Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
11374results about How to "Facilitated release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
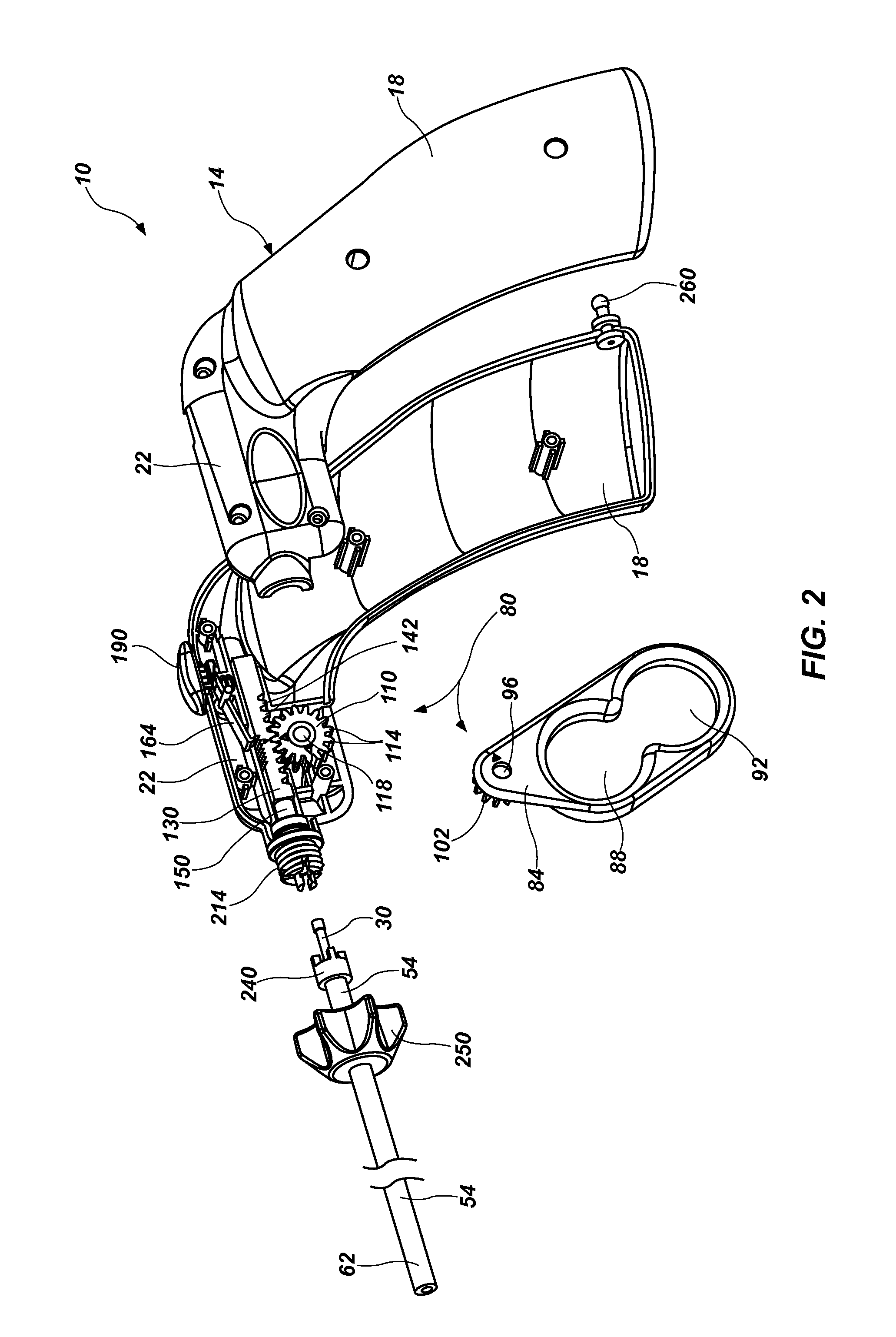
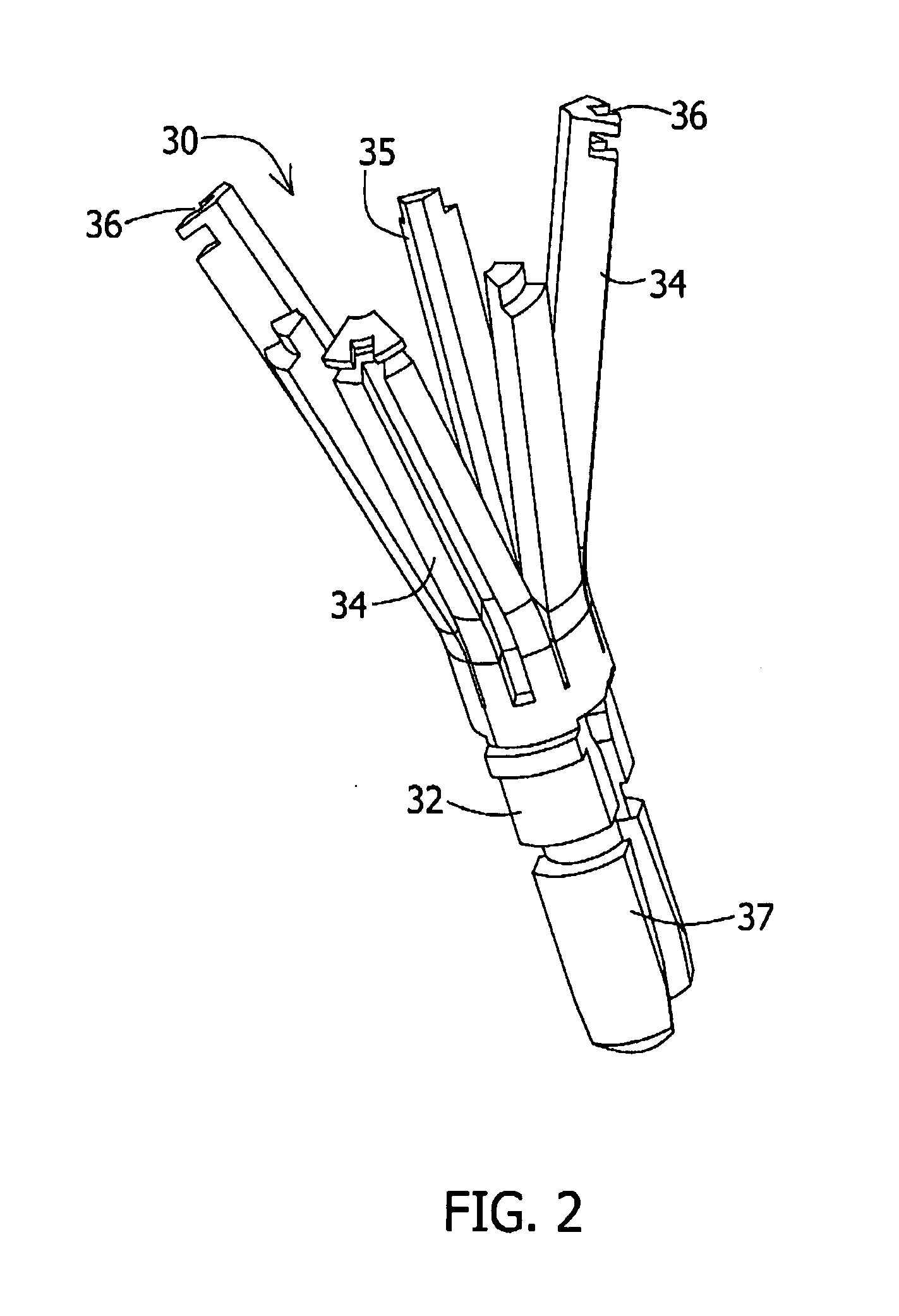
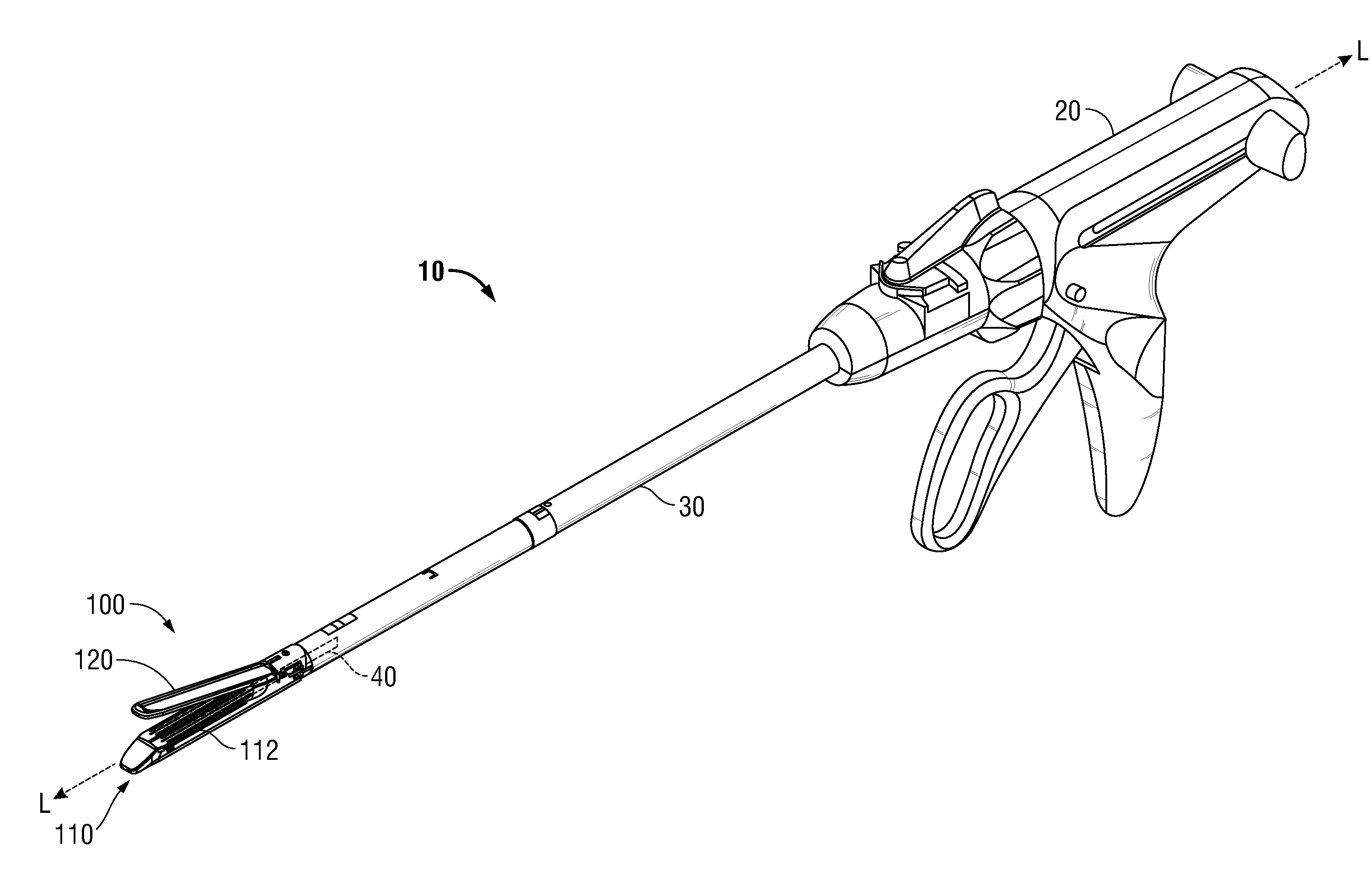
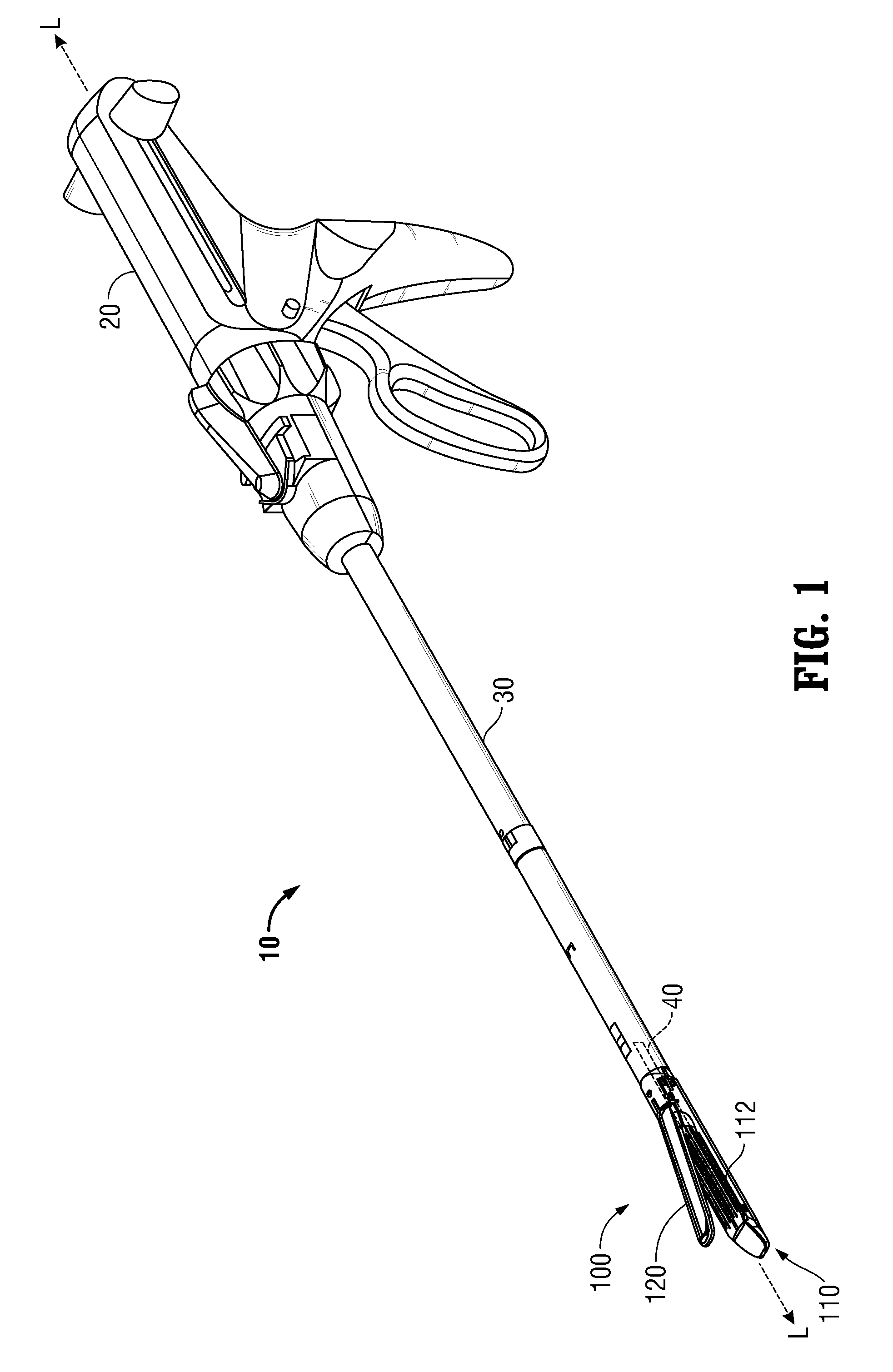
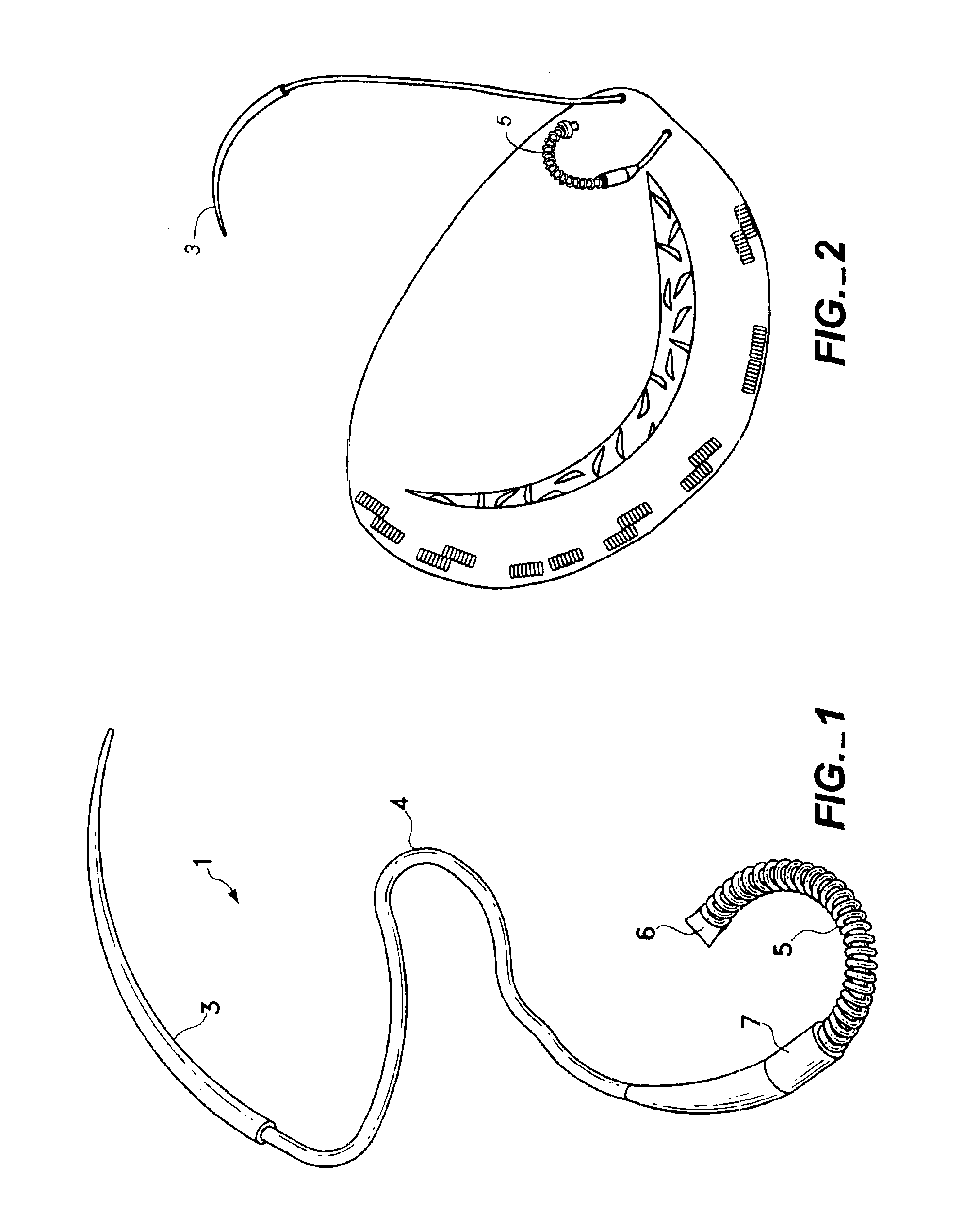
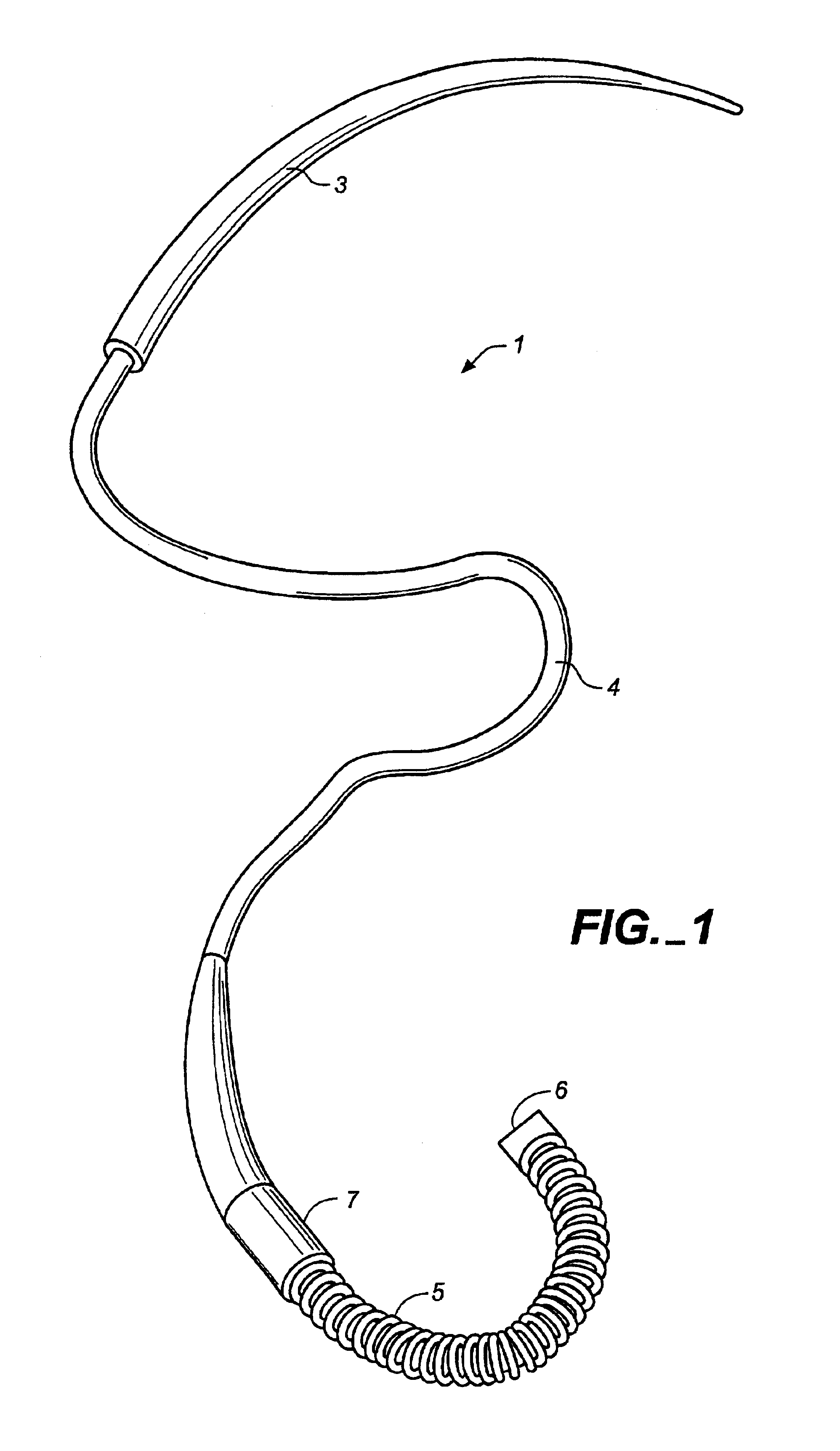
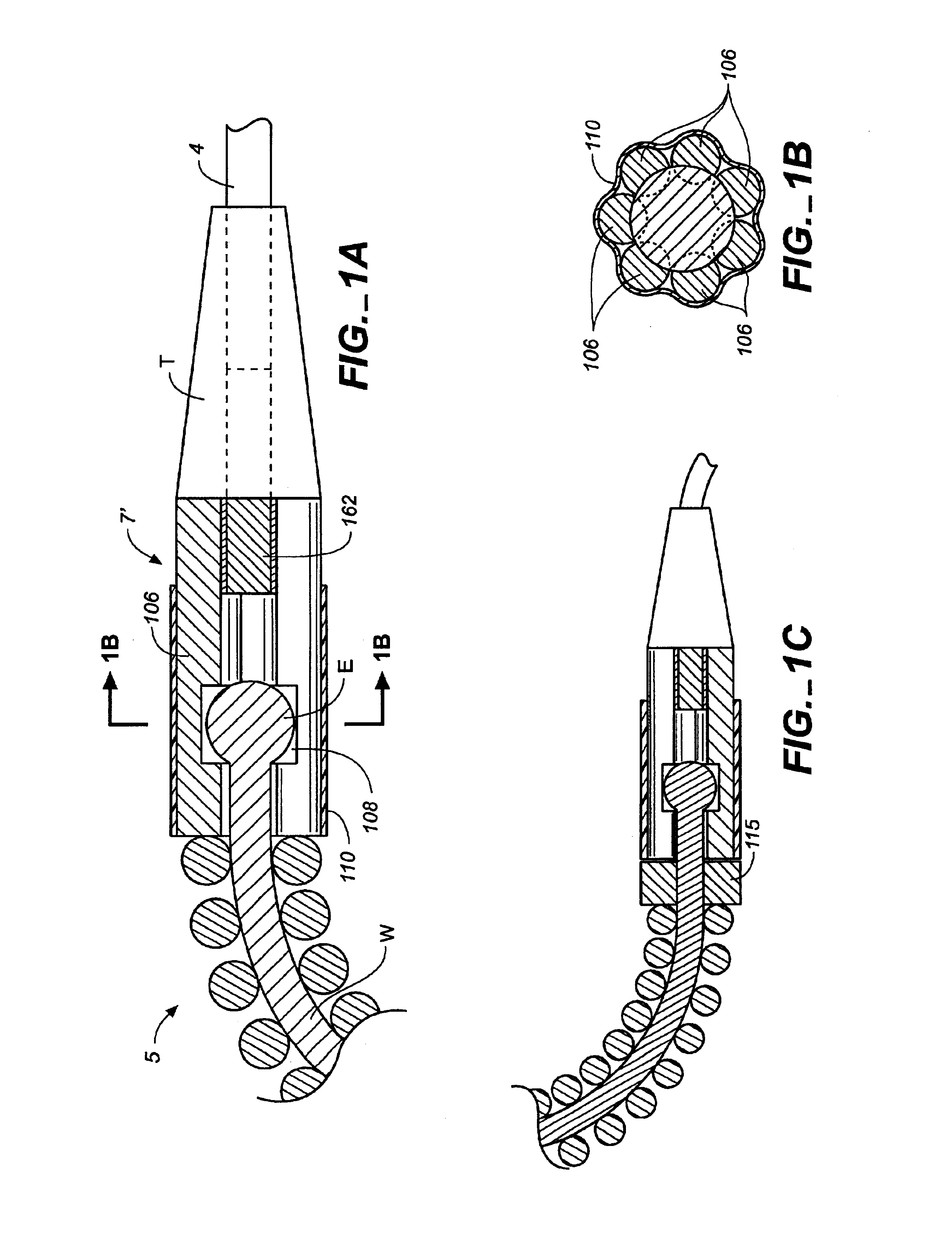
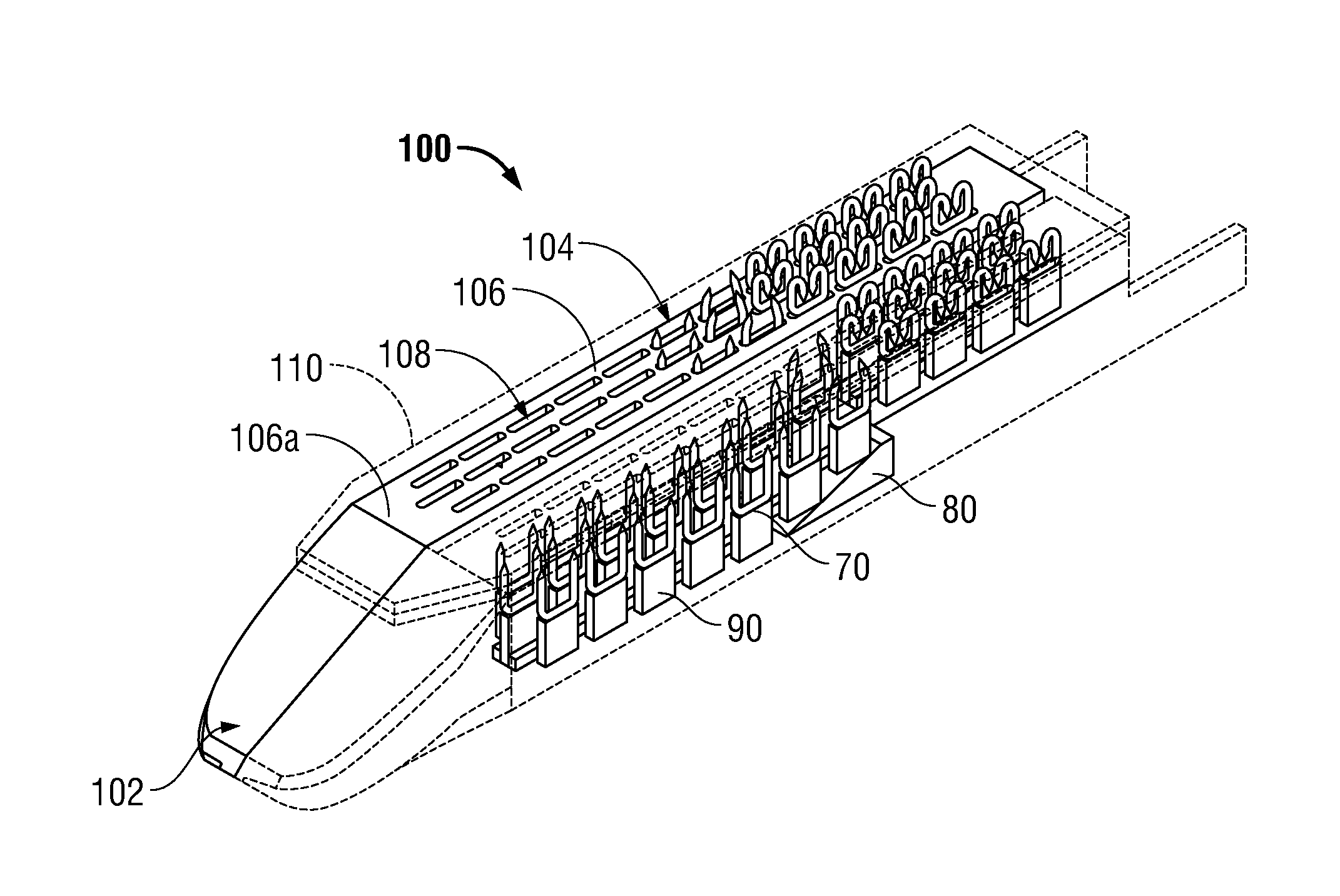
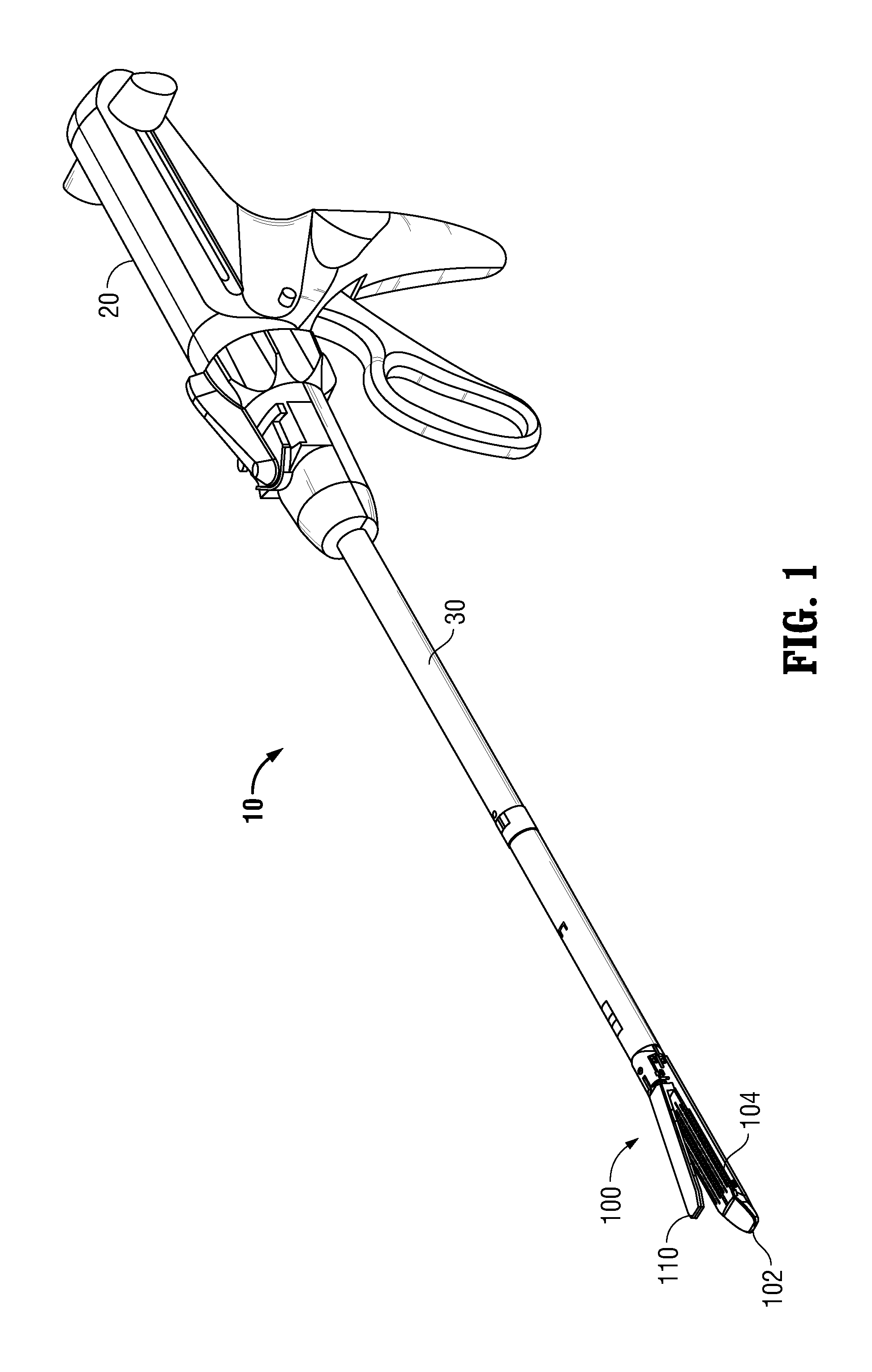
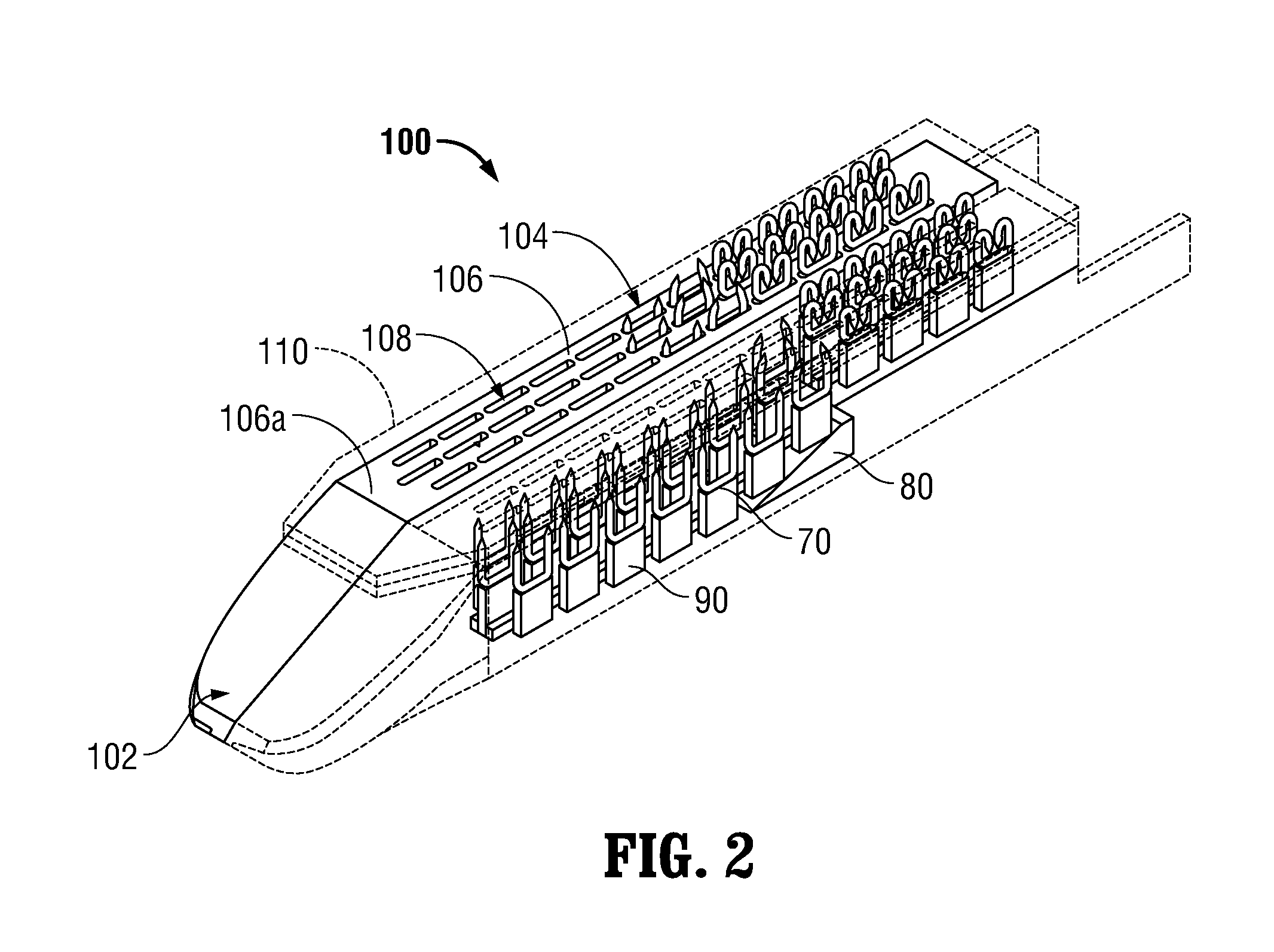
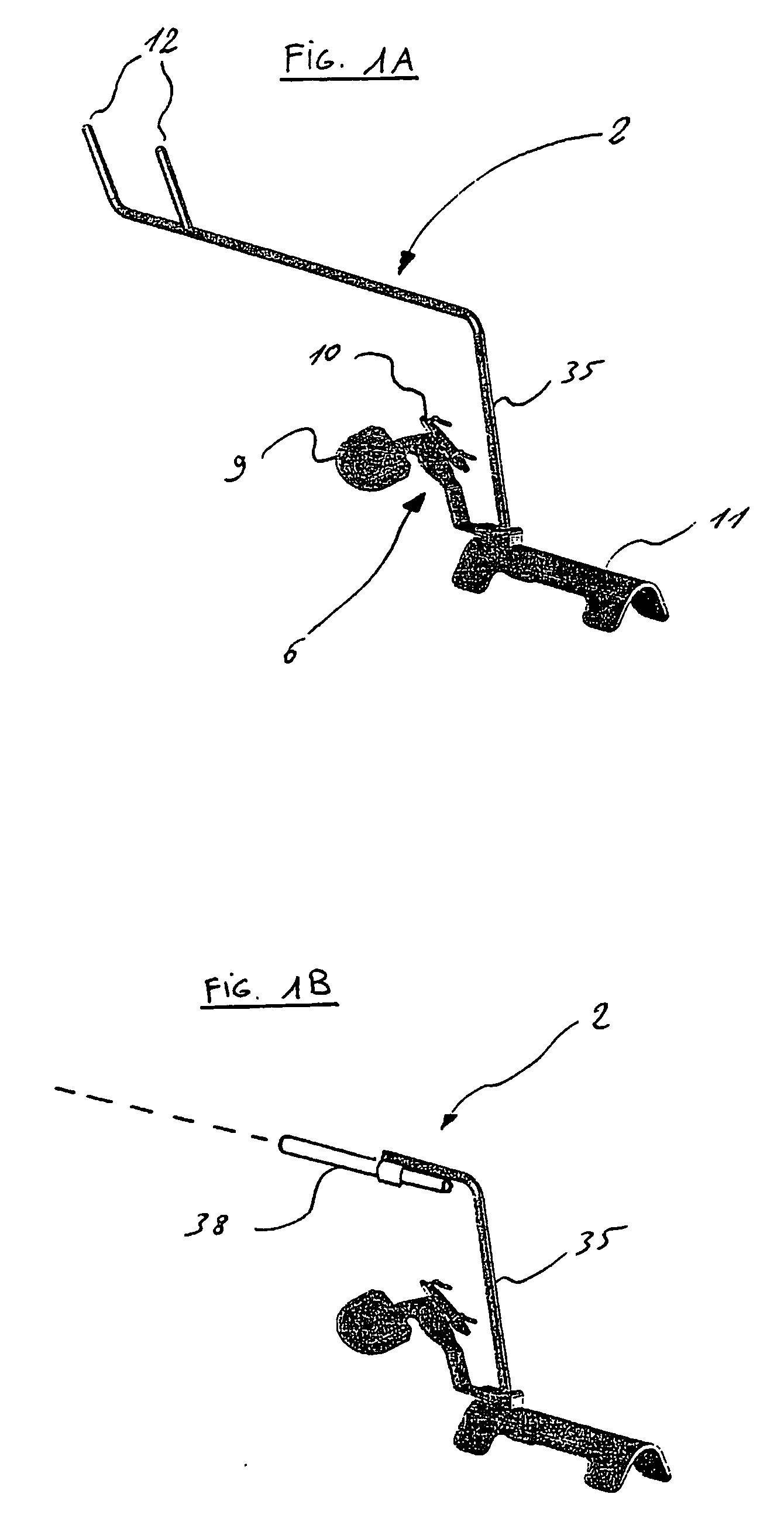
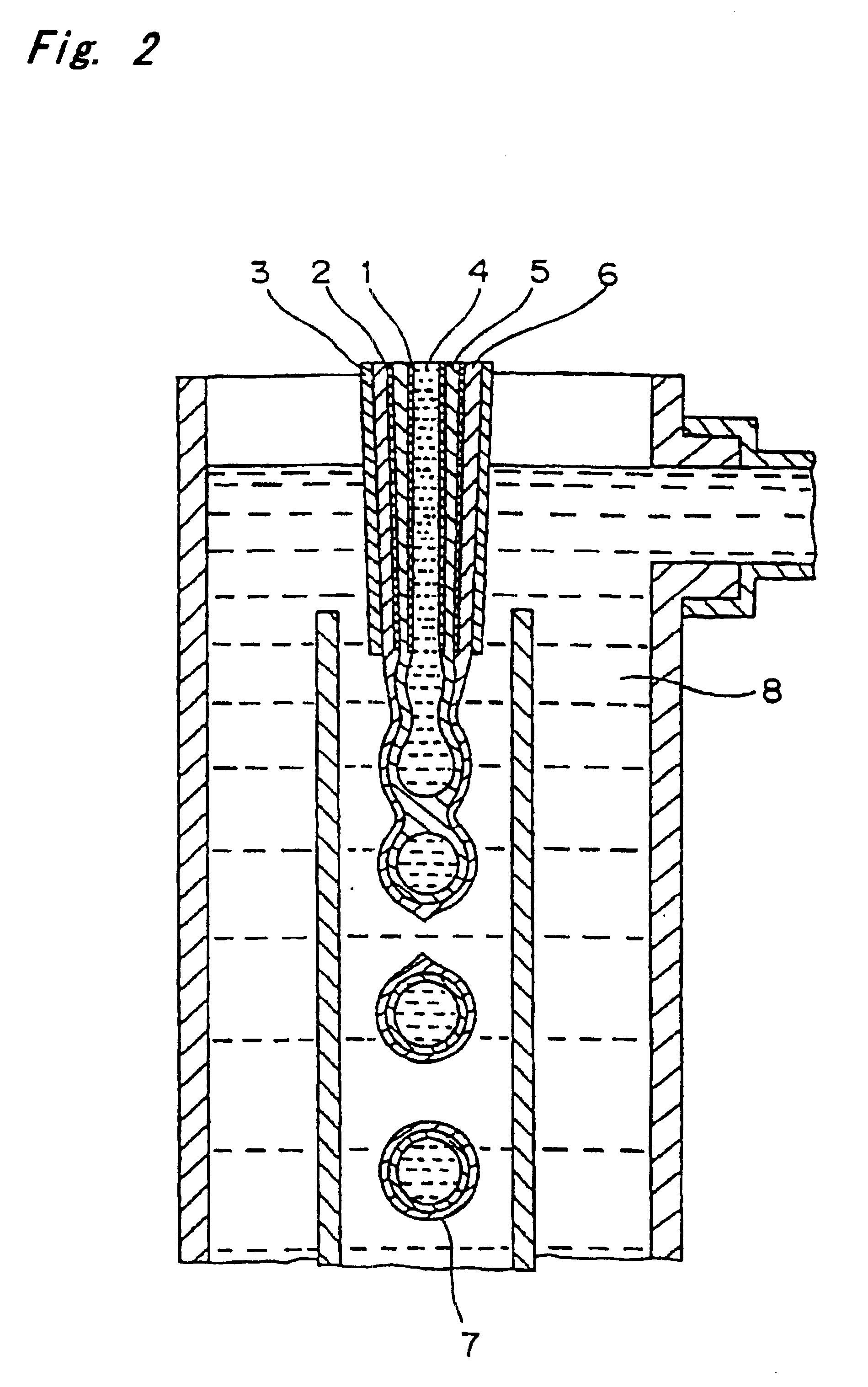
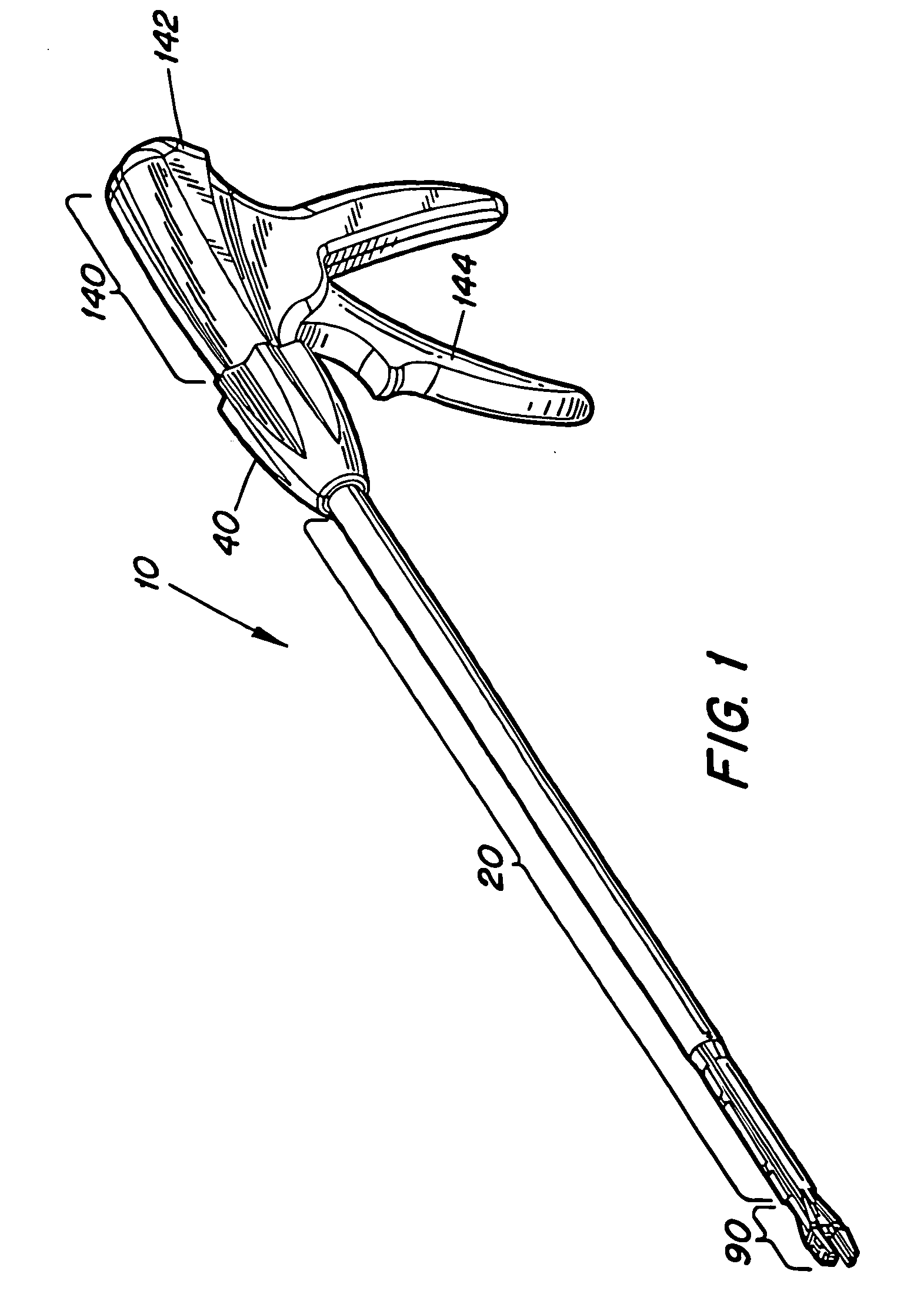
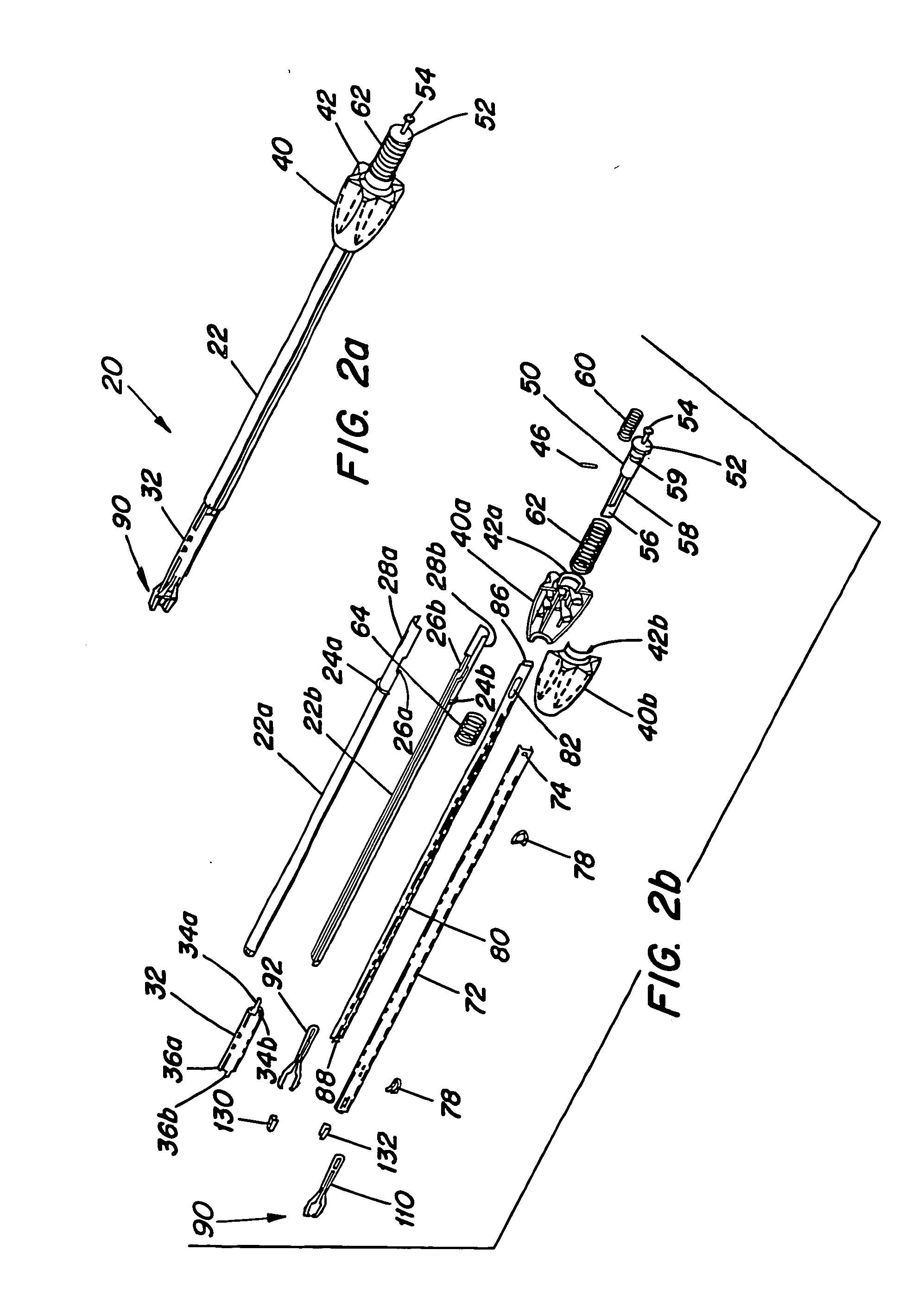
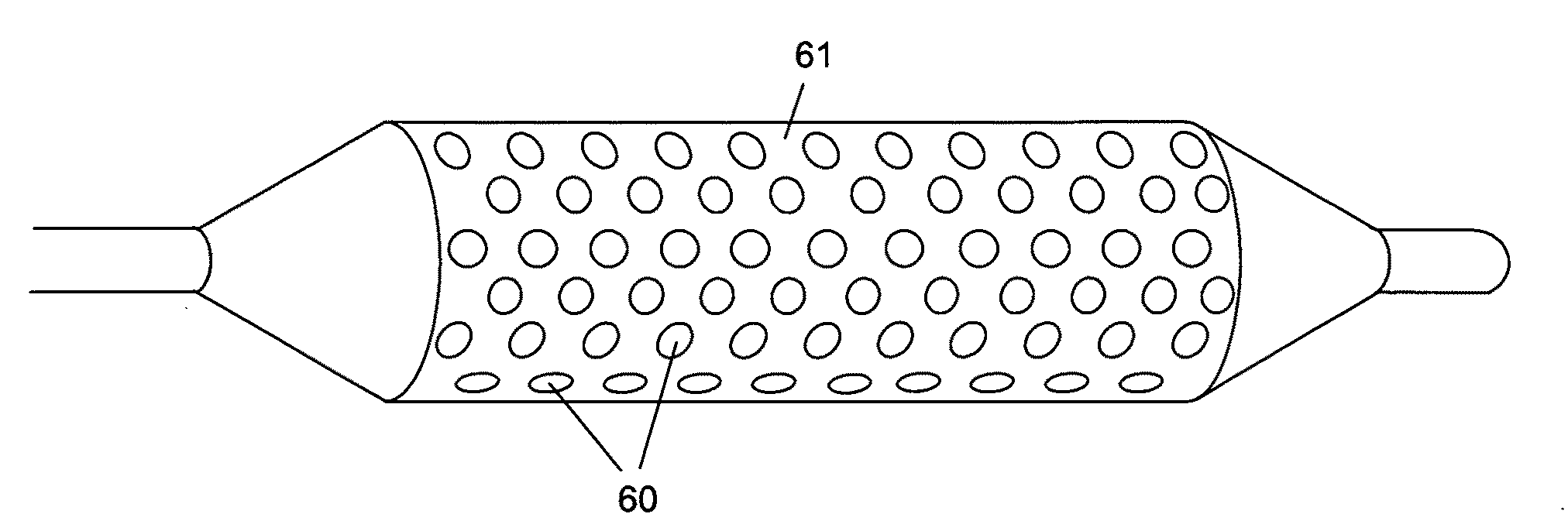
