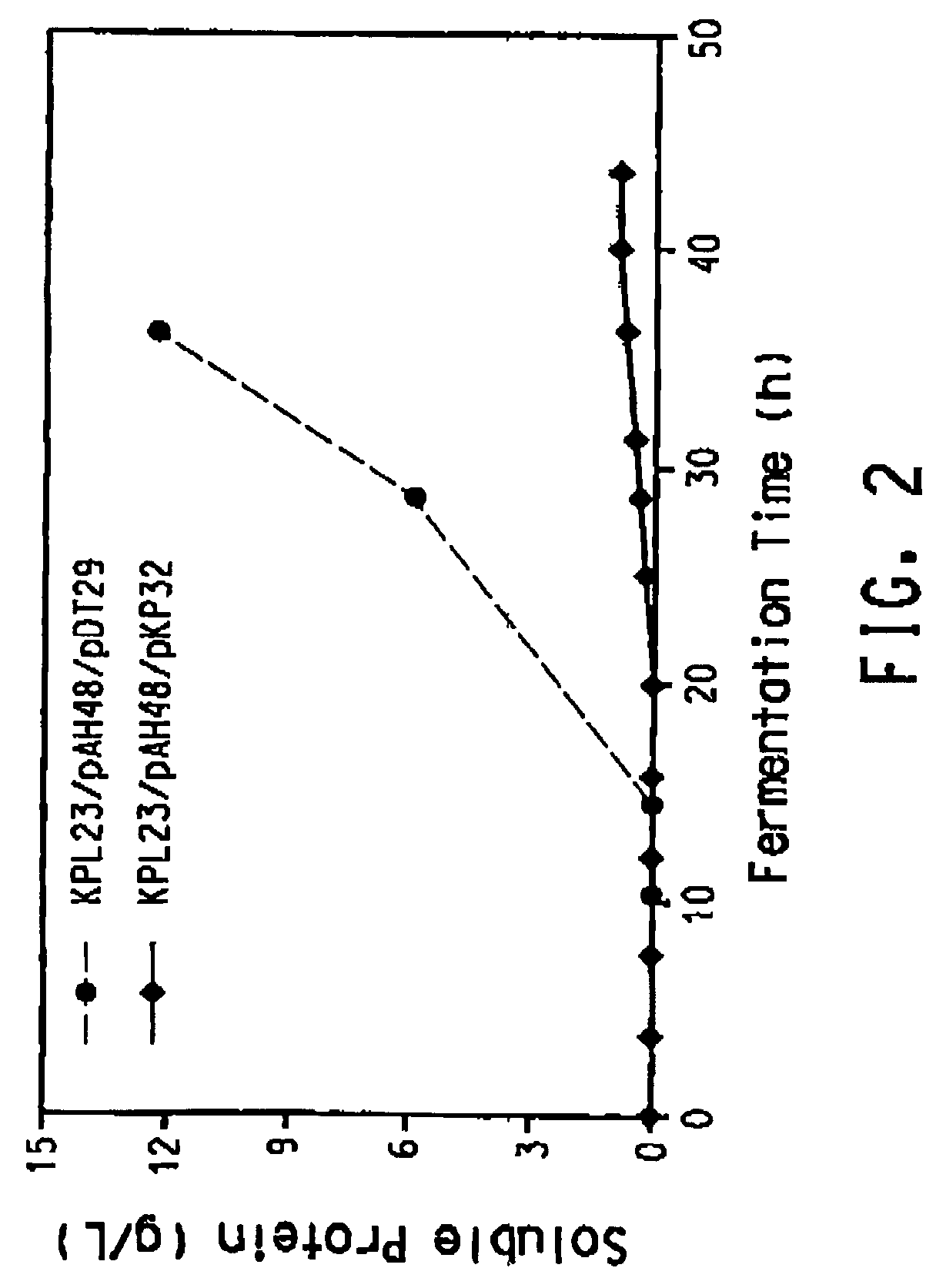
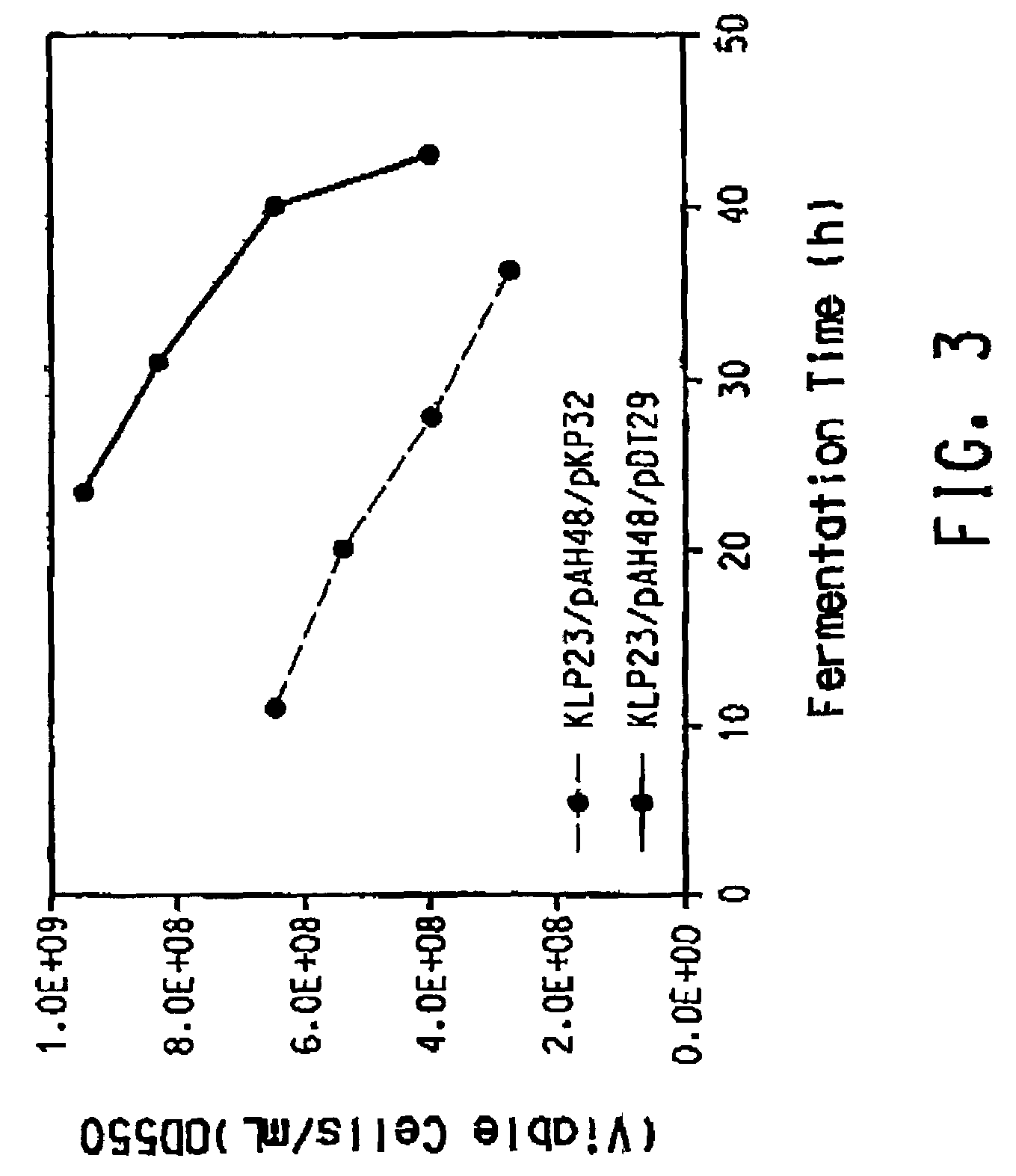
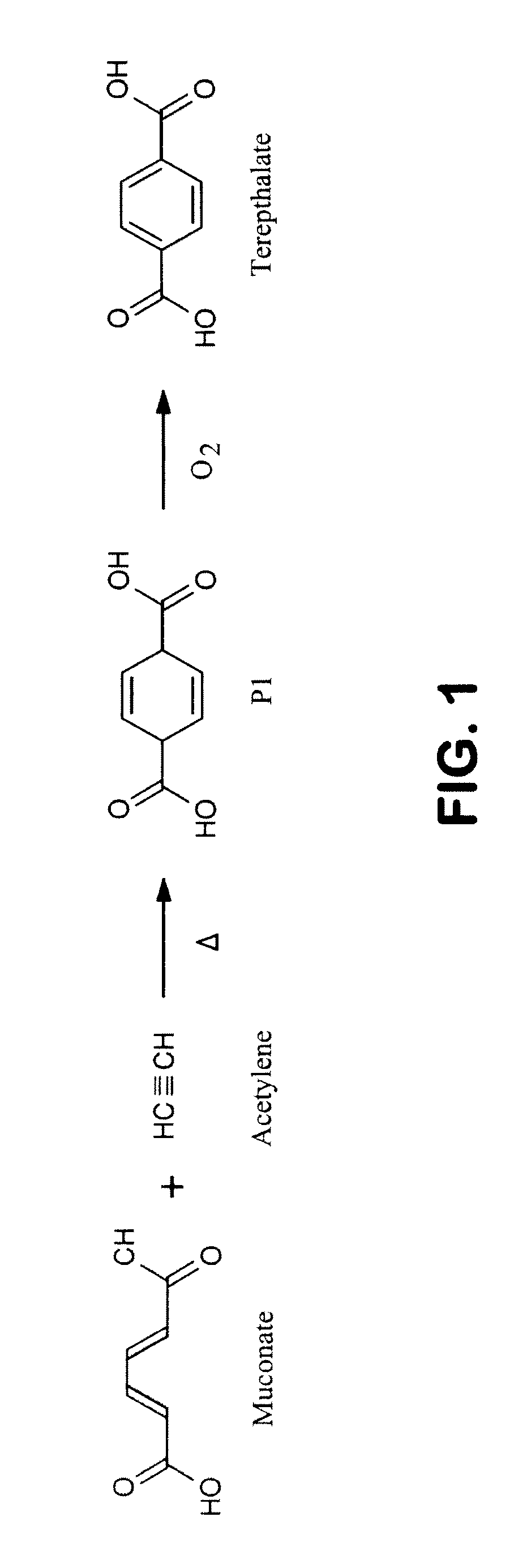
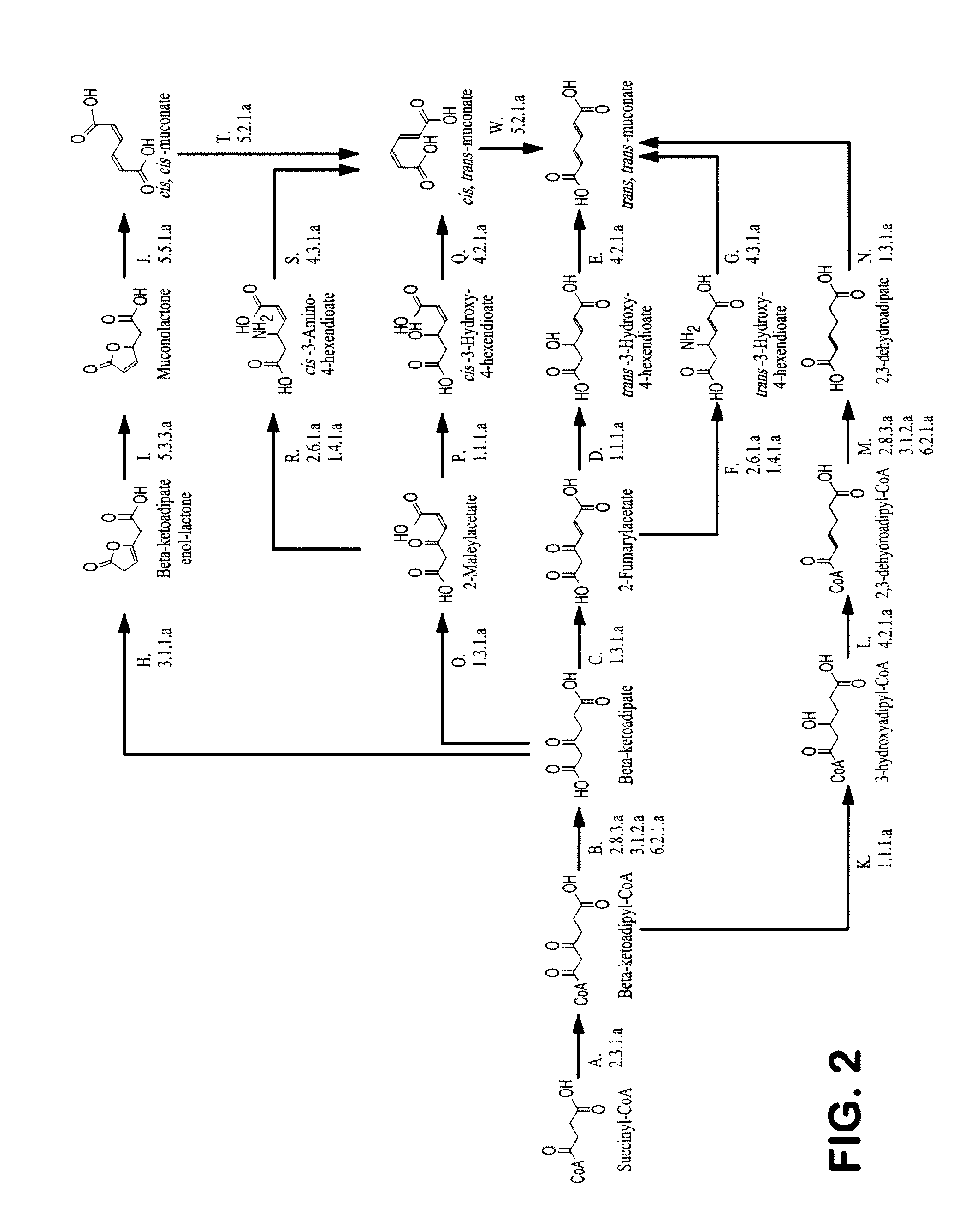
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
176 results about "Dehydratase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dehydratases are a group of lyase enzymes that form double and triple bonds in a substrate through the removal of water. They can be found in many places including the mitochondria, peroxisome and cytosol. There are more than 150 different dehydratase enzymes that are classified into four groups. Dehydratases can act on hydroxyacyl-CoA with or without cofactors, and some have a metal and non-metal cluster act as their active site.
Fermentive production of four carbon alcohols
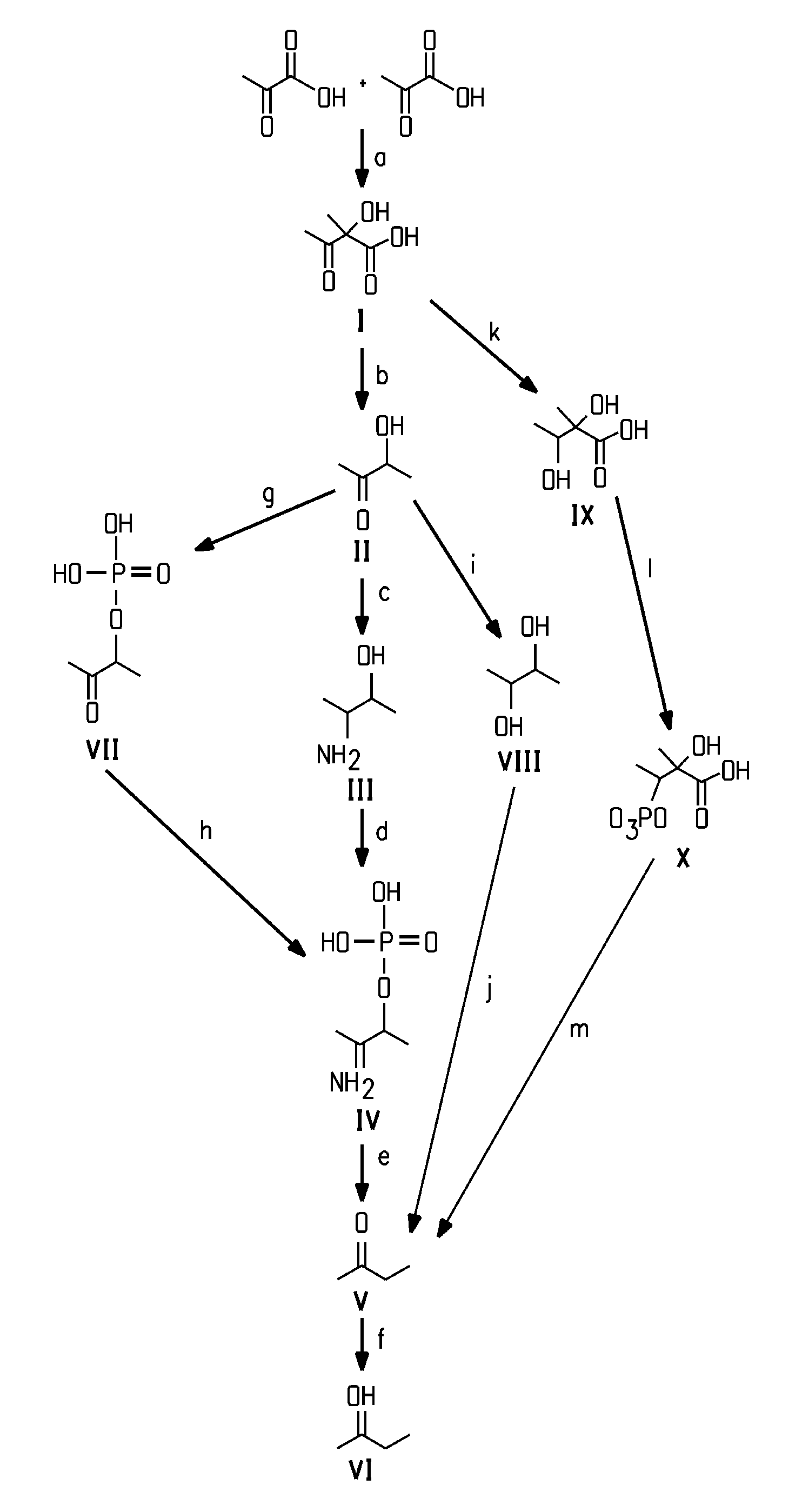
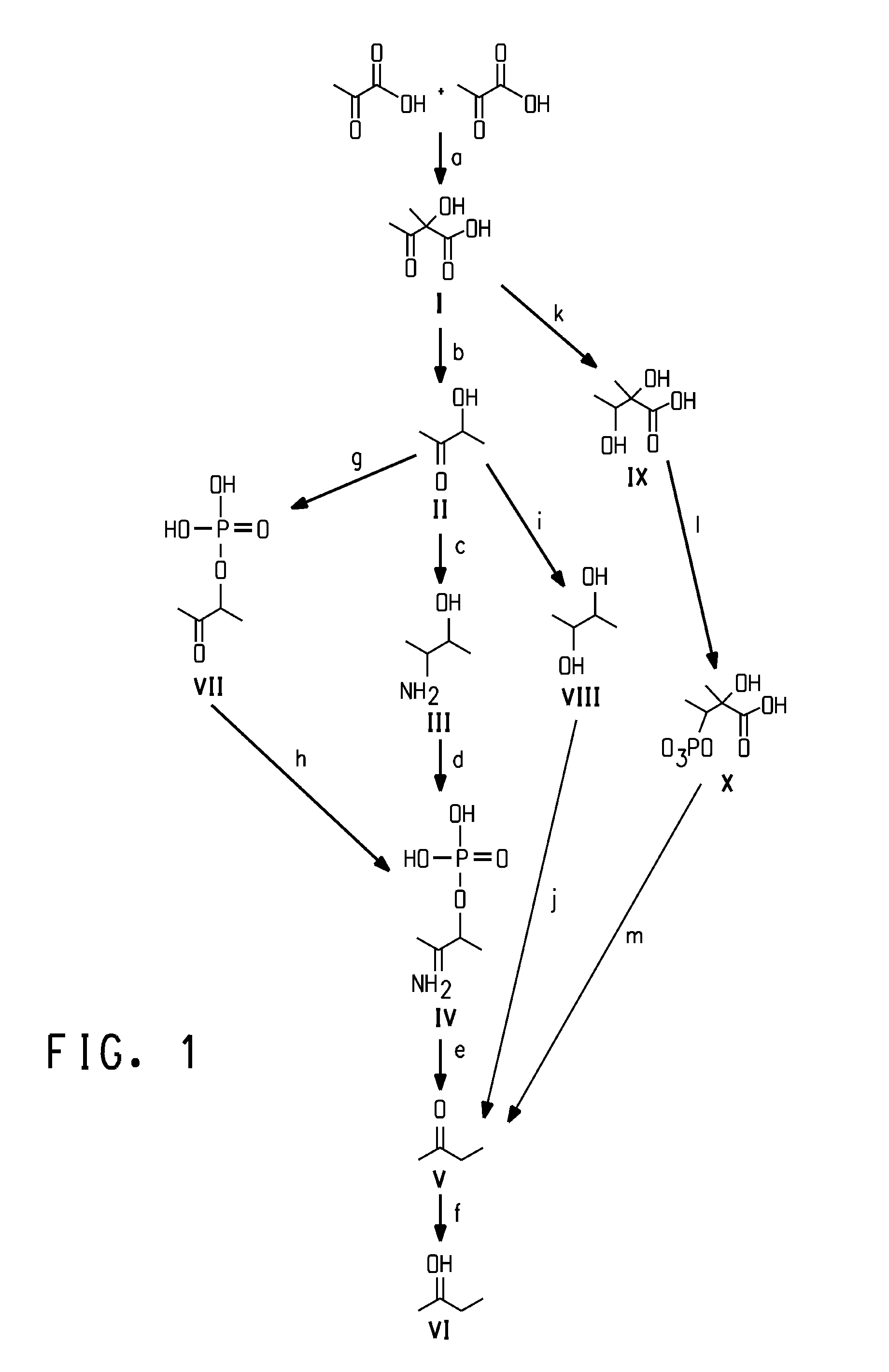
Methods for the fermentive production of four carbon alcohols are provided. Specifically, butanol, preferably 2-butanol is produced by the fermentive growth of a recombinant bacteria expressing a 2-butanol biosynthetic pathway. The recombinant microorganisms and methods of the invention can also be adapted to produce 2-butanone, an intermediate in the 2-butanol biosynthetic pathways disclosed herein. Specifically disclosed herein are the use of coenzyme B12-independent butanediol dehydratases that catalyzes the substrate to product conversion of 2,3-butanediol to 2-butanone in the process of producing 2-butanol and 2-butanone.
