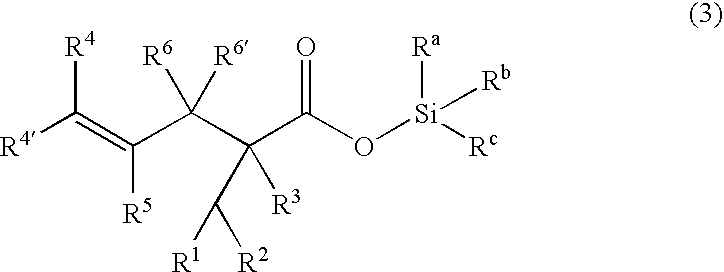
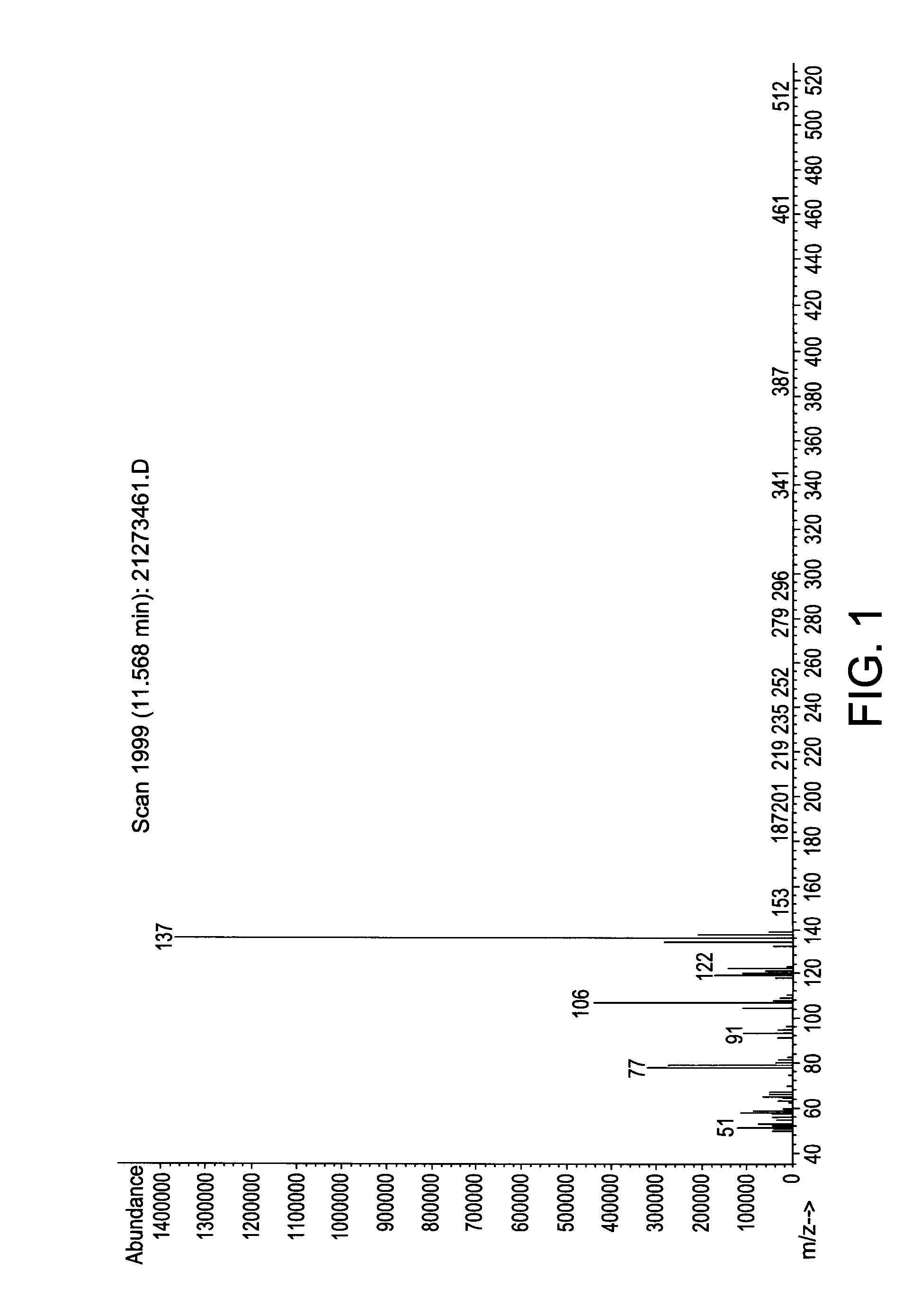
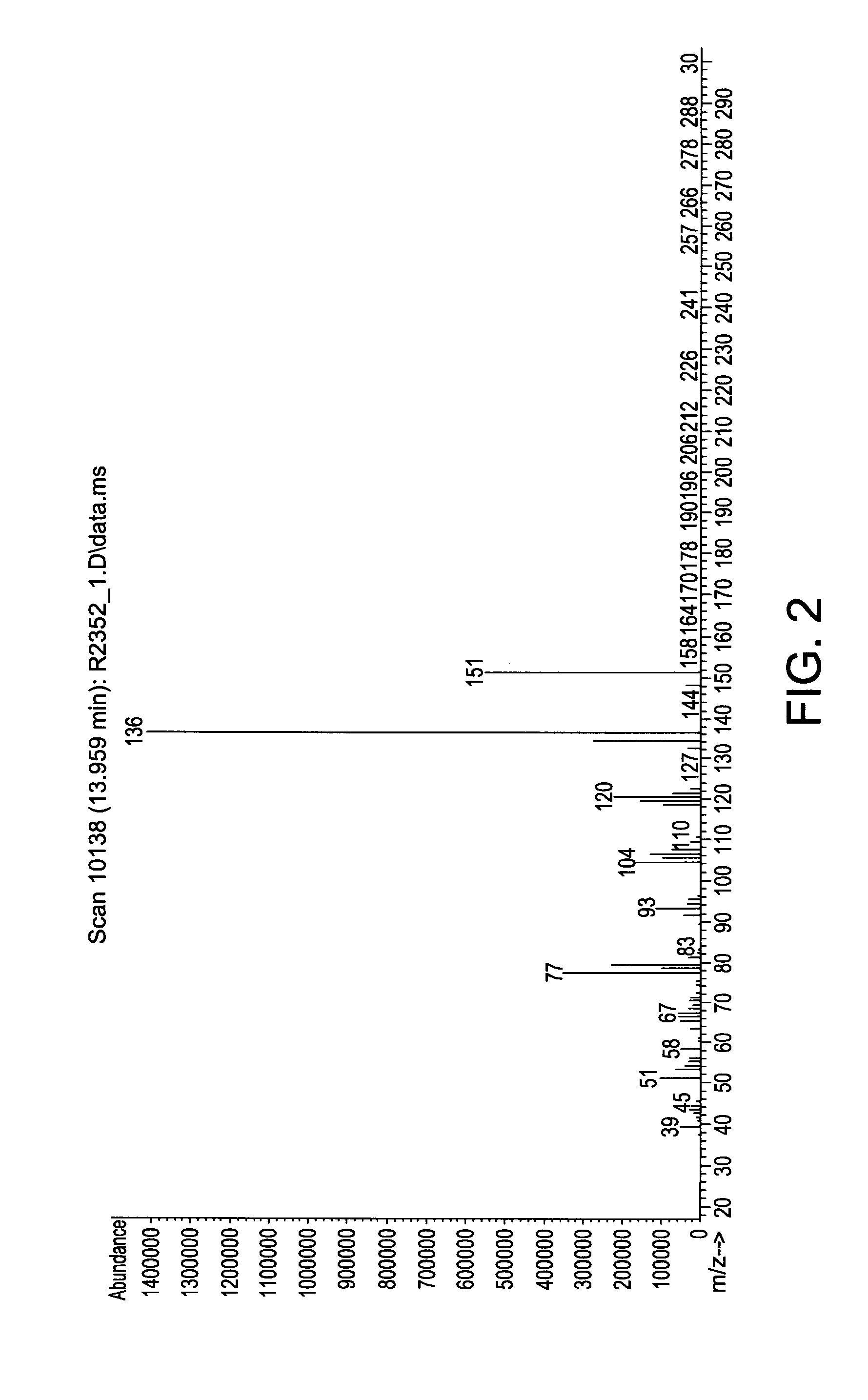
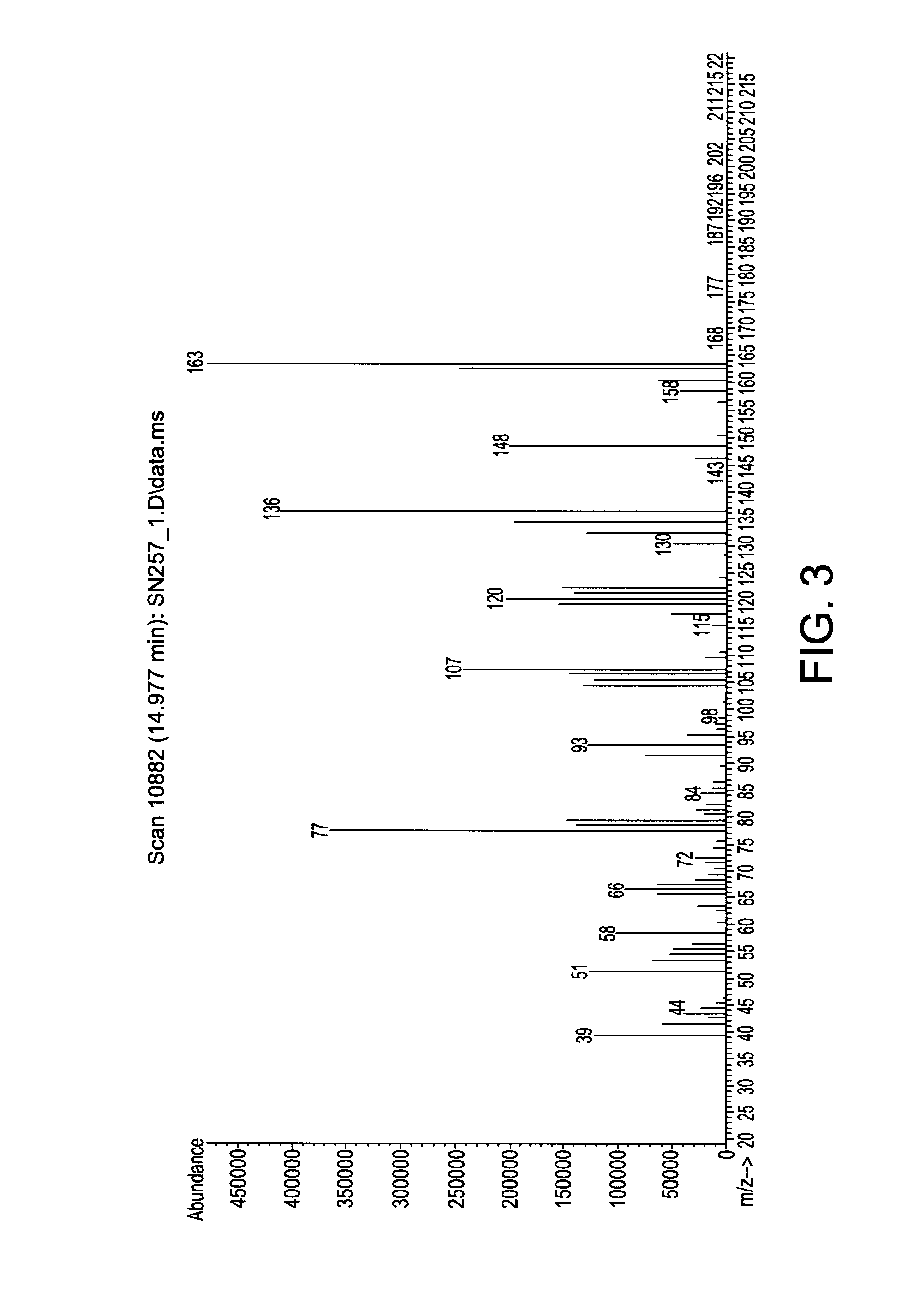
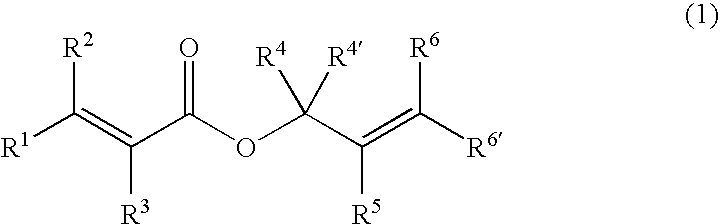
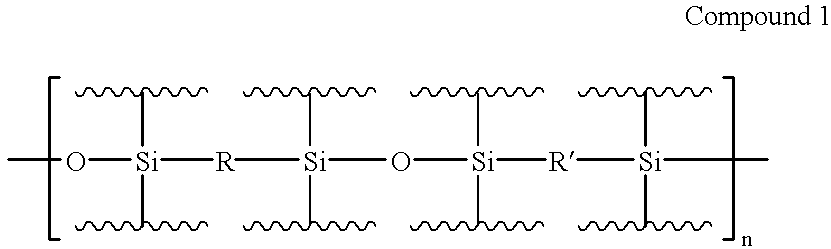
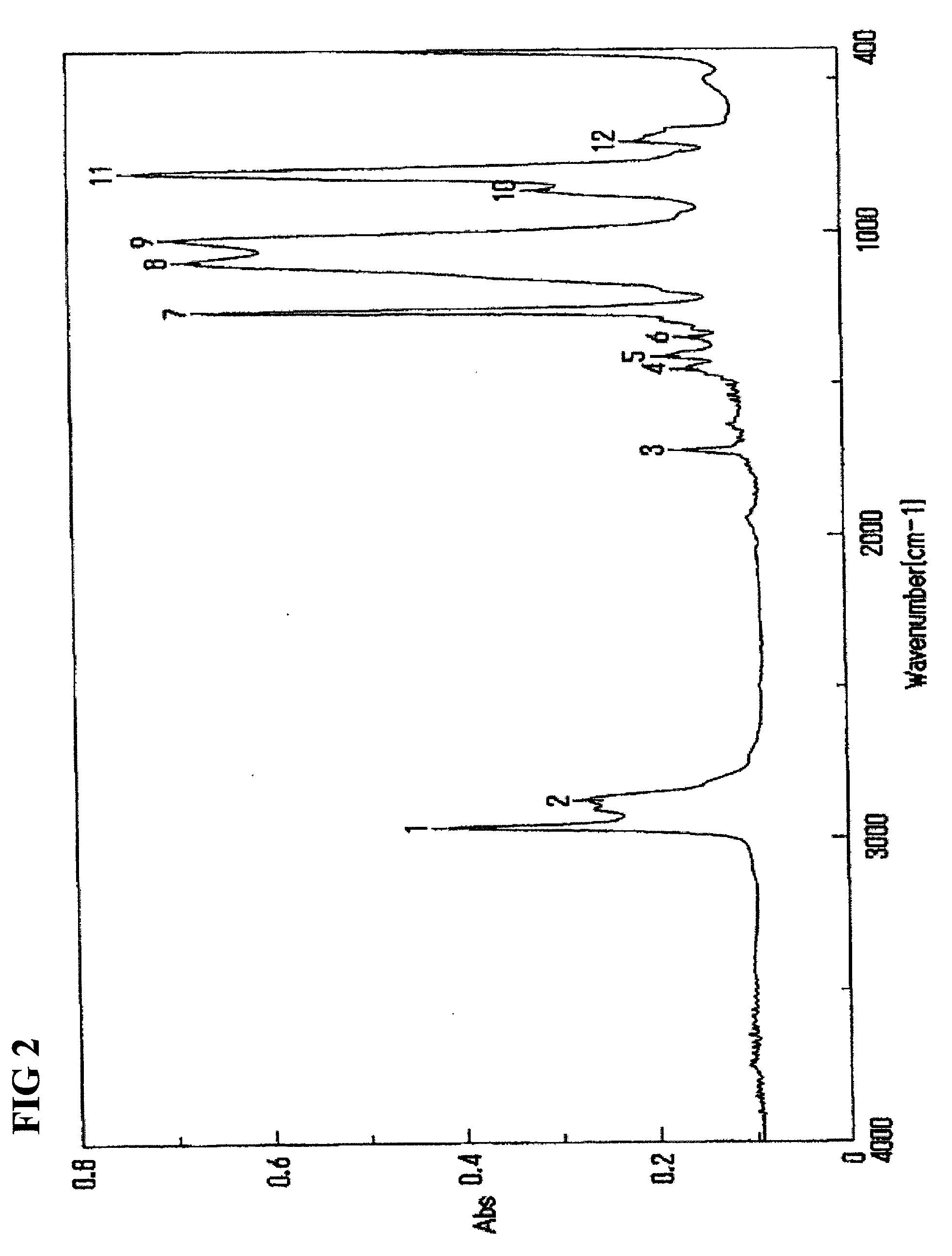
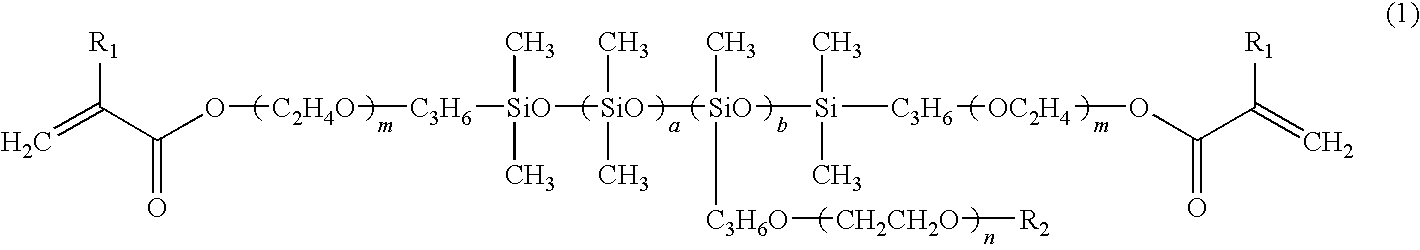
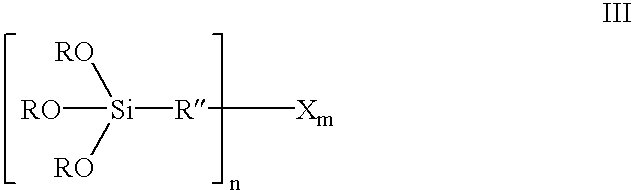Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8529results about "Silicon organic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
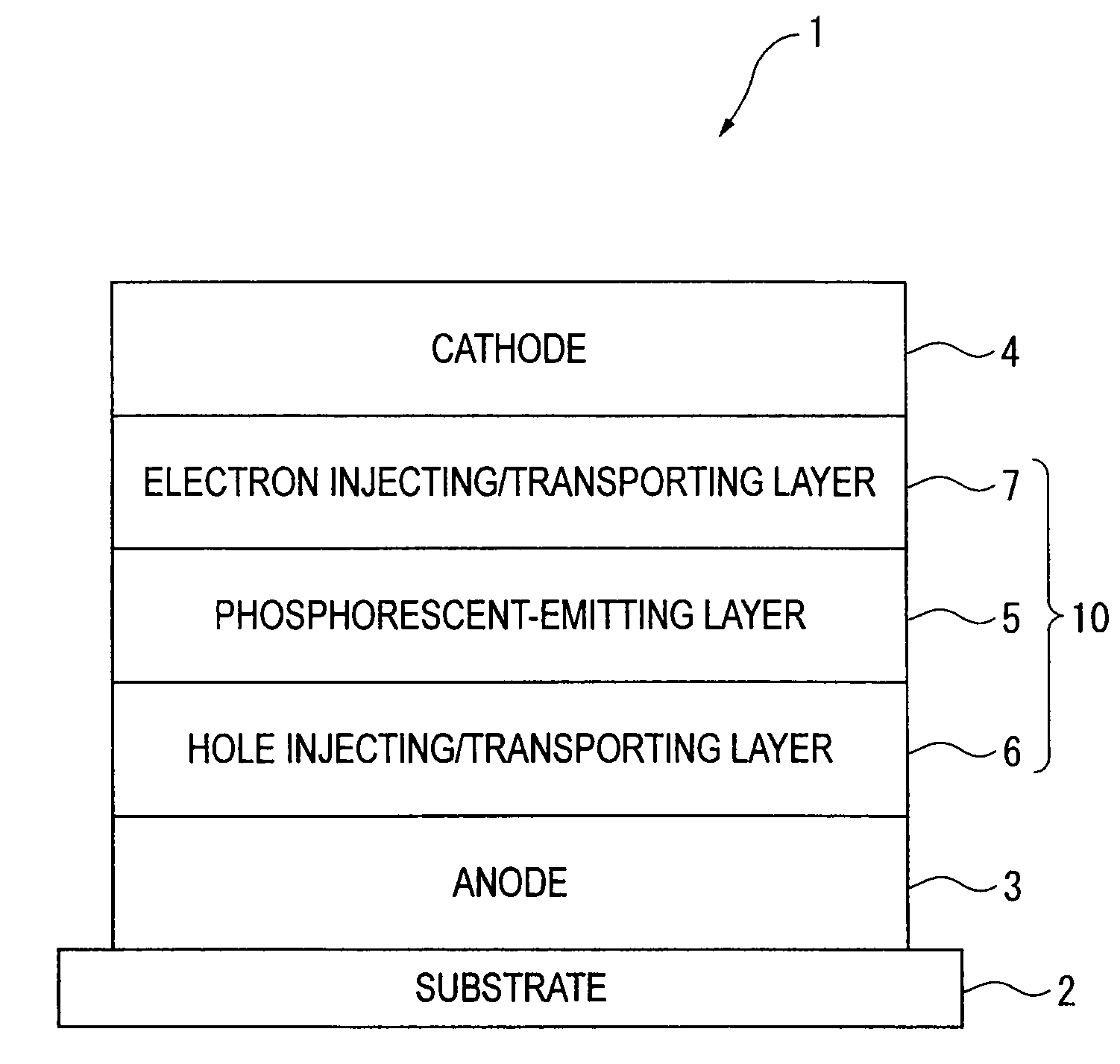
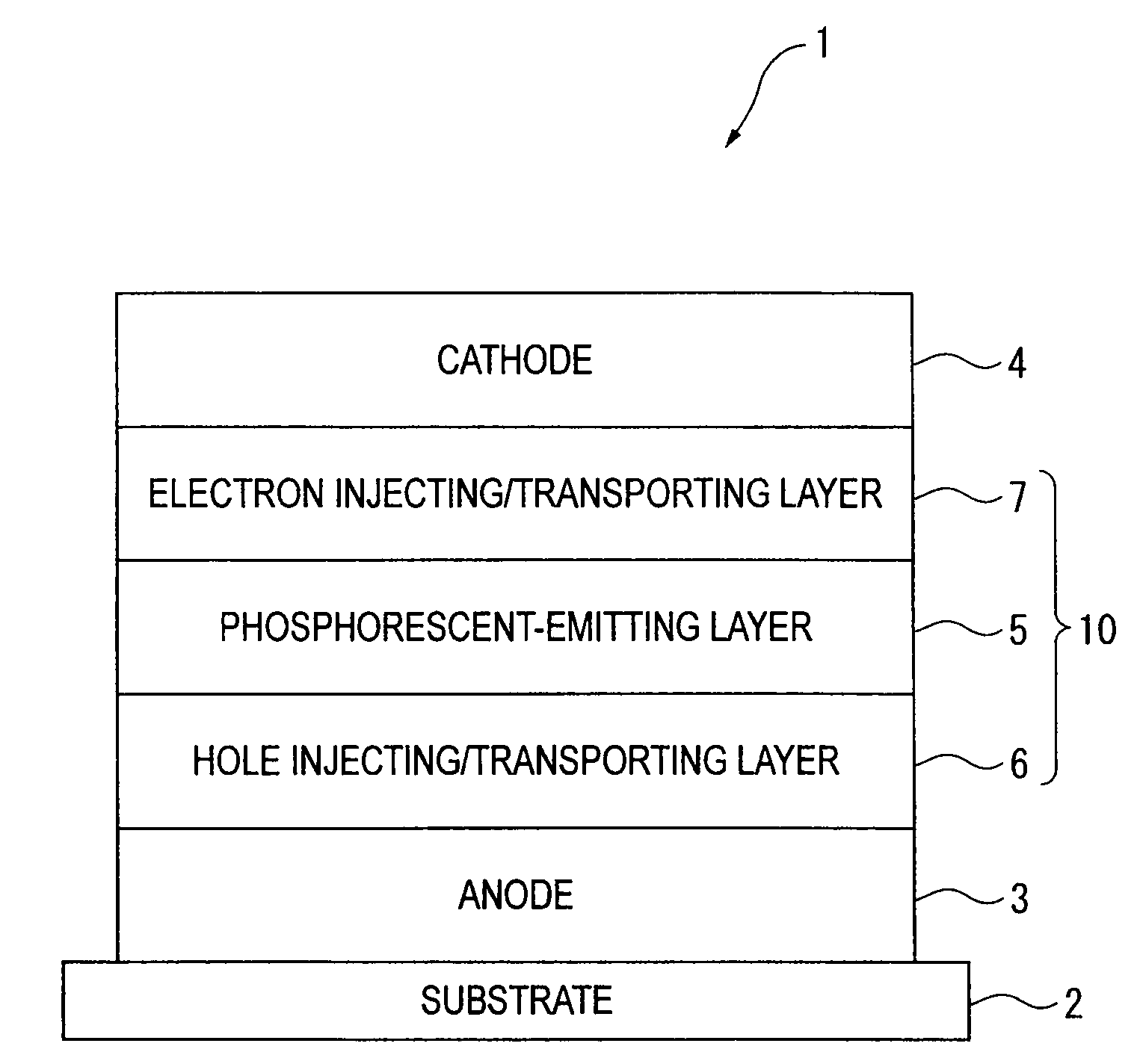
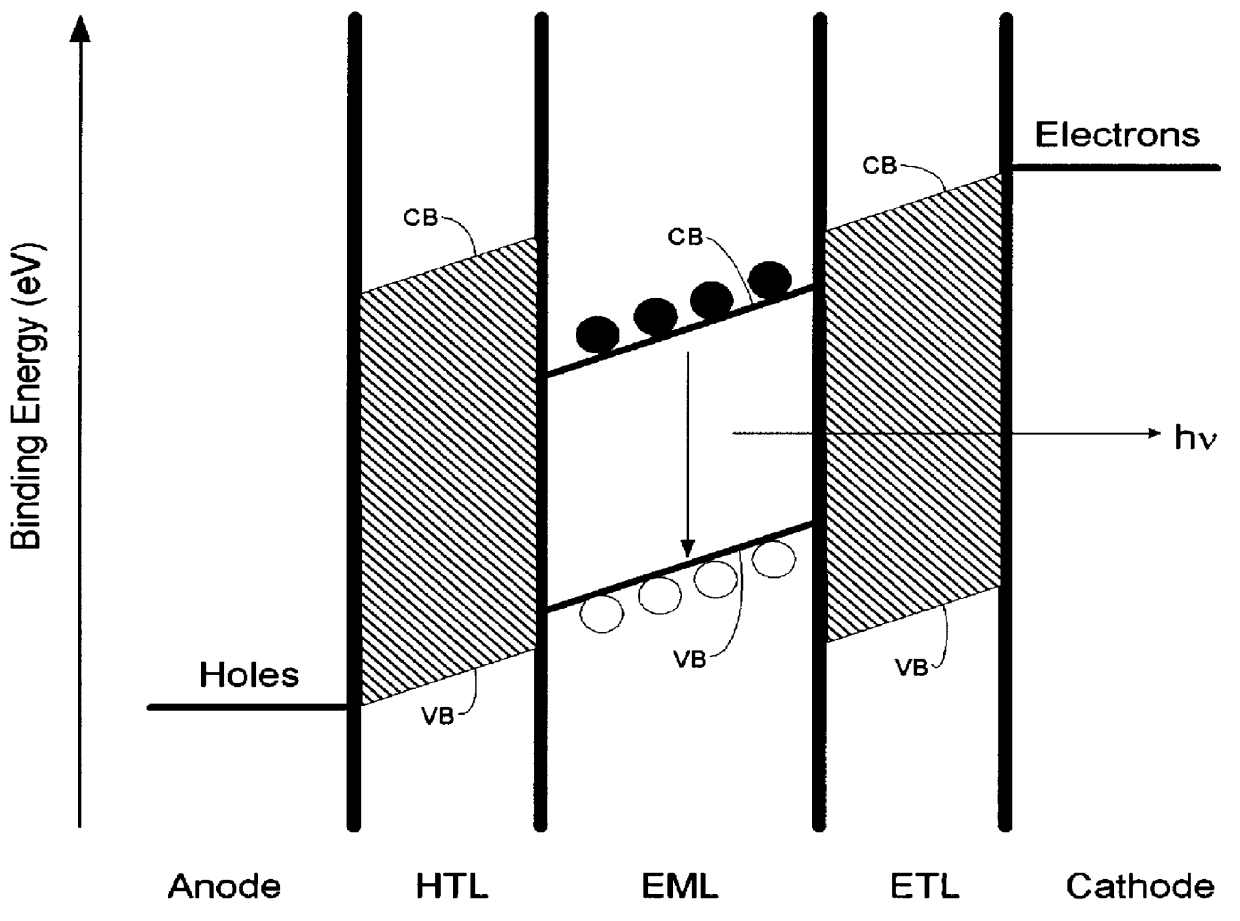
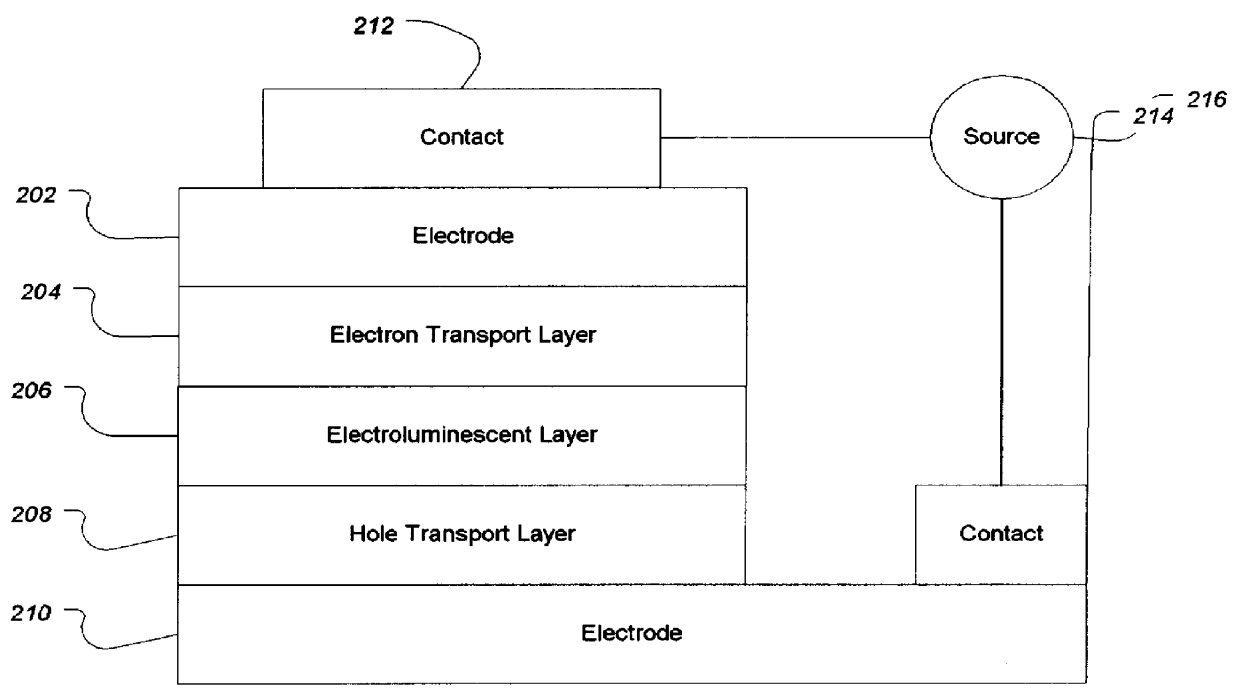
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009065A1Improve efficiencyLong lastingSilicon organic compoundsDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Electroluminescent (EL) devices
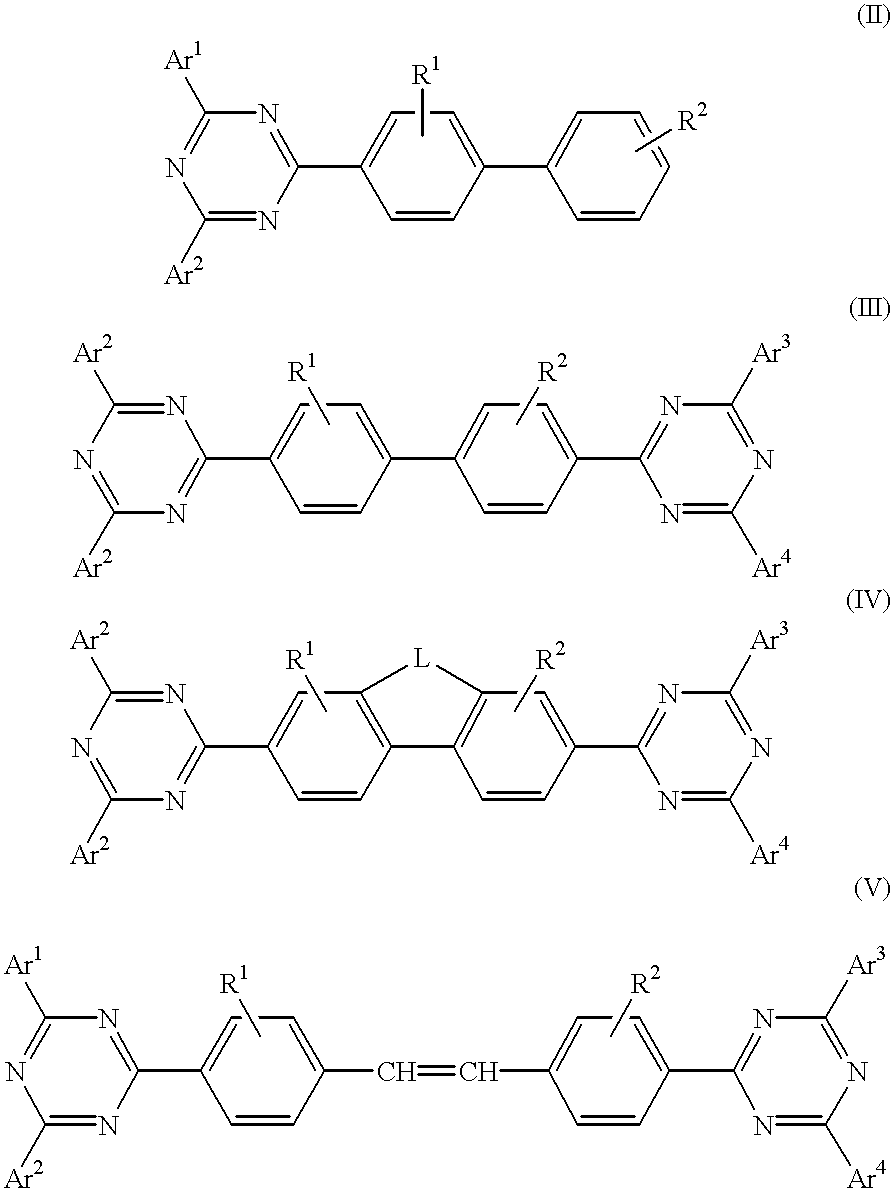
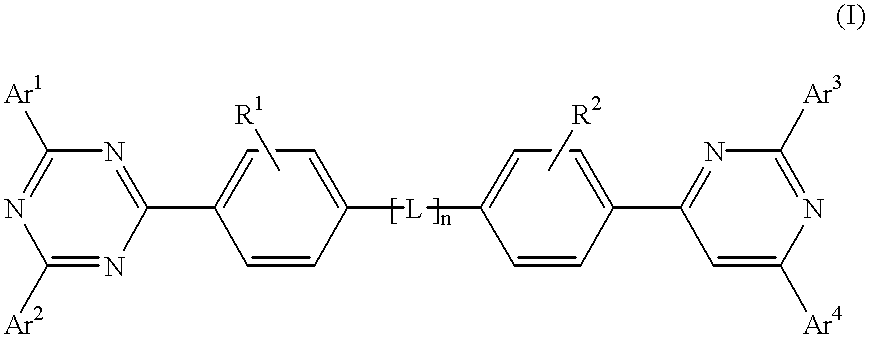
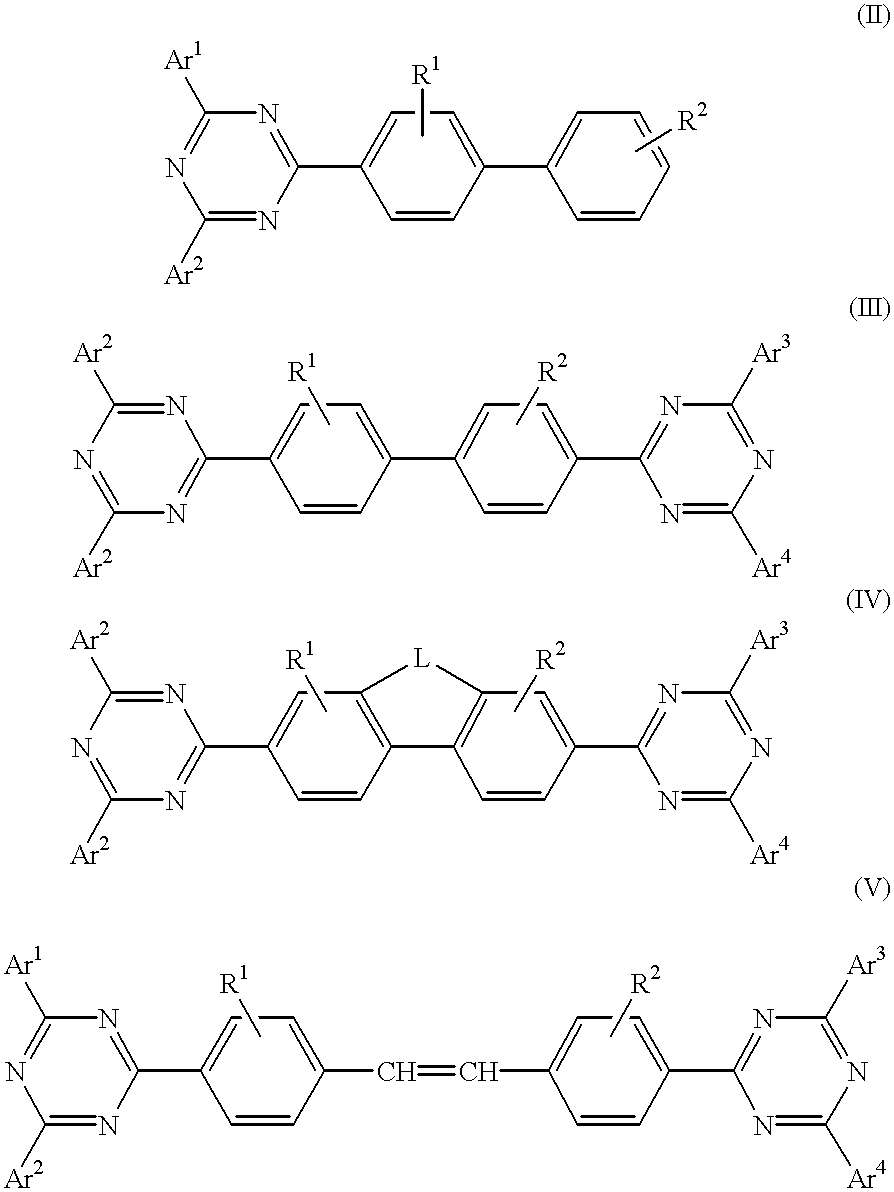
InactiveUS6225467B1Improve efficiencyIncreased durabilitySilicon organic compoundsElectroluminescent light sourcesArylHalogen
The triazinewherein Ar1, Ar2, Ar3, and Ar4 are each independently an aryl; R1 and R2 are substituents selected from the group consisting of hydrogen, an alkyl, an aryl, an alkoxy, a halogen atom, and a cyano; R3 and R4 are each a divalent group L selected from the group consisting of -C(R'R'')-, alkylene, an oxygen atom, a sulfur atom, and -Si(R'R'')-, wherein R' and R'' are selected from the group consisting of hydrogen, alkyl, alkoxy, and aryl.
