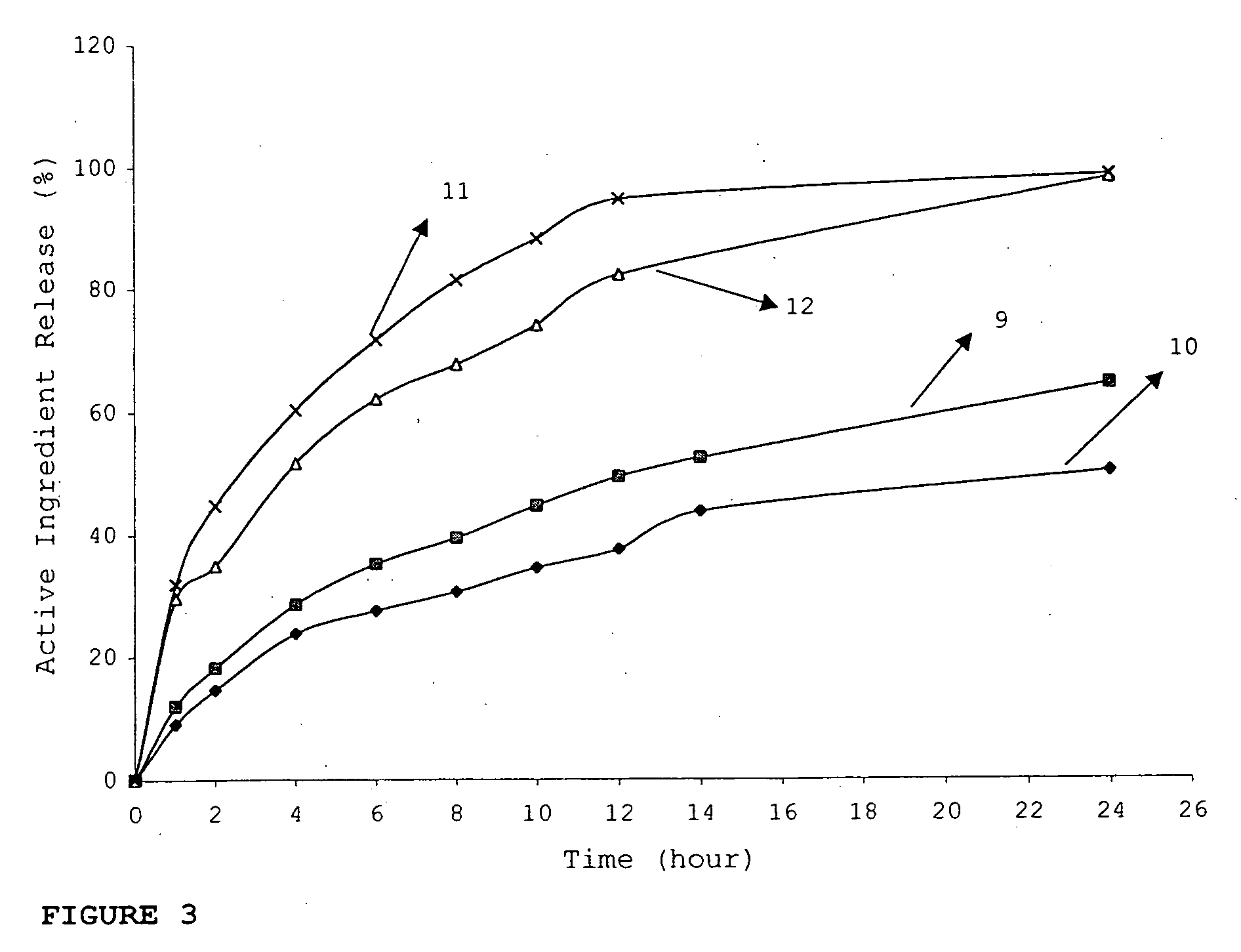
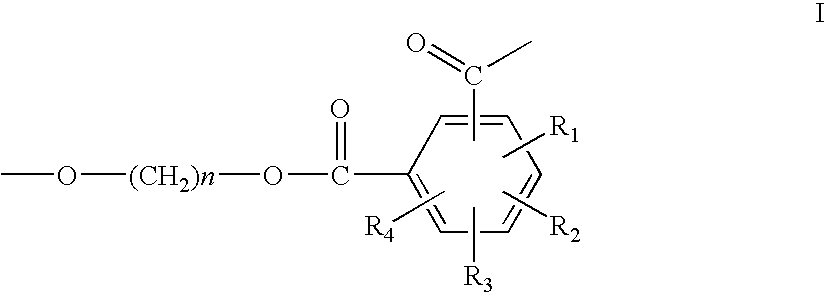
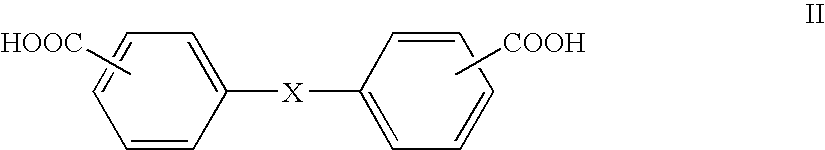
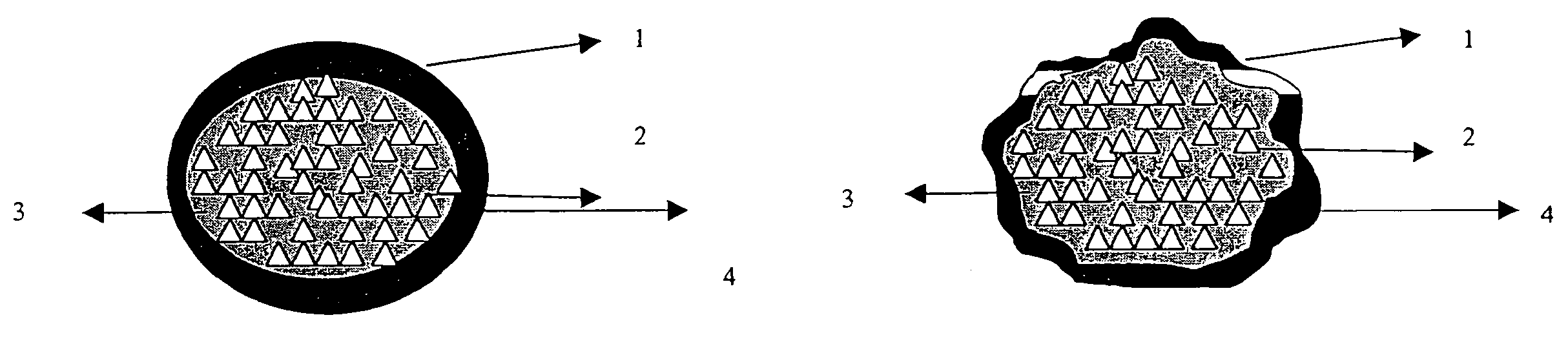
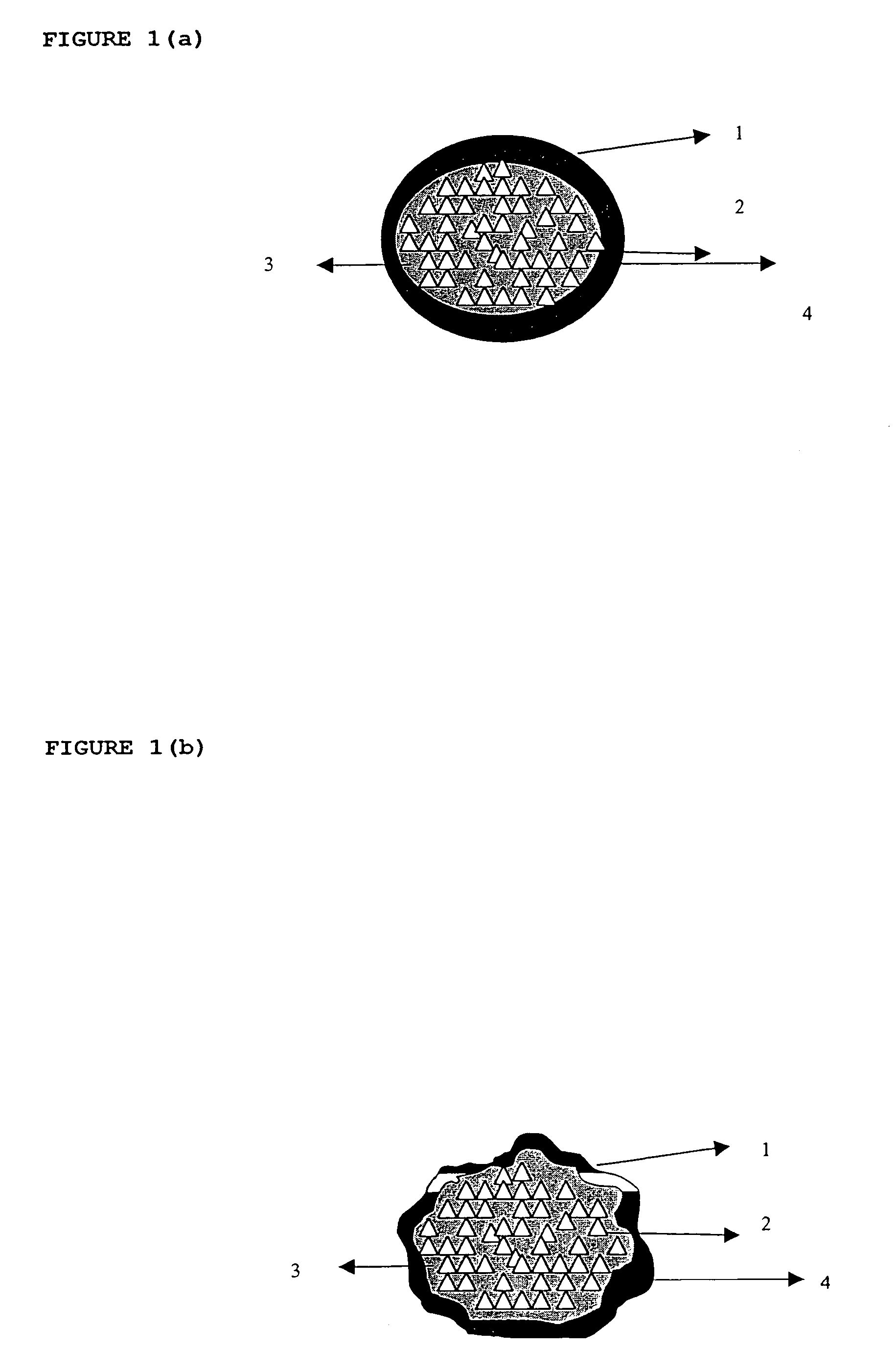
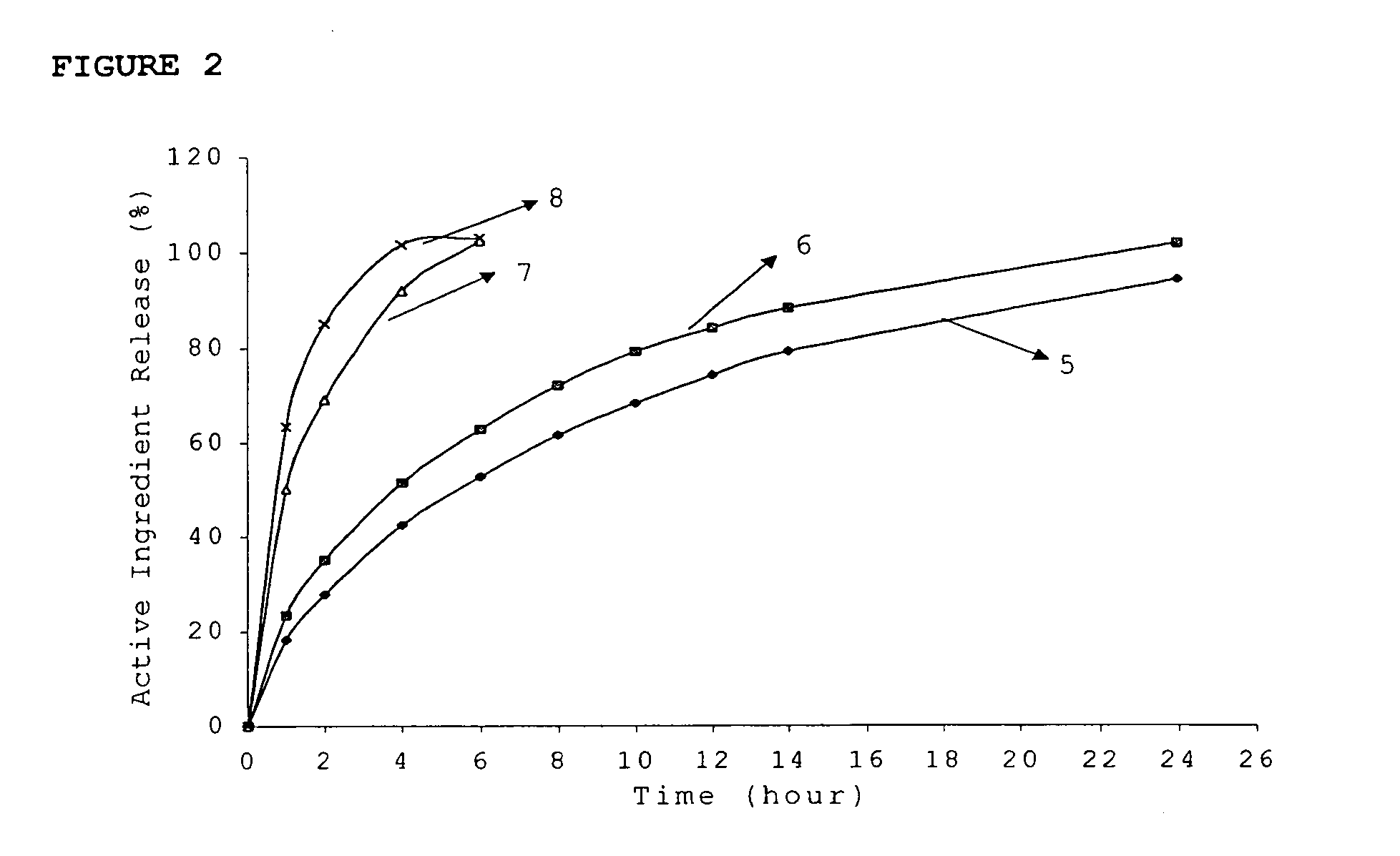
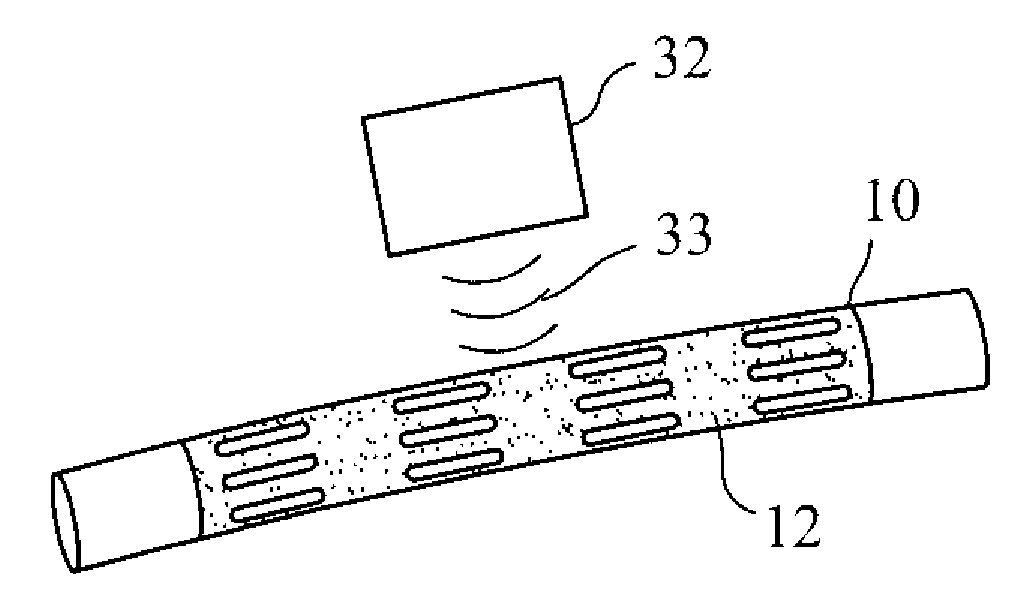
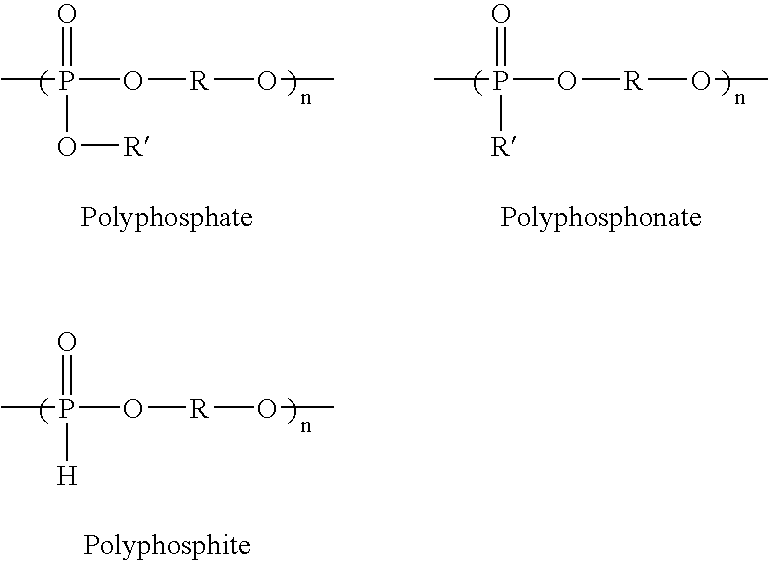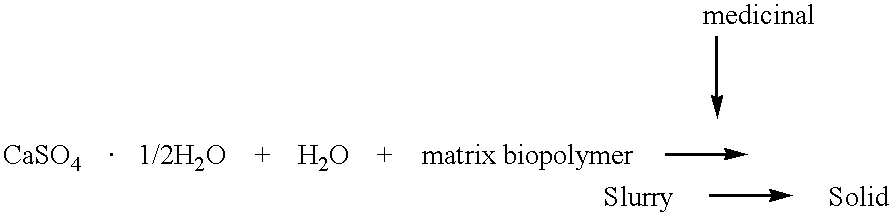Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1702results about How to "Control release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
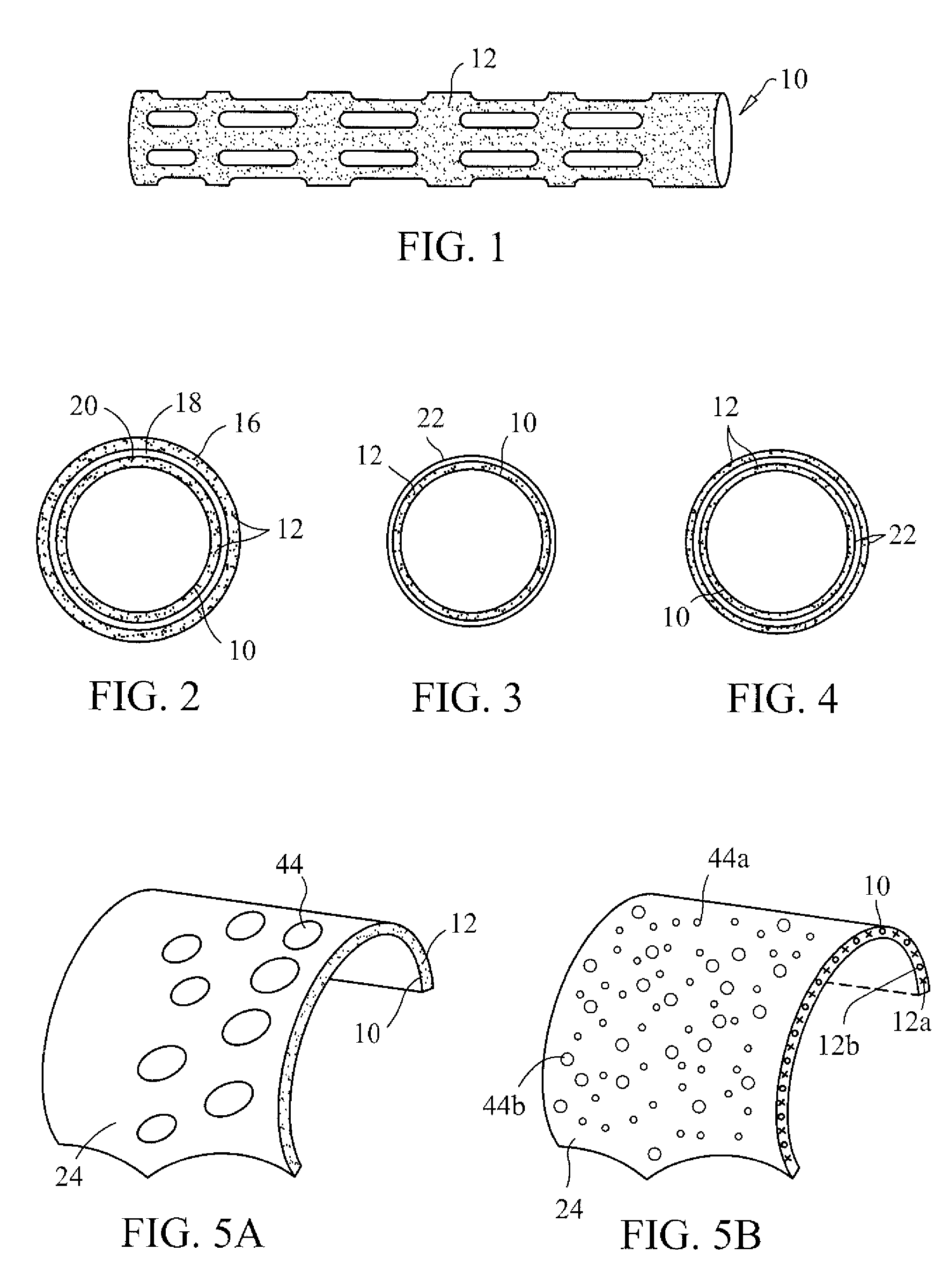
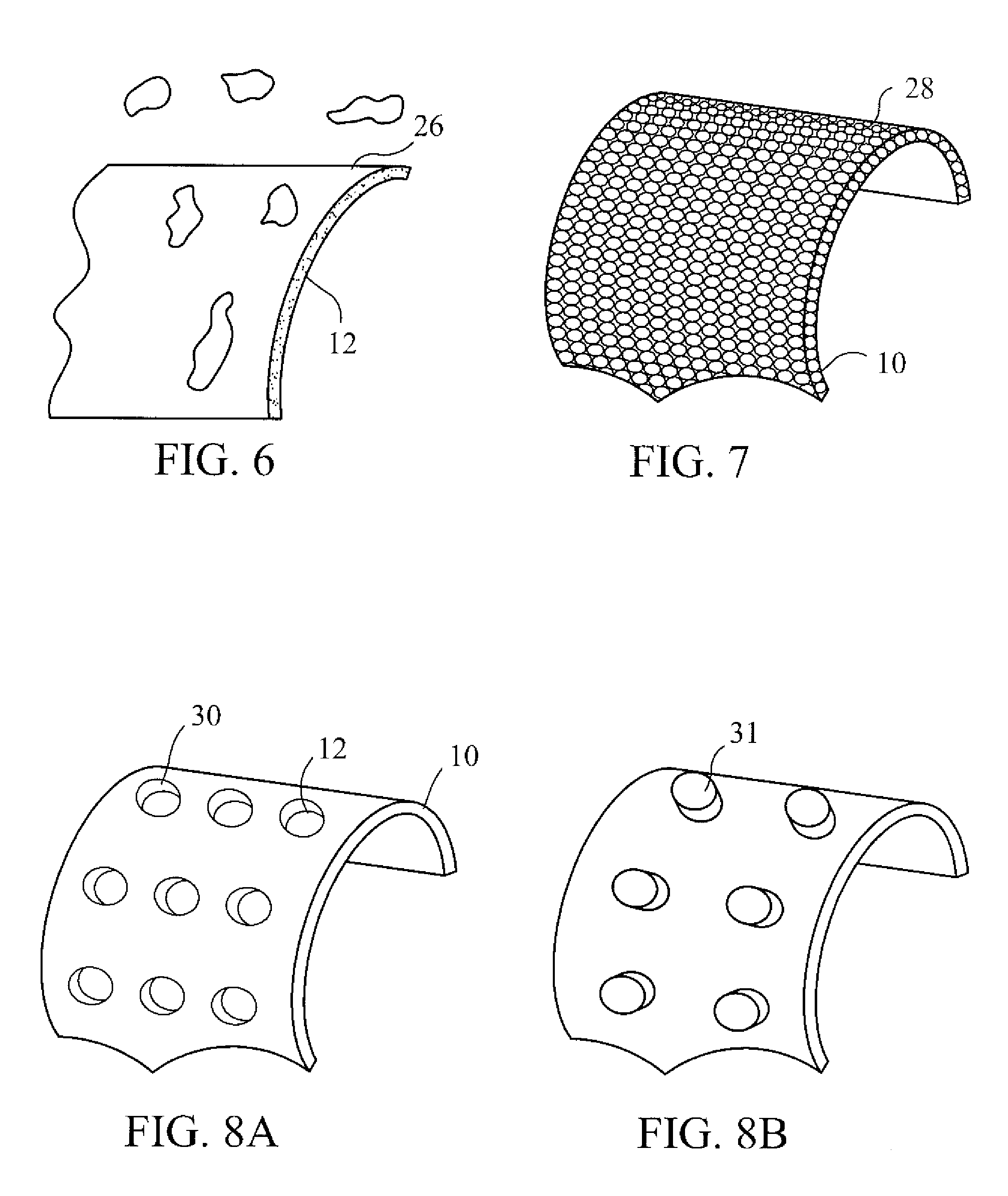
Three dimensional cell protector/pore architecture formation for bone and tissue constructs
