Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3513 results about "Carboxyl radical" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carboxyl group. carboxyl group (kɑːˈbɒksaɪl; -sɪl) or carboxyl radical n (Elements & Compounds) the monovalent group –COOH, consisting of a carbonyl group bound to a hydroxyl group: the functional group in organic acids [C19 carboxyl, from carbo- + oxy-2 + -yl]
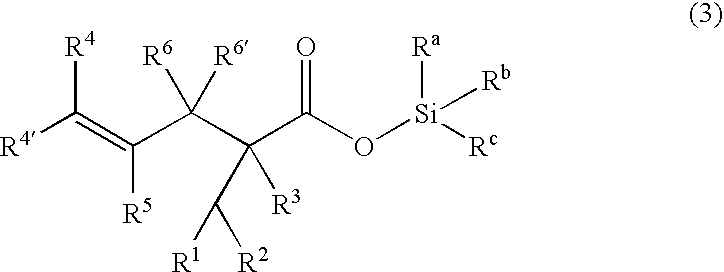
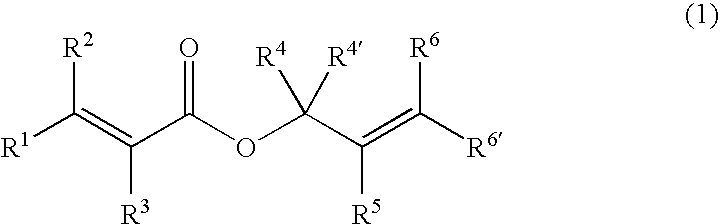
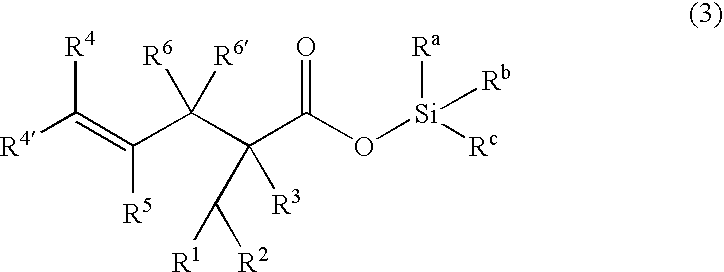
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS7307178B2Few stepsHigh yieldSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM CO LTD
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS20050070729A1High yieldFew stepsSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM IND CO LTD
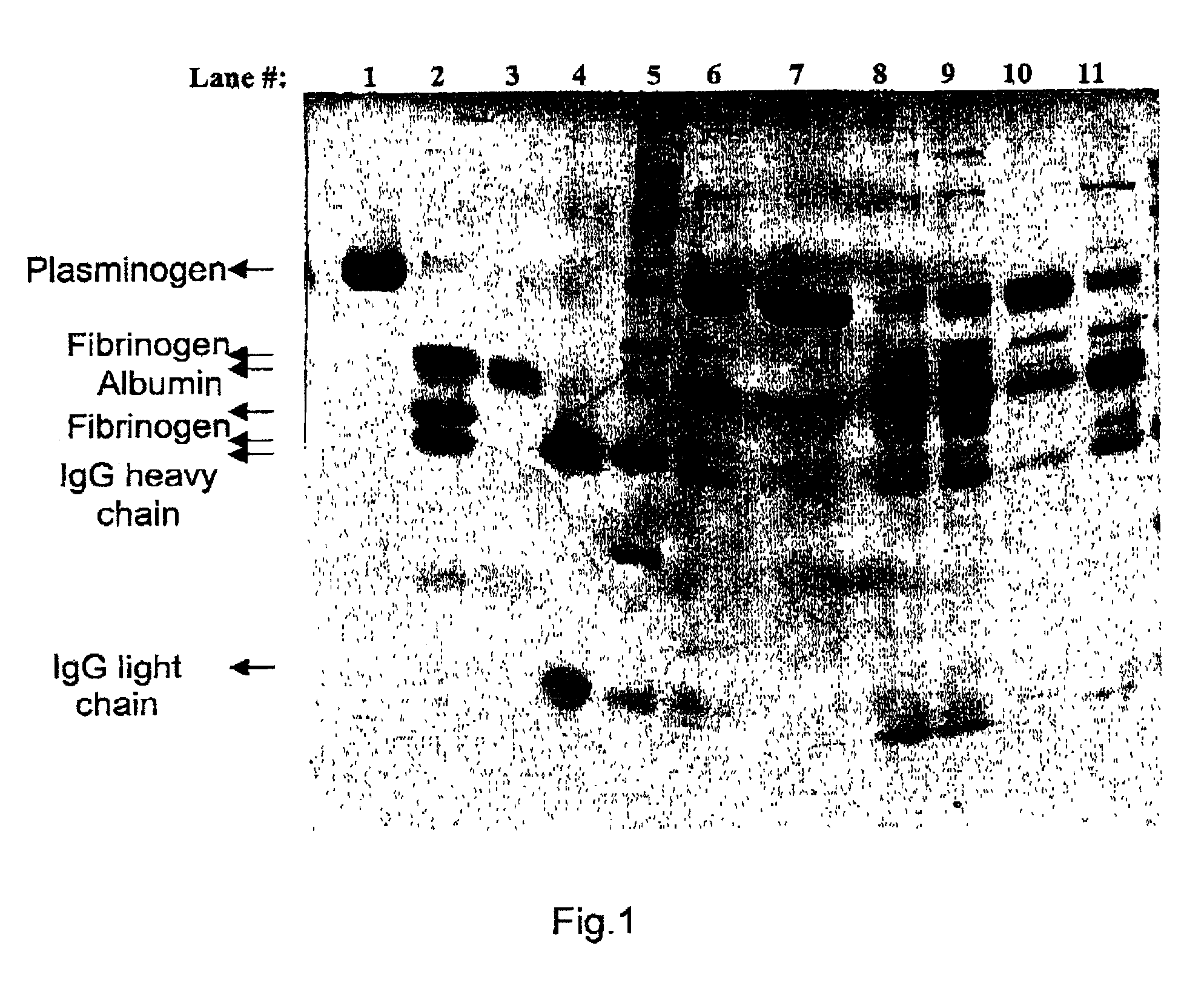
Removal of plasmin(ogen) from protein solutions
A method for specifically removing or isolating plasmin(ogen) or plasmin in presence of fibrinogen from a mixture containing plasmin(ogen) or plasmin by contacting the mixture with a rigid amino acid wherein the amino group of the amino acid and the carboxylic group of the amino acid are about 6–8 Angstroms, preferably about 7 Angstroms apart and the rigid amino acid is covalently bound to the support via the amino group of the amino acid.
Owner:OMRIX BIOPHARM
Luminescent element material and luminescent element comprising the same
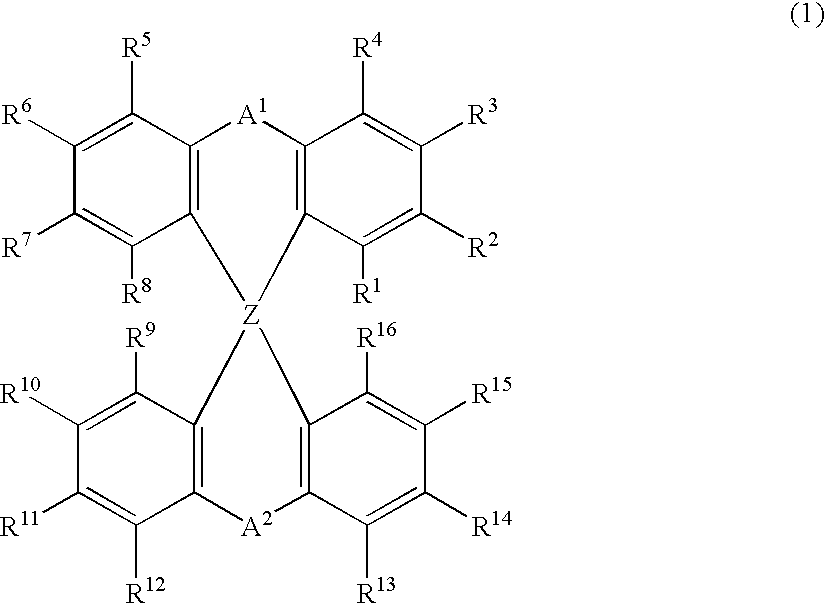
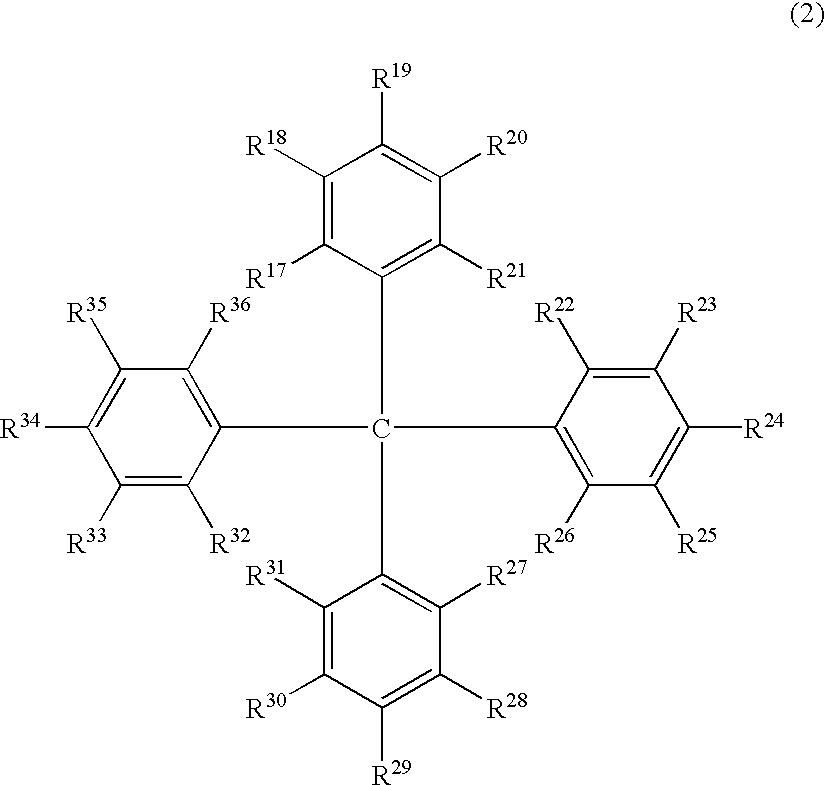
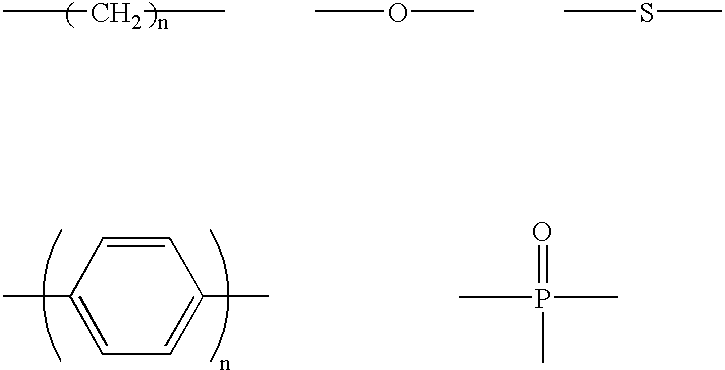
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
Polymerizable chain-extended polysiloxanes with pendant hydrophilic groups
The invention provide a class of chain-extended polysiloxane crosslinkers which comprises (1) at least two polysiloxane segments, wherein each pair of adjacent polysiloxane segments is linked by one divalent organic radical which includes at least one pendant hydrophilic group (hydroxyl and / or carboxyl groups) or at least one dangling hydrophilic polymer chain and a di-thioether linkage —S-DR-S— in which DR is a divalent organic radical; and (2) two terminal ethylenically unsaturated groups. The present invention is also related to a polymer comprising crosslinking units derived from chain-extended polysiloxane crosslinker of the invention and to ophthalmic lenses comprising such a polymer.
Owner:ALCON INC
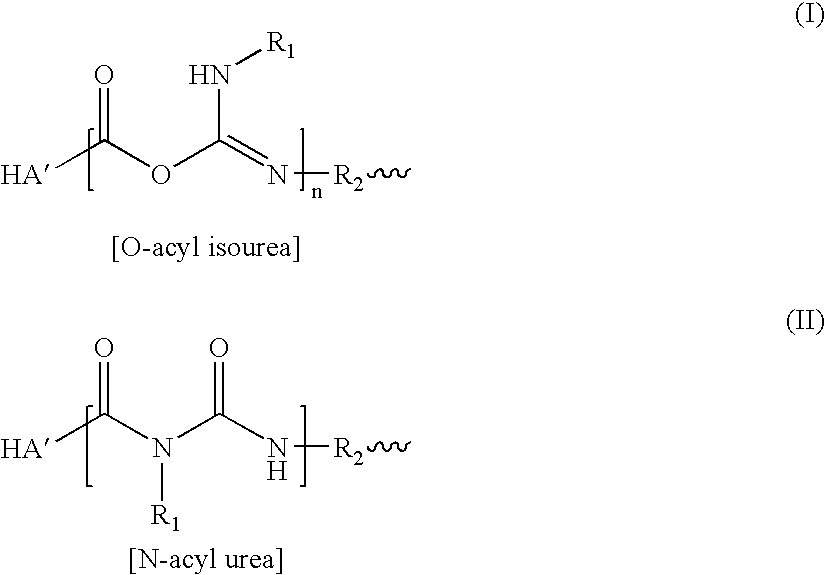
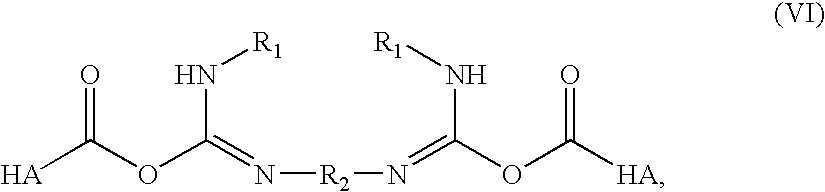
Treatment of arthritis and other musculoskeletal disorders with crosslinked hyaluronic acid
InactiveUS20070203095A1Reduce frequencyFast pain reliefBiocideOrganic active ingredientsDiseaseCarboxyl radical
A method of treating a subject having a musculoskeletal disorder includes administering to a subject's articular site in need thereof an effective amount of a hyaluronic acid (HA) composition. In one embodiment, the HA composition includes an HA derivative, wherein carboxyl functionalities of the hyaluronic acid derivative are each independently derivatized to include an N-acylurea or 0-acyl isourea, or both N-acylurea and 0-acyl isourea. In another embodiment, the HA composition includes a crosslinked HA gel that is prepared by reacting an uncrosslinked HA with a biscarbodiimide in the presence of pH buffer in a range of between about 4 and about 8. The composite can optionally include at least one second bioactive agent other than the HA derivative, such as a steroid.