Owner:GEVO INC
Process for the biological production of 1,3-propanediol with high titer
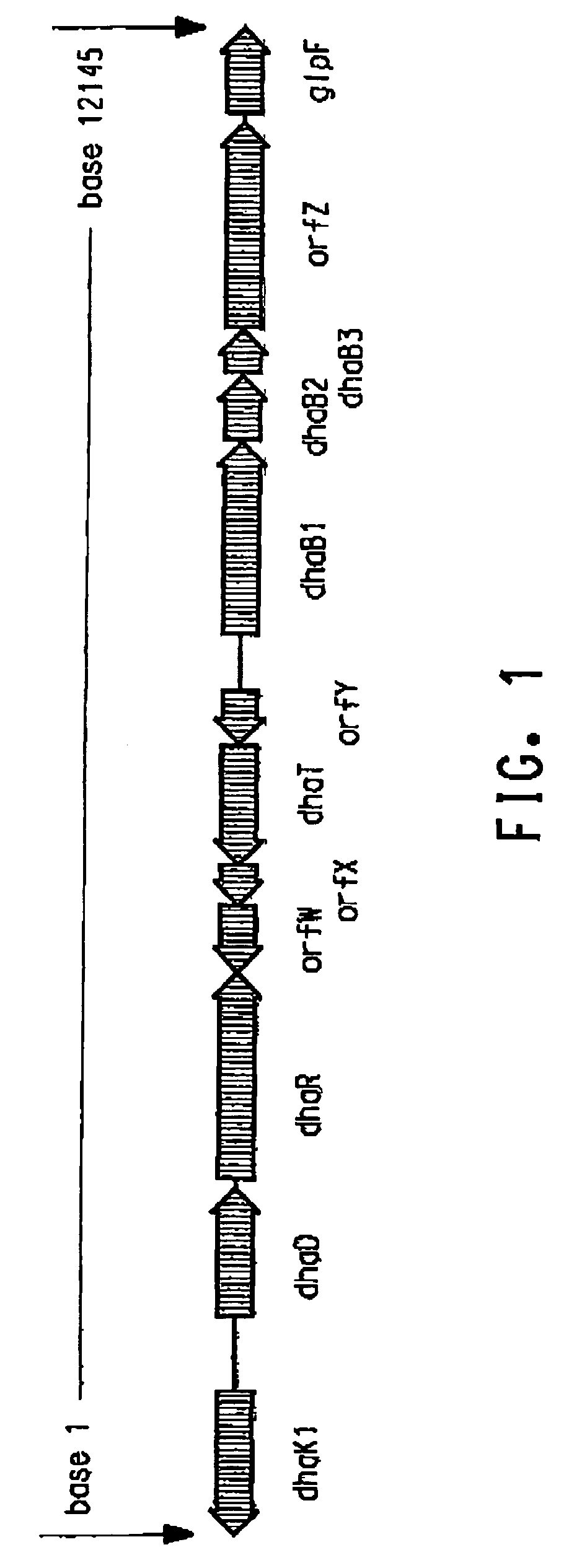
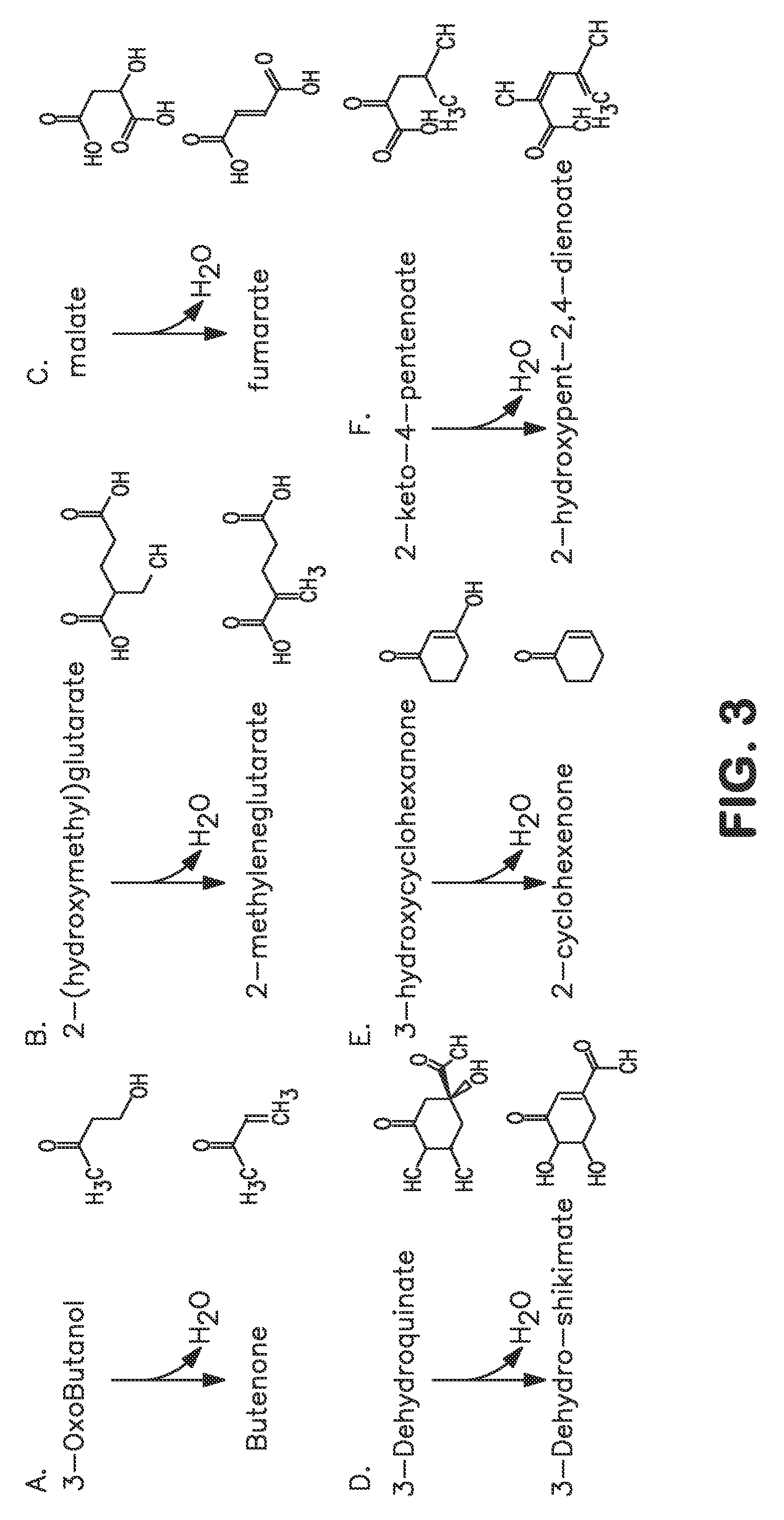
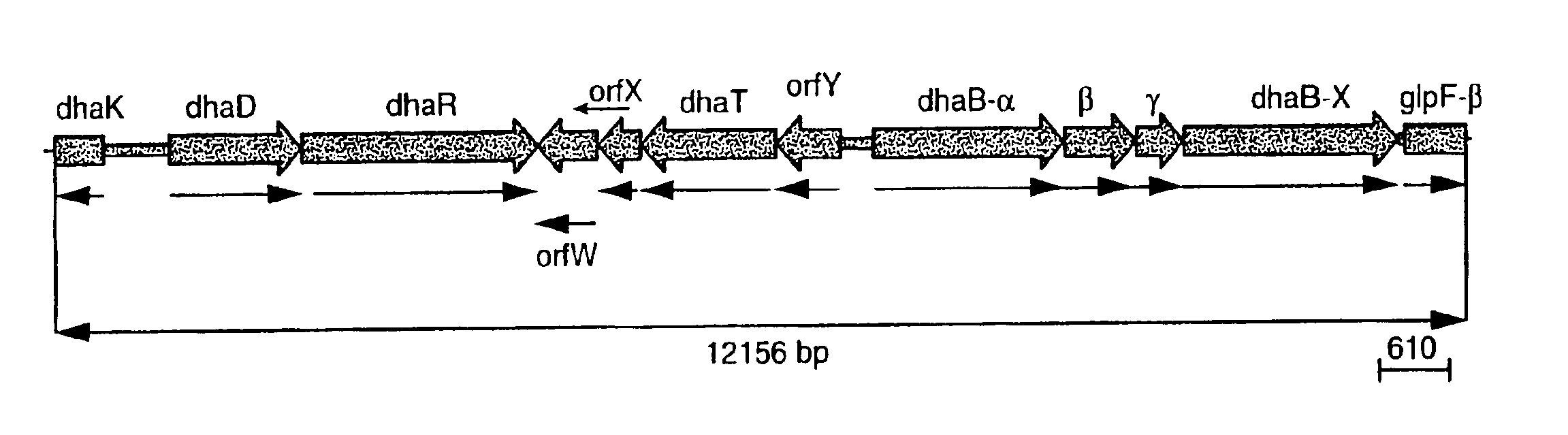
The present invention provides an improved method for the biological production of 1,3-propanediol from a fermentable carbon source in a single microorganism. In one aspect of the present invention, an improved process for the conversion of glucose to 1,3-propanediol is achieved by the use of an E. coli transformed with the Klebsiella pneumoniae dha regulon genes dhaR, orfY, dhaT, orfX, orfW, dhaB1, dhaB2, dhaB3, and orfZ, all these genes arranged in the same genetic organization as found in wild type Klebsiella pneumoniae. In another aspect of the present invention, an improved process for the production of 1,3-propanediol from glucose using a recombinant E. coli containing genes encoding a G3PDH, a G3P phosphatase, a dehydratase, and a dehydratase reactivation factor compared to an identical process using a recombinant E. coli containing genes encoding a G3PDH, a G3P phosphatase, a dehydratase, a dehydratase reactivation factor and a 1,3-propanediol oxidoreductase (dhaT). The dramatically improved process relies on the presence in E. coli of a gene encoding a non-specific catalytic activity sufficient to convert 3-hydroxypropionaldehyde to 1,3-propanediol.
Owner:DUPONT US HLDG LLC
Method for producing L-amino acid using methylotroph
InactiveUS20040142435A1High activityHigh expressionBacteriaSugar derivativesMicroorganismMethylotroph
The present invention describes a method for producing an L-amino acid comprising culturing a microorganism having an ability to produce an L-amino acid in a medium, whereby the L-amino acid accumulates in the medium, and collecting the L-amino acid from the medium, whereby said microorganism comprises a methanol-utilizing bacterium having the Entner-Doudoroff pathway in which 6-phosphogluconate dehydratase activity and / or 2-keto-3-dexoy-6-phosphogluconate aldolase activity is enhanced.
Owner:AJINOMOTO CO INC
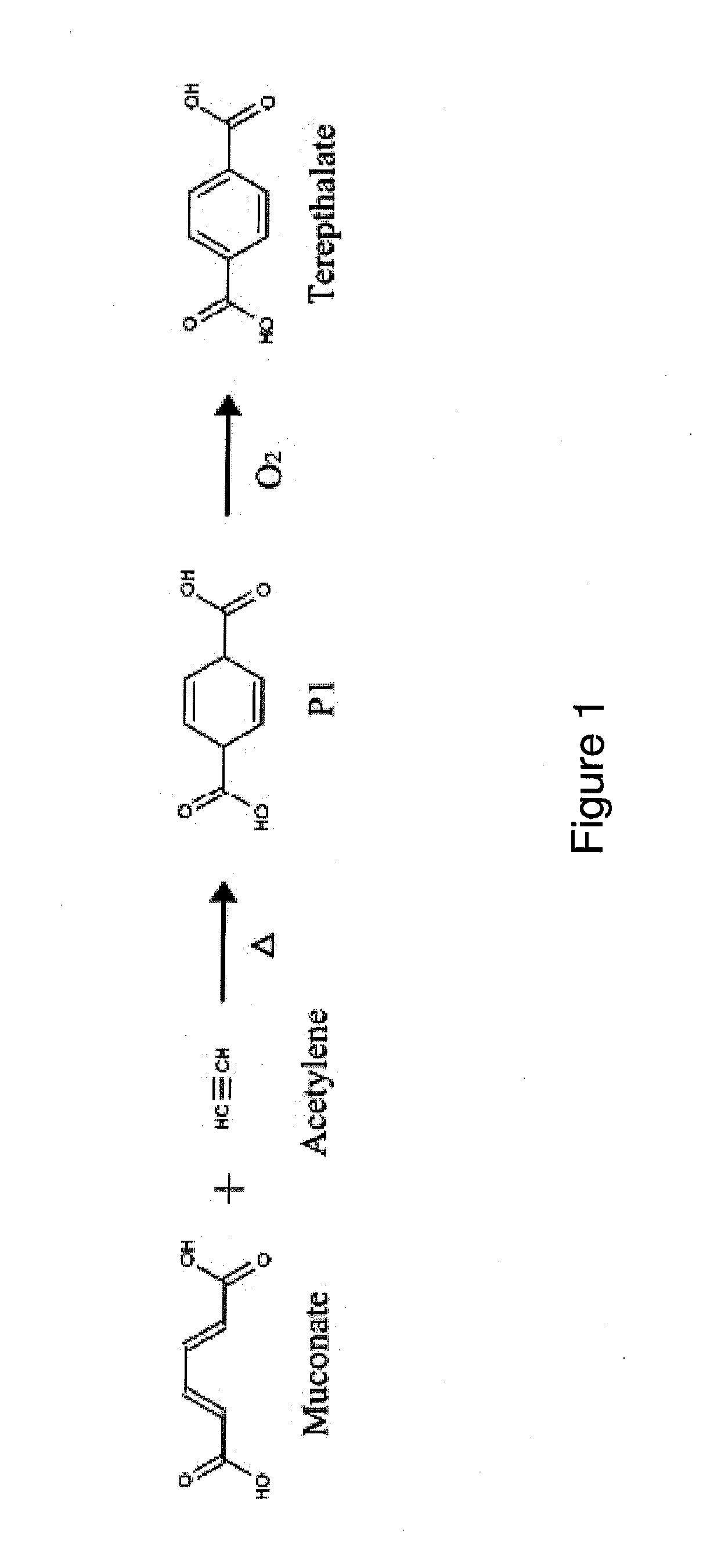
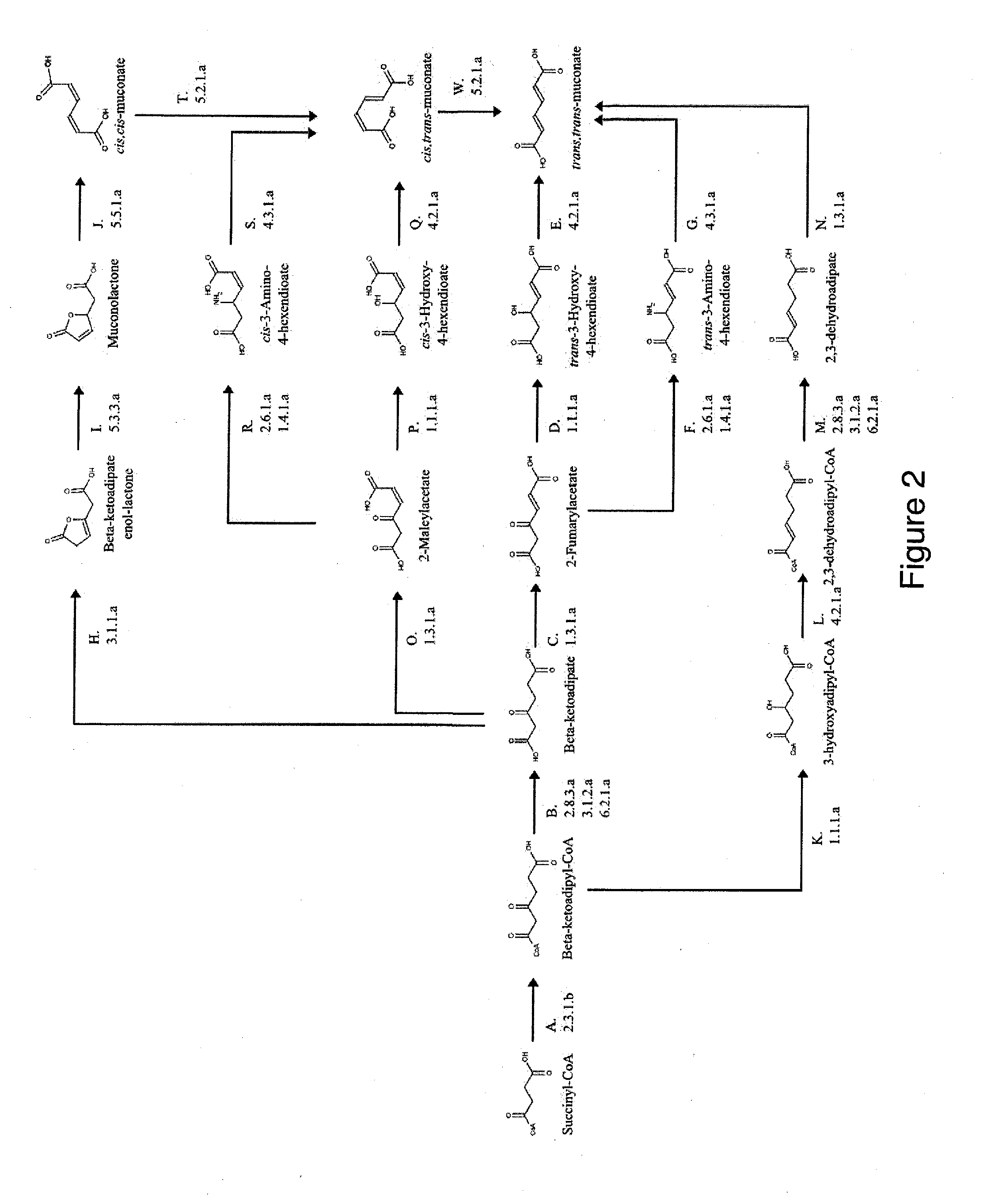
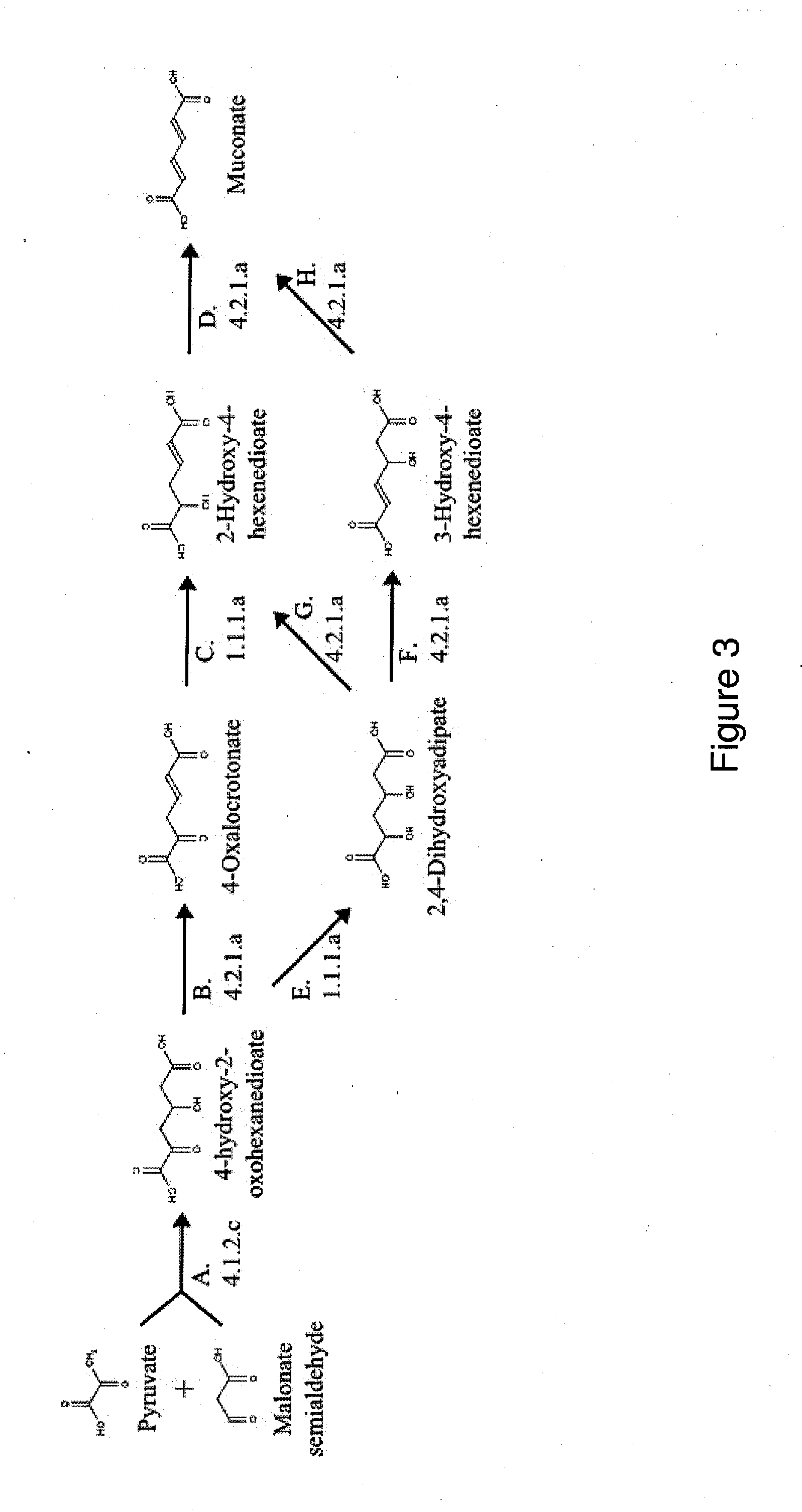
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
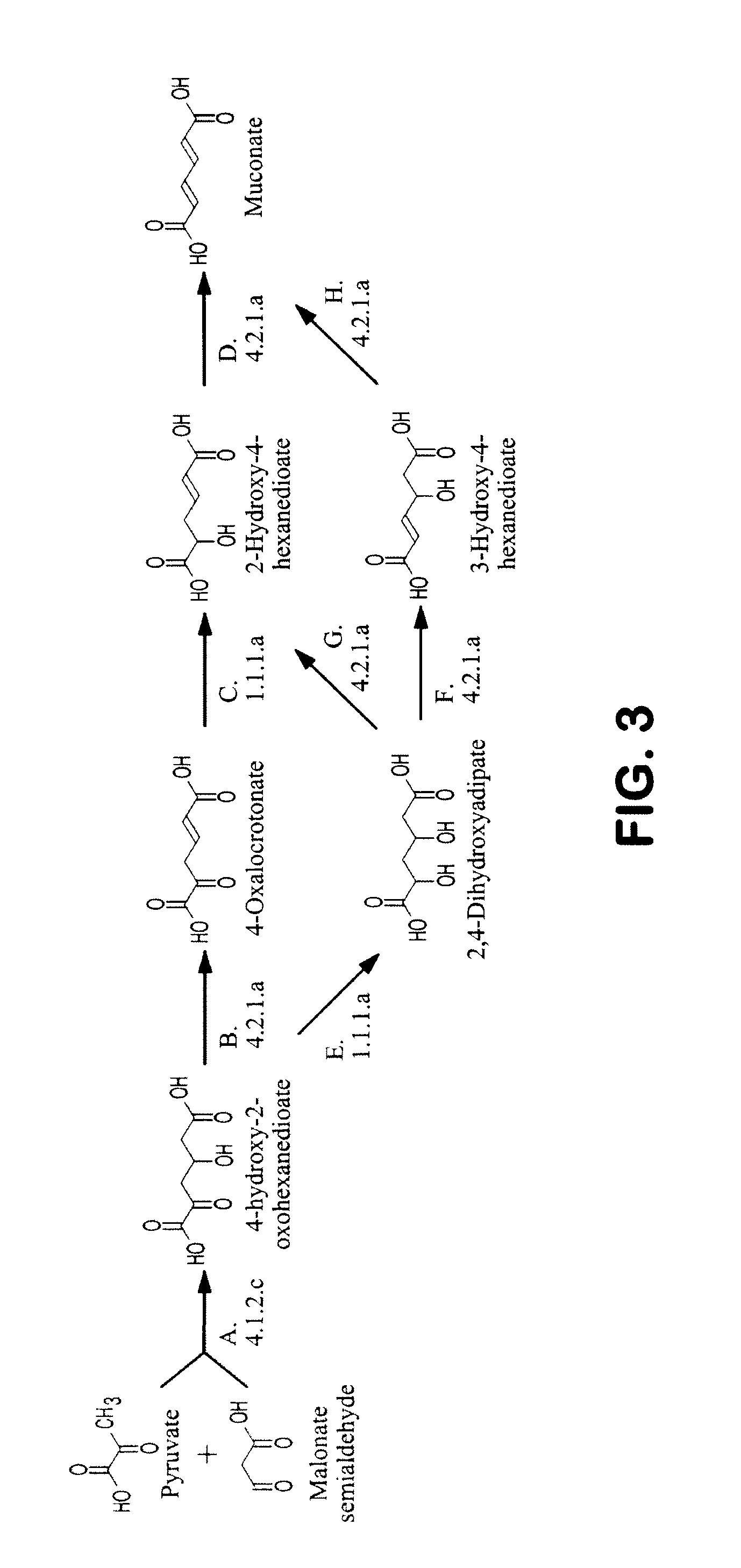
The invention provides a non-naturally occurring microbial organism having a muconate pathway having at least one exogenous nucleic acid encoding a muconate pathway enzyme expressed in a sufficient amount to produce muconate. The muconate pathway including an enzyme selected from the group consisting of a beta-ketothiolase, a beta-ketoadipyl-CoA hydrolase, a beta-ketoadipyl-CoA transferase, a beta-ketoadipyl-CoA ligase, a 2-fumarylacetate reductase, a 2-fumarylacetate dehydrogenase, a trans-3-hydroxy-4-hexendioate dehydratase, a 2-fumarylacetate aminotransferase, a 2-fumarylacetate aminating oxidoreductase, a trans-3-amino-4-hexenoate deaminase, a beta-ketoadipate enol-lactone hydrolase, a muconolactone isomerase, a muconate cycloisomerase, a beta-ketoadipyl-CoA dehydrogenase, a 3-hydroxyadipyl-CoA dehydratase, a 2,3-dehydroadipyl-CoA transferase, a 2,3-dehydroadipyl-CoA hydrolase, a 2,3-dehydroadipyl-CoA ligase, a muconate reductase, a 2-maleylacetate reductase, a 2-maleylacetate dehydrogenase, a cis-3-hydroxy-4-hexendioate dehydratase, a 2-maleylacetate aminoatransferase, a 2-maleylacetate aminating oxidoreductase, a cis-3-amino-4-hexendioate deaminase, and a muconate cis / trans isomerase. Other muconate pathway enzymes also are provided. Additionally provided are methods of producing muconate.