InactiveUS7713542B2Greatly multiplied in vitroProtection from damagePowder deliveryBiocideIn vivoLiving cell
Living cellular material is encapsulated or placed in a protective material (cell protector) which is biocompatible, biodegradable and has a three-dimensional form. The three dimensional form is incorporated into a matrix that maybe implanted in vivo, ultimately degrade and thereby by replaced by living cell generated material.
Owner:ADA FOUND
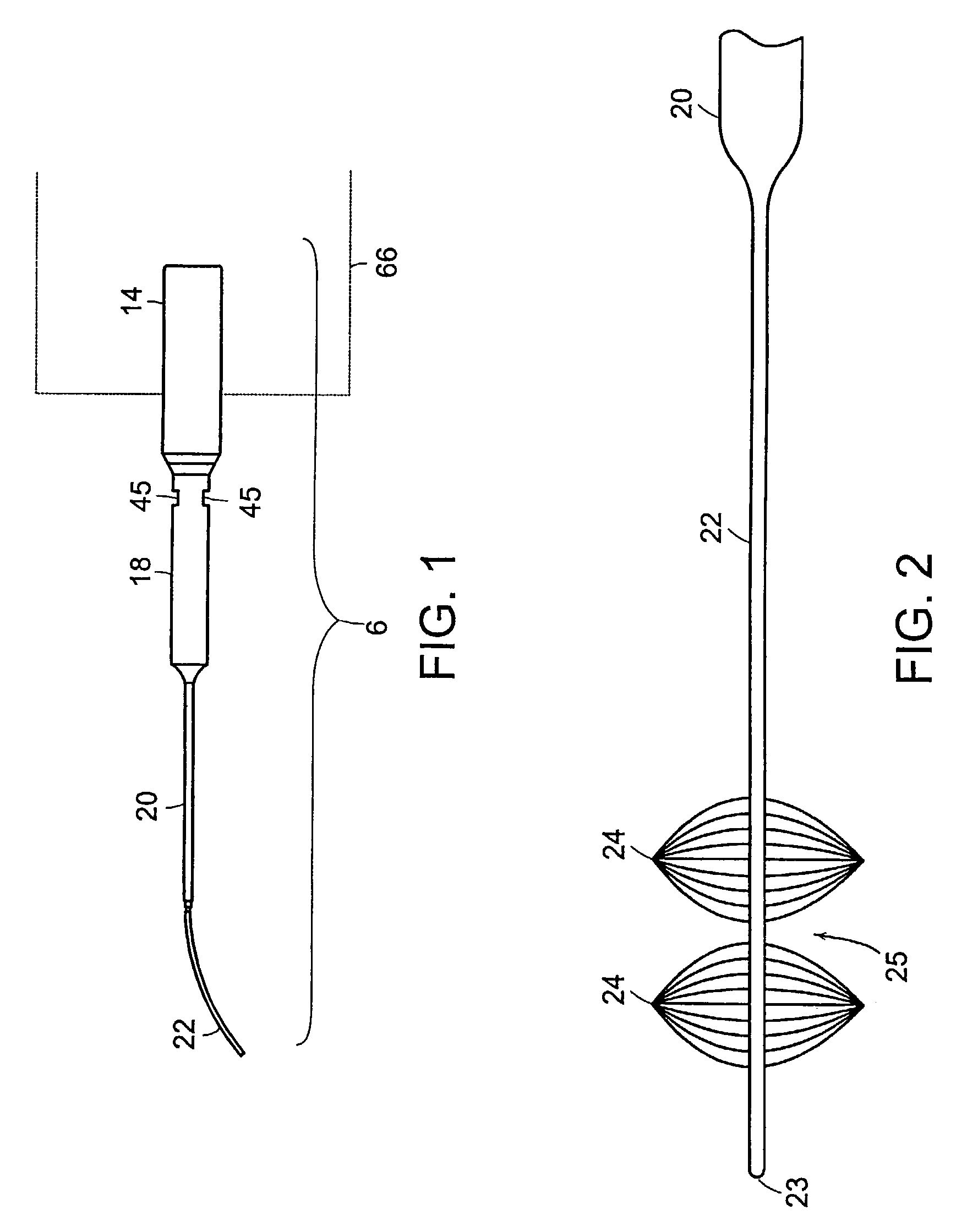
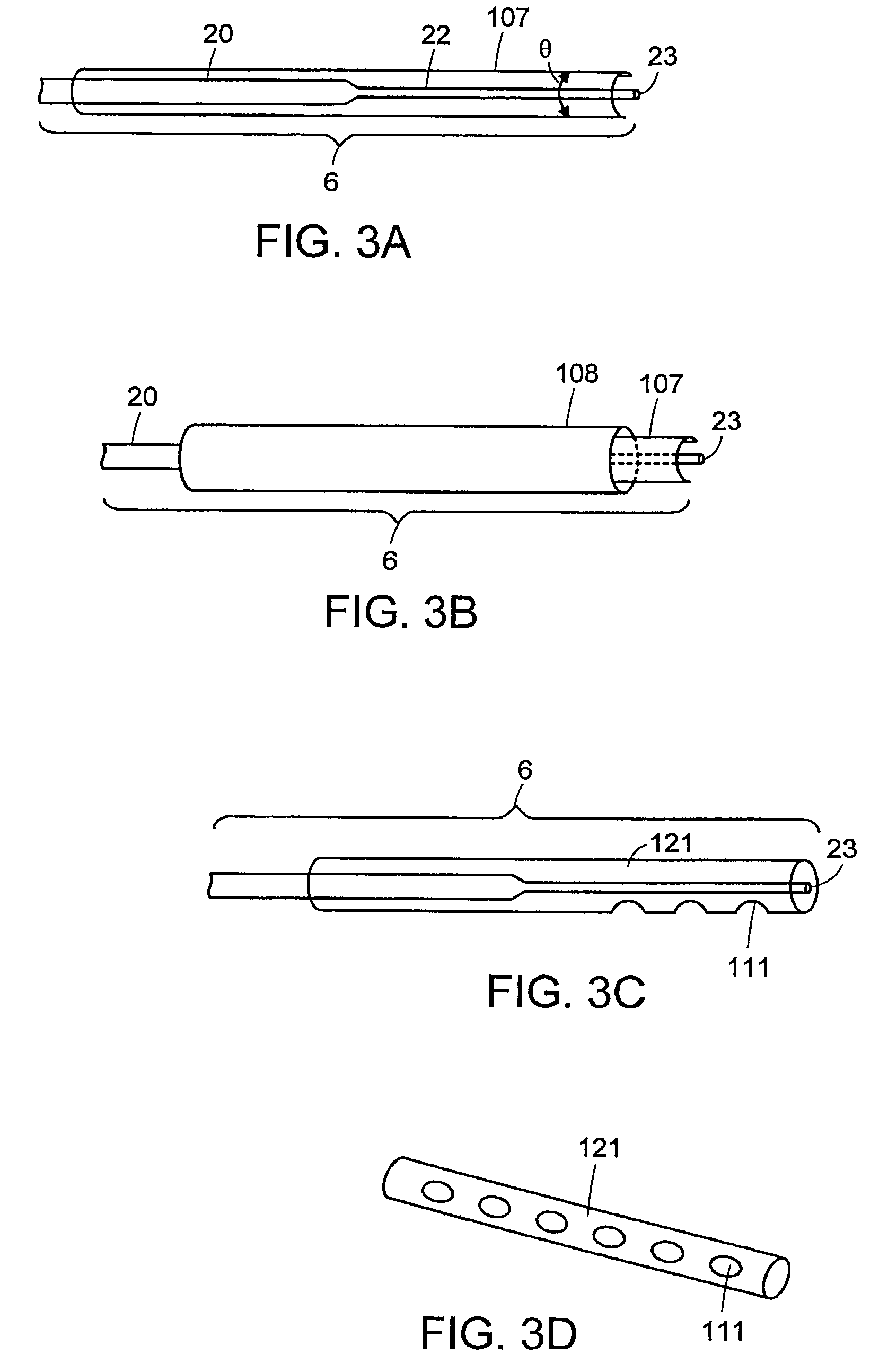
Ultrasonic device for tissue ablation and sheath for use therewith
InactiveUS7503895B2Prevent relapseImprove permeabilityUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyCavitationTransverse mode
A transverse mode ultrasonic probe is provided which creates a cavitation area along its longitudinal length, increasing the working surface of the probe. Accessory sheaths are also provided for use with the probe to enable a user to select from features most suited to an individual medical procedure. The sheaths provide acoustic enhancing and aspiration enhancing properties, and / or can be used as surgical tools or as medical access devices, protecting tissue from physical contact with the probe.
