Clindamycin isomer, analytical preparation method and application thereof
A technology of clindamycin and isomers, applied in the field of pharmaceutical analytical chemistry and analytical chemistry, can solve the problems of lack of understanding of importance, little consideration of the adverse effects of impurities on drug safety, and insufficient consideration of impurity limits, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] LC-MS Determination of Clindamycin API and Crude Products
[0079] Liquid-mass spectrometer: HPLC Waters 2486, MS Waters micromass ZQ 4000. Chromatographic column: Diamonsil C 18 (5 μ 250 × 4.6mm); mobile phase is acetonitrile-tetrahydrofuran-water-formic acid (18%: 3%: 79%: 0.2%), ammonia water adjusts the pH value to 5.45; column temperature is room temperature; detection Wavelength 210nm; flow rate 1.0mL / min, split into mass spectrometer. The mass spectrometry conditions are electrospray ionization source positive ion (ESI+) detection mode; source temperature 80°C; cone voltage 35v.
[0080] LC-MS detection of raw materials
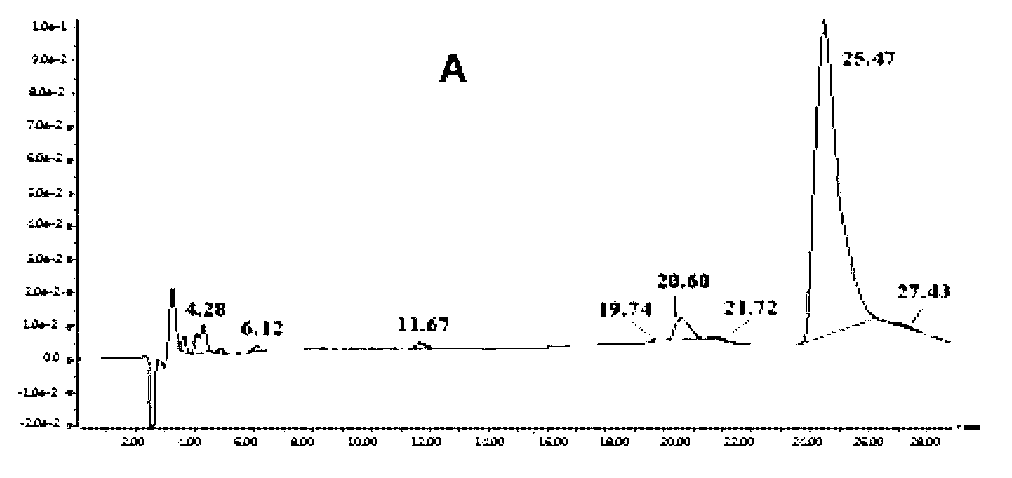
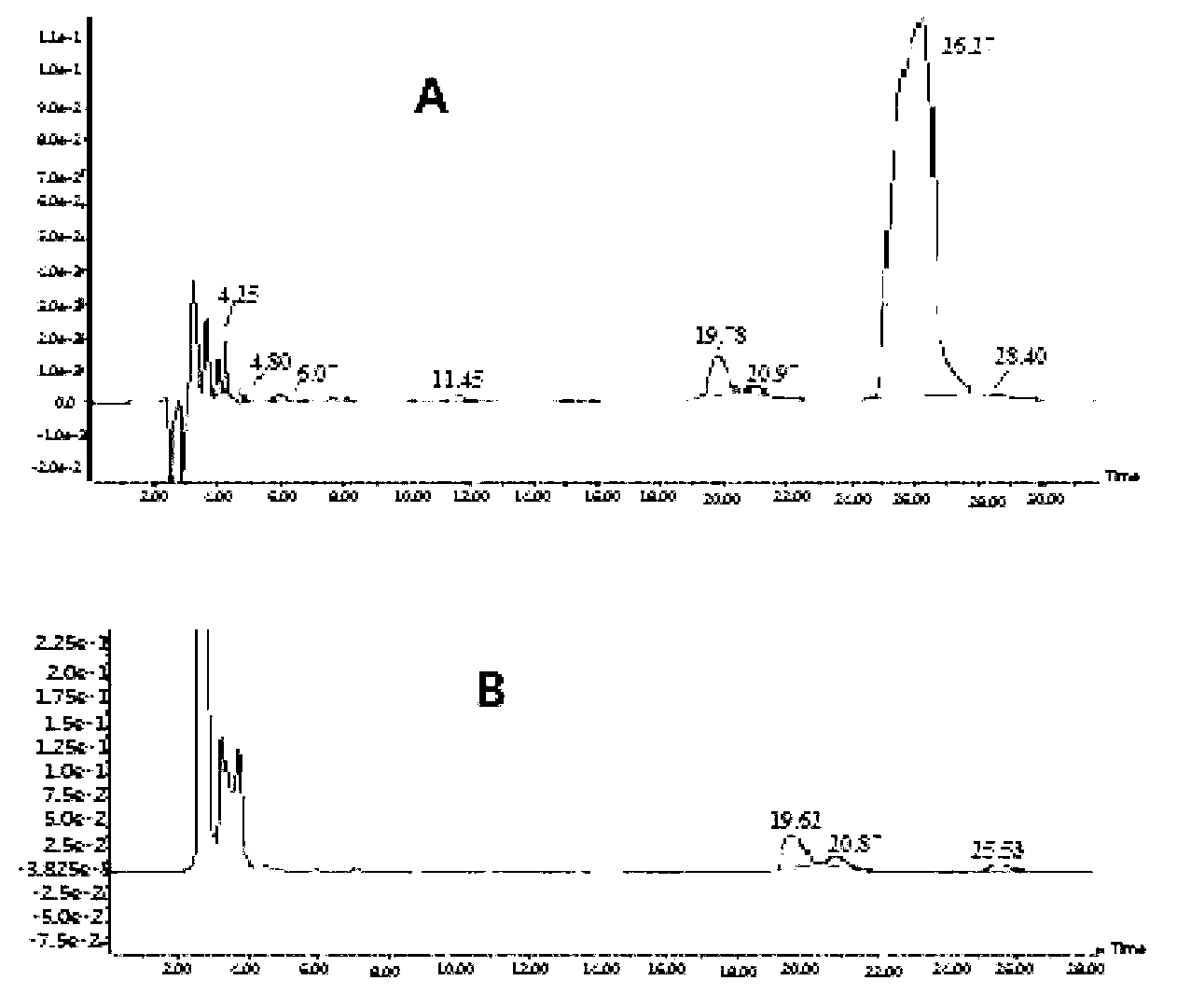
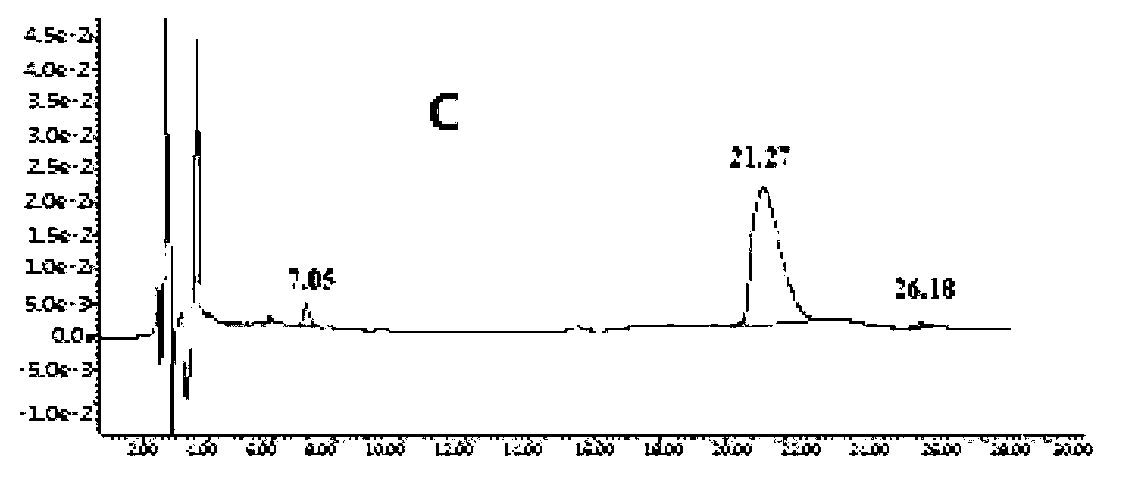
[0081] Take the mobile phase of the raw material medicine with batch number 090303×7 and dissolve it into a solution with a concentration of 2 mg / mL, and the injection volume is 20 μL. LC-MS detection results such as figure 1 shown.
[0082] Six related substances except clindamycin in clindamycin hydrochloride API were detected by liqu...
Embodiment 2
[0118] Structural identification of target impurity-clindamycin isomer shown in formula II
