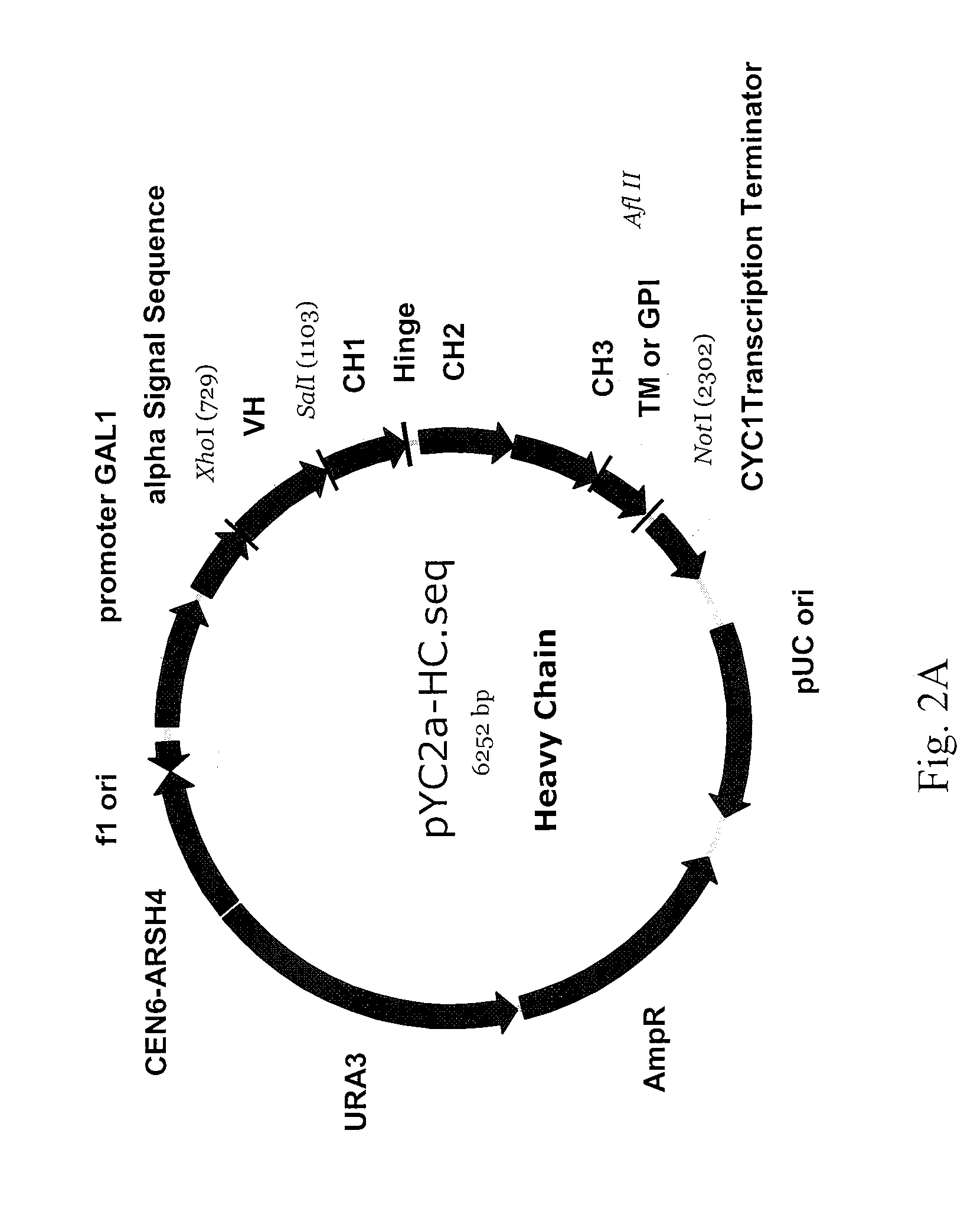
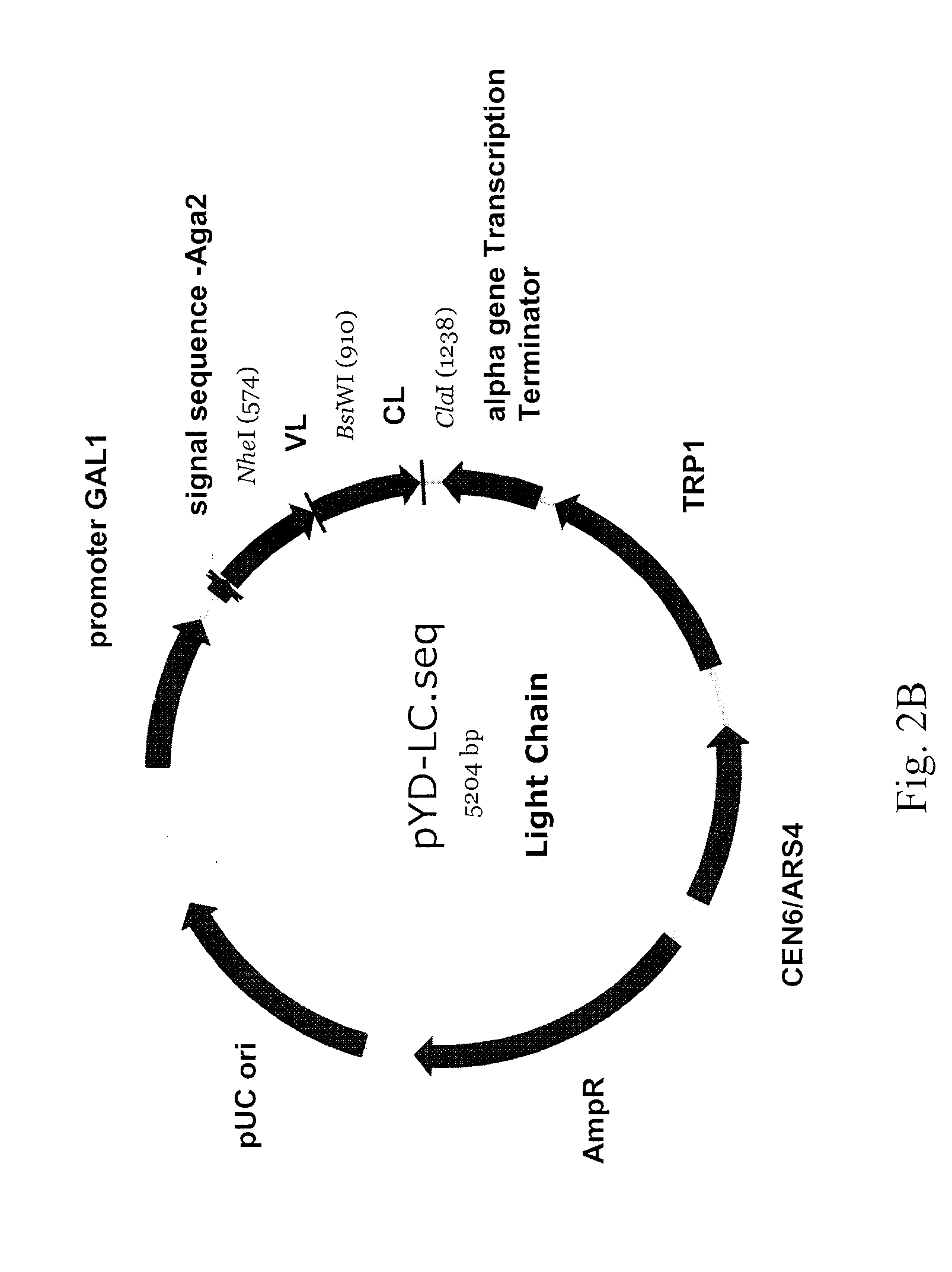
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1113 results about "Extracellular" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In cell biology, molecular biology and related fields, the word extracellular (or sometimes extracellular space) means "outside the cell". This space is usually taken to be outside the plasma membranes, and occupied by fluid (see extracellular matrix). The term is used in contrast to intracellular (inside the cell).
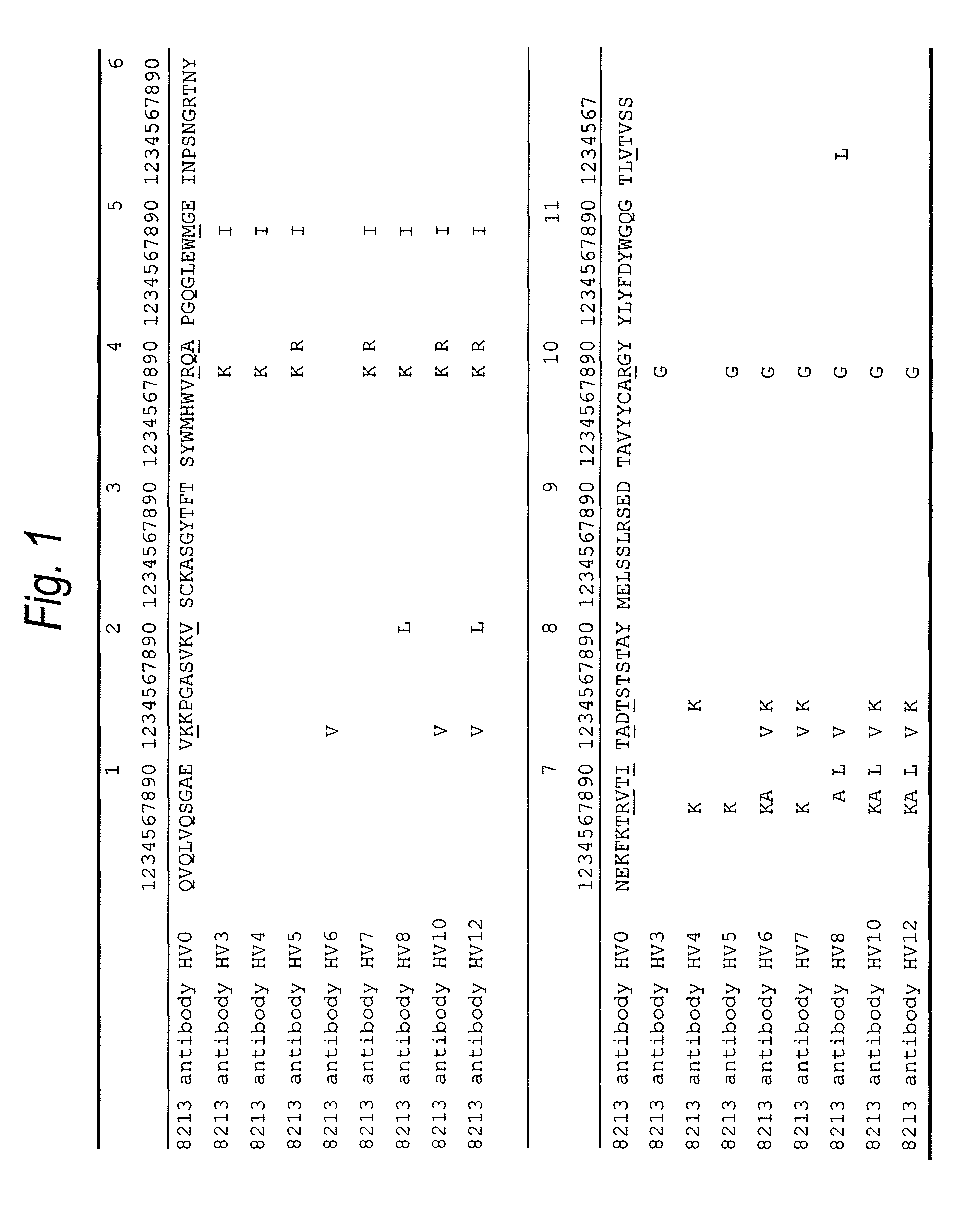
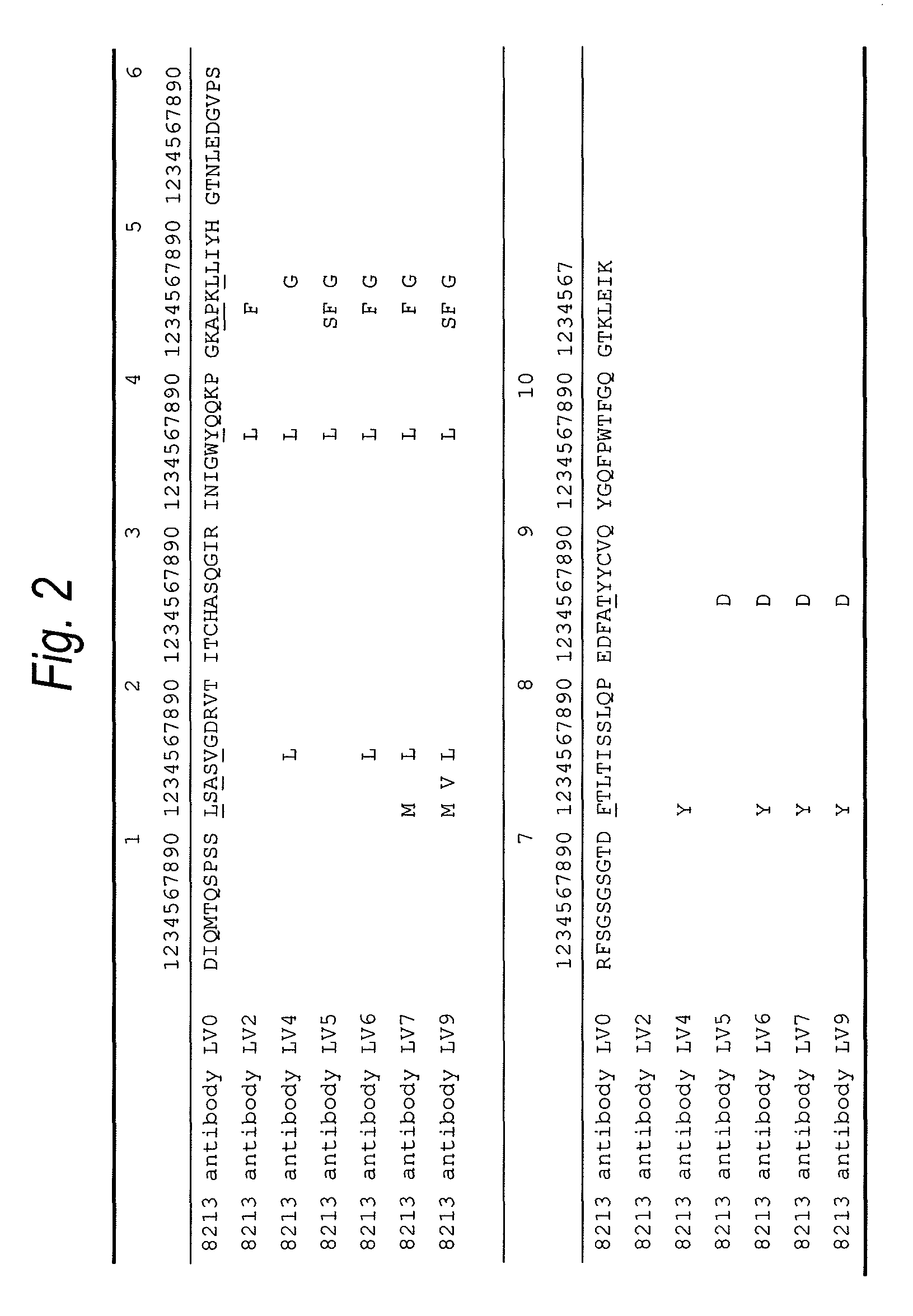
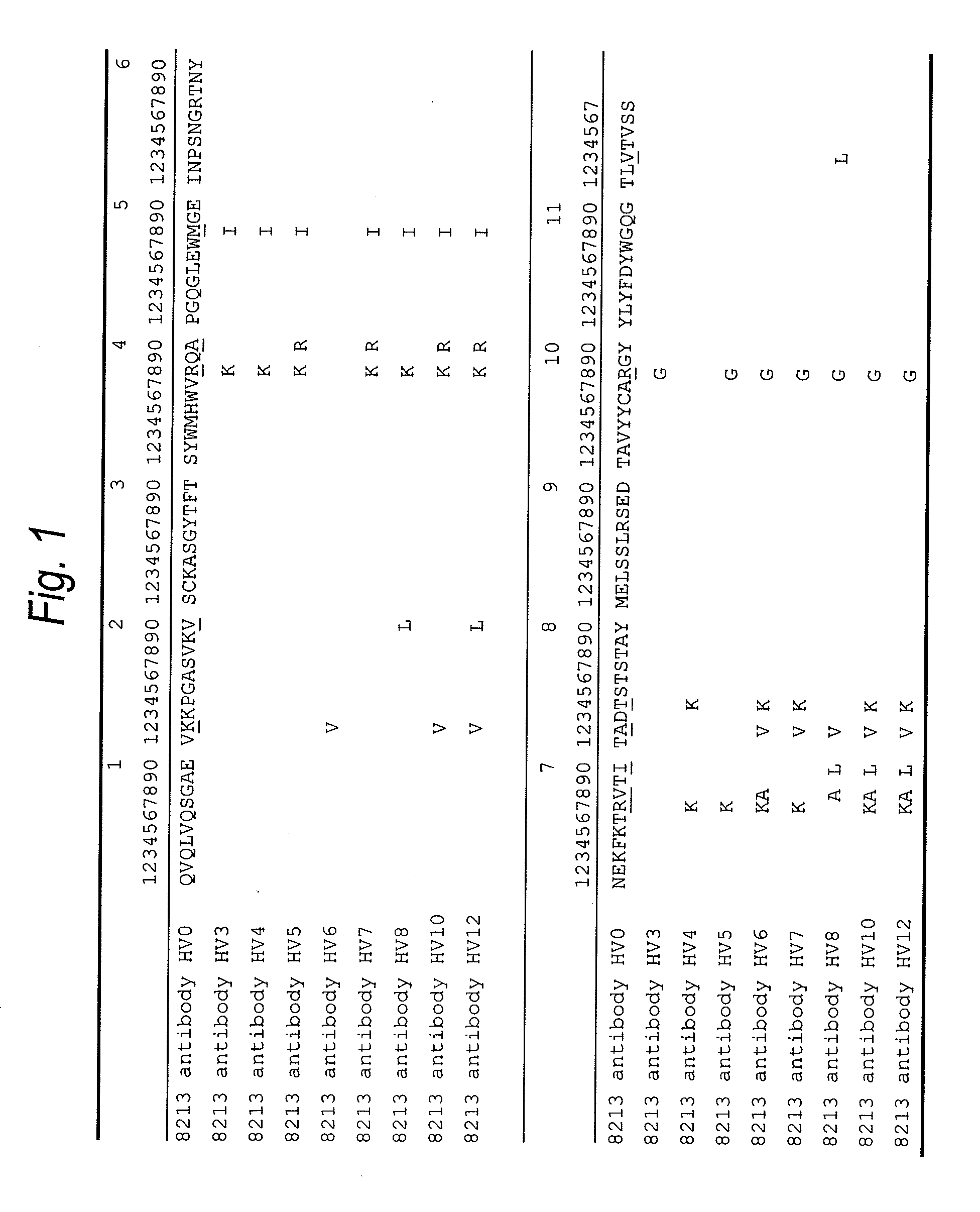
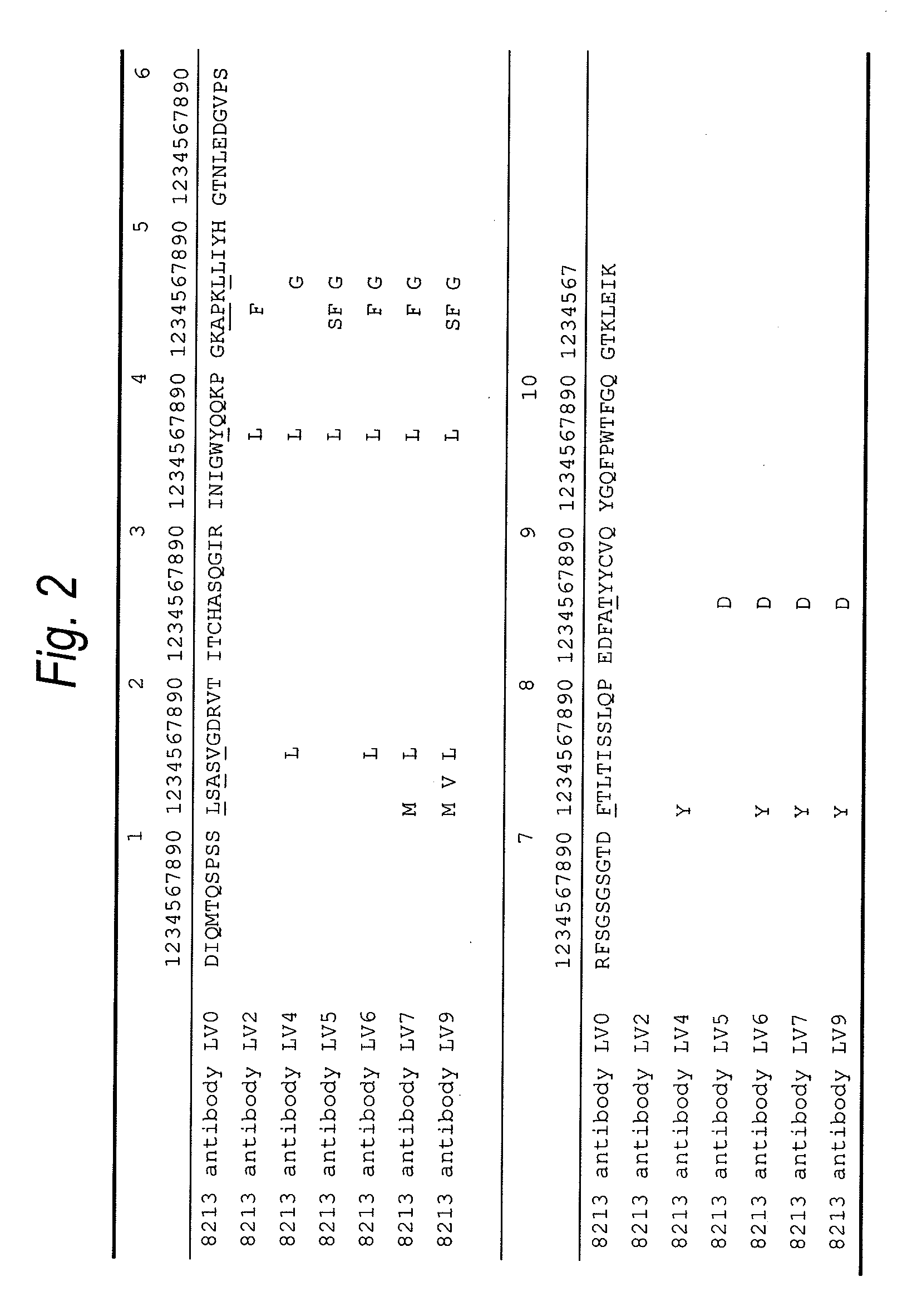
Anti-TIM-3 antibody
The present invention provides an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYUSHU UNIV +1
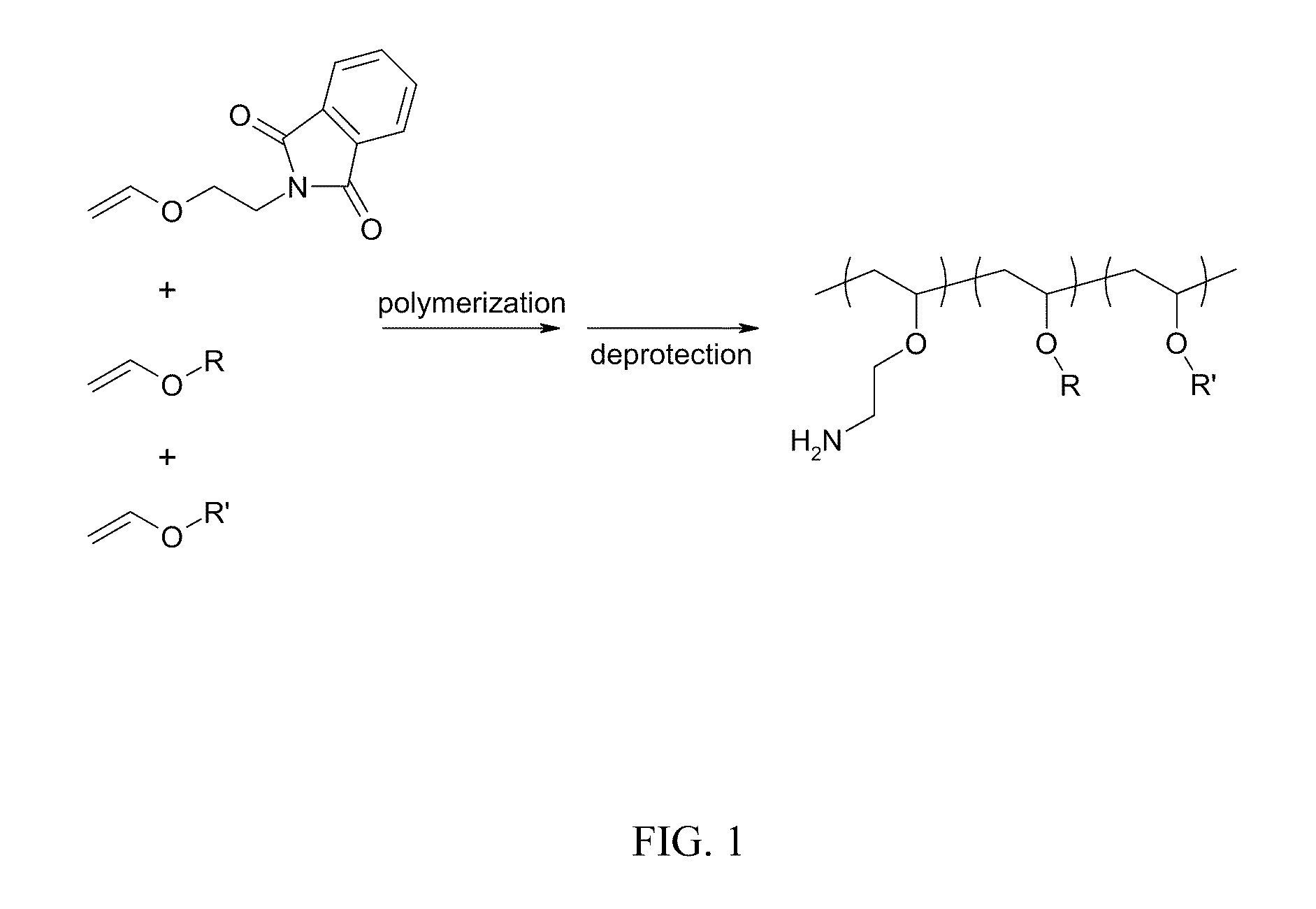
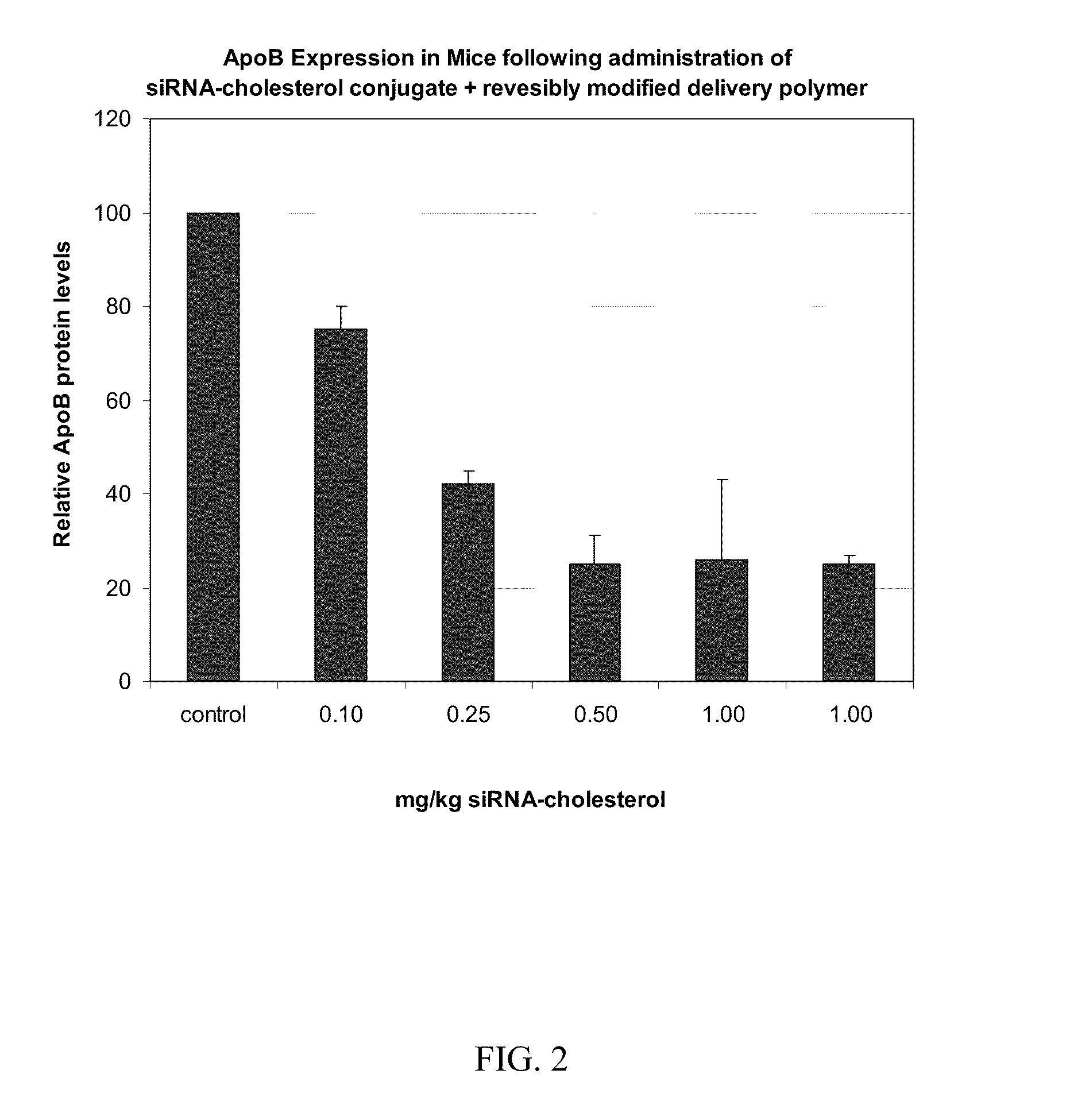
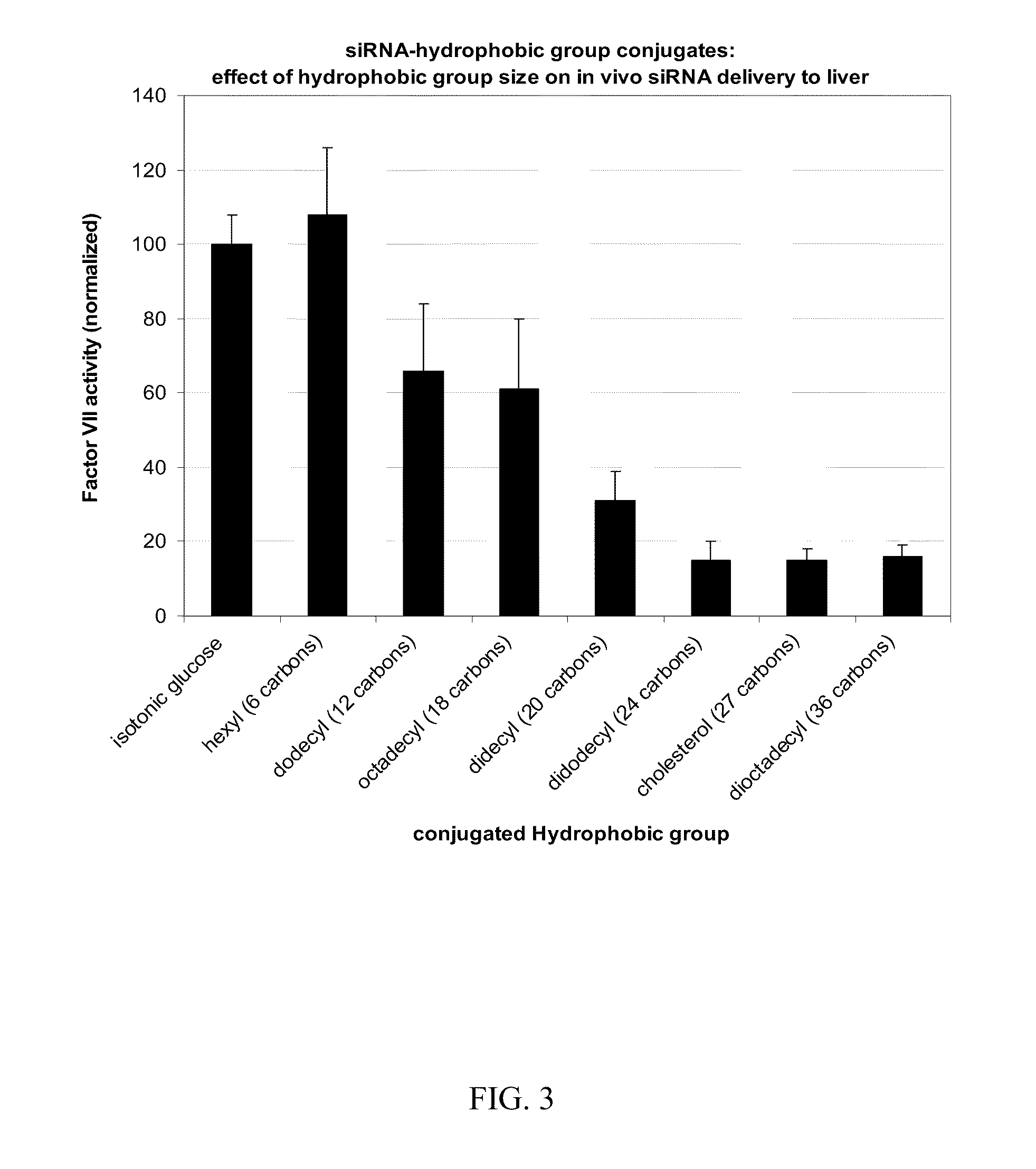
Compositions for targeted delivery of siRNA
The present invention is directed compositions for targeted delivery of RNA interference (RNAi) polynucleotides to hepatocytes in vivo. Targeted RNAi polynucleotides are administered together with co-targeted delivery polymers. Delivery polymers provide membrane penetration function for movement of the RNAi polynucleotides from outside the cell to inside the cell. Reversible modification provides physiological responsiveness to the delivery polymers.
Owner:ARROWHEAD MADISON
Anti-tim-3 antibody
ActiveUS20140044728A1High activityPeptide/protein ingredientsAntipyreticExtracellularDiagnostic agent
Disclosed are an anti-human TIM-3 antibody having high ADCC activity or antibody fragment thereof by screening a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; and a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
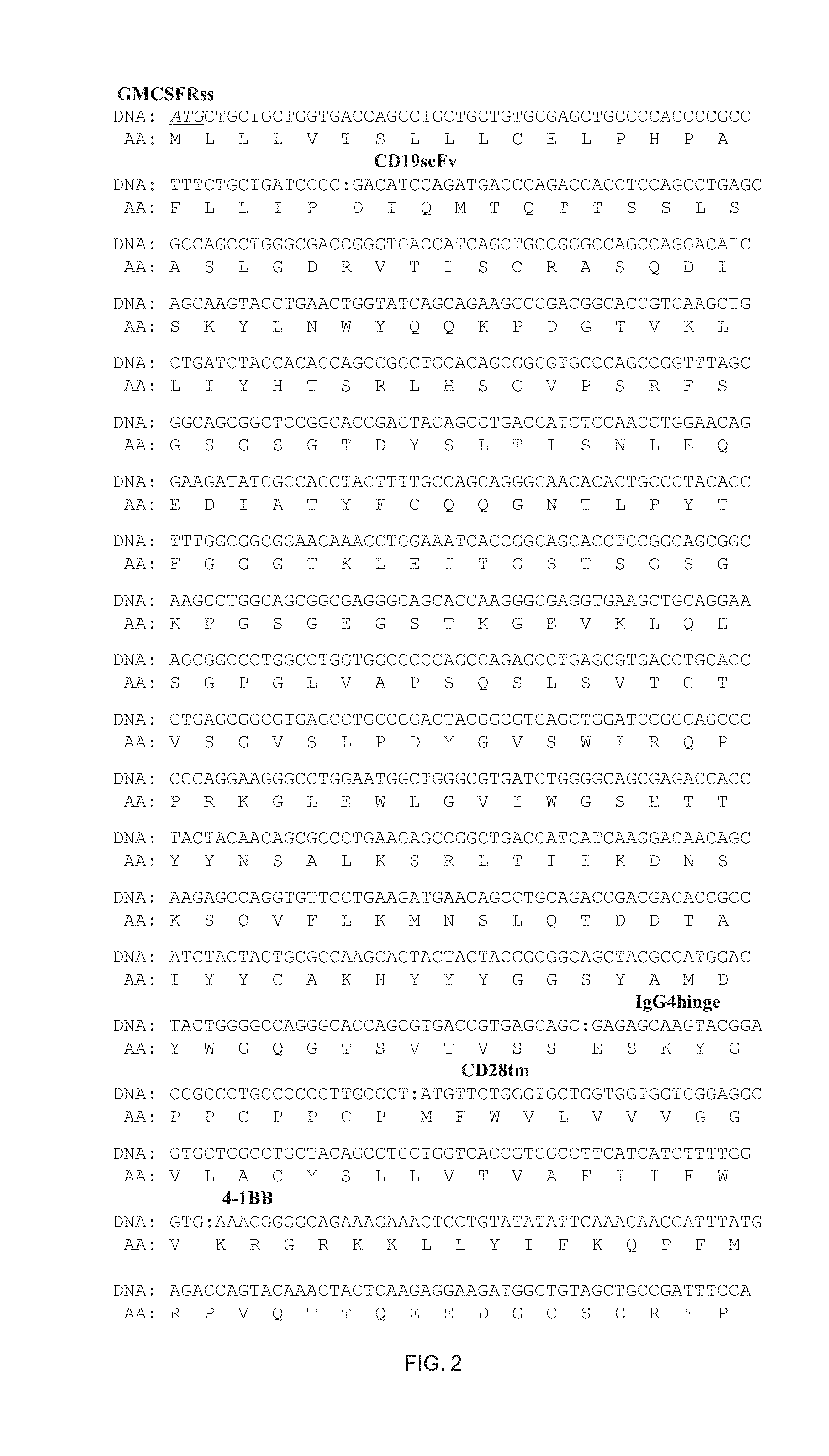
Single chain recombinant T cell receptors
InactiveUS7569664B2Improve stabilityUseful purposeCompound screeningApoptosis detectionExtracellularC-terminus
A single chain T cell receptor (scTCR) comprising an a segment constituted by a TCR α chain variable region sequence fused to the N terminus of a TCR α chain constant region extracellular sequence, a β segment constituted by a TCR β chain variable region fused to the N terminus of a TCR β chain constant region extracellular sequence, and a linker sequence linking the C terminus of the α segment to the N terminus of the β segment, or vice versa, the constant region extracellular sequences of the α and β segments being linked by a disulfide bond, the length of the linker sequence and the position of the disulfide bond being such that the variable region sequences of the α and β segments are mutually orientated substantially as in native αβ T cell receptors. Complexes of two or more such scTCRs, and use of the scTCRs in therapy and in various screening applications are also disclosed.
Owner:IMMUNOCORE LTD +1
Anti-tim-3 antibody
ActiveUS20120189617A1High ADCC activityHigh activityBacteriaPeptide/protein ingredientsDiseaseAntibody fragments
