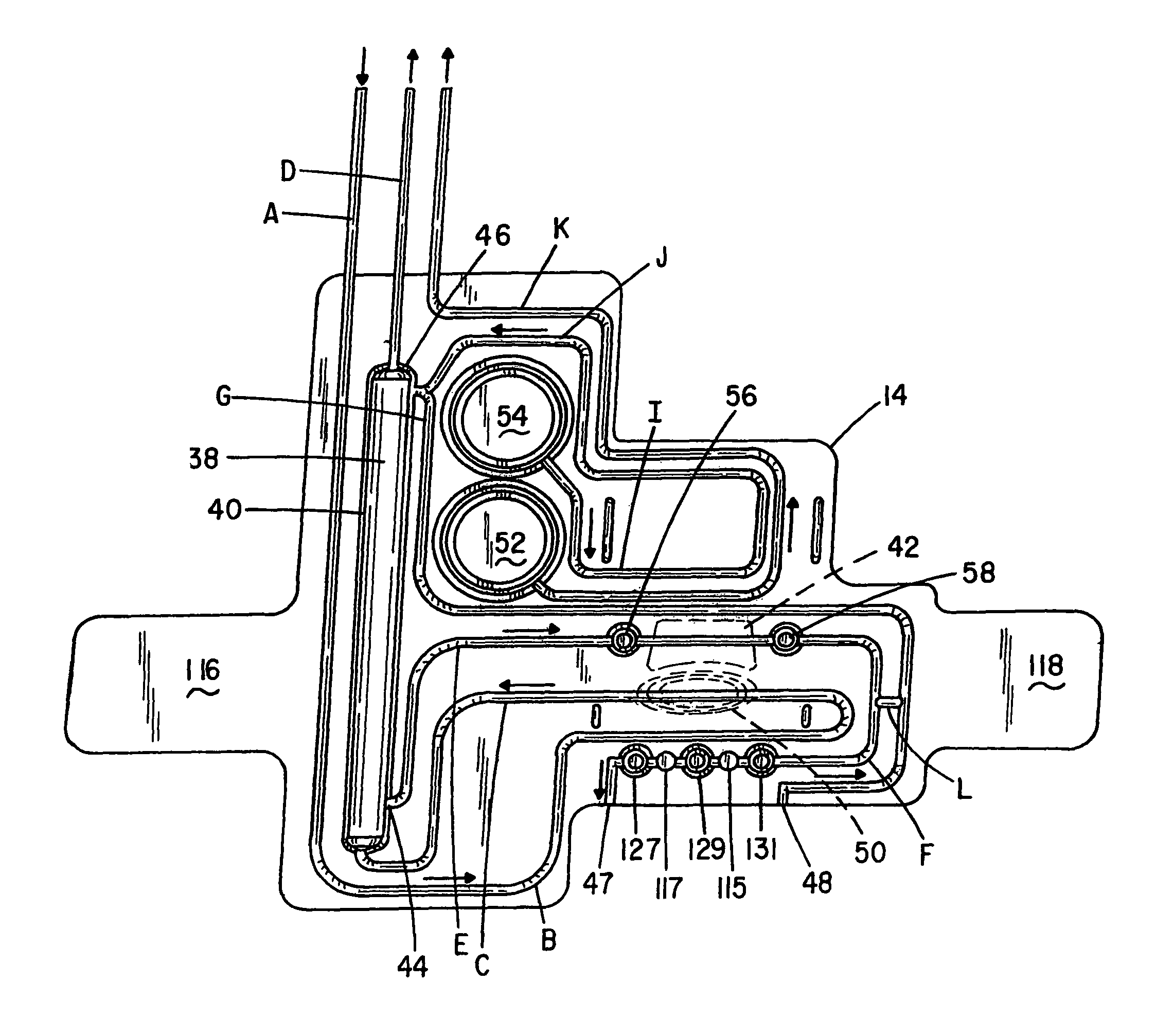
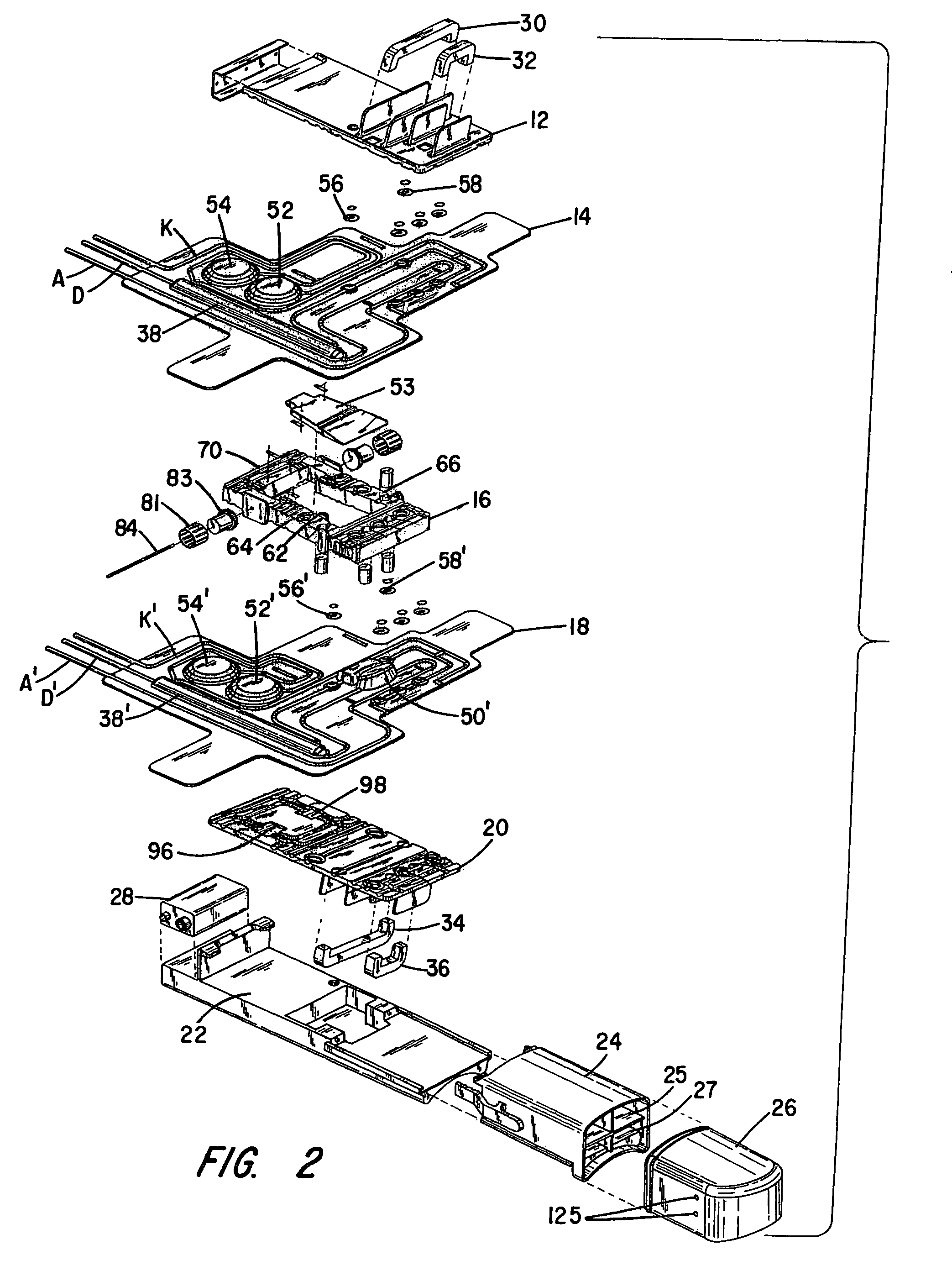
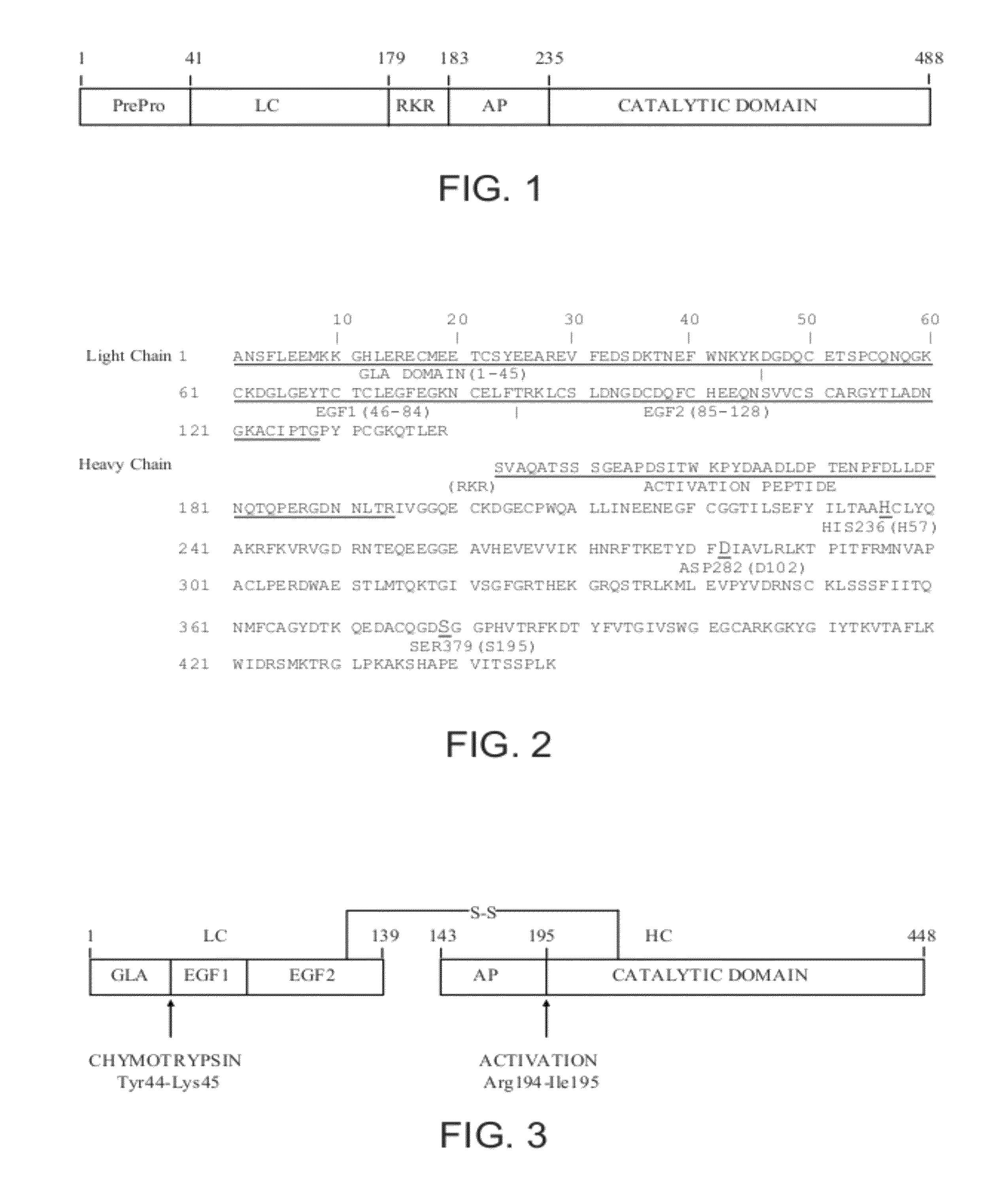
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
880 results about "Anticoagulant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, and dialysis equipment. They can also be used as rodenticides.
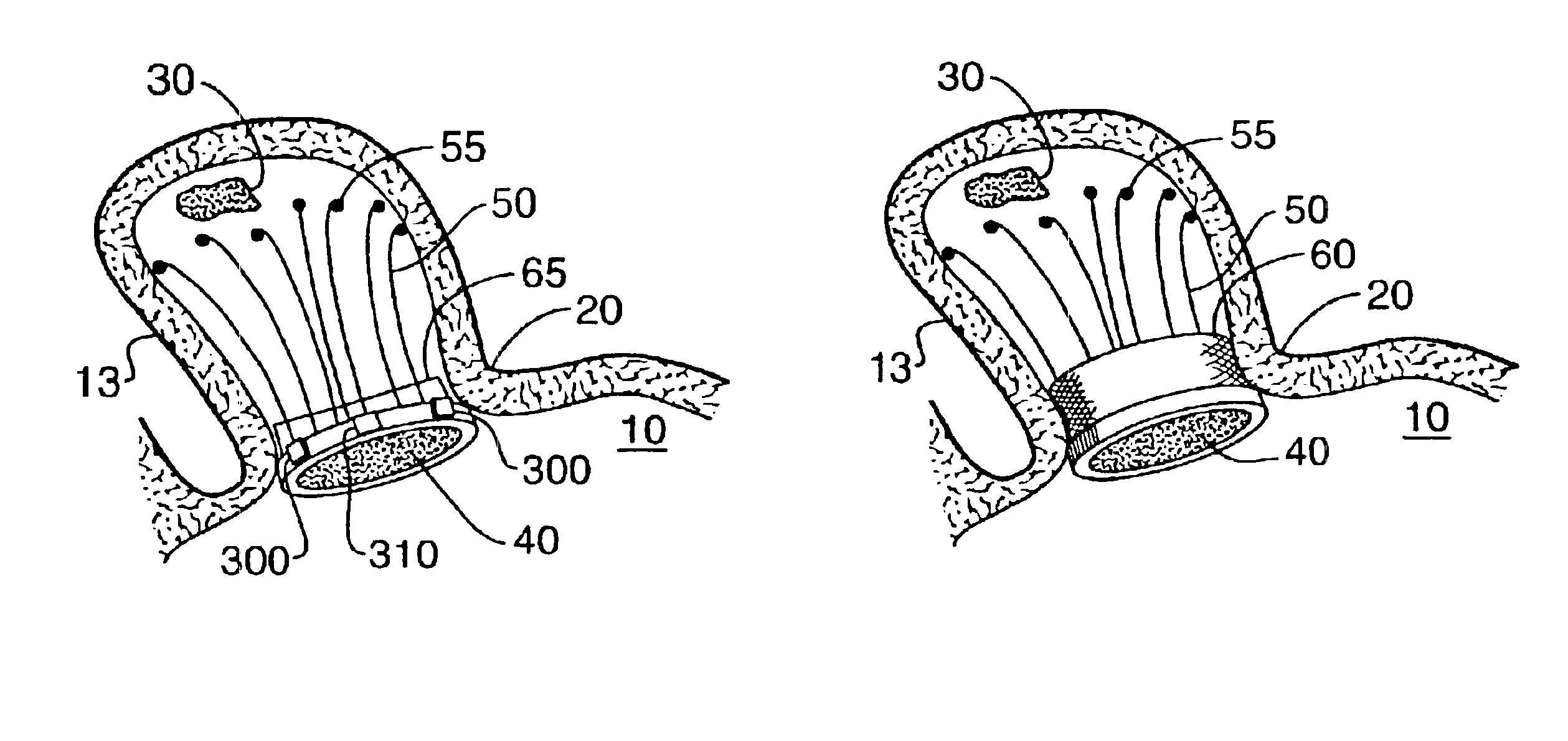
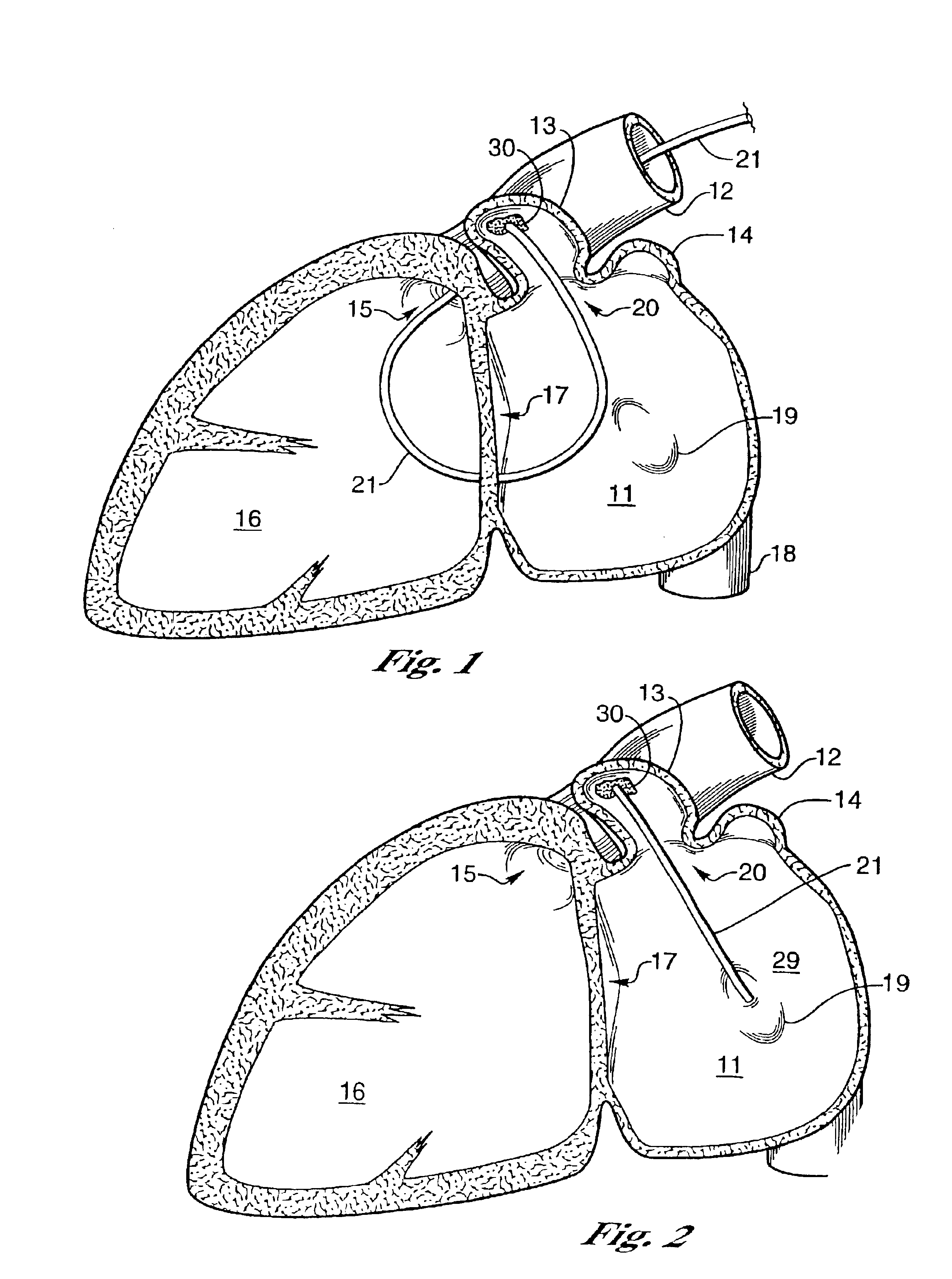
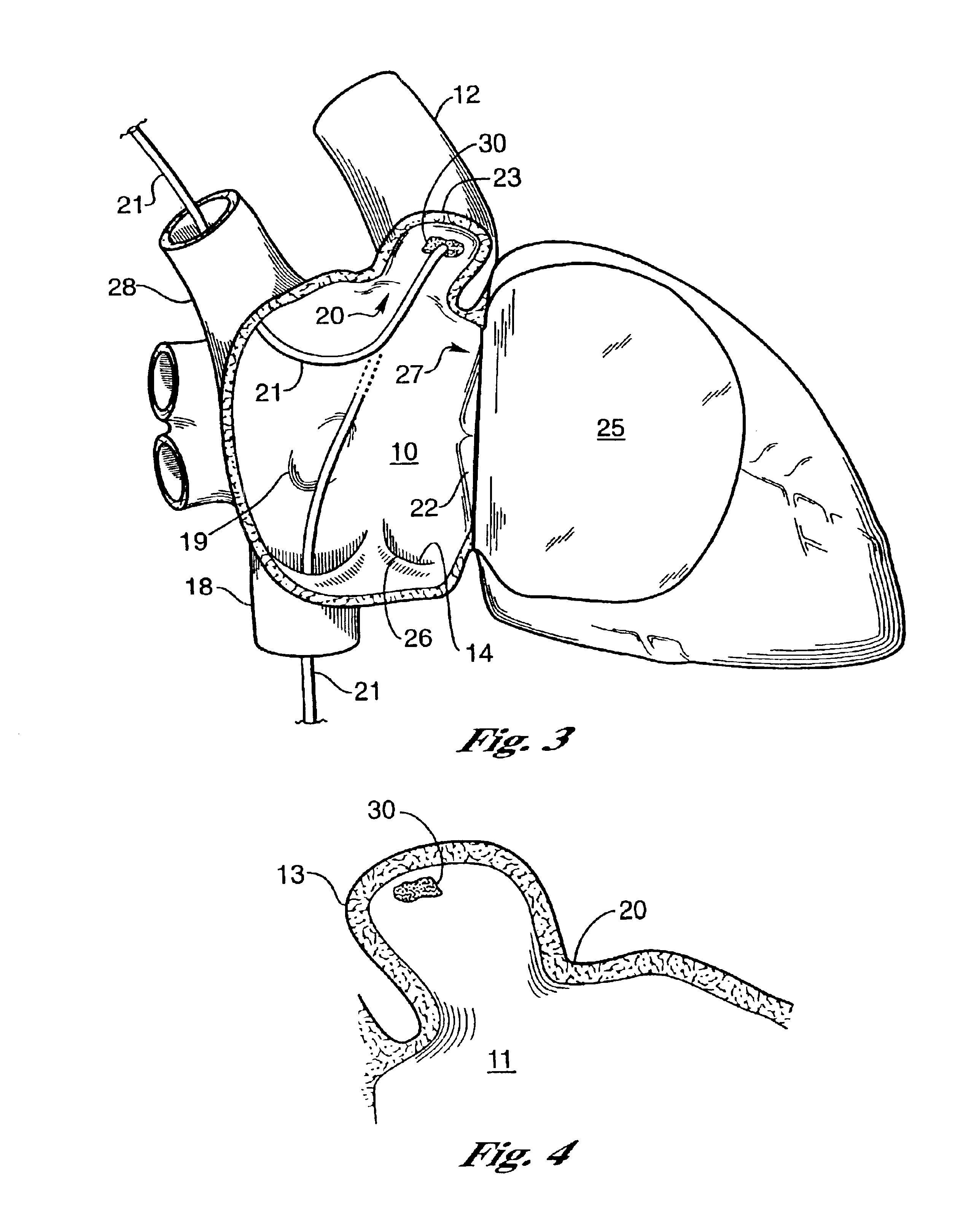
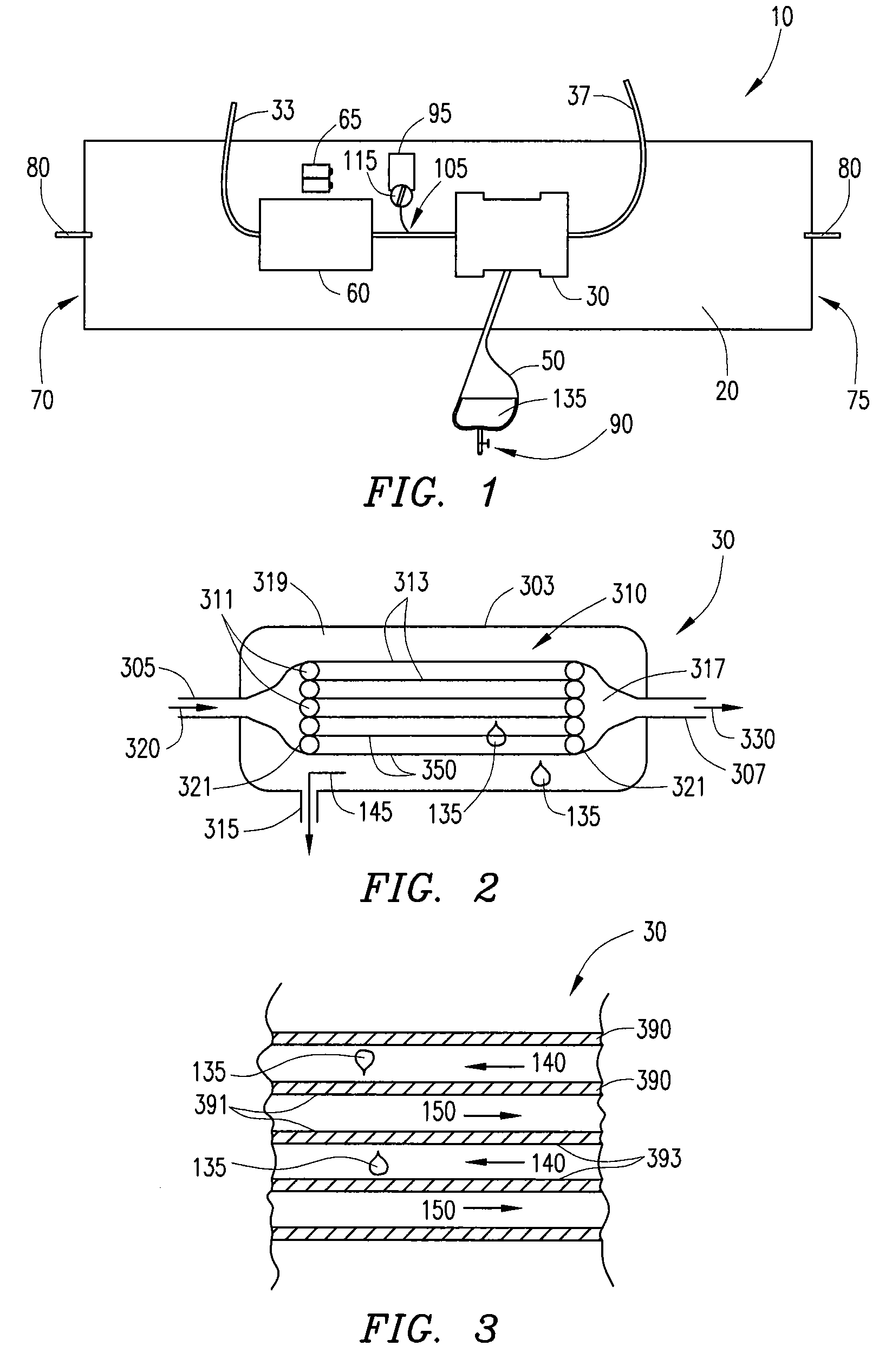
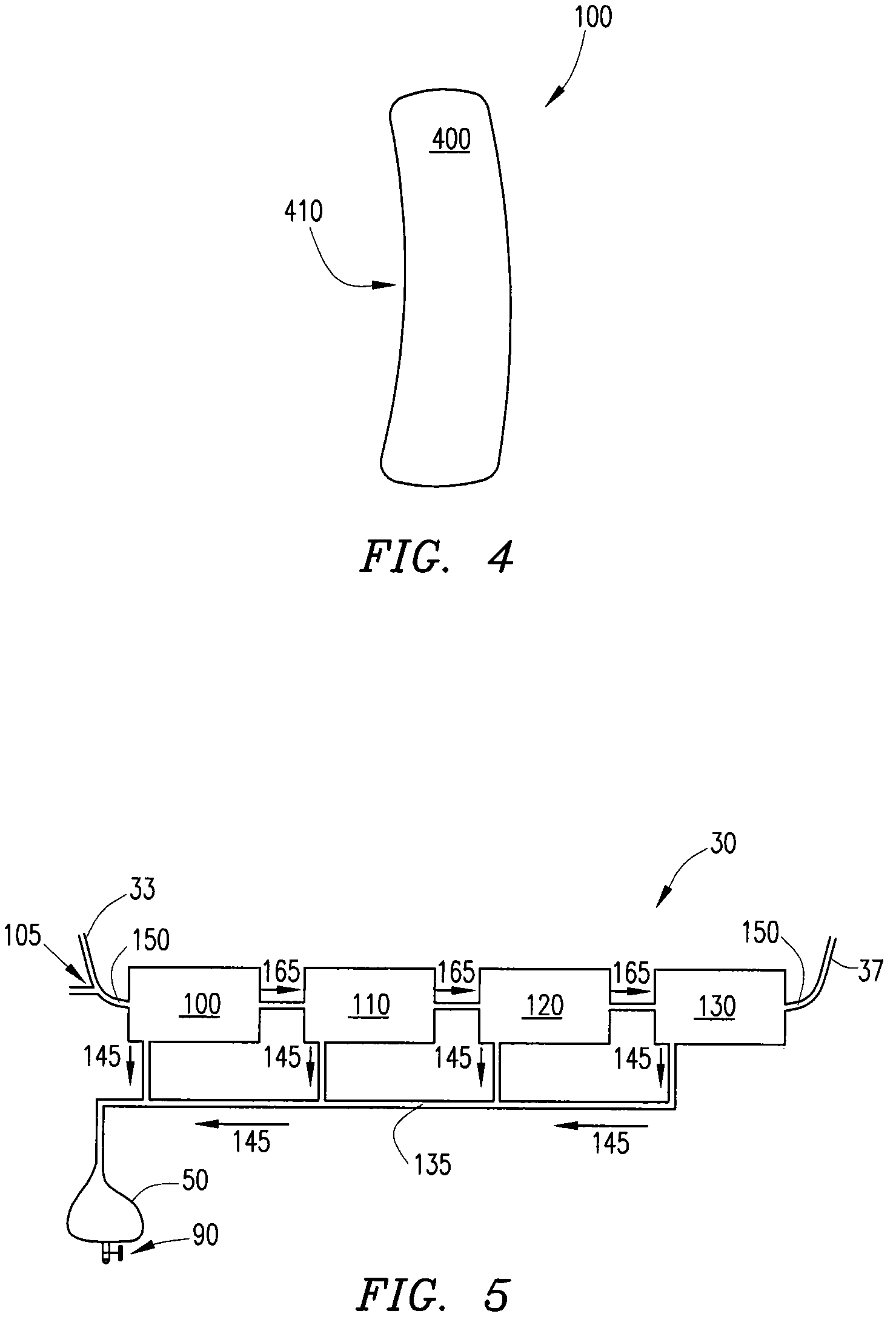
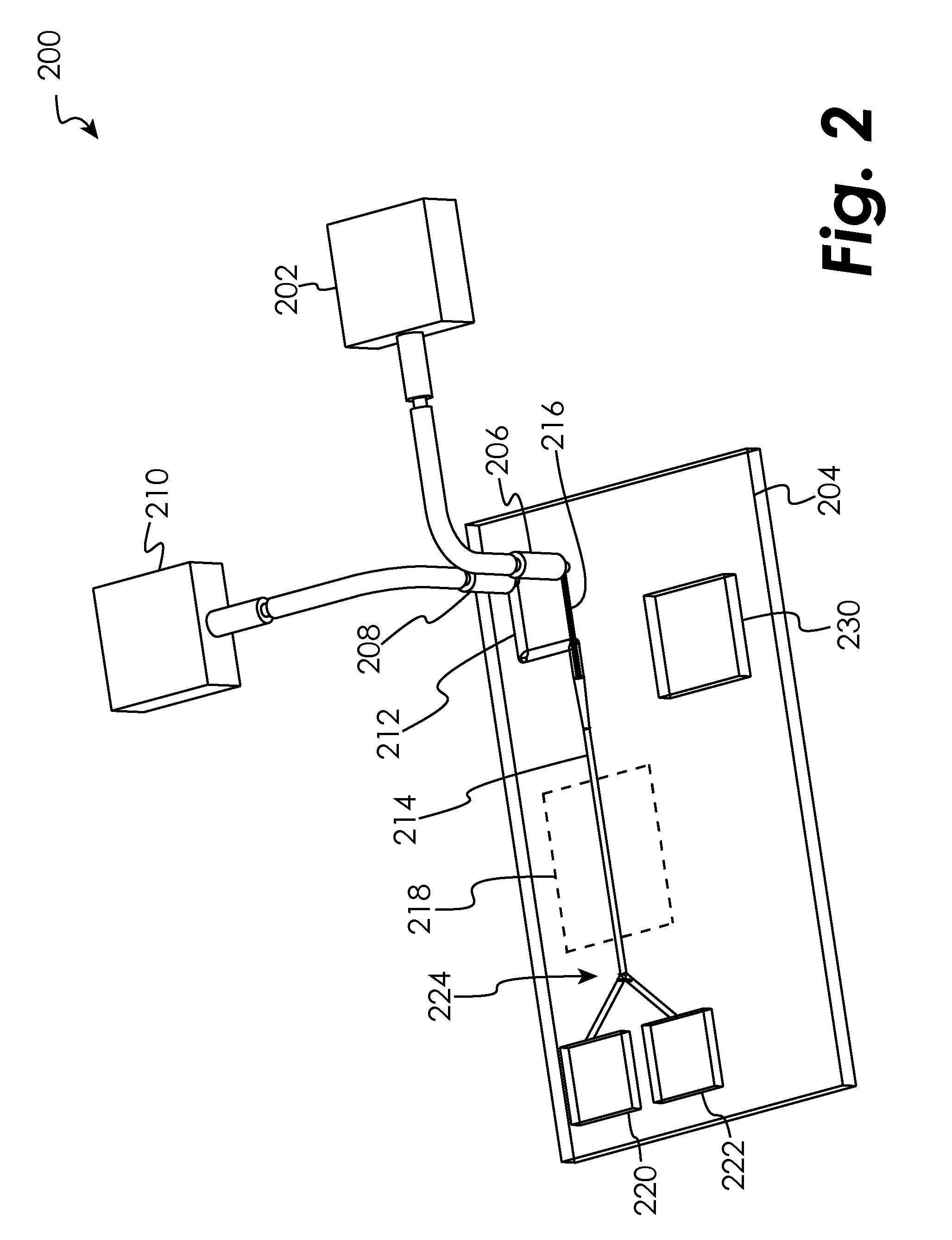
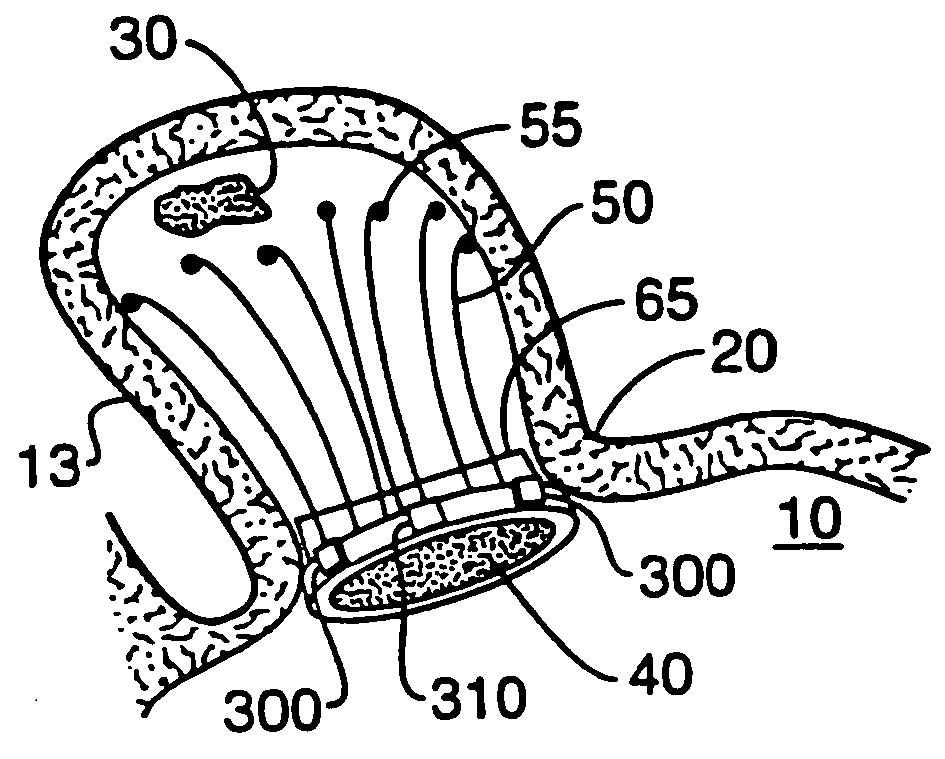
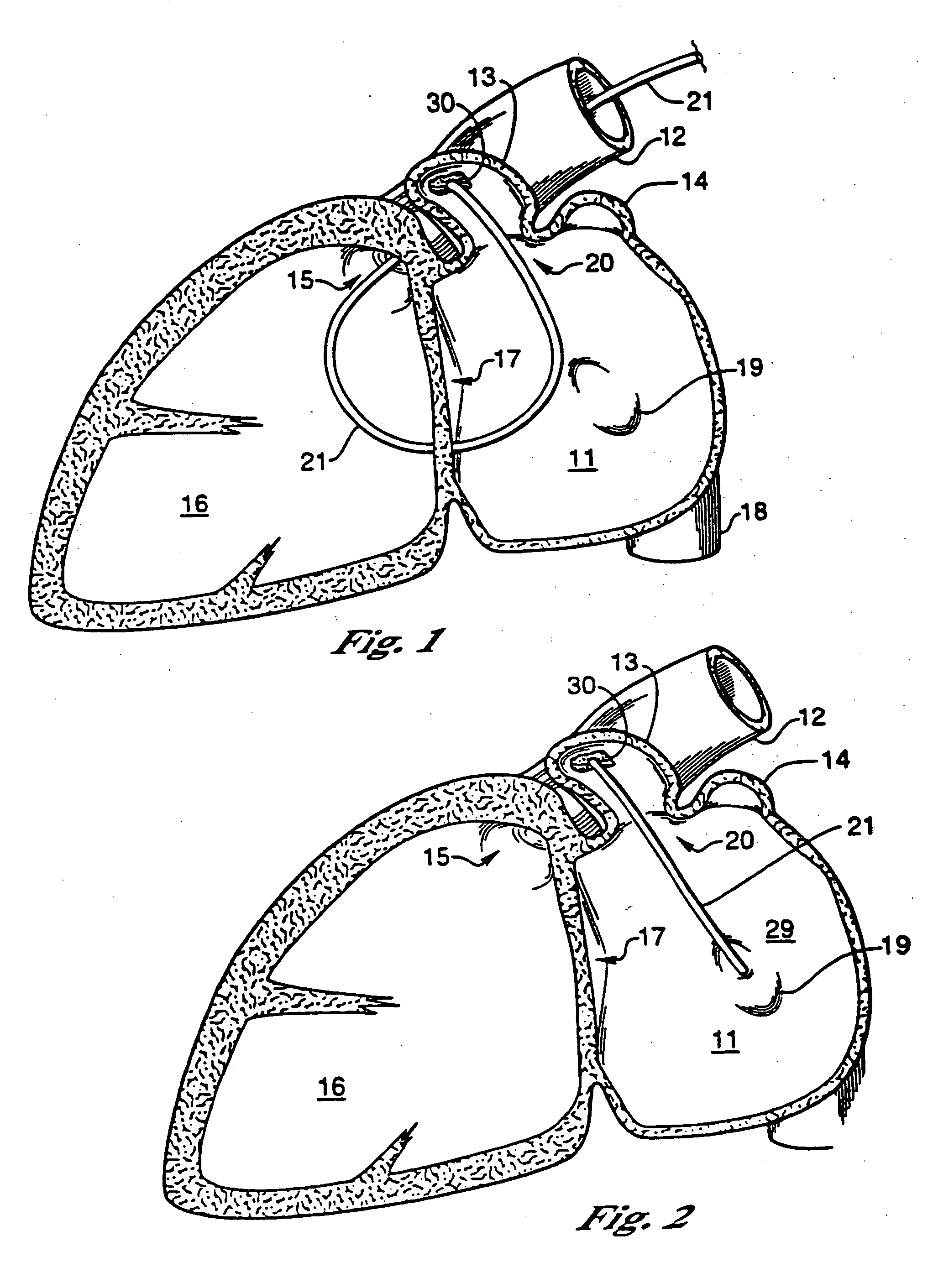
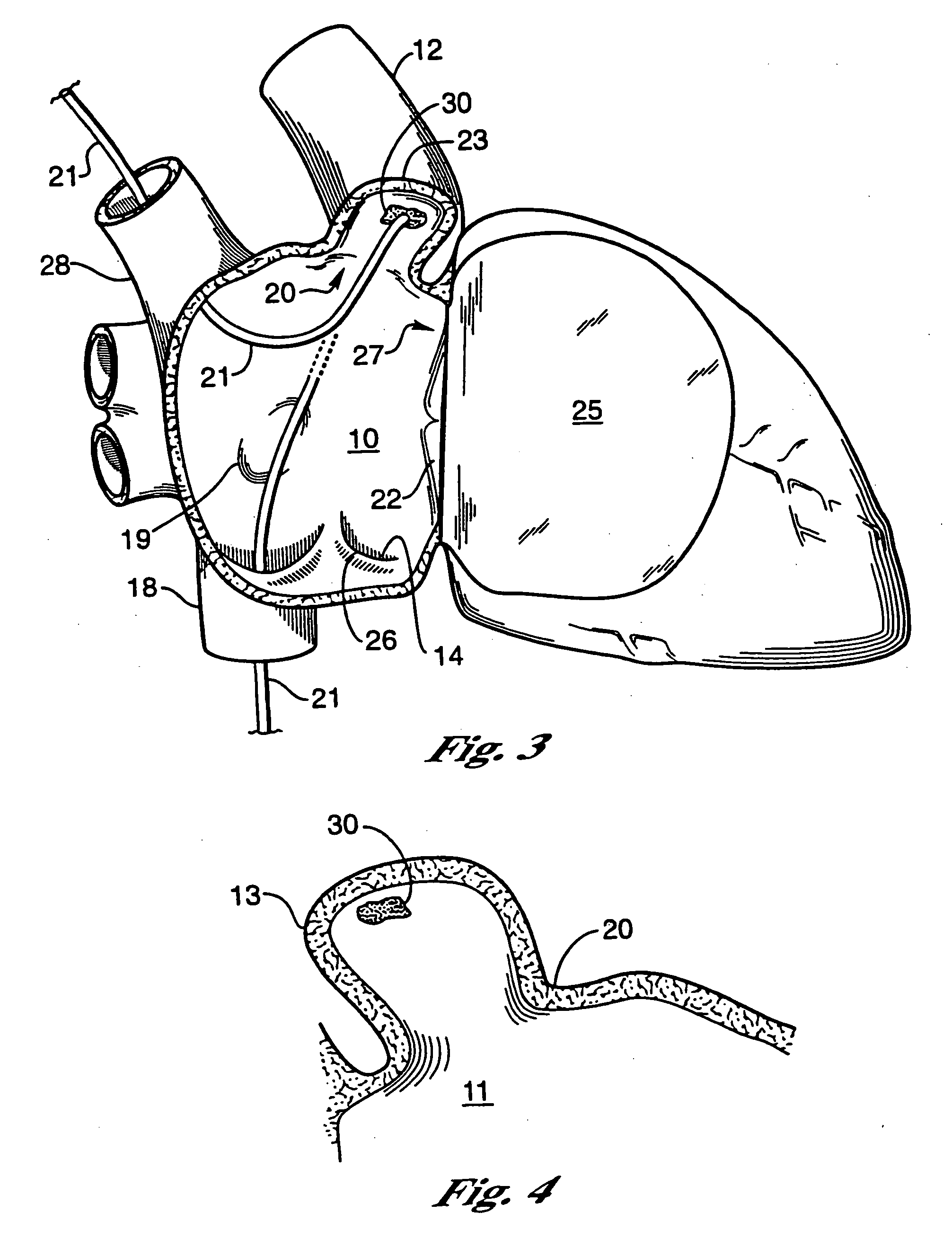
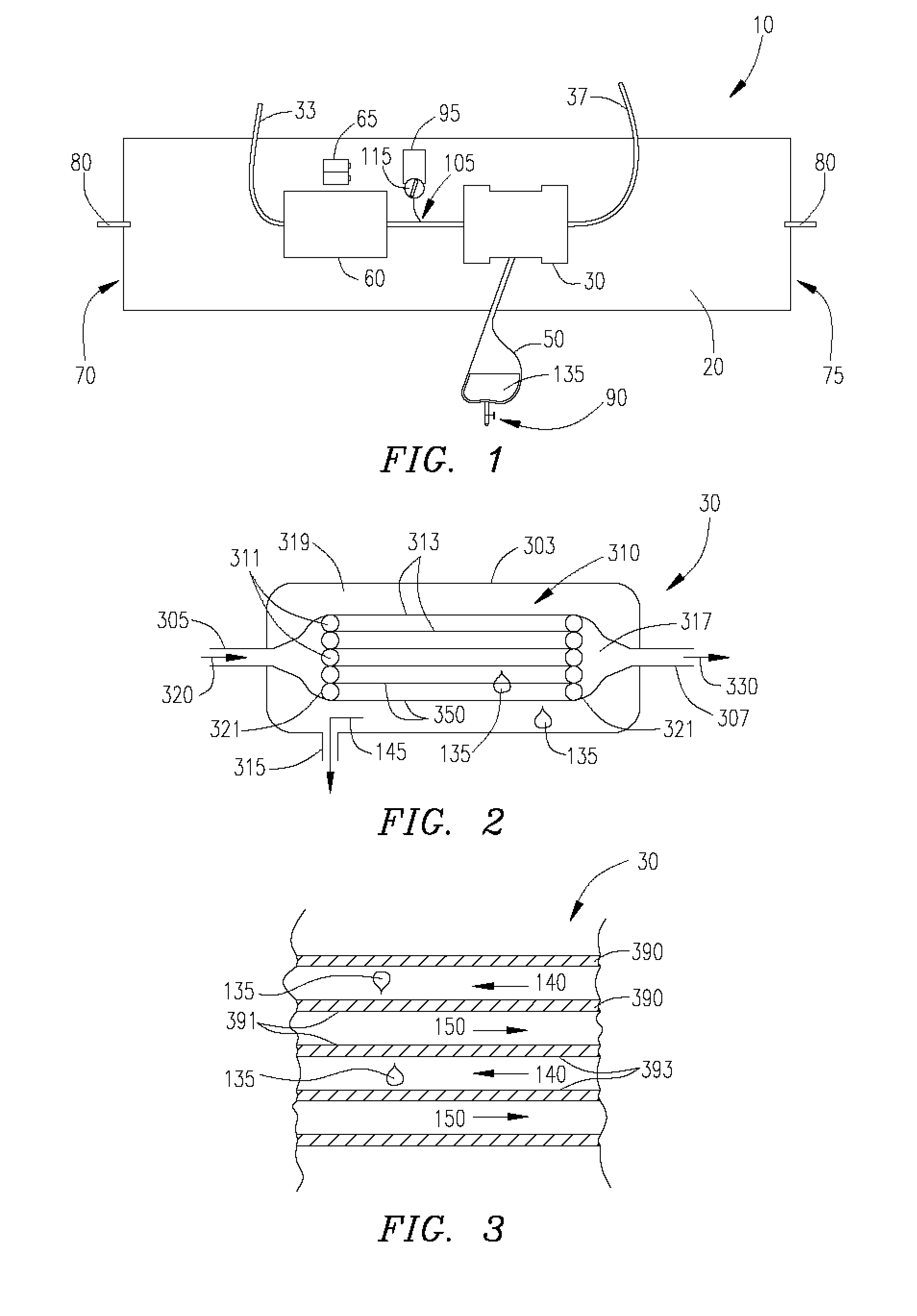
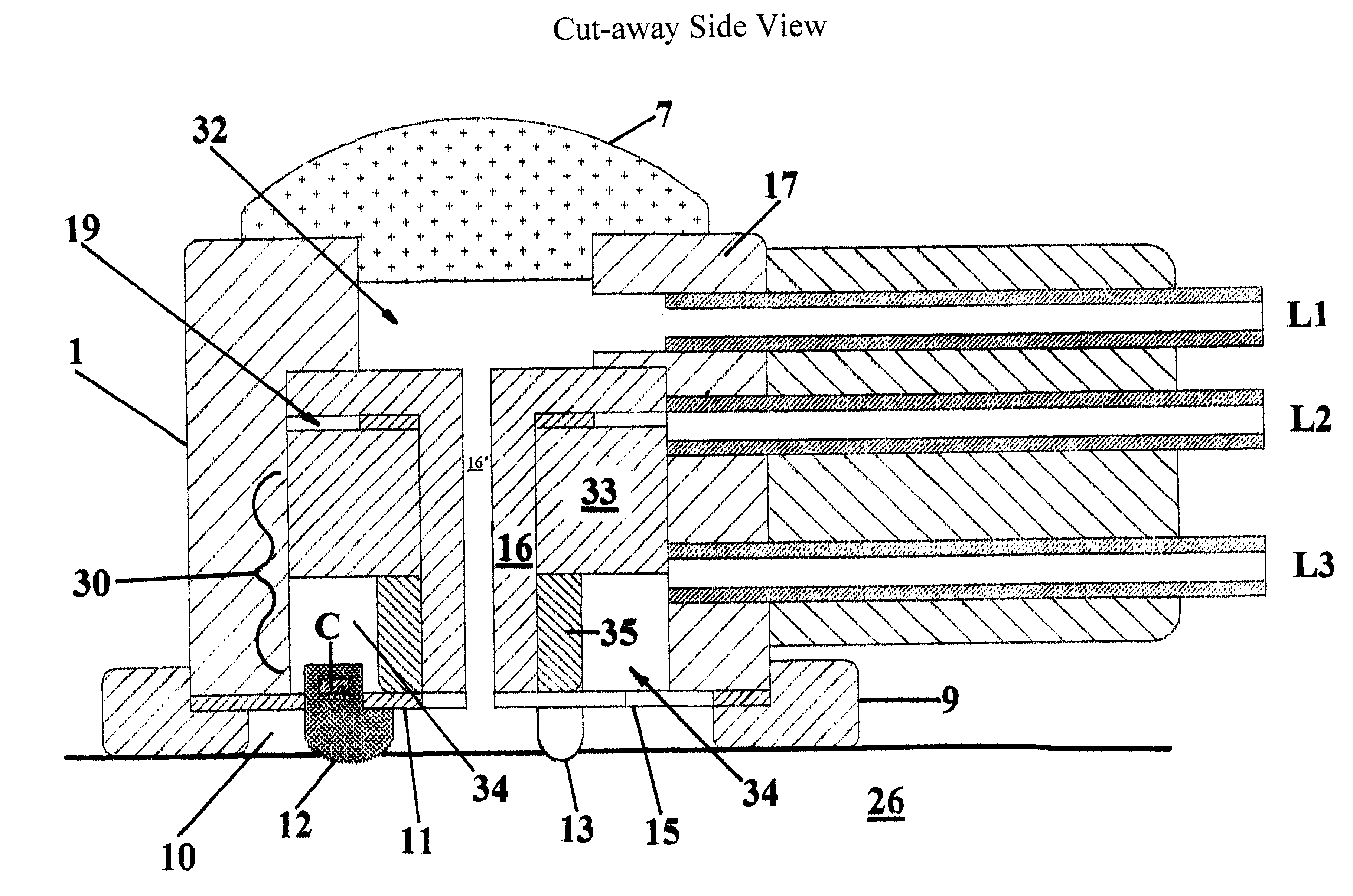
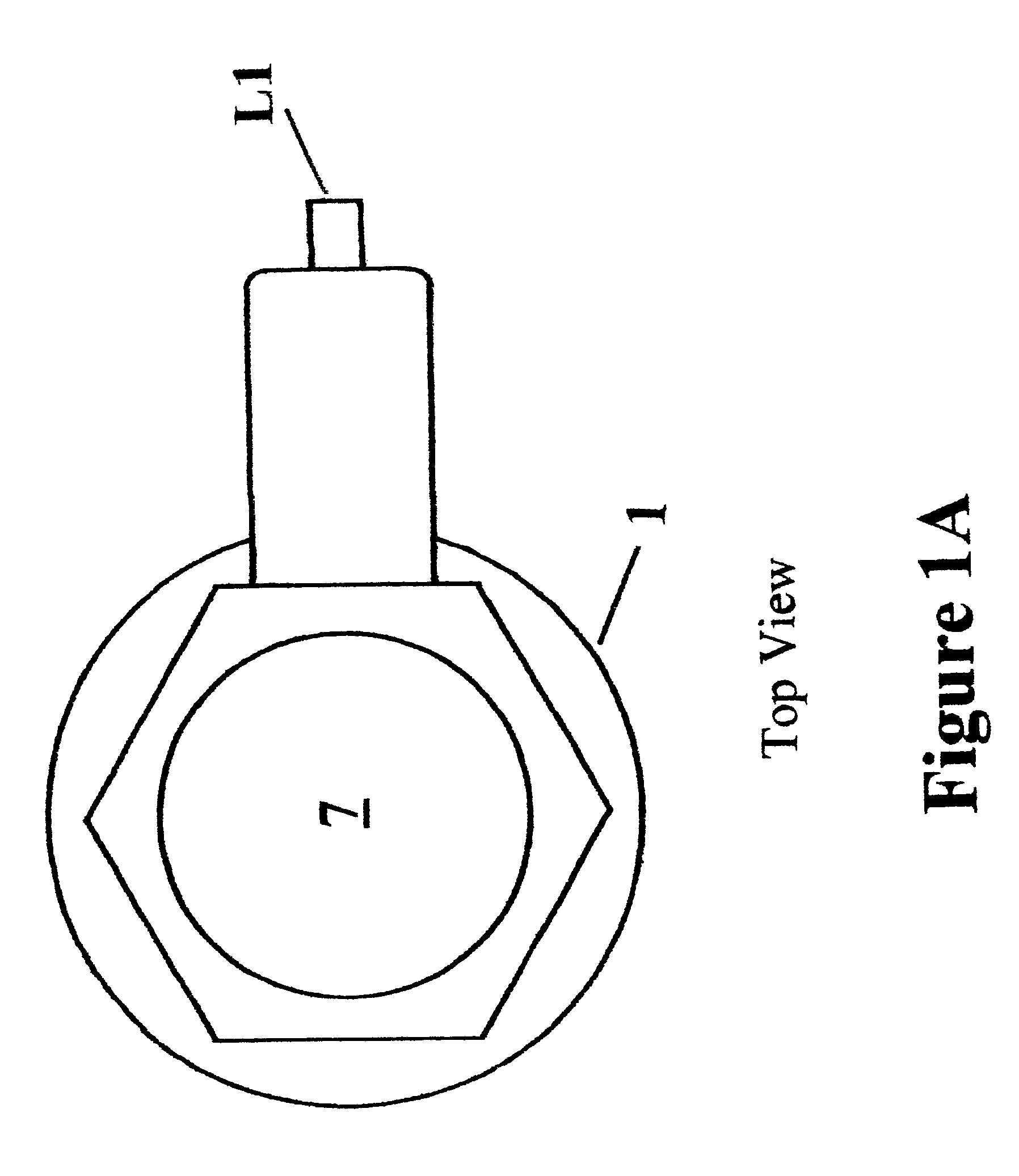
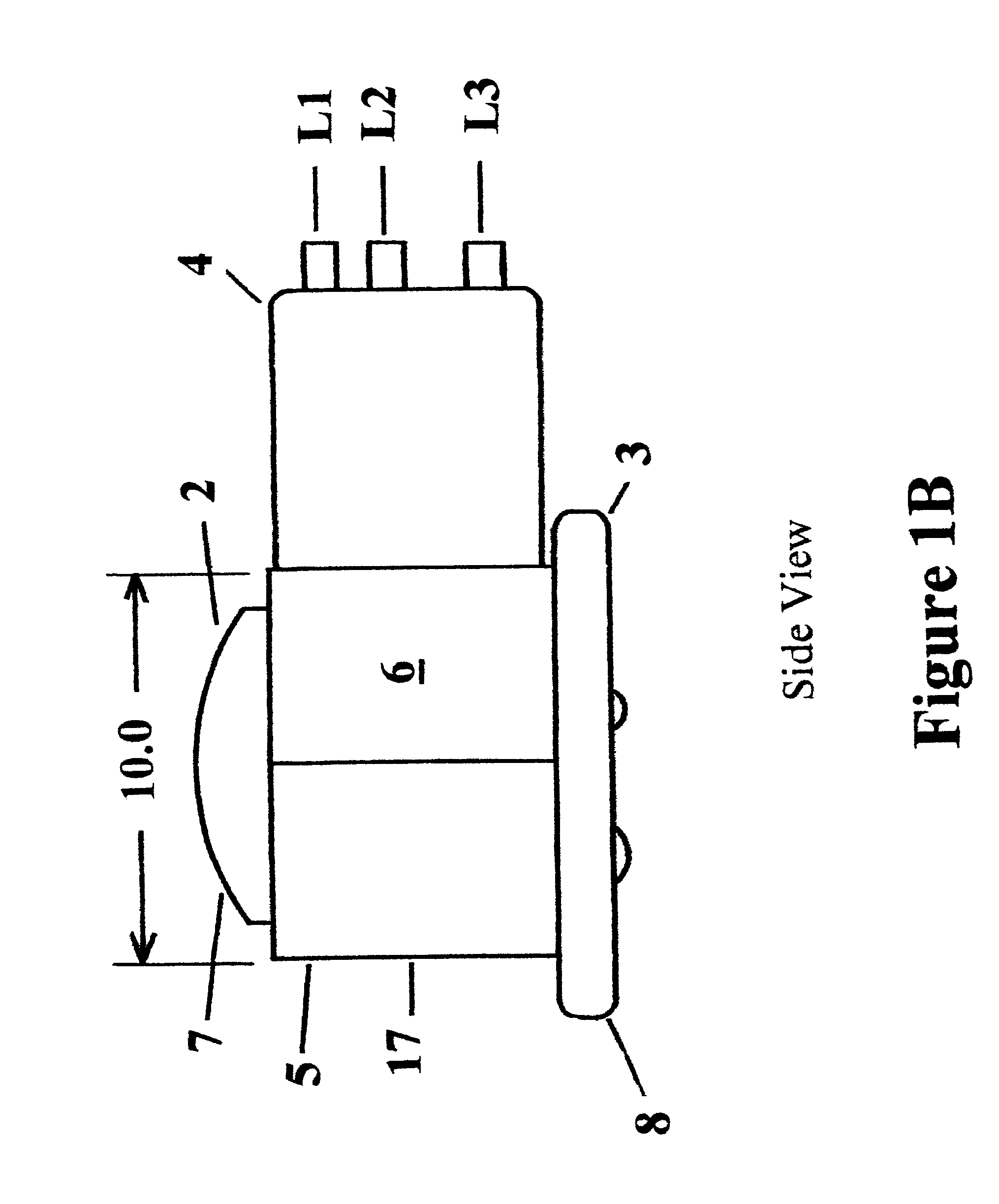
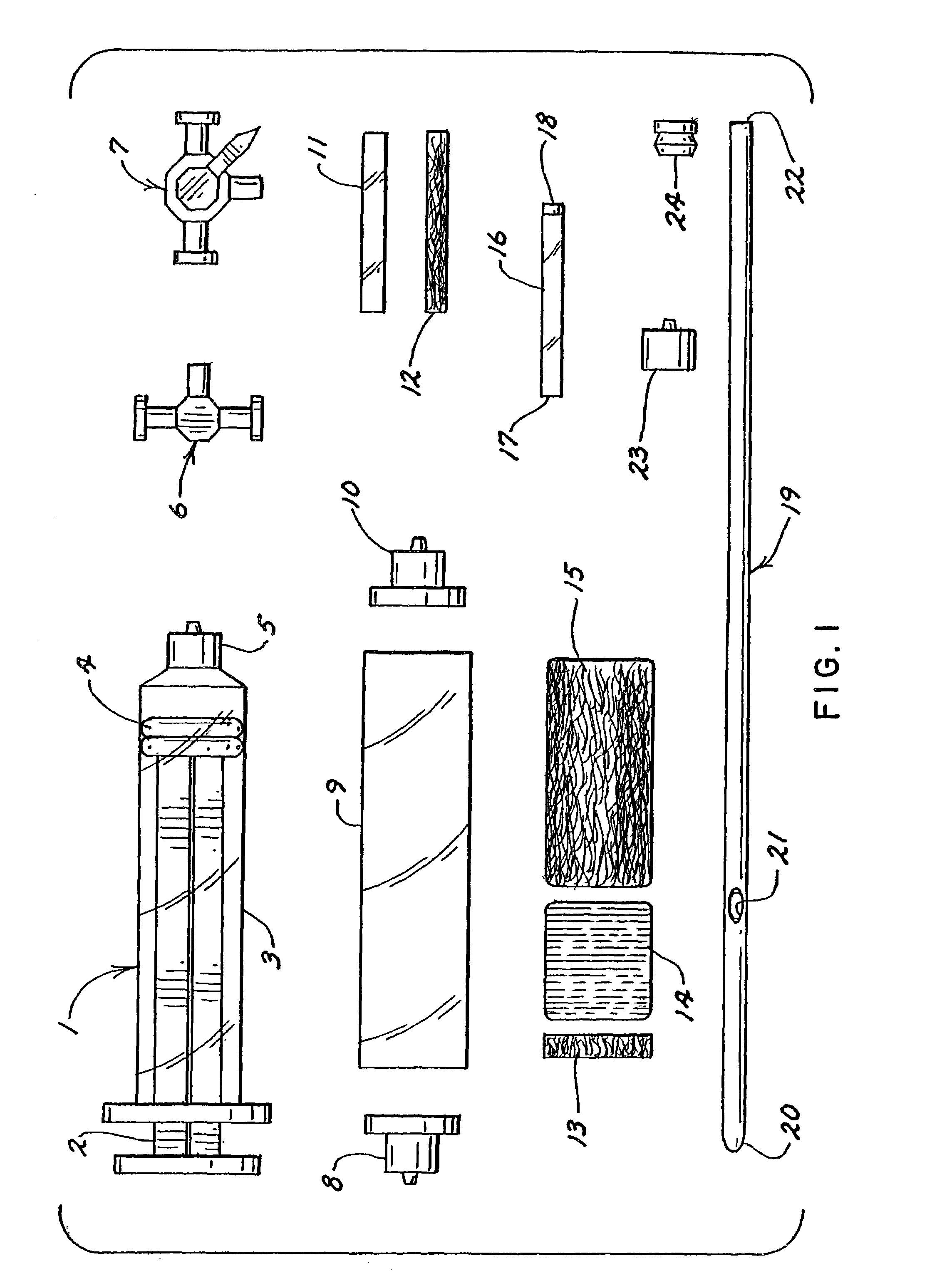
Barrier device for ostium of left atrial appendage
