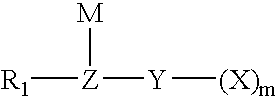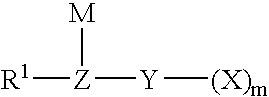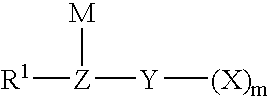Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
453 results about "Monovalent Cations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monovalent cations are ions carrying one valence electron (+1) to form an ionic bond with something else. This is the primary interaction occurring in ionic compounds.
Preparation method for sodium alginate-acrylamide-based hydrogel
InactiveCN103396562AOvercome the shortcomings of generating air bubbles and forming uneven hydrogelsEasy to operateCross-linkPolyacrylamide Hydrogel
The invention discloses a preparation method for sodium alginate-acrylamide-based hydrogel. The preparation method comprises the following steps of: dissolving sodium alginate powder in deionized water; then sequentially adding an acrylamide monomer, a methylene diacrylamide cross-linking agent, ammonium persulfate and an N,N,N',N'-tetramethylethylenediamine catalyst; uniformly stirring the materials, pouring the mixture into a glass die, and heating the mixture to obtain a hydrogel; completely soaking the hydrogel in a 0.01-1 mol / L non-monovalent cation aqueous solution for 1-10 hours, wherein cations diffuse and enter in a hydrogel network structure, and induce sodium alginate to cross-link, so as to generate the high-strength and high-toughness sodium alginate-acrylamide-based hydrogel during the process. The hydrogel disclosed by the invention has the following performances: the highest tensile strength can achieve 1 MPa, and the highest tensile elasticity modulus can achieve 250 KPa; a loading-unloading test is performed on the hydrogel, and when the great tensile multiple before unloading is 8, the highest dissipated energy can achieve 2180 KJ / m<3>.
Owner:XI AN JIAOTONG UNIV
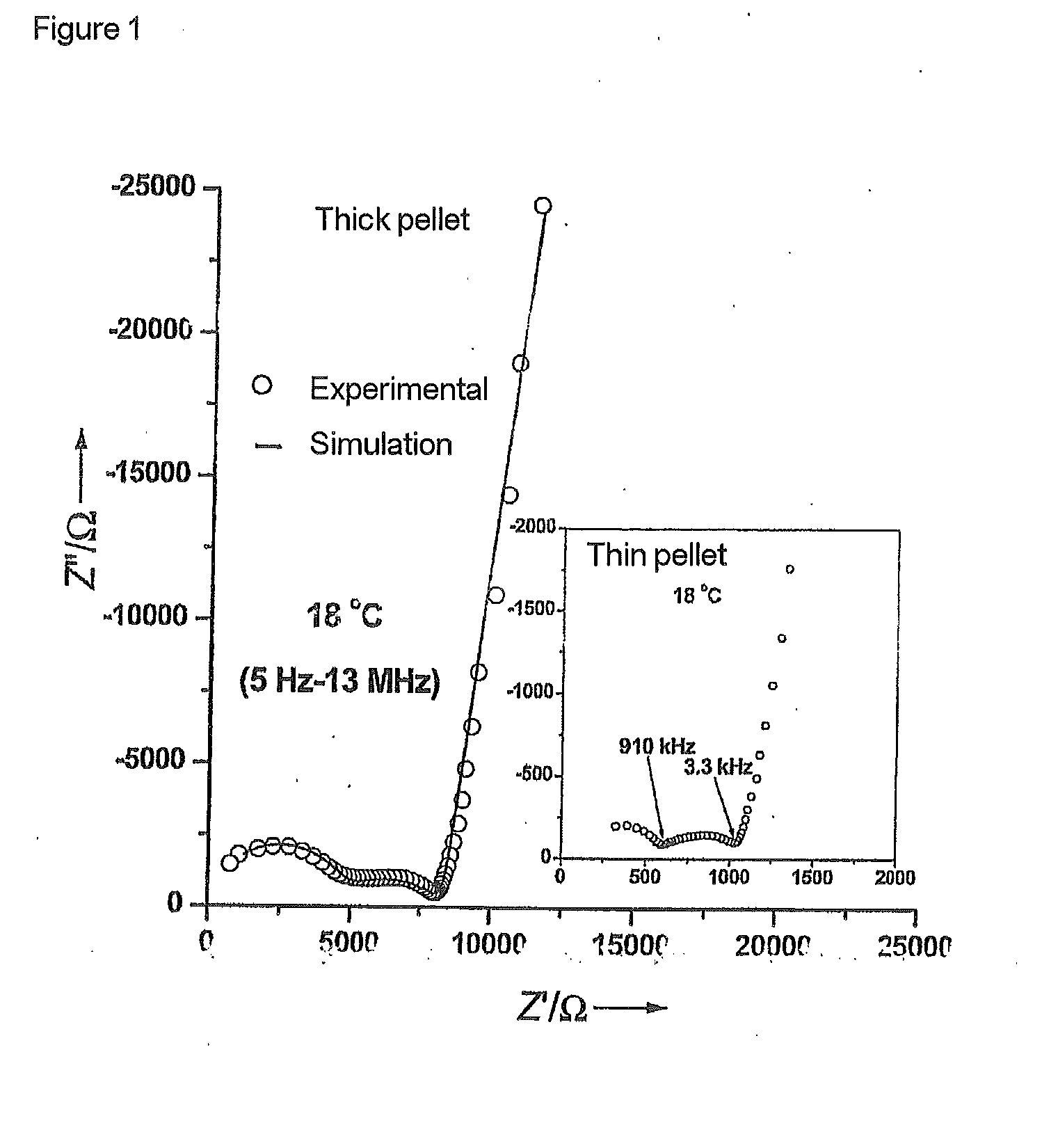
Solid ion conductor which has a garnet-like crystal structure and has the stoichiometric composition L7+XAXG3-XZr2O12
ActiveUS8658317B2Easily employedImprove ionic conductivityFinal product manufactureCell electrodesElectrical conductorCrystal structure
