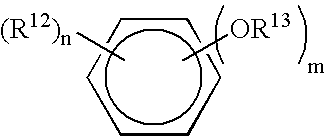
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
14652results about "Medical packaging" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
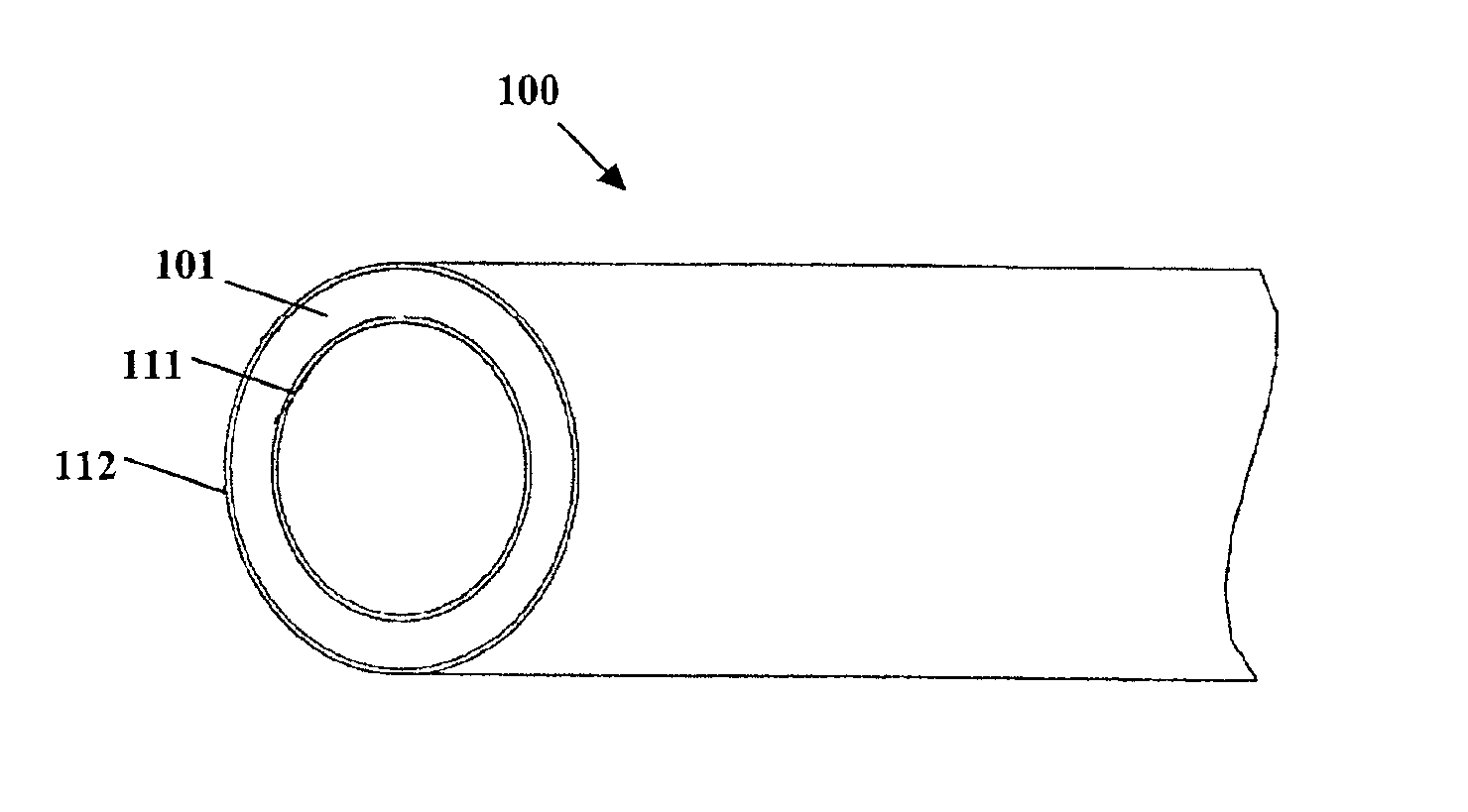
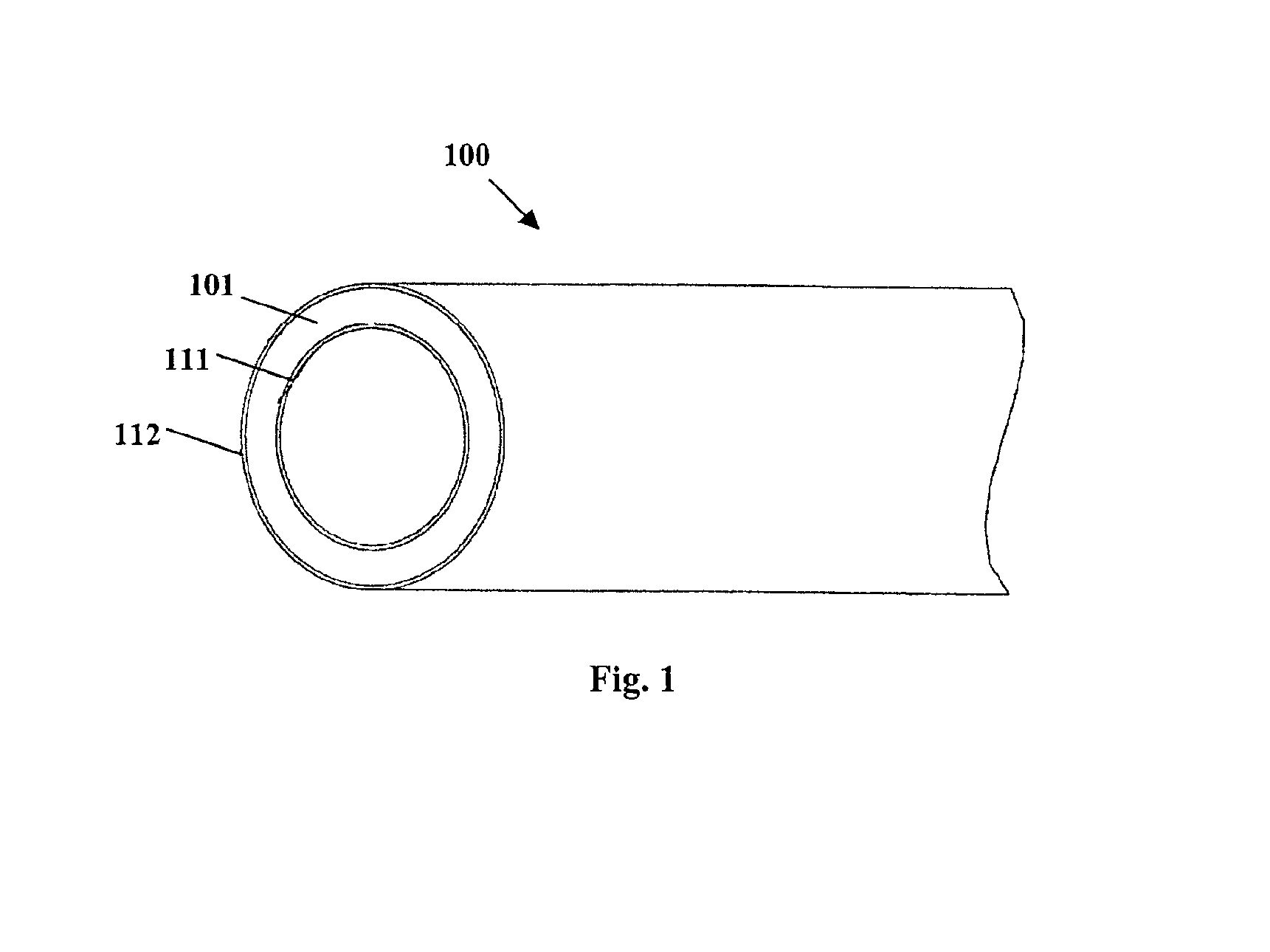
Method for preparing two-layer bicomposite collagen material for preventing post-operative adhesions
InactiveUS6596304B1Improve propertiesAvoid stickingPeptide/protein ingredientsSurgerySurgical operationPost operative
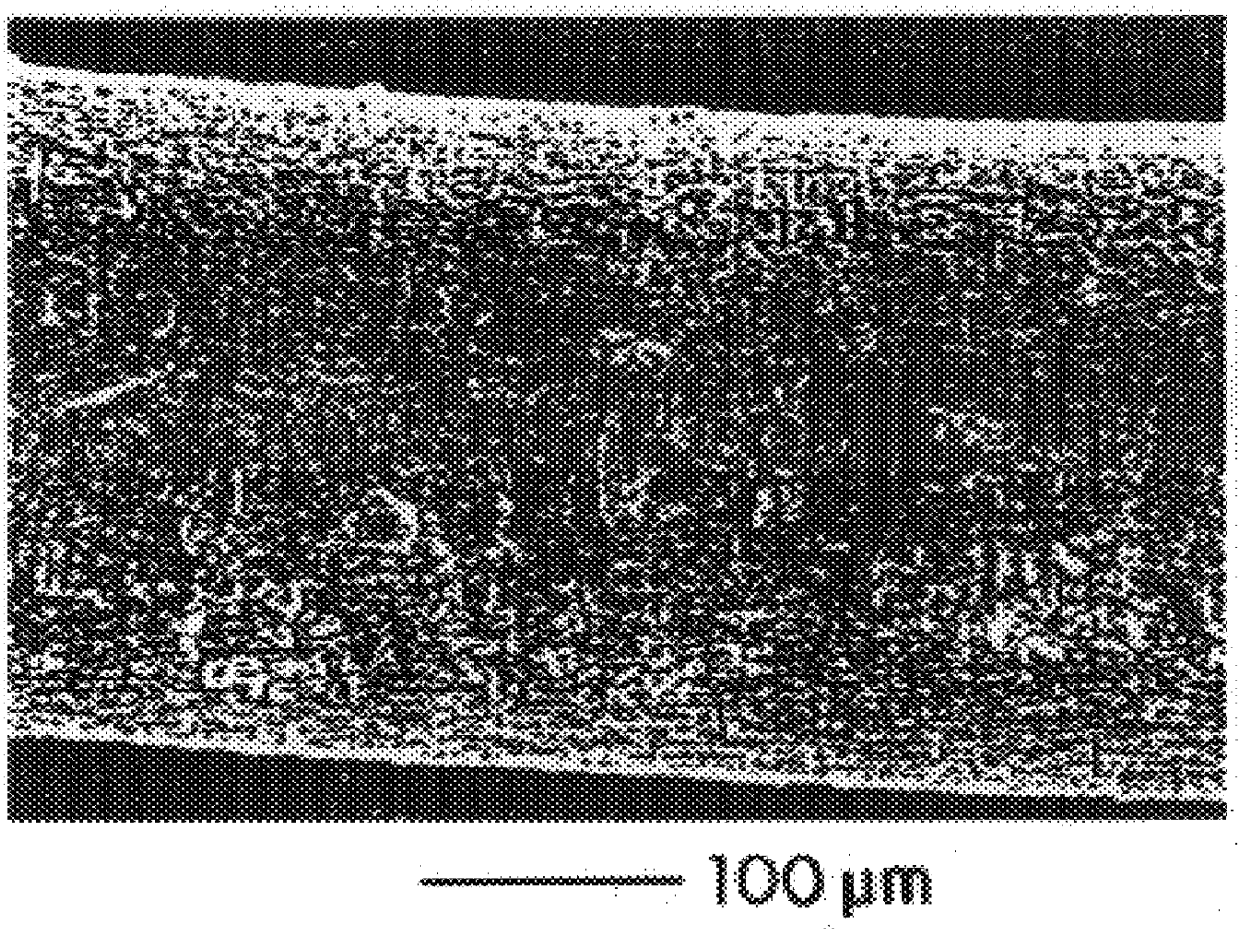
A bicomposite material based on collagen is prepared which has two closely bound layers and is biocompatible, non-toxic, hemostatic and biodegradable in less than a month, and can be used in surgery to achieve hemostasis and prevent post-surgical adhesion. To prepare the material, a solution of collagen or gelatin, which may contain glycerine and a hydrophilic additive such as polyethylene glycol or a polysaccharide, is poured onto an inert support to form a layer 30 .mu.m to less than 100 .mu.m thick. Then a polymeric porous fibrous layer is applied during gelling of the collagen or gelatin, and the resultant material is dried. The polymeric porous fibrous layer may be made of collagen or a polysaccharide, and have a density of not more than 75 mg / cm.sup.2, a pore size from 30 .mu.m to 300 .mu.m and a thickness of 0.2 cm to 1.5 cm.
Owner:IMEDEX BIOMATERIAUX CHAPONOST
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Medical devices having durable and lubricious polymeric coating
A medical device having a contact surface exposed repeatedly to bodily tissue is disclosed. The contact surface is coated with a silicone polymer and one or more non-silicone hydrophobic polymers. The preferred medical device is a surgical needle, and the preferred coating is a polydimethylsiloxane and polypropylene wax hydrocarbon mixture. The incorporation of the non-silicone hydrophobic polymer increases the durability of the coating on the device without sacrificing lubricity.
