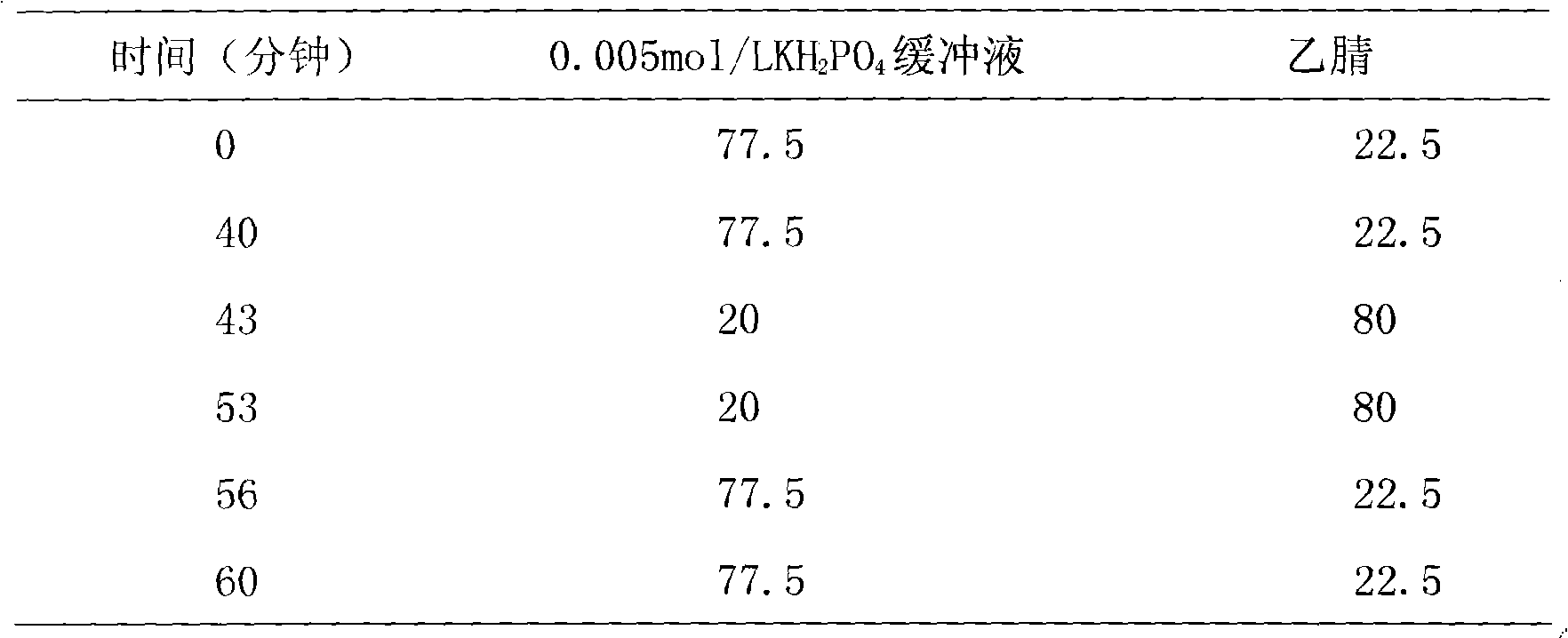
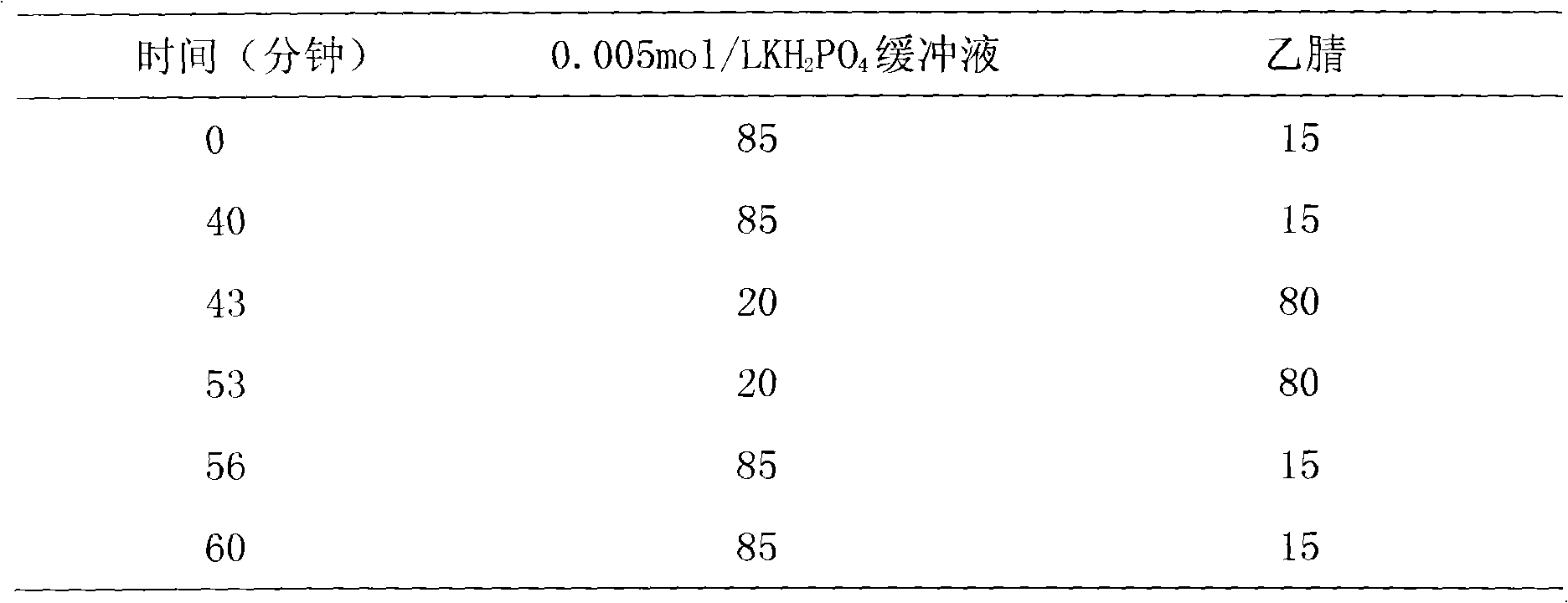
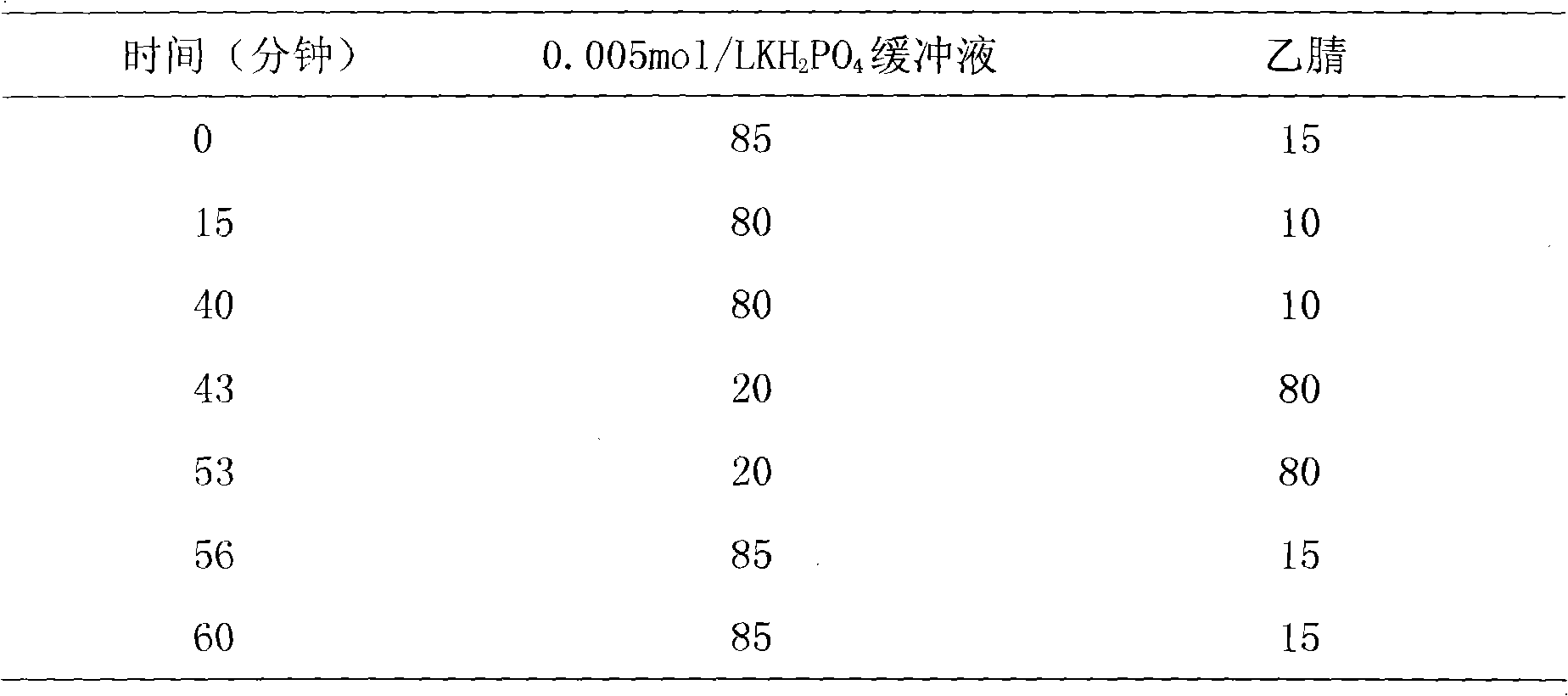
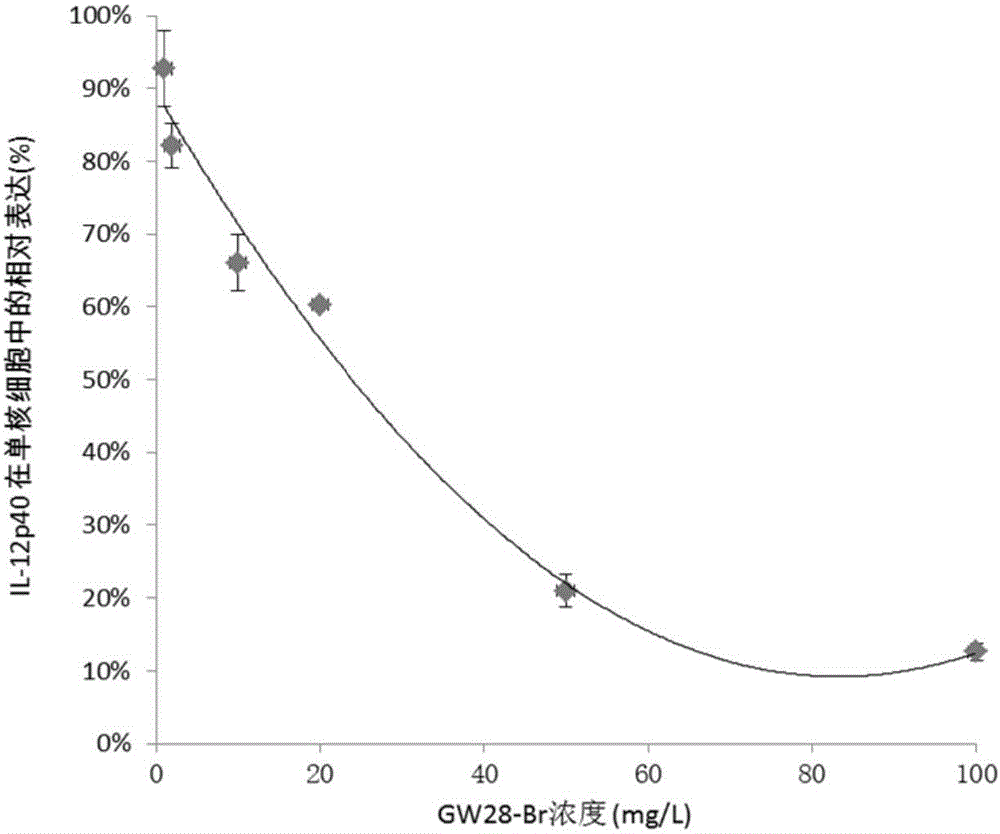
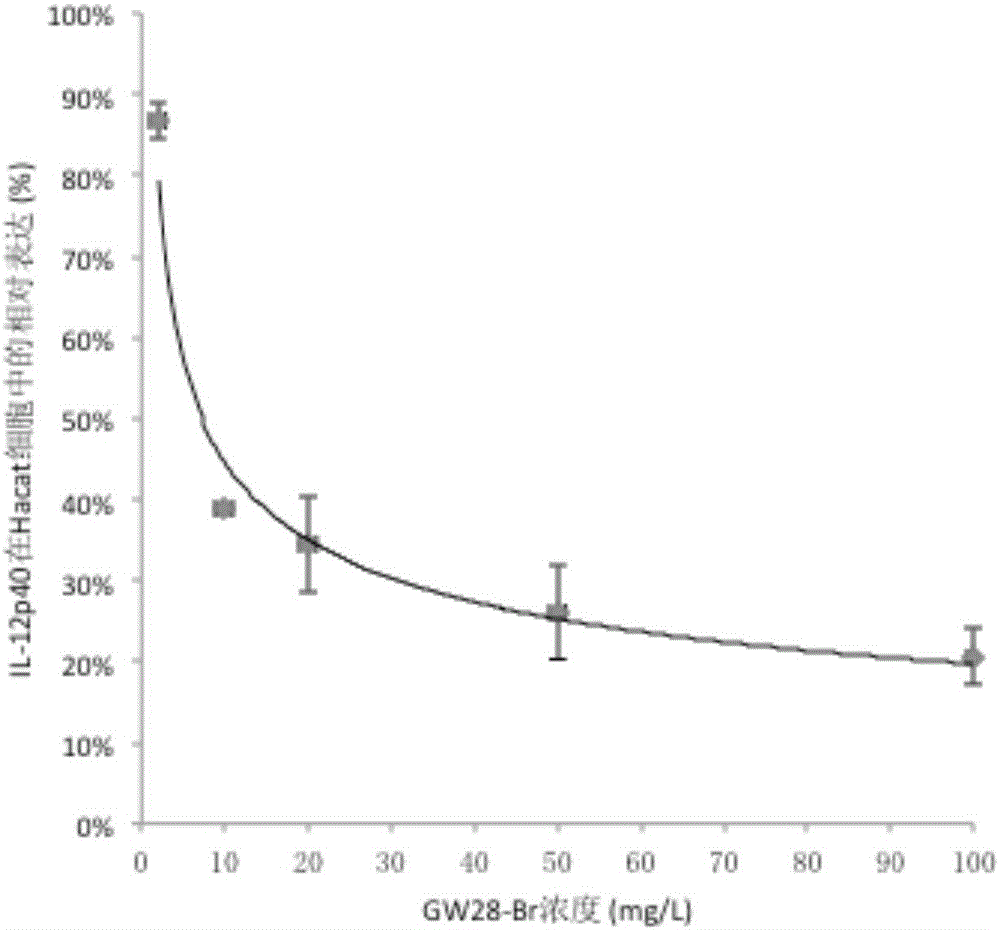
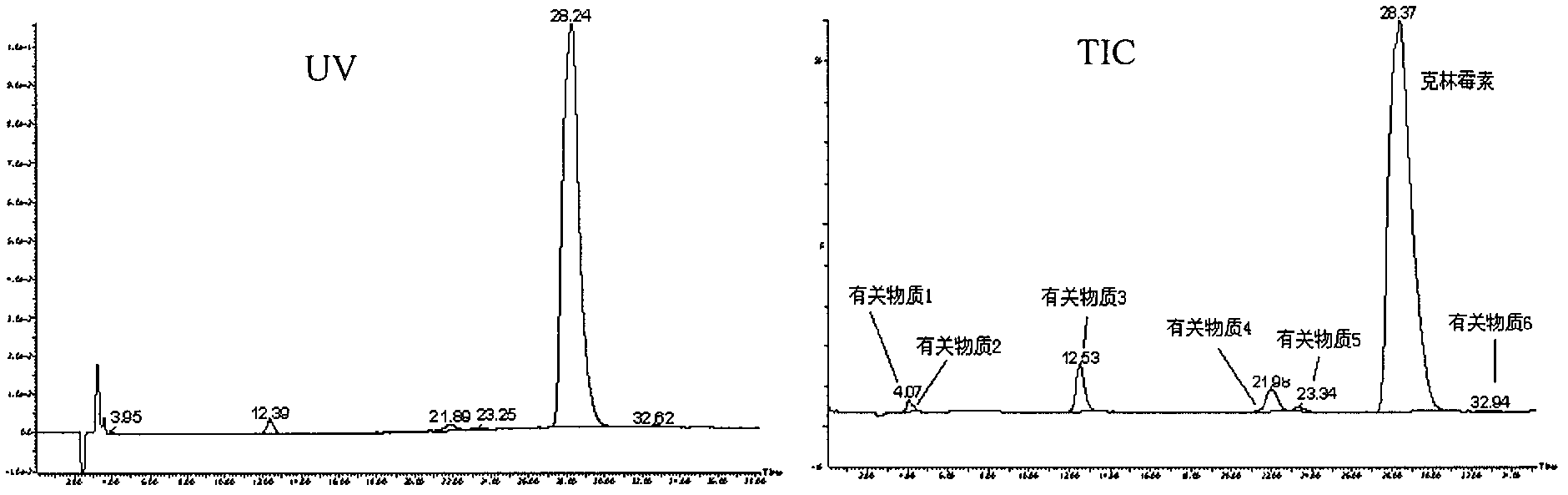
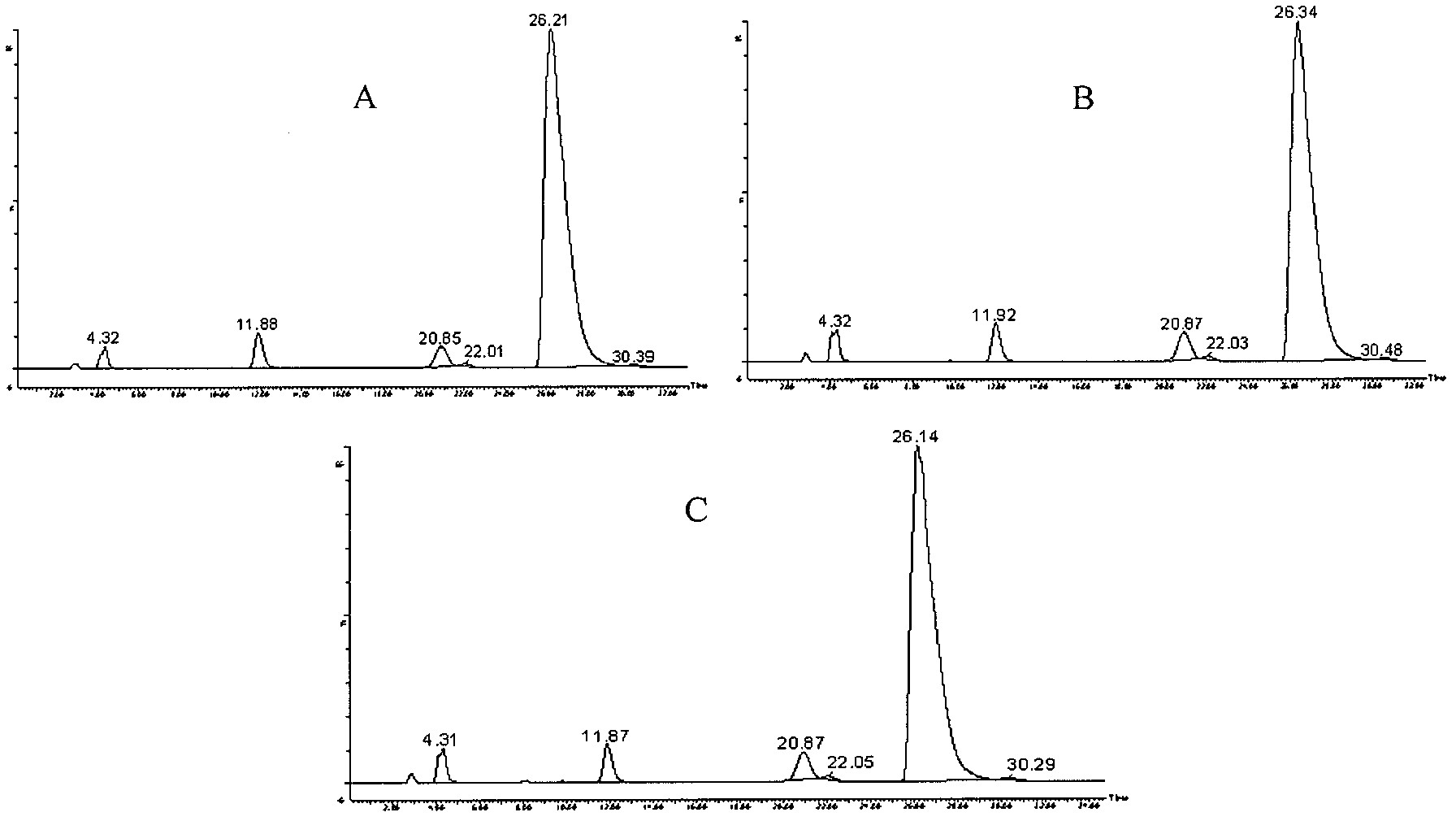
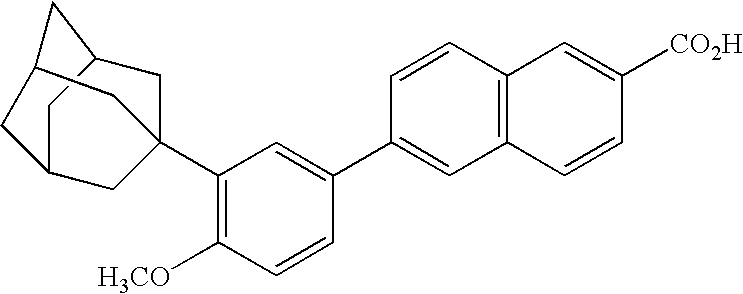
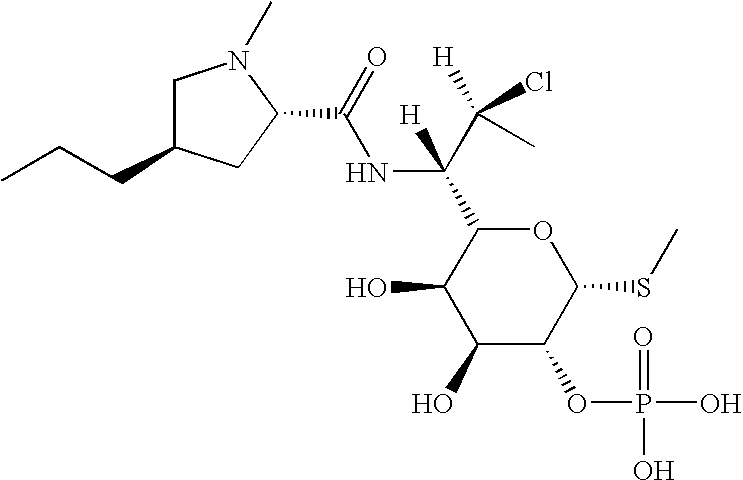
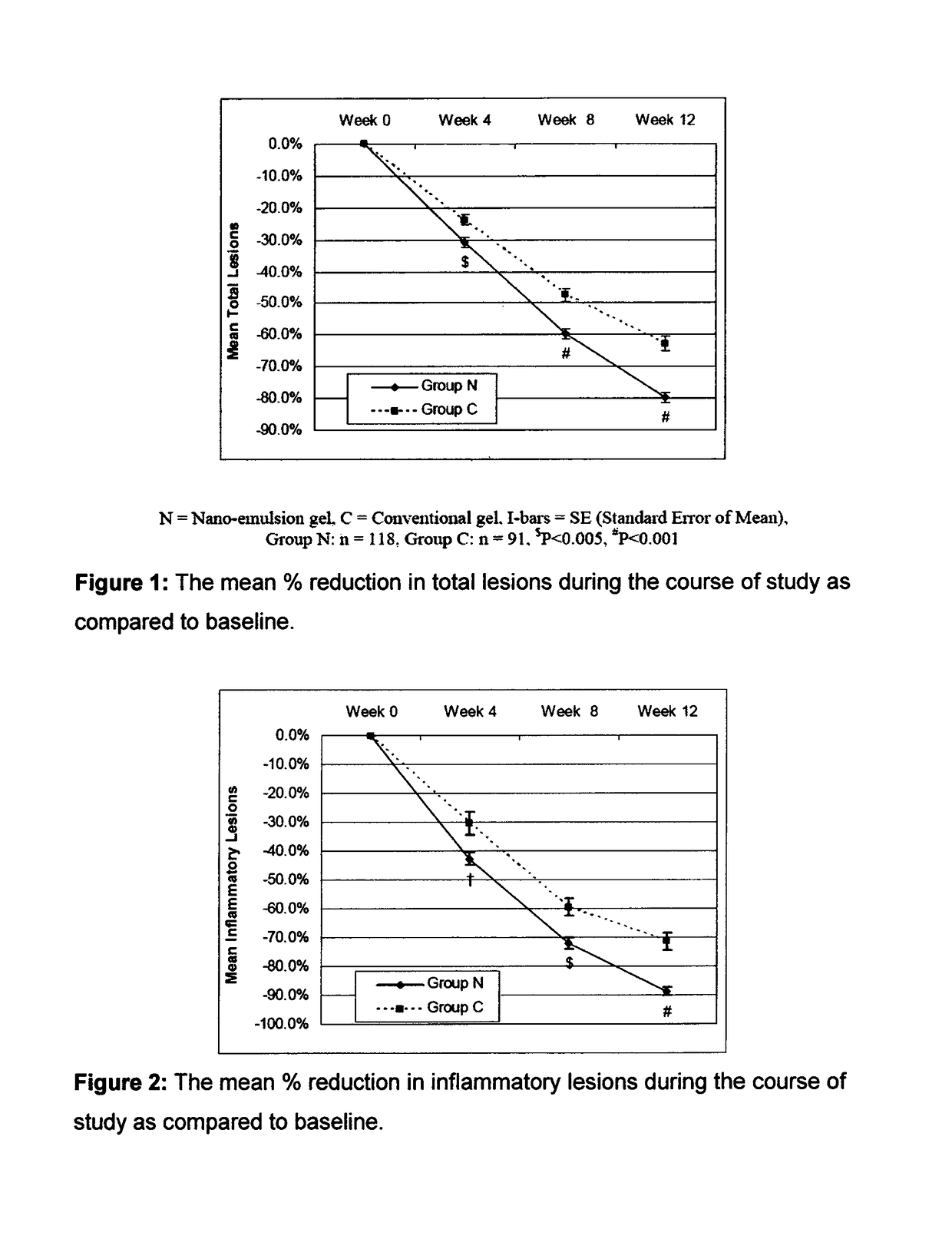
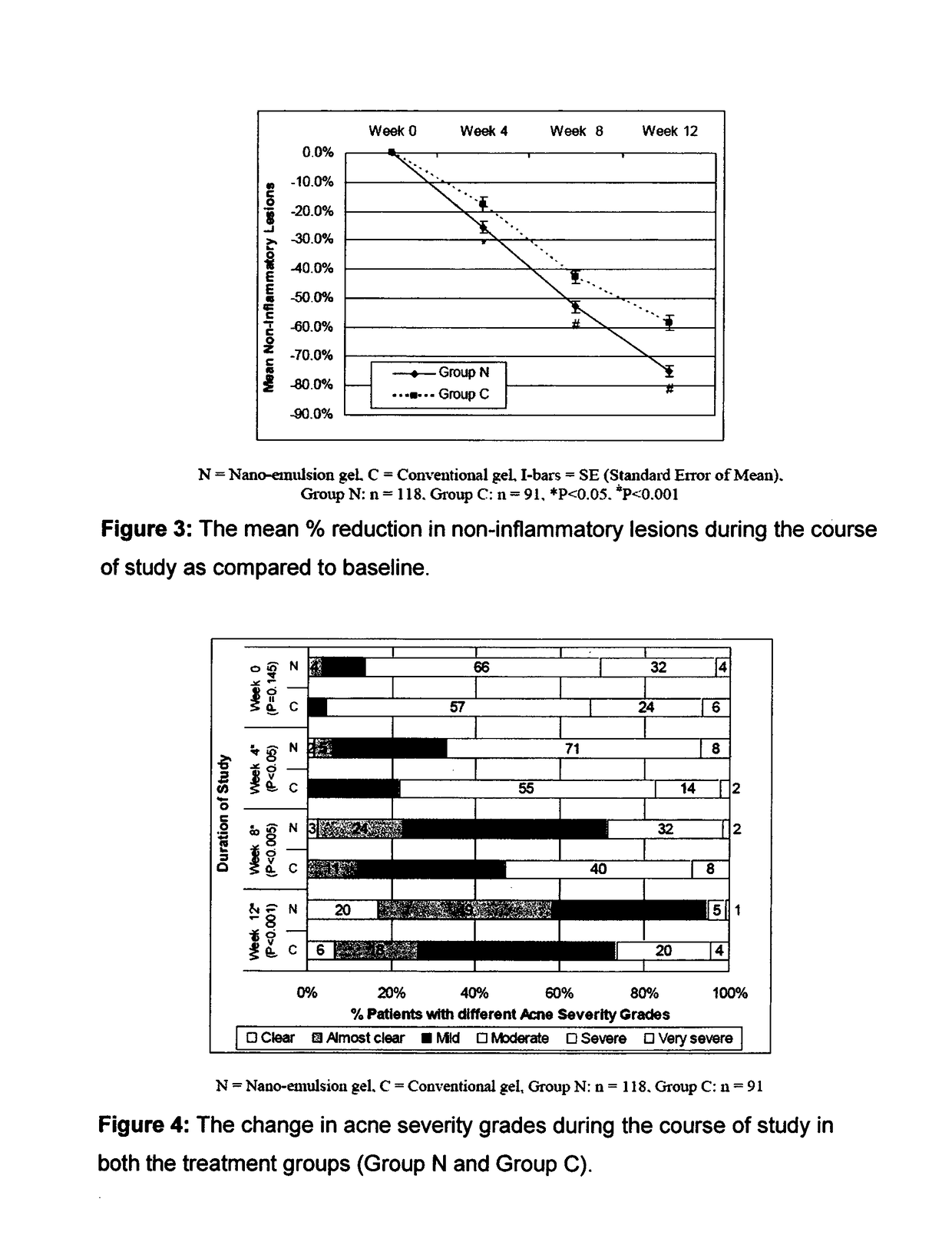
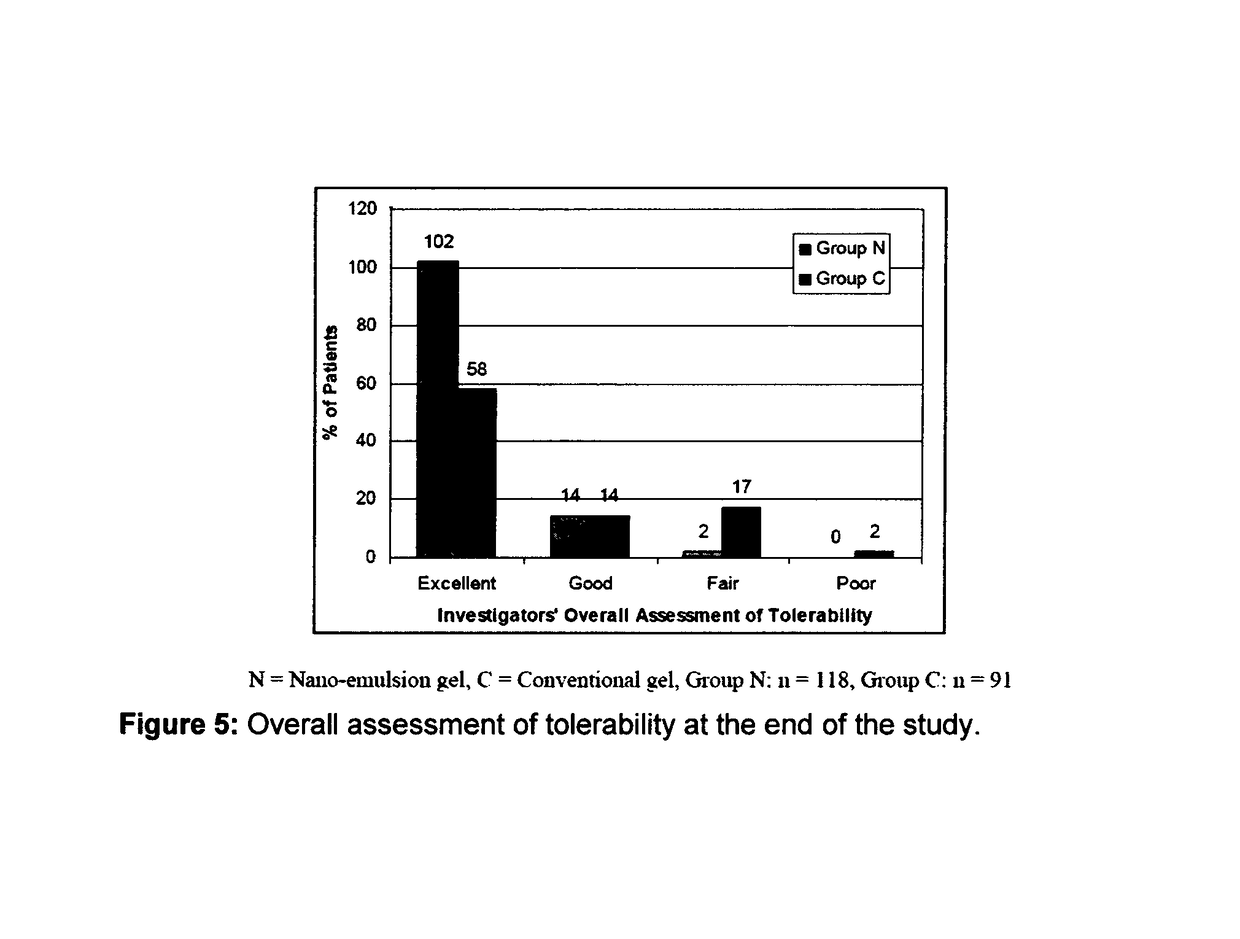
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
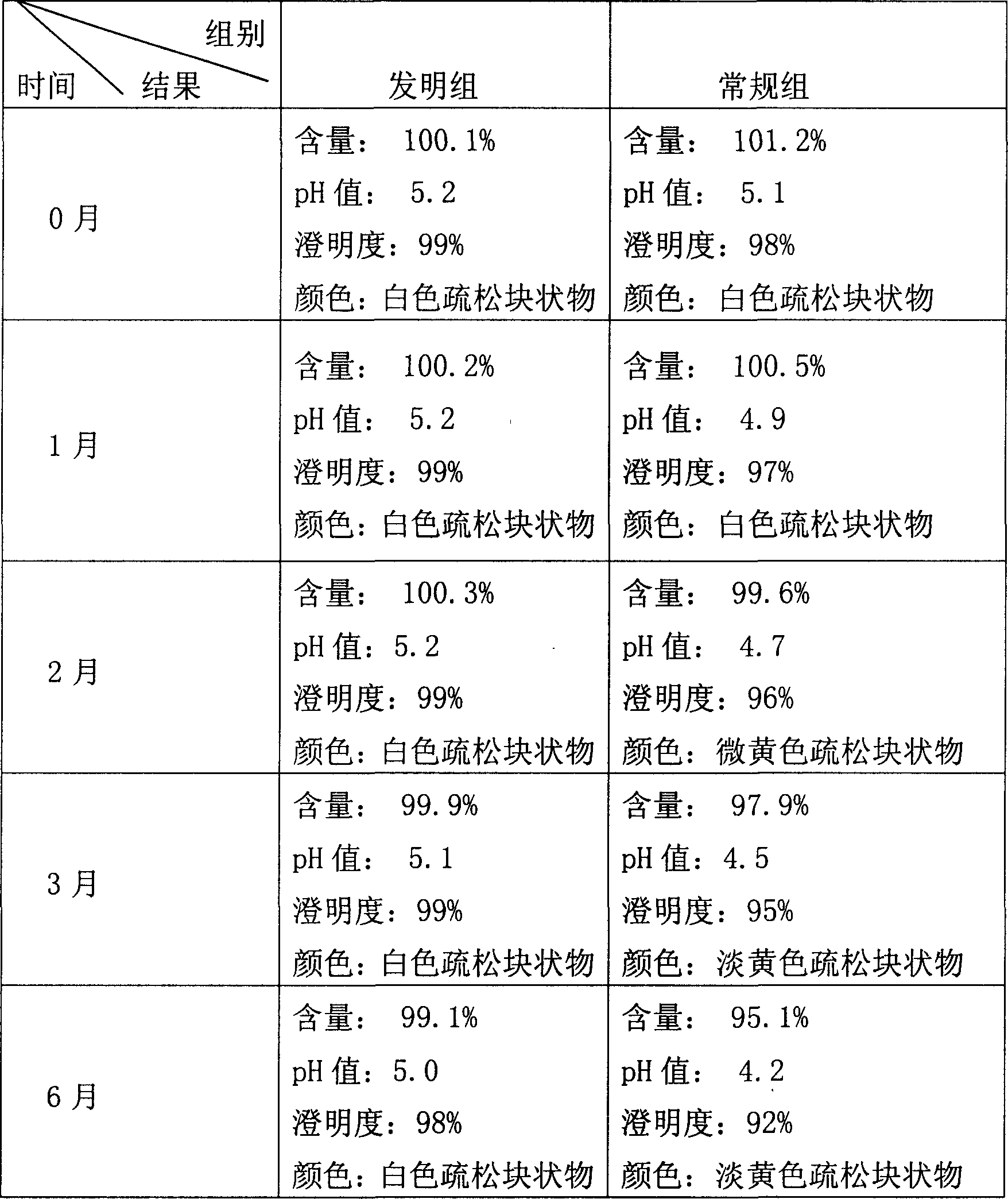
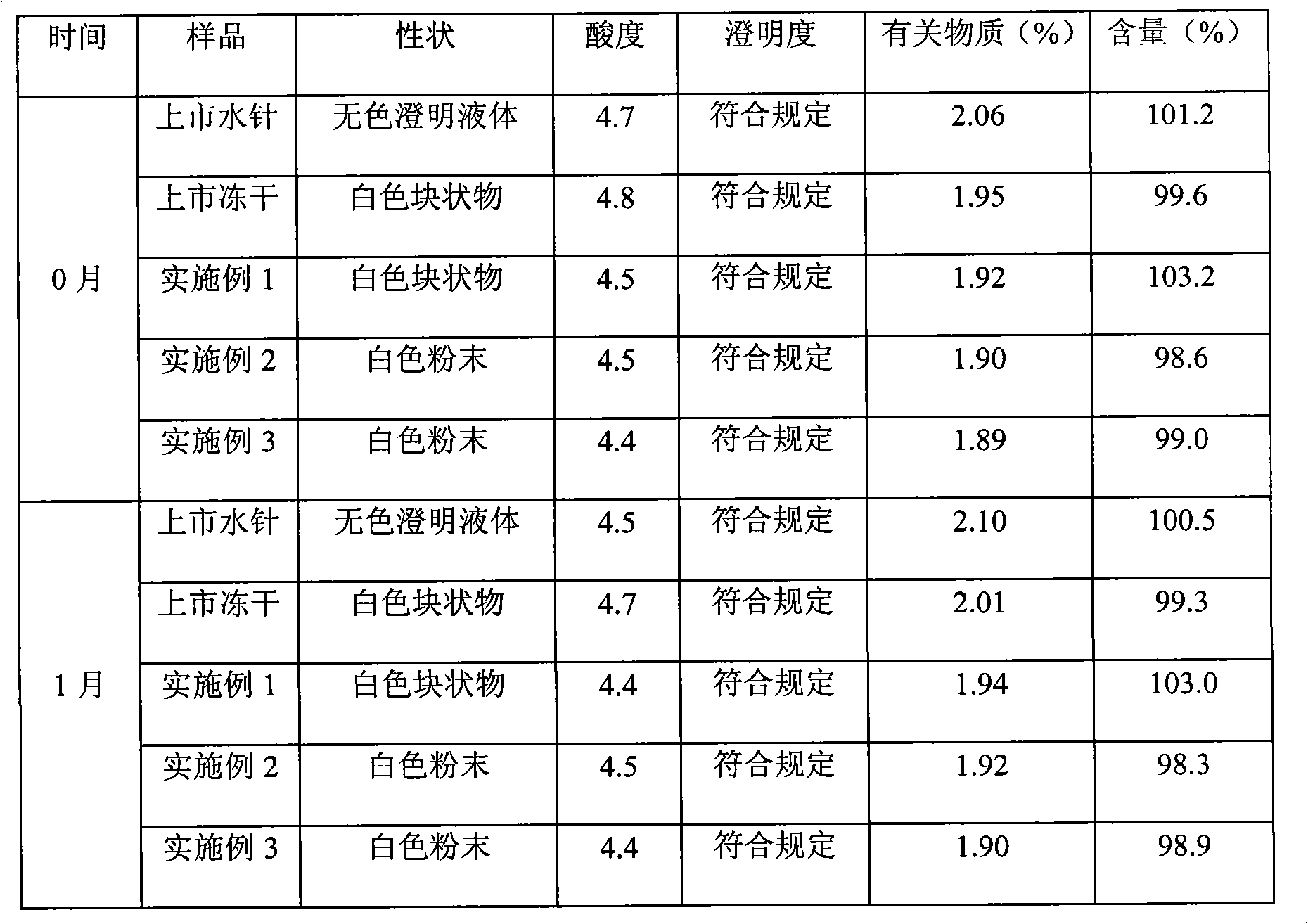
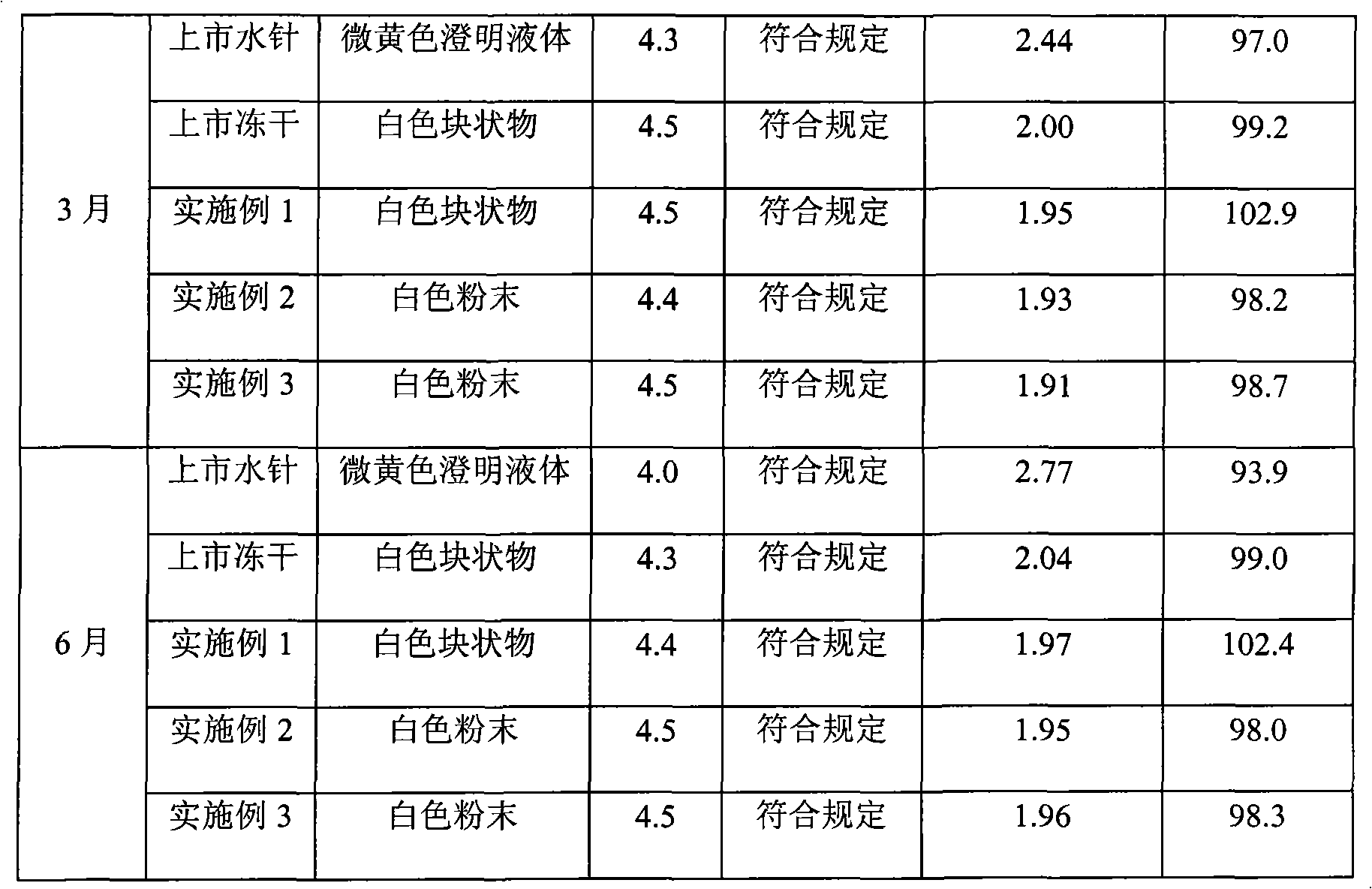
57 results about "Clindamycinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compound gelling agent containing clindamycinum
ActiveCN101292991AAvoid interferenceImprove detection qualityHydroxy compound active ingredientsPharmaceutical delivery mechanismTretinoinClindamycinum
