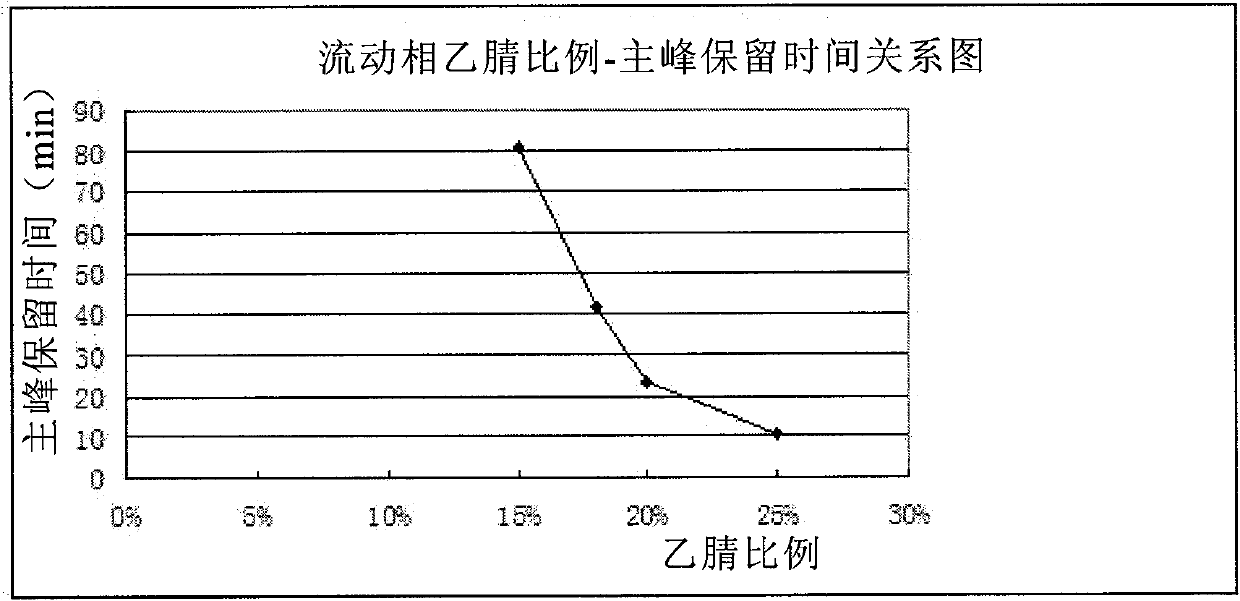
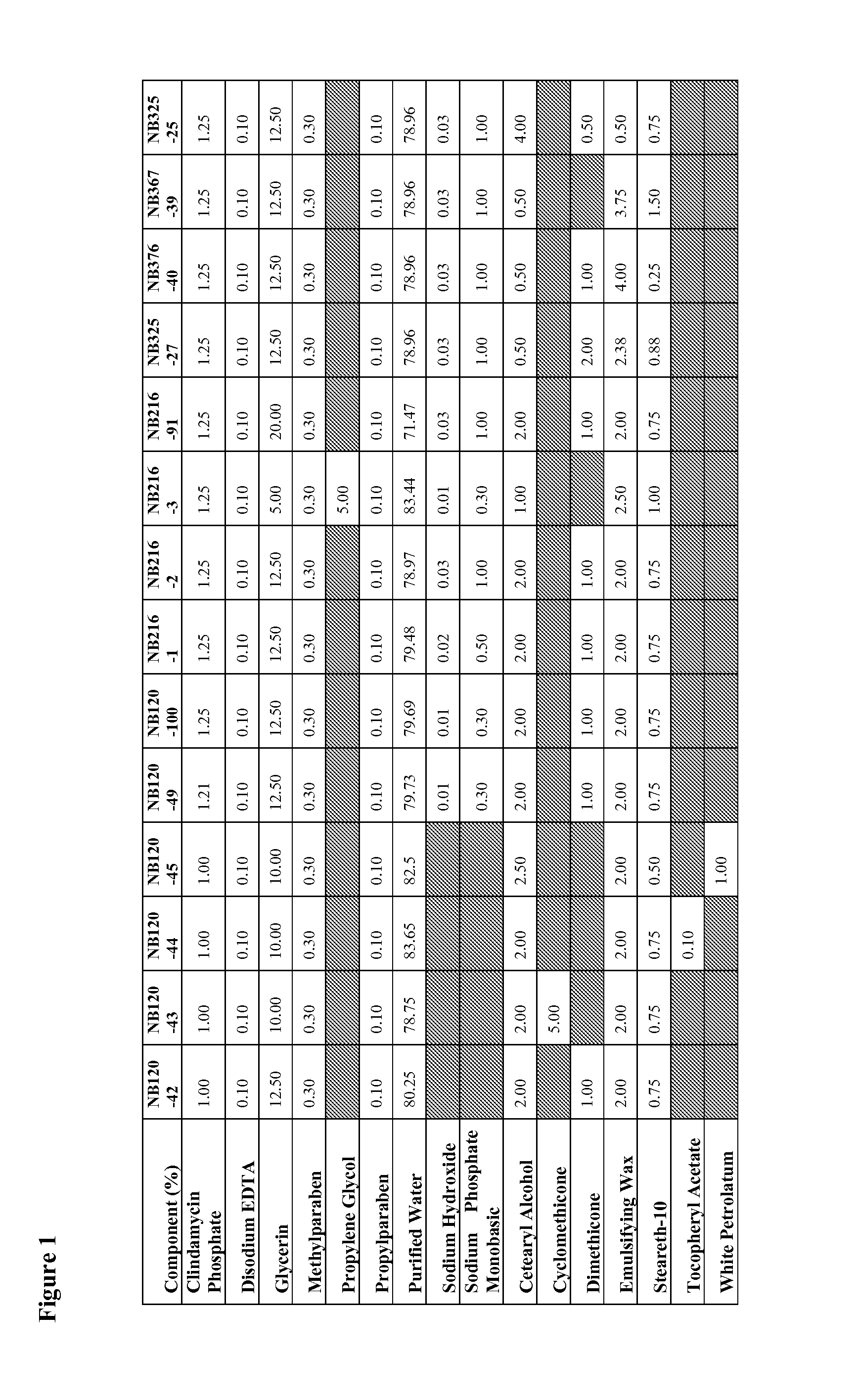
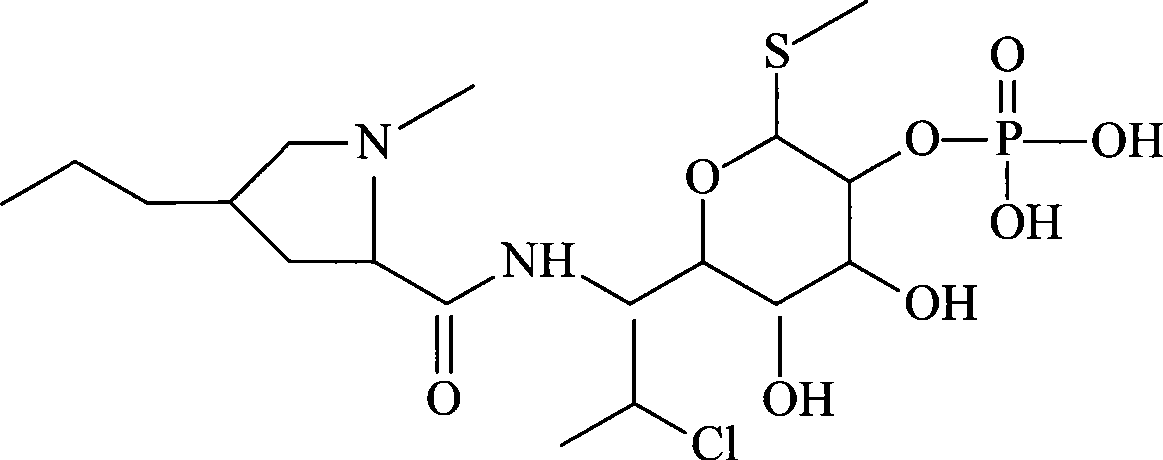
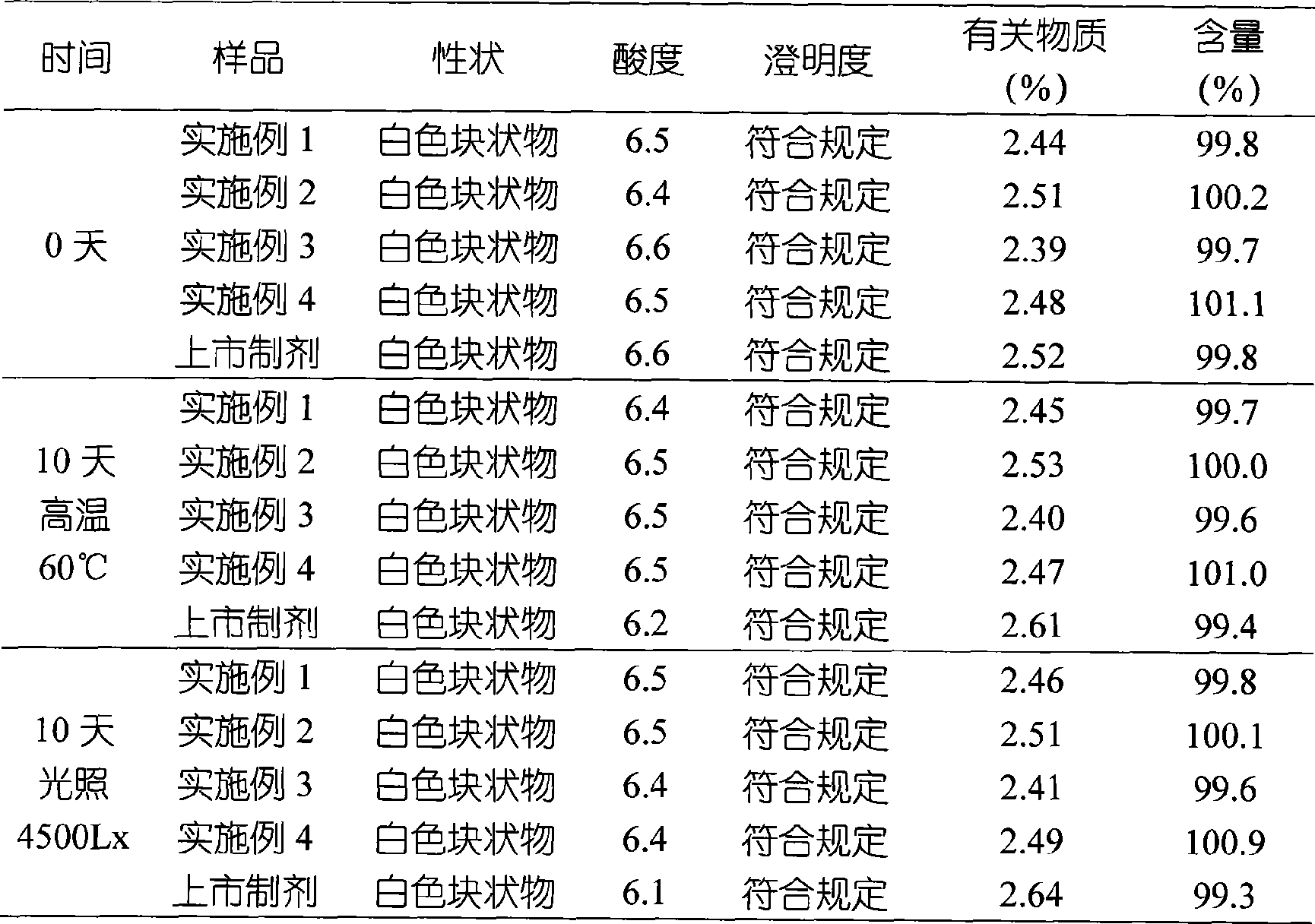
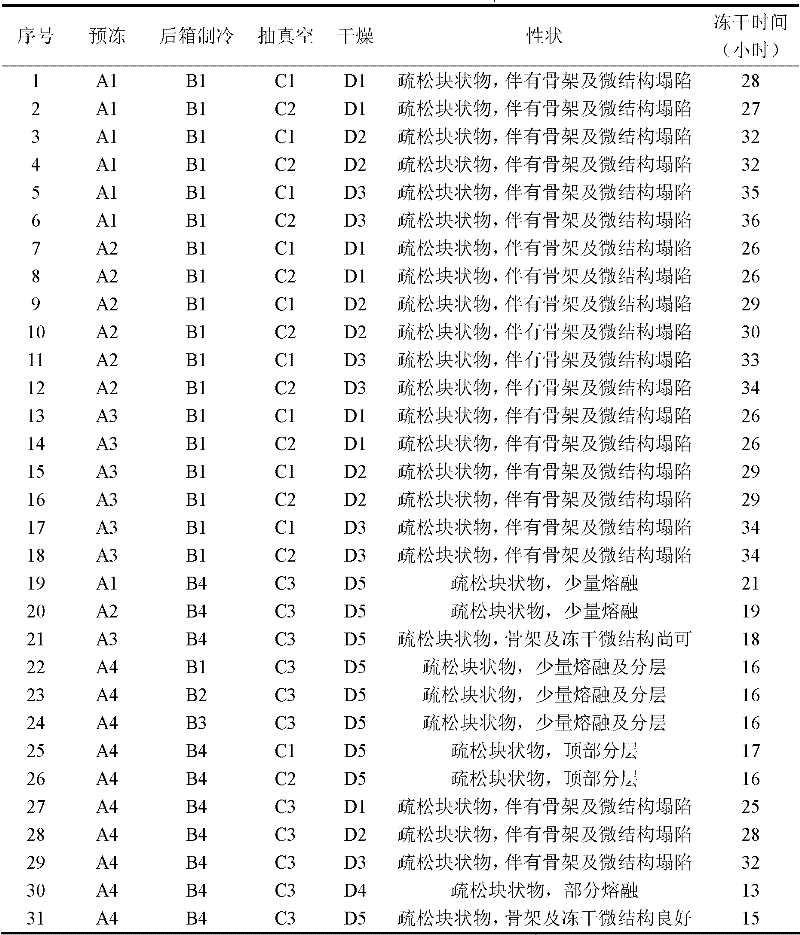
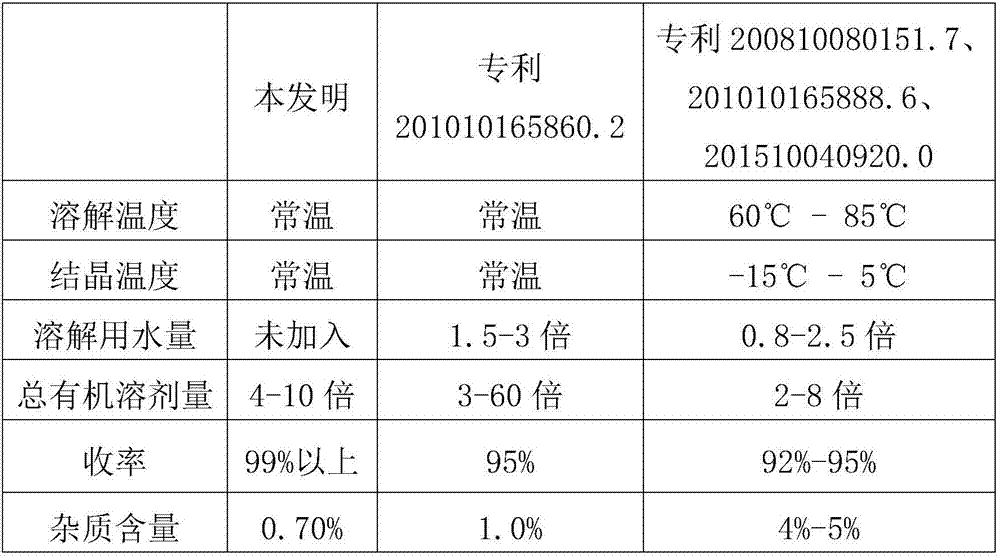
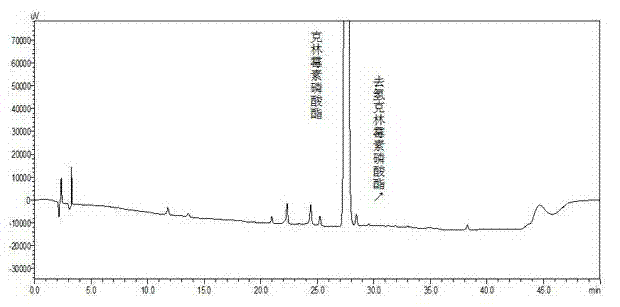
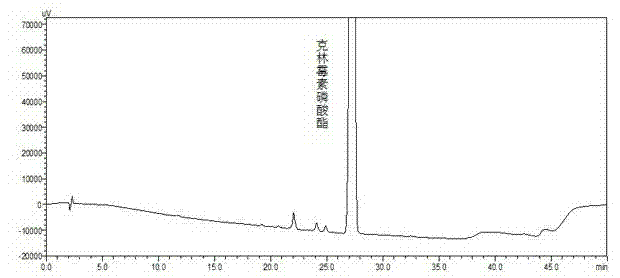
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153 results about "Clindamycin Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
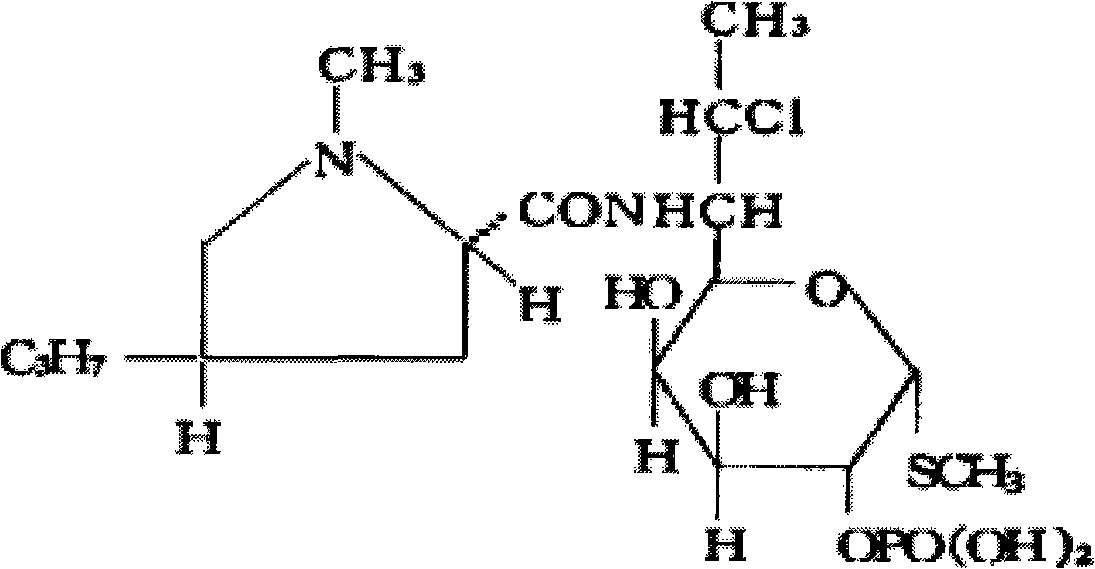
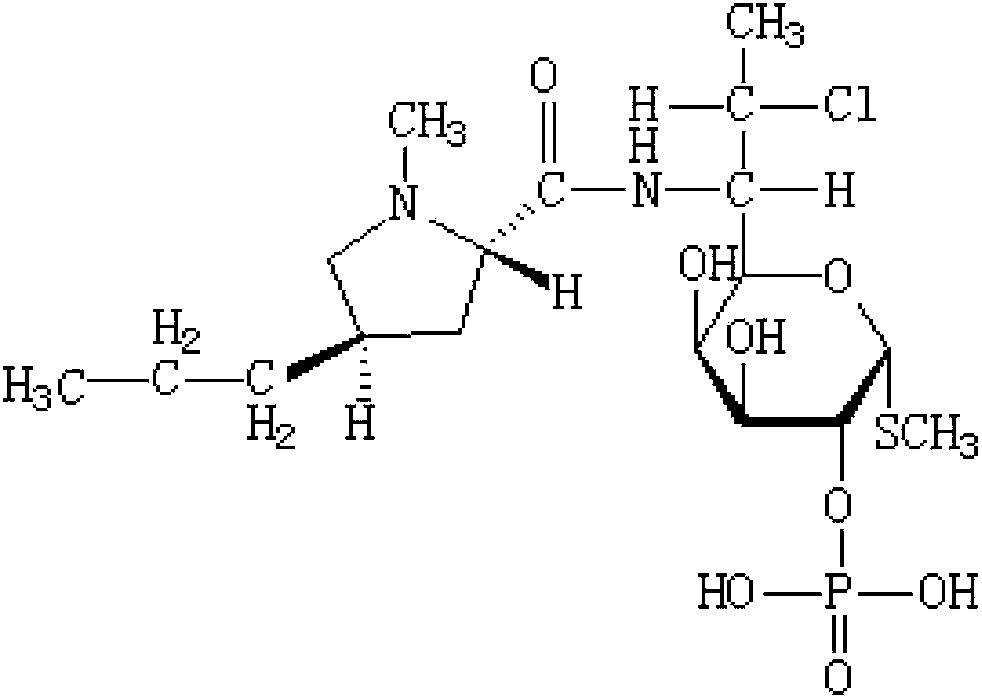
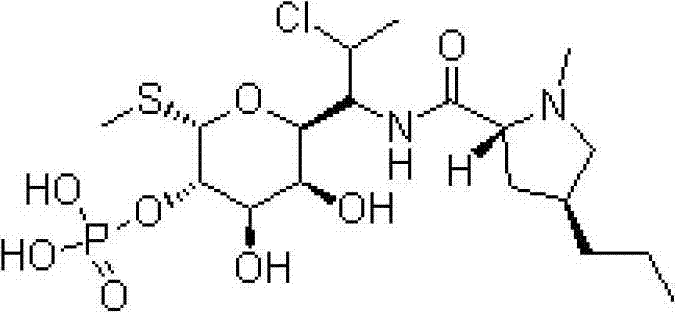
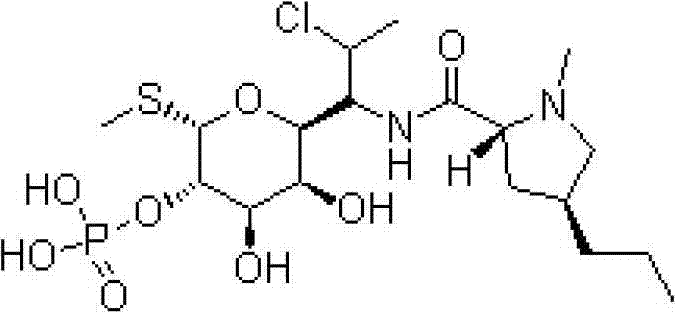
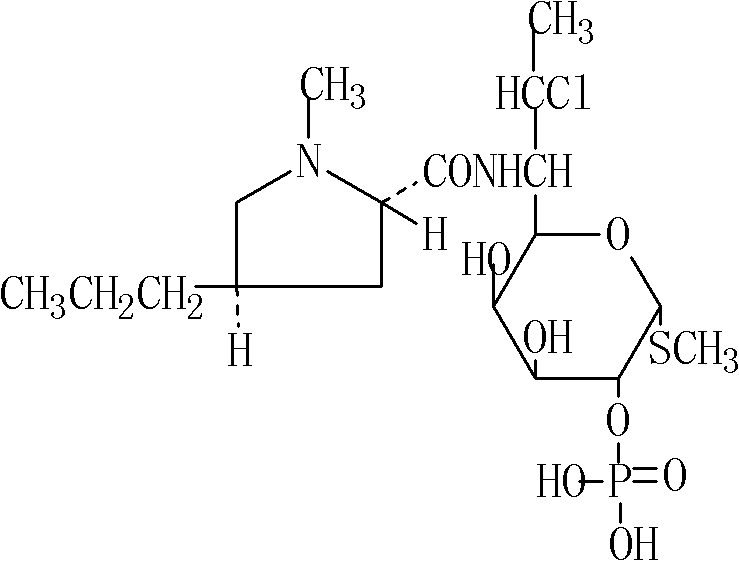