Owner:CYBERSONICS
Resorbable matrices for delivery of bioactive compounds
This invention relates generally to the production and use of inorganic-conditioning agent complexes for the controlled release of compounds including medicinals. Advantageously, the inorganic used is calcium sulfate and the conditioning agent is calcium stearate.
Owner:ROYER BIOMEDICAL INC
Novel drug delivery system
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Biodegradable implantable medical devices, methods and systems
InactiveUS20060018948A1Risk minimizationMinimize activitySurgeryProsthesisMedical deviceBiomedical engineering
Owner:SURMODICS INC
Medical devices comprising spray dried microparticles
InactiveUS20050037047A1Premature degradationControl drug releaseSurgeryCatheterBiomedical engineeringMedical treatment
An implantable or insertable medical device which includes (a) a tacky polymeric region and (b) spray dried microparticles, which are adhered to the tacky polymeric region. The present invention is further directed to methods of forming such medical devices, and methods of releasing a therapeutic agent within a patient using such medical devices.
Owner:BOSTON SCI SCIMED INC
Novel drug delivery system
InactiveUS20060018934A1Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Modified release composition for highly soluble drugs
InactiveUS8268352B2Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Drug eluting implant
InactiveUS20070141106A1Increase release rateControl releaseSuture equipmentsStentsBiomedical engineeringMedical systems
The present invention provides a medical system for the administration of a pharmaceutical agent in vivo to a patient. The medical system includes a medical implant positionable in a body of a patient. A pharmaceutical agent in disposed on the medical implant and at least partially coated with a reactive coating. The reactive coating act to controls the release of the pharmaceutical agent. An energy unit is provided for transmitting an energy signal to the reactive coating, wherein the reactive coating reacts to the energy signal to increase the release rate of the pharmaceutical agent.
Owner:P TECH
Progenitor endothelial cell capturing with a drug eluting implantable medical device
InactiveUS20050271701A1Increased formationSmooth migrationStentsHeart valvesProgenitorDrug-Coated Stents
A medical device for implantation into vessels or luminal structures within the body is provided. The medical device, such as a stent and a synthetic graft, is coated with a pharmaceutical composition consisting of a controlled-release matrix and one or more pharmaceutical substances for direct delivery of drugs to surrounding tissues. The coating on the medical device further comprises a ligand such as an antibody or a small molecule for capturing progenitor endothelial cells in the blood contacting surface of the device for restoring an endothelium at the site of injury. In particular, the drug-coated stents are for use, for example, in balloon angioplasty procedures for preventing or inhibiting restenosis.
Owner:ORBUSNEICH MEDICAL PTE LTD
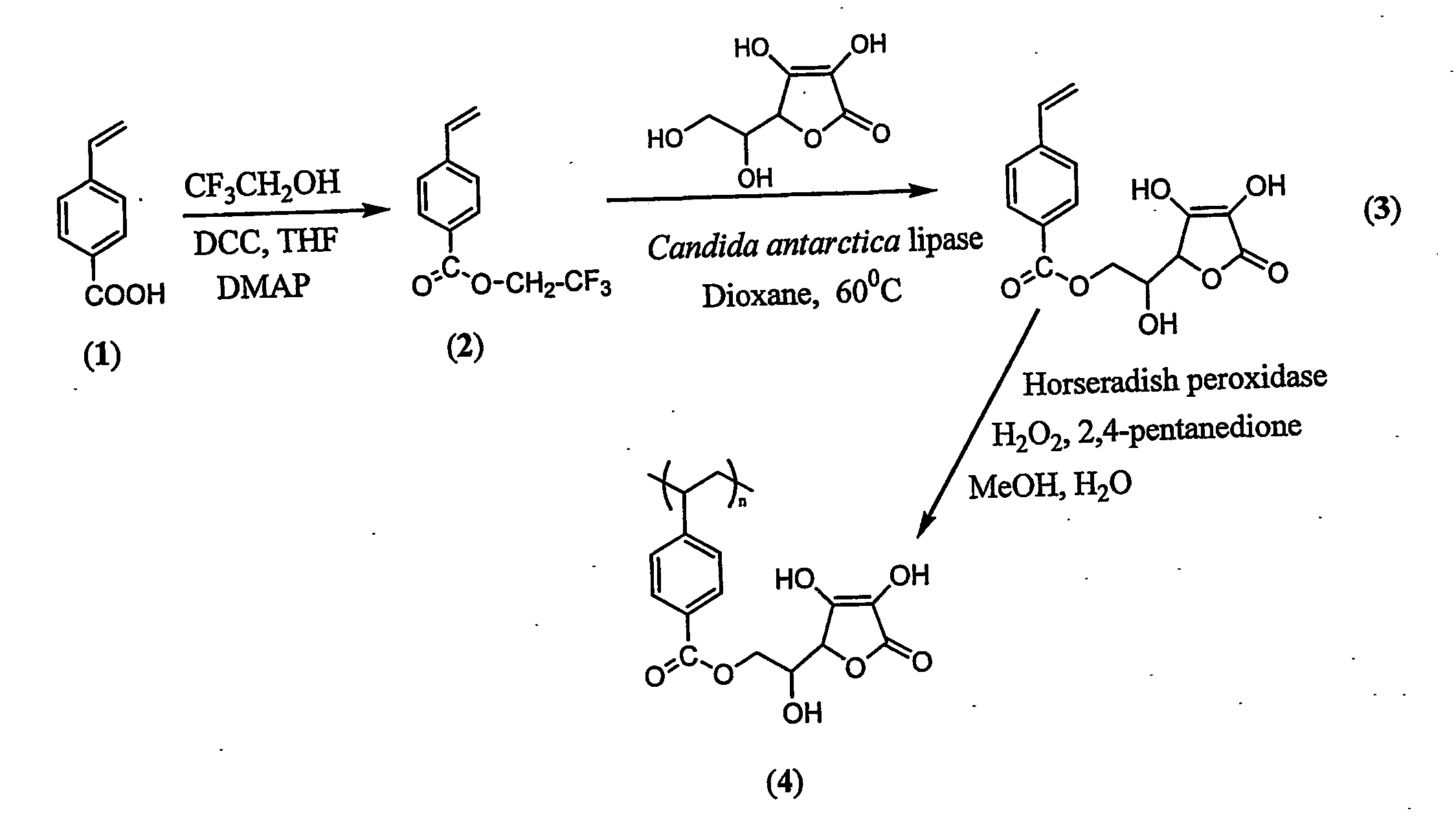
