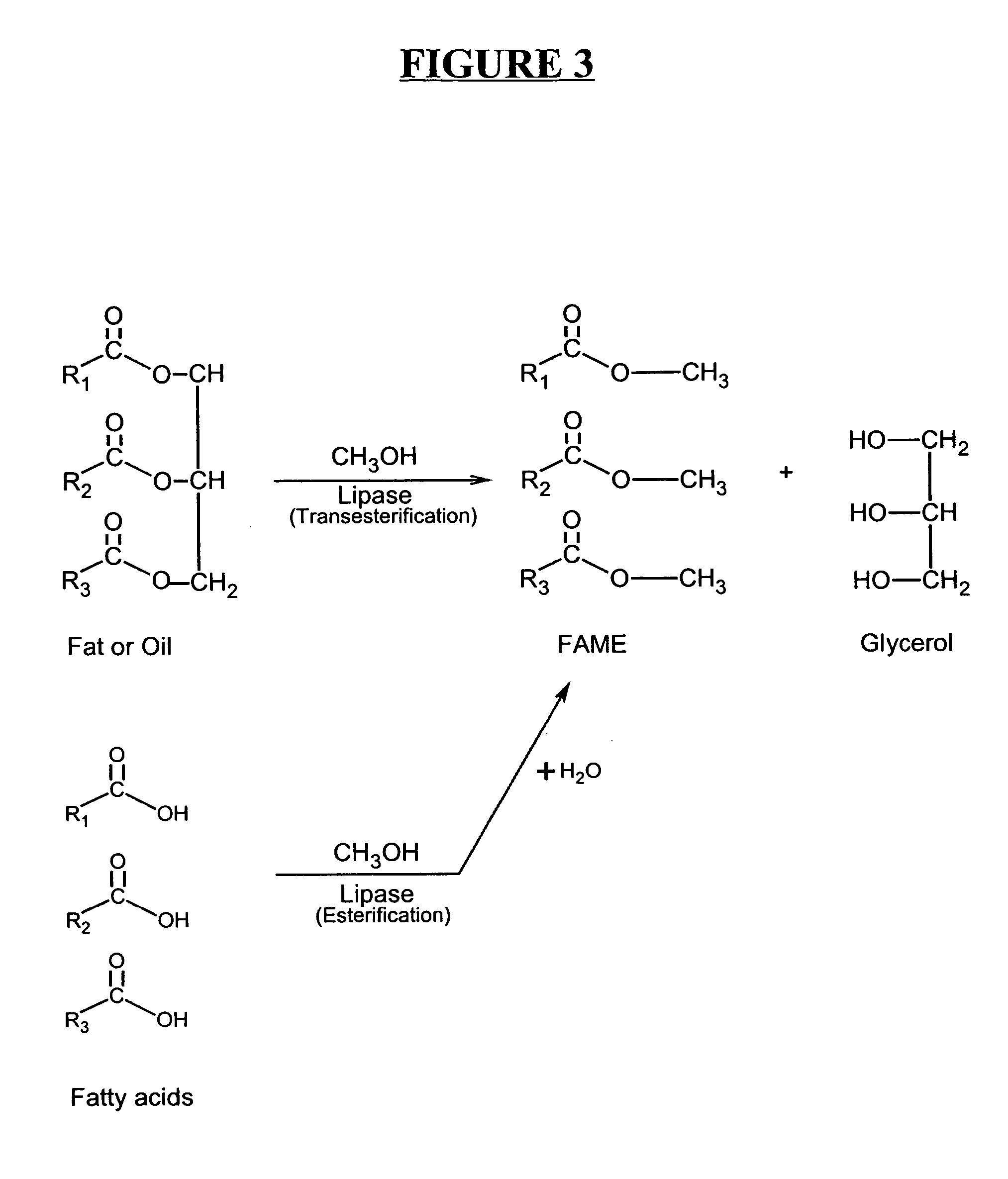
Production of biodiesel and other valuable chemicals from wastewater treatment plant sludges
ActiveUS20050112735A1Reduce the environmentPromote digestionBio-organic fraction processingByproduct vaporizationLipid formationSludge
A process for producing biodiesel has been invented by first extracting lipids from the sludges generated during primary and / or biological treatment of municipal, agricultural, and industrial wastewaters using primary, secondary, and tertiary treatments followed by the transesterification of the extracted lipids using transesterification conversion into alcohol-based esters. The resulting products from this process include biodiesel, glycerol, lipid-free proteins, various other useful chemicals and an aqueous-based substrate well suited for optimized digestion within subsequent biological digestion (either aerobic or anaerobic). The lipids extracted from the sludges containing high levels of microorganisms are phospholipids which can also be directly used as lecithin. The extraction of the lipids from the sludges will be performed using chemical extraction techniques with the transesterification of the extracted lipids accomplished using basic, acidic, and / or a combination of the two transesterification techniques.
Owner:MISSISSPPI STATE UNIV RES & TECH
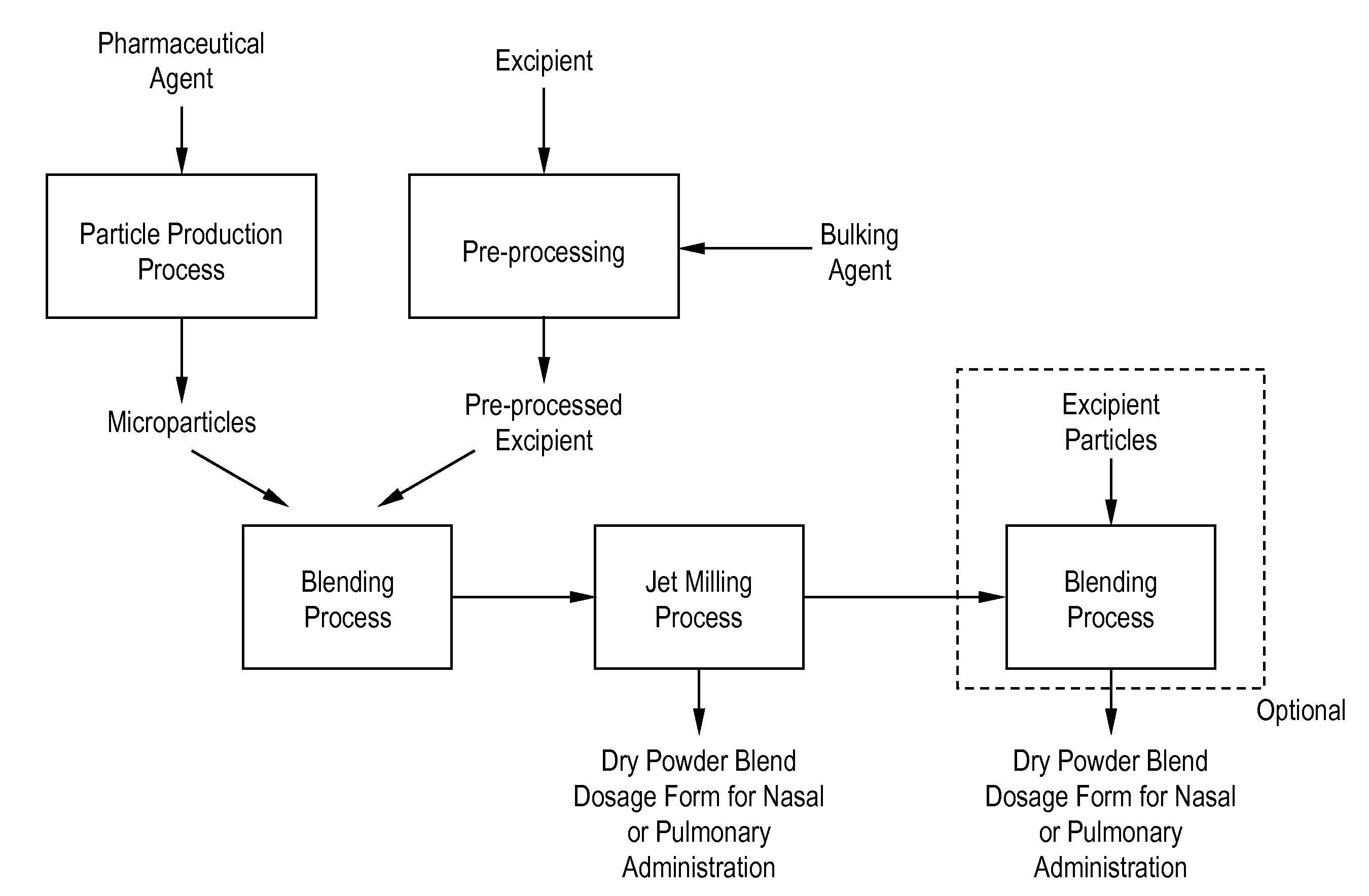
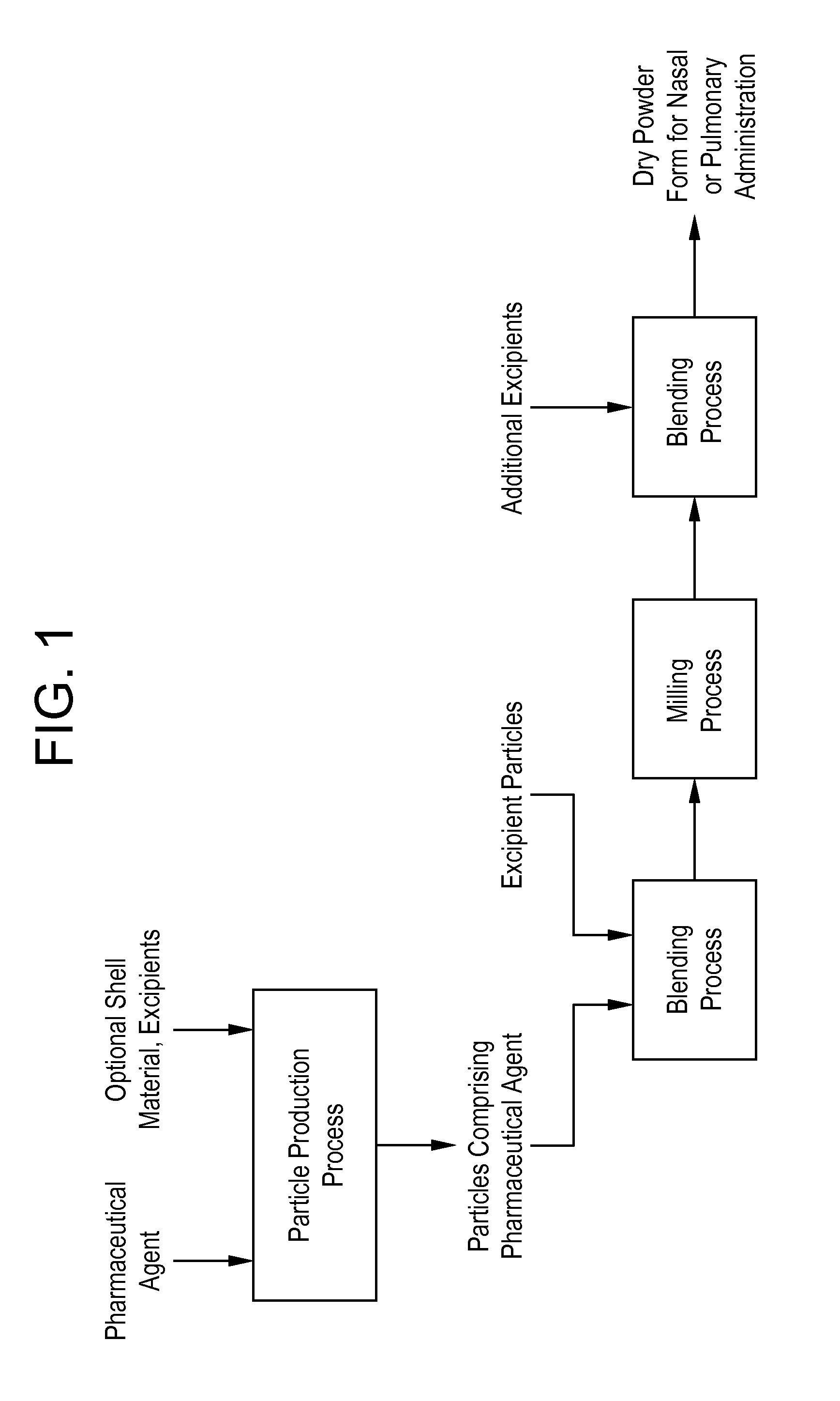
Processes for making particle-based pharmaceutical formulations for pulmonary or nasal administration
InactiveUS20070178166A1Improve stabilityStability storage conditionPowder deliverySpray deliveryPowder mixtureNanoparticle
Dry powder pharmaceutical formulations for pulmonary or nasal administration are made to provide an improved respired dose. These formulations may be blends of milled blends and may include a phospholipid, alone or in combination with other excipient materials. In one case, the process includes the steps of (a) providing particles which comprise a pharmaceutical agent, (b) blending the particles with particles of at least one first excipient to form a first powder blend; (c) milling the first powder blend to form a milled blend which comprises microparticles or nanoparticles of the pharmaceutical agent; and (d) blending the milled blend with particles of a second excipient to form a blended dry powder blend pharmaceutical formulation suitable for pulmonary or nasal administration.
