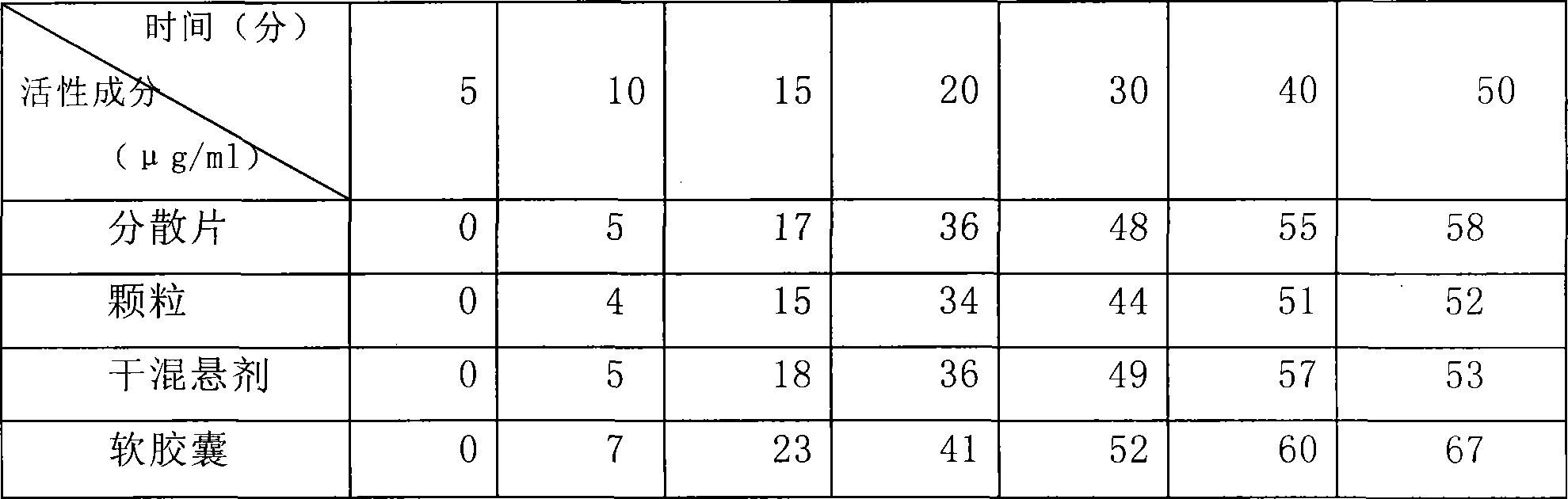
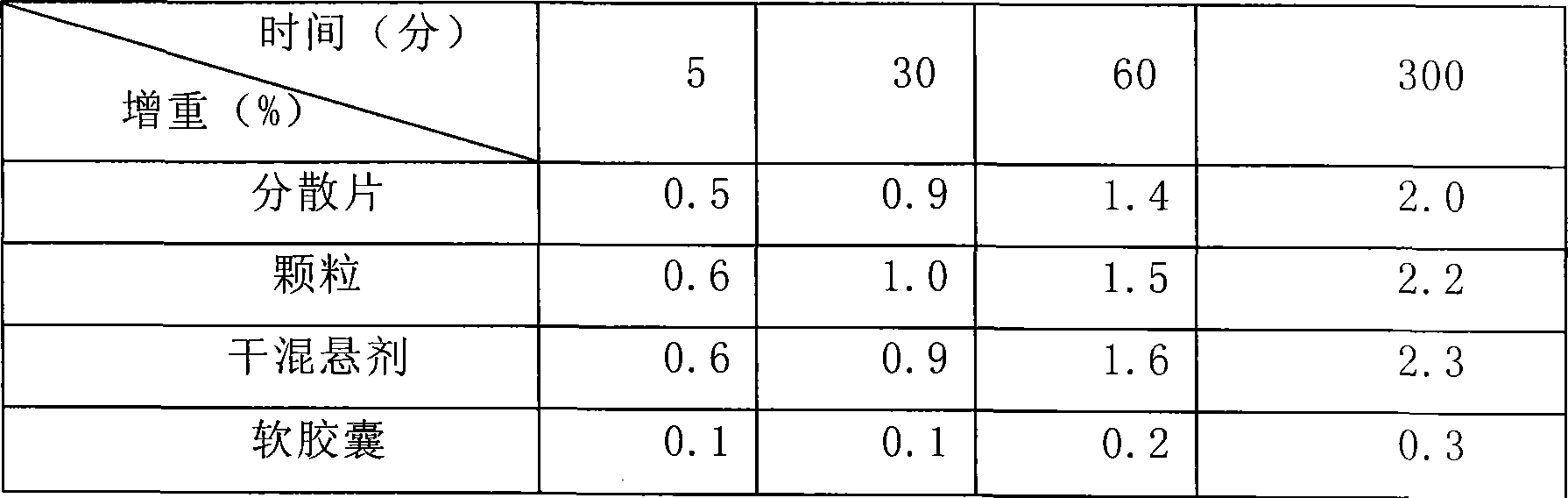
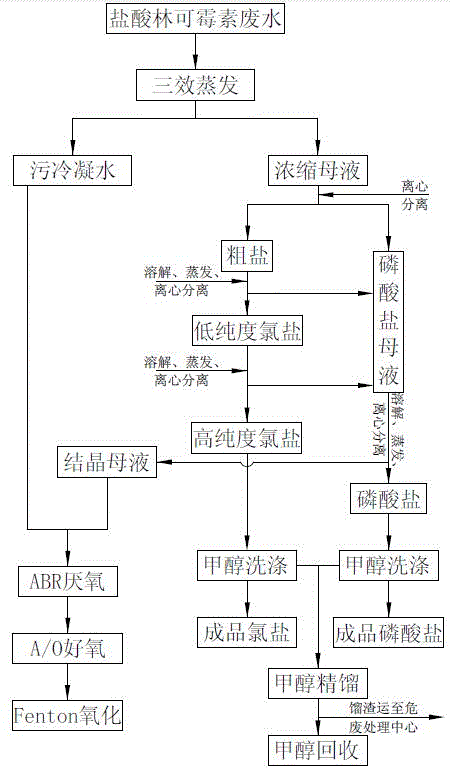
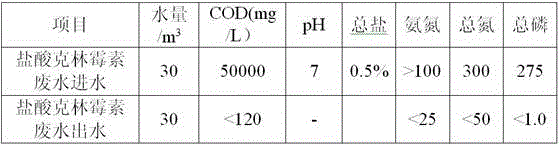
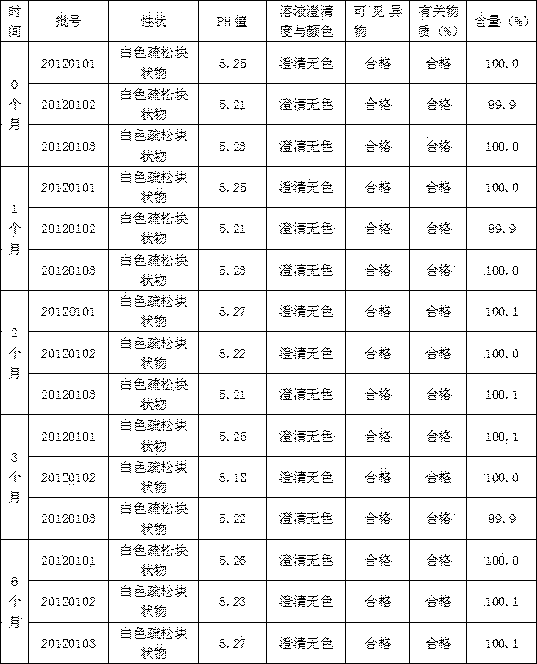
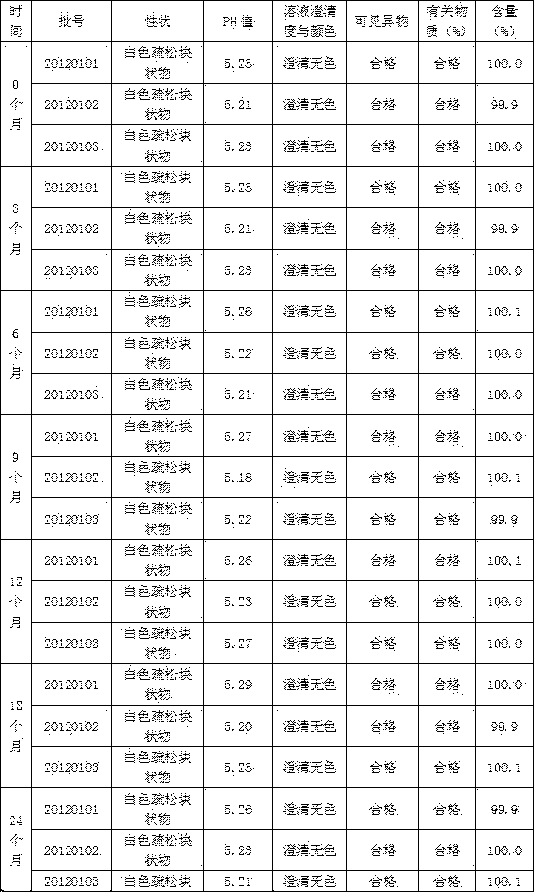
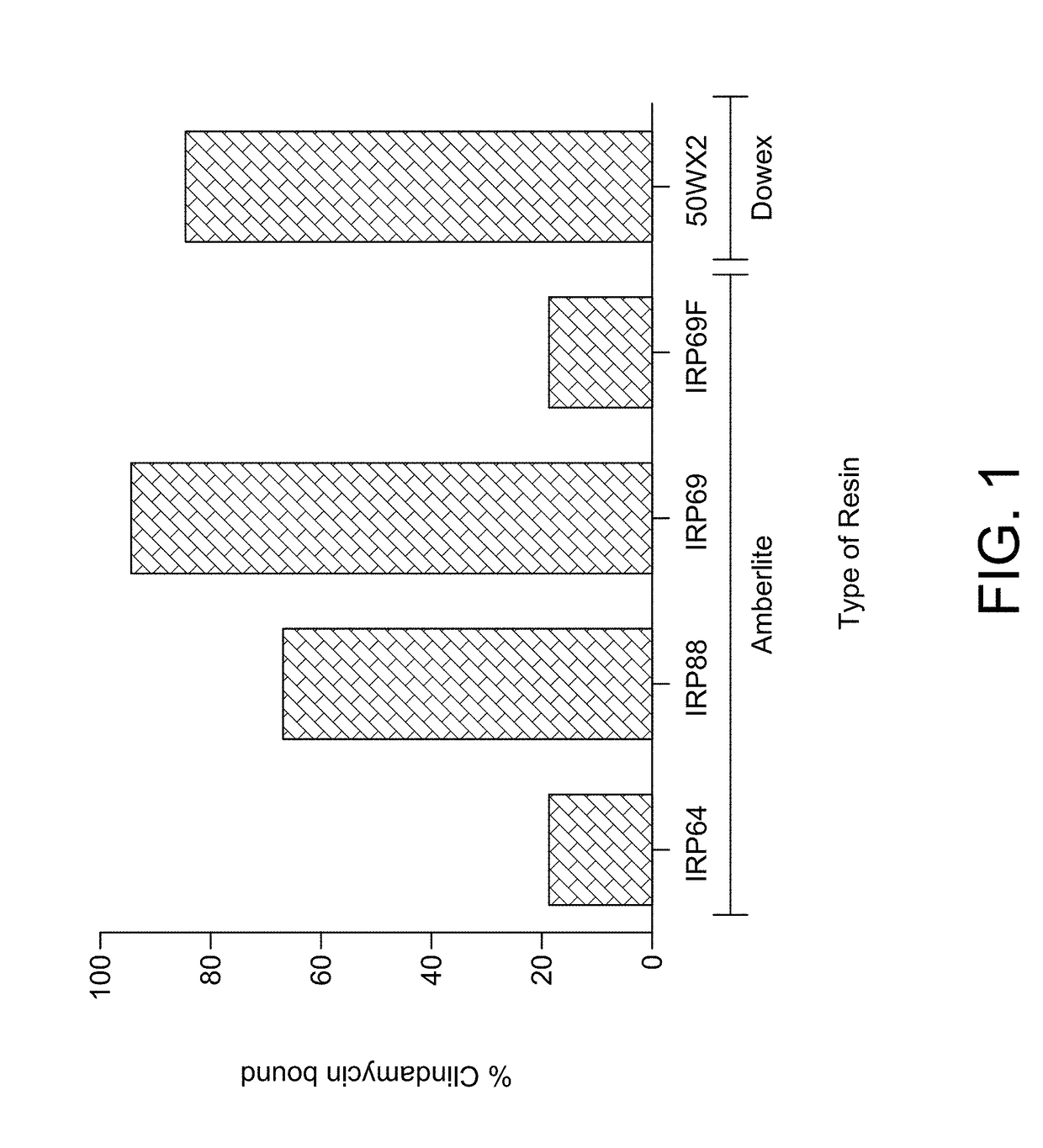
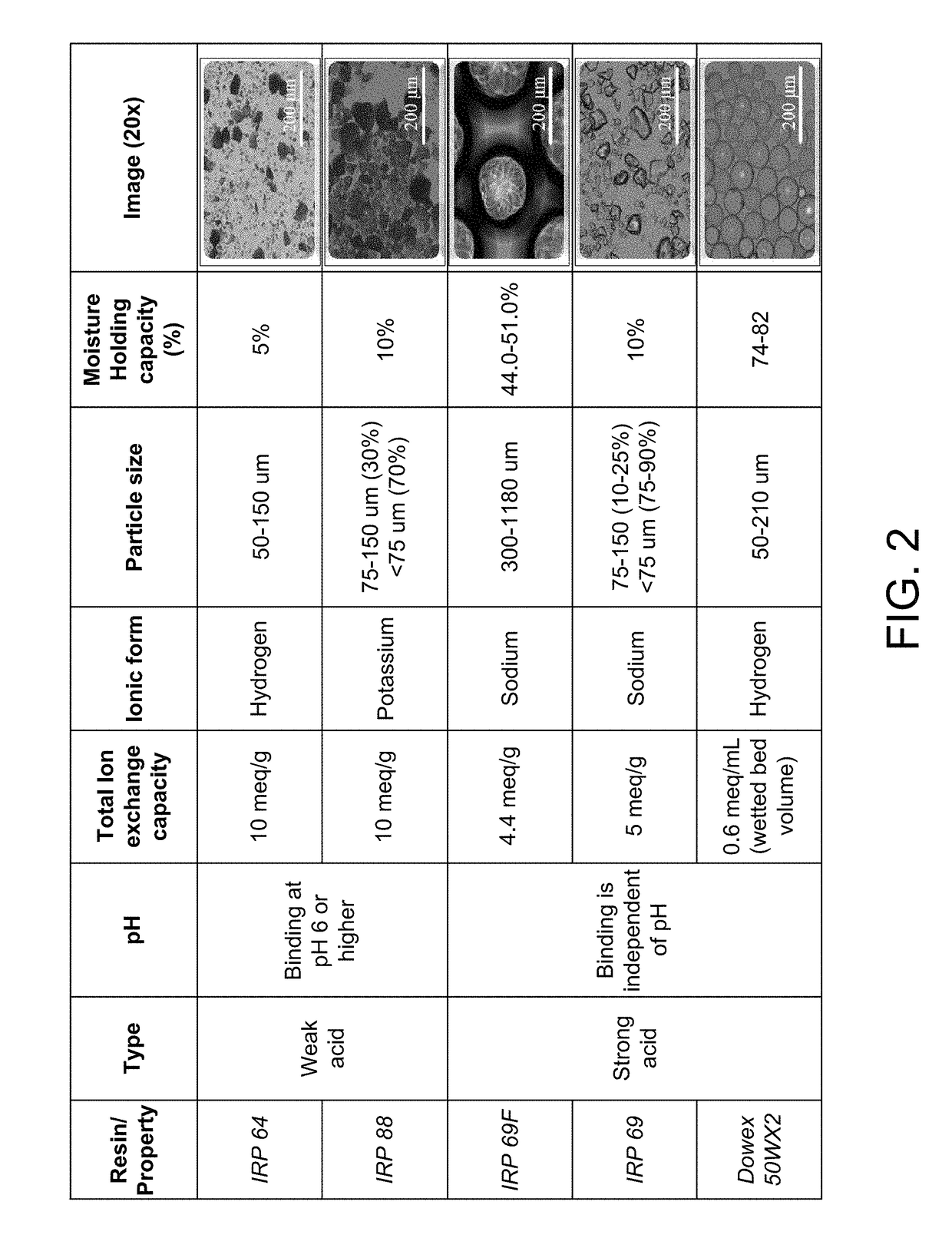
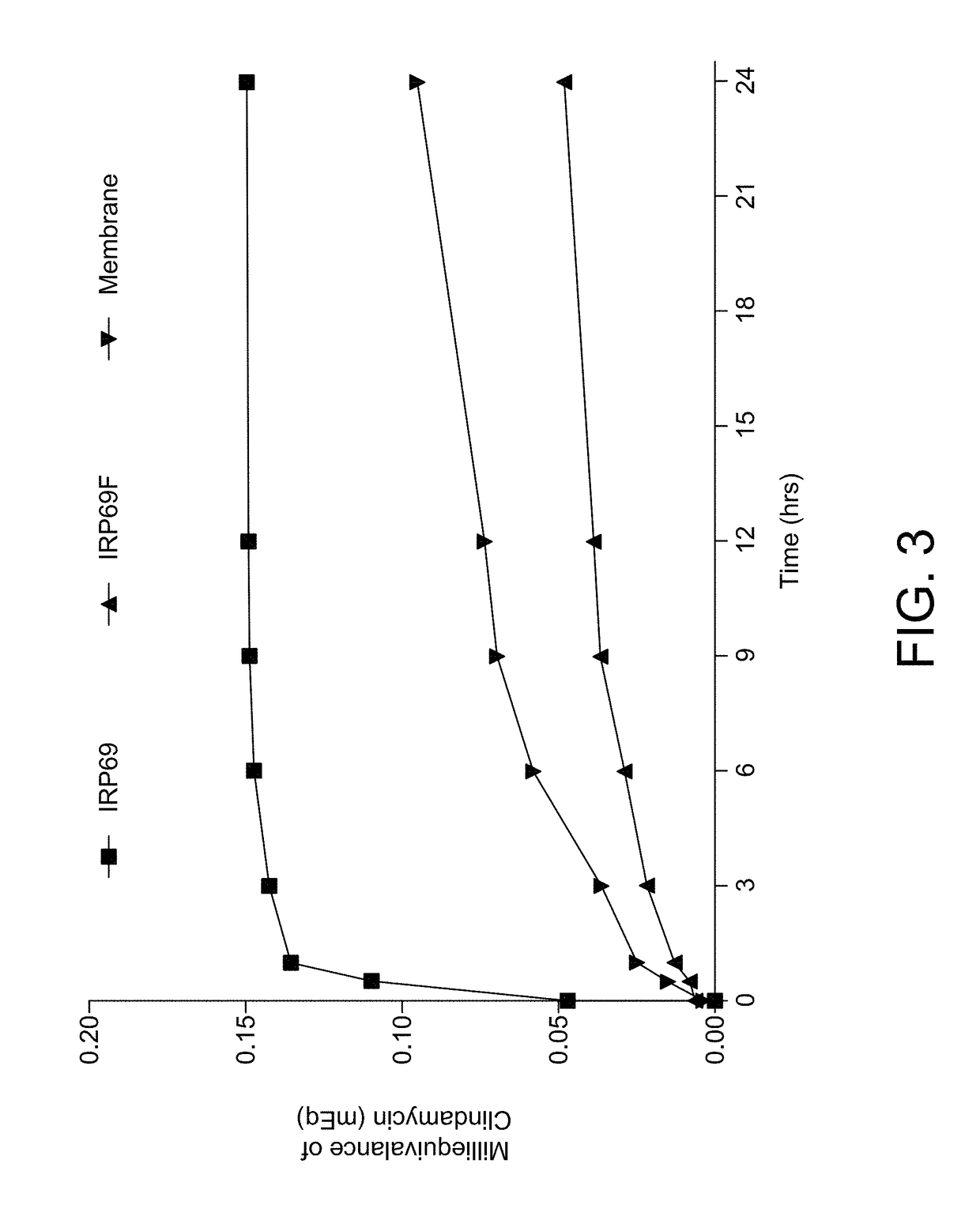
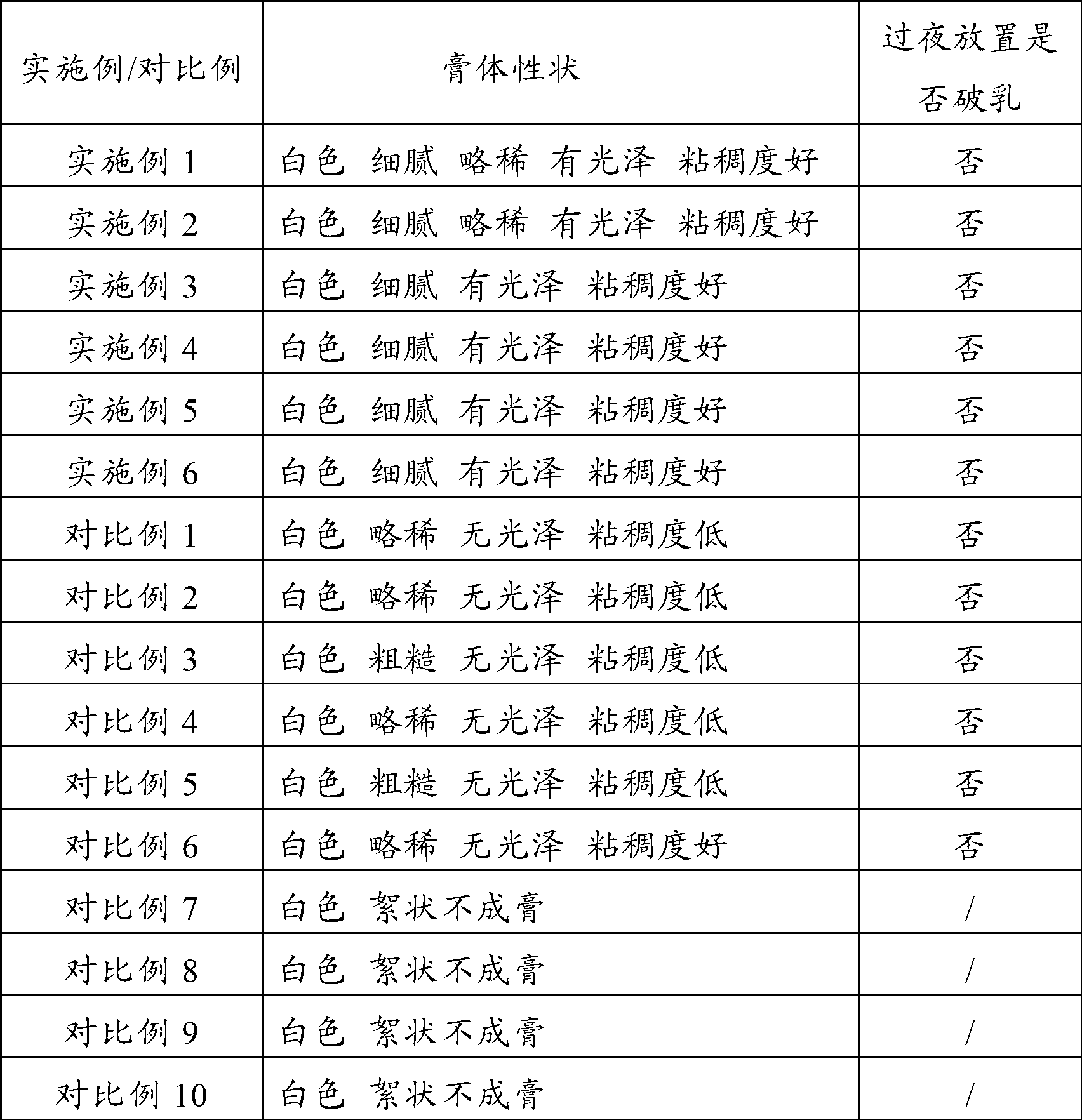
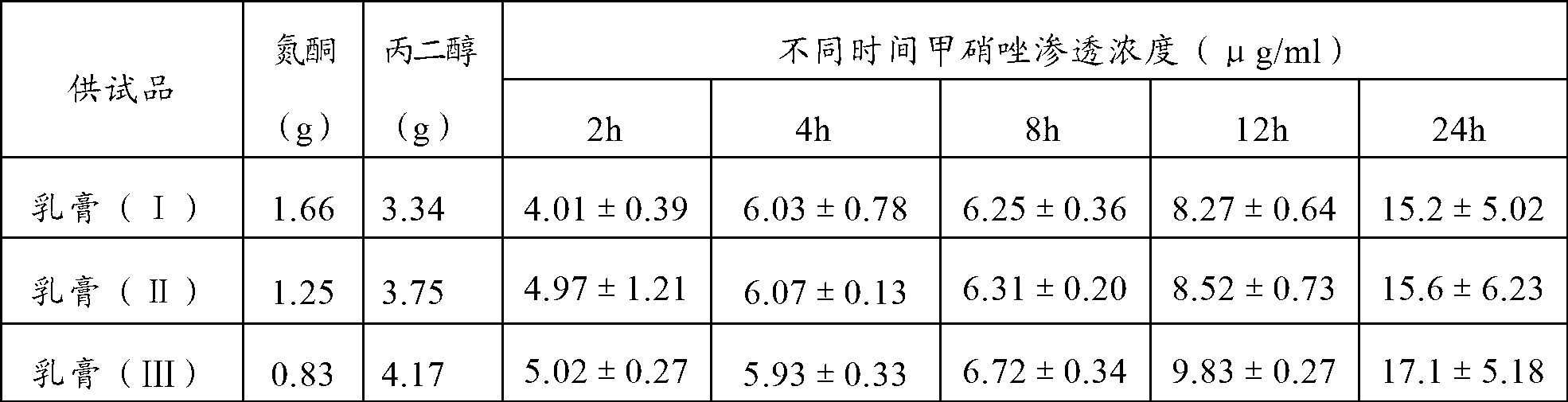
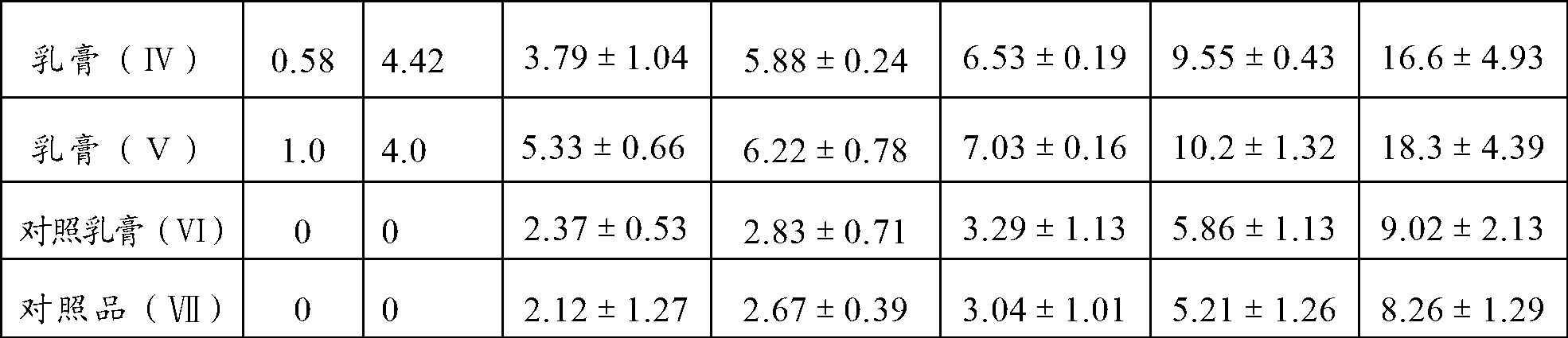Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Clindamycin Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
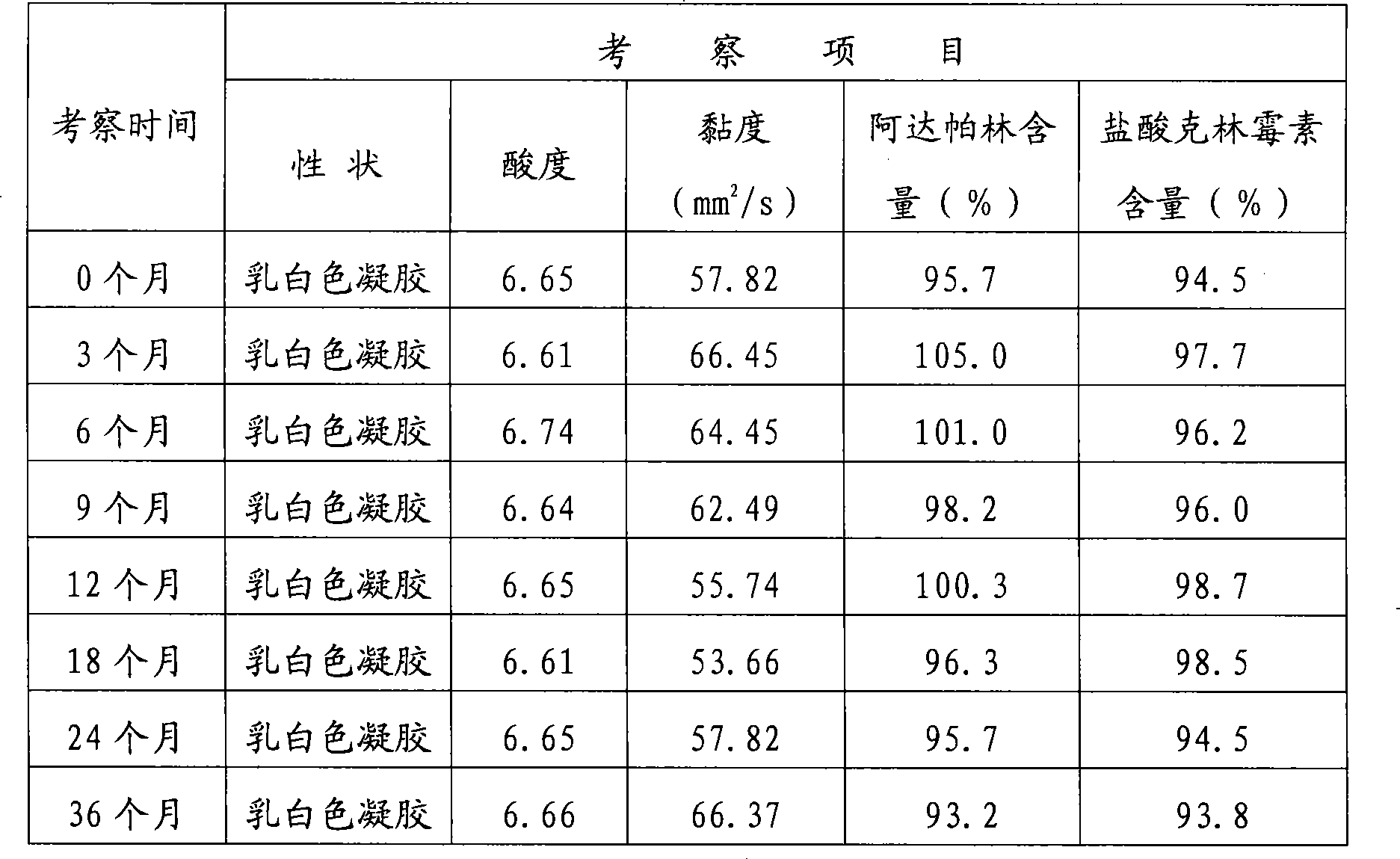
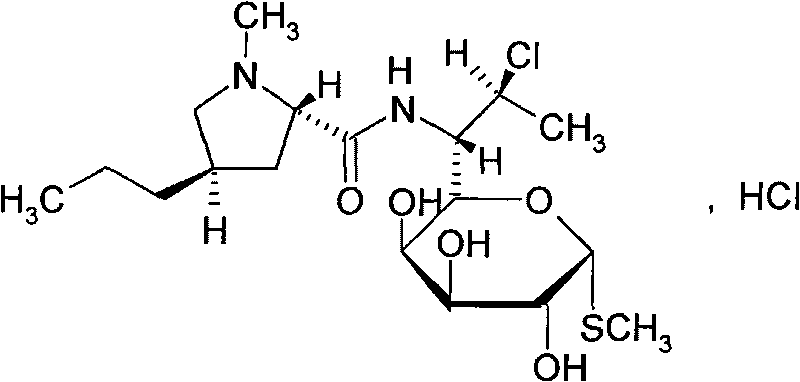
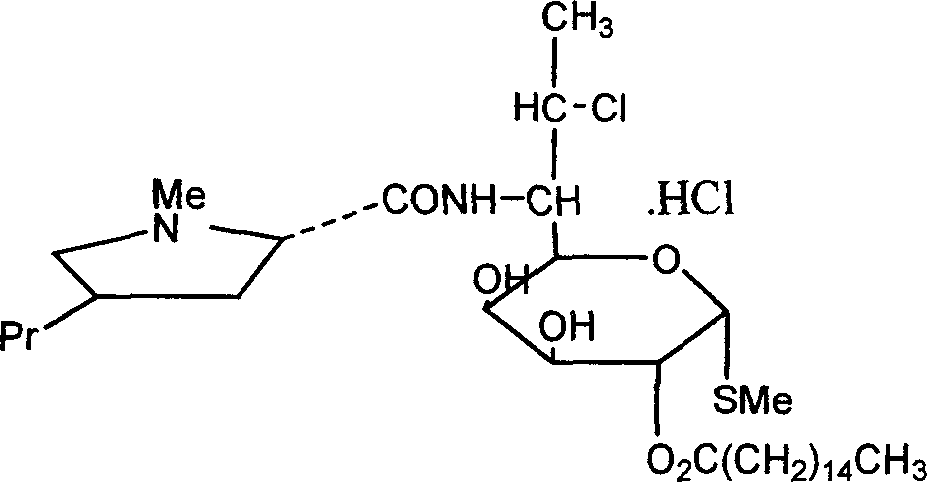
The hydrochloride salt form of clindamycin, a semi-synthetic, chlorinated broad spectrum antibiotic produced by chemical modification of lincomycin. Clindamycin hydrochloride is used as a solid in capsules.
Method for synthesizing clindamycin phosphate
InactiveCN101830946AImprove conversion rateIncrease contentSugar derivativesSugar derivatives preparationSolubilityHydrolysis
The invention discloses a method for synthesizing clindamycin phosphate, which comprises the following steps of: performing ketal protection reaction on clindamycin hydrochloride alcoholate at the temperature of between 2.0 below zero and 2.0 DEG C under the action of acetone and phosphorus oxychloride to form propylidene clindamycin; and performing esterification, hydrolysis, adsorption, washing, deabsorption, concentration, coarse crystallization, decoloration, refining and drying to obtain the finished product of clindamycin phosphate. Because a new catalyst 4-dimethylaminopyridine participates in the esterification in the rection system, the phosphorylating reaction is