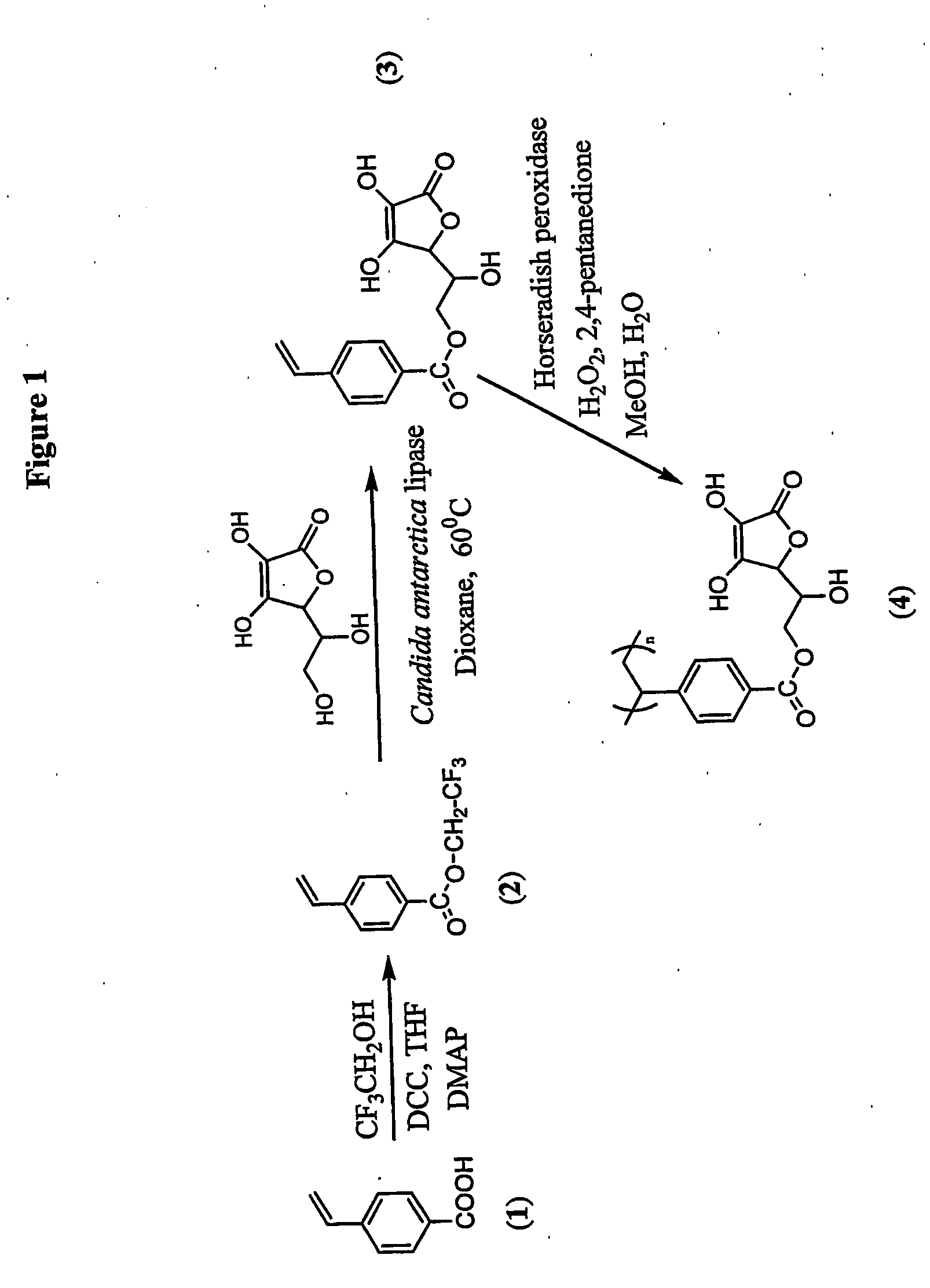
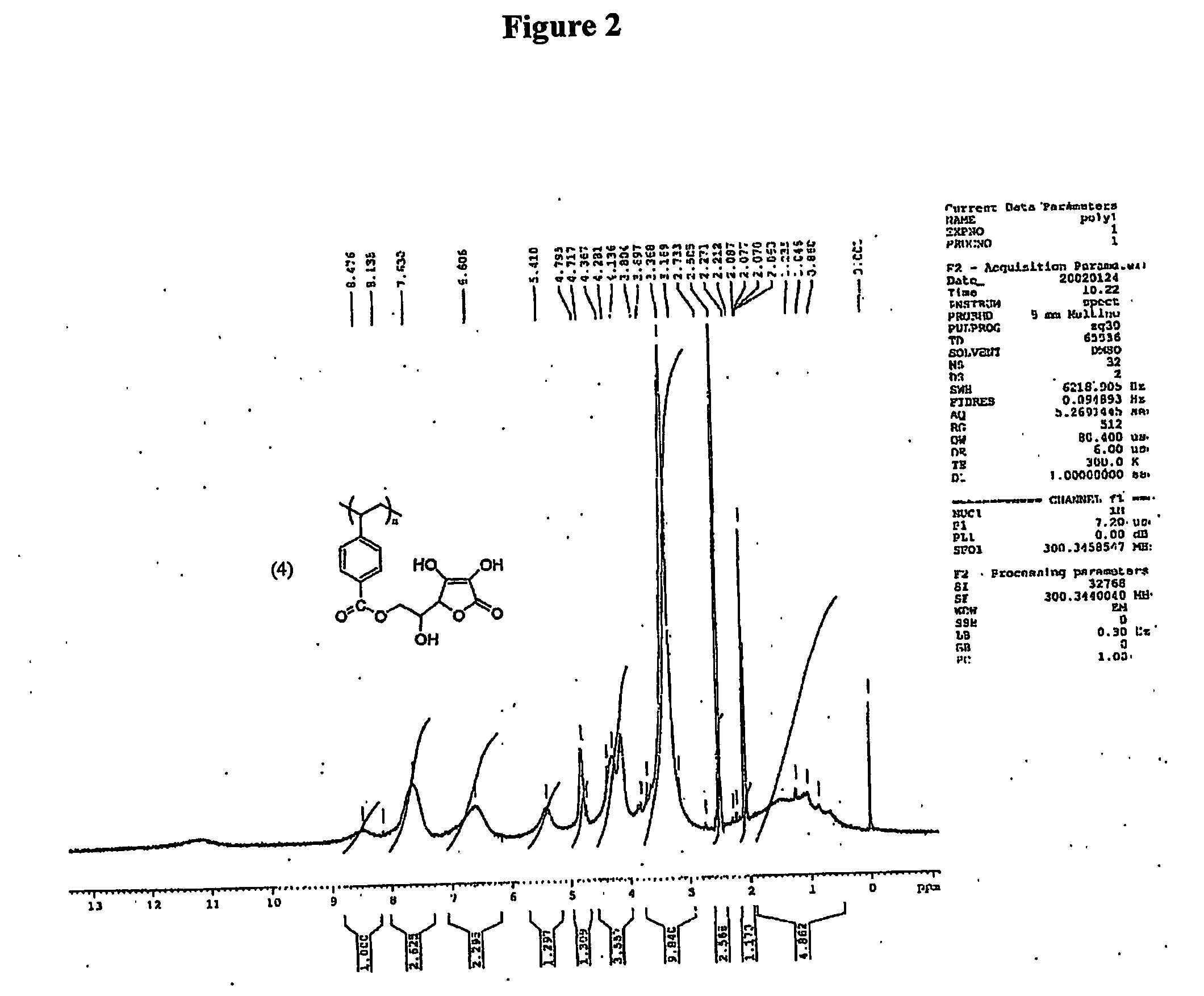
Antioxidant-functionalized polymers
InactiveUS20070010632A1Low yieldReduce sensitivitySuture equipmentsOrganic chemistryAntioxidantOxygen
Methods and compositions are disclosed for the preparation of free radical scavenging polymers and polymer films functionalized with antioxidants. Enzymatic and chemical tailoring of monomers with antioxidants followed by enzymatic polymerization is described. These antioxidant functionalized polymers can increase shelf life and quality of food products, as well as, increase effectiveness of pharmaceutical agents when used as packaging or as coatings on packaging for oxygen sensitive materials. The novel enzymatic covalent coupling of antioxidants to a polymer enhances the free radical scavenging ability of packaging while also inhibiting the escape of the antioxidants, and thus limiting exposure and / or absorption by an individual. In addition to its use in food or pharmaceutical packaging, methods are disclosed for using the antioxidant coupled polymers in a variety of applications including as coatings on the inside of medical devices, such as stents and catheters, which would substantially reduce free radical damage and / or oxygen depletion during medical procedures. Furthermore, through the coupling of antioxidants to biodegradable polymers, controlled delivery and sustained release of an antioxidant to a subject is possible.
Owner:TRUSTEES OF TUFTS COLLEGE
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Method and device for minimally invasive implantation of biomaterial
A minimally invasive method of placing a delivery device substantially adjacent to vascular tissue and a device for use with such a method are disclosed. The delivery device may be a flexible biological construct with a flexible tethering means. The delivery device may be percutaneously inserted near vascular tissue such as, for example, peritoneal tissue. When the delivery device has been inserted, the tether may be used to pull the delivery device toward the vascular tissue and secure the device thereto. Contact between the front surface of the delivery device and the vascular tissue may be maintained by making and keeping the tether substantially taut. The delivery device may serve accomplish sustained delivery of active agents.
Owner:ETHICON ENDO SURGERY INC
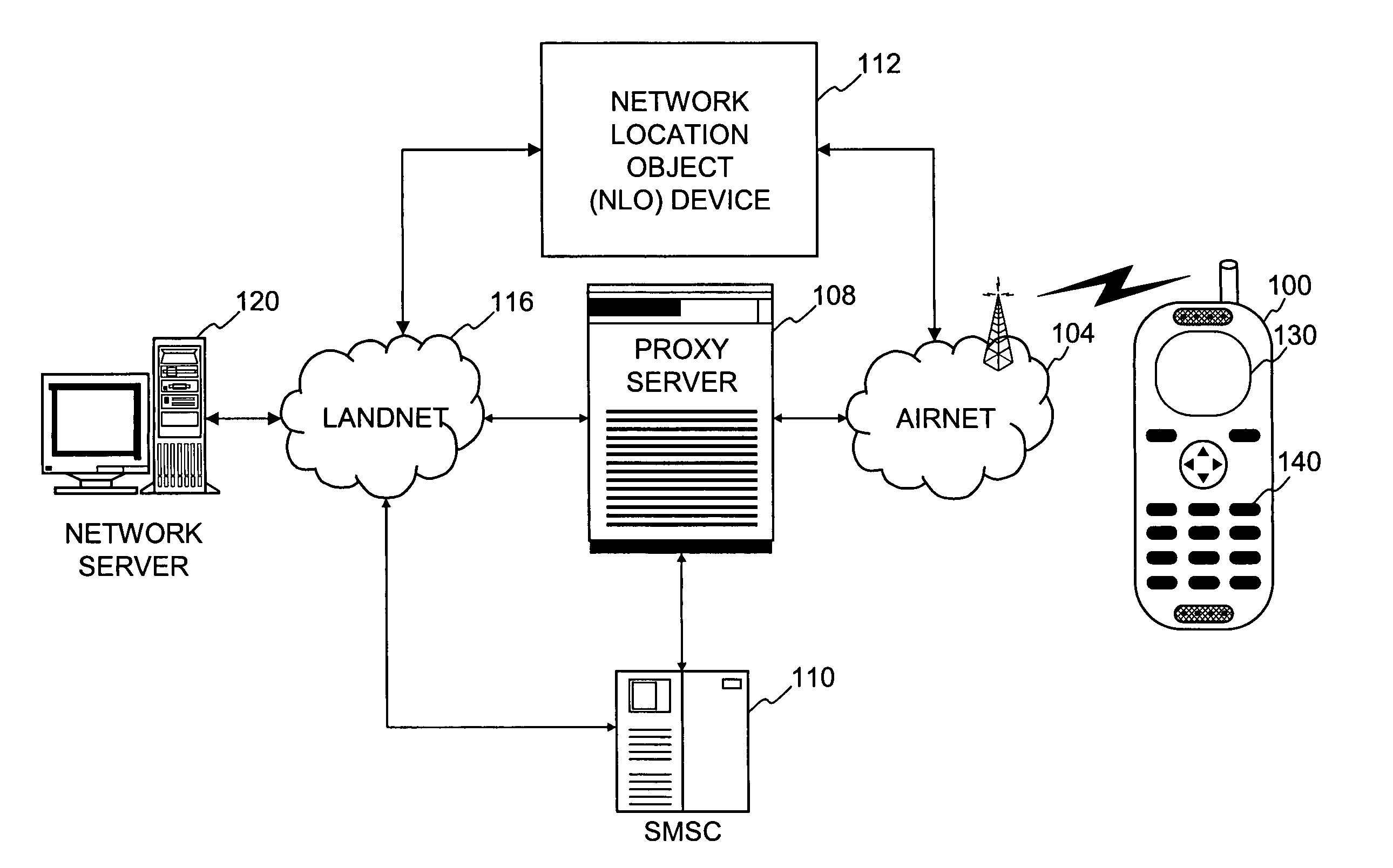
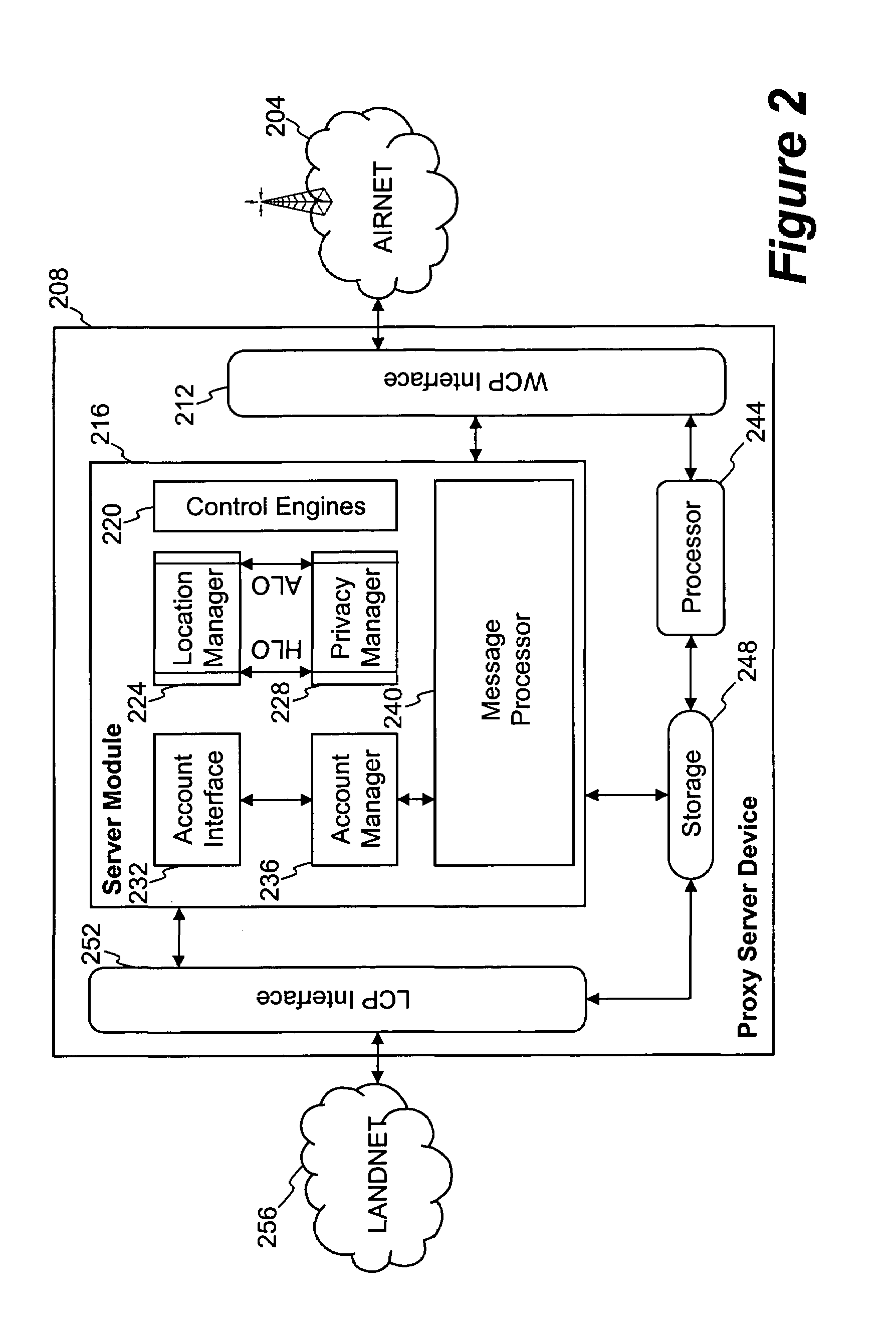
Method and system for exchanging sensitive information in a wireless communication system
InactiveUS7093286B1Control releaseEnhanced informationNetwork traffic/resource managementDigital data processing detailsCommunications systemInternet privacy
Improved techniques that enable the exchange of sensitive information between client devices and server devices are disclosed. The exchange, as well as the use and nature, of sensitive information released can be governed by one or more privacy agreements established between the principle parties, namely, a client device and a content server. A proxy server can be used to establish privacy agreements with content servers (service providers).