Owner:ETHICON INC
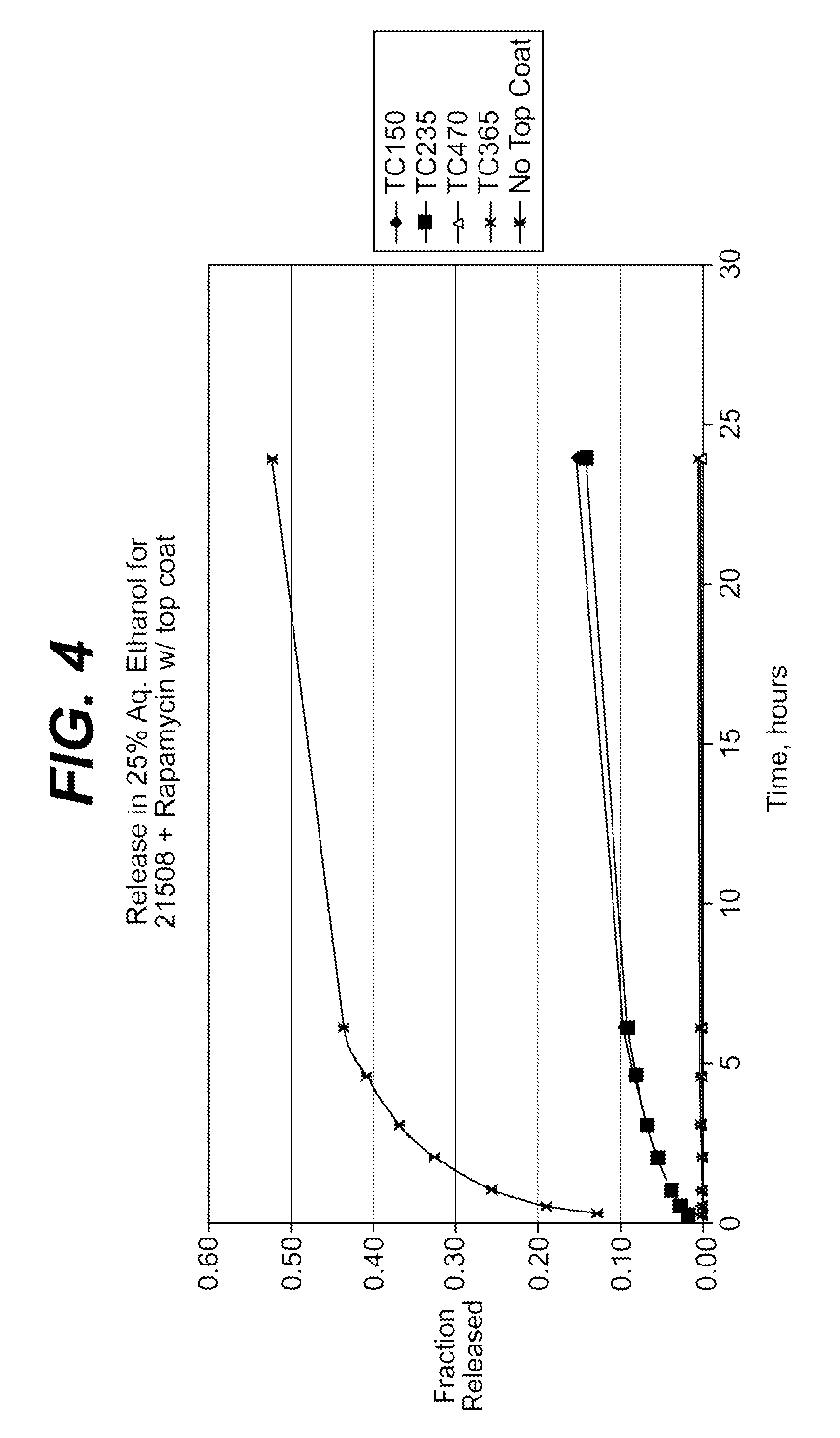
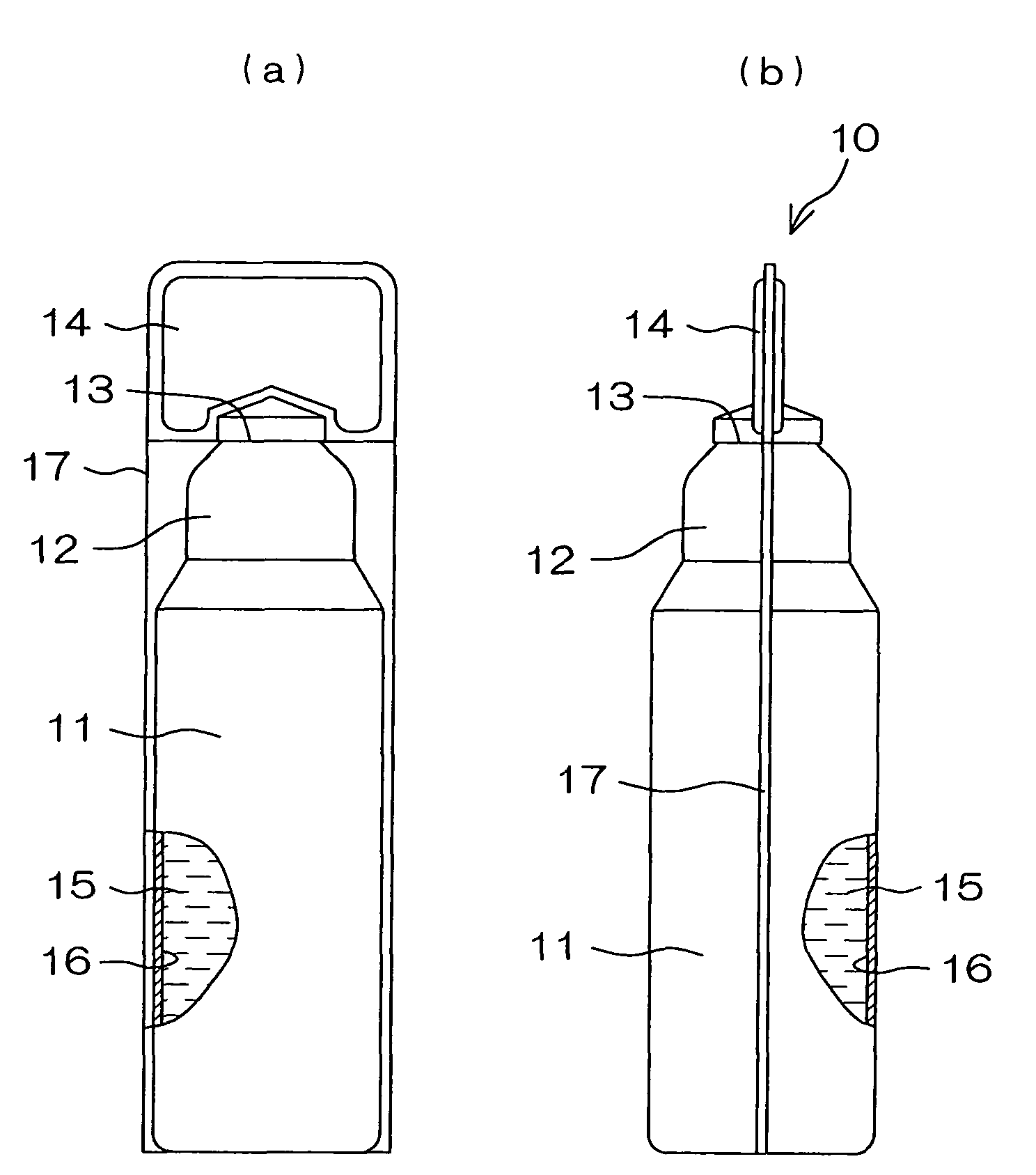
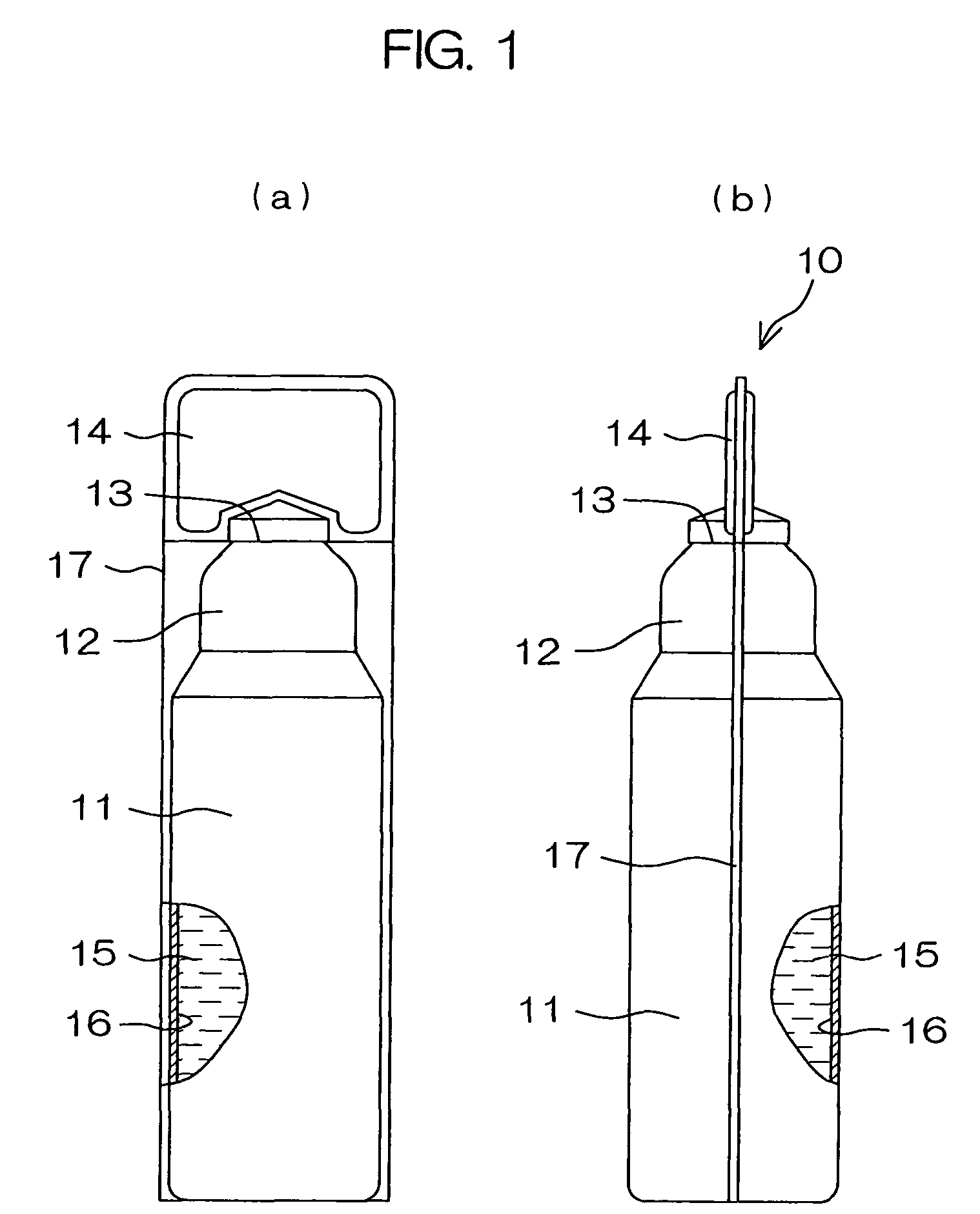
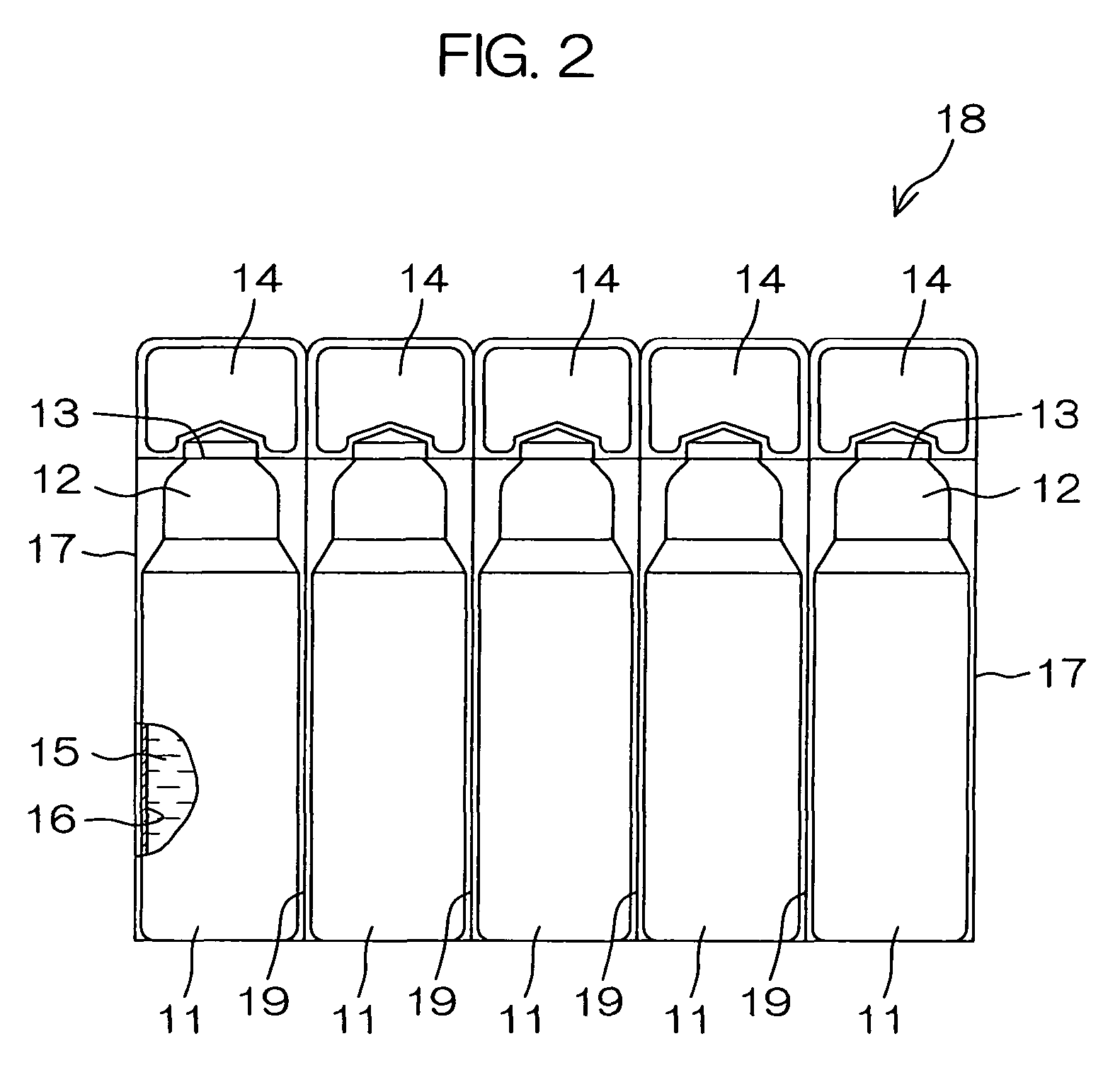
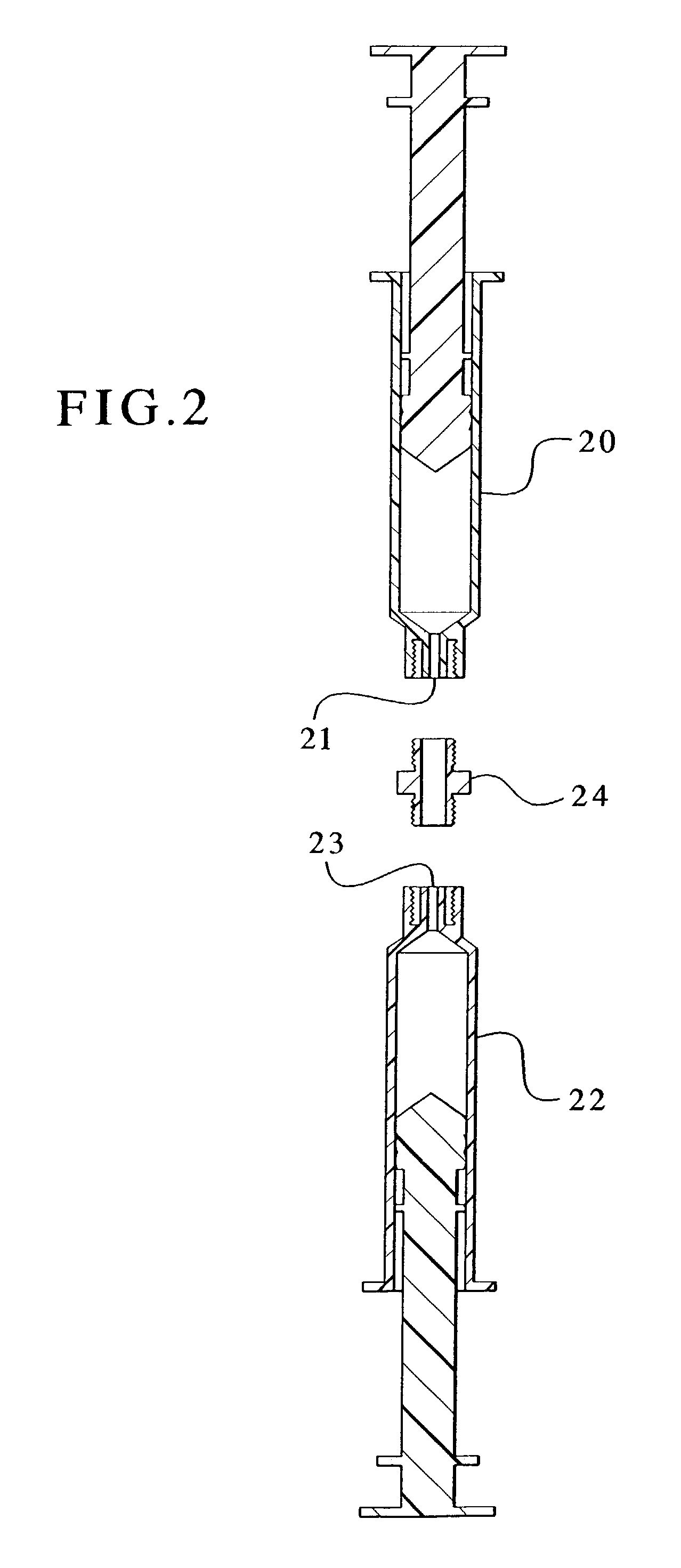
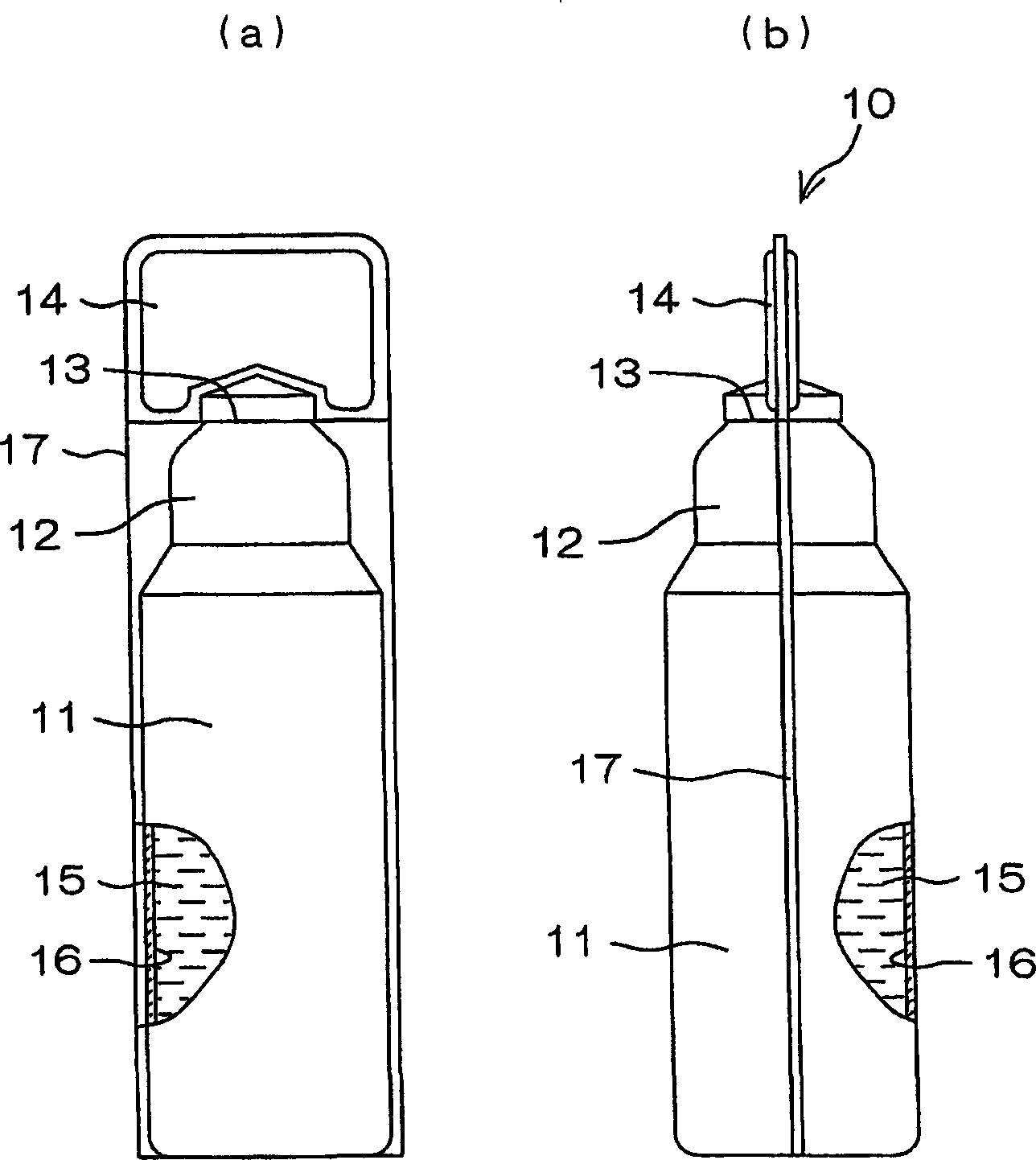
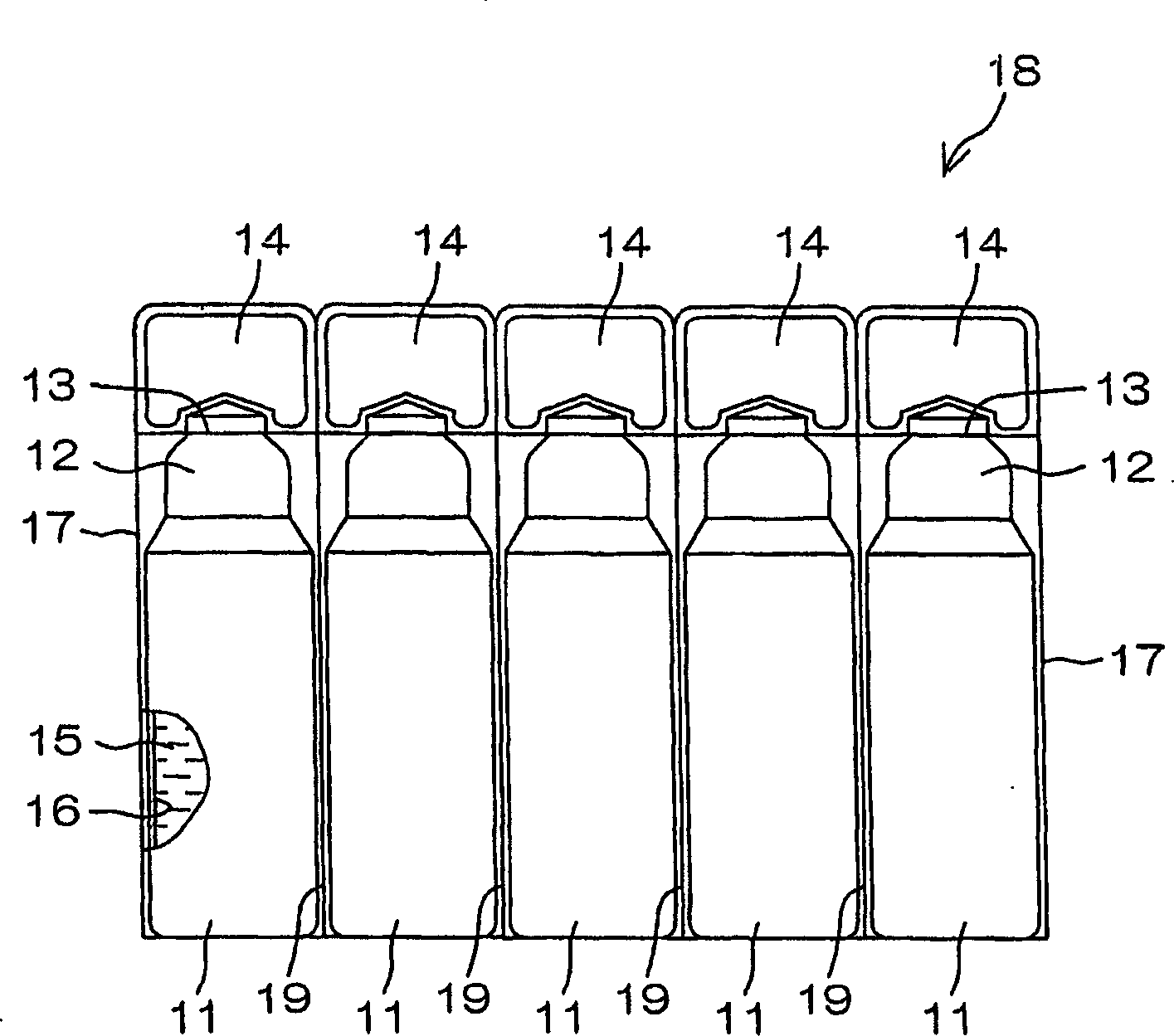
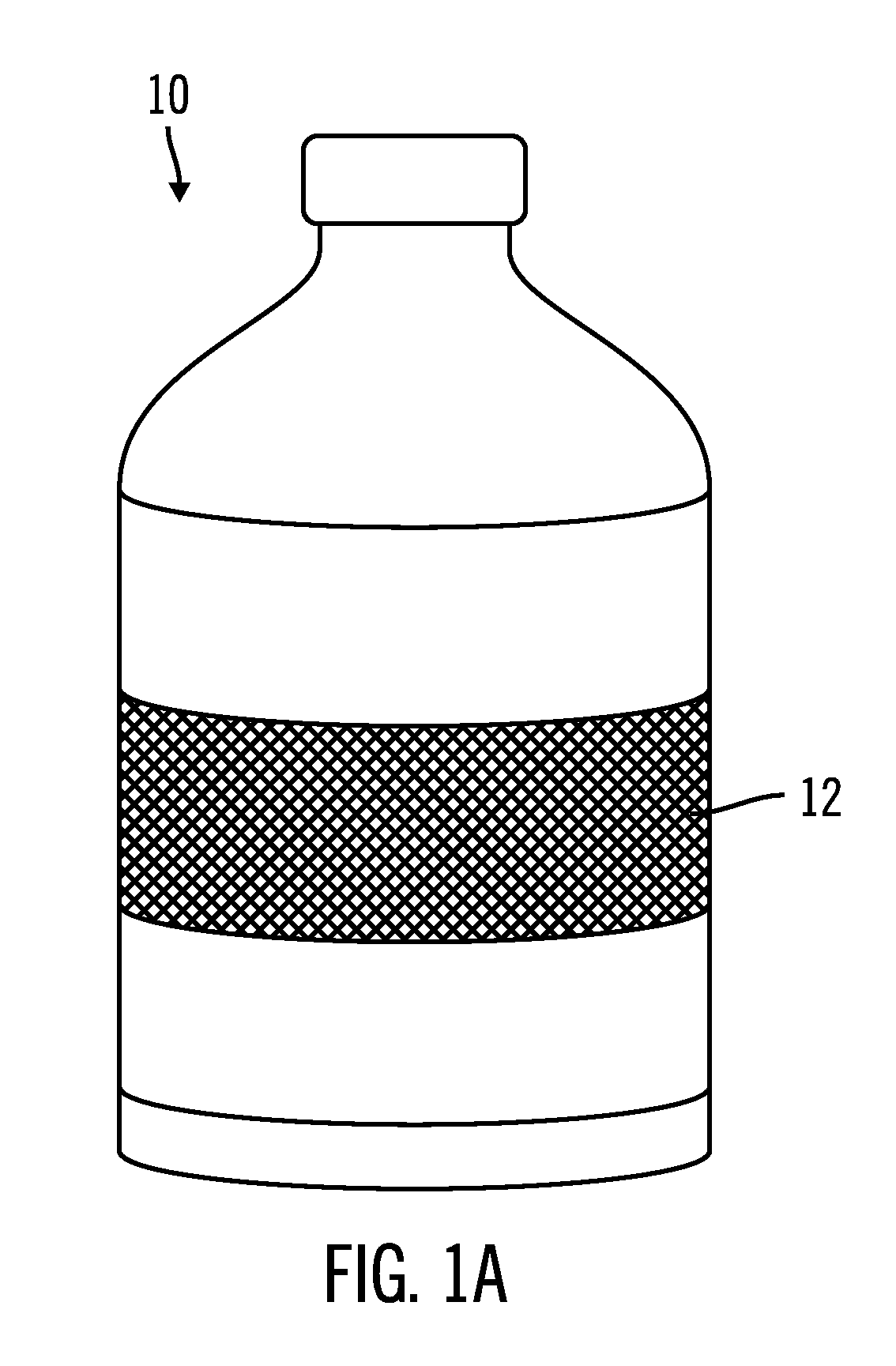
Drug solution filling plastic ampoule and process for producing the same
A drug solution filling plastic ampoule having gas, steam and light ray barrier properties, a drug permeation preventing capability and an absorption / adsorption preventing capability, and a production method for the plastic ampoule. The drug solution filling plastic ampoule includes a container body, a fusion-bonded portion which seals a mouth of the container body, and a wrench-off holder tab connected to the fusion-bonded portion. The ampoule is formed from a parison including two or more layers, at least one of which is a functional layer having at least one characteristic property selected from the group consisting of a gas permeation preventing capability, a steam permeation preventing capability, a light ray permeation preventing capability, a drug permeation preventing capability and a drug absorption / adsorption preventing capability.
Owner:OTSUKA PHARM FAB INC
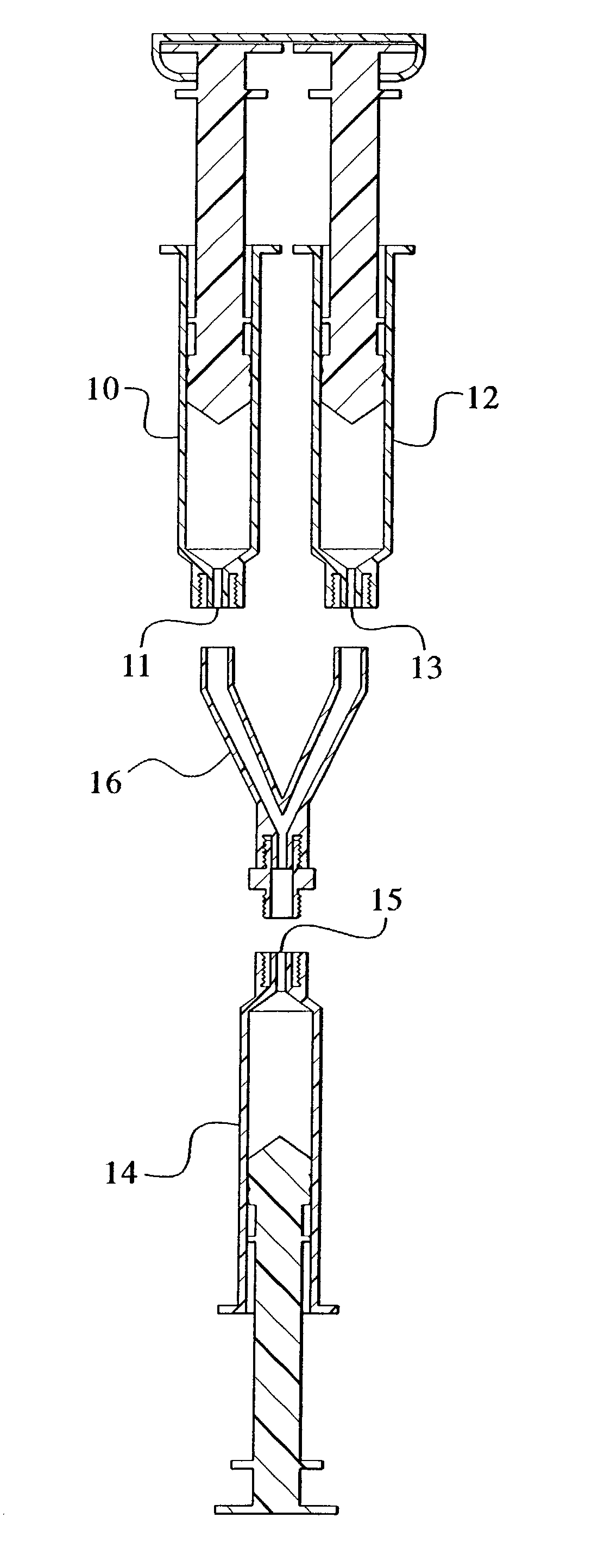
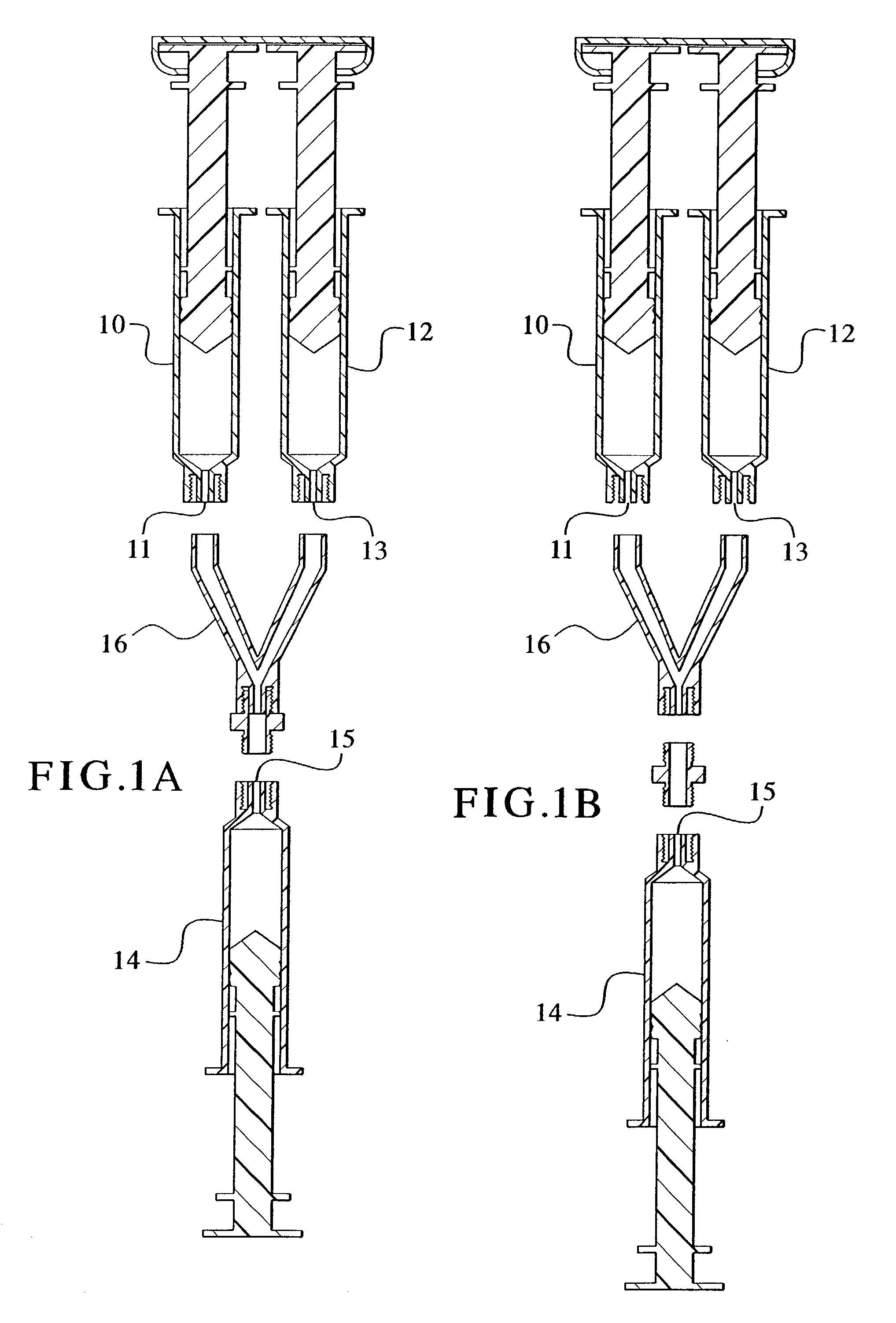
Devices and methods for mixing and extruding medically useful compositions
This invention provides devices and methods for mixing and extruding compositions which are medically and non-medically useful. The devices are particularly useful for mixing substances which are relatively inert when alone but become reactive when mixed. A common feature of all of the devices is that they allow the user to mix and ultimately extrude a composition from a single device which includes a single container or multiple interconnected containers.