Owner:LG DISPLAY CO LTD
Organosilicon compounds
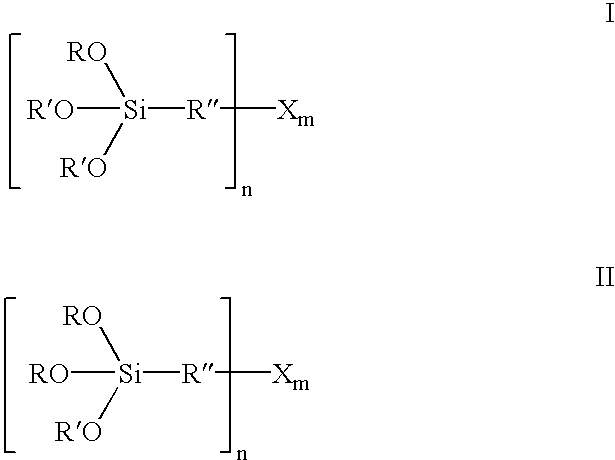
InactiveUS6849754B2Improve bindingAvoid large quantitiesSilicon organic compoundsOrganic chemistry methodsAlcoholSilanes
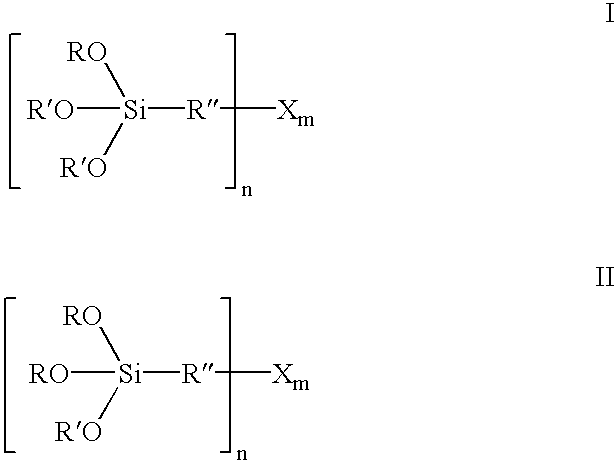
Organosilicon compounds of the general formula I and / or II are produced by reacting silanes of the general formula III with alcohols of the general formula R′—OH, with elimination of R—OH, wherein R—OH may be continuously separated from the reaction mixture by distillation. The organosilicon compounds may be used in rubber mixtures. Compounds, such as rubbers, produced using such organosilicon compounds.
Owner:EVONIK DEGUSSA GMBH
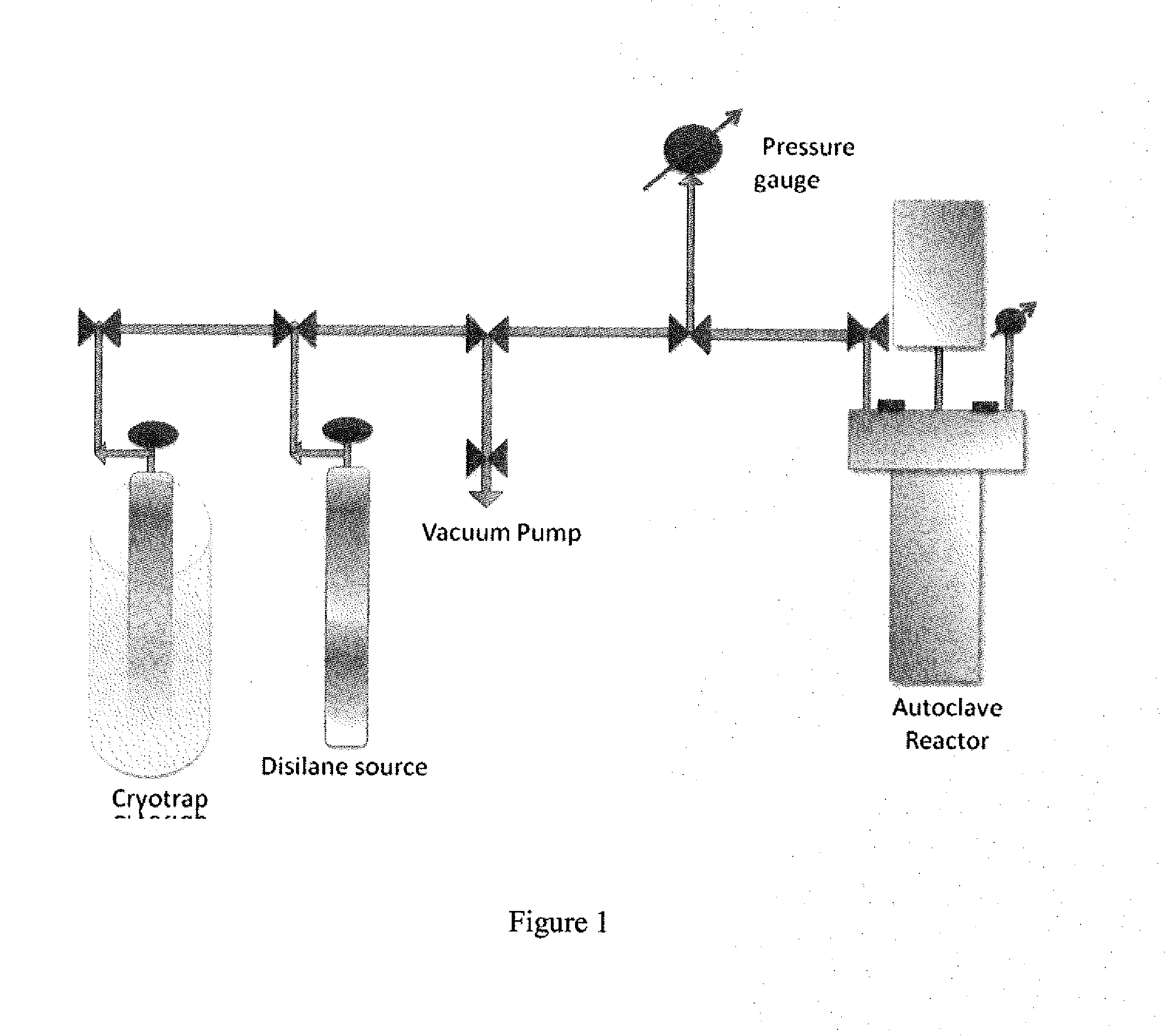
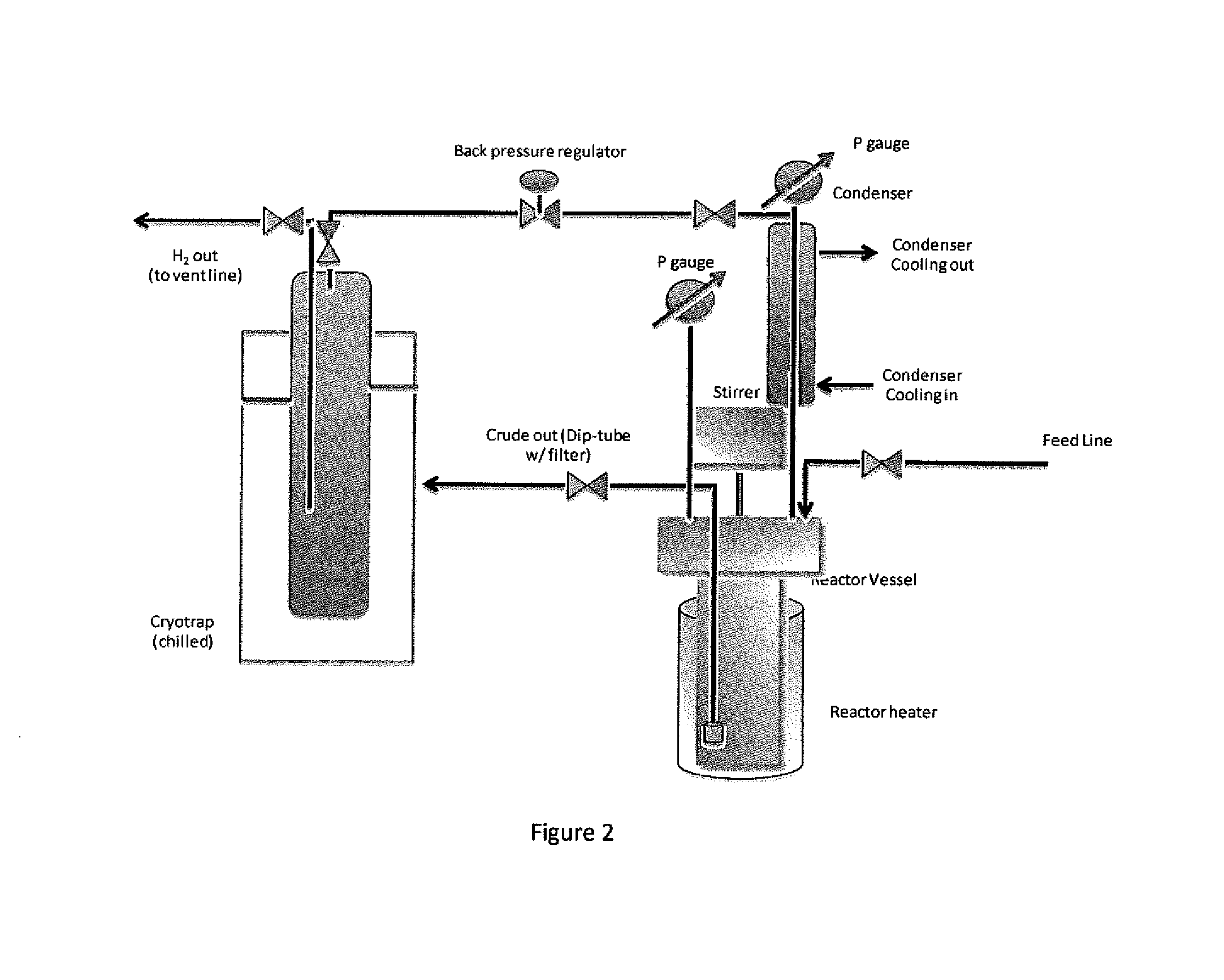
Precursors for depositing silicon containing films and processes thereof
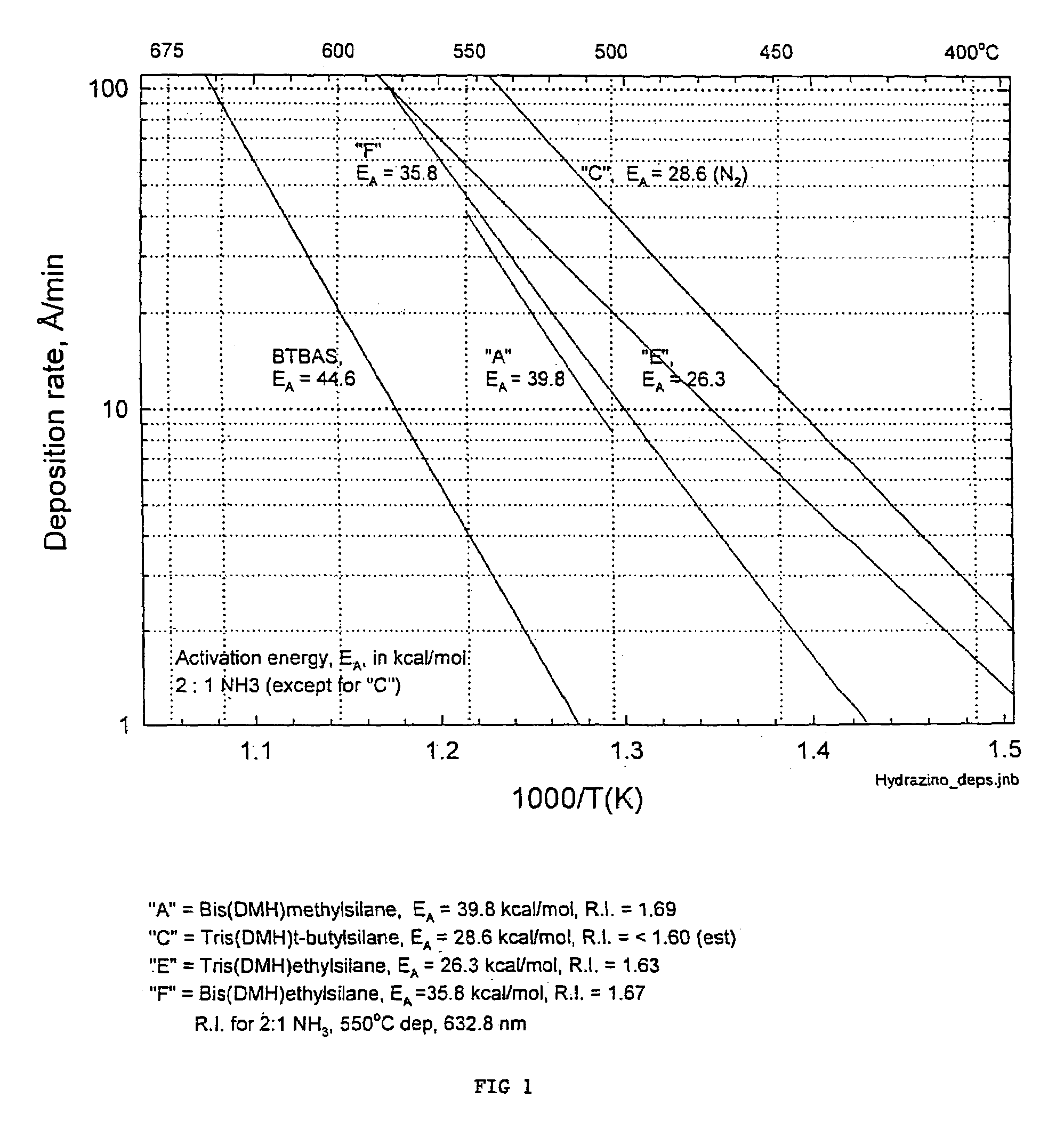
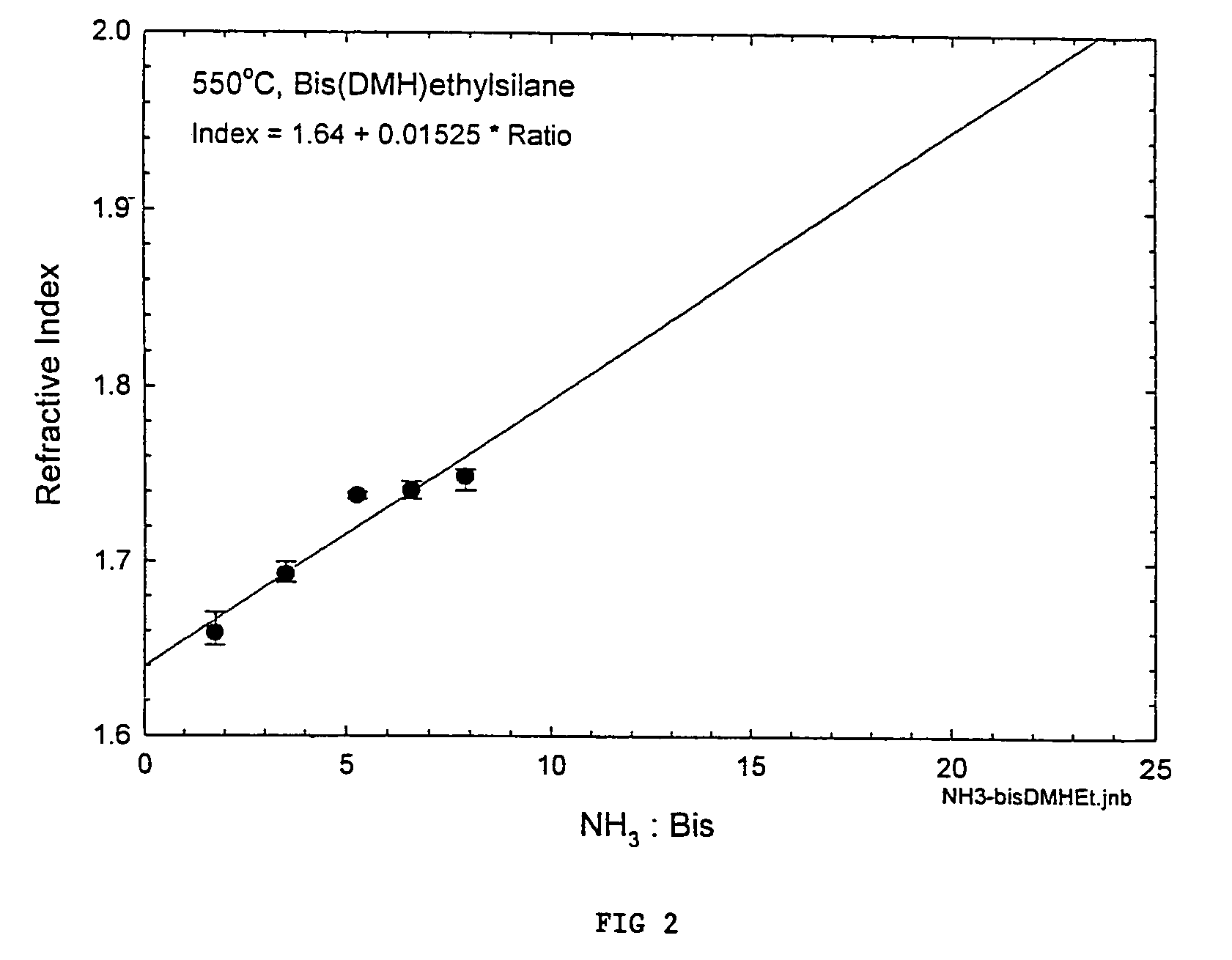
Processes for precursors for silicon dielectric depositions of silicon nitride, silicon oxide and silicon oxynitride on a substrate using a hydrazinosilane of the formula:[R12N—NH]nSi(R2)4−nwhere each R1 is independently selected from alkyl groups of C1 to C6; each R2 is independently selected from the group consisting of hydrogen, alkyl, vinyl, allyl, and phenyl; and n=1–4. Some of the hydrazinosilanes are novel precursors.