performed completely, and the conversion rate of raw materials is improved. Meanwhile, due to the secondary crystallization method, the problems of poor color grade and poor powder solubility are solved, and the operating conditions are mild and simple. By adopting triethylamine to replace partial pyridine, the esterification is pushed forwards; the reaction period is shortened; and importantly, related impurities in the finished product and the production cost are reduced, and the content is improved.
Owner:南阳普康药业有限公司 +1
Compound emulsifiable paste for treating acne and preparation method thereof
InactiveCN101884642AImprove solubilityPromote absorptionHydroxy compound active ingredientsAerosol deliveryVitamin CGlycerol
The invention relates to compound emulsifiable paste for treating acne and a preparation method thereof, in particular to a medicament for treating acne and a preparation method thereof. The compound emulsifiable paste solves the problems of long treatment course and skin oxidation, dim skin and formation of color spots in the healing process in the conventional medicament for treating the acne. The compound emulsifiable paste is prepared from clindamycin hydrochloride, metronidazole, vitamin A, vitamin C, an emulsifying agent, stearic acid, albolene, glycerol monostearate, lanolinum, glycerol and purifying water. The preparation method comprises the following steps of: adding the vitamin A into uniform solution prepared from the stearic acid, the albolene, the glycerol monostearate and the lanolinum to obtain an oil phase; dissolving the glycerol and the emulsifying agent into the purifying water, adding the clindamycin hydrochloride, the metronidazole and the vitamin C, and stirring uniformly to obtain a aqueous phase; and adding the oil phase