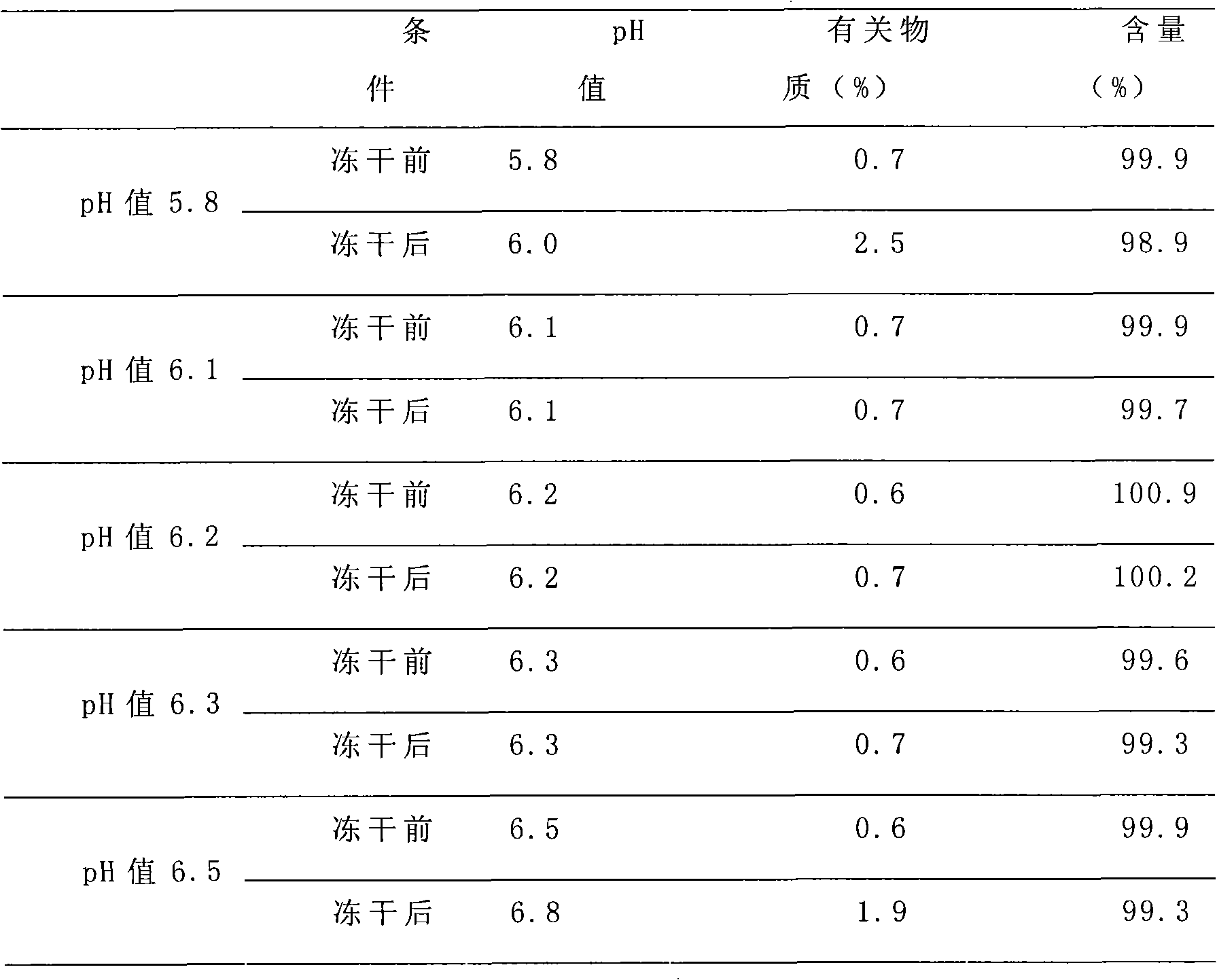
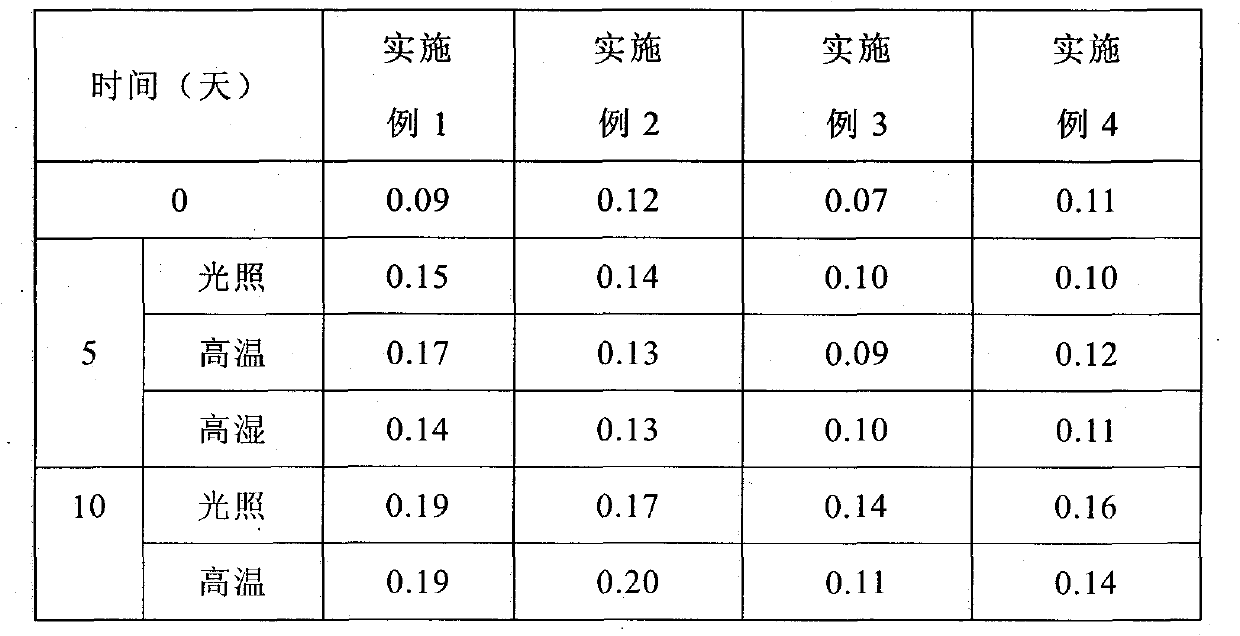
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4622 results about "Pharmaceutical Aids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
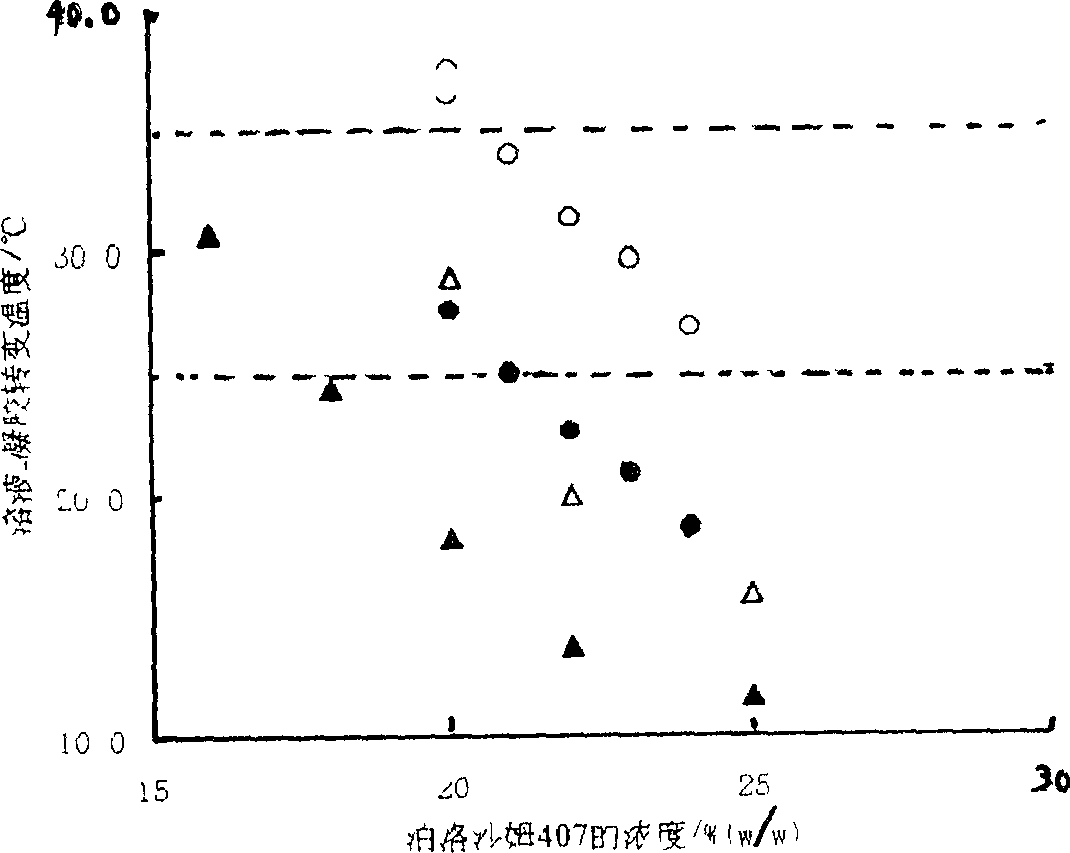
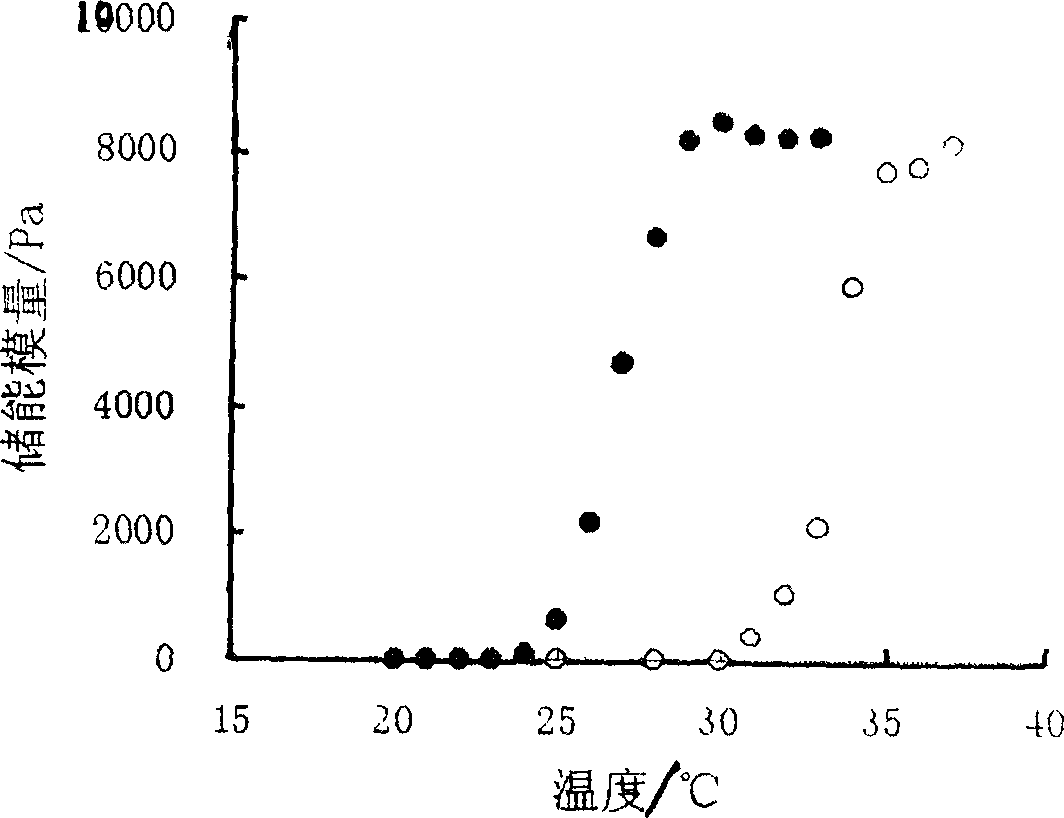
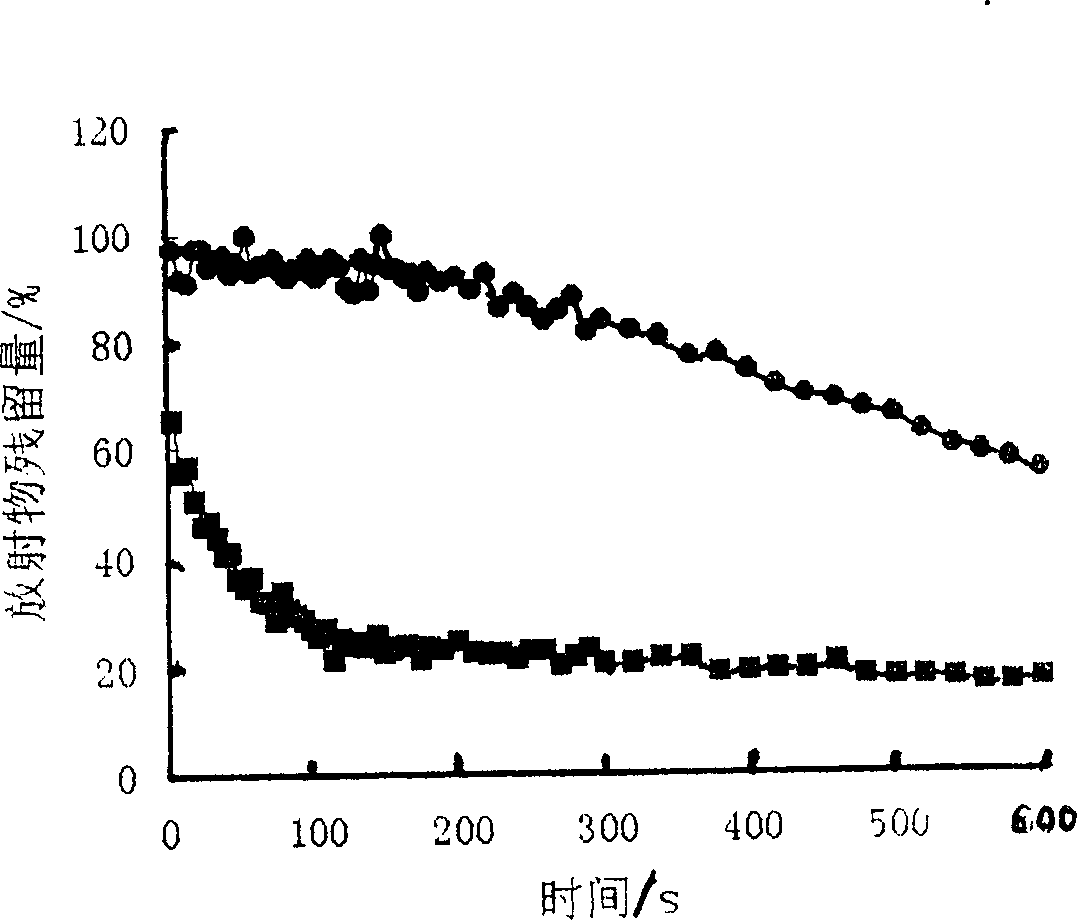
Ocular in-situ gel preparatino with proper phase conversion temperature
InactiveCN1377706AMedication convenienceReduce eliminateSenses disorderPharmaceutical delivery mechanismGel preparationTopical bioavailability
The gel preparation has the merits of both solution and gel. By means of the combination of different type poloxamers, ocular in-situ gel preparation containing medicine and water soluble polymer supplementary material is prepared. The preparation contains poloxamer 407 and poloxament 188 as well as water soluble polymer supplementary material less than 3%. The preparation has proper phase conversion temperatur and may form gel on the surface of cornea of living body after being applied in liquid state at room temperature. The present invention can delay the disappearance of medicine and raise the biological utilization in some local area and is suitable for various ocular medicines.
Owner:SHENYANG PHARMA UNIVERSITY
Human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive
InactiveCN101804218AIncrease usageImprove usabilityAbsorbent padsBandagesDressing changeCurative effect
The invention discloses a human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive, which is a novel medicine-carried dressing or a novel formulation of Yunnan white drug powder. The invention has the following remarkable characteristics: (1) the dressing can be absorbed by human bodies to lessen the pain added by dressing change and reduce the treatment cost; (2) the dressing can be made into a film solid dressing or an aquagel dressing so as to expand the use modes, the scope of applications and the drug effect of the Yunnan white drug powder; and (3) the curative effect of the dressing is enhanced by selecting a carrier material, auxiliary medicaments and functional accessories and adjusting the microstructure structure. The novel absorbable Yunnan white drug powder dressing overcomes the defects of the traditional Yunnan white drug powder in use and has economic and social values.
Owner:王艳
Composition for treating hyperlipemia
InactiveCN1425374AGood effectLarge range of dosage optionsMetabolism disorderHeavy metal compound active ingredientsActive componentCurative effect
The composition for treating hyperlipemia contains one or more nicotinic acids or the derivatives and one or more Tatin compound as 3-hydroxy-3-methyl glutaryl coenzyme inhibitor in the effective amount and the active component weight ratio of 5-100 as well as medicinal supplementary material. The present invention is used in preventing and treating hyperlipemia and has long acting period and high comprehensive treating effect.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical preparation containing a gestagen, and kit and method for treating endometriosis using the preparation