The invention is directed to a solid ion conductor which has a garnet-like crystal structure and has the stoichiometric composition L7+xAxG3−xZr2O12, whereinL is in each case independently a monovalent cation,A is in each case independently a divalent cation,G is in each case independently a trivalent cation,0≦x≦3 andO can be partly or completely replaced by divalent or trivalent anion.
Owner:BASF AG
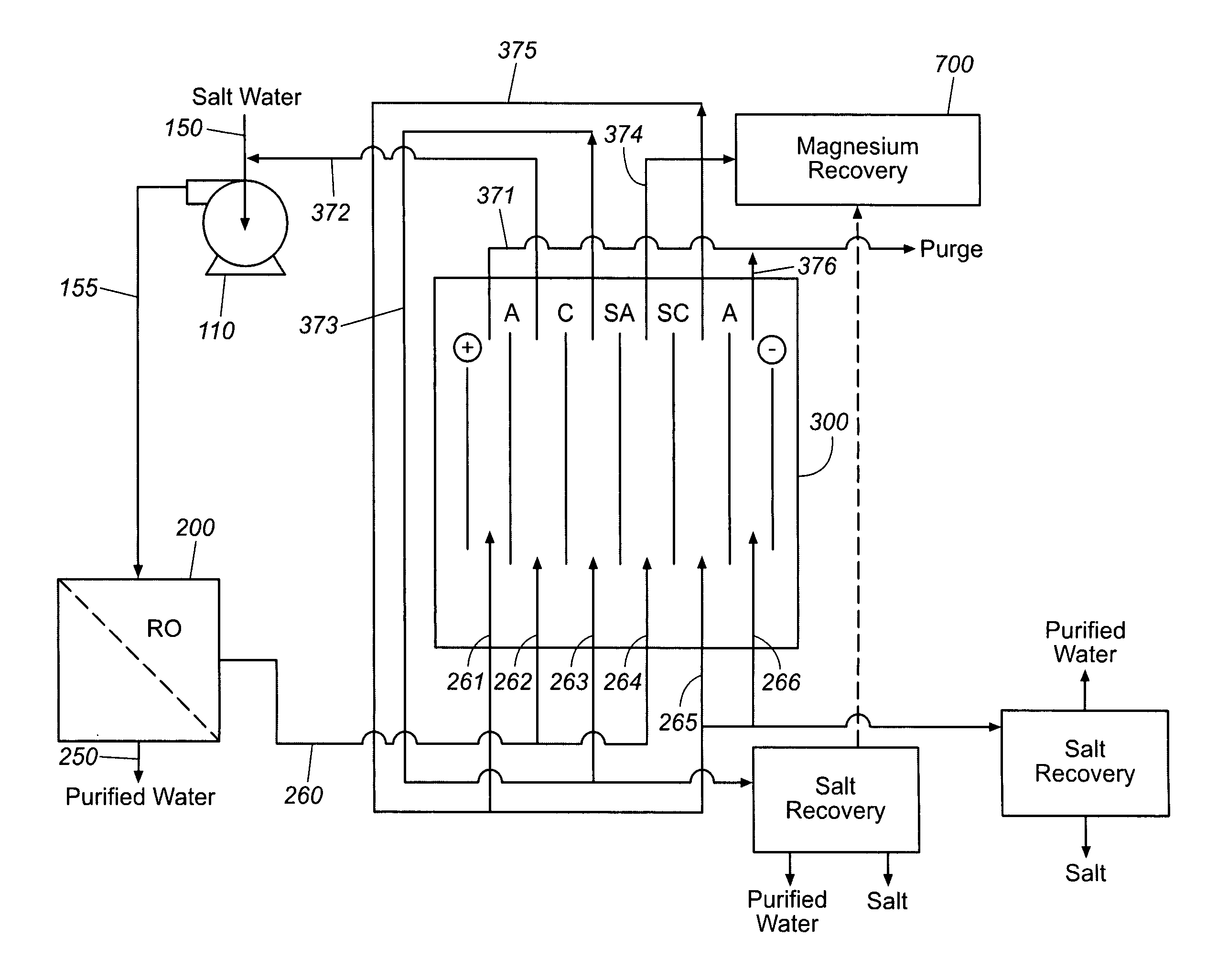
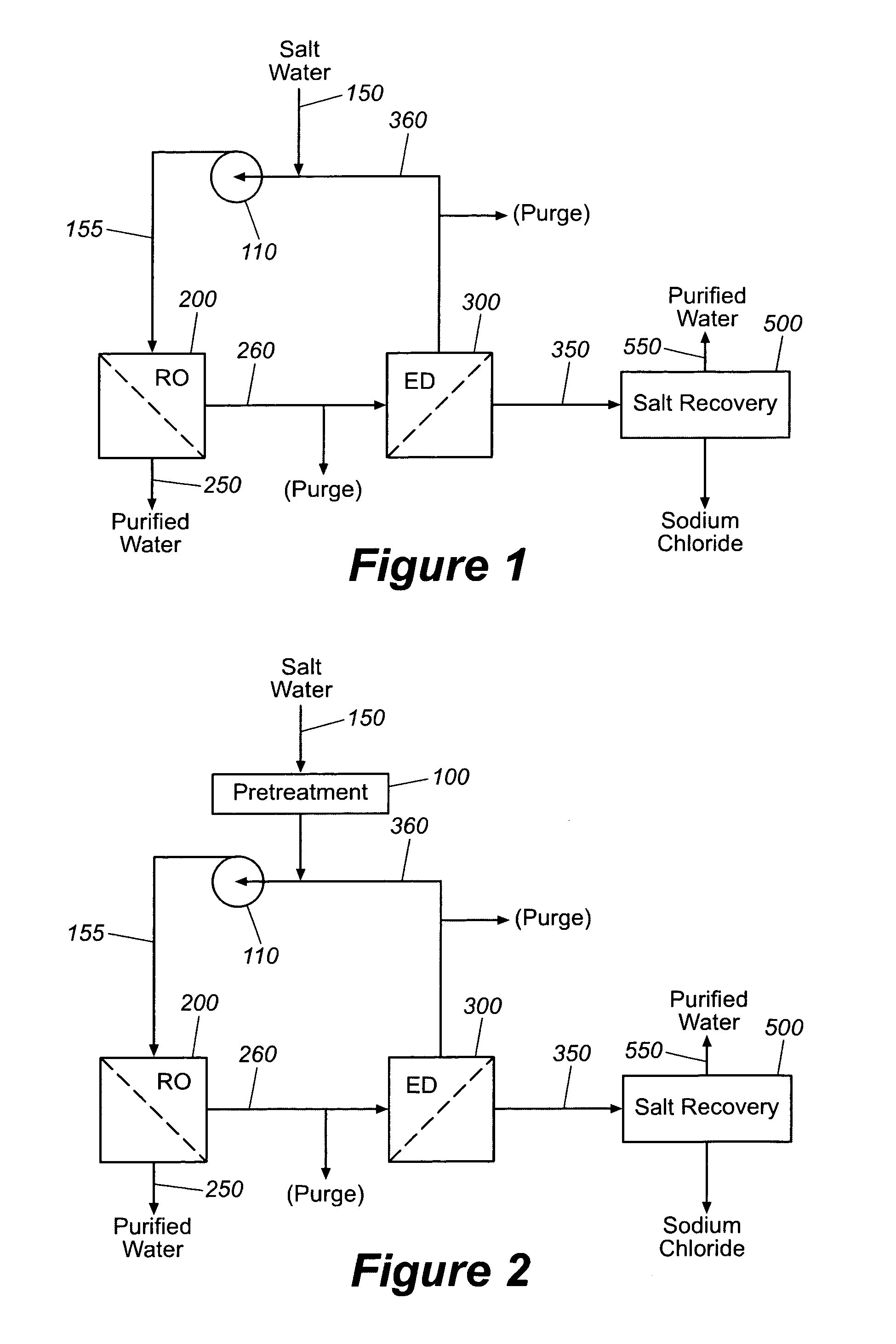
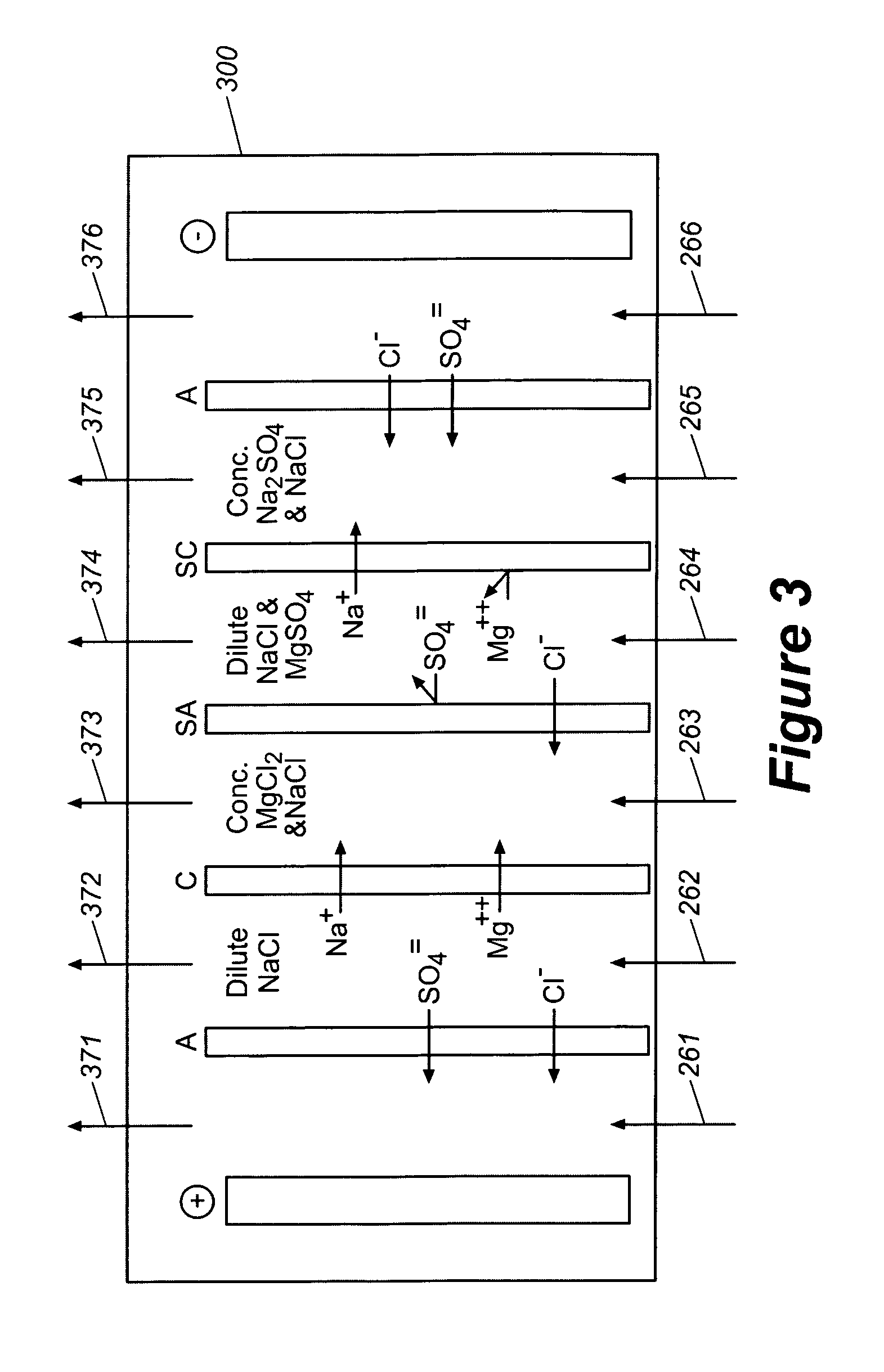
Production of purified water and high value chemicals from salt water
ActiveUS7083730B2Effective recoveryElectrolysis componentsSolvent extractionReverse osmosisHigh pressure
Sodium chloride and purified water are recovered by treating salt water that contains sodium chloride with an integrated reverse osmosis and electrodialysis system, which includes an efficiency-enhancing feature that is one or more of the following: the use of univalent anion and univalent cation selective membranes in the electrodialysis unit; the addition of a nanofiltration unit to process the diluate from the electrodialysis unit; or operation of the electrodialysis unit at an elevated pressure. Magnesium and bromine can optionally be produced when the salt water contains these materials.
Owner:SOUTH CAROLINA UNIV OF
Perovskite-type metal oxide compounds
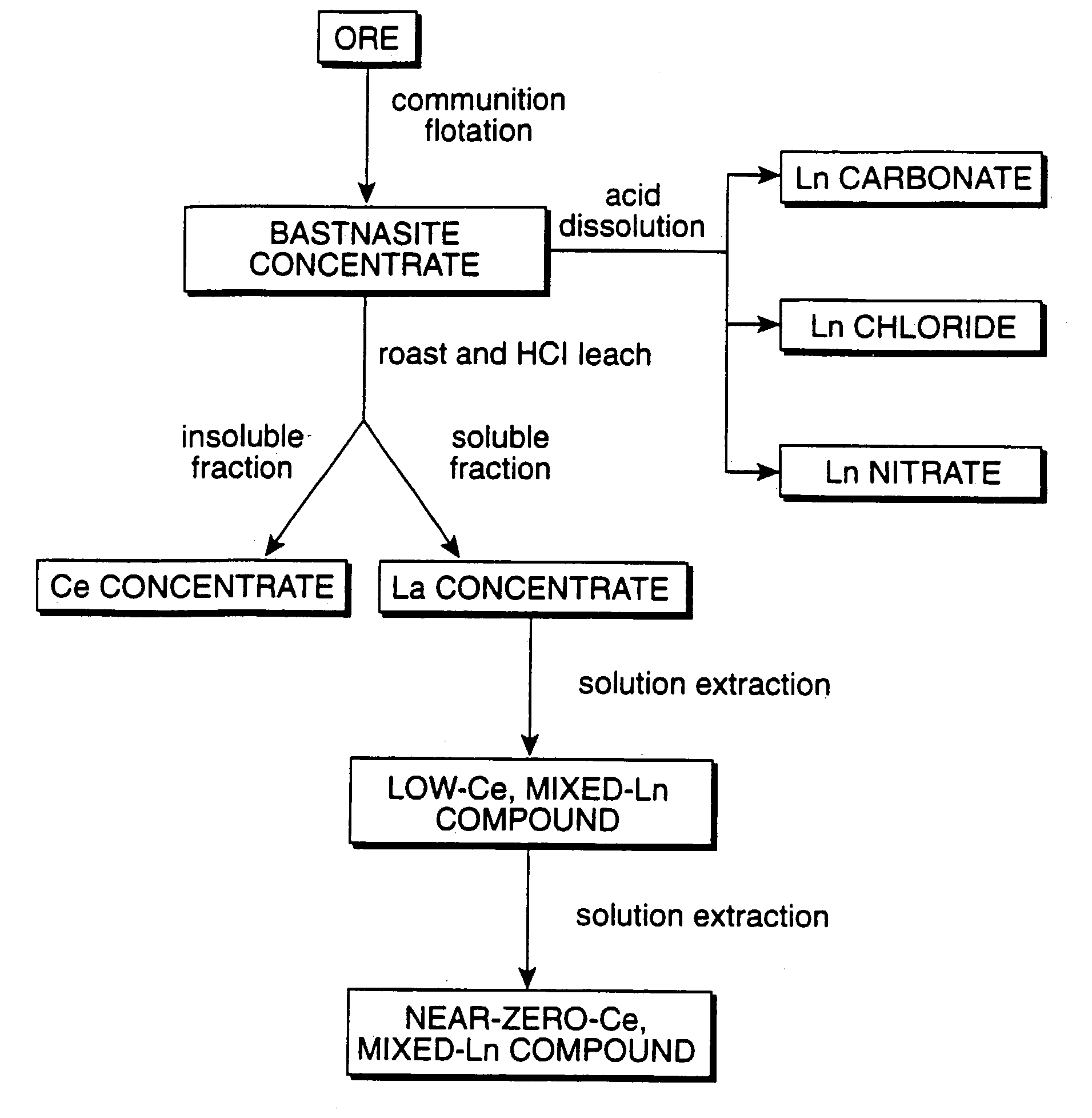
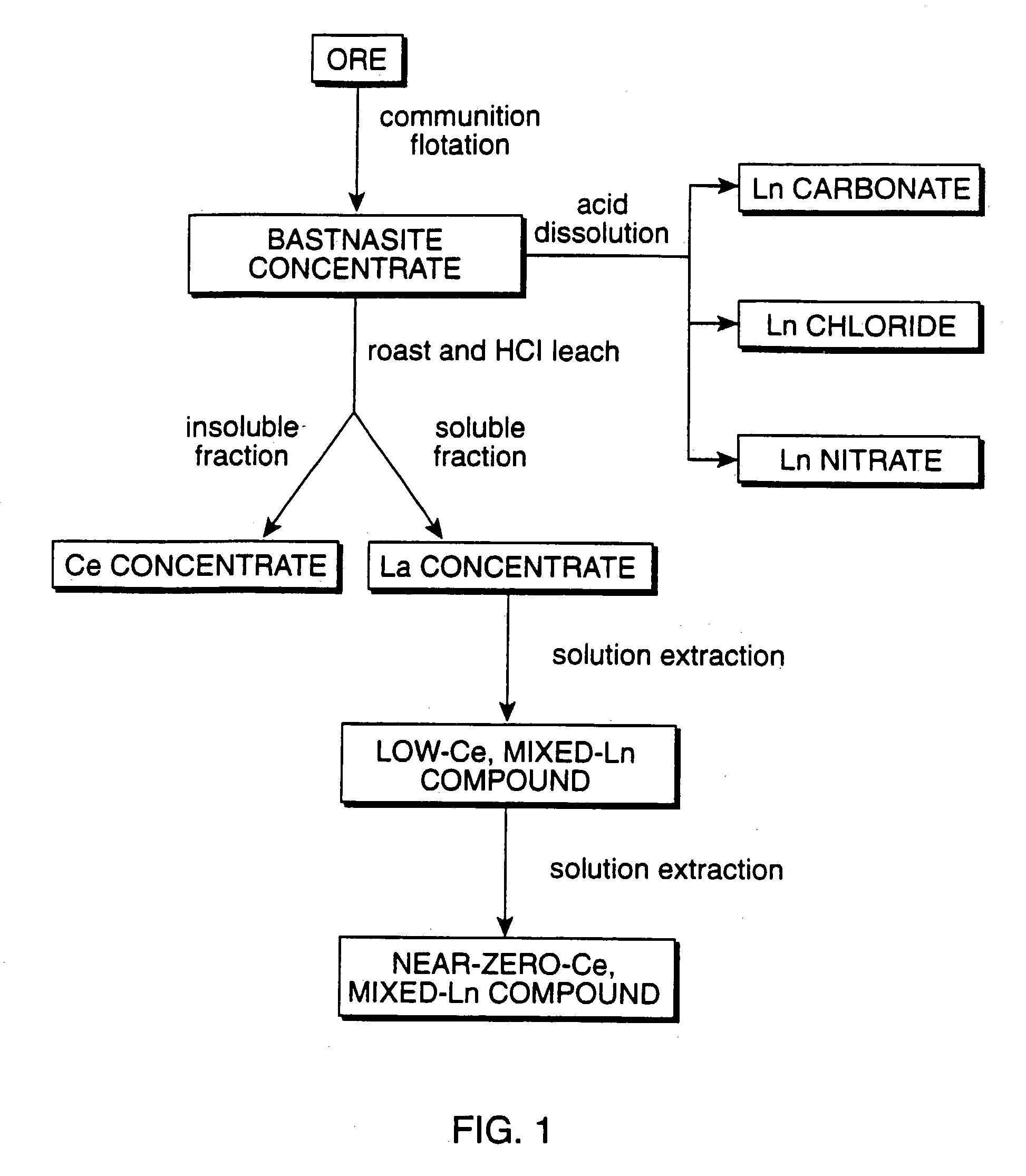
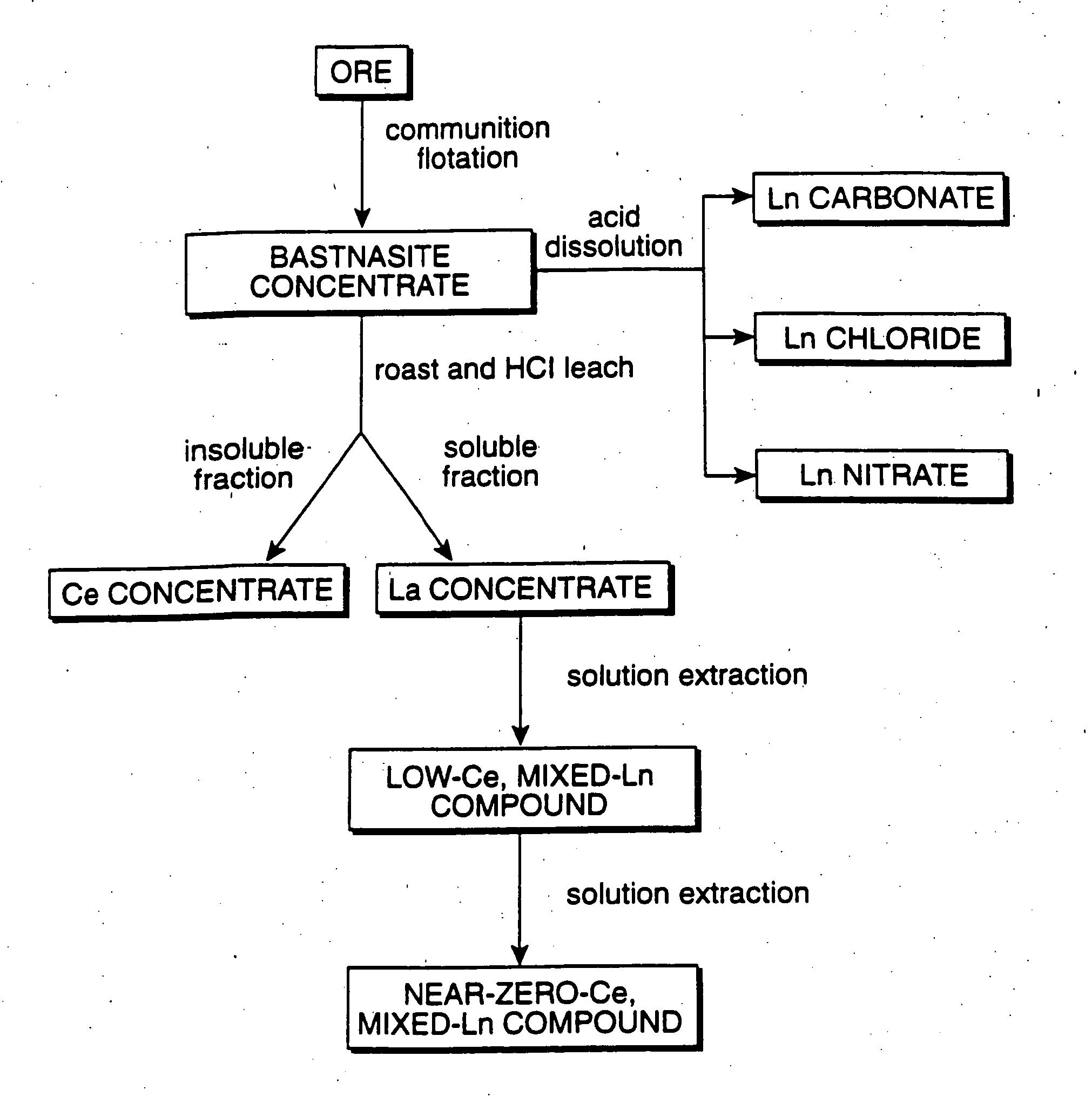
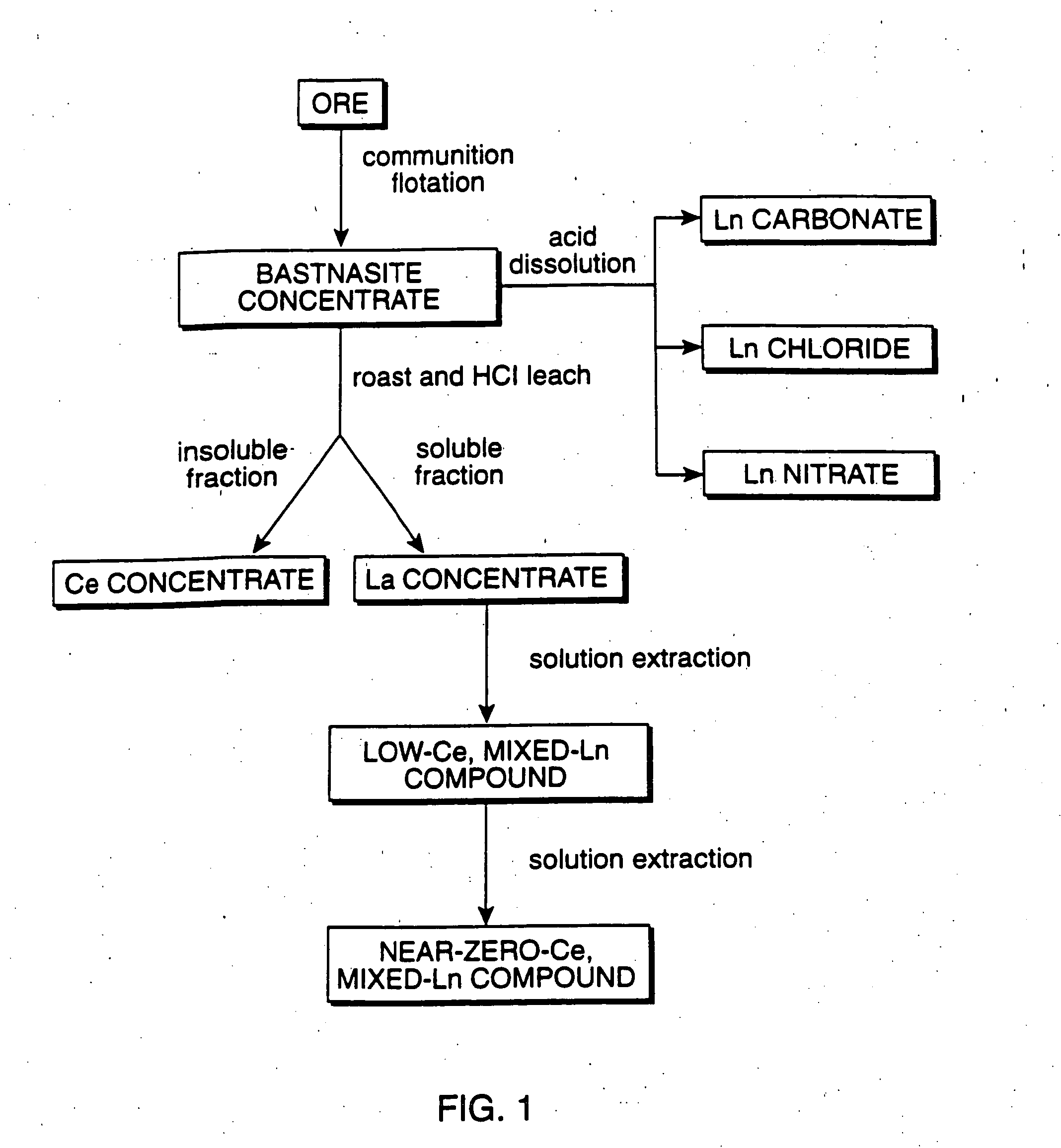
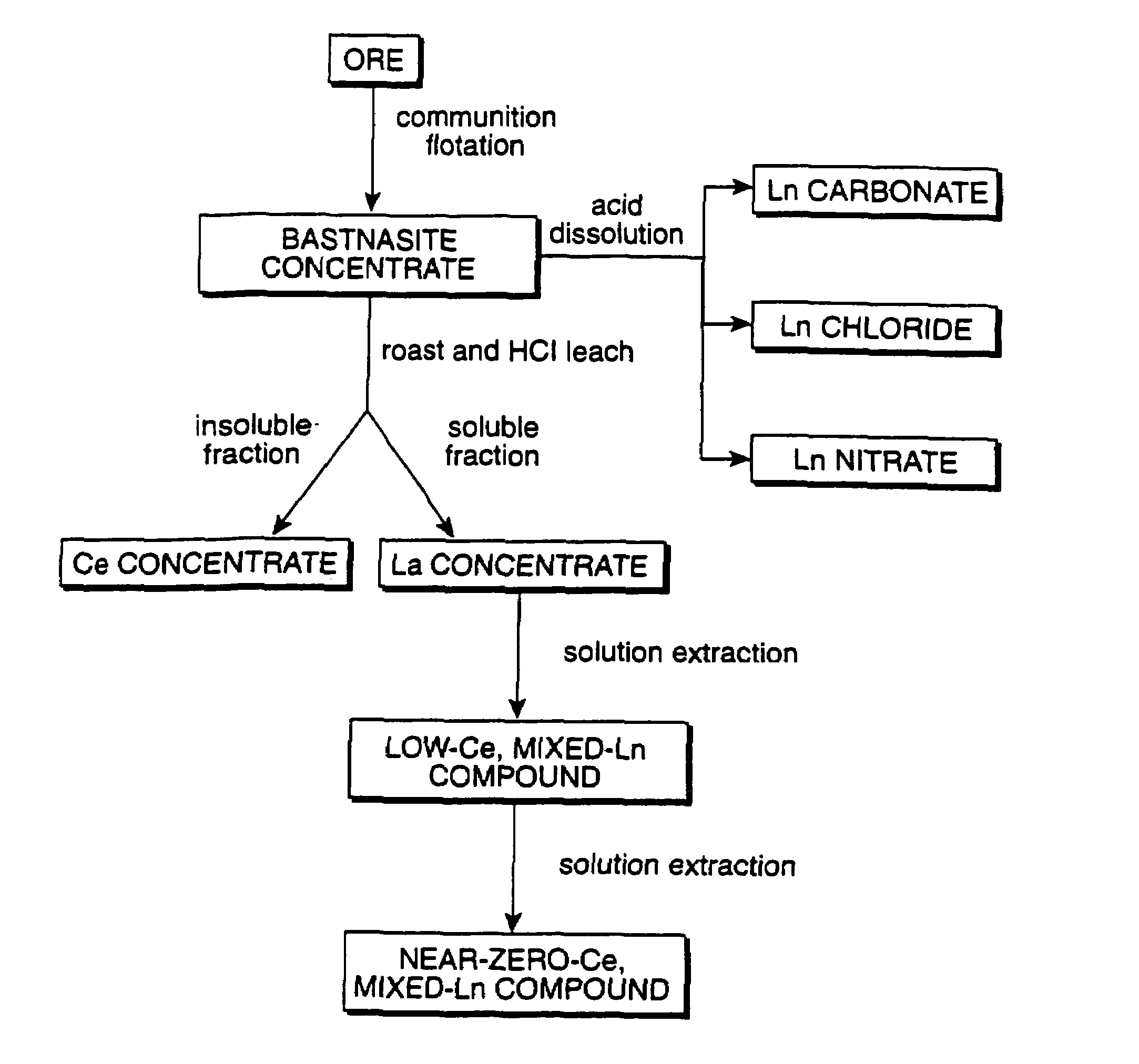
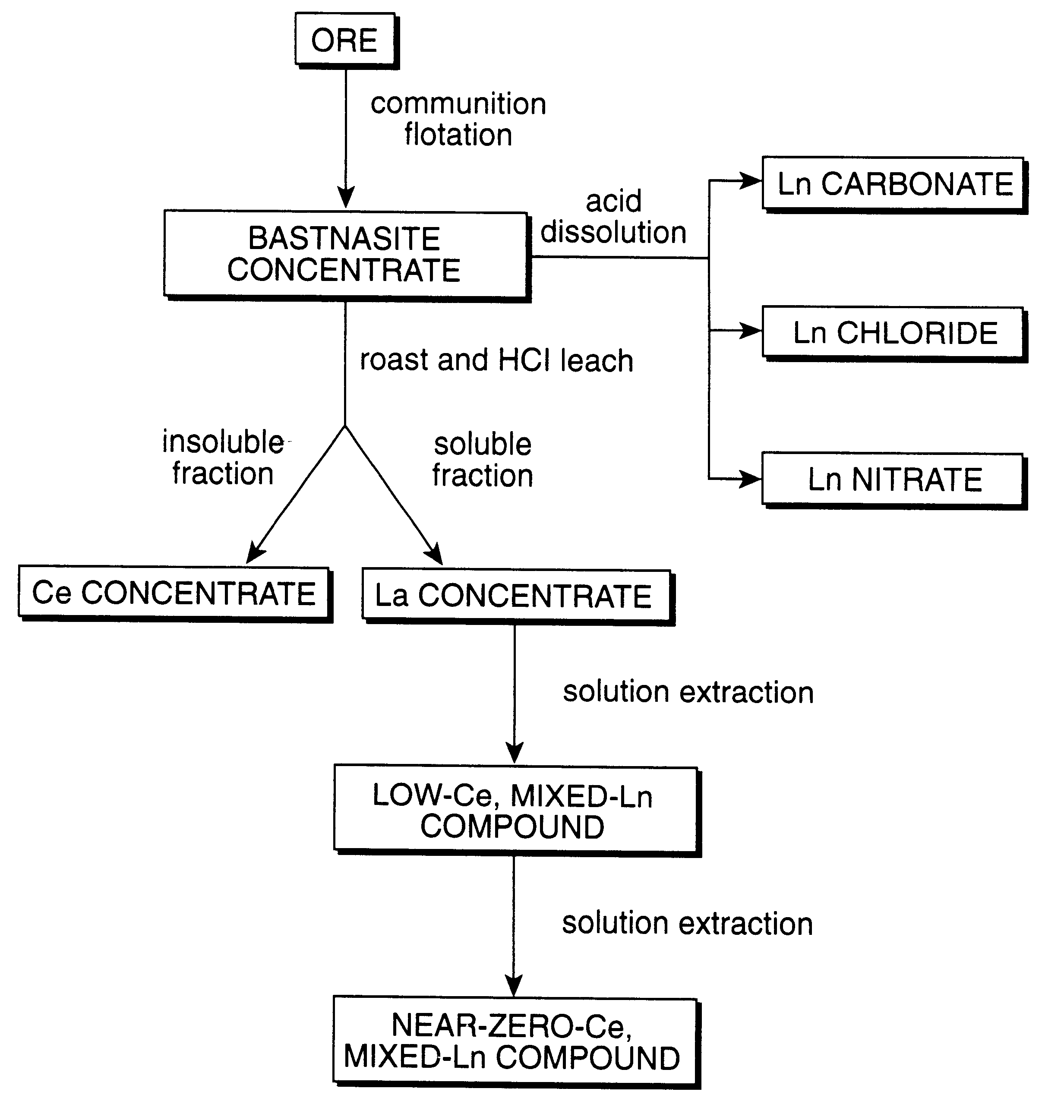
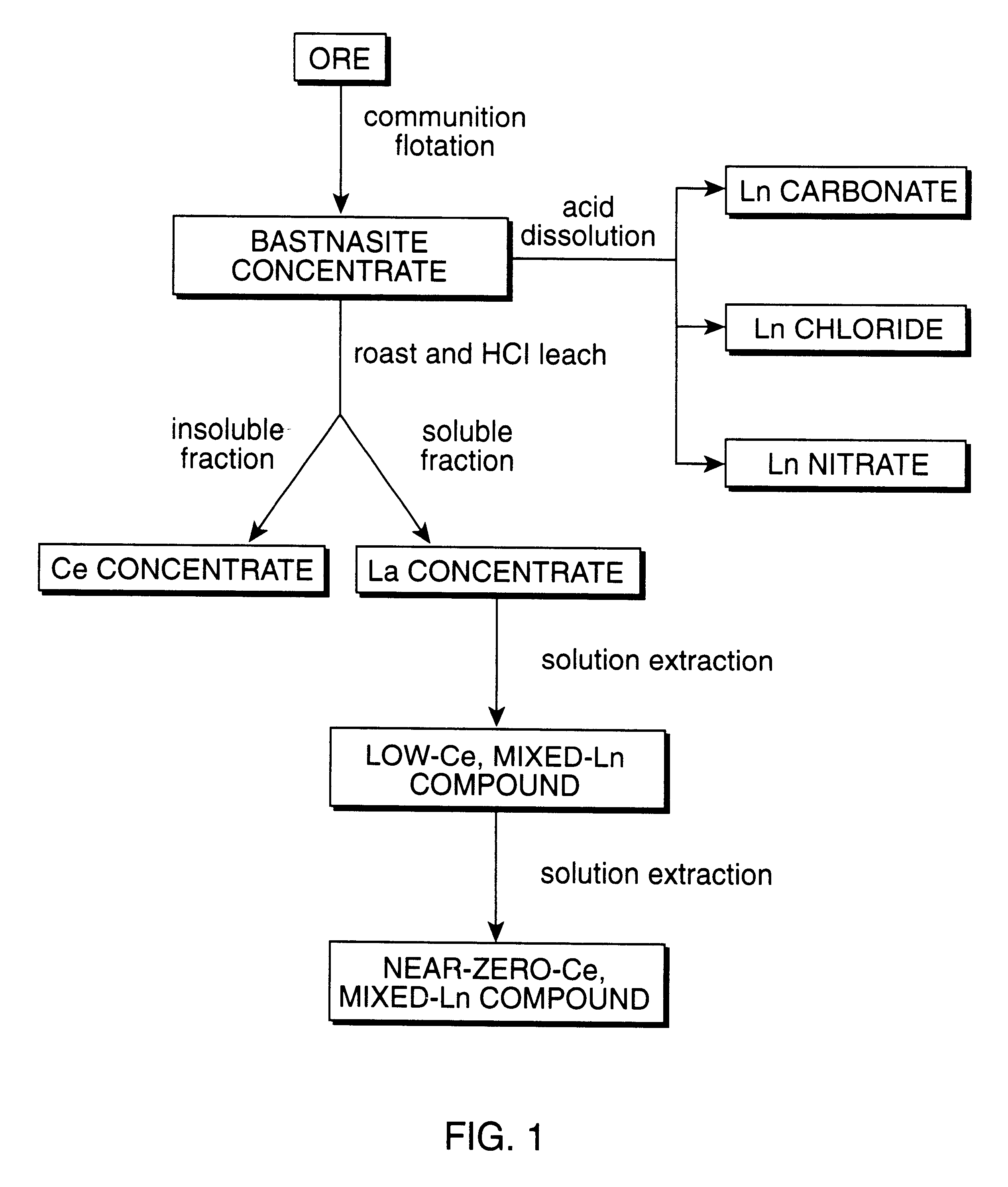
Perovskite-type catalyst consists essentially of a metal oxide composition is provided. The metal oxide composition is represented by the general formula A.sub.1-x B.sub.x MO.sub.3, in which A is a mixture of elements originally in the form of single phase mixed lanthanides collected from bastnasite; B is a divalent or monovalent cation; M is at least one element selected from the group consisting of elements of an atomic number of from 22 to 30, 40 to 51, and 73 to 80; and x is a number defined by 0.ltoreq.x<0.5.
