Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2752results about "Solvent extraction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
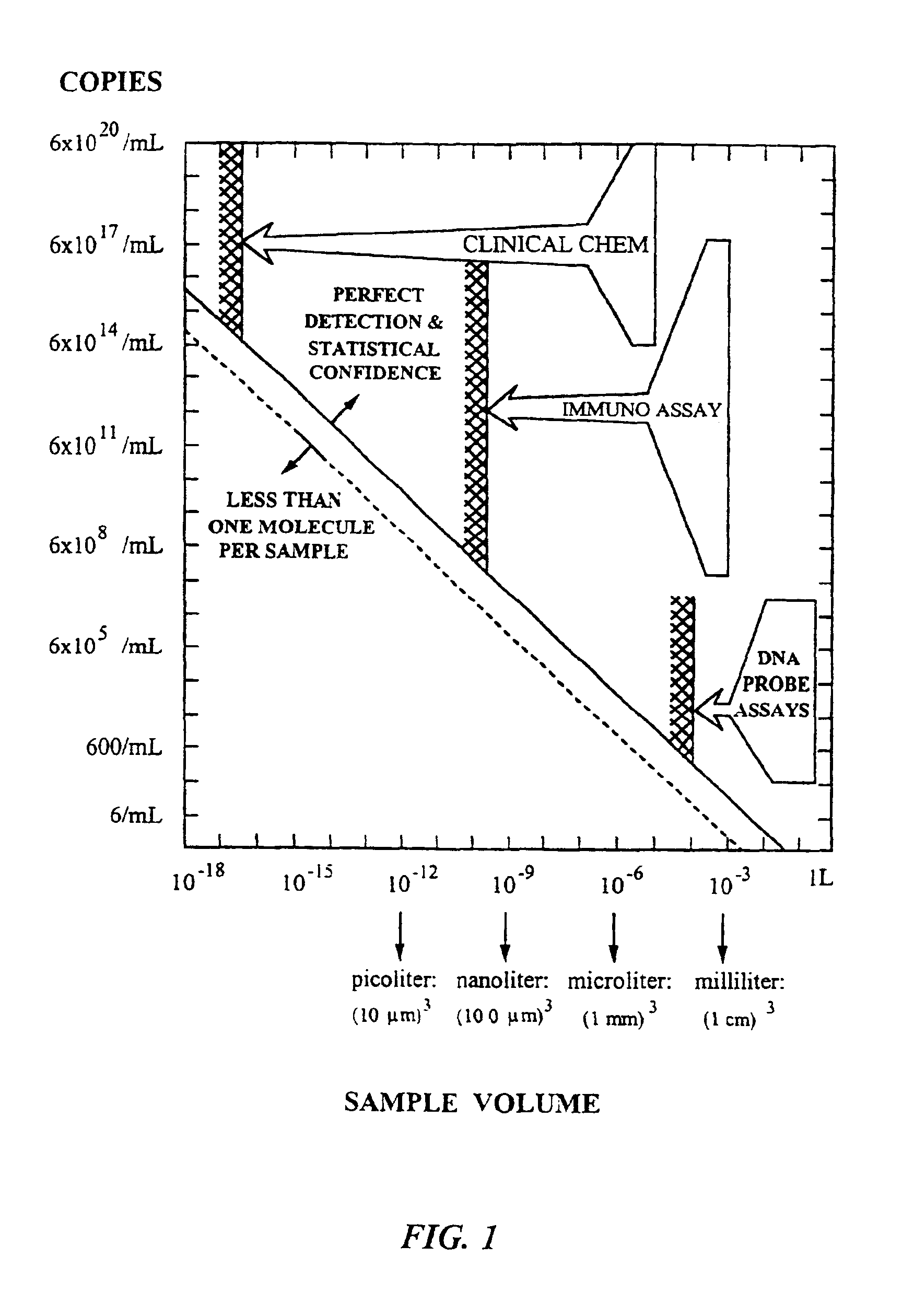
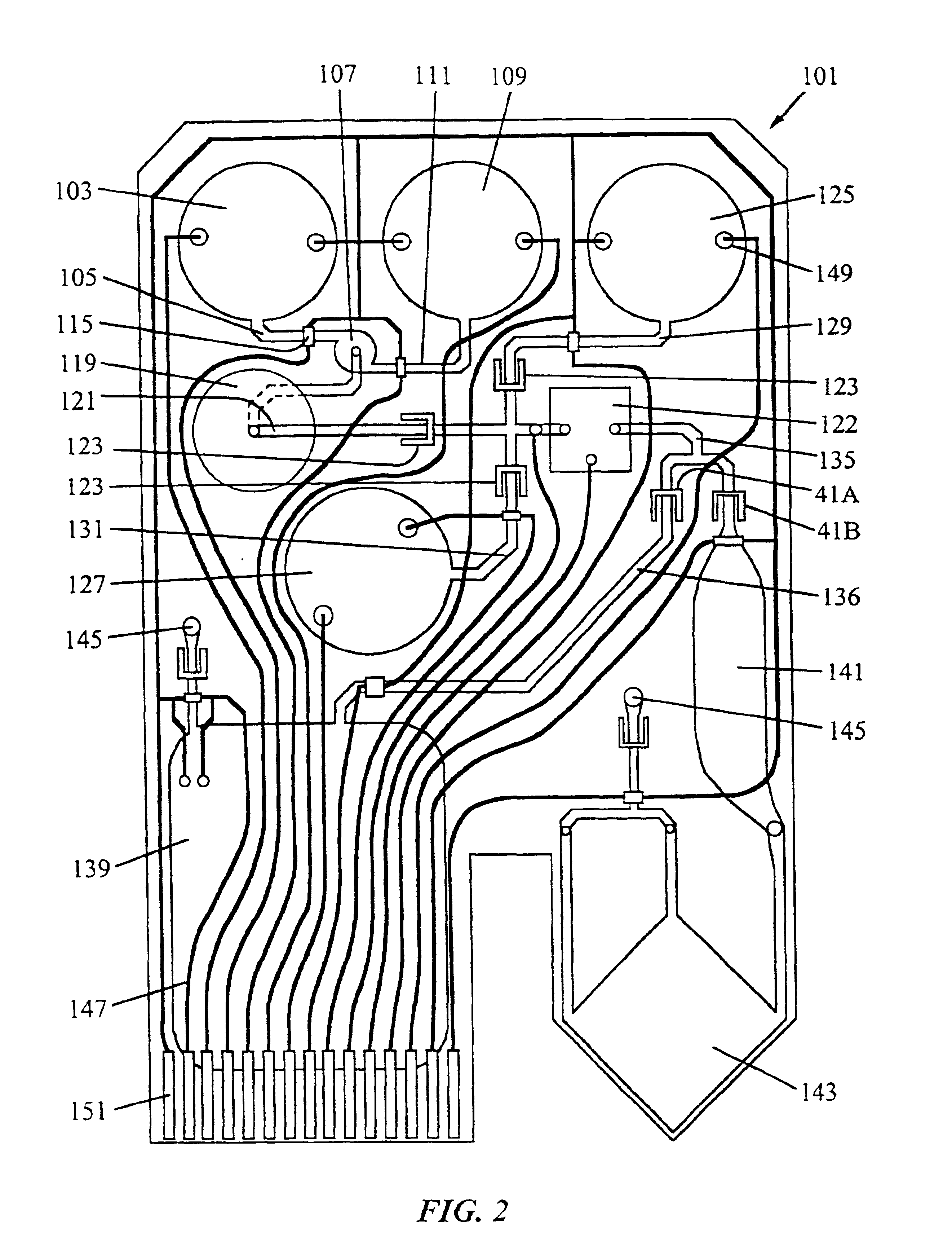
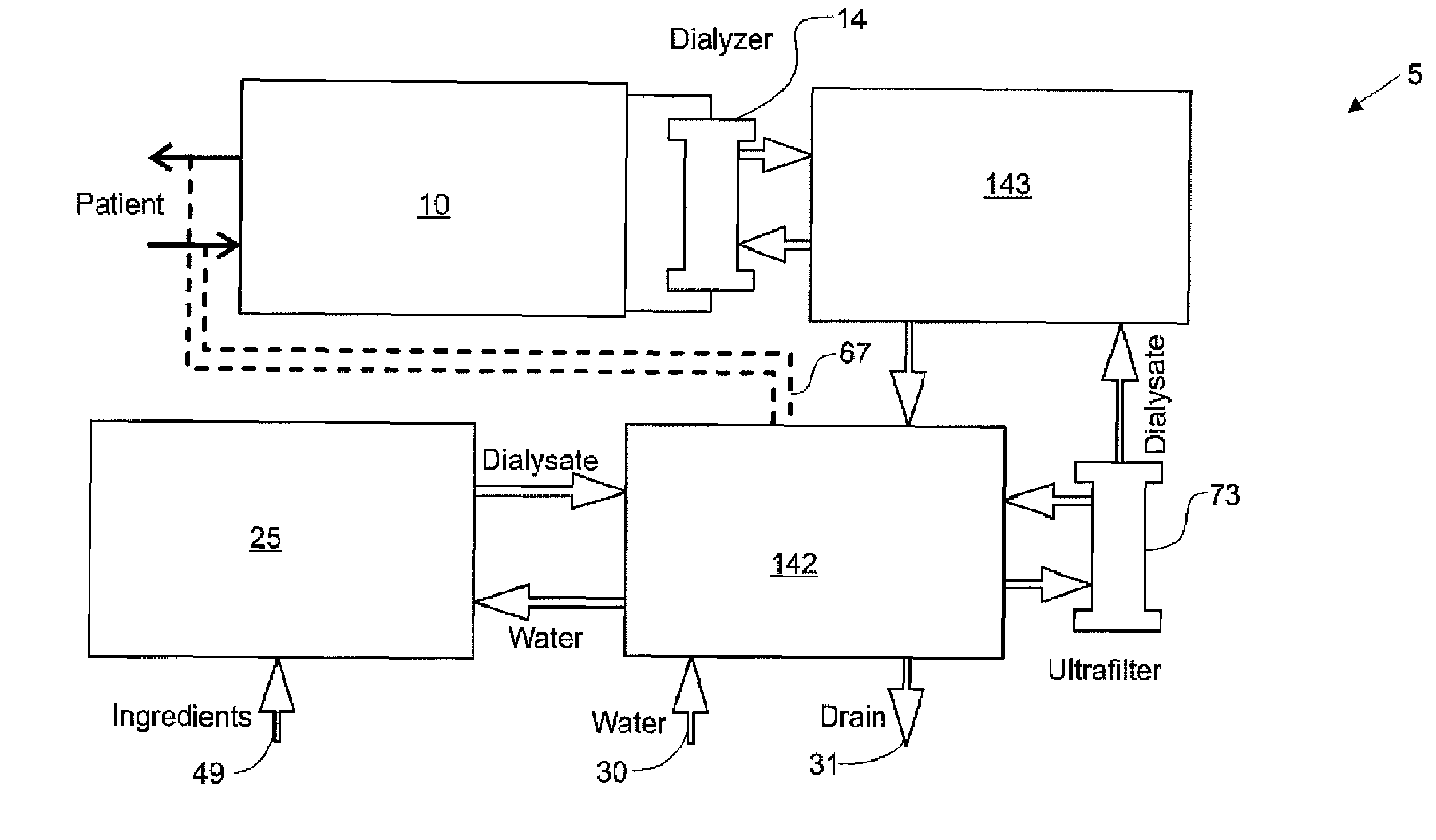
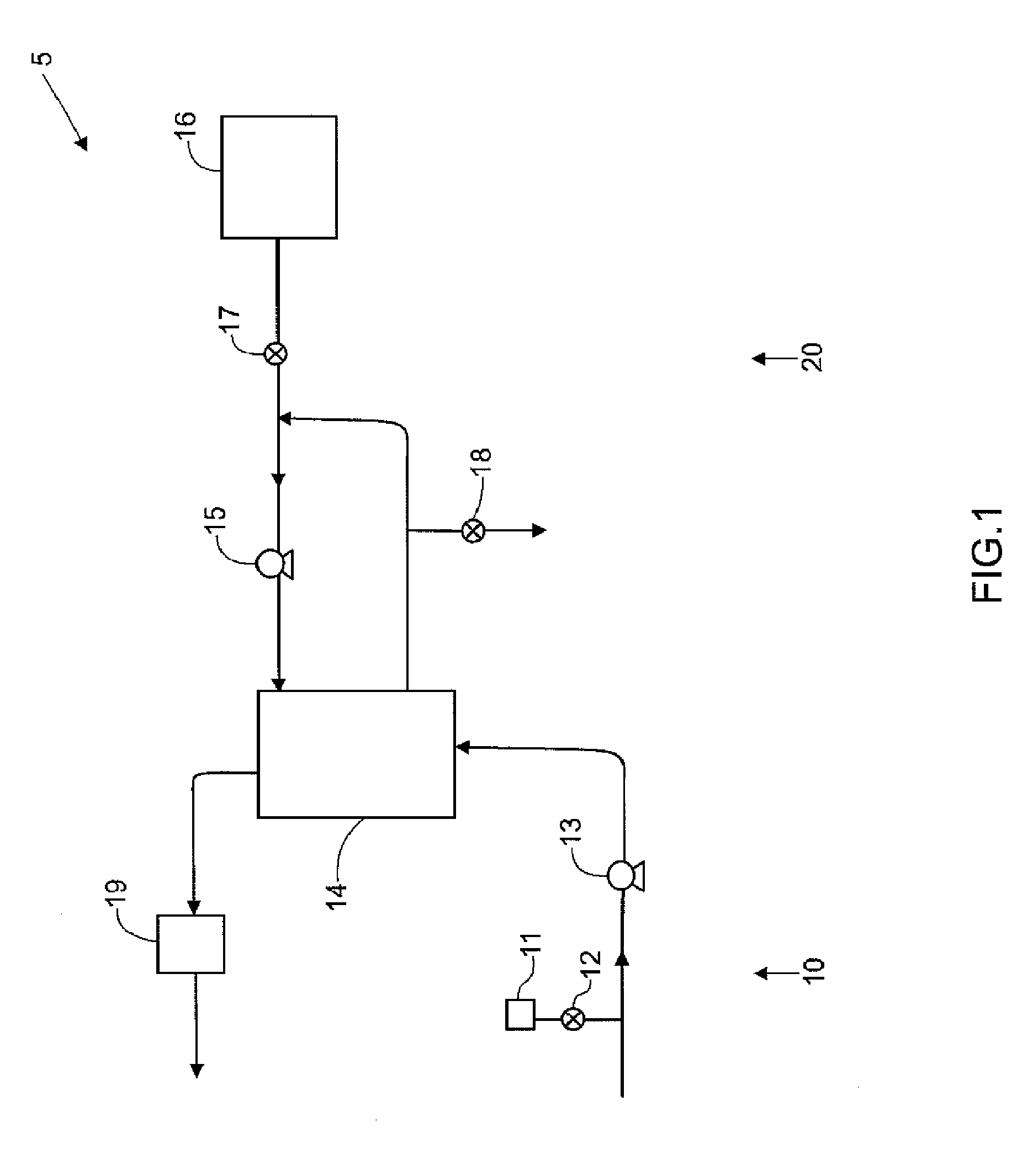
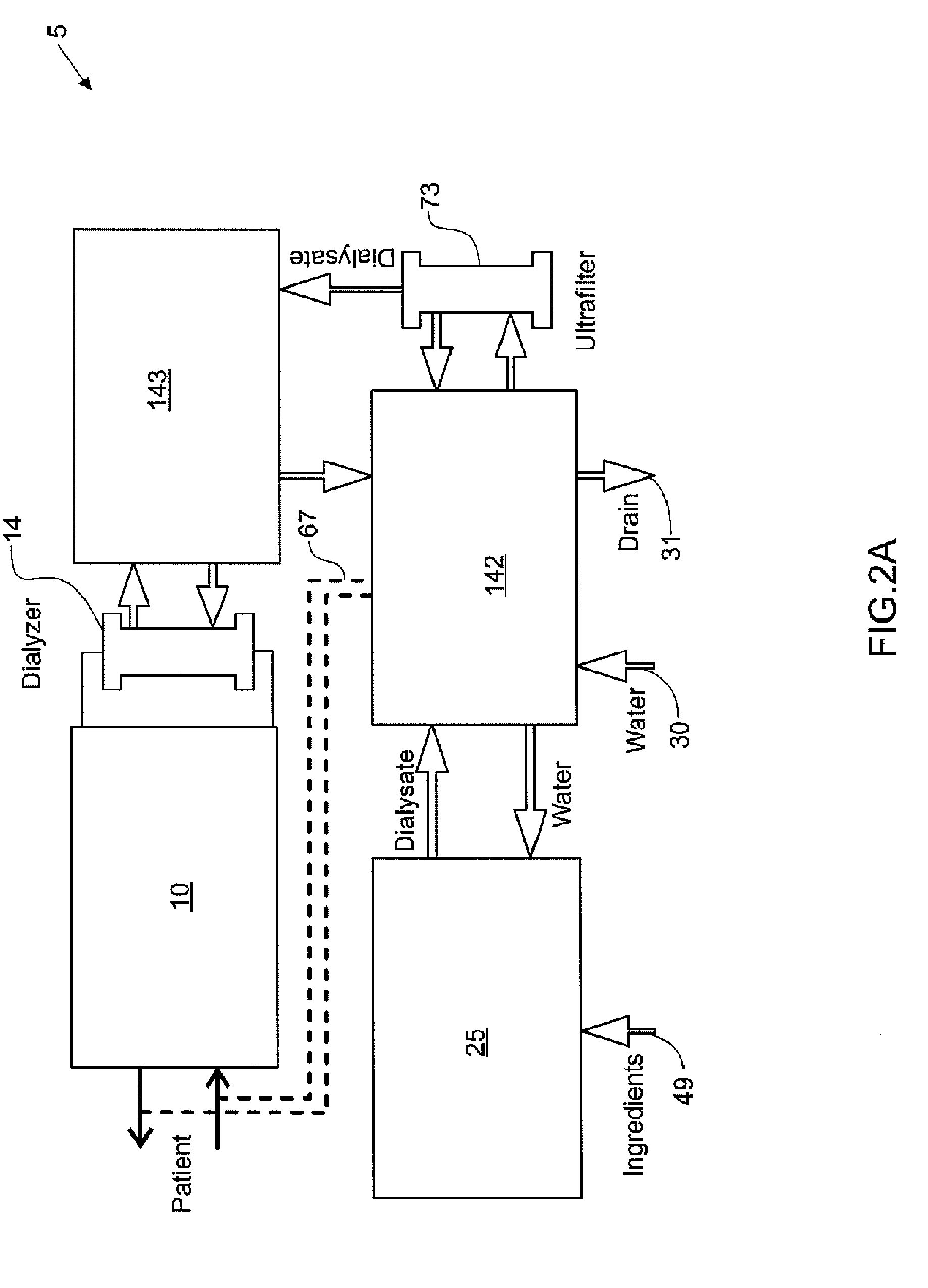
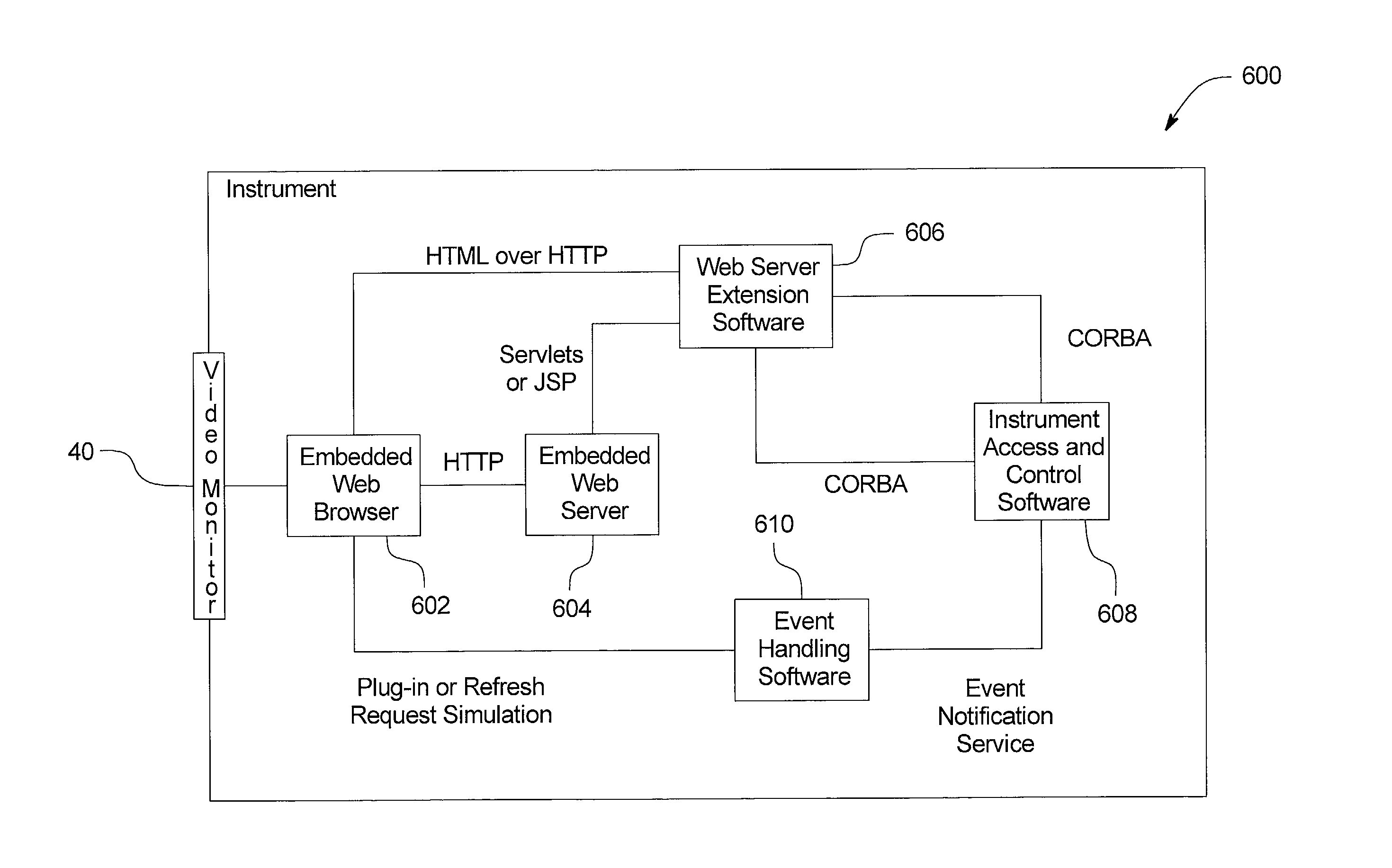
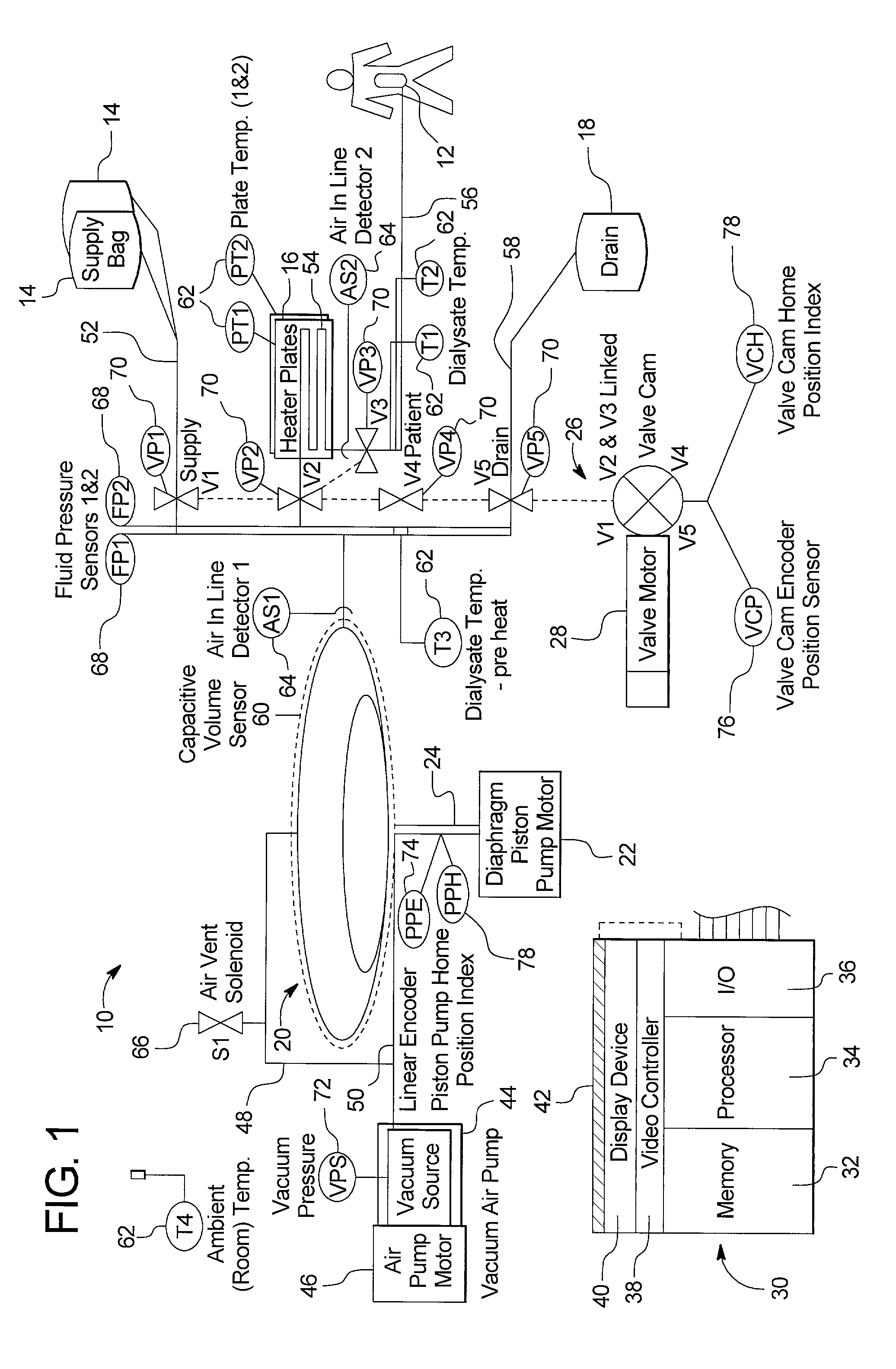
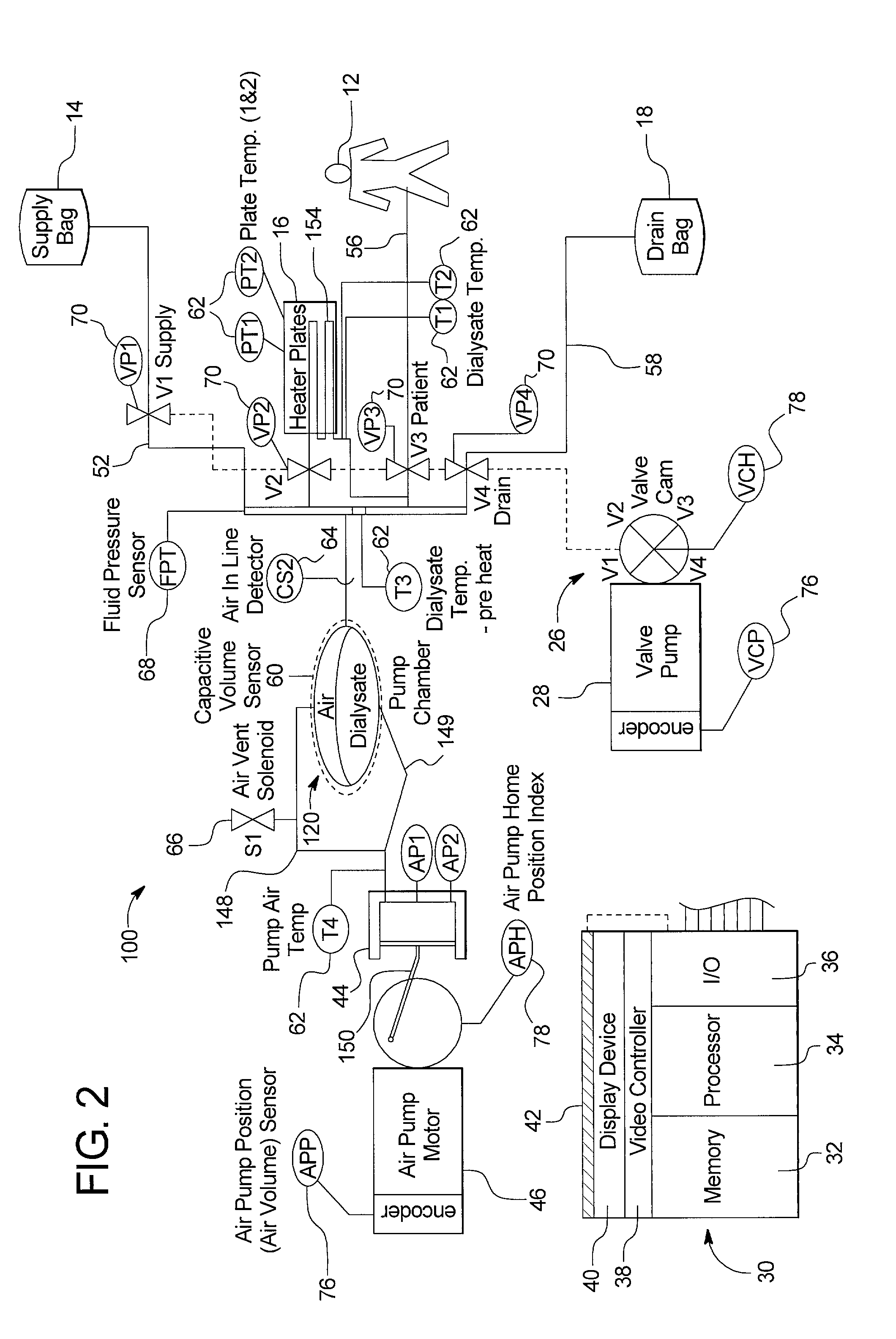
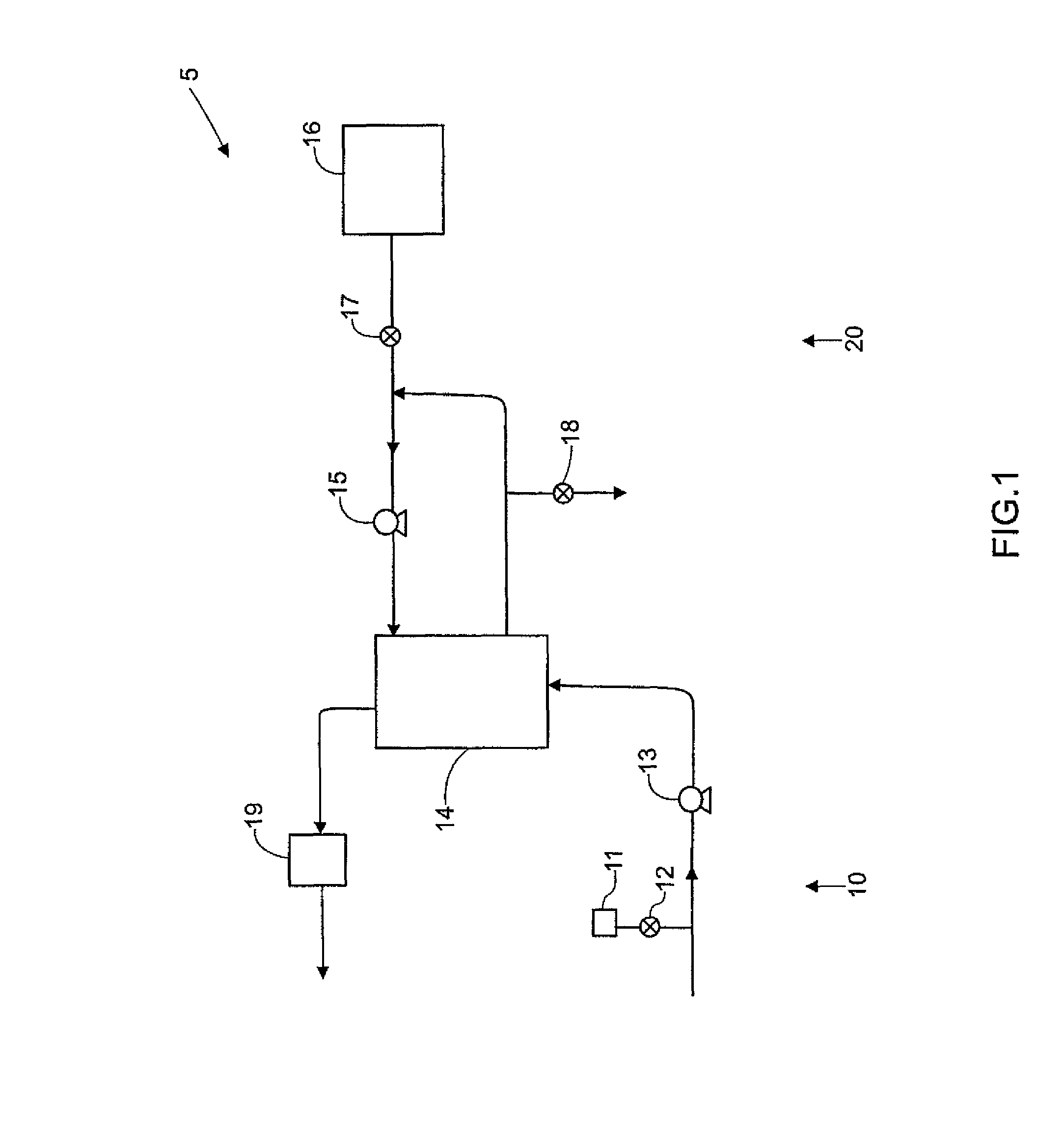
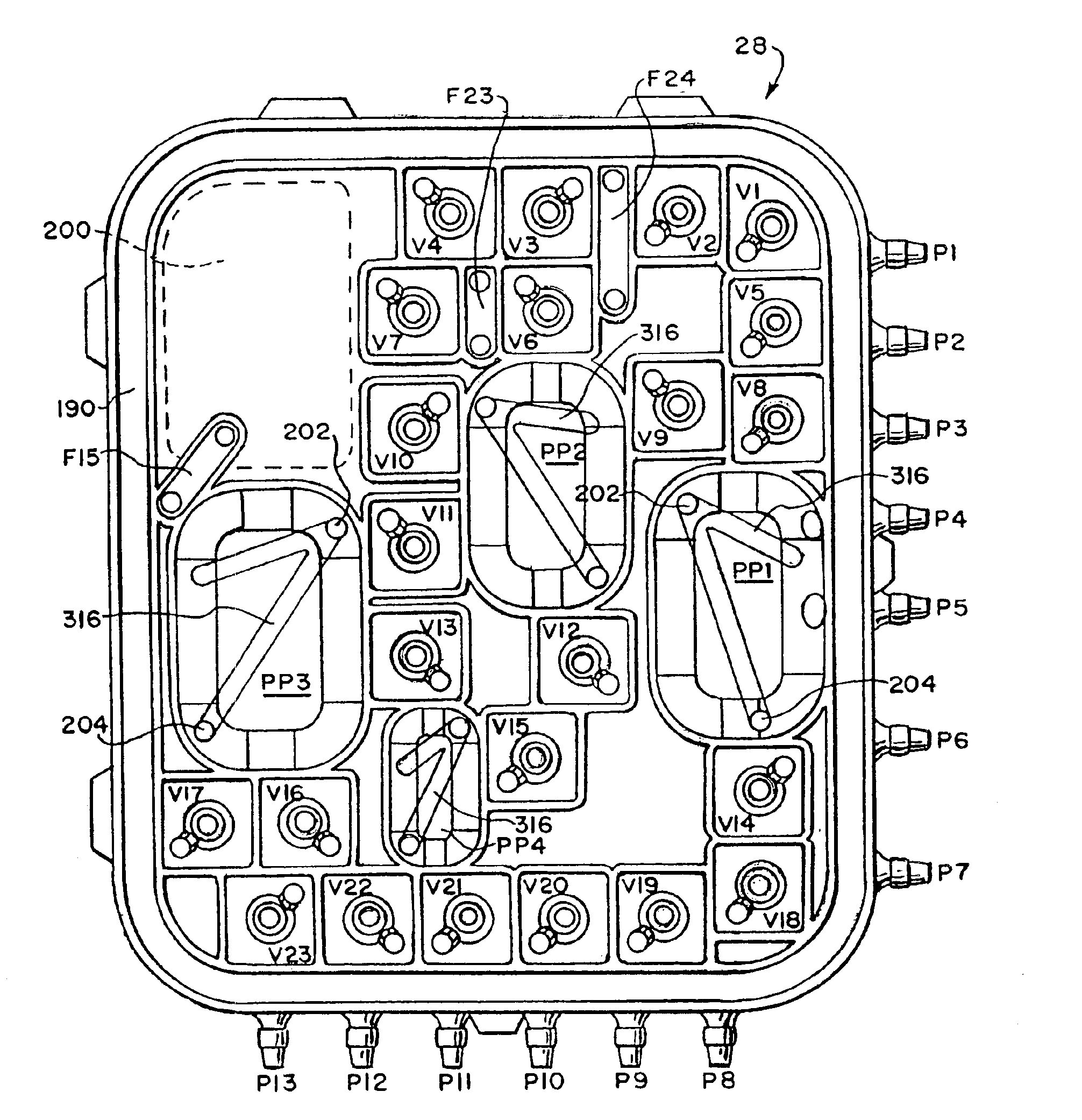
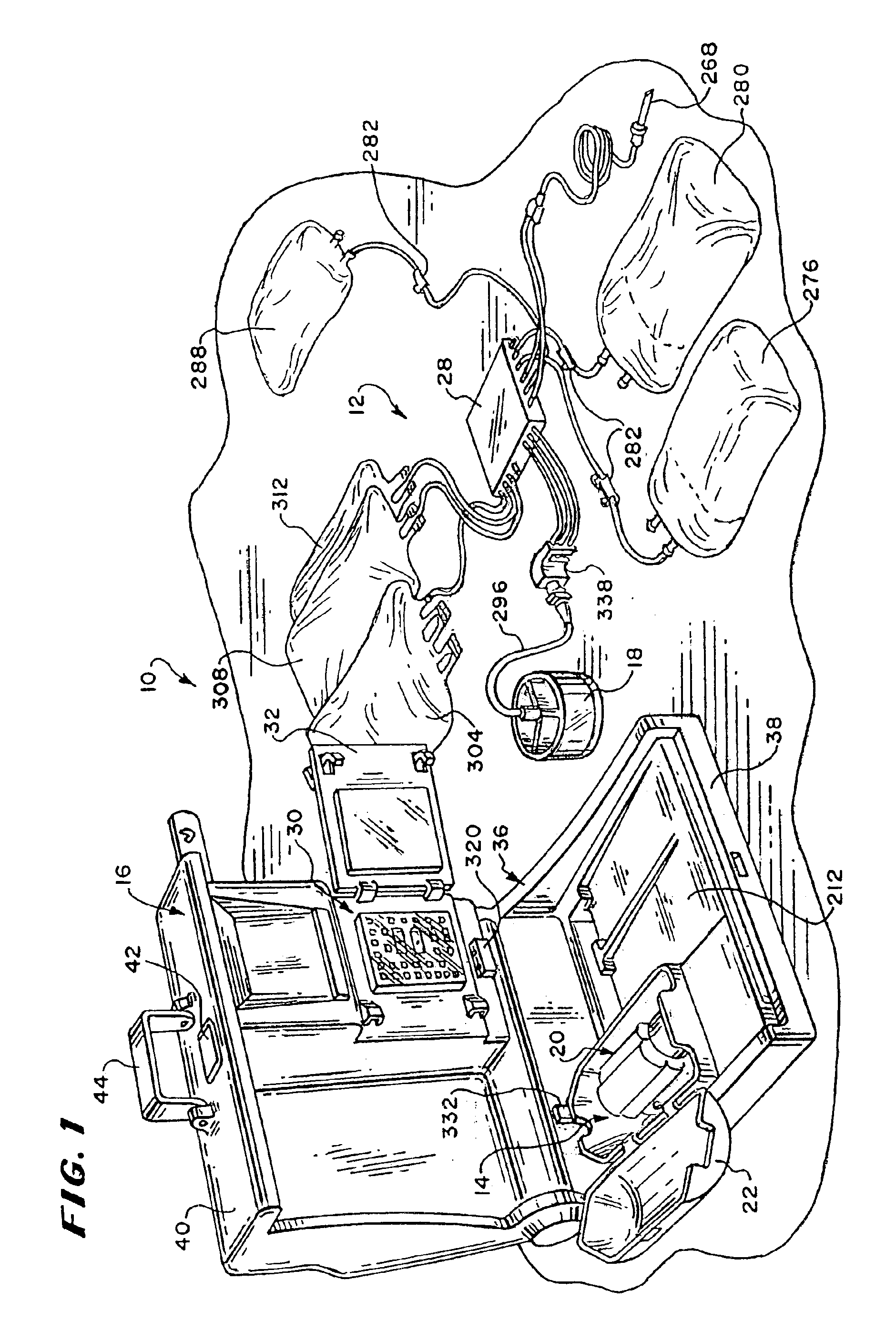
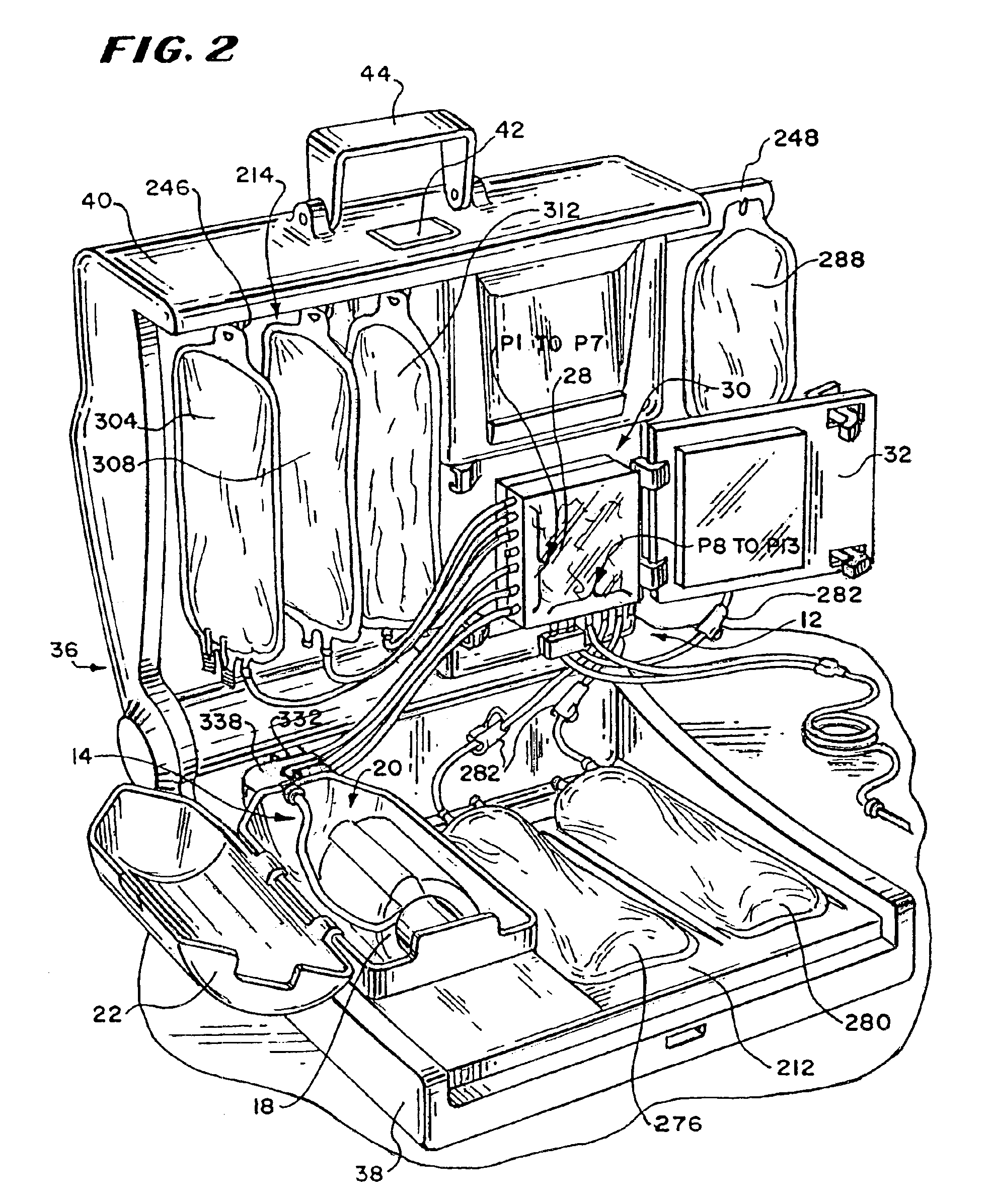