Owner:GENOMATICA INC
Organisms for the production of cyclohexanone
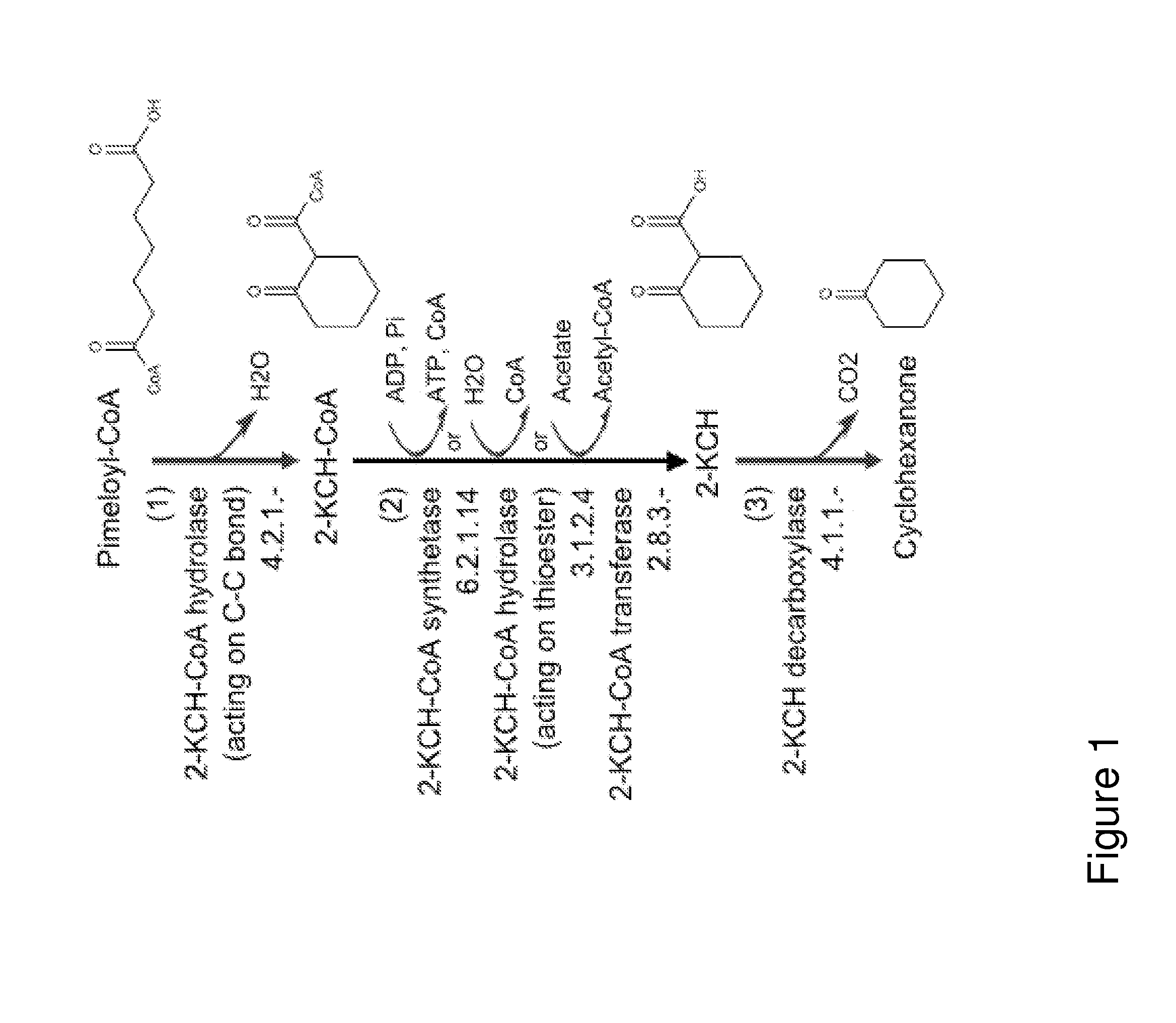
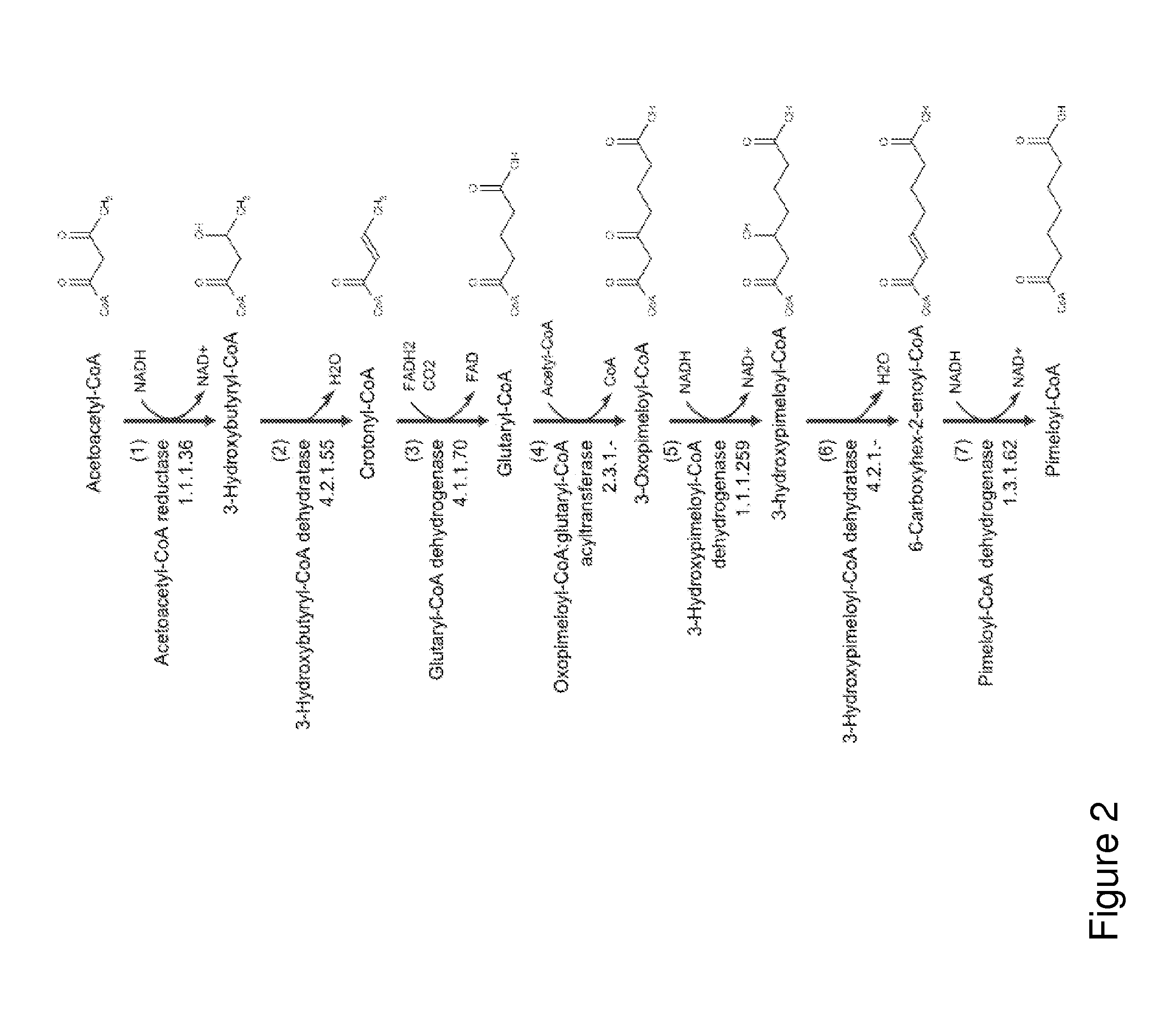
A non-naturally occurring microbial organism has cyclohexanone pathways that include at least one exogenous nucleic acid encoding a cyclohexanone pathway enzyme. A pathway includes a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on C—C bond), a 2-ketocyclohexane-1-carboxylate decarboxylase and an enzyme selected from a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxyl-CoA transferase, and a 2-ketocyclohexane-1-carboxyl-CoA synthetase. A pathway includes an enzyme selected from a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on C—C bond), a 6-ketocyclohex-1-ene-1-carboxyl-CoA synthetase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on thioester), a 6-ketocyclohex-1-ene-1-carboxyl-CoA transferase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA reductase, a 6-ketocyclohex-1-ene-1-carboxylate decarboxylase, a 6-ketocyclohex-1-ene-1-carboxylate reductase, a 2-ketocyclohexane-1-carboxyl-CoA synthetase, a 2-ketocyclohexane-1-carboxyl-CoA transferase, a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxylate decarboxylase, and a cyclohexanone dehydrogenase. A pathway includes an adipate semialdehyde dehydratase, a cyclohexane-1,2-diol dehydrogenase, and a cyclohexane-1,2-diol dehydratase. A pathway includes a 3-oxopimelate decarboxylase, a 4-acetylbutyrate dehydratase, a 3-hydroxycyclohexanone dehydrogenase, a 2-cyclohexenone hydratase, a cyclohexanone dehydrogenase and an enzyme selected from a 3-oxopimeloyl-CoA synthetase, a 3-oxopimeloyl-CoA hydrolase (acting on thioester), and a 3-oxopimeloyl-coA transferase. Each these pathways can include a PEP carboxykinase. A method for producing cyclohexanone includes culturing these non-naturally occurring microbial organisms.
Owner:GENOMATICA INC
Fermentive production of four carbon alcohols
Methods for the fermentive production of four carbon alcohols are provided. Specifically, butanol, preferably 2-butanol is produced by the fermentive growth of a recombinant bacteria expressing a 2-butanol biosynthetic pathway. The recombinant microorganisms and methods of the invention can also be adapted to produce 2-butanone, an intermediate in the 2-butanol biosynthetic pathways disclosed herein. Specifically disclosed herein are the use of coenzyme B12-independent butanediol dehydratases that catalyzes the substrate to product conversion of 2,3-butanediol to 2-butanone in the process of producing 2-butanol and 2-butanone.
Owner:GEVO INC
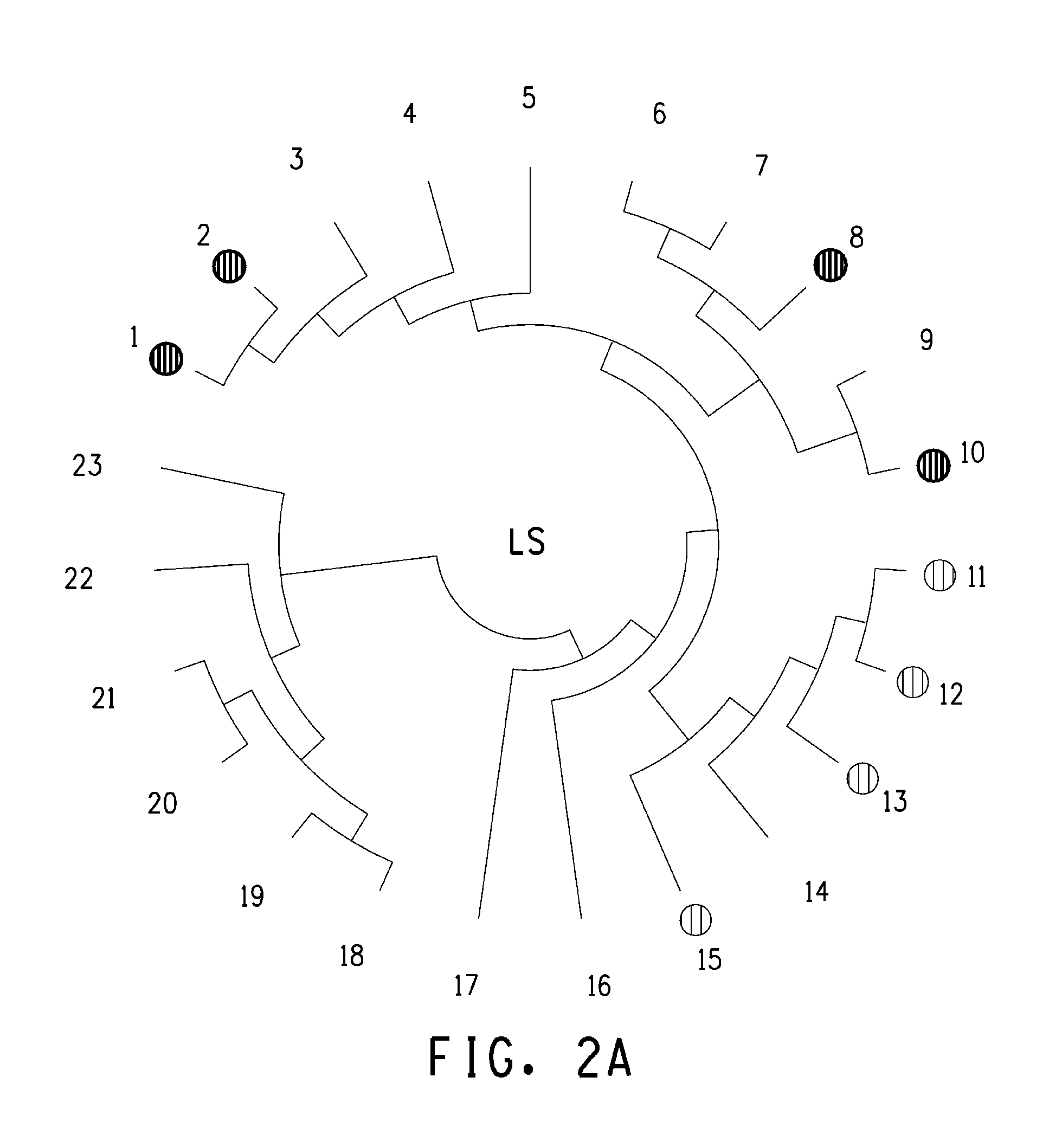
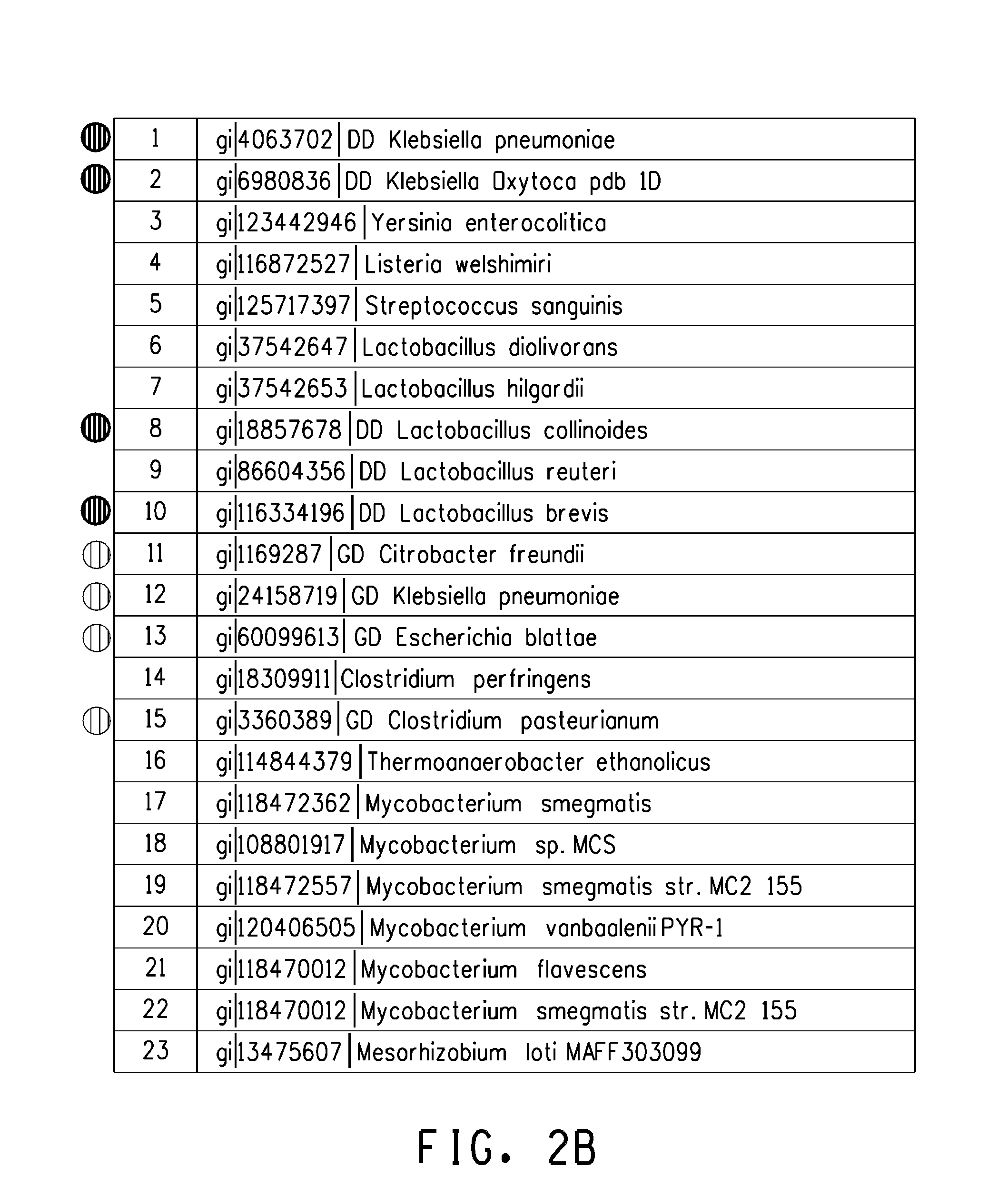
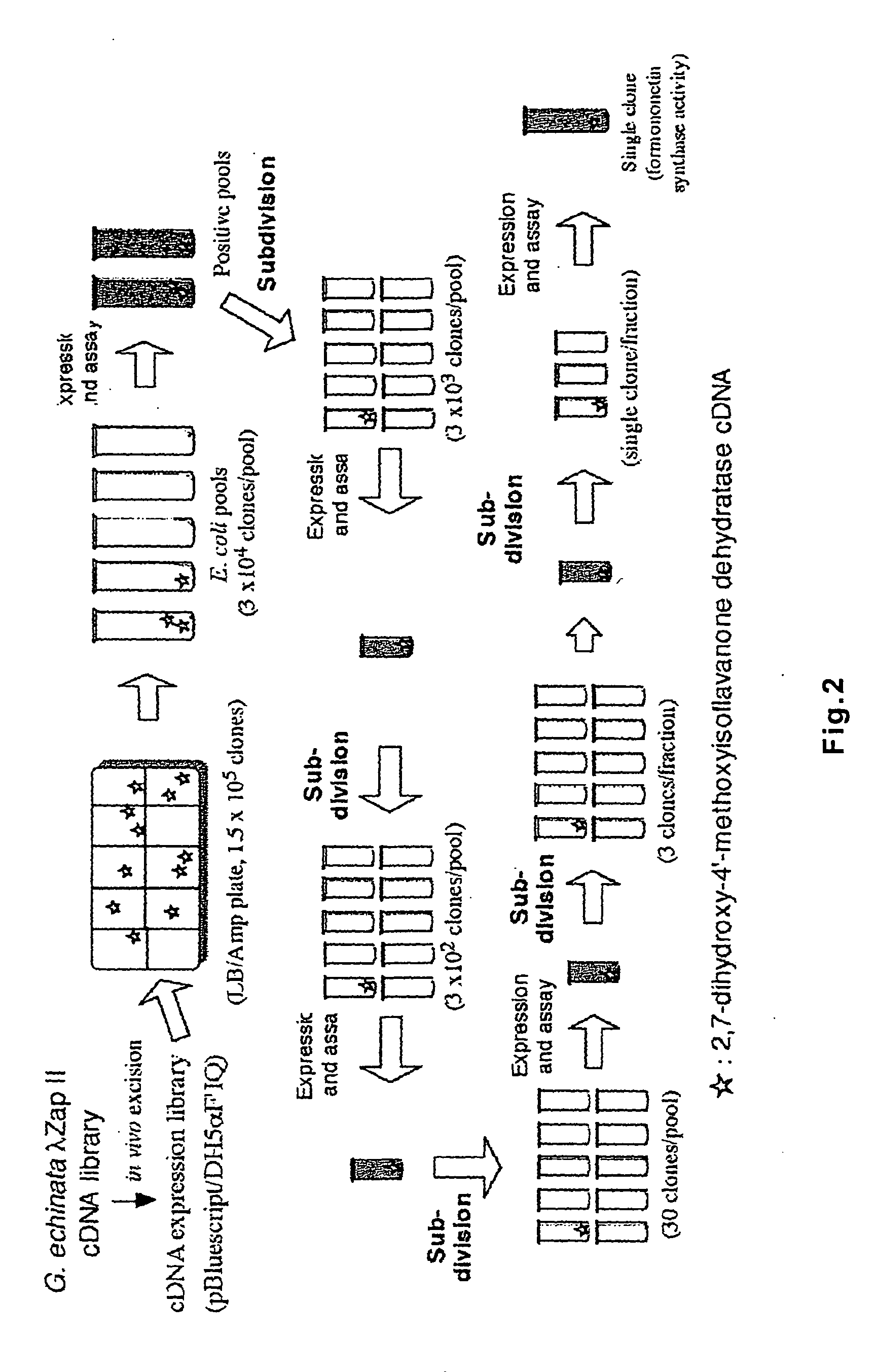
Polynucleotide encoding 2-hydorxyisoflavanone dehydratase and application of the same
2-Hydroxyisoflavanone dehydratase substantially having the amino acid sequence represented by SEQ ID NO: 1 is isolated from licorice. Further, a polynucleotide encoding 2-hydroxyisoflavanone dehydratase of the SEQ ID NO: 2 is obtained. Furthermore, the amino acid sequence of 2-hydroxyisoflavanone dehydratase is identified from soybean and a polynucleotide encoding 2-hydroxyisoflavanone dehydratase is obtained.