InactiveUS20080214512A1Significant positive effectEndometriosis can be reducedOrganic active ingredientsBiocideSide effectBone density
The pharmaceutical preparation for treating endometriosis contains at least 28, preferably 30, daily dose units, each of which contain dienogest, cyproterone acetate, or chlormadinone acetate at a daily dose that is at most twice that required to inhibit ovulation together with one or more pharmaceutical aids and / or carriers. The daily dose units are administered in a method of prophylaxis and / or therapy of endometriosis continuously during a time interval of at least 169 days or 25 weeks, preferably more than two years. The method effectively reduces endometriosis and associated pain, while undesirable side effects including bone density decrease are reduced or eliminated.
Owner:BAYER SCHERING PHARMA AG
A traditional Chinese medicine composition for enhancing human immune function
InactiveCN102274258AImmunological disordersDermatological disorderAMERICAN GINSENG ROOTPharmaceutical medicine
A traditional Chinese medicine composition for enhancing the human immune function. The traditional Chinese medicine composition consists of 5-150 parts of American ginseng by weight, 5-160 parts of Ganoderma lucidum by weight, 1-90 parts of fermented Cordyceps powder by weight and / or 1-120 parts of Cordyceps sinensis by weight, wherein the sources of raw material are Chinese medicinal materials or Chinese herb extracts, the amount of the latter being equivalent to the amount of the crude version of the above-mentioned Chinese medicinal materials; additionally, 5-90 parts of rose flower by weight can also be added. To the above-mentioned composition is added any pharmaceutically acceptable excipient, and any conventional dosage form including but not limited to tablets, granules, capsules, oral liquids, syrups and pills is prepared using conventional methods of traditional Chinese medicine preparation.
Owner:JIANGZHONG PHARMA
Mouth cavity quick dissolving quick disintegrating freeze-dried tablet and its preparing method
InactiveCN1473562AFast disintegrationPrevent "throat stuck" phenomenonAntibacterial agentsPill deliveryThroatFreeze-drying
The oral cavity quick dissolving and quick disintegrating freeze dried tablet for children includes at least one medicinal active component and at least one medicinal stuffing, adhesive and other supplementary material. It is loose and porous tablet in network structure and prepared through common freeze drying process. The said medicinal active component may be different children's medicines, such as antibiotic, antipyretic, analgesic, cough stopping and phlegm eliminating medicine, cold medicine, etc. Bitter or excitant medicine may be coated and water insoluble medicine is prepared intofine powder of 50 micron below size for stable dispersion in liquid. The present invention has fast disintegration, no jamming in throat, simple preparation process and low cost.