Owner:UNWIRED PLANET
Methods for eyeglass lens curing using ultraviolet light
InactiveUS6022498AControl generationControl releaseOther chemical processesOptical articlesUV curingRadiation pulse
Method and apparatus for making [and coating] a plastic lens is provided. [Oxygen barrier containing photoinitiator is used to cure incompletely cured lens portions. Radiation pulses are used to control lens curing rate. Lens is postcured while in a mold cavity using a conductive heat source.] More particularly, the invention relates to applying alternating periods of ultraviolet light to lens forming composition. Such composition is cured while controlling the rate of heat generation and / or dissipation via manipulation of the duration of the radiation or the cooling in the curing chamber. The ultraviolet light is directed toward the lens forming composition which is preferably disposed in a mold cavity formed by two mold members. The ultraviolet light may be directed in pulses or continuously.
Owner:Q2100
Biodegradable protrusions on inflatable device
ActiveUS20120041412A1Protection from damageFacilitated releaseStentsBalloon catheterSurgeryMedical device
A medical device for insertion and expansion in a body passageway. The medical device includes an inflatable device such as a balloon that is designed to be inflated and deflated while positioned in the body passageway. The inflatable device is inflatable by inserting a fluid in an internal cavity of the inflatable device. The inflatable device includes an outer surface that has a surface structure or micro-surface structure which is designed to at least partially penetrate into an inner wall of the body passageway when the inflatable device is inflated.
Owner:MIRUS LLC
Biodegradable ocular devices, methods and systems
InactiveUS20060024350A1Volume maximizationControl releaseSurgeryPharmaceutical delivery mechanismLimited accessActive agent
The invention provides implantable medical devices that are fabricated of biodegradable materials for delivery of bioactive agent to limited access regions of a patient's body, such as the eye. The invention further provides methods of treatment utilizing the devices.
Owner:SURMODICS INC
Methods And Apparatus For The Treatment Of Metabolic Disorders
InactiveUS20090234417A1Lower the volumeAffecting weight lossUltrasound therapyElectrotherapySmall intestineHormones production
Systems and methods are disclosed for treatment of metabolic disorders such as type 2 diabetes and obesity by stimulation of the small intestine to modulate hormone production. Methods include applying an appropriate signal to a region of the small intestine to modulate hormone production. The method may involve applying a signal to the nerves that innervate the small intestine. Devices for delivering the signal are disclosed.
Owner:ELECTROCORE
Cannabinoid active pharmaceutical ingredient for improved dosage forms
Pharmaceutical compositions comprising the cannabinoid active pharmaceutical ingredient, crystalline trans-(±)-Δ9-tetrahydrocannabinol, and formulations thereof are disclosed. The invention also relates to methods for treating or preventing a condition such as pain comprising administering to a patient in need thereof an effective amount of crystalline trans-(±)-Δ9-tetrahydrocannabinol. In specific embodiments, the crystalline trans-(±)-Δ9-tetrahydrocannabinol administered according to the methods for treating or preventing a condition such as pain can have a purity of at least about 98% based on the total weight of cannabinoids.
Owner:SVC PHARMA
Porous silica having substance carried thereon
InactiveUS20070003492A1Improve adsorption capacityMild desorbabilityCosmetic preparationsBiocideMentholSilicon dioxide
The present invention relates to a substance-supporting porous silica, wherein a porous silica supports a substance selected from the group consisting of menthols, volatile substances, thermal substances, plant polyphenols and organic colorants.
Owner:TAIYO KAGAKU CO LTD +1
Black-odor river pollution treatment method
InactiveCN101417840AEfficient removalControl and reduce the total amount receivedTreatment using aerobic processesWater aerationMicrobial agentSelf purification
The invention discloses a method for managing town black and olid river pollution in situ by utilizing biological repair technology, which takes the steps and measures of aerating and oxygenizing in riverway, adding a complex microbial agent, biologically repairing bottom sediment, arranging a bio-nest system and the like, so as to control and eliminate the exogenous and endogenous pollution of the river, purify the water quality of the river, improve the self-purification ability of the water body and achieve the purpose of eliminating black and olid. The method does not need to build structures in the riverway or lead the river water out of the riverway to be treated, or dredge the bottom sediment in the river, therefore the method is generally applicable to incomplete sewage interception and dredging or the rivers which can not be intercepted sewage or dredged temporarily, and the in situ treatment to the riverway which is affected by tide and has complicated fluid variation. Therefore, the method which can lead the water body to recover normal ecological function provides the practical, economic and convenient treatment method for various town black and olid rivers.
Owner:黎赓桓 +1
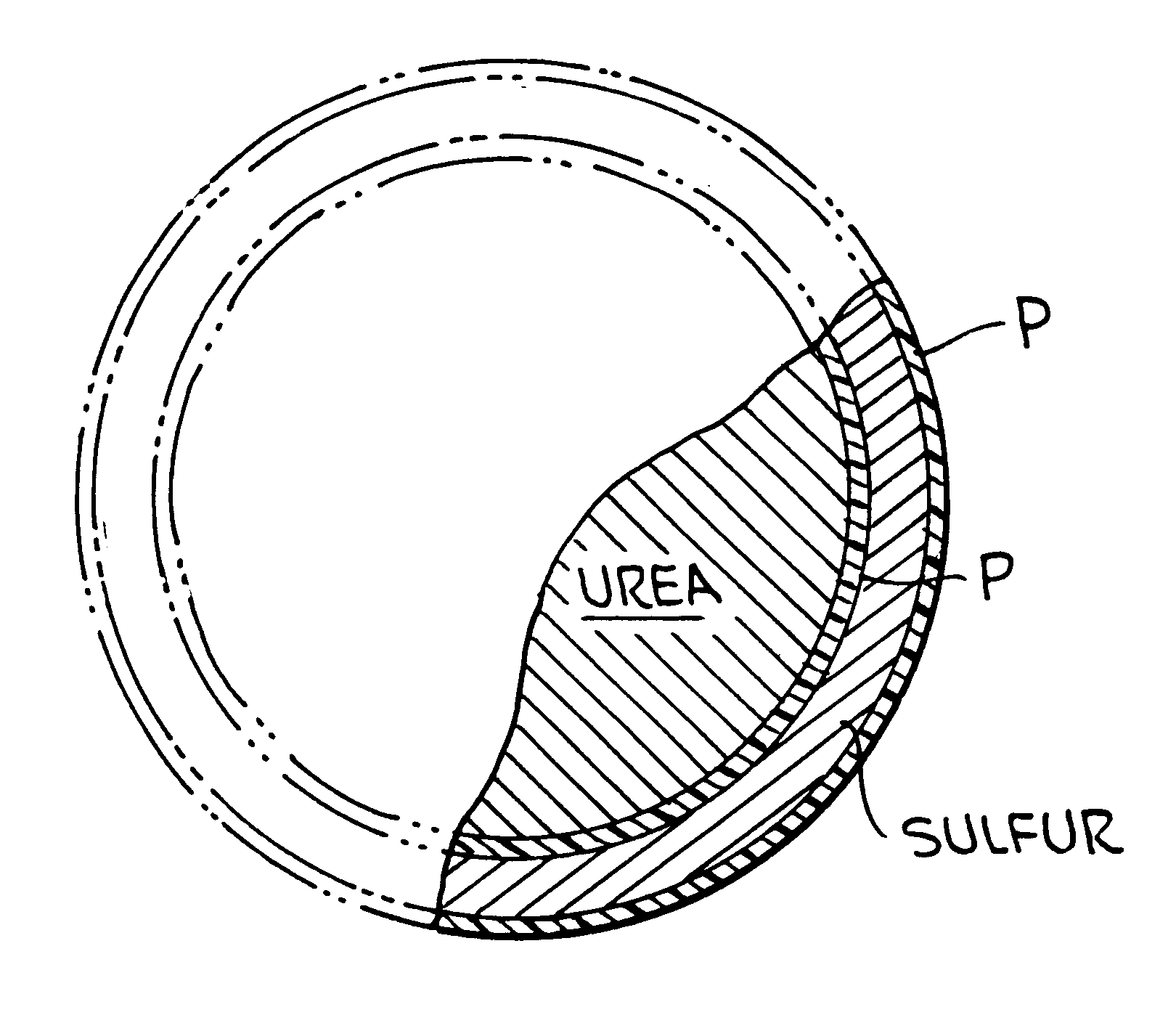
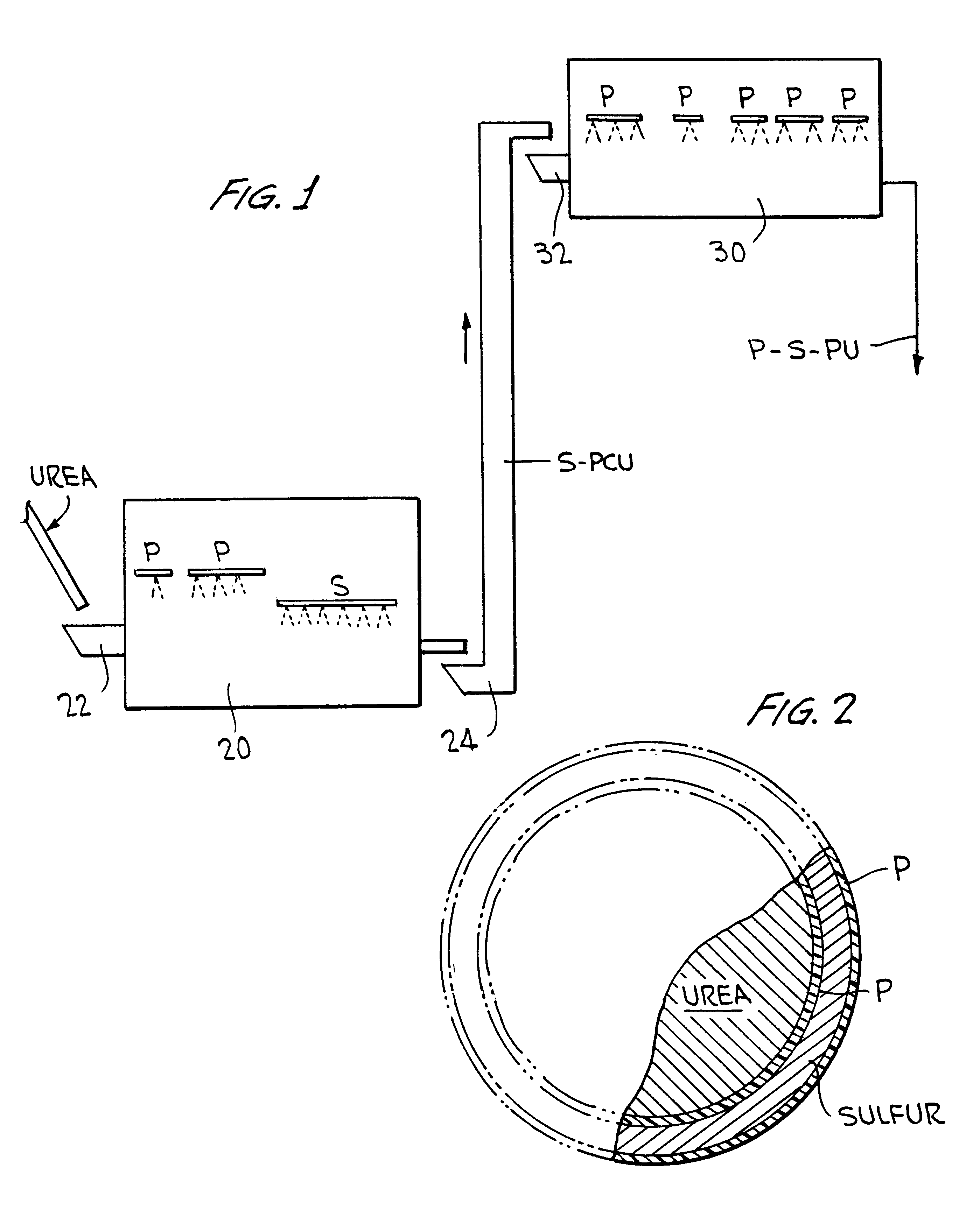
Polymer-sulfur-polymer coated fertilizers
InactiveUS6338746B1Good resistance to abrasionAvoid serious impactBiocideMaterial granulationControlled releasePositive control
The present invention described a polymer coated fertilizer, such as urea, subsequently coated with a layer of sulfur and thereafter a further coating of polymer. Preferably, the polymer coatings are formed by the direct in situ co-polymerization of the components of the polymer on the fertilizer and on the sulfur coating. The compositions provide positive controlled release characteristics, are abrasion and impact resistant and are substantially more economical to produce than polymer coated fertilizers.