into the aqueous phase, stirring, homogenizing and standing the mixture to obtain the compound emulsifiable paste for treating the acne. The treatment period of the compound emulsifiable paste is between 14 and 24 days, skin is bright and clean after healing, and the compound emulsifiable paste can treat the acne, seborrheic dermatitis, acne rosacea and folliculitis.
Owner:哈尔滨乐泰生物科技有限公司
Process for synthesizing clindamycin phosphate
InactiveCN101891779ASimple processing methodPractical applicationSugar derivativesSugar derivatives preparationHydrolysisPotassium carbonate
The invention relates to a process for synthesizing clindamycin phosphate. The process comprises the following steps: 1) ketal reaction, wherein clindamycin hydrochloride alcoholate is used as a basic raw material to prepare a 3.4-clindamycin condensation compound through selective hydroxyl protection; and 2) phosphatidic reaction, wherein the 3.4-clindamycin condensation compound is used as a raw material, phosphorus oxychloride is used as a phosphatidic agent, and anhydrous potassium carbonate is used as an acid binding agent to complete the phosphatidic reaction under double actions of esterification catalyst dimethylamino pyridine and phase-transfer catalyst benzyl triethyl ammonium chloride, and a target compound, namely the clindamycin phosphate, can be obtained through hydrolysis deprotection. The process has the advantages that: 1, the process has simple method and practical application; 2, the content of pyridine / triethylamine in wastewater of phosphatidic reacting process can be controlled, so that the pressure of high COD wastewater drainage can be greatly reduced; and 3, the weight yield reaches over 95 percent, and the product quality fulfills the requirement of WS1-(X-322)-2003Z.