The present invention provides an anti-human TIM-3 antibody which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure thereof and exhibits higher effector activity such as an antibody-dependent cellular cytotoxicity (ADCC activity) for diseases relating to a human TIM-3 expressing cell. The present invention provides a monoclonal antibody or antibody fragment thereof which binds to the amino acid sequence of the extracellular region of TIM-3 or its three-dimensional structure and exhibits ADCC activity; a hybridoma which produces the antibody; a DNA encoding the antibody; a vector comprising the DNA; a transformant which is obtainable by introducing the vector; a method for producing the antibody or the antibody fragment thereof which comprises using the hybridoma or the transformant; a therapeutic agent and a diagnostic agent comprising the antibody or the antibody fragment thereof as an active ingredient. In addition, the present invention provides an anti-human TIM-3 antibody having high ADCC activity by screening an anti-human TIM-3 antibody which competes with the monoclonal antibody or the antibody fragment thereof.
Owner:KYUSHU UNIV +1
Method enabling use of extracellular RNA extracted from plasma or serum to detect, monitor or evaluate cancer
InactiveUS20020106684A1Low tumor burdenImmunologic function is relatively intactSugar derivativesMicrobiological testing/measurementA lipoproteinNeoplasm
This invention relates to the use of tumor-derived or associated extracellular ribonucleic acid (RNA) found circulating in the plasma or serum fraction of blood for the detection, monitoring, or evaluation of cancer or premalignant conditions. Extracellular RNA may circulate as non-bound RNA, protein-bound RNA, lipid-RNA complexes, lipoprotein (proteolipid)-RNA complexes, protein-RNA complexes including within or in association with ribonucleoprotein complexes, nucleosomes, or within apoptotic bodies. Any intracellular RNA found in plasma or serum can additionally be detected by this invention. Specifically, this invention enables the extraction of circulating RNA from plasma or serum and utilizes nucleic acid amplification assays for the identification, detection, inference, monitoring, or evaluation of any neoplasm, benign, premalignant, or malignant, in humans or other animals, which might be associated with that RNA. Further, this invention allows the qualitative or quantitative detection of tumor-derived or associated extracellular RNA circulating in the plasma or serum of humans or animals with or without any prior knowledge of the presence of cancer or premalignant tissue.
Owner:ONCOMEDX
Method and apparatus for sealing access
InactiveUS20060004408A1Rapid and safe and effective sealingReduce chanceGuide needlesCannulasExtracellularConnective tissue fiber
The present invention relates to an apparatus and a method for sealing a puncture in a tubular tissue structure or the wall of a body cavity. More specifically, the present invention is directed to an apparatus and method for sealing a puncture site in the wall of a tubular tissue structure, or in the wall of a body cavity with a bioabsorbable material such as submucosal tissue, another extracellular or matrix-derived tissue, or a synthetic material capable of remodeling endogenous connective tissue in vivo. The bioabsorbable material is inserted into the puncture site as a sheet on an introducer element used to access the lumen of a tubular tissue structure or used to access a body cavity.
Owner:MORRIS INNOVATIVE RES
Extracellular adhesive proteins
InactiveUS20010010913A1High viscosityHighly concentrated solutionAntibacterial agentsFungiExtracellularAgonist
The invention provides human extracellular adhesive proteins (EXADH) and polynucleotides which identify and encode EXADH. The invention also provides expression vectors, host cells, antibodies, agonists, and antagonists. The invention also provides methods for diagnosing, treating or preventing disorders associated with expression of EXADH.