Owner:刘辉
Healthcare food capable of strengthening immunity and preparing method thereof
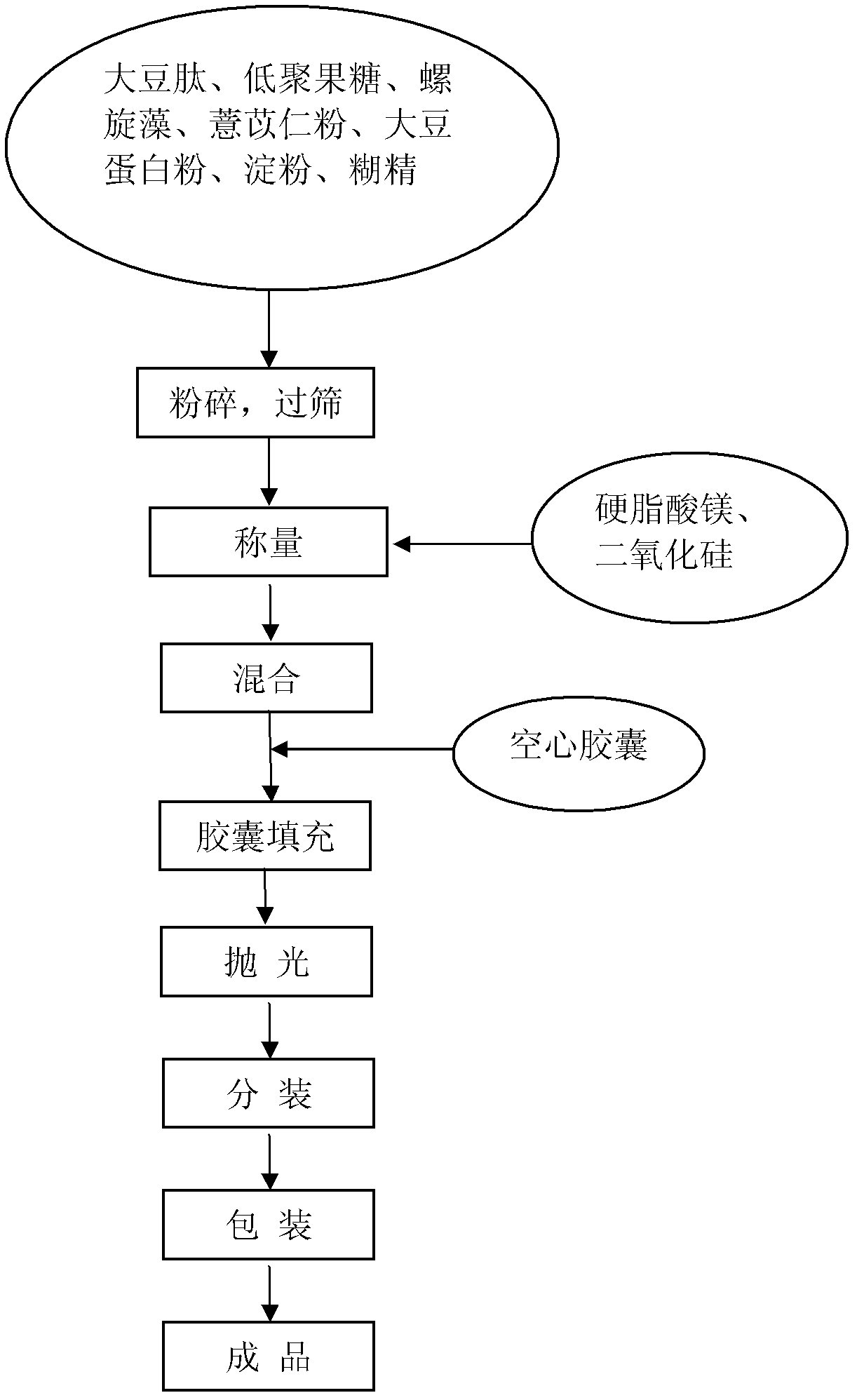
The invention discloses a healthcare food capable of strengthening immunity. The healthcare food comprises, by weight percentage, 1% to 50% of soybean peptide, 0.5% to 30% of protein powder, 1% to 30% of fructo-oligosaccharide, 0.1% to 30% of coix seed powder, 0.1% to 10% of spirulina and 15% to 85% of auxiliary materials. According to the healthcare food, materials which are nutritious and effective are selected to be subjected to scientific formulating prescription, nutrition, safety, palatability and efficacy are taken as principles, and effects of strengthening cell immune function and humoral immune function are achieved, thereby immunity strengthening is achieved.
Owner:PERFECT CHINA
Thermo-sensitive in-situ gel pharmaceutical composition
InactiveCN102125516APharmaceutical delivery mechanismMacromolecular non-active ingredientsAdjuvantPharmaceutical drug
The invention relates to an in-situ gel pharmaceutical composition, and in particular relates to a thermo-sensitive in-situ gel pharmaceutical composition, and a method for administering the pharmaceutical composition to treat and / or prevent diseases in mammals, particularly human beings. The pharmaceutical composition comprises drugs, chitosan, auxiliary gel matrix materials and pharmaceutically acceptable adjuvants. The pharmaceutical composition is superior in biocompatibility and biodegradability. The pharmaceutical composition provided by the invention has a simple preparation method, a transparent appearance and good stability, and is non-irritant and non-toxic to the mucosa.
Owner:PEKING UNIV
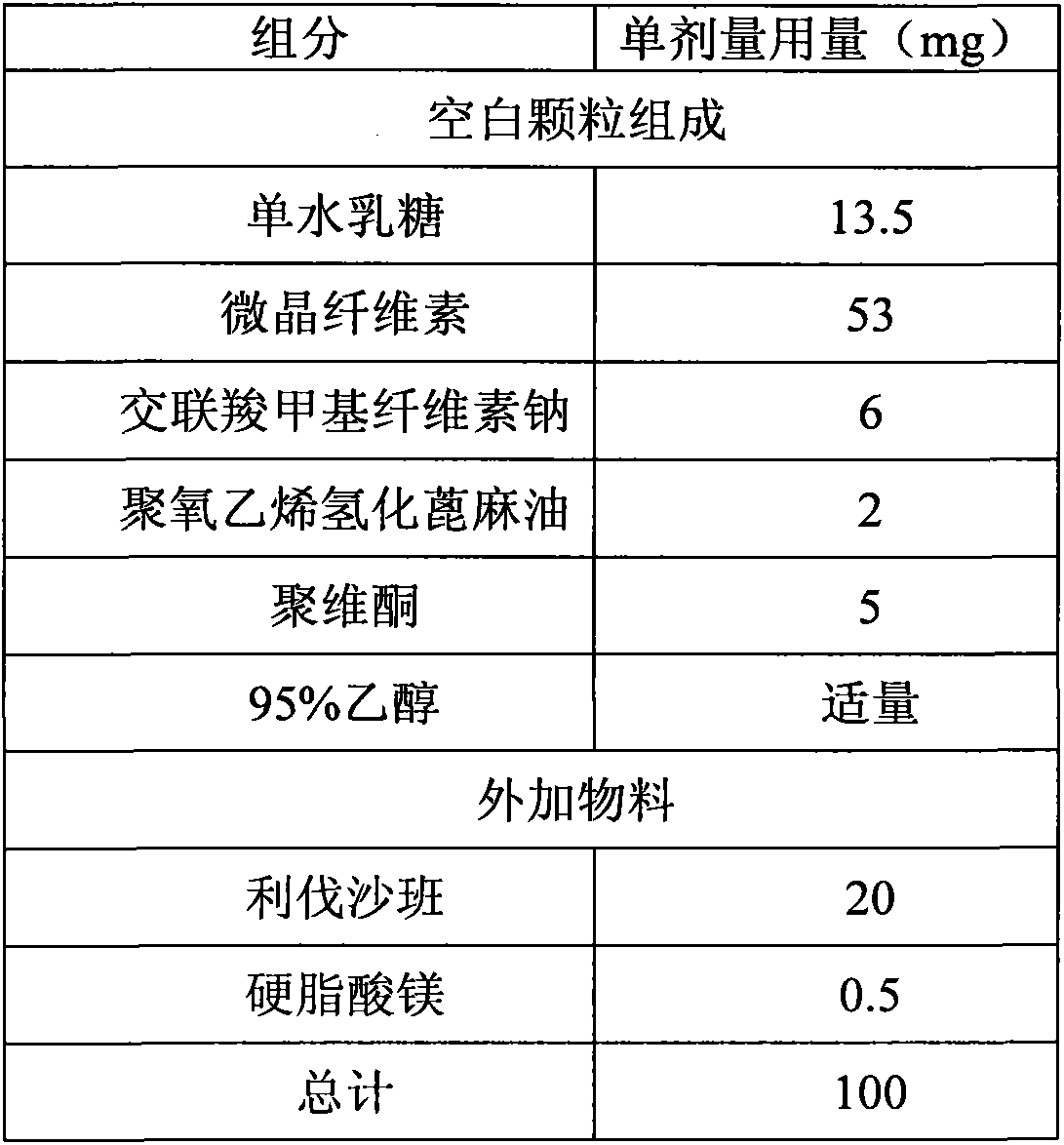
Medicinal composition containing rivaroxaban and preparation method thereof
ActiveCN103550165ASimple compositionEasy to manufactureOrganic active ingredientsPill deliveryActive componentRivaroxaban
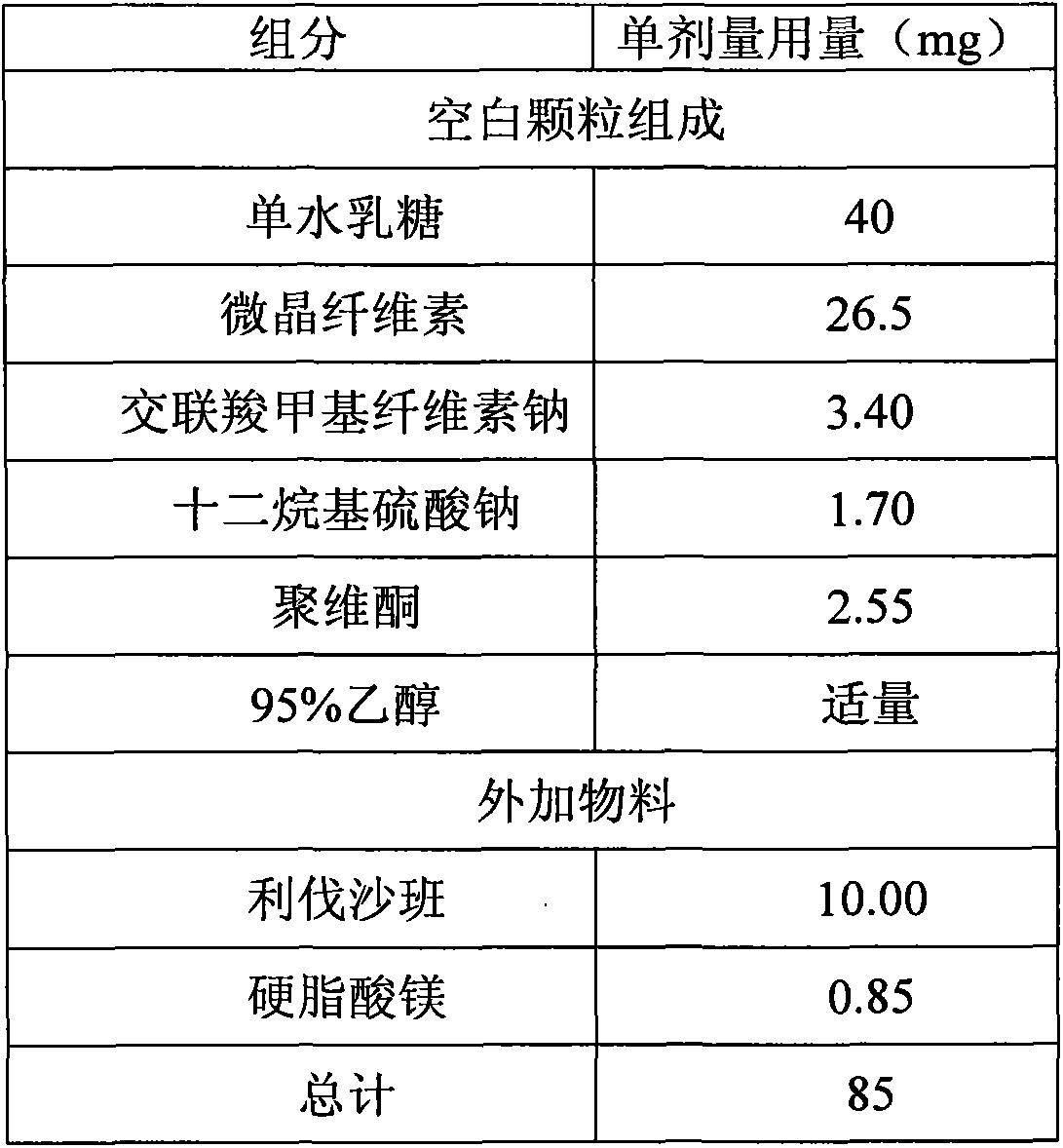
The invention relates to a quickly releasing oral solid medicinal composition containing rivaroxaban and a preparation method thereof. Proper medical auxiliaries are treated by a wet granulation process, and are mixed with active components of rivaroxaban to obtain the medicinal composition with remarkably improved dissolution. The preparation method has the characteristics of simple process and industrial production, and solves the problem of complicated preparation process in the prior art.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
PLA/PLGA shell-core microballoons prepared by oil in water-solid in oil method, and preparation method thereof
InactiveCN101461786ASmooth and rounded surfaceDisadvantages of Avoiding PollutionPharmaceutical non-active ingredientsGranular deliveryControlled releaseAcetic acid
The invention relates to a PLA / PLGA shell-core microsphere prepared by solid-in-oil-in-water in the technical field of pharmacy and a preparation method thereof. The microsphere comprises 0.01 to 50 percent of medicine, 20 to 99.99 percent of polylactic acid and / or polylactic acid-glycolic acid, or / and 0 to 30 percent of pharmaceutical excipient (weight percentage). The method comprises the steps of: adding medicine particles into a PLA and / or PLGA organic solution to be emulsified, then selecting a hydrophilic organic solvent to re-emulsify to form unhardened balls, finally hardening in another large oil phase, removing the organic solvent and collecting micropheres. The method overcomes the disadvantages of low envelope rate of the prior W / O and W / O / W, serious burst release of S / O / O, and environmental pollution, controls the grain diameter of the microsphere according to the need, does not pollute the environment, and can be applied to the preparation of slow release or controlled release microspheres of various medicines and adjuvants of vaccines.