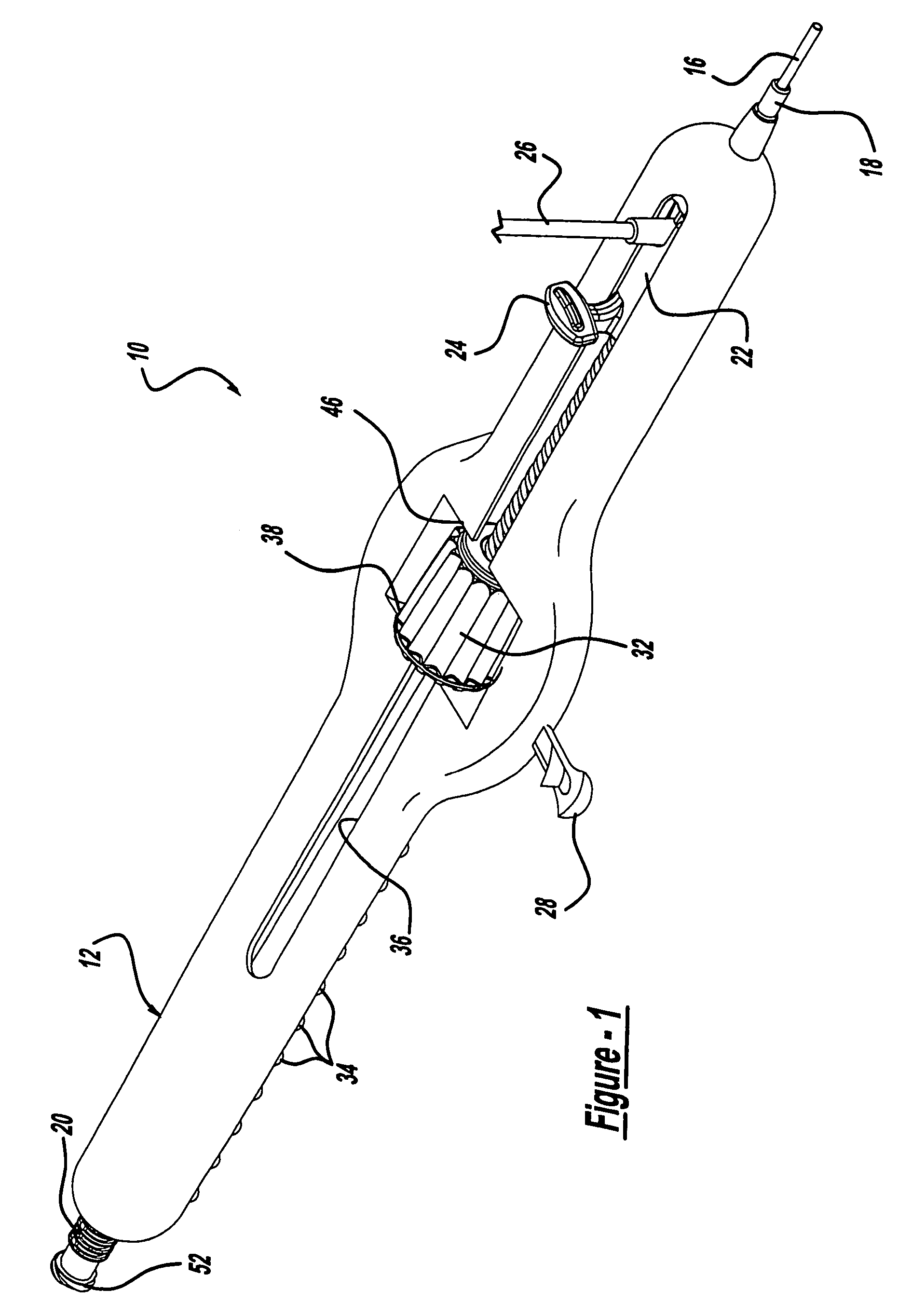
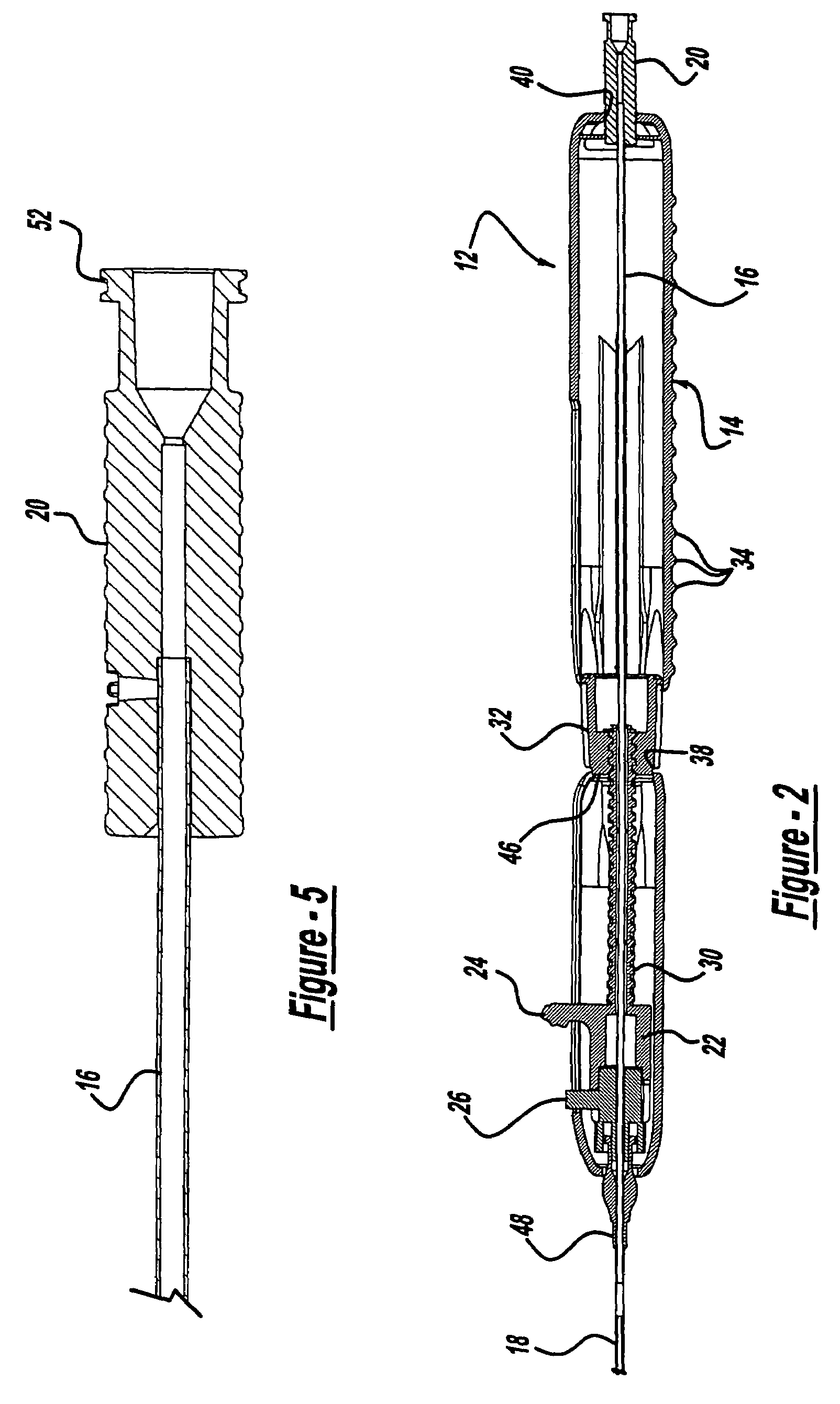
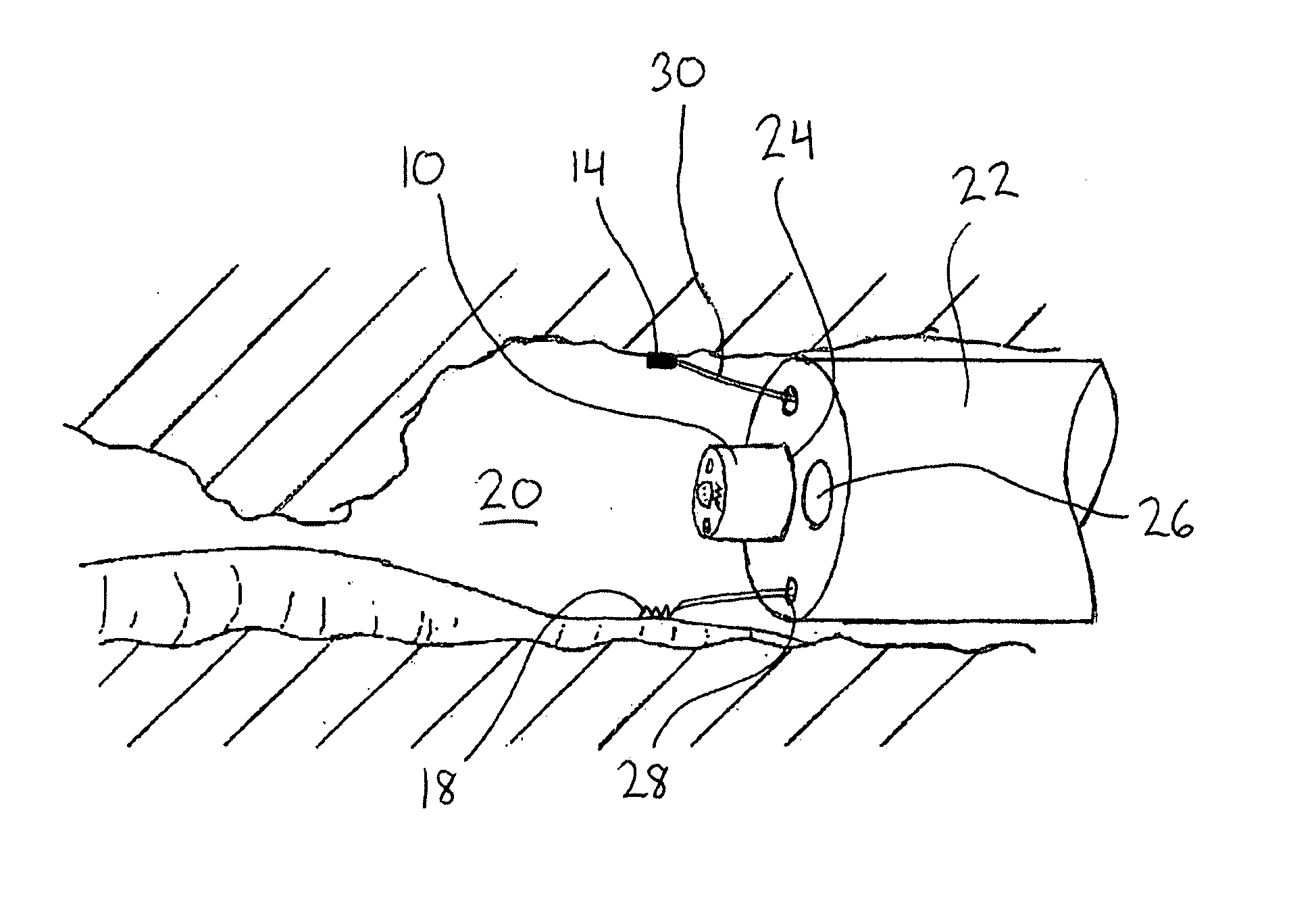
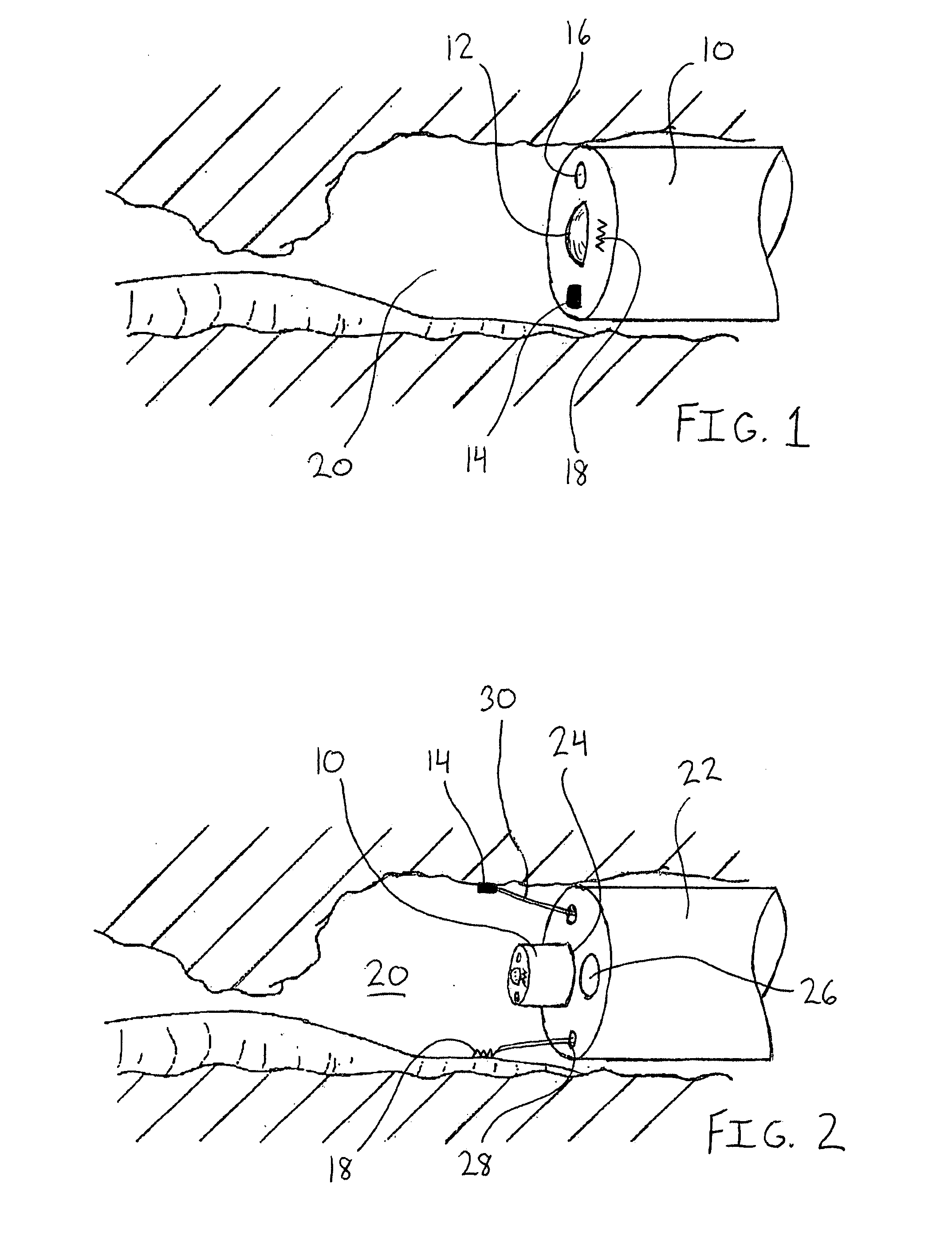
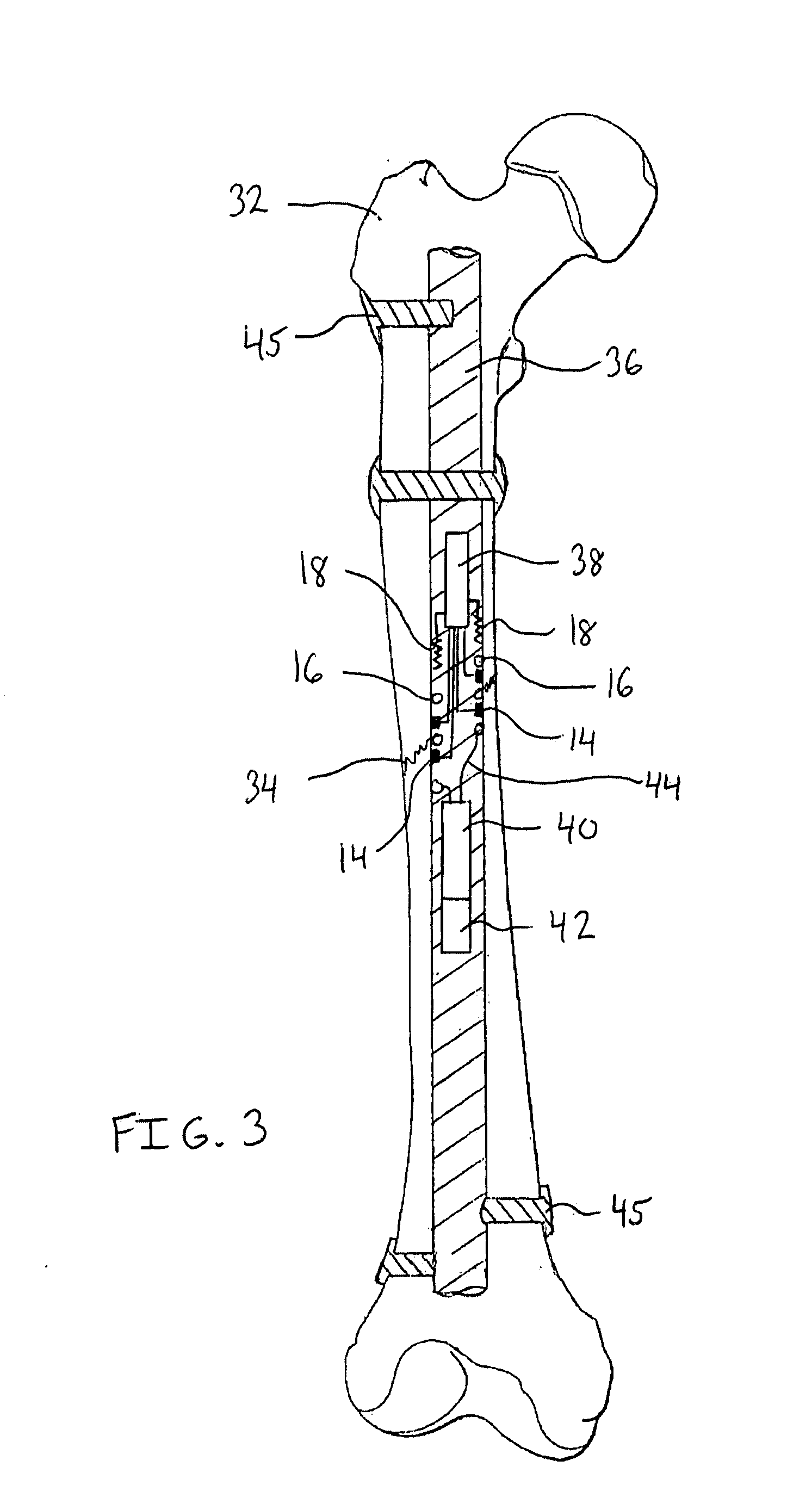
Surgical apparatus including surgical buttress
ActiveUS9433420B2Facilitated releaseImprove securityDomestic articlesSurgical staplesButtressActuator
Owner:TYCO HEALTHCARE GRP LP
Energetically-controlled delivery of biologically active material from an implanted medical device
InactiveUS7101394B2Facilitated releaseReduce deliveryOrganic active ingredientsElectrotherapyMedical deviceBiomedical engineering
A medical device and system capable of providing on-demand delivery of biologically active material to a body lumen patient, and a method of making such medical device. A first coating layer comprising a biologically active material and optionally a polymeric material is disposed on the surface of the medical device. A second coating layer comprising magnetic particles and a polymeric material is disposed on the first coating layer. The second coating layer, which is substantially free of a biologically active material, protects the biologically active material prior to delivery. The system includes the medical device and a source of energy, such as an electromagnetic or mechanical vibrational energy. When the patient is exposed to the energy source, the magnetic particles move out of the second coating layer and create channels therein through which the biologically active material can be released.