Owner:VERSUM MATERIALS US LLC
Polymerization catalysts for producing high melt index polymers without the use of hydrogen
ActiveUS7026494B1High porositySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPolyolefinHydrogen
Various catalyst compositions including the contact product of at least one ansa-metallocene compound, at least one organoaluminum compound, and at least one activator-support are disclosed. Processes for forming such compositions and for forming polyolefins with such compositions are also disclosed. Metallocene compounds are also presented.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
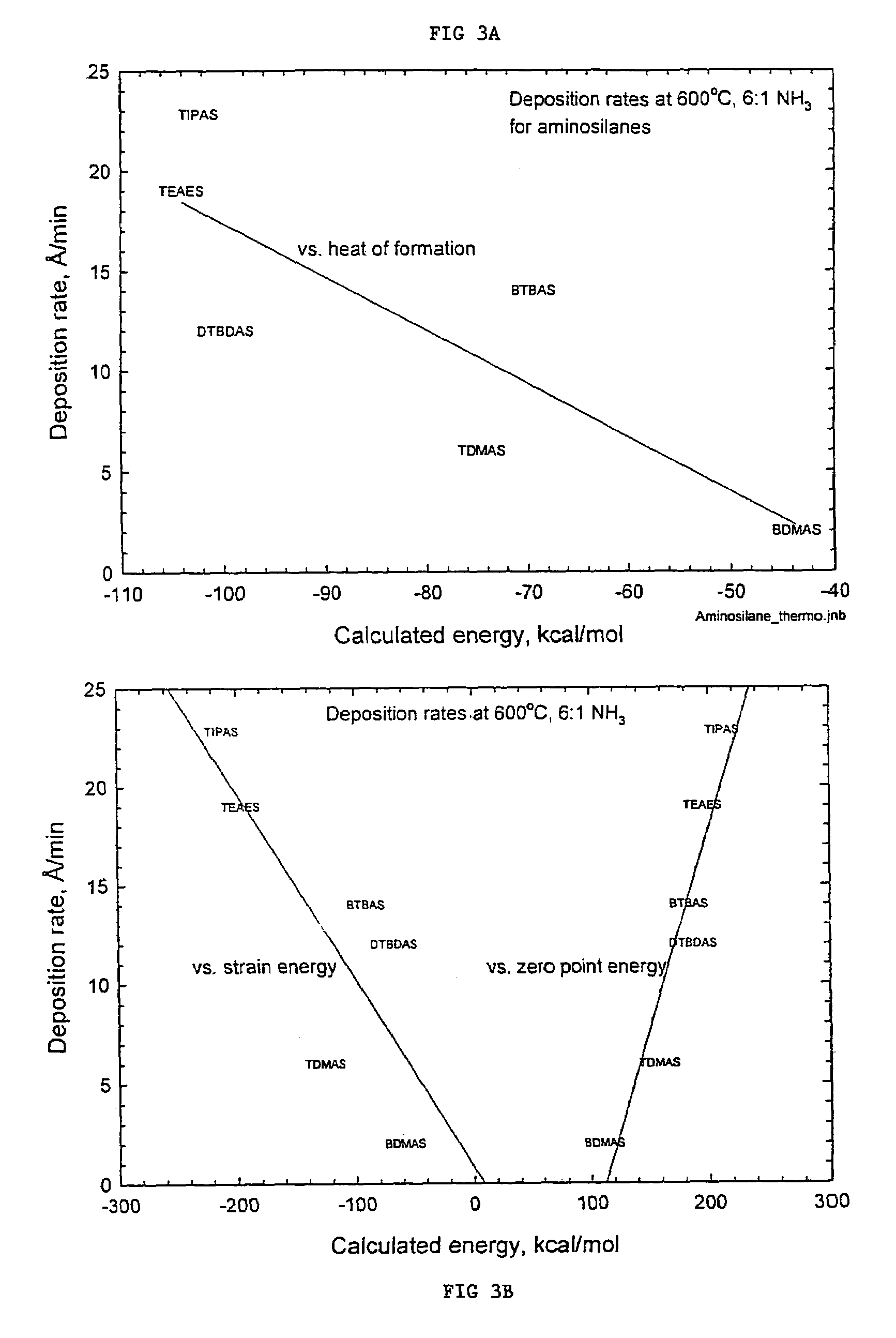
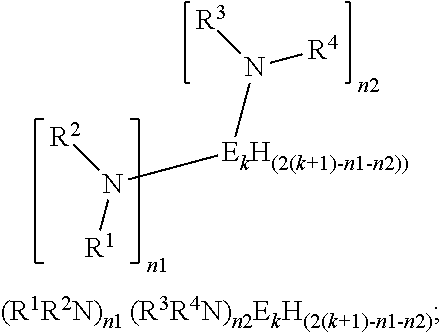
Organoaminodisilane precursors and methods for depositing films comprising same
Described herein are precursors and methods for forming silicon-containing films. In one aspect, there is a precursor of following Formula I:wherein R1 and R3 are independently selected from linear or branched C3 to C10 alkyl group, a linear or branched C3 to C10 alkenyl group, a linear or branched C3 to C10 alkynyl group, a C1 to C6 dialkylamino group, an electron withdrawing and a C6 to C10 aryl