Owner:ANIKA THERAPEUTICS INC
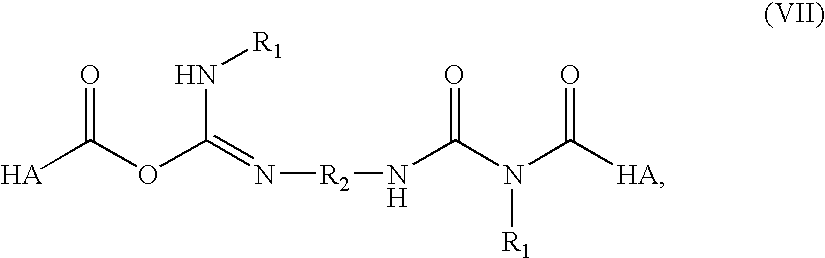
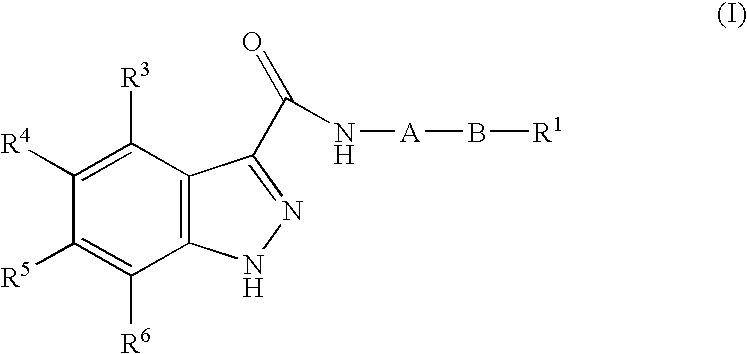
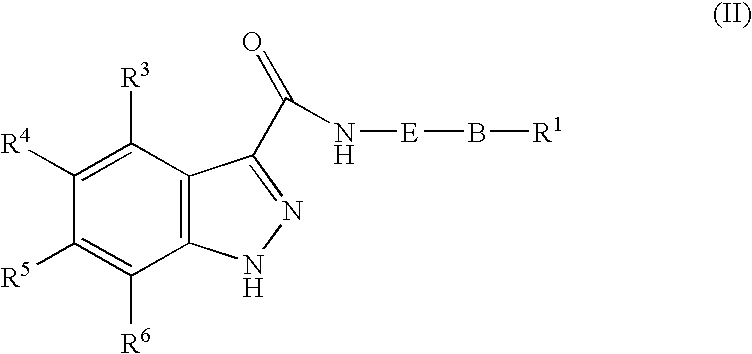
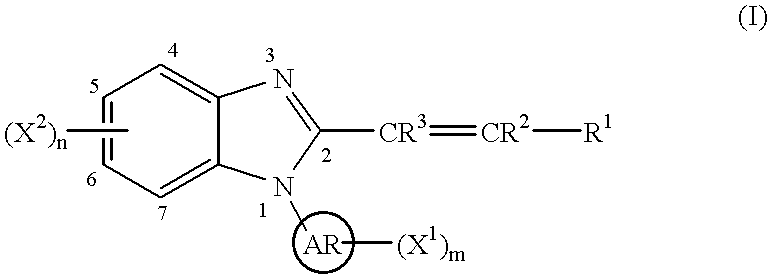
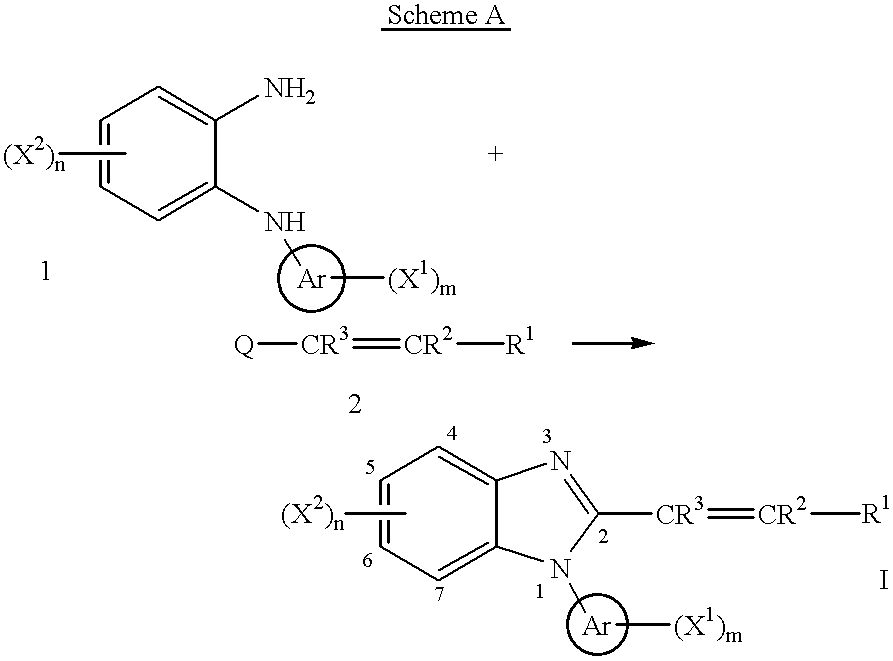
1h-Indazole-3-carboxamide compounds as cyclin dependent kinase (cdk) inhibitors
The invention provides a compound of the formula (I) for use in the prophylaxis or treatment of a disease state or condition mediated by a cyclin dependent kinase: wherein A is a group R2 or CH2—R2 where R2 is a carbocyclic or heterocyclic group having from 3 to 12 ring members; B is a bond or an acyclic linker group having a linking chain length of up to 3 atoms selected from C, N, S and O; R1 is hydrogen or a group selected from SO2Rb, SO2NR7R8, CONR7R8, NR7R9 and carbocyclic and heterocyclic groups having from 3 to 7 ring members; R3, R4, R5 and R6 are the same or different and are each selected from hydrogen, halogen, hydroxy, trifluoromethyl, cyano, nitro, carboxy, amino, carbocyclic and heterocyclic groups having from 3 to 12 ring members; a group Ra—Rb wherein Ra is a bond, O, CO, X1C(X2), C(X2)X1, X1C(X2)X1, S, SO, SO2, NRc, SO2NRc or NRcSO2; and Rb is selected from hydrogen, carbocyclic and heterocyclic groups having from 3 to 12 ring members, and a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from hydroxy, oxo, halogen, cyano, nitro, amino, mono- or di-C1-4 hydrocarbylamino, carbocyclic and heterocyclic groups having from 3 to 12 ring members and wherein one or more carbon atoms of the C1-8 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NRc, X1C(X2), C(X2)X1 or X1C(X2)X1; Rc is hydrogen or C1-4 hydrocarbyl; X1 is O, S or NRc and X2 is ═O, ═S or ═NRc; R7 is selected from hydrogen and a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from hydroxy, oxo, halogen, cyano, nitro, amino, mono- or di-C1-4 hydrocarbylamino, carbocyclic and heterocyclic groups having from 3 to 12 ring members and wherein one or more carbon atoms of the C1-8 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NRc, X1C(X2), C(X2)X1 or X1C(X2)X1; R8 is selected from R7 and carbocyclic and heterocyclic groups having from 3 to 12 ring members; R9 is selected from R8, COR8 and SO2R8; or NR7R8 or NR7R9 may each form a heterocyclic group having from 5 to 12 ring members; but excluding the compounds N-[(morpholin-4-yl)phenyl-1H-indazole-3-carboxamide and N-[4-(acetylaminosulphonyl)phenyl-1H-indazole-3-carboxamide.
Owner:ASTEX THERAPEUTICS LTD
Polymerizable chain-extended polysiloxanes with pendant hydrophilic groups
The invention provide a class of chain-extended polysiloxane crosslinkers which comprises (1) at least two polysiloxane segments, wherein each pair of adjacent polysiloxane segments is linked by one divalent organic radical which includes at least one pendant hydrophilic group (hydroxyl and / or carboxyl groups) or at least one dangling hydrophilic polymer chain and a di-thioether linkage —S-DR—S— in which DR is a divalent organic radical; and (2) two terminal ethylenically unsaturated groups. The present invention is also related to a polymer comprising crosslinking units derived from chain-extended polysiloxane crosslinker of the invention and to ophthalmic lenses comprising such a polymer.
Owner:ALCON INC
Oxy substituted 4-carboxyamino-2-methyl-1,2,3,4-tetrahydroquinolines
Cholesteryl ester transfer protein inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC
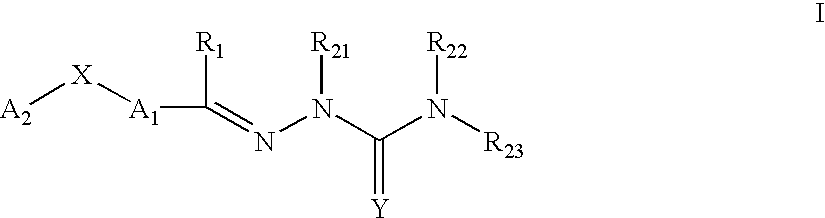
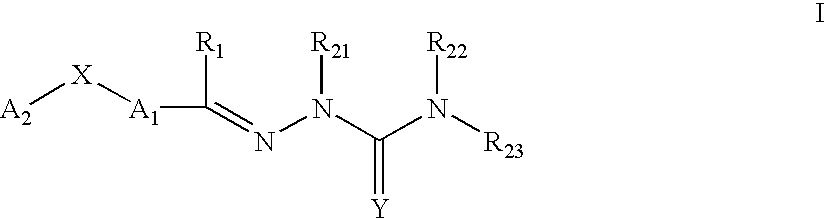
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
Hydrosilylation catalysts and silicone compositions using the same
InactiveUS6140446AShort pot lifeReduce concentrationMetal/metal-oxides/metal-hydroxide catalystsHydrosilylationSiloxane
A hydrosilylation catalyst is provided wherein a platinum catalyst is enclosed in a heat-fusible compound having a melting point of 40-200 DEG C. and containing an aliphatic unsaturated bond, carbonyl, carboxyl or thioether radical in a molecule. The catalyst is blended in organo-polysiloxane to form a silicone composition having both shelf stability and fast-curing capability.