InactiveUS6949113B2Effective isolationPrevent escapeElectrocardiographyDilatorsBlood vessel occlusionThrombus
A membrane applied to the ostium of an atrial appendage for blocking blood from entering the atrial appendage which can form blood clots therein is disclosed. The membrane also prevents blood clots in the atrial appendage from escaping therefrom and entering the blood stream which can result in a blocked blood vessel, leading to strokes and heart attacks. The membranes are percutaneously installed in patients experiencing atrial fibrillations and other heart conditions where thrombosis may form in the atrial appendages. A variety of means for securing the membranes in place are disclosed. The membranes may be held in place over the ostium of the atrial appendage or fill the inside of the atrial appendage. The means for holding the membranes in place over the ostium of the atrial appendages include prongs, stents, anchors with tethers or springs, disks with tethers or springs, umbrellas, spiral springs filling the atrial appendages, and adhesives. After the membrane is in place a filler substance may be added inside the atrial appendage to reduce the volume, help seal the membrane against the ostium or clot the blood in the atrial appendage. The membranes may have anticoagulants to help prevent thrombosis. The membranes be porous such that endothelial cells cover the membrane presenting a living membrane wall to prevent thrombosis. The membranes may have means to center the membranes over the ostium. Sensors may be attached to the membrane to provide information about the patient.
Owner:BOSTON SCI SCIMED INC
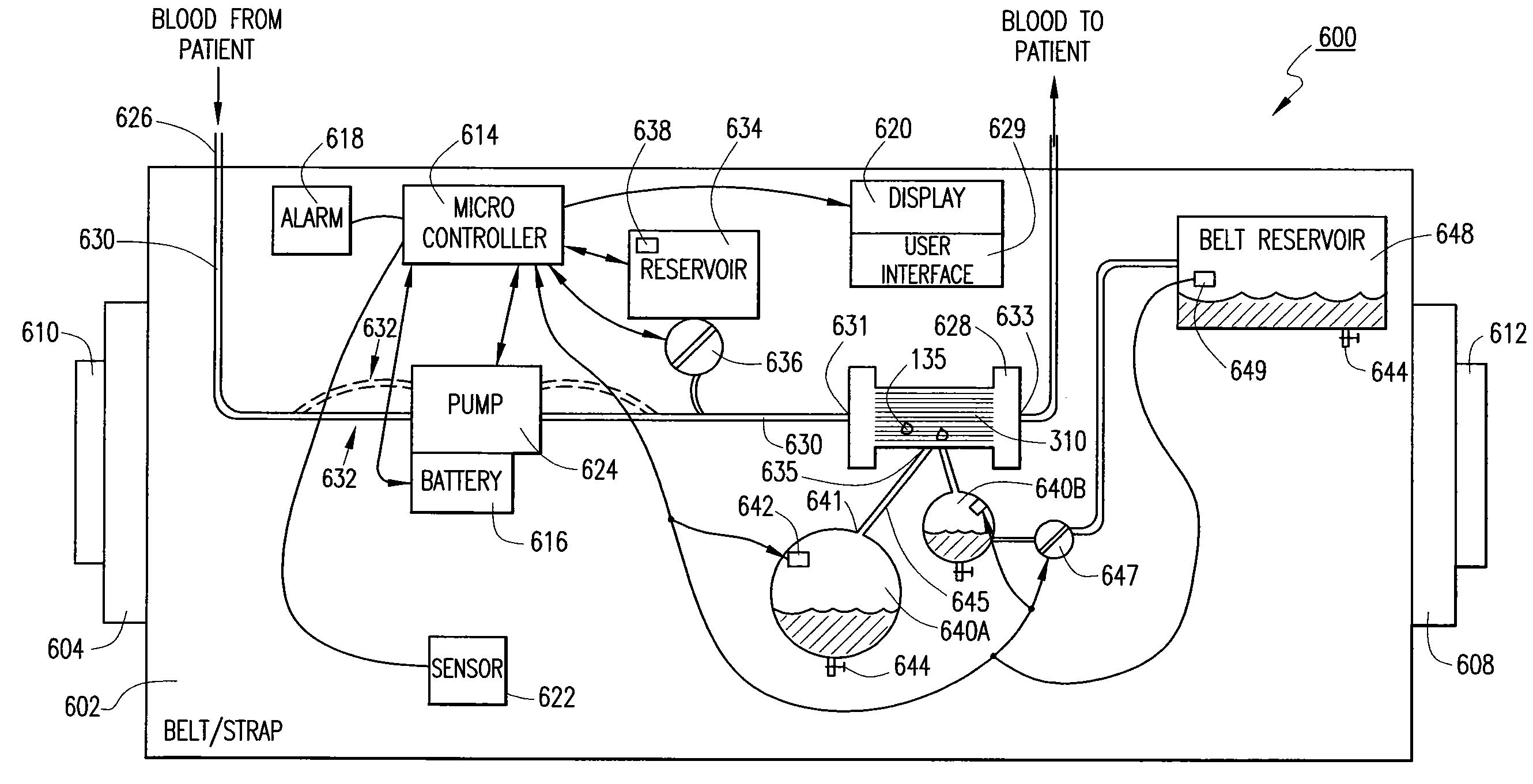
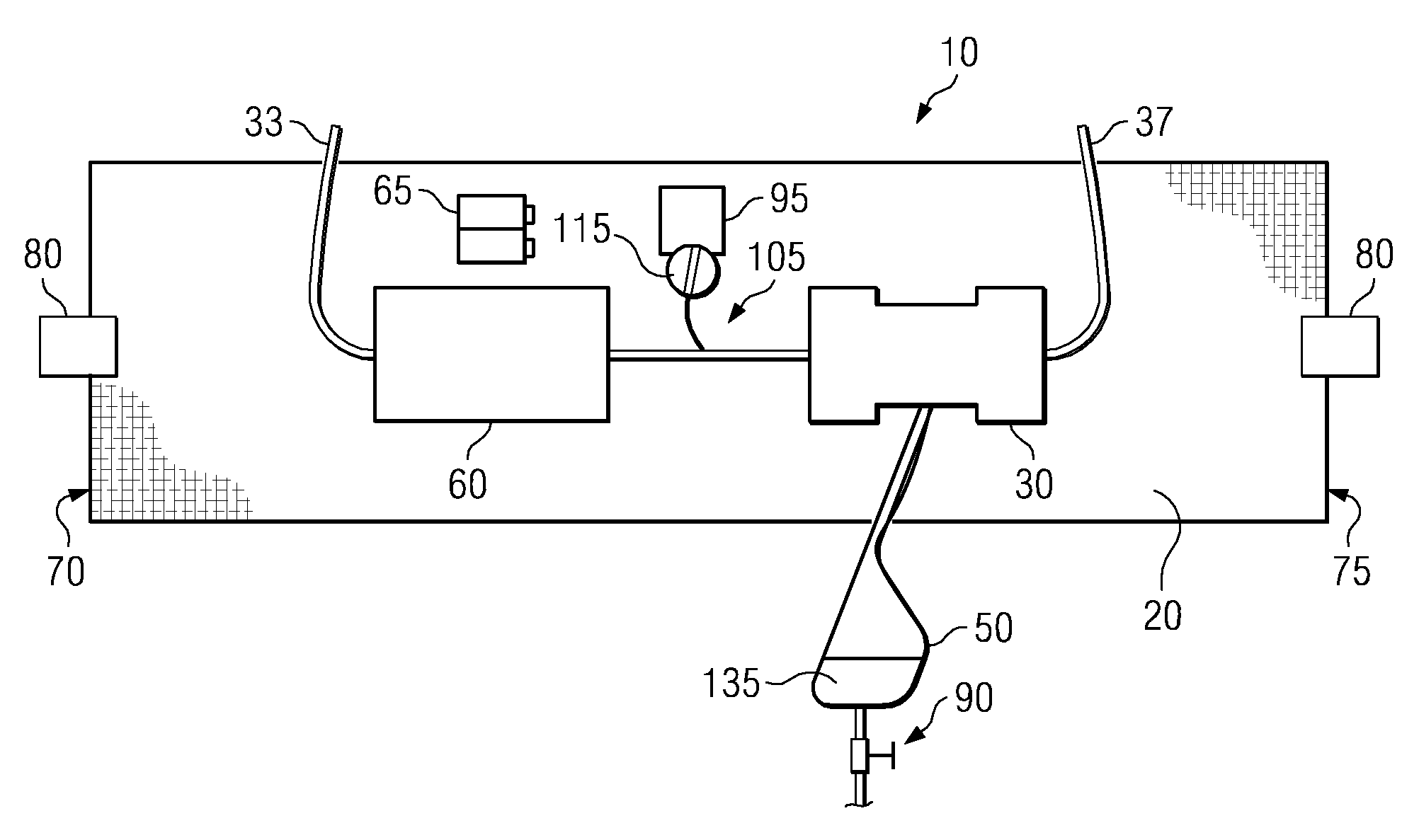
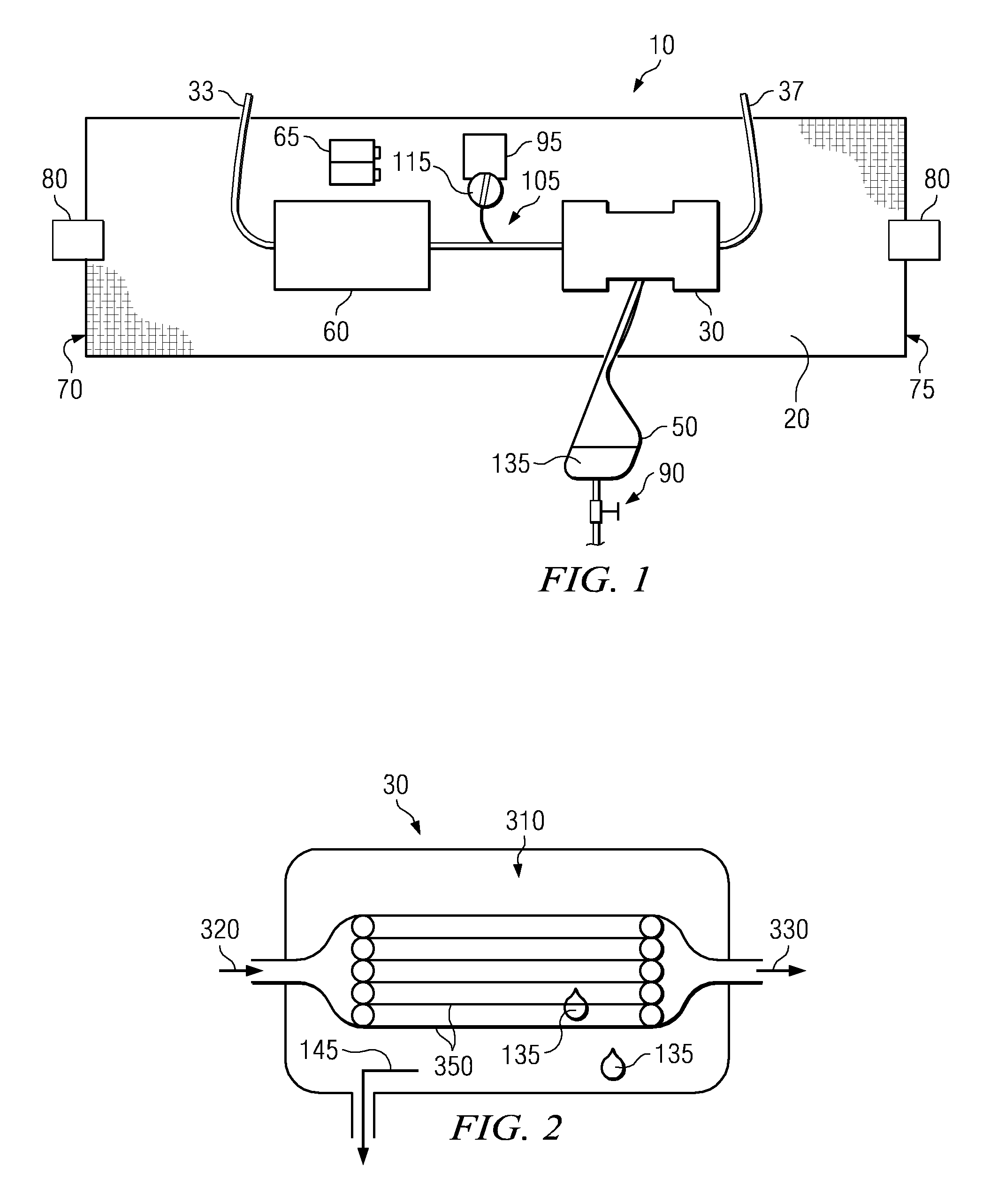
Wearable ultrafiltration device
InactiveUS7645253B2Steady and smooth removalPreventing the shortness of breath and swellingMembranesSemi-permeable membranesUltrafiltrationBlood pump
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Device and process for extracorporeal treatment by citrate anticoagulant
ActiveUS7351218B2Inhibit blood coagulationSimple structureOther blood circulation devicesHaemofiltrationExtracorporeal circulationVein
A device for treating blood by extracorporeal circulation comprising a filter having a first chamber and a second chamber separated by a semi-permeable membrane. The device has an arterial line connected to the first chamber of the filter, a venous line issuing from the first chamber of the filter, a channel used for pre-infusion of a substance for regional anticoagulation and linked to the arterial line, a purge channel issuing from the second chamber of the filter, and an air separator on the venous line. The device also includes a line for post-infusion of a solution at least partially re-establishing the ionic equilibrium of the blood downstream of the air separator, the post-infusion line being configured immediately upstream of an air detector.