The phosphate salt form of clindamycin, a semi-synthetic, chlorinated broad spectrum antibiotic produced by chemical modification of lincomycin. Clindamycin phosphate is used in topical preparations.
Aerosol foams comprising clindamycin phosphate
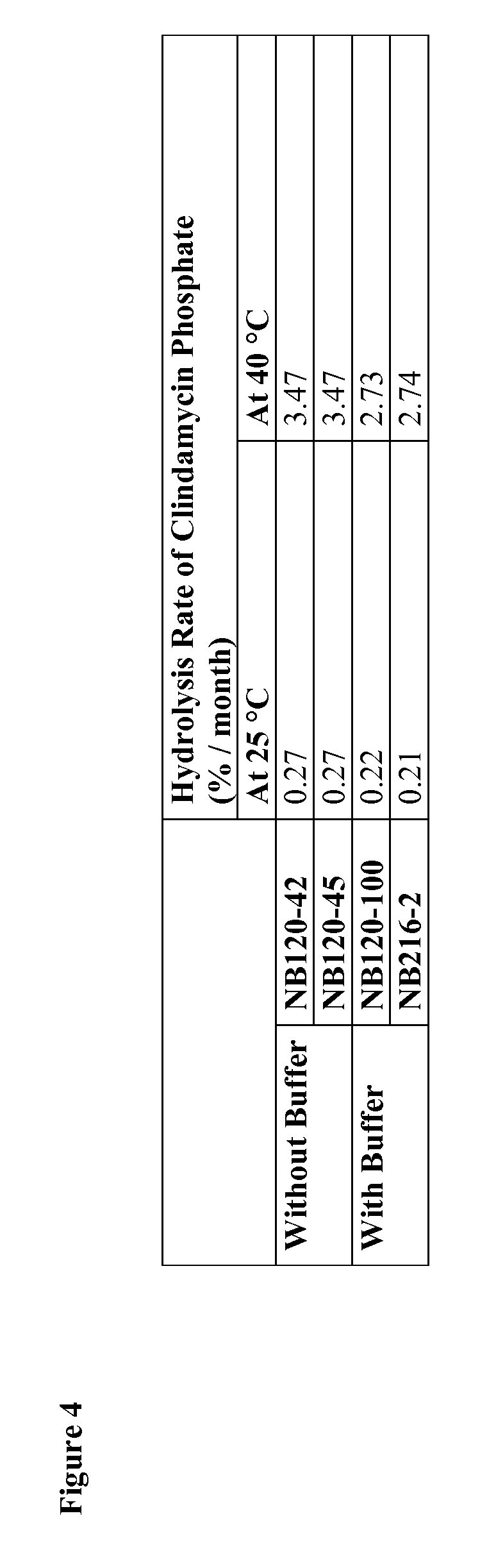
Described herein are emulsions and compositions for the treatment of acne vulgaris. The emulsions may be formulated as aerosol compositions. The aerosol propellant may be a hydrofluoroalkane propellant. The emulsions or compositions may comprise clindamycin phosphate and a buffer salt, and may exhibit decreased rates of clindamycin phosphate hydrolysis. Also described are methods of treating acne vulgaris, comprising the step of applying to an affected area of a subject in need thereof a therapeutically-effective amount of an inventive emulsion or aerosol composition.