Owner:SHIN ETSU CHEM IND CO LTD
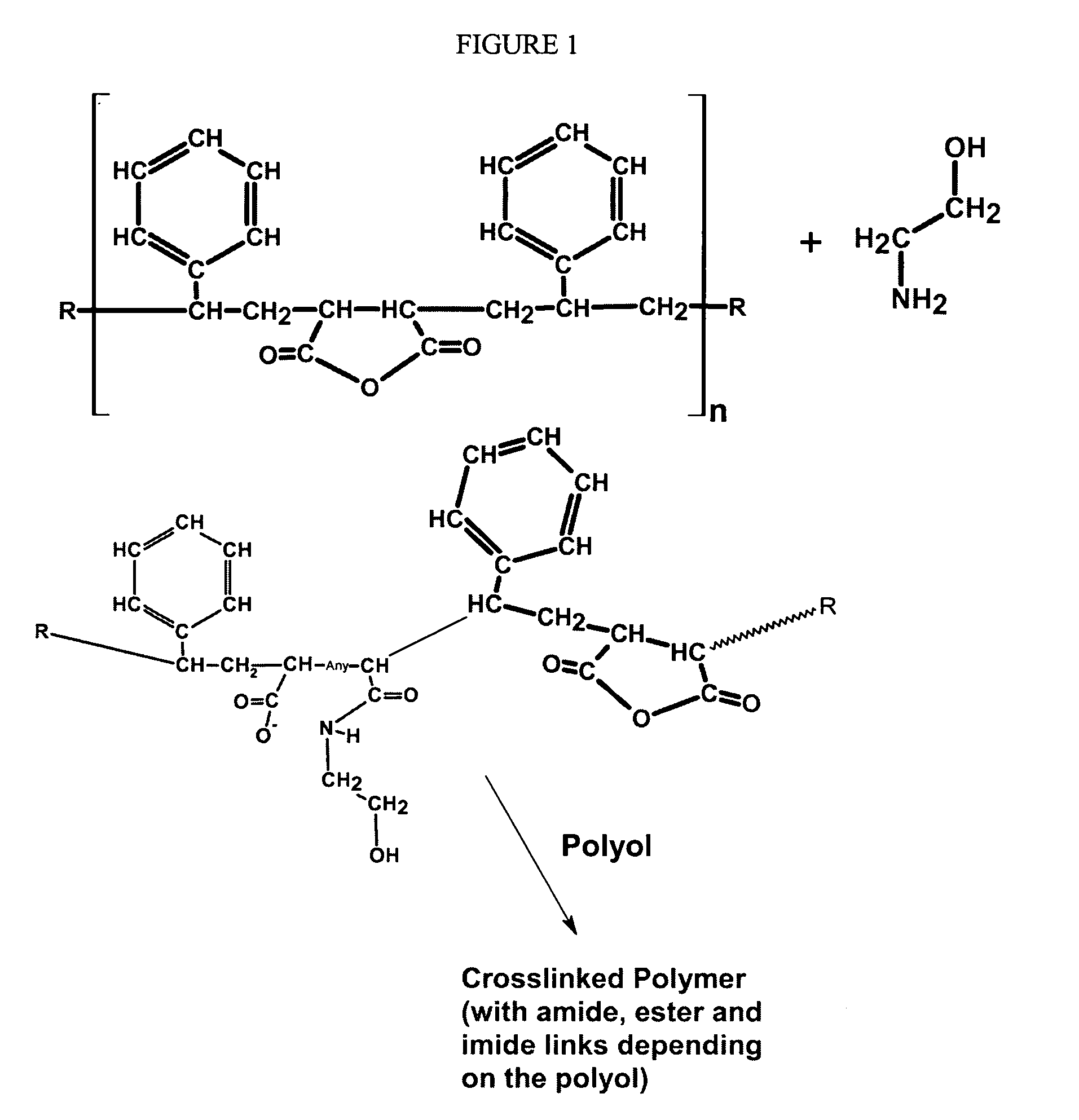
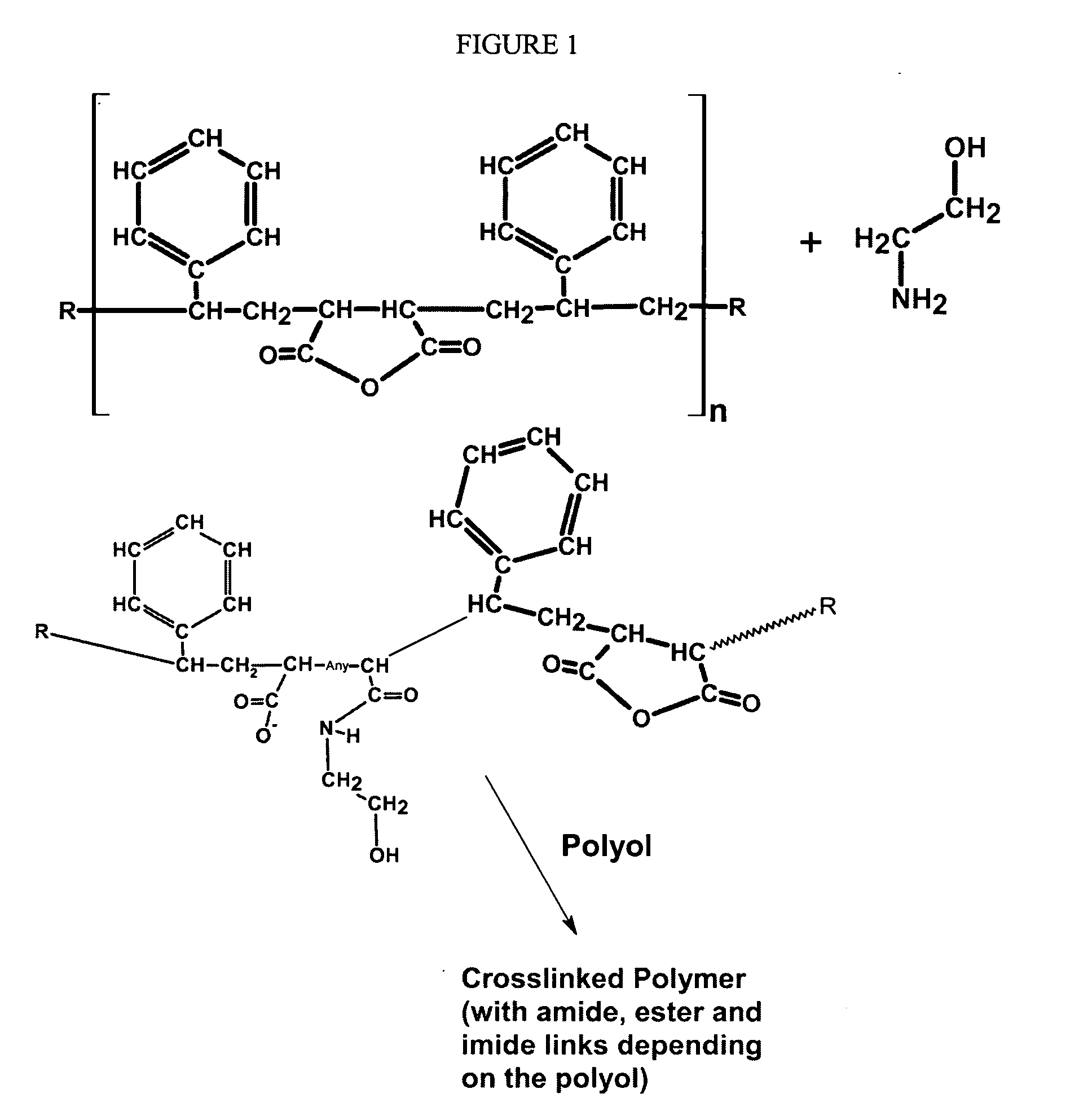
Formaldehyde free binder
An aqueous, formaldehyde-free binder composition comprising a modified copolymer of maleic anhydride and a vinyl aromatic compound such as styrene, the copolymer being modified by reaction with a primary alkanolamine, such as monoethanolamine (MEA), to produce a modified copolymer that is self-curing and cures as a consequence of cross-linking, esterification reactions between pendant carboxyls and hydroxyl groups on the solubilized (hydrolyzed) modified copolymer chains; the invention also relates to the corresponding method of using the binder composition for making fiber products, especially fiberglass insulation.
Owner:GEORGIA PACIFIC CHEM LLC
Formaldehyde free binder
An aqueous, formaldehyde-free binder composition comprising a modified copolymer of maleic anhydride and a vinyl aromatic compound such as styrene, the copolymer being modified by reaction with a primary alkanolamine, such as monoethanolamine (MEA), to produce a modified copolymer that is self-curing and cures as a consequence of cross-linking, esterification reactions between pendant carboxyls and hydroxyl groups on the solubilized (hydrolyzed) modified copolymer chains; the invention also relates to the corresponding method of using the binder composition for making fiber products, especially fiberglass insulation.
Owner:GEORGIA PACIFIC CHEM LLC
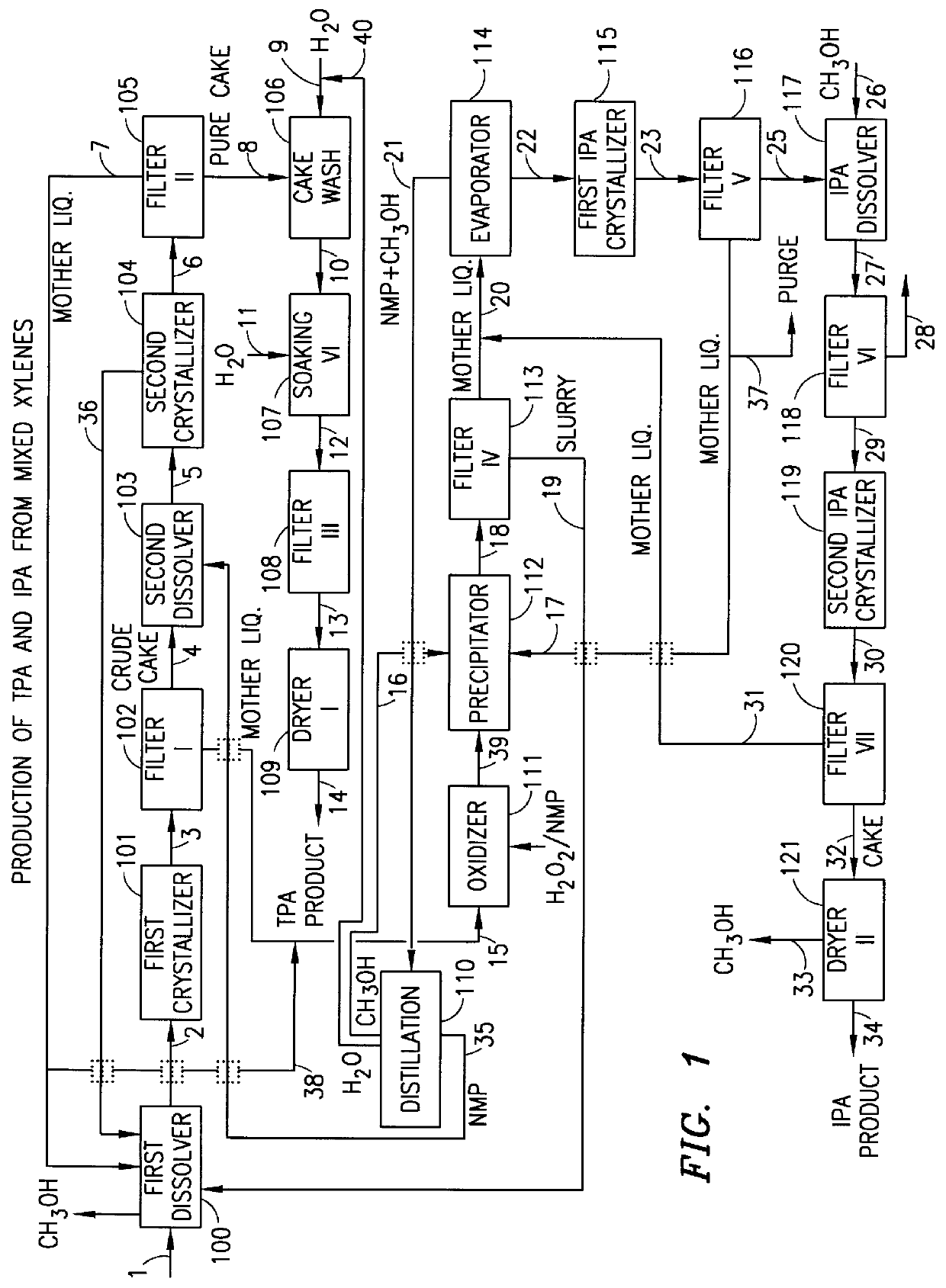
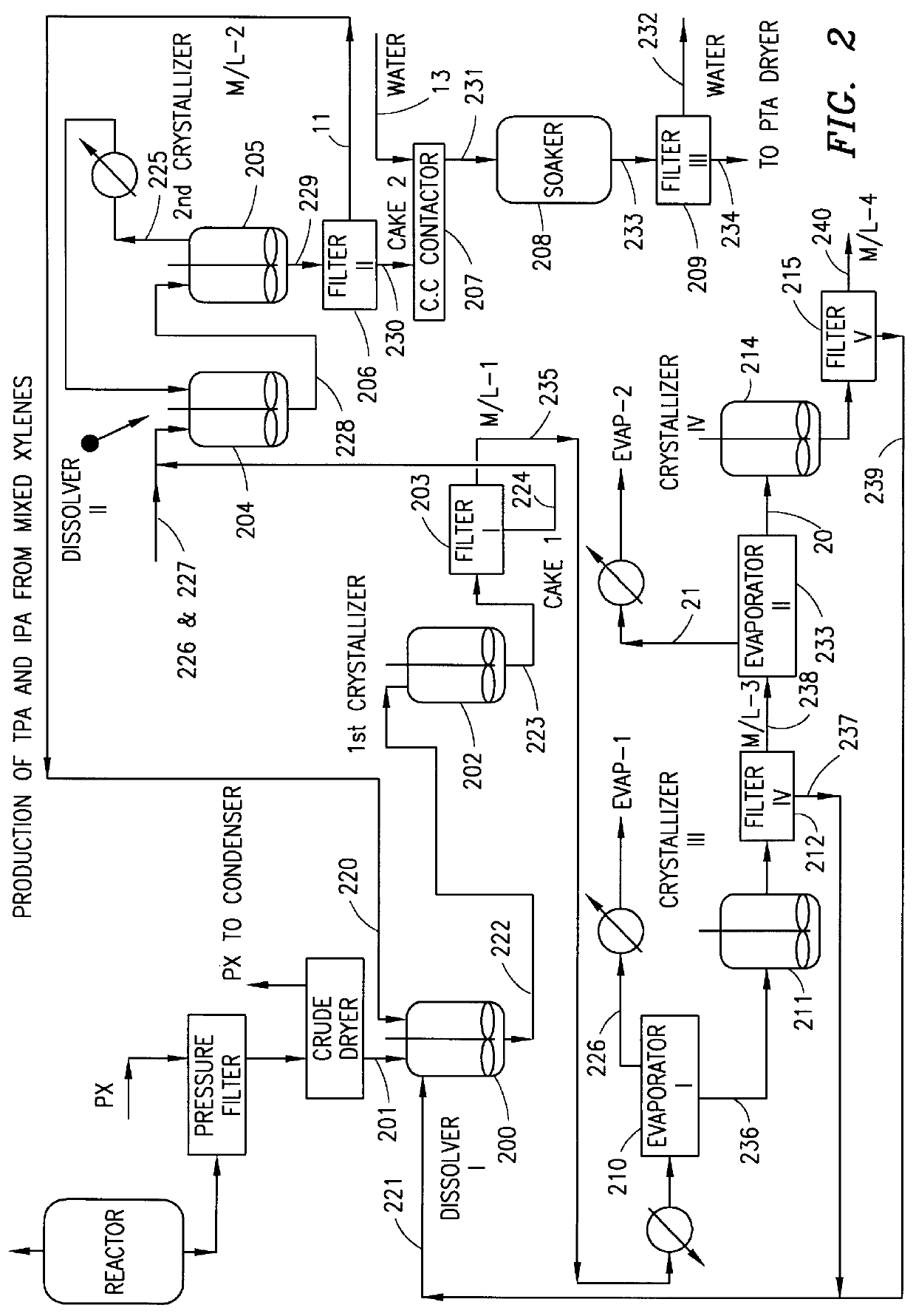
Method and apparatus for preparing purified terephthalic acid and isophthalic acid from mixed xylenes
InactiveUS6054610AReduce pressureReduce the temperatureOrganic compound preparationChemical industryIsophthalic acidXylene
A method and apparatus for preparing purified terephthalic acid and, optionally, isophthalic acid from mixed xylenes. The method of the present invention purifies the oxidation reactor effluent containing a mixture of terephthalic acid and isophthalic acid as well as minor amounts of 4-carboxybenzaldehyde (4-CBA), 3-carboxybenzaldehyde (3-CBA), and toluic acid isomers, to produce purified terephthalic acid and, optionally, purified isophthalic acid in an integrated process.