Owner:CATALYTIC SOLUTIONS INC
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
Process for preparing fluoropolymer
ActiveUS20070149733A1Improve production efficiencyTransportation and packagingFibre treatmentCarbon numberPolymer science
The present invention relates to a process for preparing a fluoropolymer containing at least one kind of fluoroolefin, which comprises carrying out polymerization in the presence of a surfactant represented by the formula (1): (wherein R1 and R2 may be the same or different respectively, and represent an alkyl group or an alkenyl group, R3 is a hydrogen atom, an alkyl group or an alkenyl group, the total carbon number of R1 to R3 is 2 to 25, L− is a group represented by —SO3−, —OSO3−, —PO3−, —OPO3− or —COO−, and M+ is a monovalent cation). Thereby, polymerization can be carried out with excellent production efficiency in the presence of a small amount of a surfactant, and a fluoropolymer can be prepared without lowering various physical properties such as water resistance by the surfactant.
Owner:DAIKIN IND LTD
Tobacco Raw Material
PendingUS20170020183A1Improve smellGreat tasteTobacco preparationTobacco treatmentAlginate matrixSmokeless tobacco
An oral smokeless tobacco product comprises bleached tobacco raw material with less than about 4 weight-% fermentable carbohydrates, calculated on dry total weight of the bleached tobacco raw material, with an ISO brightness not less than about 60; nicotine selected from nicotine from tobacco plants and / or synthetic nicotine; and an alginate composition. The alginate composition is distributed in the product and comprises at least water, alginate and an added substance intended to be released from the product when used. The composition contains an alginate matrix that retains at least a major proportion of the added substance so long as the matrix is intact, the alginate matrix being formed so as to disintegrate and / or dissolve in the chemical and physical environment existing in a user's mouth. The alginate contains an alginate salt of monovalent cations and is soluble in cold water. The product has a water content of 5 to 55%.