Owner:BAXTER INT INC +1
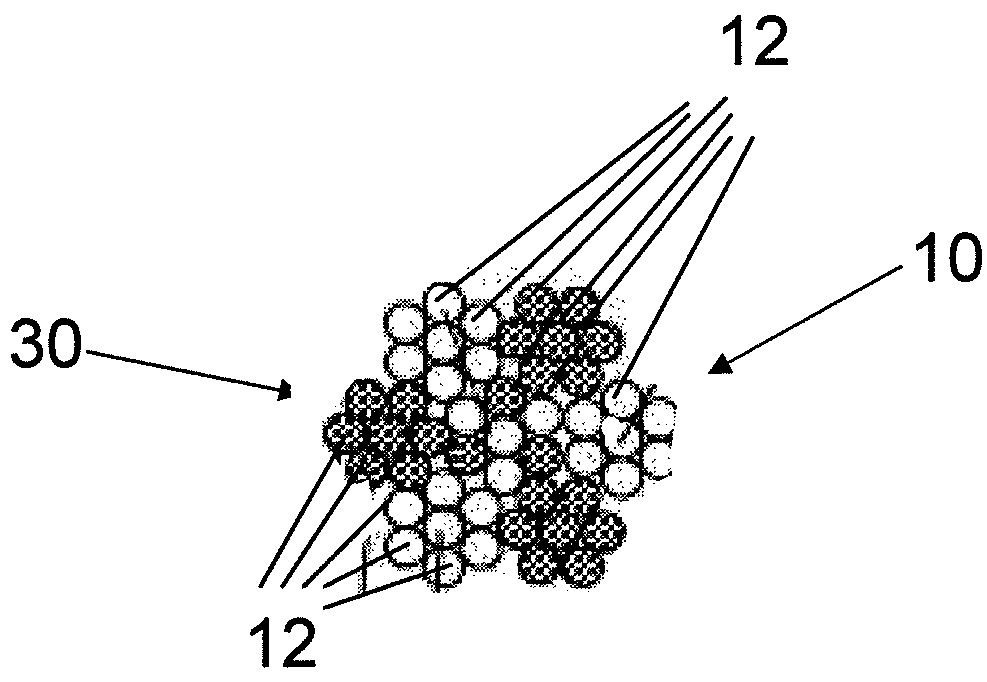
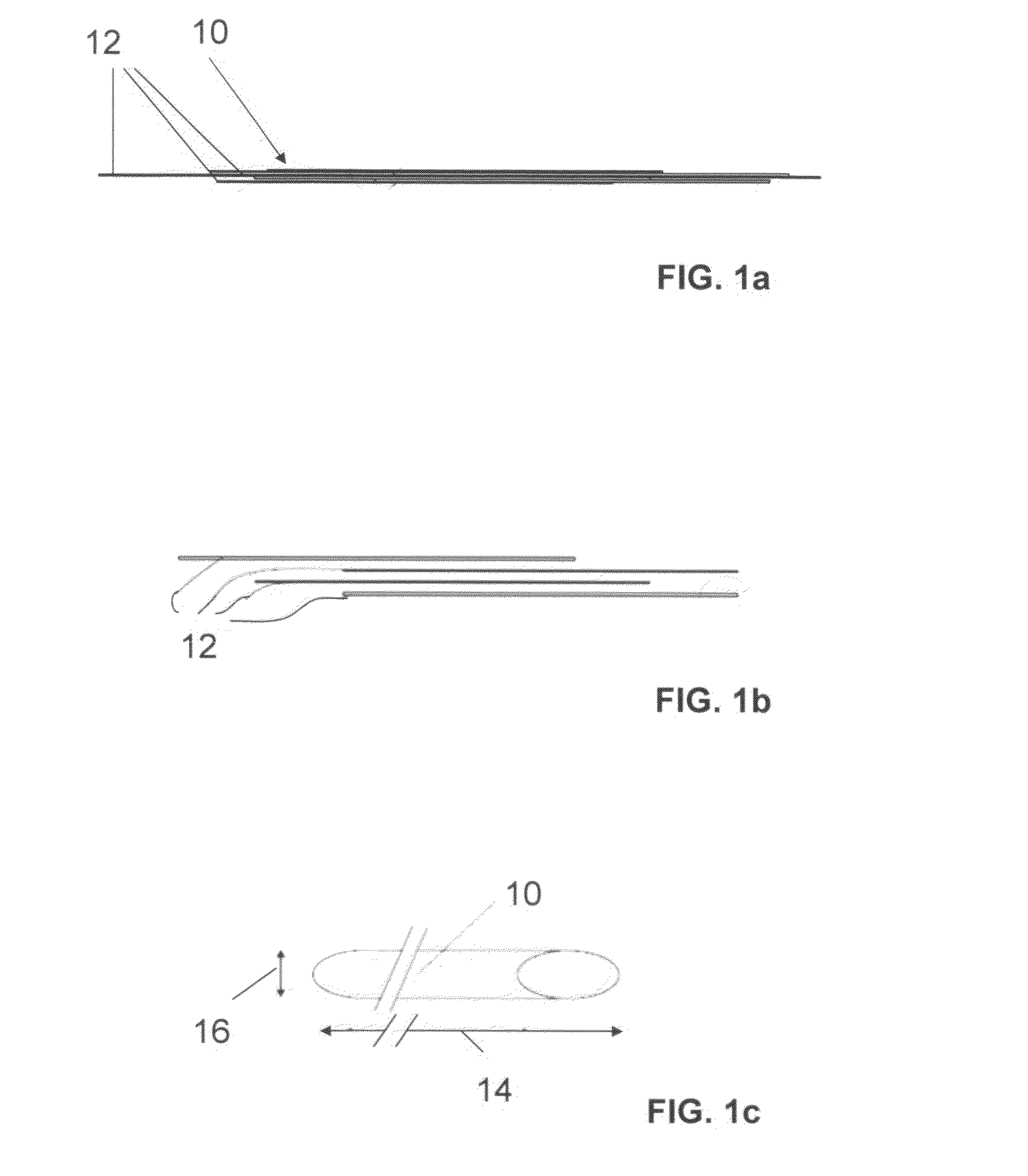
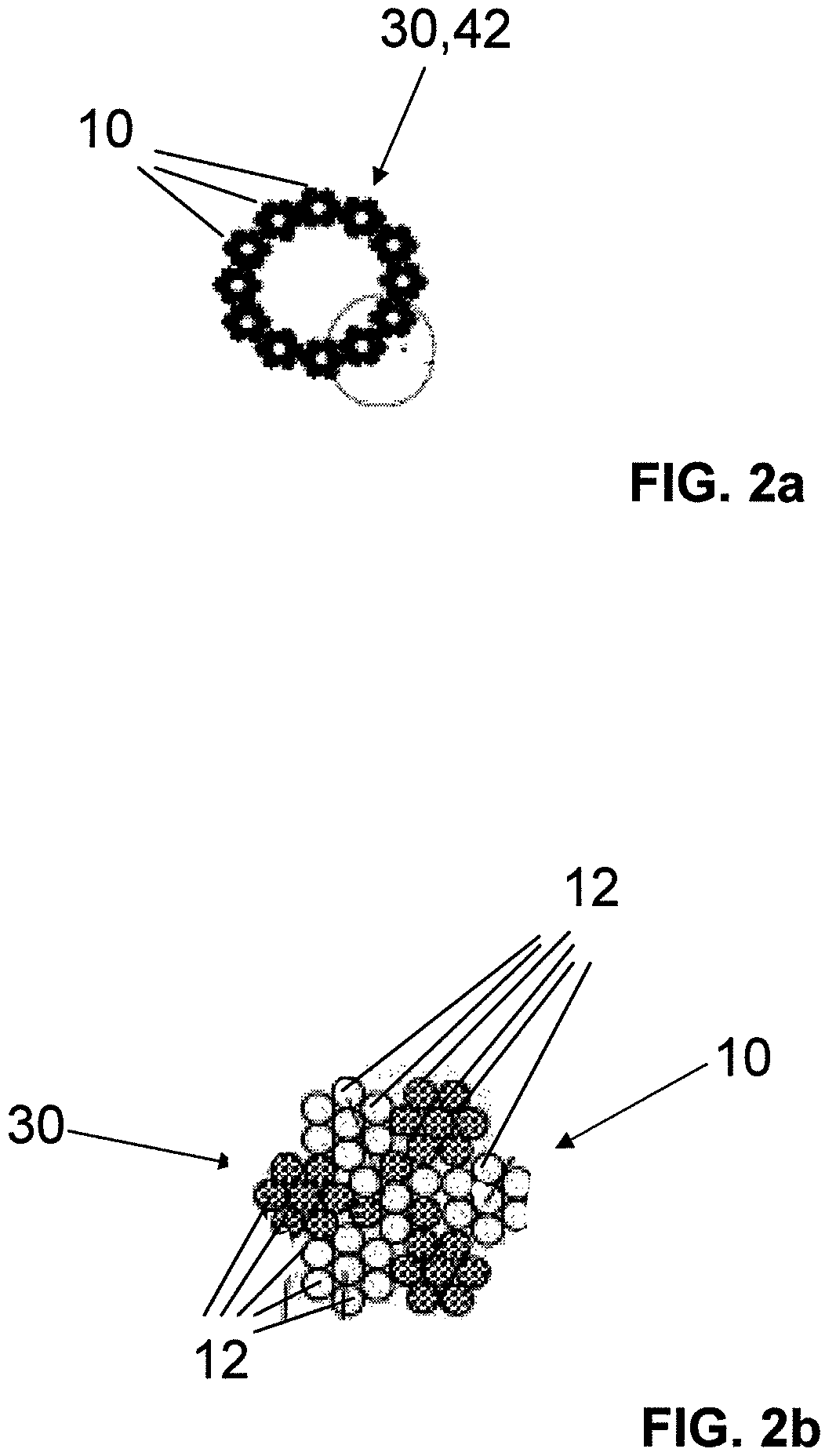
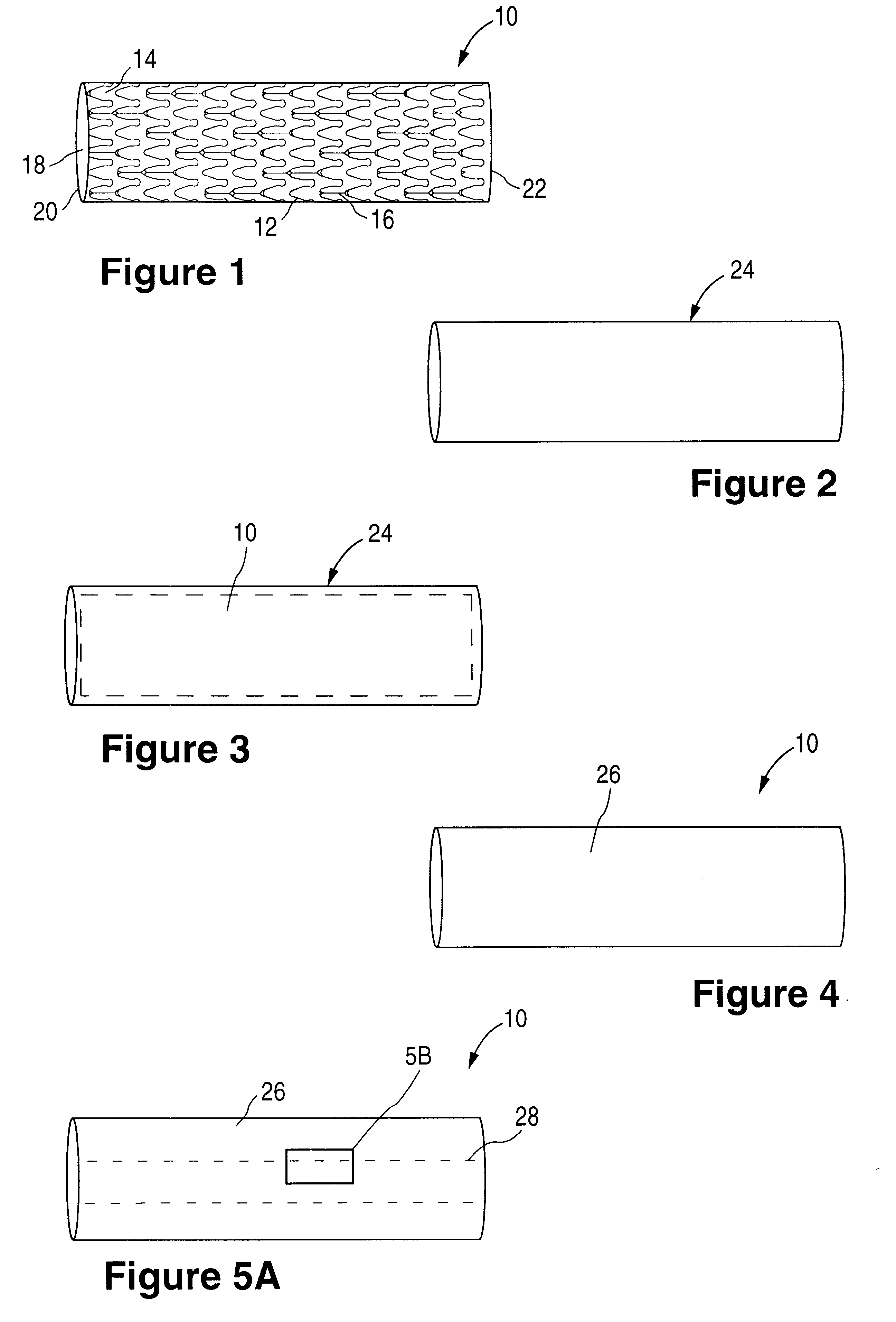
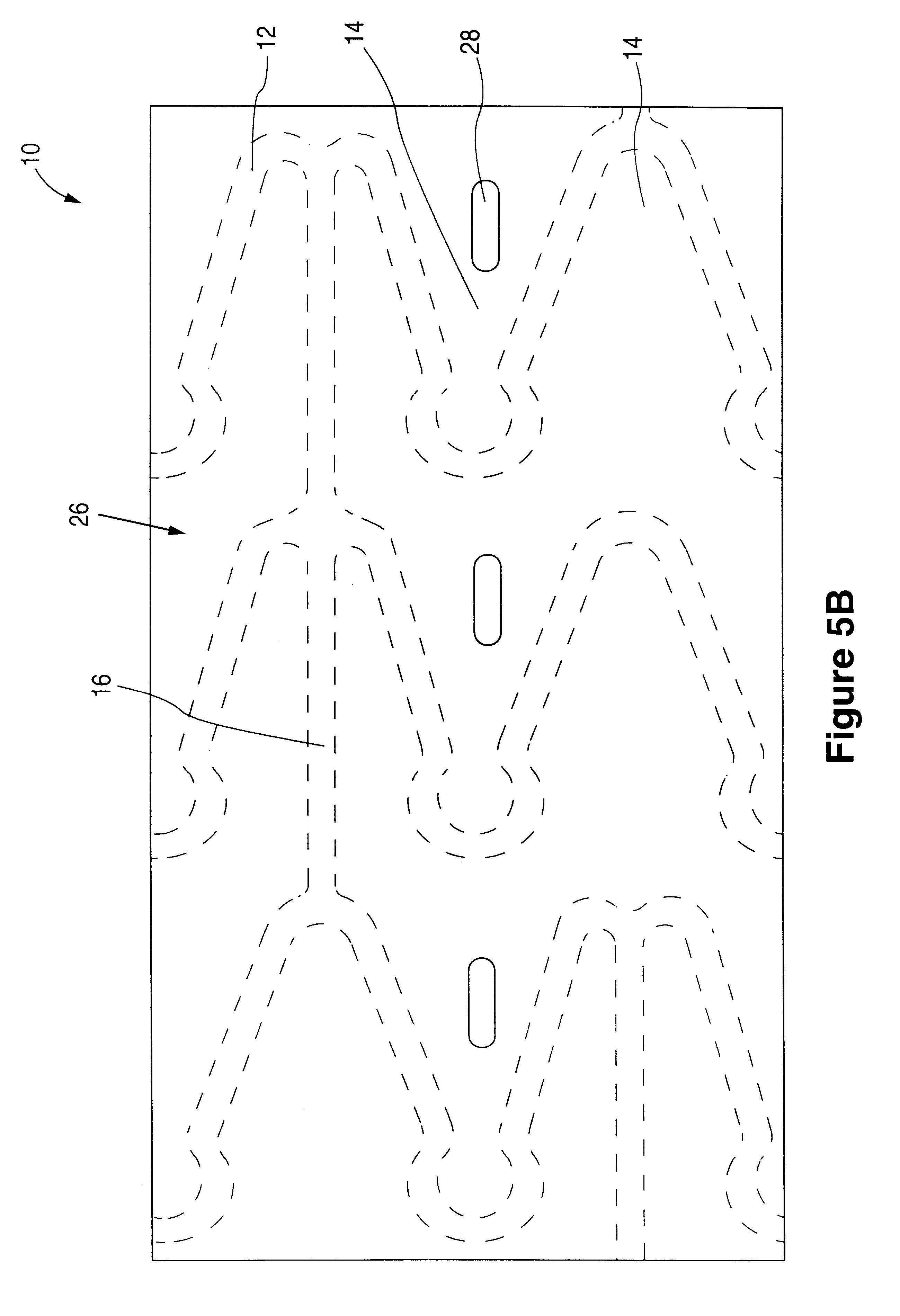
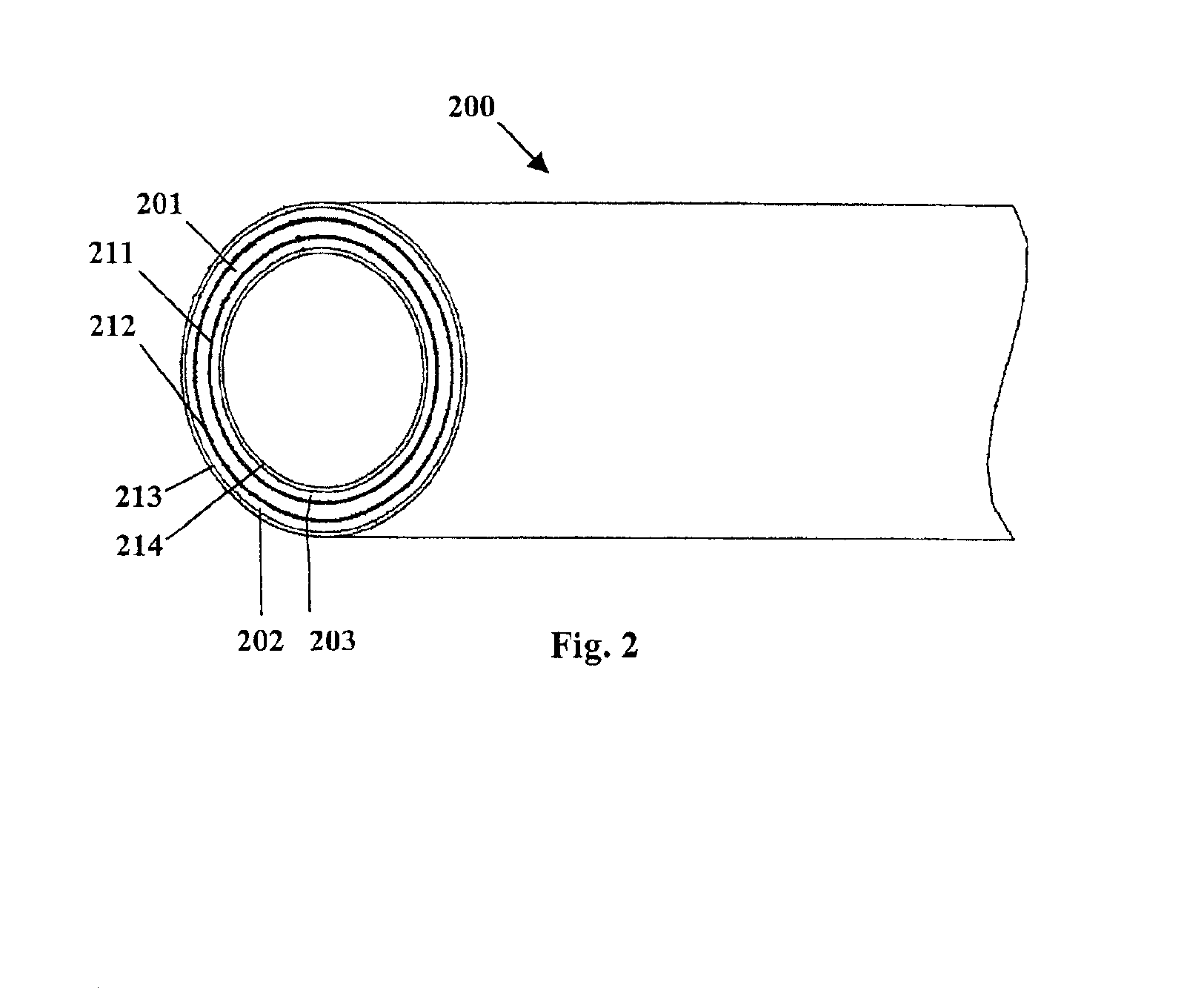
Fiber strand and implantable supporting body having a fiber strand
InactiveUS7997054B2Desired mechanical properties can be adjusted especially easilySignificant positive effectPowder deliveryStentsFiberBiomedical engineering
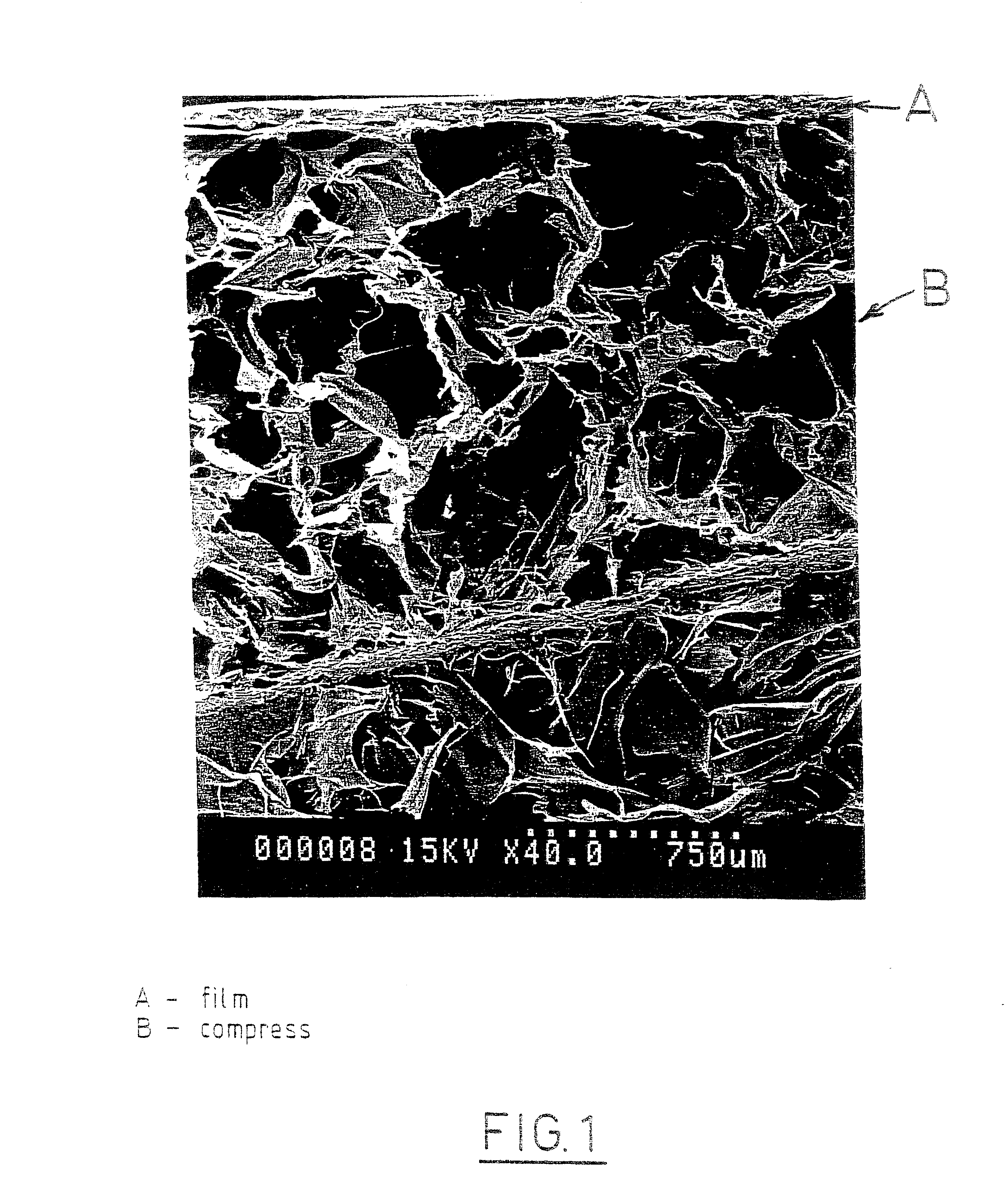
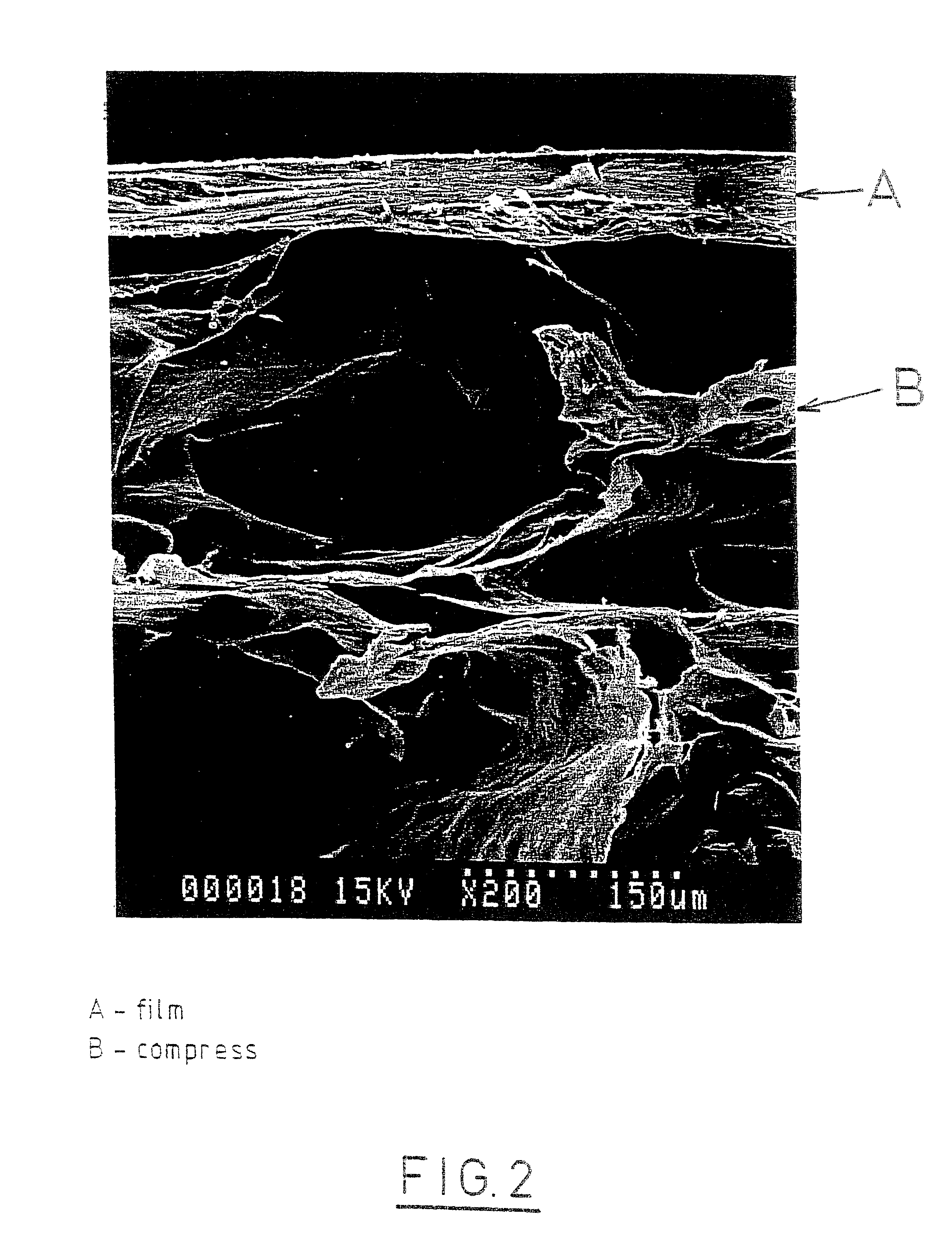
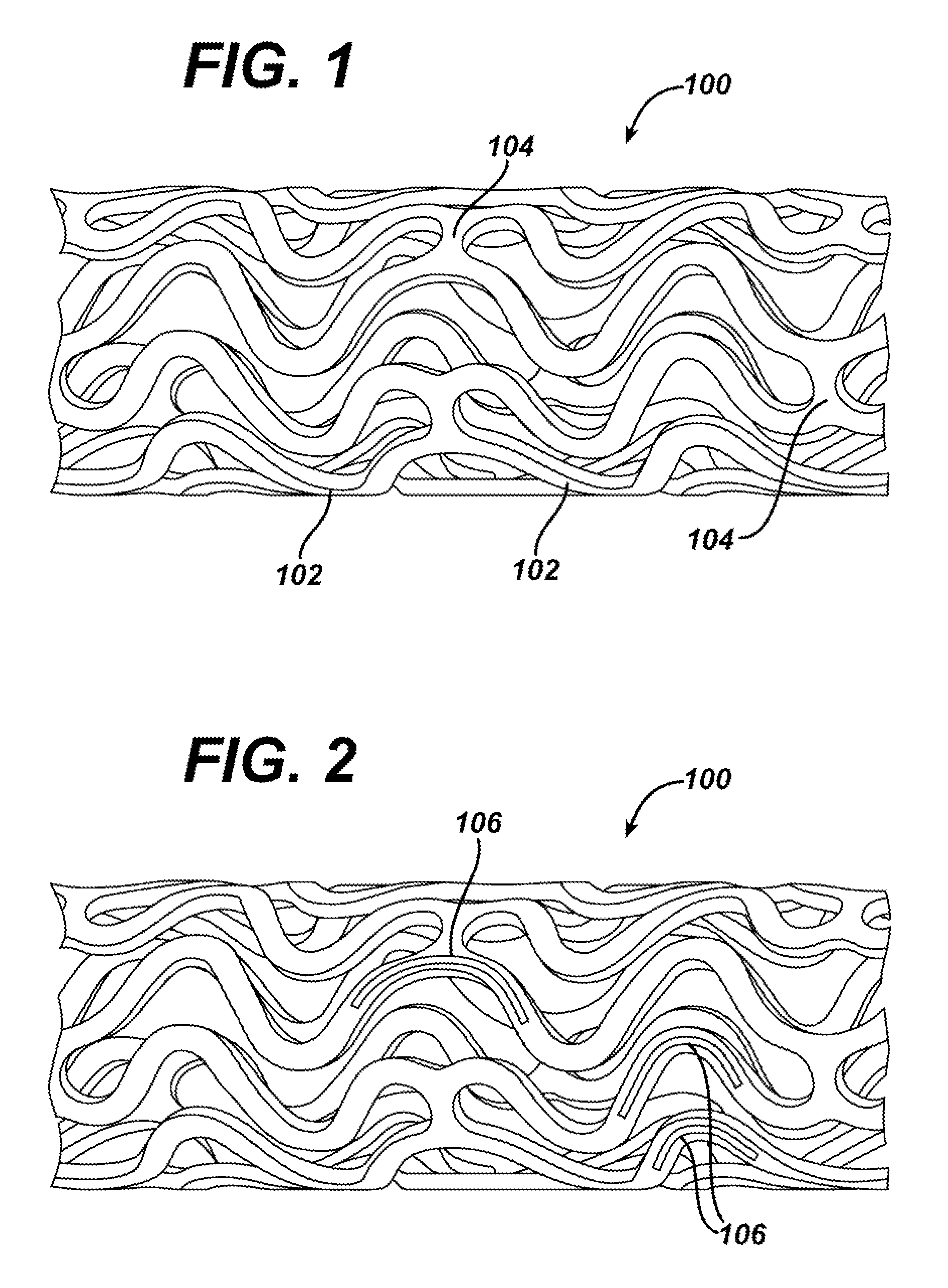
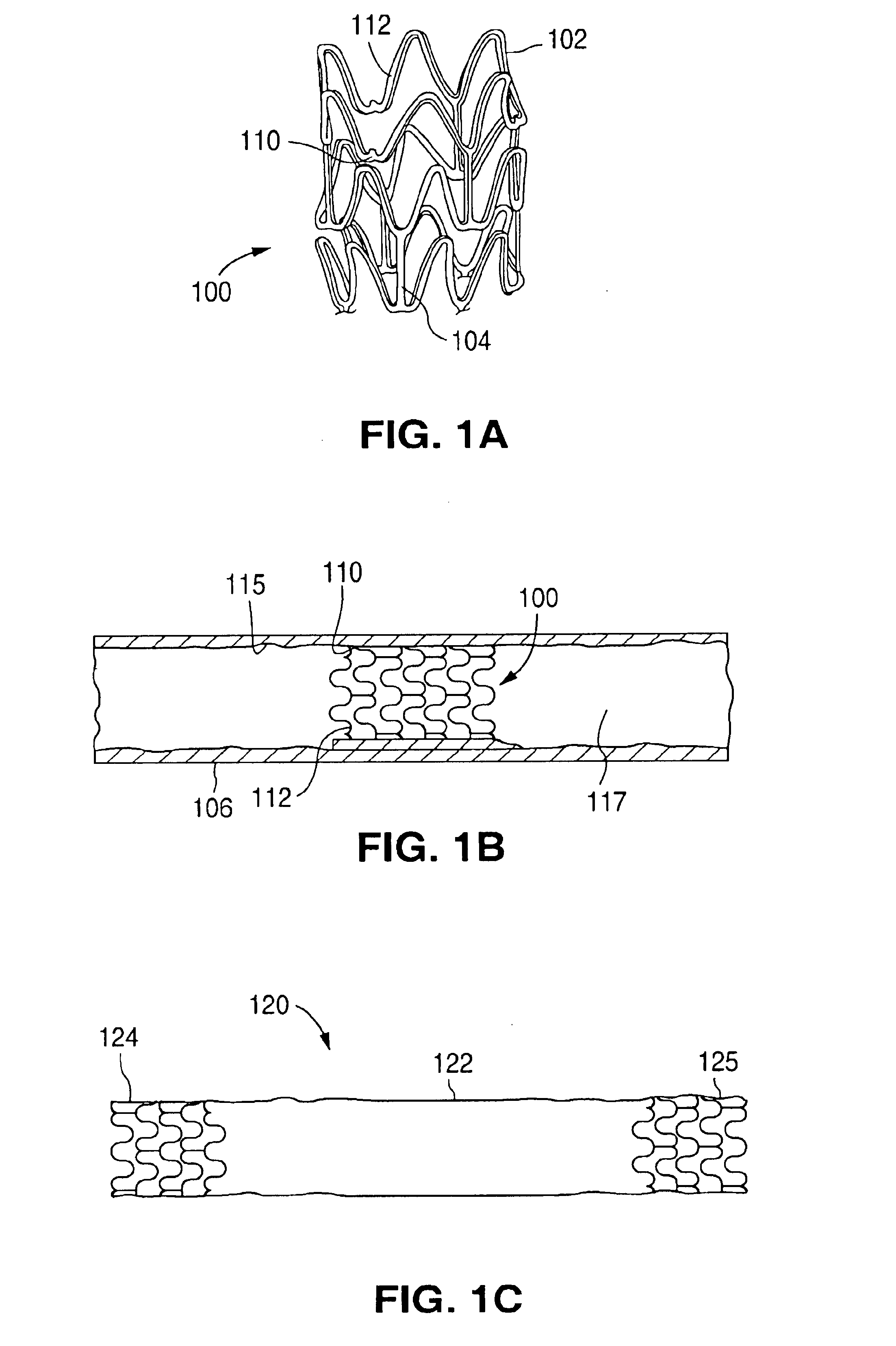
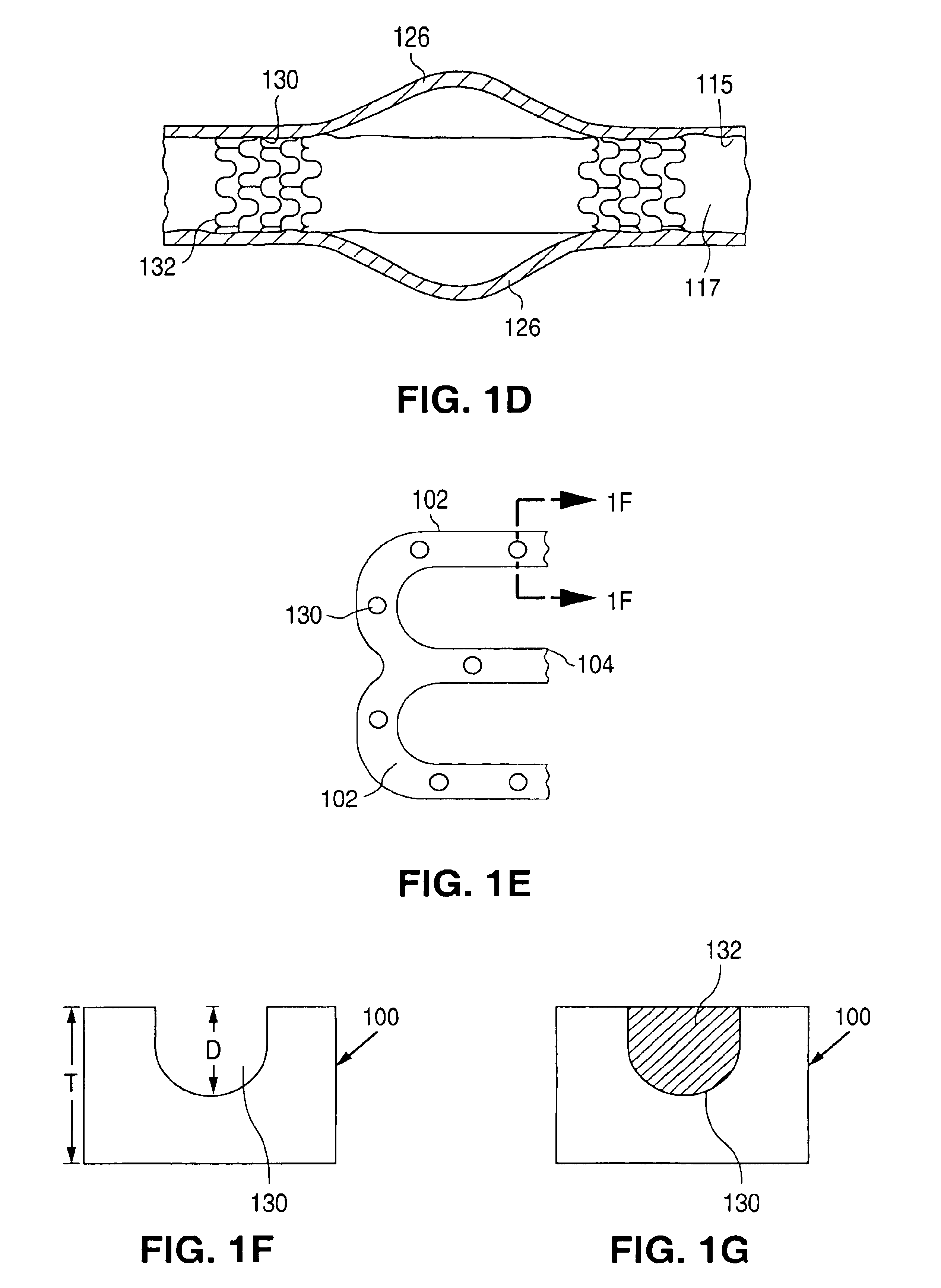
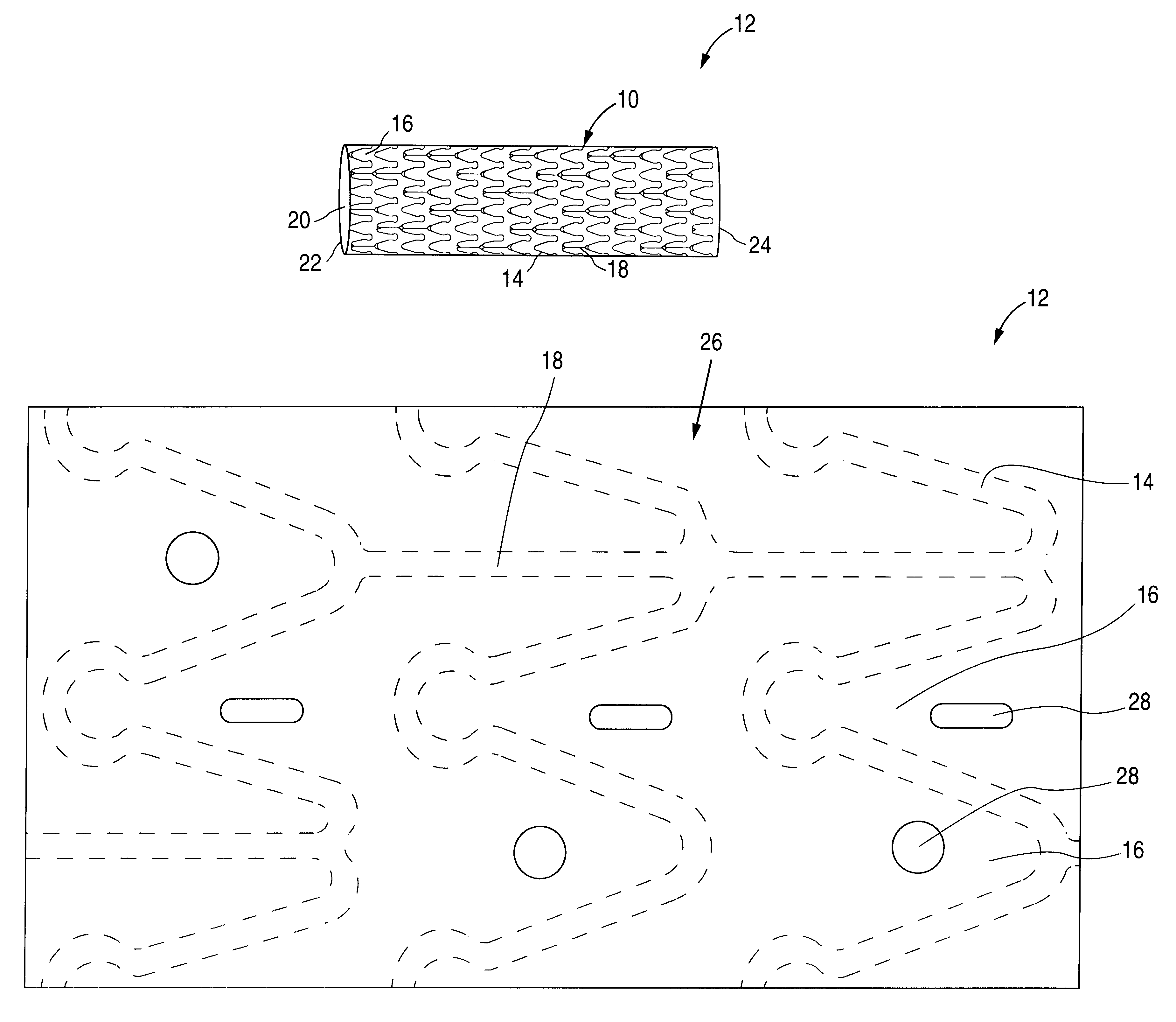
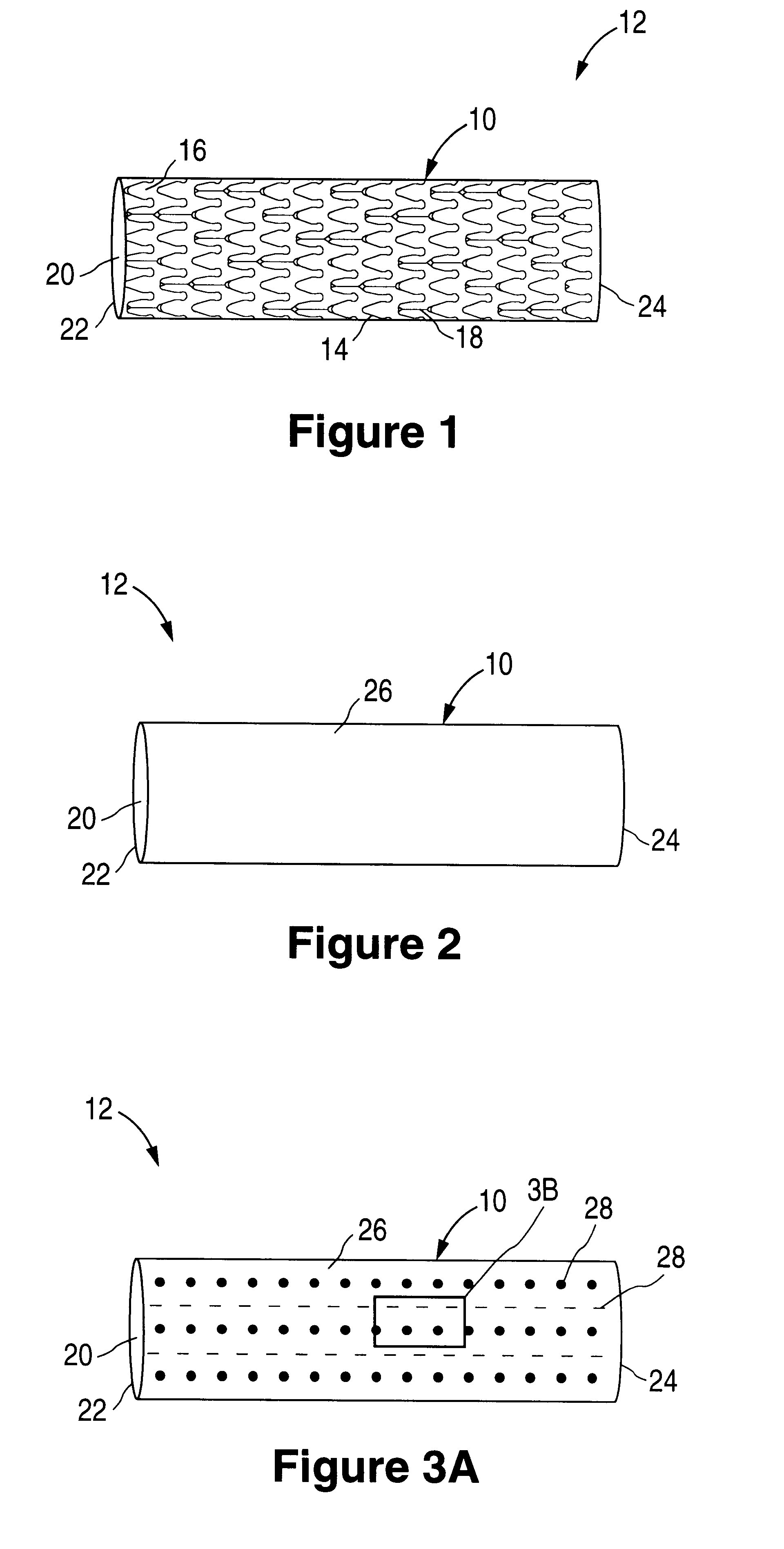
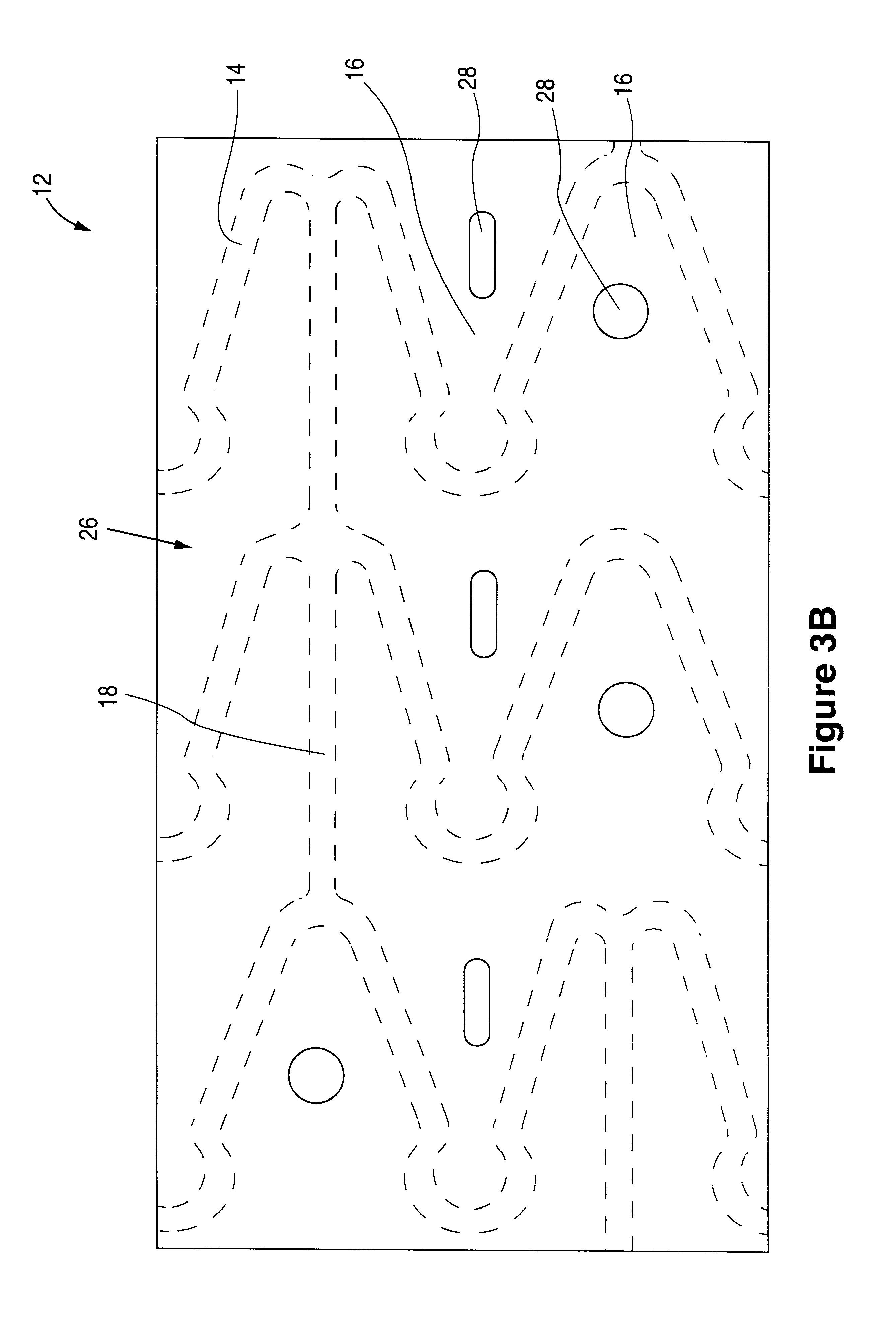
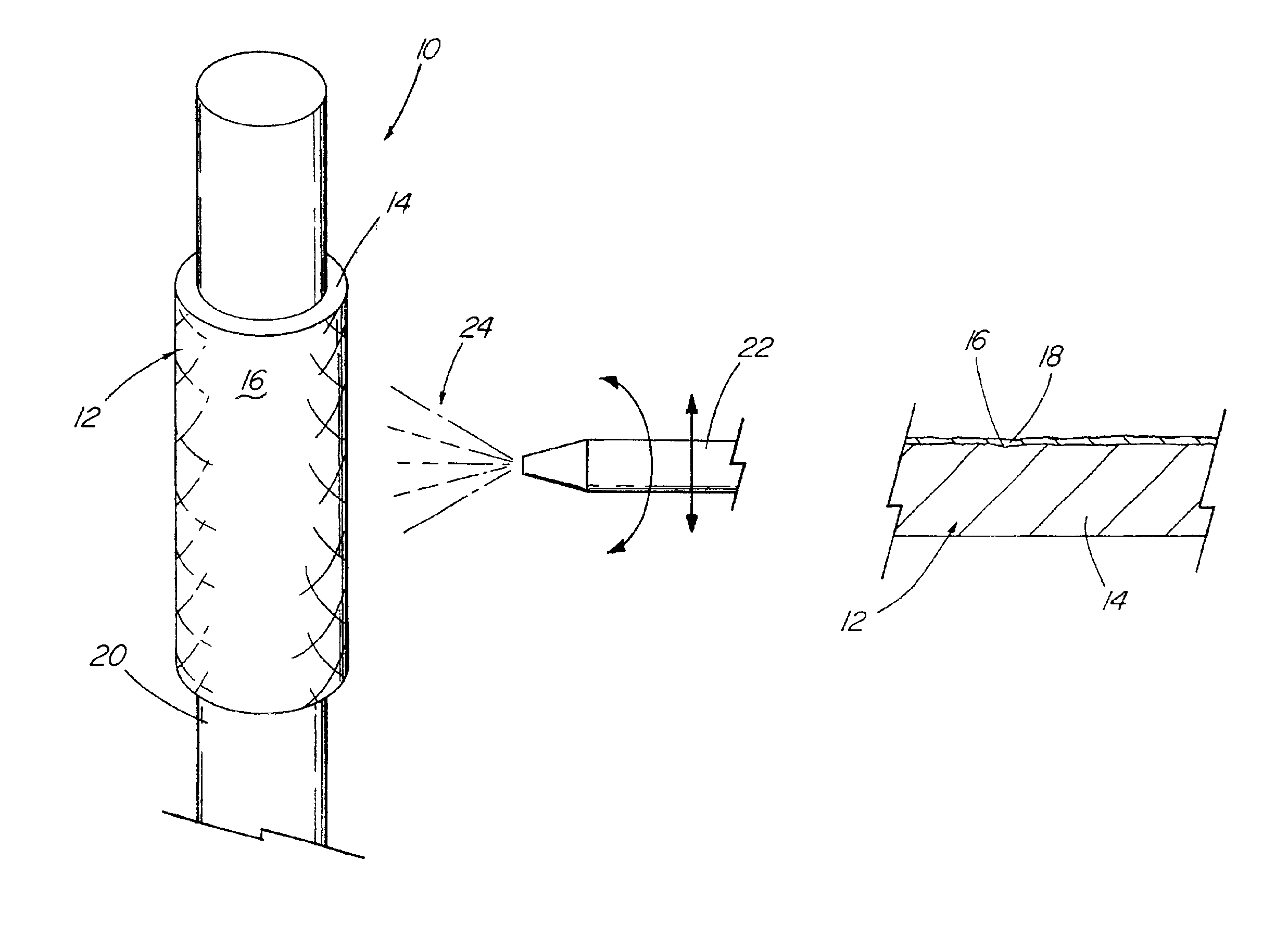
The invention relates to a fiber strand (10) for an implantable supporting body (100) comprising at least two individual fibers (12). The at least two individual fibers (12) are each shorter in their longitudinal extent than the longitudinal extent (14) of the fiber strand, and in their transverse extent they are each thinner than the transverse extent (16) of the fiber strand.
Owner:BIOTRONIK AG
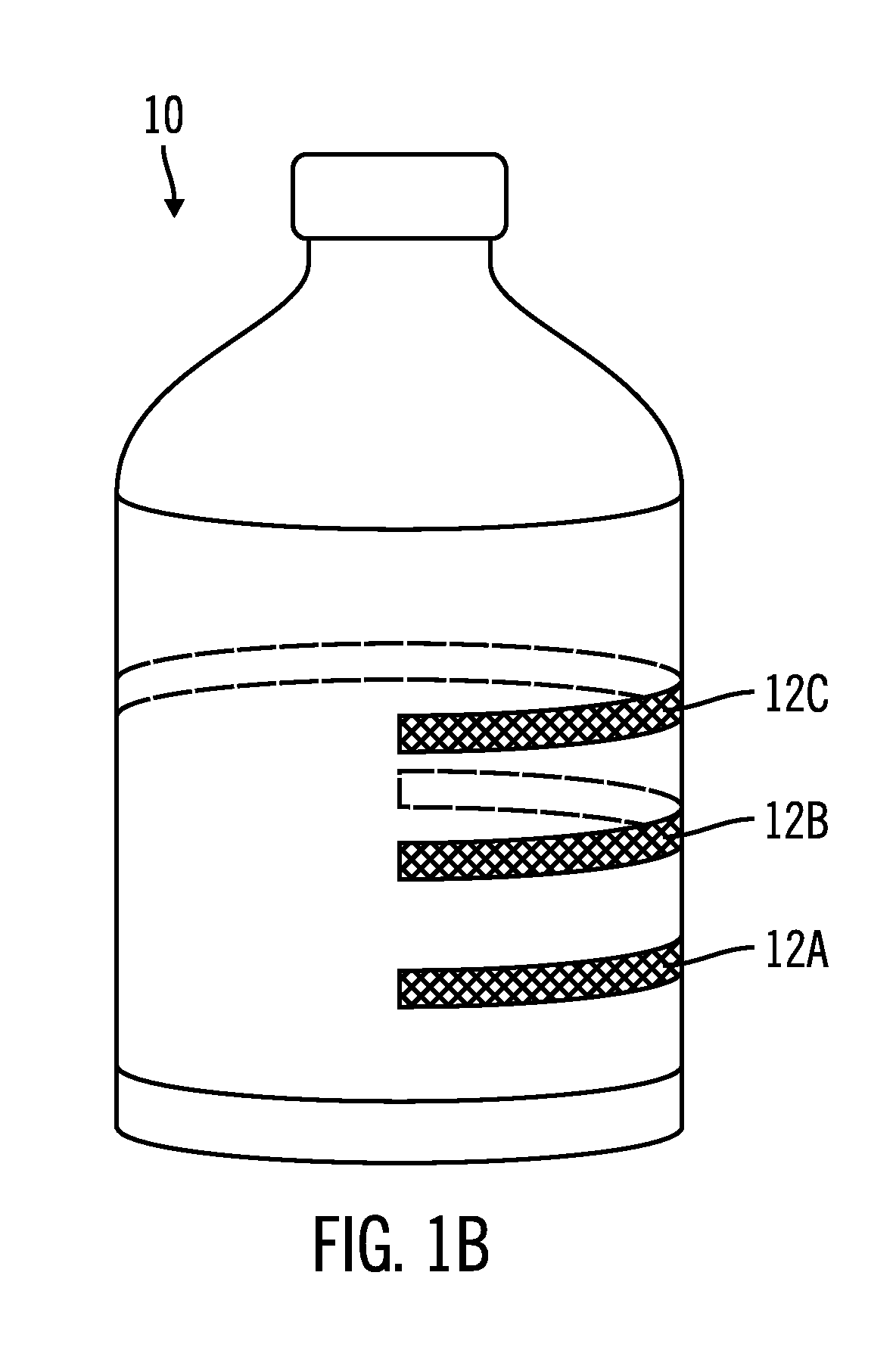
Drug solution filling plastic ampoule and production method therefor