Owner:BOSTON SCI SCIMED INC
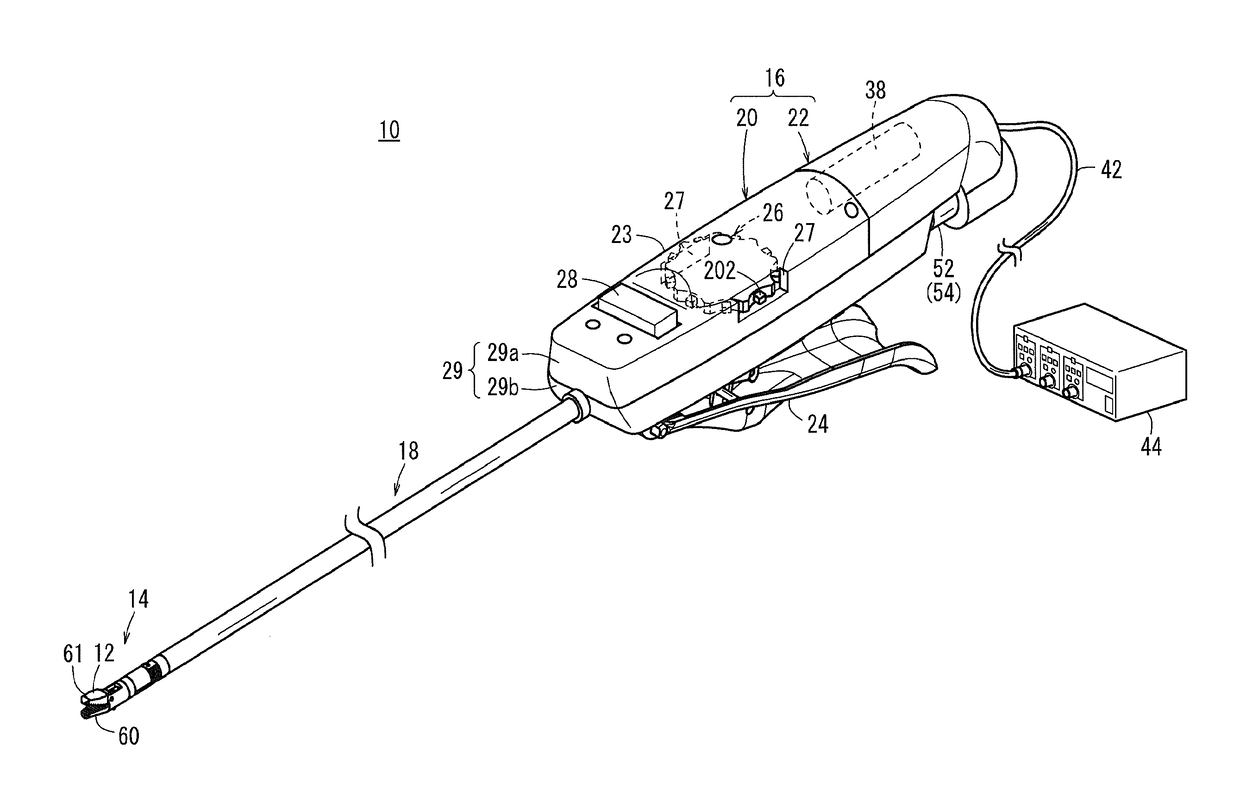
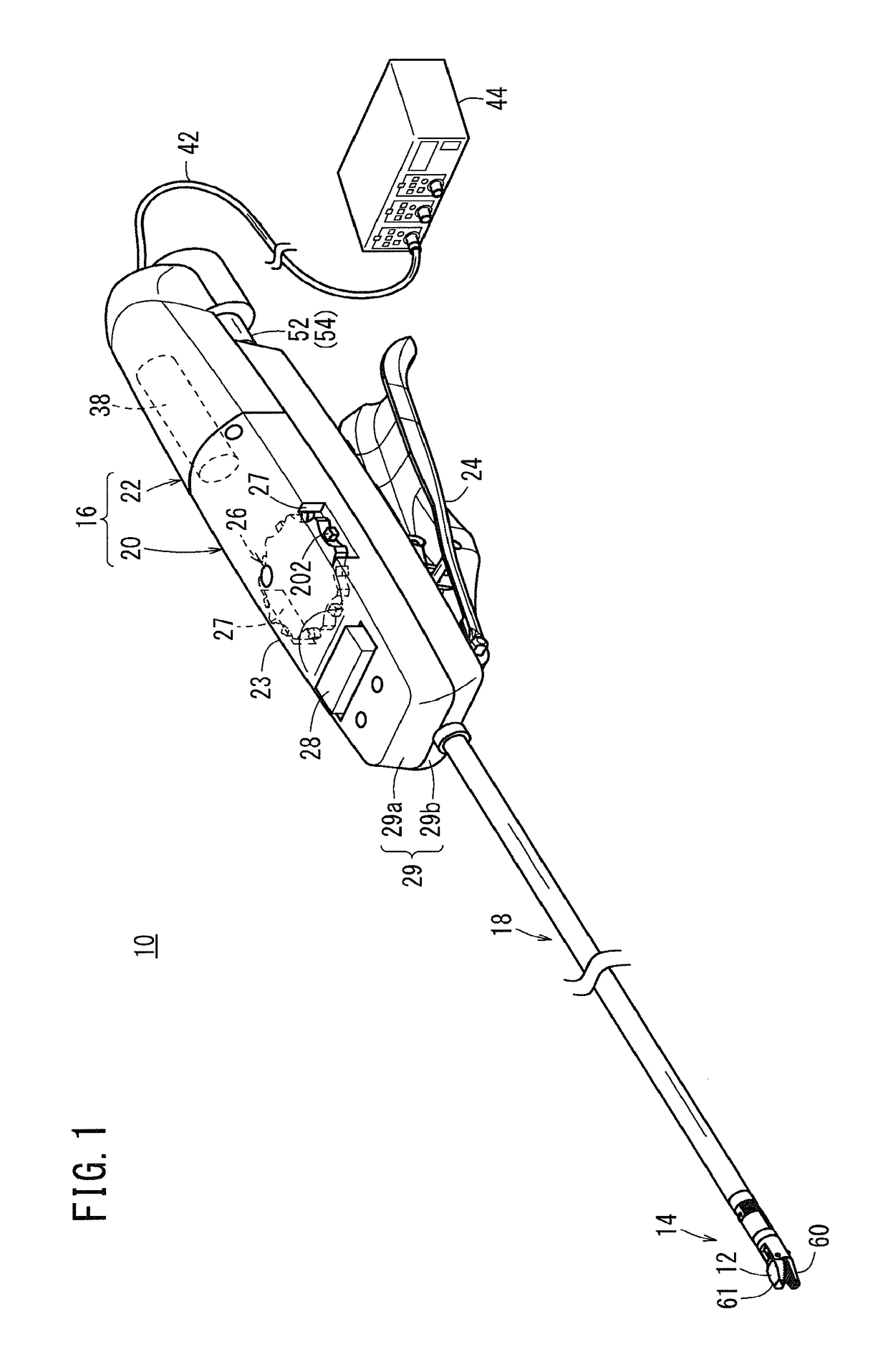
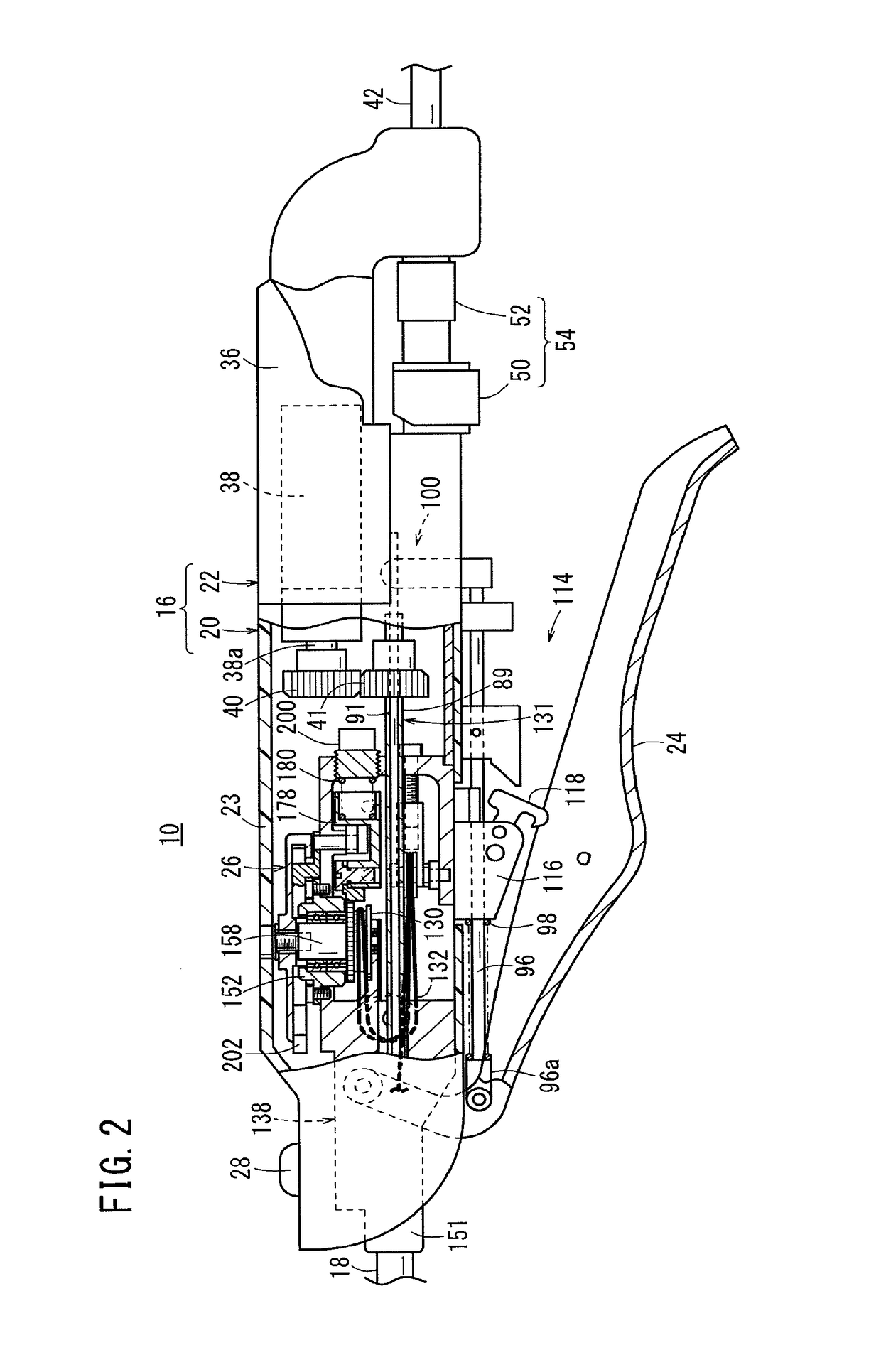
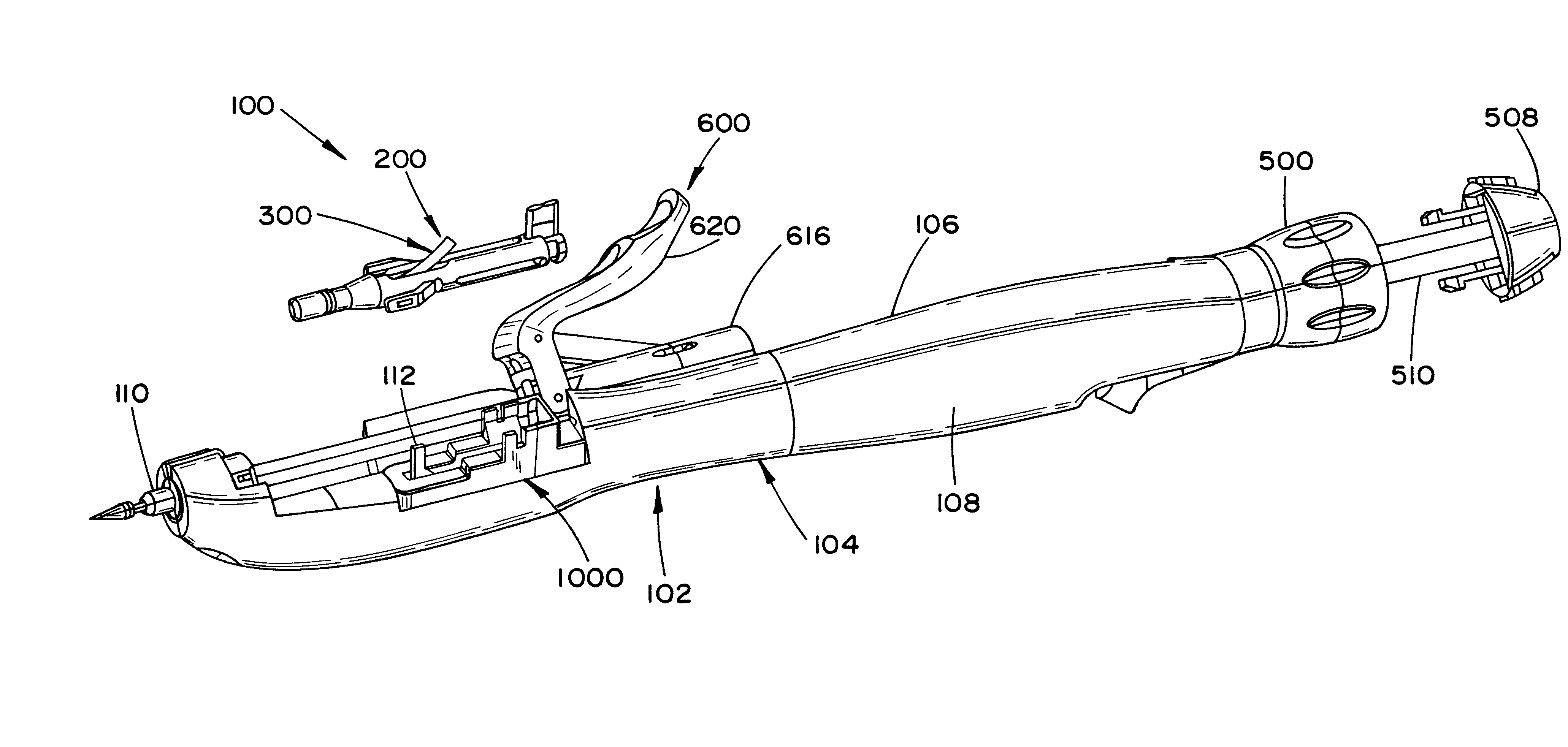
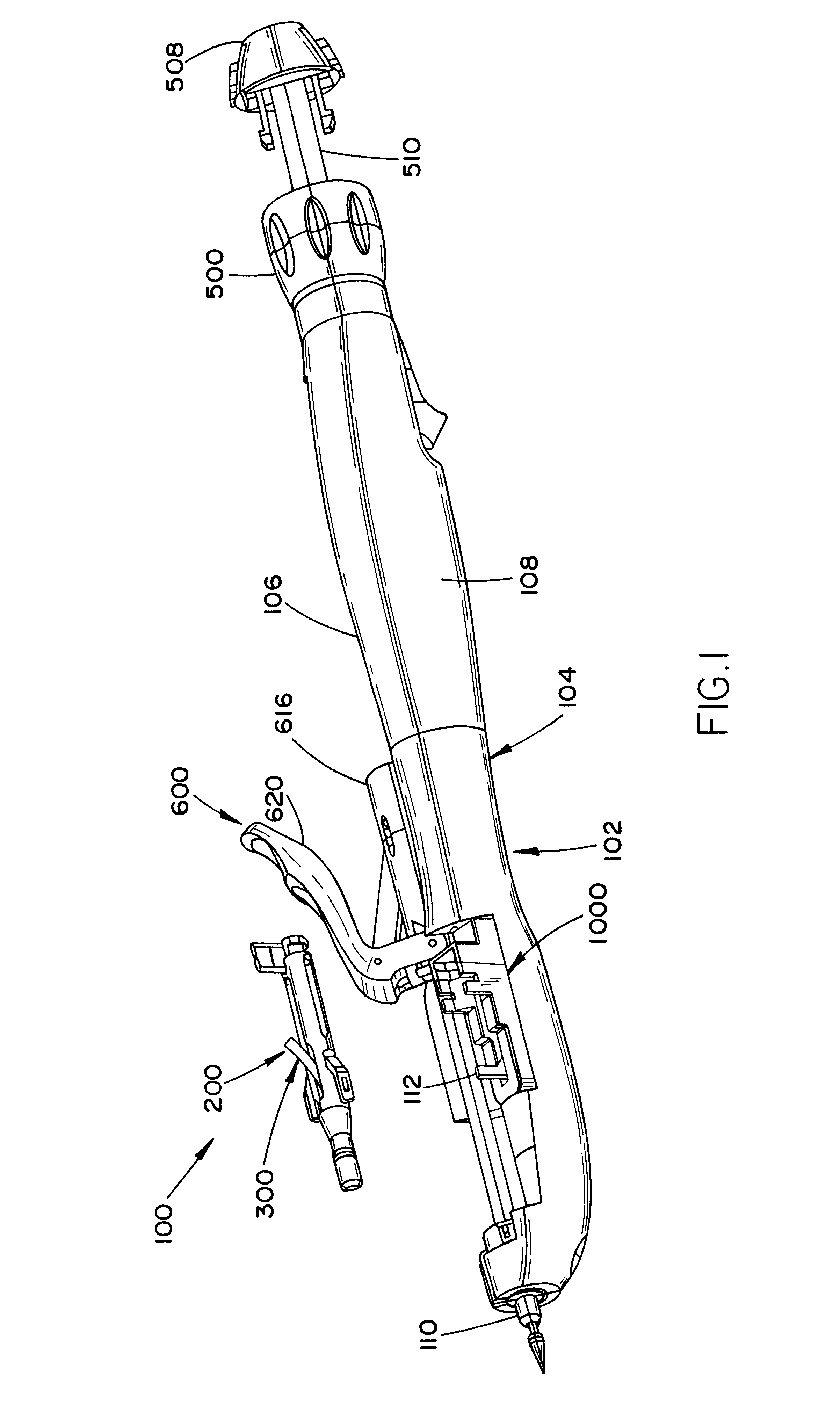
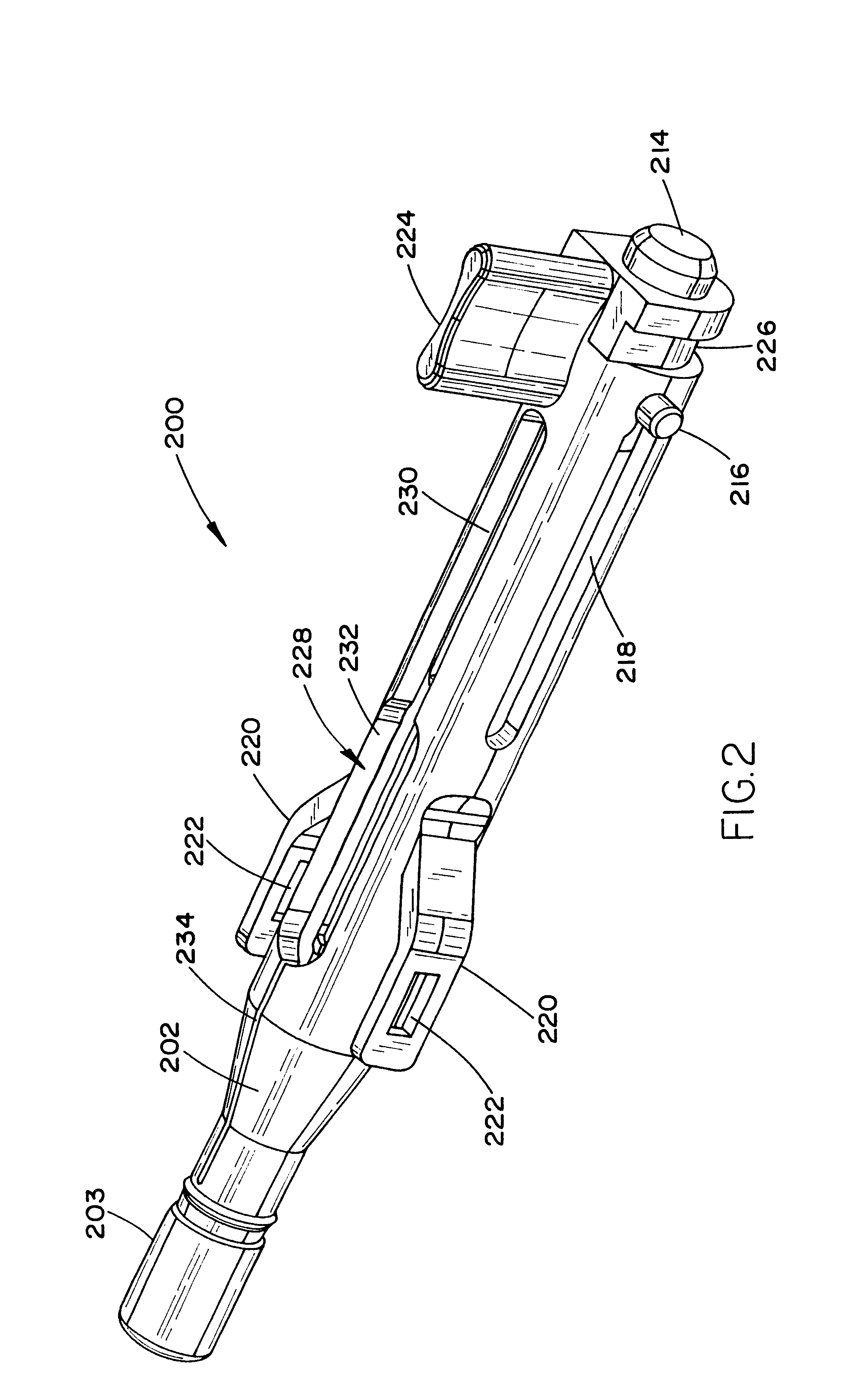
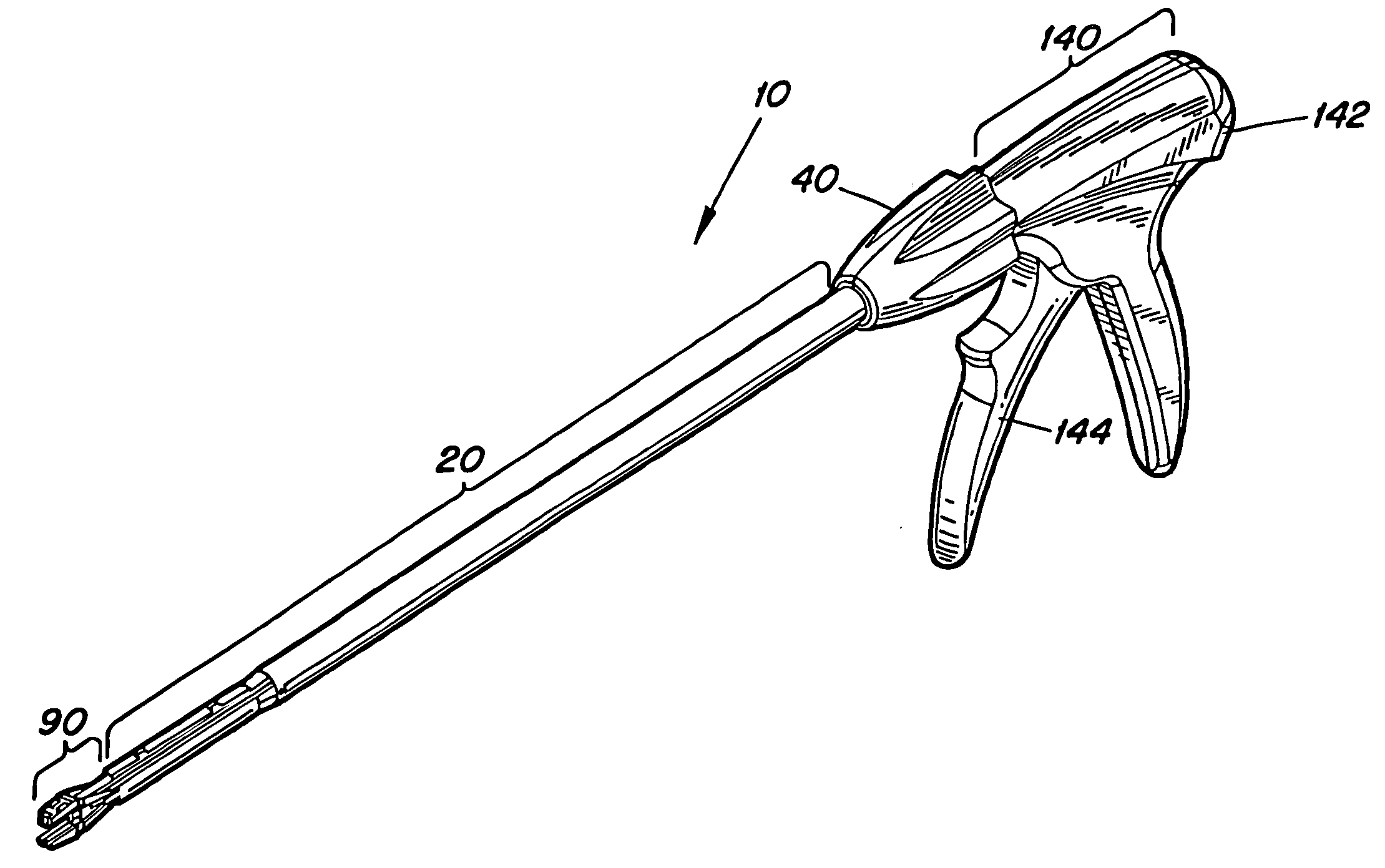
Laparoscopic surgical instrument
A laparoscopic surgical instrument configured to be ergonomic and anthropometrically correct, the laparoscopic surgical instrument comprising: (a) an ergonomic handle configured to orient a hand of a surgeon in a functional position, the handle comprising a handle grip; (b) an actuating mechanism actuatable by a finger and supported by the handle, the actuating mechanism comprising an actuator shaft and a gearing assembly operable to displace the actuator shaft with a mechanical advantage upon actuation of a trigger by the surgeon; (c) a locking mechanism configured to lock the actuating mechanism in one of a plurality of positions, the locking mechanism comprising a release located in an anthropometrically correct position; and (d) a working shaft having a proximal end coupled to and operable with the actuator shaft, the working shaft having an elongate configuration and a distal working end configured to couple a surgical tool to be manipulated by the surgeon via the handle and the actuating mechanism to perform a surgical function.
Owner:SHORTI RAMI +1
Brake release mechanism and medical manipulator provided with same
ActiveUS10064639B2Easily and swiftly releasingFacilitated releaseSurgical needlesDrum brakesEngineeringManipulator
A medical manipulator is provided with a brake release mechanism. The brake release mechanism is provided with a release button which is provided on a tilt wheel, and a lever mechanism which has at least one portion arranged on the inside of the tilt wheel and which is pressed when the release button moves inwards. By the action of the lever mechanism when the release button is operated, braking by a brake mechanism is released.
Owner:KARL STORZ GMBH & CO KG
Drug delivery system and method
InactiveUS6041252AFacilitated releaseFast wayBioreactor/fermenter combinationsElectrotherapyLiposomeBiomedical engineering
A method for delivering a therapeutic agent to a predetermined location in a host is disclosed, wherein a liposome-encapsulated therapeutic agent is administered to the host, and an electrical field which encompasses a predetermined region within the host is established, such that as the liposome-encapsulated agent is exposed to the electrical field the release of the agent from the liposome to the predetermined region is enhanced.