Owner:ACUSPHERE INC
Liposomal products
InactiveUS6060080AHigh encapsulation efficiencyEasy to packLiposomal deliveryCholesterolWater soluble drug
A liposomal aqueous dispersion and method of making the liposomal aqueous dispersion is useful for encapsulation of drugs. The liposomal aqueous dispersion comprises: an aqueous suspension medium; multilamellar liposomes comprising an anionic phospholipid and cholesterol as essential components; neutral phospholipid in a mole ratio of 0 to 40% based on the total amount of said multilamellar liposomes; and a cation moiety-containing water-soluble drug, wherein the electrolyte concentration of said aqueous suspension medium is not more than 40 mM.
Owner:DAIICHI PHARMA CO LTD
Emulsion formulations for hydrophilic active agents
This invention includes emulsion formulations comprising an aqueous carrier and the following components: triglyceride, cholesterol, phospholipid, at least one charged lipid, at least one hydrophilic biologically active molecule and, optionally, cholesteryl ester, and / or apoprotein; methods of preparing these emulsions; and the use of these emulsions for the delivery of hydrophilic biologically active molecules to cells.
Owner:PITTSBURGH UNIV OF
Phosphoryl choline coating compositions
A polymer comprising phospholipid moieties and a biocompatible polymer backbone, a composition comprising the polymer and optionally a bioactive agent, an implantable devices such as a DES comprising thereon a coating comprising the polymer and optionally a bioactive agent, and a method of using the device for the treatment of a disorder in a human being are provided.
Owner:ABBOTT CARDIOVASCULAR
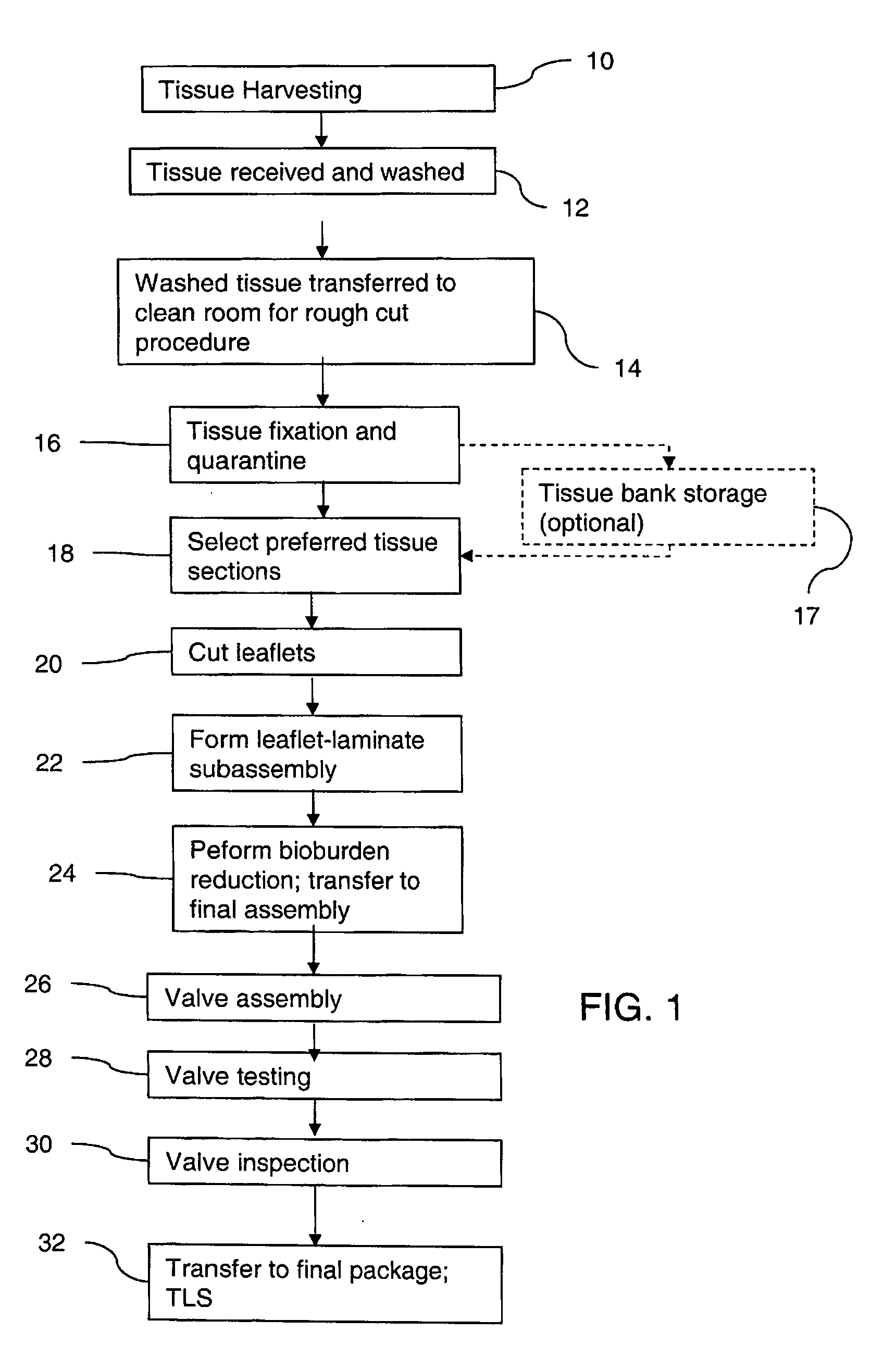
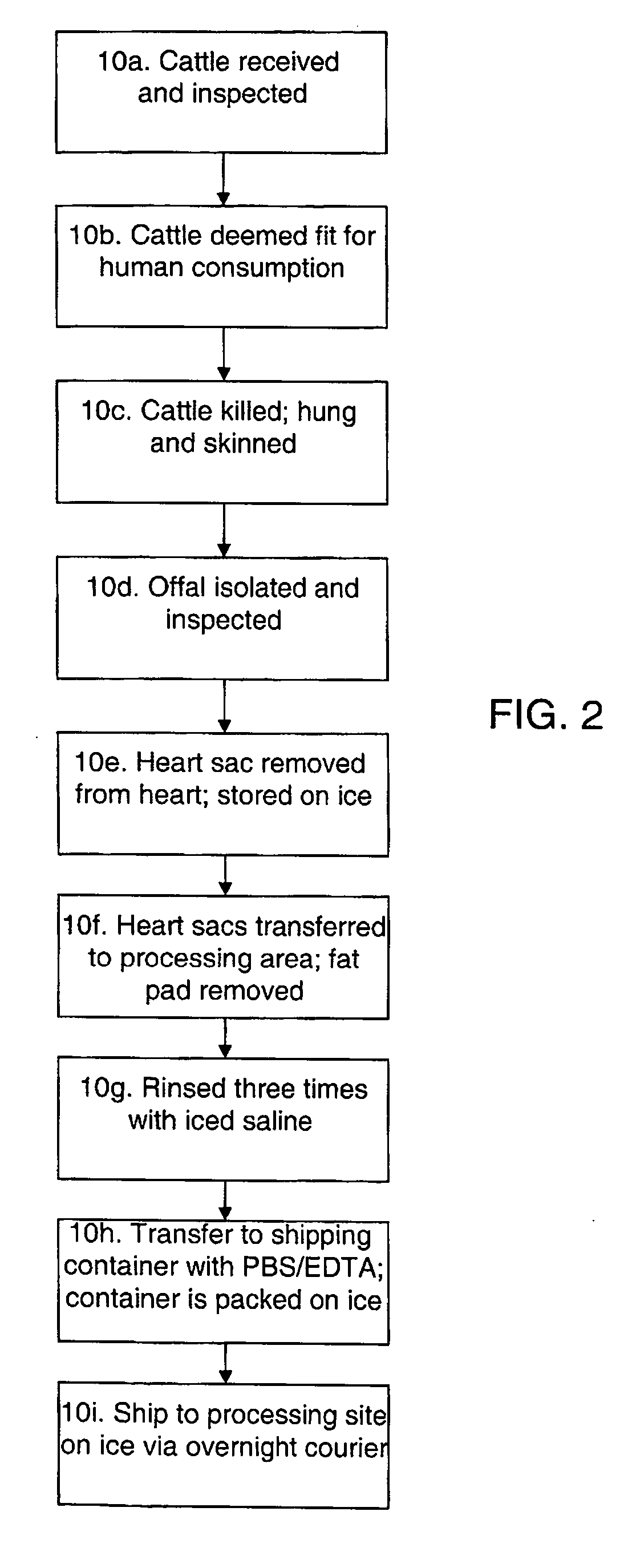
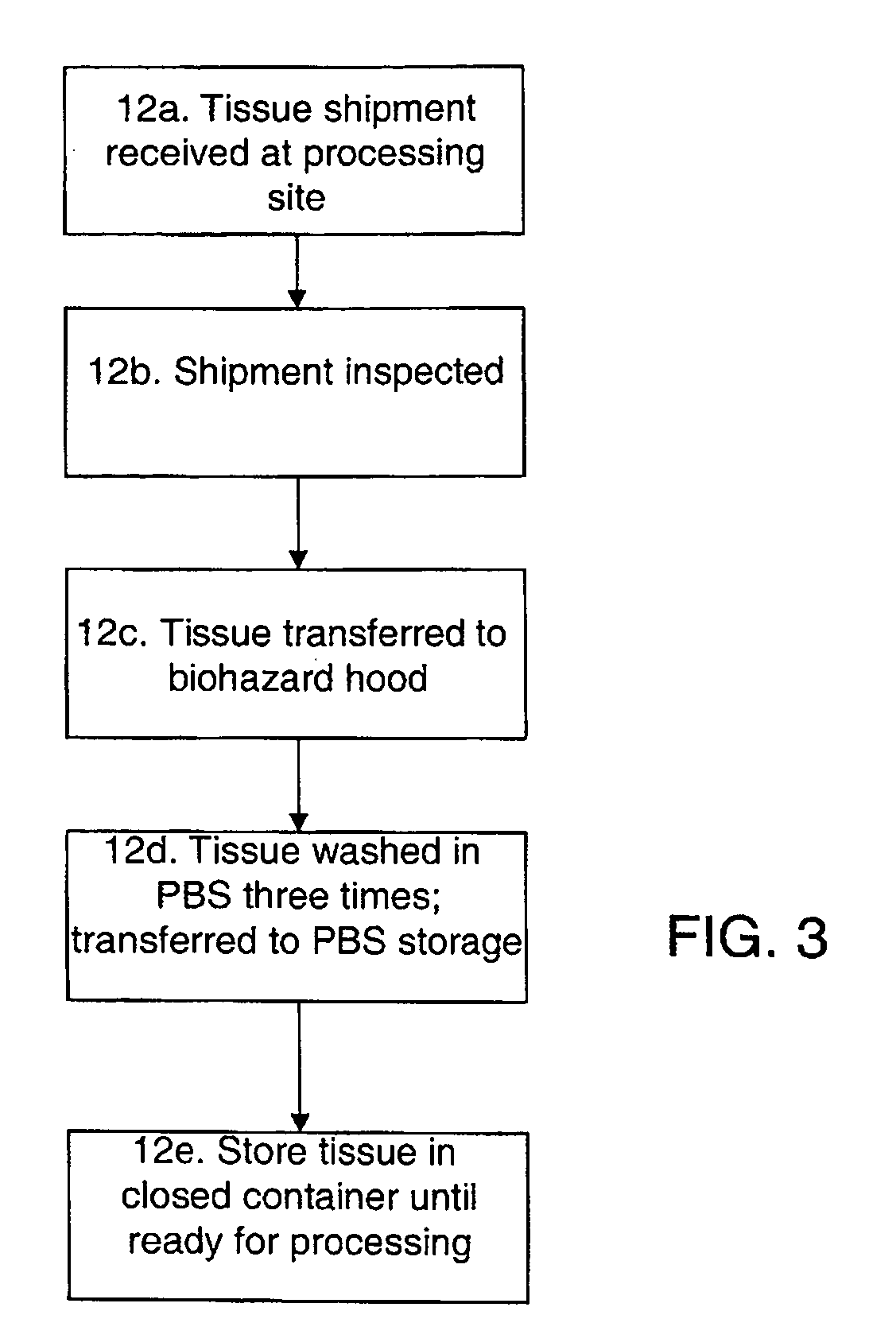
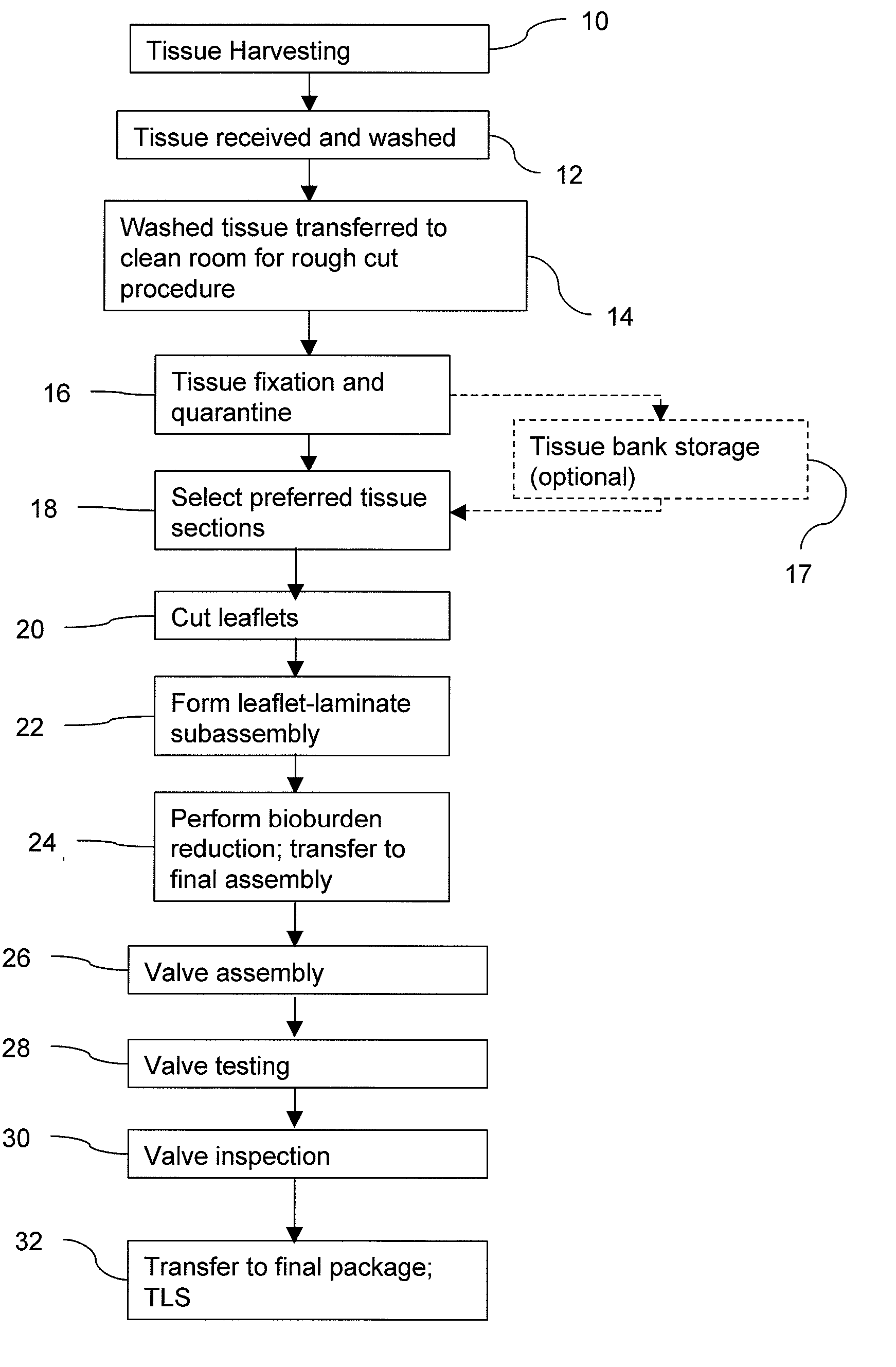
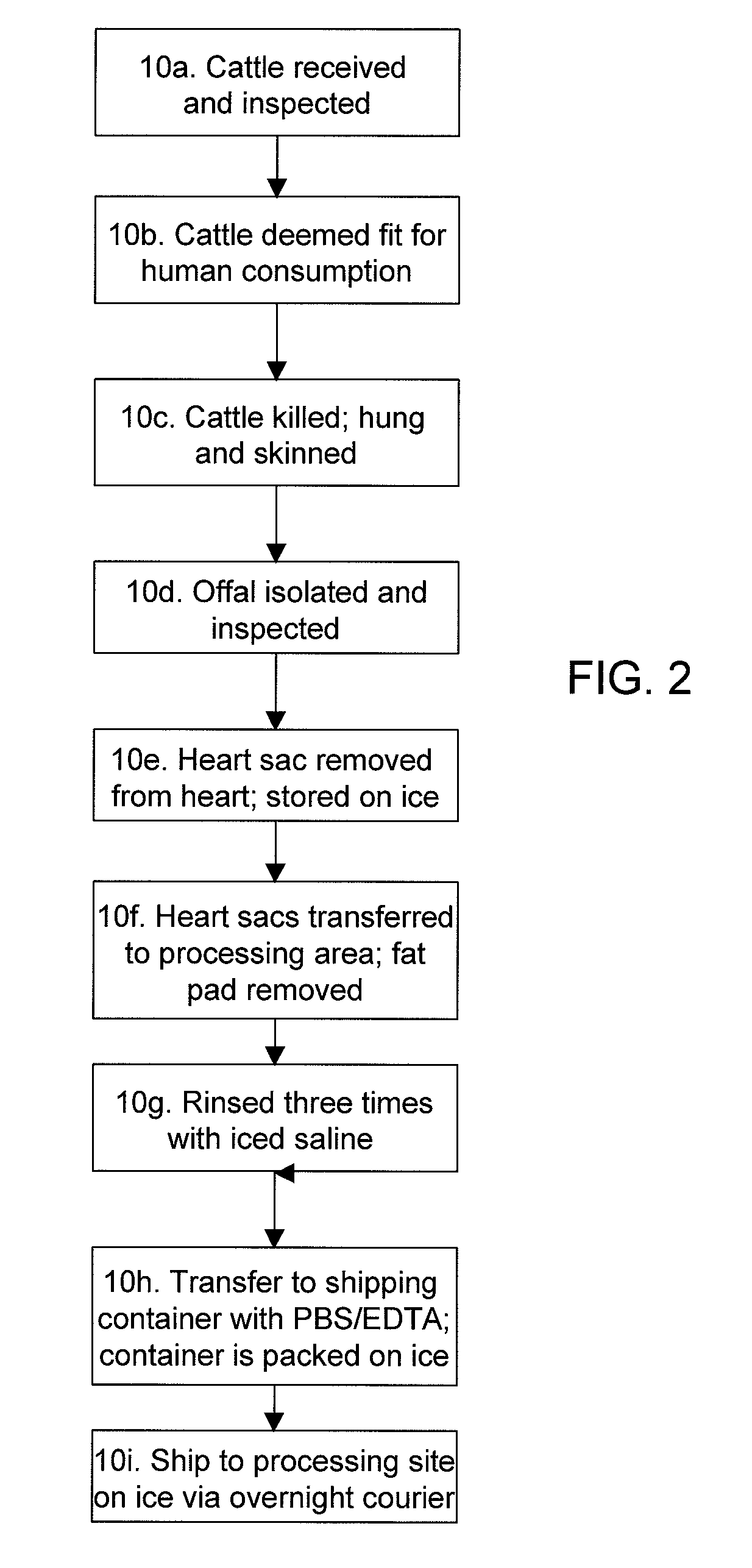
Methods for processing biological tissue
ActiveUS20060154230A1Remove background levelAvoid degradationDead animal preservationPhosphateCalcification
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Bioactive Complexes Compositions and Methods of Use Thereof
InactiveUS20070085059A1Enhanced soluble complexesGood fluidityCosmetic preparationsNervous disorderDiseaseCholesterol blood
A bioactive complex composition having enhanced oxidative stability, emulsion stability, mineral rich transparent beverages and a wide range of functional health benefits. The composition may include as a base composition individual ingredients or a synergistic blend of mineral salts, Omega-3 rich oils, phospholipids, chitosan, and alpha-casein, beta-casein, kappa-casein or protein fragments, glycopeptides, phosphopeptides. The composition may optionally be further utilized for the prevention of hypercholesterolemia, bone (and teeth) mineral loss, treatment of mental health diseases, heart health, additional nutritional supplementation, and treatment of additional medical conditions.