Owner:PRECISION DERMATOLOGY
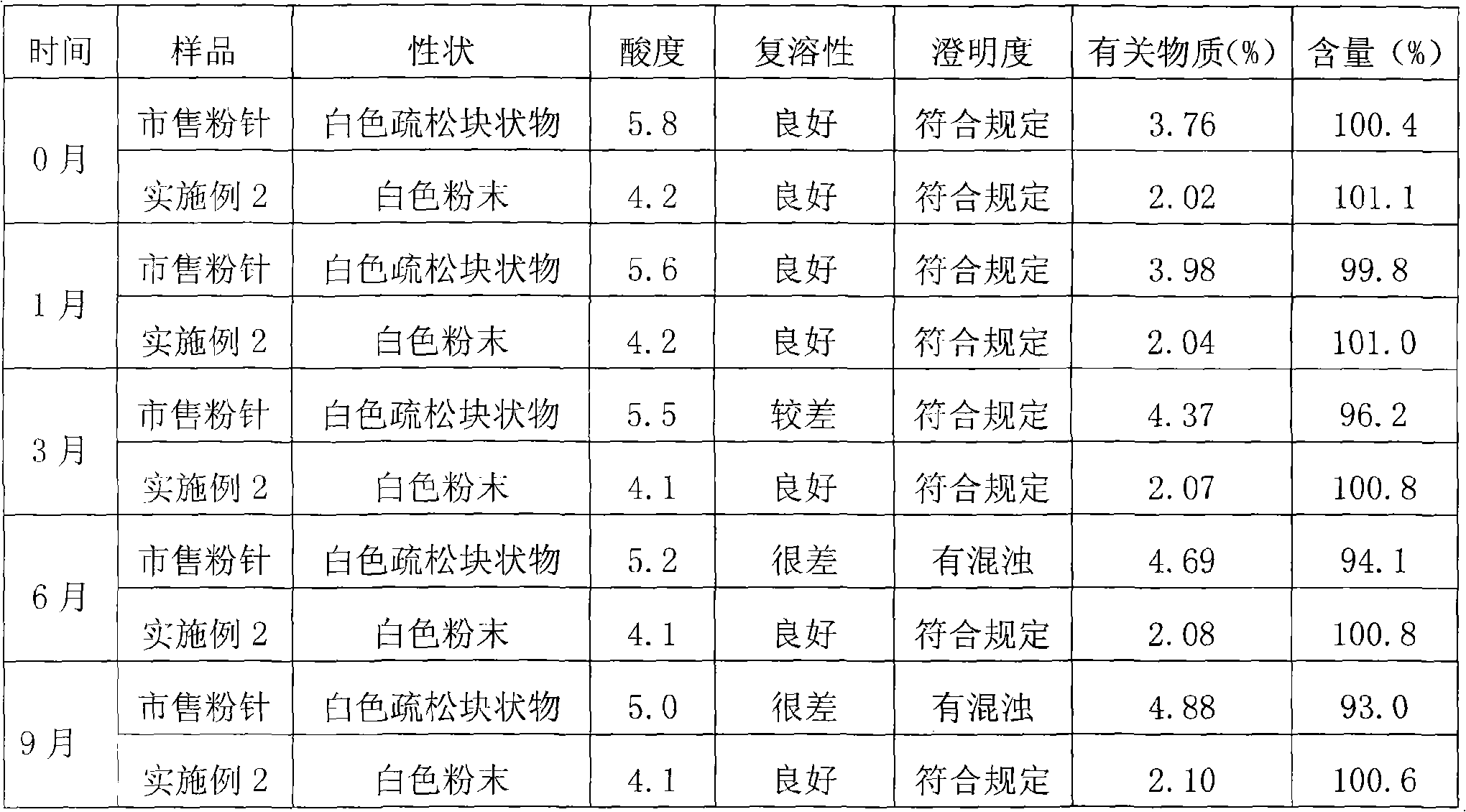
Method for preparing powder injection using superfine communication technique and prepared products
InactiveCN101332188AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsLatamoxefClindamycin Phosphate
The present invention relates to a method of using a superfine pulverizing technology to prepare sterile powder for injection (powder injection) of chemical medicine and the prepared medicine powder injection. Invert sugar, clindamycin phosphate, cefpiramide sodium, cefepime hydrochloride, latamoxef sodium or cefmetazole sodium are preferable as the chemical medicine.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of clindamycin phosphate powder injection
ActiveCN1602889AHigh purityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsHemolysisBlood vessel
The invention relates to a method for preparing clindamycin phosphate powder filling agent, including: adding and dissolve clindamycin phosphate in alcohol solution; adding in activated charcoal to make decolorization, then roughly filtering, finely filtering, and placing to crystallize, filtering to eliminate supernatant and obtaining clindamycin phosphate crystal; then making transfer-solution for the second time, recrystallizing once, filtering and obtaining the recrystallized clindamycin phosphate crystal; drying and crushing, making split charging, capping and packaging in aseptic condition and making it. In the preparing course, it needs no high temperature treatment, need not add in additive, and its powder has good fluidity, high purity, few impurities, high bio-capability and good stability. It has no anaphylaxis and hemolysis and has no stimulation to blood vessels by intravenous injection. It adds a new clinic form of clindamycin phosphate, meeting clinic requirement.
Owner:ZHUHAI EBANG PHARMA
Preparation of clindamycinum phosphoester
ActiveCN101298463APhosphorous to avoidReduce pressure on environmental protectionSugar derivativesSolid lightClindamycin Phosphate
The invention provides a preparation method of clindamycin phosphate; the method of the invention includes the following processes: muriatic acid clindamycin and a solid light (C[3]Cl[6]O[3]) carry out chlorination reaction in a chloroform solvent under the temperature of 50 to 80 DEG C; then are alcoholized and ketonized; finally under the co-catalysis of naphthyridine and triethylamine, the muriatic acid clindamycin and phosphorus oxychloride are esterified, hydrolyzed, absorbed and crystallized in an acetone solvent to obtain the clindamycin phosphate. The solid light is adopted to take part in the chlorination reaction in the reaction system of the invention, thus lightening the pressure of environment protection; simultaneously the content of epiclindamycin in the finished product is reduced; moreover, partial naphthyridine is replaced by the triethylamine, thus reducing the cost and improving the yield to a large extent.
Owner:浙江天台药业股份有限公司
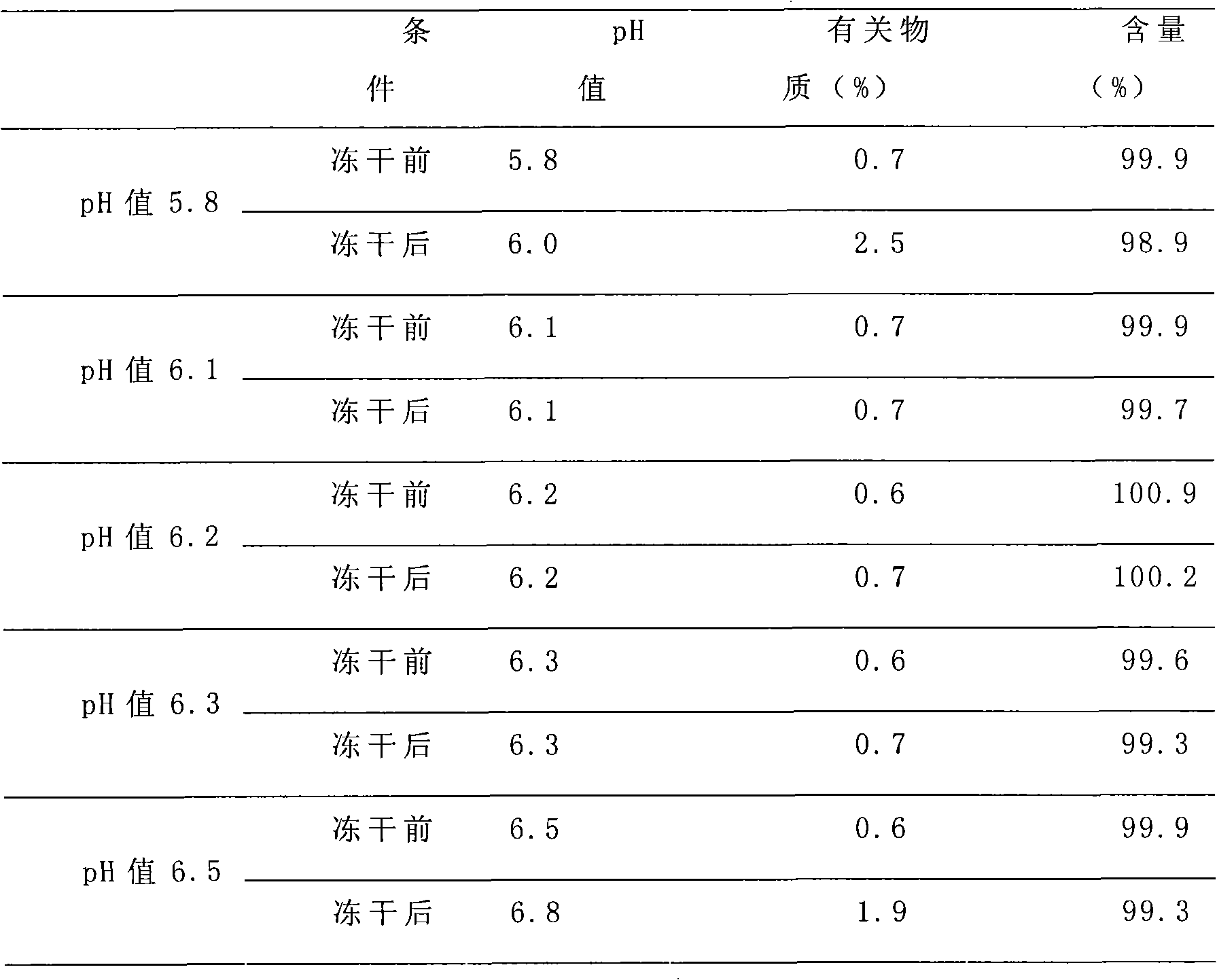
Clindamycin phosphate freeze-dried powder needle and preparation thereof
InactiveCN101301278ASimple recipeLittle side effectsAntibacterial agentsPowder deliverySide effectFreeze-drying
The invention provides a clindamycin phosphate freeze-dried powder injection, which is prepared by the steps that: clindamycin phosphate solution is added with NaOH and is freeze-dried, wherein, the weight ratio of clindamycin phosphate to the NaOH is between 12 and 18 to 1, and the preferred weight ratio is 16.5 to 1. The clindamycin phosphate freeze-dried powder injection has simple formula and less auxiliary materials, overcomes side effects due to the fact that the auxiliary materials are excessively added, and ensures that patients are safer for use.
Owner:BEIJING JINGWEI SHUNKANG MEDICAL TECH DEV
Process for preparing clindamycin phosphate injection
InactiveCN1969875AImprove production conditionsReduce the chance of infectionAntibacterial agentsOrganic active ingredientsPhosphateClindamycin Phosphate
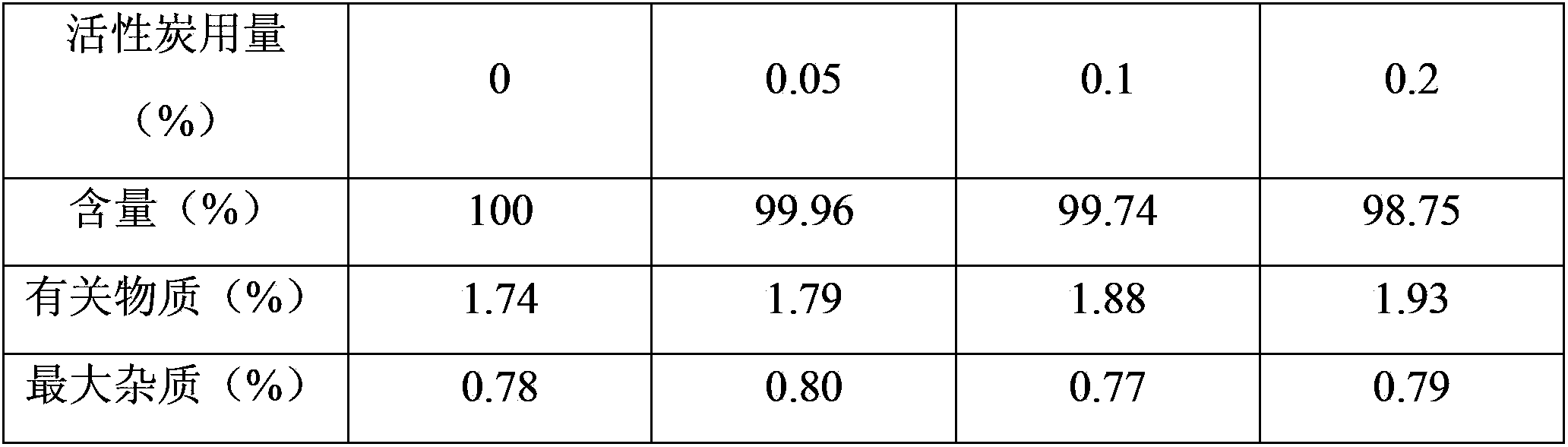
The invention discloses a making method of clindamycinum phosphate injection, which is characterized by the following: dissolving certain quantity of clindamycinum phosphate into water; adding clindamycinum phosphate and sodium hydroxide alternately; maintaining pH value between 6.0 and 6.4; stirring evenly; setting constant volume to the density of injection; adding 0.05% needle active carbon to adsorb the injection; filtering through 0.45um micropore film or corresponding cylinder filter; sterilizing; canning; sealing; obtaining the product.
Owner:沈阳金峰医药科技有限公司 +1
Method for preparing clindamycin phosphate powder injection raw medicine
ActiveCN101439022AChange the dosage ratioChange centrifugationAntibacterial agentsPowder deliverySolubilityClindamycin Phosphate
The invention discloses a preparation method for a clindamycin phosphate powder injection raw medicine which comprises the steps: (a) purification is carried out on a crude product of clindamycin phosphate; (b) crystallization and crystal growth are carried out on filter liquor after the filter liquor is arranged in a crystallization tank; (c) centrifugal separation and washing are carred on crystallization liquor; (d) fast temperature raising and drying are carried out on a clindamycin phosphate wet finished product. The preparation method for the clindamycin phosphate powder injection raw medicine has the advantages of favorable water solubility, uniform crystal form particles, small specific volume, big bulk density, low solvent residue, and the like. The method also simplifies the process operation, and dramatically enhances the product yield.
Owner:华北制药华胜有限公司
Crystal form of clindamycin phosphate and preparation method thereof
The invention belongs to the technical field of medicine, in particular relating to a crystal form of clindamycin phosphate and a preparation method thereof as well as amorphous clindamycin phosphate with stable character and high safety of clinic medication, wherein the crystal form is composed of clindamycin phosphate crystal form I, clindamycin phosphate crystal form II and clindamycin phosphate crystal form III.
Owner:ZHUHAI EBANG PHARMA
Impurity analysis and preparation method for clindamycin phosphate
The invention provides an impurity analysis and preparation method for clindamycin phosphate, which is used for analyzing a clindamycin phosphate raw material and separating and preparing impurities from the clindamycin phosphate raw material. The method comprises the following steps of: measuring the clindamycin phosphate raw material by using liquid chromatography-mass spectrometry (LC-MS), and determining one or more impurities in the raw material according to the relative retention time and / or molecular weight of each analyzed component; and determining the conditions of preparative chromatography according to chromatographic retention behaviors displayed by the relative retention time of each impurity, and collecting the one or more impurities corresponding to the relative retention time and / or molecular weights by using the preparative chromatography.