Owner:INCYTE PHARMA INC
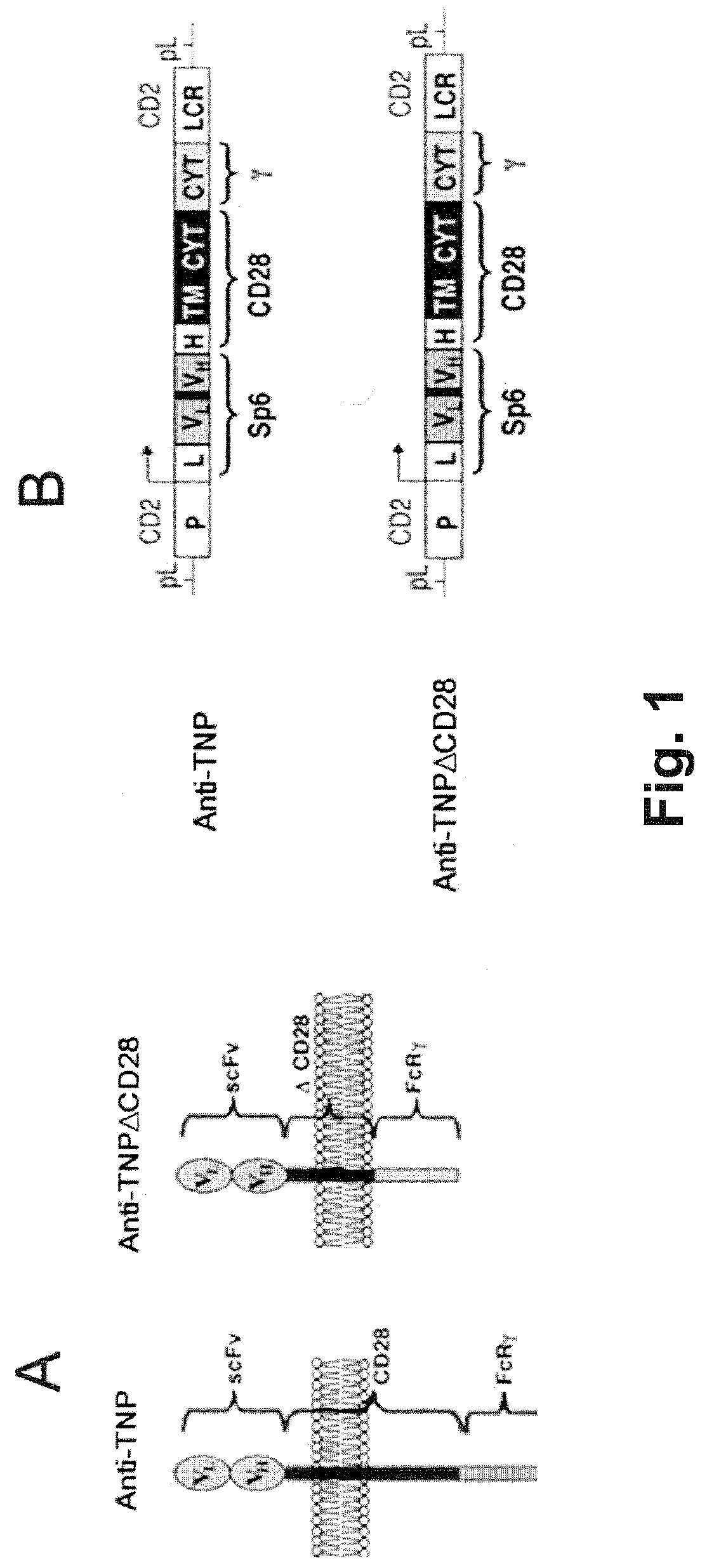
Redirected, genetically-engineered t regulatory cells and their use in suppression of autoimmune and inflammatory disease
InactiveUS20100135974A1Effective quantityOvercome scarcityBiocideAntipyreticIntracellular signallingInflammatory Bowel Diseases
A redirected Treg cell is endowed with specificity toward a selected target antigen or ligand. The cell contains a chimeric receptor polypeptide that is expressed in a single, continuous chain, with an extracellular recognition region displayed on the surface of the cell, a transmembrane region and an intracellular signaling region. The extracellular recognition region is specific for the selected target antigen or ligand. The intracellular signaling region includes a combination of T-cell signaling polypeptide moieties, which combination, upon binding of the extracellular recognition region to the selected target antigen or ligand, triggers activation of the redirected Treg cells to cause suppression of T-cell mediated immunity. Such redirected Treg cells may be used to suppress undesired activity of T effector cells thereby mediating an immune or inflammatory response. They are particularly useful in treating T effector cell-mediated diseases, such as inflammatory bowel disease, transplant rejection and GVH disease.
Owner:YEDA RES & DEV CO LTD
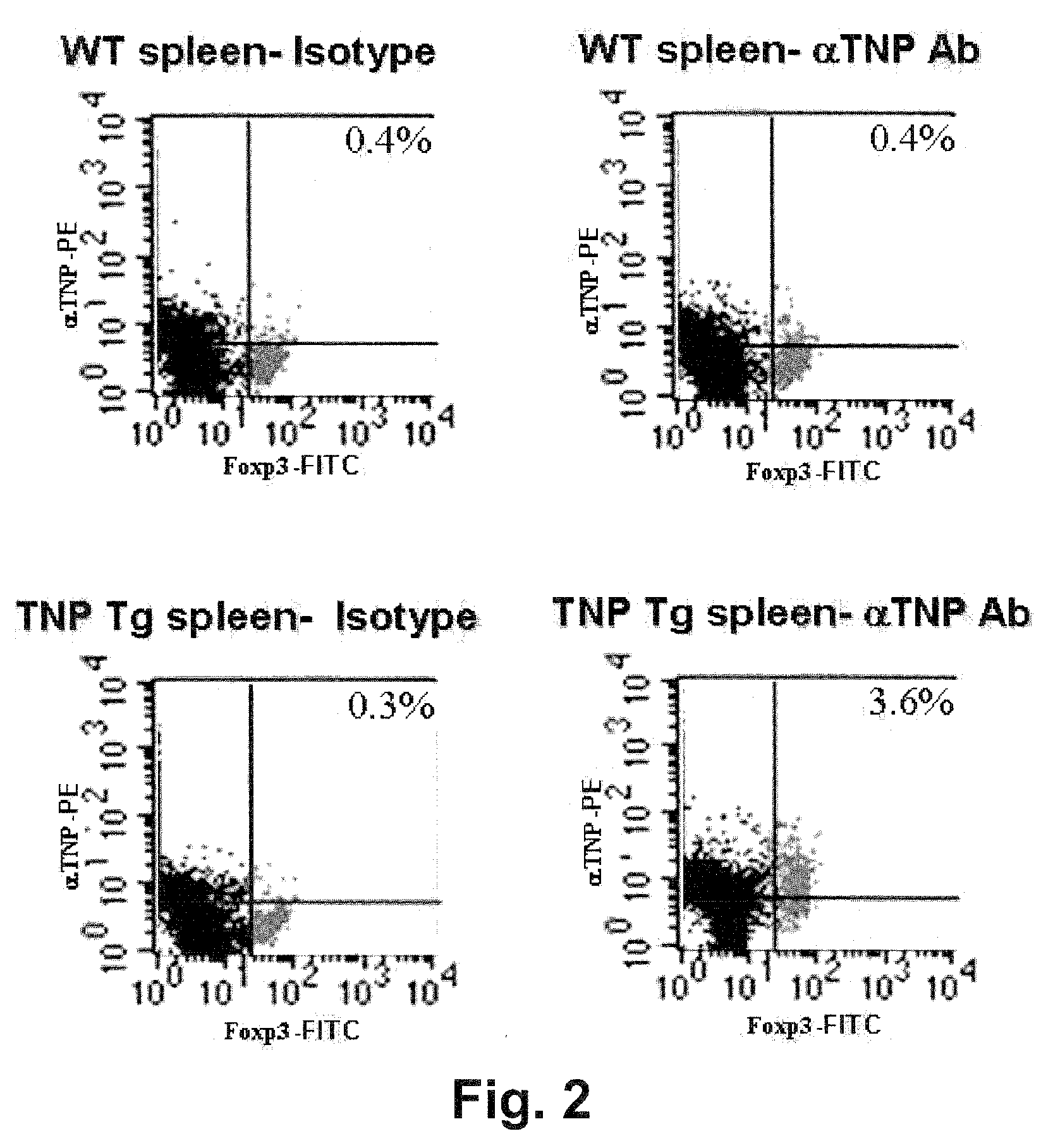
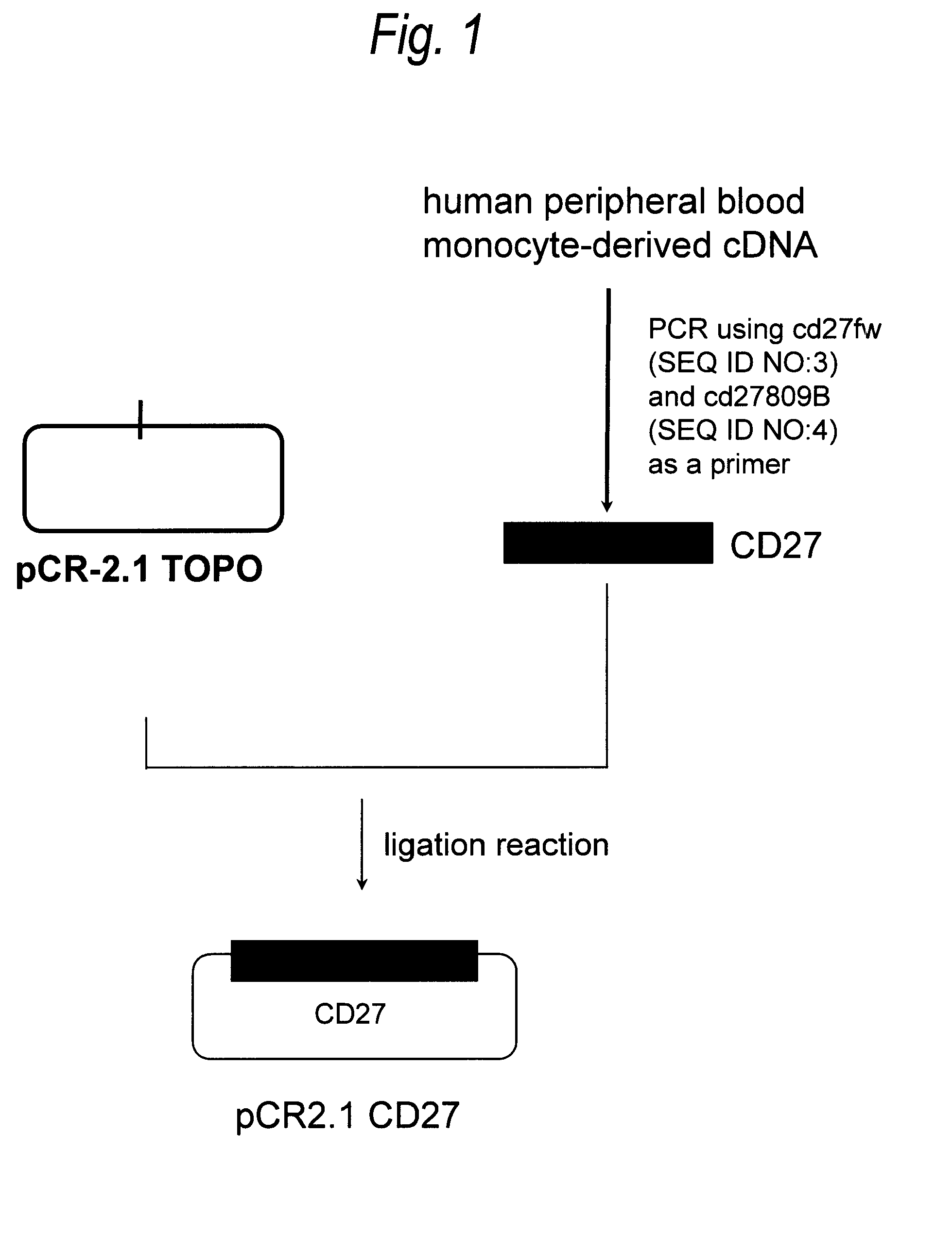
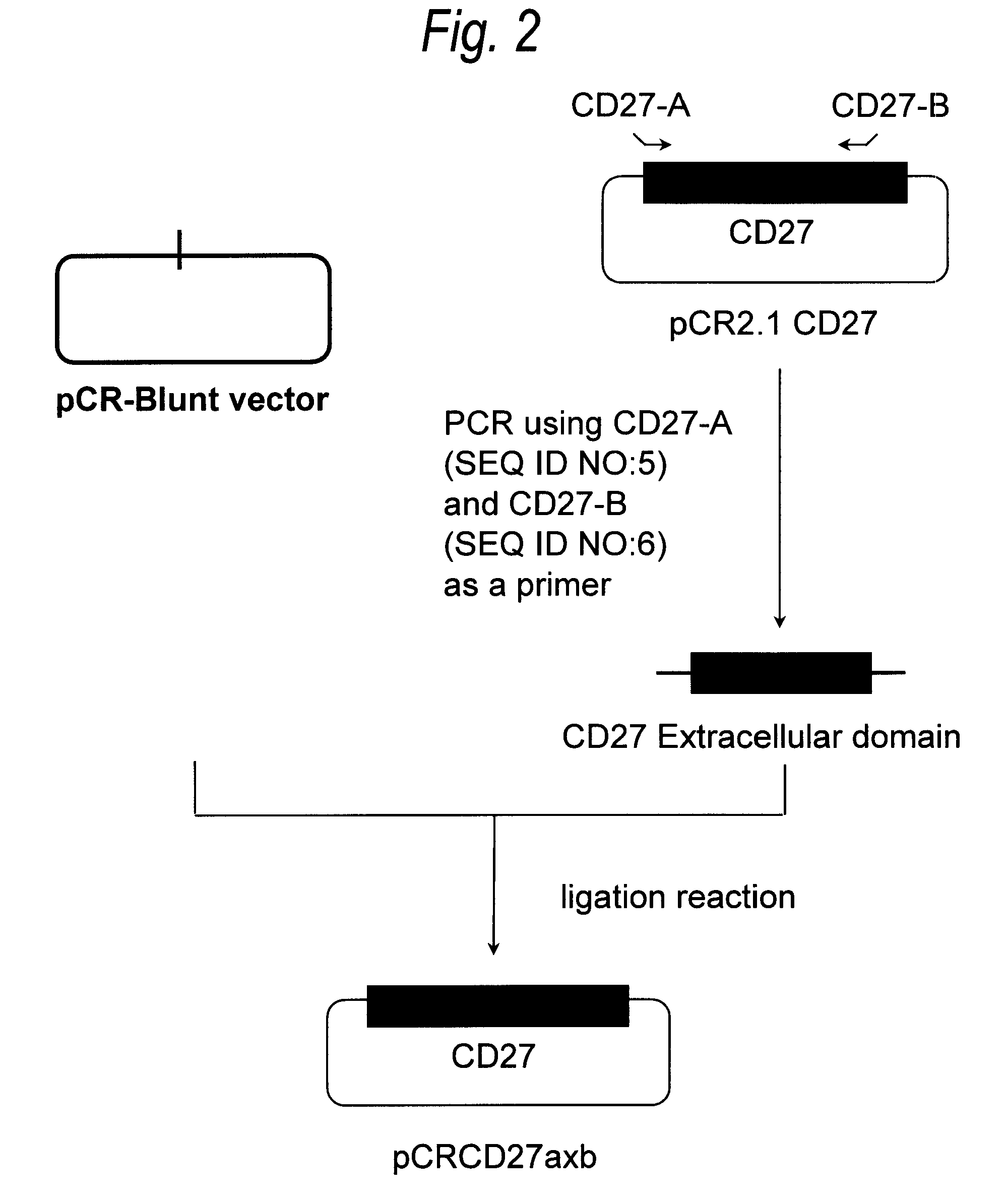
Anti-cd27 antibody
The present invention provides a monoclonal antibody which specifically recognizes CD27 containing an O-linked sugar chain to which galactose is not bound and binds to its extracellular region, or a method for using the same.The present invention can provide a monoclonal antibody or an antibody fragment thereof, which specifically recognizes a polypeptide encoded by CD27 gene containing an O-linked sugar chain to which galactose is not bound, and binds to its extracellular region; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which comprises the DNA; a transformant obtainable by transforming the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a diagnostic agent or a therapeutic agent comprising the antibody or the antibody fragment thereof as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
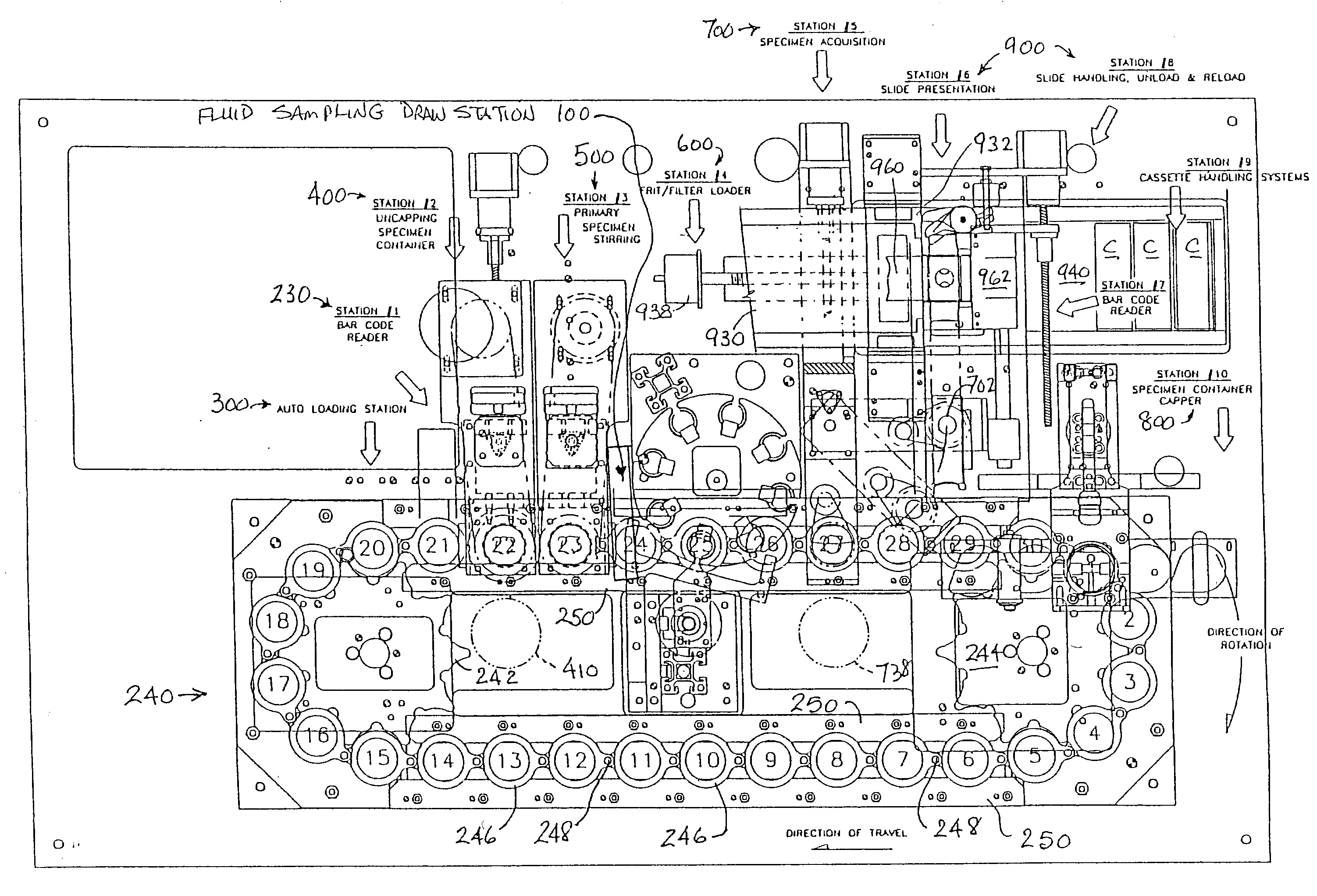
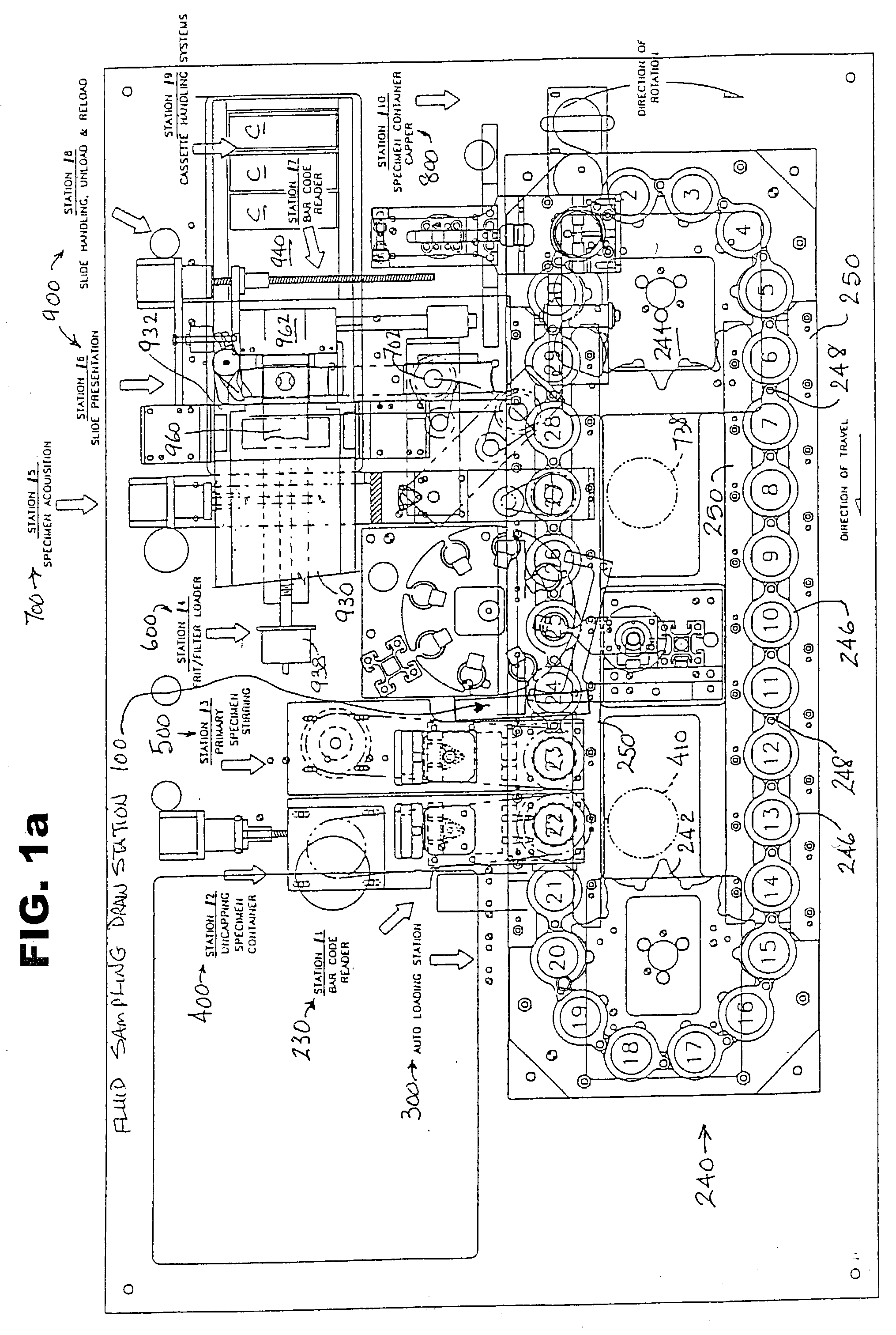
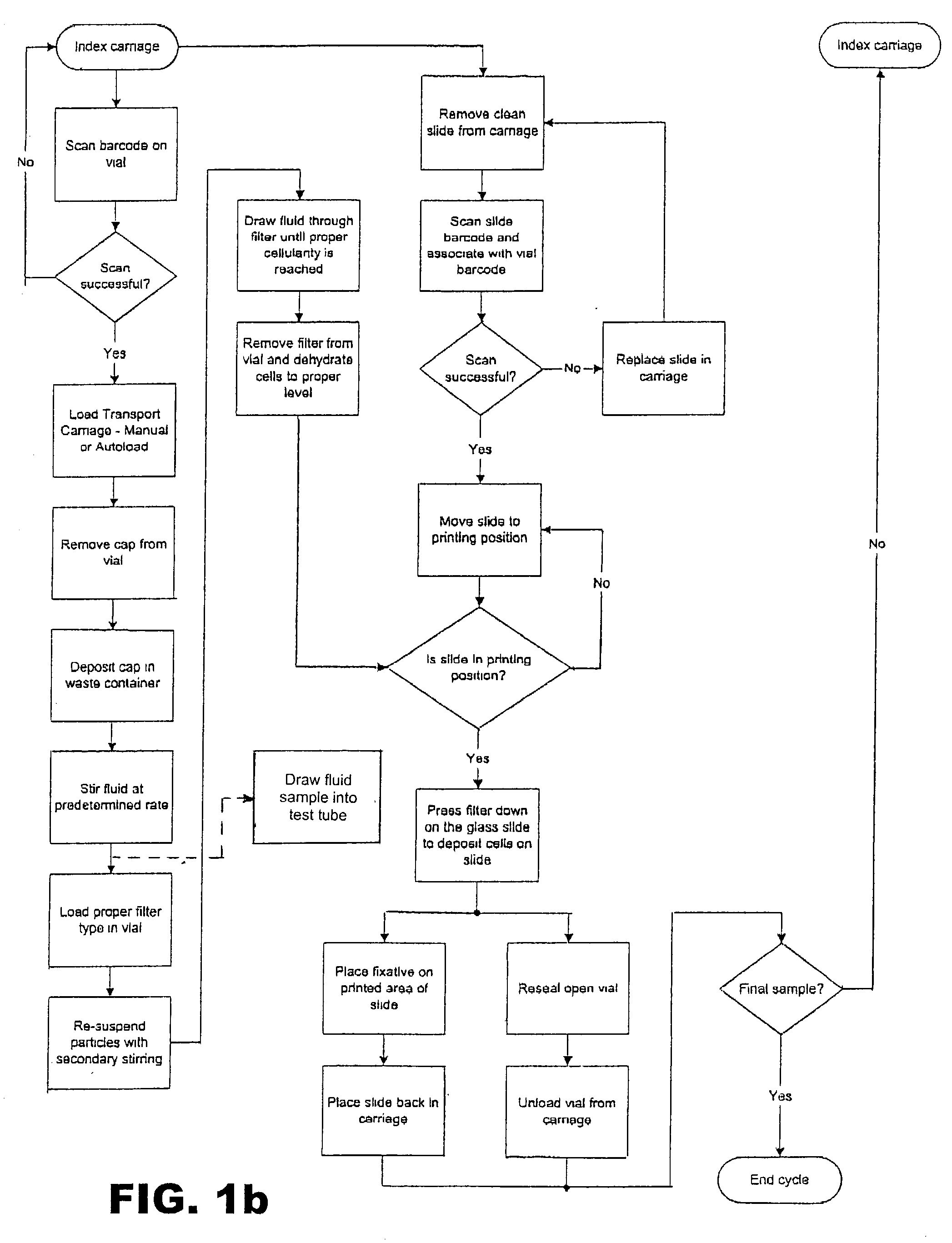
