Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Coa transferase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CoA-transferases are found in organisms from all kingdoms of life. They catalyse reversible transfer reactions of coenzyme A groups from CoA-thioesters to free acids. There are at least three families of CoA-transferases, which differ in sequence and reaction mechanism:
Biological systems for manufacture of polyhydroxyalkanoate polymers containing 4-hydroxyacids
The gene encoding a 4-hydroxybutyryl-CoA transferase has been isolated from bacteria and integrated into the genome of bacteria also expressing a polyhydroxyalkanoate synthase, to yield an improved production process for 4HB-containing polyhydroxyalkanoates using transgenic organisms, including both bacteria and plants. The new pathways provide means for producing 4HB containing PHAs from cheap carbon sources such as sugars and fatty acids, in high yields, which are stable. Useful strains are obtaining by screening strains having integrated into their genomes a gene encoding a 4HB-CoA transferase and / or PHA synthase, for polymer production. Processes for polymer production use recombinant systems that can utilize cheap substrates. Systems are provided which can utilize amino acid degradation pathways, α-ketoglutarate, or succinate as substrate.
Owner:CJ CHEILJEDANG CORP
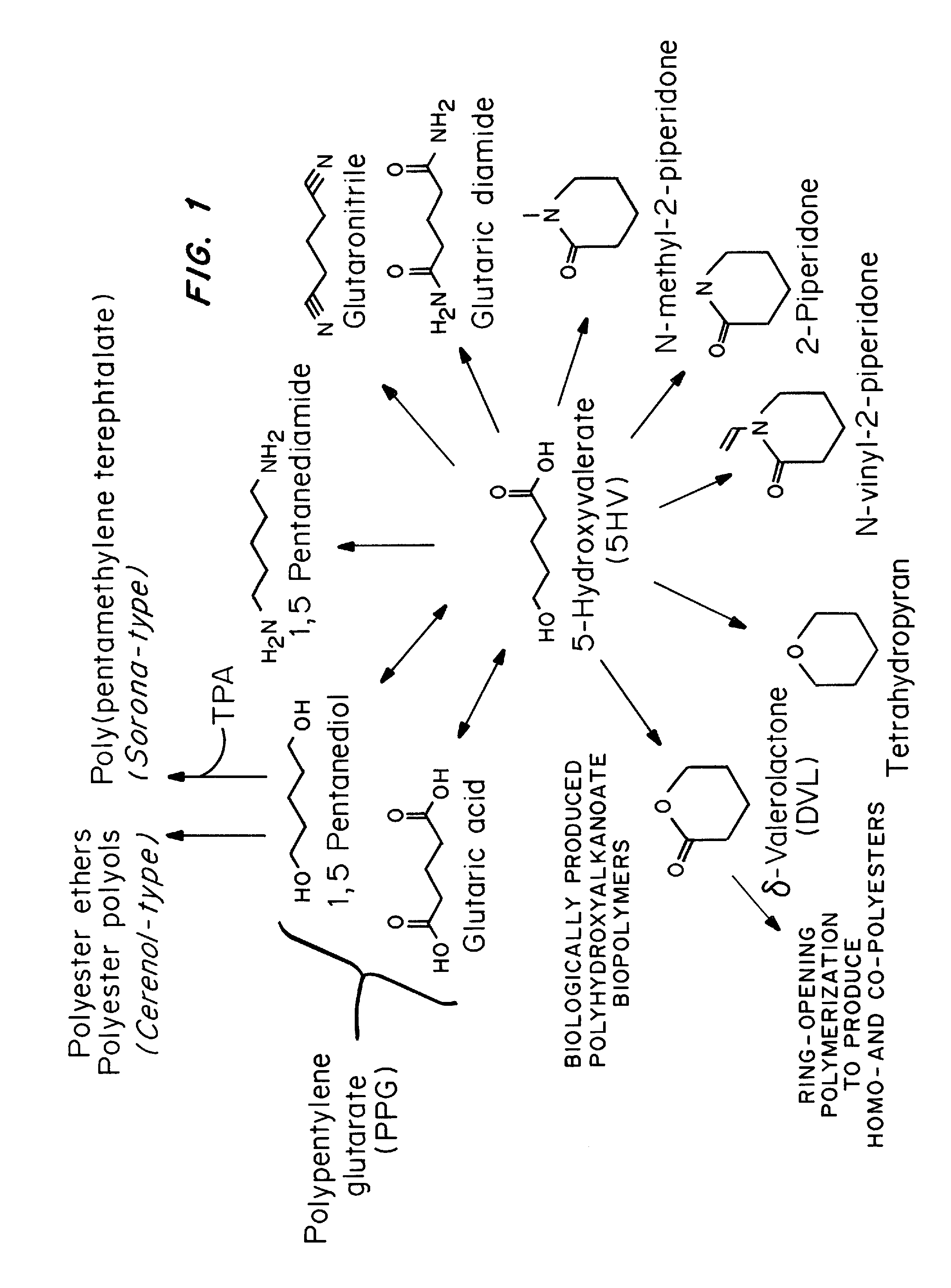
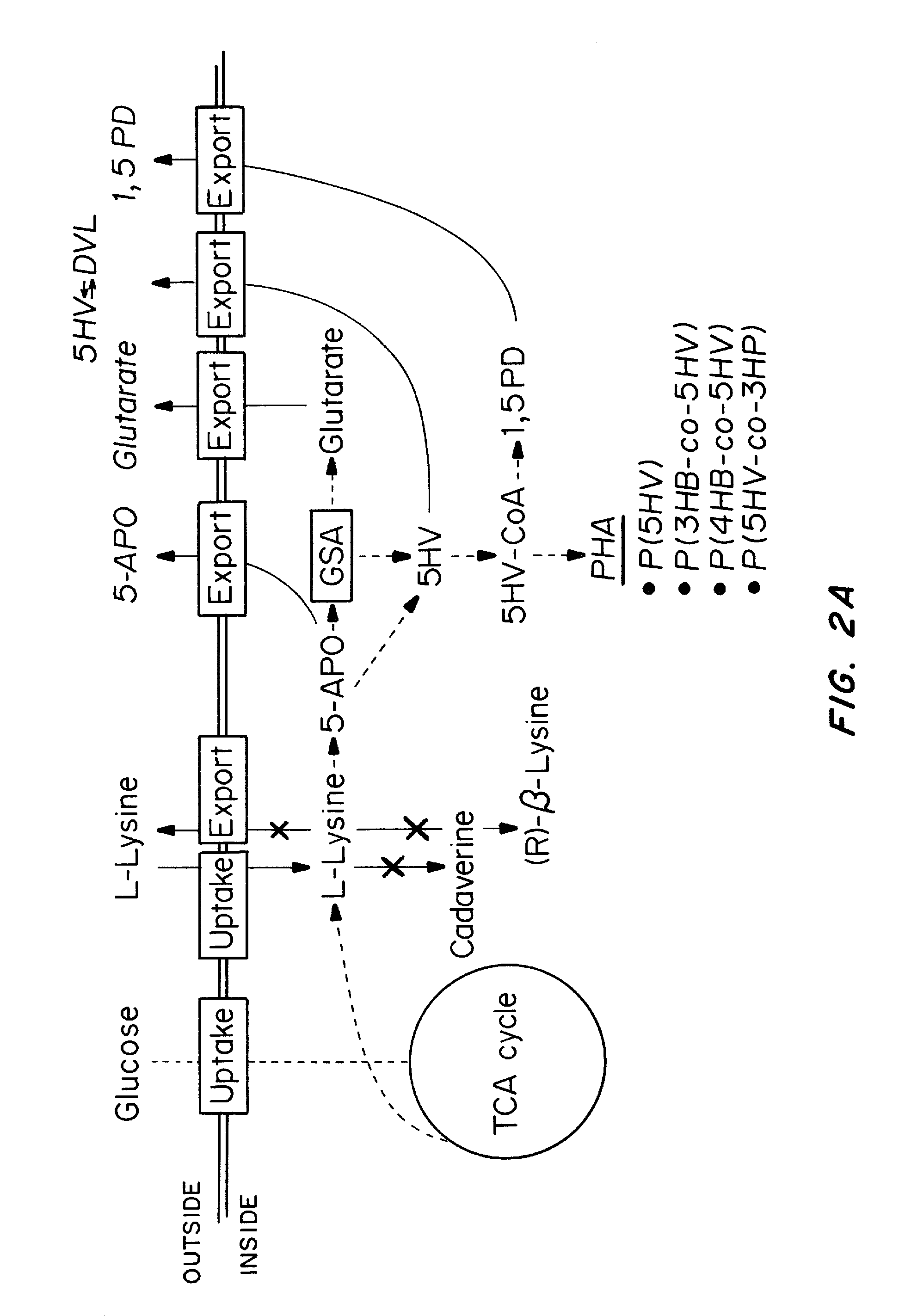
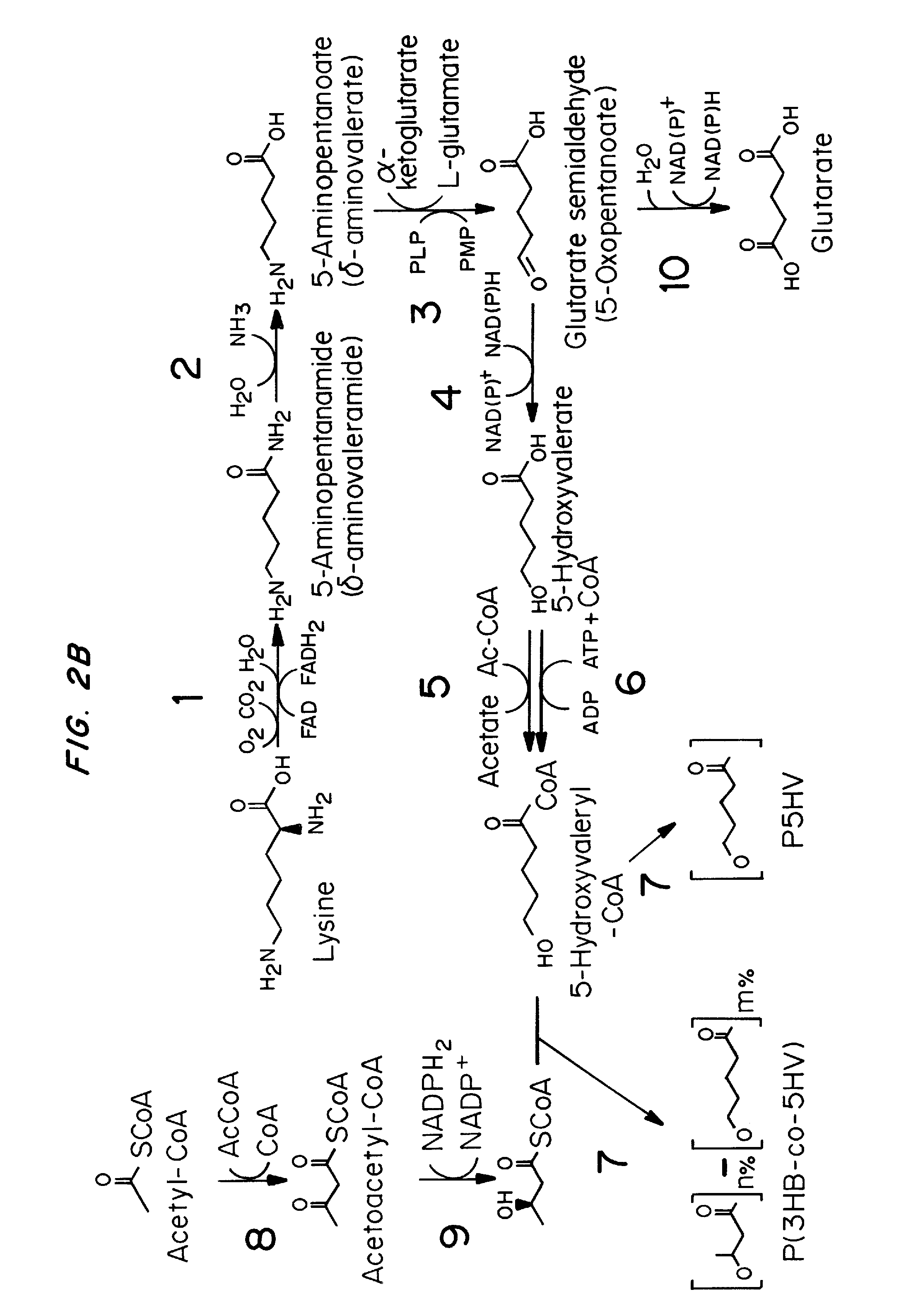
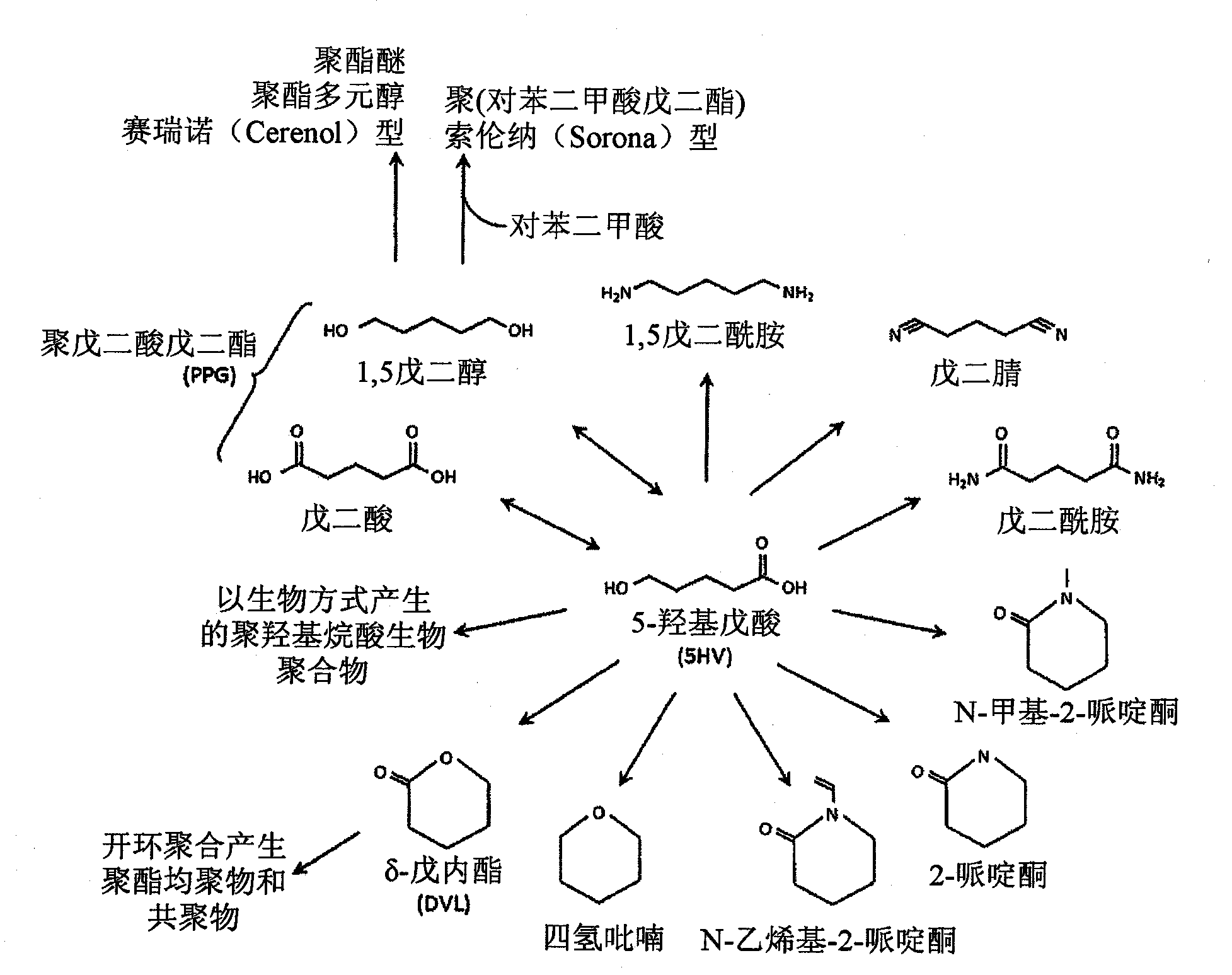
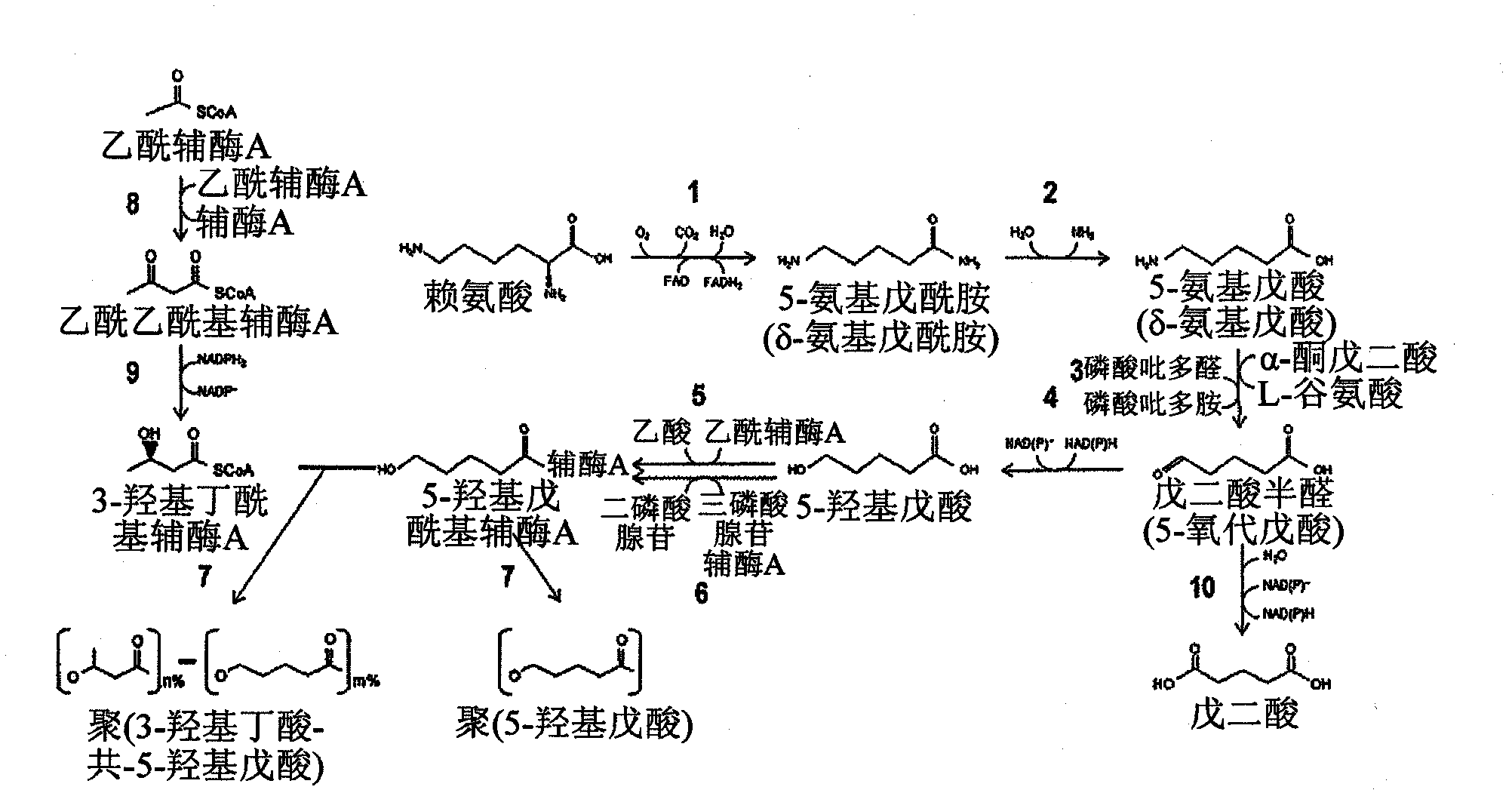
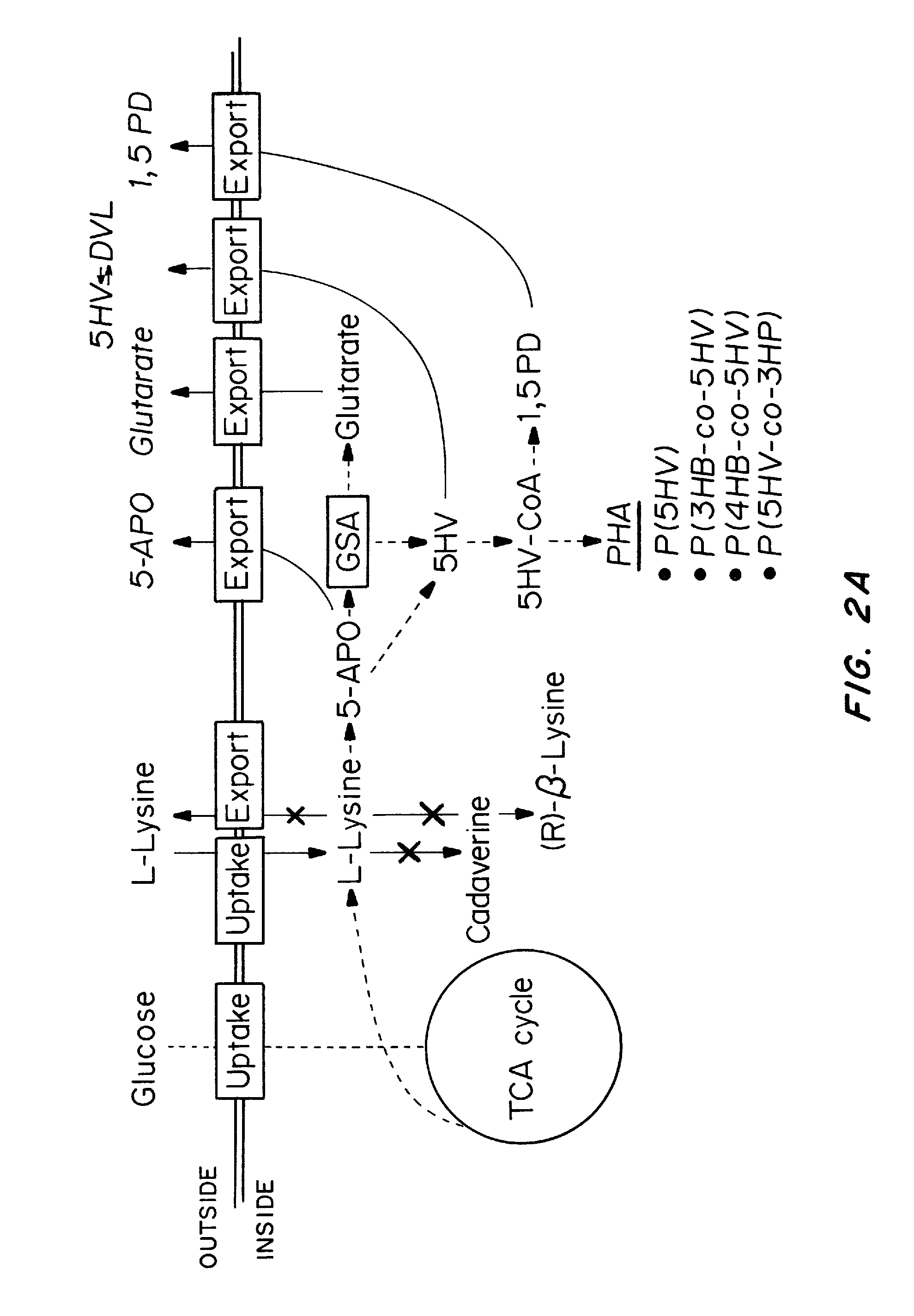
Green process and compositions for producing poly(5HV) and 5 carbon chemicals
Recombinant hosts for producing polyhydroxyalkanoates and methods of producing polyhydroxyalkanoates from renewable carbon substrates are provided. Certain recombinant hosts that produce 5 carbon chemicals such as 5-aminopentanoate (5AP), 5-hydroxyvalerate (5HV), glutarate, and 1,5 pentanediol (PDO) are also provided. One embodiment provides a recombinant host expressing a gene encoding a heterologous enzyme selected from the group consisting of a polyhydroxyalkanoate synthase and a 5-hydroxyvalerate-CoA (5HV-CoA) transferase, wherein the host produces a polymer containing 5-hydroxyvalerate. Preferably, the host expresses both a polyhydroxyalkanoate synthase and a 5HV-CoA transferase. The host can be prokaryotic or eukaryotic. A preferred prokaryotic host is E. coli. The polymers produced by the recombinant hosts can be homopolymers or copolymers of 5-hydroxyvalerate. A preferred copolymer is poly(3-hydroxybutyrate-co-5-hydroxyvalerate).