Owner:ZHANGJIAGANG XINYI CHEM
Process for synthesizing clindamycin hydrochloride
InactiveCN101891778ASimple processPractical applicationSugar derivativesSugar derivatives preparationChemical reactionHydrolysis
The invention relates to a process for synthesizing new clindamycin hydrochloride. The process comprises the following steps of: 1) finishing chlorination reaction by using lincomycin hydrochloride as a basic raw material and using low-C halogenated hydrocarbon as a solvent; 2) finishing hydrolysis reaction of sodium hydroxide in an aqueous phase by using a product obtained in the step 1), and demixing the solution to obtain clindamycin free alkali; and 3) in a solvent system of acetone, performing salt forming reaction on the clindamycin free alkali obtained in the step 2) and hydrochloric acid, and crystallizing the reaction product to obtain the clindamycin hydrochloride. The invention has the advantages that: 1, the process is simple; 2, the process reduces one-step chemical reaction on chemical unit reaction; 3, the process reduces one raw material and one intermediate on materials and intermediate links; and 4, the yield of the product is greatly improved, the epimer clint content of impurities is reduced by 80 percent, the process has high yield, and the yield is improved by over 5 percent compared with a four-step method.
Owner:ZHANGJIAGANG XINYI CHEM
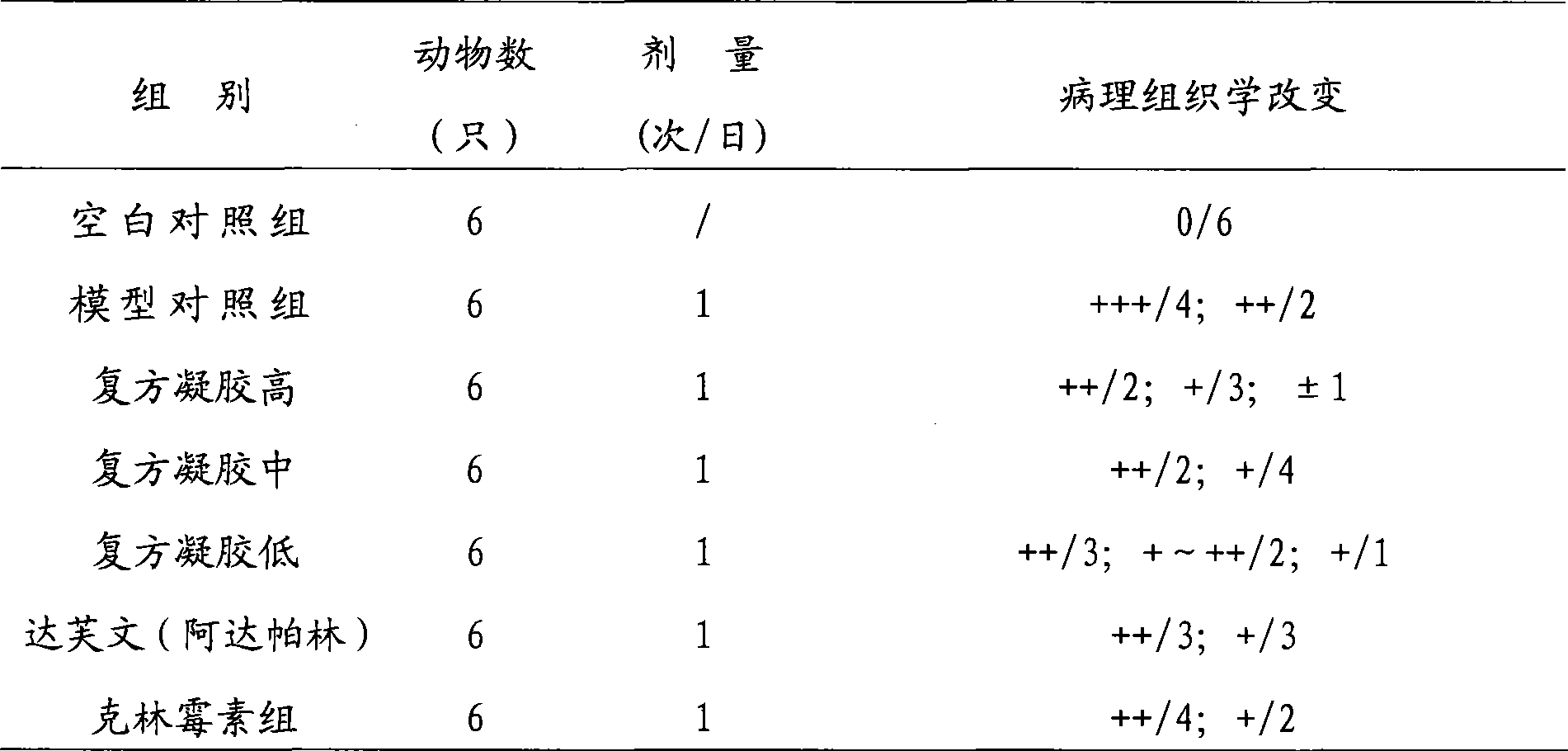
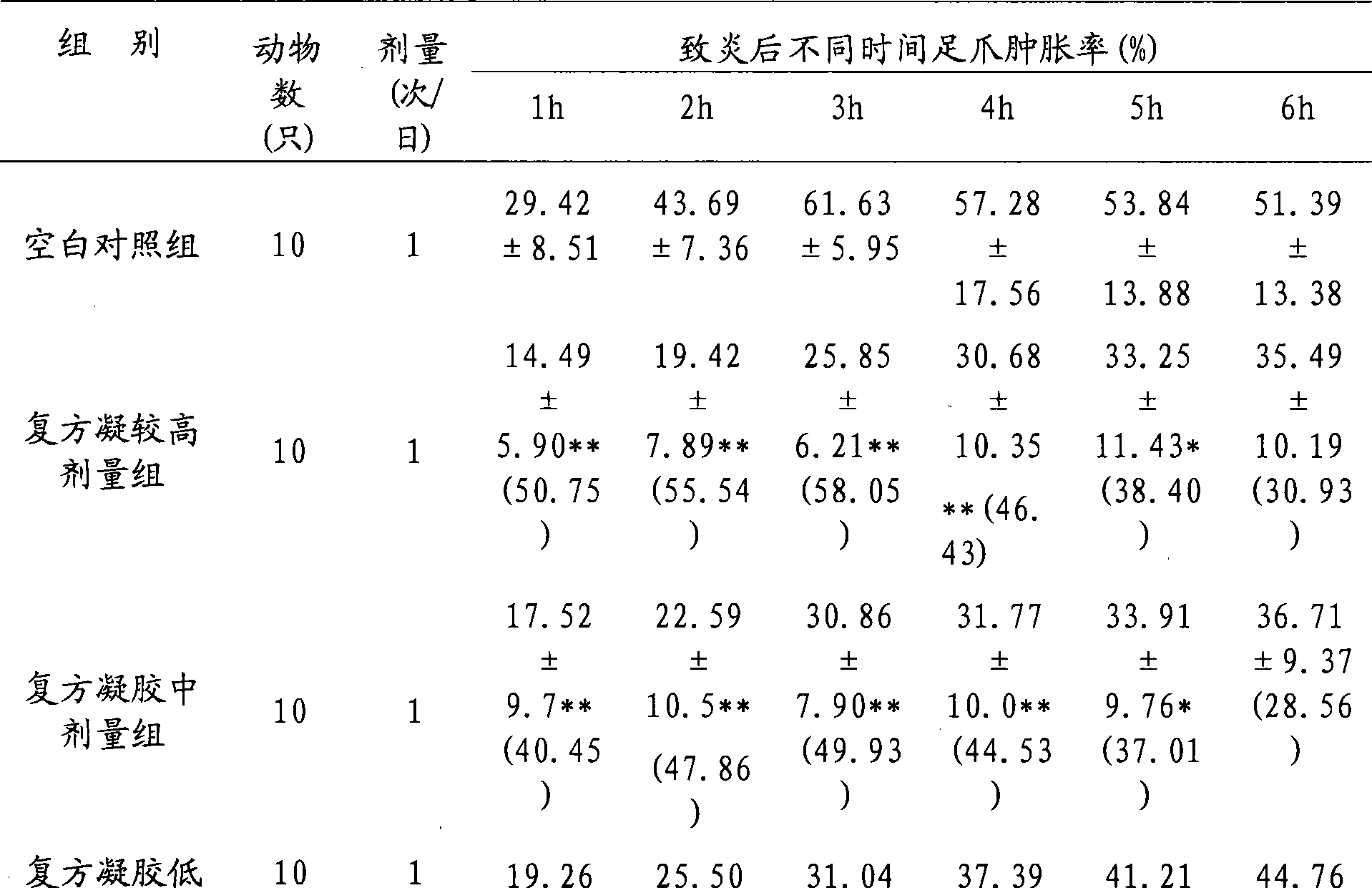
Adapalene and hydrochloric clindamycin compound gel preparation and preparation method thereof
ActiveCN101485675AEffective treatmentThe therapeutic effect of the preparation is remarkableOrganic active ingredientsPharmaceutical delivery mechanismClindamycin HydrochlorideGel matrix
The invention discloses an adapalene clindamycin hydrochloride compound gel preparation and a preparation method thereof. The preparation contains 0.08 to 0.12 percent of adapalene and 0.8 to 1.2 percent of clindamycin hydrochloride according to the weight percentage. The preparation method for the adapalene clindamycin hydrochloride compound gel comprises the steps of: preparing a gel matrix, swelling, sterilizing, dissolving the adapalene and the clindamycin hydrochloride into the gel matrix respectively, and using triethanolamine to adjust the pH value to form the steady gel. The product can effectively treat acne.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Method for preparing clindamycin hydrochloride
InactiveCN102702279AQuality improvementReduce solubilitySugar derivativesSugar derivatives preparationActivated carbonAlcohol
The invention discloses a method for preparing clindamycin hydrochloride. The method comprises the following steps of: a) adding purified water into a chloroformic solution of clindamycin hydrochloride alcohol complex and washing; b) adding purified water into the washed organic phase, dripping hydrochloric acid to adjust PH value to be 2 to 3, standing and layering; c) adding activated carbon into a water phase obtained through separation and decoloring, and filtering; d) adsorbing filtrate by using a resin, and washing by using deionized water; e) desorbing by using methanol of which the mass concentration is more than 95 percent; f) concentrating a desorption solution under reduced pressure; g) adding acetone into concentrated dried materials, and stirring at the temperature of between 55 and 58 DEG C until the solution is clarified; h) dripping hydrochloric acid to adjust PH value to be 2 to 2.5; and i) reducing temperature to 0 to 5 DEG C, performing suction filtration under negative pressure to obtain web powder, washing by using iced acetone, and after suction filtration, drying to obtain the clindamycin hydrochloride. According to the method, the step of alcohol complex crystallization in the conventional process is eliminated; the process is simplified; production cost is reduced; and the weight yield and the quality of the whole batch of clindamycin hydrochloride are improved simultaneously.
Owner:ANHUI WANBEI PHARMA
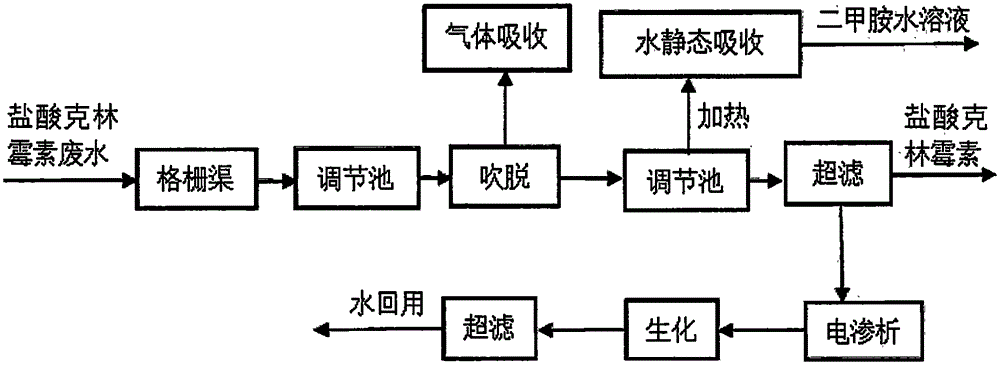
Clindamycin hydrochloride waste water processing technology
ActiveCN105417888AAchieve recyclingStrong targetingWater contaminantsDispersed particle separationUltrafiltrationWater quality
The invention relates to a clindamycin hydrochloride waste water processing technology. The processing technology comprises the following steps: firstly, waste water is intercepted, and floaters and block impurities are removed; secondary, water volume, water quality and pH of waste water are adjusted in an adjusting tank; thirdly, volatile liquids of chloroform, acetone, ethanol and the like are removed through air stripping; fourthly, dimethylamine gas generated from a heating reaction is absorbed by water in a static state and a dimethylamine agueous solution is generated; fifthly, residual clindamycin and other small particle substances are recovered through an ultrafiltration device; sixthly, the waste water after ultrafiltration goes into an electrodialysis device and is subjected to desalting processing; seventhly, the filtrate after electrodialysis is subjected to biochemistry treatment; eighthly, the filtrate after biochemistry treatment is filtered and recycled. The provided processing technology has advantages of simple technology, simple operation, high processing efficiency, low device operation expense, resource recycle, solves the standard meeting discharge and recycle problems of clindamycin hydrochloride waste water, and can bring about extra economic benefits.
