Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Renal replacement therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renal replacement therapy (RRT) is therapy that replaces the normal blood-filtering function of the kidneys. It is used when the kidneys are not working well, which is called renal failure and includes acute kidney injury and chronic kidney disease. Renal replacement therapy includes dialysis (hemodialysis or peritoneal dialysis), hemofiltration, and hemodiafiltration, which are various ways of filtration of blood with or without machines. Renal replacement therapy also includes kidney transplantation, which is the ultimate form of replacement in that the old kidney is replaced by a donor kidney.
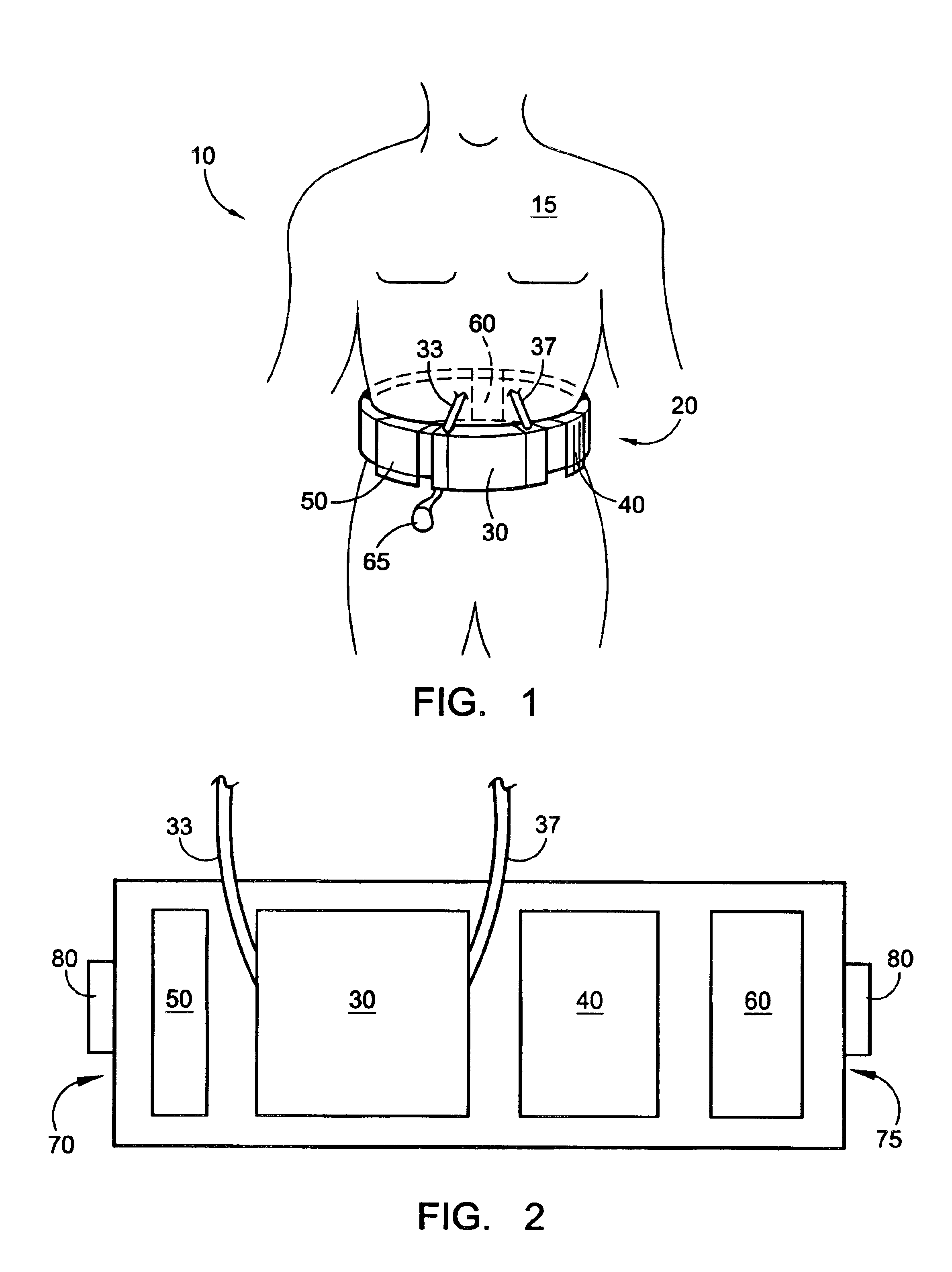
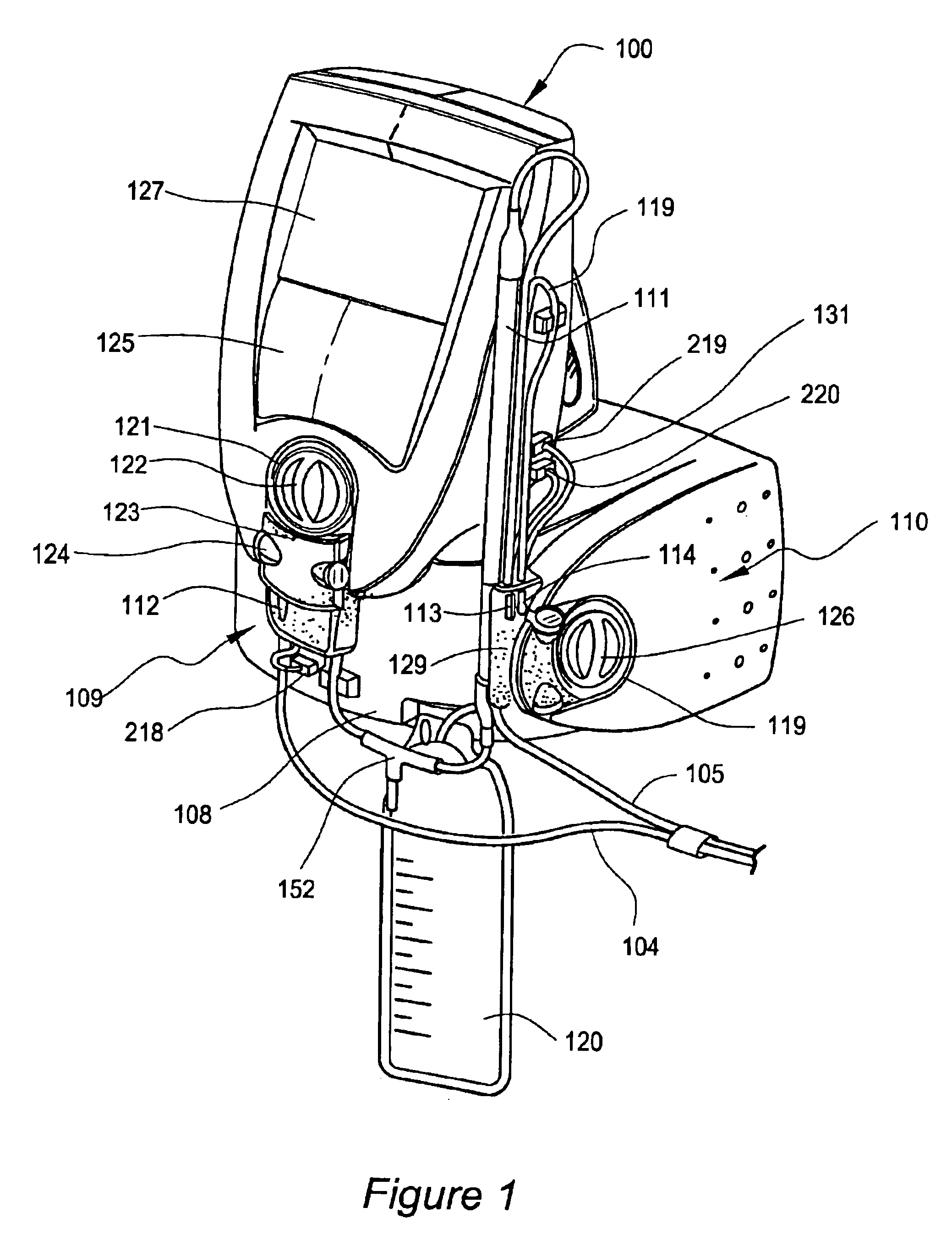
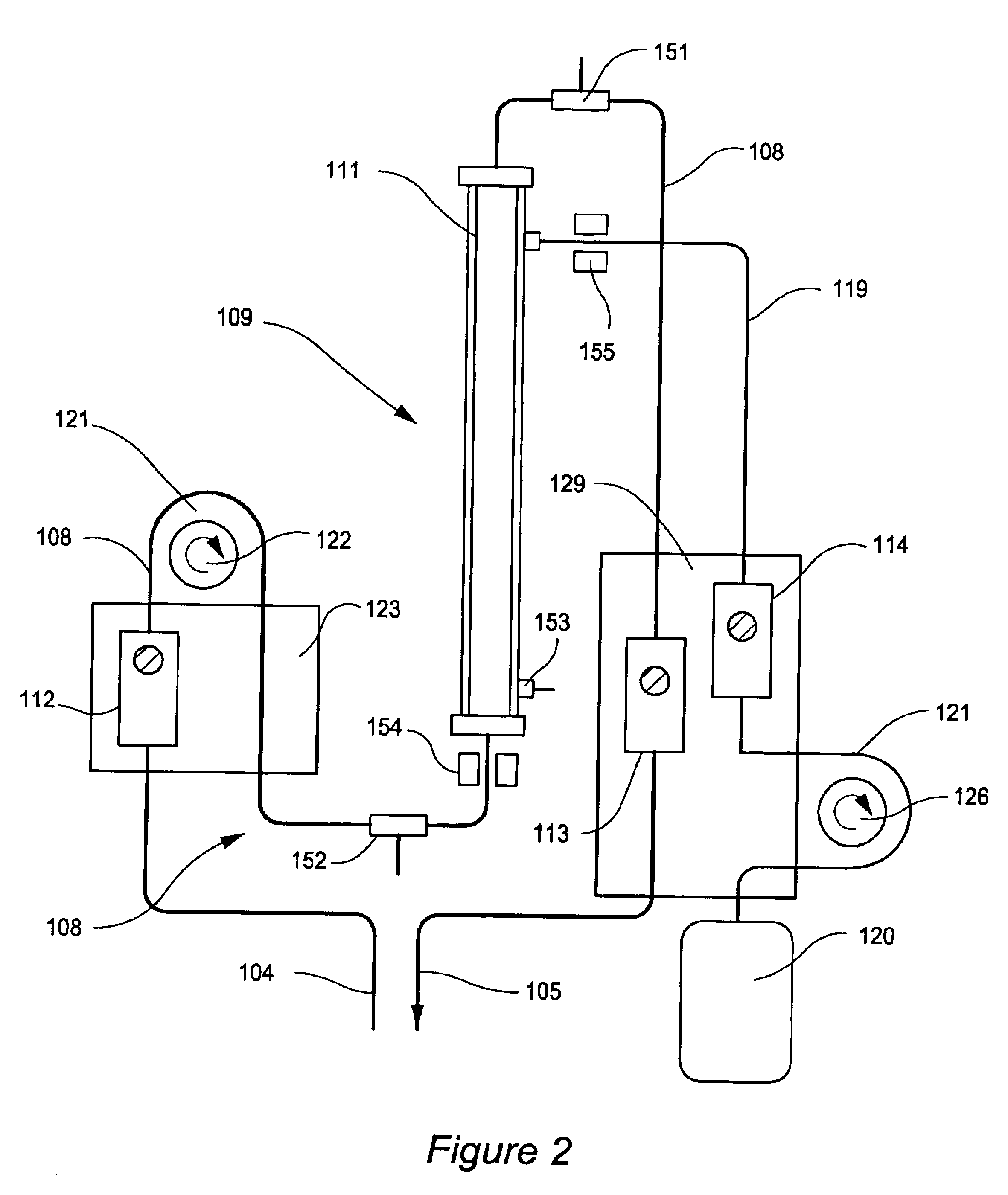
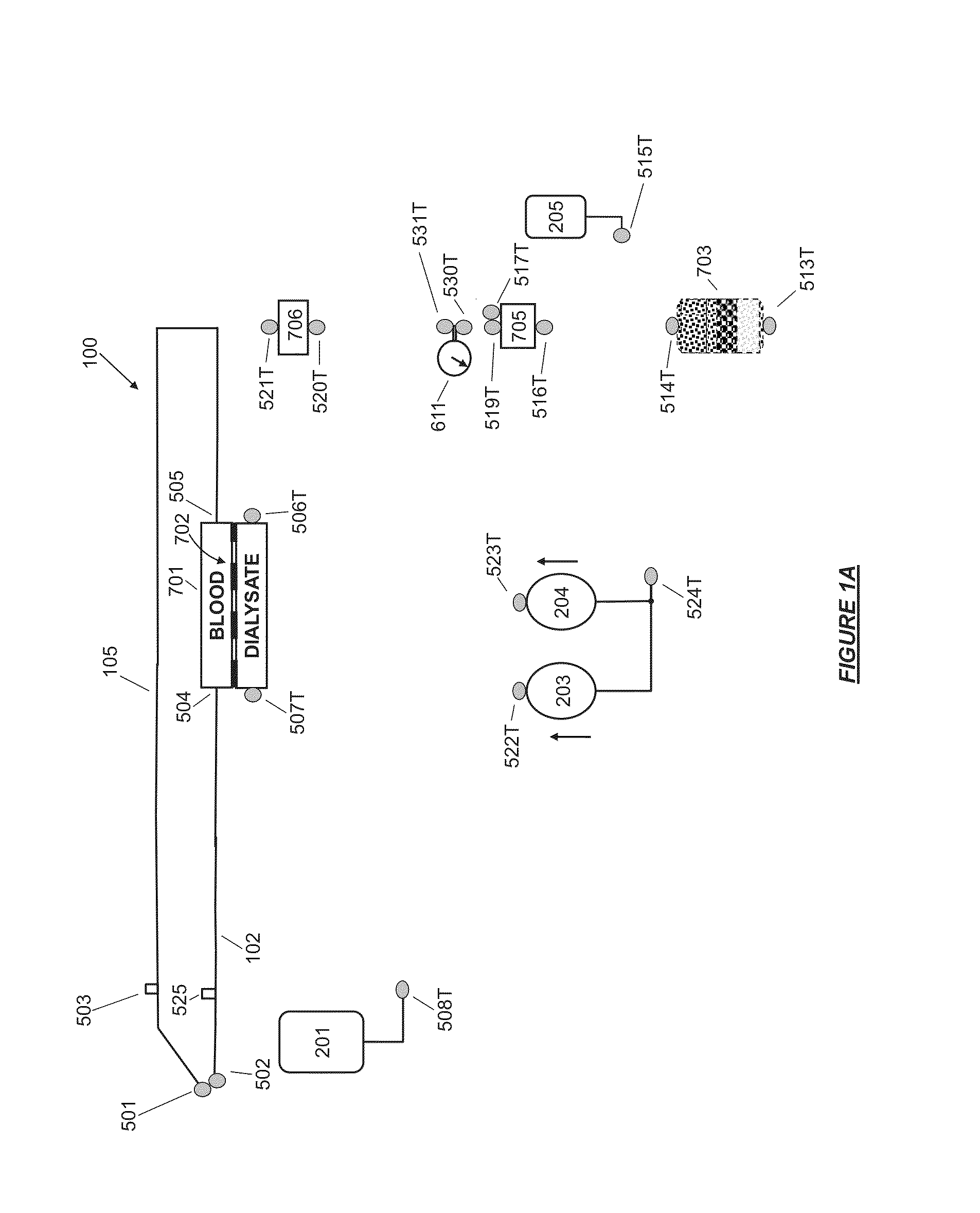
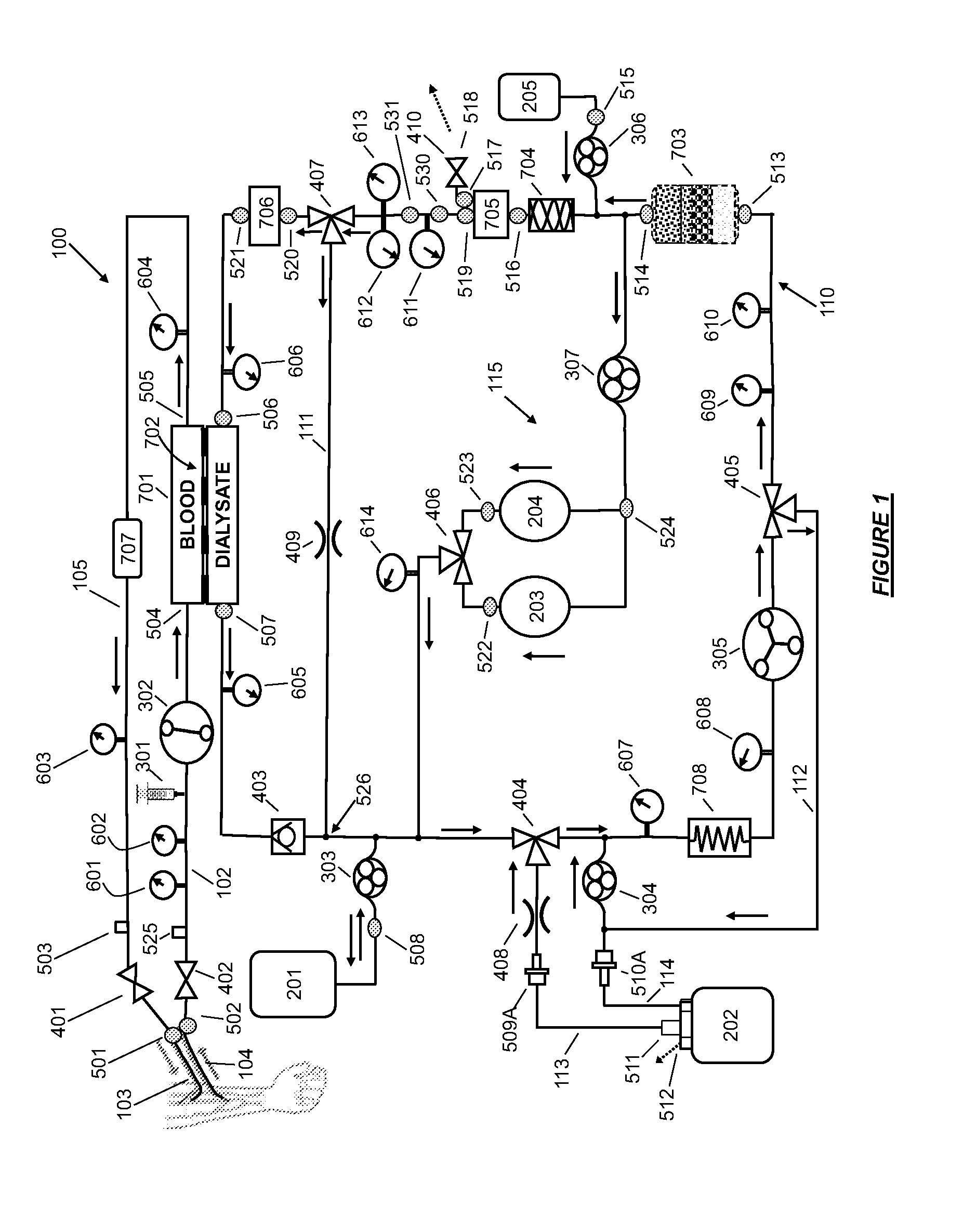
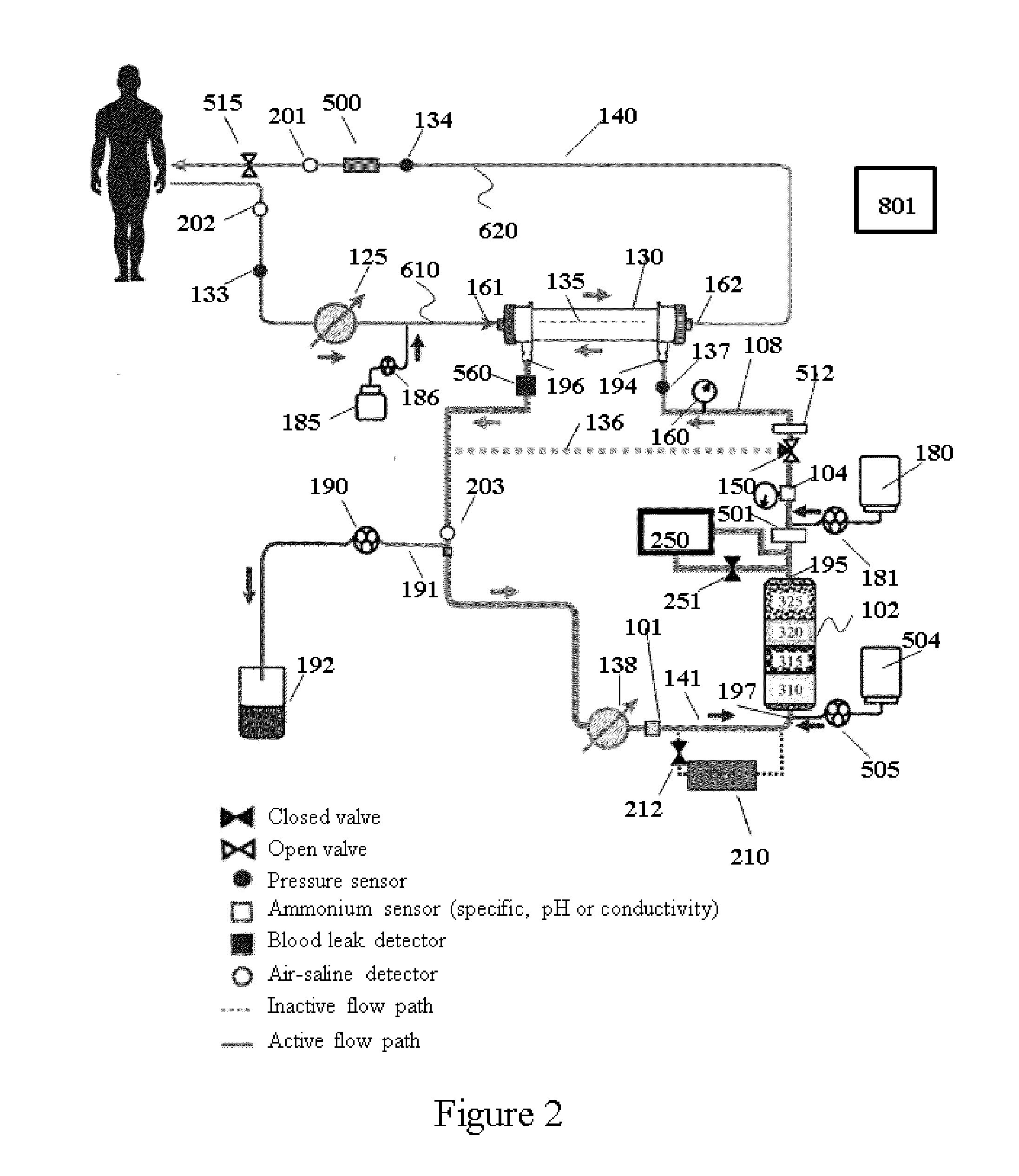
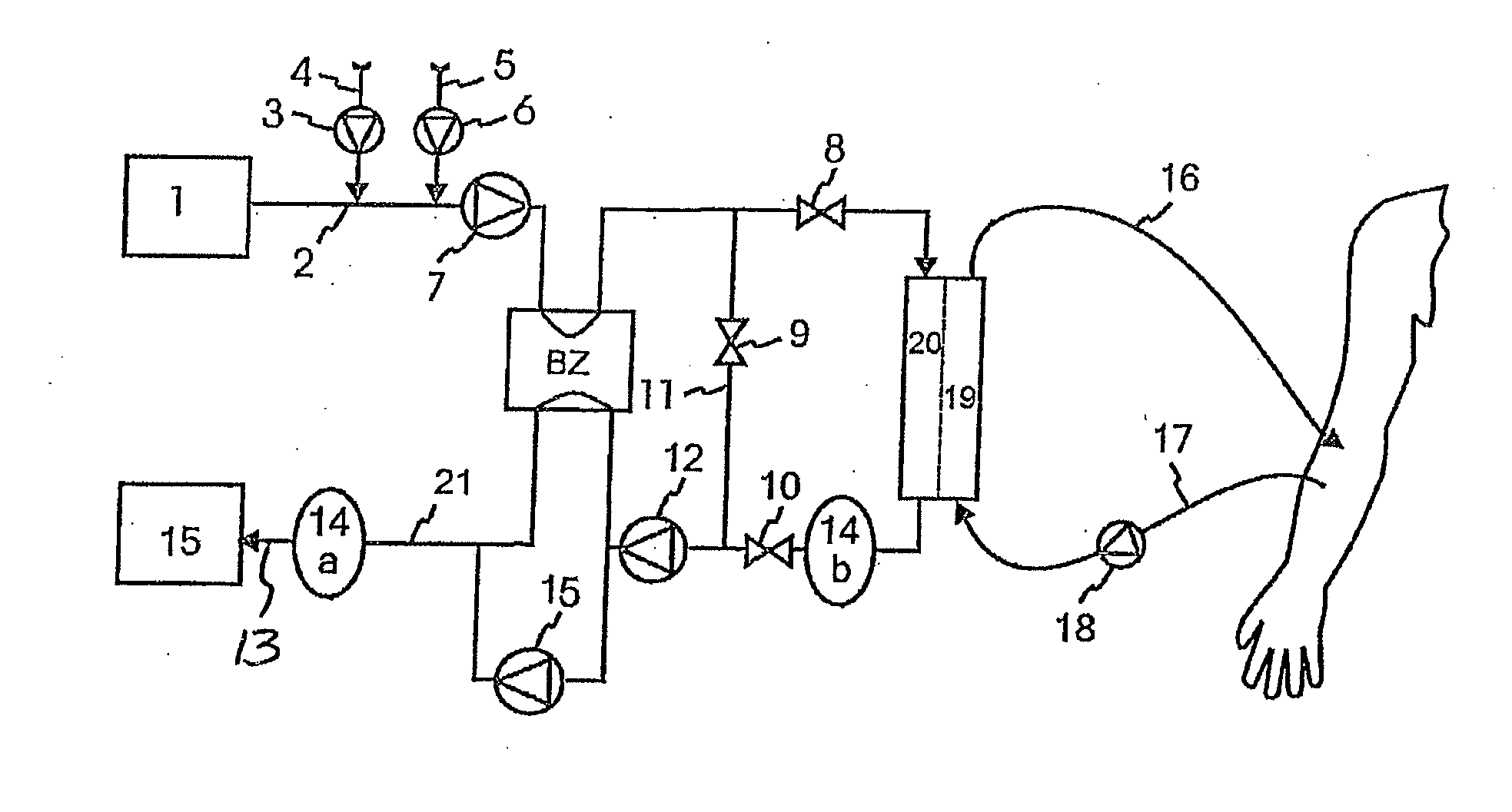
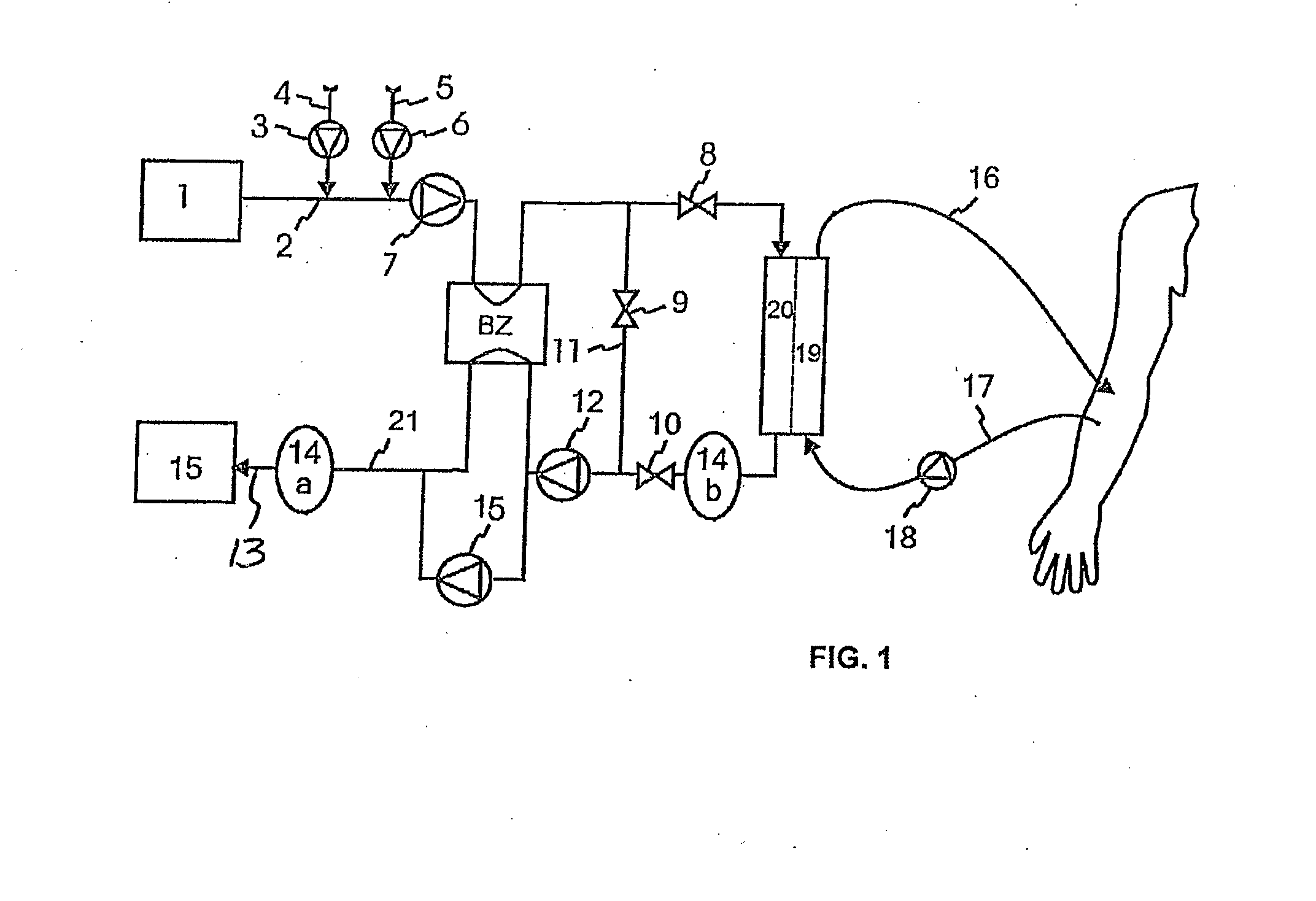
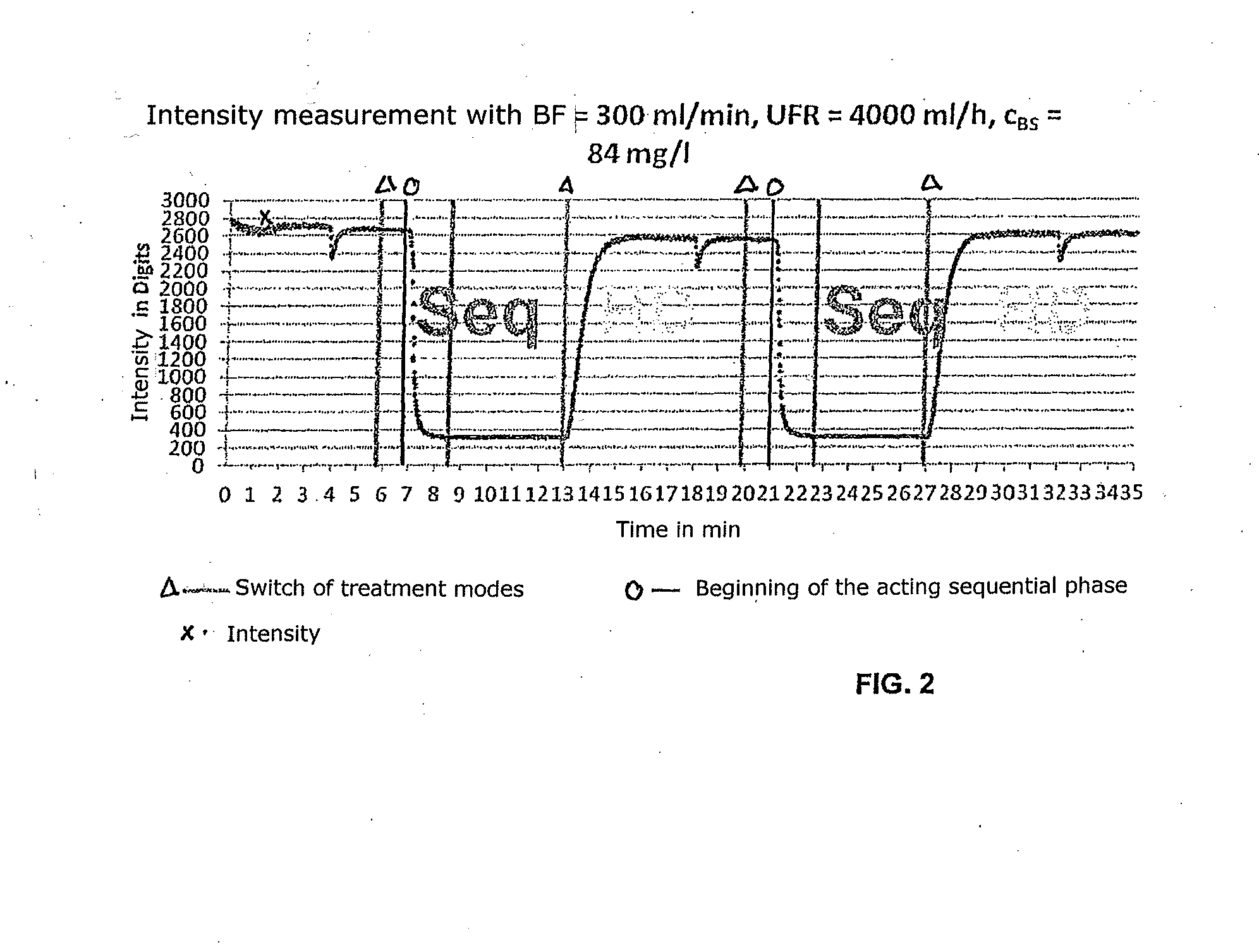
High convection home hemodialysis/hemofiltration and sorbent system
InactiveUS20050131332A1Easily set up sterile blood therapy systemImprove efficiencySemi-permeable membranesHaemofiltrationPositive pressureSorbent
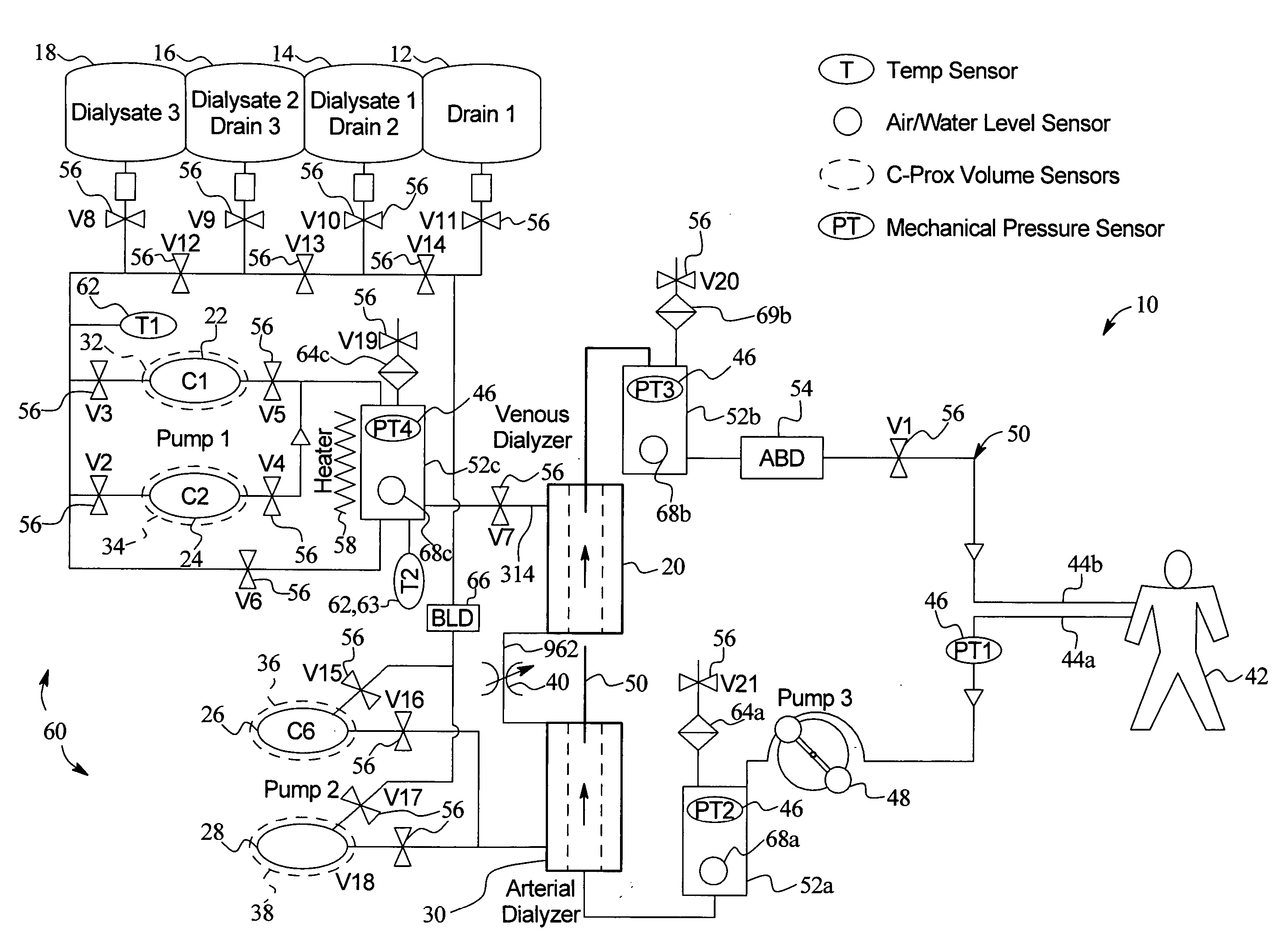
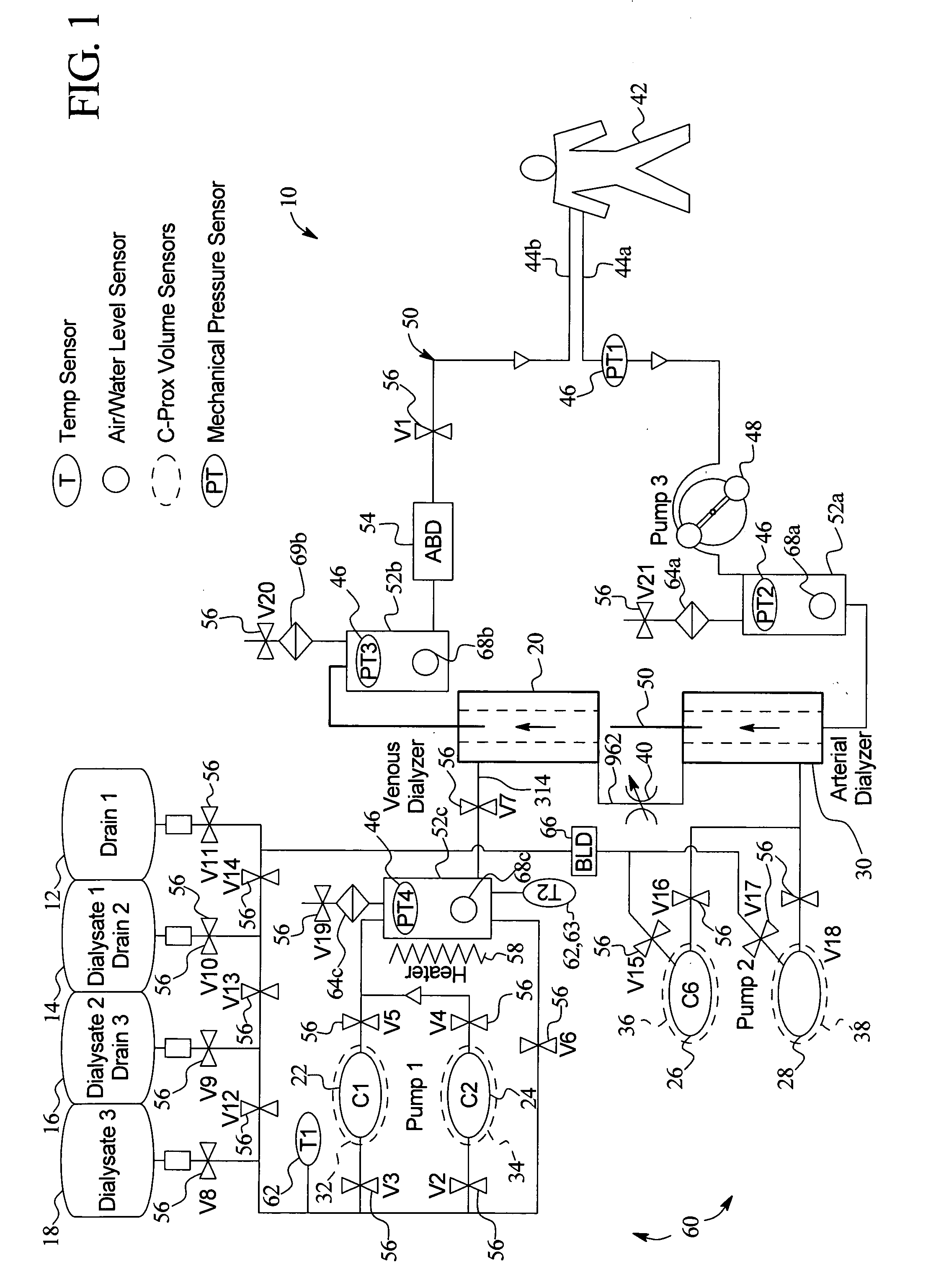
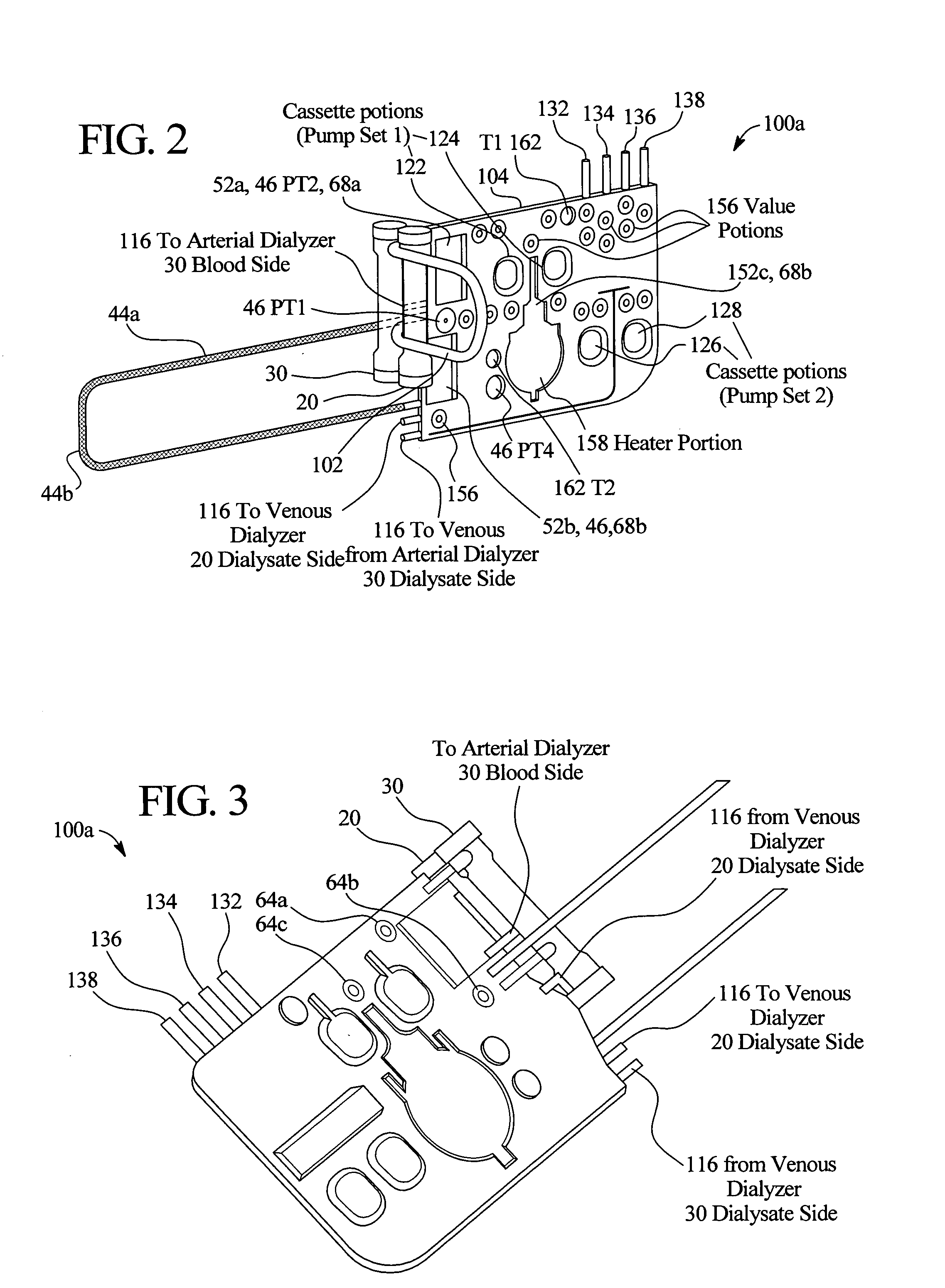