Owner:CJ CHEILJEDANG CORP
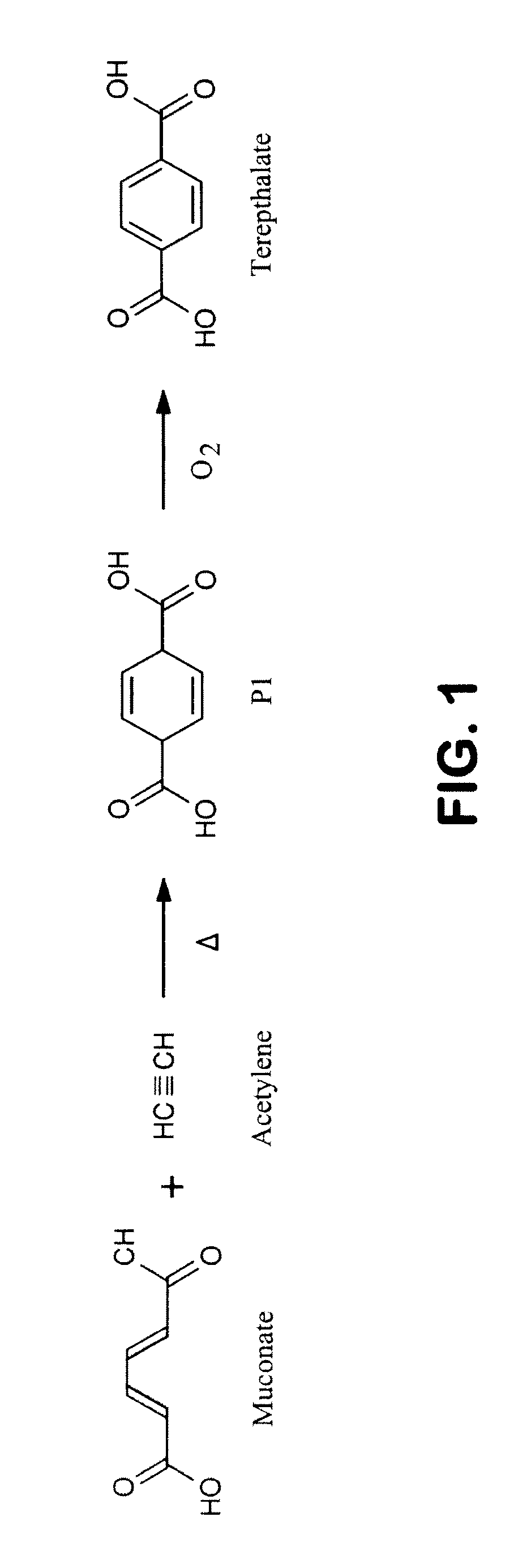
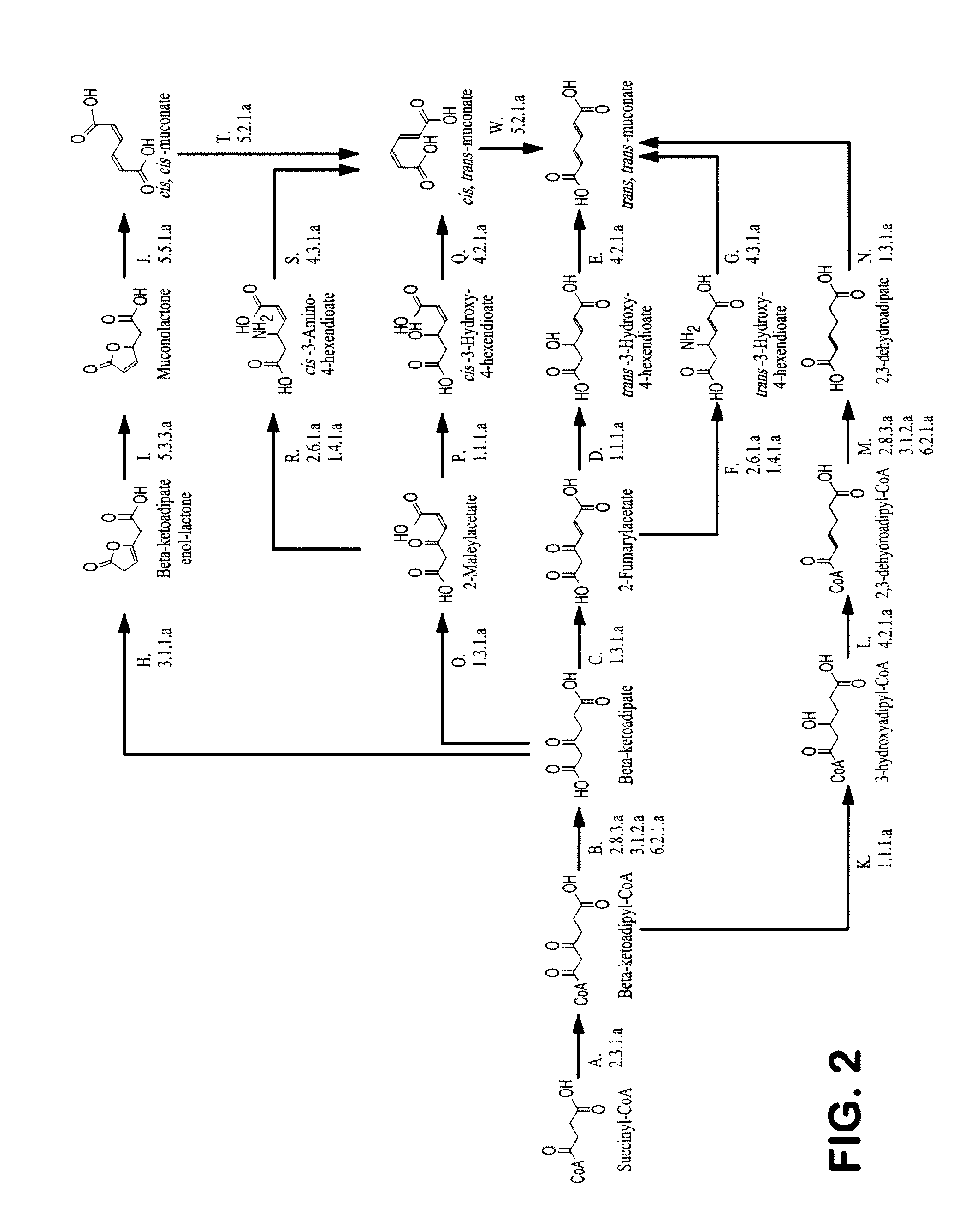
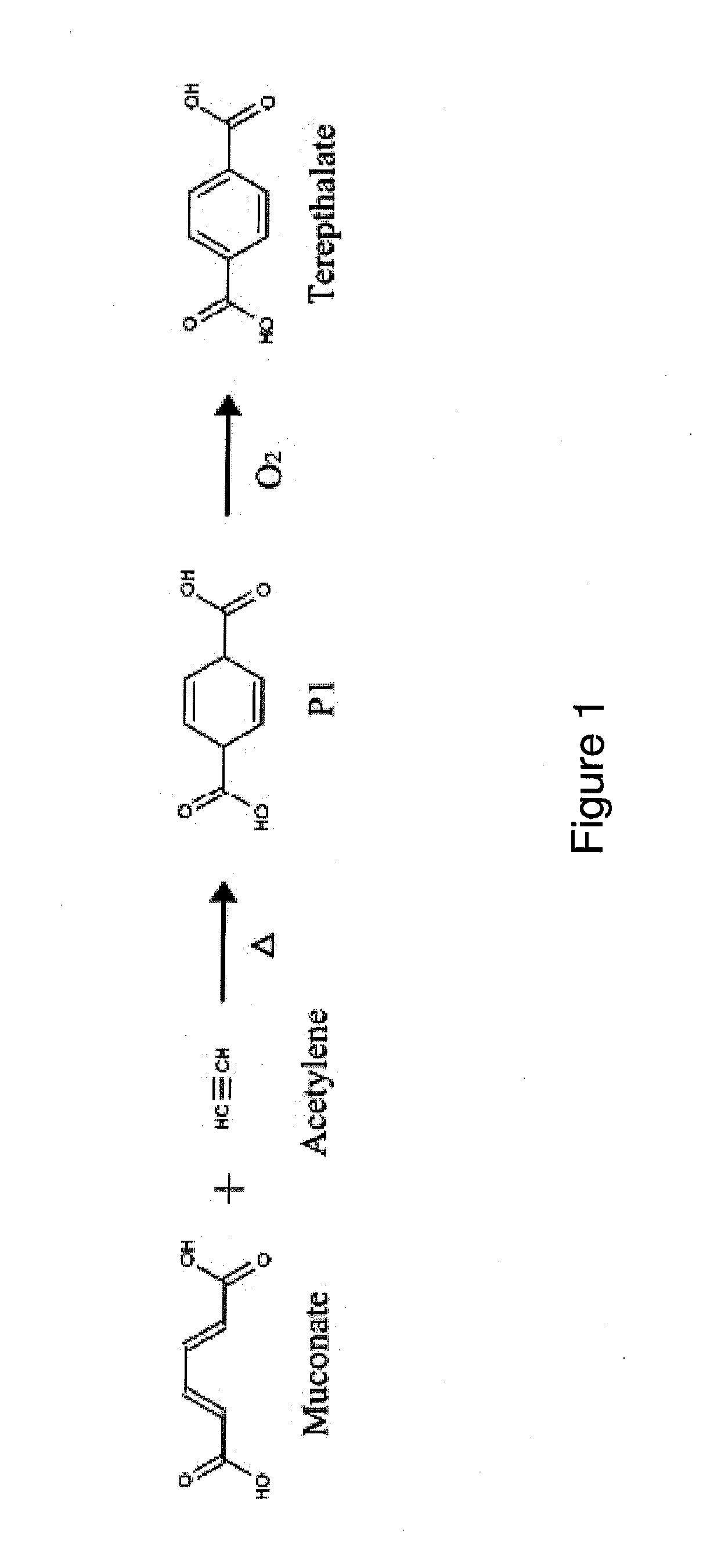
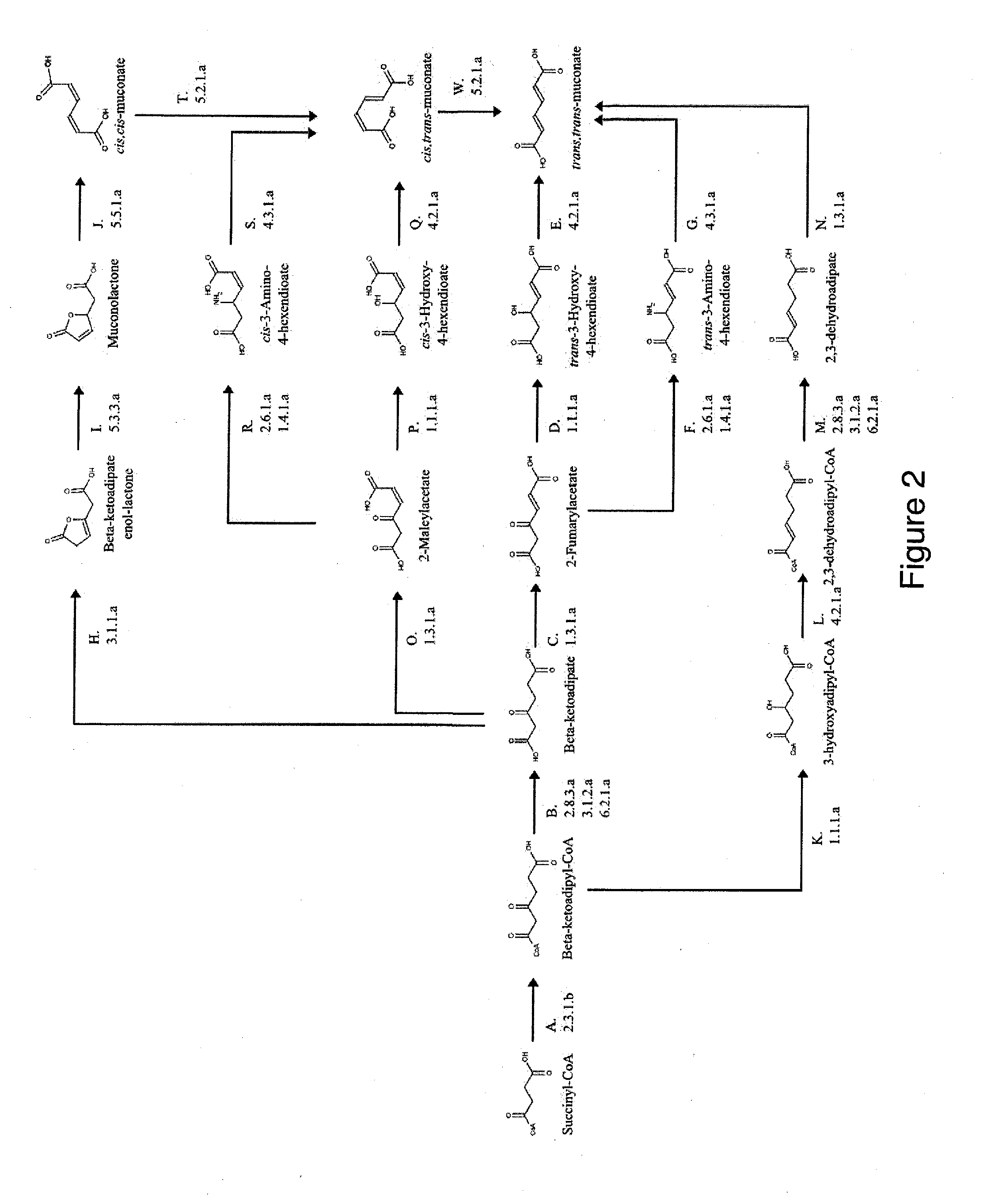
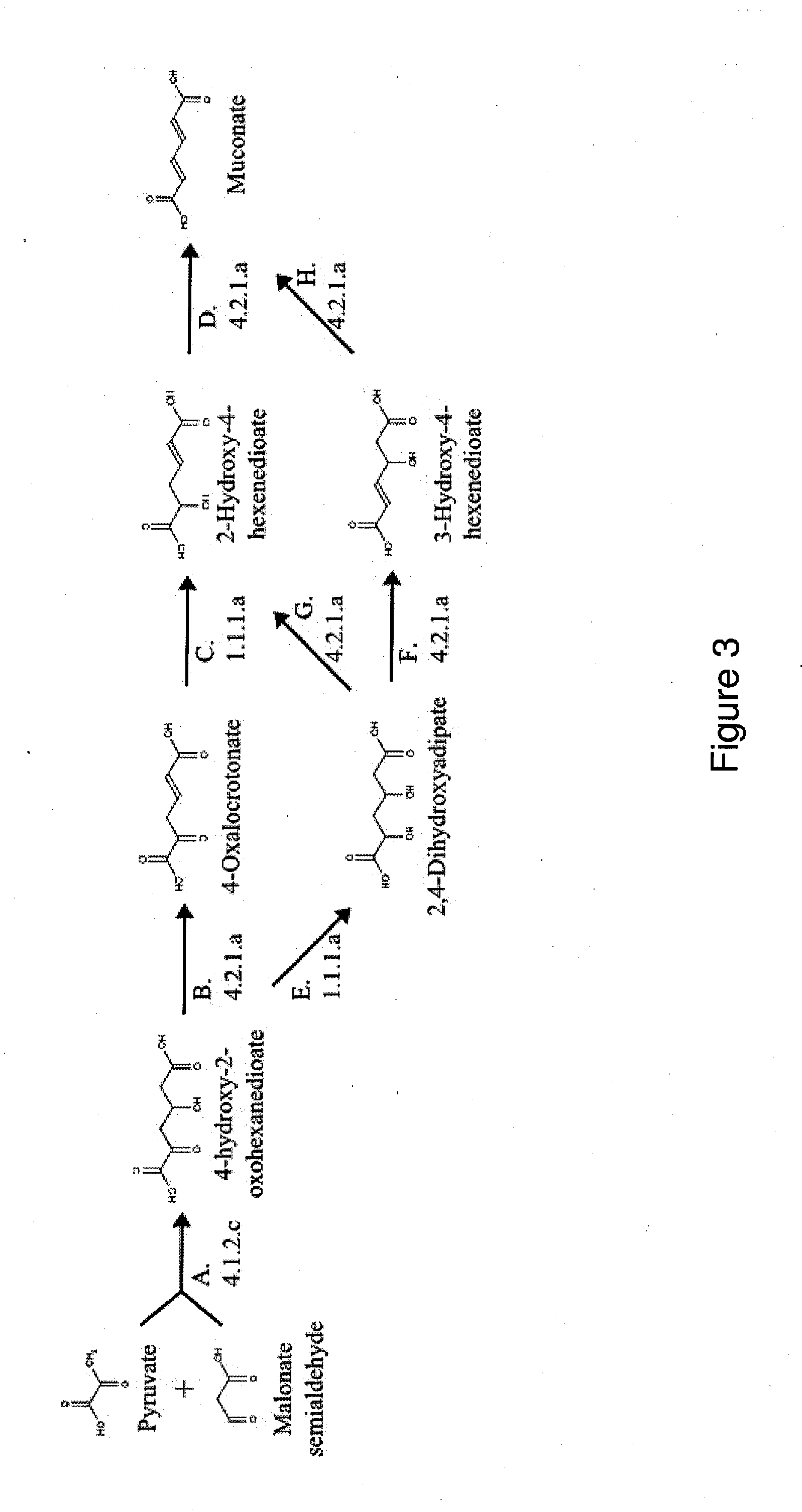
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
The invention provides a non-naturally occurring microbial organism having a muconate pathway having at least one exogenous nucleic acid encoding a muconate pathway enzyme expressed in a sufficient amount to produce muconate. The muconate pathway including an enzyme selected from the group consisting of a beta-ketothiolase, a beta-ketoadipyl-CoA hydrolase, a beta-ketoadipyl-CoA transferase, a beta-ketoadipyl-CoA ligase, a 2-fumarylacetate reductase, a 2-fumarylacetate dehydrogenase, a trans-3-hydroxy-4-hexendioate dehydratase, a 2-fumarylacetate aminotransferase, a 2-fumarylacetate aminating oxidoreductase, a trans-3-amino-4-hexenoate deaminase, a beta-ketoadipate enol-lactone hydrolase, a muconolactone isomerase, a muconate cycloisomerase, a beta-ketoadipyl-CoA dehydrogenase, a 3-hydroxyadipyl-CoA dehydratase, a 2,3-dehydroadipyl-CoA transferase, a 2,3-dehydroadipyl-CoA hydrolase, a 2,3-dehydroadipyl-CoA ligase, a muconate reductase, a 2-maleylacetate reductase, a 2-maleylacetate dehydrogenase, a cis-3-hydroxy-4-hexendioate dehydratase, a 2-maleylacetate aminoatransferase, a 2-maleylacetate aminating oxidoreductase, a cis-3-amino-4-hexendioate deaminase, and a muconate cis / trans isomerase. Other muconate pathway enzymes also are provided. Additionally provided are methods of producing muconate.