Owner:ICHOR MEDICAL SYST
Three dimensional cell protector/pore architecture formation for bone and tissue constructs
InactiveUS7713542B2Greatly multiplied in vitroProtection from damagePowder deliveryBiocideIn vivoLiving cell
Living cellular material is encapsulated or placed in a protective material (cell protector) which is biocompatible, biodegradable and has a three-dimensional form. The three dimensional form is incorporated into a matrix that maybe implanted in vivo, ultimately degrade and thereby by replaced by living cell generated material.
Owner:ADA FOUND
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Stent loading tool and method for use thereof
ActiveUS20090054976A1The method is simple and reliableEasy to pushStentsHeart valvesVALVE PORTCatheter device
A loading tool for withdrawing, crimping, and loading a stent-mounted valve into a delivery catheter, and for pushing the stent-mounted valve from the delivery catheter into a native heart valve orifice. The loading tool comprises at least one connector adapted for being removably connected to the stent of the stent-mounted valve. A crimping tool having a generally converging shape is adapted for use with the loading tool. Following connection of the loading tool to the stent-mounted valve, the loading tool operates to allow the stent-mounted valve to be drawn through the crimping tool, and loaded, in a crimped state, into a delivery catheter. Also disclosed is a kit of the of the various components for effecting the delivery of the stent-mounted valve and a method for withdrawing, crimping, and loading a stent-mounted valve from a storage container into a delivery catheter for the performance of a transcatheter valve implantation procedure.
Owner:MEDTRONIC VENTOR TECH
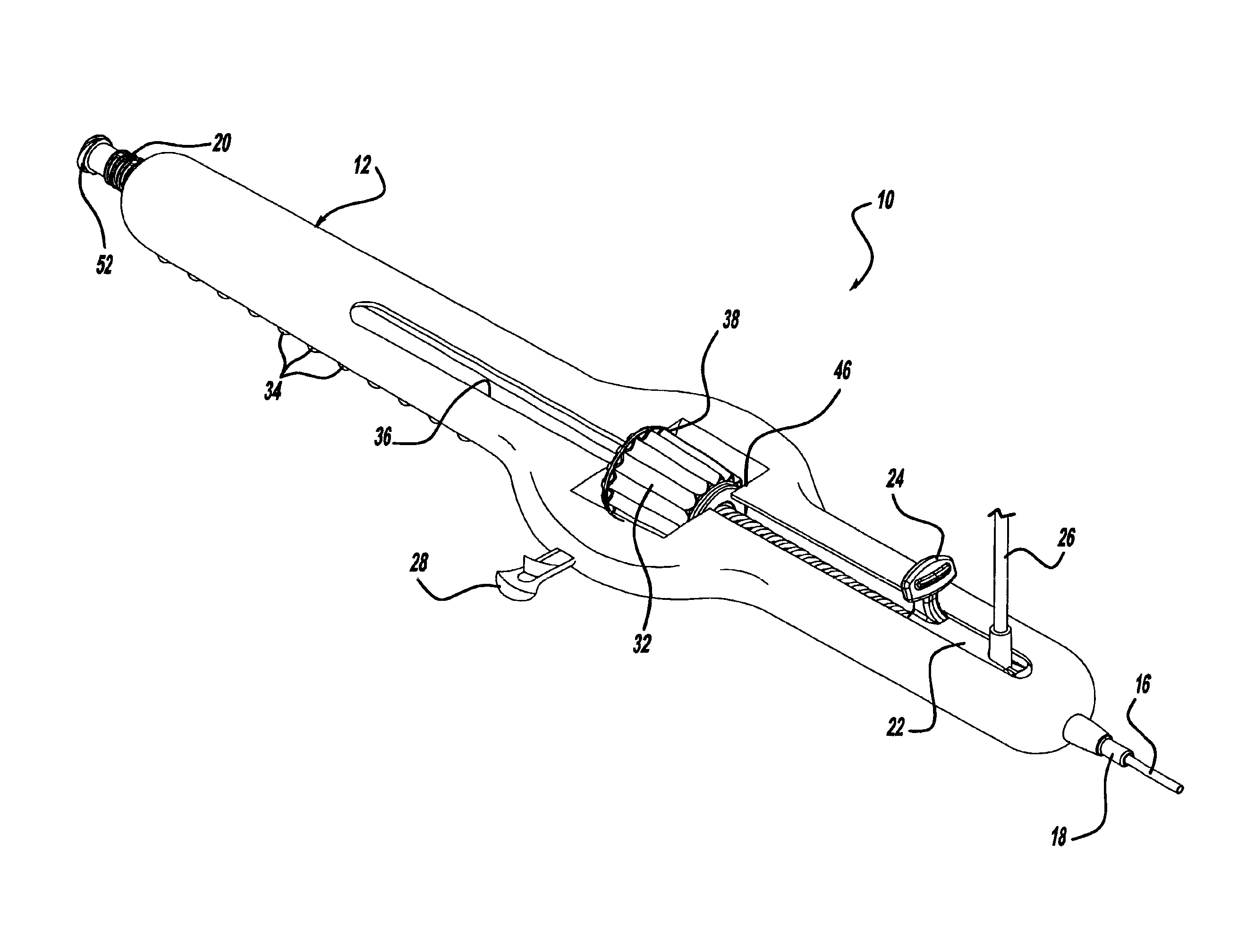
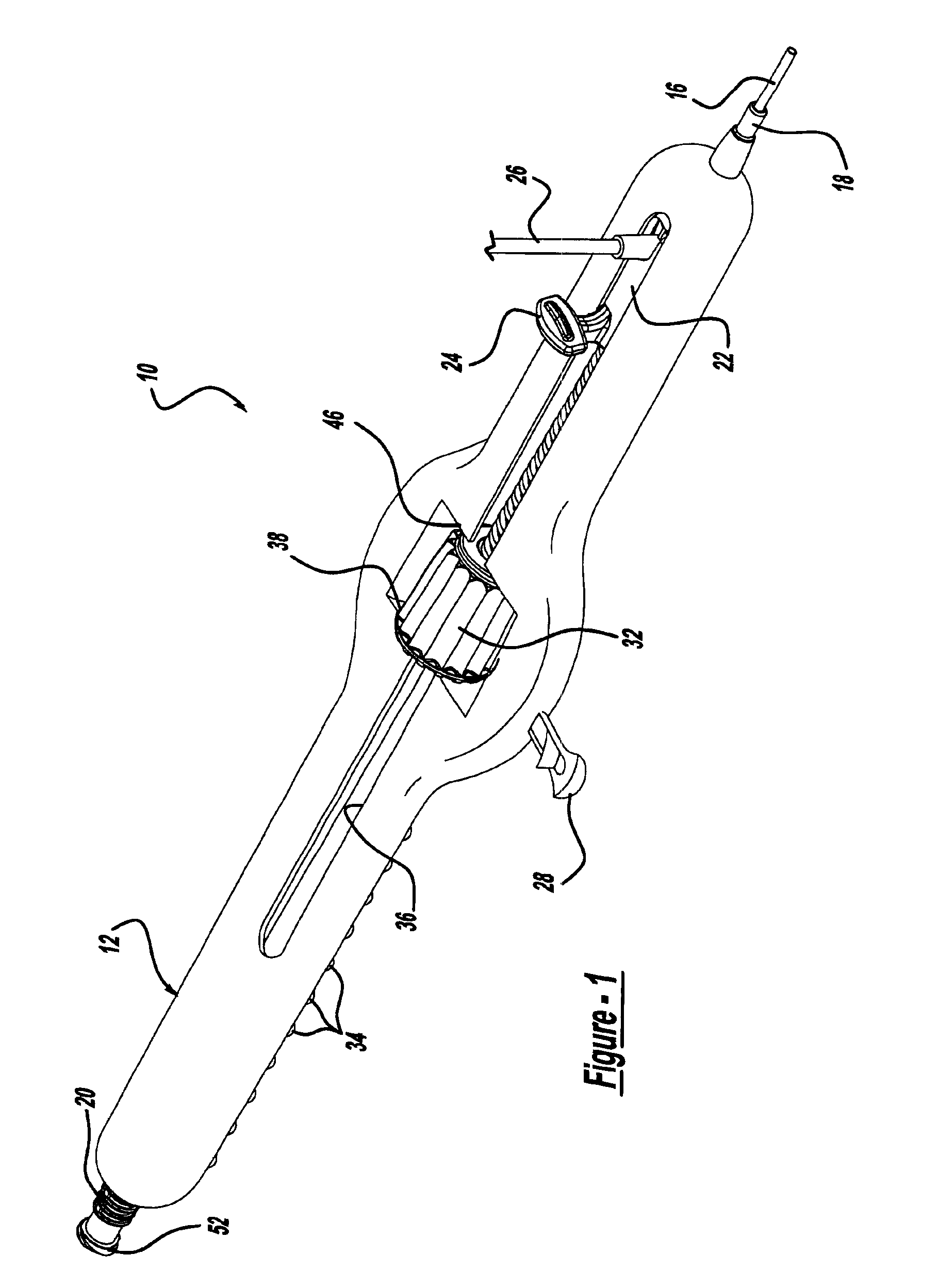
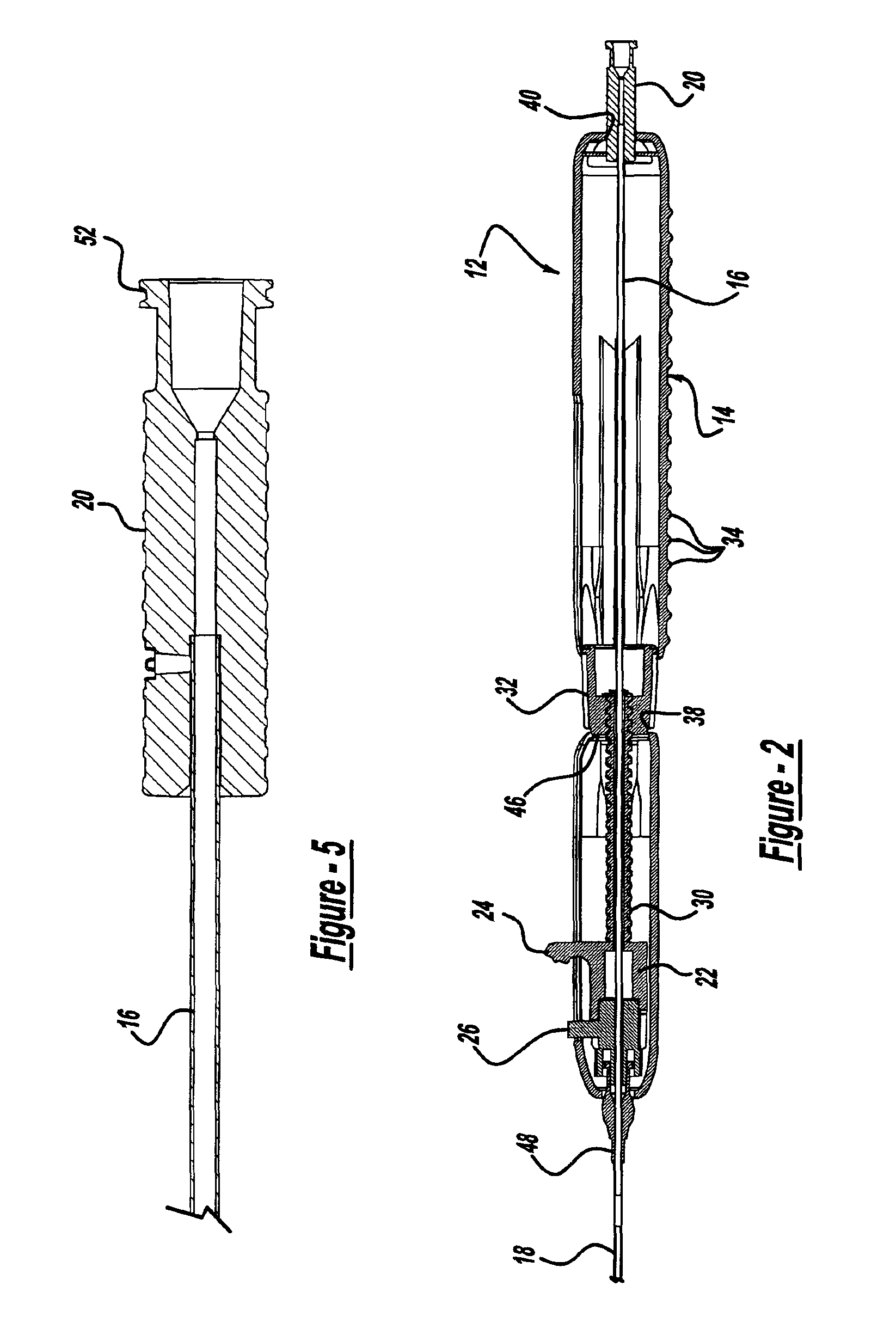
Locking handle deployment mechanism for medical device and method
InactiveUS6866669B2Easy and accurate deployment and positioningEasy to operateStentsEar treatmentLocking mechanismMedical device
A system for delivering at least one medical device to a desired location for treatment, and then selectively deploy it in position, includes an improved handle. One of the possible features of the handle may be to selectively hold the delivery system components at any desired configuration during deployment and positioning of the medical device. Another possible feature of the handle may be more than one mode of operation, in which the deployment of the medical device can selectively proceed at more than one speed. Yet another possible feature of the handle may be a locking mechanism that resists inadvertent or accidental movement or retraction of the stent delivery system components during packaging, sterilization, shipping, storage, handling and preparation of the stent delivery system.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
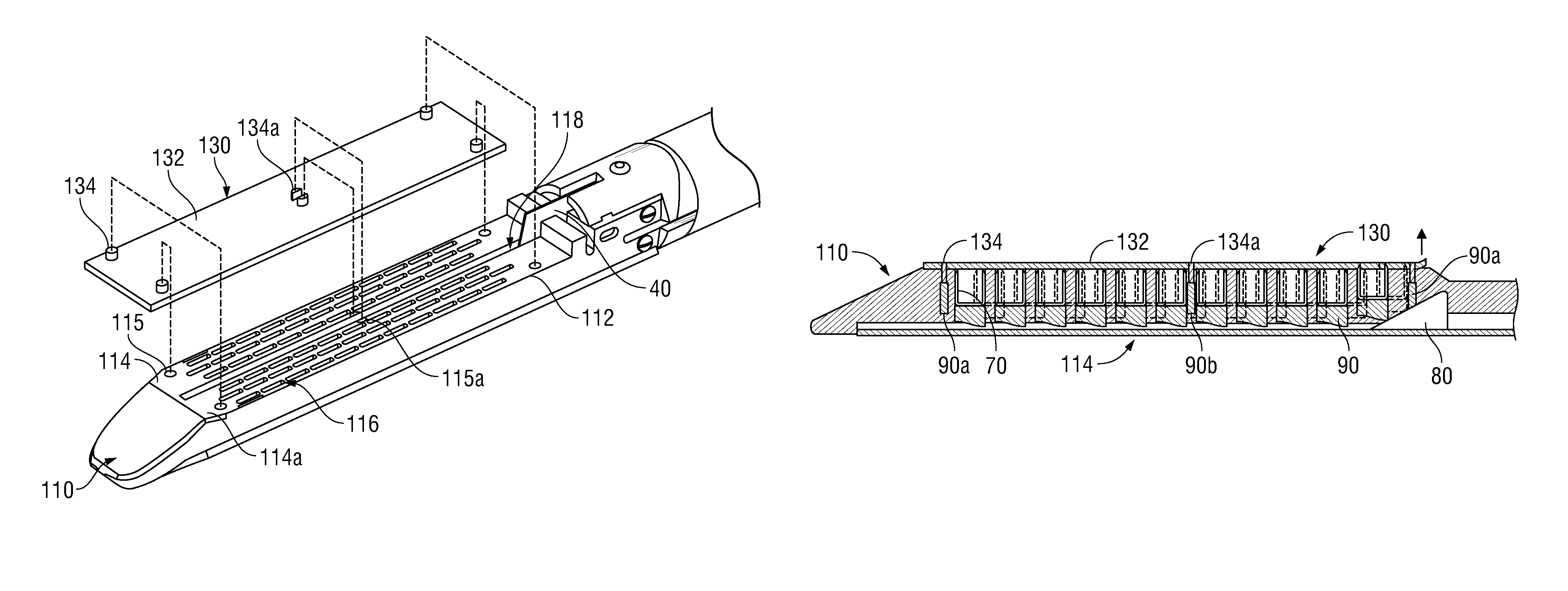
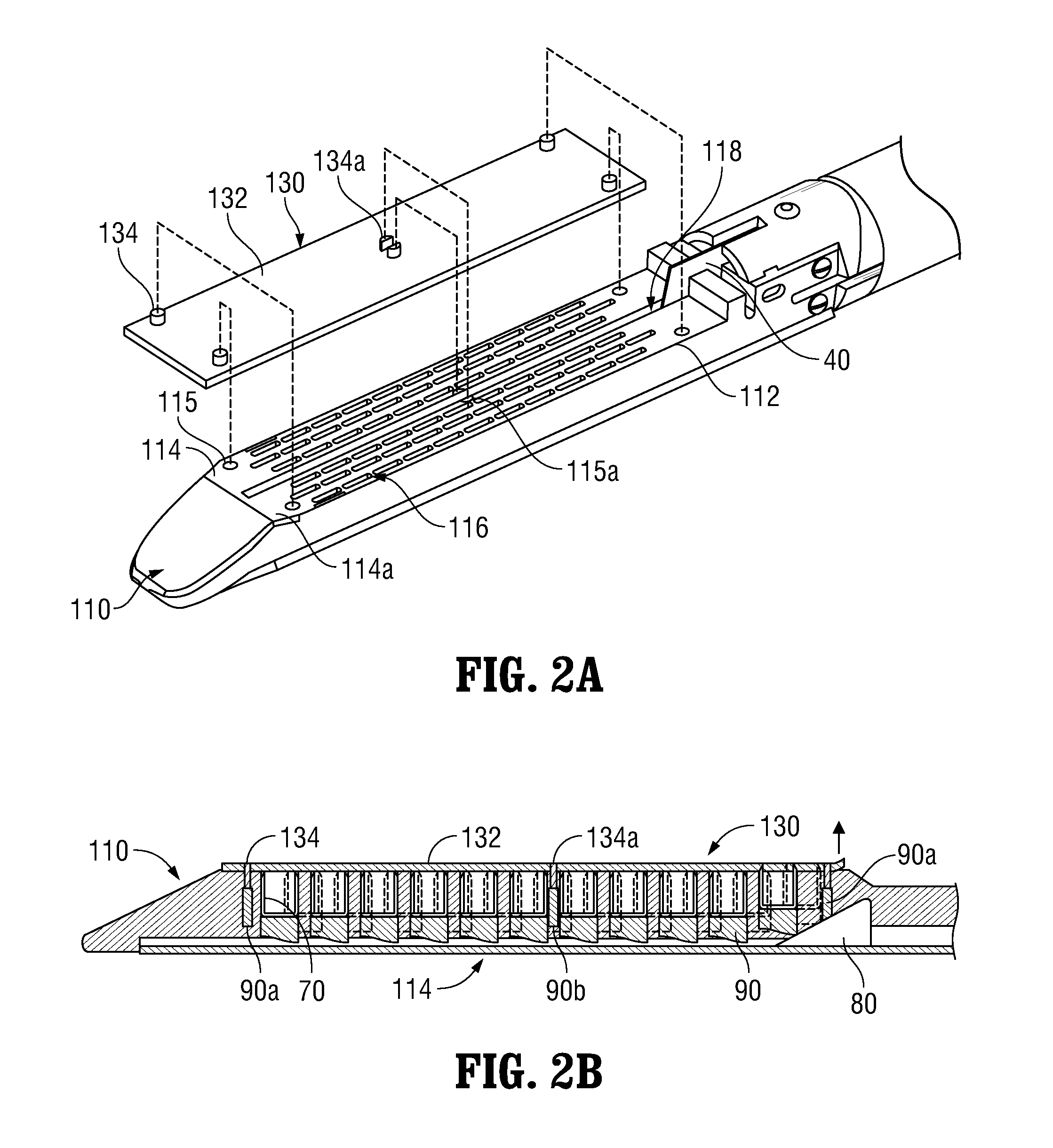
Surgical Apparatus Including Surgical Buttress
ActiveUS20140203061A1Facilitated releaseImprove securitySuture equipmentsStapling toolsButtressRobot end effector
A surgical stapling apparatus includes a housing, an end effector, fasteners, and one or more surgical buttresses. The end effector is secured to the housing and has first and second jaw assemblies. The first jaw assembly defines slots and the second jaw assembly defines pockets. One or both of the first and second jaw assemblies define recesses. The fasteners are disposed in the slots of the first jaw assembly and are formed by the pockets of the second jaw assembly. The one or more surgical buttresses have a body that includes plugs that are integrally formed with the body for insertion into the recesses to secure the surgical buttress(es) to one or both of the first and second jaw assemblies. The body substantially overlies at least some of the slots of the first jaw assembly and / or at least some of the pockets of the second jaw assembly.
Owner:TYCO HEALTHCARE GRP LP
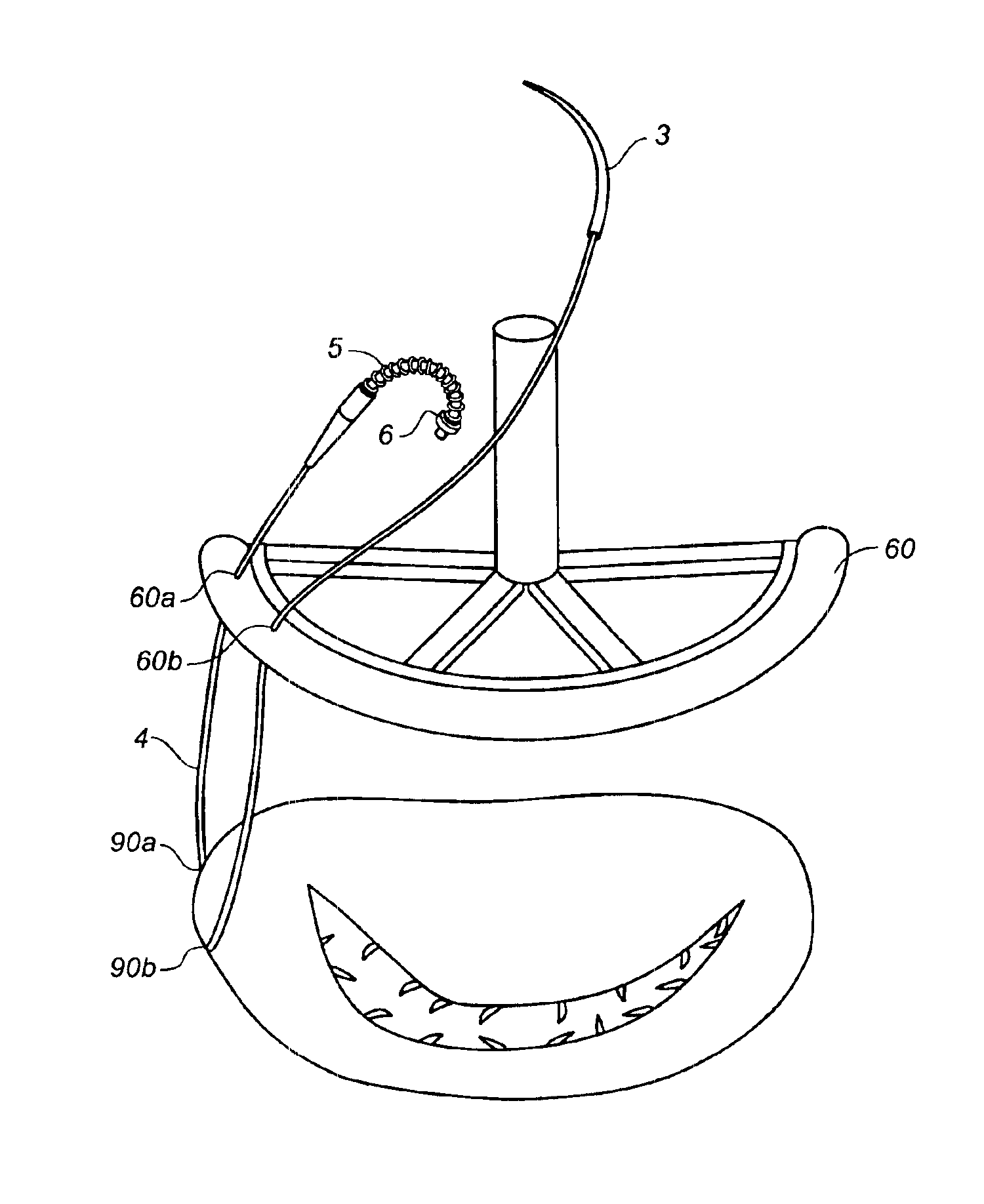
Minimally invasive annuloplasty procedure and apparatus
InactiveUS6918917B1Reduce diameterEffectively useSuture equipmentsSurgical needlesRing annuloplastyBiomedical engineering
Clips of a self-closing type are used in annuloplasty procedures. Each clip is generally U-shaped with two end points separated from each other when it is constrained to be in an open configuration, but tends to coil up to assume its naturally closed configuration if the constraint is removed. A plurality of such clips in open configurations penetrate the tissue around the annulus circumferentially and then the constraint keeping them in the open configuration is removed such that they pull the tissue together between their two end points and this tends to reduce the diameter of the annulus. Such clips may be deployed each in the form of a clip assembly, having at least one of its end points connected to a needle through a flexible member and a release mechanism by which the clip can be easily released. The needle is caused to penetrate the tissue at one position and to come out therefrom at another circumferentially separated positions. Alternatively, a clip delivery device may be used with a plurality of such clips loaded to a clip-holder serving to keep them in open configurations. A pusher pushes the loaded clips out of the device one at a time. Self-closing clips can be used efficiently also in ring annuloplasty and valve replacement procedures.