Owner:GAMBRO LUNDIA AB
Kit for flushing medical devices and method of preparation
InactiveUS6187768B1Easy and inexpensiveEasy to packBiocideTetracycline active ingredientsAntithrombotic AgentAnticoagulant
A kit and for flushing a medical device a method of preparing the kit is disclosed. The kit includes a container containing a mixed solution a unit dose of a pharmacologically effective amount of an antimicrobial agent and a second agent. The mixed solution has been mixed in a carrier solution and lyophilized. The second agent is an anticoagulant, an antithrombotic agent or a chelating agent. The kit and method are useful for maintaining the patency of indwelling medical devices such as catheters and for preventing infections caused by bacterial growth in catheters.
Owner:BECTON DICKINSON & CO
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
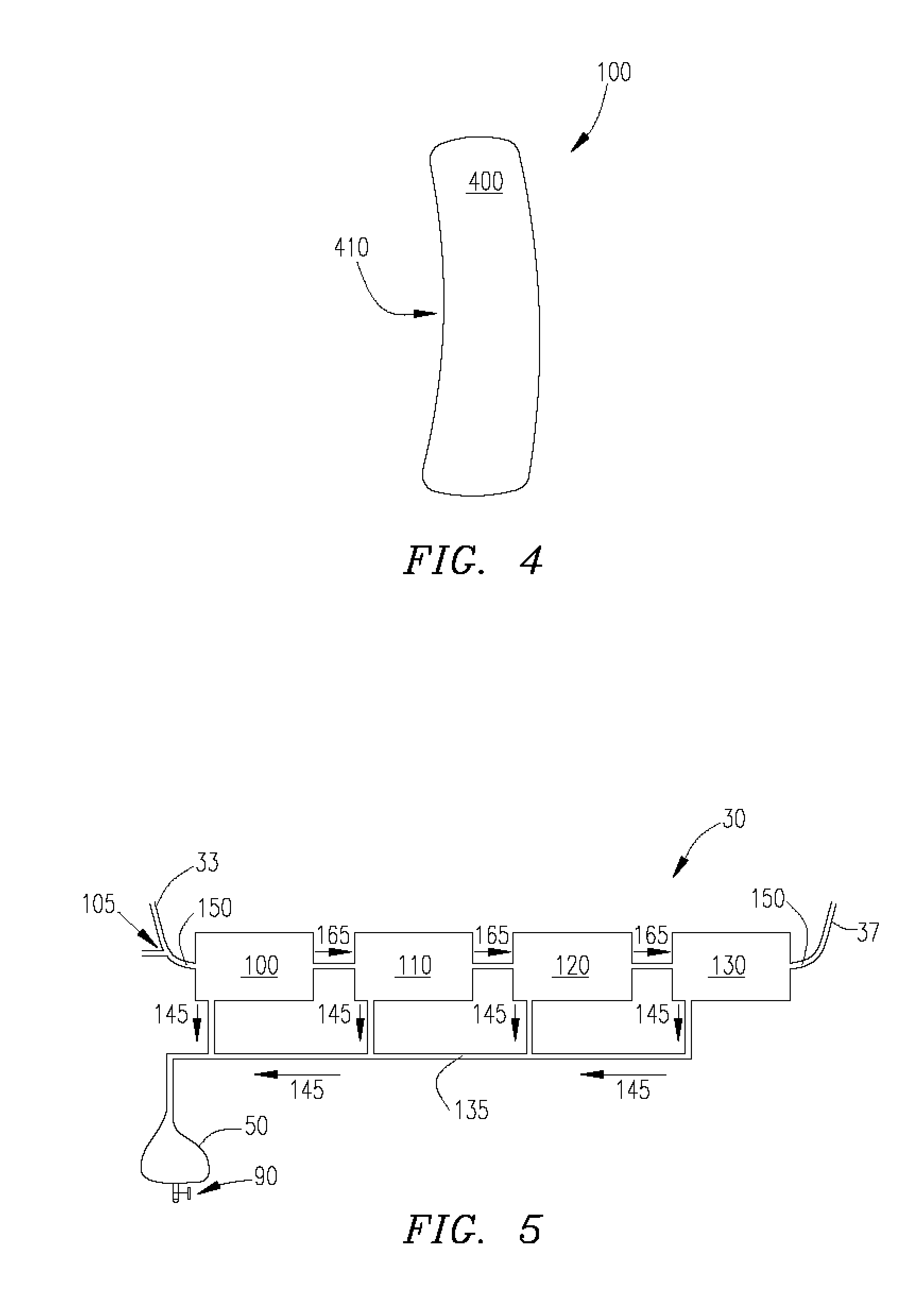
Infusion device for medical fluids
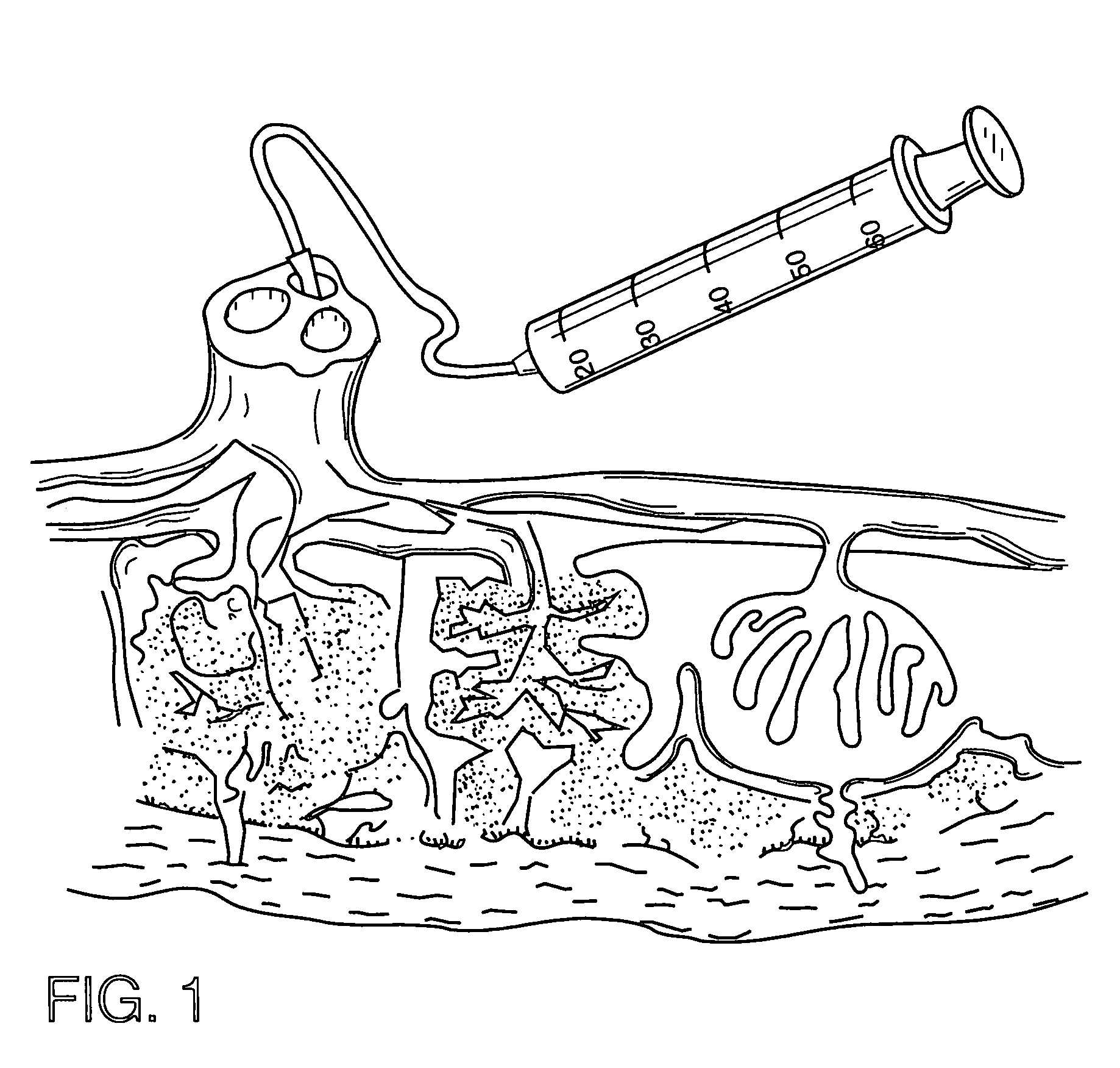
ActiveUS7517332B2Highly reliable and very precise.Other blood circulation devicesFlexible member pumpsMedicineAnticoagulant
An infusion device comprises a pusher for a plunger of a syringe containing a liquid to be infused. A load cell measures the push force. An encoder associated to a motor commanding the pusher measures the displacement of the pusher. A controller signals an alarm when the ratio between the variation of the push force and the displacement exceeds a predetermined threshold. The device, which serves for infusing an anticoagulant into an extracorporeal blood circuit in a dialysis apparatus, is able to signal an onset of an anomalous situation of lack of infusion in good time.
Owner:GAMBRO LUNDIA AB
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
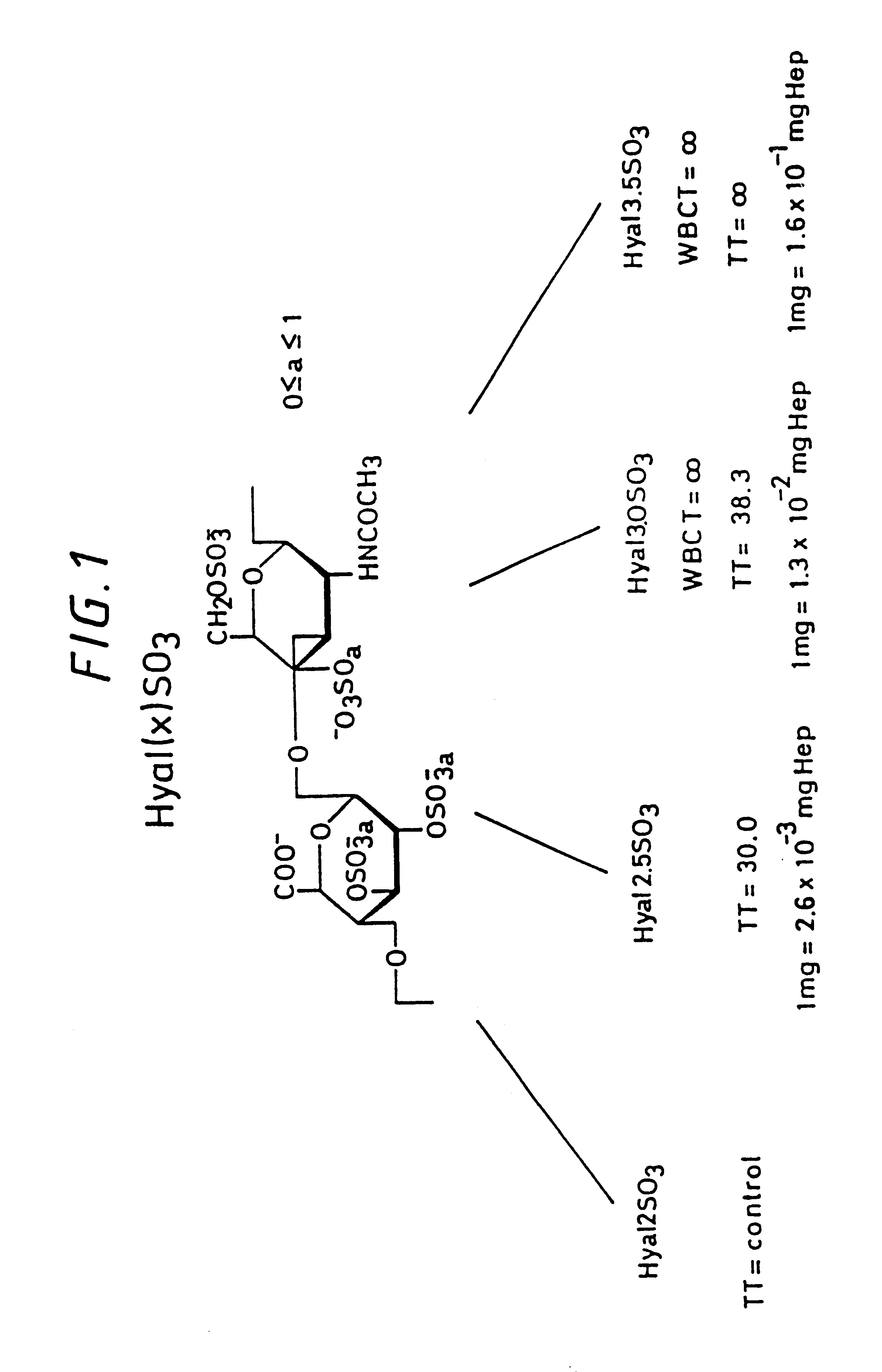
Sulfated hyaluronic acid and esters thereof
Hyaluronic acid, hyaluronate esters and salts thereof are sulfated such that the number of sulfate groups per monomeric unit is in the range of from 0.5 to 3.5. The sulfated derivatives exhibit anticoagulant and cell adhesion reduction properties, and may be used to prepare biomaterials.