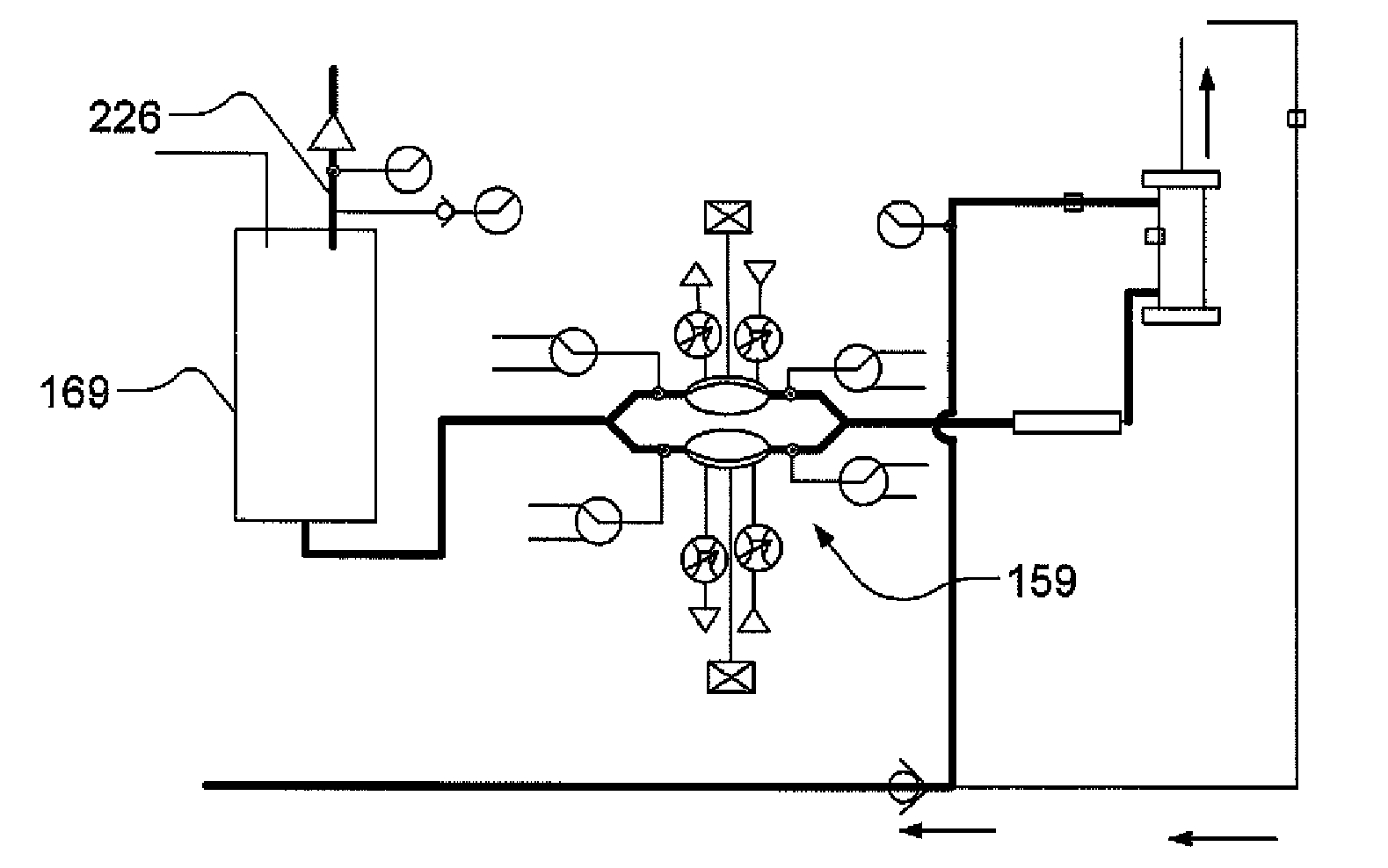
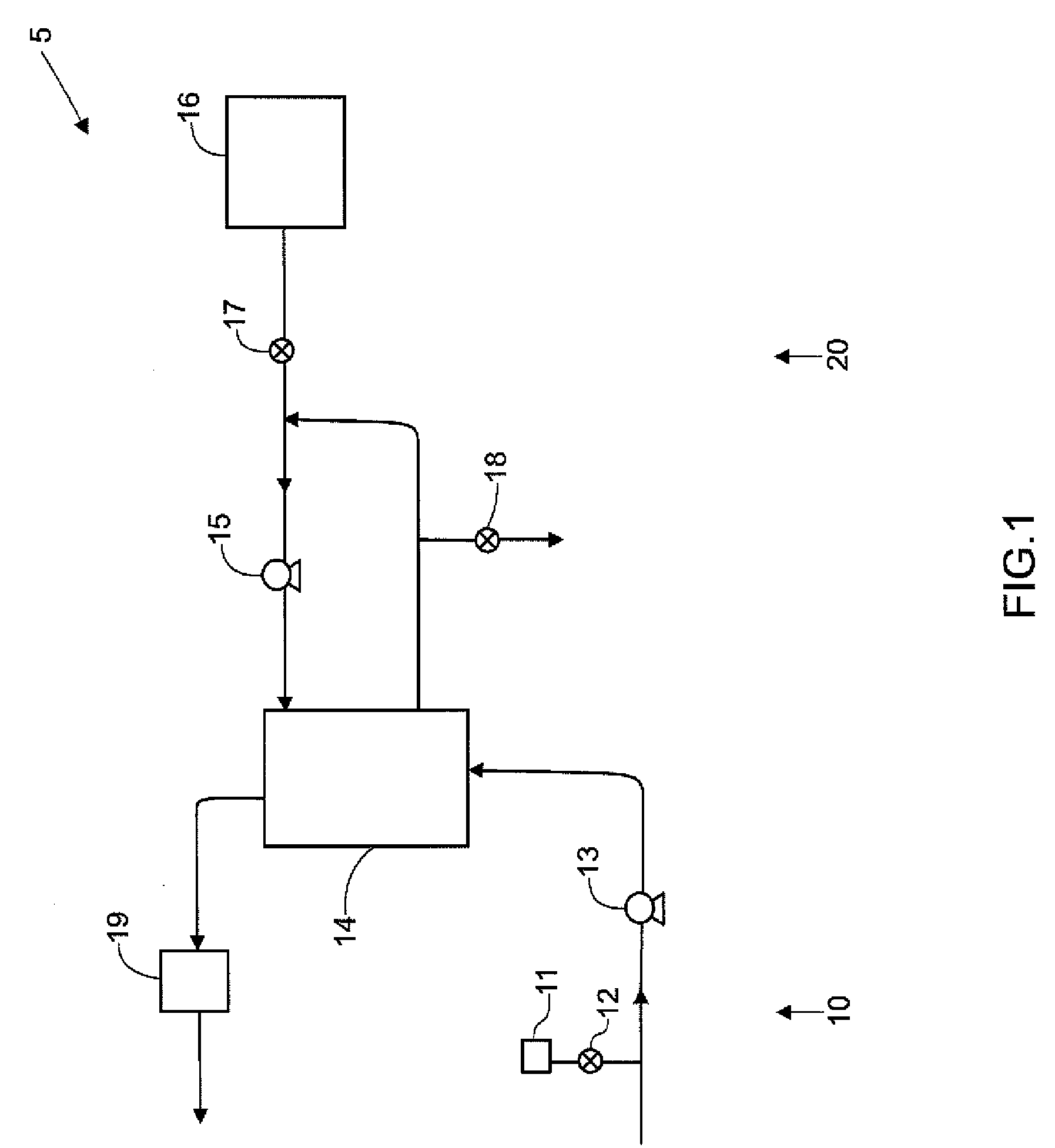
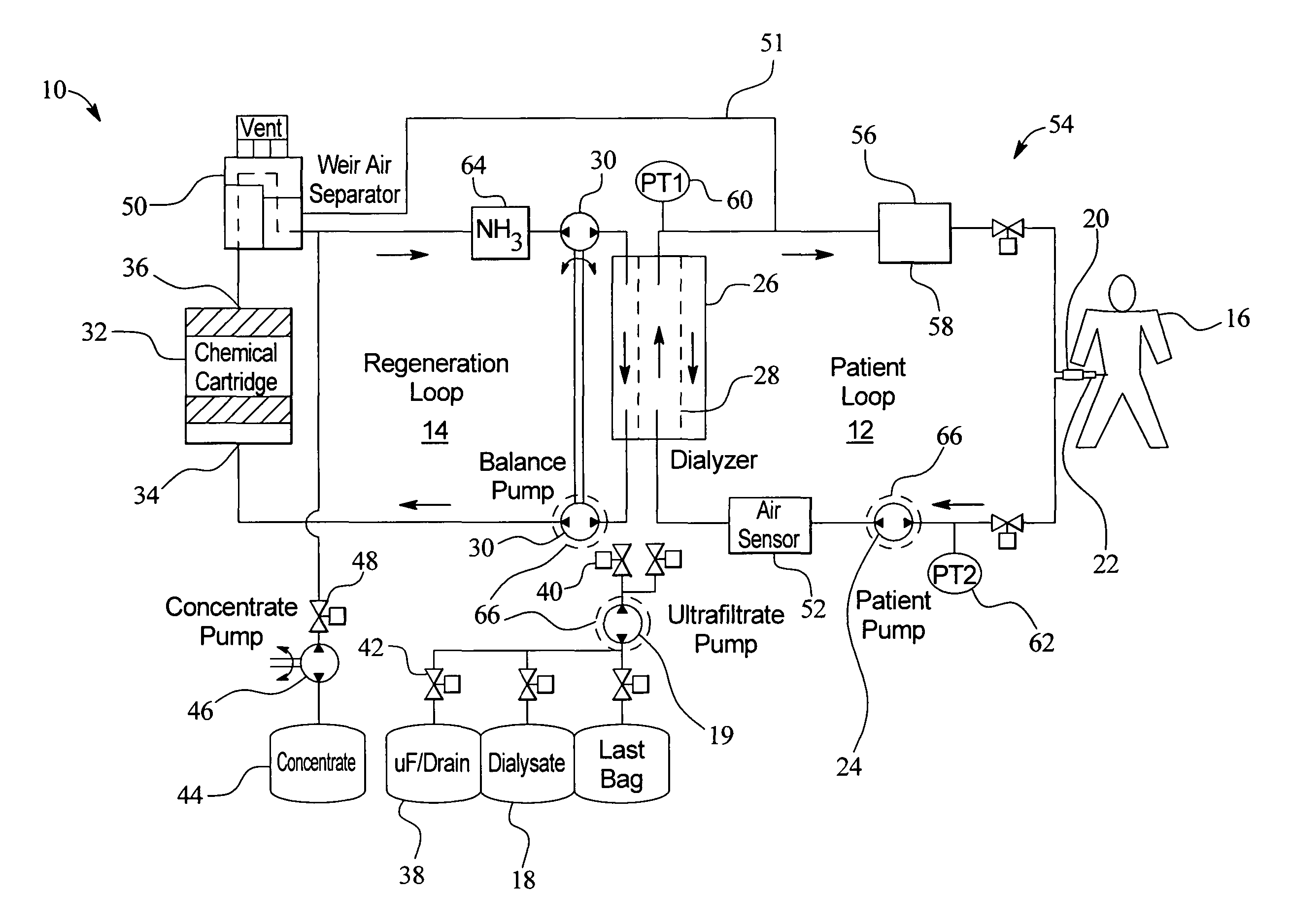
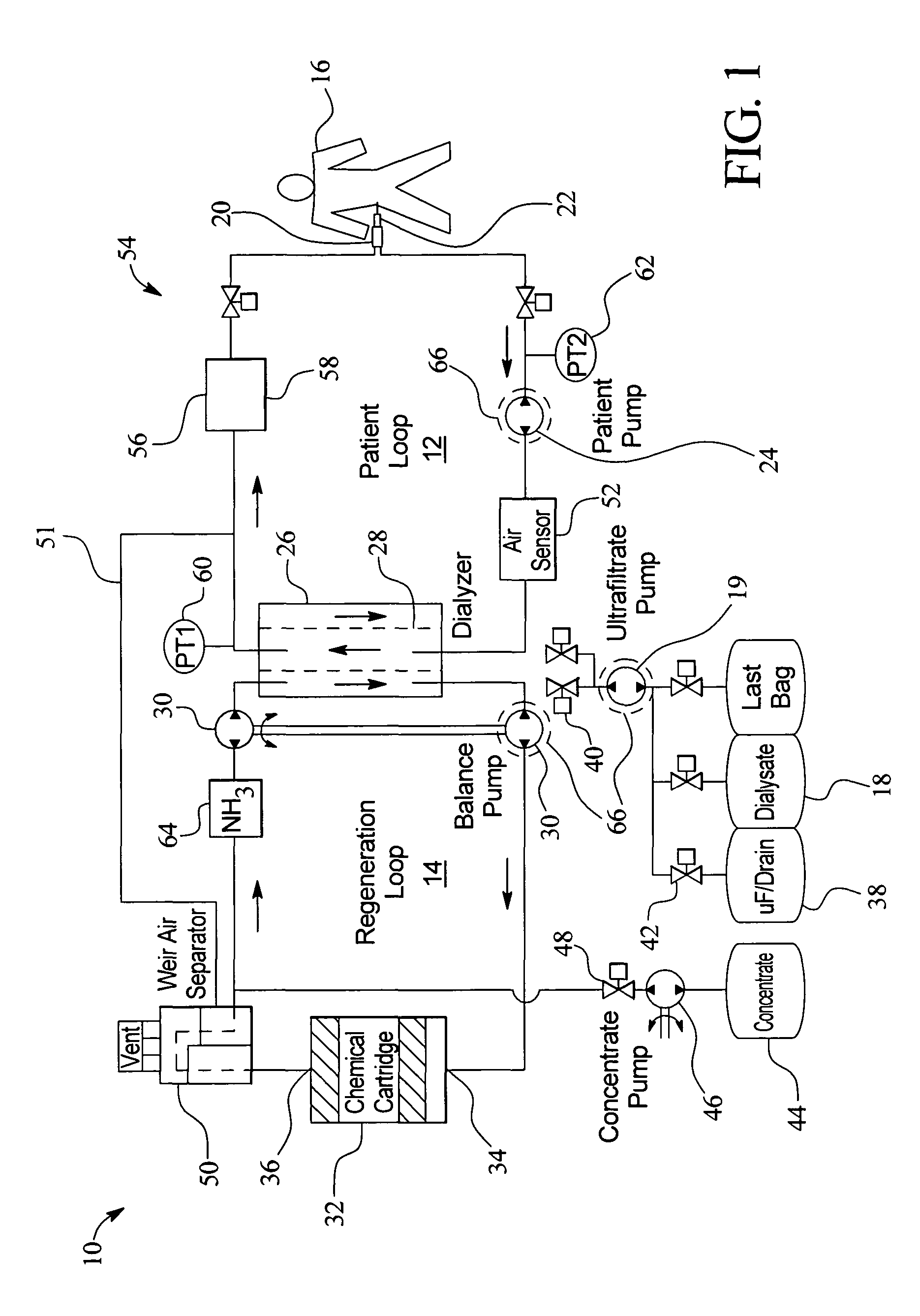
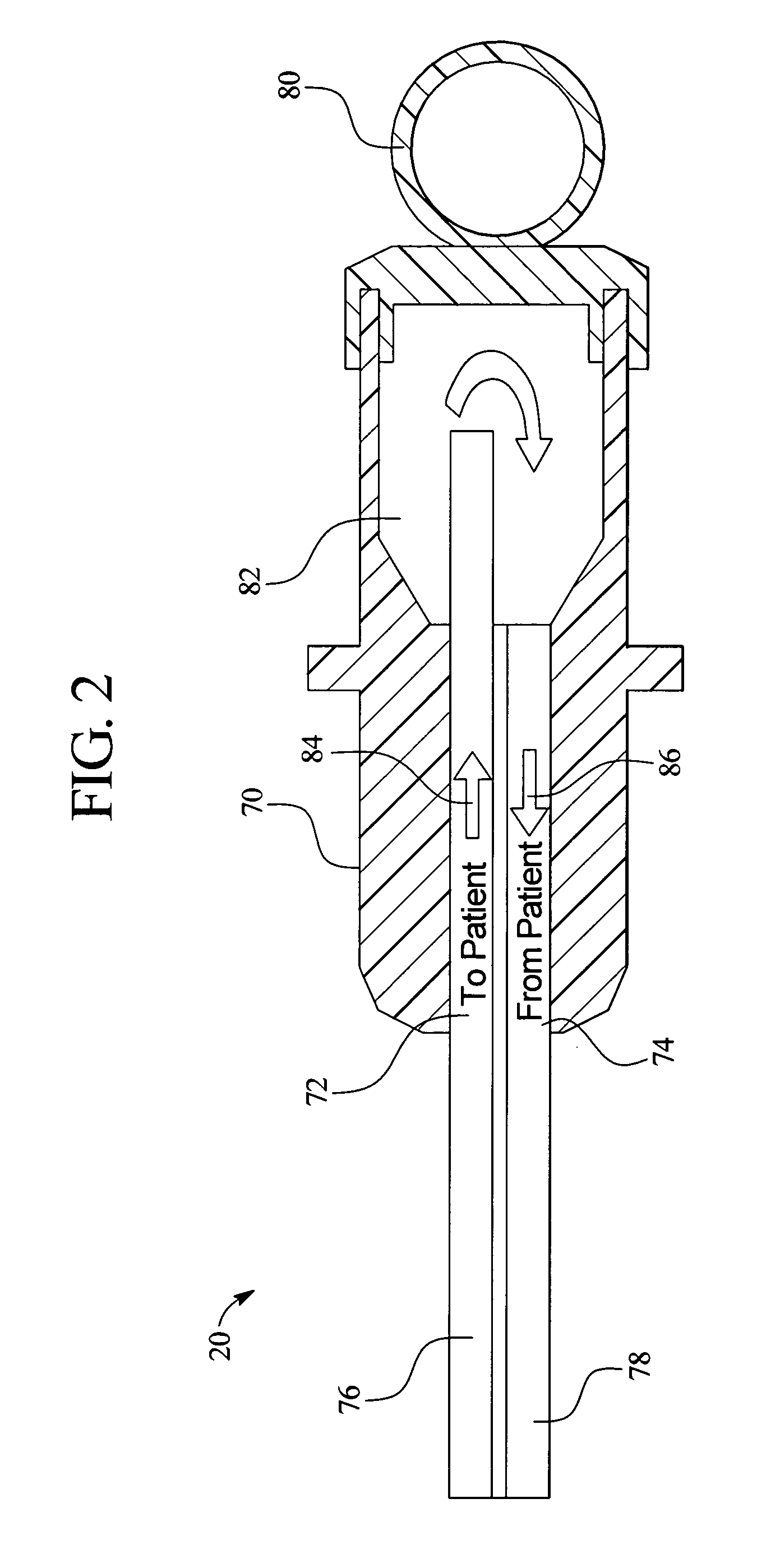
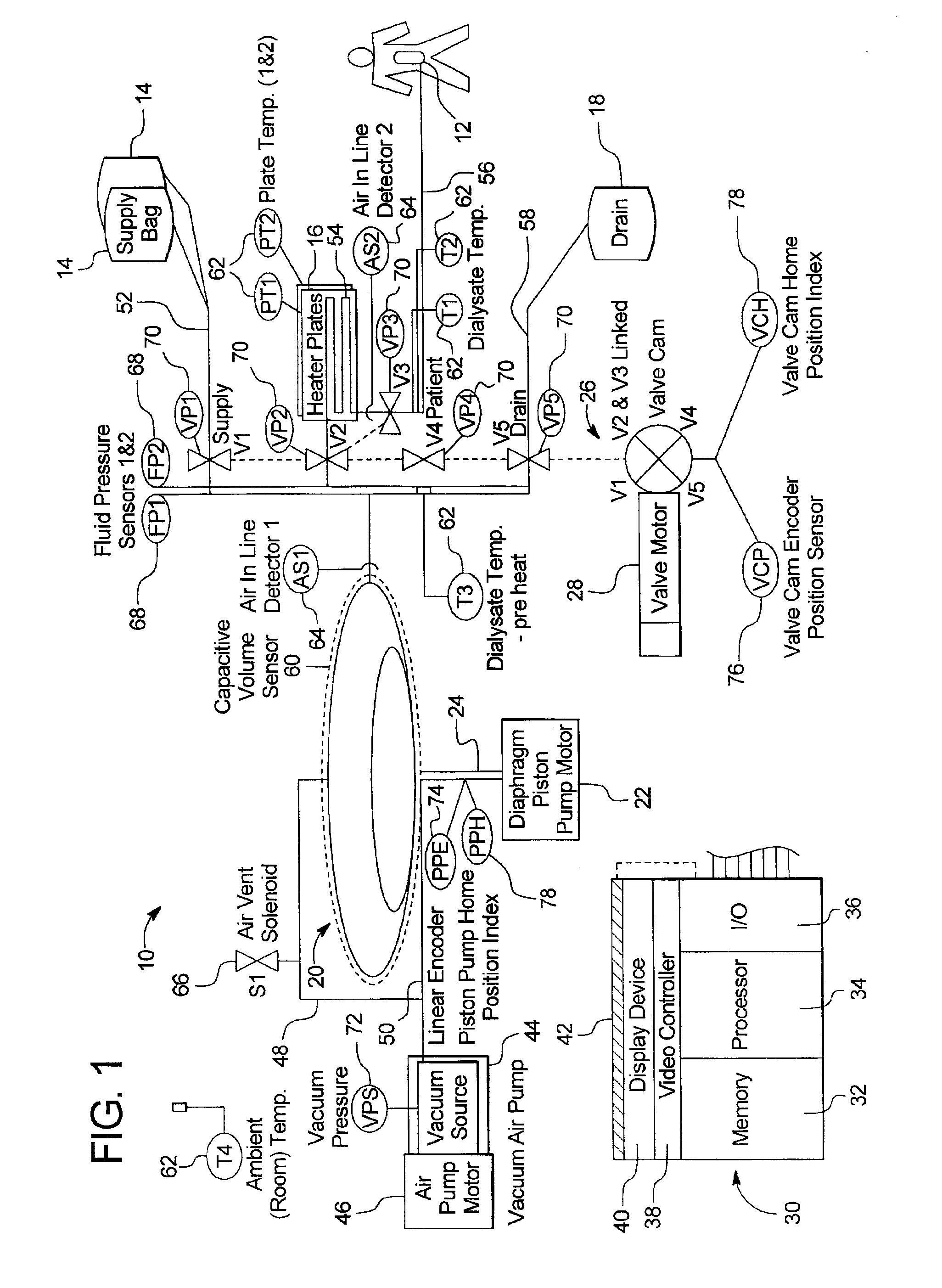
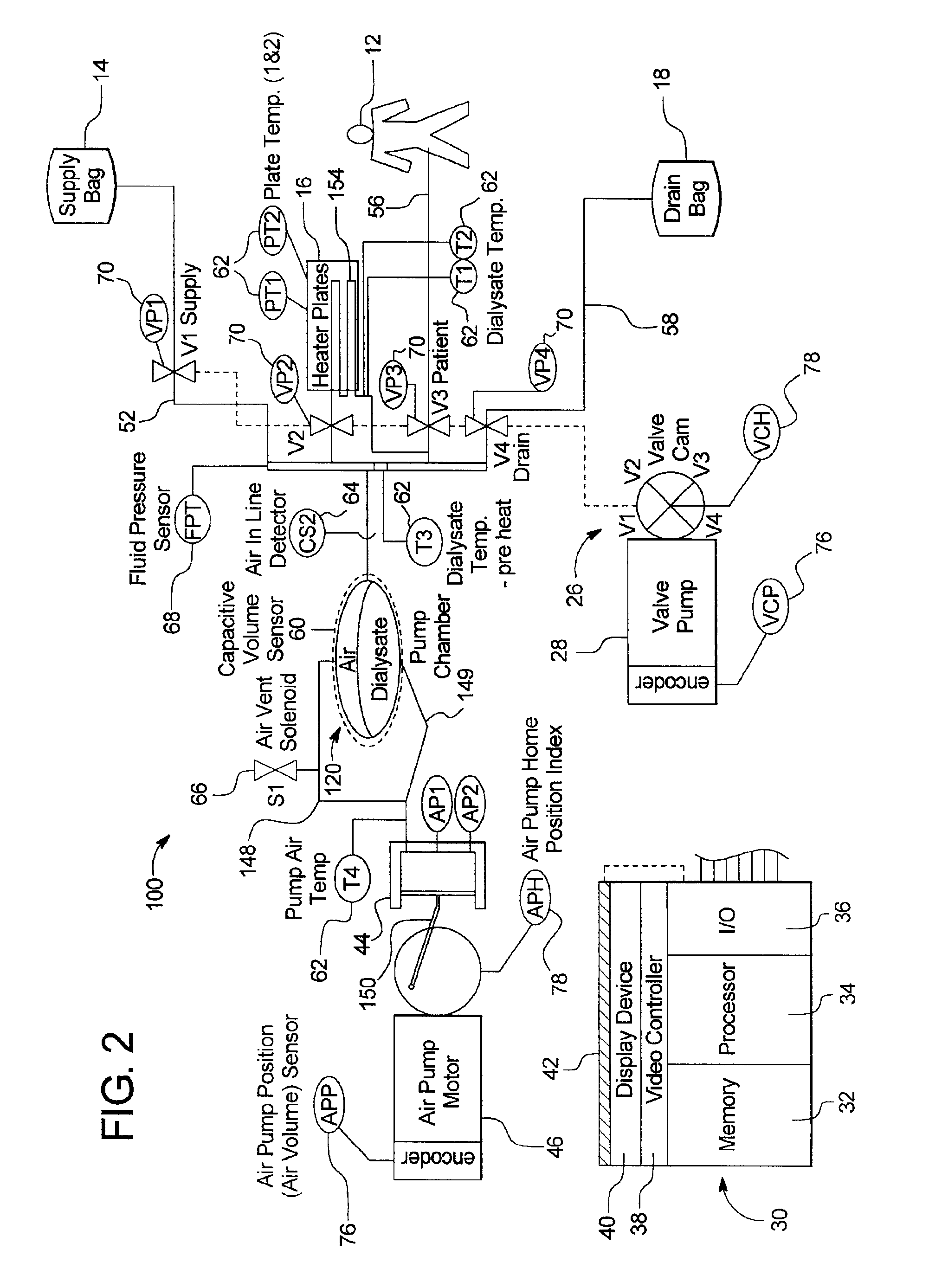
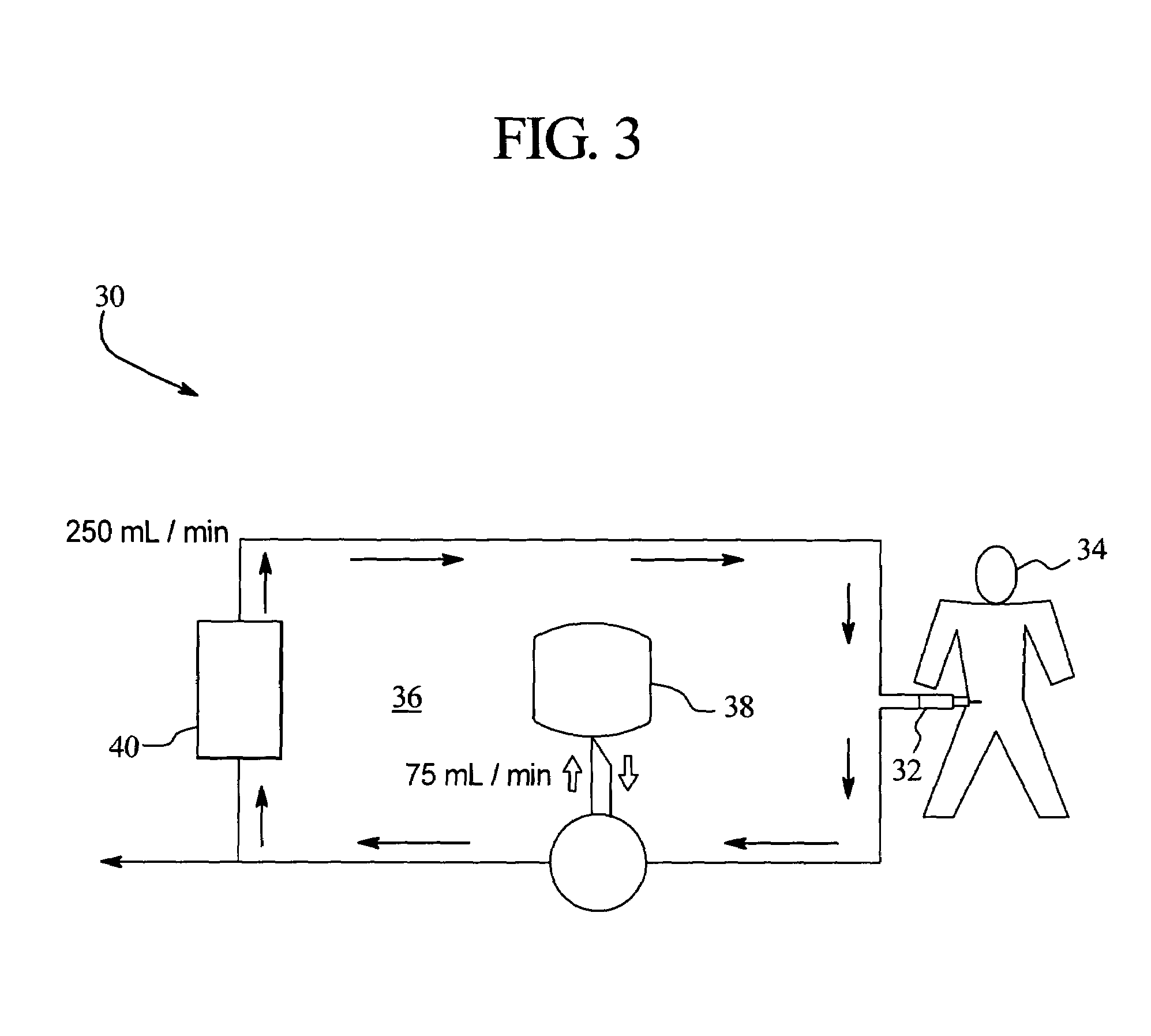
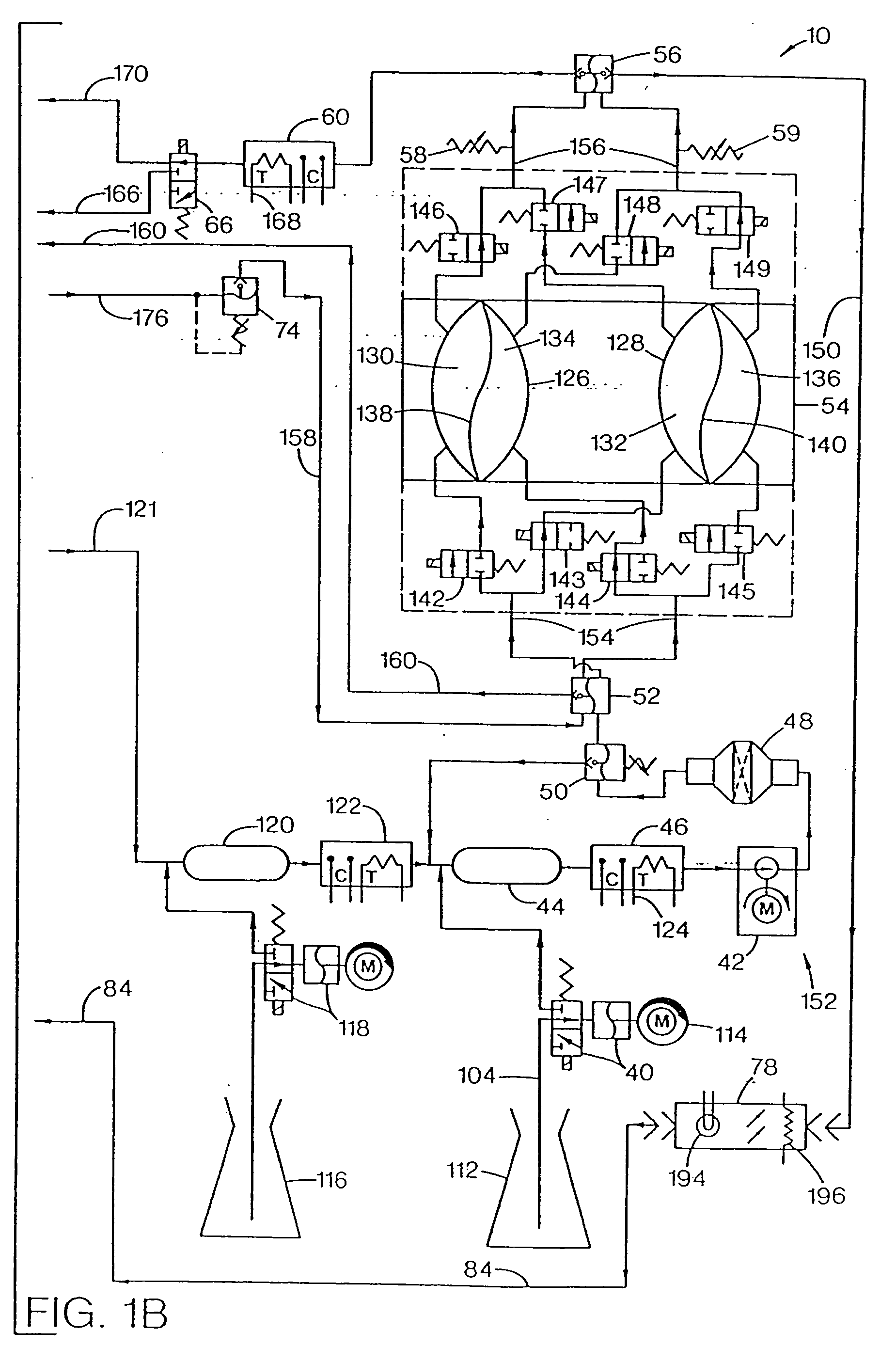
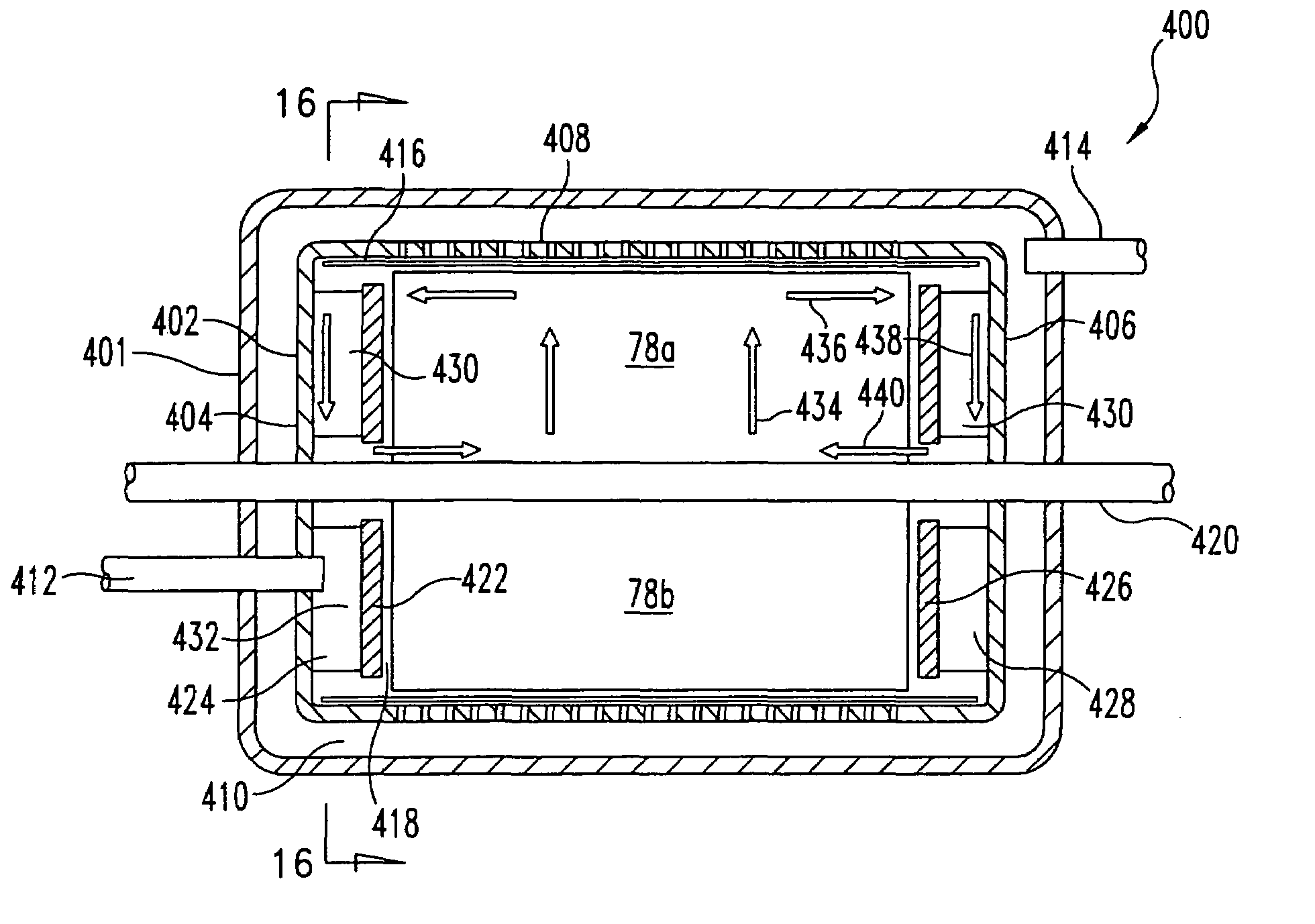
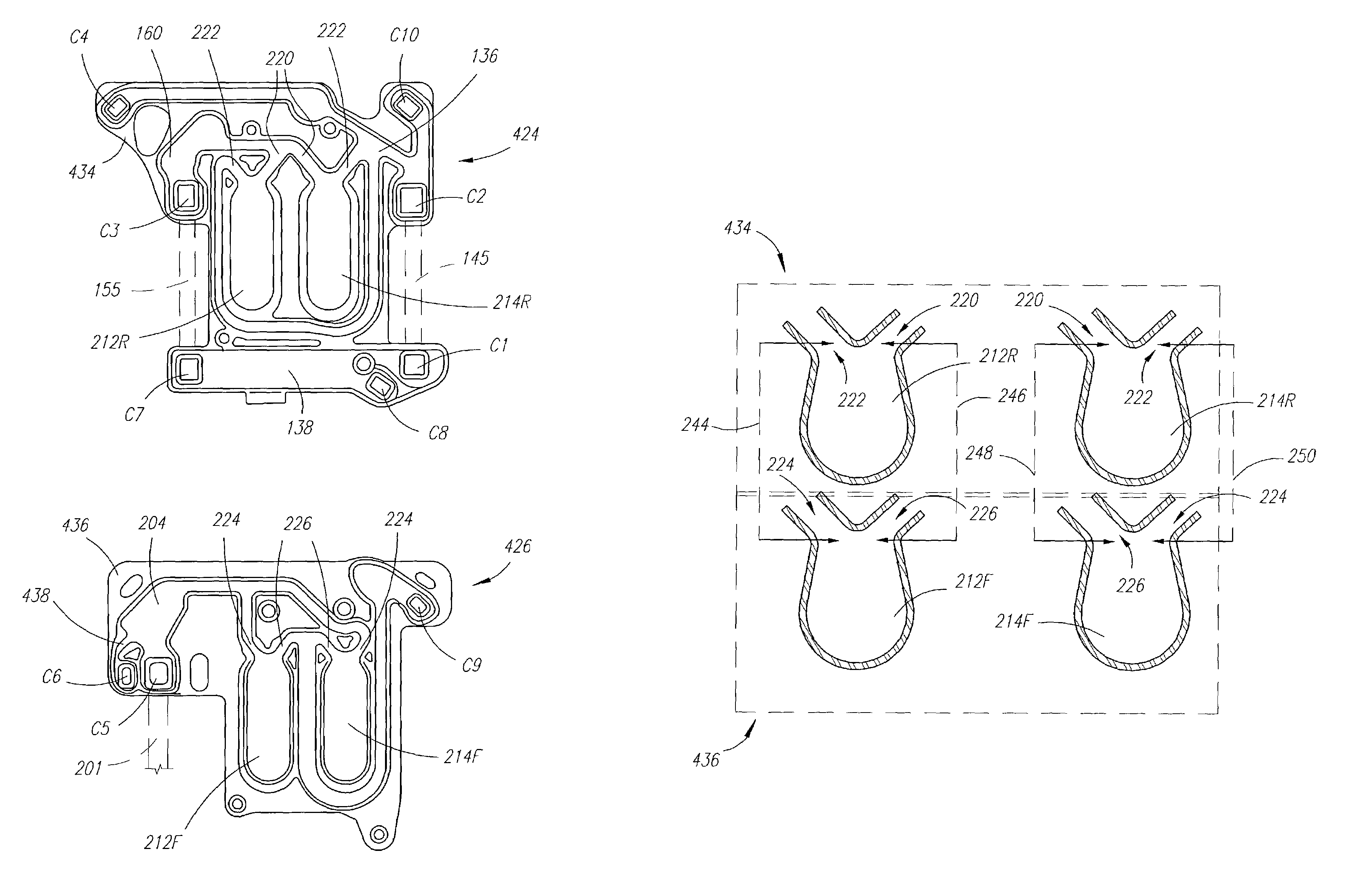
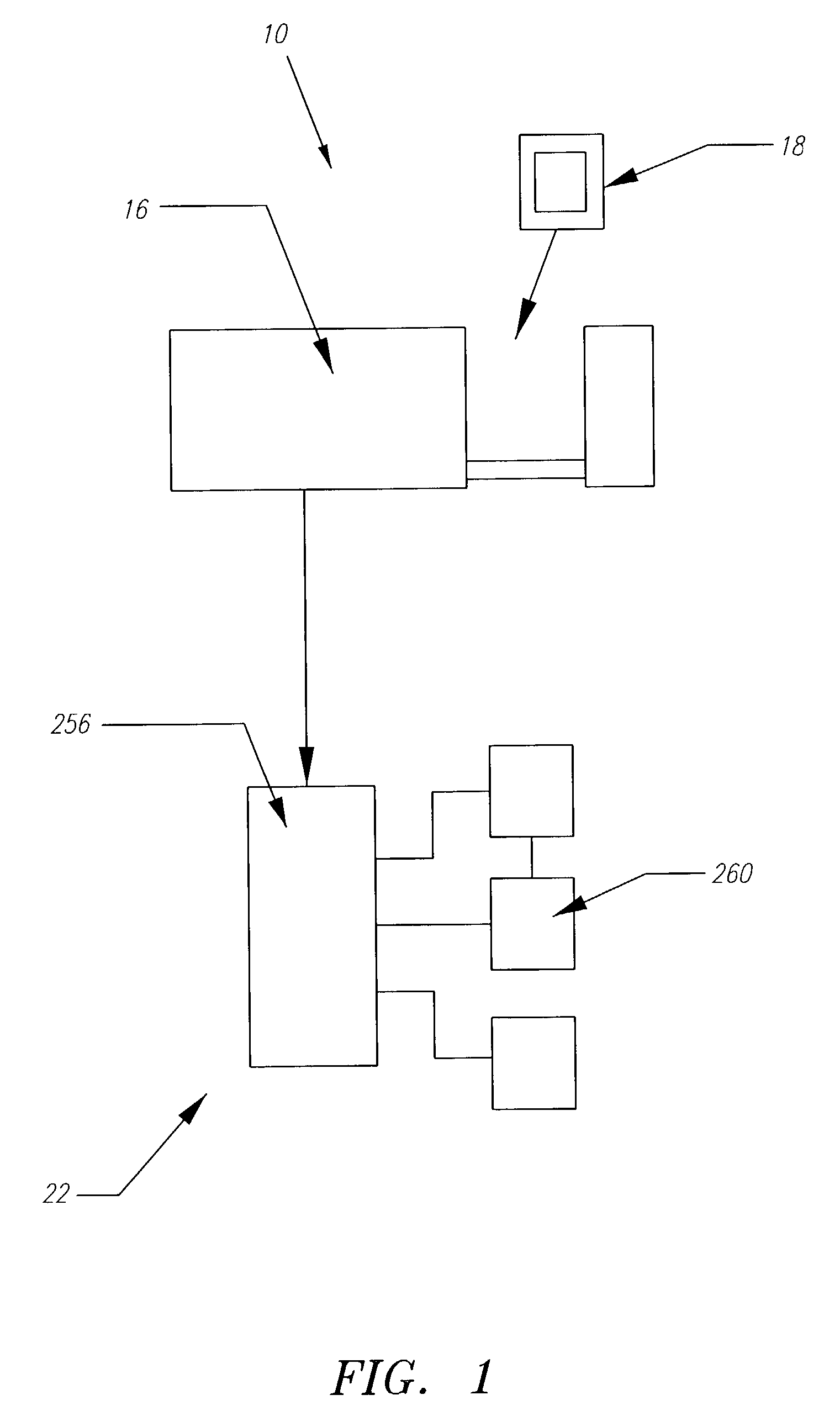
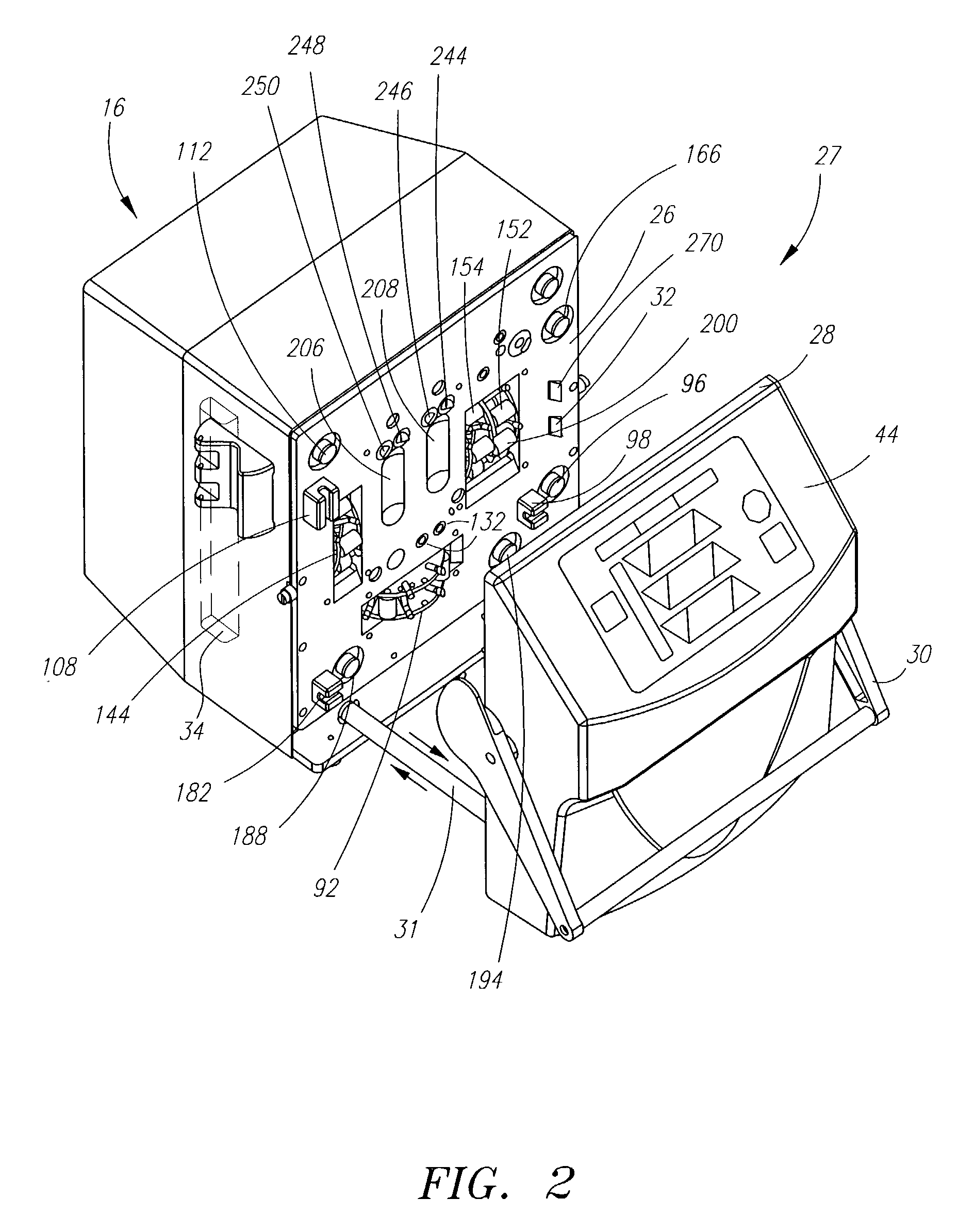
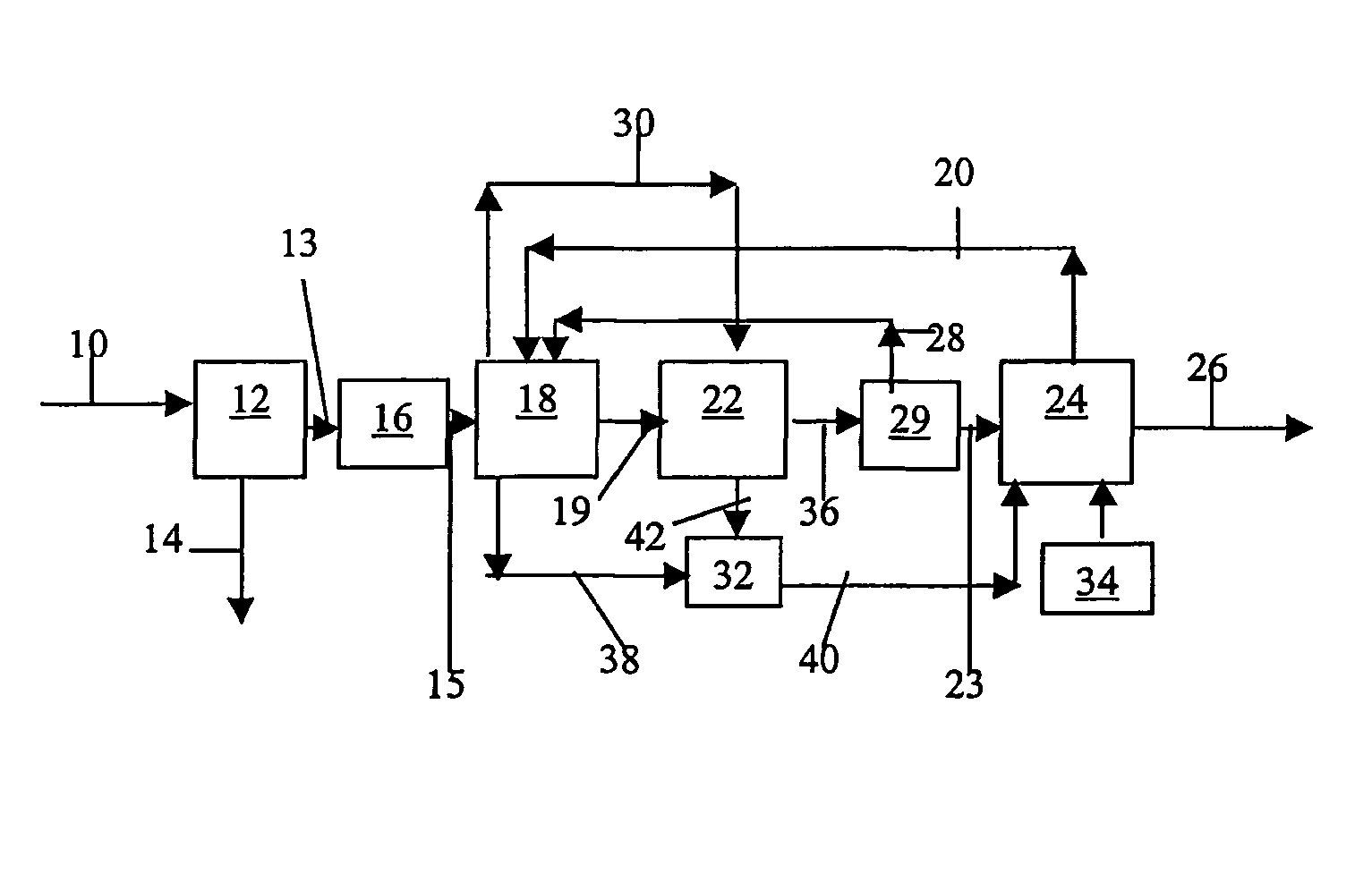
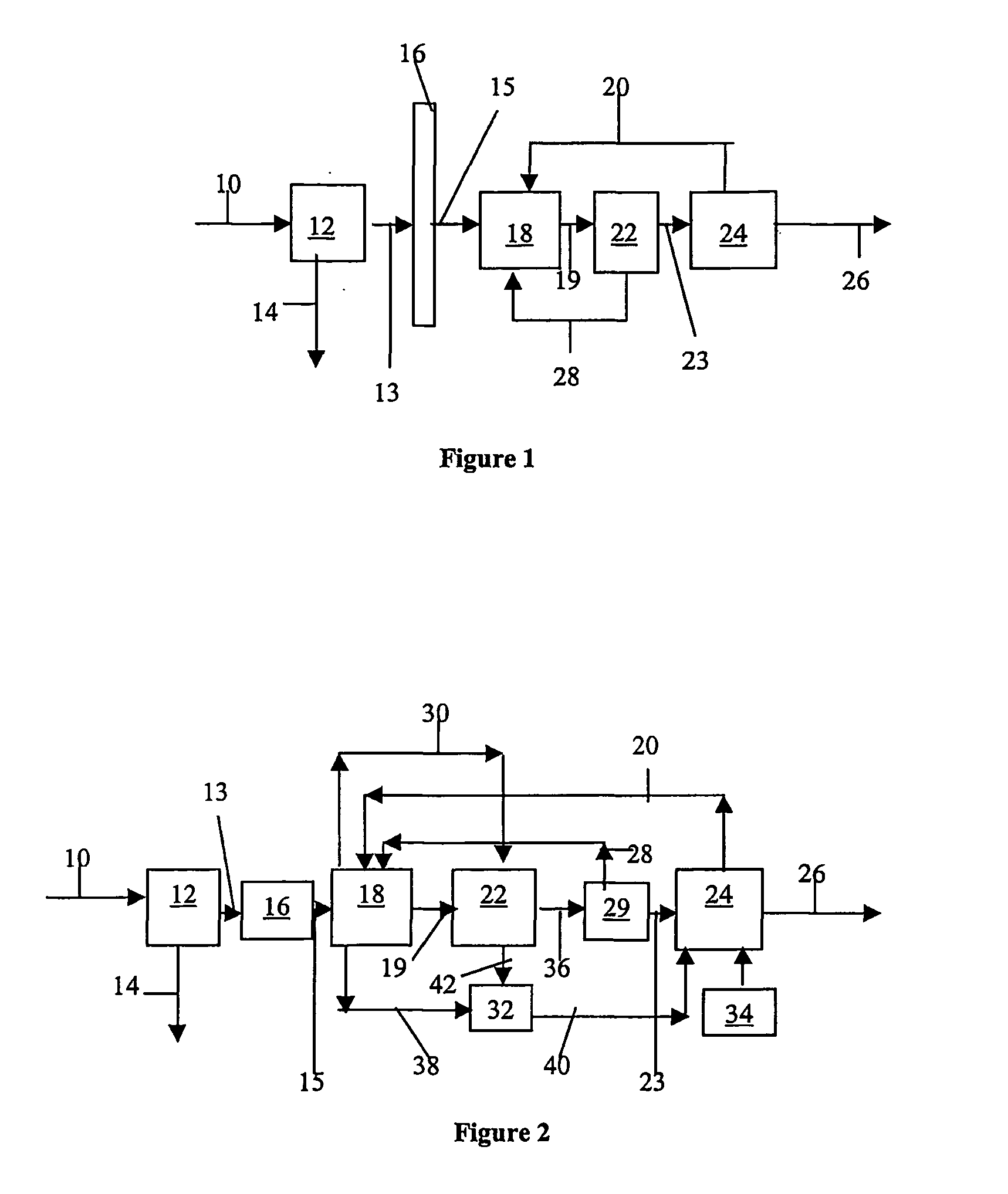
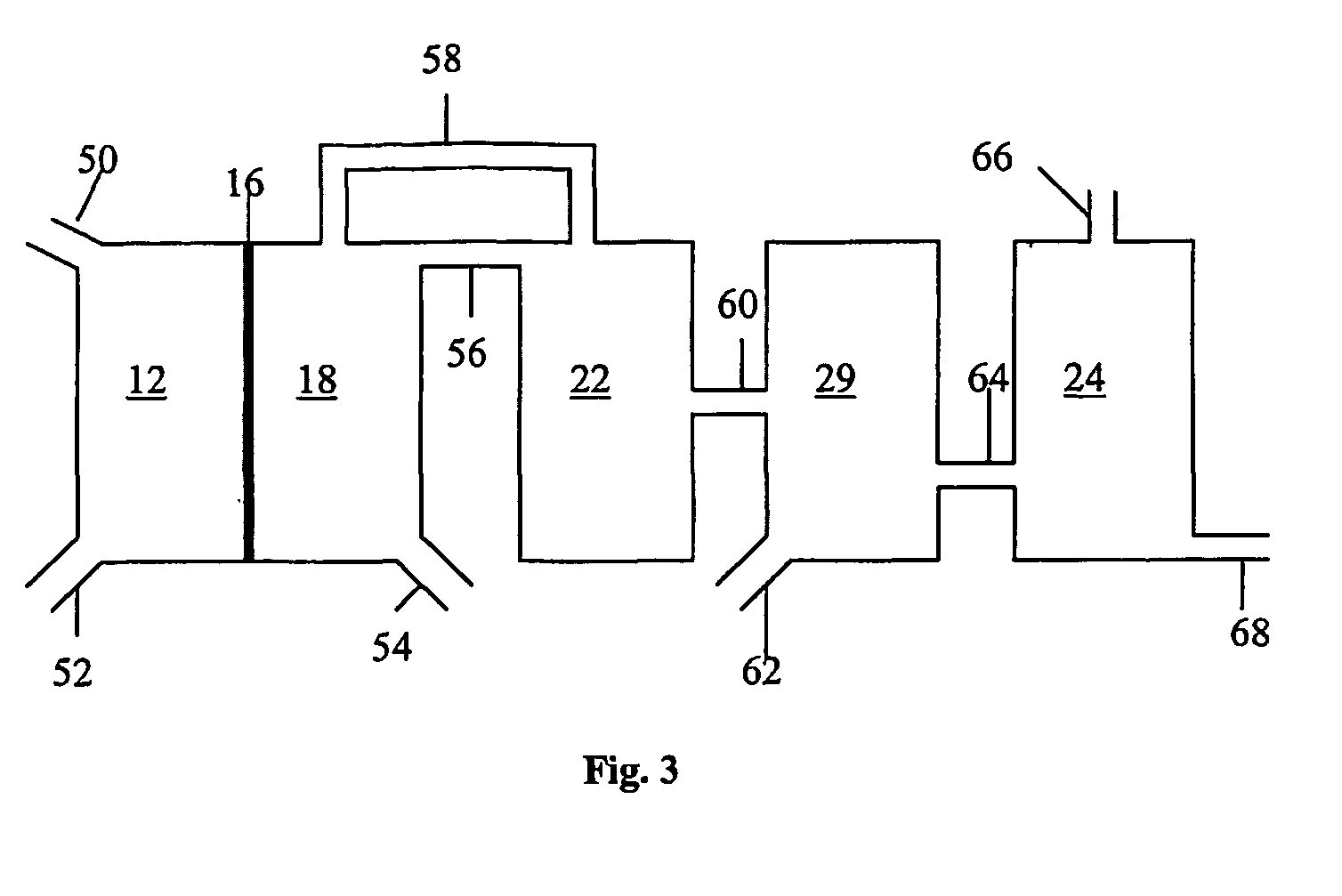
