Hydrochloride clindamycin palmitate soft capsule and the preparing method thereof
A technology of clindamycin hydrochloride and palmitate, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the problems of perishable, unsuitable age patients, low dissolution rate and bioavailability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
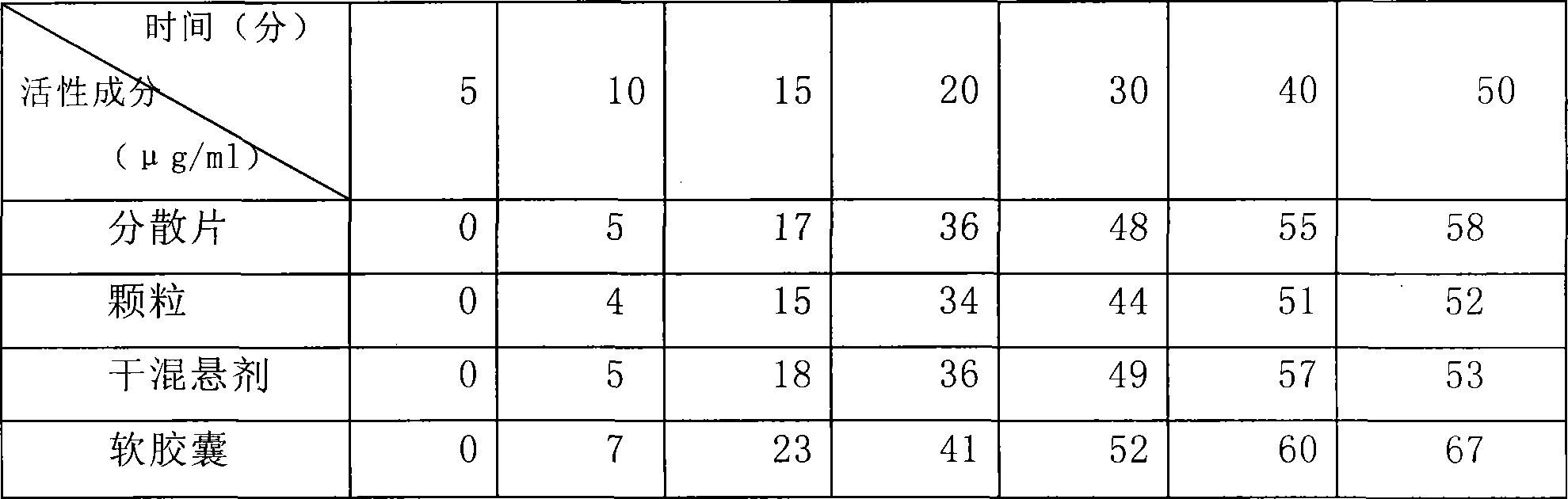
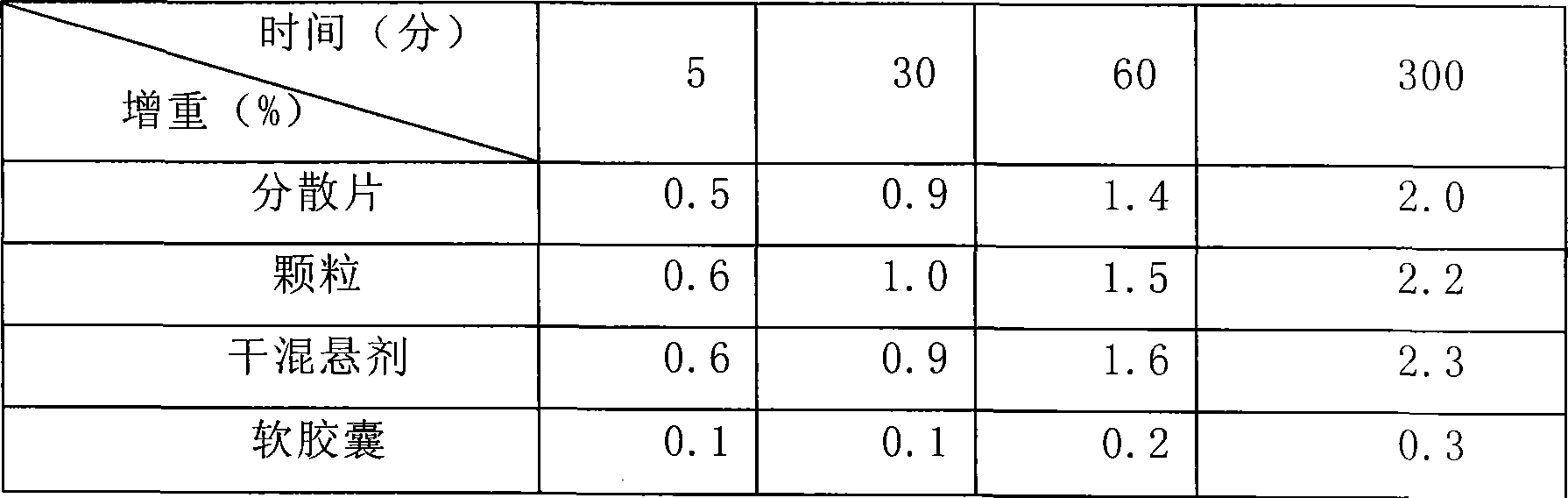
Image
Examples
Embodiment 1
[0042] Weigh 1 kg of gelatin, 0.4 kg of glycerin, and 1 kg of pure water, mix 0.8 kg of water with the gelatin to make the gelatin absorb water and swell; mix the remaining water and glycerin and heat to 50 degrees Celsius, mix well and add the expanded gelatin and heat to 70 degrees Celsius, Stir, melt into a uniform glue, keep warm, stand still, defoam, and filter for subsequent use; Accurately weigh 500 g of clindamycin hydrochloride palmitate in terms of clindamycin, dissolve it in 1.2 kg polyethylene glycol 400, and Heating to 50 degrees centigrade to make it into a uniform medicinal solution; making 6,600 soft capsules with the above-mentioned glue and medicinal solution by the rotating membrane method, drying the obtained soft capsules, cleaning, and drying to obtain a finished product.
Embodiment 2
[0044] Weigh 1 kg of gum arabic, 0.4 kg of glycerin, and 1 kg of pure water, mix 0.8 kg of water with gelatin to make the gelatin absorb water and swell; mix the remaining water and glycerin and heat to 50 degrees Celsius, mix well