Owner:FIDIA ADVANCED BIOPOLYMERS SRL
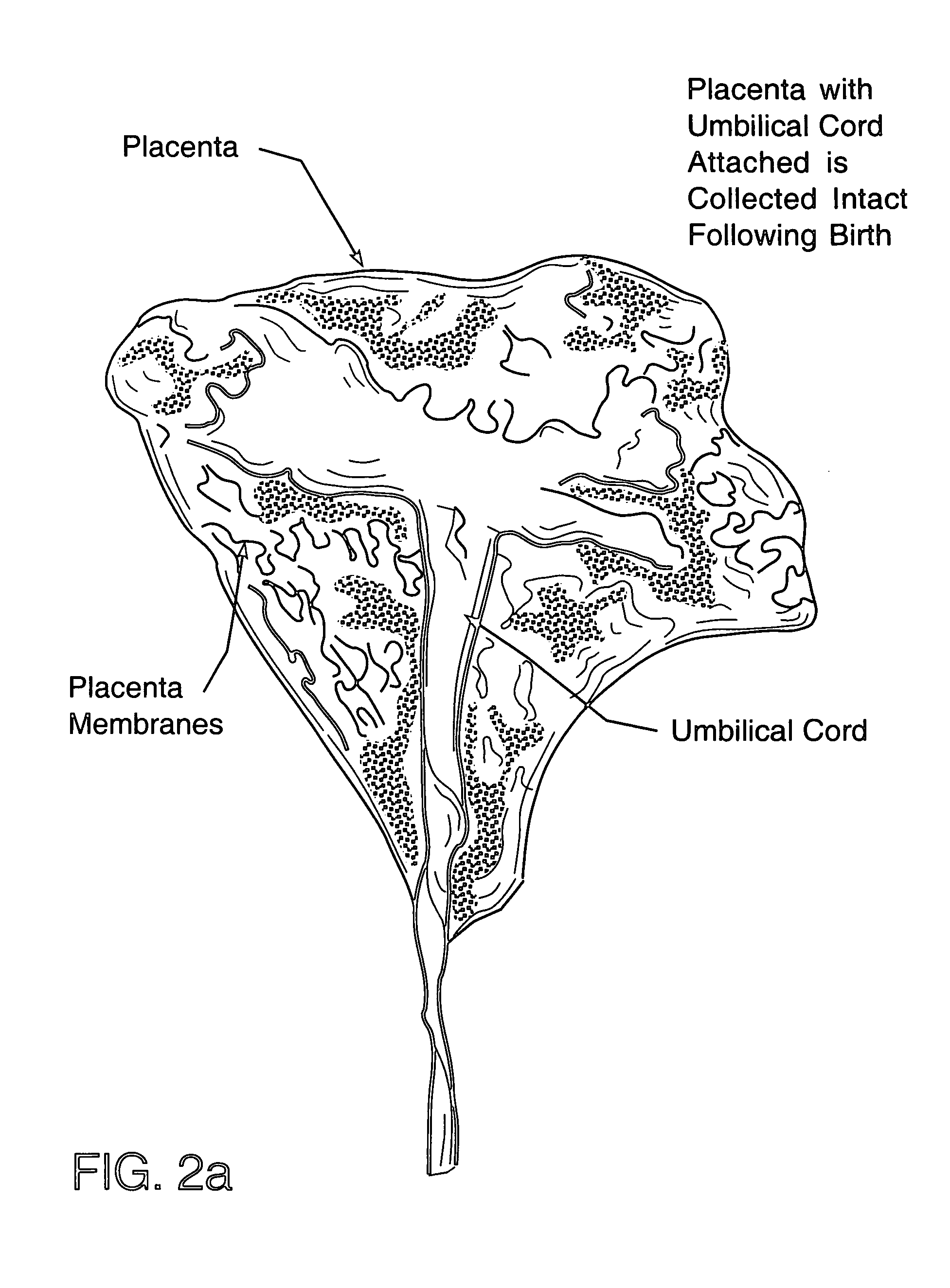
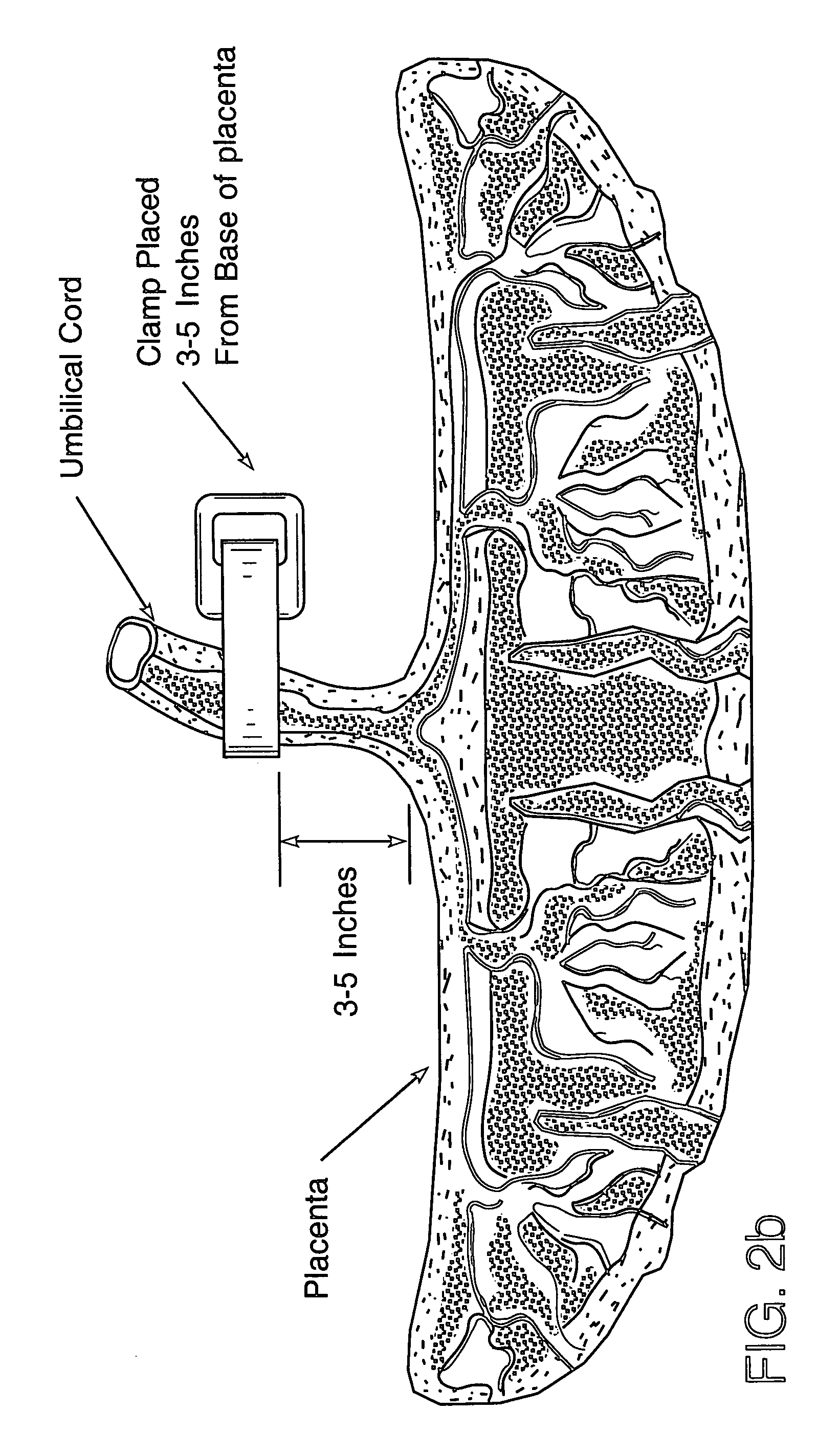
Method of collecting placental stem cells
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
Microfluidic device
InactiveUS20110003330A1Bioreactor/fermenter combinationsBiological substance pretreatmentsTemperature controlTest channel
The present disclosure relates to microfluidic devices adapted for facilitating cytometry analysis of particles flowing therethrough. In certain embodiments, the microfluidic devices have onboard data storage capabilities. In certain other embodiments, the microfluidic devices have onboard anticoagulants. In certain other embodiments, the microfluidic devices have onboard test and control channels. In certain other embodiments, the microfluidic devices have integrated collection media. In certain other embodiments, the microfluidic devices have multiple onboard test channels. In certain other embodiments, the microfluidic devices have localized temperature control. In certain other embodiments, the microfluidic devices have anatomy simulating regions. In certain other embodiments, the microfluidic devices have complete assay capabilities. In certain other embodiments, the microfluidic devices have dissociable sections. In certain other embodiments, the microfluidic devices have means for performing functional assays.
Owner:SONY CORP +1
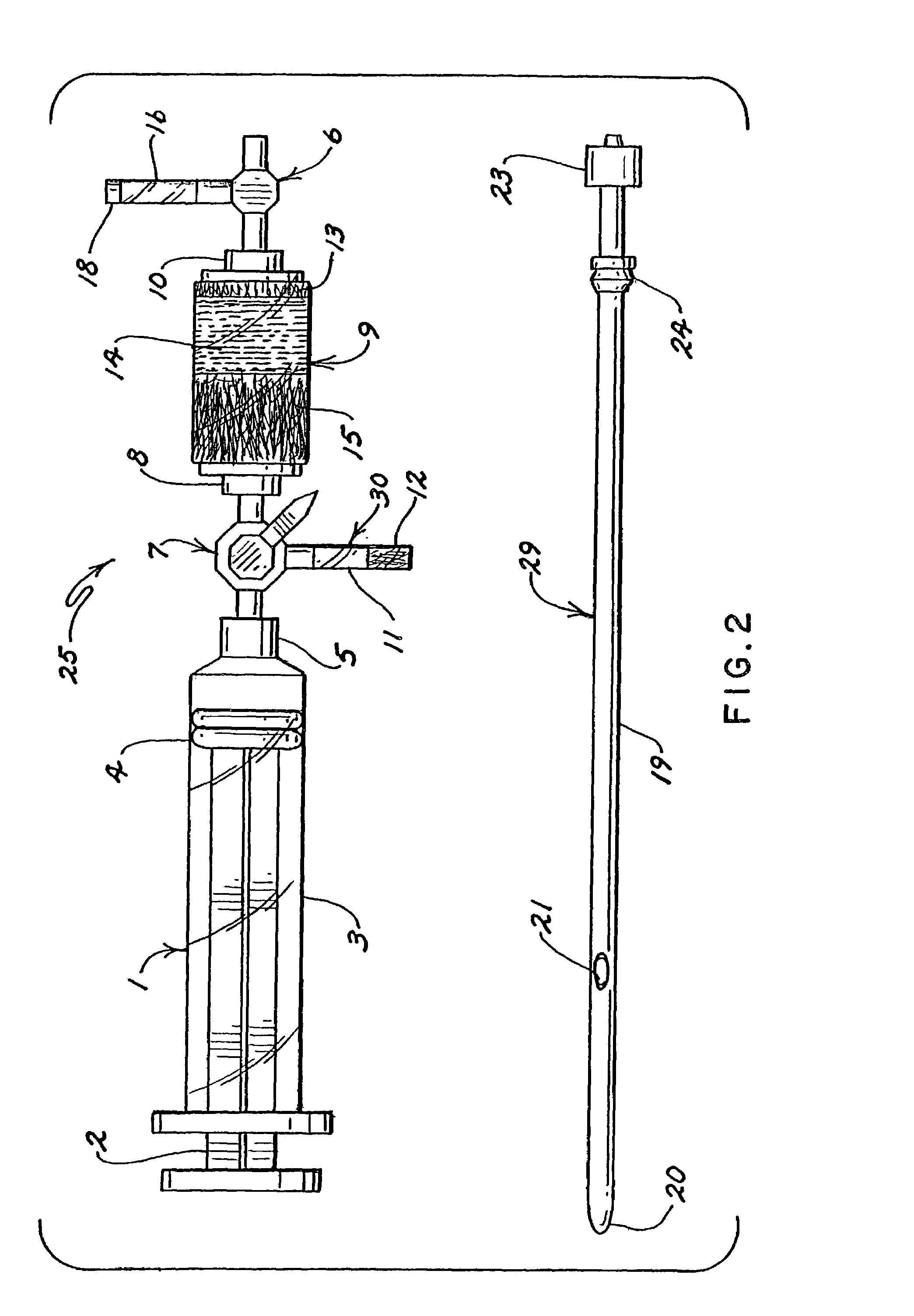
Barrier device for ostium of left atrial appendage
InactiveUS20050049573A1Accurate placementPrevent escapeElectrocardiographyMedical devicesThrombusRisk stroke
A membrane applied to the ostium of an atrial appendage for blocking blood from entering the atrial appendage which can form blood clots therein is disclosed. The membrane also prevents blood clots in the atrial appendage from escaping therefrom and entering the blood stream which can result in a blocked blood vessel, leading to strokes and heart attacks. The membranes are percutaneously installed in patients experiencing atrial fibrillations and other heart conditions where thrombosis may form in the atrial appendages. A variety of means for securing the membranes in place are disclosed. The membranes may be held in place over the ostium of the atrial appendage or fill the inside of the atrial appendage. The means for holding the membranes in place over the ostium of the atrial appendages include prongs, stents, anchors with tethers or springs, disks with tethers or springs, umbrellas, spiral springs filling the atrial appendages, and adhesives. After the membrane is in place a filler substance may be added inside the atrial appendage to reduce the volume, help seal the membrane against the ostium or clot the blood in the atrial appendage. The membranes may have anticoagulants to help prevent thrombosis. The membranes be porous such that endothelial cells cover the membrane presenting a living membrane wall to prevent thrombosis. The membranes may have means to center the membranes over the ostium. Sensors may be attached to the membrane to provide information about the patient.
Owner:BOSTON SCI SCIMED INC
Wearable ultrafiltration device
InactiveUS7597677B2Steady and smooth removalPreventing the shortness of breath and swellingSolvent extractionHaemofiltrationUltrafiltrationBlood pump
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Wearable ultrafiltration device
InactiveUS20080051689A1Inhibit swellingSteady and smooth removalMembranesHaemofiltrationUltrafiltrationBlood pump
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
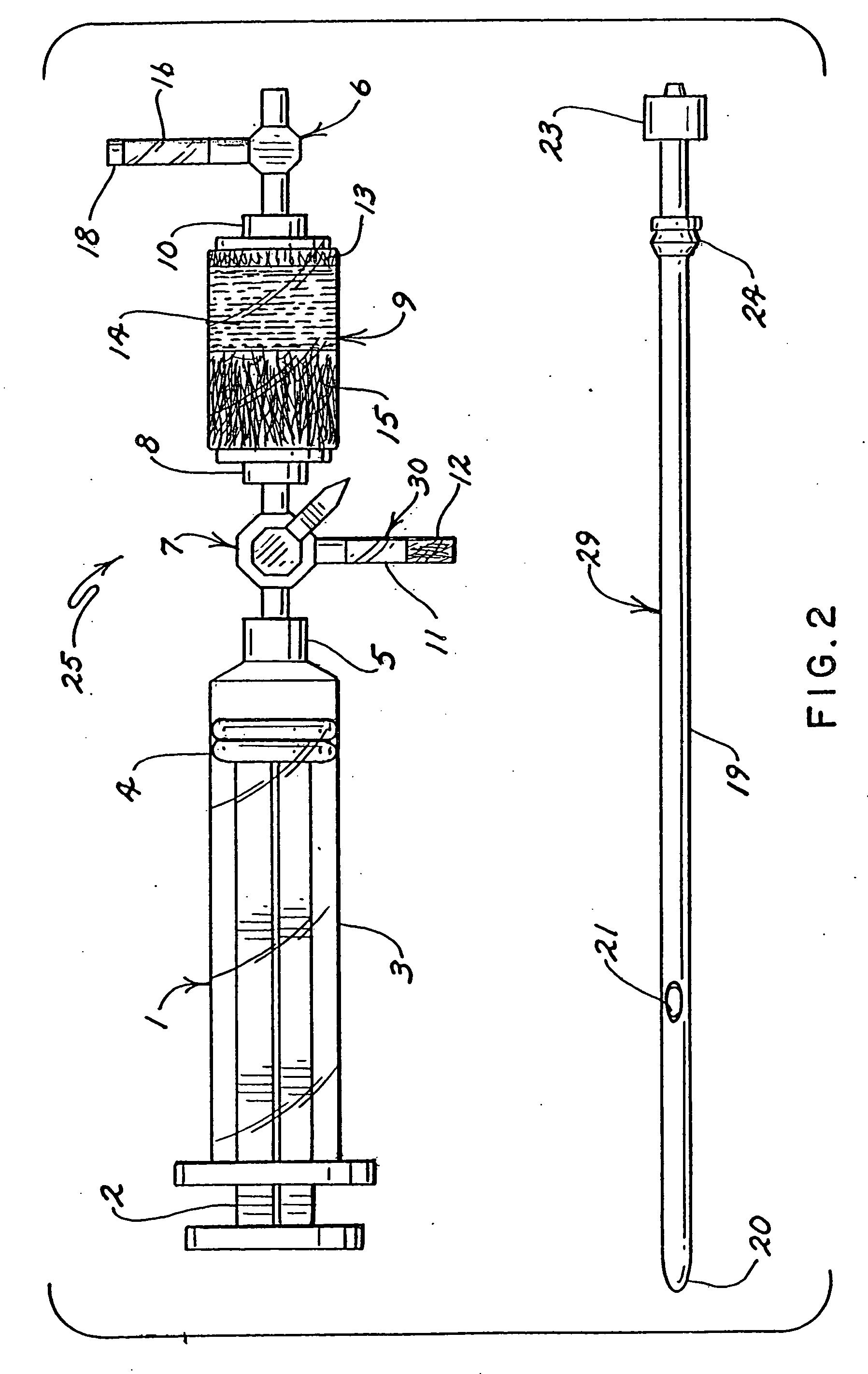
Blood collection and measurement apparatus
InactiveUS20060228258A1Material analysis by optical meansLaboratory glasswaresBlood collectionMeasuring instrument
Some embodiments of the invention provide one apparatus that is suitable for both the collection and measurement of a blood sample. Once a blood sample is drawn into such an apparatus the blood sample can be analyzed, without having to transfer any portion of the blood sample into another vessel. Also, in some very specific embodiments, the apparatus is provided with an optical chamber that is specifically designed to reduce the average attenuation of electromagnetic radiation (EMR) due to scattering of EMR by the red blood cells in a blood sample, without having to hemolyze the red blood cells by using sound waves or by adding reagents to the blood sample. Moreover, as a result of the time saved by using a single apparatus for blood sample collection and measurement, the addition of an anticoagulant is not required to prevent clotting. Moreover, in such embodiments the optical chamber is designed to spread blood into a thin film, thereby reducing the incidences of trapped air bubbles in the collected blood sample in the optical chamber. Instead air bubbles are easily pushed through the optical chamber and guided out of the apparatus through a vent. Optionally, at least one biosensor may be provided within a second flow path in the apparatus.
Owner:CHROMEDX
Bone void filling tube and shear mechanism
Owner:ARTHREX
"blood sample collection apparatus and kits"
Blood sample collection apparatus and kits are devised as easy-to-use, reduced pain devices operable by persons not having medical training or other expertise or knowledge. A flexible strip having adhesives on two portions of a first side accommodate therebetween and absorbent pad and substrate with microstructures. The substrate includes microstructures arranged to gently penetrate or pierce skin and cause a small amount of blood to pass to the external surface of a user's flesh. An absorbent pad receives and absorbs a blood sample therein. The absorbent pad may additionally support a plurality of chemical agents which further advances mobility of blood in the collection system. These chemical agents may include anticoagulants and blood anticlotting agents to facilitate blood transfer from a donor to the absorbent pad. Additionally, these systems may also include a blood preservative to stabilize blood collected for an extended shipping and transfer period. These blood sample collection systems may further include a numbing agent to improve comfort of use.