Owner:浙江天台药业股份有限公司
Aerosol Foams Comprising Clindamycin Phosphate
Described herein are emulsions and compositions for the treatment of acne vulgaris. The emulsions may be formulated as aerosol compositions. The aerosol propellant may be a hydrofluoroalkane propellant. The emulsions or compositions may comprise clindamycin phosphate and a buffer salt, and may exhibit decreased rates of clindamycin phosphate hydrolysis. Also described are methods of treating acne vulgaris, comprising the step of applying to an affected area of a subject in need thereof a therapeutically-effective amount of an inventive emulsion or aerosol composition.
Owner:PRECISION DERMATOLOGY
Antibacterial clindamycin phosphate powder injection and its preparing process
InactiveCN1338258AImprove stabilityNon-irritatingPowder deliveryAntimycoticsWestern medicinePhosphate
An antibacterial clidamycin phosphate powder injection for treating infective diseases caused by gram-positive bacteria or anaerobic bacteria contains clindamycin phosphate (77-97.3%), the surfactant(Poloxamet 188 or Tween 80) for speeding up dissolving of clindamycin phosphate, and optional sodium hydroxide (25-8%) for regulating pH value. Its advantages are high safety and stability, high dissolving speed and high clearness of its solution.
Owner:JIANGSU JIUXU PHARMA
Clindamycin phosphate lipidosome freeze-dried preparation and preparation method thereof
InactiveCN101530393AAchieve vitrificationAvoid damageAntibacterial agentsOrganic active ingredientsFreeze-dryingCholesterol
The invention relates to a clindamycin phosphate lipidosome freeze-dried preparation and a preparation method thereof. The clindamycin phosphate lipidosome freeze-dried preparation is characterized by comprising the following components in portion by weight: 15 to 25 portions of clindamycin phosphate, 10 to 40 portions of dimyristyl acid lecithin, 1 to 10 portions of cholesterol, 1 to 5 portions of antioxidant, and 5 to 25 portions of cryoprotectant.
Owner:HAINAN LINGKANG PHARMA CO LTD
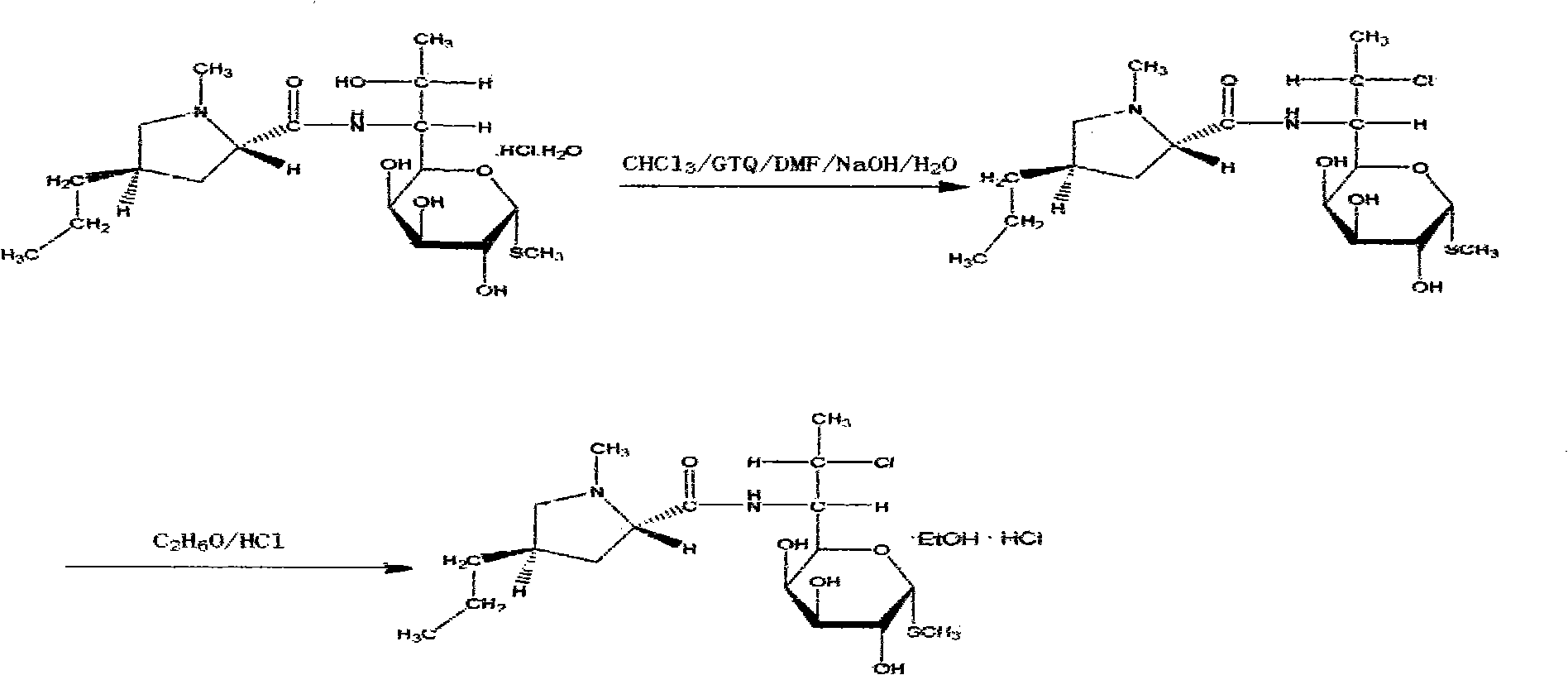
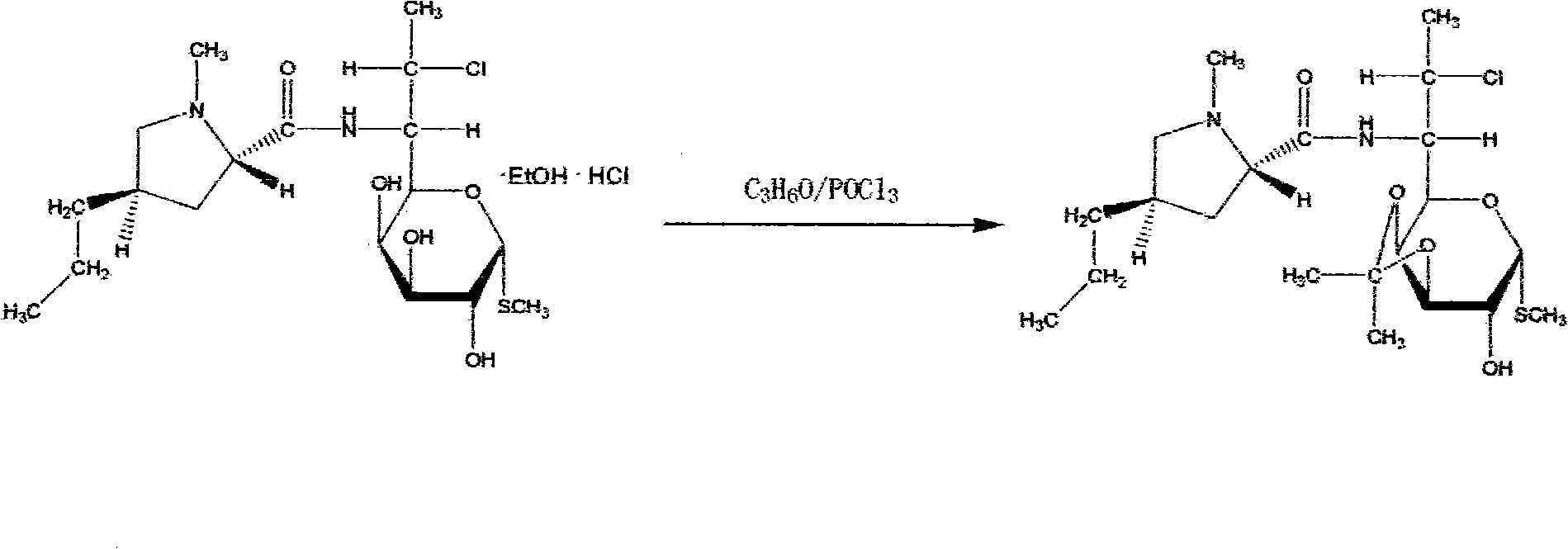
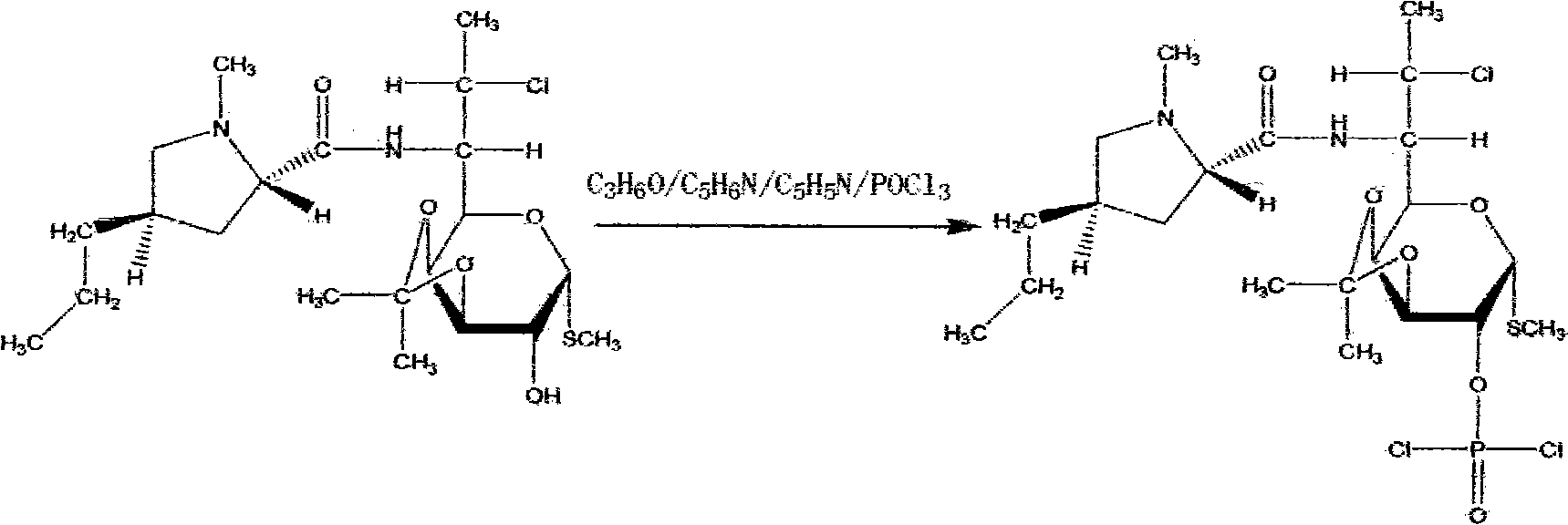
Method for synthesizing clindamycin phosphate
InactiveCN101830946AImprove conversion rateIncrease contentSugar derivativesSugar derivatives preparationSolubilityHydrolysis
The invention discloses a method for synthesizing clindamycin phosphate, which comprises the following steps of: performing ketal protection reaction on clindamycin hydrochloride alcoholate at the temperature of between 2.0 below zero and 2.0 DEG C under the action of acetone and phosphorus oxychloride to form propylidene clindamycin; and performing esterification, hydrolysis, adsorption, washing, deabsorption, concentration, coarse crystallization, decoloration, refining and drying to obtain the finished product of clindamycin phosphate. Because a new catalyst 4-dimethylaminopyridine participates in the esterification in the rection system, the phosphorylating reaction is performed completely, and the conversion rate of raw materials is improved. Meanwhile, due to the secondary crystallization method, the problems of poor color grade and poor powder solubility are solved, and the operating conditions are mild and simple. By adopting triethylamine to replace partial pyridine, the esterification is pushed forwards; the reaction period is shortened; and importantly, related impurities in the finished product and the production cost are reduced, and the content is improved.
Owner:南阳普康药业有限公司 +1
Method for simultaneously detecting methylparaben, propylparaben and dibutyl hydroxy toluene in gel
The invention provides a method for simultaneously analyzing and detecting methylparaben (Me-PHBA), propylparaben (Pr-PHBA) and dibutyl hydroxy toluene (BHT) in clindamycin phosphate gel. According to the method, Agilent ZORBAX SB-C18 (4.6mm*250mm, 5 mu-m) is adopted as a chromatographic column, an acetonitrile-1% acetic acid aqueous solution is used as a moving phase for gradient elution, double wavelengths (the detecting wavelength for detecting Pr-PHBA and Me-PHBA is 250-260nm, and the wavelength for detecting BHT is 270-286nm) are adopted for analyzing and detecting. The analyzing method is quick, has the advantages of good specificity and high sensitivity, is suitable for detecting contents of Me-PHBA, Pr-PHBA and BHT in a medicine preparation, in particular a gel.