Owner:TEXAS A&M UNIVERSITY
Glycoconjugation of polypeptides using oligosaccharyltransferases
The current invention provides polypeptides and polypeptide conjugates that include an exogenous N-linked glycosylation sequence. The N-linked glycosylation sequence is preferably a substrate for an oligosaccharyltransferase (e.g., bacterial PgIB), which can catalyze the transfer of a glycosyl moiety from a lipid-bound glycosyl donor molecule (e.g., a lipid-pyrophosphate-linked glycosyl moiety) to an asparagine (N) residue of the glycosylation sequence. In one example, the asparagine residue is part of an exogenous N-linked glycosylation sequence of the invention. The invention further provides methods of making the polypeptide conjugates that include contacting a polypeptide having an N-linked glycosylation sequence of the invention and a lipid-pyrophosphate-linked glycosyl moiety (or phospholipid-linked glycosyl moiety) in the presence of an oligosaccharyltransferase under conditions sufficient for the enzyme to transfer the glycosyl moiety to an asparagine residue of the N-linked glycosylation sequence. Exemplary glycosyl moieties that can be conjugated to the glycosylation sequence include GlcNAc, GlcNH, bacillosamine, 6-hydroybacillosamine, GalNAc, GaINH, GlcNAc-GlcNAc, GlcNAc-GlcNH, GlcNAc-Gal, GlcNAc-GlcNAc-Gal-Sia, GlcNAc-Gal-Sia, GlcNAc-GlcNAc-Man, and GlcNAc-GlcNAc-Man(Man)2. The transferred glycosyl moiety is optionally modified with a modifying group, such as a polymer (e.g., PEG). In one example, the modified glycosyl moiety is a GIcNAc or a sialic acid moiety.
Owner:RATIOPHARM GMBH +1
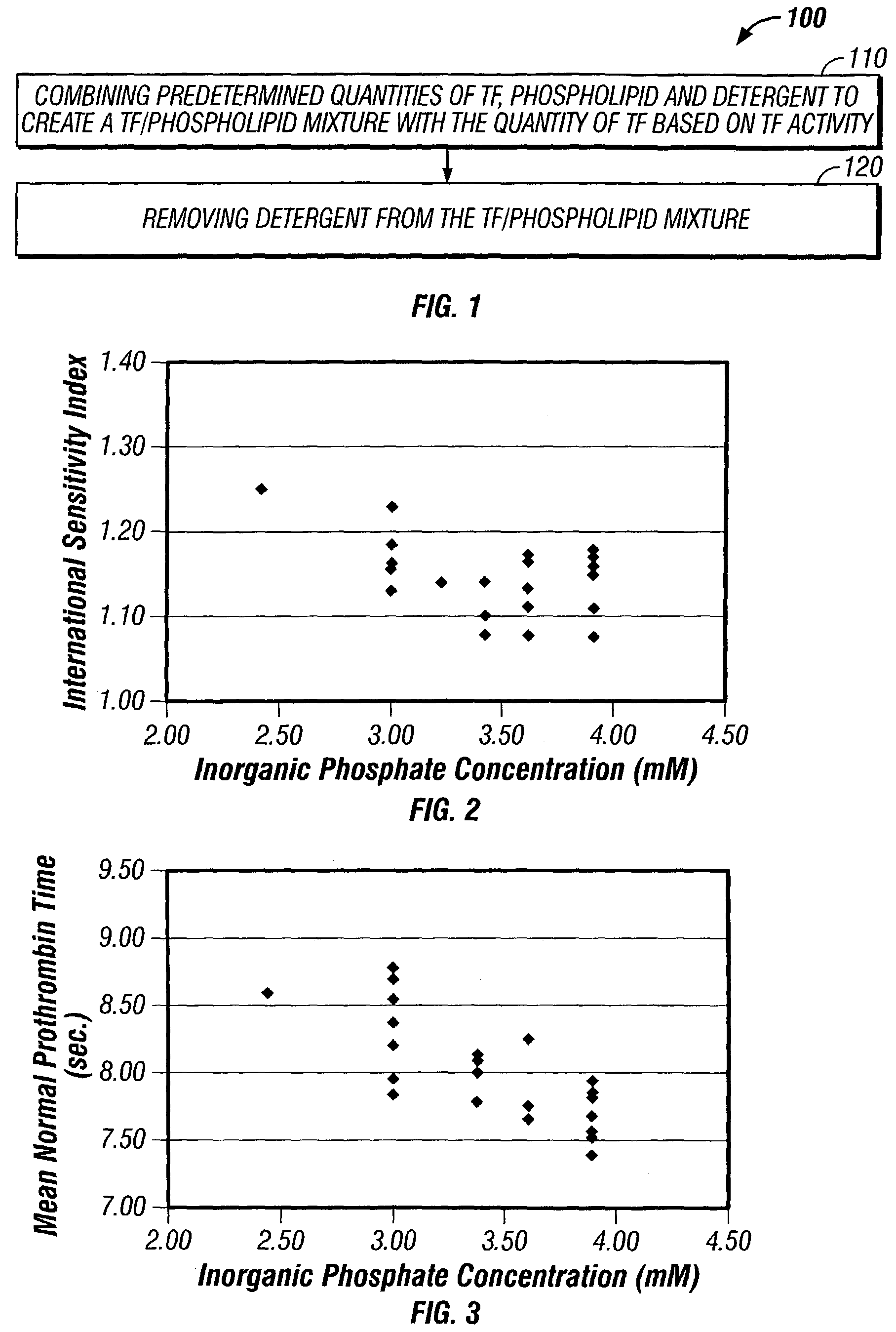
Method for manufacturing a tissue factor-based prothrombin time reagent
ActiveUS7049087B2Microbiological testing/measurementBiological material analysisTissue factorPhospholipid
Owner:LIFESCAN IP HLDG LLC
Aerosol drug formulations containing hydrofluoroalkanes and alkyl saccharides
InactiveUS6932962B1Function increaseGood dispersionPowder deliveryDispersion deliveryAerosol drugsActive agent
Aerosol formulations suitable for use in pressurised metered dose inhalers comprise a hydrofluoroalkane propellant, an medicament for inhalation and a surfactant which is a a C8–C16 fatty acid or salt thereof, a bile salt, a phospholipid, or an alkyl saccharide.