The invention provides a plastic ampoule filled with medicinal liquid, which has the functions of blocking gas, water vapor and light and preventing drug penetration, absorption and adsorption, and a preparation method thereof. The plastic ampoule 10 for filling medicinal solution of the present invention comprises a container main body 11, a fused portion 13 closing its opening 12 and a holding portion 14 connected thereto for twisting. The ampoule 10 is formed using a parison having two or more layers, at least one of which is a material selected from the group consisting of gas transmission, water vapor transmission, light transmission, drug transmission and A functional layer that prevents at least one of drug absorption and adsorption properties. That is, the parison is extruded from a multi-layer blow mold, clamped by the lower parting die to form the main body part 11 of the container, and after filling the liquid medicine 15 therein, the opening part 12 is clamped by the upper parting die to form a fusion joint. Part 13 and holding part 14, thereby making the product of the present invention.
Owner:OTSUKA PHARM FAB INC
Methods of forming a coating for a prosthesis
InactiveUS6503556B2Increase the amount addedIncrease the number ofRadiation applicationsGlovesProsthesisImplanted device
Methods of forming a coating onto an implantable device or endoluminal prosthesis, such as a stent, are provided. The coating may be used for the delivery of an active ingredient. The coating may have a selected pattern of interstices for allowing a fluid to seep through the coating in the direction of the pattern created.