Automated system and method for processing specimens to extract samples for both liquid-based and slide-based testing
InactiveUS20030087443A1Risk minimizationBioreactor/fermenter combinationsBiological substance pretreatmentsExtracellularParticulates
Apparatus and method for processing specimens, e.g., biological specimens, to extract samples for both liquid (i.e., extracellular) and slide-based (i.e., intracellular) testing. Both types of samples can be obtained from a single fluid specimen. A fluid sampling station removes fluid from a specimen container and places it in a sample receptacle. A specimen acquisition station removes fluid from the container, separates particulate matter (e.g., cells) from the removed fluid, and forms a sample layer of particulate matter, which is transferred to a slide. The two sampling operations can be carried out in any order. The fluid sample receptacle may have a special one-way valve arrangement. The apparatus can be automated so as to process multiple fluid specimens in their respective containers. The machine according to this invention is a "platform instrument" that can produce all required liquid samples and slide-based samples for essentially all cytopathology tests.
Owner:HOLOGIC INC
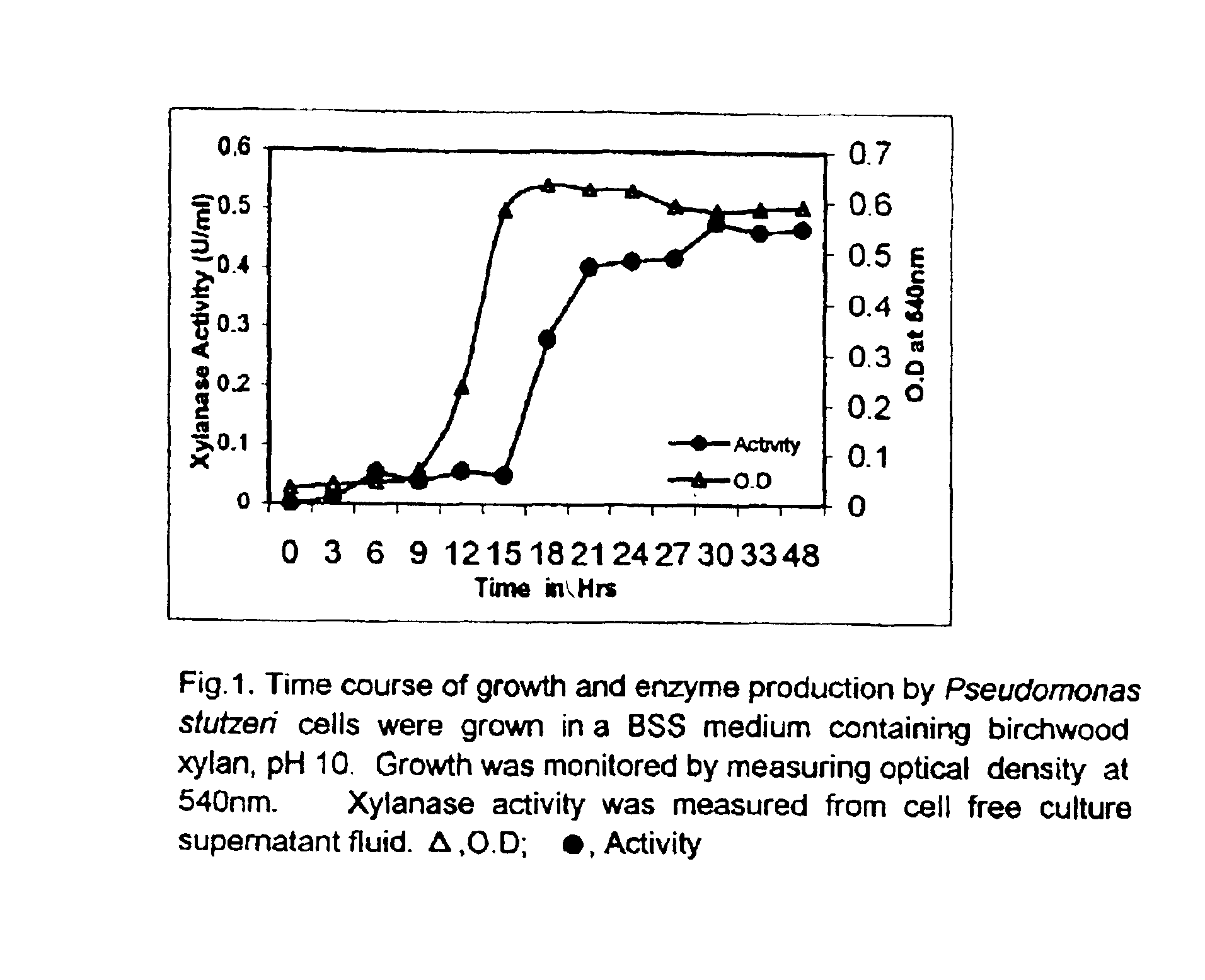
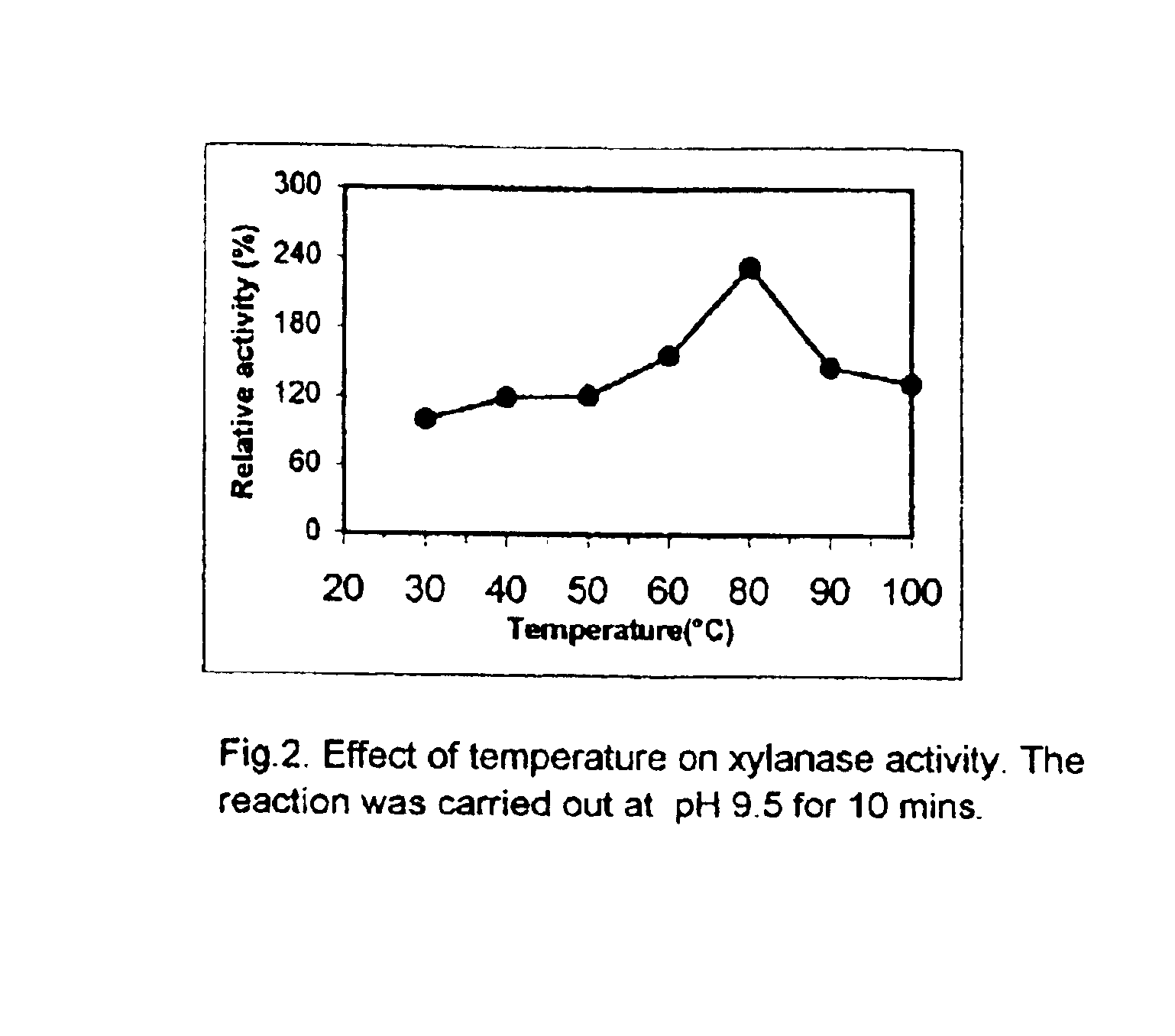
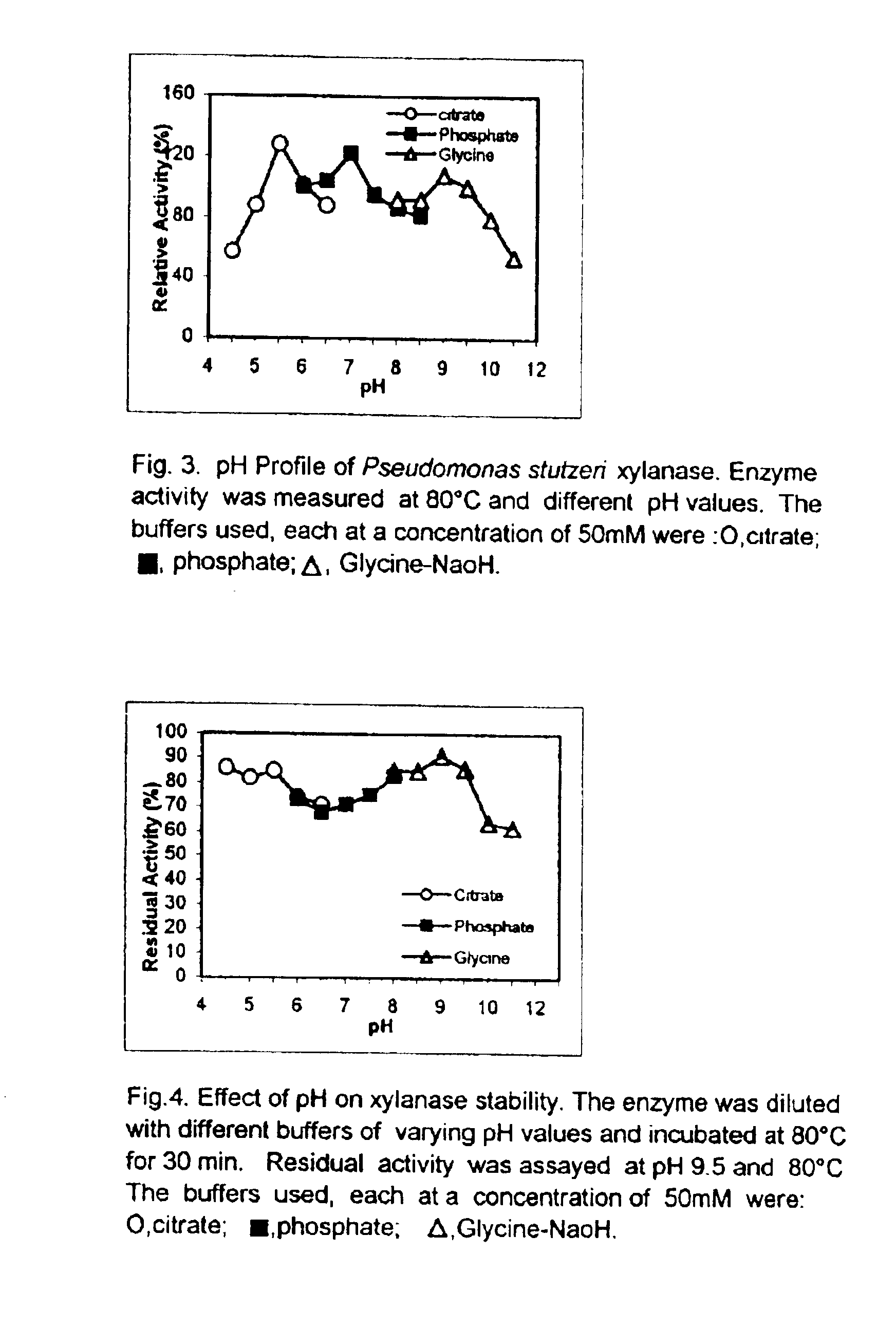
'Pseudomonas stutzeri' strain and process for preparation of xylanase
Owner:COUNCIL OF SCI & IND RES
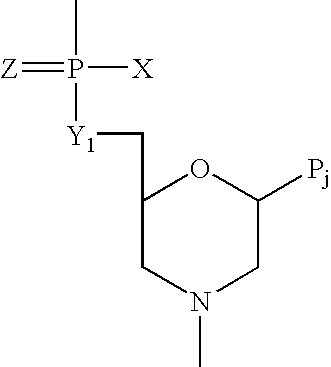
Antisense oligomers and methods for inducing immune tolerance and immunosuppression
ActiveUS20060276425A1Reduce capacityIncrease productionBiocideOrganic active ingredientsExtracellularDendritic cell
A method and composition for inducing human dendritic cells to a condition of reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10 is disclosed. A population of dendritic cells is exposed to a substantially uncharged antisense compound, including partially positively charged, containing 12-40 subunits and a base sequence effective to hybridize to an expression-sensitive region of a preprocessed or processed human CD86 transcript identified, in its processed form, by SEQ ID NO:33, to form a duplex structure between said compound and transcript having a Tm of at least 45° C. Formation of the duplex blocks expression of full-length CD86 in said cells, which in turn leads to reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10.