Owner:GTC TECHNOLOGY INC
Novel fused heterocyclic compound and use thereof
The compound represented by the general formula (I): wherein, a fused ring AB represents a 5- to 10-membered fused heterocyclic ring; R1 represents (1) a hydrogen atom, (2) a halogen atom, (3) a cyano group, (4) an oxo group, (5) an optionally protected hydroxyl group, (6) an optionally protected carboxyl group, (7) an optionally protected amino group, (8) a cyclic group which may have a substituent (s), (9) an aliphatic hydrocarbon group which may have a substituent (s), or (10) an optionally protected thiol group; n represents 0 or an integer of 1 to 8; provided that n represents an integer of not less than 2, plural R1 are the same or different; a salt thereof, a solvate thereof or a prodrug thereof has a kinase (especially c-Jun N-terminal kinase) inhibitory activity and an inhibitory activity of a function of AP-1 as a transcription factor, it is useful as a preventive and / or therapeutic agent for a for example, a diabetes of metabolic disease, etc., a rheumatoid arthritis of inflammatory, etc.
Owner:ONO PHARMA CO LTD
Light-emitting device material and light-emitting device
ActiveUS20090096356A1High film stabilitySolve low luminous efficiencyOrganic chemistryDischarge tube luminescnet screensCarboxyl radicalHydrogen
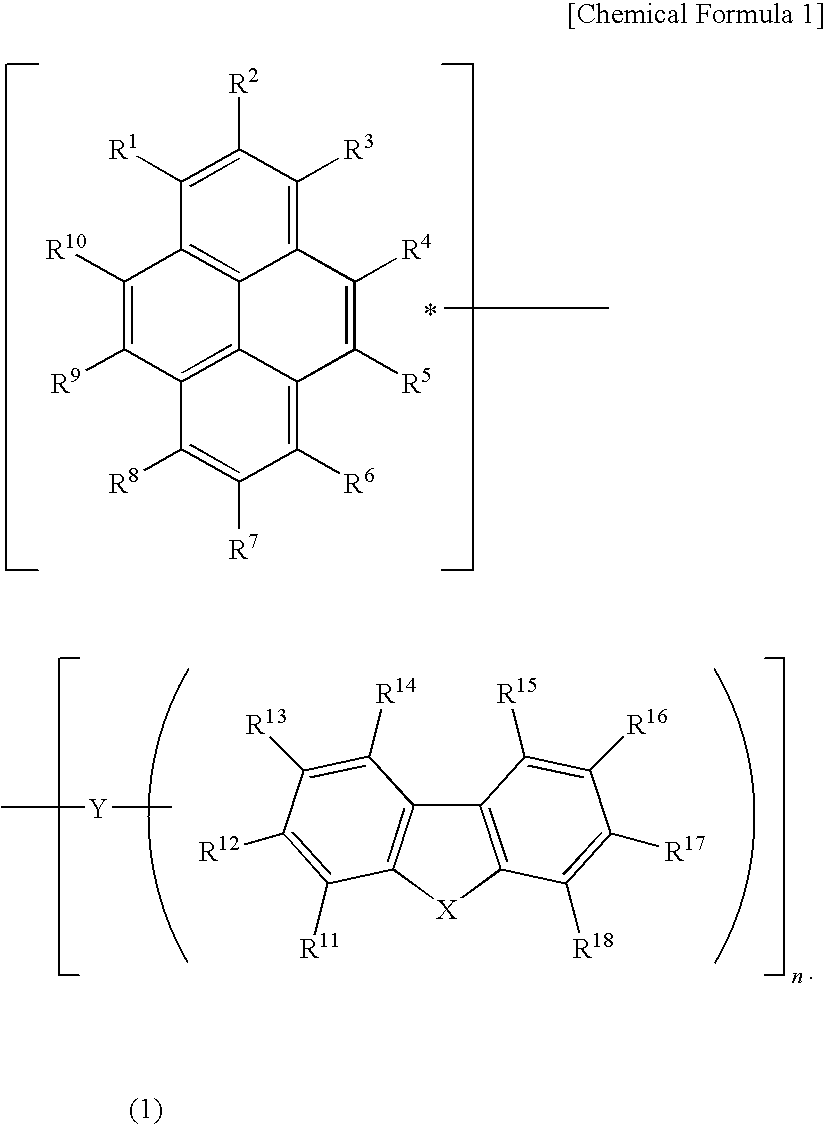
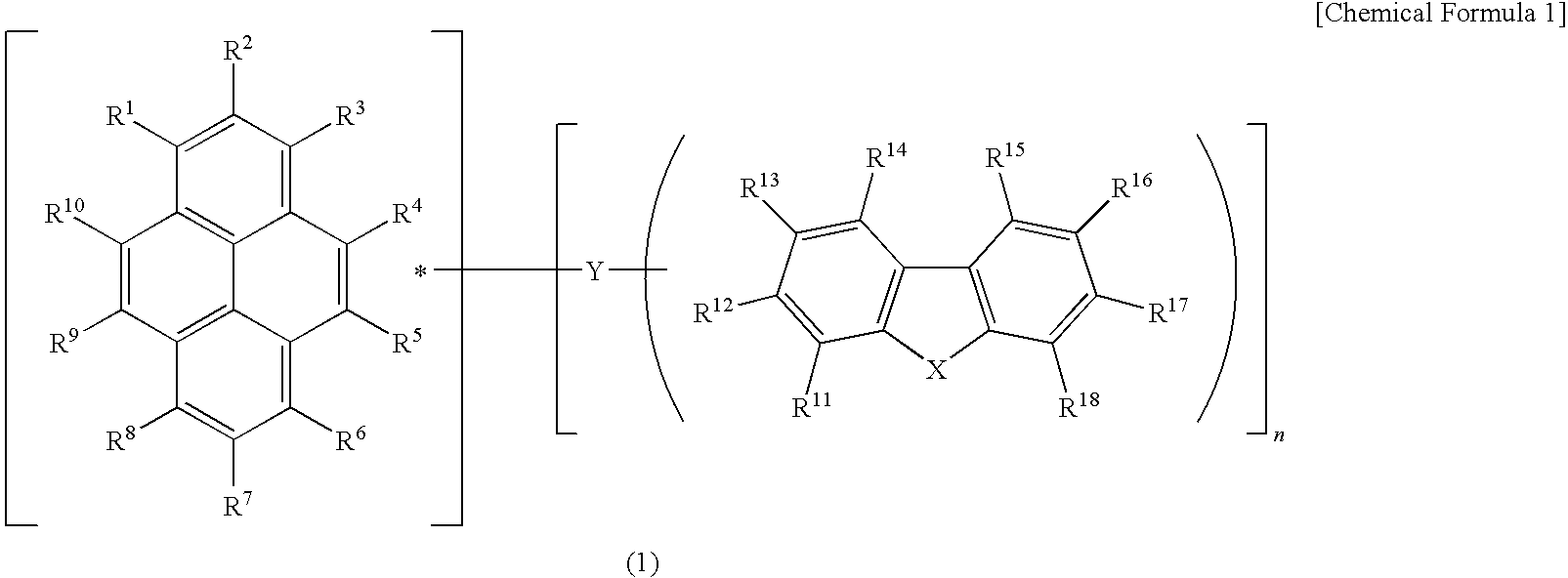
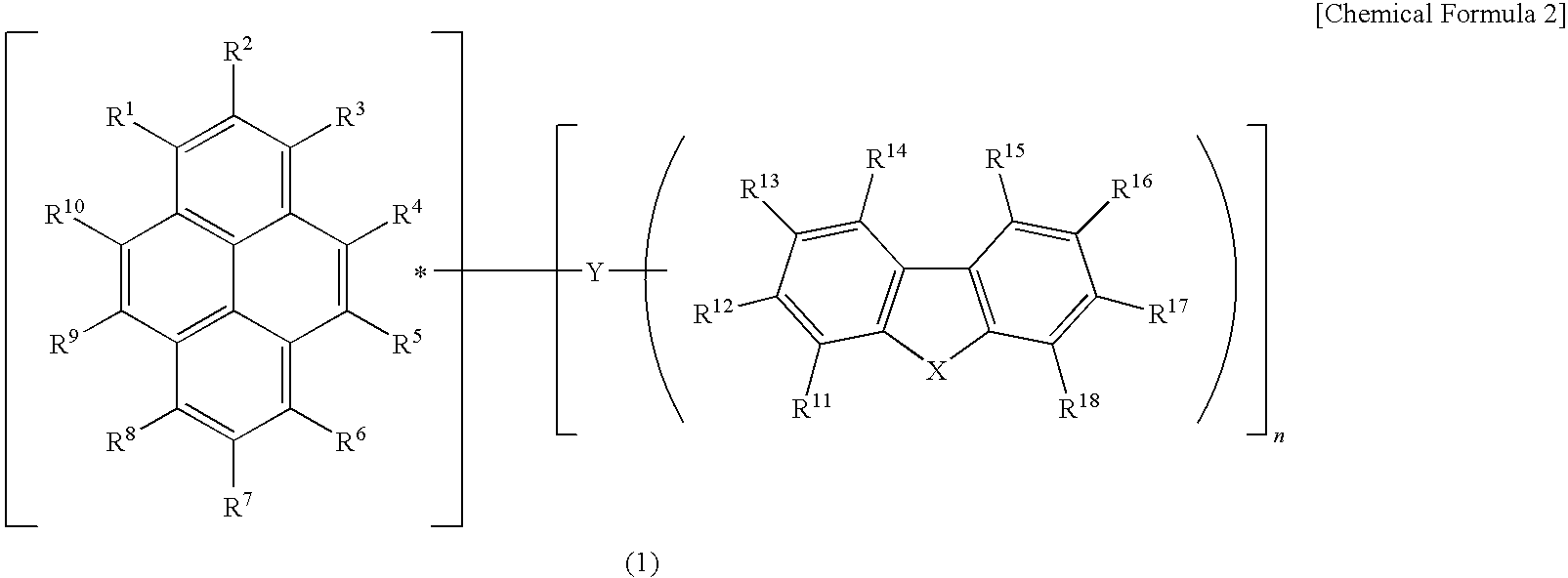
A light emitting device material containing a pyrene compound of formula (1) and a light emitting device. In formula (1), R1 to R18 are the same or different and are selected from hydrogen, alkyl, cycloalkyl, heterocyclic, alkenyl, cycloalkenyl, alkynyl, alkoxy, alkylthio, arylether, arylthioether, aryl, heteroaryl, halogen, carbonyl, carboxyl, oxycarbonyl, carbamoyl, amino, phosphine oxide and silyl; adjacent substituents among R1 to R18 may be combined with each other to form a ring; n represents an integer of 1 to 3; X is —O—, —S— or —NR19—; R19 is selected from hydrogen, alkyl, cycloalkyl, heterocyclic, alkenyl, cycloalkenyl, alkynyl, aryl, heteroaryl or amino; R19 may be combined with R11 or R18 to form a ring; Y is a single bond, arylene or heteroarylene; and n substituents among R1 to R10 and any one of R11 to R19 are used for linkage with Y
Owner:TORAY IND INC
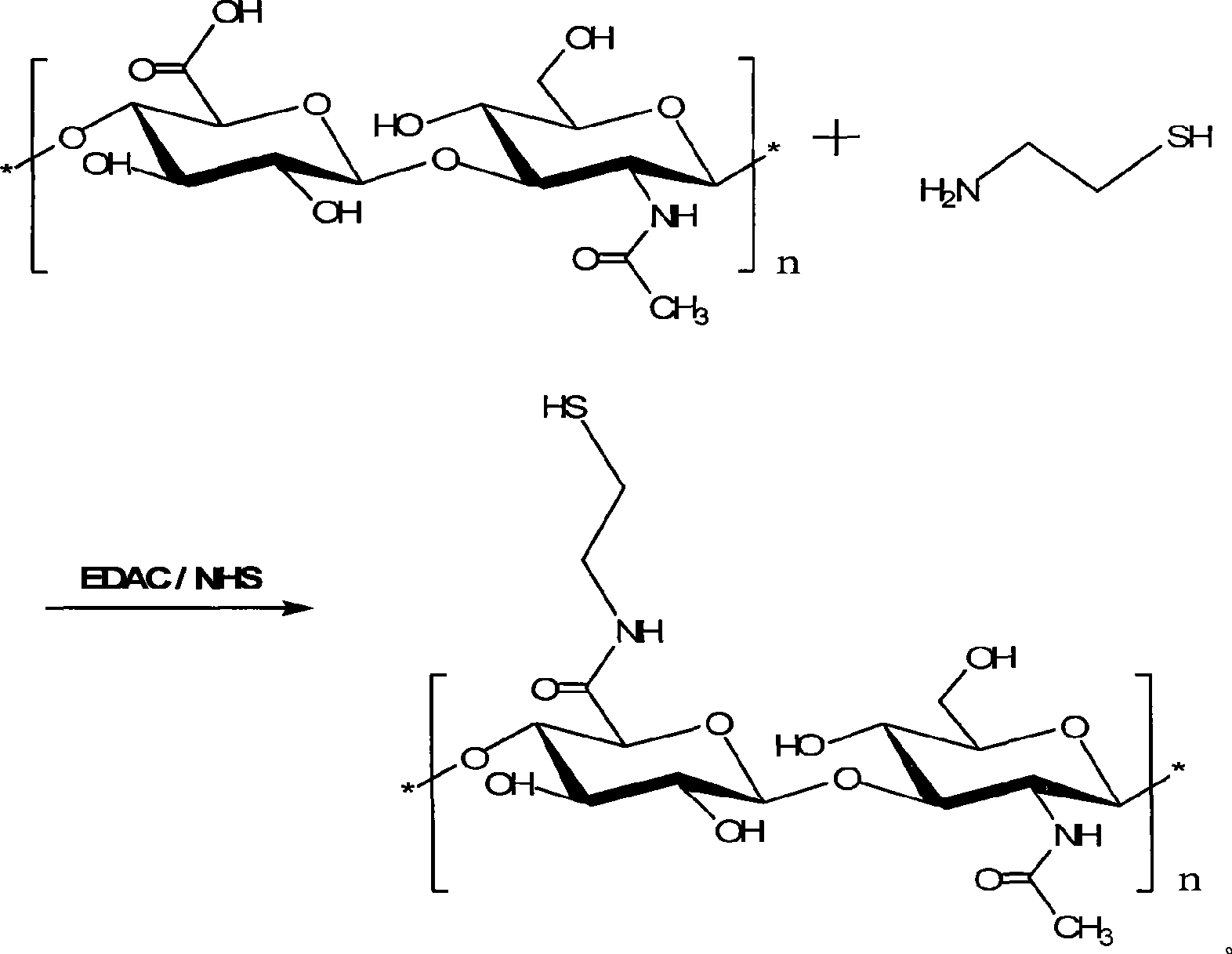
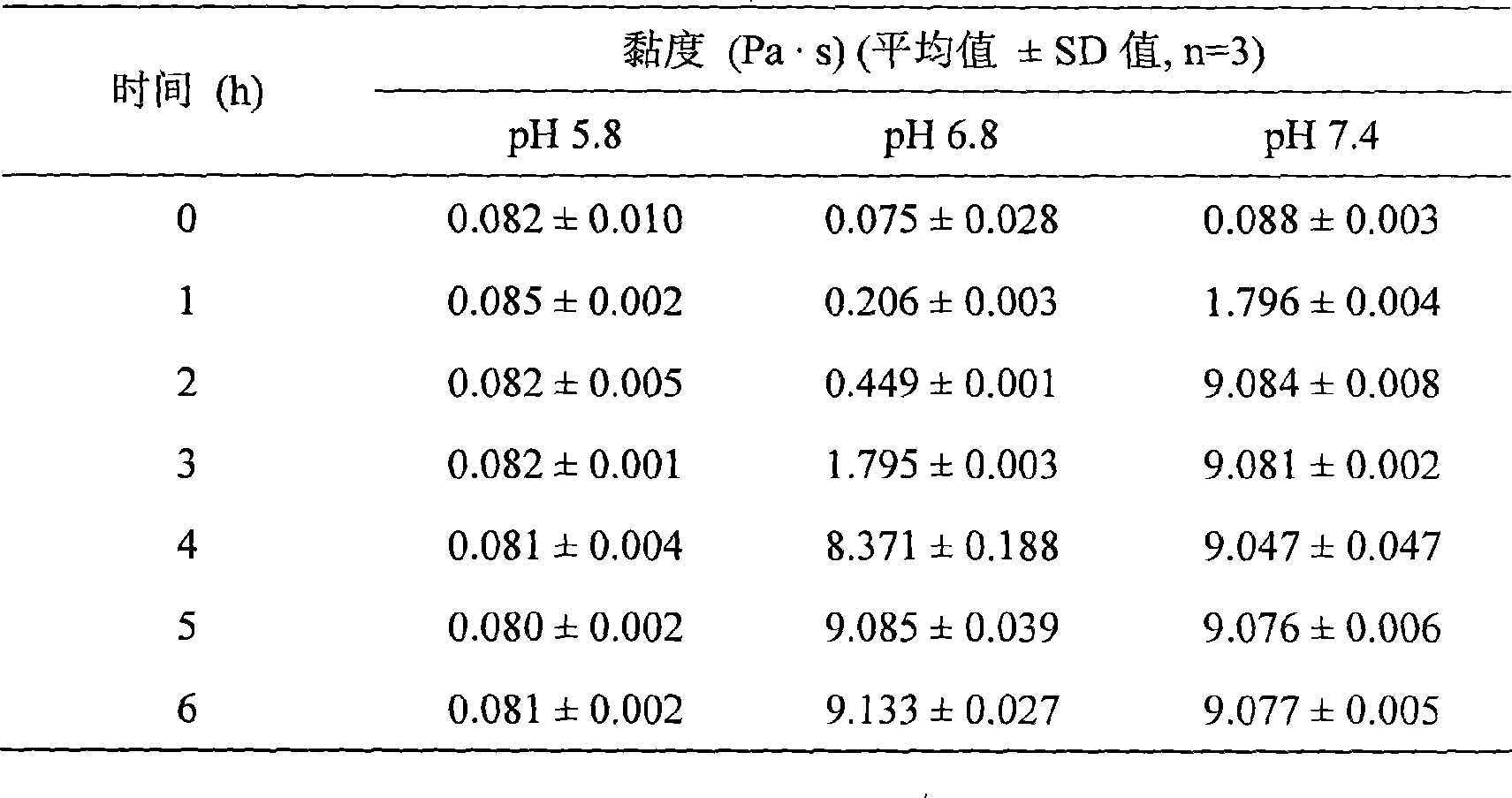
Cysteamine modified sulfhydryl hyaluronic acid couplet, preparation and application thereof
InactiveCN101367884ANon-antigenic and immunogenicGood biocompatibilityAnimal cellsPharmaceutical non-active ingredientsCYSTEAMINE HYDROCHLORIDEChemistry