A system, method and apparatus for performing a renal replacement therapy is provided. In one embodiment, two small high flux dialyzers are connected in series. A restriction is placed between the two dialyzers in the dialysate flow path. The restriction is variable and adjustable in one preferred embodiment. The restriction builds a positive pressure in the venous dialyzer, causing a high degree of intentional backfiltration. That backfiltration causes a significant flow of dialysate through the high flux venous membrane directly into the patient's blood. That backfiltered solution is subsequently ultrafiltered from the patient from the arterial dialyzer. The diffusion of dialysate into the venous filter and removal of dialysate from the arterial dialyzer causes a convective transport of toxins from the patient. Additionally, the dialysate that does not diffuse directly into the patient but instead flows across the membranes of both dialyzers provides a diffusive clearance of waste products.
Owner:BAXTER HEALTHCARE SA +1
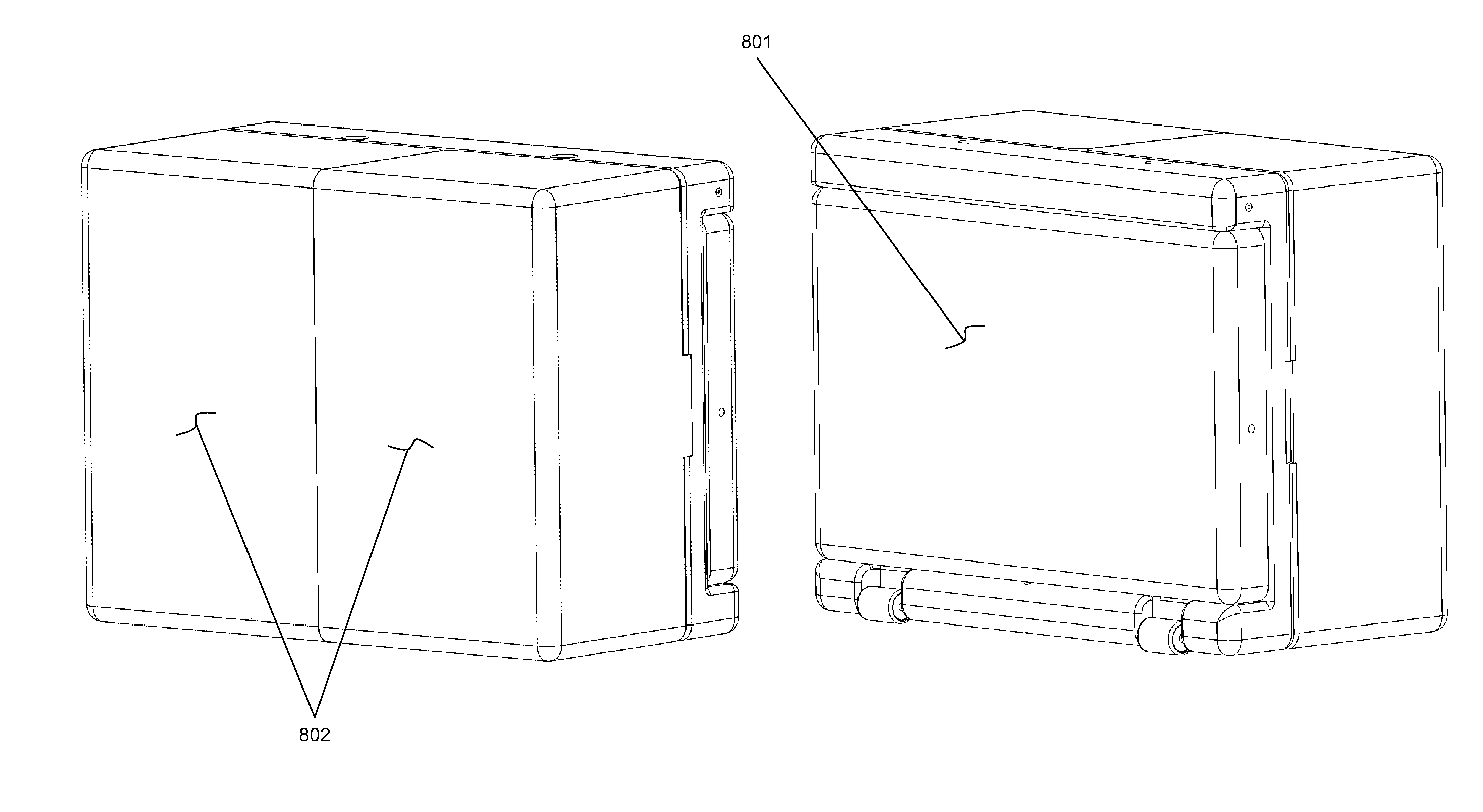
Wearable continuous renal replacement therapy device
A continuous renal replacement therapy device adapted to be worn on a portion of the body of a patient, including: a plurality of contoured dialyzers, which are connected in series and utilize dialysate to remove impurities from the blood of the patient; and a plurality of contoured sorbent device, which are connected in series and are for regenerating the spent dialysate.