Owner:ABBOTT CARDIOVASCULAR
Compositions and methods for coating medical devices
InactiveUS6143037AStimulates and promotesImproved wound healing characteristicSuture equipmentsInternal osteosythesisDiseaseMedical device
Owner:MICHIGAN REGENTS THE UNIV OF
Continuous analyte sensors and methods of making same
InactiveUS20110027453A1Low production costMinimize changesHot-dipping/immersion processesPharmaceutical containersAnalyteSolvent
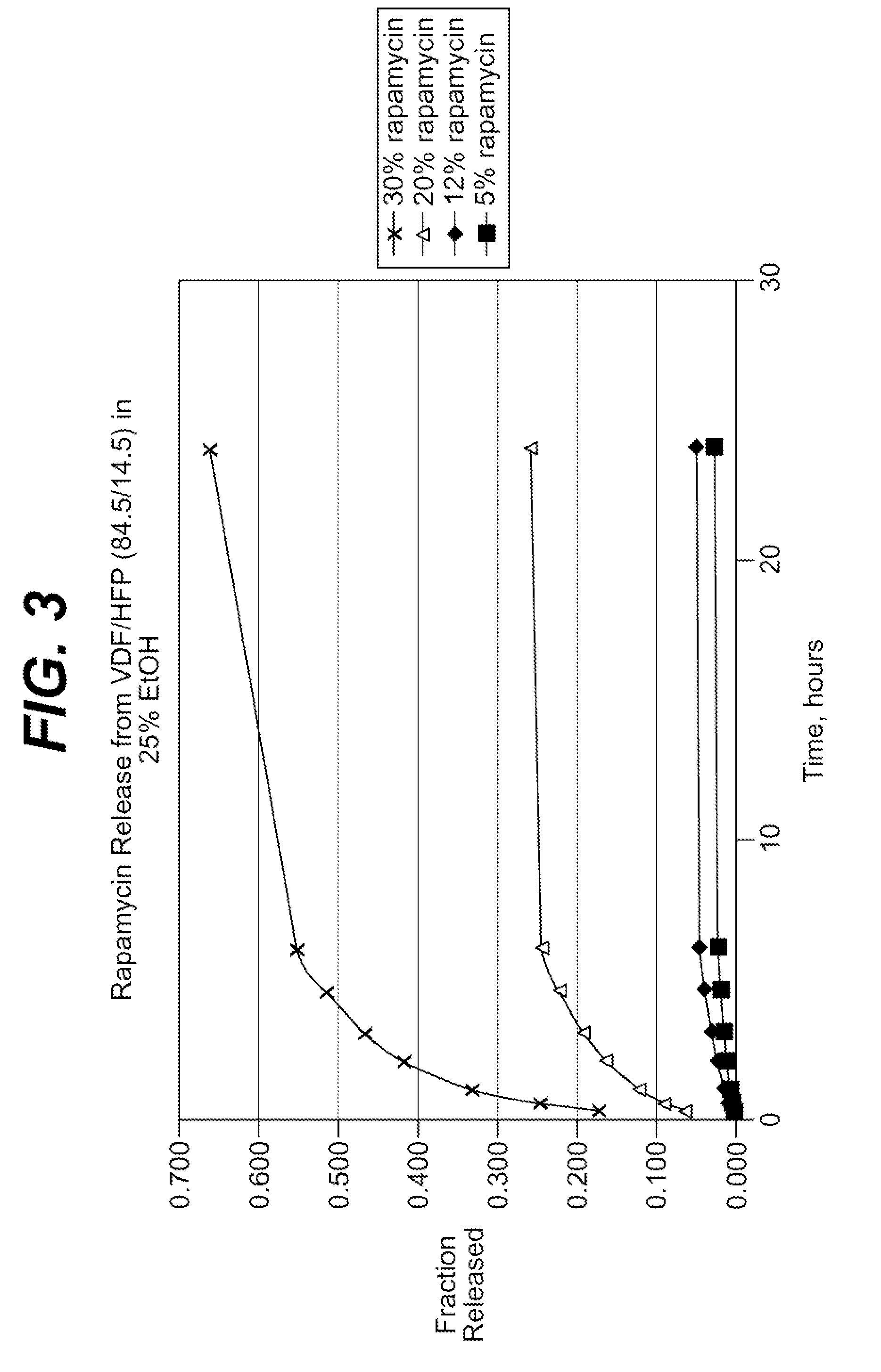
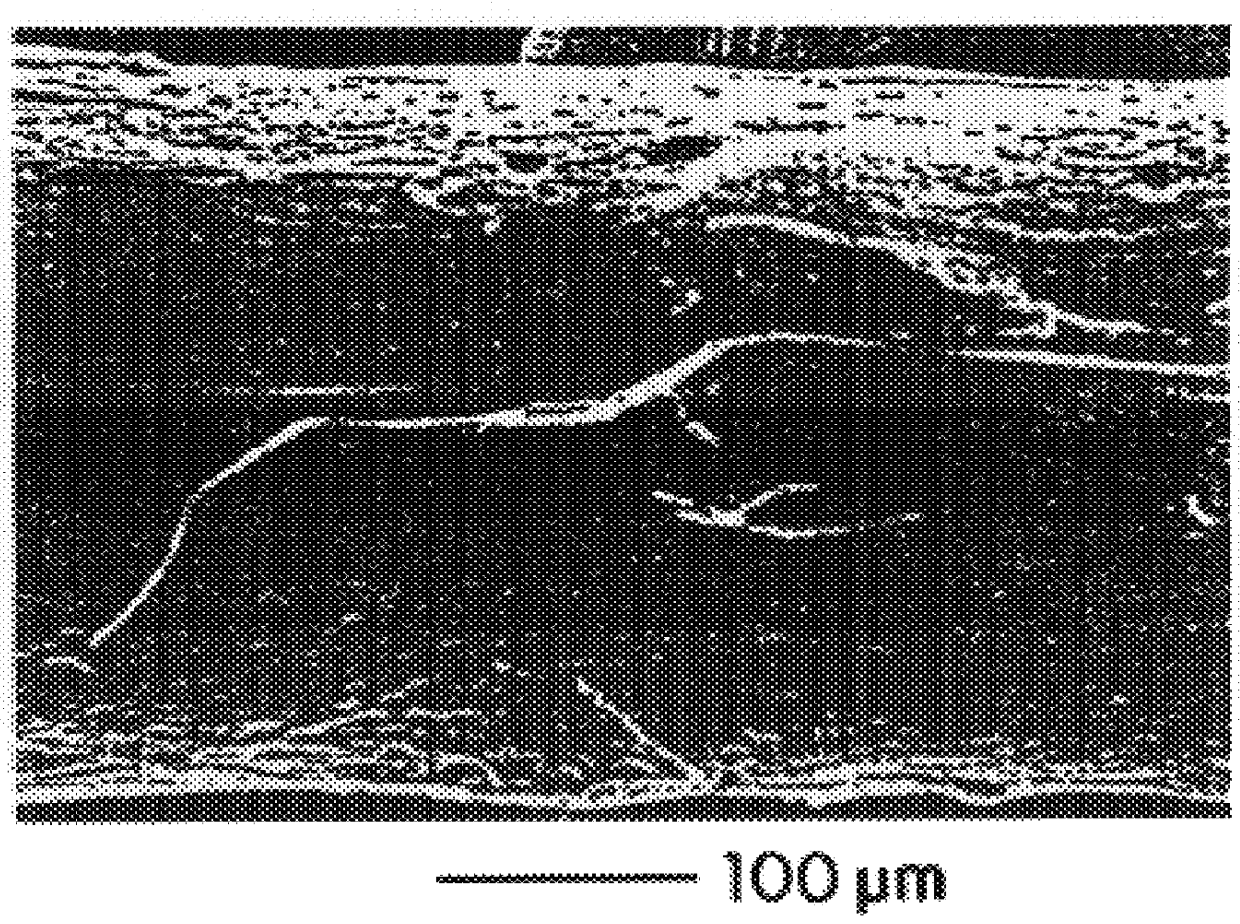
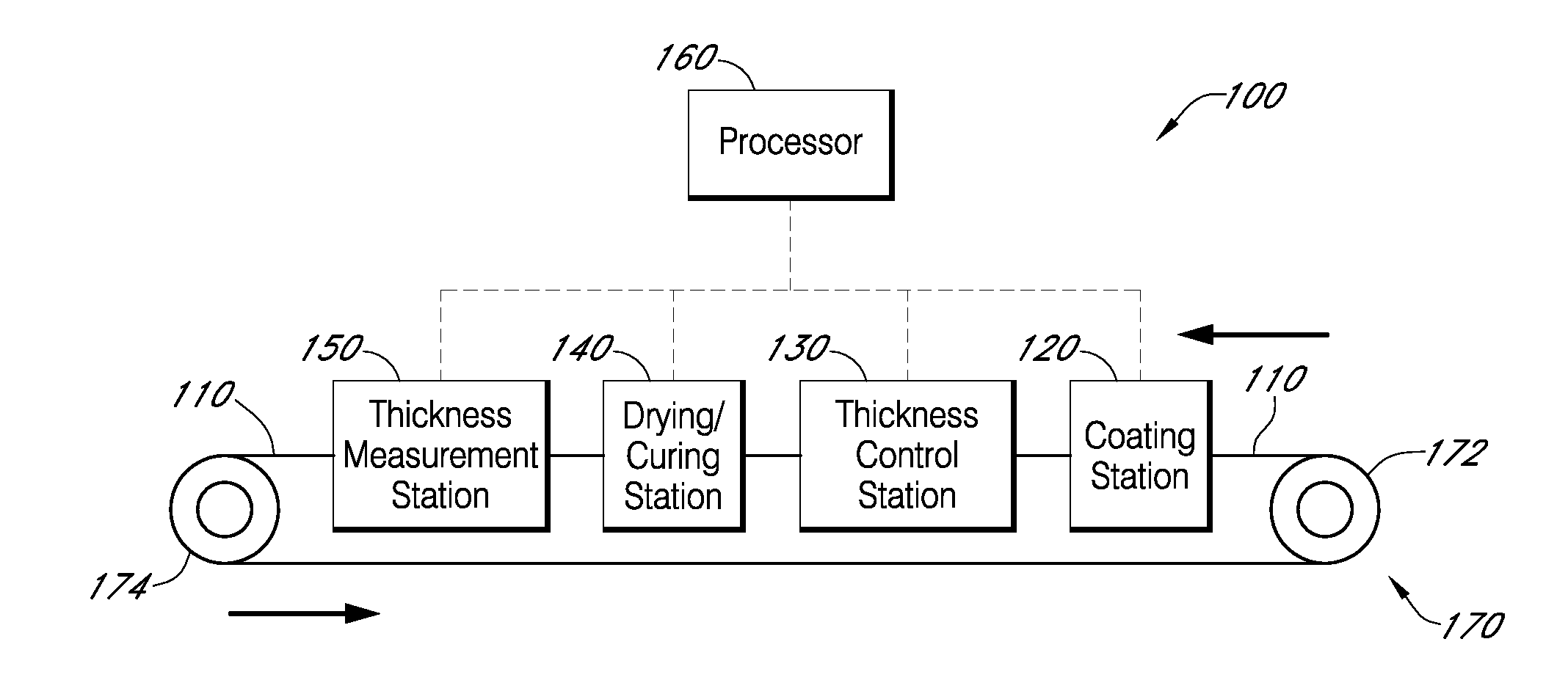
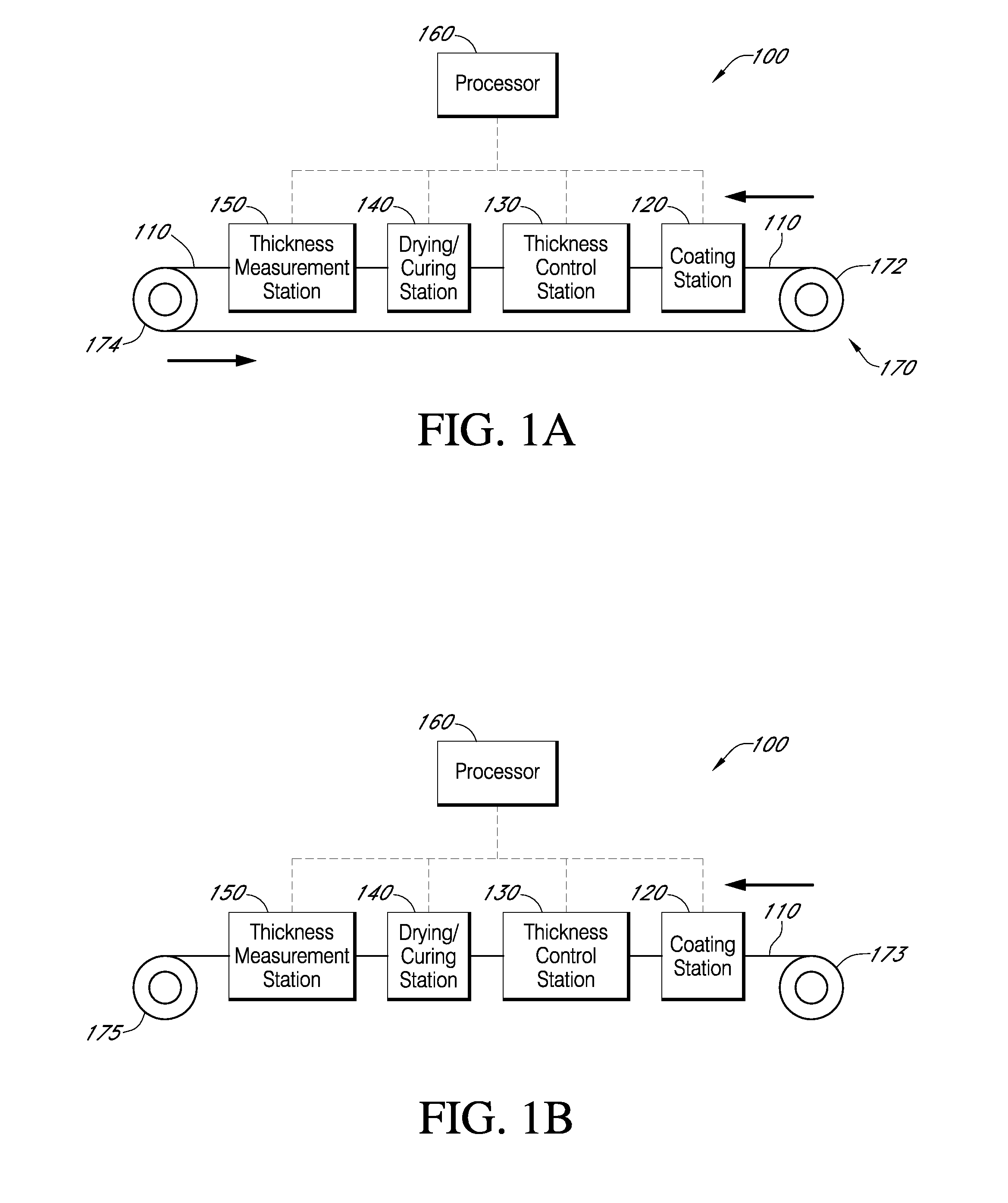
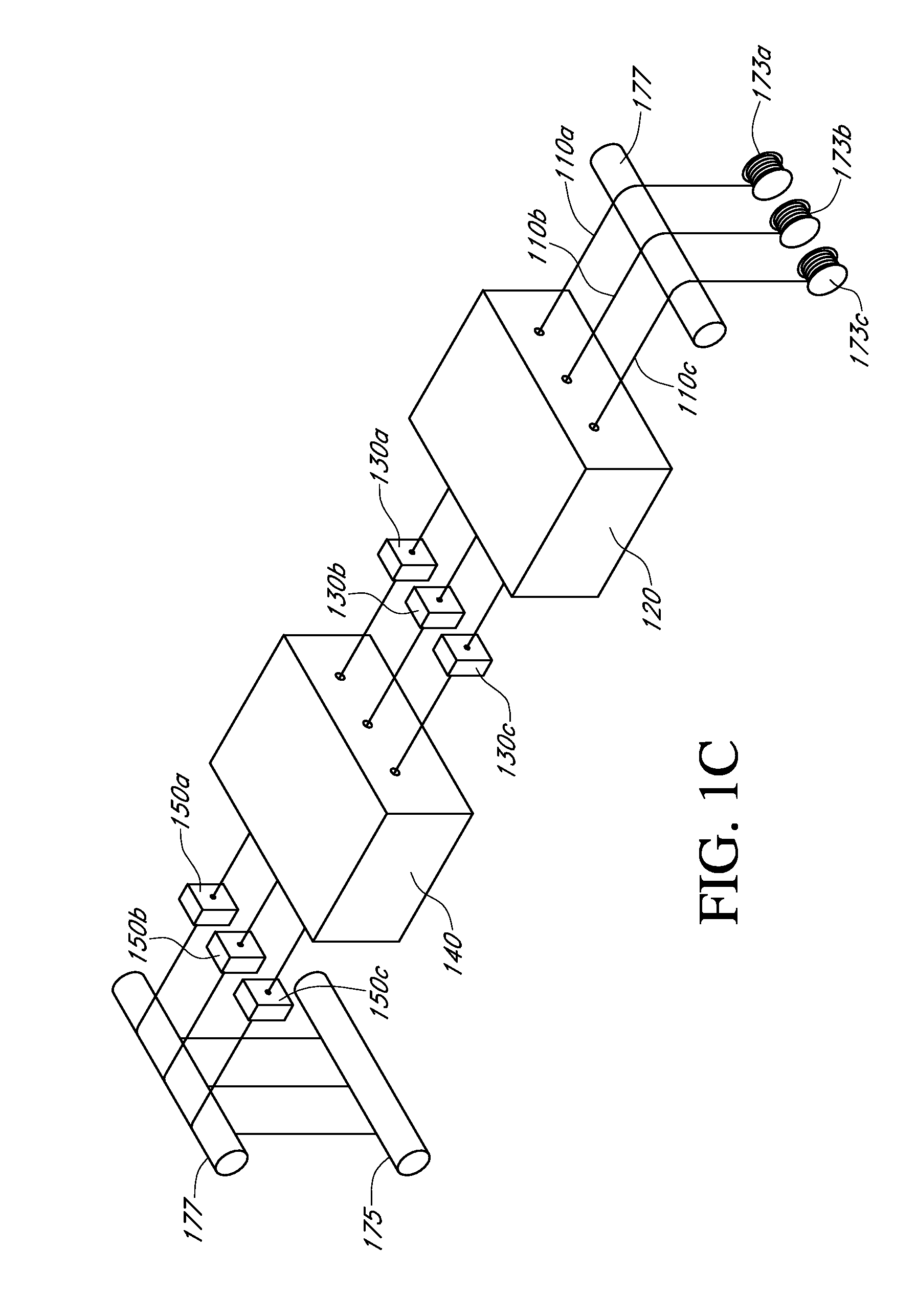
Described here are embodiments of processes and systems for the continuous manufacturing of implantable continuous analyte sensors. In some embodiments, a method is provided for sequentially advancing an elongated conductive body through a plurality of stations, each configured to treat the elongated conductive body. In some of these embodiments, one or more of the stations is configured to coat the elongated conductive body using a meniscus coating process, whereby a solution formed of a polymer and a solvent is prepared, the solution is continuously circulated to provide a meniscus on a top portion of a vessel holding the solution, and the elongated conductive body is advanced through the meniscus. The method may also comprise the step of removing excess coating material from the elongated conductive body by advancing the elongated conductive body through a die orifice. For example, a provided elongated conductive body 510 is advanced through a pre-coating treatment station 520, through a coating station 530, through a thickness control station 540, through a drying or curing station 550, through a thickness measurement station 560, and through a post-coating treatment station 570.
Owner:DEXCOM
Surface features of an implantable medical device
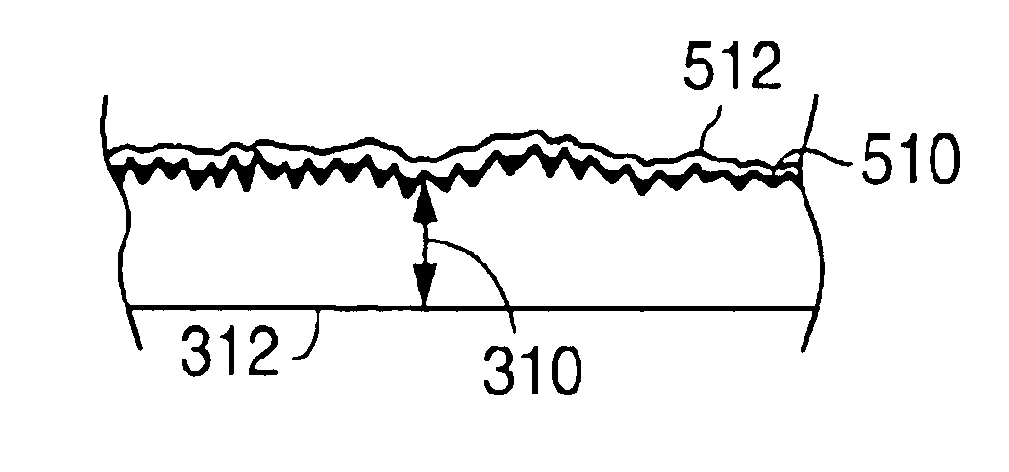
An implantable medical device, such as a stent or graft, having asperities on a designated region of its outer surface is disclosed. The asperities can serve to improve retention of one or more layers of a coating on the device and to increase the amount of coating that can be carried by the device. The asperities can be formed by using a stream of pressurized grit to roughen the surface. The asperities can also be formed by removing material from the outer surface, for example, by chemical etching with or without a patterned mask. Alternatively, the asperities can be formed by adding material to the outer surface, for example, by welding powder particles to the outer surface or sputtering.
Owner:ABBOTT CARDIOVASCULAR
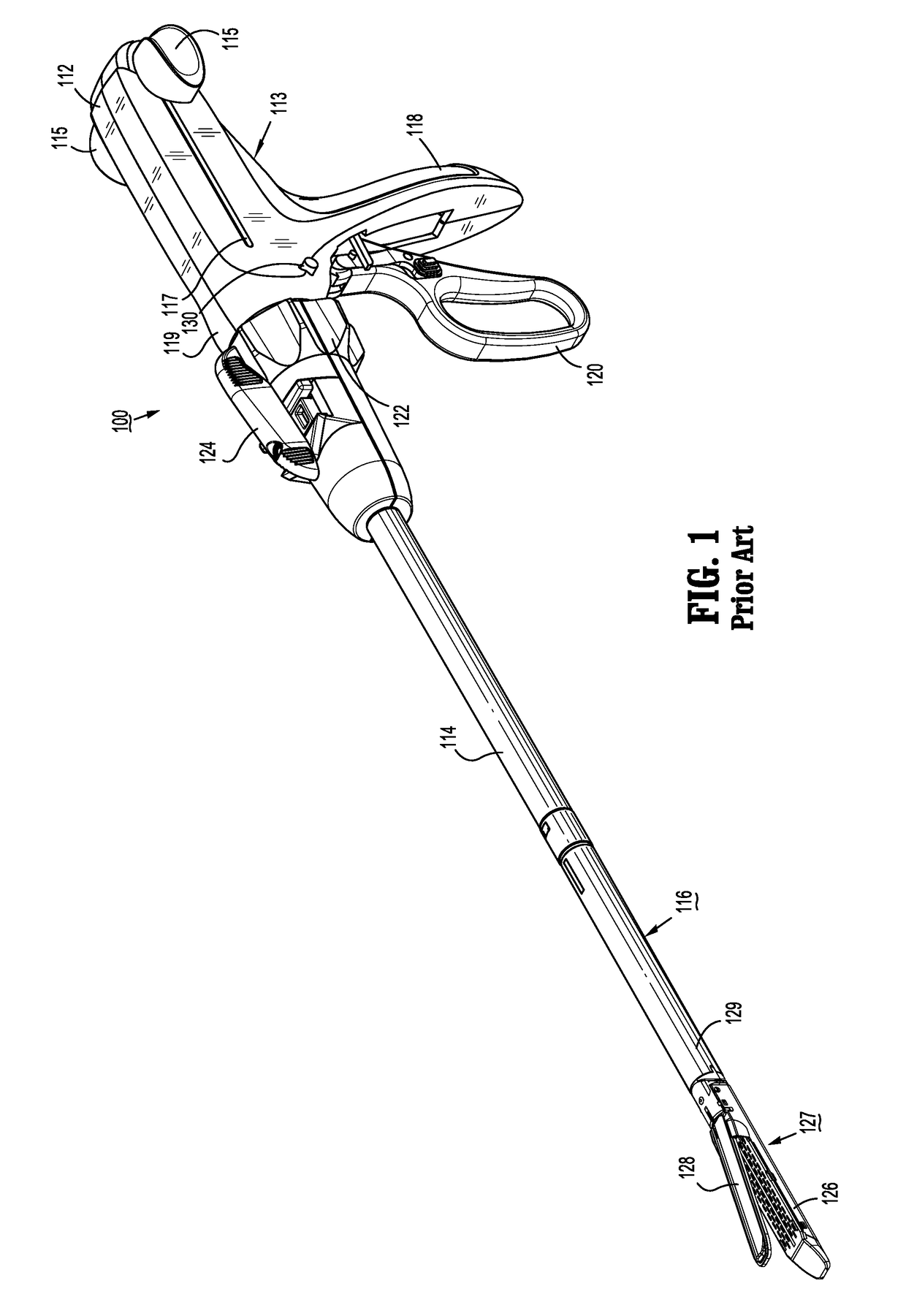
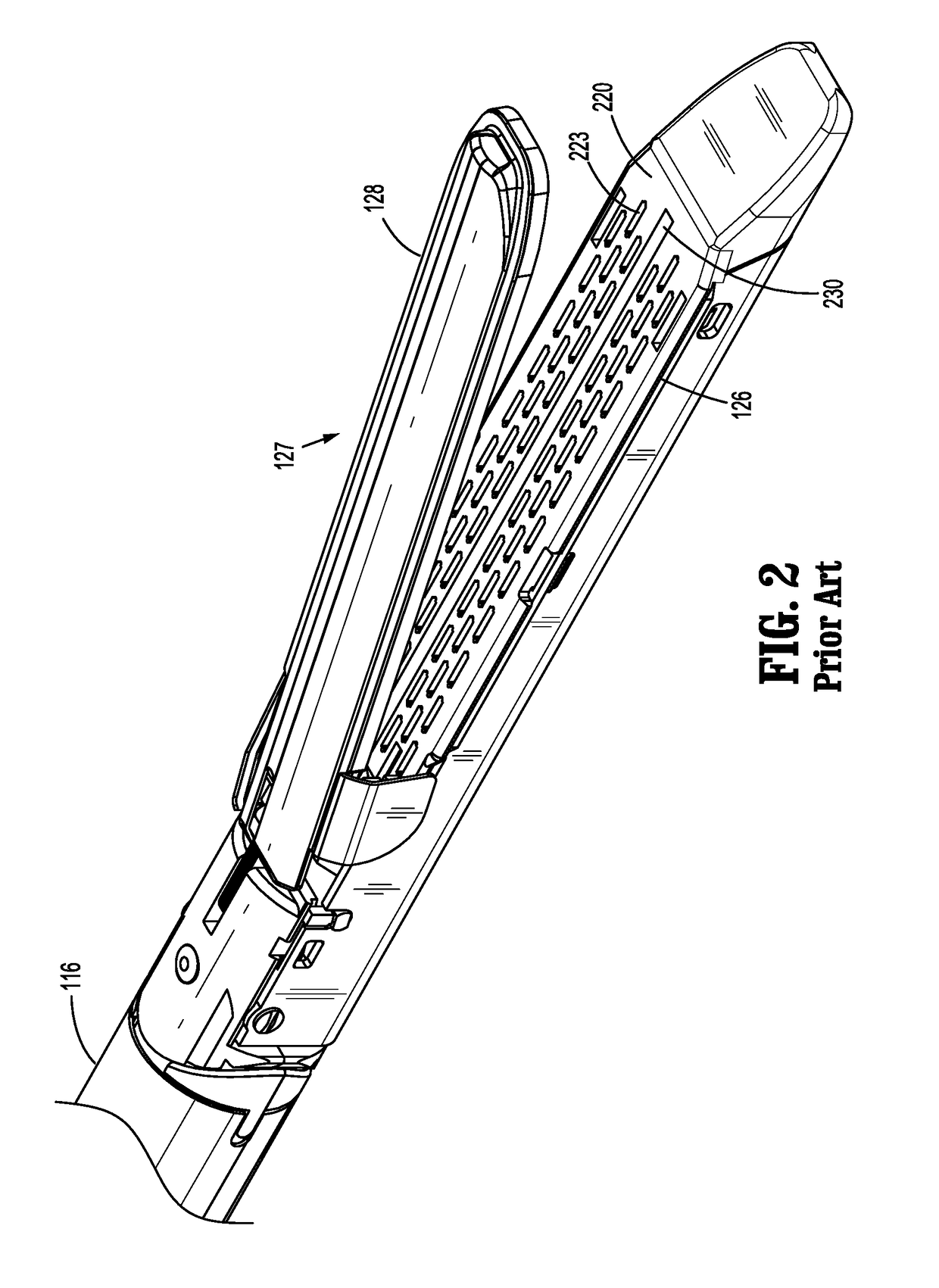
Surgical stapler flexible distal tip
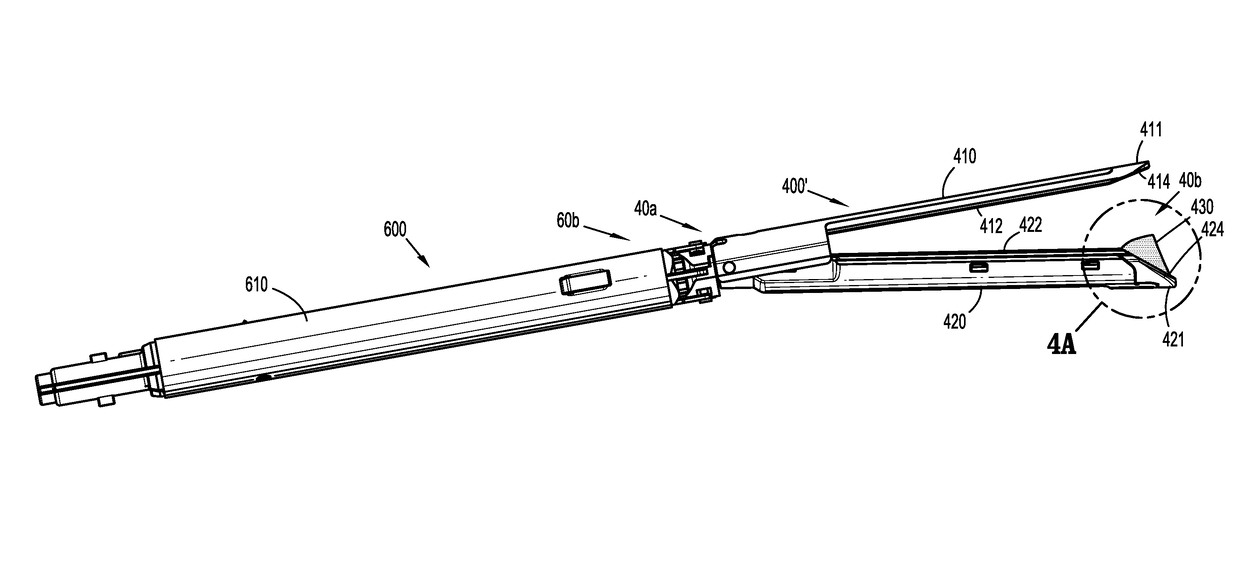
A tool assembly for a surgical device includes a first jaw member defining a first tissue contact surface and a second jaw member defining a second tissue contact surface. The first jaw member and the second jaw member are movable in relation to each other between open and closed positions wherein, in the closed position, the first and second tissue contact surfaces are spaced apart from each other and define a tissue gap. A compressible material is secured to a distal end of the first jaw member. The compressible material engages the second jaw member when the first and second jaw members are in the closed position. A surgical device includes the aforementioned tool assembly. A kit includes the compressible material secured on a tool assembly of a surgical device. A method of manufacturing the tool assembly and securing the compressible material is disclosed.