The invention belongs to the technical field of high polymer materials, in particular to a thiolated hyaluronic acid conjugate modified by cysteamine, a preparation method and an application thereof. The method comprises the steps of adding 1-ethyl-3-(3-dimethylamino propyl)-carbodiimide (EDAC) and N-hydroxysuccinimide (NHS) in the hyaluronic acid solution to activate the carboxyl of the hyaluronic acid solution, adding cysteamine hydrochloride to make the amino of cysteamine hydrochloride and the carboxyl of hyaluronic acid to form amido link, regulating the proportions of each material and reaction conditions to obtain the thiolated hyaluronic acid conjugates with different sulfhdryl contents. The invention widens the application range of the thiolated reaction. The adhesive properties of the mucosa and the biocompatibility of the conjugate are good. The invention has strong in-situ gel property and supplies good bio-adhesive materials for non-injected preparations, tissue engineering and the like.
Owner:FUDAN UNIV
Sulfonate group-containing copolymers and manufacturing method thereof
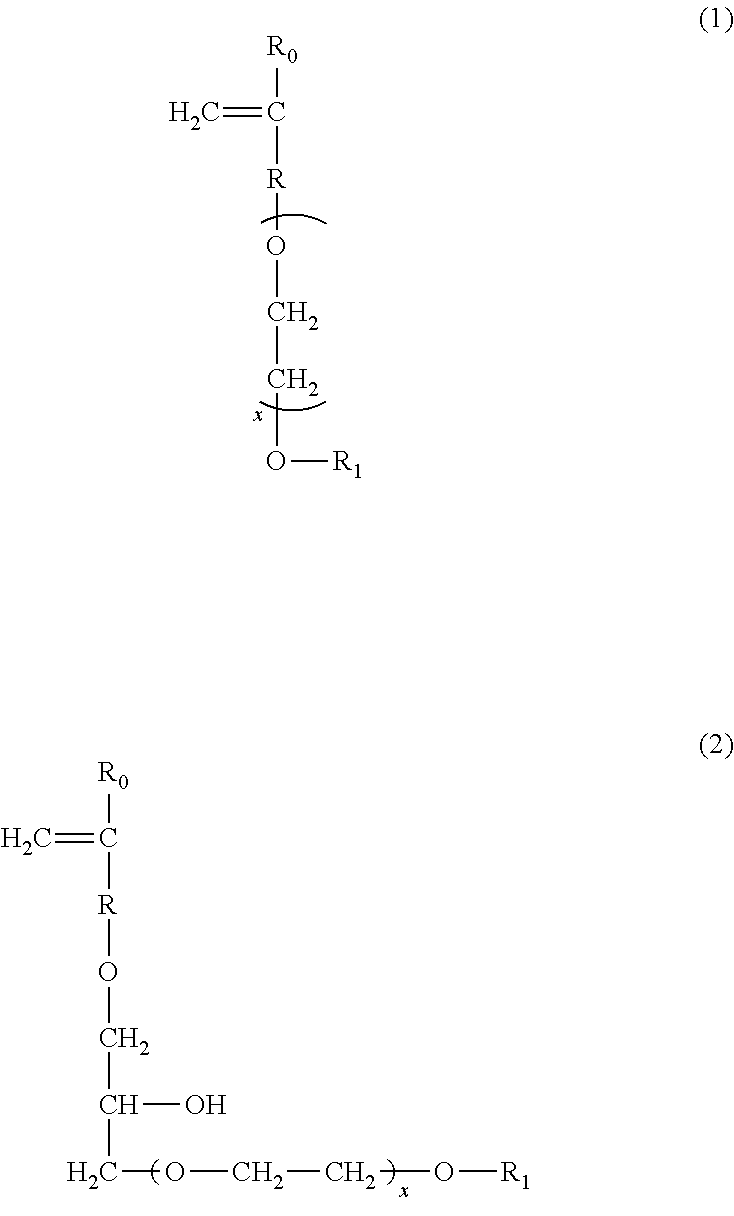
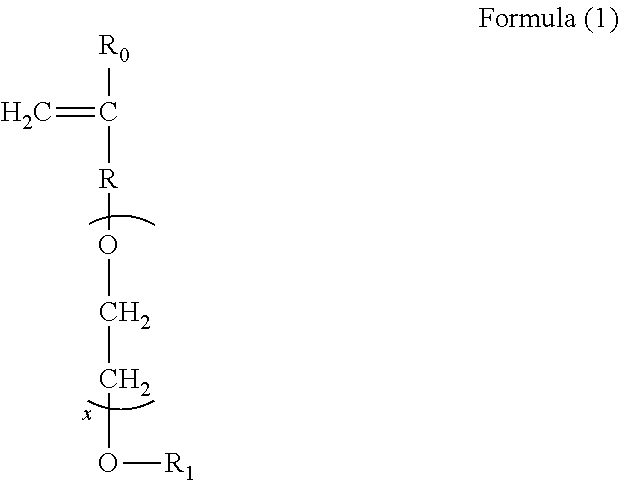
ActiveUS20110245132A1Inhibition is effectiveSuperior deposition-inhibiting abilityNon-ionic surface-active compoundsOrganic detergent compounding agentsSulfonateCarboxyl radical
[Problem] To provide detergent compositions with superior surfactant deposition-inhibiting ability and anti-gelling properties that exhibit good cleaning effectiveness even when laundering under harsh conditions such as laundering in residual bath water.[Solution] A laundry detergent or cleaning composition which comprises a copolymer containing sulfonate groups containing from 1 to 50 mass percent of structural units (a) derived from 1 or more kinds of monomers (A) selected from ether bond-containing monomers represented by Formulas (1) and (2), 50 mass % or more and less than 98 mass % of structural units (b) derived from a carboxyl group-containing monomer (B), and 1 mass % or more and less than 50 mass % of structural units (c) de-rived from a sulfonate group-containing monomer (C).
Owner:THE PROCTER & GAMBLE COMPANY
Carboxylic acid derivatives and drugs containing the same
InactiveUS6884821B1Effective preventionEffective treatmentBiocideOrganic chemistryCarboxyl radicalDouble bond
Novel carboxylic acid derivatives of general formula (I), salts of the same, esters thereof, or hydrates of them, which are useful as insulin resistance improvers; and drugs containing the derivatives as the active ingredient. In said formula, R1 is hydrogen, hydroxyl, alkyl, or the like; L is a single bond, a double bond, alkylene, or the like; M is a single bond, alkylene, or the like; T is a single bond, alkylene, or the like; W is carboxyl, —CON(RW1)RW2, or the like; represents a single or double bond; X is oxygen, alkenylene, or the like; Y is an aromatic hydrocarbon group which may contain a heteroatom, or the like; and Z is an aromatic hydrocarbon group which may contain a heteroatom.