Owner:SUNSHINE LAKE PHARM CO LTD
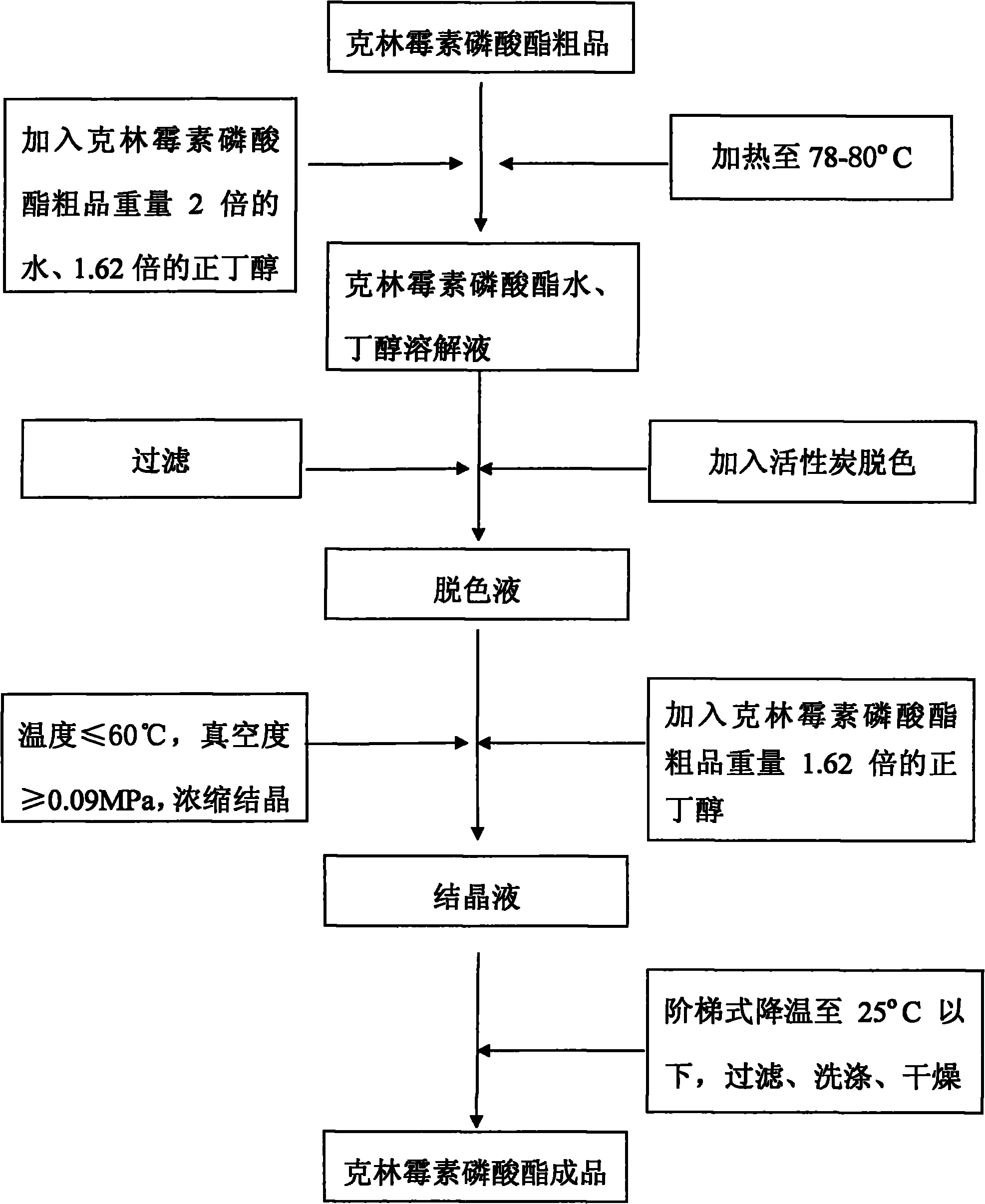
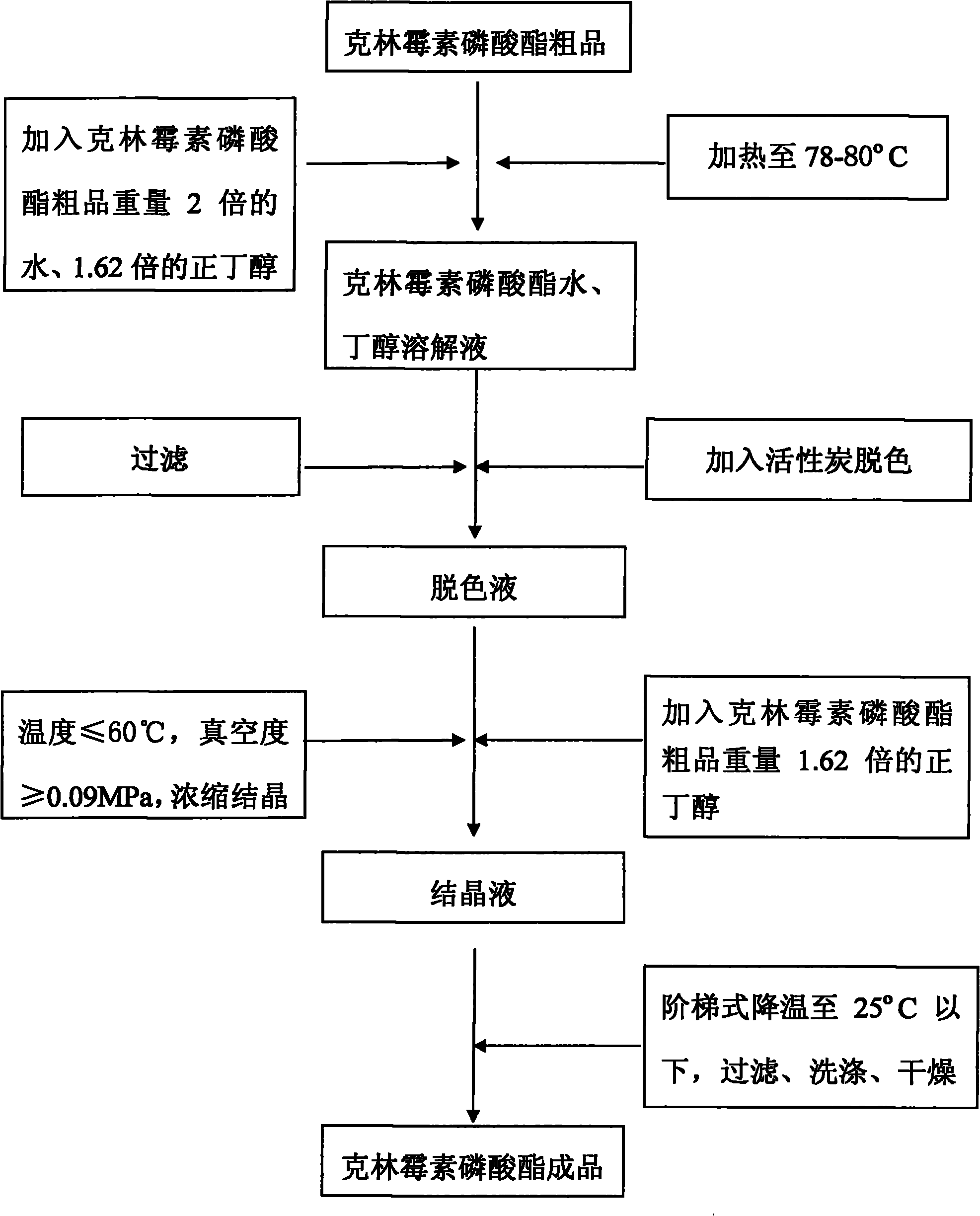
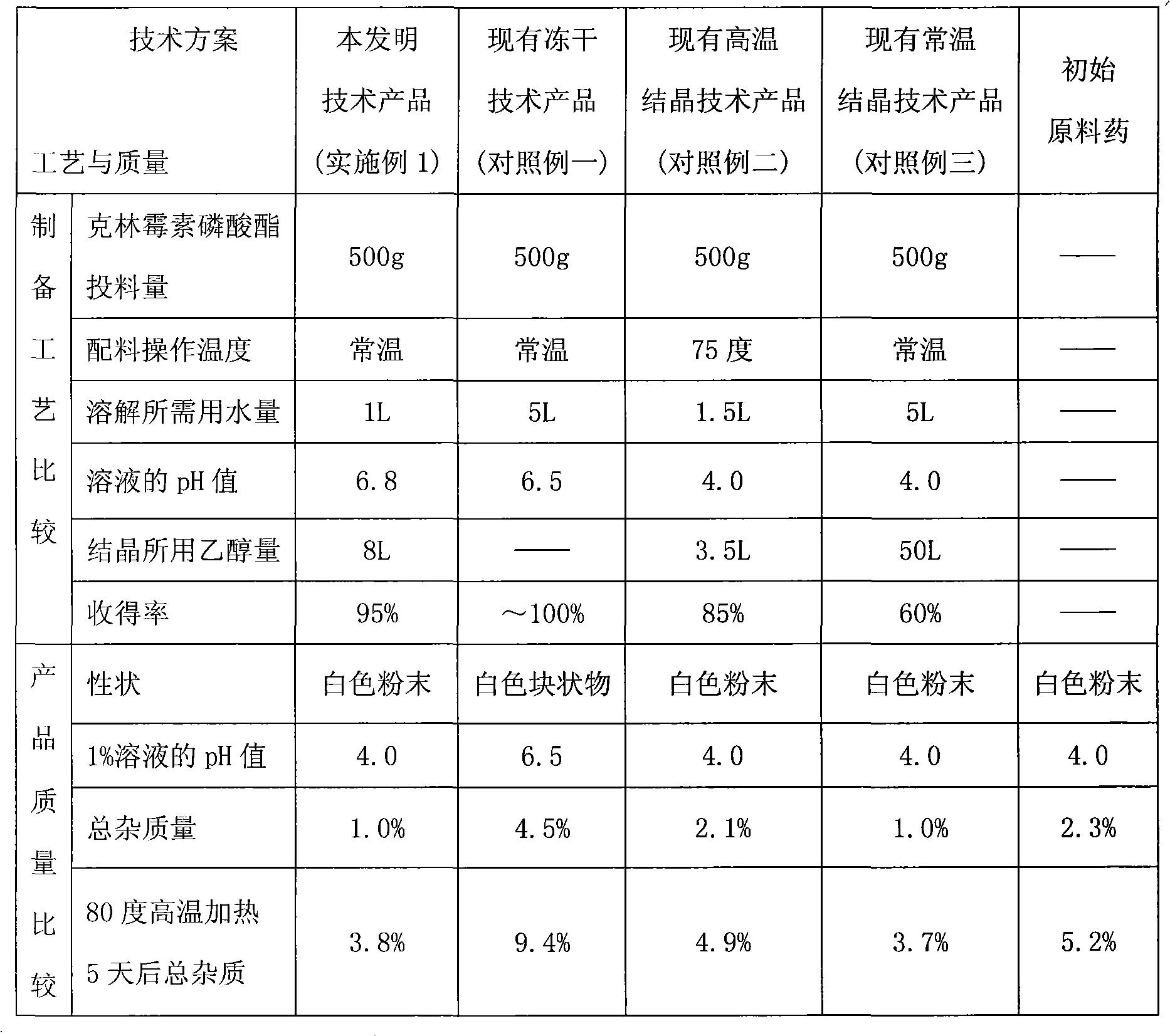
Crystallization method of clindamycin phosphate
ActiveCN101928307AHigh yieldHigh puritySugar derivativesSugar derivatives preparationClindamycin PhosphateN-Butanol
The invention discloses a crystallization method of clindamycin phosphate, comprising the following steps: firstly dissolving the clindamycin phosphate in n-butanol aqueous solution; then performing crystallization after decoloration, concentration and stepped temperature reduction to below 25 DEG C, filtering, washing, and drying to obtain the finished product. The crystallization method of the clindamycin phosphate of the invention has high crystallization yield, saves the time and reduces the cost; and the clindamycin phosphate finished product has high purity, good mobility and less possibility of agglomeration.
Owner:XINYU PHARM CO LTD
Preparation method of clindamycin phosphate powder for injection
InactiveCN101829060AReduce the amount addedHigh yieldAntibacterial agentsOrganic active ingredientsSterile environmentHigh concentration
The invention discloses a preparation method of clindamycin phosphate powder for injection, comprising the following steps of: taking clindamycin phosphate, adding alkaline solution at room temperature, stirring for dissolving the clindamycin phosphate to prepare sterile solution having a high concentration; in the sterile environment, adding inorganic acid or organic acid to adjust the pH value of the solution, adding inert organic solvent to crystallize, depressurizing, filtering and drying to obtain sterile powder of clindamycin phosphate; under the sterile condition, packing the powder in a sterile vial, covering the vial with a butyl latex plug, rolling a aluminum cover so as to make sterile powder. Compared with a prior art, the invention has the advantages of low cost, simple production device, short production cycle and the like; and dissolution of samples at a high temperature is unnecessary in the production process, which prevents the clindamycin phosphate from being dissolved or damaged in the production process, so that the final product has the advantages of high purity (low impurity), little toxicity and side effects and stable quality. The invention is particularly suitable for large-scale production.
Owner:HUBEI HOPE PHARMA
Clindamycin phosphate solvate crystal and preparation method thereof
The invention belongs to the technical field of medicine, in particular relating to a clindamycin phosphate solvate crystal and a preparation method thereof. The clindamycin phosphate solvate crystal comprises a clindamycin phosphate isobutanol-water solvate crystal and a clindamycin phosphate dimethyl sulfoxide-water solvate crystal which have good chemical stability and stable property in the storage process.
Owner:ZHUHAI EBANG PHARMA
Process for synthesizing clindamycin phosphate
InactiveCN101891779ASimple processing methodPractical applicationSugar derivativesSugar derivatives preparationHydrolysisPotassium carbonate
The invention relates to a process for synthesizing clindamycin phosphate. The process comprises the following steps: 1) ketal reaction, wherein clindamycin hydrochloride alcoholate is used as a basic raw material to prepare a 3.4-clindamycin condensation compound through selective hydroxyl protection; and 2) phosphatidic reaction, wherein the 3.4-clindamycin condensation compound is used as a raw material, phosphorus oxychloride is used as a phosphatidic agent, and anhydrous potassium carbonate is used as an acid binding agent to complete the phosphatidic reaction under double actions of esterification catalyst dimethylamino pyridine and phase-transfer catalyst benzyl triethyl ammonium chloride, and a target compound, namely the clindamycin phosphate, can be obtained through hydrolysis deprotection. The process has the advantages that: 1, the process has simple method and practical application; 2, the content of pyridine / triethylamine in wastewater of phosphatidic reacting process can be controlled, so that the pressure of high COD wastewater drainage can be greatly reduced; and 3, the weight yield reaches over 95 percent, and the product quality fulfills the requirement of WS1-(X-322)-2003Z.
Owner:ZHANGJIAGANG XINYI CHEM
Clindamycin phosphate injection composition and preparation method thereof
InactiveCN103565755AFix stability issuesAntibacterial agentsOrganic active ingredientsActivated carbonClindamycin Phosphate
The invention provides a clindamycin phosphate injection composition and a preparation method thereof. The clindamycin phosphate injection composition comprises the following components in parts by weight: 178-179 parts of clindamycin phosphate, 12-13 parts of sodium hydroxide and 1000 parts of water for injection. The preparation method of the clindamycin phosphate injection composition comprises the following steps: adding water for injection; adding sodium hydroxide into the water for injection; adding clindamycin phosphate into the solution added with sodium hydroxide, so as to obtain a mixed solution, wherein pH of the mixed solution is 6.0-7.0; adding activated carbon into the mixed solution, realizing constant volume by adopting the water for injection, stirring, filtering, and decarbonizing; sterilizing and filtering the decarbonized mixed solution, and carrying out split charging and freeze drying, thus the clindamycin phosphate injection composition is obtained. The clindamycin phosphate injection composition has good stability, contains less impurities and is simple to prepare and applicable to industrial production.