Owner:NIHON UNIVERSITY
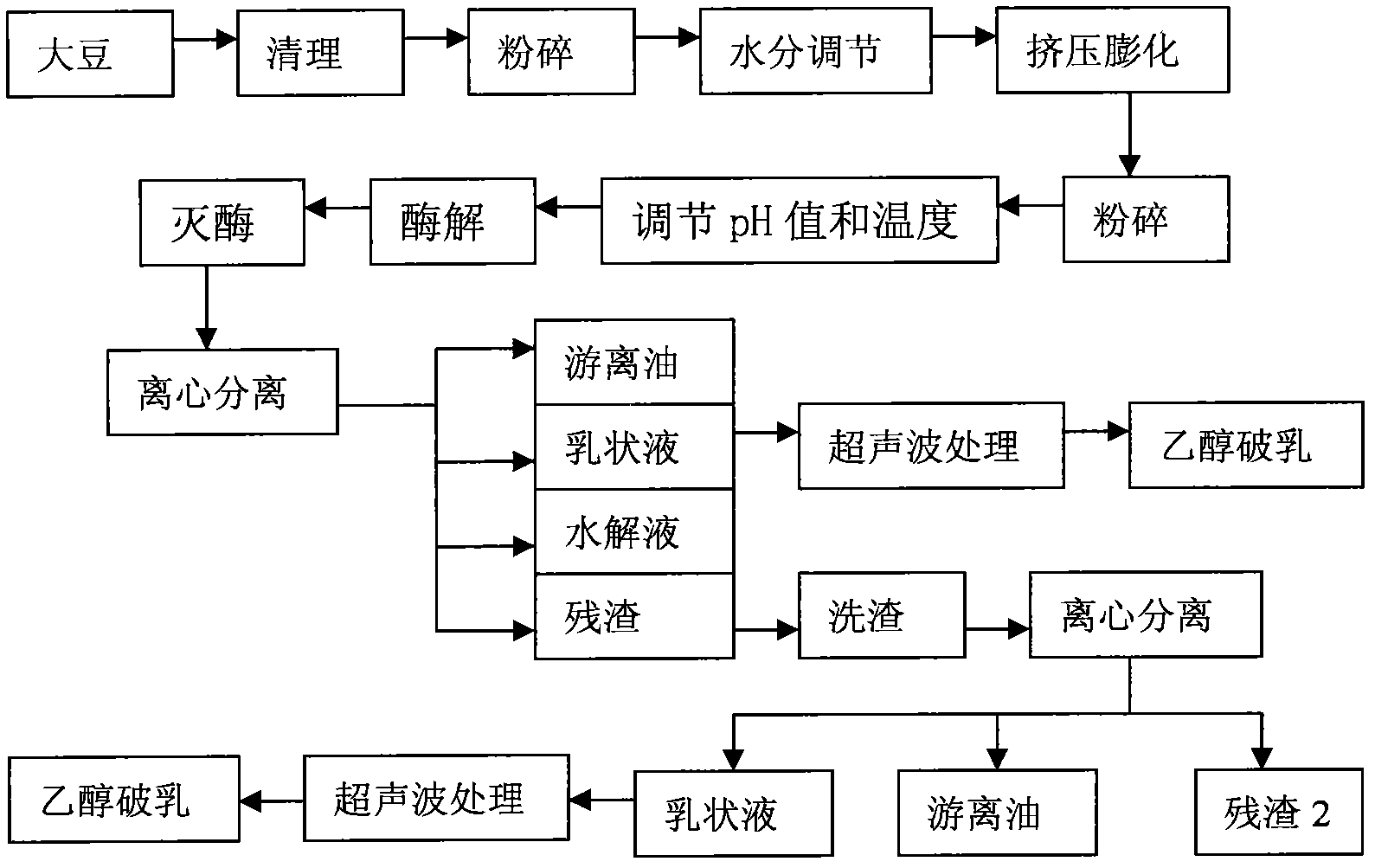
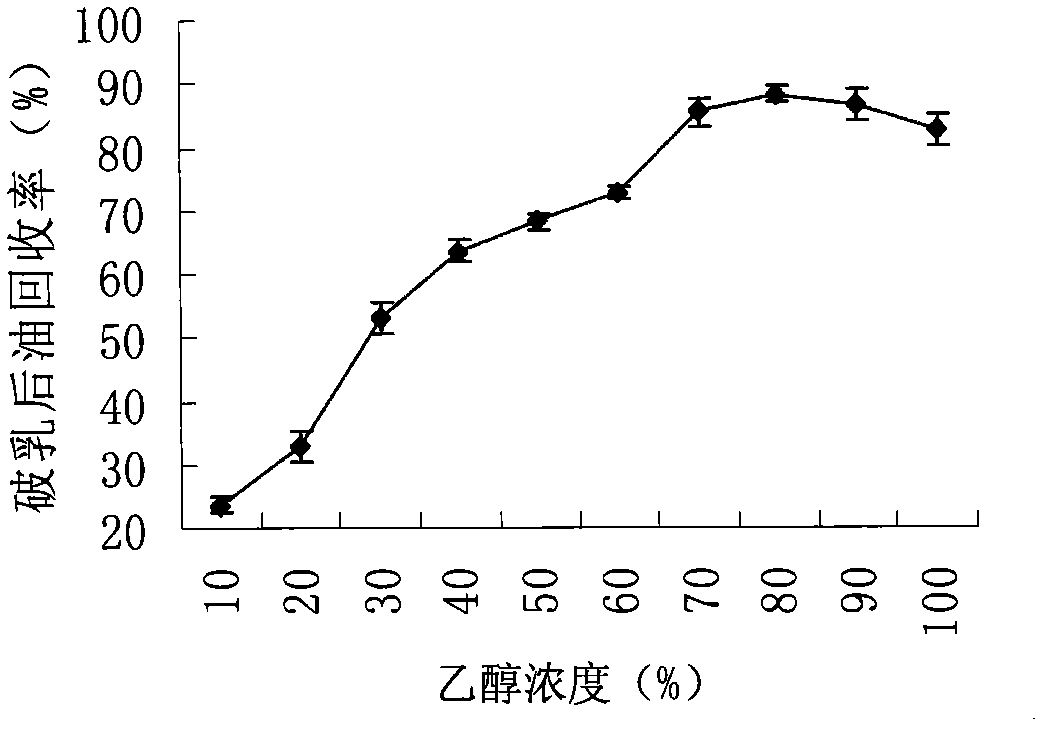
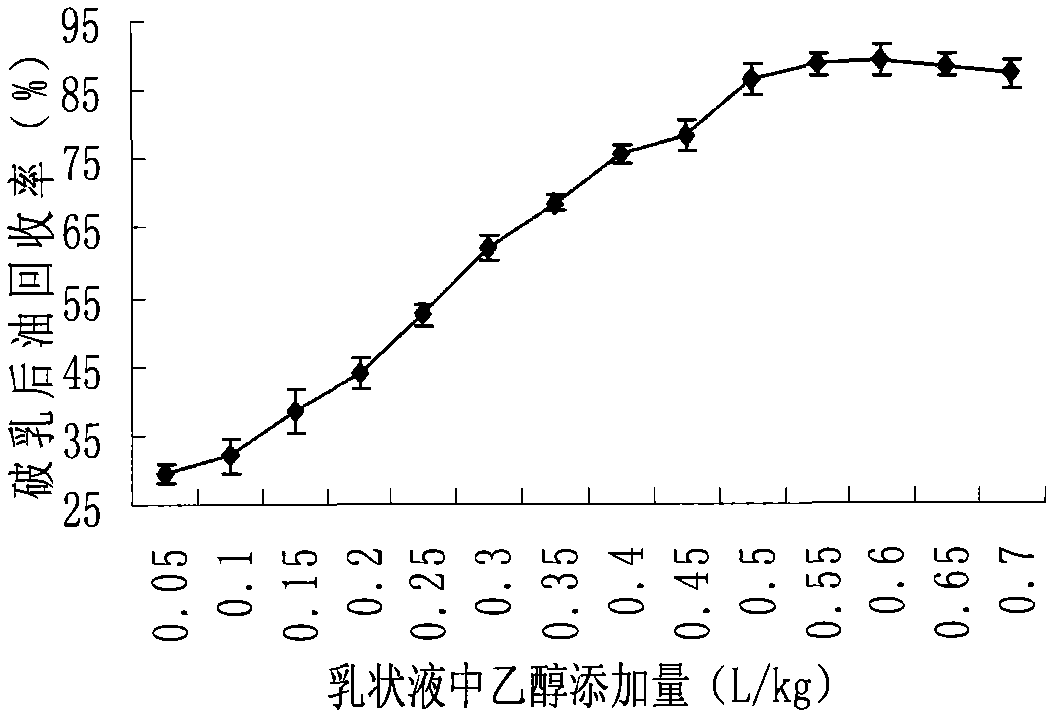
Method for extracting soybean grease from soybean emulsion
InactiveCN101974364AImprove qualityQuality improvementFatty-oils/fats productionNutritive valuesUltrasonic assisted
The invention discloses a method for extracting soybean grease from a soybean emulsion, which comprises the following steps of (1) carrying out the demulsification preprocessing of the soybean emulsion by utilizing ultrasonic processing parameters, centrifuging and collecting the supernatant; (2) adding ethanol to the supernatant for demulsification processing, centrifuging and collecting the supernatant to obtain the soybean grease. The method for assisting ethanol demulsification by utilizing ultrasonic can demulsificate the emulsion formed in the extraction course of a soybean dehydratase method so as to obtain high-quality soybean grease. The method has the advantages of simple required equipment, safe operation, no solvent residual of soybean oil, high-quality and high-nutritive-value grease obtainment. The recovery rate after demulsification under the demulsification technological conditions can reach about 98 to 100% through verification and comparison tests.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY +1
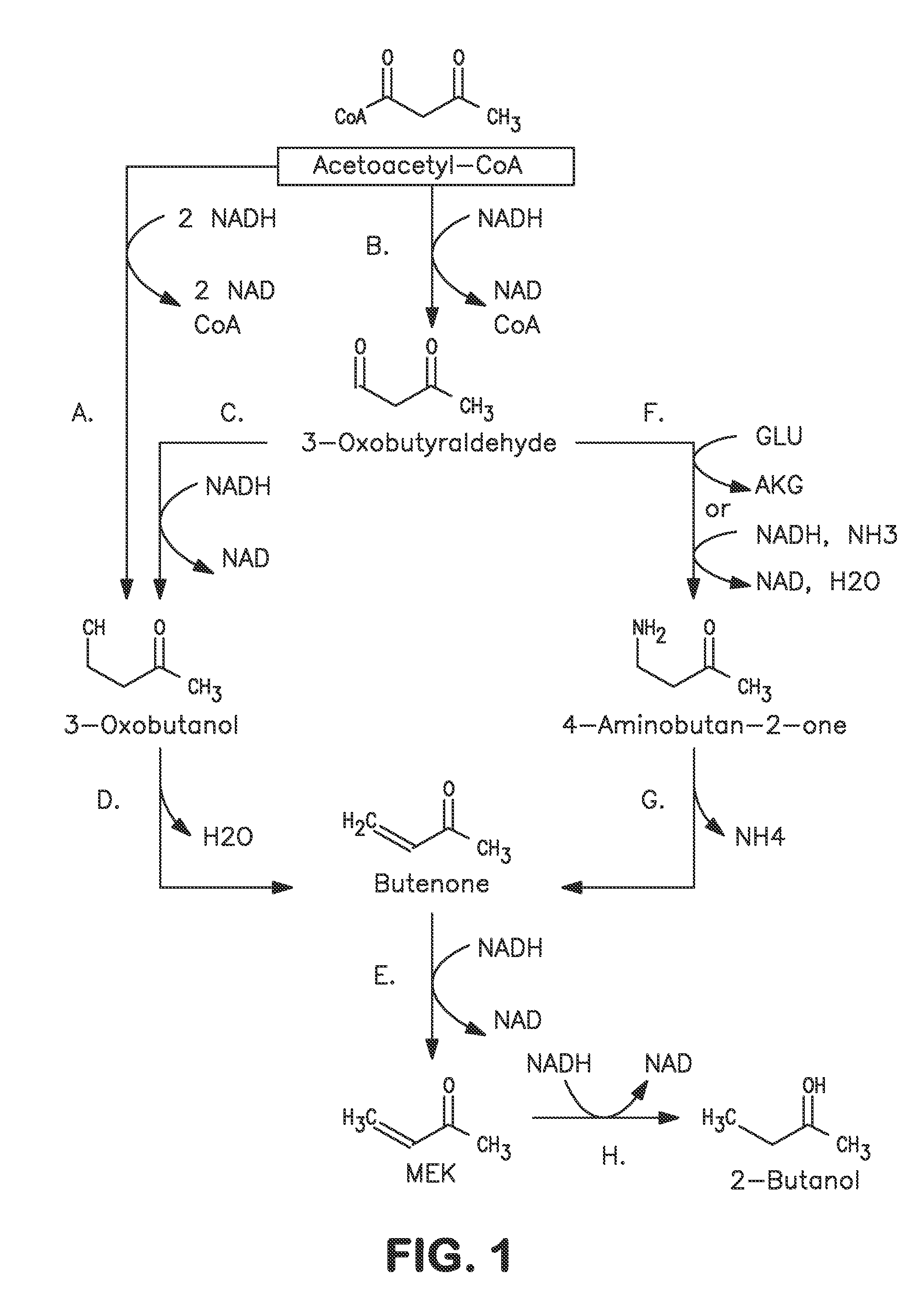
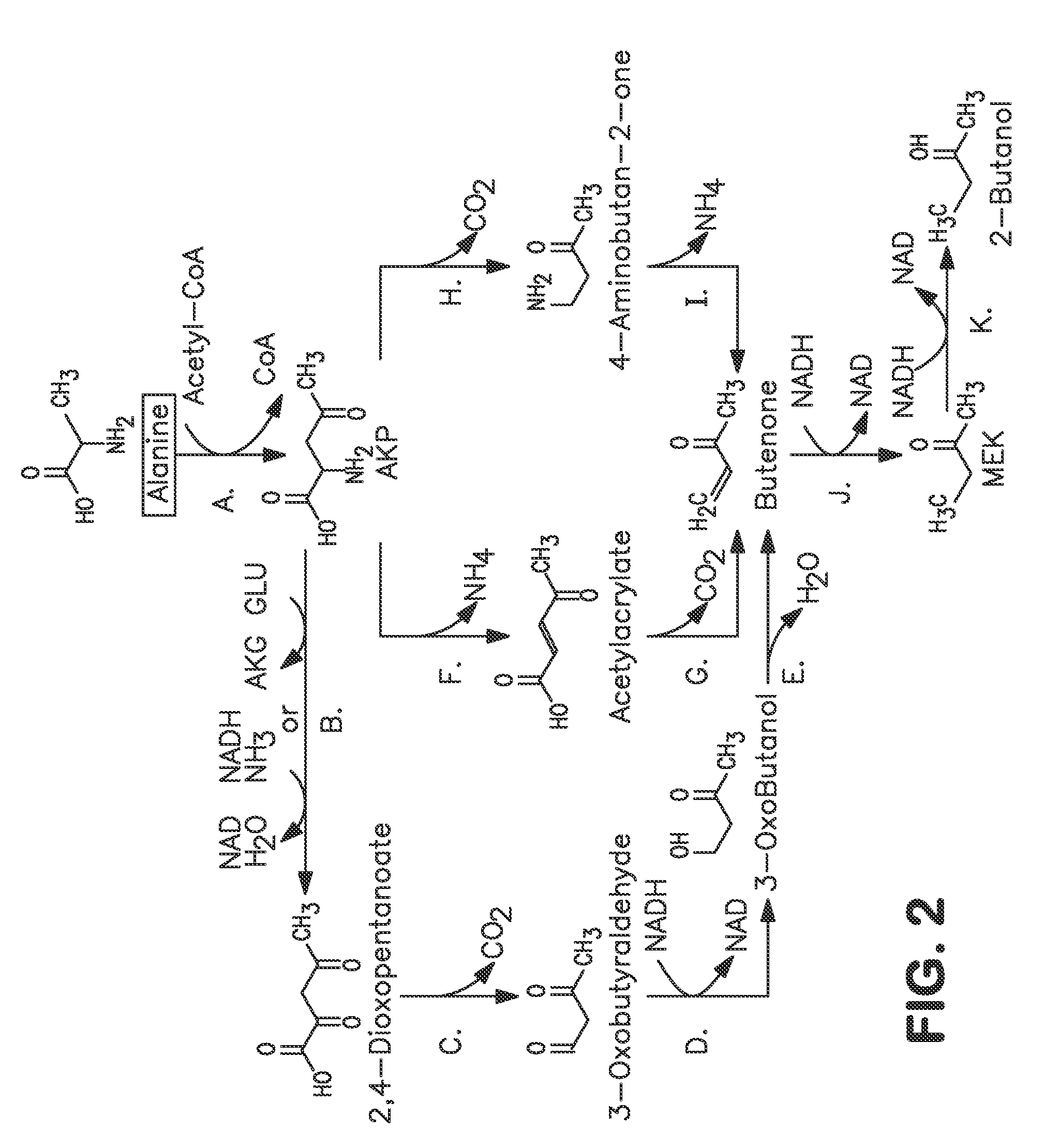
Microorganisms and methods for carbon-efficient biosynthesis of MEK and 2-butanol
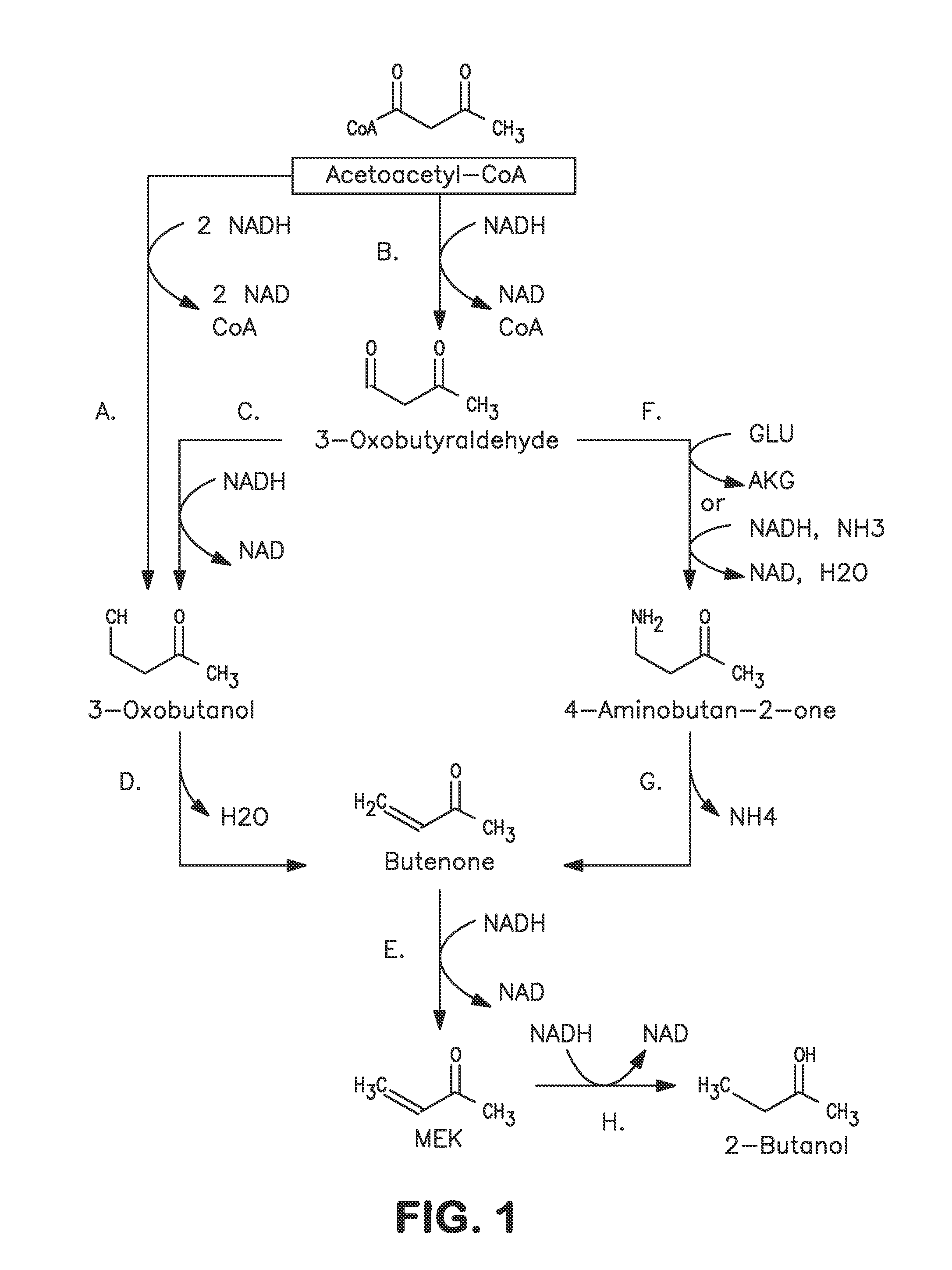
A non-naturally occurring microbial organism has at least one exogenous nucleic acid encoding a MEK pathway enzyme expressed in a sufficient amount to produce MEK. The MEK pathway includes an enzyme selected from an acetoacetyl-CoA dehydrogenase (bifunctional), an acetoacetyl-CoA aldehyde dehydrogenase, a 3-oxobutyraldehyde reductase, a 3-oxobutanol dehydratase, an MEK oxidoreductase, a 3-oxobutyraldehyde aminotransferase, a 4-aminobutan-2-one deaminase, a 2-amino-4-ketopentanoate (AKP) thiolase, an AKP aminotransferase, a 2,4-dioxopentanoate decarboxylase, an AKP deaminase, an acetylacrylate decarboxylase, an AKP decarboxylase, a glutamate dehydrogenase, a 3-oxobutyraldehyde oxidoreductase (aminating) and an AKP oxidoreductase (aminating). A 2-butanol pathway further includes an MEK reductase. A method for producing MEK or 2-butanol includes culturing these organisms under conditions and for a sufficient period of time to produce MEK or 2-butanol.