Owner:GENOMATICA INC
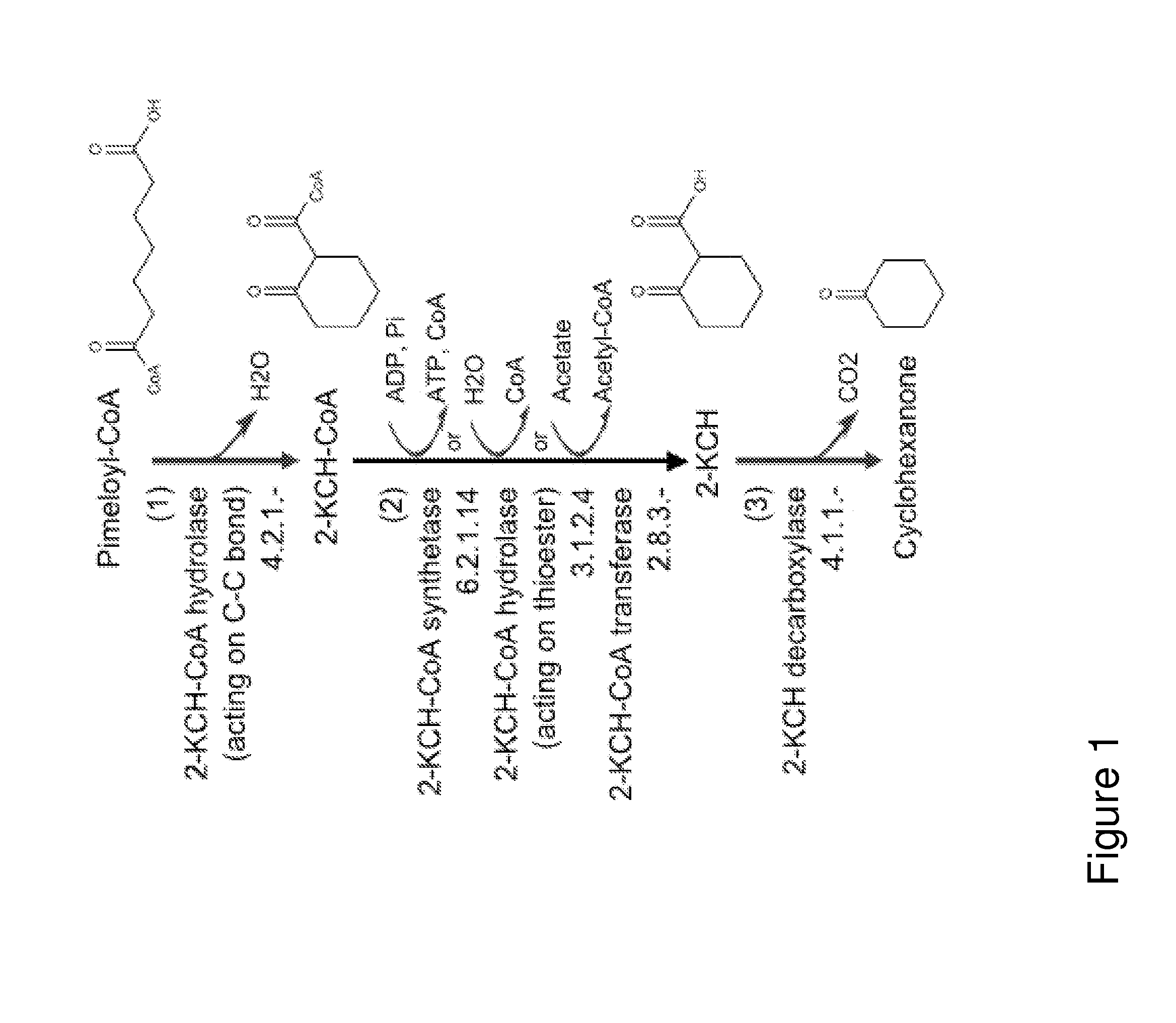
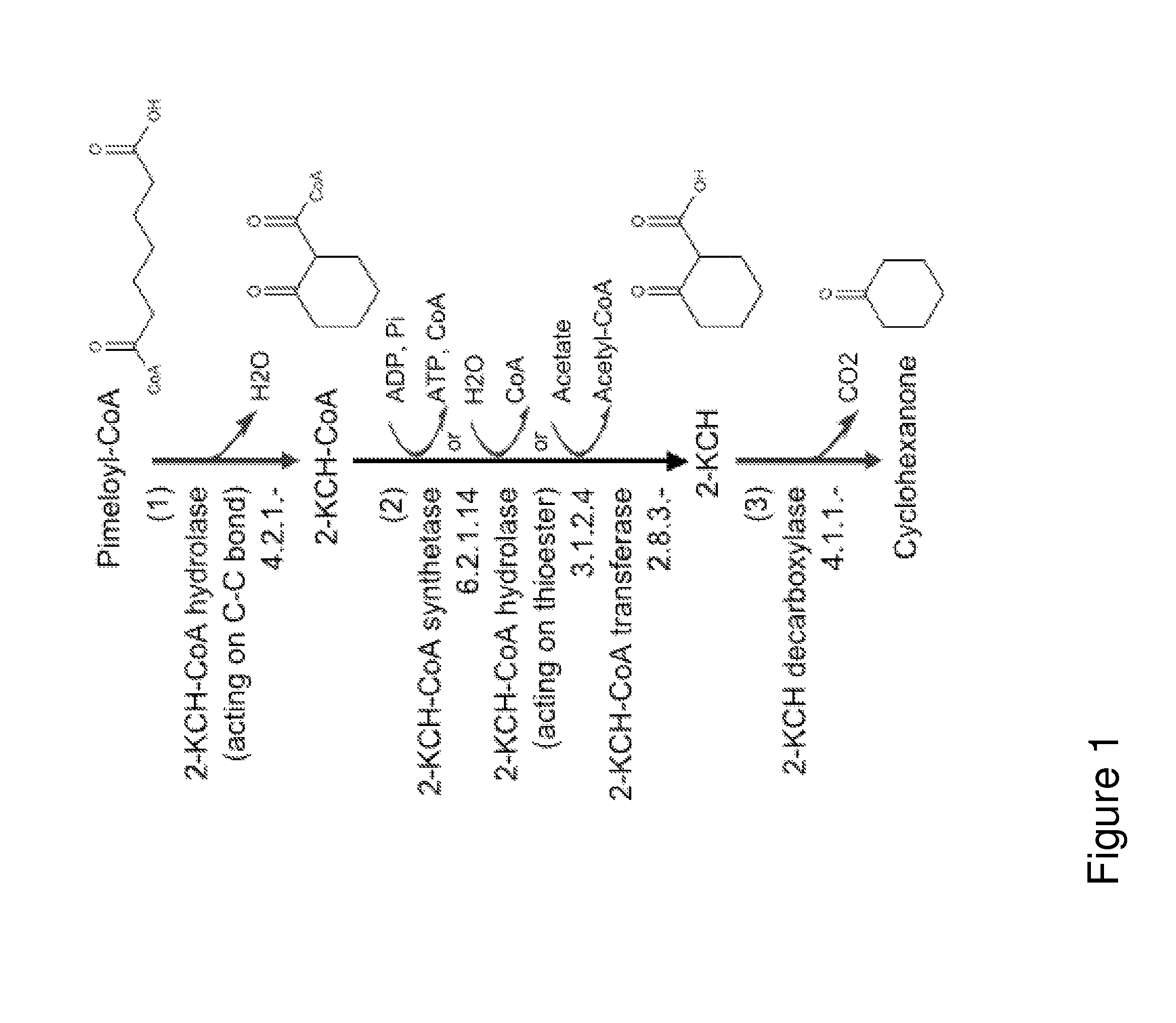
Organisms for the production of cyclohexanone
A non-naturally occurring microbial organism has cyclohexanone pathways that include at least one exogenous nucleic acid encoding a cyclohexanone pathway enzyme. A pathway includes a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on C—C bond), a 2-ketocyclohexane-1-carboxylate decarboxylase and an enzyme selected from a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxyl-CoA transferase, and a 2-ketocyclohexane-1-carboxyl-CoA synthetase. A pathway includes an enzyme selected from a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on C—C bond), a 6-ketocyclohex-1-ene-1-carboxyl-CoA synthetase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on thioester), a 6-ketocyclohex-1-ene-1-carboxyl-CoA transferase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA reductase, a 6-ketocyclohex-1-ene-1-carboxylate decarboxylase, a 6-ketocyclohex-1-ene-1-carboxylate reductase, a 2-ketocyclohexane-1-carboxyl-CoA synthetase, a 2-ketocyclohexane-1-carboxyl-CoA transferase, a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxylate decarboxylase, and a cyclohexanone dehydrogenase. A pathway includes an adipate semialdehyde dehydratase, a cyclohexane-1,2-diol dehydrogenase, and a cyclohexane-1,2-diol dehydratase. A pathway includes a 3-oxopimelate decarboxylase, a 4-acetylbutyrate dehydratase, a 3-hydroxycyclohexanone dehydrogenase, a 2-cyclohexenone hydratase, a cyclohexanone dehydrogenase and an enzyme selected from a 3-oxopimeloyl-CoA synthetase, a 3-oxopimeloyl-CoA hydrolase (acting on thioester), and a 3-oxopimeloyl-coA transferase. Each these pathways can include a PEP carboxykinase. A method for producing cyclohexanone includes culturing these non-naturally occurring microbial organisms.
Owner:GENOMATICA INC
Biological systems for manufacture of polyhydroxyalkanoate polymers containing 4-hydroxyacids
InactiveUS20060084155A1Increase productionHigh activitySugar derivativesBacteriaBiotechnologyDegradation pathway
The gene encoding a 4-hydroxybutyryl-Co A transferase has been isolated from bacteria and integrated into the genome of bacteria also expressing a polyhydroxyalkanoate synthase, to yield an improved production process for 4HB-containing polyhydroxyalkanoates using transgenic organisms, including both bacteria and plants. The new pathways provide means for producing 4HB containing PHAs from cheap carbon sources such as sugars and fatty acids, in high yields, which are stable. Useful strains are obtaining by screening strains having integrated into their genomes a gene encoding a 4HB-CoA transferase and / or PHA synthase, for polymer production. Processes for polymer production use recombinant systems that can utilize cheap substrates. Systems are provided which can utilize amino acid degradation pathways, α-ketoglutarate, or succinate as substrate.
Owner:CJ CHEILJEDANG CORP
A group of glycosyl transferase, and applications thereof
InactiveCN105177100AHigh anticancer activityResolve source issuesTransferasesPlant peptidesTriterpeneTriterpenoid
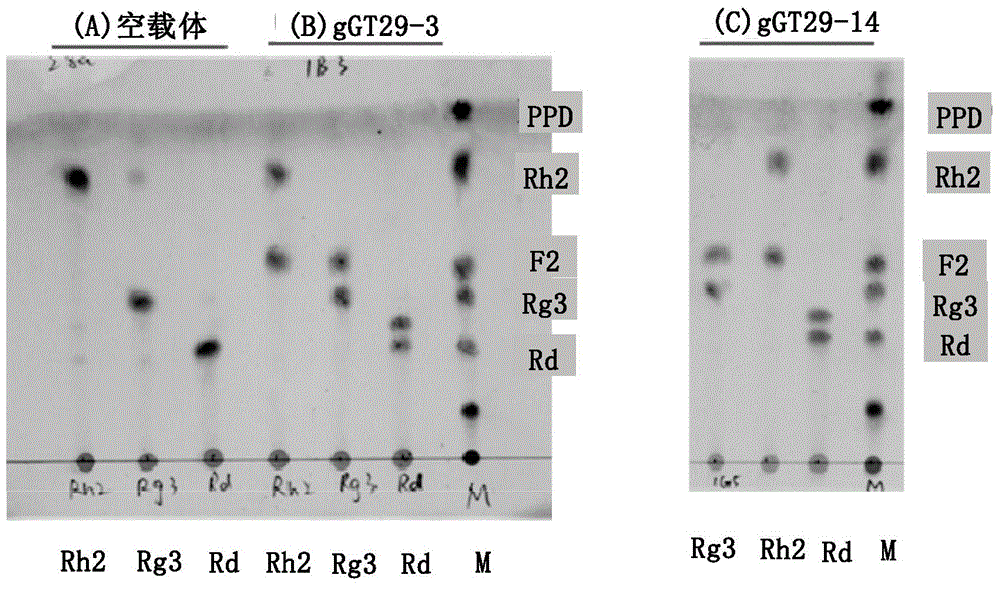
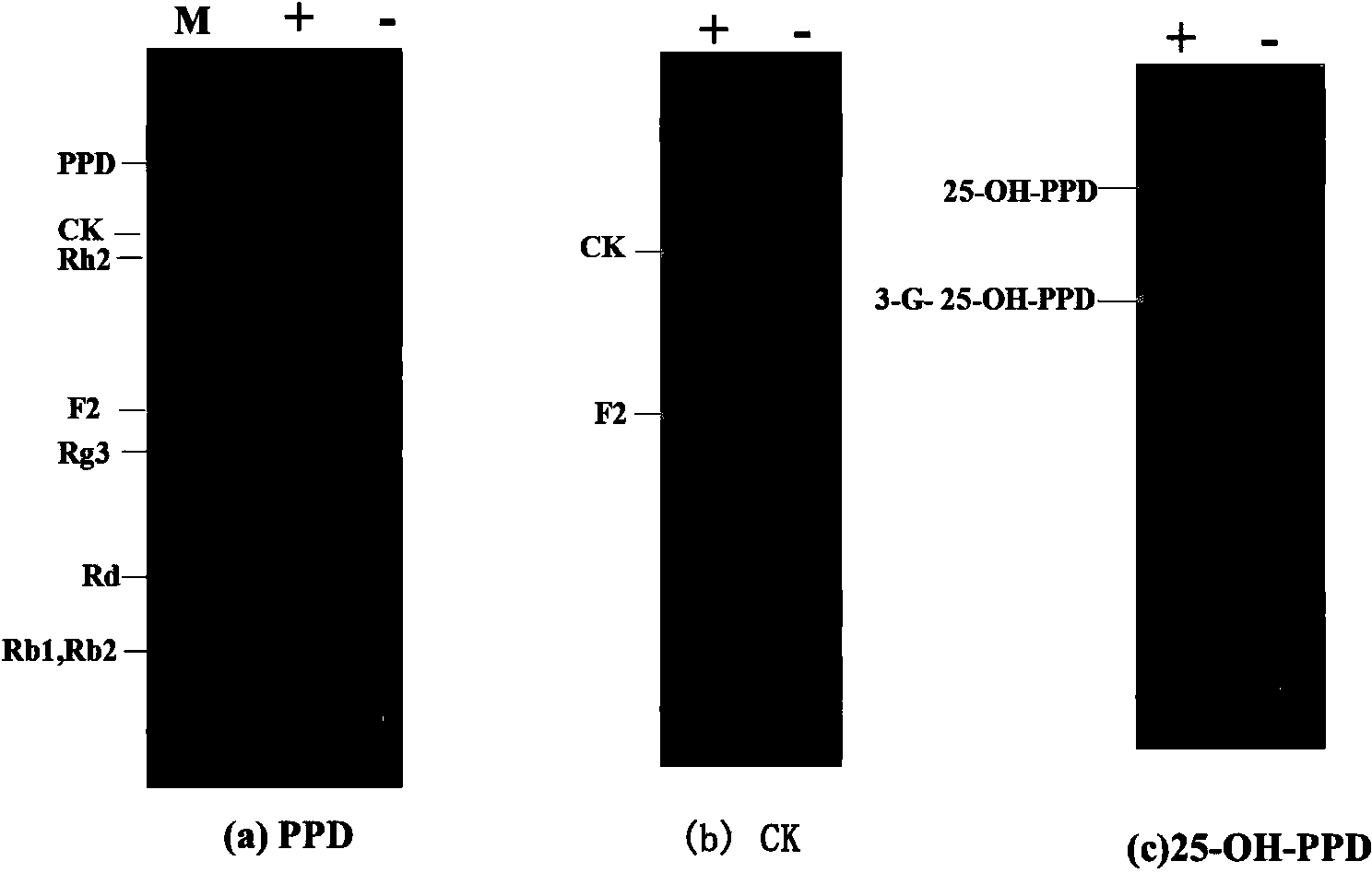
The present invention relates to a group of glycosyl transferase and applications thereof, and specifically provides applications of the glycosyl transferase gGT29-7 and a derived polypeptide thereof in terpene compound glycosylation catalysis and new saponin synthesis, wherein the glycosyl transferase can specifically and efficiently transfer the glycosyl from a glycosyl donor to the first glycosyl on C-3 site and / or C-6 site of a tetracyclic triterpene compound so as to extend the sugar chain. The glycosyl transferase of the present invention can further be used for constructing artificial ly-synthesized rare ginsenosides and a variety of new ginsenosides and derivatives thereof.
Owner:周志华
Glycosyl transferases and applications of glycosyl transferases
ActiveCN104232723ASpecific and efficient transferHas anticancer activityTransferasesFermentationTriterpeneCoa transferase
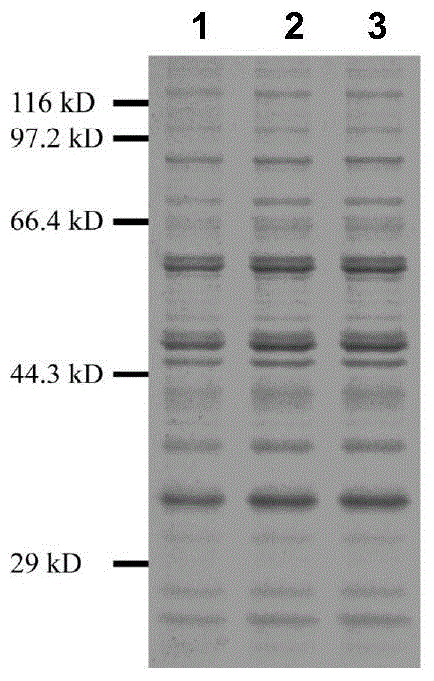
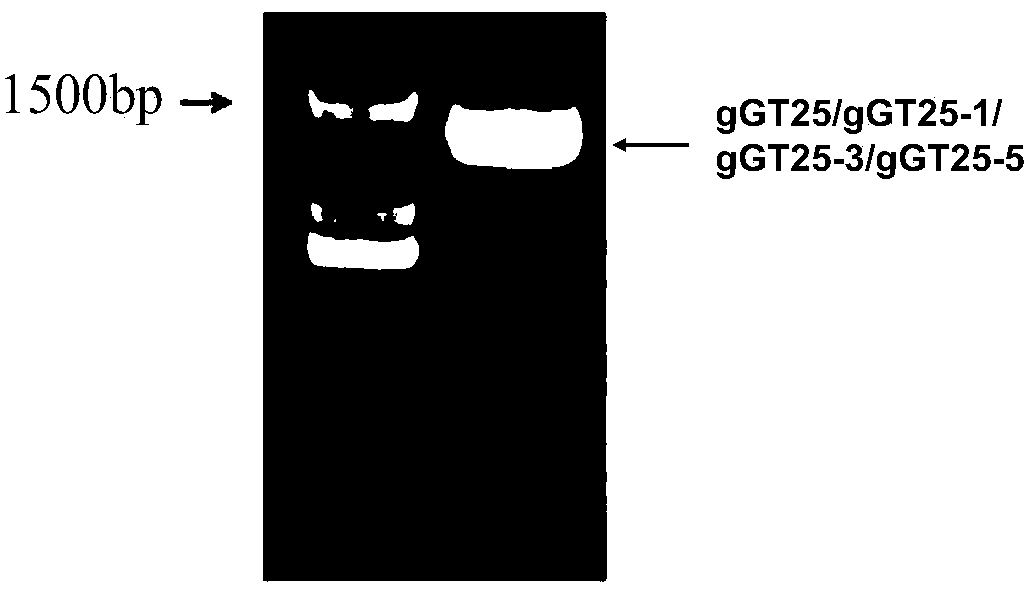
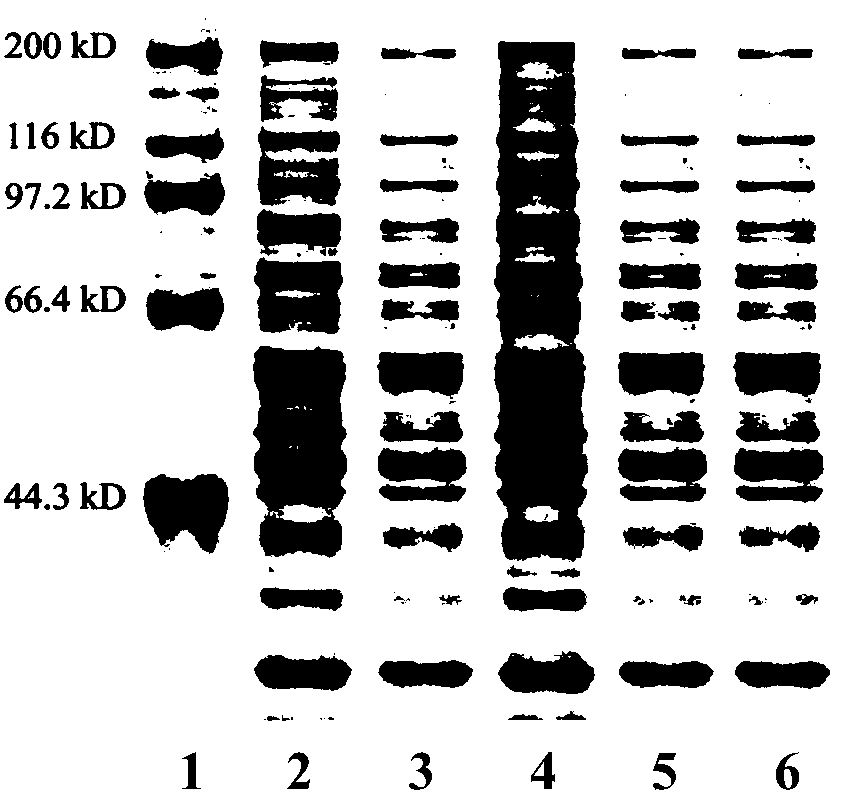
The invention relates to a group of glycosyl transferases and applications of the glycosyl transferases. Particularly, the invention provides applications of glycosyl transferases gGT25, gGT13, gGT30, gGT25-1, gGT25-3, gGT25-5, gGT29, gGT29-3, gGT29-4, gGT29-5, gGT29-6, gGT29-7, 3GT1, 3GT2, 3GT3 and 3GT4, and derived peptides of the glycosyl transferases in glycosylation catalysis and new saponin synthesis of terpenoids. The glycosyl transferases can specifically and efficiently catalyze hydroxyl glycosylation of C-20 bit and / or C-6 bit and / or C-3 of a tetracyclic triterpenoid compound substrate, and / or transfers the glycosyl groups from glycosyl donors to the first glycosyl groups of the C-3 bit and C-6 bit of the tetracyclic triterpenoid compound to extend a carbohydrate chain. The glycosyl transferases disclosed by the invention can also be applied to building synthetic rare ginsenosides and a plurality of novel ginsenosides and derivatives of the ginsenosides.