Owner:WINNINGTON AB
Prodrug comprising beta-keto carboxylic acid, beta-keto carboxylic acid salt or beta-keto carboxylic acid ester for drug delivery
InactiveUS20120065179A1Improve bioavailabilityGood water solubilityBiocideOrganic compound preparationActive agentCarboxylic acid
There is provided a prodrug of a pharmaceutically active agent, such prodrug comprising a beta-keto carboxylic acid, a beta-keto carboxylic acid salt or a beta-keto carboxylic acid ester functional group, a pharmaceutical composition comprising the prodrug, and to the use of the prodrug or composition for treatment of a mammalian subject suffering from a condition which can be cured or alleviated by administration of the pharmaceutically active agent. There is further provided a method of inhibiting decarboxylation of a compound comprising a beta-keto carboxylic acid or a salt thereof with a monovalent cation, characterized in that a dry salt of the beta-keto carboxylic acid with a divalent or polyvalent cation is prepared.
Owner:INFINITON
Sulfonic acid group-containing polymer, method for producing the same, resin composition containing such sulfonic acid group-containing polymer, polymer electrolyte membrane, polymer electrolyte membrane/electrode assembly, and fuel cell
InactiveUS20090075147A1Imparting conspicuous performance in ion conductivity and durabilityImprove conductivitySolid electrolytesFinal product manufacturePolymer electrolytesFuel cells
The present invention relates to a sulfonic acid group-containing polymer excellent in ion conductivity and durability, a method for producing the same, a resin composition containing the sulfonic acid group-containing polymer, a polymer electrolyte membrane, a polymer electrolyte membrane / electrode assembly, and a fuel cell. The sulfonic acid group-containing polymer of the present invention, in a first embodiment, includes a constituent represented by the following chemical formula 1:wherein X represents hydrogen or a monovalent cation species; Y represents a sulfone group or a ketone group; and n represents an arbitrary integer not less than 2.