Owner:GENOMATICA INC
Processes for the bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism
A process is provided for the bioconversion of a carbon substrate to 1,3-propanediol by a single organism utilizing microorganisms, such as, Citrobacter, Enterobacter, Clostridium, Klebsiella, Aerobacter, Lactobacillus, Aspergillus, Saccharomyces, Zygosaccharomyces, Pichia, Kluyveromyces, Candida, Hansenula, Debaryomyces, Mucor, Torulopsis, Methylobacter, Escherichia, Salmonella, Bacillus, Streptomyces and Pseudomonas, containing the genes encoding for an active glycerol or diol dehydratase enzyme by contacting these organisms with a carbon substrate under the appropriate fermentation conditions. Specifically, Citrobacter and, Klebsiella provide the source of exogenous genes for such active dehydratase enzyme.
Owner:EI DU PONT DE NEMOURS & CO +1
Method for producing 1,3-propylene glycol through fermentation via recombinant microbes
PendingCN106906248AAddressing Biosecurity IssuesLow costMicroorganism based processesFermentationPhosphoric acidBacterial strain
The invention provides a method for producing 1,3-propylene glycol through fermentation via recombinant microbes. According to the invention, an endogenous dihydroxypropanone phosphatein phosphatase gene hdpA is over-expressed in Corynebacterium glutamicum to reinforce removal of phosphoric acid from dihydroxypropanone phosphatein so as to produce dihydroxypropanone; exogenous glycerol dehydrogenase is introduced to convert dihydroxypropanone into glycerin; and glycerin finally produces 1,3-propylene glycol under the action of exogenous glycerol dehydratase and an activator thereof and alcohol dehydrogenase. Corynebacterium glutamicum can use different cheap raw materials for fermentation, and cheap corn steep liquor can be used as a nutritional component to replace expensive yeast powder, so cost for raw materials is further reduced, and the problems in biosecurity and tolerance of the bacterial strain to a substrate and a product are overcome; and thalli obtained in the process of fermentation can be used as a product for a feed additive. The method provided by the invention produces few by-products and can further simplify the separating process of 1,3-propylene glycol.
Owner:GUANGDONG TSINGDA SMART BIOTECH CO LTD
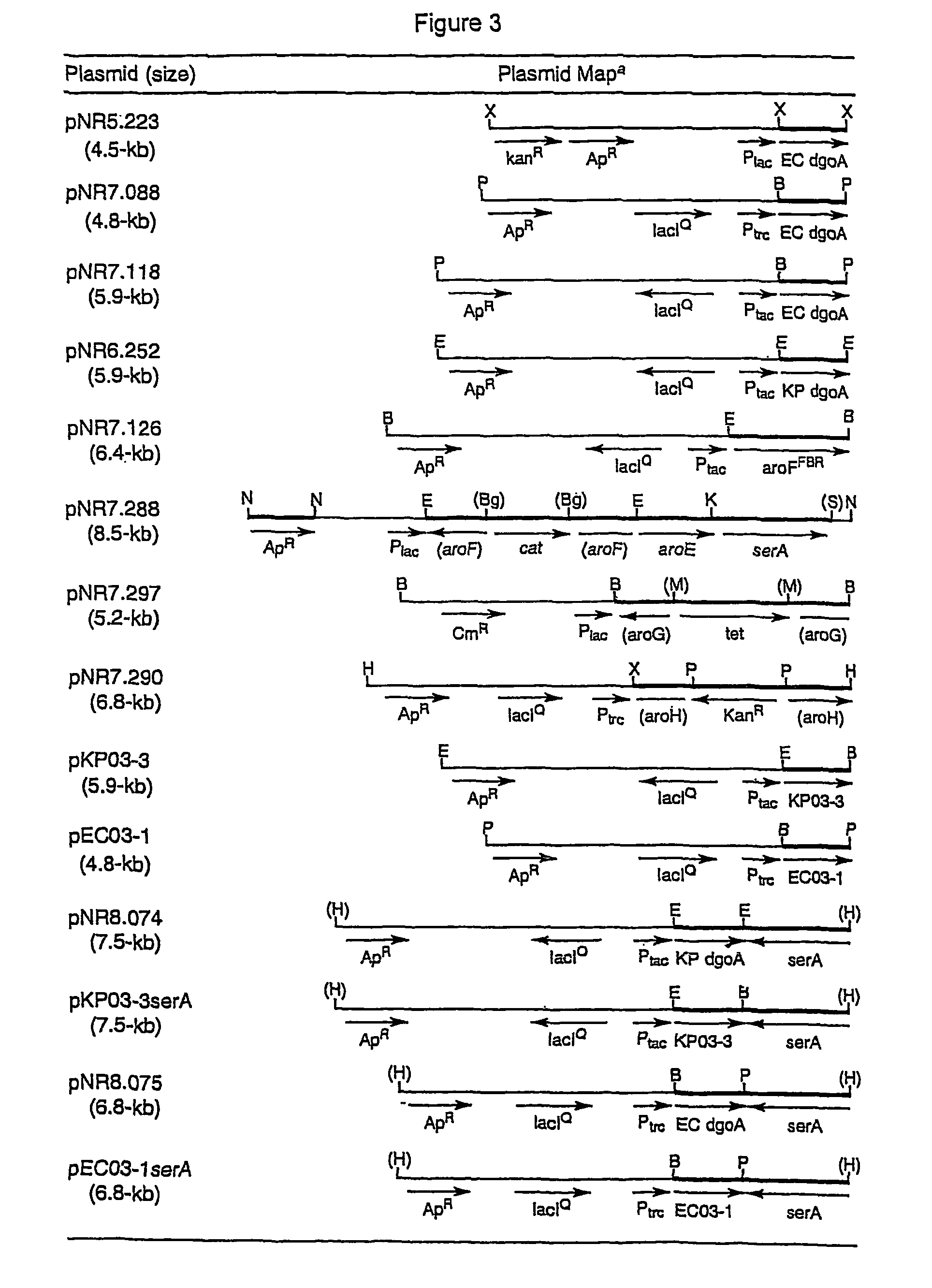
Methods and materials for the production of shikimic acid
InactiveUS7790431B2BacteriaSugar derivatives3-dehydroquinate synthase3-deoxy-D-arabino-heptulosonate-7-phosphate
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Microorganisms and methods for carbon-efficient biosynthesis of mek and 2-butanol
A non-naturally occurring microbial organism has at least one exogenous nucleic acid encoding a MEK pathway enzyme expressed in a sufficient amount to produce MEK. The MEK pathway includes an enzyme selected from an acetoacetyl-CoA dehydrogenase (bifunctional), an acetoacetyl-CoA aldehyde dehydrogenase, a 3-oxobutyraldehyde reductase, a 3-oxobutanol dehydratase, an MEK oxidoreductase, a 3-oxobutyraldehyde aminotransferase, a 4-aminobutan-2-one deaminase, a 2-amino-4-ketopentanoate (AKP) thiolase, an AKP aminotransferase, a 2,4-dioxopentanoate decarboxylase, an AKP deaminase, an acetylacrylate decarboxylase, an AKP decarboxylase, a glutamate dehydrogenase, a 3-oxobutyraldehyde oxidoreductase (aminating) and an AKP oxidoreductase (aminating). A 2-butanol pathway further includes an MEK reductase. A method for producing MEK or 2-butanol includes culturing these organisms under conditions and for a sufficient period of time to produce MEK or 2-butanol.
Owner:GENOMATICA INC
Saccharomyces cerevisiae and application thereof in food fermentation
InactiveCN107937295AHigh-yield β-phenylethanol characteristicsGood alcoholic propertiesFungiAlcoholic beverage preparationBiotechnologyAlcohol ethyl
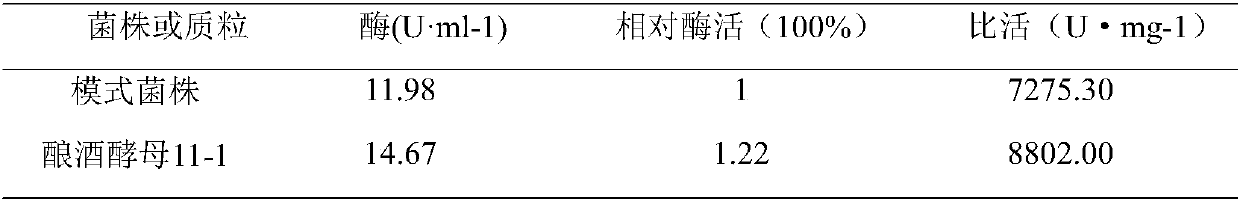
Belonging to the technical field of industrial microbiology, the invention discloses a saccharomyces cerevisiae and application thereof in food fermentation. The saccharomyces cerevisiae strain 11-1 is preserved in China Center for Type Culture Collection on September 11, 2017 at Wuhan University, Wuhan, China, and the preservation number is CCTCC NO:M 2017488. Sense mutation occurs to the key gene pha2 in the path of synthesizing beta-phenylethyl alcohol from the strain, and the catalytic ability of mutated prephenate dehydratase PHA2P is strengthened by 22%. The invention also finds that thesaccharomyces cerevisiae pha2p suffers from the feedback inhibition of L-phenylalanine, when the strain is used for fermenting yellow rice wine, the alcoholic strength can reach 13 degrees, and the yield of beta-phenylethyl alcohol can reach 120mg / L.
Owner:JIANGNAN UNIV
Recombinant bacteria using xylose to produce glycollic acid and building method and application of recombinant bacteria
The invention discloses recombinant bacteria using xylose to produce glycollic acid and a building method and application of the recombinant bacteria and belongs to the technical field of genetic engineering. The xylose dehydrogenase gene, xylonic acid lactonase gene, xylonic acid dehydratase gene, 3-deoxygenation-D-glycerin ketopentose acid aldolase gene and glycolic aldehyde dehydrogenase gene in the recombinant bacteria using the xylose to produce the glycollic acid are overexpressed. Meanwhile, the invention further provides a preparation method of the recombinant bacteria and a method using the recombinant bacteria to produce the glycollic acid. By the recombinant bacteria, the biological synthesizing path using D-xylose as the carbon source to form the glycollic acid through glycolic aldehyde conversion is achieved for the first time.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
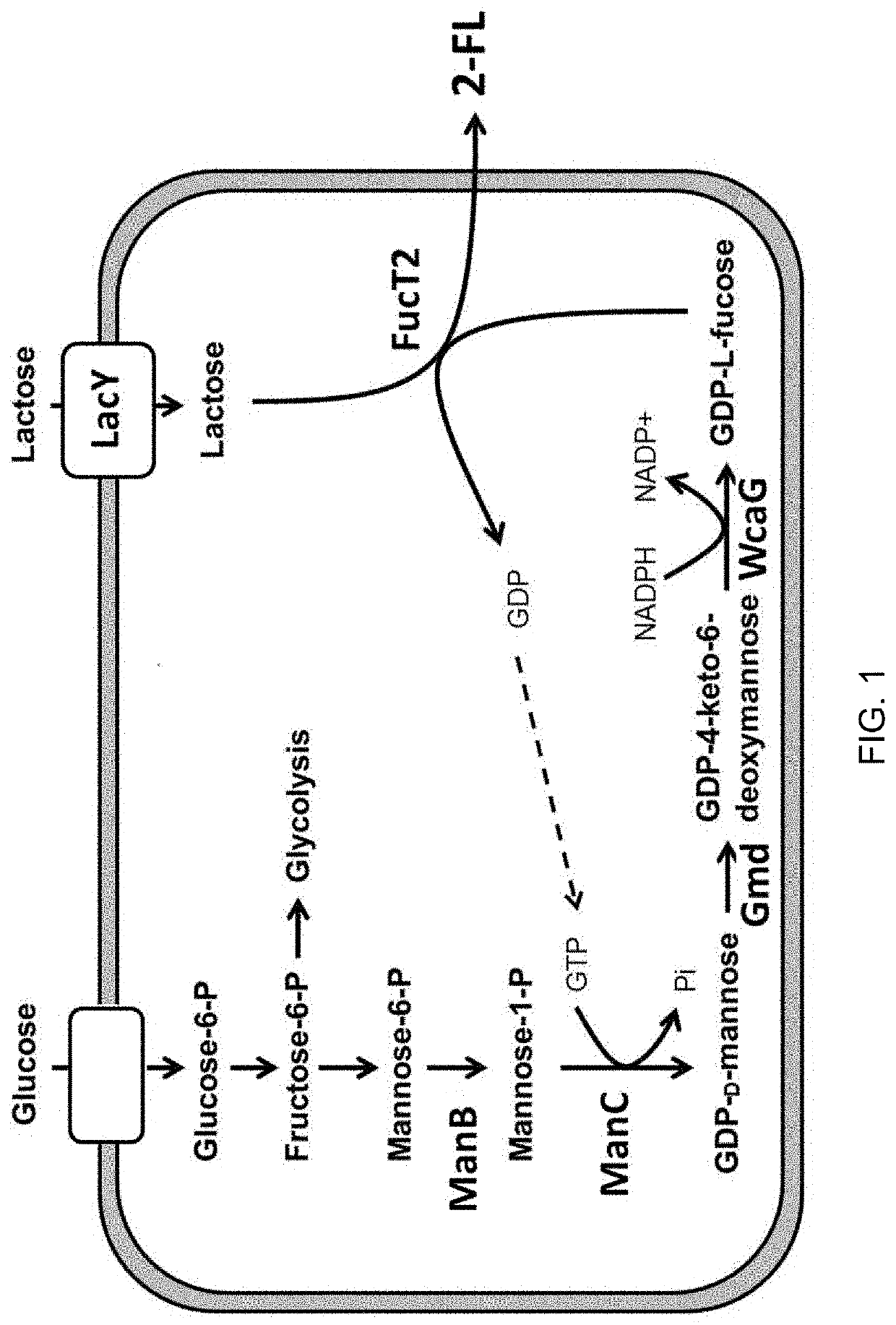
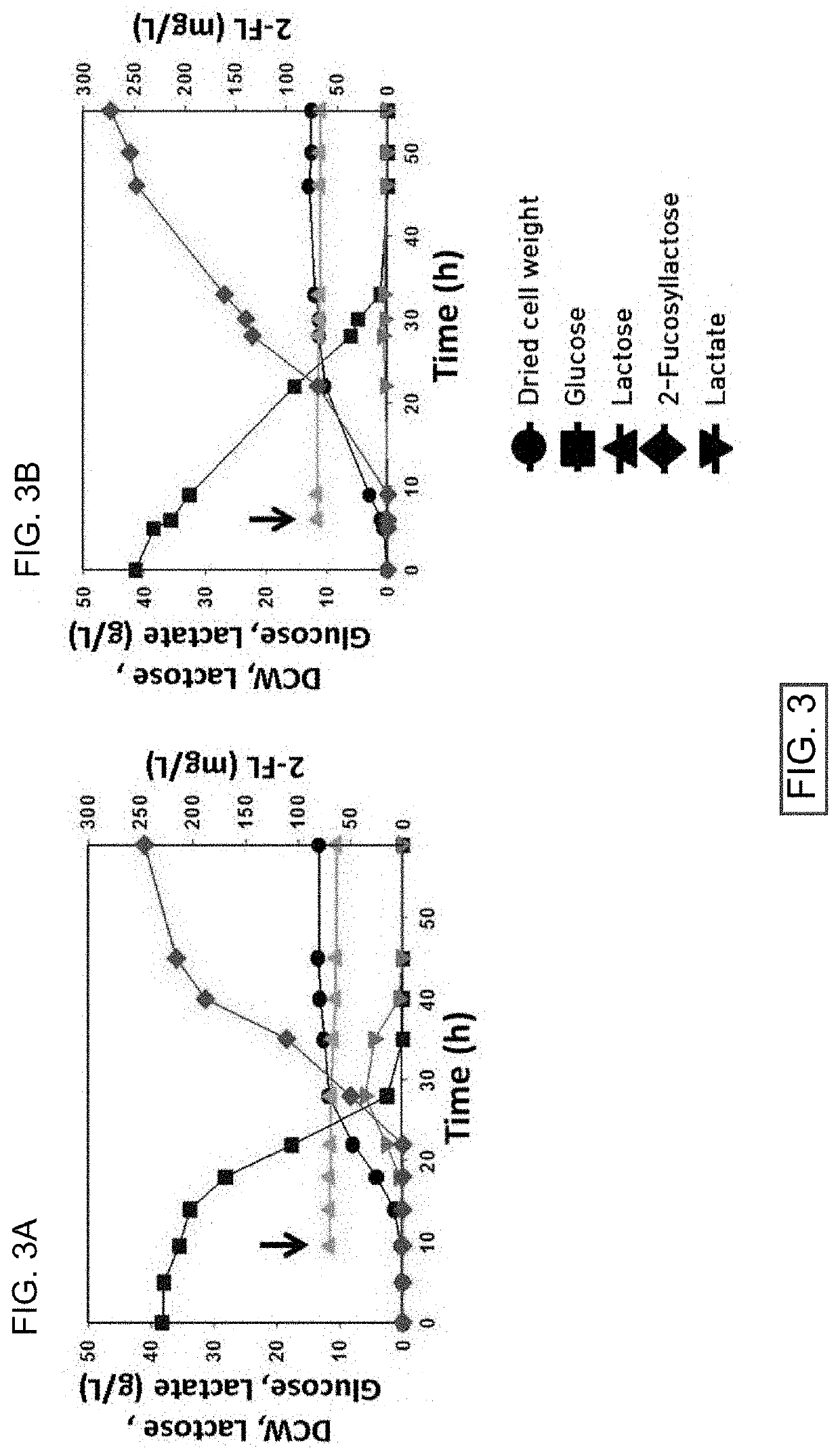
Corynebacterium glutamicum for use in producing 2'-fucosyllactose
ActiveUS20200048640A1Increase concentrationHigh yieldDepsipeptidesOxidoreductasesPhosphomannomutaseFucosyltransferase
Owner:ADVANCED PROTEIN TECH CORP
Plant lactobacillus feed capable of relieving tilapia lead toxicity
InactiveCN105285514AIncrease growth rateReduce contentClimate change adaptationAnimal feeding stuffFood additiveAgricultural science
The invention discloses a plant lactobacillus feed capable of relieving tilapia lead toxicity, and belongs to the technical field of feed. The feed is a finished feed probiotics granulated feed having the viable bacterial number of more than 10<8> cfu / g and prepared by a way of adding a bacterial agent obtained by carrying out centrifuging concentration or freeze drying and microcapsule embedding on CGMCC 5494 strains into an aquatic animal feed pre-mix material in a form of a feed additive, and then granulating or pre-granulating and then spraying probiotics on a carrier to obtain the feed. The feed obtained by the method can increase the growth rate of tilapia, increases the intestinal tract digestive enzyme activity of the lead-exposure tilapia, reduces the lead content in bodies of the lead-exposure tilapia, relieves the oxidative stress damage in the bodies of the lead-exposure tilapia, improves the activity of aminolevtllinic acid dehydratase in the bodies of the lead-exposure tilapia, improves the lysozyme activity and myeloperoxidase activity of the lead-exposure tilapia, relieves the cell and genetic toxicity of the lead-exposure tilapia, and better has application prospects.