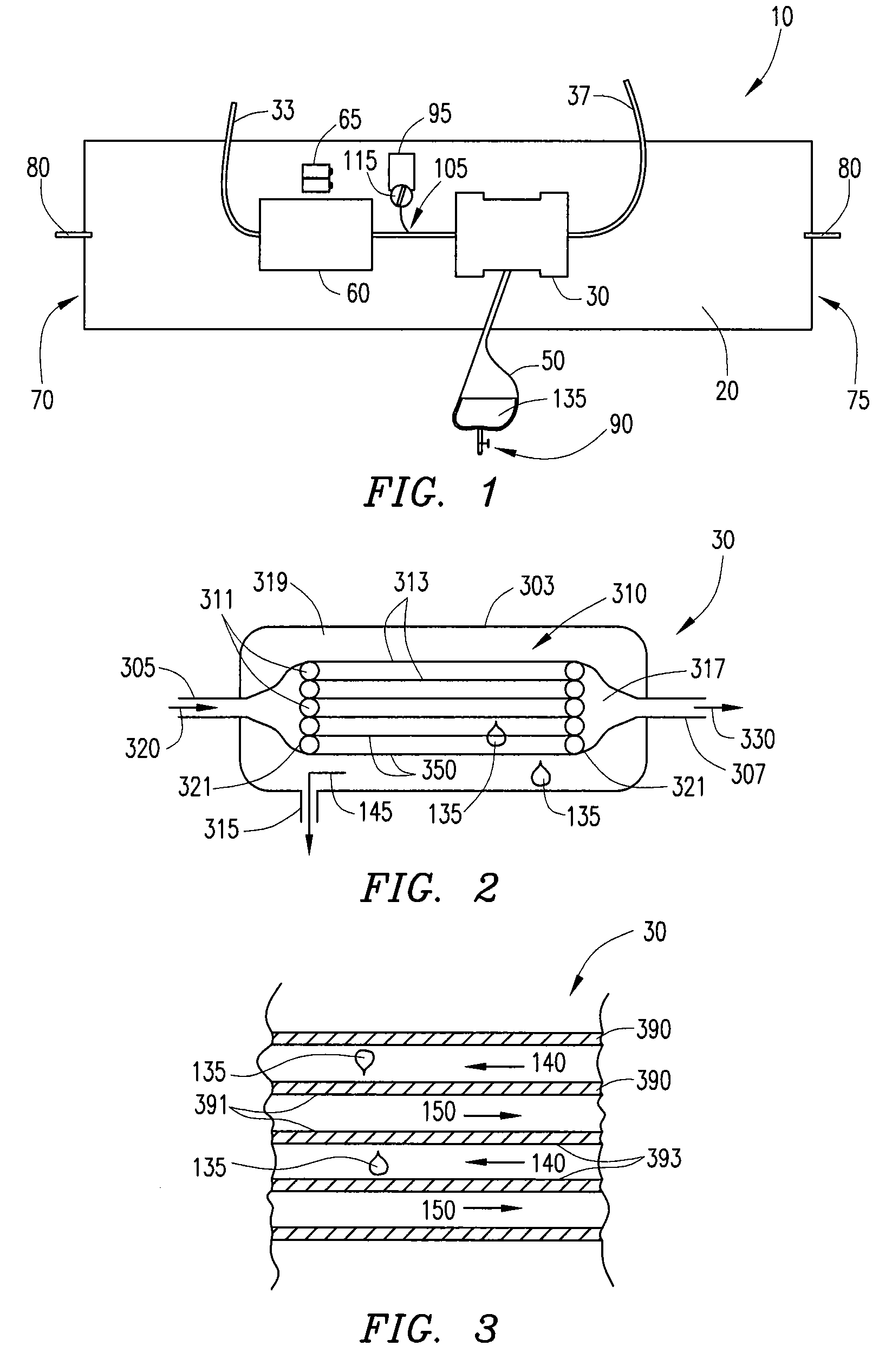
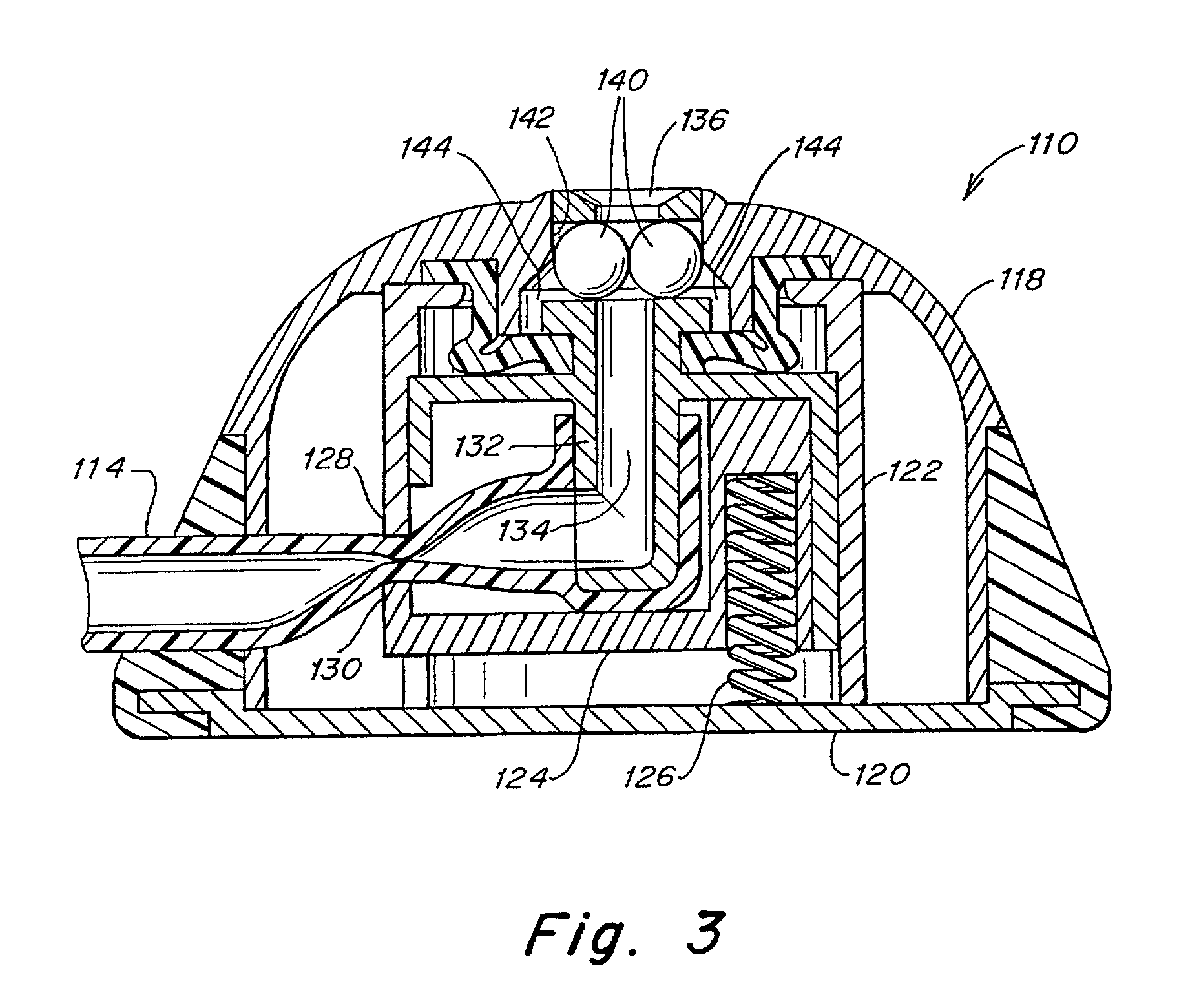
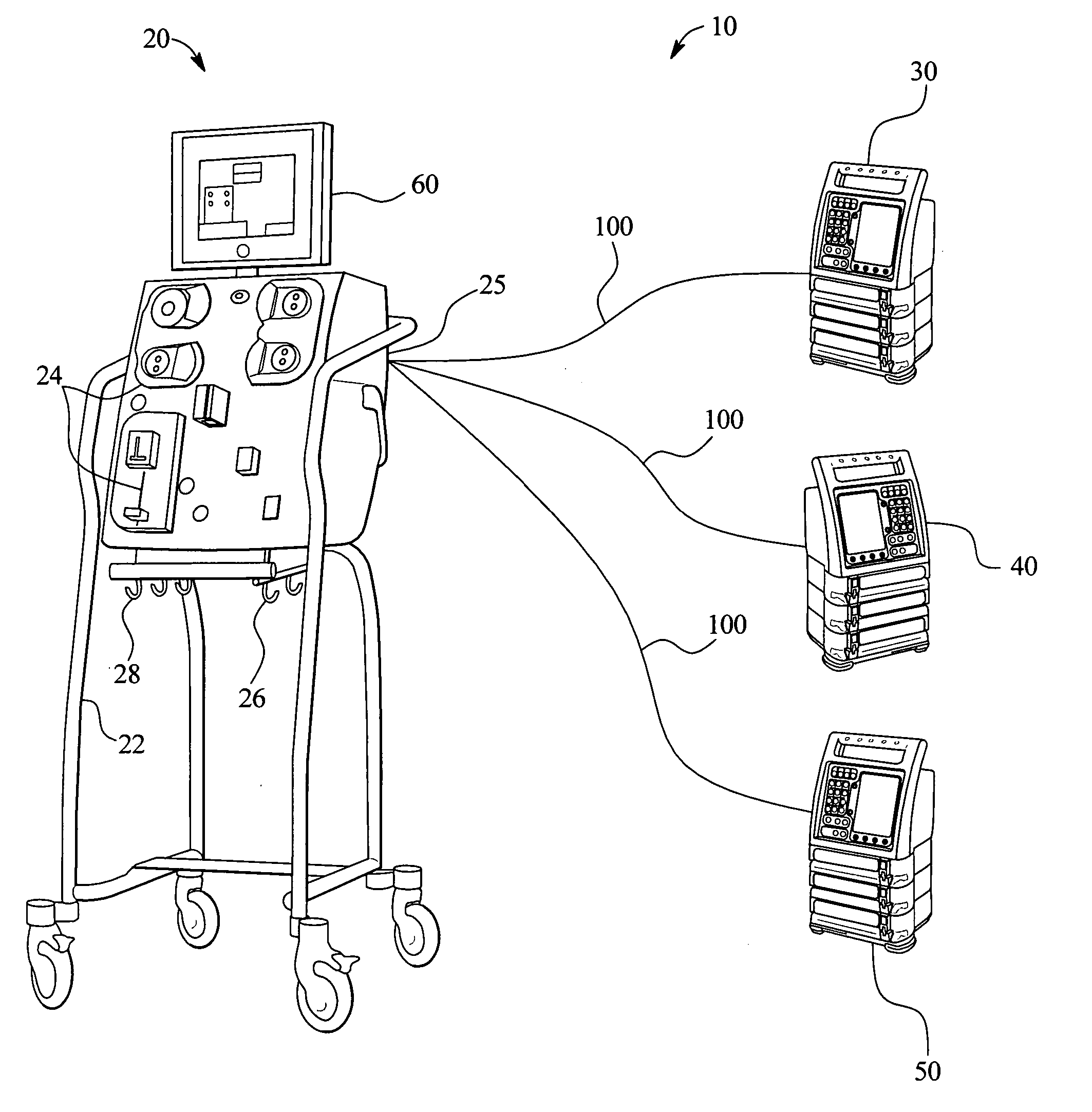
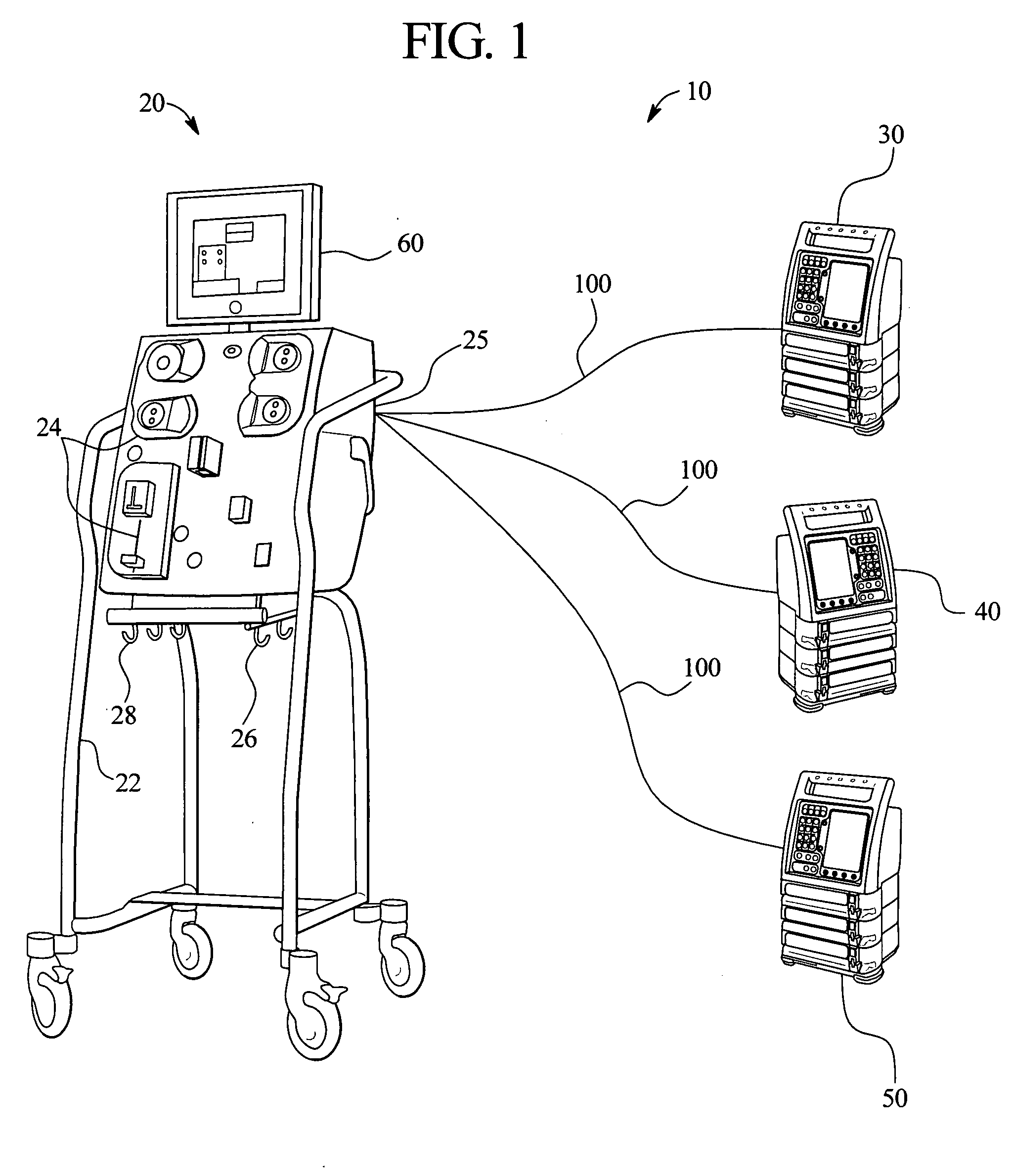
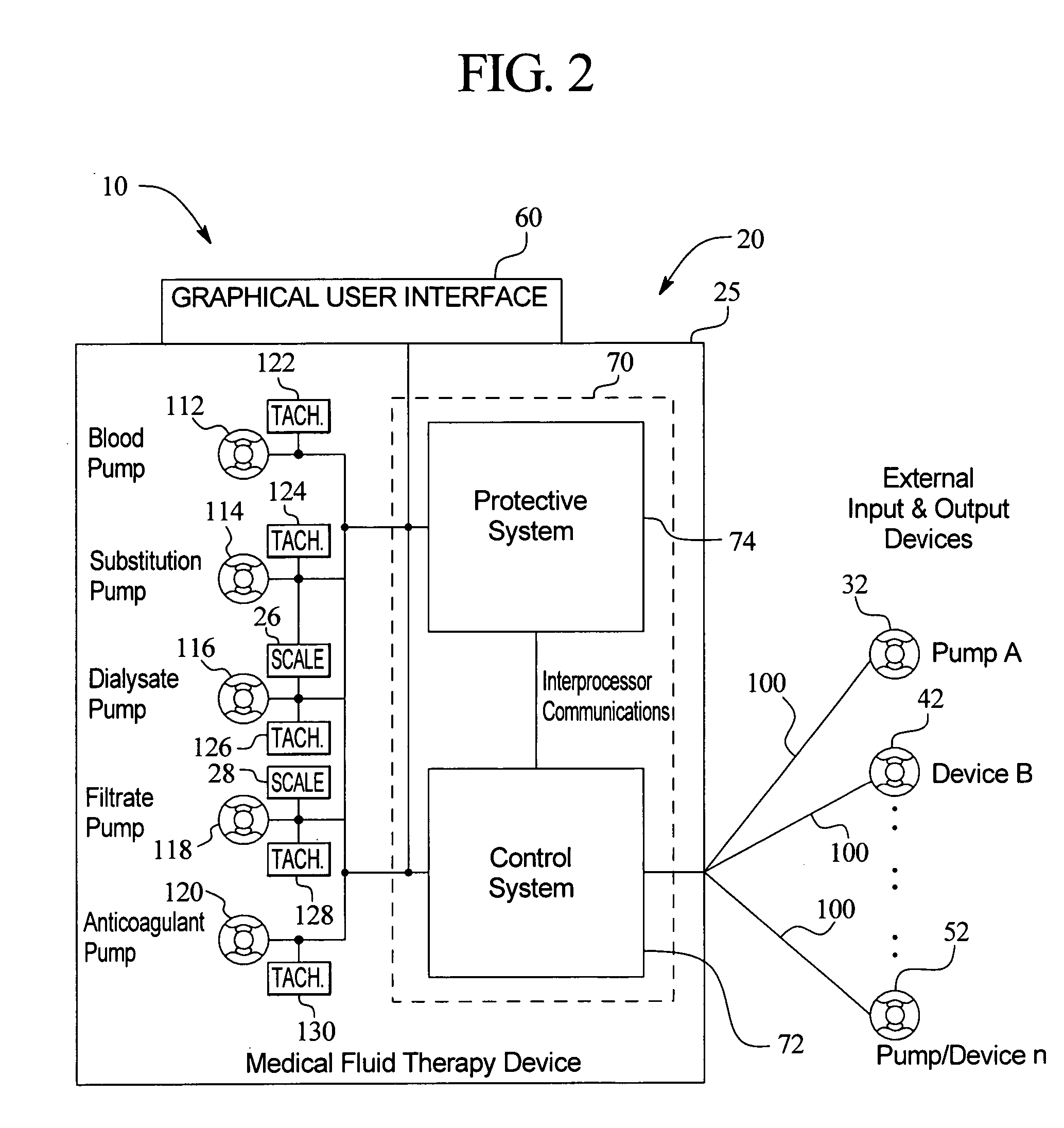
Systems, methods and apparatuses for pumping cassette-based therapies
InactiveUS7238164B2Simple materialSemi-permeable membranesSolvent extractionMultiplexingAir separation
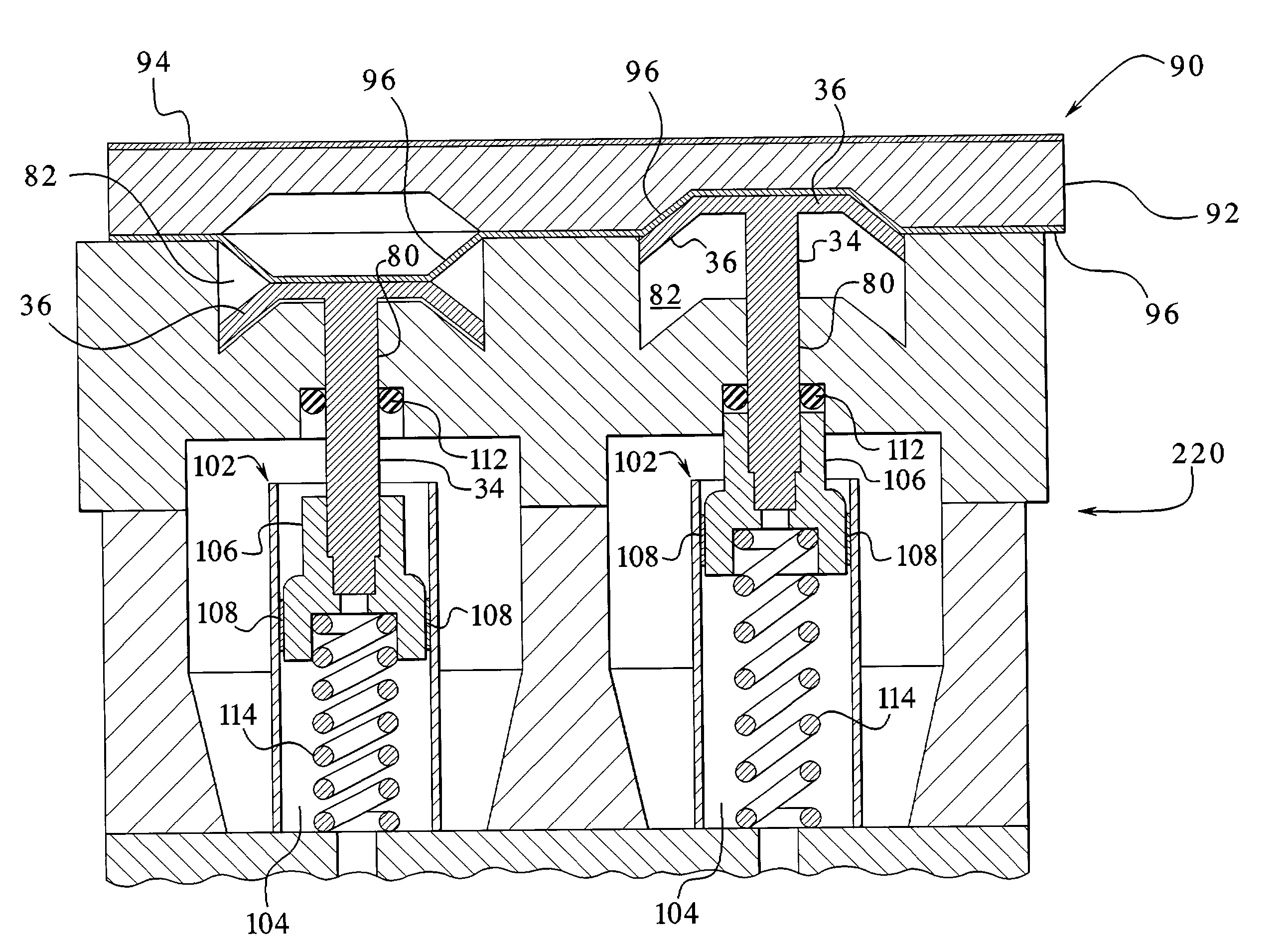
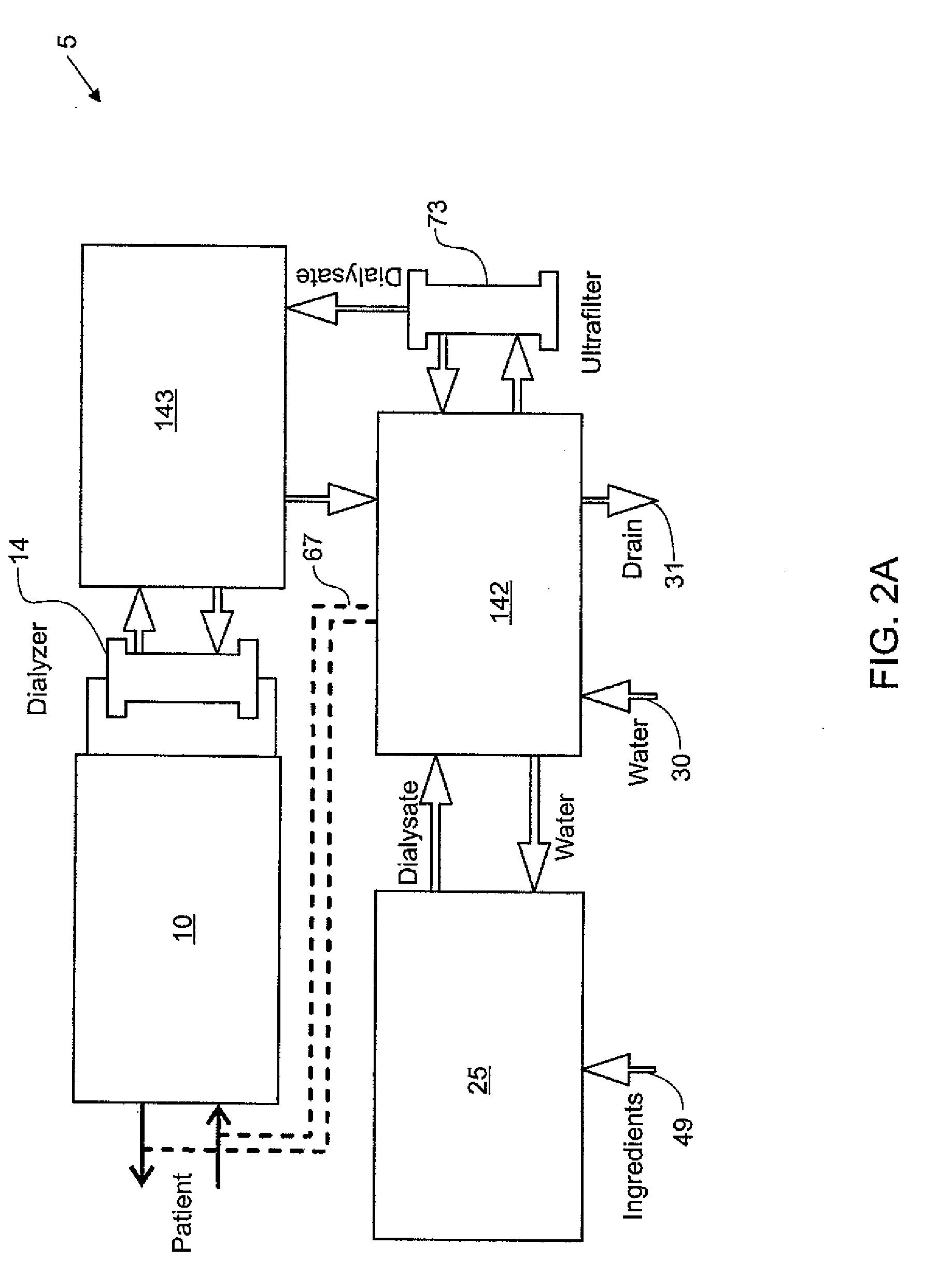
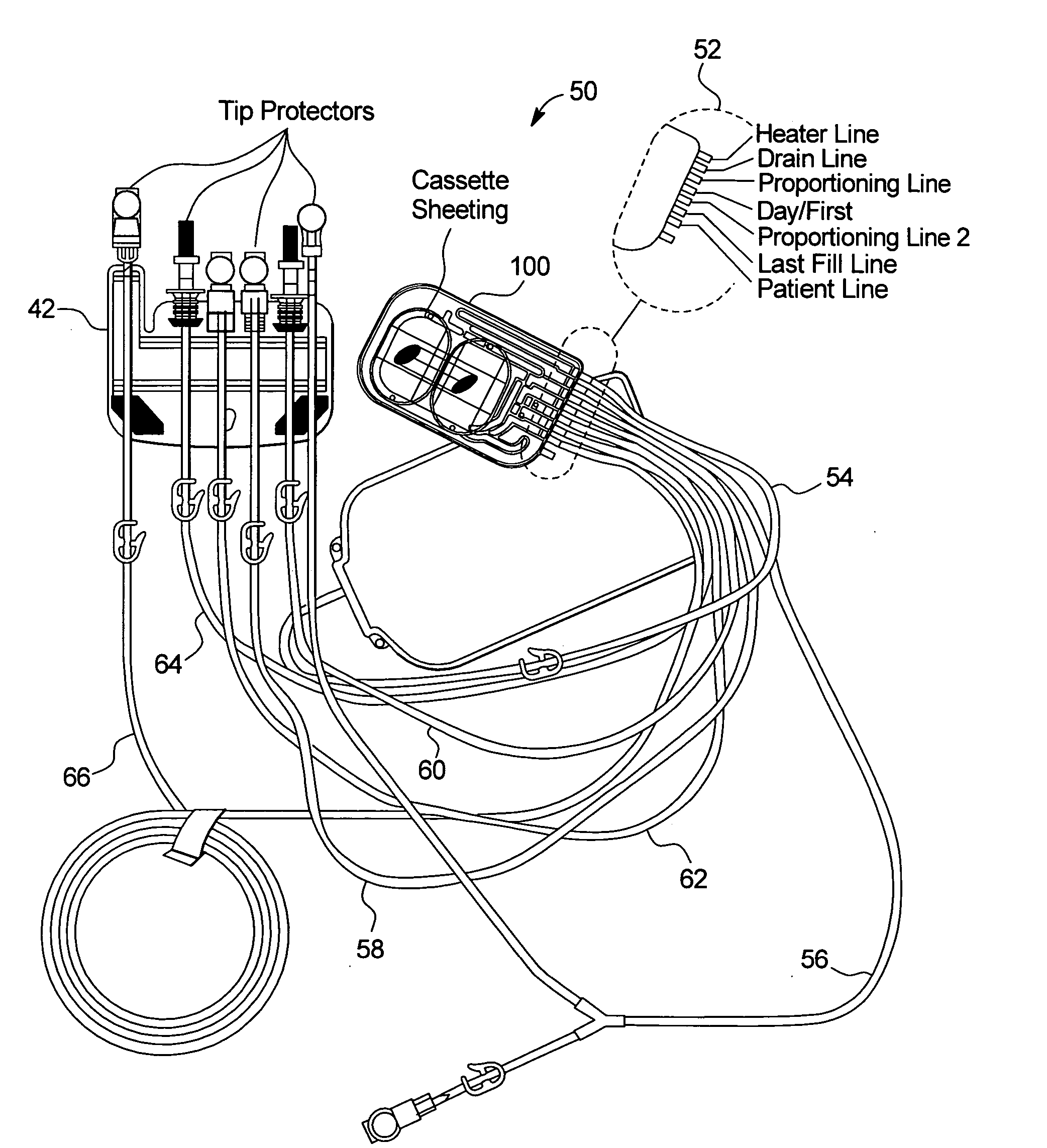
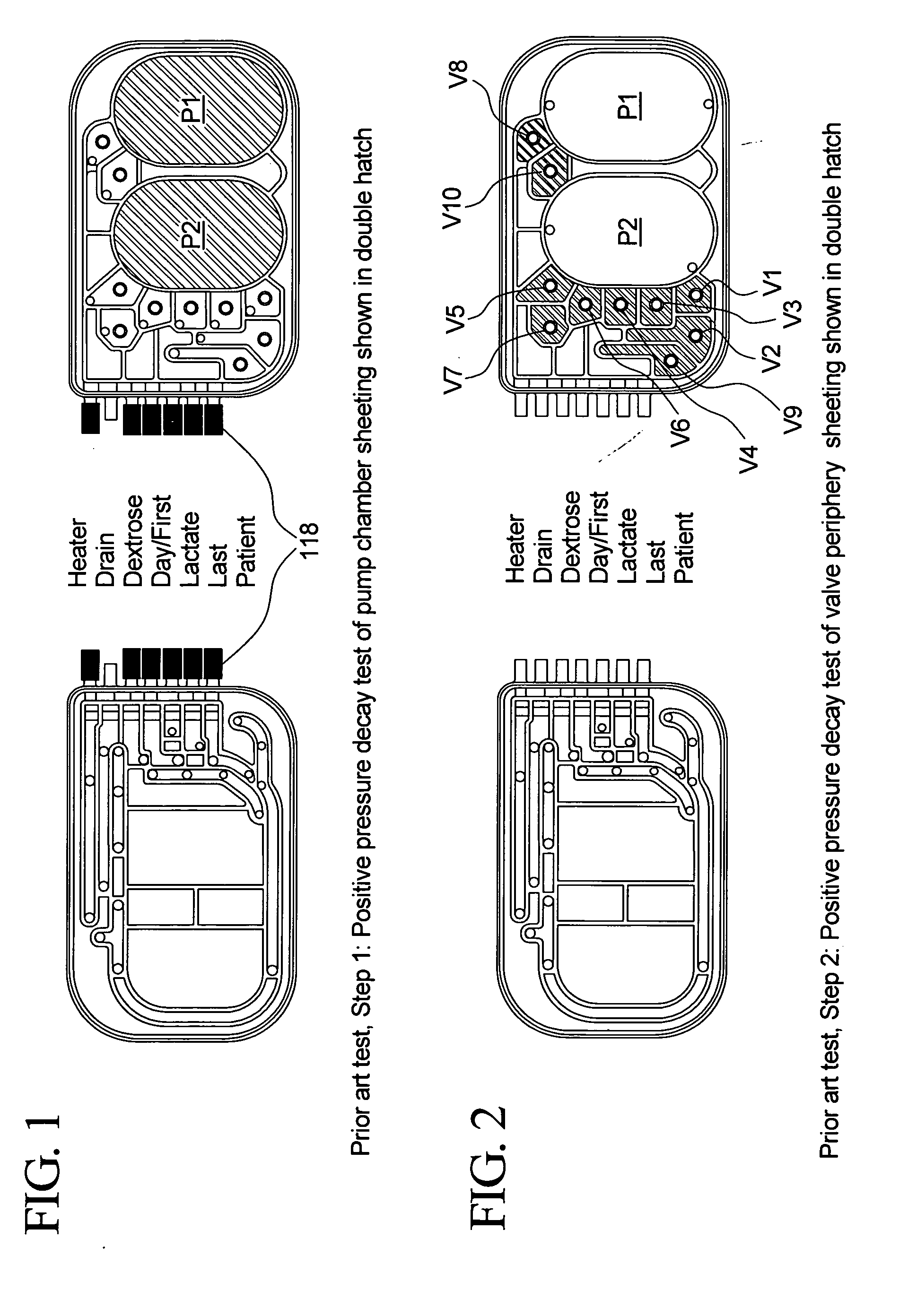
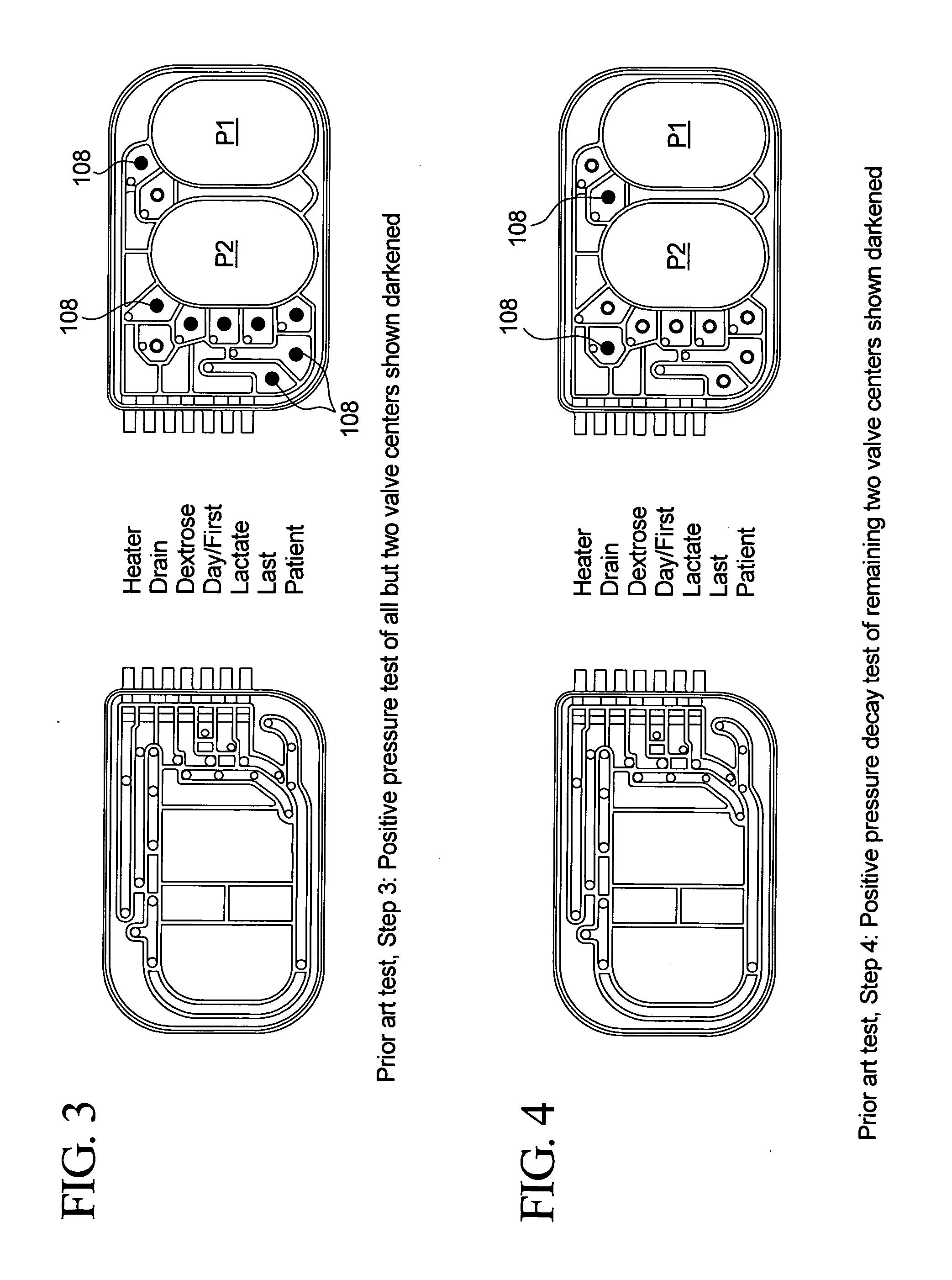
The present invention provides systems, methods and apparatuses for medical fluid delivery systems that employ a pumping cassette. In particular, the present invention provides systems, methods and apparatuses for cassette-based dialysis therapies including hemodialysis, hemofiltration, APD (including tidal modalities) and CFPD. The embodiments described include a combined pump / valve housing, a fail safe pump / valve arrangement, a cassette auto-alignment feature, a pumping membrane material, a multiplexing valve arrangement, an expert fluid pumping management system, an integral port vent and an in-line air separation chamber and combinations of each of these.