Owner:TOYOBO CO LTD
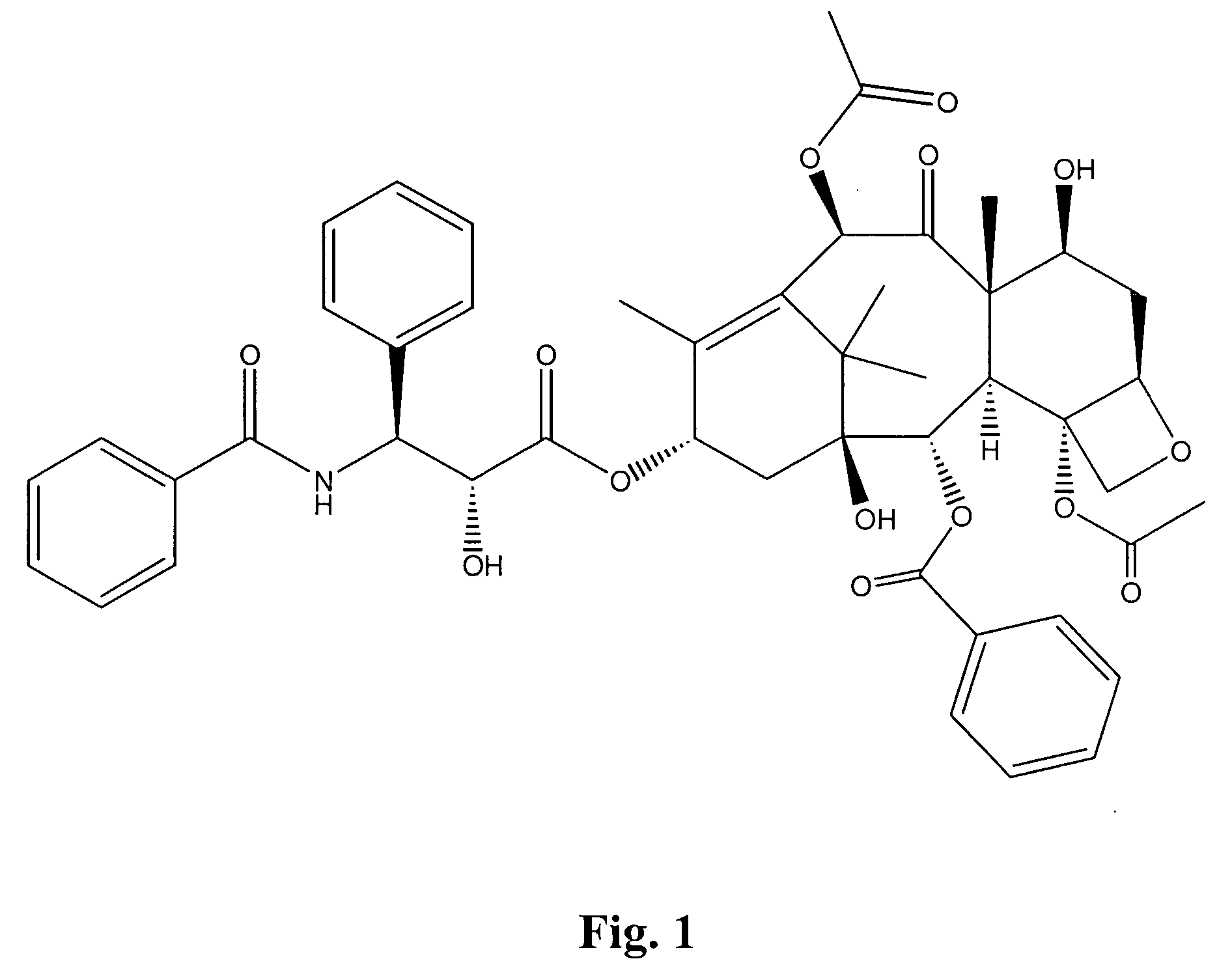
Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations
A compound of an angiotensin receptor antagonist (ARB), a neutral endopeptidase inhibitor (NEPi) and one or more monovalent cations are useful for the treatment of hypertension and / or heart failure. ARB includes S—N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine in the anion form, NEPi includes (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester in the anion form and cation includes monovalent cations such as Na+. The compound includes trisodium [3-((1S,3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3′-methyl-2′-(pentanoyl{2″-(tetrazol-5-ylate)biphenyl-4′-ylmethyl}amino)butyrate] hemipentahydrate.
Owner:NOVARTIS PHARM CORP
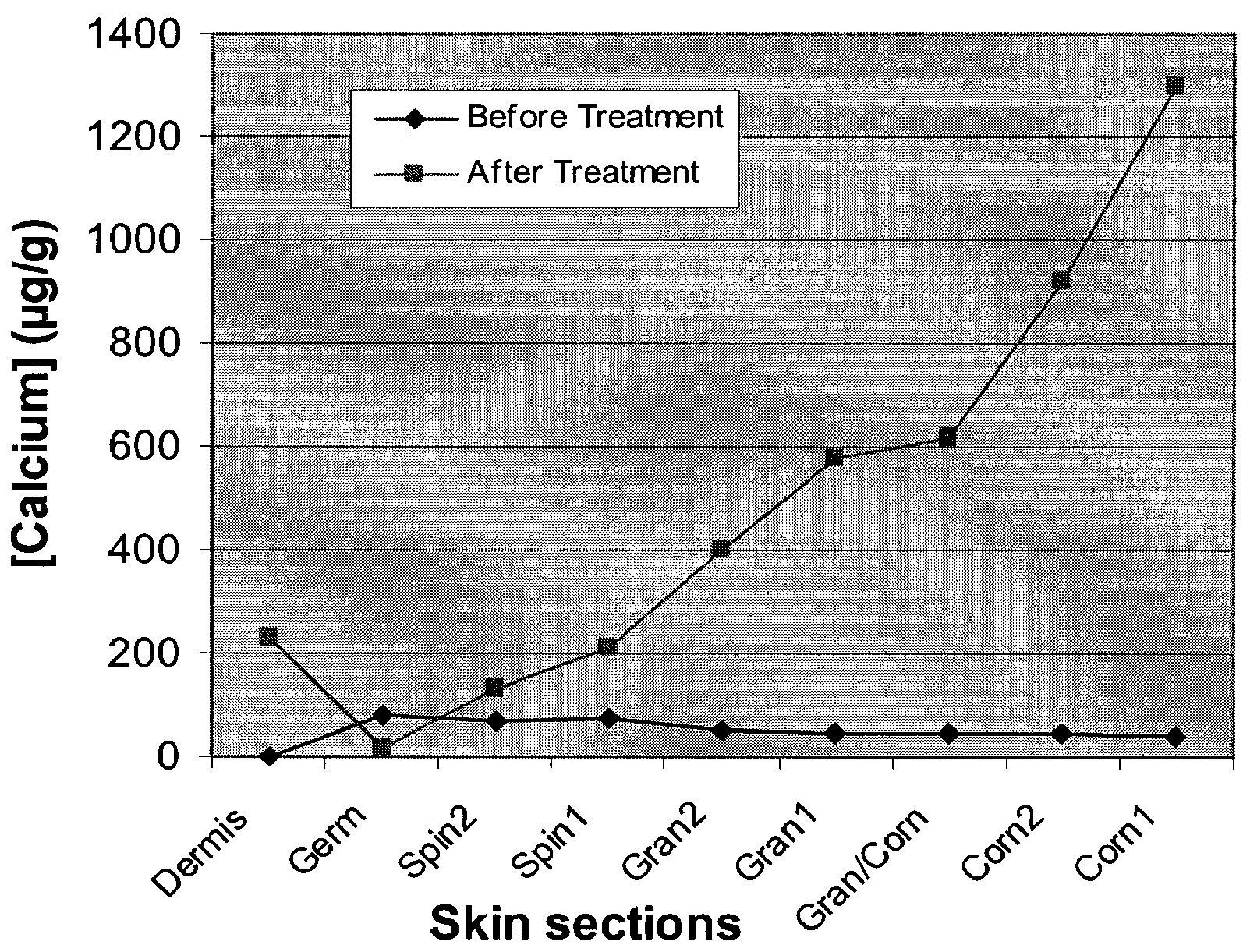
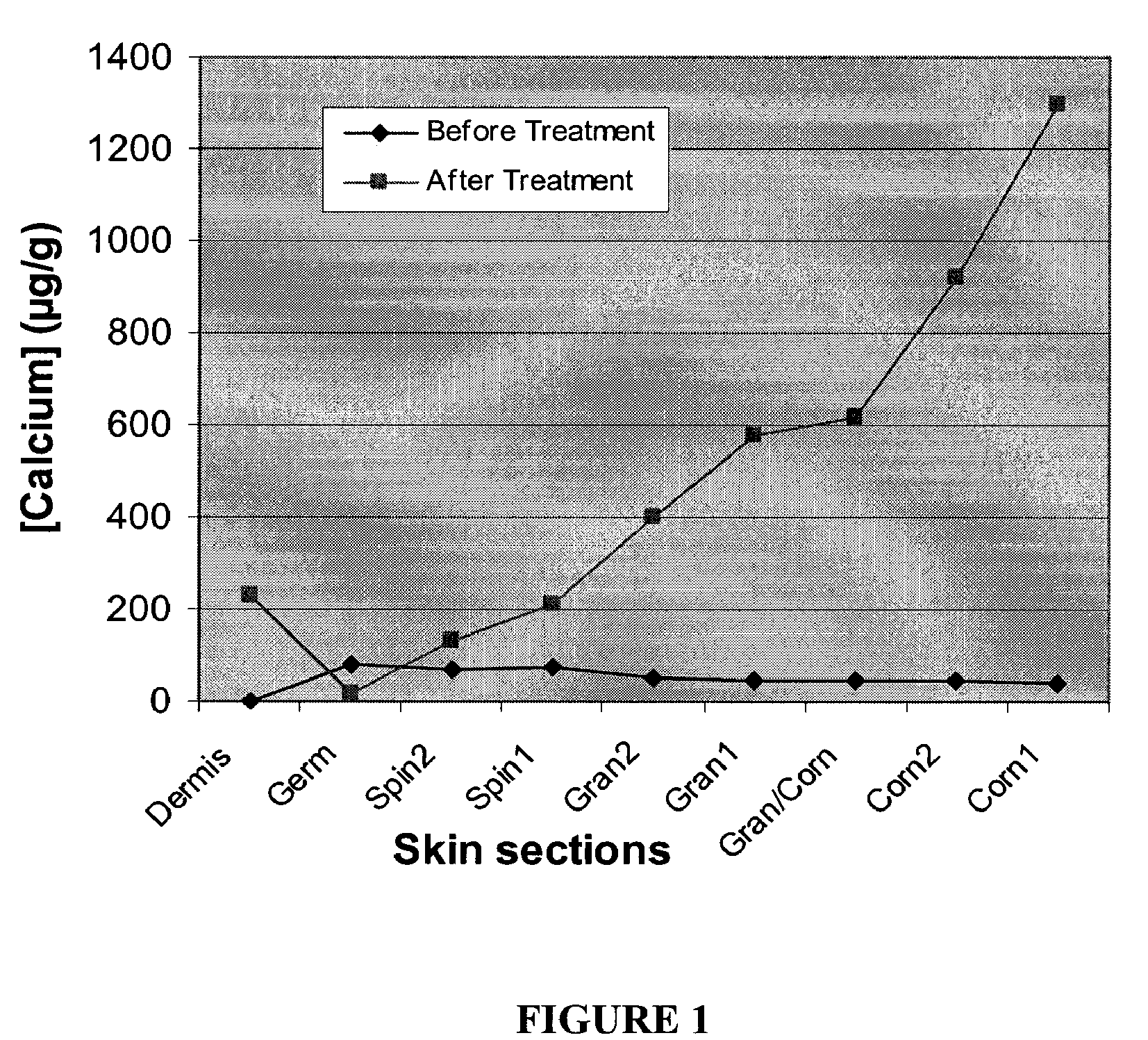
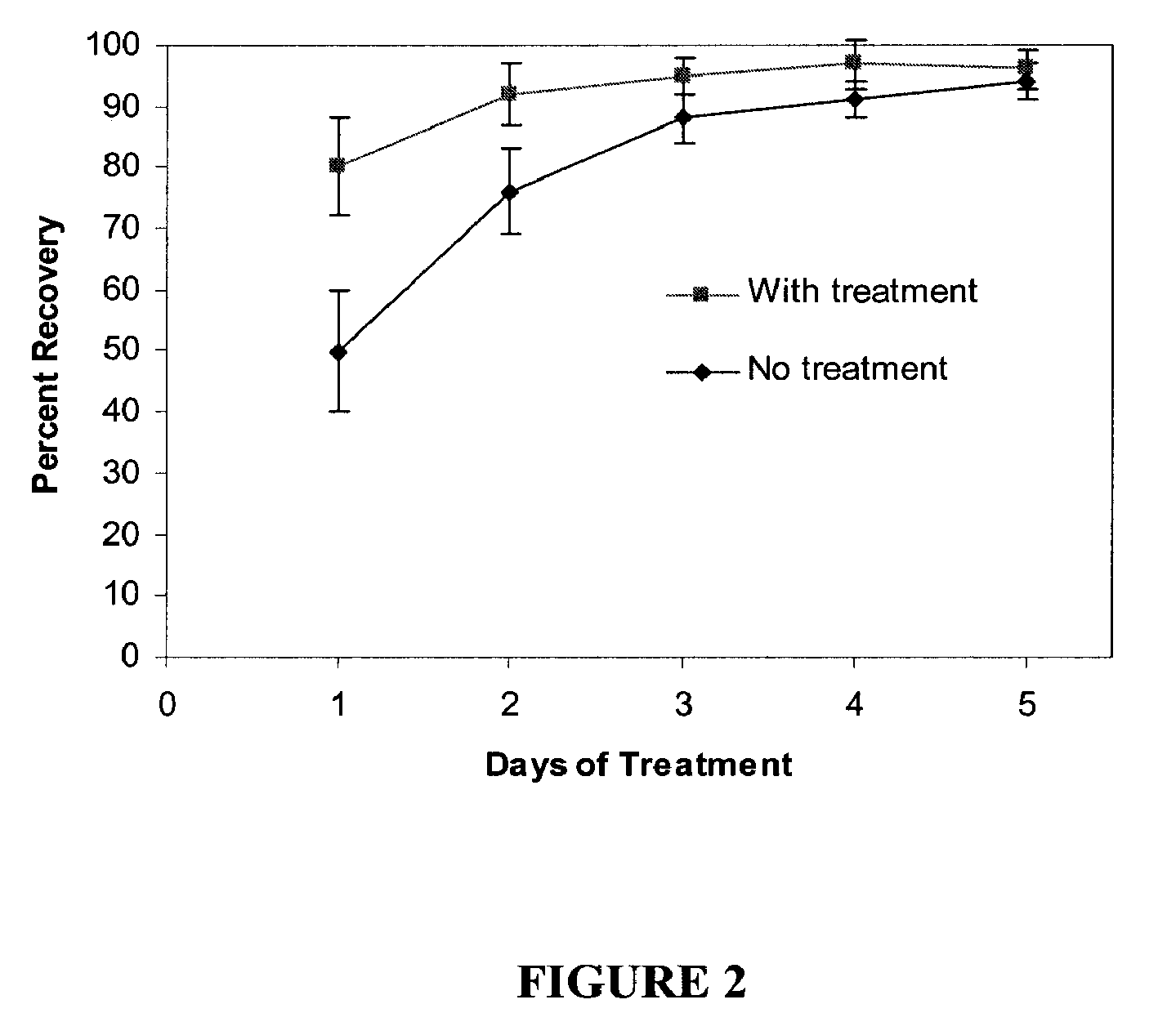
Cosmetic and cosmeceutical compositions for restoration of skin barrier function
Compositions, kits and methods are provided for restoring skin barrier function to skin exposed to environmental elements and / or in a pathological condition. In general, divalent cations such as calcium ions and / or magnesium ions are included in a physiologically acceptable medium. In some embodiments, divalent cations are balanced with monovalent cations such as sodium and potassium ions at appropriate ratios in order to maintain the homeostasis of skin barrier. The compositions, kits and methods can be used as cosmetics, cosmeceuticals or pharmaceuticals for improving skin condition, and preventing or treating dermatological diseases and skin disorders.