Owner:江苏金丝利药业股份有限公司
Clindamycin phosphate for injection and preparation method thereof
ActiveCN101569589AImprove stabilityAvoid decompositionPharmaceutical product form changePharmaceutical delivery mechanismClindamycin PhosphateFreeze-drying
The invention relates to a method for preparing clindamycin phosphate for injection. The invention also provides the clindamycin phosphate for injection which has safe quality and good stability. The invention mainly provides a freeze-dried powder pre-freezing solution and a preparation method thereof, and overcomes the disadvantage of unsteady preheating of the clindamycin phosphate.
Owner:CISEN PHARMA
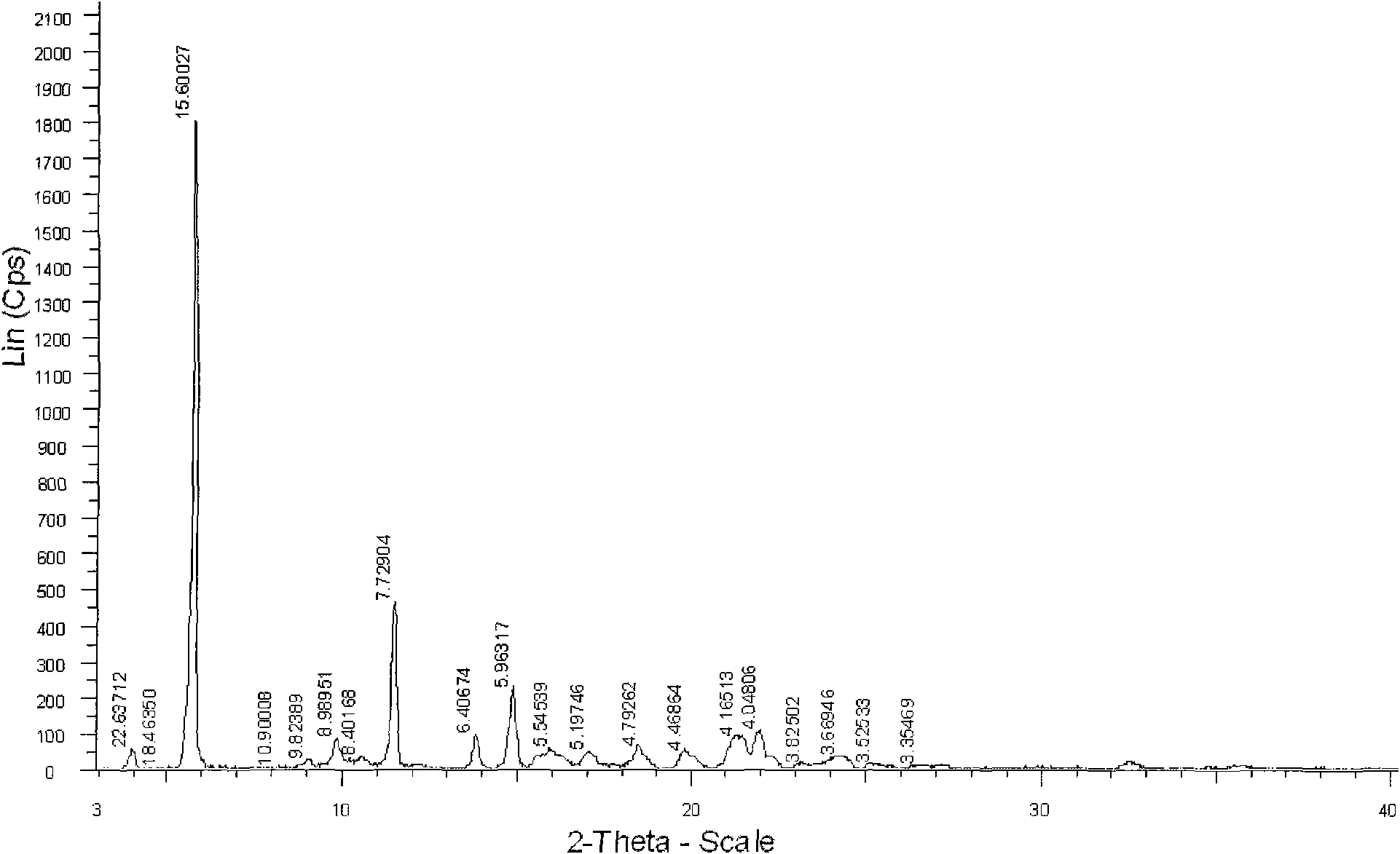
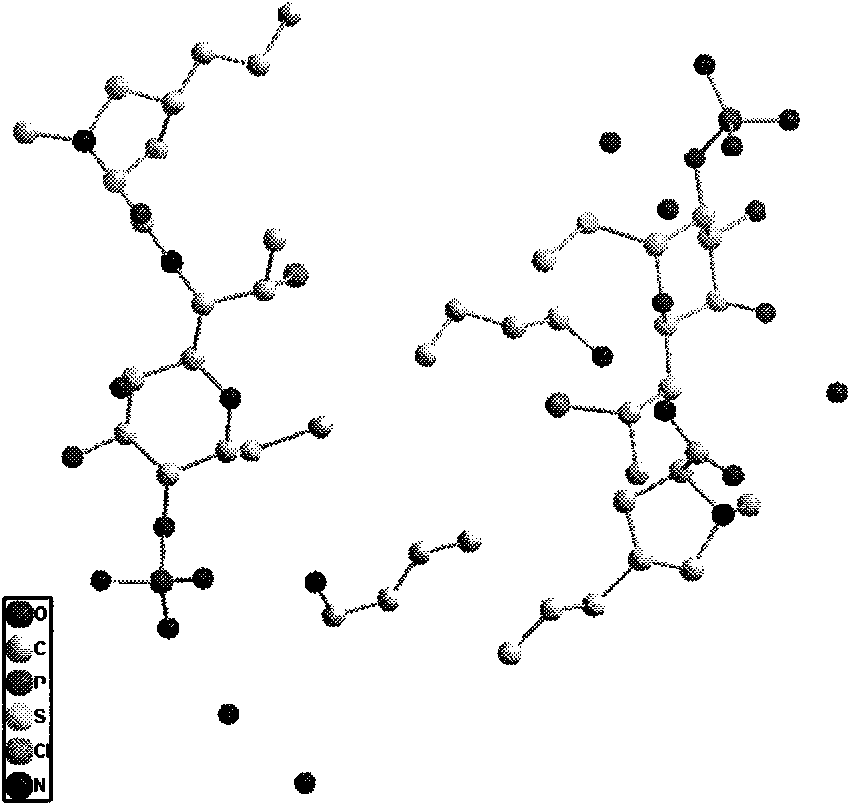
New active clindamycin phosphate compound and medicinal composition thereof
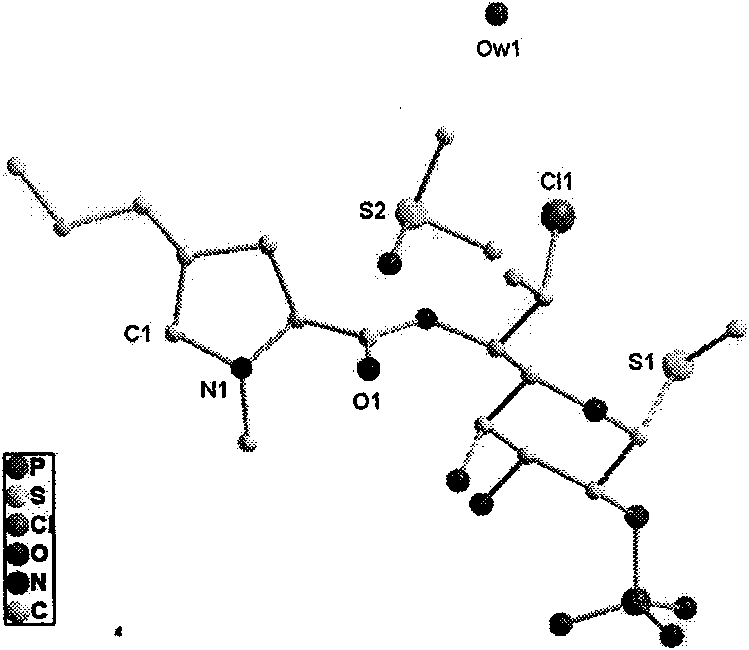
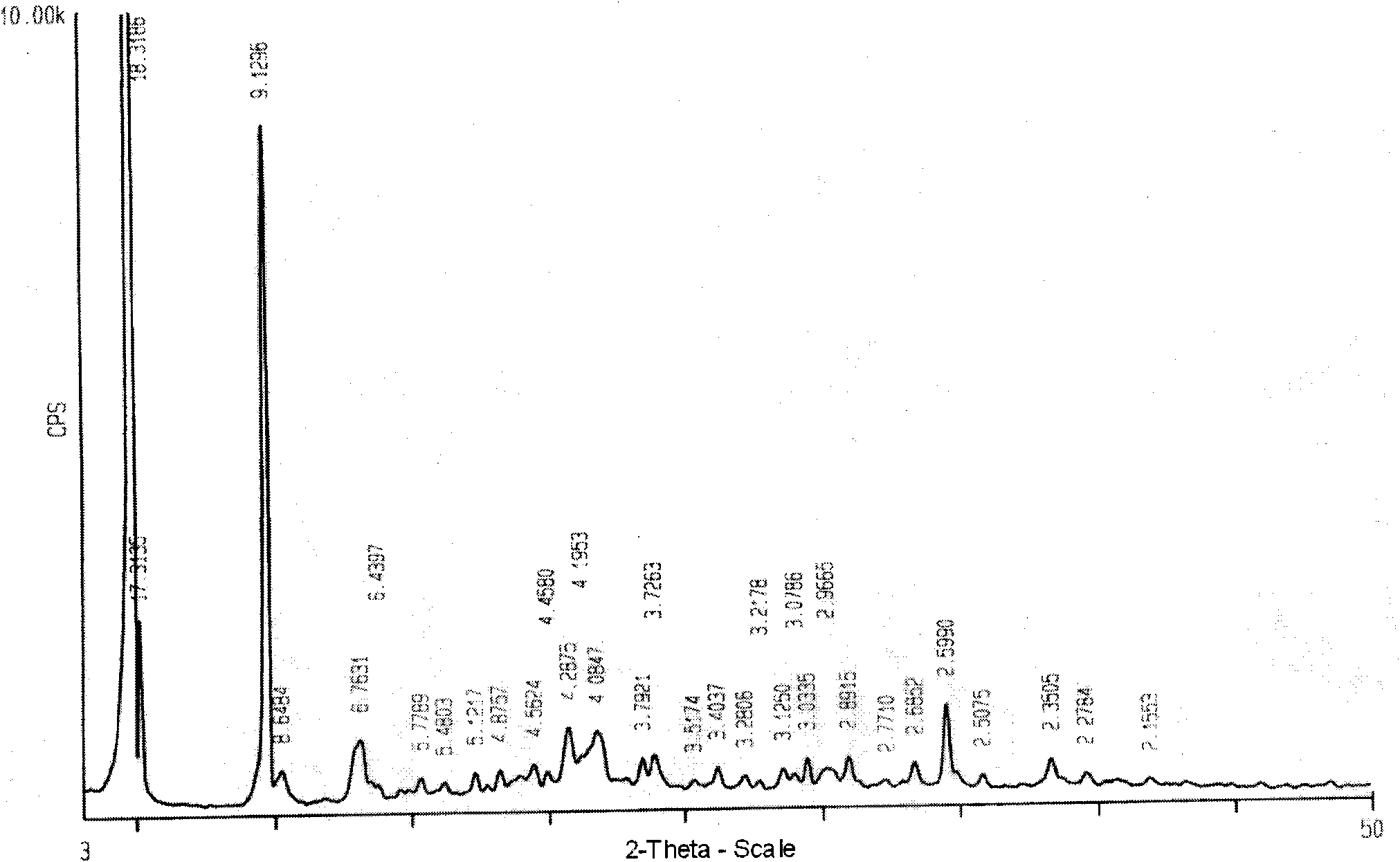
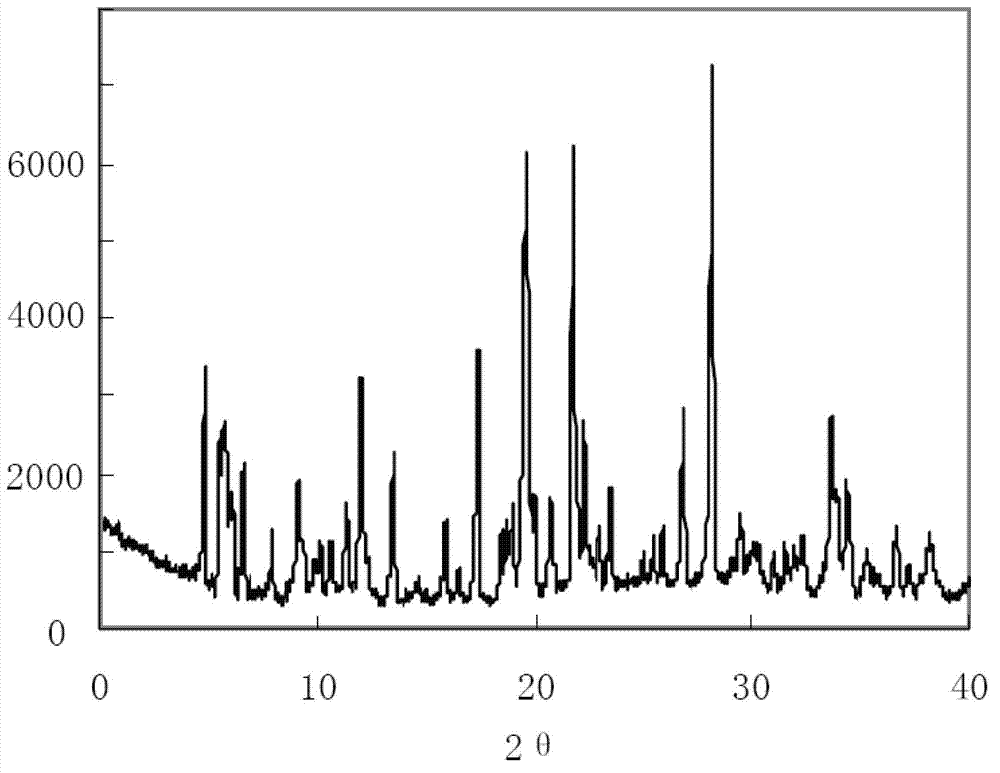
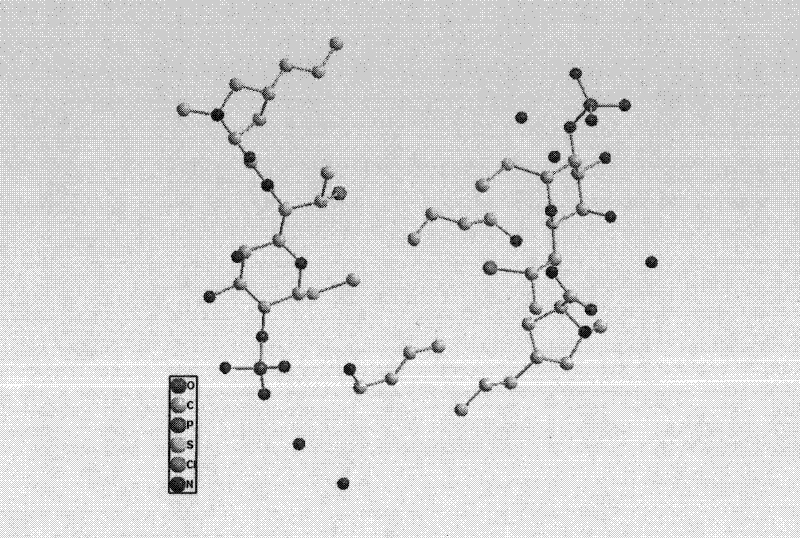
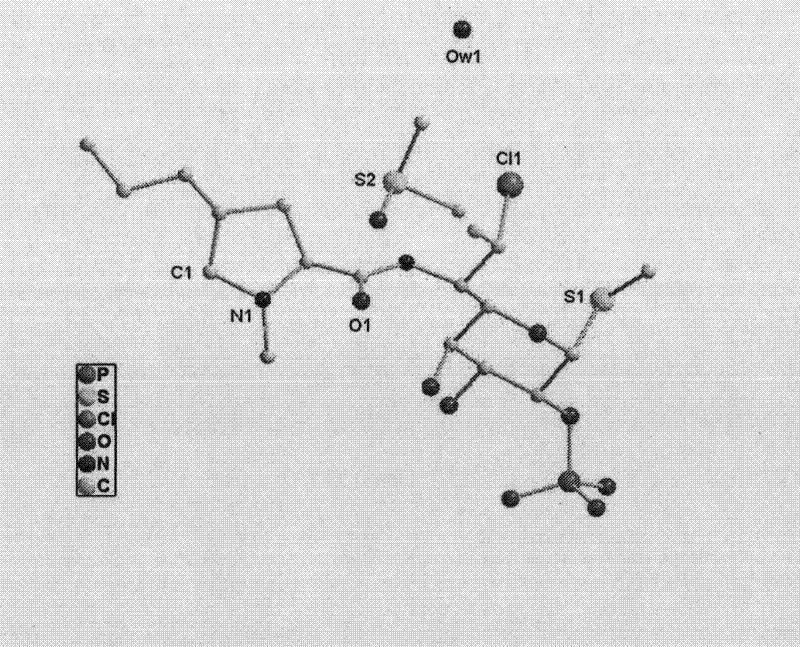
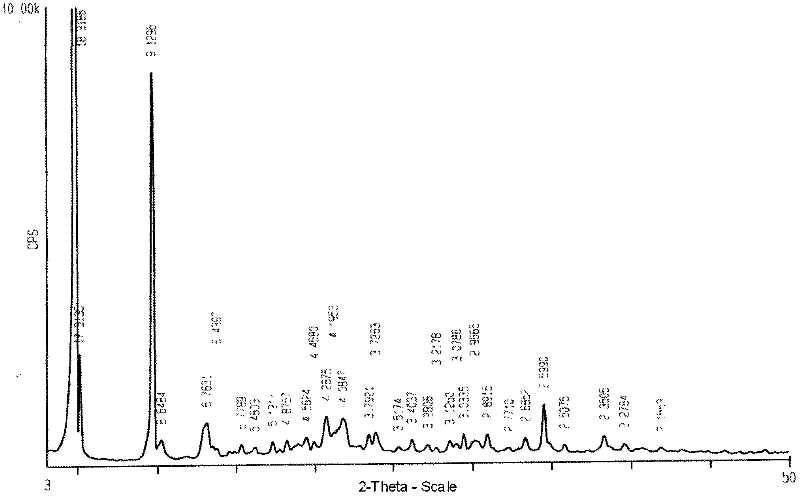
ActiveCN102731585ASimple manufacturing methodGood reproducibilityAntibacterial agentsOrganic active ingredientsClindamycin PhosphateX-ray
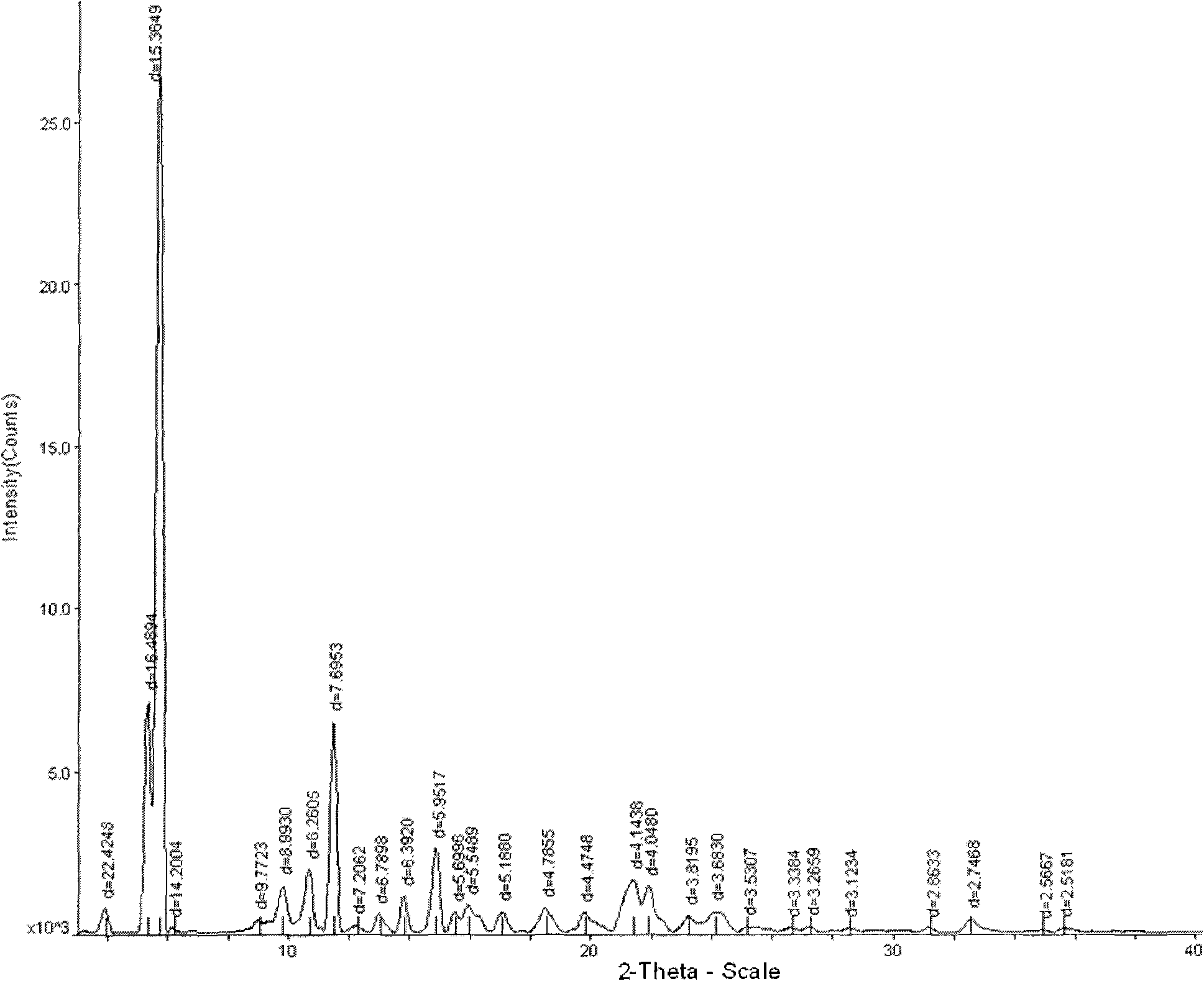
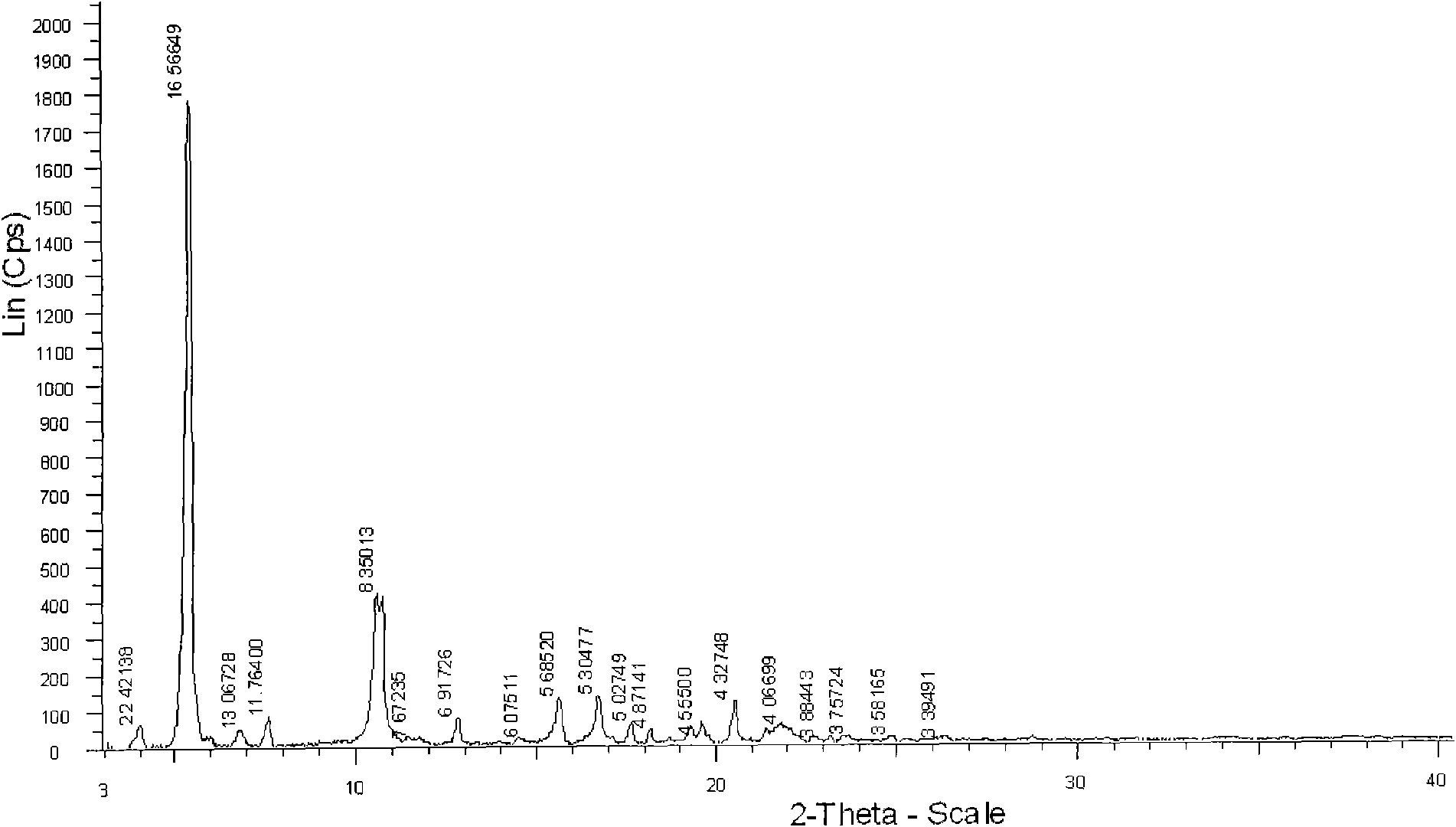
The invention relates a new active clindamycin phosphate compound and a medicinal composition of the compound. When the clindamycin phosphate compound is determined by using powder X-ray diffraction, characteristic diffraction peaks appear at the