Owner:JIANGNAN UNIV
Genetically engineered bacteria and application thereof in production of BT (D-1,2,4-butanetriol)
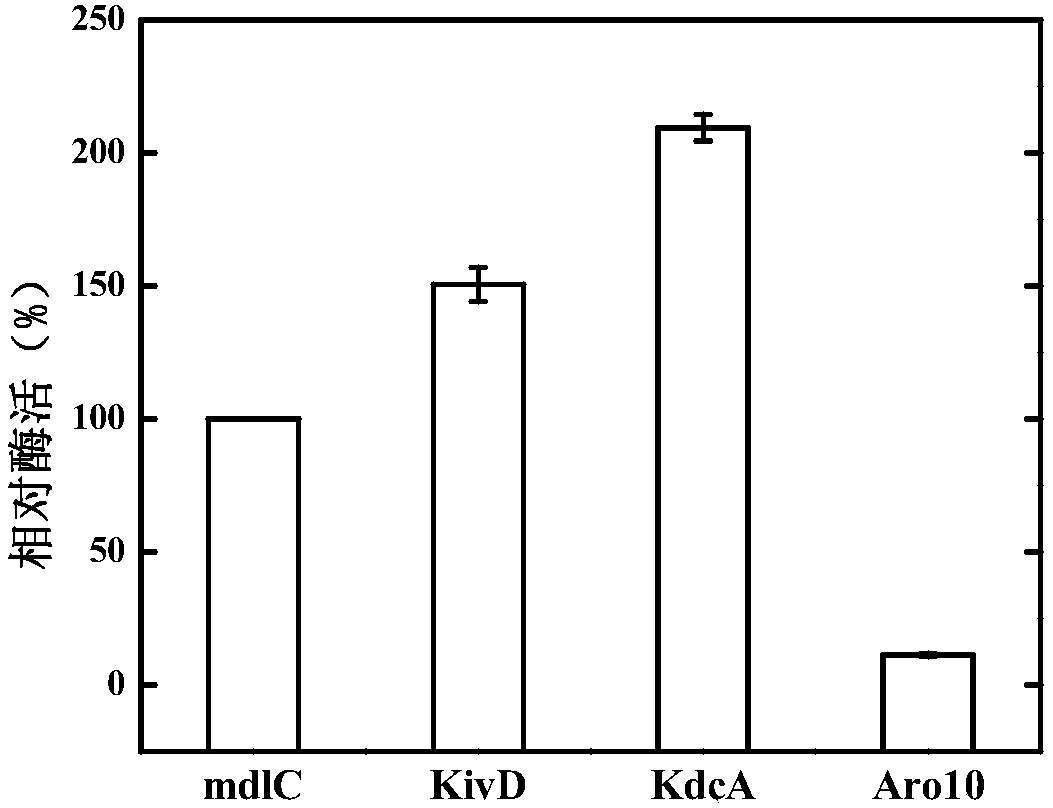
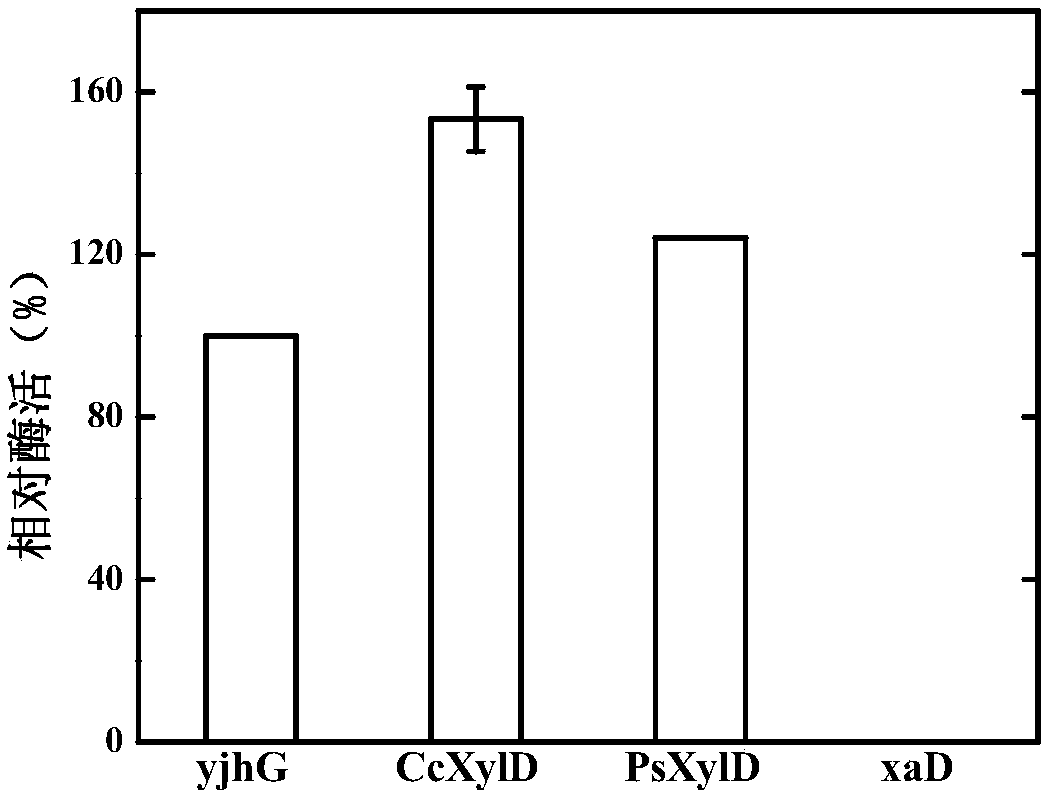
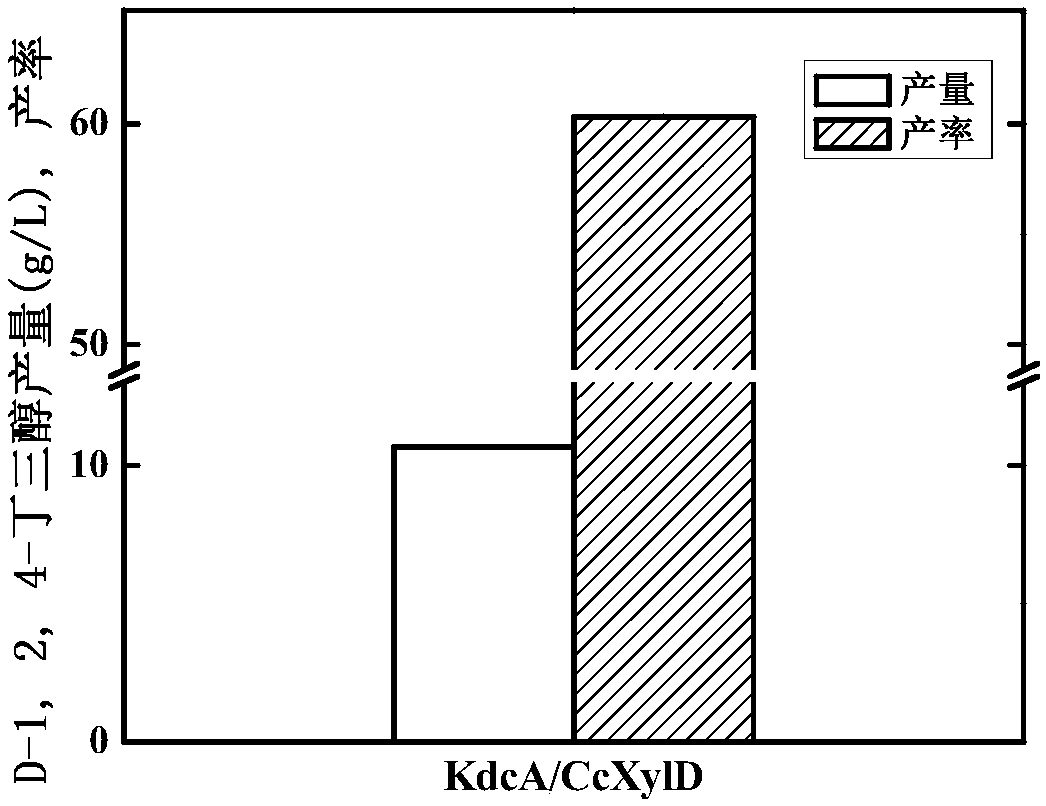
ActiveCN107699536AIncrease productionOptimization pathBacteriaMicroorganism based processesBiotechnologyKetoacid decarboxylase
The invention discloses genetically engineered bacteria and an application thereof in production of BT (D-1,2,4-butanetriol). The genetically engineered bacteria are novel genetically engineered bacteria which are obtained as follows: a 2-keto acid decarboxylase gene MalC, an xylose dehydrogenase gene XylB, an xylonic acid dehydratase gene YjhG and an alcohol dehydrogenase gene YqhD are constructed, cloned and expressed, the genes are transferred into cells of host bacteria BL21(DE3), genetically engineered bacteria BL21-02 are obtained, and new xylonic acid dehydratase is screened on the basis of the genetically engineered bacteria; the genetically engineered bacteria are subjected to fermenting culture for production of BT. The capability of synthesizing BT from D-xylose can be improvedby screening the provided xylonic acid dehydratase gene CcXylD. The optimal xylonic acid dehydratase gene CcXylD and alpha-keto acid decarboxylase gene KdcA from lactococcus lactis are applied to theproduction process of BT, the optimal strain BL21-15 is obtained, and finally, the BT yield can reach 10.66 g / L.
Owner:NANJING UNIV OF TECH
2 '-fucosyllactose high-yield strain and preparation method and application thereof
The invention discloses a 2 '-fucosyllactose high-yield strain as well as a preparation method and application thereof. The preservation number of the strain is CGMCC No.19557. Escherichia coli E.coliBL21 (DE3) is used as a production host, and phosphomannose mutase (manB), mannose-1-phosphate phosphate guanylyltransferase (manC), GDP-mannose-4, 6-dehydratase (gmd), GDP-L-fucose synthase (flc) and alpha-1,2- fucosyltransferase (futC) are overexpressed so as to construct a synthesis path of the 2'-FL; substrate supply is improved by overexpressing sucrose transporter and lactose transporter onthe basis, the production capacity of 2 '-FL is further improved, efficient expression of 2'-FL in escherichia coli is realized, the 2 '-FL high-yield strain is improved.
Owner:江苏华燕集团有限公司
Process for the biological production of 1,3-propanediol with high titer
The present invention provides an improved method for the biological production of 1,3-propanediol from a fermentable carbon source in a single microorganism. In one aspect of the present invention, an improved process for the conversion of glucose to 1,3-propanediol is achieved by the use of an E. coli transformed with the Klebsiella pneumoniae dha regulon genes dhaR, orfY, dhaT, orfX, orfW, dhaB1, dhaB2, dhaB3, and orfZ, all these genes arranged in the same genetic organization as found in wild type Klebsiella pneumoniae. In another aspect of the present invention, an improved process for the production of 1,3-propanediol from glucose using a recombinant E. coli containing genes encoding a G3PDH, a G3P phosphatase, a dehydratase, and a dehydratase reactivation factor compared to an identical process using a recombinant E. coli containing genes encoding a G3PDH, a G3P phosphatase, a dehydratase, a dehydratase reactivation factor and a 1,3-propanediol oxidoreductase (dhaT). The dramatically improved process relies on the presence in E. coli of a gene encoding a non-specific catalytic activity sufficient to convert 3-hydroxypropionaldehyde to 1,3-propanediol.
Owner:DUPONT US HLDG LLC
Genetically engineered bacterium used for producing 3-hydroxypropionic acid (3-HP) by fermentation, constructing method and application of genetically engineered bacterium
PendingCN106479947AIncrease productionStrong industrial application valueBacteriaMicroorganism based processesBiotechnology3-Hydroxypropionic acid