Owner:TYCO HEALTHCARE GRP LP
Wound closure material
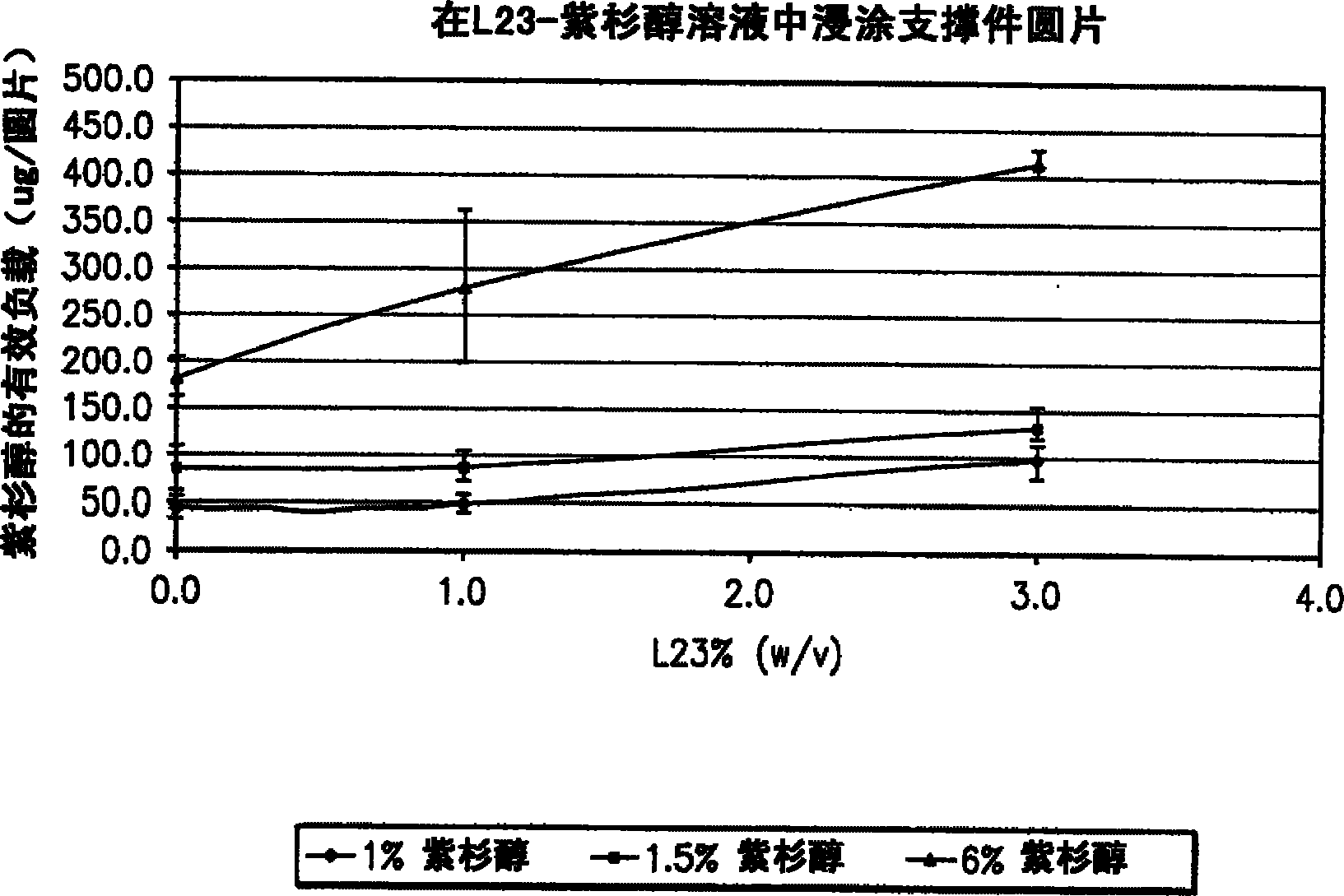
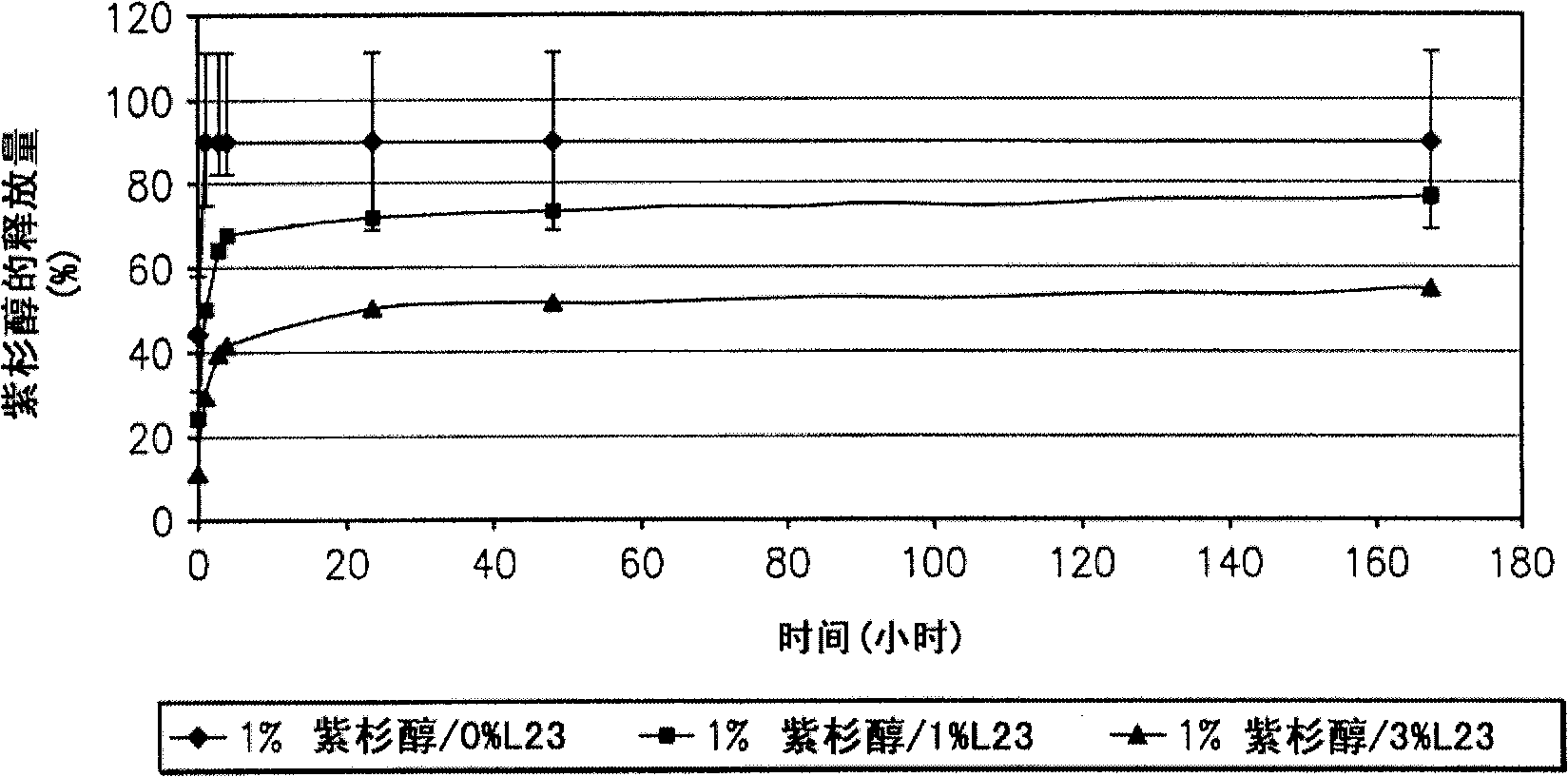
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:COVIDIEN LP
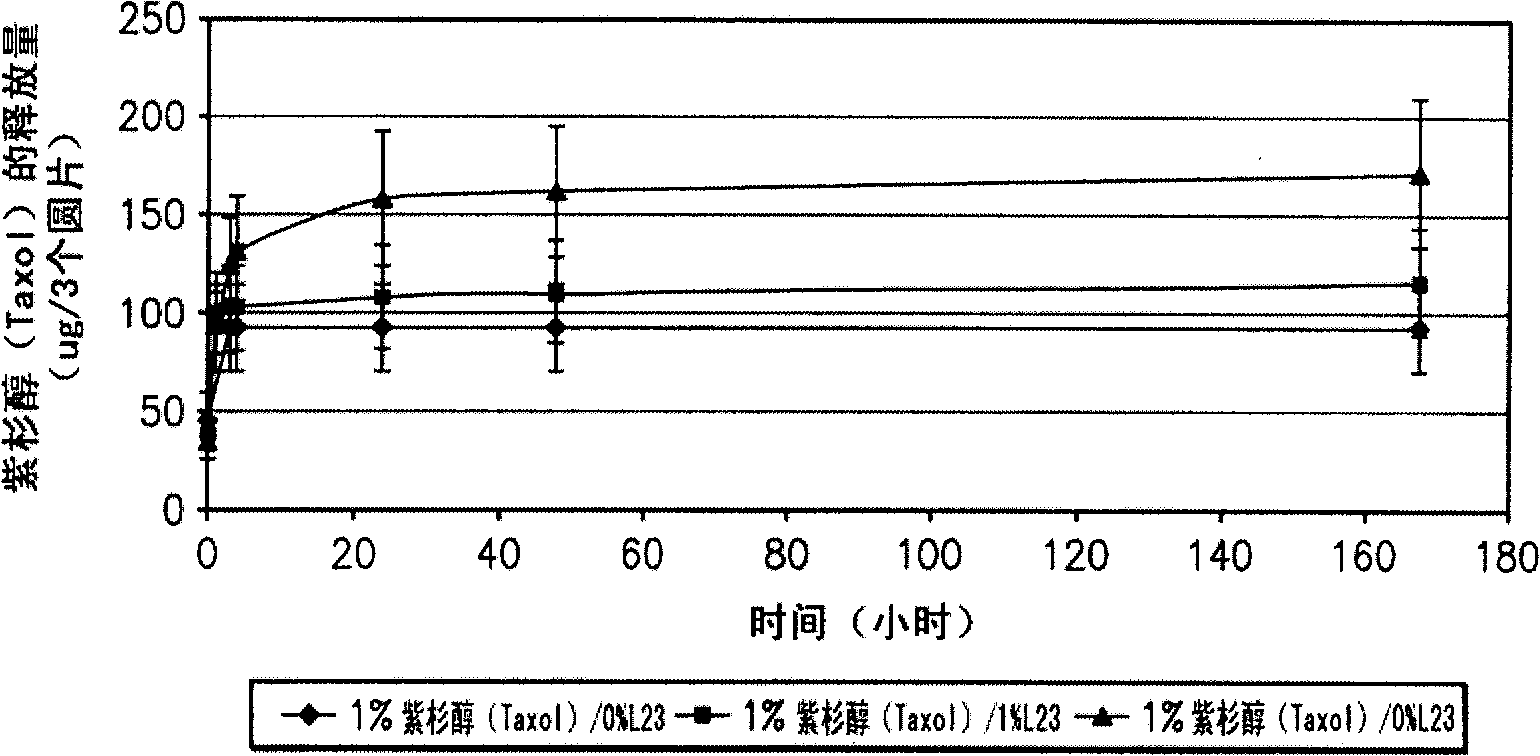
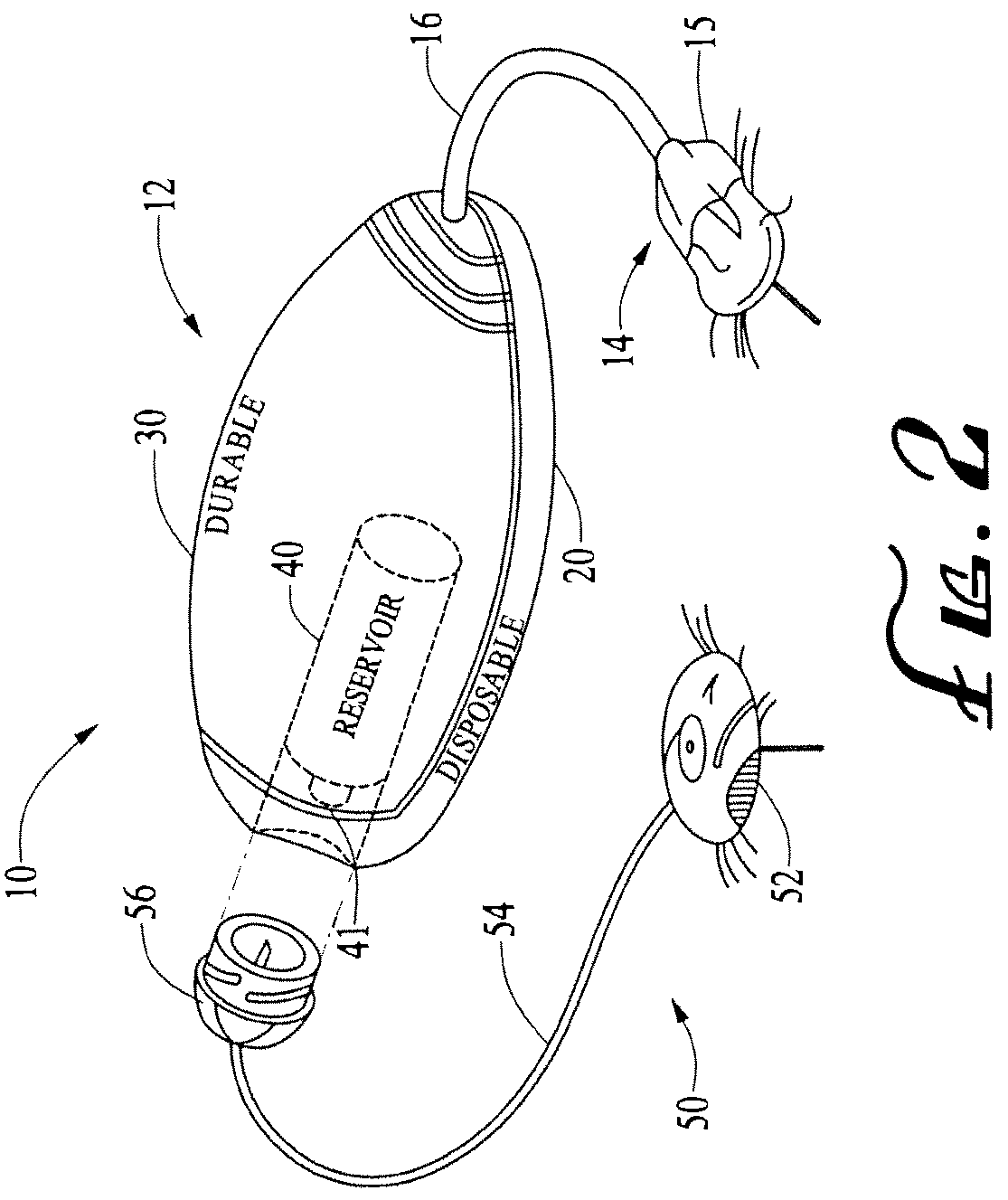
Systems and methods allowing for reservoir filling and infusion medium delivery
ActiveUS7828764B2Reduce internal volumeIncrease the internal volumePharmaceutical containersMedical devicesSurgeryMechanical engineering
A system includes a durable portion with a durable housing and a separable disposable portion with a disposable housing that selectively engage with and disengage from each other. The disposable housing secures to a patient and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient or with an infusion medium may be part of the disposable portion to allow for disposal after a prescribed use. A reservoir for holding the infusion medium may be part of the disposable portion, and may be supported by the disposable housing. The durable portion may include other components such as electronics for controlling delivery of the infusion medium from the reservoir, and a drive device including a motor and drive linkage.
Owner:MEDTRONIC MIMIMED INC
Biocompatibly coated medical implants
InactiveUS20050079200A1Easy to controlProperty variableStentsHeart valvesCarbon layerBiocompatible coating
Implantable medical devices with biocompatible coatings and processes for their production are described. The present invention relates in particular to medical implantable devices coated with a carbon-containing layer which devices are produced by at least partially coating the device with a polymer film and heating the polymer film in an atmosphere which is essentially free from oxygen to temperatures in the region of 200° C. to 2500° C., a carbon-containing layer being produced on the implantable medical device.
Owner:CINVENTION AG
Sheath for a prosthesis and methods of forming the same
InactiveUS6540776B2Increase the amount addedIncrease the number ofStentsPharmaceutical containersProsthesisImplanted device
Owner:ABBOTT CARDIOVASCULAR
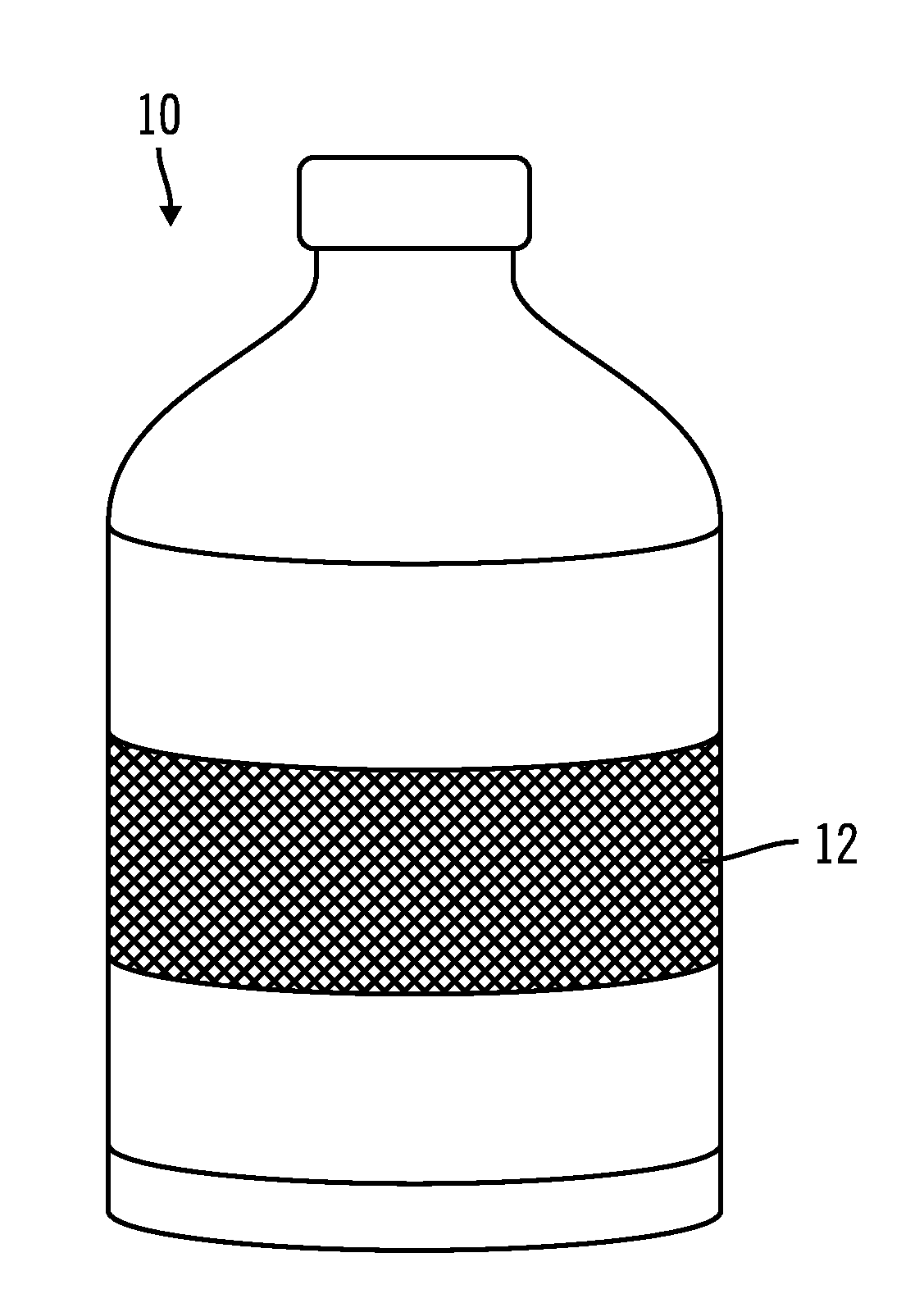
Coated implantable medical device
InactiveUS6918927B2Good worker safetyLow costOrganic active ingredientsSurgerySodium bicarbonateMedicine
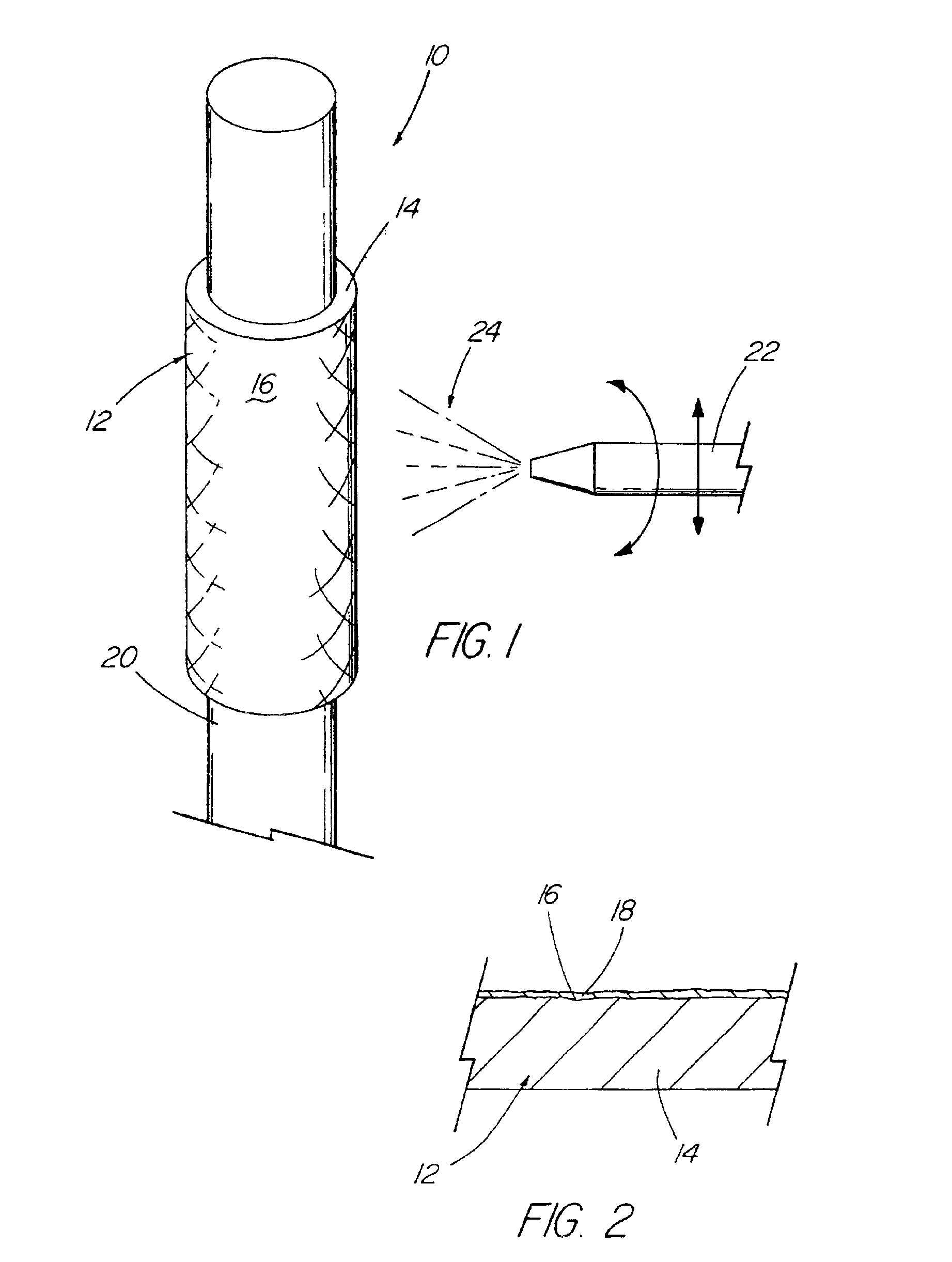
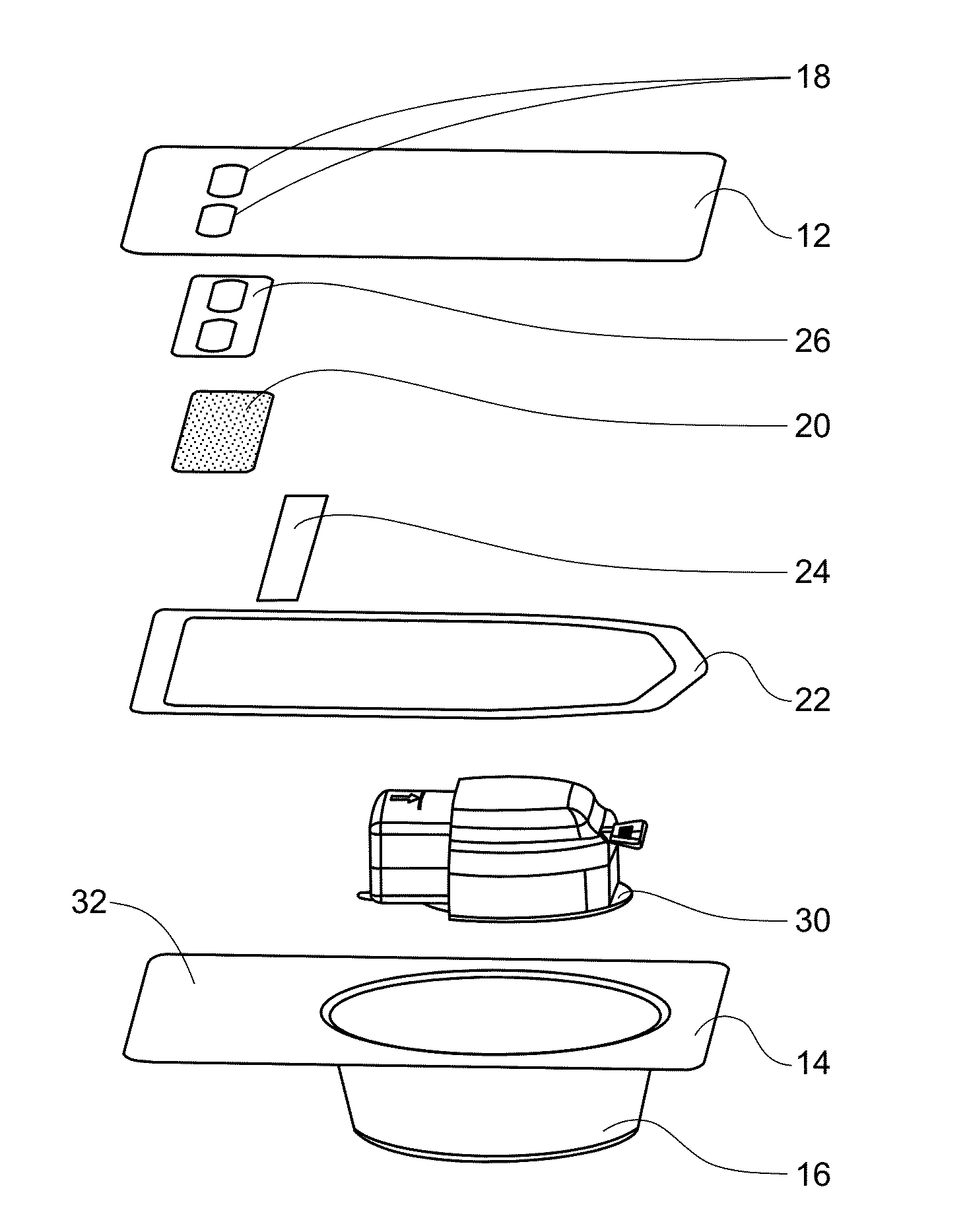
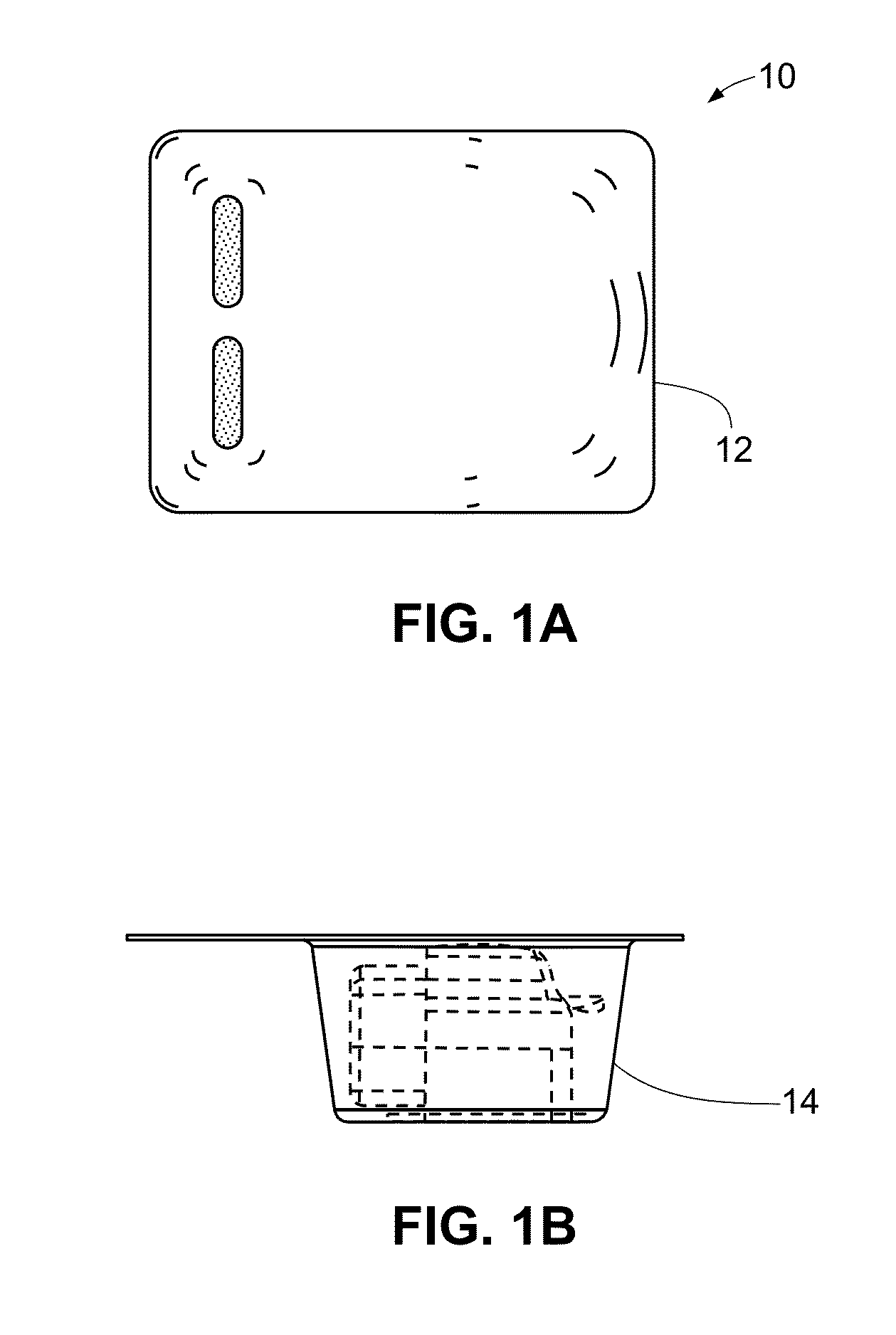
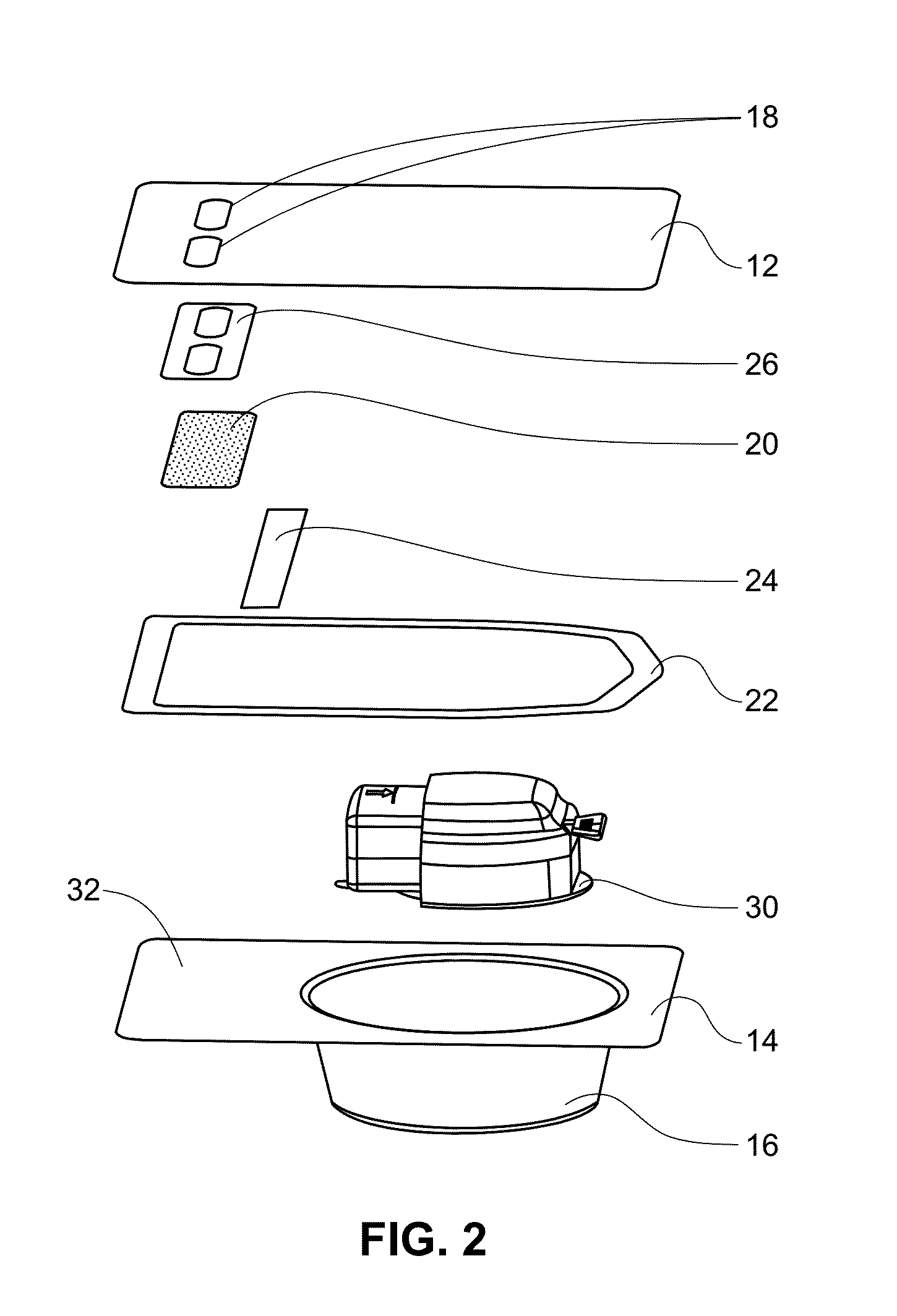
A medical device (10) includes a structure (12) adapted for introduction into a patient, the structure (12) being formed of a preferably non-porous base material (14) having a roughened or textured surface (16). The structure (12) is conveniently configured as a vascular stent with a base material (14) of stainless steel, nitinol or another suitable material. The medical device (10) also includes a layer (18) of a bioactive material posited directly upon the roughened or textured surface (16) of the base material (14) of the structure (12). The surface (16) of the base material (14) is roughened or textured by etching or by abrasion with sodium bicarbonate or another suitable grit. A preferred roughened or textured surface (16) is thought to have a mean surface roughness of about 10 μin. (about 250 nm) and a surface roughness range between about 1 μin. and about 100 μin. (about 25 nm and about 2.5 μm). The particularly preferred use of sodium bicarbonate as the abrasive to provide roughness or texture to the surface (16) of the base material (14) of the structure (12) is additionally advantageous in the low toxicity of the sodium bicarbonate to production workers, the ease of product and waste cleanup, and the biocompatibility of any residual sodium bicarbonate.
