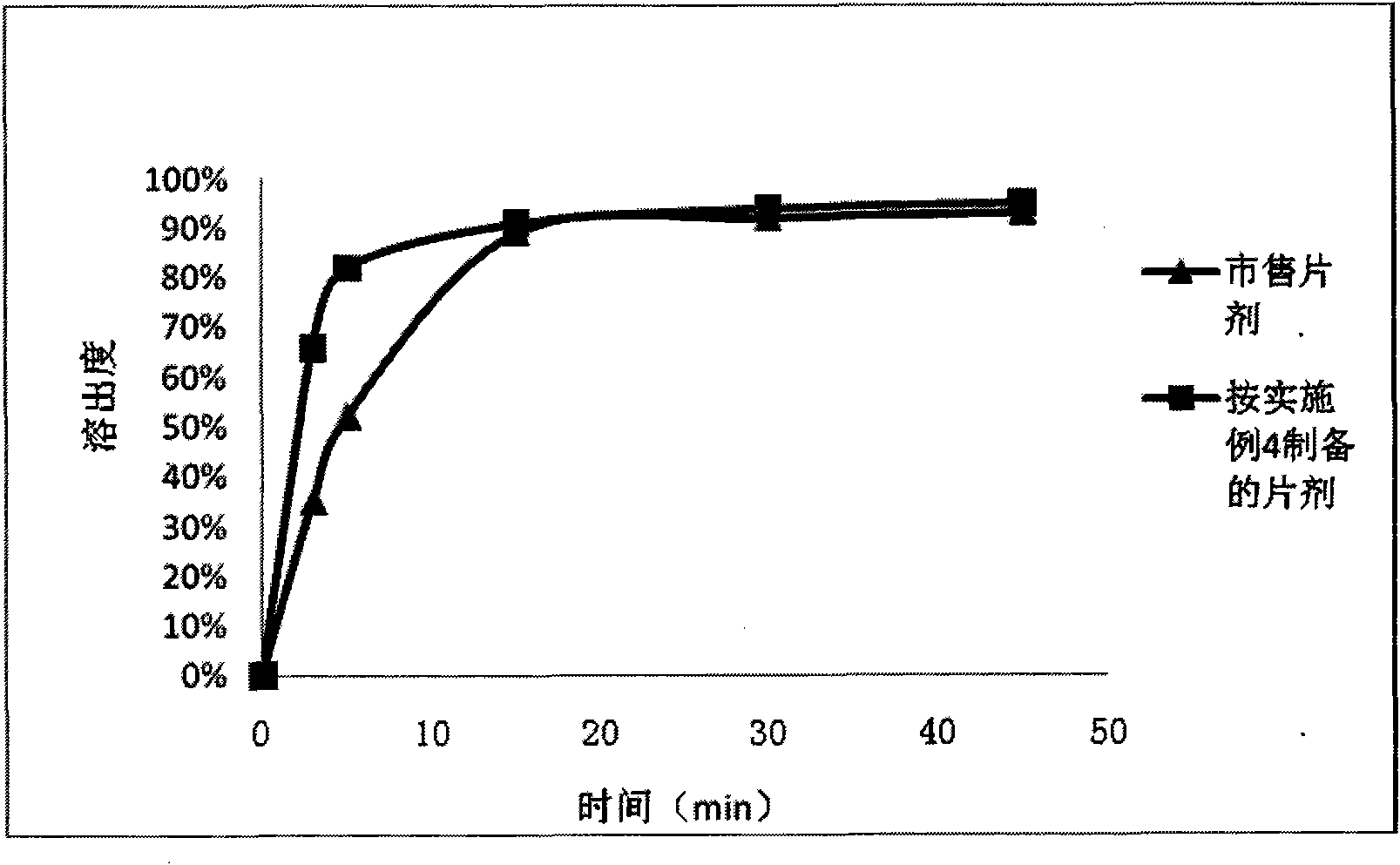
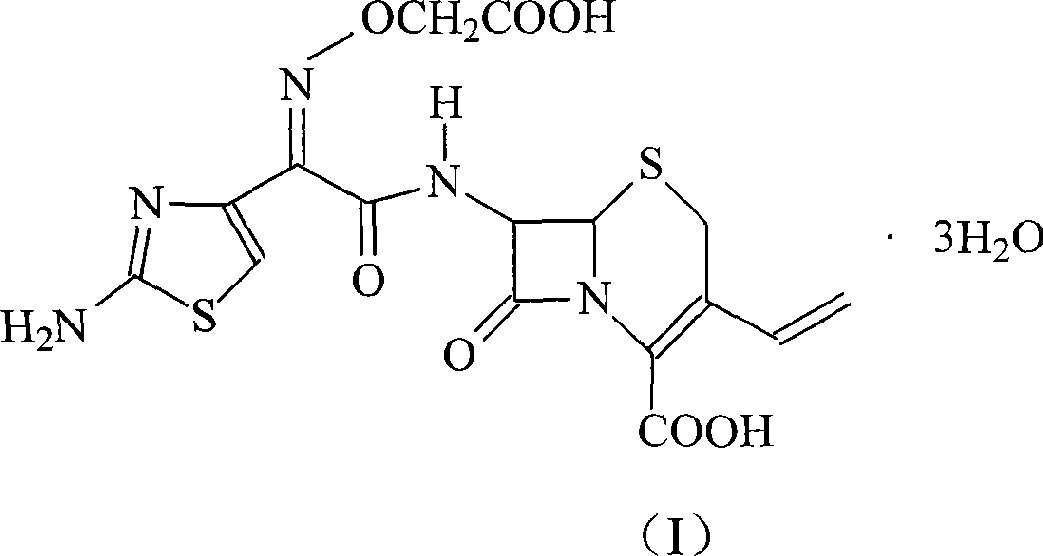
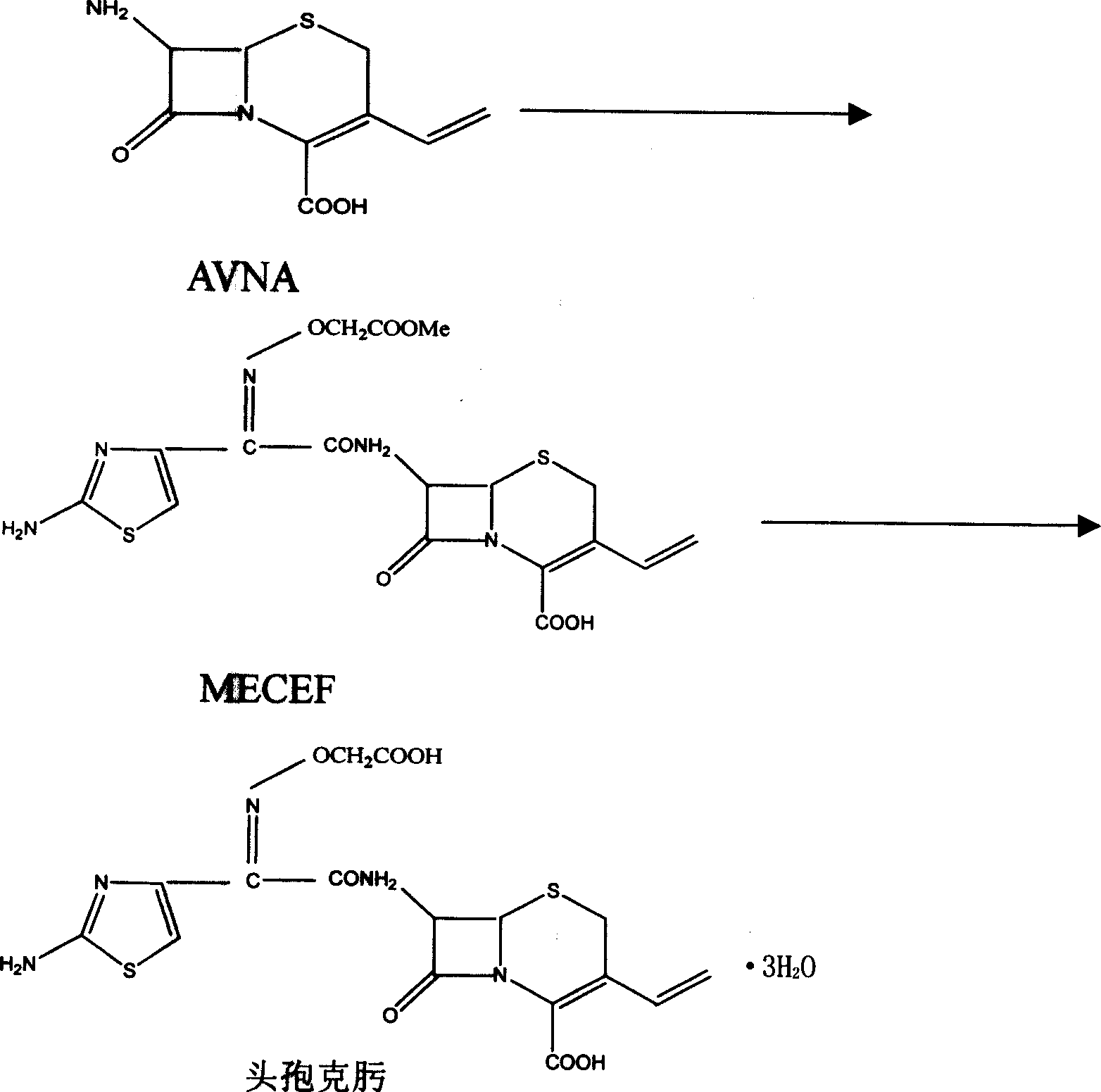
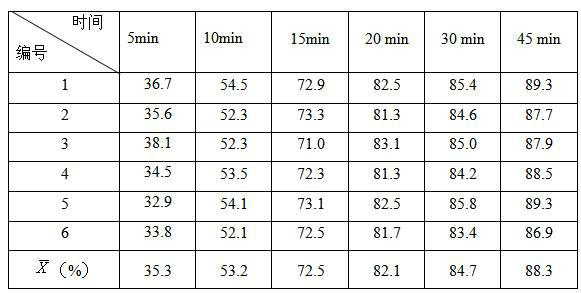
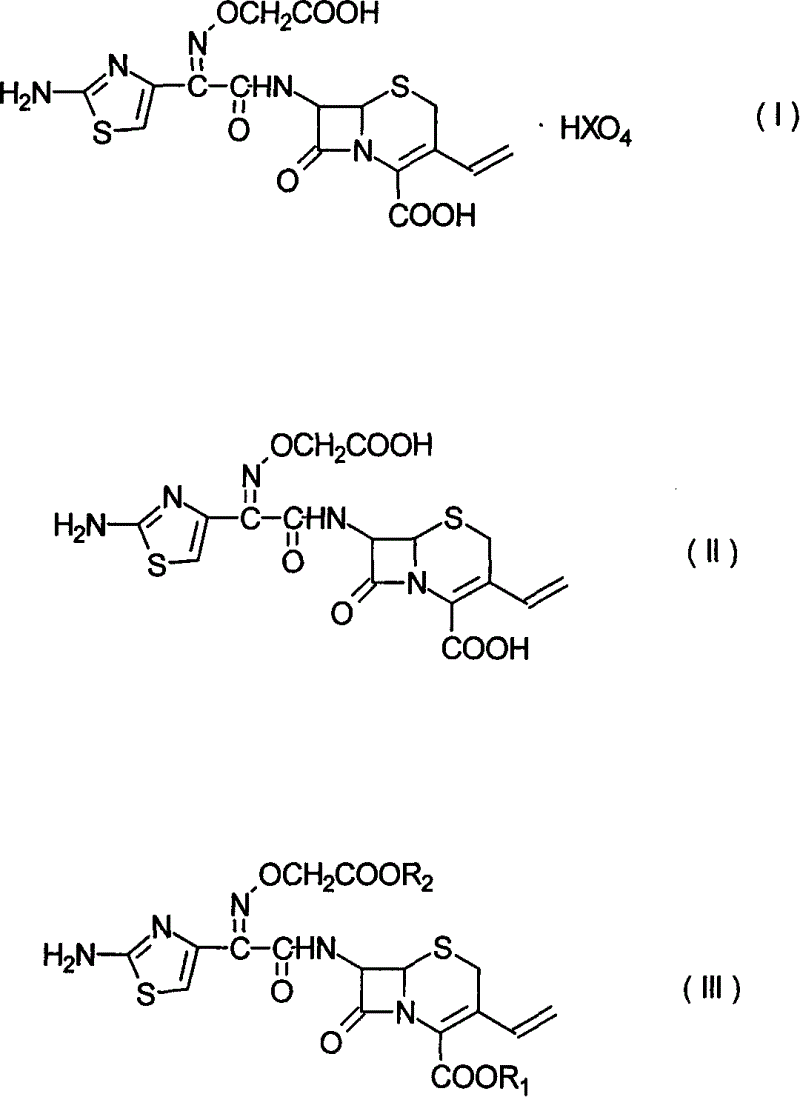
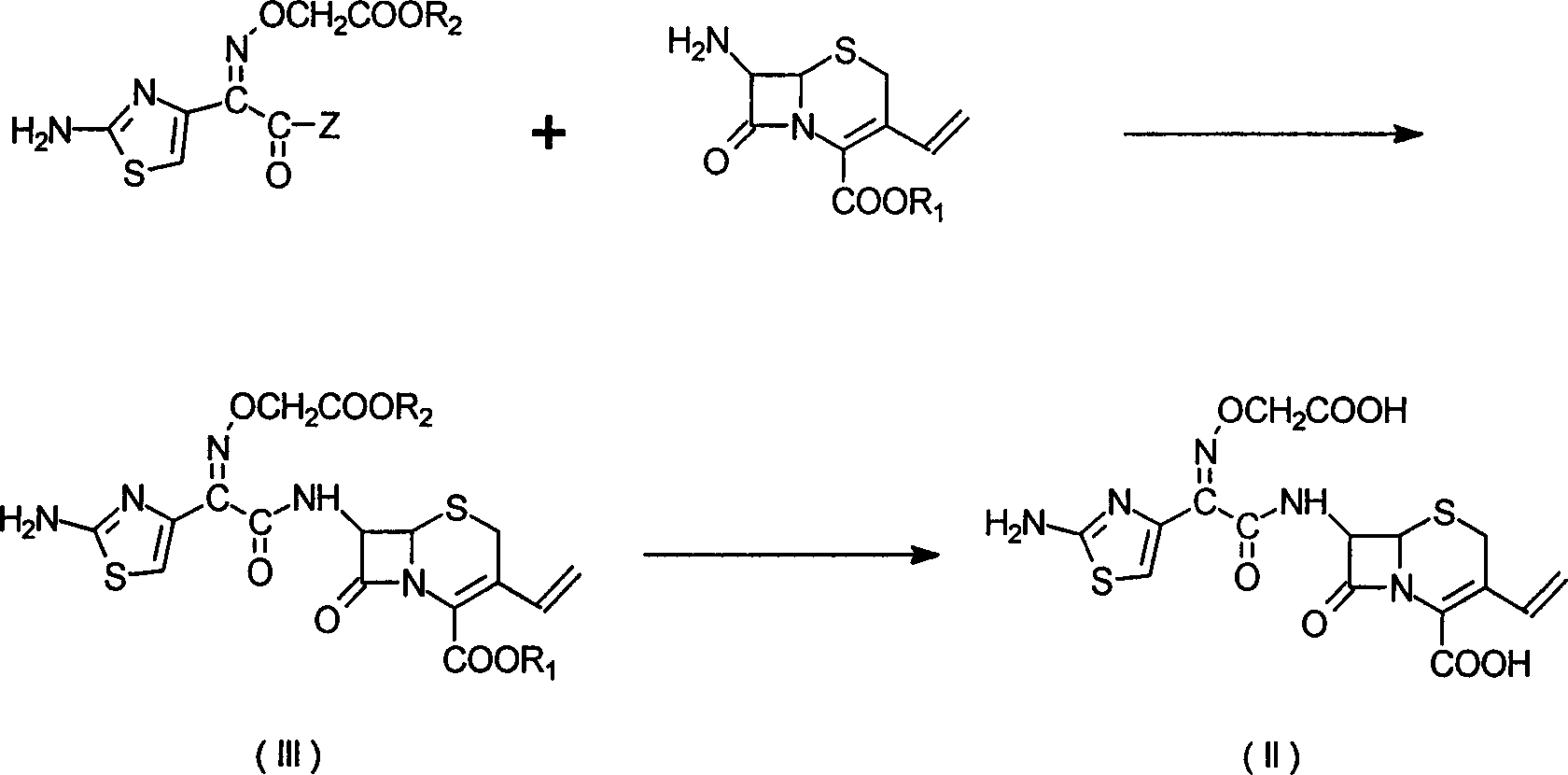
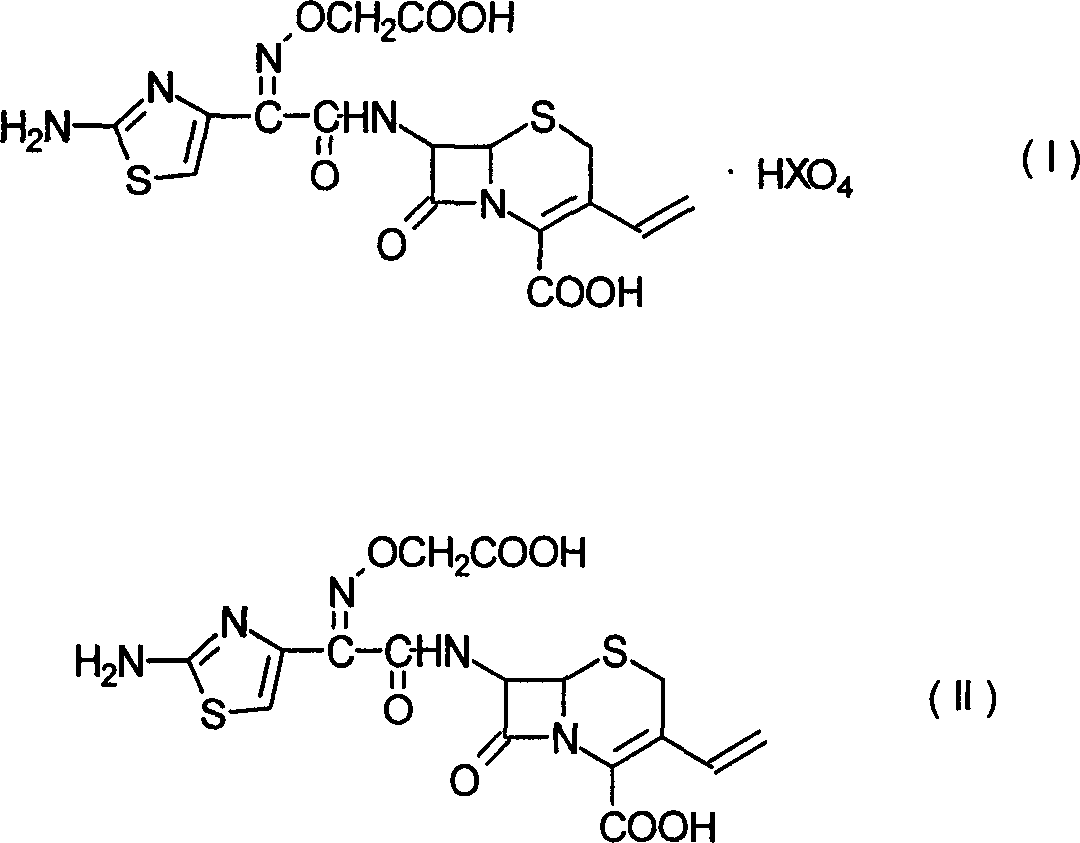
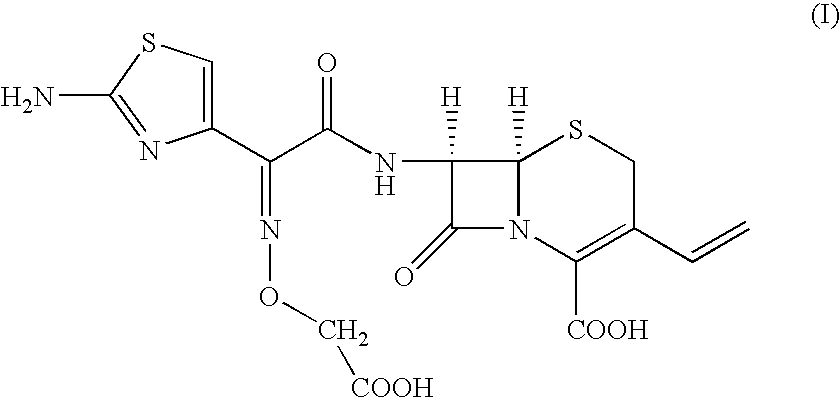
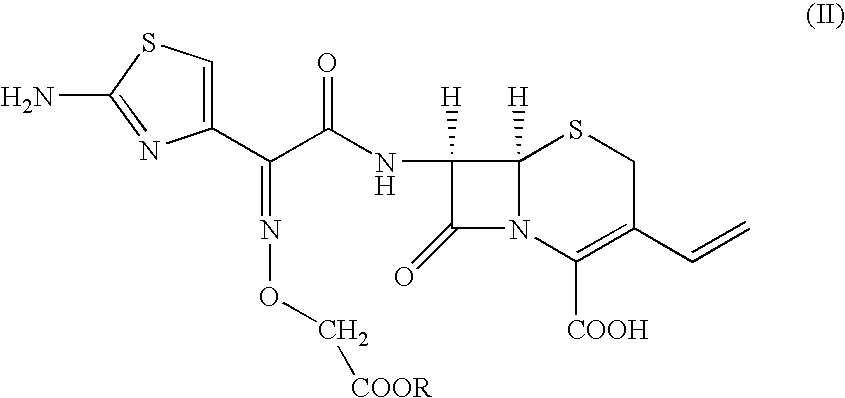
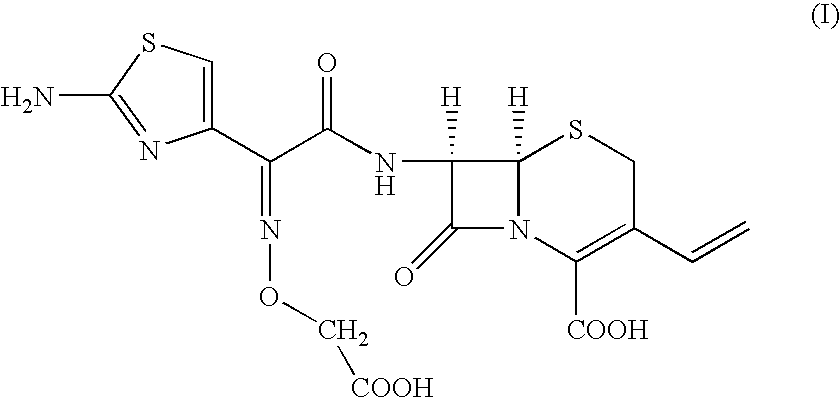
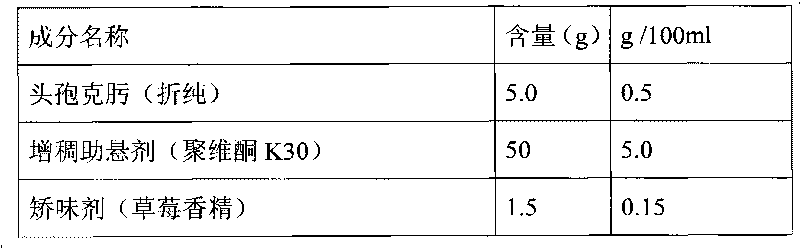
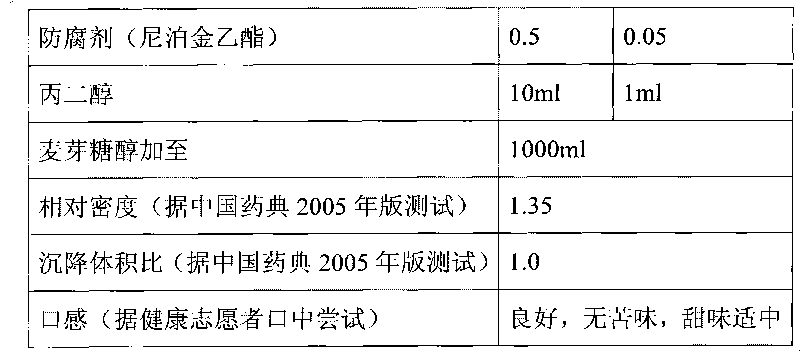
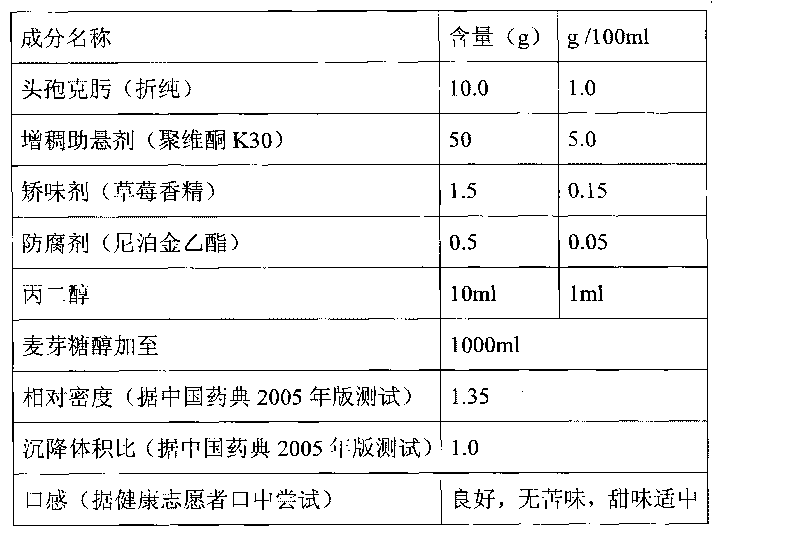
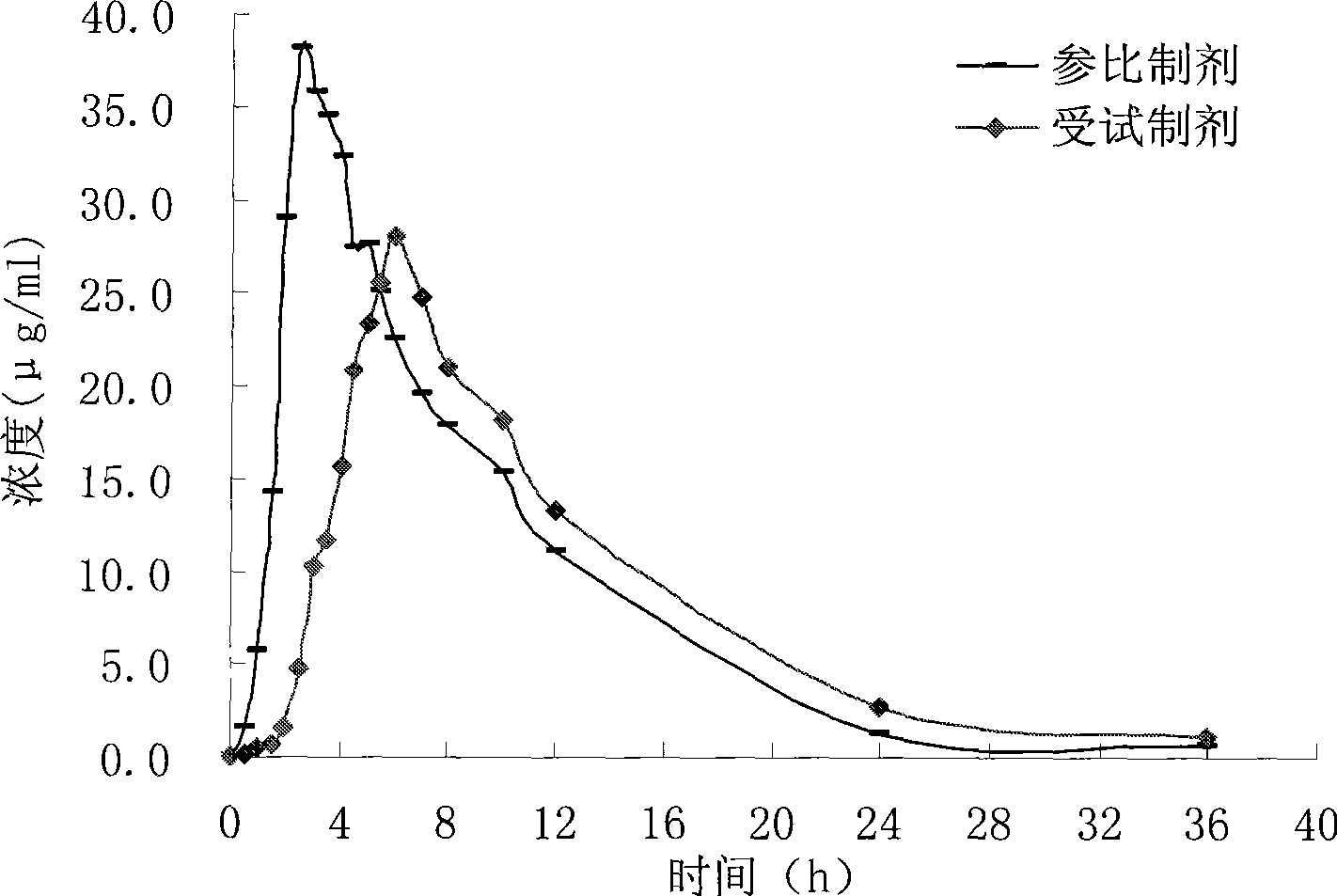
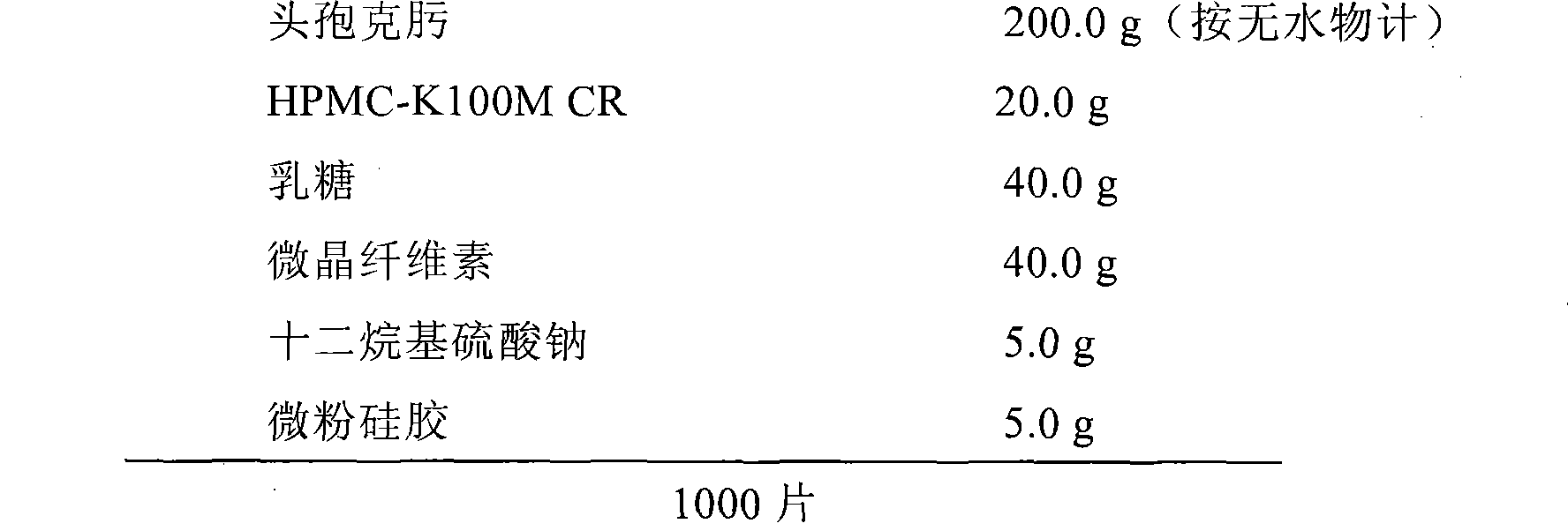
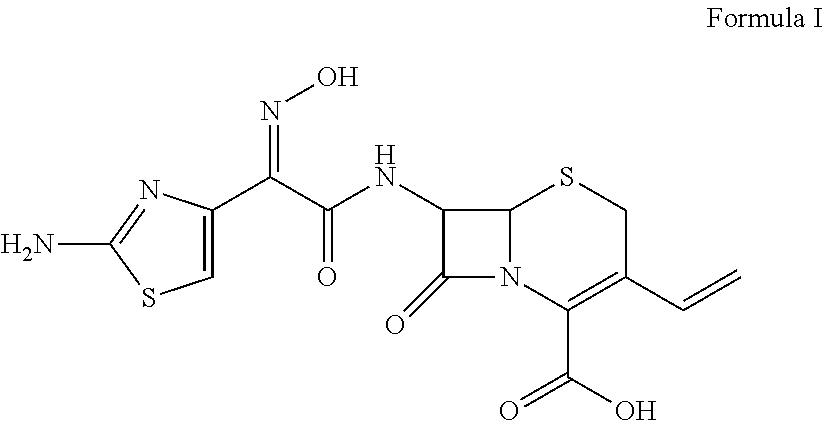