Owner:SAREPTA THERAPEUTICS INC
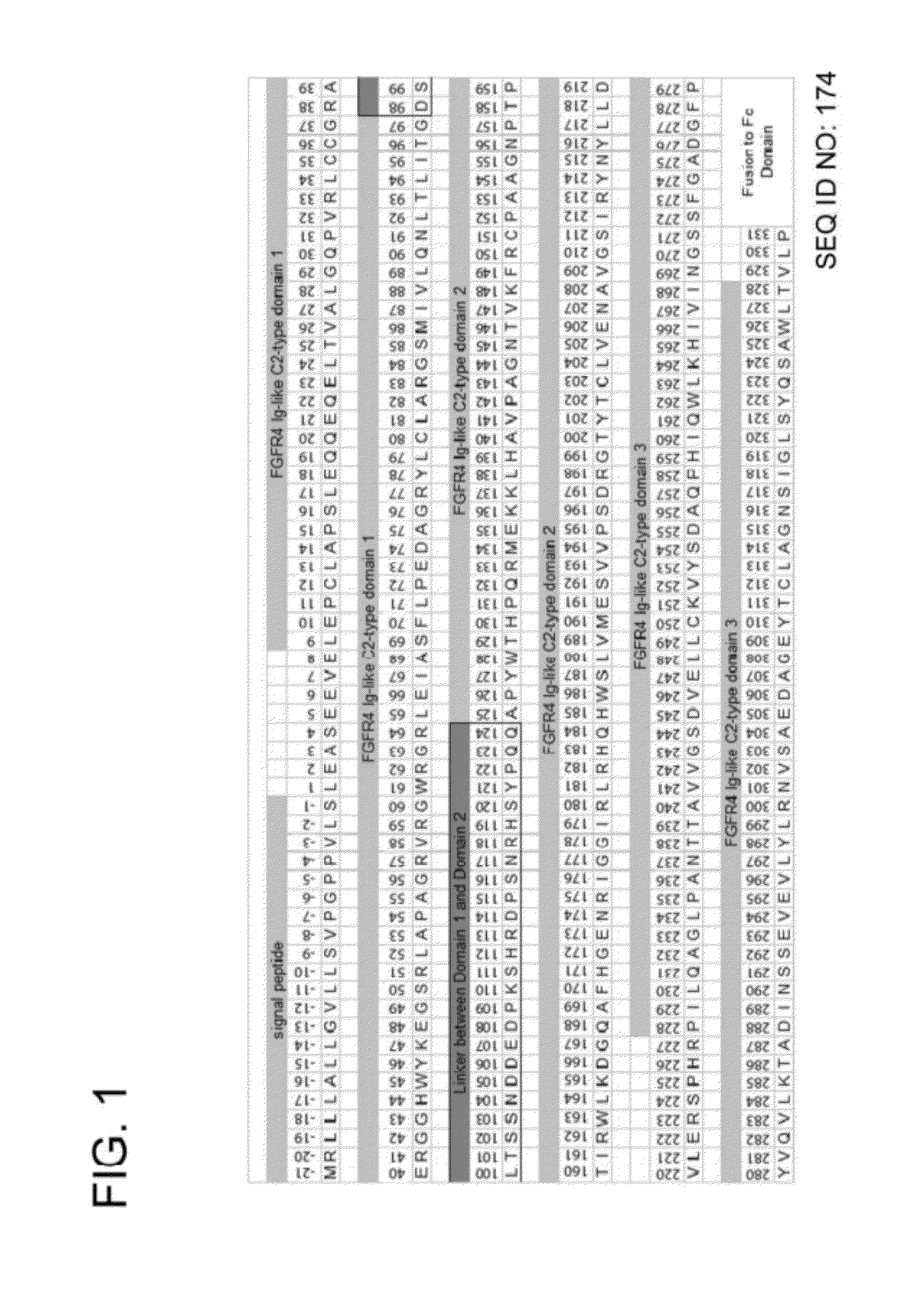
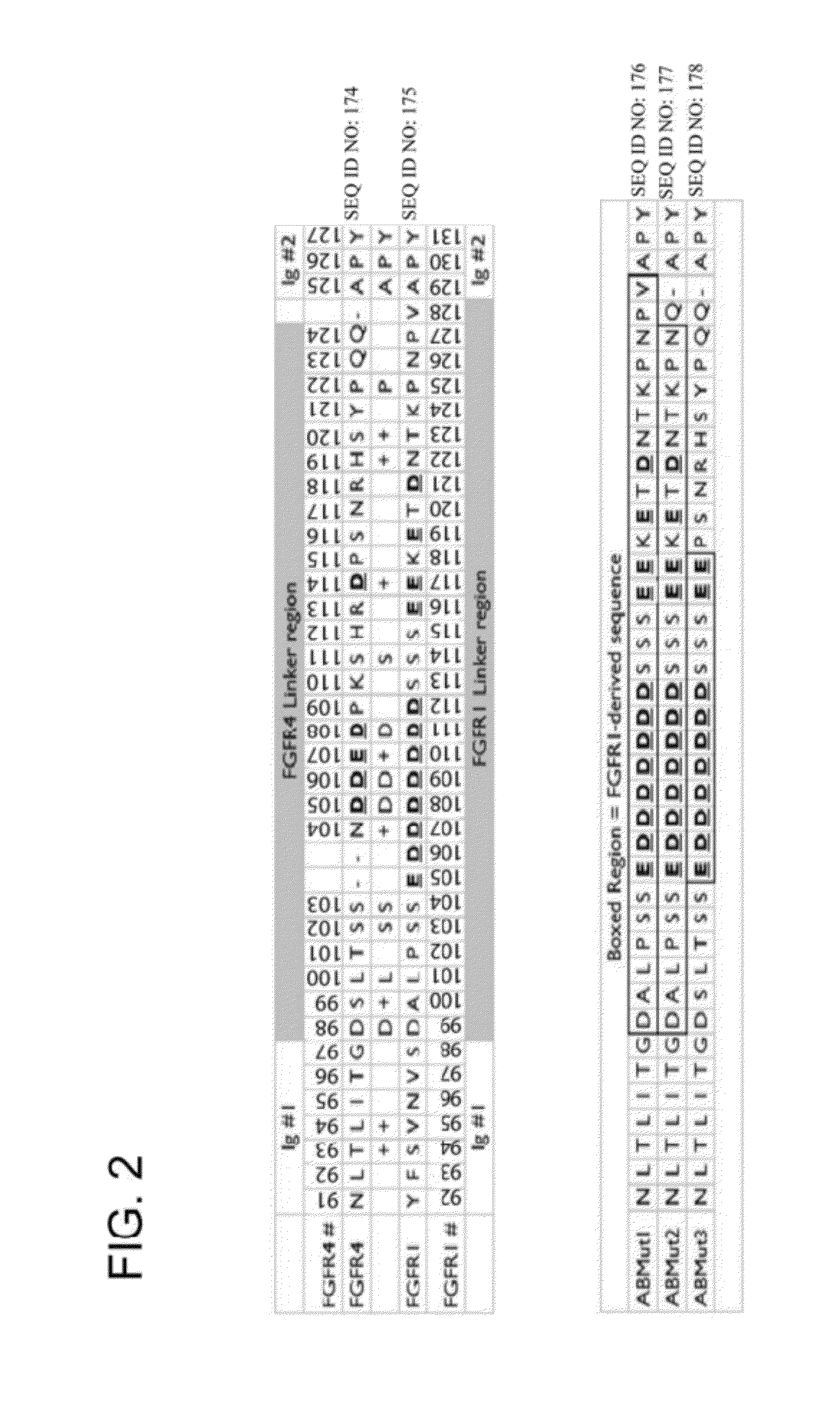
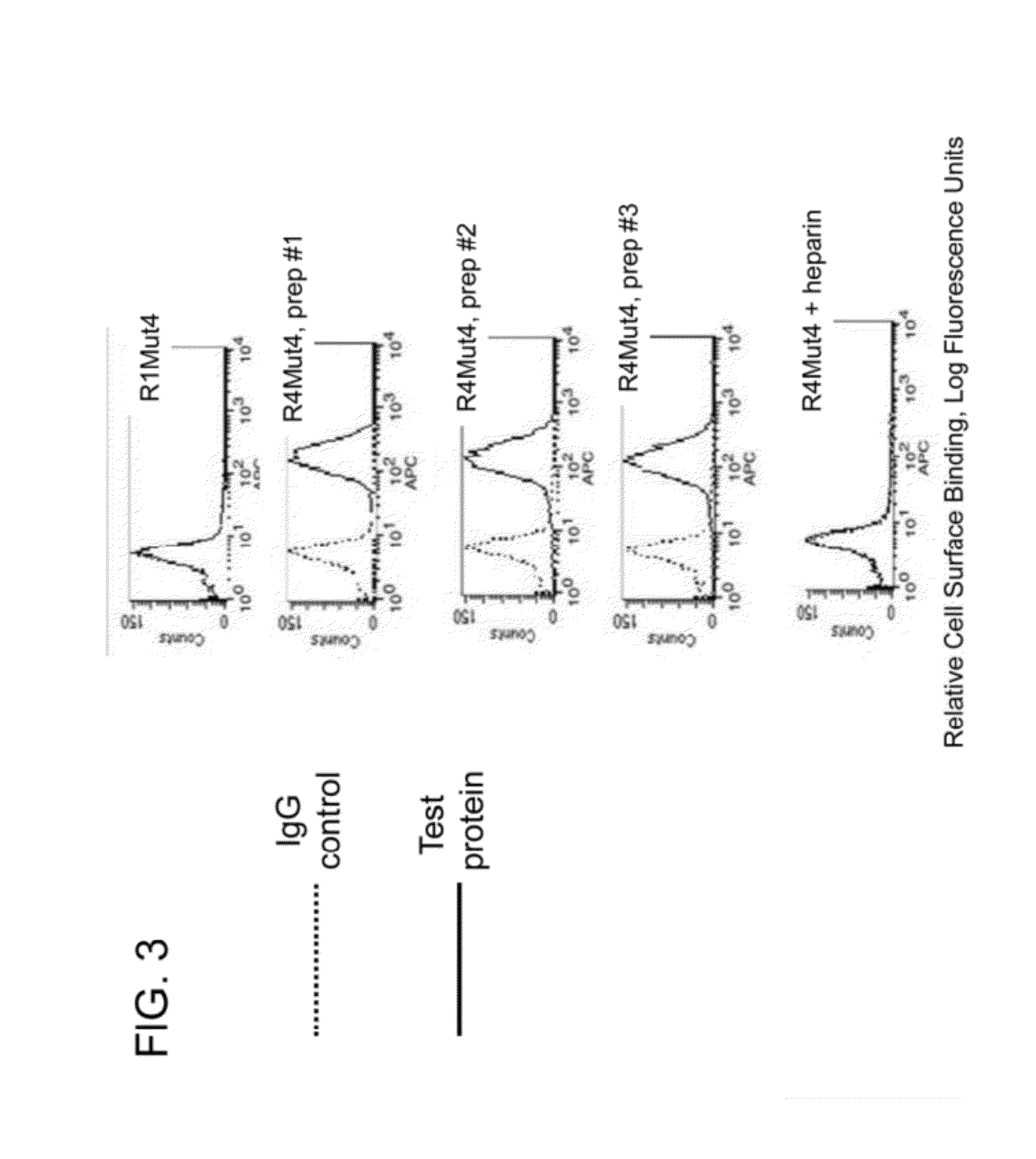
FGFR Extracellular Domain Acidic Region Muteins
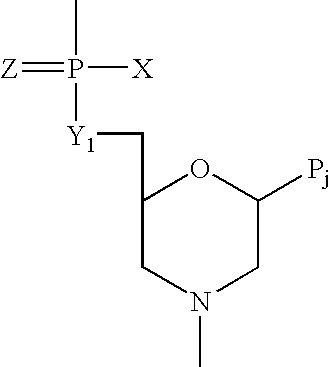
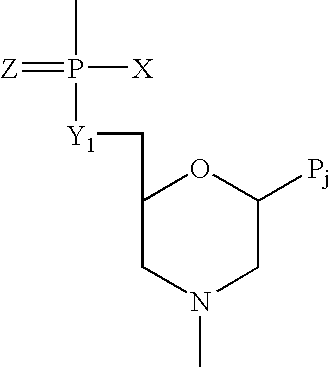
InactiveUS20100087627A1Decreased ECM bindingImprove bioavailabilitySenses disorderPeptide/protein ingredientsMutated proteinPolynucleotide
Fibroblast growth factor receptor (FGFR) extracellular domain (ECD) acidic region muteins that have been engineered to exhibit decreased tissue binding by increasing the number of acidic amino acid residues within the D1-D2 linker region are provided. Polynucleotides encoding FGFR ECD acidic region muteins are also provided. Methods of making FGFR ECD acidic region muteins, and methods of using such molecules to treat proliferative disorders, including cancers, disorders of angiogenesis, and macular degeneration, are also provided.
Owner:FIVE PRIME THERAPEUTICS
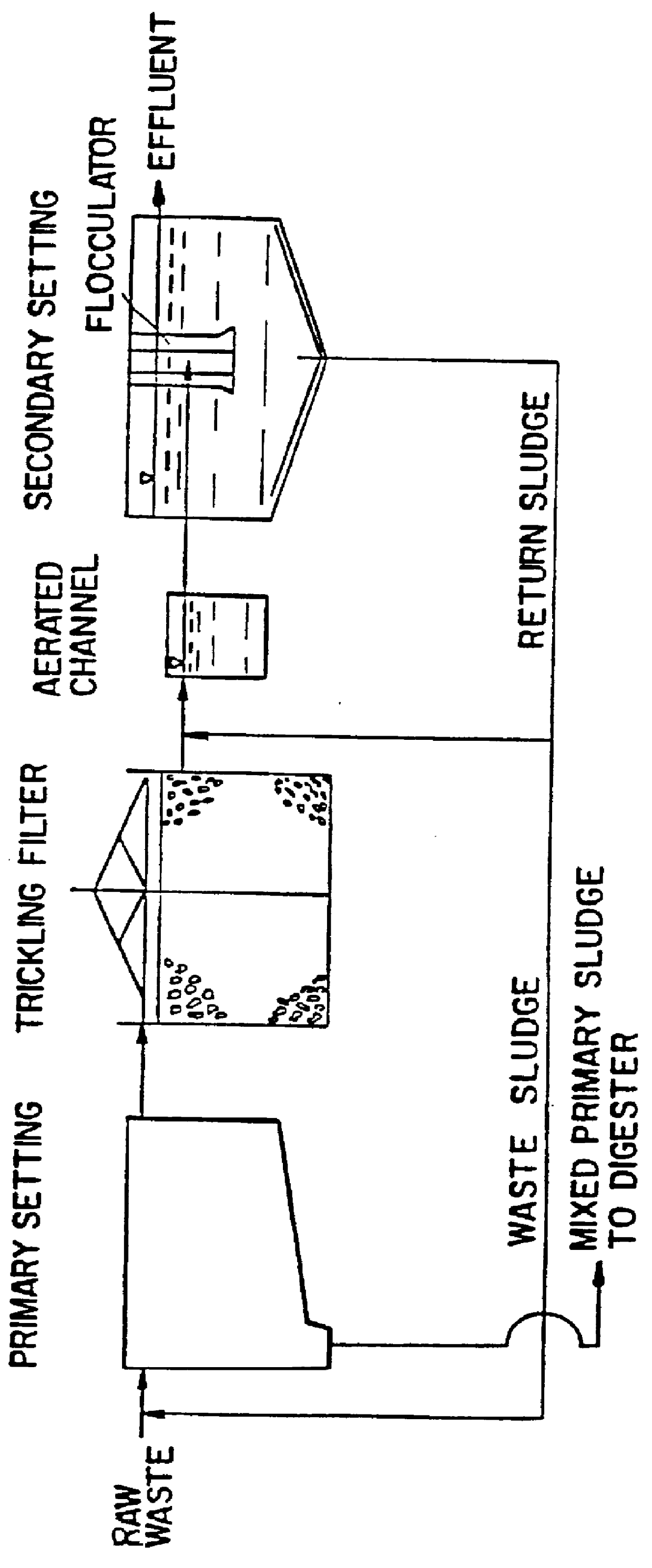
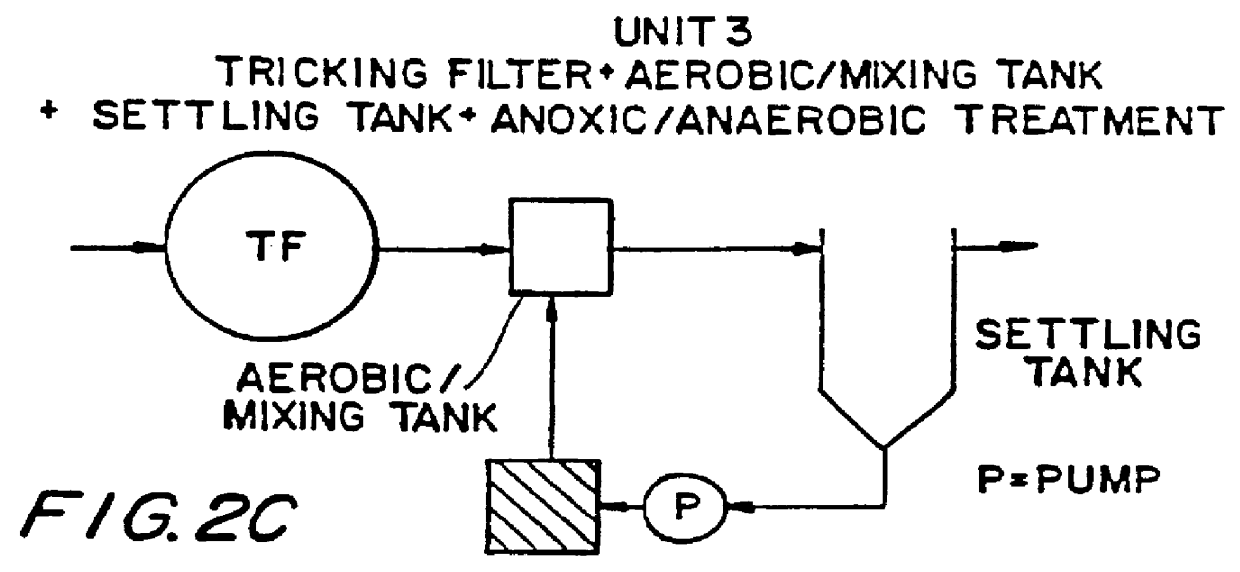
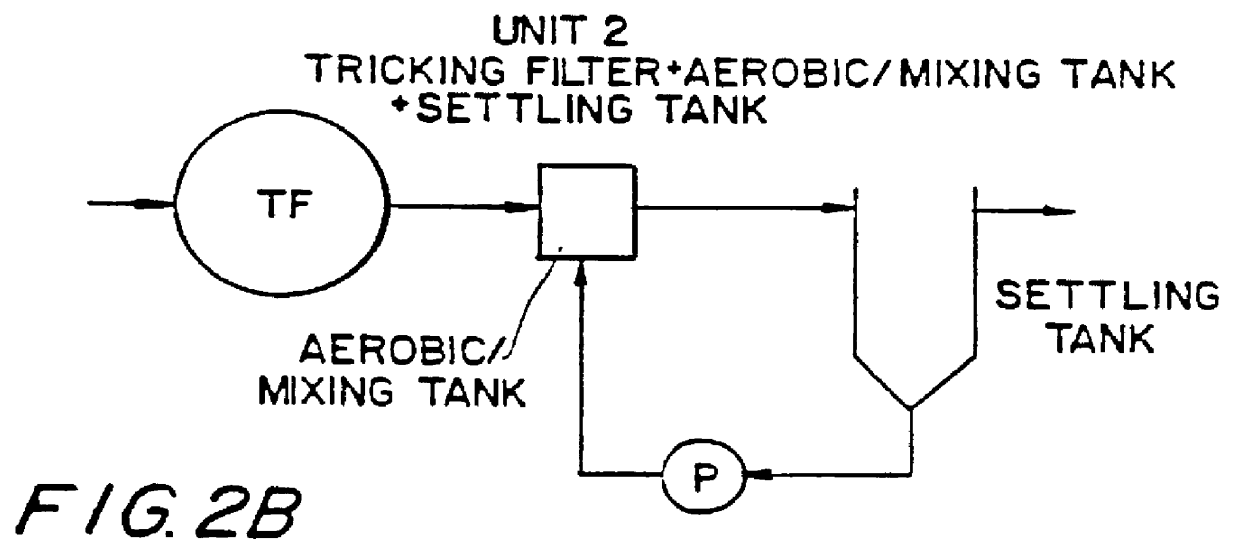
Wastewater treatment process
InactiveUS6113788AImprove efficiencyFacilitated releaseTreatment using aerobic processesSeparation devicesSludgePhosphate
A wastewater treatment process having improved solids separation characteristics and reduced biochemical oxygen demand (BOD) in the purified wastewater comprising the steps of: passing wastewater through a main aerobic biological oxidation zone and therein oxidizing a portion of the BOD a portion of the ammonia nitrogen content (NH3-N); passing the effluent from said aerobic biological oxidation zone to an aerobic / mixing zone and therein mixing said effluent with effluent from the anoxic / anaerobic zone; passing the effluent from said aerobic / mixing zone to a settling zone and therein separating purified wastewater having reduced BOD and suspended solids, and sludge containing suspended solids; passing a portion of the sludge formed in the settling zone and volatile acids to an anoxic / anaerobic zone and therein increasing the extracellular polymer content of said sludge, the release of phosphorus into solution and the reduction of nitrate nitrogen to molecular nitrogen gas; and recycling an effective amount of the effluent from said anoxic / anaerobic zone to said aerobic / mixing zone. In an alternative embodiment, a volatile acid is added to a zone to which no additional oxygen has been added that is in the flow path from the main aerobic biological oxidation zone or, alternatively, it may be added to the anoxic / anaerobic zone and the thus-treated effluent is passed to the aerobic / mixing zone wherein phosphate is removed from the effluent.
Owner:POLYTECHNIC INST OF NEW YORK
Single chain recombinant t cell receptors
InactiveUS20060166875A1Improve stabilityUseful purposeCompound screeningApoptosis detectionDisulfide bondingExtracellular
A single chain T cell receptor (scTCR) comprising an a segment constituted by a TCR α chain variable region sequence fused to the N terminus of a TCR α chain constant region extracellular sequence, a β segment constituted by a TCR β chain variable region fused to the N terminus of a TCR β chain constant region extracellular sequence, and a linker sequence linking the C terminus of the a segment to the N terminus of the β segment, or vice versa, the constant region extracellular sequences of the α and β segments being linked by a disulfide bond, the length of the linker sequence and the position of the disulfide bond being such that the variable region sequences of the α and β segments are mutually orientated substantially as in native αβ T cell receptors. Complexes of two or more such scTCRs, and use of the scTCRs in therapy and in various screening applications are also disclosed.
Owner:IMMUNOCORE LTD +1
FGFR extracellular domain acidic region muteins
InactiveUS8338569B2Preventing tissue bindingGreater ECM bindingSenses disorderPeptide/protein ingredientsMutated proteinPolynucleotide
Fibroblast growth factor receptor (FGFR) extracellular domain (ECD) acidic region muteins that have been engineered to exhibit decreased tissue binding by increasing the number of acidic amino acid residues within the D1-D2 linker region are provided. Polynucleotides encoding FGFR ECD acidic region muteins are also provided. Methods of making FGFR ECD acidic region muteins, and methods of using such molecules to treat proliferative disorders, including cancers, disorders of angiogenesis, and macular degeneration, are also provided.
Owner:FIVE PRIME THERAPEUTICS
VE-PTP Extracellular Domain Antibodies Delivered by a Gene Therapy Vector
InactiveUS20160082129A1Genetic material ingredientsAntibody ingredientsExtracellularPhosphatase inhibitor
The disclosure provides compositions and methods for the treatment of ocular conditions associated with angiogenesis, comprising administering a nucleic acid that encodes for a tyrosine phosphatase suppressor to a subject.