[0119] The obtained pure products of clindamycin isomers were determined by high-resolution mass spectrometry, and then determined by nuclear magnetic resonance hydrogen spectrum, carbon spectrum and two-dimensional spectrum DEPT, HMBC, HMQC, COZY and NOESY The structure of isomers. The instruments used are Micromass Q-TOF mass spectrometer and American Varian nuclear magnetic resonance spectrometer (400MHz). The following structural formula is the structure of clindamycin, and Table 4 is the data of clindamycin nuclear magnetic resonance spectrum.
[0120]
[0121] Table 4. Clindamycin 1 H-NMR spectrum, 13 C-NMR spectrum, HMQC spectrum, COZY spectrum,
[0122] NOESY spectral attribution
[0123]
[0124] There are four chiral centers in the structure of clindamycin, and their configurations are 6S, 7S, 1’S, 3’R.
[0125] Impurity 2-Structure Identification of Clindamyc...
Embodiment 3
[0141] Antibacterial experiment of clindamycin hydrochloride and impurity 2 of the present invention
[0142] The tests used to determine the effectiveness of antibacterial drugs in inhibiting bacterial growth in vitro are called bacteriostatic tests. In this experiment, the antibacterial activity of clindamycin isomers with an apparent content of more than 0.1% in clindamycin hydrochloride raw materials was investigated. Antibacterial activity control of raw materials.
[0143] Preparation of the test solution
[0144] Clindamycin hydrochloride (090303×7 batches, Zhejiang Tiantai Pharmaceutical Co., Ltd.): 1.091mg, dissolved in 1mL water;
[0145] Impurity 2: 1.200mg, dissolved in 0.5mL water;
[0146] Experimental strain
[0147] Staphylococcus aureus (Gram-positive bacteria), Bacillus subtilis (bacteria), Candida albicans (fungi); provided by the Department of Biology, Shanghai Pharmaceutical Industry Research Institute.
[0148] Preparation of culture medium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com