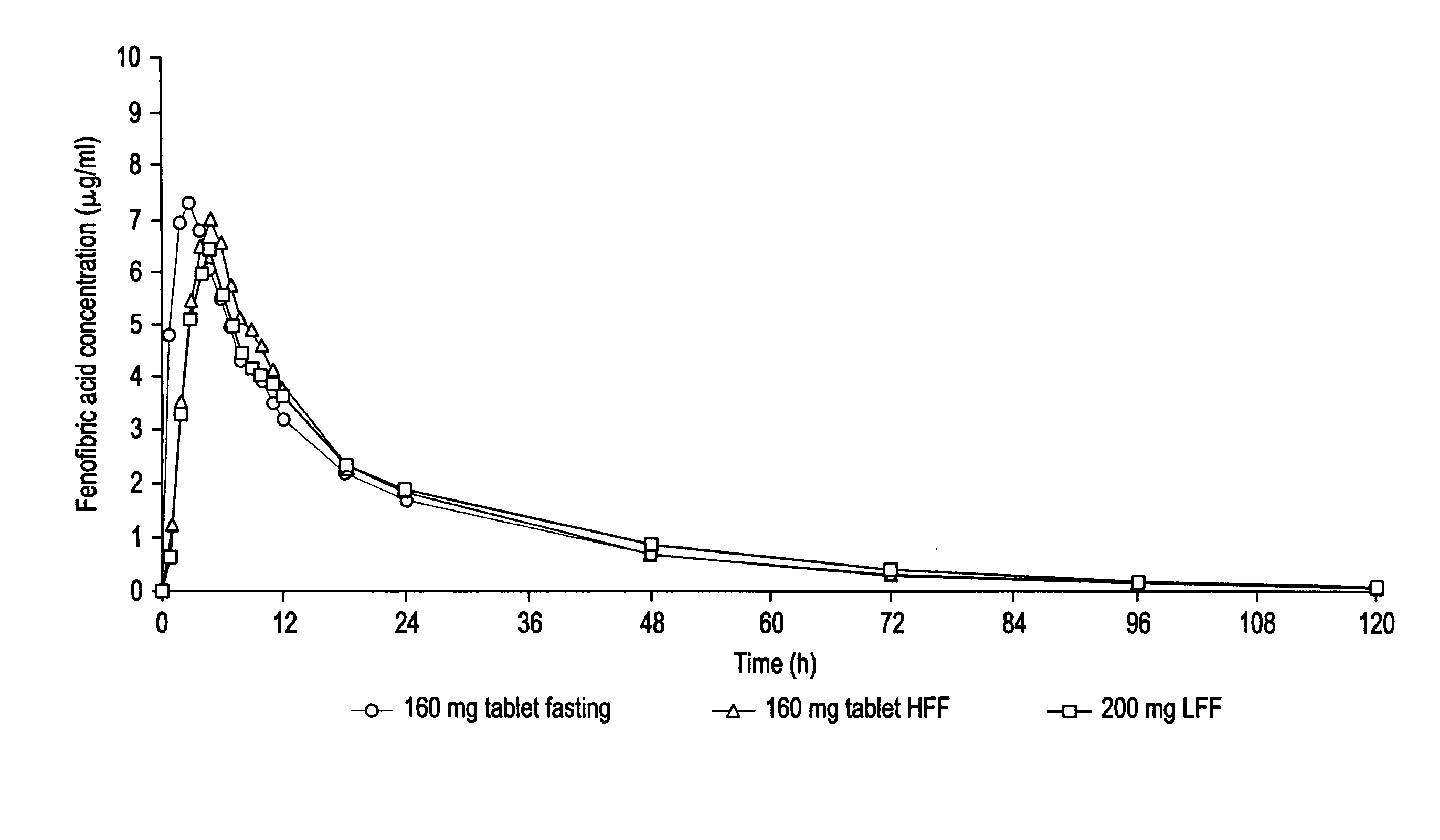
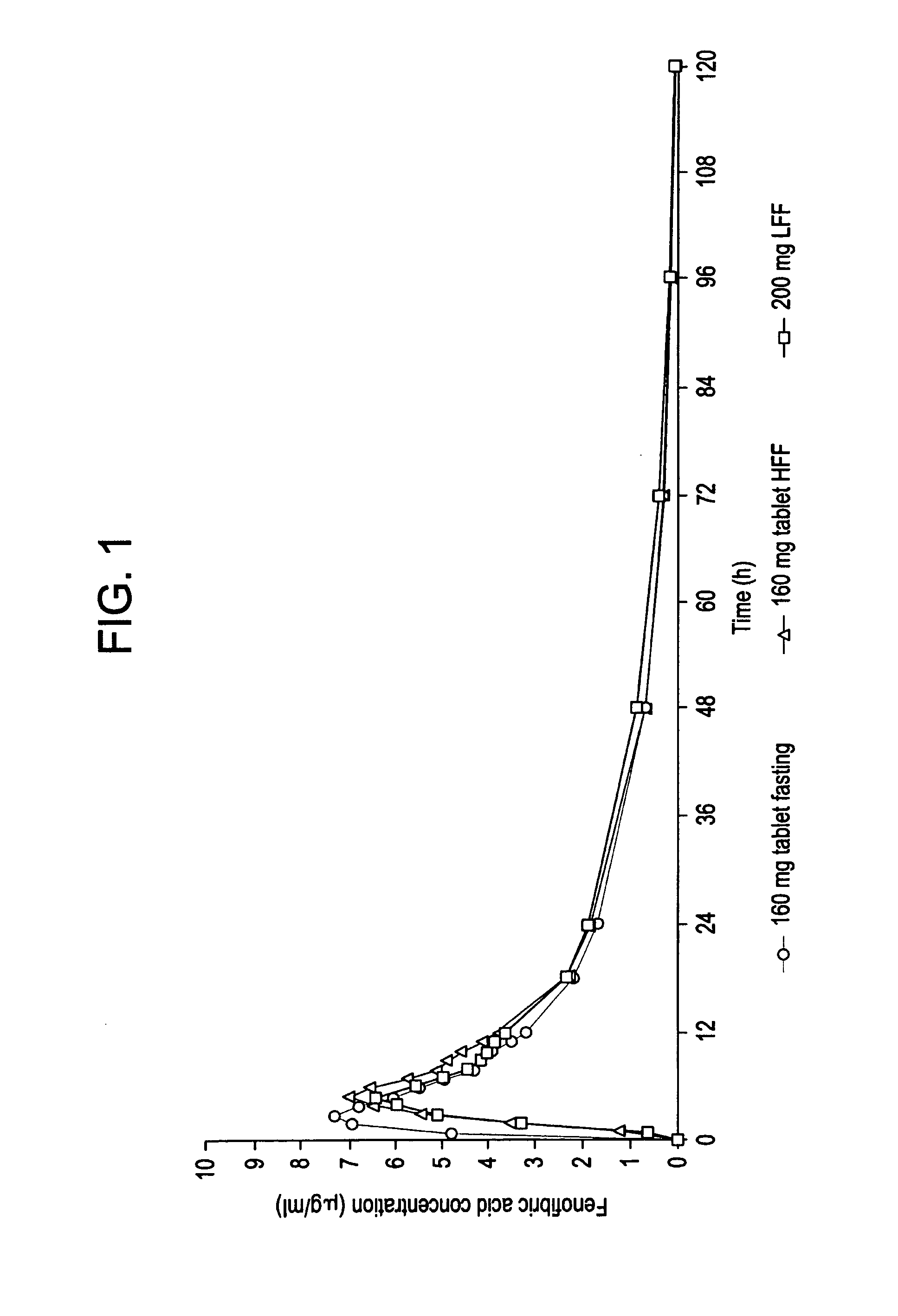
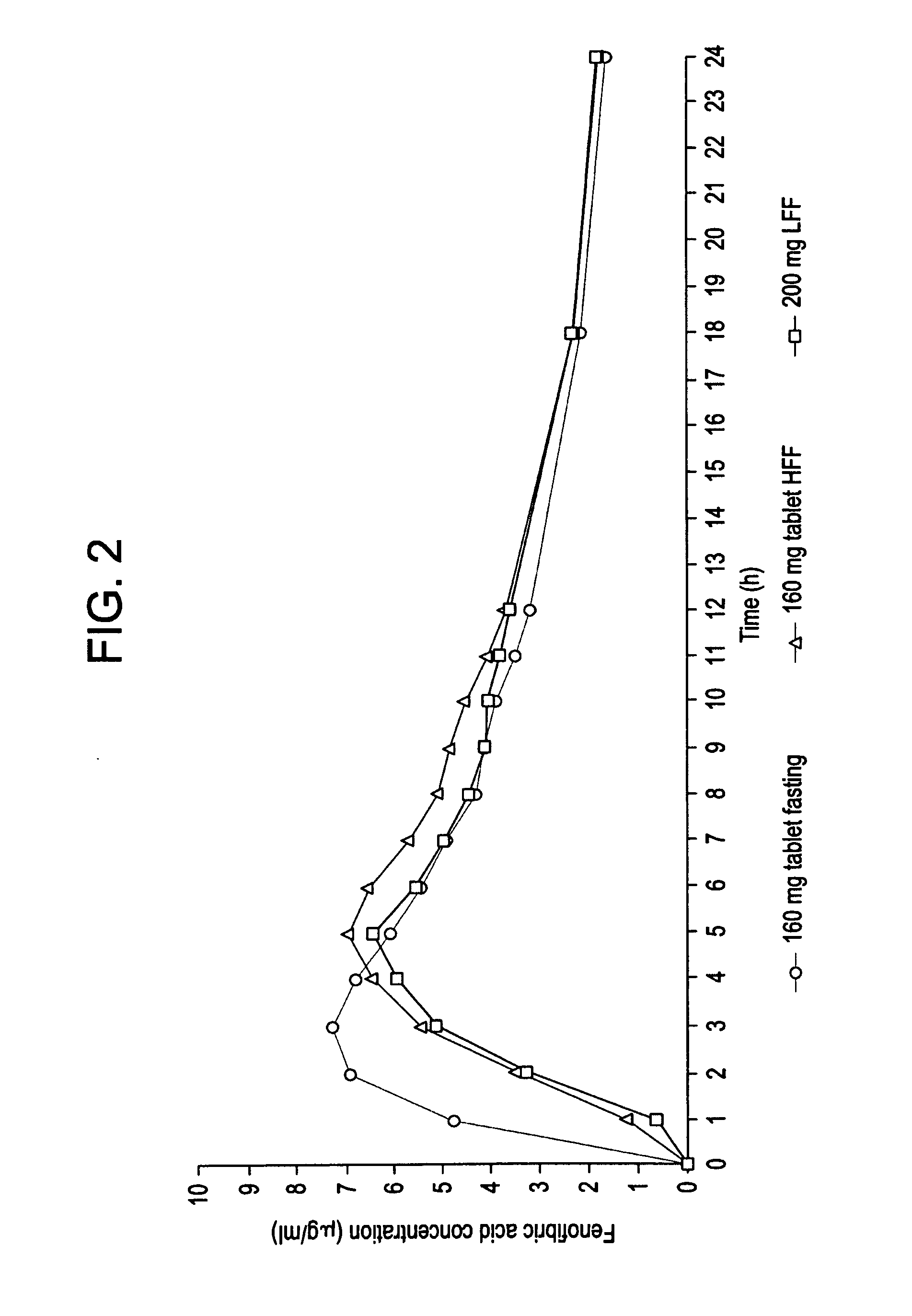
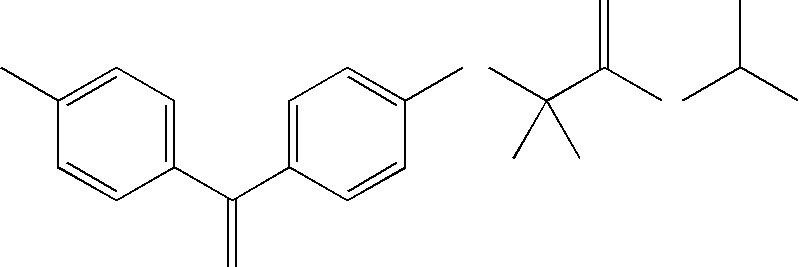
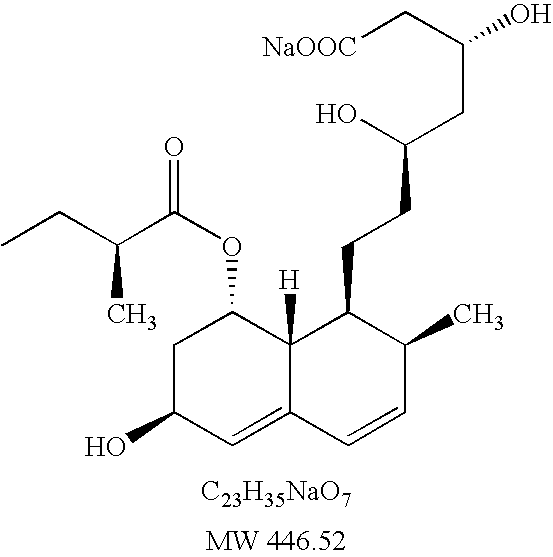
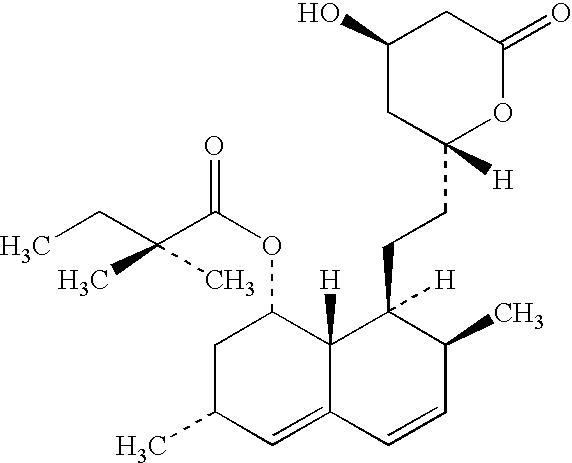
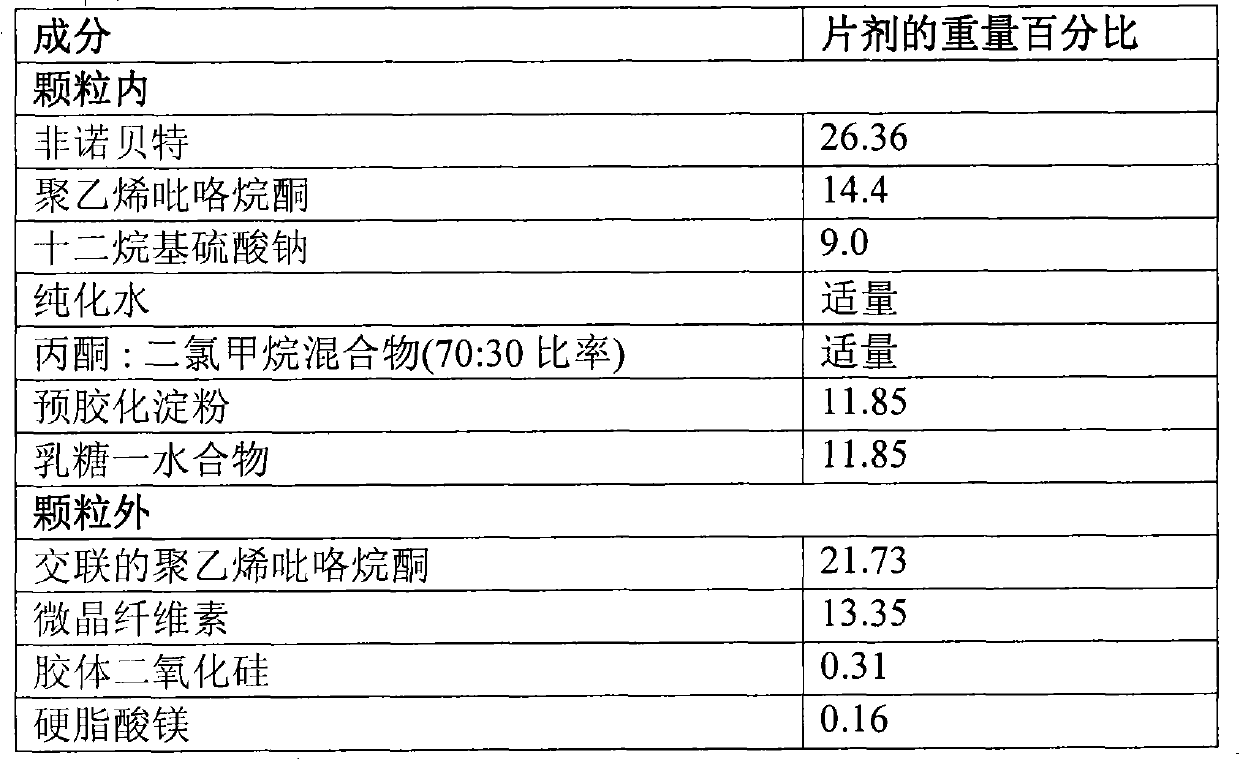
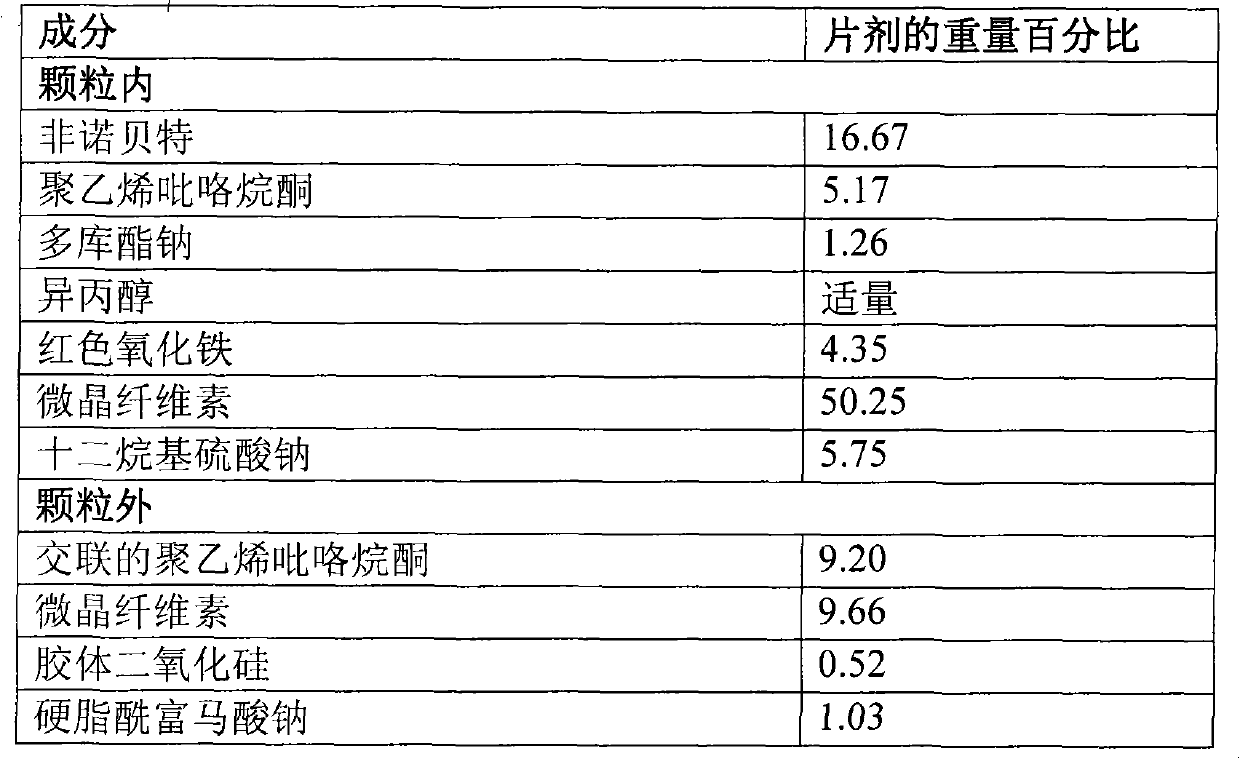
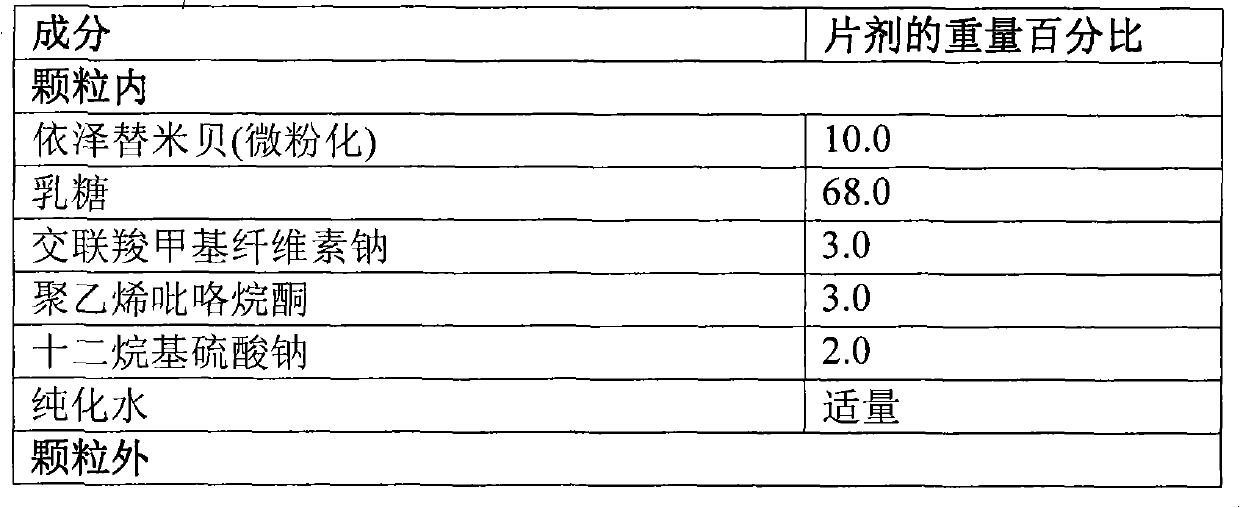
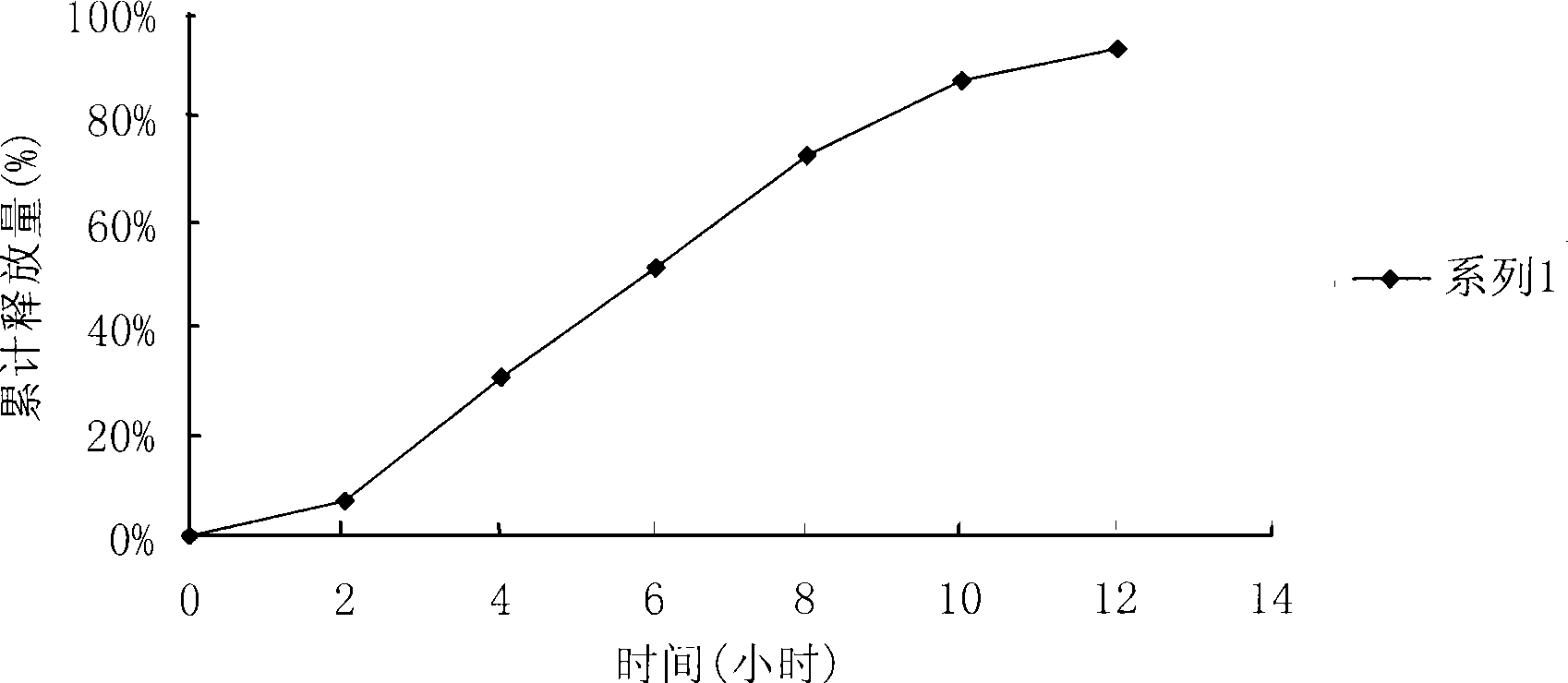
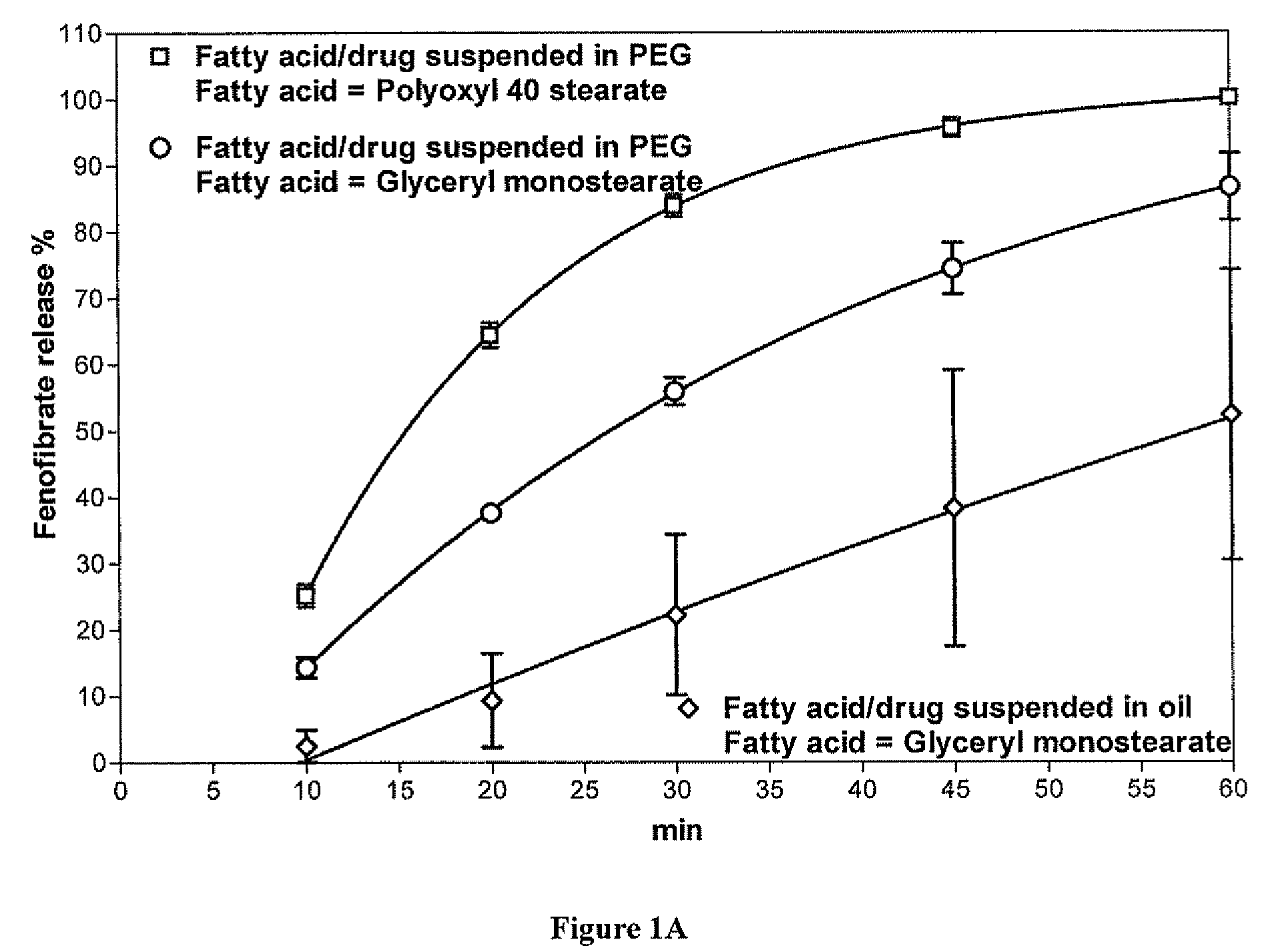
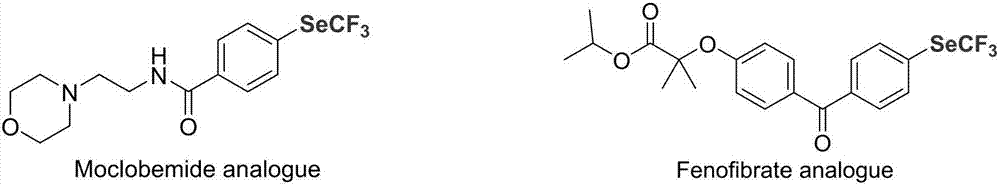Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
159 results about "Fenofibrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
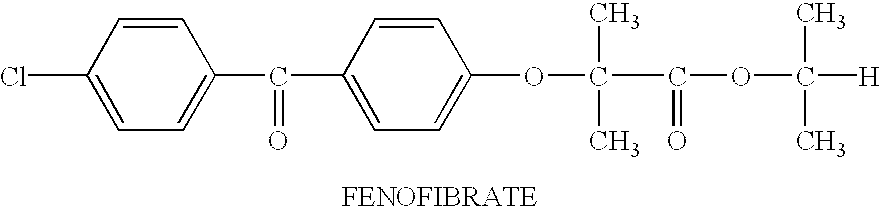
Fenofibrate is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood.
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs, particularly fenofibrate, are provided. The compositions comprise a therapeutically