group; R2 and R4 are independently selected from hydrogen, a linear or branched C3 to C10 alkyl group, a linear or branched C3 to C10 alkenyl group, a linear or branched C3 to C10 alkynyl group, a C1 to C6 dialkylamino group, an electron withdrawing, and a C6 to C10 aryl group; and wherein any one, all, or none of R1 and R2, R3 and R4, R1 and R3, or R2 and R4 are linked to form a ring.
Owner:VERSUM MATERIALS US LLC
Organoaminodisilane precursors and methods for depositing films comprising same
Described herein are precursors and methods for forming silicon-containing films. In one aspect, there is provided a precursor of Formula I:wherein R1 is selected from linear or branched C3 to C10 alkyl group, linear or branched C3 to C10 alkenyl group, linear or branched C3 to C10 alkynyl group, C1 to C6 dialkylamino group, electron withdrawing group, and C6 to C10 aryl group; R2 is selected from hydrogen, linear or branched C1 to C10 alkyl group, linear or branched C3 to C6 alkenyl group, linear or branched C3 to C6 alkynyl group, C1 to C6 dialkylamino group, C6 to C10 aryl group, linear or branched C1 to C6 fluorinated alkyl group, electron withdrawing group, and C4 to C10 aryl group; optionally wherein R1 and R2 are linked together to form ring selected from substituted or unsubstituted aromatic ring or substituted or unsubstituted aliphatic ring; and n=1 or 2.