Owner:PATHWAY GENOMICS
Automated compulsory blood extraction system
InactiveUS6340354B1Cost-effectively mass producedPromote circulationIncision instrumentsWound drainsVeinHead size
A method and apparatus for the treatment of thrombosis, venous insufficiency, and the like, and in particular to an Automated Compulsory Blood Extraction System (ACBES) configured to provide an efficient and safe means for the measured extraction of blood utilizing a device providing, in effect, an artificial leech, but without the infection, control, care, and other limitations associated with the medicinal leech. The preferred embodiment of the present invention utilizes recent micro technological advances to provide a micro mechanical device which mimics and improves upon the bloodletting properties of the medicinal leech utilizing a micro mechanical valve, micropump, and micro sensor arraignment cooperating with a tertiary jaw array having teeth situated thereon. The preferred embodiment of the present invention contemplates an extraction device which may have a head size of one centimeter or less, and which may be utilized in number about the affected area of the patient to provide controlled, precision, pulsed blood extraction via vacuum induction, supplying a controlled dosage of anticoagulant, histamine anesthetic, or the like. Alternative embodiments of the present invention include an independent, single needle, stationary design configured primarily for emergency use, a multi-needle piston design, a large extraction area array design including concentric needles of adjustable depth, and a deep extraction needle design.
Owner:RAMBIN CHRISTOPHER L
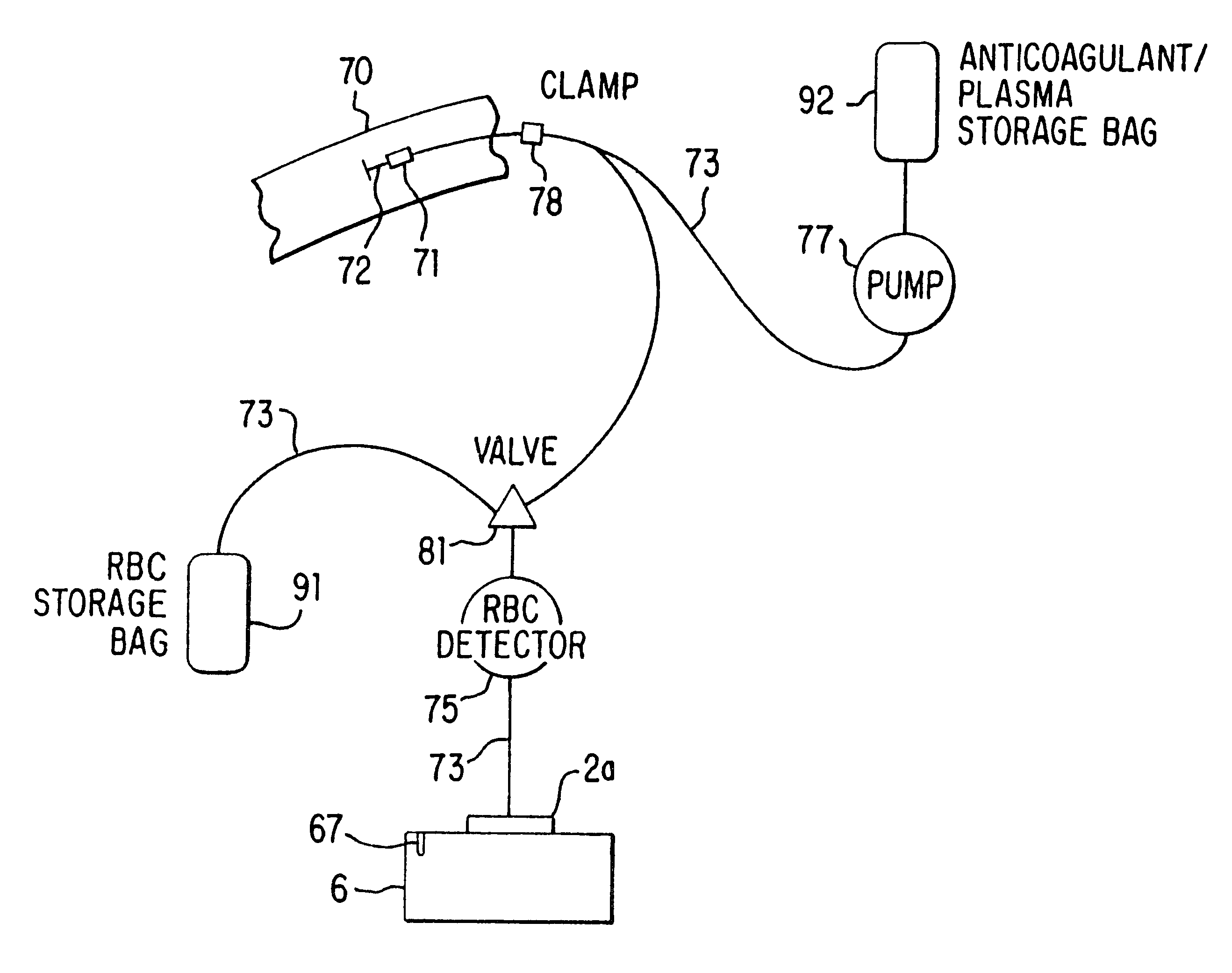
Blood collection and separation system
InactiveUS6379322B1Water/sewage treatment by centrifugal separationOther blood circulation devicesBlood collectionVein
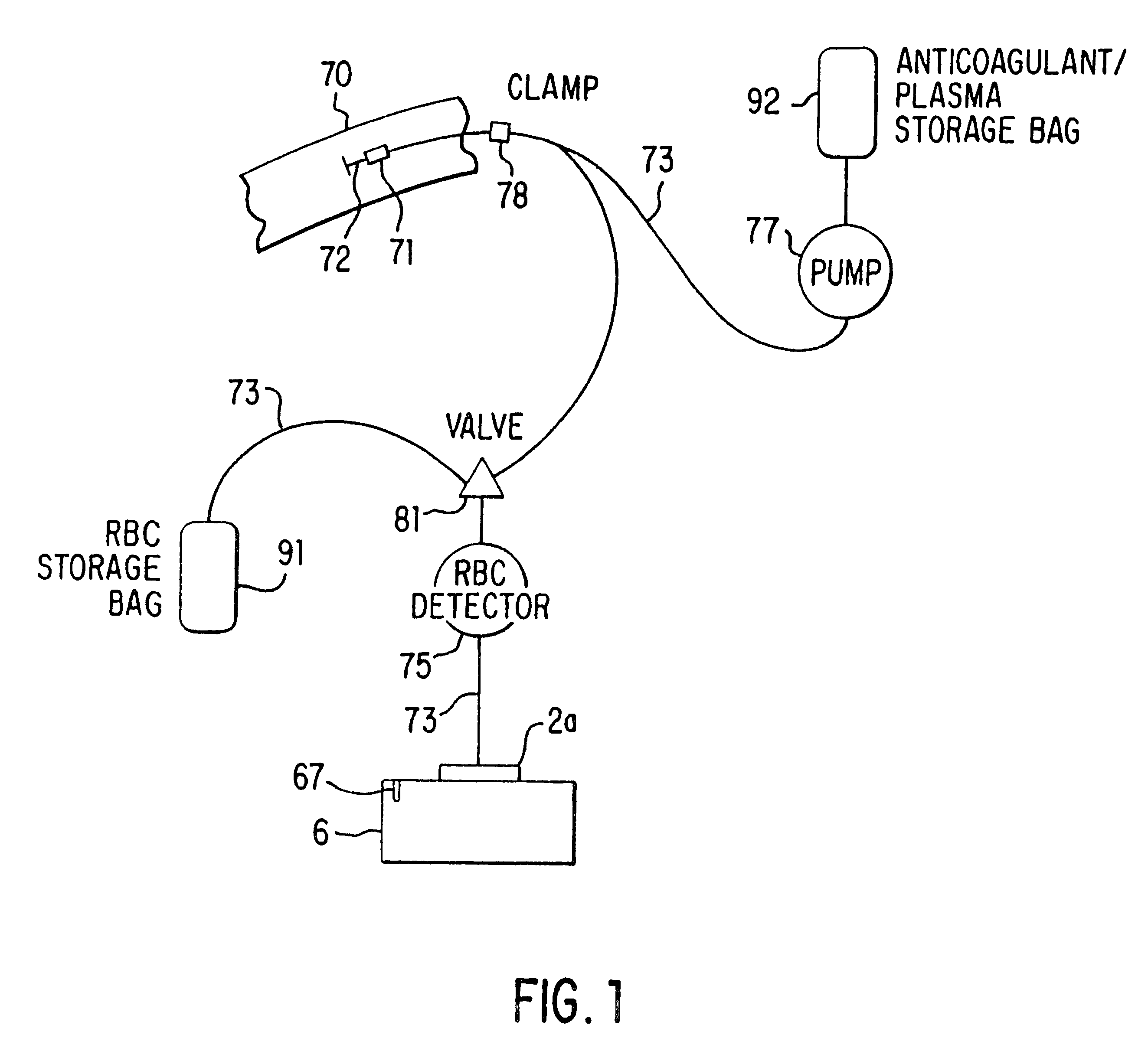
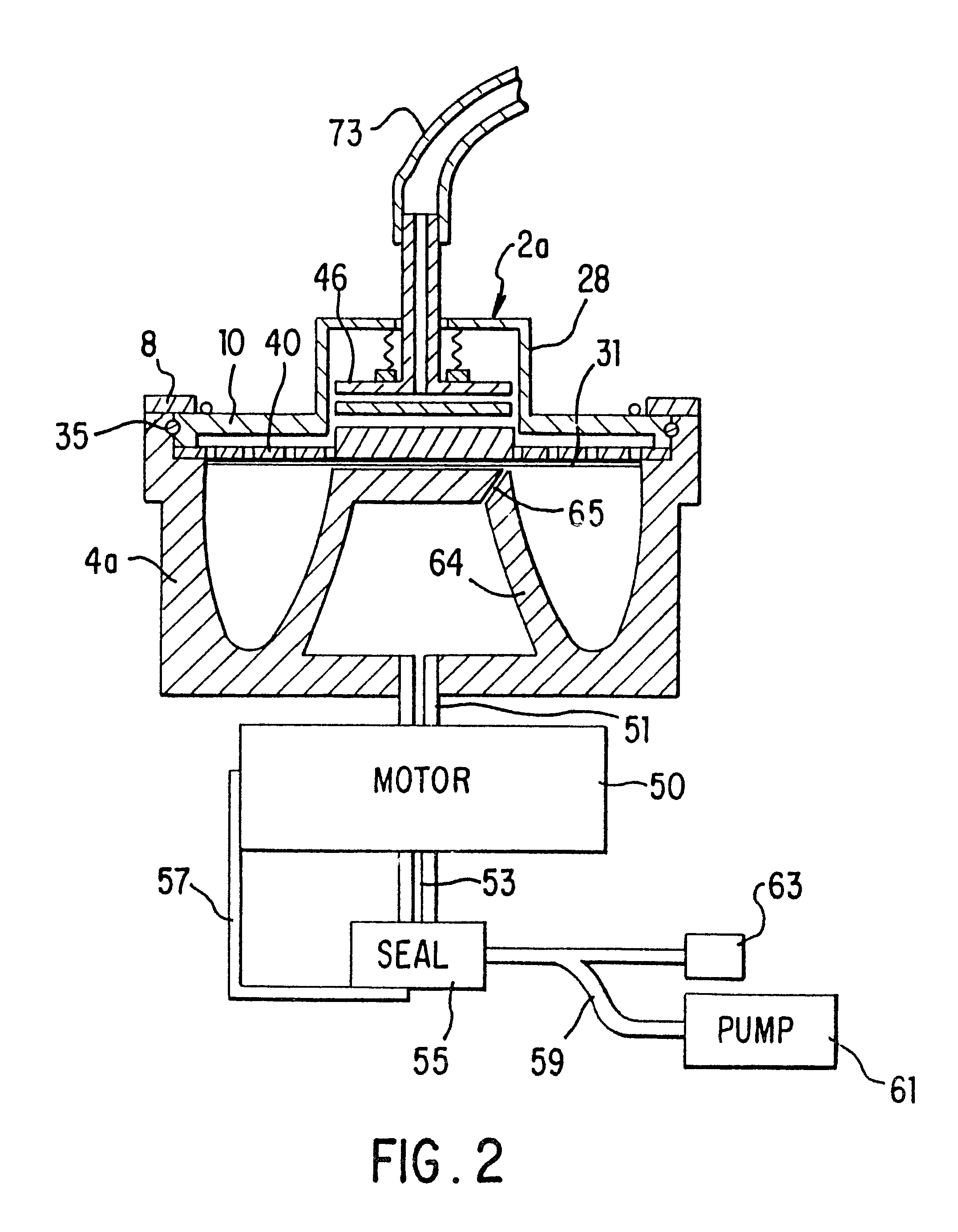
A system for collecting and processing blood from a donor (70), wherein the system may be compact enough to be located entirely beside the donor's chair, and be able to process the blood while the donor is still resting in the chair after having donated the blood. Thus, the separated blood components (plasma and red blood cells) may be stored in their individual optimum environments immediately after the whole blood is drawn, and the blood does not need to be transported back to a separation laboratory for processing. The system includes a needle (72) (or other cannula-like device) for insertion into a vein of the donor and drawing whole blood therethrough, a variable-volume rotor (2a) for holding the blood after it is drawn, and a motor (50) for spinning the rotor so as to cause the blood to separate into components, for example, plasma and red blood cells. The system also provides for a container for collecting a separated component. In a preferred embodiment two containers are used: the first container (92) for containing an anticoagulant, which is preferably added to the whole blood as it is drawn from the donor, and then for storing the plasma after it has been separated from the red blood cells, and the second container (91) for storing the separated red blood cells. The system further includes tubing (73), which may have valving (81) built into it and which may be acted on externally, so as to direct the blood components in the desired manner.
Owner:HAEMONETICS
Vacuum blood collection tube and method thereof capable of directly separating blood plasma
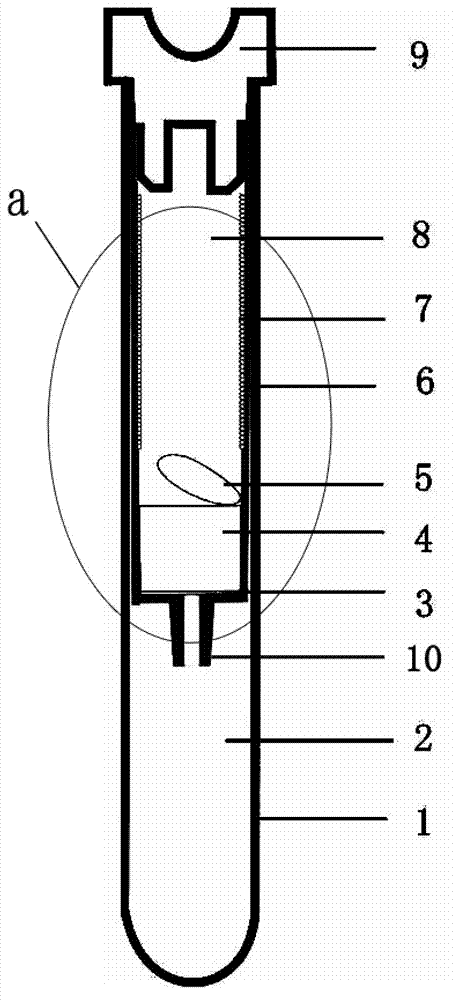
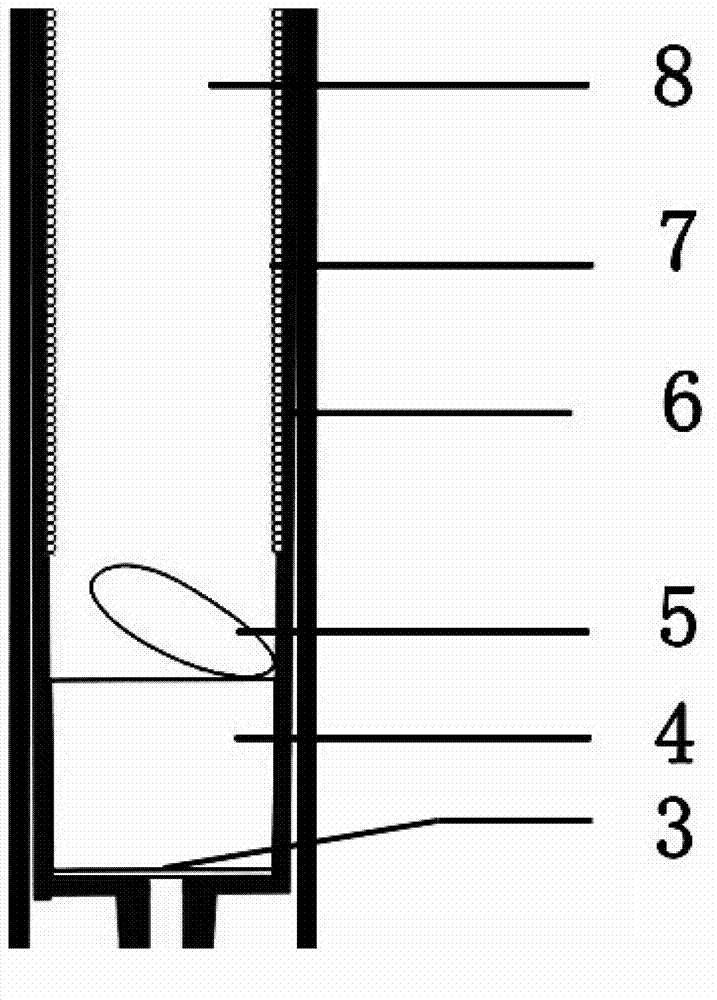
InactiveCN102764133AEasy to getLow costPreparing sample for investigationDiagnostic recording/measuringBlood Collection TubeAnticoagulant
The invention relates to the technical field of clinical medical instruments, in particular to a vacuum blood collection tube and a method thereof capable of directly separating blood plasma. The vacuum blood collection tube comprises a blood collection tube body, an inner tube, a rubber plug and a filter element. The vacuum blood collection tube is characterized in that the blood collection tube inner tube is arranged in the blood collection tube body, the rubber plug is disposed at the top of the blood collection tube body, the structure of the rubber plug is close to a plug shape, a semicircle groove is disposed at the center of the upper portion of the rubber plug structure, left and right ends of the lower portion of the rubber plug are embedded into the blood collection tube body to be connected with the blood collection tube inner tube, a blood plasma outlet is disposed in the middle of the bottom of the blood collection tube inner tube, a blood plasma collecting chamber is mounted below the blood plasma outlet, a filtering membrane is arranged on the upper portion of the blood plasma outlet of the blood collection tube inner tube, the filter element is arranged on the upper portion of the filtering membrane, a buffer plate is mounted on the filter element, consequently a blood collection chamber is formed in the blood collection tube inner tube, and a layer of anticoagulant is disposed on the inner wall of the blood collection tube inner tube. The vacuum blood collection tube and the method thereof capable of directly separating the blood plasma have the advantages that physical form differences of blood visible components and plasma components are utilized, the blood plasma can be rapidly separated, and the vacuum blood collection tube and the method thereof are simple, convenient, safe and rapid.