Owner:KOCH AGRONOMIC SERVICES LLC
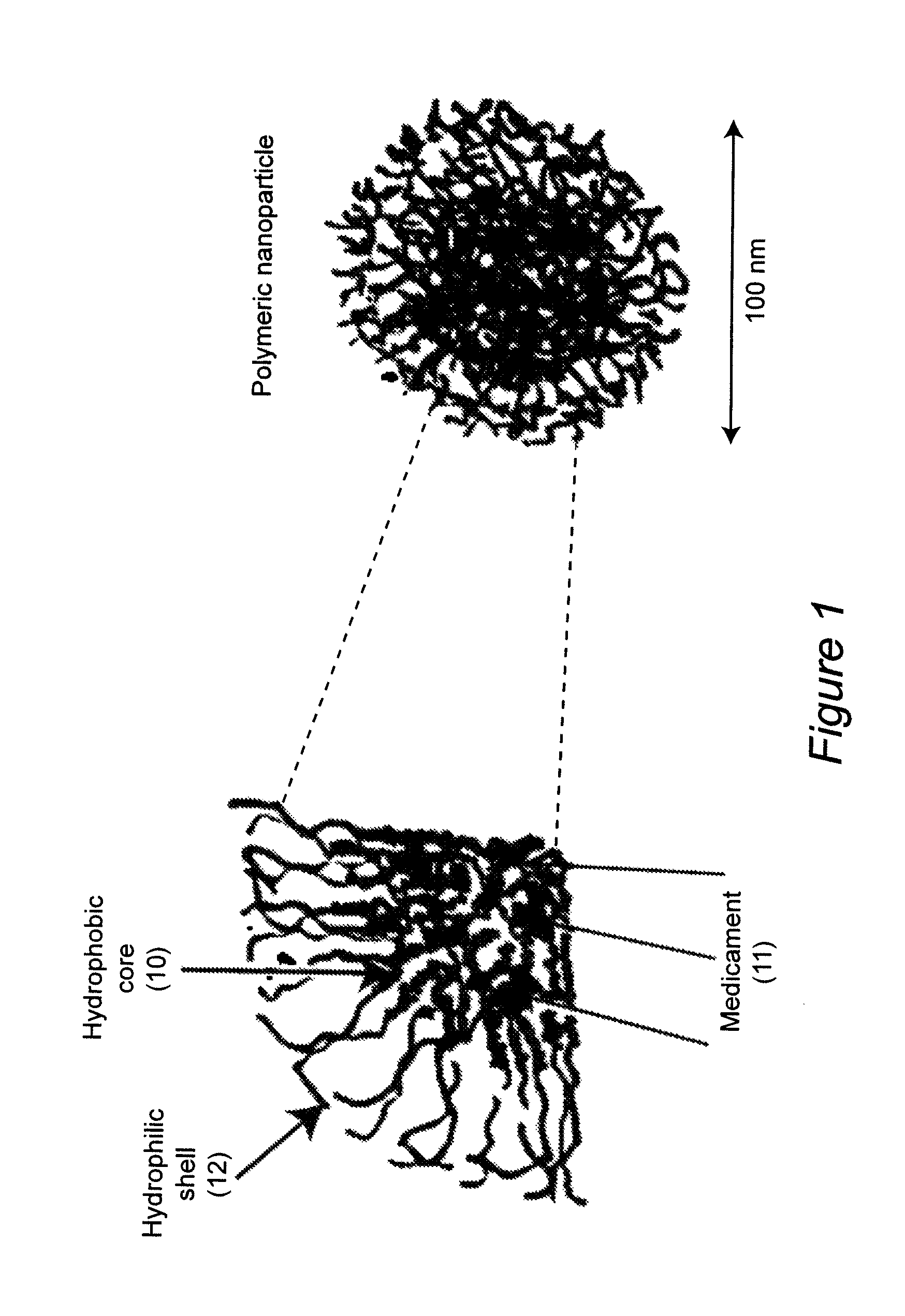
Water-dispersible oral, parenteral, and topical formulations for poorly water soluble drugs using smart polymeric nanoparticles
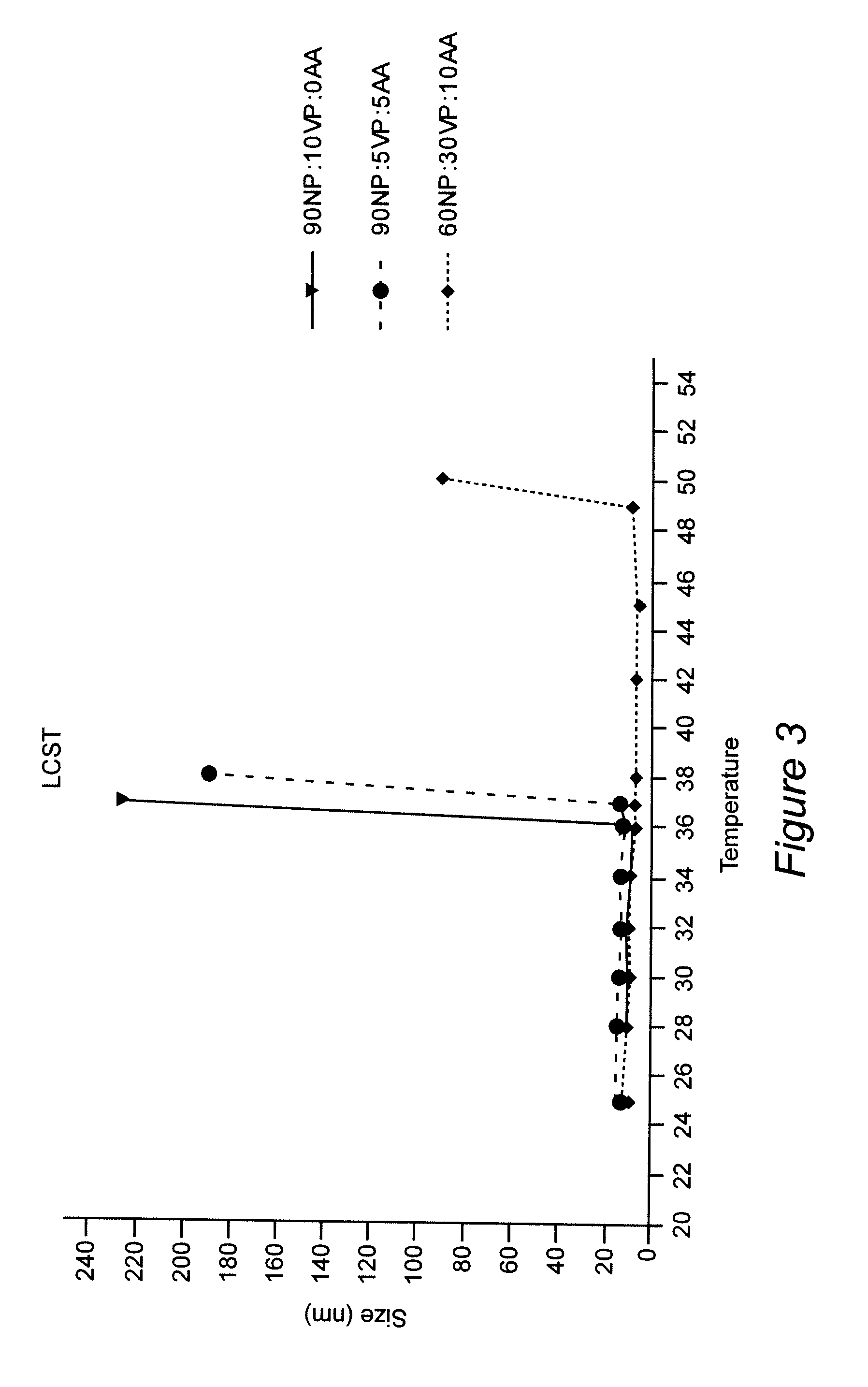
ActiveUS8313777B2Control releaseImprove bioavailabilityPowder deliveryNervous disorderWater dispersibleSmart polymer
Polymeric nanoparticles with a hydrophobic core and a hydrophilic shell are formed from: 1) N-isopropyl acrylamide (NIPAAM), at a molar ratio of about 50% to about 90%, and preferably 60% for specific delivery routes such as oral or parenteral; either water-soluble vinyl derivatives like vinylpyrolidone (VP) or vinyl acetate (VA), or water insoluble vinyl derivatives like methyl methacrylate (MMA) or styrene (ST), at a molar ratio of about 10% to about 30%; and acrylic acid (AA), at a molar ratio of about 10% to about 30%. The formed nanoparticles may be optionally surface functionalized using reactive groups present in AA, including PEGylation, or conjugation of moieties such as chemotherapeutics, contrasting agents, antibodies, radionucleides, ligands, and sugars, for diagnostic, therapeutic, and imaging purposes. The polymeric nanoparticles are preferably dispersed in aqueous solutions. The polymeric nanoparticles incorporate one or more types of medicines or bioactive agents in the hydrophobic core; on occasion, the medicine or bioactive agent may be conjugated to the nanoparticle surface via reactive functional groups. The polymeric nanoparticles are capable of delivering the said medicines or bioactive agents through oral, parenteral, or topical routes. The polymeric nanoparticles allow poorly water soluble medicines or bioactive agents, or those with poor oral bioavailability, to be formulated in an aqueous solution, and enable their convenient delivery into the systemic circulation.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Granular fertilizer coated with decomposable coating film and process for producing the same
A coated granular fertilizer which comprises a degradable film containing 10% by weight or more of a polyolefin and / or petroleum wax having a weight-average molecular weight of 300-10,000 and an inorganic filler and / or a surface active agent and a granular fertilizer coated with said degradable film.