angels of 4.85 degrees, 5.73 degrees, 6.64 degrees, 9.12 degrees, 11.98 degrees, 13.51 degrees, 17.37 degrees, 19.61 degrees, 21.76 degrees, 22.23 degrees, 23.46 degrees, 26.82 degrees, 28.16 degrees, 33.68 degrees and 34.29 degrees in a X-ray diffraction pattern represented in 2theta + / -0.2 degree diffraction angles. The stability of the clindamycinphosphate compound provided by the invention is improved remarkably; the influences on the appearance, content and other aspects of the clindamycin phosphate compound caused by long-term storage can be ignored, thereby ensuring the drug safety for patients. In addition, the new clindamycin phosphate compound is suitable to be popularized and applied to various clindamycin phosphate preparations.
Owner:南城第二医院
Novel route for clindamycin phosphate compounds
InactiveCN101704852AReduce dosageGood effectSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsPhosphorylationClindamycin Phosphate
The invention provides a novel route for clindamycin phosphate compounds. In particular, clindamycin serving as an initial reaction raw material undergoes hydroxyl protection, phosphorylation and hydrolysis reaction to obtain the clindamycin phosphate compounds. In the new route, a specific catalyst is used and phosphorus oxychloride is used as a phosphorylating agent, so that compared with the prior art, the new route has the advantages of greatly lowering consumption of phosphorus oxychloride, lowering production cost and being more suitable for industrialization.