effective amount of an active agent and a solubilizer. The solubilizer is selected to effectively solubilize active agent in the composition. The solubilizers employed as part of the invention include: a vitamin E substance; monohydric alcohol esters such as trialkyl citrates, lactones and lower alcohol fatty acid esters; nitrogen-containing solvents; phospholipids; glycerol acetates such as acetin, diacetin and triacetin; glycerol fatty acid esters such as mono-, di- and triglycerides and acetylated mono- and diglycerides; propylene glycol esters; ethylene glycol esters; and combinations thereof. The pharmaceutical dosage forms contain the compositions in a suitable dosage form unit such as a capsule. Methods of treating patients comprising administering the compositions are also provided.
Owner:LIPOCINE
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
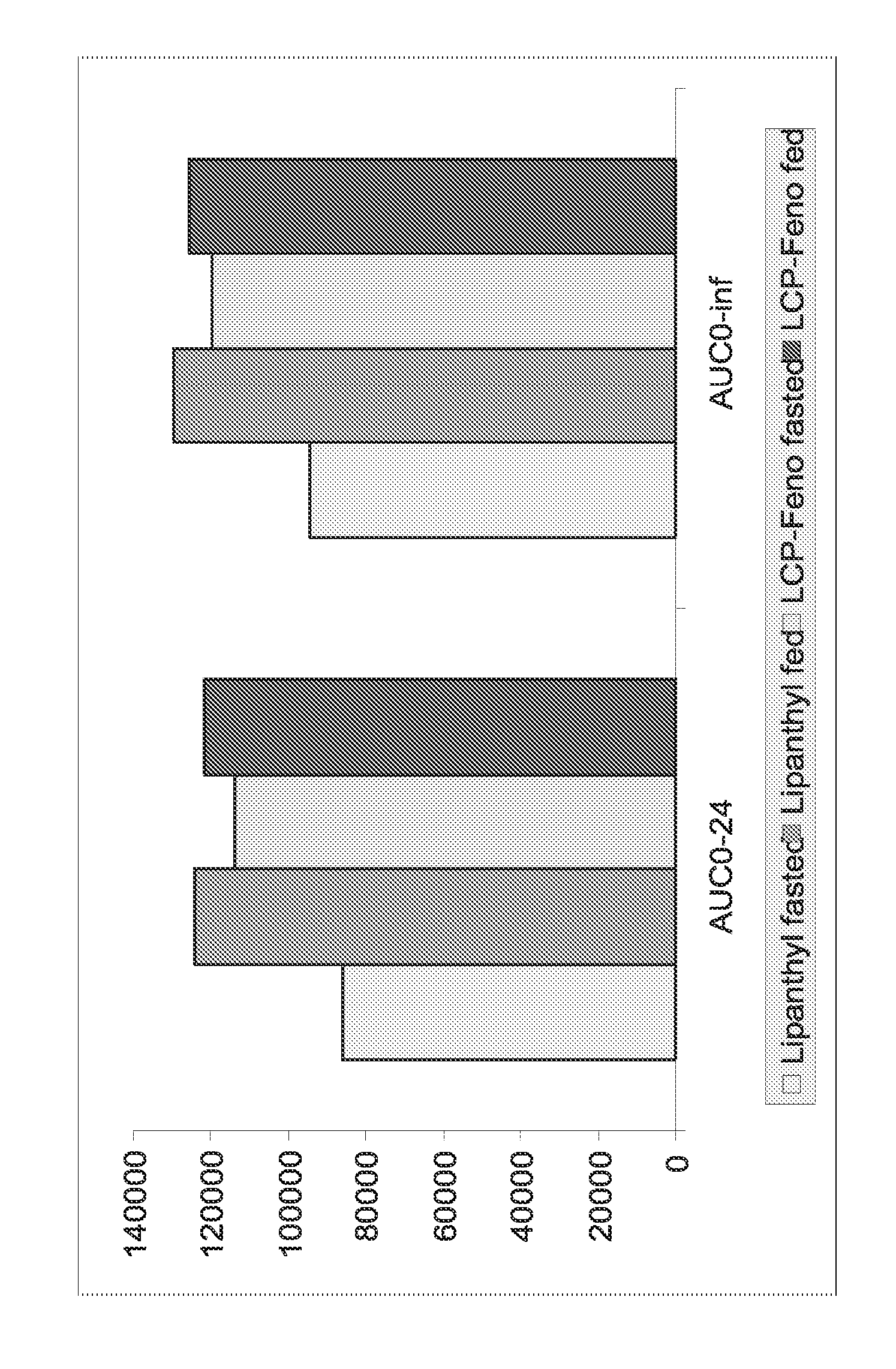
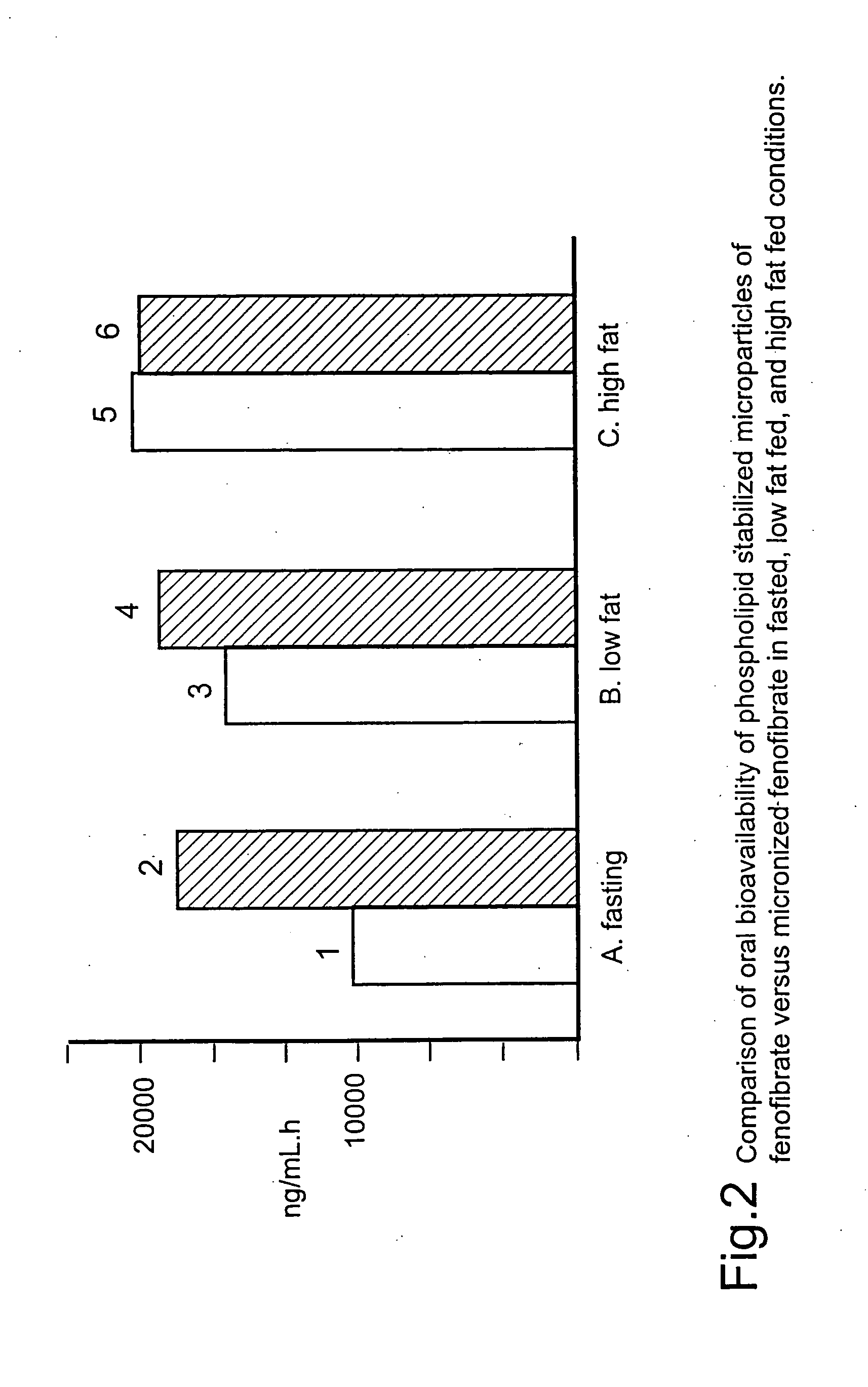
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Pharmaceutical compositions comprising fenofibrate and atorvastatin
InactiveUS20070014846A1Improve bioavailabilityReducing inter-individual variationBiocidePill deliveryParticulatesHMG-CoA reductase
Pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor atorvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCatorvastatin) of between about 250 and about 10,000. The solid compositions are manufactured without any need of addition of water or aqueous medium. Atorvastatin is optionally provided as a controlled release or a delayed release formulation resulting in a maintained LDL-lowering effect at a reduced dosage, and fenofibrate is provided in a formulation having increasing bioavailability and reduced food effect.