Owner:ZHENGZHOU UNIV MULTI-FUNTIONAL DESIGN & RES ACAD CO LTD
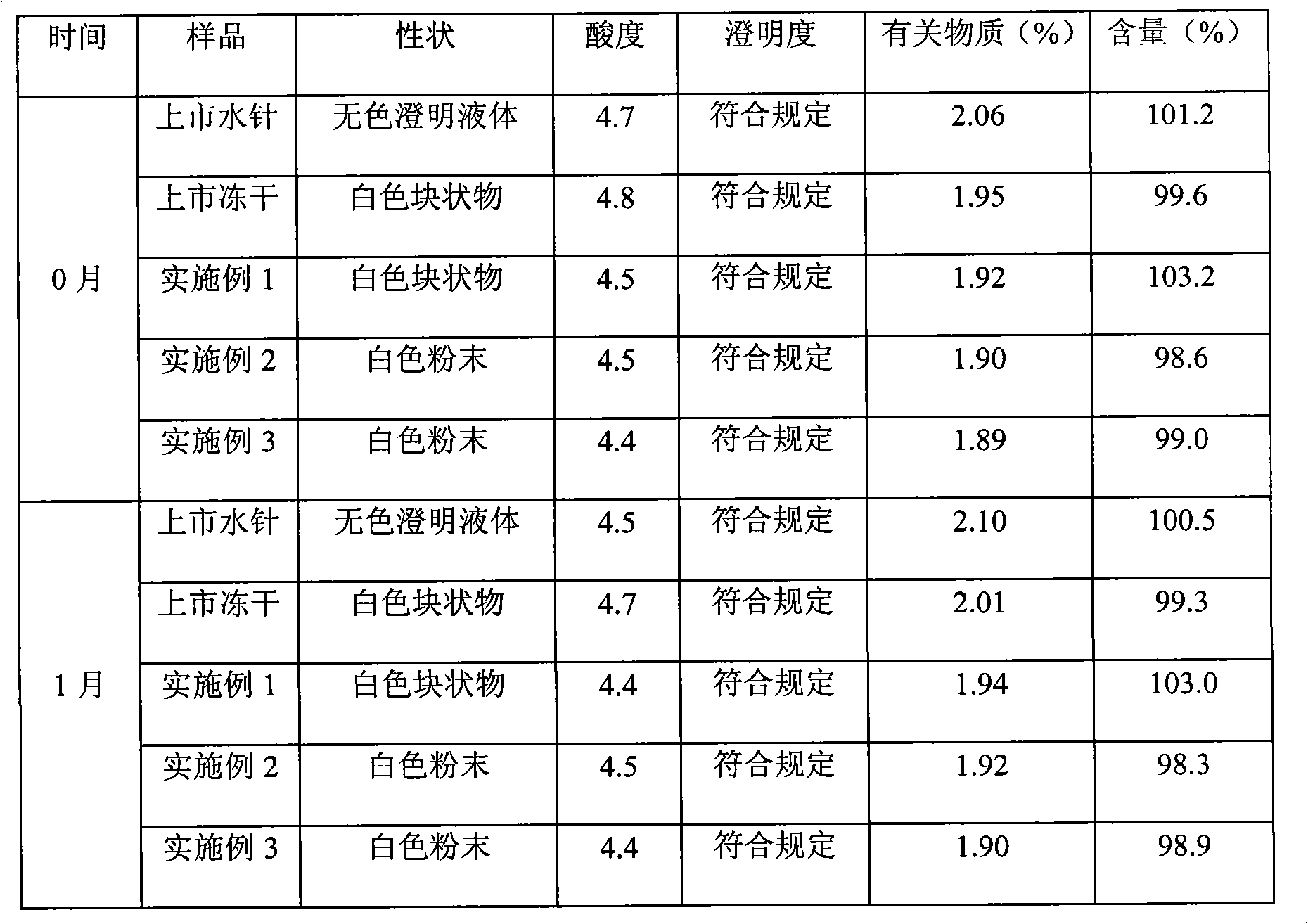
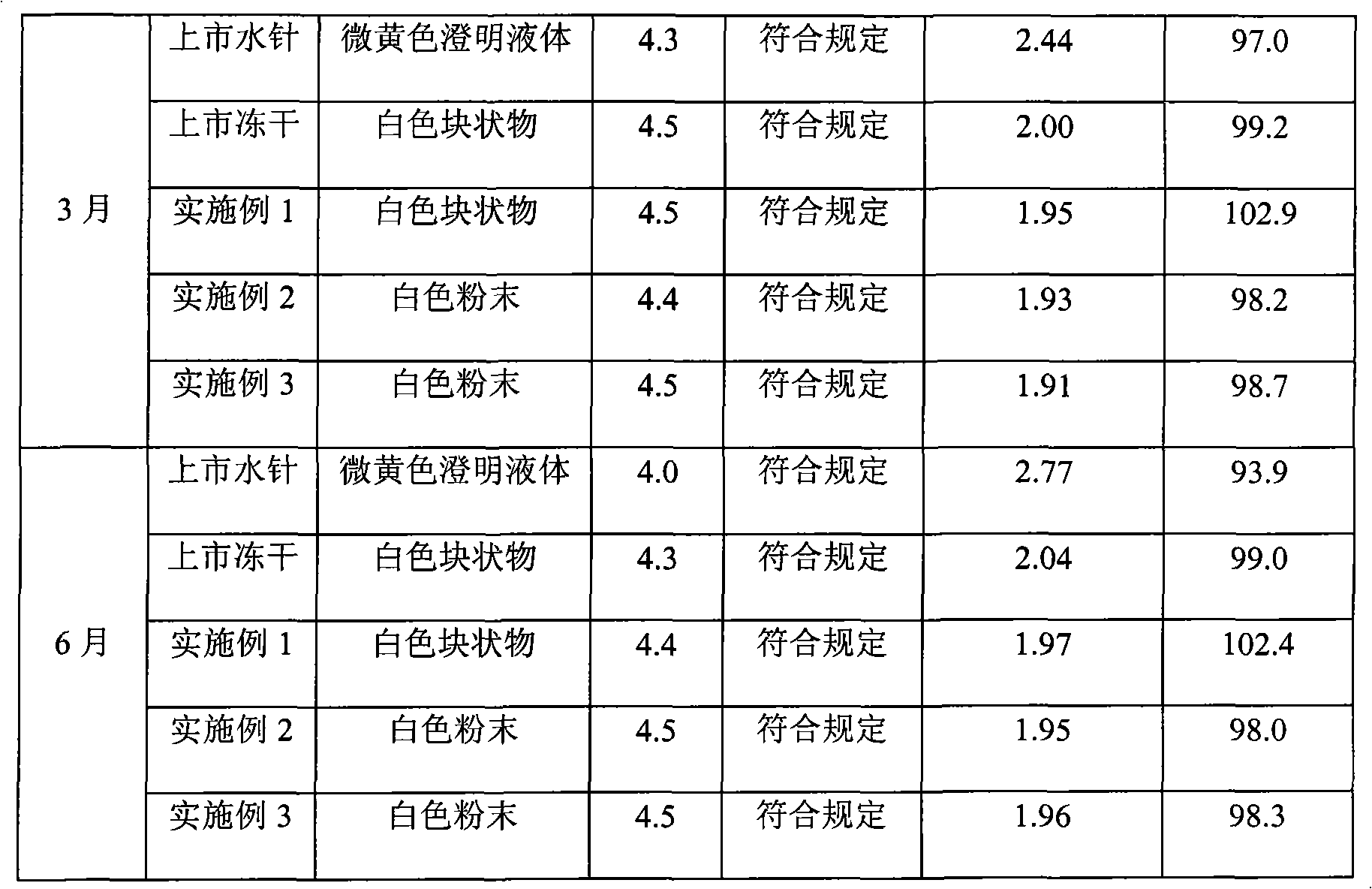
Clindamycin hydrochloride injection and preparation method thereof
ActiveCN101756897AReduce manufacturing costReduce the risk of adverse reactionsAntibacterial agentsOrganic active ingredientsUse medicationHigh volume manufacturing
The invention discloses a clindamycin hydrochloride injection and a preparation method thereof. The preferred preparation formulation is injection and lyophilized powder. The clindamycin hydrochloride injection composition consists of clindamycin hydrochloride as an active ingredient and moderate buffer salt. The solvent of the invention is only water for injection and has no any other organic solvents, and the lyophilized powder does not contain excipients. The clindamycin hydrochloride injection has the advantages that: firstly, the formula is simple, the species of excipients are few, and the excipients belong to the medicinal injection grade, are commonly used medicinal excipients, meet the requirements of intravenous injection medication of human bodies, and greatly reduce the risk of potential adverse reaction in clinical use; secondly, the product quality is very stable, the long-term 2-year experiment shows that the product quality has a little change, so that the product can meet the needs of industrialized mass production.
Owner:HAINAN LEVTEC PHARMA
Emulsifiable paste for curing acne and preparation thereof
InactiveCN101297814AImprove stabilityProlong the action timeOrganic active ingredientsAerosol deliveryPropanoic acidBacillus acnes
The invention discloses a cream for treatment for acnes and a preparation method thereof, which relates to a drug for the treatment for acnes and the preparation method thereof. The invention can solve the shortcomings that the existing drugs for the treatment for acnes can cause dry skin after usage, even peeling, cracking and short action time of the drug. The cream for the treatment for acnes of the invention is prepared by clindamycin hydrochloride, metronidazole, stearic acid, glycerol monostearate, white Vaseline, polysorbate 80, glycerin and water; the preparation method is carried out according to the following steps: (1) the oil phase is prepared; (2) the water phase is prepared; and (3) the oil phase is added into the water phase; thus obtaining the cream for treatment for acnes. The cream for treatment for acnes can kill propionibacterium acnes, have long action time of the drug and have no peeling and cracking phenomena after the usage.
Owner:哈尔滨乐泰生物科技有限公司
Preparation method of clindamycin hydrochloride freeze dried powder injection
InactiveCN1830452AAvoid degradationEnsure complete removalAntibacterial agentsOrganic active ingredientsAcute bronchitisUltrafiltration
A freeze-dried powder injection of clindamycin hydrochloride for treating various infectious diseases, tonsillitis, tympanitis, acute bronchitis, pneumonia, etc is prepared from clindamycin hydrochloride through ultrafiltration, mixing with filtered mannitol in dark condition and N2 atmosphere, pouring in containers, freeze drying, and sealing.
Owner:巴里莫尔制药(通化)有限公司
Hydrochloric clindamycinum palmitate capsule and method for preparing the same
InactiveCN101152161ASolve mass productionSolve the problem that it cannot be prepared into capsulesAntibacterial agentsOrganic active ingredientsMedicineDiluent
The invention discloses a clindamycin hydrochloride palmitate capsule and the preparation method. Each capsule contains clindamycin hydrochloride palmitate with a content equal to 35 to 300 milligram of clindamycin hydrochloride. The capsule also contains pharmaceutical excipients such as diluent, disintegrant, solubilizer and seed coating agents. The invention produces granules by dry granulating method, wraps the granules with moisture proof agricultural film, and performs the process of capsule loading. The invention solves the problems of easy moisture absorbing, caking and breaking of capsule shell caused by moisture absorbing. The invention also solves the problems with the product that the formulation is unitary and only granules and dispersible tables are provided, enriches the formulation of the capsule, offers patients with more choices of formulation and the compliance is good. Due to the addition of solubilizer, the clindamycin hydrochloride palmitate is stripped more rapidly and the bioavailability of the invention is enhanced. And the invention adopts the process of moisture proof agricultural film wrapping after dry granulating method, so the activity of the clindamycin hydrochloride palmitate is preserved better, the effect of the drug is better and the drug is much safer; thereby the invention is fit for industrial production.