Owner:JCAM AGRI
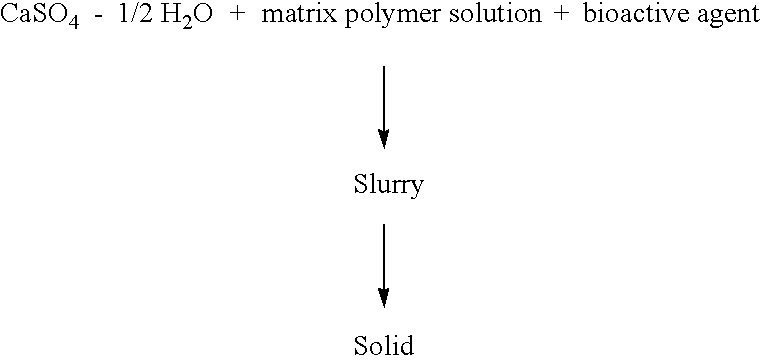
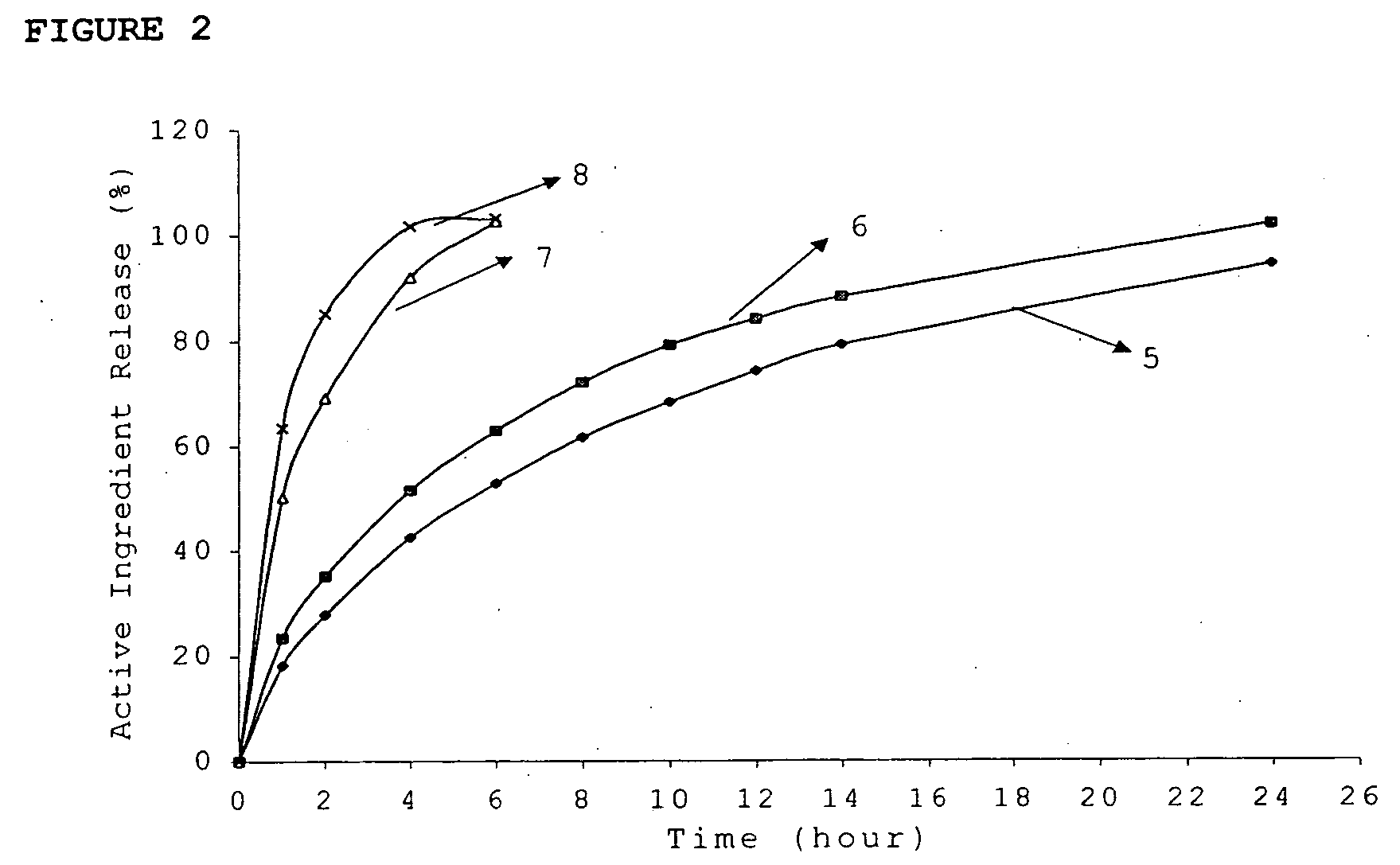
Inorganic-polymer complexes for the controlled release of compounds including medicinals
InactiveUS6391336B1Control releasePowder deliveryPeptide/protein ingredientsControlled releaseActive agent
This invention relates generally to the production and use of inorganic-polymer complexes for the controlled release of compounds including medicinals. The inorganic compound used is advantageously calcium sulfate-hemihydrate. The invention includes a composition for the controlled release of an active agent comprising: a) a hydrated or crystallized inorganic compound, and b) a matrix polymer which slows the release of the active agent, wherein the composition is a solid matrix due to the hydration or crystallization of the inorganic compound. Further included is a composition for the controlled release of an active agent comprising: a) a hydrated or crystallized inorganic compound, and b) a complexing agent which forms a salt or conjugate with the active agent, wherein the composition is a solid matrix due to the hydration or crystallization of the inorganic compound.
Owner:ROYER BIOMEDICAL INC
Controlled degradation magnesium alloy coating bracket and preparation thereof
InactiveCN101214396AImprove mechanical propertiesExcellent pharmacological propertiesAnodisationStentsSurface cleaningPolymer chemistry
The invention relates to a controlled degradation magnesium alloy coating stent and a preparation method. The stent body is made of medical high purity magnesium or magnesium alloy by mechanical processing or laser carving; the stent body is provided with a drug-loading coating which bears curative drug; the surface of the stent body is provided with an anti-corrosive coating; the surface of the anti-corrosive coating is provided with a degradable polymer film drug-loading coating; the preparation method includes surface cleaning, preparation of the degradable polymer film drug-loading coating, and application of curative drug; through (1)surface cleaning, (2)preparation of the degradable polymer film drug-loading coating, and (3)application of curative drug, an oxide film is formed on the surface; different drugs and dosage can be fixed by regulating the molecular weight and the thickness of the polymer layer, the drug-loading quantity is more than 30 percent, which improves the fixed stability of the drug, greatly reduces the degradation speed of the magnesium alloy and controls release of the drug, delays corrosion of the magnesium alloy, extends the service life of the stent, is safe in use, and meets the clinical requirement.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Misuse Preventative, Controlled Release Formulation
ActiveUS20090175937A1Control releasePowder deliveryOrganic active ingredientsActive agentMicroparticle
Disclosed is a misuse preventative, controlled release formulation comprising a core comprising a superabsorbent material (for example, polycarbophil), a controlled release coat surrounding the core, and a plurality of controlled release microparticles having a pharmaceutically active agent (for example, an opioid analgesic) disposed within the core, the coat, or both the core and the coat. When crushed, either intentionally or accidentally, and exposed to an aqueous medium, the superabsorbent material present in the core swells to encapsulate the microparticles, which remain substantially intact thereby retarding the release of the pharmaceutically active agent from the formulation. Also disclosed is a method of using the misuse preventative, controlled release formulation to deliver a pharmaceutically active agent to a mammal, for example, a human, in need thereof.
Owner:LABOPHARM BARBADOS LTD 36646
Biodegradable coating compositions including multiple layers
InactiveUS20060147491A1Avoiding toxic levelRate of releaseSurgeryCoatingsBiodegradable coatingBiomedical engineering
The invention provides devices for treatment of a patient, wherein at least a portion of the device is provided with a biodegradable coating composed of multiple coated layers of biodegradable material. The invention further provides methods of treatment utilizing the devices.
Owner:SURMODICS INC
Non-ionic non-aqueous vehicles for topical and oral administration of carrier-complexed active agents
InactiveUS20070036843A1Control releaseLow costOrganic active ingredientsPharmaceutical non-active ingredientsActive agentNon ionic
An improved controlled release composition for non-parenteral administration of active agents and other therapeutics, particularly for oral or topical administration, has been developed. The composition is made by dispersing a complex formed of an active agent bound to an ion-exchange resin or to another form of resin or carrier, in a non-ionic non-aqueous (“NINA”) vehicle. The complexes are optionally coated with one or more layers of coating material to provide a controlled pattern of release of active agent from the carrier. Replacing the usual aqueous vehicle with a NINA vehicle, such as an oil or an ointment, allows the active agent-carrier complexes, with or without coatings, to be both orally and topically administered. The compositions can be formulated as powders, liquids, liquid suspensions, gels, capsules, soft gelatin capsules, tablets, chewable tablets, topical ointments, lotions, pourable or pumpable fluids, semisolid, crushable tablets, and unit-of-use sachets or capsules for reconstitution or direct application. The combination of multiple active agents is possible with this system, in which one or more active agents are bound to particles and one or more active agents are dissolved or dispersed in the NINA vehicle. This allows the combination of two or more active agents, which are otherwise incompatible, into a single dosage form.
Owner:COLLEGIUM PHARMA INC
Methods and apparatus for eyeglass lens curing using ultraviolet light and improved cooling
InactiveUS6086799AControl generationControl releaseOther chemical processesOptical articlesUltraviolet lightsRadiation pulse
Method and apparatus for making and coating a plastic lens. Oxygen barrier containing photoinitiator is used to cure incompletely cured lens portions. Radiation pulses are used to control lens curing rate. Lens is postcured while in a mold cavity using a conductive heat source. Air may be directed toward the mold cavity to help remove heat from the lens. An in-mold scratch resistant coating may be formed from two separate material which both contain a photoinitiator.
Owner:Q2100
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com