Owner:VELOXIS PHARMA
Stable Pharmaceutical Composition Comprising a Fixed Dose Combination of Fenofibrate and an Hmg-Coa Reductase Inhibitor
InactiveUS20080131503A1Avoid interactionImprove stabilityBiocideDrug compositionsHMG-CoA reductaseAdditive ingredient
Owner:VELOXIS PHARMA
Fenofibrate pharmaceutical composition having high bioavailability and method for preparing it
The invention provides an immediate-release fenofibrate composition comprising (a) an inert hydrosoluble carrier covered with at least one layer containing fenofibrate in a micronized form having a size less than 20 μm, a hydrophilic polymer and, optionally, a surfactant, the polymer making up at least 20% by weight of (a); and (b) optionally one or several outer phase(s) or layer(s).The invention also provides a method for preparing said composition.
Owner:LABES FOURNIER
Novel fenofibrate formulations and related methods of treatment
InactiveUS20090149533A1Readily bioavailableProperty is unexpectedBiocideOrganic active ingredientsFOOD EFFECTGram
The invention provides novel omega-3 ester-based oil solutions of fenofibrate. These solutions are substantially free of any food effect, effective in small volumes, and readily bioavailable. Notably, because the solutions of the invention contain an omega-3 ester-based oil as the major ingredient, they not only provide an antihyperlipidemic effect due to the fenofibrate active ingredient, they also provide recommended daily dosages of omega-3 oils (i.e., approximately 1 gram of omega-3 oil per day), or a portion thereof.
Owner:ALMBURG
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Fenofibrate dosage forms
InactiveUS20080241070A1Good redispersibilityImprove bioavailabilityBiocideOrganic active ingredientsCost effectivenessPhysiology
Disclosed are redispersible fibrate, such as fenofibrate, dosage forms. Also disclosed are in vitro methods for evaluating the in vivo effectiveness of fibrate, such as fenofibrate, dosage forms. The methods utilize media representative of in vivo human physiological conditions.
Owner:ABBOTT LAB IRELAND +1
Fenofibrate microparticles
Fenofibrate microparticles are prepared using a combination of surface modifiers with a phospholipid. Particle size growth and stability are controlled while significantly smaller sized fenofibrate particles are produced.
Owner:JAGOTEC AG
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
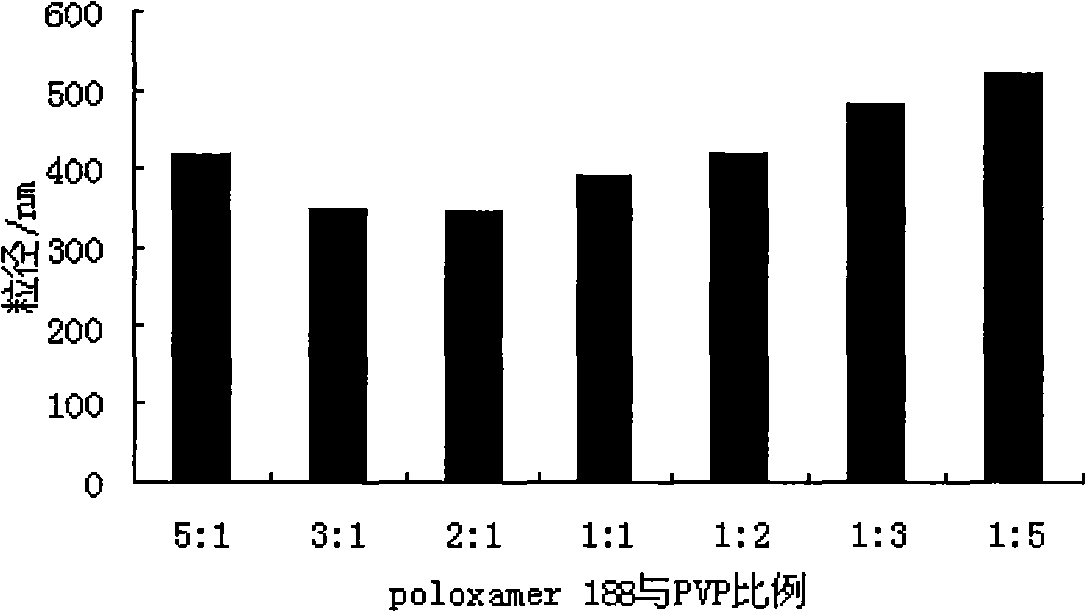
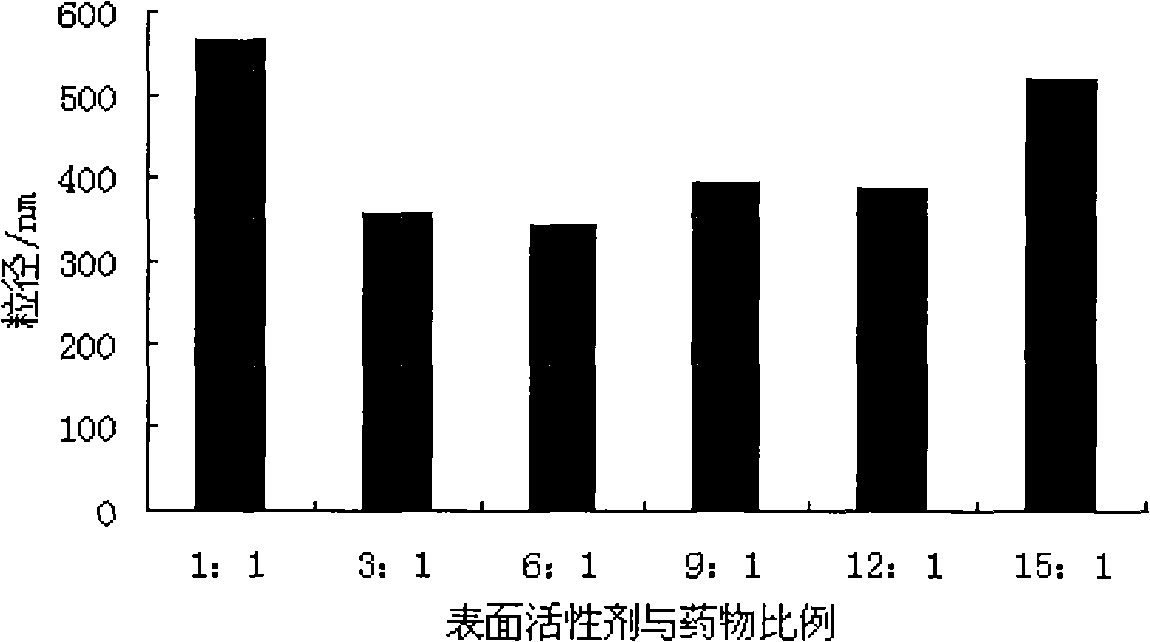
Fenofibrate nanometer suspension and preparation method thereof
InactiveCN101283982AOral bioavailability is lowSolve solubilityOrganic active ingredientsPowder deliveryPharmaceutical formulationSURFACTANT BLEND
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a fenofibrate nanosuspension and a lyophilized powder thereof. The fenofibrate nanosuspension is characterized in that poloxamer 188 and polyvinylpyrrolidone (PVP), with a weight ratio of (1-3):1, are used as surfactant. The fenofibrate nanosuspension has stable quality and particle diameter of about 190-380nm. The invention further discloses a preparation method thereof.
Owner:NANJING UNIV OF TECH
Pharmaceutical formulations of fenofibrate having improved bioavailability
Provided are pharmaceutical compositions of fenofibrate, and dosage forms containing them, that include fenofibrate, a polyethylene glycol, and a polyethylene-polypropylene glycol; wherein the compositions are made by subliming a sublimable carrier from a combination of fenofibrate, the polyethylene glycol, and the polyethylene-polypropylene glycol with the sublimable carrier, for example menthol.
Owner:TEVA PHARM USA INC
Self-emulsifying formulations of fenofibrate and/or fenofibrate derivatives with improved oral bioavailability and/or reduced food effect
InactiveUS7022337B2Improve bioavailabilityBiocideCapsule deliveryTG - TriglyceridePolyethylene glycol
A fibrate self-emulsifying oral formulation with improved bioavailability when compared to commercially available formulations containing a therapeutically effective dose of fenofibrate, derivative of fenofibrate or mixtures thereof dissolved in a fibrate solubilizer selected from N-alkyl derivative of 2-pyrrolidone, mono- or di- or polyethylene glycol monoethers, C8-12 fatty acid mono- or di-esters of propylene glycol, or combinations thereof, one or more surfactants and optionally one or more stabilizers useful in the treatment of hypercholesterolaemia or hypertriglyceridaemia in mammals in the fed or fasted state.
Owner:SUPERNUS PHARM INC
Treating metabolic syndrome with fenofibrate
ActiveUS20060083783A1Reduce in quantityOvercome problemsBiocidePill deliveryAfter treatmentTreatment period
A method of treating metabolic syndrome in a human diagnosed with metabolic syndrome by administering a therapeutically effective amount of fenofibrate over a treatment period. The results can include identifying a human as not having clinical metabolic syndrome after treatment, as compared to having metabolic syndrome before treatment.
Owner:LUPIN INC
Solid dosage form comprising a fibrate and a statin
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of a fibrate, notably fenofibrate, and a statin (also known as a HMG CoA reductase inhibitors), which compositions are manufactured without any need of addition of water or an aqueous medium and wherein at least 80% of the active substances (i.e. the fibrate and the statin) are present in the composition in dissolved form in order to ensure suitable bioavailability of both active ingredients upon oral administration
Owner:VELOXIS PHARMA
Stable compositions of fenofibrate with fatty acid esters
A pharmaceutical composition in unit dose form of fenofibrate and a solvent system of fatty acid esters, wherein the fenofibrate is substantially dissolved in the solvent system.
Owner:RELIANT PHARMACEUTICALS INC
Compositions comprising fenofibrate and simvastatin
InactiveUS20060105050A1Substance may accumulateImprove bioavailabilityPowder deliveryBiocideParticulatesHMG-CoA reductase
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor simvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCsimvastatin) of between about 800 and about 29,300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and simvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Antilipidemic pharmaceutical compositions and process for preparation thereof
The present invention relates to a new process for preparing an oral pharmaceutical composition comprising fenofibrate alone or in combination with at least one other antilipidemic agent in a single dosage form that can be conveniently administered once or twice in a day.