Owner:重庆智上医药科技有限公司
Clindamycin palmitate hydrochloride dispersion tablet and its preparation method
ActiveCN1823808AFast absorptionImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPalmitatesClindamycin Hydrochloride
A dispersing tablet of clindamycin hydrochloride palmitate contains proportionally clindamycin hydrochloride palmitate, diluent, disintegrant, solubilizer, flavouring and lubricant. Its preparing process is also disclosed, which features use of empty particles and dry granulating.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Hydrochloride clindamycin palmitate soft capsule and the preparing method thereof
The invention discloses a muriatic acid clindamycinum palmitate soft capsule preparation and preparing method, which is characterized by the following: comprising muriatic acid clindamycinum palmitate, dissolvent to dissolve muriatic acid clindamycinum palmitate and capsule shell solution; choosing the dissolvent from carbowax, glycerine, soil temperature and dimethyl carbinol; choosing the capsule shell solution from dope or acacia gum, glycerine and water; incorporating 35-200mg clindamycinum of muriatic acid clindamycinum palmitate in each soft capsule. This invention possesses quick effect and best stability.
Owner:PKU HEALTHCARE CORP LTD
Integrated treatment process for clindamycin hydrochloride production wastewater
ActiveCN106348545AReduce landfill volumeRational use of resourcesWater/sewage treatment by centrifugal separationWater treatment compoundsPhosphateDistillation
The invention discloses an integrated treatment process for clindamycin hydrochloride production wastewater. The process comprises the following steps: performing triple-effect evaporation and concentration on the clindamycin hydrochloride production wastewater, performing centrifugal separation, feeding coarse salts produced by centrifugal separation into a salt refining workshop, collecting the mother solution to be used for extracting phosphate, and repeatedly performing dissolution, evaporation and centrifugal separation on the coarse salts in the salt refining workshop, thereby obtaining high-purity chlorine salts; washing by ethanol, thereby obtaining the finished chlorine salts; collecting the produced mother solution to be used for extracting phosphate; performing evaporation and concentration on the phosphate mother solution, centrifuging and washing, thereby obtaining the phosphate; rectifying the scrubbing solution, recycling the methanol, feeding the distillation residues into a hazardous waste treatment center, merging the foul condensate produced by multiple-effect evaporation and crystallization mother liquor produced by centrifugal separation, homogenizing, and performing anaerobic and aerobic biological treatment and advanced treatment. The method provided by the invention is an integrated method for combining multiple-effect evaporation, methanol rectification and biological treatment and has the characteristics of reasonable resource utilization, low cost, simplicity and convenience in operation, stable operation and the like.
Owner:河南君和环保科技有限公司
Disperse tablet contg. klinemycin hydrochloride palmitate, and its prepn. method
InactiveCN1559431AEasy to takeOvercome the shortcomings of easy moisture absorption and deteriorationOrganic active ingredientsAntisepticsPalmitatesClindamycin Hydrochloride
Owner:重庆凯林制药有限公司
Clindamycin palmitate hydrochloride particle and preparation method thereof
The invention provides a clindamycin palmitate hydrochloride particle and a preparation method thereof. The clindamycin palmitate hydrochloride particle provided by the invention consists of a drug pill core and a coating layer covering the outside of the drug pill core, wherein the drug pill core comprises clindamycin palmitate hydrochloride, a carrier and a water-soluble pore-forming agent; andthe coating layer comprises a pH-dependent coating material. According to the clindamycin palmitate hydrochloride particle provided by the invention, a soft material is prepared by virtue of a wet process / the drug pill core is prepared by virtue of an extrusion-spheronisation process firstly, and then the soft material or the drug pill core is coated by coating liquid by virtue of a spray coatingprocess. The clindamycin palmitate hydrochloride particle provided by the invention is applicable to children; the integrity of the particle can be kept before the particle is taken, and the particle,after entering human bodies, can be slowly released, so that a stable and lasting blood concentration can be provided, and clinical demands of reducing the times of administration, reducing dose-related toxicity and improving patient compliance can be satisfied; and meanwhile, the bad taste of the medicine (the particle) can be masked without adding a great amount of flavoring agents.
Owner:GUANGZHOU DAGUANG PHARMA
Clindamycin palmitate hydrochloride chewing tablet andi its preparing method
InactiveCN1977856AFully dissolvedFast absorptionAntibacterial agentsOrganic active ingredientsClindamicinPalmitates
The present invention relates to a clindamicin palmitate masticatory tablet and its preparation method. It is characterized by that it adopts dry granulation process so as to maximally retain the activity of clindamicin palmitate, it is easy to implement industrial production and its preparation process is simple.
Owner:张文静
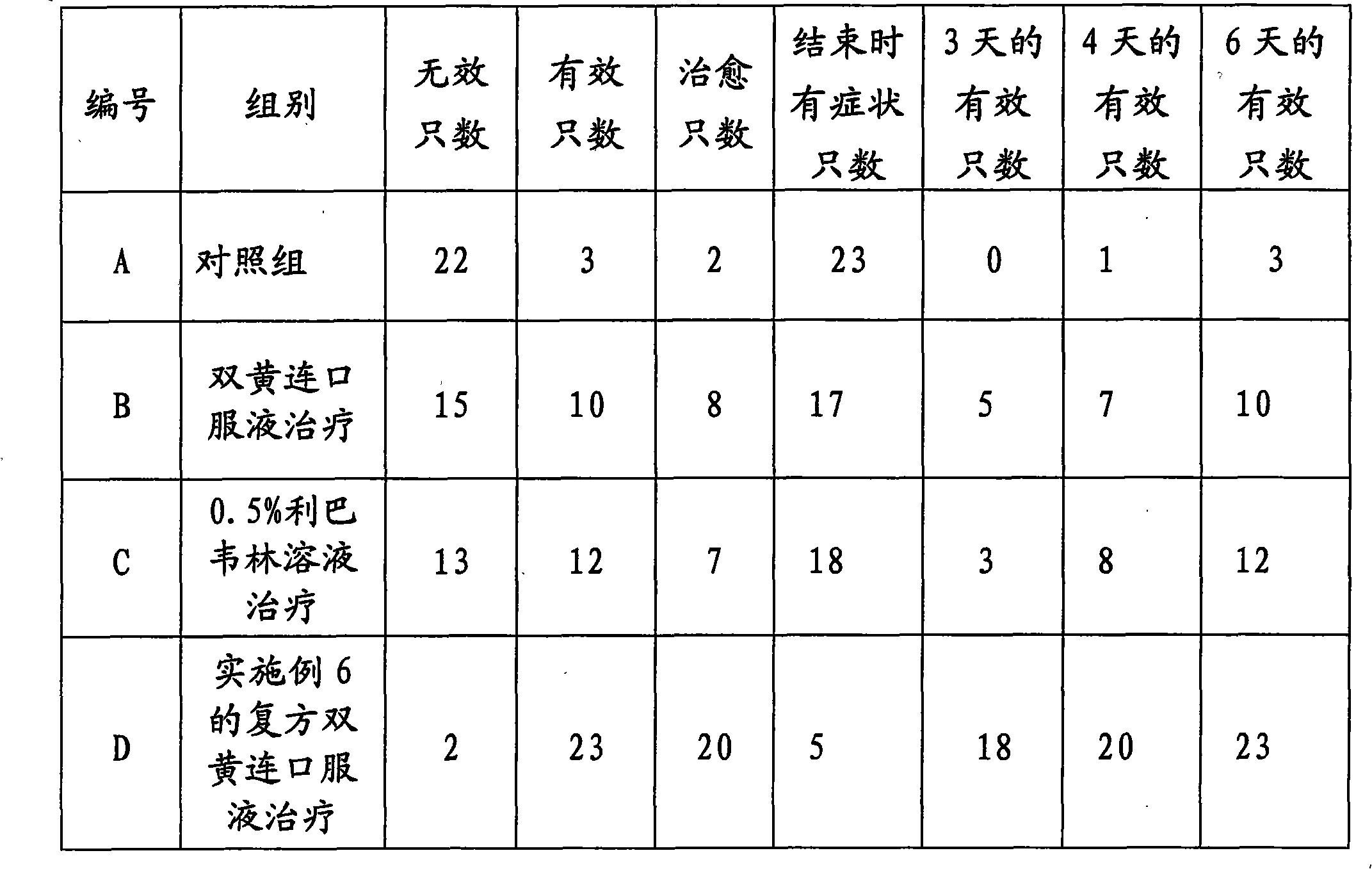
Compound shuanghuanglian oral solution used for treating cold of dog and preparation method thereof
InactiveCN101879218AGreat tasteImprove smellOrganic active ingredientsPharmaceutical delivery mechanismDiseaseForsythia
The invention discloses compound shuanghuanglian oral solution used for controlling the cold of a dog and a preparation method thereof, and aims to provide compound shuanghuanglian oral solution used for treating the cold of a dog, which has the advantages of convenient use, high cure rate, quick response and treatment of both principal and secondary aspect of disease, and a preparation method thereof, which has the advantages of simple process and easy implementation. Each 100L of oral solution comprises 0.05 to 5kg of baicalin, 0.5 to 10kg of honey suckle and weeping forsythia extract, 0.1 to 5kg of clindamycin hydrochloride, 5 to 20kg of glucose, 0.2 to 5kg of analginum, 0.005 to 0.05kg of chlortrimeton, 0.1 to 5kg of ribavirin, 0.1kg of sodium glutamate and 0.1kg of sodium hydroxide. The compound shuanghuanglian oral solution is a compound preparation; the honey suckle and weeping forsythia extract, the baicalin, the ribavirin, and the clindamycin hydrochloride have the effect of inhibiting viruses and bacteria so as to control the cold; and by combining the glucose for supplementing energy, the antipyretic and anti-inflammatory analginum, the anti-allergic chlortrimeton and the sodium glutamate for improving the taste and smell of the oral solution, the cold of the dog is multilaterally treated.
Owner:天津市天合力药物研发有限公司
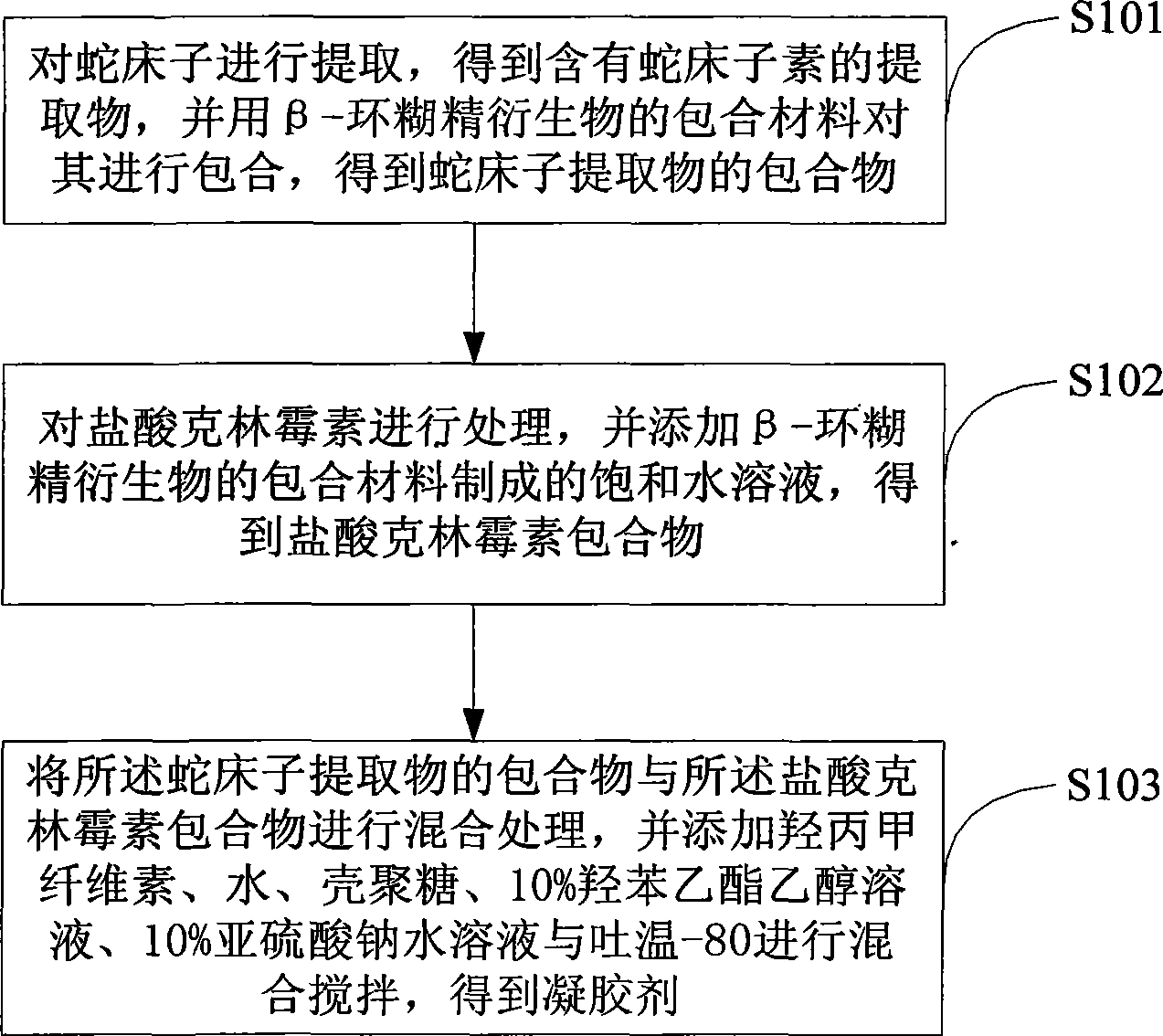
Preparation method for gel
InactiveCN103520232ALasting effectIncrease contact areaAntibacterial agentsOrganic active ingredientsIrritationCnidium monnieri
The invention discloses a preparation method for a gel, belonging to the field of preparation of medicines. The method comprises the following steps: extracting common cnidium fruit to obtain an extract containing osthole and carrying out clathration on the extract by using beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin so as to obtain a clathrate of the extract of common cnidium fruit; treating clindamycin hydrochloride and adding a saturated aqueous solution prepared from beta-cyclodextrin so as to obtain a clindamycin hydrochloride clathrate; and mixing the clathrate of the extract of common cnidium fruit with the clindamycin hydrochloride clathrate, adding hydroxypropyl methylcellulose, water, glycerin, chitosan, a 10% ethylparaben ethanol solution, a 10% aqueous sodium hydrosulphite solution and Tween-80 and carrying out mixing with stirring so as to obtain the gel. With the preparation method provided by the invention, the gel can safely and effectively perform curative effects and has reduced irritation, improved solubility, an accelerated dissolution speed and enhanced bioavailability.