Owner:ASTRAZENECA AB
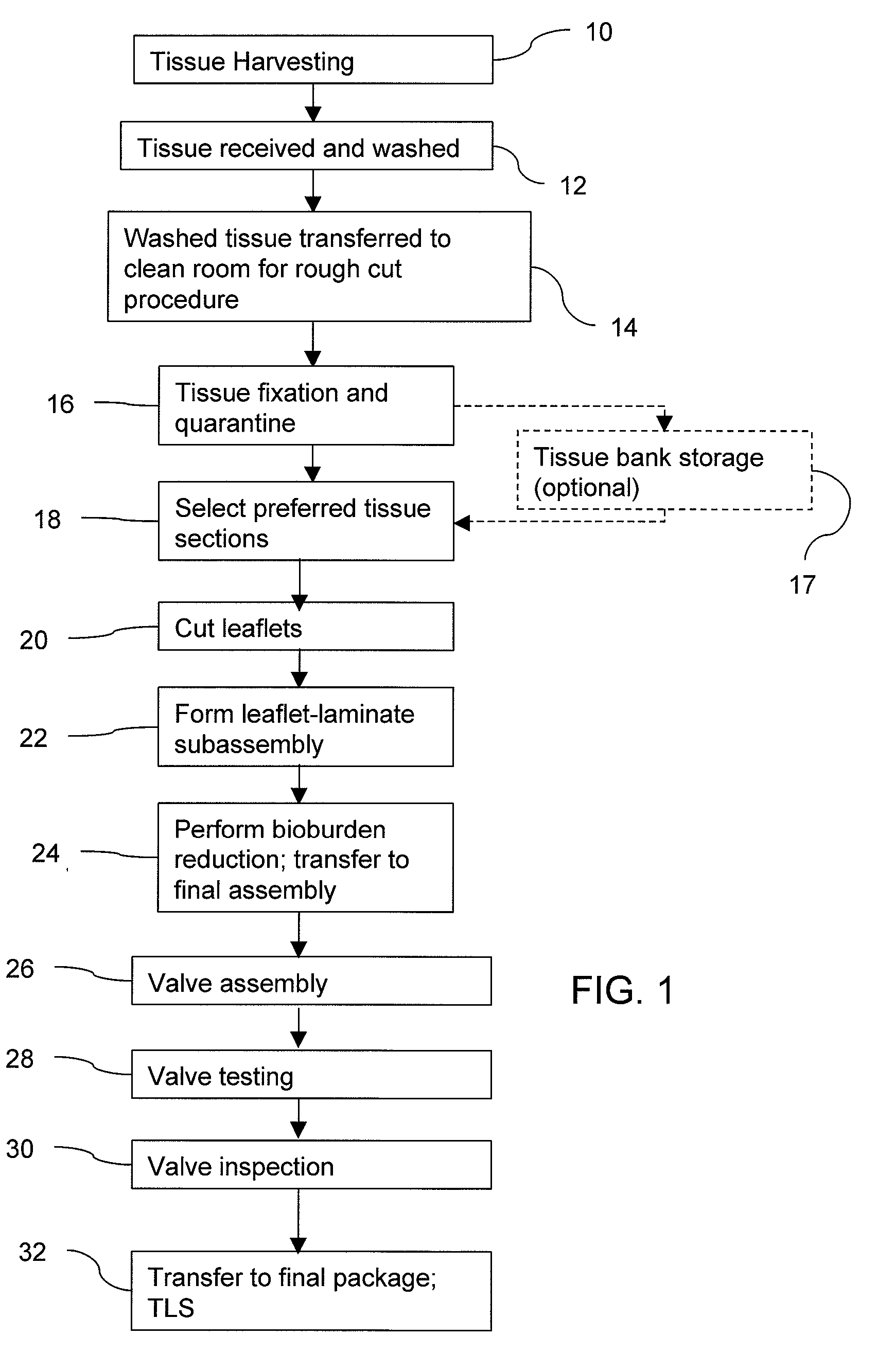
Methods for processing biological tissue
ActiveUS20060207031A1Remove background levelAvoid degradationSuture equipmentsDead animal preservationCalcificationPhospholipid
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Electrochemical luminescence composite material with anti-biofouling properties
InactiveUS20060029979A1Easy to optimizeBioreactor/fermenter combinationsBiological substance pretreatmentsMeth-Phosphorylcholine
The present invention relates to the preparation ad application of a high-sensitive electrochemical luminescent composite material which has anti-biofouling properties useful as a sensor material. This material is prepared by immobilization of electrochemical luminescent material into polymer containing phospholipid groups, wherein, the electrochemical luminescent material including ruthenium complex, osmium complex, etc.; the phospholipid containing polymer is the copolymer of 2-methacryloyloxyethyl phosphorylcholine (MPC) and other polymerisable monomers. Animal experiment results revealed that this composite material has good anti-biofouling properties; it can be used in producing various sensors for bio-related detections.
Owner:FUDAN UNIV
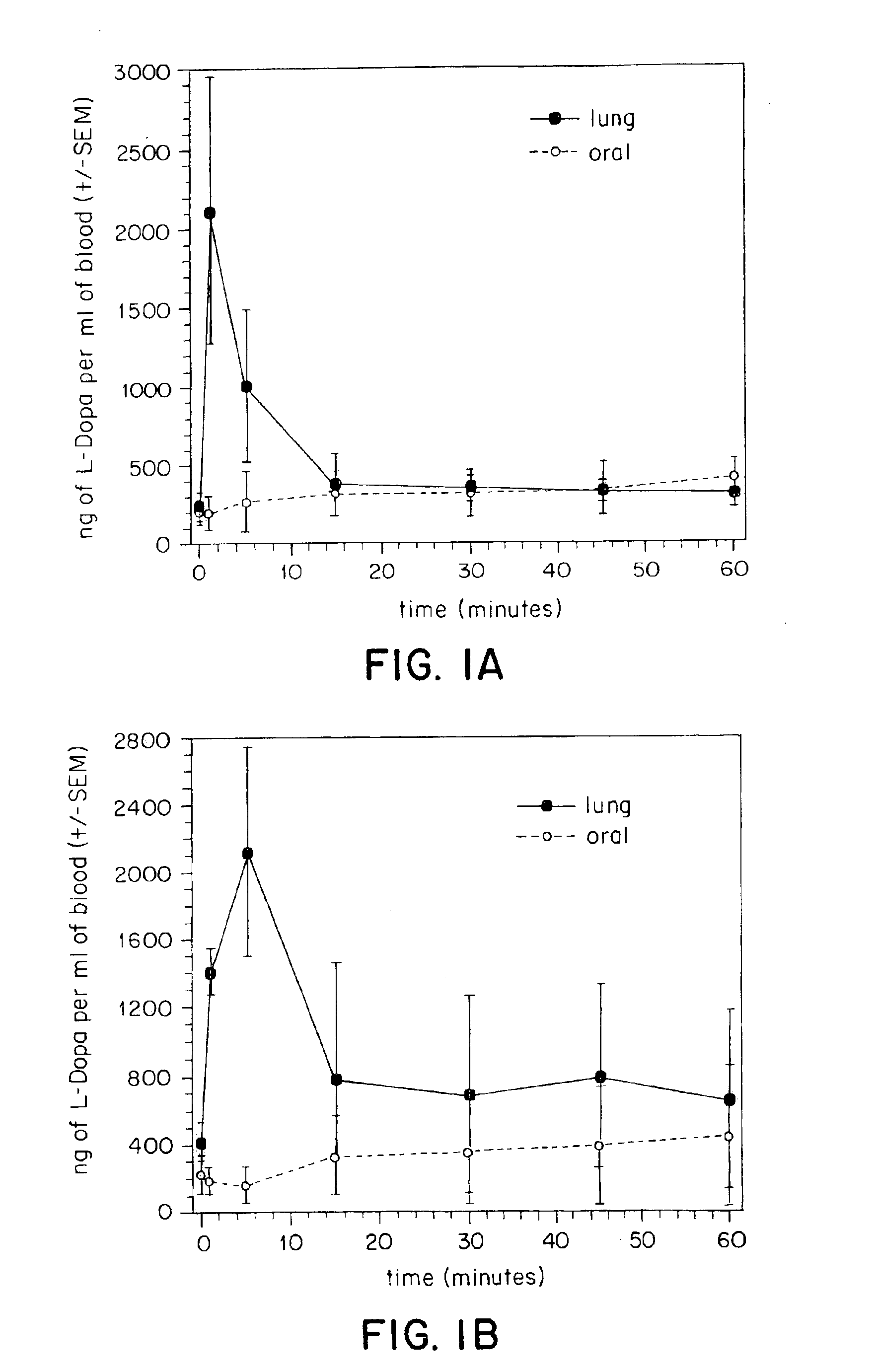
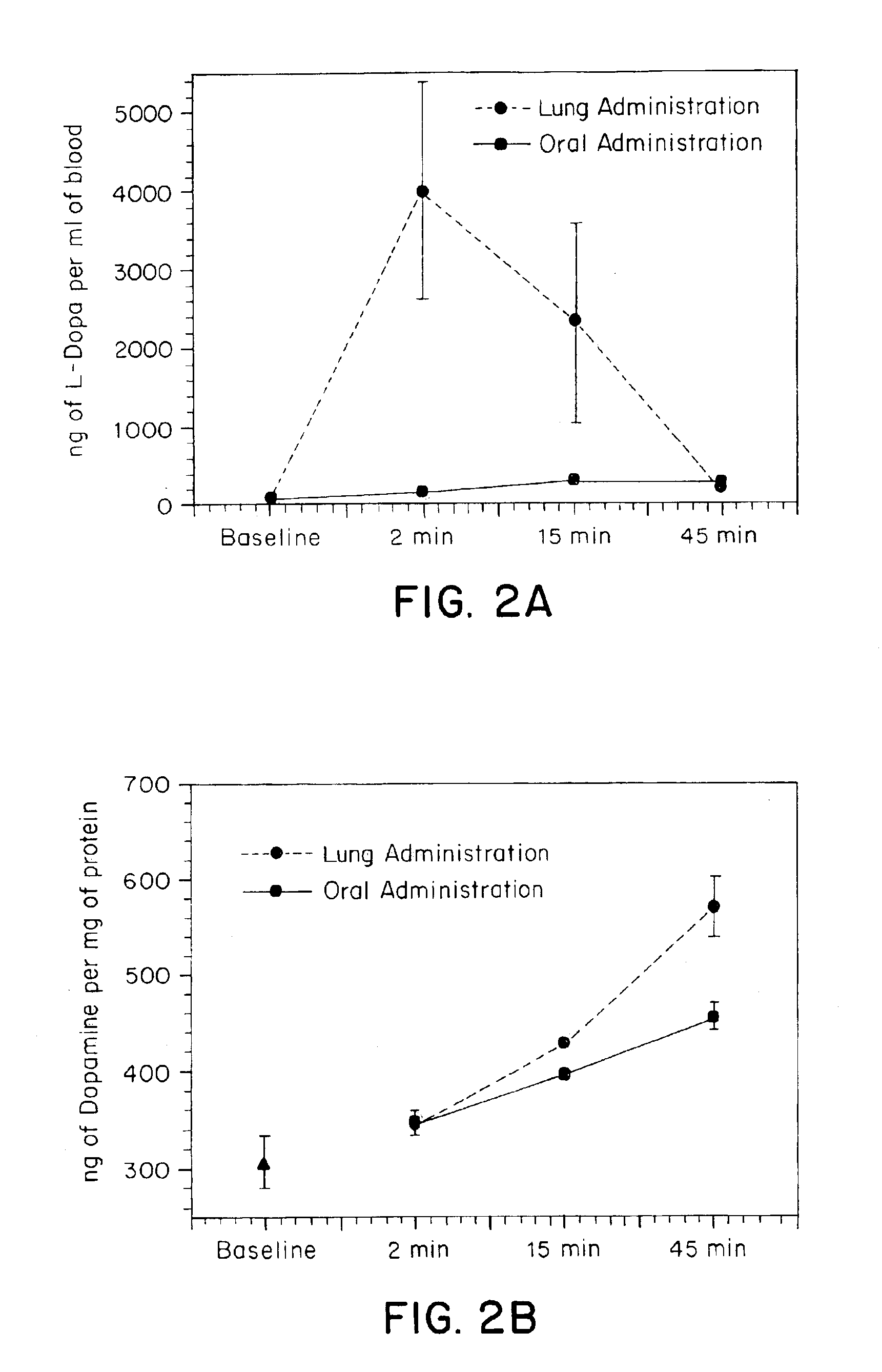
Pulmonary delivery in treating disorders of the central nervous system
A method for treating a disorder of the central nervous system includes administering to the respiratory tract of a patient a drug which is delivered to the pulmonary system, for instance to the alveoli or the deep lung. The drug is administered at a dose which is at least about two-fold less than the dose required by oral administration. Particles that include the drug can be employed. Preferred particles have a tap density of less than about 0.4 g / cm3. In addition to the medicament, the particles can include other materials such as, for example, phospholipids, amino acids, combinations thereof and others.