Owner:GENEPHARM
Electrode Which Has Been Coated With A Solid Ion Conductor Which Has A Garnet-Like Crystal Structure And Has The Stoichiometric Composition L7+XAXG3-XZr2O12
ActiveUS20140205910A1Stable crystal structureGood chemical stabilityHybrid capacitor electrodesElectrolytic capacitorsCrystal structureStoichiometric composition
The invention is directed to an electrode which has been coated with the solid ion conductor which has a garnet-like crystal structure and has the stoichiometric composition L7+xAxG3−xZr2O12, whereinL is in each case independently a monovalent cation,A is in each case independently a divalent cation,G is in each case independently a trivalent cation,0≦x≦3 andO can be partly or completely replaced by divalent or trivalent anion.
Owner:BASF SE
Bis(thio-hydrazide amide) salts for treatment of cancers
Disclosed are bis(thio-hydrazide amide) disalts, which are represented by Structural Formula (I):Y is a covalent bond or a substituted or unsubstituted straight chained hydrocarbyl group. R1-R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. Z is —O or —S. M+ is a pharmaceutically acceptable monovalent cation and M2+ is a pharmaceutically acceptable divalent cation.Also, disclosed are pharmaceutical compositions comprising a bis(thio-hydrazide amide) disalt described above. Further disclosed are methods of treating a subject with cancer. The methods comprise the step of administering an effective amount of a bis(thio-hydrazide amide) disalt described above.
Owner:SYNTA PHARMA CORP
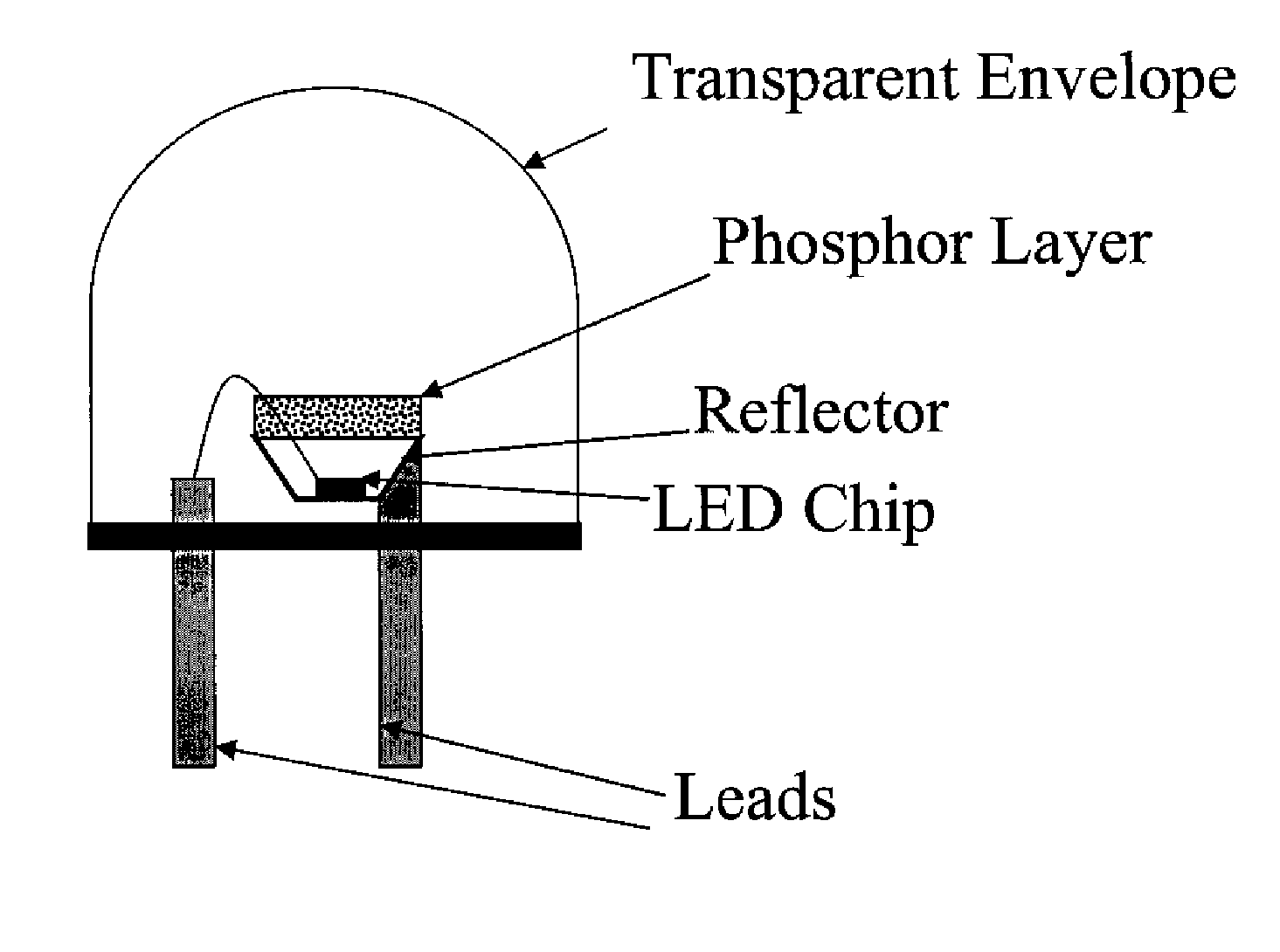
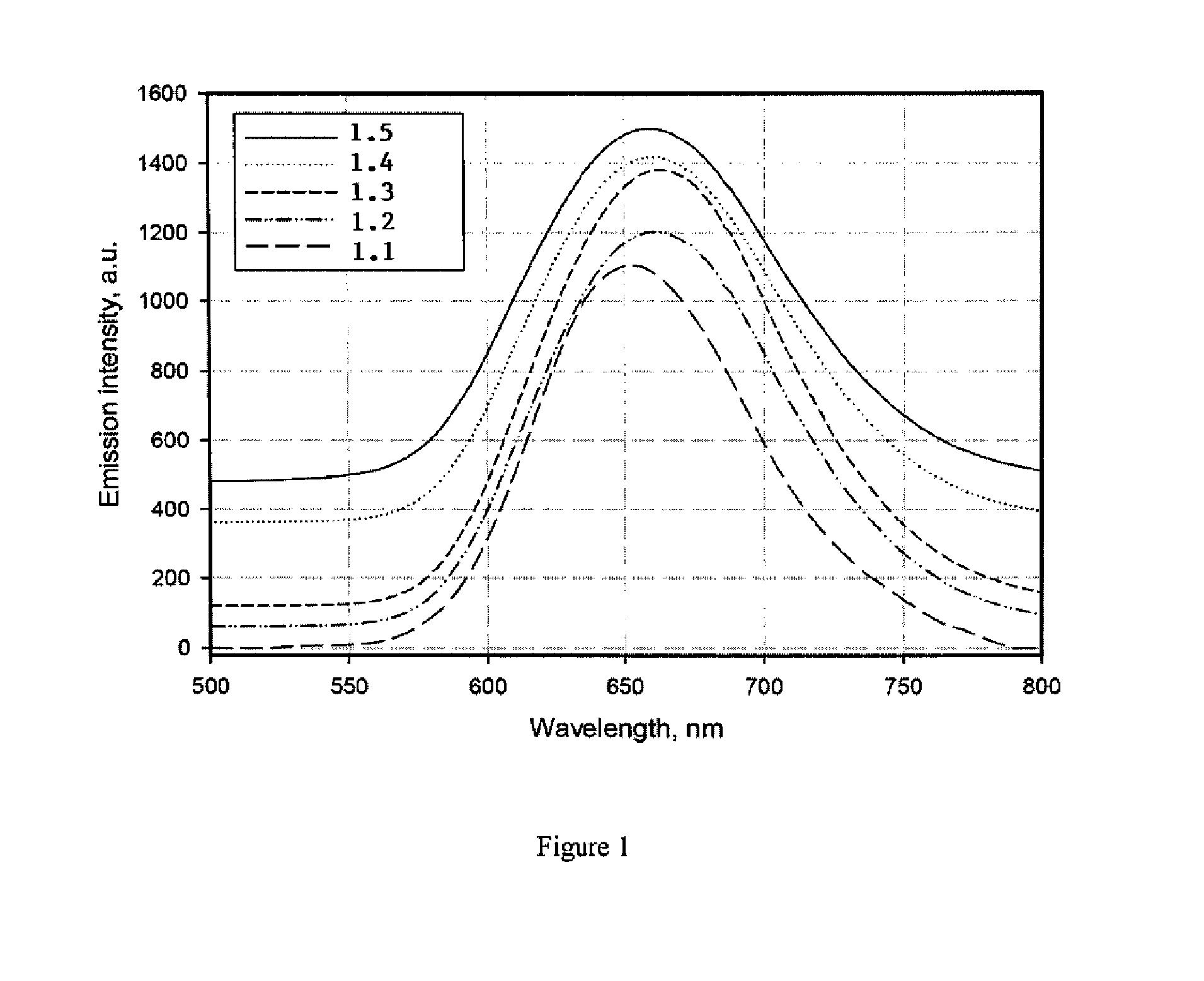
Carbonitride based phosphors and light emitting devices using the same
Disclosed herein is a novel group of carbidonitride phosphors and light emitting devices which utilize these phosphors. In certain embodiments, the present invention is directed to a novel family of carbidonitride-based phosphors expressed as follows:Ca1-xAlx-xySi1-x+xyN2-x-xyCxy:A (1);Ca1-x-zNazM(III)x-xy-zSi1-x+xy+zN2-x-xyCxy:A (2);M(II)1-x-zM(I)zM(III)x-xy-zSi1-x+xy+zN2-x-xyCxy:A (3);M(II)1-x-zM(I)zM(III)x-xy-zSi1-x+xy+zN2-x-xy-2w / 3CxyOw-v / 2Hv:A (4); andM(II)1-x-zM(I)zM(III)x-xy-zSi1-x+xy+zN2-x-xy-2w / 3-v / 3CxyOwHv:A (4a),wherein 0<x<1, 0<y<1, 0≦z<1, 0≦v<1, 0<w<1, x+z<1, x>xy+z, and 0<x-xy-z<1, M(II) is at least one divalent cation, M(I) is at least one monovalent cation, M(III) is at least one trivalent cation, H is at least one monovalent anion, and A is a luminescence activator doped in the crystal structure.