The invention discloses a genetically engineered bacterium used for producing 3-hydroxypropionic acid (3-HP) by fermentation, a constructing method and application of the genetically engineered bacterium. An acetaldehyde dehydrogenase gene in Saccharomyces cerevisiae and a glycerol dehydratase gene in Klebsiella pneumoniae are connected onto a prokaryotic expression vector pET30a(+) so as to construct a Pet30a-ALDH-DHAB double-gene prokaryotic expression vector, and then the constructed Pet30a-ALDH-DHAB double-gene prokaryotic expression vector is transformed in to an escherichia coli BL21(DE3) competent cell to construct the genetically engineered bacterium used for effectively producing the 3-HP by fermentation. The problems of high energy consumption and sever pollution in chemical industrial synthesis of the 3-HP and the problem of low yield rate in microbial fermentation production of the 3-HP are solved. By utilizing the genetically engineered bacterium to produce the 3-HP by fermentation, a yield of the 3-HP can reach to 8.443g / L, and a production process is simple and convenient for industrialized application.
Owner:XUZHOU UNIV OF TECH
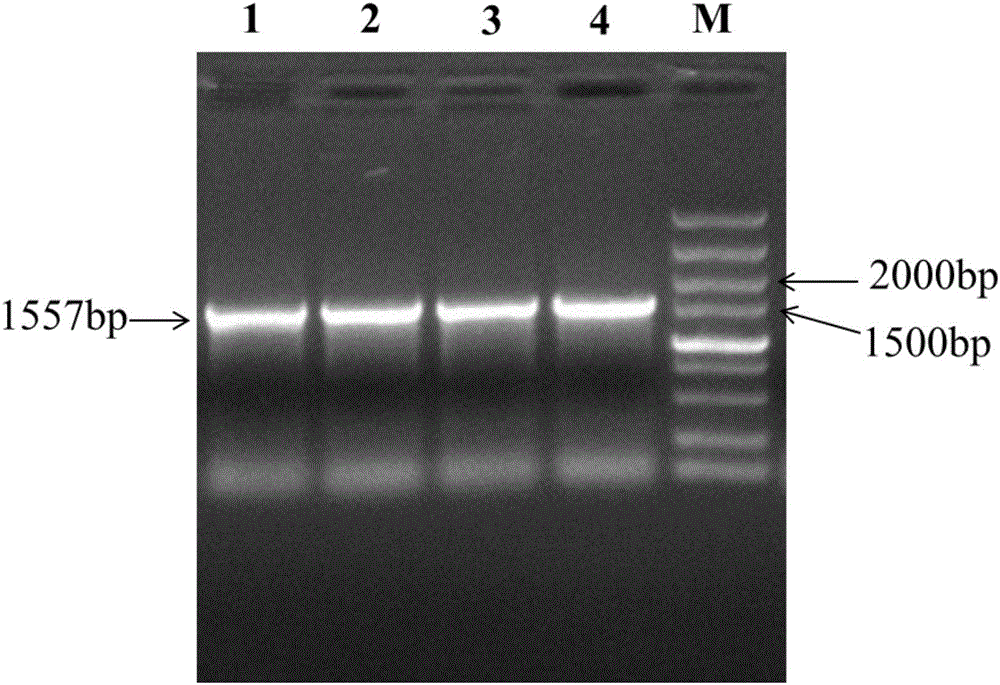
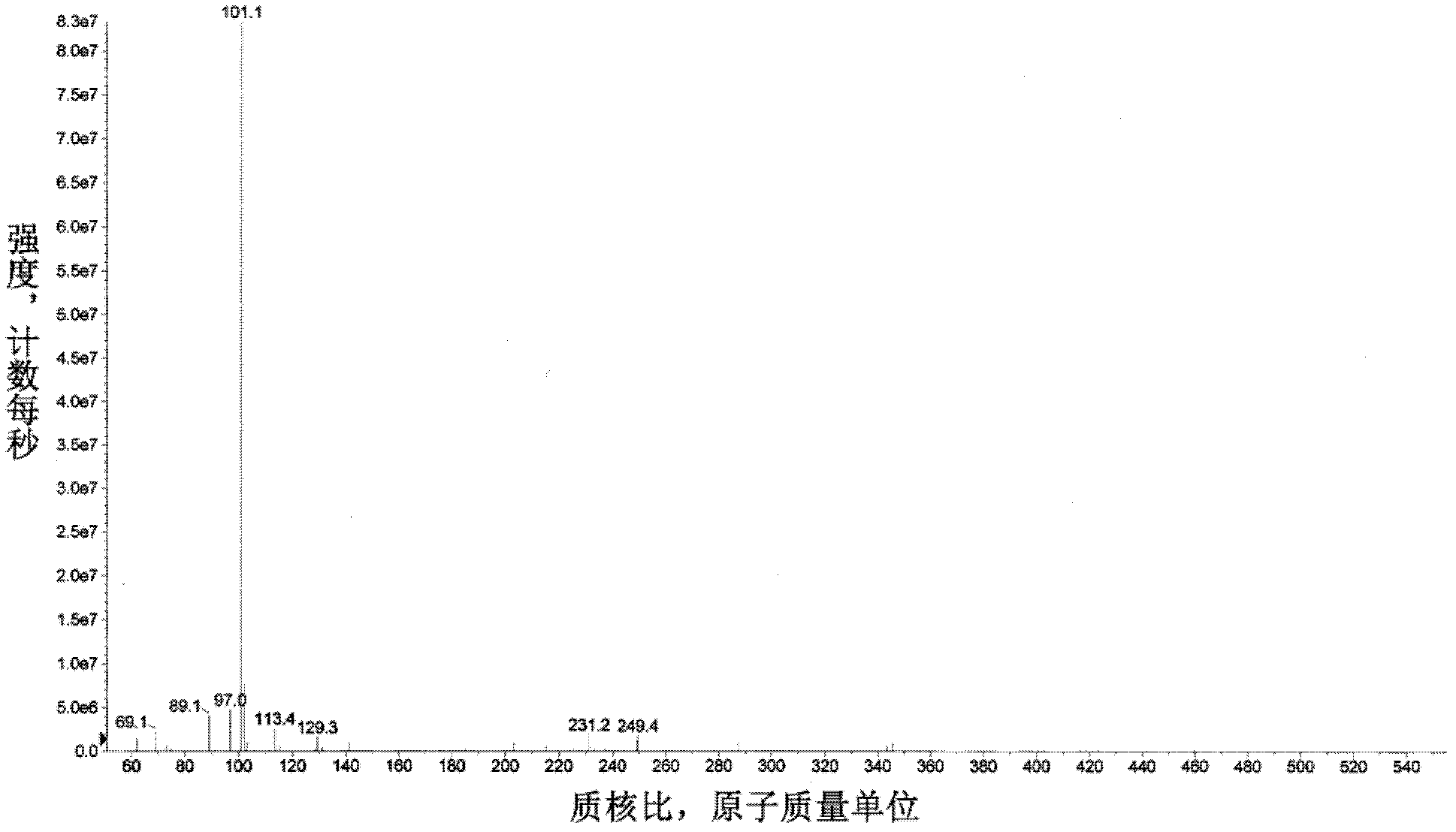
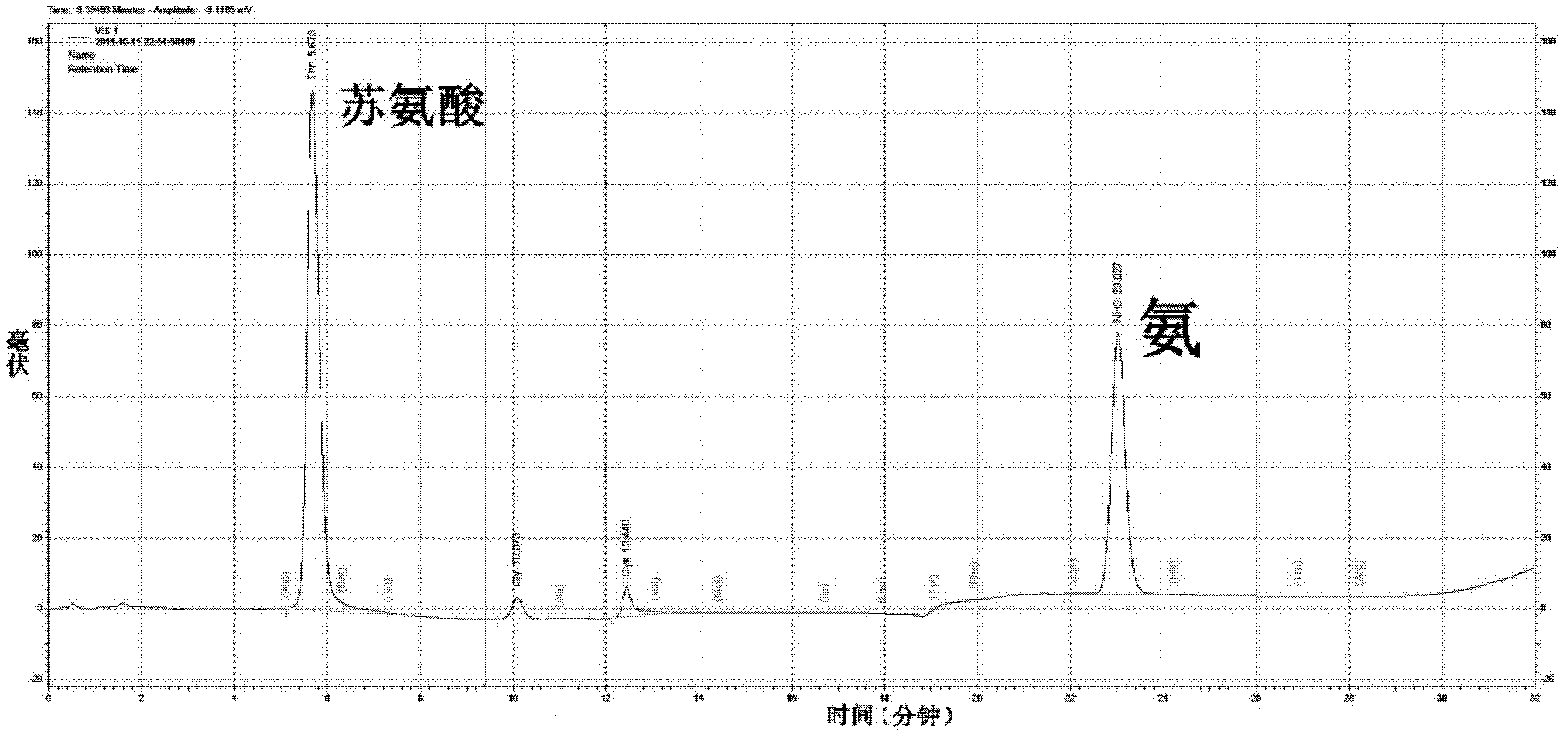
Method for preparing alpha-ketobutyric acid by using L-threonine as substrate
ActiveCN102433360AEfficient productionImprove conversion rateMicroorganism based processesFermentationHigh concentrationThreonine
Owner:上海肆芃科技有限公司
Process for prodn. of L-Thr
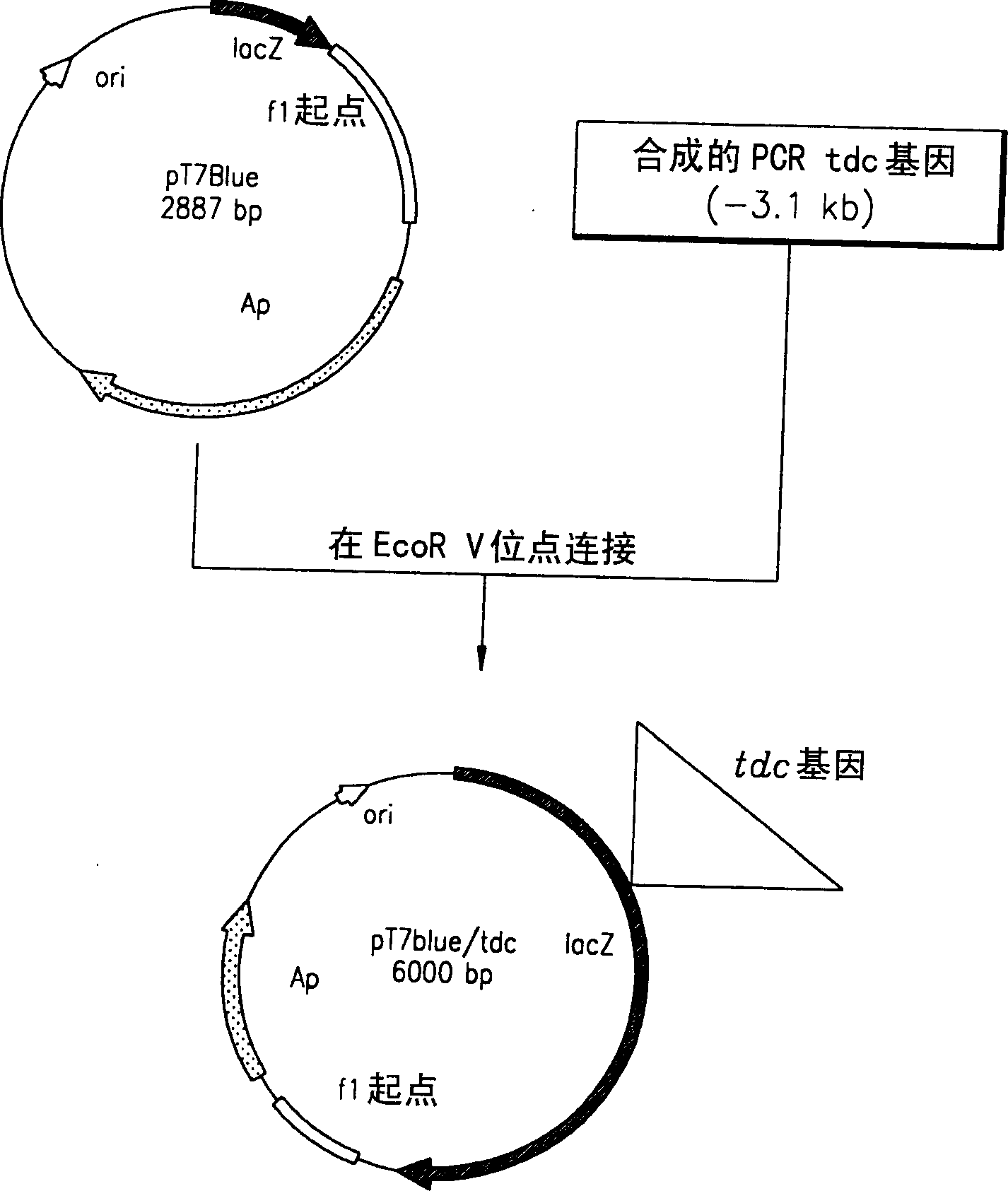
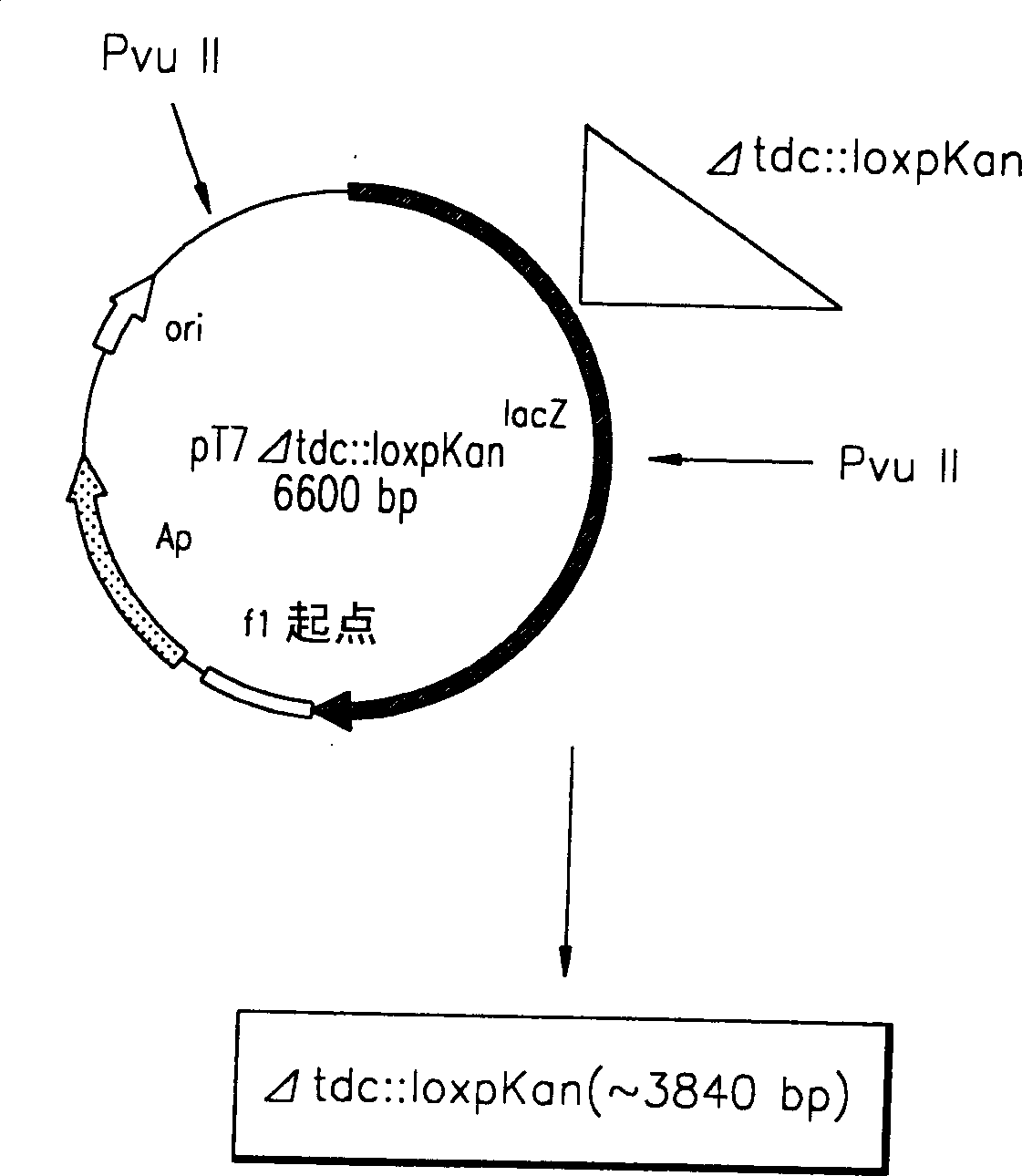
A method for producing L-threonine using a microorganism is provided. In the method, the threonine dehydratase (tdc) gene existing in the genomic DNA of the microorganism is partially deactivated using a recombination technique. For a microorganism strain with enhanced activity of threonine operon-containing enzymes and the phosphoenolpyruvate carboxylase (ppc) gene, the tdc gene engaged in one of the four threonine metabolic pathways is specifically deactivated, thereby markedly increasing the yield of L-threonine.
Owner:CJ CHEILJEDANG CORP
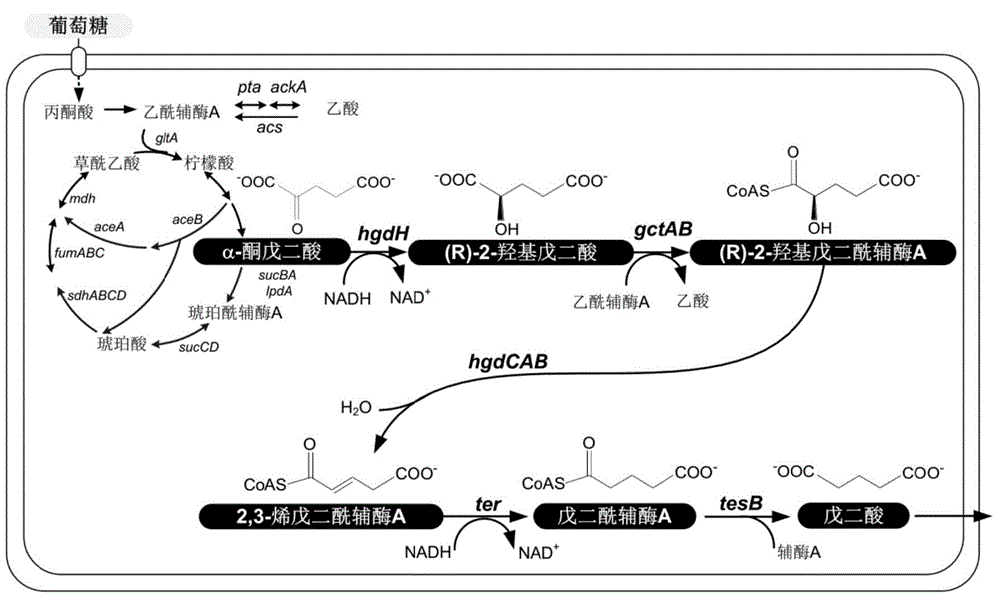
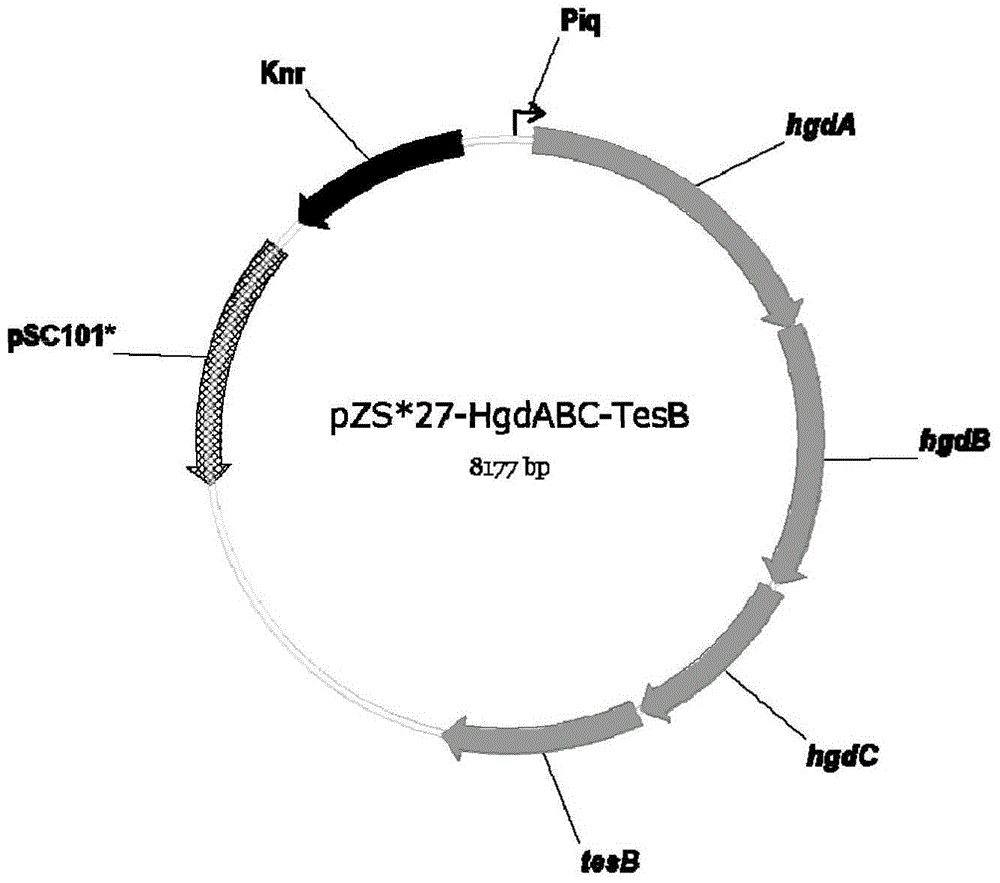
Biological improvement synthesis method of glutaric acid
The invention relates to a biological improvement synthesis method of glutaric acid. According to the present invention, the method is achieved by expressing 2-hydroxyglutaric acid dehydrogenase, glutaryl-coenzyme A transferase, 2-hydroxyglutaryl coenzyme A dehydratase, trans-CoA reductase and thioesterase in a recombinant host Escherichia coli, the recombinant Escherichia coli expressing the genes is subjected to fermentation production, and the generation of the target product glutaric acid and the by-products such as glutaconic acid and 2-hydroxyglutaric acid is successfully detected in the fermentation broth; and the constructed biological synthesis approach provides the new method for the utilization of the renewable resources to synthesize the glutaric acid.
Owner:SHANGHAI JIAO TONG UNIV
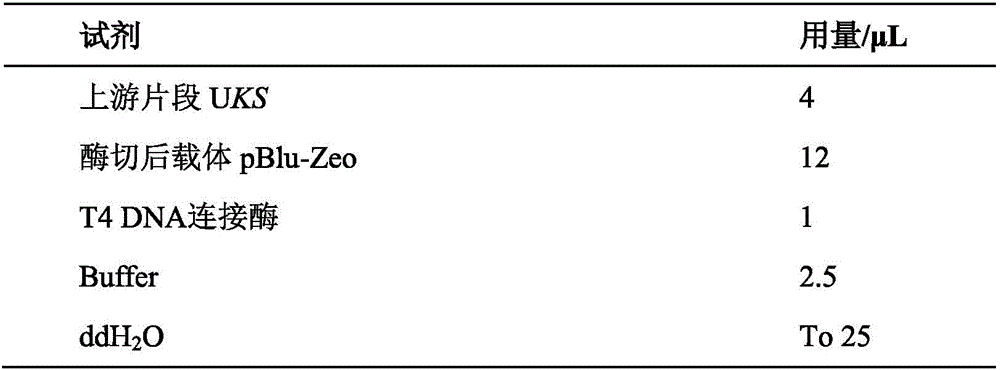
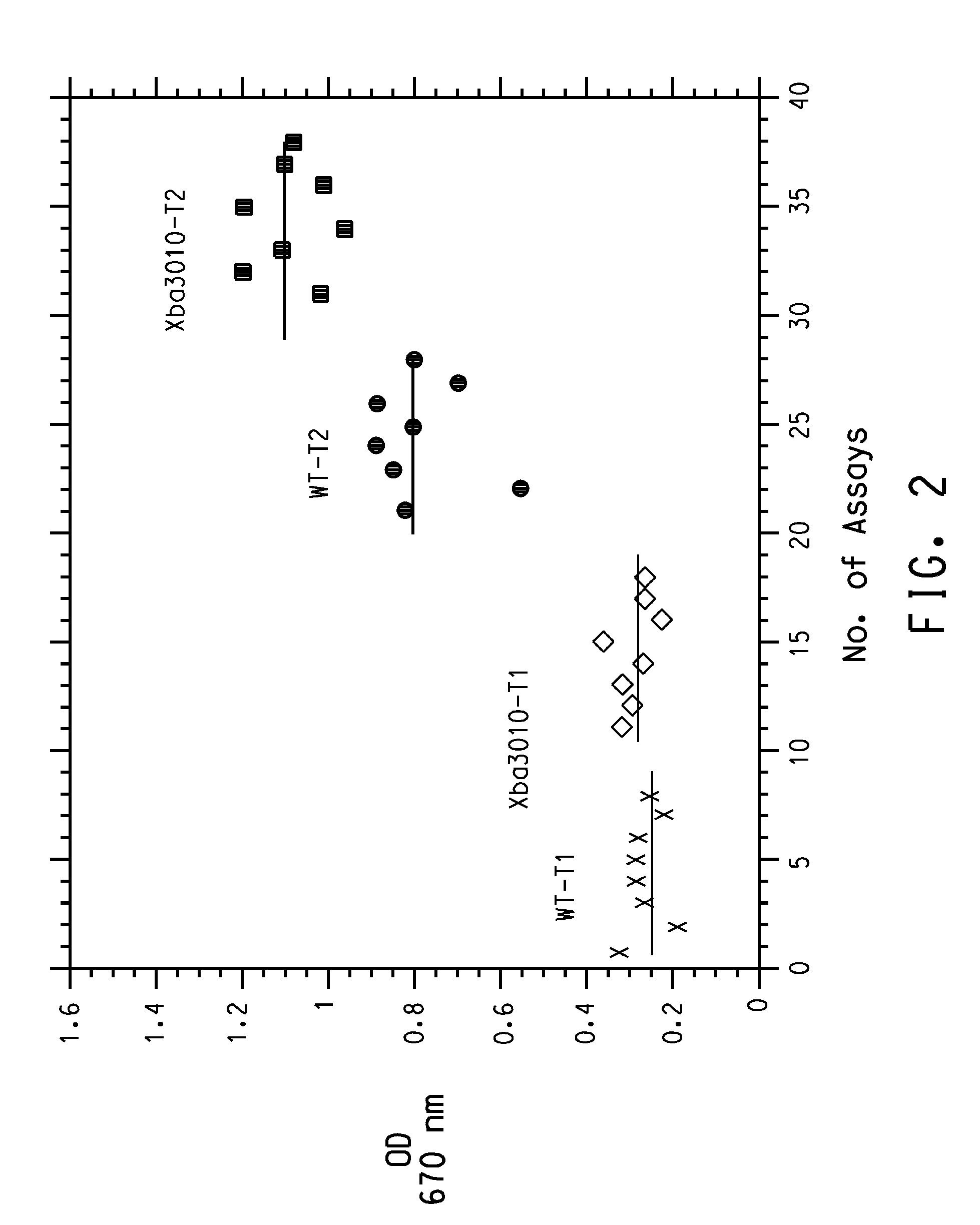
Construction method and application of schizochytrium limacinum engineering bacteria for KS (Beta-ketoacyl Synthase) gene knockout
ActiveCN106676127ACompositing unaffectedIncrease productionMicroorganism based processesNucleic acid vectorBiotechnologyBeta-ketoacyl synthase
The invention discloses a construction method and application of schizochytrium limacinum engineering bacteria for KS (Beta-ketoacyl Synthase) gene knockout. The construction method comprises the following steps: (1) using a genome of schizochytrium limacinum as a DNA (Deoxyribonucleic Acid) template, and respectively carrying out PCR (Polymerase Chain Reaction) amplification on an upstream fragment UKS and a downstream fragment DKS of a KS gene by using an upstream primer pair and a downstream primer pair; (2) connecting the upstream fragment UKS and the downstream fragment DKS with a knockout carrier, and constructing recombinant knockout plasmids; (3) electrically converting the recombinant knockout plasmids into the schizochytrium limacinum, screening by using a resistant plate, and verifying through a PCR resistant gene sequence, thus obtaining a converter, i.e., the schizochytrium limacinum engineering bacteria for the KS gene knockout. According to the construction method of the schizochytrium limacinum engineering bacteria for the KS gene knockout, provided by the invention, schizochytrium limacinum recombinant bacteria for knocking out FabA (Beta-Hydroxydecanoyl-Acyl Carrier Protein-Dehydratase) genes are constructed; compared with a proportion of total oil and fat in original strains, the polyunsaturated fatty acid production ability of the engineering bacteria is reduced by 43 percent, and the production of C16 and C18 saturated fatty acids is increased by 29 percent.