Owner:EYEPOINT PHARMA INC
Method for treating systemic bacterial, fungal and protozoan infection
ActiveUS8431123B2Low-toxicLower Level RequirementsHydrolasesPeptide/protein ingredientsBacteroidesProtozoa
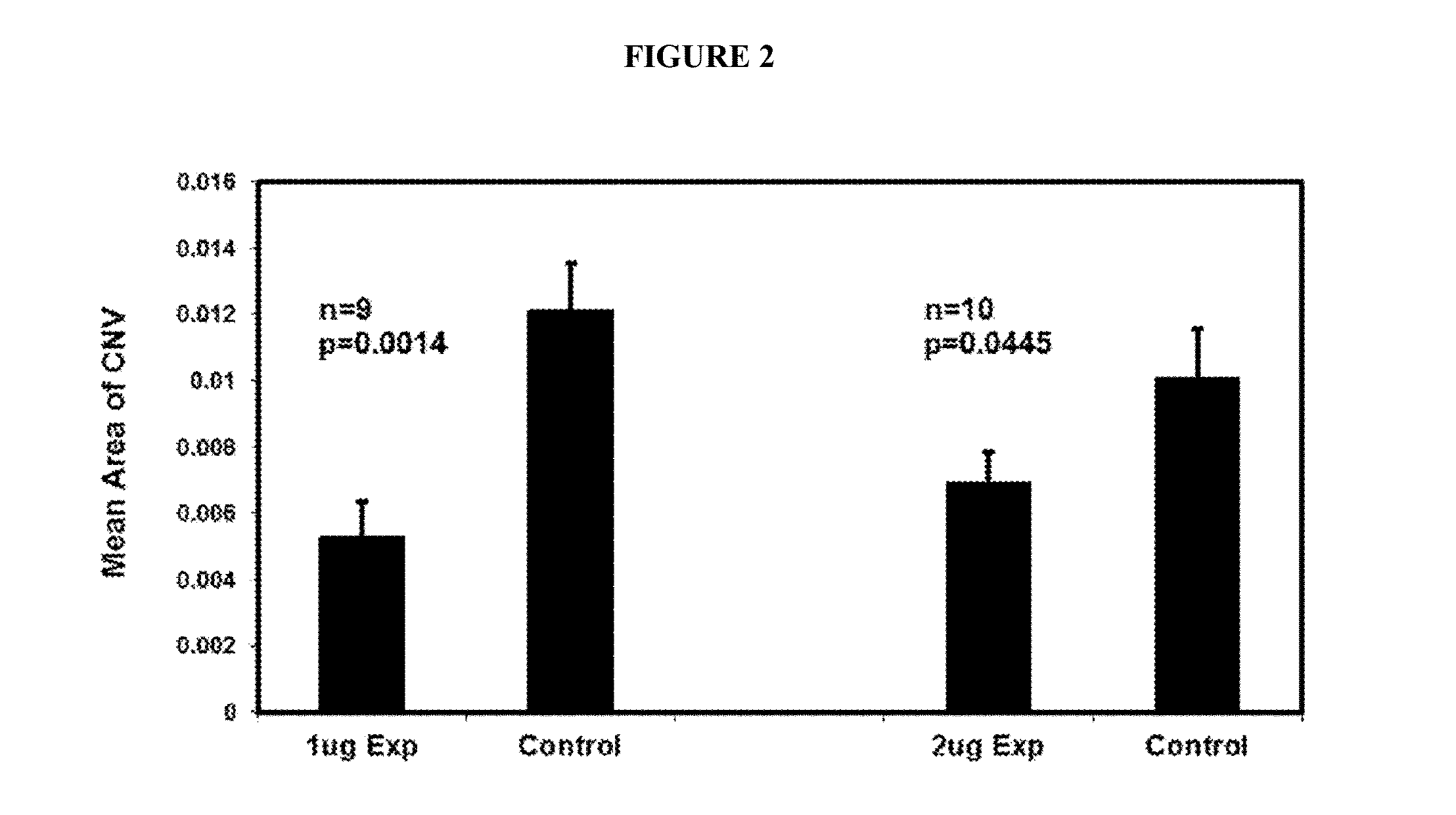
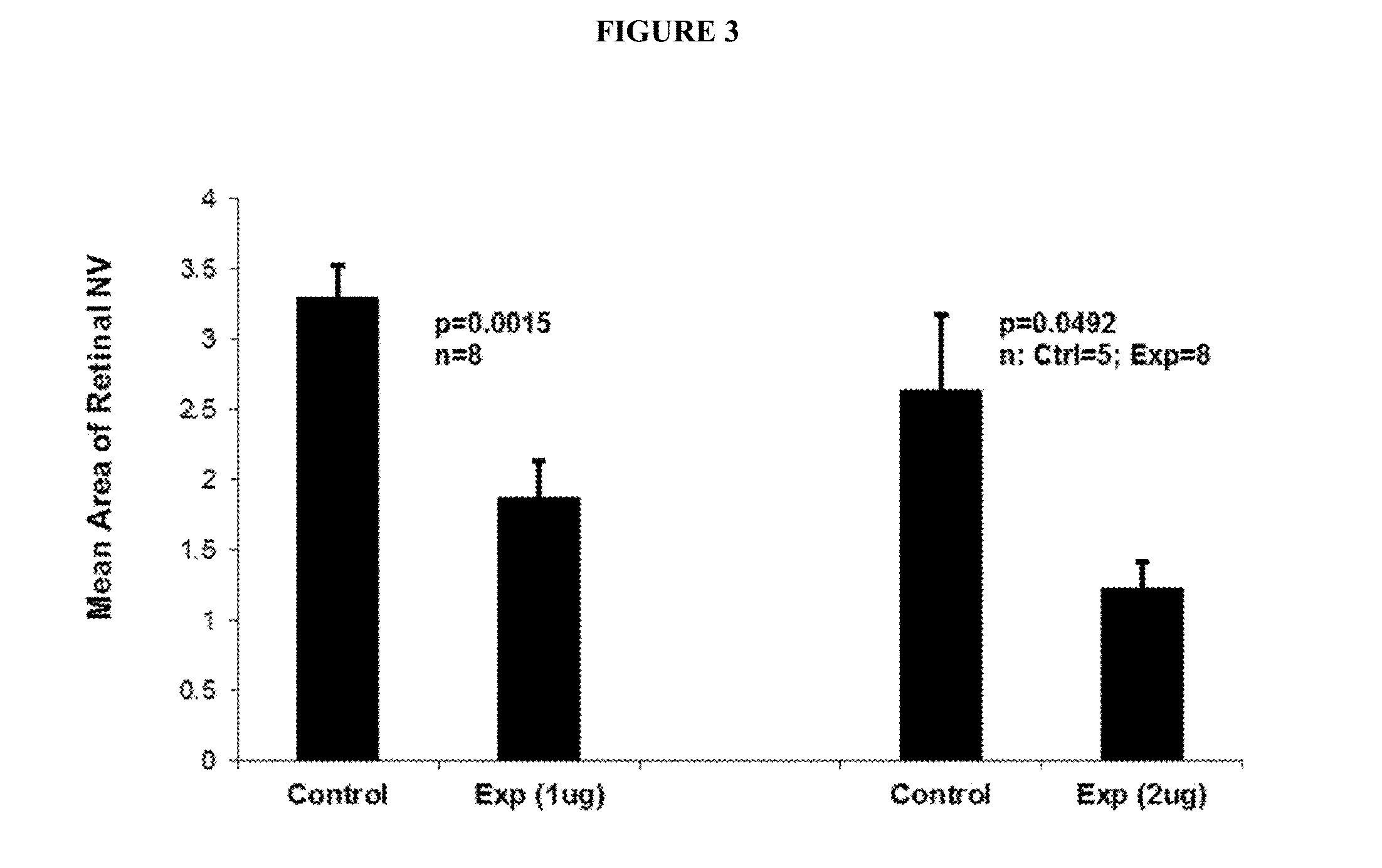
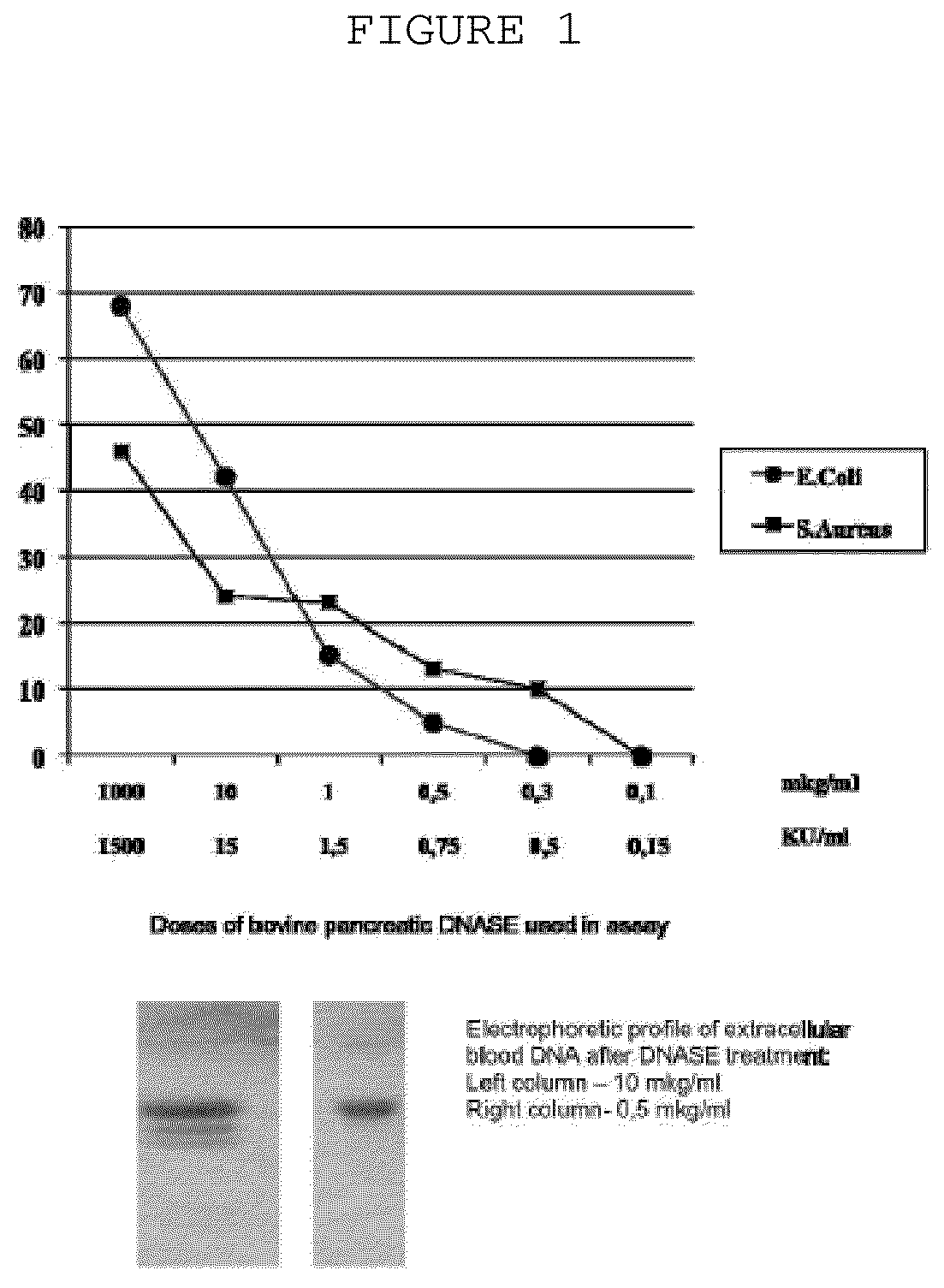
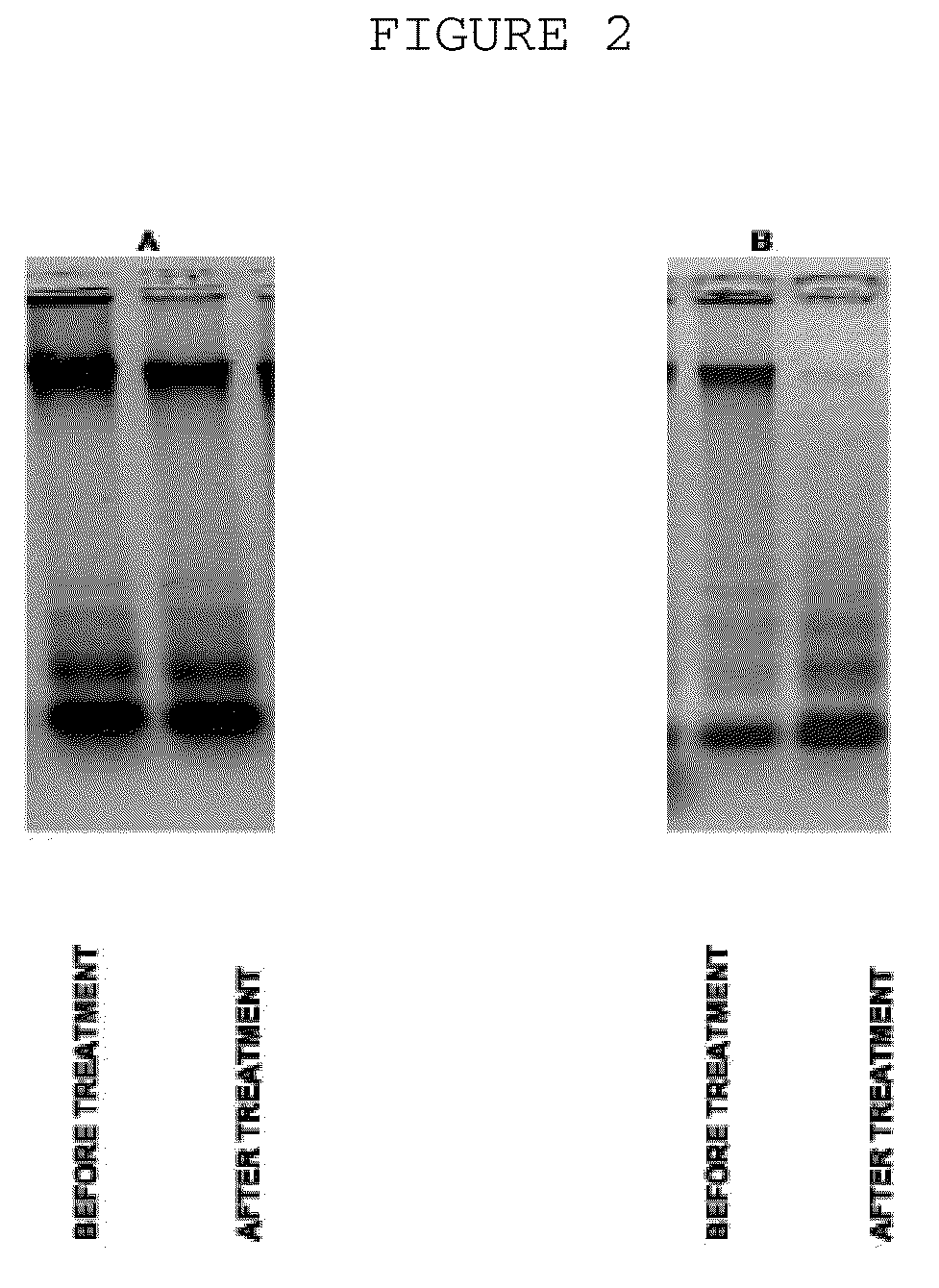
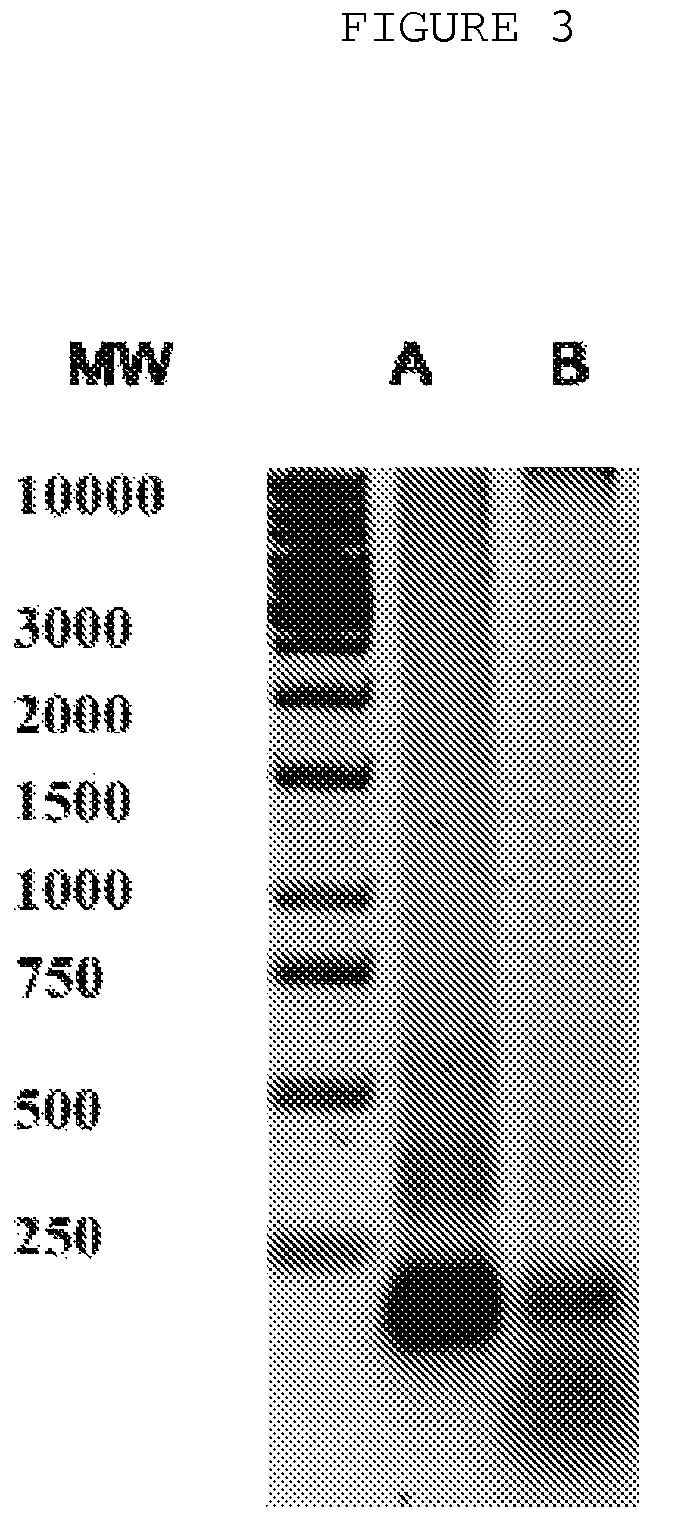
The invention is directed to a treatment of diseases that are accompanied by quantitative and / or qualitative changes of blood extracellular DNA and, more particularly, to a treatment of systemic bacterial, fungal and protozoan infections. The inventive method comprises introducing a treatment agent into a circulating blood system of a patient diagnosed with systemic infection caused by bacteria, fungi or protozoa, wherein said treatment agent destroys extracellular DNA in said blood of said patient and wherein said treatment agent used to destroy said extracellular DNA is a DNase enzyme: said agent being administered in doses and regimens which are sufficient to decrease the average molecular weight of circulating extracellular blood DNA in the blood of said patient; such decrease in the average molecular weight can be measured by gel electrophoresis of extracellular blood DNA fraction from the blood of said patient. A DNase enzyme may be applied in a dose and regimen that provide a DNase DNA hydrolytic activity measured in blood plasma that exceeds 1.5 Kunitz units per 1 ml of blood plasma for more than 12 hours within a period of 24 hours.