Owner:SUIHUA UNIV
Hydrochloric clindamycin nano granule formulation for injections and preparation thereof
InactiveCN101322692AGood biocompatibilityLow costAntibacterial agentsOrganic active ingredientsExcipientSodium sulfate
The invention discloses a clindamycin hydrochloride nano-particle preparation; the preparation is prepared by the raw materials with the following parts by weight: 75-900 parts of clindamycin hydrochloride, 10-300 parts of stabilizing agent, 25-450 parts of sodium sulfate, 150-1,800 parts of polyalkylcyano-acrylate and 10-1,000 parts of excipient. The invention further discloses a preparation method thereof. The preparation is prepared by nano-particles and has fine stability and high safety.
Owner:HAINAN YONGTIAN PHARMA INST
Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
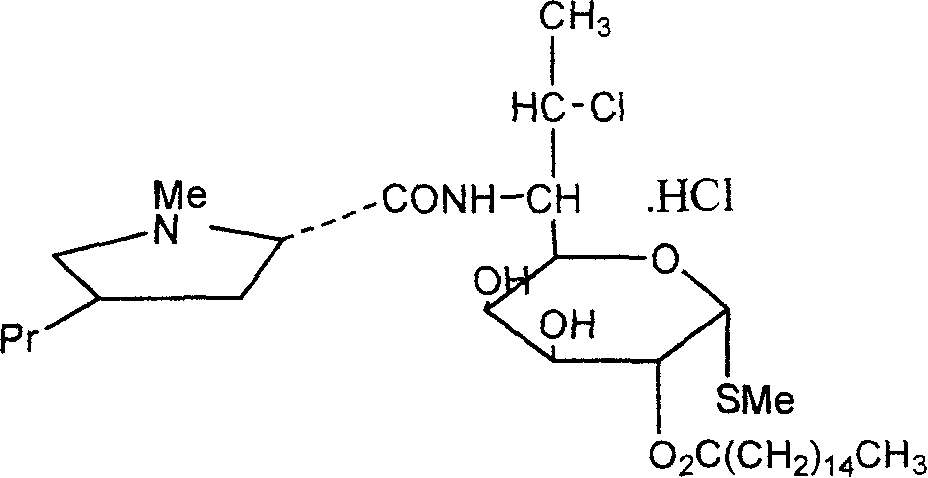
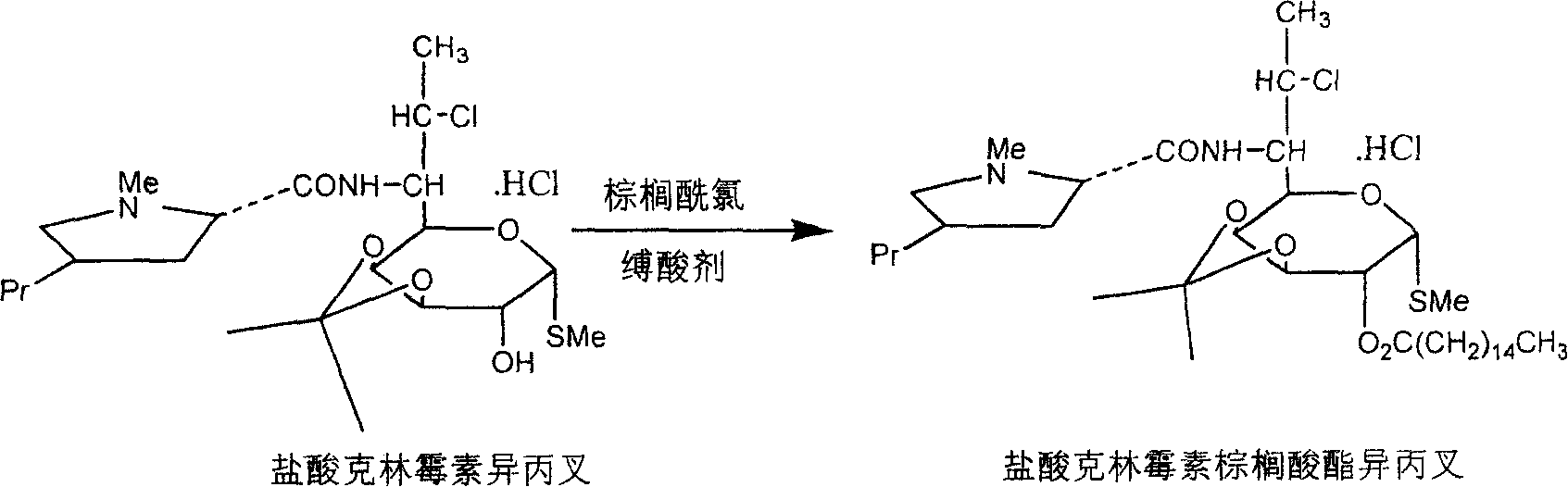
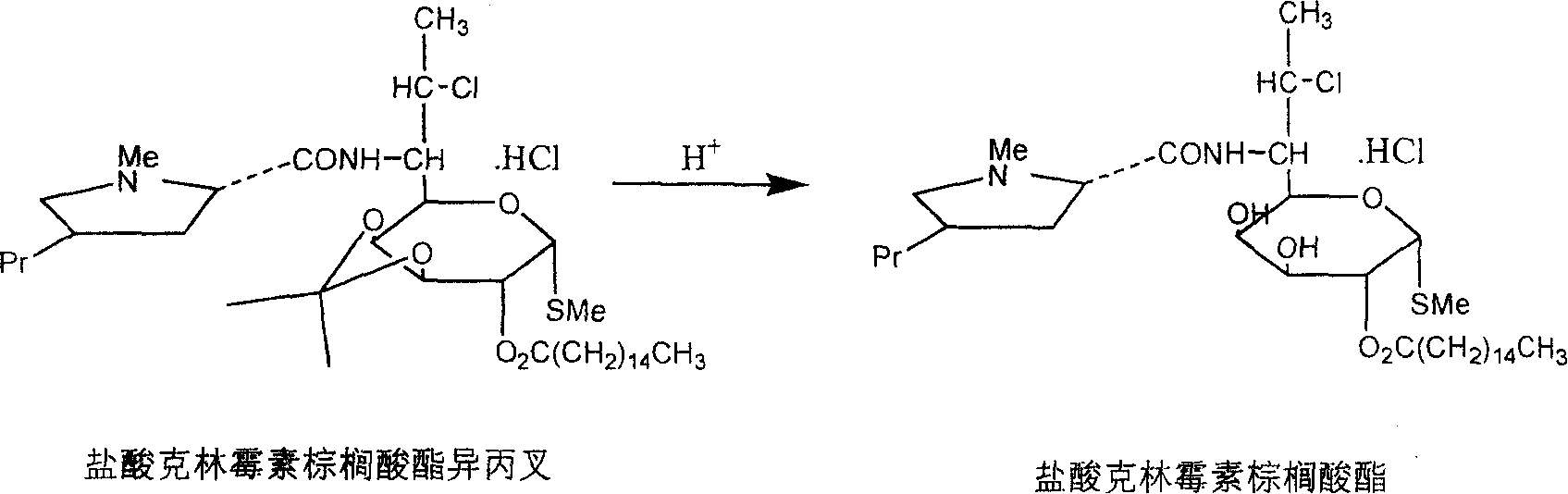
Industrial production process of palmitate of clindamycin hydrochloride
ActiveCN100368419CAvoid filter effectsPrevent drynessSugar derivativesCarbohydrate active ingredientsPalmitoyl chloridePalmitates
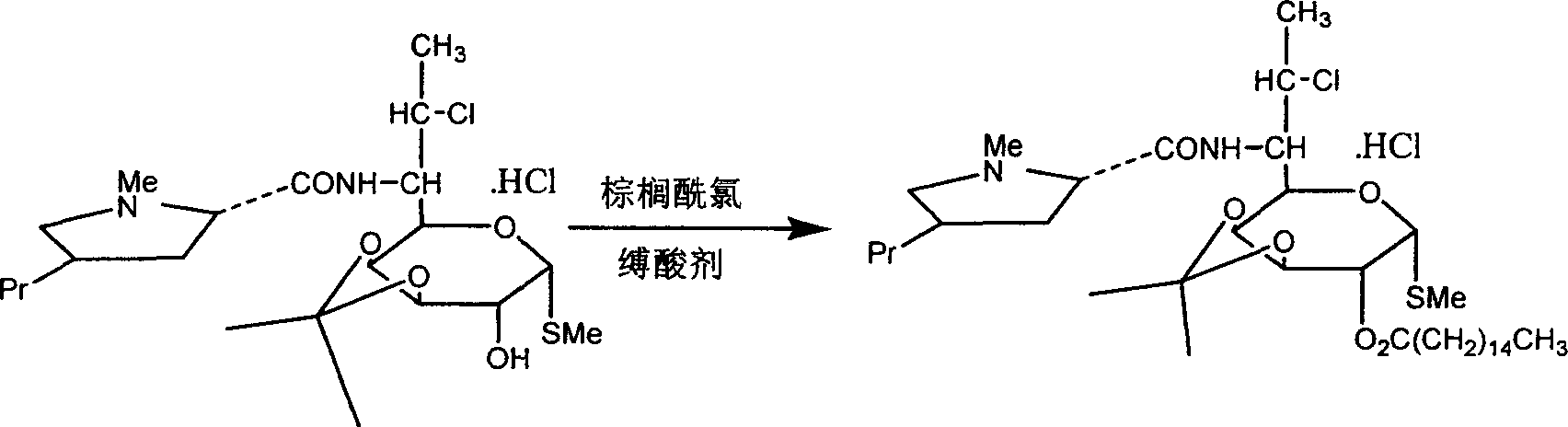
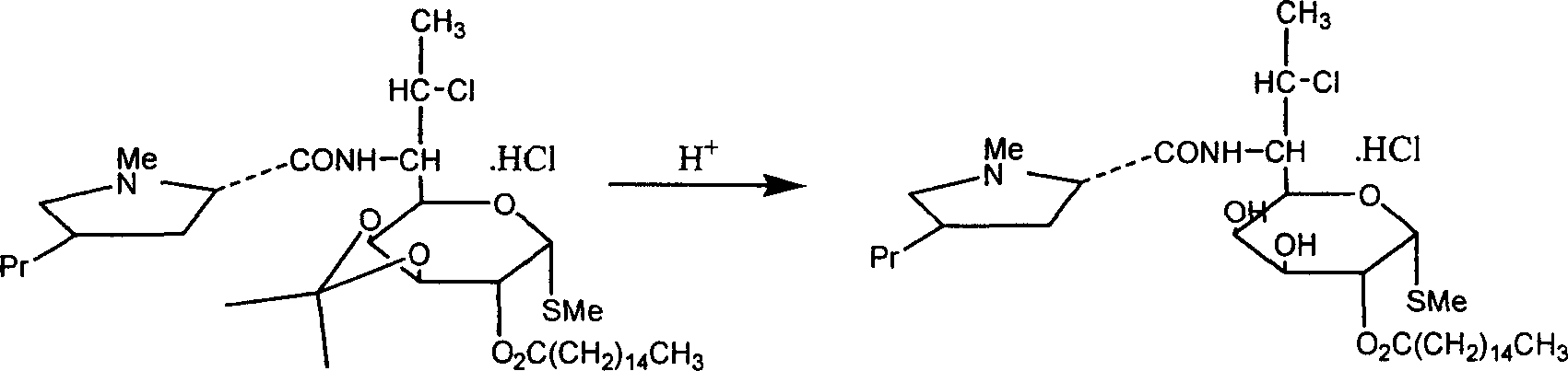
The industrial production process of palmitate of clindamycin hydrochloride includes the following main steps: the reaction of isopropylylidene clindamycin hydrochloride and palmitoyl chloride in the presence of acid-binding agent to prepare palmitate of isopropylylidene clindamycin hydrochloride; eliminating protecting radical to obtain coarse palmitate of clindamycin hydrochloride; and final re-crystallization and separation to obtain palmitate of clindamycin hydrochloride product. The present invention has the advantages of facile materials, simple and mild technological process and low power consumption, can obtain white product with HPLC content up to 97.1 %, and is suitable for industrial production.