Owner:FRESENIUS MEDICAL CARE HLDG INC
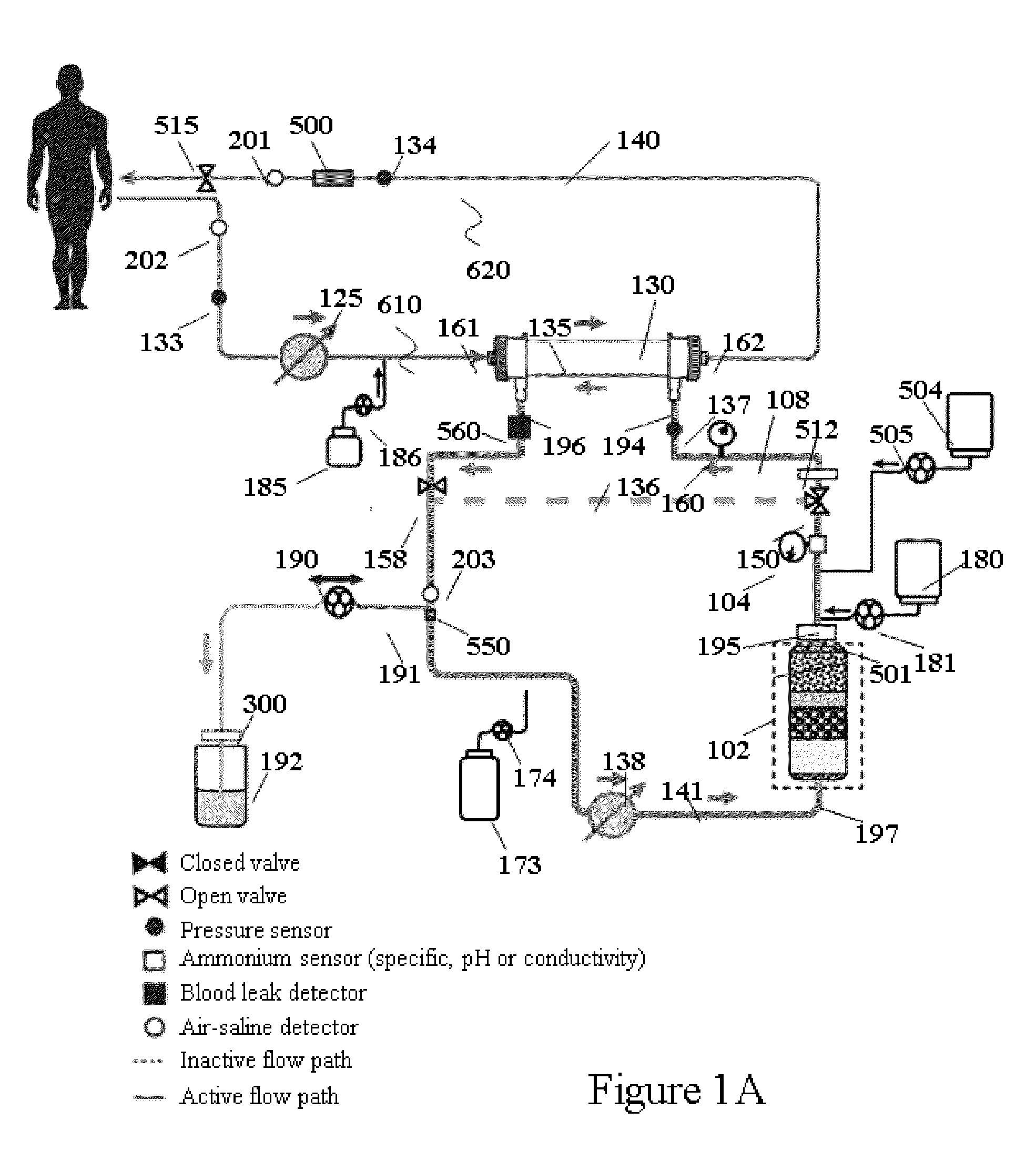
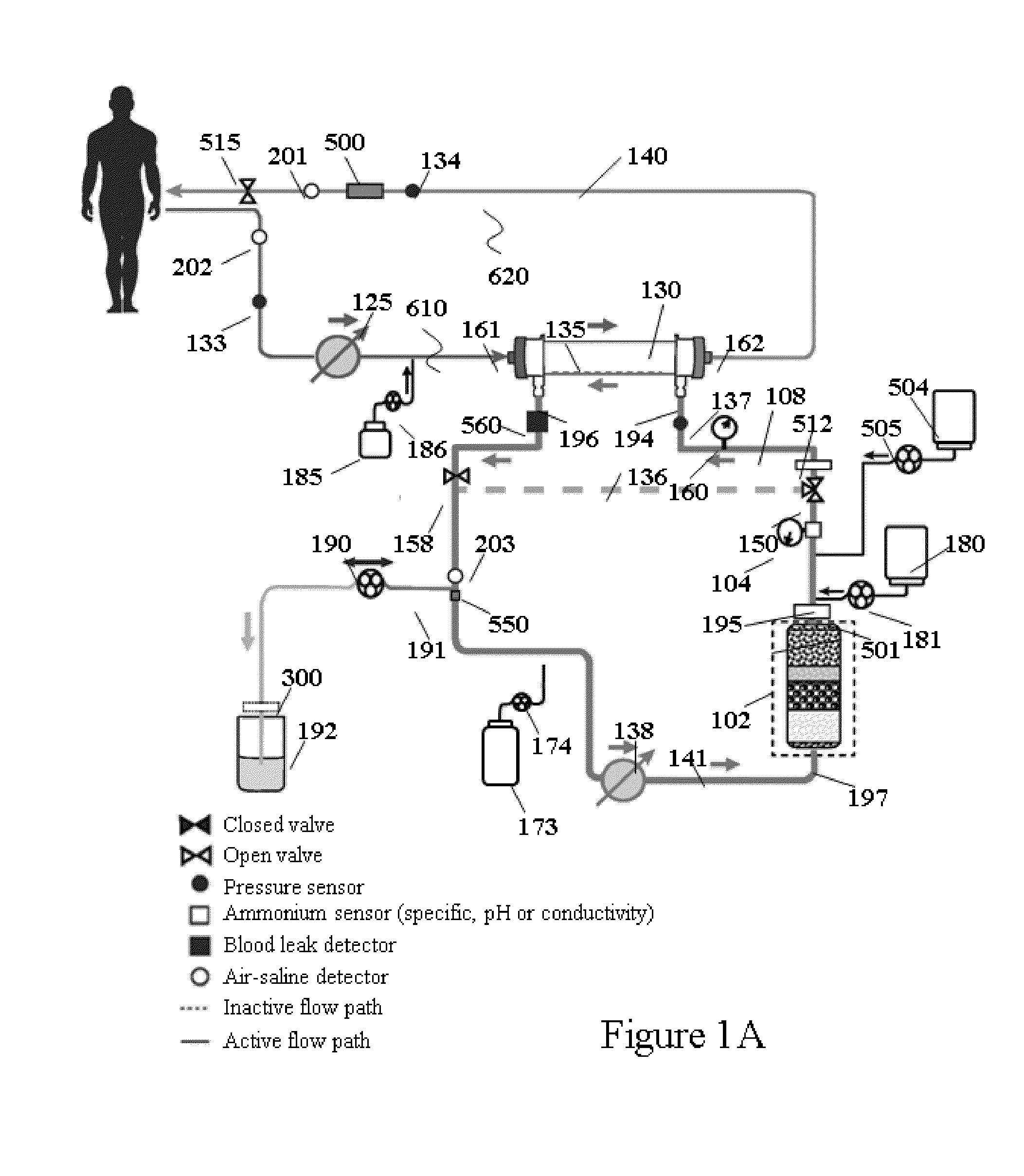
Hemodialysis system having a flow path with a controlled compliant volume
ActiveUS20130199998A1Enhanced convective clearanceReduce riskSemi-permeable membranesSolvent extractionDialysis membranesHaemodialysis machine
Systems and methods for the performance of kidney replacement therapy having or using a dialyzer, control components, sorbent cartridge and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. The system for performing kidney replacement therapy has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. The dialysis circuit and an extracorporeal circuit for circulating blood are in fluid communication through the dialysis membrane. The flux of fluid moving between the extracorporeal circuit and the dialysis circuit is modified by the rate at which the control pump is operating such that a rate of ultrafiltration and convective clearance can be controlled. The system provides for the monitoring of an inlet and outlet conductivity of the sorbent cartridge to provide a facility to quantify or monitor the removal of urea by the sorbent cartridge.
Owner:MOZARC MEDICAL US LLC
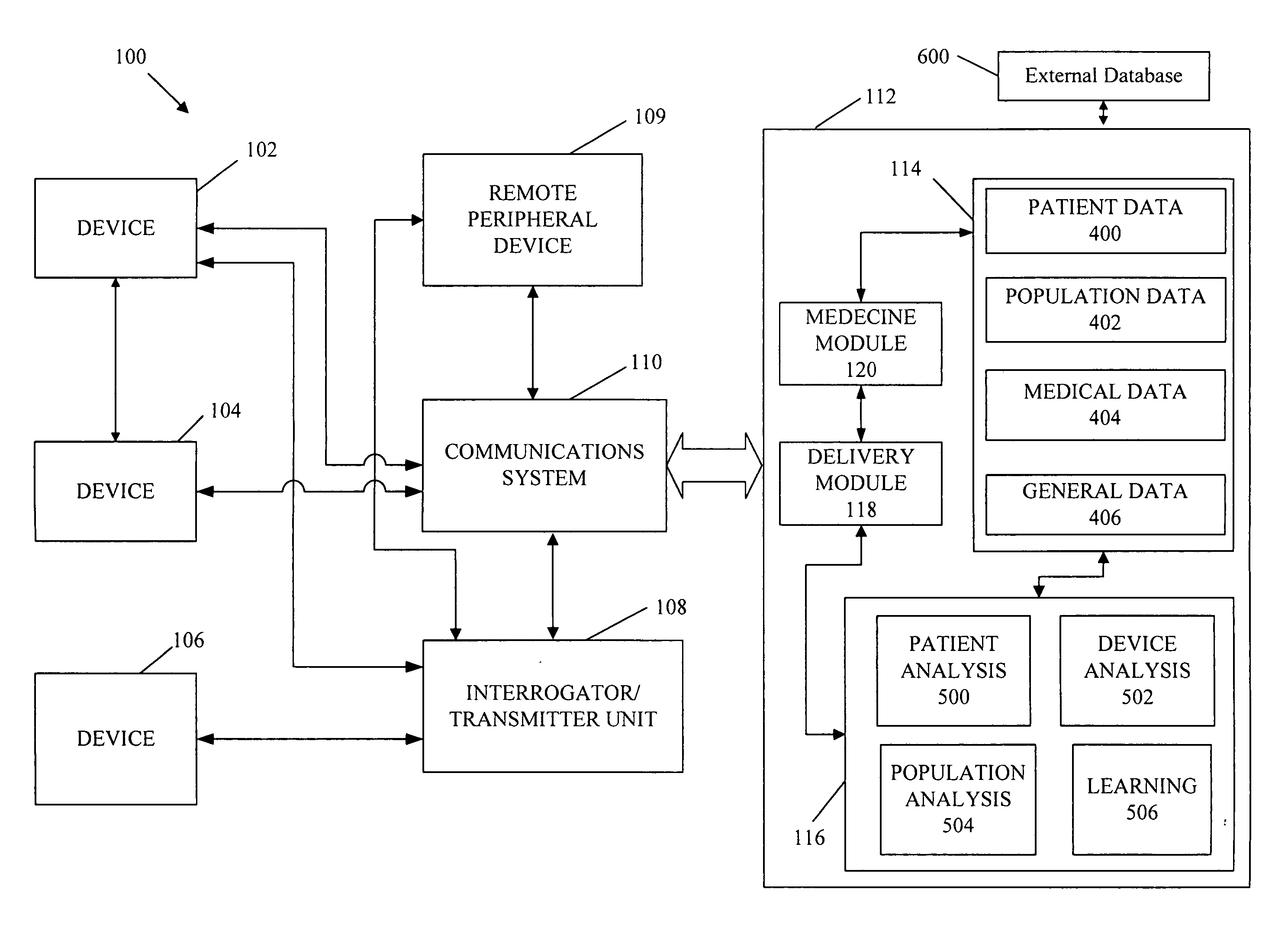
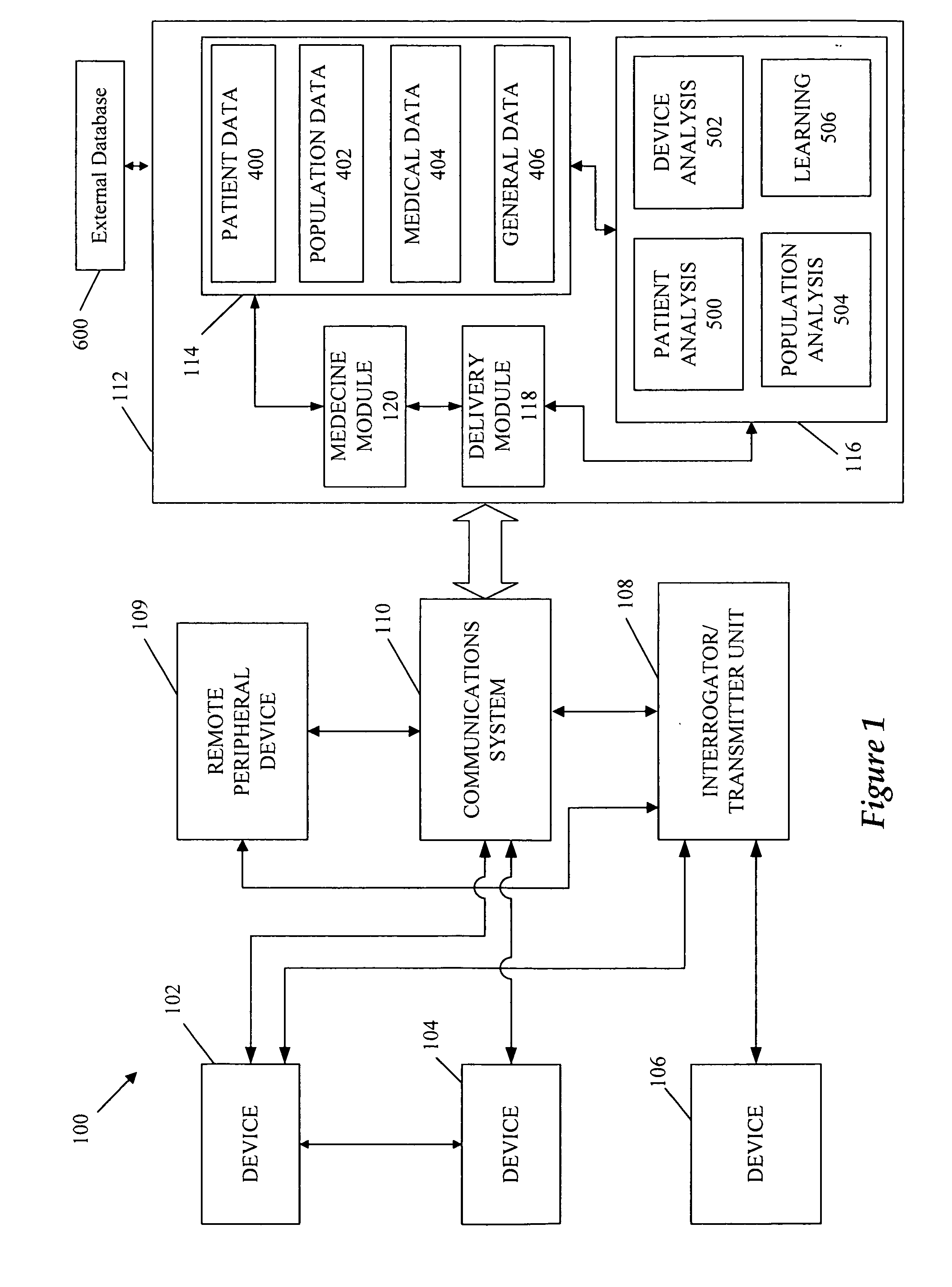
Cardiac rhythm management device and sensor-suite for the optimal control of ultrafiltration and renal replacement therapies
ActiveUS20070175827A1ElectrotherapyMechanical/radiation/invasive therapiesUltrafiltrationOptimal control
A cardiorenal patient monitoring system comprising, either implanted or non-implanted device(s), remote peripheral device(s), computer network(s), host, and communication means between the device(s), computer network(s), and host. The preferred embodiment shows an advanced patient monitoring system for using an implanted cardiac device and a dialysis machine in renal therapy. In addition, the method of advanced patient monitoring is in conjunction with the advanced patient monitoring system is disclosed.
Owner:CARDIAC PACEMAKERS INC
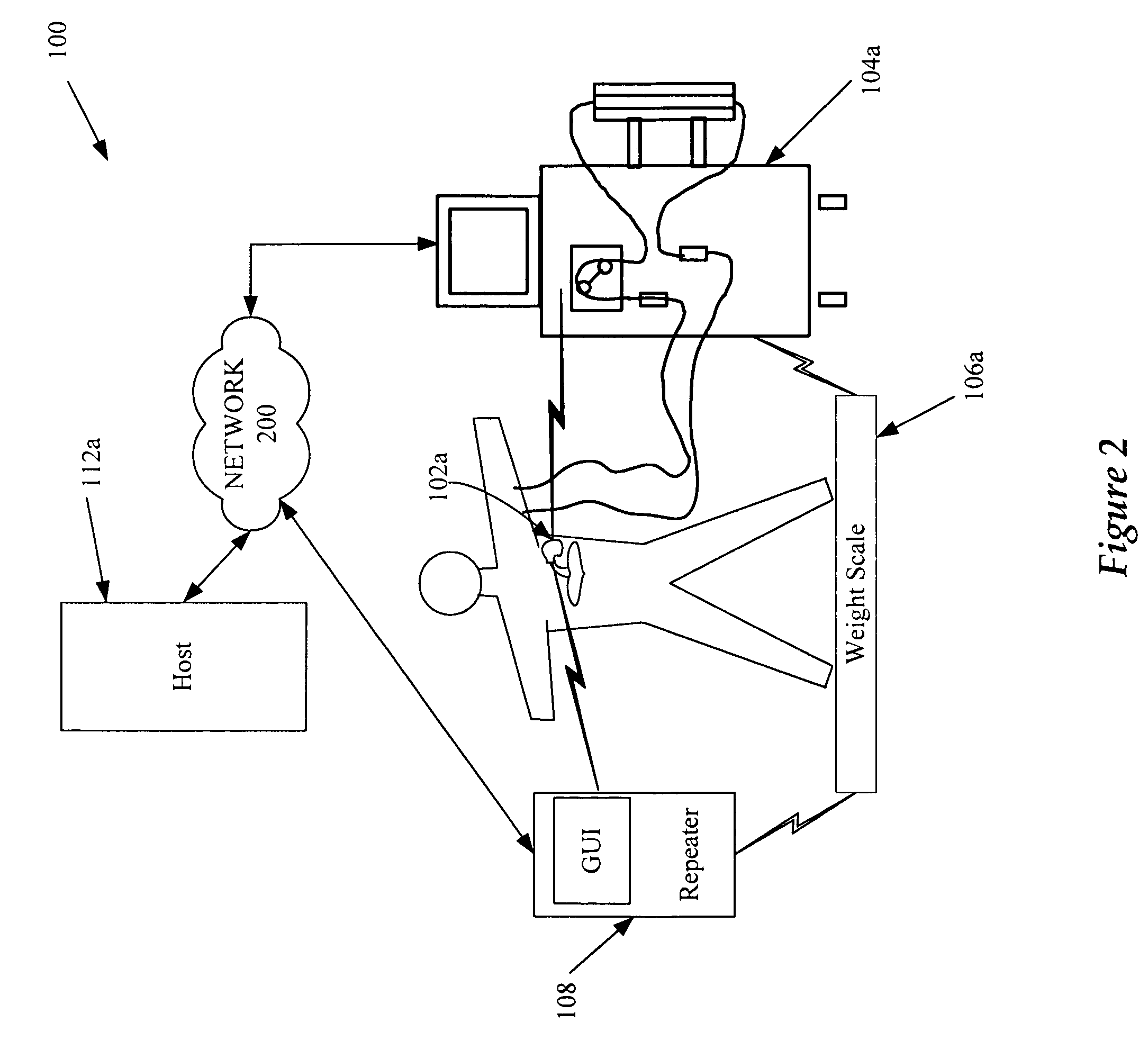
User interface for blood treatment device
InactiveUS6923782B2Accurate insertionSemi-permeable membranesMechanical/radiation/invasive therapiesGraphicsBlood treatments
A graphical user interface for a medical instruments for a Renal Replacement Therapy includes a pictogram representation of cthe fluid path of an extracorporeal blood circuit that represents fluid lines, pumps and sensors. To assist the user in responding to alarms and rectifying faults in the system the source of a potential trouble is animated. The location of a trouble spot is easily identified by flashing of the corresponding element of the pictogram, change of color or thickness of a corresponding line.