The invention relates to compound gel containing clindamycin. The compound gels comprise effective dose of the clindamycin or medicinal salt or ester, Tretinoin and gel matrix. The invention is characterized in that the gel matrix is celluloses derivative, a preparation method as well as the detection and analysis methods of the gel are also related. The gel of the invention has the advantages of easy quality control, simple preparation technique and being suitable for industrial production.
Owner:CHONGQING PHARMA RES INST +1
Polypeptide having anti-bacterial and anti-inflammatory activity and application thereof
ActiveCN106589088AHigh activityEfficient broad-spectrum antibacterial effectAntibacterial agentsCosmetic preparationsBacteroidesBacillus acnes
The invention provides a polypeptide having the molecular weight of 3321.6 Da and having an amino acid sequence as shown in SEQ ID NO.1. The invention also provides six derivatives of the polypeptide. The polypeptide and the derivatives thereof provided by the invention have efficient broad-spectrum bacteriostatic effect on gram positive bacteria and gram negative bacteria (especially having good killing effect on anti-clindamycin anaerobic propionibacterium acnes), fungi and yeasts, and have high activity (the concentration for killing the propionibacterium acnes in vitro can be only 0.125 mg / L). The polypeptide and the derivatives thereof can be applied in preparation of microbial bacterial and fungal infection-controlling and anti-inflammatory products, wherein the products include externally-applied disinfectants, anti-bacterial agents, wound-protection or infection-prevention dressings, acne treating drugs, skin care products, cosmetics and the like. The polypeptide is designed and synthesized based on a tryptophan bromide-containing fragment of a marine biological sea urchin, has stronger bacteria inhibiting or killing and anti-inflammatory effects and better stability, and can be used as an antibiotic substitute.
Owner:ZHEJIANG UNIV
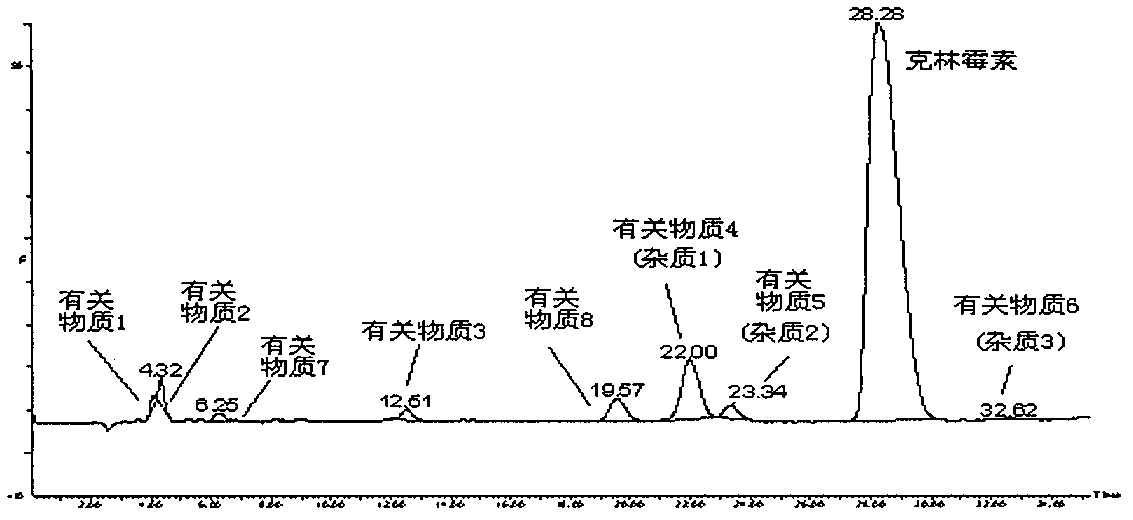
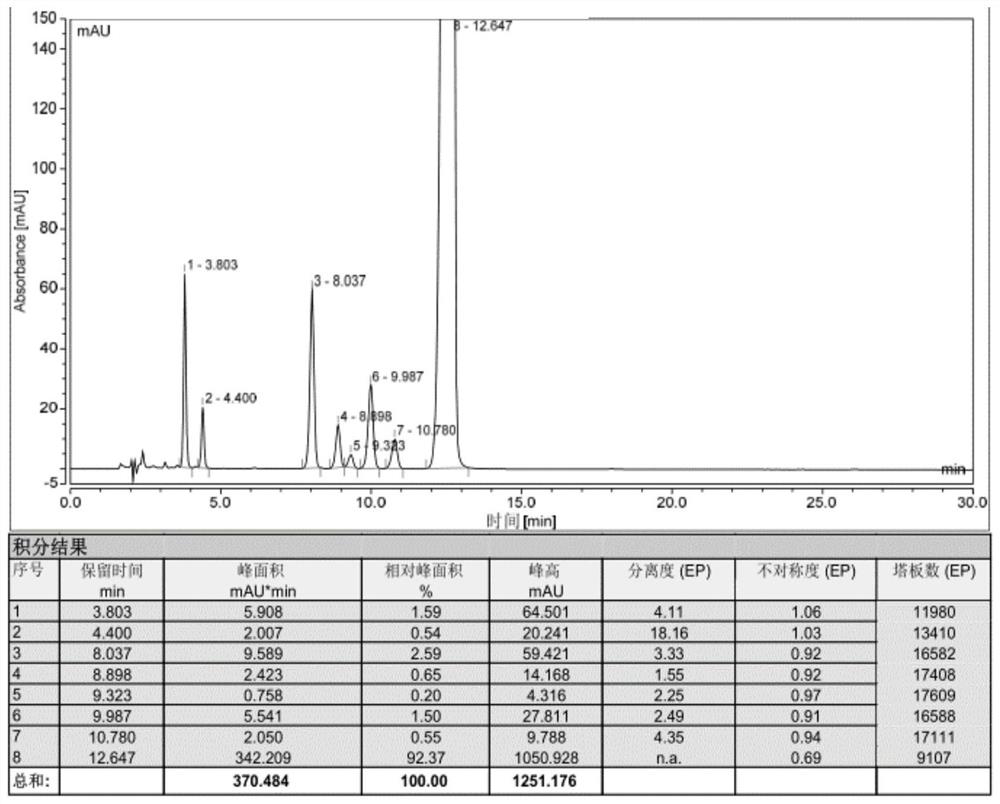
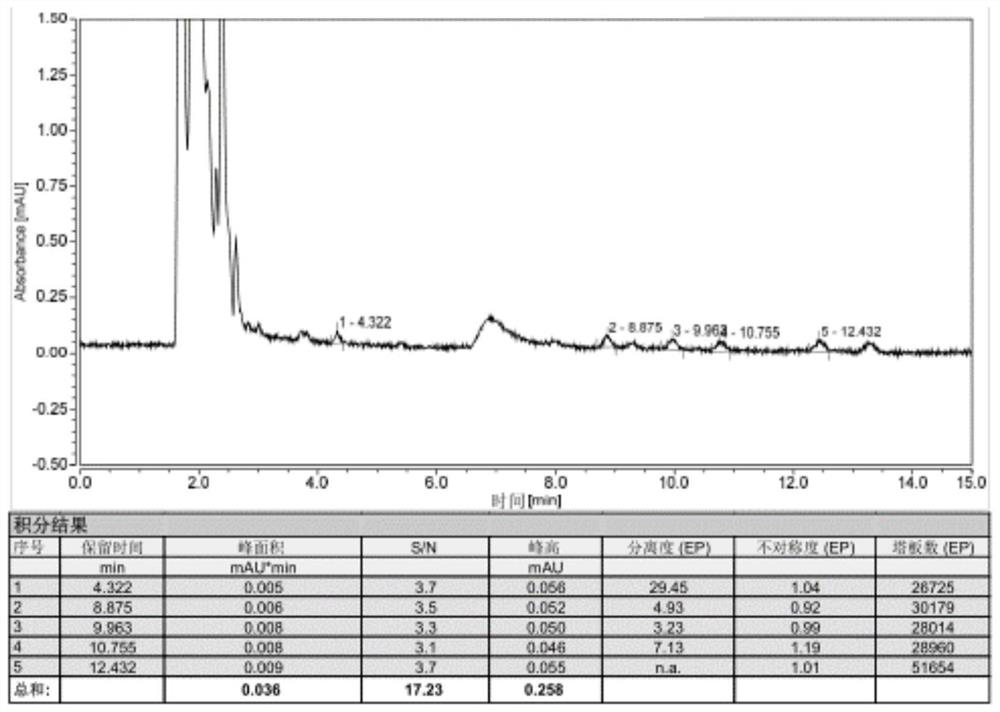
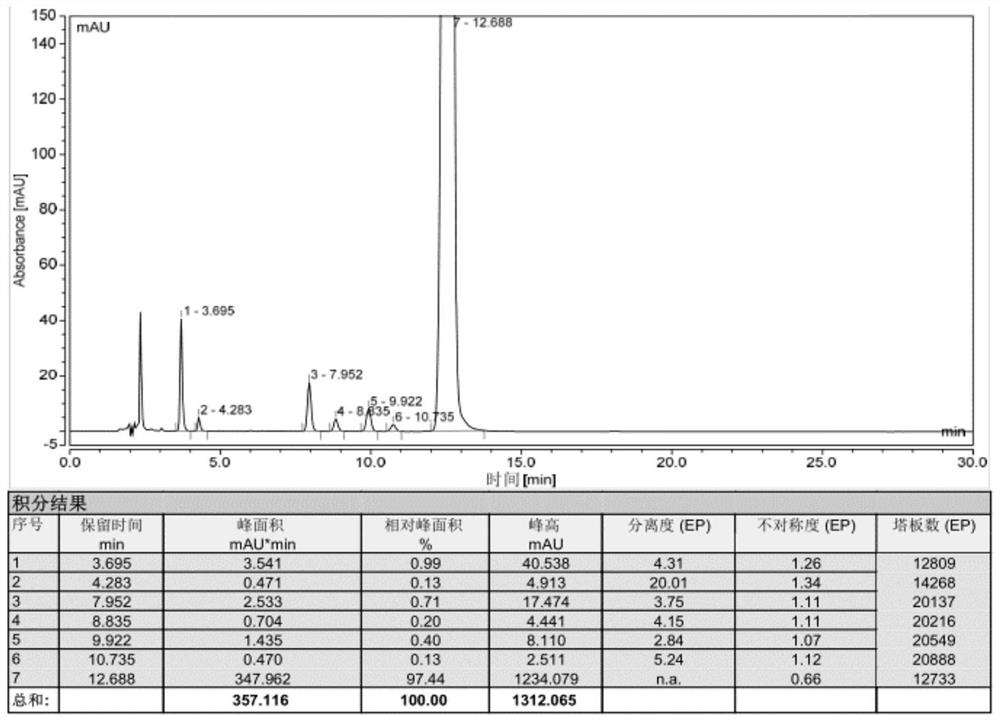
Impurity analysis preparation method for clindamycin
The invention provides an impurity analysis preparation method for clindamycin. The method is used for analyzing clindamycin materials and separating and preparing impurities from the materials, and comprises the following steps of: (a) measuring the clindamycin materials by an LC-MS (Liquid Chromatography-Mass Spectrometry) method, and determining one or more impurities in the materials according to the relative retention time and / or the molecular weight of the analyzed component; (b) determining the conditions of a column chromatography method according to the relative retention time and / or the molecular weight of one or more impurities, and gathering one or more impurities corresponding to the relative retention time and / or the molecular weight by the normal phase silica gel column chromatography method; and (c) determining the conditions of a preparative chromatography method according to the chromatographic retention behavior displayed by the relative retention time of one or more impurities in the step (a), and collecting one or more impurities corresponding to the retention time by the preparative chromatography method.