Owner:EISIA R&D MANAGEMENT CO LTD
Carboxylic acid derivative and medicine comprising salt or ester of the same
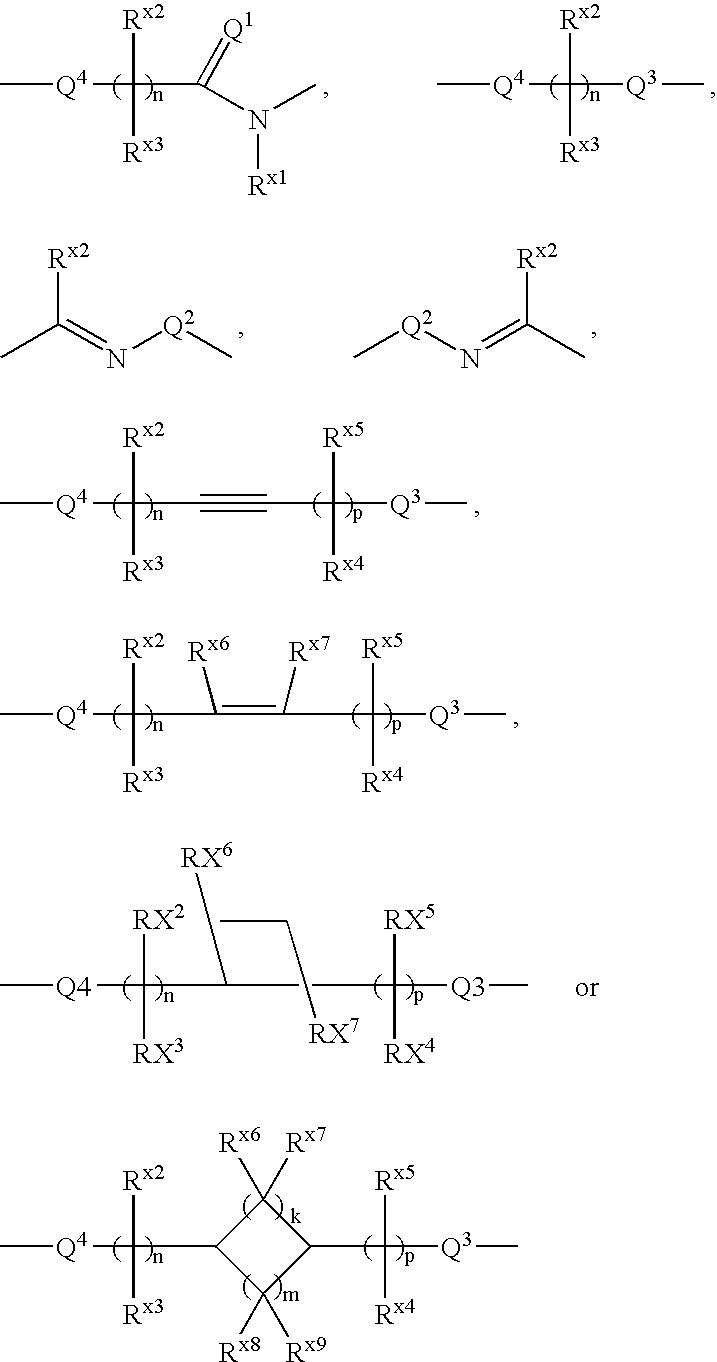
The present invention provides novel carboxylic acid derivatives useful as an insulin sensitizer, a salt thereof or a hydrate of them, and a medicament comprising the derivative as the active ingredient. Specifically, it provides a carboxylic acid derivative represented by the following formula: (wherein L represents a single bond, or a C1 to C6 alkylene group, a C2 to C6 alkenylene group or a C2 to C6 alkynylene group, each of which may have one or more substituent groups; M represents a single bond, or a C1 to C6 alkylene group, a C2 to C6 alkenylene group or a C2 to C6 alkynylene group, each of which may have one or more substituent groups; T represents a single bond, or a C1 to C3 alkylene group, a C2 to C3 alkenylene group or a C2 to C3 alkynylene group, each of which may have one or more substituent groups; W represents a carboxyl group; X represents a single bond, an oxygen atom, or a group represented by the various substituent groups including -NR<X1>CQ<1>O- (wherein Q<1 >represents an oxygen atom or a sulfur atom; R<X1 >represents a hydrogen atom, a cyano group, a formyl group, or various groups including a C1 to C6 alkyl group and a C1 to C6 hydroxyalkyl group, each of which may have one or more substituent groups), ONR<X1>CQ<1>-, -NR<X1>CQ<1>-, -CQ<1>NR<X1>-, -NR<X1a>CQ<1>NR<X1b>-, -Q<2>SO2- and -SO2Q<2>-; Y represents a 5 to 14-membered aromatic group which may have one or more substituent groups and one or more hetero atoms, or a C3 to C7 alicyclic hydrocarbon group; and the rings Z and U may be the same as or different from each other and each represents a 5 to 14-membered aromatic group which may have 1 to 4 substituent groups and one or more hetero atoms, and the ring may be partially saturated.), a salt thereof, an ester thereof or a hydrate of them.
Owner:EISIA R&D MANAGEMENT CO LTD
Benzimidazole cyclooxygenase-2 inhibitors
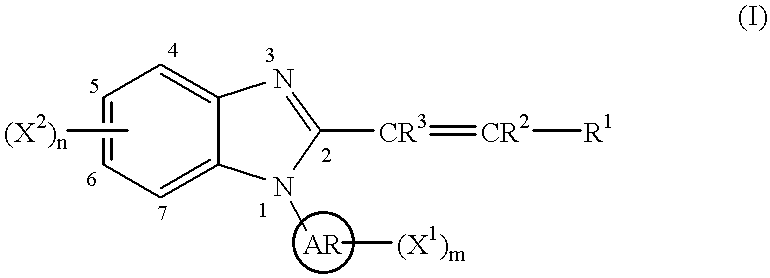
This invention provides a compound of the following formula:or the pharmaceutically acceptable salts thereof, whereinAr is heteroaryl; X1 and X2 are independently selected from halo, C1-C4 alkyl, hydroxy, C1-C4 alkoxy, amino, C1-C4 alkanoyl, carboxy, carbamoyl, cyano, nitro, mercapto, (C1-C4 alkyl)thio, (C1-C4 alkyl)sulfinyl, (C1-C4 alkyl)sulfonyl, aminosulfonyl, or the like; R1 is selected from hydrogen, straight or branched C1-C4 alkyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, phenyl , heteroaryl and the like; R2 and R3 are independently selected from hydrogen, halo, C1-C4 alkyl, phenyl and the like; or R1 and R2 can form, together with the carbon atom to which they are attached, a C5-C7 cycloalkyl ring; and m and n are independently 0, 1, 2 or 3.These compounds and pharmaceutical compositions containing such compounds are useful as analgesics and anti-inflammatory agents.
Owner:PFIZER INC
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
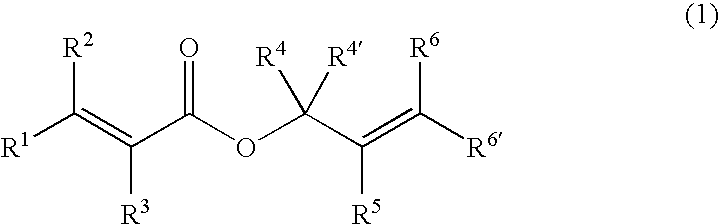
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
Method for preparing viscosity-reduction-type polycarboxylic acid superplasticizer and application of viscosity-reduction-type polycarboxylic acid superplasticizer
The invention provides a method for preparing a viscosity-reduction-type polycarboxylic acid superplasticizer and application of the viscosity-reduction-type polycarboxylic acid superplasticizer. The method is simple in operating process, and the prepared polycarboxylic acid superplasticizer is applied to high- and ultrahigh-strength concrete, can be used for effectively lowering the viscosity of concrete and improving the placeability and flow velocity of the concrete and is beneficial to pumping construction. According to the method, the viscosity-reduction-type polycarboxylic acid superplasticizer is prepared through carrying out free-radical copolymerization on a carboxylic monomer a, a branched side chain containing monomer b and a rigid cyclic group containing monomer c which are in the mole ratio of (4-15): 1: (0.5-2). The polycarboxylic acid superplasticizer prepared by the method provided by the invention can serve as a cement dispersant so as to greatly lower the water-cement ratio of concrete; and the polycarboxylic acid superplasticizer can be used for effectively lowering the viscosity of the high- and ultrahigh-strength concrete and improving the placeability and flow velocity of the concrete, so that the pumpability is excellent.
Owner:JIANGSU SOBUTE NEW MATERIALS +2
Method for extracting carbon quantum dots from activated carbon
ActiveCN101973541AUniform particle sizeImprove photoelectric performanceNanostructure manufactureLuminescent compositionsActivated charcoal powderCarboxyl radical
The invention provides a method for extracting carbon quantum dots from activated carbon, which comprises the following steps of: adding dry activated carbon powder to salpeter solution and stirring for backflow; performing reduced pressure distillation for evaporating suspension obtained by backflow to dryness; dispersing obtained black solids in water, and neutralizing obtained solution with sodium hydroxide; and finally, centrifugating neutralized black suspension for removing precipitation, separating supernatant fluid by using an ultrafiltration centrifugal tube or an ultrafiltration membrane, collecting filtrate, and drying the filtrate to obtain carbon quantum dots. The method uses cheap and available activated carbon as carbon sources, and can obtain a large number of carbon quantum dots by simple chemical oxidation process and simple subsequent processes of evaporation, saturation, centrifugation and ultrafiltration. The carbon quantum dots are graphite structure nanocrystals with the grain diameter of 3 to 5 nm, the surfaces of which have a large amount of hydroxide radicals. The carbon quantum dots have good fluorescence and electrochemiluminescence.
Owner:FUZHOU UNIV
L-Threonine derivatives of high therapeutic index
The present invention is directed to a derivative comprised of an L-Threonine bonded to a medicament or drug having a hydroxy, amino, carboxy or acylating derivative thereon. The derivative has the same utility as the drug from which it is made, but it has enhanced therapeutic properties. In fact, the derivatives of the present invention enhance at least one or more therapeutic qualities, as defined herein. The present invention is also directed to pharmaceutical compositions containing same.
Owner:SIGNATURE R & D HLDG LLC
Magnetic nanometer ion liquid composite particles as well as preparation method and application thereof
InactiveCN103100358ASimple and fast operationLow costMicroballoon preparationWater/sewage treatment by extractionCarboxyl radicalSilicic acid
The invention discloses magnetic nanometer ion liquid composite particles as well as a preparation method and application of the magnetic nanometer ion liquid composite particles in removing pollutants in water bodies. The preparation method of the magnetic nanometer ion liquid composite particle comprises the following steps of: firstly synthesizing ferric oleate into magnetic Fe3O4 nanometer particles by taking ferric oleate as an iron source and adopting a chemical precipitation method; preparing nanometer nuclear shell type magnetic silicon dioxide with surface amino-functionalization by utilizing the nanometer magnetic particles as a kern, taking ethyl orthosilicate and a silane coupling agent as silicon sources, and utilizing a colloidal sol-gel method; and synthesizing a functionalized ion liquid by utilizing reaction between N, N-carbonyldimidazole (CDI) and an ion liquid containing carboxyl, and thus preparing the magnetic nanometer ion liquid composite particle by utilizing reaction between the functionalized ion liquid and amino on the surface of the magnetic nanometer silicon dioxide; and the method is used for removing pollutants in the water bodies. The method provided by the invention has the advantages that the operation is simple and convenient, the cost is low, the treatment process is simple, and the removal efficiency is high.