Owner:GAMBRO LUNDIA AB
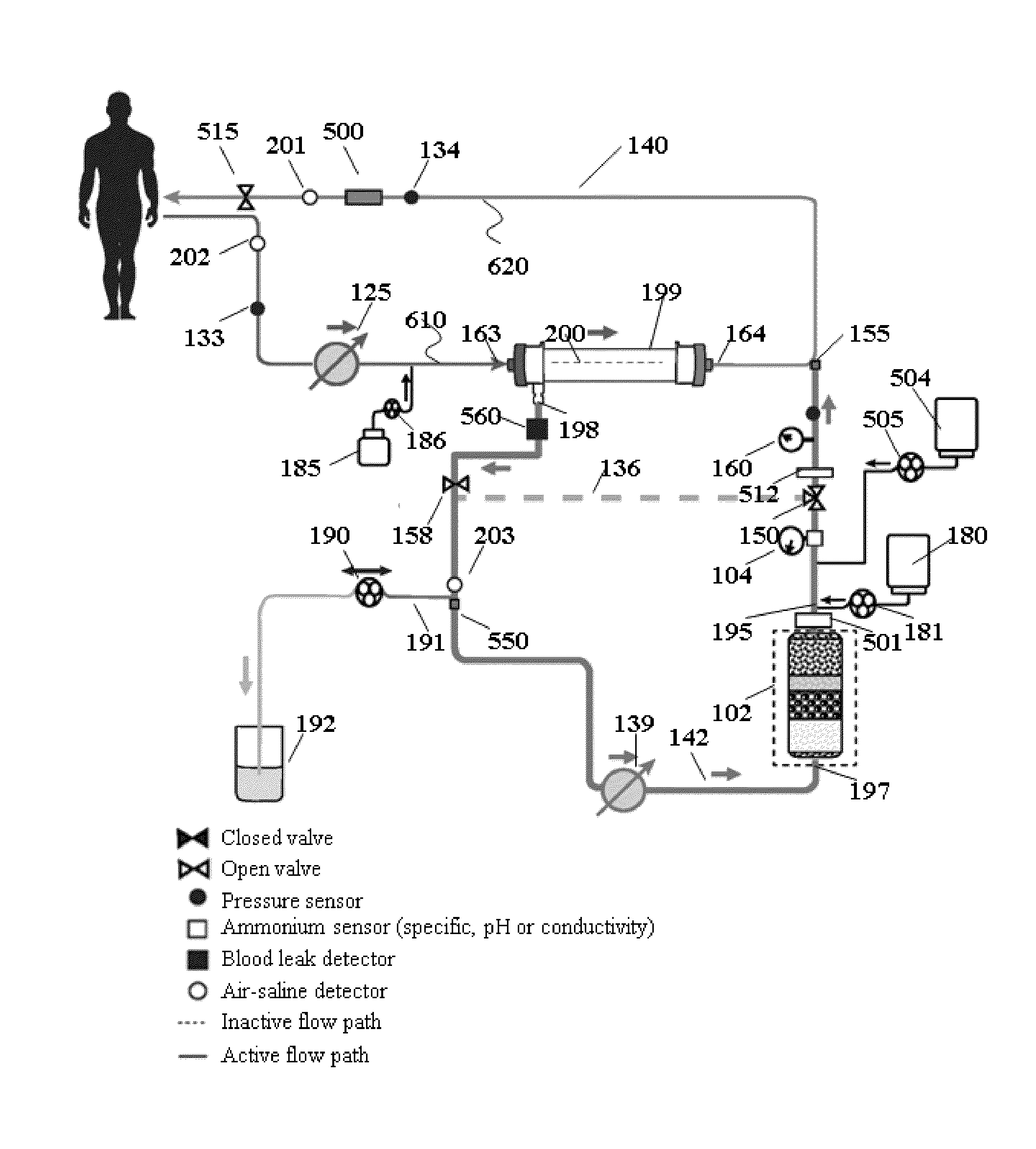
Modular hemodialysis system
ActiveUS20130213890A1Mechanical/radiation/invasive therapiesSolvent extractionDialysis membranesActivated carbon
Apparatuses, systems, and methods for the performance of kidney replacement therapy having or using a dialyzer, control components, sorbent cartridge, and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. The system has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. A first sorbent cartridge is provided for use in a portable treatment module having activated carbon and zirconium oxide. The system also provides for the monitoring of an inlet and outlet conductivity of a sorbent cartridge containing urease to provide a facility to quantify or monitor the removal of urea by a detachable urea removal module.
Owner:MOZARC MEDICAL US LLC
Fluid circuits, systems, and processes for extracorporeal blood processing
InactiveUS7214312B2Avoid pollutionLiquid separation auxillary apparatusSolvent extractionMedicineFluid management
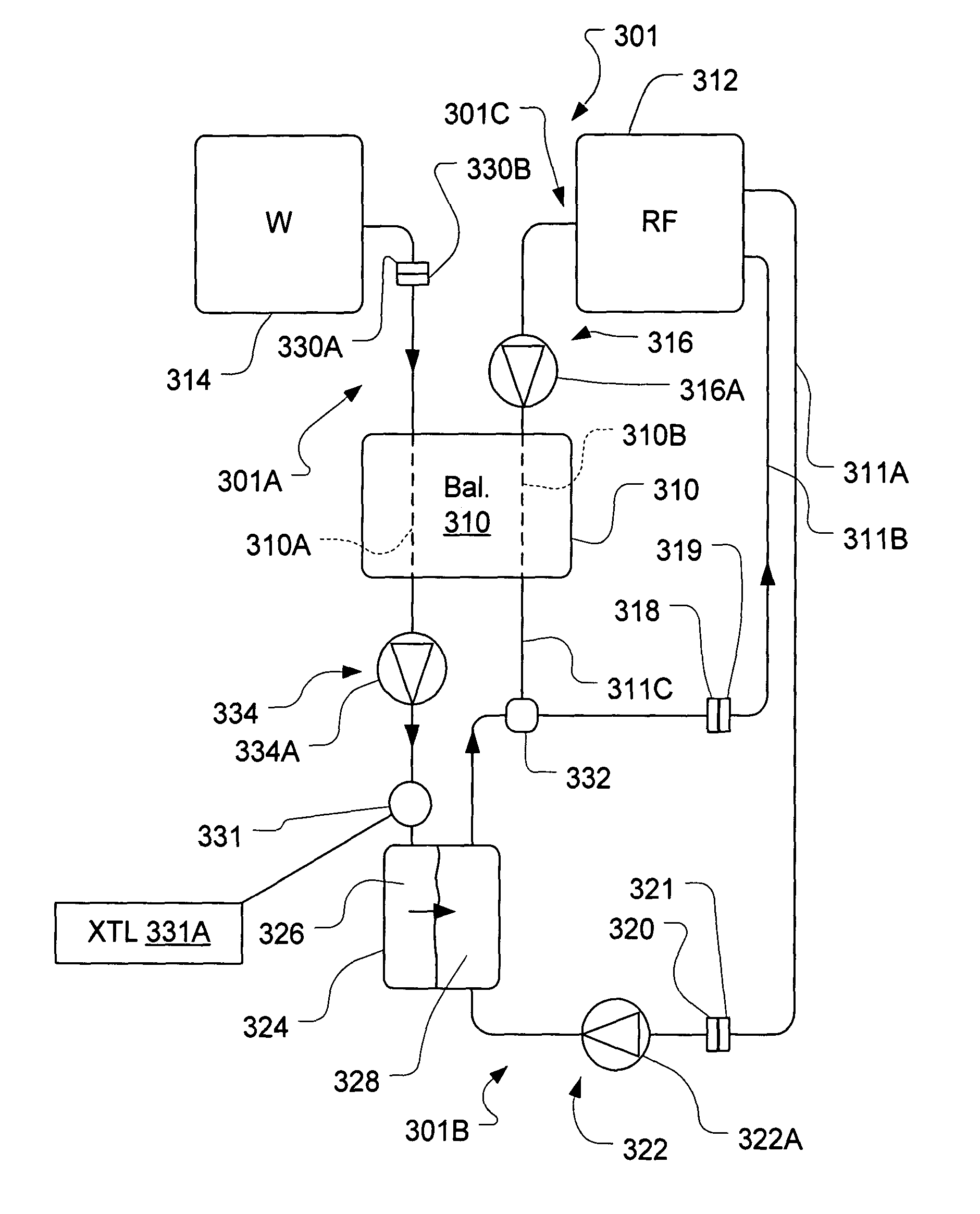
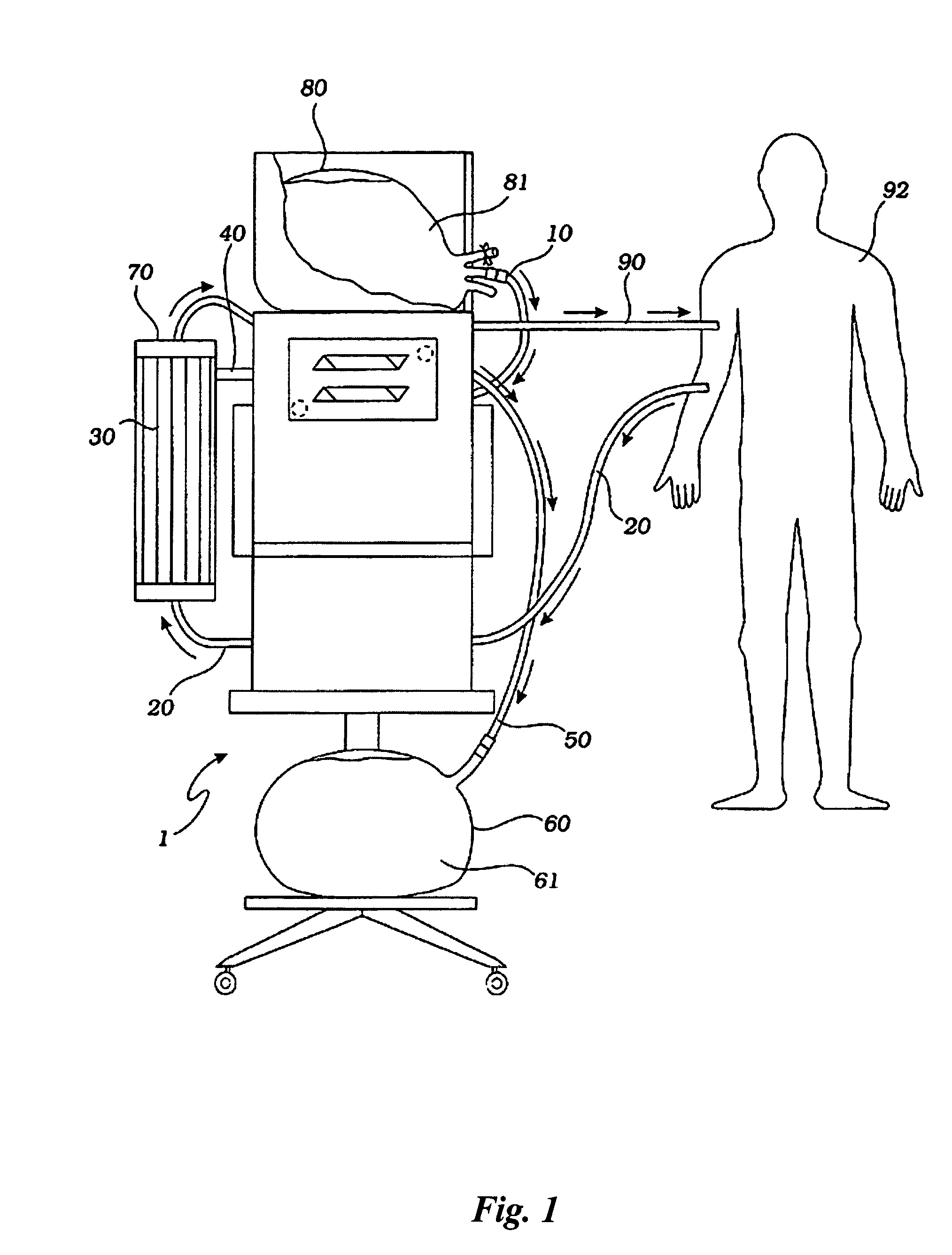
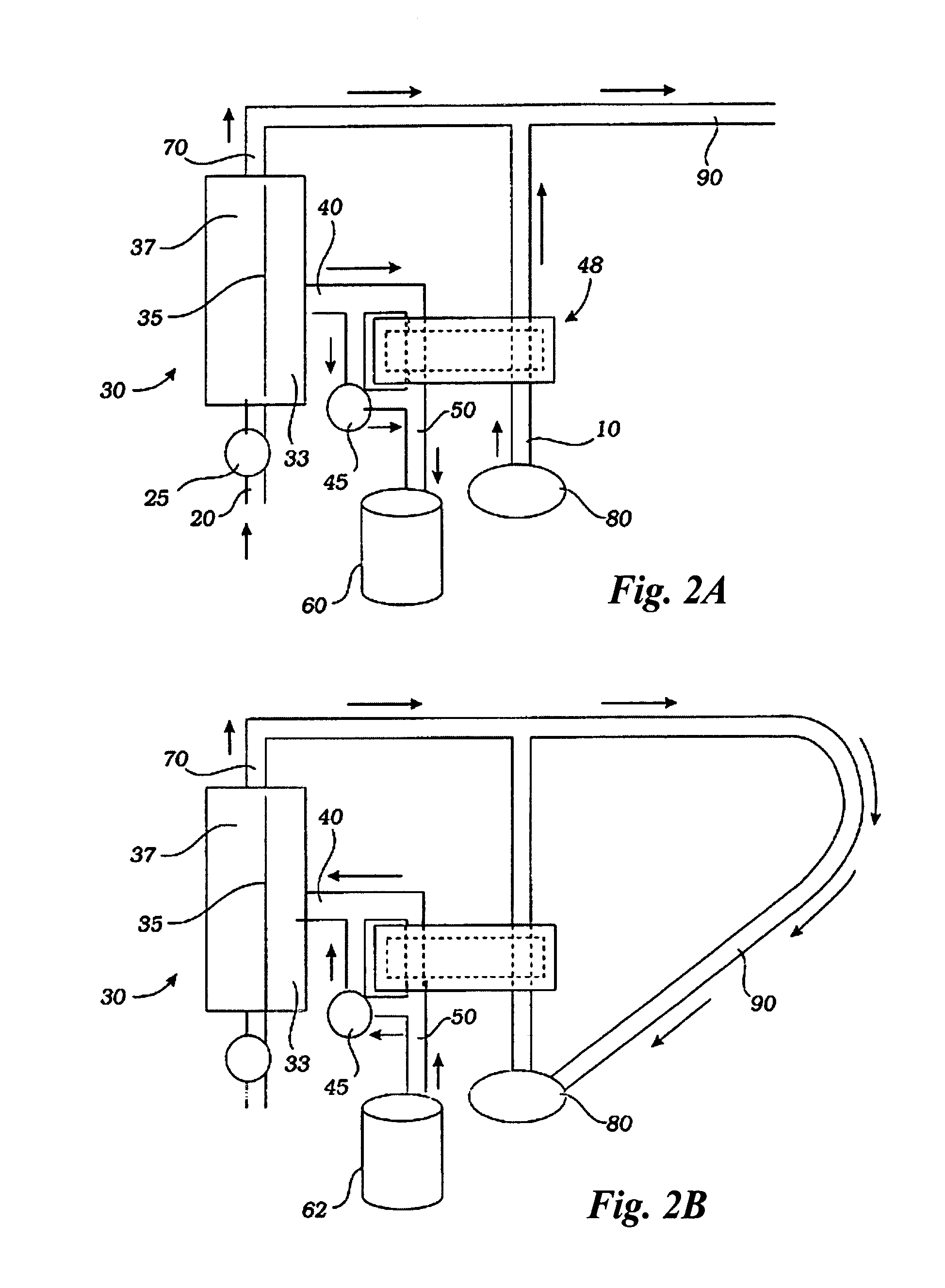
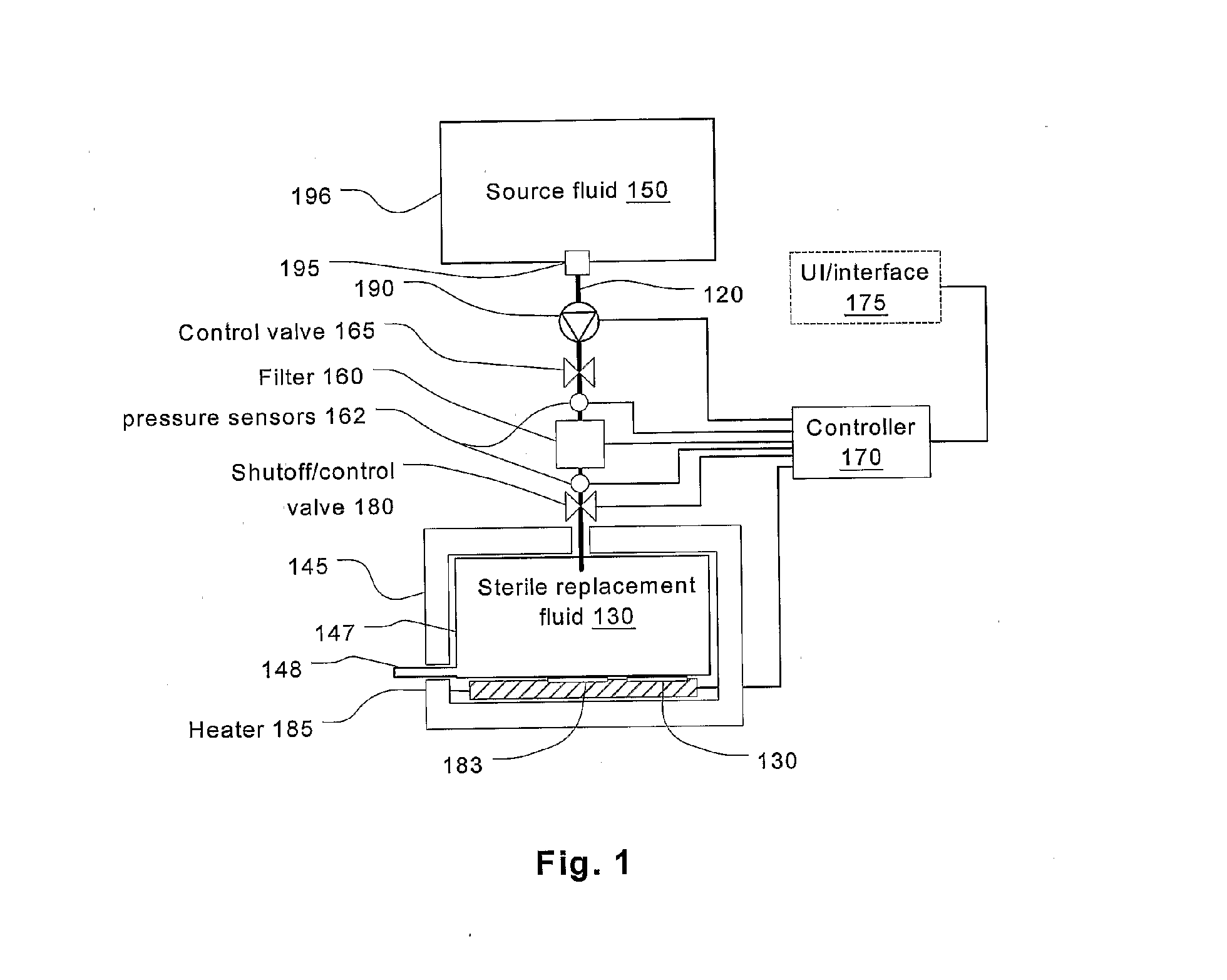
A sterilized fluid management kit for use in renal replacement therapy isolates a replacement fluid container from touch contamination and provides for sterilization of replacement fluid by filtering using an installed filter that is hermetically connected to the replacement fluid container. A method is described where replacement fluid is automatically generated before treatment.
Owner:NXSTAGE MEDICAL
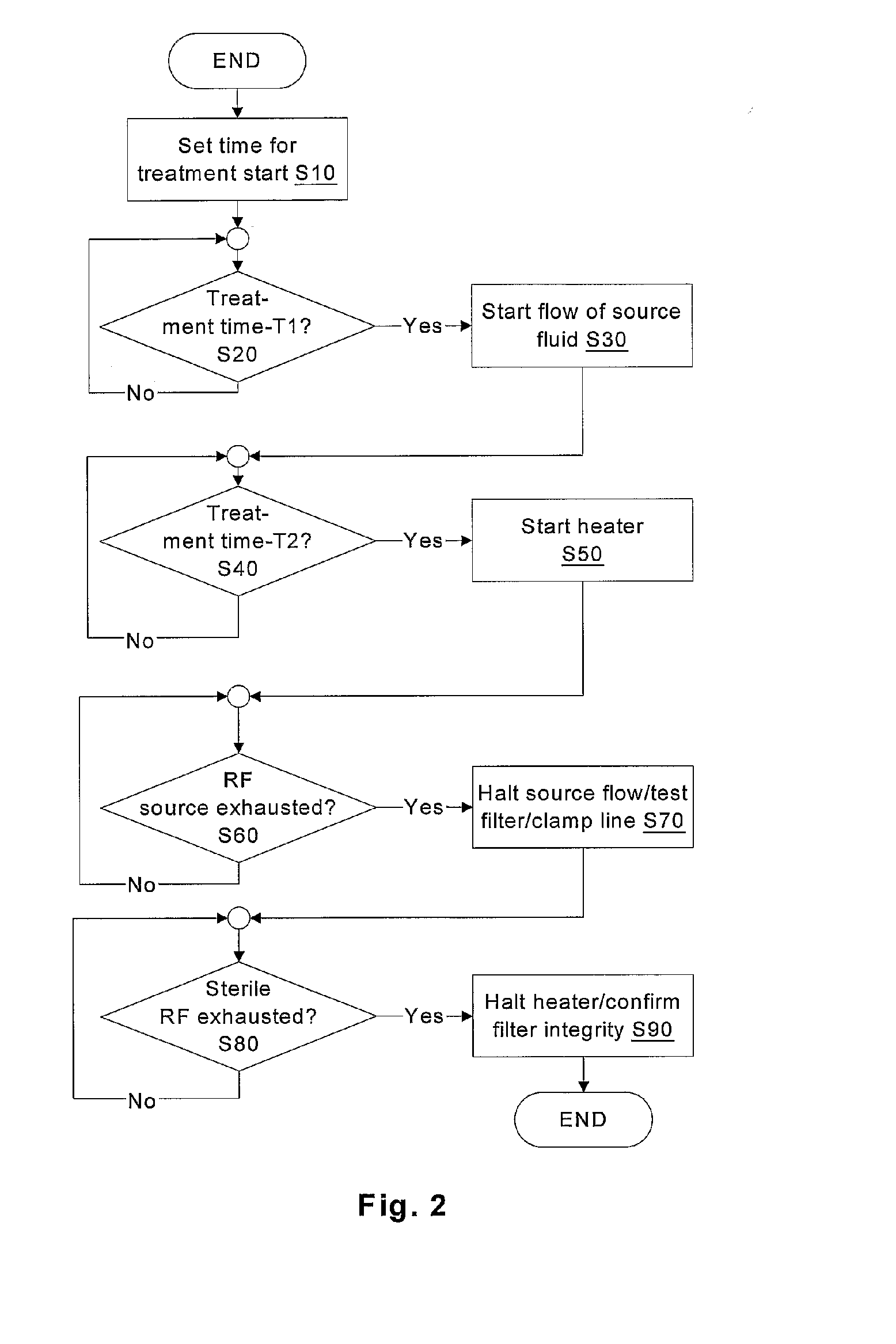
Batch filtration system for preparation of sterile fluid for renal
ActiveUS20080053905A9Solve insufficient capacityPrevent heat lossSolvent extractionMedical devicesBlood treatmentsSterile filtration
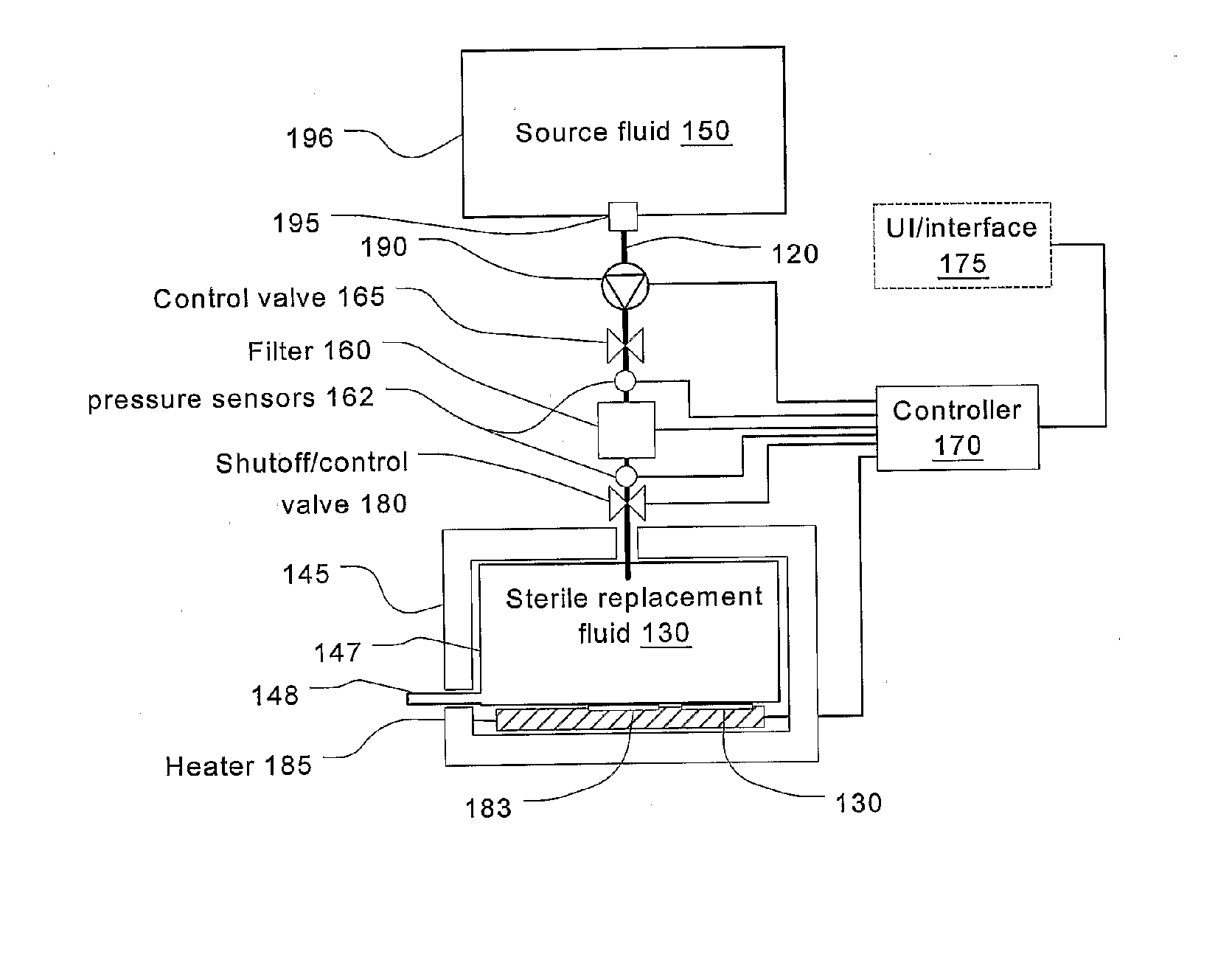
A method and device for blood treatments that use fluids such as dialysate and replacement fluid for renal replacement therapy. In an embodiment, fluid is passed either by pump or passively by gravity feed, through a microporous sterilization filter from a fluid source to a replacement fluid container. The latter forms a batch that may be used during treatment. The advantage of forming the batch before treatment is that the rate of filtering needn't match the rate of consumption during treatment. As a result, the sterilization filter can have a small capacity. In another embodiment, a filter is placed immediately prior to the point at which the sterile fluid is consumed by the treatment process. The latter may be used in combination with the former embodiment as a last-chance guarantee of sterility and / or that the fluid is free of air bubbles. It may also be used as the primary means of sterile-filtration.