Owner:SHANGHAI JIAO TONG UNIV
Composite type emulsifier, and emulsion prepared by using the emulsifier, and preparation method
InactiveCN101091890AGood chemical stabilityLess irritatingCosmetic preparationsToilet preparationsPolymer scienceOfficinal
The invention belongs to the medicine area, which involves a multi-emulsifier and a emulsion which be used by the multi-emulsifier and can resist high temperature and low temperature. This multi-emulsifier can combine by two or more then three emulsifiers. When combining, we can use two or more then three emulsifiers at will. The multi-emulsifier includes pharmaceutic adjuvant, oil and water. The emulsion includes drugs, oil phase, multi-emulsifier, the water and so on. The emulsion can be made after combining the oil and the water by isotropic device. The invention has some advantages: 1, the emulsion can resist high temperature and frost thawing. 2, easy to produce the average granularity smaller than the 150nm. 3, obtains the aseptic preparation without the high temperature. 4, produces the emulsion under the low pH value, so expand the application area. 5, increases the chemical stability of the medicine. 6, reduces the irritating quality.
Owner:SHENYANG PHARMA UNIVERSITY +1
Prescription and preparation method of rejoicing powder having new dosage form
InactiveCN102895432AWide range of pharmacological effectsNootropic AntioxidantNervous disorderInanimate material medical ingredientsOrganic solventModern medicine
The invention relates to a prescription and a preparation method of rejoicing powder having a new dosage form and used for preventing or treating the senile dementia, and belongs to the biological medicine field. The rejoicing powder having a new dosage form is based on rejoicing powder having an ancient classic prescription and treats Radix Polygalae, Acorus gramineus and the like as primary medicines, the primary medicines are pretreated, partial or all medicines are extracted with water or an organic solvent in necessity to obtain effective components, the primary medicines or the effective components are mixed with proper medicinal auxiliary materials, and the rejoicing powder having the new dosage form is prepared through a certain preparation technology. The traditional prescription and the traditional technology for preparing the powder through simply crushing all medicines are changed in the invention, a new method and a new technology are applied against modern clinic medicine use characteristics to prepare the novel powder, and technological products have the characteristics of safety, effectiveness, controllable quality, convenient taking and the like of modern medicines, have the advantages of high biological utilization degrees of the effective components, fast effectiveness, substantial improvement of the product quality, and effective guarantee of the basic efficacies comprising qi benefiting, heart nourishing, nerve calming and tranquilizing, and have unique clinic application values in the prevention and the treatment of the senile dementia.
Owner:王登之
Aweto micropowder tablet and preparation method thereof
ActiveCN101332212AIncrease concentrationFully activePill deliveryImmunological disordersMedicineTableting
The present invention relates to a cordyceps sinensis powder tablet and a preparation method thereof, which pertains to the field of medicines and health products. The technical problem the present invention aims at providing a tablet containing cordyceps sinensis medicinal powder and no auxiliary material; the appearance, shape and harness of the tablet is consistent with the tablet quality standard. The cordyceps sinensis powder tablet only adopts cordyceps sinensis powder with the water content of 8 to 18 percent and the granule diameter of 1 to 150mum; no auxiliary material is added in the preparation process and the cordyceps sinensis powder is the only component. The aim of direct tablet forming can be achieved by controlling the water of the powder or by the process of second tabletting or dry granule tabletting. The process ensures that the tablet appearance is good; pockmark surface rate and fracture rate are low; tablet harness, disintegration rate and friability are consistent with the tablet quality requirement; the harness can also ensure that the tablet can not fracture in the preparation process when coating and film covering is carried out in the later stage.
Owner:QINGHAI SPRING MEDICINAL RESOURCES TECHNOLOGY CO LTD
Application of traditional Chinese medicine composition in preparation of health food or medicament for preventing and relieving physical fatigue
The purpose of the invention is to provide applications of a traditional Chinese medicine composition in the preparation of health food or medicaments for preventing and relieving physical fatigue; the traditional Chinese medicine composition comprises 5-90 parts by weight of American ginseng, 5-160 parts by weight of lucid ganoderma, 1-90 parts by weight of fermented cordyceps sinensis powder and / or 1-120 parts by weight of cordyceps sinensis; the sources of the raw materials are traditional Chinese medicines or traditional Chinese medicine extracts equivalent to the crude drug amounts of the above traditional Chinese medicines; and 5-90 parts by weight of roses can be added into the composition; the invention also relates to an application of the composition in the preparation of health food or medicaments for preventing high blood lipid and reducing blood lipid; the invention also relates to an application of the composition in the preparation of health food or medicaments for preventing and resisting oxidation; the invention also relates to an application of the composition in the preparation of health food or medicaments for improving hypoxia tolerance; the pharmaceutical composition of the invention can be added with any one of pharmaceutically-acceptable auxiliary materials so as to be prepared into any routine dosage form.
Owner:JIANGZHONG PHARMA
Lutein ester health care product for protecting eyesight and preparation method thereof
InactiveCN102144780AFree from destructionStrengthen the immune systemSenses disorderSulfur/selenium/tellurium inorganic active ingredientsDiseaseAlpha-Lipoic Acid
The invention discloses a lutein ester health care product for protecting eyesight. The lutein ester health care product is prepared by taking lutein ester as a main material, and adding any one or more materials including taurine, selenium, zinc, alpha-lipoic acid, docosahexaenoic acid (DHA), vitamin A, vitamin E, vitamin B1, vitamin B2 and bilberry extract, and pharmaceutically acceptable formulation accessories. The outstanding innovation of the invention is the implementation of the method for preparing a functional eye health care product with the lutein ester instead of lutein, so that the bioavailability, the stability and the health care effect of the product are improved, and production cost can be greatly reduced. The health care product can be prepared into common dosage forms, such as tablets, capsula, granules and soft capsules. The lutein ester health care product is mainly used for the treatment on diseases, such as myopia, amblyopia, hyperopia, presbyopia, cataract, vitreous opacity, retinal pigment degeneration, macular degeneration, asthenopia, and retinopathy caused by diabetes, and the like. The lutein ester health care product adopts a unique formulation of multiple constituents, measures and mechanisms, and has significant treatment effects and excellent social and economic benefits.
Owner:崔晓廷
Total flavone glycoside extract of Radix scutellariae, Rodix scutellariae monomer flavone glycoside, its preparation and use
This is a kind of baicalin total flavones glucoside distillation, baicalin monomer flavones glucoside and its preparation method and application, belonging to the Chinese native medicine drugs manufacturing technology field. The invention obtains the baical in total flavones glucoside distillation mainly containing biacalein, or baical in flavones glucoside monomer compound mainly containing biacalein or wogonoside or oroxylin; comminute baixcal, add water, have alcoholysis reaction at 41 -46 Deg. C, the baialinase and baicalin flavones glucoside naturally existing in baixcal directly have alcoholysis reaction without distillation and separation. The pharmacy preparation includes various pharmacy preparations comprised of officinal auxiliary materials with baicalin total flavones glucoside distillation or monomer flavones glucoside as pharmacy active components, and are used to prepare drugs for curing liver diseases and AIDS. The reaction condition of the invention is mild, and it has high distilling rate, good product quality, low energy waste simple craft, no pollution and low production cost.
Owner:SHANDONG UNIV
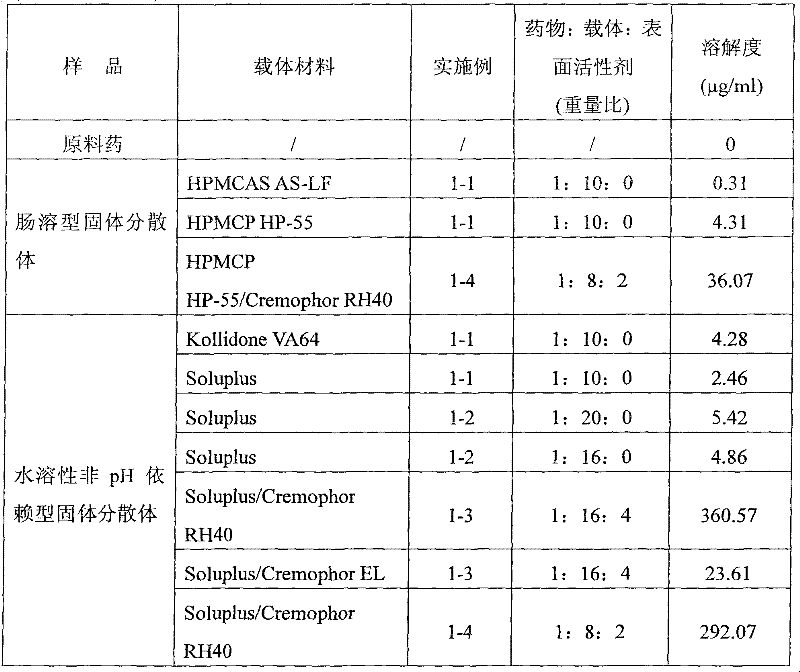
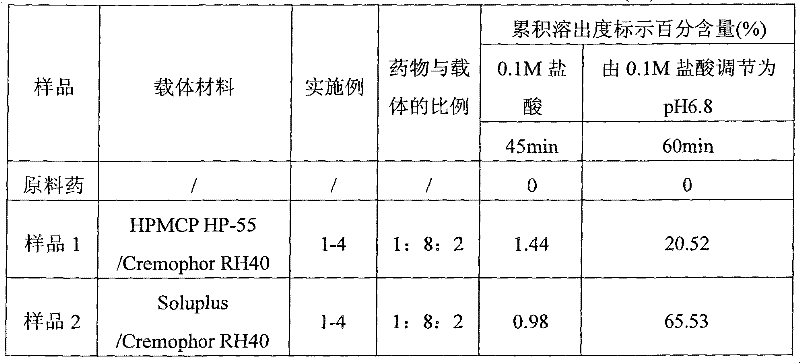
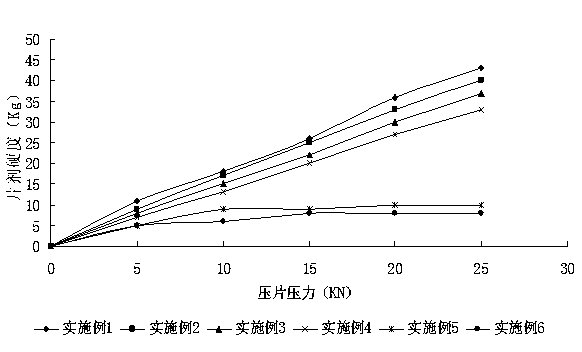
Enteric solid preparation containing lycopene, resveratrol or melatonin and preparation method of enteric solid preparation
The invention relates to the field of medical preparations, in particular to an enteric solid preparation containing lycopene, resveratrol or melatonin and a preparation method of the enteric solid preparation. The enteric solid preparation comprises one or more of lycopene, resveratrol and melatonin as an active ingredient, water-soluble and / or enteric carrier adjuvants or other pharmaceutic adjuvants. The water-soluble carrier adjuvants can be used as water-soluble solid dispersoid carriers; and the enteric carrier adjuvants are enteric polymers and can be used as enteric solid dispersoid carriers or enteric coating film materials. The lycopene, the resveratrol and the melatonin of the enteric solid preparation have favorable dissolubility in the intestinal tract, so that the medicament, namely, the enteric solid preparation, can be rapidly dissolved and released in the intestinal tract, and thus absorption and bioavailability of the lycopene, the resveratrol and the melatonin are increased. The enteric solid preparation containing the lycopene, the resveratrol and the melatonin can be suitable for application and industrial production of oral preparations, such as tablets, particles, pellets, capsules, enteric capsules, enteric coating tablets, enteric coating pellets, enteric coating particles and the like.