Owner:CIVITAS THERAPEUTICS
Enriched infant formulas
InactiveUS20080003329A1Reduce riskShorten the construction periodFood preparationSialic acidPhospholipid
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis (A) at least about 5 mg / L of gangliosides, (B) at least about 150 mg / L of phospholipids, and (C) lactoferrin, and (D) at least about 70 mg / L of sialic acid, with at least about 2.5% by weight of the sialic acid as lipid-bound sialic acid. Also disclosed are methods of using the formula to reduce the risk of diarrhea infants, and to produce a gut microflora profile similar to that of breast-fed infants.
Owner:ABBOTT LAB INC
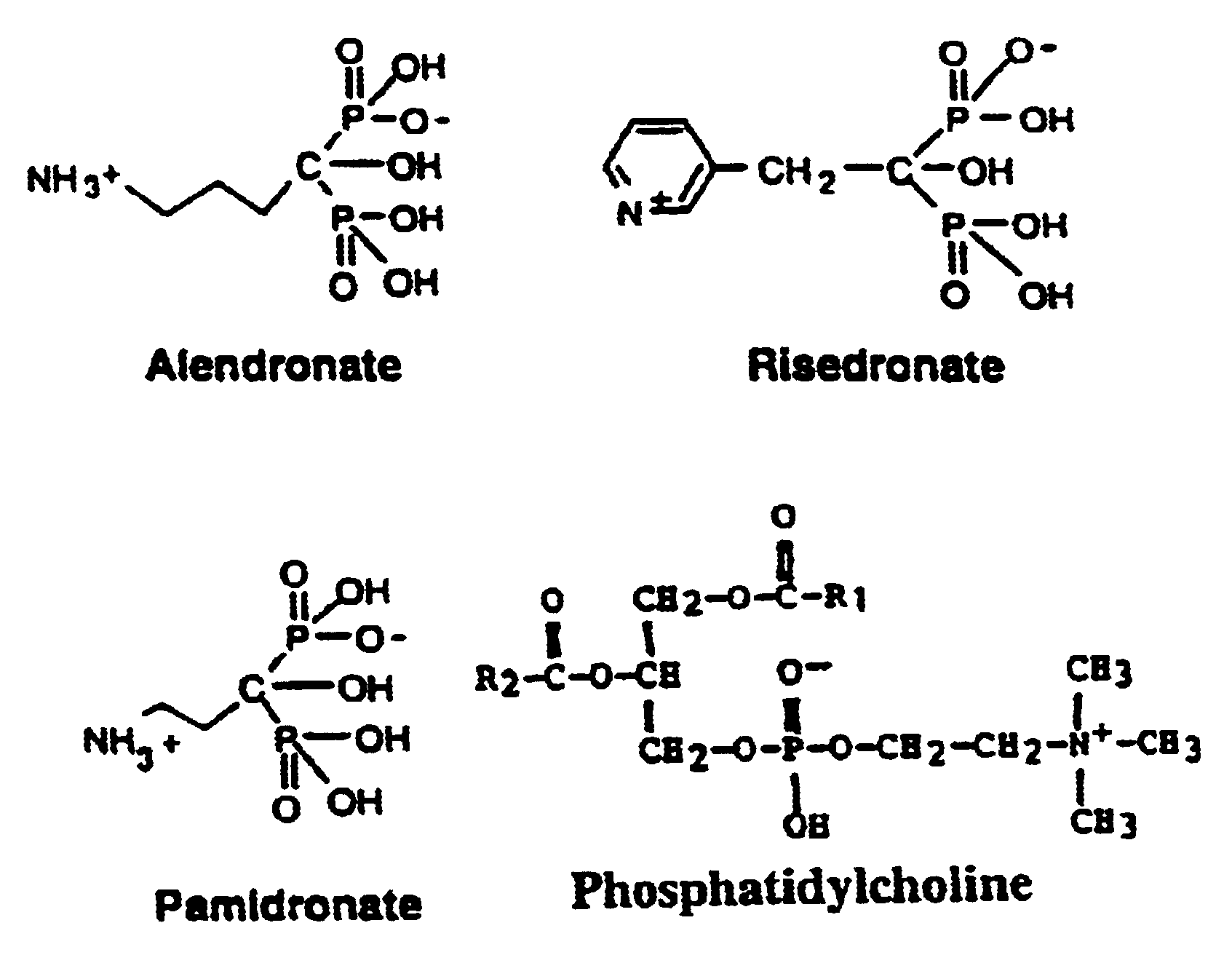
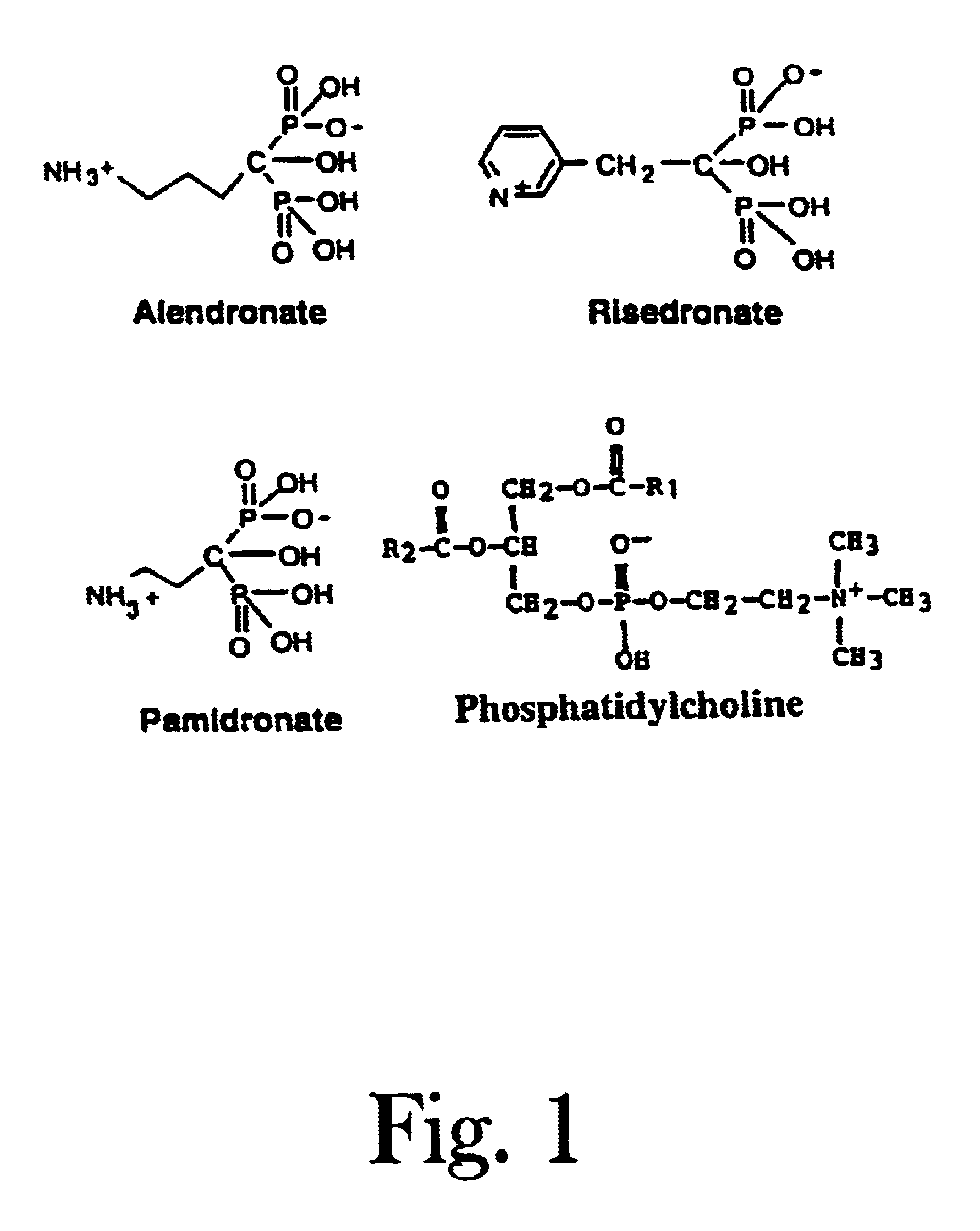
Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity
InactiveUS6943155B2Reduce GI toxicityImprove bioavailabilityBiocideInorganic active ingredientsDiphosphonatesMedicine
Compositions and methods for treating osteoporosis using the compositions are disclosed where the compositions have reduced GI toxicity and improved bio-availability and include a bisphosphonate and zwitterionic phospholipid.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for extracting krill oil with high phosphatide content from Antarctic krills
InactiveCN102041166AImprove enrichment effectImprove extraction efficiencyFatty-oils/fats productionOrganic solventPrawn
The invention discloses a method for extracting krill oil with high phosphatide content from Antarctic krills. The method comprises the following steps: (1) drying fresh Antarctic krills to obtain dry Antarctic krills; (2) performing extraction to dry Antarctic krills for 3 times with organic solvent; (3) mixing the extracting solutions, evaporating the mixed extracting solution to obtain Antarctic krill oil with the phosphatide content of 30-35%; then introducing nitrogen or carbon dioxide to remove the residual organic solvent; and adding polar organic solvent in the Antarctic krill oil, mixing evenly, standing to ensure that the mixed solution performs natural layering; and evaporating the lower solution to obtain the Antarctic krill oil with high phosphatide content, and then introducing nitrogen or carbon dioxide to remove the residual polar organic solvent so as to obtain a product. The extraction technology is performed at a low temperature, thus the red color of krill oil can be maintained and the beneficial ingredients in the product can not be damaged. The invention has high extraction efficiency and good phosphatide accumulation effect. Therefore, the Antarctic krill oil with high phosphatide content can be obtained and other byproducts can also be obtained.
Owner:SHANDONG NORMAL UNIV +1
Systems, apparatuses, and methods for extracting non-polar lipids from an aqueous algae slurry and lipids produced therefrom
InactiveUS20110095225A1Increase volume flowEasy extractionLiquid separation by electricityMicroorganism lysisLipid formationPhospholipid
Methods, systems, and apparatuses for extracting non-polar lipids from microalgae are achieved using a lipid extraction device having an anode and a cathode that forms a channel and defines a fluid flow path through which an aqueous slurry is passed. An electromotive force is applied across the channel at a gap distance in a range from 0.5 mm to 200 mm to cause the non-polar lipids to be released from the algae cells. The non-polar lipids can be extracted at a high throughput rate and with low concentrations of polar lipids such as phospholipids and chlorophyll.
Owner:ORGINOIL INC
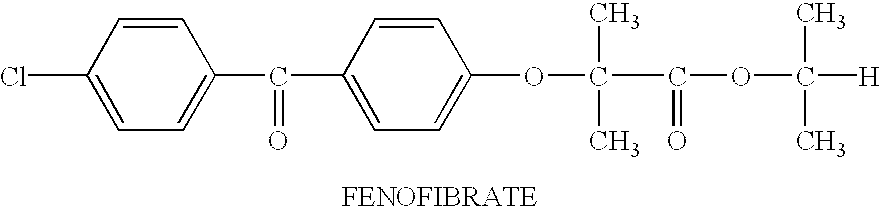
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs, particularly fenofibrate, are provided. The compositions comprise a therapeutically effective amount of an active agent and a solubilizer. The solubilizer is selected to effectively solubilize active agent in the composition. The solubilizers employed as part of the invention include: a vitamin E substance; monohydric alcohol esters such as trialkyl citrates, lactones and lower alcohol fatty acid esters; nitrogen-containing solvents; phospholipids; glycerol acetates such as acetin, diacetin and triacetin; glycerol fatty acid esters such as mono-, di- and triglycerides and acetylated mono- and diglycerides; propylene glycol esters; ethylene glycol esters; and combinations thereof. The pharmaceutical dosage forms contain the compositions in a suitable dosage form unit such as a capsule. Methods of treating patients comprising administering the compositions are also provided.