Owner:NXSTAGE MEDICAL INC +1
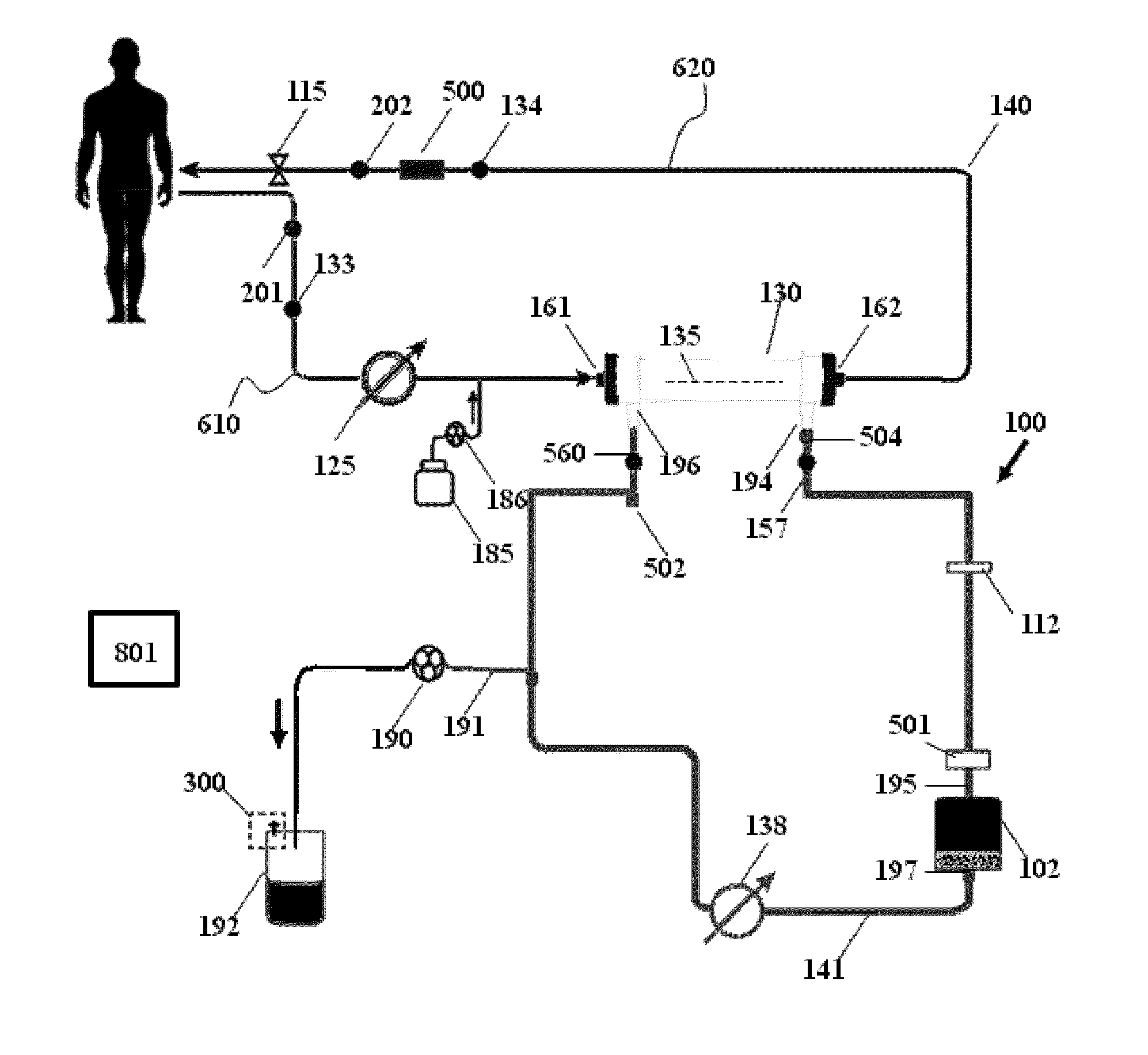
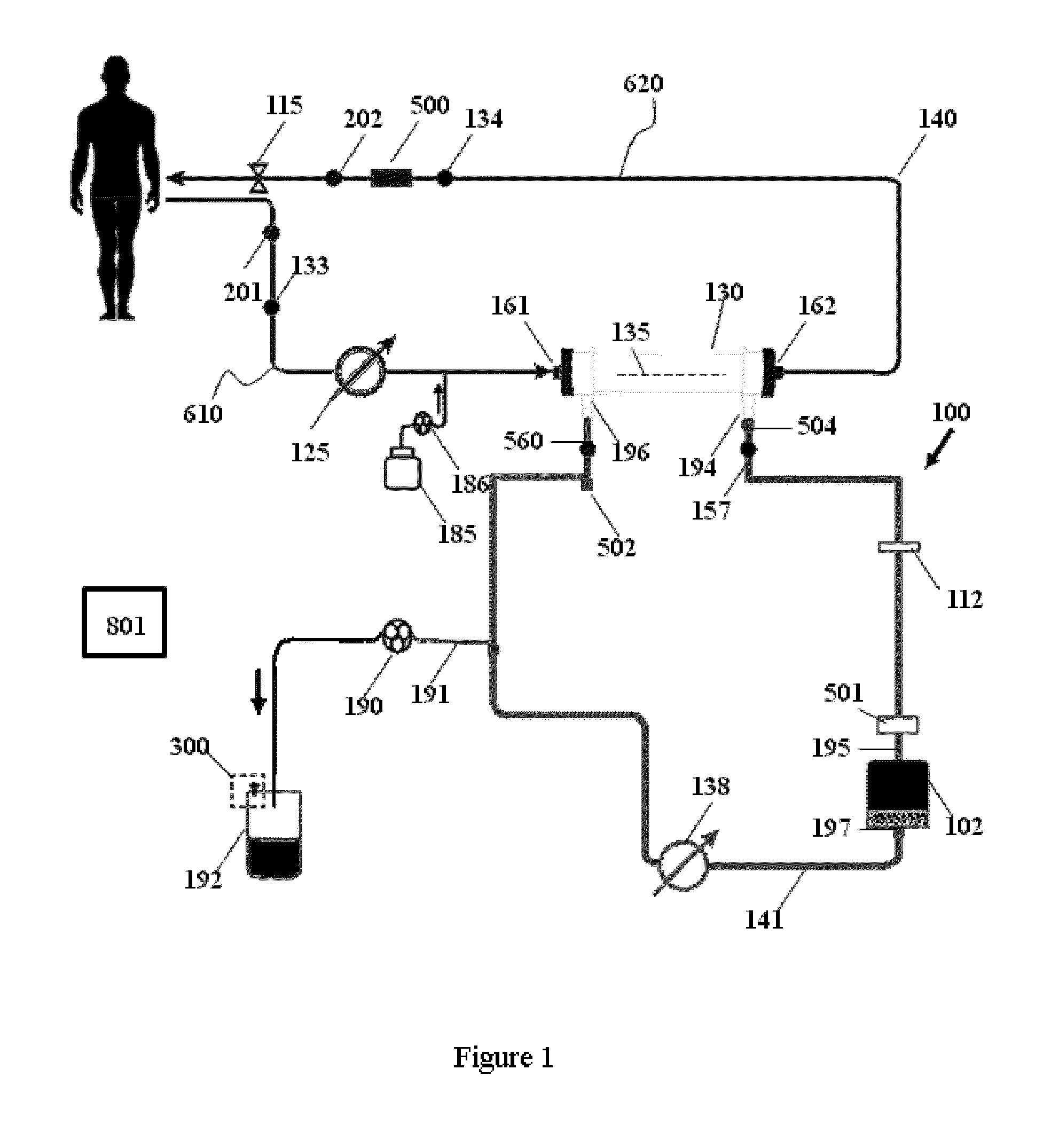
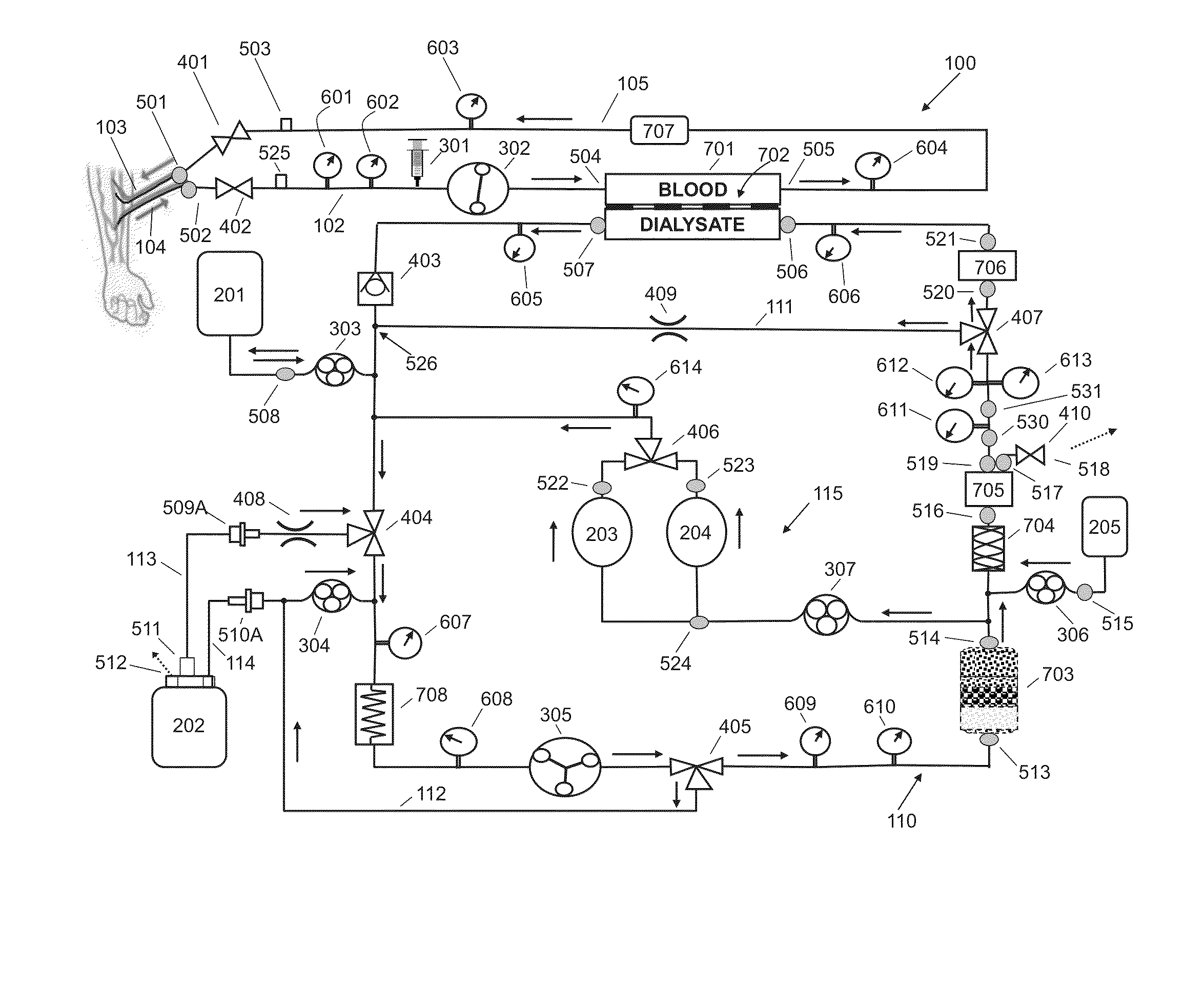
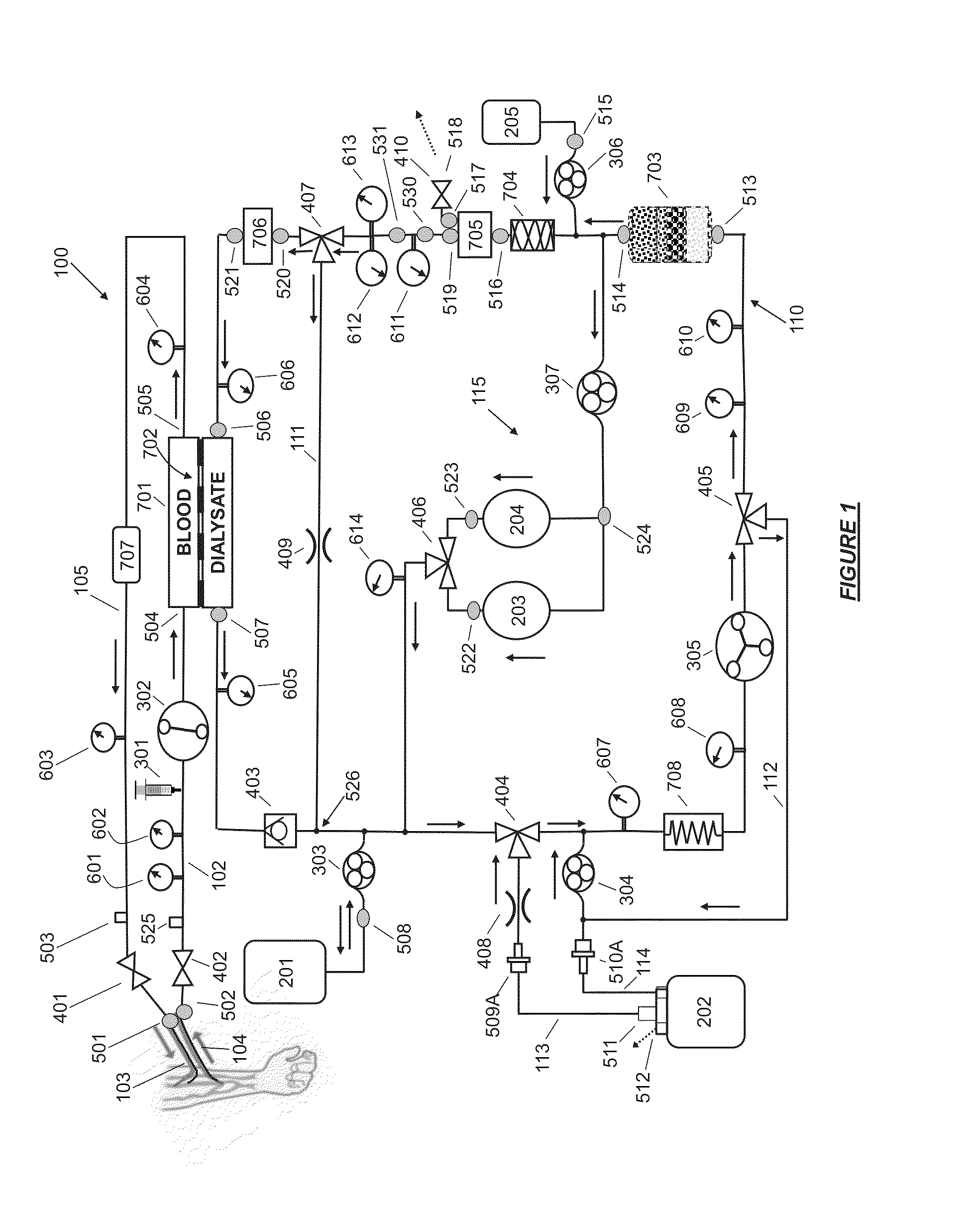
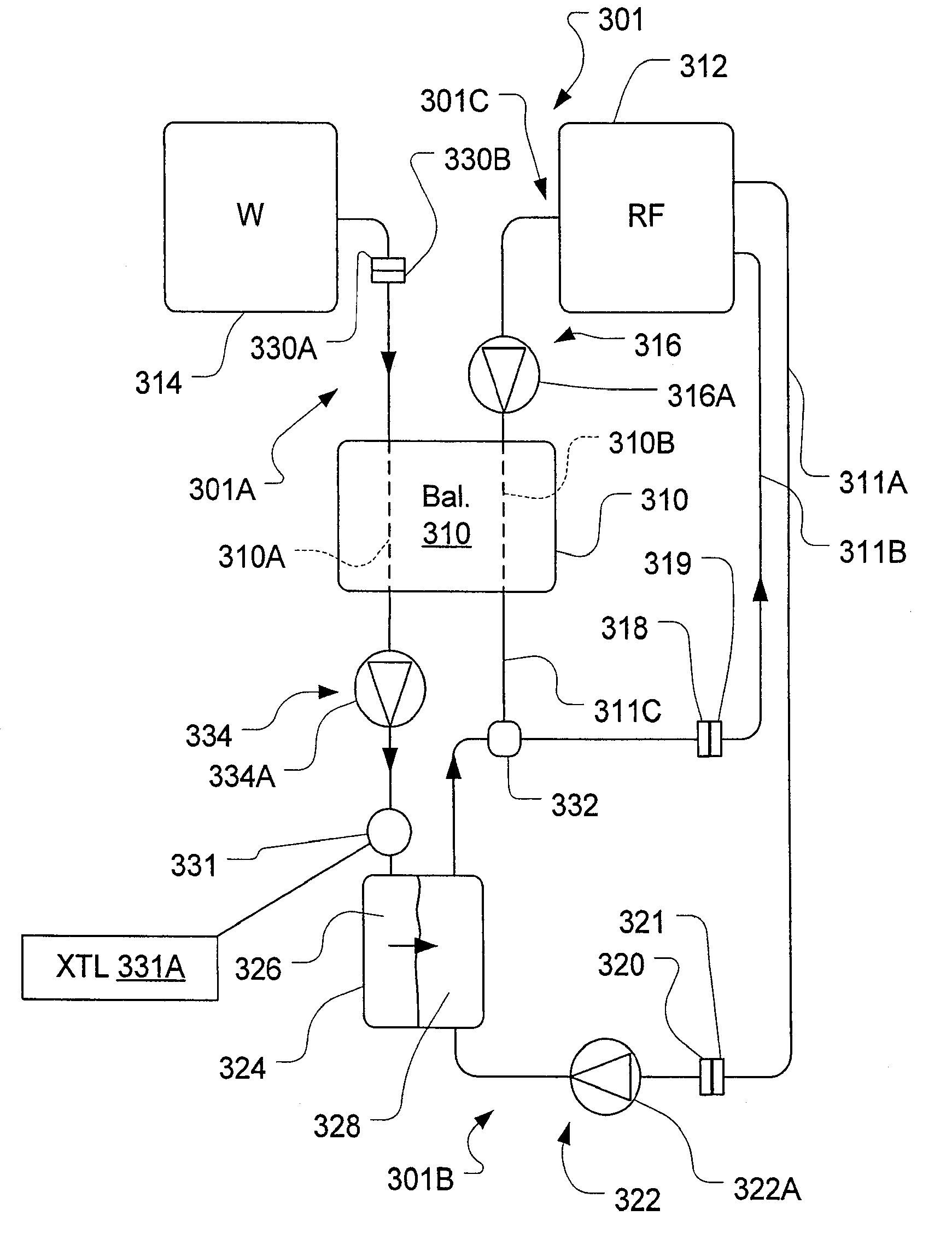
Fluid circuit for delivery of renal replacement therapies
A flow loop for hemodialysis, hemodiafiltration and hemofiltration for the treatment of pathological conditions such as End Stage Renal Disease (ESRD) that has a controlled compliant flow path for preparing fluids required for a hemodialysis therapy session from water. The controlled compliant flow path modifies water into any one of a solution for priming a hemodialysis system, a physiologically compatible solution for contacting blood, a physiologically compatible solution for infusion to a subject, and a solution for blood rinse back to a subject. The controlled compliant flow path has a means for selectively metering in and metering out fluid from the flow path.
Owner:MOZARC MEDICAL US LLC
Composition
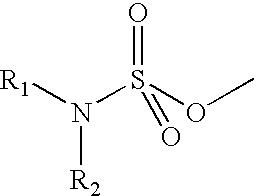
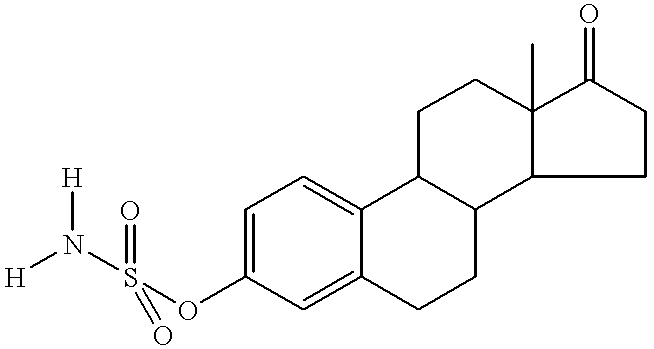
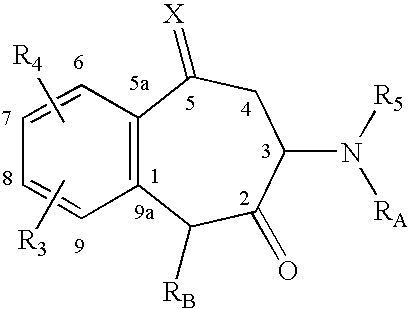
InactiveUS6653298B2Low hepatic metabolismImprove bioavailabilityOrganic active ingredientsBiocideHormone replacementHormone
Disclosed and claimed are methods for oral contraception, or a hormone replacement therapy, or for treating breast cancer, in a patient in need thereof involving administering to the patient, at a dosage of no greater than 200 mug / day per 70 kg subject, a compound having Formula (I):wherein X in combination with K form a steroidal ring and R5 is a sulphamate group that has of the formula:wherein each of R1 and R2 is H.
