Aweto micropowder tablet and preparation method thereof
A Cordyceps micropowder and micropowder technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, plant raw materials, etc., can solve sticking, de-powdering, splitting, falling blocks, loose flakes, loss of heat-sensitive components of Cordyceps sinensis, and tablets Uneven drug content and other problems, to achieve the effect of improving curative effect, clinical significance and resource utilization, and reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] (3) Preparation of the test solution: filter the filtrate obtained by measuring the total dissolution rate in the above test 1 with a microporous membrane (0.45 μm), and take the subsequent filtrate as the test solution.
[0025] (4) Linear relationship investigation
[0026] Precisely draw 5, 10, 15, 20, and 25 μl of the above-mentioned reference substance solution and inject them sequentially, measure according to the above-mentioned chromatographic conditions, take the concentration as the abscissa, and the integrated value of the peak area as the ordinate, draw a standard curve, and the regression equation is: Y=33.815 X-19.792r=0.9996. It shows that the linear relationship of adenosine is good in the range of 0.011~0.302μg.
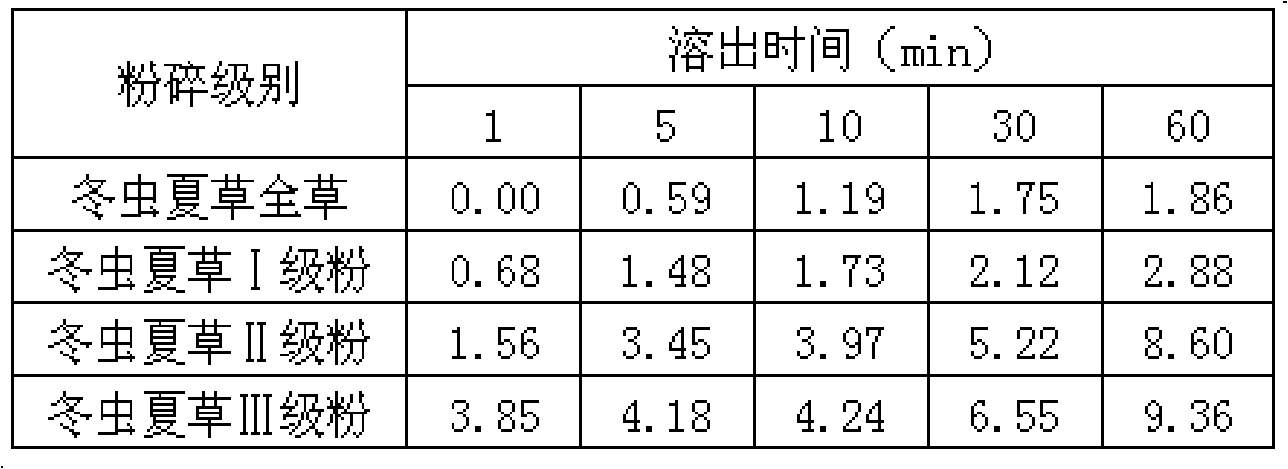
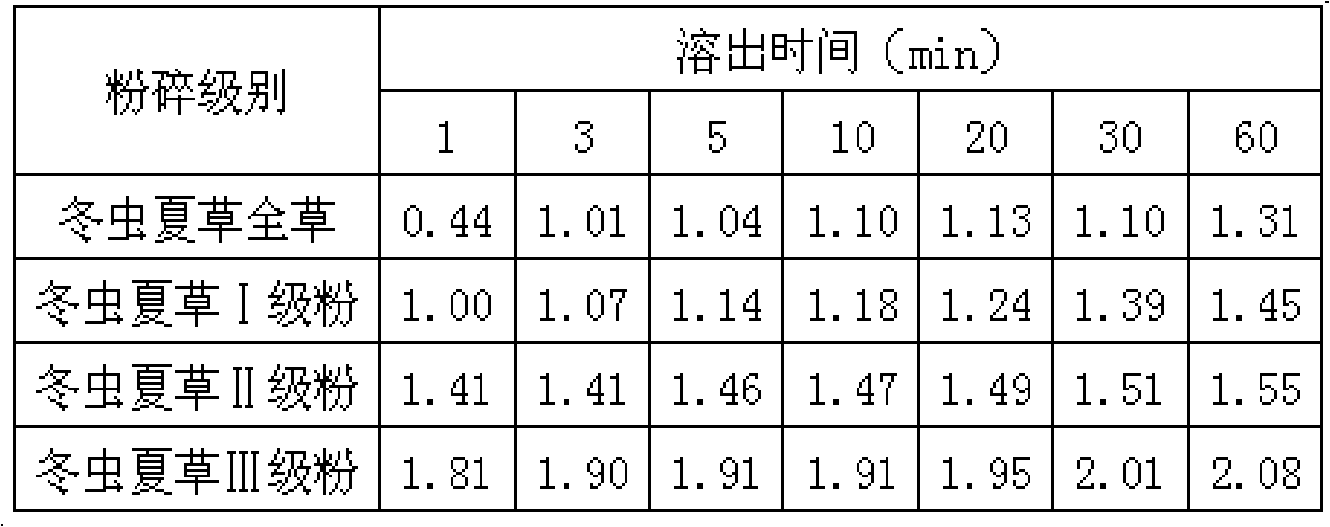
[0027] The dissolution rate (%) of adenosine over time in the Cordyceps sinensis of different crushing grades in table 2
[0028]
[0029]The determination results of adenosine in Cordyceps sinensis of different crushed grades showed that...
Embodiment 1
[0082] Embodiment 1 micropowder direct powder tableting process
[0083] 1. Raw material: superfine powder of Cordyceps sinensis (particle size: D90=15-20 μm; moisture content about 9.5%).
[0084] 2. The speed of the tablet press is 15 / h, the pre-compression pressure is 0.8kN, and the pressure is 48kN.
[0085] 3. Adopt round rod type pulsator feeder (to increase the fluidity of powder).
[0086] 4. The ambient temperature is 23°C; the ambient humidity is 50%.
[0087] Under the above process conditions, the powder is directly compressed into tablets, the tablet specification is 0.25g / tablet, 100 tablets.
Embodiment 2
[0088] Embodiment 2 Micropowder secondary tableting process:
[0089] 1. Raw materials: superfine powder of Cordyceps sinensis with a particle size of D90=15-20 μm and a moisture content of about 9.5%.
[0090] 2. The speed of the tablet press is 15 / h, the pre-compression pressure of the first tablet compression is 28kN, and the main pressure is 28kN; the pre-compression pressure of the second tablet compression is 28kN, and the main pressure is 28kN.
[0091] 3. Adopt round rod type pulsator feeder (to increase the fluidity of powder).
[0092] 4. The ambient temperature is 23°C; the ambient humidity is 50%.
[0093] Under the above-mentioned process conditions, the first tablet is made into a first tablet with an average hardness of 2.57Kg (the specification of the first tablet is about 0.25g / tablet), and then crushed into a fine powder that can pass through a 100-mesh sieve. Tablets, ready to use, tablet specification 0.25g / tablet, 100 tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com