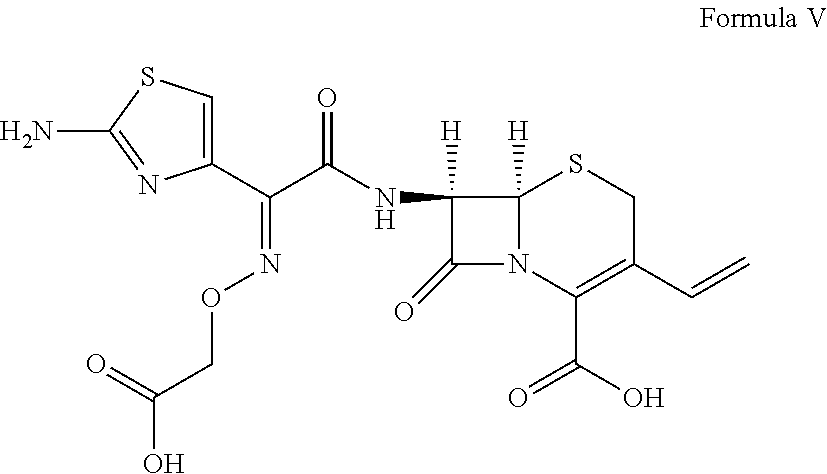
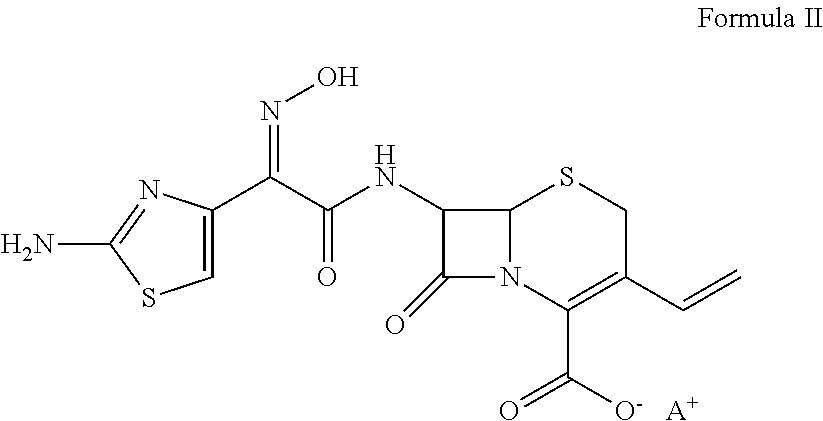
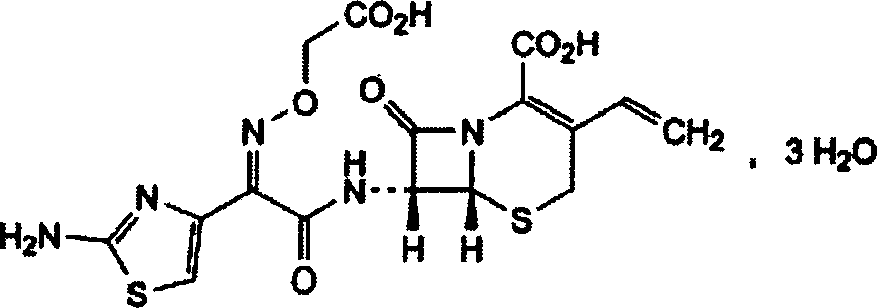
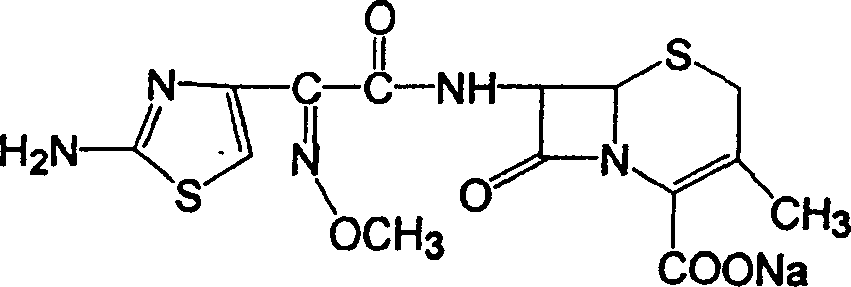
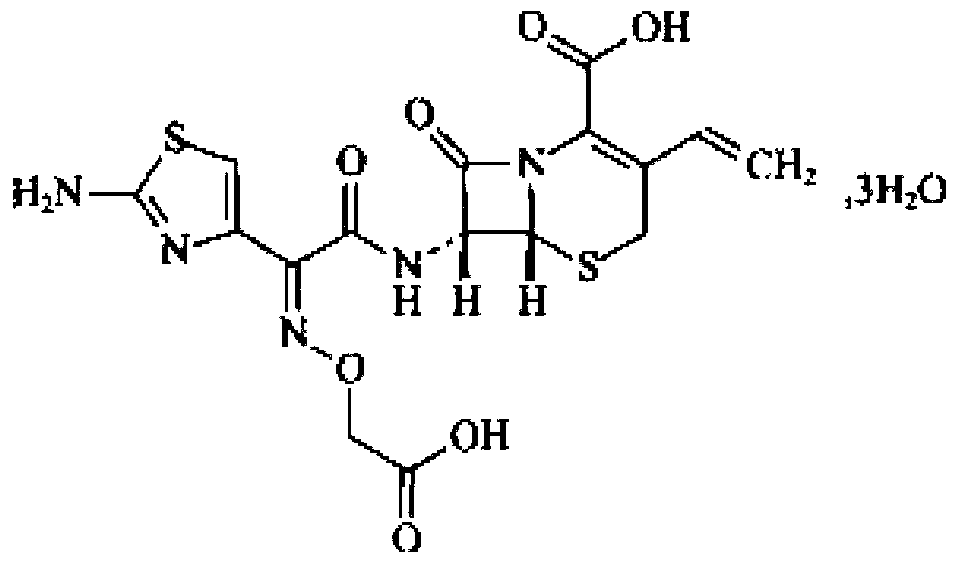
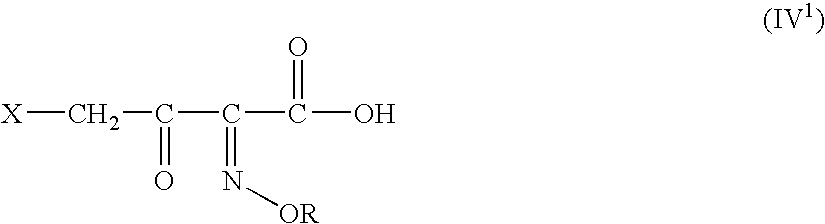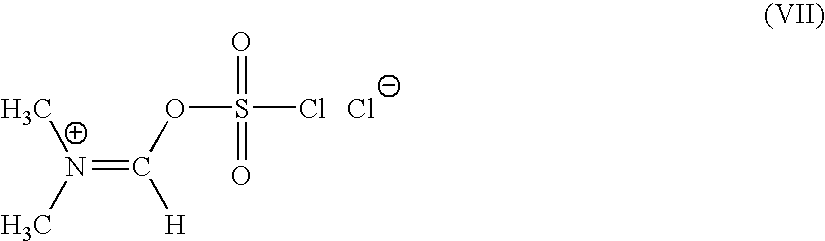Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
166 results about "Cefixime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
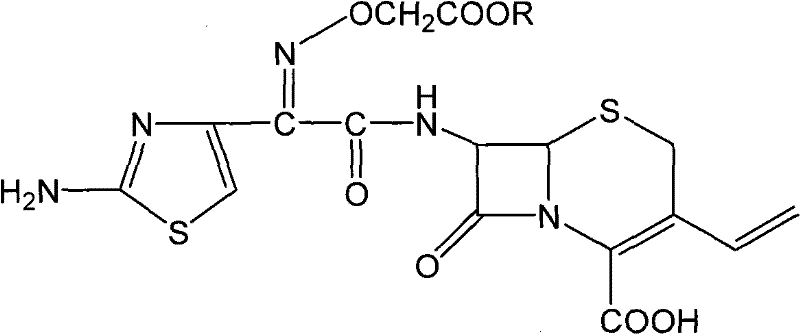
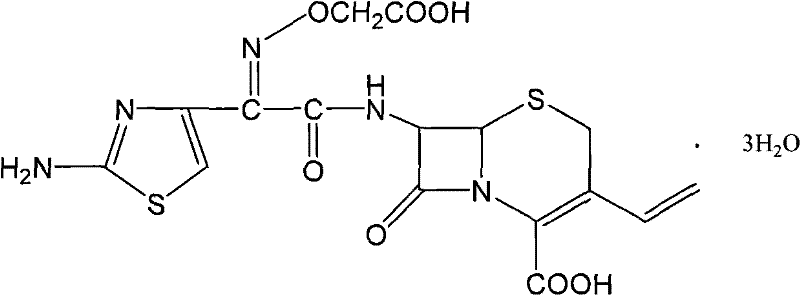
Cefixime is used to treat a wide variety of bacterial infections.
Cephalosporin suspension granule and preparation method thereof
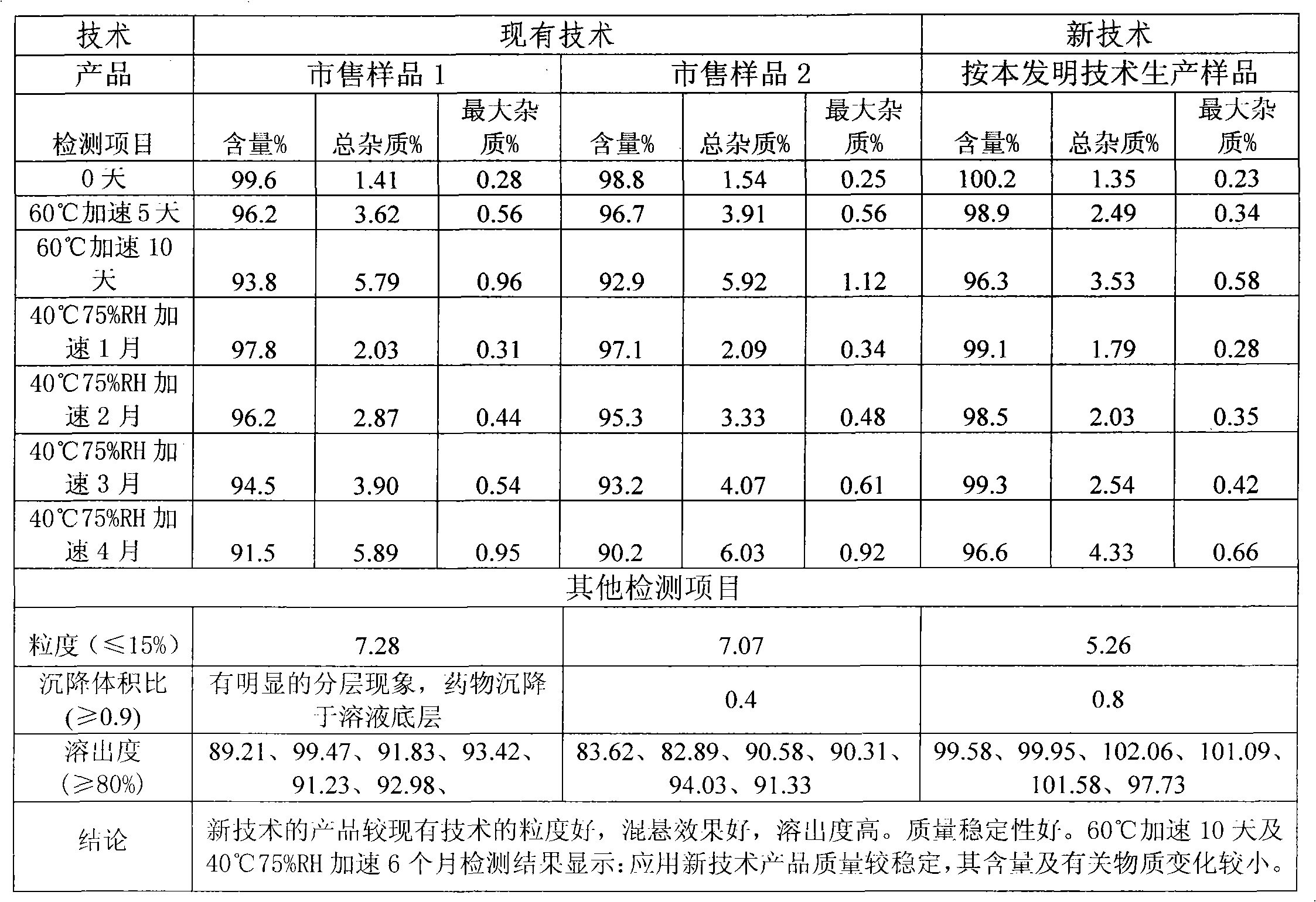
ActiveCN101816635AGranularityAvoid damageAntibacterial agentsOrganic active ingredientsFluidized bed dryingAdditive ingredient
The invention discloses a cephalosporin suspension granule of which the formula composition comprises drug active ingredients, excipient, suspending agent, disintegrating agent, flavoring agent, coloring agent, stabilizing agent, adhesive and spice. The granule takes cefixime, cefprozil, cefaclor or cefdinir as the main drug ingredient, disintegrating agent and suspending agent with a certain quantity are added into the formula to ensure that the effective ingredients are dissolved out, and the solution has favorable suspending effect. Meanwhile, the invention also discloses a preparation method of the cephalosporin suspension granule, which adopts the wet granulation method to prepare granules and adopts the fluidized bed drying method to dry. The invention adopts the fluidized bed drying technology, so that compared with the traditional oven drying, the invention has the advantages of short drying time, small influence on active matter quality and the like, not only ensures product quality but also improves production efficiency. The granule prepared by the formula of the invention has solid granularity and lowers damage to the granule by fluidized drying.