Owner:VERSUM MATERIALS US LLC
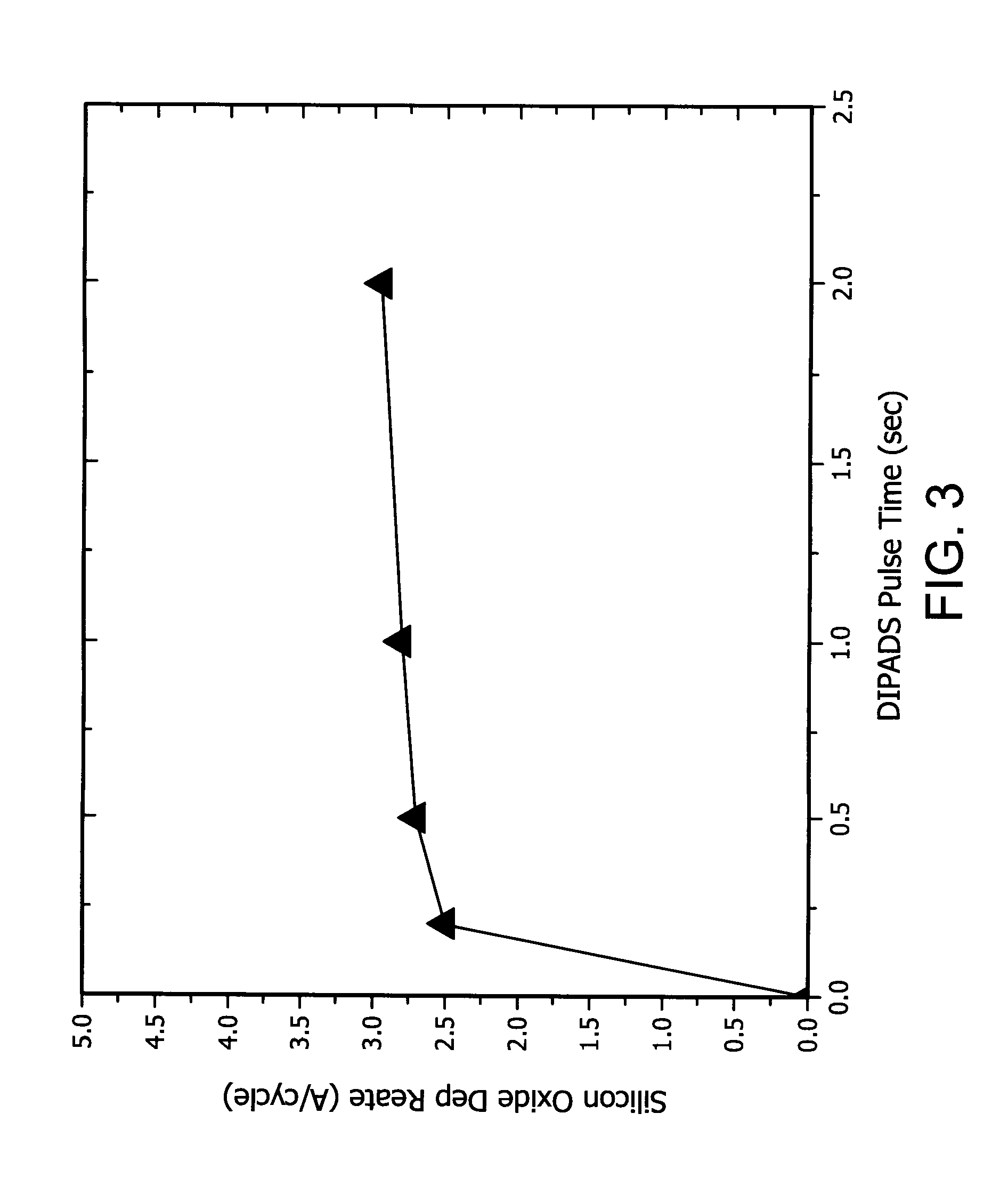
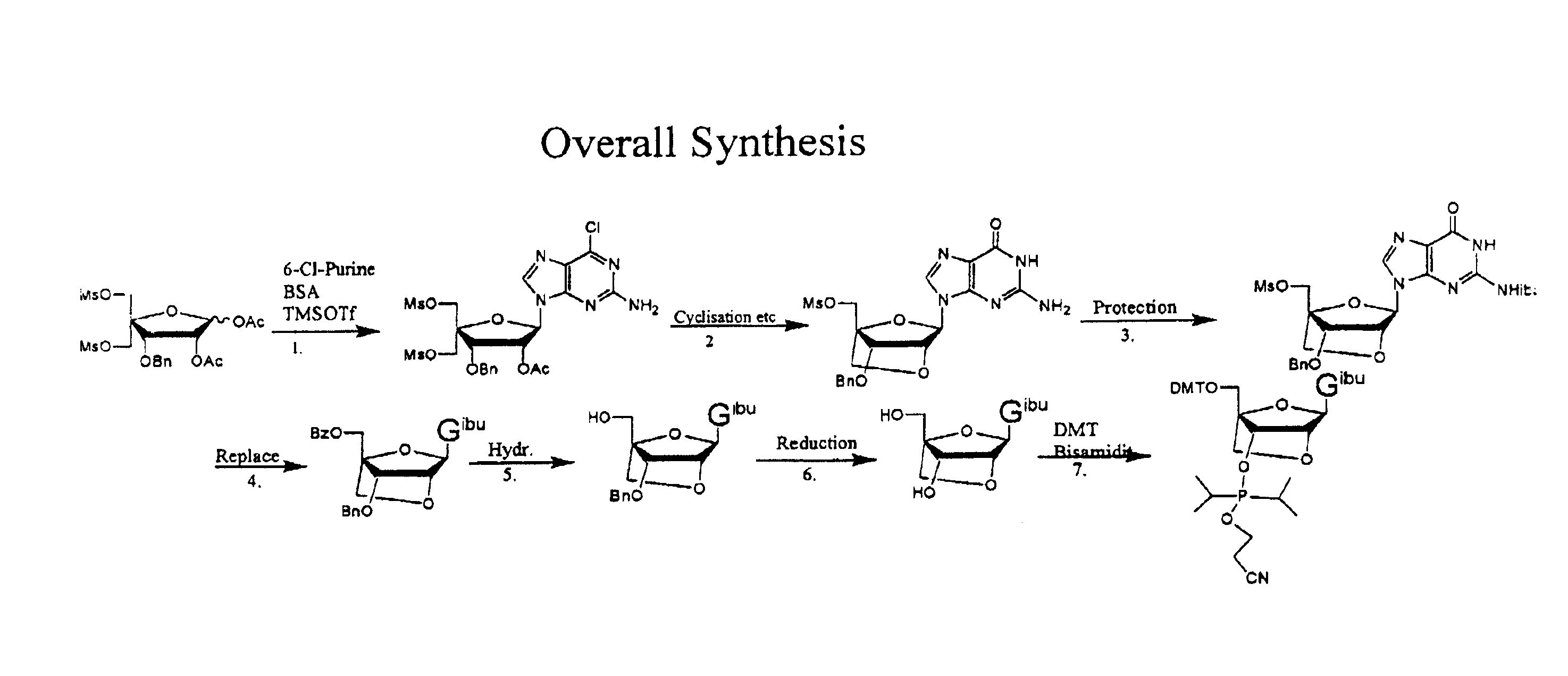
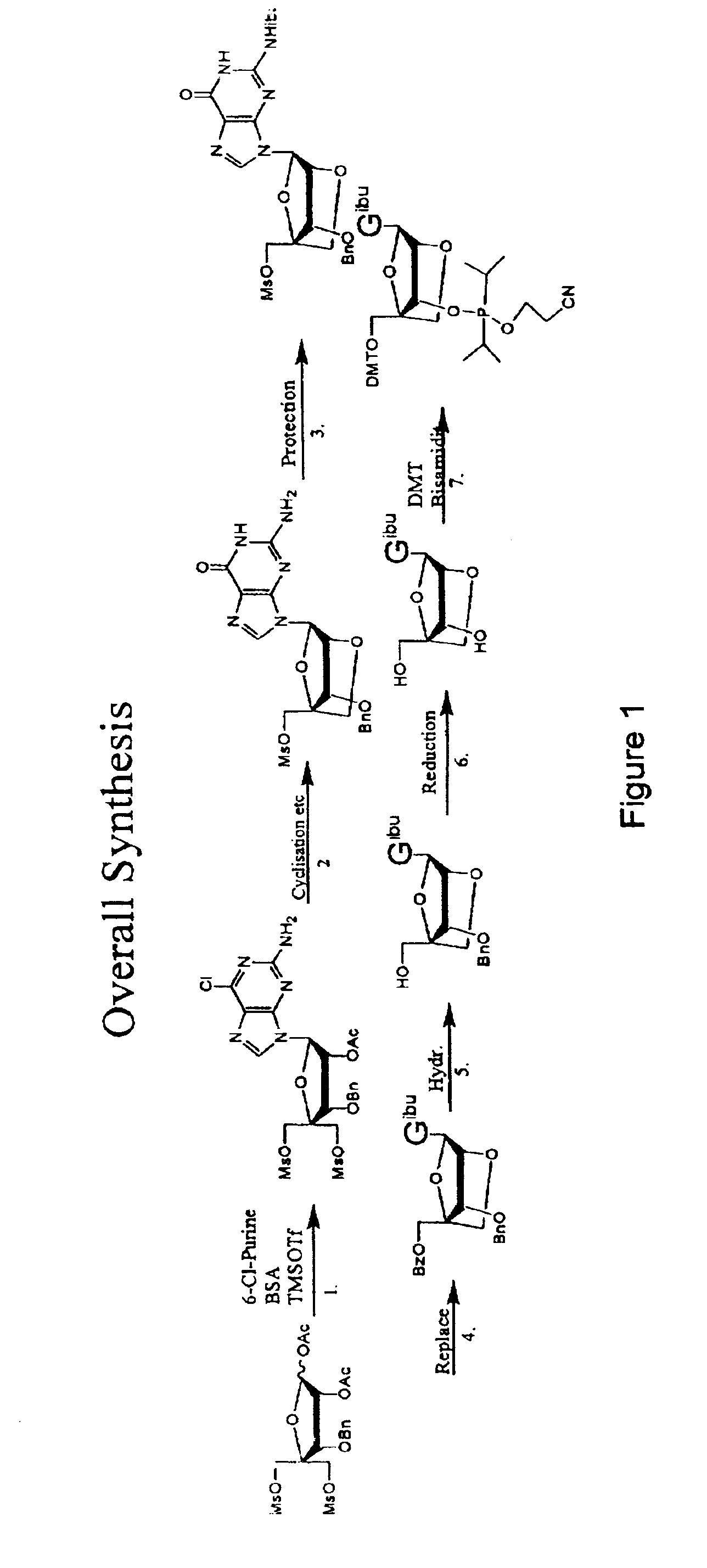
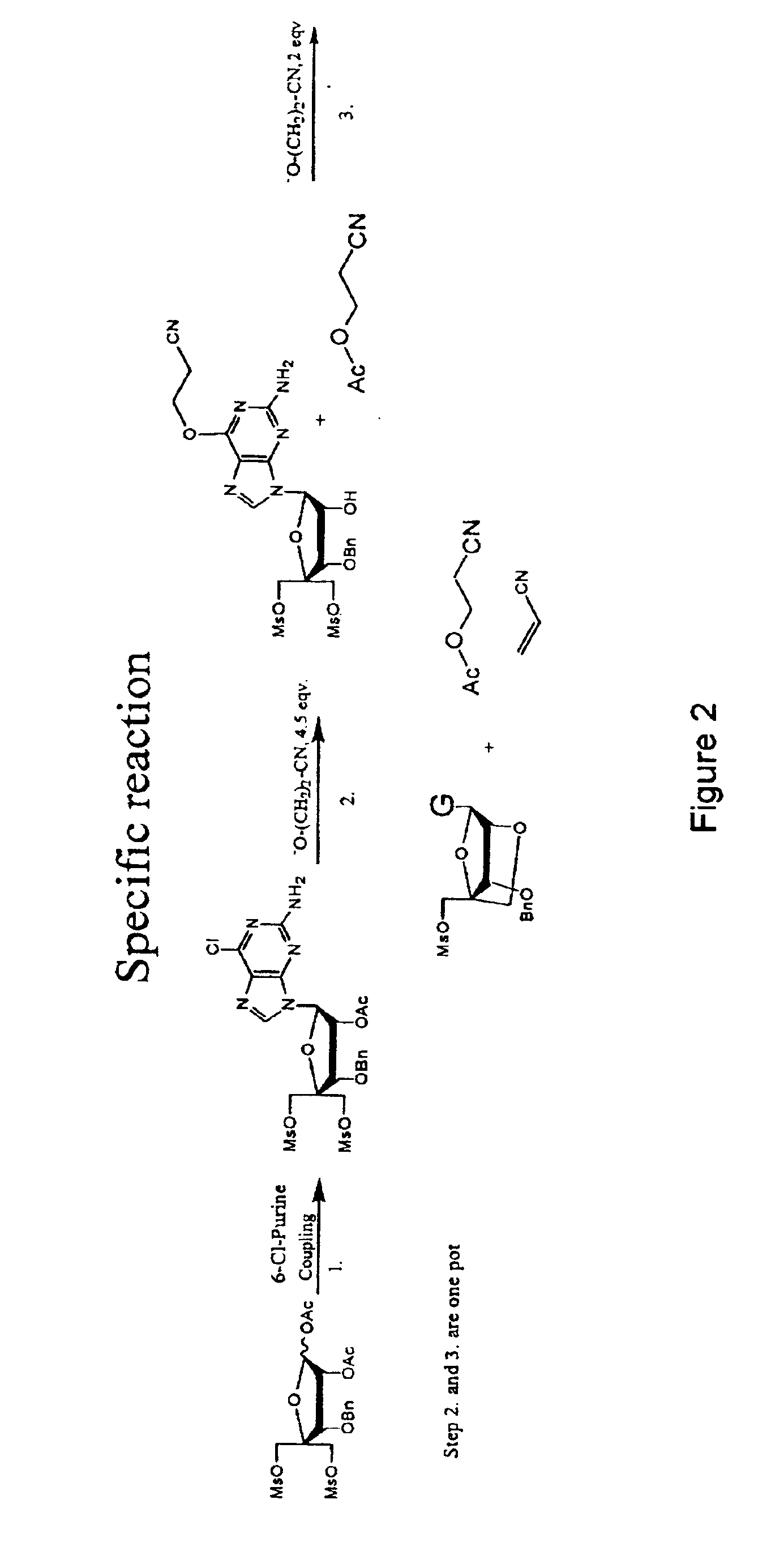
Synthesis of purine locked nucleic acid analogues
InactiveUS6998484B2Easy to convertEasy to liftSilicon organic compoundsSugar derivativesPurineLocked nucleic acid
The present invention relates to a new method for the synthesis of purine LNA (Locked Nucleic Acid) analogues which provides a higher overall yield. The method comprising a regioselective 9-N purine glycosylation reaction followed by a one-pot nucleophilic aromatic substitution reaction of the 6-substituent in the purine ring and simultaneous nucleophile-induced intramolecular ring closure of the C-branched carbohydrate to form novel purine LNA analogues. The novel strategy is illustrated by the synthesis of the novel compound (1S,3R,4R,7S)-7-benzyloxy-1-methanesulfonylmethyl-3-(guanin-9-yl)-2,5-dioxabicyclo[2.2.1]heptane which is easily converted into (1S,3R,4R,7S)-7-hydroxy-1-hydroxymethyl-3-((2-N-isobutyrylguanin-9-yl)-2,5-dioxabicyclo[2.2.1]heptane after isobutyryl protection of the 2-amino purine group and subsequent substitution of 1-methanesulfonyl with benzoate, debenzoylation and debenzylation.