Owner:浙江天台药业股份有限公司
Method for preparing clindamycin hydrochloride
InactiveCN102702279AQuality improvementReduce solubilitySugar derivativesSugar derivatives preparationActivated carbonAlcohol
The invention discloses a method for preparing clindamycin hydrochloride. The method comprises the following steps of: a) adding purified water into a chloroformic solution of clindamycin hydrochloride alcohol complex and washing; b) adding purified water into the washed organic phase, dripping hydrochloric acid to adjust PH value to be 2 to 3, standing and layering; c) adding activated carbon into a water phase obtained through separation and decoloring, and filtering; d) adsorbing filtrate by using a resin, and washing by using deionized water; e) desorbing by using methanol of which the mass concentration is more than 95 percent; f) concentrating a desorption solution under reduced pressure; g) adding acetone into concentrated dried materials, and stirring at the temperature of between 55 and 58 DEG C until the solution is clarified; h) dripping hydrochloric acid to adjust PH value to be 2 to 2.5; and i) reducing temperature to 0 to 5 DEG C, performing suction filtration under negative pressure to obtain web powder, washing by using iced acetone, and after suction filtration, drying to obtain the clindamycin hydrochloride. According to the method, the step of alcohol complex crystallization in the conventional process is eliminated; the process is simplified; production cost is reduced; and the weight yield and the quality of the whole batch of clindamycin hydrochloride are improved simultaneously.
Owner:ANHUI WANBEI PHARMA
Oral compositions of clindamycin
A taste masked pharmaceutical composition of clindamycin, or a pharmaceutically acceptable salt(s), hydrate(s), solvate(s) and physiologically functional derivative(s) and precursors thereof, which includes all polymorphic forms, whether crystalline or amorphous comprising polyhydric alcohol(s); and one or more other pharmaceutically acceptable excipient(s). A process for preparation of a taste masked pharmaceutical composition of clindamycin or a pharmaceutically acceptable salt(s) thereof the said process comprising the steps of a) dry mixing clindamycin, polyhydric alcohol and other pharmaceutically acceptable excipient(s) to get a dry mixture; b) granulating the dry mixture above with a granulating liquid prepared by mixing the suitable pharmaceutically acceptable excipient(s) with aqueous / non-aqueous fluid to obtain a wet mass; c) drying the wet mass to obtain the discrete particles; d) lubricating the discrete particles obtained with a suitable lubricating agent and / or flavour(s).
Owner:LUPIN LTD
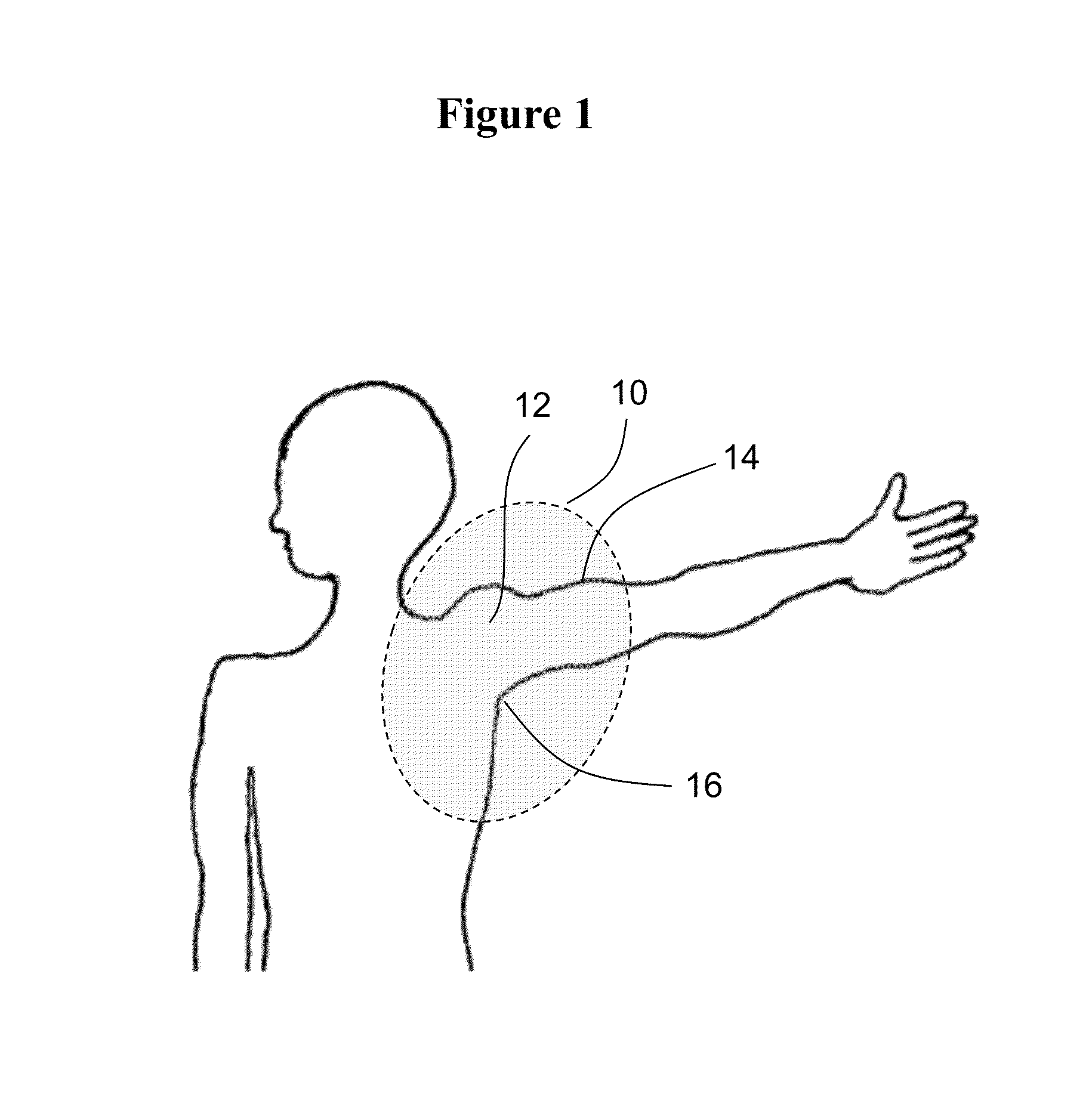
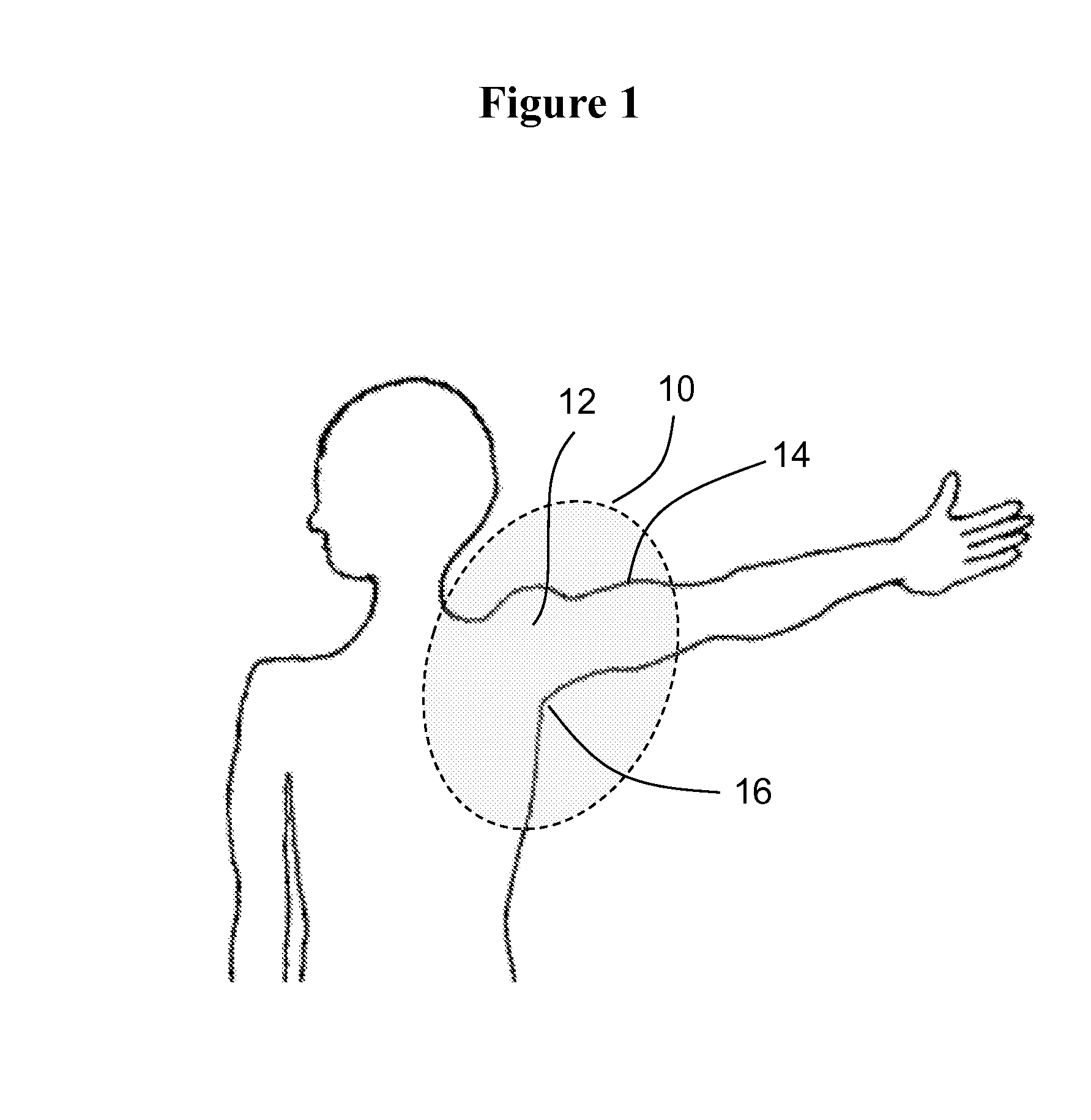
Methods and materials for reducing the risk of infections
ActiveUS9176487B2Reduce infectionReduce riskAntibacterial agentsOrganic active ingredientsCvd riskMedical procedure
This document relates to methods and materials for reducing the risk of infection after a shoulder surgery or medical procedure. For example, this document relates to methods and materials for using a topical composition containing clindamycin or erythromycin to reduce the risk of or to prevent infection associated with shoulder surgeries or medical procedures.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Anti-acne composition and methods of use thereof
The present invention provides for a topical dermatological composition that includes diclofenac and clindamycin, in a pharmaceutically acceptable medium, wherein at least one of the diclofenac and clindamycin is at least partially microencapsulated.
Owner:RAYUDU SREEDHAR RAO
Compound vitamin A acid gel preparation for treating acne and its preparing method
InactiveCN1850101AObvious superiorityEasy to usePharmaceutical delivery mechanismDermatological disorderGel preparationAlcohol
The present invention discloses a compound gel preparation-compound tretinoin gel preparation for curing acnes. It is made up by using carbomer, glycerine, propylene alcohol, Tween, triethanolamine, clindamycin phosphate, tretinoin, EDTA and distilled water as raw material through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:李海涛
Preparation method of clindamycin hydrochloride freeze dried powder injection
InactiveCN1830452AAvoid degradationEnsure complete removalAntibacterial agentsOrganic active ingredientsAcute bronchitisUltrafiltration
A freeze-dried powder injection of clindamycin hydrochloride for treating various infectious diseases, tonsillitis, tympanitis, acute bronchitis, pneumonia, etc is prepared from clindamycin hydrochloride through ultrafiltration, mixing with filtered mannitol in dark condition and N2 atmosphere, pouring in containers, freeze drying, and sealing.