Owner:MEDTRONIC INC
Minimally invasive annuloplasty procedure and apparatus
InactiveUS6921407B2Reduce the overall diameterFacilitated releaseSuture equipmentsBone implantComing outRing annuloplasty
Clips of a self-closing type are used in annuloplasty procedures. Each clip is generally U-shaped with two end points separated from each other when it is constrained to be in an open configuration, but tends to coil up to assume its naturally closed configuration if the constraint is removed. A plurality of such clips in open configurations penetrate the tissue around the annulus circumferentially and then the constraint keeping them in the open configuration is removed such that they pull the tissue together between their two end points and this tends to reduce the diameter of the annulus. Such clips may be deployed each in the form of a clip assembly, having at least one of its end points connected to a needle through a flexible member and a release mechanism by which the clip can be easily released. The needle is caused to penetrate the tissue at one position and to come out therefrom at another circumferentially separated positions. Alternatively, a clip delivery device may be used with a plurality of such clips loaded to a clip-holder serving to keep them in open configurations. A pusher pushes the loaded clips out of the device one at a time. Self-closing clips can be used efficiently also in ring annuloplasty and valve replacement procedures.
Owner:MEDTRONIC INC
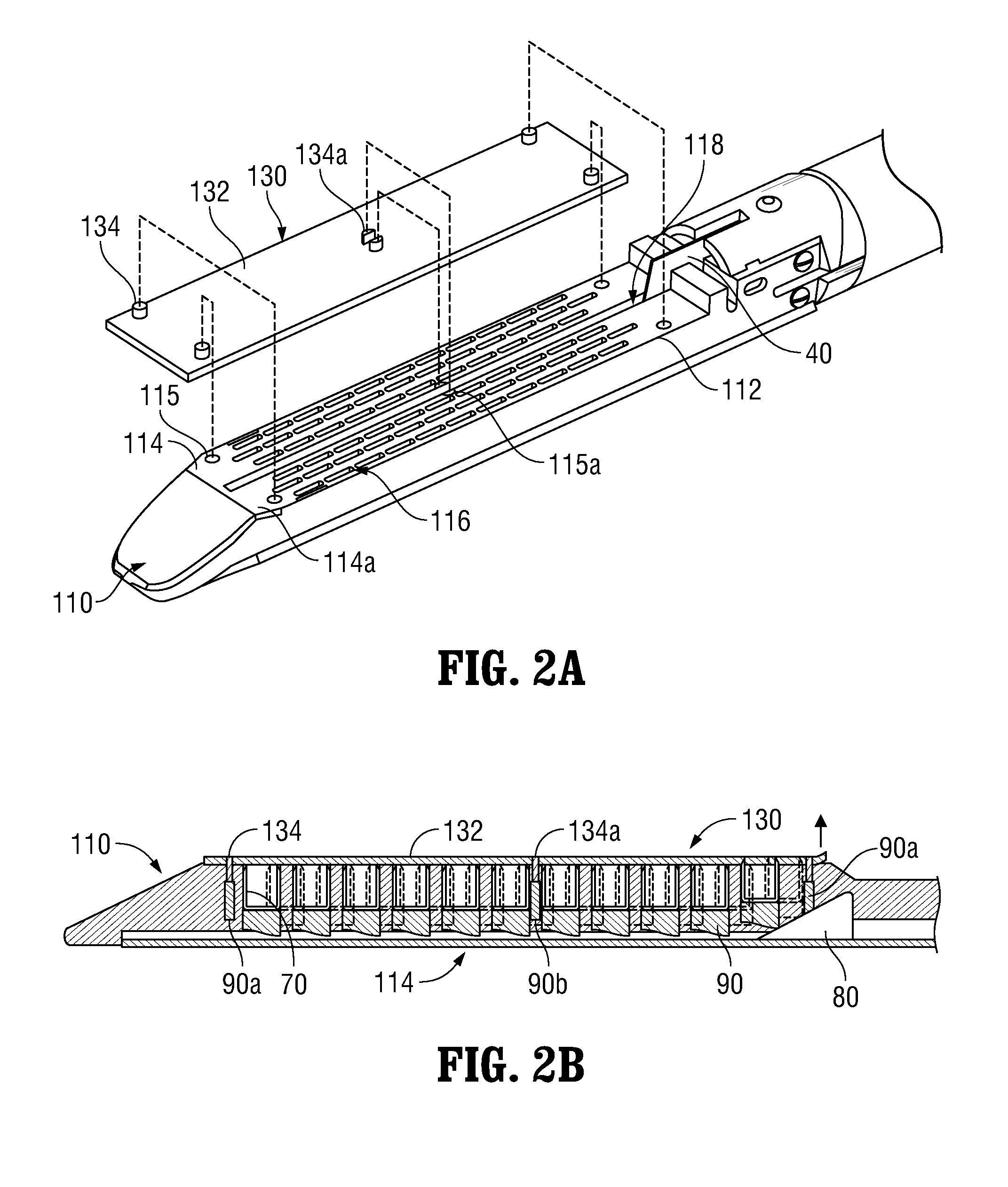
Surgical apparatus including surgical buttress
An end effector for a surgical stapling apparatus is provided which includes an anvil assembly and a surgical buttress. The anvil assembly includes an anvil body and an anvil plate. The anvil plate includes a bottom surface that defines a plurality of staple forming pockets. The anvil body and the anvil plate are selectively connectable. The surgical buttress includes a buttress body and a plurality of arms extending from the body. The arms are disposable between a top surface of the anvil plate and a bottom surface of the anvil body to support the buttress body against the bottom surface of the anvil plate when the anvil plate and the anvil body are connected to one another.
Owner:TYCO HEALTHCARE GRP LP
Surgical device for creating an anastomosis between first and second hollow organs
A surgical device including: a punch slidingly disposed in a housing for forming a hole in a first vessel; a cartridge movably disposed in the housing between a cutting and deploying positions, the cartridge having a second vessel and a coupler for coupling the first and second vessel loaded therein; a punch actuator for sliding the punch between the cutting and deploying positions; a cartridge actuator for moving the cartridge between the cutting and deploying positions, wherein while in the cutting position, the punch and cartridge are in position to permit the punch to form the hole in the first vessel and while in the deploying position, they are in position to deploy the second vessel; and a deployment mechanism for deploying the second vessel and coupler into the hole of the first vessel while the punch and cartridge are in the deploying position to create an anastomosis.
Owner:ETHICON INC
Orientation device for surgical implement
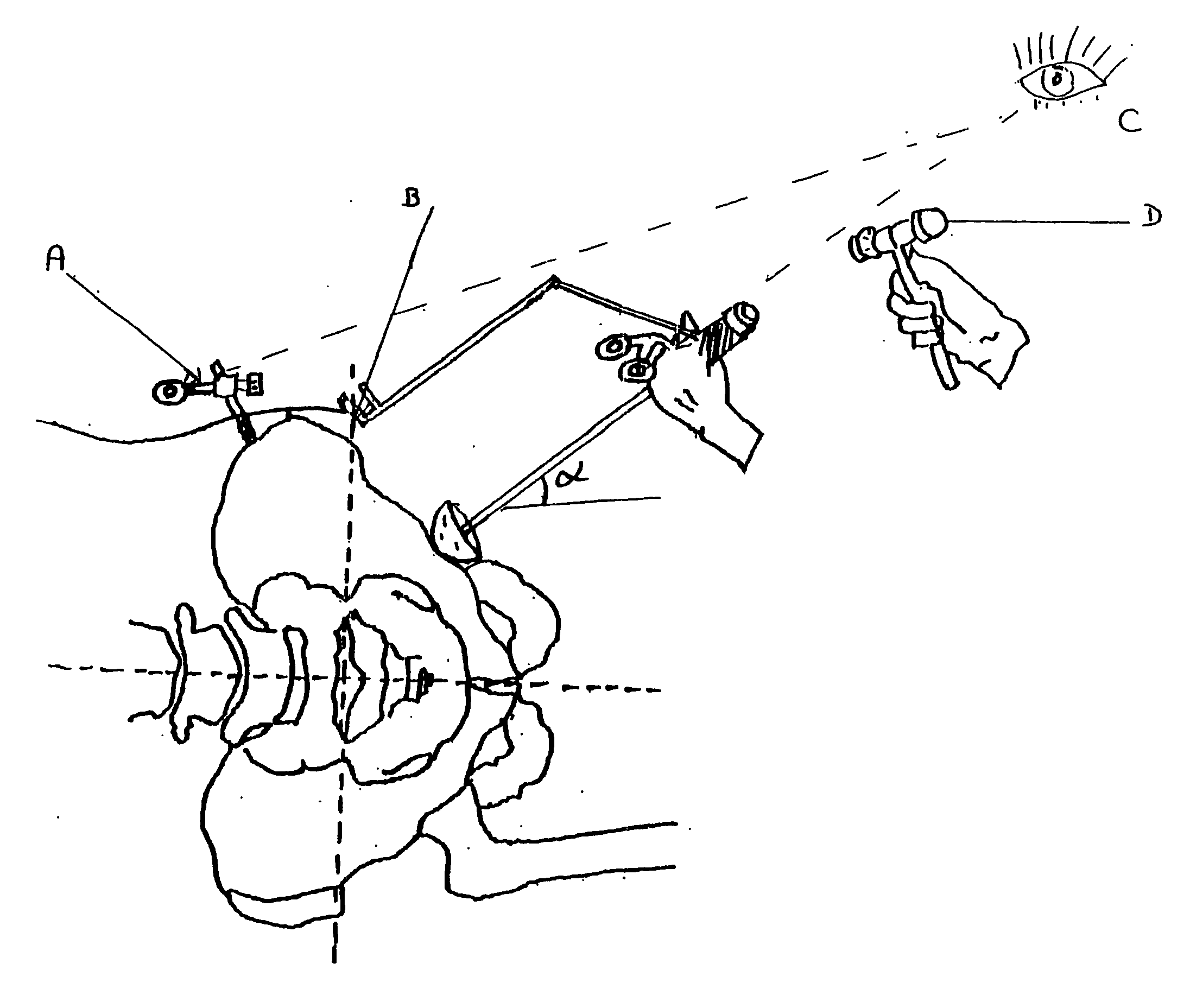
InactiveUS20060184177A1Improve objectivityFacilitates and improves trainingDiagnosticsJoint implantsSurgical operationPhysical medicine and rehabilitation
Orientation device for surgical use includes a frame and a level device attached to the frame. The feature may be oriented with a reference such as the Antero Superior Iliac Spine, the acetabulum or the operating table to position the pelvis or the device. The level device is adapted to define a reference plane. The device thus allows precise orientation with respect to the pelvis through use of a reference plane.
Owner:SAN TECH SURGICAL
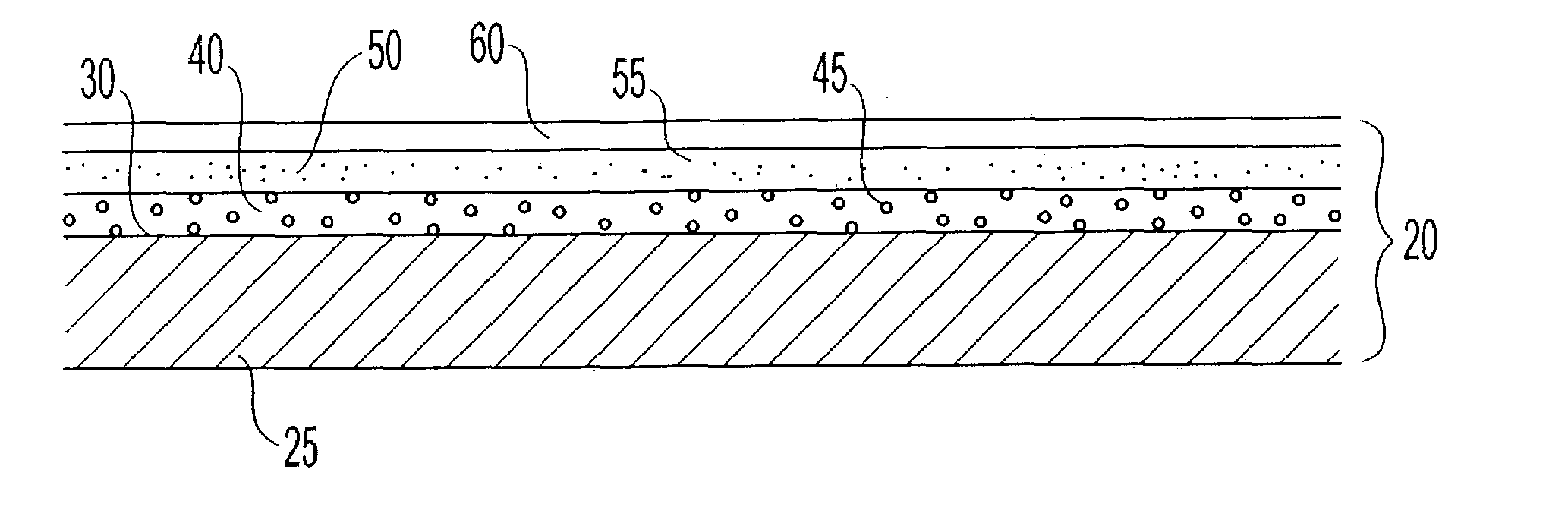
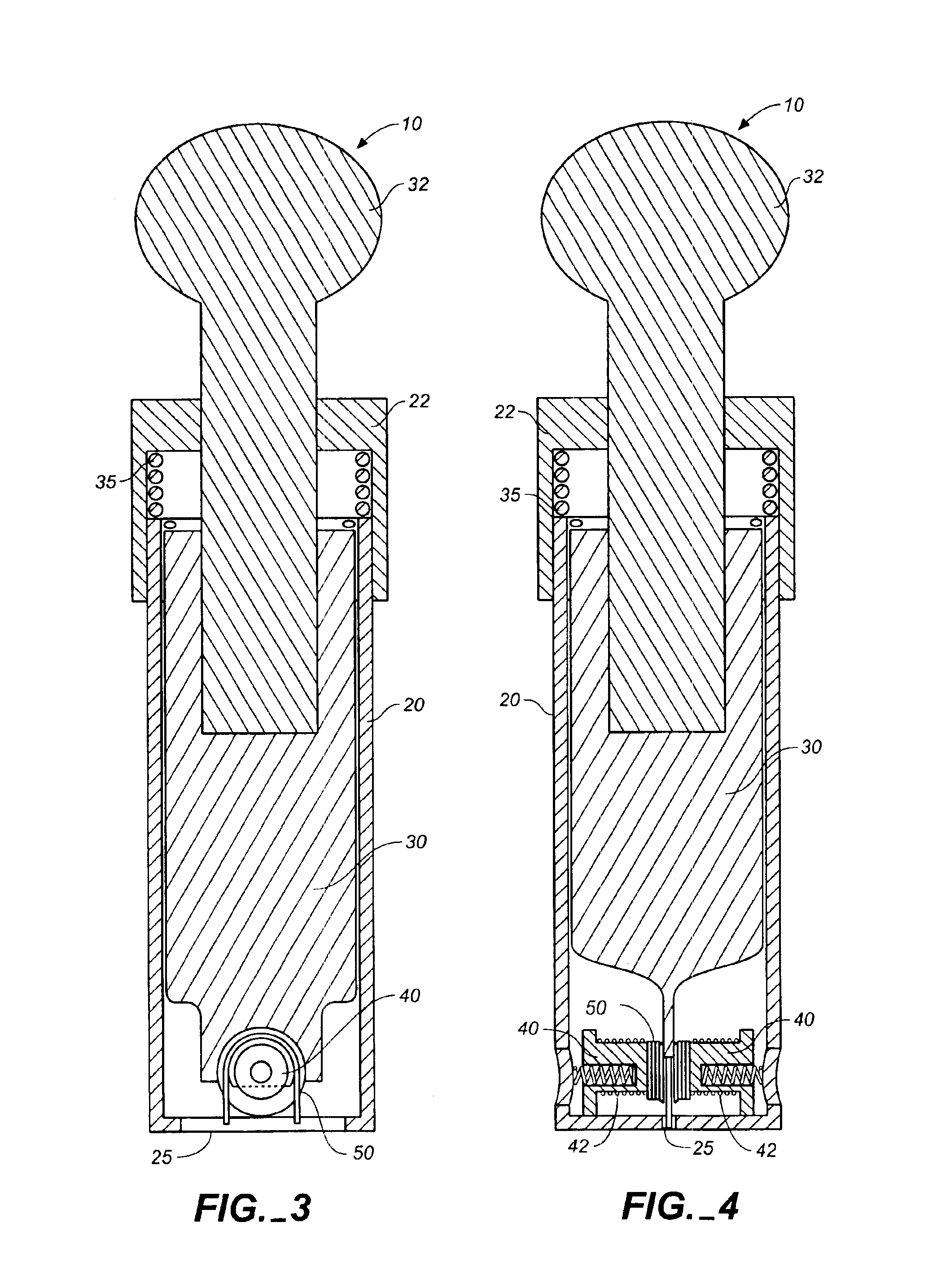
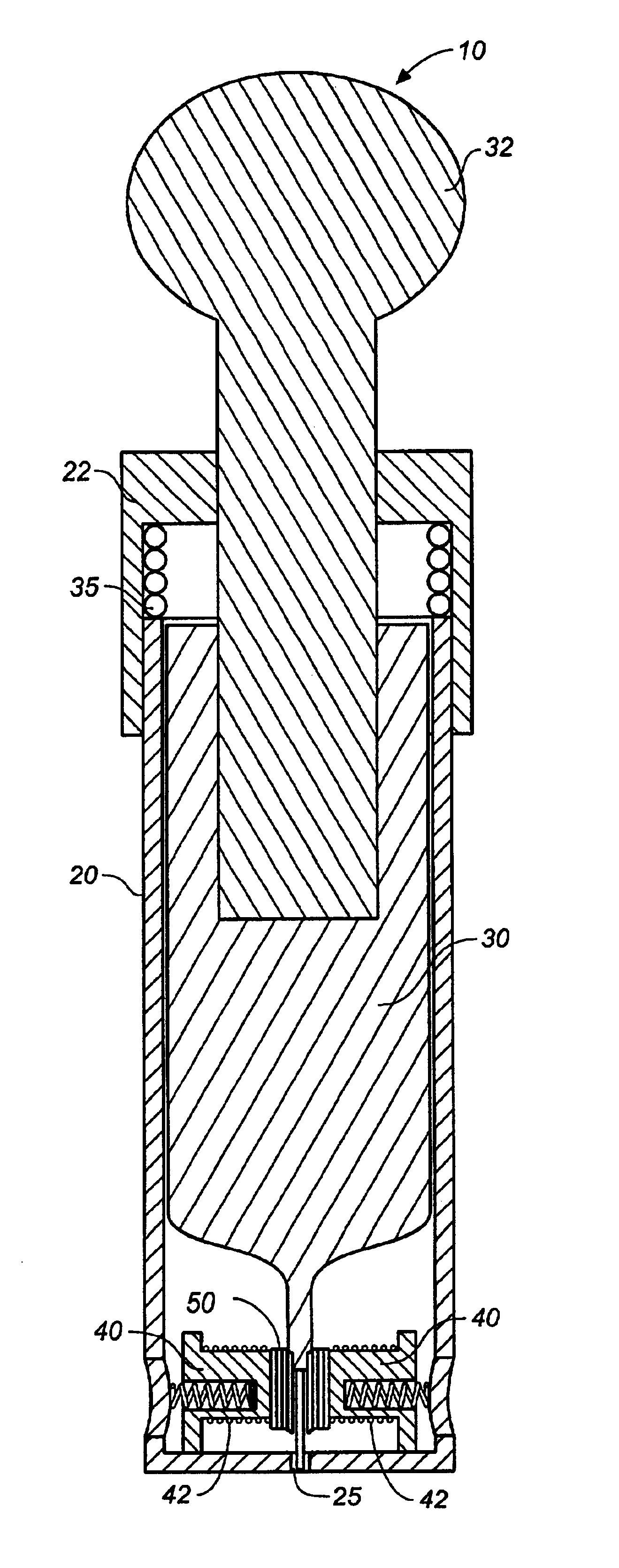
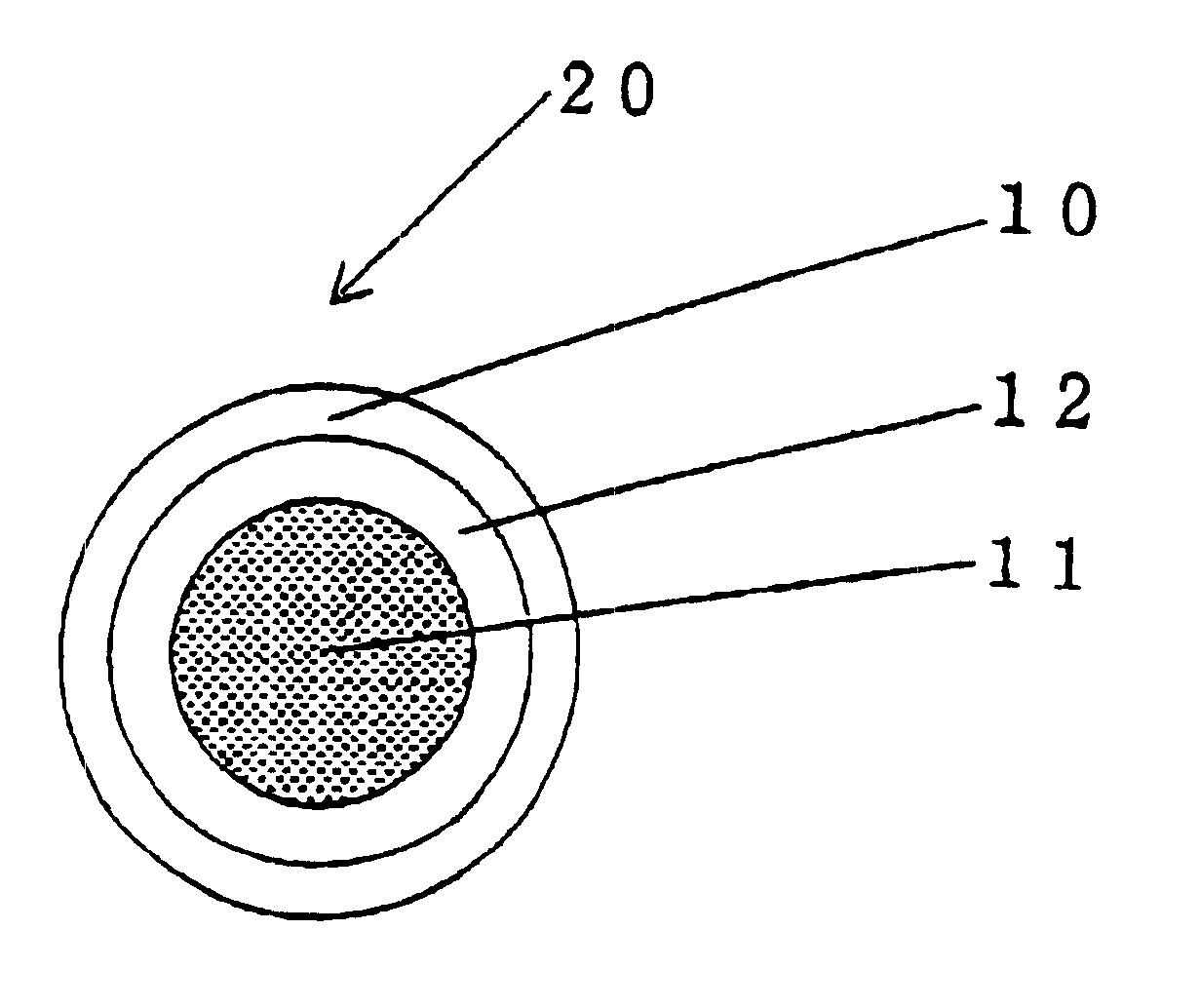
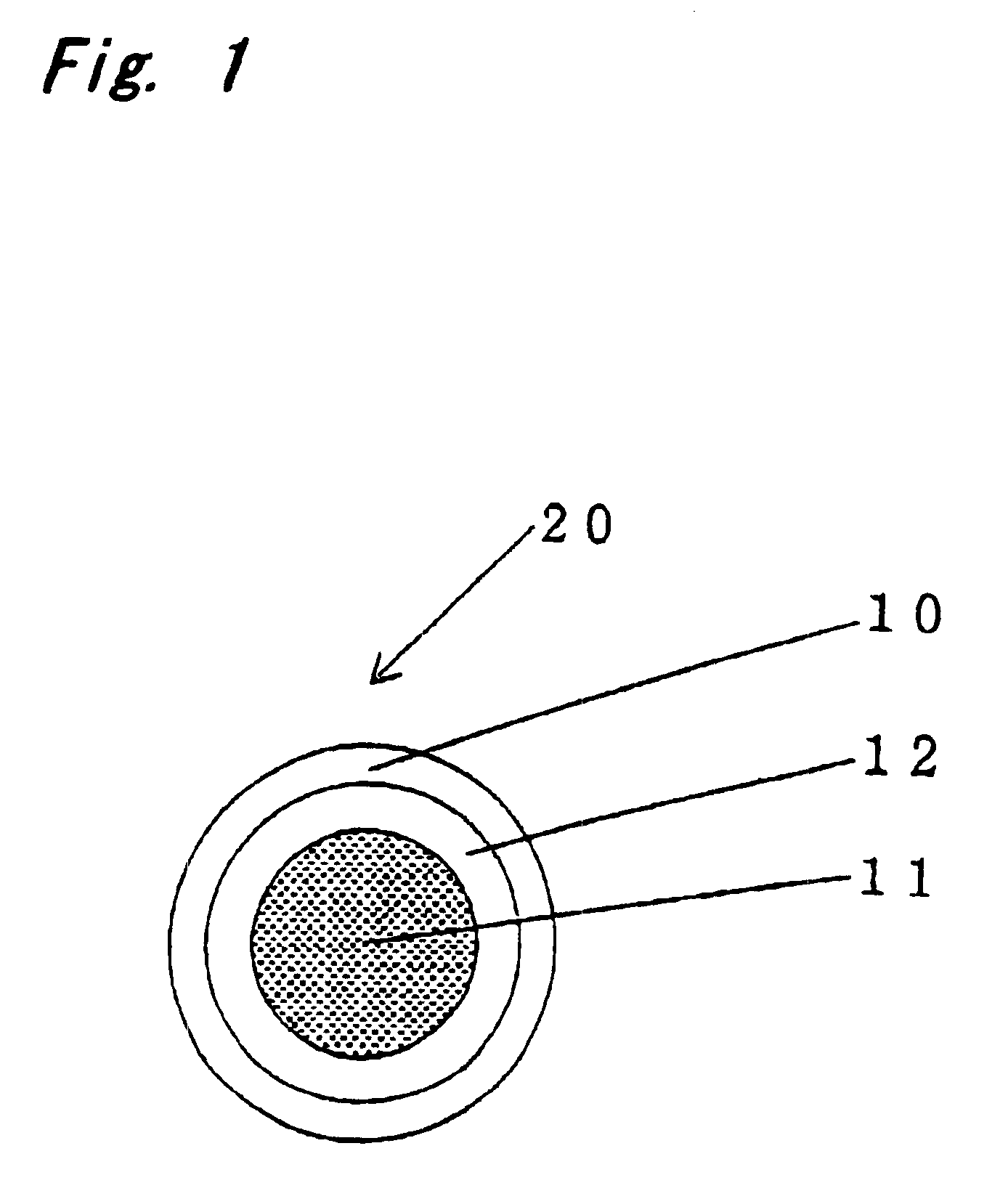
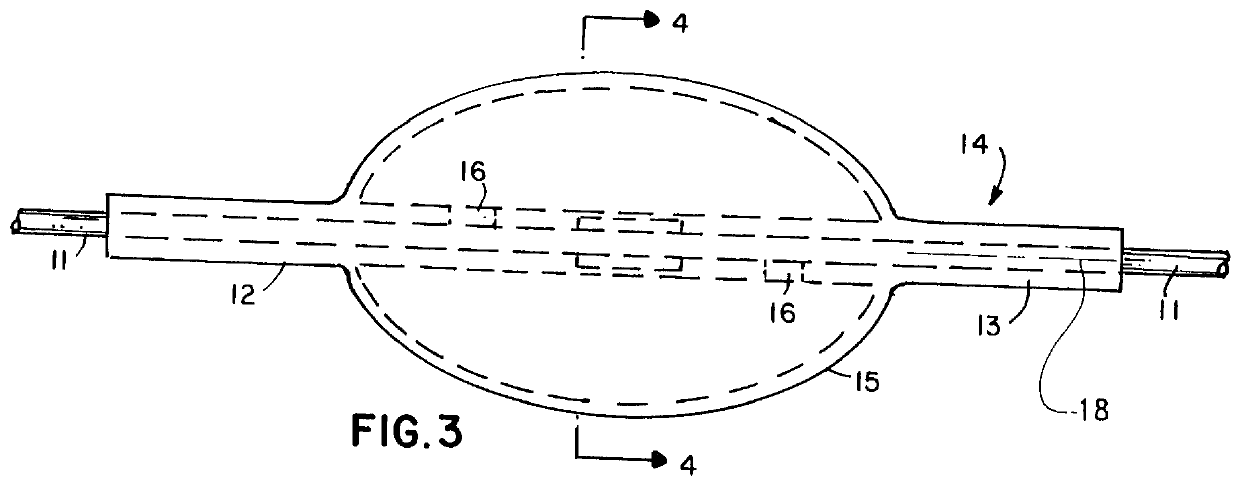
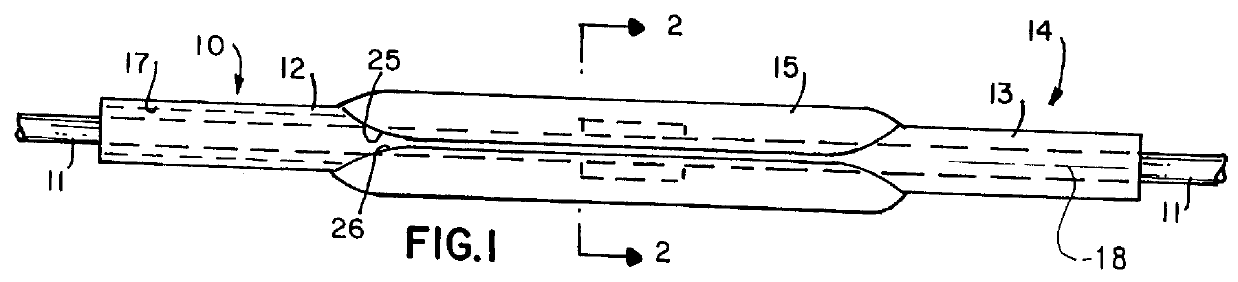
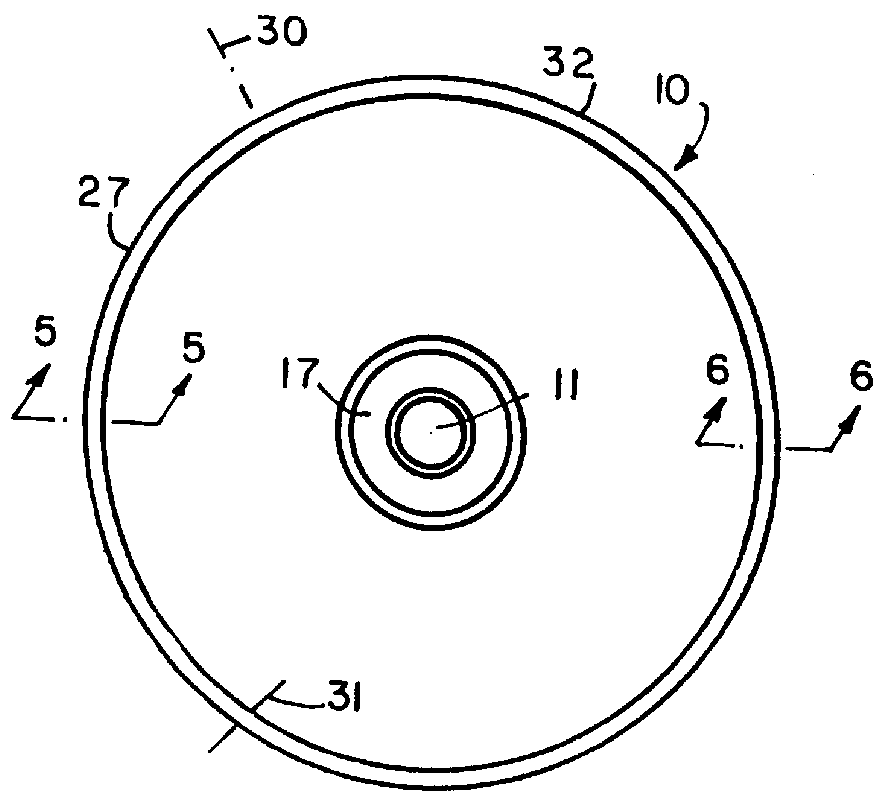
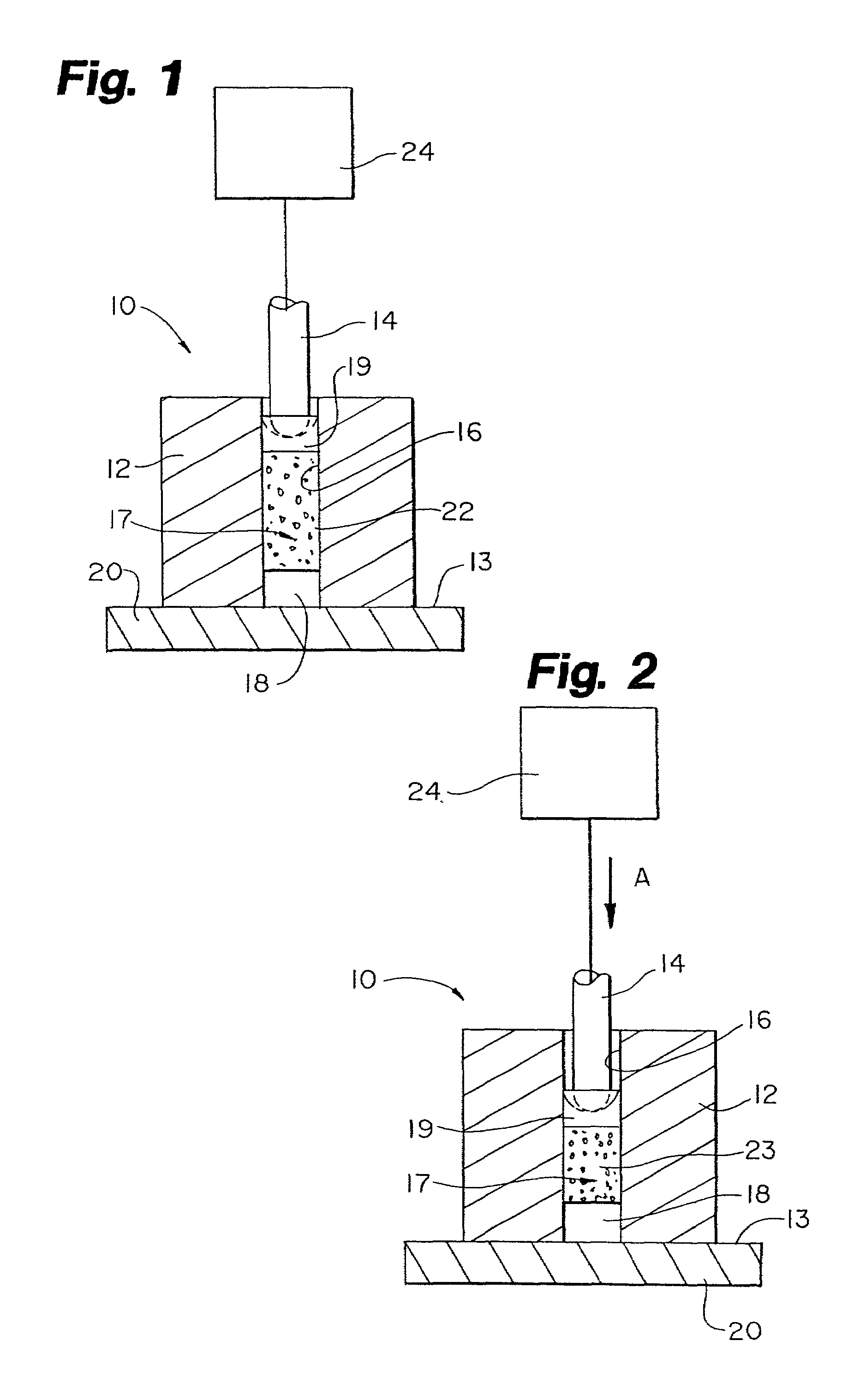
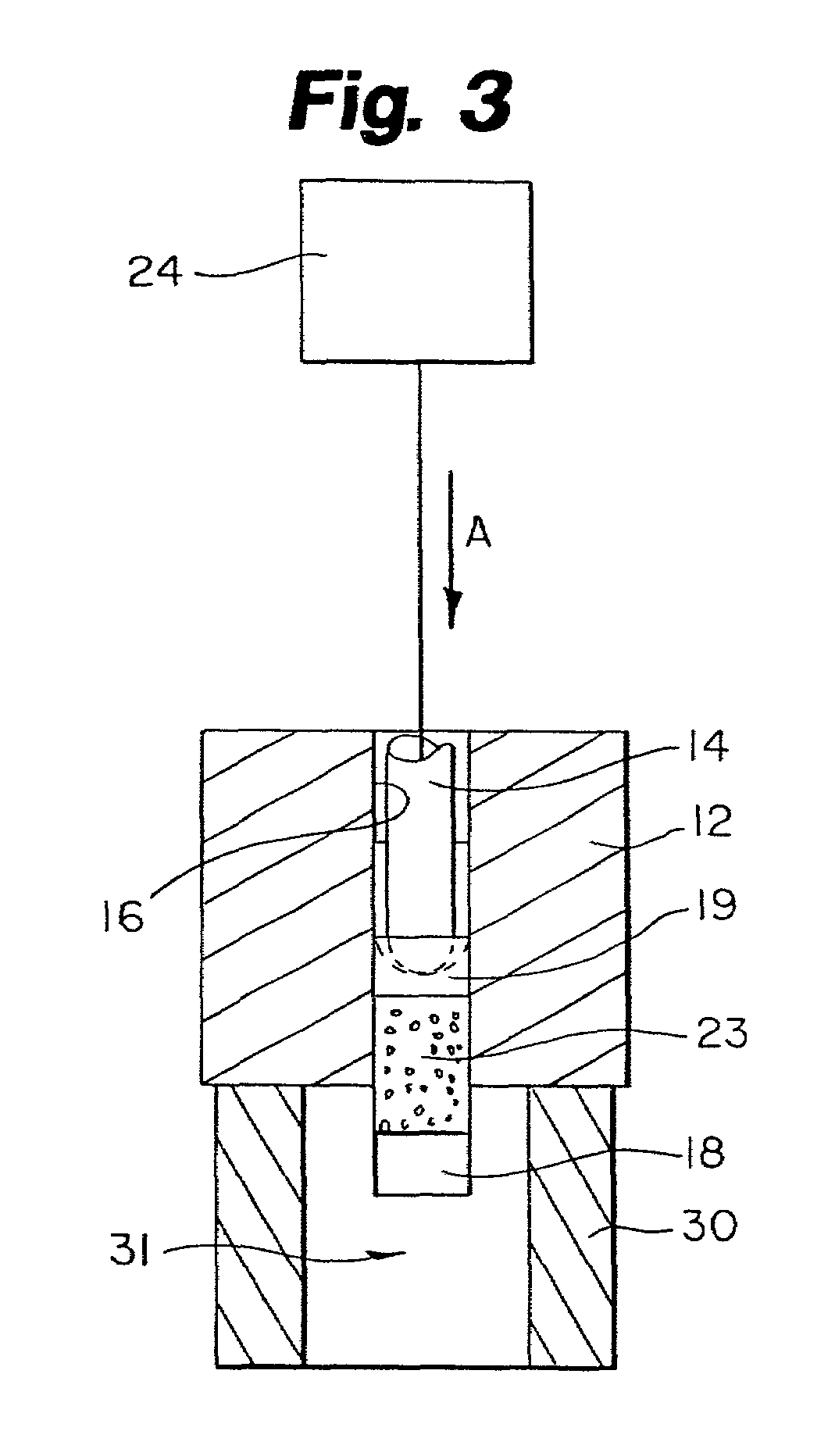
Encapsulated unsaturated fatty acid substance and method for producing the same
InactiveUS6531150B1Improve product qualityInhibit oxygen-permeabilityPowder deliveryGranular deliveryWater solubleGelatin