Owner:BAXTER INT INC +1
Method for separating analyte from a sample
InactiveUS6893879B2Improve elution efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsSporeChemical reaction
An analyte is separated from a fluid sample by introducing the sample into a cartridge having an extraction chamber containing capture material for capturing the analyte. The sample is forced to flow through the extraction chamber to capture the analyte with the capture material in the extraction chamber. The captured analyte is then eluted from the extraction chamber by forcing an elution fluid to flow through the extraction chamber. The cartridge may optionally include a lysing region for lysing sample components (e.g., cells spores, or microorganisms), a waste chamber for storing waste fluid, and reaction or detection chambers for chemically reacting or detecting the eluted analyte.
Owner:CEPHEID INC
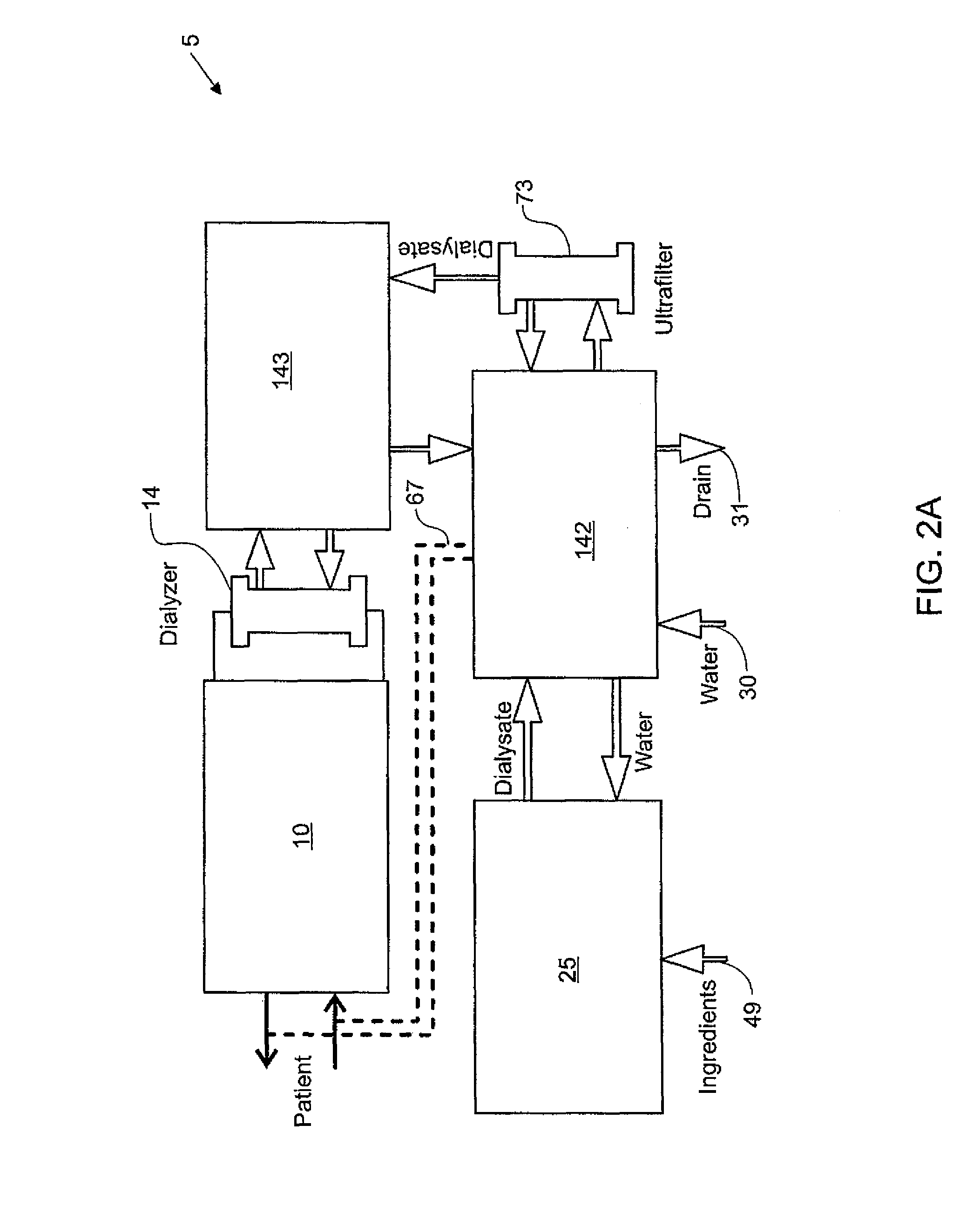
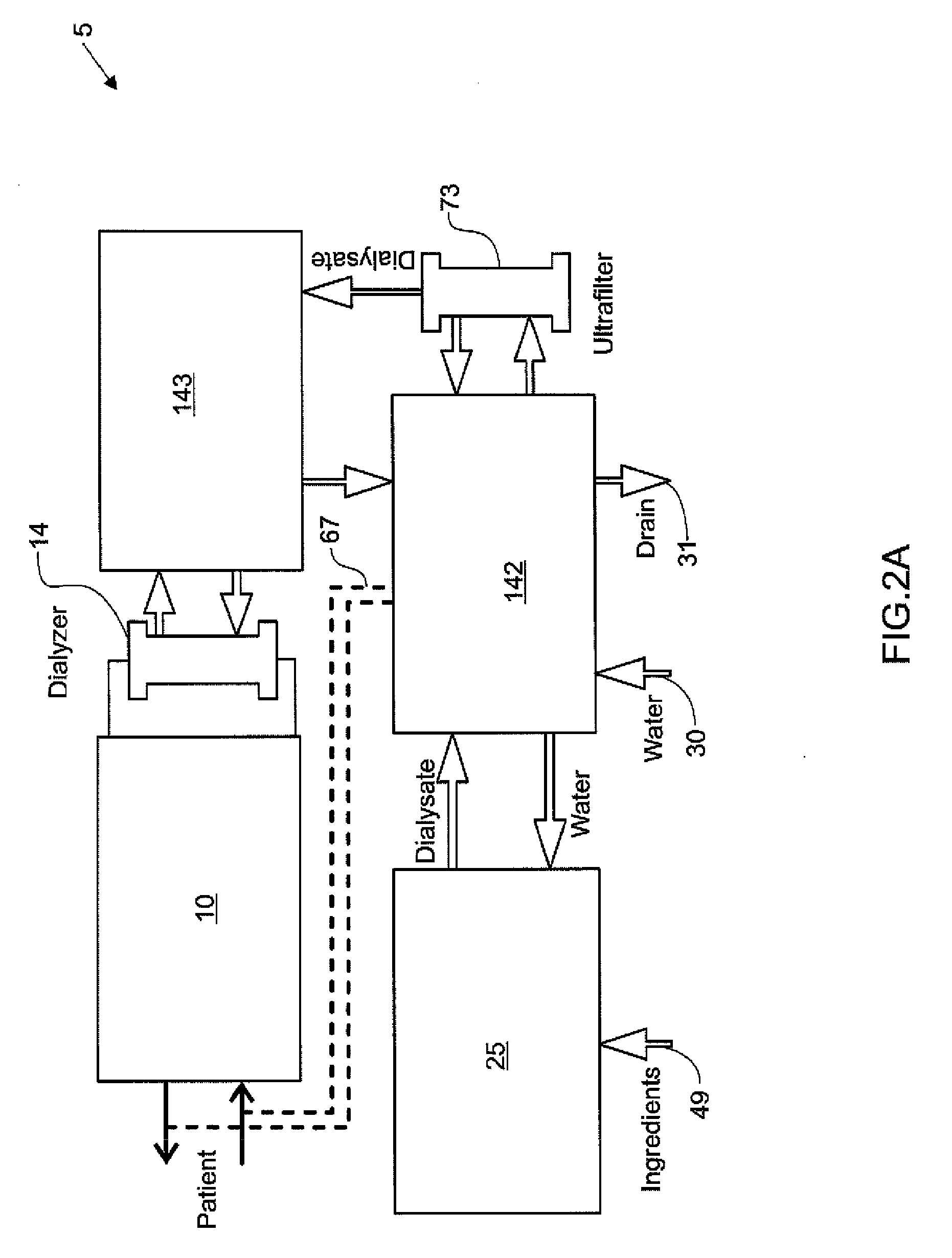
Blood treatment systems and methods
ActiveUS20100192686A1Increase pressureMechanical/radiation/invasive therapiesSolvent extractionBlood treatmentsGraphics
Dialysis systems comprising actuators that cooperate to perform dialysis functions and sensors that cooperate to monitor dialysis functions are disclosed. According to one aspect, such a hemodialysis system comprises a user interface model layer, a therapy layer, below the user interface model layer, and a machine layer below the therapy layer. The user interface model layer is configured to manage the state of a graphical user interface and receive inputs from a graphical user interface. The therapy layer is configured to run state machines that generate therapy commands based at least in part on the inputs from the graphical user interface. The machine layer is configured to provide commands for the actuators based on the therapy commands.
Owner:DEKA PROD LLP
Priming, integrity and head height methods and apparatuses for medical fluid systems
ActiveUS20050126998A1Less timeLeakage is detectedDetection of fluid at leakage pointOther blood circulation devicesDelivery systemFluid system
Improved integrity test, priming sequence and bag height detection tests, apparatuses and methods for a medical fluid delivery system are provided. The integrity test includes a plurality of air pressure decay tests, using positive and negative pressure. The priming sequence includes pumping fluid through a portion of a patient line to be primed to overcome air in the line and other potential obstacles. The head height test measures a pressure build-up or drop-off within a pump chamber of a membrane pump. The measured pressure corresponds to a head height between a fluid supply and the pump or between the pump and a fluid drain. A determination is made whether the corresponding head height is acceptable.
Owner:BAXTER INT INC +1
Hemodialysis systems and methods
ActiveUS20090095679A1Mechanical/radiation/invasive therapiesSolvent extractionHaemodialysis machineDialysate flow
Hemodialysis dialysis systems are disclosed. Hemodialysis systems of the invention may include a dialysate flow path including a balancing circuit, a mixing circuit, and / or a directing circuit. The circuits may be defined within one or more cassettes. The fluid circuits may be at least partially isolated, spatially and / or thermally, from electrical components of the system. A gas supply may be provided in fluid communication with the dialysate flow path and / or the dialyzer to urge dialysate through the dialyzer and blood back to the patient. The hemodialysis systems may include fluid handling devices, actuated using a control fluid, optionally delivered using a detachable pump. Fluid handling devices may be generally rigid and of a spheroid shape, optionally with a diaphragm dividing the device into compartments.
Owner:DEKA PROD LLP
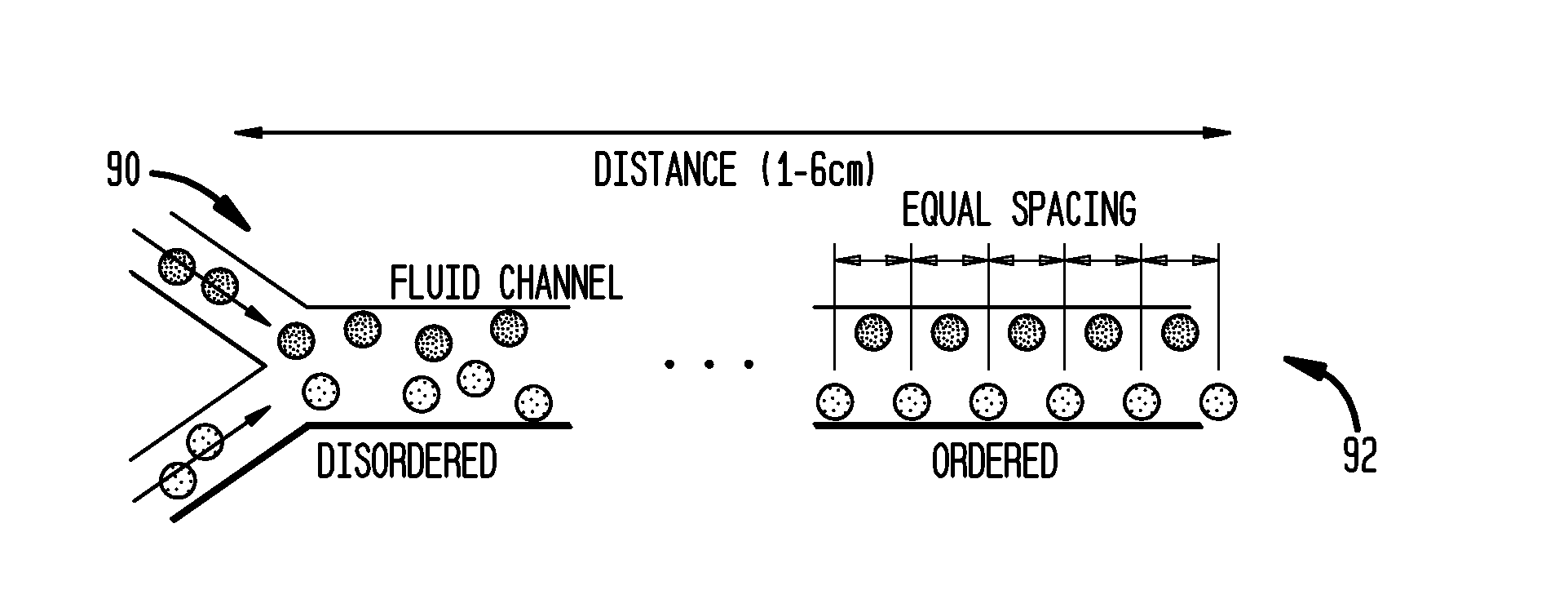
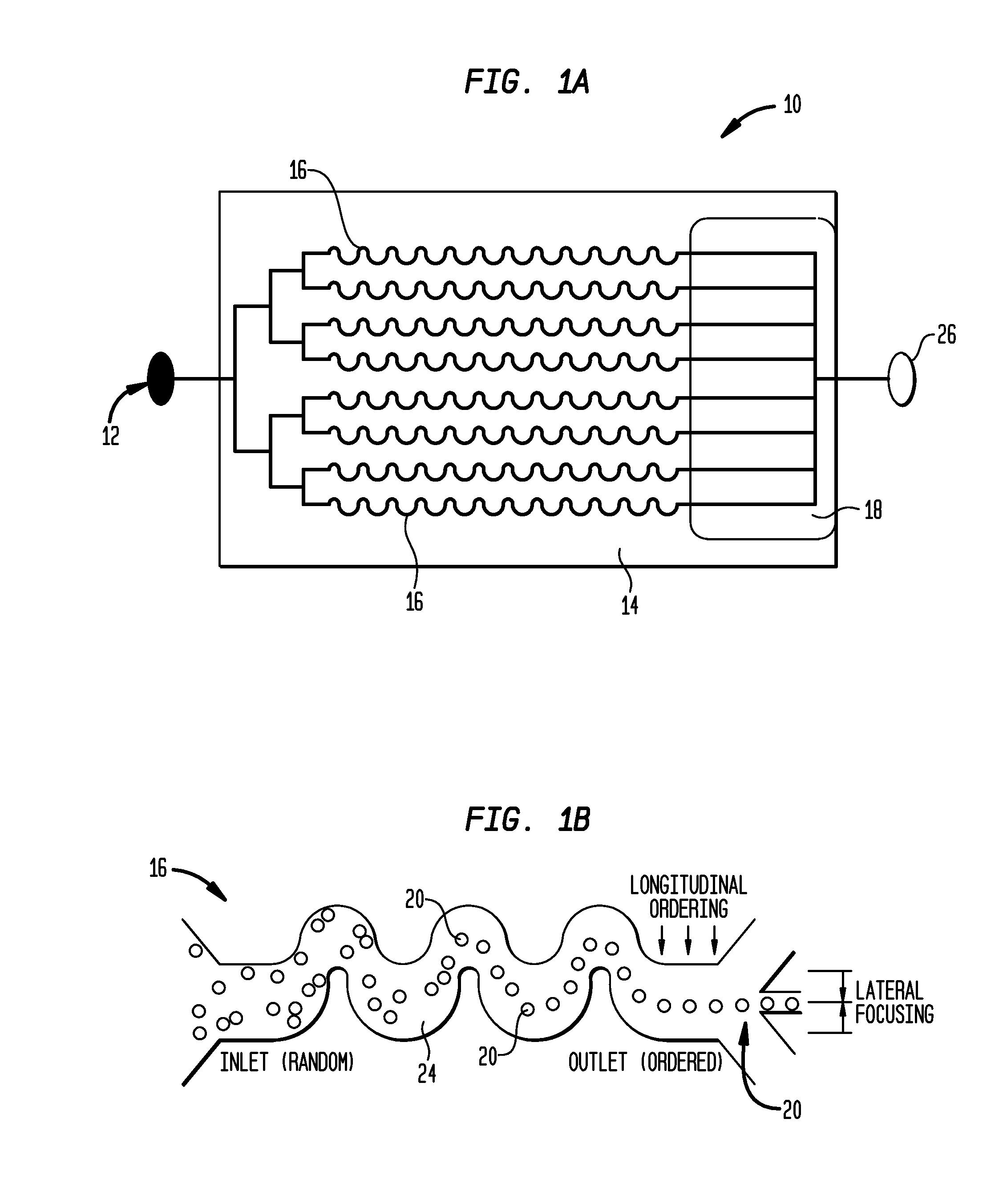
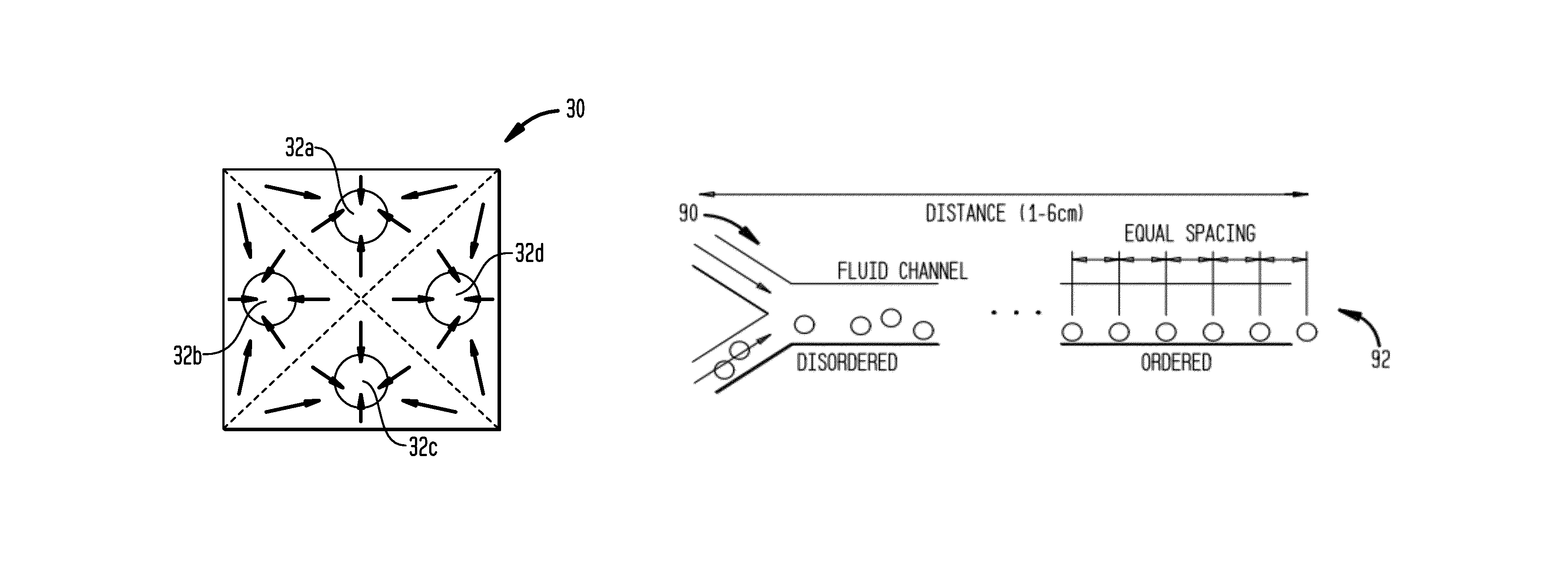
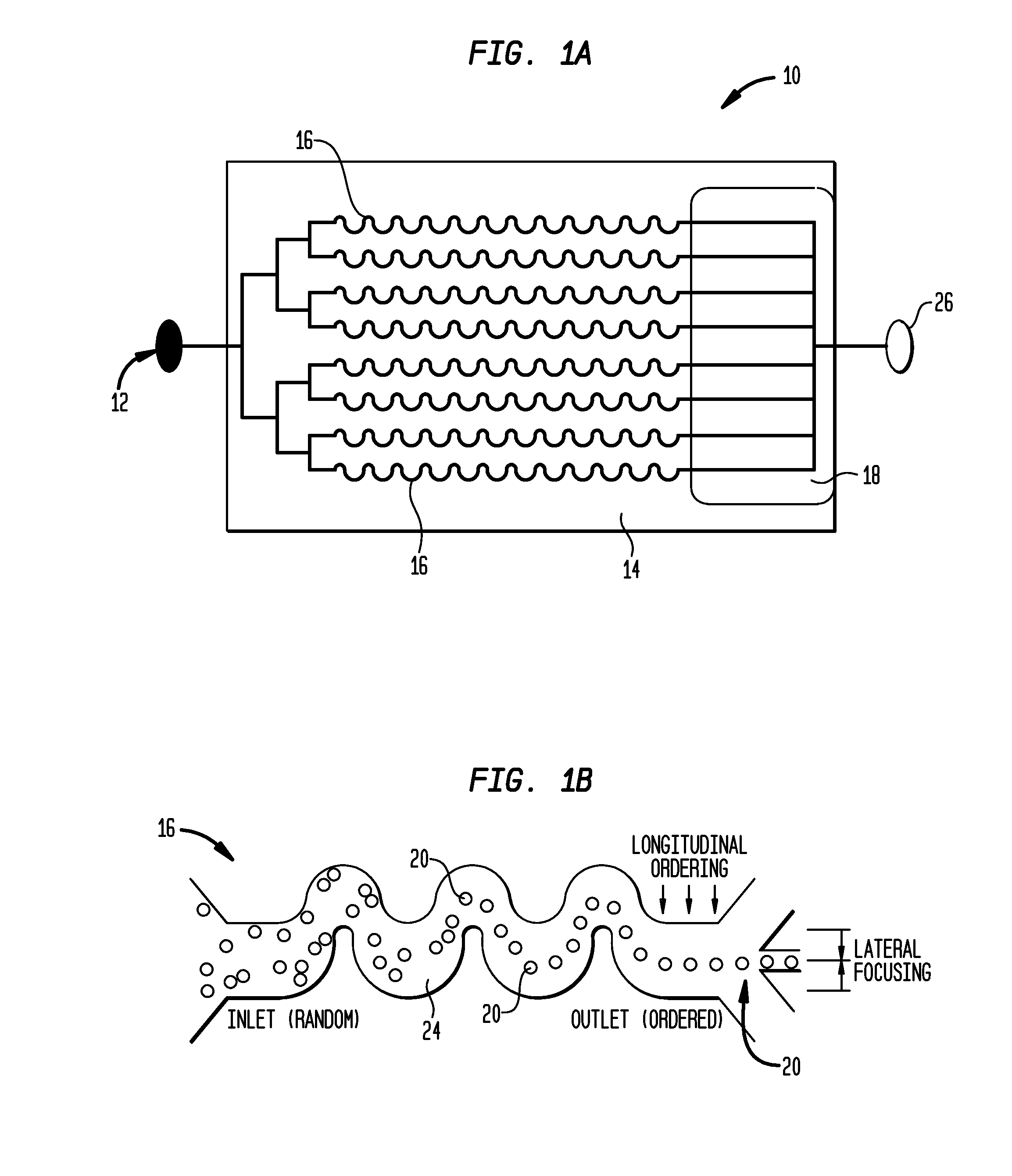
Systems and methods for particle focusing in microchannels
ActiveUS20090014360A1Increase in sizeImprove concentrationImmobilised enzymesSolvent extractionEngineeringSuspended particles
Various systems, methods, and devices are provided for focusing particles suspended within a moving fluid into one or more localized stream lines. The system can include a substrate and at least one channel provided on the substrate having an inlet and an outlet. The system can further include a fluid moving along the channel in a laminar flow having suspended particles and a pumping element driving the laminar flow of the fluid. The fluid, the channel, and the pumping element can be configured to cause inertial forces to act on the particles and to focus the particles into one or more stream lines.
Owner:THE GENERAL HOSPITAL CORP
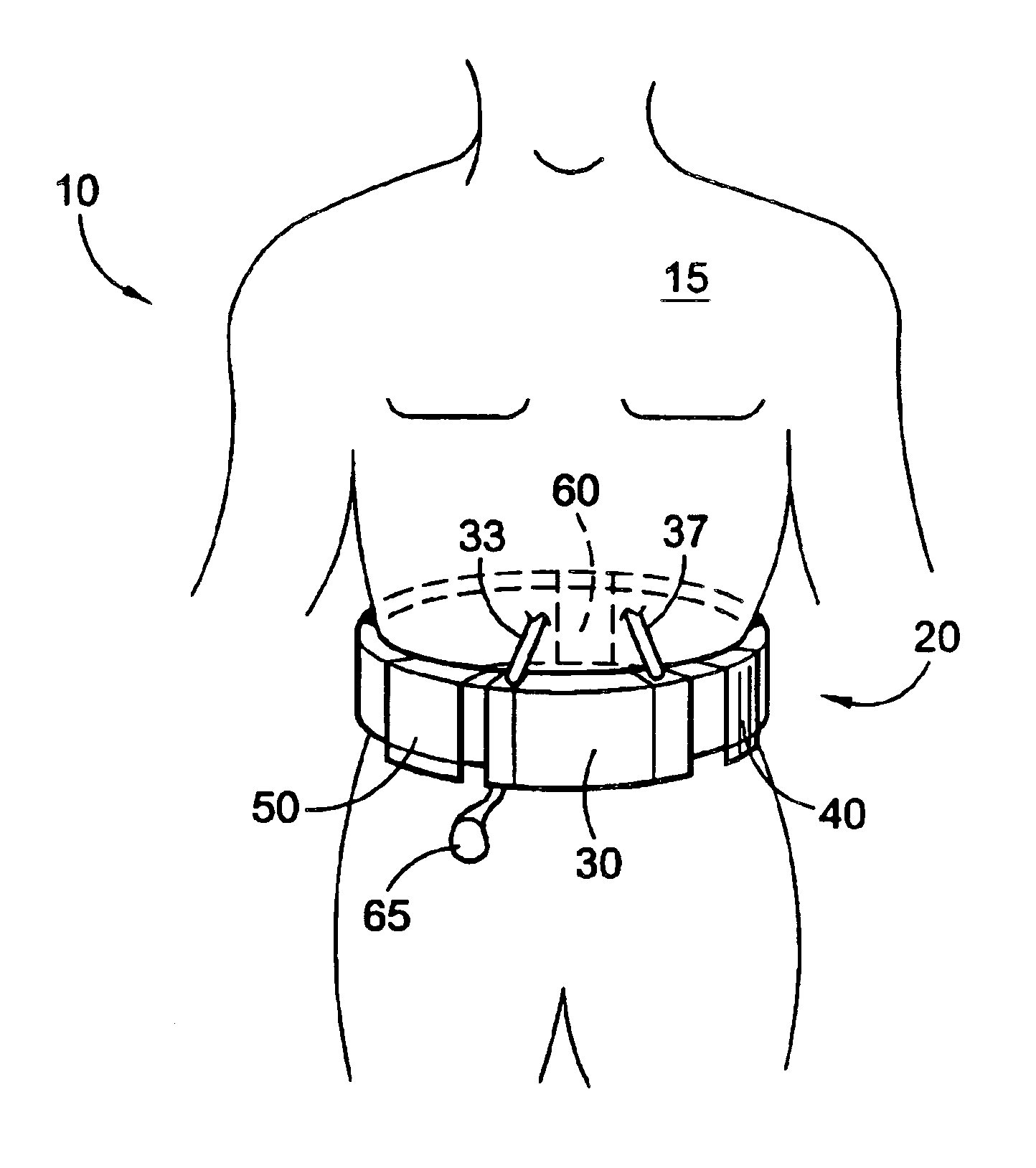
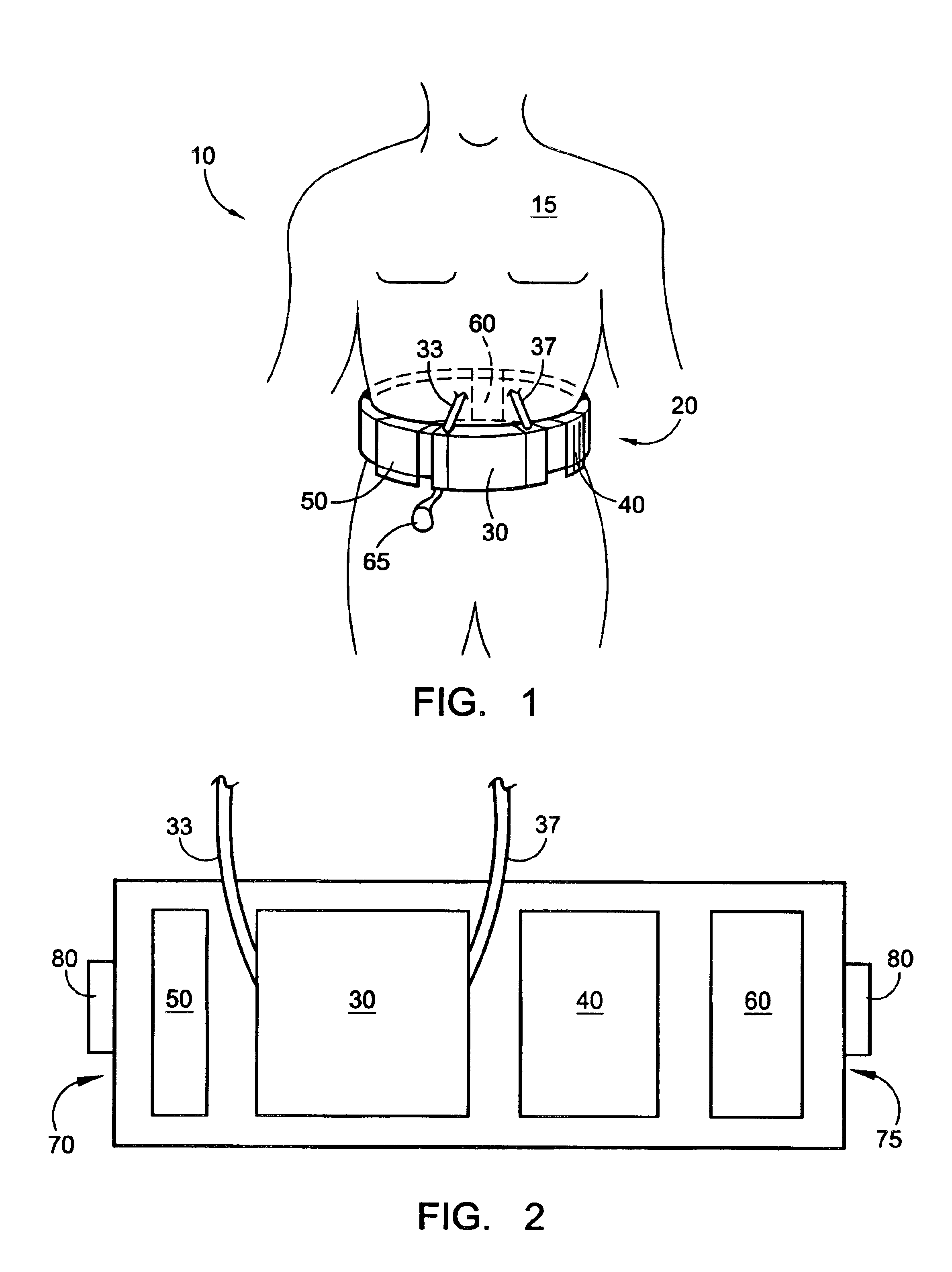
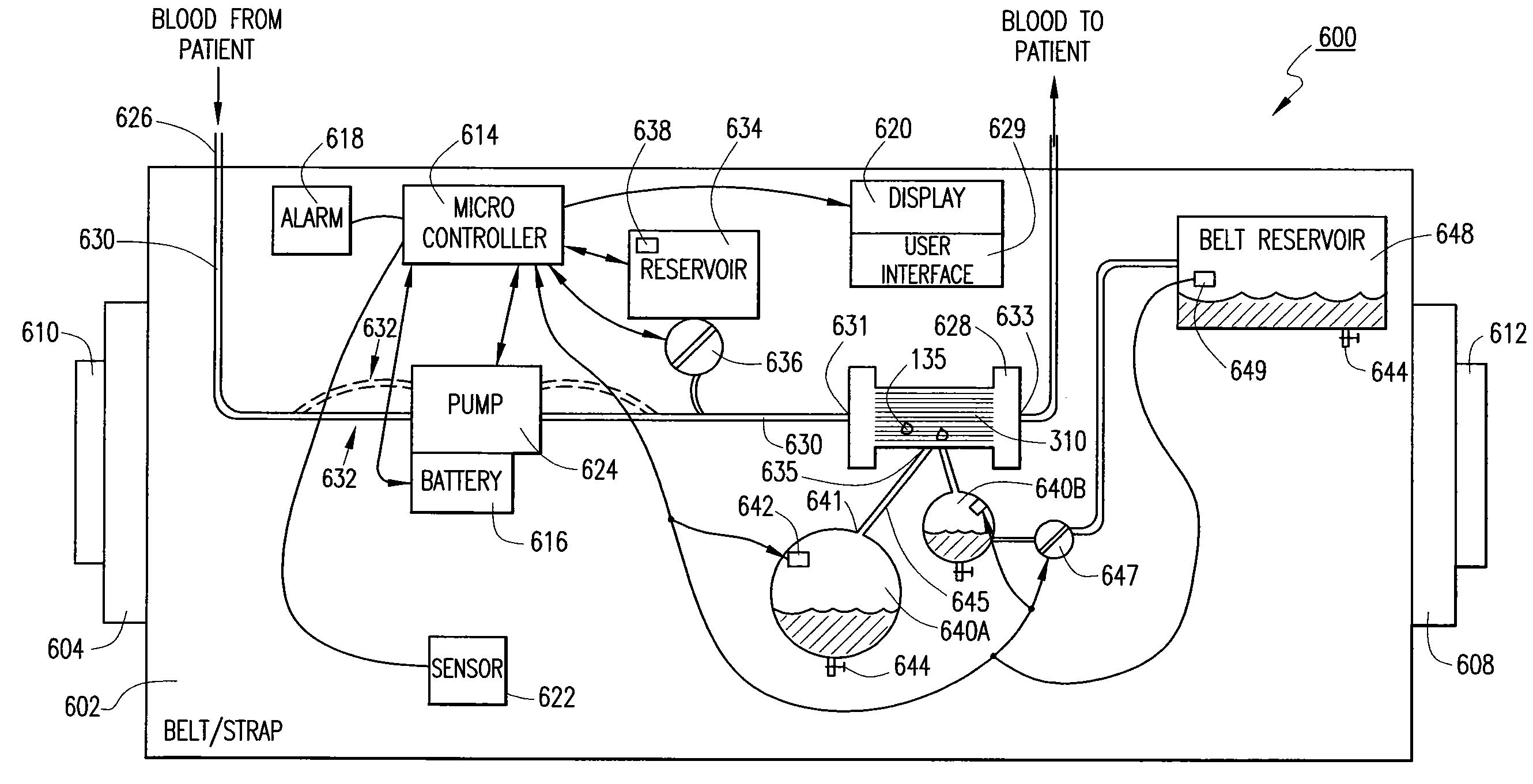
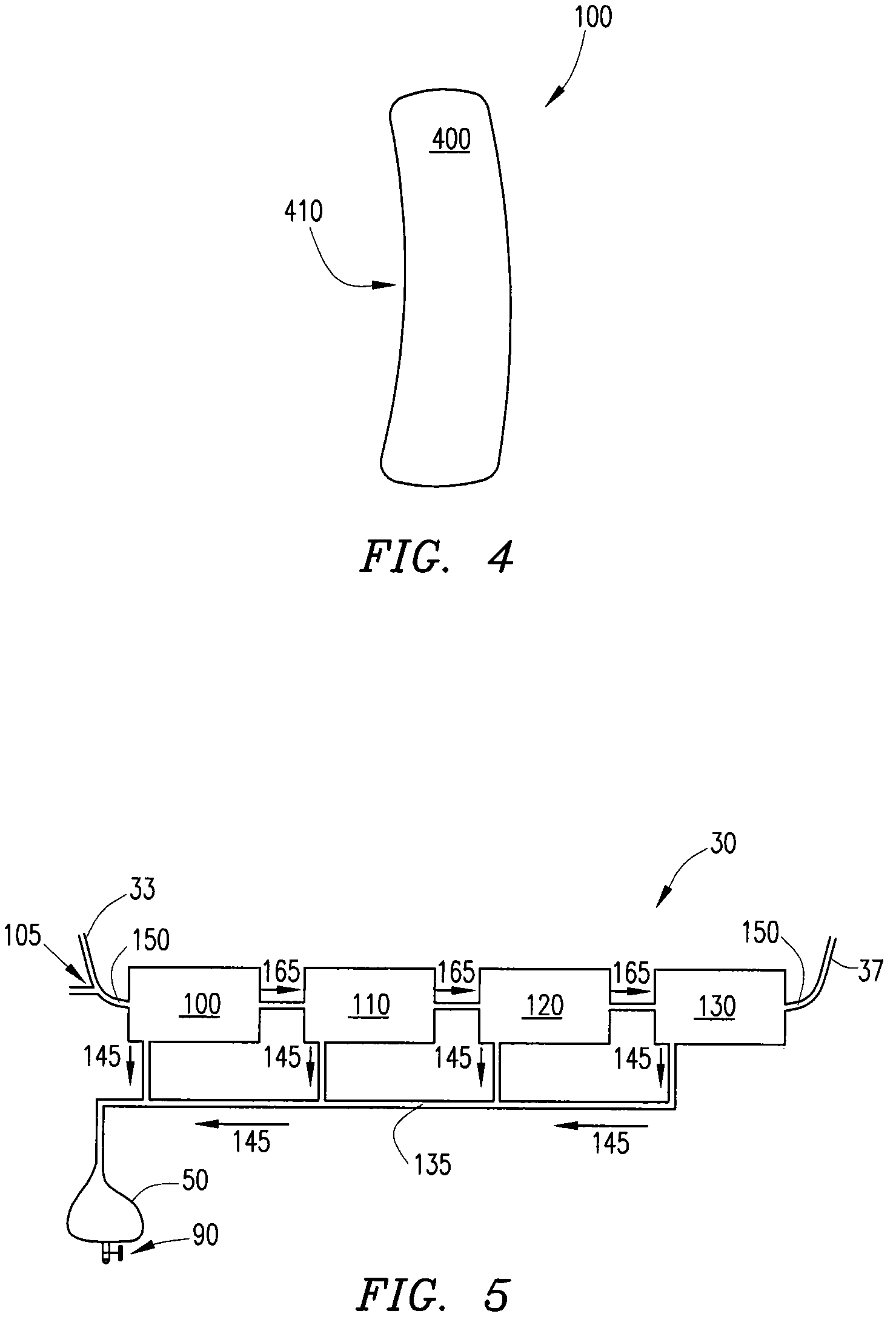
Wearable continuous renal replacement therapy device
A continuous renal replacement therapy device adapted to be worn on a portion of the body of a patient, including: a plurality of contoured dialyzers, which are connected in series and utilize dialysate to remove impurities from the blood of the patient; and a plurality of contoured sorbent device, which are connected in series and are for regenerating the spent dialysate.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Graphical user interface for automated dialysis system
ActiveUS7033539B2Semi-permeable membranesMechanical/radiation/invasive therapiesGraphicsPeritoneal dialysis
A method, system and apparatus for performing peritoneal dialysis are provided. To this end, in part, a dialysis system is provided. The dialysis system includes a display device and a web browser and web server embedded in the dialysis system. The browser and the server operate with the display device to display a number of dialysis therapy set-up procedure screens that require operator input, and to display a number of dialysis treatment screens that graphically illustrate the progress of at least one step in the dialysis therapy in at least substantially real time.