Owner:CLS THERAPEUTICS
Determining the hydration status of a patient
InactiveUS7133716B2The process is simple and convenientSimple methodDiagnostic recording/measuringSensorsExtracellularWater volume
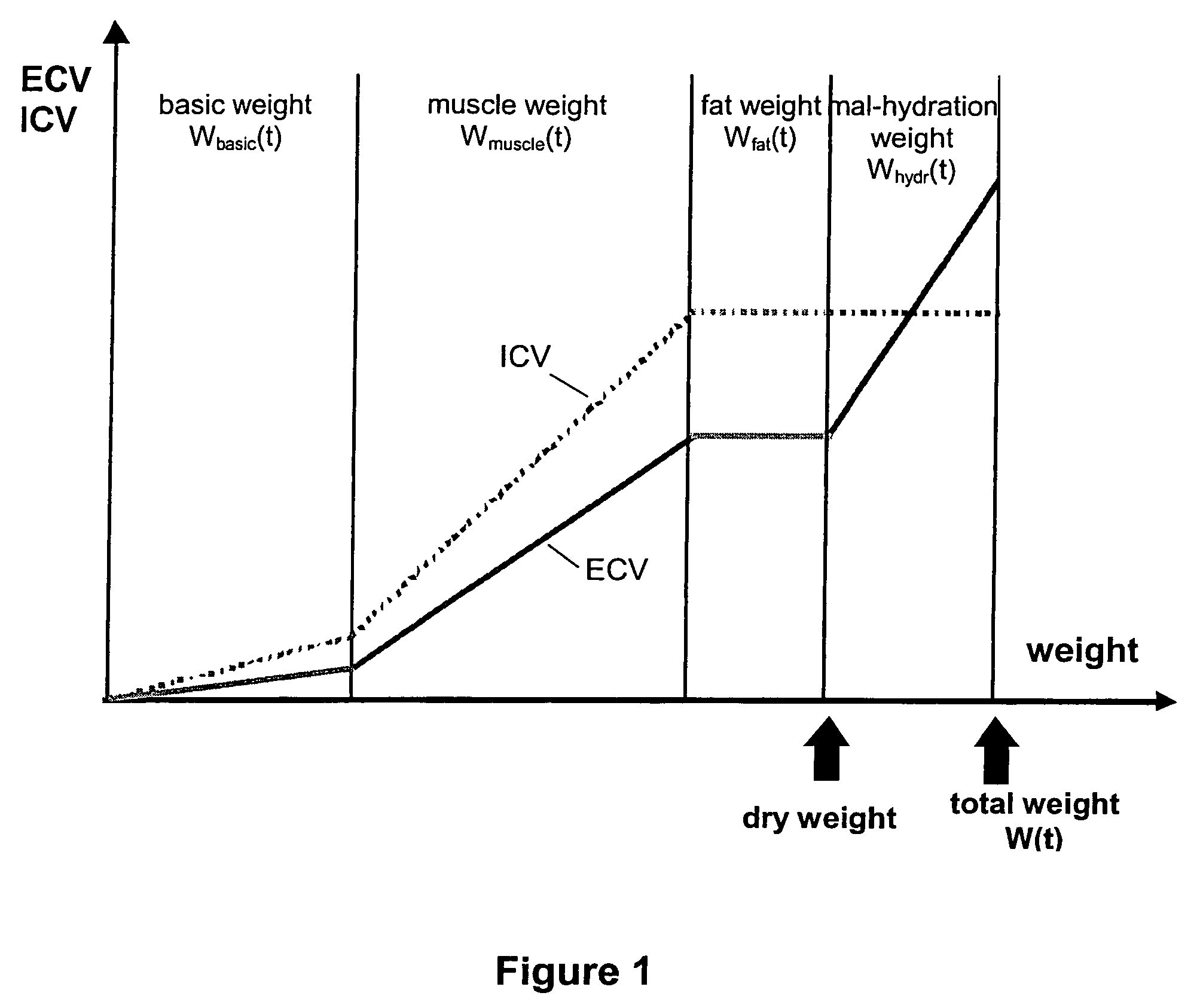
A device and method are provided for determining the volume ECVhydr(t) of a body compartment of a patient at a time t by conducting measurements at the time t of the patient to determine at least one anthropometric measure X(t), the extracellular water volume ECV(t), and the intracellular water volume ICV (t) of the patient. The extracellular water volume ECVbasic(t) of a first compartment with weight Wbasic(t) of the patient at the time t is derived by using X (t), the extracellular water volume ECVsec(t) of a second compartment of the patient at the time t is derived by using ICV (t), and ECVhydr(t) as the extracellular water volume of a third compartment of the patient is derived with weight Whydr(t). The extracellular volume ECVhydr(t) is a measure for the hydration status of the patient.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
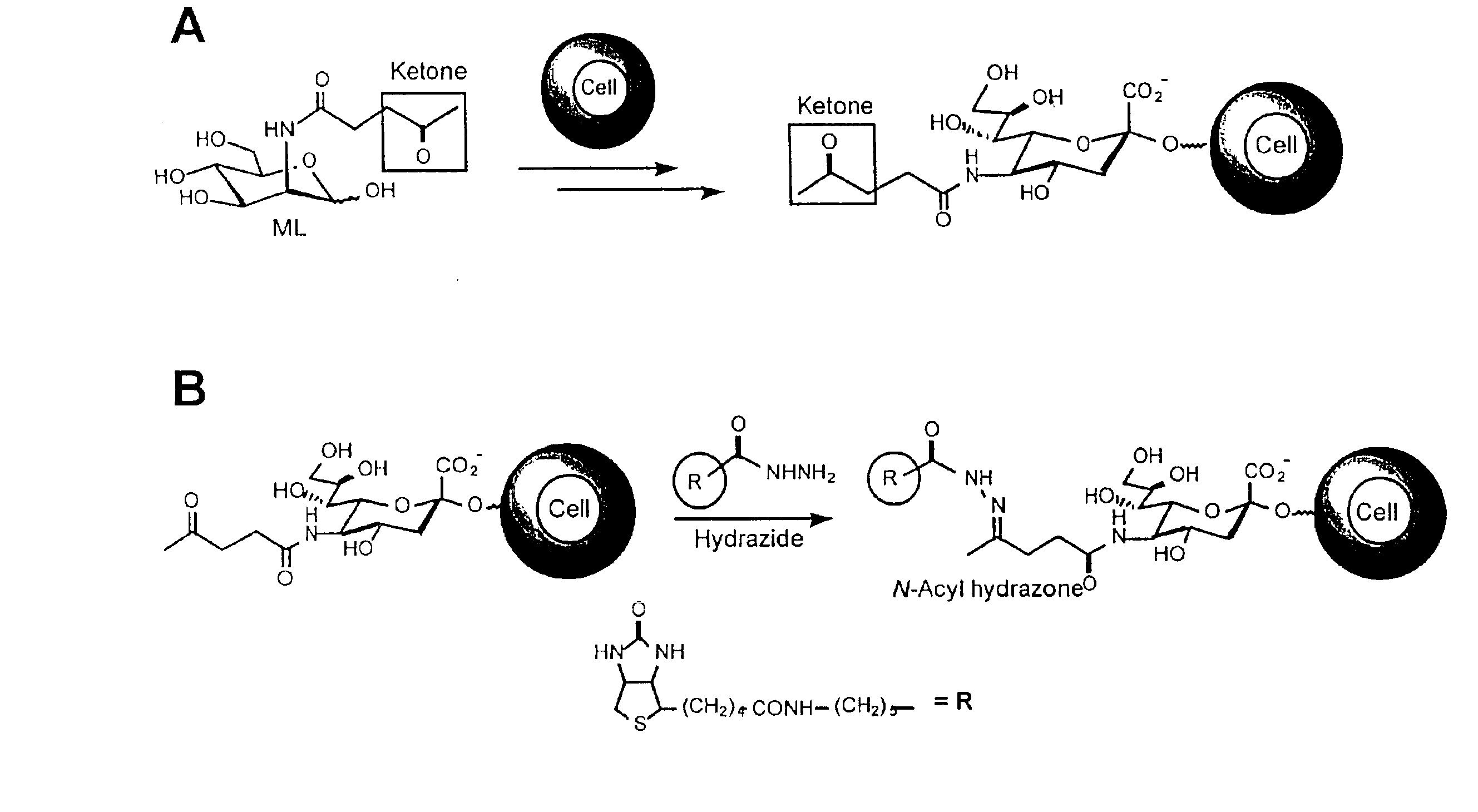
Glycoconjugates and methods
Methods for making the functionalized glycoconjugates include (a) contacting a cell with a first monosaccharide, and (b) incubating the cell under conditions whereby the cell (i) internalizes the first monosaccharide, (ii) biochemically processes the first monosaccharide into a second saccharide, (iii) conjugates the saccharide to a carrier to form a glycoconjugate, and (iv) extracellularly expresses the glycoconjugate to form an extracellular glycoconjugate comprising a selectively reactive functional group. Methods for forming products at a cell further comprise contacting the functional group of the extracellularly expressed glycoconjugate with an agent which selectively reacts with the functional group to form a product. Subject compositions include cyto-compatible monosaccharides comprising a nitrogen or ether linked functional group selectively reactive at a cell surface and compositions and cells comprising such saccharides.
Owner:RGT UNIV OF CALIFORNIA
Antibody library display by yeast cell plasma membrane
The present invention relates to antibodies or antibody fragments that may be displayed on the extracellular surface of the plasma membrane when expressed in a host cell. The present invention provides libraries comprising a plurality of plasma membrane displayed antibodies and methods of screening the libraries for antibodies or antibody fragments with desired characteristics.
Owner:MEDIMMUNE LLC
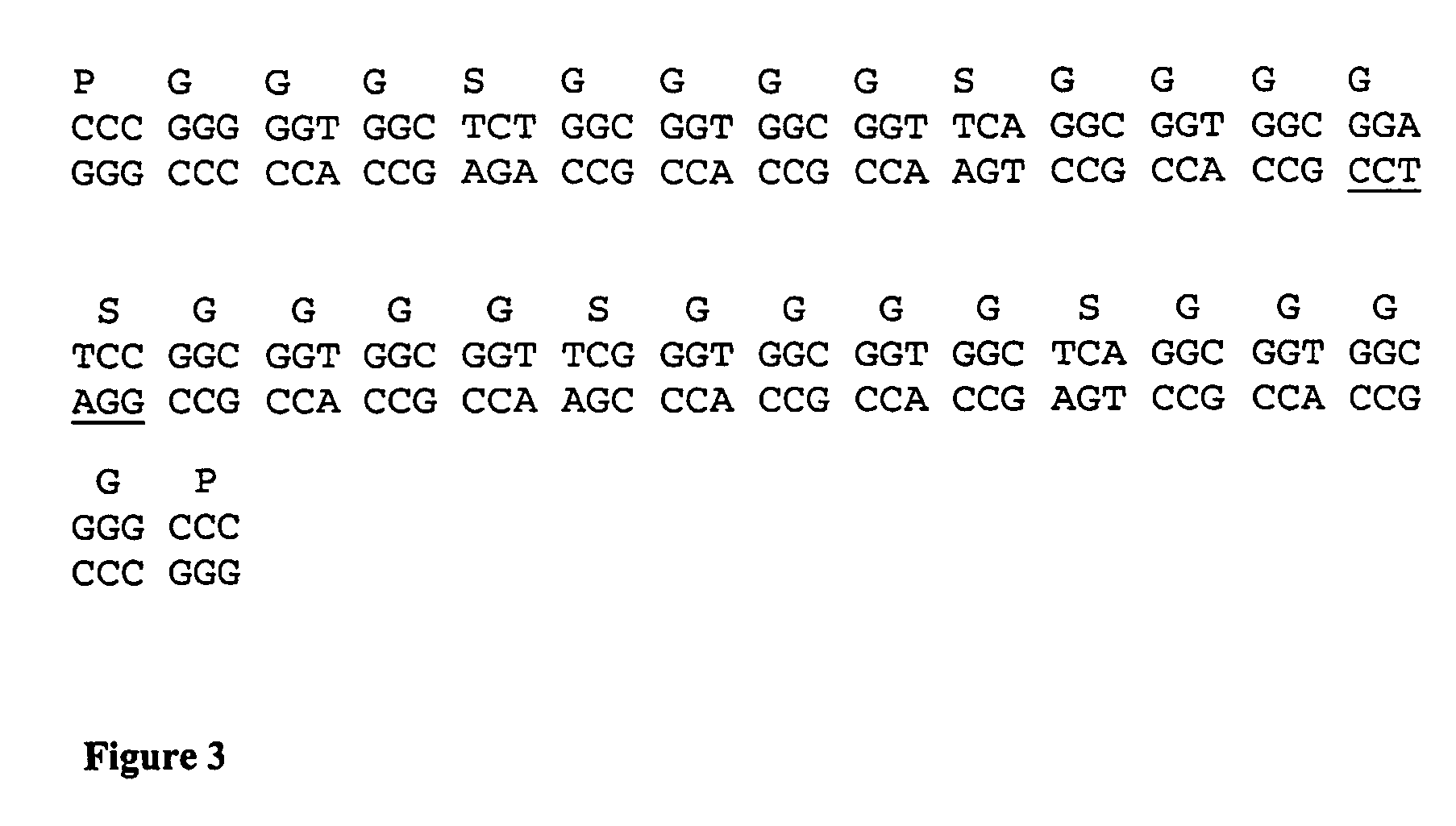
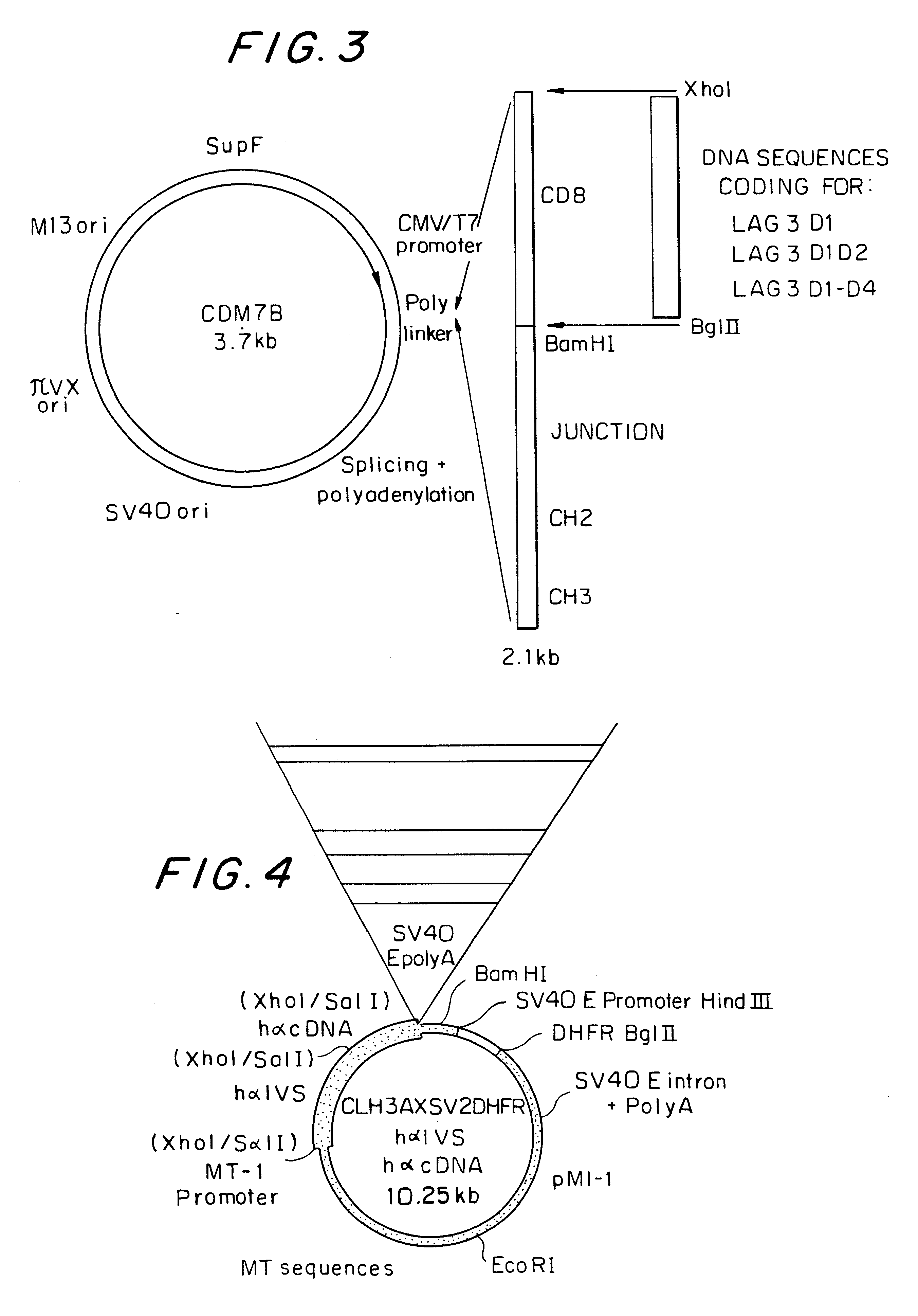
Soluble polypeptide fractions of the LAG-3 protein, production method, therapeutic composition, anti-idiotype antibodies
Soluble polypeptide fraction consisting of all or part .[.one.]. .Iadd.of .Iaddend.at least .Iadd.one .Iaddend.of the four immunoglobulin-type extracellular LAG-3 protein domains (amino acids 1-159, 160-.[.230.]. .Iadd.239.Iaddend., 240-330 and 331-412 of the SEQ ID NO:1 sequence) or consisting of one peptide sequence derived from these domains by replacement, addition or deletion of one or more amino acids. The fraction of the invention has a specificity at least equal to that of LAG-3 in relation to its ligand.
Owner:MERCK SERONO SA
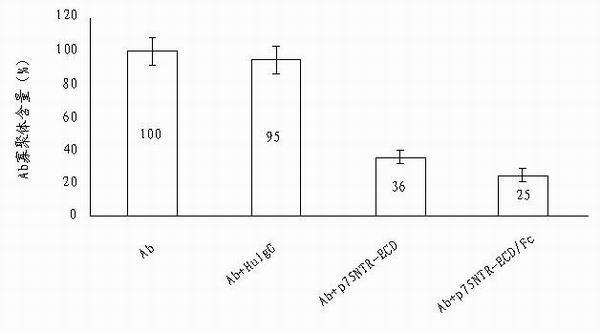
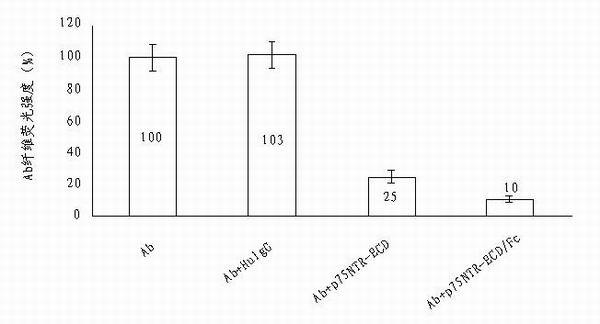
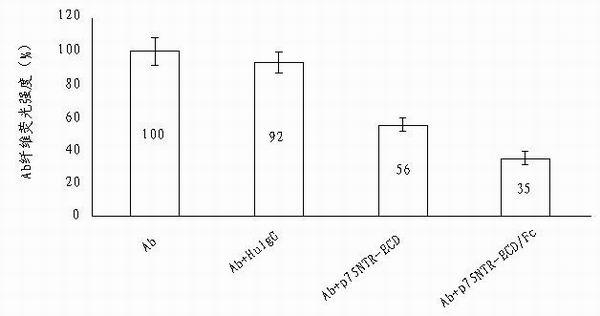
Use of p75neurotrophin receptor-extracellular domain (p75NTR-ECD) in medicine for preventing and treating Alzheimer disease
The invention discloses a medicine for preventing and treating Alzheimer disease. The medicine may comprise p75NTR-ECD of which the nucleotide sequence is represented by the nucleotide sequence SEQ ID No.1 in a sequence table and of which the amino acid sequence is represented by amino acid sequence SEQ ID No.2 in the sequence table. The medicine also may be p75NTR-ECD / Fc of which the nucleotide sequence is represented by the nucleotide sequence SEQ ID No.3 in the sequence table and of which the amino acid sequence is represented by amino acid sequence SEQ ID No.4 in the sequence table. The medicine has the effects of inhibiting Abeta from aggregating into oligomer and fiber, promoting the depolymerization of Abeta fibers, eliminating Abeta in head and resisting Abeta neurotoxicity. The invention provides the new medicine for treating Alzheimer disease, which has a plurality of effects and has a bright clinic application prospect.
Owner:SUZHOU AUZONE BIOLOGICAL TECH CO LTD
Detection of extracellular tumor-associated nucleic acid in blood plasma or serum using nucleic acid amplification assays
InactiveUS6939675B2Antibody mimetics/scaffoldsMicrobiological testing/measurementNeoplasmBlood plasma
This invention relates to detection of specific extracellular nucleic acid in plasma or serum fractions of human or animal blood associated with neoplastic or proliferative disease. Specifically, the invention relates to detection of nucleic acid derived from mutant oncogenes or other tumor-associated DNA, and to those methods of detecting and monitoring extracellular mutant oncogenes or tumor-associated DNA found in the plasma or serum fraction of blood by using rapid DNA extraction followed by nucleic acid amplification with or without enrichment for mutant DNA. In particular, the invention relates to the detection, identification, or monitoring of the existence, progression or clinical status of benign, premalignant, or malignant neoplasms in humans or other animals that contain a mutation that is associated with the neoplasm through detection of the mutated nucleic acid of the neoplasm in plasma or serum fractions. The invention permits the detection of extracellular, tumor-associated nucleic acid in the serum or plasma of humans or other animals recognized as having a neoplastic or proliferative disease or in individuals without any prior history or diagnosis of neoplastic or proliferative disease. The invention provides the ability to detect extracellular nucleic acid derived from genetic sequences known to be associated with neoplasia, such as oncogenes, as well as genetic sequences previously unrecognized as being associated with neoplastic or proliferative disease. The invention thereby provides methods for early identification of colorectal, pancreatic, lung, breast, bladder, ovarian, lymphoma and all other malignancies carrying tumor-related mutations of DNA and methods for monitoring cancer and other neoplastic disorders in humans and other animals.
Owner:PENN STATE RES FOUND
Method for treating systemic DNA mutation disease
ActiveUS8388951B2Lower Level RequirementsGood treatment effectPeptide/protein ingredientsHydrolasesDiseaseA-DNA
A treatment for systemic DNA mutation diseases accompanied with development of somatic mosaicism and elevation of blood extracellular DNA and, more particularly, to a treatment of diabetes mellitus and atherosclerosis. The inventive method consist from introducing a treatment agent into a circulating blood system of a patient diagnosed with systemic DNA mutation diseases when said treatment agent destroys extracellular DNA in said blood of said patient and wherein said treatment agent used to destroy said extracellular DNA is a DNASE enzyme: said agent might be administered in doses and regimens which sufficient to decrease number average molecular weight of circulating extracellular blood DNA in the blood of said patient; such decrease of number average molecular weight might be measured by gel electrophoresis of extracellular blood DNA fraction from the blood of said patient. A DNASE enzyme may be further applied in a dose and regime that provide a DNA hydrolytic activity measured in blood plasma exceeding 1.5 Kunitz units per 1 ml of blood plasma for more than 12 hours within a period of 24 hours.