The present invention relates to an encapsulated unsaturated fatty acid substance in a form of a three-layered capsule, comprising an unsaturated fatty acid or a derivative thereof (11) as a content and a coating layer (10) mainly containing gelatin, encapsulating the content (11), wherein a water-soluble gel layer (12) containing an acid or an acid salt thereof is present between the coating layer (10) and the content (11). The encapsulated unsaturated fatty acid substance of the present invention is characterized by that it has neither insolubility nor deterioration with time, and that it is enteric.
Owner:MORISHITA JINTAN CO LTD
Balloon catheter
InactiveUS6010480AImprovement factorReduce coefficient of frictionStentsBalloon catheterBalloon catheterLesion
An expansible balloon catheter has at least a first exterior surface with a given coefficient of friction and a second exterior surface with a greater coefficient of friction. In a compact form only the first exterior surface is exposed to produce one coefficient of friction during transfer of the collapsed or uninflated balloon to and across a lesion. When inflated, the second surface dominates the first surface and produces a second coefficient of friction.
Owner:BOSTON SCI SCIMED INC
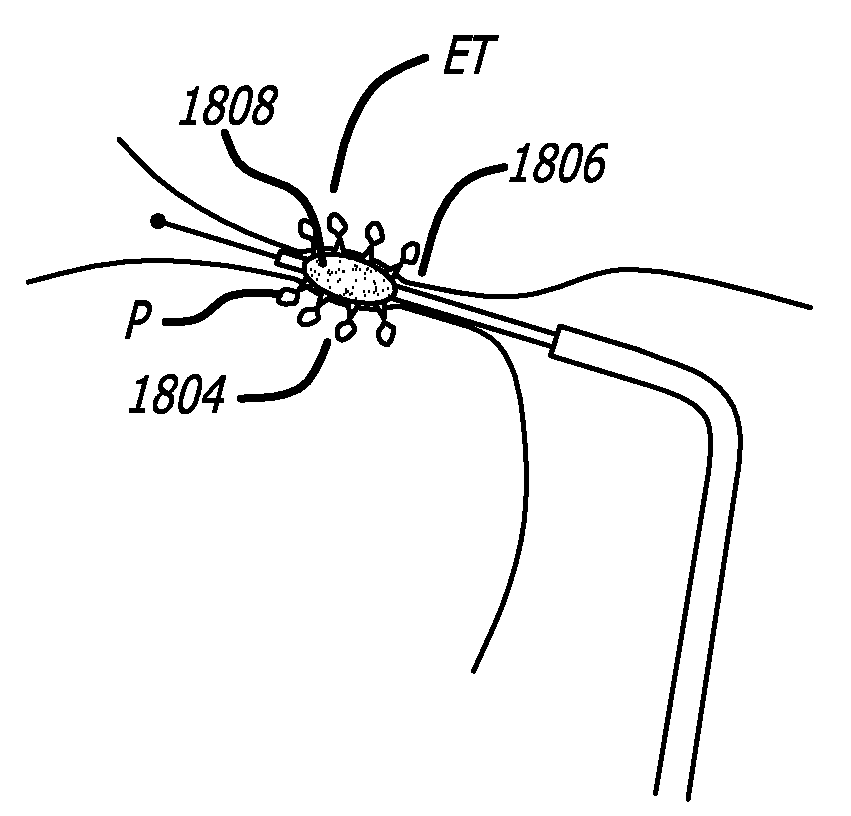
Method and system for treating target tissue within the eustachian tube
Methods and systems for accessing a Eustachian tube of a patient are disclosed. The system includes a guide configured for passing into a nasal passage of the patient to position a distal tip of the catheter at or near a Eustachian tube, the guide having a distal tip with a bend having an angle between 30 and 90 degrees; and a guidewire configured to pass through the guide into the Eustachian tube. A device for providing therapy to the Eustachian tube is passed through the guide.
Owner:ACCLARENT INC
Foam carrier containing amphiphilic copolymeric gelling agent
InactiveUS20050069566A1Broaden applicationEvenly distributedAntibacterial agentsCosmetic preparationsAlcohol freeWater soluble
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier including water, a hydrophobic solvent, a surface-active agent and a gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Endoscopic clip applying apparatus with improved aperture for clip release and related method
ActiveUS20050171560A1Increase widthFacilitated releaseSurgical forcepsWound clampsEndoscopic clippingSacroiliac joint
An apparatus for applying surgical clips includes a jaw assembly and a jaw opening member. The jaw assembly includes first and second opposing pivotable jaw members that define a variable-width jaw aperture therebetween for receiving a clip. The jaw opening member is movable into engagement with the first and second jaw members for increasing the width of the jaw aperture, thereby improving the release of a clip from the jaw assembly.
Owner:TELEFLEX MEDICAL INC
Protein matrix materials, devices and methods of making and using thereof
InactiveUS7662409B2Enhances strength and durabilityFacilitated releaseBiocidePowder deliveryActive agentProtein materials
The present invention relates to protein matrix materials and devices and the methods of making and using protein matrix materials and devices. More specifically the present invention relates to protein matrix materials and devices that may be utilized for various medical applications including, but not limited to, drug delivery devices for the controlled release of pharmacologically active agents, encapsulated or coated stent devices, vessels, tubular grafts, vascular grafts, wound healing devices including protein matrix suture material and meshes, skin / bone / tissue grafts, biocompatible electricity conducting matrices, clear protein matrices, protein matrix adhesion prevention barriers, cell scaffolding and other biocompatible protein matrix devices. Furthermore, the present invention relates to protein matrix materials and devices made by forming a film comprising one or more biodegradable protein materials, one or more biocompatible solvents and optionally one or more pharmacologically active agents. The film is then partially dried, rolled or otherwise shaped, and then compressed to form the desired protein matrix device.
Owner:PETVIVO HLDG INC
Insertable medical devices having microparticulate-associated elastic substrates and methods for drug delivery
ActiveUS20090246252A1Optimize quantityReduce lossesBiocideOrganic active ingredientsDrug deliveryElastic substrate
The present invention provides insertable medical devices having elastic surfaces associated with bioactive agent-containing microparticulates and a coating material. Upon expansion of the elastic surfaces the microparticulates can be released to a subject.