Owner:LIGHTSCAPE MATERIALS
Fluorinated ionomers with reduced amounts of carbonyl end groups
The present invention is a fluoropolymer comprising a plurality of pendent groups terminating in —CF2SO3X, —CF2SO2F, or combinations thereof, where X is selected from a group consisting of H+ and a monovalent cation, and at least one —CF2Y end group, where Y is selected from a group consisting of a chlorine atom, a bromine atom, an iodine atom, a nitrile group, and an —SO3X group.
Owner:3M INNOVATIVE PROPERTIES CO
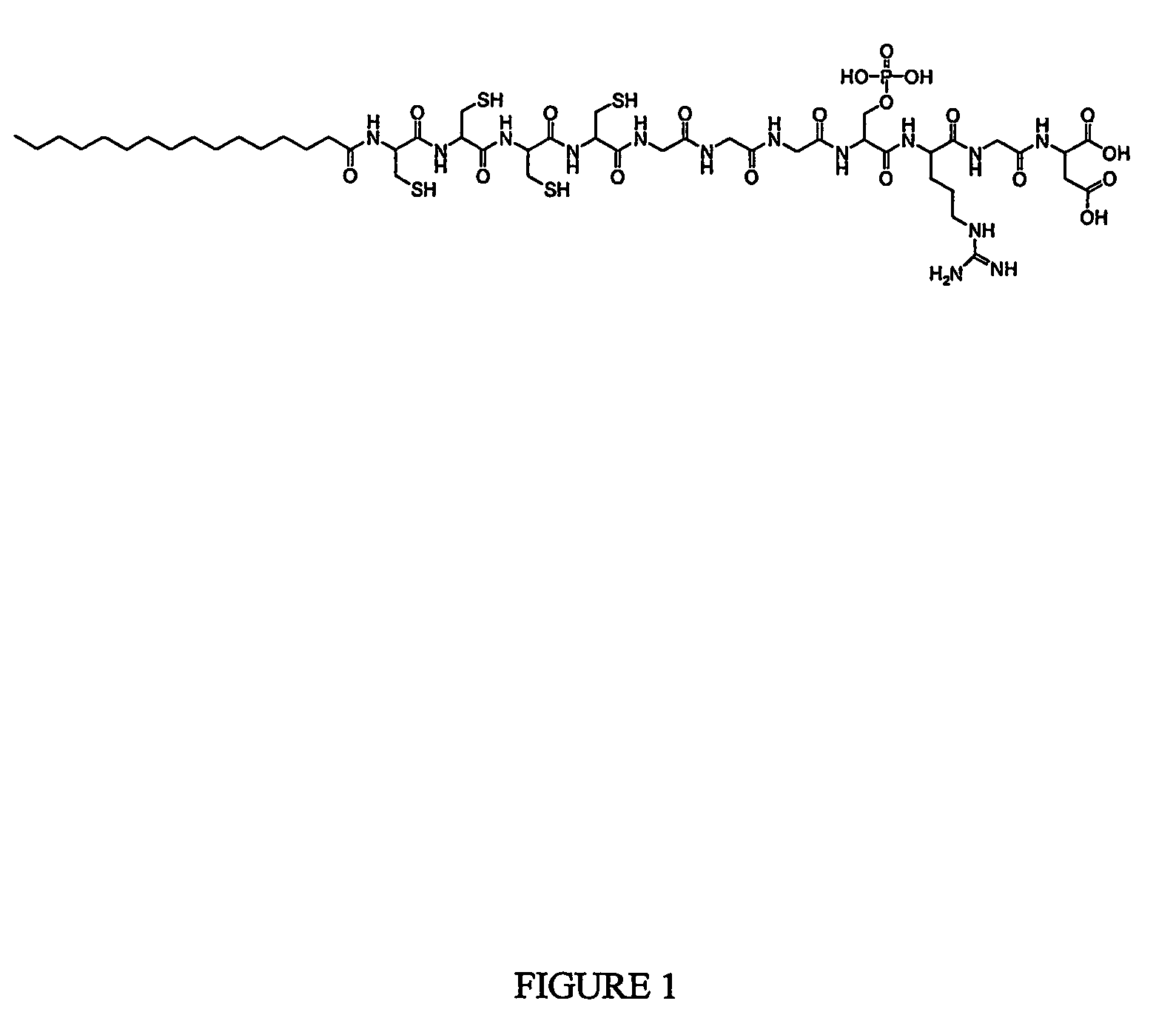
Composition and method for self-assembly and mineralizatin of peptide amphiphiles
The present invention is directed to a composition useful for making homogeneously mineralized self assembled peptide-amphiphile nanofibers and nanofiber gels. The composition is generally a solution comprised of a positively or negatively charged peptide-amphiphile and a like signed ion from the mineral. Mixing this solution with a second solution containing a dissolved counter-ion of the mineral and / or a second oppositely charged peptide amphiphile, results in the rapid self assembly of the peptide-amphiphiles into a nanofiber gel and templated mineralization of the ions. Templated mineralization of the initially dissolved mineral cations and anions in the mixture occurs with preferential orientation of the mineral crystals along the fiber surfaces within the nanofiber gel. One advantage of the present invention is that it results in homogenous growth of the mineral throughout the nanofiber gel. Another advantage of the present invention is that the nanofiber gel formation and mineralization reactions occur in a single mixing step and under substantially neutral or physiological pH conditions. These homogeneous nanostructured composite materials are useful for medical applications especially the regeneration of damaged bone in mammals. This invention is directed to the synthesis of peptide-amphiphiles with more than one amphiphilic moment and to supramolecular compositions comprised of such multi-dimensional peptide-amphiphiles. Supramolecular compositions can be formed by self assembly of multi-dimensional peptide-amphiphiles by mixing them with a solution comprising a monovalent cation.
Owner:NORTHWESTERN UNIV
Semi-batch process for producing fluoroelastomers
A novel semi-batch emulsion polymerization process for the production of fluoroelastomers is disclosed wherein a hydrocarbon anionic surfactant is employed as the dispersing agent. The surfactant has the formula R-L-M wherein R is an alkyl group having between 6 and 17 carbon atoms, L is selected from the group consisting of ArSO3−, SO3−, SO4−, PO3− and COO− and M is a univalent cation. Preferred surfactants are sulfonates of the formula CH3—(CH2)n—SO3M, CH3—(CH2)n—C6H4—SO3M, or CH3—(CH2)n—CH═CHCH2—SO3M, where n is an integer from 6 to 17, or mixtures thereof, and M is a cation having a valence of 1. Surfactant is not introduced to the reactor until shortly after the polymerization reaction has begun.
Owner:THE CHEMOURS CO FC LLC
Isomerized alpha olefin sulfonate and method of making the same
The present invention is directed to an isomerized alpha olefin sulfonate and a method of making the same wherein the isomerized alpha olefin sulfonate is derived from sulfonating an isomerized alpha olefin with sulfur trioxide in the presence of air thereby producing an isomerized alpha olefin sulfonic acid, wherein the isomerized alpha olefin is derived from the isomerization of C12-C40 normal alpha olefins; and neutralizing the isomerized alpha olefin sulfonic acid with a source of a mono-valent cation.
Owner:CHEVRON ORONITE CO LLC
Composition and method for self-assembly and mineralization of peptide amphiphiles
The present invention is directed to a composition useful for making homogeneously mineralized self assembled peptide-amphiphile nanofibers and nanofiber gels. The composition is generally a solution comprised of a positively or negatively charged peptide-amphiphile and a like signed ion from the mineral. Mixing this solution with a second solution containing a dissolved counter-ion of the mineral and / or a second oppositely charged peptide amphiphile, results in the rapid self assembly of the peptide-amphiphiles into a nanofiber gel and templated mineralization of the ions. Templated mineralization of the initially dissolved mineral cations and anions in the mixture occurs with preferential orientation of the mineral crystals along the fiber surfaces within the nanofiber gel. One advantage of the present invention is that it results in homogenous growth of the mineral throughout the nanofiber gel. Another advantage of the present invention is that the nanofiber gel formation and mineralization reactions occur in a single mixing step and under substantially neutral or physiological pH conditions. These homogeneous nanostructured composite materials are useful for medical applications especially the regeneration of damaged bone in mammals. This invention is directed to the synthesis of peptide-amphiphiles with more than one amphiphilic moment and to supramolecular compositions comprised of such multi-dimensional peptide-amphiphiles. Supramolecular compositions can be formed by self assembly of multi-dimensional peptide-amphiphiles by mixing them with a solution comprising a monovalent cation.
Owner:NORTHWESTERN UNIV
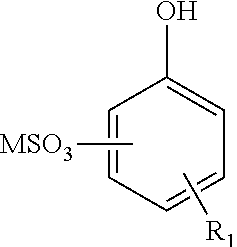
Enhanced oil recovery surfactant composition and method of making the same
ActiveUS20110046024A1Reduce the amount requiredLess solventFlushingDrilling compositionSulfonateSolvent
The present invention is directed to an enhanced oil recovery composition comprising (a) a surfactant comprising an alkylated hydroxyaromatic sulfonate having the general formula:wherein R1 is an alkyl group containing from about 8 to 40 carbon atoms and having from about 20% to about 50% methyl branching, and M is a mono-valent cation; (b) a solvent; (c) a passivator; and (d) a polymer.
Owner:CHEVRON ORONITE CO LLC
Method for preparing novel biological microcapsule for biological fluidized bed
ActiveCN102351320AImprove mass transfer effectEnhanced mass transferSustainable biological treatmentBiological water/sewage treatmentFluidized bedIon exchange
The invention discloses a method for preparing a novel biological microcapsule for a biological fluidized bed. The microcapsule is prepared through treating sodium alginate, powdery active carbon, calcium chloride and chitosan as capsule materials, and a dominant degradation bacterium as a capsule core, preparing calcium alginate gel beads through carrying out ion exchange on sodium alginate and calcium chloride, coating the surface of the gel beads with chitosan, liquefying the gel beads in sodium citrate, and coating the gel beads with sodium alginate. The diameter of the prepared microcapsule is 3.0-4.0mm, the membrane thickness of the prepared microcapsule is 10-20mum, the density of the prepared microcapsule is 1.05-1.08g / mL, and the maximum interception molecular weight of the prepared microcapsule is about PEG 4000, so a micromolecular substance with the relative molecular weight of less than PEG 1500 can freely go through the capsule membrane. Acidic conditions and most divalent cations allow the microcapsule to be stable, and monovalent cations and most anions are bad for the stability of the microcapsule.
Owner:江苏科易达环保科技股份有限公司
Perovskite-type metal oxide compounds and methods of making and using thereof
Perovskite-type catalyst consists essentially of a metal oxide composition and methods of using them are provided. The metal oxide composition is represented by the general formula Aa−xBxMOb, in which A is a mixture of elements originally in the form of single phase mixed lanthanides collected from bastnasite; B is a divalent or monovalent cation; M is at least one element selected from the group consisting of elements of an atomic number of from 23 to 30, 40 to 51, and 73 to 80; a is 1 or 2; b is 3 when a is 1 or b is 4 when a is 2; and x is a number defined by 0≦x<0.5. Methods of making and using the perovskite-type catalysts are also provided. The perovskite-type catalyst may be used to reduce nitrogen oxides, oxidize carbon monoxide, and oxidize hydrocarbons in an exhaust stream from a motor vehicle. Methods of such a use are provided.