Owner:SCHERING AG +1
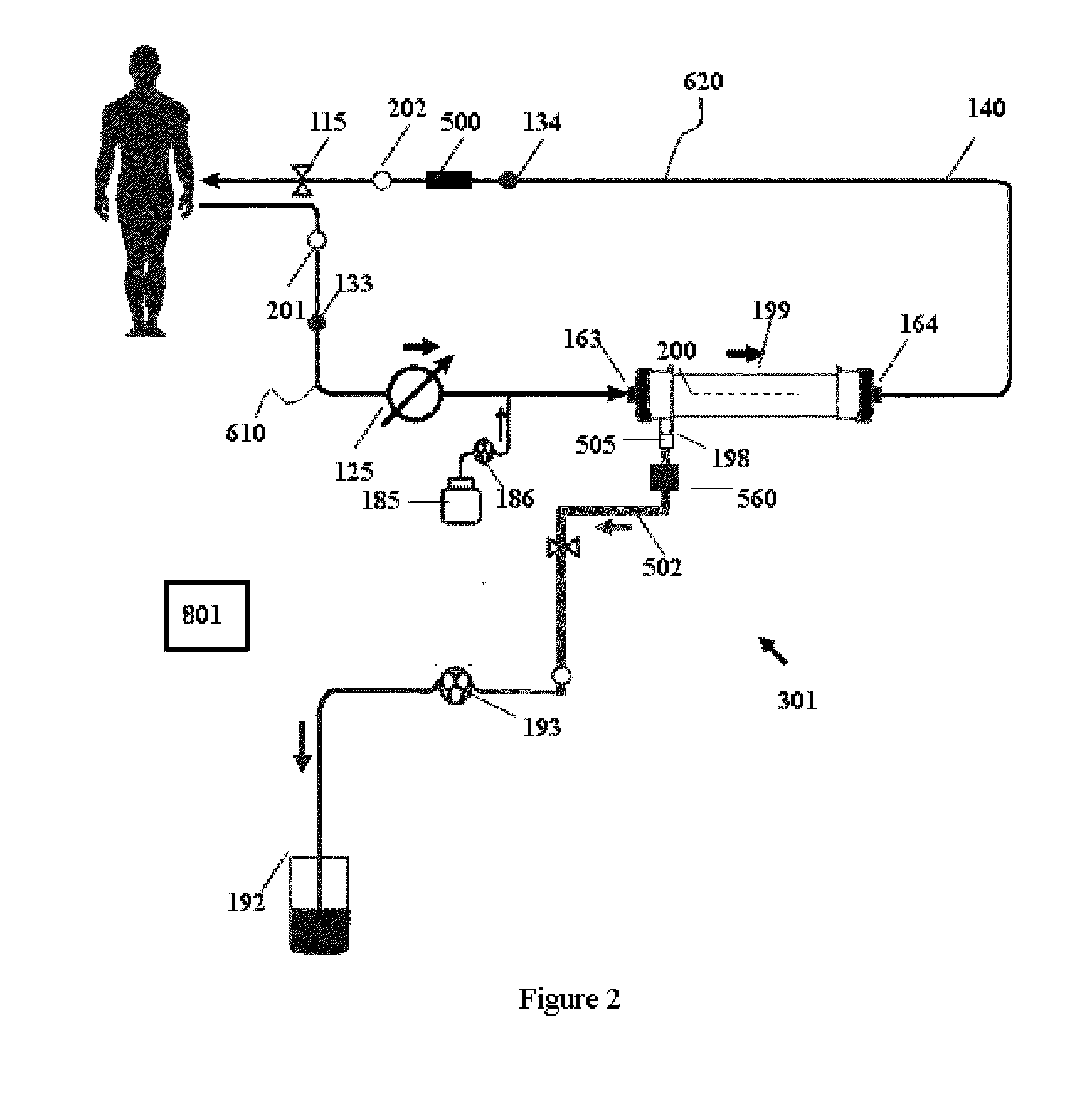
Fluid circuit for delivery of renal replacement therapies
Owner:MOZARC MEDICAL US LLC
Fluid, circuits, systems, and processes for extracorporeal blood processing
InactiveUS7419597B2Avoid pollutionEnsure sterilityLiquid separation auxillary apparatusSolvent extractionIntensive care medicineBlood processing
A method for renal replacement therapy includes priming a fluid circuit and recirculating the sterile fluid during priming to permit gas to float out of the sterile fluid into a fluid reservoir. The fluid is preferably either warmed during circulation or vibrated to promote the removal of gas.
Owner:NXSTAGE MEDICAL INC
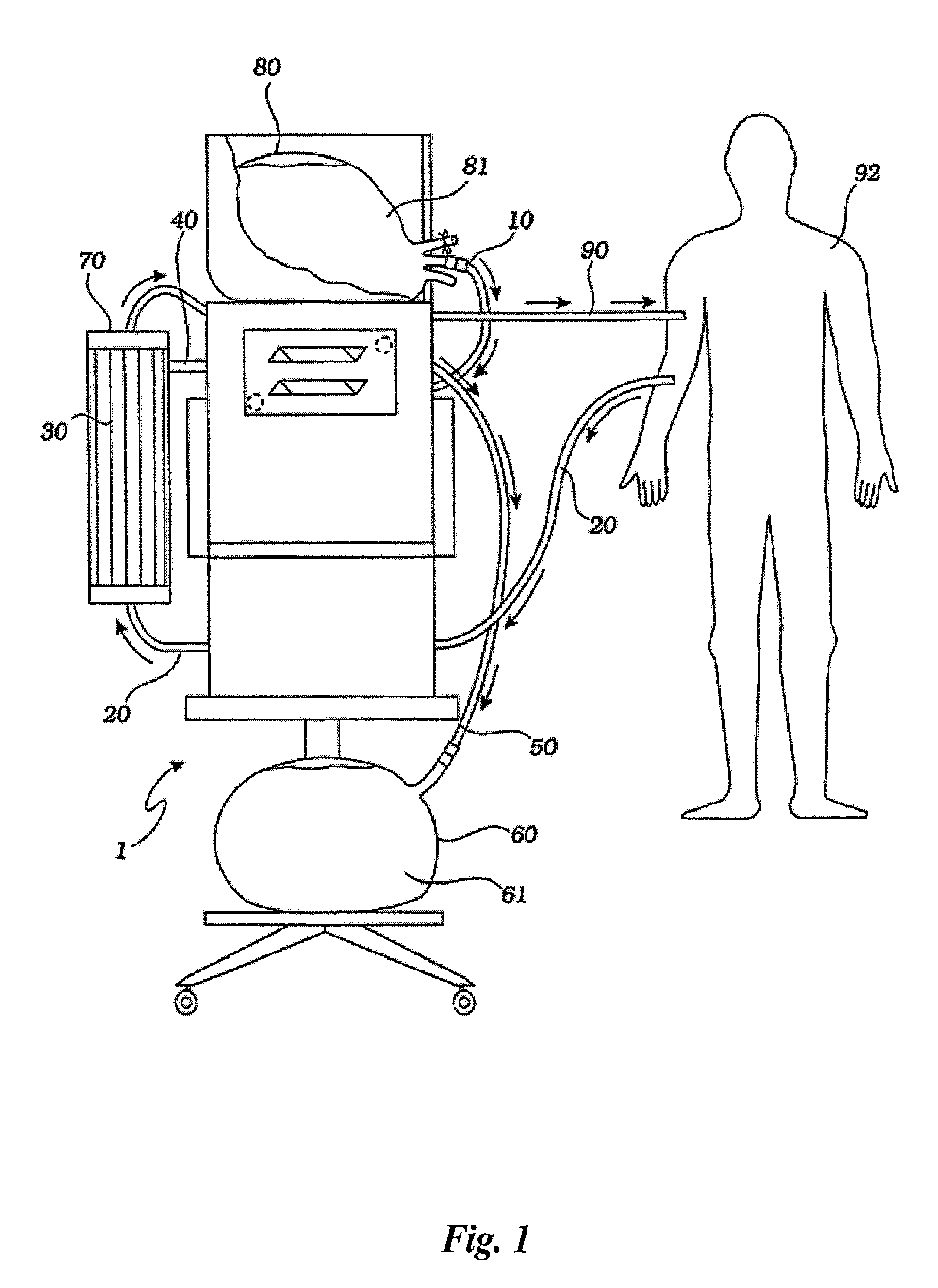
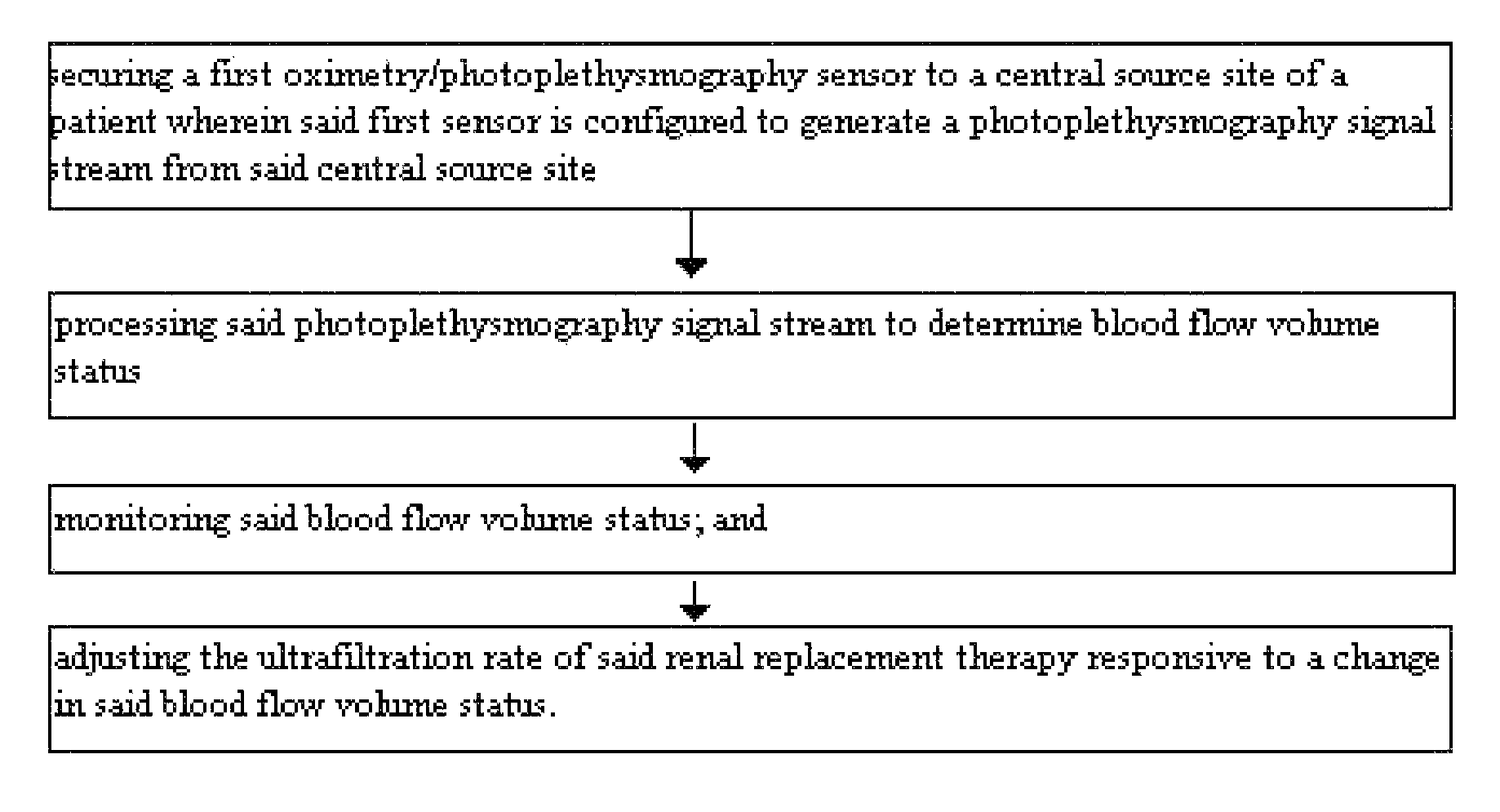
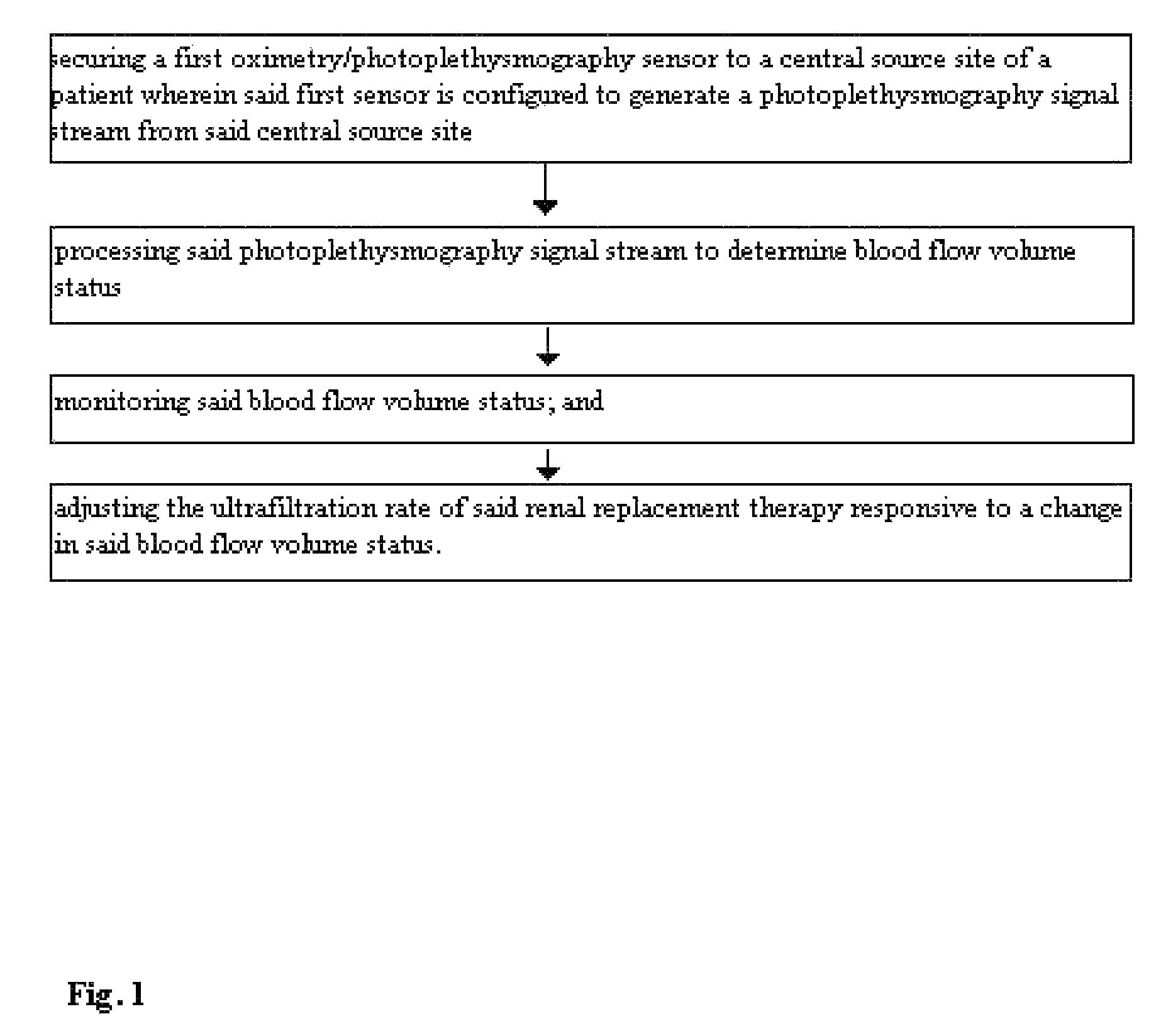
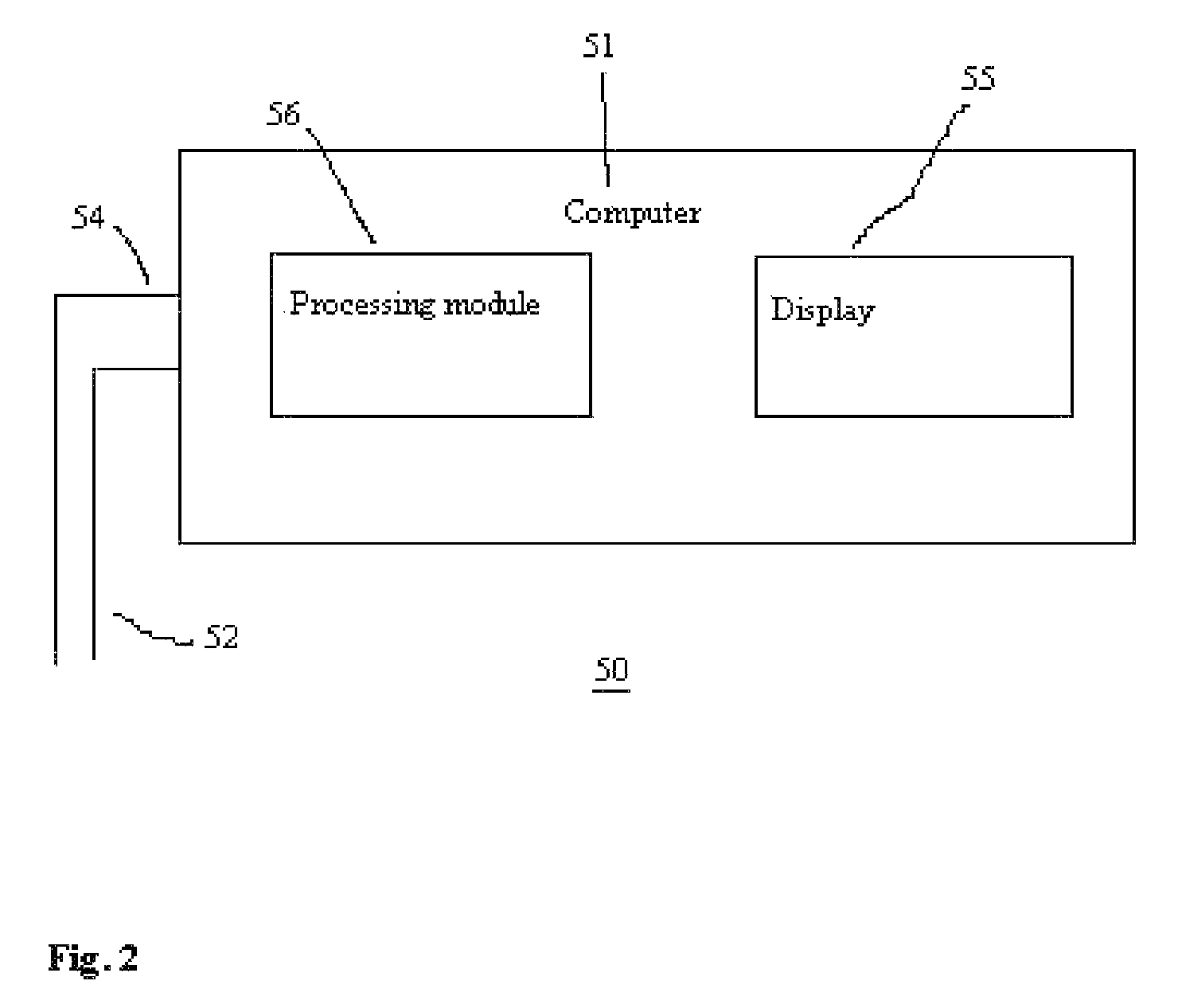
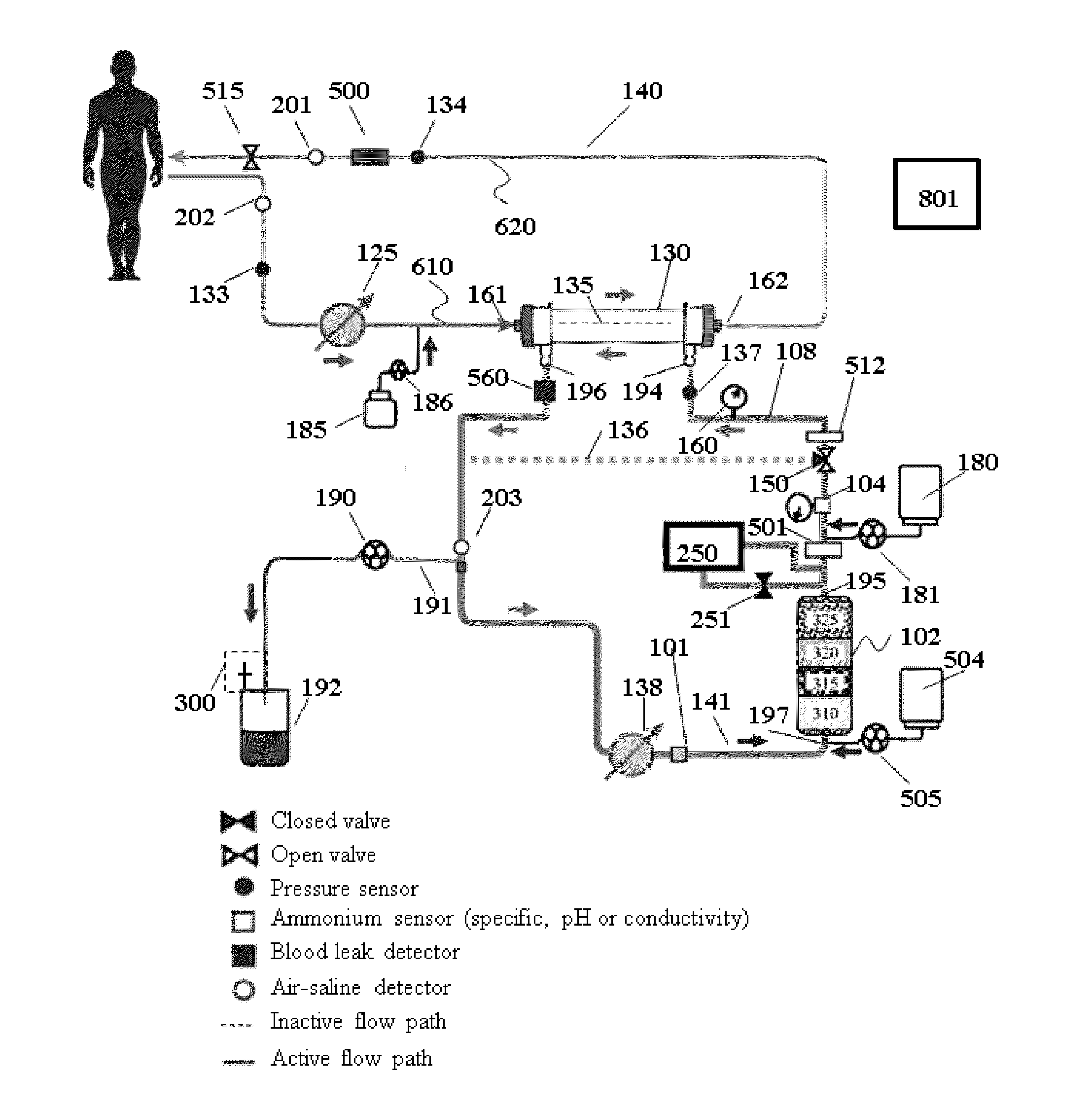
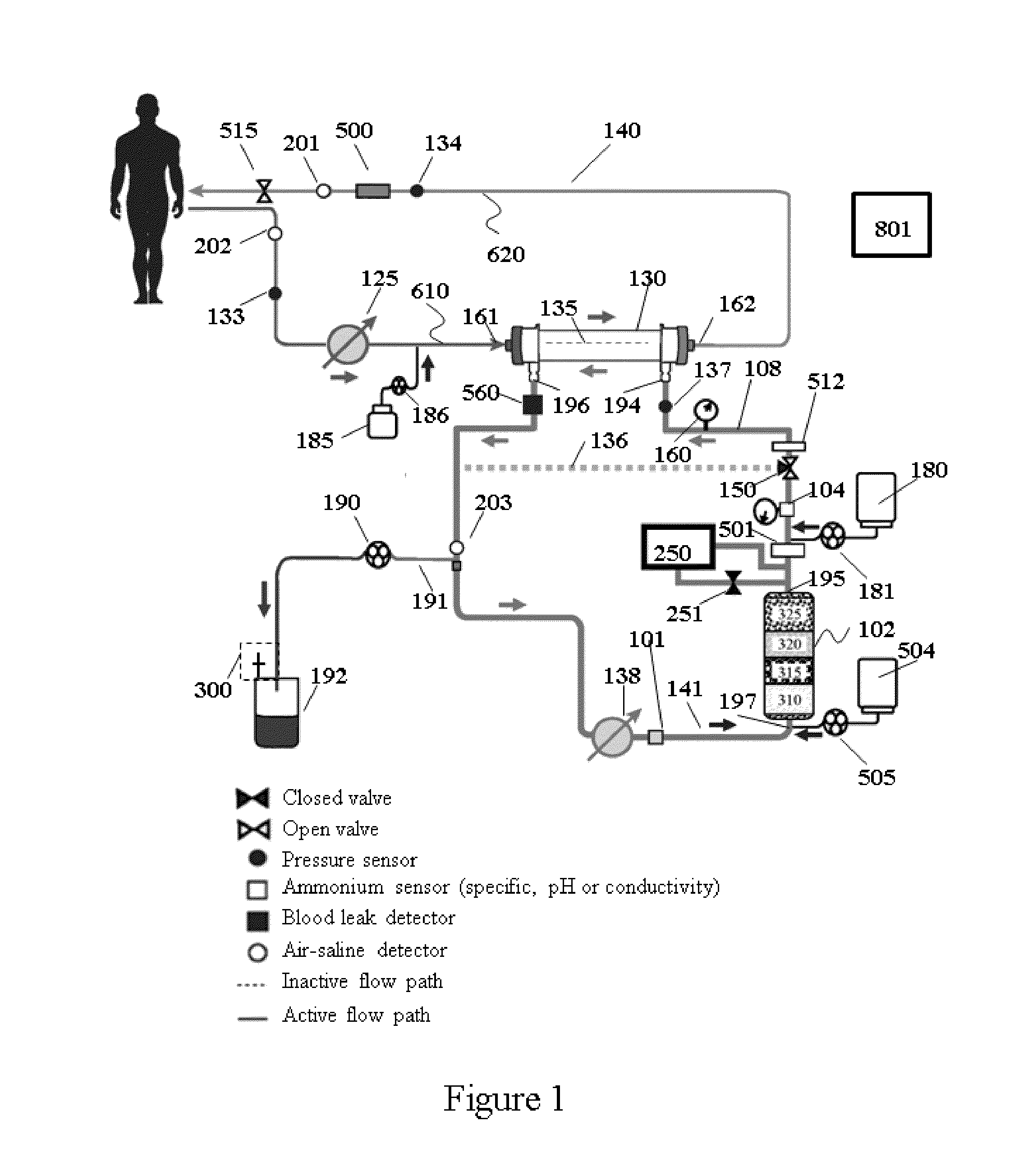
Method for using photoplethysmography to optimize fluid removal during renal replacement therapy by hemodialysis or hemofiltration
Disclosed herein are methods, systems and devices to monitor vascular volume status during renal replacement therapy utilizing at least one oximetry / photoplethysmography sensor. The methods, systems and devices provide an alternative to conventional vascular volume monitoring methods during renal replacement therapy while enabling reliable, non-invasive, and automatic monitoring of vascular volume to avert patient hypotension. The methods, systems and devices may be employed in the context of both inpatient and outpatient dialysis facilities and may also be incorporated into conventional hemodialysis and hemofiltration techniques and equipment.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Polystyrene sulfonate resin for use with a hemodialysis system having a controlled compliance dialysis circuit
InactiveUS20130256227A1Semi-permeable membranesOther blood circulation devicesDialysis membranesPolystyrene
Sorbent cartridges having a polystyrene sulfonate resin saturated with calcium ions for the performance of kidney replacement therapy are disclosed. Systems and methods having or using a sorbent cartridge, a dialyzer, control components, a cartridge having a polystyrene sulfonate resin, and fluid reservoirs configured to be of a weight and size suitable to be worn or carried by an individual requiring treatment are disclosed. A system for performing kidney replacement therapy has a controlled compliance dialysis circuit, where a control pump controls the bi-directional movement of fluid across a dialysis membrane. The system provides for the monitoring of an inlet and outlet conductivity of the sorbent cartridge to quantify or monitor the removal of urea by the sorbent cartridge.