Owner:LIPOCINE
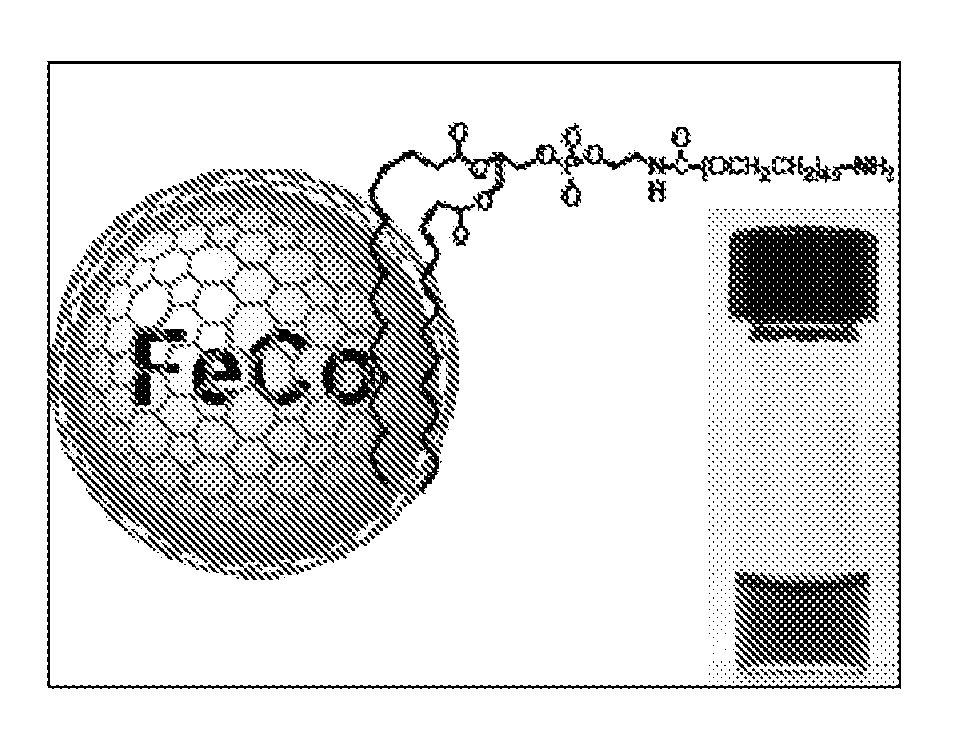
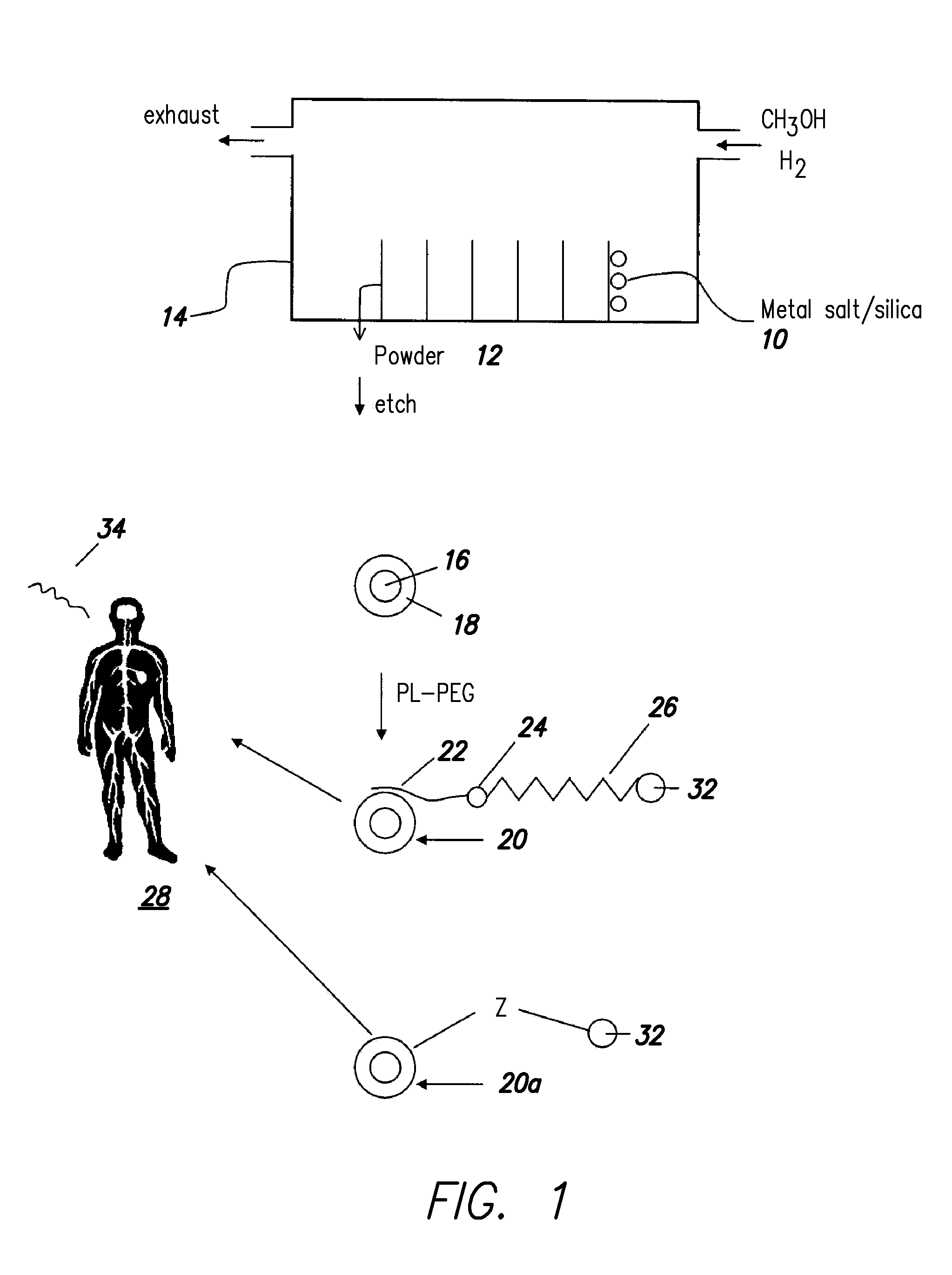
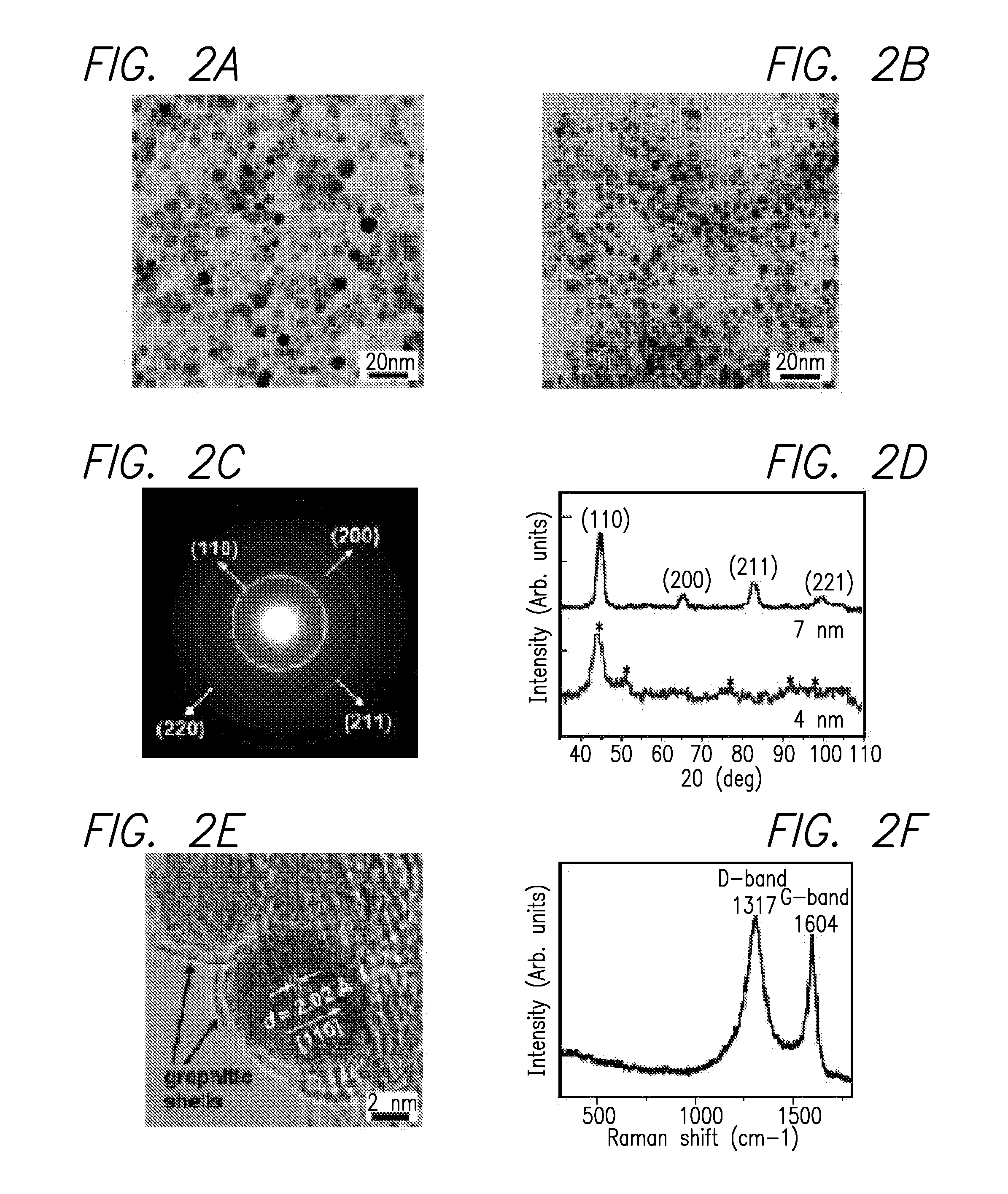
Multifunctional metal-graphite nanocrystals
InactiveUS20080213189A1High resolutionShrink tumorPowder deliveryInorganic active ingredientsMRI contrast agentPolyethylene glycol
Disclosed are nanocrystals comprising metals and metal alloys, which are formed by a process that results in a layer of graphite in direct contact with the metallic core. The nanocrystals may be used in vivo as MRI contrast agents, X-ray contrast agents, near IR (NIR) heating agents, drug delivery, protein separation, catalysis etc. The nanocrystals may be further functionalized with a hydrophilic coating, e.g., phospholipid-polyethylene glycol, which improves in vivo stability. The process comprises chemical vapor deposition of metals adsorbed onto silica as a fine powder, in conjunction with a carbon containing gas, which coats the metal particles. The silica is then etched away. Preferred metals include iron, gold, cobalt, platinum, ruthenium and mixtures thereof, e.g., FeCo and AuFe. The process permits control of the alloy compositions, size, and other characteristics.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