Owner:SINOTHERAPEUTICS
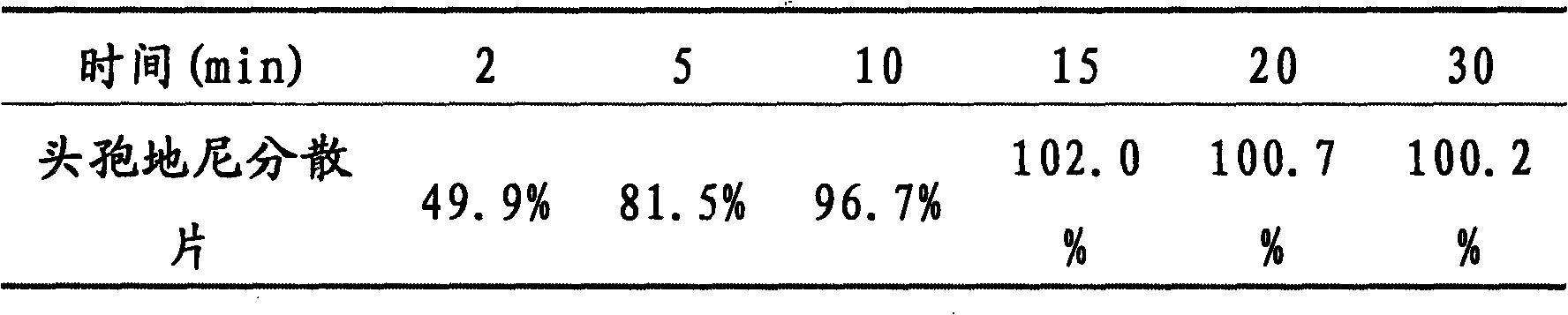
Cefixime dispersing tablet and preparation methods thereof
ActiveCN101606913AIncrease contentEasy to takeAntibacterial agentsOrganic active ingredientsMagnesium stearatePharmaceutical preservatives
The invention discloses a cefixime dispersing tablet and preparation methods thereof, belonging to the technical field of pharmaceutical preparation. The cefixime dispersing tablet comprises 40-420 mg of cefixime, 0-100 mg of starch, 0-250 mg of amylum pregelatinisatum, 10-80 mg of mannite, 0-150 mg of microcrystalline cellulose, 10-60 mg of carboxyrnethyl starch sodium, 2-20 mg of polyvidone K30, 0.4-10 mg of magnesium stearate, 0-10 mg of Steviosin and 0-10 mg of orange compound perfume. The preparation method comprises the following steps: evenly mixing basic remedies and excipients; adding adhesive to prepare granulates; drying; sorting and then adding with other excipients; and tabletting. The other preparation method is described as follows: the basic remedies are added after granulating. The invention aims to solve the technical problem that cefixime preparation has shorter disintegration time, better leachability, higher content of the basic remedies, production cost reduction, quality detection time reduction and production environment pollution reduction.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Oral solid composition of abiraterone and preparation method thereof
The invention relates to an oral solid composition of abiraterone and a preparation method thereof. The composition contains abiraterone acetate with a particle size of 1-30 micrometers and pharmaceutic adjuvants.
Owner:CHONGQING PHARMA RES INST
Long-circulating solid lipid docetaxel nanoparticles and preparation method thereof
InactiveCN101653414AGood water solubilityImprove stabilityOrganic active ingredientsAntineoplastic agentsLipid formationSolubility
The invention discloses long-circulating solid lipid docetaxel nanoparticles and a preparation method thereof. The long-circulating solid lipid docetaxel nanoparticles comprise the following materialsin therapeutic effective dose: docetaxel, lipid materials, long-circulating auxiliary materials and an emulsifier. The long-circulating solid lipid docetaxel nanoparticles have small particle size, high encapsulation rate and good stability, and not only improve the solubility and the stability of the docetaxel, reduce the toxicity of the docetaxel, but also prolong the circulating time of a medicament in blood, and improve the therapeutic index of the medicament, so that the preparation has the characteristics of low toxicity, low allergy, high efficiency and targeting in clinical application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
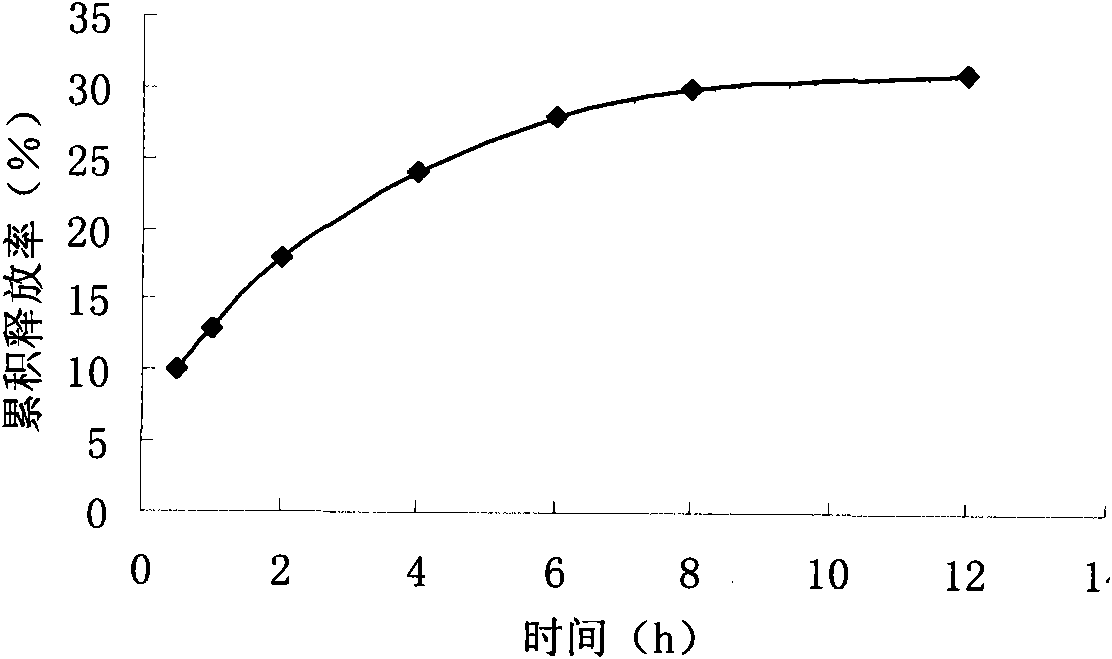
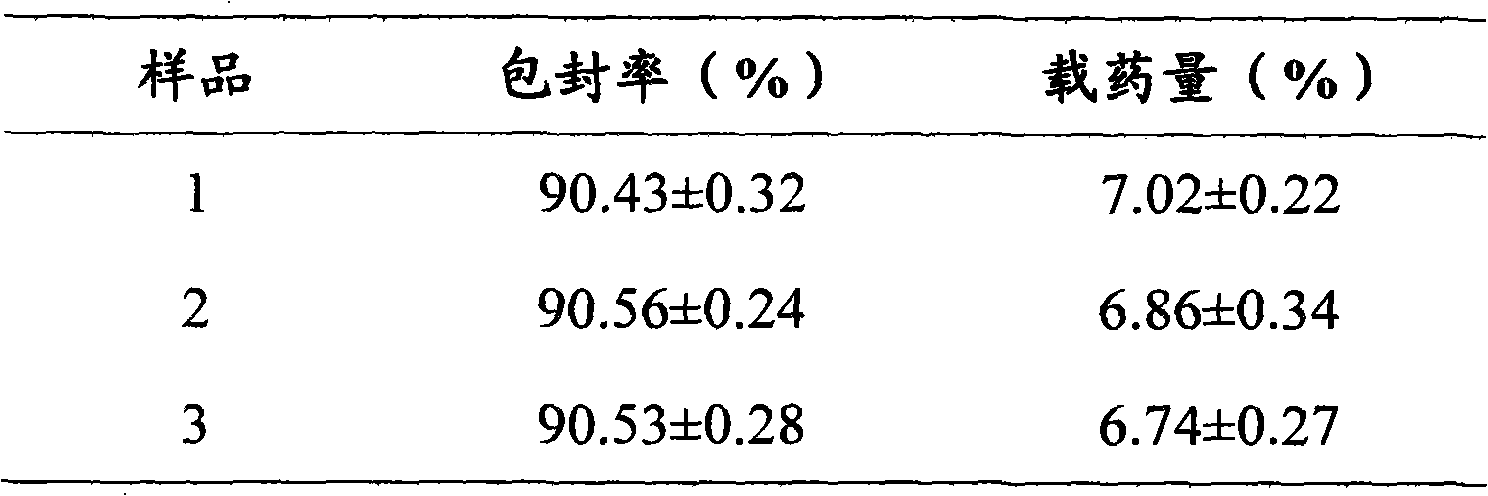
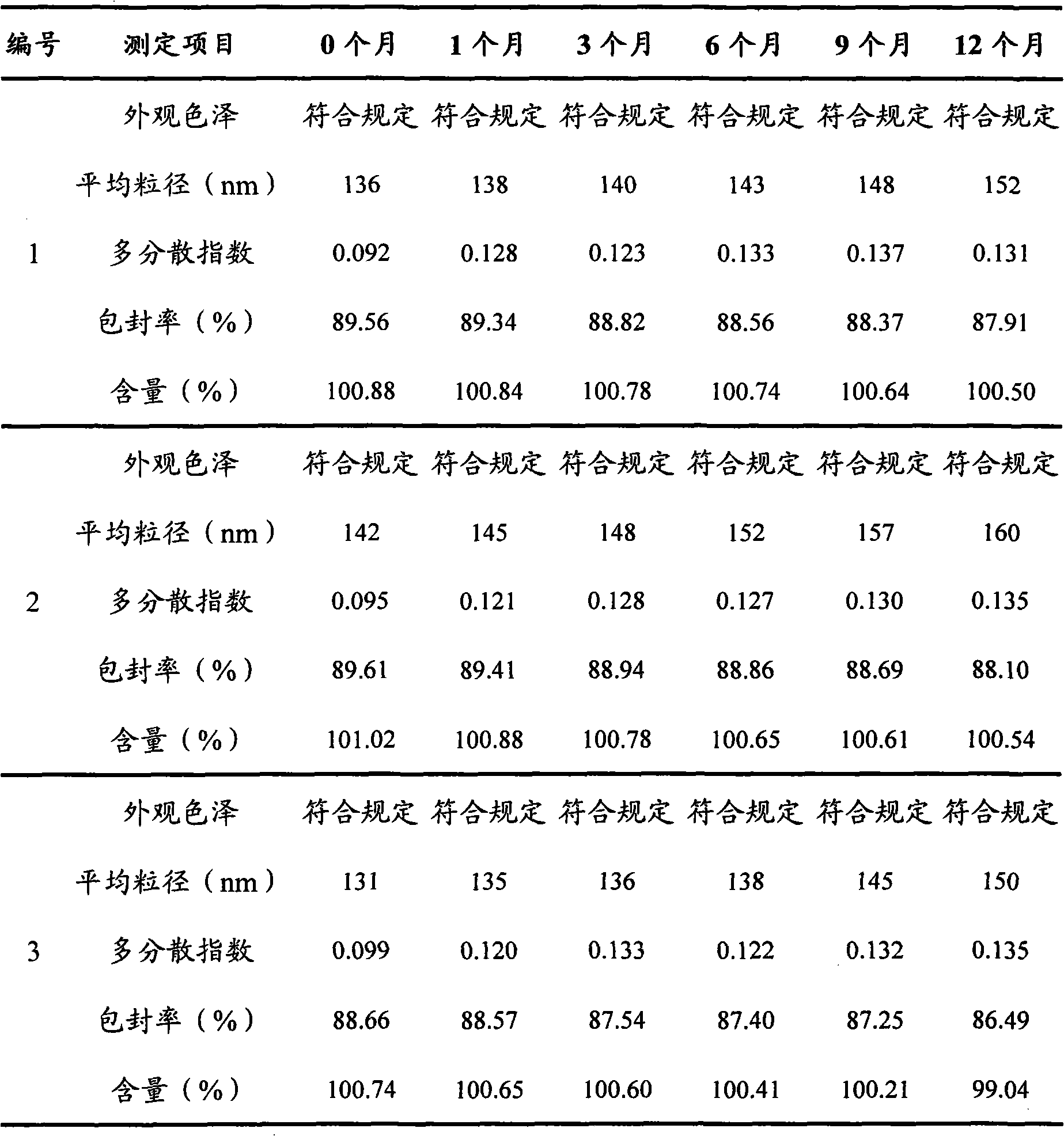
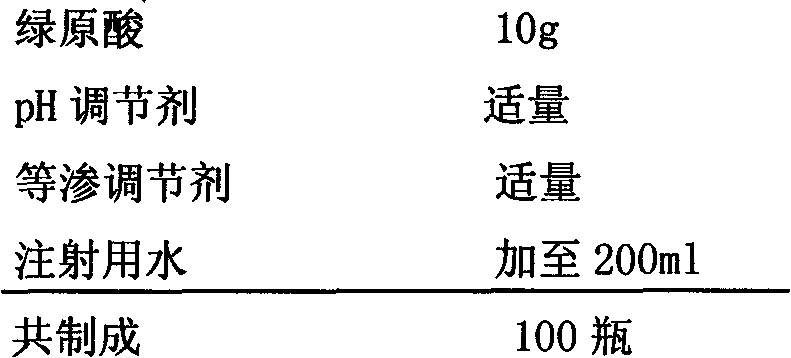
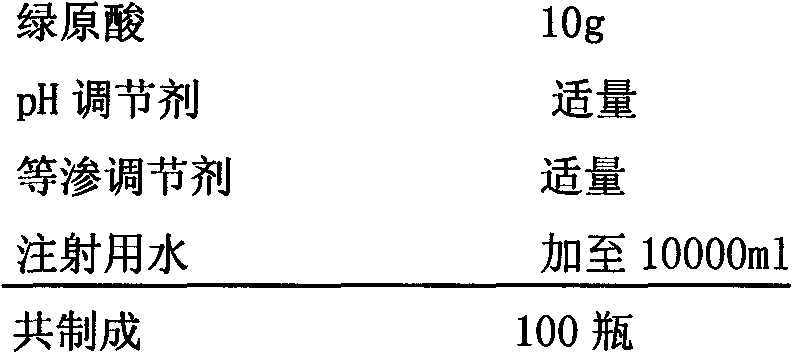
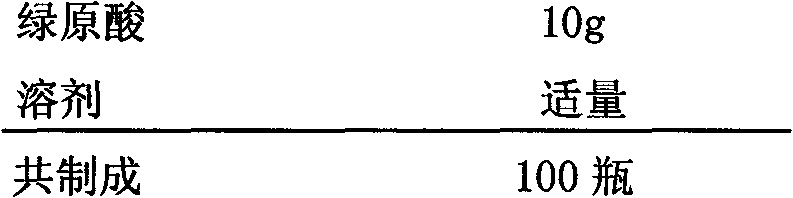
Preparation of high purity chlorogenic acid preparation and clinical application thereof
InactiveCN102391119AAntibacterialSuppressor mutationAntibacterial agentsOrganic active ingredientsDiseaseChlorogenic acid