Owner:MEDTRONIC INC
Fluid, circuits, systems, and processes for extracorporeal blood processing
A sterilized fluid management kit for use in renal replacement therapy isolates a replacement fluid container from touch contamination and provides for sterilization of replacement fluid by filtering using an installed filter that is hermetically connected to the replacement fluid container. A method is described where replacement fluid is automatically generated before treatment.
Owner:NXSTAGE MEDICAL INC
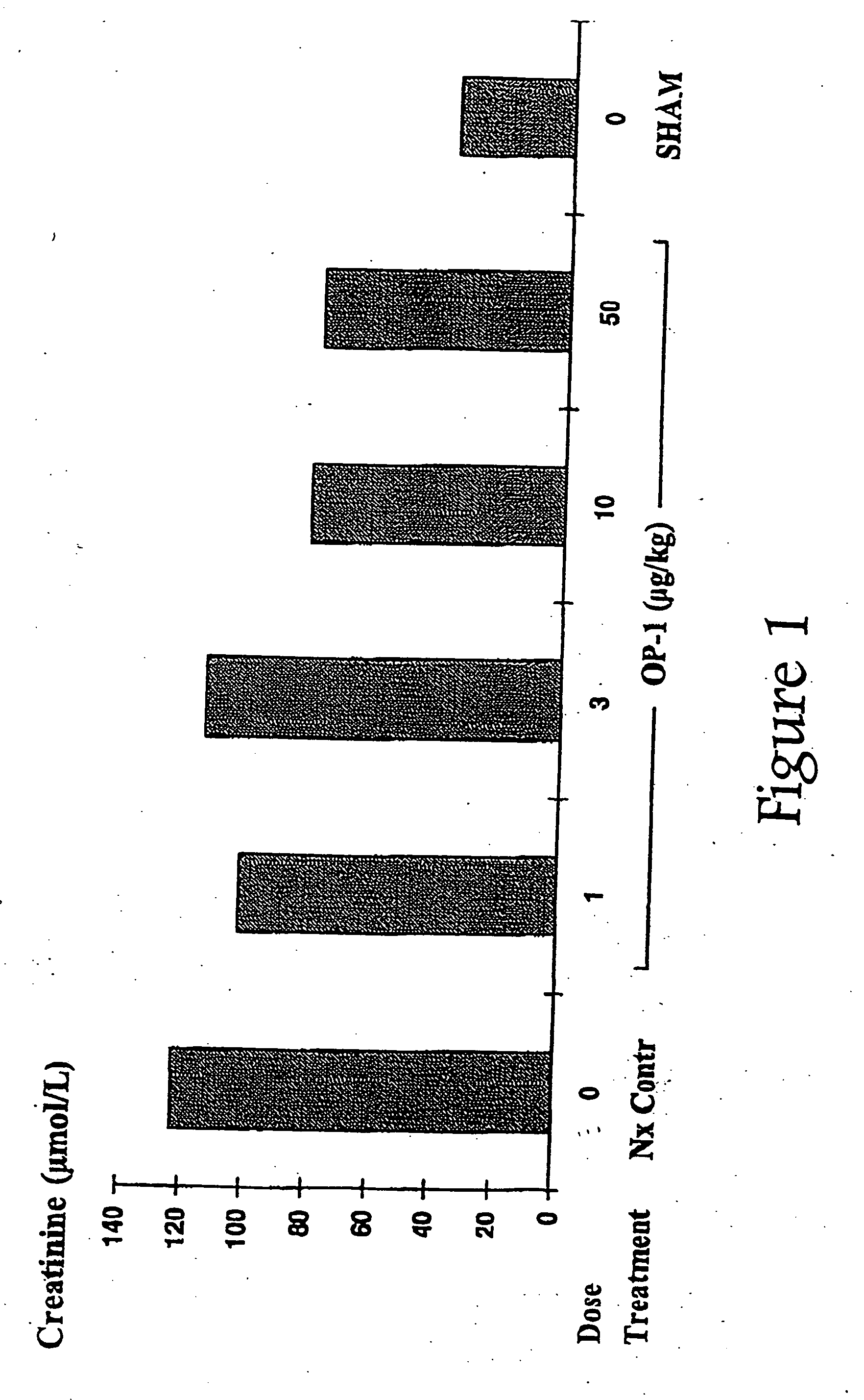
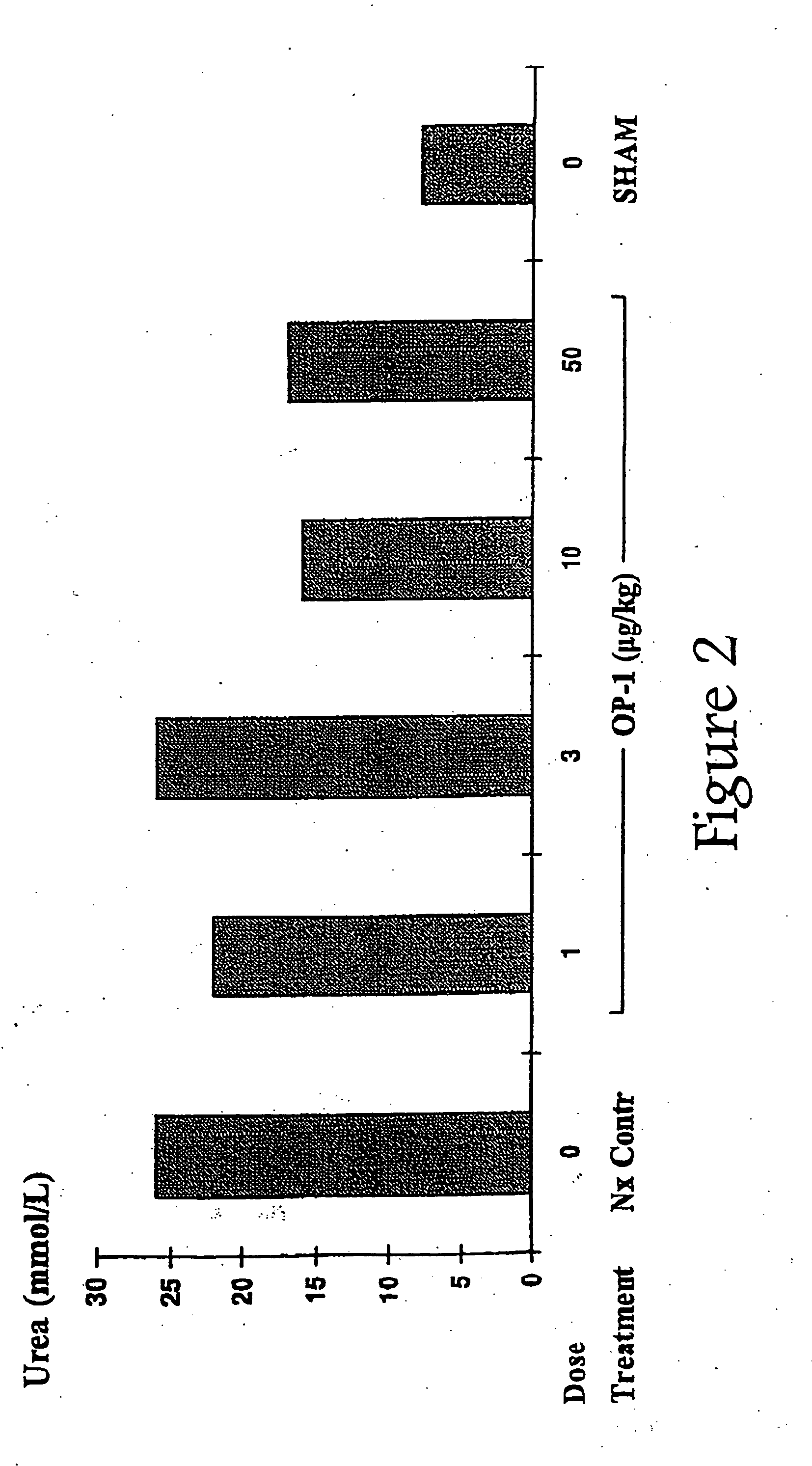
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
Method for the early identification and prediction of kidney injury
InactiveUS20090239242A1Convenient treatmentTransferasesDisease diagnosisCreatinine riseElevated blood
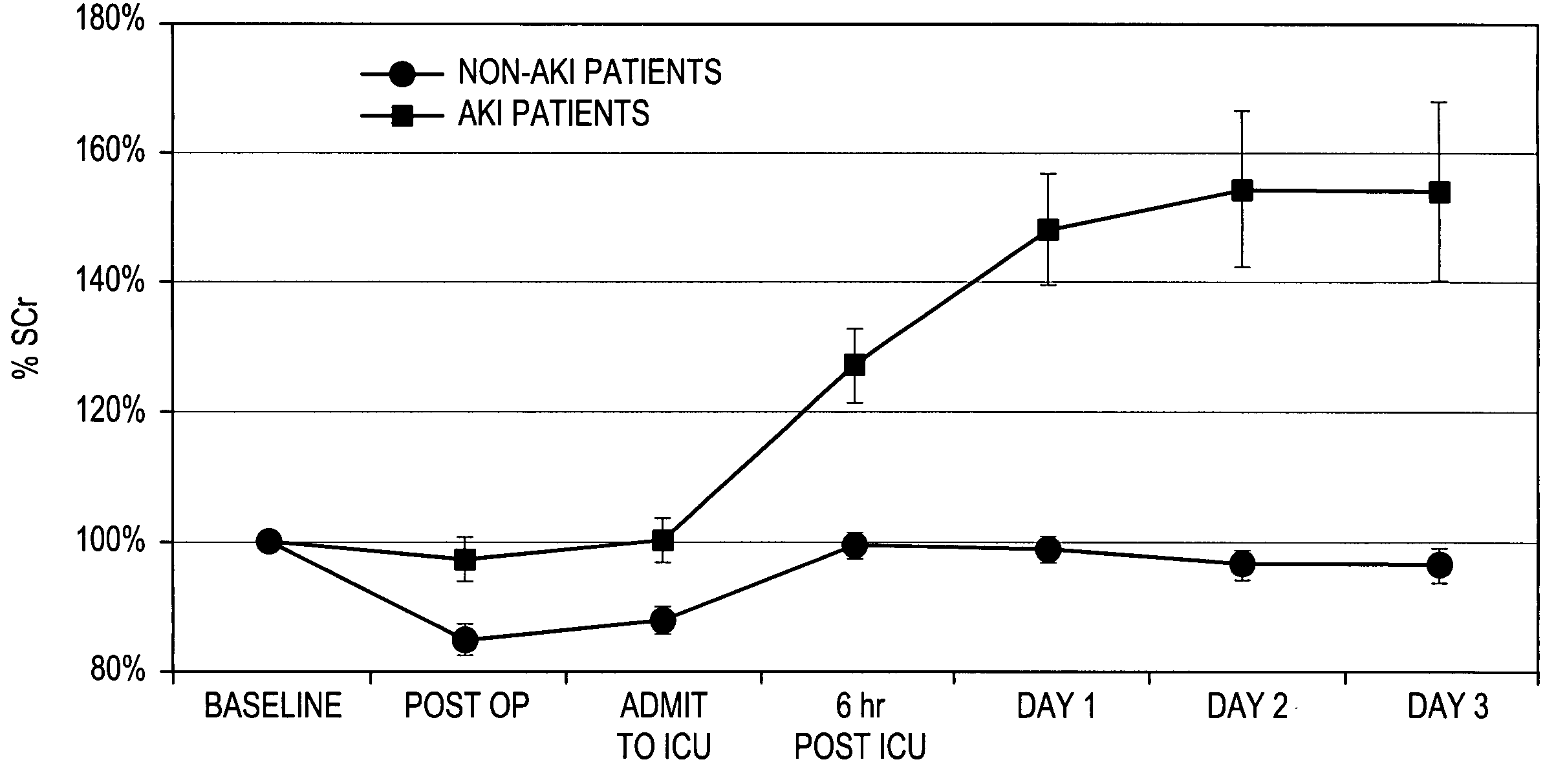
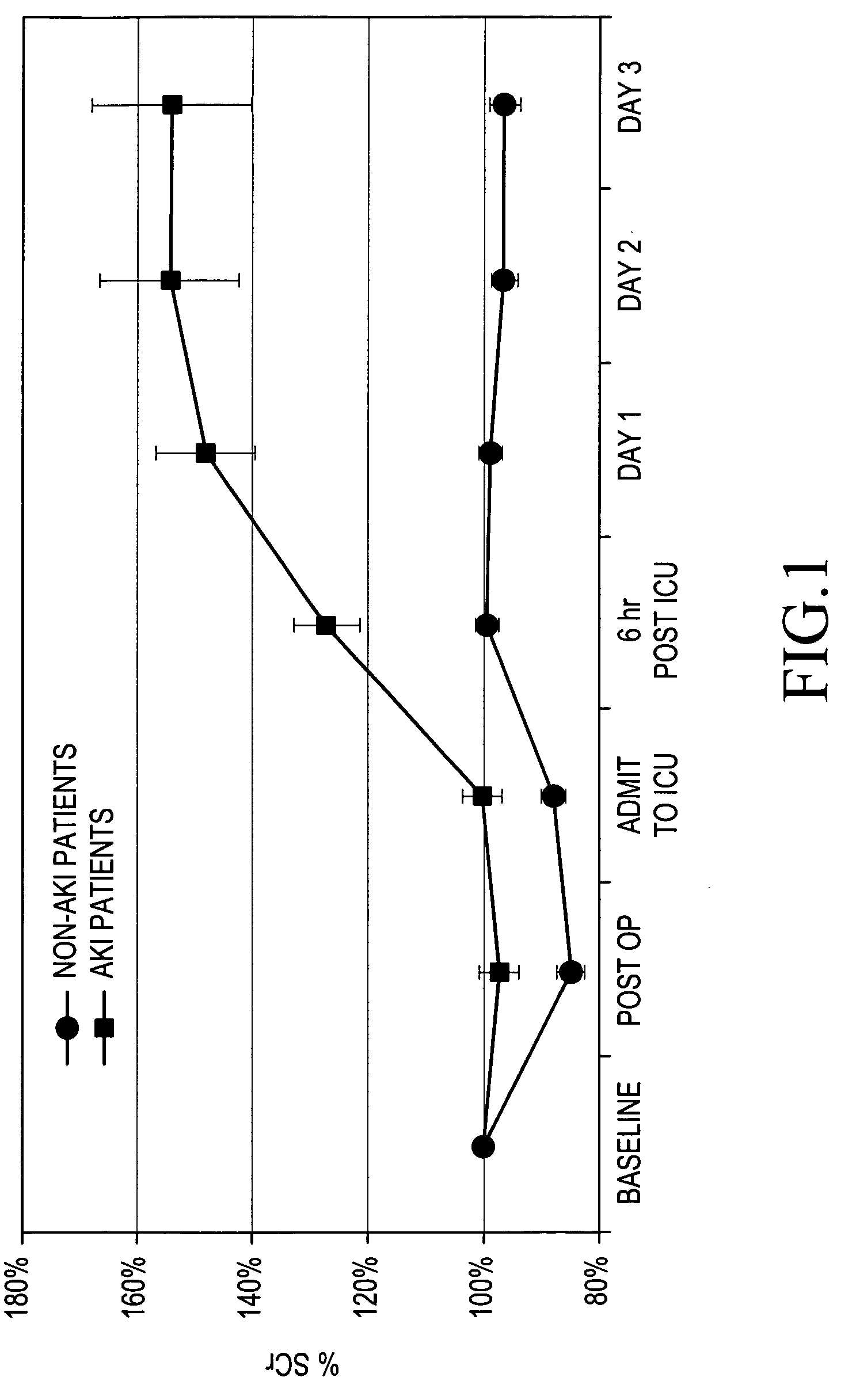
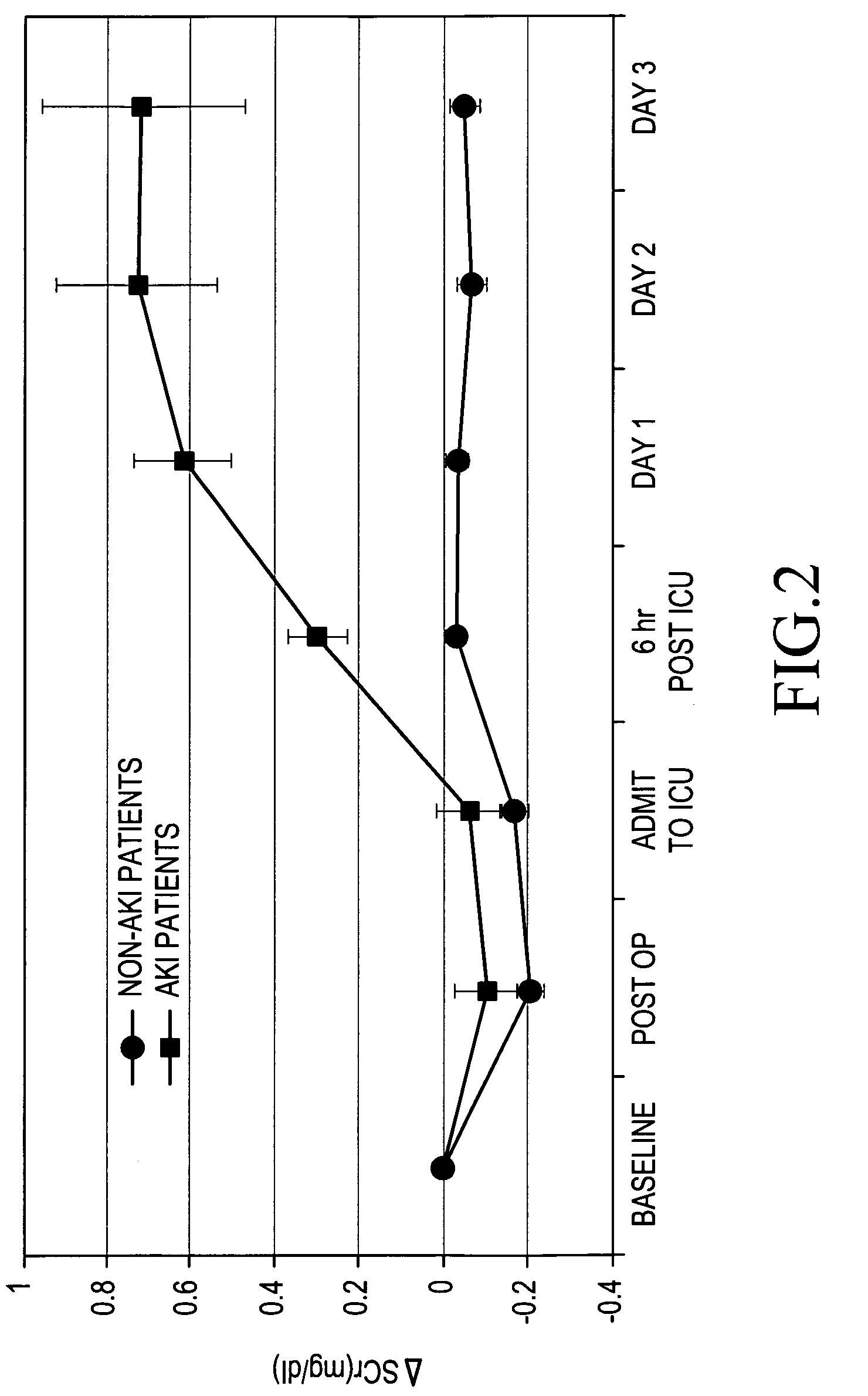
A method for the early identification and prediction of elevated blood creatinine levels resulting from a reduction in kidney function in a subject, comprises contacting a urine sample from the subject with a capture molecule for a biomarker specific for the distal region of the renal tubule and which biomarker is released from said region when there is damage to said region indicative and predictive of elevated blood creatinine levels resulting from a reduction in kidney function. The method can be used to detect Acute Kidney Injury (AKI) caused by many conditions or diseases or through the administration of drugs. The method can indicate and / or predict a reduction in kidney function significantly earlier than the current standard creatinine test. Methods for predicting a need for renal replacement therapy (RRT) are also disclosed.
Owner:ARGUTUS INTPROP
Bicarbonate-based solution in two parts for peritoneal dialysis or substitution in continuous renal replacement therapy
InactiveCN1336825ASo as not to damageAddressing the need to maintain the physical integrity of gas barrier materialsPharmaceutical containersPharmaceutical product form changePeritoneal dialysisMedicine
The present invention provides devices and methods for stabilizing bicarbonate-based solutions for peritoneal dialysis or hemofiltration. The bicarbonate-based solutions of the present invention are formulated and stored in at least two parts - an alkaline bicarbonate concentrate and an acidic concentrate. The alkaline bicarbonate concentrate is adjusted to have a pH of about 8.6 to 10.0. The acidic concentrate is adjusted to have a stable, acidic pH ranging from about 1.0 to 3.0. Upon mixing, although some variation in the pH of the mixed bicarbonate solution exists, the inventors have discovered that with the appropriate selection of the parameters of the concentrates, the pH of the mixed solution is always within an acceptable physiological range.