Owner:BAXTER HEALTHCARE SA +1
Blood treatment systems and methods
ActiveUS8409441B2Increase pressureMechanical/radiation/invasive therapiesSolvent extractionBlood treatmentsGraphics
Dialysis systems comprising actuators that cooperate to perform dialysis functions and sensors that cooperate to monitor dialysis functions are disclosed. According to one aspect, such a hemodialysis system comprises a user interface model layer, a therapy layer, below the user interface model layer, and a machine layer below the therapy layer. The user interface model layer is configured to manage the state of a graphical user interface and receive inputs from a graphical user interface. The therapy layer is configured to run state machines that generate therapy commands based at least in part on the inputs from the graphical user interface. The machine layer is configured to provide commands for the actuators based on the therapy commands.
Owner:DEKA PROD LLP
Methods and Systems for Controlling Ultrafiltration Using Central Venous Pressure Measurements
The volume of fluid removed from a patient during ultrafiltration is controlled automatically on the basis of central venous pressure (CVP) measurements. In one embodiment, a central venous catheter (CVC) is used for accessing blood during dialysis. A sensor located at the tip of the catheter or inside the dialysis machine is used to periodically measure CVP. CVP feedback data helps prevent the excessive removal of fluids from the patient.
Owner:FRESENIUS MEDICAL CARE HLDG INC
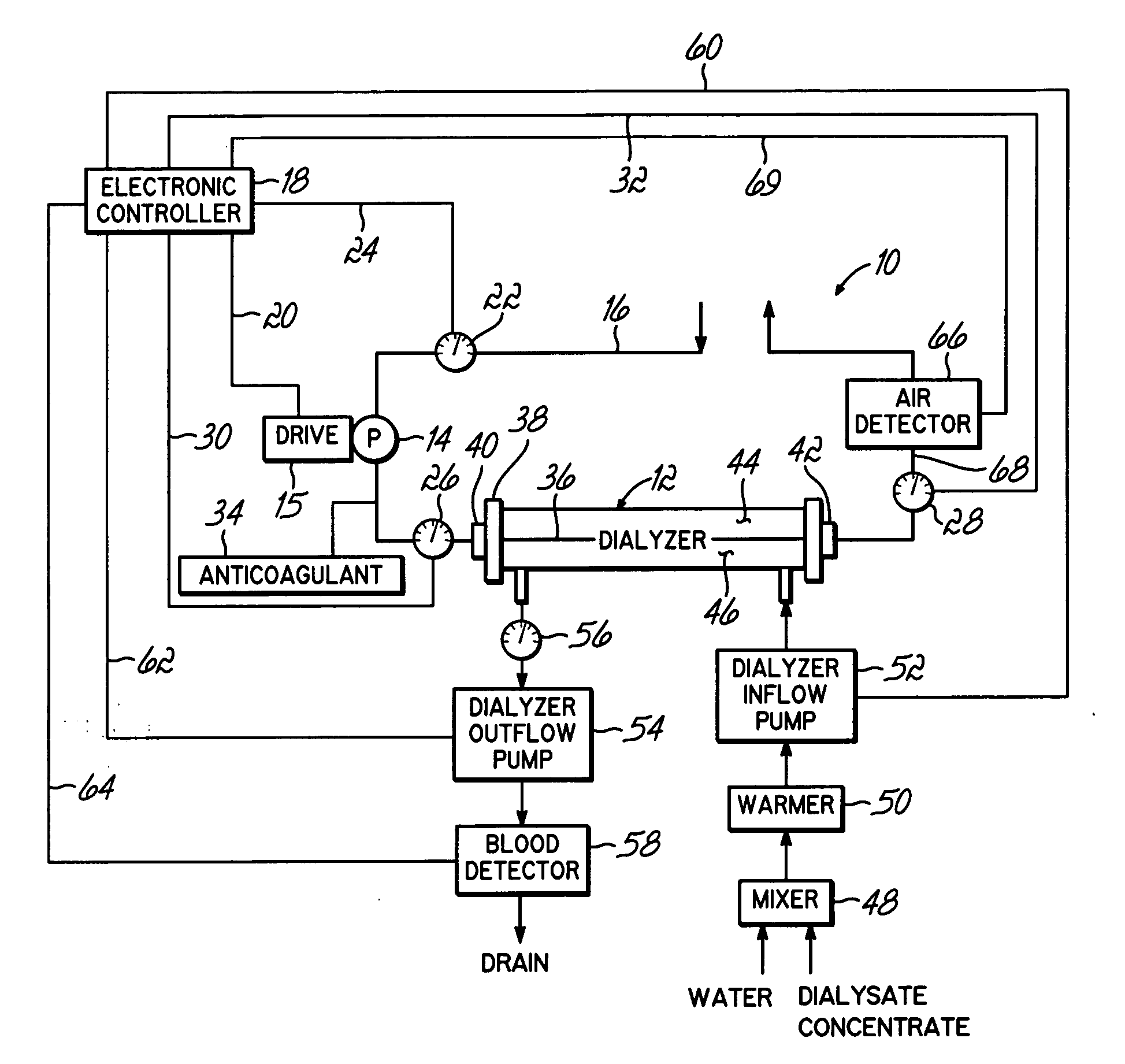
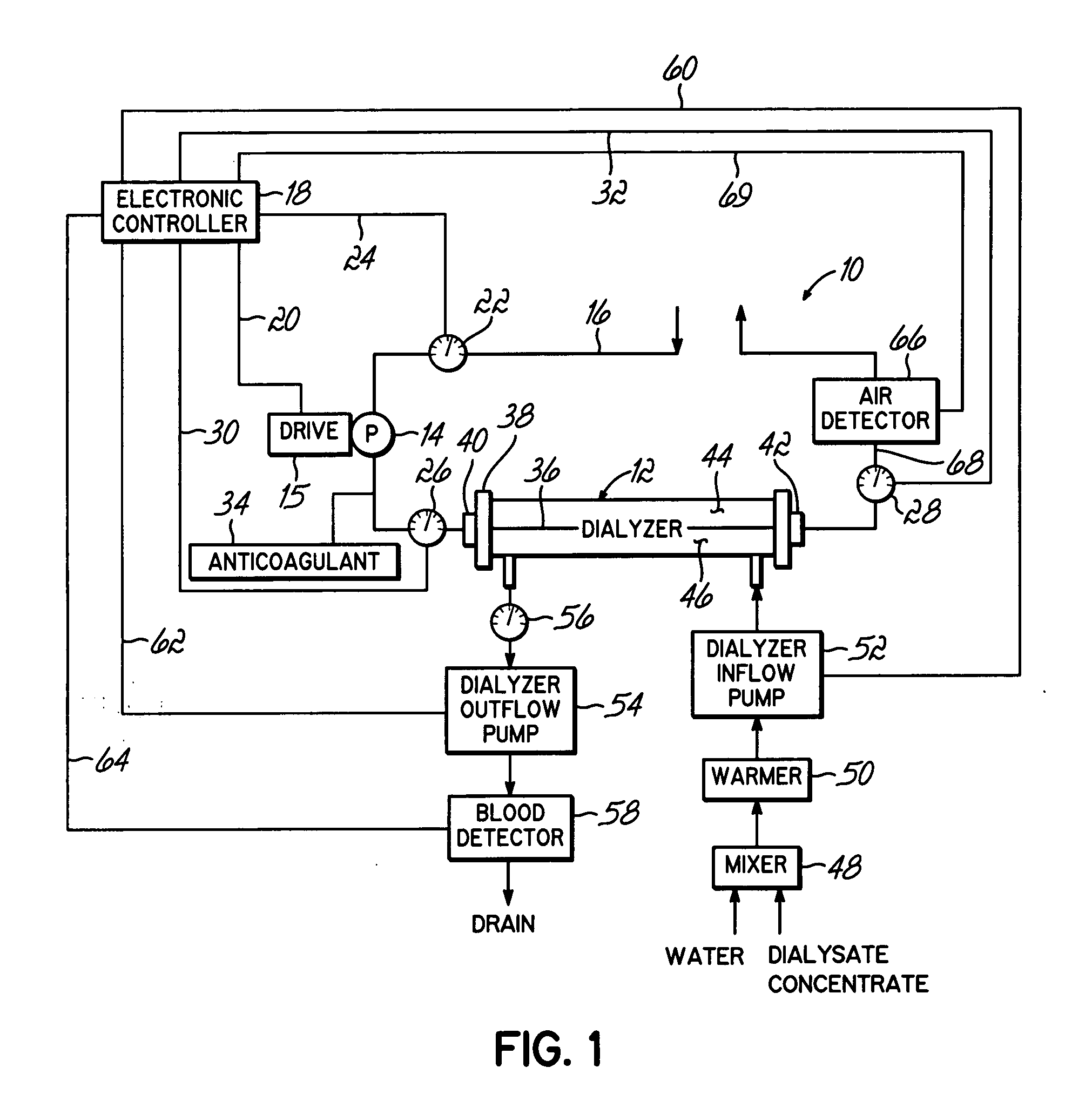
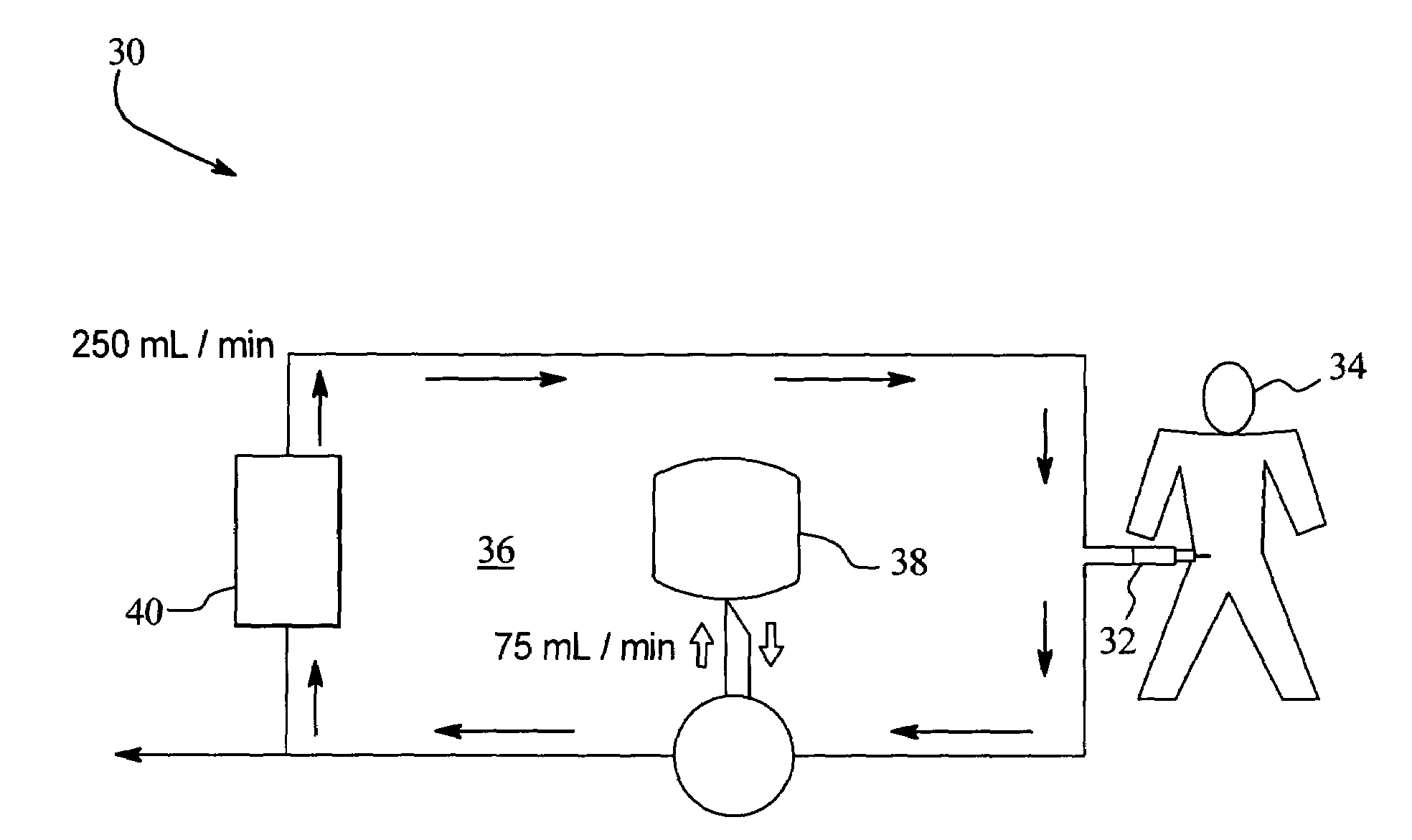
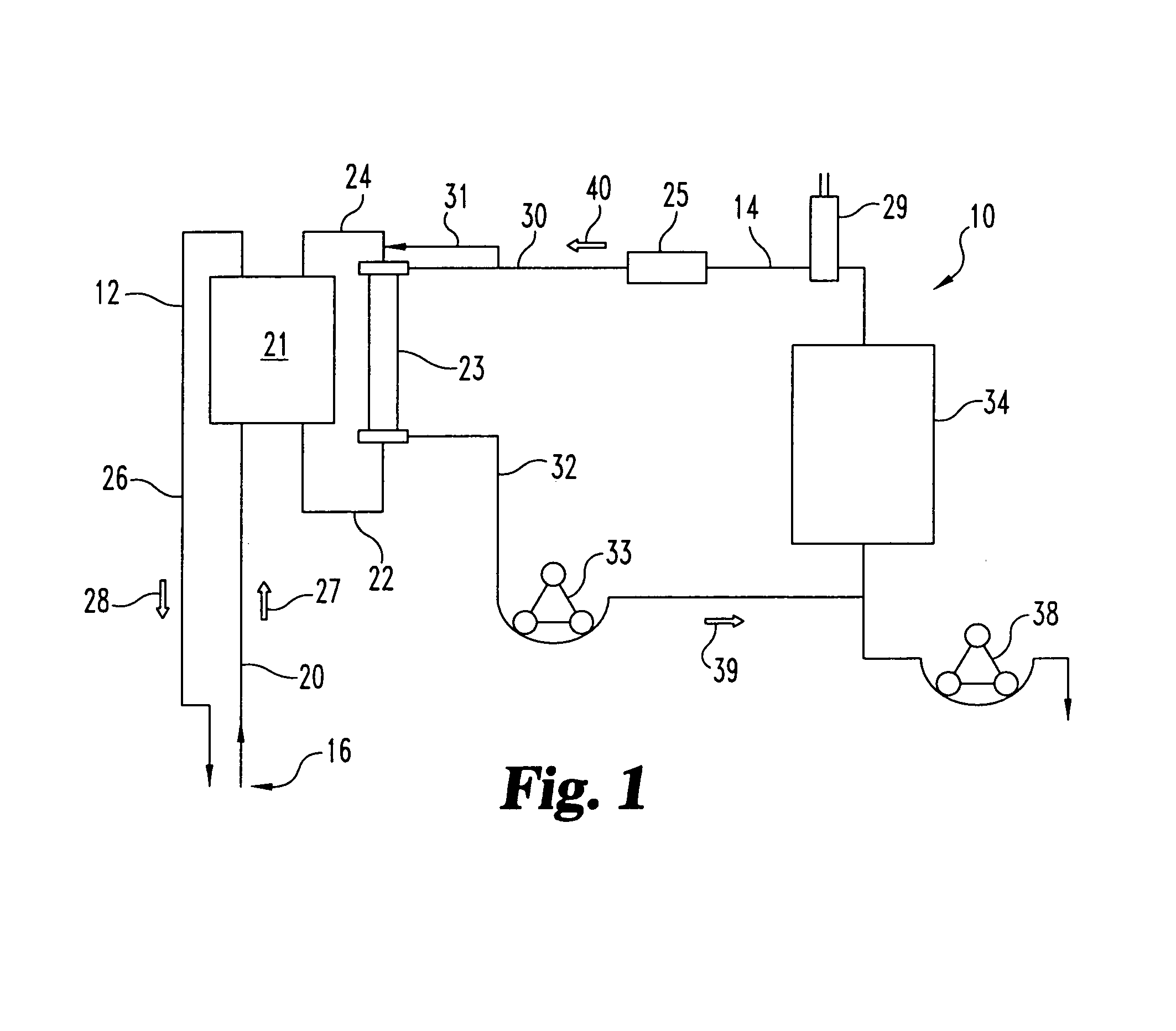
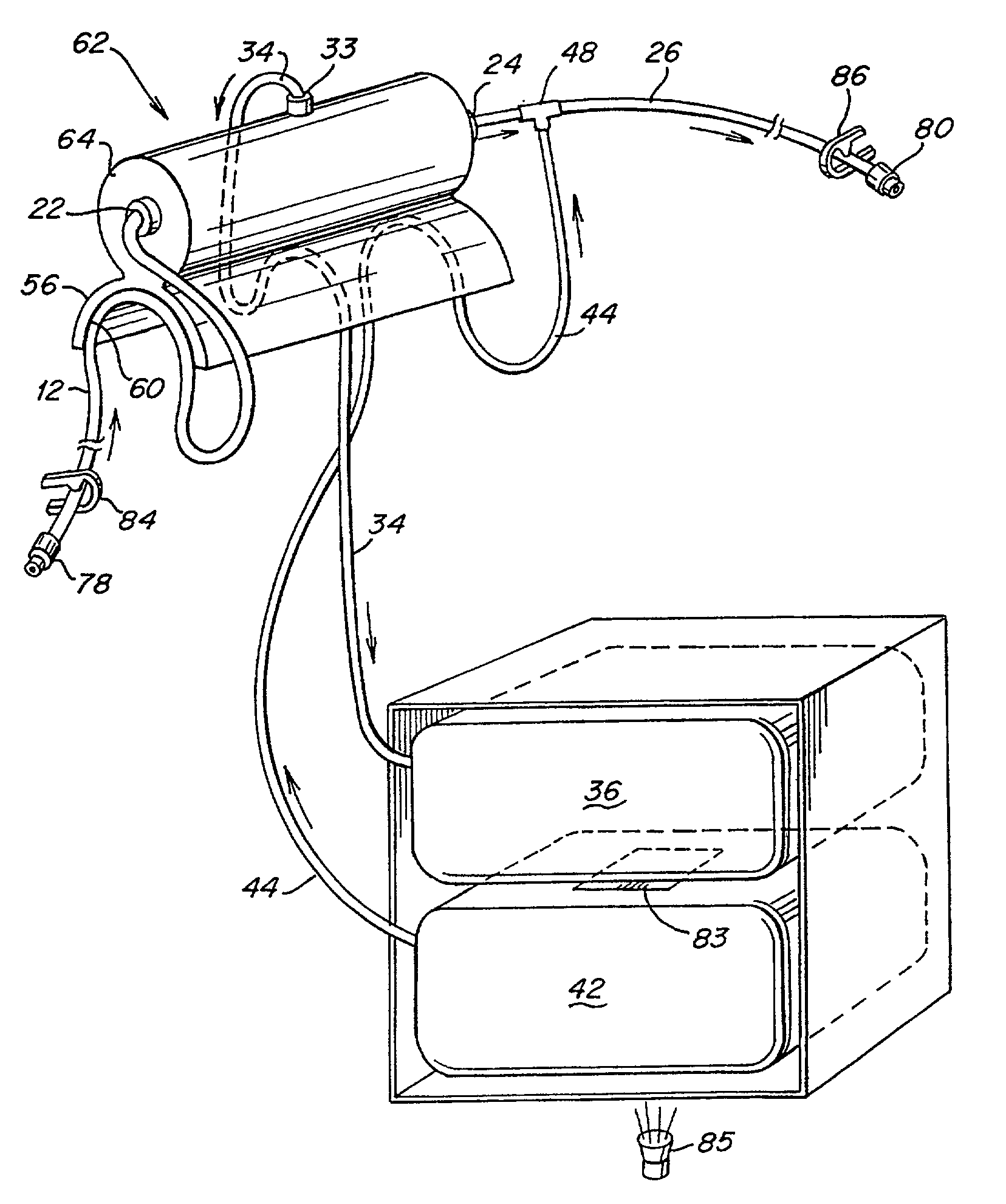
Hemofiltration system
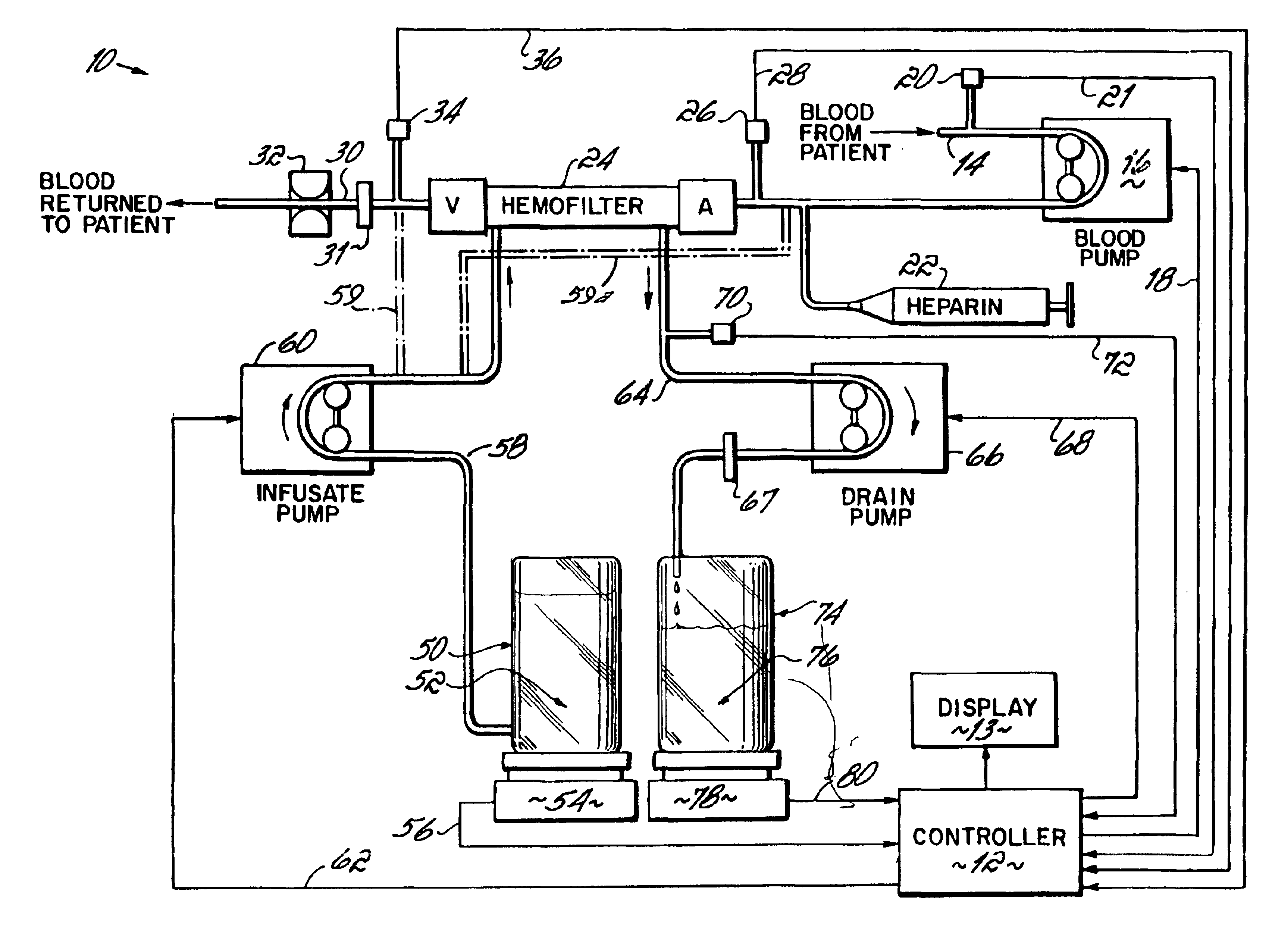
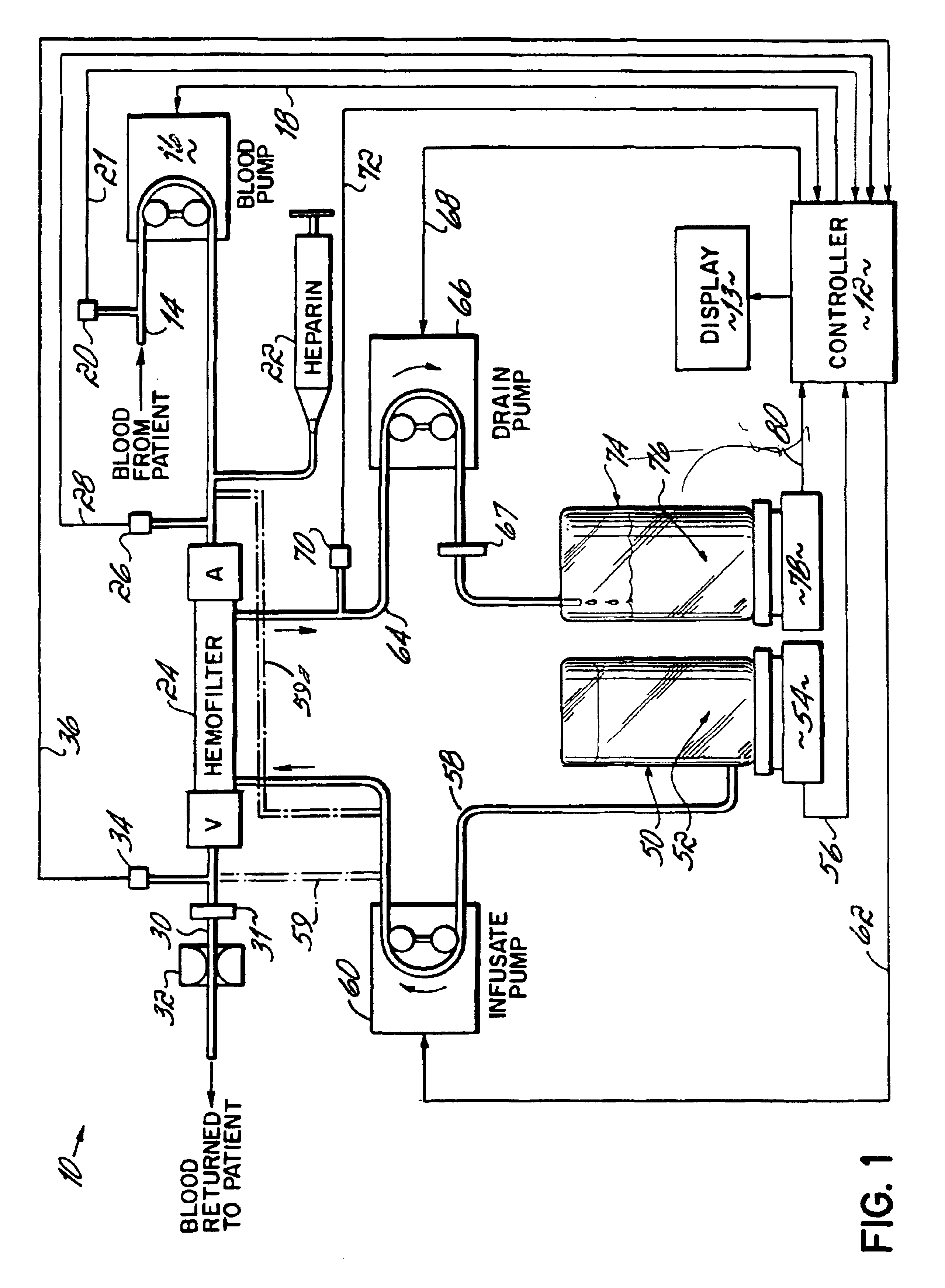
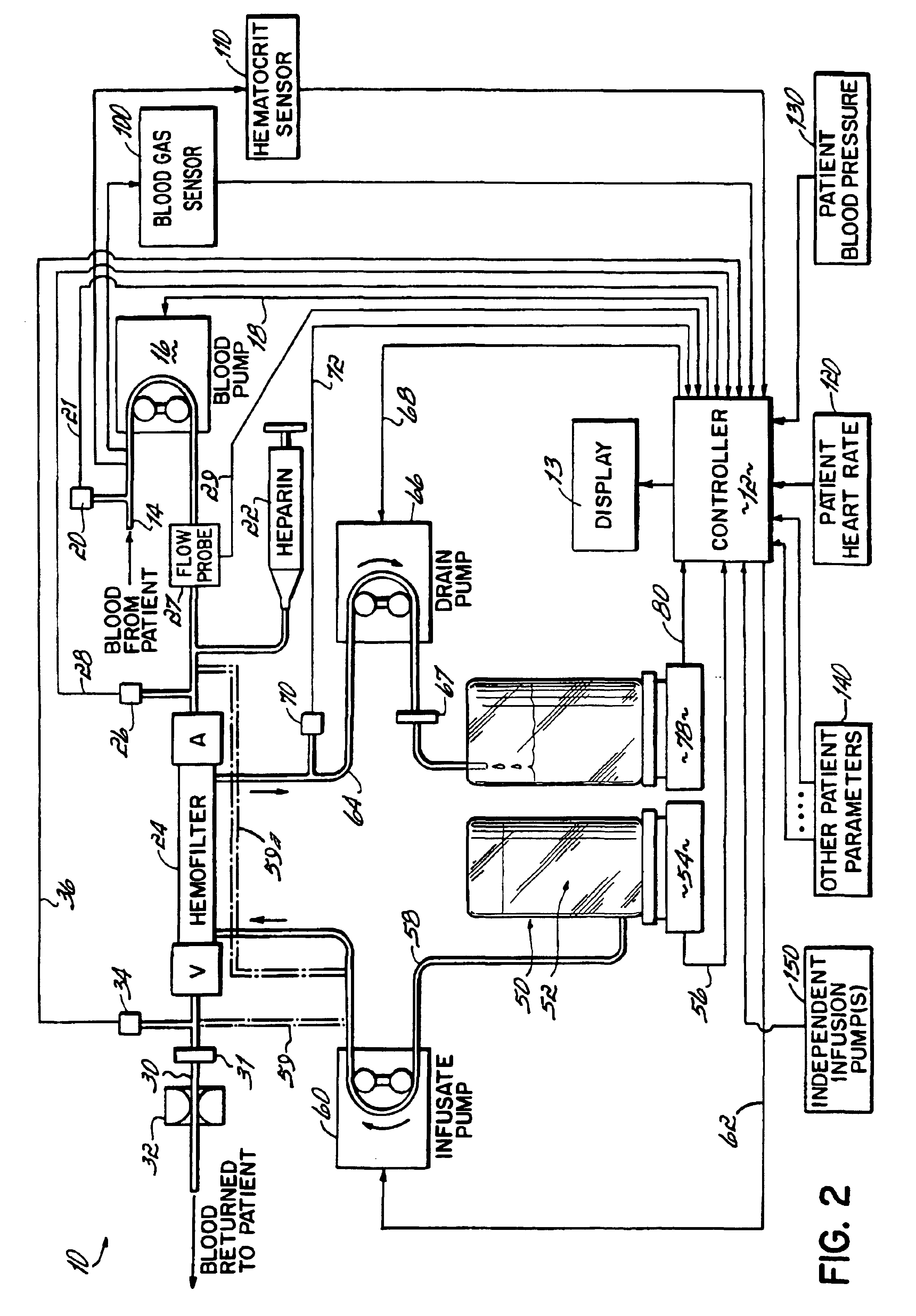
InactiveUS6780322B1Reliable and long-term operationImprove accuracySolvent extractionUltrafiltrationMedicineTime changes
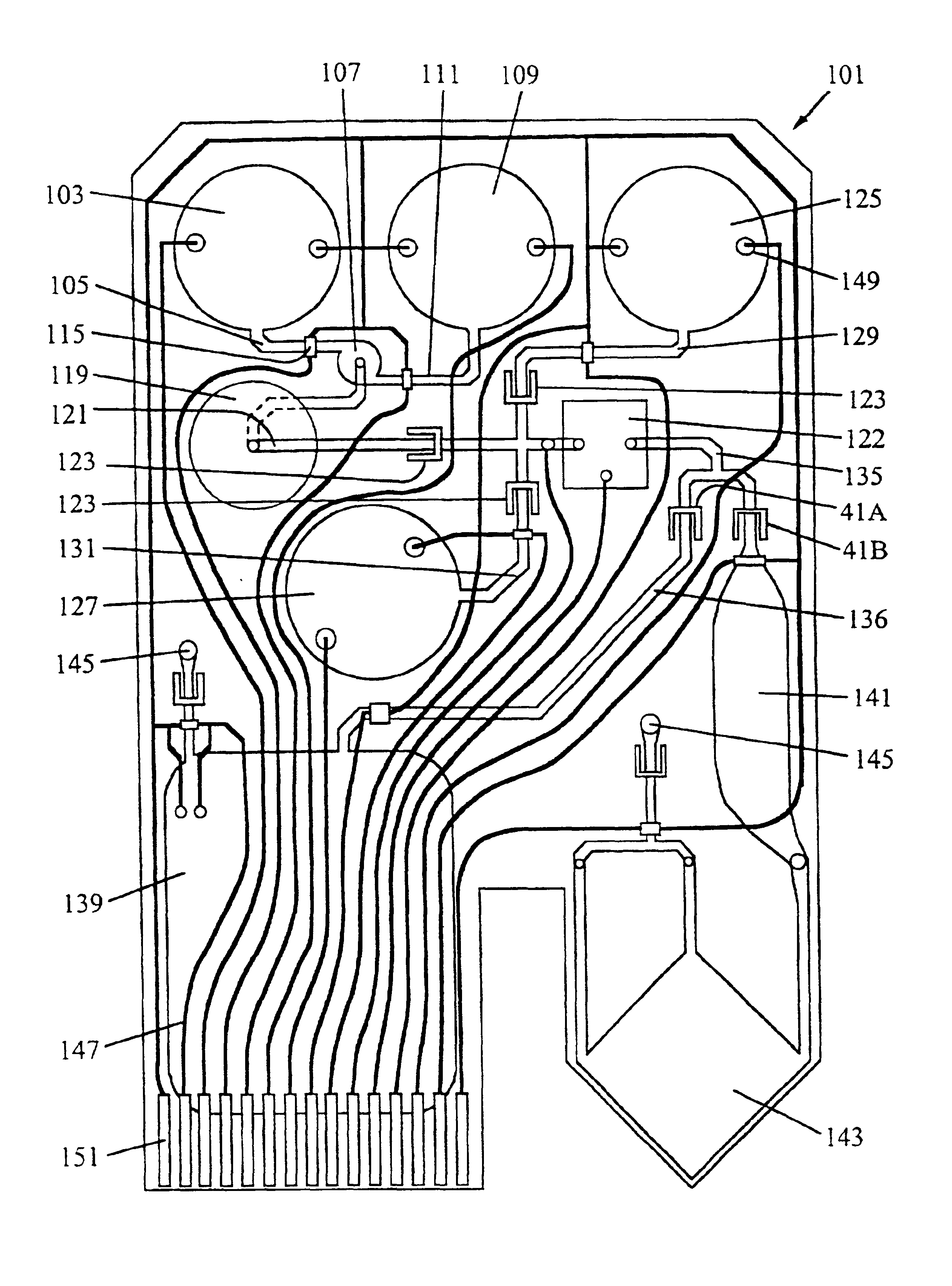
A multipurpose hemofiltration system (10) and method are disclosed for the removal of fluid and / or soluble waste from the blood of a patient. The system (10) continuously monitors the flow rates of drained fluid, blood, and infusate. When necessary, the pumping rates of the infusate, drained fluid and blood are adjusted to remove a preselected amount of fluid from the blood in a preselected time period. A supervisory controller (160) can monitor patient parameters, such as heart rate (120) and blood pressure (130), and adjust the pumping rates accordingly. The supervisory controller (160) uses fuzzy logic to make expert decisions, based upon a set of supervisory rules, to control each pumping rate to achieve a desired flow rate and to respond to fault conditions. An adaptive controller (162) corrects temporal variations in the flow rate based upon an adaptive law and a control law.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
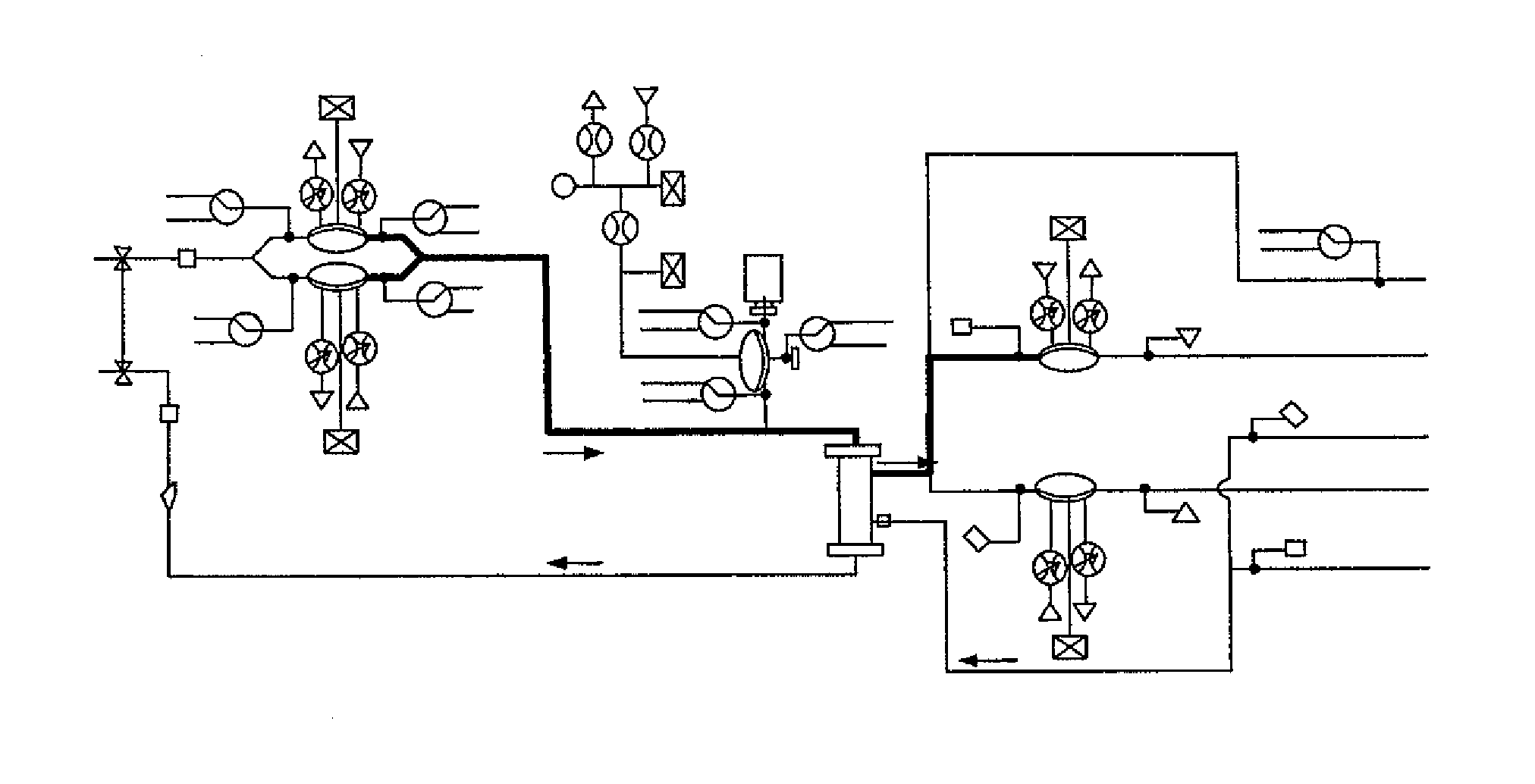
Programmable, fluid pressure actuated blood processing systems and methods
InactiveUS6949079B1Solvent extractionOther blood circulation devicesBlood separation deviceEngineering
Owner:FENWAL
Hemodialysis systems and methods
The present invention generally relates to hemodialysis and similar dialysis systems, including a variety of systems and methods that would make hemodialysis more efficient, easier, and / or more affordable. One aspect of the invention is generally directed to new fluid circuits for fluid flow. In one set of embodiments, a hemodialysis system may include a blood flow path and a dialysate flow path, where the dialysate flow path includes one or more of a balancing circuit, a mixing circuit, and / or a directing circuit. Preparation of dialysate by the preparation circuit, in some instances, may be decoupled from patient dialysis. In some cases, the circuits are defined, at least partially, within one or more cassettes, optionally interconnected with conduits, pumps, or the like. In one embodiment, the fluid circuit and / or the various fluid flow paths may be at least partially isolated, spatially and / or thermally, from electrical components of the hemodialysis system. In some cases, a gas supply may be provided in fluid communication with the dialysate flow path and / or the dialyzer that, when activated, is able to urge dialysate to pass through the dialyzer and urge blood in the blood flow path back to the patient. Such a system may be useful, for example, in certain emergency situations (e.g., a power failure) where it is desirable to return as much blood to the patient as possible. The hemodialysis system may also include, in another aspect of the invention, one or more fluid handling devices, such as pumps, valves, mixers, or the like, which can be actuated using a control fluid, such as air. In some cases, the control fluid may be delivered to the fluid handling devices using an external pump or other device, which may be detachable in certain instances. In one embodiment, one or more of the fluid handling devices may be generally rigid (e.g., having a spheroid shape), optionally with a diaphragm contained within the device, dividing it into first and second compartments.
Owner:DEKA PROD LLP
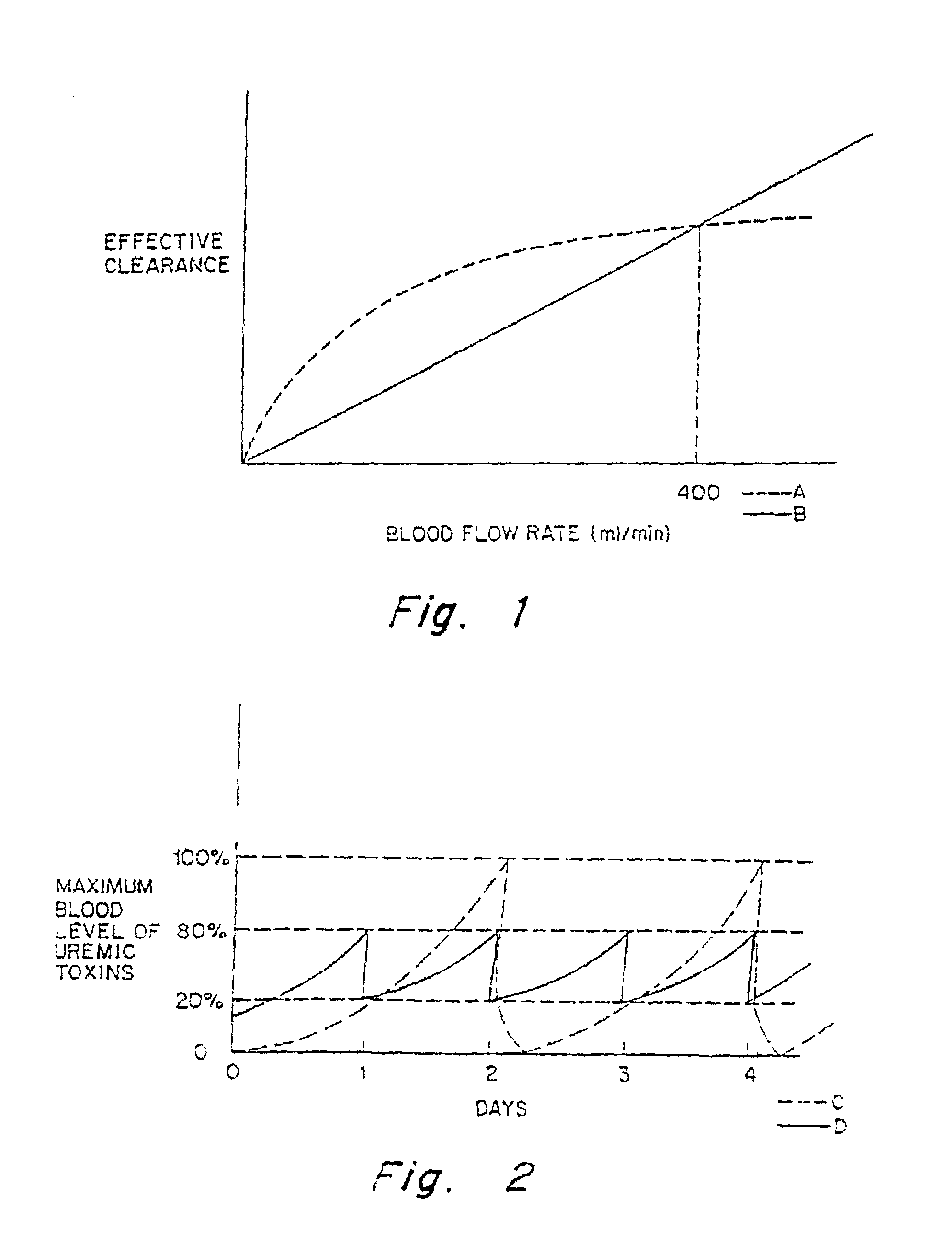
Extracorporeal renal replacement modeling system
InactiveUS20070215545A1AccuracyReduce decreaseSolvent extractionDialysis systemsUltrafiltrationMathematical model
A system, program product and method continuously optimize an ultrafiltration rate during an extracorporeal renal replacement process by modeling physiological and actual rate data. The system maps the sensed, physiological data to a mathematical model to assess the data in terms of the ultrafiltration rate. The model provides parameters used to predict where the treatment is headed based on current conditions. The system processes the parameters in terms of preset criteria to generate the optimized ultrafiltration rate. Where the system is networked, communication of the data may be accomplished using remote and online communication techniques.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Hemodialysis system having a flow path with a controlled compliant volume