Owner:SANTARIS PHARMA AS
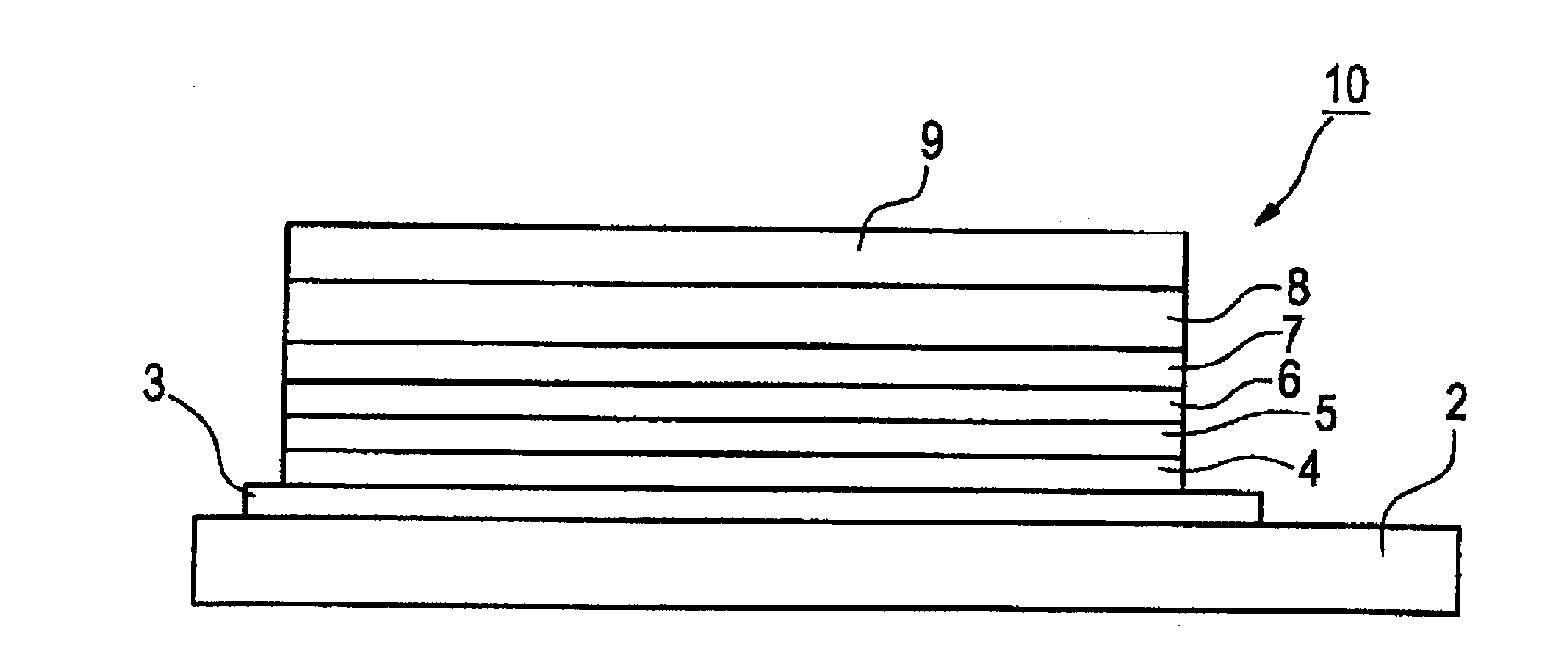
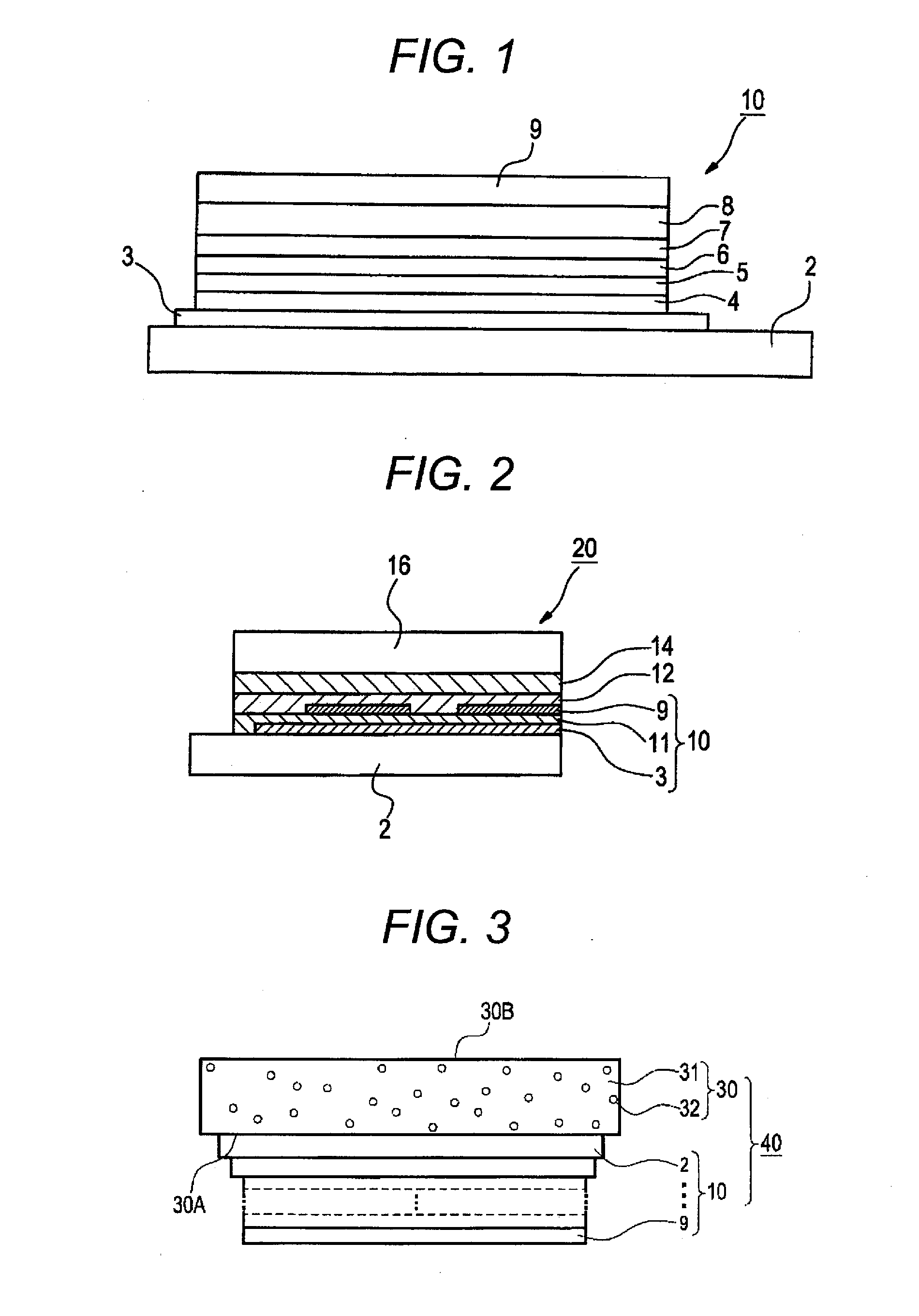
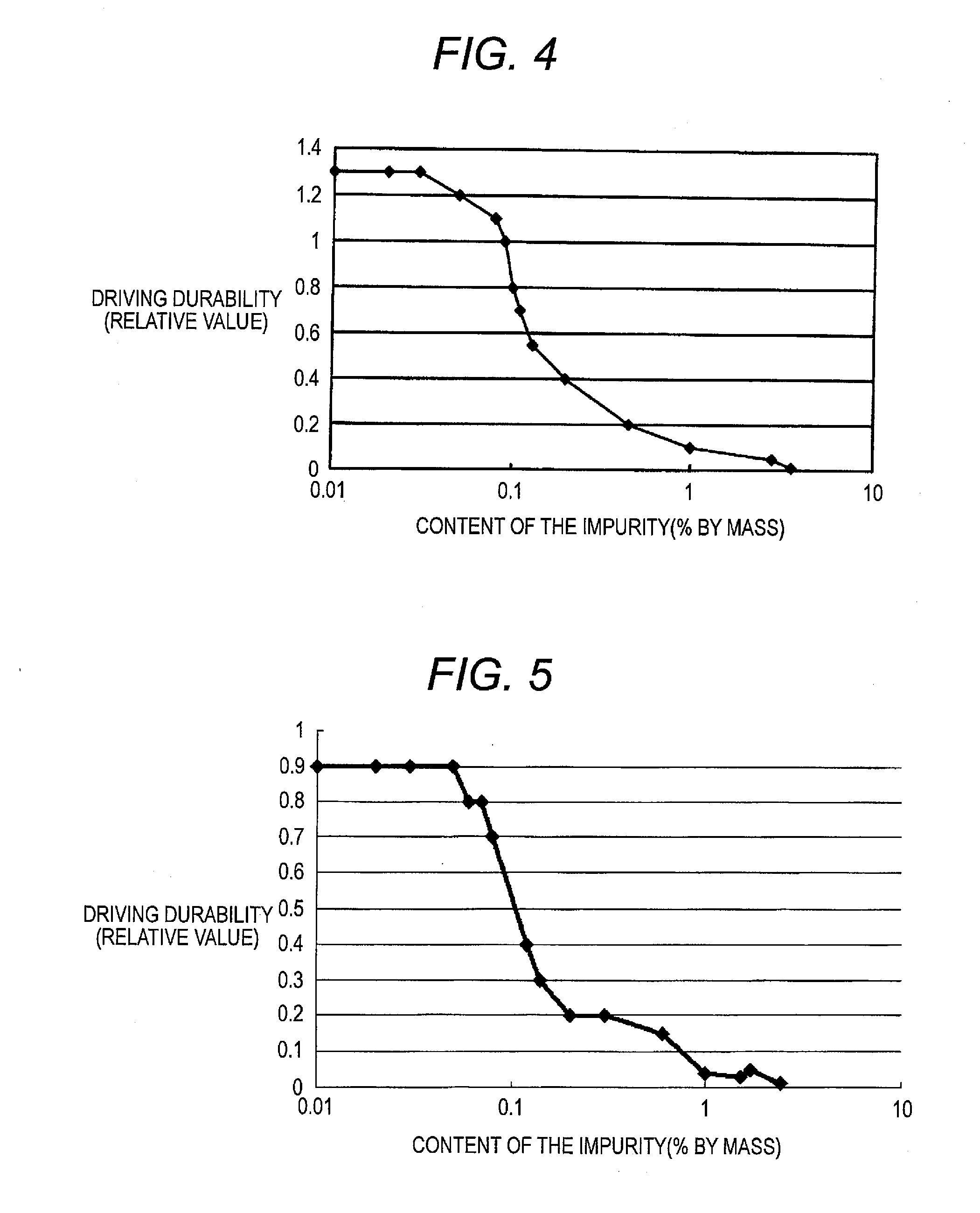
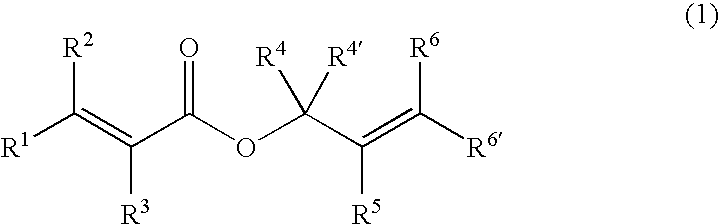
Charge-transporting material and organic electroluminescence device
InactiveUS20120126221A1Improve light emission efficiencyIncreased durabilitySilicon organic compoundsSolid-state devicesHalogenOrganic layer
A charge-transporting material contains a compound represented by the following formula (1) in an organic layer, in which the contents of specific halogen-containing compounds are 0.1% or less to the compound represented by formula (1). In formula (1), each of A1 and A2 independently represents N, —CH or —CR; R represents a substituent; L represents a single bond, an arylene group, a cycloalkylene group or an aromatic heterocyclic group; each of R1 to R5 independently represents a substituent; each of n1, n2 and n3 independently represents an integer of 0 to 4; each of n4 and n5 independently represents an integer of 0 to 5; and each of p and q independently represents an integer of 1 to 4.
Owner:UDC IRELAND
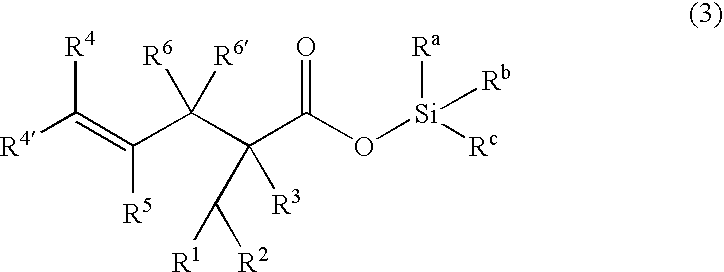
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS7307178B2Few stepsHigh yieldSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM CO LTD
Method of depositing catalyst assisted silicates of high-k materials
InactiveUS20080113096A1Reduce the temperatureSilicon organic compoundsChemical vapor deposition coatingSoaking ProcedureHafnium
A high-k silicate atomic layer deposition method is disclosed. To produce a hafnium silicate layer, a substrate may be exposed to a pulse of a hafnium precursor, a pulse of an oxidizer, a pulse of a silicon precursor, and a pulse of another oxidizer. A catalyst may additionally be co-flowed with one or more reactants into the chamber through a separate inlet. Alternatively, the catalyst may be flowed to the chamber before the reactant is introduced in a soaking procedure. By either co-flowing the catalyst through separate inlets or by performing a catalyst soak, hafnium silicate formation may proceed at a fast rate and / or at a low temperature.
Owner:APPLIED MATERIALS INC
Organoaminosilane Precursors and Methods for Depositing Films Comprising Same
Owner:VERSUM MATERIALS US LLC
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS20050070729A1High yieldFew stepsSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM IND CO LTD
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
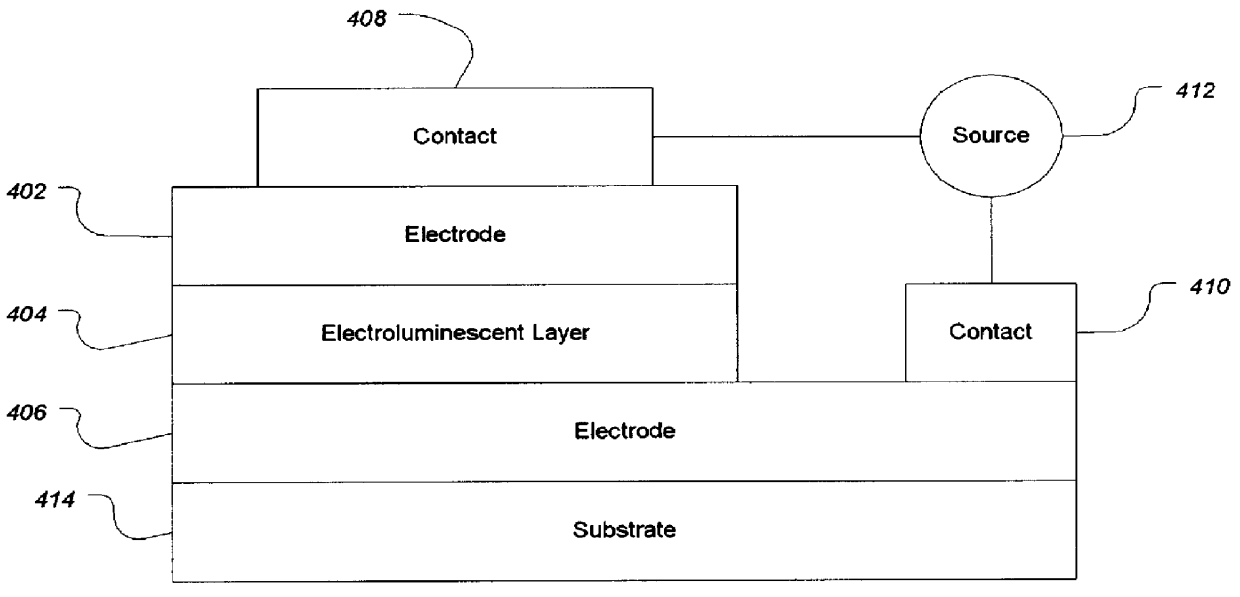
Organic electroluminescent materials and device made from such materials
Electroluminescent devices and materials including an electroluminescent organo-siloxane polymer. The main chain of the organo-siloxane polymer comprises an organic component that can be alkenyl, alkynyl, aralkyl, aryl, heteroaralkyl, and heteroaryl which can be substituted optionally with hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, or sulfonyloxy. The organic component includes at least two covalent bonds coupling the organic component to the main chain of the organo-siloxane polymer. Such devices provide superior performance and mechanical stability compared with conventional organic electroluminescent materials and devices made from such materials.
Owner:ORGANIC DISPLAY TECH
Organoaminosilane precursors and methods for making and using same
Described herein are organoaminosilane precursors which can be used to deposit silicon containing films which contain silicon and methods for making these precursors. Also disclosed herein are deposition methods for making silicon-containing films or silicon containing films using the organoaminosilane precursors described herein. Also disclosed herein are the vessels that comprise the organoaminosilane precursors or a composition thereof that can be used, for example, to deliver the precursor to a reactor in order to deposit a silicon-containing film.