Owner:COOK MEDICAL TECH LLC
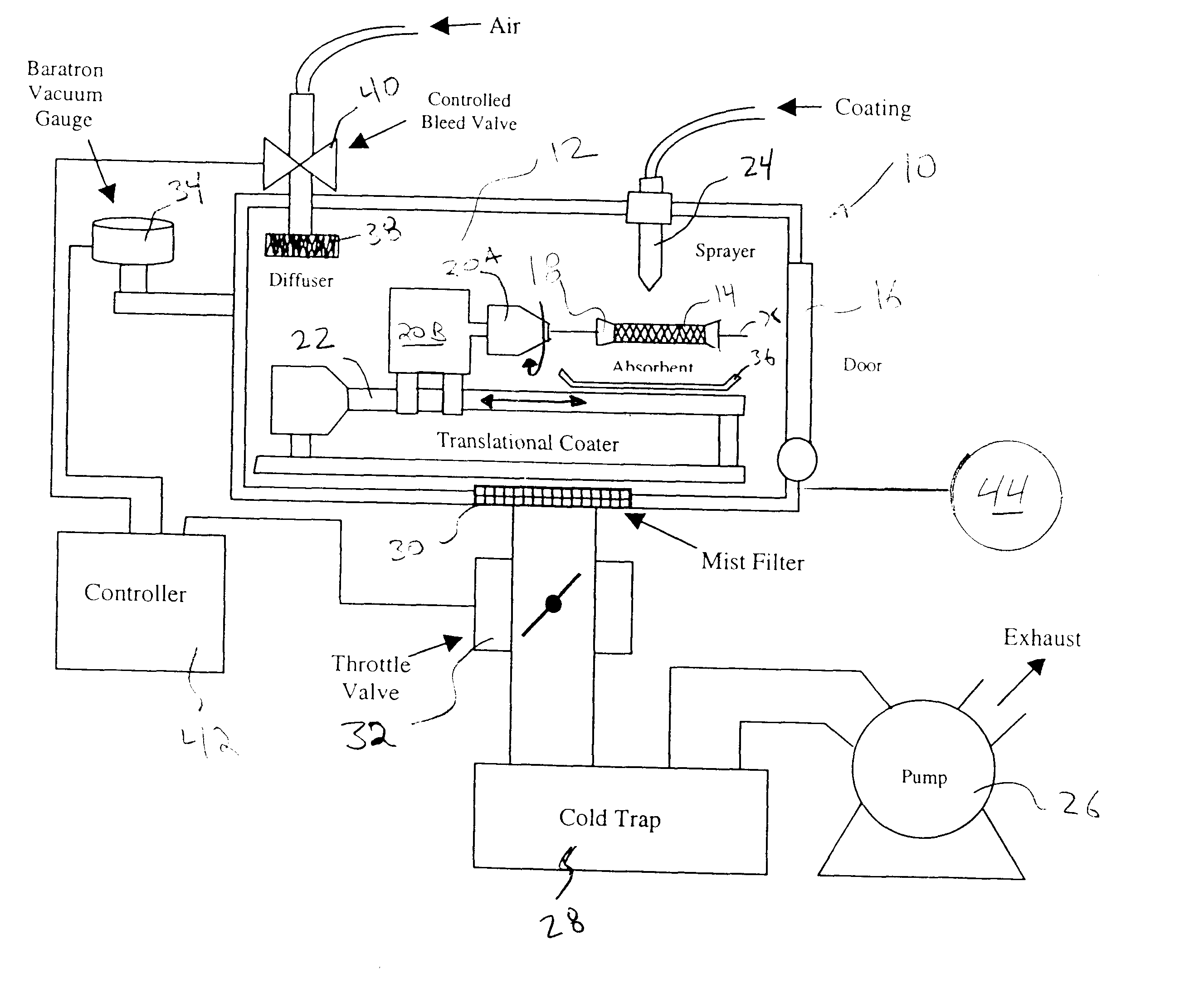
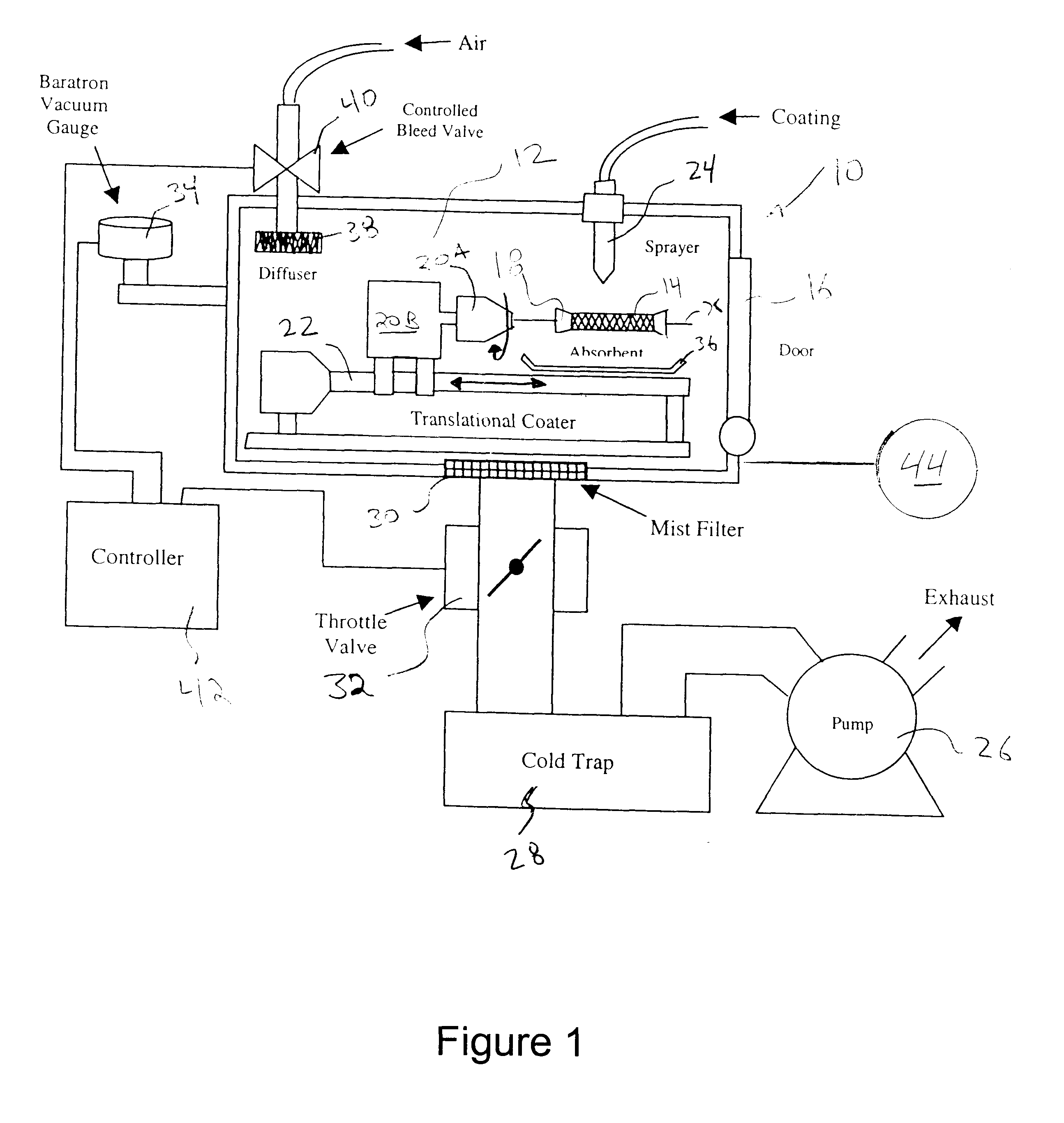
Apparatus and method for coating implantable devices
A method of forming a coating for an implantable medical device, such as a stent, is provided which includes applying a composition to the device in an environment having a selected pressure. An apparatus is also provided for coating the devices. The apparatus comprises a chamber for housing the device wherein the pressure of the chamber can be adjusted during the coating process.
Owner:ABBOTT CARDIOVASCULAR
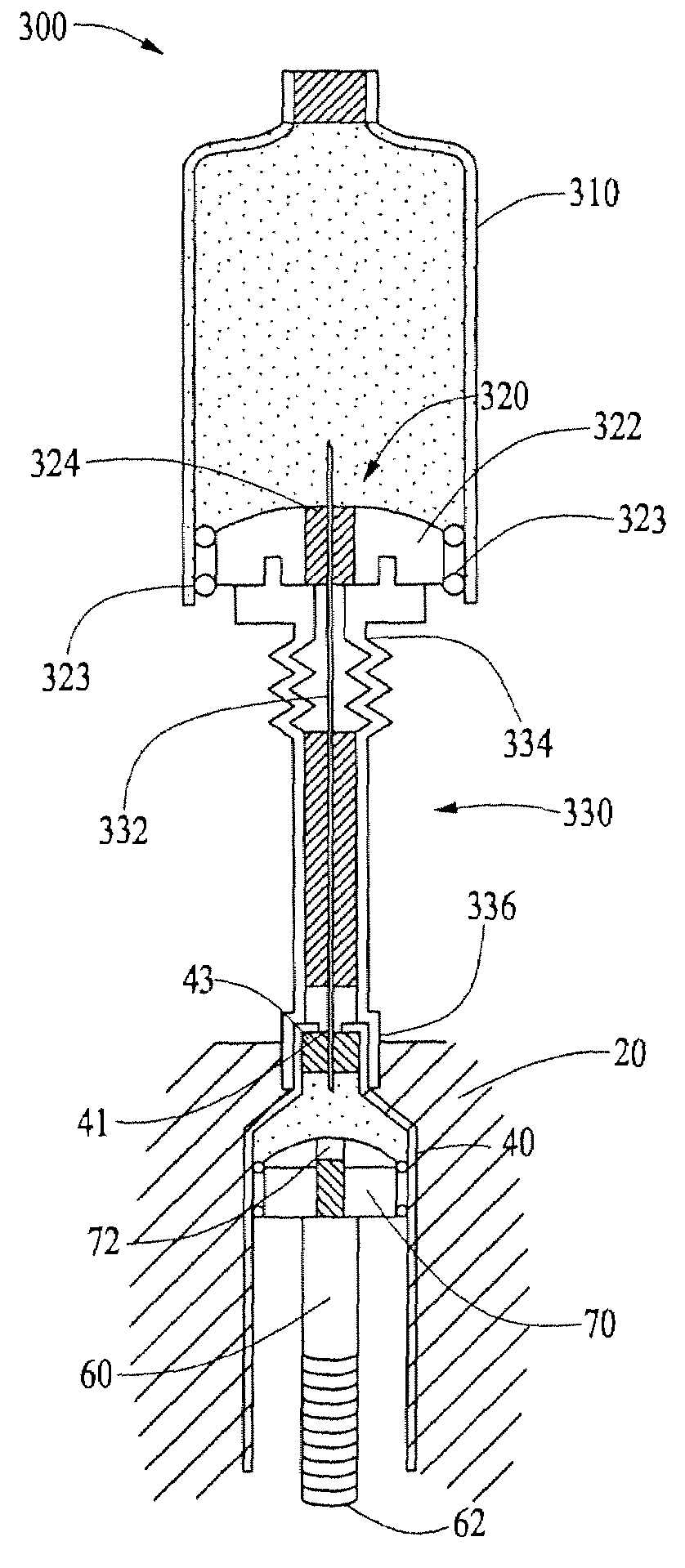
Infusion medium delivery device and method with drive device for driving plunger in reservoir
A delivery device includes a durable housing portion and a separable disposable portion that selectively engage and disengage from each other. The disposable housing portion secures to the patient-user and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient-user or with infusion medium are supported by the disposable housing portion, while the durable housing portion supports other components such as electronics and a drive device. A reservoir is supported by the disposable housing portion and has a moveable plunger that operatively couples to the drive device, when the disposable and durable housing portions are engaged.
Owner:MEDTRONIC MIMIMED INC
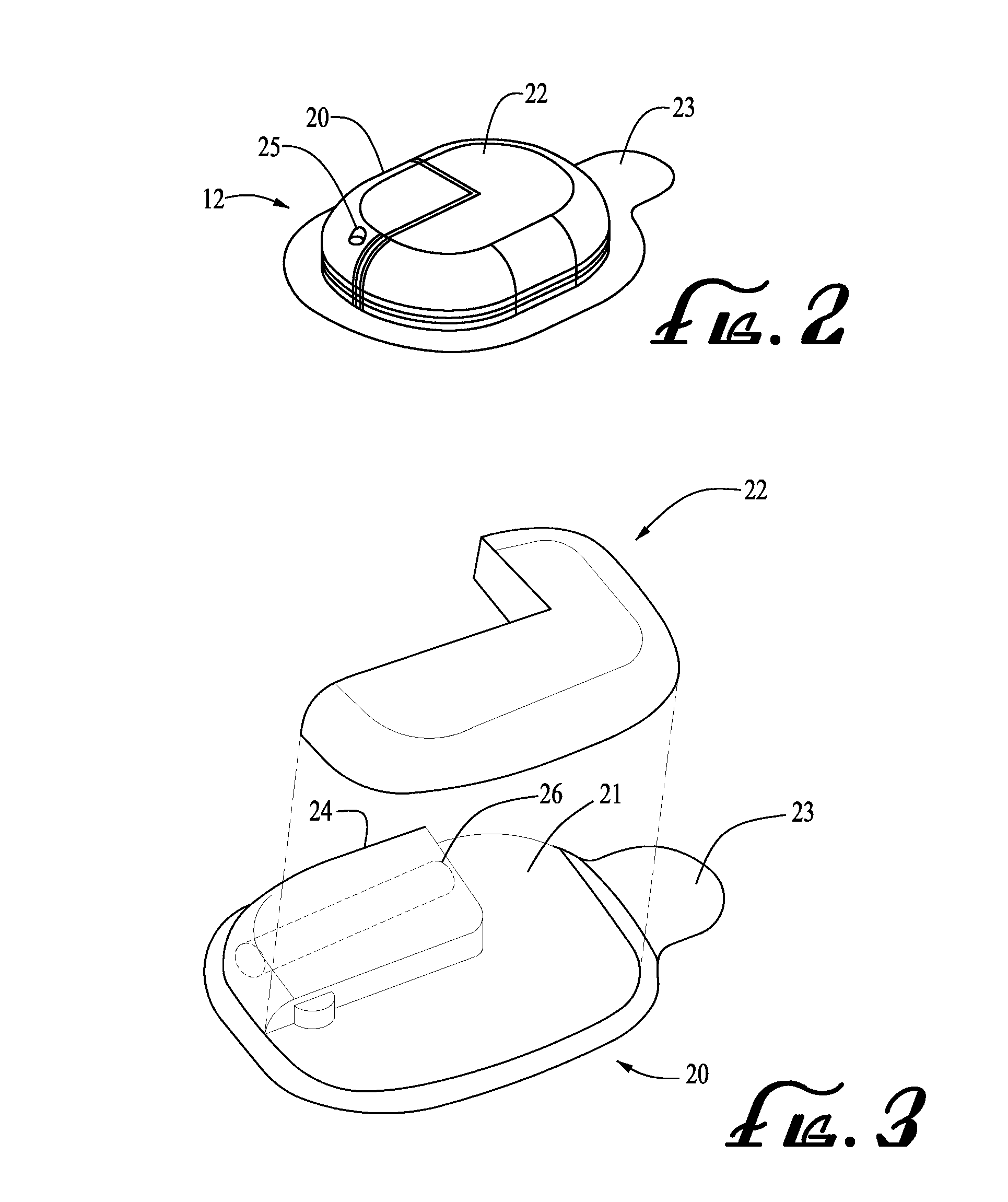
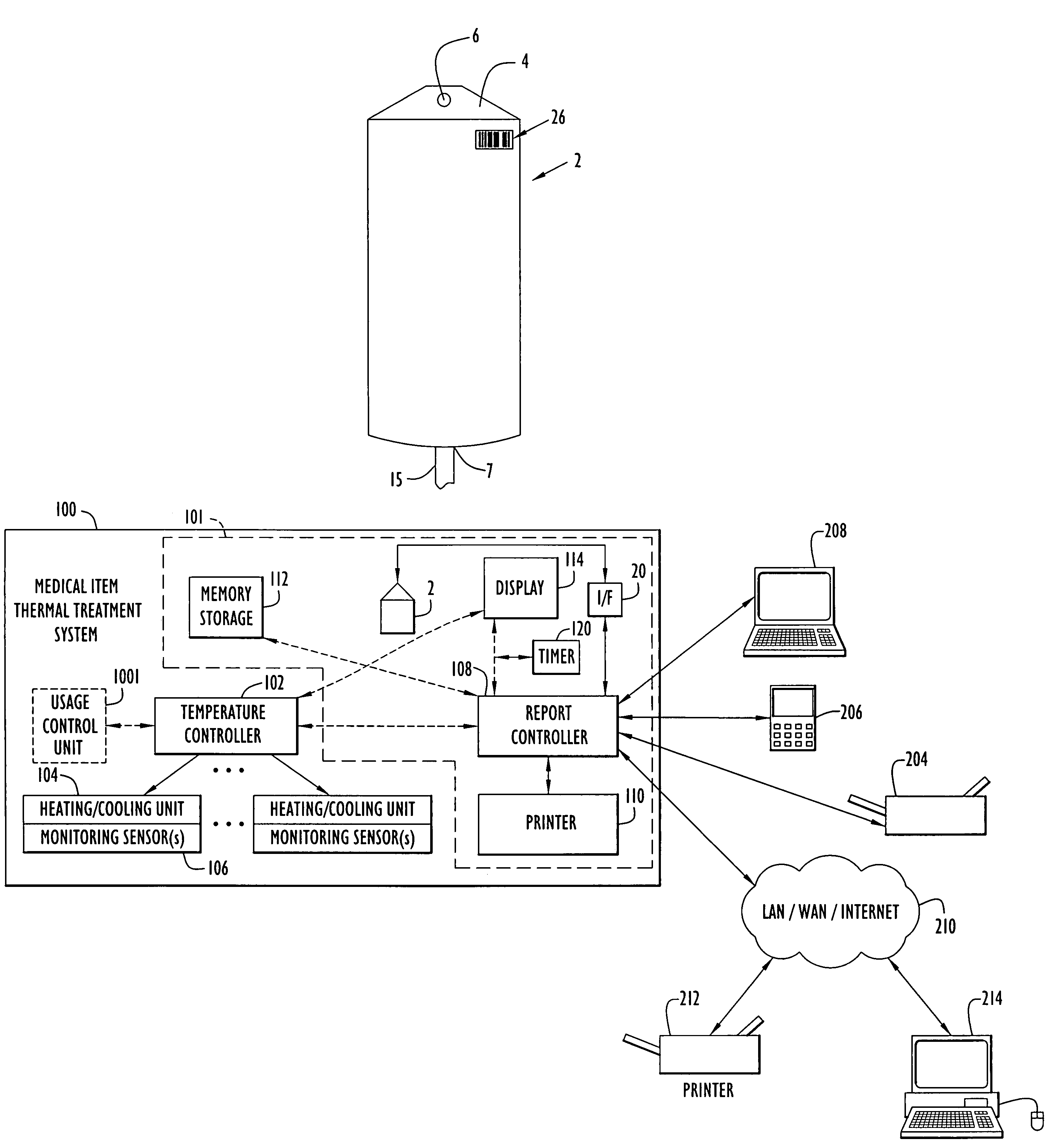
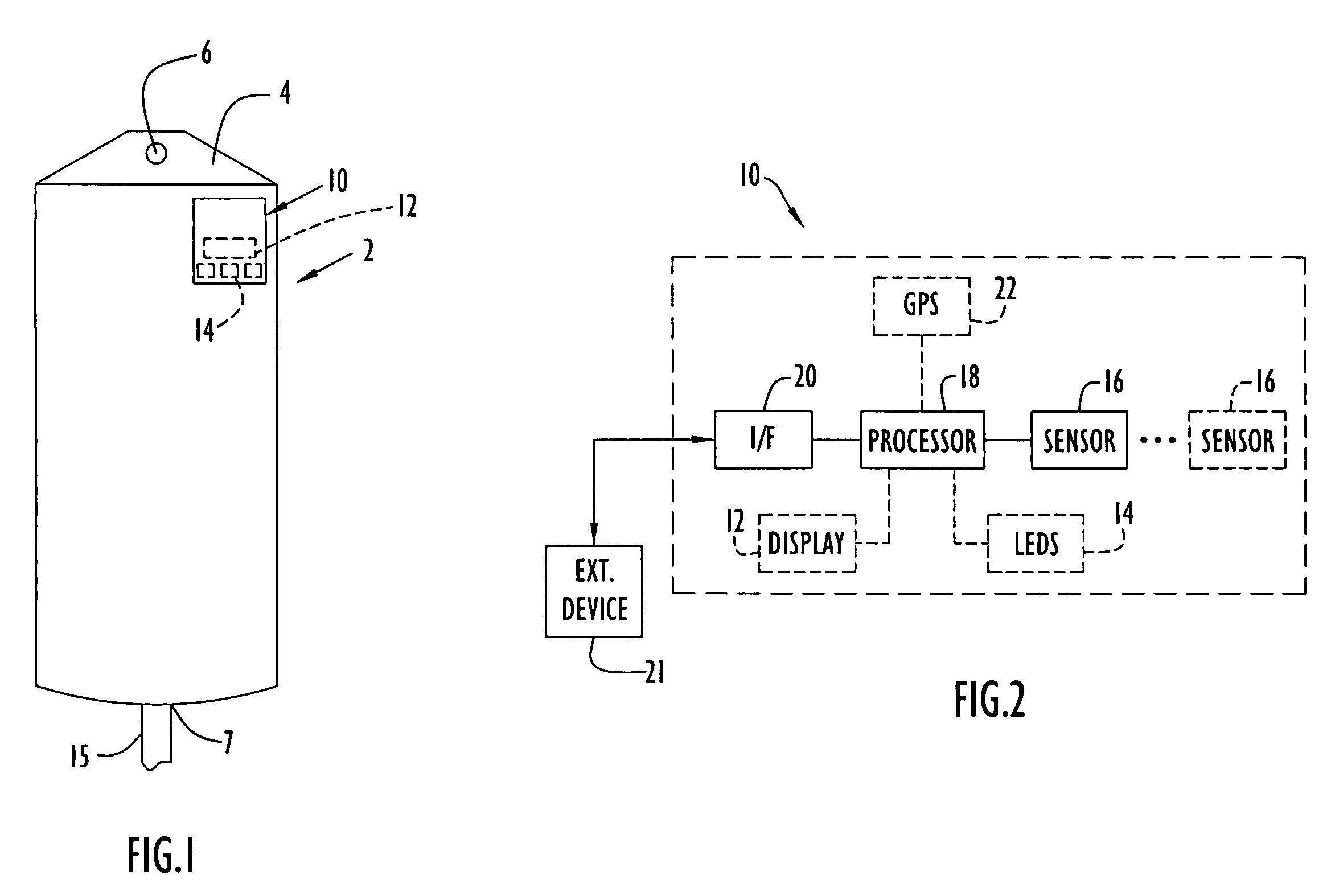
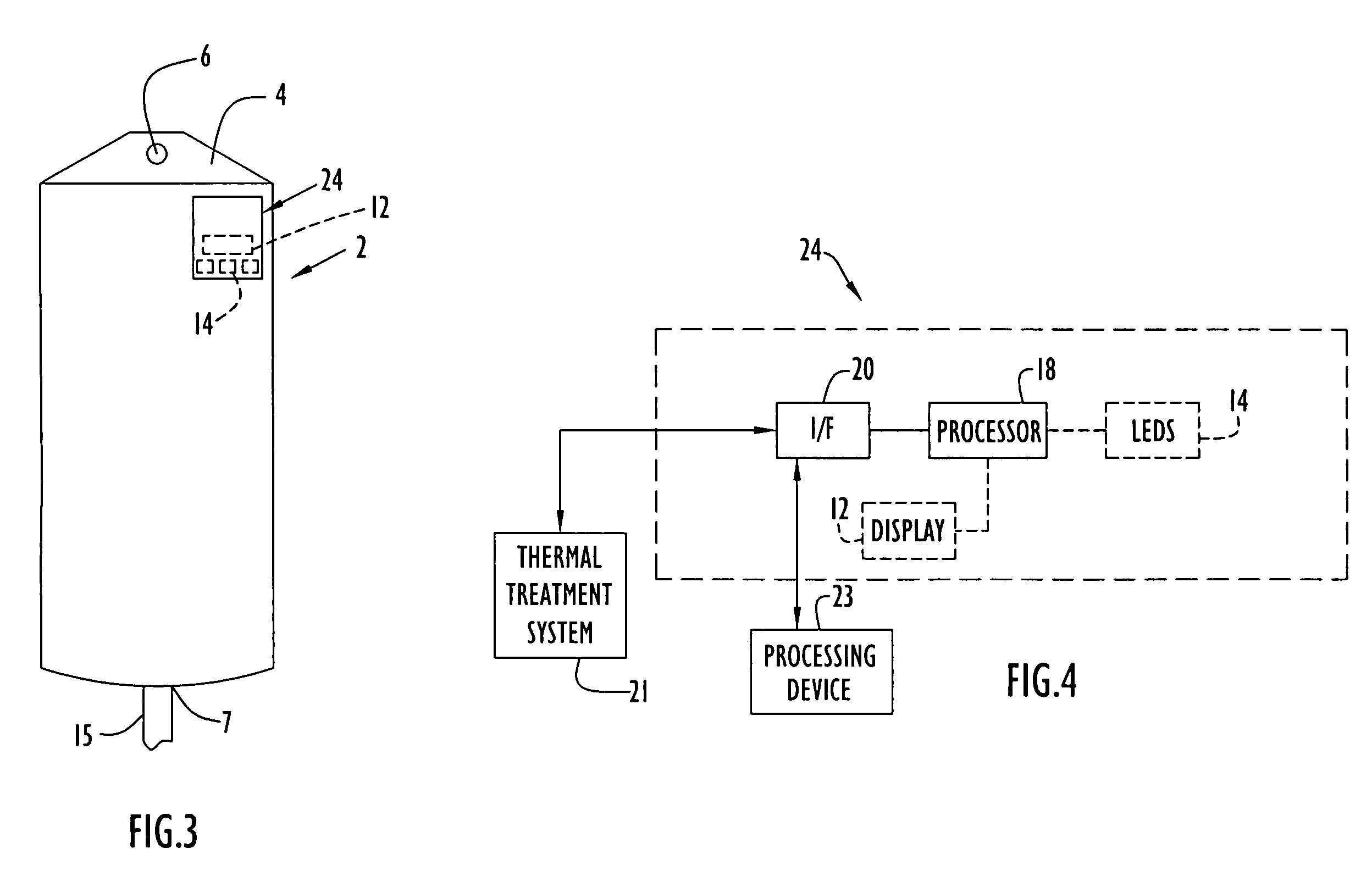
Medical item thermal treatment systems and method of monitoring medical items for compliance with prescribed requirements
Various present invention devices enable adherence to requirements for medical items. A medical item of the present invention includes a monitoring or data recording device to monitor and / or record medical solution conditions. The device may further include indicators to indicate compliance of the medical solution with prescribed requirements (e.g., manufacturer, medical standard or regulation, etc.). The medical item may alternatively include a barcode or transponder to uniquely identify the medical item to a thermal treatment system measuring and storing conditions in a central database. The present invention further includes various thermal treatment systems that monitor medical items for prescribed requirements and display the monitored parameters to medical personnel. In addition, the present invention may place time stamp information on medical items to enable determination by medical personnel of compliance with prescribed requirements.