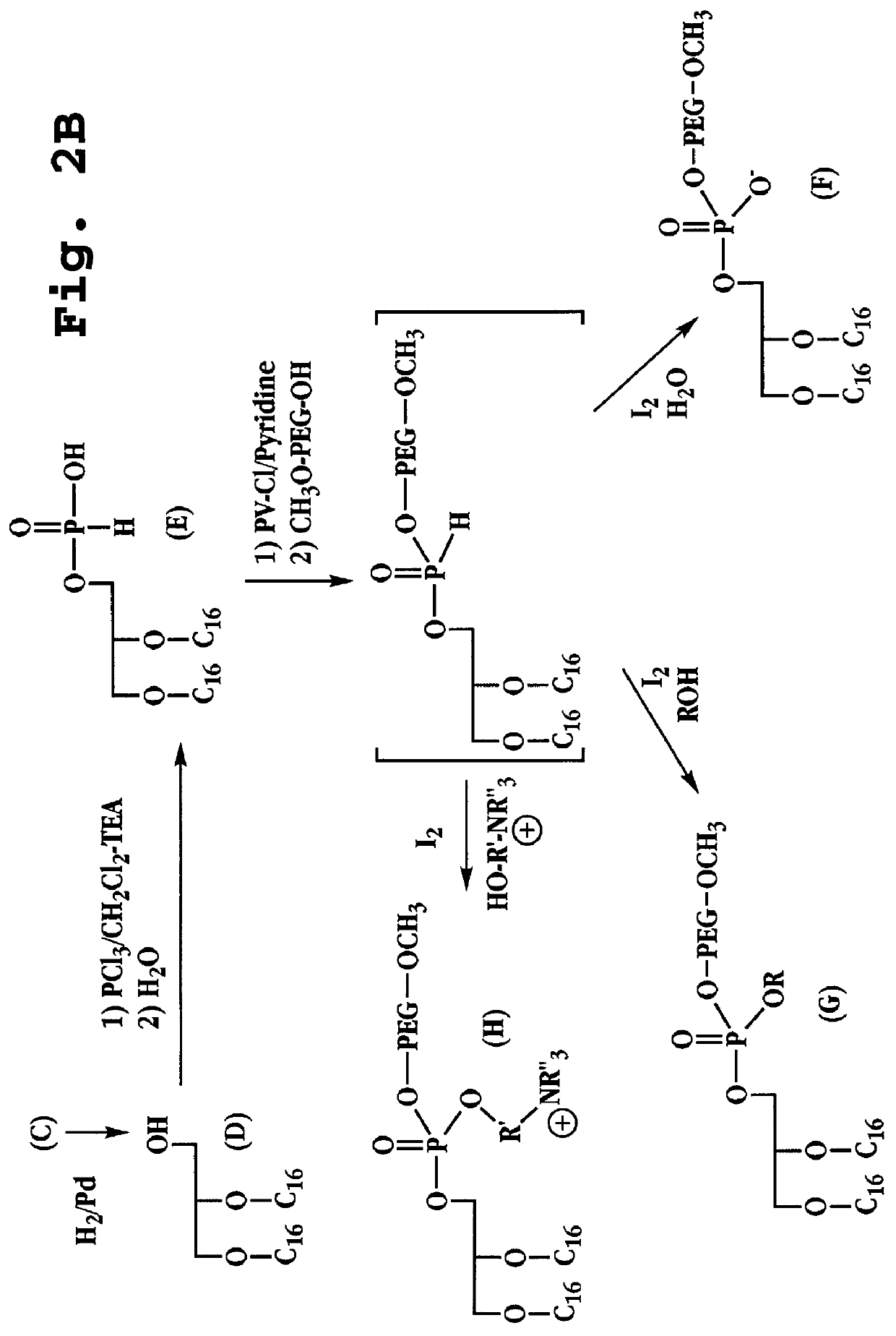
Radiation-protective phospholipid and method
InactiveUS6165501AImprove Oxidation StabilityCosmetic preparationsToilet preparationsLipid formationEther
Ether-linked phospholipids, derivatized at the polar head group with polyethylene glycol chains having molecular weights greater than 2,000 daltons, are disclosed. Lipid bilayers containing these phospholipids show high oxidative stability. Also disclosed is the use of PEG-derivatized ether-linked lipids in moisturizing and radiation-protective cosmetic compositions.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
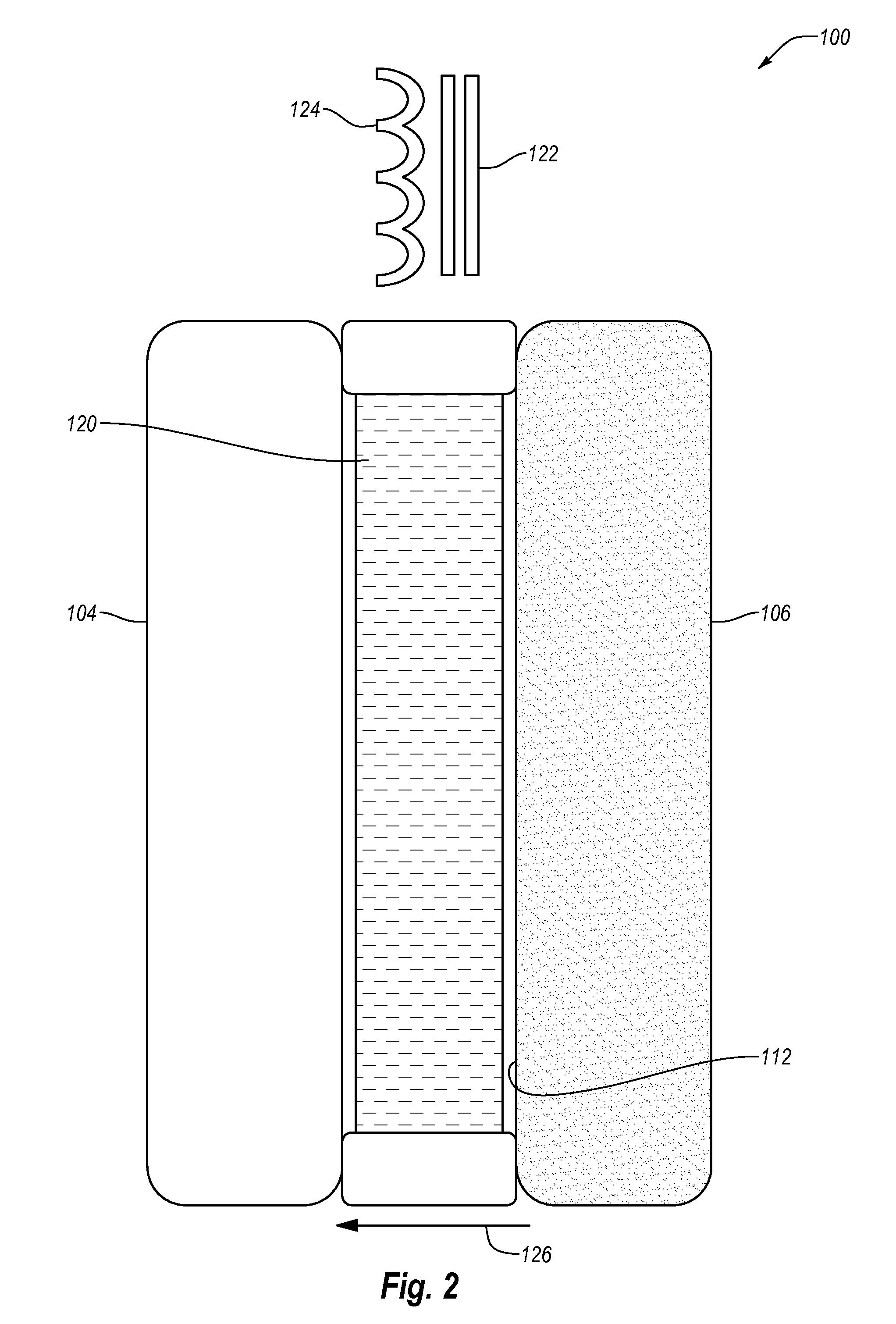
Phospholipid-based powders for drug delivery
InactiveUS7442388B2Raise the inlet temperatureRaise the outlet temperaturePowder deliveryBiocidePrillMedicine
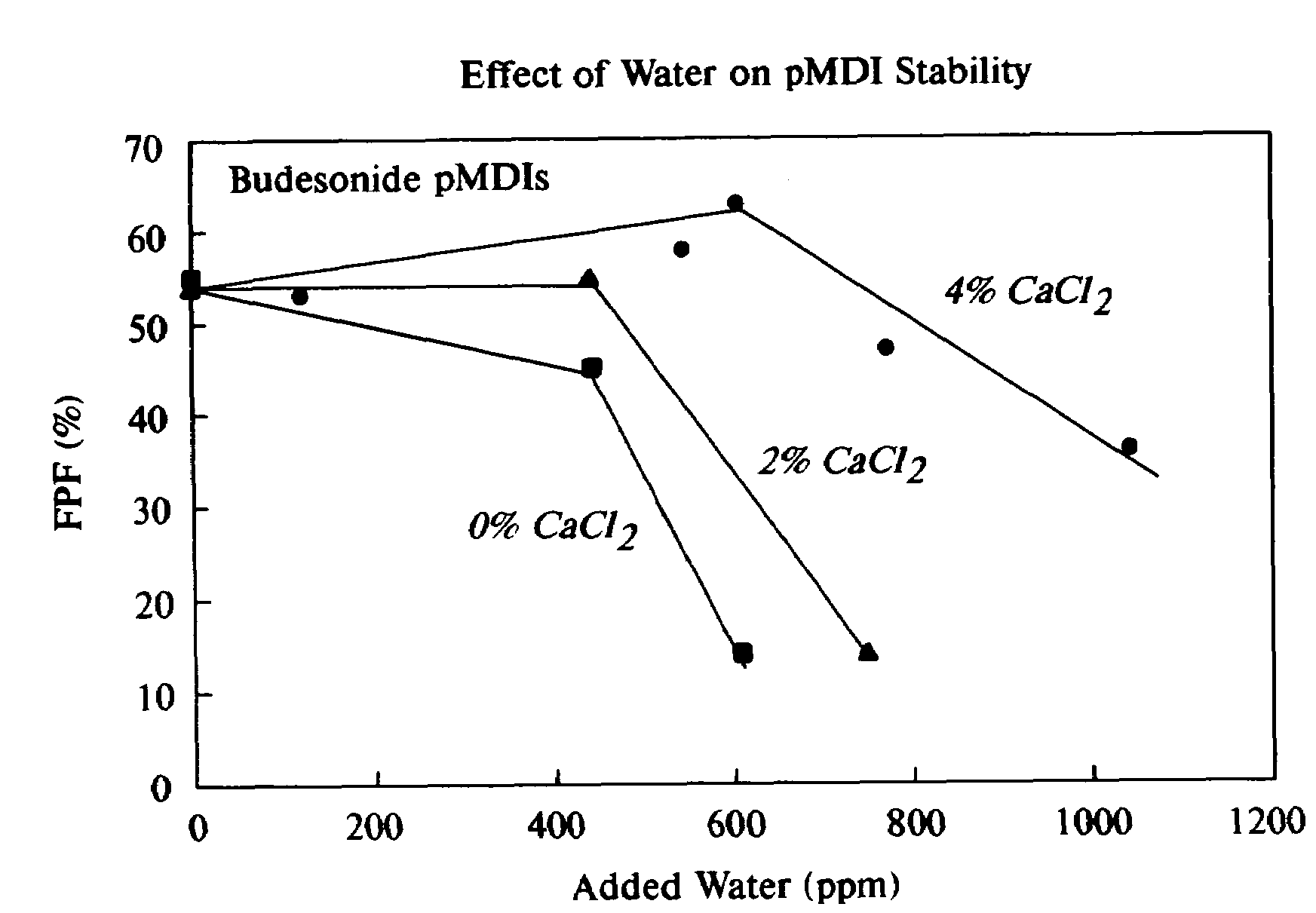
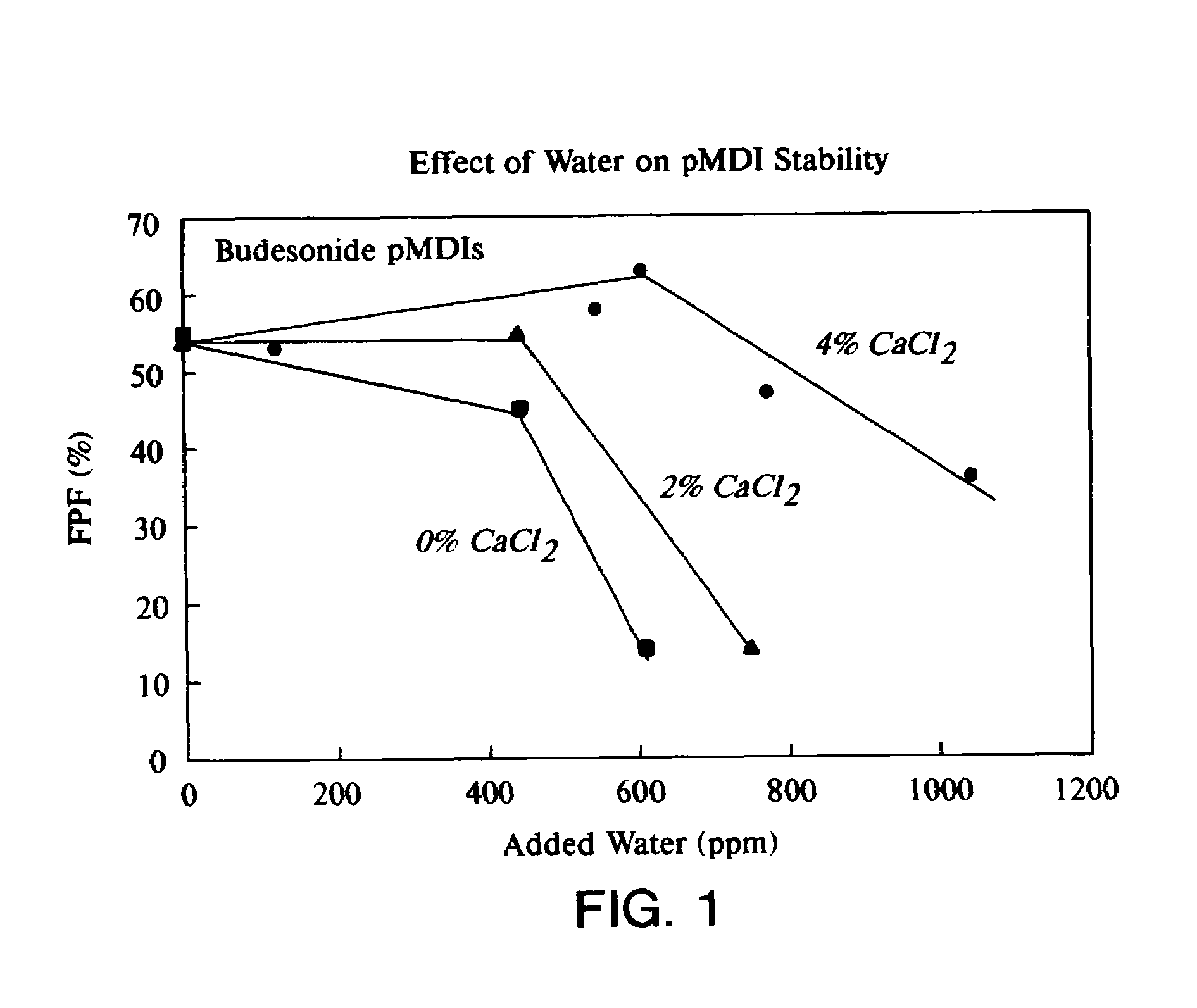
Phospholipid based powders for drug delivery applications are disclosed. The powders comprise a polyvalent cation in an amount effective to increase the gel-to-liquid crystal transition temperature of the particle compared to particles without the polyvalent cation. The powders are hollow and porous and are preferably administered via inhalation.