Owner:SHANGHAI KEHUADIAGNOSITIC MEDICAL PRODS
Chronic access system for extracorporeal treatment of blood including a continously wearable hemodialyzer
ActiveUS8109893B2More convenientEliminates the need for time-consuming, sedentary, hospital or clinic dialysisSemi-permeable membranesSolvent extractionIntravenous cannulaHaemodialysis machine
Owner:LANDE ARNOLD J
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8268783B2Reduces and removes anticoagulant effectReduced activityBiocidePeptide/protein ingredientsFactor XAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Inorganic Coagulation Accelerators for Individuals taking Platelet Blockers or Anticoagulants
InactiveUS20090047366A1Good coagulationAccelerate blood clottingBiocideInorganic non-active ingredientsMolecular sieveMontmorillonite
The present invention is a method to accelerate the coagulation of blood through the application of inorganic materials to the wound of a patient on anticoagulant or platelet blocker therapy. The method comprises contacting such wounds with a substance selected from the group consisting of zeolitic molecular sieves and non-zeolitic molecular sieves, diatomaceous earth, glass powder or fibers, precipitated or fumed silica, kaolin and montmorillonite clays and Ca exchanged permutites.
Owner:HONEYWELL INT INC
Preparation method of copper ion mediated anticoagulant coating with function of in situ catalysis of NO release
ActiveCN104208760ALong-acting sustained releaseImprove researchPharmaceutical containersMedical packagingNo donorsBlood vessel
The invention discloses a preparation method of a copper ion mediated anticoagulant coating with function of in situ catalysis of NO release. The method includes steps of preparing an acidic buffer solution, and adding a compound with pyrogallol structure and certain concentration, a compound with amino or thiol, and copper ion soluble salt. The method of the invention has the advantages of simple operation, mild reaction conditions and easiness. The prepared coating has the advantages of controllable content of the units containing multiple amino or thiol compounds, and easily controlled loading amount of copper ion. The modified coating prepared by the method has excellent adhesion with a base material, and through act of the copper ions in the coating with the blood, the coating can conduct in situ catalyze the NO donor molecules in blood to continuously decompose and release NO molecules, thus realizing the inhibition of platelet activation and aggregation, inhibiting smooth muscle cell proliferation and migration, and protecting vascular endothelial layer function.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
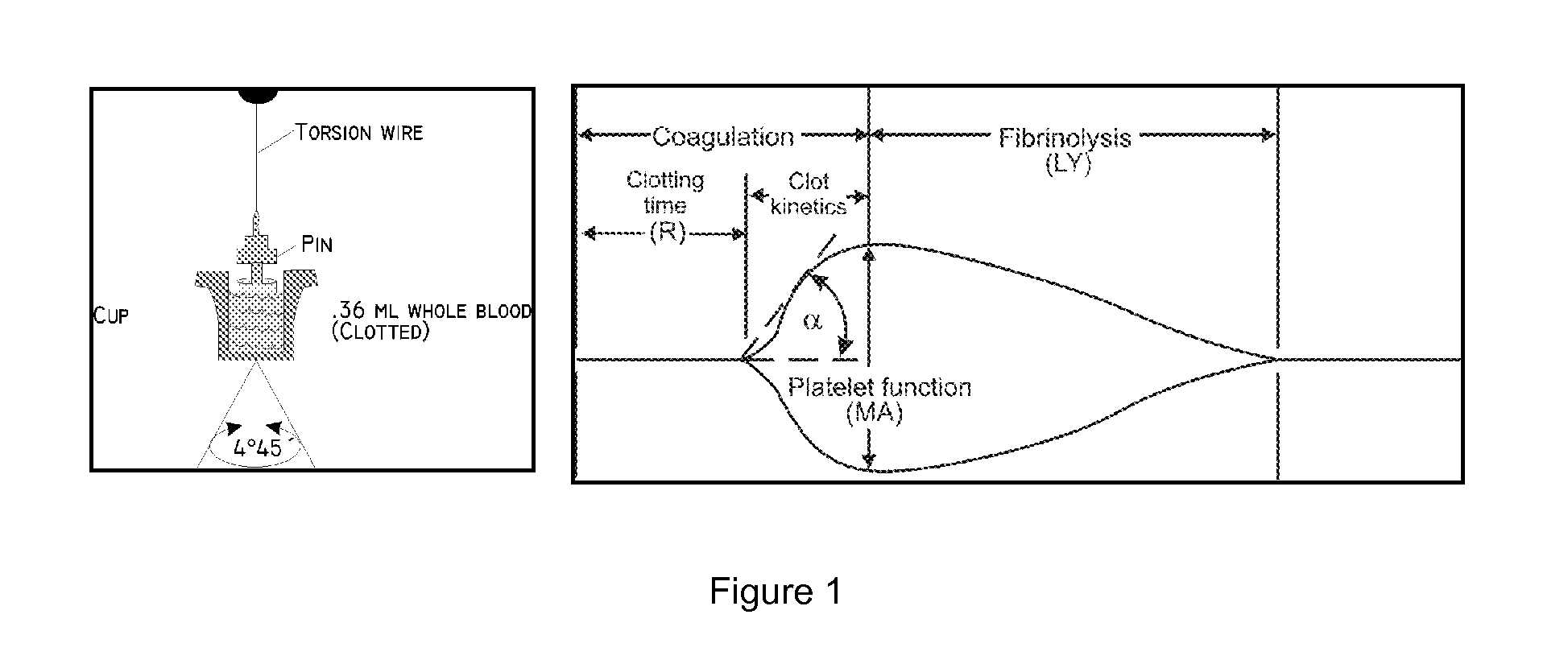
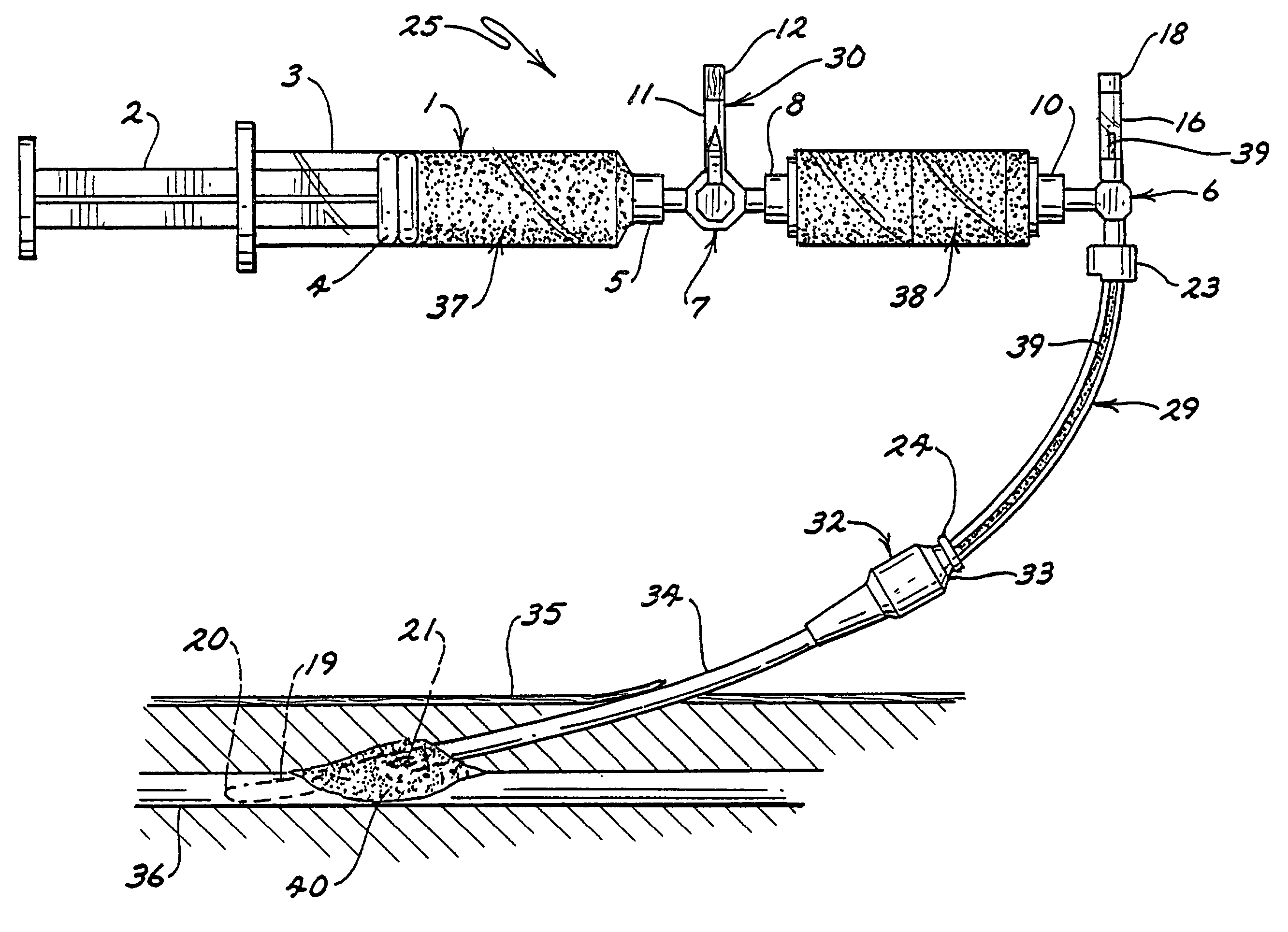
Clotting cascade initiating apparatus and methods of use and methods of closing wounds
InactiveUS20060178610A1Avoid injuryPractical and convenientGuide needlesSurgical adhesivesClosing woundAnticoagulant
Wound closure methods and apparata are provided which utilize blood fluid by activating the clotting cascade of the blood fluid outside the body within a substantially enclosed sterile container then introducing the blood fluid to the wound site to complete clotting. Methods and apparata for providing ways of inhibiting anticoagulants and slowing fibrin clot degradation are also disclosed. Kits for practicing the invention singularly or in combination with and / or associated with preferred procedures are also disclosed.
Owner:CLOSYS CORP
Methods of treatment of patients at increased risk of development of ischemic events and compounds hereof
InactiveUS20130040898A1Impair thrombus formationIncreased riskElcosanoid active ingredientsInorganic active ingredientsBeta blockerPlatelet inhibitors
The present invention relates to compounds for treatment that protects the endothelium, prevents pathologic thrombus formation in the microcirculation and preserves platelet number and function and thus may be related to treatment or prevention of ischemic events in patients with cardiovascular disease. The present invention is particularly useful for patients having or being at increased risk of development of an ischemic event such as an acute myocardial infarction and / or no-reflow phenomena and / or ischemia-reperfusion injury by administration of agent(s) modulating and / or preserving endothelial integrity. The compounds may be administered in combination with standard treatment of acute cardiovascular ischemic events such as Platelet inhibitors such as aspirin (ASA), Thienopyridins, GPIIb / IIIa inhibitors), Parenteral anticoagulants such as unfractioned heparin (UFH), bivalirudin, enoxaparin, and fondaparinux, Verapamil, Adenosine, Sodium nitroprusside, Nitroglycerin, Epinephrine, Beta-blockers and surgical methods such as percutaneous coronary intervention (PCI), PCI with thrombus aspiration, PCI with stents.
Owner:THROMBOLOGIC
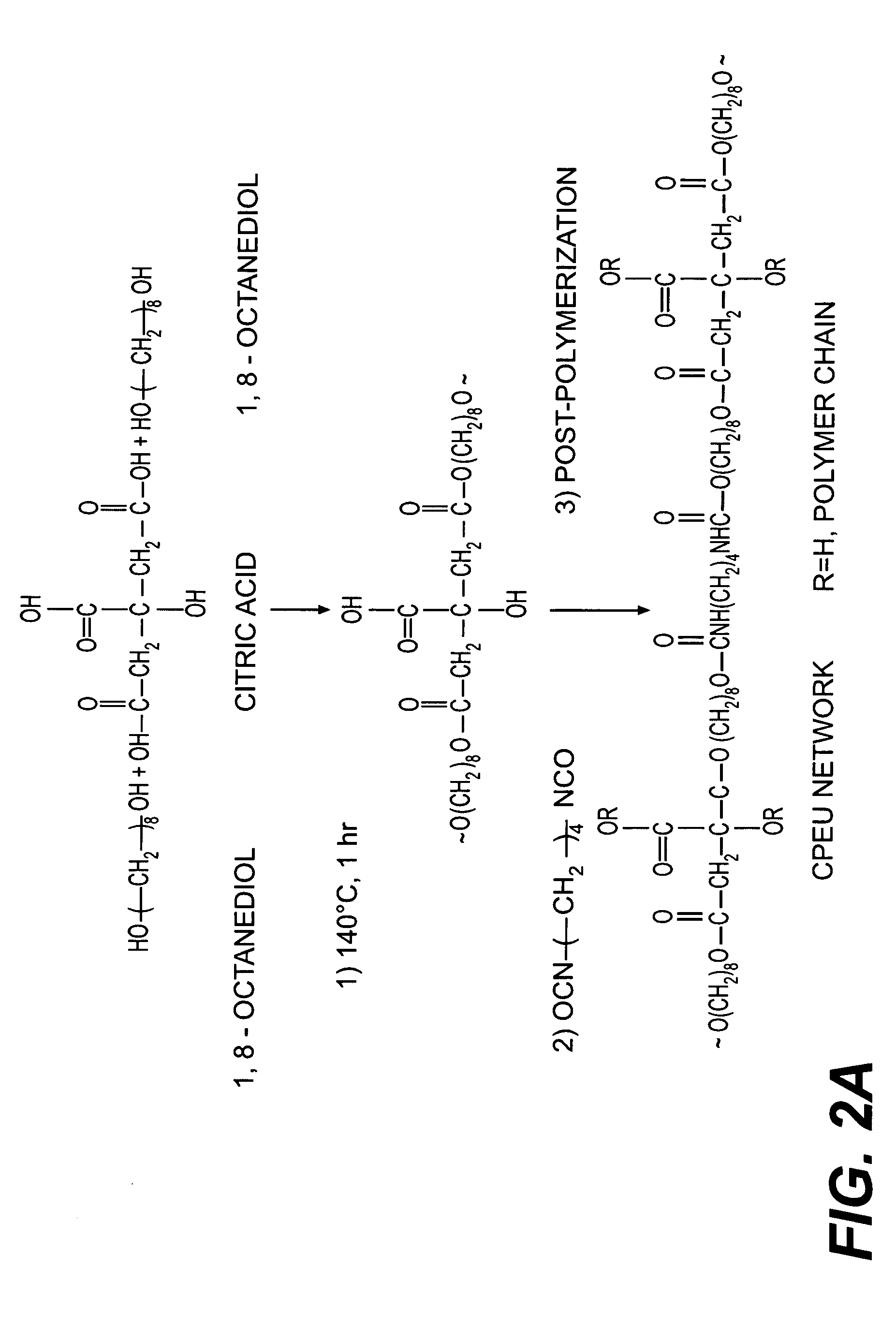
Bio-polymer and scaffold-sheet method for tissue engineering
ActiveUS20090093565A1Easy to divideAvoid communicationAdditive manufacturing apparatusSurgical adhesivesElastomerPolyester
A method of making a new type of biomaterials, biodegrable crosslinked urethane-containing polyester (CUPE) elastomers and a scaffold-sheet engineering method for tissue engineering applications is provided. CUPEs can be synthesized by forming a linear pre-polymer, which is a polyester, introducing the urethane bonds into polyester using a diisocyanate as a linker, and crosslinking the resulting urethane containing linear polymers to form CUPEs via post-polymerization. This family of polymers, CUPEs, exhibit excellent biocompatibility with desired degradation. Tissue engineering scaffolds made of CUPEs are soft and elastic, and have good mechanical strength. Complex tissue grafts can be constructed by a novel layer-by-layer (LBL) scaffold-sheet engineering design using CUPE sheets. CUPE scaffolds can provide openings for cell to cell communication across scaffold layers and angiogenesis into the depth of the construct. Biomolecules, such as anticoagulants, can be incorporated into the CUPE polymers, increasing their viability as vascular graft scaffolds.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Wearable ultrafiltration device
InactiveUS20080021366A1Inhibit swellingSteady and smooth removalHaemofiltrationUltrafiltrationMedicineUltrafiltration
An ultrafiltration device adapted to be worn on a portion of the body of a patient includes a blood inlet tube leading from a first blood vessel, a blood pump, an anticoagulant reservoir for infusing anticoagulants into the blood, a blood filter including a substrate through which the blood is circulated and filtered, a fluid bag for storing the excess fluid and a blood outlet tube leading to a second blood vessel.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Clotting cascade initiating apparatus and methods of use and methods of closing wounds
Wound closure methods and apparatus are provided which utilize blood fluid by activating the clotting cascade of the blood fluid outside the body within a substantially enclosed sterile container then introducing the blood fluid to the wound site to complete clotting. Methods and apparatus for providing ways of inhibiting anticoagulants and slowing fibrin clot degradation are also disclosed. Kits for practicing the invention singularly or in combination with and / or associated with preferred procedures are also disclosed.
Owner:CLOSYS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com