Owner:RANBAXY LAB LTD
Fenfibrate masticatory and its preparation
InactiveCN1509711AThe particle size of the main drug is smallSmall particle sizeOrganic active ingredientsMetabolism disorderWater bathsSURFACTANT BLEND
A chewing tablet of fenofibrate is prepared from fenofibrate, solid disperser as carrier, filler, disintegrant, flowing aid, lubricant, surfactant and sweetening agent through proportioning, heating the fenofibrate and carrier by water bath, quick cooling for solidifying, drying, breaking, mixing with others, and tabletting.
Owner:四川维奥制药有限公司
Fenofibrate osmotic pump controlled release preparation and preparation method thereof
InactiveCN101422443AAdjust the rate of constant releaseImprove complianceOrganic active ingredientsMetabolism disorderSide effectFoaming agent
The invention belongs to the field of medicament preparation and discloses a fenofibrate osmotic pump type controlled release preparation and a preparation method thereof. The preparation is formed by an osmotic pump tablet core and a controlled release coat coated outside the tablet core. The weight of the osmotic pump controlled release tablet core is as follows: 250mg / tablet of fenofibrate, 25mg to 100mg / tablet of osmotic active matter, 200mg of 300mg / tablet of accessory which can lead the medicament in a medicine containing layer to be easily released, 75 to 150mg / tablet of accessory which leads the medicament to be easily released in a promoting layer and proper amount of other accessories. The weight of the osmotic pump coat is as follows: 15g to 30g / 100 tablets of semi-transparent membrane coat macromolecule material and 2 to 10g / 100 tablets of pore-foaming agent. The invention can effectively adjust the release speed of the medicament by adjusting the tablet core and the prescription of the coat and obtain more stable and durable effective blood medicine concentration, thereby reducing the side effect and the taking times of the medicament, ensuring that the suction of themedicament is not affected by whether the patient eats or not and the environment in a body, and improving the compliance of a sufferer. The invention can be broadly applied to curing hyperlipemia.
Owner:SHENYANG PHARMA UNIVERSITY
Solid preparation containing highly dispersed fenofibrate
The invention provides a solid preparation containing highly dispersed fenofibrate, wherein the fenofibrate stably exists in carrier materials in a highly dispersed form. The carrier materials are water-soluble carrier materials and are selected from polyethylene glycol, povidone, a surfactant containing a polyoxyethylene group, a water-soluble cellulose derivative, organic acid, organic sugar and organic alcohol. The carriers can be used separately or in a combination manner. The solid preparation containing the highly dispersed fenofibrate can be in a form of capsules or tablets. The bioavailability of the fenofibrate can be improved. The dosage of the single preparation of the fenofibrate can be reduced.
Owner:SHANGHAI TIANLONG PHARMA
Solid dosage forms of fenofibrate
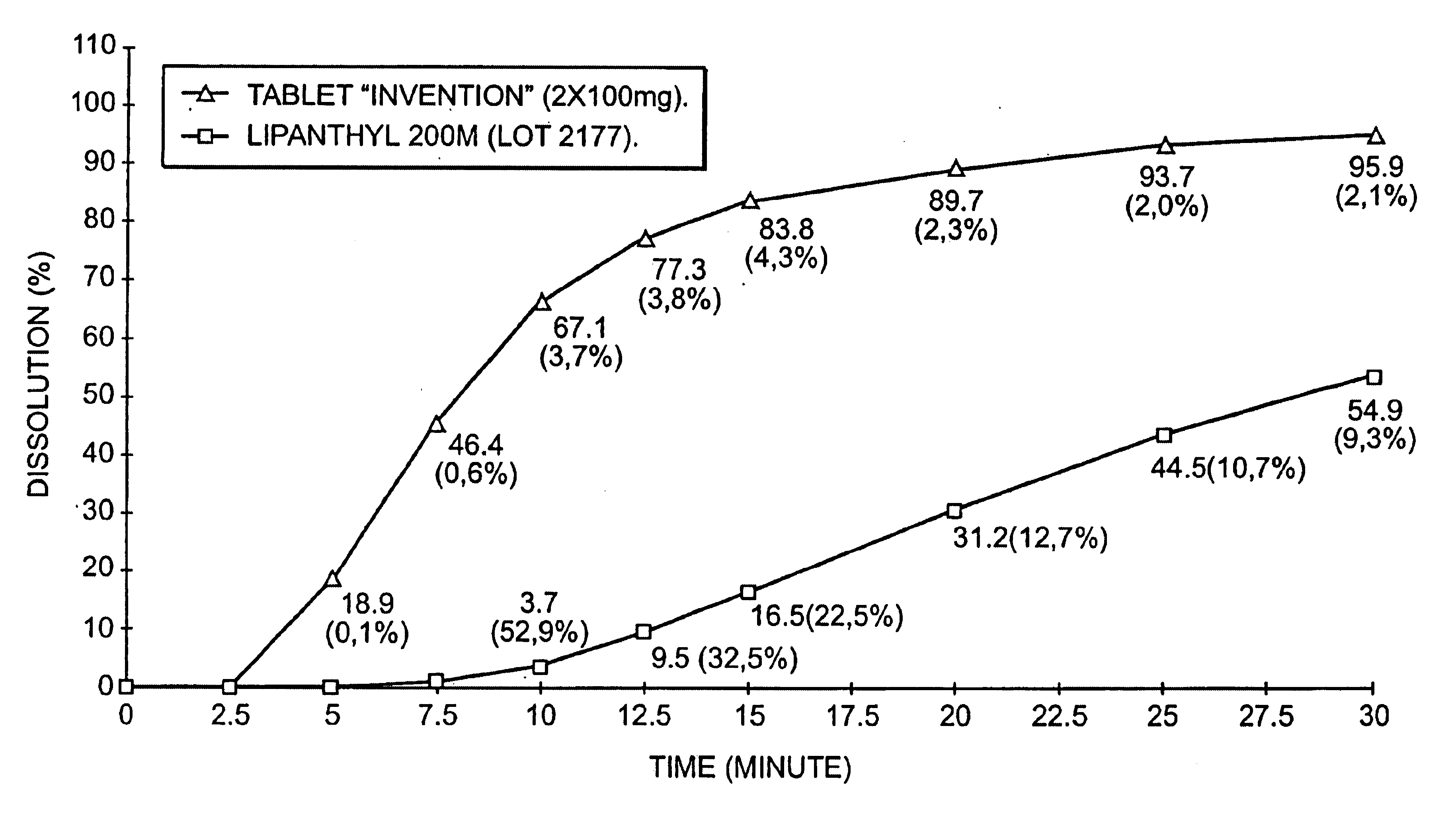
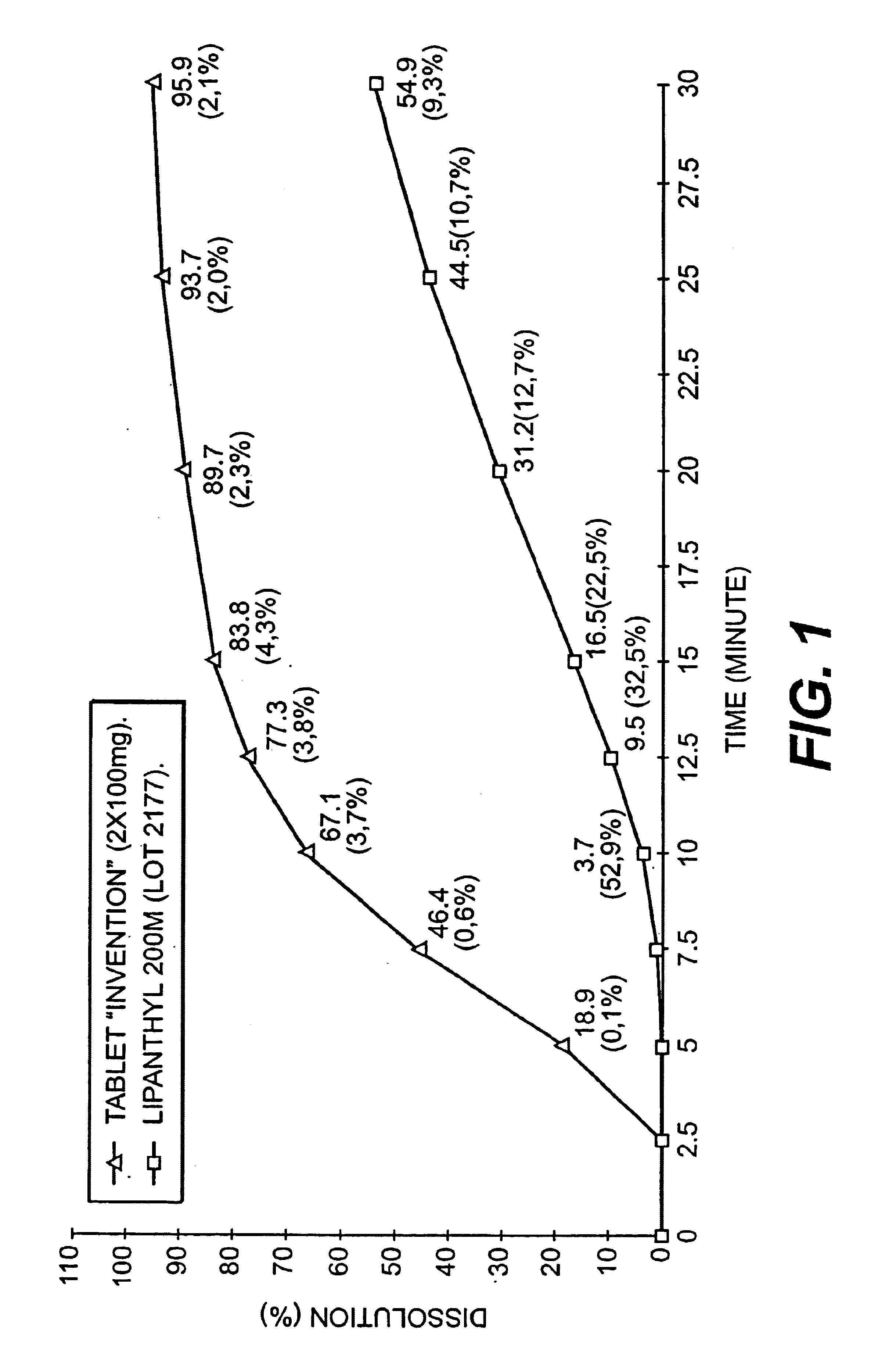
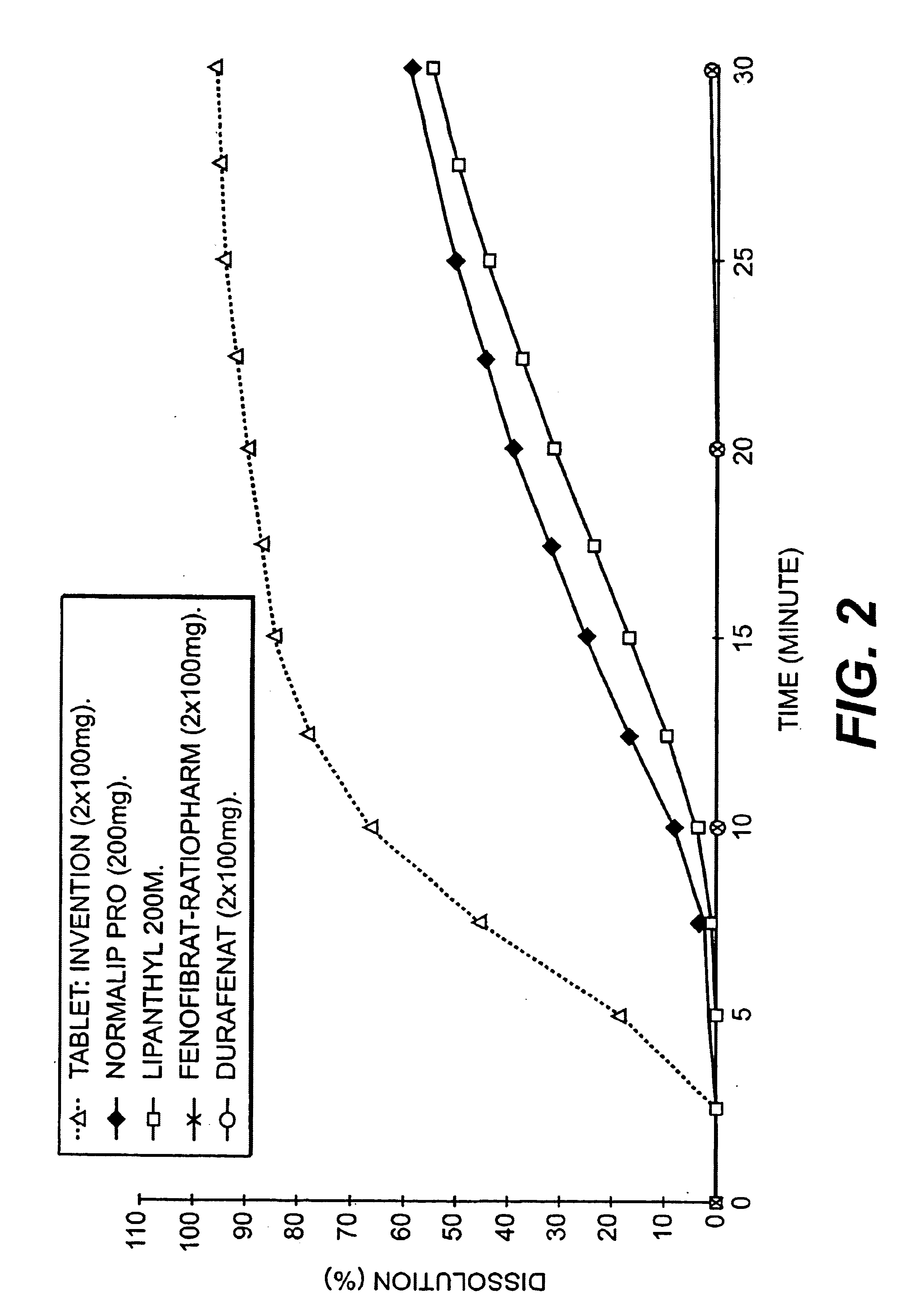
An improved solid dosage form of fenofibrate which exhibits improved dissolution properties leading to increased bioavailability of fenofibrate. A novel core-shell approach to the composition is provided as well as a process for the preparation of the improved solid dosage forms.