Owner:巴里莫尔制药(通化)有限公司
Orally taken medicament formulation of clindamycin phosphate and preparing method thereof
InactiveCN101401814AIncrease concentrationQuick effectAntibacterial agentsOrganic active ingredientsMedicineClindamycin Phosphate
The invention discloses a clindamycin phosphate oral pharmaceutical preparation, which is mainly prepared from raw materials in the following weight ratio: (1) a buccal tablet which comprises 1 to 100 portions of clindamycin phosphate counted on clindamycin, 0 to 100 portions of disintegrant, and 5 to 500 portions of filler; (2) a filmogen which comprises 1 to 100 portions of the clindamycin phosphate counted on the clindamycin, and 5 to 500 portions of film-forming material; and (3) an oral patch which comprises 1 to 100 portions of the clindamycin phosphate counted on the clindamycin, 5 to 500 portions of the filler, and 1 to 100 portions of adhesive material. The clindamycin phosphate oral pharmaceutical preparation is directly applied to a local infection and has high local drug concentration and fast effect, and the dose is low and adverse reactions are few.
Owner:张宏宇
Hydrochloric clindamycinum palmitate capsule and method for preparing the same
InactiveCN101152161ASolve mass productionSolve the problem that it cannot be prepared into capsulesAntibacterial agentsOrganic active ingredientsMedicineDiluent
The invention discloses a clindamycin hydrochloride palmitate capsule and the preparation method. Each capsule contains clindamycin hydrochloride palmitate with a content equal to 35 to 300 milligram of clindamycin hydrochloride. The capsule also contains pharmaceutical excipients such as diluent, disintegrant, solubilizer and seed coating agents. The invention produces granules by dry granulating method, wraps the granules with moisture proof agricultural film, and performs the process of capsule loading. The invention solves the problems of easy moisture absorbing, caking and breaking of capsule shell caused by moisture absorbing. The invention also solves the problems with the product that the formulation is unitary and only granules and dispersible tables are provided, enriches the formulation of the capsule, offers patients with more choices of formulation and the compliance is good. Due to the addition of solubilizer, the clindamycin hydrochloride palmitate is stripped more rapidly and the bioavailability of the invention is enhanced. And the invention adopts the process of moisture proof agricultural film wrapping after dry granulating method, so the activity of the clindamycin hydrochloride palmitate is preserved better, the effect of the drug is better and the drug is much safer; thereby the invention is fit for industrial production.
Owner:重庆智上医药科技有限公司
Hydrochloric clindamycin nano granule formulation for injections and preparation thereof
InactiveCN101322692AGood biocompatibilityLow costAntibacterial agentsOrganic active ingredientsExcipientSodium sulfate
The invention discloses a clindamycin hydrochloride nano-particle preparation; the preparation is prepared by the raw materials with the following parts by weight: 75-900 parts of clindamycin hydrochloride, 10-300 parts of stabilizing agent, 25-450 parts of sodium sulfate, 150-1,800 parts of polyalkylcyano-acrylate and 10-1,000 parts of excipient. The invention further discloses a preparation method thereof. The preparation is prepared by nano-particles and has fine stability and high safety.
Owner:HAINAN YONGTIAN PHARMA INST
Method for purifying clindamycin hydrochloride
ActiveCN105949253AHigh synthetic yieldReduce lossSugar derivativesSugar derivatives preparationDistillationEthylic acid
The invention relates to a method for purifying clindamycin hydrochloride. The process comprises process steps as follows: clindamycin hydrochloride adduct reaction liquid is subjected to macroporous resin adsorption and methyl alcohol desorption; obtained desorbed solution is subjected to reduced pressure distillation and concentration, then is dissolved by butyl acetate solvent, and is subjected to alkaline hydrolysis, alkaline extraction and activated carbon decoloration, and clindamycin alkali decolored solution is obtained; a clindamycin hydrochloride product is obtained after acetone crystallization conversion. According to the method, the macroporous resin is utilized in the clindamycin hydrochloride adduct reaction liquid, so as to facilitate alkaline hydrolysis and alkaline extraction and separation processes, reduce an emulsification effect, improve the clindamycin hydrochloride synthesis yield by more than 6%, reduce a solvent loss, lower production costs, and increase economic benefits; the DMF recovery is facilitated, the cost for raw materials is saved, the influence of DMF on the environment is reduced, and the ecological environment is preserved.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
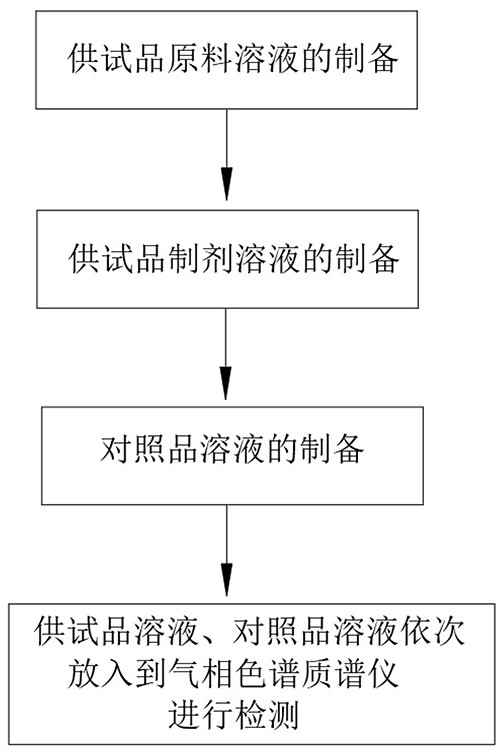
Intelligent detection method for trichloromethyl carbonate residues in clindamycin hydrochloride
ActiveCN113252816AImprove leveling efficiencyQuality improvementComponent separationTest samplePhysical chemistry
The invention relates to the technical field of intelligent measurement and control of trichloromethyl carbonate residues, in particular to an intelligent detection method for trichloromethyl carbonate residues in clindamycin hydrochloride. The method is completed by adopting a gas chromatography-mass spectrometer convenient for automatically leveling a column head of a chromatographic column in a matching manner. The detection method comprises the following steps: 1) preparing a raw material solution of a test sample; 2) preparing a test sample preparation solution; 3) preparing a reference substance solution; and 4) respectively and independently putting the prepared test sample raw material solution, the test sample preparation solution and the reference substance solution into a gas chromatography-mass spectrometer for detection. According to the invention, through the arranged cutting and leveling structure, the chromatographic column head can be automatically cut and leveled when the chromatographic column head of the chromatographic mass spectrometer body is replaced, and compared with manual leveling, the leveling efficiency and the leveling quality are greatly improved.
Owner:广州国标检验检测有限公司
Specific clindamycin hydrochloride superfine powdered lyophilized preparation and preparation method thereof
InactiveCN104138358AHigh clarityImprove stabilityAntibacterial agentsPowder deliveryAllergy preventionClindamycinum
The invention discloses a specific clindamycin hydrochloride superfine powdered lyophilized preparation and a preparation method thereof. The preparation method uses clindamycin hydrochloride and triphosgene as raw materials, and comprises the following steps: performing chlorination reaction in the system of trichloromethane, N,N-dimethylformamide and antioxidant; hydrolyzing and alcoholizing to obtain clindamycin hydrochloride ethylate; decoloring and dealcoholizing to obtain high-purity clindamycin hydrochloride; and performing air-jet superfine crushing, and lyophilizing to obtain the specific clindamycin hydrochloride superfine powdered lyophilized preparation. The specific clindamycin hydrochloride superfine powdered lyophilized preparation has the advantages of good clarity, high stability, high purity, few impurities, small particles, large specific surface area, good solubility, small toxic and side effect, allergy prevention and the like.
Owner:浙江磐谷药源有限公司
Stable Fixed Dose Topical Formulation
The present invention relates to stable fixed dose topical formulations comprising an antiacne agent and an antibiotic, which exhibit storage stability at a temperature of about 40° C. and relative humidity of about 75% for a period of at least 3 months. Particularly, the present invention relates to stable fixed dose topical formulations comprising therapeutically effective amounts of (a) adapalene-containing microspheres and (b) clindamycin, a process for their preparation thereof and their use for the treatment of acne.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Antibacterial active dressing and preparation method thereof
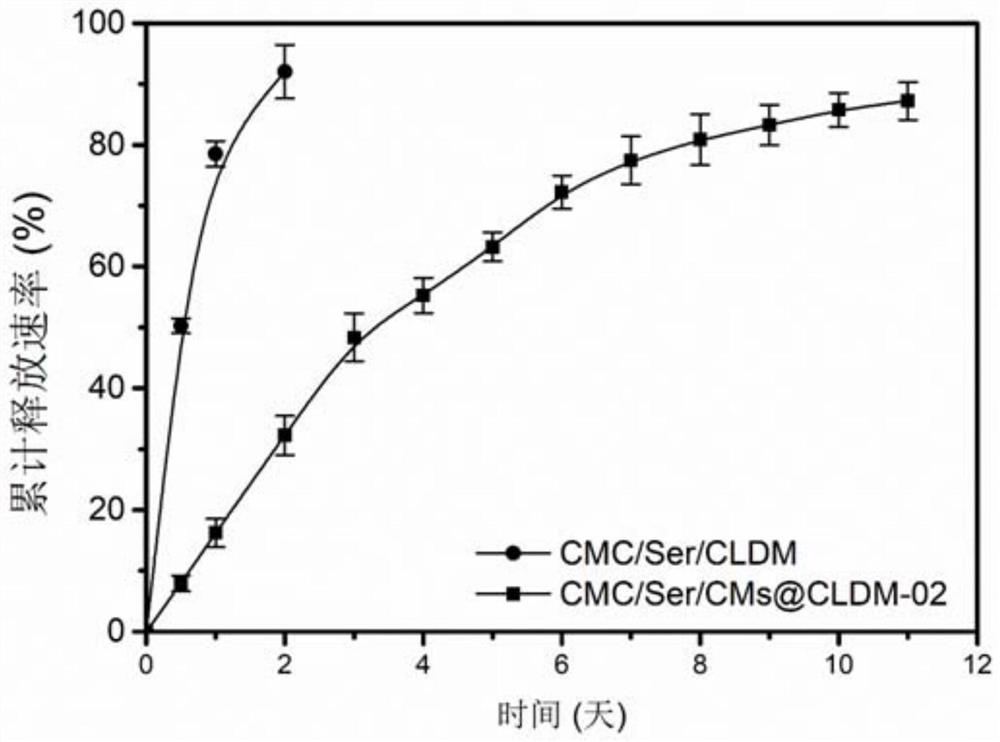
InactiveCN111686297AImprove mechanical propertiesGood biocompatibilityAbsorbent padsMicrocapsulesCarboxymethyl celluloseCell adhesion
The invention relates to an antibacterial active dressing. The dressing is prepared from the following components in percentage by mass: 1%-4% of sodium carboxymethyl cellulose, 0.2%-2% of sericin, 0.01%-0.2% of clindamycin-containing carboxymethyl cellulose microspheres and the balance of deionized water. Clindamycin is loaded on carboxymethyl cellulose microspheres (CMs) to obtain the carboxymethyl cellulose microspheres (CMs@CLDM) with the uniform particle size so as to develop synergistically effective antibacterial microspheres, so that the addition amount of antibiotics is reduced underthe condition of not reducing the antibacterial property, and meanwhile the biocompatibility of materials is improved; and based on a sericin / carboxymethyl cellulose dressing, the easy-to-degrade performance of a pure carboxymethyl cellulose (CMC) dressing can be improved, and the water stability and cell adhesion performance of the CMC dressing are improved by adding the sericin.
Owner:GUANGZHOU CHUANGSAI BIOLOGICAL MEDICAL MATERIALS CO LTD
Methods and materials for reducing the risk of infections
ActiveUS20160022722A1Reduce infectionReduce riskAntibacterial agentsBiocideClindamycinumInfection risks
This document relates to methods and materials for reducing the risk of infection after a shoulder surgery or medical procedure. For example, this document relates to methods and materials for using a topical composition containing clindamycin or erythromycin to reduce the risk of or to prevent infection associated with shoulder surgeries or medical procedures.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Medicine and preparation for treating allergic rhinitis and preparation method of same
InactiveCN108096308AQuick fixEliminate allergy symptomsHydroxy compound active ingredientsMammal material medical ingredientsWestern medicineAllergy
The invention relates to a medicine and a preparation for treating allergic rhinitis and a preparation method of same. The medicine includes Western medicines and traditional Chinese medicines, wherein the Western medicines include, by weight, 38-42 parts of clindamycin and 27-33 parts of lidocaine, and the traditional Chinese medicines include, by weight, 50-100 parts of storax, 15-30 parts of borneol, 10-30 parts of artificial musk, and 50-80 parts of centipeda minima. Through combination of the Western medicines and traditional Chinese medicines, damaged nasal mucosa can be rapidly repairedand allergy symptoms are eliminated. The medicine is convenient to use, has reliable and significant curative effects, is good in safety, and is suitable for adults and childrens.
Owner:袁影丽
Lincosamides universal monoclonal antibody hybridoma cell strain and application thereof
ActiveCN108165532AStable secretionHigh affinityBiological material analysisMicroorganism based processesBALB/cBovine serum albumin
The invention relates to a lincosamides universal monoclonal antibody hybridoma cell strain and an application thereof, which belong to the technical field of food safety immunology detection. The lincosamides universal monoclonal antibody hybridoma cell strain employs a lincosamide substituted derivative as semiantigen, semiantigen is coupled with bovine serum albumin (BSA) through an activated ester method to obtain immunization antigen, the immunization antigen and a freund's adjuvant are uniformly mixed, a mixture is used for immunization BALB / c mice through hypodermic injection, clindamycin is coupled with albumin from chicken egg white (OVA) by employing a carbonyl diimidazole (CDI) method, and the material is used for detecting mice serum and cell supernatant as coating antigen, thespleen cell of the immunization mice is subjected to fusion with mice myeloma cells through a PEG method, and through indirect ELISA and indirect competition ELISA screening and three-time subclone,the group selected hybridoma cell strain can be obtained. The cell has good inhibition effect for clindamycin, lincomycin and pirlimycin, and can satisfy the requirement on lincosamides stumpy immunodetection products on the market.