ActiveUS20130199998A1Enhanced convective clearanceReduce riskSemi-permeable membranesSolvent extractionDialysis membranesHaemodialysis machine
Systems and methods for the performance of kidney replacement therapy having or using a dialyzer, control components, sorbent cartridge and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. The system for performing kidney replacement therapy has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. The dialysis circuit and an extracorporeal circuit for circulating blood are in fluid communication through the dialysis membrane. The flux of fluid moving between the extracorporeal circuit and the dialysis circuit is modified by the rate at which the control pump is operating such that a rate of ultrafiltration and convective clearance can be controlled. The system provides for the monitoring of an inlet and outlet conductivity of the sorbent cartridge to provide a facility to quantify or monitor the removal of urea by the sorbent cartridge.
Owner:MOZARC MEDICAL US LLC
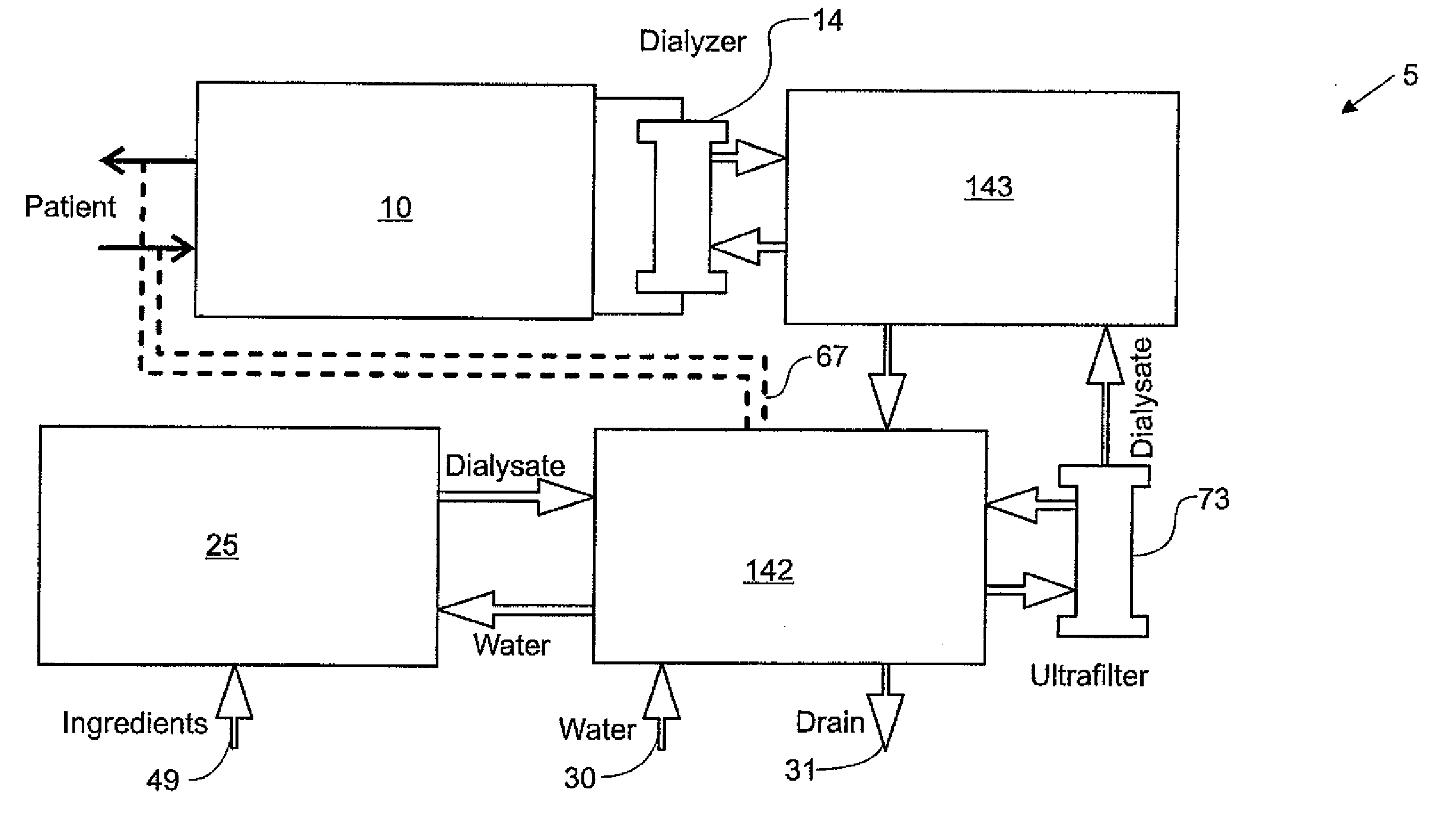
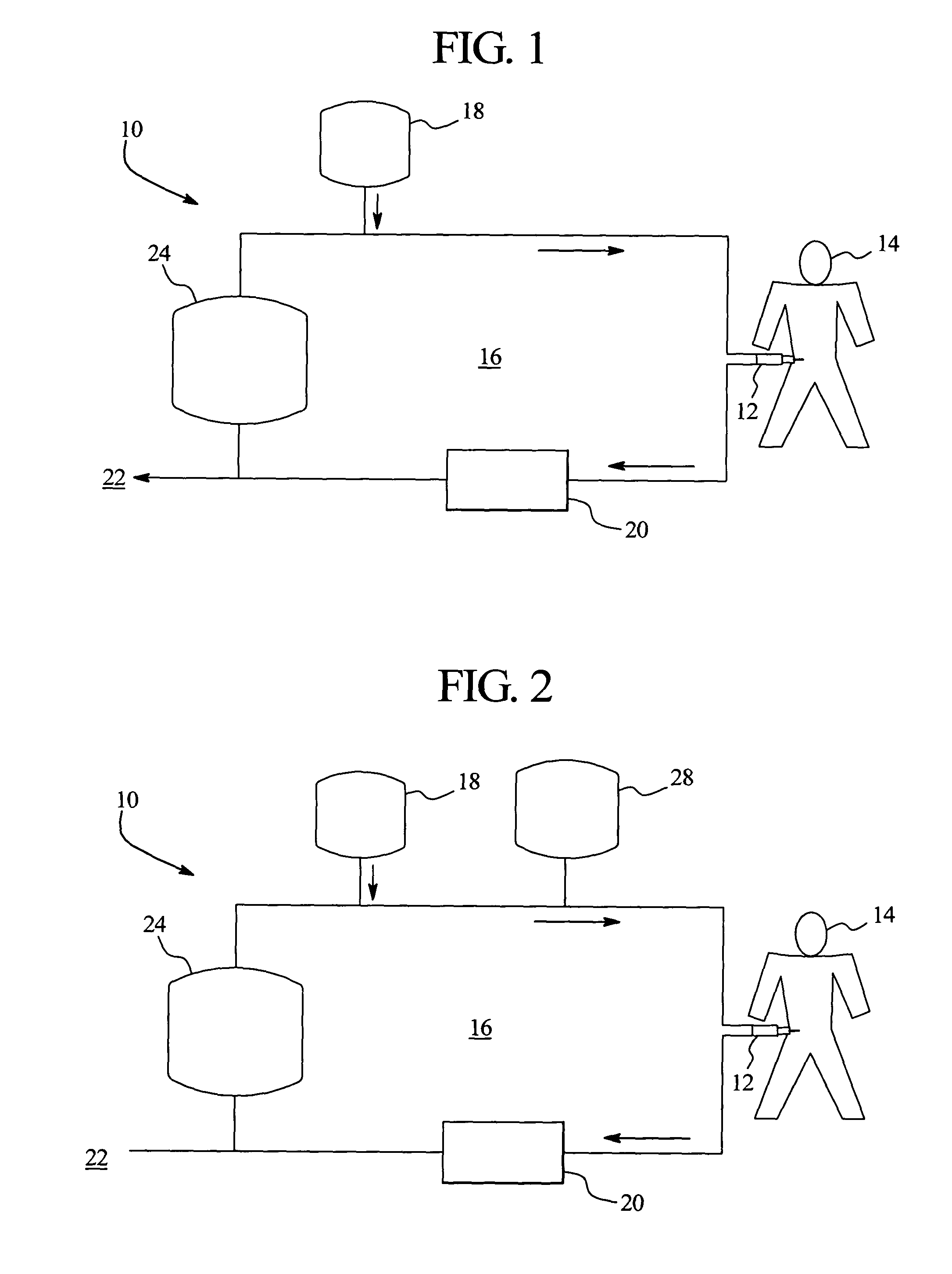
Systems and methods for performing peritoneal dialysis
ActiveUS7867214B2Strengthen the systemImprove methodSolvent extractionIon-exchanger regenerationMetabolic wasteSorbent
In a peritoneal dialysis embodiment of the present invention, spent dialysate from the patient's peritoneal cavity passes, along a patient loop, through a dialyzer having a membrane that separates waste components from the spent dialysate, wherein the patient loop returns fresh dialysate to the patient's peritoneal cavity. The waste components are carried away in a second regeneration loop to a regeneration unit or sorbent cartridge, which absorbs the waste components. The regeneration unit removes undesirable components in the dialysate that were removed from the patient loop by the dialyzer, for example, excess water (ultrafiltrate or UF), toxins and metabolic wastes. Desirable components can be added to the dialysate by the system, such as glucose and electrolytes. The additives assist in maintaining the proper osmotic gradients in the patient to perform dialysis and provide the necessary compounds to the patient.
Owner:BAXTER INT INC
Vented medical fluid tip protector methods
InactiveUS6929751B2Semi-permeable membranesSolvent extractionPeritoneal dialysisBiomedical engineering
A method, system and apparatus for performing peritoneal dialysis are provided. To this end, in part, a member for removably securing a medical fluid tube is provided. The member includes a body providing a first fluid pathway and an opening for removably receiving an end of the medical tube. The first fluid pathway is placed in fluid communication with a second fluid pathway defined by the tube when the end of the tube is removably receiving. The member further includes a filter so positioned and arranged so as to cause fluid flowing through the first fluid pathway to pass through the filter, the filter allowing air but not liquid to flow through the first fluid pathway.
Owner:BAXTER HEALTHCARE SA +1
Systems and methods for peritoneal dialysis
ActiveUS7208092B2Strengthen the systemImprove methodSemi-permeable membranesSolvent extractionContinuous flowIntensive care medicine
Systems and methods relating to dialysis therapy, particularly continuous flow dialysis therapy, are provided. The present invention includes a single closed fluid path along which a minimal amount of therapy fluid including dialysate is fed into, continuously circulated and cleaned such that a therapeutic effective amount of solutes, excess water and the like can be removed from the patient connected to the closed fluid loop during treatment.
Owner:BAXTER INT INC +1
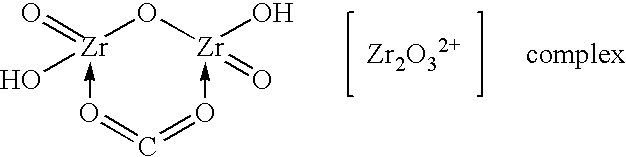
Method of synthesizing zirconium phosphate particles
InactiveUS7566432B2Inhibition of agglomerationReduce moistureSemi-permeable membranesPhosphatesO-Phosphoric AcidZirconium oxychloride
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one oxygen-containing additive to the solution, the oxygen-containing additive being selected to form a complex with zirconium ions in the solution of zirconium oxychloride and thereby reduce hydration of the zirconium ions, and combining this solution with phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:RENAL SOLUTIONS
Method and apparatus for kidney dialysis
InactiveUS20050045540A1Mechanical/radiation/invasive therapiesSolvent extractionUltrafiltrationElectrical resistivity and conductivity
A number of improvements relating to methods and apparatuses for kidney dialysis are disclosed. These include checking of dialysate bypass status using flow measurement; using a flow sensor to confirm the absence of ultrafiltration during bypass; automatic testing of ultrafiltration function by removal of a discrete volume from a portion of the dialysate flow path coupled with a pressure test of that part of the flow path; using a touch screen user interface; bar graph profile programming of ultrafiltration, sodium, and bicarbonate parameters; using a RAM card to upload treatment instructions to, and to download treatment data from, the machine; automatic setting of proportioning mode (acetate or bicarbonate) based on connections of concentrate lines; predicting dialysate conductivity values based on brand and formulation of concentrates; minimizing no-flow dead time between dialysate pulses; initiating operation in a timed mode from a machine power-off condition; preserving machine mode during machine power-fail condition; calibration scheduling and reminding; automatic level adjusting; and blood leak flow rate detecting.