Owner:广东恒健制药有限公司
Process for preparing cefixime
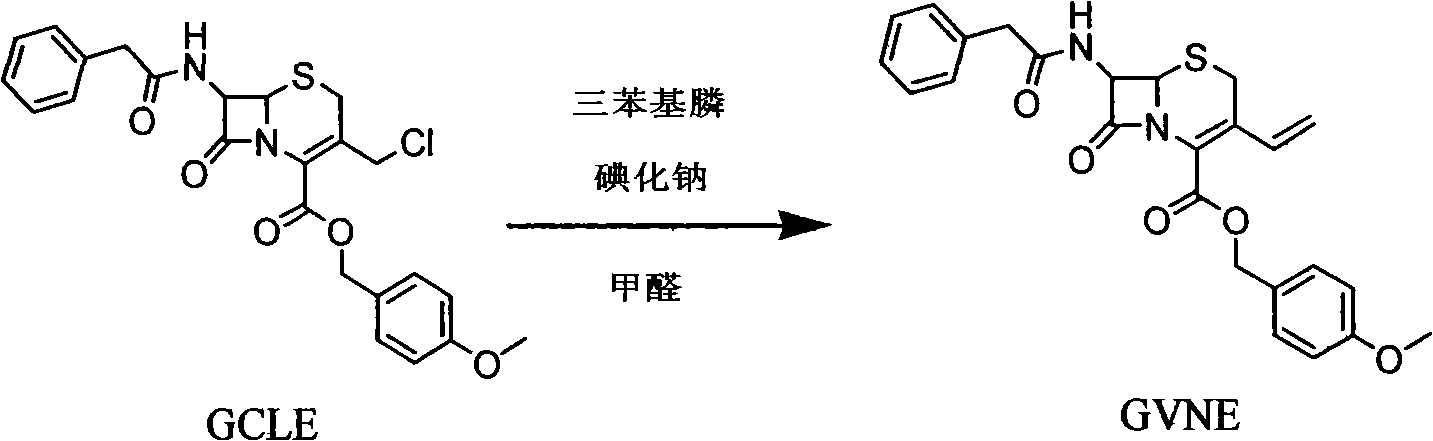
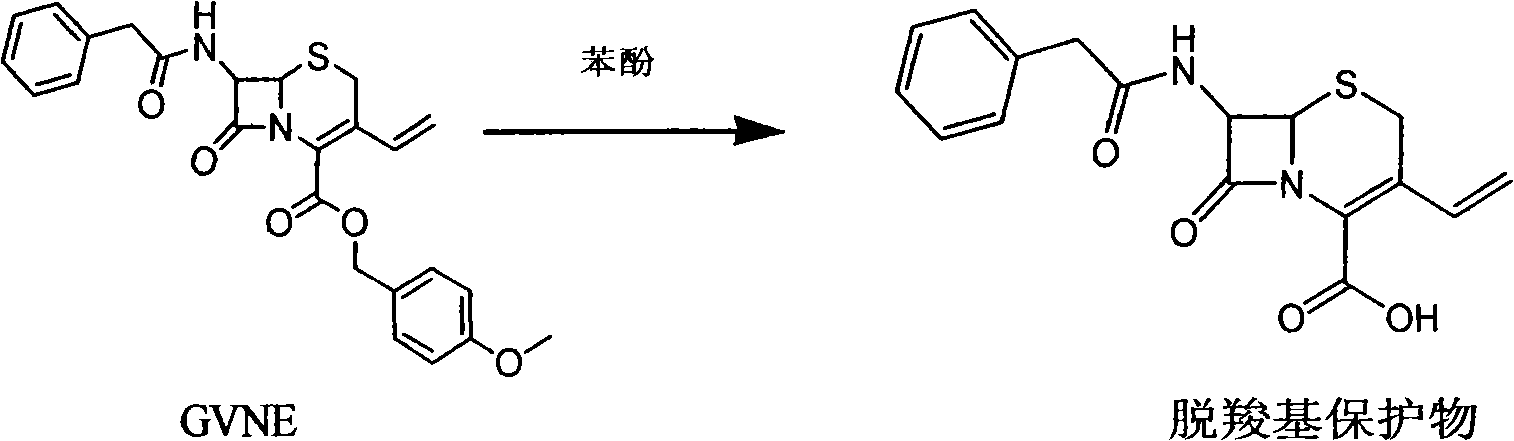
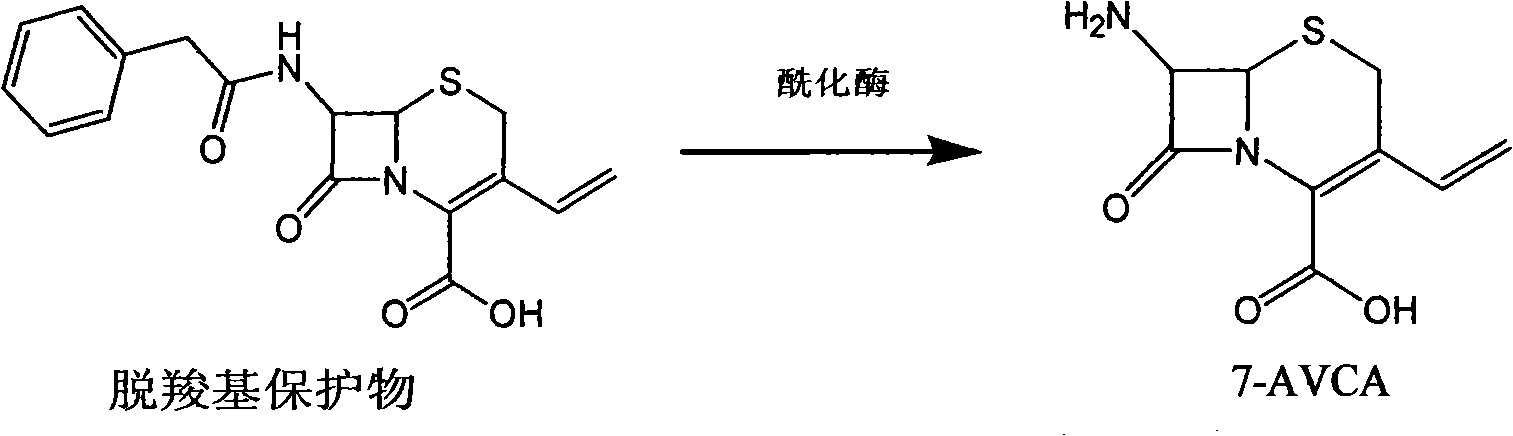
The invention provides a method for preparing cefixime, which belongs to the medicine synthesis technical field and comprises the steps of preparation of 7-AVCA, preparation of cefixime methyl ester, preparation of the cefixime and so on. The method aims at the prior amino de-protection to make an improvement, and uses penicillin acylase to substitute trifluoroacetic acid and so on in the prior process to ensure that an amino de-protection reaction is greatly improved, the use of organic solvents can be completely avoided, and acylase can also be reused at the same time. Methylene dichloride and tetrahydrofuran solvents in the prior process are substituted by adopting alcohol, ketone or ester solvents, so that an amidation reaction is greatly improved, which ensures that reaction solvents are easy to recover and reuse, can reduce production cost, reduce the discharge of waste liquid, reduce the pollution to the environment, can completely recover a byproduct, namely mercaptobenzothiazole produced by the reaction, improve the atom utilization rate of reaction raw-materials, and greatly improve the hydrolyzation and crystallization of subsequent products and the quality of a finished product.
Owner:ZHEJIANG ANGLIKANG PHARMA
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Effervescent tablet containing cefixime and its preparing method
InactiveCN100417383CEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
Dispersible tablet containing cefixime and preparation method thereof
The invention belongs to the technical field of pharmaceutical preparation, and in particular relates to a cefixime dispersible tablet and a method for preparing the same. The method comprises the following steps: firstly, weighing cefixime, starch, microcrystalline cellulose, partially cross-linked polyvinylpyrrolidone, partially cross-linked sodium carboxymethyl cellulose, and partially low substituted-hydroxypropyl cellulose, and mixing the components evenly; secondly, dripping 5 percent polyvinylpyrrolidone K30 ethanol solution into the mixture prepare a soft material, and performing granulating through a sieve of between 18 and 24 meshes; thirdly, drying wet particles at a temperature of between 50 and 80 DEG C, palletizing the particles through the sieve of between 18 and 24 meshes, adding the remaining cross-linked polyvinylpyrrolidone, the remaining cross-linked sodium carboxymethyl cellulose, and the remaining low substituted-hydroxypropyl cellulose, magnesium stearate and superfine silica gel powder into the mixture to be mixed evenly, and tabletting the mixture to obtain the required cefixime dispersible tablet. The cefixime dispersible tablet has the characteristics of rapid disintegration with water, even dispersion, high dissolution, and convenient taking and carrying around.
Owner:BEIJING TRADE STAR MEDICAL TECH
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
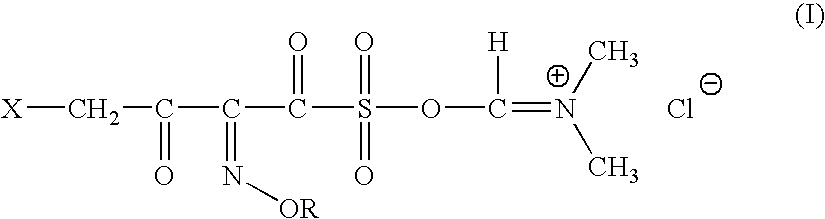
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
Method for preparing novel cefixime tablets and cefixime capsules
ActiveCN101889987AImprove stabilityIncrease dissolution rateAntibacterial agentsOrganic active ingredientsCurative effectWater soluble
The invention provides a method for preparing novel cefixime tablets and cefixime capsules. In the method, cefixime, a solubilizer and water soluble auxiliary materials are micronized and then mixed with other auxiliary materials, and the obtained products are granulated by a dry method, so that the stability of cefixime tablets and cefixime capsules is improved, and the dissolution rate of cefixime is also raised. The cefixime can be quickly absorbed by a patient after the patient takes the cefixime tablets and cefixime capsules, the plasma peak time is shortened and the plasma concentration can be quickly stabilized at a higher level, so that the treating effect is improved.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Cefixime compound and preparation method thereof
InactiveCN101544660AAddressing Purity IssuesSettlement yieldAntibacterial agentsOrganic chemistryOrganic solventFiltration
Owner:郑仙锋
Method of synthesizing cefixime
InactiveCN101016305AEliminate the dissolution processReduce usageOrganic chemistryCefuroximeCefixime
The invention discloses a synthesizing method of cefuroxime, which is characterized by the following: crystallizing cefuroxime intermediate MECEF; synthesizing cefuroxime through water-phased extract of MECEF without dissolving MECEF; saving cost to improve receiving rate effectively; shortening manufacturing period.