Owner:SYNBIOTECH (SUZHOU) CO LTD
Glycosyl transferase participating in neoandrographolide biosynthesis as well as coding genes and application thereof
ActiveCN108728422AHelps in parsingAntibody mimetics/scaffoldsTransferasesBULK ACTIVE INGREDIENTPlant growth
The invention discloses glycosyl transferase participating in neoandrographolide biosynthesis as well as coding genes and application thereof. The protein provided by the invention is derived from herba andrographitis, and is (a1) or (a5) as follows: (a1) a protein composed of an amino acid sequence shown as a sequence 1 in a sequence table; and (a5) a protein composed of an amino acid sequence shown as a sequence 3 in the sequence table. The invention further discloses application of the protein serving as glycosyl transferase. The glycosyl transferase participates in plant growth and development and secondary metabolism processes. The discovery of the glycosyl transferase and coding genes thereof has important significance. The invention provides a basis for further describing biosynthesis of glycoside compounds in the herba andrographitis, contributes to analyzing a biosynthetic pathway of glycoside active ingredients in the herba andrographitis, and provides novel material basis and gene resources for new drug research and development.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
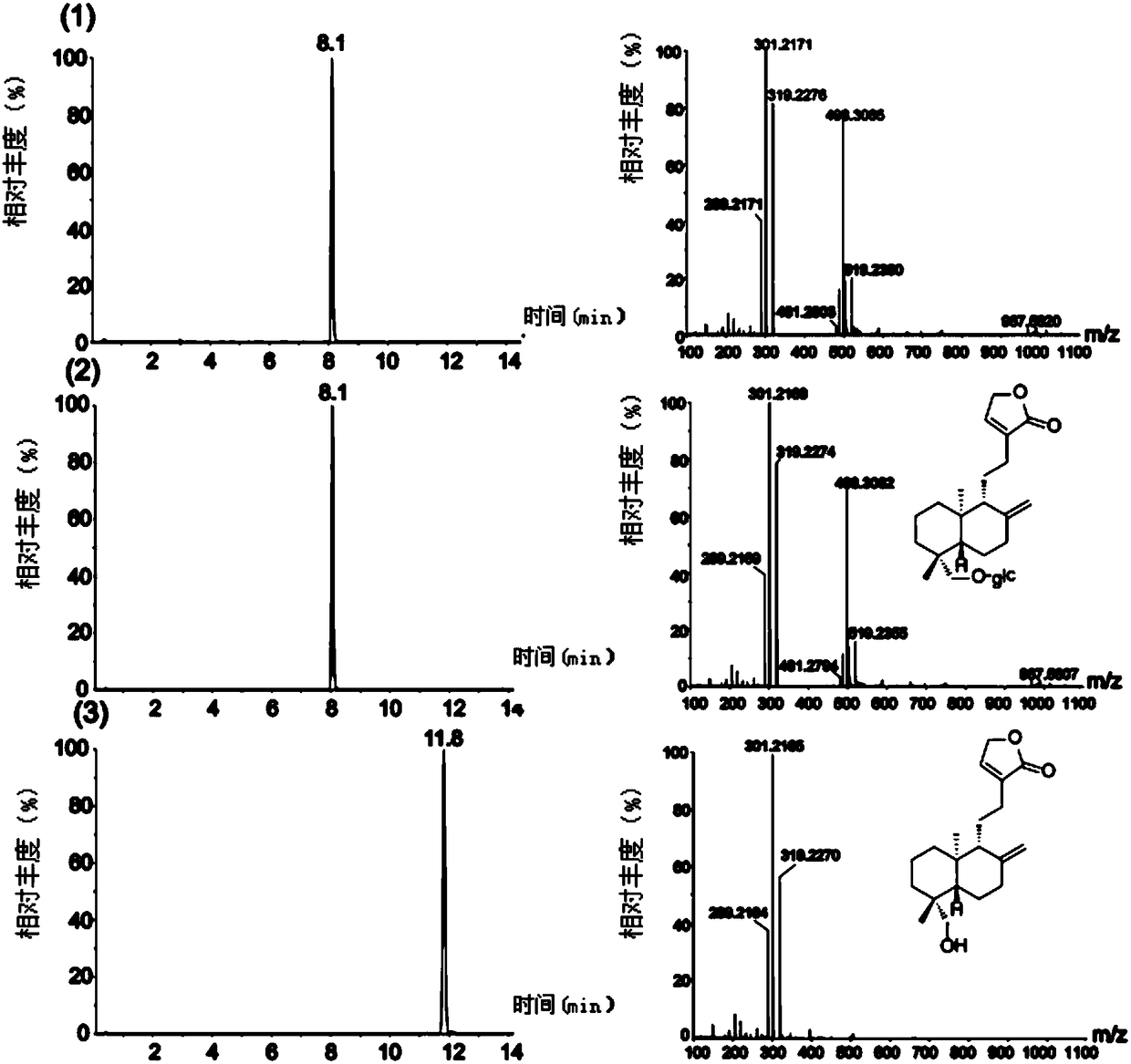
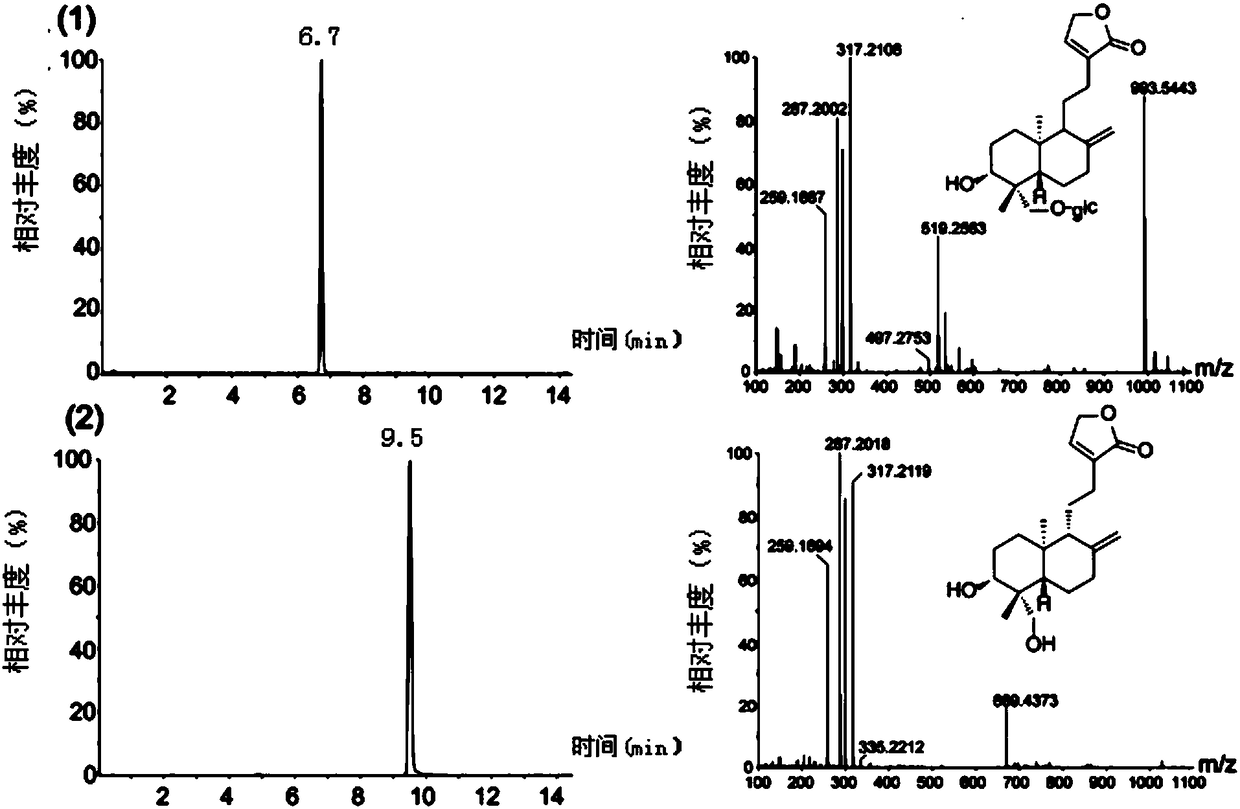
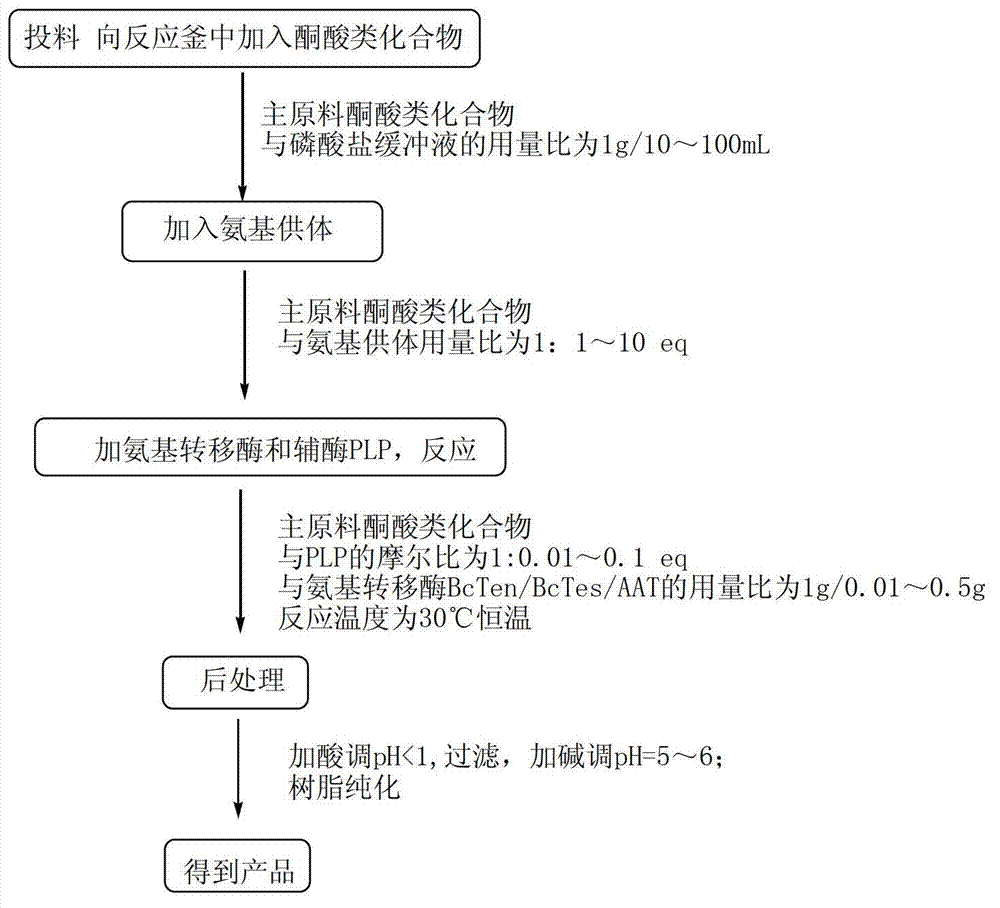
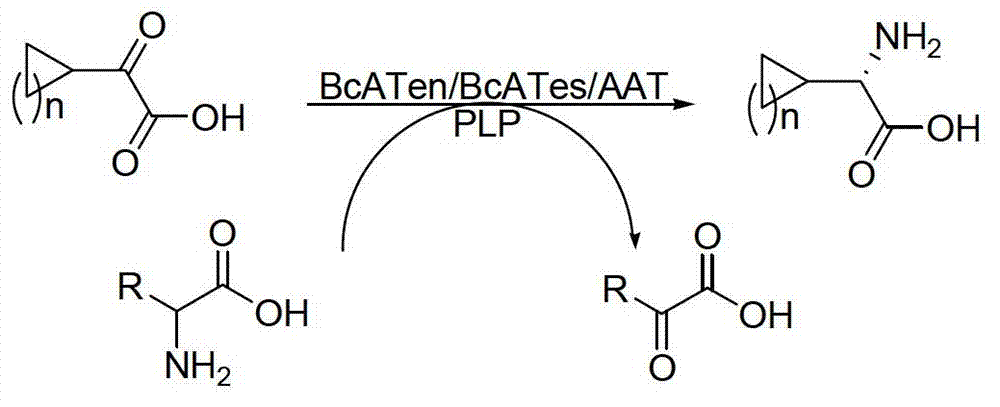
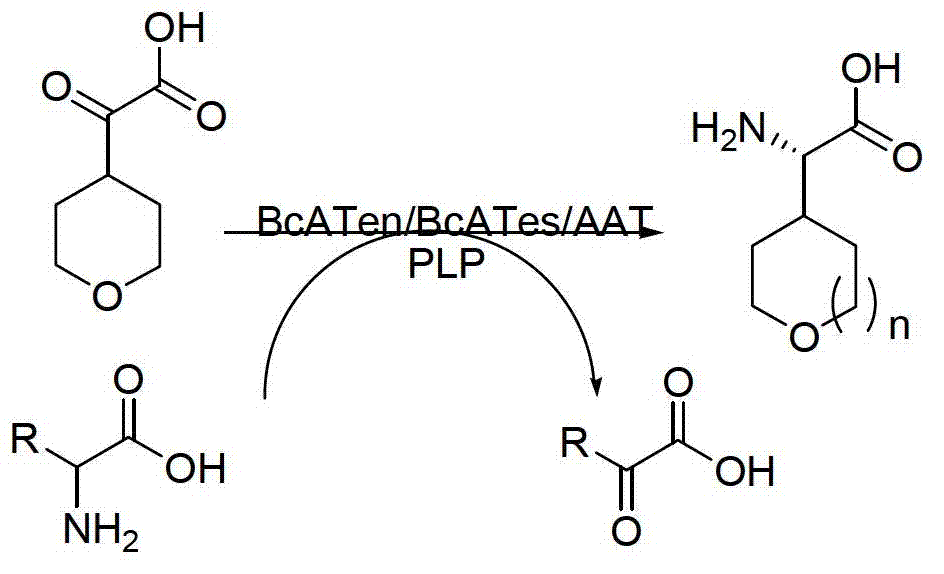
Method for synthetizing chiral cyclic alkyl amino acid by amino transferase
The invention discloses a method for synthetizing chiral cyclic alkyl amino acid by amino transferase. The commercialized material ketonic acid or corresponding soluble ketonic acid salt compound in the market is selected as an initial material; the initial material is dissolved into phosphate buffer, and added to an amino supply body; pyridoxal phosphate (PLP) and amino transferase main enzyme are added to a system containing the amino supply body and main material ketonic acid or corresponding soluble ketonic acid salt compound to react under constant temperature, and obtaining a product with a high ee value, wherein n is equal to 1, 2, 3, 4, 5, or obtaining a product wherein n is equal to 0 and 1. The method is stable in technological condition, simple to operate, high in yield, low in cost, and suitable for large-scale production, and beneficial for environmental protection; and a novel train of thought and a method are provided for the preparation of chiral cyclic alkyl amino acid compound.
Owner:ASYMCHEM LAB TIANJIN +5
Green process and compositions for producing poly(5HV) and 5 carbon chemicals
Recombinant hosts for producing polyhydroxyalkanoates and methods of producing polyhydroxyalkanoates from renewable carbon substrates are provided. Certain recombinant hosts that produce 5 carbon chemicals such as 5-amino?entanoate (5AP), 5 -hydroxy valerate (5HV), glutarate, and 1,5 pentanediol (PDO) are also provided. One embodiment provides a recombinant host expressing a gene encoding a heterologous enzyme selected from the group consisting of a polyhydroxyalkanoate synthase and a 5-hydroxyvalerate-CoA (5HV-CoA) transferase, wherein the host produces a polymer containing 5-hydroxyvalerate. Preferably, the host expresses both a polyhydroxyalkanoate synthase and a 5HV-CoA transferase. The host can be prokaryotic or eukaryotic. A preferred prokaryotic host is E. coli. The polymers produced by the recombinant hosts can be homopolymers or copolymers of 5-hydroxyvalerate.; A preferred copolymer is poly(3-hydroxybutyrate-co-5-hydroxyvalerate).