Owner:CATALYTIC SOLUTIONS INC
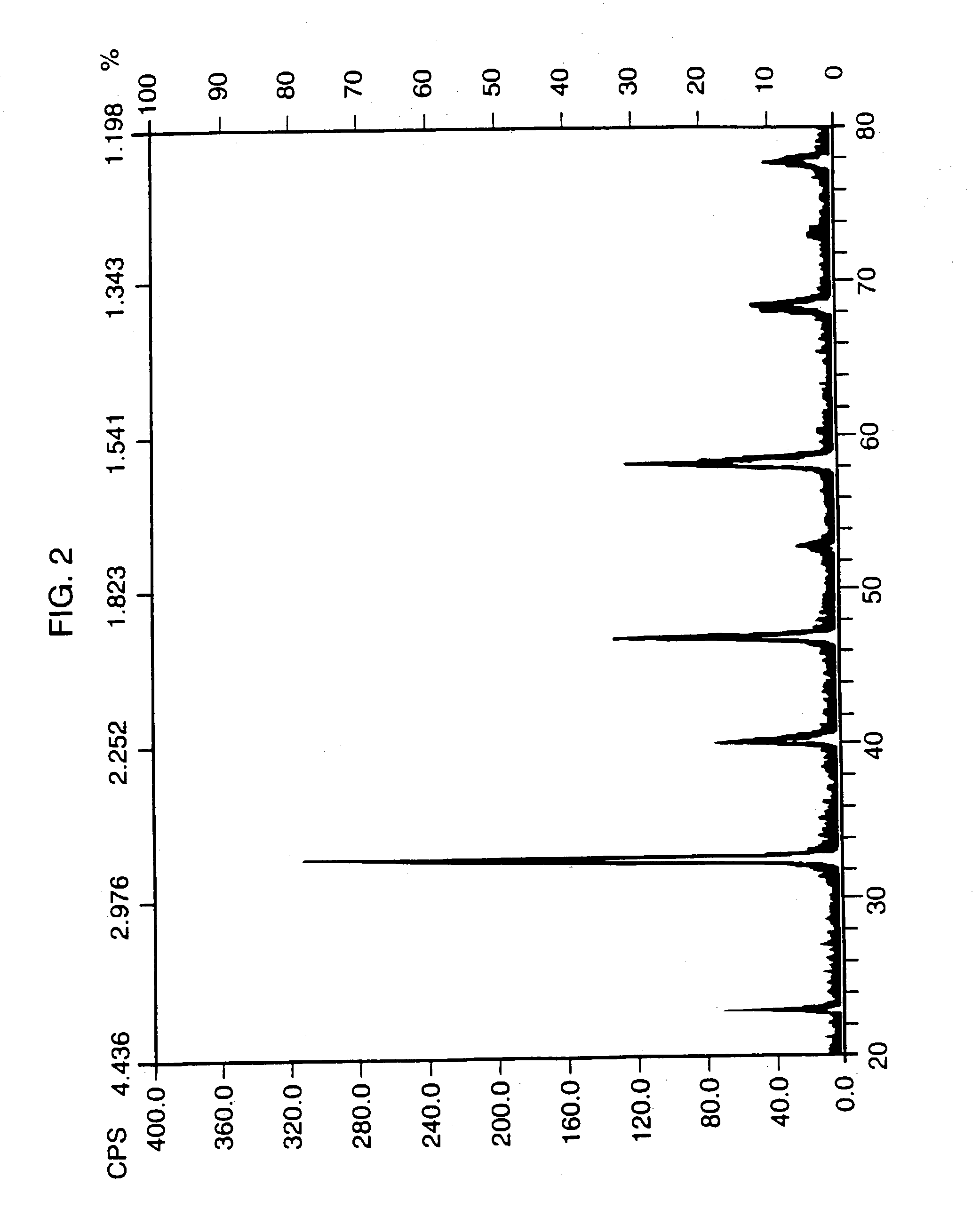
Method for fluorinating a compound comprising a halosulphonyl or dihalophosphonyl group
The invention relates to a fluorination process for producing fluorinated compounds.The process consists in reacting a compound (I) corresponding to the formulawith an ionic fluoride of a monovalent cation. M represents H, an alkali metal, a quaternary phosphonium group or a quaternary ammonium group. Y represents SO2 and m is 1, or else Y is PO and m is 2. Z represents CR2, N or P. R1 represents an electron-withdrawing group which has a Hammet σP parameter of greater than 0.4. R2 represents a carbonaceous and / or electron-withdrawing group. X represents a halogen other than a fluorine.The fluorinated compounds obtained are of use in particular as electrolytes in lithium batteries.
Owner:HYDRO QUEBEC CORP
Method of controlling emissions from a diesel cycle internal combustion engine with perovskite-type metal oxide compounds
Methods of controlling emissions from a diesel engine are provided. The method includes contacting the emissions with a perovskite-type catalyst consisting essentially of a metal oxide composition represented by the general formula Aa-xBxMOb, in which A is a mixture originally in the form of single phase mixed lanthanides collected from bastnasite; B is a divalent or monovalent cation; M is at least one element selected from the group consisting of M is at least one element selected from the group consisting of elements of an atomic number of from 22 to 30, 40 to 51, and 73 to 80; a is 1 or 2; b is 3 when a is 1 or b is 4 when a is 2; and x is a number defined by 0<x<0.7. The perovskite-type catalyst may be used to oxidize hydrocarbons and carbon monoxide and to control particulate emissions in the diesel exhaust.
Owner:CATALYTIC SOLUTIONS INC
Liquid Detergent Composition
PendingUS20140228274A1Low stability of enzymeSurface-active detergent compositionsNon-surface-active detergent compositionsSubtilisinAdduct
In a liquid detergent comprising a subtilisin and optionally a second (non-subtilisin) enzyme, the combination of a peptide aldehyde (or hydrosulfite adduct thereof) with a salt of a monovalent cation and a monovalent organic anion has a synergistic stabilizing effect on the subtilisin and / or the second enzyme. The improved enzyme stability is of particular interest in liquid detergent compositions where the enzyme would otherwise have poor storage stability.
Owner:NOVOZYMES AS
Method for preparing positively charged nanofiltration membranes
InactiveCN101766962ASeparation performance is differentHigh retention rateSemi-permeable membranesCross-linkAqueous solution
The invention discloses a method for preparing positively charged nanofiltration membranes. The positively charged nanofiltration membranes are formed by porous support layers and functional layers which are formed by copolymers containing cations and hydroxy. The preparation process is as follows: firstly, obtaining functional copolymers through free radical copolymerization, preparing the copolymers into water solution with certain concentration, coating the prepared water solution on the support layers and drying the support layers; and secondly, immerging the support layers into solution containing cross-linking agents, and finally carrying out heating and curing to obtain the positively charged nanofiltration membranes. Under the operation pressure of 0.6MPa, the positively charged nanofiltration membranes have water flux of 12-18L / m<2>.h, show very high retention ratio which is generally 75-95% to bivalent cations and show retention ratio which is generally lower than 65% to monovalent cations. The prepared positively charged nanofiltration membranes have excellent separation property, and the method is simple and feasible, low in cost and easy for industrial production.
Owner:ZHEJIANG UNIV
Bis(thio-hydrazide amide) salts for treatment of cancers
InactiveUS20060135595A1Good water solubilityImprove bioavailabilityBiocideOrganic chemistryArylThio-
Disclosed are bis(thio-hydrazide amide) disalts, which are represented by Structural Formula (I): Y is a covalent bond or a substituted or unsubstituted straight chained hydrocarbyl group. R1-R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. Z is —O or —S. M+ is a pharmaceutically acceptable monovalent cation and M2+ is a pharmaceutically acceptable divalent cation. Also, disclosed are pharmaceutical compositions comprising a bis(thio-hydrazide amide) disalt described above. Further disclosed are methods of treating a subject with cancer. The methods comprise the step of administering an effective amount of a bis(thio-hydrazide amide) disalt described above.
Owner:SYNTA PHARMA CORP
Preparation method of polyvinylidene fluoride grafted p-styrenesulfonic acid proton exchange membrane
InactiveCN102496732AIncrease contact areaImprove conductivityFinal product manufactureSolid electrolyte fuel cellsNitrogen gasUltrasonic oscillation
The invention provides a preparation method of a polyvinylidene fluoride grafted p-styrenesulfonic acid proton exchange membrane. The method comprises the following steps of: firstly, adding a phase transfer catalyst into a prepared alkaline alcohol solution to obtain an alkaline alcohol solution containing the phase transfer catalyst; adding polyvinylidene fluoride into the solution and processing to obtain alkaline-treatment polyvinylidene fluoride powder; adding the obtained powder into an organic solvent to obtain an alkaline-treatment polyvinylidene fluoride powder solution; adding a p-styrene sulfonate monomer and an initiator, and reacting in a nitrogen atmosphere; performing ultrasonic oscillation, and putting the product in a polytetrafluoroethylene membrane frame; drying and stripping the membrane; replacing the monovalent cations in the membrane; removing the residual sulfuric acid; and storing the product in the deionized water to obtain the polyvinylidene fluoride grafted p-styrenesulfonic acid proton exchange membrane. Through the method, a proton exchange membrane with high electric conductivity can be prepared, and the electric conductivity is relatively approximate to that of a Nafion membrane; and the preparation method is simple and easy to implement, has relatively low cost and can be applied to large-scale production.
Owner:HUBEI UNIV
Method of controlling emissions from a diesel cycle internal combustion engine with perovskite-type metal oxide compounds
Methods of controlling emissions from a diesel engine are provided. The method includes contacting the emissions with a perovskite-type catalyst consisting essentially of a metal oxide composition represented by the general formula Aa−xBxMOb, in which A is a mixture originally in the form of single phase mixed lanthanides collected from bastnasite; B is a divalent or monovalent cation; M is at least one element selected from the group consisting of M is at least one element selected from the group consisting of elements of an atomic number of from 22 to 30, 40 to 51, and 73 to 80; a is 1 or 2; b is 3 when a is 1 or b is 4 when a is 2; and x is a number defined by 0<x<0.7. The perovskite-type catalyst may be used to oxidize hydrocarbons and carbon monoxide and to control particulate emissions in the diesel exhaust.
Owner:CATALYTIC SOLUTIONS INC
Novel Sulfonic-Acid-Group-Containing Segmented Block Copolymer, Application Thereof, and Method of Manufacturing Novel Block Copolymer
InactiveUS20110065021A1Excellent methanol inhibition propertyEnhanced inhibitory effectSolid electrolytesFinal product manufactureBenzeneFuel cells
[Object] To provide a proton exchange membrane for a fuel cell having excellent proton conductivity, lower property of swelling with hot water, and excellent durability, as well as a block copolymer forming the proton exchange membrane, and a composition, a molded product, a fuel cell proton exchange membrane electrode assembly, and a fuel cell.[Solving Means] (1) A block copolymer having a hydrophilic segment and a hydrophobic segment and having a structure expressed by Chemical Formula 1 below(where X represents H or a univalent cation, Y represents sulfonyl group or carbonyl group, each of Z and z′ independently represents any of O and S atoms, W represents one or more group selected from the group consisting of direct bond between benzenes, sulfone group and carbonyl group, each of Ar1 and Ar2 independently represents divalent aromatic group, and each of n and m independently represents an integer from 2 to 100), and a molded product, a composition, and a proton exchange membrane, as well as a fuel cell including the proton exchange membrane.
Owner:TOYO TOYOBO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



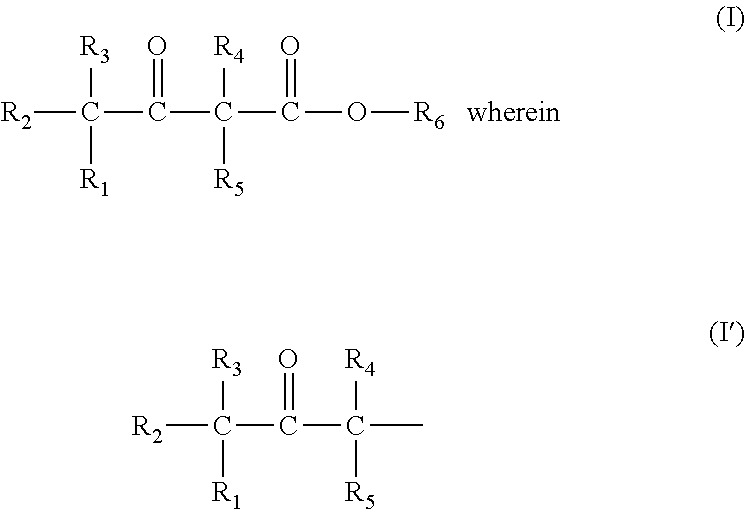


![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00000.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-D00001.png)
![Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations Compounds containing S-N-valeryl-N-{[2′-(1H-tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester moieties and cations](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eb239ae2-f451-4c9e-8101-694067a7ab8d/US08877938-20141104-C00001.png)