Owner:河源市制药工程技术研究开发中心
Crystallization method of cefixime
The invention belongs to the technical field of medicine and relates to a crystallization method of cefixime. The method comprises: adding an acid to a solution of cefixime salt in a mixture of an organic solvent and water or in water at a temperature of 0-40° C., adjusting the pH of the solution to 3.0-3.5 while stirring, and monitoring the system with an online pH meter pH, with the precipitation of cefixime crystals, the pH of the system begins to rise. At this time, by continuously adding acid and controlling the rate of acid addition, the pH of the system has been stabilized in the range of 3.0 to 3.5, so that the cefixime crystals have always been stable. Precipitate in a narrow range of pH=3.0-3.5; when the pH of the system does not fluctuate upward without adding acid dropwise and stabilizes at 3.0-3.5 for at least 30 minutes, stop adding acid, stir, then filter, wash, and dry to obtain cephalosporin Kexime crystals. The cefixime crystals obtained by the method of the invention have high bulk density and good stability, and are especially suitable for making capsule preparations.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Cefixime oral disintegration tablet and its preparation method
ActiveCN1803138AShort disintegration timeGreat tasteAntibacterial agentsOrganic active ingredientsCelluloseMANNITOL/SORBITOL
The invention discloses an orally disintegrating cefixime tablet and its preparation, wherein the ingredients include (by weight ratio): cefixime 10.0-35.0%, crystalline cellulose 0-10%, lactose starch 0-35%, mannitol 35.0-59%, cross-linked sodium carboxymethylcellulose 4.0-15.0%, Kollidon 1.0-5.0%, sodium dodecylsulfate 0.01-1.0%, and silica gel powder 0.01-0.5%, the preparing process comprises the following steps, mixing the medicinal materials homogeneously, charging water, ethanol or their mixture, granulating and drying, mixing homogeneously with miropowdered silica gel and tabletting.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
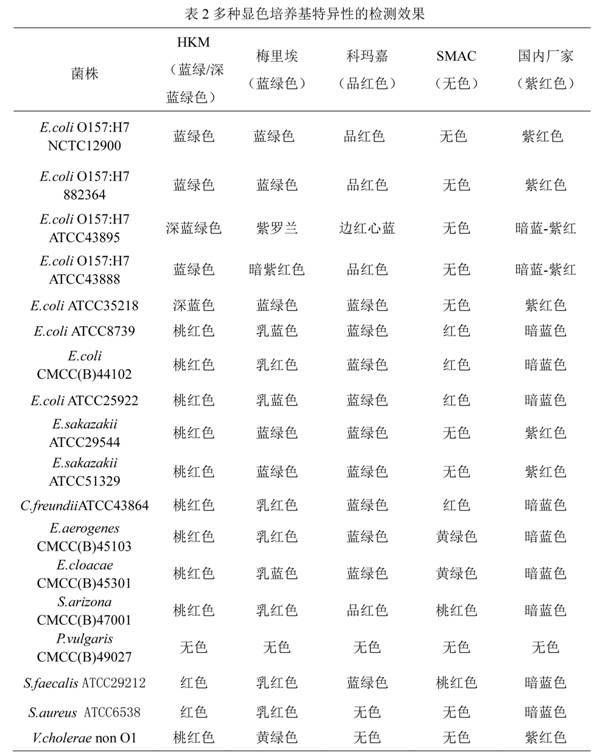
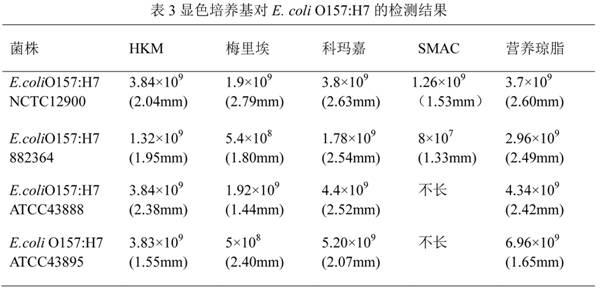
Chromogenic medium used for detecting esherichia coli O157:H7
ActiveCN102424832AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesBiotechnologyThio-
The invention discloses a chromogenic medium used for detecting esherichia coli O157:H7. The medium comprises agar, peptone, beef extract powder, sodium chloride, sorbitol, inositol, neutral red, beta-galactosidase chromogenic substrate, beta-glucuronidase chromogenic substrate, isopropyl-beta-D- thiogalactopyranoside, natrium taurocholicum, potassium tellurite and cefixime. The chromogenic medium used for detecting esherichia coli O157:H7 of the present invention has the advantages of high sensitivity, good specificity, direct bacterial strain discrimination according to colony color, short detection period and strong operationality, is suitable for treating high-reflux samples, is capable of comprehensively, systematically and accurately detecting and preliminarily identifying the esherichia coli O157:H7 in food production and environment, and provides a novel approach for rapidly detecting the microbes.
Owner:GUANGDONG HUANKAI MICROBIAL SCI & TECH
Effervescent tablet containing cefixime and its preparing method
InactiveCN1850087AEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
Method for preparing cefixime dispersible tablets
ActiveCN102670536AAntibacterial agentsOrganic active ingredientsAntiInfective DrugsPharmaceutical Aids
The invention discloses a method for preparing cefixime dispersible tablets, relates to the field of pharmaceutical preparation and particularly relates to cefixime dispersible tablets which are anti-infective agents and the method for preparing the cefixime dispersible tablets. Cefixime serves as an active pharmaceutical ingredient of the cefixime dispersible tablets and auxiliary materials are added in the cefixime dispersible tablets. The auxiliary materials include solubilizing agents, filling agents, lubricating agents, bonding agents and disintegrating agents. The cefixime dispersible tablets include, by weight, 15%-60% of the micronized cefixime, 10%-70% of the filling agents or diluents, 1%-20% of the disintegrating agents, 0.3%-5% of the lubricating agents, 0.5%-10% of the bonding agents and 0.5%-5% of the solubilizing agents, wherein grain diameters of the micronized cefixime range from 20 mu m to 120 mu m. The method has the advantages that the dissolving speed of the cefixime dispersible tablets is rapid and the dissolving is complete.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Method for preparing cefixime
ActiveCN1696134ASignificant positive effectHigh purityAntibacterial agentsOrganic chemistryCompound aOrganic solvent
A process for preparingcefixime from perhalate includes reaction between compound A, perhalognic acid and organic protonic acid to obtain cefixime perhalate, removing protective radical, and setting free.
Owner:天津市医药集团技术发展有限公司
Cefixime submicro-emulsion solid preparation and novel application thereof