and add the expanded gelatin and heat to 70 degrees Celsius , stirred, melted into a uniform glue solution, kept warm, static, defoamed, filtered for later use; accurately weighed 500g of clindamycin hydrochloride palmitate in terms of clindamycin, dissolved in 0.8kg propylene glycol 0.4kg soil temperature 80 , and heated to 50 degrees centigrade to make it a uniform medicinal solution; the above-mentioned prepared glue and medicinal solution are made into 6660 soft capsules by the rotating membrane method, and the obtained soft capsules are dried, cleaned, and dried to obtain a finished product.
Embodiment 3
[0046]Weigh 1 kg of gelatin, 0.4 kg of glycerin, and 1 kg of pure water, mix 0.8 kg of water with the gelatin to make the gelatin absorb water and swell; mix the remaining water and glycerin and heat to 50 degrees Celsius, mix well and add the expanded gelatin and heat to 70 degrees Celsius, Stir, melt into a uniform glue, keep warm, stand still, remove foam, and filter for standby; Accurately weigh 500 g of clindamycin hydrochloride palmitate in terms of clindamycin, dissolve it in 0.8 kg of glycerin and 0.4 kg of soil temperature 80, And heated to 50 degrees centigrade to make it into a uniform medicinal solution; the glue and medicinal solution prepared above were made into 6620 soft capsules by the rotating film method, and the obtained soft capsules were dried, washed and dried to obtain finished products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com