Owner:SOUTH CHINA UNIV OF TECH
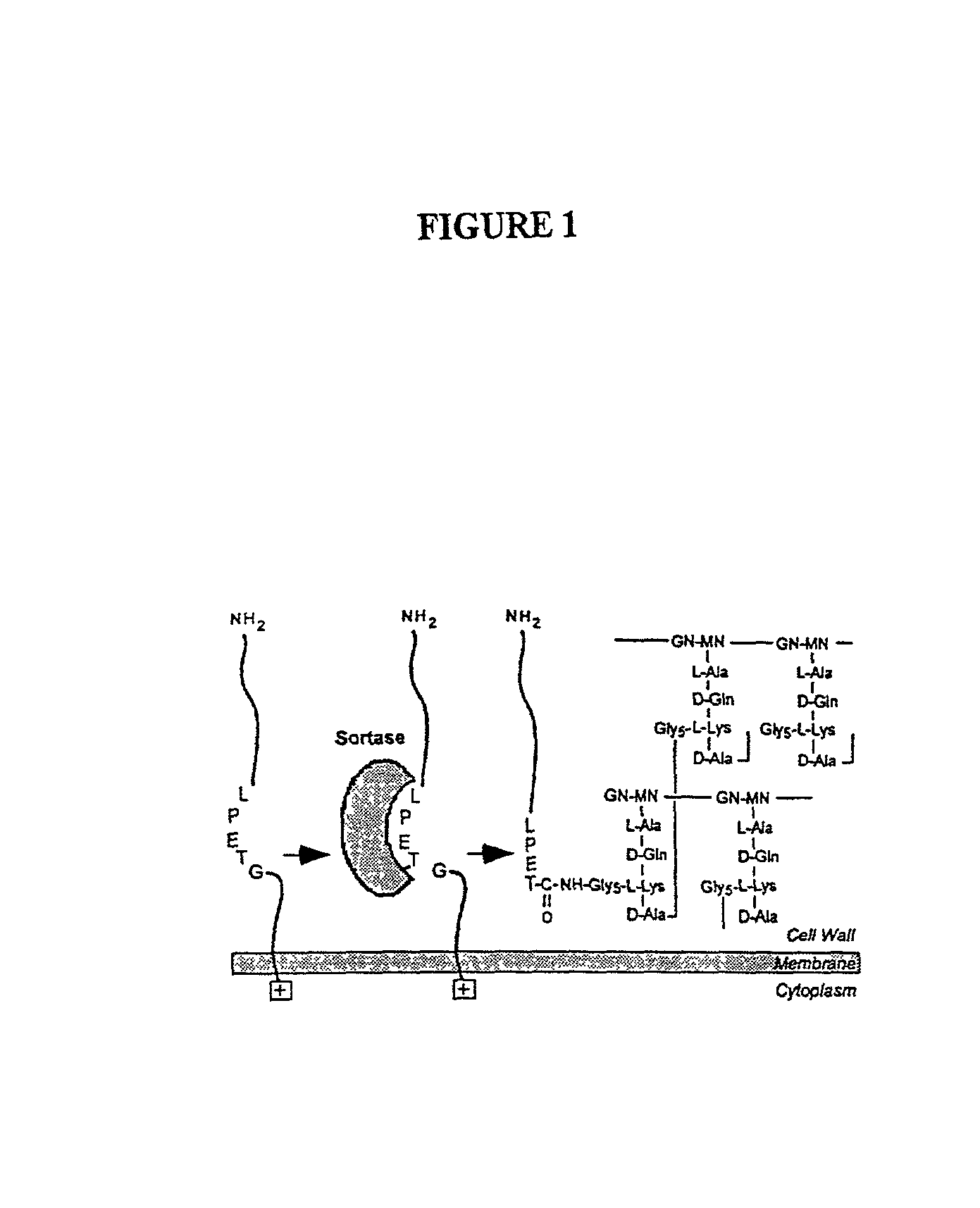
Identification of sortase gene
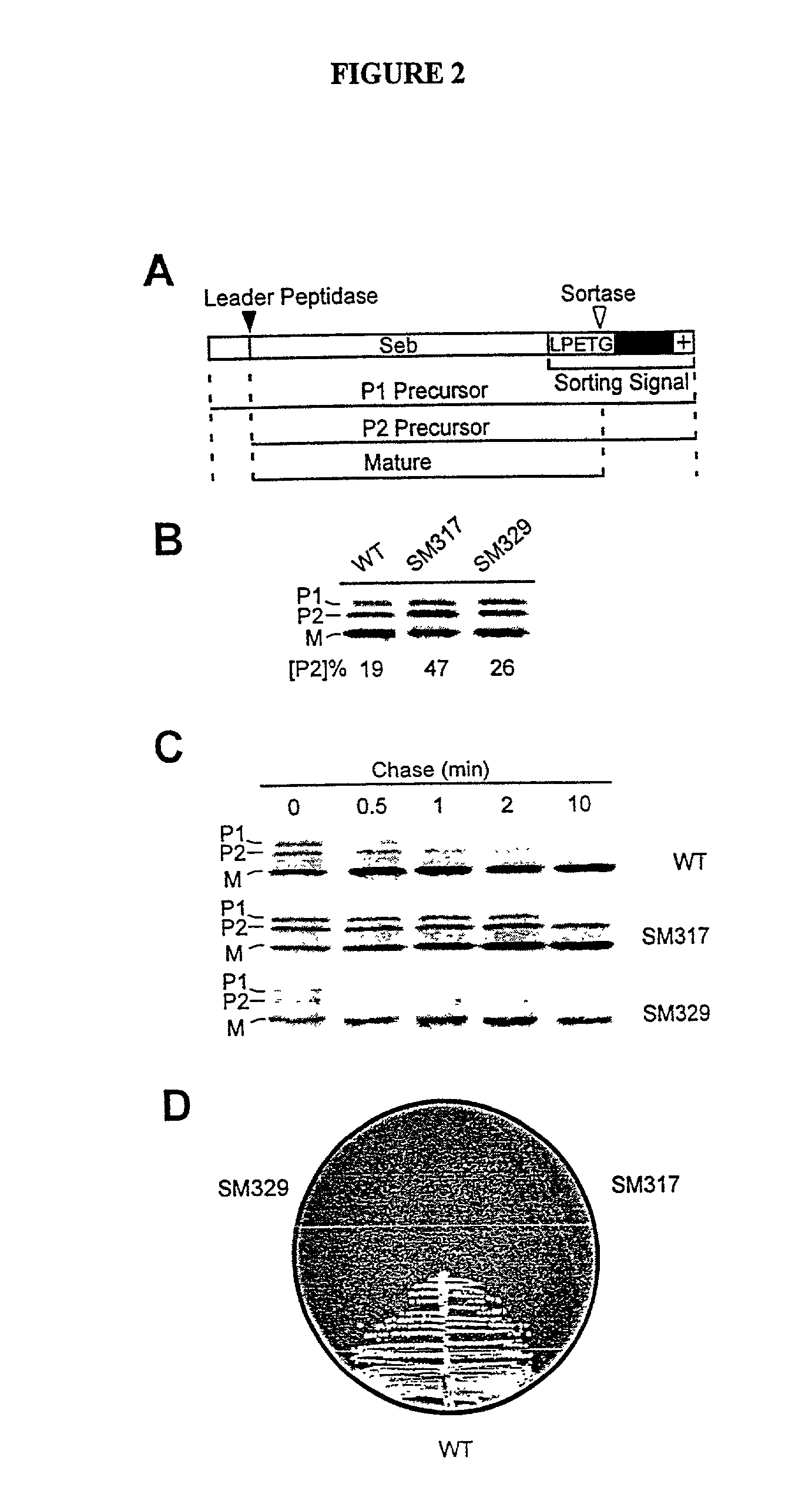
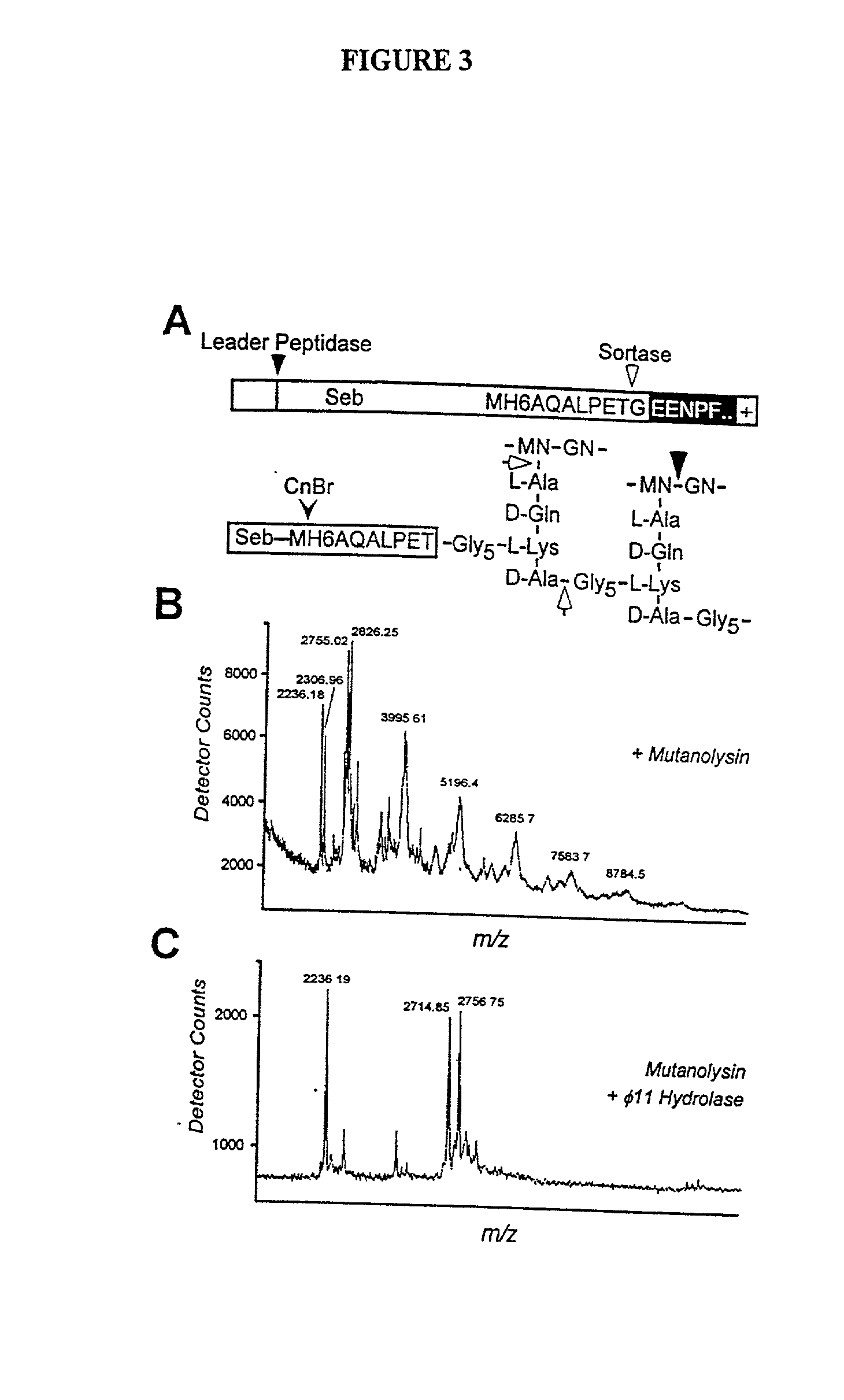
InactiveUS20030022178A1Fluorescence enhancementIncreased cleavageAntibacterial agentsFungiEnzyme GeneCarboxyl radical
The present invention is a substantially purified sortase-transamidase enzyme from Gram-positive bacteria, such as Staphylococcus aureus. The enzyme having a molecular weight of about 23,539 or about 29,076 daltons and catalyzing a reaction that covalently cross-links the carboxyl terminus of a protein having a sorting signal to the peptidoglycan of a Gram-positive bacterium, the sorting signal having: (1) a motif of LPX3X4G therein; (2) a substantially hydrophobic domain of at least 31 amino acids carboxyl to the motif; and (3) a charged tail region with at least two positively charged residues carboxyl to the substantially hydrophobic domain, at least one of the two positively charged residues being arginine, the two positively charged residues being located at residues 31-33 from the motif, wherein X3 is any of the twenty naturally-occurring L-amino acids and X4 is selected from the group consisting of alanine, serine, and threonine, and wherein sorting occurs by cleavage between the fourth and fifth residues of the LPX3X4G motif. Variants of the enzyme, methods for cloning the gene encoding the enzyme and expressing the cloned gene, and methods of use of the enzyme, including for screening for antibiotics and for display of proteins or peptides on the surfaces of Gram-positive bacteria, are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Organisms for the production of cyclohexanone
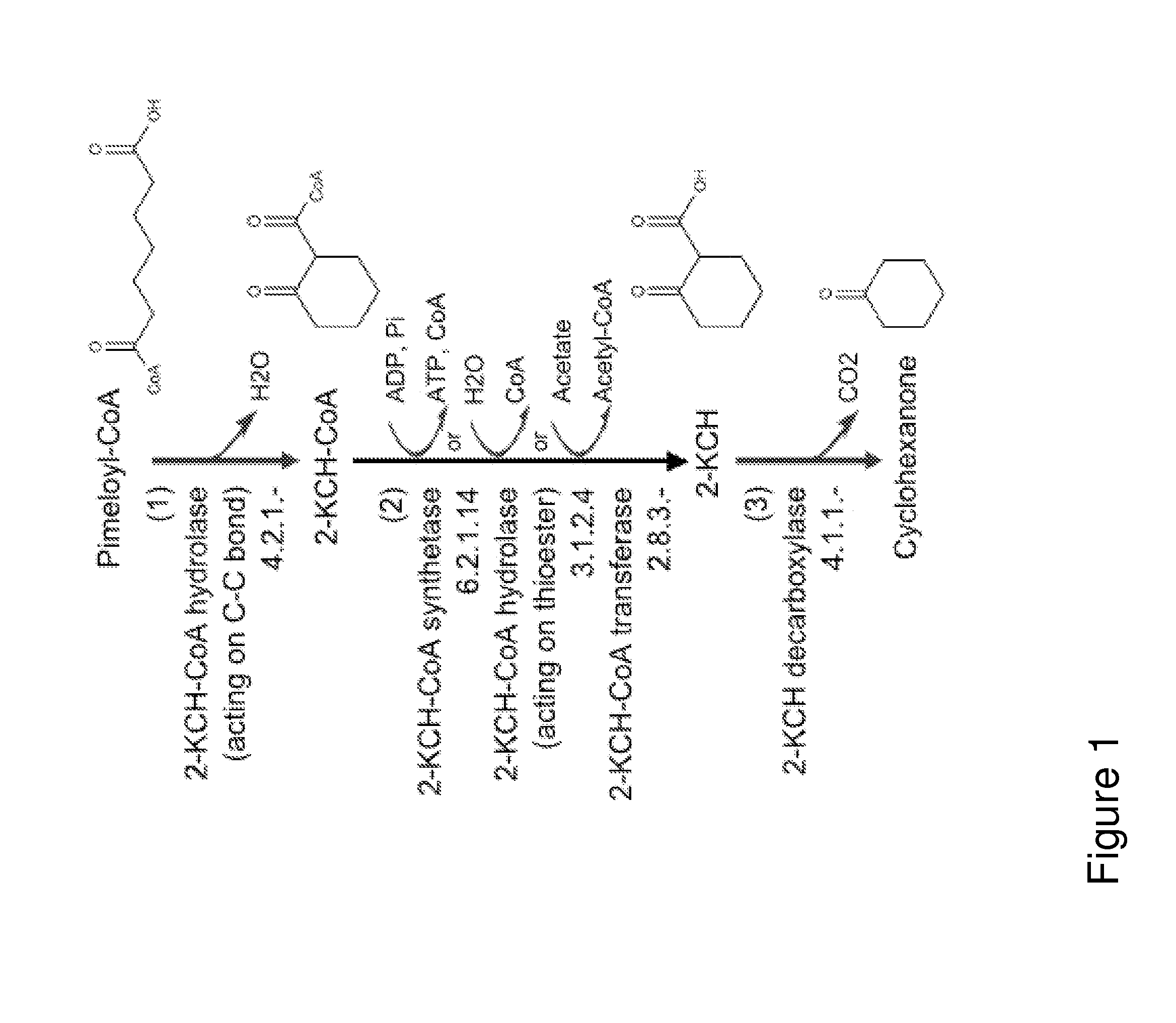
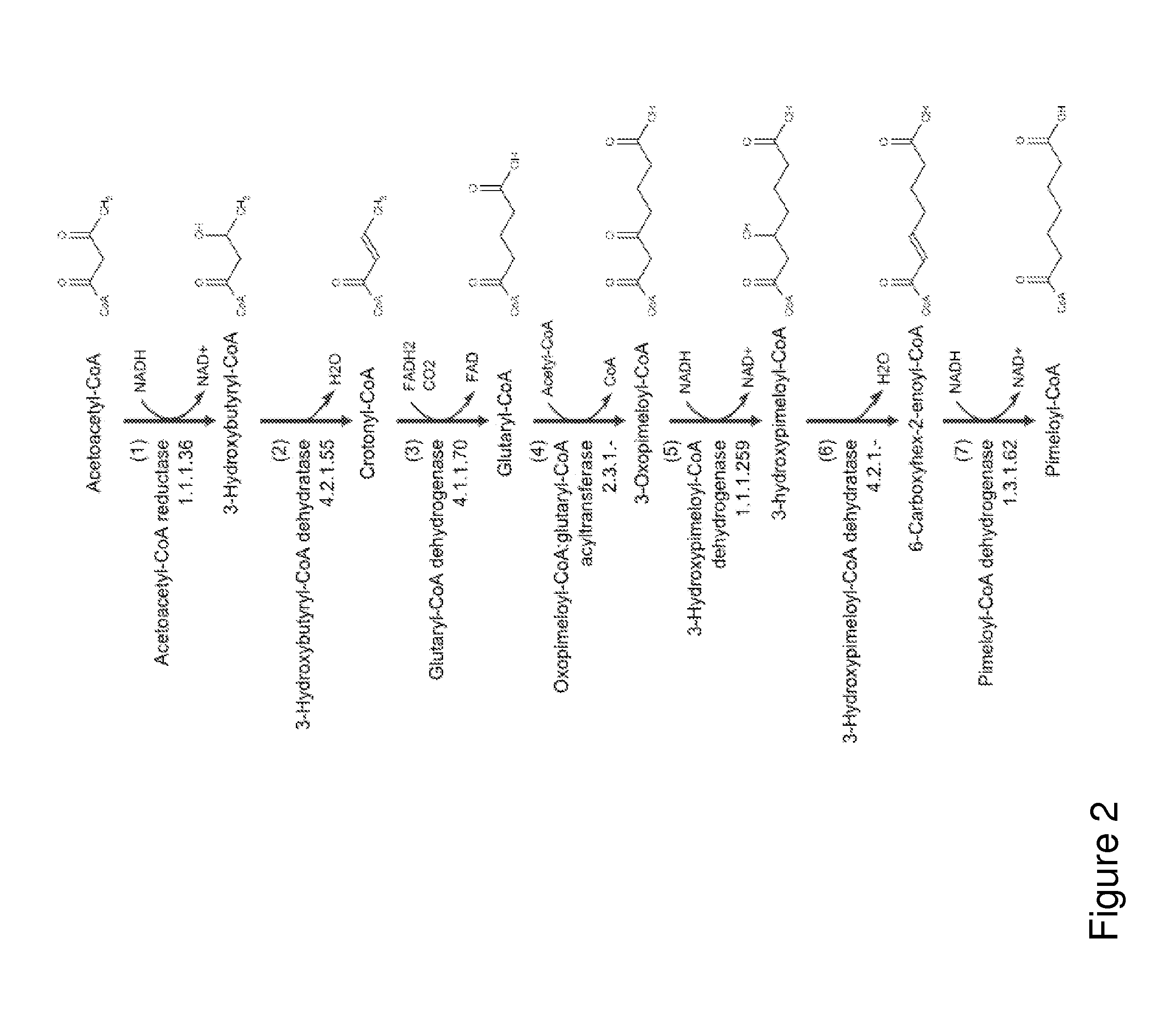
A non-naturally occurring microbial organism has cyclohexanone pathways that include at least one exogenous nucleic acid encoding a cyclohexanone pathway enzyme. A pathway includes a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on C—C bond), a 2-ketocyclohexane-1-carboxylate decarboxylase and an enzyme selected from a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxyl-CoA transferase, and a 2-ketocyclohexane-1-carboxyl-CoA synthetase. A pathway includes an enzyme selected from a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on C—C bond), a 6-ketocyclohex-1-ene-1-carboxyl-CoA synthetase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on thioester), a 6-ketocyclohex-1-ene-1-carboxyl-CoA transferase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA reductase, a 6-ketocyclohex-1-ene-1-carboxylate decarboxylase, a 6-ketocyclohex-1-ene-1-carboxylate reductase, a 2-ketocyclohexane-1-carboxyl-CoA synthetase, a 2-ketocyclohexane-1-carboxyl-CoA transferase, a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxylate decarboxylase, and a cyclohexanone dehydrogenase. A pathway includes an adipate semialdehyde dehydratase, a cyclohexane-1,2-diol dehydrogenase, and a cyclohexane-1,2-diol dehydratase. A pathway includes a 3-oxopimelate decarboxylase, a 4-acetylbutyrate dehydratase, a 3-hydroxycyclohexanone dehydrogenase, a 2-cyclohexenone hydratase, a cyclohexanone dehydrogenase and an enzyme selected from a 3-oxopimeloyl-CoA synthetase, a 3-oxopimeloyl-CoA hydrolase (acting on thioester), and a 3-oxopimeloyl-coA transferase. Each these pathways can include a PEP carboxykinase. A method for producing cyclohexanone includes culturing these non-naturally occurring microbial organisms.