Owner:AUROBINDO PHARMA LTD
Methods for enhancing the release and absorption of water insoluble active agents
InactiveUS8524280B2Improved profilePromote absorptionBiocidePowder deliveryHydrophilic polymersActive agent
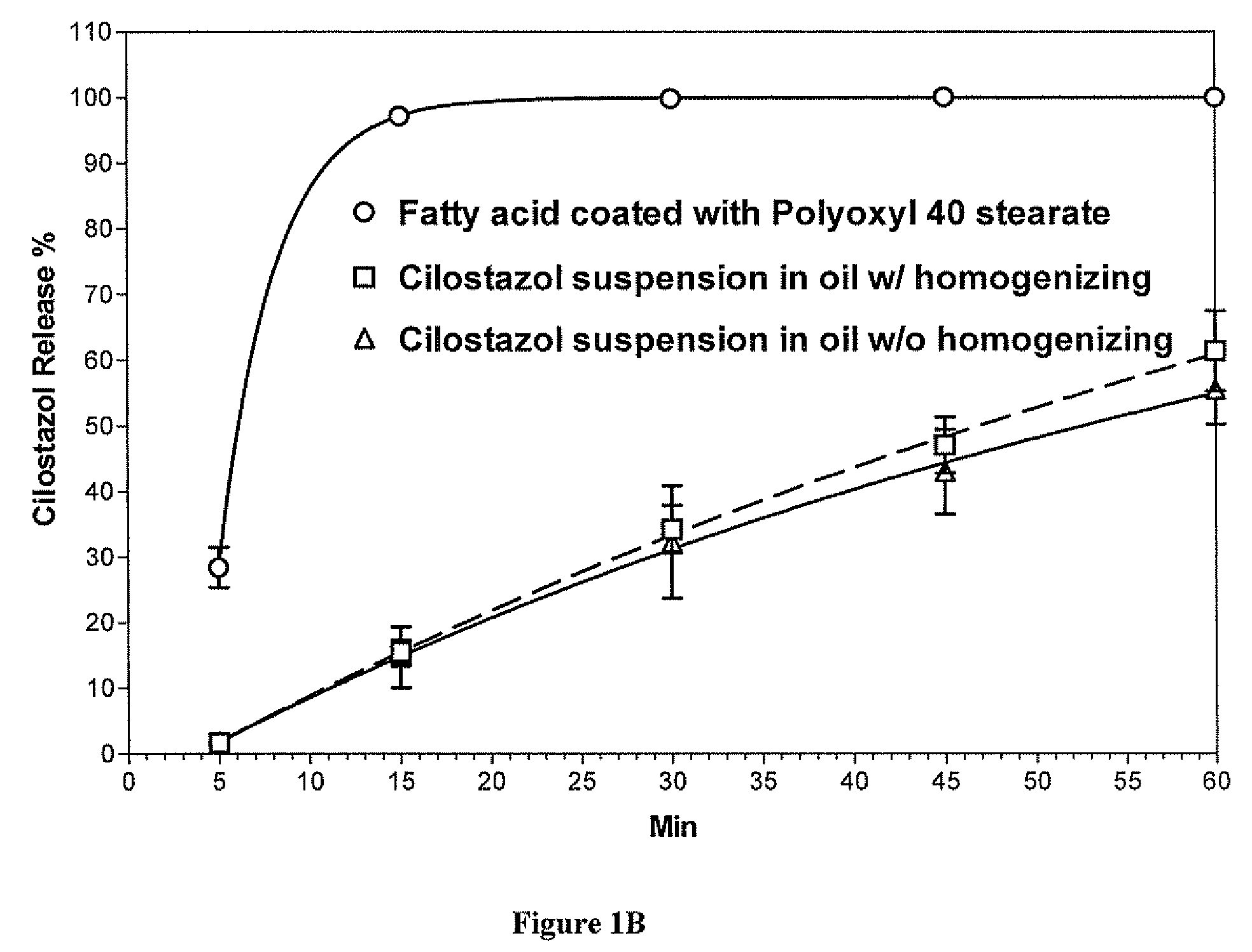
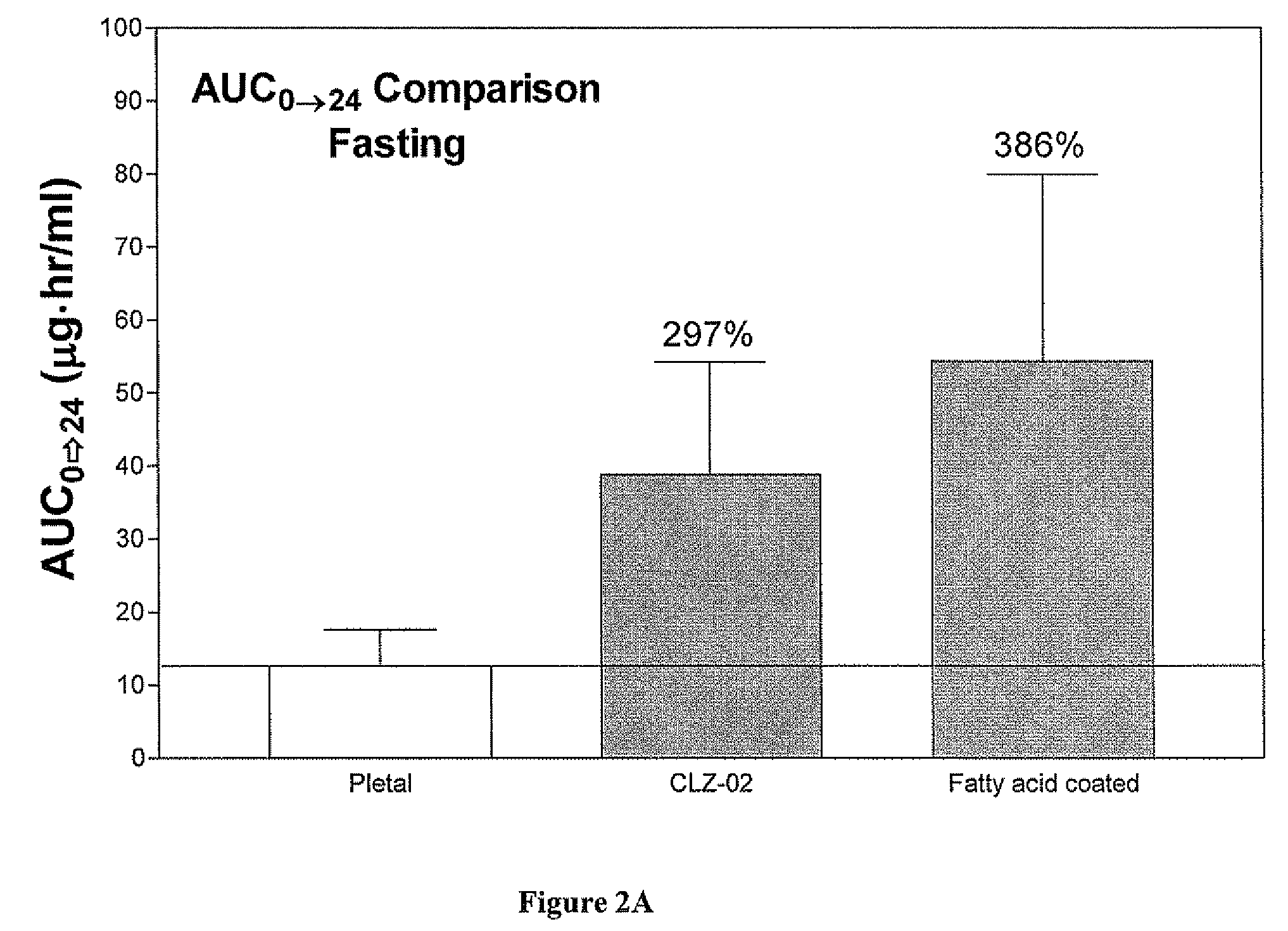
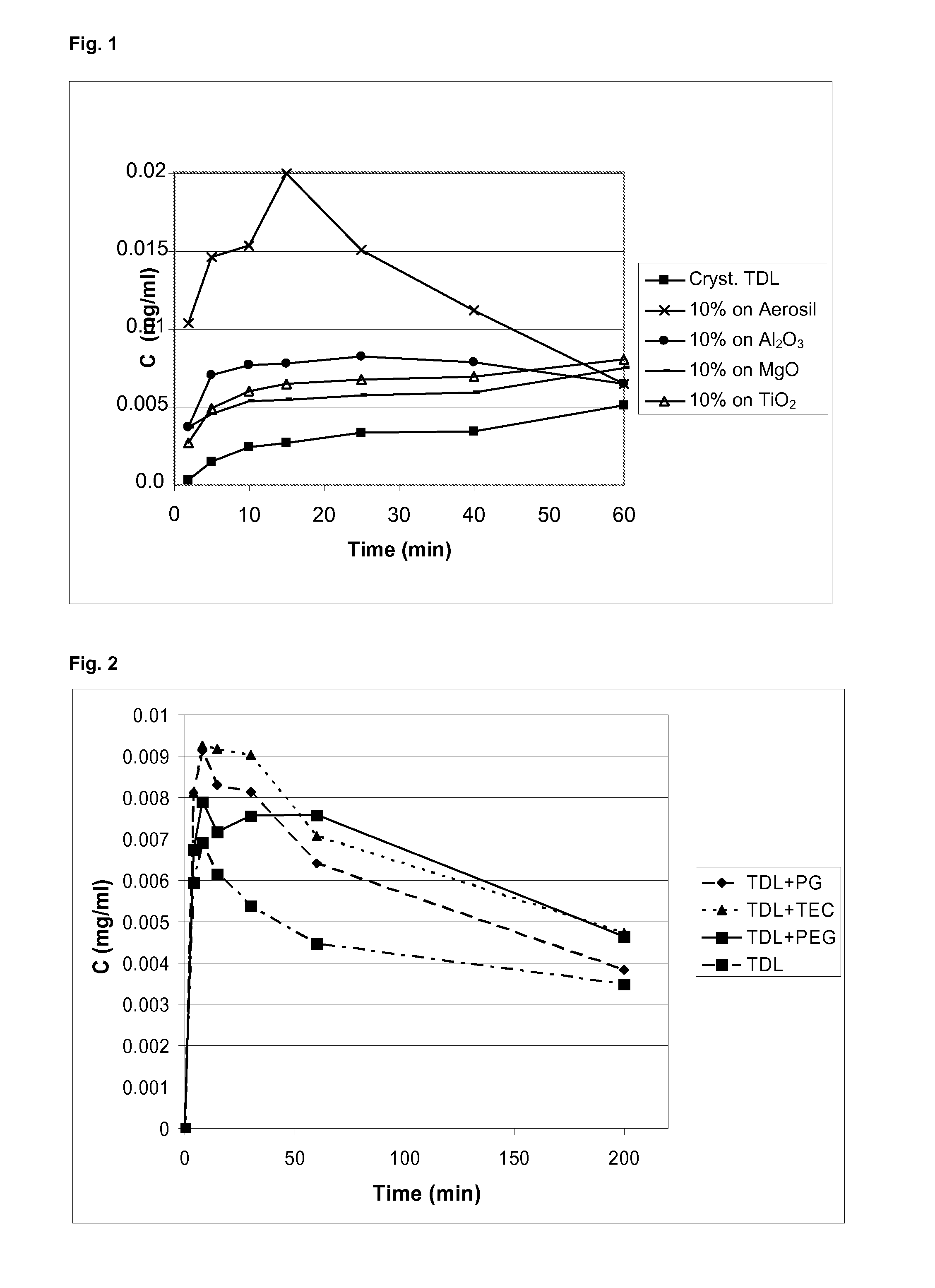
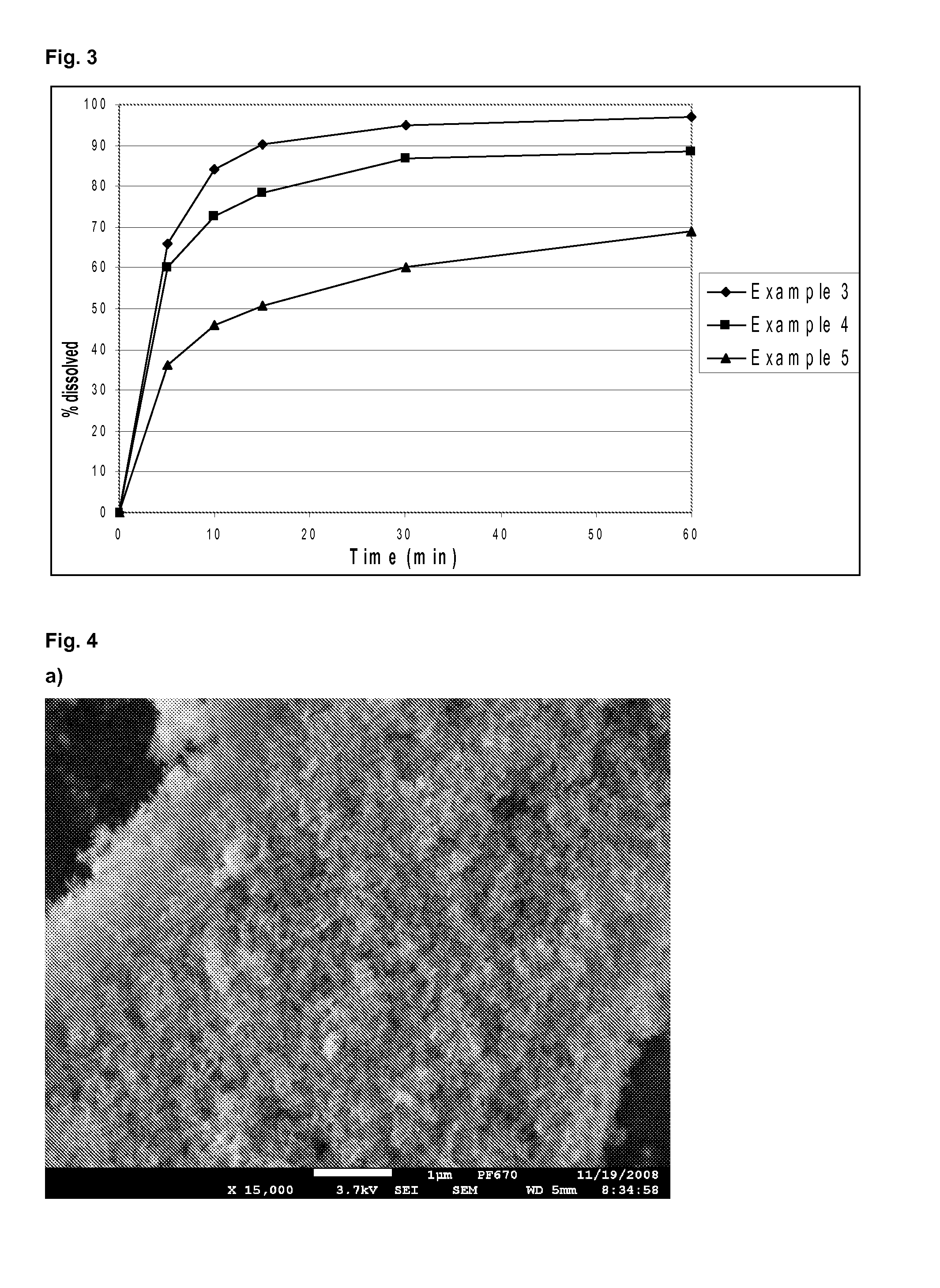
Methods for enhancing the release and / or absorption of poorly water soluble active agents are described herein. The method involves dissolving, melting, or suspending a poorly water soluble active agent in one or more molten fatty acids, conjugated fatty acids, (semi-) solid surfactants of high HLB value, and / or hydrophilic polymers. The molten active agent mixture is then suspended and homogenized in a hydrophilic or lipophilic carrier to form microparticles suspended in the hydrophilic or lipophilic carrier. The particles suspended in the hydrophilic or lipophilic carrier can be encapsulated in a hard or soft gelatin or non-gelatin capsule. It is believed that the microparticles produced by the method described above will exhibit enhanced dissolution profiles. In vitro release studies of formulations containing cilostazol and fenofibrate showed 100% dissolution of cilostazol in 15 minutes and over 90% dissolution of fenofibrate in 35 minutes.
Owner:PATHEON SOFTGELS INC
Active pharmaceutical ingredient adsorbed on solid support
InactiveUS20120088774A1Good chemical stabilityImprove physical stabilityPowder deliveryBiocideLovastatinTadalafil
The present invention belongs to the field of pharmaceutical industry and relates to dosage forms comprising active pharmaceutical ingredients (API) such as tadalafil, simvastatin, fenofibrate and lovastatin that are practically insoluble in water, adsorbed on a carrier. Furthermore it relates to an adsorbate comprising API being practically insoluble in water and to a process for the preparation of said adsorbate with non-polar solvent (s) such as chlorinated hydrocarbon, diisopropylethes and hexane. Furthermore the invention relates to a process for the preparation of the dosage form, as well as to the use of the adsorbate for the preparation of the dosage form. Moreover it relates to the dosage form for use in the treatment of erectile dysfunction, human immunodeficiency virus (HIV) infections and / or Acquired Immune Deficiency Syndrome (AIDS).
Owner:LEK PHARMA D D
Coated tablets
InactiveUS20060257494A1Improve bioavailabilityReduce the differencePowder deliveryBiocideCelluloseMicroparticle