Owner:CJ CHEILJEDANG CORP
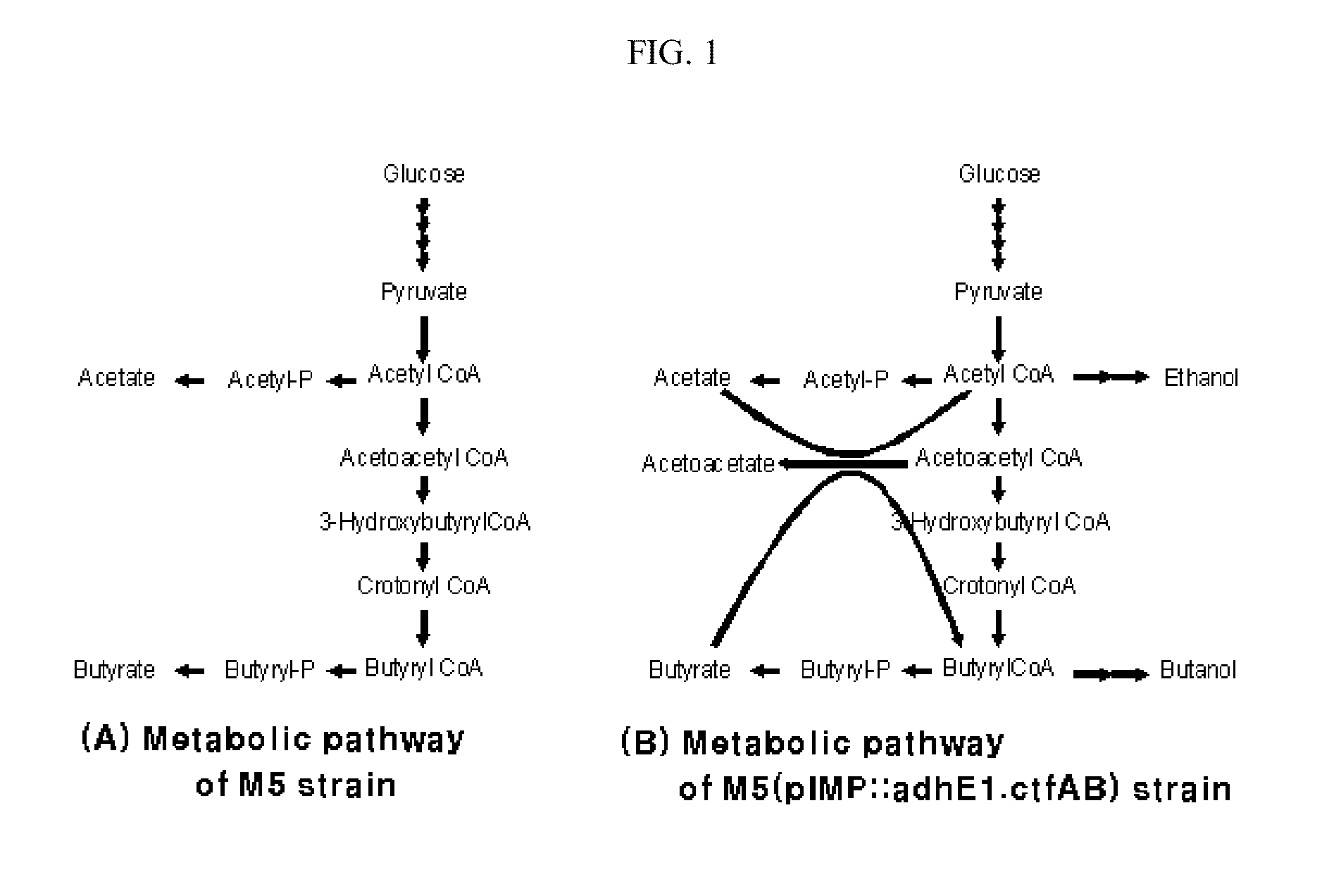
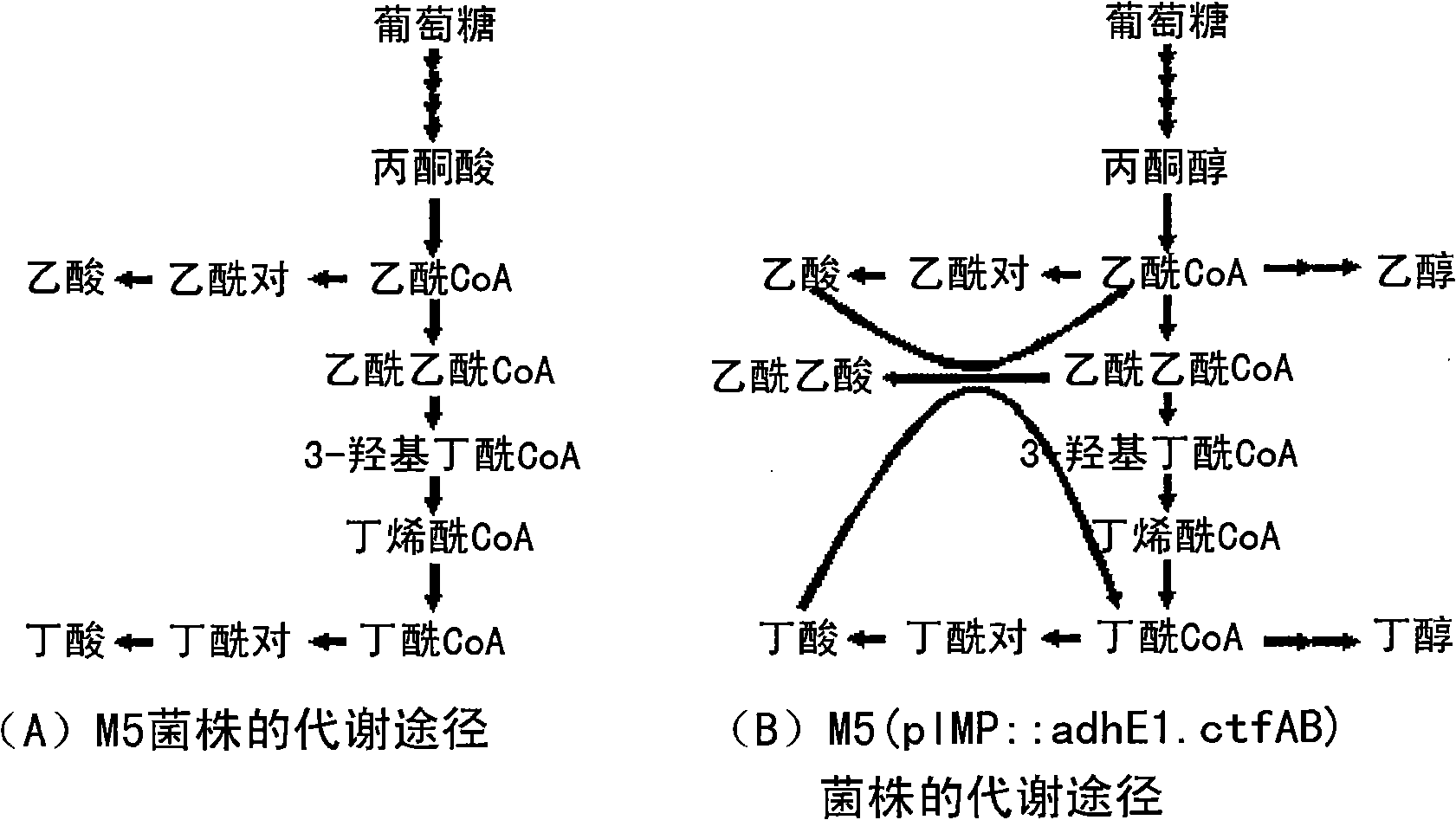
Enhanced ethanol and butanol producing microorganisms and method for preparing ethanol and butanol using the same
InactiveUS20110027845A1Improve efficiencyEnhanced ability to produce ethanolBacteriaTransferasesMicroorganismAlcohol
The present invention relates to a recombinant microorganism having an enhanced ability to produce ethanol and butanol and a method for preparing ethanol and butanol using the same, and more particularly to a recombinant microorganism having an enhanced ability to produce ethanol and butanol, into which a gene encoding CoA transferase and a gene encoding alcohol / aldehyde dehydrogenase are introduced, and to a method for preparing ethanol and butanol using the same. The recombinant microorganism according to the present invention, obtained by manipulating metabolic pathways of microorganisms, is capable of producing butanol and ethanol exclusively without producing any byproduct, and thus is useful as a microorganism producing industrial solvents and transportation fuel.
Owner:KOREA ADVANCED INST OF SCI & TECH +2
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
The invention provides a non-naturally occurring microbial organism having a muconate pathway having at least one exogenous nucleic acid encoding a muconate pathway enzyme expressed in a sufficient amount to produce muconate. The muconate pathway including an enzyme selected from the group consisting of a beta-ketothiolase, a beta-ketoadipyl-CoA hydrolase, a beta-ketoadipyl-CoA transferase, a beta-ketoadipyl-CoA ligase, a 2-fumarylacetate reductase, a 2-fumarylacetate dehydrogenase, a trans-3-hydroxy-4-hexendioate dehydratase, a 2-fumarylacetate aminotransferase, a 2-fumarylacetate aminating oxidoreductase, a trans-3-amino-4-hexenoate deaminase, a beta-ketoadipate enol-lactone hydrolase, a muconolactone isomerase, a muconate cycloisomerase, a beta-ketoadipyl-CoA dehydrogenase, a 3-hydroxyadipyl-CoA dehydratase, a 2,3-dehydroadipyl-CoA transferase, a 2,3-dehydroadipyl-CoA hydrolase, a 2,3-dehydroadipyl-CoA ligase, a muconate reductase, a 2-maleylacetate reductase, a 2-maleylacetate dehydrogenase, a cis-3-hydroxy-4-hexendioate dehydratase, a 2-maleylacetate aminoatransferase, a 2-maleylacetate aminating oxidoreductase, a cis-3-amino-4-hexendioate deaminase, and a muconate cis / trans isomerase. Other muconate pathway enzymes also are provided. Additionally provided are methods of producing muconate.
Owner:GENOMATICA INC
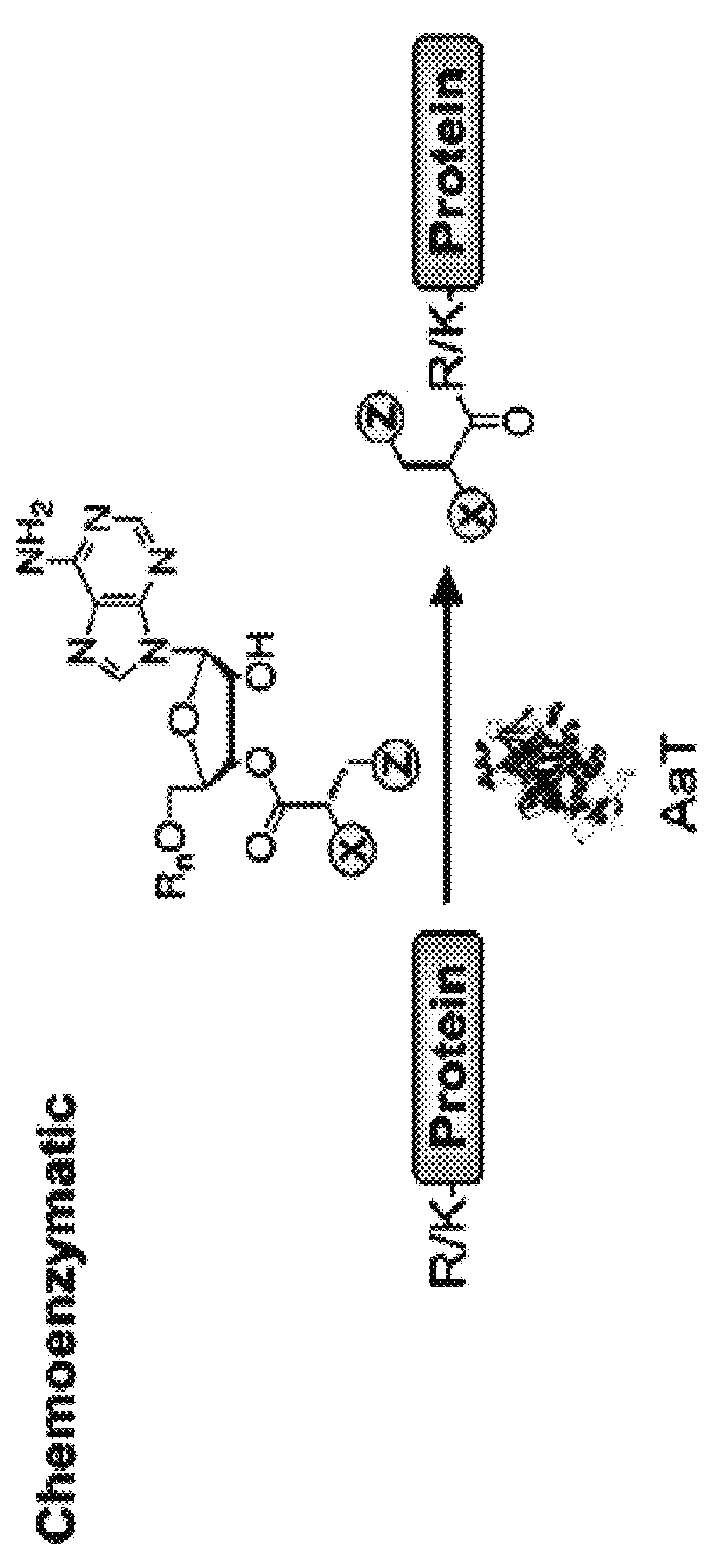
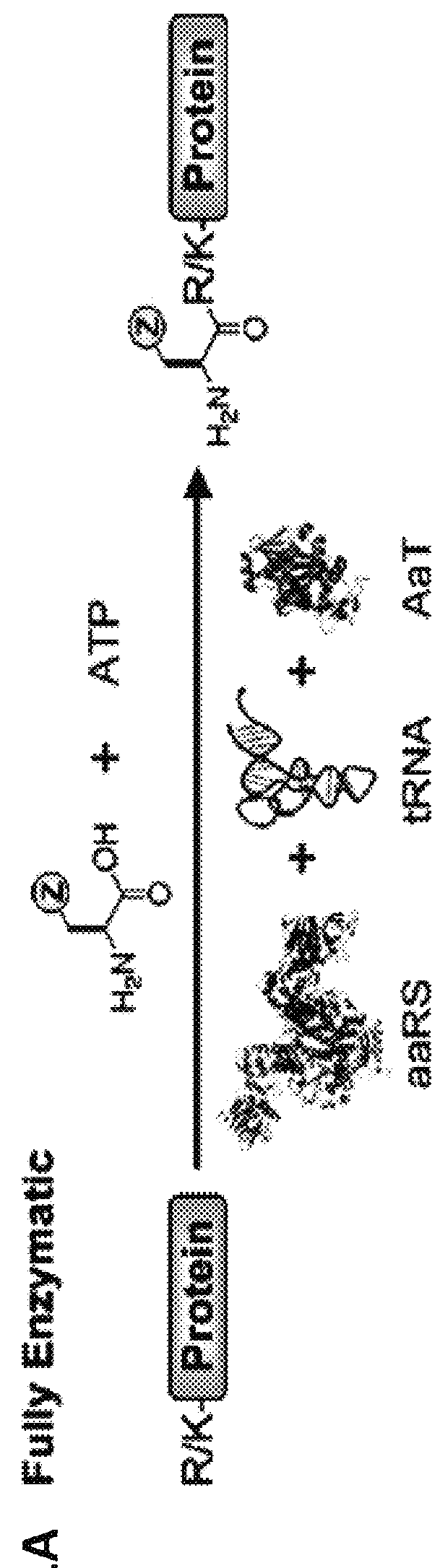
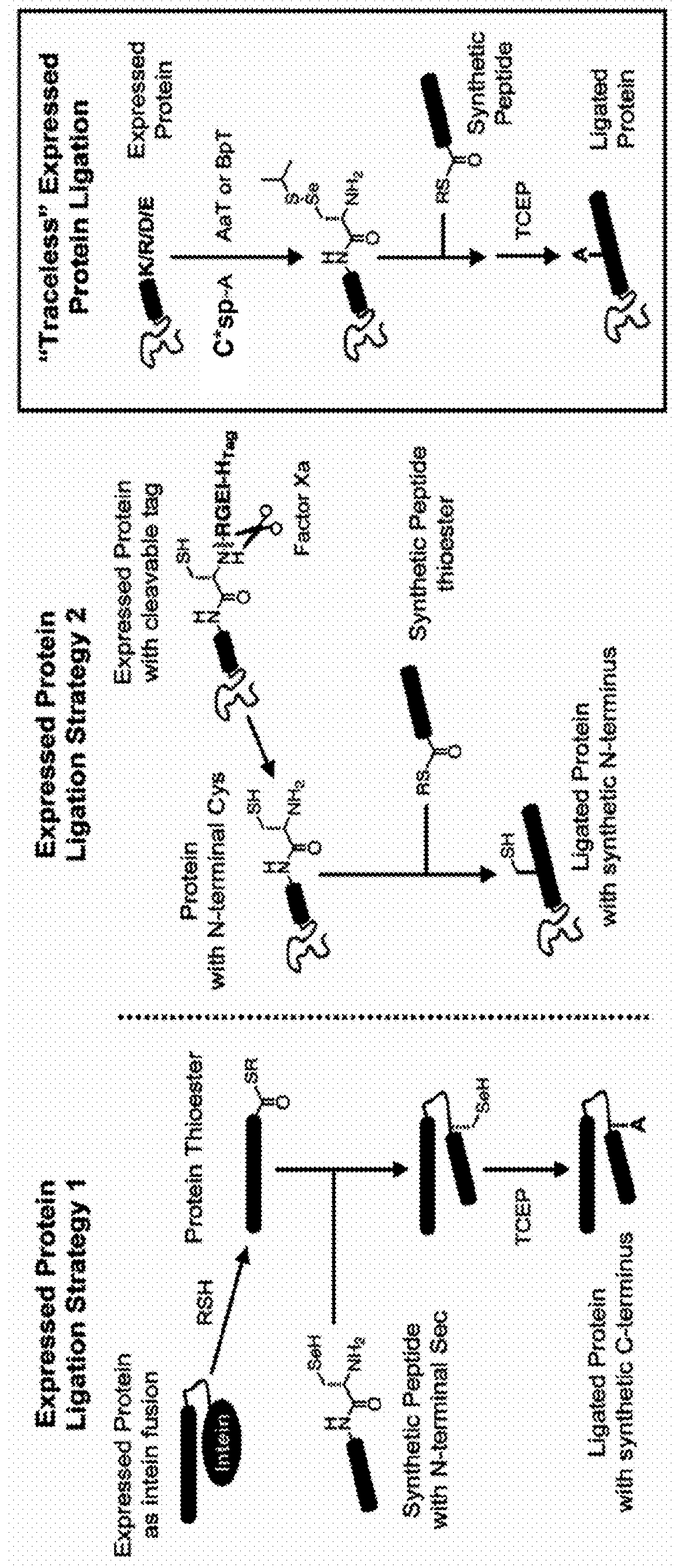
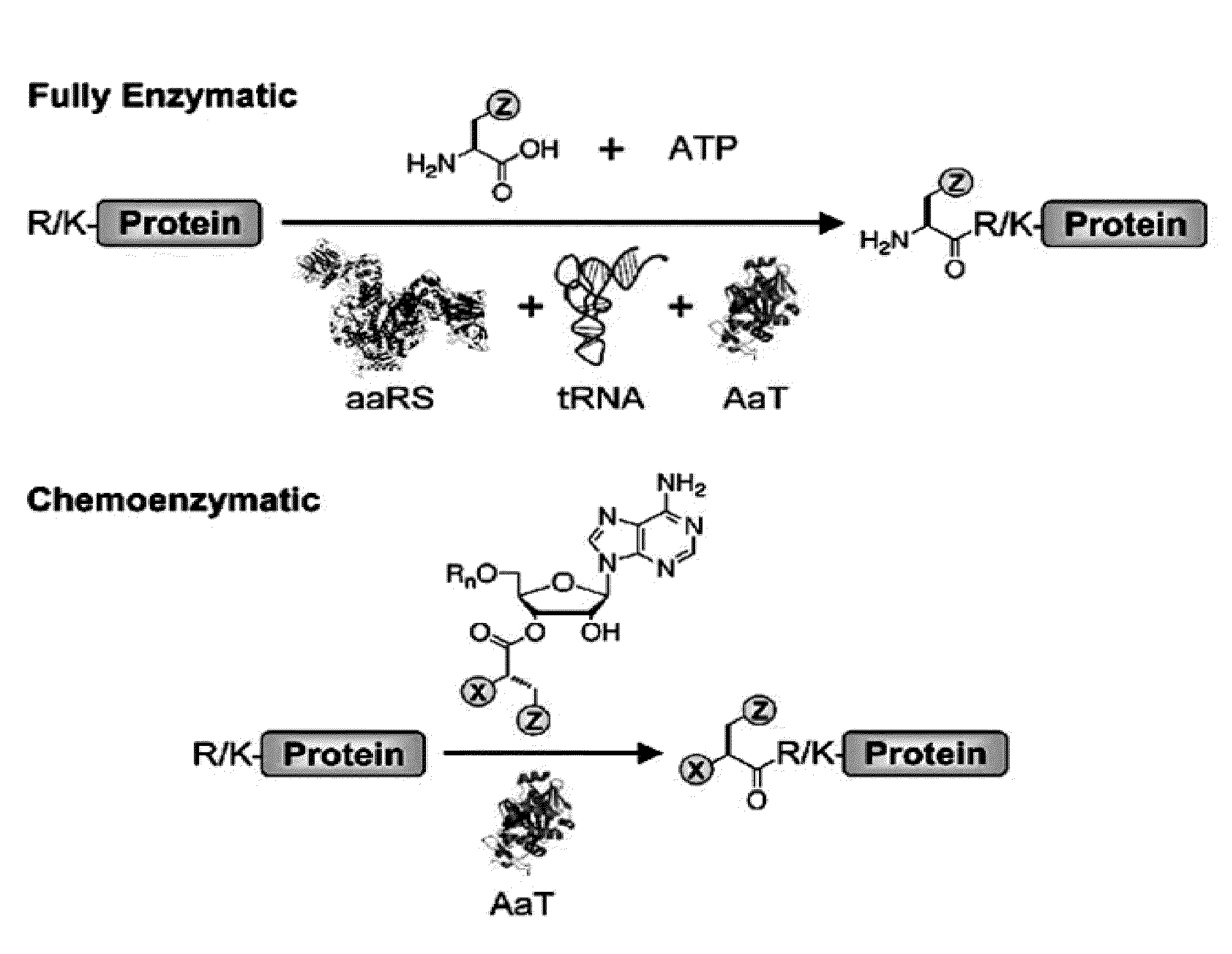
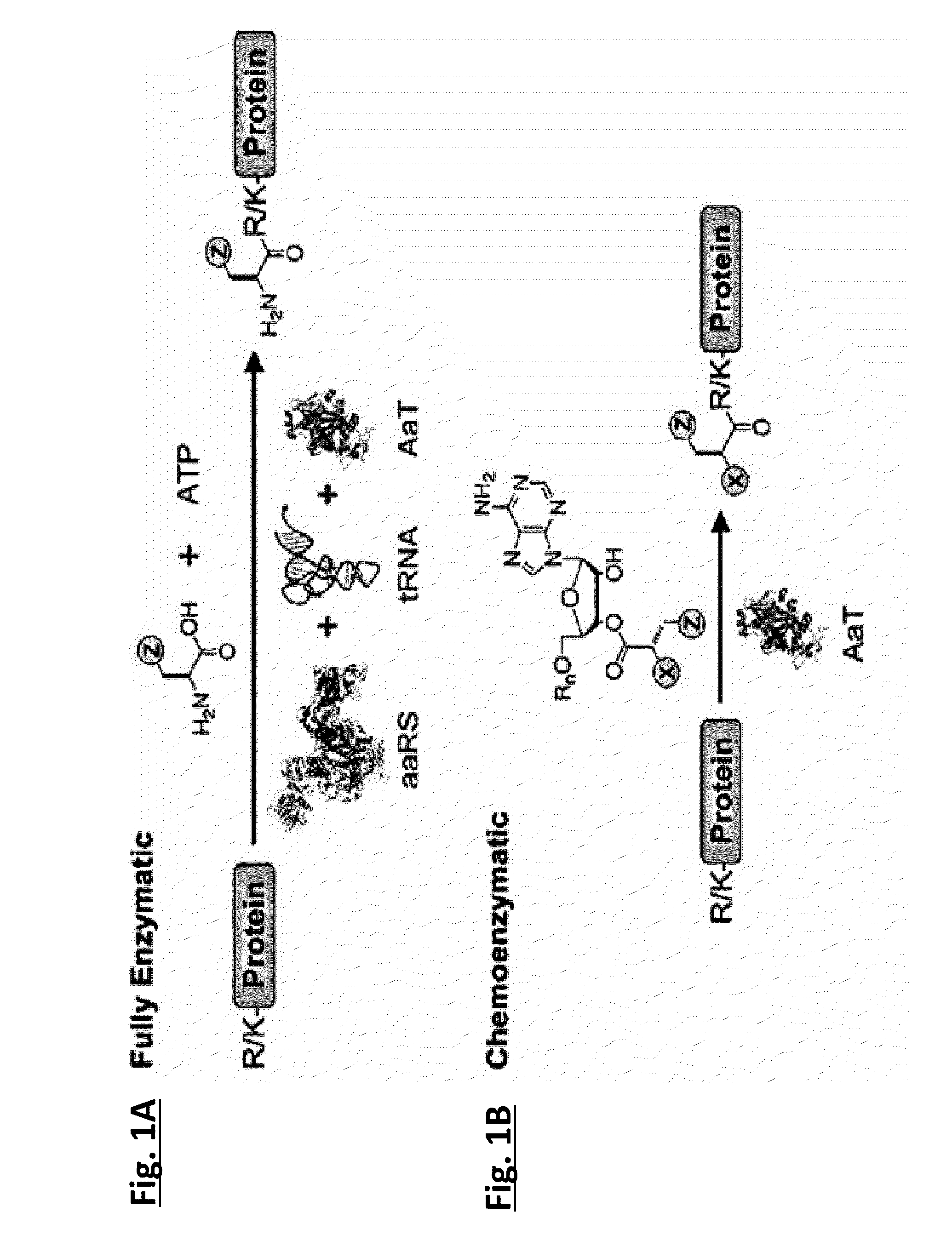
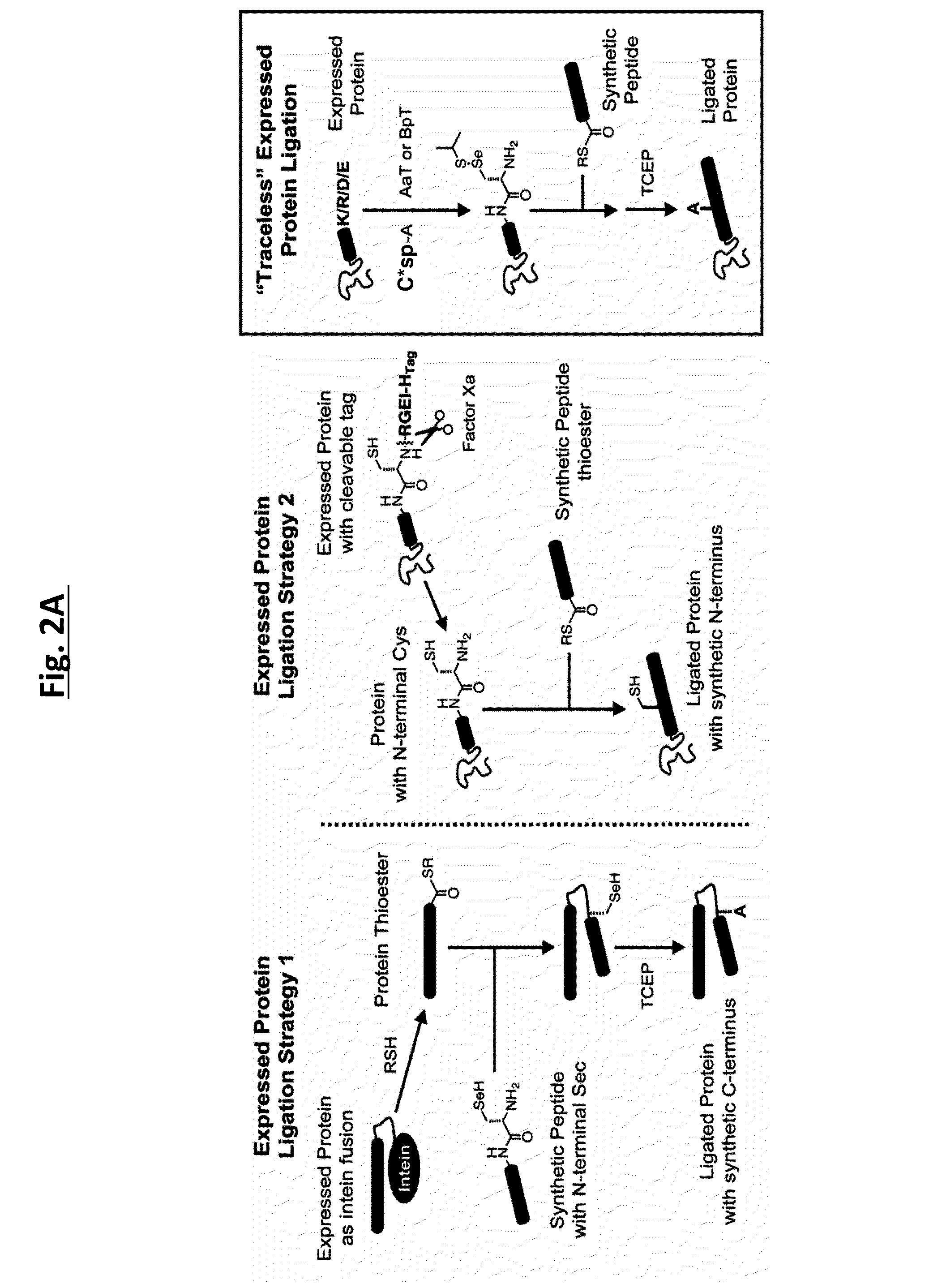
Methods of modifying N-termini of a peptide or protein using transferases
ActiveUS9376700B2Peptide/protein ingredientsPeptide preparation methodsAminoacyl-tRNAADAMTS Proteins