Owner:JIANGNAN UNIV +1
Pharmaceutical composition of compound clindamycin and tazarotene lipid complex
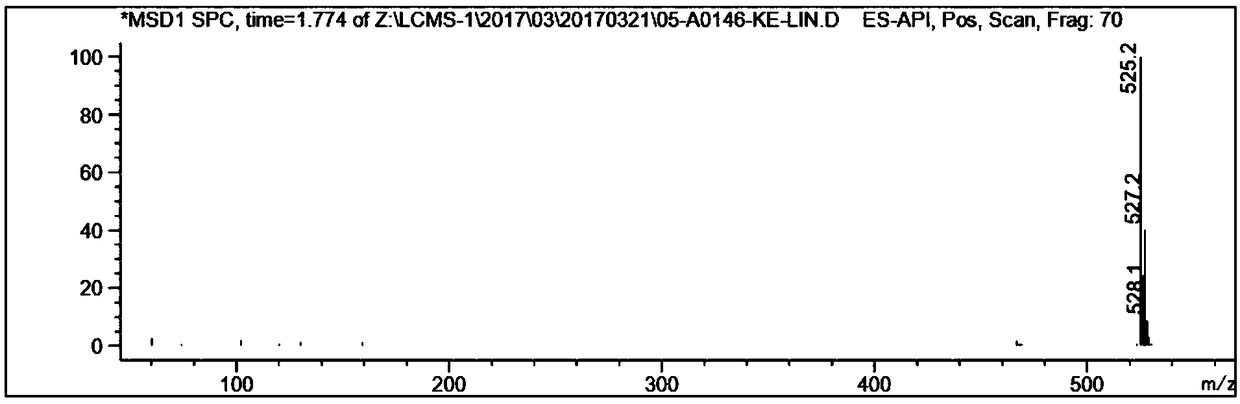
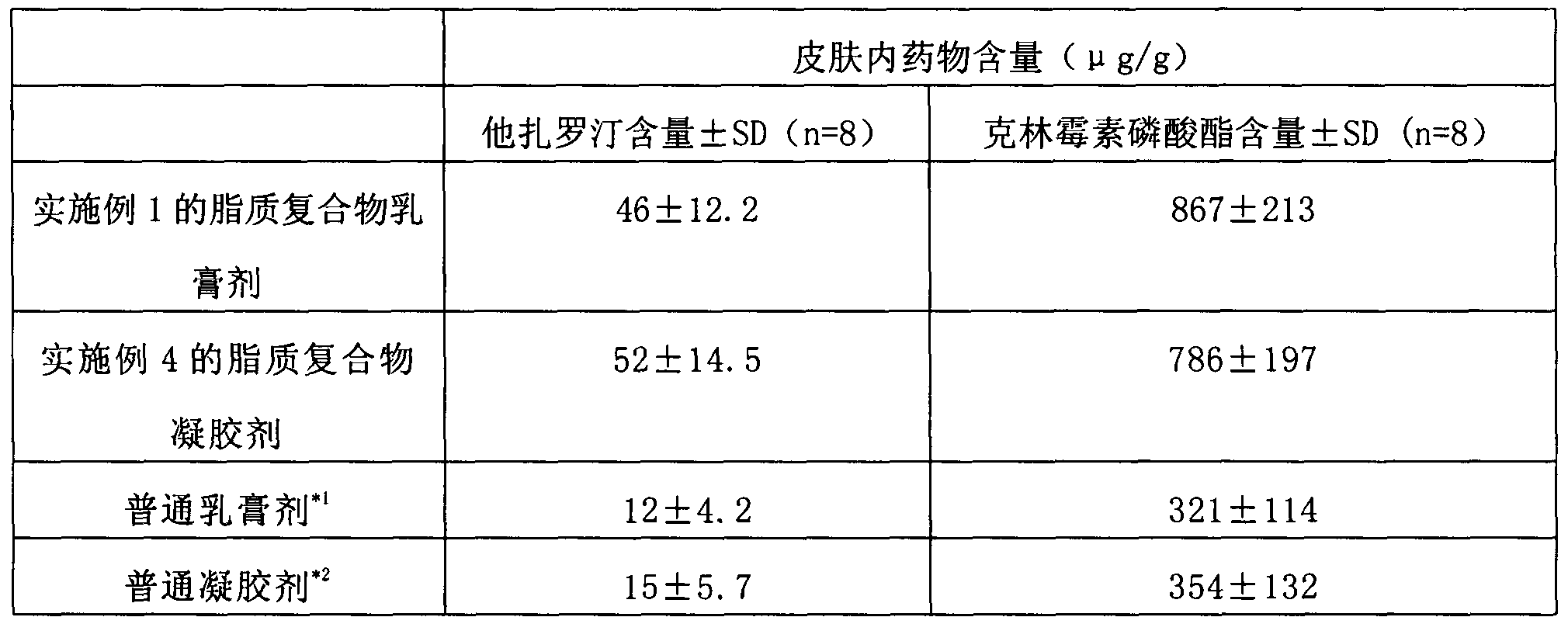
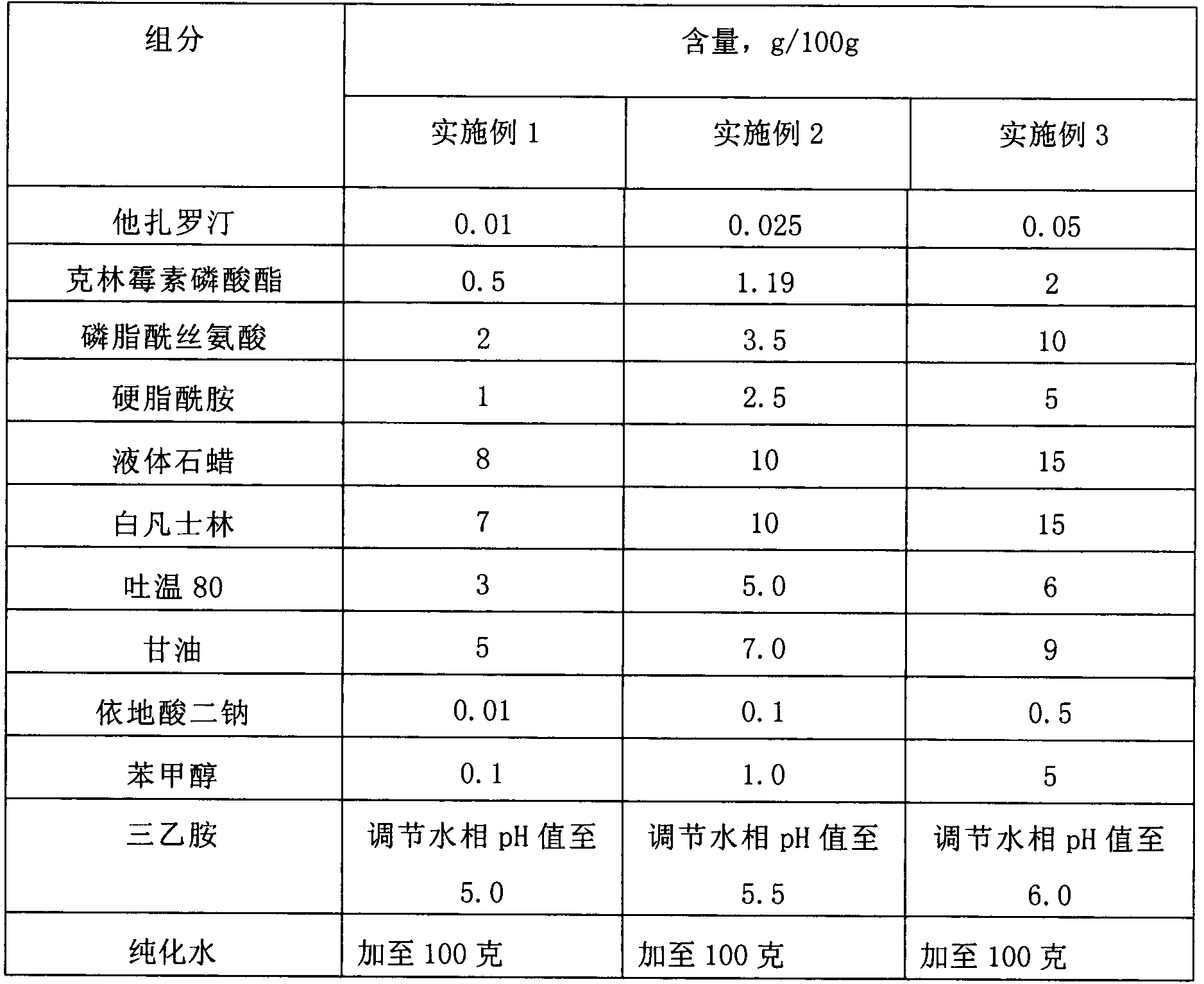
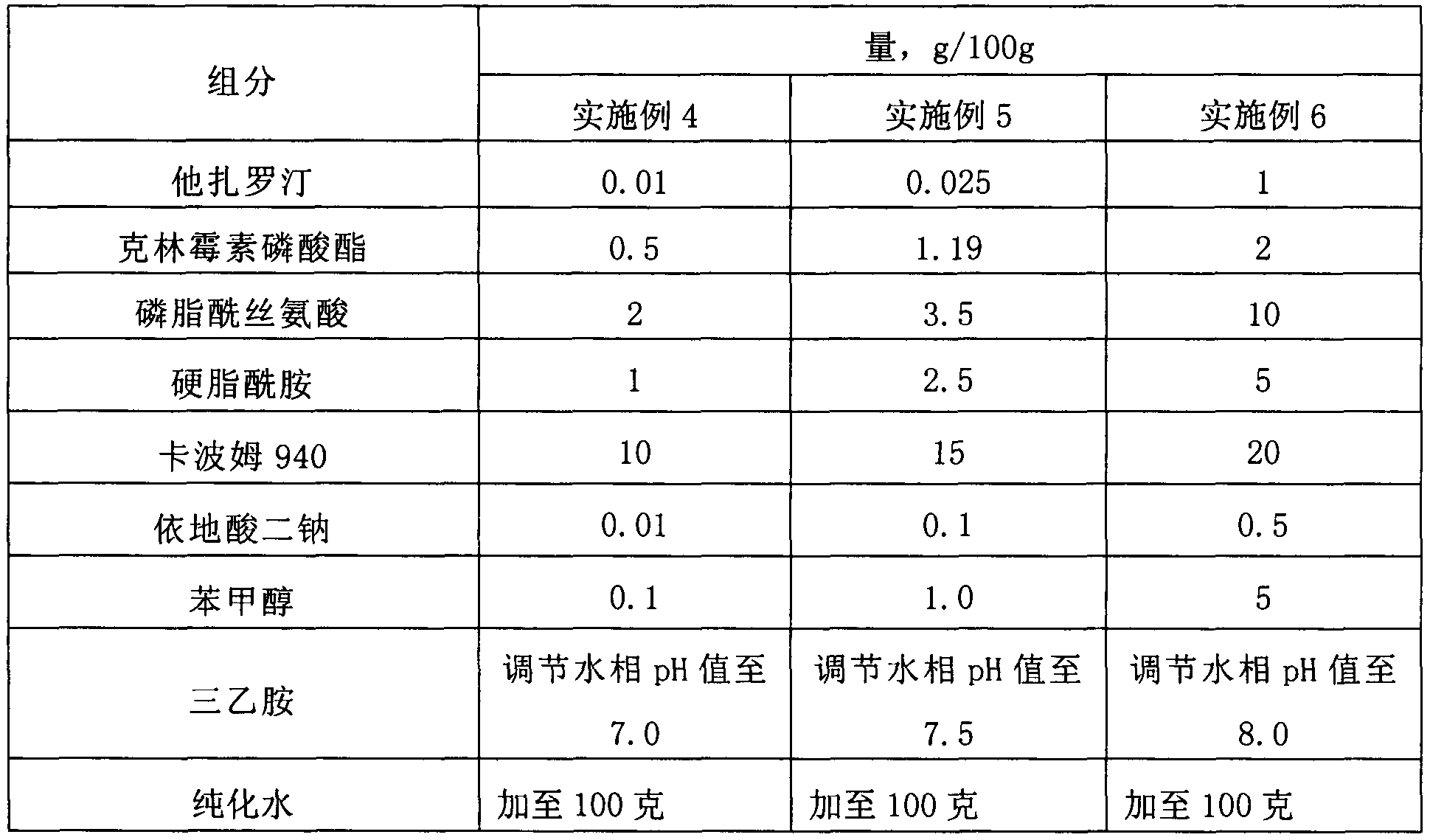
ActiveCN103405781AImprove retentionLong onset timeOrganic active ingredientsAerosol deliveryClindamycin PhosphatePhospholipid
The invention provides a lipid complex of tazarotene and clindamycin phosphate. The lipid complex is prepared mainly by utilizing synthetic phospholipids and stearamide, is wrapped with tazarotene and is further mixed with the clindamycin to prepare cream and gel which are mainly used for treating acne vulgaris. The lipid complex is simple in preparation technique, and the stability of pharmaceuticals can be ensured in the preparation process; compared with the common cream and gel, the lipid complex has the advantages that the skin medicine retention volume is higher, the content of medicine in local skins can be increased, and the therapeutic index can be improved.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Method for treatment of acne using pharmaceutical compositions of clindamycin and adapalene
InactiveUS9636353B2Reduce in quantityReduce incidenceBiocidePharmaceutical delivery mechanismAcne medicationsSkin color
The present invention relates to a method for treating, reducing or preventing acne. In particular, the present invention relates to methods for reducing the total number, incidence and severity of acne lesions on the skin which includes both inflammatory and non-inflammatory lesions. Further, the invention relates to reducing the incidence and severity of adverse events resulting from topical application of anti-acne agents resulting in improvement of skin tone. The method includes administering a novel and stable topical anti-acne pharmaceutical composition.