Owner:NOVARTIS AG
Rumen by-pass delivery system
InactiveUS20020127259A1Prevent oxidationEffective absorptionBiocideAnimal feeding stuffBiotechnologyNutrition
The present invention relates to a composition for the delivery of active agents that are protected from environmental oxidation and are resistant to ruminal fermentation degradation but not intestinal digestion and adsorption. The composition may contain biologically active ingredients such as for example, vitamins, minerals, proteins, amino acids, fatty acids, nutritional supplements, and pharmaceuticals together with natural and / or synthetic lecithin phospholipids.
Owner:ORTHOEFER FRAND T
High SPF transparent or translucent, cytoprotective, biodegradable, UV radiation resistant compositions
ActiveUS20080233060A1Protect the skinEliminating possible endocrine disruption responseCosmetic preparationsToilet preparationsCarrageenanPhospholipid
A composition comprising purified water using ozonation, ionization, or distillation or any combination thereof wherein alcohol may be substituted for, or combined with water at least one emollient including but not limited to chitosan, and aloe vera gel, individually or in any combination; an oil component with spf boosting agents including but not limited to; ethyl macadamiate, non-toxic silicone oil and essential oils, butter milk, waxes impregnated with inorganic sun-block or sunscreen agent and organic / inorganic micronized particles, wood powder and bentonite clay, keratin, either individually or in any combination; at least one inorganic sun-block or sunscreen agent including any metal oxide, glass microsphere, silica and silica compound, and optionally metal oxide pigments with particles that are micronized, submicronized, nanoparticle sized, or otherwise individually or in any combination that can be homogenized in either a water phase, a water-aloe phase, an oil phase or any phase of said composition; at least one emulsifier wherein said emulsifier includes but is not limited to a phospholipid and / or liposome or an aloe vera gel or an ester of coconut oil individually or in any combination, for emulsifying the water, water-aloe, or oil phase in combination with an homogenizer; where any of components are preferably mixed with an homogenizer and where an appropriate thickening agent including but not limited to xanthan gum, carageenan, either individually or in any combination is added as required.
Owner:GRUNE GUERRY L
Ophthalmic devices for delivery of hydrophobic comfort agents
ActiveUS20100140114A1Easy to understandPackage sterilisationPharmaceutical delivery mechanismLipid formationMonoglyceride
The invention relates to a soft hydrogel contact lens, especially a silicone hydrogel contact lens, which has a capability of delivering a hydrophobic comfort agent into the eye of a wearer. The hydrophobic comfort agent includes without limitation a monoglyceride, a diglyceride, a triglyceride, a glycolipid, a glyceroglycolipid, a sphingolipid, a sphingo-glycolipid, a phospholipid, a fatty acid, a fatty alcohol, a hydrocarbon having a C12-C28 chain in length, a mineral oil, a silicone oil, or a mixture thereof. It can be released from the soft hydrogel contact lens into the eye of a wearer when being worn so as to strengthen and stabilize the tear film lipid layer and alleviate the dryness of the eye.
Owner:ALCON INC
Natural marine source phospholipids comprising polyunsaturated fatty acids and their applications
ActiveUS8030348B2Reduce local fat depositReducing visible celluliteCosmetic preparationsOrganic active ingredientsPhospholipidMetal
A phospholipid extract from a marine or aquatic biomass possesses therapeutic properties. The phospholipid extract comprises a variety of phospholipids, fatty acid, metals and a novel flavonoid.
Owner:AKER BIOMARINE ANTARCTIC
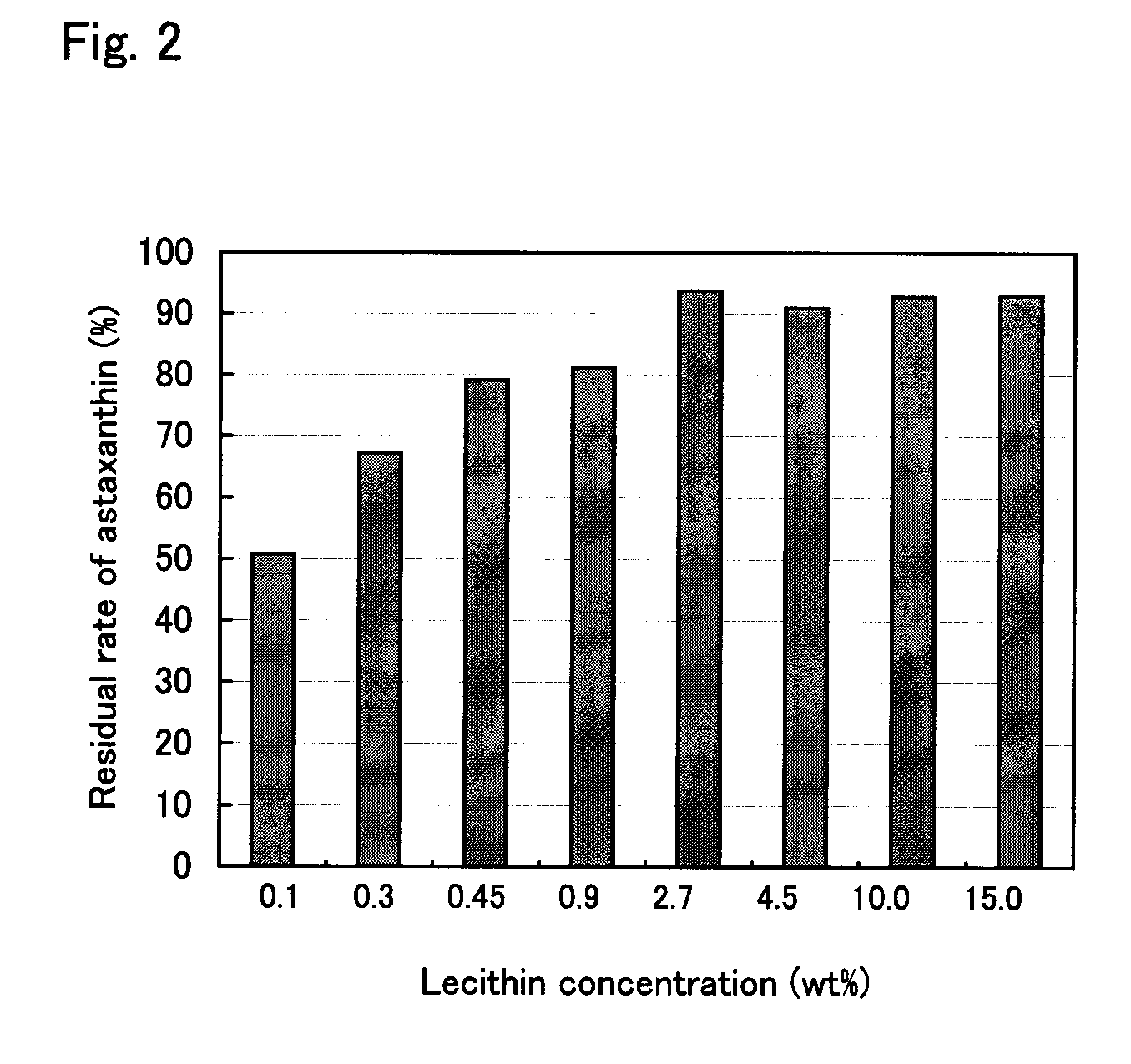
Green Algal Extract Containing Astaxanthin Having High Storage Stability
InactiveUS20070196383A1Good storage stabilityEasy to operateBiocideOrganic active ingredientsBetaxanthinsAstaxanthin
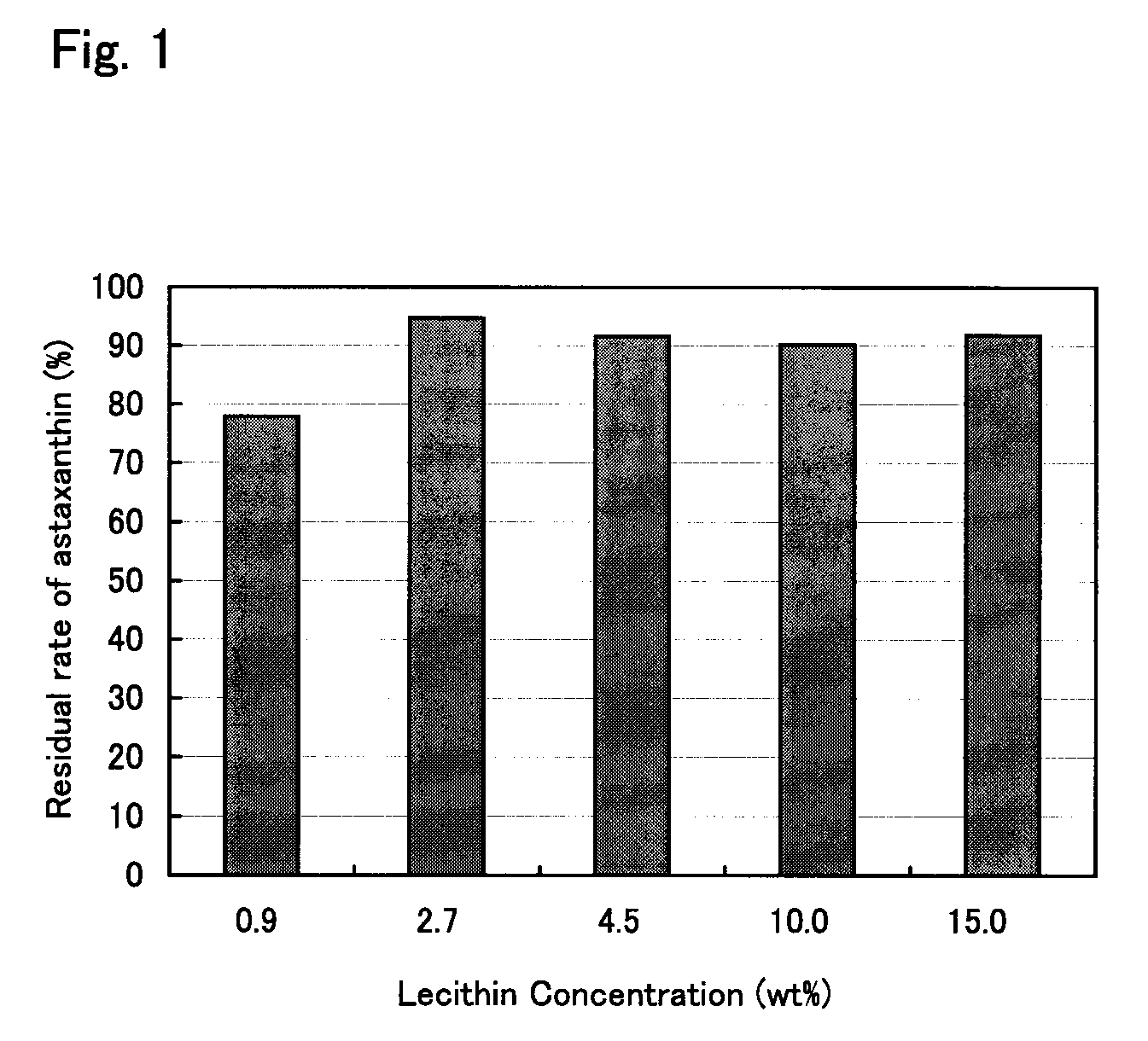
The present invention provides means for increasing the storage stability of astaxanthin in a green algal extract, and a green algal extract containing astaxanthin having high storage stability. The present invention provides a green algal extract comprising astaxanthin at a concentration of 0.5 to 20 wt % expressed in terms of free astaxanthin, and at least one phospholipid at a phospholipid concentration of 0.1 to 15 wt %. This green algal extract has an excellent ability to store stably astaxanthin, and even after storage for one week at 60° C. it is possible to retain approximately 80% or more of the astaxanthin.
Owner:YAMAHA MOTOR CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com