The invention includes a selective method of modifying the N-terminus of a protein using an aminoacyl tRNA transferase. In certain embodiments, the method comprises contacting a solution of the protein or peptide with a transferase and a derivative of a molecule, whereby the N-terminus of the protein or peptide is derivatized with the molecule.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
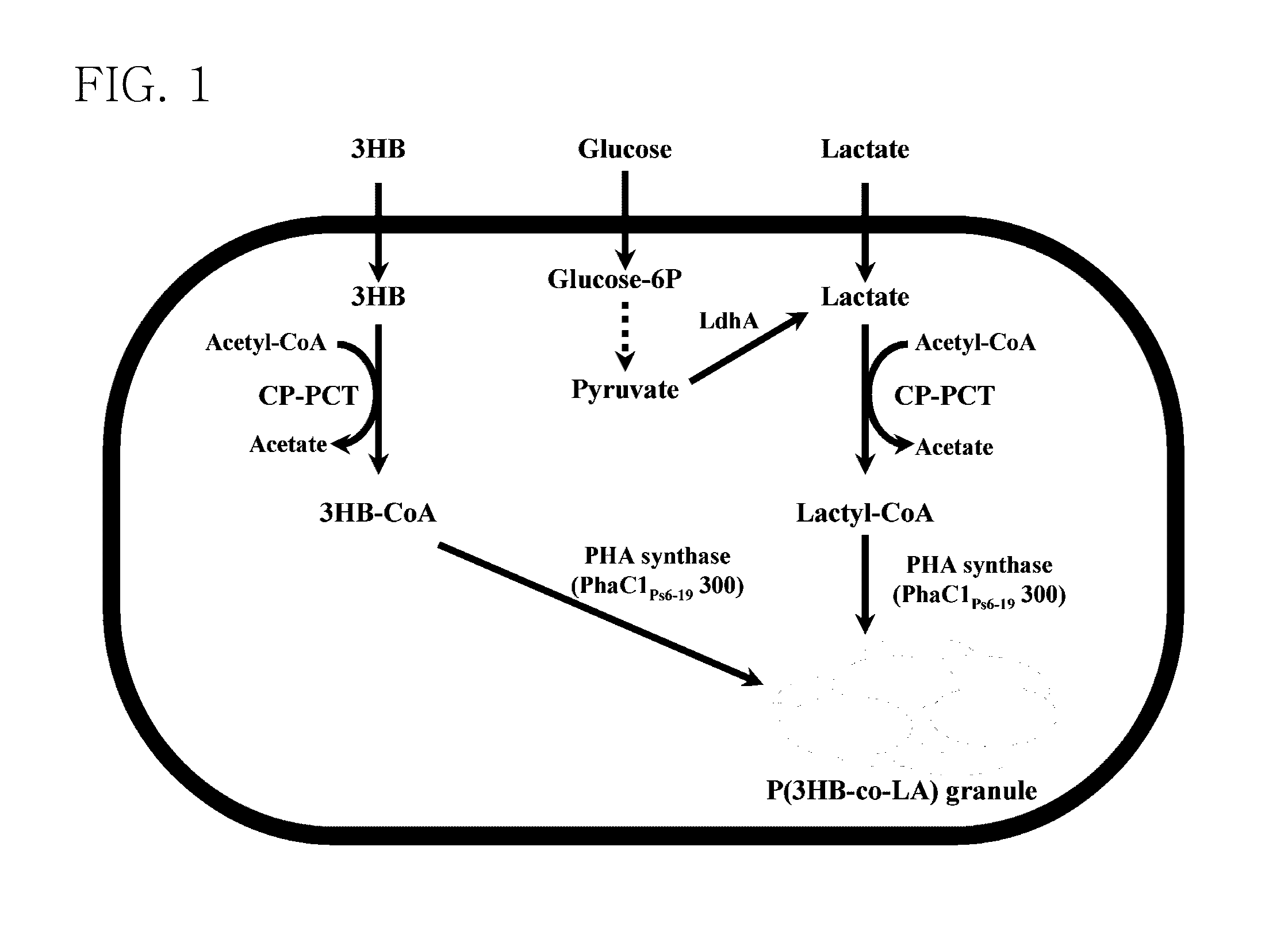
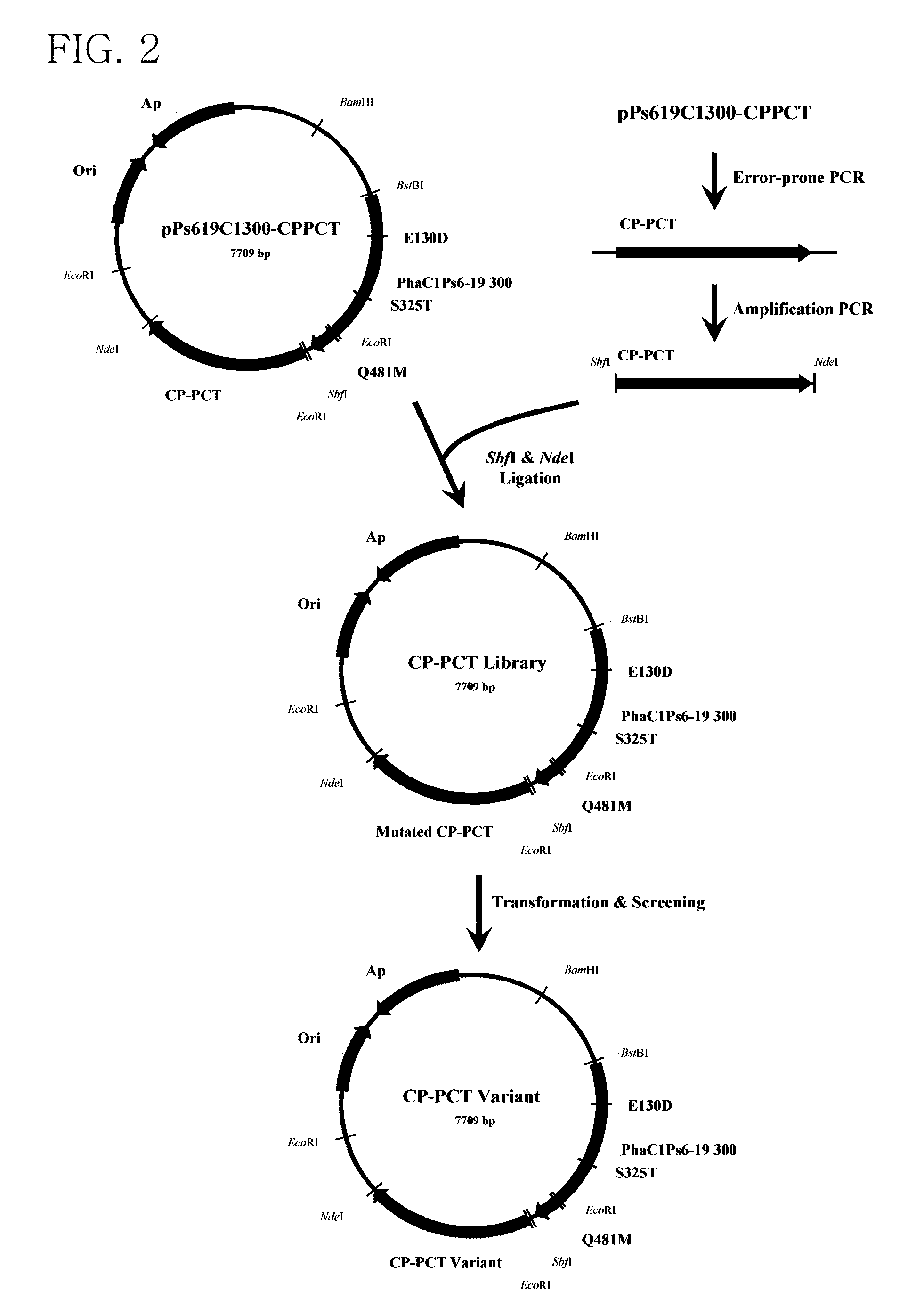
Mutant of propionyl-CoA transferase from Clostridium propionicum and preparing method for PLA or PLA copolymer using the same
ActiveUS8524478B2Increase supplyEfficient supplySugar derivativesProtozoaEscherichia coliMicroorganism
Provided is a mutant of propionyl-CoA transferase from Clostridium propionicum that can convert lactate into lactyl-CoA with high efficiency in a method of preparing a polylactate (PLA) or PLA copolymer using microorganisms. Unlike conventional propionyl-CoA transferase which is weakly expressed in E. coli, when a mutant of propiony-CoA transferase from Clostridium propionicum is introduced into recombinant E. coli, lactyl-CoA can be supplied very smoothly, thereby enabling highly efficient preparation of polylactate (PLA) and PLA copolymer.
Owner:LG CHEM LTD
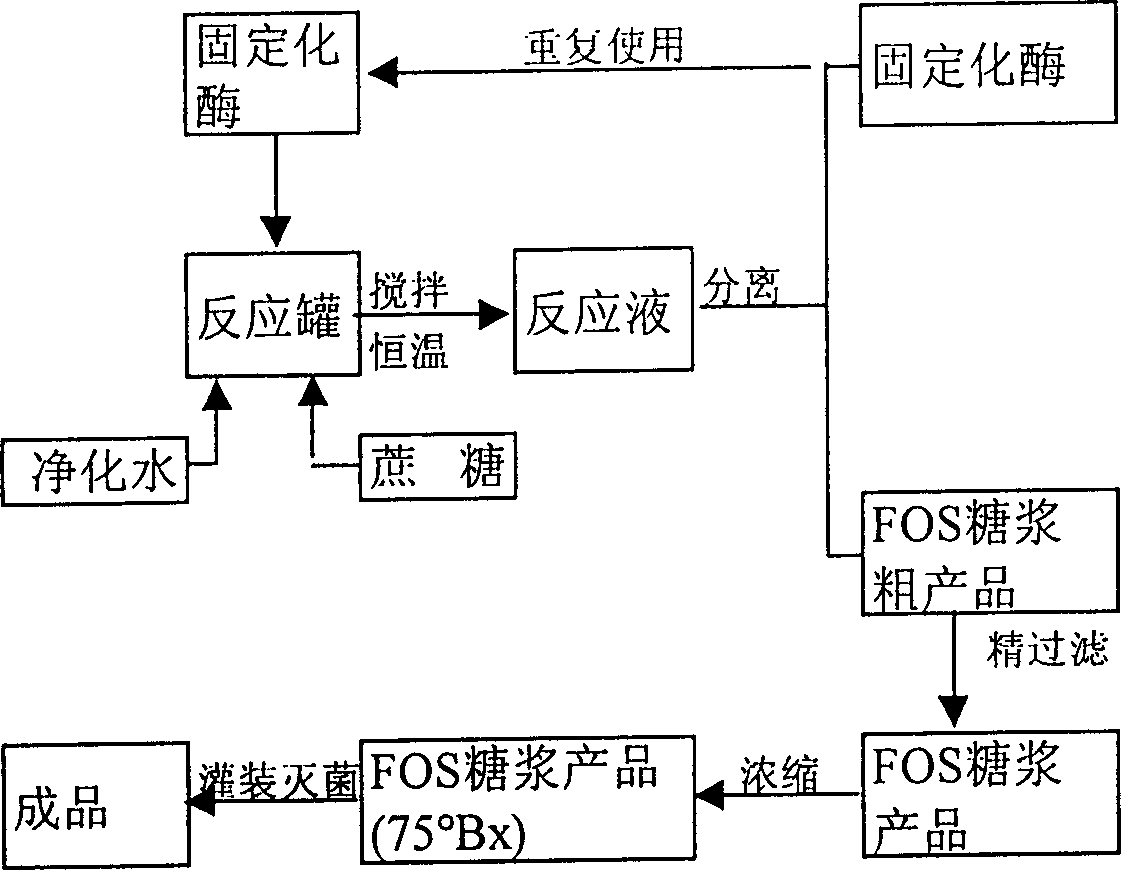
Production process of cane-fruit oligosaccharide with immobilized fructose-base transferase
The industrial production process of fruit oligosaccharide with cane sugar as material includes two parts, the industrial production of immobilized fructose-base transferase preparation and the production of fruit oligosaccharide by means of the enzyme preparation. Selected excellent strain capable of secreting fructose-base transferase is cultured in proper culture medium, the obtained mycelium is wall broken, and the enzyme is separated and purified through centrifugal process and superfiltering process and reagent immobilized. Fruit oligosaccharide is then produced through batched reaction process or column reaction process with the immobilized fructose-base transferase. The fruit oligosaccharide may be used to produce fruit oligosaccharide syrup without passing through active carbon decoloring and exchange column desalting.
Owner:GUANGXI UNIV +1
Enhanced ethanol and butanol producing microorganisms and method for preparing ethanol and butanol using the same
The present invention relates to a recombinant microorganism having an enhanced ability to produce ethanol and butanol and a method for preparing ethanol and butanol using the same, and more particularly to a recombinant microorganism having an enhanced ability to produce ethanol and butanol, into which a gene encoding CoA transferase and a gene encoding alcohol / aldehyde dehydrogenase are introduced, and to a method for preparing ethanol and butanol using the same. The recombinant microorganism according to the present invention, obtained by manipulating metabolic pathways of microorganisms, is capable of producing butanol and ethanol exclusively without producing any byproduct, and thus is useful as a microorganism producing industrial solvents and transportation fuel.