Owner:VERSUM MATERIALS US LLC
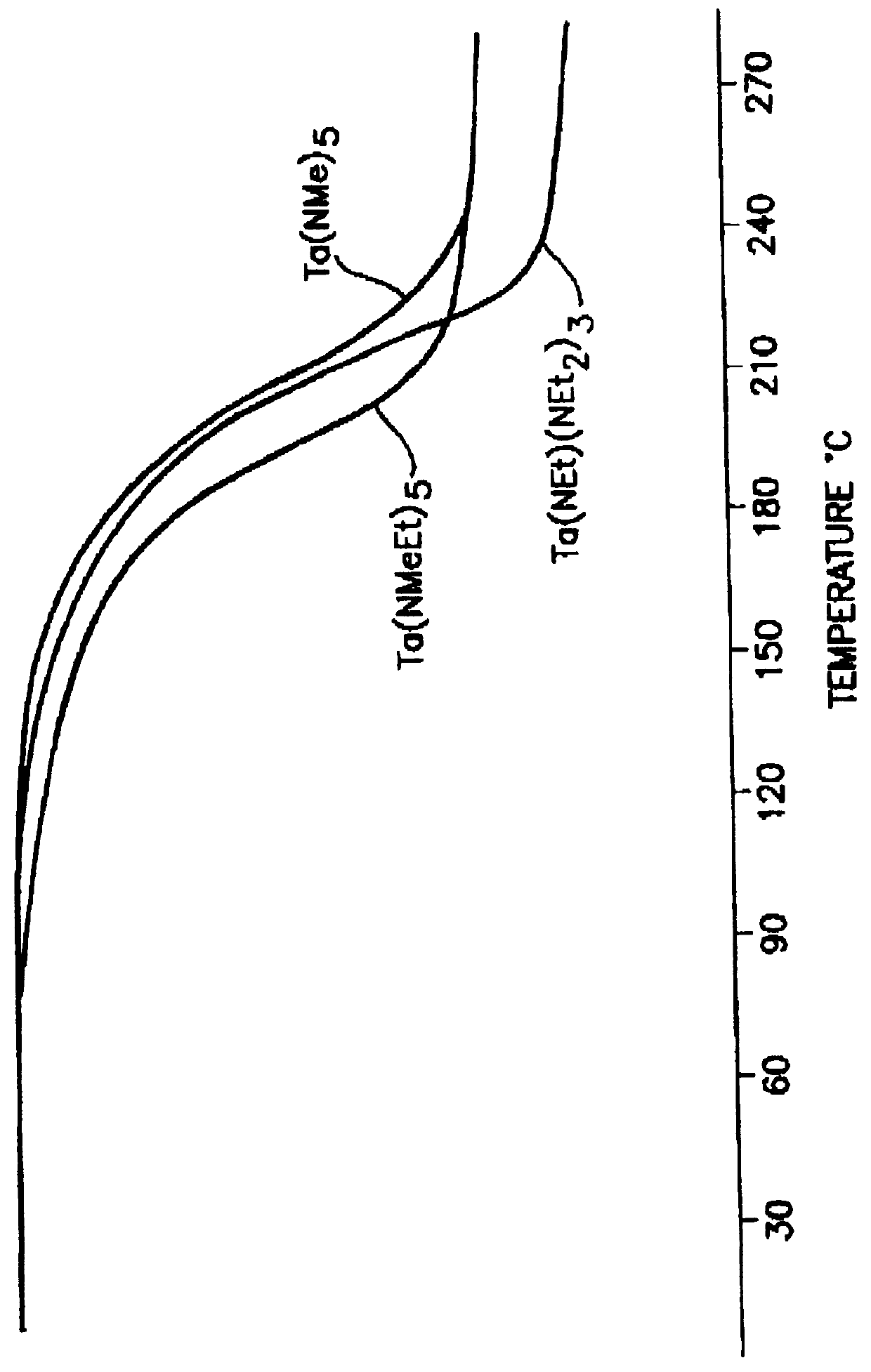
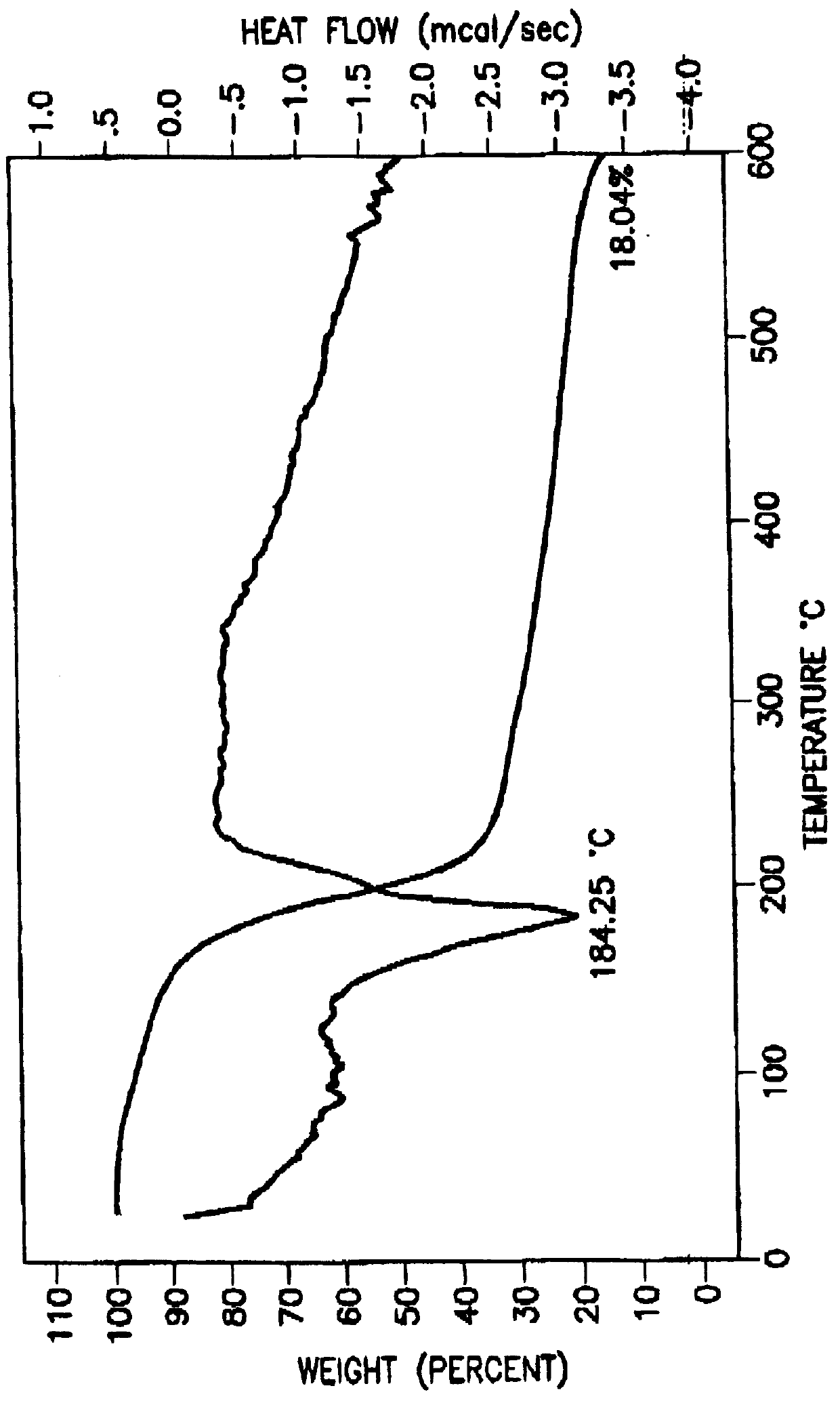
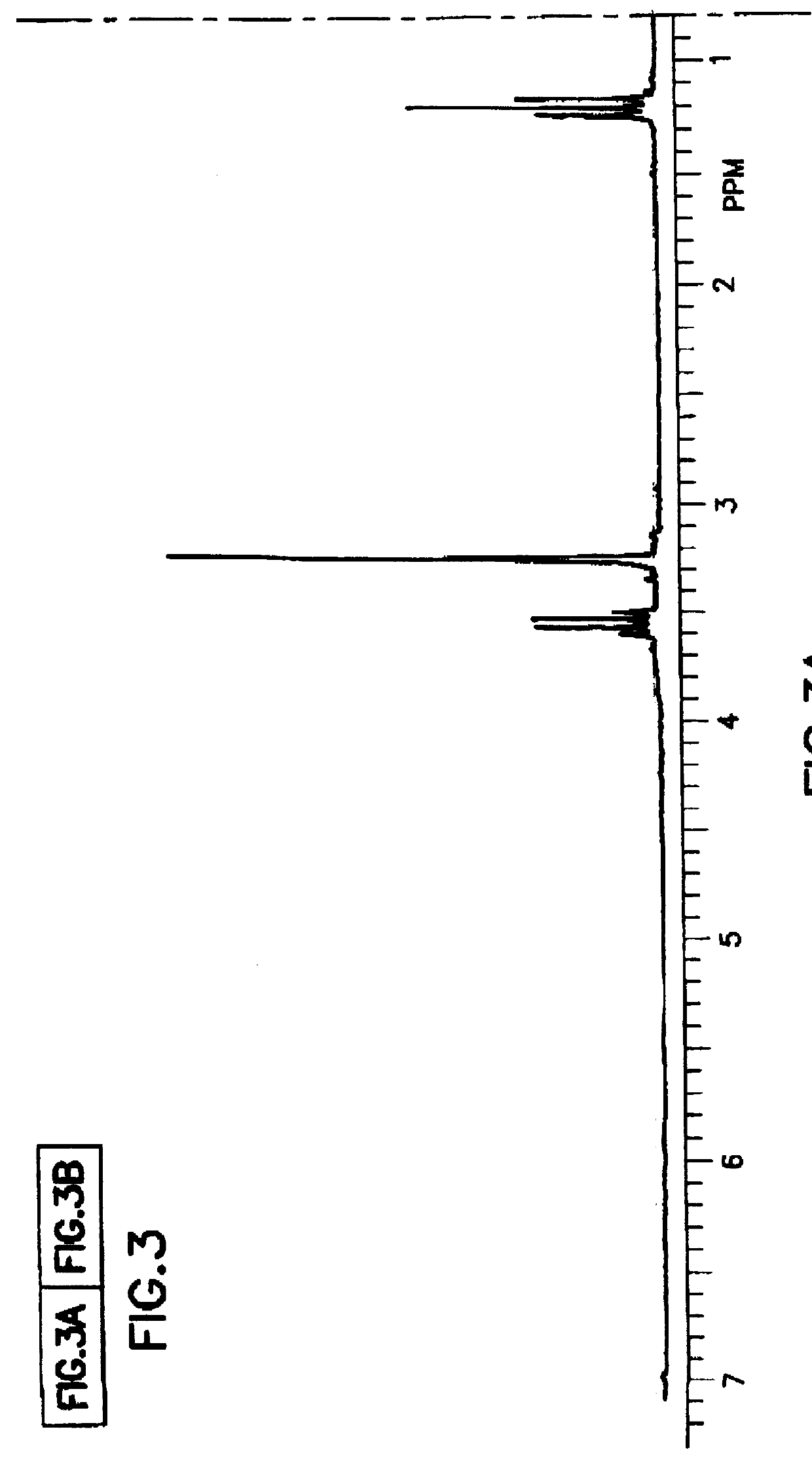
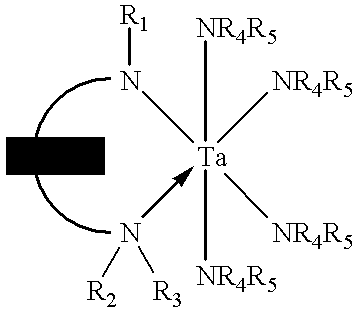
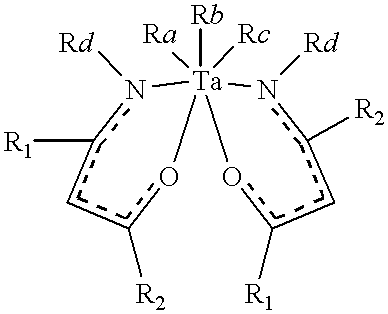
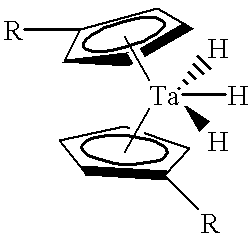
Tantalum amide precursors for deposition of tantalum nitride on a substrate
InactiveUS6015917ARapid heat treatmentSilicon organic compoundsPolycrystalline material growthFerroelectric thin filmsChemical vapor deposition
Tantalum and titanium source reagents are described, including tantalum amide and tantalum silicon nitride precursors for the deposition of tantalum nitride material on a substrate by processes such as chemical vapor deposition, assisted chemical vapor deposition, ion implantation, molecular beam epitaxy and rapid thermal processing. The precursors may be employed to form diffusion barrier layers on microlectronic device structures enabling the use of copper metallization and ferroelectric thin films in device construction.
Owner:ENTEGRIS INC
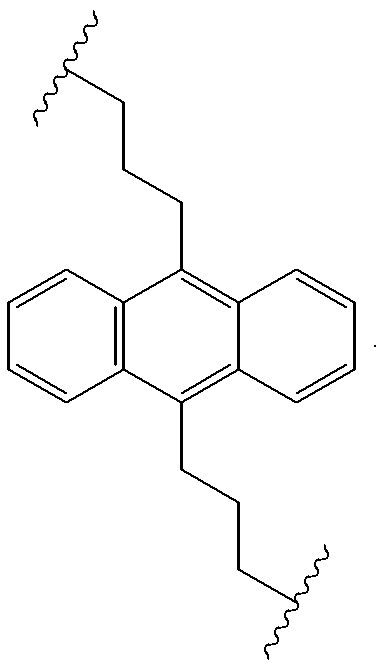
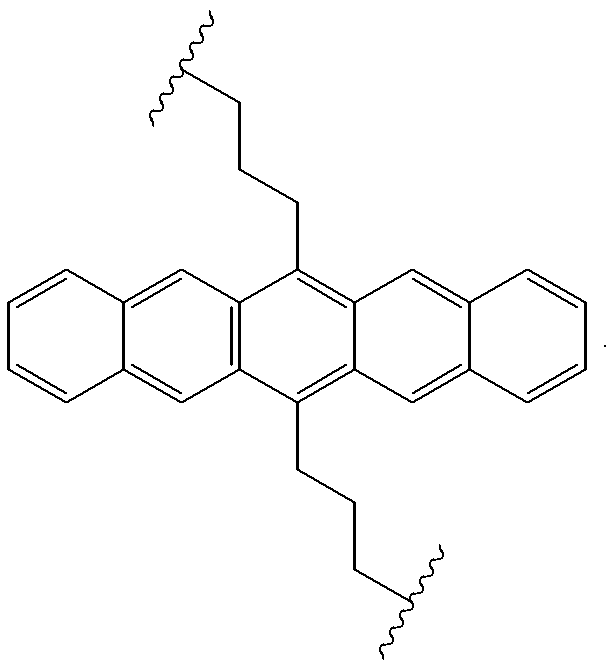
Useful precursors for organic electroluminescent materials and devices made from such materials
InactiveUS6165383ADesirable efficiencyDesirable weightSilicon organic compoundsLayered productsArylAnthracene
Precursor materials useful for making organic electroluminescent devices such as anthracene derivatives having the structure: R40-R43 and R44-R47 are selected independently from the group consisting of hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, sulfonyloxy. R48 and R49 are selected independently from the group consisting of trialkoxysilyl, dialkoxysilyl, trichlorosilyl, dichlorosilyl, heptachlorotrisiloxy, and pentachlorodisiloxy.
Owner:ORGANIC DISPLAY TECH
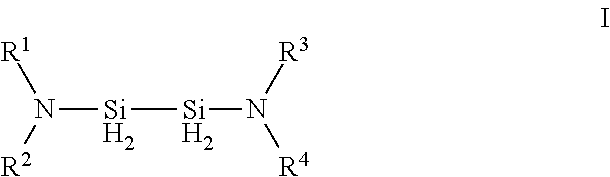
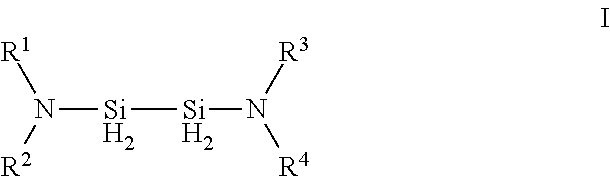
Hexakis(monohydrocarbylamino) disilanes and method for the preparation thereof
InactiveUS20050107627A1Improve featuresSilicon organic compoundsSemiconductor/solid-state device manufacturingSilane compoundsOrganic solvent
A composition and method of preparation, to provide silane compounds that are free of chlorine. The compounds are hexakis(monohydrocarbylamino)disilanes with general formula (I) ((R)HN)3—Si—Si—(NH(R))3 (I) wherein each R independently represents a C1 to C4 hydrocarbyl. These disilanes may be synthesized by reacting hexachlorodisilane in organic solvent with at least 6-fold moles of the monohydrocarbylamine RNH2 (wherein R is a C1 to C4 hydrocarbyl). Such compounds have excellent film-forming characteristics at low temperatures. These films, particularly in the case of silicon nitride and silicon oxynitride, also have excellent handling characteristics.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Hydrophilic Polysiloxane Macromonomer, and Production and Use of the same
ActiveUS20090234089A1Improve compatibilityHigh oxygen permeabilitySilicon organic compoundsOptical articlesSide chainHydrophile
Problem to be Solved To provide an ophthalmic lens, which can be more safely worn, that is, to provide a material, which is transparent and has high oxygen permeability and a high hydrophilic property, and to provide a novel monomer to be a raw material thereof.Solution A hydrophilic polysiloxane macromonomer contains polyoxyethylene as a hydrophilic side chains in a polysiloxane main chain, wherein transparency, oxygen permeability, and hydrophilic properties of the material are controlled by regulating the length of the polysiloxane main chain, the length of the hydrophilic polyoxyethylene side chains, and the number of the side chains.
Owner:COOPERVISION INT LTD
Tantalum amide precursors for deposition of tantalum nitride on a substrate
InactiveUS6379748B1Rapid heat treatmentSilicon organic compoundsPolycrystalline material growthFerroelectric thin filmsChemical vapor deposition
Tantalum and titanium source reagents are described, including tantalum amide and tantalum silicon nitride precursors for the deposition of tantalum nitride material on a substrate by processes such as chemical vapor deposition, assisted chemical vapor deposition, ion implantation, molecular beam epitaxy and rapid thermal processing. The precursors may be employed to form diffusion barrier layers on microelectronic device structures enabling the use of copper metallization and ferroelectric thin films in device construction.
Owner:ADVANCED TECH MATERIALS INC
Material for organic electroluminescence device and organic electroluminescence device using the same
InactiveUS20070224446A1Improve current efficiencyImprove heat resistanceSilicon organic compoundsDischarge tube luminescnet screensHeat resistanceOrganic electroluminescence
A material for organic electroluminescence device with specific structure. An an organic electroluminescence device comprising a cathode, an anode and an organic thin film layer which is sandwiched between the cathode and the anode and comprises at least one layer, wherein at least one layer in the organic thin film layer contains a material for the organic electroluminescence device described above. An organic electroluminescence device with excellent efficiency of light emission, without pixel defects, which is superior in heat resistance and prolonged lifetime is obtained.
Owner:IDEMITSU KOSAN CO LTD
Compositions and methods for the deposition of silicon oxide films
InactiveUS20150275355A1Silicon organic compoundsSemiconductor/solid-state device manufacturingHydrogenSilicon oxide
Described herein are compositions and methods for forming silicon oxide films. In one aspect, the film is deposited from at least one precursor having the following formula:R1nSi(NR2R3)mH4-m-n wherein R1 is independently selected from a linear C1 to C6 alkyl group, a branched C2 to C6 alkyl group, a C3 to C6 cyclic alkyl group, a C2 to C6 alkenyl group, a C3 to C6 alkynyl group, and a C4 to C10 aryl group; wherein R2 and R3 are each independently selected from hydrogen, a C1 to C6 linear alkyl group, a branched C2 to C6 alkyl group, a C3 to C6 cyclic alkyl group, a C2 to C6 alkenyl group, a C3 to C6 alkynyl group, and a C4 to C10 aryl group, wherein R2 and R3 are linked or, are not linked, to form a cyclic ring structure; n=1, 2, 3; and m=1, 2.