Owner:HAINAN LINGKANG PHARMA CO LTD
Clindamycin phosphate injection preparation and preparation method thereof
InactiveCN101780032AThe preparation method is simple and easyQuality improvementAntibacterial agentsOrganic active ingredientsClindamycin PhosphateSolvent
The invention provides a clindamycin phosphate injection preparation and a prescription thereof. According to the prescription, the clindamycin phosphate injection preparation comprises clindamycin phosphate, a cosolvent, a metal ion complexing agent, a solvent of raw materials and auxiliary materials and the like. The invention aims to provide a method for preparing the clindamycin phosphate injection preparation with more advanced technology and better product quality.
Owner:双鹤药业(海南)有限责任公司
Clindamycin phosphate solvate crystal and preparation method thereof
The invention belongs to a medical technology field, more specifically relates to clindamycin phosphate solvate crystal and a preparation method thereof. The crystal comprises clindamycin phosphate n-butanol-water solvate crystal and clindamycin phosphate dimethyl sulfoxide-water solvate crystal. Chemical stabilities of the two crystals are good and properties thereof in the storage process are stable.
Owner:ZHUHAI EBANG PHARMA +1
A kind of clindamycin phosphate composition for injection and preparation method thereof
ActiveCN102258488AExcellent freeze-dried structureExcellent average particle sizeAntibacterial agentsPowder deliveryPorosityClindamycin Phosphate
The invention relates to a clindamycin phosphate composition for injection. The composition is freeze-dried powder which consists of clindamycin phosphate and sodium hydroxide, wherein the weight ratio of the clindamycin phosphate to the sodium hydroxide is (24-26):1; the average grain diameter of the freeze-dried powder is 70-130nm; and the porosity is 92-98 percent. A preparation method of the composition comprises the following steps of: (1) preparing: weighing the clindamycin phosphate and the sodium hydroxide, filling in a preparation tank, adding water for injection, stirring to fully dissolve the clindamycin phosphate and the sodium hydroxide and uniformly mixing; (2) decarbonizing and performing sterile filtration; (3) performing sterile subpackaging; and (4) freeze-drying under vacuum. The composition has the advantages of simple formula, advanced process, uniform quality and superior stability and meanwhile has better redissolving performance and clinical medication safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Refining method of clindamycin phosphate
InactiveCN107880083AAvoid hydrolytic degradationReduce contentSugar derivativesSugar derivatives preparationAlcoholPharmaceutical industry
The invention relates to a refining method of clindamycin phosphate and is suitable for the pharmaceutical industry. The refining method comprises the following steps: dispersing clindamycin phosphatein a low alcohol of 4-10 times; adding organic alkali till the clindamycin phosphate is fully dissolved while stirring; decoloring, degerming and filtering the mixture; adding a degermed and filteredhydrogen chloride ethanol solution till the pH is 3.5-4.5; crystallizing the mixture for 1h; and filtering, washing and drying the mixture in vacuum. The clindamycin phosphate is prepared in an anhydrous system, so that the probability of hydrolytic degradation is avoided. The refining method is operated at normal temperature, is low in energy consumption, short in production period and high in yield by adopting the low alcohol, and the yield reaches 99% or above.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Preparation method of clindamycin phosphate injection
ActiveCN102144966AImprove stabilityGood curative effectAntibacterial agentsOrganic active ingredientsMedicineClindamycin Phosphate
The invention discloses a chemical injection, and particularly relates to a preparation method of a clindamycin phosphate injection. The clindamycin phosphate injection is prepared from the following raw materials in grams: clindamycin phosphate 300-400 g and sodium hydroxide 24.3-25.5 g. The preparation method comprises the following steps: dissolving 24.3-25.5g of sodium hydroxide in 1500-1900 g of water for injection, dissolving 300-400 g of clindamycin phosphate in the sodium hydroxide solution, stirring uniformly, adding water for injection to a constant volume of 2,000 mL, performing coarse filtering with a 0.65 mu m microporous filter membrane, performing fine filtering with a 0.22 mu m microporous filter membrane for sterilizing, and filling to obtain the clindamycin phosphate injection. The preparation method provided by the invention has the characteristics of applicability to mass production, high product yield, stable drug quality, and good therapeutic effect of drugs.
Owner:辽宁格林生物药业集团股份有限公司
Method for preparing clindamycin phosphate
ActiveCN102964401ALow impurity contentQuality improvementAntibacterial agentsOrganic active ingredientsHydrogenAlcohol
The invention relates to a method for preparing clindamycin phosphate, belonging to the field of medicine technologies. According to the method, clindamycin phosphate containing dehydro-clindamycin phosphate is taken as a raw material and dissolved into water or water-containing alcoholic liquid, 1 / 5-1 / 100 of the raw material amount of palladium carbon is added, hydrogen is introduced to react for 1-48 hours while stirring, the dehydro-clindamycin phosphate is enabled to fully produce the clindamycin phosphate, and then crystallization is carried out. The method has the advantages that the dehydro-clindamycin phosphate can be enabled to fully produce the clindamycin phosphate, so that the content of impurities in drugs is reduced, and the quality of the drugs is improved.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Detection method of related substances in clindamycin phosphate gel
ActiveCN106950290AImprove accuracyStrong specificityComponent separationClindamycin PhosphateRepeatability
The invention provides a detection method of related substances in clindamycin phosphate gel. The detection method is established on the basis of screening of preparation conditions of a sample solution, purification in pretreatment of the gel and screening of chromatographic conditions. Methodology validation research proves that the method has high accuracy and specificity and is good in repeatability and durability. By controlling the related substances in the clindamycin phosphate gel, the quality of a product is improved.
Owner:NINGXIA DUOWEI PHARMA
Preparation and application of clindamycin phosphate in-situ gel
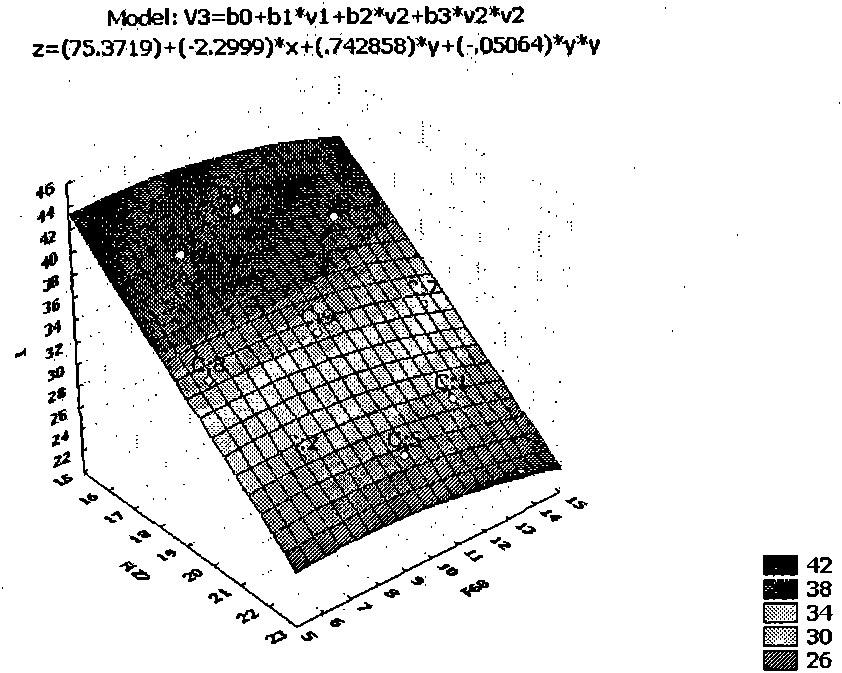
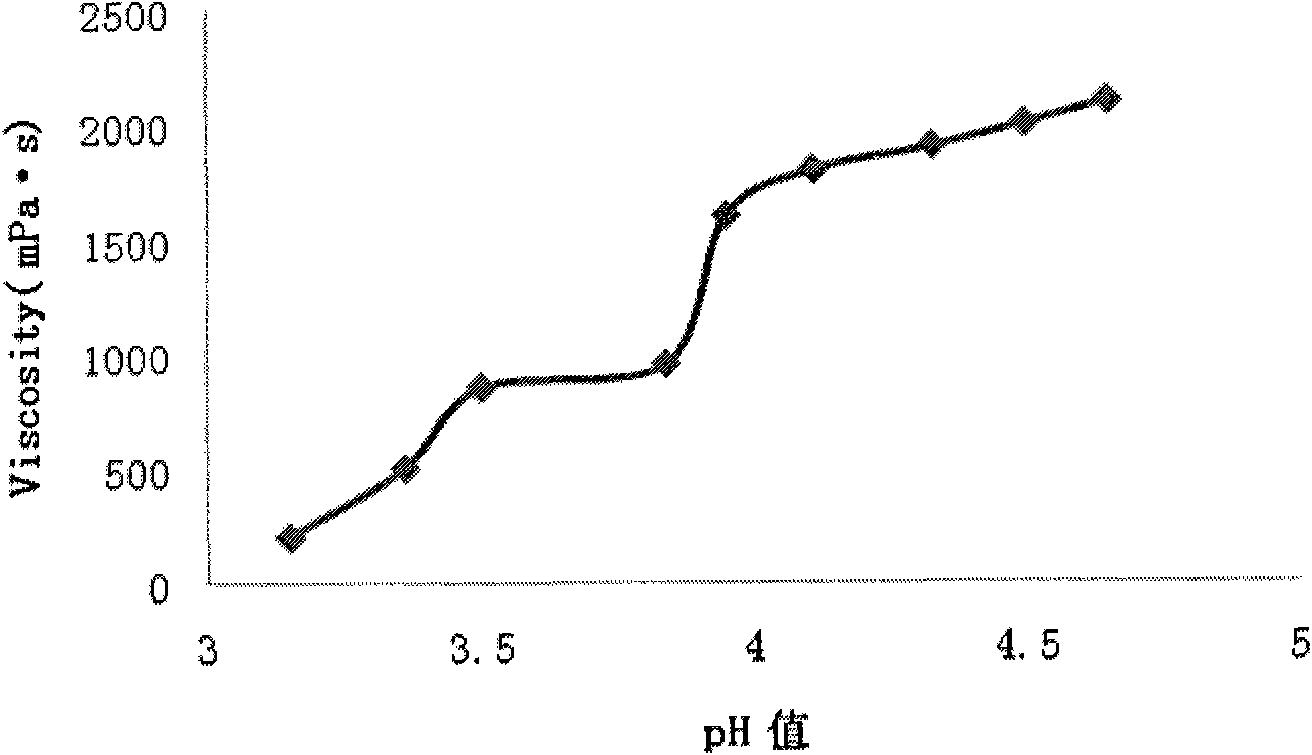
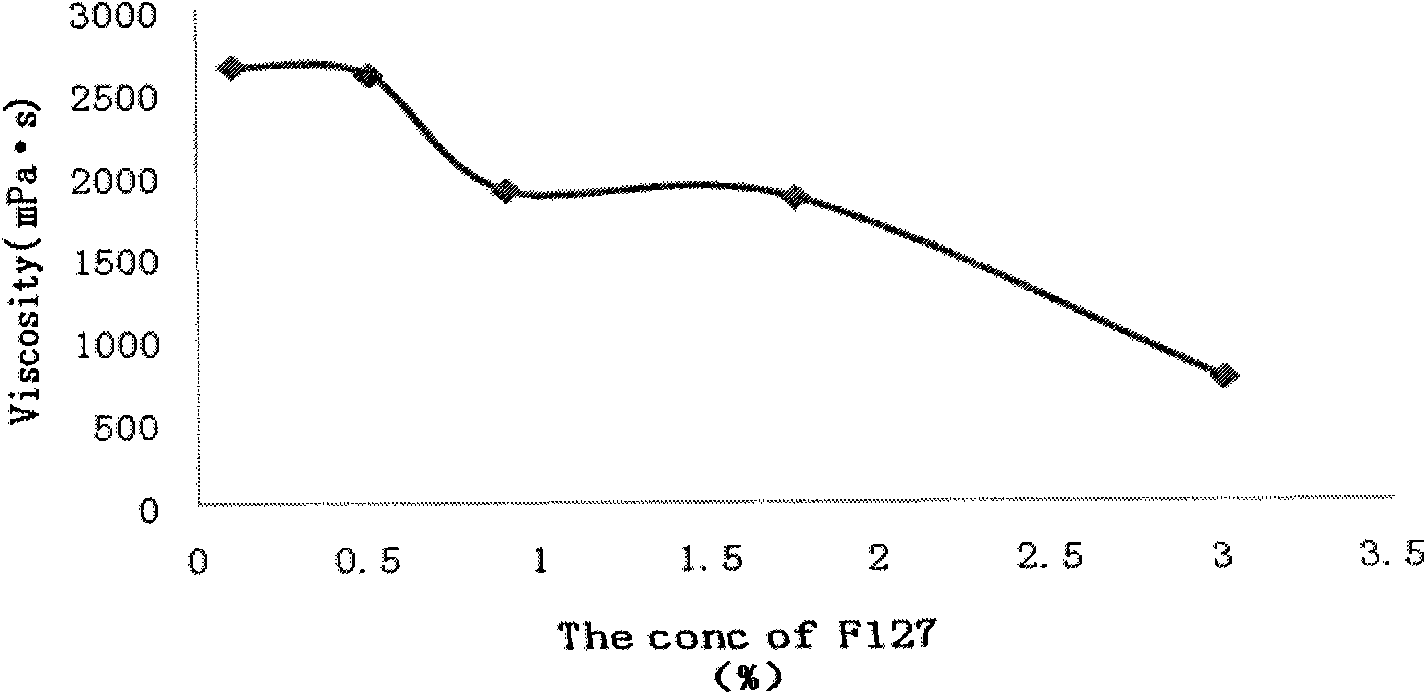
Relating to the field of medical technologies, the invention provides a new dosage form of clindamycin phosphate, i.e. preparation and application of temperature sensitive and pH sensitive clindamycin phosphate in-situ gel. The gel is characterized by preparation and application of the gel that is a solution under a nonphysiological state and is gel through phase transition under a physiological state. The temperature sensitive and pH sensitive clindamycin phosphate gel is finally prepared with Poloxamer 407 (F127 for short) and Poloxamer 188 (F68 for short) in a proper ratio. The pH sensitive in situ gel is prepared by Carbopol 980 and Poloxamer F127 in a proper ratio. The gel has viscosity of less than 1100mpa.s under a nonphysiological condition and viscosity of greater than 2500mpa.s under a physiological condition with pH of 4.5. The gel prolongs the detention time of clindamycin phosphate in the vagina and improves the bioavailability and curative effects, thus boasting good application prospects.
Owner:胡容峰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com