Owner:GS CALTEX CORP
Organisms for the production of cyclohexanone
Owner:GENOMATICA INC
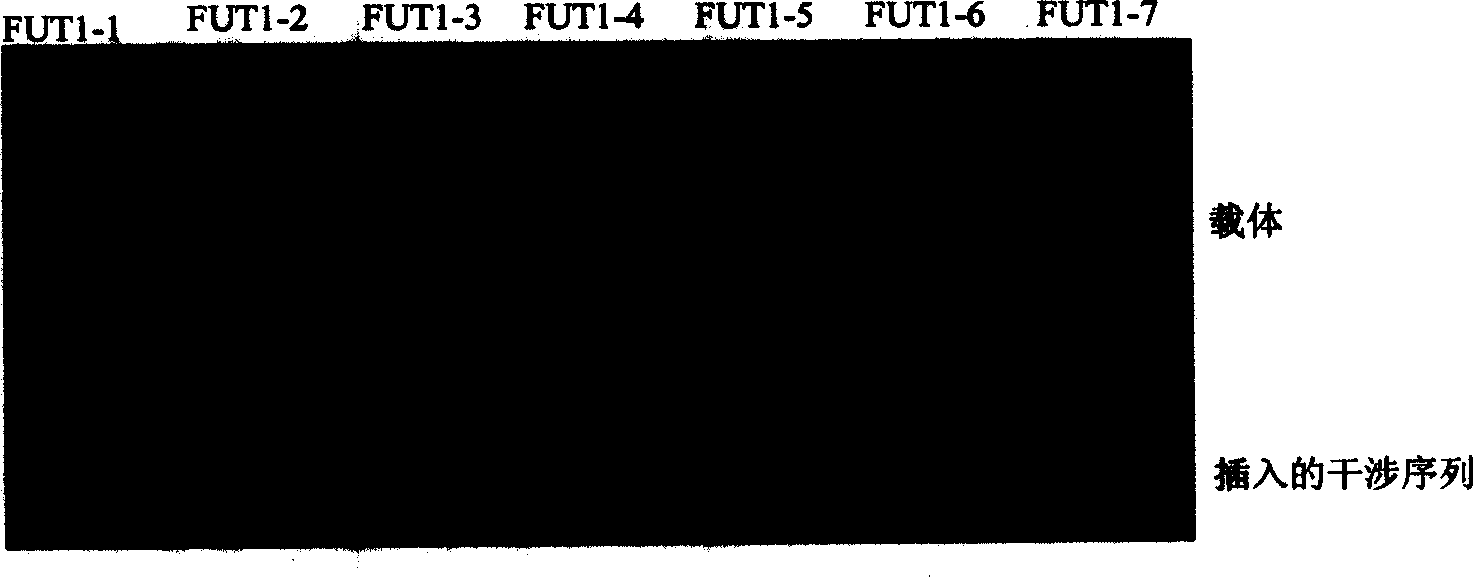
RNA interference sequence and recombinant interference plasmid of fucosyl transferase I and IV for suppressing synthesis of LeY carbohydrate antigen
InactiveCN101225383AShort treatment cycleGood effectOrganic active ingredientsSugar derivativesFucosyltransferase IVAntigen
The invention relates to a RNA interference sequence and a restructuring interference plasmid of the fucosyltransferase I and IV used for inhibiting the synthesis of LeY saccharide antigen, which mainly comprises: the DNA sequence of seven FUT1 / FUT4 SiRNA templates of fucosyltransferase I and seven FUT1 / FUT4 SiRNA templates of fucosyltransferase IV are screened, seven FUT1 and seven FUT4 restructuring interference plasmid are respectively constructed on the base of polyclonal specific enzyme cutting sites of the RNA interference carried (pSilencer-4.1-CMV neo), and the interference plasmid is used as antitumor drugs. The RNA interference sequence and a restructuring interference plasmid of the fucosyltransferase I and IV used for inhibiting the synthesis of LeY saccharide antigen has the advantages of obvious function of inhibiting tumor growing, high efficiency, high safety and economic tumor gene therapeutic mode.
Owner:燕秋
Methods, reagents and cells for biosynthesizing compounds
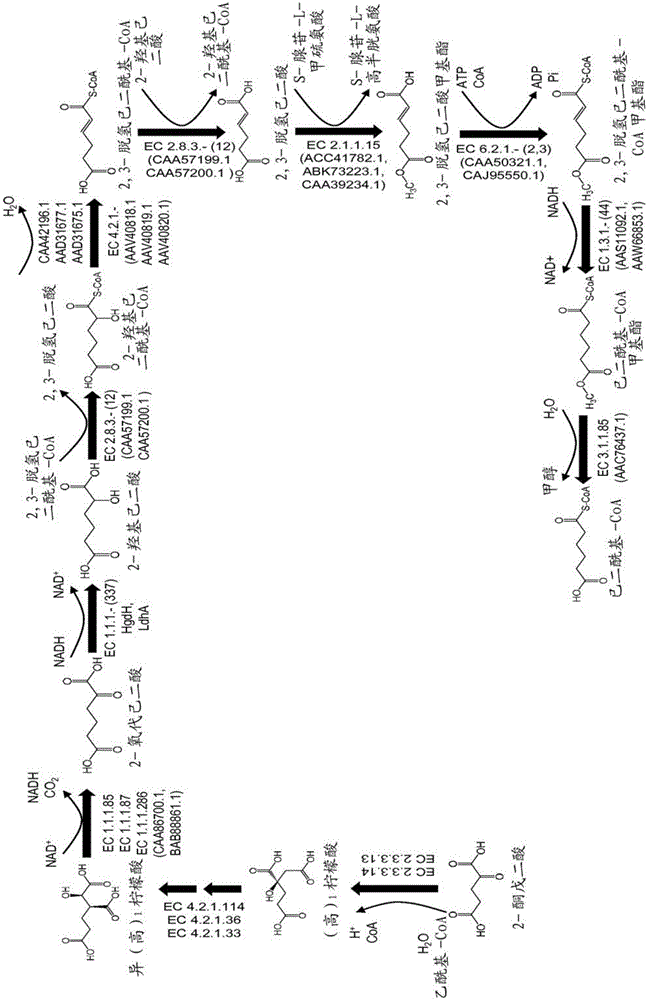
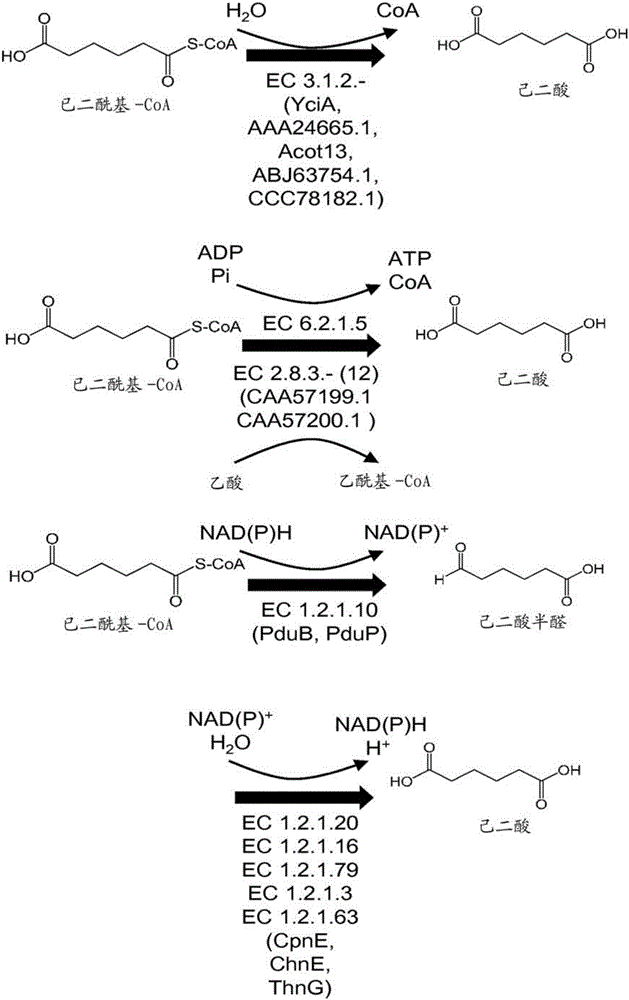
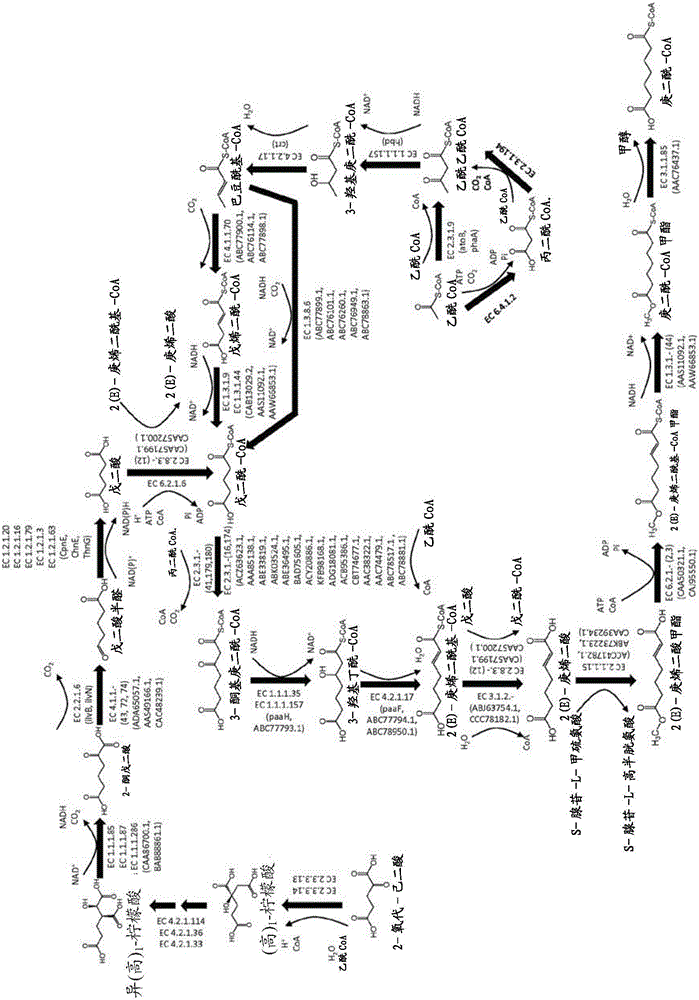
This document describes biochemical pathways for producing 2,3-dehydroadipyl-CoA methyl ester from precursors such as 2-oxoglutarate using one or more of a fatty acid O-methyltransferase, a thioesterase, a CoA-transferase and a CoA ligase, as well as recombinant hosts expressing one or more of such enzymes. 2,3-dehydroadipyl-CoA methyl ester can be enzymatically converted to adipyl-CoA using a trans-2-enoyl-CoA reductase, and a methylesterase, which in turn can be enzymatically converted to adipic acid, 6-aminohexanoate, 6-hydroxyhexanoate, caprolactam, hexamethylenediamine, or 1,6-hexanediol.
Owner:INVISTA TEXTILES (U K) LTD
Emulsifier prepared using a glycosyl transferase
ActiveUS8178323B2Improve emulsion stabilityCosmetic preparationsNon-fibrous pulp additionEsterification reactionCoa transferase
The invention relates to an emulsifier, a method for preparing said emulsifier, and to its use in various applications, primarily food and cosmetic applications. The invention also relates to the use of said emulsifier for the creation of an elastic, gelled foam. An emulsifier according to the invention is based on a starch which is enzymatically converted, using a specific type of enzyme, and modified in a specific esterification reaction.
Owner:COOP AVEBE U A
Green process and compositions for producing poly(5HV) and 5 carbon chemicals
Owner:CJ CHEILJEDANG CORP
Composite stabilizing agent and enzyme activity determination method of NMN transferase with composite stabilizing agent
InactiveCN106085996AImprove stabilitySimple methodMicrobiological testing/measurementBiological material analysisWater bathsTransferase
The invention provides a composite stabilizing agent and an enzyme activity determination method of an NMN transferase with the composite stabilizing agent. The method comprises 1, preparing a crude enzyme liquid, 2, carrying out split charging on the crude enzyme liquid through multiple centrifuge tubes, adding a protective agent into one part of the centrifuge tubes and adding double distilled water into the other part of the centrifuge tubes, 3, adding a buffer solution, a substrate and metal ions into the two parts of the centrifuge tubes, uniformly mixing the materials, putting the centrifuge tubes with the mixtures into a water bath kettle, carrying out a reaction process, adding EDTA into the reaction systems, carrying out a reaction process, carrying out centrifugation, removing precipitates and collecting the supernatant, 4, taking the supernatant and determining a light absorption value of the reaction product and 5, determining relative enzyme activity through a control group which is a crude enzyme liquid without the protective agent. The composite stabilizing agent comprises common simple ingredients, has a low cost, can effectively improve NMN transferase stability and has a wide application prospect. The enzyme activity determination method of the NMN transferase is simple and practical and has visual effects.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
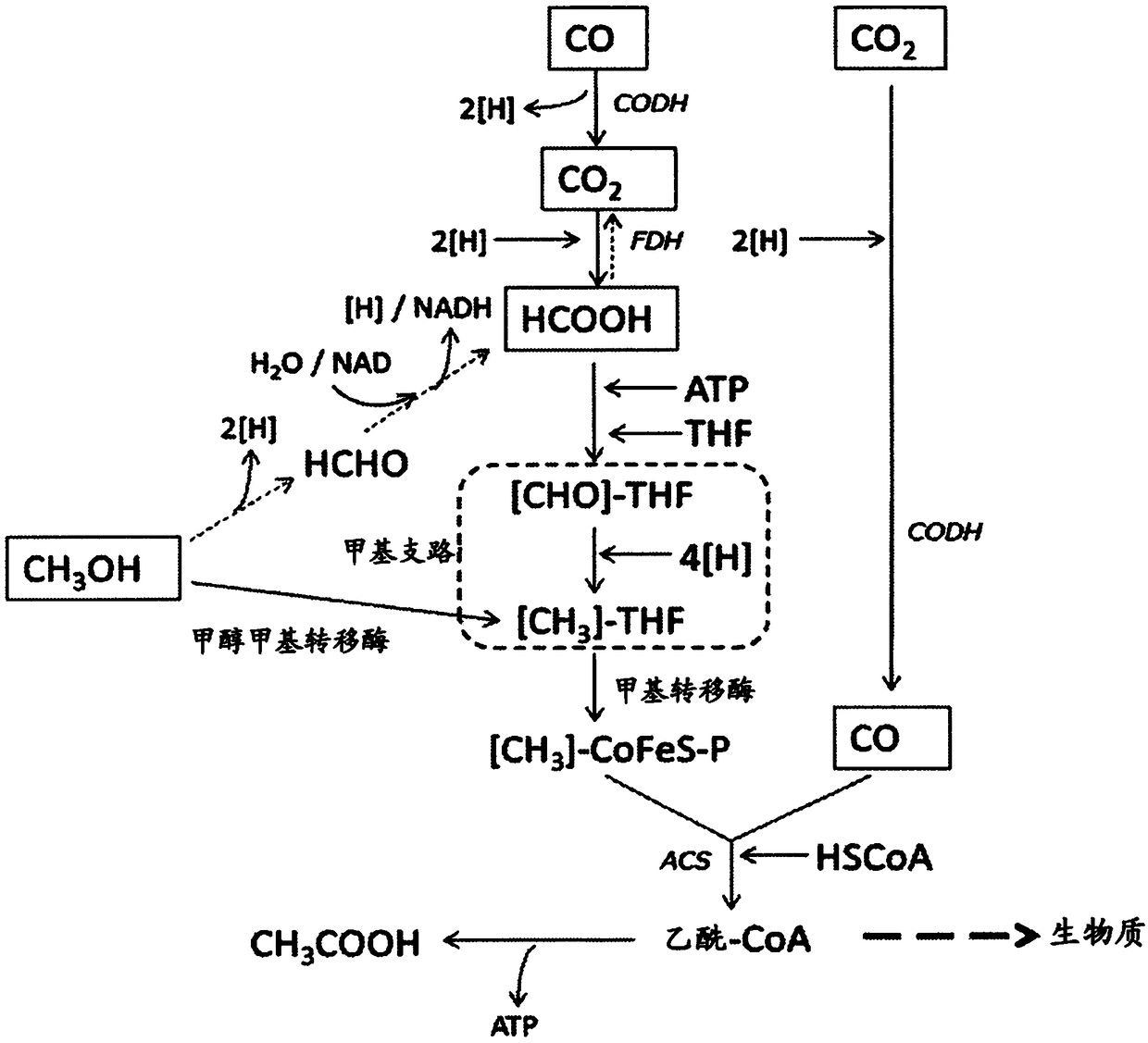
Recombinant microorganisms and uses therefor
The invention provides recombinant microorganisms and methods for the production of acetone from gaseous substrates. For example, the recombinant microorganism may be modified to express an exogenous thiolase, an exogenous CoA transferase, and an exogenous decarboxylase.
Owner:LANZATECH NEW ZEALAND LTD
Methods, reagents and cells for biosynthesizing compound
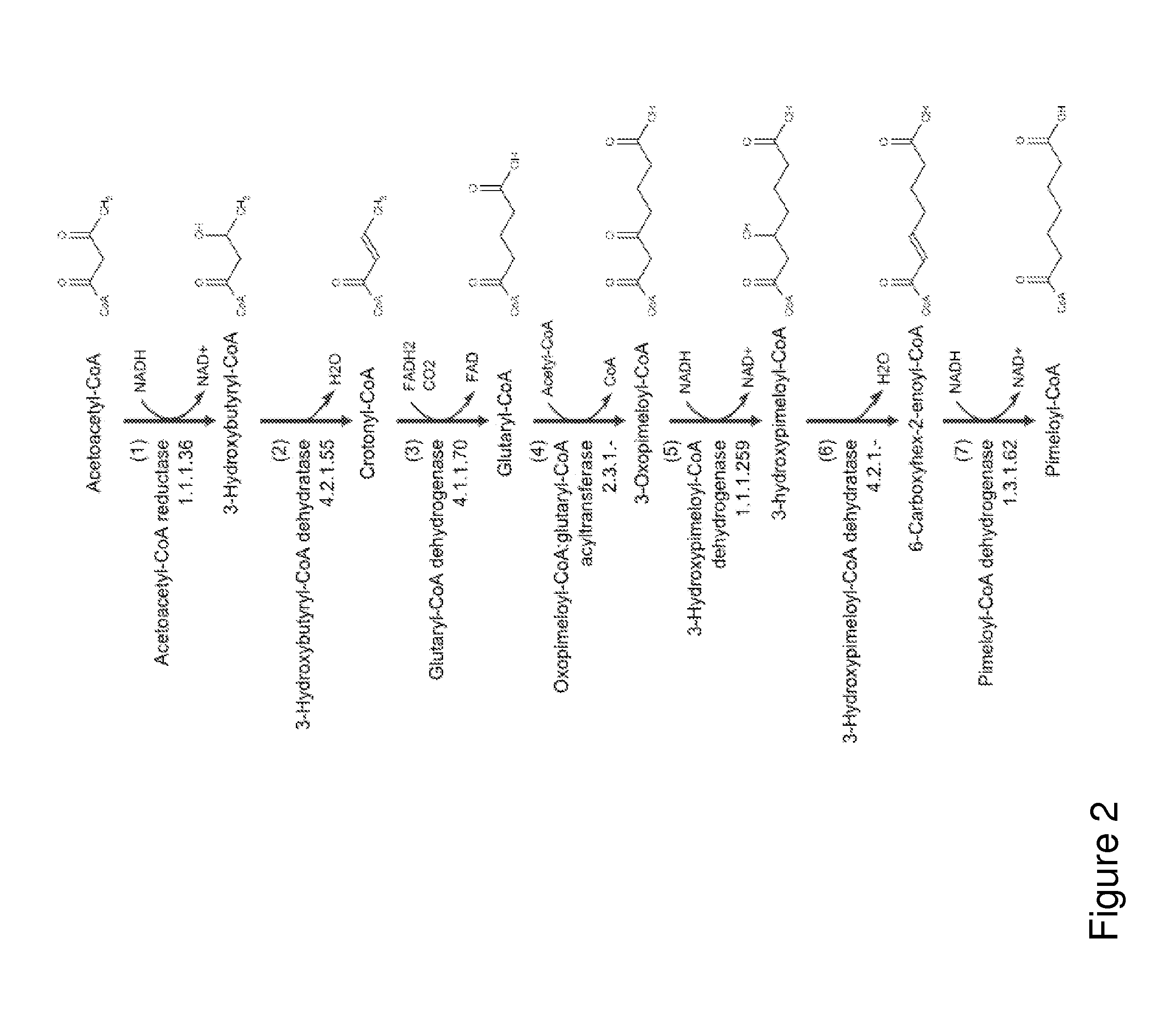
This document describes biochemical pathways for producing 2(E)-heptenedioyl-CoA methyl ester from precursors such as 2-oxo-glutarate, acetyl-CoA, or succinyl-CoA using one or more of a fatty acid O-methyltransferase, a thioesterase, a CoA-transferase, a CoA ligase, as well as recombinant hosts expressing one or more of such enzymes. 2(E)-heptenedioyl-CoA methyl ester can be enzymatically converted to pimeloyl-CoA using a trans-2-enoyl-CoA reductase, and a methylesterase. Pimeloyl-CoA can be enzymatically converted to pimelic acid, 7-aminoheptanoate, 7-hydroxyheptanoate, heptamethylenediamine, or 1,7-heptanediol.