Chlorogenic acid, i.e. 1, 3, 4, 5-tetrahydroxycyclohexanecarboxylic acid-(3, 4-dihydroxycinnamic acid ester), exists in a plurality of plants, and also, high purity chlorogenic acid can be prepared through synthesis. Chlorogenic acid has a lot of biological activity, such as antibacterium, antivirus, antioxidation, antitumor and the like. However, there exists no report and research on oral preparations prepared by high purity chlorogenic acid and application of the preparations in clinics. In the invention, high purity chlorogenic acid is extracted from plants or obtained through synthesis, and is then added with a proper amount of accessories, thus obtaining oral preparations like tablets, capsules, granules, oral solutions, etc. The preparations provided in the invention can be applied in clinics for treating cardio-cerebrovascular diseases, infections, hepatitis B, tumors and other diseases, and also can be used in health care medicines for heat clearing and detoxifying, face nursing and skin moistening, as well as hangover relieving, etc.
Owner:肖文辉 +1
Paris total saponins extract with tumor metastasis resisting action and pharmaceutical formulation thereof
InactiveCN101214342ASmall side effectsSignificant transfer effectPowder deliveryOrganic active ingredientsPharmaceutical formulationSaponin
The present invention provides a paris total saponin extract for resisting tumor metastasis and a pharmaceutical preparation thereof. The pharmaceutical preparation is composed of paris total saponin extract as the active component and medicinal auxiliary materials; wherein, the weight percentage of paris total saponin extract in the total weight of the pharmaceutical preparation is 60 to 98 percent. The paris total saponin extract for resisting tumor metastasis and the pharmaceutical preparation provided by the present invention have good inhabitation on the metastasis of animal transplanted tumor cells, demonstrated in (1) significant anti-metastasis function for inbred mice and nude mice metastasis model of vaccinated mouse lung adenocarcinoma, human lung cancer, human gastric cancer, human intestinal cancer, human liver cancer and other tumor strains; (2) significant effect on relative albumen metastasis; (3) significant effect on relative gene metastasis and no obvious toxicity on major viscera. In addition, the main active component of the pharmaceutical preparation is a Chinese medicine extract, so compared with chemical drugs, the present invention has the advantage of small toxicity.
Owner:TIANJIN UNIV
Taxine kind anti-cancer slow release injection
InactiveCN1923189AOrganic active ingredientsPharmaceutical delivery mechanismCelluloseAcetic acid ethenyl ester
The invention relates to a slow-release injection of taxine anti-cancer drug, which comprises anti-cancer drug, slow-release finding, suspension and / or solvent. Wherein, said anti-cancer drug is taxine, 2'-hydroxy Paclitaxel, etc; the slow-release finding is polymer of hydroxyl, glycollic acid and glycolic acid, one of acetic acid ethyenyl ester polymer and polyphony; the suspension is polyphenyl (sodium), and mannite; the solvent is distilled water, injection water, absolute ethyl alcohol, etc. The invention can be injected to reduce the toxicity effect of drug, and improve the density locally to strengthen the treatment effect of chemotherapy and radiation therapy.