Owner:SURMODICS INC
Method for locking handle deployment mechanism with medical device
InactiveUS7381216B2Easy and accurate deployment and positioningFacilitated releaseStentsEar treatmentLocking mechanismEngineering
Methods for delivering at least one medical device to a desired location for treatment, and then selectively deploy it in position, include an improved handle. One of the possible features of the handle may be to selectively hold the delivery system components at any desired configuration during deployment and positioning of the medical device. Another possible feature of the handle may be more than one mode of operation, in which the deployment of the medical device can selectively proceed at more than one speed. Yet another possible feature of the handle may be a locking mechanism that resists inadvertent or accidental movement or retraction of the stent delivery system components during packaging, sterilization, shipping, storage, handling and preparation of the stent delivery system.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
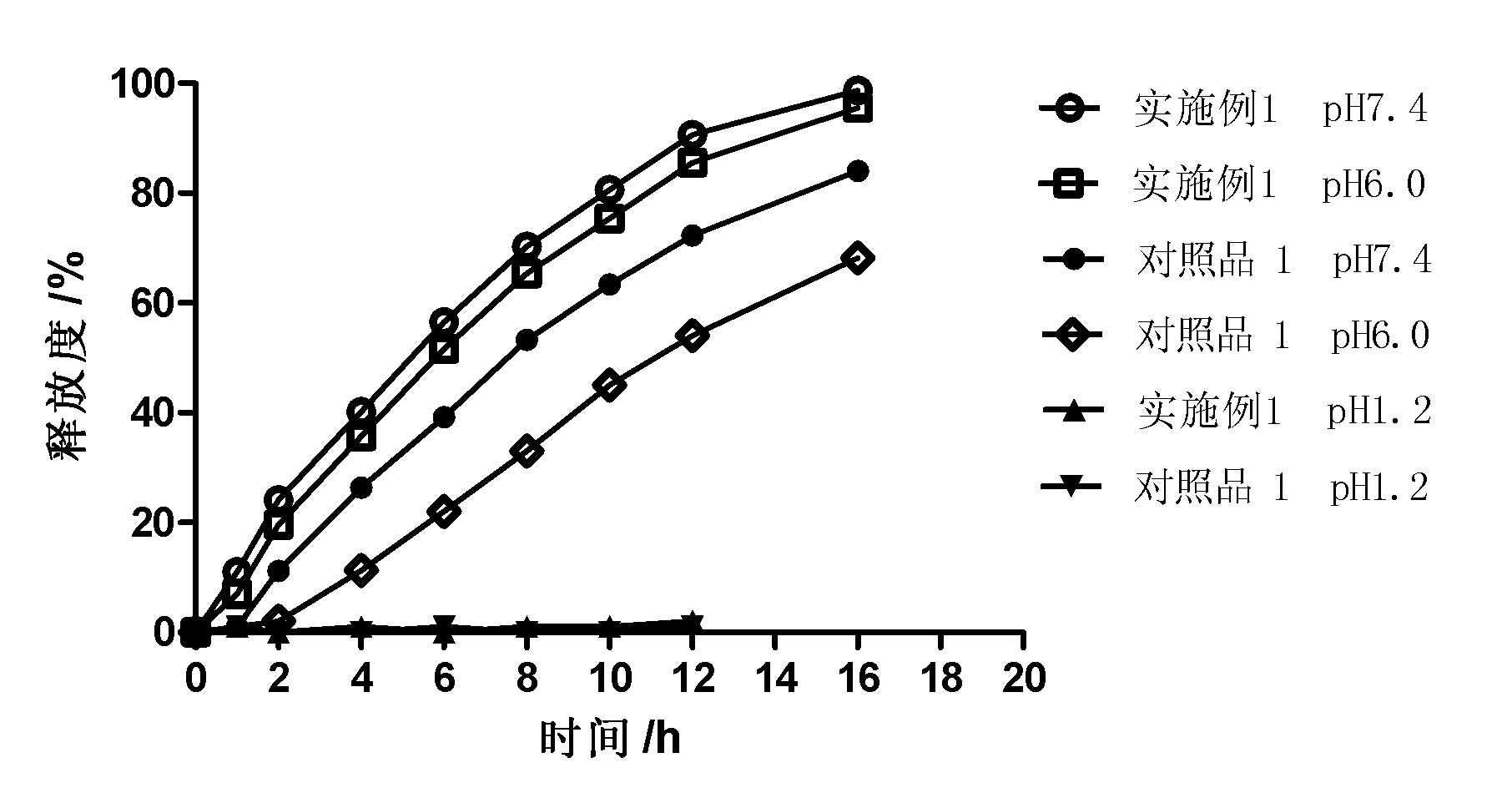
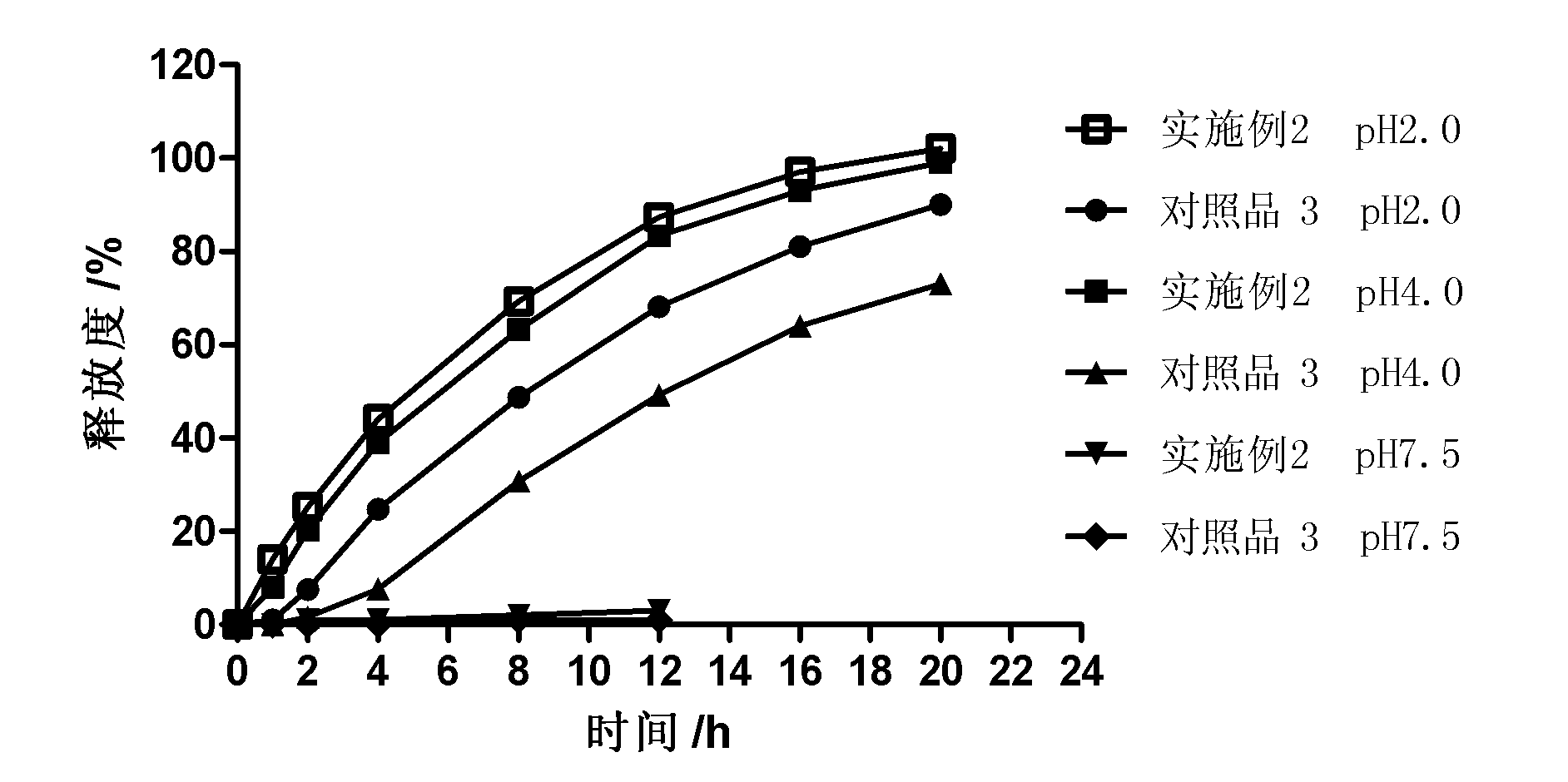
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Low pressure, extended coverage, upright fire protection sprinkler
InactiveUS6976543B1Increased heat release rateDeplete supplySpray nozzlesFire rescueExtended coverageFire protection
A low pressure, extended coverage, fire protection sprinkler, e.g., of the upright type, suitable for use in protection of at least extra hazard and high piled storage occupancies, in accordance with the 1999 Edition of NFPA 13, has a body with an internal passageway extending between an inlet end and an opposite outlet end, and a deflector mounted to the body by at least one support arm and disposed in alignment with the axis and generally spaced from the outlet end of the internal passageway. The sprinkler has a predetermined K-factor, e.g., of greater than about 16.0. The sprinkler is configured and arranged to deflect flow of water generally radially outwardly and downwardly of the sprinkler in a predetermined spray pattern. Preferably, the predetermined spray pattern has a generally polygonal shape, e.g., a rectangular shape, when viewed at a predetermined distance below the deflector.
Owner:TYCO FIRE PRODS LP
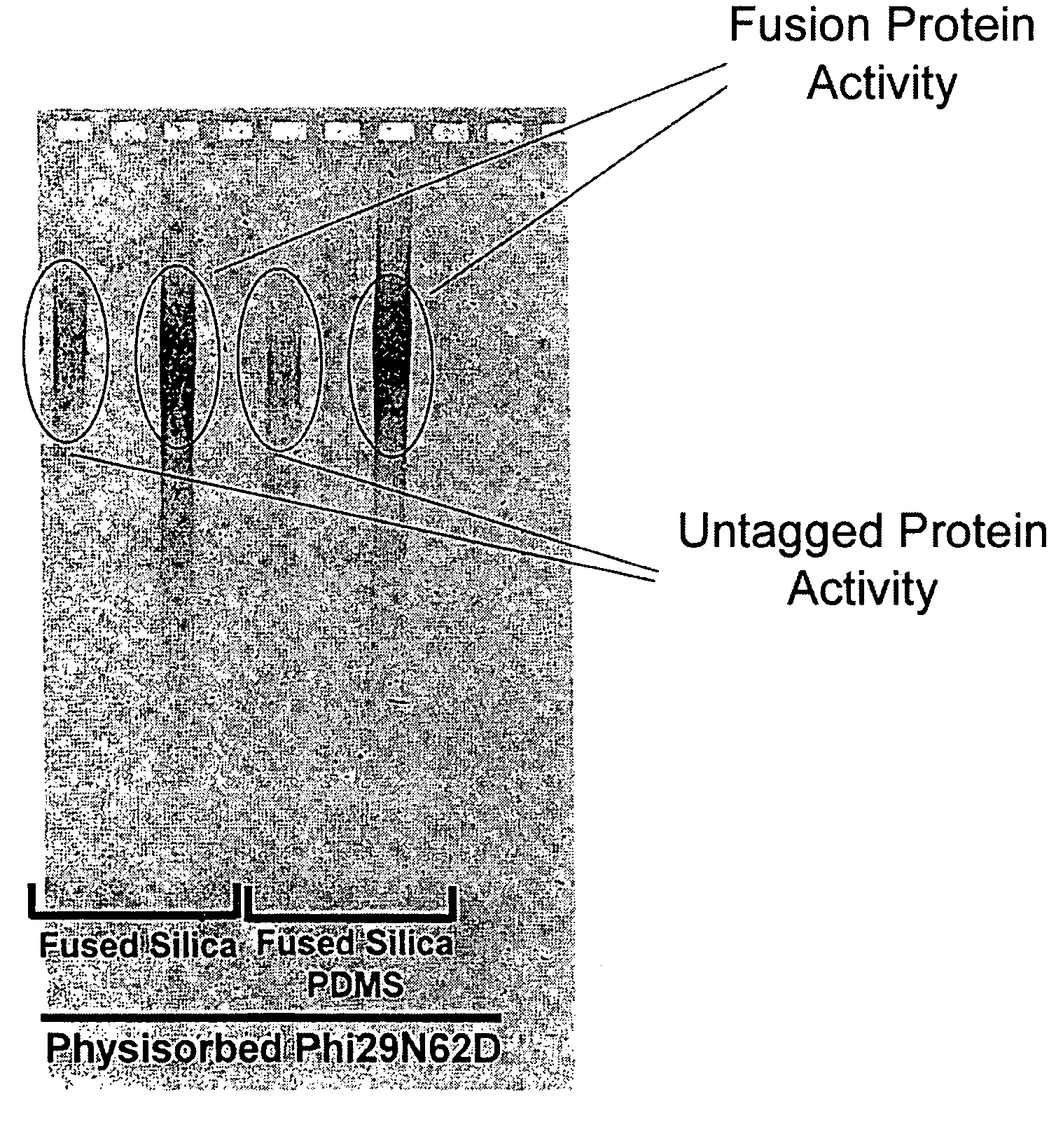
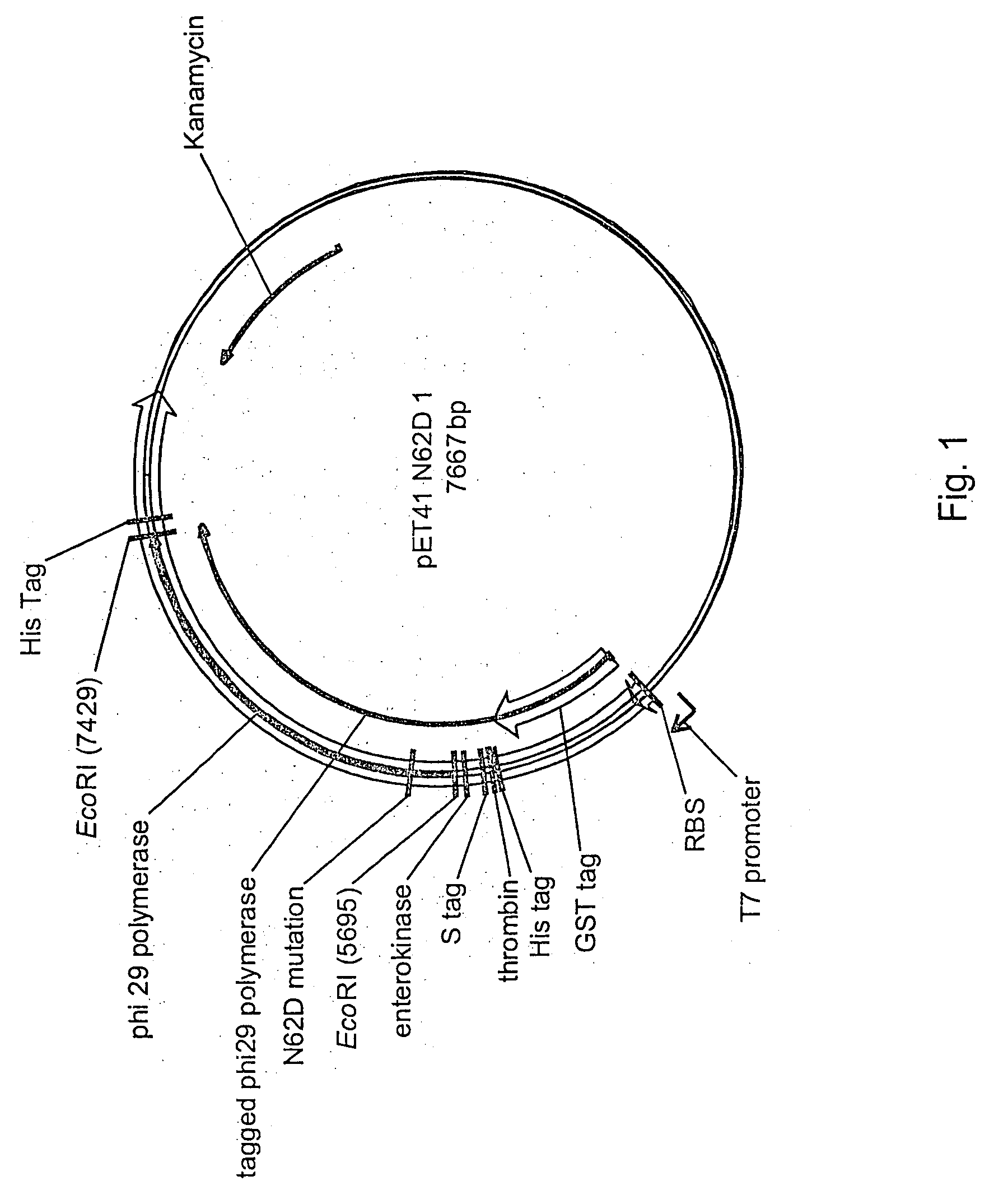
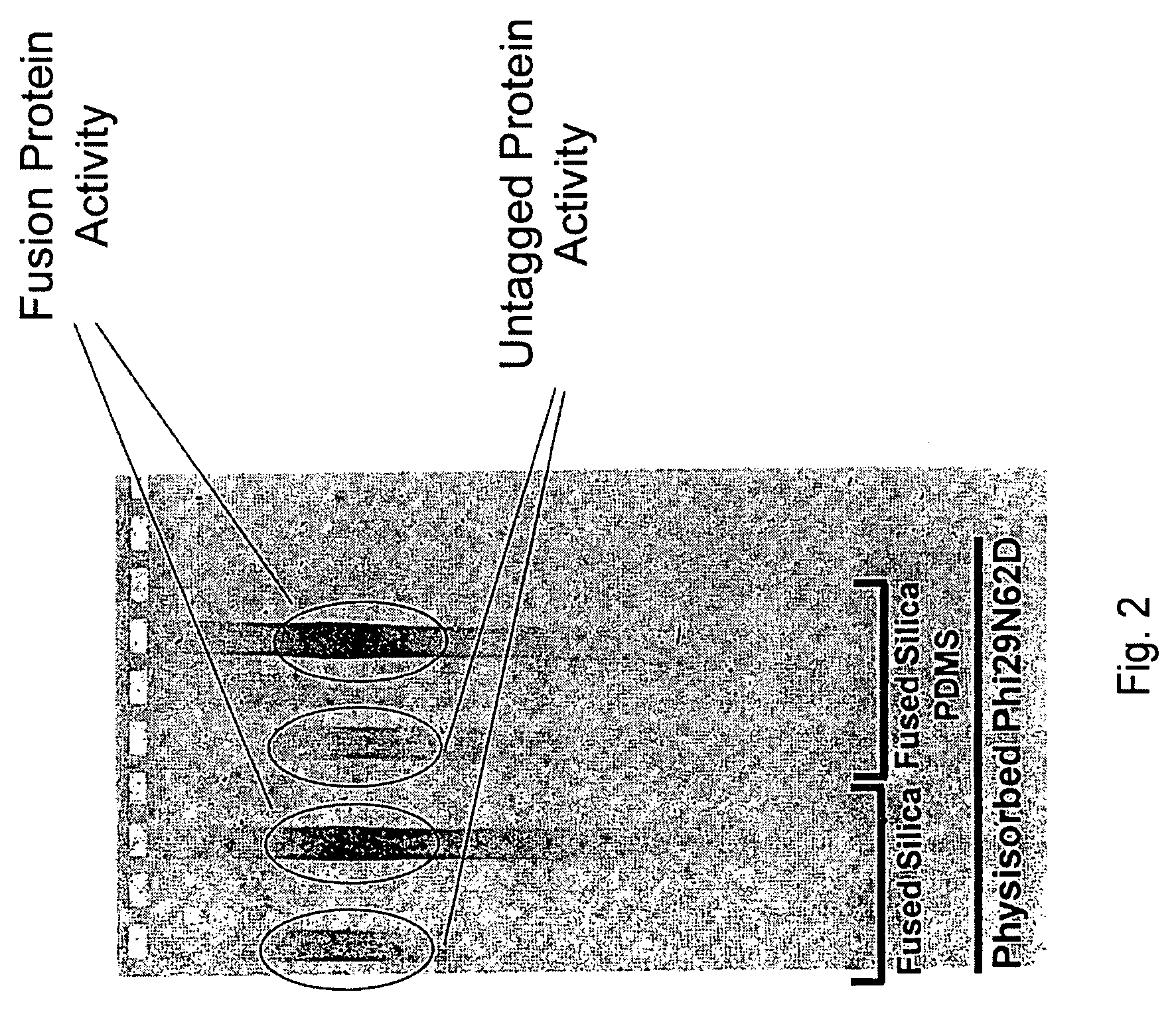
Active surface coupled polymerases
ActiveUS20080199932A1Without substantial loss of activityHigh binding affinityMicrobiological testing/measurementOn/in organic carrierPolymerase LBiomedical engineering
Active surface coupled polymerases, surfaces that include such polymerases, and methods of making and using surface-attached polymerases are provided.
Owner:PACIFIC BIOSCIENCES
Methods and devices for controlling biologic microenvironments
InactiveUS20080086072A1Enhance and improve effectImprove interventionElectrotherapySurgical furnitureBiological bodyMoisture
A microenvironment of a biological body is controlled, and more particularly, is measured, changed, and monitored with respect to temperature, pH level, moisture and other tissue parameters of a region of the body while, optionally, administering a therapeutic agent to that region.
Owner:P TECH
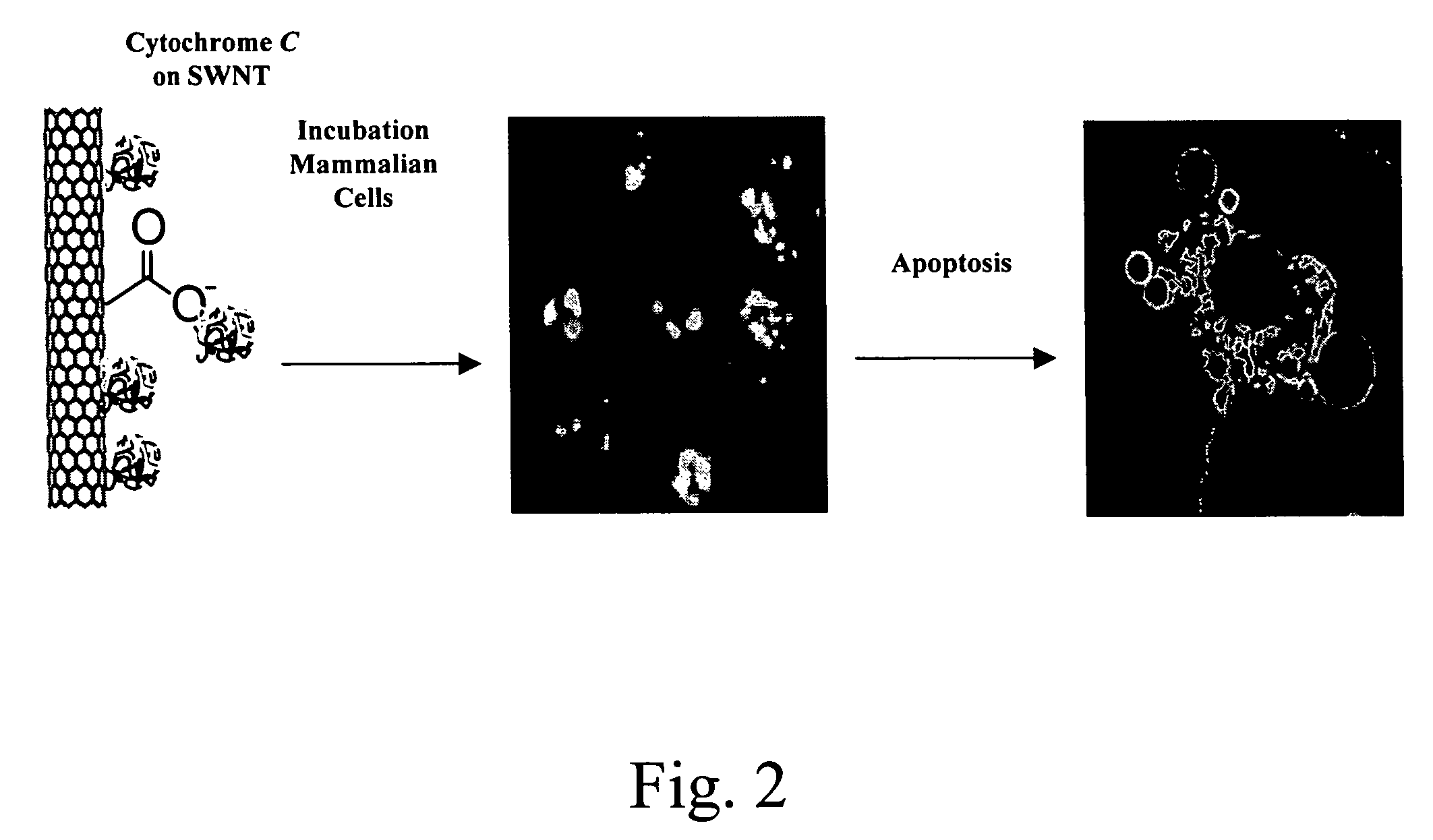
Hydrophobic nanotubes and nanoparticles as transporters for the delivery of drugs into cells
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Non-stochastic generation of genetic vaccines
InactiveUS6479258B1Improve abilitiesPotent and direct effect on proliferation and Ig-synthesisHydrolasesLibrary screeningAntigenMutagenic Process
This invention provides methods of obtaining vaccines by use of non-stochastic methods of directed evolution (DirectEvolution(TM)). These methods include non-stochastic polynucleotide site-satuaration mutagenesis (Gene Site Saturation Mutagenesis(TM)) and non-stochastic polynucleotide reassembly (GeneReassembly(TM)). Through use of the claimed methods, vectors can be obtained which exhibit increased efficacy for use as genetic vaccines. Vectors obtained by using the methods can have, for example, enhanced antigen expression, increased uptake into a cell, increased stability in a cell, ability to tailor an immune response, and the like.
Owner:VERENIUM CORPORATION
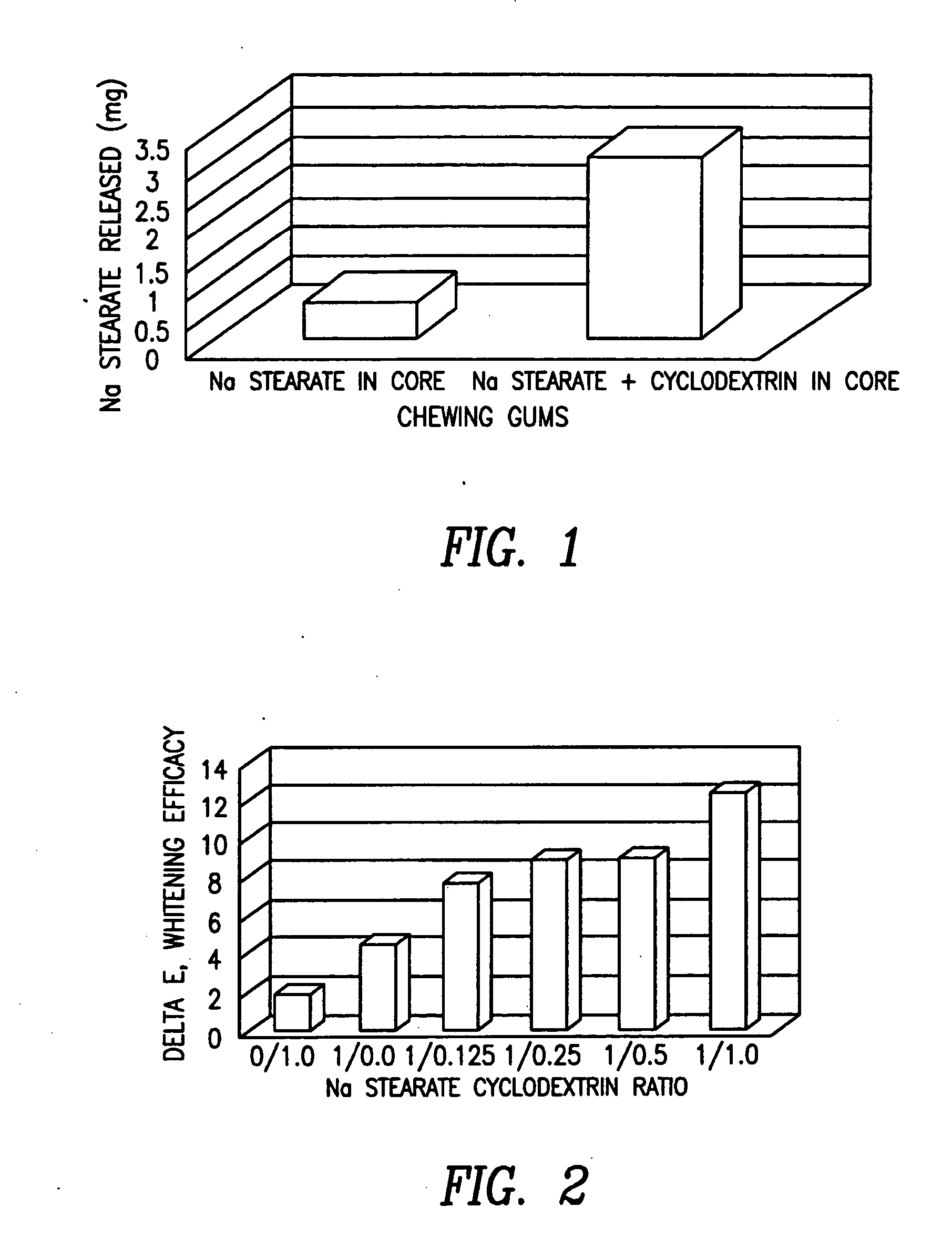
Chewing gum and confectionery compositions containing a stain removing complex, and methods of making and using the same
InactiveUS20050008732A1Enhancing release and deliveryMaintaining desirable organoleptic and taste propertyContainers for annular articlesConfectioneryChewing gumCyclodextrin
A composition in the form of a chewing gum composition or a confectionery composition containing a stain removing complex of a stain removing agent and a cyclodextrin compound and methods of preparing and using the same to remove stains from dental material including teeth.
Owner:KRAFT FOODS GLOBAL BRANDS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com