Owner:INVISTA TEXTILES (U K) LTD
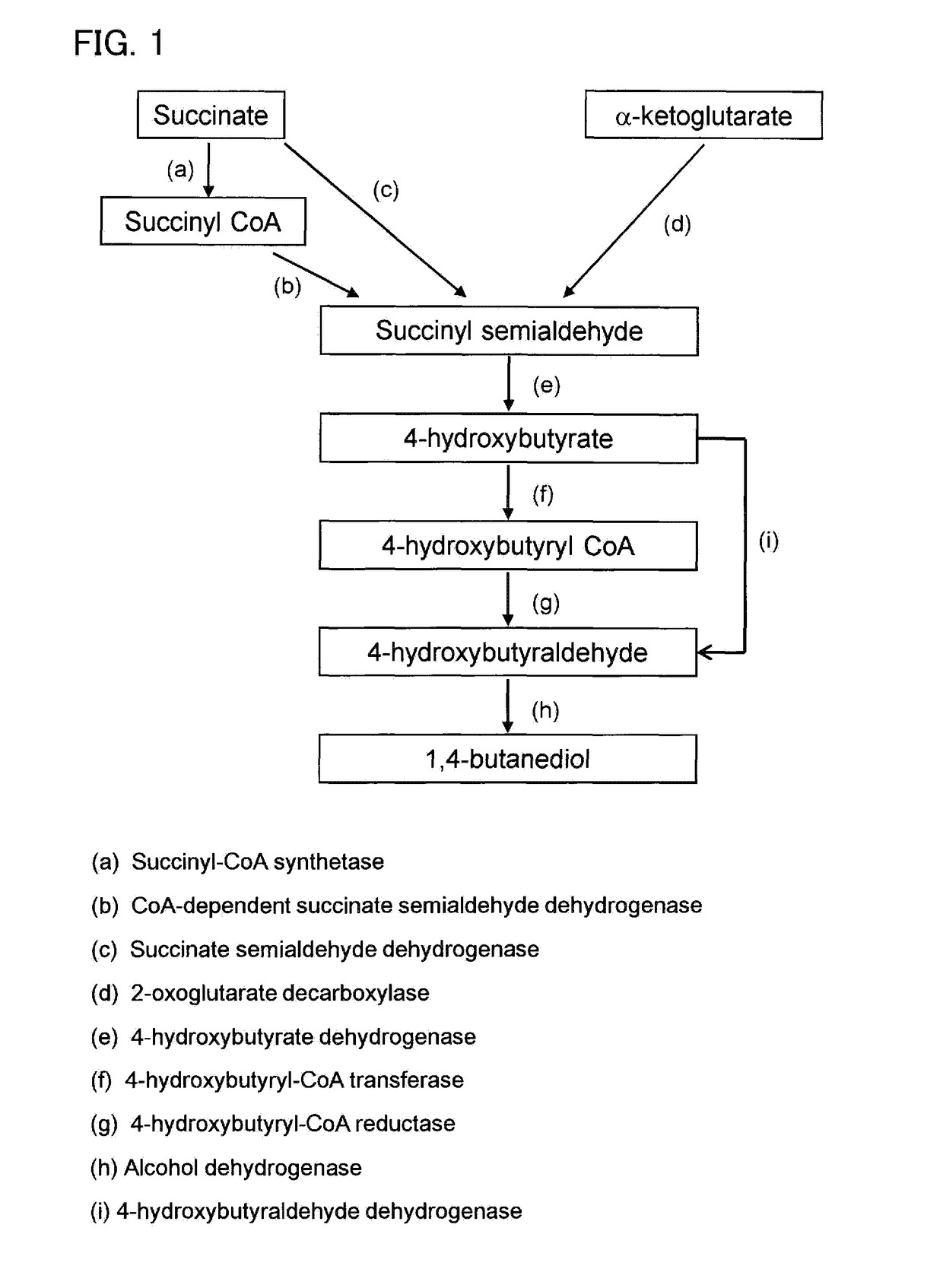
Recombinant cells, method for producing recombinant cells, and method for producing 1,4-butanediol
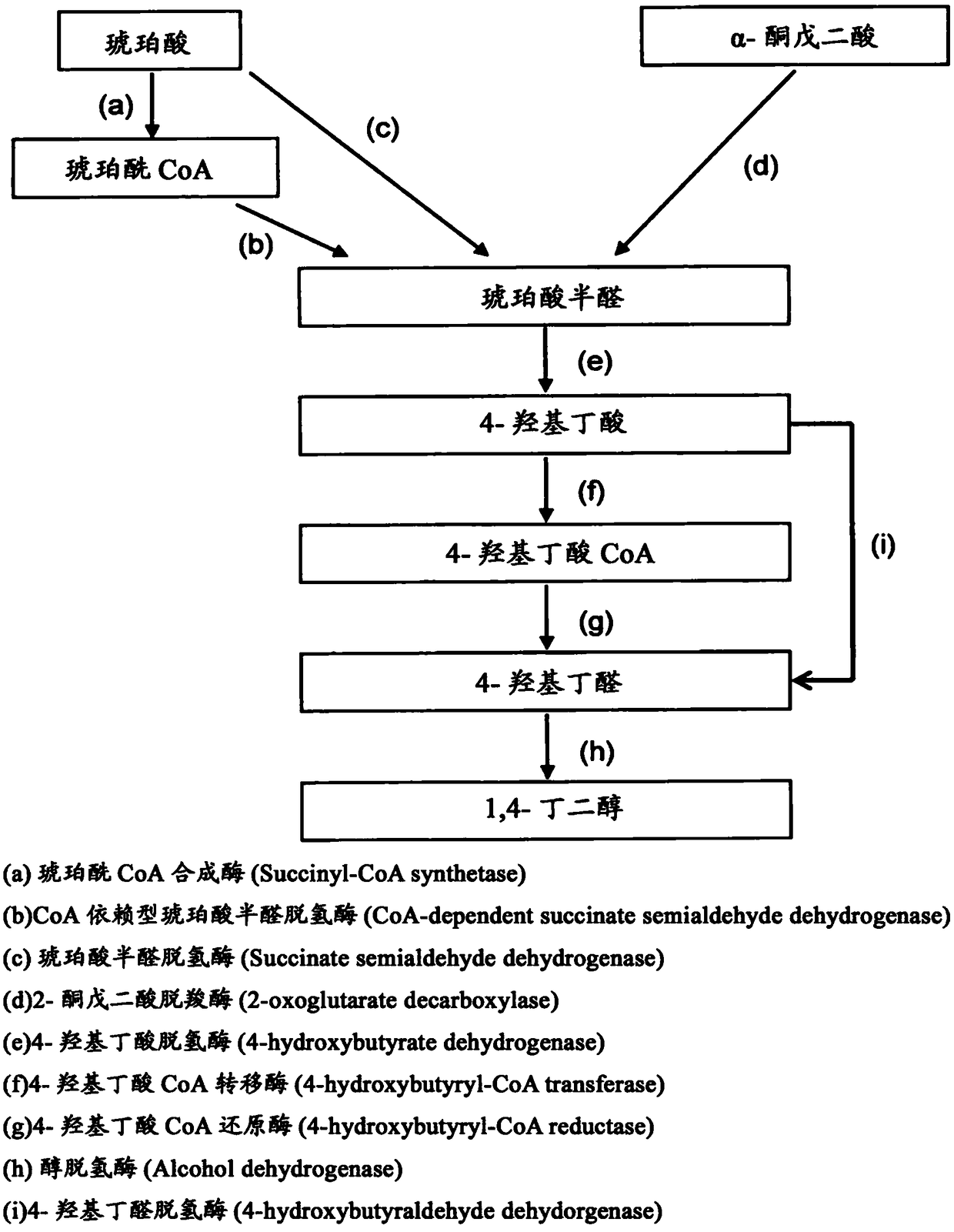
The present invention addresses the problem of providing a technique for producing 1,4-butanediol by recombinant cells. Recombinant cells having obligate anaerobicity and acetic acid production capacity, wherein the recombinant cells have a gene that encodes at least one enzyme selected from the group comprising succinate semialdehyde dehydrogenase, succinyl CoA synthetase, CoA-dependent succinatesemialdehyde dehydrogenase, 4-hydroxybutyrate dehydrogenase, 4-hydroxybutyrate CoA transferase, 4-hydroxybutyrate CoA reductase, 4-hydroxybutyraldehyde dehydrogenase, and alcohol dehydrogenase, express the gene, and are capable of producing 1,4-butanediol.
Owner:SEKISUI CHEM CO LTD +1
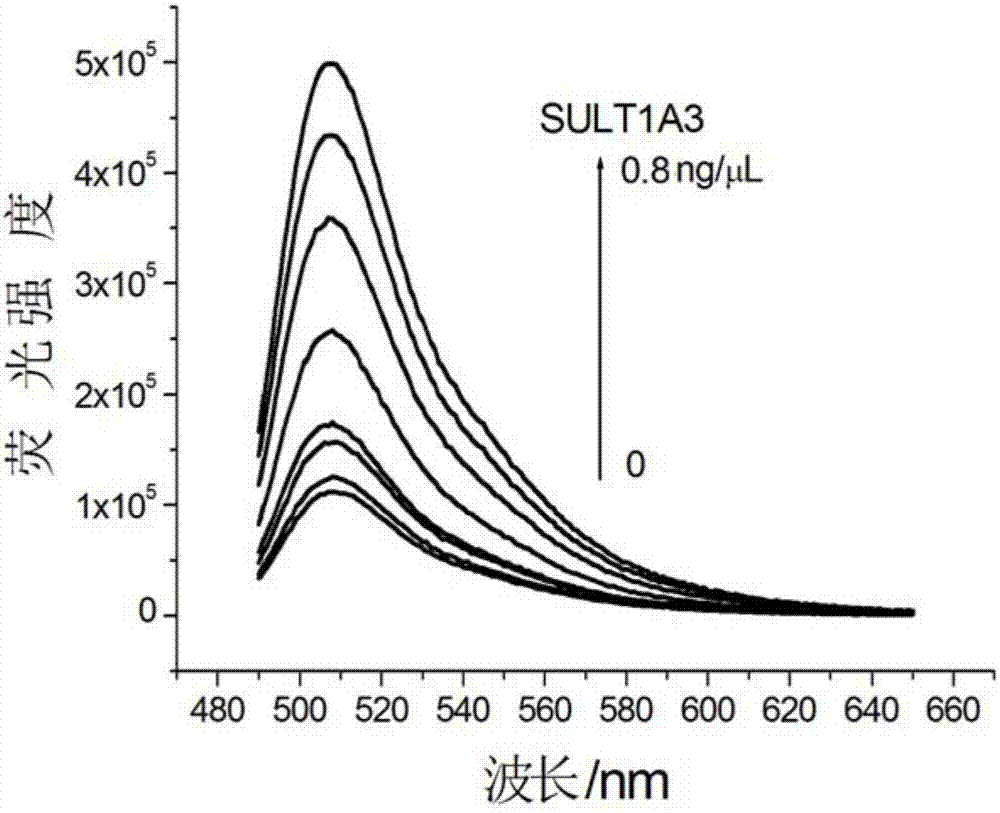
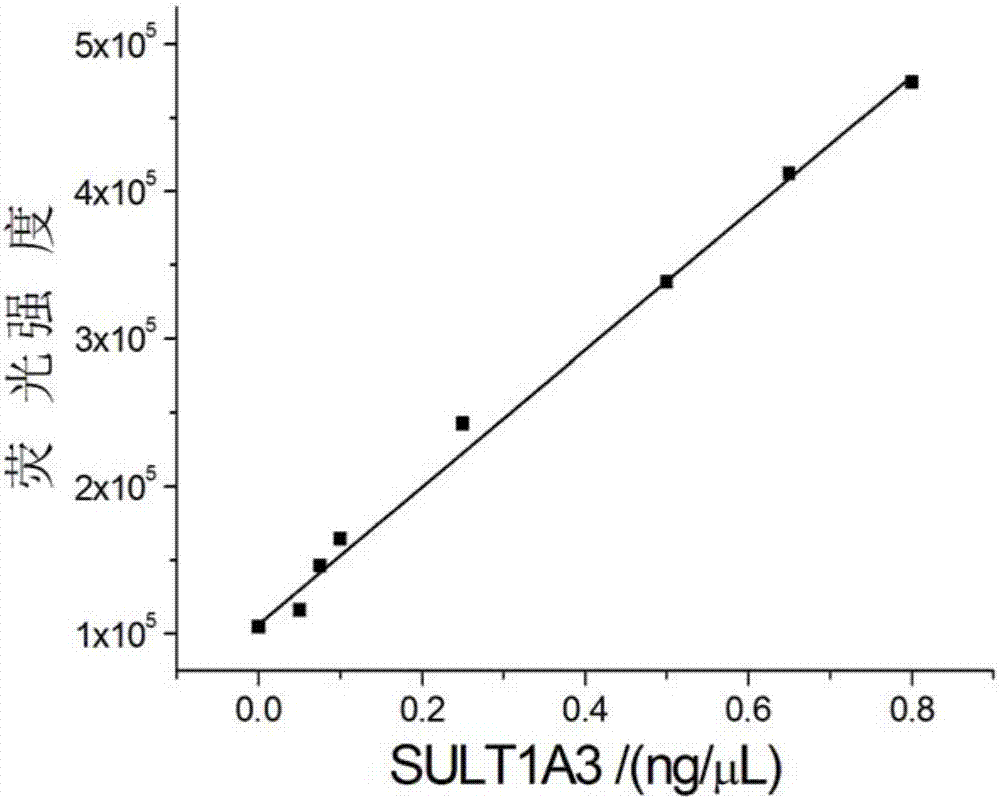
Universal sulfo-group transferase activity analysis method
ActiveCN107356577ARealize detectionGood general applicabilityFluorescence/phosphorescencePhosphateFluorescence
The invention discloses a universal sulfo-group transferase activity analysis method. According to the method, 3'-phosphoadenosine-5'-phosphosulfate (PAPS), sulfo-group transferase and a sulfo-group transferase specific substrate are mixed to be subjected to a sulfonation reaction to generate 3'-phosphoadenosine-phosphate, an IMPAD1 enzyme can specifically hydrolyze 3'-phosphoadenosine-5'-phosphosulfate to release a phosphate group (Pi), and Pi is subjected to fluorogenic quantitative detection through a mixed system composed of metal ions (Mn+) which has a high combining capacity with Pi and a specific fluorescent probe (FP), and quantitative analysis of an sulfo-group transferase activity can be achieved. According to the system, the combination of Mn+ and FP causes that a fluorescence system is effectively quenched, Pi generated in the reaction process effectively shields Mn+, therefore the fluorescence signal of FP is restored. Therefore, by monitoring the change situation of the fluorescence signal in the system, fluorescence reinforced sensing of the sulfo-group transferase activity can be achieved. The universal sulfo-group transferase activity analysis method is generally applicable to detection of the sulfo-group transferase activity with PASA being a sulfonic group donor.
Owner:SHAANXI NORMAL UNIV
Recombinant cells, method for producing recombinant cells, and method for producing 1,4-butanediol
An object of the present invention is to provide a technique for producing 1,4-butanediol by recombinant cells. Provided is a recombinant cell that is acetogenic and obligatory anaerobic, wherein the recombinant cell includes a gene encoding at least one enzyme selected from the group consisting of succinate semialdehyde dehydrogenase, succinyl-CoA synthase, CoA-dependent succinate semialdehyde dehydrogenase, 4-hydroxybutyrate dehydrogenase, 4-hydroxybutyryl-CoA transferase, 4-hydroxybutyryl-CoA reductase, 4-hydroxybutyraldehyde dehydrogenase, and alcohol dehydrogenase, the gene is expressed in the recombinant cell, and the recombinant cell produces 1,4-butandiol.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV +1
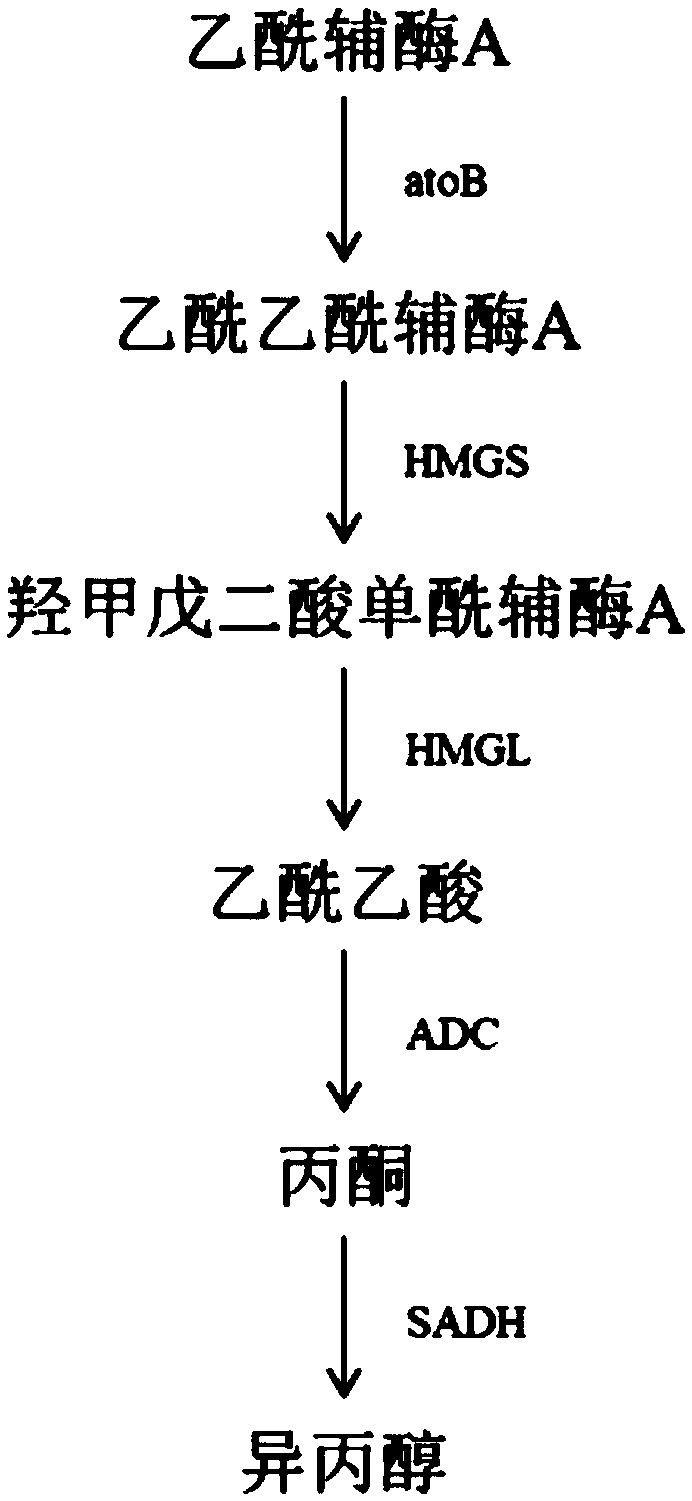
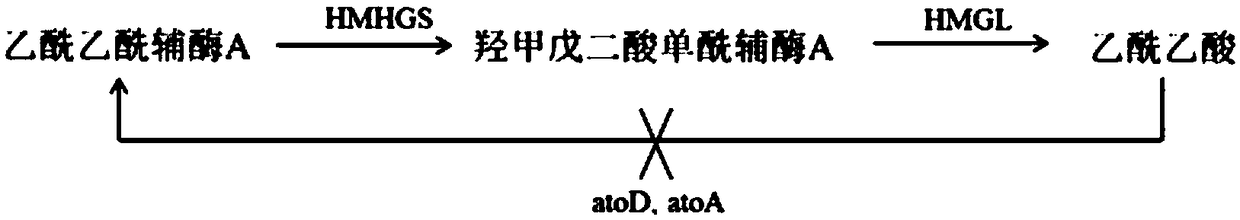
Acetic acid CoA transferase gene-deficient engineered Escherichia coli strain and application thereof
The invention discloses an acetic acid CoA transferase gene-deficient engineered Escherichia coli strain and application thereof. The engineered Escherichia coli strain constructs an engineering strain of an isopropanol synthesis pathway composed of a plurality of genes, and is obtained by knocking out encoding genes of acetic acid CoA transferase of host escherichia coli. The isopropanol synthesis pathway provided by the invention consists of acetoacetyl ketase CoA synthetase, hydroxymethylglutaryl CoA synthetase, hydroxymethylglutaryl CoA lyase, acetoacetate decarboxylase and secondary ethanol dehydrogenase, and can achieve the transformation from acetyl-CoA as starting metabolite to isopropanol in vivo. The mutant strain provided by the invention is obtained by expressing a gene encoding all enzymes constituting the isopropanol anabolic pathway. The recombinant escherichia coli strain constructed in the invention can synthesize 364mg / L isopropanol from a rich medium-containing glucose or glycerol under facultative anaerobic conditions.
Owner:扬州麦塔瑞思生物科技有限公司
One step n-terminal tagging of proteins
The invention includes a selective method of modifying the N-terminus of a protein using an aminoacyl tRNA transferase. In certain embodiments, the method comprises contacting a solution of the protein or peptide with a transferase and a derivative of a molecule, whereby the N-terminus of the protein or peptide is derivatized with the molecule.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for producing polyhydroxyalkanoate using 2-hydroxyisocaproate-coa transferase
The present invention relates to a recombinant microorganism to which a gene coding for 2-hydroxyisocaproate-CoA transferase and a gene coding for polyhydroxyalkanoate synthase are introduced and which has a potential of producing polyhydroxyalkanoate bearing an aromatic monomer or a long-chain 2-HA monomer and a method for producing polyhydroxyalkanoate bearing an aromatic monomer or a long-chain 2-HA monomer, using the recombinant microorganism. According to the present invention, a biodegradable polymer bearing an aromatic monomer or a long-chain 2-HA monomer can be produced.
Owner:KOREA ADVANCED INST OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com