Disclosed is a pharmaceutically acceptable oral dosage form comprising fenofibrate, phospholipid, a buffer salt, a water-soluble bulking agent selected from maltodextrin, mannitol, and combinations thereof, a cellulosic additive, beads or crystals of a pharmaceutically acceptable water-soluble excipient support material, a polyvinylpyrrolidone or crospovidone, croscarmellose sodium, granular mannitol, sodium dodecyl sulfate, silicon dioxide, and a stearate, wherein the fenofibrate is in the form of microparticles, and wherein at least a portion of the phospholipid is coated on the surfaces of the fenofibrate microparticles, the phospholipid coated microparticles are embedded in a matrix comprising the water-soluble bulking agent, phospholipid that is not coated on the microparticles, the buffer salt and the cellulosic additive, and the matrix is coated on up to 100% of the surfaces of the beads or crystals of the excipient support material.
Owner:JAGOTEC AG
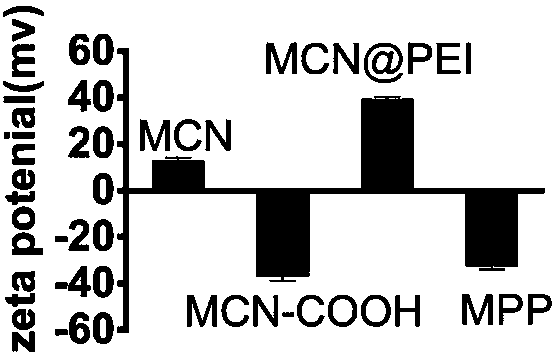
Polymer-modified mesoporous carbon nanoparticle and preparation and application thereof
ActiveCN109806240AOrganic active ingredientsPharmaceutical non-active ingredientsPolymer modifiedDissolution
The invention belongs to the technical field of medicine, and relates to preparation of mesoporous carbon with an adjustable particle size, and preparation of a Polymer-modified mesoporous carbon nanoparticle and application thereof as a poorly soluble drug carrier to promoting oral absorption. First, the mesoporous carbon with a particle size ranging from 50 to 1000 nm is prepared by adjusting the formulation process using a 'one-step method'. After the prepared mesoporous carbon is carboxylated, polyethyleneimine-polyacrylic acid is modified on the surface of the carboxylated carbon sphere by a classical EDC-NHS amide reaction to obtain the polymer-modified mesoporous carbon nanoparticle. The polymer-modified mesoporous carbon prepared by the invention has no stimulating effect on the gastrointestinal tract and is non-cytotoxic, and is suitable for use as an oral drug carrier. The poorly soluble drug (fenofibrate) is loaded into the polymer-modified mesoporous carbon by a solvent evaporation method to achieve high drug dispersion. The drug carrying system can significantly increase the dissolution rate and oral bioavailability of the drug.
Owner:SHENYANG PHARMA UNIVERSITY
Bioavailable fenofibrate compositions, methods for treating hyperlipidemia and hypercholesterolemia and processes for the preparation of such compositions
InactiveUS20040142903A1Improve bioavailabilityHighly suitablePowder deliveryBiocideSecondary hyperlipidemiaFenofibrate
Pharmaceutical compositions for treating hyperlipidemia or hypercholesterolemia in mammals are described which comprise: A therapeutically effective amount of the compositions are orally administered to mammals to treat hyperlipidemia or hypercholesterolemia.