Owner:PATENTED MEDICAL SOLUTIONS LLC
Method for applying an LbL coating onto a medical device
The present invention provides an improved LbL-coating process for modifying the surface of a medical device, preferably an ophthalmic device, more preferably a contact lens. An LbL coating on a contact lens, which is prepared according to the process of the invention, can have increased hydrophilicity characterized by an averaged contact angle of about 80 degree or less, preferably about 50 degrees or less, while maintaining the desired bulk properties such as oxygen permeability and ion permeability of lens material.
Owner:ALCON INC
Medical device
InactiveUS20050002981A1Reduce connective tissue hyperplasiaReduce restenosisStentsPeptide/protein ingredientsBiological propertyConnective tissue fiber
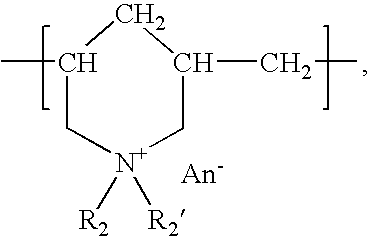
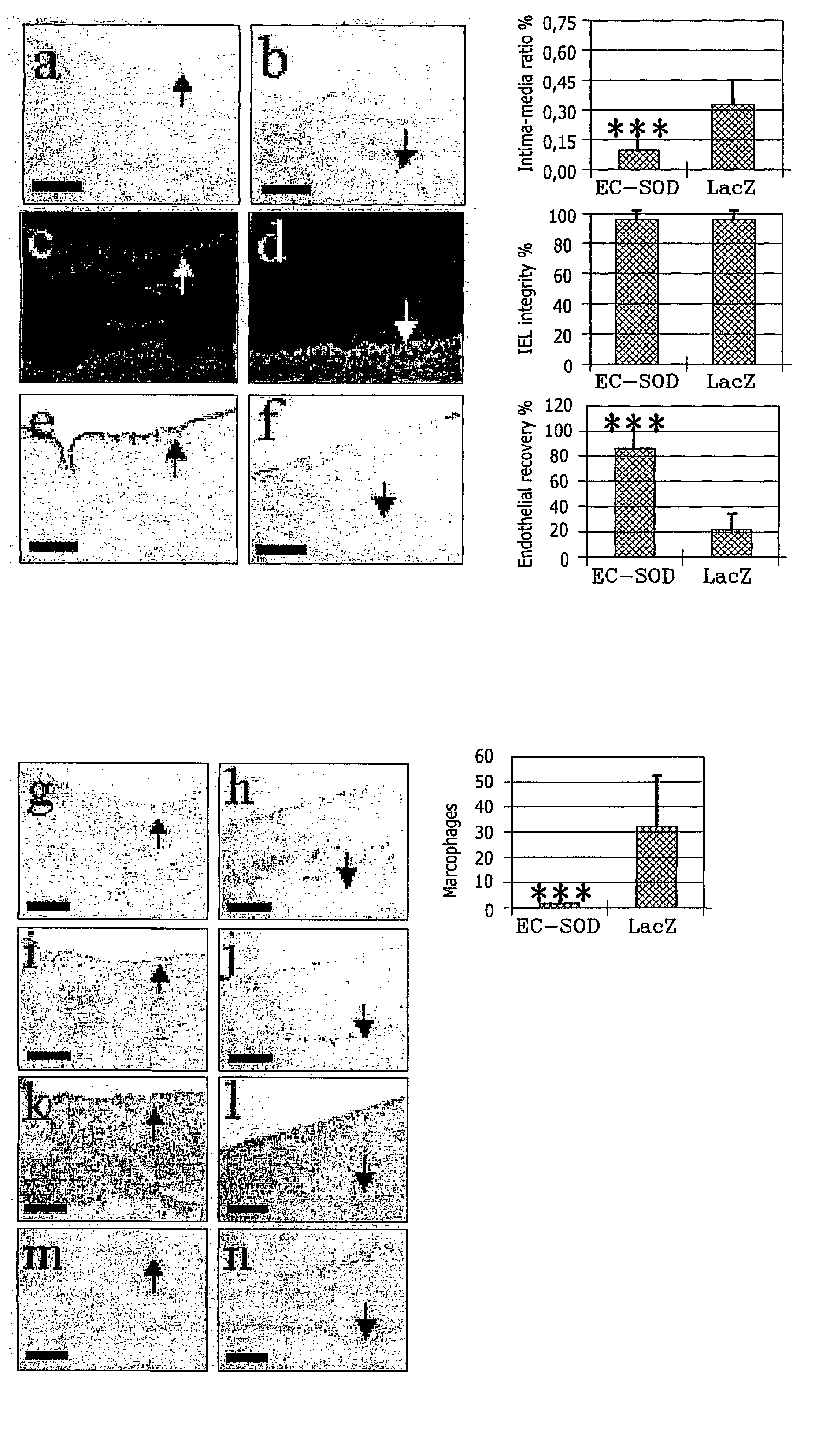
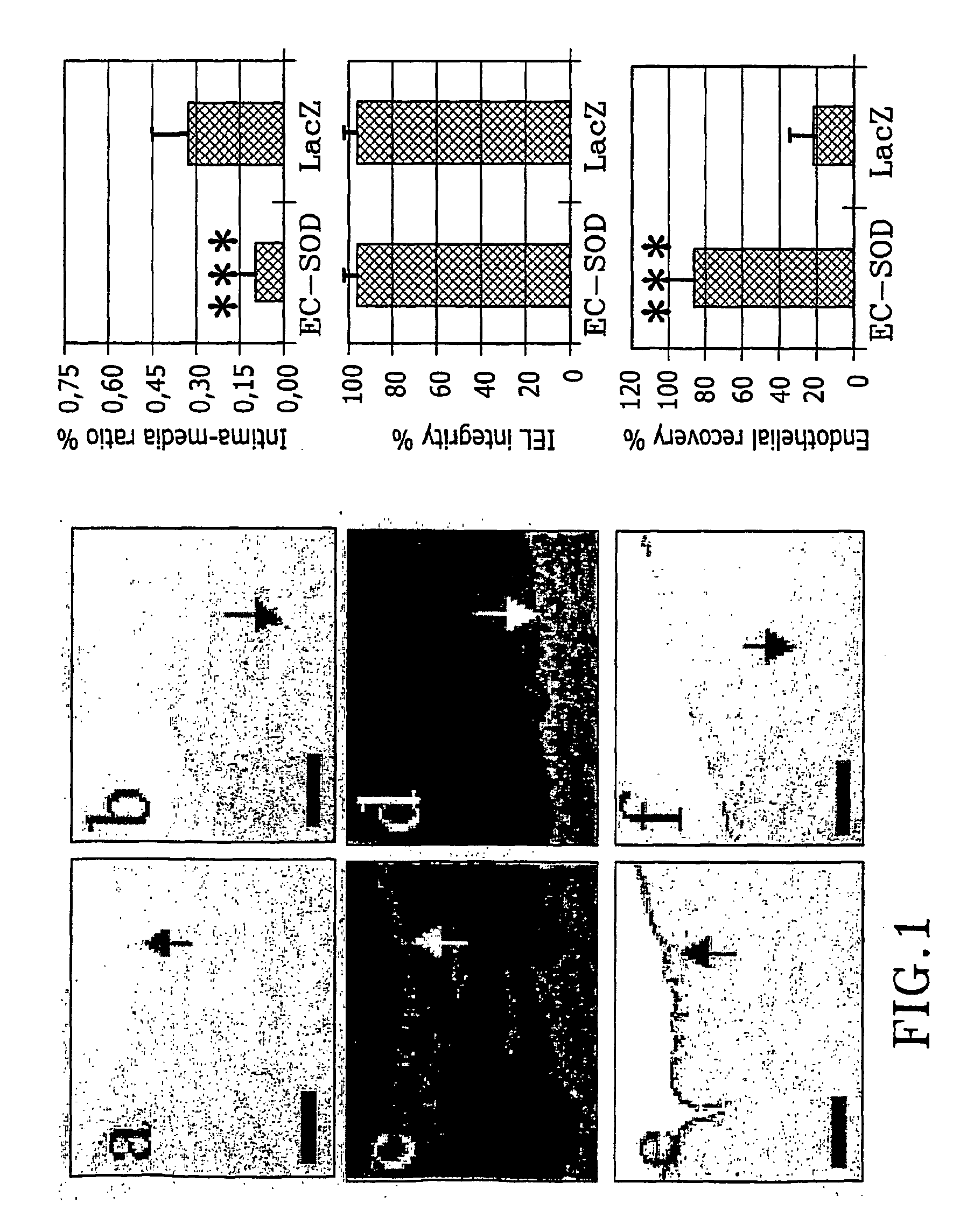
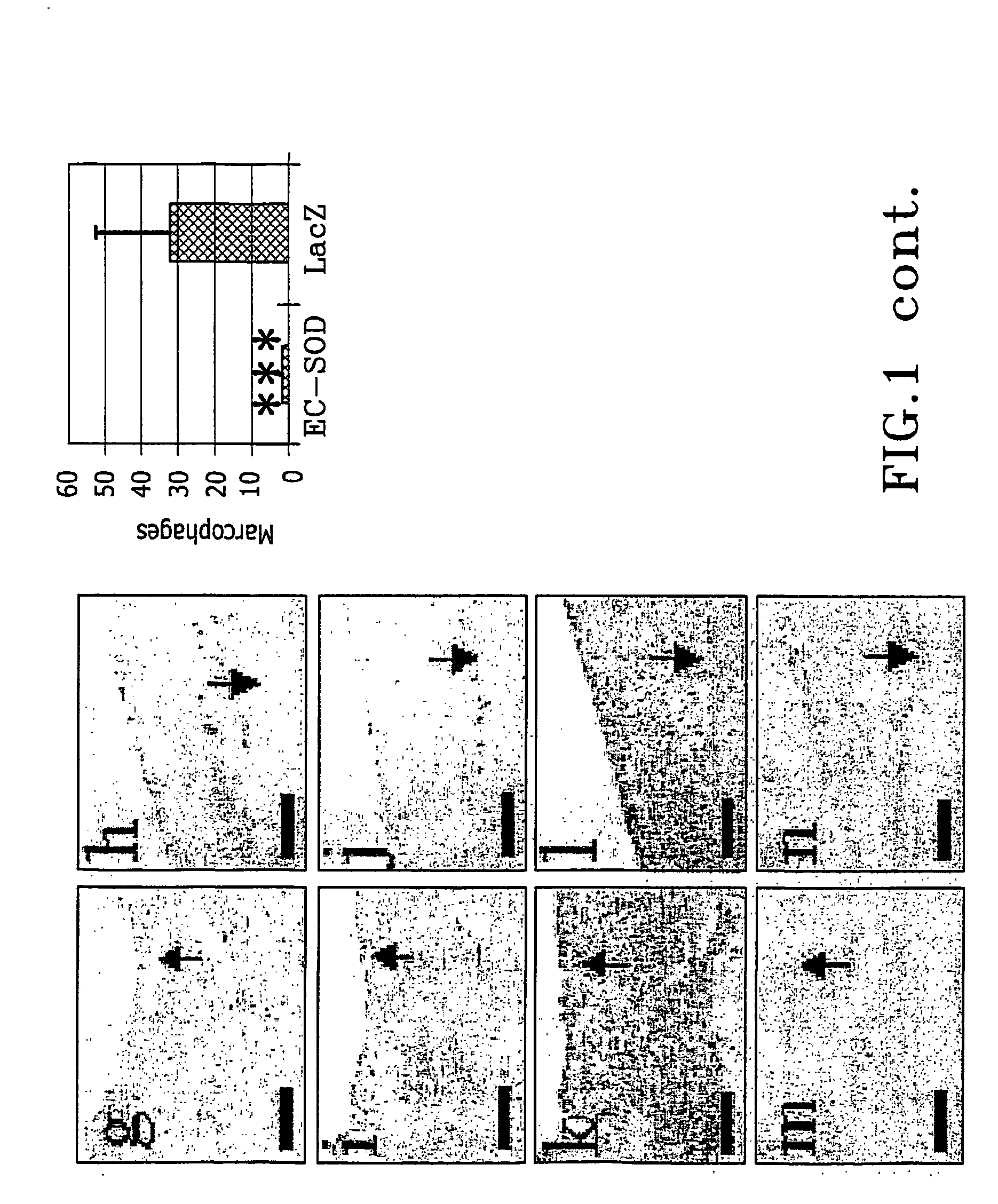
The present invention relates to the use of a gene transfer product to reduce hyperplastic connective tissue growth after tissue trauma or implantation of a medical device. The present invention also relates to a medical device with improved biological properties for an at least partial contact with blood, bodily fluids and / or tissues when introduced in a mammalian body, which device comprises a core and a nucleic acid, encoding a product capable of leading to production of extracellular superoxide dismutase present in a biologically compatible medium. Said nucleic acid encodes a translation or transcription product, which is capable of inhibiting hyperplastic connective tissue growth and promoting endothelialisation in vivo at least partially on a synthetic surface of said core. The present invention also relates to a method of producing a medical device according to the invention.
Owner:FIT BIOTECH OY PLC
Implantable or insertable medical device resistant to microbial growth and biofilm formation
InactiveUS6887270B2Prevent preferential partitioningPrevent chemical modificationAntipyreticAnalgesicsActive agentMicrobial adhesion
Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion / biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents.
Owner:BOSTON SCI SCIMED INC
Color detection system for detecting reservoir presence and content in device
A system for identifying a reservoir used with a fluid delivery device. The system includes a reservoir comprising a housing for holding fluid and a colored marking located on a surface of the housing. A fluid delivery device includes a compartment for receiving and operatively coupling with the reservoir. A light source shines light on the colored marking of the reservoir. A color sensor detects wavelengths reflected and / or refracted from the colored marking due to the light shined on the colored marking. A processor of the delivery device determines a color of the colored marking from the detected wavelengths and ascertains information related to the reservoir or the fluid in the reservoir corresponding to the determined color, wherein the processor operates the fluid delivery device according to the ascertained information, wherein the information may include a reservoir type and / or medication type.
Owner:MEDTRONIC MIMIMED INC
Adhesion barriers applicable by minimally invasive surgery and methods of use thereof
Biocompatible crosslinked polymers, and methods for their preparation and use with minimally invasive surgery applicators are disclosed. The disclosure includes compositions and methods for in situ formation of hydrogels using minimally invasive surgical techniques.
Owner:INCEPT LLC
Infusion medium delivery device and method with drive device for driving plunger in reservoir
ActiveUS20080051711A1Prevent rotationObstruct passageInfusion syringesPharmaceutical containersEngineeringMechanical engineering
A delivery device includes a durable housing portion and a separable disposable portion that selectively engage and disengage from each other. The disposable housing portion secures to the patient-user and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient-user or with infusion medium are supported by the disposable housing portion, while the durable housing portion supports other components such as electronics and a drive device. A reservoir is supported by the disposable housing portion and has a moveable plunger that operatively couples to the drive device, when the disposable and durable housing portions are engaged.
Owner:MEDTRONIC MIMIMED INC
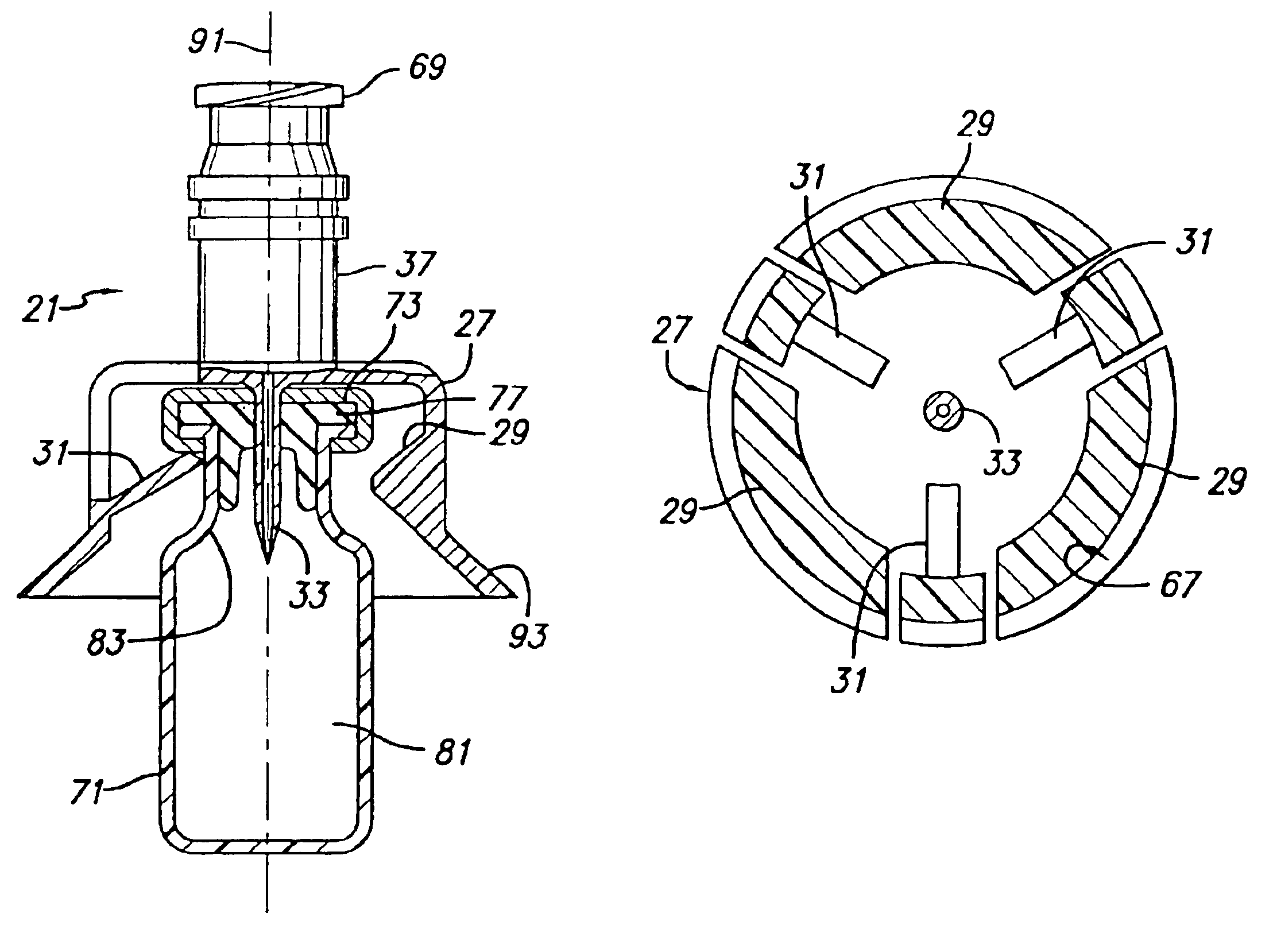
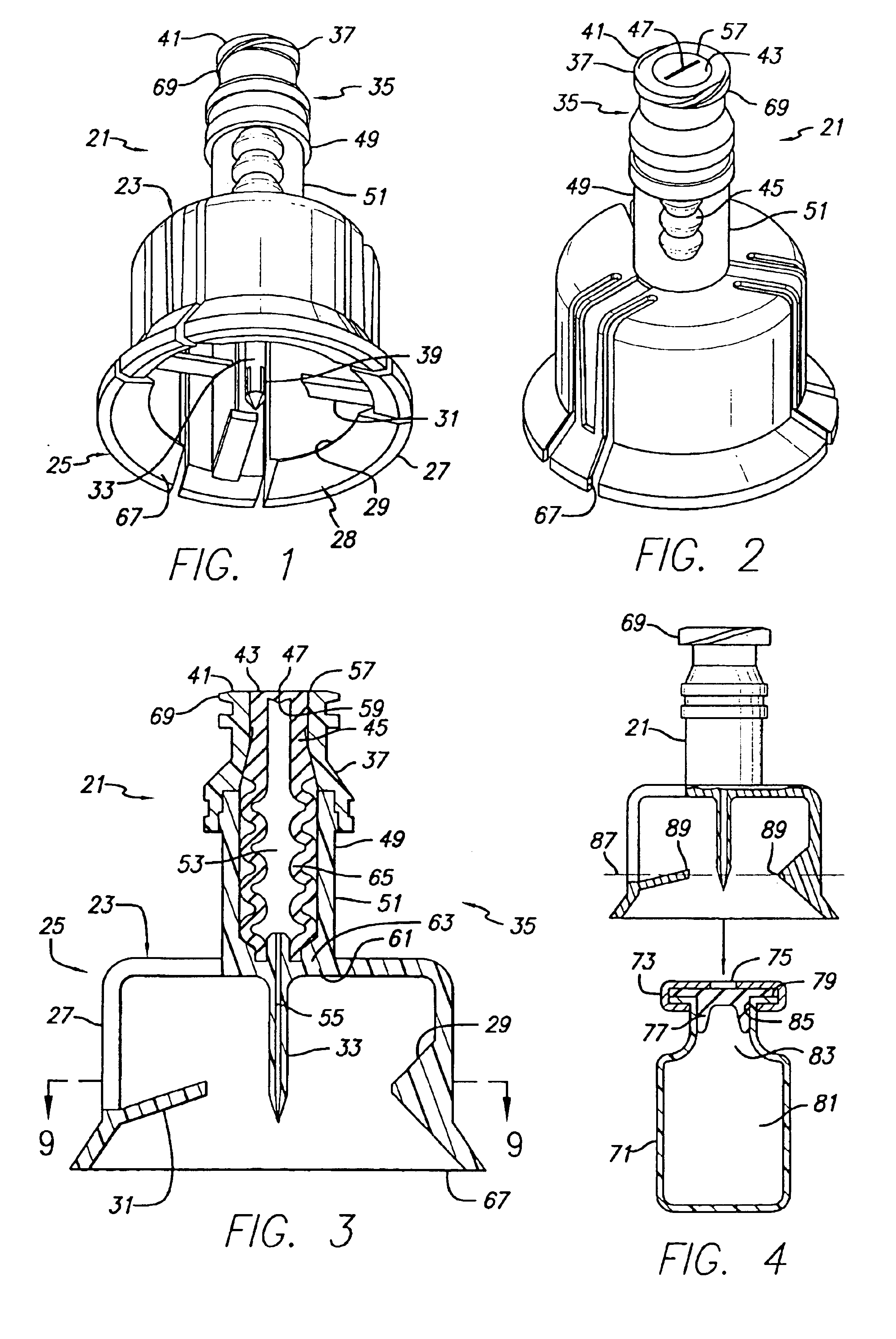
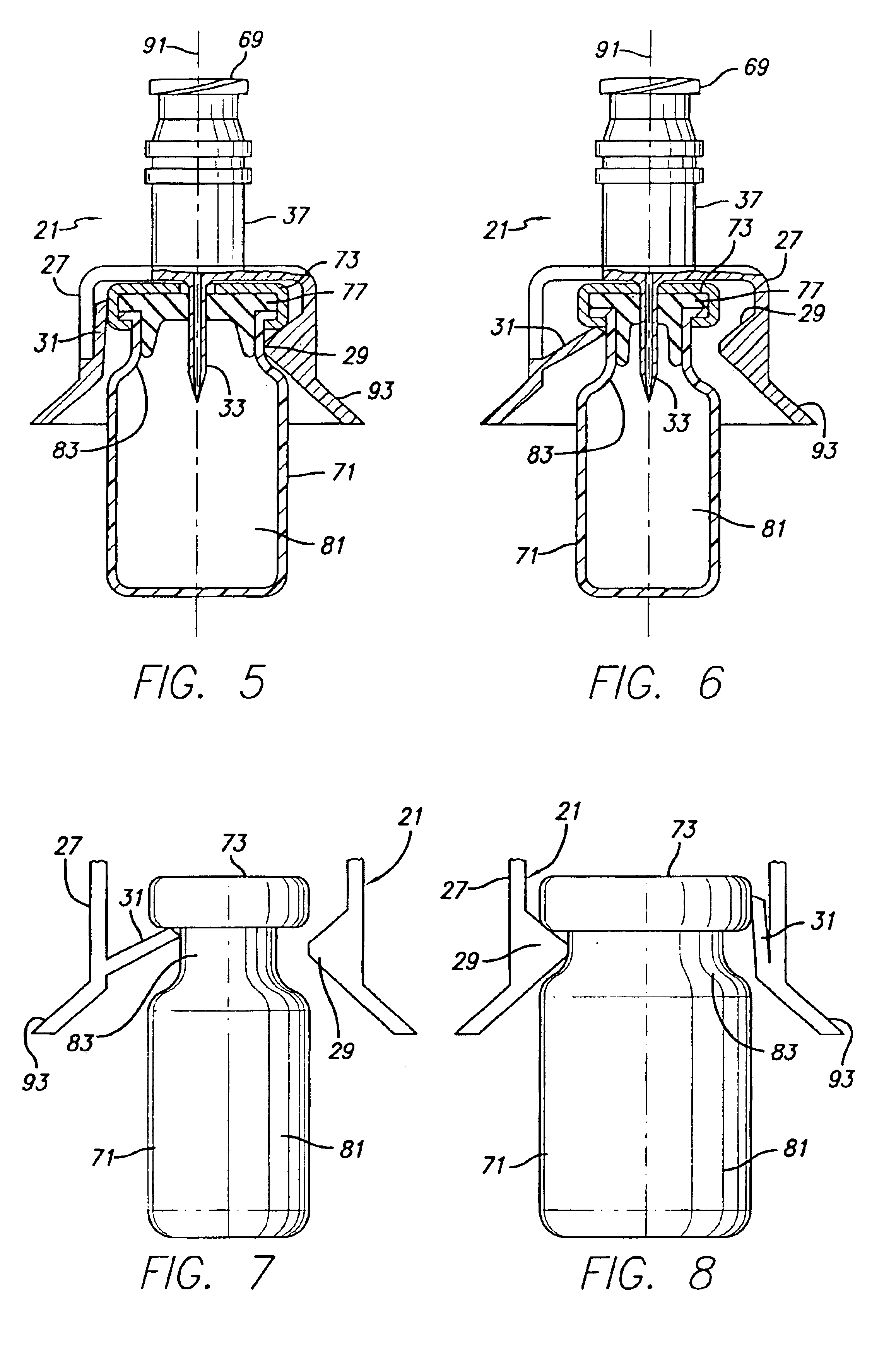
Vial adapter having a needle-free valve for use with vial closures of different sizes
A vial adapter having a needle-free valve, a sharpened cannula used to perforate a vial's rubber stopper, and a circular array of claws of different lengths to engage vial closures of different diameters. The array of claws includes a first set of claws each having a first length extending inwardly from the periphery of the housing of the adapter and a second set of claws alternating with the first set of claws and each having a longer length. The second set of claws are mounted so that they deflect and plastically deform out of the way in the case where the adapter is engaged with a vial that exceeds a predetermined size. The housing includes a shroud that is at least as long as the sharpened cannula to protect medical personnel who use the adapter from inadvertent punctures. The needle-free valve includes a resiliently deformable piston element with a naturally open bore. The interior of the piston provides a fluid flow path through the adapter. In one embodiment, the first set of claws of the adapter may be used with a vial closure of at approximately 20 mm in diameter and the second set of claws may be used with a vial closure of approximately 13 to 17 mm in diameter.
Owner:CAREFUSION 303 INC
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Articles having bioactive surfaces and solvent-free methods of preparation thereof
Methods for preparing articles having a bioactive surface comprising treating a substrate to form free reactive groups, depositing a monomer onto the treated substrate, and covalently immobilizing a biologically functional molecule onto the deposited monomer. Additional embodiments include methods for the deposition of the monomer onto the treated substrate in a solvent-free environment. Further embodiments include articles having surfaces prepared using the methods described herein. Additional embodiments include articles prepared using the methods described herein.
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com