Owner:VERSUM MATERIALS US LLC
Organoaminosilane precursors and methods for depositing films comprising same
ActiveUS20150087139A1Low temperature efficientlySilicon organic compoundsOrganic compound preparationSilanesPhysical chemistry
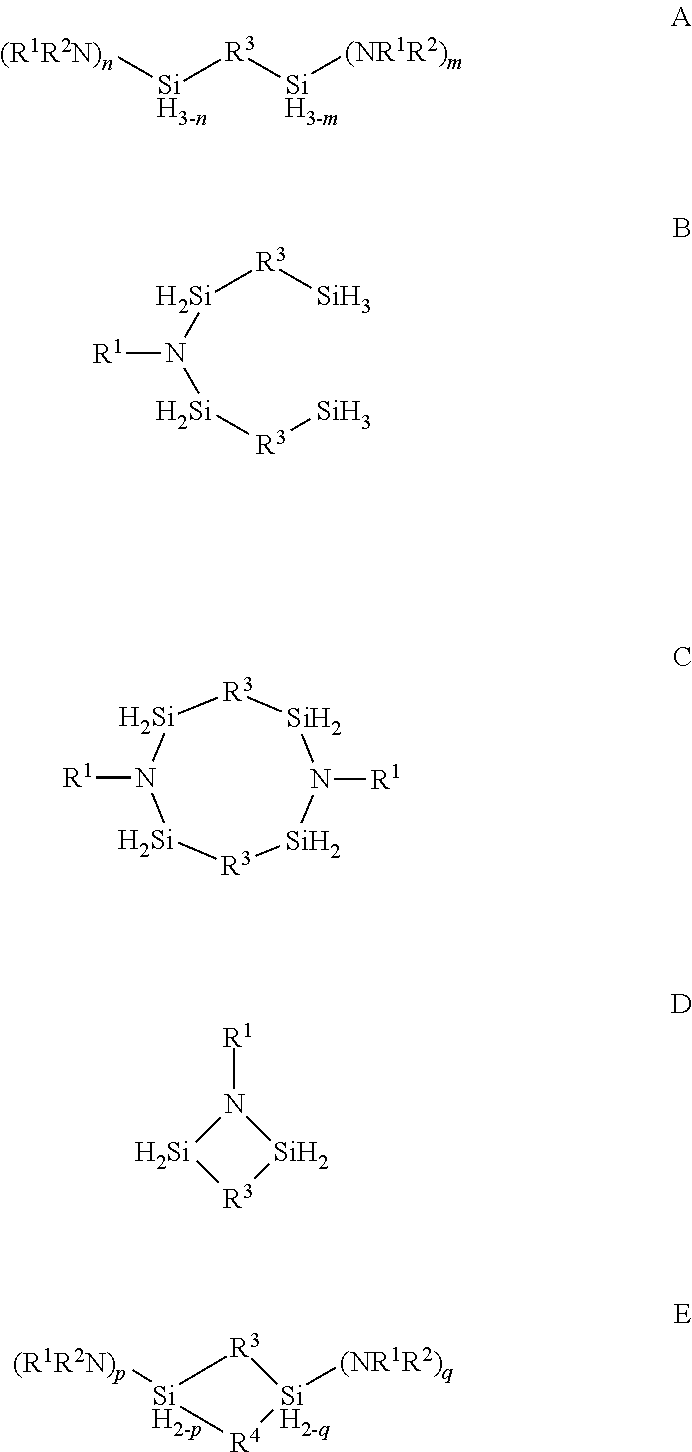
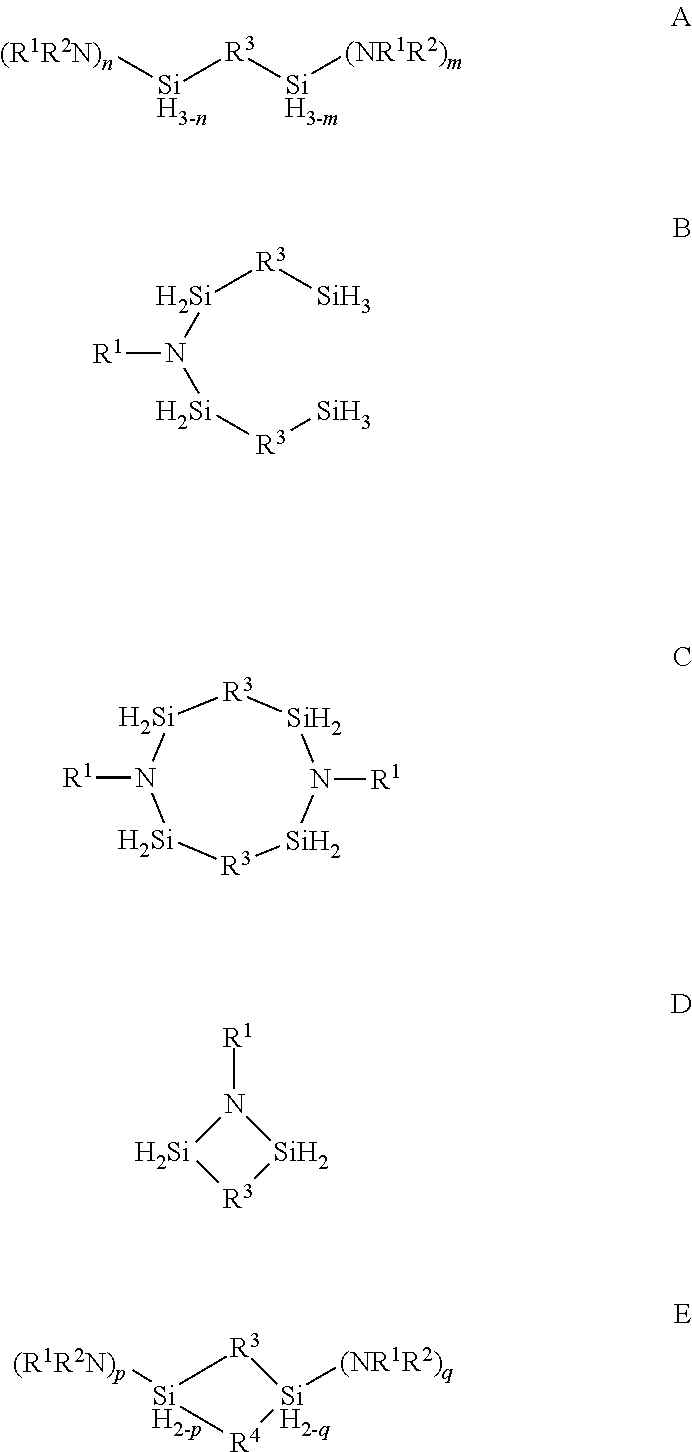
Described herein are precursors and methods for forming silicon-containing films. In one aspect, the precursor comprises a compound represented by one of following Formulae A through E below:In one particular embodiment, the organoaminosilane precursors are effective for a low temperature (e.g., 350° C. or less), atomic layer deposition (ALD) or plasma enhanced atomic layer deposition (PEALD) of a silicon-containing film. In addition, described herein is a composition comprising an organoaminosilane described herein wherein the organoaminosilane is substantially free of at least one selected from the amines, halides (e.g., Cl, F, I, Br), higher molecular weight species, and trace metals.
Owner:VERSUM MATERIALS US LLC
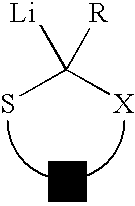
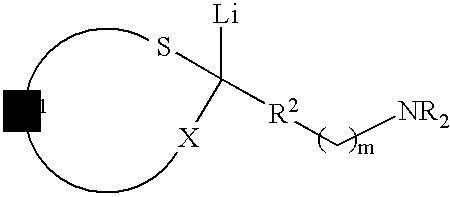
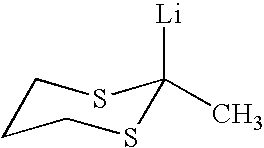
Use of sulfur containing initiators for anionic polymerization of monomers
InactiveUS7153919B2Reduced hysteresis lossSilicon organic compoundsLithium organic compoundsElastomerRolling resistance
An initiator is presented for anionically polymerizing monomers, to provide a functional head group on the polymer. A polymer having a functional head group derived from a sulfur containing anionic initiator, and optionally as additional functional group resulting from the use of a functional terminating reagent, coupling agent or linking agent is also provided. A method is presented for anionically polymerizing monomers comprising the step of polymerizing the monomers with a sulfur containing anionic initiator to provide a functional head group on the polymer. An elastomeric compound, comprising a functional polymer and filler is also described. Also provided is a tire having decreased rolling resistance resulting from a tire component containing a vulcanizable elastomeric compound.
Owner:BRIDGESTONE CORP
Halogen free syntheses of aminosilanes by catalytic dehydrogenative coupling
ActiveUS20150094470A1Silicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenGas phase
Compounds and method of preparation of Si—X and Ge—X compounds (X═N, P, As and Sb) via dehydrogenative coupling between the corresponding unsubstituted silanes and amines (including ammonia) or phosphines catalyzed by metallic catalysts is described. This new approach is based on the catalytic dehydrogenative coupling of a Si—H and a X—H moiety to form a Si—X containing compound and hydrogen gas (X═N, P, As and Sb). The process can be catalyzed by transition metal heterogenous catalysts such as Ru(0) on carbon, Pd(0) on MgO) as well as transition metal organometallic complexes that act as homogeneous catalysts. The —Si—X products produced by dehydrogenative coupling are inherently halogen free. Said compounds can be useful for the deposition of thin films by chemical vapor deposition or atomic layer deposition of Si-containing films.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Luminescence device, display apparatus and metal coordination compound
InactiveUS20020064681A1Solid-state devicesSemiconductor/solid-state device manufacturingOrganic compoundLuminescence
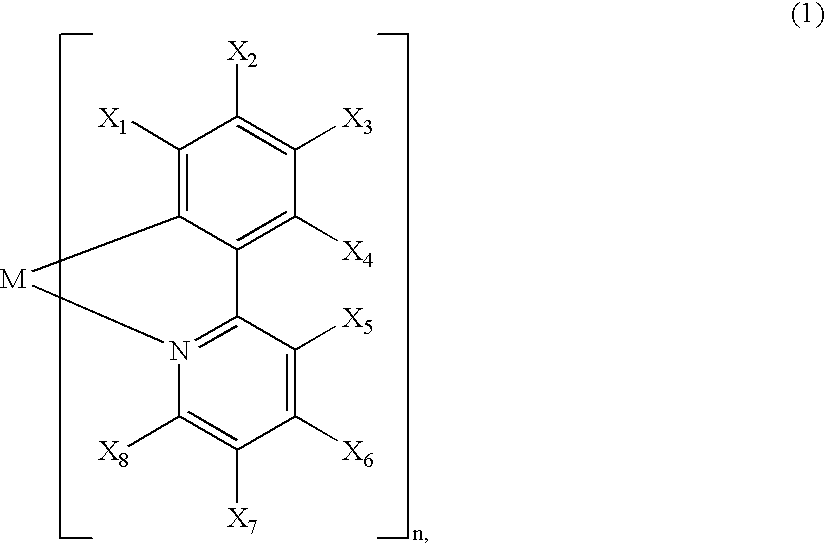
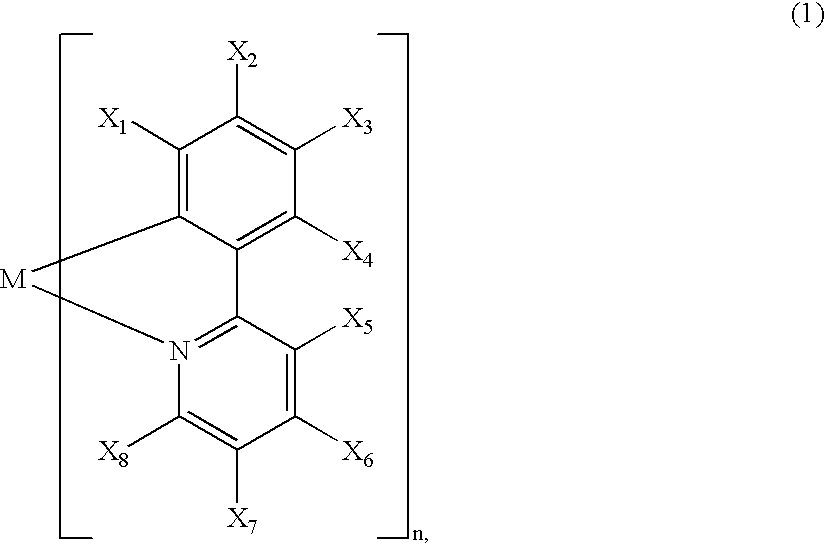
A luminescence device is principally constituted by a pair of electrodes and an organic compound layer disposed therebetween. The layer contains a metal coordination compound represented by the following formula (1): wherein M denotes Ir, Rh or Pd; n is 2 or 3; and X1 to X8 independently denote hydrogen atom or a substituent selected from the group consisting of halogen atom; nitro group; trifluoromethyl group trialkylsilyl group having three linear or branched alkyl groups each independently having 1-8 carbon atoms; and a linear or branched alkyl group having 2-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom; with the proviso that at least one of X1 to X8 is a substituent other than hydrogen atom, and X2 and X3 cannot be fluorine atom at the same time.
Owner:CANON KK
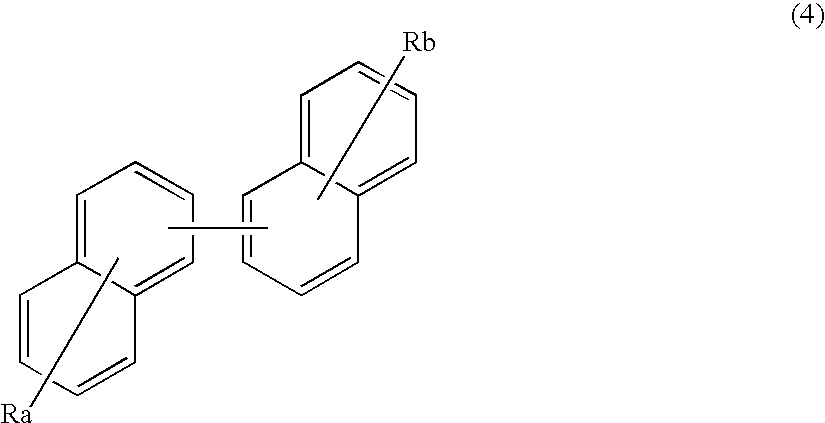
Benzanthracene derivative and electroluminescence device using the same
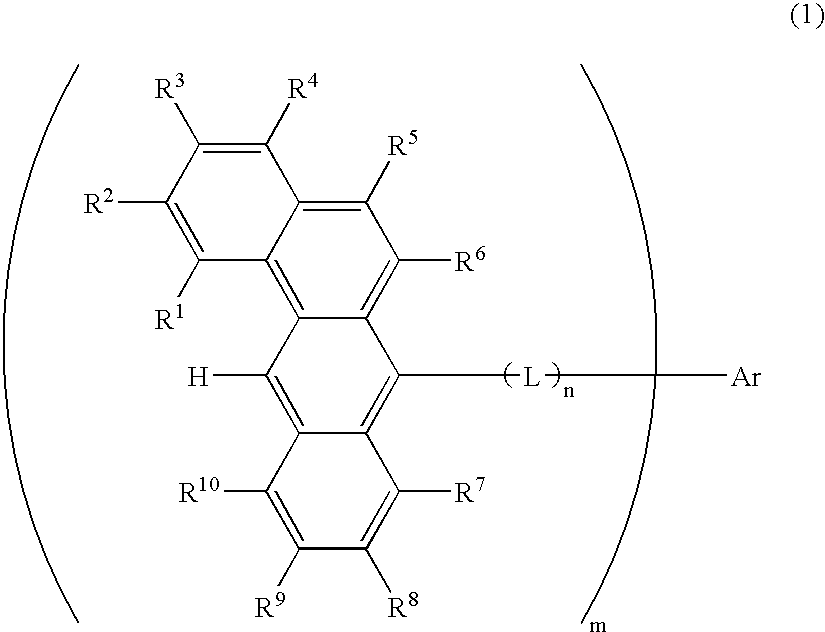
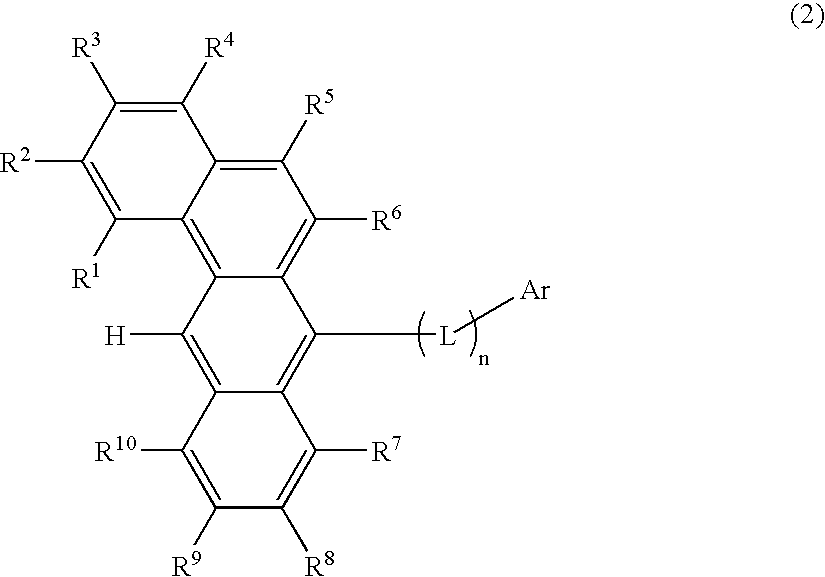
ActiveUS20070273272A1Improve efficiencyGood chromaSilicon organic compoundsDischarge tube luminescnet screensOrganic filmOrganic electroluminescence
A benzanthracene derivative having hydrogen atom at the 12-position, and an organic electroluminescence device having an organic thin film layer, which has one layer or a plurality of layers including at least a light emitting layer, is disposed between a cathode and an anode and contains the benzanthracene derivative in at least one layer in the organic thin film layer singly or as a component of a mixture. The electroluminescence device provides a great efficiency of light emission, has a long life and exhibits an excellent chromaticity.
Owner:IDEMITSU KOSAN CO LTD
Cyclic diol-derived blocked mercaptofunctional silane compositions
ActiveUS20060041063A1Reduce volatilityReducing VOC levelSilicon organic compoundsPolymer scienceOrganic chemistry
Diol derived blocked mercaptofunctional silane compositions in which the silanes comprise cyclic and bridged alkoxy groups derived from hydrocarbon-based diols and processes for their preparation are provided. Also provided are rubber compositions comprising the cyclic diol-derived blocked mercaptofunctional silanes, processes for their preparation and articles of manufacture comprising the rubber compositions, in particular, automotive tires and components thereof.
Owner:GENERAL ELECTRIC CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com