Owner:PAR PHARMA
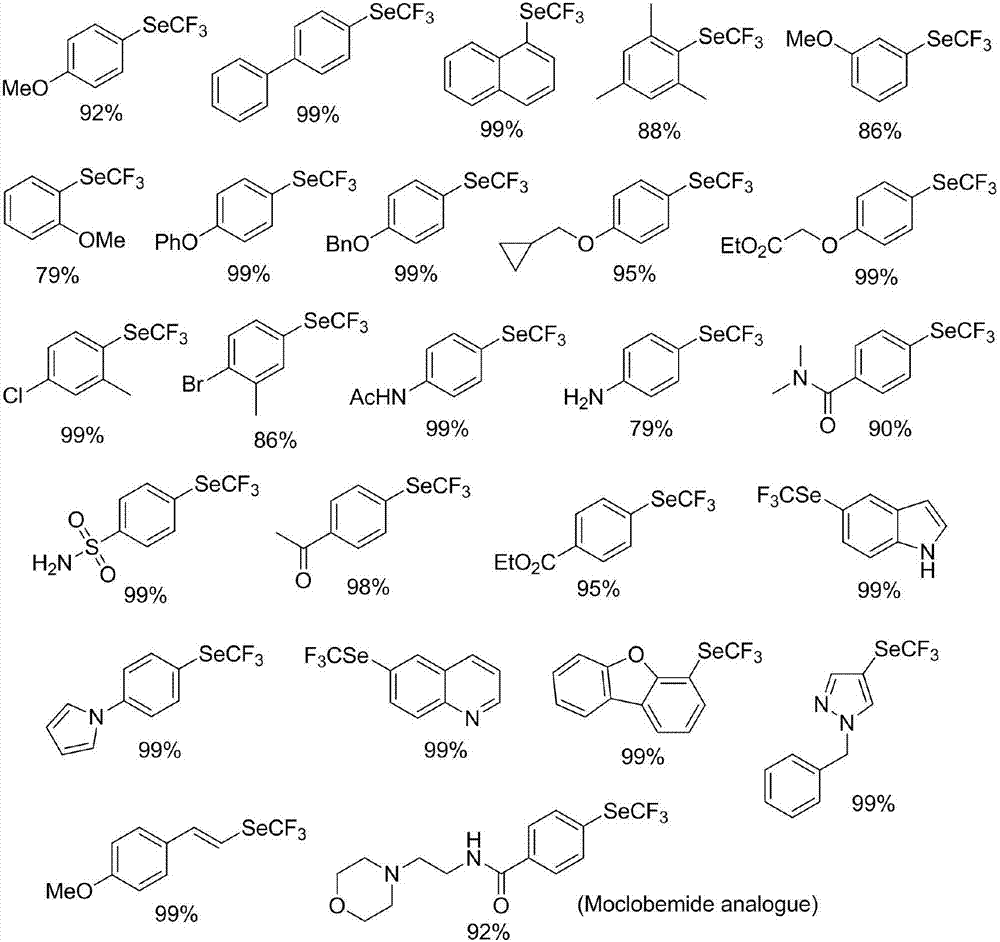
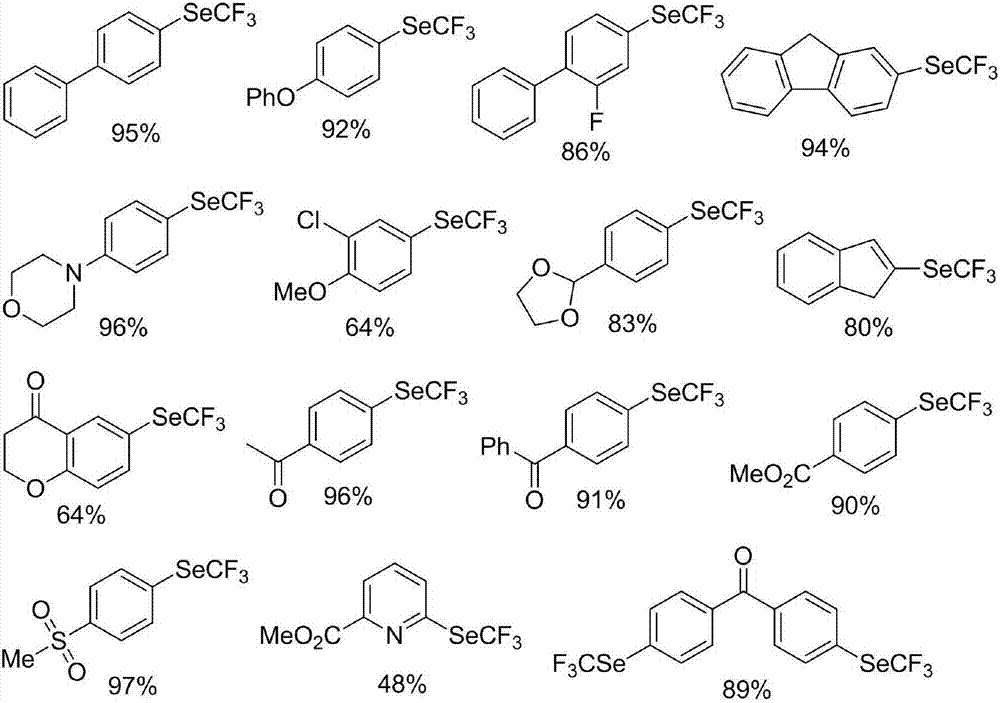
Preparation method of nickel-catalyzed trifluoromethyl diaryliodonium
The invention discloses a preparation method of nickel-catalyzed trifluoromethyl diaryliodonium. The method comprises the following steps of mixing aryl halide, trifluoromethyl selenium-based tetramethyl ammonium, a nickel catalyst and an organic phosphine ligand or dipyridyl compound, reacting at -80 DEG C to 10 DEG C for 5min to 48h, separating and purifying to obtain the trifluoromethyl diaryliodonium. The aryl halide comprises an antidepressant moclobemide or fenofibrate for treating adult hyperlipidaemia. The preparation method is available in raw materials, mild in condition, good in reaction selectivity, high in yield, good in functional group compatibility, wide in substrate application range (suitable for aryl chloride), low in demands on instruments and equipment and simple in technological operation.
Owner:WUHAN UNIV OF TECH
Novel formulations comprising fenofibrate and a statin, and related methods of treatment
The invention provides novel omega-3 oil formulations comprising fenofibrate and a statin. These formulations are effective in small volumes. Related methods of treatment are also described.
Owner:ALMBURG
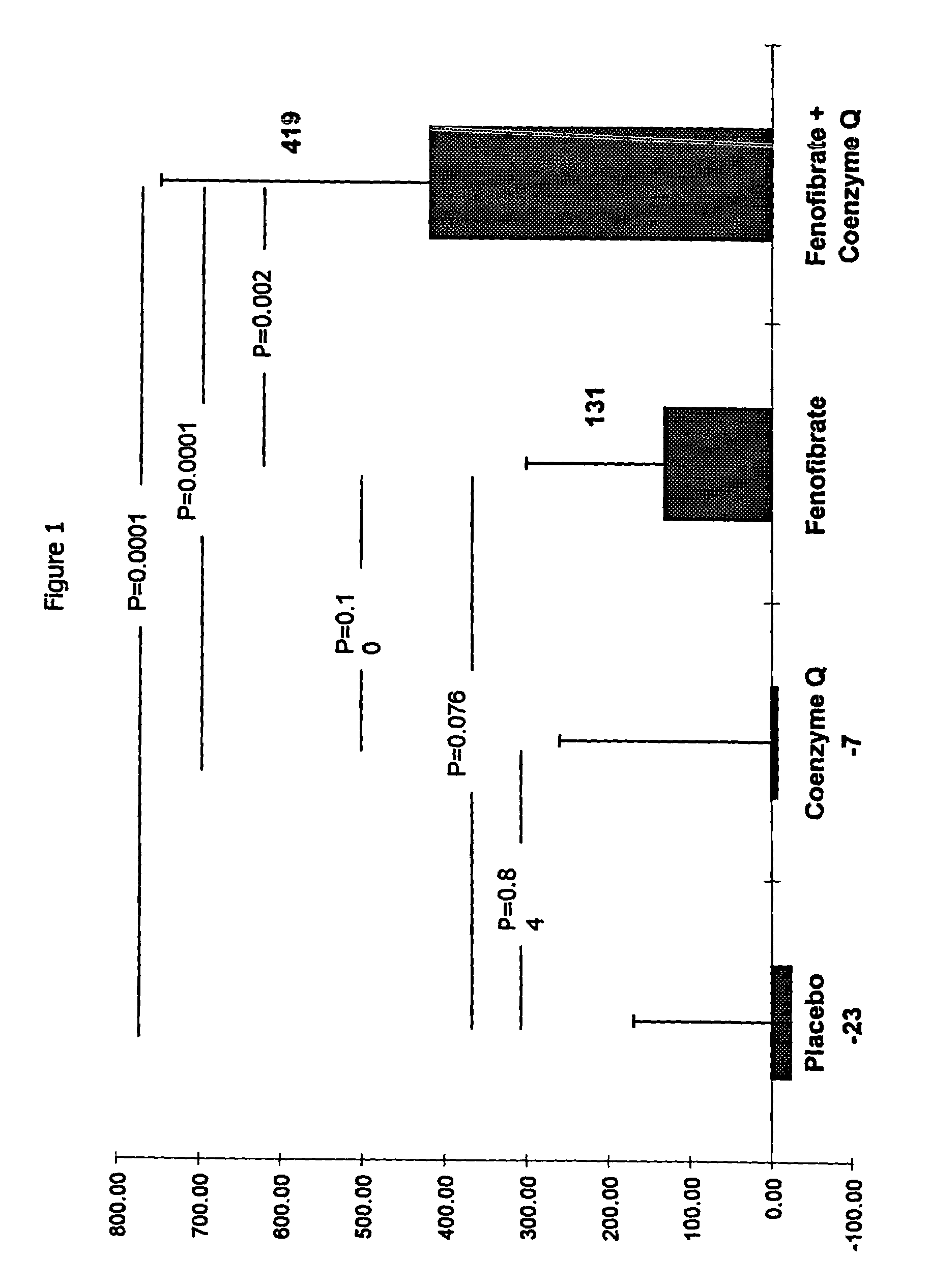
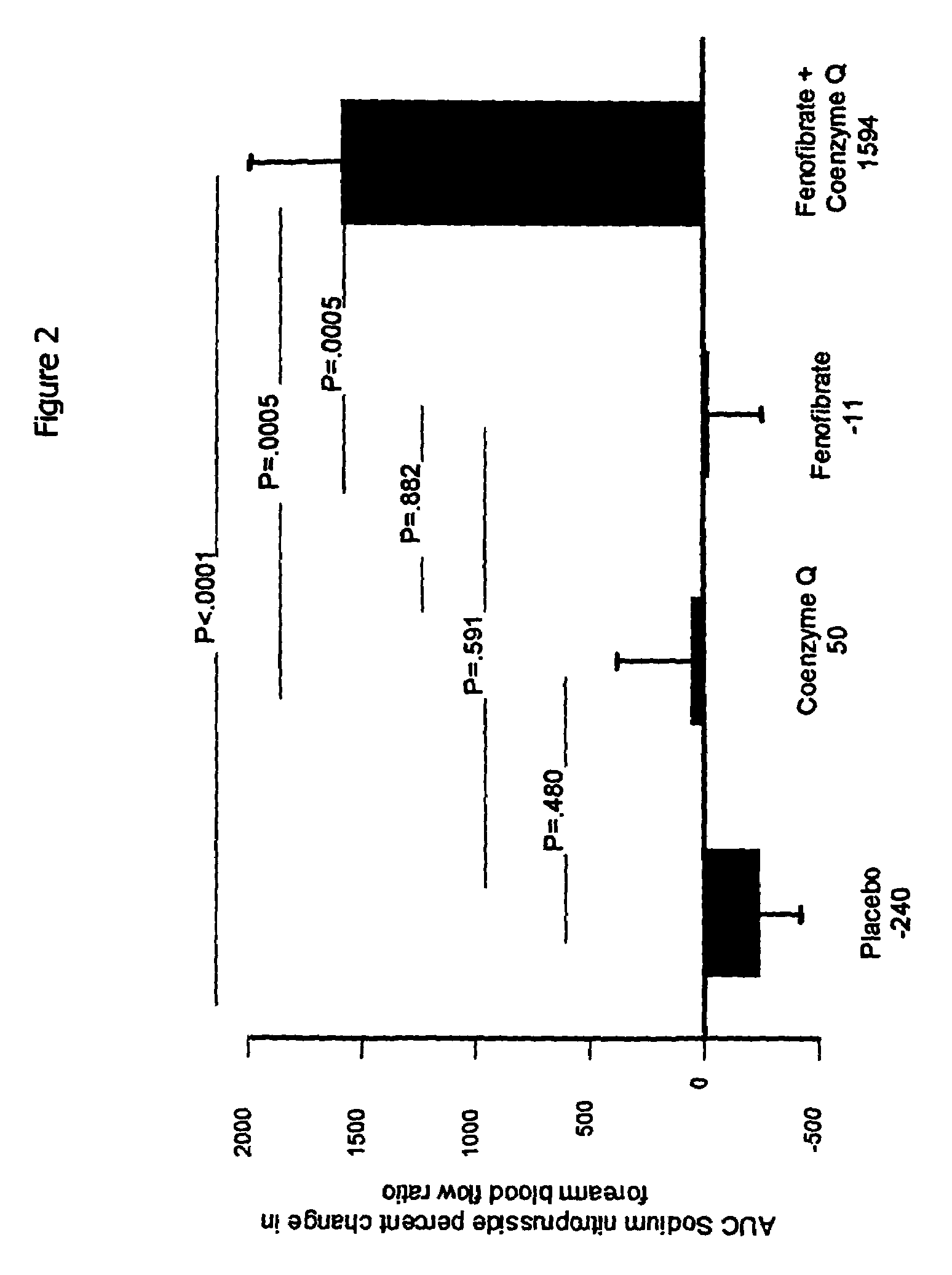
Combination of fenofibrate and coenzyme q10 for the treatment of endothelial dysfunction
The present invention relates to a combination of a peroxisome proliferator activated receptor (PPAR) activator and a benzoquinone and their use in treating and / or preventing disorders characterized by endothelial dysfunction, such as cardiovascular disease, strokes and myocardial infarction. According to a preferred embodiment of the invention the benzoquinone or precursor thereof is a ubiquinone or precursor thereof, more preferably, coenzyme Q10 or a precursor thereof, and the PPAR activator is a fibrate or a thiazolidinedione, more preferably fenofibrate.
Owner:FOURNIER LAB IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com