InactiveCN101711741AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityEmulsion
The invention relates to a cefixime submicro-emulsion solid preparation and a novel application thereof, in particular to a solid preparation of cefixime processed by micro-emulsification and a novel application thereof. The cefixime submicro-emulsion solid preparation comprises cefixime, an emulsifying agent and an emulsifying aid agent, wherein the active component of the cefixime is processed by applying a micro-emulsification technology. The stability is enhanced, the solubility is increased, and the problem of low leaching degree is solved.
Owner:HAINAN MEIDA PHARMA
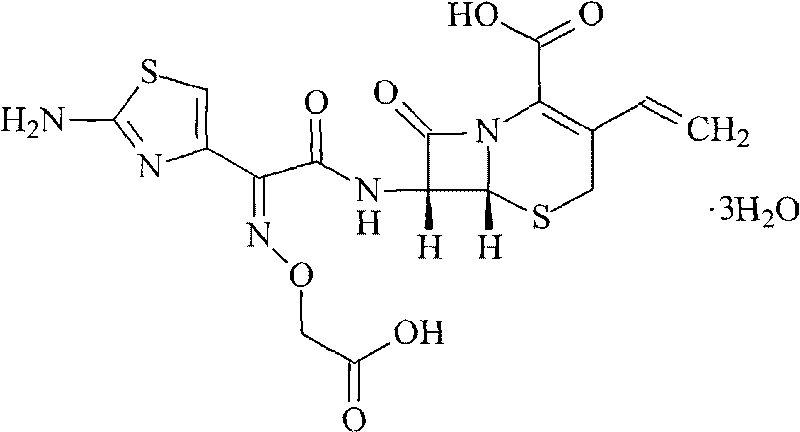
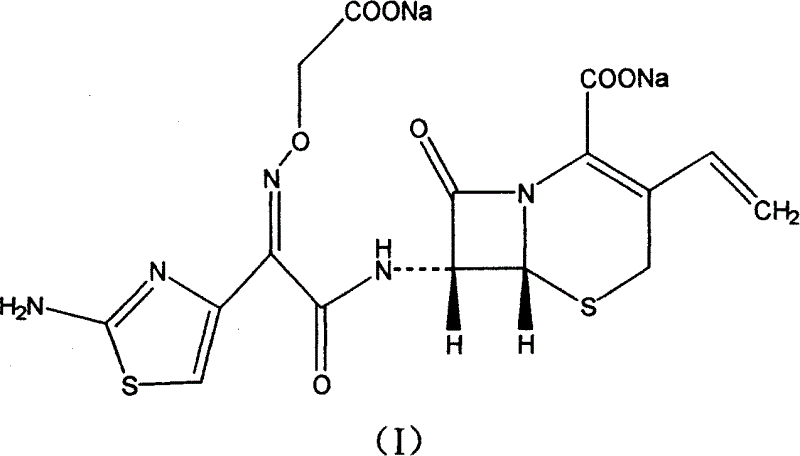
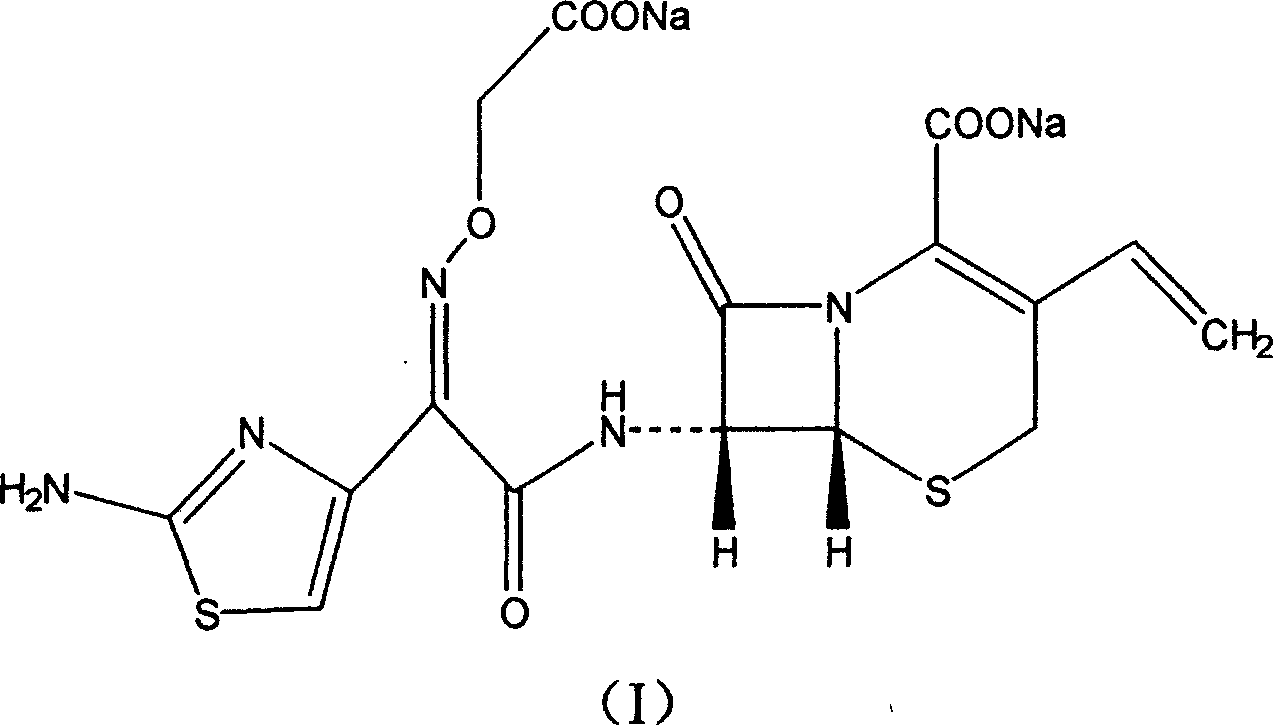
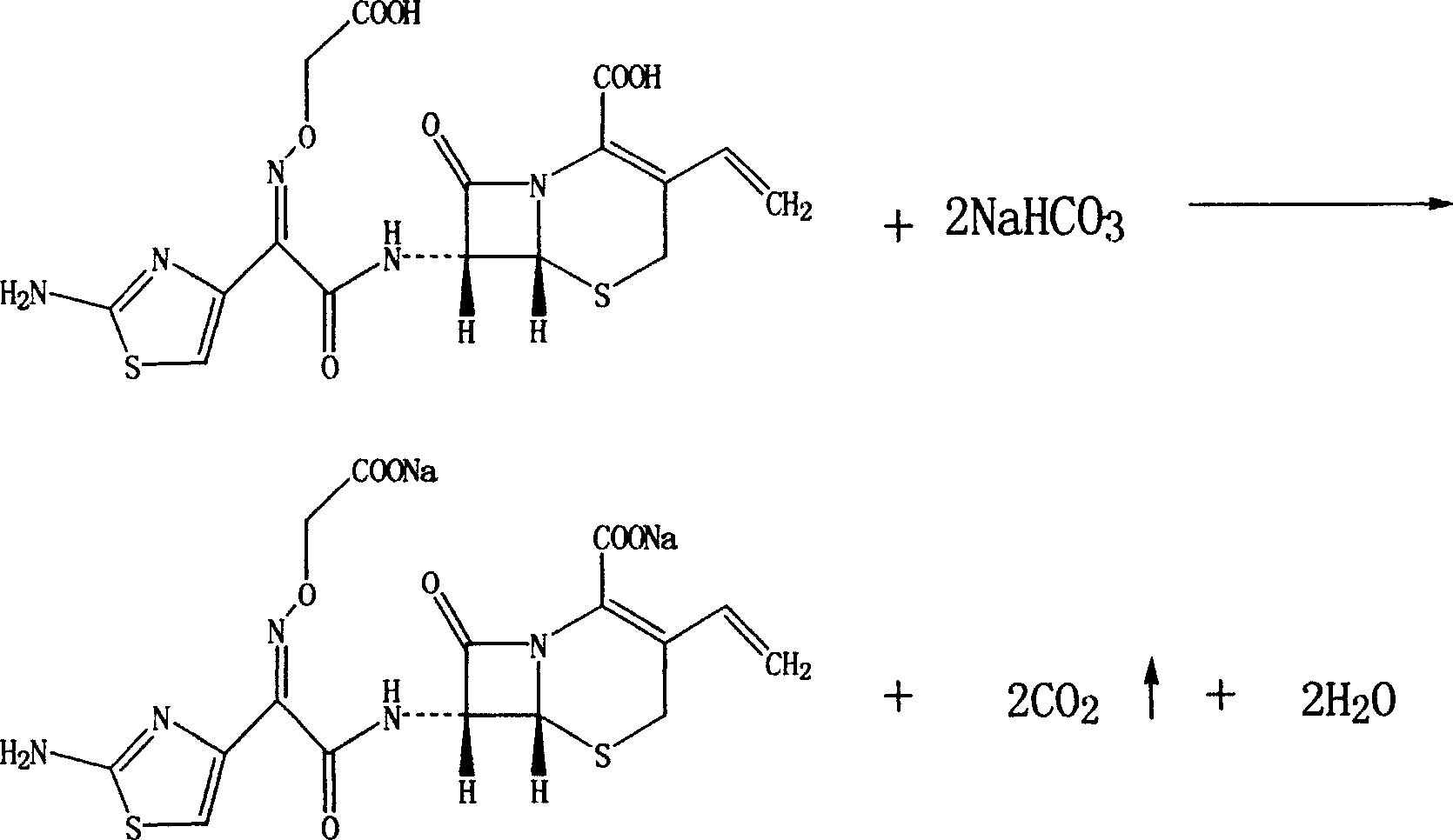
Cefixime sodium pharmaceutical composition and its preparation and application
InactiveCN1594322AEasy to synthesizeLow costAntibacterial agentsOrganic active ingredientsSodium bicarbonateDrug compound
The invention discloses a Ceftazidime pharmaceutical compound with a molecular formula of C16H13N5Na2O7S2, whose molecular weight is 497.5, the pharmaceutical compound is prepared by reacting 1 mol of Ceftzaidime with 2mol of sodium bicarbonate under normal temperature and ordinary pressure, charging ethanol for crystallization, recrystallization, and vacuum drying at below 60 deg. C.
Owner:余安国
Process for the preparation of cefixime
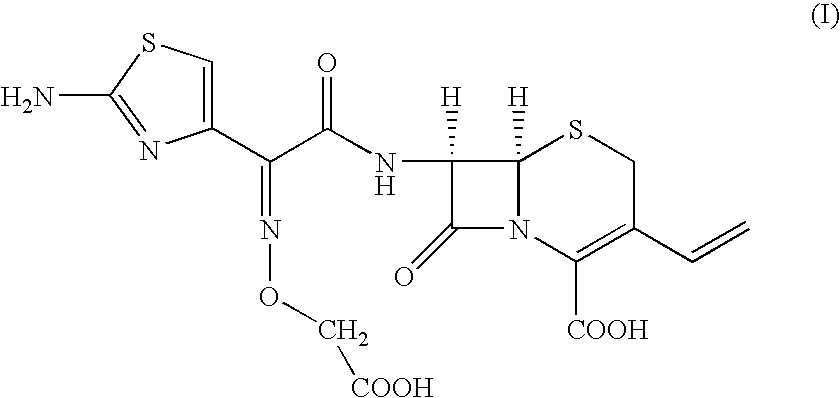
InactiveUS20040082560A1Quality improvementSpeed up the processOrganic active ingredientsOrganic chemistrySolubilityMedicinal chemistry
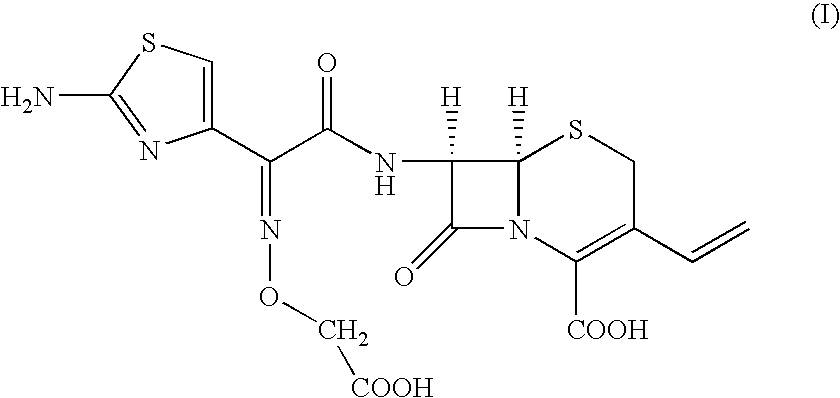
The present invention provides an improved process for the preparation of cefixime of formula (I), with an improved quality having / possessing better color and solubility.
Owner:ORCHID CHEM & PHARM LTD
Cefixime oral administration mixed suspension and preparation method thereof
ActiveCN101721363AGreat tasteFix stability issuesAntibacterial agentsOrganic active ingredientsLiquid mediumDispersed media
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
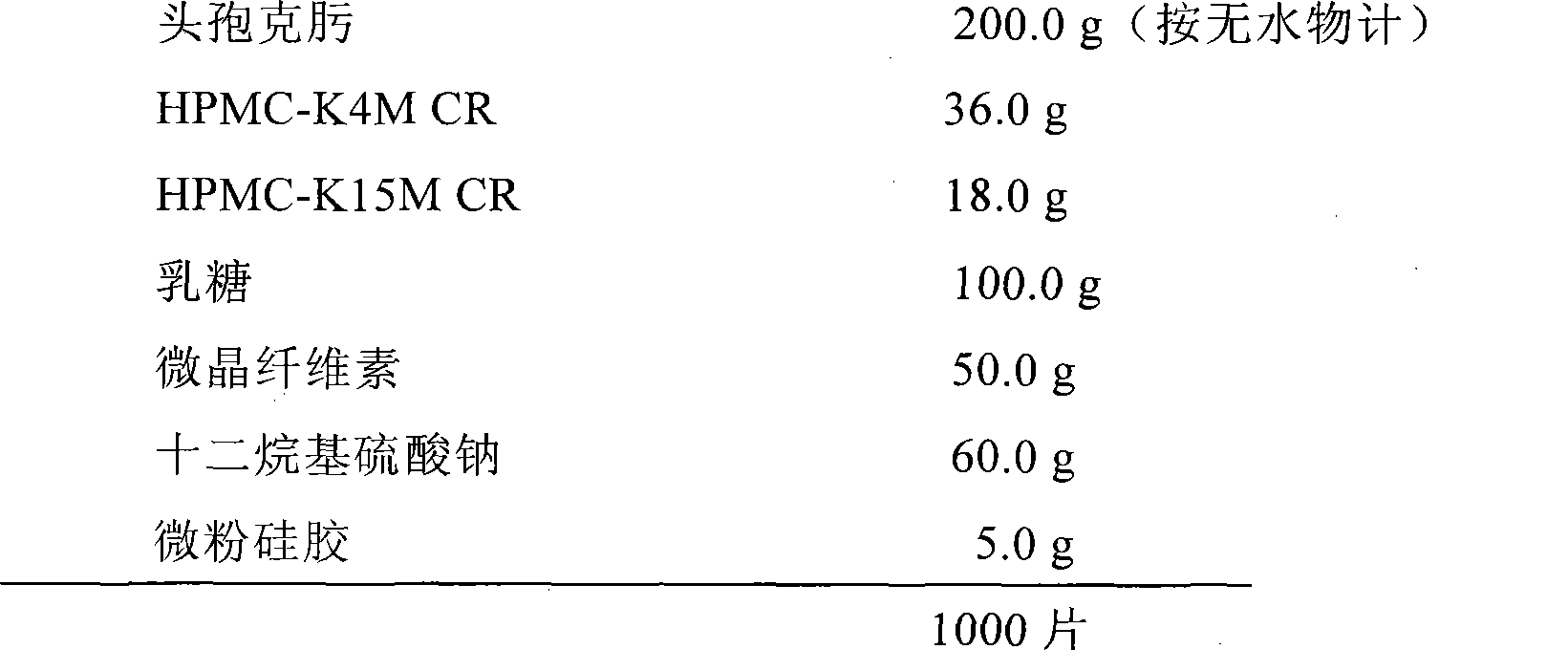
Cefixime sustained-release tablets and preparation method thereof
The invention relates to a slow-release tablet prepared from cefixime and pharmaceutically acceptable auxiliary materials. The slow-release tablet is characterized in that: the slow-release tablet consists of the following raw materials and auxiliary materials in portion by weight: 200 portions of cefixime(counted as an anhydride), 20 to 200 portions of at least one pharmaceutically acceptable slow-release material which can control the drug to continuously and completely release, 20 and 400 portions of a pharmaceutically acceptable excipient and 5 to 100 portions of at least one solubilizer which can effectively improve the release rate of the drug. The slow-release tablet is easy to prepare and facilitates the industrialized production.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Cefixime granule and preparation method thereof
InactiveCN102697736AGood quality and stabilityGood suspension effectAntibacterial agentsOrganic active ingredientsFluidized bedAdhesive
The invention relates to the field of pharmaceutical preparations, especially to a cefixime granule and a one-step granulating method of the cefixime granule. The cefixime granule comprises the following components by weight: 1-20% of cefixime, 56-95% of diluent, 0.5-1.0% of a buffer agent, 0.5-10% of adhesive and 0.1-5% of flavoring. The cefixime granule has the advantages of good quality stability, good suspension property and good content uniformity, and good taste simultaneously, so that the cefixime granule is especially suitable for children and crowd of dysphagia, and medication compliance is good. A preparation method of the cefixime granule further is provided by the invention; due to use of the one-step granulating method, steps of mixing, granulating and drying are finished in the same one fluidized bed by one step; operation method is simple, and production efficiency is high, so that the preparation method is especially suitable for industrial production.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Process for the preparation of cefixime
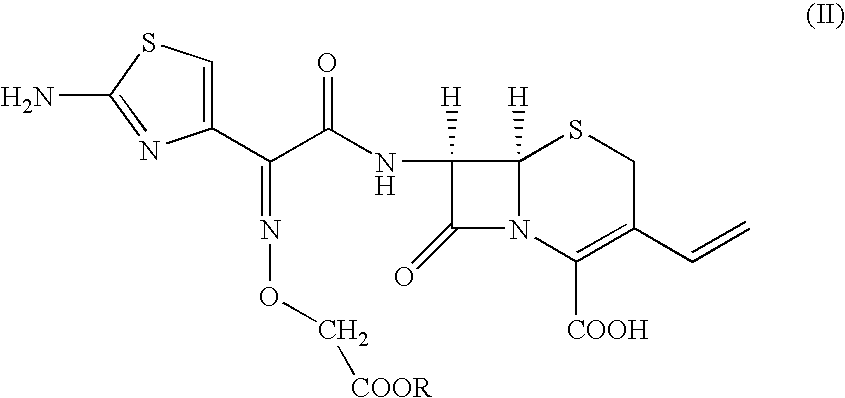
InactiveUS6800755B2Quality improvementSpeed up the processOrganic active ingredientsOrganic chemistrySolubilitySodium bicarbonate
The present invention provides an improved process for the preparation of cefixime of formula (I), with an improved quality having / possessing better color and solubility:the process includes the steps of dissolving the compound of formula (II)in water / water immisible solvent using sodium bicarbonate at a temperature in the range of 0° C. to the 35° C., hydrolyzing with sodium hydroxide at a temperature in the range of 0° C. to 25° C., and acidifying the resultant mass to 2.3 to 3.0 with dilute acid in the presence or absence of solvent at a temperature in the range of 10° C. to 45° C.