Owner:重庆凯林制药有限公司
Method for preparing clindamycin hydrochloride powder for injection
InactiveCN1813785AHigh purityImprove bioavailabilityAntibacterial agentsPowder deliverySterile environmentSolvent
The present invention discloses a preparation method of clindamycin hydrochloride powder injection. Said preparation method includes the following steps: (1) adding solvent to dissolve clindamycin hydrochloride; (2), decoloring the dissolved solution and filtering; (3), fine filtering the above-mentioned filtrate under the condition of sterile environment to obtain fine filtrate; (4), adding acetone into the fine filtrate, heating, dissolving, adding active carbon and filtering; (5), standing still to obtain clindamycin crystal; and (6), drying, pulverizing and filling so as to obtain the invented product.
Owner:王冕
Method for purifying clindamycin hydrochloride
ActiveCN105949253AHigh synthetic yieldReduce lossSugar derivativesSugar derivatives preparationDistillationEthylic acid
The invention relates to a method for purifying clindamycin hydrochloride. The process comprises process steps as follows: clindamycin hydrochloride adduct reaction liquid is subjected to macroporous resin adsorption and methyl alcohol desorption; obtained desorbed solution is subjected to reduced pressure distillation and concentration, then is dissolved by butyl acetate solvent, and is subjected to alkaline hydrolysis, alkaline extraction and activated carbon decoloration, and clindamycin alkali decolored solution is obtained; a clindamycin hydrochloride product is obtained after acetone crystallization conversion. According to the method, the macroporous resin is utilized in the clindamycin hydrochloride adduct reaction liquid, so as to facilitate alkaline hydrolysis and alkaline extraction and separation processes, reduce an emulsification effect, improve the clindamycin hydrochloride synthesis yield by more than 6%, reduce a solvent loss, lower production costs, and increase economic benefits; the DMF recovery is facilitated, the cost for raw materials is saved, the influence of DMF on the environment is reduced, and the ecological environment is preserved.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
Industrial production process of palmitate of clindamycin hydrochloride
ActiveCN1810821AAvoid filter effectsPrevent drynessSugar derivativesAntiinfectivesPalmitoyl chloridePalmitates
The industrial production process of palmitate of clindamycin hydrochloride includes the following main steps: the reaction of isopropylylidene clindamycin hydrochloride and palmitoyl chloride in the presence of acid-binding agent to prepare palmitate of isopropylylidene clindamycin hydrochloride; eliminating protecting radical to obtain coarse palmitate of clindamycin hydrochloride; and final re-crystallization and separation to obtain palmitate of clindamycin hydrochloride product. The present invention has the advantages of facile materials, simple and mild technological process and low power consumption, can obtain white product with HPLC content up to 97.1 %, and is suitable for industrial production.
Owner:重庆凯林制药有限公司
Intelligent detection method for trichloromethyl carbonate residues in clindamycin hydrochloride
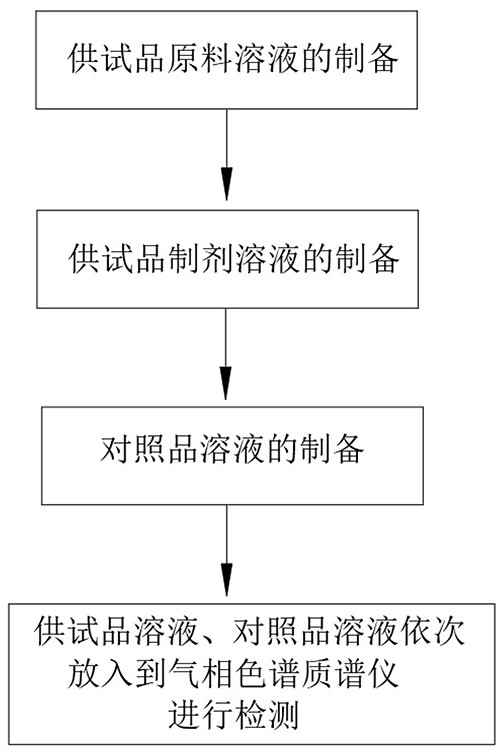
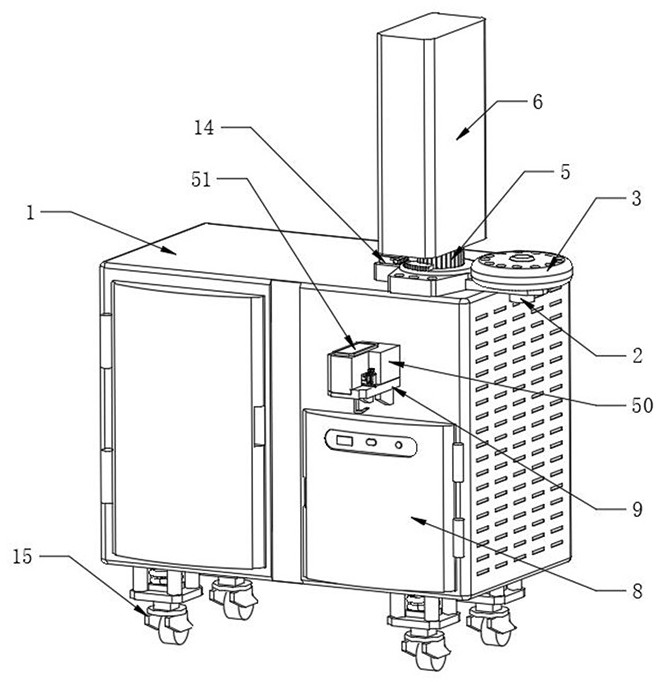
ActiveCN113252816AImprove leveling efficiencyQuality improvementComponent separationTest samplePhysical chemistry
The invention relates to the technical field of intelligent measurement and control of trichloromethyl carbonate residues, in particular to an intelligent detection method for trichloromethyl carbonate residues in clindamycin hydrochloride. The method is completed by adopting a gas chromatography-mass spectrometer convenient for automatically leveling a column head of a chromatographic column in a matching manner. The detection method comprises the following steps: 1) preparing a raw material solution of a test sample; 2) preparing a test sample preparation solution; 3) preparing a reference substance solution; and 4) respectively and independently putting the prepared test sample raw material solution, the test sample preparation solution and the reference substance solution into a gas chromatography-mass spectrometer for detection. According to the invention, through the arranged cutting and leveling structure, the chromatographic column head can be automatically cut and leveled when the chromatographic column head of the chromatographic mass spectrometer body is replaced, and compared with manual leveling, the leveling efficiency and the leveling quality are greatly improved.
Owner:广州国标检验检测有限公司
Specific clindamycin hydrochloride superfine powdered lyophilized preparation and preparation method thereof
InactiveCN104138358AHigh clarityImprove stabilityAntibacterial agentsPowder deliveryAllergy preventionClindamycinum
The invention discloses a specific clindamycin hydrochloride superfine powdered lyophilized preparation and a preparation method thereof. The preparation method uses clindamycin hydrochloride and triphosgene as raw materials, and comprises the following steps: performing chlorination reaction in the system of trichloromethane, N,N-dimethylformamide and antioxidant; hydrolyzing and alcoholizing to obtain clindamycin hydrochloride ethylate; decoloring and dealcoholizing to obtain high-purity clindamycin hydrochloride; and performing air-jet superfine crushing, and lyophilizing to obtain the specific clindamycin hydrochloride superfine powdered lyophilized preparation. The specific clindamycin hydrochloride superfine powdered lyophilized preparation has the advantages of good clarity, high stability, high purity, few impurities, small particles, large specific surface area, good solubility, small toxic and side effect, allergy prevention and the like.
Owner:浙江磐谷药源有限公司
Immediate release clindamycin delivery composition and formulation
InactiveUS20180256746A1Improve palatabilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsImmediate releaseSubject matter
Owner:IONO PHARMA LLC
Compound emulsifiable paste for treating acne and preparation method thereof
ActiveCN103006681ASimple processReduce manufacturing costOrganic active ingredientsAerosol deliveryGlycerolColor changes
The invention relates to a compound emulsifiable paste for treating acne and a preparation method thereof. The compound emulsifiable paste comprises the following ingredients by weight percent: 1% of clindamycin hydrochloride, 0.8% of metronidazole, 7%-16% of albolene, 4%-12% of glycerol, 1%-3% of liquid paraffin, 4%-6% of simethicone, 3%-8% of emulsifying agent, 4%-6% of penetration enhancer and the balance of distilled water. The compound emulsifiable paste solves the difficulties that indissolvable drug can not be distributed uniformly, the drug with heat instability is decomposed and subjected to color change when being heated, the possibility of paste preparation in normal temperature can be realized, and the paste is ideal in appearance and is stable. Meanwhile, according to the compound emulsifiable paste, the permeability of corneum of the skin is improved, the effective dose of the drug achieving the wound part is ensured, the bioavailability of effective ingredients of the drug can be improved, the effective dose is reduced, and the toxicity and the skin irritation are low.
Owner:乐泰药业有限公司
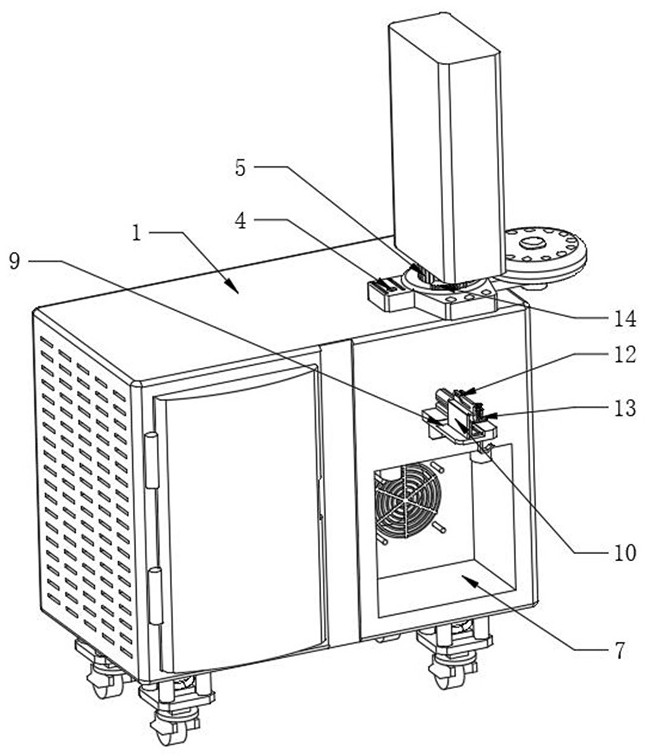
Material control device for tableting clindamycin hydrochloride palmitate
The invention relates to a material control device for tableting clindamycin hydrochloride palmitate. The material control device comprises a feed pipe body and a motor, wherein a feed hole is formed in the feed pipe body; a material baffle is arranged in the feed hole; a rotating shaft is arranged at the left end of the material baffle; a base is arranged at the lower end of the motor; a signal sensor is arranged at the upper end of the motor; a frequency modulation controller is arranged at the left upper end of the signal sensor; the frequency modulation controller and the signal sensor exchange signals through a connecting line; a control panel is arranged on a casing of the frequency modulation controller; and a power plug is arranged at the upper end of the frequency modulation controller. According to the material control device, the material baffle is arranged in the feed hole, the other end of the material baffle is fixedly connected with the rotating shaft of the motor, and the frequency modulation controller controls the time that the motor spends in rotating once and the rotation frequency of the motor to further control the weight of medicine powder which enters a middle mold hole through the feed hole; and the material control device has very high accuracy and improves yield of medicines.
Owner:江苏海阔生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com