Owner:CONNELL MARK E +9
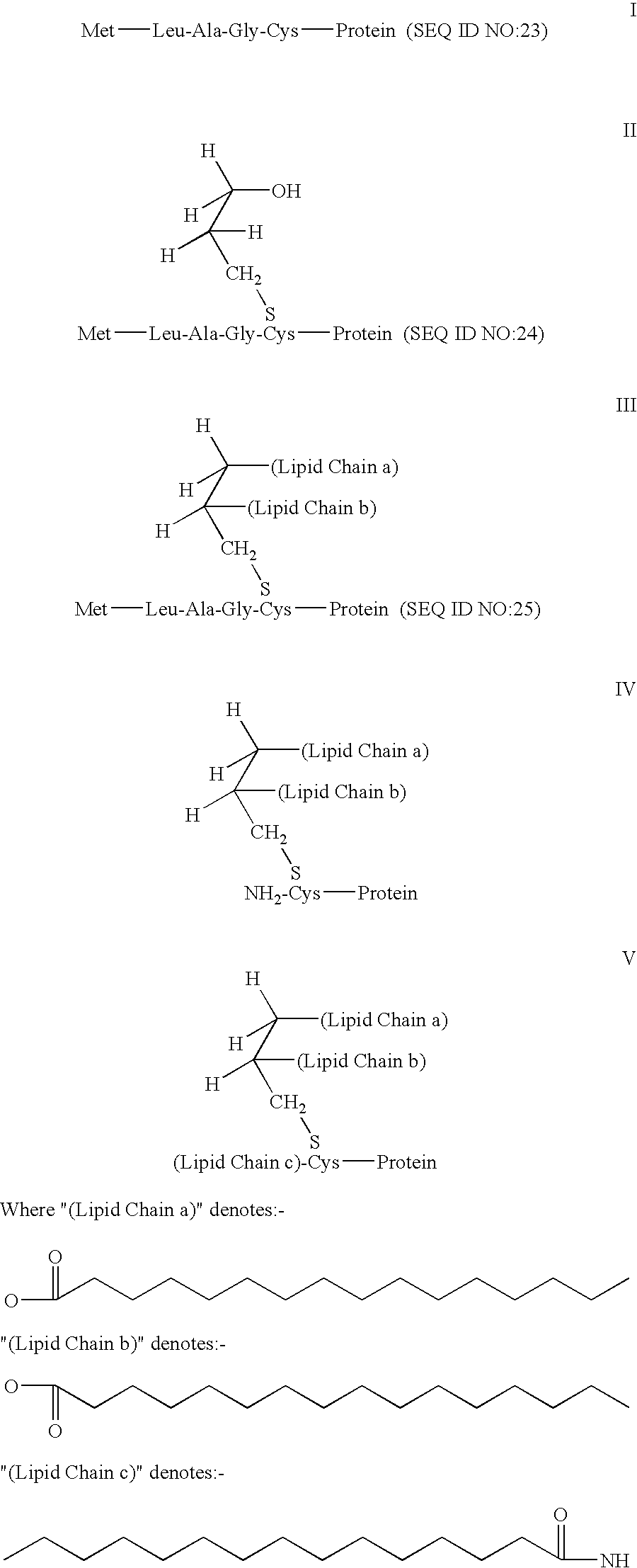
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
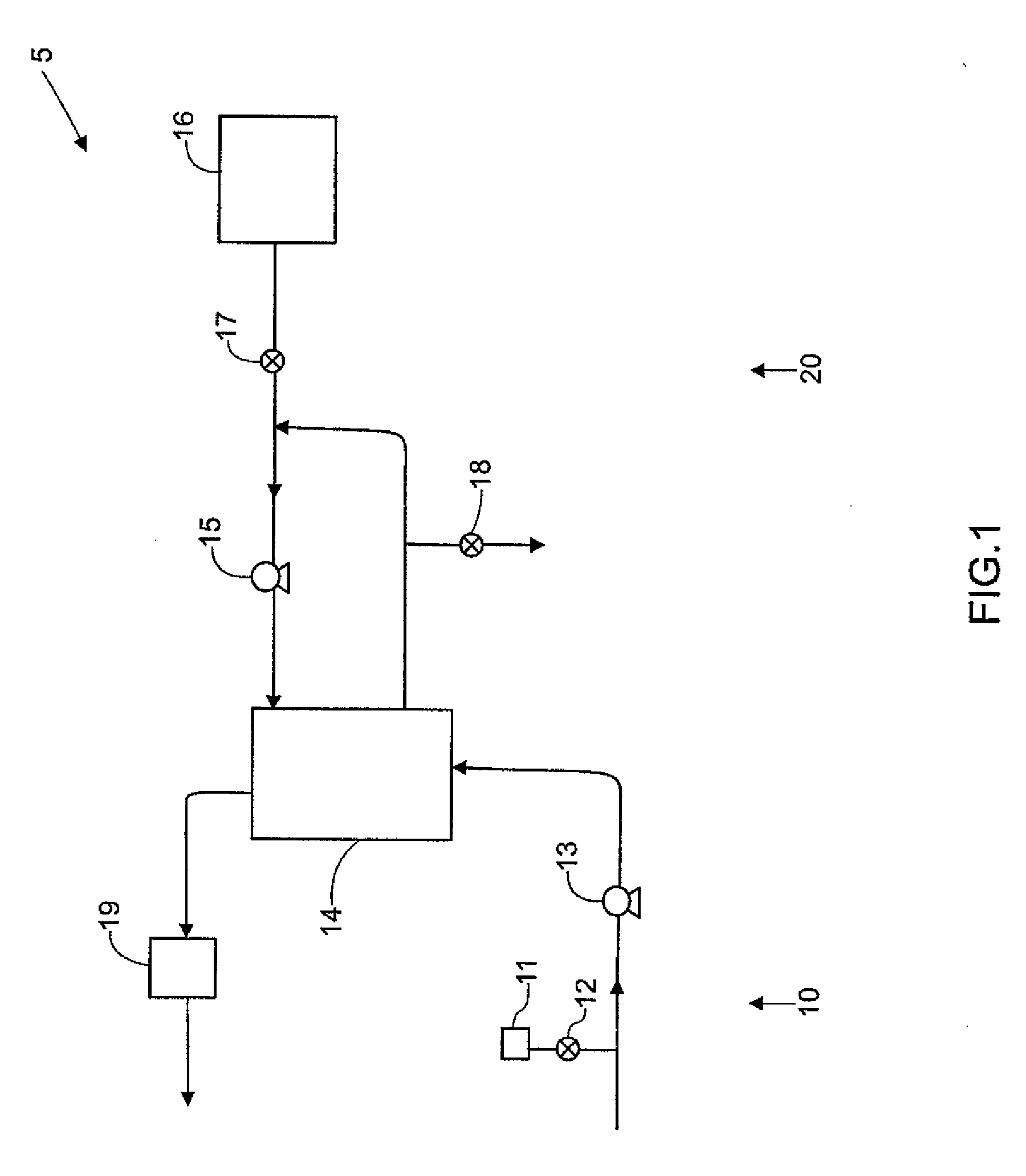
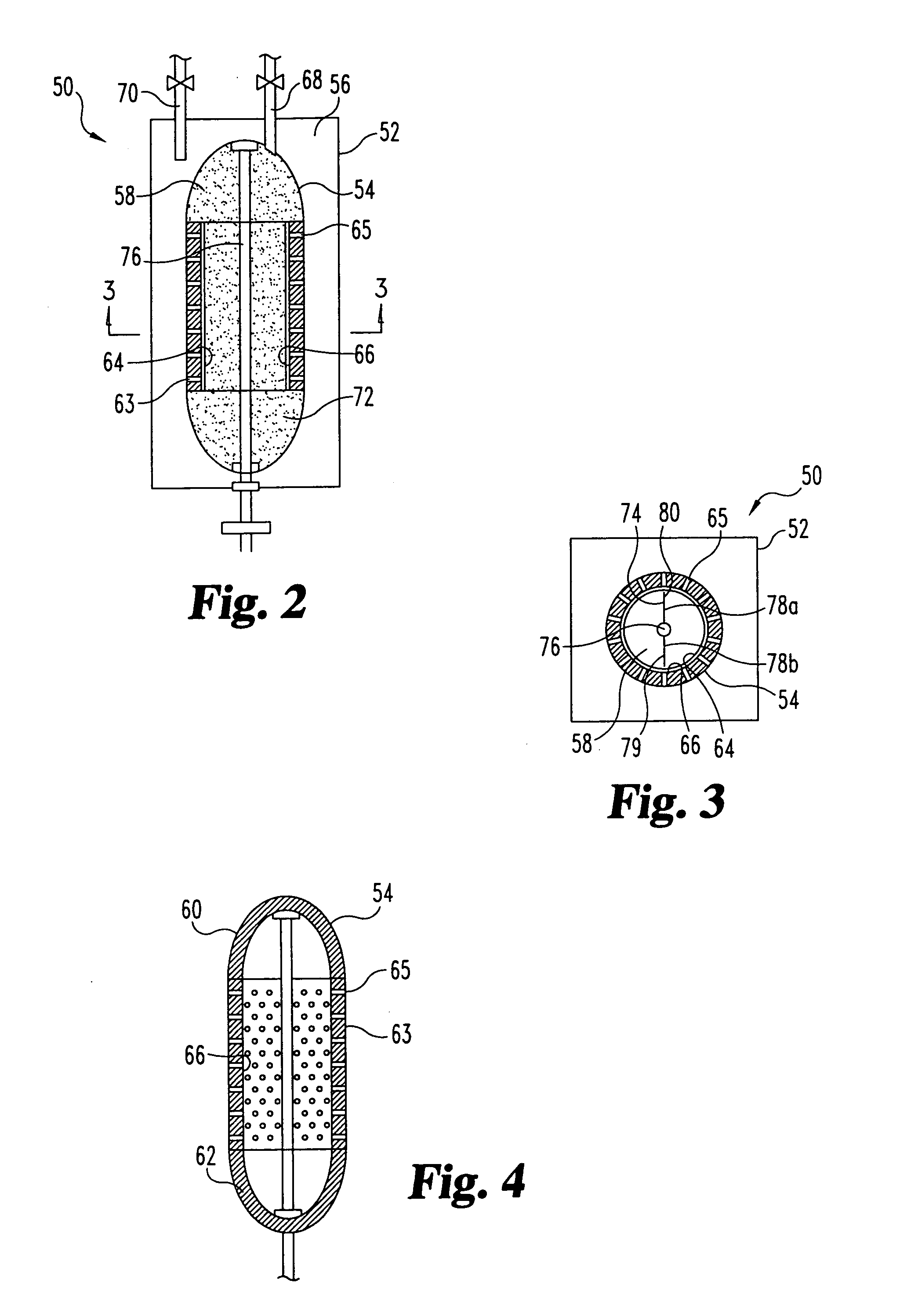
Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems
InactiveUS7169303B2Facilitate homogeneous suspensionReduce probabilitySolvent extractionHaemofiltrationFluid balancePeritoneal dialysis
Systems and methods for extracorporeal processing of blood or other body fluid for the treatment of conditions, such as sepsis, autoimmune disease, or toxemia related to kidney failure, liver failure, or drug overdose are provided. In an extracorporeal treatment system, a fraction of a body fluid is passed into a treatment fluid, at least a portion of which is then passed through a sorbent suspension reactor for treatment by a sorbent suspension. The treatment fluid circuit can be maintained at a fixed volume, which enables accurate fluid balance between the patient and the extracorporeal circuit. Some or all of the treatment fluid, optionally also containing nutrients and / or therapeutic agents, is returned to the patient. In a peritoneal dialysis system, dialysate is passed into a patient's peritoneal cavity, recovered from the cavity, passed through a sorbent suspension reactor in accordance with the invention, and returned to the cavity.
Owner:HEMOCLEANSE TECH
Systems and methods for performing blood processing and/or fluid exchange procedures
InactiveUS6979309B2Fast and convenient and one step process for loading processingPotent inhibitionSemi-permeable membranesSolvent extractionBlood treatmentsEngineering
A flow management system for extracorporeal blood treatment application helps to ensure proper balance of incoming and outgoing fluids by precise balancing of relatively small balance chambers. The invention employs combinations of features that help to ensure accuracy including underfilling of the waste flow side of a fixed volume chamber and mechanical connections to synchronize valves and pumps.
Owner:NXSTAGE MEDICAL
Systems and methods for particle focusing in microchannels
ActiveUS8186913B2Improve concentrationEvenly distributedImmobilised enzymesSolvent extractionSuspended particlesEngineering
Various systems, methods, and devices are provided for focusing particles suspended within a moving fluid into one or more localized stream lines. The system can include a substrate and at least one channel provided on the substrate having an inlet and an outlet. The system can further include a fluid moving along the channel in a laminar flow having suspended particles and a pumping element driving the laminar flow of the fluid. The fluid, the channel, and the pumping element can be configured to cause inertial forces to act on the particles and to focus the particles into one or more stream lines.
Owner:THE GENERAL HOSPITAL CORP
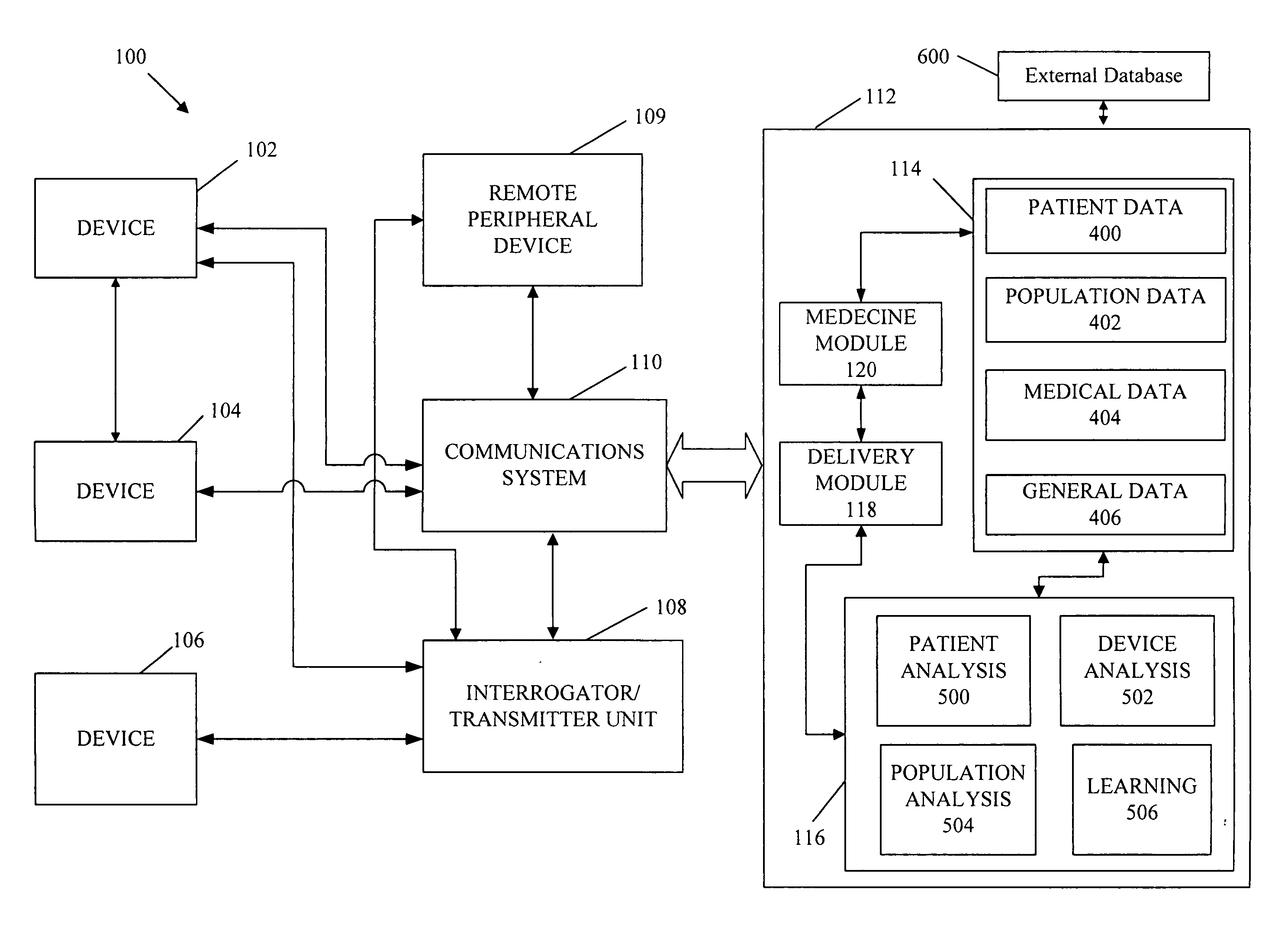
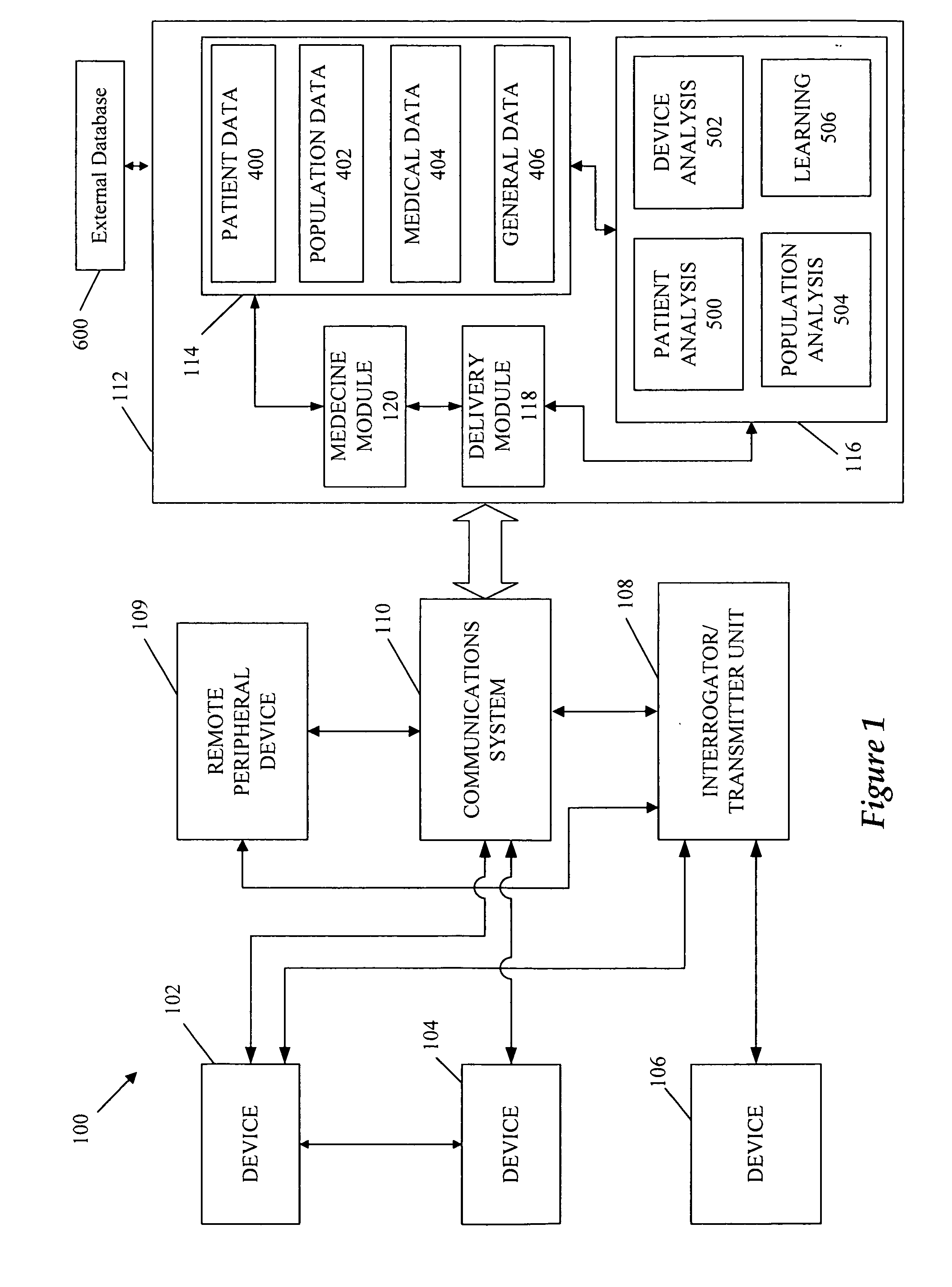
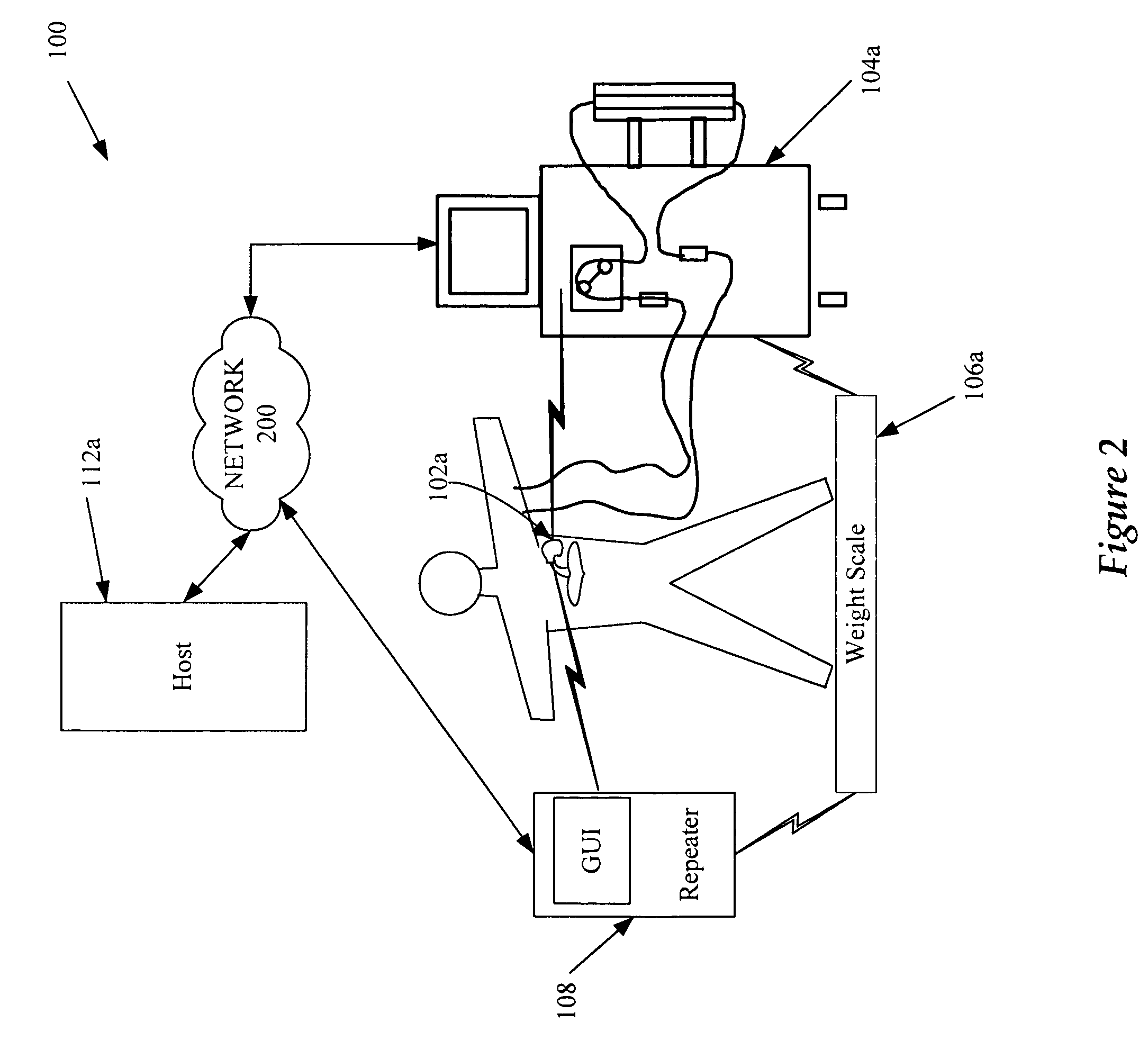
Cardiac rhythm management device and sensor-suite for the optimal control of ultrafiltration and renal replacement therapies
ActiveUS20070175827A1ElectrotherapyMechanical/radiation/invasive therapiesUltrafiltrationOptimal control
A cardiorenal patient monitoring system comprising, either implanted or non-implanted device(s), remote peripheral device(s), computer network(s), host, and communication means between the device(s), computer network(s), and host. The preferred embodiment shows an advanced patient monitoring system for using an implanted cardiac device and a dialysis machine in renal therapy. In addition, the method of advanced patient monitoring is in conjunction with the advanced patient monitoring system is disclosed.
Owner:CARDIAC PACEMAKERS INC
Osmotic desalination process
InactiveUS20050145568A1High yieldImprove concentrationSolvent extractionGeneral water supply conservationPresent methodDesalination
An energy efficient desalination process that does not produce waste products involves the extraction of water from a first solution, such as seawater, by using a second concentrated solution to draw the water from the first solution across a semi-permeable membrane. By manipulating the equilibrium of the soluble and insoluble species of solute within the second solution in favor of the soluble species of the solute, a saturated second solution can be used to generate osmotic pressure on the first solution. Also, by adjusting the equilibrium in favor of the less soluble species after the water has been drawn from the first solution, a portion of the solute can easily be precipitated out. Heating the second solution decomposes the solute into its constituent gasses. The constituent gasses and precipitated solute may be recycled through the process to affect the changes in equilibrium and eliminate waste products. Additionally, by using the waste steam from industrial sources and a heat pump to effectively distribute heat through the present method, the present method exhibits greater energy efficiency than prior art methods.
Owner:YALE UNIV
Wearable ultrafiltration device
InactiveUS7645253B2Steady and smooth removalPreventing the shortness of breath and swellingMembranesSemi-permeable membranesUltrafiltrationBlood pump
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Hemofiltration system
InactiveUS6955655B2High rateSemi-permeable membranesOther blood circulation devicesFluid replacementCatheter
A hemofiltration system and method is provided that allows for high flow rate, accurate determination of net fluid withdrawal from or addition to a patient, and simple and reliable home operation. A removable, disposable assembly includes a filter housing and pump member including one or more fluid conduits mounted against the pump member. When the disposable filter / pump member assembly is attached to the treatment system, a pump roller mechanism associated with the system actuates the conduits mounted against the pump member. A disposable waste receptacle and fluid replacement (infusate) reservoir can be provided as an integral part of the disposable filter / pump member assembly.
Owner:NXSTAGE MEDICAL
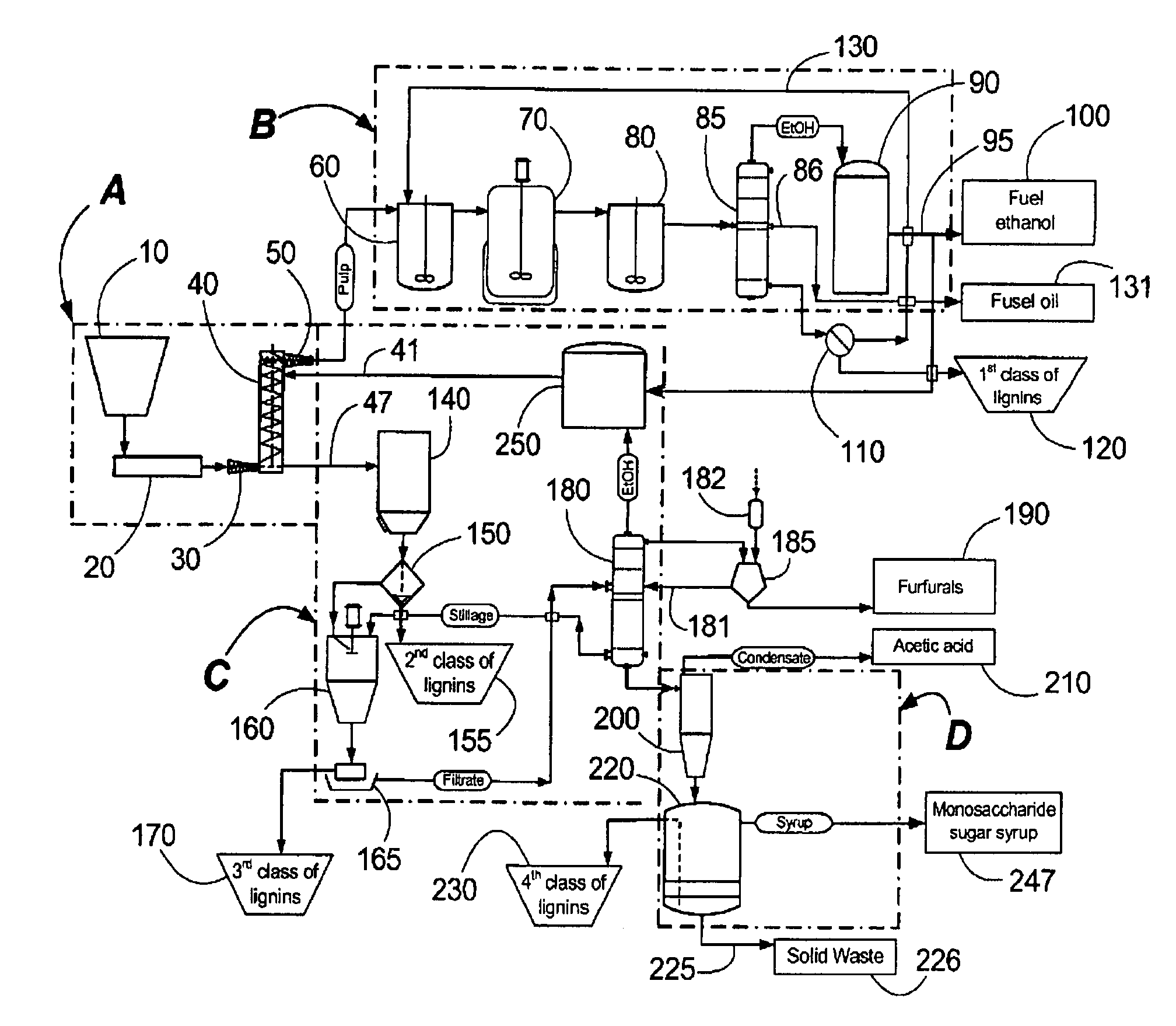
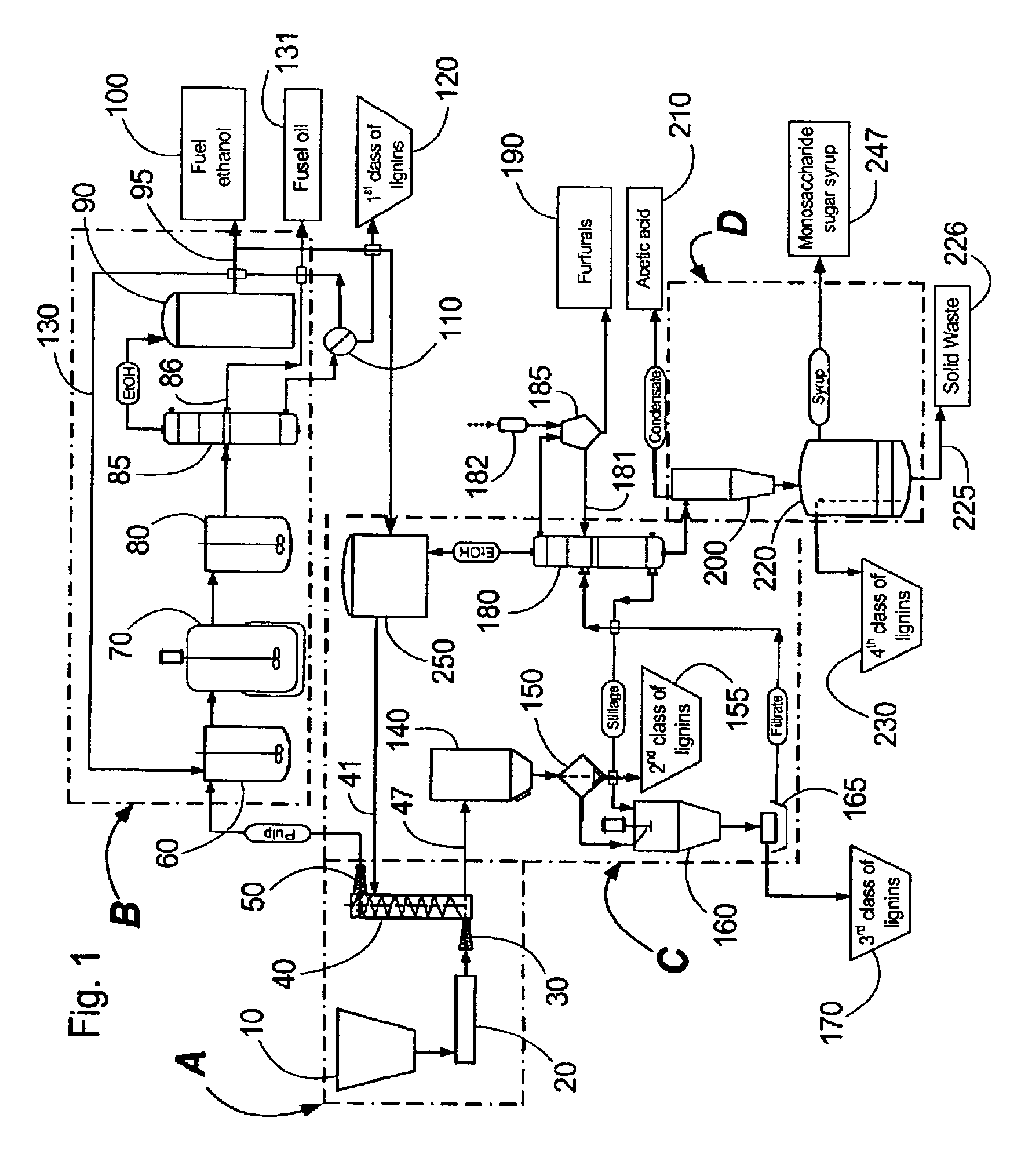
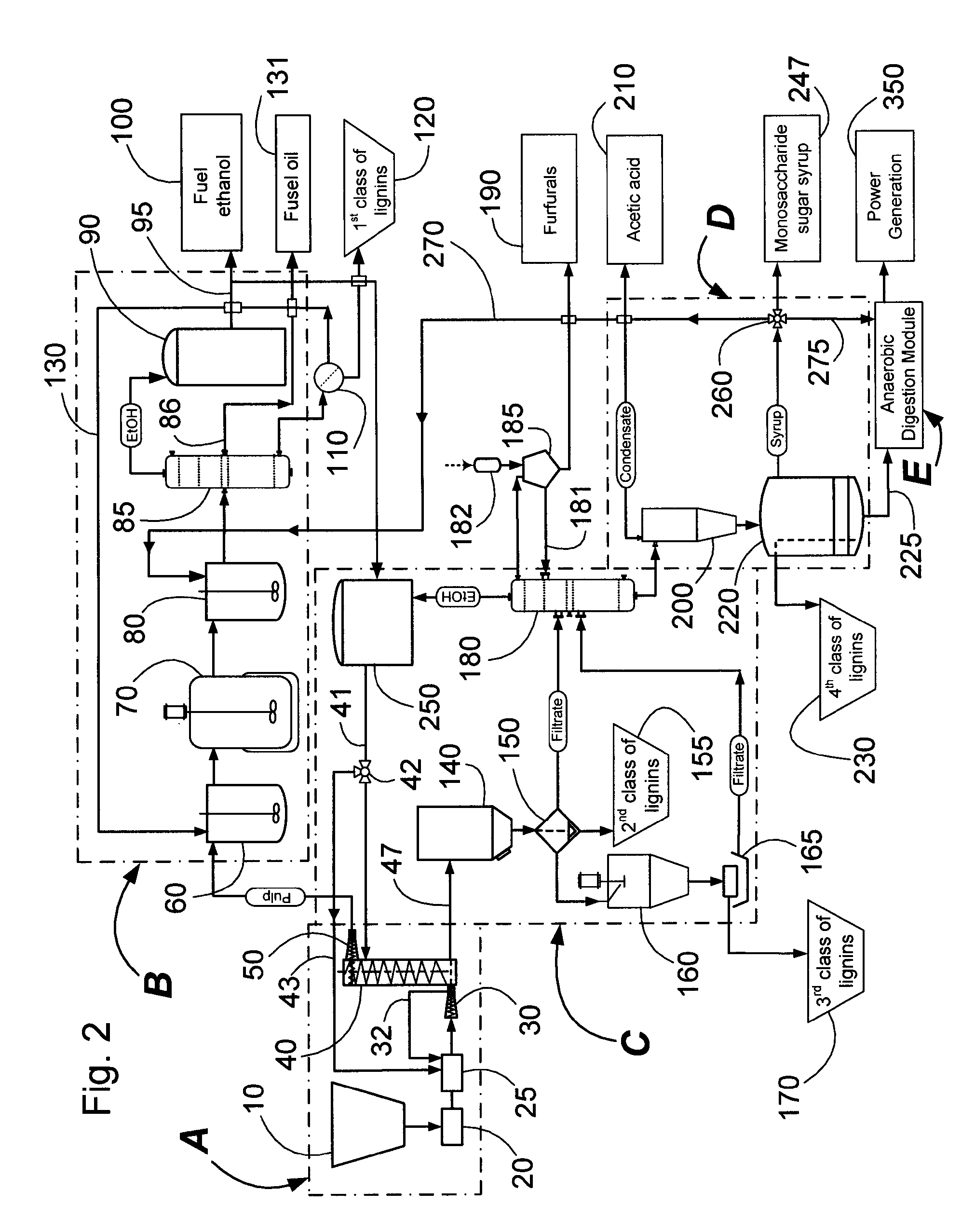
Continuous counter-current organosolv processing of lignocellulosic feedstocks
InactiveUS7465791B1Low viscosityNon-fibrous pulp additionBiological substance pretreatmentsFractionationOrganosolv
A modular process for organosolv fractionation of lignocellulosic feedstocks into component parts and further processing of said component parts into at least fuel-grade ethanol and four classes of lignin derivatives. The modular process comprises a first processing module configured for physico-chemically digesting lignocellulosic feedstocks with an organic solvent thereby producing a cellulosic solids fraction and a liquid fraction, a second processing module configured for producing at least a fuel-grade ethanol and a first class of novel lignin derivatives from the cellulosic solids fraction, a third processing module configured for separating a second class and a third class of lignin derivatives from the liquid fraction and further processing the liquid fraction to produce a distillate and a stillage, a fourth processing module configured for separating a fourth class of lignin derivatives from the stillage and further processing the stillage to produce a sugar syrup.
Owner:SUZANO CANADA INC
Medical fluid therapy flow balancing and synchronization system
ActiveUS20050085760A1Simplify the startup processSimplify fluid flow maintenanceSemi-permeable membranesSolvent extractionFluid therapyBiomedical engineering
Systems, apparatus and methods that allow external infusion, IV or administration pumps to be synchronized with the internal pumps for a medical fluid therapy machine are provided. The system reduces the time and effort needed to calculate, set-up, enter and maintain flowrates of various fluids, maintained internally or externally with respect to the medical fluid therapy machine. The system also automatically follows therapy requirements, for example, a requirement that one pump / fluid be running / flowing for another pump / fluid to be enabled to run / flow. The system further automatically adjusts for variations in flowrate of one fluid with respect to another. In short, the system provides a more “hands-off”, safe and effective method and apparatus for medical fluid therapy fluid delivery.
Owner:BAXTER INT INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com