Owner:ORCHID CHEM & PHARM LTD
Cefixime pharmaceutical composition for injection
InactiveCN1593423ATo avoidFacilitated releaseAntibacterial agentsOrganic active ingredientsSolubilityOrganic acid
The invention discloses a cefixime pharmaceutical composition for injection, which is prepared from active component cefixime and solubility promoter by the weight proportion of 1-15 : 1 thus fully mixing in asepsis environment, the solubility promoter is selected from alkaline amino acids, alkali metals, or the inorganic or organic acid salt of alkaline-earth metals.
Owner:余安国
Cefdinir and cefixime formulations and uses thereof
The invention features pharmaceutically acceptable salts of cefdinir, including primary, secondary, and tertiary amine salts of cefdinir, and preparation methods, and pharmaceutical compositions including cefdinir. The invention also features water dispersible pharmaceutical dosage forms including cefdinir as active agent and methods for preparing the dosages. The invention also features tablet forms of cefixime characterized in that the tablets are in effervescent form. The invention also features the process for preparing effervescent tablet forms with cefdinir as active agents and pharmaceutical formulations obtained by the process.
Owner:BILGIC MAHMUT
Cefixime dry suspension and preparation method thereof
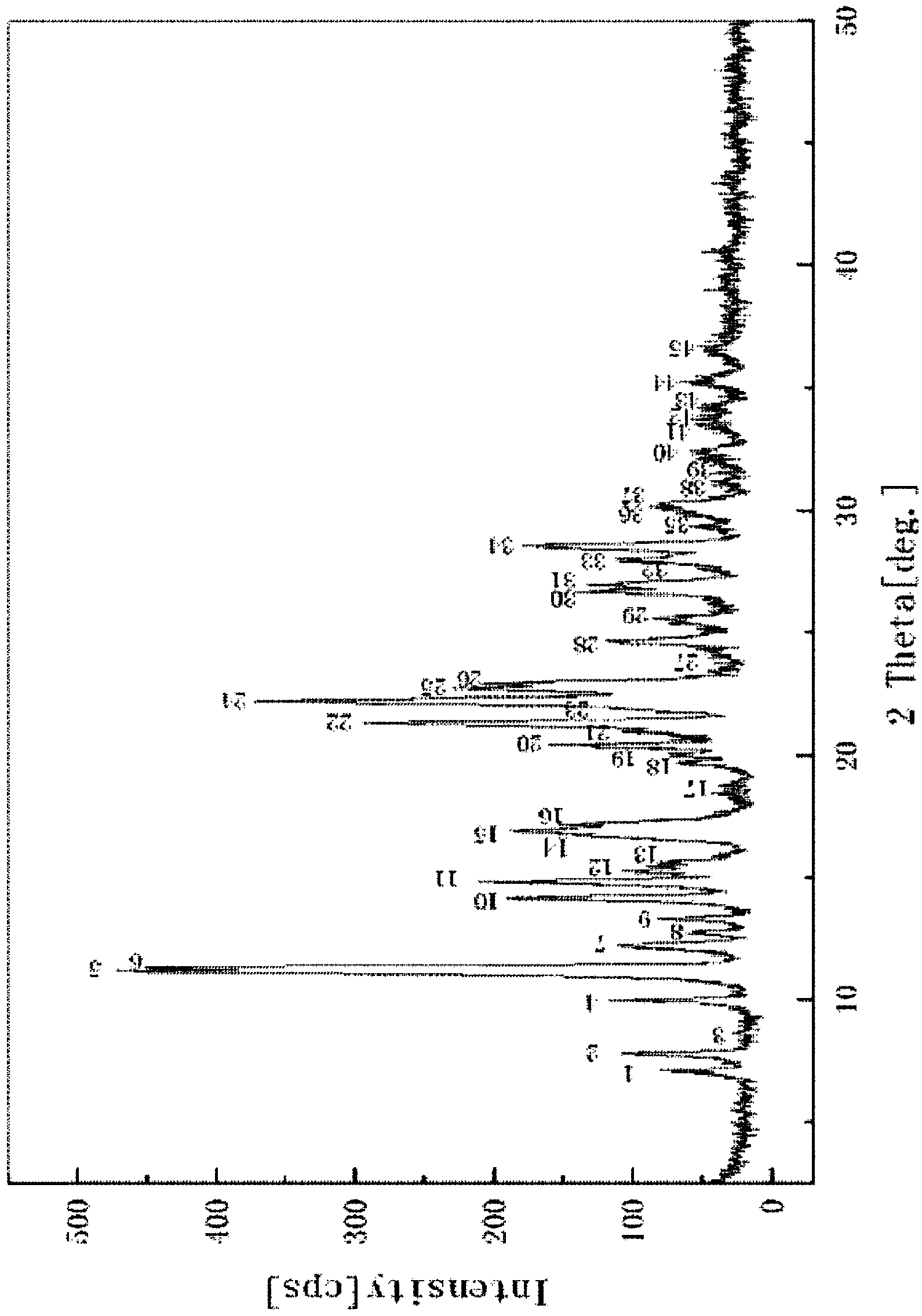
ActiveCN103622916AImprove stabilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsCurative effectX-ray
The invention relates to a cefixime dry suspension and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The cefixime dry suspension contains 40-60 parts by weight of cefixime crystal form, wherein the X-ray powder diffraction pattern of the cefixime crystal form has characteristic absorption peaks at reflection angles 2theta of 11.14 degrees, 11.50 degrees, 14.14 degrees, 16.60 degrees, 16.90 degrees, 17.18 degrees, 20.50 degrees, 21.40 degrees, 22.20 degrees, 22.80 degrees, 22.94 degrees and 28.54 degrees. As the cefixime crystal form is used by the cefixime dry suspension, absorption of the cefixime dry suspension in human bodies is accelerated and the bioavailability is improved, thus the curative effects are improved. The cefixime dry suspension has better stability and dissolution effect and improves the use efficiency of unit dose.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Cefixime crystal, preparation method thereof and tablet composition containing same
ActiveCN102311452AGood disintegrationHigh dissolution rateAntibacterial agentsOrganic active ingredientsCarboxymethyl starchSolubility
The invention relates to a cefixime crystal, a preparation method thereof and a tablet composition containing the crystal. The cefixime crystal can significantly improve the stability and dissolvability. The tablet core of the cefixime tablet composition is prepared by the following components: 35-65 parts of cefixime crystals, 17-29 parts of starch, 3-6 parts of hydroxypropyl cellulose, 3-5 parts of carboxymethyl starch sodium, 7-13.5 parts of microcrystalline cellulose, 0.3-0.7 parts of starch sodium, and 0.3-0.7 parts of magnesium stearate. The cefixime tablet combination prepared by the invention has good stability, and stable effective component contents, and is an ideal cefixime preparation.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Chromogenic culture medium for separating and detecting shigella
ActiveCN102703565AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesBiotechnologyStreptonivicin
The invention discloses a chromogenic culture medium for separating and detecting shigella; the culture medium comprises yeast powder, tryptone, soy peptone, sodium chloride, lactose, cane sugar, 2-deoxy-D-ribose, agar powder, beta-glucosaccharase chromogenic substrate, beta-pyranfucosidase chromogenic substrate, beta-galactosidase chromogenic substrate, phenol red, 3# cholate, novobiocin sodium salt, cefsulodin and cefixime; residue is water; the chromogenic culture medium provided by the invention can be used for separating and detecting four species of shigella and has advantages of high specificity, high sensitivity, easy operation, simple result judgment and the like; the chromogenic culture medium is suitable for detecting each sample and has wide application prospect; and detecting effect of the chromogenic culture medium can achieve level of like products in an import R&F company and is superior to inland like products.
Owner:GUANGDONG HUANKAI MICROBIAL SCI & TECH
Compound medicinal preparation for treating pneumonia infection disease and its preparation method
InactiveCN1650868AIncrease concentrationPlay an antibacterial roleAntibacterial agentsOrganic active ingredientsTreatment effectInfection disease
A medicine in the form of tablet, capsule, particle, oral liquid, or suspension for treating pulmonary infectious diseases is prepared from ambroxol hydrochloride and one of cefixime, cefetamet sodium and cefetamet hydrochloride.
Owner:四川川投医药生物技术有限责任公司
Granular formulation containing cefixime liposomes and preparation method thereof
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
Suspension granules for cefixime composition and preparation method thereof
ActiveCN103301075AOptimizing the composition formulaImprove stabilityAntibacterial agentsOrganic active ingredientsDrug productXanthan gum
The invention relates to the field of a pharmaceutical preparation, and particularly discloses suspension granules of a cefixime composition and a preparation method thereof. The suspension granules of the cefixime composition disclosed by the invention include effective components of herbs: cefminox, xylitol, 50% ethanol, xanthan gum, sodium polyacrylate and apple essence; and the weight-average molecular weight of the sodium polyacrylate is 90000-540000. Preferably, the cefminox, the xylitol, 50% of ethanol, the xanthan gum, the sodium polyacrylate and the apple essence are taken as effective compositions of herbs of cefminox suspension granules. Stable performance and suspension property of the cefixime are improved by synergistic effects; the medication compliance of a sufferer is improved; the safety use and long-term storage of a clinical drug are facilitated.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com