Owner:CLS THERAPEUTICS
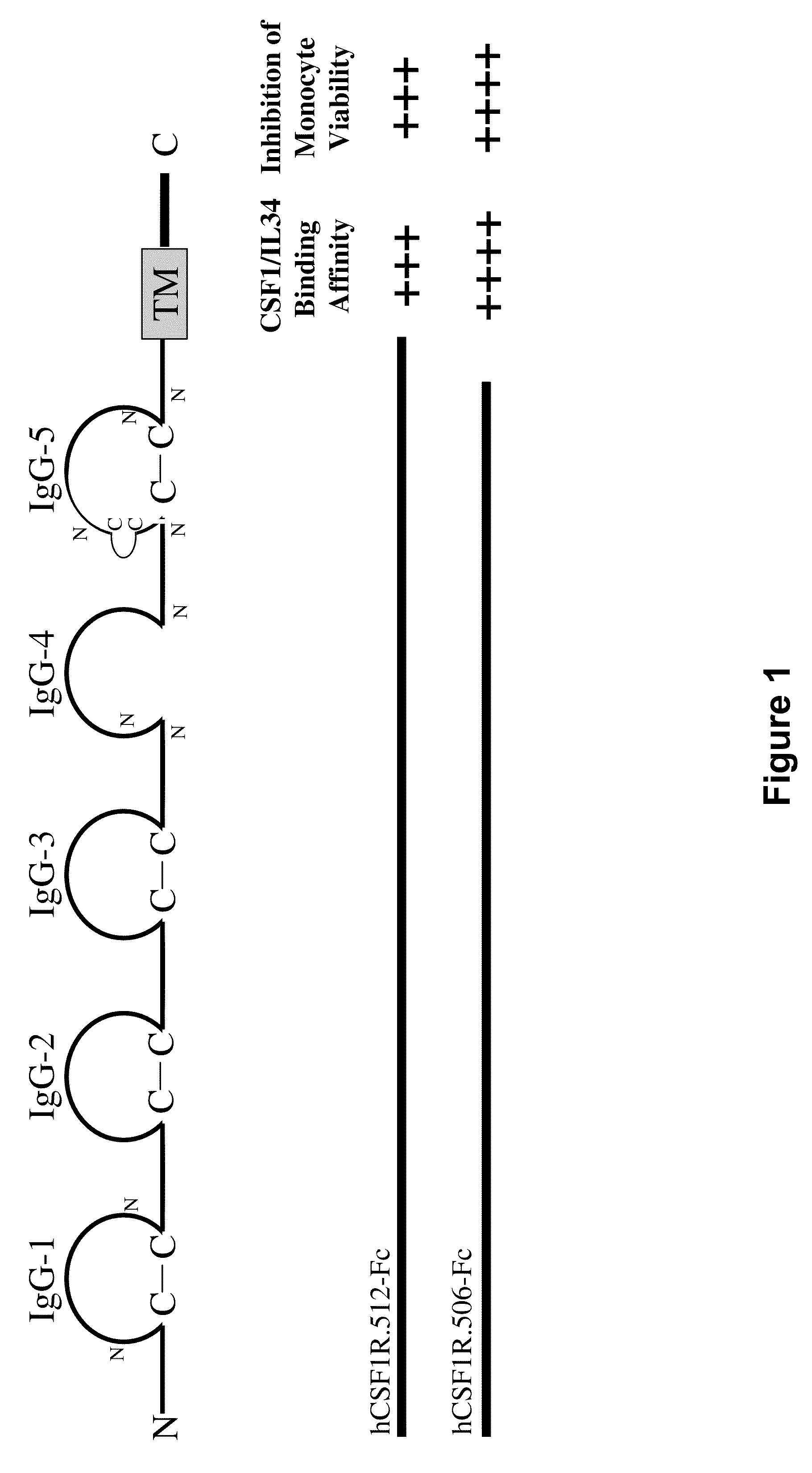
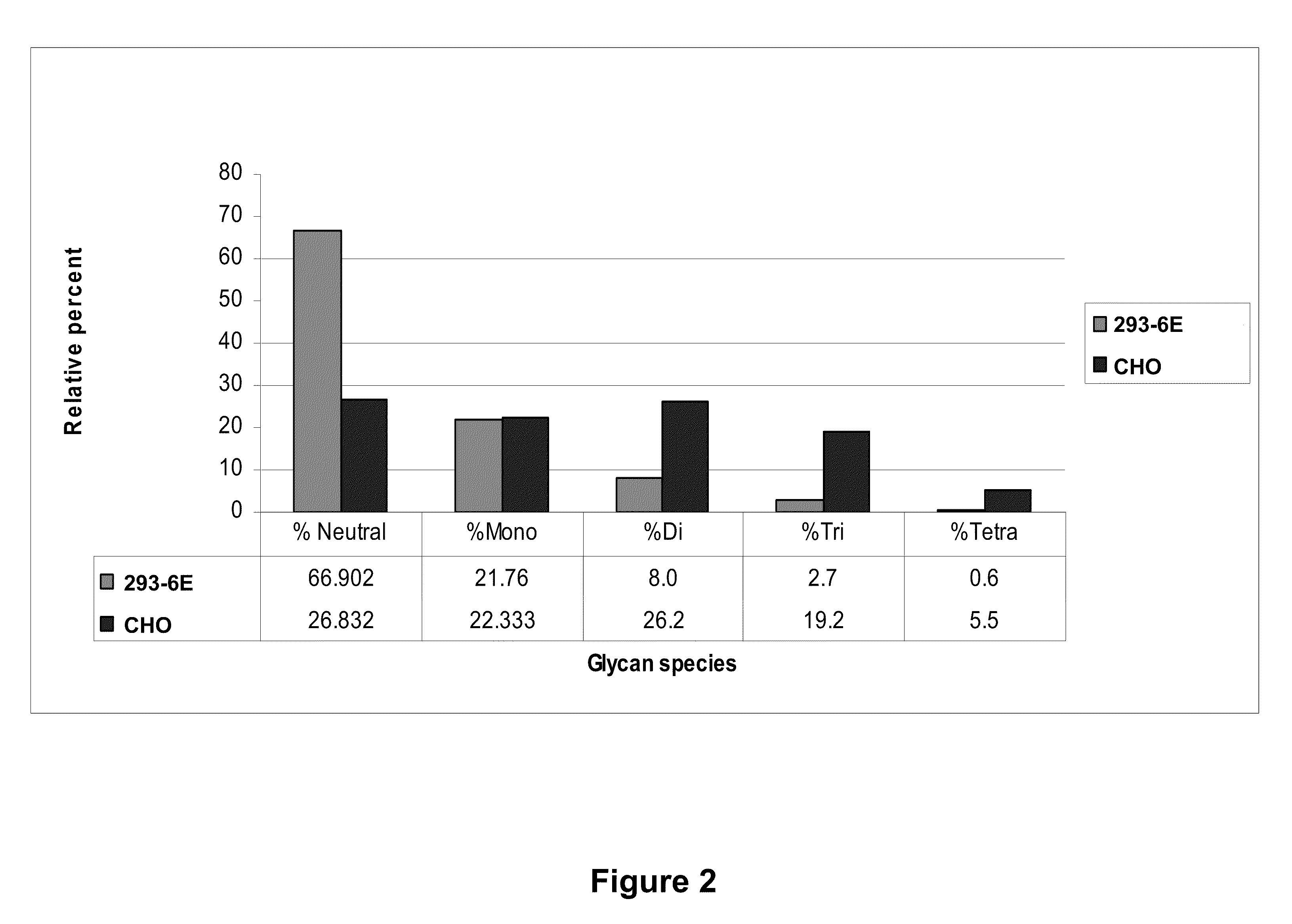
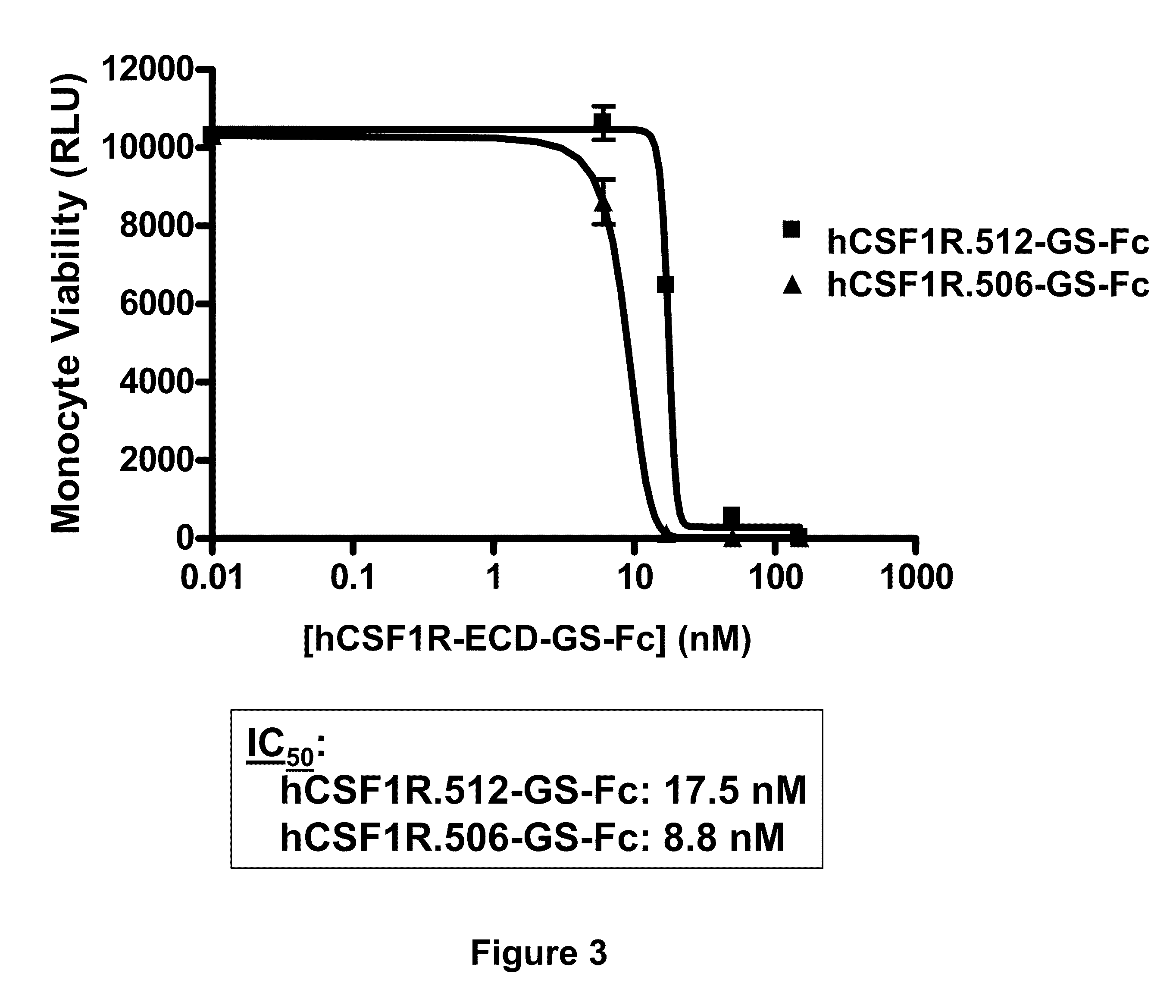
Csf1r extracellular domain fusion molecules and treatments using same
InactiveUS20100136007A1Improve propertiesGrowth inhibitionNervous disorderPeptide/protein ingredientsExtracellularTreatment use
The present invention relates to specific CSF1R ECD fusion molecules that exhibit improved therapeutic properties. The invention also relates to polypeptide and polynucleotide sequences, vectors, host cells, and compositions comprising or encoding such molecules. The invention also relates to methods of making and using the CSF1R ECD fusion molecules. The invention further relates to methods of treatment using the CSF1R ECD fusion molecules. For example, certain CSF1R ECDs of the invention may be used to treat rheumatoid arthritis (RA) or multiple sclerosis (MS).
Owner:FIVE PRIME THERAPEUTICS
Novel Strain of Schizochytrium limacinum useful in the production of lipids and Extracellular Polysaccharides and process thereof
The present disclosure provides a novel mutant strain of Schizochytrium limacinum having the Accession No. MTCC 5249, which produces lipids and extracellular polysaccharide (EPS) simultaneously. The disclosure further provides a process for simultaneous production of lipids and extracellular polysaccharide (EPS) from the novel mutant strain of Schizochytrium limacinum. The lipids produced from the novel mutant strain of Schizochytrium limacinum comprises docosahexaenoic acid (DHA). The disclosure also provides a food, feed, cosmetic, nutritional or therapeutic supplement for humans or animals comprising the cell biomass and extracellular polysaccharides (EPS) of the mutant strain of Schizochytrium limacinum. A cosmetic composition comprising the extracellular polysaccharides (EPS) of Schizochytrium limacinum is also provided that is useful as a base for cosmetics for topical application. The present disclosure further provides a pickle composition and a fat product having improved nutritive value.
Owner:ABL BIOTECH
Modified hematopoietic stem/progenitor and non-t effector cells, and uses thereof
Hematopoeitic stem / progenitor cells (HSPC) and / or non-T effector cells are genetically modified to express (i) an extracellular component including a ligand binding domain that binds a cellular marker preferentially expressed on an unwanted cell; and (ii) an intracellular component comprising an effector domain. Among other uses, the modified cells can be administered to patients to target unwanted cancer cells without the need for immunological matching before administration.
Owner:SEATTLE CHILDRENS HOSPITAL +1
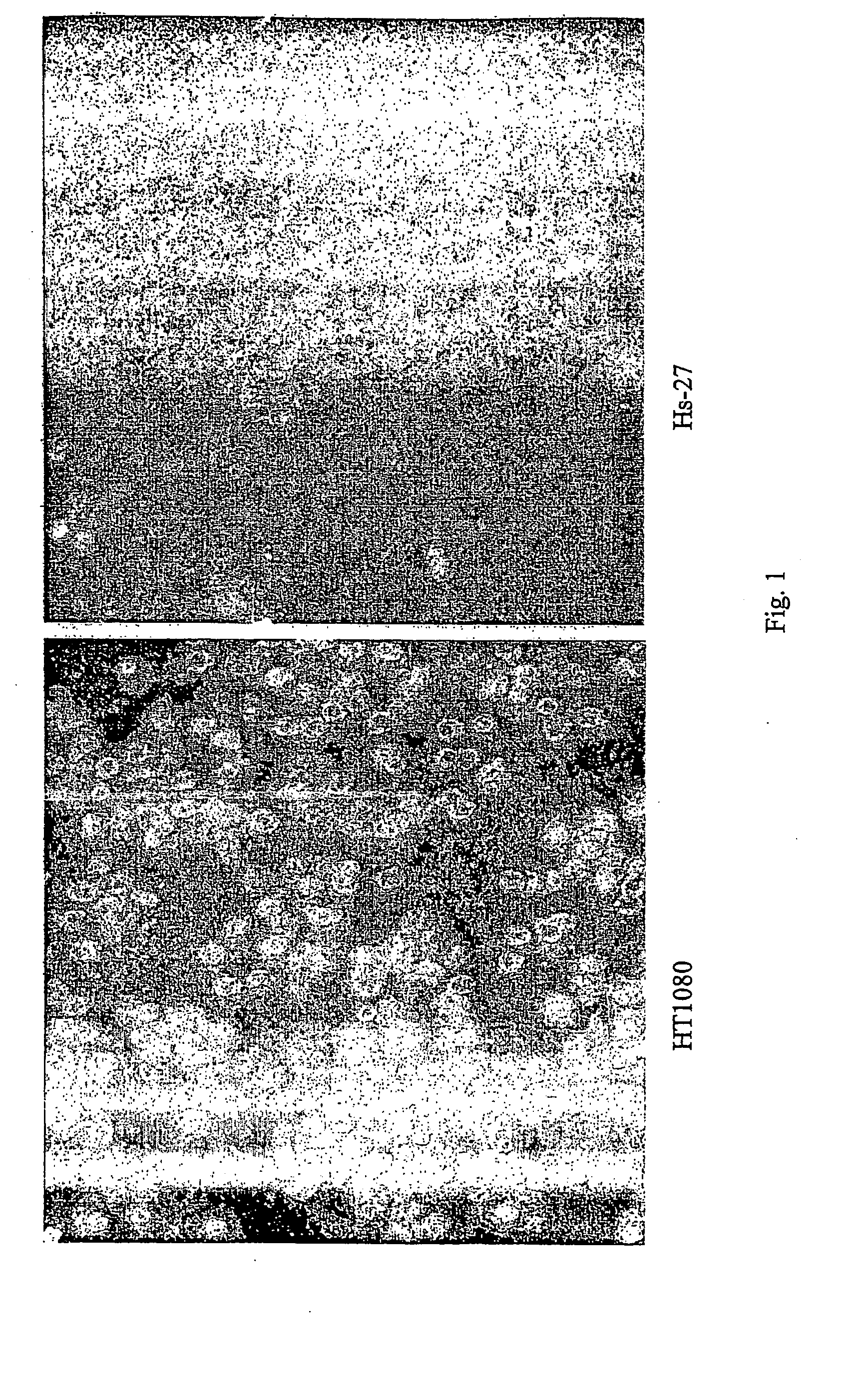
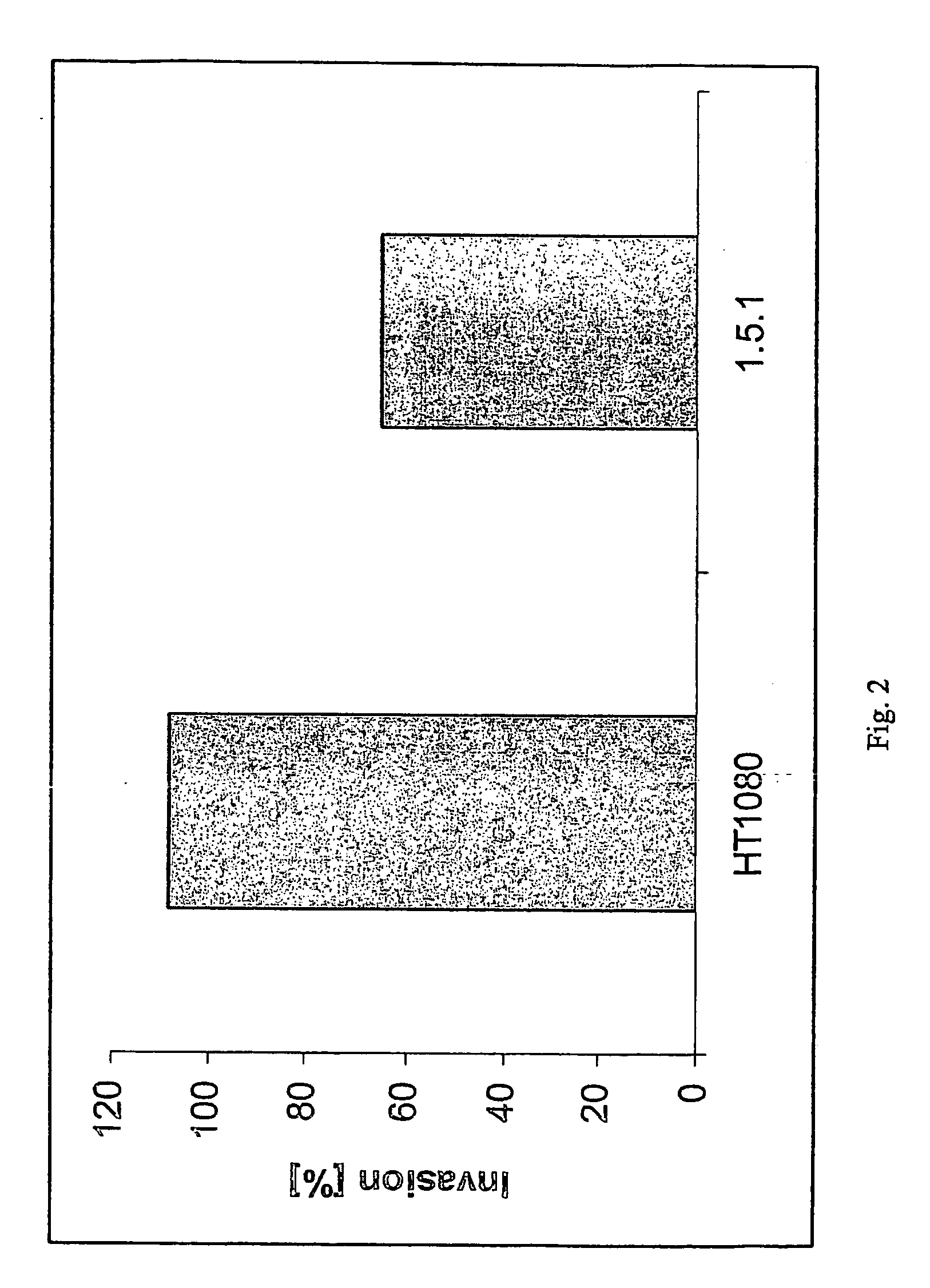
Inhibitors of extracellular Hsp90
ActiveUS20060073151A1Increased toxicityEasy to synthesizePowder deliveryBiocideExtracellularCancer cell
The present invention describes inhibitors of extracellular Hsp90. The inhibition of extracellular Hsp90 leads to a reduction of the invasiveness of the tumor cells. Furthermore, the invention relates to the use of molecules inhibiting extracellular Hsp90 function for the manufacture of a medicament for the treatment or prevention of invasion and / or metastatic potential of cancer cells.
Owner:TUFTS UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com