Owner:GENOMATICA INC
Algin oligosaccharides and the derivatives thereof as well as the manufacture and the use of the same
The present invention provides an alginate oligosaccharide and its derivatives with the degree of polymerization ranging from 2 to 22. The alginate oligosaccharide is composed of β-D-mannuronic acid linked by 1,4 glycosidic bonds. The derivatives with the reduced terminal in position 1 of carboxyl radical can be prepared by oxidative degradation. The present invention also provides a process for preparing the alginate oligosaccharide and its derivatives, which includes the procedures that an alginate solution is reacted for 2 to 6 h in an autoclave at pH 2-6 and the temperature of 100-120° C., and pH is adjusted to 7 after the reaction is stopped, after which the resultant oligosaccharide is oxidized in the presence of an oxidant to obtain an oxidative degradation product. The alginate oligosaccharide and its derivatives of the invention can be used in the manufacture of a medicament for the prophylaxis and treatment of AD and diabetes.
Owner:OCEAN UNIV OF CHINA
Cellulose acetate and dope containing the same
InactiveUS7122660B1Good release effectSatisfactory optical propertyArtificial filaments from cellulose derivativesCarboxyl radicalCellulose diacetate
Using the dope containing the following cellulose acetate (1), (2), or (3), a film is prepared by the casting process:(1) a cellulose acetate having carboxyl groups binding to at least one member selected from the group consisting of a cellulose acetate and a hemicellulose acetate, wherein at least a part of said carboxyl groups are in an acidic form;(2) a cellulose acetate containing at least one member selected from the group consisting of an acid having an acid dissociation exponent pKa of 1.93 to 4.50 in water, an alkali metal salt of said acid and an alkaline earth metal of said acid; or(3) a cellulose acetate containing an alkali metal or an alkaline earth metal wherein the total content of an alkaline metal and an alkaline earth metal in 1 gram of the cellulose acetate is from an effective amount to 5.5×10−6 equivalent (in terms of ion equivalent). The above cellulose acetate is also useful for spinning process. The cellulose acetate includes a cellulose diacetate and a cellulose triacetate. The cellulose acetate of the present invention has high film-releasability from a support and excellent optical characteristics.
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


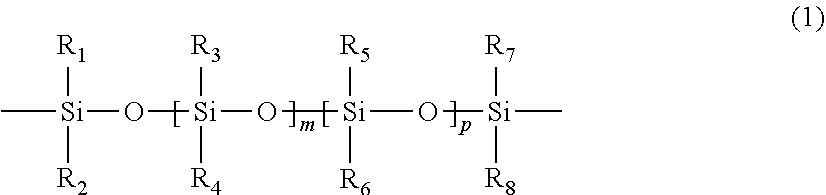
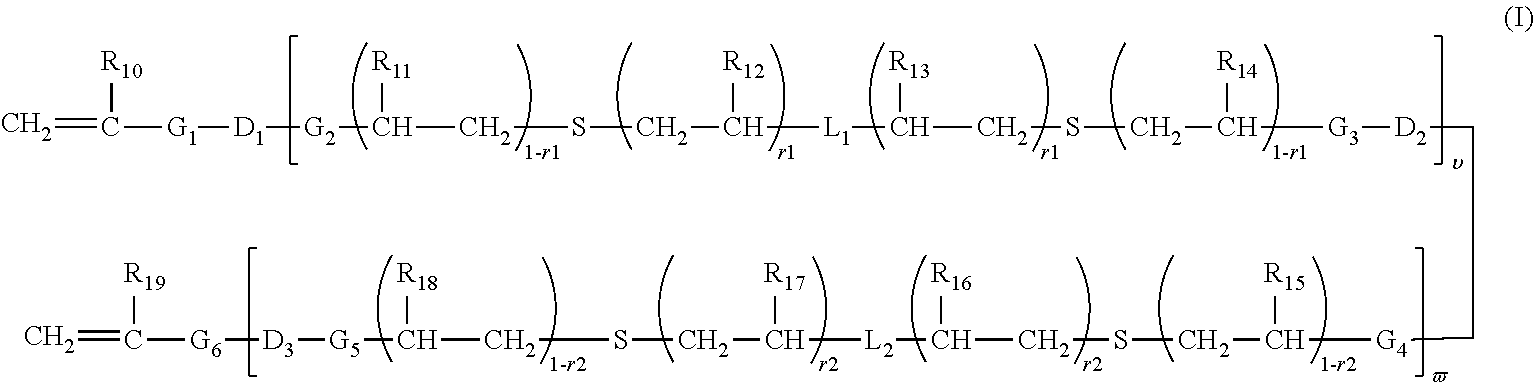

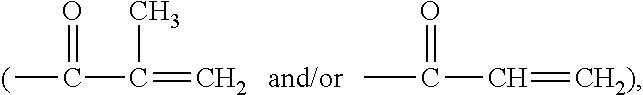
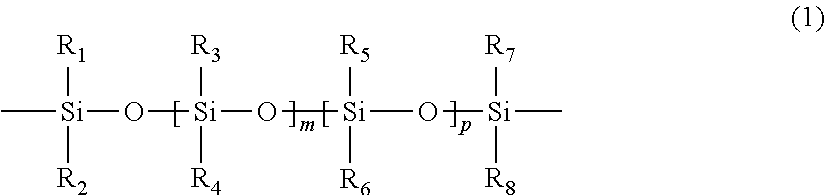

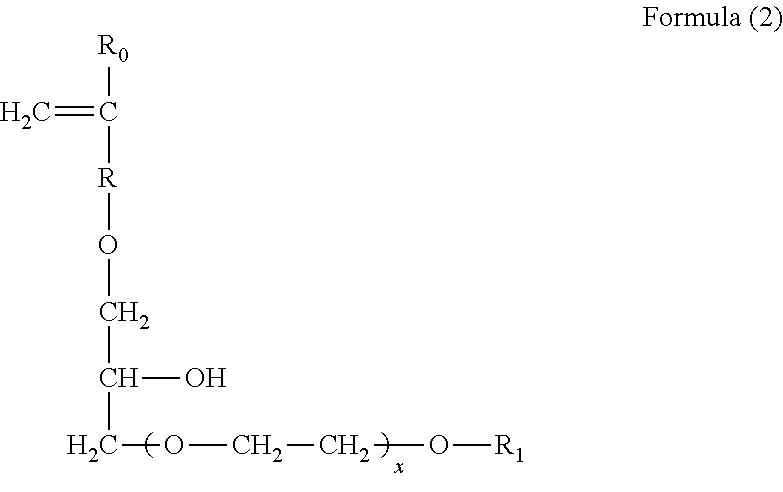
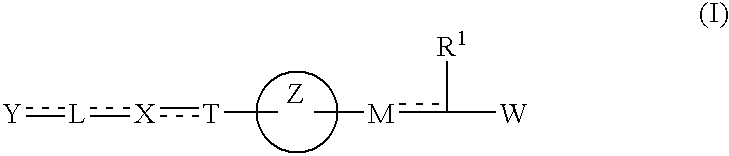
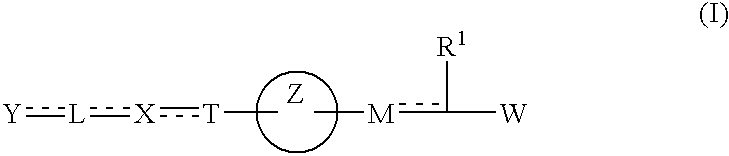
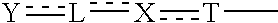
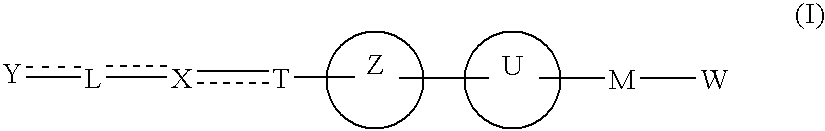
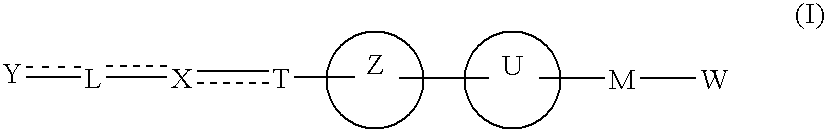
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)