Owner:XIAMEN UNIV
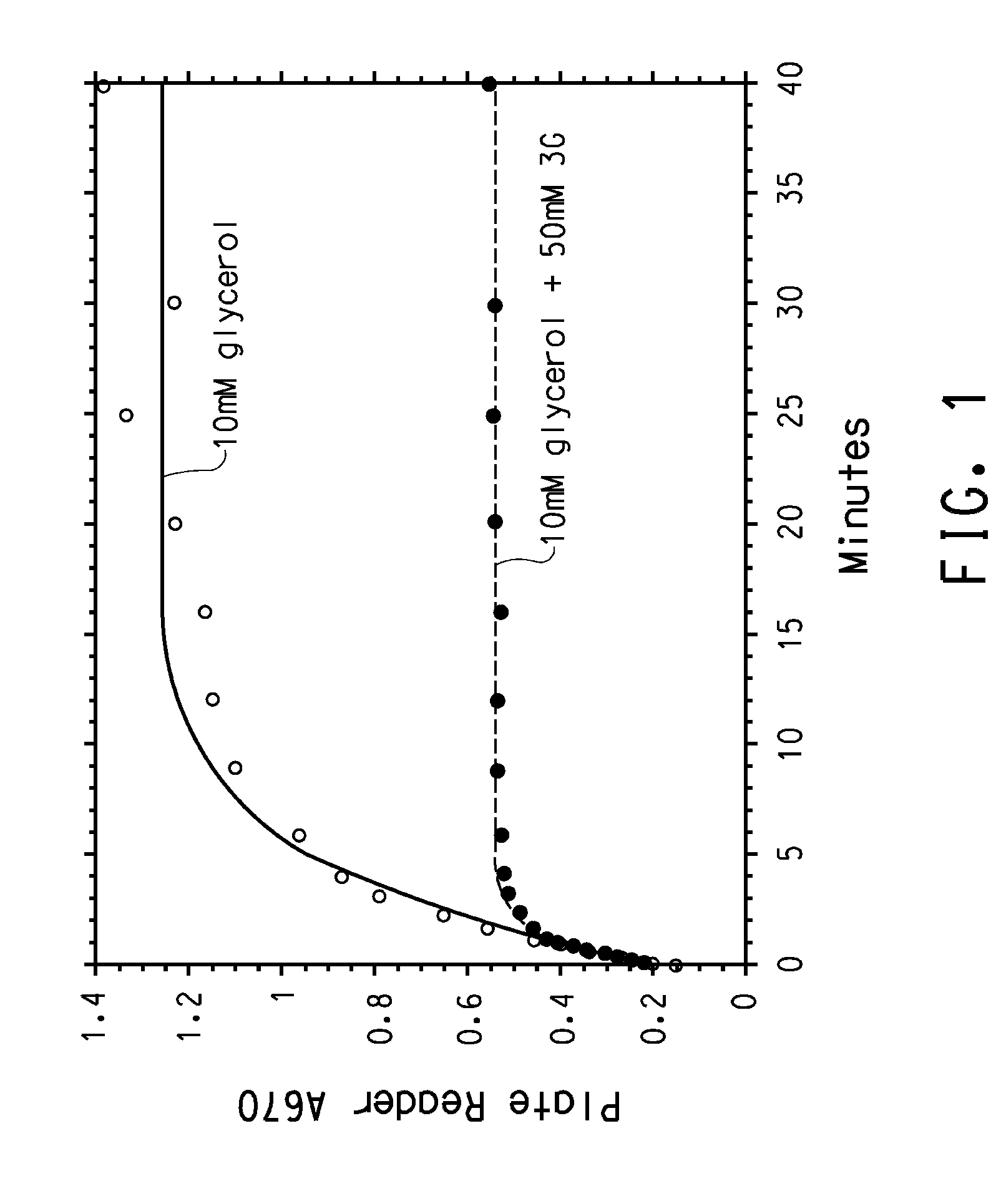
B12 dependent dehydratases with improved reaction kinetics
InactiveUS20080293119A1Improve stabilitySugar derivativesMicrobiological testing/measurementOligonucleotide-Directed MutagenesisPropanediol
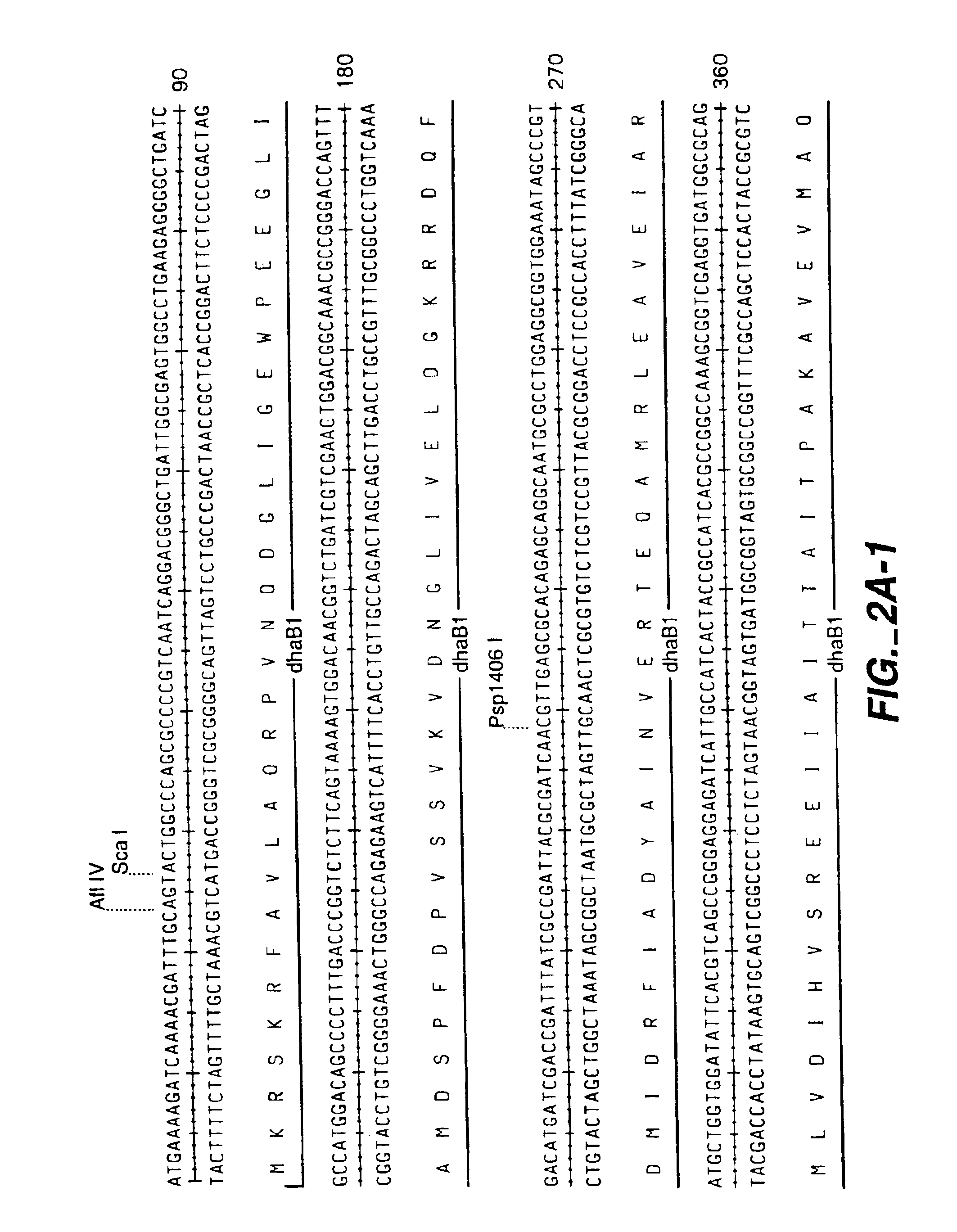
Sequences of B12-dependent dehydratases with improved reaction kinetics are presented. Use of these B12-dependent dehydratases reduce the rate of the enzyme's suicide inactivation in the presence of glycerol and 1,3-propanediol. The enzymes were created using error-prone PCR and oligonucleotide-directed mutagenesis to target the DhaB1 gene, which encodes the α-subunit of glycerol dehydratase. Mutants with improved reaction kinetics were rapidly identified using high throughput assays.
Owner:EI DU PONT DE NEMOURS & CO
Method for the recombinant production of 1,3-propanediol
The present invention provides a microorganism for the production of 1,3-propanediol from a variety of carbon sources in an organism capable of 1,3-propanediol production and comprising a) at least one gene encoding a dehydratase activity; b) at least one gene encoding a glycerol-3-phosphatase; and c) at least one gene encoding protein X. The protein X may be derived from a Klebsiella or Citrobacter gene cluster. The recombinant microorganism may further comprise d) at least one gene encoding a protein having at least 50% similarity to a protein selected from the group consisting of protein 1 (SEQ ID NO:60 or SEQ ID NO:61), of protein 2 (SEQ ID NO:62 or SEQ ID NO:63) and of protein 3 (SEQ ID NO:64 or SEQ ID NO:65) from Klebsiella or Citrobacter.
Owner:GENENCOR INT INC
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
The invention provides a non-naturally occurring microbial organism having a muconate pathway having at least one exogenous nucleic acid encoding a muconate pathway enzyme expressed in a sufficient amount to produce muconate. The muconate pathway including an enzyme selected from the group consisting of a beta-ketothiolase, a beta-ketoadipyl-CoA hydrolase, a beta-ketoadipyl-CoA transferase, a beta-ketoadipyl-CoA ligase, a 2-fumarylacetate reductase, a 2-fumarylacetate dehydrogenase, a trans-3-hydroxy-4-hexendioate dehydratase, a 2-fumarylacetate aminotransferase, a 2-fumarylacetate aminating oxidoreductase, a trans-3-amino-4-hexenoate deaminase, a beta-ketoadipate enol-lactone hydrolase, a muconolactone isomerase, a muconate cycloisomerase, a beta-ketoadipyl-CoA dehydrogenase, a 3-hydroxyadipyl-CoA dehydratase, a 2,3-dehydroadipyl-CoA transferase, a 2,3-dehydroadipyl-CoA hydrolase, a 2,3-dehydroadipyl-CoA ligase, a muconate reductase, a 2-maleylacetate reductase, a 2-maleylacetate dehydrogenase, a cis-3-hydroxy-4-hexendioate dehydratase, a 2-maleylacetate aminoatransferase, a 2-maleylacetate aminating oxidoreductase, a cis-3-amino-4-hexendioate deaminase, and a muconate cis / trans isomerase. Other muconate pathway enzymes also are provided. Additionally provided are methods of producing muconate.
Owner:GENOMATICA INC
Method for synthesizing of 2'-fucosyllactose
InactiveCN110172486AReduce manufacturing costIncrease productivityMicroorganism based processesFermentationBiotechnologyStaphylococcus lactis
The invention belongs to the field of genetic engineering and relates to a method for producing 2'-fucosyllactose, in particular to a method for synthesizing 2'-fucosyllactose by co-catalysis of a genetically engineered strain and enzyme. The method of the invention comprises the steps of using a recombinant strain of lactococcus lactis which efficiently expresses hexokinase, phosphomannomutase and mannose-1-phosphate guanyl transferase as a production strain, and using mannose as a substrate to synthesize GDP-mannose, and then using GDP-mannose 4,6-dehydratase, GDP-4-keto-6-deoxymannose 3,5-mutarotase / 4-reductase, alpha1,2-fucosyltransferase to catalyze GDP-mannose in vitro to synthesize the 2'-fucosyllactose, thus providing a new method for the industrial production of the 2'-fucosyllactose.
Owner:TIANJIN UNIV OF SCI & TECH
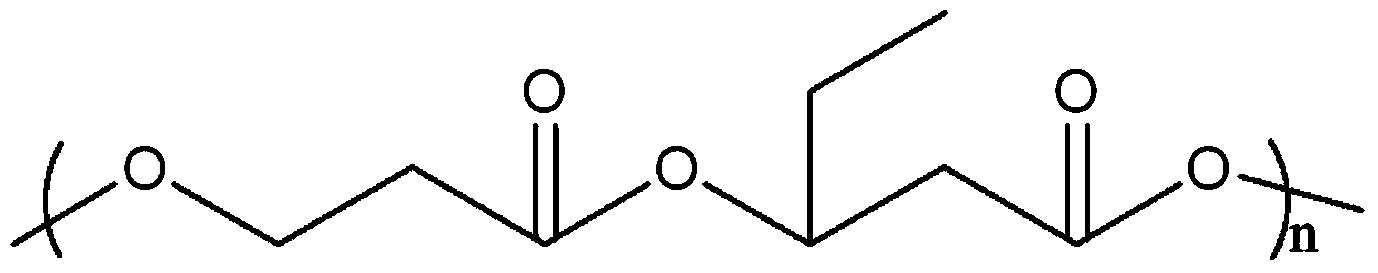
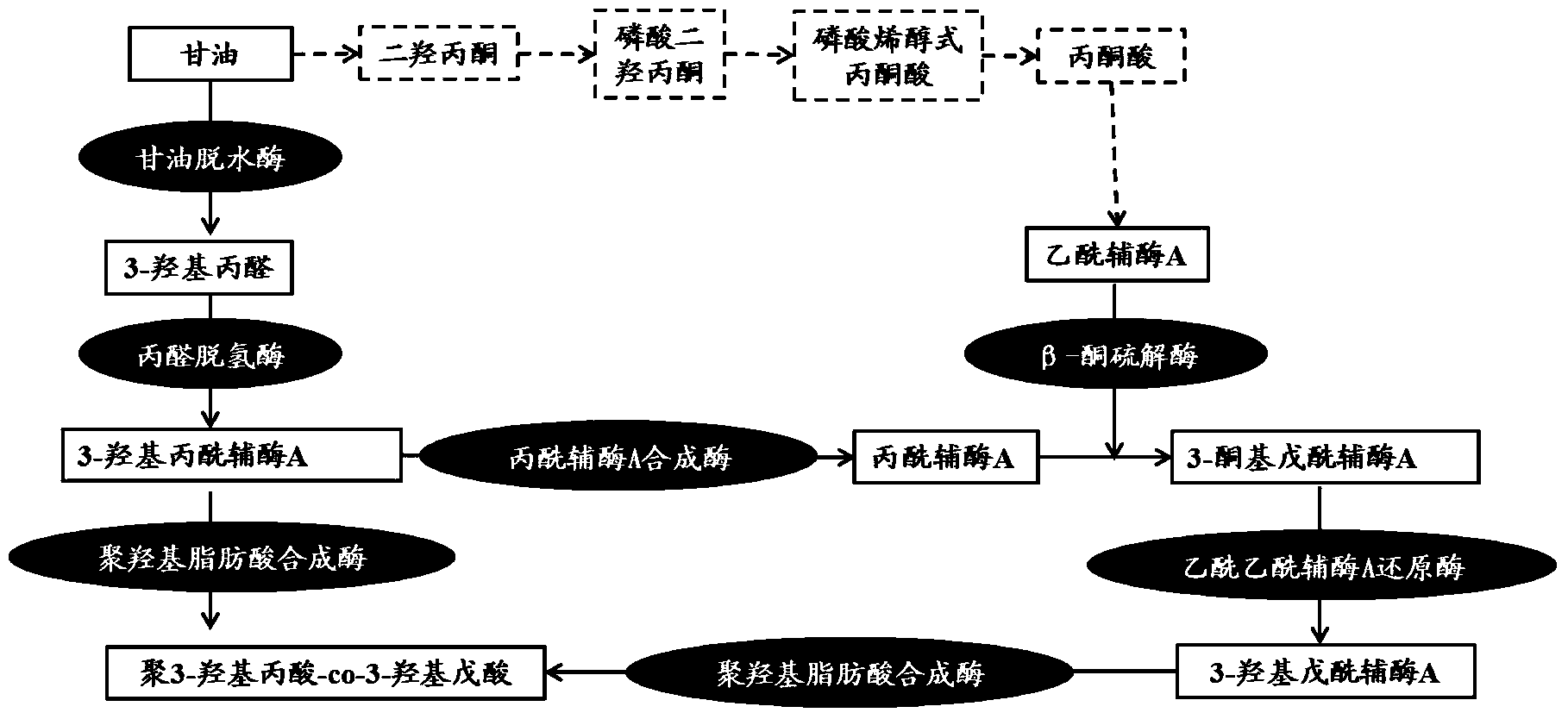
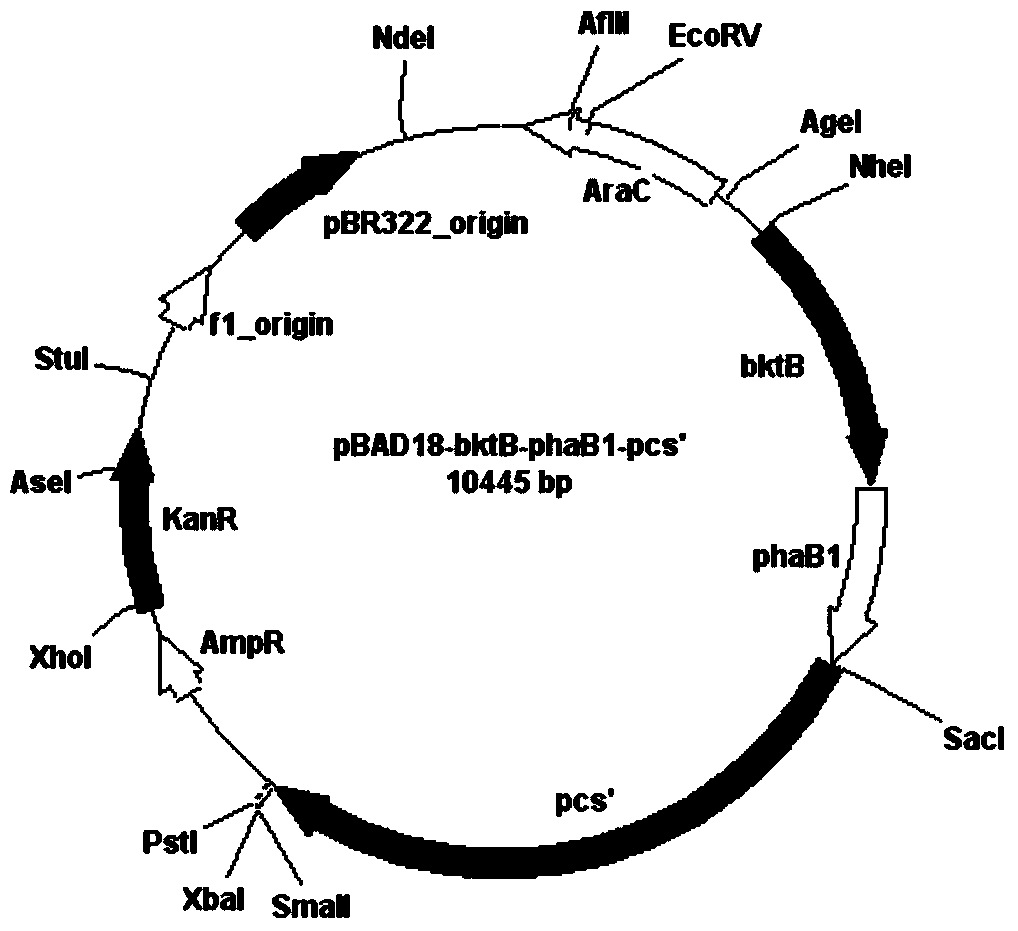
Poly-3-hydroxy propionic acid copolymer and production method thereof
ActiveCN104046659AHigh melting pointReduce crystallinityBacteriaMicroorganism based processes3-Hydroxypropionic acidPropanoic acid
The invention discloses a poly-3-hydroxy propionic acid copolymer and a production method thereof and belongs to the technical field of genetic engineering. According to the poly-3-hydroxy propionic acid copolymer and the production method thereof, a glycerol dehydratase gene and a glycerol dehydratase re-activating enzyme gene are integrated with a host strain genome by a gene integration technology, a polyhydroxy fatty acid synthase gene, a propionaldehyde dehydrogenase gene, a beta-ketoacyl coenzyme A thiolase gene, an acetoacetyl coenzyme A reductase gene and a propionyl coenzyme A synthetase gene are introduced, and a recombinant gene engineering strain has the ability of biologically synthesizing poly-3-hydracrylic acid-co-3-hydroxyvaleric acid. According to the poly-3-hydroxy propionic acid copolymer and the production method thereof, the poly-3-hydracrylic acid-co-3-hydroxyvaleric acid is obtained in a biosynthesis manner for the first time; compared with poly-3-hydracrylic acid, the obtained poly 3-hydracrylic acid-co-3-hydroxyvaleric acid has a higher melting point and lower crystallinity, has good degradability and can serve as a packaging material, a medical implant material, a drug sustained-release material and an electrochemical material.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com