Owner:孔庆忠
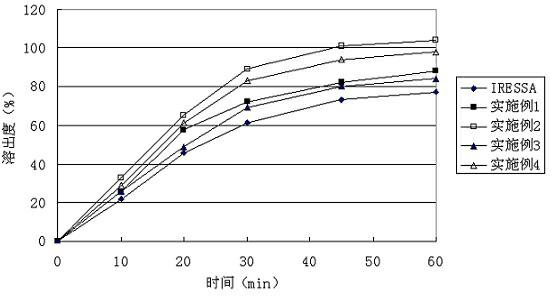
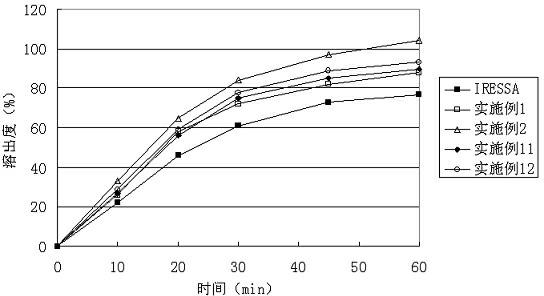
Gefinitib medicinal composite and method for preparing same
ActiveCN102631347ABreak the use limitWill not increase the burdenOrganic active ingredientsAntineoplastic agentsAdjuvantAdhesive
The invention relates to a gefinitib medicinal composite, which consists of the following components in proportion by weight: 20%-65% gefinitib, 20%-75% diluent, 0.1%-3% solubilizer, 1%-5% adhesive, 2%-10% disintegrant and 0.4%-2% lubricant. The gefinitib in the composite provided in the invention also can exist in an amorphous state, and accordingly, the dissolution efficiency is further improved. The gefinitib medicinal composite provided in the invention adopts conventional adjuvant, has a simple and easy preparing technology, and dissolution experiments prove that a better dissolution effect can be achieved, and the quality of the gefinitib medicinal composite is obviously better than products on the market.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
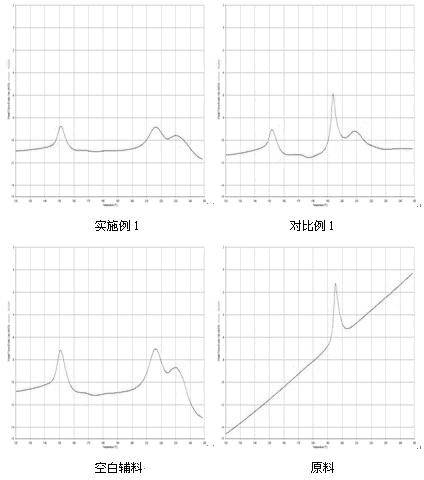
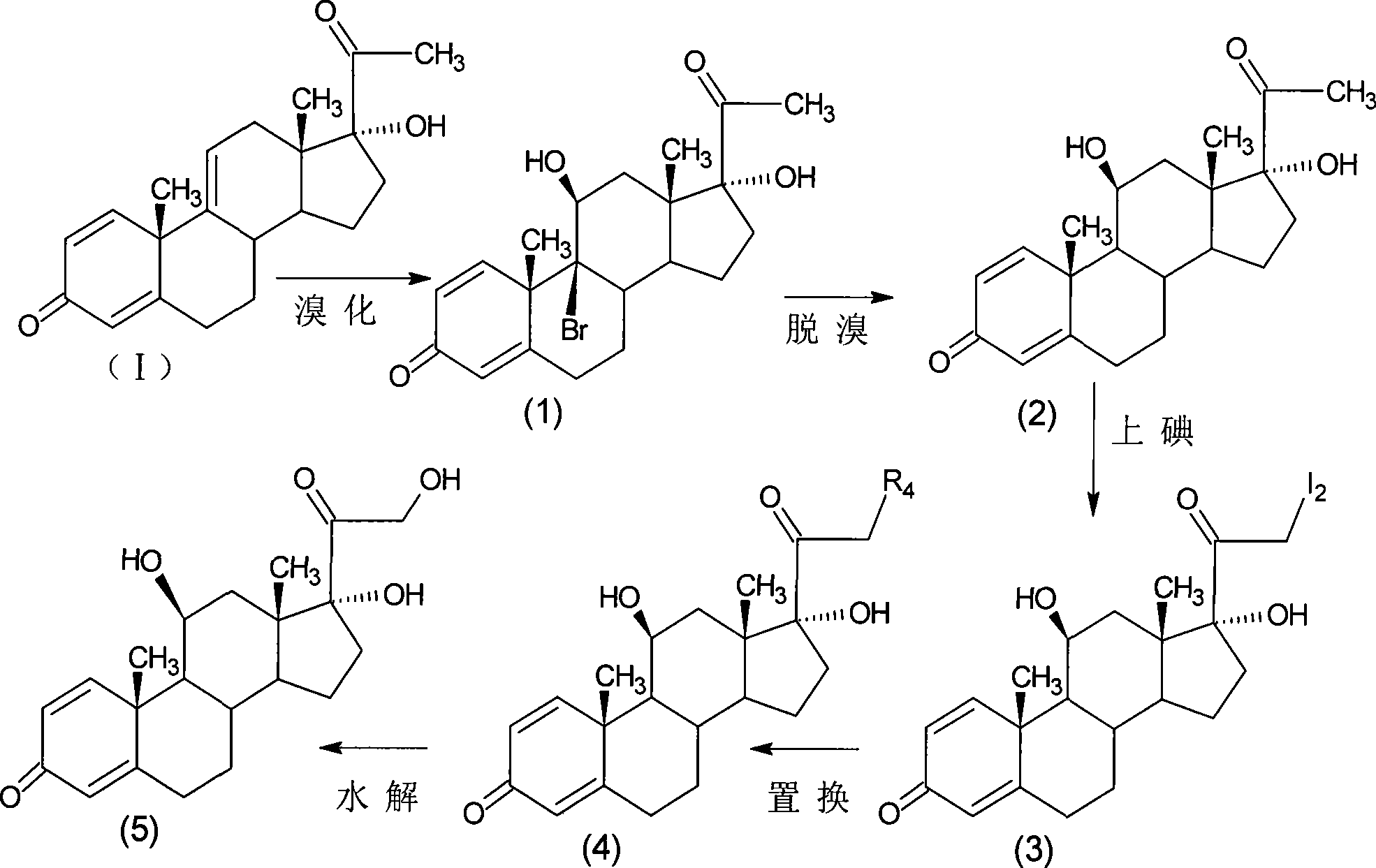
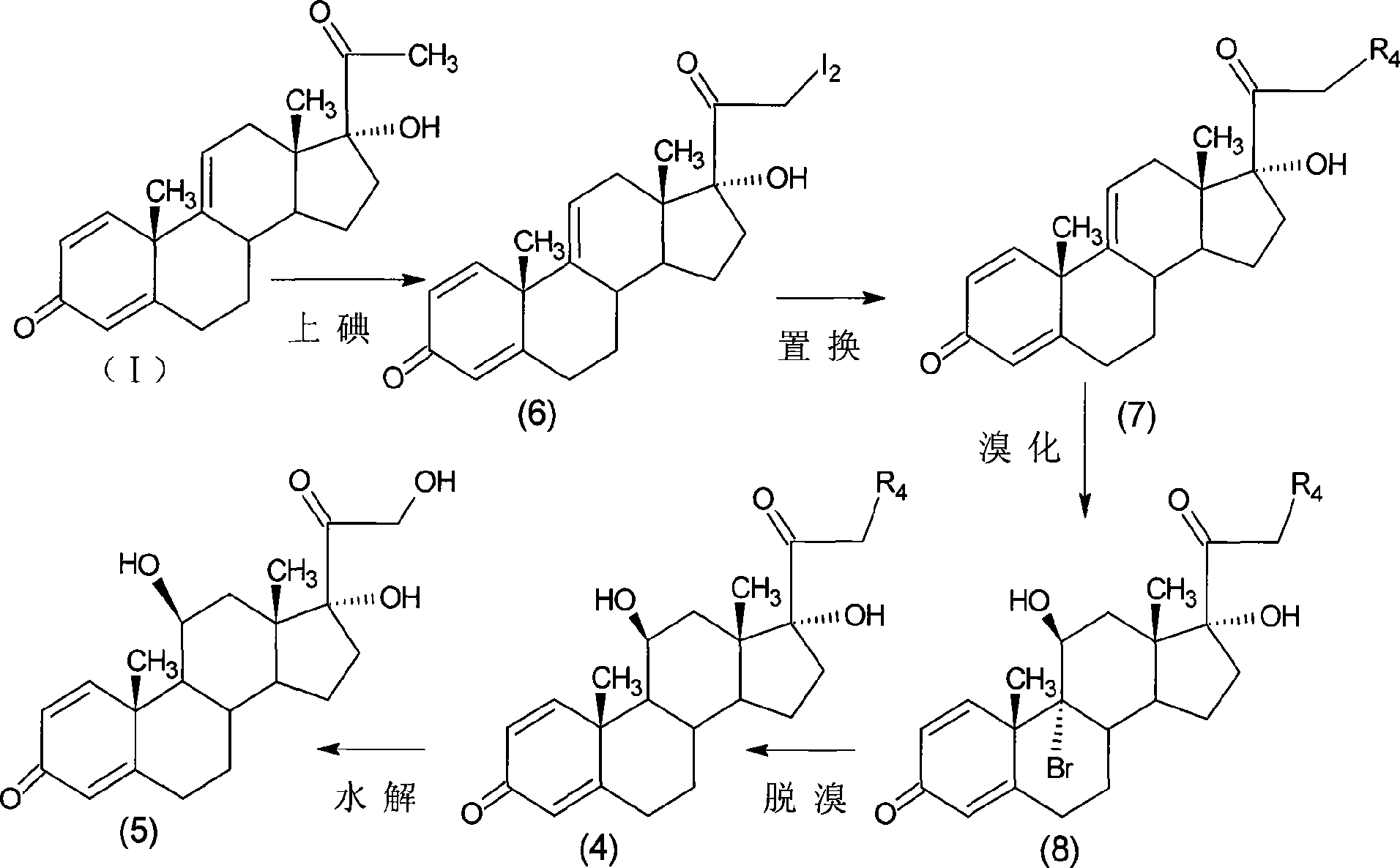
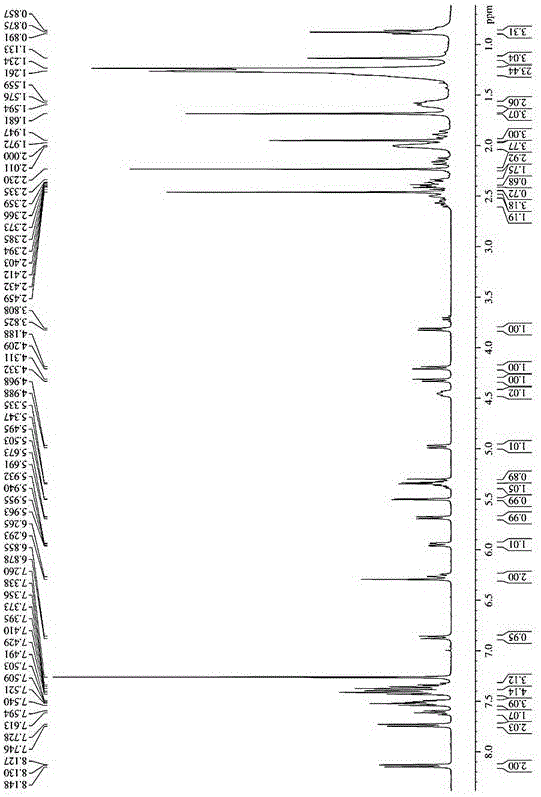
Preparation of metacortandralone and derivatives thereof
The invention relates to a preparation method of a steroid compound, in particular to the preparation for prednisolone and the derivative thereof, which takes 17-hydroxyl-1, 4, 9-triene- pregna-3, 20-diketone as the initiator and is improved by 9, 11th and 21st to obtain the prednisolone and the derivative thereof, such as prednisolone acetic ester, isoflupredone, and the like. The invention further provides the application of a compound (I) in the preparation of a compound (II). As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the prednisolone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the triamcinolone products and the prednisolone products, thus greatly reducing the production cost and industrial conditions. R1 is equal to H, F, Cl and Br; R2 is equal to H, OH and OCOR3, wherein, R3 is equal to the alkyl with less than 11carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
Construction of paclitaxel-oleic acid small-molecular prodrug self-assembled nanoparticles
ActiveCN105833284AAchieve specific drug releasePromote enrichmentPowder deliveryOrganic active ingredientsSide effectPolyethylene glycol
The invention designs and synthesizes a series of paclitaxel-oleic acid small-molecular prodrugs; with the application of a chemical connecting arm which is sensitive to an oxidation-deoxidation environment, the rapid release of the drugs in tumor cells is promoted. On the basis, small-molecular prodrug self-assembled nano-drug delivery systems are prepared. The small-molecular prodrug self-assembled nano-drug delivery systems have the advantages that by virtue of a one-step nano-precipitation method, nano-drug self-assembled nanoparticles are simple in preparation process and easy for industrialization; the nano-drug self-assembled nanoparticles are small and uniform in grain size (to 100nm), and the nano-drug self-assembled nanoparticles are enriched in a tumor part by virtue of an EPR (enhanced permeability and retention) effect; an ultrahigh drug-loading capacity is guaranteed, which is beneficial for reducing adverse reactions caused by auxiliary materials and biological materials; surface modification is easy to implement, and the intake of a reticuloendothelial system can be effectively avoided and the intake of the tumor cells to the nanoparticles can be improved by virtue of PEG (polyethylene glycol) and active targeting modification; and on the basis of the sensitivity of the chemical connecting arm to the oxidation-deoxidation microenvironment of the tumor cells, the specific drug release of paclitaxel in the tumor part is achieved, a curative effect is improved and toxic and side effects are reduced.