Owner:BAXTER INT INC
Prenatal enzyme replacement therapy
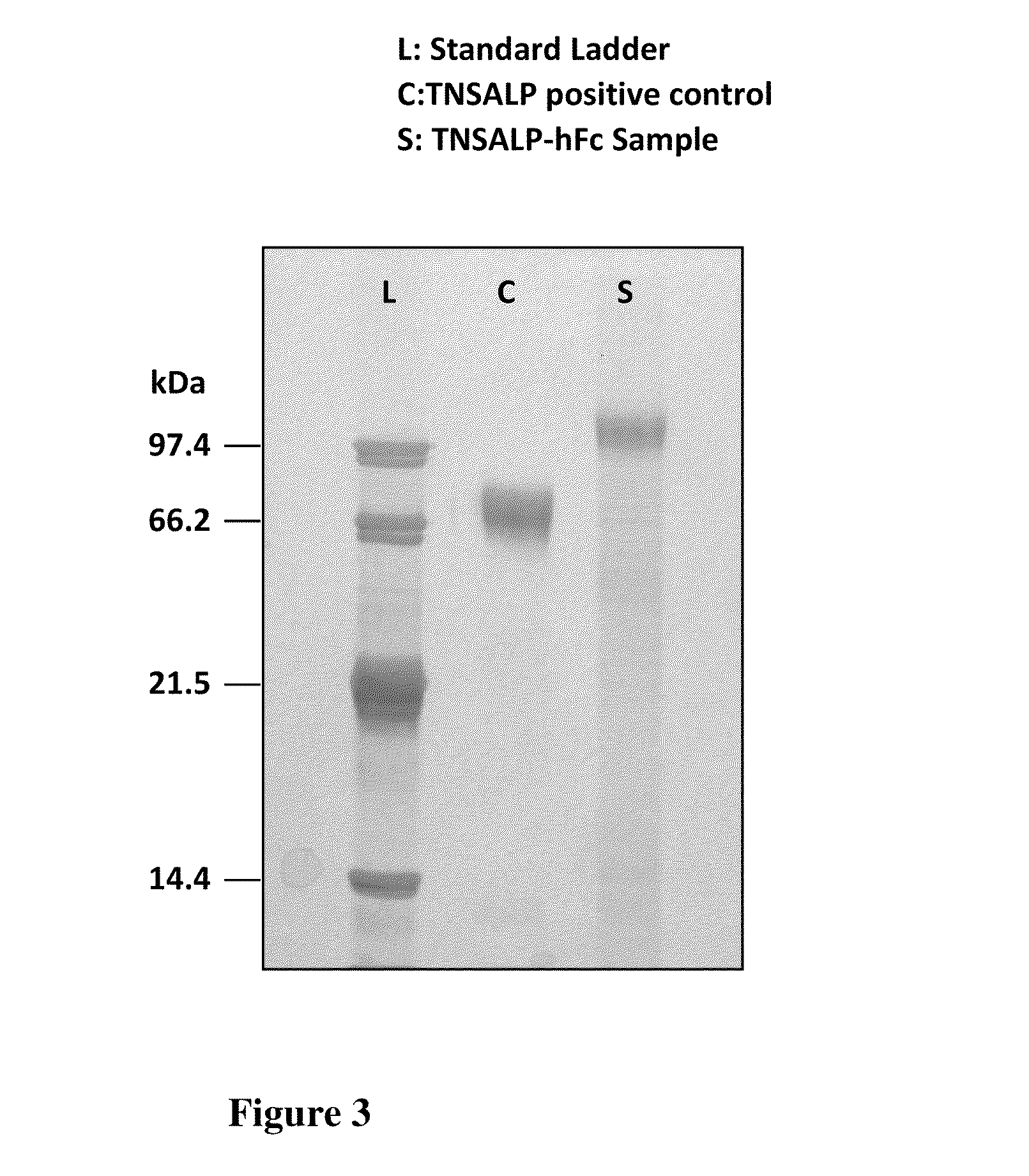
The invention contemplates transplacental enzyme replacement therapy (ERT) for deficiency of a polypeptide such as a tissue-nonspecific alkaline phosphatase (TNSALP) by administering a before-described pharmaceutical composition to a pregnant animal whose fetus or embryo is in need of such therapy. The fusion protein of such a composition comprises a water-soluble TNSALP portion, e.g., C-terminus-truncated TNSALP peptide-bonded to an IgG1 antibody Fc portion.The invention also contemplates a method for treating a metabolic disorder, such as HPP, in a fetus or embryo were a protein is administered to a pregnant mother. The fusion protein comprises a Fc fragment of an IgG1 antibody peptide-bonded to TNSALP. The protein crosses the placenta of the mother and enters the fetal blood stream. The protein is taken up into fetal tissue such that the TNSALP restores normal metabolic activity in the fetus.
Owner:SAINT LOUIS UNIVERSITY
Systems for utilizing the water content in fluid from a renal replacement therapy process
ActiveUS20170065762A1High Salt RejectionReduce energy consumptionHaemofiltrationUltrafiltrationHaemodialysis machineNephropathy
The present invention relates to systems, methods and uses for recycling at least a part of water lost during various renal replacement therapy processes, e.g. in the preparation of a fresh dialysate solution or fresh reconstitution fluid for kidney disease dialysis and hemofiltration by utilizing water from the spent fluids. The system of the invention is useful in hemodialysis and in peritoneal dialysis as well as in hemofiltration for reuse of water from filtrates and spent fluids. In addition, the system of the invention is useful in the development of a renal assist device or artificial kidney.
Owner:AQUAPORIN AS
Novel therapies for chronic renal failure
InactiveUS20050143304A1Preventing delaying needReducing necessary frequencyOrganic active ingredientsPeptide/protein ingredientsOsteogenic proteinsMorphogenesis
The present invention provides methods for the treatment, and pharmaceuticals for use in the treatment, of mammalian subjects in, or at risk of chronic renal failure, or at risk of a need for renal replacement therapy. The methods involve the administration of certain proteins of, or based upon, the osteogenic protein / bone morphogenetic protein (OP / BMP) family within the TGF-β superfamily of proteins.
Owner:MARIEL THERAPEUTICS
Method for improving regional citric acid anticoagulation
InactiveCN102309785AStable levelExtended service lifeOther blood circulation devicesAluminium/calcium/magnesium active ingredientsExtracorporeal circulationMathematical model
The invention belongs to the field of blood purification and relates to a method for improving the regional citric acid anticoagulation, in particular to the method for improving the regional citric acid anticoagulation based on a mathematical model. The method comprises two stages of quantificational calculation of calcium supplement amounts for citric acid anticoagulation, namely, in the first stage, quantificationally calculating the sum of in-vitro circulation clearing amount and in-vivo accumulation amount of calcium, and in the second stage, quantificationally calculating the in-vitro circulation clearing amount of the calcium only required to be supplemented. By the method, the infusion speeds of the citric acid and the calcium during regional citric acid anticoagulation during continuous renal replacement therapy (CRRT) can be quantificationally calculated, and the application range of the regional citric acid anticoagulation is greatly expanded. Corresponding application and verification are performed on critical patients requiring regional citric acid anticoagulation CRRT treatment. A result shows that the method is safe and effective and can stabilize in-vivo and in-vitro accumulation ionized calcium level in a proper range; the service life of a filter is prolonged; and the monitoring times are obviously reduced.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Dual-ventricle pump cartridge, pump and method of use in a wearable continuous renal replacement therapy device
InactiveUS7854718B2Semi-permeable membranesSolvent extractionBiomedical engineeringRenal replacement therapy
Owner:FRESENIUS MEDICAL CARE HLDG INC
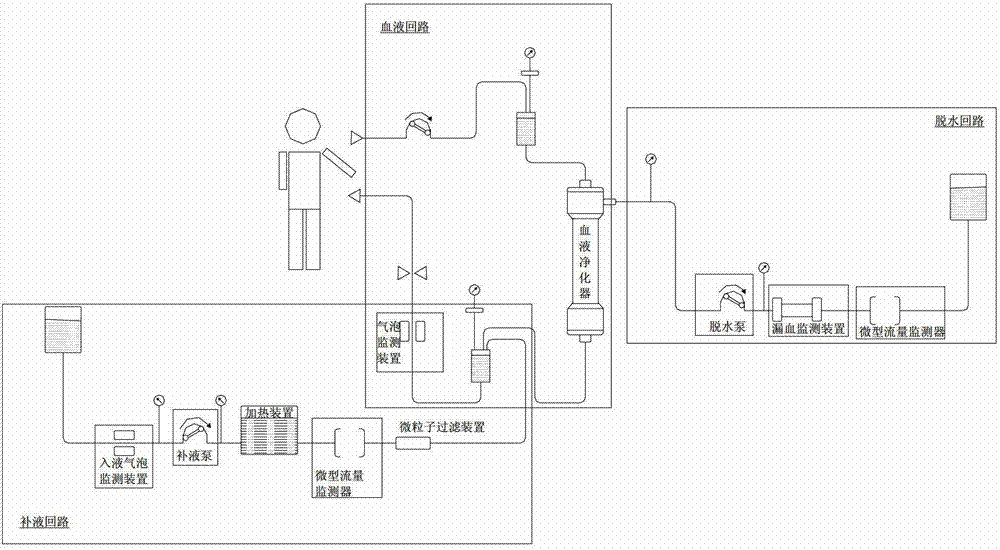
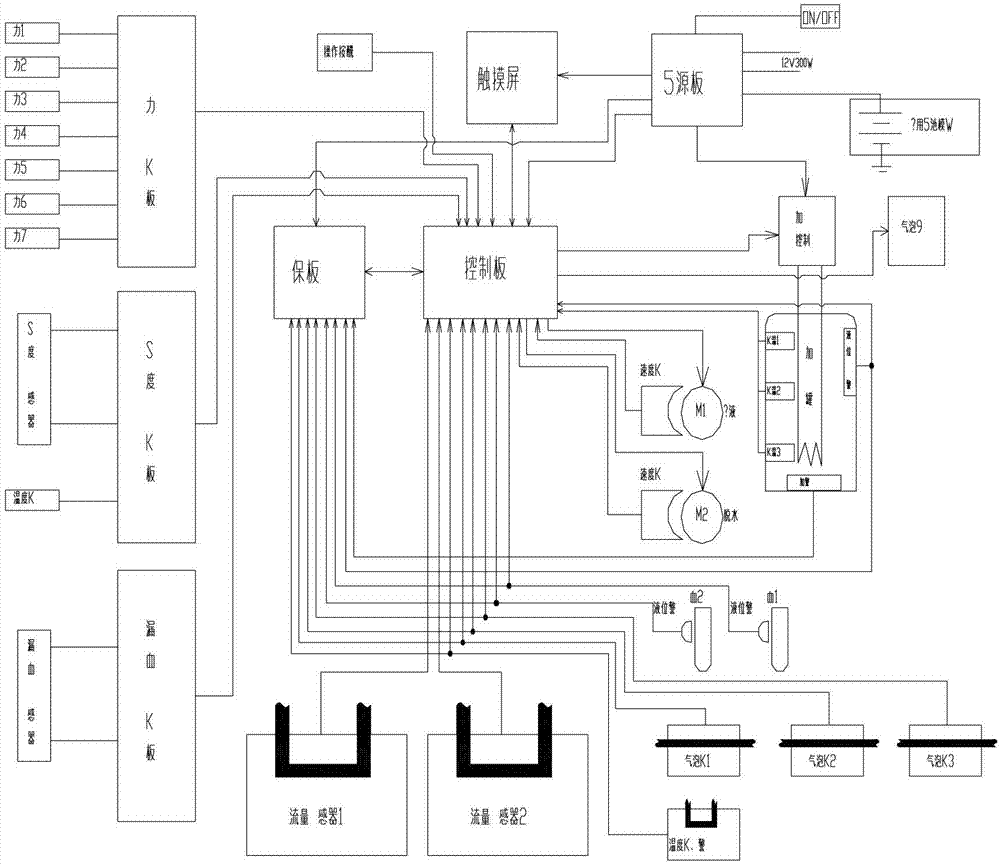
Device for continuous renal replacement therapy (CRRT)
InactiveCN102895711AGuaranteed to be constantEnsure accurateDialysis systemsBlood pumpDialysis fluid
The invention relates to a device for continuous renal replacement therapy (CRRT). The device comprises a blood loop, a blood purifier, a blood pump, a dialysate loop, a dialysate pump, a controller and a flow monitor; wherein the controller is used for adjusting a solution supply pump or a dewatering pump or uttering an alarm according to results of the flow monitor in monitoring of the dialysate loop. The problem that the accuracy of a CRRT machine in a volumetric metering technology is affected by multiple factors can be solved, and the constant and precise liquid flow can be ensured in the dialysate loop.
Owner:王雪 +2
Intelligent heparin pushing injector used for CRRT (continuous renal replacement therapy) machine
InactiveCN105854101AInput speed control is preciseInput speed control stableOther blood circulation devicesBall screw driveEngineering
The invention relates to structural design of parts of a CRRT (continuous renal replacement therapy) machine, and in particular relates to an intelligent heparin pushing injector used for the CRRT machine. The intelligent heparin pushing injector comprises an injection syringe, a controller, a motor, a ball screw, an installation base plate, and a pushing base with an inner cavity, wherein a support base with a first groove in the bottom is fixedly arranged on the top of the installation base plate, and a through hole communicated with the first groove is formed in the bottom of the installation base plate; a fixed base is fixedly arranged on the top of the installation base plate, the pushing base is positioned between the support base and the fixed base, a nut of the ball screw is mounted in the inner cavity of the pushing base; a tube of the injection syringe is arranged on the top of the support base, the tail end of a handle of the injection syringe is connected with the pushing base, the output end of the motor extends towards the through hole, and is connected with the end, extending into the first groove of the support base, of a screw rod of the ball screw through a transmission mechanism, the controller is connected with the motor through a circuit to control the operation of the motor, and the nut of the ball screw drives the pushing base to push the tail end of the handle of the injection syringe to carry out pushing injection. The intelligent heparin pushing injector has the beneficial effects that the input speed is accurate to control and is stable, the operation is convenient, and the degree of automation is high.
Owner:CHONGQING AOKLAND MEDICAL EQUIP RES
Portable equipment for continuous renal replacement therapy and blood purification method
InactiveCN101347644AGuaranteed measurement accuracyAvoid uneven temperatureDialysis systemsEngineeringBlood purification
The invention provides a portable and continuous kidney replacing therapy apparatus and a method for purifying blood. The therapy apparatus comprises a blood channel, a displacement liquid channel and an ultrafiltrate channel. The apparatus also comprises a first displacement pump, a second displacement pump and a controller, wherein, the first displacement pump is arranged in the displacement liquid channel, and liquid of fixed volume can be output per revolution; the second displacement pump is arranged in the ultrafiltrate channel, and liquid of fixed volume can be output per revolution; the controller calculates the input flow of the displacement liquid channel and the output flow of the ultrafiltrate channel in a volume metering method, controls the rotational speed of the first displacement pump and the second displacement pump and maintains the balance of the input flow and the output flow. The therapy apparatus of the invention can be used in the environments of turbulence, rocking and vibration, and the like, such as on the sea, and can be suitable for corrosion of salt-fog on the sea and a plurality of external power supplies.
Owner:BEIJING MEDER MEDICAL SCI & TECH
Method for predicting heparin dosage in continuous renal replacement therapy process
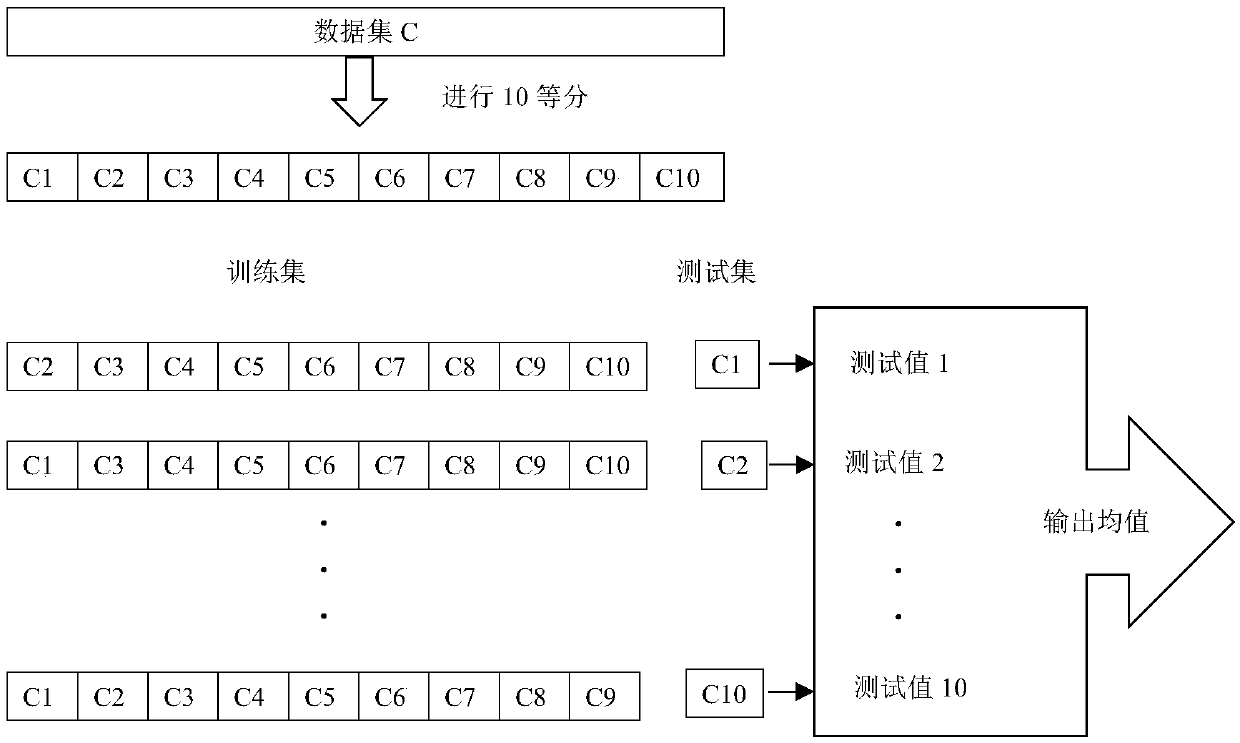
ActiveCN108831556AEconomical and reliableImprove the quality of surgeryMedical data miningMedical automated diagnosisData setTest set
The invention relates to the technical field of machine learning and to a method for predicting a heparin dosage in a continuous renal replacement therapy (CRPT) process, comprising the following steps: (1) installing relevant software and libraries; (2) analyzing and understanding the relevant information of a data set collected by a hospital; (3) preprocessing data; (4) extracting features, (5)determining a training set and a test set and processing unbalanced data; (6) predicting and evaluating a model; and (7) processing the abnormal values of predicted values. The method predicts the heparin dosage in the CRRT process by using a machine learning algorithm such as a gradient boosting regression model, and can give a reasonable and reliable heparin dosage reference value in the CRRT process by further processing the predicted value according to the trained model, thereby assisting the doctors, improving surgery quality, and achieving good economical efficiency and reliability.
Owner:DALIAN UNIV OF TECH
Dialysis optimizing method
InactiveUS20140102983A1Stop the flowRapid determinationSemi-permeable membranesSolvent extractionPower flowHaemodialysis machine
A method as well as an apparatus is disclosed for determining the efficiency of a currently performed kidney replacement therapy on the dialysis side making use of a dialysis machine which in a first step is operated in a hemodialysis or hemodiafiltration process and in a second step is sequentially changed over to a hemofiltration process or is changed over to a sequential mode in which merely a flow from the blood compartment via the semipermeable membrane to the dialysis fluid compartment of the dialyser is generated in which, according to a third step, a sensor for determining or measuring the concentration at least of uremic toxins in the saturated dialysate or ultra-filtrate which is connected downstream of a dialyser on the dialysis side outputs corresponding measuring signals that are representative of the current concentration at least of uremic toxins in the blood to a calculation or determination unit.
Owner:B BRAUN AVITUM
Method of reducing titers of antibodies specific for a therapeutic agent
The present invention relates, in general, to a method of treating patients undergoing enzyme replacement therapy (ERT) or other therapy involving the administration of a proteinaceous therapeutic agent as well gene replacement therapy with non-viral or viral vectors, or other therapeutic modality or modalities, used alone or in combination, which involve the administration of exogenous substances for potential therapeutic benefit, including, but not limited to DNA vaccines, siRNA, splice-site switching oligomers (SSOs) as well as RNA-based nanoparticles (RNPs) and nanovaccines. The invention further relates to compounds and compositions suitable for use in such methods.
Owner:SYNPAC NORTH CAROLINA INC
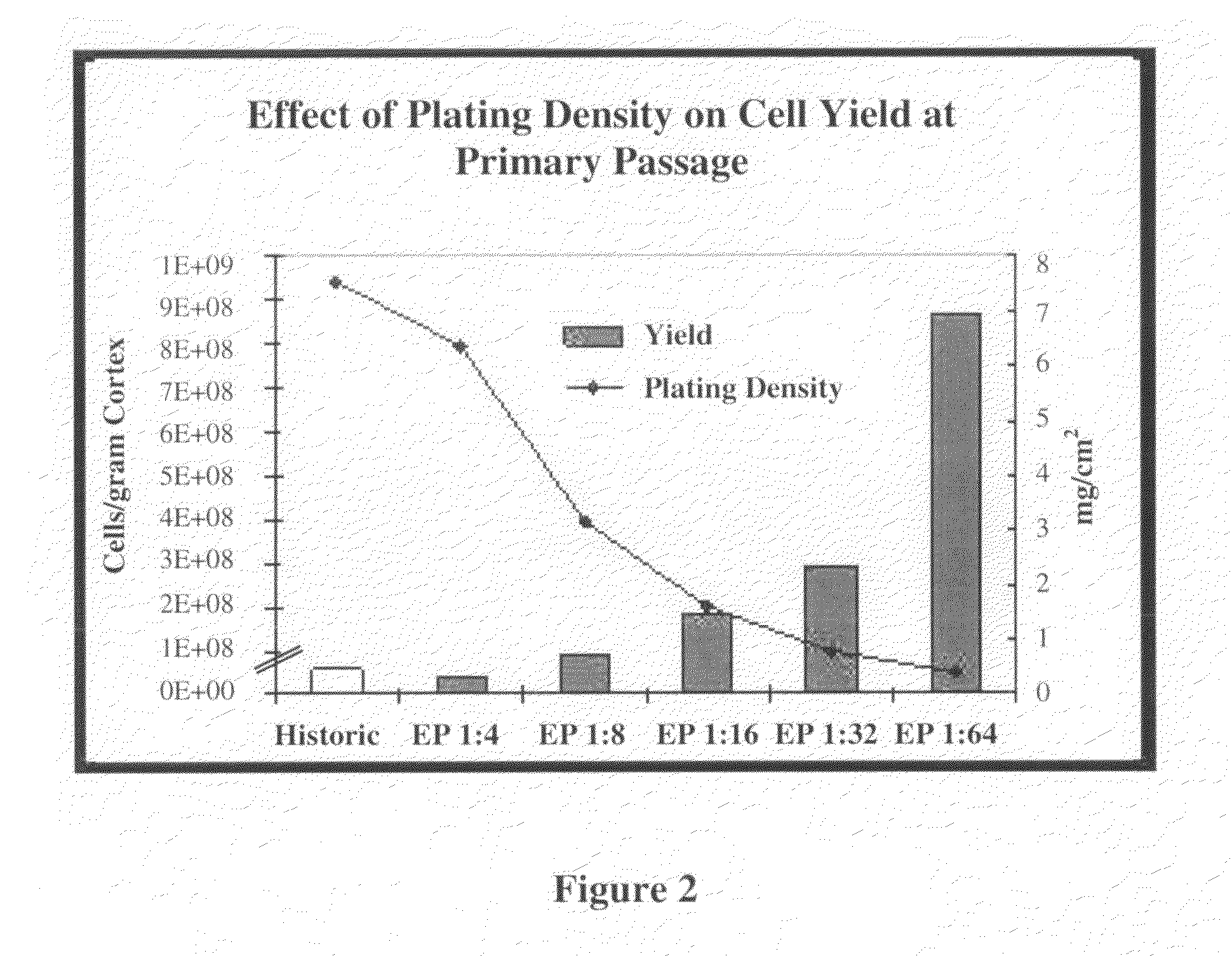
Methods for Enhanced Propagation of Cells
ActiveUS20100136687A1Enhancing kidney cell expansionQuality improvementCell culture active agentsUrinary disorderEnd stage renal diseaseMulti organ
The present invention relates generally to methods for the isolation and propagation of cells. For example, embodiments of the present invention relate to isolation and propagation methods for the manufacture of a large number of cells for use, for example, in biotherapeutic devices, such as devices for renal replacement therapy for the treatment of acute renal failure (ARF), acute tubular necrosis (ATN), multi-organ failure (MOF), sepsis, cardiorenal syndrome (CRS) and end-stage renal disease (ESRD).
Owner:SEASTAR MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com