Owner:CADILA HEALTHCARE LTD
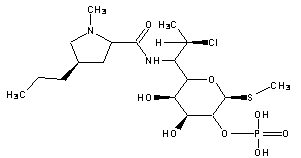
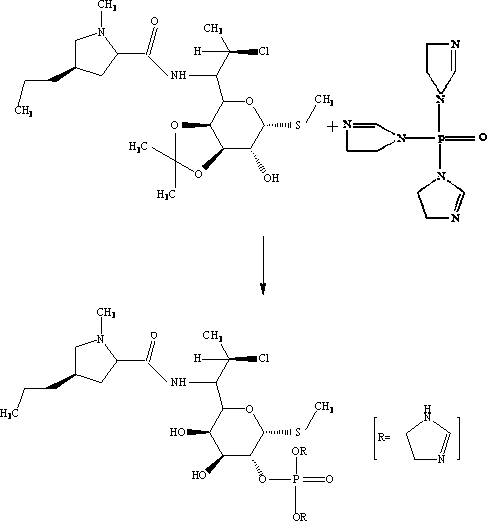
A kind of synthetic method of clindamycin phosphate
ActiveCN103483399BEliminate potential safety hazardsReduce generationSugar derivativesSugar derivatives preparationChemical synthesisKetonic acids
The invention belongs to the technical field of chemical synthesis and specifically relates to a synthetic method of clindamycin phosphate. The method comprises the following steps of synthesis of clindamycin free alkali, ketonization reaction, phosphorylation reaction and aftertreatment. According to the invention, the weight yield of the clindamycin phosphate can reach over 80% by changing a charging sequence, steps and a catalyst.
Owner:TOPFOND PHARMA CO LTD
Composition for topical treatment of mixed vaginal infections
Owner:DRAGTEK CORP
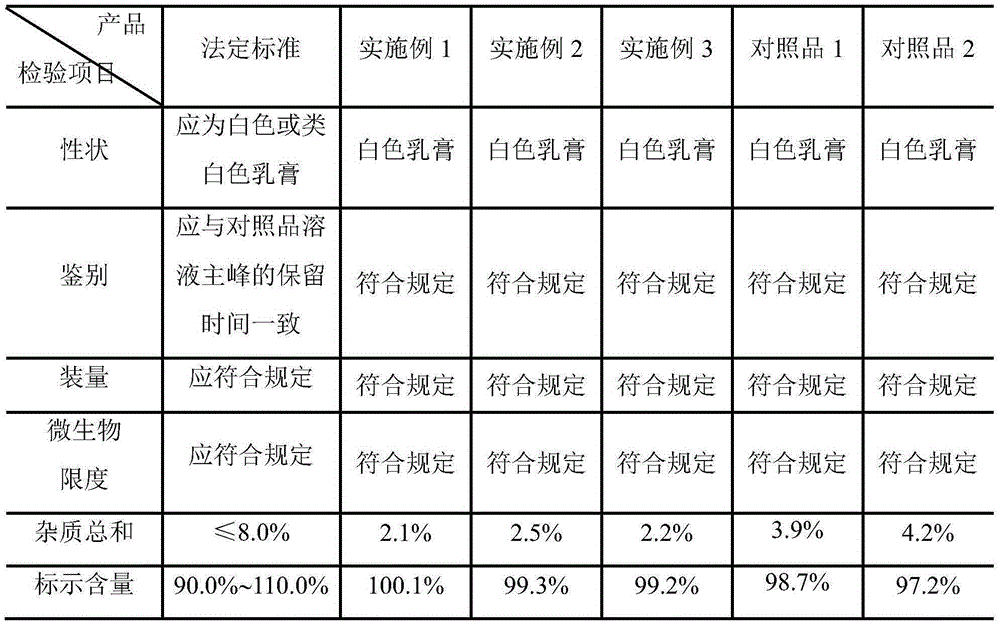
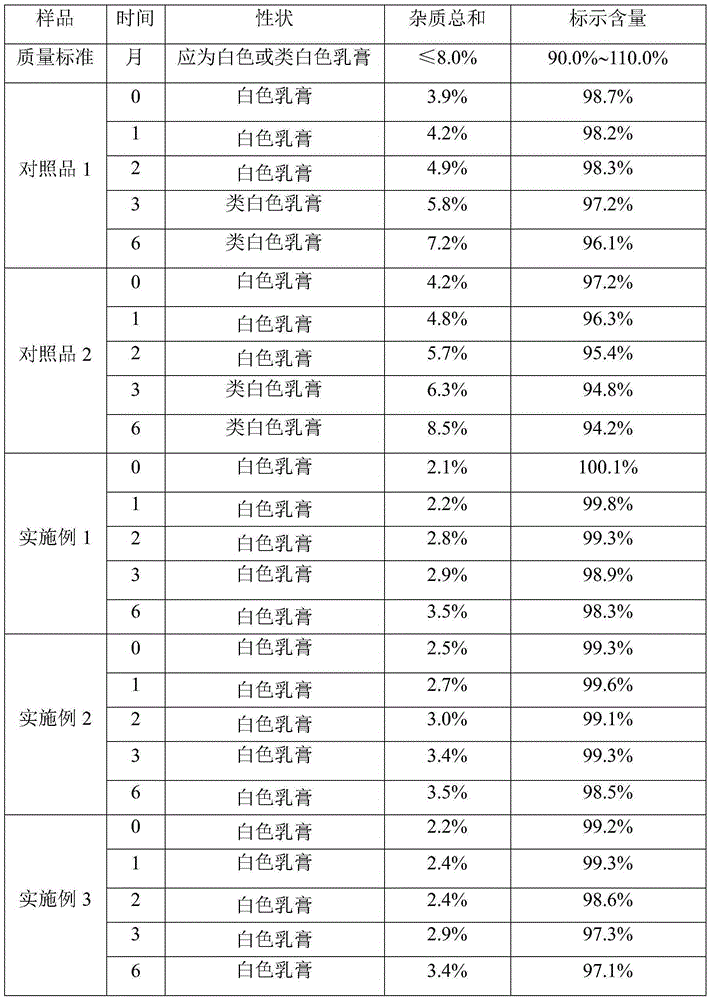
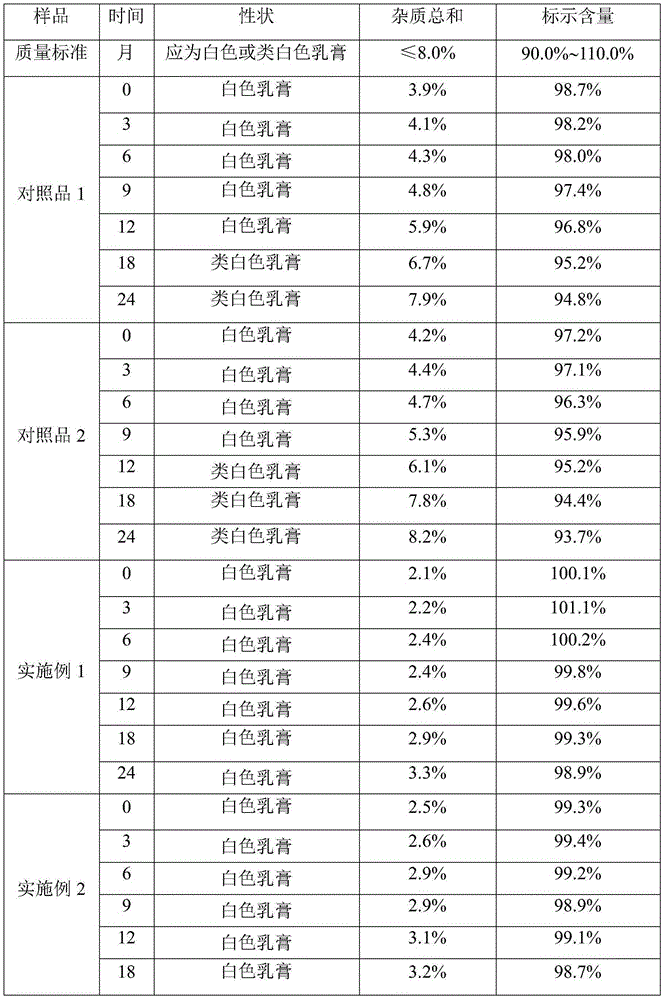
Impurity and quality detection method of clindamycin hydrochloride capsules
PendingCN112379021AHigh sensitivityMeet analysis requirementsComponent separationPhysical chemistryClindamycinum
The invention discloses an impurity and quality detection method of clindamycin hydrochloride capsules. According to the method, related substances, dissolution rate and content in the clindamycin hydrochloride capsule are measured, and impurities such as lincomycin, 7-epilincomycin, clindamycin B, dehydroclindamycin, 7-epilincomycin, clindamycin isomers and the like can be detected. Methodological verification is carried out on related substances, including quantification limit, detection limit, accuracy and durability. Results show that the method is high in sensitivity and can meet analysisrequirements.
Owner:乐泰药业有限公司
Clindamycin hydrochloride emulsifiable paste and preparation method thereof
ActiveCN105395473AQuality improvementAvoid hydrolysisAntibacterial agentsOrganic active ingredientsOil phaseButylated hydroxytoluene
The invention discloses a clindamycin hydrochloride emulsifiable paste and a preparation method thereof. The clindamycin hydrochloride emulsifiable paste is composed of clindamycin hydrochloride used as an active component and a pharmaceutical adjuvant. The pharmaceutical adjuvant comprises an oil-phase matrix, a hygroscopic agent, an emulsifier, an antiseptic, essence a pH value conditioning agent and purified water and is characterized in that the oil-phase matrix is a mixture of cetanol, lightweight liquid paraffin and albolene, the hygroscopic agent is propylene glycol, the emulsifier is Span-60, and the antiseptic is a mixture of ethylparaben, potassium sorbate and 2,6-di-t-butyl p-cresol. According to the invention, clindamycin hydrochloride is dispersed in inertial albolene during preparation of the emulsifiable paste, albolene is used for clathration of the raw material clindamycin hydrochloride, so hydrolysis of the raw material is avoided; moreover, the pH value of the prepared emulsifiable paste maintains 3.0 to 4.5, which enables the hydrolysis process of clindamycin to be effectively inhibited.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Stable fixed dose topical formulation
The present invention relates to stable fixed dose topical formulations comprising an antiacne agent and an antibiotic, which exhibit storage stability at a temperature of about 40° C. and relative humidity of about 75% for a period of at least 3 months. Particularly, the present invention relates to stable fixed dose topical formulations comprising therapeutically effective amounts of (a) adapalene-containing microspheres and (b) clindamycin, a process for their preparation thereof and their use for the treatment of acne.
Owner:GLENMARK PHARMACEUTICALS LTD
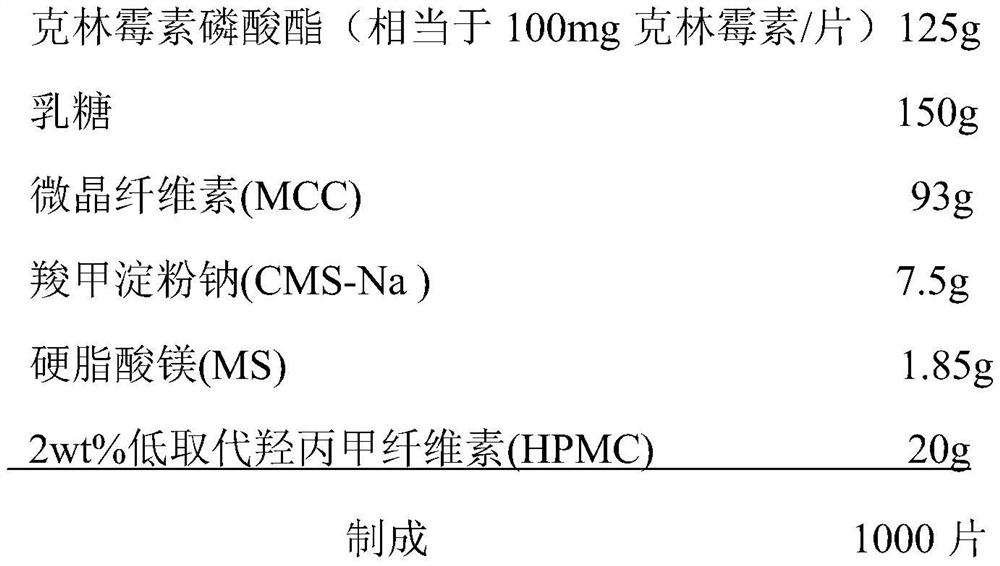
Clindamycin phosphate vaginal tablets and preparation process thereof
ActiveCN112569196AGood swelling rateImprove adhesionAntibacterial agentsOrganic active ingredientsMagnesium stearateStearic acid
The invention provides a clindamycin phosphate vaginal tablet and a preparation process thereof. The clindamycin phosphate vaginal tablet is prepared from the following raw materials in parts by weight: 125 parts of clindamycin phosphate, 140-160 parts of lactose, 90-95 parts of microcrystalline cellulose, 7-8 parts of carboxymethyl starch sodium, 1.5-2.0 parts of magnesium stearate and 10-20 parts of a 1.8-2.2 wt% hydroxypropyl methylcellulose aqueous solution. The clindamycin phosphate vaginal tablet prepared by the preparation method disclosed by the invention is good in swelling rate and relatively large in adhesive force, better exerts the drug effect of clindamycin, shortens the drug use time and improves the use compliance of a patient.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
Clindamycin isomer, analytical preparation method and application thereof
The invention provides a clindamycin isomer which has a structure shown in the formula II. The invention further provides an analytical preparation method of the clindamycin isomer. The method is characterized in that the clindamycin raw materials are analyzed and the clindamycin isomer is separated and prepared from the raw materials. The method comprises the following steps of: a) determining the clindamycin raw materials by the LC-MS (liquid chromatography-mass spectrometry) method, and determining the clindamycin isomer in the raw materials according to relative retaining time and / or molecular weight of analyzed components; b) determining conditions of column chromatography according to the relative retaining time and / or molecular weight of the clindamycin isomer, obtained in the step a, and enriching analyzed components corresponding to the relative retaining time and / or molecular weight by positive phase silica gel column chromatography; and c) determining condition of the preparation liquid phase method according to chromatographic retention behavior displayed by the relative retaining time of the clindamycin isomer, obtained in the step a, and collecting the analyzed components corresponding to the relative retaining time by the liquid phase method.
Owner:SHANGHAI INST OF PHARMA IND +1
Application of a rifamycin-quinazinone dual target molecule
ActiveCN106902116BHas broad-spectrum antimicrobial activityActiveAntibacterial agentsOrganic active ingredientsDiseaseBacterial vaginosis
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com