Owner:SHENYANG PHARMA UNIVERSITY +1
Clindamycin phosphate freeze-dried powder needle and preparation thereof
InactiveCN101301278ASimple recipeLittle side effectsAntibacterial agentsPowder deliverySide effectFreeze-drying
The invention provides a clindamycin phosphate freeze-dried powder injection, which is prepared by the steps that: clindamycin phosphate solution is added with NaOH and is freeze-dried, wherein, the weight ratio of clindamycin phosphate to the NaOH is between 12 and 18 to 1, and the preferred weight ratio is 16.5 to 1. The clindamycin phosphate freeze-dried powder injection has simple formula and less auxiliary materials, overcomes side effects due to the fact that the auxiliary materials are excessively added, and ensures that patients are safer for use.
Owner:BEIJING JINGWEI SHUNKANG MEDICAL TECH DEV
Levamlodipine beaylate tablets and preparation method thereof
ActiveCN101766582AImprove stabilityRapid dissolutionOrganic active ingredientsPharmaceutical delivery mechanismActive componentLevamlodipine
The invention belongs to the technical field of medicament, and provides levamlodipine beaylate tablets and a preparation method thereof. The tablets consist of tablet cores using the levamlodipine beaylate as an active component and film coatings coated on the outer layer, wherein diluent in the tablet cores contains diatomite or aerosil, or a mixture of diatomite and aerosil, and contains other pharmaceutically acceptable supplementary materials; and the outer film coating accounts for 8 to 12 percent of the weight of the tablets, and can play a role in resisting humidity and avoiding light to ensure that the medicinal stability can be greatly improved, and related substances are obviously reduced. Furthermore, the tablets have small specification, so the tablets ensure uniform content, and improve dissolution; and the method is simple and controllable, and ensures that the hygroscopicity of the medicament is obviously reduced.
Owner:鲁南新时代生物技术有限公司
Sustained releasing preparation of vilamin C and its preparing method
InactiveCN1582922AGood sustained release effectOrganic active ingredientsMetabolism disorderVitamin CPlasticizer
A slow-releasing VC in the form of micropills is composed of pill and coated layer. Said pill is prepared from core, VC, antioxidizing agent, synergistic, adhesive and other auxiliary. Said coated layer is prepared from slow-releasing material, plasticizer, pore forming agent and defoaming agent.
Owner:范敏华
Cefdinir dispersible tablet and preparation method thereof
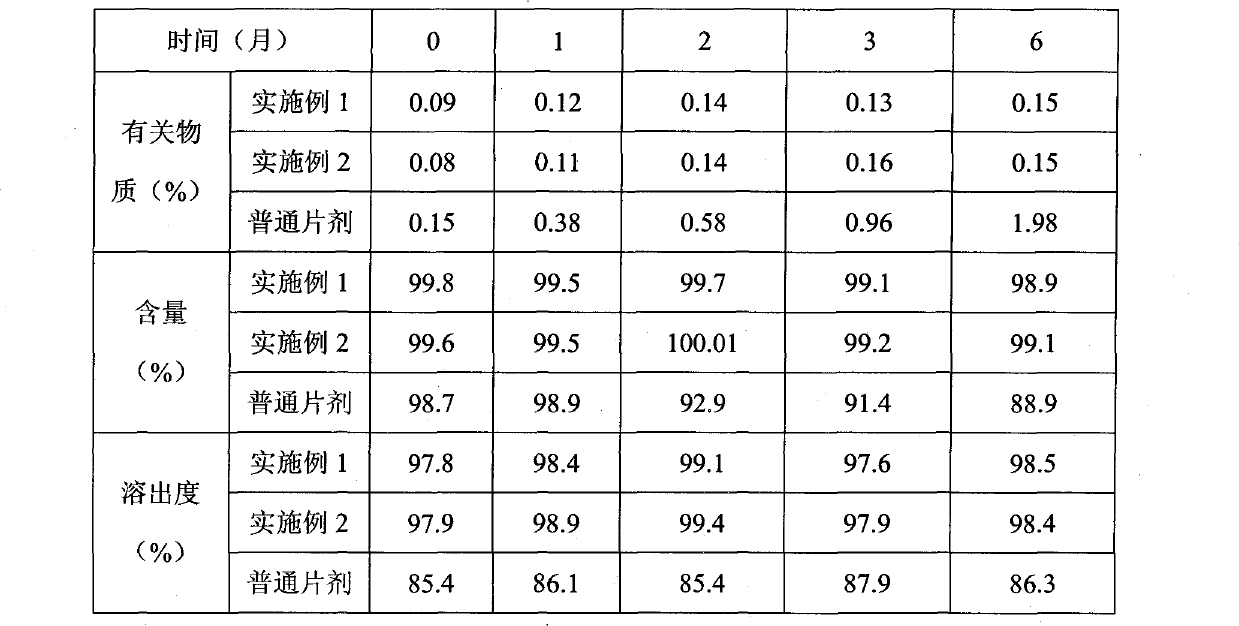
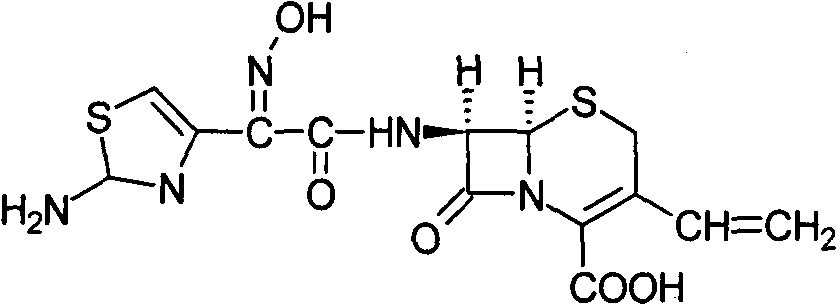
InactiveCN101352424AImprove solubilityLarge distribution areaAntibacterial agentsOrganic active ingredientsCefdinirPharmaceutic Adjuvant
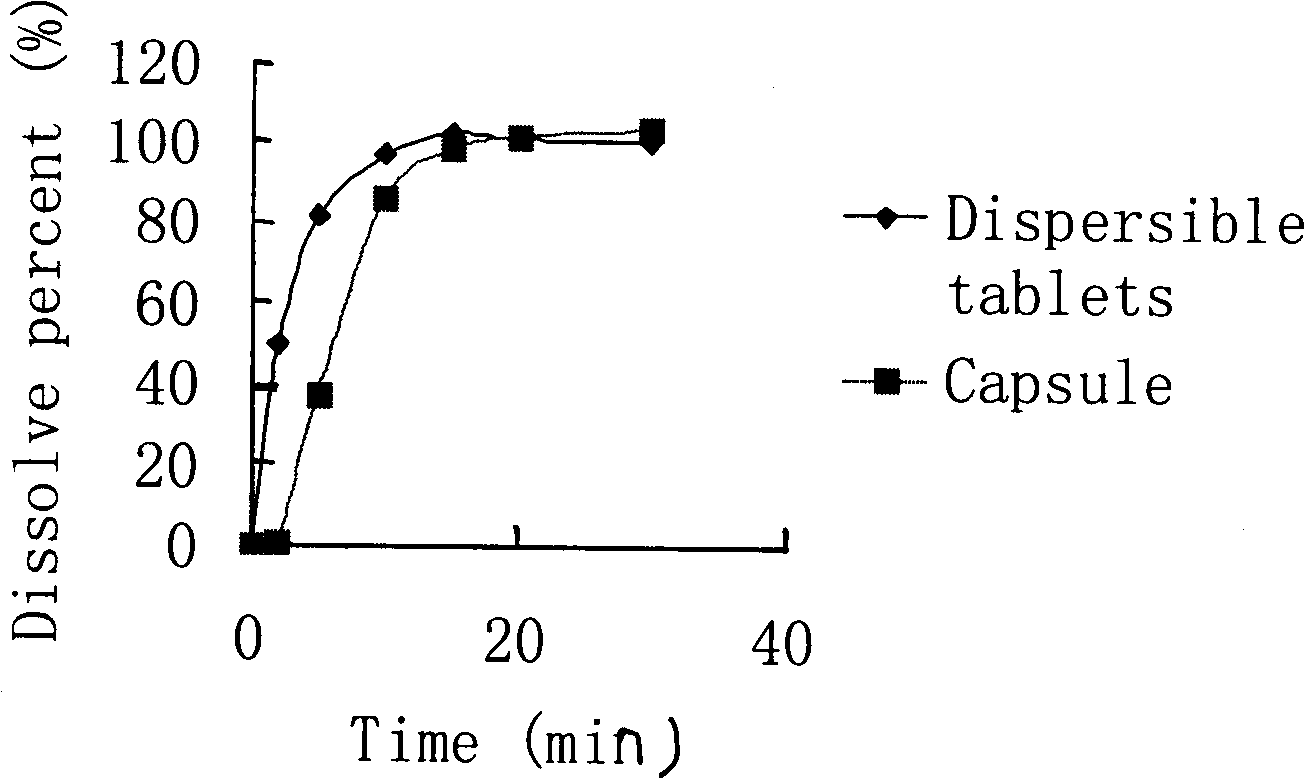
The invention provides a cefdinir dispersible tablet and a preparation method thereof, and the cefdinir dispersible tablet contains cefdinir with effective dose and pharmaceutic adjuvant which includes disintegrant and disintegrant-promoting aerosol; in every 100 parts of cefdinir, the dosage of the disintegrant is 2-60 parts, and the dosage of the disintegrant-promoting aerosol is 0.1-45 parts; after being taken orally, the cefdinir dispersible tablet of the invention can quickly disintegrate, and the cefdinir which is the active ingredient of the dispersible tablet has the accumulating dissolution rate of over 102.0% within 15 minutes. Compared with other medicines, the accumulating dissolution rate of cefdinir capsule is 98.3%. The cefdinir dispersible tablet are evenly dispersed into fine particles, so as to have remarkable disintegration and leachability capacity compared with the compressed tablets, realize the rapid absorption of medicine, the function of rapid onset, and improve the bioavailability of human body.
Owner:TIANJIN CENT PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com